Vibronic Coupling in Quantum Wires: Applications to Polydiacetylene
|
|
|
- Gerald Bond
- 5 years ago
- Views:
Transcription
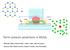
1 Vibronic Coupling in Quantum Wires: Applications to Polydiacetylene An Exhaustively Researched Report by Will Bassett and Cole Johnson Overall Goal In order to elucidate the absorbance spectra of different polymer semiconductors such as Polydiacetylene (PDA), Yamagata and Spano have put forth a theoretical study including not only the electronic transitions, but also their coupling to vibrational modes to yield vibronic excitons 1. This requires determination of the one dimensional direct band gap energy and structure. These polymers are long linear chains that are treated as quantum wires, simplifying calculations by reducing them to one dimensional problem analogous to the one dimensional particle in a box. Using the 2-MO approximation, to be described later, simplifies the calculations more as well as improves cost. The main focus of this study is to model the vibronic excitons and determine the energy loss from coupling a vibrational mode to an electronic mode for energy dissipation. In this paper the model for Wannier-Mott excitons was improved using the multi-particle basis set for Frenkel excitons, including the charge transfer states, and incorporating linear vibrational modes. From an emission model, the ratio of intensities of the first two vibronic bands formed from an exciton collapsing back into its hole and producing a photon provides information about exciton coherence as well as the effective mass. To further understand the quantum chemistry involved in the described paper, we will detail many quantum phenomena including: excitons, the 2-MO approximation, the Hamiltonian constructed for this model, the basis sets used, the absorption and emission models, the line strength ratio, phonons, k space, and aspects of vibronic coupling in general. Exciton In the simplest description, an exciton is the particle created upon exciting an electron to form an electron-hole pair. The Frenkel model dictates that the electron-hole pair is constrained to the same unit cell where the HOMO/LUMO of each unit cell is weakly coupled to neighboring HOMO/LUMOs. Another way to describe this is to define the Bohr radius of a Frenkel exciton as smaller than the lattice spacing in a crystal where the Bohr radius of an exciton is the average distance between the electron and hole. The coupling of the exciton between two adjacent unit cells is defined as the off-diagonal Hamiltonian elements of each HOMO/LUMO to adjacent HOMO/LUMOs and so a weak coupling ensures the orbitals are far enough apart spatially to prevent the electron from hopping to another unit cell. Conversely, the Wannier-Mott model for an exciton allows for electron-hole hopping to other unit cells, further and further away depending on the magnitude of the transfer integral to be described later. The Bohr radius of Wannier-Mott excitons is therefore on the same order or larger than the lattice spacing, which allows coupling. The two models for excitons are shown in figure 1. In the present study an intermediate coupling Figure 1 Left: An electron-hole pair confined to a single unit-cell. Middle and Right: An electron-hole pair in a charge seperated state with the hole and electron at middle and right respectively.
2 strength is used for the Wannier-Mott exciton and weak coupling for the Frenkel exciton with these strengths to be described later. The simplest exciton is purely electronic in that the formation of an electron-hole pair is simply the excitation of electron from a ground state to an excited electronic state. More correctly, excitons can couple to nuclear vibrations in vibronic transitions, thus removing the applicability of the Born-Oppenheimer approximation. The vibronic transitions allow for non-adiabatic relaxations from excited states to both the ground vibrational and electronic state. Vibronic Transitions The reason we must take into account the coupling of the vibrational and electronic modes of these molecules is that for conjugated polymers, electronic transitions can result in large molecular shifts. Consider the example given in figure 2 for polyacetylene, where the electronic transition from the ground state to the first excited state effects the pi-electron density, and thus shifts the molecule 2. These shifts alter the overlap of the nuclear potential energy surfaces between ground and excited states, which in turn changes the way electronic transitions occur. When this potential shifts, the probability for each electronic transition shifts due to the ability for vertical transitions between ground state vibrational modes of excited states and the ground state to occur, as will be described later in the absorption and emission model section. Thus the vibrational modes of the polymer crystal strongly affect the electronic transitions. For electrons to relax from the excited electronic state, the entire crystal must have a collective vibration to return to ground state geometry, known as a phonon. This coupling of the relaxation to a phonon is what makes it a vibronic transition. 2-Molecular Orbital Approximation Figure 2 Electronic effects on the geometry of polyacetylene. In the model used, a polymer is treated as a one-dimensional semiconductor in which each unit cell is represented by only it s HOMO and LUMO as in figure 3. This approximation is known as the two-molecular-orbital approximation 3 and is used here to greatly simplify calculations. The dominant excitonic transition will be long axis polarized therefore the two-state model will include all necessary components. In principal what this means is that out of the two excitons created, the 1 1 B u - and the 1 A g +, the 1 1 B u - exciton is the only significant contributor due to being created along the axis of the molecule. In solids the most reasonable HOMO and LUMO to use Figure 3 HOMO and LUMO for each unit cell.
3 for each unit cell are not the typical MOs, but rather Wannier functions, which are crystal orbitals. This function includes an extra phase factor to account for the periodicity of the crystal as described in the section on k-space. Using only the HOMO and LUMO makes intuitive sense because the excitation from HOMO to LUMO is the lowest energy and most probable transition. Model Frenkel Hamiltonian The simple Hamiltonian for describing a Frenkel exciton is constructed by simplifying the more complex Hamiltonian describing a Wannier-Mott exciton. The Wannier-Mott Hamiltonian is made up of the summation of an electronic and vibrational component. The electronic Hamiltonian is given in equation 1: (1) where c and d represent the creation and annihilation operators for the hole electron and hole respectively, where the double dagger notates a creation operator and the lack of double dagger is the annihilation operator and t represents the transfer integral. The h.c. in the first summation stands for higher corrections such as the component given by separating the electron and hole more than a single unit cell. The transfer integrals give the overlap between each HOMO and LUMO and its adjacent orbital and give a measure of the energy required for an exciton to jump from one orbital to another and is given by equation 2: (2) where Ĥ is the full Hamiltonian. The values of the transfer integral divide the interactions between unit cells into one of two classifications: weak and intermediate coupling. The weak coupling is defined as when the transfer integral is much less than the potential difference between the unit cell where the exciton resides and neighboring unit cells, and the intermediate coupling is when the transfer integral is roughly equal to the same potential difference as elucidated in equation 3. { (3) The potential interaction in the polymer crystal is given in equation 4: { (4) where U > 0, V 1 > 0, and U > V 1, U is the potential within the unit cell, and V 1 is related to the dielectric constant of the material. Thus e e + e h + V(0) is the energy required to create the electron/hole pair in a unit cell. At V(0) the terms in the second summation simply become the potential within unit cell n times the number operator used to count the electrons and holes in that unit cell.
4 To describe the number operator which is defined as the product of creation and annihilation operators,, we use the harmonic oscillator for an example in equations 5-7: (5) (6) (7) where clearly the result is counting the number of vibrational quanta in this case, or in our case counting the electrons and holes. The basis sets used herein only ever involve the creation of one electron/hole pair and so the number operator always equals one. The vibrational component of the Hamiltonian is given in equation 8 (note: ħ=1). { ( ) } { ( ) ( ) } (8) The symmetric vinyl stretching mode is the only considered mode and has a frequency of ω 0 = 1390 cm -1. The new operators represented with b are associated with the creating and destroying a vibrational quanta in the ground state nuclear potential well corresponding to the absence of electrons and holes in unit cell n. The second term in the summation corresponds to the nuclear stretch when an electron/hole pair is created in nth unit cell (The number operators on the end ensure this, acting as Dirac delta functions) and the third term summation accounts for the same stretching mode when in a charge separated state. The stretches are characterized by Huang-Rhys (HR) factors associated with the neutral unit cell, cationic unit cell, and anionic unit cell given by λ 0, λ +, λ - respectively as shown in figure 4. All nuclear potentials are defined to be harmonic wells with symmetric and equal curvature. The shifts are parameterized to account for nuclear shifts and correspond to experimental values calculated from the vinyl stretching mode. The parenthetical term (b ǂ n + b n ) is known as the position operator that when multiplied by the nuclear shift parameter defines shift of the nuclei in unit cell n during the excitonic transition. Figure 4 The Huang-Rhys factors and corresponding nuclear states. The much simpler Hamiltonian used to describe Frenkel excitons eliminates all components from the charge separated states and combines the electronic and vibrational Hamiltonians into a single Hamiltonian as seen in equation 9. ( ) (9) J mn is a matrix representing couplings between excitons in unit cells m and n, and the creation and annihilation operators B ǂ and B are for Frenkel excitons. The diagonal components of the J mn matrix are the energies of the excitons, and the off-diagonal elements correspond to the transfer integrals between unit cells m and n. The energies for the exciton, as stated before, are just the summation of the energies of the electron, hole, and potential V(0). The number operator again
5 makes an appearance counting both the number of vibrational quanta (b) and the number of excitons (B). The latter ensuring there is only a nuclear shift when there is actually an excitonic transition in unit cell n. Basis Set The set of basis functions used to determine band structure are a combination of five different types of basis functions as seen in Figure 5. The five types are, in order of increasing complexity: the One-Particle Frenkel- State (F-state), Two-Particle F-state, Three- Particle F-state, Charge-Separated Exciton, and Charge-Separated/ Vibrational Exciton. A particle is described as a vibronic or purely vibrational excitation. As you can see in the Figure 5 The five types of basis functions. Three-Particle F-state in figure 5, the n-1 orbital contains a vibronic exciton where the n and n+1 orbitals contain purely vibrational excitations. Similarly the charge-separated exciton contains an orbital with the electron in the ground vibrational state and the hole in a vibronic state. The F-state basis functions represent the Frenkel excitons in that the electron/hole pair is confined to a single unit cell, and the charge separated states compensate for possible Wannier-Mott behavior of the exciton where the electron can migrate from the hole. In order to use the Hamiltonian to completely describe the system, we must rigorously define these basis functions in equations (10) In the equation above we have n as the unit cell, as the number of vibrational quanta in the excited state potential (S 1 ), is the ground electronic state being operated on by creation operators to form an exciton in the same unit cell as the vibrational quanta as shown by the direct product between the electronic state and the vibrational ket. This ensures electronic and vibrational coupling since our basis functions are now a product of electronic and vibrational states. When we extend this basis set to include the vibrationally coupled exciton and one or two pure vibrational excitations, we define other unit cells p and r as well as the ground state vibrational modes ν p and ν r to generate the two and three particle F-state basis functions. (11) (12) In addition to basis functions to describe Frenkel excitons, we need to include basis functions for charge transfer states, with and without additional vibrational quanta. The vibrational quanta in charged states are in the potential states S - and S + for the electron and hole respectively. These potential states are then associated with the λ - and λ + Huang-Rhys factors respectively.
6 (13) (14) The basis sets provided here only go up to two additional vibrational quanta for F-states and one for charge separated states and so is not a complete set. The complete set would require additional basis sets containing more vibrational quanta until you have a vibrational quanta on every unit cell. The number of basis functions increases dramatically as you increase vibrational quanta by N!/(N-ν)!ν! where ν is the number of quanta and N is the number of unit cells. So even with a relatively low number of unit cells such as 100, to account for two additional vibrational quanta requires roughly a million basis functions. The use of a limited number of basis functions is validated by experimental verification that optical responses in weak to intermediately coupled systems are reproducible with high accuracy. This is due to the oscillator strength, and thus the majority of optical responses, primarily being a function of the one-particle F-state. The Hamiltonian is factorable into blocks representing each unit cell and energies of the eigenstate, k and α respectively. k represents the momentum of the crystal and α represents the electronic state. Thus the lowest energy exciton occurs in the k=0 block when α=1. Thus the eigenstate in the k th block is denoted and has energy ε kα for harmonic oscillators where the again the frequency is ω 0 = 1390 cm -1 corresponding the symmetric vinyl stretch. This exciton, as described above, is the 1 1 B u exciton. A non-zero transfer integral is associated mostly with the Wannier-Mott exciton in that the Frenkel exciton is essentially confined to a unit cell by having such a low energy transfer integral relative to the potential between wells, but this does not completely bar a Frenkel exciton from migrating. As shown in figure 6, even with a very small value transfer integral relative to the potential between unit cells, a migration has a small possibility due to quantum effects. As is to be expected, a larger energy for the transfer integral allows greater migration of the hole due to the transfer energy being comparable to the energy required for that transfer. Absorption and Emission Model Figure 6 Probability of an exciton to move to a charge separated state based on transfer integral energy. U = 2eV, V 1 = 1eV. Once the basis sets and Hamiltonian have been described, the authors move to calculate the eigenstates and energies so that absorption and emission can be modeled. These models are used to calculate relative line strengths between peaks in the spectra, which are utilized for determining exciton coherence and effective mass. In the absorption model we neglect the possibility that a unit cell is not in its ground vibrational state because the energy required to be in an excited state is generally higher than thermal energy. The absorption model is described first in equation 15.
7 ( ) ( ) (15) This equation is described by the long-axis polarized dipole moment associated with the S 0 S 1 transition along the vinyl group, μ, the dipole moment operator shown in equation 16, (16) the vibrationless ground state of the polymer shown in equation 17, (17) and the line shape function shown in equation 18, where the gamma function is chosen to mediate line width and is taken as Γ=0.3ω 0 for all measurements herein. In order to define the emission model, we must first define the line strength which depends on the number of vibrational quanta in all unit cells, which are accessed after an electron has been promoted to an excited state seen in equation 19. (19) As you can see the line strength looks similar to the absorption model with the exception that you now have access to the vibrational quanta. As before, the sum of all ν n must equal ν t. With this line strength we can now define the emission spectra in equation 20: (20) where the total emission model differs from the absorption model in three ways. The line strength depends on the vibrational quanta rather than the vibrationless ground state, the third term in the line strength function accounts for a stokes shift, and the emission must be thermally averaged to account for accessibility of all thermally excited states. This thermal average can be seen in equation 21 (18) (21) where Z is the standard partition function Z Σ k,α and beta is 1/kT. The line strength ratio represents the sum of all possible transitions from the state k,α decaying to the ground electronic state with ν t vibrational quanta. Hence the line-strength for the decay of any state to the vibrationless ground state of the ground electronic state as seen in figure 7 can only occur for the case when k=0 creating the 1 1 B u exciton. This is important because it defines the 0-0 transition as uniquely dependent on a single exciton, allowing studies on that exciton with confidence. As shown in figure 7, the 0-2 transition would be the transition from any excited state to the ground electronic state requiring relaxation of two vibrational modes to arrive at the ground vibrational state. As the nuclear potentials shift and increase the overlap of the ground
8 vibrational state of the excited and ground state, other transitions become less likely. The probabilities of each transition, and thus their intensity, are due to the nature of the vibronic transitions which is why we must include the coupling between vibrational modes and electronic when dealing with conjugated polymers as described in the above section on vibronic transitions. Plotting the absorption and emission for the case of weak and intermediate coupling as a function of unit cells reveals the dependence of the emission on the number of unit cells as seen in figure 8. The absorption in the Frenkel model (weak coupling) is relatively unaffected and the Wannier- Mott (intermediate coupling) shows a dependence due to charge transfer states being allowed. In the calculations used to generate the intensity plots in figure 8, several parameters are used: U=2eV, V 1 =1eV,,. The x-axis in the figure is a function of the frequency minus the energy required to create the exciton and normalized to the frequency of the symmetric vinyl stretch. It is worthy to note the large red shift in the intermediate coupling case as a function of the number of unit cells whereas the red shift in the weak coupling case is miniscule due to the ability of the exciton to be more diffuse as the coupling between unit cells increases. Figure 8 The 0-0 emission as noted by the dottod lines. The 0-2 transition is shown by the green line. k-space In order to fully understand the Figure 7 Absoprtion spectra (black) and emission spectra (red) at T=0K. The spectra exactly overlap, but the absorption has been manually blue shifted for clarity.
9 intensity plots and their implications, we must investigate the use of k-space, commonly used in crystallographic studies. In k-space, k is the momentum of the crystal and defines the phase between the Wannier orbitals of each unit cell according to equation 22. (22) The phase factor accounts for rotations in k-space of each unit cell relative to the adjacent unit cells, where the phase in each unit cell is dependent on the crystal momentum. For a vibrational wave function where k=0 all vibrations are in phase. The result of this is that only optical phonons exist, as opposed to acoustic phonons 4. k-space requires boundary conditions such that when you move a distance equal to the lattice spacing, a through a crystal you arrive at the same spatial coordinate. Line Strength Ratio The line strength ratio of the 0-0 to 0-1 transitions is shown at T=0K to be approximately linear with the number of unit cells and is corrected by kappa, where kappa can be calculated from equation 23 which was demonstrated in a previous publication 5 and values the line strength ratio calculated in figure 8. Calculating thermodynamic parameters as a function of the number of unit cells, N, has been shown in a previous paper through the Boltzmann thermal average and integrating in k-space from -π to π to give equation 24 at the thermodynamic limit of high temperature and unit cell count, (23) (24) where ω c is the curvature of the 1B u exciton band which is given in equation 25 and is outside the scope of this paper. Theoretical calculations of the line strength ratio based on the plotted emission at T=0K and the thermodynamic change of the line strength, experimental values have been accurately reproduced. This line strength ratio is instrumental in calculating the coherence of the 1B u exciton and its effective mass, both of which are very important properties of these sorts of systems but will not be covered in this discussion. Conclusions The theoretical study put forth by Yamagata and Spano has developed an improved Hamiltonian and basis set used to study vibronic transitions in long conjugated polymers, referred to as quantum wires. Extending the Frenkel exciton model to include charge separation as in the (25)
10 Wannier-Mott model, a more accurate system is used to simulate absorption and emission spectra. From these simulated spectra and previous knowledge on the temperature dependence of the system, line strength ratios are calculated at a variety of temperatures, which are used to calculate the effective mass and coherence of the lowest energy exciton which is responsible for these transitions. References (1) Yamagata, H.; Spano, F. C. The Journal of Chemical Physics 2011, 135. (2) Research, C. f. M. a. D. f. I. T. In Photonics Wiki; University of Washington: (3) Barford, W.; Bursill, R. J.; Smith, R. W. Physical Review B 2002, 66, (4) Kittel, C. Introduction to Solid State Physics; 7th ed.; John Wiley & Sons, (5) Spano, F. C.; Yamagata, H. The Journal of Physical Chemistry B 2010, 115, 5133.
Optical Properties of Lattice Vibrations
 Optical Properties of Lattice Vibrations For a collection of classical charged Simple Harmonic Oscillators, the dielectric function is given by: Where N i is the number of oscillators with frequency ω
Optical Properties of Lattice Vibrations For a collection of classical charged Simple Harmonic Oscillators, the dielectric function is given by: Where N i is the number of oscillators with frequency ω
Born-Oppenheimer Approximation
 Born-Oppenheimer Approximation Adiabatic Assumption: Nuclei move so much more slowly than electron that the electrons that the electrons are assumed to be obtained if the nuclear kinetic energy is ignored,
Born-Oppenheimer Approximation Adiabatic Assumption: Nuclei move so much more slowly than electron that the electrons that the electrons are assumed to be obtained if the nuclear kinetic energy is ignored,
I 2 Vapor Absorption Experiment and Determination of Bond Dissociation Energy.
 I 2 Vapor Absorption Experiment and Determination of Bond Dissociation Energy. What determines the UV-Vis (i.e., electronic transitions) band appearance? Usually described by HOMO LUMO electron jump LUMO
I 2 Vapor Absorption Experiment and Determination of Bond Dissociation Energy. What determines the UV-Vis (i.e., electronic transitions) band appearance? Usually described by HOMO LUMO electron jump LUMO
ELEMENTARY BAND THEORY
 ELEMENTARY BAND THEORY PHYSICIST Solid state band Valence band, VB Conduction band, CB Fermi energy, E F Bloch orbital, delocalized n-doping p-doping Band gap, E g Direct band gap Indirect band gap Phonon
ELEMENTARY BAND THEORY PHYSICIST Solid state band Valence band, VB Conduction band, CB Fermi energy, E F Bloch orbital, delocalized n-doping p-doping Band gap, E g Direct band gap Indirect band gap Phonon
5.74 Introductory Quantum Mechanics II
 MIT OpenCourseWare http://ocw.mit.edu 5.74 Introductory Quantum Mechanics II Spring 009 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. Andrei Tokmakoff,
MIT OpenCourseWare http://ocw.mit.edu 5.74 Introductory Quantum Mechanics II Spring 009 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. Andrei Tokmakoff,
ψ s a ˆn a s b ˆn b ψ Hint: Because the state is spherically symmetric the answer can depend only on the angle between the two directions.
 1. Quantum Mechanics (Fall 2004) Two spin-half particles are in a state with total spin zero. Let ˆn a and ˆn b be unit vectors in two arbitrary directions. Calculate the expectation value of the product
1. Quantum Mechanics (Fall 2004) Two spin-half particles are in a state with total spin zero. Let ˆn a and ˆn b be unit vectors in two arbitrary directions. Calculate the expectation value of the product
Luminescence. Photoluminescence (PL) is luminescence that results from optically exciting a sample.
 Luminescence Topics Radiative transitions between electronic states Absorption and Light emission (spontaneous, stimulated) Excitons (singlets and triplets) Franck-Condon shift(stokes shift) and vibrational
Luminescence Topics Radiative transitions between electronic states Absorption and Light emission (spontaneous, stimulated) Excitons (singlets and triplets) Franck-Condon shift(stokes shift) and vibrational
I 2 Vapor Absorption Experiment and Determination of Bond Dissociation Energy.
 I 2 Vapor Absorption Experiment and Determination of Bond Dissociation Energy. What determines the UV-Vis (i.e., electronic transitions) band appearance? Usually described by HOMO LUMO electron jump LUMO
I 2 Vapor Absorption Experiment and Determination of Bond Dissociation Energy. What determines the UV-Vis (i.e., electronic transitions) band appearance? Usually described by HOMO LUMO electron jump LUMO
Exciton spectroscopy
 Lehrstuhl Werkstoffe der Elektrotechnik Exciton spectroscopy in wide bandgap semiconductors Lehrstuhl Werkstoffe der Elektrotechnik (WW6), Universität Erlangen-Nürnberg, Martensstr. 7, 91058 Erlangen Vortrag
Lehrstuhl Werkstoffe der Elektrotechnik Exciton spectroscopy in wide bandgap semiconductors Lehrstuhl Werkstoffe der Elektrotechnik (WW6), Universität Erlangen-Nürnberg, Martensstr. 7, 91058 Erlangen Vortrag
Semiconductor Physics and Devices
 Introduction to Quantum Mechanics In order to understand the current-voltage characteristics, we need some knowledge of electron behavior in semiconductor when the electron is subjected to various potential
Introduction to Quantum Mechanics In order to understand the current-voltage characteristics, we need some knowledge of electron behavior in semiconductor when the electron is subjected to various potential
What dictates the rate of radiative or nonradiative excited state decay?
 What dictates the rate of radiative or nonradiative excited state decay? Transitions are faster when there is minimum quantum mechanical reorganization of wavefunctions. This reorganization energy includes
What dictates the rate of radiative or nonradiative excited state decay? Transitions are faster when there is minimum quantum mechanical reorganization of wavefunctions. This reorganization energy includes
Review of Optical Properties of Materials
 Review of Optical Properties of Materials Review of optics Absorption in semiconductors: qualitative discussion Derivation of Optical Absorption Coefficient in Direct Semiconductors Photons When dealing
Review of Optical Properties of Materials Review of optics Absorption in semiconductors: qualitative discussion Derivation of Optical Absorption Coefficient in Direct Semiconductors Photons When dealing
7 Conjugated Polymers
 7 Conjugated Polymers The large majority of polymers, first of all the broadly used commodity materials polyethylene, polypropylene, poly(ethylene terephthalate) or polystyrene, have similar electrical
7 Conjugated Polymers The large majority of polymers, first of all the broadly used commodity materials polyethylene, polypropylene, poly(ethylene terephthalate) or polystyrene, have similar electrical
Luminescence Process
 Luminescence Process The absorption and the emission are related to each other and they are described by two terms which are complex conjugate of each other in the interaction Hamiltonian (H er ). In an
Luminescence Process The absorption and the emission are related to each other and they are described by two terms which are complex conjugate of each other in the interaction Hamiltonian (H er ). In an
Spectroscopy of. Semiconductors. Luminescence OXFORD IVAN PELANT. Academy ofsciences of the Czech Republic, Prague JAN VALENTA
 Luminescence Spectroscopy of Semiconductors IVAN PELANT Institute ofphysics, v.v.i. Academy ofsciences of the Czech Republic, Prague JAN VALENTA Department of Chemical Physics and Optics Charles University,
Luminescence Spectroscopy of Semiconductors IVAN PELANT Institute ofphysics, v.v.i. Academy ofsciences of the Czech Republic, Prague JAN VALENTA Department of Chemical Physics and Optics Charles University,
Lecture 3: Optical Properties of Insulators, Semiconductors, and Metals. 5 nm
 Metals Lecture 3: Optical Properties of Insulators, Semiconductors, and Metals 5 nm Course Info Next Week (Sept. 5 and 7) no classes First H/W is due Sept. 1 The Previous Lecture Origin frequency dependence
Metals Lecture 3: Optical Properties of Insulators, Semiconductors, and Metals 5 nm Course Info Next Week (Sept. 5 and 7) no classes First H/W is due Sept. 1 The Previous Lecture Origin frequency dependence
Quantum Chemistry. NC State University. Lecture 5. The electronic structure of molecules Absorption spectroscopy Fluorescence spectroscopy
 Quantum Chemistry Lecture 5 The electronic structure of molecules Absorption spectroscopy Fluorescence spectroscopy NC State University 3.5 Selective absorption and emission by atmospheric gases (source:
Quantum Chemistry Lecture 5 The electronic structure of molecules Absorption spectroscopy Fluorescence spectroscopy NC State University 3.5 Selective absorption and emission by atmospheric gases (source:
Chapter 3 Properties of Nanostructures
 Chapter 3 Properties of Nanostructures In Chapter 2, the reduction of the extent of a solid in one or more dimensions was shown to lead to a dramatic alteration of the overall behavior of the solids. Generally,
Chapter 3 Properties of Nanostructures In Chapter 2, the reduction of the extent of a solid in one or more dimensions was shown to lead to a dramatic alteration of the overall behavior of the solids. Generally,
NPTEL/IITM. Molecular Spectroscopy Lectures 1 & 2. Prof.K. Mangala Sunder Page 1 of 15. Topics. Part I : Introductory concepts Topics
 Molecular Spectroscopy Lectures 1 & 2 Part I : Introductory concepts Topics Why spectroscopy? Introduction to electromagnetic radiation Interaction of radiation with matter What are spectra? Beer-Lambert
Molecular Spectroscopy Lectures 1 & 2 Part I : Introductory concepts Topics Why spectroscopy? Introduction to electromagnetic radiation Interaction of radiation with matter What are spectra? Beer-Lambert
Charge and Energy Transfer Dynamits in Molecular Systems
 Volkhard May, Oliver Kühn Charge and Energy Transfer Dynamits in Molecular Systems Second, Revised and Enlarged Edition WILEY- VCH WILEY-VCH Verlag GmbH & Co. KGaA Contents 1 Introduction 19 2 Electronic
Volkhard May, Oliver Kühn Charge and Energy Transfer Dynamits in Molecular Systems Second, Revised and Enlarged Edition WILEY- VCH WILEY-VCH Verlag GmbH & Co. KGaA Contents 1 Introduction 19 2 Electronic
Vibronic quantum dynamics of exciton relaxation/trapping in molecular aggregates
 Symposium, Bordeaux Vibronic quantum dynamics of exciton relaxation/trapping in molecular aggregates Alexander Schubert Institute of Physical and Theoretical Chemistry, University of Würzburg November
Symposium, Bordeaux Vibronic quantum dynamics of exciton relaxation/trapping in molecular aggregates Alexander Schubert Institute of Physical and Theoretical Chemistry, University of Würzburg November
Electronic Structure Models
 Electronic Structure Models Hückel Model (1933) Basic Assumptions: (a) One orbital per atom contributes to the basis set; all orbitals "equal" (b) The relevant integrals involving the Hamiltonian are α
Electronic Structure Models Hückel Model (1933) Basic Assumptions: (a) One orbital per atom contributes to the basis set; all orbitals "equal" (b) The relevant integrals involving the Hamiltonian are α
Atomic Structure and Processes
 Chapter 5 Atomic Structure and Processes 5.1 Elementary atomic structure Bohr Orbits correspond to principal quantum number n. Hydrogen atom energy levels where the Rydberg energy is R y = m e ( e E n
Chapter 5 Atomic Structure and Processes 5.1 Elementary atomic structure Bohr Orbits correspond to principal quantum number n. Hydrogen atom energy levels where the Rydberg energy is R y = m e ( e E n
Ψ t = ih Ψ t t. Time Dependent Wave Equation Quantum Mechanical Description. Hamiltonian Static/Time-dependent. Time-dependent Energy operator
 Time Dependent Wave Equation Quantum Mechanical Description Hamiltonian Static/Time-dependent Time-dependent Energy operator H 0 + H t Ψ t = ih Ψ t t The Hamiltonian and wavefunction are time-dependent
Time Dependent Wave Equation Quantum Mechanical Description Hamiltonian Static/Time-dependent Time-dependent Energy operator H 0 + H t Ψ t = ih Ψ t t The Hamiltonian and wavefunction are time-dependent
Appendix A. The Particle in a Box: A Demonstration of Quantum Mechanical Principles for a Simple, One-Dimensional, One-Electron Model System
 Appendix A The Particle in a Box: A Demonstration of Quantum Mechanical Principles for a Simple, One-Dimensional, One-Electron Model System Real quantum mechanical systems have the tendency to become mathematically
Appendix A The Particle in a Box: A Demonstration of Quantum Mechanical Principles for a Simple, One-Dimensional, One-Electron Model System Real quantum mechanical systems have the tendency to become mathematically
Minimal Update of Solid State Physics
 Minimal Update of Solid State Physics It is expected that participants are acquainted with basics of solid state physics. Therefore here we will refresh only those aspects, which are absolutely necessary
Minimal Update of Solid State Physics It is expected that participants are acquainted with basics of solid state physics. Therefore here we will refresh only those aspects, which are absolutely necessary
Modern Physics for Frommies IV The Universe - Small to Large Lecture 4
 Fromm Institute for Lifelong Learning University of San Francisco Modern Physics for Frommies IV The Universe - Small to Large Lecture 4 3 February 06 Modern Physics IV Lecture 4 Agenda Administrative
Fromm Institute for Lifelong Learning University of San Francisco Modern Physics for Frommies IV The Universe - Small to Large Lecture 4 3 February 06 Modern Physics IV Lecture 4 Agenda Administrative
Chem 442 Review of Spectroscopy
 Chem 44 Review of Spectroscopy General spectroscopy Wavelength (nm), frequency (s -1 ), wavenumber (cm -1 ) Frequency (s -1 ): n= c l Wavenumbers (cm -1 ): n =1 l Chart of photon energies and spectroscopies
Chem 44 Review of Spectroscopy General spectroscopy Wavelength (nm), frequency (s -1 ), wavenumber (cm -1 ) Frequency (s -1 ): n= c l Wavenumbers (cm -1 ): n =1 l Chart of photon energies and spectroscopies
Fluorescence Spectroscopy
 Fluorescence Spectroscopy Frequency and time dependent emission Emission and Excitation fluorescence spectra Stokes Shift: influence of molecular vibrations and solvent Time resolved fluorescence measurements
Fluorescence Spectroscopy Frequency and time dependent emission Emission and Excitation fluorescence spectra Stokes Shift: influence of molecular vibrations and solvent Time resolved fluorescence measurements
Correlation spectroscopy
 1 TWO-DIMENSIONAL SPECTROSCOPY Correlation spectroscopy What is two-dimensional spectroscopy? This is a method that will describe the underlying correlations between two spectral features. Our examination
1 TWO-DIMENSIONAL SPECTROSCOPY Correlation spectroscopy What is two-dimensional spectroscopy? This is a method that will describe the underlying correlations between two spectral features. Our examination
Phonons I - Crystal Vibrations (Kittel Ch. 4)
 Phonons I - Crystal Vibrations (Kittel Ch. 4) Displacements of Atoms Positions of atoms in their perfect lattice positions are given by: R 0 (n 1, n 2, n 3 ) = n 10 x + n 20 y + n 30 z For simplicity here
Phonons I - Crystal Vibrations (Kittel Ch. 4) Displacements of Atoms Positions of atoms in their perfect lattice positions are given by: R 0 (n 1, n 2, n 3 ) = n 10 x + n 20 y + n 30 z For simplicity here
Notes on x-ray scattering - M. Le Tacon, B. Keimer (06/2015)
 Notes on x-ray scattering - M. Le Tacon, B. Keimer (06/2015) Interaction of x-ray with matter: - Photoelectric absorption - Elastic (coherent) scattering (Thomson Scattering) - Inelastic (incoherent) scattering
Notes on x-ray scattering - M. Le Tacon, B. Keimer (06/2015) Interaction of x-ray with matter: - Photoelectric absorption - Elastic (coherent) scattering (Thomson Scattering) - Inelastic (incoherent) scattering
Introduction to Franck-Condon Factors
 Introduction to Franck-Condon Factors Theresa Julia Zielinski Monmouth University Department of Chemistry, Medical Technology, and Physics West Long Branch, NJ 07764 tzielins@monmouth.edu and La Salle
Introduction to Franck-Condon Factors Theresa Julia Zielinski Monmouth University Department of Chemistry, Medical Technology, and Physics West Long Branch, NJ 07764 tzielins@monmouth.edu and La Salle
Density of states for electrons and holes. Distribution function. Conduction and valence bands
 Intrinsic Semiconductors In the field of semiconductors electrons and holes are usually referred to as free carriers, or simply carriers, because it is these particles which are responsible for carrying
Intrinsic Semiconductors In the field of semiconductors electrons and holes are usually referred to as free carriers, or simply carriers, because it is these particles which are responsible for carrying
CHAPTER 13 Molecular Spectroscopy 2: Electronic Transitions
 CHAPTER 13 Molecular Spectroscopy 2: Electronic Transitions I. General Features of Electronic spectroscopy. A. Visible and ultraviolet photons excite electronic state transitions. ε photon = 120 to 1200
CHAPTER 13 Molecular Spectroscopy 2: Electronic Transitions I. General Features of Electronic spectroscopy. A. Visible and ultraviolet photons excite electronic state transitions. ε photon = 120 to 1200
In-class exercises. Day 1
 Physics 4488/6562: Statistical Mechanics http://www.physics.cornell.edu/sethna/teaching/562/ Material for Week 8 Exercises due Mon March 19 Last correction at March 5, 2018, 8:48 am c 2017, James Sethna,
Physics 4488/6562: Statistical Mechanics http://www.physics.cornell.edu/sethna/teaching/562/ Material for Week 8 Exercises due Mon March 19 Last correction at March 5, 2018, 8:48 am c 2017, James Sethna,
October Entrance Examination: Condensed Matter Multiple choice quizzes
 October 2013 - Entrance Examination: Condensed Matter Multiple choice quizzes 1 A cubic meter of H 2 and a cubic meter of O 2 are at the same pressure (p) and at the same temperature (T 1 ) in their gas
October 2013 - Entrance Examination: Condensed Matter Multiple choice quizzes 1 A cubic meter of H 2 and a cubic meter of O 2 are at the same pressure (p) and at the same temperature (T 1 ) in their gas
Figure 1 Relaxation processes within an excited state or the ground state.
 Excited State Processes and Application to Lasers The technology of the laser (Light Amplified by Stimulated Emission of Radiation) was developed in the early 1960s. The technology is based on an understanding
Excited State Processes and Application to Lasers The technology of the laser (Light Amplified by Stimulated Emission of Radiation) was developed in the early 1960s. The technology is based on an understanding
Particle nature of light & Quantization
 Particle nature of light & Quantization A quantity is quantized if its possible values are limited to a discrete set. An example from classical physics is the allowed frequencies of standing waves on a
Particle nature of light & Quantization A quantity is quantized if its possible values are limited to a discrete set. An example from classical physics is the allowed frequencies of standing waves on a
Today: general condition for threshold operation physics of atomic, vibrational, rotational gain media intro to the Lorentz model
 Today: general condition for threshold operation physics of atomic, vibrational, rotational gain media intro to the Lorentz model Laser operation Simplified energy conversion processes in a laser medium:
Today: general condition for threshold operation physics of atomic, vibrational, rotational gain media intro to the Lorentz model Laser operation Simplified energy conversion processes in a laser medium:
Molecular orbitals, potential energy surfaces and symmetry
 Molecular orbitals, potential energy surfaces and symmetry mathematical presentation of molecular symmetry group theory spectroscopy valence theory molecular orbitals Wave functions Hamiltonian: electronic,
Molecular orbitals, potential energy surfaces and symmetry mathematical presentation of molecular symmetry group theory spectroscopy valence theory molecular orbitals Wave functions Hamiltonian: electronic,
CONTENTS. vii. CHAPTER 2 Operators 15
 CHAPTER 1 Why Quantum Mechanics? 1 1.1 Newtonian Mechanics and Classical Electromagnetism 1 (a) Newtonian Mechanics 1 (b) Electromagnetism 2 1.2 Black Body Radiation 3 1.3 The Heat Capacity of Solids and
CHAPTER 1 Why Quantum Mechanics? 1 1.1 Newtonian Mechanics and Classical Electromagnetism 1 (a) Newtonian Mechanics 1 (b) Electromagnetism 2 1.2 Black Body Radiation 3 1.3 The Heat Capacity of Solids and
Thermal and Statistical Physics Department Exam Last updated November 4, L π
 Thermal and Statistical Physics Department Exam Last updated November 4, 013 1. a. Define the chemical potential µ. Show that two systems are in diffusive equilibrium if µ 1 =µ. You may start with F =
Thermal and Statistical Physics Department Exam Last updated November 4, 013 1. a. Define the chemical potential µ. Show that two systems are in diffusive equilibrium if µ 1 =µ. You may start with F =
Chapter 8 Problem Solutions
 Chapter 8 Problem Solutions 1. The energy needed to detach the electron from a hydrogen atom is 13.6 ev, but the energy needed to detach an electron from a hydrogen molecule is 15.7 ev. Why do you think
Chapter 8 Problem Solutions 1. The energy needed to detach the electron from a hydrogen atom is 13.6 ev, but the energy needed to detach an electron from a hydrogen molecule is 15.7 ev. Why do you think
Principles of Molecular Spectroscopy
 Principles of Molecular Spectroscopy What variables do we need to characterize a molecule? Nuclear and electronic configurations: What is the structure of the molecule? What are the bond lengths? How strong
Principles of Molecular Spectroscopy What variables do we need to characterize a molecule? Nuclear and electronic configurations: What is the structure of the molecule? What are the bond lengths? How strong
PHYS 172: Modern Mechanics Fall 2009
 PHYS 172: Modern Mechanics Fall 2009 Lecture 14 Energy Quantization Read 7.1 7.9 Reading Question: Ch. 7, Secs 1-5 A simple model for the hydrogen atom treats the electron as a particle in circular orbit
PHYS 172: Modern Mechanics Fall 2009 Lecture 14 Energy Quantization Read 7.1 7.9 Reading Question: Ch. 7, Secs 1-5 A simple model for the hydrogen atom treats the electron as a particle in circular orbit
wbt Λ = 0, 1, 2, 3, Eq. (7.63)
 7.2.2 Classification of Electronic States For all diatomic molecules the coupling approximation which best describes electronic states is analogous to the Russell- Saunders approximation in atoms The orbital
7.2.2 Classification of Electronic States For all diatomic molecules the coupling approximation which best describes electronic states is analogous to the Russell- Saunders approximation in atoms The orbital
Quantum mechanics can be used to calculate any property of a molecule. The energy E of a wavefunction Ψ evaluated for the Hamiltonian H is,
 Chapter : Molecules Quantum mechanics can be used to calculate any property of a molecule The energy E of a wavefunction Ψ evaluated for the Hamiltonian H is, E = Ψ H Ψ Ψ Ψ 1) At first this seems like
Chapter : Molecules Quantum mechanics can be used to calculate any property of a molecule The energy E of a wavefunction Ψ evaluated for the Hamiltonian H is, E = Ψ H Ψ Ψ Ψ 1) At first this seems like
Optical Characterization of Solids
 D. Dragoman M. Dragoman Optical Characterization of Solids With 184 Figures Springer 1. Elementary Excitations in Solids 1 1.1 Energy Band Structure in Crystalline Materials 2 1.2 k p Method 11 1.3 Numerical
D. Dragoman M. Dragoman Optical Characterization of Solids With 184 Figures Springer 1. Elementary Excitations in Solids 1 1.1 Energy Band Structure in Crystalline Materials 2 1.2 k p Method 11 1.3 Numerical
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester Christopher J. Cramer. Lecture 30, April 10, 2006
 Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 20056 Christopher J. Cramer Lecture 30, April 10, 2006 Solved Homework The guess MO occupied coefficients were Occupied
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 20056 Christopher J. Cramer Lecture 30, April 10, 2006 Solved Homework The guess MO occupied coefficients were Occupied
Chapter 2 Energy Transfer Review
 Chapter 2 Energy Transfer Review In this chapter, we discuss the basic concepts of excitation energy transfer, making the distinction between radiative and nonradiative, and giving a brief overview on
Chapter 2 Energy Transfer Review In this chapter, we discuss the basic concepts of excitation energy transfer, making the distinction between radiative and nonradiative, and giving a brief overview on
Physics 828 Problem Set 7 Due Wednesday 02/24/2010
 Physics 88 Problem Set 7 Due Wednesday /4/ 7)a)Consider the proton to be a uniformly charged sphere of radius f m Determine the correction to the s ground state energy 4 points) This is a standard problem
Physics 88 Problem Set 7 Due Wednesday /4/ 7)a)Consider the proton to be a uniformly charged sphere of radius f m Determine the correction to the s ground state energy 4 points) This is a standard problem
5.74 Introductory Quantum Mechanics II
 MIT OpenCourseWare http://ocw.mit.edu 5.74 Introductory Quantum Mechanics II Spring 009 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. Andrei Tokmakoff,
MIT OpenCourseWare http://ocw.mit.edu 5.74 Introductory Quantum Mechanics II Spring 009 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. Andrei Tokmakoff,
Neutron scattering from quantum materials
 Neutron scattering from quantum materials Bernhard Keimer Max Planck Institute for Solid State Research Max Planck UBC UTokyo Center for Quantum Materials Detection of bosonic elementary excitations in
Neutron scattering from quantum materials Bernhard Keimer Max Planck Institute for Solid State Research Max Planck UBC UTokyo Center for Quantum Materials Detection of bosonic elementary excitations in
Physical Chemistry Laboratory II (CHEM 337) EXPT 9 3: Vibronic Spectrum of Iodine (I2)
 Physical Chemistry Laboratory II (CHEM 337) EXPT 9 3: Vibronic Spectrum of Iodine (I2) Obtaining fundamental information about the nature of molecular structure is one of the interesting aspects of molecular
Physical Chemistry Laboratory II (CHEM 337) EXPT 9 3: Vibronic Spectrum of Iodine (I2) Obtaining fundamental information about the nature of molecular structure is one of the interesting aspects of molecular
Fermi polaron-polaritons in MoSe 2
 Fermi polaron-polaritons in MoSe 2 Meinrad Sidler, Patrick Back, Ovidiu Cotlet, Ajit Srivastava, Thomas Fink, Martin Kroner, Eugene Demler, Atac Imamoglu Quantum impurity problem Nonperturbative interaction
Fermi polaron-polaritons in MoSe 2 Meinrad Sidler, Patrick Back, Ovidiu Cotlet, Ajit Srivastava, Thomas Fink, Martin Kroner, Eugene Demler, Atac Imamoglu Quantum impurity problem Nonperturbative interaction
Chapter 39. Particles Behaving as Waves
 Chapter 39 Particles Behaving as Waves 39.1 Electron Waves Light has a dual nature. Light exhibits both wave and particle characteristics. Louis de Broglie postulated in 1924 that if nature is symmetric,
Chapter 39 Particles Behaving as Waves 39.1 Electron Waves Light has a dual nature. Light exhibits both wave and particle characteristics. Louis de Broglie postulated in 1924 that if nature is symmetric,
SUPPLEMENTARY INFORMATION
 Supporting online material SUPPLEMENTARY INFORMATION doi: 0.038/nPHYS8 A: Derivation of the measured initial degree of circular polarization. Under steady state conditions, prior to the emission of the
Supporting online material SUPPLEMENTARY INFORMATION doi: 0.038/nPHYS8 A: Derivation of the measured initial degree of circular polarization. Under steady state conditions, prior to the emission of the
Quantum mechanics (QM) deals with systems on atomic scale level, whose behaviours cannot be described by classical mechanics.
 A 10-MINUTE RATHER QUICK INTRODUCTION TO QUANTUM MECHANICS 1. What is quantum mechanics (as opposed to classical mechanics)? Quantum mechanics (QM) deals with systems on atomic scale level, whose behaviours
A 10-MINUTE RATHER QUICK INTRODUCTION TO QUANTUM MECHANICS 1. What is quantum mechanics (as opposed to classical mechanics)? Quantum mechanics (QM) deals with systems on atomic scale level, whose behaviours
N independent electrons in a volume V (assuming periodic boundary conditions) I] The system 3 V = ( ) k ( ) i k k k 1/2
![N independent electrons in a volume V (assuming periodic boundary conditions) I] The system 3 V = ( ) k ( ) i k k k 1/2 N independent electrons in a volume V (assuming periodic boundary conditions) I] The system 3 V = ( ) k ( ) i k k k 1/2](/thumbs/90/101212070.jpg) Lecture #6. Understanding the properties of metals: the free electron model and the role of Pauli s exclusion principle.. Counting the states in the E model.. ermi energy, and momentum. 4. DOS 5. ermi-dirac
Lecture #6. Understanding the properties of metals: the free electron model and the role of Pauli s exclusion principle.. Counting the states in the E model.. ermi energy, and momentum. 4. DOS 5. ermi-dirac
Composite Model for LENR in linear defects of a lattice
 Composite Model for LENR in linear defects of a lattice A. Meulenberg a) and K. P. Sinha b) a) Science for Humanity Trust, Inc. Tucker, GA, USA mules333@gmail.com b) Department of Physics, Indian Institute
Composite Model for LENR in linear defects of a lattice A. Meulenberg a) and K. P. Sinha b) a) Science for Humanity Trust, Inc. Tucker, GA, USA mules333@gmail.com b) Department of Physics, Indian Institute
Summary lecture VI. with the reduced mass and the dielectric background constant
 Summary lecture VI Excitonic binding energy reads with the reduced mass and the dielectric background constant Δ Statistical operator (density matrix) characterizes quantum systems in a mixed state and
Summary lecture VI Excitonic binding energy reads with the reduced mass and the dielectric background constant Δ Statistical operator (density matrix) characterizes quantum systems in a mixed state and
is the minimum stopping potential for which the current between the plates reduces to zero.
 Module 1 :Quantum Mechanics Chapter 2 : Introduction to Quantum ideas Introduction to Quantum ideas We will now consider some experiments and their implications, which introduce us to quantum ideas. The
Module 1 :Quantum Mechanics Chapter 2 : Introduction to Quantum ideas Introduction to Quantum ideas We will now consider some experiments and their implications, which introduce us to quantum ideas. The
CHARGE CARRIERS PHOTOGENERATION. Maddalena Binda Organic Electronics: principles, devices and applications Milano, November 23-27th, 2015
 CHARGE CARRIERS PHOTOGENERATION Maddalena Binda Organic Electronics: principles, devices and applications Milano, November 23-27th, 2015 Charge carriers photogeneration: what does it mean? Light stimulus
CHARGE CARRIERS PHOTOGENERATION Maddalena Binda Organic Electronics: principles, devices and applications Milano, November 23-27th, 2015 Charge carriers photogeneration: what does it mean? Light stimulus
Angle-Resolved Two-Photon Photoemission of Mott Insulator
 Angle-Resolved Two-Photon Photoemission of Mott Insulator Takami Tohyama Institute for Materials Research (IMR) Tohoku University, Sendai Collaborators IMR: H. Onodera, K. Tsutsui, S. Maekawa H. Onodera
Angle-Resolved Two-Photon Photoemission of Mott Insulator Takami Tohyama Institute for Materials Research (IMR) Tohoku University, Sendai Collaborators IMR: H. Onodera, K. Tsutsui, S. Maekawa H. Onodera
Quantum Mechanics - I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras. Lecture - 9 Introducing Quantum Optics
 Quantum Mechanics - I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras Lecture - 9 Introducing Quantum Optics (Refer Slide Time: 00:07) In the last lecture I gave
Quantum Mechanics - I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras Lecture - 9 Introducing Quantum Optics (Refer Slide Time: 00:07) In the last lecture I gave
Introduction to Sources: Radiative Processes and Population Inversion in Atoms, Molecules, and Semiconductors Atoms and Molecules
 OPTI 500 DEF, Spring 2012, Lecture 2 Introduction to Sources: Radiative Processes and Population Inversion in Atoms, Molecules, and Semiconductors Atoms and Molecules Energy Levels Every atom or molecule
OPTI 500 DEF, Spring 2012, Lecture 2 Introduction to Sources: Radiative Processes and Population Inversion in Atoms, Molecules, and Semiconductors Atoms and Molecules Energy Levels Every atom or molecule
Vibrational-Rotational Spectroscopy. Spectroscopy
 Applied Spectroscopy Vibrational-Rotational Spectroscopy Recommended Reading: Banwell and McCash Section 3.2, 3.3 Atkins Section 6.2 Harmonic oscillator vibrations have the exact selection rule: and the
Applied Spectroscopy Vibrational-Rotational Spectroscopy Recommended Reading: Banwell and McCash Section 3.2, 3.3 Atkins Section 6.2 Harmonic oscillator vibrations have the exact selection rule: and the
(a) What are the probabilities associated with finding the different allowed values of the z-component of the spin after time T?
 1. Quantum Mechanics (Fall 2002) A Stern-Gerlach apparatus is adjusted so that the z-component of the spin of an electron (spin-1/2) transmitted through it is /2. A uniform magnetic field in the x-direction
1. Quantum Mechanics (Fall 2002) A Stern-Gerlach apparatus is adjusted so that the z-component of the spin of an electron (spin-1/2) transmitted through it is /2. A uniform magnetic field in the x-direction
Perturbation Theory. Andreas Wacker Mathematical Physics Lund University
 Perturbation Theory Andreas Wacker Mathematical Physics Lund University General starting point Hamiltonian ^H (t) has typically noanalytic solution of Ψ(t) Decompose Ĥ (t )=Ĥ 0 + V (t) known eigenstates
Perturbation Theory Andreas Wacker Mathematical Physics Lund University General starting point Hamiltonian ^H (t) has typically noanalytic solution of Ψ(t) Decompose Ĥ (t )=Ĥ 0 + V (t) known eigenstates
5.80 Small-Molecule Spectroscopy and Dynamics
 MIT OpenCourseWare http://ocw.mit.edu 5.80 Small-Molecule Spectroscopy and Dynamics Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 5.80 Lecture
MIT OpenCourseWare http://ocw.mit.edu 5.80 Small-Molecule Spectroscopy and Dynamics Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 5.80 Lecture
Experiment 6: Vibronic Absorption Spectrum of Molecular Iodine
 Experiment 6: Vibronic Absorption Spectrum of Molecular Iodine We have already seen that molecules can rotate and bonds can vibrate with characteristic energies, each energy being associated with a particular
Experiment 6: Vibronic Absorption Spectrum of Molecular Iodine We have already seen that molecules can rotate and bonds can vibrate with characteristic energies, each energy being associated with a particular
Computational Chemistry Workshops West Ridge Research Building-UAF Campus 9:00am-4:00pm, Room 009
 Computational Chemistry Workshops West Ridge Research Building-UAF Campus 9:00am-4:00pm, Room 009 Electronic Structure - July 19-21, 2016 Molecular Dynamics - July 26-28, 2016 Why are the Azulene and Naphthalene
Computational Chemistry Workshops West Ridge Research Building-UAF Campus 9:00am-4:00pm, Room 009 Electronic Structure - July 19-21, 2016 Molecular Dynamics - July 26-28, 2016 Why are the Azulene and Naphthalene
Quantum Physics in the Nanoworld
 Hans Lüth Quantum Physics in the Nanoworld Schrödinger's Cat and the Dwarfs 4) Springer Contents 1 Introduction 1 1.1 General and Historical Remarks 1 1.2 Importance for Science and Technology 3 1.3 Philosophical
Hans Lüth Quantum Physics in the Nanoworld Schrödinger's Cat and the Dwarfs 4) Springer Contents 1 Introduction 1 1.1 General and Historical Remarks 1 1.2 Importance for Science and Technology 3 1.3 Philosophical
Last Name or Student ID
 12/05/18, Chem433 Final Exam Last Name or Student ID 1. (2 pts) 12. (3 pts) 2. (6 pts) 13. (3 pts) 3. (3 pts) 14. (2 pts) 4. (3 pts) 15. (3 pts) 5. (4 pts) 16. (3 pts) 6. (2 pts) 17. (15 pts) 7. (9 pts)
12/05/18, Chem433 Final Exam Last Name or Student ID 1. (2 pts) 12. (3 pts) 2. (6 pts) 13. (3 pts) 3. (3 pts) 14. (2 pts) 4. (3 pts) 15. (3 pts) 5. (4 pts) 16. (3 pts) 6. (2 pts) 17. (15 pts) 7. (9 pts)
Summary lecture IX. The electron-light Hamilton operator reads in second quantization
 Summary lecture IX The electron-light Hamilton operator reads in second quantization Absorption coefficient α(ω) is given by the optical susceptibility Χ(ω) that is determined by microscopic polarization
Summary lecture IX The electron-light Hamilton operator reads in second quantization Absorption coefficient α(ω) is given by the optical susceptibility Χ(ω) that is determined by microscopic polarization
Model Answer (Paper code: AR-7112) M. Sc. (Physics) IV Semester Paper I: Laser Physics and Spectroscopy
 Model Answer (Paper code: AR-7112) M. Sc. (Physics) IV Semester Paper I: Laser Physics and Spectroscopy Section I Q1. Answer (i) (b) (ii) (d) (iii) (c) (iv) (c) (v) (a) (vi) (b) (vii) (b) (viii) (a) (ix)
Model Answer (Paper code: AR-7112) M. Sc. (Physics) IV Semester Paper I: Laser Physics and Spectroscopy Section I Q1. Answer (i) (b) (ii) (d) (iii) (c) (iv) (c) (v) (a) (vi) (b) (vii) (b) (viii) (a) (ix)
Optical Lattices. Chapter Polarization
 Chapter Optical Lattices Abstract In this chapter we give details of the atomic physics that underlies the Bose- Hubbard model used to describe ultracold atoms in optical lattices. We show how the AC-Stark
Chapter Optical Lattices Abstract In this chapter we give details of the atomic physics that underlies the Bose- Hubbard model used to describe ultracold atoms in optical lattices. We show how the AC-Stark
V( x) = V( 0) + dv. V( x) = 1 2
 Spectroscopy 1: rotational and vibrational spectra The vibrations of diatomic molecules Molecular vibrations Consider a typical potential energy curve for a diatomic molecule. In regions close to R e (at
Spectroscopy 1: rotational and vibrational spectra The vibrations of diatomic molecules Molecular vibrations Consider a typical potential energy curve for a diatomic molecule. In regions close to R e (at
van Quantum tot Molecuul
 10 HC10: Molecular and vibrational spectroscopy van Quantum tot Molecuul Dr Juan Rojo VU Amsterdam and Nikhef Theory Group http://www.juanrojo.com/ j.rojo@vu.nl Molecular and Vibrational Spectroscopy Based
10 HC10: Molecular and vibrational spectroscopy van Quantum tot Molecuul Dr Juan Rojo VU Amsterdam and Nikhef Theory Group http://www.juanrojo.com/ j.rojo@vu.nl Molecular and Vibrational Spectroscopy Based
Metals: the Drude and Sommerfeld models p. 1 Introduction p. 1 What do we know about metals? p. 1 The Drude model p. 2 Assumptions p.
 Metals: the Drude and Sommerfeld models p. 1 Introduction p. 1 What do we know about metals? p. 1 The Drude model p. 2 Assumptions p. 2 The relaxation-time approximation p. 3 The failure of the Drude model
Metals: the Drude and Sommerfeld models p. 1 Introduction p. 1 What do we know about metals? p. 1 The Drude model p. 2 Assumptions p. 2 The relaxation-time approximation p. 3 The failure of the Drude model
Introduction to Electronic Structure Theory
 Introduction to Electronic Structure Theory C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology June 2002 Last Revised: June 2003 1 Introduction The purpose of these
Introduction to Electronic Structure Theory C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology June 2002 Last Revised: June 2003 1 Introduction The purpose of these
NERS 311 Current Old notes notes Chapter Chapter 1: Introduction to the course 1 - Chapter 1.1: About the course 2 - Chapter 1.
 NERS311/Fall 2014 Revision: August 27, 2014 Index to the Lecture notes Alex Bielajew, 2927 Cooley, bielajew@umich.edu NERS 311 Current Old notes notes Chapter 1 1 1 Chapter 1: Introduction to the course
NERS311/Fall 2014 Revision: August 27, 2014 Index to the Lecture notes Alex Bielajew, 2927 Cooley, bielajew@umich.edu NERS 311 Current Old notes notes Chapter 1 1 1 Chapter 1: Introduction to the course
Physics 213. Practice Final Exam Spring The next two questions pertain to the following situation:
 The next two questions pertain to the following situation: Consider the following two systems: A: three interacting harmonic oscillators with total energy 6ε. B: two interacting harmonic oscillators, with
The next two questions pertain to the following situation: Consider the following two systems: A: three interacting harmonic oscillators with total energy 6ε. B: two interacting harmonic oscillators, with
Triplet state diffusion in organometallic and organic semiconductors
 Triplet state diffusion in organometallic and organic semiconductors Prof. Anna Köhler Experimental Physik II University of Bayreuth Germany From materials properties To device applications Organic semiconductors
Triplet state diffusion in organometallic and organic semiconductors Prof. Anna Köhler Experimental Physik II University of Bayreuth Germany From materials properties To device applications Organic semiconductors
CHEM6416 Theory of Molecular Spectroscopy 2013Jan Spectroscopy frequency dependence of the interaction of light with matter
 CHEM6416 Theory of Molecular Spectroscopy 2013Jan22 1 1. Spectroscopy frequency dependence of the interaction of light with matter 1.1. Absorption (excitation), emission, diffraction, scattering, refraction
CHEM6416 Theory of Molecular Spectroscopy 2013Jan22 1 1. Spectroscopy frequency dependence of the interaction of light with matter 1.1. Absorption (excitation), emission, diffraction, scattering, refraction
Non-stationary States and Electric Dipole Transitions
 Pre-Lab Lecture II Non-stationary States and Electric Dipole Transitions You will recall that the wavefunction for any system is calculated in general from the time-dependent Schrödinger equation ĤΨ(x,t)=i
Pre-Lab Lecture II Non-stationary States and Electric Dipole Transitions You will recall that the wavefunction for any system is calculated in general from the time-dependent Schrödinger equation ĤΨ(x,t)=i
Physics 221 Lecture 31 Line Radiation from Atoms and Molecules March 31, 1999
 Physics 221 Lecture 31 Line Radiation from Atoms and Molecules March 31, 1999 Reading Meyer-Arendt, Ch. 20; Möller, Ch. 15; Yariv, Ch.. Demonstrations Analyzing lineshapes from emission and absorption
Physics 221 Lecture 31 Line Radiation from Atoms and Molecules March 31, 1999 Reading Meyer-Arendt, Ch. 20; Möller, Ch. 15; Yariv, Ch.. Demonstrations Analyzing lineshapes from emission and absorption
Ab initio calculations of f-orbital electron-phonon interaction in laser cooling
 Ab initio calculations of f-orbital electron-phonon interaction in laser cooling Jedo Kim and Massoud Kaviany* Department of Mechanical Engineering, University of Michigan, Ann Arbor, Michigan 48109-2125,
Ab initio calculations of f-orbital electron-phonon interaction in laser cooling Jedo Kim and Massoud Kaviany* Department of Mechanical Engineering, University of Michigan, Ann Arbor, Michigan 48109-2125,
Quantum Mechanics-I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras. Lecture - 21 Square-Integrable Functions
 Quantum Mechanics-I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras Lecture - 21 Square-Integrable Functions (Refer Slide Time: 00:06) (Refer Slide Time: 00:14) We
Quantum Mechanics-I Prof. Dr. S. Lakshmi Bala Department of Physics Indian Institute of Technology, Madras Lecture - 21 Square-Integrable Functions (Refer Slide Time: 00:06) (Refer Slide Time: 00:14) We
ECE440 Nanoelectronics. Lecture 07 Atomic Orbitals
 ECE44 Nanoelectronics Lecture 7 Atomic Orbitals Atoms and atomic orbitals It is instructive to compare the simple model of a spherically symmetrical potential for r R V ( r) for r R and the simplest hydrogen
ECE44 Nanoelectronics Lecture 7 Atomic Orbitals Atoms and atomic orbitals It is instructive to compare the simple model of a spherically symmetrical potential for r R V ( r) for r R and the simplest hydrogen
Theoretical Photochemistry WiSe 2017/18
 Theoretical Photochemistry WiSe 2017/18 Lecture 7 Irene Burghardt (burghardt@chemie.uni-frankfurt.de) http://www.theochem.uni-frankfurt.de/teaching/ Theoretical Photochemistry 1 Topics 1. Photophysical
Theoretical Photochemistry WiSe 2017/18 Lecture 7 Irene Burghardt (burghardt@chemie.uni-frankfurt.de) http://www.theochem.uni-frankfurt.de/teaching/ Theoretical Photochemistry 1 Topics 1. Photophysical
Condensed matter physics FKA091
 Condensed matter physics FKA091 Ermin Malic Department of Physics Chalmers University of Technology Henrik Johannesson Department of Physics University of Gothenburg Teaching assistants: Roland Jago &
Condensed matter physics FKA091 Ermin Malic Department of Physics Chalmers University of Technology Henrik Johannesson Department of Physics University of Gothenburg Teaching assistants: Roland Jago &
Quantum Physics & From Ideas to Implementation. Underlying concepts in the syllabus
 Quantum Physics & From Ideas to Implementation Underlying concepts in the syllabus 1 1 What is Quantum Physics? Wave-particle duality Tells us that energy comes in packets, particles are wave-like. Systems
Quantum Physics & From Ideas to Implementation Underlying concepts in the syllabus 1 1 What is Quantum Physics? Wave-particle duality Tells us that energy comes in packets, particles are wave-like. Systems
Topic 6: Light Absorption and Color in Biomolecules
 1 6.1 INTRODUCTION Topic 6: Light Absorption and Color in Biomolecules Why are trees green? Blood red? Carrots orange? Most colors in biological tissues arise from natural pigments. A pigment is a molecule
1 6.1 INTRODUCTION Topic 6: Light Absorption and Color in Biomolecules Why are trees green? Blood red? Carrots orange? Most colors in biological tissues arise from natural pigments. A pigment is a molecule
General NMR basics. Solid State NMR workshop 2011: An introduction to Solid State NMR spectroscopy. # nuclei
 : An introduction to Solid State NMR spectroscopy Dr. Susanne Causemann (Solid State NMR specialist/ researcher) Interaction between nuclear spins and applied magnetic fields B 0 application of a static
: An introduction to Solid State NMR spectroscopy Dr. Susanne Causemann (Solid State NMR specialist/ researcher) Interaction between nuclear spins and applied magnetic fields B 0 application of a static
Quantum dynamics in complex environments towards biological and nanostructured systems
 Quantum dynamics in complex environments towards biological and nanostructured systems Chris Engelbrecht Summer School on Quantum Biology Lecture 4 Irene Burghardt Department of Physical and Theoretical
Quantum dynamics in complex environments towards biological and nanostructured systems Chris Engelbrecht Summer School on Quantum Biology Lecture 4 Irene Burghardt Department of Physical and Theoretical
( ) x10 8 m. The energy in a mole of 400 nm photons is calculated by: ' & sec( ) ( & % ) 6.022x10 23 photons' E = h! = hc & 6.
 Introduction to Spectroscopy Spectroscopic techniques are widely used to detect molecules, to measure the concentration of a species in solution, and to determine molecular structure. For proteins, most
Introduction to Spectroscopy Spectroscopic techniques are widely used to detect molecules, to measure the concentration of a species in solution, and to determine molecular structure. For proteins, most
Physics PhD Qualifying Examination Part I Wednesday, August 26, 2015
 Physics PhD Qualifying Examination Part I Wednesday, August 26, 2015 Name: (please print) Identification Number: STUDENT: Designate the problem numbers that you are handing in for grading in the appropriate
Physics PhD Qualifying Examination Part I Wednesday, August 26, 2015 Name: (please print) Identification Number: STUDENT: Designate the problem numbers that you are handing in for grading in the appropriate
SOLID STATE PHYSICS. Second Edition. John Wiley & Sons. J. R. Hook H. E. Hall. Department of Physics, University of Manchester
 SOLID STATE PHYSICS Second Edition J. R. Hook H. E. Hall Department of Physics, University of Manchester John Wiley & Sons CHICHESTER NEW YORK BRISBANE TORONTO SINGAPORE Contents Flow diagram Inside front
SOLID STATE PHYSICS Second Edition J. R. Hook H. E. Hall Department of Physics, University of Manchester John Wiley & Sons CHICHESTER NEW YORK BRISBANE TORONTO SINGAPORE Contents Flow diagram Inside front
