Non-Newtonian Fluids How slow can you go?
|
|
|
- Osborn Chandler
- 5 years ago
- Views:
Transcription
1 Non-Newtonian Fluids How slow can you go? Background Have you heard of Sir Isaac Newton? Newton ( ) is a famous scientist and mathematician who discovered gravity! In addition, he created the three laws of motion, contributed to creating calculus and predicted the shape of the earth. Newton also did a lot of work with fluids. Newton described how normal fluids, like water, behave. For example, these fluids have a constant viscosity. For Newtonian fluids (or normal fluids), viscosity only changes with temperature. Viscosity refers to a fluid s resistance to flow. A highly viscous substance flows slowly. For example, peanut butter is more viscous than water because it is more difficult to pour. There are some fluids that do not have a constant viscosity, and these are called non-newtonian fluids. These fluids can be affected by other factors besides temperature, such as stress. Applying a force to non-newtonian fluids can cause them to get thicker and act like a solid or get thinner and less viscous than they were before. If the force or stress is removed, the fluid will return to its natural state. Watch this clip to see an example of a shear thickening non-newtonian fluid and to see someone walk on a liquid! - 1
2 Procedure: Timer: Reader/Recorder: Measurer: Handler: 1. Choose one version of Non-Newtonian fluid that you will test. Write that version on the line. A. B. C. Glue Borax Solution Glue Borax Solution Water Water Corn Starch 2. The measurer will measure how far the silly putty has fallen from its original spot on a vertical surface at 30 seconds, 1 minute, 3 minutes, 5 minutes and 3 additional times decided by your group. These times must be less than 10 minutes long. Write your three times in the boxes below. 3. Order your times from smallest to greatest in the table on the next page. 4. Create your version of the non-newtonian liquid. 5. Place non-newtonian liquid on a vertical surface (window, white board, chalk board) by holding the it against the surface for 10 seconds. BEFORE LETTING GO of the non-newtonian liquid, MARK where the bottom of the non-newtonian liquid is with a marker (make sure the line is wide enough that you can see it even after the silly putty drips over it). Once the handler let s go of the non-newtonian liquid, the Timer starts the timer. 6. Measure the distance the non-newtonian liquid has fallen from its starting point using a ruler at each designated time and record your results in the table. 7. Once you are done, throw out the non-newtonian liquid, along with the cup, mixing stick, and any excess materials used to make the non-newtonian liquid Directions: Record how far your silly putty has fallen from it s original location. Be sure - 2
3 to include the time of your recording. TIME 0 minutes DISTANCE 0 cm Directions: Plot the data on the graph below. Label your axis, scale and units. - 3
4 Analysis 1. If you did the experiment again, would you get the same results? Why or why not? 2. Compare your results to groups who measured the different versions of non-newtonian fluids. Which version had the most viscosity? Support your answer with evidence. 3. Which version had the least viscosity? Support your answer with evidence. 4. In your own words, describe what a non-newtonian fluid is. Include an example of a non-newtonian fluid, and where you might find an example of one. - 4
5 Procedures for Silly Putty Non-Newtonian Fluid Version A: Glue and Borax 1. Fill the cup half-way with glue. 2. Add one drop of food coloring to the glue and stir well with the craft stick. 3. Add one dropper full of sodium tetraborate (Borax solution) into the glue and stir. Notice what happens to the glue and Borax solution. 4. Continue adding a dropper full of sodium tetraborate (Borax solution) at a time and stirring well after each addition until the glue solution takes on a silly-putty texture (this should take 3-6 times). You will need to really stir it for about 2 minutes with the stick. 5. Remove the solid glob and roll it around in your hands to dry it off. It will remain sticky for one or two minutes and then will take on the elastic quality of putty. Non-Newtonian Fluid Version B: Glue, Water, and Borax 1. Fill the cup ¼ of the way with glue. 2. Add one drop of food coloring to the glue and stir well with the craft stick. 3. Add water until the cup is ½ full. Stir with the craft stick until the glue and water are well combined. 4. Add one dropper full of sodium tetraborate (Borax solution) into the glue and stir. Notice what happens to the glue and Borax solution. 5. Continue adding a dropper full of sodium tetraborate (Borax solution) at a time and stirring well after each addition until the glue solution takes on a silly-putty texture. You will need to really stir it for about 2 minutes with the stick. Be careful not to add too much borax to the solution, or the silly putty will become too stiff. A good rule of thumb is to quit adding the borax solution when there is still a little glue-water mixture left in the bottom of the cup. This way you will not add too much borax. 6. Remove the solid glob and roll it around in your hands to dry it off. It will remain sticky for one or two minutes and then will take on the elastic quality of putty. - 5
6 Non-Newtonian Fluid Version C: Water and Corn Starch 1. Fill the cup half-way with water. 2. Add one drop of food coloring to the water and stir well with the craft stick. 3. Add one spoonful of cornstarch into the water and stir. Notice what happens to the water and cornstarch. 4. Continue adding a spoonful of cornstarch at a time and stirring well after each addition until the water solution takes on a gooey fluid-like/silly putty consistency. (You will need to add about twice the amount of cornstarch as water.) Notice what the new consistency of the water solution is like. - 6
A SLIPPERY SLIMY SUBSTANCE
 A SLIPPERY SLIMY SUBSTANCE PRE LAB DISCUSSION Scientists must use exact language to communicate their observations and ideas to other scientists. Many words have different meanings to different people.
A SLIPPERY SLIMY SUBSTANCE PRE LAB DISCUSSION Scientists must use exact language to communicate their observations and ideas to other scientists. Many words have different meanings to different people.
Fluids: How thick are liquids?
 Fluids: How thick are liquids? Student Advanced Version Introduction: Fluids are substances that can flow under an applied force. What are some examples of fluids? We often think of fluids as substances
Fluids: How thick are liquids? Student Advanced Version Introduction: Fluids are substances that can flow under an applied force. What are some examples of fluids? We often think of fluids as substances
NON-NEWTONIAN FLUIDS. What are they? AND POLYMERS
 NON-NEWTONIAN FLUIDS What are they? AND POLYMERS VOCABULARY Non-Newtonian Dilatant rigid Thixotropic does NOT follow the laws of physics as described by Newton adding energy (shear force) makes a liquid
NON-NEWTONIAN FLUIDS What are they? AND POLYMERS VOCABULARY Non-Newtonian Dilatant rigid Thixotropic does NOT follow the laws of physics as described by Newton adding energy (shear force) makes a liquid
Save time by mixing the two solutions below in advance of the activity. You could do this with the participants if you have plenty of time.
 CREEPY PUTTY Grades 3 5, 6 8 30 45 minutes DESIGN CHALLENGE Experiment with the properties of materials as you manipulate a Silly Putty-like material to have different degrees of viscoelasticity. Create
CREEPY PUTTY Grades 3 5, 6 8 30 45 minutes DESIGN CHALLENGE Experiment with the properties of materials as you manipulate a Silly Putty-like material to have different degrees of viscoelasticity. Create
MiSP Weathering and Erosion Worksheet #1 L1
 MiSP Weathering and Erosion Worksheet #1 L1 Weathering and Erosion Worksheet#1L1 Name Date L 1, 2, 3 MASS WASTING - GLACIAL CREEPING Advance Preparation Making Glacial Ooze Recipe for each lab group: The
MiSP Weathering and Erosion Worksheet #1 L1 Weathering and Erosion Worksheet#1L1 Name Date L 1, 2, 3 MASS WASTING - GLACIAL CREEPING Advance Preparation Making Glacial Ooze Recipe for each lab group: The
What Do You Think? Investigate GOALS
 Activity 7 Polymers GOALS In this activity you will: Make a polymer-based material that has properties different from other states of matter that you have studied. Observe the material s properties and
Activity 7 Polymers GOALS In this activity you will: Make a polymer-based material that has properties different from other states of matter that you have studied. Observe the material s properties and
Objective Students will gain an understanding of how the properties of a solid material can affect how it interacts with water.
 OOBLECK! (1 Hour) Addresses NGSS Level of Difficulty: 4 Grade Range: K-2 OVERVIEW Students will examine the behavior of different types of solids when they are dissolved in water and explain those behaviors
OOBLECK! (1 Hour) Addresses NGSS Level of Difficulty: 4 Grade Range: K-2 OVERVIEW Students will examine the behavior of different types of solids when they are dissolved in water and explain those behaviors
Castle Challenge Teacher Instructions (First Third Grade)
 Castle Challenge Teacher Instructions (First Third Grade) The set of experiments explores the concept of surface tension. Students are asked to help Prince Charming save his princess from the castle by
Castle Challenge Teacher Instructions (First Third Grade) The set of experiments explores the concept of surface tension. Students are asked to help Prince Charming save his princess from the castle by
Modeling Conservation of Matter
 Modeling Conservation of Matter Imagine that you and two of your classmates want to make a strawberry banana smoothie. You lay out the ingredients: one banana, five strawberries and two scoops of ice cream.
Modeling Conservation of Matter Imagine that you and two of your classmates want to make a strawberry banana smoothie. You lay out the ingredients: one banana, five strawberries and two scoops of ice cream.
Partnerships Implementing Engineering Education Worcester Polytechnic Institute Worcester Public Schools Supported by: National Science Foundation
 Introduction to Engineering: 1.A.III Chemical Engineering Grade Level 1 Sessions Seasonality Instructional Mode(s) Team Size MA Frameworks WPS Benchmarks Key Words Session I: What do chemical engineers
Introduction to Engineering: 1.A.III Chemical Engineering Grade Level 1 Sessions Seasonality Instructional Mode(s) Team Size MA Frameworks WPS Benchmarks Key Words Session I: What do chemical engineers
Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from
 Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from http://www.csun.edu/chemteach/activities/beginninganalysis.pdf ] In this lab, you will be observing physical and chemical properties
Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from http://www.csun.edu/chemteach/activities/beginninganalysis.pdf ] In this lab, you will be observing physical and chemical properties
Oil and Natural Gas in Arkansas Fossil Fuel Resources from the Natural State
 NS.1.7.1 NS.1.6.4 PS.5.5.2 PS.5.6.5 ESS.8.5.7 Oil and Natural Gas in Arkansas Fossil Fuel Resources from the Natural State Middle School Lesson Plan Lesson 3 : Oil and Natural Gas Deposits Science Grades
NS.1.7.1 NS.1.6.4 PS.5.5.2 PS.5.6.5 ESS.8.5.7 Oil and Natural Gas in Arkansas Fossil Fuel Resources from the Natural State Middle School Lesson Plan Lesson 3 : Oil and Natural Gas Deposits Science Grades
Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from
 Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from http://www.csun.edu/chemteach/activities/beginninganalysis.pdf ] In this lab, you will be observing physical and chemical properties
Name: Date: Period: Identifying & Analyzing Powders Lab [Adapted from http://www.csun.edu/chemteach/activities/beginninganalysis.pdf ] In this lab, you will be observing physical and chemical properties
PREPARE FOR THE ACTIVITY. Activity Sheet Chapter 6, Lesson 8 ph and Color Change
 Activity Sheet Chapter 6, Lesson 8 ph and Color Change Name Date DEMONSTRATION 1. Your teacher poured green universal indicator into each of two cups. What does the change in color of the indicator solution
Activity Sheet Chapter 6, Lesson 8 ph and Color Change Name Date DEMONSTRATION 1. Your teacher poured green universal indicator into each of two cups. What does the change in color of the indicator solution
Saturday Science Lesson Plan Fall 2008
 Saturday Science Lesson Plan Fall 2008 LEARNING OBJECTIVES STANDARDS 1.1.1 Observe, describe, draw, and sort objects carefully to learn about them. 1.2.6 Describe and compare objects in terms of number,
Saturday Science Lesson Plan Fall 2008 LEARNING OBJECTIVES STANDARDS 1.1.1 Observe, describe, draw, and sort objects carefully to learn about them. 1.2.6 Describe and compare objects in terms of number,
Name Period Date. Lab: Introduction to Stoichiometry
 Name Period Date Lab: Introduction to Stoichiometry Introduction: Reactants are not always present in the exact ratio required by a balanced chemical equation. In planning any cost-effective production
Name Period Date Lab: Introduction to Stoichiometry Introduction: Reactants are not always present in the exact ratio required by a balanced chemical equation. In planning any cost-effective production
Oobleck: A Program about States of Matter Presented by the Sciencenter in Ithaca, NY. Program Overview
 Oobleck: A Program about States of Matter Presented by the Sciencenter in Ithaca, NY Program Overview Oobleck introduces students to states of matter and scientific observation. The program is designed
Oobleck: A Program about States of Matter Presented by the Sciencenter in Ithaca, NY Program Overview Oobleck introduces students to states of matter and scientific observation. The program is designed
Virtual Library Lesson: Oobleck, Gloop, and Glurch
 Oobleck, Gloop, and Glurch Lesson Overview Throughout this lesson, students will use inquiry skills to identify states of matter, describe physical properties, and modify the recipe to change physical
Oobleck, Gloop, and Glurch Lesson Overview Throughout this lesson, students will use inquiry skills to identify states of matter, describe physical properties, and modify the recipe to change physical
Section 1: Four States of Matter (pages 60-67)
 Name: Sci Number: Period: Parent Signature: My little book of: Word: Pg found States of matter Solid Four States of Matter: Section 1 definitions: (pg60) My definition Chp 3 Draw/paste examples of all
Name: Sci Number: Period: Parent Signature: My little book of: Word: Pg found States of matter Solid Four States of Matter: Section 1 definitions: (pg60) My definition Chp 3 Draw/paste examples of all
Station 1 Water is a polar molecule and has a very unique structure
 Station 1 Water is a polar molecule and has a very unique structure A water molecule, because of its shape, is a polar molecule. That is, it has one side that is positively charged and one side that is
Station 1 Water is a polar molecule and has a very unique structure A water molecule, because of its shape, is a polar molecule. That is, it has one side that is positively charged and one side that is
Surface Tension: Liquids Stick Together Student Version
 Surface Tension: Liquids Stick Together Student Version In this lab you will learn about properties of liquids, specifically cohesion, adhesion, and surface tension. These principles will be demonstrated
Surface Tension: Liquids Stick Together Student Version In this lab you will learn about properties of liquids, specifically cohesion, adhesion, and surface tension. These principles will be demonstrated
WHAT EXACTLY IS SLIME???
 SLIME Pre-Lab - Read the following passages below out loud with your partner. After you are done reading answer the questions that follow in complete sentences. WHAT EXACTLY IS SLIME??? The mixture of
SLIME Pre-Lab - Read the following passages below out loud with your partner. After you are done reading answer the questions that follow in complete sentences. WHAT EXACTLY IS SLIME??? The mixture of
States of Matter: A Solid Lesson where Liquids Can be a Gas!
 TEACHER GUIDE STATES OF MATTER 60 Minute Physical Science Lesson Science- to- Go! Program Grades: 1-3 States of Matter: A Solid Lesson where Liquids Can be a Gas! Description Your classroom will be converted
TEACHER GUIDE STATES OF MATTER 60 Minute Physical Science Lesson Science- to- Go! Program Grades: 1-3 States of Matter: A Solid Lesson where Liquids Can be a Gas! Description Your classroom will be converted
Table of Contents. Diagnostic Pre-test... 5 Lesson 1: What Is an Atom? Lesson 5: Gases. Lesson 6: Melting and Freezing. Lesson 2: What Are Molecules?
 Table of Contents Diagnostic Pre-test................. 5 Lesson 1: What Is an Atom? What Can You See?................ 11 What Is Matter Made Of?........... 12 Describing the Atom............... 13 What
Table of Contents Diagnostic Pre-test................. 5 Lesson 1: What Is an Atom? What Can You See?................ 11 What Is Matter Made Of?........... 12 Describing the Atom............... 13 What
Chromatography Lab # 4
 Chromatography Lab # 4 Chromatography is a method for separating mixtures based on differences in the speed at which they migrate over or through a stationary phase which means that a complex mixture will
Chromatography Lab # 4 Chromatography is a method for separating mixtures based on differences in the speed at which they migrate over or through a stationary phase which means that a complex mixture will
Earth s Tectonic Plates
 Earth s Tectonic Plates Lesson 3 The Earth is known as the blue planet because two-thirds of its surface lies beneath the ocean. Before the 19 th century most people thought the ocean floors were flat
Earth s Tectonic Plates Lesson 3 The Earth is known as the blue planet because two-thirds of its surface lies beneath the ocean. Before the 19 th century most people thought the ocean floors were flat
Surface Tension: Liquids Stick Together Teacher Version
 Surface Tension: Liquids Stick Together Teacher Version In this lab you will learn about properties of liquids, specifically cohesion, adhesion, and surface tension. These principles will be demonstrated
Surface Tension: Liquids Stick Together Teacher Version In this lab you will learn about properties of liquids, specifically cohesion, adhesion, and surface tension. These principles will be demonstrated
Cell Membranes and Permeability Laboratory
 Cell Membranes and Permeability Laboratory Do all chemical substances pass in and out of a cell membrane with equal ease? Do chemical substances move from areas of high concentration to areas of low concentration
Cell Membranes and Permeability Laboratory Do all chemical substances pass in and out of a cell membrane with equal ease? Do chemical substances move from areas of high concentration to areas of low concentration
We may have a general idea that a solid is hard and a fluid is soft. This is not satisfactory from
 Chapter 1. Introduction 1.1 Some Characteristics of Fluids We may have a general idea that a solid is hard and a fluid is soft. This is not satisfactory from scientific or engineering point of view. In
Chapter 1. Introduction 1.1 Some Characteristics of Fluids We may have a general idea that a solid is hard and a fluid is soft. This is not satisfactory from scientific or engineering point of view. In
T h e M yster y Pow der s L ab: Ph ysical an d Ch em ical Ch an ges
 T h e M yster y Pow der s L ab: Ph ysical an d Ch em ical Ch an ges Background Information: You have learned how to describe matter based on its physical and chemical properties. You have also learned
T h e M yster y Pow der s L ab: Ph ysical an d Ch em ical Ch an ges Background Information: You have learned how to describe matter based on its physical and chemical properties. You have also learned
Density. weight: a measure of the pull of gravity on an object
 Imagine that it is a very hot day. You decide to cool a glass of water by placing several ice cubes in the drink. What happens when you drop the ice into the water? Likely, when you place the first ice
Imagine that it is a very hot day. You decide to cool a glass of water by placing several ice cubes in the drink. What happens when you drop the ice into the water? Likely, when you place the first ice
How Can We Describe Liquids?
 How Can We Describe Liquids? Focus: Students explore and describe various liquids, then sequence liquids according to a variety of properties. Specific Curriculum Outcomes Students will be expected to:
How Can We Describe Liquids? Focus: Students explore and describe various liquids, then sequence liquids according to a variety of properties. Specific Curriculum Outcomes Students will be expected to:
Mixtures. Part 2 Add 50 ml of water (one full syringe) to each cup. Stir and observe. Write your observations on the opposite page.
 Mixtures Part 1 Prepare three cups. Put 1 level spoon (5 ml) of each solid material in each cup. Observe the three solid materials. Fill in the property chart below. Color Texture Particle shape Particle
Mixtures Part 1 Prepare three cups. Put 1 level spoon (5 ml) of each solid material in each cup. Observe the three solid materials. Fill in the property chart below. Color Texture Particle shape Particle
Real Science-4-Kids. Level I. Laboratory Workbook. Dr. R. W. Keller
 Real Science-4-Kids Level I Laboratory Workbook Dr. R. W. Keller Cover design: David Keller Opening page: David Keller, Rebecca Keller Illustrations: Rebecca Keller Copyright 2004, 2007 Gravitas Publications,
Real Science-4-Kids Level I Laboratory Workbook Dr. R. W. Keller Cover design: David Keller Opening page: David Keller, Rebecca Keller Illustrations: Rebecca Keller Copyright 2004, 2007 Gravitas Publications,
1. The Properties of Fluids
 1. The Properties of Fluids [This material relates predominantly to modules ELP034, ELP035] 1.1 Fluids 1.1 Fluids 1.2 Newton s Law of Viscosity 1.3 Fluids Vs Solids 1.4 Liquids Vs Gases 1.5 Causes of viscosity
1. The Properties of Fluids [This material relates predominantly to modules ELP034, ELP035] 1.1 Fluids 1.1 Fluids 1.2 Newton s Law of Viscosity 1.3 Fluids Vs Solids 1.4 Liquids Vs Gases 1.5 Causes of viscosity
Energy Transformations Activities Students engage in different activities to observe energy changing form!
 Energy Transformations Activities Students engage in different activities to observe energy changing form! Name: Energy Labs and Activities Activity 1: Static Electricity Glue this side down Procedure:
Energy Transformations Activities Students engage in different activities to observe energy changing form! Name: Energy Labs and Activities Activity 1: Static Electricity Glue this side down Procedure:
Creative Classroom Lessons
 Matter, Matter Everywhere! Solids, Liquids and Gases Unit Creative Classroom Lessons Contents: 1. Solids, Liquids and gases student folder cover 2. Introduction Activity: Mystery Box 3. Solids, Liquids
Matter, Matter Everywhere! Solids, Liquids and Gases Unit Creative Classroom Lessons Contents: 1. Solids, Liquids and gases student folder cover 2. Introduction Activity: Mystery Box 3. Solids, Liquids
LAB Exercise #10 What controls rheology?
 LAB Exercise #10 What controls rheology? Based on lab exercise developed by Dyanna Czeck Exercises are in two parts. The Lab exercise is to be completed and submitted today. The Homework Problems are Due
LAB Exercise #10 What controls rheology? Based on lab exercise developed by Dyanna Czeck Exercises are in two parts. The Lab exercise is to be completed and submitted today. The Homework Problems are Due
5 th GRADE PHYSICAL AND CHEMICAL TESTS
 5 th GRADE PHYSICAL AND CHEMICAL TESTS Summary: Students investigate 5 unknown white powders. They gather clues by observing the physical and chemical changes of the powders. At the end of the activity,
5 th GRADE PHYSICAL AND CHEMICAL TESTS Summary: Students investigate 5 unknown white powders. They gather clues by observing the physical and chemical changes of the powders. At the end of the activity,
Checkpoint Identify matter and non-matter from the following air, temperature, table, thoughts, emotions, water, pen, prayer and phone.
 1 World of Matter What is matter? Definition Matter is something that occupies space and has mass. Checkpoint Identify matter and non-matter from the following air, temperature, table, thoughts, emotions,
1 World of Matter What is matter? Definition Matter is something that occupies space and has mass. Checkpoint Identify matter and non-matter from the following air, temperature, table, thoughts, emotions,
Section 3. What Drives the Plates? What Do You See? Think About It. Investigate. Learning Outcomes
 Section 3 What Drives the Plates? What Do You See? Learning Outcomes In this section, you will Calculate the density of liquids and compare their densities with their position in a column of liquid. Observe
Section 3 What Drives the Plates? What Do You See? Learning Outcomes In this section, you will Calculate the density of liquids and compare their densities with their position in a column of liquid. Observe
mixtures reflect What properties could you use to separate the ingredients in these mixtures?
 reflect Every day, we interact with many different kinds of matter. We look at it, feel it, taste it, and even breathe it. Sometimes different types of matter are combined. For example, a salad might have
reflect Every day, we interact with many different kinds of matter. We look at it, feel it, taste it, and even breathe it. Sometimes different types of matter are combined. For example, a salad might have
Predict the effect of increased competition for abiotic and biotic resources on a food web. colored pencils graph paper ruler
 Edit File QUICK LAB Effect of Abiotic and Biotic Factors No organism exists in isolation. Organisms depend on and compete for the abiotic, or non-living, factors in its environment. For example, organisms
Edit File QUICK LAB Effect of Abiotic and Biotic Factors No organism exists in isolation. Organisms depend on and compete for the abiotic, or non-living, factors in its environment. For example, organisms
Surface Tension: Liquids Stick Together Student Advanced Version
 Surface Tension: Liquids Stick Together Student Advanced Version In this lab you will learn about properties of liquids, specifically cohesion, adhesion, and surface tension. These principles will be demonstrated
Surface Tension: Liquids Stick Together Student Advanced Version In this lab you will learn about properties of liquids, specifically cohesion, adhesion, and surface tension. These principles will be demonstrated
APPENDIX A USEFUL EQUATIONS (METRIC AND IMPERIAL SYSTEMS) THE DEFINITION OF VISCOSITY RHEOLOGICAL (VISCOUS BEHAVIOR) PROPERTIES OF FLUIDS
 APPENDIX A USEFUL EQUATIONS (METRIC AND IMPERIAL SYSTEMS) THE DEFINITION OF VISCOSITY RHEOLOGICAL (VISCOUS BEHAVIOR) PROPERTIES OF FLUIDS A APPENDIX A APPENDIX A Flow vs. velocity Specific gravity vs.
APPENDIX A USEFUL EQUATIONS (METRIC AND IMPERIAL SYSTEMS) THE DEFINITION OF VISCOSITY RHEOLOGICAL (VISCOUS BEHAVIOR) PROPERTIES OF FLUIDS A APPENDIX A APPENDIX A Flow vs. velocity Specific gravity vs.
Cambridge International Examinations Cambridge International General Certificate of Secondary Education
 Cambridge International Examinations Cambridge International General Certificate of Secondary Education *4923060500* CO-ORDINATED SCIENCES 0654/61 Paper 6 Alternative to Practical October/November 2014
Cambridge International Examinations Cambridge International General Certificate of Secondary Education *4923060500* CO-ORDINATED SCIENCES 0654/61 Paper 6 Alternative to Practical October/November 2014
1/7. 4-1) Introduction
 1/7 Measurement Technology Monthly Periodical Article for the February 2013 issue Classification Products and technology 1) Title Vibration Rheometer RV-10000 2) Subtitle Viscosity of various liquids measured
1/7 Measurement Technology Monthly Periodical Article for the February 2013 issue Classification Products and technology 1) Title Vibration Rheometer RV-10000 2) Subtitle Viscosity of various liquids measured
Chapter 1, Lesson 3: The Ups and Downs of Thermometers
 Chapter 1, Lesson 3: The Ups and Downs of Thermometers Key Concepts The way a thermometer works is an example of heating and cooling a liquid. When heated, the molecules of the liquid in the thermometer
Chapter 1, Lesson 3: The Ups and Downs of Thermometers Key Concepts The way a thermometer works is an example of heating and cooling a liquid. When heated, the molecules of the liquid in the thermometer
THE LAWS OF MOTION. Mr. Banks 7 th Grade Science
 THE LAWS OF MOTION Mr. Banks 7 th Grade Science MOTION Motion is a change in position over a certain amount of time. When you say that something has moved you are describing motion. SPEED Speed is the
THE LAWS OF MOTION Mr. Banks 7 th Grade Science MOTION Motion is a change in position over a certain amount of time. When you say that something has moved you are describing motion. SPEED Speed is the
Surface Tension: Liquids Stick Together Advanced Student Version
 Surface Tension: Liquids Stick Together Advanced Student Version Image from www.eyefetch.com In this lab you will learn about surface tension. Surface tension is a special property of liquids that allows
Surface Tension: Liquids Stick Together Advanced Student Version Image from www.eyefetch.com In this lab you will learn about surface tension. Surface tension is a special property of liquids that allows
Activity Sheet Transferring thermal energy by dissolving salts
 Student Name: Date: Activity Sheet Transferring thermal energy by dissolving salts 1) Define Thermal energy and temperature in the boxes below. Thermal Energy Temperature Practice Experiment: Aim: To practice
Student Name: Date: Activity Sheet Transferring thermal energy by dissolving salts 1) Define Thermal energy and temperature in the boxes below. Thermal Energy Temperature Practice Experiment: Aim: To practice
Procedure: 1. On your wax paper, place 5-10 drops of water in one area. 3. What do you notice the water does on the wax paper?
 Properties Lab NAME: Date: Background: is everywhere. It makes up about 3/4ths of the surface of the earth. It makes up 50-95% of the weight of living organisms. It is in the air we breathe, the sinks
Properties Lab NAME: Date: Background: is everywhere. It makes up about 3/4ths of the surface of the earth. It makes up 50-95% of the weight of living organisms. It is in the air we breathe, the sinks
Redhound Day 2 Assignment (continued)
 Redhound Day 2 Assignment (continued) Directions: Watch the power point and answer the questions on the last slide Which Law is It? on your own paper. You will turn this in for a grade. Background Sir
Redhound Day 2 Assignment (continued) Directions: Watch the power point and answer the questions on the last slide Which Law is It? on your own paper. You will turn this in for a grade. Background Sir
Friction. Objectives. Assessment. Assessment. Physics terms. Equations 5/20/14. Models for friction
 Objectives Friction Calculate friction forces from equation models for static, kinetic, and rolling friction. Solve one-dimensional force problems that include friction. 1. A box with a mass of 10 kg is
Objectives Friction Calculate friction forces from equation models for static, kinetic, and rolling friction. Solve one-dimensional force problems that include friction. 1. A box with a mass of 10 kg is
Applications in Forensic Science. T. Trimpe
 Applications in Forensic Science T. Trimpe 2006 http://sciencespot.net/ What is chromatography? From Wikipedia... Chromatography (from Greek word for chromos for colour) is the collective term for a family
Applications in Forensic Science T. Trimpe 2006 http://sciencespot.net/ What is chromatography? From Wikipedia... Chromatography (from Greek word for chromos for colour) is the collective term for a family
3. Kira makes 93 greeting cards for a craft fair. She sells the cards in packs of 5. How many full packs of greeting cards does Kira make?
 Chapter 4 Test 1. 2. 3. Kira makes 93 greeting cards for a craft fair. She sells the cards in packs of 5. How many full packs of greeting cards does Kira make? packs Work Space: 5. A kennel is moving 160
Chapter 4 Test 1. 2. 3. Kira makes 93 greeting cards for a craft fair. She sells the cards in packs of 5. How many full packs of greeting cards does Kira make? packs Work Space: 5. A kennel is moving 160
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education CHEMISTRY
 Centre Number Candidate Number Name UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education CHEMISTRY 06/06 Paper 6 Alternative to Practical Candidates
Centre Number Candidate Number Name UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education CHEMISTRY 06/06 Paper 6 Alternative to Practical Candidates
A STUDY OF REACTION RATES
 CH095 A Study of Reaction Rates Page 1 A STUDY OF REACTION RATES Chemical reaction rates are affected by changes in concentration of the reactants and temperature. How concentration and temperature affect
CH095 A Study of Reaction Rates Page 1 A STUDY OF REACTION RATES Chemical reaction rates are affected by changes in concentration of the reactants and temperature. How concentration and temperature affect
Test Review- Final- Physical Science- Strausser Chapters 1-5. Due May 5, 2014
 Test Review- Final- Physical Science- Strausser 2013-2014- Chapters 1-5 Due May 5, 2014 Name Date Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Technology
Test Review- Final- Physical Science- Strausser 2013-2014- Chapters 1-5 Due May 5, 2014 Name Date Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Technology
Rheometer: Procedure: Part A: Viscosity v Time
 Rheometer A fluid is defined as a substance that deforms continuously under the action of a shear stress, no matter how small the shear stress may be. Without shear stress, there will be no deformation.
Rheometer A fluid is defined as a substance that deforms continuously under the action of a shear stress, no matter how small the shear stress may be. Without shear stress, there will be no deformation.
Handle Food Samples with Care for Reliable Rheological Results
 Handle Food Samples with Care for Reliable Rheological Results Dr. Klaus Oldörp The world leader in serving science Overview Food and rheology Sample handling before the measurement The right measuring
Handle Food Samples with Care for Reliable Rheological Results Dr. Klaus Oldörp The world leader in serving science Overview Food and rheology Sample handling before the measurement The right measuring
Physical & Chemical PROPERTIES
 Name Test Date Hour Group Chem #4 Notebook Properties & Changes LEARNING TARGETS I can give examples of physical and chemical properties. I can give examples of physical and chemical changes. I can identify
Name Test Date Hour Group Chem #4 Notebook Properties & Changes LEARNING TARGETS I can give examples of physical and chemical properties. I can give examples of physical and chemical changes. I can identify
STRUCTURAL GEOLOGY. Deformation
 eformation STRUCTURAL GEOLOGY Styles of deformation These items can be used to demonstrate different styles of deformation to the class. To show elastic deformation: elastic band, sponge or plastic ruler,
eformation STRUCTURAL GEOLOGY Styles of deformation These items can be used to demonstrate different styles of deformation to the class. To show elastic deformation: elastic band, sponge or plastic ruler,
Newton s Laws of Motion Discovery
 Student handout Since the first caveman threw a rock at a sarer- toothed tiger, we ve been intrigued by the study of motion. In our quest to understand nature, we ve looked for simple, fundamental laws
Student handout Since the first caveman threw a rock at a sarer- toothed tiger, we ve been intrigued by the study of motion. In our quest to understand nature, we ve looked for simple, fundamental laws
LEVEL ZERO VOICE CATALYST (10 minutes, individual work): 1. Counting atoms
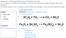 Assignment 4 Chemical Detectives at the Molecular Level LO: To explain the differences between chemical and physical changes through observable evidence. EQ: What evidence can be used to tell the difference
Assignment 4 Chemical Detectives at the Molecular Level LO: To explain the differences between chemical and physical changes through observable evidence. EQ: What evidence can be used to tell the difference
Forces. A force is a push or a pull on an object
 Forces Forces A force is a push or a pull on an object Arrows are used to represent forces. The direction of the arrow represent the direction the force that exist or being applied. Forces A net force
Forces Forces A force is a push or a pull on an object Arrows are used to represent forces. The direction of the arrow represent the direction the force that exist or being applied. Forces A net force
Lesson Plan: Diffusion
 Lesson Plan: Diffusion Background Particles in cells show rapid back and forth movement, or Brownian motion, which is also known as diffusion. The back and forth motion consists of random steps from a
Lesson Plan: Diffusion Background Particles in cells show rapid back and forth movement, or Brownian motion, which is also known as diffusion. The back and forth motion consists of random steps from a
Predictions: I predicted that when we combine the Sodium Borate with the Poly Alcohol that a thick slime will be made and the volume will change.
 Braden Monteyro Due:February 7, 2016 Polymers and Cross Linking Introduction: This lab was completed February 7, 2017. My lab partner was David. In Phase 1 of this lab we combined two reactants, Polyvinyl
Braden Monteyro Due:February 7, 2016 Polymers and Cross Linking Introduction: This lab was completed February 7, 2017. My lab partner was David. In Phase 1 of this lab we combined two reactants, Polyvinyl
Fluid Mechanics Abdusselam Altunkaynak
 Fluid Mechanics Abdusselam Altunkaynak 1. Unit systems 1.1 Introduction Natural events are independent on units. The unit to be used in a certain variable is related to the advantage that we get from it.
Fluid Mechanics Abdusselam Altunkaynak 1. Unit systems 1.1 Introduction Natural events are independent on units. The unit to be used in a certain variable is related to the advantage that we get from it.
Chemistry 1B Experiment 14 65
 Chemistry 1B Experiment 14 65 14 Electrochemistry Introduction In this experiment you will observe some spontaneous and non-spontaneous oxidation-reduction reactions, and see how the spontaneous reactions
Chemistry 1B Experiment 14 65 14 Electrochemistry Introduction In this experiment you will observe some spontaneous and non-spontaneous oxidation-reduction reactions, and see how the spontaneous reactions
Thermal Convection of a Fluid
 C04 Thermal Convection of a Fluid http://web.ics.purdue.edu/~braile/edumod/convect/convect.htm Focus on Inquiry The students will calculate the velocity of convection currents using vegetable oil and thyme
C04 Thermal Convection of a Fluid http://web.ics.purdue.edu/~braile/edumod/convect/convect.htm Focus on Inquiry The students will calculate the velocity of convection currents using vegetable oil and thyme
Blast off and enjoy these Space Activities. ~Holly
 Blast off and enjoy these Space Activities ~Holly Astronaut Song Tune: If You're Happy and You Know It" Outer space is where I really like to go. I ride inside a spaceship, don't you know? I like to travel
Blast off and enjoy these Space Activities ~Holly Astronaut Song Tune: If You're Happy and You Know It" Outer space is where I really like to go. I ride inside a spaceship, don't you know? I like to travel
The Laws of Motion. Gravity and Friction
 The Laws of Motion Gravity and Friction Types of Forces Think about all the things you pushed or pulled today. You might have pushed toothpaste out of a tube. Maybe you pulled out a chair to sit down.
The Laws of Motion Gravity and Friction Types of Forces Think about all the things you pushed or pulled today. You might have pushed toothpaste out of a tube. Maybe you pulled out a chair to sit down.
September 16, Read & Annotate the reading individually. When you finish... Scientific Method Foldable: -Cut all dotted lines Glue into the
 September 16, 2016 : Glue into the RIGHT side. Then, try to answer the questions using the following as your guide. Quantitative vs. Qualitative Observations: Quantitative= Quantity= #s Qualitative= Quality=
September 16, 2016 : Glue into the RIGHT side. Then, try to answer the questions using the following as your guide. Quantitative vs. Qualitative Observations: Quantitative= Quantity= #s Qualitative= Quality=
Merrily we roll along
 Merrily we roll along Name Period Date Lab partners Overview Measuring motion of freely falling objects is difficult because they acclerate so fast. The speed increases by 9.8 m/s every second, so Galileo
Merrily we roll along Name Period Date Lab partners Overview Measuring motion of freely falling objects is difficult because they acclerate so fast. The speed increases by 9.8 m/s every second, so Galileo
Investigation 2. Physical properties and physical change in solids. How can you tell if crystals that look the same are really the same or different?
 Investigation 2 Physical properties and physical change in solids How can you tell if crystals that look the same are really the same or different? Summary In this investigation, students compare the properties
Investigation 2 Physical properties and physical change in solids How can you tell if crystals that look the same are really the same or different? Summary In this investigation, students compare the properties
Make Your Own Rock Grade: 3-5 Time: 1 class period
 Make Your Own Rock Grade: 3-5 Time: 1 class period Lesson #4A: What Are Minerals? Overview: Students explore rock composition by making simulated edible or non-edible rocks using a variety of materials
Make Your Own Rock Grade: 3-5 Time: 1 class period Lesson #4A: What Are Minerals? Overview: Students explore rock composition by making simulated edible or non-edible rocks using a variety of materials
The Laws of Motion. Gravity and Friction
 The Laws of Motion Gravity and Friction What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with the statement
The Laws of Motion Gravity and Friction What do you think? Read the two statements below and decide whether you agree or disagree with them. Place an A in the Before column if you agree with the statement
Read & Learn. Read the provided article. Use the information in the reading to answer the questions on the task cards on your answer sheet.
 Read & Learn Read the provided article. Use the information in the reading to answer the questions on the task cards on your answer sheet. Make sure your answers in the correct spot on the answer sheet.
Read & Learn Read the provided article. Use the information in the reading to answer the questions on the task cards on your answer sheet. Make sure your answers in the correct spot on the answer sheet.
The Curious Case of Soft Matter Ranjini Bandyopadhyay Raman Research Institute
 The Curious Case of Soft Matter Ranjini Bandyopadhyay Raman Research Institute ranjini@rri.res.in. My research interests: structure, dynamics, phase behavior and flow of soft materials Panta rei: everything
The Curious Case of Soft Matter Ranjini Bandyopadhyay Raman Research Institute ranjini@rri.res.in. My research interests: structure, dynamics, phase behavior and flow of soft materials Panta rei: everything
Stoichiometry Question Paper
 Stoichiometry Question Paper Level Subject Exam Board Topic Sub-Topic Paper Type Booklet IGCSE Chemistry CIE Stoichiometry Alternative to Practical Question Paper Time Allowed: 65 minutes Score: /54 Percentage:
Stoichiometry Question Paper Level Subject Exam Board Topic Sub-Topic Paper Type Booklet IGCSE Chemistry CIE Stoichiometry Alternative to Practical Question Paper Time Allowed: 65 minutes Score: /54 Percentage:
Name per due date mail box. Friction Lab. Challenge - move your wooden block from start to finish with the least amount of force.
 Name per due date mail box Friction Lab Challenge - move your wooden block from start to finish with the least amount of force. Procedure - 1. Make sure your spring scale pointer is zeroed - if not see
Name per due date mail box Friction Lab Challenge - move your wooden block from start to finish with the least amount of force. Procedure - 1. Make sure your spring scale pointer is zeroed - if not see
Earth s Layers Activity
 Earth s Layers Activity Purpose: To visualize the basic structure of the Earth and how we study it. Materials: Procedure: A piece of poster board shaped like a pizza slice with radius of about 16 cm Compass
Earth s Layers Activity Purpose: To visualize the basic structure of the Earth and how we study it. Materials: Procedure: A piece of poster board shaped like a pizza slice with radius of about 16 cm Compass
GG170L: RHEOLOGY Handout (Material to read instead of a Lab Book chapter you might want to print this out and bring it to lab)
 GG170L: RHEOLOGY Handout (Material to read instead of a Lab Book chapter you might want to print this out and bring it to lab) Rheology is the study of how materials flow, and includes two main properties.
GG170L: RHEOLOGY Handout (Material to read instead of a Lab Book chapter you might want to print this out and bring it to lab) Rheology is the study of how materials flow, and includes two main properties.
Thermal Energy and Temperature Lab. Experiment Question: How can the difference between thermal energy and temperature be experimentally observed?
 Thermal Energy and Temperature Lab Name 7 th Grade PSI Grade / 20 Experiment Question: How can the difference between thermal energy and temperature be experimentally observed? Hypothesis Starters: 1.
Thermal Energy and Temperature Lab Name 7 th Grade PSI Grade / 20 Experiment Question: How can the difference between thermal energy and temperature be experimentally observed? Hypothesis Starters: 1.
LAB 1 PRE-LAB. residuals (cm)
 LAB 1 PRE-LAB 1. The table below records measurements of the lengths l of five goldfish. Calculate the average length l avg of this population of goldfish, and the residual, or deviation from average length
LAB 1 PRE-LAB 1. The table below records measurements of the lengths l of five goldfish. Calculate the average length l avg of this population of goldfish, and the residual, or deviation from average length
Chemistry 1B Experiment 17 89
 Chemistry 1B Experiment 17 89 17 Thermodynamics of Borax Solubility Introduction In this experiment, you will determine the values of H and S for the reaction which occurs when borax (sodium tetraborate
Chemistry 1B Experiment 17 89 17 Thermodynamics of Borax Solubility Introduction In this experiment, you will determine the values of H and S for the reaction which occurs when borax (sodium tetraborate
NEWTON S LAWS OF MOTION. Review
 NEWTON S LAWS OF MOTION Review BACKGROUND Sir Isaac Newton (1643-1727) an English scientist and mathematician famous for his discovery of the law of gravity also discovered the three laws of motion. He
NEWTON S LAWS OF MOTION Review BACKGROUND Sir Isaac Newton (1643-1727) an English scientist and mathematician famous for his discovery of the law of gravity also discovered the three laws of motion. He
Level 4 Investigative Skills
 John Buchan Middle School Level 4 Investigative Skills 69 min 59 marks John Buchan Middle School 1 1. Travelling sounds (a) Jill investigated whether or not sound travelled through different materials.
John Buchan Middle School Level 4 Investigative Skills 69 min 59 marks John Buchan Middle School 1 1. Travelling sounds (a) Jill investigated whether or not sound travelled through different materials.
Bay Area Scientists in Schools Presentation Plan
 Bay Area Scientists in Schools Presentation Plan Lesson Name Presenter(s) Chemical Reactions Mercedes Taylor, Nick Settineri, Jessica Ziegler, Tyler Hurlburt, Parker Deal Grade Level 5 Standards Connection(s)
Bay Area Scientists in Schools Presentation Plan Lesson Name Presenter(s) Chemical Reactions Mercedes Taylor, Nick Settineri, Jessica Ziegler, Tyler Hurlburt, Parker Deal Grade Level 5 Standards Connection(s)
Introduction to Geology Spring 2008
 MIT OpenCourseWare http://ocw.mit.edu 12.001 Introduction to Geology Spring 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. RHEOLOGICAL MODELS Rheology
MIT OpenCourseWare http://ocw.mit.edu 12.001 Introduction to Geology Spring 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. RHEOLOGICAL MODELS Rheology
Fluid Mechanics Prof. T.I. Eldho Department of Civil Engineering Indian Institute of Technology, Bombay. Lecture - 17 Laminar and Turbulent flows
 Fluid Mechanics Prof. T.I. Eldho Department of Civil Engineering Indian Institute of Technology, Bombay Lecture - 17 Laminar and Turbulent flows Welcome back to the video course on fluid mechanics. In
Fluid Mechanics Prof. T.I. Eldho Department of Civil Engineering Indian Institute of Technology, Bombay Lecture - 17 Laminar and Turbulent flows Welcome back to the video course on fluid mechanics. In
Lesson 1 Solids, Liquids, and Gases
 Lesson 1 Student Labs and Activities Page Launch Lab 8 Content Vocabulary 9 Lesson Outline 10 MiniLab 12 Content Practice A 13 Content Practice B 14 School to Home 15 Key Concept Builders 16 Enrichment
Lesson 1 Student Labs and Activities Page Launch Lab 8 Content Vocabulary 9 Lesson Outline 10 MiniLab 12 Content Practice A 13 Content Practice B 14 School to Home 15 Key Concept Builders 16 Enrichment
MECHANICAL PROPERTIES
 MECHANICAL PROPERTIES Rheology S.C. BAYNE, 1 J.Y. Thompson 2 1 University of Michigan School of Dentistry, Ann Arbor, MI 48109-1078 sbayne@umich.edu 2 Nova Southeastern College of Dental Medicine, Ft.
MECHANICAL PROPERTIES Rheology S.C. BAYNE, 1 J.Y. Thompson 2 1 University of Michigan School of Dentistry, Ann Arbor, MI 48109-1078 sbayne@umich.edu 2 Nova Southeastern College of Dental Medicine, Ft.
Physical Science Bingo Book
 ~A BINGO BOOK~ Physical Science Bingo Book COMPLETE BINGO GAME IN A BOOK Written by Rebecca Stark Educational Books n Bingo TITLE: Physical Science Bingo AUTHOR: Rebecca Stark 2016 Barbara M. Peller, also
~A BINGO BOOK~ Physical Science Bingo Book COMPLETE BINGO GAME IN A BOOK Written by Rebecca Stark Educational Books n Bingo TITLE: Physical Science Bingo AUTHOR: Rebecca Stark 2016 Barbara M. Peller, also
Substances and Mixtures:Separating a Mixture into Its Components
 MiraCosta College Introductory Chemistry Laboratory Substances and Mixtures:Separating a Mixture into Its Components EXPERIMENTAL TASK To separate a mixture of calcium carbonate, iron and sodium chloride
MiraCosta College Introductory Chemistry Laboratory Substances and Mixtures:Separating a Mixture into Its Components EXPERIMENTAL TASK To separate a mixture of calcium carbonate, iron and sodium chloride
Kinetics of an Iodine Clock Reaction
 Kinetics of an Iodine Clock Reaction Introduction: In this experiment, you will determine the rate law for a reaction and the effect of concentration on the rate of the reaction by studying the initial
Kinetics of an Iodine Clock Reaction Introduction: In this experiment, you will determine the rate law for a reaction and the effect of concentration on the rate of the reaction by studying the initial
Describing Matter Laboratory
 Describing Matter Laboratory Name: 5 th Grade PSI Science Score: / 5 Experiment Question: How is matter identified? What are the observable properties of matter? Hypothesis Starters: 1. Your eyes help
Describing Matter Laboratory Name: 5 th Grade PSI Science Score: / 5 Experiment Question: How is matter identified? What are the observable properties of matter? Hypothesis Starters: 1. Your eyes help
Lesson 1 Matter and Its Properties
 Lesson 1 Student Labs and Activities Page Launch Lab 8 Content Vocabulary 9 Lesson Outline 10 MiniLab 12 Content Practice A 13 Content Practice B 14 Math Skills 15 School to Home 16 Key Concept Builders
Lesson 1 Student Labs and Activities Page Launch Lab 8 Content Vocabulary 9 Lesson Outline 10 MiniLab 12 Content Practice A 13 Content Practice B 14 Math Skills 15 School to Home 16 Key Concept Builders
Non-Newtonian Fluids: The Weissenberg Effect Experiment. Sagir Kadiwala, Josh Mcbride, Joseph Mondok. University of California Merced
 Non-Newtonian Fluids: The Weissenberg Effect Experiment 1 Non-Newtonian Fluids: The Weissenberg Effect Experiment Sagir Kadiwala, Josh Mcbride, Joseph Mondok University of California Merced Non-Newtonian
Non-Newtonian Fluids: The Weissenberg Effect Experiment 1 Non-Newtonian Fluids: The Weissenberg Effect Experiment Sagir Kadiwala, Josh Mcbride, Joseph Mondok University of California Merced Non-Newtonian
