79035 Soil breccia 2806 grams
|
|
|
- Jeffery Payne
- 5 years ago
- Views:
Transcription
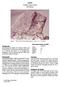
1 Soil breccia 2806 grams Figure 1: Photo of,1. Lines limit band of apparent coarse material. NASA S Sample about 8 cm across. Figure 2: Photo of,2. NASA S Cube is 1 cm.
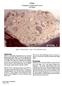
2 Figure 3: Photo of additional pieces of,3. NASA S Smallest is about 1 c x 2 cm. Mineralogical Mode for (Simon et al. 1989) Matrix 58.1 % micron micron Mare Basalt KREEP Basalt Feld. Basalt Plutonic Granulitic Breccia Olivine Pyroxene Plagioclase Opaques Glass Agglutinate Figure 4: Location of from near trench and drive tube. Introduction is a large friable regolith breccia that was probably exposed to moisture after splashdown in the Pacific. It broke in several large pieces (figures 1 3), which are rounded, yielding a lot of fines. It was collected near Van Serg Crater (figures 4) near the drive tube and trench. It has an exposure age of about 600 m.y. has been the source of material for many studies related to the implantation of solar wind into lunar regolith particles particularly ilmenite. Petrography Heuer et al. (1974) described as a dark, porous, fine-grained breccia containing clasts of ilmenite-rich basalt, fine-grained vesicular breccias and spheres and fragments of glass. Basalt fragments show moderate deformation, indicated by undulatory extinction in plagioclase and abundant twinning in ilmenite. The fine-grained matrix was examined by HVEM and found to consist of very porous glassy and crystalline fragmental material, including angular fragments of both homogeneous and highly vesicular glass and glassy spherules. The matrix glass which cements the crystalline and glassy fragments is commonly homogeneous. In some clasts, a high concentration of fossil particle tracks is preserved, indicating that the average temperature of the cementing glass was never sufficiently high to anneal these features. Housely et al. (1976) showed that has very high ferromagnetic resonance and that this was correlated with high agglutinate content with associated reduction of Fe-glass to fine metal particles. This has since been referred to as high maturity.
3 Figure 5a: Transmitted light photo of thin section of. Figure 5b: Reflected light photo of thin section of. FeO Apollo soils breccia sample/ chondrite Al2O3 Figure 6: Composition of compared with that of lunar soil samples. 0.1 La Ce Pr Nd Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Figure 7: Normalized rare-earth-element diagram for. Fruland (1983) and Simon et al. (1990) included in their study of regolith breccias (figure 8). It has the highest percentage of agglutinate fragments of the various Apollo regolith breccias. Haggerty (1974), Fredriksson et al. (1974), Simon et al. (1990) and Shearer et al. (1991) analyzed the glass in. Chemistry Wanke et al. (1974), Laul et al. (1974), Miller (1974) and Simon et al. (1990) analyzed, finding high Ni, Ir and Au, meteoritic siderophiles, typical of soil. According to Becker and Epstein (1981), has 128 ppm carbon and 74 ppm nitrogen (figure 9). Frick et al. (1987) reported 114 ppm N.
4 Table 1. Chemical composition of. Laul74 Miller74 Wanke74 Morgan74 Garg76 Simon89 reference mg weight SiO2 % TiO Al2O FeO MnO MgO CaO Na2O K2O P2O S % sum Sc ppm V Cr Co Ni Cu 11 Zn Ga 5.5 Ge ppb As 14 Se 300 Rb Sr Y Zr Nb Mo Ru Rh Pd ppb 10 Ag ppb 19 Cd ppb 71 In ppb Sn ppb Sb ppb 0.89 Te ppb 18.6 Cs ppm Ba La Ce Pr 4 Nd Sm Eu Gd Tb Dy Ho 3 Er 7.4 Tm 0.87 Yb Lu Hf Ta W ppb 0.12 Re ppb Os ppb Ir ppb Pt ppb Au ppb Th ppm U ppm technique: (a) INAA, (b)
5 Figure 8: Comparison of lithologies of Apollo 17 soil breccias (Simon et al. 1990). Cosmogenic isotopes and exposure ages Hintenberger et al. (1974, 1975) determined a 21 Ne exposure age for of 660 ± 50 m.y. Other Studies Hintenberger et al. (1974, 1975) were the first to report that had very high rare gas content due to implantation of solar wind. Frick et al. (1987) included nitrogen and went on to show this in great detail including temperature release and direct comparison to lunar soil (figure 10). Clayton and Thiemens (1980) showed the evolution of nitrogen isotopes (figure 11). Becker and Pepin (1989) started a long line of rare gas studies of individual ilmenite grains. Benkert et al. (1991, 1993), Kerridge et al. (1992, 1993), Wiens et al. (1992), Heber (2001, 2003), etc. have all used this sample to gain an understanding of solar wind implantation. Rock CMB Regolith Breccia soil carbon ppm Figure 9: Carbon content of compared with that of other lunar samples.
6 Figure 11: Nitrogen isotopes as function of thermal release (Clayton and Thiemens 1980). Figure 10: Thermal release of nitrogen and rare gases from (Frick et al. 1987). bags, and were found broken in pieces. One can only surmise that the SSLSS bag was somehow initially forgotten, and walked on while the rest of the Command Module was unloaded!! Processing This large friable soil breccia was brought back in the BSLSS which contained other large and tough samples. The large pieces of (figures 1 3) were nicely rounded, without zap pits, and was probably abraded during transit, yielding numerous fines and coarse-fines in the residue ( ) from the BSLSS (see below). The 4 10 mm particles from this bag are described in the catalog by Meyer (1973). Actually it appears that was rather abused. The BSLSS bag was found, after 10 hrs, sitting in a ¼ inch of water on the floor of the Command Module and it took a long time to dry out the sample bag in a dry nitrogen cabinet (Butler 1973). Worse yet, the four large rocks in the bag were without individual doc.
7 CMeyer 2011 BSLSS 2806 g residue outer portion of?,3 61 g fines,6 PB,60,10,17 PB,66,45, g,65,137,180 TS,72 TS, g, g, g, g References for Becker R.H. and Epstein S. (1981) Carbon isotopic ratios in some low-d l5 N lunar breccias. Proc. 12 th Lunar Planet. Sci. Conf Becker R.H. and Pepin R.O. (1989) Long-term changes in solar wind elemental and isotopic ratios: A comparison of two lunar ilmenites of different antiquities. Geochim. Cosmochim. Acta 53, Benkert J.P., Kerridge J.F., Kim J.S., Kim Y., Marti K., Signer P. and Wieler R. (1991) Evolution of isotopic signatures in lunar regolith nitrogen: Noble gases and N in ilmenite grainsize fractions from regolith breccia (abs). Lunar Planet. Sci. XXII, Lunar Planetary Institute, Houston. Benkert J.P., Baur H., Signer P. and Wieler R. (1993) He, Ne, and Ar from the solar wind and solar energetic particles in lunar ilmenites and pyroxenes. J. Geophys. Res. 98, Butler P. (1973) Lunar Sample Information Catalog Apollo 17. Lunar Receiving Laboratory. MSC Curator s Catalog. pp Christie J.M., Griggs D.T., Heuer A.H., Nord G.L., Radcliffe S.V., Lally J.S. and Fischer R.M. (1973) Electron petrography of Apollo 14 and 15 breccias and shockproduced analogs. Proc. 4 th Lunar Sci. Conf Clayton R.N. and Thiemens M.H. (1980) Lunar nitrogen: Evidence for secular change in the solar wind. In The Ancient Sun (eds Pepin, Eddy, Merrill) Frick U., Becker R.H. and Pepin R.O. (1987) Solar wind record in the lunar regolith: nitrogen and noble gases. Proc. 18 th Lunar Planet. Sci. Conf Lunar Planetary Institute, Houston Fredriksson K., Brenner P., Nelen J., Noonan A., Dube A. and Reid A. (1974) Comparative studies of impact glasses and breccias (abs). Lunar Sci. V, Lunar Planetary Institute, Houston. Fruland R.M. (1983) Regolith Breccia Workbook. Curatorial Branch Publication # 66. JSC Garg A.N. and Ehmann W.N. (1976a) Zr-Hf fractionation in chemically defined lunar rock groups. Proc. 7 th Lunar Sci. Conf Haggerty S.E. (1974) Apollo 17 orange glass: Textural and morphological characteristics of devitrification. Proc. 5 th Lunar Sci. Conf Hashizume K., Claussidon M., Marty B. and Robert F. (2000) Solar wind record on the Moon: Deciphering presolar from planetary nitrogen. Science 290, Heber V.S., Baur H. and Wieler R. (2001) High resolution solar He record in lunar samples: Evidence for a temporal variation of the solar wind composition with time? (abs#1847) Lunar Planet. Sci. XXXII, Lunar Planetary Institute, Houston. Heber Veronika S., Baur H. and Wieler R. (2003) Helium in lunar samples analyzed by high-resolution stepwise etching: Implications for the temporal constancy of solar wind isotopic composition. Astrophysical J. 597,
8 Heuer A.H., Christie J.M., Lally J.S. and Nord G.L. (1974) Electron petrographic study of some Apollo 17 breccias. Proc. 5 th Lunar Sci. Conf Hintenberger H., Weber H.W. and Schultz L. (1974a) Solar, spallenogenic, and radiogenic rare gases in Apollo 17 soils and breccias. Proc. 5 th Lunar Sci. Conf Hintenberger H., Weber H.W. and Schultz L. (1974b) Solar, spallenogenic, and radiogenic rare gases in Apollo 17 soils and breccias (abs). Lunar Sci. V, Lunar Planetary Institute, Houston Hintenberger H., Schultz L. and Weber H.W. (1975a) A comparison of noble gases in lunar fines and soil breccias: Implications for the origin of soil breccias. Proc. 6 th Lunar Sci. Conf Hintenberger H., Schultz L. and Weber H.W. (1975b) Rare gases in ilmenite and bulk samples of Apollo 17 soils and breccias (abs). Lunar Sci. VI, Lunar Planetary Institute, Houston. Housley R.M., Cirlin E.H., Goldberg I.B. and Crowe H. (1976) Ferromagnetic resonance studies of lunar core stratigraphy. Proc. 7 th Lunar Sci. Conf Kerridge J.F., Kim J.S., Kim Y. and Marti K. (1992) Evolution of isotopic signatures in lunar-regolith nitrogen: Noble gases and nitrogen in grain-size fractions from regolith breccia. Proc. 22 nd Lunar Planet. Sci Lunar Planetary Institute, Houston. Kerridge J.F., Kim Y., Kim J. and Marti K. (1993) Nitrogen isotopic signatures in agglutinates from breccia (abs). Lunar Planet. Sci. XXIV, Lunar Planetary Institute, Houston Laul J.C., Hill D.W. and Schmitt R.A. (1974d) Chemical studies of Apollo 16 and 17 samples. Proc. 5 th Lunar Sci. Conf Meyer C. (1973) Apollo 17 Coarse Fines (4-10 mm) Sample Location, Classification and Photo Index. Curator Report. pp Miller M.D., Pacer R.A., Ma M.-S., Hawke B.R., Lookhart G.L. and Ehmann W.D. (1974) Compositional studies of the lunar regolith at the Apollo 17 site. Proc. 5 th Lunar Sci. Conf Morgan J.W., Ganapathy R., Higuchi H., Krahenbuhl U. and Anders E (1974a) Lunar basins: Tentative characterization of projectiles, from meteoritic dements in Apollo 17 boulders. Proc. 5 th Lunar Sci. Conf Neal C.R. and Taylor L.A. (1993) Catalog of Apollo 17 rocks, central valley. Volumes 2 and 3. Curators Office #26088 JSC, Houston. Shearer C.K., Papike J.J., Galbreath K.C. and Shimizu N. (1991) Exploring the lunar mantle with secondary ion mass spectometry: A comparison of lunar picritic glass beads from the Apollo 14 and Apollo 17 sites. Earth Planet. Sci. Lett. 102, Simon S.B., Papike J.J., Gosselin D.C., Laul J.C., Hughes S.S. and Schmitt R.A. (1990) Petrology and chemistry of Apollo 17 regolith breccias: A history of mixing of highland and mare regolith. Proc. 20 th Lunar Planet. Sci Lunar Planetary Institute, Houston. Wänke H., Palme H., Baddenhausen H., Dreibus G., Jagoutz E., Kruse H., Spettel B., Teschke F. and Thacker R. (1974) Chemistry of Apollo 16 and 17 samples: bulk composition, late-stage accumulation and early differentiation of the Moon. Proc. 5 th Lunar Sci. Conf Wänke H., Palme H., Baddenhausen H., Dreibus G., Jagoutz E., Kruse H., Palme C., Spettel B., Teschke F. and Thacker R. (1975a) New data on the chemistry of lunar samples: Primary matter in the lunar highlands and the bulk composition of the moon. Proc. 6 th Lunar Sci. Conf Wiens R.C., Burnett D.S., Neugebauer M. and Pepin R.O. (1992) A comparison of solar wind and estimated solar system xenon abundances: A test for solid/gas fractionation in the solar nebula. Proc. 22 nd Lunar Planet. Sci Lunar Planetary Institute, Houston. Wieler R., Etique P., Signer P. and Poupean G. (1983) Decrease of the solar flare/solar wind flux ratio in the past several aeons deduced from solar neon and tracks in lunar soil plagioclases. Proc. 13 th Lunar Planet. Sci. Conf. in J. Geophys. Res. A713 -A724. Wieler R., Baur H. and Signer P. (1986) Noble gases from solar energetic particles revealed by closed system step wise etching of lunar soil minerals. Geochim. Cosmichim. Acta 50, Wolfe E.W., Bailey N.G., Lucchitta B.K., Muehlberger W.R., Scott D.H., Sutton R.L and Wilshire H.G. (1981) The geologic investigation of the Taurus-Littrow Valley: Apollo 17 Landing Site. US Geol. Survey Prof. Paper, 1080, pp. 280.
72320 Partially Shadowed Soil (portion frozen) grams. boulder # 2 station 2 South Massif
 Partially Shadowed Soil (portion ) 106.31 grams Nansen Crater boulder # 2 station 2 South Massif Figure 1: Location of soil sample in shadow of boulder 2, at station 2, Apollo 17. NASA photo #AS17-137-20925.
Partially Shadowed Soil (portion ) 106.31 grams Nansen Crater boulder # 2 station 2 South Massif Figure 1: Location of soil sample in shadow of boulder 2, at station 2, Apollo 17. NASA photo #AS17-137-20925.
grams g g g g Regolith Breccia
 15306 134.2 grams 15315 35.6 g 15324 32.3 g 15325 57.8 g 15330 57.8 g Regolith Breccia Figure 1a,b: Photo of dust-covered 15306. Sample is 7 cm across. S71-43064 and 067. Introduction 15306 is a regolith
15306 134.2 grams 15315 35.6 g 15324 32.3 g 15325 57.8 g 15330 57.8 g Regolith Breccia Figure 1a,b: Photo of dust-covered 15306. Sample is 7 cm across. S71-43064 and 067. Introduction 15306 is a regolith
,1,2,3,4,9, Impact Melt Breccia 65.4 grams
 63335 Impact Melt Breccia 65.4 grams,1,2,3,4,9,10,8 Figure 2: 63335,6. Cube is 1 cm. S75-33389.,7 Introduction 63335 is a sample chipped off of Shadow Rock (Ulrich 1973). It was collected as several fragments
63335 Impact Melt Breccia 65.4 grams,1,2,3,4,9,10,8 Figure 2: 63335,6. Cube is 1 cm. S75-33389.,7 Introduction 63335 is a sample chipped off of Shadow Rock (Ulrich 1973). It was collected as several fragments
grams grams Double Drive tube 61 cm
 68002 583.5 grams 68001 840.7 grams Double Drive tube 61 cm Figure 1a: Surface photo for double drive tube 68001-2. AS16-108-17684. Introduction Station 8 was the closest to South Ray Crater so premission
68002 583.5 grams 68001 840.7 grams Double Drive tube 61 cm Figure 1a: Surface photo for double drive tube 68001-2. AS16-108-17684. Introduction Station 8 was the closest to South Ray Crater so premission
72335 Impact melt Breccia grams
 Impact melt Breccia 108.9 grams Figure 1: Location of on boulder #2 on landslide off of South Massif. Boulder is ~ 2-3 meters high. AS17-137-20918. Transcript Hey, that s a different rock, Gene (station
Impact melt Breccia 108.9 grams Figure 1: Location of on boulder #2 on landslide off of South Massif. Boulder is ~ 2-3 meters high. AS17-137-20918. Transcript Hey, that s a different rock, Gene (station
14041, and Regolith Breccia 166.3, and 65.2 grams
 14041, and 14045 Regolith Breccia 166.3, 103.2 and 65.2 grams Figure 1a: Sample 14041-14045 on lunar surface. MET and LM in distance. AS14-68-9409. Introduction Samples 14041 14046 are fragments from a
14041, and 14045 Regolith Breccia 166.3, 103.2 and 65.2 grams Figure 1a: Sample 14041-14045 on lunar surface. MET and LM in distance. AS14-68-9409. Introduction Samples 14041 14046 are fragments from a
DRAFT Coarse-fines 569 grams
 10085 Coarse-fines 569 grams DRAFT Figure 1: Selected coarse fines from 10085. Scale is in mm. Photo from Wood et al. 1969. Introduction!0085 and 10084 were created during the Apollo 11 preliminary examination
10085 Coarse-fines 569 grams DRAFT Figure 1: Selected coarse fines from 10085. Scale is in mm. Photo from Wood et al. 1969. Introduction!0085 and 10084 were created during the Apollo 11 preliminary examination
grams grams Regolith Breccia
 10019 297 grams 10066 60 grams Regolith Breccia Figure 1: Photo of 10019,1. Cube is 1 inch and scale is in cm. NASA S76-23354. Introduction Kramer et al. (1077) reported that 10019 and 10066 appeared to
10019 297 grams 10066 60 grams Regolith Breccia Figure 1: Photo of 10019,1. Cube is 1 inch and scale is in cm. NASA S76-23354. Introduction Kramer et al. (1077) reported that 10019 and 10066 appeared to
74235 Vitrophric Basalt 59 grams
 74235 Vitrophric Basalt 59 grams Figure 1: Photo of 74235. Cube is 1 cm. S73-16017 Figure 2: Map of station 4, Apollo 17. Transcript (while the LMP was discussing the core with CC, the CDR made a discovery)
74235 Vitrophric Basalt 59 grams Figure 1: Photo of 74235. Cube is 1 cm. S73-16017 Figure 2: Map of station 4, Apollo 17. Transcript (while the LMP was discussing the core with CC, the CDR made a discovery)
65055 Basaltic Impact Melt grams
 65055 Basaltic Impact Melt 500.8 grams Figure 1: Photo of 65055 showing large zap pit. Cube is 1 cm. S72-43869. Introduction According to the Apollo 16 Catalog by Ryder and Norman, 65055 is an aluminous,
65055 Basaltic Impact Melt 500.8 grams Figure 1: Photo of 65055 showing large zap pit. Cube is 1 cm. S72-43869. Introduction According to the Apollo 16 Catalog by Ryder and Norman, 65055 is an aluminous,
14315 Unusual Regolith Breccia 115 grams
 Unusual Regolith Breccia 115 grams Figure 1: Photo of,0 after chipping and dusting. Sample is 5 cm across. NASA S86-36340. Introduction was collected as a grab sample from the North Boulder Field (station
Unusual Regolith Breccia 115 grams Figure 1: Photo of,0 after chipping and dusting. Sample is 5 cm across. NASA S86-36340. Introduction was collected as a grab sample from the North Boulder Field (station
grams grams Poikilitic Impact Melt Breccia
 64567 13.8 grams 64569-14.3 grams Poikilitic Impact Melt Breccia Figure 1: Photo of 64567. Scale in mm. S72-55386 Mineralogical Mode by Simonds et al. (1973) 64567 64569 Plagioclase 69% 57 Pyroxene 10
64567 13.8 grams 64569-14.3 grams Poikilitic Impact Melt Breccia Figure 1: Photo of 64567. Scale in mm. S72-55386 Mineralogical Mode by Simonds et al. (1973) 64567 64569 Plagioclase 69% 57 Pyroxene 10
61015 Dimict Breccia 1789 grams
 61015 Dimict Breccia 1789 grams Figure 1: Photo of 61015. NASA # S72-37758. Scale and cube in cm. Note the zap pits on the T1 surface and the residual black glass coating on the sides. Introduction Breccia
61015 Dimict Breccia 1789 grams Figure 1: Photo of 61015. NASA # S72-37758. Scale and cube in cm. Note the zap pits on the T1 surface and the residual black glass coating on the sides. Introduction Breccia
grams grams Crystalline matrix Breccia
 14069 24.87 grams 14070-36.56 grams Crystalline matrix Breccia Figure 1: Photo of 14069,0. Scale in cm and mm. S78-28808. 14070 Figure 2: Photo of 14070. Scale is in cm. S71-22168. Introduction 14069 and
14069 24.87 grams 14070-36.56 grams Crystalline matrix Breccia Figure 1: Photo of 14069,0. Scale in cm and mm. S78-28808. 14070 Figure 2: Photo of 14070. Scale is in cm. S71-22168. Introduction 14069 and
DRAFT Cataclastic Dunite grams
 72415-72418 Cataclastic Dunite 58.74 grams DRAFT Figure 1: Boulder 3 at Station 2, with large dunite clast (72415-72418). The bright spot is the location of samples. A sample of the boulder matrix (72435)
72415-72418 Cataclastic Dunite 58.74 grams DRAFT Figure 1: Boulder 3 at Station 2, with large dunite clast (72415-72418). The bright spot is the location of samples. A sample of the boulder matrix (72435)
15556 Vesicular Olivine-normative Basalt grams
 15556 Vesicular Olivine-normative Basalt 1542.3 grams Figure 1: Photo of 15556,0. Largest vesicles are about 6 mm. NASA S87-48187. Introduction 15556 was collected about 60 meters from the edge of Hadley
15556 Vesicular Olivine-normative Basalt 1542.3 grams Figure 1: Photo of 15556,0. Largest vesicles are about 6 mm. NASA S87-48187. Introduction 15556 was collected about 60 meters from the edge of Hadley
76215 Vesicular Micropoikilitic Impact Melt Breccia 644 grams
 76215 Vesicular Micropoikilitic Impact Melt Breccia 644 grams 76215 76215 Figure 1: Photo of station 6 boulder with location of 76215 indicated. AS17-140-21421 Figure 2: Photo of 76215. Sample is about
76215 Vesicular Micropoikilitic Impact Melt Breccia 644 grams 76215 76215 Figure 1: Photo of station 6 boulder with location of 76215 indicated. AS17-140-21421 Figure 2: Photo of 76215. Sample is about
67016 Feldspathic Fragmental Breccia 4262 grams
 67016 Feldspathic Fragmental Breccia 4262 grams Figure 1: Photo of 67016,1. Cube is 1 inch. NASA # S81-26050. Introduction 67016 is a feldspathic fragmental breccia with both light and dark clasts (figures
67016 Feldspathic Fragmental Breccia 4262 grams Figure 1: Photo of 67016,1. Cube is 1 inch. NASA # S81-26050. Introduction 67016 is a feldspathic fragmental breccia with both light and dark clasts (figures
15009 Single Drive Tube Station 6
 15009 Single Drive Tube Station 6 Figure 2: Location of soil samples, trench and drive tube at station 6, Apollo 15. Figure 1: Photo of drive tube 15009 driven in all the way. AS15-86-11565. Introduction
15009 Single Drive Tube Station 6 Figure 2: Location of soil samples, trench and drive tube at station 6, Apollo 15. Figure 1: Photo of drive tube 15009 driven in all the way. AS15-86-11565. Introduction
62255 Anorthosite with melt 1239 grams
 62255 Anorthosite with melt 1239 grams Figure 1: Photo of 62255 showing glass splash on anorthosite. Cube is 1 cm. NASA S # S72-38309. Introduction Lunar sample 62255 is significant because it is largely
62255 Anorthosite with melt 1239 grams Figure 1: Photo of 62255 showing glass splash on anorthosite. Cube is 1 cm. NASA S # S72-38309. Introduction Lunar sample 62255 is significant because it is largely
12052 Pigeonite Basalt 1866 grams
 12052 Pigeonite Basalt 1866 grams Revised Figure 1: Photo of broken side of 12052 showing vugs. Sample is 6.5 cm high. NASA # S70-44633. Summary of Age Data for 12052 Ar/Ar Rb/Sr Nyquist 1977 (recalculated)
12052 Pigeonite Basalt 1866 grams Revised Figure 1: Photo of broken side of 12052 showing vugs. Sample is 6.5 cm high. NASA # S70-44633. Summary of Age Data for 12052 Ar/Ar Rb/Sr Nyquist 1977 (recalculated)
14049 Regolith Breccia grams
 Regolith Breccia 200.1 grams Figure 1: Photo of. Edge of cube is 1 inch. NASA S71-29143. Figure 2: Map of Apollo 14 showing traverse for EVA 2. Introduction The Apollo 14 regolith breccias (vitric matrix
Regolith Breccia 200.1 grams Figure 1: Photo of. Edge of cube is 1 inch. NASA S71-29143. Figure 2: Map of Apollo 14 showing traverse for EVA 2. Introduction The Apollo 14 regolith breccias (vitric matrix
Element Cube Project (x2)
 Element Cube Project (x2) Background: As a class, we will construct a three dimensional periodic table by each student selecting two elements in which you will need to create an element cube. Helpful Links
Element Cube Project (x2) Background: As a class, we will construct a three dimensional periodic table by each student selecting two elements in which you will need to create an element cube. Helpful Links
15595 and Vuggy Vitrophyric Pigeonite Basalt and grams
 and 15596 Vuggy Vitrophyric Pigeonite Basalt 237.6 and 224.8 grams Figure 1: Photo of. Sample is about 9 cm across. NASA S71-44491. Figure 2: Photo of 15596 showing surface that was exposed to micrometeorites.
and 15596 Vuggy Vitrophyric Pigeonite Basalt 237.6 and 224.8 grams Figure 1: Photo of. Sample is about 9 cm across. NASA S71-44491. Figure 2: Photo of 15596 showing surface that was exposed to micrometeorites.
Ilmenite Basalt grams
 Ilmenite Basalt 737.6 grams Figure 1: Picute of 5 meter boulder on rim of Shorty Crater showing where was collected. Trench in forground is location of orange soil sample 74220. Photo number AS17-137-20990
Ilmenite Basalt 737.6 grams Figure 1: Picute of 5 meter boulder on rim of Shorty Crater showing where was collected. Trench in forground is location of orange soil sample 74220. Photo number AS17-137-20990
60016 Ancient Regolith Breccia 4307 grams
 60016 Ancient Regolith Breccia 4307 grams Figure 1: Photo of 60016 showing large glass-lined micrometeorite crater with significant spall zone. Glass lining about 0.8 cm in size. Sample is about 15 cm
60016 Ancient Regolith Breccia 4307 grams Figure 1: Photo of 60016 showing large glass-lined micrometeorite crater with significant spall zone. Glass lining about 0.8 cm in size. Sample is about 15 cm
The Periodic Table of Elements
 The Periodic Table of Elements 8 Uuo Uus Uuh (9) Uup (88) Uuq (89) Uut (8) Uub (8) Rg () 0 Ds (9) 09 Mt (8) 08 Hs (9) 0 h () 0 Sg () 0 Db () 0 Rf () 0 Lr () 88 Ra () 8 Fr () 8 Rn () 8 At (0) 8 Po (09)
The Periodic Table of Elements 8 Uuo Uus Uuh (9) Uup (88) Uuq (89) Uut (8) Uub (8) Rg () 0 Ds (9) 09 Mt (8) 08 Hs (9) 0 h () 0 Sg () 0 Db () 0 Rf () 0 Lr () 88 Ra () 8 Fr () 8 Rn () 8 At (0) 8 Po (09)
Radiometric Dating (tap anywhere)
 Radiometric Dating (tap anywhere) Protons Neutrons Electrons Elements on the periodic table are STABLE Elements can have radioactive versions of itself called ISOTOPES!! Page 1 in your ESRT has your list!
Radiometric Dating (tap anywhere) Protons Neutrons Electrons Elements on the periodic table are STABLE Elements can have radioactive versions of itself called ISOTOPES!! Page 1 in your ESRT has your list!
Nucleus. Electron Cloud
 Atomic Structure I. Picture of an Atom Nucleus Electron Cloud II. Subatomic particles Particle Symbol Charge Relative Mass (amu) protons p + +1 1.0073 neutrons n 0 1.0087 electrons e - -1 0.00054858 Compare
Atomic Structure I. Picture of an Atom Nucleus Electron Cloud II. Subatomic particles Particle Symbol Charge Relative Mass (amu) protons p + +1 1.0073 neutrons n 0 1.0087 electrons e - -1 0.00054858 Compare
12042 Soil 255 grams
 Soil 255 grams Figure 1: Photo of location of (Halo Crater). AS12-48-7072 Introduction are fines collected in documented bag 12. They were from the outer flank of Surveyor Crater (figure 2). Figure 2:
Soil 255 grams Figure 1: Photo of location of (Halo Crater). AS12-48-7072 Introduction are fines collected in documented bag 12. They were from the outer flank of Surveyor Crater (figure 2). Figure 2:
Atoms and the Periodic Table
 Atoms and the Periodic Table Parts of the Atom Proton Found in the nucleus Number of protons defines the element Charge +1, mass 1 Parts of the Atom Neutron Found in the nucleus Stabilizes the nucleus
Atoms and the Periodic Table Parts of the Atom Proton Found in the nucleus Number of protons defines the element Charge +1, mass 1 Parts of the Atom Neutron Found in the nucleus Stabilizes the nucleus
70035 Ilmenite Basalt 5765 grams
 Ilmenite Basalt 5765 grams Figure 1: Photograph of top surface of illustrating vugs and vesicles. Note the smooth, rounded surface shaped by micrometeorite bombardment. Small cube is 1 cm. NASA photo #
Ilmenite Basalt 5765 grams Figure 1: Photograph of top surface of illustrating vugs and vesicles. Note the smooth, rounded surface shaped by micrometeorite bombardment. Small cube is 1 cm. NASA photo #
The Periodic Table of the Elements
 The Periodic Table of the Elements All matter is composed of elements. All of the elements are composed of atoms. An atom is the smallest part of an element which still retains the properties of that element.
The Periodic Table of the Elements All matter is composed of elements. All of the elements are composed of atoms. An atom is the smallest part of an element which still retains the properties of that element.
The Periodic Table. Periodic Properties. Can you explain this graph? Valence Electrons. Valence Electrons. Paramagnetism
 Periodic Properties Atomic & Ionic Radius Energy Electron Affinity We want to understand the variations in these properties in terms of electron configurations. The Periodic Table Elements in a column
Periodic Properties Atomic & Ionic Radius Energy Electron Affinity We want to understand the variations in these properties in terms of electron configurations. The Periodic Table Elements in a column
IV. Governador Valadares clinopyroxenite, 158 grams find
 IV. Governador Valadares clinopyroxenite, 158 grams find Figure IV-1. Photograph of Governador Valadares (158 grams) from Dr. Fernanda Ferrucci via Dr. Giuseppe Cavarretta. Photo taken by L. Spinozzi.
IV. Governador Valadares clinopyroxenite, 158 grams find Figure IV-1. Photograph of Governador Valadares (158 grams) from Dr. Fernanda Ferrucci via Dr. Giuseppe Cavarretta. Photo taken by L. Spinozzi.
Chemistry 431 Practice Final Exam Fall Hours
 Chemistry 431 Practice Final Exam Fall 2018 3 Hours R =8.3144 J mol 1 K 1 R=.0821 L atm mol 1 K 1 R=.08314 L bar mol 1 K 1 k=1.381 10 23 J molecule 1 K 1 h=6.626 10 34 Js N A = 6.022 10 23 molecules mol
Chemistry 431 Practice Final Exam Fall 2018 3 Hours R =8.3144 J mol 1 K 1 R=.0821 L atm mol 1 K 1 R=.08314 L bar mol 1 K 1 k=1.381 10 23 J molecule 1 K 1 h=6.626 10 34 Js N A = 6.022 10 23 molecules mol
DO NOW: Retrieve your projects. We will be reviewing them again today. Textbook pg 23, answer questions 1-3. Use the section 1.2 to help you.
 DO NOW: Retrieve your projects. We will be reviewing them again today. Textbook pg, answer questions. Use the section. to help you. Chapter test is FRIDAY. The Periodic Table of Elements 8 Uuo Uus Uuh
DO NOW: Retrieve your projects. We will be reviewing them again today. Textbook pg, answer questions. Use the section. to help you. Chapter test is FRIDAY. The Periodic Table of Elements 8 Uuo Uus Uuh
CHEM 10113, Quiz 5 October 26, 2011
 CHEM 10113, Quiz 5 October 26, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
CHEM 10113, Quiz 5 October 26, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
DRAFT Regolith Breccia 155 grams
 12034 Regolith Breccia 155 grams DRAFT Figure 1: Lunar sample 12034 after first saw cut in 1970. NASA S75-34235. Cube is 1 cm. Introduction 12034 was collected from the bottom of the same trench as soil
12034 Regolith Breccia 155 grams DRAFT Figure 1: Lunar sample 12034 after first saw cut in 1970. NASA S75-34235. Cube is 1 cm. Introduction 12034 was collected from the bottom of the same trench as soil
Using the Periodic Table
 MATH SKILLS TRANSPARENCY WORKSHEET Using the Periodic Table 6 Use with Chapter 6, Section 6.2 1. Identify the number of valence electrons in each of the following elements. a. Ne e. O b. K f. Cl c. B g.
MATH SKILLS TRANSPARENCY WORKSHEET Using the Periodic Table 6 Use with Chapter 6, Section 6.2 1. Identify the number of valence electrons in each of the following elements. a. Ne e. O b. K f. Cl c. B g.
Solutions and Ions. Pure Substances
 Class #4 Solutions and Ions CHEM 107 L.S. Brown Texas A&M University Pure Substances Pure substance: described completely by a single chemical formula Fixed composition 1 Mixtures Combination of 2 or more
Class #4 Solutions and Ions CHEM 107 L.S. Brown Texas A&M University Pure Substances Pure substance: described completely by a single chemical formula Fixed composition 1 Mixtures Combination of 2 or more
5 questions, 3 points each, 15 points total possible. 26 Fe Cu Ni Co Pd Ag Ru 101.
 Physical Chemistry II Lab CHEM 4644 spring 2017 final exam KEY 5 questions, 3 points each, 15 points total possible h = 6.626 10-34 J s c = 3.00 10 8 m/s 1 GHz = 10 9 s -1. B= h 8π 2 I ν= 1 2 π k μ 6 P
Physical Chemistry II Lab CHEM 4644 spring 2017 final exam KEY 5 questions, 3 points each, 15 points total possible h = 6.626 10-34 J s c = 3.00 10 8 m/s 1 GHz = 10 9 s -1. B= h 8π 2 I ν= 1 2 π k μ 6 P
Figure of rare earth elemental abundances removed due to copyright restrictions.
 Figure of rare earth elemental abundances removed due to copyright restrictions. See figure 3.1 on page 26 of Tolstikhin, Igor and Jan Kramers. The Evolution of Matter: From the Big Bang to the Present
Figure of rare earth elemental abundances removed due to copyright restrictions. See figure 3.1 on page 26 of Tolstikhin, Igor and Jan Kramers. The Evolution of Matter: From the Big Bang to the Present
Made the FIRST periodic table
 Made the FIRST periodic table 1869 Mendeleev organized the periodic table based on the similar properties and relativities of certain elements Later, Henri Moseley organized the elements by increasing
Made the FIRST periodic table 1869 Mendeleev organized the periodic table based on the similar properties and relativities of certain elements Later, Henri Moseley organized the elements by increasing
Last 4 Digits of USC ID:
 Chemistry 05 B Practice Exam Dr. Jessica Parr First Letter of last Name PLEASE PRINT YOUR NAME IN BLOCK LETTERS Name: Last 4 Digits of USC ID: Lab TA s Name: Question Points Score Grader 8 2 4 3 9 4 0
Chemistry 05 B Practice Exam Dr. Jessica Parr First Letter of last Name PLEASE PRINT YOUR NAME IN BLOCK LETTERS Name: Last 4 Digits of USC ID: Lab TA s Name: Question Points Score Grader 8 2 4 3 9 4 0
CLASS TEST GRADE 11. PHYSICAL SCIENCES: CHEMISTRY Test 4: Matter and materials 1
 CLASS TEST GRADE PHYSICAL SCIENCES: CHEMISTRY Test 4: Matter and materials MARKS: 45 TIME: hour INSTRUCTIONS AND INFORMATION. Answer ALL the questions. 2. You may use non-programmable calculators. 3. You
CLASS TEST GRADE PHYSICAL SCIENCES: CHEMISTRY Test 4: Matter and materials MARKS: 45 TIME: hour INSTRUCTIONS AND INFORMATION. Answer ALL the questions. 2. You may use non-programmable calculators. 3. You
(please print) (1) (18) H IIA IIIA IVA VA VIA VIIA He (2) (13) (14) (15) (16) (17)
 CHEM 10113, Quiz 3 September 28, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
CHEM 10113, Quiz 3 September 28, 2011 Name (please print) All equations must be balanced and show phases for full credit. Significant figures count, show charges as appropriate, and please box your answers!
12013 Breccia with granite 82.3 grams
 12013 Breccia with granite 82.3 grams Figure 1: Close-up photo of 12013,11. Field of view is 4.5 cm. NASA# S70-43636 Introduction 12013 is a unique lunar sample, but it did not appear to be remarkable
12013 Breccia with granite 82.3 grams Figure 1: Close-up photo of 12013,11. Field of view is 4.5 cm. NASA# S70-43636 Introduction 12013 is a unique lunar sample, but it did not appear to be remarkable
Spin Cut-off Parameter of Nuclear Level Density and Effective Moment of Inertia
 Commun. Theor. Phys. (Beijing, China) 43 (005) pp. 709 718 c International Academic Publishers Vol. 43, No. 4, April 15, 005 Spin Cut-off Parameter of Nuclear Level Density and Effective Moment of Inertia
Commun. Theor. Phys. (Beijing, China) 43 (005) pp. 709 718 c International Academic Publishers Vol. 43, No. 4, April 15, 005 Spin Cut-off Parameter of Nuclear Level Density and Effective Moment of Inertia
Part 2. Multiple choice (use answer card). 90 pts. total. 3 pts. each.
 1 Exam I CHEM 1303.001 Name (print legibly) Seat no. On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Part 1. Nomenclature. 10 pts. total. 2 pts. each. Fill in
1 Exam I CHEM 1303.001 Name (print legibly) Seat no. On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Part 1. Nomenclature. 10 pts. total. 2 pts. each. Fill in
Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
PERIODIC TABLE OF THE ELEMENTS
 Useful Constants and equations: K = o C + 273 Avogadro's number = 6.022 x 10 23 d = density = mass/volume R H = 2.178 x 10-18 J c = E = h = hc/ h = 6.626 x 10-34 J s c = 2.998 x 10 8 m/s E n = -R H Z 2
Useful Constants and equations: K = o C + 273 Avogadro's number = 6.022 x 10 23 d = density = mass/volume R H = 2.178 x 10-18 J c = E = h = hc/ h = 6.626 x 10-34 J s c = 2.998 x 10 8 m/s E n = -R H Z 2
MANY ELECTRON ATOMS Chapter 15
 MANY ELECTRON ATOMS Chapter 15 Electron-Electron Repulsions (15.5-15.9) The hydrogen atom Schrödinger equation is exactly solvable yielding the wavefunctions and orbitals of chemistry. Howev er, the Schrödinger
MANY ELECTRON ATOMS Chapter 15 Electron-Electron Repulsions (15.5-15.9) The hydrogen atom Schrödinger equation is exactly solvable yielding the wavefunctions and orbitals of chemistry. Howev er, the Schrödinger
Ch. 9 NOTES ~ Chemical Bonding NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics.
 Ch. 9 NOTES ~ Chemical Bonding NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Review: Comparison of ionic and molecular compounds Molecular compounds Ionic
Ch. 9 NOTES ~ Chemical Bonding NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Review: Comparison of ionic and molecular compounds Molecular compounds Ionic
CHEM 130 Exp. 8: Molecular Models
 CHEM 130 Exp. 8: Molecular Models In this lab, we will learn and practice predicting molecular structures from molecular formulas. The Periodic Table of the Elements IA 1 H IIA IIIA IVA VA VIA VIIA 3 5
CHEM 130 Exp. 8: Molecular Models In this lab, we will learn and practice predicting molecular structures from molecular formulas. The Periodic Table of the Elements IA 1 H IIA IIIA IVA VA VIA VIIA 3 5
CHM 101 PRACTICE TEST 1 Page 1 of 4
 CHM 101 PRACTICE TEST 1 Page 1 of 4 Please show calculations (stuffed equations) on all mathematical problems!! On the actual test, "naked answers, with no work shown, will receive no credit even if correct.
CHM 101 PRACTICE TEST 1 Page 1 of 4 Please show calculations (stuffed equations) on all mathematical problems!! On the actual test, "naked answers, with no work shown, will receive no credit even if correct.
(C) Pavel Sedach and Prep101 1
 (C) Pavel Sedach and Prep101 1 (C) Pavel Sedach and Prep101 1 (C) Pavel Sedach and Prep101 2 (C) Pavel Sedach and Prep101 2 (C) Pavel Sedach and Prep101 3 (C) Pavel Sedach and Prep101 3 (C) Pavel Sedach
(C) Pavel Sedach and Prep101 1 (C) Pavel Sedach and Prep101 1 (C) Pavel Sedach and Prep101 2 (C) Pavel Sedach and Prep101 2 (C) Pavel Sedach and Prep101 3 (C) Pavel Sedach and Prep101 3 (C) Pavel Sedach
Grade 11 Science Practice Test
 Grade 11 Science Practice Test Nebraska Department of Education 2012 Directions: On the following pages of your test booklet are multiple-choice questions for Session 1 of the Grade 11 Nebraska State Accountability
Grade 11 Science Practice Test Nebraska Department of Education 2012 Directions: On the following pages of your test booklet are multiple-choice questions for Session 1 of the Grade 11 Nebraska State Accountability
Guide to the Extended Step-Pyramid Periodic Table
 Guide to the Extended Step-Pyramid Periodic Table William B. Jensen Department of Chemistry University of Cincinnati Cincinnati, OH 452201-0172 The extended step-pyramid table recognizes that elements
Guide to the Extended Step-Pyramid Periodic Table William B. Jensen Department of Chemistry University of Cincinnati Cincinnati, OH 452201-0172 The extended step-pyramid table recognizes that elements
Circle the letters only. NO ANSWERS in the Columns!
 Chemistry 1304.001 Name (please print) Exam 5 (100 points) April 18, 2018 On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Circle the letters only. NO ANSWERS in
Chemistry 1304.001 Name (please print) Exam 5 (100 points) April 18, 2018 On my honor, I have neither given nor received unauthorized aid on this exam. Signed Date Circle the letters only. NO ANSWERS in
February 20, Joe Cerniglia The International Group for Historic Aircraft Recovery (TIGHAR) Job Number: S0CHG688. Dear Joe:
 February 20, 2012 Joe Cerniglia The International Group for Historic Aircraft Recovery (TIGHAR) Subject: ICP-MS Report Job Number: S0CHG688 Dear Joe: Please find enclosed the procedure report for the analysis
February 20, 2012 Joe Cerniglia The International Group for Historic Aircraft Recovery (TIGHAR) Subject: ICP-MS Report Job Number: S0CHG688 Dear Joe: Please find enclosed the procedure report for the analysis
Chapter 12 The Atom & Periodic Table- part 2
 Chapter 12 The Atom & Periodic Table- part 2 Electrons found outside the nucleus; negatively charged Protons found in the nucleus; positive charge equal in magnitude to the electron s negative charge Neutrons
Chapter 12 The Atom & Periodic Table- part 2 Electrons found outside the nucleus; negatively charged Protons found in the nucleus; positive charge equal in magnitude to the electron s negative charge Neutrons
Why all the repeating Why all the repeating Why all the repeating Why all the repeating
 Why all the repeating Why all the repeating Why all the repeating Why all the repeating Patterns What Patterns have you observed in your life? Where to Get Help If you don t understand concepts in chapter
Why all the repeating Why all the repeating Why all the repeating Why all the repeating Patterns What Patterns have you observed in your life? Where to Get Help If you don t understand concepts in chapter
Secondary Support Pack. be introduced to some of the different elements within the periodic table;
 Secondary Support Pack INTRODUCTION The periodic table of the elements is central to chemistry as we know it today and the study of it is a key part of every student s chemical education. By playing the
Secondary Support Pack INTRODUCTION The periodic table of the elements is central to chemistry as we know it today and the study of it is a key part of every student s chemical education. By playing the
Atomic Emission Spectra. and. Flame Tests. Burlingame High School Chemistry
 Atomic Structure Atomic Emission Spectra and Flame Tests Flame Tests Sodium potassium lithium When electrons are excited they bump up to a higher energy level. As they bounce back down they release energy
Atomic Structure Atomic Emission Spectra and Flame Tests Flame Tests Sodium potassium lithium When electrons are excited they bump up to a higher energy level. As they bounce back down they release energy
HANDOUT SET GENERAL CHEMISTRY II
 HANDOUT SET GENERAL CHEMISTRY II Periodic Table of the Elements 1 2 3 4 5 6 7 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 IA VIIIA 1 2 H He 1.00794 IIA IIIA IVA VA VIA VIIA 4.00262 3 Li 6.941 11 Na 22.9898
HANDOUT SET GENERAL CHEMISTRY II Periodic Table of the Elements 1 2 3 4 5 6 7 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 IA VIIIA 1 2 H He 1.00794 IIA IIIA IVA VA VIA VIIA 4.00262 3 Li 6.941 11 Na 22.9898
HANDOUT SET GENERAL CHEMISTRY I
 HANDOUT SET GENERAL CHEMISTRY I Periodic Table of the Elements 1 2 3 4 5 6 7 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 IA VIIIA 1 2 H He 1.00794 IIA IIIA IVA VA VIA VIIA 4.00262 3 Li 6.941 11 Na 22.9898
HANDOUT SET GENERAL CHEMISTRY I Periodic Table of the Elements 1 2 3 4 5 6 7 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 IA VIIIA 1 2 H He 1.00794 IIA IIIA IVA VA VIA VIIA 4.00262 3 Li 6.941 11 Na 22.9898
Atomic Structure & Interatomic Bonding
 Atomic Structure & Interatomic Bonding Chapter Outline Review of Atomic Structure Atomic Bonding Atomic Structure Atoms are the smallest structural units of all solids, liquids & gases. Atom: The smallest
Atomic Structure & Interatomic Bonding Chapter Outline Review of Atomic Structure Atomic Bonding Atomic Structure Atoms are the smallest structural units of all solids, liquids & gases. Atom: The smallest
Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
If anything confuses you or is not clear, raise your hand and ask!
 CHM 1045 Dr. Light s Section December 10, 2002 FINAL EXAM Name (please print) Recitation Section Meeting Time This exam consists of six pages. Make sure you have one of each. Print your name at the top
CHM 1045 Dr. Light s Section December 10, 2002 FINAL EXAM Name (please print) Recitation Section Meeting Time This exam consists of six pages. Make sure you have one of each. Print your name at the top
Modified from: Larry Scheffler Lincoln High School IB Chemistry 1-2.1
 Modified from: Larry Scheffler Lincoln High School IB Chemistry 1-2.1 The development of the periodic table brought a system of order to what was otherwise an collection of thousands of pieces of information.
Modified from: Larry Scheffler Lincoln High School IB Chemistry 1-2.1 The development of the periodic table brought a system of order to what was otherwise an collection of thousands of pieces of information.
02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr
 Chemistry 05 B First Letter of PLEASE PRINT YOUR NAME IN BLOCK LETTERS Exam last Name Name: 02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr Lab TA s Name: Question Points Score Grader 2 2 9 3 9 4 2
Chemistry 05 B First Letter of PLEASE PRINT YOUR NAME IN BLOCK LETTERS Exam last Name Name: 02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr Lab TA s Name: Question Points Score Grader 2 2 9 3 9 4 2
8. Relax and do well.
 CHEM 1314.03 Exam I John I. Gelder September 25, 1997 Name TA's Name Lab Section Please sign your name below to give permission to post, by the last 4 digits of your student I.D. number, your course scores
CHEM 1314.03 Exam I John I. Gelder September 25, 1997 Name TA's Name Lab Section Please sign your name below to give permission to post, by the last 4 digits of your student I.D. number, your course scores
Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
7) Applications of Nuclear Radiation in Science and Technique (1) Analytical applications (Radiometric titration)
 7) Applications of Nuclear Radiation in Science and Technique (1) (Radiometric titration) The radioactive material is indicator Precipitation reactions Complex formation reactions Principle of a precipitation
7) Applications of Nuclear Radiation in Science and Technique (1) (Radiometric titration) The radioactive material is indicator Precipitation reactions Complex formation reactions Principle of a precipitation
Chapter 3: Stoichiometry
 Chapter 3: Stoichiometry Chem 6A Michael J. Sailor, UC San Diego 1 Announcements: Thursday (Sep 29) quiz: Bring student ID or we cannot accept your quiz! No notes, no calculators Covers chapters 1 and
Chapter 3: Stoichiometry Chem 6A Michael J. Sailor, UC San Diego 1 Announcements: Thursday (Sep 29) quiz: Bring student ID or we cannot accept your quiz! No notes, no calculators Covers chapters 1 and
610B Final Exam Cover Page
 1 st Letter of Last Name NAME: 610B Final Exam Cover Page No notes or calculators of any sort allowed. You have 3 hours to complete the exam. CHEM 610B, 50995 Final Exam Fall 2003 Instructor: Dr. Brian
1 st Letter of Last Name NAME: 610B Final Exam Cover Page No notes or calculators of any sort allowed. You have 3 hours to complete the exam. CHEM 610B, 50995 Final Exam Fall 2003 Instructor: Dr. Brian
9/20/2017. Elements are Pure Substances that cannot be broken down into simpler substances by chemical change (contain Only One Type of Atom)
 CAPTER 6: TE PERIODIC TABLE Elements are Pure Substances that cannot be broken down into simpler substances by chemical change (contain Only One Type of Atom) The Periodic Table (Mendeleev) In 1872, Dmitri
CAPTER 6: TE PERIODIC TABLE Elements are Pure Substances that cannot be broken down into simpler substances by chemical change (contain Only One Type of Atom) The Periodic Table (Mendeleev) In 1872, Dmitri
EMMR25 Mineralogy: Ol + opx + chlorite + cpx + amphibole + serpentine + opaque
 GSA Data Repository 2017365 Marshall et al., 2017, The role of serpentinite derived fluids in metasomatism of the Colorado Plateau (USA) lithospheric mantle: Geology, https://doi.org/10.1130/g39444.1 Appendix
GSA Data Repository 2017365 Marshall et al., 2017, The role of serpentinite derived fluids in metasomatism of the Colorado Plateau (USA) lithospheric mantle: Geology, https://doi.org/10.1130/g39444.1 Appendix
6.3 Classifying Elements with the Periodic Table
 6.3 Classifying Elements with the Periodic Table The Periodic Table was developed by scientists to organize elements in such a way as to make sense of the growing information about their properties. The
6.3 Classifying Elements with the Periodic Table The Periodic Table was developed by scientists to organize elements in such a way as to make sense of the growing information about their properties. The
Chemistry 1 First Lecture Exam Fall Abbasi Khajo Levine Mathias Mathias/Ortiz Metlitsky Rahi Sanchez-Delgado Vasserman
 Chemistry 1 First Lecture Exam Fall 2011 Page 1 of 9 NAME Circle the name of your recitation/lab instructor(s) Abbasi Khajo Levine Mathias Mathias/Ortiz Metlitsky Rahi Sanchez-Delgado Vasserman Before
Chemistry 1 First Lecture Exam Fall 2011 Page 1 of 9 NAME Circle the name of your recitation/lab instructor(s) Abbasi Khajo Levine Mathias Mathias/Ortiz Metlitsky Rahi Sanchez-Delgado Vasserman Before
8. Relax and do well.
 CHEM 1515 Exam II John II. Gelder October 14, 1993 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last two pages include a periodic table, a
CHEM 1515 Exam II John II. Gelder October 14, 1993 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last two pages include a periodic table, a
Summary of test results for Daya Bay rock samples. by Patrick Dobson Celia Tiemi Onishi Seiji Nakagawa
 Summary of test results for Daya Bay rock samples by Patrick Dobson Celia Tiemi Onishi Seiji Nakagawa October 2004 Summary A series of analytical tests were conducted on a suite of granitic rock samples
Summary of test results for Daya Bay rock samples by Patrick Dobson Celia Tiemi Onishi Seiji Nakagawa October 2004 Summary A series of analytical tests were conducted on a suite of granitic rock samples
Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
INSTRUCTIONS: CHEM Exam I. September 13, 1994 Lab Section
 CHEM 1314.05 Exam I John I. Gelder September 13, 1994 Name TA's Name Lab Section Please sign your name below to give permission to post, by the last 4 digits of your student I.D. number, your course scores
CHEM 1314.05 Exam I John I. Gelder September 13, 1994 Name TA's Name Lab Section Please sign your name below to give permission to post, by the last 4 digits of your student I.D. number, your course scores
K. 27 Co. 28 Ni. 29 Cu Rb. 46 Pd. 45 Rh. 47 Ag Cs Ir. 78 Pt.
 1 IA 1 H Hydrogen 1.01 Atomic number Element symbol Element name Atomic mass VIIIA 1 H 1.01 IIA IIIA IVA VA VIA VIIA 2 He 4.00 Metalloids 3 Li 6.94 4 Be 9.01 5 B 10.81 6 C 12.01 7 N 14.01 8 O 16.00 9 F
1 IA 1 H Hydrogen 1.01 Atomic number Element symbol Element name Atomic mass VIIIA 1 H 1.01 IIA IIIA IVA VA VIA VIIA 2 He 4.00 Metalloids 3 Li 6.94 4 Be 9.01 5 B 10.81 6 C 12.01 7 N 14.01 8 O 16.00 9 F
8. Relax and do well.
 CHEM 1014 Exam I John I. Gelder September 16, 1999 Name TA's Name Lab Section Please sign your name below to give permission to post your course scores on homework, laboratories and exams. If you do not
CHEM 1014 Exam I John I. Gelder September 16, 1999 Name TA's Name Lab Section Please sign your name below to give permission to post your course scores on homework, laboratories and exams. If you do not
Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
SCIENCE 1206 UNIT 2 CHEMISTRY. September 2017 November 2017
 SCIENCE 1206 UNIT 2 CHEMISTRY September 2017 November 2017 UNIT OUTLINE 1. Review of Grade 9 Terms & the Periodic Table Bohr diagrams Evidence for chemical reactions Chemical Tests 2. Naming & writing
SCIENCE 1206 UNIT 2 CHEMISTRY September 2017 November 2017 UNIT OUTLINE 1. Review of Grade 9 Terms & the Periodic Table Bohr diagrams Evidence for chemical reactions Chemical Tests 2. Naming & writing
NAME: FIRST EXAMINATION
 1 Chemistry 64 Winter 1994 NAME: FIRST EXAMINATION THIS EXAMINATION IS WORTH 100 POINTS AND CONTAINS 4 (FOUR) QUESTIONS THEY ARE NOT EQUALLY WEIGHTED! YOU SHOULD ATTEMPT ALL QUESTIONS AND ALLOCATE YOUR
1 Chemistry 64 Winter 1994 NAME: FIRST EXAMINATION THIS EXAMINATION IS WORTH 100 POINTS AND CONTAINS 4 (FOUR) QUESTIONS THEY ARE NOT EQUALLY WEIGHTED! YOU SHOULD ATTEMPT ALL QUESTIONS AND ALLOCATE YOUR
7. Relax and do well.
 CHEM 1215 Exam II John II. Gelder October 7, 1998 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 5 different pages. The last page includes a periodic table and a solubility
CHEM 1215 Exam II John II. Gelder October 7, 1998 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 5 different pages. The last page includes a periodic table and a solubility
Laser Spectroscopy on Bunched Radioactive Ion Beams
 Laser Spectroscopy on Bunched Radioactive Ion Beams Jon Billowes University of Manchester Balkan School on Nuclear Physics, Bodrum 2004 Lecture 1. 1.1 Nuclear moments 1.2 Hyperfine interaction in free
Laser Spectroscopy on Bunched Radioactive Ion Beams Jon Billowes University of Manchester Balkan School on Nuclear Physics, Bodrum 2004 Lecture 1. 1.1 Nuclear moments 1.2 Hyperfine interaction in free
PHYSICAL SCIENCES GRADE : 10
 PHYSICAL SCIENCES GRADE : 0 TIME : hour TOTAL : 75 INSTRUCTIONS AND INFORMATION. Write your full name on your answer book in the appropriate place. 2. The question paper consists of SEVEN questions. Answer
PHYSICAL SCIENCES GRADE : 0 TIME : hour TOTAL : 75 INSTRUCTIONS AND INFORMATION. Write your full name on your answer book in the appropriate place. 2. The question paper consists of SEVEN questions. Answer
BROOKLYN COLLEGE Department of Chemistry. Chemistry 1 Second Lecture Exam Nov. 27, Name Page 1 of 5
 BROOKLYN COLLEGE Department of Chemistry Chemistry 1 Second Lecture Exam Nov. 27, 2002 Name Page 1 of 5 Circle the name of your lab instructor Kobrak, Zhou, Girotto, Hussey, Du Before you begin the exam,
BROOKLYN COLLEGE Department of Chemistry Chemistry 1 Second Lecture Exam Nov. 27, 2002 Name Page 1 of 5 Circle the name of your lab instructor Kobrak, Zhou, Girotto, Hussey, Du Before you begin the exam,
Essential Chemistry for Biology
 1 Chapter 2 Essential Chemistry for Biology Biology and Society: More Precious than Gold A drought is a period of abnormally dry weather that changes the environment and one of the most devastating disasters.
1 Chapter 2 Essential Chemistry for Biology Biology and Society: More Precious than Gold A drought is a period of abnormally dry weather that changes the environment and one of the most devastating disasters.
Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
[ ]:543.4(075.8) 35.20: ,..,..,.., : /... ;. 2-. ISBN , - [ ]:543.4(075.8) 35.20:34.
![[ ]:543.4(075.8) 35.20: ,..,..,.., : /... ;. 2-. ISBN , - [ ]:543.4(075.8) 35.20:34. [ ]:543.4(075.8) 35.20: ,..,..,.., : /... ;. 2-. ISBN , - [ ]:543.4(075.8) 35.20:34.](/thumbs/74/69911105.jpg) .. - 2-2009 [661.87.+661.88]:543.4(075.8) 35.20:34.2373-60..,..,..,..,.. -60 : /... ;. 2-. : -, 2008. 134. ISBN 5-98298-299-7 -., -,,. - «,, -, -», - 550800,, 240600 «-», -. [661.87.+661.88]:543.4(075.8)
.. - 2-2009 [661.87.+661.88]:543.4(075.8) 35.20:34.2373-60..,..,..,..,.. -60 : /... ;. 2-. : -, 2008. 134. ISBN 5-98298-299-7 -., -,,. - «,, -, -», - 550800,, 240600 «-», -. [661.87.+661.88]:543.4(075.8)
Chem Exam 1. September 26, Dr. Susan E. Bates. Name 9:00 OR 10:00
 Chem 1711 Exam 1 September 26, 2013 Dr. Susan E. Bates Name 9:00 OR 10:00 N A = 6.022 x 10 23 mol 1 I A II A III B IV B V B VI B VII B VIII I B II B III A IV A V A VI A VII A inert gases 1 H 1.008 3 Li
Chem 1711 Exam 1 September 26, 2013 Dr. Susan E. Bates Name 9:00 OR 10:00 N A = 6.022 x 10 23 mol 1 I A II A III B IV B V B VI B VII B VIII I B II B III A IV A V A VI A VII A inert gases 1 H 1.008 3 Li
CHEM 167 FINAL EXAM MONDAY, MAY 2 9:45 11:45 A.M GILMAN HALL
 PROF. JOHN VERKADE SPRING 2005 THIS EXAM CONSISTS OF 12 QUESTIONS ON 9 PAGES CHEM 167 HOUR EXAM IV APRIL 20, 2005 SEAT NO. NAME RECIT. INSTR. RECIT. SECT. GRADING PAGE Page 2 Page 3 Page 4 Page 5 Page
PROF. JOHN VERKADE SPRING 2005 THIS EXAM CONSISTS OF 12 QUESTIONS ON 9 PAGES CHEM 167 HOUR EXAM IV APRIL 20, 2005 SEAT NO. NAME RECIT. INSTR. RECIT. SECT. GRADING PAGE Page 2 Page 3 Page 4 Page 5 Page
Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
