CHAPTER 8 RESULTS AND DISCUSSION - FACTSAGE MODEL
|
|
|
- Isabel Sherman
- 5 years ago
- Views:
Transcription
1 CHAPTER 8 RESULTS AND DISCUSSION - FACTSAGE MODEL In the next section (8.1) the FactSage results for high-temperature equilibria of the inorganic material from the feed coal, float fraction, sink fraction as well as selected clinker and heated rock fragment particles are discussed. 8.1 FactSage Model FactSage results for the feed coal The expected equilibrium phases in the coal ash were predicted by using the "Equilibrium" module of FactSage (Bale et al., 2002), considering as possible phases: liquid slag, several solid-solution phases (including spinel, anorthite, monoxide, wollastonite, pyroxenes and olivine) and all pure compound solids between the inputs. In first calculations, to show broad trends, the following oxide composition was assumed: 56.0% SiO 2, 26.1% Al 2 O 3, 9.1% CaO, 3.4% FeO, 3.0% MgO, 1.1% TiO 2, 0.9% K 2 O and 0.4% Na 2 O (note that it is effectively assumed that all the pyrite in the coal is oxidised to FeO in the lower regions of the gasifier). The composition is the average of the gasifier ash originating from the coal minerals and the rock fragments. By using this composition, it is effectively assumed that there is full interaction between the coal minerals and the rock fragments. In fact, there is only partial interaction, as was explored in further calculations. Based on other models of the gasifier, the temperature at the ash grate is predicted to be 335 C, with the peak temperature in the combustion zone being some 1390 C, with the gas composition changing from an O 2 -H 2 O mixture at the ash discharge point to H 2 O-CO 2 in the combustion zone. This does imply that much of the iron would be in the trivalent form at equilibrium. Since FeO is a strong fluxing agent for silica, oxidation to Fe 3+ would affect the formation of liquid slag. However, in the calculations presented here, it was assumed that all the iron is present as FeO. The presence of glass (supercooled molten silicate) indicates that the ash and clinker formed from phases which included a large amount of liquid slag. Hence, the equilibrium calculations were performed for the temperature range C, where a substantial amount of liquid Chapter 8- Results and discussion 180
2 slag was predicted. The results are given in Figure 8.1. As Figure 8.1 shows, the mineral matter of the coal is predicted to be largely molten at the peak temperature (1390 C), with anorthite the main solid phase upon cooling below 1300 C (the "anorthite" was modelled as the CaO.Al 2 O 3.2SiO 2 -Na 2 O.Al 2 O 3.6SiO 2 solid solution, here containing 93-94% of CaO.Al 2 O 3.2SiO 2 ). In comparison with this, CCSEM or XRD did not detect any appreciable levels of cordierite (2MgO.2Al 2 O 3.5SiO 2 ) or leucite (KAlSi 2 O 6 ), but confirmed the occurrence of glass, anorthite, mullite and quartz. Comparing the CCSEM, EMP, SEM-EDS results (Figure 7.4, Figure 7.5, Figure 7.6, Figure 7.16,,Figure 7.14, Figure 7.15) to FactSage model (Figure 8.1), it is proposed that rising steam and oxygen quenched the molten slag, preventing the crystallisation of cordierite, effectively freezing the structure which had been present at temperatures estimated to be between 1220 C and 1390 C. This is confirmed by the occurrence of crushed ash fragments ranging from pure glass, with no evidence of any anorthite crystals, to fragments in which anorthite is the dominant phase (Figure 7.4). Note that the quartz in the coarse ash originated from coarse-grained sandstone rock fragments or included quartz in siltstone/mudstone rock fragments, not from crystallisation during cooling of the ash slag. Hence, the observation of quartz in the coarse ash is consistent with the proposal that the phases in the ash represent the structure as quenched from temperatures above 1220 C, where quartz and cordierite are not stable under equilibrium conditions slag Mass % anorthite cordierite mullite quartz leucite T ( C) Figure 8.1: Predicted mass percentages of phases in ash (composition 56.0% SiO 2, 26.1% Al 2 O 3, 9.1% CaO, 3.4% FeO, 3.0% MgO, 1.1% TiO 2, 0.9% K 2 O and 0.4% Na 2 O). Chapter 8- Results and discussion 181
3 8.1.2 Predicted equilibrium phases in the float and sink fractions of the feed coal As was stated earlier in this study, the included minerals in the coal are responsible for the clinkering and slagging of mineral matter during coal gasification, hence the equilibrium phases in the float fraction (containing a significantly higher proportion of included minerals) and sink fraction were calculated by FactSage. The chemical compositions of ash samples of the float and sink fractions used in the calculation were given in Table The FactSage results (Figures 8.2 and 8.3) indicate broadly similar trends, with anorthite and mullite the dominant high-temperature solid phases, but with higher proportions of anorthite and a smaller proportion of slag in the float fraction in comparison with the sink fraction. Significant proportions of Fe deriving from the pyrite mineral and Si from silicate minerals in the rock fragment present in the sink fraction, contribute to the predicted fayalite and ilmenite. These minerals (and cordierite) are not generally observed in the gasifier ash, but significant proportions of glass are observed. This is in line with the suggestion that rapid cooling in the lower part of the gasifier prevents crystallisation beyond the formation of anorthite and mullite. Figure 8.2: Predicted mass percentage in the ash of the float fraction (<1.5g/cm 3 ). Chapter 8- Results and discussion 182
4 Figure 8.3: Predicted mass percentage in the ash of the sink fraction (>1.8g/cm 3 ) Predicted equilibrium phases in the hand-picked dig-out samples FactSage 5.5 was used to predict the expected equilibrium phases present in the selected fragments from the dig-out samples 6D and 7D. In the present study area analyses (Tables 8.1) of the selected areas of these dig-out samples (determined by SEM-EDS) were used for the input compositions. The predicted equilibrium phases formed at the different temperatures after using the chemical compositions of the selected areas in the heated rock fragments were compared to minerals in the selected areas of the coal ash that were detected by SEM-EDS (Figure 8.4). Note that the major differences in composition are the high CaO content in the coal ash, compared with low CaO (but higher Al 2 O 3 ) in the rock fragment. Chapter 8- Results and discussion 183
5 Table 8.1: Area analysis of the selected spot fragments of samples taken from the gasifier during the dig-out test (wt %) Metal oxide 7.4D 6.7D Na 2 O Al 2 O SiO TiO CaO MgO FeO K 2 O P 2 O Total Note: 7.4D = Image of selected region of the dig-out sample 7D shows reacted coal ash (Figure 8.4) 6.7D = Area analysis of rock fragment in Figure 8.4 (bottom right) Figure 8.4: Left: BSE image of sample 7.4D showing reacted coal ash, containing anorthite crystals in glass; the region at the bottom left is a heated rock fragment (not included in the area analysis). Right: Reaction interface in sample 6.7D, showing reacted ash and a rock fragment; the area analysis was taken from the rock fragment at bottom right. The FactSage results show that mullite is the main solid phase in the heated rock fragment (Figure 8.5), persisting at 1400 C. In contrast, the main high-temperature solid in the coal ash (Figure 8.6) is anorthite. This difference in the identity of the primary solid phase, results from the differences in oxide composition: higher CaO levels favour anorthite formation, while higher Al 2 O 3 levels favour mullite formation. Chapter 8- Results and discussion 184
6 Substantial liquid formation requires high temperatures: for the two examples shown here, approximately 50% liquid is predicted to be presented at 1400 C. The extent of liquid formation depends on the oxide composition. As Figures 8.1 to 8.3 indicated, substantial liquid formation can occur at lower temperatures, for compositions with lower CaO/SiO 2 ratios. Figure 8.5: Predicted mass percentages of phases in the heated stone 6.7D. Chapter 8- Results and discussion 185
7 Figure 8.6: Predicted mass percentages of phases in the heated stone 7.4D. Chapter 8- Results and discussion 186
8 CHAPTER 9 CONCLUSIONS AND RECOMMENDATIONS The feed coal to gasification consists of coarse coal particles mixed with rock fragments (>6mm coal fraction) derived from the various sources. During coal gasification or combustion, minerals with fluxing elements (Mg, Ca and Fe 2+ ) in the rock fragments and coal macerals interacted with kaolinite at elevated temperatures and pressures to form a melt. On cooling the melt with steam, the anorthite and mullite phases crystallised out from the melt and the heated rock fragments attached to the cooled melt to form clinkers in the gasifier. The clinker formation could significantly affect the permeability for syngas (mixture of carbon monoxide and hydrogen), which is produced by gasification. In some cases, the carbon particles that are supposed to be converted to syngas were encapsulated by the melt and reported to the ash particles during coal gasification. The principal objective of this study was to test the hypothesis that the included fluxing elements-bearing minerals that are associated with the included kaolinite, lowered the ash fusion temperature of this clay at an elevated temperature of greater than 1000 C, to form a melt. The other objective of this study was to use conventional and advanced analytical techniques to qualify and quantify mineral associations that are responsible for the sintering and slagging of mineral matter in the coal during the gasification process. From this, advances in the management of clinker formation are envisaged, which include decreasing carbon loss in the ash fraction and understanding the influences on the production efficiency of a mixture of carbon monoxide and hydrogen. This thesis describes the detailed characterisation of coals from mines in the Highveld coalfield, feed coal, gasification ash particles (heated rock fragment and clinker particles), coal fractions from density separation, coal and corresponding ash samples from the selected gasifier, gas liquor and char samples from the pyrolysis experiments and, turn-out and dig-out samples from the different gasifiers, as well as coal and liquid samples from the chemical fractionation method. As stated earlier in this thesis, the different forms of mineral matter are responsible for many of the problems (e.g. Chapter 9- Conclusions and recommendations 187
9 abrasion, stickiness, slagging, sintering, corrosion and pollution) associated with coal handling and use. The conclusions of results for coal and ash samples from the different experiments are discussed in this section. Chemical analyses of coals from six different Highveld coal mines and feed coal to the coal conversion process The primary constituents in the ashes of coals from the six different Highveld coal mines including the feed coal were SiO 2, Al 2 O 3, CaO, Fe 2 O 3, MgO, SO 3 and TiO 2. Minor proportions (<1.0%) of K 2 O, Na 2 O, P 2 O 5, SrO, BaO and Mn 3 O 4 were also present in both cases. Mineralogical analyses of LTA of coals from the different mines and feed coal Low-temperature oxygen-plasma ashing indicated proportions of mineral matter in the coals ranging from 25 to 45%. These are higher than the proportions of ash indicated by conventional proximate analysis (22-30%) reflecting the breakdown of the different mineral structures at the higher temperatures used in the proximate analysis process. XRD analysis of the low temperature ash indicates that kaolinite (Al 2 Si 2 O 5 (OH) 4 ) and to a lesser extent quartz (SiO 2 ), dolomite (CaMg(CO 3 ) 2 ), illite and/or mica are the major minerals present. Minor proportions of fluxing elements-bearing minerals such as calcite (CaCO 3 ), pyrite (FeS 2 ) and siderite (FeCO 3 ) were also found in some LTA samples. The XRD technique also detected small amounts of the goyazite group (aluminophosphate) minerals in the LTA samples, along with small proportions of anatase (TiO 2 ) and bassanite (CaSO 4.½H 2 O). More detailed XRD analysis of the clay fraction of the LTA samples, using ethylene glycol and heat treatment further identified the nature of the expandable clay minerals (smectite and interstratified illite/smectite) present in the coal samples. QEMSCAN analysis of turn-out sample 32T feed coal confirmed high proportions of kaolinite (15.81%), quartz (6.5%), dolomite (2.67%), calcite (2.18%), organic matter (68.76%) and low proportions of pyrite (1.63%), siderite (0.02%), rutile (0.94%), illite (0.42%), apatite (0.07%) and organic sulphur (0.94%)). QEMSCAN and SEM-EDS Chapter 9- Conclusions and recommendations 188
10 show that calcite grains are strongly associated with the carbon-rich fraction and are also associated with dolomite, kaolinite, quartz and apatite. Due to the presence of the fluxing elements-bearing minerals in the coal, this mineral association could contribute significantly to the sintering and slagging of mineral matter at elevated temperatures during coal gasification or combustion. Petrographic analysis Petrographic analysis indicates that coals are relatively low in vitrinite (20-25%) and rich in inertinite (70-75%), with minor proportions of liptinite also present. Sample from mine 5 had slightly higher proportions of vitrinite and less inertinite than the other coal samples. Mean maximum vitrinite (telocollinite) reflectance was 0.7 to 0.75%. Elemental composition of individual coal macerals in the coals from the six different Highveld coal mines by electron microprobe The vitrinite had significantly higher concentrations of nitrogen and organic sulphur than the inertinite macerals in the same coal samples. Minor proportions of organically-bound inorganic elements, including calcium, aluminium, silicon, magnesium and titanium, were identified in the macerals, especially (for Ti) in the vitrinite components. The overall proportions of carbon, oxygen and nitrogen for the whole-coal samples, expressed to a dry, ash-free basis (Table 4.2) are intermediate between the carbon, oxygen and nitrogen contents for the vitrinite macerals on the one hand and the inertinite macerals on the other (Table 4.8), as determined by the electron microprobe study. The sulphur in the macerals is also significantly lower than the total sulphur in the coals, as it does not incorporate the additional sulphur present separately as pyrite particles. Chapter 9- Conclusions and recommendations 189
11 Chemical fractionation method Water washing had no particular effect on the mineral matter in the bituminous feed coal sample. Treatment with ammonium acetate (at 70ºC) removed most of the carbonate minerals from the bituminous coals. The removal of carbonates by the acetate suggests that the common assumption of such solutions providing only an ion-exchange reaction, needs to be re-evaluated. However, hydrochloric acid treatment did remove almost all the Ca and Fe from the coal samples. Close evaluation of the ash analysis data suggests that some of the Al in the coals was also removed by the selective leaching process. This may reflect a solution of organicallyassociated Al from the maceral components (cf. Matjie et al., 2007; Li et al., 2007). The loss of Ca and other fluxing elements from the coals, induced by the different selective leaching processes, is associated with an increase in the different ash fusion temperatures in all cases. With high pyrite coals, in some cases, the sulfides may oxidise during the extraction process, resulting in a decrease in the ph of the solution, causing the extraction of acid-soluble elements from the minerals. In this leaching step iron, vanadium, titanium and chromium were not dissolved in ammonium acetate solution, indicating that these coals used in the chemical fractionation experiments were not oxidised. These elements are present in Highveld coals in the form of minerals that are insoluble in ammonium acetate. Characterisation of coal size fractions and density fractions and feed coal by analytical techniques The mm size fraction contains high proportions of pyrite, calcite and dolomite and a low proportion of kaolinite, compared with the other coal fractions. Pyrite predominantly occurs as extraneous cleat fragments and to a lesser extent associated with medium to coarse-grained sandstone rock fragments. Calcite in this size fraction also occurs as extraneous cleat fragments and is to a lesser extent included in carbonrich matrix. Chapter 9- Conclusions and recommendations 190
12 The CCSEM results for the coal size fractions indicated that the proportion of extraneous particles decreases with a decrease in size, while the proportion of carbonrich particles containing included minerals increase with a decrease in size. In this context, extraneous particles are principally derived from extraneous rock fragments ( stone ), coarse (>1mm) pyrite and/or calcite rich cleats transecting the coal and possibly from mineral-rich layers in the coal. The mineralogical and petrographic analyses of the density fractions of coal analysed in this study showed the following interesting trends: The increase in SiO 2, K 2 O and Fe 2 O 3 proportions to the >1.8g/cm 3 density fraction is attributed to the increase in the proportion of quartz (Si), microcline (K) and pyrite (Fe), from arkosic sandstone fragments in the 1.8g/cm 3 density fraction. The >1.8g/cm 3 density fraction contains a higher proportion of calcite (CaCO 3 ) than dolomite (CaMg(CO 3 ) 2 ). This is attributed to calcite occurring as coarse extraneous cleat fragments, whereas dolomite tends to mainly occur as inclusions in carbon matrix or cleats transecting the carbon matrix. Kaolinite, which is a predominant mineral in the feed coal, concentrates in the g/cm 3 density fraction and to a lesser extent in the >1.8 g/cm 3 density fraction. A high proportion of kaolinite occurs as fine inclusions in carbon matrix and to a lesser extent as a component of mudstone and siltstone rock fragments. Pyrolysis of coal samples from Highveld coal mines The mineralogical and FactSage results of the coal and char samples show that some of the inorganic elements in the minerals transformed to form new minerals without significant volatilisation, reporting to pyrolysed coal. Further, the XRD analysis of the char samples indicates that some coal minerals are still contained in the char samples produced during the pyrolysis of coal at 600 o C and 26bar under reducing conditions. Chapter 9- Conclusions and recommendations 191
13 ICP-MS and IC analyses of all gas liquor samples indicate that Mg, Ca, Fe, Al, Si, Ti, Na, K, NH + 4, SO 2-4, HCO 2-3, Cl -, F - 2- and CO 3 are present in these liquid samples produced during the pyrolysis experiments. Most of the elements were found to volatilise in relatively high amounts from the mine 5 and mine 6 coals (which had higher inertinite and lower vitrinite contents than the other four coals). Except for some of the Na, all the metallic elements volatilised from the coals studied during the pyrolysis tests, appear to be associated with the carbon matrix in the coals. Chemical and mineralogical analyses of gasification ash samples The mineralogical signature of coarse feed coal and corresponding coarse ash/clinker samples offers an insight into clinker formation. The first stage of clinker formation is the dehydration of kaolinite and muscovite/illite in the carbon-rich particles and rock fragments and devolatisation of coarse pyrite (yielding SO 2, S 2 or H 2 S) and carbonate (yielding CO 2 ) cleat fragments. Dehydrated rock fragments, Fe-S-oxide and Ca-Mg-oxide particles are formed. As the ash-bed gravitates towards the hotter zone of the gasifier, the fluxing effect of Ca/Mg and Fe (derived from respectively included carbonates/organically bound cations and pyrite) lowers the melting points of included kaolinite and quartz, causing a CaO- and FeObearing aluminosilicate melts to form. The reducing conditions (maintaining iron in the divalent form) and higher particle temperatures of carbon-rich particles promote the formation the molten phase. The molten material encapsulates devolatilised rock fragments, coarse Fe-oxide of rich particles, coarse Ca-Mg-oxide particles and char fragments. Sintering is initiated by the crystallisation of anorthite and mullite from the molten material upon cooling. Mullite crystallisation starts at approximately 1400 C and anorthite at 1360 C, depending on melt composition. As the combustion of the carbon progresses, the residual molten/rock fragment ash load gravitates to the rotating grate and is cooled/quenched by the rising steam and oxygen that are introduced at the base of the gasifier. With a decrease in temperature, crystallisation of anorthite and mullite Chapter 9- Conclusions and recommendations 192
14 is halted and the remnant molten material solidifies to form the CaO/MgO- and FeObearing aluminosilicate glass. Predicted quenching temperatures range from 1220 C to as high as 1390 C. This is the final stage of clinker formation. The bulk gasification ash, heated stone and clinker samples produced by the gasification process contain fragments of heat-altered rock materials, set in an often vesicular, partly crystalline glassy matrix. Quartz and possibly K-feldspar in the feed coal or admixed non-coal rocks may pass unaltered into the bulk gasification ash, along perhaps with titanium or iron-titanium oxides and aluminophosphate minerals. Although products of solid-state reactions may be preserved in some of the heataltered fragments, anorthite that has crystallised from molten aluminosilicate material is contained within a glassy matrix in a manner analogous to the formation of igneous rocks. The main objective of this study was to test all hypotheses mentioned in Chapter 2 of this thesis. The CCSEM results indicated that feed coal to gasification consists of three components: Carbon-rich coal particles with included mineral matter: The proportion of mineral matter can vary significantly. The major minerals are kaolinite, quartz, dolomite, calcite and pyrite; Extraneous rock fragments or stone : These rock fragments are derived from footwall, hanging wall and in-seam partings. The major rock fragments are mudstone, siltstone, carbonaceous shale and arkosic sandstone. These fragments have varying proportions of kaolinite, quartz, muscovite/illite and microcline; Extraneous calcite-rich and pyrite-rich cleat fragments: These are derived from coarse cleats transecting the coal seam, footwall and hanging wall. The coarse ash sample is characterised by black porous glass, partially or totally enclosing rock fragments. The included rock fragments are significantly finer than the original rock fragments in the coal feedstock. A significant proportion of the minerals in the rock fragments transform in-situ. Typically, kaolinite dehydrates and forms a devolatilised aluminosilicate. Mullite was Chapter 9- Conclusions and recommendations 193
15 detected by the scanning electron microscope in the rock fragments. Quartz tends to crack. Muscovite/illite transforms into K-bearing glass with an Al/Si ratio similar to muscovite/illite or microcline. Based on the CCSEM results for the turn-out samples it is clear that carbonates (dolomite or ankerite, siderite and calcite) associated with kaolinite lower the ash fusion temperature of kaolinite at elevated temperatures, forming a melt. Interestingly, a low proportion of iron-bearing phases derived from the reaction between kaolinite and pyrite was found in the turn-out samples. This implies that pyrite and its composition products contribute minimally to sintering or slagging of mineral matter at elevated temperatures during coal gasification. FactSage results of coal and ash particles taken from the dig-out test FactSage results predict that it is not possible for Fe, Na, Al and Si to be removed from the coal during pyrolysis as volatile inorganic compounds. Some of the halogens (chlorine and fluorine) are predicted to be removed from the coal as HCl and HF during pyrolysis. The area SEM-EDS analysis of the selected spots of clinker and heated rock fragments taken from the dig-out samples were used in the FactSage model to predict equilibrium phases such as anorthite and mullite at temperature between 800 C and 1200 C. In most cases, these phases completely melted at 1400 C. The presence of the equilibrium anorthite and mullite phases are in agreement with the anorthite and mullite crystals produced from the cooled melt, which was formed by the interaction between high temperature transformation products of the included dolomite or ankerite and calcite, associated with the included kaolinite during coal gasification. RECOMMENDATIONS This study has highlighted a number of mineralogical features and described a few techniques, which could be used to further understand clinker formation in the rock fragments present in the feed coal to gasification. The following is recommended: Chapter 9- Conclusions and recommendations 194
16 The types of rock fragments (siltstones, sandstone, mudstone and carbonaceous shale) in the feed coal to gasification, as well as the heated rock fragment particles in the ash, should be examined, e.g. by a scanning electron microscope (such as a QEMSCAN) to determine the proportions of fluxing elementsbearing minerals (minerals containing calcite, dolomite, siderite and pyrite) as well as mineral associations that are responsible for the slagging of mineral matter during heat treatment. The impact of rock fragments on the crystallisation of the anorthite and mullite should be determined. The heated rock fragments may initiate the crystallisation of mullite and anorthite, depending on the proportions of calcium, silicon and aluminium in the melt. It is also proposed that some anorthite and mullite particles in the heated rock fragments may form by solid-state reaction. This needs to be confirmed. The proportion and mineralogical compositions of the stone in the coal feedstock and the stone in the corresponding gasifier coarse ash should be measured. High temperature XRD should be used to monitor the transformation of individual minerals in the stones under high pressures and oxidising and reducing conditions. It should also be used to analyse molten glass beads formed by heating coal particles under pressure. This data is extremely important for predicting transformation, solidus and liquidus temperatures. Combine all the analytical data and methods and develop a clinker prediction model. This model should be used to predict the clinker formation propensity of varying coal feedstocks and whether it is possible to manage clinker formation. A detailed characterisation of South African coals using electron microprobe and QEMSCAN equipment should be done in order to determine the proportions and the chemical structures of the organically-associated inorganic elements present in the individual macerals in these coals. The organically-associated inorganic elements in the coal macerals could contribute significantly to the volatilisation of inorganic species at low temperature under the reducing and oxidising conditions. Chapter 9- Conclusions and recommendations 195
17 The significant volatilisation of inorganic elements derived from the organicallyassociated inorganic elements is responsible for precipitation of the dissolved metal ions from the gas liquor in the pipe and heat exchangers during the treatment of this liquid. Chapter 9- Conclusions and recommendations 196
PREDICTING THE SLAGGING PROPENSITY OF SASOL-LURGI GASIFIER COAL FEEDSTOCK
 PREDICTING THE SLAGGING PROPENSITY OF SASOL-LURGI GASIFIER COAL FEEDSTOCK Chris van Alphen and Henry Matjie Indaba Conference 10-11 th October 2006 Objective present a new ash formation and slag prediction
PREDICTING THE SLAGGING PROPENSITY OF SASOL-LURGI GASIFIER COAL FEEDSTOCK Chris van Alphen and Henry Matjie Indaba Conference 10-11 th October 2006 Objective present a new ash formation and slag prediction
THE MULTI-TECHNIQUE APPROACH
 CHARACTERISATION OF INORGANIC MATTER IN COAL : THE MULTI-TECHNIQUE APPROACH Sanja Potgieter-Vermaak Maledi, N., Wagner, N., Godoi, R. H. M., Potgieter, J. H. Minerals for LIFE conference, 7-9 June 23 COAL
CHARACTERISATION OF INORGANIC MATTER IN COAL : THE MULTI-TECHNIQUE APPROACH Sanja Potgieter-Vermaak Maledi, N., Wagner, N., Godoi, R. H. M., Potgieter, J. H. Minerals for LIFE conference, 7-9 June 23 COAL
Determination of mineral matter and elemental composition of individual macerals in coals from Highveld mines
 http://dx.doi.org/10.17159/2411-9717/2016/v116n2a8 Determination of mineral matter and elemental composition of individual macerals in coals from Highveld mines by R.H. Matjie*, Z. Li, C.R. Ward, J.R.
http://dx.doi.org/10.17159/2411-9717/2016/v116n2a8 Determination of mineral matter and elemental composition of individual macerals in coals from Highveld mines by R.H. Matjie*, Z. Li, C.R. Ward, J.R.
Sedimentary Rocks and Processes
 Sedimentary Rocks and Processes Weathering Sedimentary Processes Breakdown of pre-existing rock by physical and chemical processes Transport Movement of sediments from environments of relatively high potential
Sedimentary Rocks and Processes Weathering Sedimentary Processes Breakdown of pre-existing rock by physical and chemical processes Transport Movement of sediments from environments of relatively high potential
CERAMIC GLAZING as an IGNEOUS PROCESS
 GEOL 640: Geology through Global Arts and Artifacts CERAMIC GLAZING as an IGNEOUS PROCESS GLAZE COMPONENTS A glaze is a waterproof silica glass on the surface of a ceramic pot, and was first produced by
GEOL 640: Geology through Global Arts and Artifacts CERAMIC GLAZING as an IGNEOUS PROCESS GLAZE COMPONENTS A glaze is a waterproof silica glass on the surface of a ceramic pot, and was first produced by
organisms CaCO 3 + H 2 O + CO 2 shallow water
 Weathering and Reverse weathering Step I:Weathering of igneous rocks 1. Igneous rocks are mainly composed of Al, Si and O 2 with minor and varying quantities of Na, K, Ca and Mg composing pheldspar minerals
Weathering and Reverse weathering Step I:Weathering of igneous rocks 1. Igneous rocks are mainly composed of Al, Si and O 2 with minor and varying quantities of Na, K, Ca and Mg composing pheldspar minerals
WEATHERING. Turning Rock to Sediment and Solutions 10/22/2012
 WEATHERING Turning Rock to Sediment and Solutions Igneous rocks form at high temperatures; at the Earth s surface they are chemically unstable and will begin to disintegrate and decompose in a process
WEATHERING Turning Rock to Sediment and Solutions Igneous rocks form at high temperatures; at the Earth s surface they are chemically unstable and will begin to disintegrate and decompose in a process
muscovite PART 4 SHEET SILICATES
 muscovite PART 4 SHEET SILICATES SHEET SILICATES = PHYLLOSILICATES Phyllon = leaf Large group of mineral including many common minerals: muscovite, biotite, serpentine, chlorite, talc, clay minerals Structure:
muscovite PART 4 SHEET SILICATES SHEET SILICATES = PHYLLOSILICATES Phyllon = leaf Large group of mineral including many common minerals: muscovite, biotite, serpentine, chlorite, talc, clay minerals Structure:
Minerals and Rocks Chapter 20
 Minerals and Rocks Chapter 20 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
Minerals and Rocks Chapter 20 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
23/9/2013 ENGINEERING GEOLOGY. Chapter 2: Rock classification:
 ENGINEERING GEOLOGY Chapter 2: Rock classification: ENGINEERING GEOLOGY Chapter 1.0: Introduction to engineering geology Chapter 2.0: Rock classification Igneous rocks Sedimentary rocks Metamorphic rocks
ENGINEERING GEOLOGY Chapter 2: Rock classification: ENGINEERING GEOLOGY Chapter 1.0: Introduction to engineering geology Chapter 2.0: Rock classification Igneous rocks Sedimentary rocks Metamorphic rocks
About Earth Materials
 Grotzinger Jordan Understanding Earth Sixth Edition Chapter 3: EARTH MATERIALS Minerals and Rocks 2011 by W. H. Freeman and Company About Earth Materials All Earth materials are composed of atoms bound
Grotzinger Jordan Understanding Earth Sixth Edition Chapter 3: EARTH MATERIALS Minerals and Rocks 2011 by W. H. Freeman and Company About Earth Materials All Earth materials are composed of atoms bound
An Investigation into
 An Investigation into MINERALOGICAL CHARACTERIZATION OF FOUR TAILINGS SAMPLES prepared for LISHEEN MINE LR 11527-001 MI5002-APR07 May 28, 2007 NOTE: This report refers to the samples as received. The practice
An Investigation into MINERALOGICAL CHARACTERIZATION OF FOUR TAILINGS SAMPLES prepared for LISHEEN MINE LR 11527-001 MI5002-APR07 May 28, 2007 NOTE: This report refers to the samples as received. The practice
I.S : What s in it and the role of the Geologist
 Institute of Geologists of Ireland Pyrite Course I.S. 398-1: What s in it and the role of the Geologist Michael L.J. Maher 4 December, 2013 Responsibilities of Geologist You re only the messenger! Classification
Institute of Geologists of Ireland Pyrite Course I.S. 398-1: What s in it and the role of the Geologist Michael L.J. Maher 4 December, 2013 Responsibilities of Geologist You re only the messenger! Classification
1/31/2013. Weathering Includes Physical, Chemical, Biological processes. Weathering Mechanisms. Wind abrasion forming Ventifacts
 Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes Weathering Mechanisms Physical
Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes Weathering Mechanisms Physical
2.2 Acid mine drainage
 PROBLEM SOLVING 2. CHEMICAL REACTIONS AND EQUILIBRIA 1 2.2 Acid mine drainage Problem 2.2 The weathering of iron sulphide minerals produces acidified water, leading to major environmental problems from
PROBLEM SOLVING 2. CHEMICAL REACTIONS AND EQUILIBRIA 1 2.2 Acid mine drainage Problem 2.2 The weathering of iron sulphide minerals produces acidified water, leading to major environmental problems from
Emily and Megan. Earth System Science. Elements of Earth by weight. Crust Elements, by weight. Minerals. Made of atoms Earth is mostly iron, by weight
 Emily and Megan Chapter 20 MINERALS AND ROCKS Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
Emily and Megan Chapter 20 MINERALS AND ROCKS Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Elements of Earth by weight Made of atoms Earth
PETROGRAPHIC MINERALOGICAL ANALYSIS OF AGGREGATES FROM DEVOLL HYDROPOWER PROJECT
 PETROGRAPHIC MINERALOGICAL ANALYSIS OF AGGREGATES FROM DEVOLL HYDROPOWER PROJECT Rezarta QEMALLAJ 1,dr. ing. Alma GOLGOTA 1 Authors Affiliations: Prof.Dr. Marie Koçi 1 ( KIBE1 Laborator, Durres, Albania)
PETROGRAPHIC MINERALOGICAL ANALYSIS OF AGGREGATES FROM DEVOLL HYDROPOWER PROJECT Rezarta QEMALLAJ 1,dr. ing. Alma GOLGOTA 1 Authors Affiliations: Prof.Dr. Marie Koçi 1 ( KIBE1 Laborator, Durres, Albania)
Lecture 6. Physical Properties. Solid Phase. Particle Composition
 Lecture 6 Physical Properties Solid Phase Particle Composition 1 Questions What are tetrahedrons and octahedrons? How do silica tetrahedra bonds affect mineral weathering? Difference between primary and
Lecture 6 Physical Properties Solid Phase Particle Composition 1 Questions What are tetrahedrons and octahedrons? How do silica tetrahedra bonds affect mineral weathering? Difference between primary and
Igneous Rocks. Sedimentary Rocks. Metamorphic Rocks
 Name: Date: Igneous Rocks Igneous rocks form from the solidification of magma either below (intrusive igneous rocks) or above (extrusive igneous rocks) the Earth s surface. For example, the igneous rock
Name: Date: Igneous Rocks Igneous rocks form from the solidification of magma either below (intrusive igneous rocks) or above (extrusive igneous rocks) the Earth s surface. For example, the igneous rock
Copyright SOIL STRUCTURE and CLAY MINERALS
 SOIL STRUCTURE and CLAY MINERALS Soil Structure Structure of a soil may be defined as the mode of arrangement of soil grains relative to each other and the forces acting between them to hold them in their
SOIL STRUCTURE and CLAY MINERALS Soil Structure Structure of a soil may be defined as the mode of arrangement of soil grains relative to each other and the forces acting between them to hold them in their
Weathering Cycle Teacher Notes
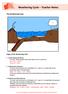 The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
Rocks Rock- A group of minerals, glass, mineroid bound together in some way.
 Rocks Rock- A group of minerals, glass, mineroid bound together in some way. All rocks fit into one of three categories: Igneous- formed by the cooling and hardening of hot molten rock Sedimentary- formed
Rocks Rock- A group of minerals, glass, mineroid bound together in some way. All rocks fit into one of three categories: Igneous- formed by the cooling and hardening of hot molten rock Sedimentary- formed
A Rock is a solid aggregate of minerals.
 Quartz A Rock is a solid aggregate of minerals. Orthoclase Feldspar Plagioclase Feldspar Biotite Four different minerals are obvious in this piece of Granite. The average automobile contains: Minerals
Quartz A Rock is a solid aggregate of minerals. Orthoclase Feldspar Plagioclase Feldspar Biotite Four different minerals are obvious in this piece of Granite. The average automobile contains: Minerals
Weathering and Erosion
 Weathering and Erosion Weathering the disintegration and decomposition of material at the surface Erosion the transportation of weathered material by water, wind, or ice Weathering Two kinds of weathering
Weathering and Erosion Weathering the disintegration and decomposition of material at the surface Erosion the transportation of weathered material by water, wind, or ice Weathering Two kinds of weathering
Sedimentary Geology. Strat and Sed, Ch. 1 1
 Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
9/4/2015. Feldspars White, pink, variable Clays White perfect Quartz Colourless, white, red, None
 ENGINEERING GEOLOGY Chapter 1.0: Introduction to engineering geology Chapter 2.0: Rock classification Igneous rocks Sedimentary rocks Metamorphic rocks Chapter 3.0: Weathering & soils Chapter 4.0: Geological
ENGINEERING GEOLOGY Chapter 1.0: Introduction to engineering geology Chapter 2.0: Rock classification Igneous rocks Sedimentary rocks Metamorphic rocks Chapter 3.0: Weathering & soils Chapter 4.0: Geological
TWO COMPONENT (BINARY) PHASE DIAGRAMS. Experimental Determination of 2-Component Phase Diagrams
 Page 1 of 12 EENS 211 Earth Materials Tulane University Prof. Stephen A. Nelson TWO COMPONENT (BINARY) PHASE DIAGRAMS This document last updated on 08-Oct-2003 Experimental Determination of 2-Component
Page 1 of 12 EENS 211 Earth Materials Tulane University Prof. Stephen A. Nelson TWO COMPONENT (BINARY) PHASE DIAGRAMS This document last updated on 08-Oct-2003 Experimental Determination of 2-Component
Minerals and Rocks. Environmental Learning Community CORC 1332 Sept 21, 2010
 Minerals and Rocks Environmental Learning Community CORC 1332 Sept 21, 2010 Outline Quiz More on minerals Twinkies Rocks How can you identify one mineral from another? Distinguishing One Mineral from Another
Minerals and Rocks Environmental Learning Community CORC 1332 Sept 21, 2010 Outline Quiz More on minerals Twinkies Rocks How can you identify one mineral from another? Distinguishing One Mineral from Another
Crust Elements. Elements of Earth. Minerals. Crystals. Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air
 Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Made of atoms Earth is mostly iron, by weight Elements of Earth Made of atoms
Emily and Megan Earth System Science Interconnected Rocks and minerals Interior processes Erosion and deposition Water and air Made of atoms Earth is mostly iron, by weight Elements of Earth Made of atoms
Potential Geochemical Releases to Groundwater from an In-Situ Oil Shale Retort
 Potential Geochemical Releases to Groundwater from an In-Situ Oil Shale Retort Earl D. Mattson 1, Carl D. Palmer 1, Robert B. Perkins 2 1 Idaho National Laboratory, 2 Portland State University www.inl.gov
Potential Geochemical Releases to Groundwater from an In-Situ Oil Shale Retort Earl D. Mattson 1, Carl D. Palmer 1, Robert B. Perkins 2 1 Idaho National Laboratory, 2 Portland State University www.inl.gov
10/8/15. Earth Materials Minerals and Rocks. I) Minerals. Minerals. (A) Definition: Topics: -- naturally occurring What are minerals?
 minerals Earth Materials Minerals and Rocks I) Minerals Minerals Topics: What are minerals? Basic Chemistry Amethysts in geode: minerals Characteristics of Minerals Types of Minerals -- orderly arrangement
minerals Earth Materials Minerals and Rocks I) Minerals Minerals Topics: What are minerals? Basic Chemistry Amethysts in geode: minerals Characteristics of Minerals Types of Minerals -- orderly arrangement
Chemical Variation of Feed Coal and Coal Combustion Products from an Indiana Power Plant Utilizing Low Sulfur Powder River Basin Coal
 Chemical Variation of Feed Coal and Coal Combustion Products from an Indiana Power Plant Utilizing Low Sulfur Powder River Basin Coal Ronald H. Affolter, Michael E. Brownfield, and James D. Cathcart U.S.
Chemical Variation of Feed Coal and Coal Combustion Products from an Indiana Power Plant Utilizing Low Sulfur Powder River Basin Coal Ronald H. Affolter, Michael E. Brownfield, and James D. Cathcart U.S.
Quartz. ! Naturally occurring - formed by nature. ! Solid - not liquid or gas. Liquid water is not a mineral
 GEOL 110 - Minerals, Igneous Rocks Minerals Diamond Azurite Quartz Why Study Minerals?! Rocks = aggregates of minerals! Importance to Society?! Importance to Geology? 5 part definition, must satisfy all
GEOL 110 - Minerals, Igneous Rocks Minerals Diamond Azurite Quartz Why Study Minerals?! Rocks = aggregates of minerals! Importance to Society?! Importance to Geology? 5 part definition, must satisfy all
Chemistry and the Earth. Martin F. Schmidt, Jr.
 Chemistry and the Earth Martin F. Schmidt, Jr. How were the elements made? How stars make elements. http://www.valdosta.edu/~cbarnbau/astro_ demos/stellar_evol/home_stellar.html Low mass - do #3 first,
Chemistry and the Earth Martin F. Schmidt, Jr. How were the elements made? How stars make elements. http://www.valdosta.edu/~cbarnbau/astro_ demos/stellar_evol/home_stellar.html Low mass - do #3 first,
Aggregates for Concrete
 Fine Aggregate Sand and/or crushed stone < 5 mm (0.2 in.) F.A. content usually 35% to 45% by mass or volume of total aggregate Coarse Aggregate Gravel and crushed stone 5 mm (0.2 in.) typically between
Fine Aggregate Sand and/or crushed stone < 5 mm (0.2 in.) F.A. content usually 35% to 45% by mass or volume of total aggregate Coarse Aggregate Gravel and crushed stone 5 mm (0.2 in.) typically between
Weathering and mineral equilibria. Seminar at NGU 23 May 2016 Håkon Rueslåtten
 Weathering and mineral equilibria Seminar at NGU 23 May 2016 Håkon Rueslåtten Weathering is the breakdown of rocks and minerals that are exposed to surface processes (climatically controlled). Water is
Weathering and mineral equilibria Seminar at NGU 23 May 2016 Håkon Rueslåtten Weathering is the breakdown of rocks and minerals that are exposed to surface processes (climatically controlled). Water is
Minerals. Atoms, Elements, and Chemical Bonding. Definition of a Mineral 2-1
 Minerals In order to define a what we mean by a mineral we must first make some definitions: 2-1 Most of the Earth s surface is composed of rocky material. An element is a substance which cannot be broken
Minerals In order to define a what we mean by a mineral we must first make some definitions: 2-1 Most of the Earth s surface is composed of rocky material. An element is a substance which cannot be broken
Block: Igneous Rocks. From this list, select the terms which answer the following questions.
 Geology 12 Name: Mix and Match: Igneous Rocks Refer to the following list. Block: porphyritic volatiles mafic glassy magma mixing concordant discontinuous reaction series igneous vesicular partial melting
Geology 12 Name: Mix and Match: Igneous Rocks Refer to the following list. Block: porphyritic volatiles mafic glassy magma mixing concordant discontinuous reaction series igneous vesicular partial melting
1 What Is a Mineral? Critical Thinking 2. Apply Concepts Glass is made up of silicon and oxygen atoms in a 1:2 ratio. The SiO 2
 CHAPTER 5 1 What Is a Mineral? SECTION Minerals of Earth s Crust KEY IDEAS As you read this section, keep these questions in mind: What is a mineral? What are the two main groups of minerals? What are
CHAPTER 5 1 What Is a Mineral? SECTION Minerals of Earth s Crust KEY IDEAS As you read this section, keep these questions in mind: What is a mineral? What are the two main groups of minerals? What are
PLATE TECTONICS, VOLCANISM AND IGNEOUS ROCKS
 PLATE TECTONICS, VOLCANISM AND IGNEOUS ROCKS PLATE TECTONICS TO IGNEOUS ROCKS Internal Heat Seafloor Spreading/Plate Tectonics Volcanism Plate Boundary Intra-plate (hot spot) Divergent Convergent Igneous
PLATE TECTONICS, VOLCANISM AND IGNEOUS ROCKS PLATE TECTONICS TO IGNEOUS ROCKS Internal Heat Seafloor Spreading/Plate Tectonics Volcanism Plate Boundary Intra-plate (hot spot) Divergent Convergent Igneous
PREPARATION OF SYNTHETIC ZEOLITES FROM COAL FLY ASH. Shamsul Kamal Sulaiman
 Solid State Science and Technology, Vol. 16, No 1 (28) 17-113 ISSN 128-7389 PREPARATION OF SYNTHETIC ZEOLITES FROM COAL FLY ASH Shamsul Kamal Sulaiman Mineral Research Centre, Minerals and Geoscience Department,
Solid State Science and Technology, Vol. 16, No 1 (28) 17-113 ISSN 128-7389 PREPARATION OF SYNTHETIC ZEOLITES FROM COAL FLY ASH Shamsul Kamal Sulaiman Mineral Research Centre, Minerals and Geoscience Department,
Engineering Geology ECIV 2204
 Engineering Geology ECIV 2204 Instructor : Dr. Jehad Hamad 2017-2016 Chapter (3) Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth
Engineering Geology ECIV 2204 Instructor : Dr. Jehad Hamad 2017-2016 Chapter (3) Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth Igneous Rocks Chapter 3: Rocks: Materials of the Solid Earth
Atoms, Molecules and Minerals
 Atoms, Molecules and Minerals Atoms Matter The smallest unit of an element that retain its properties Molecules - a small orderly group of atoms that possess specific properties - H 2 O Small nucleus surrounded
Atoms, Molecules and Minerals Atoms Matter The smallest unit of an element that retain its properties Molecules - a small orderly group of atoms that possess specific properties - H 2 O Small nucleus surrounded
Sediment and sedimentary rocks Sediment
 Sediment and sedimentary rocks Sediment From sediments to sedimentary rocks (transportation, deposition, preservation and lithification) Types of sedimentary rocks (clastic, chemical and organic) Sedimentary
Sediment and sedimentary rocks Sediment From sediments to sedimentary rocks (transportation, deposition, preservation and lithification) Types of sedimentary rocks (clastic, chemical and organic) Sedimentary
How many hydrogen atoms are there in the empirical formula of propene, C 3 H 6? How many neutrons are there in one atom of 24 Mg?
 1 A 2 B 3 C The atomic number of Na is 11. How many electrons are there in a sodium ion, Na +? How many hydrogen atoms are there in the empirical formula of propene, C 3 H 6? What is the mass in grams
1 A 2 B 3 C The atomic number of Na is 11. How many electrons are there in a sodium ion, Na +? How many hydrogen atoms are there in the empirical formula of propene, C 3 H 6? What is the mass in grams
12 Chemistry (Mg,Fe) 2 SiO 4 Olivine is forms what is called an isomorphous solid solution series that ranges between two end members: Forsterite Mg
 11 Olivine Structure Olivine is a common green or brown rock forming minerals which consists of a solid-solution series between Forsterite (Fo) and Fayalite (Fa). It is an orthorhombic orthosilicate with
11 Olivine Structure Olivine is a common green or brown rock forming minerals which consists of a solid-solution series between Forsterite (Fo) and Fayalite (Fa). It is an orthorhombic orthosilicate with
Properties of Compounds
 Chapter 6. Properties of Compounds Comparing properties of elements and compounds Compounds are formed when elements combine together in fixed proportions. The compound formed will often have properties
Chapter 6. Properties of Compounds Comparing properties of elements and compounds Compounds are formed when elements combine together in fixed proportions. The compound formed will often have properties
Field Trips. Field Trips
 Field Trips Saturday field trips have been scheduled October 9, October 23 and December 4 Last all day (9:00 AM to 4:00 PM) Bus transportation provided from campus Joint with GG101 laboratory, GG101 Section
Field Trips Saturday field trips have been scheduled October 9, October 23 and December 4 Last all day (9:00 AM to 4:00 PM) Bus transportation provided from campus Joint with GG101 laboratory, GG101 Section
ENVI.2030L - Minerals
 ENVI.2030L - Minerals Name I. Minerals Minerals are crystalline solids - the particles (atoms) that make-up the solid have a regular arrangement. In glasses, on the other hand, the atoms are not arranged
ENVI.2030L - Minerals Name I. Minerals Minerals are crystalline solids - the particles (atoms) that make-up the solid have a regular arrangement. In glasses, on the other hand, the atoms are not arranged
F321: Atoms, Bonds and Groups Structure & Bonding
 F321: Atoms, Bonds and Groups Structure & Bonding 1. This question is about different models of bonding and molecular shapes. Magnesium sulfide shows ionic bonding. What is meant by the term ionic bonding?
F321: Atoms, Bonds and Groups Structure & Bonding 1. This question is about different models of bonding and molecular shapes. Magnesium sulfide shows ionic bonding. What is meant by the term ionic bonding?
Minerals. What are minerals and how do we classify them?
 Minerals What are minerals and how do we classify them? 1 Minerals! Minerals are the ingredients needed to form the different types of rocks! Rock - is any naturally formed solid that is part of Earth
Minerals What are minerals and how do we classify them? 1 Minerals! Minerals are the ingredients needed to form the different types of rocks! Rock - is any naturally formed solid that is part of Earth
Wikipedia.org BUILDING STONES. Chapter 4. Materials of Construction-Building Stones 1
 Wikipedia.org BUILDING STONES Chapter 4 Materials of Construction-Building Stones 1 What is Stone? Stone is a concretion of mineral matter. Used either as a; Construction material, Manufacture of other
Wikipedia.org BUILDING STONES Chapter 4 Materials of Construction-Building Stones 1 What is Stone? Stone is a concretion of mineral matter. Used either as a; Construction material, Manufacture of other
CLASS EXERCISE 5.1 List processes occurring in soils that cause changes in the levels of ions.
 5 SIL CHEMISTRY 5.1 Introduction A knowledge of the chemical composition of a soil is less useful than a knowledge of its component minerals and organic materials. These dictate the reactions that occur
5 SIL CHEMISTRY 5.1 Introduction A knowledge of the chemical composition of a soil is less useful than a knowledge of its component minerals and organic materials. These dictate the reactions that occur
Mole Conversions Worksheet
 Mole Conversions Worksheet There are three mole equalities. They are: 1 mol = 6.02 x 10 particles 1 mol = g-formula-mass (periodic table) 1 mol = 22.4 L for a gas at STP Each equality can be written as
Mole Conversions Worksheet There are three mole equalities. They are: 1 mol = 6.02 x 10 particles 1 mol = g-formula-mass (periodic table) 1 mol = 22.4 L for a gas at STP Each equality can be written as
Earth Science 232 Petrography
 Earth Science 232 Petrography Course notes by Shaun Frape and Alec Blyth Winter 2002 1 Petrology - Introduction Some Definitions Petra Greek for rock Logos Greek for disclosure or explanation Petrology
Earth Science 232 Petrography Course notes by Shaun Frape and Alec Blyth Winter 2002 1 Petrology - Introduction Some Definitions Petra Greek for rock Logos Greek for disclosure or explanation Petrology
Cation Exchange Capacity, CEC
 Cation Exchange Capacity, CEC The basic building blocks of clay minerals are: silicon atoms surrounded by four oxygen atoms (tetrahedra), and aluminium atoms surrounded by six hydroxide groups (dioctahedra),
Cation Exchange Capacity, CEC The basic building blocks of clay minerals are: silicon atoms surrounded by four oxygen atoms (tetrahedra), and aluminium atoms surrounded by six hydroxide groups (dioctahedra),
Unit 2: Minerals and Rocks Practice Questions
 Name: Date: 1. Which mineral is white or colorless, has a hardness of 2.5, and splits with cubic cleavage? 6. Base your answer(s) to the following question(s) on the photograph of a sample of gneiss below.
Name: Date: 1. Which mineral is white or colorless, has a hardness of 2.5, and splits with cubic cleavage? 6. Base your answer(s) to the following question(s) on the photograph of a sample of gneiss below.
Classification of Igneous Rocks
 Classification of Igneous Rocks Textures: Glassy- no crystals formed Aphanitic- crystals too small to see by eye Phaneritic- can see the constituent minerals Fine grained- < 1 mm diameter Medium grained-
Classification of Igneous Rocks Textures: Glassy- no crystals formed Aphanitic- crystals too small to see by eye Phaneritic- can see the constituent minerals Fine grained- < 1 mm diameter Medium grained-
Environments of Mineral Formation. Stability Diagrams
 Environments of Mineral Formation Unary, Binary, and Ternary Mineral Stability Diagrams Minerals of differing composition (or polymorphs of the same mineral) that coexist at a set of pressure (P) temperature
Environments of Mineral Formation Unary, Binary, and Ternary Mineral Stability Diagrams Minerals of differing composition (or polymorphs of the same mineral) that coexist at a set of pressure (P) temperature
UNIT 4 SEDIMENTARY ROCKS
 UNIT 4 SEDIMENTARY ROCKS WHAT ARE SEDIMENTS Sediments are loose Earth materials (unconsolidated materials) such as sand which are transported by the action of water, wind, glacial ice and gravity. These
UNIT 4 SEDIMENTARY ROCKS WHAT ARE SEDIMENTS Sediments are loose Earth materials (unconsolidated materials) such as sand which are transported by the action of water, wind, glacial ice and gravity. These
Chapter 4 Rocks & Igneous Rocks
 Chapter 4 Rocks & Igneous Rocks Rock Definition A naturally occurring consolidated mixture of one or more minerals e.g, marble, granite, sandstone, limestone Rock Definition Must naturally occur in nature,
Chapter 4 Rocks & Igneous Rocks Rock Definition A naturally occurring consolidated mixture of one or more minerals e.g, marble, granite, sandstone, limestone Rock Definition Must naturally occur in nature,
Log Interpretation Parameters Determined by Analysis of Green River Oil Shale Samples: Initial Steps
 Log Interpretation Parameters Determined by Analysis of Green River Oil Shale Samples: Initial Steps Michael M. Herron Susan L. Herron Malka Machlus Schlumberger-Doll Research Log Interpretation in Green
Log Interpretation Parameters Determined by Analysis of Green River Oil Shale Samples: Initial Steps Michael M. Herron Susan L. Herron Malka Machlus Schlumberger-Doll Research Log Interpretation in Green
EPS 50 Lab 4: Sedimentary Rocks
 Name: EPS 50 Lab 4: Sedimentary Rocks Grotzinger and Jordan, Chapter 5 Introduction In this lab we will classify sedimentary rocks and investigate the relationship between environmental conditions and
Name: EPS 50 Lab 4: Sedimentary Rocks Grotzinger and Jordan, Chapter 5 Introduction In this lab we will classify sedimentary rocks and investigate the relationship between environmental conditions and
Chapter 4 8/27/2013. Igneous Rocks. and Intrusive Igneous Activity. Introduction. The Properties and Behavior of Magma and Lava
 Introduction Chapter 4 Igneous rocks form by the cooling of magma (or lava). Large parts of the continents and all the oceanic crust are composed of. and Intrusive Igneous Activity The Properties and Behavior
Introduction Chapter 4 Igneous rocks form by the cooling of magma (or lava). Large parts of the continents and all the oceanic crust are composed of. and Intrusive Igneous Activity The Properties and Behavior
Trinitite the Atomic Rock
 Trinitite the Atomic Rock Nelson Eby, EEAS, University of Massachusetts, Lowell, MA Norman Charnley, Earth Sciences, University of Oxford, Oxford, UK John Smoliga, Roxbury, CT Special thanks to Robert
Trinitite the Atomic Rock Nelson Eby, EEAS, University of Massachusetts, Lowell, MA Norman Charnley, Earth Sciences, University of Oxford, Oxford, UK John Smoliga, Roxbury, CT Special thanks to Robert
Engineering Geology. Igneous rocks. Hussien Al - deeky
 Igneous rocks Hussien Al - deeky 1 The Geology Definition of Rocks In Geology Rock is defined as the solid material forming the outer rocky shell or crust of the earth. There are three major groups of
Igneous rocks Hussien Al - deeky 1 The Geology Definition of Rocks In Geology Rock is defined as the solid material forming the outer rocky shell or crust of the earth. There are three major groups of
Chapter 6. Weathering, Erosion, and Soil
 Chapter 6 Weathering, Erosion, and Soil Introduction Rocks and minerals disintegrate and decompose by the processes of physical and chemical weathering. This breakdown occurs because the parent material
Chapter 6 Weathering, Erosion, and Soil Introduction Rocks and minerals disintegrate and decompose by the processes of physical and chemical weathering. This breakdown occurs because the parent material
it must be it must be it must have been formed by it must have it must have
 6. Minerals II (p. 78-87) What is a mineral? The five characteristics required in order for a compound to be a mineral are: it must be it must be it must have been formed by it must have it must have Characteristics
6. Minerals II (p. 78-87) What is a mineral? The five characteristics required in order for a compound to be a mineral are: it must be it must be it must have been formed by it must have it must have Characteristics
CERAMIC MATERIALS I. Asst. Prof. Dr. Ayşe KALEMTAŞ
 CERAMIC MATERIALS I akalemtas@mu.edu.tr, akalemtas@gmail.com, Phone: 211 19 17 Metallurgical and Materials Engineering Department Traditional Ceramics Clay products Main Components Clay Feldspar Silica
CERAMIC MATERIALS I akalemtas@mu.edu.tr, akalemtas@gmail.com, Phone: 211 19 17 Metallurgical and Materials Engineering Department Traditional Ceramics Clay products Main Components Clay Feldspar Silica
Common non-silicate planetary minerals
 Common non-silicate planetary minerals Many of the non-silicate minerals are simple oxides. Corundum Al2O3 Al2+3 O3-2 Rutile Ti2O3 Ti2+3 O3-2 Ilmenite FeTiO3 Fe+3Ti+3O3-2 Hematite Fe2O3 Fe2+3 O3-2 Families
Common non-silicate planetary minerals Many of the non-silicate minerals are simple oxides. Corundum Al2O3 Al2+3 O3-2 Rutile Ti2O3 Ti2+3 O3-2 Ilmenite FeTiO3 Fe+3Ti+3O3-2 Hematite Fe2O3 Fe2+3 O3-2 Families
Rocks Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3
 Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification VI. Additional sub-classes
Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification VI. Additional sub-classes
Rocks Environmental Significance. Rocks Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3. Rocks Definition of a rock
 Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 Environmental Significance I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification
Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 Environmental Significance I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification
APPENDIX F PHOTOGRAPHS OF CORE SAMPLES
 EKATI DIAMOND MINE LONG LAKE CONTAINMENT FACILITY INVESTIGATION 2013 EBA FILE: E14103013-01.002 MAY 2014 ISSUED FOR USE APPENDIX F PHOTOGRAPHS OF CORE SAMPLES LLCF Geotechnical Site Investigation Report_IFU.docx
EKATI DIAMOND MINE LONG LAKE CONTAINMENT FACILITY INVESTIGATION 2013 EBA FILE: E14103013-01.002 MAY 2014 ISSUED FOR USE APPENDIX F PHOTOGRAPHS OF CORE SAMPLES LLCF Geotechnical Site Investigation Report_IFU.docx
The Nucleus. Protons. Positive electrical charge The number of protons in the nucleus determines the atomic number
 Matter Atoms The smallest unit of an element that retain its properties Small nucleus surrounded by a cloud of electrons The nucleus contains protons and neutrons The Nucleus Protons Positive electrical
Matter Atoms The smallest unit of an element that retain its properties Small nucleus surrounded by a cloud of electrons The nucleus contains protons and neutrons The Nucleus Protons Positive electrical
Current State of Extraction Don t Be Deceived! Sharon F. Webb, Ph.D. Director of Quality Program
 Current State of Extraction Don t Be Deceived! Sharon F. Webb, Ph.D. Director of Quality Program Overview Factors Purpose of Dissolution Quality Objectives of Program Effectiveness of Dissolution Technique
Current State of Extraction Don t Be Deceived! Sharon F. Webb, Ph.D. Director of Quality Program Overview Factors Purpose of Dissolution Quality Objectives of Program Effectiveness of Dissolution Technique
Feldspars. Structure. The feldspars are by far the most abundant group of minerals and are found in igneous, metamorphic and many sedimentary rocks.
 Feldspars The feldspars are by far the most abundant group of minerals and are found in igneous, metamorphic and many sedimentary rocks. Structure Felsdpars are framework silicates where each silica tetrahedra
Feldspars The feldspars are by far the most abundant group of minerals and are found in igneous, metamorphic and many sedimentary rocks. Structure Felsdpars are framework silicates where each silica tetrahedra
ENVI.2030L Rock Identification
 ENVI.2030L Rock Identification Name I. Introduction The bulk of the earth's crust is composed of relatively few minerals. These can be mixed together, however, to give an endless variety of rocks - aggregates
ENVI.2030L Rock Identification Name I. Introduction The bulk of the earth's crust is composed of relatively few minerals. These can be mixed together, however, to give an endless variety of rocks - aggregates
Textures of Igneous Rocks
 Page 1 of 6 EENS 212 Prof. Stephen A. Nelson Petrology Tulane University This document last updated on 12-Feb-2004 Introduction to Igneous Rocks An igneous rock is any crystalline or glassy rock that forms
Page 1 of 6 EENS 212 Prof. Stephen A. Nelson Petrology Tulane University This document last updated on 12-Feb-2004 Introduction to Igneous Rocks An igneous rock is any crystalline or glassy rock that forms
The Lithosphere. Definition
 10/14/2014 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2014 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
10/14/2014 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2014 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
Minerals: Building Blocks of Rocks Chapter 2. Based on: Earth Science, 10e
 Minerals: Building Blocks of Rocks Chapter 2 Based on: Earth Science, 10e Minerals: the building blocks of rocks Definition of a mineral Solid Inorganic Natural Crystalline Structure - Possess an orderly
Minerals: Building Blocks of Rocks Chapter 2 Based on: Earth Science, 10e Minerals: the building blocks of rocks Definition of a mineral Solid Inorganic Natural Crystalline Structure - Possess an orderly
KISS Resources for NSW Syllabuses & Australian Curriculum.
 Discusssion / Activity 1 Structure of the Earth Student Name... 1. Outline how we think the Sun & planets formed. The solar system formed from a cloud of gas & dust. Part of the cloud collapsed under gravity
Discusssion / Activity 1 Structure of the Earth Student Name... 1. Outline how we think the Sun & planets formed. The solar system formed from a cloud of gas & dust. Part of the cloud collapsed under gravity
This is how we classify minerals! Silicates and Non-Silicates
 Why are some minerals harder than others? Their atomic structure and chemical formula. This is how we classify minerals! Silicates and Non-Silicates Part #1 - Silicates: Silicon and Oxygen make up 70%
Why are some minerals harder than others? Their atomic structure and chemical formula. This is how we classify minerals! Silicates and Non-Silicates Part #1 - Silicates: Silicon and Oxygen make up 70%
Practice Questions for Lecture 5 Geology 1200
 Practice Questions for Lecture 5 Geology 1200 Use these questions to test your knowledge of Lecture5. The exams will be similar in format, except that they will deal with more than one chapter, and will
Practice Questions for Lecture 5 Geology 1200 Use these questions to test your knowledge of Lecture5. The exams will be similar in format, except that they will deal with more than one chapter, and will
Instructor: Ms. Terry J. Boroughs Geology 8 INTRODUCTION TO ROCKS AND THE ROCK CYCLE
 DATE DUE: Name: Instructor: Ms. Terry J. Boroughs Geology 8 INTRODUCTION TO ROCKS AND THE ROCK CYCLE Instructions: Read each question carefully before selecting the BEST answer Provide specific and detailed
DATE DUE: Name: Instructor: Ms. Terry J. Boroughs Geology 8 INTRODUCTION TO ROCKS AND THE ROCK CYCLE Instructions: Read each question carefully before selecting the BEST answer Provide specific and detailed
Imagine the first rock and the cycles that it has been through.
 A rock is a naturally formed, consolidated material usually composed of grains of one or more minerals The rock cycle shows how one type of rocky material gets transformed into another The Rock Cycle Representation
A rock is a naturally formed, consolidated material usually composed of grains of one or more minerals The rock cycle shows how one type of rocky material gets transformed into another The Rock Cycle Representation
Matter and Minerals Earth: Chapter Pearson Education, Inc.
 Matter and Minerals Earth: Chapter 3 Minerals: Building Blocks of Rocks By definition a mineral is: Naturally occurring An inorganic solid Ordered internal molecular structure Definite chemical composition
Matter and Minerals Earth: Chapter 3 Minerals: Building Blocks of Rocks By definition a mineral is: Naturally occurring An inorganic solid Ordered internal molecular structure Definite chemical composition
Geos 306, Mineralogy Final Exam, Dec 12, pts
 Name: Geos 306, Mineralogy Final Exam, Dec 12, 2014 200 pts 1. (9 pts) What are the 4 most abundant elements found in the Earth and what are their atomic abundances? Create a reasonable hypothetical charge-balanced
Name: Geos 306, Mineralogy Final Exam, Dec 12, 2014 200 pts 1. (9 pts) What are the 4 most abundant elements found in the Earth and what are their atomic abundances? Create a reasonable hypothetical charge-balanced
Sediment. Weathering: mechanical and chemical decomposition and disintegration of rock and minerals at the surface
 Sediment Some basic terminology Weathering: mechanical and chemical decomposition and disintegration of rock and minerals at the surface Erosion: removal of weathered rock and minerals from one place to
Sediment Some basic terminology Weathering: mechanical and chemical decomposition and disintegration of rock and minerals at the surface Erosion: removal of weathered rock and minerals from one place to
Chapter 3. Atoms and Minerals. Earth Materials
 Chapter 3 Atoms and Minerals Earth Materials Atoms and Elements: Isotopes and Ions A Review of Chemistry Atoms Atoms are composed of Protons, Neutrons and Electrons A proton has an electric charge of +1
Chapter 3 Atoms and Minerals Earth Materials Atoms and Elements: Isotopes and Ions A Review of Chemistry Atoms Atoms are composed of Protons, Neutrons and Electrons A proton has an electric charge of +1
The Martian Sedimentary Mass: Constraints on its Composition, Age and Size. Scott McLennan Department of Geosciences, SUNY Stony Brook
 The Martian Sedimentary Mass: Constraints on its Composition, Age and Size Scott McLennan Department of Geosciences, SUNY Stony Brook Exploring Mars Habitability Lisbon 14 June, 2011 Martian Crustal Chemistry
The Martian Sedimentary Mass: Constraints on its Composition, Age and Size Scott McLennan Department of Geosciences, SUNY Stony Brook Exploring Mars Habitability Lisbon 14 June, 2011 Martian Crustal Chemistry
Chapter 6 9/25/2012. Weathering, Erosion and Soils. Introduction. How Are Earth Materials Altered? Introduction. How Are Earth Materials Altered?
 Chapter 6 Introduction Rocks and minerals are disintegrated and decomposed by the processes of mechanical and chemical weathering. Weathering, Erosion and Soils This breakdown occurs because the parent
Chapter 6 Introduction Rocks and minerals are disintegrated and decomposed by the processes of mechanical and chemical weathering. Weathering, Erosion and Soils This breakdown occurs because the parent
Lecture 6 Flotation 1. Chapter 8_Flotation of Non-Sulphide Minerals
 Lecture 6 Flotation 1 Chapter 8_Flotation of Non-Sulphide Minerals Contents: Oxides & silicates. Adsorption & chemisorption of collectors. Activation & depression. Silica, feldspar, calcite, phosphates.
Lecture 6 Flotation 1 Chapter 8_Flotation of Non-Sulphide Minerals Contents: Oxides & silicates. Adsorption & chemisorption of collectors. Activation & depression. Silica, feldspar, calcite, phosphates.
EPSC 233. Compositional variation in minerals. Recommended reading: PERKINS, p. 286, 41 (Box 2-4).
 EPSC 233 Compositional variation in minerals Recommended reading: PERKINS, p. 286, 41 (Box 2-4). Some minerals are nearly pure elements. These are grouped under the category of native elements. This includes
EPSC 233 Compositional variation in minerals Recommended reading: PERKINS, p. 286, 41 (Box 2-4). Some minerals are nearly pure elements. These are grouped under the category of native elements. This includes
Tailings and Mineral Carbonation: The Potential for Atmospheric CO 2 Sequestration
 Tailings and Mineral Carbonation: The Potential for Atmospheric CO 2 Sequestration H. Andrew Rollo Lorax Environmental Services Ltd. Heather. E. Jamieson Department of Geological Sciences and Geological
Tailings and Mineral Carbonation: The Potential for Atmospheric CO 2 Sequestration H. Andrew Rollo Lorax Environmental Services Ltd. Heather. E. Jamieson Department of Geological Sciences and Geological
1. Base your answer to the following question on The diagram below represents a part of the crystal structure of the mineral kaolinite.
 1. Base your answer to the following question on The diagram below represents a part of the crystal structure of the mineral kaolinite. An arrangement of atoms such as the one shown in the diagram determines
1. Base your answer to the following question on The diagram below represents a part of the crystal structure of the mineral kaolinite. An arrangement of atoms such as the one shown in the diagram determines
UNIQUE MINERALOGY OF OIL SHALE FROM THE PICEANCE BASIN, COLORADO
 UNIQUE MINERALOGY OF OIL SHALE FROM THE PICEANCE BASIN, COLORADO 27th Oil Shale Symposium Golden, Colorado Marcus Wigand (Presenter) Steve Chipera Giday Woldegabriel J. William Carey John Kaszuba Doug
UNIQUE MINERALOGY OF OIL SHALE FROM THE PICEANCE BASIN, COLORADO 27th Oil Shale Symposium Golden, Colorado Marcus Wigand (Presenter) Steve Chipera Giday Woldegabriel J. William Carey John Kaszuba Doug
Geology for Engineers Rocks
 89.325 Geology for Engineers Rocks Name I. Introduction The bulk of the earth's crust is composed of relatively few minerals. These can be mixed together, however, to give an endless variety of rocks -
89.325 Geology for Engineers Rocks Name I. Introduction The bulk of the earth's crust is composed of relatively few minerals. These can be mixed together, however, to give an endless variety of rocks -
Chapter: Earth Materials
 Table of Contents Chapter: Earth Materials Section 1: Minerals Section 2: Igneous Rocks Section 3: Sedimentary Rocks Section 4: Metamorphic Rocks and the Rock Cycle 1 Minerals Common Elements Composition
Table of Contents Chapter: Earth Materials Section 1: Minerals Section 2: Igneous Rocks Section 3: Sedimentary Rocks Section 4: Metamorphic Rocks and the Rock Cycle 1 Minerals Common Elements Composition
Earth Materials. The Crust and its Composition. Igneous Rocks. Sediments and Sedimentary Rocks. Metamorphic Rocks. The Cycle of Rock Change
 Earth Materials The Crust and its Composition Igneous Rocks Sediments and Sedimentary Rocks Metamorphic Rocks The Cycle of Rock Change The Crust and its Composition oxygen and silicon account for about
Earth Materials The Crust and its Composition Igneous Rocks Sediments and Sedimentary Rocks Metamorphic Rocks The Cycle of Rock Change The Crust and its Composition oxygen and silicon account for about
Sedimentology & Stratigraphy. Thanks to Rob Viens for slides
 Sedimentology & Stratigraphy Thanks to Rob Viens for slides Sedimentology The study of the processes that erode, transport and deposit sediments Sedimentary Petrology The study of the characteristics and
Sedimentology & Stratigraphy Thanks to Rob Viens for slides Sedimentology The study of the processes that erode, transport and deposit sediments Sedimentary Petrology The study of the characteristics and
