EQUILIBRIUM VERSUS KINETICS IN C02 DOMINATED HYDROTHERMAL ALTERATION F. MAY
|
|
|
- Eustacia Lambert
- 5 years ago
- Views:
Transcription
1 189 Proceedings 18th NZ Geothermal Workshop 1996 EQUILIBRIUM VERSUS KINETICS IN C02 DOMINATED HYDROTHERMAL ALTERATION F. MAY Nuclear Sciences Group, IGNS, Lower Hutt, NZ Geod pmik/physik der LithOsphk, Ftheinische Friedrich-Wilhelms UniversiGt, BOM, D SUMMARY - Compositional differences between unaltered protoliths and altered rocks fiom mineral water wells can be related to the compositional variations between groundwater and highly concentrated bicarbonate waters in order to reveal the alteration reactions. One basic process is sufficient to explain the range of bicarbonate waters occurring in the Rhenish Massif: Carbonic acid, produced by the dissolution of magmatic CO, in groundwater, gradually dissolves unstable primary minerals of the wallrock, exposed on fiesh fracture surfaces along the flow path, while the successive formation of stable alumosilicates and carbonates precipitates the surplus of dissolved species. Thermodynamic reaction progress simulations including in-eversible and equilibrium steps are suitable to quanti@ water rock ratios as well as the amounts of reaction products. 1. INTRODUCTION The understanding of hydrothermal alteration reactions at temperatures below 100 "C is made difficult, because of slow reaction velocities. Alteration reactions, especially those involving complex silicate minerals often do not reach thermodynamic equilibria with the surrounding fluids. Protolith minerals formed at higher temperatures may remain unaffected in metastable states of stability even for long periods of time. Thus, calculations of fluid compositions and secondary phase assemblages, based on thermodynamic equilibrium assumptions, often fail to reproduce the observations. Chemical equilibrium considerations between rock and water, useful in metamorphic or high temperature geothermal systems, do not apply to cool hydrothermal alteration reactions. Mineral waters from the Rhenish Massif do not plot along the solid line in Fig. 1, that marks the composition of waters in equilibrium with average crustal rocks (Giggenbach 1988). To overcome the difficulties of slow reaction kinetics it is necessary to identi@ and to separate the equilibrium reactions fiom those alteration steps which are limited by reaction velocities. The consideration of both reaction types in a reaction progress concept allows to calculate the compositions of the altered rocks and those of the associated mineral waters as well. One single reaction progress model is sufficient to explain different degrees of hydrothermal alteration and the occurrence of various mineral water types. This will be demonstrated for the kaolinisation of silicate rocks by cool CO, rich waters. CO, rich waters often occur in regions of Quaternary volcanic activity. They are frequently found in areas of continental rifting, associated with alkalibasaltic volcanism. In areas with subduction volcanism they also occur in hydrothermal systems related. to degassing magmas in subvolcanic intrusions. 2. STUDY AREA The Rhenish Massif is part of a larger region characterized by carbonic springs, recent crustal extension, uplift, rifting, alkalibasaltic volcanism and high fluxes of mantle helium, that extends fiom the French Massif Central to the Bohemian Massif. Its geodynamic features and the relation between mineral waters and mantle processes have been discussed by May (1994a) and May et al. (in press). The predominant rock types in the massif are Palaeozoic shales, greywackes and sandstones, that form the fiactured aquifers for the mineral waters. Within the massif, HCO, waters discharge fiom numerous small springs or wells, with frequency maxima in the Quaternary Eifel volcanic fields. In mineral waters from the margins of the massif, C1' is the dominant anion. The C0,-rich waters reach well-head temperatures of up to 73 OC, while most of the spring waters are only slightly warmer than the mean annual air temperature of 10 "C. Thermal waters discharge in the major valleys and along the margins of the massif fiom topography driven flow systems. Since many springs are used for drinking and health purposes, they are analysed frequently and a representative set of almost 800 mainly published water analyses has been available for this study. The siliciclastic rocks of the massif are often bleached and kaolinised. This alteration is partly the result of weathering under subtropical conditions in the Mesozoic and in the Early Tertiary. The recent hydrothermal alteration by C0,-rich groundwaters also
2 190. Y... rn IvIs-Ca-HcQ 0 IVIS-Ca-Na-Hrn o ca Fig. 1. Mass ratios of alkali and alkaline earth element concentrations in mineral waters from the Rhenish Massif results in kaolinisation of wallrocks. Iron minerals and the topographical position of the altered rocks allow a distinction between both types of alteration (May 1994b). In the altered rocks encountered in mineral water wells chlorite has been replaced by kaolinite. Their albite contents are lower than in the protoliths. Dolomite/ankerite and (Mg-) siderite are common secondary phases in the altered rocks. Alunite and smectite occur occasionally but barite, cristobalite and halloysite rarely exceed the XRD detection limits. Abundant quartz veins with euhedral crystals indicate that silica precipitates during alteration too. The degree of alteration varies within individual wells, according to the structure of the fractured aquifers. Significant trends with depth (temperature) are the exception in the sampled wells, which are 100 to 600 m deep. Further properties of the secondary phases are given by May et al. (in press). Matching rock and water analyses from these wells provides the opportunity to test possible alteration reactions using both, the solid reaction products that remain in the aquifer and the dissolved reaction products that emerge in the mineral springs. Suitable reactions have to fulfil mass balances for the changes in composition of rocks and water, resulting from the alteration reactions: protolith + carbon dioxide + groundwater = bicarbonate-water + altered rock (1) 3. ANALYTICAL RESULTS Dissolved C02(aq.), H,CO, and HCO,' are the main carbon species in the slightly acidic waters. The dissociation of H2C03 provides H,O+ for the dissolution of the primary minerals. The conversion of H2C0, to HCO,' proceeds continuously with progressive rock alteration. Thus, the HCO,' concentrations reflect the amount of rock altered by the mineral waters, while CO, and H2C03 remaining in,the water represent the potential for further rock alteration. Relative HCO,' concentrations in mineral waters from the Rhenish Massif vary from to 1 to 95 mol % of total Cy indicating quite different degrees of reaction progress and neutralisation of carbonic acid. The cation concentrations provide information on the minerals participating in the alteration reactions. Variograms of the major cations against HCO,- concentrations of Ca-
3 oo 1 Y 200 3oo E 3oo g I Rhenish Massif Ca-Nlg-HCO3-vvaters. 5c I sad.i m -.. I.. a 90 Fig. 2. Variation of major cation concentrations with bicarbonate concentrations in Ca-Mg-Na-HCO, waters. Mg-Na-HCO, waters exhibit nearly linear trends with positive slopes, indicating that the acid neutralization is proportional to whole rock dissolution. The Ca, Mg and Na concentrations are in the range expected for the dissolution of average shale or sandstone wallrocks. The dissolution of primary quartz is neglegible in the mineral waters, which are usually supersaturated with respect to this mineral. The K contents however, are considerably lower, indicating; that illite, the main K phase is stable and only K-feldspar contributes to the K content. Since the mineral waters are mostly undersaturated with respcet to illite, its dissolution reaction appears to be slow. The lines in Fig. 2 have been calculated for complete leaching of all the Ca, Mg and Na of average unaltered sandstones (dotted) and siltstones (solid) from the Palaeozoic basement, and for the dissolution of 5 wt.-% of their K, according to their illite and K-feldspar contents. An equivalent amount of HCOi is added to maintain the solutions charge-balance. The main cation contents in one kg of the most highly concentrated waters are 'equal to their amounts contained in the soluble minerals of 35 g shale or 90 g sandstone. The congruent dissolution of chlorite should liberate substantial amounts of Fe, A1 and Si too. These elements usually have low concentrations in mineral waters, and they do not correlate with the HCO; concentrations, as the major cations do, but they are constituents of the secondary phases kaolinite, siderite and (vein-)quartz. The unstable primary minerals chlorite, albite, K-feldspar and calcite dissolve in proportion to their abundance in the wallrocks. The Fe, A1 and Si phases precipitate in fast reactions, approaching to thermodynamic equilibrium. The dissolution reactions of the soluble primary minerals do not reach equilibrium instead, as indicated by the excess of aggressive H,CO, still present in the mineral waters. The dissolution of wallrock in CO, charged water and the state of reaction progress are limited by the amount of fresh wallrock available along the waters flow path. 4. NUMERICAL RESULTS The combination of irreversible and equilibrium reactions in reaction progress simulations is a suitable way to quantify the water compositions as well as the associated degrees of wallrock alteration. The computer code PHREEQC of Parkhurst (1995), which is based on an ion association concept, has been used for the numerical models. The total C (initial CO,) contents of the Rhenish HCO,- waters reach 160 mmol/kg. The first equilibrium step in the simulation shown in Fig. 3, is the dissolution of CO, at a partial pressure of 0.2 Mpa in pure water, at 20 "C, at ph 6, resulting in a carbonic acid solution of ph 3.8. The lower Devonian siliciclastic wallrocks are represented by silty shales, 100 g containing 8.5 g chlorite (thuringite - ripidolite), 27.5 g illite, 0.35 g kaolinite, 58.5 g quartz,, 4 g albite, 0.3 g K-feldspar, and 0.6 g carbonate. The 'soluble' minerals chlorite, feldspar and carbonate comprise 13.8 % b.w. of the rock. S has been added as FeSO, without further assumptions about actual redox reactions, which involve Fe and organic C species too. The elements produced by the dissolution of unstable primary minerals are added stepwise to the carbonated water, up to 100 g whole rock. The precipitation of secondary phases and the species distribution in the resulting solution are calculated for equilibrium conditions again.
4 192 At first, the element concentrations increase linearly with progressive rock alteration (see Fig. 3). Carbonic acid dissociates to bicarbonate and hydronium ions are consumed in the silicate hydrolysis. After the alteration of about 0.3 g rock by one kg of carbonated water, the solution becomes saturated with silica and chalcedony (to allow for some supersaturation of the solution with respect to quartz) starts to precipitate. Further on the Si content of the water remains almost constant at about 6.9 ppm. Alunite follows at 0.4 g and halloysite (a metastable kaolinite precursor) precipitates beyond 0.5 g whole rock alteration. The predominant supply of SiO, from silicate dissolution is sufficient to maintain an excess of silica to precipitate, even during halloysite precipitation, so that the SiO, concentration remains at the level of chalcedony saturation. The next mineral to precipitate is siderite at 3 g of rock alteration. At this stage only 390 mg of primary minerals have been dissolved yet and the maximum iron content of 76 ppm is reached. Between siderite and dolomite saturation, a quasi-steady state of reaction progress is attained. The amount of secondary phases produced is proportional to the amount of dissolved rock. Most of the Mg-Ca-HCO, waters are within this range of steady state alteration progress, which explains the linear trends in Fig. 2. The alkali and alkaline earth element concentrations continue to increase linearly, until 50 g of rock have reacted with the water and the saturation of dolomite is reached. Now Ca and Mg begin to precipitate, the MgKa ratio increases and the solution evolves forward to become a Na-HCO, water. Higher CO, pressures which can occur at depth will not change the general pattern of reaction progress, but they will increase the amounts of dissolved elements forming bicarbonate complexes, and the precipitation of siderite and dolomite will start at higher degrees of rock alteration. At a CO, partial pressure of 20 Mpa 3 kg whole rock can react until the solution becomes saturated with respect to chlorite. The high CO, content will prevent the precipitation of other secondary silicates and increase the stability field of alunite. Thus, the principal reaction is the same, if we assume dilution of C0,-saturated water before - or after reaction with the wall rocks. The reaction temperature influences the order in which the saturation of the secondary minerals is reached during progressive rock alteration, but not the relative amounts that precipitate. At a temperature of 40 "C, the solubility of CO, is lower and the carbonates precipitate earlier than at a reaction temperature of 20 "C. At 40 "Cy carbonic acid will be used up earlier and halloysite as well as alunite will finally dissolve in the alkaline carbonate solution. At initial stages of the alteration reactions (low rockwater ratios), the cation concentrations of the C0,-rich but HC0,--poor solutions are close to the composition of the local groundwaters (see Fig. 1). Variations in the composition of clastic rocks will affect the alteration reactions to a lesser extent, e.g. increased SO:- concentrations can result from S-rich shales. Locally pyroclastic rocks and carbonates form parts of the aquifers and modify the alteration reactions and the resulting solutions. In case of the irreversible alteration of West-Eifel foidites, goethite, montmorillonite and other sheet silicates will precipitate. The general pattern of alteration progress is maintained however, since chalcedony, halloysite, siderite and dolomite remain major secondary phases. Montmorillonite and illite may be secondary phases produced by alteration of the siliciclastic rocks too. They will not replace kaolinite as the predominant clay mineral, but they can replace alunite, at low CO, pressures or at higher temperatures, and they can limit the K concentrations. 5. DISCUSSION The calculated alteration reactions, as described above, confm the reaction progress concept derived from the considerations of mass balances. The amounts of primary mineral dissolution and the precipitation of secondary phases are in accordance with the modal compositions of rocks from the sampled wells. The calculated water compositions are similar to those observed in the Rhenish Massif. The analytical element concentrations plot on the calculated reaction progress curves within a narrow range of rock alteration, as shown for two examples: the low-concentrated Fe-Mg-HCO, water fiom Darscheid and the more highly concentrated Na-Mg-Ca-HCO, water fiom the well Walburgisquelle in Bad Neuenahr (see Fig. 3). Low degrees of wallrock alteration produce the Fe rich bicarbonate waters, typical of cool, fiee discharging springs. No dissolution of iron sulfides, as often stated, is required for low-concentrattion Fe-HCO, waters. The non-monotonous evolution of the Si, A1 and Fe concentrations explains their bad correlations with the other elements, which significantly correlate with each other. The precipitation of siderite does not cause kinks in the linear Ca/HCO, and Mg/HCO, trends (see Fig. 2), because it occurs early in the alteration process at very low total contents of dissolved solids (400 ppm), whereas most of the analysed waters have higher concentrations. Compositional differences among waters at one location, or even within one well, can be explained by different 'access' to fiesh wall rocks and various degrees of reaction progress, without the need of hydraulic isolation of different aquifer domains. These differences correspond to varying degrees of alteration within wells, reflecting the nature of the fiactured auifers. 'Access' is used as a bulk term integrating the volumes of water, the rock surface area, the contact time and diffusion through secondary phases on fracture surfaces. The generation of
5 E-2 1 E-3 1 E4 1 E-5 1 E-6 1 E-I 1 E-2 1 E-3.- c 1 E4 I E-5 1 E-6 Dameid + Wburgisquelle 1 E-? o mole Rod< Alteration [g] Fig. 3. Progressive alteration of average SiegenEms siltstones: rock mass balance (top), secondary phase assemblage (center), element and species concentrations in solution (bottom).
6 194 Na-HCO, waters is favoured on active faults along the Middle Rhine area, which continuously create new porosity and fresh fracture surfaces. While the reaction progress simulations show that Na- HCO, waters result from advanced wallrock alteration, they are usually seen as products of ion exchange reactions, an explanation made popular by Schwille (1955). Though Plum (1989) recognized that silicate alteration and carbonate precipitation can produce Na- HCO, waters, he did not believe that they could form by these reactions alone and stressed the importance of ion exchange. In none of the papers suggesting ion exchange for Rhenish HCO, waters, have the exchange reactions been quantified. Their authors did not demonstrate the effects of ion exchange on specific exchange phases in the wallrocks either. 6. CONCLUSIONS One single genetic model is sufficient to explain many features of wall rock alteration and the range of different HCO, waters found in the Rhenish massif. The variety of individual processes proposed by previous workers to explain the compositions of different water types or the abundance of individual elements can be restricted. Local peculiarities modify the reactions, but they do not alter the general principle of progressive wallrock alteration. Numerical simulations help to check the general probability of possible reactions and they allow to quantify the water chemistry and the wallrock composition as a function of water-rock ratios and physical aquifer conditions. A prerequisite for successful numerical simulations is the identification of irreversible and equilibrium reactions. When dolomite saturation is exceeded, the mineral mass balance becomes positive. The mass of minerals precipitated exceeds the mass of dissolved minerals. (Volume balances depend on the actual mineral densities, but they are similar to the mass changes.) At this stage the alteration process may come to an end by self-sealing of the pore and fracture space. This can be the reason why no further evolved alkaline Na-CO, waters, which are expected at further alteration progress, occur in the Rhenish Massif, despite of the remaining alteration potential in the Na-HCO, waters. The reaction progress simulations allow to determine ranges where the cation ratios depend on the amount of wall rock alteration, limiting the applicability of certain geothermometers. While the silica geothermometer is only affected at very low concentrations, thermometers using Ca and Mg are limited to less than -50 g whole rock alteration per kg water. The simulations provide a way to obtain waterhock ratios from actual water compositions and to test the sensitivity of values obtained for different elements to changes in reaction progress. The numerical results show the possibility of illite and montmorillonite precipitation. Unknown reaction kinetics, variable mineral compositions and uncertainties of thermodynamical data are reasons not to draw conclusions about their actual formation, based on numerical simulations alone. The detection of low amounts of montmorillonite and secondary illite, among abundant primary illite, by conventional XRD is difficult too. Thus, more detailed clay mineralogical studies are required to reveal the role of illite and montmorillonite in the alteration reactions. The validity of the equilibrium assumptions, for their formation at the time scales of deep groundwater flow, should be tested by hydrothermal experiments. 7. ACKNOWLEDGEMENTS The results presented here were obtained during a postdoctoral visit at IGNS, Lower Hutt, sponsored by the Deutsche Forschungsgemeinschaft. 8. REFERENCES Giggenbach, W.F. (1 988). Geothermal solute equilibria. Derivation of Na-K-Mg-Ca geoindicators. Geochim. Cosmochim. Acta, Vol. 52, May, F. (1994a). Zur Entstehung der Mineralwasser des Rheinischen Masivs. PhD Thesis, Bonn. May, F. (1994b). Weathering or hydrothermal alteration? Examples from the Rhenish Massif, Germany. Mineral. Mag., Vol. 58(A), May, F., Hoernes, S. and Neugebauer, H.J. (1996). Genesis and distribution of mineral waters as a consequence of recent lithospheric dynamics: The Rhenish Massif, Central Europe. Geologische Rundschau (in press). Parkhurst, D.L. (1995). User's guide to PHREEQC - A computer program for speciation, reaction-path, advective transport, and inverse geochemical calculations. USGS Water-Res. Invest. Rep Plum, H. (1989). Genetische Klassifikation und geochemische Interpretation der Mineral- und ThermalwSisser der Eifel und Ardennen. Mitt. Ing.- u. Hydrogeol., Vol. 34, Schwille, F. (1955). Ionenumtausch und der Chemismus von Grund- und Mineralasern. Zeitschr. Dt. Geol. Ges., Vol. 106 (l),
Effect of chemical composition to large scale CO 2 Injection in Morrow Sandstone, Farnsworth Hydrocarbon Field, Texas, USA
 Effect of chemical composition to large scale CO 2 Injection in Morrow Sandstone, Farnsworth Hydrocarbon Field, Texas, USA Bulbul Ahmmed Martin Appold Department of Geological Sciences University of Missouri-Columbia
Effect of chemical composition to large scale CO 2 Injection in Morrow Sandstone, Farnsworth Hydrocarbon Field, Texas, USA Bulbul Ahmmed Martin Appold Department of Geological Sciences University of Missouri-Columbia
Search and Discovery Article #50999 (2014)** Posted August 18, Abstract
 Oil Degradation in the Gullfaks Field (Norway): How Hydrogeochemical Modeling can Help to Decipher Organic- Inorganic Interactions Controlling CO 2 Fate and Behavior* Wolfgang van Berk 1, Yunjiao Fu 2,
Oil Degradation in the Gullfaks Field (Norway): How Hydrogeochemical Modeling can Help to Decipher Organic- Inorganic Interactions Controlling CO 2 Fate and Behavior* Wolfgang van Berk 1, Yunjiao Fu 2,
predictive mineral discovery*cooperative Research Centre A legacy for mineral exploration science Mineral Systems Q3 Fluid reservoirs
 Mineral Systems Q3 Fluid reservoirs 1 Key Parameter Mineral System Exploration is reflected in scale-dependent translation A. Gradient in hydraulic potential B. Permeability C. Solubility sensitivity to
Mineral Systems Q3 Fluid reservoirs 1 Key Parameter Mineral System Exploration is reflected in scale-dependent translation A. Gradient in hydraulic potential B. Permeability C. Solubility sensitivity to
WAMUNYU EDWARD MUREITHI I13/2358/2007
 WAMUNYU EDWARD MUREITHI I13/2358/2007 Olkaria geothermal area is situated south of Lake Naivasha on the floor of the southern segment of the Kenya rift. The geology of the Olkaria Geothermal area is subdivided
WAMUNYU EDWARD MUREITHI I13/2358/2007 Olkaria geothermal area is situated south of Lake Naivasha on the floor of the southern segment of the Kenya rift. The geology of the Olkaria Geothermal area is subdivided
CHEMICAL GEOTHERMOMETERS FOR GEOTHERMAL EXPLORATION
 Presented at Short Course III on Exploration for Geothermal Resources, organized by UNU-GTP and KenGen, at Lake Naivasha, Kenya, October 4 - November 17, 008. GEOTHERMAL TRAINING PROGRAMME Kenya Electricity
Presented at Short Course III on Exploration for Geothermal Resources, organized by UNU-GTP and KenGen, at Lake Naivasha, Kenya, October 4 - November 17, 008. GEOTHERMAL TRAINING PROGRAMME Kenya Electricity
Geochemical Modelling of Low-Temperature Geothermal Fields from Bihor County, Romania
 Proceedings World Geothermal Congress 2015 Melbourne, Australia, 19-25 April 2015 Geochemical Modelling of Low-Temperature Geothermal Fields from Bihor County, Romania Oana Stǎnǎşel, Iulian Stǎnǎşel University
Proceedings World Geothermal Congress 2015 Melbourne, Australia, 19-25 April 2015 Geochemical Modelling of Low-Temperature Geothermal Fields from Bihor County, Romania Oana Stǎnǎşel, Iulian Stǎnǎşel University
(4) Give an example of important reactions that are responsible for the composition of river water.
 Lecture 12 Global Biogeochemical Cycles (1) If rivers are the chief source of the dissolved salts in seawater, why is seawater not simply a concentrated version of average composition of all rivers? The
Lecture 12 Global Biogeochemical Cycles (1) If rivers are the chief source of the dissolved salts in seawater, why is seawater not simply a concentrated version of average composition of all rivers? The
Geochemical Characteristics of Reservoir Fluid from NW-Sabalan Geothermal Field, Iran
 Proceedings World Geothermal Congress 2010 Bali, Indonesia, 25-29 April 2010 Geochemical Characteristics of Reservoir Fluid from NW-Sabalan Geothermal Field, Iran Svetlana Strelbitskaya and Behnam Radmehr
Proceedings World Geothermal Congress 2010 Bali, Indonesia, 25-29 April 2010 Geochemical Characteristics of Reservoir Fluid from NW-Sabalan Geothermal Field, Iran Svetlana Strelbitskaya and Behnam Radmehr
Redox, ph, pe OUTLINE 9/12/17. Equilibrium? Finish last lecture Mineral stability Aquatic chemistry oxidation and reduction: redox
 Redox, ph, pe Equilibrium? OUTLINE Finish last lecture Mineral stability Aquatic chemistry oxidation and reduction: redox Reading: White p555-563 1 Question of the day? So what about the CO 2 system? CO
Redox, ph, pe Equilibrium? OUTLINE Finish last lecture Mineral stability Aquatic chemistry oxidation and reduction: redox Reading: White p555-563 1 Question of the day? So what about the CO 2 system? CO
FLUID-MINERAL EQUILIBRIA AND SUBSURFACE TEMPERATURE EVALUATION OF SELECTED HOT SPRINGS, JIANGXI PROVINCE, SE-CHINA
 PROCEEDINGS, Thirty-First Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, California, January 30-February 1, 2006 SGP-TR-179 FLUID-MINERAL EQUILIBRIA AND SUBSURFACE TEMPERATURE
PROCEEDINGS, Thirty-First Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, California, January 30-February 1, 2006 SGP-TR-179 FLUID-MINERAL EQUILIBRIA AND SUBSURFACE TEMPERATURE
Weathering and mineral equilibria. Seminar at NGU 23 May 2016 Håkon Rueslåtten
 Weathering and mineral equilibria Seminar at NGU 23 May 2016 Håkon Rueslåtten Weathering is the breakdown of rocks and minerals that are exposed to surface processes (climatically controlled). Water is
Weathering and mineral equilibria Seminar at NGU 23 May 2016 Håkon Rueslåtten Weathering is the breakdown of rocks and minerals that are exposed to surface processes (climatically controlled). Water is
WEATHERING. Weathering breakdown of rock materials Erosion transport of broken-down materials
 WEATHERING the interacting physical, chemical & biological processes that progressively alter the original lithologic character of rocks to produce secondary minerals (e.g. clays) & unconsolidated regolith
WEATHERING the interacting physical, chemical & biological processes that progressively alter the original lithologic character of rocks to produce secondary minerals (e.g. clays) & unconsolidated regolith
organisms CaCO 3 + H 2 O + CO 2 shallow water
 Weathering and Reverse weathering Step I:Weathering of igneous rocks 1. Igneous rocks are mainly composed of Al, Si and O 2 with minor and varying quantities of Na, K, Ca and Mg composing pheldspar minerals
Weathering and Reverse weathering Step I:Weathering of igneous rocks 1. Igneous rocks are mainly composed of Al, Si and O 2 with minor and varying quantities of Na, K, Ca and Mg composing pheldspar minerals
Petrological Studies by Terry Leach at the North Carlin Trend, Nevada. Keith Bettles October 17, 2008
 Petrological Studies by Terry Leach at the North Carlin Trend, Nevada Keith Bettles October 17, 2008 North Carlin Trend From 1999 to 2003 Terry Leach studied the Betze and Meikle ore bodies for Barrick
Petrological Studies by Terry Leach at the North Carlin Trend, Nevada Keith Bettles October 17, 2008 North Carlin Trend From 1999 to 2003 Terry Leach studied the Betze and Meikle ore bodies for Barrick
1/31/2013. Weathering Includes Physical, Chemical, Biological processes. Weathering Mechanisms. Wind abrasion forming Ventifacts
 Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes Weathering Mechanisms Physical
Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes Weathering Mechanisms Physical
The Martian Sedimentary Mass: Constraints on its Composition, Age and Size. Scott McLennan Department of Geosciences, SUNY Stony Brook
 The Martian Sedimentary Mass: Constraints on its Composition, Age and Size Scott McLennan Department of Geosciences, SUNY Stony Brook Exploring Mars Habitability Lisbon 14 June, 2011 Martian Crustal Chemistry
The Martian Sedimentary Mass: Constraints on its Composition, Age and Size Scott McLennan Department of Geosciences, SUNY Stony Brook Exploring Mars Habitability Lisbon 14 June, 2011 Martian Crustal Chemistry
Lecture 4 What Controls the Composition of Seawater
 Lecture 4 What Controls the Composition of Seawater Seawater is salty! Why? What controls the composition of seawater? Do Chemical Equilibrium reactions control the composition of the Ocean? What is meant
Lecture 4 What Controls the Composition of Seawater Seawater is salty! Why? What controls the composition of seawater? Do Chemical Equilibrium reactions control the composition of the Ocean? What is meant
Chapter 4 Rocks & Igneous Rocks
 Chapter 4 Rocks & Igneous Rocks Rock Definition A naturally occurring consolidated mixture of one or more minerals e.g, marble, granite, sandstone, limestone Rock Definition Must naturally occur in nature,
Chapter 4 Rocks & Igneous Rocks Rock Definition A naturally occurring consolidated mixture of one or more minerals e.g, marble, granite, sandstone, limestone Rock Definition Must naturally occur in nature,
Graduate School of Science and Engineering, Kagoshima University
 20 +- -,+ +. / +- Geochemical Interpretation on Hydrothermal Formation Mechanism of the Thermal Water in the Iwodani Region and the Vicinity of the Hayashida Region of the Kirishima Volcanic Area Shun-ichi
20 +- -,+ +. / +- Geochemical Interpretation on Hydrothermal Formation Mechanism of the Thermal Water in the Iwodani Region and the Vicinity of the Hayashida Region of the Kirishima Volcanic Area Shun-ichi
Fluid Geochemistry at the Nir Geothermal Field, Nw-Iran
 Proceedings World Geothermal Congress 2015 Melbourne, Australia, 19-25 April 2015 Fluid Geochemistry at the Nir Geothermal Field, Nw-Iran Mohammad Reza Rahmani Renewable Energy Organization of Iran (SUNA),
Proceedings World Geothermal Congress 2015 Melbourne, Australia, 19-25 April 2015 Fluid Geochemistry at the Nir Geothermal Field, Nw-Iran Mohammad Reza Rahmani Renewable Energy Organization of Iran (SUNA),
CO 2 sequestration via direct mineral carbonation of Mg-silicates. Natalie Johnson GCEP Symposium 4 October 2011
 CO 2 sequestration via direct mineral carbonation of Mg-silicates Natalie Johnson GCEP Symposium 4 October 2011 CO 2 release/year (Gt) 2 CCS: Part of climate change mitigation Projection based on current
CO 2 sequestration via direct mineral carbonation of Mg-silicates Natalie Johnson GCEP Symposium 4 October 2011 CO 2 release/year (Gt) 2 CCS: Part of climate change mitigation Projection based on current
Geochemical Monitoring of the Lakes District Area
 Proceedings World Geothermal Congress 25 Antalya, Turkey, 24-29 April 25 Geochemical Monitoring of the Lakes District Area Kibret Beyene and Meseret Teklemariam Geological Survey of Ethiopia, P.O.Box 469,
Proceedings World Geothermal Congress 25 Antalya, Turkey, 24-29 April 25 Geochemical Monitoring of the Lakes District Area Kibret Beyene and Meseret Teklemariam Geological Survey of Ethiopia, P.O.Box 469,
INTRODUCTION TO GEOCHEMICAL AND REACTIVE TRANSPORT MODELING. Ondra Sracek
 INTRODUCTION TO GEOCHEMICAL AND REACTIVE TRANSPORT MODELING Ondra Sracek Principal types of geochemical modeling: 1. Speciation (for exemple, for lead); 2. Inverse geochemical modeling; 3. Direct geochemical
INTRODUCTION TO GEOCHEMICAL AND REACTIVE TRANSPORT MODELING Ondra Sracek Principal types of geochemical modeling: 1. Speciation (for exemple, for lead); 2. Inverse geochemical modeling; 3. Direct geochemical
4 Carbonates and CO2
 PhreeqcI Introductory Course (Exercises booklet, chapters 4 and 5) by Manuel Prieto Department of Geology, University of Oviedo, Spain mprieto@ @geol.uniovi.es http://wwwbrr.cr.usgs.gov/projects/gwc_coupled/phreeqci/
PhreeqcI Introductory Course (Exercises booklet, chapters 4 and 5) by Manuel Prieto Department of Geology, University of Oviedo, Spain mprieto@ @geol.uniovi.es http://wwwbrr.cr.usgs.gov/projects/gwc_coupled/phreeqci/
Lecture 13. Hydrothermal Circulation
 Lecture 13. Hydrothermal Circulation The discovery of hot springs on the ocean floor during the 1970s was one of the most exciting events in the history of oceanography. Although hydrothermal activity
Lecture 13. Hydrothermal Circulation The discovery of hot springs on the ocean floor during the 1970s was one of the most exciting events in the history of oceanography. Although hydrothermal activity
Big Island Field Trip
 Big Island Field Trip Space Still Available Group Airline Tickets May be available if enough people sign on If interested send email to Greg Ravizza Planning Meeting Next Week Will
Big Island Field Trip Space Still Available Group Airline Tickets May be available if enough people sign on If interested send email to Greg Ravizza Planning Meeting Next Week Will
Luigi Marini and Claudio Pasqua. ELC-Electroconsult S.p.A. Via 1 Maggio 41, I Baranzate, Milano, Italy
 Proceedings 5 th African Rift geothermal Conference Arusha, Tanzania, 29-31 October 2014 Geochemical modeling of water-rock interaction processes in the geothermal systems of the continental rift zone
Proceedings 5 th African Rift geothermal Conference Arusha, Tanzania, 29-31 October 2014 Geochemical modeling of water-rock interaction processes in the geothermal systems of the continental rift zone
Geochemical monitoring of the response ofgeothermal reservoirs to production load examples from Krafla, Iceland
 International Geothermal Conference, Reykjavík, Sept. 23 Session #7 Geochemical monitoring of the response ofgeothermal reservoirs to production load examples from Krafla, Iceland Stefán Arnórsson 1 and
International Geothermal Conference, Reykjavík, Sept. 23 Session #7 Geochemical monitoring of the response ofgeothermal reservoirs to production load examples from Krafla, Iceland Stefán Arnórsson 1 and
Continent-Ocean Interaction: Role of Weathering
 Institute of Astrophysics and Geophysics (Build. B5c) Room 0/13 email: Guy.Munhoven@ulg.ac.be Phone: 04-3669771 28th February 2018 Organisation of the Lecture 1 Carbon cycle processes time scales modelling:
Institute of Astrophysics and Geophysics (Build. B5c) Room 0/13 email: Guy.Munhoven@ulg.ac.be Phone: 04-3669771 28th February 2018 Organisation of the Lecture 1 Carbon cycle processes time scales modelling:
Copyright SOIL STRUCTURE and CLAY MINERALS
 SOIL STRUCTURE and CLAY MINERALS Soil Structure Structure of a soil may be defined as the mode of arrangement of soil grains relative to each other and the forces acting between them to hold them in their
SOIL STRUCTURE and CLAY MINERALS Soil Structure Structure of a soil may be defined as the mode of arrangement of soil grains relative to each other and the forces acting between them to hold them in their
Chapter 7 Metamorphism, Metamorphic Rocks, and Hydrothermal Rocks
 Chapter 7 Metamorphism, Metamorphic Rocks, and Hydrothermal Rocks Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Metamorphism What happens to rocks that are
Chapter 7 Metamorphism, Metamorphic Rocks, and Hydrothermal Rocks Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Metamorphism What happens to rocks that are
GROUNDWATER GEOCHEMISTRY AND ASSOCIATED HARDPANS IN SOUTHWESTERN AUSTRALIA
 254 GROUNDWATER GEOCHEMISTRY AND ASSOCIATED HARDPANS IN SOUTHWESTERN AUSTRALIA Sam Lee CRC LEME, Department of Applied Geology, Curtin University of Technology, Bentley, Western Australia, 6485 INTRODUCTION
254 GROUNDWATER GEOCHEMISTRY AND ASSOCIATED HARDPANS IN SOUTHWESTERN AUSTRALIA Sam Lee CRC LEME, Department of Applied Geology, Curtin University of Technology, Bentley, Western Australia, 6485 INTRODUCTION
muscovite PART 4 SHEET SILICATES
 muscovite PART 4 SHEET SILICATES SHEET SILICATES = PHYLLOSILICATES Phyllon = leaf Large group of mineral including many common minerals: muscovite, biotite, serpentine, chlorite, talc, clay minerals Structure:
muscovite PART 4 SHEET SILICATES SHEET SILICATES = PHYLLOSILICATES Phyllon = leaf Large group of mineral including many common minerals: muscovite, biotite, serpentine, chlorite, talc, clay minerals Structure:
MULTICOMPONENT EQUILIBRIUM MODELS FOR TESTING GEOTHERMOMETRY APPROACHES
 PROCEEDINGS, Thirty-Eighth Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, California, February -3, 203 SGP-TR-98 MULTICOMPONENT EQUILIBRIUM MODELS FOR TESTING GEOTHERMOMETRY
PROCEEDINGS, Thirty-Eighth Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, California, February -3, 203 SGP-TR-98 MULTICOMPONENT EQUILIBRIUM MODELS FOR TESTING GEOTHERMOMETRY
Lab: Metamorphism: minerals, rocks and plate tectonics!
 Introduction The Earth s crust is in a constant state of change. For example, plutonic igneous rocks are exposed at the surface through uplift and erosion. Many minerals within igneous rocks are unstable
Introduction The Earth s crust is in a constant state of change. For example, plutonic igneous rocks are exposed at the surface through uplift and erosion. Many minerals within igneous rocks are unstable
Hydrothermal Chemistry/ Reverse Weathering. Marine Chemistry Seminar
 Hydrothermal Chemistry/ Reverse Weathering Marine Chemistry Seminar 1974 Drever, The Sea Chapter 10:The Magnesium Problem 1979 Edmonds et al., Ridge Crest Hydrothermal Activity and the Balances of Major
Hydrothermal Chemistry/ Reverse Weathering Marine Chemistry Seminar 1974 Drever, The Sea Chapter 10:The Magnesium Problem 1979 Edmonds et al., Ridge Crest Hydrothermal Activity and the Balances of Major
Capabilities of TOUGH Codes for Modeling Geologic Sequestration and Leakage of CO 2
 Capabilities of TOUGH Codes for Modeling Geologic Sequestration and Leakage of CO 2 Karsten Pruess Earth Sciences Division Lawrence Berkeley National Laboratory Presented at Workshop on Leakage Modeling
Capabilities of TOUGH Codes for Modeling Geologic Sequestration and Leakage of CO 2 Karsten Pruess Earth Sciences Division Lawrence Berkeley National Laboratory Presented at Workshop on Leakage Modeling
Carbonates and Silica Scaling Model Applied to Hot Water Pipes for Sanitary Use at Hotel Río Perlas, Costa Rica
 PROCEEDINGS, 43rd Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, California, February 12-14, 2018 SGP-TR-213 Carbonates and Silica Scaling Model Applied to Hot Water Pipes
PROCEEDINGS, 43rd Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, California, February 12-14, 2018 SGP-TR-213 Carbonates and Silica Scaling Model Applied to Hot Water Pipes
Acid Soil. Soil Acidity and ph
 Acid Soil Soil Acidity and ph ph ph = - log (H + ) H 2 O H + + OH - (H + ) x (OH - )= K w = 10-14 measures H + activity with an electrode (in the lab), solutions (in the field) reflects the acid intensity,
Acid Soil Soil Acidity and ph ph ph = - log (H + ) H 2 O H + + OH - (H + ) x (OH - )= K w = 10-14 measures H + activity with an electrode (in the lab), solutions (in the field) reflects the acid intensity,
Sedimentary Rocks and Processes
 Sedimentary Rocks and Processes Weathering Sedimentary Processes Breakdown of pre-existing rock by physical and chemical processes Transport Movement of sediments from environments of relatively high potential
Sedimentary Rocks and Processes Weathering Sedimentary Processes Breakdown of pre-existing rock by physical and chemical processes Transport Movement of sediments from environments of relatively high potential
Constitution of Magmas. Magmas. Gas Law. Composition. Atomic Structure of Magma. Structural Model. PV = nrt H 2 O + O -2 = 2(OH) -
 Constitution of Magmas Magmas Best, Ch. 8 Hot molten rock T = 700-1200 degrees C Composed of ions or complexes Phase Homogeneous Separable part of the system With an interface Composition Most components
Constitution of Magmas Magmas Best, Ch. 8 Hot molten rock T = 700-1200 degrees C Composed of ions or complexes Phase Homogeneous Separable part of the system With an interface Composition Most components
Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Mechanisms
 Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes WEATHERING CHAPTER 7 Weathering
Monument Valley, Utah. What weathering processes contributed to the development of these remarkable rock formations? Weathering Includes Physical, Chemical, Biological processes WEATHERING CHAPTER 7 Weathering
2.2 Acid mine drainage
 PROBLEM SOLVING 2. CHEMICAL REACTIONS AND EQUILIBRIA 1 2.2 Acid mine drainage Problem 2.2 The weathering of iron sulphide minerals produces acidified water, leading to major environmental problems from
PROBLEM SOLVING 2. CHEMICAL REACTIONS AND EQUILIBRIA 1 2.2 Acid mine drainage Problem 2.2 The weathering of iron sulphide minerals produces acidified water, leading to major environmental problems from
GEOCHEMISTRY, GROUNDWATER AND POLLUTION,
 GEOCHEMISTRY, GROUNDWATER AND POLLUTION, 2 ND EDITION C.A.J. APPELO Hydrochemical Consultant, Amsterdam, the Netherlands D. POSTMA Environment & Resources DTU, Technical University of Denmark, Kgs. Lyngby,
GEOCHEMISTRY, GROUNDWATER AND POLLUTION, 2 ND EDITION C.A.J. APPELO Hydrochemical Consultant, Amsterdam, the Netherlands D. POSTMA Environment & Resources DTU, Technical University of Denmark, Kgs. Lyngby,
CO 2 -water-rock reactivity at hydrothermal temperatures: The BigRig2 experiment
 CO 2 -water-rock reactivity at hydrothermal temperatures: The BigRig2 experiment C.A. ROCHELLE 1 *, K. BATEMAN 1, A. LACINSKA 1, D. WAGNER 1, J. LIONS 2 AND I. GAUS 2 1 British Geological Survey, Keyworth,
CO 2 -water-rock reactivity at hydrothermal temperatures: The BigRig2 experiment C.A. ROCHELLE 1 *, K. BATEMAN 1, A. LACINSKA 1, D. WAGNER 1, J. LIONS 2 AND I. GAUS 2 1 British Geological Survey, Keyworth,
Chapter 13. Groundwater
 Chapter 13 Groundwater Introduction Groundwater is all subsurface water that completely fills the pores and other open spaces in rocks, sediments, and soil. Groundwater is responsible for forming beautiful
Chapter 13 Groundwater Introduction Groundwater is all subsurface water that completely fills the pores and other open spaces in rocks, sediments, and soil. Groundwater is responsible for forming beautiful
Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions
 Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions OCN 623 Chemical Oceanography Reading: Libes, Chapters 15 and 16 Outline I. Deep sea sedimentation Detrital sediments
Making Sediments: Biogenic Production, Carbonate Saturation and Sediment Distributions OCN 623 Chemical Oceanography Reading: Libes, Chapters 15 and 16 Outline I. Deep sea sedimentation Detrital sediments
Weathering and Erosion
 Weathering and Erosion Weathering the disintegration and decomposition of material at the surface Erosion the transportation of weathered material by water, wind, or ice Weathering Two kinds of weathering
Weathering and Erosion Weathering the disintegration and decomposition of material at the surface Erosion the transportation of weathered material by water, wind, or ice Weathering Two kinds of weathering
Sedimentary Geology. Strat and Sed, Ch. 1 1
 Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
Sedimentary Geology Strat and Sed, Ch. 1 1 Sedimentology vs. Stratigraphy Sedimentology is the study of the origin and classification of sediments and sedimentary rocks Mostly the physical and chemical
Lecture 11: Petrology of Mineralizing Aqueous Solutions GY303 Igneous & Metamorphic Petrology
 Lecture 11: Petrology of Mineralizing Aqueous Solutions GY303 Igneous & Metamorphic Petrology H2O Dominated Fluids Excluding Magmatic and Metamorphic systems leaves hydrothermal H2O fluids as the most
Lecture 11: Petrology of Mineralizing Aqueous Solutions GY303 Igneous & Metamorphic Petrology H2O Dominated Fluids Excluding Magmatic and Metamorphic systems leaves hydrothermal H2O fluids as the most
Geology 560, Prof. Thomas Johnson, Fall 2003 Unit II: Chemical reactions: Guide Questions
 Geology 560, Prof. Thomas Johnson, Fall 2003 Unit II: Chemical reactions: Guide Questions Goals: Refresh your memory on the basics of chemical reactions Ponder the chaos that underlies chemical reactions;
Geology 560, Prof. Thomas Johnson, Fall 2003 Unit II: Chemical reactions: Guide Questions Goals: Refresh your memory on the basics of chemical reactions Ponder the chaos that underlies chemical reactions;
WHAT CAN CLAY MINERALOGY TELL US ABOUT ALTERATION ENVIRONMENTS ON MARS?
 WHAT CAN CLAY MINERALOGY TELL US ABOUT ALTERATION ENVIRONMENTS ON MARS? David Bish and David Vaniman Indiana University Los Alamos National Laboratory Products of Mineralogical Studies Mars surface mineralogy
WHAT CAN CLAY MINERALOGY TELL US ABOUT ALTERATION ENVIRONMENTS ON MARS? David Bish and David Vaniman Indiana University Los Alamos National Laboratory Products of Mineralogical Studies Mars surface mineralogy
Geochemical Modeling of Acidic Geothermal Fluids using SOLVEQ and CHIM-xpt
 Proceedings World Geothermal Congress 2015 Melbourne, Australia, 19-25 April 2015 Geochemical Modeling of Acidic Geothermal Fluids using SOLVEQ and CHIM-xpt Emily Ann A. Bartolo 1 and Mark H. Reed 2 1
Proceedings World Geothermal Congress 2015 Melbourne, Australia, 19-25 April 2015 Geochemical Modeling of Acidic Geothermal Fluids using SOLVEQ and CHIM-xpt Emily Ann A. Bartolo 1 and Mark H. Reed 2 1
HYDROTHERMAL FLUID FLOW AND MINERAL ALTERATION IN A FRACTURED ROCK UNDER MULTIPHASE H 2O- CO 2 MIXTURE CONDITIONS
 HYDROTHERMAL FLUID FLOW AND MINERAL ALTERATION IN A FRACTURED ROCK UNDER MULTIPHASE H 2O- CO 2 MIXTURE CONDITIONS Tianfu Xu and Karsten Pruess Earth Sciences Division, Lawrence Berkeley National Laboratory,
HYDROTHERMAL FLUID FLOW AND MINERAL ALTERATION IN A FRACTURED ROCK UNDER MULTIPHASE H 2O- CO 2 MIXTURE CONDITIONS Tianfu Xu and Karsten Pruess Earth Sciences Division, Lawrence Berkeley National Laboratory,
WEATHERING. Turning Rock to Sediment and Solutions 10/22/2012
 WEATHERING Turning Rock to Sediment and Solutions Igneous rocks form at high temperatures; at the Earth s surface they are chemically unstable and will begin to disintegrate and decompose in a process
WEATHERING Turning Rock to Sediment and Solutions Igneous rocks form at high temperatures; at the Earth s surface they are chemically unstable and will begin to disintegrate and decompose in a process
Sedimentary Rocks, Stratigraphy, and Geologic Time
 Sedimentary Rocks, Stratigraphy, and Geologic Time A rock is any naturally formed, nonliving, coherent aggregate mass of solid matter that constitutes part of a planet, asteroid, moon, or other planetary
Sedimentary Rocks, Stratigraphy, and Geologic Time A rock is any naturally formed, nonliving, coherent aggregate mass of solid matter that constitutes part of a planet, asteroid, moon, or other planetary
Cation Exchange Capacity, CEC
 Cation Exchange Capacity, CEC The basic building blocks of clay minerals are: silicon atoms surrounded by four oxygen atoms (tetrahedra), and aluminium atoms surrounded by six hydroxide groups (dioctahedra),
Cation Exchange Capacity, CEC The basic building blocks of clay minerals are: silicon atoms surrounded by four oxygen atoms (tetrahedra), and aluminium atoms surrounded by six hydroxide groups (dioctahedra),
SoilGen2 model: data requirements and model output
 SoilGen2 model: data requirements and model output 1. Essential plot data... 1 2. Essential soil data... 2 3. Precipitation data (for a typical year)... 2 4. Evapotranspiration and Air temperature data
SoilGen2 model: data requirements and model output 1. Essential plot data... 1 2. Essential soil data... 2 3. Precipitation data (for a typical year)... 2 4. Evapotranspiration and Air temperature data
Practice Questions for Lecture 5 Geology 1200
 Practice Questions for Lecture 5 Geology 1200 Use these questions to test your knowledge of Lecture5. The exams will be similar in format, except that they will deal with more than one chapter, and will
Practice Questions for Lecture 5 Geology 1200 Use these questions to test your knowledge of Lecture5. The exams will be similar in format, except that they will deal with more than one chapter, and will
CLASS EXERCISE 5.1 List processes occurring in soils that cause changes in the levels of ions.
 5 SIL CHEMISTRY 5.1 Introduction A knowledge of the chemical composition of a soil is less useful than a knowledge of its component minerals and organic materials. These dictate the reactions that occur
5 SIL CHEMISTRY 5.1 Introduction A knowledge of the chemical composition of a soil is less useful than a knowledge of its component minerals and organic materials. These dictate the reactions that occur
Chapter 21: Metamorphism. Fresh basalt and weathered basalt
 Chapter 21: Metamorphism Fresh basalt and weathered basalt Chapter 21: Metamorphism The IUGS-SCMR proposed this definition: Metamorphism is a subsolidus process leading to changes in mineralogy and/or
Chapter 21: Metamorphism Fresh basalt and weathered basalt Chapter 21: Metamorphism The IUGS-SCMR proposed this definition: Metamorphism is a subsolidus process leading to changes in mineralogy and/or
Rocks Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3
 Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification VI. Additional sub-classes
Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification VI. Additional sub-classes
Rocks Environmental Significance. Rocks Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3. Rocks Definition of a rock
 Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 Environmental Significance I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification
Reading this week: Ch. 2 and App. C Reading for next week: Ch. 3 Environmental Significance I. Environmental significance II. Definition III. 3 major classes IV. The Rock Cycle V. Secondary classification
ALTERATION ZONATION OF SILICA MINERALS IN A GEOTHERMAL SYSTEM A NUMERICAL SIMULATION BASED ON REACTION-TRANSPORT MODEL
 Proceedings 20th NZ GeothermalWorkshop 1998 ALTERATION ZONATION OF SILICA MINERALS IN A GEOTHERMAL SYSTEM A NUMERICAL SIMULATION BASED ON REACTION-TRANSPORT MODEL N. T. J.W. Survey of Japan, Japan TechnologiesInc.
Proceedings 20th NZ GeothermalWorkshop 1998 ALTERATION ZONATION OF SILICA MINERALS IN A GEOTHERMAL SYSTEM A NUMERICAL SIMULATION BASED ON REACTION-TRANSPORT MODEL N. T. J.W. Survey of Japan, Japan TechnologiesInc.
Overview. Open system flush with kinetic constraints Closed system with kinetic constraints
 Rationale Minerals associated with lung diseases How do lungs function? Health threats of minerals The paper researched Approach and methodology Saturation indices Activity-Ratio diagrams Reaction-path
Rationale Minerals associated with lung diseases How do lungs function? Health threats of minerals The paper researched Approach and methodology Saturation indices Activity-Ratio diagrams Reaction-path
Geochemistry of storing CO 2 and NO x in the deep Precipice Sandstone
 Geochemistry of storing CO 2 and NO x in the deep Precipice Sandstone Sid Fe Mn K Qz J.K Pearce a G.K.W. Dawson a, S.D. Golding a, D. Kirste b, J. Underschultz a a University of Queensland, Australia b
Geochemistry of storing CO 2 and NO x in the deep Precipice Sandstone Sid Fe Mn K Qz J.K Pearce a G.K.W. Dawson a, S.D. Golding a, D. Kirste b, J. Underschultz a a University of Queensland, Australia b
Lecture 15: Adsorption; Soil Acidity
 Lecture 15: Adsorption; Soil Acidity Surface Complexation (Your textbook calls this adsorption ) Surface Complexation Both cations and anions can bind to sites on the external surfaces of soil minerals
Lecture 15: Adsorption; Soil Acidity Surface Complexation (Your textbook calls this adsorption ) Surface Complexation Both cations and anions can bind to sites on the external surfaces of soil minerals
At the beginning. Matter + antimatter. Matter has the advantage. baryons quarks, leptons, electrons, photons (no protons or neutrons)
 At the beginning Matter + antimatter Matter has the advantage baryons quarks, leptons, electrons, photons (no protons or neutrons) Hadrons protons, neutrons Hydrogen, helium (:0 H:He) Origin of the Universe
At the beginning Matter + antimatter Matter has the advantage baryons quarks, leptons, electrons, photons (no protons or neutrons) Hadrons protons, neutrons Hydrogen, helium (:0 H:He) Origin of the Universe
Weathering Cycle Teacher Notes
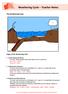 The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
The Weathering Cycle Stages of the Weathering Cycle: 1. Carbon Dioxide and Water In clouds, carbon dioxide reacts with water to form a weak acid. H 2 O + CO 2 --> H 2 CO 3 H 2 CO 3 H + + HCO 3-2. Acid
Types of Metamorphism!
 Types of Metamorphism! The Types of Metamorphism 2 different approaches to classification 1. Based on principal process or agent Dynamic Metamorphism Thermal Metamorphism Dynamo-thermal Metamorphism The
Types of Metamorphism! The Types of Metamorphism 2 different approaches to classification 1. Based on principal process or agent Dynamic Metamorphism Thermal Metamorphism Dynamo-thermal Metamorphism The
Igneous Rocks. Sedimentary Rocks. Metamorphic Rocks
 Name: Date: Igneous Rocks Igneous rocks form from the solidification of magma either below (intrusive igneous rocks) or above (extrusive igneous rocks) the Earth s surface. For example, the igneous rock
Name: Date: Igneous Rocks Igneous rocks form from the solidification of magma either below (intrusive igneous rocks) or above (extrusive igneous rocks) the Earth s surface. For example, the igneous rock
The Lithosphere. Definition
 10/14/2014 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2014 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
10/14/2014 www.komar.de The Lithosphere Ben Sullivan, Assistant Professor NRES 765, Biogeochemistry October 14th, 2014 Contact: bsullivan@cabnr.unr.edu Definition io9.com tedquarters.net Lithos = rocky;
Lecture 6. Physical Properties. Solid Phase. Particle Composition
 Lecture 6 Physical Properties Solid Phase Particle Composition 1 Questions What are tetrahedrons and octahedrons? How do silica tetrahedra bonds affect mineral weathering? Difference between primary and
Lecture 6 Physical Properties Solid Phase Particle Composition 1 Questions What are tetrahedrons and octahedrons? How do silica tetrahedra bonds affect mineral weathering? Difference between primary and
Block: Igneous Rocks. From this list, select the terms which answer the following questions.
 Geology 12 Name: Mix and Match: Igneous Rocks Refer to the following list. Block: porphyritic volatiles mafic glassy magma mixing concordant discontinuous reaction series igneous vesicular partial melting
Geology 12 Name: Mix and Match: Igneous Rocks Refer to the following list. Block: porphyritic volatiles mafic glassy magma mixing concordant discontinuous reaction series igneous vesicular partial melting
APPLICATION OF GEOCHEMICAL METHODS IN GEOTHERMAL EXPLORATION. Halldór Ármannsson November 2007
 APPLICATION OF GEOCHEMICAL METHODS IN GEOTHERMAL EXPLORATION Halldór Ármannsson November 2007 Geochemical Exploration Subsurface composition Temperature Origin and flow direction Reservoir location Equilibrium
APPLICATION OF GEOCHEMICAL METHODS IN GEOTHERMAL EXPLORATION Halldór Ármannsson November 2007 Geochemical Exploration Subsurface composition Temperature Origin and flow direction Reservoir location Equilibrium
Earthquakes. Earthquakes are caused by a sudden release of energy
 Earthquakes Earthquakes are caused by a sudden release of energy The amount of energy released determines the magnitude of the earthquake Seismic waves carry the energy away from its origin Fig. 18.1 Origin
Earthquakes Earthquakes are caused by a sudden release of energy The amount of energy released determines the magnitude of the earthquake Seismic waves carry the energy away from its origin Fig. 18.1 Origin
Igneous Rock Classification, Processes and Identification Physical Geology GEOL 100
 Igneous Rock Classification, Processes and Identification Physical Geology GEOL 100 Ray Rector - Instructor Major Concepts 1) Igneous rocks form directly from the crystallization of a magma or lava 2)
Igneous Rock Classification, Processes and Identification Physical Geology GEOL 100 Ray Rector - Instructor Major Concepts 1) Igneous rocks form directly from the crystallization of a magma or lava 2)
Mineral Stability and Phase Diagrams Introduction
 1 of 10 10/10/2002 2:50 PM Prof. Stephen A. Nelson Geology 211 Tulane University Mineralogy and Phase Diagrams Introduction This document last updated on 10-Oct-2002 As we discussed previously, there are
1 of 10 10/10/2002 2:50 PM Prof. Stephen A. Nelson Geology 211 Tulane University Mineralogy and Phase Diagrams Introduction This document last updated on 10-Oct-2002 As we discussed previously, there are
RATIO ANALYSIS IN LITHOGEOCHEMISTRY: QUANTIFYING ALTERATION AND INTEGRATING RESULTS INTO EXPLORATION EFFORTS. Elura GER - Discrimination Diagram
 RATIO ANALYSIS IN LITHOGEOCHEMISTRY: QUANTIFYING ALTERATION AND INTEGRATING RESULTS INTO EXPLORATION EFFORTS Y Index/Z Index 5.0 4.5 4.0 3.5 3.0 2.5 2.0 Altered! Elura GER - Discrimination Diagram Muscovite,
RATIO ANALYSIS IN LITHOGEOCHEMISTRY: QUANTIFYING ALTERATION AND INTEGRATING RESULTS INTO EXPLORATION EFFORTS Y Index/Z Index 5.0 4.5 4.0 3.5 3.0 2.5 2.0 Altered! Elura GER - Discrimination Diagram Muscovite,
Lecture 5 Sedimentary rocks Recap+ continued. and Metamorphic rocks!
 Lecture 5 Sedimentary rocks Recap+ continued and Metamorphic rocks! Metamorphism Process that leads to changes in: Mineralogy Texture Sometimes chemical composition Metamorphic rocks are produced from
Lecture 5 Sedimentary rocks Recap+ continued and Metamorphic rocks! Metamorphism Process that leads to changes in: Mineralogy Texture Sometimes chemical composition Metamorphic rocks are produced from
BIBLIOGRAPHIC REFERENCE
 BIBLIOGRAPHIC REFERENCE Chambefort, I; Bignall, G. 2013. Preliminary stable isotope study on the Lahendong geothermal system, Indonesia, GNS Science Report 2013/14. 9p. I. Chambefort, GNS Science, Wairakei
BIBLIOGRAPHIC REFERENCE Chambefort, I; Bignall, G. 2013. Preliminary stable isotope study on the Lahendong geothermal system, Indonesia, GNS Science Report 2013/14. 9p. I. Chambefort, GNS Science, Wairakei
23/9/2013 ENGINEERING GEOLOGY. Chapter 2: Rock classification:
 ENGINEERING GEOLOGY Chapter 2: Rock classification: ENGINEERING GEOLOGY Chapter 1.0: Introduction to engineering geology Chapter 2.0: Rock classification Igneous rocks Sedimentary rocks Metamorphic rocks
ENGINEERING GEOLOGY Chapter 2: Rock classification: ENGINEERING GEOLOGY Chapter 1.0: Introduction to engineering geology Chapter 2.0: Rock classification Igneous rocks Sedimentary rocks Metamorphic rocks
9/4/2015. Feldspars White, pink, variable Clays White perfect Quartz Colourless, white, red, None
 ENGINEERING GEOLOGY Chapter 1.0: Introduction to engineering geology Chapter 2.0: Rock classification Igneous rocks Sedimentary rocks Metamorphic rocks Chapter 3.0: Weathering & soils Chapter 4.0: Geological
ENGINEERING GEOLOGY Chapter 1.0: Introduction to engineering geology Chapter 2.0: Rock classification Igneous rocks Sedimentary rocks Metamorphic rocks Chapter 3.0: Weathering & soils Chapter 4.0: Geological
Simulation of Mineral Precipitation and Dissolution in the 5-km Deep Enhanced Geothermal Reservoir at Soultz-sous-Forêts, France
 Proceedings World Geothermal Congress 2005 Antalya, Turkey, 24-29 April 2005 Simulation of Mineral Precipitation and Dissolution in the 5-km Deep Enhanced Geothermal Reservoir at Soultz-sous-Forêts, France
Proceedings World Geothermal Congress 2005 Antalya, Turkey, 24-29 April 2005 Simulation of Mineral Precipitation and Dissolution in the 5-km Deep Enhanced Geothermal Reservoir at Soultz-sous-Forêts, France
Real-Life Applications: Economic Mineral Deposits
 Real-Life Applications: Economic Mineral Deposits Economic Minerals Economic minerals are minerals that can be extracted, processed and marketed for a profit. Various factors determine if a mineral is
Real-Life Applications: Economic Mineral Deposits Economic Minerals Economic minerals are minerals that can be extracted, processed and marketed for a profit. Various factors determine if a mineral is
Model Study of Formation of the Cap Rocks for Geothermal System Using ChemTOUGH2
 Proceedings World Geothermal Congress 2005 Antalya, Turkey, 24-29 April 2005 Model Study of Formation of the Cap Rocks for Geothermal System Using ChemTOUGH2 Sato, T. +, Sato, M. +. Ueda, A. ++, Kato,
Proceedings World Geothermal Congress 2005 Antalya, Turkey, 24-29 April 2005 Model Study of Formation of the Cap Rocks for Geothermal System Using ChemTOUGH2 Sato, T. +, Sato, M. +. Ueda, A. ++, Kato,
Quiz 1. 3) Which of the following planetary bodies has the least number of impact craters on its surface? A) Mercury B) Mars C) the Moon D) Earth
 Quiz 1 1) Earth's atmosphere is unique among the moons and planets in that A) it has a nitrogen (N2) rich atmosphere. B) it is rich in oxygen (O2) and nitrogen (N2). C) it is rich in carbon dioxide because
Quiz 1 1) Earth's atmosphere is unique among the moons and planets in that A) it has a nitrogen (N2) rich atmosphere. B) it is rich in oxygen (O2) and nitrogen (N2). C) it is rich in carbon dioxide because
About Earth Materials
 Grotzinger Jordan Understanding Earth Sixth Edition Chapter 3: EARTH MATERIALS Minerals and Rocks 2011 by W. H. Freeman and Company About Earth Materials All Earth materials are composed of atoms bound
Grotzinger Jordan Understanding Earth Sixth Edition Chapter 3: EARTH MATERIALS Minerals and Rocks 2011 by W. H. Freeman and Company About Earth Materials All Earth materials are composed of atoms bound
DIFFERENTIATION OF MAGMAS BY FRACTIONAL CRYSTALLIZATION THE M&M MAGMA CHAMBER
 Geol 2312 Igneous and Metamorphic Petrology Spring 2009 Name DIFFERENTIATION OF MAGMAS BY FRACTIONAL CRYSTALLIZATION THE M&M MAGMA CHAMBER Objective: This exercise is intended to improve understanding
Geol 2312 Igneous and Metamorphic Petrology Spring 2009 Name DIFFERENTIATION OF MAGMAS BY FRACTIONAL CRYSTALLIZATION THE M&M MAGMA CHAMBER Objective: This exercise is intended to improve understanding
Hydrogeological Modelling of Some Geothermal Waters of Havran, Ivrindi and Gonen in the Province Capital of Balikesir, Western Anatolia, Turkey
 PROCEEDINGS, 43rd Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, California, February 12-14, 2018 SGP-TR-213 Hydrogeological Modelling of Some Geothermal Waters of Havran,
PROCEEDINGS, 43rd Workshop on Geothermal Reservoir Engineering Stanford University, Stanford, California, February 12-14, 2018 SGP-TR-213 Hydrogeological Modelling of Some Geothermal Waters of Havran,
Chemical Hydrogeology
 Physical hydrogeology: study of movement and occurrence of groundwater Chemical hydrogeology: study of chemical constituents in groundwater Chemical Hydrogeology Relevant courses General geochemistry [Donahoe]
Physical hydrogeology: study of movement and occurrence of groundwater Chemical hydrogeology: study of chemical constituents in groundwater Chemical Hydrogeology Relevant courses General geochemistry [Donahoe]
Quartz Cementation in Mudrocks: How Common Is It?
 Quartz Cementation in Mudrocks: How Common Is It? Kitty L. Milliken Barnett Shale SE/CL image Woodford Shale SE/CL image Cements are Pore-filling Precipitates Specific definition differs with research
Quartz Cementation in Mudrocks: How Common Is It? Kitty L. Milliken Barnett Shale SE/CL image Woodford Shale SE/CL image Cements are Pore-filling Precipitates Specific definition differs with research
Geology 252, Historical Geology, California State University, Los Angeles - professor: Dr. Alessandro Grippo
 LAB # 1 - CLASTIC ROCKS Background: - Mechanical and Chemical Weathering - Production of Clastic Sediment - Classification of Sediment according to size: Gravel, Sand, Silt, Clay - Erosion, Transportation
LAB # 1 - CLASTIC ROCKS Background: - Mechanical and Chemical Weathering - Production of Clastic Sediment - Classification of Sediment according to size: Gravel, Sand, Silt, Clay - Erosion, Transportation
Classification of Igneous Rocks
 Classification of Igneous Rocks Textures: Glassy- no crystals formed Aphanitic- crystals too small to see by eye Phaneritic- can see the constituent minerals Fine grained- < 1 mm diameter Medium grained-
Classification of Igneous Rocks Textures: Glassy- no crystals formed Aphanitic- crystals too small to see by eye Phaneritic- can see the constituent minerals Fine grained- < 1 mm diameter Medium grained-
Prediction of Calcite Scaling at the Oguni Geothermal Field, Japan: Chemical Modeling Approach
 Todaka et Prediction of Calcite Scaling at the Oguni Geothermal Field, Japan: Chemical Modeling Approach Norifumi Yoshiyuki Hideo and Nobuyuki Electric Power Development Co., Ltd. 6-15-1, Ginza, Chuo-ku,
Todaka et Prediction of Calcite Scaling at the Oguni Geothermal Field, Japan: Chemical Modeling Approach Norifumi Yoshiyuki Hideo and Nobuyuki Electric Power Development Co., Ltd. 6-15-1, Ginza, Chuo-ku,
Alkaline Seafloor Hydrothermal Systems: Experimental Simulation of CO 2 -Peridotite-Seawater Reactions
 Alkaline Seafloor Hydrothermal Systems: Experimental Simulation of CO 2 -Peridotite-Seawater Reactions Thomas M. Carpenter John P. Kaszuba Melissa Fittipaldo Michael Rearick Los Alamos National Laboratory
Alkaline Seafloor Hydrothermal Systems: Experimental Simulation of CO 2 -Peridotite-Seawater Reactions Thomas M. Carpenter John P. Kaszuba Melissa Fittipaldo Michael Rearick Los Alamos National Laboratory
Activity and Concentration
 Activity and Concentration Activity effective concentration Ion-ion and ion-h 2 O interactions (hydration shell) cause number of ions available to react chemically ("free" ions) to be less than the number
Activity and Concentration Activity effective concentration Ion-ion and ion-h 2 O interactions (hydration shell) cause number of ions available to react chemically ("free" ions) to be less than the number
New Mexico Geological Society
 New Mexico Geological Society Downloaded from: http://nmgs.nmt.edu/publications/guidebooks/33 Geochemical studies of discharge water from a uranium acid-leach process Patrick A. Longmire and Douglas G.
New Mexico Geological Society Downloaded from: http://nmgs.nmt.edu/publications/guidebooks/33 Geochemical studies of discharge water from a uranium acid-leach process Patrick A. Longmire and Douglas G.
Igneous, Metamorphic & Sedimentary. Chapter 5 & Chapter 6
 Igneous, Metamorphic & Sedimentary Chapter 5 & Chapter 6 Section 5.1 What are Igneous Rocks? Compare and contrast intrusive and extrusive igneous rocks. Describe the composition of magma Discuss the factors
Igneous, Metamorphic & Sedimentary Chapter 5 & Chapter 6 Section 5.1 What are Igneous Rocks? Compare and contrast intrusive and extrusive igneous rocks. Describe the composition of magma Discuss the factors
CEE 437 Lecture 10 Rock Classification. Thomas Doe
 CEE 437 Lecture 10 Rock Classification Thomas Doe Igneous Origins Intrusive Batholithic or plutonic: phaneritic Dikes or sills that chill rapidly: aphanitic Extrusive deposition as melt (lava) pyroclastic
CEE 437 Lecture 10 Rock Classification Thomas Doe Igneous Origins Intrusive Batholithic or plutonic: phaneritic Dikes or sills that chill rapidly: aphanitic Extrusive deposition as melt (lava) pyroclastic
