AJNISH GUPTA & BHARTI GUPTA
|
|
|
- Stephany Holmes
- 5 years ago
- Views:
Transcription
1
2 Performance Booster Organic Chemistry For Board Examinations By AJNISH GUPTA & BHARTI GUPTA Professor of Organic Chemistry
3 Copyright 2015 by Ajnish Gupta All rights reserved. No part of this publication may be reproduced, stored in a database or retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior written Permission of the writter. First Indian Print: September 2015 This edition is manufactured in Indian and is authorized for sale only in India, Bangladesh, Pakistan, Nepal, Sri Lanka & Maldeves. Printed & Distributed by: Udaan Classes Pvt. Ltd. Rainbow building, Patna & Madhur Satyapushpa Shubhash Nagar, Kota Price: Rs. 300/-
4 Preface The guiding principle in writing this book was to create a textbook for students- a textbook that presents the material in a way that they learn to solve all the questions of previous year board questions by themself at appropriate space. This spacing area help the students to think for the length of the answer which they have to write. As the school examination plays the most important role in the life of Student and their marks are counted in many espect of life and from todays scenario, the importance of school exa mina tion is ma ximise by government a s fixed w eighta ge of these examinations are counted in the process of admission to any engineering or higher level educational programme. Now one question arises, what should be the way to study to get maximum percentage. The answer is so simple and we know it well. We should plan our way of studies in such a way that along with the school studies we should also keep an eye on what types of questions are being asked in the real examination. We had prepared this workbook in chapterwise manner which are further divided into topics, in the same way in which we taught in our classroom so that you should feel easy to understand & practice correctly. For better picture of the topic, the questions in each topic have been arranged as per their marks and in ascending order i.e i.e. 1 marks questions, 2 Marks questions, 3 Marks questions & 5 Marks questions. We had tried our best to answer all the questions according to CBSE marking scheme so that student can learn correct way to get more marks in exam. So this book will surely help you to get a high success in 12th class board examination. However we had put our best efforts in preparing this book, but if any error or what so ever has been skipped out, we will by heart welcome your suggestion. We would like to thank the editors and production staff for their efforts in bringing out the book. Suggestions from readers for the improvement of the book are welcome. Ajnish Kumar Gupta & Bharti Gupta Ajnish@OrganicChemistry
5 Acknowledgement We are thankful to all the teachers who taught us during the concept building session of our life specially Dr. Nizamuddin sir, senior Chandra Vijay Rao and Dr. Vijay Pratima Mittal madam. We have written this book to remove the fever of organic chemistry from mind of students. We particularly want to thank many wonderful and talented students whom we have taught over the years who in turn taught us how to be a good teacher and how we can help others. We want to make this book as user friendly as possible, and we will appreciate any comments that will help us to achieve this goal in future editions. Finally, this edition has been presented before you with efforts to make it error free. Any that remain are our responsibility; if you find any, please let us know so they can be corrected in future printing. Ajnish Gupta & Bharti Gupta Professors of Organic Chemistry Ajnish@OrganicChemistry
6 Table of Contents Chapter Title Page 1. Haloalkanes and Haloarenes : 1 Mark Questions Marks Questions Marks Question Alcohols, Phenols and Ethers : 1 Mark Questions & 23 2 Marks Questions & 23 3 Marks Question & Aldehydes, Ketones and Carboxylic Acids : 1 Mark Questions & Marks Questions & Marks Question & Marks Questions & Organic Compounds containing Nitrogen : 1 Mark Questions Marks Questions Marks Question Biomolecules : 1 Mark Questions & Marks Questions & Marks Question & Polymers : 1 Mark Questions Marks Questions Marks Question Chemistry in Everyday Life : 1 Mark Questions Marks Questions Marks Question Answers :
7 Dedication Dedicated to all those students who are in fever of Organic Chemistry.
8 Marking Scheme in Board Examinations Theory : Chapters Marks Organic Inorganic Physical Unit I Unit II Unit III Unit IV Unit V Unit VI Unit VII Unit VIII Unit IX Unit X Unit XI Unit XII Unit XIII Unit XIV Unit XV Unit XVI Solid State Solutions Electrochemistry Chemical Kinetics Surface Chemistry General Principles and Processes of Isolation of Elements p -Block Elements d -and f -Block Elements Coordination Compounds Haloalkanes and Haloarenes Alcohols, Phenols and Ethers Aldehydes, Ketones and Carboxylic Acids Organic Compounds containing Nitrogen Biomolecules Polymers Chemistry in Everyday Life Total Marks : 70 Practical : Evaluation Scheme Marks Volumetric Analysis Salt Analysis Content Based Experiment Project Work Class Record and Viva Total Marks : 30
9 i <+ksdghal sl h[ ksa; ghal s
10 Haloalkanes and Haloarenes Chapter 01 Haloalkanes and Haloarenes 1 Mark Questions: 1. Write the IUPAC name of the following compound: (CH 3 ) 3CCH 2Br. [Delhi 2011] 2. Write the IUPAC name of the following compound: CH 2=CHCH 2Br. [All India 2011] 3. Draw the structure of 1,4-Dibromobut-2-ene. [Delhi 2011] 4. Draw the structure of 2-(2-Bromophenyl)butane. [Delhi 2011] 5. Draw the structure of 2-(2-Chlorophenyl)-1-iodooctane. [All India 2011] 6. Draw the structure of the following compound: 1-bromo-4-sec-butyl-2-methylbenzene. [All India 2011] 1
11 Haloalkanes and Haloarenes 7. Draw the structure of the following organic compound: 2-Chloro-3-methylpentane [All India 2011] 8. Which will react faster in S N 2 displacement: 1-Bromopentane or 2-Bromopentane and why? [Foreign 2011] 9. Draw the structure of the following compound: 1-(4-Chlorophenyl)-2-methylpropane [Delhi 2011] CH 2=C CH2Br 10. Give the IUPAC name of the following compound: [Delhi 2012] CH What happens when bromine attacks over CH 2=CH CH 2 C CH? [All India 2012] 12. Write the IUPAC name of (CH 3) 2CH.CH(Cl)CH 3 [Delhi 2013] 13. Write the IUPAC name of CH 3 CH CH 2 CH=CH 2 Cl [Delhi 2013] 14. What happens when CH 3 Br is treated with KCN? [Delhi 2013] 2
12 Haloalkanes and Haloarenes 15. What happens when Ethyl chloride is treated with aqueous KOH? [Delhi 2013] CH Write the IUPAC name of CH 3CH=CH C CH 3 Br [Delhi 2013] 17. Which compound in the following pair undergoes faster towards S N 1 reaction? (i) Cl (ii) Cl [Delhi 2013] CH 3 C CH CH Write the IUPAC name of the following compound: CH 3 Cl CH 3 [All India 2013] 19. Write the IUPAC name of the following compound: CH [All India 2013] 3 Cl 20. Write the IUPAC name of the following compound: Cl CH 3 CH CH 2 CH CH 3 Br Cl [All India 2013] 21. How Methylbromide be preferentially converted to methyl isocyanide? [Delhi 2013] 3
13 Haloalkanes and Haloarenes 22. Identify the chiral molecule in the following pair: Cl Cl [Delhi 2014] 23. Draw the structure of 2-Bromopentane: [Delhi 2014] 24. Which would undergo S N 2 reaction faster in the following pair and why? CH 3 CH 3 CH 2 Br and CH 3 C CH3 Br [Delhi 2015] 25. Which would undergo S N 1 reaction faster in the following pair? CH 3 CH 2 CH 2 Br and CH 3 CH CH 3 Br [All India 2015] 2 Marks Questions: 26. Explain why (i) alkyl halides, though polar, are immiscible with water? (ii) Grignard s reagent should be prepared under anhydrous conditions? [Foreign 2012] 4
14 27. Account for the following: (i) Haloalkanes and Haloarenes The C Cl bond length in chlorobenzene is shorter than that in CH 3 Cl. (ii) Chloroform is stored in closed dark brown bottles. [Delhi 2013] 28. Chlorobenzene is extremely less reactive towards a nucleophilic substitution reaction. Give two reasons for the same. [Delhi 2013] 29. Write the mechanism of the following reaction: HBr CH CH OH CH CH Br + H O [Delhi 2014] 5
15 Haloalkanes and Haloarenes 30. (i) Which alkyl halide from the following pair is chiral and undergoes faster S N 2 reactions? (ii) (a) Br (b) Out of S N 1 and S N 2, which reaction occurs with (a) inversion of configuration? (b) racemisation? [All India 2014] 31. Draw the structure of major monohalo product in each of the following reactions: SOCl2 (i) OH (ii) CH 2 CH=CH 2 + HBr Peroxide Br [All India 2014] 32. Write chemical equations when (i) Ethylchloride is treated with aqueous KOH. (ii) Chlorobenzene is treated with CH 3 COCl in the presence of anhydrous AlCl 3.[Foreign 2014] 6
16 Haloalkanes and Haloarenes 33. (i) Which alkyl halide from the following pairs would you expect to react more rapidly by an S N 2 mechanism and why? CH 3 CH 2 CH CH 3 Br CH 3 CH 2 CH 2 CH 2 Br (ii) Racemisation occurs in S N 1 reactions. Why? [Foreign 2014] 34. Write the structures of the following organic halogen compounds: (i) p-bromochlorobenzene (ii) 1-Chloro-4-ethylcyclohexane [All India 2014] 35. What are ambident nucleophiles? Explain with an example. [All India 2014] 7
17 Haloalkanes and Haloarenes 36. Draw the structures of the following organic halogen compounds: (i) 4-Tert-butyl-3-iodoheptane (ii) 4-Bromo-3-methylpent-2-ene [All India Write the IUPAC names of the following compounds: (i) CH 2 = CHCH 2 Br (ii) (CCl 3 ) 3 CCl [All India 2014] 3 Marks Questions: 38. Answer the following: (i) (ii) Haloalkanes easily dissolve in organic solvents than haloarene. Why? What is known as racemic mixture? Give an example. (iii) Of the two bromo derivatives, C 6 H 5 CH(CH 3 )Br and (C 6 H 5 )CH(C 6 H 5 )Br, which one is more reactive in S N 1 substitution reaction and why? [Delhi 2011] 8
18 Haloalkanes and Haloarenes 39. Rearrange the compounds of each of the following sets in order of reactivity towards S N 2 displacement. (i) (ii) 2-Bromo-2-methylbutane, 1-Bromopentane, 2-Bromopentane. 1-Bromo-3-methylbutane, 2-Bromo-2-methylbutane, 3-Bromo-2-methylbutane. (iii) 1-Bromobutane, 1-Bromo-2, 2-Dimethylpropane, 1-Bromo-2-methylbutane, 1-Bromo-3-methylbutane. [All India 2011] 40. (i) Write a chemical test to distinguish between: (a) Chlorobenzene and Benzyl chloride. (b) Chloroform and Carbontetrachloride. (ii) Why is Methylchloride hydrolysed more easily than Chlorobenzene? [All India 2011] 41. Complete the following reactions: H H H + HBr [Foreign 2011] 9
19 Haloalkanes and Haloarenes 42. Although chlorine is an electron w ithdraw ing group, yet it is ortho, para-directing in electrophilic aromatic substitution reactions. Explain, why is it so? [Delhi 2012] 43. Answer the following questions: (i) (ii) What is meant by chirality of a compound? Give an example. Which one of the following compounds is more easily hydrolysed by KOH and why? CH 3CHClCH 2CH 3 or CH3CH 2CH2CH2Cl [All India 2012] 10
20 44. Give reasons for the following: (i) Haloalkanes and Haloarenes Ethyl iodide undergoes S N 2 reaction faster than ethyl bromide. (ii) ( ) 2-butanol is optically inactive. (iii) C X bond length in halobenzene is smaller than C X bond length in CH 3 X.[All India 2013] 45. Explain the following: The dipole moment of Chlorobenzene is lower than that of Cyclohexylchloride. [Delhi 2013] 11
21 Haloalkanes and Haloarenes 46. (i) Draw the structures of major monohalo products in each of the following reactions: (a) CH 2OH PCl 5 (b) CH 2 CH=CH 2 + HBr (ii) Which halogen compound in each of the following pairs will react faster in S N 2 reaction? (a) CH3Br or CH3I (b) (CH 3) 3C Cl or CH 3 Cl [Delhi 2014] 47. Write the product of the following reactions: (a) CH 3 Cl + KCN? (b) Cl + CH Cl 3 Anhyd. AlCl 3? +? [Delhi 2014] 12
22 48. Give reasons: (i) (ii) Haloalkanes and Haloarenes n-butyl bromide has higher boiling point than t-butyl bromide. Racemic mixture is optically inactive. (iii) The presence of nitro group ( NO 2 ) at o/p positions increases the reactivity of haloarenes towards nucleophilic substitution reactions. [Delhi 2015] 13
23 Haloalkanes and Haloarenes 49. How can the following conversions be carried out? (i) Aniline to Bromobenzene (ii) Chlorobenzene to 2-Chloroacetophenone (iii) Chloroethane to Butane Or What happens when (i) Chlorobenzene is treated with Cl 2 /FeCl 3? (ii) Ethyl chloride is treated with AgNO 2? (iii) 2-Bromopentane is treated with alcoholic KOH? Write the chemical equations in support of your answer. [All India 2015] 14
HALOALKANES AND HALOARENES
 Unit - 10 HALOALKANES AND HALOARENES 1. Write the IUPAC names of the following compounds. Br CH = CH C CH (vi) (vii) (ix) (CCl 3 ) 3 CCl 103 XII Chemistry 2. Write the structure of following halogen compounds
Unit - 10 HALOALKANES AND HALOARENES 1. Write the IUPAC names of the following compounds. Br CH = CH C CH (vi) (vii) (ix) (CCl 3 ) 3 CCl 103 XII Chemistry 2. Write the structure of following halogen compounds
Chapter: Haloalkanes and Haloarenes
 Characteristics of halo compounds. Chapter: Haloalkanes and Haloarenes Question 1 Write the IUPAC name of the following compound:(ch 3 ) 3 CCH 2 Br The IUPAC name of the given structure is 2, 2-Dimethylbromopropane.
Characteristics of halo compounds. Chapter: Haloalkanes and Haloarenes Question 1 Write the IUPAC name of the following compound:(ch 3 ) 3 CCH 2 Br The IUPAC name of the given structure is 2, 2-Dimethylbromopropane.
Chapter 10- Halo Alkanes and Halo arenes LEVEL-1 QUESTIONS
 Chapter 10- Halo Alkanes and Halo arenes LEVEL-1 QUESTIONS 1. Out of PCl5 and SOCl2 which one is better reagent for conversion of alcohol to alkylchloride. Why? Answer: SOCl2.Because all the biproducts
Chapter 10- Halo Alkanes and Halo arenes LEVEL-1 QUESTIONS 1. Out of PCl5 and SOCl2 which one is better reagent for conversion of alcohol to alkylchloride. Why? Answer: SOCl2.Because all the biproducts
DAV CENTENARY PUBLIC SCHOOL, PASCHIM ENCLAVE, NEW DELHI-87
 (iv) benzene 1 Chloromethyl 3 (2, 2 dimethylpropyl) Q1 Name the following compounds according to IUPAC system. (CH 3 ) 3 CCH 2 CH(Br)C 6 H 5 CH 3 C(C 2 H 5 ) 2 CH 2 Br CH 3 CH=CHC(Br)(CH 3 ) 2 (iv) o-br-c
(iv) benzene 1 Chloromethyl 3 (2, 2 dimethylpropyl) Q1 Name the following compounds according to IUPAC system. (CH 3 ) 3 CCH 2 CH(Br)C 6 H 5 CH 3 C(C 2 H 5 ) 2 CH 2 Br CH 3 CH=CHC(Br)(CH 3 ) 2 (iv) o-br-c
Halo Alkanes and Halo Arenes
 alo Alkanes and alo Arenes Short Answer Questions: **1. Write the isomers of the compound having formula C 4 9 Br? Sol. There are five isomers of C 4 9 Br. These are: 2-bromobutane is expected to exhibit
alo Alkanes and alo Arenes Short Answer Questions: **1. Write the isomers of the compound having formula C 4 9 Br? Sol. There are five isomers of C 4 9 Br. These are: 2-bromobutane is expected to exhibit
a. (CH 3 ) 2 CHCH(Cl)CH 3 b. CH 3 CH 2 CH(CH 3 )CH(C 2 H 5 )Cl c. CH 3 CH 2 C(CH 3 ) 2 CH 2 I d. (CH 3 ) 3 CCH 2 CH(Br)C 6 H 5.
 1. Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides: a. (CH 3 ) 2 CHCH(Cl)CH 3 b. CH 3 CH 2 CH(CH 3 )CH(C
1. Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides: a. (CH 3 ) 2 CHCH(Cl)CH 3 b. CH 3 CH 2 CH(CH 3 )CH(C
 Question 10.1: Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides: (i) (CH 3 ) 2 CHCH(Cl)CH 3 CH 3 CH 2
Question 10.1: Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides: (i) (CH 3 ) 2 CHCH(Cl)CH 3 CH 3 CH 2
UNIT-10 HALOALKANES AND HALOARENES
 Important terms and concepts: UNIT-10 HALOALKANES AND HALOARENES Alkyl halide or Haloalkane is the compound obtained by replacing one or more hydrogen atoms of an alkane by same number of halogen atoms.
Important terms and concepts: UNIT-10 HALOALKANES AND HALOARENES Alkyl halide or Haloalkane is the compound obtained by replacing one or more hydrogen atoms of an alkane by same number of halogen atoms.
Indian School Muscat. Chemistry IIT - JEE
 Indian School Muscat Chemistry IIT - JEE HALOALKANES AND HALOARENES 1. Which one of the following is not formed when a mixture of methyl bromide and bromobenzene is heated with sodium metal in the presence
Indian School Muscat Chemistry IIT - JEE HALOALKANES AND HALOARENES 1. Which one of the following is not formed when a mixture of methyl bromide and bromobenzene is heated with sodium metal in the presence
UNIT.10 HALOALKANES AND HALOARENES
 UNIT.10 ALOALKANES AND ALOARENES ONE MARKS QUESTIONS 1. What are haloalkanes? aloalkane is a derivative obtained by replacing hydrogen atom of alkane by halogen atom. 2. What is the hybridization of the
UNIT.10 ALOALKANES AND ALOARENES ONE MARKS QUESTIONS 1. What are haloalkanes? aloalkane is a derivative obtained by replacing hydrogen atom of alkane by halogen atom. 2. What is the hybridization of the
I. Multiple Choice Questions (Type-I)
 Unit 13 HYDROCARBONS I. Multiple Choice Questions (Type-I) 1. Arrange the following in decreasing order of their boiling points. (A) n butane (B) 2 methylbutane (C) n-pentane (D) 2,2 dimethylpropane A
Unit 13 HYDROCARBONS I. Multiple Choice Questions (Type-I) 1. Arrange the following in decreasing order of their boiling points. (A) n butane (B) 2 methylbutane (C) n-pentane (D) 2,2 dimethylpropane A
Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat #
 Test 4 Name Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat # Part 1. Multiple Choice. Choose the one best answer, and clearly mark that answer
Test 4 Name Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat # Part 1. Multiple Choice. Choose the one best answer, and clearly mark that answer
A4 Alcohols form H-bonds with water due to the presence of OH group. However, hydrocarbons cannot form H-bonds with water.
 Q1 p-dichlorobenzene has higher m.p. and lower solubility than those of o- and m-isomers. Discuss. A4 Alcohols form H-bonds with water due to the presence of OH group. However, hydrocarbons cannot form
Q1 p-dichlorobenzene has higher m.p. and lower solubility than those of o- and m-isomers. Discuss. A4 Alcohols form H-bonds with water due to the presence of OH group. However, hydrocarbons cannot form
 Unit - 11 ALCOHOLS, PHENOLS AND ETHERS 1. Write IUPAC names of the following compounds : (ix) C 6 H 5 OC 3 H 7 (x) O Cl 2. Write the structures of the compounds whose names are given below : (i) 3, 5-dimethoxyhexane-1,
Unit - 11 ALCOHOLS, PHENOLS AND ETHERS 1. Write IUPAC names of the following compounds : (ix) C 6 H 5 OC 3 H 7 (x) O Cl 2. Write the structures of the compounds whose names are given below : (i) 3, 5-dimethoxyhexane-1,
DAV CENTENARY PUBLIC SCHOOL, PASCHIM ENCLAVE, NEW DELHI - 87
 HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
C h a p t e r N i n e t e e n Aromatics II: Reactions of Benzene & Its Derivatives
 C h a p t e r N i n e t e e n Aromatics II: Reactions of Benzene & Its Derivatives Arenium ion from addition of tert-butyl cation to benzene (blue is δ+and red δ-) Note: Problems with italicized numbers
C h a p t e r N i n e t e e n Aromatics II: Reactions of Benzene & Its Derivatives Arenium ion from addition of tert-butyl cation to benzene (blue is δ+and red δ-) Note: Problems with italicized numbers
REASONING QUESTIONS FROM ORGANIC CHEMISTRY (CH. 1 & 2)
 REASONING QUESTIONS FROM ORGANIC CHEMISTRY (CH. 1 & 2) 1.) Why do haloalkenes under go nucleophillic substitution whereas haloarenes under go electophillic substitution. Ans. Due to more electro negative
REASONING QUESTIONS FROM ORGANIC CHEMISTRY (CH. 1 & 2) 1.) Why do haloalkenes under go nucleophillic substitution whereas haloarenes under go electophillic substitution. Ans. Due to more electro negative
CHEM 203 Exam 1. Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question.
 CHEM 203 Exam 1 Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Which of the following elements is a large percentage of both the earth's
CHEM 203 Exam 1 Name Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Which of the following elements is a large percentage of both the earth's
MUNISH KAKAR s INSTITUTE OF CHEMISTRY
 REACTONS & PREPARATON OF HALOGENATED COMPDS. WS#3 Q1. Reagent for preparing a chloroalkane from an alcohol is (a) SOCl 2 (b) HCl/ZnCl 2 (c) PCl 3 (d) All of these Q2. n the addition of H to propene in
REACTONS & PREPARATON OF HALOGENATED COMPDS. WS#3 Q1. Reagent for preparing a chloroalkane from an alcohol is (a) SOCl 2 (b) HCl/ZnCl 2 (c) PCl 3 (d) All of these Q2. n the addition of H to propene in
1. CONCEPTS IN ORGANIC CHEMISTRY 2. SYNTHETIC ORGANIC CHEMISTRY 3. ISOMERISM II 4. HYDROCARBONS II 5. HALOALKANES. Vikasana - CET 2012
 CET OBJECTIVE QUESTION ON 1. CONCEPTS IN ORGANIC CHEMISTRY 2. SYNTHETIC ORGANIC CHEMISTRY 3. ISOMERISM II 4. HYDROCARBONS II 5. HALOALKANES 1.The inductive effect a. Implies the atoms ability to cause
CET OBJECTIVE QUESTION ON 1. CONCEPTS IN ORGANIC CHEMISTRY 2. SYNTHETIC ORGANIC CHEMISTRY 3. ISOMERISM II 4. HYDROCARBONS II 5. HALOALKANES 1.The inductive effect a. Implies the atoms ability to cause
Chapter 3. Organic Compounds: Alkanes and Their Stereochemistry
 Chapter 3. Organic Compounds: Alkanes and Their Stereochemistry Functional Group: Be able to identify and name any of the functional groups listed on Table 3.1, pages 76-77. Summary of important functional
Chapter 3. Organic Compounds: Alkanes and Their Stereochemistry Functional Group: Be able to identify and name any of the functional groups listed on Table 3.1, pages 76-77. Summary of important functional
Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2
 Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2 Key Terms For Unit 3 Free Radical Chain Reaction Homolytic Cleavage Free Radical Initiation Propagation
Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2 Key Terms For Unit 3 Free Radical Chain Reaction Homolytic Cleavage Free Radical Initiation Propagation
3.2.8 Haloalkanes. Elimination. 148 minutes. 145 marks. Page 1 of 22
 3.2.8 Haloalkanes Elimination 148 minutes 145 marks Page 1 of 22 Q1. Reaction of 2-bromobutane with potassium hydroxide can produce two types of product depending on the solvent used. In aqueous solution,
3.2.8 Haloalkanes Elimination 148 minutes 145 marks Page 1 of 22 Q1. Reaction of 2-bromobutane with potassium hydroxide can produce two types of product depending on the solvent used. In aqueous solution,
Organic Chemistry Worksheets
 Highlight the single longest, continuous carbon-carbon chain. Note the alkyl branches that are connected to the root chain. Count the carbons in the root chain, starting from the end closest to the alkyl
Highlight the single longest, continuous carbon-carbon chain. Note the alkyl branches that are connected to the root chain. Count the carbons in the root chain, starting from the end closest to the alkyl
3.2.8 Haloalkanes. Nucleophilic Substitution. 267 minutes. 264 marks. Page 1 of 36
 3.2.8 Haloalkanes Nucleophilic Substitution 267 minutes 264 marks Page 1 of 36 Q1. (a) The equation below shows the reaction of 2-bromopropane with an excess of ammonia. CH 3 CHBrCH 3 + 2NH 3 CH 3 CH(NH
3.2.8 Haloalkanes Nucleophilic Substitution 267 minutes 264 marks Page 1 of 36 Q1. (a) The equation below shows the reaction of 2-bromopropane with an excess of ammonia. CH 3 CHBrCH 3 + 2NH 3 CH 3 CH(NH
1. When methane is photochlorinated a small amount of ethane is found in the product. Give a full mechanism to account for presence of the ethane.
 Chemistry 51 DS Quiz 2 1. When methane is photochlorinated a small amount of ethane is found in the product. Give a full mechanism to account for presence of the ethane. 2. When 2,3-dimethylbutane is monochlorinated
Chemistry 51 DS Quiz 2 1. When methane is photochlorinated a small amount of ethane is found in the product. Give a full mechanism to account for presence of the ethane. 2. When 2,3-dimethylbutane is monochlorinated
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION
 REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
When 2-bromopentane reacts with ethanolic KOH, two structurally isomeric alkenes are formed.
 Q1. Organic reaction mechanisms help chemists to understand how the reactions of organic compounds occur. The following conversions illustrate a number of different types of reaction mechanism. (a) When
Q1. Organic reaction mechanisms help chemists to understand how the reactions of organic compounds occur. The following conversions illustrate a number of different types of reaction mechanism. (a) When
Unit Title Marks HOURS Starting Date I Basic Concepts of Chemistry April-2019 II Structure of Atom
 Class 11+12, Classes Schedule & Syllabus [ CBSE ] Chemistry Class 11 Unit Title Marks HOURS Starting Date I Basic Concepts of Chemistry 11 18 1-April- II Structure of Atom III Classification of Elements
Class 11+12, Classes Schedule & Syllabus [ CBSE ] Chemistry Class 11 Unit Title Marks HOURS Starting Date I Basic Concepts of Chemistry 11 18 1-April- II Structure of Atom III Classification of Elements
CBSE Class-12 Chemistry Quick Revision Notes Chapter-10: Haloalkanes and Haloarenes
 CBSE Class-12 Chemistry Quick Revision Notes Chapter-10: Haloalkanes and Haloarenes Nature of C-X bond in alkyl halides: X is more electronegative than carbon. So, the C-X bond is polarized with C having
CBSE Class-12 Chemistry Quick Revision Notes Chapter-10: Haloalkanes and Haloarenes Nature of C-X bond in alkyl halides: X is more electronegative than carbon. So, the C-X bond is polarized with C having
CHEM Chapter 16. Chemistry of Benzene: Electrophilic Aromatic Substitution (homework) W
 CHEM 2425. Chapter 16. Chemistry of Benzene: Electrophilic Aromatic Substitution (homework) W Short Answer Exhibit 16-1 MATCH a structure or term from the following list with each description below. Place
CHEM 2425. Chapter 16. Chemistry of Benzene: Electrophilic Aromatic Substitution (homework) W Short Answer Exhibit 16-1 MATCH a structure or term from the following list with each description below. Place
Overview : Table Of Content :
 Book Title:-Complete Chemistry for NEET (National Eligibilitycum-Entrance Test) Author :-BASE ISBN :-9788131531648 Price :-INR 775 Pages :-976 Edition :-1 Binding :-Paperback Imprint :-CL India Year :-2016
Book Title:-Complete Chemistry for NEET (National Eligibilitycum-Entrance Test) Author :-BASE ISBN :-9788131531648 Price :-INR 775 Pages :-976 Edition :-1 Binding :-Paperback Imprint :-CL India Year :-2016
Author :-Vishal Joshi ISBN : Price :-INR 475. Pages :-486. Edition :-4. Binding :-Paperback. Imprint :-CL India.
 Book Title:-Problems and Solutions in Inorganic Chemistry for JEE (Main & Advanced) Author :-Vishal Joshi ISBN :-9788131531402 Price :-INR 475 Pages :-486 Edition :-4 Binding :-Paperback Imprint :-CL India
Book Title:-Problems and Solutions in Inorganic Chemistry for JEE (Main & Advanced) Author :-Vishal Joshi ISBN :-9788131531402 Price :-INR 475 Pages :-486 Edition :-4 Binding :-Paperback Imprint :-CL India
Chemistry 2.5 AS WORKBOOK. Working to Excellence Working to Excellence
 Chemistry 2.5 AS 91165 Demonstrate understanding of the properties of selected organic compounds WORKBOOK Working to Excellence Working to Excellence CONTENTS 1. Writing Excellence answers to Cis-Trans
Chemistry 2.5 AS 91165 Demonstrate understanding of the properties of selected organic compounds WORKBOOK Working to Excellence Working to Excellence CONTENTS 1. Writing Excellence answers to Cis-Trans
Homework problems Chapters 6 and Give the curved-arrow formalism for the following reaction: CH 3 OH + H 2 C CH +
 omework problems hapters 6 and 7 1. Give the curved-arrow formalism for the following reaction: : 3 - : 2 : 3 2-3 3 2. In each of the following sets, arrange the compounds in order of decreasing pka and
omework problems hapters 6 and 7 1. Give the curved-arrow formalism for the following reaction: : 3 - : 2 : 3 2-3 3 2. In each of the following sets, arrange the compounds in order of decreasing pka and
CHE 275 NUCLEOPHILIC SUBSTITUTUION CHAP 8 ASSIGN. 1. Which best depicts the partial charges on methyl bromide and sodium methoxide?
 CHE 275 NUCLEPHILIC SUBSTITUTUIN CHAP 8 ASSIGN 1. Which best depicts the partial charges on methyl bromide and sodium methoxide? 2. Which of the following would be the best (most reactive) nucleophile
CHE 275 NUCLEPHILIC SUBSTITUTUIN CHAP 8 ASSIGN 1. Which best depicts the partial charges on methyl bromide and sodium methoxide? 2. Which of the following would be the best (most reactive) nucleophile
Candidate number. Centre number
 Oxford Cambridge and RSA A Level Chemistry A H432/02 Synthesis and analytical techniques Practice paper Set 2 Time allowed: 2 hours 15 minutes *PRACTICE2016* You must have: the Data Sheet for Chemistry
Oxford Cambridge and RSA A Level Chemistry A H432/02 Synthesis and analytical techniques Practice paper Set 2 Time allowed: 2 hours 15 minutes *PRACTICE2016* You must have: the Data Sheet for Chemistry
1. CONCEPTS IN ORGANIC CHEMISTRY 2. SYNTHETIC ORGANIC CHEMISTRY 3. ISOMERISM II 4. HYDROCARBONS II 5. HALOALKANES. Vikasana - CET 2012
 CET OBJECTIVE QUESTION ON 1. CONCEPTS IN ORGANIC CHEMISTRY 2. SYNTHETIC ORGANIC CHEMISTRY 3. ISOMERISM II 4. HYDROCARBONS II 5. HALOALKANES 1.The inductive effect a. Implies the atoms ability to cause
CET OBJECTIVE QUESTION ON 1. CONCEPTS IN ORGANIC CHEMISTRY 2. SYNTHETIC ORGANIC CHEMISTRY 3. ISOMERISM II 4. HYDROCARBONS II 5. HALOALKANES 1.The inductive effect a. Implies the atoms ability to cause
Q1. Pentanenitrile can be made by reaction of 1-bromobutane with potassium cyanide.
 Q1. Pentanenitrile can be made by reaction of 1-bromobutane with potassium cyanide. Which of these is the correct name for the mechanism of this reaction? A B C D Electrophilic addition Electrophilic substitution
Q1. Pentanenitrile can be made by reaction of 1-bromobutane with potassium cyanide. Which of these is the correct name for the mechanism of this reaction? A B C D Electrophilic addition Electrophilic substitution
QUESTIONSHEETS ORGANIC REACTION MECHANISMS I FREE RADICAL SUBSTITUTION I FREE RADICAL SUBSTITUTION II ELECTROPHILIC ADDITION TO SYMMETRICAL ALKENES
 CHEMISTRY QUESTIONSHEETS AS Level AS TOPIC 14 ORGANIC REACTION MECHANISMS I Questionsheet 1 Questionsheet 2 Questionsheet 3 Questionsheet 4 Questionsheet 5 Questionsheet 6 Questionsheet 7 Questionsheet
CHEMISTRY QUESTIONSHEETS AS Level AS TOPIC 14 ORGANIC REACTION MECHANISMS I Questionsheet 1 Questionsheet 2 Questionsheet 3 Questionsheet 4 Questionsheet 5 Questionsheet 6 Questionsheet 7 Questionsheet
EXTRA QUESTIONS FOR 2.8 HALOALKANES. 1. Methylbenzene is converted into (chloromethyl)benzene in a free radical substitution reaction....
 EXTRA QUESTIONS FOR 2.8 HALOALKANES 1. Methylbenzene is converted into (chloromethyl)benzene in a free radical substitution reaction. C 6 H 5 3 + Cl 2 C 6 H 5 2 Cl + HCl Write an equation for the initiation
EXTRA QUESTIONS FOR 2.8 HALOALKANES 1. Methylbenzene is converted into (chloromethyl)benzene in a free radical substitution reaction. C 6 H 5 3 + Cl 2 C 6 H 5 2 Cl + HCl Write an equation for the initiation
FOURTH EXAMINATION. (1)..20 pts... (2)..24 pts... (3)..18 pts... (4)..12 pts... (5)..12 pts... (6).. 6 pts... (7).. 8 pts... Bonus 4 pts...
 Name: CHEM 331 FURTH EXAMINATIN All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer
Name: CHEM 331 FURTH EXAMINATIN All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided; if I have to guess as to what or where your answer
Aryl Halides. Structure
 Aryl Halides Structure Aryl halides are compounds containing halogen attached directly to an aromatic ring. They have the general formula ArX, where Ar is phenyl, substituted phenyl. X= F,Cl,Br,I An aryl
Aryl Halides Structure Aryl halides are compounds containing halogen attached directly to an aromatic ring. They have the general formula ArX, where Ar is phenyl, substituted phenyl. X= F,Cl,Br,I An aryl
The C-X bond gets longerand weakergoing down the periodic table.
 Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
Organic Chemistry, 7 L. G. Wade, Jr. Chapter , Prentice Hall
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds 2010, Prentice Hall Electrophilic Aromatic Substitution Although h benzene s pi electrons are in a stable aromatic
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds 2010, Prentice Hall Electrophilic Aromatic Substitution Although h benzene s pi electrons are in a stable aromatic
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
MOSTLY ALCOHOLS. Question 2, 2017 The structure of a molecule of an organic compound, threonine, is shown below.
 MOSTLY ALCOHOLS Modified Question 1, 2017 A chemistry class was learning about the chemistry of haloalkanes. They were researching the effect of heat and concentrated potassium hydroxide in ethanol, conc.
MOSTLY ALCOHOLS Modified Question 1, 2017 A chemistry class was learning about the chemistry of haloalkanes. They were researching the effect of heat and concentrated potassium hydroxide in ethanol, conc.
Class Revision on Intro to Organic, Alkanes and Alkenes
 Class Revision on Intro to Organic, Alkanes and Alkenes 2015 Term 1 Week 1 169 min 110 marks ~ Section A: Multiple Choice Questions Circle the best answer 1. What is the correct name of this compound?
Class Revision on Intro to Organic, Alkanes and Alkenes 2015 Term 1 Week 1 169 min 110 marks ~ Section A: Multiple Choice Questions Circle the best answer 1. What is the correct name of this compound?
AMINES. Unit Write IUPAC names of the following :
 Unit - 13 AMINES 1. Write IUPAC names of the following : 2. Giving an example of each, describe the following reactions : Hoffman bromamide reaction Gabriel phthanlimide synthesis Gatterman reaction Coupling
Unit - 13 AMINES 1. Write IUPAC names of the following : 2. Giving an example of each, describe the following reactions : Hoffman bromamide reaction Gabriel phthanlimide synthesis Gatterman reaction Coupling
Dr. Anand Gupta Mr Mahesh Kapil
 Dr. Anand Gupta Mr Mahesh Kapil 09356511518 09888711209 anandu71@yahoo.com mkapil_foru@yahoo.com Preparation of Haloalkanes From Alkanes Alkenes Alcohols Carboxylic Acids (Hundsdicker Reaction) Halide
Dr. Anand Gupta Mr Mahesh Kapil 09356511518 09888711209 anandu71@yahoo.com mkapil_foru@yahoo.com Preparation of Haloalkanes From Alkanes Alkenes Alcohols Carboxylic Acids (Hundsdicker Reaction) Halide
Class XII Chapter 11 Alcohols Phenols and Ethers Chemistry. Question 11.1: Write IUPAC names of the following compounds: (i) (ii) (iii) (iv) (v) (vi)
 Question 11.1: Write IUPAC names of the following compounds: (iii) (iv) (v) (vi) (vii) (viii) Page 1 of 37 (ix) (x) (xi) (xii) 2, 2, 4-Trimethylpentan-3-ol 5-Ethylheptane-2, 4-diol (iii) Butane-2, 3-diol
Question 11.1: Write IUPAC names of the following compounds: (iii) (iv) (v) (vi) (vii) (viii) Page 1 of 37 (ix) (x) (xi) (xii) 2, 2, 4-Trimethylpentan-3-ol 5-Ethylheptane-2, 4-diol (iii) Butane-2, 3-diol
Chem 2320 Final 210 points Dr. Luther Giddings
 Chem 2320 Final 210 points Dr. Luther Giddings Name Phone or E-Mail Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam,
Chem 2320 Final 210 points Dr. Luther Giddings Name Phone or E-Mail Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam,
SECOND TERMINAL EXAMINATION, 2017 CHEMISTRY Time - 3:00 hrs. Class XI M.M. - 70
 SECOND TERMINAL EXAMINATION, 2017 CHEMISTRY Time - 3:00 hrs. Class XI M.M. - 70 Date 23.02.2017 (Thursday) Name of the student Section General instructions : All questions are compulsory. Q. Nos. 1 to
SECOND TERMINAL EXAMINATION, 2017 CHEMISTRY Time - 3:00 hrs. Class XI M.M. - 70 Date 23.02.2017 (Thursday) Name of the student Section General instructions : All questions are compulsory. Q. Nos. 1 to
CHE1502. Tutorial letter 201/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry CHE1502/201/1/2016
 CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
1. Name the following compound. Use the IUPAC system and include stereochemical designations.
 Chemistry 51 Exam 1, Fall 2004, Evening Division This is a closed book exam. The exam lasts 90 minutes. All answers must appear on the answer sheet. Only the answer sheet will be collected. Put your name
Chemistry 51 Exam 1, Fall 2004, Evening Division This is a closed book exam. The exam lasts 90 minutes. All answers must appear on the answer sheet. Only the answer sheet will be collected. Put your name
Organic Chemistry Practice Problems: Solutions
 rganic Chemistry Practice Problems: Solutions 1. 2. a. B, A b. D, B c. A, D d. D, A a. Resonance b. Electronegativity of fluorine atoms F F c. Neither is very acidic, but the oxygen will help stabilise
rganic Chemistry Practice Problems: Solutions 1. 2. a. B, A b. D, B c. A, D d. D, A a. Resonance b. Electronegativity of fluorine atoms F F c. Neither is very acidic, but the oxygen will help stabilise
TOPIC 13 ANSWERS & MARK SCHEMES QUESTIONSHEET 1 ALKANES
 QUESTIONSHEET 1 ALKANES a) (i) UV light / temperatures > 500 ºC (ii) CH 4 (g) + Cl 2 (g) Cl(g) + HCl(g) Cl(g) + Cl 2 (g) Cl 2 (l) + HCl(g) Cl 2 (l) + Cl 2 (g) CHCl 3 (l) + HCl(g) CHCl 3 (l) + Cl 2 (g)
QUESTIONSHEET 1 ALKANES a) (i) UV light / temperatures > 500 ºC (ii) CH 4 (g) + Cl 2 (g) Cl(g) + HCl(g) Cl(g) + Cl 2 (g) Cl 2 (l) + HCl(g) Cl 2 (l) + Cl 2 (g) CHCl 3 (l) + HCl(g) CHCl 3 (l) + Cl 2 (g)
Benzenes & Aromatic Compounds
 Benzenes & Aromatic Compounds 1 Structure of Benzene H H C C C H C 6 H 6 H C C C H H A cyclic conjugate molecule Benzene is a colourless odourless liquid, boiling at 80 o C and melting at 5 o C. It is
Benzenes & Aromatic Compounds 1 Structure of Benzene H H C C C H C 6 H 6 H C C C H H A cyclic conjugate molecule Benzene is a colourless odourless liquid, boiling at 80 o C and melting at 5 o C. It is
Pearson Edexcel Level 3 GCE Chemistry Advanced Paper 2: Advanced Organic and Physical Chemistry
 Write your name here Surname Other names Pearson Edexcel Level 3 GCE Centre Number Candidate Number Chemistry Advanced Paper 2: Advanced Organic and Physical Chemistry Specimen Paper for first teaching
Write your name here Surname Other names Pearson Edexcel Level 3 GCE Centre Number Candidate Number Chemistry Advanced Paper 2: Advanced Organic and Physical Chemistry Specimen Paper for first teaching
C. CH CH COH CH CCH. 6. Which substance(s) could be formed during the incomplete combustion of a hydrocarbon?
 1. Which of the structures below is an aldehyde? O O A. CH 3CH 2 CH B. CH 3CCH3 C. CH 3CH 2 COH D. CH COCH 2. What product results from the reaction of CH 2 ==CH 2 with Br 2? A. CHBrCHBr B. CH 2 CHBr C.
1. Which of the structures below is an aldehyde? O O A. CH 3CH 2 CH B. CH 3CCH3 C. CH 3CH 2 COH D. CH COCH 2. What product results from the reaction of CH 2 ==CH 2 with Br 2? A. CHBrCHBr B. CH 2 CHBr C.
CHEM 303 Organic Chemistry II Problem Set III Chapter 14 Answers
 CHEM 303 rganic Chemistry II Problem Set III Chapter 14 Answers 1) Give the major products of each of the following reactions. If a mixture is expected, identify the major product. + H 3 CHC CHCH 3 H 2
CHEM 303 rganic Chemistry II Problem Set III Chapter 14 Answers 1) Give the major products of each of the following reactions. If a mixture is expected, identify the major product. + H 3 CHC CHCH 3 H 2
HSSLiVE.IN HALOALKANES AND HALOARENES
 HALOALKANES AND HALOARENES These are compounds containing halogen atoms attached to an alkyl or aryl group. The general representation of haloalkanes is R-X and that of haloarenes is Ar-X [where X = F,
HALOALKANES AND HALOARENES These are compounds containing halogen atoms attached to an alkyl or aryl group. The general representation of haloalkanes is R-X and that of haloarenes is Ar-X [where X = F,
Class: 12 Subject: chemistry Topic: Organic Chemistry of O compounds No. of Questions: 20 Duration: 60 Min Maximum Marks: 60
 Class: 12 Subject: chemistry Topic: Organic Chemistry of O compounds No. of Questions: 20 Duration: 60 Min Maximum Marks: 60 1. Ethylene is passed through conc. H 2 SO 4 and the product obtained is diluted
Class: 12 Subject: chemistry Topic: Organic Chemistry of O compounds No. of Questions: 20 Duration: 60 Min Maximum Marks: 60 1. Ethylene is passed through conc. H 2 SO 4 and the product obtained is diluted
CHAPTER 7. Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination
 CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
Class XI Chapter 13 Hydrocarbons Chemistry
 Question 13.1: How do you account for the formation of ethane during chlorination of methane? Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the
Question 13.1: How do you account for the formation of ethane during chlorination of methane? Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. A) I B) II C) III D) IV E) V
 Practice Questions : Chem 226 / Exam 3 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements about benzene is correct?
Practice Questions : Chem 226 / Exam 3 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following statements about benzene is correct?
IB Topics 10, 20 & 21 MC Practice
 IB Topics 10, 20 & 21 MC Practice 1. What is the major product of the reaction between HCl and but-2-ene? 1,2-dichlorobutane 2,3-dichlorobutane 1-chlorobutane 2-chlorobutane 2. Which compound can be oxidized
IB Topics 10, 20 & 21 MC Practice 1. What is the major product of the reaction between HCl and but-2-ene? 1,2-dichlorobutane 2,3-dichlorobutane 1-chlorobutane 2-chlorobutane 2. Which compound can be oxidized
C 3 H 6 ClBr represent a saturated alkyl halide. The following structural isomers are possible for the given compound. Among the above 5 structural is
 Q. No. 1 In CH 3 CH 2 Br, % of Br is 80 73 70 7 Molecular mass of CH 3 CH 2 Br = 109 At Wt. of Br = 80 80 % of Br is = 100 = 73.3 109 Q. No. 2 Which of the following is primary halide Isopropyl iodide
Q. No. 1 In CH 3 CH 2 Br, % of Br is 80 73 70 7 Molecular mass of CH 3 CH 2 Br = 109 At Wt. of Br = 80 80 % of Br is = 100 = 73.3 109 Q. No. 2 Which of the following is primary halide Isopropyl iodide
CHEMISTRY (Theory) tt, "1 Pc. 1"1 ( oiilki&i) CLASS-XII
 CBSE QUESTION PAPER CHEMISTRY (Theory) tt, "1 Pc. 1"1 ( oiilki&i) CLASS-XII 1. Write a feature which will distinguish a metallic solid from an ionic solid. 1 2. Define order of a reaction. 1 3. What is
CBSE QUESTION PAPER CHEMISTRY (Theory) tt, "1 Pc. 1"1 ( oiilki&i) CLASS-XII 1. Write a feature which will distinguish a metallic solid from an ionic solid. 1 2. Define order of a reaction. 1 3. What is
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS
 AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
Chapter 17. Reactions of Aromatic Compounds
 Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Although benzene s pi electrons are in a stable aromatic system, they are available to attack a strong electrophile to give
Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Although benzene s pi electrons are in a stable aromatic system, they are available to attack a strong electrophile to give
Q. No. 4 Chlorination of toluene in the presence of light and heat followed by treatment with aqueous NaOH gives o cresol p cresol 2, 4 dihydroxy tolu
 Q. No. 1 Glycerine has One primary and two secondary OH groups One secondary and two primary OH groups Three primary OH groups Three secondary OH groups Q. No. 2 The structural formula of cyclohexanol
Q. No. 1 Glycerine has One primary and two secondary OH groups One secondary and two primary OH groups Three primary OH groups Three secondary OH groups Q. No. 2 The structural formula of cyclohexanol
Paper Reference. Advanced Unit Test 6B (Synoptic) Thursday 18 June 2009 Morning Time: 1 hour 30 minutes
 Centre No. Paper Reference Surname Initial(s) Candidate No. 6 2 4 6 0 2 Signature Paper Reference(s) 6246/02 Edexcel GCE Chemistry Examiner s use only Team Leader s use only Advanced Unit Test 6B (Synoptic)
Centre No. Paper Reference Surname Initial(s) Candidate No. 6 2 4 6 0 2 Signature Paper Reference(s) 6246/02 Edexcel GCE Chemistry Examiner s use only Team Leader s use only Advanced Unit Test 6B (Synoptic)
ORGANIC MOLECULES (LIVE) 10 APRIL 2015 Section A: Summary Notes and Examples Naming and Functional Groups
 ORGANIC MOLECULES (LIVE) 10 APRIL 2015 Section A: Summary Notes and Examples Naming and Functional Groups Important Features of Carbon There are different allotropes (same element, same phase, different
ORGANIC MOLECULES (LIVE) 10 APRIL 2015 Section A: Summary Notes and Examples Naming and Functional Groups Important Features of Carbon There are different allotropes (same element, same phase, different
Organic Chemistry. Second Edition. Chapter 19 Aromatic Substitution Reactions. David Klein. Klein, Organic Chemistry 2e
 Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
Organic Chemistry Second Edition David Klein Chapter 19 Aromatic Substitution Reactions Copyright 2015 John Wiley & Sons, Inc. All rights reserved. Klein, Organic Chemistry 2e 19.1 Introduction to Electrophilic
3 free radical is most stable. Q.5. A + Cl 2 hv monochloro product To maximize the yield of monochloro product in the above reaction? Cl 2 must be add
 Q.1. I II III IV IV III II I I III II IV IV II III I I II III IV The decreasing order of the anti knocking value of octane number of the following is: (I) CH 4 (II) C 2 H 6 (III) C 3 H 8 (IV) C 4 H 10
Q.1. I II III IV IV III II I I III II IV IV II III I I II III IV The decreasing order of the anti knocking value of octane number of the following is: (I) CH 4 (II) C 2 H 6 (III) C 3 H 8 (IV) C 4 H 10
CHEM 242 REACTIONS OF ARENES: CHAP 12 ASSIGN ELECTROPHILIC AROMATIC SUBSTITUTION A B C D E
 CHEM 242 REACTIONS OF ARENES: CHAP 12 ASSIGN ELECTROPHILIC AROMATIC SUBSTITUTION 1. Consider carefully the mechanism of the following electrophilic aromatic substitution reaction and indicate which of
CHEM 242 REACTIONS OF ARENES: CHAP 12 ASSIGN ELECTROPHILIC AROMATIC SUBSTITUTION 1. Consider carefully the mechanism of the following electrophilic aromatic substitution reaction and indicate which of
What is the major product of the following reaction?
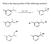 What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
Nuggets of Knowledge for Chapter 12 Alkenes (II) Chem reaction what is added to the C=C what kind of molecule results addition of HX HX only
 I. Addition Reactions of Alkenes Introduction Nuggets of Knowledge for Chapter 12 Alkenes (II) Chem 2310 An addition reaction always involves changing a double bond to a single bond and adding a new bond
I. Addition Reactions of Alkenes Introduction Nuggets of Knowledge for Chapter 12 Alkenes (II) Chem 2310 An addition reaction always involves changing a double bond to a single bond and adding a new bond
AMINES. 3. From your knowledge of the effects involved, predict or explain experimental results. Important areas include:
 AMINES A STUDENT SHOULD BE ABLE TO: 1. Name given the structure, and draw the structure given the name of amines and common nitrogen heterocycles (pyrrole and pyridine). Also, give the classification of
AMINES A STUDENT SHOULD BE ABLE TO: 1. Name given the structure, and draw the structure given the name of amines and common nitrogen heterocycles (pyrrole and pyridine). Also, give the classification of
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
Chemistry Class 12 th NCERT Solutions
 This e-book is prepared by the CBSE board exam experts of jagranjosh.com, an online educational portal of Dainik Jagran. The purpose of providing solutions for CBSE class 12 th Science and Mathematics
This e-book is prepared by the CBSE board exam experts of jagranjosh.com, an online educational portal of Dainik Jagran. The purpose of providing solutions for CBSE class 12 th Science and Mathematics
Part I. Multiple choice. (4 points each.) Choose the one best answer and mark your answer on the ScanTron sheet.
 Test 3 Chemistry 2321 April 25, 2003 McMurry, Chapters 9-11 103 Total Points Name Please Print Part I. Multiple choice. (4 points each.) Choose the one best answer and mark your answer on the ScanTron
Test 3 Chemistry 2321 April 25, 2003 McMurry, Chapters 9-11 103 Total Points Name Please Print Part I. Multiple choice. (4 points each.) Choose the one best answer and mark your answer on the ScanTron
CHAPTER HYDROCARBONS. Chapterwise Previous year Qs. (a) Na (b) HCl in H2O (c) KOH in C2H5OH (d) Zn in alcohol. Ans: (c)
 122 CHAPTER HYDROCARBONS 1. Acetylenic hydrogens are acidic because [1989] Sigma electron density of C Hbond in acetylene is nearer to carbon, which has 50% s- character Acetylene has only open hydrogen
122 CHAPTER HYDROCARBONS 1. Acetylenic hydrogens are acidic because [1989] Sigma electron density of C Hbond in acetylene is nearer to carbon, which has 50% s- character Acetylene has only open hydrogen
1. Name the following compound. Use the IUPAC system and include the stereochemical designations.
 Chemistry 51 Exam 1, Summer 2004 This is a closed book exam. The exam lasts 100 minutes. All answers must appear on the answer sheet. Only the answer sheet will be collected. Put your name on the answer
Chemistry 51 Exam 1, Summer 2004 This is a closed book exam. The exam lasts 100 minutes. All answers must appear on the answer sheet. Only the answer sheet will be collected. Put your name on the answer
Organic Halogen Compounds
 8 Organic alogen ompounds APTER SUMMARY 8.1 Introduction Although organic halogen compounds are rarely found in nature, they do have a variety of commercial applications including use as insecticides,
8 Organic alogen ompounds APTER SUMMARY 8.1 Introduction Although organic halogen compounds are rarely found in nature, they do have a variety of commercial applications including use as insecticides,
Level 3 Chemistry, 2015
 91391 913910 3SUPERVISOR S Level 3 Chemistry, 2015 91391 Demonstrate understanding of the properties of organic compounds 2.00 p.m. Wednesday 11 November 2015 Credits: Five Achievement Achievement with
91391 913910 3SUPERVISOR S Level 3 Chemistry, 2015 91391 Demonstrate understanding of the properties of organic compounds 2.00 p.m. Wednesday 11 November 2015 Credits: Five Achievement Achievement with
Chemistry 52 Exam #1. Name: 22 January This exam has six (6) questions, two cover pages, six pages, and 2 scratch pages.
 Chemistry 52 Exam #1 Name: 22 January 2003 This exam has six (6) questions, two cover pages, six pages, and 2 scratch pages. Please check before beginning to make sure no questions are missing. 65 minutes
Chemistry 52 Exam #1 Name: 22 January 2003 This exam has six (6) questions, two cover pages, six pages, and 2 scratch pages. Please check before beginning to make sure no questions are missing. 65 minutes
1. Which of the structures below is an aldehyde? O A. CH CH CH O B. CH CCH O C. CH CH COH O D. CH COCH
 1. Which of the structures below is an aldehyde? O A. CH CH CH 3 2 O B. CH CCH 3 3 O C. CH CH COH 3 2 O D. CH COCH 3 3 2. What product results from the reaction of CH 2 ==CH 2 with Br 2? A. CHBrCHBr B.
1. Which of the structures below is an aldehyde? O A. CH CH CH 3 2 O B. CH CCH 3 3 O C. CH CH COH 3 2 O D. CH COCH 3 3 2. What product results from the reaction of CH 2 ==CH 2 with Br 2? A. CHBrCHBr B.
Organic Chemistry CHM 224
 rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
rganic Chemistry CM 224 Final Exam Review Questions This is a compilation example final exam questions. Provide IUPAC names for each of the structures below. 2 ! Propose a structure for the compound that
Alkanes, Alkenes and Alkynes
 Alkanes, Alkenes and Alkynes Hydrocarbons Hydrocarbons generally fall into 2 general groupings, aliphatic hydrocarbons and aromatic hydrocarbons. Aliphatic hydrocarbons contain chains and rings of hydrocarbons,
Alkanes, Alkenes and Alkynes Hydrocarbons Hydrocarbons generally fall into 2 general groupings, aliphatic hydrocarbons and aromatic hydrocarbons. Aliphatic hydrocarbons contain chains and rings of hydrocarbons,
Downloaded from
 1 Class XII Chemistry Chapter: Alcohols, Phenols And Ethers Top concepts: 1. Structure of alcohols, phenols and ethers: 2. Preparation of alcohols: 3. Preparation of phenols: 2 4. Physical properties of
1 Class XII Chemistry Chapter: Alcohols, Phenols And Ethers Top concepts: 1. Structure of alcohols, phenols and ethers: 2. Preparation of alcohols: 3. Preparation of phenols: 2 4. Physical properties of
STEREOCHEMISTRY A STUDENT SHOULD BE ABLE TO:
 A STUDENT SHOULD BE ABLE TO: STEREOCHEMISTRY 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define the following terms, and give examples of pairs
A STUDENT SHOULD BE ABLE TO: STEREOCHEMISTRY 1. Determine the relationship between two given structures (which may be any of the kinds below). Also, define the following terms, and give examples of pairs
AMINES. 3. Secondary When two hydrogen atoms are replaced by two alkyl or aryl groups.
 AMINES Amine may be regarded as derivative of ammonia formed by replacement of one or more hydrogen atoms by corresponding number of alkyl or aryl group CLASSIFICATION 1. Ammonia 2. Primary amine 3. Secondary
AMINES Amine may be regarded as derivative of ammonia formed by replacement of one or more hydrogen atoms by corresponding number of alkyl or aryl group CLASSIFICATION 1. Ammonia 2. Primary amine 3. Secondary
Chem 3719 Example Exams. Chemistry 3719 Practice Exams
 Chem 3719 Example Exams Chemistry 3719 Practice Exams Fall 2018 Chemistry 3719, Fall 2017 Exam 1 Student Name: Y Number: This exam is worth 100 points out of a total of 700 points for Chemistry 3719/3719L.
Chem 3719 Example Exams Chemistry 3719 Practice Exams Fall 2018 Chemistry 3719, Fall 2017 Exam 1 Student Name: Y Number: This exam is worth 100 points out of a total of 700 points for Chemistry 3719/3719L.
Ethers. Synthesis of Ethers. Chemical Properties of Ethers
 Page 1 of 6 like alcohols are organic derivatives of water, but lack the labile -OH group. As a result, ethers, except for epoxides, are usually not very reactive and are often used as solvents for organic
Page 1 of 6 like alcohols are organic derivatives of water, but lack the labile -OH group. As a result, ethers, except for epoxides, are usually not very reactive and are often used as solvents for organic
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
CHEM 242 ETHERS, EPOXIDES, AND SULFIDES CHAP 17 ASSIGN
 CHEM 242 ETHERS, EPOXIDES, AND SULFIDES CHAP 17 ASSIGN 1. Which of the following is the correct name of the compound below? A) benzyl ethyl ether B) ethyl phenyl ether C) ethylbenzene ether D) ethoxy phenoxy
CHEM 242 ETHERS, EPOXIDES, AND SULFIDES CHAP 17 ASSIGN 1. Which of the following is the correct name of the compound below? A) benzyl ethyl ether B) ethyl phenyl ether C) ethylbenzene ether D) ethoxy phenoxy
-SQA- SCOTTISH QUALIFICATIONS AUTHORITY HIGHER NATIONAL UNIT SPECIFICATION GENERAL INFORMATION
 -SQA- SCOTTISH QUALIFICATIONS AUTHORITY HIGHER NATIONAL UNIT SPECIFICATION GENERAL INFORMATION -Unit Number- 3481996 -Superclass- -Title- RD CONCEPTS IN ORGANIC CHEMISTRY -------------------------------
-SQA- SCOTTISH QUALIFICATIONS AUTHORITY HIGHER NATIONAL UNIT SPECIFICATION GENERAL INFORMATION -Unit Number- 3481996 -Superclass- -Title- RD CONCEPTS IN ORGANIC CHEMISTRY -------------------------------
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
 Unit - 12 ALDEHYDES, KETONES AND CARBOXYLIC ACIDS 1. Indicate the electrophilic and nucleophilic centres in acetaldehyde. 2. Write the IUPAC names of the following organic compounds : 122 XII Chemistry
Unit - 12 ALDEHYDES, KETONES AND CARBOXYLIC ACIDS 1. Indicate the electrophilic and nucleophilic centres in acetaldehyde. 2. Write the IUPAC names of the following organic compounds : 122 XII Chemistry
