Chapter: Haloalkanes and Haloarenes
|
|
|
- Hugo Stewart
- 6 years ago
- Views:
Transcription
1 Characteristics of halo compounds. Chapter: Haloalkanes and Haloarenes Question 1 Write the IUPAC name of the following compound:(ch 3 ) 3 CCH 2 Br The IUPAC name of the given structure is 2, 2-Dimethylbromopropane. Question 2 Question 3 Give the common name of the following. (i) 1- Bromopropane (ii) 2-chloro propane (iii) 1-chloro-2-methylpropane (i) Common name of 1- Bromopropane is n-propylbromide (ii) Common name of 2-chloro propane is Isopropyl chloride (iii) Common name of 1-chloro-2-methylpropane Isobutyl chloride
2 Question 4 Classify the following as alkyl, allyl, benzyl, vinyl or aryl halides: (i) (CH 3 ) 2 CHCH(Cl)CH 3 (ii) ( CH 3 ) 3 CCH 2 CH(Br)C 6 H 5 (iii) CH 3 CH=C(Cl)CH 2 CH(CH 3 ) 2 (iv) CH 3 CH=CHC(Br)(CH 3 ) 2 (v) p-clc 6 H 4 CH 2 CH(CH 3 ) 2 (i) Alkyl halide: (CH 3 ) 2 CHCH (Cl) CH 3 (ii) Allyl halide: CH 3 CH=CHC (Br) (CH 3 ) 2 (iii) Benzyl halide: (CH3) 3 CCH 2 CH (Br) C 6 H 5 (iv) Vinyl halide: CH 3 CH=C (Cl) CH 2 CH (CH 3 ) 2 (v) Aryl halide: p-clc 6 H 4 CH 2 CH (CH 3 ) 2 Question 5 Write the isomers of the compound having formula C 4 H 9 Br. i. CH 3 CH 2 CH 2 CH 2 Br ii. CH 3 CH 2 CH (Br)CH 3 iii. CH 3 CH(CH 3 ) CH 2 Br iv. CH 3 CBr(CH 3 ) CH 3 Question 6 Define racemisation? A mixture containing two enantiomers in equal proportions is known as racemic mixture and the process of conversion of enantiomers into a racemic mixture is known as racemisation. Question 7 What are enantiomers? Give example. The stereoisomers related to each other as nonsuperimposable mirror images are called enantiomers. For example 2-chloro butane (CH 3 CH 2 C * H (Cl) CH 3 ). Here the carbon atom marked is the chiral centre and posses four different group.
3 Question 8 What is benzylic halide? Give the structure. The compounds in which the halogen atom is bonded to an sp 3 -hybridised carbon atom next to an aromatic ring is called benzylic halide. Question 9 What is the common name of 1, 3-Dibromobenzene? m- Dibromobenzene. Question 10 What is the general formula for alkyl halide? C n H 2n + 1 X, where X is halogen atom and n is the number of carbon atom. Question 11 Write the structures of the following organic halogen compound. 2-Chloro-3-methylpentane. CH 3 CH (Cl) CH (CH 3 ) CH 2 CH 3 Question 12 Give the IUPAC name of the compound CH 3 CH (Cl) CH (Br) CH 3. 2-bromo-3-chloro-Butane
4 General methods of preparation Question 1 Write the structure of the major organic product in each of the following reactions: (i) CH 3 CH 2 CH 2 OH + SOCl 2 (ii) CH 3 CH 2 CH = CH 2 + HBr (iii) CH 3 CH = C (CH 3 ) 2 + HBr (i) CH 3 CH 2 CH 2 Cl (ii) CH 3 CH 2 CH 2 CH 2 Br (iii) CH 3 CH 2 CBr(CH 3 ) 2 Question 2 Write the equations for the preparation of 1-iodobutane from (i) 1-butanol (ii) 1-chlorobutane (iii) But-1-ene. (i) CH 3 CH 2 CH 2 CH 2 OH 1-butanol (ii) CH 3 CH 2 CH 2 CH 2 Cl + NaI 1-chlorobutane CH 3 CH 2 CH 2 CH 2 I 1-iodobutane CH 3 CH 2 CH 2 CH 2 I + NaCl 1-iodobutane (iii) CH 3 CH 2 CH = CH 2 + HI But-1-ene. CH 3 CH 2 CH 2 CH 2 I 1-iodobutane Question 3 Complete the following reactions: (i)
5 (ii) (iii) CH 3 CH 2 Br + NaI (i) + SO 2 + HCl (ii) (iii) CH 3 CH 2 Br + NaI CH 3 CH 2 I + NaBr Question 4 p-dichlorobenzene has higher melting point and solubility than those of orthoand meta-isomers. Discuss. Due to its symmetry p-dichlorobenzene fits in crystal lattice better as compared to o- and m-isomers. As a result the melting point and solubility of p-dichlorobenzene is higher than ortho- and meta-isomers. Question 5 Explain the statement "Alkyl halides, though polar, are immiscible with water". Energy is required to overcome the attractions between the haloalkane molecules and to break the hydrogen bonds between water molecules. The energy released during the formation of new attractive force between Haloalkane and the water molecule is very less as the attractions between the haloalkane and the water molecule is not as strong as the original hydrogen bonds in water. Thus Alkyl halides, though polar, are immiscible with water.
6 Question 6 Why is sulphuric acid not used during the reaction of alcohols with KI? H 2 SO 4 converts KI to corresponding HI and then oxidizes it to I 2. Hence it cannot be used during the reaction of alcohols with KI. Question 7 Name the aromatic salt formed as intermediate during the Sandmeyer's Reaction. Benzene diazonium halide also known as diazonium salt is formed as intermediate during the Sandmeyer's Reaction. Question 8 Which alkyl halides show colour on exposure to light? Alkyl Bromide and Alky iodide. Question 9 Give the Swarts reaction for the synthesis of fluoromethane. Question 10 Give the order of reactivity of alcohols with a given haloacid. The order of reactivity of alcohols with a given haloacid is 3 >2 >1. Chemical properties Question 1 Answer the following: (i) Haloalkanes easily dissolve in organic solvents, why? (ii) What is known as a racemic mixture? Give an example. (iii) Of the two bromo derivatives, C 6 H 5 CH(CH 3 )Br and C 6 H 5 CH(C 6 H 5 )Br, which one is more reactive in S N 1 substitution reaction and why?
7 (i) Haloalkanes can easily dissolve in organic solvents of low polarity because the new forces of attraction set up between haloalkanes and the solvent molecules are of same strength as the forces of attraction being broken. (ii) A mixture of equal amounts of two enantiomers is known as racemic mixture. For example: When a 3 o halide undergoes substitution with KOH, the reaction proceeds through S N 1 mechanism forming the racemic mixture in which one of the products has the same configuration as a reactant, while the product has an inverted configuration. (iii) The S N 1 substitution reaction involves the formation of carbocation, which is not affected by the presence of bulky groups. Thus, C 6 H 5 CH(C 6 H 5 )Br will be more reactive towards S N 1 substitution reaction forming racemic mixture. Question 2 A solution of KOH hydrolyses CH 3 CHClCH 2 CH 3 and CH 2 CH 2 CH 2 CH 2 Cl.Which one of these is more easily hydrolysed? than undergoes hydrolysis more easily than. being a primary alkyl halide has less steric hindrance which is secondary alkyl halide.
8 Question 3 Question 4 Give reasons for the following: (i) Grignard reagent should be prepared under anhydrous conditions. (ii) Neo-pentyl bromide undergoes nucleophilic substituting reactions very slowly. (iii) p-methoxybenzyl bromide reacts faster than p-nitrobenzyl bromide with ethanol to form an ether product. (i) Grignard reagent reacts with water and gets decomposed, so it is produced in anhydrous conditions. (ii) Neo-pentyl bromide being a primary halide reacts slowly through SN 1, and being a sterically hindered halide reacts slowly even through SN 2 mechanism. (iii) Methoxy (-OCH 3 ) group being an electron releasing group stabilizes the intermediate carbocation. On the other hand nitro group (-NO 2 ) is an electron withdrawing group and hence it destabalises the intermediate carbocation. Thus p-methoxy benzyl bromide reacts faster than p-nitrobenzyl bromide because in its case the reaction proceeds via more stable intermediate carbocation. Question 5 A primary alkyl halide (A) C 4 H 9 reacts with alcoholic KOH to give a compound (B). The compound (B) reacts with HBr to give the compound C which is an isomer of A. When A reacts with sodium metal it give a compound (D) whose molecular formula is C 8 H 18. The compound D is different from the compound formed when n-butyl bromide reacts with sodium. Give the structural formula of A and write all the equations involved in the reaction.
9 For C 4 H 9 Br, two primary halides are possible CH 3 CH 2 CH 2 CH 2 Br (n-butyl bromide) and (CH 3 ) 2 CHCH 2 Br (iso-butyl bromide). Since A is not normal butyl bromide, it must be isobutyl bromide. Question 6 Give reasons for the following: i) Haloalkanes react with KCN to form alkyl cyanides as main product while AgCN forms isocyanide as the chief products. ii) Alcohols do not react with NaBr, but when H 2 SO 4 is added, they form alkyl bromides. i) KCN is predominantly ionic and provides cyanide ions in solution. Although both carbon and nitrogen atoms of CN can donate electron pairs but the attack takes place mainly through carbon atom and not through nitrogen atom because C-C bond is more stable than C-N bond. However AgCN is mainly covalent in nature and nitrogen is free to donate electron pair forming isocyanide as the main product. ii) Br - is a weak base. So it cannot displace the strong base OH - when H 2 SO 4 is added, it leads to protonation of alcohol, as a result of which water molecule is formed. Since water molecule is a very weak base it is easily replaced by Br -.
10 Question 7 What is Saytzeff rule? Illustrate with suitable example. According to Saytzeff rule in dehydrohalogenation reactions the Haloalkane can form more than one alkene due to the availability of more than one alpha hydrogen atoms. In such a case the preferred alkene is that alkene which has greater number of alkyl groups attached to the doubly bonded carbon atoms. For example: the dehydrohalogenation of 2-bromobutane yields two products 1 butene and 2 butene. Out of these 2 butene is the major product (80%) as it is more highly substituted and it is more stable Question 8 Give the nitration and sulphonation reaction of chlorobenzene.
11 Question 9 Which compound in each of the following pairs will react faster in S N 2 reaction with - OH? (i) CH 3 Br or CH 3 I (ii) (CH 3 ) 3 CCl or CH 3 Cl (i) CH3I will react faster than CH3Br because the order of reactivity is as follows R-I > R-Br > R-Cl > R-F (ii) CH 3 Cl will react faster than (CH 3 ) 3 CCl because the order of reactivity is as follows in SN 2 reaction. Question 10 What is the directive influence of chlorine in chlorobenzene? The -Cl group is ortho and para directing in chlorobenzene. Question 11 What is Grignard reagent? It is an organometallic compound having formula RMgX also called alkyl magnesium halide.
12 Question 12 Give the order of reactivity for S N 1 reaction in methyl, primary, secondary and tertiary halide. Methyl halide >Primary halide > secondary halide> Tertiary halide Question 13 What are ambident nucleophiles? Nucleophiles that possess two nucleophilic centres are called ambident nucleophiles. Example cyanide Polyhalogen compounds uestion 1 (i) State one use each of DDT and iodoform. (ii) Which compound in the following couples will react faster in S N 2 displacement and why? (a) 1-Bromopentane or 2-bromopentane (b) 1-Bromo-2-methylbutane or 2-bromo-2methylbutane. (i) Use of DDT: It is used as an insecticide. Use of iodoform: It is used as an antiseptic. (ii) (a) 1 Bromopentane will undergo faster S N 2 displacement reaction than 2- bromopentane because 1- bromopentane has less steric hindrance than 2 bromopentane. This is because 1- bromopentane is a primary alkyl halide whereas 2- bromopentane is a secondary alkyl halide. (b) 1- Bromo-2-methylbutane will undergo S N 2 reaction faster than 2- Bromo-2- methylbutane because 1- Bromo-2-methylbutane has less steric hindrance than 2- Bromo-2-methylbutane. This is because 1- Bromo-2-methylbutane is a primary alkyl halide whereas 2- Bromo-2-methylbutane is a tertiary alkyl halide.
13 Question 2 What is DDT and why its use is banned in United States? DDT is p, p'-dichlorodiphenyltrichloroethane and is used as insecticide. It is banned for the following reason (i) Its high toxicity towards fish. (ii) DDT is not metabolized very rapidly by animals but get deposited and stored in the fatty tissues. Question 3 Give the three uses of each of the following: (i) Dichloromethane (ii) Tetrachloromethane Dichloromethane: It can be used (i) As solvent (ii) As paint remover (iii) As aerosol propellant Tetrachloro methane: It can be used (i) As a solvent (ii) As a fire extinguisher (iii) As a degreasing agent Question 4 What is the reason that Haloarenes are less reactive than haloalkane towards nucleophilic substitution reaction? In haloalkane, the halogen is attached to sp 2 hybridised carbon atom while in case of haloarene it is attached to sp 2 hybridised carbon atom. The sp 2 hybridized carbon have greater s-character than sp 3 -hybridised carbon and thus more electronegative and can hold the electron pair of C X bond more tightly than in haloalkane. The, C Cl bond length in haloalkane is greater than in haloarene. Since it is difficult to break a shorter bond than a longer bond, therefore, Haloarenes are less reactive than haloalkanes towards nucleophilic substitution reaction. Question 5 Why chloroform is kept closed in dark colored bottles? Chloroform is oxidized slowly by air in the presence of light to an extremely poisonous gas, carbonyl chloride, also known as Phosgene
14 Hence it is kept closed in dark colored bottles. Question 6 Give the role of carbon tetrachloride and Freon on environmental degradation. Both the carbon tetrachloride and Freon causes depletion of ozone layer when released in air because they remain unchanged and diffuses into stratosphere. The depletion of ozone layer increases the exposure of UV rays to human being which results in skin cancer, eye disorder and immune system diseases. Question 7 Why the use of chloroform as anesthetic is decreasing? Inhaling chloroform vapors depresses the central nervous system and its chronic exposure may cause damage to the liver and kidneys due to metabolism of chloroform to phosgene gas. Hence the use of chloroform as anesthetic is decreasing. Question 8 Give two hazardous effects of methylene dichloride. i) It harms the central nervous system. (ii) On direct contact with skin causes severe burning. Question 9 What is iodoform? Give one use of it. Triodomethane is called iodoform and can be used as antiseptic due to liberation of free iodine. Question 10 What are freons? Give one example. Freons are chloro fluoro carbons used as refrigerants. Example CCl 2 F 2. Give the full name of DDT. p,p
HALOALKANES AND HALOARENES
 Unit - 10 HALOALKANES AND HALOARENES 1. Write the IUPAC names of the following compounds. Br CH = CH C CH (vi) (vii) (ix) (CCl 3 ) 3 CCl 103 XII Chemistry 2. Write the structure of following halogen compounds
Unit - 10 HALOALKANES AND HALOARENES 1. Write the IUPAC names of the following compounds. Br CH = CH C CH (vi) (vii) (ix) (CCl 3 ) 3 CCl 103 XII Chemistry 2. Write the structure of following halogen compounds
a. (CH 3 ) 2 CHCH(Cl)CH 3 b. CH 3 CH 2 CH(CH 3 )CH(C 2 H 5 )Cl c. CH 3 CH 2 C(CH 3 ) 2 CH 2 I d. (CH 3 ) 3 CCH 2 CH(Br)C 6 H 5.
 1. Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides: a. (CH 3 ) 2 CHCH(Cl)CH 3 b. CH 3 CH 2 CH(CH 3 )CH(C
1. Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides: a. (CH 3 ) 2 CHCH(Cl)CH 3 b. CH 3 CH 2 CH(CH 3 )CH(C
Halo Alkanes and Halo Arenes
 alo Alkanes and alo Arenes Short Answer Questions: **1. Write the isomers of the compound having formula C 4 9 Br? Sol. There are five isomers of C 4 9 Br. These are: 2-bromobutane is expected to exhibit
alo Alkanes and alo Arenes Short Answer Questions: **1. Write the isomers of the compound having formula C 4 9 Br? Sol. There are five isomers of C 4 9 Br. These are: 2-bromobutane is expected to exhibit
Chapter 10- Halo Alkanes and Halo arenes LEVEL-1 QUESTIONS
 Chapter 10- Halo Alkanes and Halo arenes LEVEL-1 QUESTIONS 1. Out of PCl5 and SOCl2 which one is better reagent for conversion of alcohol to alkylchloride. Why? Answer: SOCl2.Because all the biproducts
Chapter 10- Halo Alkanes and Halo arenes LEVEL-1 QUESTIONS 1. Out of PCl5 and SOCl2 which one is better reagent for conversion of alcohol to alkylchloride. Why? Answer: SOCl2.Because all the biproducts
UNIT.10 HALOALKANES AND HALOARENES
 UNIT.10 ALOALKANES AND ALOARENES ONE MARKS QUESTIONS 1. What are haloalkanes? aloalkane is a derivative obtained by replacing hydrogen atom of alkane by halogen atom. 2. What is the hybridization of the
UNIT.10 ALOALKANES AND ALOARENES ONE MARKS QUESTIONS 1. What are haloalkanes? aloalkane is a derivative obtained by replacing hydrogen atom of alkane by halogen atom. 2. What is the hybridization of the
DAV CENTENARY PUBLIC SCHOOL, PASCHIM ENCLAVE, NEW DELHI-87
 (iv) benzene 1 Chloromethyl 3 (2, 2 dimethylpropyl) Q1 Name the following compounds according to IUPAC system. (CH 3 ) 3 CCH 2 CH(Br)C 6 H 5 CH 3 C(C 2 H 5 ) 2 CH 2 Br CH 3 CH=CHC(Br)(CH 3 ) 2 (iv) o-br-c
(iv) benzene 1 Chloromethyl 3 (2, 2 dimethylpropyl) Q1 Name the following compounds according to IUPAC system. (CH 3 ) 3 CCH 2 CH(Br)C 6 H 5 CH 3 C(C 2 H 5 ) 2 CH 2 Br CH 3 CH=CHC(Br)(CH 3 ) 2 (iv) o-br-c
REASONING QUESTIONS FROM ORGANIC CHEMISTRY (CH. 1 & 2)
 REASONING QUESTIONS FROM ORGANIC CHEMISTRY (CH. 1 & 2) 1.) Why do haloalkenes under go nucleophillic substitution whereas haloarenes under go electophillic substitution. Ans. Due to more electro negative
REASONING QUESTIONS FROM ORGANIC CHEMISTRY (CH. 1 & 2) 1.) Why do haloalkenes under go nucleophillic substitution whereas haloarenes under go electophillic substitution. Ans. Due to more electro negative
 Question 10.1: Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides: (i) (CH 3 ) 2 CHCH(Cl)CH 3 CH 3 CH 2
Question 10.1: Name the following halides according to IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides: (i) (CH 3 ) 2 CHCH(Cl)CH 3 CH 3 CH 2
UNIT-10 HALOALKANES AND HALOARENES
 Important terms and concepts: UNIT-10 HALOALKANES AND HALOARENES Alkyl halide or Haloalkane is the compound obtained by replacing one or more hydrogen atoms of an alkane by same number of halogen atoms.
Important terms and concepts: UNIT-10 HALOALKANES AND HALOARENES Alkyl halide or Haloalkane is the compound obtained by replacing one or more hydrogen atoms of an alkane by same number of halogen atoms.
Q1. Pentanenitrile can be made by reaction of 1-bromobutane with potassium cyanide.
 Q1. Pentanenitrile can be made by reaction of 1-bromobutane with potassium cyanide. Which of these is the correct name for the mechanism of this reaction? A B C D Electrophilic addition Electrophilic substitution
Q1. Pentanenitrile can be made by reaction of 1-bromobutane with potassium cyanide. Which of these is the correct name for the mechanism of this reaction? A B C D Electrophilic addition Electrophilic substitution
HSSLiVE.IN HALOALKANES AND HALOARENES
 HALOALKANES AND HALOARENES These are compounds containing halogen atoms attached to an alkyl or aryl group. The general representation of haloalkanes is R-X and that of haloarenes is Ar-X [where X = F,
HALOALKANES AND HALOARENES These are compounds containing halogen atoms attached to an alkyl or aryl group. The general representation of haloalkanes is R-X and that of haloarenes is Ar-X [where X = F,
CBSE Class-12 Chemistry Quick Revision Notes Chapter-10: Haloalkanes and Haloarenes
 CBSE Class-12 Chemistry Quick Revision Notes Chapter-10: Haloalkanes and Haloarenes Nature of C-X bond in alkyl halides: X is more electronegative than carbon. So, the C-X bond is polarized with C having
CBSE Class-12 Chemistry Quick Revision Notes Chapter-10: Haloalkanes and Haloarenes Nature of C-X bond in alkyl halides: X is more electronegative than carbon. So, the C-X bond is polarized with C having
3.2.8 Haloalkanes. Elimination. 148 minutes. 145 marks. Page 1 of 22
 3.2.8 Haloalkanes Elimination 148 minutes 145 marks Page 1 of 22 Q1. Reaction of 2-bromobutane with potassium hydroxide can produce two types of product depending on the solvent used. In aqueous solution,
3.2.8 Haloalkanes Elimination 148 minutes 145 marks Page 1 of 22 Q1. Reaction of 2-bromobutane with potassium hydroxide can produce two types of product depending on the solvent used. In aqueous solution,
Organic Halogen Compounds
 8 Organic alogen ompounds APTER SUMMARY 8.1 Introduction Although organic halogen compounds are rarely found in nature, they do have a variety of commercial applications including use as insecticides,
8 Organic alogen ompounds APTER SUMMARY 8.1 Introduction Although organic halogen compounds are rarely found in nature, they do have a variety of commercial applications including use as insecticides,
 1 Class XII Chemistry Chapter 10 Haloalkanes and Haloarenes 1. Nature of C-X bond in alkyl halides: X is more electronegative than carbon. So, the C-X bond is polarized with C having a partial positive
1 Class XII Chemistry Chapter 10 Haloalkanes and Haloarenes 1. Nature of C-X bond in alkyl halides: X is more electronegative than carbon. So, the C-X bond is polarized with C having a partial positive
3.2.8 Haloalkanes. Nucleophilic Substitution. 267 minutes. 264 marks. Page 1 of 36
 3.2.8 Haloalkanes Nucleophilic Substitution 267 minutes 264 marks Page 1 of 36 Q1. (a) The equation below shows the reaction of 2-bromopropane with an excess of ammonia. CH 3 CHBrCH 3 + 2NH 3 CH 3 CH(NH
3.2.8 Haloalkanes Nucleophilic Substitution 267 minutes 264 marks Page 1 of 36 Q1. (a) The equation below shows the reaction of 2-bromopropane with an excess of ammonia. CH 3 CHBrCH 3 + 2NH 3 CH 3 CH(NH
Alkyl halides. Substitutive nomenclature. Nomenclature of Alkyl halides. Primary, Secondary and Tertiary Alkyl halides 3/12/2012
 Alkyl halides A compound with a halogen atom bonded to one of the sp 3 hybrid carbon atoms of an alkyl group + - C Primary, Secondary and Tertiary Alkyl halides Alkyl: alogen,, is directly bonded to sp3
Alkyl halides A compound with a halogen atom bonded to one of the sp 3 hybrid carbon atoms of an alkyl group + - C Primary, Secondary and Tertiary Alkyl halides Alkyl: alogen,, is directly bonded to sp3
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION
 REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
REACTIONS OF HALOALKANES - SUBSTITUTION AND ELIMINATION Haloalkanes (also known as halogenoalkanes and alkyl halides) are organic compounds where one of the hydrogens of an alkane or cycloalkane has been
MUNISH KAKAR s INSTITUTE OF CHEMISTRY
 REACTONS & PREPARATON OF HALOGENATED COMPDS. WS#3 Q1. Reagent for preparing a chloroalkane from an alcohol is (a) SOCl 2 (b) HCl/ZnCl 2 (c) PCl 3 (d) All of these Q2. n the addition of H to propene in
REACTONS & PREPARATON OF HALOGENATED COMPDS. WS#3 Q1. Reagent for preparing a chloroalkane from an alcohol is (a) SOCl 2 (b) HCl/ZnCl 2 (c) PCl 3 (d) All of these Q2. n the addition of H to propene in
Aryl Halides. Structure
 Aryl Halides Structure Aryl halides are compounds containing halogen attached directly to an aromatic ring. They have the general formula ArX, where Ar is phenyl, substituted phenyl. X= F,Cl,Br,I An aryl
Aryl Halides Structure Aryl halides are compounds containing halogen attached directly to an aromatic ring. They have the general formula ArX, where Ar is phenyl, substituted phenyl. X= F,Cl,Br,I An aryl
Indian School Muscat. Chemistry IIT - JEE
 Indian School Muscat Chemistry IIT - JEE HALOALKANES AND HALOARENES 1. Which one of the following is not formed when a mixture of methyl bromide and bromobenzene is heated with sodium metal in the presence
Indian School Muscat Chemistry IIT - JEE HALOALKANES AND HALOARENES 1. Which one of the following is not formed when a mixture of methyl bromide and bromobenzene is heated with sodium metal in the presence
A4 Alcohols form H-bonds with water due to the presence of OH group. However, hydrocarbons cannot form H-bonds with water.
 Q1 p-dichlorobenzene has higher m.p. and lower solubility than those of o- and m-isomers. Discuss. A4 Alcohols form H-bonds with water due to the presence of OH group. However, hydrocarbons cannot form
Q1 p-dichlorobenzene has higher m.p. and lower solubility than those of o- and m-isomers. Discuss. A4 Alcohols form H-bonds with water due to the presence of OH group. However, hydrocarbons cannot form
AJNISH GUPTA & BHARTI GUPTA
 Performance Booster Organic Chemistry For Board Examinations By AJNISH GUPTA & BHARTI GUPTA Professor of Organic Chemistry Copyright 2015 by Ajnish Gupta All rights reserved. No part of this publication
Performance Booster Organic Chemistry For Board Examinations By AJNISH GUPTA & BHARTI GUPTA Professor of Organic Chemistry Copyright 2015 by Ajnish Gupta All rights reserved. No part of this publication
Preparation of Alkyl Halides, R-X. Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): R + X X 2.
 Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
Preparation of Alkyl alides, R-X Reaction of alkanes with Cl 2 & Br 2 (F 2 is too reactive, I 2 is unreactive): UV R + X 2 R X or heat + X This mechanism involves a free radical chain reaction. A chain
The C-X bond gets longerand weakergoing down the periodic table.
 Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
Chapter 10: Organohalides Organic molecules containing halogen atoms (X) bonded to carbon are useful compounds in synthesis and on their own. 10.2 Structure of alkyl halides The C-X bond gets longerand
Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2
 Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2 Key Terms For Unit 3 Free Radical Chain Reaction Homolytic Cleavage Free Radical Initiation Propagation
Name: Unit 3 Packet: Activation Energy, Free Radical Chain Reactions, Alkane Preparations, S N 2, E 2 Key Terms For Unit 3 Free Radical Chain Reaction Homolytic Cleavage Free Radical Initiation Propagation
Classes of Halides. Chapter 6 Alkyl Halides: Nucleophilic Substitution and Elimination. Polarity and Reactivity. Classes of Alkyl Halides
 rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 6 Alkyl alides: Nucleophilic Substitution and Elimination lasses of alides Alkyl: alogen, X, is directly bonded to sp 3 carbon. Vinyl: X is bonded to
rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 6 Alkyl alides: Nucleophilic Substitution and Elimination lasses of alides Alkyl: alogen, X, is directly bonded to sp 3 carbon. Vinyl: X is bonded to
C 3 H 6 ClBr represent a saturated alkyl halide. The following structural isomers are possible for the given compound. Among the above 5 structural is
 Q. No. 1 In CH 3 CH 2 Br, % of Br is 80 73 70 7 Molecular mass of CH 3 CH 2 Br = 109 At Wt. of Br = 80 80 % of Br is = 100 = 73.3 109 Q. No. 2 Which of the following is primary halide Isopropyl iodide
Q. No. 1 In CH 3 CH 2 Br, % of Br is 80 73 70 7 Molecular mass of CH 3 CH 2 Br = 109 At Wt. of Br = 80 80 % of Br is = 100 = 73.3 109 Q. No. 2 Which of the following is primary halide Isopropyl iodide
I. Multiple Choice Questions (Type-I)
 Unit 13 HYDROCARBONS I. Multiple Choice Questions (Type-I) 1. Arrange the following in decreasing order of their boiling points. (A) n butane (B) 2 methylbutane (C) n-pentane (D) 2,2 dimethylpropane A
Unit 13 HYDROCARBONS I. Multiple Choice Questions (Type-I) 1. Arrange the following in decreasing order of their boiling points. (A) n butane (B) 2 methylbutane (C) n-pentane (D) 2,2 dimethylpropane A
10. Alkyl Halides. What Is an Alkyl Halide. An organic compound containing at least one carbonhalogen
 10. Alkyl Halides What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain many C-X bonds Properties and some uses Fire-resistant
10. Alkyl Halides What Is an Alkyl Halide An organic compound containing at least one carbonhalogen bond (C-X) X (F, Cl, Br, I) replaces H Can contain many C-X bonds Properties and some uses Fire-resistant
DAV CENTENARY PUBLIC SCHOOL, PASCHIM ENCLAVE, NEW DELHI - 87
 HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
HYDROCARBONS 1. Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain. 2. Alkynes on reduction with sodium in liquid ammonia
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
CHE 275 NUCLEOPHILIC SUBSTITUTUION CHAP 8 ASSIGN. 1. Which best depicts the partial charges on methyl bromide and sodium methoxide?
 CHE 275 NUCLEPHILIC SUBSTITUTUIN CHAP 8 ASSIGN 1. Which best depicts the partial charges on methyl bromide and sodium methoxide? 2. Which of the following would be the best (most reactive) nucleophile
CHE 275 NUCLEPHILIC SUBSTITUTUIN CHAP 8 ASSIGN 1. Which best depicts the partial charges on methyl bromide and sodium methoxide? 2. Which of the following would be the best (most reactive) nucleophile
When 2-bromopentane reacts with ethanolic KOH, two structurally isomeric alkenes are formed.
 Q1. Organic reaction mechanisms help chemists to understand how the reactions of organic compounds occur. The following conversions illustrate a number of different types of reaction mechanism. (a) When
Q1. Organic reaction mechanisms help chemists to understand how the reactions of organic compounds occur. The following conversions illustrate a number of different types of reaction mechanism. (a) When
Chapter 8. Substitution reactions of Alkyl Halides
 Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
1. CONCEPTS IN ORGANIC CHEMISTRY 2. SYNTHETIC ORGANIC CHEMISTRY 3. ISOMERISM II 4. HYDROCARBONS II 5. HALOALKANES. Vikasana - CET 2012
 CET OBJECTIVE QUESTION ON 1. CONCEPTS IN ORGANIC CHEMISTRY 2. SYNTHETIC ORGANIC CHEMISTRY 3. ISOMERISM II 4. HYDROCARBONS II 5. HALOALKANES 1.The inductive effect a. Implies the atoms ability to cause
CET OBJECTIVE QUESTION ON 1. CONCEPTS IN ORGANIC CHEMISTRY 2. SYNTHETIC ORGANIC CHEMISTRY 3. ISOMERISM II 4. HYDROCARBONS II 5. HALOALKANES 1.The inductive effect a. Implies the atoms ability to cause
CHAPTER 7. Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination
 CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
CHAPTER 7 Further Reactions of Haloalkanes: Unimolecular Substitution and Pathways of Elimination 7-1 Solvolysis of Tertiary and Secondary Haloalkanes The rate of S N 2 reactions decrease dramatically
CHE1502. Tutorial letter 201/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry CHE1502/201/1/2016
 CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions
 Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Chapter 6: Organic Halogen Compounds; Substitution and Elimination Reactions Halogen compounds are important for several reasons. Simple alkyl and aryl halides, especially chlorides and bromides, are versatile
Organic Chemistry. Unit 10
 Organic Chemistry Unit 10 Halides Primary Carbons Secondary Carbons Tertiary Carbons IMPORTANCE?? REACTIONS!! Benzene C6H6 Aromatic functional group - C6H5 (IUPAC name - phenyl) Substitution Reactions
Organic Chemistry Unit 10 Halides Primary Carbons Secondary Carbons Tertiary Carbons IMPORTANCE?? REACTIONS!! Benzene C6H6 Aromatic functional group - C6H5 (IUPAC name - phenyl) Substitution Reactions
1. CONCEPTS IN ORGANIC CHEMISTRY 2. SYNTHETIC ORGANIC CHEMISTRY 3. ISOMERISM II 4. HYDROCARBONS II 5. HALOALKANES. Vikasana - CET 2012
 CET OBJECTIVE QUESTION ON 1. CONCEPTS IN ORGANIC CHEMISTRY 2. SYNTHETIC ORGANIC CHEMISTRY 3. ISOMERISM II 4. HYDROCARBONS II 5. HALOALKANES 1.The inductive effect a. Implies the atoms ability to cause
CET OBJECTIVE QUESTION ON 1. CONCEPTS IN ORGANIC CHEMISTRY 2. SYNTHETIC ORGANIC CHEMISTRY 3. ISOMERISM II 4. HYDROCARBONS II 5. HALOALKANES 1.The inductive effect a. Implies the atoms ability to cause
Organic Chemistry, 7 L. G. Wade, Jr. Chapter , Prentice Hall
 Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds 2010, Prentice Hall Electrophilic Aromatic Substitution Although h benzene s pi electrons are in a stable aromatic
Organic Chemistry, 7 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds 2010, Prentice Hall Electrophilic Aromatic Substitution Although h benzene s pi electrons are in a stable aromatic
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
Basic Organic Chemistry Course code : CHEM (Pre-requisites : CHEM 11122)
 Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Basic Organic Chemistry Course code : CHEM 12162 (Pre-requisites : CHEM 11122) Chapter 01 Mechanistic Aspects of S N2,S N1, E 2 & E 1 Reactions Dr. Dinesh R. Pandithavidana Office: B1 222/3 Phone: (+94)777-745-720
Homework problems Chapters 6 and Give the curved-arrow formalism for the following reaction: CH 3 OH + H 2 C CH +
 omework problems hapters 6 and 7 1. Give the curved-arrow formalism for the following reaction: : 3 - : 2 : 3 2-3 3 2. In each of the following sets, arrange the compounds in order of decreasing pka and
omework problems hapters 6 and 7 1. Give the curved-arrow formalism for the following reaction: : 3 - : 2 : 3 2-3 3 2. In each of the following sets, arrange the compounds in order of decreasing pka and
Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat #
 Test 4 Name Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat # Part 1. Multiple Choice. Choose the one best answer, and clearly mark that answer
Test 4 Name Chemistry 2321 Last name, First name (please print) April 22, 2008 McMurry, Chapters 10 and 11 Row # Seat # Part 1. Multiple Choice. Choose the one best answer, and clearly mark that answer
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
Chapter 17. Reactions of Aromatic Compounds
 Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Although benzene s pi electrons are in a stable aromatic system, they are available to attack a strong electrophile to give
Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Although benzene s pi electrons are in a stable aromatic system, they are available to attack a strong electrophile to give
AMINES. 3. Secondary When two hydrogen atoms are replaced by two alkyl or aryl groups.
 AMINES Amine may be regarded as derivative of ammonia formed by replacement of one or more hydrogen atoms by corresponding number of alkyl or aryl group CLASSIFICATION 1. Ammonia 2. Primary amine 3. Secondary
AMINES Amine may be regarded as derivative of ammonia formed by replacement of one or more hydrogen atoms by corresponding number of alkyl or aryl group CLASSIFICATION 1. Ammonia 2. Primary amine 3. Secondary
ALCOHOLS AND PHENOLS
 ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
Haloalkanes. Isomers: Draw and name the possible isomers for C 5 H 11 Br
 Haloalkanes The basics: The functional group is a halogen atom: F, Cl, Br or I General formula C n H 2n+1 X Use the prefixes: fluoro, chloro, bromo and iodo. Isomers: Draw and name the possible isomers
Haloalkanes The basics: The functional group is a halogen atom: F, Cl, Br or I General formula C n H 2n+1 X Use the prefixes: fluoro, chloro, bromo and iodo. Isomers: Draw and name the possible isomers
Class XI Chapter 13 Hydrocarbons Chemistry
 Question 13.1: How do you account for the formation of ethane during chlorination of methane? Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the
Question 13.1: How do you account for the formation of ethane during chlorination of methane? Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the
Essential Organic Chemistry. Chapter 9
 Essential Organic Chemistry Paula Yurkanis Bruice Chapter 9 Substitution and Elimination Reactions of Alkyl Halides 9.1 How Alkyl Halides React Substitution Reactions One group takes the place of another.
Essential Organic Chemistry Paula Yurkanis Bruice Chapter 9 Substitution and Elimination Reactions of Alkyl Halides 9.1 How Alkyl Halides React Substitution Reactions One group takes the place of another.
Organic Chemistry. M. R. Naimi-Jamal. Faculty of Chemistry Iran University of Science & Technology
 Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 7-1. Alkyl Halides Based on McMurry s Organic Chemistry, 6 th edition What Is an Alkyl Halide? An
Organic Chemistry M. R. Naimi-Jamal Faculty of Chemistry Iran University of Science & Technology Chapter 7-1. Alkyl Halides Based on McMurry s Organic Chemistry, 6 th edition What Is an Alkyl Halide? An
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
Class XII Chapter 11 Alcohols Phenols and Ethers Chemistry. Question 11.1: Write IUPAC names of the following compounds: (i) (ii) (iii) (iv) (v) (vi)
 Question 11.1: Write IUPAC names of the following compounds: (iii) (iv) (v) (vi) (vii) (viii) Page 1 of 37 (ix) (x) (xi) (xii) 2, 2, 4-Trimethylpentan-3-ol 5-Ethylheptane-2, 4-diol (iii) Butane-2, 3-diol
Question 11.1: Write IUPAC names of the following compounds: (iii) (iv) (v) (vi) (vii) (viii) Page 1 of 37 (ix) (x) (xi) (xii) 2, 2, 4-Trimethylpentan-3-ol 5-Ethylheptane-2, 4-diol (iii) Butane-2, 3-diol
Alkyl Halides. Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp 3 orbital of an alkyl group.
 Alkyl Halides Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp 3 orbital of an alkyl group. CHCl 3 (Chloroform: organic solvent) CF 2 Cl 2 (Freon-12: refrigerant
Alkyl Halides Alkyl halides are a class of compounds where a halogen atom or atoms are bound to an sp 3 orbital of an alkyl group. CHCl 3 (Chloroform: organic solvent) CF 2 Cl 2 (Freon-12: refrigerant
Reactions of Haloalkanes
 Reactions of aloalkanes N Goalby chemrevise.org aloalkanes ontain a halogen atom covalently bonded to a carbon atom General formula: R-X where X is a halogen atom (F, l,, I) and R is the carbon chain.
Reactions of aloalkanes N Goalby chemrevise.org aloalkanes ontain a halogen atom covalently bonded to a carbon atom General formula: R-X where X is a halogen atom (F, l,, I) and R is the carbon chain.
Nucleophilic Substitution Synthesis of 1-Iodobutane.
 Nucleophilic Substitution Synthesis of 1-Iodobutane Nucleophilic Substitution of Alkyl alides: Synthesis of 1-Iodobutane C 4 9 Br MW 137.02 d 1.28 g/ml BP 100-104 o C Br + NaI I + NaBr 1-Bromobutane is
Nucleophilic Substitution Synthesis of 1-Iodobutane Nucleophilic Substitution of Alkyl alides: Synthesis of 1-Iodobutane C 4 9 Br MW 137.02 d 1.28 g/ml BP 100-104 o C Br + NaI I + NaBr 1-Bromobutane is
Lesmahagow High School CfE Advanced Higher Chemistry
 Lesmahagow High School AHChemistry Organic Chemistry& Instrumental Analysis Lesmahagow High School CfE Advanced Higher Chemistry Unit 2 Organic Chemistry and Instrumental Analysis Alkanes, Alkenes and
Lesmahagow High School AHChemistry Organic Chemistry& Instrumental Analysis Lesmahagow High School CfE Advanced Higher Chemistry Unit 2 Organic Chemistry and Instrumental Analysis Alkanes, Alkenes and
Test Date: (Sunday) Test Time: 1:00 pm to 2:00 pm Test Venue: Lajpat Bhawan, Madhya Marg, Sector 15-B, Chandigarh
 Test ate: 1.09.015 (Sunday) Test Time: 1:00 pm to :00 pm Test Venue: Lajpat hawan, Madhya Marg, Sector 15-, handigarh r. Sangeeta Khanna Ph. 1 EMISTRY OING IRLE G:\+ Grand test-4 L-1.doc r. Sangeeta Khanna
Test ate: 1.09.015 (Sunday) Test Time: 1:00 pm to :00 pm Test Venue: Lajpat hawan, Madhya Marg, Sector 15-, handigarh r. Sangeeta Khanna Ph. 1 EMISTRY OING IRLE G:\+ Grand test-4 L-1.doc r. Sangeeta Khanna
Chapter 1 Reactions of Organic Compounds. Reactions Involving Hydrocarbons
 Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 12. Reactions of Arenes: Electrophilic Aromatic Substitution. Class Notes. A. The method by which substituted benzenes are synthesized
 Chapter 12 Reactions of Arenes: Electrophilic Aromatic Substitution Chapter 12 suggested problems: 22, 23, 26, 27, 32, 33 Class Notes I. Electrophilic aromatic substitution reactions A. The method by which
Chapter 12 Reactions of Arenes: Electrophilic Aromatic Substitution Chapter 12 suggested problems: 22, 23, 26, 27, 32, 33 Class Notes I. Electrophilic aromatic substitution reactions A. The method by which
Mechanisms. . CCl2 F + Cl.
 Mechanisms 1) Free radical substitution Alkane à halogenoalkane Initiation: Propagation: Termination: Overall: 2) Ozone depletion UV light breaks the C Cl bond releasing chlorine radical CFCl 3 F à. CCl2
Mechanisms 1) Free radical substitution Alkane à halogenoalkane Initiation: Propagation: Termination: Overall: 2) Ozone depletion UV light breaks the C Cl bond releasing chlorine radical CFCl 3 F à. CCl2
General A haloalkane is a compound in which one or more H atoms of an alkane are replaced by halogen atoms.
 aloalkanes General A haloalkane is a compound in which one or more atoms of an alkane are replaced by halogen atoms. If one hydrogen atom is replaced, the general formula is n 2n+1 X where X = F, l, Br
aloalkanes General A haloalkane is a compound in which one or more atoms of an alkane are replaced by halogen atoms. If one hydrogen atom is replaced, the general formula is n 2n+1 X where X = F, l, Br
Organic Chemistry Lecture 2 - Hydrocarbons, Alcohols, Substitutions
 ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
ALKANES Water-insoluble, low density C-C single bonds Higher MW -> higher BP, higher MP Branching -> lower BP, higher MP Forms cycloalkanes which can have ring strain Cyclohexane: chair vs. boat configuration
Chapter 11, Part 1: Polar substitution reactions involving alkyl halides
 hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
hapter 11, Part 1: Polar substitution reactions involving alkyl halides Overview: The nature of alkyl halides and other groups with electrophilic sp 3 hybridized leads them to react with nucleophiles and
Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 1 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen
Chapter 15 1 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen
12/27/2010. Chapter 15 Reactions of Aromatic Compounds
 Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Chapter 15 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Arene (Ar-H) is the generic term for an aromatic hydrocarbon The aryl group (Ar) is derived by removal of a hydrogen atom
Organic Chemistry. REACTIONS Grade 12 Physical Science Mrs KL Faling
 Organic Chemistry REACTIONS Grade 12 Physical Science Mrs KL Faling SUBSTITUTION REACTIONS This is a reaction where an atom or group of atoms is replaced by another atom or group of atoms Substitution
Organic Chemistry REACTIONS Grade 12 Physical Science Mrs KL Faling SUBSTITUTION REACTIONS This is a reaction where an atom or group of atoms is replaced by another atom or group of atoms Substitution
Lecture Topics: I. Electrophilic Aromatic Substitution (EAS)
 Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Reactions of Aromatic Compounds Reading: Wade chapter 17, sections 17-1- 17-15 Study Problems: 17-44, 17-46, 17-47, 17-48, 17-51, 17-52, 17-53, 17-59, 17-61 Key Concepts and Skills: Predict and propose
Haloalkanes and Haloar
 Unit 10 Objectives After studying this Unit, you will be able to name haloalkanes and haloarenes according to the IUPAC system of nomenclature from their given structures; describe the reactions involved
Unit 10 Objectives After studying this Unit, you will be able to name haloalkanes and haloarenes according to the IUPAC system of nomenclature from their given structures; describe the reactions involved
HALOALKANES (HALOGENOALKANES) Structure Contain the functional group C-X where X is a halogen (F, Cl, Br or I)
 alogenoalkanes 1 ALOALKANES (ALOGENOALKANES) Structure ontain the functional group X where X is a halogen (F, l, or I) Types aloalkanes halogen is attached to an aliphatic skeleton alkyl group aloarenes
alogenoalkanes 1 ALOALKANES (ALOGENOALKANES) Structure ontain the functional group X where X is a halogen (F, l, or I) Types aloalkanes halogen is attached to an aliphatic skeleton alkyl group aloarenes
7. Haloalkanes (text )
 2009, Department of hemistry, The University of Western Ontario 7.1 7. aloalkanes (text 7.1 7.10) A. Structure and Nomenclature Like hydrogen, the halogens have a valence of one. Thus, a halogen atom can
2009, Department of hemistry, The University of Western Ontario 7.1 7. aloalkanes (text 7.1 7.10) A. Structure and Nomenclature Like hydrogen, the halogens have a valence of one. Thus, a halogen atom can
HALOGENOALKANES (HALOALKANES) Structure Contain the functional group C-X where X is a halogen (F,Cl,Br or I)
 aloalkanes 2812 1 ALOGENOALKANES (ALOALKANES) Structure ontain the functional group X where X is a halogen (F,l, or I) Types alogenoalkanes halogen is attached to an aliphatic skeleton alkyl group aloarenes
aloalkanes 2812 1 ALOGENOALKANES (ALOALKANES) Structure ontain the functional group X where X is a halogen (F,l, or I) Types alogenoalkanes halogen is attached to an aliphatic skeleton alkyl group aloarenes
CHAPTER HYDROCARBONS. Chapterwise Previous year Qs. (a) Na (b) HCl in H2O (c) KOH in C2H5OH (d) Zn in alcohol. Ans: (c)
 122 CHAPTER HYDROCARBONS 1. Acetylenic hydrogens are acidic because [1989] Sigma electron density of C Hbond in acetylene is nearer to carbon, which has 50% s- character Acetylene has only open hydrogen
122 CHAPTER HYDROCARBONS 1. Acetylenic hydrogens are acidic because [1989] Sigma electron density of C Hbond in acetylene is nearer to carbon, which has 50% s- character Acetylene has only open hydrogen
Aromatic Compounds II
 2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
2302272 Org Chem II Part I Lecture 2 Aromatic Compounds II Instructor: Dr. Tanatorn Khotavivattana E-mail: tanatorn.k@chula.ac.th Recommended Textbook: Chapter 17 in Organic Chemistry, 8 th Edition, L.
HALO ALKANES AND HALO ARENES
 HALO ALKANES AND HALO ARENES Halo alkanes and Halo Arenes find wide applications in industry as well as in day-to-day life. They are used as solvents for relatively non-polar compounds and as starting
HALO ALKANES AND HALO ARENES Halo alkanes and Halo Arenes find wide applications in industry as well as in day-to-day life. They are used as solvents for relatively non-polar compounds and as starting
Paper Reference. Advanced Unit Test 6B (Synoptic) Thursday 18 June 2009 Morning Time: 1 hour 30 minutes
 Centre No. Paper Reference Surname Initial(s) Candidate No. 6 2 4 6 0 2 Signature Paper Reference(s) 6246/02 Edexcel GCE Chemistry Examiner s use only Team Leader s use only Advanced Unit Test 6B (Synoptic)
Centre No. Paper Reference Surname Initial(s) Candidate No. 6 2 4 6 0 2 Signature Paper Reference(s) 6246/02 Edexcel GCE Chemistry Examiner s use only Team Leader s use only Advanced Unit Test 6B (Synoptic)
PAPER No. 5: REACTION MECHANISM MODULE No. 2: Types of Organic Reaction Mechanisms
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper No. 5:Organic Chemistry-II Module No. 2: Overview of different types of Organic Reaction Mechanisms CHE_P5_M2 TABLE OF CONTENTS
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper No. 5:Organic Chemistry-II Module No. 2: Overview of different types of Organic Reaction Mechanisms CHE_P5_M2 TABLE OF CONTENTS
11. Nucleophilic Substitution Reactions
 11. Nucleophilic Substitution Reactions A. Introduction It would be beneficial if you review the chapter on substitution reactions in your textbook prior to lab. This is Ch. 11 in the 9 th edition McMurry
11. Nucleophilic Substitution Reactions A. Introduction It would be beneficial if you review the chapter on substitution reactions in your textbook prior to lab. This is Ch. 11 in the 9 th edition McMurry
TOPIC 13 ANSWERS & MARK SCHEMES QUESTIONSHEET 1 ALKANES
 QUESTIONSHEET 1 ALKANES a) (i) UV light / temperatures > 500 ºC (ii) CH 4 (g) + Cl 2 (g) Cl(g) + HCl(g) Cl(g) + Cl 2 (g) Cl 2 (l) + HCl(g) Cl 2 (l) + Cl 2 (g) CHCl 3 (l) + HCl(g) CHCl 3 (l) + Cl 2 (g)
QUESTIONSHEET 1 ALKANES a) (i) UV light / temperatures > 500 ºC (ii) CH 4 (g) + Cl 2 (g) Cl(g) + HCl(g) Cl(g) + Cl 2 (g) Cl 2 (l) + HCl(g) Cl 2 (l) + Cl 2 (g) CHCl 3 (l) + HCl(g) CHCl 3 (l) + Cl 2 (g)
Chapter 10 Radical Reactions"
 Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
Downloaded from
 1 Class XII Chemistry Chapter: Alcohols, Phenols And Ethers Top concepts: 1. Structure of alcohols, phenols and ethers: 2. Preparation of alcohols: 3. Preparation of phenols: 2 4. Physical properties of
1 Class XII Chemistry Chapter: Alcohols, Phenols And Ethers Top concepts: 1. Structure of alcohols, phenols and ethers: 2. Preparation of alcohols: 3. Preparation of phenols: 2 4. Physical properties of
dihalogenoalkane H 2, Nickel Catalyst KOH alcoholic HBr, HCl Br Cl Elimination KOH aqueous heat under reflux Nucleophilic substitution
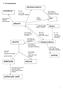 7 AS mechanisms dihalogenoalkane poly(alkene) Br 2, 2 KO aqueous room temp Electrophilic addition heat under reflux Nucleophilic substitution high pressure atalyst polymerization alkene KMnO 4 oxidation
7 AS mechanisms dihalogenoalkane poly(alkene) Br 2, 2 KO aqueous room temp Electrophilic addition heat under reflux Nucleophilic substitution high pressure atalyst polymerization alkene KMnO 4 oxidation
Class XII: Chemistry Chapter 13: Amines Top concepts
 Class XII: Chemistry Chapter 13: Amines Top concepts 1. Amines are regarded as derivatives of ammonia in which one, two or all three hydrogen atoms are replaced by alkyl or aryl group 2. Classification
Class XII: Chemistry Chapter 13: Amines Top concepts 1. Amines are regarded as derivatives of ammonia in which one, two or all three hydrogen atoms are replaced by alkyl or aryl group 2. Classification
ST. JOSEPH S COLLEGE OF ARTS & SCIENCE (AUTONOMOUS) ST. JOSEPH S COLLEGE ROAD, CUDDALORE CH101T ORGANIC CHEMISTRY I (SEMESTER-I)
 UNIT I 1. The hybridization involved in the formation of acetylene is a) sp b) sp 2 c) sp 3 d) sp 3 d 2. The IUPAC name of is 1. 3-hexene b) 4-hexene c) 3-hexyne d) 4-hexyne 3. -------- is the type of
UNIT I 1. The hybridization involved in the formation of acetylene is a) sp b) sp 2 c) sp 3 d) sp 3 d 2. The IUPAC name of is 1. 3-hexene b) 4-hexene c) 3-hexyne d) 4-hexyne 3. -------- is the type of
18.8 Oxidation. Oxidation by silver ion requires an alkaline medium
 18.8 Oxidation Oxidation by silver ion requires an alkaline medium Test for detecting aldehydes Tollens reagent to prevent precipitation of the insoluble silver oxide, a complexing agent is added: ammonia
18.8 Oxidation Oxidation by silver ion requires an alkaline medium Test for detecting aldehydes Tollens reagent to prevent precipitation of the insoluble silver oxide, a complexing agent is added: ammonia
Haloalkanes and Haloar
 Unit 10 Objectives After studying this Unit, you will be able to name haloalkanes and haloarenes according to the IUPAC system of nomenclature from their given structures; describe the reactions involved
Unit 10 Objectives After studying this Unit, you will be able to name haloalkanes and haloarenes according to the IUPAC system of nomenclature from their given structures; describe the reactions involved
 Question 13.1: Classify the following amines as primary, secondary or tertiary: (i) (ii) (iii) (C 2 H 5 ) 2 CHNH 2 (iv) (C 2 H 5 ) 2 NH Primary: (i) and (iii) Secondary: (iv) Tertiary: (ii) Question 13.2:
Question 13.1: Classify the following amines as primary, secondary or tertiary: (i) (ii) (iii) (C 2 H 5 ) 2 CHNH 2 (iv) (C 2 H 5 ) 2 NH Primary: (i) and (iii) Secondary: (iv) Tertiary: (ii) Question 13.2:
BENZENE & AROMATIC COMPOUNDS
 BENZENE & AROMATIC COMPOUNDS Dr. Zainab M Almarhoon 2 Learning Objectives By the end of chapter four the students will: Understand the resonance description of structure of benzene Understand the hybridization
BENZENE & AROMATIC COMPOUNDS Dr. Zainab M Almarhoon 2 Learning Objectives By the end of chapter four the students will: Understand the resonance description of structure of benzene Understand the hybridization
Topic 6 Alkyl halide and carbonyl compounds Organic compounds containing a halogen
 Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene (aryl
Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene (aryl
Topic 6 Alkyl halide and carbonyl compounds Organic compounds containing a halogen
 Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way, eg: 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene
Topic 6 Alkyl halide and carbonyl compounds rganic compounds containing a halogen ompounds are named in standard way, eg: 3 1 3 3 2 3 2-iodo-2-methylpropane (tertiary alkyl halide) l 3 4-chlorotoluene
Chapter 22 Amines. Nomenclature Amines are classified according to the degree of substitution at nitrogen.
 CH. 22 Chapter 22 Amines Amines are very important in biological chemistry. Most of the bases in biological acid-base reactions are amines. They are also very important nucleophiles in biochemical reactions.
CH. 22 Chapter 22 Amines Amines are very important in biological chemistry. Most of the bases in biological acid-base reactions are amines. They are also very important nucleophiles in biochemical reactions.
William H. Brown & Christopher Foote
 William H. Brown & Christopher Foote Requests for permission to make copies of any part of the work should be mailed to:permissions Department, Harcourt Brace & Company, 6277 Sea Harbor Drive, Orlando,
William H. Brown & Christopher Foote Requests for permission to make copies of any part of the work should be mailed to:permissions Department, Harcourt Brace & Company, 6277 Sea Harbor Drive, Orlando,
HALOGENOALKANES (HALOALKANES) Structure Contain the functional group C-X where X is a halogen (F, Cl, Br or I)
 alogenoalkanes F322 1 ALOGENOALKANES (ALOALKANES) Structure ontain the functional group X where X is a halogen (F, l, or I) Types alogenoalkanes halogen is attached to an aliphatic skeleton alkyl group
alogenoalkanes F322 1 ALOGENOALKANES (ALOALKANES) Structure ontain the functional group X where X is a halogen (F, l, or I) Types alogenoalkanes halogen is attached to an aliphatic skeleton alkyl group
Chemistry 2.5 AS WORKBOOK. Working to Excellence Working to Excellence
 Chemistry 2.5 AS 91165 Demonstrate understanding of the properties of selected organic compounds WORKBOOK Working to Excellence Working to Excellence CONTENTS 1. Writing Excellence answers to Cis-Trans
Chemistry 2.5 AS 91165 Demonstrate understanding of the properties of selected organic compounds WORKBOOK Working to Excellence Working to Excellence CONTENTS 1. Writing Excellence answers to Cis-Trans
ALKYNE, AROMATIC HYDROCARBONS OR ARENES ALKYNES. Alkynes are unsaturated hydrocarbon with at least one triple bond between two carbon atoms ( C = C)
 ALKYNES Alkynes are unsaturated hydrocarbon with at least one triple bond between two carbon atoms ( C = C) Their general formula is Cn H2n-2 They are also called as a acetylenes STRUCTURE OF ALKYNES Each
ALKYNES Alkynes are unsaturated hydrocarbon with at least one triple bond between two carbon atoms ( C = C) Their general formula is Cn H2n-2 They are also called as a acetylenes STRUCTURE OF ALKYNES Each
Chemistry of Benzene: Electrophilic Aromatic Substitution
 Chemistry of Benzene: Electrophilic Aromatic Substitution Why this Chapter? Continuation of coverage of aromatic compounds in preceding chapter focus shift to understanding reactions Examine relationship
Chemistry of Benzene: Electrophilic Aromatic Substitution Why this Chapter? Continuation of coverage of aromatic compounds in preceding chapter focus shift to understanding reactions Examine relationship
Reaction of alkanes with bromine / chlorine in UV light. This is the overall reaction, but a more complex mixture of products is actually formed
 8. The aloalkanes opyright N Goalby Bancroft's School Synthesis of chloroalkanes Reaction of alkanes with bromine / chlorine in UV light In the presence of UV light alkanes react with chlorine to form
8. The aloalkanes opyright N Goalby Bancroft's School Synthesis of chloroalkanes Reaction of alkanes with bromine / chlorine in UV light In the presence of UV light alkanes react with chlorine to form
