ECN-144 Nov /027/ pdf
|
|
|
- Jayson Barrie Griffin
- 5 years ago
- Views:
Transcription
1 ECN-144 Nov /027/ pdf RADIATION DOSE DEPOSITION AND ENERGY ACCUMULATION IN A ROCK SALT WASTE REPOSITORY BY J. BERGSMA RJ. HIJBOER ECN does not assume any liability with respect to the use of, or for damages resulting from the use of any information, apparatus, method or process disclosed in this document. CONTENTS 1. INTRODUCTION 7 2. IRRADIATION EFFECTS IN ALKALI HALIDES 9 3. CALCULATION OF GAMMA DOSIS AND ENERGY STORAGE General description Calculation of the radioactive source Calculations of the energy deposition in the salt The cases considered Calculation of energy storage CONCLUDING REMARKS 23 REFERENCES 24 FIGURE LEGEND IRRADIATION EFFECTS IN ALKALI HALIDES Mechanisms of defect creation by the irradiation of alkali halides and of their annealing afterwards have been subject of many theoretical and experimental studies l2,13j during the last decade. Such studies became of technological interest when geological rock salt formations were considered for use as nuclear waste repository medium. It is in view of such applications that the presently available knowledge and information will be briefly reviewed. Based on this information calculated values for irradiation dose deposition will be used to estimate such effects as build-up of stored energy and formation of colloidal alkali metal. It seems to be evident that a relation exists between these two effects although this may not be a simple one. Most experimental investigations have been carried out by means of high energy electron beams from an accelerator facility or by means of gamma radiation or X-rays. It is important to understand that the effects in the alkali halide structure of gamma-ray produced Compton and
2 photo-electric recoil electrons are very similar to those of highenergy electrons. Of course, the transmission properties through the material are very much different for the various types of radiation. In a study of stored energy effects Delgado and Alvarez Rivas [6] pointed out that there may occur appreciable quantitative differences in defect creation by gamma radiation and high energy electrons and for the latter there are very noticeable differences if the electron energy has different values. Considering processes of irradiation damage, different stages of effects should be distinguished. The primary effect of electron or gamma radiation penetrating into the alkali halide lattice is ionization, i.e. the formation of a free electron and an electron hole. In many cases they will quickly afterwards recombine leaving an undamaged lattice in which the energy will be released as heat. In other cases the ionization products will be trapped in the lattice. Due to this ionization the chlorine anions in sodium chloride may become positively charged and are then easily expelled from their lattice positions. This may give rise to two effects of irradiations. (1) The anion vacancy will catch an electron thus forming an F-centre and (2) the chlorine ion will occupy an interstitional position. Associating with another chlorine ion located at a lattice site it can form a pair of chlorine ions now located around the lattice position and aligning its molecular axis either along the direction [110] or [111]. Defects in the form of such molecular centres are generally characterized as H-centres [14]. Depending on temperature, impurities and dislocation density both the F- centres and the H-centres may become mobile and then give rise either to the formation of conglomerates or to annealing and restoring of the original lattice. (1) Conglomeration of F-centres will lead to local regions with excess of sodium ions and if such regions become sufficiently large it is probable that due to gradually changed atom potentials the electron energy bands will be rearranged according to a scheme for sodium metal. In that case one may speak of particles of colloidal sodium. (2) Similarly a mechanism of coagulation of chlorine molecular centres may be expected, giving rise to minor chlorine bubbles. Although the processes for the primary defect creation are now reasonably well understood, the complexity of secondary processes is still such that no unified description has been accomplished.
3 With the purpose of making estimates of the build-up of stored energy due to radiation damage and the possible hazards of this phenonenon when using salt mines as waste repositories, an experimental programme was carried out at Oak Ridge National Laboratory by Jenks and Bopp 3,4,5. They measured the stored energy build-up in rock salt specimens from a variety of sources and irradiated by gamma-radiation to several dose values and they studied the kinetics of the defect creation of the simultaneously occurring back-reaction and a following annealing process. It is important to note that they carried out their investigations in the range of dose levels that will be encountered in rock salt directly surrounding waste containers in a repository. Their observations and conclusions can be summarized as follows. The rate of formation of stored radiation energy can be expressed by de/dt = k1 I (2.1) Here E is the amount of stored energy, I is the dose rate and k1 is the formation constant. Taking into account a term for radiation induced annealing the expression becomes de/dt = k1 l - k2 I E (2.2) where k2 is an annealing constant. The formation term k1 I can be understood on the basis of defectformation as has been described above. The form of the back-reaction term can be explained if it is assumed that a fraction of the freshly formed primary radiation defects react with existing defects to form undamaged NaCl. That fraction is proportional to the number of defects already present in the crystal. The reaction constant k2 varies with temperature since the back reaction term in expression (2.2) depends on the state of aggregation of these defects. At lower temperatures the defects will be more in the form of F-centres whereas at higher temperatures they will have coagulated into colloidal sodium. It is evident that mobilities associated with the two states of defects will be different. Expression (2.2) leads to the integral E = k1/k2 (1-exp(-k2 D)) + Eo exp(-k2 D). (2.3) Here D is the collected radiation dose D = Integral(I dt) and Eo is the stored energy at zero time, which usually can be taken equal to zero. Expression (2.3) shows that this phenomenology leads to a saturation for the amount of stored energy.
4 At temperatures above 150 C the annealing apparently occurs according to an additional term in equation (2.2), which turned out to be of zerokinetic order: de/dt = -K. This means that the rate of annealing is independent of the amount of stored energy. This is a rather rarely occurring process, which can be explained by assuming a rapidly adjusting equilibrium between coagulated and single defects, e.g. colloidal sodium and F-centres. The rate determining step is some kind of an "evaporation" process due to which the concentration of evaporated single defects does not depend on the amount of conglomerated defects. This concentration will be maintained at a constant value until e.g. the colloidal sodium has disappeared. The evaporation process is thermally activated and consequently the single defect concentration is temperature dependent. For temperatures significantly higher than 150 C the effect of this annealing process is such that build-up of stored energy can be neglected. With this additional process taken into account expression (2.2) becomes de/dt = k1 l - k2 I E - K (2.4) and by integration E = (k1 - (K/I)) / k2 (l - exp(-k2 D)). (2.5) Here the zero-time stored energy has been neglected. The saturation level associated with expression (2.5) depends strongly on the temperature regime. Between 100 and 150 C it amounts to about 75 J/g and below 100 C it rises to 245 J/g. Assuming a stored energy of 4.25 ev per defect pair l2 these saturation values correspond to a defect concentration of 1.1% and 3.5% mole fraction. Lidiard 12 pointed out that such large concentrations can only be retained by colloids since F-centre concentrations saturate at much lower values. However, he argues that according to the Jain-Lidiard theory 7 the rate of energy release is not compatible with the evaporation of F- centres from colloids being the rate-determining step, but that it is rather the recombination of F-centres with molecular centres. This can only be a valid interpretation if the molecular centres are clustered together, because of the zero-order character of the reaction. The F-centres apparently maintain an equilibrium by relatively rapid evaporation from colloids. In agreement with the above-mentioned theory it was found by Jenks
5 and Bopp [4]: i) that the formation of stored energy in the dose regime when saturation may be reached is insensitive for dose rate and ii) that beyond a maximum temperature and a minimum dose rate the build-up of stored energy is negligible. The measurements of the energy release were confirmed by chemical analysis determining the amounts of hydrogen and Cl-, that were formed by solution of specimens in water CALCULATION OF ENERGY STORAGE Based on the calculations of radiation dose deposition in rock salt around radioactive waste containers it has been attempted to make estiå0ö2 mates of the build-up of energy storage. It has been pointed out in chapter 2 that this energy build-up is strongly dependent on the temå0ö2 perature of the rock salt. For the configurations that were considered and that have been specified in table 3.1. the development of temperå0ö2 ature with time has been calculated at several distances from the waste containers. These calculations show that in only a few configurations the material directly surrounding a container will reach temperatures above 150 ß and then only during a limited period of time. (*) Therefore, it has been assumed that the zero-order annealing process according to the third term in expression (2.4) may be neglected. In the few cases in which this process may be active it will have the effect to decrease both the build-up of stored energy and the formation of colloidal sodium. (*) The calculations have been carried out according to expressions (2.2) and (2.3) with the assumption that the zero-time stored energy Eo is equal to zero. (*) From the investigations by Jenks and Bopp 4,5 it follows that the energy build-up is strongly temperature dependent. From their results the following data have been used in the calculations. For T > 100 C : k1 = 6.95 * 10^-9 J/(g rad) k2 = 9.3 * 10^-11 rad^-1 For T < 100 C : k1 = 20.9 * 10^-9 J/(g rad) k2 = 8.5 * 10^-11 rad^-1
6 ====================== REFERENCES [1] J. Hamstra, J.W.A.M. Kevenaar, Temperature calculations on different configurations for disposal of high-level reprocessing waste in a salt dome model, ECN-42 (1978) [2] P. Plousmen and G. Strickmann, Berechnung der zeitlichen und raumlichen Temperaturverteilung bei der så0ä1kularen Lagerung hochradioaktiver AbfÅ0ä1lle in SalzstÅ0ã2cken, Aachen, Rheinisch- WestfÅ0ä1lische Technische Hochschule, [3] G.H. Jenks, C.D. Bopp, ORNL-TM-4449 (1974). 4 G.H. Jenks, C.D. Bopp, ORNL-5058 (1977). [5] G.H. Jenks, E. Sonder, CD. Bopp, J.R. Walton and S. Lindenbaum, J. Chem. Phys. 79 (1975), 871. [6] L. Delgado and J.L. Alvarez Rivas, J. Phys. C. Solid State Phys. 13 (1980), [7] L. Jain and A.B. Lidiard, Phil. Mag. 35 (1977), [8] L. Delgado and J.L. Alvarez Rivas, J. Phys. C. Solid State Phys. 12 (1979), [9] P.W. Levy, K.J. Swyler, and R.W. Klaffky, Journal de Physique, suppl. 7 (1980) C [10] J.M. Loman, P.W. Levy and K.J. Swyler, Proc. 4th Math. Res. Sc. Symp. on the Scientific Basis for Nuclear Waste Management, Boston, Mass Nov [11] B. Verkerk, Energiespectrum 1 (1977), [12] A.B. Lidiard, Phil. Mag. A39 (1979), [13] C.R.A. Catlow, K.M. Diller and L.W. Hobbs, Phil. Mag. A42 (1980), [14] P.W. Levy, Encyclopedic of Physics, R.G. Lerner and G.L. Trigg, editors (Addison-Wesley, Reading Mass., 1981) pp [15] P.W. Levy, Nucl. Techn. 60 (1983), [16] M.J. Bell, ORIGEN, the ORNL isotope generation and depletion code, report ORNL-4628 (1973).
7 [17] J. Hamstra, J.W.A.M. Kevenaar, J. Prij, Proc. Conf. Underground Disposal of Radioactive wastes IAEA, Vienna 1980, [18] R.G. Jaeger et al., Engineering Compendium on Radiation Shielding, Vol.I, Shielding Fundamentals and Methods, Springer, Berlin (1968) [19] J.J. Taylor, Application of gamma-ray build-up data to shield design, WAPD-RM-217 (1954) [20] S. Buscaglione and R. Manzini, Buildup factors: Coefficients of the equation of J.J. Taylor, report ORNL-TR-80(Rev) (1965) Version: Address of this Page: Bergsma&Heijboer.pdf J. Gruber (
Radiation induced color center and colloid formation in synthetic NaCl and natural rock salt
 Radiation induced color center and colloid formation in synthetic NaCl and natural rock salt P. Levy, K. Swyler, R. Klaffky To cite this version: P. Levy, K. Swyler, R. Klaffky. Radiation induced color
Radiation induced color center and colloid formation in synthetic NaCl and natural rock salt P. Levy, K. Swyler, R. Klaffky To cite this version: P. Levy, K. Swyler, R. Klaffky. Radiation induced color
Introduction. Neutron Effects NSEU. Neutron Testing Basics User Requirements Conclusions
 Introduction Neutron Effects Displacement Damage NSEU Total Ionizing Dose Neutron Testing Basics User Requirements Conclusions 1 Neutron Effects: Displacement Damage Neutrons lose their energy in semiconducting
Introduction Neutron Effects Displacement Damage NSEU Total Ionizing Dose Neutron Testing Basics User Requirements Conclusions 1 Neutron Effects: Displacement Damage Neutrons lose their energy in semiconducting
Safety Assessment on the Storage of Irradiated Graphite Waste Produced from the Decommissioning of KRR-2
 Safety Assessment on the Storage of Irradiated Graphite Waste Produced from the Decommissioning of KRR-2 D.G. Lee, G.H. Jeong, W.Z. Oh, K.W. Lee Korea Atomic Energy Research Institute Korea ABSTRACT Irradiated
Safety Assessment on the Storage of Irradiated Graphite Waste Produced from the Decommissioning of KRR-2 D.G. Lee, G.H. Jeong, W.Z. Oh, K.W. Lee Korea Atomic Energy Research Institute Korea ABSTRACT Irradiated
Joint ICTP-IAEA Workshop on Physics of Radiation Effect and its Simulation for Non-Metallic Condensed Matter.
 2359-3 Joint ICTP-IAEA Workshop on Physics of Radiation Effect and its Simulation for Non-Metallic Condensed Matter 13-24 August 2012 Electrically active defects in semiconductors induced by radiation
2359-3 Joint ICTP-IAEA Workshop on Physics of Radiation Effect and its Simulation for Non-Metallic Condensed Matter 13-24 August 2012 Electrically active defects in semiconductors induced by radiation
RADIATION EFFECTS AND DAMAGE
 RADIATION EFFECTS AND DAMAGE The detrimental consequences of radiation are referred to as radiation damage. To understand the effects of radiation, one must first be familiar with the radiations and their
RADIATION EFFECTS AND DAMAGE The detrimental consequences of radiation are referred to as radiation damage. To understand the effects of radiation, one must first be familiar with the radiations and their
SIMULATION OF COMPETING FADING AND IRRADIATION EFFECTS IN THERMOLUMINESCENCE (TL) DOSIMETRY: 1 st ORDER KINETICS
 IMULATION OF COMPETING FADING AND IRRADIATION EFFECT IN THERMOLUMINECENCE (TL) DOIMETRY: 1 st ORDER KINETIC C.Furetta 1, J.Azorin 1, T.Rivera 1, G.Kitis 2 1. Dep.to de Fisica, Edif. T, cub. 331 Universidad
IMULATION OF COMPETING FADING AND IRRADIATION EFFECT IN THERMOLUMINECENCE (TL) DOIMETRY: 1 st ORDER KINETIC C.Furetta 1, J.Azorin 1, T.Rivera 1, G.Kitis 2 1. Dep.to de Fisica, Edif. T, cub. 331 Universidad
Determination of Photon Ambient Dose Buildup Factors for Radiological Applications for Points and Plaque Source Configurations Using MCNP5
 Determination of Photon Ambient Dose Buildup Factors for Radiological Applications for Points and Plaque Source Configurations Using MCNP5 P. Deatanyah 1, C.C. Arwui 1, S. Wotorchi- Gordon 1, H. Lawluvi
Determination of Photon Ambient Dose Buildup Factors for Radiological Applications for Points and Plaque Source Configurations Using MCNP5 P. Deatanyah 1, C.C. Arwui 1, S. Wotorchi- Gordon 1, H. Lawluvi
Modeling of charge collection efficiency degradation in semiconductor devices induced by MeV ion beam irradiation
 Modeling of charge collection efficiency degradation in semiconductor devices induced by MeV ion beam irradiation Ettore Vittone Physics Department University of Torino - Italy 1 IAEA Coordinate Research
Modeling of charge collection efficiency degradation in semiconductor devices induced by MeV ion beam irradiation Ettore Vittone Physics Department University of Torino - Italy 1 IAEA Coordinate Research
Interaction of ion beams with matter
 Interaction of ion beams with matter Introduction Nuclear and electronic energy loss Radiation damage process Displacements by nuclear stopping Defects by electronic energy loss Defect-free irradiation
Interaction of ion beams with matter Introduction Nuclear and electronic energy loss Radiation damage process Displacements by nuclear stopping Defects by electronic energy loss Defect-free irradiation
Transmissive Final Optic for Laser IFE
 Transmissive Final Optic for Laser IFE S. A. Payne, J. F. Latkowski, A. Kubota, M. J. Caturla, S. N. Dixit, and J. A. Speth Lawrence Livermore National Laboratory April 4, 2002 HAPL Program Workshop General
Transmissive Final Optic for Laser IFE S. A. Payne, J. F. Latkowski, A. Kubota, M. J. Caturla, S. N. Dixit, and J. A. Speth Lawrence Livermore National Laboratory April 4, 2002 HAPL Program Workshop General
Lecture 15: Optoelectronic devices: Introduction
 Lecture 15: Optoelectronic devices: Introduction Contents 1 Optical absorption 1 1.1 Absorption coefficient....................... 2 2 Optical recombination 5 3 Recombination and carrier lifetime 6 3.1
Lecture 15: Optoelectronic devices: Introduction Contents 1 Optical absorption 1 1.1 Absorption coefficient....................... 2 2 Optical recombination 5 3 Recombination and carrier lifetime 6 3.1
Multiscale modelling of D trapping in W
 CMS Multiscale modelling of D trapping in W Kalle Heinola, Tommy Ahlgren and Kai Nordlund Department of Physics and Helsinki Institute of Physics University of Helsinki, Finland Contents Background Plasma-wall
CMS Multiscale modelling of D trapping in W Kalle Heinola, Tommy Ahlgren and Kai Nordlund Department of Physics and Helsinki Institute of Physics University of Helsinki, Finland Contents Background Plasma-wall
Electrically active defects in semiconductors induced by radiation
 Electrically active defects in semiconductors induced by radiation Ivana Capan Rudjer Boskovic Institute, Croatia http://www.irb.hr/users/capan Outline Radiation damage Capacitance transient techniques
Electrically active defects in semiconductors induced by radiation Ivana Capan Rudjer Boskovic Institute, Croatia http://www.irb.hr/users/capan Outline Radiation damage Capacitance transient techniques
The outermost container into which vitrified high level waste or spent fuel rods are to be placed. Made of stainless steel or inert alloy.
 Glossary of Nuclear Waste Terms Atom The basic component of all matter; it is the smallest part of an element having all the chemical properties of that element. Atoms are made up of protons and neutrons
Glossary of Nuclear Waste Terms Atom The basic component of all matter; it is the smallest part of an element having all the chemical properties of that element. Atoms are made up of protons and neutrons
EEE4106Z Radiation Interactions & Detection
 EEE4106Z Radiation Interactions & Detection 2. Radiation Detection Dr. Steve Peterson 5.14 RW James Department of Physics University of Cape Town steve.peterson@uct.ac.za May 06, 2015 EEE4106Z :: Radiation
EEE4106Z Radiation Interactions & Detection 2. Radiation Detection Dr. Steve Peterson 5.14 RW James Department of Physics University of Cape Town steve.peterson@uct.ac.za May 06, 2015 EEE4106Z :: Radiation
Radiation Detection for the Beta- Delayed Alpha and Gamma Decay of 20 Na. Ellen Simmons
 Radiation Detection for the Beta- Delayed Alpha and Gamma Decay of 20 Na Ellen Simmons 1 Contents Introduction Review of the Types of Radiation Charged Particle Radiation Detection Review of Semiconductor
Radiation Detection for the Beta- Delayed Alpha and Gamma Decay of 20 Na Ellen Simmons 1 Contents Introduction Review of the Types of Radiation Charged Particle Radiation Detection Review of Semiconductor
Evaluation of Radiation Characteristics of Spent RBMK-1500 Nuclear Fuel Storage Casks during Very Long Term Storage
 SESSION 7: Research and Development Required to Deliver an Integrated Approach Evaluation of Radiation Characteristics of Spent RBMK-1500 Nuclear Fuel Storage Casks during Very Long Term Storage A. Šmaižys,
SESSION 7: Research and Development Required to Deliver an Integrated Approach Evaluation of Radiation Characteristics of Spent RBMK-1500 Nuclear Fuel Storage Casks during Very Long Term Storage A. Šmaižys,
RESULTS OF UNDERGROUND DOSE RATES USING TL DETECTORS AND PRELIMINARY TL CHARACTERISTICS OF IRRADIATED SALT ROCK *
 Romanian Reports in Physics, Vol. 63, No. 1, P. 220 225, 2011 RESULTS OF UNDERGROUND DOSE RATES USING TL DETECTORS AND PRELIMINARY TL CHARACTERISTICS OF IRRADIATED SALT ROCK * ANA MARIA APOSTU 2, AL. CHIROSCA
Romanian Reports in Physics, Vol. 63, No. 1, P. 220 225, 2011 RESULTS OF UNDERGROUND DOSE RATES USING TL DETECTORS AND PRELIMINARY TL CHARACTERISTICS OF IRRADIATED SALT ROCK * ANA MARIA APOSTU 2, AL. CHIROSCA
Neutron Dose near Spent Nuclear Fuel and HAW after the 2007 ICRP Recommendations
 Neutron Dose near Spent Nuclear Fuel and HAW after the 2007 ICRP Recommendations Gunter Pretzsch Gesellschaft fuer Anlagen- und Reaktorsicherheit (GRS) mbh Radiation and Environmental Protection Division
Neutron Dose near Spent Nuclear Fuel and HAW after the 2007 ICRP Recommendations Gunter Pretzsch Gesellschaft fuer Anlagen- und Reaktorsicherheit (GRS) mbh Radiation and Environmental Protection Division
Iodine-131, Iodine-125 and heavy charged particles
 lucas heights 1971 Strict limits for exposure to all kinds of radiation are set and applied worldwide in an attempt to ensure the safe use of nuclear energy and nuclear techniques. These limits are kept
lucas heights 1971 Strict limits for exposure to all kinds of radiation are set and applied worldwide in an attempt to ensure the safe use of nuclear energy and nuclear techniques. These limits are kept
Introduction to Radiation Testing Radiation Environments
 http://www.maxwell.com/microelectronics/products/radtest/intro.html Introduction to Radiation Testing Quick links: Radiation Environments Total Dose Testing Single Event Effects Testing Neutron Testing
http://www.maxwell.com/microelectronics/products/radtest/intro.html Introduction to Radiation Testing Quick links: Radiation Environments Total Dose Testing Single Event Effects Testing Neutron Testing
A. Husain Kinectrics Inc. 800 Kipling Avenue, Toronto, Ontario, CANADA M8Z 6C4
 WM 03 Conference, February 23-27, 2003, Tucson, AZ ESTIMATION OF RADIOLYTIC GAS GENERATION RATE FOR CYLINDRICAL RADIOACTIVE WASTE PACKAGES APPLICATION TO SPENT ION EXCHANGE RESIN CONTAINERS A. Husain Kinectrics
WM 03 Conference, February 23-27, 2003, Tucson, AZ ESTIMATION OF RADIOLYTIC GAS GENERATION RATE FOR CYLINDRICAL RADIOACTIVE WASTE PACKAGES APPLICATION TO SPENT ION EXCHANGE RESIN CONTAINERS A. Husain Kinectrics
Semiconductor Detectors
 Semiconductor Detectors Summary of Last Lecture Band structure in Solids: Conduction band Conduction band thermal conductivity: E g > 5 ev Valence band Insulator Charge carrier in conductor: e - Charge
Semiconductor Detectors Summary of Last Lecture Band structure in Solids: Conduction band Conduction band thermal conductivity: E g > 5 ev Valence band Insulator Charge carrier in conductor: e - Charge
EE 212 FALL ION IMPLANTATION - Chapter 8 Basic Concepts
 EE 212 FALL 1999-00 ION IMPLANTATION - Chapter 8 Basic Concepts Ion implantation is the dominant method of doping used today. In spite of creating enormous lattice damage it is favored because: Large range
EE 212 FALL 1999-00 ION IMPLANTATION - Chapter 8 Basic Concepts Ion implantation is the dominant method of doping used today. In spite of creating enormous lattice damage it is favored because: Large range
Kinetics of alkali ion exchange of irradiated glasses
 Mat. Res. Soc. Symp. Proc. Vol. 792 2004 Materials Research Society R2.5.1 Kinetics of alkali ion echange of irradiated glasses Michael I. Ojovan, William E. Lee Immobilisation Science Laboratory, Department
Mat. Res. Soc. Symp. Proc. Vol. 792 2004 Materials Research Society R2.5.1 Kinetics of alkali ion echange of irradiated glasses Michael I. Ojovan, William E. Lee Immobilisation Science Laboratory, Department
Experience with Moving from Dpa to Changes in Materials Properties
 Experience with Moving from Dpa to Changes in Materials Properties Meimei Li, Argonne National Laboratory N. V. Mokhov, Fermilab 46 th ICFA Advanced Beam Dynamics Workshop Sept. 27 Oct. 1, 2010 Morschach,
Experience with Moving from Dpa to Changes in Materials Properties Meimei Li, Argonne National Laboratory N. V. Mokhov, Fermilab 46 th ICFA Advanced Beam Dynamics Workshop Sept. 27 Oct. 1, 2010 Morschach,
Chapter Four (Interaction of Radiation with Matter)
 Al-Mustansiriyah University College of Science Physics Department Fourth Grade Nuclear Physics Dr. Ali A. Ridha Chapter Four (Interaction of Radiation with Matter) Different types of radiation interact
Al-Mustansiriyah University College of Science Physics Department Fourth Grade Nuclear Physics Dr. Ali A. Ridha Chapter Four (Interaction of Radiation with Matter) Different types of radiation interact
Chapter 4. Surface defects created by kev Xe ion irradiation on Ge
 81 Chapter 4 Surface defects created by kev Xe ion irradiation on Ge 4.1. Introduction As high energy ions penetrate into a solid, those ions can deposit kinetic energy in two processes: electronic excitation
81 Chapter 4 Surface defects created by kev Xe ion irradiation on Ge 4.1. Introduction As high energy ions penetrate into a solid, those ions can deposit kinetic energy in two processes: electronic excitation
Stable isotope. Relative atomic mass. Mole fraction. Chlorine isotopes in Earth/planetary science
 Stable isotope Relative atomic mass Mole fraction 35 Cl 34.968 8527 0.7576 37 Cl 36.965 9026 0.2424 / Chlorine isotopes in Earth/planetary science Because molecules, atoms, and ions of the stable isotopes
Stable isotope Relative atomic mass Mole fraction 35 Cl 34.968 8527 0.7576 37 Cl 36.965 9026 0.2424 / Chlorine isotopes in Earth/planetary science Because molecules, atoms, and ions of the stable isotopes
Supporting Information
 Temperature Effect on Transport, Charging and Binding of Low-Energy Electrons Interacting with Amorphous Solid Water Films Roey Sagi, Michelle Akerman, Sujith Ramakrishnan and Micha Asscher * Institute
Temperature Effect on Transport, Charging and Binding of Low-Energy Electrons Interacting with Amorphous Solid Water Films Roey Sagi, Michelle Akerman, Sujith Ramakrishnan and Micha Asscher * Institute
Molecular Dynamics Simulations of Fusion Materials: Challenges and Opportunities (Recent Developments)
 Molecular Dynamics Simulations of Fusion Materials: Challenges and Opportunities (Recent Developments) Fei Gao gaofeium@umich.edu Limitations of MD Time scales Length scales (PBC help a lot) Accuracy of
Molecular Dynamics Simulations of Fusion Materials: Challenges and Opportunities (Recent Developments) Fei Gao gaofeium@umich.edu Limitations of MD Time scales Length scales (PBC help a lot) Accuracy of
D DAVID PUBLISHING. Transport Properties of InAs-InP Solid Solutions. 2. Experiment. 1. Introduction. 3. Results and Discussion
 Journal of Electrical Engineering 2 (2014) 207-212 doi: 10.17265/2328-2223/2014.05.002 D DAVID PUBLISHING Nodar Kekelidze 1, 2, 3, Elza Khutsishvili 1, 2, Bella Kvirkvelia 1, 2, 3, David Kekelidze 2, Vugar
Journal of Electrical Engineering 2 (2014) 207-212 doi: 10.17265/2328-2223/2014.05.002 D DAVID PUBLISHING Nodar Kekelidze 1, 2, 3, Elza Khutsishvili 1, 2, Bella Kvirkvelia 1, 2, 3, David Kekelidze 2, Vugar
Analysis of recombination and relaxation of non-equilibrium air plasma generated by short time energetic electron and photon beams
 22 nd International Symposium on Plasma Chemistry July 5-10, 2015; Antwerp, Belgium Analysis of recombination and relaxation of non-equilibrium air plasma generated by short time energetic electron and
22 nd International Symposium on Plasma Chemistry July 5-10, 2015; Antwerp, Belgium Analysis of recombination and relaxation of non-equilibrium air plasma generated by short time energetic electron and
Chapiter VII: Ionization chamber
 Chapiter VII: Ionization chamber 1 Types of ionization chambers Sensitive volume: gas (most often air direct measurement of exposure) ionization chamber Sensitive volume: semiconductor (silicon, germanium,
Chapiter VII: Ionization chamber 1 Types of ionization chambers Sensitive volume: gas (most often air direct measurement of exposure) ionization chamber Sensitive volume: semiconductor (silicon, germanium,
Study on Nuclear Transmutation of Nuclear Waste by 14 MeV Neutrons )
 Study on Nuclear Transmutation of Nuclear Waste by 14 MeV Neutrons ) Takanori KITADA, Atsuki UMEMURA and Kohei TAKAHASHI Osaka University, Graduate School of Engineering, Division of Sustainable Energy
Study on Nuclear Transmutation of Nuclear Waste by 14 MeV Neutrons ) Takanori KITADA, Atsuki UMEMURA and Kohei TAKAHASHI Osaka University, Graduate School of Engineering, Division of Sustainable Energy
A new protocol to evaluate the charge collection efficiency degradation in semiconductor devices induced by MeV ions
 Session 12: Modification and Damage: Contribute lecture O-35 A new protocol to evaluate the charge collection efficiency degradation in semiconductor devices induced by MeV ions Ettore Vittone Physics
Session 12: Modification and Damage: Contribute lecture O-35 A new protocol to evaluate the charge collection efficiency degradation in semiconductor devices induced by MeV ions Ettore Vittone Physics
Production of Fluorine-18 by Small Research Reactor
 Journal of NUCLEAR SCIENCE and TECHNOLOGY, 4[4], p.185~189 (April 1967). 185 Production of Fluorine-18 by Small Research Reactor Yoshiaki MARUYAMA* Received October 4, 1966 High purity 18F was prepared
Journal of NUCLEAR SCIENCE and TECHNOLOGY, 4[4], p.185~189 (April 1967). 185 Production of Fluorine-18 by Small Research Reactor Yoshiaki MARUYAMA* Received October 4, 1966 High purity 18F was prepared
AR-7781 (Physical Chemistry)
 Model Answer: B.Sc-VI th Semester-CBT-602 AR-7781 (Physical Chemistry) One Mark Questions: 1. Write a nuclear reaction for following Bethe s notation? 35 Cl(n, p) 35 S Answer: 35 17Cl + 1 1H + 35 16S 2.
Model Answer: B.Sc-VI th Semester-CBT-602 AR-7781 (Physical Chemistry) One Mark Questions: 1. Write a nuclear reaction for following Bethe s notation? 35 Cl(n, p) 35 S Answer: 35 17Cl + 1 1H + 35 16S 2.
7 The atomic number is the number of protons in the nucleus. The mass number is the total number of protons and neutrons.
 Chapter 1 The atom Unit 1.1 1 proton, neutron, electron 2 Rutherford s discovery was that the atom contained a small positive nucleus surrounded by a cloud of electrons. He also found that atoms are mostly
Chapter 1 The atom Unit 1.1 1 proton, neutron, electron 2 Rutherford s discovery was that the atom contained a small positive nucleus surrounded by a cloud of electrons. He also found that atoms are mostly
Temperature effect on lyoluminescence of potassium halide microcrystals in luminol solution
 Indian Journal of Pure & Applied Physics Vol. 44, July 2006, pp. 519-523 Temperature effect on lyoluminescence of potassium halide microcrystals in luminol solution R S Chandok*, R Kaur**, G K Chandok
Indian Journal of Pure & Applied Physics Vol. 44, July 2006, pp. 519-523 Temperature effect on lyoluminescence of potassium halide microcrystals in luminol solution R S Chandok*, R Kaur**, G K Chandok
Outline. Radiation Interactions. Spurs, Blobs and Short Tracks. Introduction. Radiation Interactions 1
 Outline Radiation Interactions Introduction Interaction of Heavy Charged Particles Interaction of Fast Electrons Interaction of Gamma Rays Interactions of Neutrons Radiation Exposure & Dose Sources of
Outline Radiation Interactions Introduction Interaction of Heavy Charged Particles Interaction of Fast Electrons Interaction of Gamma Rays Interactions of Neutrons Radiation Exposure & Dose Sources of
Radiation damage in alkali halides
 Radiation damage in alkali halides RuG-IMA-2002-11 by: Prof. Dr. H.W. den Hartog Solid State Physics Laboratory State University of Groningen 4 Nijenborgh 9747 AG Groningen the Netherlands Contents Abstract
Radiation damage in alkali halides RuG-IMA-2002-11 by: Prof. Dr. H.W. den Hartog Solid State Physics Laboratory State University of Groningen 4 Nijenborgh 9747 AG Groningen the Netherlands Contents Abstract
Analysis of Thermoluminescence Glow-Curves for. General-Order Kinetics Using Mathematica
 Adv. Studies Theor. Phys., Vol. 4, 2010, no. 1, 3-53 Analysis of Thermoluminescence Glow-Curves for General-Order Kinetics Using Mathematica Md. Shah Alam 1,2 and Sabar Bauk 1 1 Physics Section, School
Adv. Studies Theor. Phys., Vol. 4, 2010, no. 1, 3-53 Analysis of Thermoluminescence Glow-Curves for General-Order Kinetics Using Mathematica Md. Shah Alam 1,2 and Sabar Bauk 1 1 Physics Section, School
Sample Questions Chem 22 Student Chapters Page 1 of 5 Spring 2016
 Sample Questions Chem 22 Student Chapters 13-18 Page 1 of 5 1. The vapor pressure of a liquid is the pressure, at equilibrium, of the a) solid above its liquid. b) liquid above its solid. c) gas above
Sample Questions Chem 22 Student Chapters 13-18 Page 1 of 5 1. The vapor pressure of a liquid is the pressure, at equilibrium, of the a) solid above its liquid. b) liquid above its solid. c) gas above
A.M. THURSDAY, 19 June hour 40 minutes
 Candidate Name Centre Number 2 Candidate Number GCE A level 335/01 CHEMISTRY CH5 A.M. THURSDAY, 19 June 2008 1 hour 40 minutes JD*(S08-335-01) 4 B 5 ADDITIONAL MATERIALS TOTAL MARK In addition to this
Candidate Name Centre Number 2 Candidate Number GCE A level 335/01 CHEMISTRY CH5 A.M. THURSDAY, 19 June 2008 1 hour 40 minutes JD*(S08-335-01) 4 B 5 ADDITIONAL MATERIALS TOTAL MARK In addition to this
Creation and annealing of point defects in germanium crystal lattices by subthreshold energy events
 Creation and annealing of point defects in germanium crystal lattices by subthreshold energy events University of Sevilla 203 Sergio M. M. Coelho, Juan F. R. Archilla 2 and F. Danie Auret Physics Department,
Creation and annealing of point defects in germanium crystal lattices by subthreshold energy events University of Sevilla 203 Sergio M. M. Coelho, Juan F. R. Archilla 2 and F. Danie Auret Physics Department,
C. Perform the following calculations and Round into correct scientific notation.
 Name Hour Honors Chemistry Final Exam Review 2018 - HERBERHOLZ *Due on the day of the exam! No photocopying or copying other classmate s review. Must be handwritten and show work for calculations. Chapter
Name Hour Honors Chemistry Final Exam Review 2018 - HERBERHOLZ *Due on the day of the exam! No photocopying or copying other classmate s review. Must be handwritten and show work for calculations. Chapter
Energetic particles and their detection in situ (particle detectors) Part II. George Gloeckler
 Energetic particles and their detection in situ (particle detectors) Part II George Gloeckler University of Michigan, Ann Arbor, MI University of Maryland, College Park, MD Simple particle detectors Gas-filled
Energetic particles and their detection in situ (particle detectors) Part II George Gloeckler University of Michigan, Ann Arbor, MI University of Maryland, College Park, MD Simple particle detectors Gas-filled
Steady-state diffusion is diffusion in which the concentration of the diffusing atoms at
 Chapter 7 What is steady state diffusion? Steady-state diffusion is diffusion in which the concentration of the diffusing atoms at any point, x, and hence the concentration gradient at x, in the solid,
Chapter 7 What is steady state diffusion? Steady-state diffusion is diffusion in which the concentration of the diffusing atoms at any point, x, and hence the concentration gradient at x, in the solid,
Today, I will present the first of two lectures on neutron interactions.
 Today, I will present the first of two lectures on neutron interactions. I first need to acknowledge that these two lectures were based on lectures presented previously in Med Phys I by Dr Howell. 1 Before
Today, I will present the first of two lectures on neutron interactions. I first need to acknowledge that these two lectures were based on lectures presented previously in Med Phys I by Dr Howell. 1 Before
4. Inelastic Scattering
 1 4. Inelastic Scattering Some inelastic scattering processes A vast range of inelastic scattering processes can occur during illumination of a specimen with a highenergy electron beam. In principle, many
1 4. Inelastic Scattering Some inelastic scattering processes A vast range of inelastic scattering processes can occur during illumination of a specimen with a highenergy electron beam. In principle, many
- 1 - Core Chemistry Topic 2 Atomic Structure
 - 1 - Core Chemistry REVIEW a model for matter (10 mins) 1. On your own take 5 minutes to construct a concept map organizing what you know about the structure of matter. 2. Take 5 more minutes and share
- 1 - Core Chemistry REVIEW a model for matter (10 mins) 1. On your own take 5 minutes to construct a concept map organizing what you know about the structure of matter. 2. Take 5 more minutes and share
3 Radioactivity - Spontaneous Nuclear Processes
 3 Radioactivity - Spontaneous Nuclear Processes Becquerel was the first to detect radioactivity. In 1896 he was carrying out experiments with fluorescent salts (which contained uranium) and found that
3 Radioactivity - Spontaneous Nuclear Processes Becquerel was the first to detect radioactivity. In 1896 he was carrying out experiments with fluorescent salts (which contained uranium) and found that
INTRODUCTION TO THE DEFECT STATE IN MATERIALS
 INTRODUCTION TO THE DEFECT STATE IN MATERIALS DEFECTS, DEFECTS, DEFECTS CAN T LIVE WITH THEM!!! CAN T LIVE WITHOUT THEM!!! INTRODUCTION TO DEFECT STATE IN MATERIALS DEFECTS, DEFECTS, DEFECTS Perfect crystals
INTRODUCTION TO THE DEFECT STATE IN MATERIALS DEFECTS, DEFECTS, DEFECTS CAN T LIVE WITH THEM!!! CAN T LIVE WITHOUT THEM!!! INTRODUCTION TO DEFECT STATE IN MATERIALS DEFECTS, DEFECTS, DEFECTS Perfect crystals
Interaction of Ionizing Radiation with Matter
 Interaction of Ionizing Radiation with Matter Interaction of neutrons with matter Neutral particles, no repulsion with the positively charged nucleus: important projectile Origin of the neutrons: Nuclear
Interaction of Ionizing Radiation with Matter Interaction of neutrons with matter Neutral particles, no repulsion with the positively charged nucleus: important projectile Origin of the neutrons: Nuclear
You have two samples of water each made up of different isotopes of hydrogen: one contains
 Chapter 2 Nuclear Chemistry Concept Check 2. You have two samples of water each made up of different isotopes of hydrogen: one contains H2O and the other, H2O. a. Would you expect these two water samples
Chapter 2 Nuclear Chemistry Concept Check 2. You have two samples of water each made up of different isotopes of hydrogen: one contains H2O and the other, H2O. a. Would you expect these two water samples
Activities of the neutron standardization. at the Korea Research Institute of Standards and Science (KRISS)
 Activities of the neutron standardization at the Korea Research Institute of Standards and Science (KRISS) I. Introduction The activities of neutron standardization in KRISS have been continued for last
Activities of the neutron standardization at the Korea Research Institute of Standards and Science (KRISS) I. Introduction The activities of neutron standardization in KRISS have been continued for last
GCE AS and A Level. Physics A. AS exams 2009 onwards A2 exams 2010 onwards. Unit 5: Approved specimen question paper. Version 1.3
 GCE AS and A Level Physics A AS exams 2009 onwards A2 exams 2010 onwards Unit 5: Approved specimen question paper Version 1.3 Surname Other Names Leave blank Centre Number Candidate Number Candidate Signature
GCE AS and A Level Physics A AS exams 2009 onwards A2 exams 2010 onwards Unit 5: Approved specimen question paper Version 1.3 Surname Other Names Leave blank Centre Number Candidate Number Candidate Signature
The annealing of interstitial carbon atoms in high resistivity n-type silicon after proton irradiation
 ROSE/TN/2002-01 The annealing of interstitial carbon atoms in high resistivity n-type silicon after proton irradiation M. Kuhnke a,, E. Fretwurst b, G. Lindstroem b a Department of Electronic and Computer
ROSE/TN/2002-01 The annealing of interstitial carbon atoms in high resistivity n-type silicon after proton irradiation M. Kuhnke a,, E. Fretwurst b, G. Lindstroem b a Department of Electronic and Computer
8/24/2018. Bio 1101 Lecture 2 (guided) Chapters 2: Essential Chemistry. Chapter 2: Essential Chemistry for Biology
 1 2 3 4 5 Bio 1101 Lecture 2 (guided) Chapters 2: Essential Chemistry Chapter 2: Essential Chemistry for Biology Levels of biological organization Ecosystem (e.g. savanna) Community (e.g. the organisms
1 2 3 4 5 Bio 1101 Lecture 2 (guided) Chapters 2: Essential Chemistry Chapter 2: Essential Chemistry for Biology Levels of biological organization Ecosystem (e.g. savanna) Community (e.g. the organisms
ION IMPLANTATION - Chapter 8 Basic Concepts
 ION IMPLANTATION - Chapter 8 Basic Concepts Ion implantation is the dominant method of doping used today. In spite of creating enormous lattice damage it is favored because: Large range of doses - 1 11
ION IMPLANTATION - Chapter 8 Basic Concepts Ion implantation is the dominant method of doping used today. In spite of creating enormous lattice damage it is favored because: Large range of doses - 1 11
Chapter IV: Radioactive decay
 Chapter IV: Radioactive decay 1 Summary 1. Law of radioactive decay 2. Decay chain/radioactive filiation 3. Quantum description 4. Types of radioactive decay 2 History Radioactivity was discover in 1896
Chapter IV: Radioactive decay 1 Summary 1. Law of radioactive decay 2. Decay chain/radioactive filiation 3. Quantum description 4. Types of radioactive decay 2 History Radioactivity was discover in 1896
Application of positrons in materials research
 Application of positrons in materials research Trapping of positrons at vacancy defects Using positrons, one can get defect information. R. Krause-Rehberg and H. S. Leipner, Positron annihilation in Semiconductors,
Application of positrons in materials research Trapping of positrons at vacancy defects Using positrons, one can get defect information. R. Krause-Rehberg and H. S. Leipner, Positron annihilation in Semiconductors,
Outlook: Application of Positron Annihilation for defects investigations in thin films. Introduction to Positron Annihilation Methods
 Application of Positron Annihilation for defects investigations in thin films V. Bondarenko, R. Krause-Rehberg Martin-Luther-University Halle-Wittenberg, Halle, Germany Outlook: Introduction to Positron
Application of Positron Annihilation for defects investigations in thin films V. Bondarenko, R. Krause-Rehberg Martin-Luther-University Halle-Wittenberg, Halle, Germany Outlook: Introduction to Positron
Radioactivity III: Measurement of Half Life.
 PHY 192 Half Life Spring 2010 1 Radioactivity III: Measurement of Half Life. Introduction This experiment will once again use the apparatus of the first experiment, this time to measure radiation intensity
PHY 192 Half Life Spring 2010 1 Radioactivity III: Measurement of Half Life. Introduction This experiment will once again use the apparatus of the first experiment, this time to measure radiation intensity
The Reference Atomic Weight
 How to Calculate Molecular Weights of Compounds The Molecular Weight (also referred to as the Formula Weight) of a chemical compound is calculated by adding the atomic masses (weights) of the atoms (elements)
How to Calculate Molecular Weights of Compounds The Molecular Weight (also referred to as the Formula Weight) of a chemical compound is calculated by adding the atomic masses (weights) of the atoms (elements)
X-Ray transitions to low lying empty states
 X-Ray Spectra: - continuous part of the spectrum is due to decelerated electrons - the maximum frequency (minimum wavelength) of the photons generated is determined by the maximum kinetic energy of the
X-Ray Spectra: - continuous part of the spectrum is due to decelerated electrons - the maximum frequency (minimum wavelength) of the photons generated is determined by the maximum kinetic energy of the
DEVELOPMENT OF A NEW POSITRON LIFETIME SPECTROSCOPY TECHNIQUE FOR DEFECT CHARACTERIZATION IN THICK MATERIALS
 Copyright JCPDS - International Centre for Diffraction Data 2004, Advances in X-ray Analysis, Volume 47. 59 DEVELOPMENT OF A NEW POSITRON LIFETIME SPECTROSCOPY TECHNIQUE FOR DEFECT CHARACTERIZATION IN
Copyright JCPDS - International Centre for Diffraction Data 2004, Advances in X-ray Analysis, Volume 47. 59 DEVELOPMENT OF A NEW POSITRON LIFETIME SPECTROSCOPY TECHNIQUE FOR DEFECT CHARACTERIZATION IN
DETECTORS. I. Charged Particle Detectors
 DETECTORS I. Charged Particle Detectors A. Scintillators B. Gas Detectors 1. Ionization Chambers 2. Proportional Counters 3. Avalanche detectors 4. Geiger-Muller counters 5. Spark detectors C. Solid State
DETECTORS I. Charged Particle Detectors A. Scintillators B. Gas Detectors 1. Ionization Chambers 2. Proportional Counters 3. Avalanche detectors 4. Geiger-Muller counters 5. Spark detectors C. Solid State
Nuclear Reaction and Radiation Detectors
 King Saud University College of Applied Studies and Community Service Department of Natural Sciences Nuclear Reaction and Radiation Detectors General Physics II PHYS 111 Nouf Alkathran nalkathran@ksu.edu.sa
King Saud University College of Applied Studies and Community Service Department of Natural Sciences Nuclear Reaction and Radiation Detectors General Physics II PHYS 111 Nouf Alkathran nalkathran@ksu.edu.sa
Introduction into Positron Annihilation
 Introduction into Positron Annihilation Introduction (How to get positrons? What is special about positron annihilation?) The methods of positron annihilation (positron lifetime, Doppler broadening, ACAR...)
Introduction into Positron Annihilation Introduction (How to get positrons? What is special about positron annihilation?) The methods of positron annihilation (positron lifetime, Doppler broadening, ACAR...)
Ch : Electrochemistry and Radiochemistry AP Review Questions
 Ch. 17-21: Electrochemistry and Radiochemistry AP Review Questions Radioactivity: Zone of Stability All nuclides with 84 or more protons are unstable (radioactive). Light elements like the neutron to proton
Ch. 17-21: Electrochemistry and Radiochemistry AP Review Questions Radioactivity: Zone of Stability All nuclides with 84 or more protons are unstable (radioactive). Light elements like the neutron to proton
Simple Experimental Design for Calculation of Neutron Removal Cross Sections K. Groves 1 1) McMaster University, 1280 Main St. W, Hamilton, Canada.
 Simple Experimental Design for Calculation of Neutron Removal Cross Sections K. Groves 1 1) McMaster University, 1280 Main St. W, Hamilton, Canada. (Dated: 5 August 2017) This article proposes an experimental
Simple Experimental Design for Calculation of Neutron Removal Cross Sections K. Groves 1 1) McMaster University, 1280 Main St. W, Hamilton, Canada. (Dated: 5 August 2017) This article proposes an experimental
Calculations of the Oxygen Isotope Fractionation between Hydration Water of Cations and Free Water
 Calculations of the Oxygen Isotope Fractionation between Hydration Water of Cations and Free Water P. Bopp, K. Heinzinger, and P. C. Vogel Max-Planck-Institut für Chemie (Otto-Hahn-Institut), Mainz, Germany
Calculations of the Oxygen Isotope Fractionation between Hydration Water of Cations and Free Water P. Bopp, K. Heinzinger, and P. C. Vogel Max-Planck-Institut für Chemie (Otto-Hahn-Institut), Mainz, Germany
Large electron screening effect in different environments
 Large electron screening effect in different environments Aleksandra Cvetinović, Matej Lipoglavsek, Sabina Markelj and Jelena Vesić Jožef Stefan Institute, Jamova cesta 39, Ljubljana, Slovenia Abstract
Large electron screening effect in different environments Aleksandra Cvetinović, Matej Lipoglavsek, Sabina Markelj and Jelena Vesić Jožef Stefan Institute, Jamova cesta 39, Ljubljana, Slovenia Abstract
SO 4... [2], to an excess of dilute sulfuric acid. A student adds a sample of solid potassium carbonate, K [3]
![SO 4... [2], to an excess of dilute sulfuric acid. A student adds a sample of solid potassium carbonate, K [3] SO 4... [2], to an excess of dilute sulfuric acid. A student adds a sample of solid potassium carbonate, K [3]](/thumbs/83/87669036.jpg) 1 Chemicals called acids have been known throughout history The word acid comes from the Latin acidus meaning sour Dilute sulfuric acid, H 2 SO 4, is a common laboratory acid (a) State the formulae of
1 Chemicals called acids have been known throughout history The word acid comes from the Latin acidus meaning sour Dilute sulfuric acid, H 2 SO 4, is a common laboratory acid (a) State the formulae of
Part E. Radiation monitors. Radiation Safety - JUAS 2014, X. Queralt. Part E. Radiation monitors
 Part E. Radiation monitors 1 / 29 Part E. Radiation monitors dose rate ( Sv.h -1 ) ma 0 10 20 30 40 50 10.0 1.0 0.1 250 0 10 20 30 40 50 days since 18/01/02 200 150 100 50 0 Example: photon dose rate measurement
Part E. Radiation monitors 1 / 29 Part E. Radiation monitors dose rate ( Sv.h -1 ) ma 0 10 20 30 40 50 10.0 1.0 0.1 250 0 10 20 30 40 50 days since 18/01/02 200 150 100 50 0 Example: photon dose rate measurement
SOME ELEMENTS AND ISOTOPES OF SPECIAL CONCERN IN FUEL CYCLE C SEPARATIONS S Tc: ( 99 Tc) U: ( 3 U, 33 U, 34 U, Np: ( 37 Np) Pu: ( 38 Pu, 39 Pu, Am: (
 COMPLEXATION REACTIONS IN NUCLEAR SEPARATIONS A PRESENTATION AT THE SHORT COURSE ON INTRODUCTION TO NUCLEAR CHEMISTRY AND FUEL CYCLE SEPARATIONS BY R. G. WYMER DECEMBER 16-18, 18, 008 1/6/008 1 SOME ELEMENTS
COMPLEXATION REACTIONS IN NUCLEAR SEPARATIONS A PRESENTATION AT THE SHORT COURSE ON INTRODUCTION TO NUCLEAR CHEMISTRY AND FUEL CYCLE SEPARATIONS BY R. G. WYMER DECEMBER 16-18, 18, 008 1/6/008 1 SOME ELEMENTS
Electrostatic charging e ects in fast H interactions with thin Ar
 Nuclear Instruments and Methods in Physics Research B 157 (1999) 116±120 www.elsevier.nl/locate/nimb Electrostatic charging e ects in fast H interactions with thin Ar lms D.E. Grosjean a, R.A. Baragiola
Nuclear Instruments and Methods in Physics Research B 157 (1999) 116±120 www.elsevier.nl/locate/nimb Electrostatic charging e ects in fast H interactions with thin Ar lms D.E. Grosjean a, R.A. Baragiola
physics/ Sep 1997
 GLAS-PPE/97-6 28 August 1997 Department of Physics & Astronomy Experimental Particle Physics Group Kelvin Building, University of Glasgow, Glasgow, G12 8QQ, Scotland. Telephone: +44 - ()141 3398855 Fax:
GLAS-PPE/97-6 28 August 1997 Department of Physics & Astronomy Experimental Particle Physics Group Kelvin Building, University of Glasgow, Glasgow, G12 8QQ, Scotland. Telephone: +44 - ()141 3398855 Fax:
Chapter V: Cavity theories
 Chapter V: Cavity theories 1 Introduction Goal of radiation dosimetry: measure of the dose absorbed inside a medium (often assimilated to water in calculations) A detector (dosimeter) never measures directly
Chapter V: Cavity theories 1 Introduction Goal of radiation dosimetry: measure of the dose absorbed inside a medium (often assimilated to water in calculations) A detector (dosimeter) never measures directly
GASEOUS AND VOLATILE FISSION PRODUCT RELEASE FROM MOLTEN SALT NUCLEAR FUEL
 GASEOUS AND VOLATILE FISSION PRODUCT RELEASE FROM MOLTEN SALT NUCLEAR FUEL Ian Scott Moltex Energy LLP, 6 th Floor Remo House, 310-312 Regent St., London Q1B 3BS, UK * Email of corresponding author: ianscott@moltexenergy.com
GASEOUS AND VOLATILE FISSION PRODUCT RELEASE FROM MOLTEN SALT NUCLEAR FUEL Ian Scott Moltex Energy LLP, 6 th Floor Remo House, 310-312 Regent St., London Q1B 3BS, UK * Email of corresponding author: ianscott@moltexenergy.com
Activation of Air and Concrete in Medical Isotope Production Cyclotron Facilities
 Activation of Air and Concrete in Medical Isotope Production Cyclotron Facilities CRPA 2016, Toronto Adam Dodd Senior Project Officer Accelerators and Class II Prescribed Equipment Division (613) 993-7930
Activation of Air and Concrete in Medical Isotope Production Cyclotron Facilities CRPA 2016, Toronto Adam Dodd Senior Project Officer Accelerators and Class II Prescribed Equipment Division (613) 993-7930
At the conclusion of this lesson the trainee will be able to: a) Write a typical equation for the production of each type of radiation.
 RADIOACTIVITY - SPONTANEOUS NUCLEAR PROCESSES OBJECTIVES At the conclusion of this lesson the trainee will be able to: 1. For~, p and 7 decays a) Write a typical equation for the production of each type
RADIOACTIVITY - SPONTANEOUS NUCLEAR PROCESSES OBJECTIVES At the conclusion of this lesson the trainee will be able to: 1. For~, p and 7 decays a) Write a typical equation for the production of each type
Lattice energy of ionic solids
 1 Lattice energy of ionic solids Interatomic Forces Solids are aggregates of atoms, ions or molecules. The bonding between these particles may be understood in terms of forces that play between them. Attractive
1 Lattice energy of ionic solids Interatomic Forces Solids are aggregates of atoms, ions or molecules. The bonding between these particles may be understood in terms of forces that play between them. Attractive
1. Write the number of subatomic particles in a gold atom: a. # of Protons: b. # of Neutrons: c. # of Electrons: 2. Give one property of gold.
 Name: Date Period PART 1: Basics of Chemistry Gold - Au 1. Write the number of subatomic particles in a gold atom: a. # of Protons: b. # of Neutrons: c. # of Electrons: 2. Give one property of gold. 3.
Name: Date Period PART 1: Basics of Chemistry Gold - Au 1. Write the number of subatomic particles in a gold atom: a. # of Protons: b. # of Neutrons: c. # of Electrons: 2. Give one property of gold. 3.
Radiation Quantities and Units
 Radiation Quantities and Units George Starkschall, Ph.D. Lecture Objectives Define and identify units for the following: Exposure Kerma Absorbed dose Dose equivalent Relative biological effectiveness Activity
Radiation Quantities and Units George Starkschall, Ph.D. Lecture Objectives Define and identify units for the following: Exposure Kerma Absorbed dose Dose equivalent Relative biological effectiveness Activity
SYNCHROTRON RADIATION INDUCED X-RAY EMISSION - SRIXE W.M. KWIATEK
 Vol. 82 (1992) -ACTA PHYSICA POLONICA A No 2 Proceedings of the ISSSRNS,92, Jaszowiec 1992 SYNCHROTRON RADIATION INDUCED X-RAY EMISSION - SRIXE W.M. KWIATEK Institute of Nuclear Physics, Department of
Vol. 82 (1992) -ACTA PHYSICA POLONICA A No 2 Proceedings of the ISSSRNS,92, Jaszowiec 1992 SYNCHROTRON RADIATION INDUCED X-RAY EMISSION - SRIXE W.M. KWIATEK Institute of Nuclear Physics, Department of
Efficiencies of Some Spherical Ion Chambers in Continuous and Pulsed Radiation: A Numerical Evaluation
 Signature: Pol J Radiol, 05; 80: 55-5 DOI: 0.659/PJR.89450 ORIGINAL ARTICLE Received: 05.03.7 Accepted: 05.06.9 Published: 05..5 Authors Contribution: A Study Design B Data Collection C Statistical Analysis
Signature: Pol J Radiol, 05; 80: 55-5 DOI: 0.659/PJR.89450 ORIGINAL ARTICLE Received: 05.03.7 Accepted: 05.06.9 Published: 05..5 Authors Contribution: A Study Design B Data Collection C Statistical Analysis
PN Junction
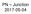 P Junction 2017-05-04 Definition Power Electronics = semiconductor switches are used Analogue amplifier = high power loss 250 200 u x 150 100 u Udc i 50 0 0 50 100 150 200 250 300 350 400 i,u dc i,u u
P Junction 2017-05-04 Definition Power Electronics = semiconductor switches are used Analogue amplifier = high power loss 250 200 u x 150 100 u Udc i 50 0 0 50 100 150 200 250 300 350 400 i,u dc i,u u
High Energy Neutron Scattering Benchmark of Monte Carlo Computations
 International Conference on Mathematics, Computational Methods & Reactor Physics (M&C 2009) Saratoga Springs, New York, May 3-7, 2009, on CD-ROM, American Nuclear Society, LaGrange Park, IL (2009) High
International Conference on Mathematics, Computational Methods & Reactor Physics (M&C 2009) Saratoga Springs, New York, May 3-7, 2009, on CD-ROM, American Nuclear Society, LaGrange Park, IL (2009) High
Radiation Dose, Biology & Risk
 ENGG 167 MEDICAL IMAGING Lecture 2: Sept. 27 Radiation Dosimetry & Risk References: The Essential Physics of Medical Imaging, Bushberg et al, 2 nd ed. Radiation Detection and Measurement, Knoll, 2 nd Ed.
ENGG 167 MEDICAL IMAGING Lecture 2: Sept. 27 Radiation Dosimetry & Risk References: The Essential Physics of Medical Imaging, Bushberg et al, 2 nd ed. Radiation Detection and Measurement, Knoll, 2 nd Ed.
Mechanisms inherent in the thermoluminescence processes
 Indian Journal of Pure & Applied Physics Vol. 42, August 2004, pp 565-571 Mechanisms inherent in the thermoluminescence processes J Prakash, S K Rai, P K Singh & H O Gupta Department of Physics, D D U
Indian Journal of Pure & Applied Physics Vol. 42, August 2004, pp 565-571 Mechanisms inherent in the thermoluminescence processes J Prakash, S K Rai, P K Singh & H O Gupta Department of Physics, D D U
Void superlattice formation as self-organization phenomemon. 1. Scaling estimates
 Void superlattice formation as self-organization phenomemon. 1. Scaling estimates V.N. Kuzovkov 1, E.A. Kotomin 1*, G.Zvejnieks 1, K.D. Li 2, L.M. Wang 2 1 Institute for Solid State Physics, University
Void superlattice formation as self-organization phenomemon. 1. Scaling estimates V.N. Kuzovkov 1, E.A. Kotomin 1*, G.Zvejnieks 1, K.D. Li 2, L.M. Wang 2 1 Institute for Solid State Physics, University
HOMEWORK 22-1 (pp )
 CHAPTER 22 HOMEWORK 22-1 (pp. 701 702) Define. 1. nucleons 2. nuclide 3. mass defect 4. nuclear binding energy Solve. Use masses of 1.0087 amu for the neutron, 1.00728 amu for the proton, and 5.486 x 10
CHAPTER 22 HOMEWORK 22-1 (pp. 701 702) Define. 1. nucleons 2. nuclide 3. mass defect 4. nuclear binding energy Solve. Use masses of 1.0087 amu for the neutron, 1.00728 amu for the proton, and 5.486 x 10
arxiv: v1 [physics.ins-det] 3 Feb 2011
![arxiv: v1 [physics.ins-det] 3 Feb 2011 arxiv: v1 [physics.ins-det] 3 Feb 2011](/thumbs/86/94571492.jpg) Nuclear Instruments and Methods in Physics Research A 00 (2018) 1 5 Alogo.pdf Nuclear Instruments and Methods in Physics Research A Scintillation decay time and pulse shape discrimination in oxygenated
Nuclear Instruments and Methods in Physics Research A 00 (2018) 1 5 Alogo.pdf Nuclear Instruments and Methods in Physics Research A Scintillation decay time and pulse shape discrimination in oxygenated
Radiation damage I. Steve Fitzgerald.
 Radiation damage I Steve Fitzgerald http://defects.materials.ox.ac.uk/ Firstly an apology Radiation damage is a vast area of research I cannot hope to cover much in any detail I will try and introduce
Radiation damage I Steve Fitzgerald http://defects.materials.ox.ac.uk/ Firstly an apology Radiation damage is a vast area of research I cannot hope to cover much in any detail I will try and introduce
Influence of High Magnetic Field on Fusion Reactor Blanket Processes
 FT/P5-7 Influence of High Magnetic Field on Fusion Reactor Blanket Processes G. Kizane, J. Tiliks, A. Vitins, E. Kolodinska Laboratory of Radiation Chemistry of Solids, University of Latvia 4 blvd. Kronvalda,
FT/P5-7 Influence of High Magnetic Field on Fusion Reactor Blanket Processes G. Kizane, J. Tiliks, A. Vitins, E. Kolodinska Laboratory of Radiation Chemistry of Solids, University of Latvia 4 blvd. Kronvalda,
Chap. 5 Ion Chambers the electroscope
 Chap. 5 Ion Chambers the electroscope Electroscope: an early device used to study static electricity continues to be used for personal dosimeters. Put a (known) charge on the central electrode, leaves
Chap. 5 Ion Chambers the electroscope Electroscope: an early device used to study static electricity continues to be used for personal dosimeters. Put a (known) charge on the central electrode, leaves
Exposure Buildup Factors for Heavy Metal Oxide Glass: A Radiation Shield
 Downloaded from orbit.dtu.dk on: Jan 16, 2019 Exposure Buildup Factors for Heavy Metal Oxide Glass: A Radiation Shield Manonara, S. R.; Hanagodimath, S. M.; Gerward, Leif; Mittal, K. C. Published in: Journal
Downloaded from orbit.dtu.dk on: Jan 16, 2019 Exposure Buildup Factors for Heavy Metal Oxide Glass: A Radiation Shield Manonara, S. R.; Hanagodimath, S. M.; Gerward, Leif; Mittal, K. C. Published in: Journal
