Name: 1: /33 Grade: /100 2: /33 3: /33 +1 free point. Midterm Exam I. CHEM 181: Introduction to Chemical Principles September 20, 2012 Answer Key
|
|
|
- Nelson Nichols
- 5 years ago
- Views:
Transcription
1 ame: 1: /33 Grade: /100 2: /33 3: /33 +1 free point Directions: Do all three problems. Midterm Exam I EM 181: Introduction to hemical Principles eptember 20, 2012 Answer Key how all of your work neatly and clearly. Do not skip steps. Partial credit will be awarded for all problems. orrect answers will not receive credit if your work is not shown. If you are not sure exactly what a question means, ask! ot all problems are of equal difficulty, but all are worth the same fraction of the overall grade. 1
2 1. Draw Lewis electron structures for the following molecules and ions. Do not exceed an octet on any atom. Mark all non-zero formal charges. Include all reasonable resonance structures. Mark which structures are, and mark major and minor structures. You can either omit extremely minor structures, or mark them as extremely minor. (a) 3 (onnect major the to an oxygen; avoidminor bonds) v. minor (b) 2 (ne, the, and the are connected to the.) minor major v.v. minor major minor Drawing the other isomer (with one each attached to and ) and its resonance structure is fine, too. 2
3 (c) v. minor v. minor minor major v.v. minor (d) 2 2 (four-membered ring, alternating,,, ) minor major v.v. minor 3
4 2. The first through sixth ionization energies of carbon, in attojoules (1 aj = J) are: Z Element I 1 I 2 I 3 I 4 I 5 I In photoelectron spectroscopy, a single photon detaches an electron from an atom A: A + photon A + + e (with kinetic energy) (a) arbon has 1s, 2s, and 2p electrons. Which of these can be detached using light with wavelength λ = 100 nm? Explain your answer. First calculate the photon energy: E = hc λ = J s m s m = J = 1.99 aj This is enough to pull of the first electron, which is in a 2p orbital. The 2s orbital is lower in energy than the 2p orbital; the question is, how much lower? It s not going to take a full 7.67 aj to pull off an electron, as that s the energy needed to remove a 2s electron after both 2p electrons are gone. n the other hand, you can eyeball the large jump between I 2 and I 3, compared to the much smaller jumps between I 1 and I 2, and I 3 and I 4, and guess that the 2s orbital is approximately (or at least) 1 aj more stable than the 2p. o 100 nm can only remove a 2p electron, as the 2s is too strongly bound. 4
5 (b) What is the maximum speed for an electron detached with 100 nm light? The kinetic energy released is KE = hc λ E binding = 1.99 aj 1.81 aj = 0.18 aj Plugging this in with the mass of the electron: KE = 1 2 m ev 2 2E v = m e = 18 J kg = J 11 kg = J kg 1 kg m2 s 2 1 J = m s 1 (c) Which electrons (1s, 2s, and/or 2p) can be detached using 28 nm light? Explain your answer. You can do another calculation, or go ahead and say that this light has 100/28 times the energy: aj = 7.09 aj Based on the previous discussion, this will be enough to detach either a 2s or 2p electron. It is almost enough to detach a 2s electron even after both 2p electrons are gone. The 1s orbital energy is going to be less than 62.8 aj, but it s not going to be too much less: the 2s and 2p electrons don t shield the 1s electrons from the nucleus much. 5
6 (d) Assume no energy is lost as heat or remains in the atom; that is, all excess energy goes into kinetic energy. If light with a wavelength of 2 nm is used, how many different electron speeds will be observed? (Explain your answer.) The energy is 50 times higher than the 1.99 aj you calculated in part (a), so almost 100 aj: plenty of energy to knock out any one of the electrons. The possible processes are: (1s 2 2s 2 2p 2 ) + photon + (1s 2 2s 2 2p 1 ) + energy (1s 2 2s 2 2p 2 ) + photon + (1s 2 2s 1 2p 2 ) + energy (1s 2 2s 2 2p 2 ) + photon + (1s 1 2s 2 2p 2 ) + energy There are 3 possible electron speeds. (2p is removed) (2s is removed) (1s is removed) 6
7 v. minor 3. The cyclopentadienyl anion, 5 5, has a five-membered ring of carbons, with one hydrogen bonded minor to each carbon. major v.v. minor (a) Draw the Lewis electron structure, including all resonance structures, for cyclopentadienyl. There are five resonance structures, all. (The molecule is aromatic.) (b) What is the bond order, and what is the average formal charge on each carbon atom? For any given bond you pick, it will be double-bonded in two of the five resonance structures, and single-bonded in the other three. That s seven bonds total over five resonance structures: bond order = 7 5 All five carbon-carbon bonds are identical in this molecule. The formal charge, by a similar calculation, is 1/5. 7
8 (c) The molecular orbitals for cyclopentadienyl look like haracterize each orbital as bonding, antibonding, or non-bonding, and write this (or b, ab, nb or the like) next to each of the above orbitals. A, B, and D are bonding, and and E are antibonding. (d) Rank the molecular orbitals in terms of energy, lowest to highest. (If two orbitals are very close in energy, do not worry overmuch about which one you list first.) lowest energy A < B = D < = E highest energy It is not obvious that B and D should be exactly the same energy, but they are. Any answer that has B and D as higher in energy than A but lower than and E is marked as correct; ditto any answer with and E as the two highest-energy orbitals. (e) Which orbitals are filled? What does M theory predict for the bond order of this molecule? There are 26 electrons total. 10 of these are taken up in the 5 sigma bonds, and another 10 in the 5 sigma bonds. There are 6 electrons left for our π molecular orbitals, shown above. All of these electrons go into bonding orbitals (A, B, and D), so they contribute an additional 3 bonds around the ring. This suggest 8 bonds total (5 σ, 3 π), and a bond order of 8/5. 8
Final Exam. CHEM 181: Introduction to Chemical Principles Answer Key. 1. The major resonance structure of 1,4-diazepine is shown below:
 Final Exam EM 181: Introduction to hemical Principles Answer Key 1. The major resonance structure of 1,4-diazepine is shown below: or Show all reasonable resonance structures; label them as major (if equivalent
Final Exam EM 181: Introduction to hemical Principles Answer Key 1. The major resonance structure of 1,4-diazepine is shown below: or Show all reasonable resonance structures; label them as major (if equivalent
Practice Test Questions 4 Molecular Orbital Theory: Polyatomic Molecules
 Practice Test Questions 4 Molecular rbital Theory: Polyatomic Molecules 1. The images below show the valence molecular orbitals obtained for the carbonate ion via a semiempirical calculation. Both side
Practice Test Questions 4 Molecular rbital Theory: Polyatomic Molecules 1. The images below show the valence molecular orbitals obtained for the carbonate ion via a semiempirical calculation. Both side
CHAPTER 9 COVALENT BONDING: ORBITALS 323
 APTER 9 OVALET BODIG: ORBITALS 323 2 3 2 2 2 3 3 2 2 3 2 3 O * * 2 o; most of the carbons are not in the same plane since a majority of carbon atoms exhibit a tetrahedral structure (19.5 bond angles).
APTER 9 OVALET BODIG: ORBITALS 323 2 3 2 2 2 3 3 2 2 3 2 3 O * * 2 o; most of the carbons are not in the same plane since a majority of carbon atoms exhibit a tetrahedral structure (19.5 bond angles).
Goals for Today. Clarify some Rydberg Concepts Absorption vs. emission
 Note: Due to recent changes the exam 2 material for these slides ends at Ionization Energy Exceptions. You can omit Lewis Structures through General Formal Charge Rules. CH301 Unit 2 QUANTUM NUMBERS AND
Note: Due to recent changes the exam 2 material for these slides ends at Ionization Energy Exceptions. You can omit Lewis Structures through General Formal Charge Rules. CH301 Unit 2 QUANTUM NUMBERS AND
Midterm Exam I. CHEM 181: Introduction to Chemical Principles September 24, 2015 Key
 Midterm Exam I CHEM 8: Introduction to Chemical Principles September 24, 205 Key. A Li 2+ ion in an unknown, highly excited electronic state, first emits a photon at a wavelength of 4.36 µm, and following
Midterm Exam I CHEM 8: Introduction to Chemical Principles September 24, 205 Key. A Li 2+ ion in an unknown, highly excited electronic state, first emits a photon at a wavelength of 4.36 µm, and following
Group Members: Your Name In Class Exercise #6. Photon A. Energy B
 Group Members: Your Name In Class Exercise #6 Shell Structure of Atoms Part II Photoelectron Spectroscopy Photoelectron spectroscopy is closely related to the photoelectric effect. When high energy photons
Group Members: Your Name In Class Exercise #6 Shell Structure of Atoms Part II Photoelectron Spectroscopy Photoelectron spectroscopy is closely related to the photoelectric effect. When high energy photons
Objective 3. Draw resonance structures, use curved arrows, determine extent of delocalization. Identify major/minor contributor.
 Objective 3 Draw resonance structures, use curved arrows, determine extent of delocalization. Identify major/minor contributor. Structure Should Fit Experimental Data The chemical formula of benzene is
Objective 3 Draw resonance structures, use curved arrows, determine extent of delocalization. Identify major/minor contributor. Structure Should Fit Experimental Data The chemical formula of benzene is
Chapter 6 Molecular Structure
 hapter 6 Molecular Structure 1. Draw the Lewis structure of each of the following ions, showing all nonzero formal charges. Indicate whether each ion is linear or bent. If the ion is bent, what is the
hapter 6 Molecular Structure 1. Draw the Lewis structure of each of the following ions, showing all nonzero formal charges. Indicate whether each ion is linear or bent. If the ion is bent, what is the
CHEMISTRY 107 Section 501 Exam #2 Version A October 20, 2017 Dr. Larry Brown
 NAME: (print) UIN #: CHEMISTRY 107 Section 501 Exam #2 Version A October 20, 2017 Dr. Larry Brown This is a 50-minute exam, and contains 23 multiple-choice questions and 3 free response problems. Point
NAME: (print) UIN #: CHEMISTRY 107 Section 501 Exam #2 Version A October 20, 2017 Dr. Larry Brown This is a 50-minute exam, and contains 23 multiple-choice questions and 3 free response problems. Point
Chemistry 181 Fall 2015 Professor S. Alex Kandel Due 10/7/2015. Problem Set 6. Draw a Lewis structure for covalently bonded XeF 2.
 Chemistry 181 Fall 2015 Professor S. Alex Kandel Due 10/7/2015 Practice problems: Problem Set 6 1. Is xenon difluoride ionic or covalent? Draw a Lewis structure for covalently bonded XeF 2. There s pretty
Chemistry 181 Fall 2015 Professor S. Alex Kandel Due 10/7/2015 Practice problems: Problem Set 6 1. Is xenon difluoride ionic or covalent? Draw a Lewis structure for covalently bonded XeF 2. There s pretty
Midterm Exam III. CHEM 181: Introduction to Chemical Principles November 25, 2014 Answer Key
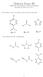 Midterm Exam III EM 181: Introduction to hemical Principles ovember 25, 2014 Answer Key 1. For reference, here are the pk a values for three weak acids: 3 pk a = 7.2 pk a = 4.8 pk a = 15 ow consider this
Midterm Exam III EM 181: Introduction to hemical Principles ovember 25, 2014 Answer Key 1. For reference, here are the pk a values for three weak acids: 3 pk a = 7.2 pk a = 4.8 pk a = 15 ow consider this
Chem Hughbanks Exam 2, March 7, 2014
 Chem 107 - Hughbanks Exam 2, March 7, 2014 Key UI # Section 504 Exam 2, Key for n the last page of this exam, you ve been given a periodic table and some physical constants. You ll probably want to tear
Chem 107 - Hughbanks Exam 2, March 7, 2014 Key UI # Section 504 Exam 2, Key for n the last page of this exam, you ve been given a periodic table and some physical constants. You ll probably want to tear
Answers to Practice Test Questions 3 Molecular Orbital Theory: Heteronuclear Diatomic Molecules
 Answers to Practice Test Questions 3 Molecular Orbital Theory: Heteronuclear Diatomic Molecules 1. The electron configuration for HH is 1ss 1, so HH has 1 valence electron. The electron configuration for
Answers to Practice Test Questions 3 Molecular Orbital Theory: Heteronuclear Diatomic Molecules 1. The electron configuration for HH is 1ss 1, so HH has 1 valence electron. The electron configuration for
CEM 351, Fall 2010 Midterm Exam 1 Friday, October 1, :50 2:40 p.m. Room 138, Chemistry
 Name (print) Signature Student # EM 351, Fall 2010 Midterm Exam 1 Friday, ctober 1, 2010 1:50 2:40 p.m. Room 138, hemistry Wright N. Swers 1.(20 2.(20.. 3.(20 4.(20 5.(20 6.(20 Section Number (2 pts extra
Name (print) Signature Student # EM 351, Fall 2010 Midterm Exam 1 Friday, ctober 1, 2010 1:50 2:40 p.m. Room 138, hemistry Wright N. Swers 1.(20 2.(20.. 3.(20 4.(20 5.(20 6.(20 Section Number (2 pts extra
Chem Hughbanks Exam 2, March 10, 2016
 Chem 107 - Hughbanks Exam 2, March 10, 2016 Name (Print) UIN # Section 502 Exam 2, On the last page of this exam, you ve been given a periodic table and some physical constants. You ll probably want to
Chem 107 - Hughbanks Exam 2, March 10, 2016 Name (Print) UIN # Section 502 Exam 2, On the last page of this exam, you ve been given a periodic table and some physical constants. You ll probably want to
Lewis Dot Structures. a. Duet Rule: 2 electrons needed to satisfy valence shell. i. What follows this rule? Hydrogen and Helium
 1. Important points about Lewis Dot: Lewis Dot Structures a. Duet Rule: 2 electrons needed to satisfy valence shell. i. What follows this rule? Hydrogen and Helium b. Octet Rule: 8 electrons needed to
1. Important points about Lewis Dot: Lewis Dot Structures a. Duet Rule: 2 electrons needed to satisfy valence shell. i. What follows this rule? Hydrogen and Helium b. Octet Rule: 8 electrons needed to
move on if you get stuck on one part
 Chem 105 Exam 2 Name This exam is schedule for 75 minutes and I anticipate it to take the full time allotted. You are free to leave if you finish. In multiple part problems, points awarded will not be
Chem 105 Exam 2 Name This exam is schedule for 75 minutes and I anticipate it to take the full time allotted. You are free to leave if you finish. In multiple part problems, points awarded will not be
Part I. Multiple choice. Circle the correct answer for each problem. 3 points each
 CEM 100 Name Exam 1 Summer 2011 Part I. Multiple choice. Circle the correct answer for each problem. 3 points each 1. Which symbols represent elements that are metals? a. X and Z b. X and Q c. P and L
CEM 100 Name Exam 1 Summer 2011 Part I. Multiple choice. Circle the correct answer for each problem. 3 points each 1. Which symbols represent elements that are metals? a. X and Z b. X and Q c. P and L
CHEMISTRY Midterm #3 November 27, 2007
 Name: The total number of points in this exam is 100. CHEMISTRY 123-01 Midterm #3 November 27, 2007 PART I: MULTIPLE CHOICE (Each multiple choice question has a 2-point value). Mass of electron = 9.11
Name: The total number of points in this exam is 100. CHEMISTRY 123-01 Midterm #3 November 27, 2007 PART I: MULTIPLE CHOICE (Each multiple choice question has a 2-point value). Mass of electron = 9.11
Chemistry 1A Spring 1998 Exam #4 KEY Chapters 9 & 10
 Chemistry 1A Spring 1998 Exam #4 KEY Chapters 9 & 10 For each of the following, write the word, words, or number in each blank that best completes each sentence. (2 points each) 1. A(n) molecular orbital
Chemistry 1A Spring 1998 Exam #4 KEY Chapters 9 & 10 For each of the following, write the word, words, or number in each blank that best completes each sentence. (2 points each) 1. A(n) molecular orbital
Unit Five Practice Test (Part I) PT C U5 P1
 Unit Five Practice Test (Part I) PT C U5 P1 Name Period LPS Standard(s): --- State Standard(s): 12.3.1 Short Answers. Answer the following questions. (5 points each) 1. Write the electron configuration
Unit Five Practice Test (Part I) PT C U5 P1 Name Period LPS Standard(s): --- State Standard(s): 12.3.1 Short Answers. Answer the following questions. (5 points each) 1. Write the electron configuration
Identification Sticker
 Chemistry 1A Midterm Exam 1 September 20, 2001 Page 1 of 10. (Closed Book, 90 minutes, 150 points) Version D Name: TA: SID: Section: Identification Sticker Test-taking strategy: PLEASE READ THIS FIRST!
Chemistry 1A Midterm Exam 1 September 20, 2001 Page 1 of 10. (Closed Book, 90 minutes, 150 points) Version D Name: TA: SID: Section: Identification Sticker Test-taking strategy: PLEASE READ THIS FIRST!
Chemistry 6 10:00 Section Time limit = 3 hours Spring There are two sections to this exam. Please read the instructions carefully.
 Chemistry 6 10:00 Section Final Exam Time limit = 3 hours Spring 2005 Name There are two sections to this exam. Please read the instructions carefully. In the first section, there are 13 multiple choice
Chemistry 6 10:00 Section Final Exam Time limit = 3 hours Spring 2005 Name There are two sections to this exam. Please read the instructions carefully. In the first section, there are 13 multiple choice
CH301 Fall 2012 Name: KEY VandenBout/LaBrake UNIT 2 READINESS ASSESSMENT QUIZ (RAQ) THIS QUIZ WILL BE PACED WITH CLICKER QUESTIONS
 CH301 Fall 2012 Name: KEY VandenBout/LaBrake UNIT 2 READINESS ASSESSMENT QUIZ (RAQ) THIS QUIZ WILL BE PACED WITH CLICKER QUESTIONS 1. A laser pulse shines for 10 s delivering a total energy of 4 mj of
CH301 Fall 2012 Name: KEY VandenBout/LaBrake UNIT 2 READINESS ASSESSMENT QUIZ (RAQ) THIS QUIZ WILL BE PACED WITH CLICKER QUESTIONS 1. A laser pulse shines for 10 s delivering a total energy of 4 mj of
n from which can be derived that
 page 1 of 10 Name Last 5 digits of Student Number: XXX X (may be the same as your social security number) Chem 103 Sample Examination #3 This exam consists of ten (10) pages, including this cover page.
page 1 of 10 Name Last 5 digits of Student Number: XXX X (may be the same as your social security number) Chem 103 Sample Examination #3 This exam consists of ten (10) pages, including this cover page.
1. What is the phenomenon that occurs when certain metals emit electrons when illuminated by particular wavelengths of light? a.
 CHEMISTRY 123-07 Midterm #3 solution key December 02, 2010 Statistics: Average: 77 p (77%); Highest: 100 p (100%); Lowest: 33 p (33%) Number of students performing at or above average: 54 (52%) Number
CHEMISTRY 123-07 Midterm #3 solution key December 02, 2010 Statistics: Average: 77 p (77%); Highest: 100 p (100%); Lowest: 33 p (33%) Number of students performing at or above average: 54 (52%) Number
Chemistry 14C Spring 2016 Final Exam Part B Solutions Page 1
 hemistry 14 Spring 2016 Final Exam Part B Solutions Page 1 Statistics: igh score, average and low score will be posted on the course web site after exam grading is complete. A note about exam keys: The
hemistry 14 Spring 2016 Final Exam Part B Solutions Page 1 Statistics: igh score, average and low score will be posted on the course web site after exam grading is complete. A note about exam keys: The
Chapter 9 Bonding 2 Polar Covalent Bond, Electronegativity, Formal Charge, Resonance. Dr. Sapna Gupta
 Chapter 9 Bonding 2 Polar Covalent Bond, Electronegativity, Formal Charge, Resonance Dr. Sapna Gupta Writing Lewis Structures 1. Draw the skeleton structure of the molecule or ion by placing the lowest
Chapter 9 Bonding 2 Polar Covalent Bond, Electronegativity, Formal Charge, Resonance Dr. Sapna Gupta Writing Lewis Structures 1. Draw the skeleton structure of the molecule or ion by placing the lowest
Name: Class: Date: 3. How many lone pairs of electrons are assigned to the carbon atom in carbon monoxide? a. 0 b. 1 c. 2 d. 3
 Class: Date: Midterm 3, Fall 2009 Record your name on the top of this exam and on the scantron form. Record the test ID letter in the top right box of the scantron form. Record all of your answers on the
Class: Date: Midterm 3, Fall 2009 Record your name on the top of this exam and on the scantron form. Record the test ID letter in the top right box of the scantron form. Record all of your answers on the
CHEMISTRY 107 Section 501 Final Exam Version A December 12, 2016 Dr. Larry Brown
 NAME: (print) UIN #: CHEMISTRY 107 Section 501 Final Exam Version A December 12, 2016 Dr. Larry Brown This is a 2-hour exam, and contains 11 problems. There should be 14 numbered pages, including this
NAME: (print) UIN #: CHEMISTRY 107 Section 501 Final Exam Version A December 12, 2016 Dr. Larry Brown This is a 2-hour exam, and contains 11 problems. There should be 14 numbered pages, including this
This is our rough sketch of the molecular geometry. Not too important right now.
 MIT OpenCourseWare http://ocw.mit.edu 3.091SC Introduction to Solid State Chemistry, Fall 2010 Transcript Exam 1 Problem 3 The following content is provided under a Creative Commons license. Your support
MIT OpenCourseWare http://ocw.mit.edu 3.091SC Introduction to Solid State Chemistry, Fall 2010 Transcript Exam 1 Problem 3 The following content is provided under a Creative Commons license. Your support
Chem 111 Exam #2 November 8, J h = c E = h E. ΔH = energies of bonds broken - energies of bonds formed SHOW ALL WORK
 General Chemistry I NAME: Answer Key Chem 111 Exam #2 November 8, 2013 Some Equations and Constants for your use: -18-2.18 10 J h = c E = h E n = = 2 n mv o ΔH = energies of bonds broken - energies of
General Chemistry I NAME: Answer Key Chem 111 Exam #2 November 8, 2013 Some Equations and Constants for your use: -18-2.18 10 J h = c E = h E n = = 2 n mv o ΔH = energies of bonds broken - energies of
PH 102 Exam III SOLUTION
 PH 102 Exam III SOLUTION Part I: Multiple choice (50%) November 30, 2007 P. LeClair 1. Answer all multiple choice problems. 2. No partial credit will be given for multiple choice questions. 1. What energy
PH 102 Exam III SOLUTION Part I: Multiple choice (50%) November 30, 2007 P. LeClair 1. Answer all multiple choice problems. 2. No partial credit will be given for multiple choice questions. 1. What energy
Final Exam. CHEM 181: Introduction to Chemical Principles December 14, 2015 Answer Key N C S C
 Final Exam EM 181: Introduction to hemical Principles December 14, 2015 Answer Key 1. The compound 4 5 NO has the connectivity shown below O N On the next page: Draw all reasonable resonance structures
Final Exam EM 181: Introduction to hemical Principles December 14, 2015 Answer Key 1. The compound 4 5 NO has the connectivity shown below O N On the next page: Draw all reasonable resonance structures
CHEM- 457: Inorganic Chemistry
 CHEM- 457: Inorganic Chemistry Midterm I March 13 th, 2014 NAME This exam is comprised of six questions and is ten pages in length. Please be sure that you have a complete exam and place your name on each
CHEM- 457: Inorganic Chemistry Midterm I March 13 th, 2014 NAME This exam is comprised of six questions and is ten pages in length. Please be sure that you have a complete exam and place your name on each
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester Christopher J. Cramer. Lecture 30, April 10, 2006
 Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 20056 Christopher J. Cramer Lecture 30, April 10, 2006 Solved Homework The guess MO occupied coefficients were Occupied
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 20056 Christopher J. Cramer Lecture 30, April 10, 2006 Solved Homework The guess MO occupied coefficients were Occupied
Chem 6 Sample exam 2 (150 points total) NAME:
 hem 6 Sample exam 2 (150 points total) @ This is a closed book exam to which the onor Principle applies. @ The last page contains equations and physical constants; you can detach it for easy reference.
hem 6 Sample exam 2 (150 points total) @ This is a closed book exam to which the onor Principle applies. @ The last page contains equations and physical constants; you can detach it for easy reference.
Bohr model and Franck-Hertz experiment
 Bohr model and Franck-Hertz experiment Announcements: Will finish up material in Chapter 5. There will be no class on Friday, Oct. 18. Will announce again! Still have a few midterms see me if you haven
Bohr model and Franck-Hertz experiment Announcements: Will finish up material in Chapter 5. There will be no class on Friday, Oct. 18. Will announce again! Still have a few midterms see me if you haven
Chemistry 4A Midterm Exam 2 Version B October 17, 2008 Professor Pines Closed Book, 50 minutes, 125 points 5 pages total (including cover)
 Chemistry 4A Midterm Exam 2 Version B October 17, 2008 Professor Pines Closed Book, 50 minutes, 125 points 5 pages total (including cover) Student Name: KEY Student ID#: GSI Name: Lab Section Day/Time:
Chemistry 4A Midterm Exam 2 Version B October 17, 2008 Professor Pines Closed Book, 50 minutes, 125 points 5 pages total (including cover) Student Name: KEY Student ID#: GSI Name: Lab Section Day/Time:
Chem 115 Sample Examination #3
 _ Chem 115 Sample Examination #3 Student Number: This exam consists of seven (7) pages, including this cover page. Be sure your copy is complete before beginning your work. If this test packet is defective,
_ Chem 115 Sample Examination #3 Student Number: This exam consists of seven (7) pages, including this cover page. Be sure your copy is complete before beginning your work. If this test packet is defective,
CHEMISTRY 31 Name: KEY Exam #1 100 pts 1. (6 pts) Provide the complete IUPAC name for each of the following compounds:
 CEMISTRY 31 ame: KEY Exam #1 100 pts 1. (6 pts) Provide the complete IUPAC name for each of the following compounds: (1S,3S)-1-bromo-3-butylcyclopentane 3,4-diethyl-2,2-dimethyloctane 1-cyclopropyl-2-methylcyclobutane
CEMISTRY 31 ame: KEY Exam #1 100 pts 1. (6 pts) Provide the complete IUPAC name for each of the following compounds: (1S,3S)-1-bromo-3-butylcyclopentane 3,4-diethyl-2,2-dimethyloctane 1-cyclopropyl-2-methylcyclobutane
CHEM 341: Organic Chemistry I at North Dakota State University Midterm Exam 01 - Fri, Feb 10, 2012!! Name:!
 CEM 341: rganic Chemistry I at North Dakota State University Midterm Exam 01 - Fri, Feb 10, 2012!! Name:! Please read through each question carefully and answer in the spaces provided. A good strategy
CEM 341: rganic Chemistry I at North Dakota State University Midterm Exam 01 - Fri, Feb 10, 2012!! Name:! Please read through each question carefully and answer in the spaces provided. A good strategy
Chem. 1C Midterm 1 Version B April 26, 2017
 First initial of last name Chem. C Midterm Version B April 6, 07 Name: Print Neatly. You will lose point if I cannot read your name or perm number. Perm Number: All work must be shown on the exam for partial
First initial of last name Chem. C Midterm Version B April 6, 07 Name: Print Neatly. You will lose point if I cannot read your name or perm number. Perm Number: All work must be shown on the exam for partial
Chem. 1C Midterm 1 Version A April 26, 2017
 First initial of last name Chem. C Midterm Version A April 6, 07 Name: Print Neatly. You will lose point if I cannot read your name or perm number. Perm Number: All work must be shown on the exam for partial
First initial of last name Chem. C Midterm Version A April 6, 07 Name: Print Neatly. You will lose point if I cannot read your name or perm number. Perm Number: All work must be shown on the exam for partial
Chem. 1B Final Practice
 Chem. 1B Final Practice Name Student Number All work must be shown on the exam for partial credit. Points will be taken off for incorrect or no units and for the incorrect number of significant figures.
Chem. 1B Final Practice Name Student Number All work must be shown on the exam for partial credit. Points will be taken off for incorrect or no units and for the incorrect number of significant figures.
Chem 105 Monday, 31 Oct 2011
 Chem 105 Monday, 31 Oct 2011 Ch 7: Ion sizes; Ionization Potential Ch 8: Drawing Lewis Formulas Formal charge Resonance 10/31/2011 1 Sizes of Ions Cations (remember ca + ion) always SMALLER than corresponding
Chem 105 Monday, 31 Oct 2011 Ch 7: Ion sizes; Ionization Potential Ch 8: Drawing Lewis Formulas Formal charge Resonance 10/31/2011 1 Sizes of Ions Cations (remember ca + ion) always SMALLER than corresponding
Dr. Steven Pedersen February 27, Chemistry 3B. Midterm 1
 r. Steven Pedersen February 7, 017 Student name: ASWER KEY Chemistry 3B Midterm 1 Student I: (Also include your SI in the top left corner of each page) Student signature: Problem 1 Problem Problem 3 Problem
r. Steven Pedersen February 7, 017 Student name: ASWER KEY Chemistry 3B Midterm 1 Student I: (Also include your SI in the top left corner of each page) Student signature: Problem 1 Problem Problem 3 Problem
AP Chemistry Chapter 7: Bonding
 AP Chemistry Chapter 7: Bonding Types of Bonding I. holds everything together! I All bonding occurs because of! Electronegativity difference and bond character A. A difference in electronegativity between
AP Chemistry Chapter 7: Bonding Types of Bonding I. holds everything together! I All bonding occurs because of! Electronegativity difference and bond character A. A difference in electronegativity between
Chapter 9. Covalent Bonding: Orbitals. Copyright 2017 Cengage Learning. All Rights Reserved.
 Chapter 9 Covalent Bonding: Orbitals Chapter 9 Table of Contents (9.1) (9.2) (9.3) (9.4) (9.5) (9.6) Hybridization and the localized electron model The molecular orbital model Bonding in homonuclear diatomic
Chapter 9 Covalent Bonding: Orbitals Chapter 9 Table of Contents (9.1) (9.2) (9.3) (9.4) (9.5) (9.6) Hybridization and the localized electron model The molecular orbital model Bonding in homonuclear diatomic
Department of Chemistry Memorial University Chemistry 1050
 Department of Chemistry Memorial University Chemistry 1050 Fall 2013 Deferred Examination Time 3 hours NAME: MUN Student Number: Circle your professor s name: Dr. R. Davis Dr. T. Fridgen Dr. C. Kozak Read
Department of Chemistry Memorial University Chemistry 1050 Fall 2013 Deferred Examination Time 3 hours NAME: MUN Student Number: Circle your professor s name: Dr. R. Davis Dr. T. Fridgen Dr. C. Kozak Read
CHEM1101 Worksheet 6: Lewis Structures
 CHEM1101 Worksheet 6: Lewis Structures Model 1: Simple Compounds of C, N, O and F The octet rule tells us that C, N, O and F will form covalent bonds so that they are surrounded by eight electrons. For
CHEM1101 Worksheet 6: Lewis Structures Model 1: Simple Compounds of C, N, O and F The octet rule tells us that C, N, O and F will form covalent bonds so that they are surrounded by eight electrons. For
AGK s Fall 2006 Chem 111 Exam 2 Review Sheet
 AGK s Fall 2006 Chem 111 Exam 2 Review Sheet *NOTE: This list is fairly comprehensive but designed only as a study aid. You are responsible for all material covered in class and in assigned readings. While
AGK s Fall 2006 Chem 111 Exam 2 Review Sheet *NOTE: This list is fairly comprehensive but designed only as a study aid. You are responsible for all material covered in class and in assigned readings. While
What is the energy of a photon with wavelength 232 nm?
 EMISTRY 110 EXAM 1 February 6, 2012 FRM A 1 ow many single covalent bonds must a sulfur atom form to have a complete octet in its valence shell? A. 3 B. 4. 1 D. 2 E. 0 2. What are the correct numbers of
EMISTRY 110 EXAM 1 February 6, 2012 FRM A 1 ow many single covalent bonds must a sulfur atom form to have a complete octet in its valence shell? A. 3 B. 4. 1 D. 2 E. 0 2. What are the correct numbers of
Chemistry 1A, Spring 2007 Midterm Exam 2 March 5, 2007 (90 min, closed book)
 Chemistry 1A, Spring 2007 Midterm Exam 2 March 5, 2007 (90 min, closed book) Name: KEY SID: TA Name: 1.) Write your name on every page of this exam. 2.) This exam has 40 multiple choice questions. Fill
Chemistry 1A, Spring 2007 Midterm Exam 2 March 5, 2007 (90 min, closed book) Name: KEY SID: TA Name: 1.) Write your name on every page of this exam. 2.) This exam has 40 multiple choice questions. Fill
Chem Discussion #13 Chapter 10. Correlation diagrams for diatomic molecules. TF s name: Your name: Discussion Section:
 Chem 101 2016 Discussion #13 Chapter 10. Correlation diagrams for diatomic molecules. TF s name: Your name: Discussion Section: 1. Below is a plot of the first 10 ionization energies for a single atom
Chem 101 2016 Discussion #13 Chapter 10. Correlation diagrams for diatomic molecules. TF s name: Your name: Discussion Section: 1. Below is a plot of the first 10 ionization energies for a single atom
The wise does at once what the fool does at last.
 hem 201 Midterm all, 2018 Beauchamp ame Problems Points redit 1. unctional Group omenclature (1 large structure) 30 2. esonance, ormal harge, Arrows 18 3. yclohexane onformations, ewman Projections 30
hem 201 Midterm all, 2018 Beauchamp ame Problems Points redit 1. unctional Group omenclature (1 large structure) 30 2. esonance, ormal harge, Arrows 18 3. yclohexane onformations, ewman Projections 30
CHEMISTRY 107 Section 501 Exam #3 Version A November 16, 2016 Dr. Larry Brown
 NAME: (print) UIN #: CHEMISTRY 107 Section 501 Exam #3 Version A November 16, 2016 Dr. Larry Brown This is a 50-minute exam, and contains 7 problems. There should be 10 numbered pages, including this one.
NAME: (print) UIN #: CHEMISTRY 107 Section 501 Exam #3 Version A November 16, 2016 Dr. Larry Brown This is a 50-minute exam, and contains 7 problems. There should be 10 numbered pages, including this one.
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Take Home Exam Chem 1A Fall 2008 - Chapters 6 to 9: You may us any resource you wish accept people. On your honor, you may not ask another person for help. Show your work on every answer. Partial credit
Take Home Exam Chem 1A Fall 2008 - Chapters 6 to 9: You may us any resource you wish accept people. On your honor, you may not ask another person for help. Show your work on every answer. Partial credit
Carbon-based molecules are held together by covalent bonds between atoms
 hapter 1: hemical bonding and structure in organic compounds arbon-based molecules are held together by covalent bonds between atoms omposition: Mainly nonmetals; especially,, O, N, S, P and the halogens
hapter 1: hemical bonding and structure in organic compounds arbon-based molecules are held together by covalent bonds between atoms omposition: Mainly nonmetals; especially,, O, N, S, P and the halogens
Midterm Exam III. CHEM 181: Introduction to Chemical Principles Solutions C HC N H 3 C. pka = 9.7
 Midterm Exam III EM 181: Introduction to hemical Principles Solutions 1. For reference, here are the pk a values for four weak acids (not all resonance structures shown): N 3 N N 3 3 2 pka = 3.5 pka =
Midterm Exam III EM 181: Introduction to hemical Principles Solutions 1. For reference, here are the pk a values for four weak acids (not all resonance structures shown): N 3 N N 3 3 2 pka = 3.5 pka =
Part I. Multiple choice. Circle the correct answer for each problem. 3 points each
 CHEM 100 Name Exam 1 Summer 2010 Part I. Multiple choice. Circle the correct answer for each problem. 3 points each 1. The observation that 20 g of hydrogen gas always combines with 160 g of oxygen gas
CHEM 100 Name Exam 1 Summer 2010 Part I. Multiple choice. Circle the correct answer for each problem. 3 points each 1. The observation that 20 g of hydrogen gas always combines with 160 g of oxygen gas
Chapter 4: Covalent Bonding and Chemical Structure Representation
 Chapter 4: Covalent Bonding and Chemical Structure Representation The Octet Rule -An atom with 8 electrons (an octet ) in its outer shell has the same number of valence electrons as the noble gas in the
Chapter 4: Covalent Bonding and Chemical Structure Representation The Octet Rule -An atom with 8 electrons (an octet ) in its outer shell has the same number of valence electrons as the noble gas in the
Name. Chapter 4 covers acid-base chemistry. That should help you get going.
 Name Chapter 4 covers acid-base chemistry. That should help you get going. 1 Use curved arrows to illustrate the transfer of a proton (i.e. an + ) from benzoic acid to phenoxide, and draw the products.
Name Chapter 4 covers acid-base chemistry. That should help you get going. 1 Use curved arrows to illustrate the transfer of a proton (i.e. an + ) from benzoic acid to phenoxide, and draw the products.
The role of atomic radius in ion channel selectivity :
 5.111 Lecture Summary #10 Readings for today: Sections 2.14-2.16 (2.15-2.17 in 3rd ed), Section 2.5 (2.6 in 3rd ed) and Section 2.6 (2.7 in 3rd ed). Read for Lecture #11: Section 2.7 (2.8 in 3rd ed) Resonance,
5.111 Lecture Summary #10 Readings for today: Sections 2.14-2.16 (2.15-2.17 in 3rd ed), Section 2.5 (2.6 in 3rd ed) and Section 2.6 (2.7 in 3rd ed). Read for Lecture #11: Section 2.7 (2.8 in 3rd ed) Resonance,
Chemistry 1411 Practice Exam 2, Chapters 5-8 Brown
 Chemistry 1411 Practice Exam 2, Chapters 5-8 Brown Some constants and equations: E = q + w q = C p T Heat = m T Cs h = 6.626 X 10 34 J. s c = 2.998 X 10 8 m/s R H = 2.18 X 10 18 J E = (2.18 X 10 18 J)(1/n
Chemistry 1411 Practice Exam 2, Chapters 5-8 Brown Some constants and equations: E = q + w q = C p T Heat = m T Cs h = 6.626 X 10 34 J. s c = 2.998 X 10 8 m/s R H = 2.18 X 10 18 J E = (2.18 X 10 18 J)(1/n
Chem 1A, Fall 2015, Midterm Exam 1. Version A September 21, 2015 (Prof. Head-Gordon) 2
 Chem 1A, Fall 2015, Midterm Exam 1. Version A September 21, 2015 (Prof. Head-Gordon) 2 Name: Student ID: TA: Contents: 9 pages A. Multiple choice (7 points) B. Stoichiometry (10 points) C. Photoelectric
Chem 1A, Fall 2015, Midterm Exam 1. Version A September 21, 2015 (Prof. Head-Gordon) 2 Name: Student ID: TA: Contents: 9 pages A. Multiple choice (7 points) B. Stoichiometry (10 points) C. Photoelectric
Chapter 1: Orbitals And Bonding
 hapter 1: rbitals And Bonding hapter 1 Topics: Bonding oncepts Look back at your General hemistry Textbook!!! Ionization Potential Ionic Bonds und s Rule Aufbau Principle Lewis Structures Electronegativity
hapter 1: rbitals And Bonding hapter 1 Topics: Bonding oncepts Look back at your General hemistry Textbook!!! Ionization Potential Ionic Bonds und s Rule Aufbau Principle Lewis Structures Electronegativity
CHEM311 FALL 2005 Practice Exam #3
 EM311 FALL 2005 Practice Exam #3 Instructions: This is a multiple choice / short answer practice exam. For the multiple-choice questions, there may be more than one correct answer. If so, then circle as
EM311 FALL 2005 Practice Exam #3 Instructions: This is a multiple choice / short answer practice exam. For the multiple-choice questions, there may be more than one correct answer. If so, then circle as
CHEM 105 Dr. Lammi EXAM II Oct. 8, (9 pts) Please provide the correct formula or name for each of the following compounds. c.
 EM 105 Dr. Lammi EXAM II Oct. 8, 2018 Name: Please show all work and/or reasoning in the space provided or on the attached scratch page. Partial credit for incorrect answers may only be awarded if work/reasoning
EM 105 Dr. Lammi EXAM II Oct. 8, 2018 Name: Please show all work and/or reasoning in the space provided or on the attached scratch page. Partial credit for incorrect answers may only be awarded if work/reasoning
Chem. 1A Final Practice Test 1
 Chem. 1A Final Practice Test 1 All work must be shown on the exam for partial credit. Points will be taken off for incorrect or no units. Calculators are allowed. Cell phones may not be used for calculators.
Chem. 1A Final Practice Test 1 All work must be shown on the exam for partial credit. Points will be taken off for incorrect or no units. Calculators are allowed. Cell phones may not be used for calculators.
Structure and Bonding of Organic Molecules
 Chem 220 Notes Page 1 Structure and Bonding of Organic Molecules I. Types of Chemical Bonds A. Why do atoms forms bonds? Atoms want to have the same number of electrons as the nearest noble gas atom (noble
Chem 220 Notes Page 1 Structure and Bonding of Organic Molecules I. Types of Chemical Bonds A. Why do atoms forms bonds? Atoms want to have the same number of electrons as the nearest noble gas atom (noble
Spectroscopy. a laboratory method of analyzing matter using electromagnetic radiation.
 Spectroscopy a laboratory method of analyzing matter using electromagnetic radiation. Mass Spectrometry Determines the relative abundance of the different isotopes of an element Used to determine the average
Spectroscopy a laboratory method of analyzing matter using electromagnetic radiation. Mass Spectrometry Determines the relative abundance of the different isotopes of an element Used to determine the average
Exam 2 Practice Problems (Key) 1. An electron has an n value of 3. What are the possible l values? What are the possible m l values?
 Exam 2 Practice Problems (Key) 1. An electron has an n value of 3. What are the possible l values? What are the possible m l values? l = 0, 1, 2 m l = 0 (for s) m l = 1, 0, +1 (for p) m l = 2, 1, 0, +1,
Exam 2 Practice Problems (Key) 1. An electron has an n value of 3. What are the possible l values? What are the possible m l values? l = 0, 1, 2 m l = 0 (for s) m l = 1, 0, +1 (for p) m l = 2, 1, 0, +1,
Spectroscopy. a laboratory method of analyzing matter using electromagnetic radiation
 Spectroscopy a laboratory method of analyzing matter using electromagnetic radiation The electromagnetic spectrum Radiation Scale of Absorption involves: Example of spectroscopy Gamma rays pm Nuclear reactions
Spectroscopy a laboratory method of analyzing matter using electromagnetic radiation The electromagnetic spectrum Radiation Scale of Absorption involves: Example of spectroscopy Gamma rays pm Nuclear reactions
Organic Chemistry I (CHE ) Examination I S ep t ember 2 9, KEY Name (PRINT LEGIBLY):
 Organic hemistry I (E 2 3 0-001 ) Examination I S ep t ember 2 9, 2 0 0 4 KEY Name (PRINT LEGIBLY): Please provide clear and concise answers to all of the following questions. Use equations and/or drawings
Organic hemistry I (E 2 3 0-001 ) Examination I S ep t ember 2 9, 2 0 0 4 KEY Name (PRINT LEGIBLY): Please provide clear and concise answers to all of the following questions. Use equations and/or drawings
Physics 221: Optical and Thermal Physics Exam 1, Sec. 500, 14 Feb Please fill in your Student ID number (UIN): IMPORTANT
 Physics 221: Optical and Thermal Physics Exam 1, Sec. 500, 14 Feb. 2005 Instructor: Dr. George R. Welch, 415 Engineering-Physics, 845-7737 Print your name neatly: Last name: First name: Sign your name:
Physics 221: Optical and Thermal Physics Exam 1, Sec. 500, 14 Feb. 2005 Instructor: Dr. George R. Welch, 415 Engineering-Physics, 845-7737 Print your name neatly: Last name: First name: Sign your name:
Chemistry 1A, Fall 2003 Midterm 1 Sept 16, 2003 (90 min, closed book)
 Name: SID: TA Name: Chemistry 1A, Fall 2003 Midterm 1 Sept 16, 2003 (90 min, closed book) This exam has 38 multiple choice questions. Fill in the Scantron form AND circle your answer on the exam. Each
Name: SID: TA Name: Chemistry 1A, Fall 2003 Midterm 1 Sept 16, 2003 (90 min, closed book) This exam has 38 multiple choice questions. Fill in the Scantron form AND circle your answer on the exam. Each
COURSE: CHEMISTRY 211, Foundations of Chemistry: Structure and Bonding
 DEPARTMENT OF CHEMISTRY COURSE SYLLABUS FALL 2014 COURSE: CHEMISTRY 211, Foundations of Chemistry: Structure and Bonding LEC DAYS TIME ROOM INSTRUCTOR OFFICE PHONE EMAIL L01 TR 11:00-12:15 EEEL161 Dr.
DEPARTMENT OF CHEMISTRY COURSE SYLLABUS FALL 2014 COURSE: CHEMISTRY 211, Foundations of Chemistry: Structure and Bonding LEC DAYS TIME ROOM INSTRUCTOR OFFICE PHONE EMAIL L01 TR 11:00-12:15 EEEL161 Dr.
CHEMpossible. 261 Exam 1 Review
 CHEMpossible 261 Exam 1 Review A. Rank the following carboxylic acids from least acidic to most acidic: B. Draw the line-angle formulas for three acylic (non-cyclic) esters with the molecular formula C
CHEMpossible 261 Exam 1 Review A. Rank the following carboxylic acids from least acidic to most acidic: B. Draw the line-angle formulas for three acylic (non-cyclic) esters with the molecular formula C
Part I. Multiple choice. Circle the correct answer for each problem. 3 points each
 CEM 100 Name Exam 1 Summer 2010 Part I. Multiple choice. Circle the correct answer for each problem. 3 points each 1. The observation that 20 g of hydrogen gas always combines with 160 g of oxygen gas
CEM 100 Name Exam 1 Summer 2010 Part I. Multiple choice. Circle the correct answer for each problem. 3 points each 1. The observation that 20 g of hydrogen gas always combines with 160 g of oxygen gas
Review Outline Chemistry 1B, Fall 2012
 Review Outline Chemistry 1B, Fall 2012 -------------------------------------- Chapter 12 -------------------------------------- I. Experiments and findings related to origin of quantum mechanics A. Planck:
Review Outline Chemistry 1B, Fall 2012 -------------------------------------- Chapter 12 -------------------------------------- I. Experiments and findings related to origin of quantum mechanics A. Planck:
"Friendship is one mind in two bodies." Mencius
 California State Polytechnic University, Pomona 1 Fall, 2014 Midterm Exam Chem 314 Beauchamp Chem 314 Name Problem Points Credit 1. Nomenclature 30 2. 2D Lewis structures 20 3. 3D Structures, Formal Charge
California State Polytechnic University, Pomona 1 Fall, 2014 Midterm Exam Chem 314 Beauchamp Chem 314 Name Problem Points Credit 1. Nomenclature 30 2. 2D Lewis structures 20 3. 3D Structures, Formal Charge
FIRST HOUR EXAMINATION
 Name: ANSWERS CEM 331 FIRST UR EXAMINATIN All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided. If I have to guess as to what or where
Name: ANSWERS CEM 331 FIRST UR EXAMINATIN All answers should be written on the exam in the spaces provided. Clearly indicate your answers in the spaces provided. If I have to guess as to what or where
5. Stereochemical Analysis. 7. Dipole Moments and Inductive versus Resonance Effects. 8. Types of isomers from a given formula. 9. Physical Properties
 hem 201 Sample Midterm Beauchamp ame Problems Points redit 1. Functional Group omenclature (1 large structure) 2. Lewis Structures, Resonance, Formal harge 3. yclohexane onformations, 2 substituents, ewman
hem 201 Sample Midterm Beauchamp ame Problems Points redit 1. Functional Group omenclature (1 large structure) 2. Lewis Structures, Resonance, Formal harge 3. yclohexane onformations, 2 substituents, ewman
Quiz 5 R = lit-atm/mol-k 1 (25) R = J/mol-K 2 (25) 3 (25) c = X 10 8 m/s 4 (25)
 ADVANCED INORGANIC CHEMISTRY QUIZ 5 and FINAL December 18, 2012 INSTRUCTIONS: PRINT YOUR NAME > NAME. QUIZ 5 : Work 4 of 1-5 (The lowest problem will be dropped) FINAL: #6 (10 points ) Work 6 of 7 to 14
ADVANCED INORGANIC CHEMISTRY QUIZ 5 and FINAL December 18, 2012 INSTRUCTIONS: PRINT YOUR NAME > NAME. QUIZ 5 : Work 4 of 1-5 (The lowest problem will be dropped) FINAL: #6 (10 points ) Work 6 of 7 to 14
 Chem 201 Page 7 of 14 SECTION II: To be graded manually (Total value 42) Answers must be written in non-erasable ink to be considered for re-grading! Show all your work for full marks. QUESTION 15 VALUE
Chem 201 Page 7 of 14 SECTION II: To be graded manually (Total value 42) Answers must be written in non-erasable ink to be considered for re-grading! Show all your work for full marks. QUESTION 15 VALUE
Practice Exam A Answer Key for Fall 2014
 Practice Exam A Answer Key for Fall 2014 First Hour Exam 5.111. Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. The exam is closed notes and closed
Practice Exam A Answer Key for Fall 2014 First Hour Exam 5.111. Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. The exam is closed notes and closed
HL Chemistry. Thursday August 20th Thursday, August 20, 15
 HL Chemistry Thursday August 20th 2015 Agenda Warm Up: Electron Configuration Practice Review Topic 2.2 Electron Configuration and begin Topic 12.1 Atomic Structure Warm Up You have 10 minutes ONLY! GO!
HL Chemistry Thursday August 20th 2015 Agenda Warm Up: Electron Configuration Practice Review Topic 2.2 Electron Configuration and begin Topic 12.1 Atomic Structure Warm Up You have 10 minutes ONLY! GO!
Drawing Ionic Compounds
 Drawing Molecules on Paper Structures (or Dot Structures) are one way we draw molecules on paper Since paper is 2-D and molecules aren t, it s not a perfect way to represent how molecules bond but it s
Drawing Molecules on Paper Structures (or Dot Structures) are one way we draw molecules on paper Since paper is 2-D and molecules aren t, it s not a perfect way to represent how molecules bond but it s
Version 188 Exam 2 mccord (51600) 1
 Version 188 Exam 2 mccord (51600) 1 This print-out should have 35 questions. Multiple-choice questions may continue on the next column or page find all choices before answering. l I l l 001 3.0 points
Version 188 Exam 2 mccord (51600) 1 This print-out should have 35 questions. Multiple-choice questions may continue on the next column or page find all choices before answering. l I l l 001 3.0 points
PHYS 3313 Section 001 Lecture #14
 PHYS 3313 Section 001 Lecture #14 Monday, March 6, 2017 The Classic Atomic Model Bohr Radius Bohr s Hydrogen Model and Its Limitations Characteristic X-ray Spectra 1 Announcements Midterm Exam In class
PHYS 3313 Section 001 Lecture #14 Monday, March 6, 2017 The Classic Atomic Model Bohr Radius Bohr s Hydrogen Model and Its Limitations Characteristic X-ray Spectra 1 Announcements Midterm Exam In class
First Name CARI / PHIL / ADAM / HEATHER. Chem 30A Fall 2004 MIDTERM #1 (50 Min) Weds October 27th
 Last First MI Student ID Number: Total Score Circle the name of your TA: CARI / PIL / ADAM / EATER Discussion Section Day: Time: / 100 Chem 30A Fall 2004 MIDTERM #1 (50 Min) Weds October 27th INTERPRETATION
Last First MI Student ID Number: Total Score Circle the name of your TA: CARI / PIL / ADAM / EATER Discussion Section Day: Time: / 100 Chem 30A Fall 2004 MIDTERM #1 (50 Min) Weds October 27th INTERPRETATION
1. My answers for this Chemistry 102 exam should be graded with the answer sheet associated with: a. Form A b. Form B c. Form C d. Form D e.
 EMISTRY 10 Fall 010 our Exam Page 1 1. My answers for this hemistry 10 exam should be graded with the answer sheet associated with: a. Form A b. Form B c. Form d. Form D e. Form E. Which of the statements
EMISTRY 10 Fall 010 our Exam Page 1 1. My answers for this hemistry 10 exam should be graded with the answer sheet associated with: a. Form A b. Form B c. Form d. Form D e. Form E. Which of the statements
Placement Exam for Exemption from Chemistry 120 Fall 2006
 Placement Exam for Exemption from Chemistry 120 Fall 2006 Name: By submitting this exam you affirm that you have not consulted any person, book, website or any other source for assistance. You may use
Placement Exam for Exemption from Chemistry 120 Fall 2006 Name: By submitting this exam you affirm that you have not consulted any person, book, website or any other source for assistance. You may use
AP PHYSICS 2011 SCORING GUIDELINES
 AP PHYSICS 2011 SCORING GUIDELINES General Notes About 2011 AP Physics Scoring Guidelines 1. The solutions contain the most common method of solving the free-response questions and the allocation of points
AP PHYSICS 2011 SCORING GUIDELINES General Notes About 2011 AP Physics Scoring Guidelines 1. The solutions contain the most common method of solving the free-response questions and the allocation of points
CHEM 163 MIDTERM October 23, 2001 Dr. John C. Vederas
 CHEM 163 MIDTERM October 23, 2001 Dr. John C. Vederas I. Structure and Nomenclature - 56 Points - A. Draw structures for which names are given, or name the given structures by any correct (systematic or
CHEM 163 MIDTERM October 23, 2001 Dr. John C. Vederas I. Structure and Nomenclature - 56 Points - A. Draw structures for which names are given, or name the given structures by any correct (systematic or
PLEASE read the questions carefully! Partial Periodic Table
 CEMISTRY 3311, Fall 1997 Professor Walba First our Exam September 25, 1997 ame scores: 1) 2) This is a closed-book "open model" exam. You may use models, but no notes 3) or books. Please put all your answers
CEMISTRY 3311, Fall 1997 Professor Walba First our Exam September 25, 1997 ame scores: 1) 2) This is a closed-book "open model" exam. You may use models, but no notes 3) or books. Please put all your answers
Nuclear magnetic resonance spectroscopy II. 13 C NMR. Reading: Pavia Chapter , 6.7, 6.11, 6.13
 Nuclear magnetic resonance spectroscopy II. 13 NMR Reading: Pavia hapter 6.1-6.5, 6.7, 6.11, 6.13 1. General - more/better/additional structural information for larger compounds -problems: a) isotopes
Nuclear magnetic resonance spectroscopy II. 13 NMR Reading: Pavia hapter 6.1-6.5, 6.7, 6.11, 6.13 1. General - more/better/additional structural information for larger compounds -problems: a) isotopes
Photoelectron Spectroscopy
 32 ChemActivity 8 Photoelectron Spectroscopy (What Is Photoelectron Spectroscopy?) From our previous examination of the ionization energies of the atoms, we proposed a shell model of the atom, and noted
32 ChemActivity 8 Photoelectron Spectroscopy (What Is Photoelectron Spectroscopy?) From our previous examination of the ionization energies of the atoms, we proposed a shell model of the atom, and noted
and the localized electron bonding model: bonds are formed by a pair of electrons being shared by two atoms.
 Lewis Structures 167 and the localized electron bonding model: bonds are formed by a pair of electrons being shared by two atoms. Bonding pairs and lone pairs: since an orbital can hold two electrons we
Lewis Structures 167 and the localized electron bonding model: bonds are formed by a pair of electrons being shared by two atoms. Bonding pairs and lone pairs: since an orbital can hold two electrons we
