Final Exam. CHEM 181: Introduction to Chemical Principles December 14, 2015 Answer Key N C S C
|
|
|
- Claude Harmon
- 5 years ago
- Views:
Transcription
1 Final Exam EM 181: Introduction to hemical Principles December 14, 2015 Answer Key 1. The compound 4 5 NO has the connectivity shown below O N On the next page: Draw all reasonable resonance structures and label them as major or minor. Label all non-zero formal charges. Rank the resonance structures: 1=best, 2=next best, etc. If two resonant structures are equivalent or very close to equivalent, you can assign them the same number in the ranking. If you prefer, you can skip writing the carbons and the hydrogens attached to N the carbons, like so: O 1
2 There is only one structure with zero formal charges; everything else is minor. Ranking of minor structures is based on electronegativity of the atom getting the positive charge. O N 1, major O N 3 O N 2, minor O N 3 O N 2 O N 4 2
3 2. A compound containing only,, and O with a molecular weight under 110 amu has the following 13 NMR spectrum (and note that peak heights in this 13 NMR spectrum are not meaningful): IR spectrum: 3
4 Dl 3 QE and 1 NMR spectrum: expanded to show detail: Draw a Lewis structure for the compound. Also mark on each spectrum: (a) which protons correspond to which peaks in the 1 NMR spectrum, and (b) labels for peaks in the IR spectrum that can be assigned unambiguously. 4
5 3. The electron configurations for the ground state and first three excited states of N 2 are shown. Use the following information about energies and bond lengths to figure out the electron configurations for the ground and excited states of N. (All answers that are logical and self-consistent will be marked as correct. You can use the MO diagram for N 2 on the next page the MO diagram for N will have states with different energies, but the order and names will be the same.) N 2 energy (aj) bond length (nm) σ 2s σ 2s π 2px, π 2py σ 2pz π 2p x, π 2p y σ 2p z N energy (aj) bond length (nm) σ 2s σ 2s π 2px, π 2py σ 2pz π 2p x, π 2p y σ 2p z
6 There are a few ways to answer this in a self-consistent manner. ere is one line of reasoning: The 0.18 aj state must come from a transition not available to N 2. Promoting a π 2px (or y) to a σ 2pz meets this requirement. Both are bonding, so the overall bond order is the same, and the bond length changes only slightly. The 1.08 aj and 1.17 aj states involve significant increases in bond length, and should involve moving a bonding electron to an antibonding orbital. Match the 1.08 aj state for N to the 1.00 aj state for N 2, and the 1.17 aj to the 1.18 aj state. The 0.51 aj also has no clear counterpart in N 2. ince bond length decreases slightly, this means either bond order must stay the same or increase. For that to be the case, an electron must move out of the σ2s orbital, and the relatively low energy of this state would place it into the σ 2pz. The 2s orbital for will be significantly higher in energy than the N 2s, which will bring the σ2s much closer in energy to the states derived from the 2p orbitals. 6
7 4. Use the following enthalpies of formation f 2 6 (g) 83.7 N 2 4 (g) and the following bond enthalpies bond N N N N N alculate the enthalpy of formation of 3 N 2 (g). N The reaction 2 6 (g) + N 2 4 (g) 2 3 N 2 (g) involves breaking a and an N N bond, and making two N bonds; viz., 2 6 (g) + N 2 4 (g) 3 (g) + 3 (g) + N 2 (g) + N 2 (g) 2 3 N 2 (g) o rxn = bond(reactants) bond(products) = bond( ) + bond(n N) 2 bond( N) = = 7 kj mol 1 But we can also express rxn in terms of formation enthalpies: rxn = f (products) f (reactants) 7 kj mol 1 = 2 f ( 3 N 2 (g)) [ f ( 2 6 (g)) + f (N 2 4 (g)) ] 7 kj mol 1 = 2 f ( 3 N 2 (g)) [ 83.7 kj mol kj mol 1] 2 f ( 3 N 2 (g)) = 7 kj mol kj mol kj mol 1 = 26 kj mol 1 f ( 3 N 2 (g)) = 13 kj mol 1 7
8 5. Molecular iodine (I 2 ) is slightly soluble in water and somewhat more soluble in cyclohexane ( 6 12 ): K 20 K 50 I 2 (s) 2O(l) I 2 (aq) M M I 2 (s) 6 12 (l) I 2 ( 6 12 sol.) M M Water and cyclohexane do not mix, and iodine will distribute between the two solvents according to the reaction: I 2 (aq) I 2 ( 6 12 sol.) For this reaction, what is K at 20, and what are and? (Assume that and are constant over this temperature range.) Our two reactions have very simple equilibrium constant expressions: K sp (aq) = [I 2 (aq)] and K sp ( 6 12 ) = [I 2 ( 6 12 sol.)] For we have I 2 (aq) I 2 ( 6 12 sol.) K = [I 2( 6 12 sol.)] [I 2 (aq)] = = 7.76 This is at 20 ; at 50, K = We can use the 20 value to find G : G = RT ln K = (8.314 J mol 1 K 1 ) (293 K) ln 7.76 = 4.99 kj mol 1 The van t off equation gives us using the values of K at the two different 8
9 temperatures: ln K 2 K 1 = R ( 1 T 2 1 T 1 ) ln = J mol 1 K (8.314 J mol 1 K 1 ) = ( K 1 ) = 1.48 kj mol 1 ( K 1 ) 293 K ince the equilibrium constant increases (albeit slightly) with temperature, Lehâtelier s principle says should be positive (albeit slightly): add heat to raise the temperature, and equilibrium shifts towards products in order to take up some of that heat. Then, G = T 4.99 kj mol 1 = 1.48 kj mol 1 (293 K) = 6.47 kj mol K = 22.1 J mol 1 K 1 9
10 6. ere are the chemical reactions to consider in the reaction of baking soda (NaO 3 ) with vinegar ( 3 OO(aq)): O 2 (g) O 2 (aq) K = M atm 1 O 2 (aq) + 2 O(l) 2 O 3 (aq) K = O 3 (aq) O 3 (aq) + + (aq) K a1 = M O 3 (aq) O 2 3 (aq) + + (aq) K a2 = M 3 OO(aq) 3 OO (aq) + + (aq) K a = M 2 O(l) + (aq) + O (aq) K w = M 2 (a) 1 L of a 0.8 M aqueous solution of 3 OO has NaO 3 added to it until it reaches p 7, at which point it is at equilibrium with respect to all of the above reactions, and with atmospheric O 2 (g) with P O2 = atm. ow many moles of NaO 3 were added to reach this equilibrium? (int: one way to do this is to figure out [Na + ] at equilibrium.) Equilibrium constant expressions for all of the above: [O 2 (aq)] P O2 (g) = M atm 1 [ 2 O 3 (aq)] [O 2 (aq)] = [ + ][O 3 (aq)] [ 2 O 3 (aq)] = M [ + ][O 2 3 (aq)] [O 3 (aq)] = M [ + ][ 3 OO (aq)] [ 3 OO(aq)] = M [ + (aq)][o (aq)] = M 2 We have P O2 in: fixed at an equilibrium value of atm. Plugging [O 2 (aq)] P O2 (g) = M atm 1 [O 2 (aq)] atm = M atm 1 [O 2 (aq)] = M [ 2 O 3 (aq)] = M 10
11 At p 7, [ + ] must be 10 7 at equilibrium, so [ + ][O 3 (aq)] [ 2 O 3 (aq)] = M 10 7 [O 3 (aq)] = M [O 3 (aq)] = M [O 2 3 (aq)] = M On the other hand, at p 7 almost all of the acetic acid is in its conjugate base form: [ + ][ 3 OO (aq)] [ 3 OO(aq)] 10 7 [ 3 OO (aq)] [ 3 OO(aq)] [ 3 OO (aq)] [ 3 OO(aq)] = M = M = 180 o at equilibrium, the negative ions and their concentrations in solution are just under 0.8 M of 3 OO, M of O 3, 10 7 M of O, and M of O 2 3. There is only 10 7 M of +, so a neutral solution requires 0.8 moles of NaO 3 were added. [Na + (aq)] 0.8 M 11
12 (b) 1 L of a 0.8 M aqueous solution of 3 OO has NaO 3 added to it until it reaches p 7, at which point it is at equilibrium with respect to all of the above reactions. In this case, the reaction takes place in a sealed 4 L container. ompared to part (a), how much NaO 3 was added: more, slightly more, exactly the same amount, slightly less, or less? Explain your answer. In a sealed container, P O2 is going to be much higher, resulting in significant concentrations of all of the carbonate species, and in particular O 3 (aq). The extra negative charge must be balanced by extra sodium, so we will need more NaO 3. Alternately, keeping higher pressures of O 2 (g) will act to acidify the solution, so you must add more base (NaO 3 ) to counteract this. (c) alculate how many moles of NaO 3 were added in part (b). (Use T = 298 K. int to simplify computations: the 3 L space left in the container will hold P /atm moles of gas at this temperature.) With a O 2 (g) pressure of P at equilibrium, the following come from our equilibrium constant expressions: [O 2 (aq)] = (P/atm) M [ 2 O 3 (aq)] = (P/atm) M [O 3 (aq)] = (P/atm) M [O 2 3 (aq)] = (P/atm) M Each O 3 that is protonated ends up either as an 2 O 3 (aq), a O 2 (aq), or a O 2 (g). ince [ 3 OO (aq)] 0.8 M, there are 0.8 moles of protons to be accounted for. The number of protons making 2 O 3 (aq) is more or less cancelled out by the number released forming O 2 3 (aq), so 0.8 mol n O2 (aq) + n O2 (g) = (P/atm) M 1 L (P/atm) mol = (P/atm) mol P = 5.1 atm The O 3 concentration must be [O 3 (aq)] = (P/atm) M = 0.45 M In contrast to the situation in part (a), our negative-ion species in solution are 12
13 just under 0.8 M of 3 OO, 0.45 M of O 3, negligible amounts of O and O 2 3. We must have added 1.25 M of NaO 3. 13
Final Exam. CHEM 181: Introduction to Chemical Principles Answer Key. 1. The major resonance structure of 1,4-diazepine is shown below:
 Final Exam EM 181: Introduction to hemical Principles Answer Key 1. The major resonance structure of 1,4-diazepine is shown below: or Show all reasonable resonance structures; label them as major (if equivalent
Final Exam EM 181: Introduction to hemical Principles Answer Key 1. The major resonance structure of 1,4-diazepine is shown below: or Show all reasonable resonance structures; label them as major (if equivalent
Midterm Exam III. CHEM 181: Introduction to Chemical Principles November 25, 2014 Answer Key
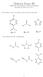 Midterm Exam III EM 181: Introduction to hemical Principles ovember 25, 2014 Answer Key 1. For reference, here are the pk a values for three weak acids: 3 pk a = 7.2 pk a = 4.8 pk a = 15 ow consider this
Midterm Exam III EM 181: Introduction to hemical Principles ovember 25, 2014 Answer Key 1. For reference, here are the pk a values for three weak acids: 3 pk a = 7.2 pk a = 4.8 pk a = 15 ow consider this
Midterm Exam III. CHEM 181: Introduction to Chemical Principles Solutions C HC N H 3 C. pka = 9.7
 Midterm Exam III EM 181: Introduction to hemical Principles Solutions 1. For reference, here are the pk a values for four weak acids (not all resonance structures shown): N 3 N N 3 3 2 pka = 3.5 pka =
Midterm Exam III EM 181: Introduction to hemical Principles Solutions 1. For reference, here are the pk a values for four weak acids (not all resonance structures shown): N 3 N N 3 3 2 pka = 3.5 pka =
Midterm Exam I. CHEM 181: Introduction to Chemical Principles September 24, 2015 Key
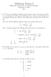 Midterm Exam I CHEM 8: Introduction to Chemical Principles September 24, 205 Key. A Li 2+ ion in an unknown, highly excited electronic state, first emits a photon at a wavelength of 4.36 µm, and following
Midterm Exam I CHEM 8: Introduction to Chemical Principles September 24, 205 Key. A Li 2+ ion in an unknown, highly excited electronic state, first emits a photon at a wavelength of 4.36 µm, and following
Lecture 21 Cations, Anions and Hydrolysis in Water:
 2P32 Principles of Inorganic Chemistry Dr. M. Pilkington Lecture 21 Cations, Anions and ydrolysis in Water: 1. ydration.energy 2. ydrolysis of metal cations 3. Categories of acidity and observable behavior
2P32 Principles of Inorganic Chemistry Dr. M. Pilkington Lecture 21 Cations, Anions and ydrolysis in Water: 1. ydration.energy 2. ydrolysis of metal cations 3. Categories of acidity and observable behavior
WYSE Academic Challenge 2004 Sectional Chemistry Solution Set
 WYSE Academic Challenge 2004 Sectional Chemistry Solution Set 1. Answer: d. Assume 100.0 g of the compound. Thus, we have 40.00 g of carbon, or 40.00/12.01 = 3.33 mol C. We have 6.71 g of hydrogen, or
WYSE Academic Challenge 2004 Sectional Chemistry Solution Set 1. Answer: d. Assume 100.0 g of the compound. Thus, we have 40.00 g of carbon, or 40.00/12.01 = 3.33 mol C. We have 6.71 g of hydrogen, or
Midterm Exam IV. CHEM 181: Introduction to Chemical Principles November 29, C(s, coal) + O 2 (g) CO 2 (g)
 Midterm Exam IV CHEM 8: Introduction to Chemical Principles November 29, 202. Use some or all of the following data: C(graphite) 0. C(diamond).9 CO 2 (g) 393.5 H 2 O(g) 24.8 CH 4 (g) 74.6 H f (kj mol )
Midterm Exam IV CHEM 8: Introduction to Chemical Principles November 29, 202. Use some or all of the following data: C(graphite) 0. C(diamond).9 CO 2 (g) 393.5 H 2 O(g) 24.8 CH 4 (g) 74.6 H f (kj mol )
CHEMISTRY 110 Final EXAM Dec 17, 2012 FORM A
 CEMISTRY 110 Final EXAM Dec 17, 2012 FORM A 1. Given the following reaction which of the following statements is true? Fe(s) + CuCl 2 (aq)! Cu(s) + FeCl 2 (aq) A. Iron is oxidized and copper is reduced.
CEMISTRY 110 Final EXAM Dec 17, 2012 FORM A 1. Given the following reaction which of the following statements is true? Fe(s) + CuCl 2 (aq)! Cu(s) + FeCl 2 (aq) A. Iron is oxidized and copper is reduced.
Cork Institute of Technology. Autumn 2005 Chemistry (Time: 3 Hours)
 Cork Institute of Technology Higher Certificate in Science in Applied Biology Stage 1 (National Certificate in Science in Applied Biology Stage 1) (NFQ Level 6) Autumn 2005 Chemistry (Time: 3 Hours) Instructions
Cork Institute of Technology Higher Certificate in Science in Applied Biology Stage 1 (National Certificate in Science in Applied Biology Stage 1) (NFQ Level 6) Autumn 2005 Chemistry (Time: 3 Hours) Instructions
1. (24 points) 5. (8 points) 6d. (13 points) 7. (6 points)
 1 of 9 Third our Exam 5.111 Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. The exam is closed notes and closed book. 1. Read all parts of each
1 of 9 Third our Exam 5.111 Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. The exam is closed notes and closed book. 1. Read all parts of each
4. Which of the following gas molecules will have the highest average velocity at 500K? a. H 2 b. He c. CH 4 d. C 2H 6
 Multiple hoice (3 pts each) 1. In which of the following processes will work be positive? Assume the pressure remains constant for all examples. a. 2(l) 2(g) at constant temperature b. 2 8 18(l) + 25 2(g)
Multiple hoice (3 pts each) 1. In which of the following processes will work be positive? Assume the pressure remains constant for all examples. a. 2(l) 2(g) at constant temperature b. 2 8 18(l) + 25 2(g)
Name. Chapter 4 covers acid-base chemistry. That should help you get going.
 Name Chapter 4 covers acid-base chemistry. That should help you get going. 1 Use curved arrows to illustrate the transfer of a proton (i.e. an + ) from benzoic acid to phenoxide, and draw the products.
Name Chapter 4 covers acid-base chemistry. That should help you get going. 1 Use curved arrows to illustrate the transfer of a proton (i.e. an + ) from benzoic acid to phenoxide, and draw the products.
The rate of reaction is defined as the change in concentration of a substance in unit time Its usual unit is mol dm -3 s -1
 16. Kinetics II The rate of reaction is defined as the change in concentration of a substance in unit time Its usual unit is mol dm -3 s -1 When a graph of concentration of reactant is plotted vs time,
16. Kinetics II The rate of reaction is defined as the change in concentration of a substance in unit time Its usual unit is mol dm -3 s -1 When a graph of concentration of reactant is plotted vs time,
The rate of reaction is defined as the change in concentration of a substance in unit time Its usual unit is mol dm -3 s -1
 11. Kinetics The rate of reaction is defined as the change in concentration of a substance in unit time Its usual unit is mol dm -3 s -1 When a graph of concentration of reactant is plotted vs time, the
11. Kinetics The rate of reaction is defined as the change in concentration of a substance in unit time Its usual unit is mol dm -3 s -1 When a graph of concentration of reactant is plotted vs time, the
Electrochemistry: Oxidation numbers. EIT Review F2006 Dr. J.A. Mack. Electrochemistry: Oxidation numbers
 EIT Review F2006 Dr. J.A. Mack Electrochemistry: Oxidation numbers In the compound potassium bromate (KBrO 3 ), the oxidation number of bromine (Br) is? www.csus.edu/indiv/m/mackj/ Part 2 38 39 +1 +2 Oxidation
EIT Review F2006 Dr. J.A. Mack Electrochemistry: Oxidation numbers In the compound potassium bromate (KBrO 3 ), the oxidation number of bromine (Br) is? www.csus.edu/indiv/m/mackj/ Part 2 38 39 +1 +2 Oxidation
M09/4/CHEMI/SP2/ENG/TZ1/XX+ CHEMISTRY. Monday 18 May 2009 (afternoon) Candidate session number. 1 hour 15 minutes INSTRUCTIONS TO CANDIDATES
 M09/4/EMI/SP2/ENG/TZ1/XX+ 22096111 EMISTRY standard level Paper 2 Monday 18 May 2009 (afternoon) 1 hour 15 minutes 0 0 andidate session number INSTRUTINS T ANDIDATES Write your session number in the boxes
M09/4/EMI/SP2/ENG/TZ1/XX+ 22096111 EMISTRY standard level Paper 2 Monday 18 May 2009 (afternoon) 1 hour 15 minutes 0 0 andidate session number INSTRUTINS T ANDIDATES Write your session number in the boxes
Chemistry 1A, Spring 2008 Final Exam, Version A May 17, 2008 (180 min, closed book)
 Chemistry 1A, Spring 2008 Final Exam, Version A May 17, 2008 (180 min, closed book) Name: SID: TA Name: 1) Write your name on every page of this exam. 2) This exam has 25 multiple-choice questions and
Chemistry 1A, Spring 2008 Final Exam, Version A May 17, 2008 (180 min, closed book) Name: SID: TA Name: 1) Write your name on every page of this exam. 2) This exam has 25 multiple-choice questions and
TERMS AND DEFINITIONS IN THERMOCHEMISTRY
 (v.2.0 16/3/90 8hrs.) TERMS AND DEFINITIONS IN THERMOCHEMISTRY (v.2.1 12/4/90 2hrs.) 1) ENTHALPY CHANGE ( H) This is the same as the heat change involved in a process (provided the initial and final states
(v.2.0 16/3/90 8hrs.) TERMS AND DEFINITIONS IN THERMOCHEMISTRY (v.2.1 12/4/90 2hrs.) 1) ENTHALPY CHANGE ( H) This is the same as the heat change involved in a process (provided the initial and final states
CHAPTER 9 COVALENT BONDING: ORBITALS 323
 APTER 9 OVALET BODIG: ORBITALS 323 2 3 2 2 2 3 3 2 2 3 2 3 O * * 2 o; most of the carbons are not in the same plane since a majority of carbon atoms exhibit a tetrahedral structure (19.5 bond angles).
APTER 9 OVALET BODIG: ORBITALS 323 2 3 2 2 2 3 3 2 2 3 2 3 O * * 2 o; most of the carbons are not in the same plane since a majority of carbon atoms exhibit a tetrahedral structure (19.5 bond angles).
Acid-Base Chemistry & Organic Compounds. Chapter 2
 Acid-Base Chemistry & Organic Compounds Chapter 2 Brønsted Lowry Acids & Bases! Brønsted-Lowry Acid: Proton (H + ) Donor! Brønsted-Lowry Base: Proton (H + ) Acceptor! General reaction: HA + B: A - + BH
Acid-Base Chemistry & Organic Compounds Chapter 2 Brønsted Lowry Acids & Bases! Brønsted-Lowry Acid: Proton (H + ) Donor! Brønsted-Lowry Base: Proton (H + ) Acceptor! General reaction: HA + B: A - + BH
CHM 151 Practice Final Exam
 CM 151 Practice Final Exam 1. ow many significant figures are there in the result of 5.52 divided by 3.745? (a) 1 (b) 2 (c) 3 (d) 4 (e) 5 2. ow many significant figures are there in the answer when 9.021
CM 151 Practice Final Exam 1. ow many significant figures are there in the result of 5.52 divided by 3.745? (a) 1 (b) 2 (c) 3 (d) 4 (e) 5 2. ow many significant figures are there in the answer when 9.021
Chemistry 231 Fall 2014 Oregon State University Final Exam December 8, 2014 Drs. Nafshun, Watson, Nyman, Barth, Burand
 Chemistry 231 Fall 2014 Oregon State University Final Exam December 8, 2014 Drs. Nafshun, Watson, Nyman, Barth, Burand Instructions: You should have with you several number two pencils, an eraser, your
Chemistry 231 Fall 2014 Oregon State University Final Exam December 8, 2014 Drs. Nafshun, Watson, Nyman, Barth, Burand Instructions: You should have with you several number two pencils, an eraser, your
Final Exam Worksheet Answers Chemistry 102
 Final Exam Worksheet Answers hemistry 102 hapter 9 hemical Bonding I The ovalent Bond 1. a. I b. I c. d. I e. f. N g. I h. I i. I j. k. N l. m. n. N o. IN p. q. I 2. For the Born aber cycle the standard
Final Exam Worksheet Answers hemistry 102 hapter 9 hemical Bonding I The ovalent Bond 1. a. I b. I c. d. I e. f. N g. I h. I i. I j. k. N l. m. n. N o. IN p. q. I 2. For the Born aber cycle the standard
Fifth Exam CHEM 1A Summer 2017
 ifth Exam EM 1A Summer 2017 Name: Last KEY irst Instructions: Read every problem carefully. Gauge your time. Use the proper number of significant figures on your results. Don t just believe the calculator,
ifth Exam EM 1A Summer 2017 Name: Last KEY irst Instructions: Read every problem carefully. Gauge your time. Use the proper number of significant figures on your results. Don t just believe the calculator,
Chapter 3. Acids and Bases
 Chapter 3 Acids and Bases 3.1 Acids and Bases Brønsted-Lowry definition Acids donate a proton Bases accept a proton Recall from General Chemistry this classic example 3-2 3.1 Conjugate Acids and Bases
Chapter 3 Acids and Bases 3.1 Acids and Bases Brønsted-Lowry definition Acids donate a proton Bases accept a proton Recall from General Chemistry this classic example 3-2 3.1 Conjugate Acids and Bases
General Chemistry I Final Exam 100 pts Fall 2010
 General Chemistry I Final Exam 100 pts Fall 2010 Name This is a closed-book exam: the only reference materials you may use are a periodic table of the elements, a table of enthalpies of formation, and
General Chemistry I Final Exam 100 pts Fall 2010 Name This is a closed-book exam: the only reference materials you may use are a periodic table of the elements, a table of enthalpies of formation, and
D. Bond making is endothermic and releases energy. (Total 1 mark) Cu(s) + 2. D (Total 1 mark)
 1. Which statement about bonding is correct? A. Bond breaking is endothermic and requires energy. B. Bond breaking is endothermic and releases energy. C. Bond making is exothermic and requires energy.
1. Which statement about bonding is correct? A. Bond breaking is endothermic and requires energy. B. Bond breaking is endothermic and releases energy. C. Bond making is exothermic and requires energy.
Chapter 2: Acids and Bases
 hapter 2: Acids and Bases 32 hapter 2: Acids and Bases Problems 2.1 Write each acid- reaction as a proton-transfer reaction. Label which reactant is the acid and which the, as well as which product is
hapter 2: Acids and Bases 32 hapter 2: Acids and Bases Problems 2.1 Write each acid- reaction as a proton-transfer reaction. Label which reactant is the acid and which the, as well as which product is
Aqueous Equilibria: Acids and Bases
 /3/014 Aqueous Equilibria: Acids and Bases Ch. 16 What is an? What is a? There are actually multiple definitions Arrhenius: Dealt with species in aqueous solutions. Most basic definition of acis. Acid:
/3/014 Aqueous Equilibria: Acids and Bases Ch. 16 What is an? What is a? There are actually multiple definitions Arrhenius: Dealt with species in aqueous solutions. Most basic definition of acis. Acid:
Chapter 11 Answers. Practice Examples
 hapter Answers Practice Examples a. There are three half-filled p orbitals on, and one half-filled 5p orbital on I. Each halffilled p orbital from will overlap with one half-filled 5p orbital of an I.
hapter Answers Practice Examples a. There are three half-filled p orbitals on, and one half-filled 5p orbital on I. Each halffilled p orbital from will overlap with one half-filled 5p orbital of an I.
1. (24 points) 2. (15 points) 4. (6 points) 6a,b,c. (20 points)
 1 of 9 Third our Exam 5.111 Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. The exam is closed notes and closed book. 1. Read all parts of each
1 of 9 Third our Exam 5.111 Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. The exam is closed notes and closed book. 1. Read all parts of each
First Law of Thermodynamics: energy cannot be created or destroyed.
 1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
ORGANIC - CLUTCH CH. 3 - ACIDS AND BASES.
 !! www.clutchprep.com CONCEPT: OVERVIEW OF CHEMICAL REACTIONS There are 4 types of common chemical reactions that we need to be familiar with in organic chemistry 1. Acid-Base Reactions: Two molecules
!! www.clutchprep.com CONCEPT: OVERVIEW OF CHEMICAL REACTIONS There are 4 types of common chemical reactions that we need to be familiar with in organic chemistry 1. Acid-Base Reactions: Two molecules
1.) The answer to question 1 is A. Bubble in A on your Scantron form.
 Part 1: Multiple Choice. (5 pts each, 145 pts total) Instructions: Bubble in the correct answer on your Scantron form AND circle the answer on your exam. Each question has one correct answer. 1.) The answer
Part 1: Multiple Choice. (5 pts each, 145 pts total) Instructions: Bubble in the correct answer on your Scantron form AND circle the answer on your exam. Each question has one correct answer. 1.) The answer
Chemistry 105: General Chemistry I Dr. Gutow and Dr. Matsuno Spring 2004 Page 1
 Page 1 1) Name You are to keep this copy of the test. Your name is in case you leave it behind. 2) Use only a #2 pencil on the answer sheet. 3) Before starting the exam fill in your student ID# (not your
Page 1 1) Name You are to keep this copy of the test. Your name is in case you leave it behind. 2) Use only a #2 pencil on the answer sheet. 3) Before starting the exam fill in your student ID# (not your
CH 222 Sample Exam Exam I Name: Lab Section:
 222 Sample Exam Exam I Name: Lab Section: Part I: Multiple hoice Questions (100 Points) Use a scantron sheet for Part I. There is only one best answer for each question. 1. Which of the following statements
222 Sample Exam Exam I Name: Lab Section: Part I: Multiple hoice Questions (100 Points) Use a scantron sheet for Part I. There is only one best answer for each question. 1. Which of the following statements
Chem GENERAL CHEMISTRY II
 GENERAL CHEMISTRY II CHEM 206 /2 51 Final Examination December 19, 2006 1900-2200 Dr. Cerrie ROGERS x x programmable calculators must be reset Chem 206 --- GENERAL CHEMISTRY II LAST NAME: STUDENT NUMBER:
GENERAL CHEMISTRY II CHEM 206 /2 51 Final Examination December 19, 2006 1900-2200 Dr. Cerrie ROGERS x x programmable calculators must be reset Chem 206 --- GENERAL CHEMISTRY II LAST NAME: STUDENT NUMBER:
Use your time wisely. Do not get stuck on one question. WORK MUST BE SHOWN CAREFULLY, WITH UNITS AT EVERY STEP OF SETUP.
 Spring 2014 CCBC-Catonsville (Wed 3/12/14) Use your time wisely. Do not get stuck on one question. WORK MUST BE SHOWN CAREFULLY, WITH UNITS AT EVERY STEP OF SETUP. PAGE TOTAL SCORE POSSIBLE YOUR SCORE
Spring 2014 CCBC-Catonsville (Wed 3/12/14) Use your time wisely. Do not get stuck on one question. WORK MUST BE SHOWN CAREFULLY, WITH UNITS AT EVERY STEP OF SETUP. PAGE TOTAL SCORE POSSIBLE YOUR SCORE
ACID-BASE EXTRACTION
 ACID-BASE EXTRACTION An acid-base extraction is a type of liquid-liquid extraction. It typically involves different solubility levels in water and an organic solvent. The organic solvent may be any carbon-based
ACID-BASE EXTRACTION An acid-base extraction is a type of liquid-liquid extraction. It typically involves different solubility levels in water and an organic solvent. The organic solvent may be any carbon-based
ORGANIC - BROWN 8E CH.4 - ACIDS AND BASES.
 !! www.clutchprep.com CONCEPT: FREE ENERGY DIAGRAMS Atoms save energy by forming bonds. Free energy diagrams show overall changes in potential energy during reactions. Free energy diagrams give us information
!! www.clutchprep.com CONCEPT: FREE ENERGY DIAGRAMS Atoms save energy by forming bonds. Free energy diagrams show overall changes in potential energy during reactions. Free energy diagrams give us information
Topics in the November 2014 Exam Paper for CHEM1101
 November 2014 Topics in the November 2014 Exam Paper for CHEM1101 Click on the links for resources on each topic. 2014-N-2: 2014-N-3: 2014-N-4: 2014-N-5: 2014-N-7: 2014-N-8: 2014-N-9: 2014-N-10: 2014-N-11:
November 2014 Topics in the November 2014 Exam Paper for CHEM1101 Click on the links for resources on each topic. 2014-N-2: 2014-N-3: 2014-N-4: 2014-N-5: 2014-N-7: 2014-N-8: 2014-N-9: 2014-N-10: 2014-N-11:
Chem. 1B Final Practice
 Chem. 1B Final Practice Name Student Number All work must be shown on the exam for partial credit. Points will be taken off for incorrect or no units and for the incorrect number of significant figures.
Chem. 1B Final Practice Name Student Number All work must be shown on the exam for partial credit. Points will be taken off for incorrect or no units and for the incorrect number of significant figures.
Chemistry 1A, Fall 2003 Midterm 2 Oct 14, 2003 (90 min, closed book)
 Name: SID: TA Name: Chemistry 1A, Fall 2003 Midterm 2 Oct 14, 2003 (90 min, closed book) This exam has 45 multiple choice questions. Fill in the Scantron form AND circle your answer on the exam. Each question
Name: SID: TA Name: Chemistry 1A, Fall 2003 Midterm 2 Oct 14, 2003 (90 min, closed book) This exam has 45 multiple choice questions. Fill in the Scantron form AND circle your answer on the exam. Each question
Name Date IB Chemistry HL-II Summer Review Unit 1 Atomic Structure IB 2.1 The nuclear atom
 Name Date IB Chemistry HL-II Summer Review Unit 1 Atomic Structure IB.1 The nuclear atom 1. State the number of protons, neutrons, and electrons in each of the following: a. 65 Cu b. 15 N 3- c. 137 Ba
Name Date IB Chemistry HL-II Summer Review Unit 1 Atomic Structure IB.1 The nuclear atom 1. State the number of protons, neutrons, and electrons in each of the following: a. 65 Cu b. 15 N 3- c. 137 Ba
KEY. Chemistry 1A, Fall2003 Midterm Exam III, Version A November 13, 2003 (90 min, closed book)
 KEY Name: SID: TA Name: hemistry 1A, Fall2003 Midterm Exam III, Version A November 13, 2003 (90 min, closed book) Write your name on every page of this exam. This exam is multiple choice. Fill in the Scantron
KEY Name: SID: TA Name: hemistry 1A, Fall2003 Midterm Exam III, Version A November 13, 2003 (90 min, closed book) Write your name on every page of this exam. This exam is multiple choice. Fill in the Scantron
Chemistry Higher level Paper 1
 M18/4/EMI/PM/ENG/TZ2/XX hemistry igher level Paper 1 Wednesday 16 May 2018 (afternoon) 1 hour Instructions to candidates Do not open this examination paper until instructed to do so. Answer all the questions.
M18/4/EMI/PM/ENG/TZ2/XX hemistry igher level Paper 1 Wednesday 16 May 2018 (afternoon) 1 hour Instructions to candidates Do not open this examination paper until instructed to do so. Answer all the questions.
Department of Chemistry Memorial University of Newfoundland Chemistry 1050
 Department of Chemistry Memorial University of Newfoundland Chemistry 1050 FINAL EXAMINATION Fall 017 TIME: 3 hours READ TE FOLLOWING CAREFULLY 1. This examination consists of 13 pages including a Data
Department of Chemistry Memorial University of Newfoundland Chemistry 1050 FINAL EXAMINATION Fall 017 TIME: 3 hours READ TE FOLLOWING CAREFULLY 1. This examination consists of 13 pages including a Data
2nd Semester Exam Review. C. K eq = [N 2][H 2 ]
![2nd Semester Exam Review. C. K eq = [N 2][H 2 ] 2nd Semester Exam Review. C. K eq = [N 2][H 2 ]](/thumbs/82/85457292.jpg) Name: ate: 1. Which pair of formulas represents the empirical formula and the molecular formula of a compound?. H 2 O, 4 H 6 O 4. HO, 6 H 12 O 6 8. Given the reaction at equilibrium: N 2 (g) + 3H 2 (g)
Name: ate: 1. Which pair of formulas represents the empirical formula and the molecular formula of a compound?. H 2 O, 4 H 6 O 4. HO, 6 H 12 O 6 8. Given the reaction at equilibrium: N 2 (g) + 3H 2 (g)
Name: 1: /33 Grade: /100 2: /33 3: /33 +1 free point. Midterm Exam I. CHEM 181: Introduction to Chemical Principles September 20, 2012 Answer Key
 ame: 1: /33 Grade: /100 2: /33 3: /33 +1 free point Directions: Do all three problems. Midterm Exam I EM 181: Introduction to hemical Principles eptember 20, 2012 Answer Key how all of your work neatly
ame: 1: /33 Grade: /100 2: /33 3: /33 +1 free point Directions: Do all three problems. Midterm Exam I EM 181: Introduction to hemical Principles eptember 20, 2012 Answer Key how all of your work neatly
Gateway 125,126,130 Fall 2006 Exam 3 p1. Section (circle one): 601 (Colin) 602 (Brannon) 603 (Mali) 604 (Xiaomu)
 Gateway 125,126,130 Fall 2006 Exam 3 p1 Gateway General Chemistry 125/126/130 Exam 3 December 5, 2006 (8:00-10:00pm) Name Section (circle one): 601 (Colin) 602 (Brannon) 603 (Mali) 604 (Xiaomu) The exam
Gateway 125,126,130 Fall 2006 Exam 3 p1 Gateway General Chemistry 125/126/130 Exam 3 December 5, 2006 (8:00-10:00pm) Name Section (circle one): 601 (Colin) 602 (Brannon) 603 (Mali) 604 (Xiaomu) The exam
[H O ][CH COO ] [CH COOH] 0.2 x
![[H O ][CH COO ] [CH COOH] 0.2 x [H O ][CH COO ] [CH COOH] 0.2 x](/thumbs/73/68762023.jpg) CEM1612 Answers to Problem Sheet 6 1. (a) 0.2 M acetic acid As acetic acid is a weak acid, [ 3 O + ] must be calculated: C 3 2 O 3 O + C 3 initial 0.2 large 0 0 change - negligible + + final 0.2 large
CEM1612 Answers to Problem Sheet 6 1. (a) 0.2 M acetic acid As acetic acid is a weak acid, [ 3 O + ] must be calculated: C 3 2 O 3 O + C 3 initial 0.2 large 0 0 change - negligible + + final 0.2 large
Announcements. Chem 7 Final Exam Wednesday, Oct 10 1:30-3:30AM Chapter or 75 multiple choice questions
 Exam III (Chapter 7-0) Wednesday, ctober 3, 202 Time 600PM - 730PM SEC A 24A and 25A SKIPPING THIS STUFF Announcements Chem 7 Final Exam Wednesday, ct 0 30-330AM Chapter -2 70 or 75 multiple choice questions
Exam III (Chapter 7-0) Wednesday, ctober 3, 202 Time 600PM - 730PM SEC A 24A and 25A SKIPPING THIS STUFF Announcements Chem 7 Final Exam Wednesday, ct 0 30-330AM Chapter -2 70 or 75 multiple choice questions
What is the energy of a photon with wavelength 232 nm?
 EMISTRY 110 EXAM 1 February 6, 2012 FRM A 1 ow many single covalent bonds must a sulfur atom form to have a complete octet in its valence shell? A. 3 B. 4. 1 D. 2 E. 0 2. What are the correct numbers of
EMISTRY 110 EXAM 1 February 6, 2012 FRM A 1 ow many single covalent bonds must a sulfur atom form to have a complete octet in its valence shell? A. 3 B. 4. 1 D. 2 E. 0 2. What are the correct numbers of
tert-butyl alcohol n-butyl alcohol methyl propyl ether (c) Explain briefly why n-butyl alcohol has a much higher bp than methyl propyl ether.
 1. (15 points) Three 4 10 isomers are shown below, along with their boiling points. ( 3 ) 3 3 2 2 2 3 2 2 3 tert-butyl alcohol n-butyl alcohol methyl propyl ether bp: 82 118 39 (a) Based on their boiling
1. (15 points) Three 4 10 isomers are shown below, along with their boiling points. ( 3 ) 3 3 2 2 2 3 2 2 3 tert-butyl alcohol n-butyl alcohol methyl propyl ether bp: 82 118 39 (a) Based on their boiling
The following practice quiz contains 26 questions. The actual quiz will also contain 26 questions valued at 1 point/question
 hem 121 Quiz 3 Practice Winter 2018 The following practice quiz contains 26 questions. The actual quiz will also contain 26 questions valued at 1 point/question Name KEY G = T S PV = nrt P 1 V 1 = P 2
hem 121 Quiz 3 Practice Winter 2018 The following practice quiz contains 26 questions. The actual quiz will also contain 26 questions valued at 1 point/question Name KEY G = T S PV = nrt P 1 V 1 = P 2
CHEMISTRY 110 EXAM 1 SEPTEMBER 20, 2010 FORM A
 CHEMISTRY 110 EXAM 1 SEPTEMBER 20, 2010 FORM A 1. What are the correct numbers of protons, neutrons and electrons in a 39 K + ion? p n e A. 20 19 18 B. 20 19 19 C. 19 20 18 D. 19 20 19 E. 20 19 20 2. Which
CHEMISTRY 110 EXAM 1 SEPTEMBER 20, 2010 FORM A 1. What are the correct numbers of protons, neutrons and electrons in a 39 K + ion? p n e A. 20 19 18 B. 20 19 19 C. 19 20 18 D. 19 20 19 E. 20 19 20 2. Which
Learning Guide for Chapter 7 - Organic Reactions I
 Learning Guide for Chapter 7 - rganic Reactions I I. Introduction to Reactions II. Principles of Kinetics III. Principles of Thermodynamics IV. Nucleophiles and Electrophiles V. Acids and Bases What a
Learning Guide for Chapter 7 - rganic Reactions I I. Introduction to Reactions II. Principles of Kinetics III. Principles of Thermodynamics IV. Nucleophiles and Electrophiles V. Acids and Bases What a
AS Demonstrate understanding of the properties of selected organic compounds
 No Brain Too Small EMISTRY AS 91165 Demonstrate understanding of the properties of selected organic compounds ollated Flow hart Type Questions / types of reaction (2017) (a) (i) omplete the following reaction
No Brain Too Small EMISTRY AS 91165 Demonstrate understanding of the properties of selected organic compounds ollated Flow hart Type Questions / types of reaction (2017) (a) (i) omplete the following reaction
CHEM J-14 June 2014
 CHEM1101 2014-J-14 June 2014 An electrochemical cell consists of an Fe 2+ /Fe half cell with unknown [Fe 2+ ] and a Sn 2+ /Sn half-cell with [Sn 2+ ] = 1.10 M. The electromotive force (electrical potential)
CHEM1101 2014-J-14 June 2014 An electrochemical cell consists of an Fe 2+ /Fe half cell with unknown [Fe 2+ ] and a Sn 2+ /Sn half-cell with [Sn 2+ ] = 1.10 M. The electromotive force (electrical potential)
Review for Final Exam
 Review for Final Exam Chapter 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Types of Changes A physical change does not alter the composition or identity
Review for Final Exam Chapter 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Types of Changes A physical change does not alter the composition or identity
AP CHEMISTRY 2009 SCORING GUIDELINES
 2009 SCORING GUIDELINES Question 1 (10 points) Answer the following questions that relate to the chemistry of halogen oxoacids. (a) Use the information in the table below to answer part (a)(i). Acid HOCl
2009 SCORING GUIDELINES Question 1 (10 points) Answer the following questions that relate to the chemistry of halogen oxoacids. (a) Use the information in the table below to answer part (a)(i). Acid HOCl
Name: Class: Date: 3. How many lone pairs of electrons are assigned to the carbon atom in carbon monoxide? a. 0 b. 1 c. 2 d. 3
 Class: Date: Midterm 3, Fall 2009 Record your name on the top of this exam and on the scantron form. Record the test ID letter in the top right box of the scantron form. Record all of your answers on the
Class: Date: Midterm 3, Fall 2009 Record your name on the top of this exam and on the scantron form. Record the test ID letter in the top right box of the scantron form. Record all of your answers on the
Chem 152 Final. You will have 1 hour and 50 minutes. Do not begin the exam until you are instructed to start. Best of luck.
 Chem 152 Final Section: Name: You will have 1 hour and 50 minutes. Do not begin the exam until you are instructed to start. Best of luck. Question 1 /80 Question 2 /20 Question 3 /20 Question 4 /20 Question
Chem 152 Final Section: Name: You will have 1 hour and 50 minutes. Do not begin the exam until you are instructed to start. Best of luck. Question 1 /80 Question 2 /20 Question 3 /20 Question 4 /20 Question
Ionic and Covalent Bonding
 Chapter 9 Ionic and Covalent Bonding Concept Check 9.1 The following are electron configurations of some ions. Which ones would you expect to see in chemical compounds? State the concept or rule you used
Chapter 9 Ionic and Covalent Bonding Concept Check 9.1 The following are electron configurations of some ions. Which ones would you expect to see in chemical compounds? State the concept or rule you used
Chemistry 201 FINAL EXAM Name Illinois Wesleyan University December 13, 2005 Fall 2005
 Chemistry 201 FINAL EXAM Name Illinois Wesleyan University December 13, 2005 Fall 2005 There are 200 points on this exam, and you have exactly two hours to complete it. 1. (12 points) a. Give the name
Chemistry 201 FINAL EXAM Name Illinois Wesleyan University December 13, 2005 Fall 2005 There are 200 points on this exam, and you have exactly two hours to complete it. 1. (12 points) a. Give the name
Chem. 1A Final Practice Test 1
 Chem. 1A Final Practice Test 1 All work must be shown on the exam for partial credit. Points will be taken off for incorrect or no units. Calculators are allowed. Cell phones may not be used for calculators.
Chem. 1A Final Practice Test 1 All work must be shown on the exam for partial credit. Points will be taken off for incorrect or no units. Calculators are allowed. Cell phones may not be used for calculators.
CHEM 341: Organic Chemistry I at North Dakota State University Midterm Exam 01 - Fri, Feb 10, 2012!! Name:!
 CEM 341: rganic Chemistry I at North Dakota State University Midterm Exam 01 - Fri, Feb 10, 2012!! Name:! Please read through each question carefully and answer in the spaces provided. A good strategy
CEM 341: rganic Chemistry I at North Dakota State University Midterm Exam 01 - Fri, Feb 10, 2012!! Name:! Please read through each question carefully and answer in the spaces provided. A good strategy
I. Multiple Choice Questions (Type-I) is K p
 Unit 7 EQUILIBRIUM I. Multiple Choice Questions (Type-I) 1. We know that the relationship between K c and K p is K p K c (RT) n What would be the value of n for the reaction NH 4 Cl (s) NH 3 (g) + HCl
Unit 7 EQUILIBRIUM I. Multiple Choice Questions (Type-I) 1. We know that the relationship between K c and K p is K p K c (RT) n What would be the value of n for the reaction NH 4 Cl (s) NH 3 (g) + HCl
Lecture 8. Polyprotic Acids often acid molecules have more than one ionizable H these are called polyprotic acids. sulfuric acid is a diprotic acid
 Lecture 8 Professor icks Inorganic Chemistry (CE152) Polyprotic Acids often acid molecules have more than one ionizable these are called polyprotic acids 1 = monoprotic, 2 = diprotic, 3 = triprotic Cl
Lecture 8 Professor icks Inorganic Chemistry (CE152) Polyprotic Acids often acid molecules have more than one ionizable these are called polyprotic acids 1 = monoprotic, 2 = diprotic, 3 = triprotic Cl
2. Explain why the ph of unbuffered water is generally around 5.7
 CHEM 108b FINAL EXAM The exam is worth a total of 100 points. Each problem is worth five points. Show all work for partial credit. Don t forget to label units and check whether equations are balanced if
CHEM 108b FINAL EXAM The exam is worth a total of 100 points. Each problem is worth five points. Show all work for partial credit. Don t forget to label units and check whether equations are balanced if
Equilibrium Practice Problems page 1
 Equilibrium Practice Problems page 1 1988 D NH 4 HS(s) NH 3 (g) + H 2 S(g) ΔHº = +93 kilojoules The equilibrium above is established by placing solid NH 4 HS in an evacuated container at 25ºC. At equilibrium,
Equilibrium Practice Problems page 1 1988 D NH 4 HS(s) NH 3 (g) + H 2 S(g) ΔHº = +93 kilojoules The equilibrium above is established by placing solid NH 4 HS in an evacuated container at 25ºC. At equilibrium,
AP Chemistry Review Packet # form B. How many grams of water are present in 1.00 mol of copper(ii) sulfate pentahydrate?
 AP Chemistry Review Packet #4 Warmup: Reaction Prediction 2010 form B (a) Solid copper(ii) sulfate pentahydrate is gently heated. How many grams of water are present in 1.00 mol of copper(ii) sulfate pentahydrate?
AP Chemistry Review Packet #4 Warmup: Reaction Prediction 2010 form B (a) Solid copper(ii) sulfate pentahydrate is gently heated. How many grams of water are present in 1.00 mol of copper(ii) sulfate pentahydrate?
Chapter 8 Basic Concepts of Chemical Bonding
 hapter 8 Basic oncepts of hemical Bonding An Important Principle in hemistry The microscopic structure defines the properties of matter at our mesoscopic level. Ex. Graphite and Diamond (both are pure
hapter 8 Basic oncepts of hemical Bonding An Important Principle in hemistry The microscopic structure defines the properties of matter at our mesoscopic level. Ex. Graphite and Diamond (both are pure
CHEMISTRY. Section II (Total time 95 minutes) Part A Time 55 minutes YOU MAY USE YOUR CALCULATOR FOR PART A.
 CHEMISTRY Section II (Total time 95 minutes) Part A Time 55 minutes YOU MAY USE YOUR CALCULATOR FOR PART A. CLEARLY SHOW THE METHOD USED AND THE STEPS INVOLVED IN ARRIVING AT YOUR ANSWERS. It is to your
CHEMISTRY Section II (Total time 95 minutes) Part A Time 55 minutes YOU MAY USE YOUR CALCULATOR FOR PART A. CLEARLY SHOW THE METHOD USED AND THE STEPS INVOLVED IN ARRIVING AT YOUR ANSWERS. It is to your
EXAM III Nov. 1, 2018
 EM 105 Dr. Lammi Name: EXAM III Nov. 1, 2018 You may work until 10:50 to complete this exam. Please show all work in the space provided or on the attached scratch page. Remember to report your final answers
EM 105 Dr. Lammi Name: EXAM III Nov. 1, 2018 You may work until 10:50 to complete this exam. Please show all work in the space provided or on the attached scratch page. Remember to report your final answers
Math Without a Calculator for AP Chemistry
 Math Without a Calculator for AP Chemistry Number Sense 6.02 1000 6.02 0.01 0.1 1000 0.02 1000 0.3 1000 0. 1000 Let fractions be your friends! Fraction Decimal Percent 3/ 0.80 Example: 3.00 1.2 3.00 6
Math Without a Calculator for AP Chemistry Number Sense 6.02 1000 6.02 0.01 0.1 1000 0.02 1000 0.3 1000 0. 1000 Let fractions be your friends! Fraction Decimal Percent 3/ 0.80 Example: 3.00 1.2 3.00 6
Group 1 Group 2 Group 3 Group 4 Group 5 Group 6 Group 7 Group 8. Na Mg Al Si P S Cl Ar
 CHM 111 Chapters 7 and 8 Worksheet and Study Guide Purpose: This is a guide for your as you work through the chapter. The major topics are provided so that you can write notes on each topic and work the
CHM 111 Chapters 7 and 8 Worksheet and Study Guide Purpose: This is a guide for your as you work through the chapter. The major topics are provided so that you can write notes on each topic and work the
AP CHEMISTRY SCORING GUIDELINES
 Mean 5.64 out of 9 pts AP CHEMISTRY Question 1 CO(g) + 1 2 O 2 (g) CO 2 (g) 1. The combustion of carbon monoxide is represented by the equation above. (a) Determine the value of the standard enthalpy change,
Mean 5.64 out of 9 pts AP CHEMISTRY Question 1 CO(g) + 1 2 O 2 (g) CO 2 (g) 1. The combustion of carbon monoxide is represented by the equation above. (a) Determine the value of the standard enthalpy change,
Chem 105 Final Exam. Here is the summary of the total 225 points plus 10 bonus points. Carefully read the questions. Good luck!
 May 3 rd, 2012 Name: CLID: Score: Chem 105 Final Exam There are 50 multiple choices that are worth 3 points each. There are 4 problems and 1 bonus problem. Try to answer the questions, which you know first,
May 3 rd, 2012 Name: CLID: Score: Chem 105 Final Exam There are 50 multiple choices that are worth 3 points each. There are 4 problems and 1 bonus problem. Try to answer the questions, which you know first,
STD-XI-Science-Chemistry Chemical Bonding & Molecular structure
 STD-XI-Science-Chemistry Chemical Bonding & Molecular structure Chemical Bonding Question 1 What is meant by the term chemical bond? How does Kessel-Lewis approach of bonding differ from the modern views?
STD-XI-Science-Chemistry Chemical Bonding & Molecular structure Chemical Bonding Question 1 What is meant by the term chemical bond? How does Kessel-Lewis approach of bonding differ from the modern views?
Name:. Correct Questions = Wrong Questions =.. Unattempt Questions = Marks =
 Name:. Correct Questions = Wrong Questions =.. Unattempt Questions = Marks = 1. (12%) Compound X contains 2.239% hydrogen, 26.681% carbon and 71.080 % oxygen by mass. The titration of 0.154 g of this compound
Name:. Correct Questions = Wrong Questions =.. Unattempt Questions = Marks = 1. (12%) Compound X contains 2.239% hydrogen, 26.681% carbon and 71.080 % oxygen by mass. The titration of 0.154 g of this compound
Chemical Reactions and Equilibrium Acid/Bases Redox. Revision What is a chemical reaction? What factors affect the rate of reaction?
 Chemistry Fundamental Topics These notes will provide a brief coverage of topics that will be important for your Course in instrumentation. The notes are supplementary to the Instrumentation notes and
Chemistry Fundamental Topics These notes will provide a brief coverage of topics that will be important for your Course in instrumentation. The notes are supplementary to the Instrumentation notes and
Final Exam Review-Honors Name Period
 Final Exam Review-Honors Name Period This is not a fully comprehensive review packet. This packet is especially lacking practice of explanation type questions!!! You should study all previous review sheets
Final Exam Review-Honors Name Period This is not a fully comprehensive review packet. This packet is especially lacking practice of explanation type questions!!! You should study all previous review sheets
ADVANCED PLACEMENT CHEMISTRY ACIDS, BASES, AND AQUEOUS EQUILIBRIA
 ADVANCED PLACEMENT CHEMISTRY ACIDS, BASES, AND AQUEOUS EQUILIBRIA Acids- taste sour Bases(alkali)- taste bitter and feel slippery Arrhenius concept- acids produce hydrogen ions in aqueous solution while
ADVANCED PLACEMENT CHEMISTRY ACIDS, BASES, AND AQUEOUS EQUILIBRIA Acids- taste sour Bases(alkali)- taste bitter and feel slippery Arrhenius concept- acids produce hydrogen ions in aqueous solution while
Write your name and date on the cover page Do not open exam until instructed to do so
 Write your name and date on the cover page Do not open exam until instructed to do so Name: Date: Exam III hem. 210 Do not open exam until told to do so. Get out your pencil, eraser, and scientific nongraphing
Write your name and date on the cover page Do not open exam until instructed to do so Name: Date: Exam III hem. 210 Do not open exam until told to do so. Get out your pencil, eraser, and scientific nongraphing
Intermolecular Forces 2 nd Semester Review Questions and Problems
 Intermolecular Forces 2 nd Semester Review Questions and Problems 1. Complete the following table: Molecule Lewis Structure Molecule Shape Polar/Nonpolar CS 2 H 3 O + CdBr 2 CHI 3 2. What makes the dipole
Intermolecular Forces 2 nd Semester Review Questions and Problems 1. Complete the following table: Molecule Lewis Structure Molecule Shape Polar/Nonpolar CS 2 H 3 O + CdBr 2 CHI 3 2. What makes the dipole
AP Chemistry Big Idea Review
 Name: AP Chemistry Big Idea Review Background The AP Chemistry curriculum is based on 6 Big Ideas and many Learning Objectives associated with each Big Idea. This review will cover all of the Big Ideas
Name: AP Chemistry Big Idea Review Background The AP Chemistry curriculum is based on 6 Big Ideas and many Learning Objectives associated with each Big Idea. This review will cover all of the Big Ideas
CHEMISTRY. Section II (Total time 95 minutes) Part A Time 55 minutes YOU MAY USE YOUR CALCULATOR FOR PART A.
 CHEMISTRY Section II (Total time 95 minutes) Part A Time 55 minutes YOU MAY USE YOUR CALCULATOR FOR PART A. CLEARLY SHOW THE METHOD USED AND THE STEPS INVOLVED IN ARRIVING AT YOUR ANSWERS. It is to your
CHEMISTRY Section II (Total time 95 minutes) Part A Time 55 minutes YOU MAY USE YOUR CALCULATOR FOR PART A. CLEARLY SHOW THE METHOD USED AND THE STEPS INVOLVED IN ARRIVING AT YOUR ANSWERS. It is to your
Chapter 16. The Danger of Antifreeze. Buffers. Aqueous Equilibrium
 hapter 16 Aqueous Equilibrium The Danger of Antifreeze Each year, thousands of pets and wildlife die from consuming antifreeze Most brands of antifreeze contain ethylene glycol sweet taste and initial
hapter 16 Aqueous Equilibrium The Danger of Antifreeze Each year, thousands of pets and wildlife die from consuming antifreeze Most brands of antifreeze contain ethylene glycol sweet taste and initial
There are five problems on the exam. Do all of the problems. Show your work.
 CHM 3410 - Physical Chemistry 1 Second Hour Exam October 22, 2010 There are five problems on the exam. Do all of the problems. Show your work. R = 0.08206 L. atm/mole. K N A = 6.022 x 10 23 R = 0.08314
CHM 3410 - Physical Chemistry 1 Second Hour Exam October 22, 2010 There are five problems on the exam. Do all of the problems. Show your work. R = 0.08206 L. atm/mole. K N A = 6.022 x 10 23 R = 0.08314
CHEM J-2 June 2006 HCO 2. Calculate the osmotic pressure of a solution of 1.0 g of glucose (C 6 H 12 O 6 ) in 1500 ml of water at 37 C.
 CEM1405 2006-J-2 June 2006 Draw Lewis structures of ozone, 3, and the formate anion, C 2, including resonance hybrids where appropriate. 3 C 2 3 C C Calculate the osmotic pressure of a solution of 1.0
CEM1405 2006-J-2 June 2006 Draw Lewis structures of ozone, 3, and the formate anion, C 2, including resonance hybrids where appropriate. 3 C 2 3 C C Calculate the osmotic pressure of a solution of 1.0
Acids and Bases. Feb 28 4:40 PM
 Acids and Bases H O s O Cl H O O H H N H Na O H H Feb 28 4:40 PM Properties of Acids 1. Taste sour 2. Conduct electrical current 3. Liberate H 2 gas when reacted with a metal. 4. Cause certain dyes to
Acids and Bases H O s O Cl H O O H H N H Na O H H Feb 28 4:40 PM Properties of Acids 1. Taste sour 2. Conduct electrical current 3. Liberate H 2 gas when reacted with a metal. 4. Cause certain dyes to
Name:. Correct Questions = Wrong Questions =.. Unattempt Questions = Marks =
 Name:. orrect Questions = Wrong Questions =.. Unattempt Questions = Marks = 1. (11%) A(g) + 3B(g) r 2(g) Use the tabulated data to answer the questions about this reaction, which is carried out in a 1.0
Name:. orrect Questions = Wrong Questions =.. Unattempt Questions = Marks = 1. (11%) A(g) + 3B(g) r 2(g) Use the tabulated data to answer the questions about this reaction, which is carried out in a 1.0
Name:. Correct Questions = Wrong Questions =.. Unattempt Questions = Marks =
 Name:. Correct Questions = Wrong Questions =.. Unattempt Questions = Marks = 1. Which salt is colorless? (A) KMn 4 (B) BaS 4 (C) Na 2 Cr 4 (D) CoCl 2 2. Which 0.10 M aqueous solution exhibits the lowest
Name:. Correct Questions = Wrong Questions =.. Unattempt Questions = Marks = 1. Which salt is colorless? (A) KMn 4 (B) BaS 4 (C) Na 2 Cr 4 (D) CoCl 2 2. Which 0.10 M aqueous solution exhibits the lowest
molality: m = = 1.70 m
 C h e m i s t r y 1 2 U n i t 3 R e v i e w P a g e 1 Chem 12: Chapters 10, 11, 12, 13, 14 Unit 3 Worksheet 1. What is miscible? Immiscible? Miscible: two or more substances blend together for form a solution
C h e m i s t r y 1 2 U n i t 3 R e v i e w P a g e 1 Chem 12: Chapters 10, 11, 12, 13, 14 Unit 3 Worksheet 1. What is miscible? Immiscible? Miscible: two or more substances blend together for form a solution
CHEMISTRY 121 AUTUMN 2009 CHAPTER 8 & 9 PROBLEMS
 Dr. Fus AU 2009 EM 121 EMISTRY 121 AUTUMN 2009 APTER 8 & 9 PROBLEMS All questions listed below are problems taken from old hemistry 121 exams given here at The Ohio State University. Read hapters 8 and
Dr. Fus AU 2009 EM 121 EMISTRY 121 AUTUMN 2009 APTER 8 & 9 PROBLEMS All questions listed below are problems taken from old hemistry 121 exams given here at The Ohio State University. Read hapters 8 and
Name AP CHEM / / Collected AP Exam Essay Answers for Chapter 16
 Name AP CHEM / / Collected AP Exam Essay Answers for Chapter 16 1980 - #7 (a) State the physical significance of entropy. Entropy (S) is a measure of randomness or disorder in a system. (b) From each of
Name AP CHEM / / Collected AP Exam Essay Answers for Chapter 16 1980 - #7 (a) State the physical significance of entropy. Entropy (S) is a measure of randomness or disorder in a system. (b) From each of
MATTER AND ITS PROPERTIES
 FINAL REVIEW MATTER AND ITS PROPERTIES VIDEO ATOM Smallest unit of an element that maintains the chemical identity of that element. ELEMENT A pure substance that cannot be broken down into simpler, stable
FINAL REVIEW MATTER AND ITS PROPERTIES VIDEO ATOM Smallest unit of an element that maintains the chemical identity of that element. ELEMENT A pure substance that cannot be broken down into simpler, stable
4 Diatomic molecules
 s manual for Burrows et.al. Chemistry 3 Third edition 4 Diatomic molecules Answers to worked examples WE 4.1 The Lewis model (on p. 174 in Chemistry 3 ) Use the Lewis model to describe the bonding in (a)
s manual for Burrows et.al. Chemistry 3 Third edition 4 Diatomic molecules Answers to worked examples WE 4.1 The Lewis model (on p. 174 in Chemistry 3 ) Use the Lewis model to describe the bonding in (a)
Chemistry 1A, Spring 2007 Midterm Exam 2 March 5, 2007 (90 min, closed book)
 Chemistry 1A, Spring 2007 Midterm Exam 2 March 5, 2007 (90 min, closed book) Name: KEY SID: TA Name: 1.) Write your name on every page of this exam. 2.) This exam has 40 multiple choice questions. Fill
Chemistry 1A, Spring 2007 Midterm Exam 2 March 5, 2007 (90 min, closed book) Name: KEY SID: TA Name: 1.) Write your name on every page of this exam. 2.) This exam has 40 multiple choice questions. Fill
