1. (24 points) 2. (15 points) 4. (6 points) 6a,b,c. (20 points)
|
|
|
- Blake Cummings
- 5 years ago
- Views:
Transcription
1 1 of 9 Third our Exam Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. The exam is closed notes and closed book. 1. Read all parts of each problem. MANY F TE LATTER PARTS F A PRBLEM CAN BE SLVED WITUT AVING SLVED EARLIER PARTS. owever, if you need a numerical result that you were not successful in obtaining for the computation of a latter part, make a physically reasonable approximation for that quantity (and indicate it as such) and use it to solve the latter parts. 2. If asked for a brief explanation, just writing an equation from the equation sheet is not sufficient. You must explain the relevance of the equation to the problem. 3. Significant figure and unit usage must be correct. 4. If you don t understand what the problem is requesting, raise your hand and a proctor will come to your desk. 5. Physical constants, formulas and a periodic table are given on the last page. You may detach this page once the exam has started. CEMICAL EQUILIBRIUM 1. (24 points) TERMDYNAMICS 2. (15 points) 3. (8 points) SLUBILITY 4. (6 points) 5. (8 points) ACID-BASE EQUILIBRIUM 6a,b,c. (20 points) 6d. (13 points) 7. (6 points) TTAL (100 points) Name TA
2 Chemical Equilibrium 1. (20 points) Predict the direction that the equilibrium will shift when each of the following changes occurs. Briefly explain your answers. N 2 4 (g) 2 N 2 (g) = +58 kj (a) N 2 (g) is removed at constant temperature (i) Circle the most likely direction toward products toward reactants no change 2 of 9 (b) the total pressure is increased by addition of N 2 (g) at constant temperature (i) Circle the most likely direction toward products toward reactants no change (c) the volume is increased at constant temperature (i) Circle the most likely direction toward products toward reactants no change (d) the temperature is decreased (i) Circle the most likely direction toward products toward reactants no change
3 Thermodynamics 2. (15 points) Predict the sign of r for the following scenarios. Briefly explain your answers in words. Writing an equation without explanation is not sufficient. 3 of 9 (a) (5 points) A reaction in which the bonds are stronger in the products than in the reactants. (i) Circle the most likely sign of r negative positive (b) (5 points) A reaction that is only spontaneous at high temperatures. (i) Circle the most likely sign of r negative positive (c) (5 points) The equilibrium shifts toward reactants when the temperature increases. (i) Circle the most likely sign of r negative positive
4 4 of 9 Thermodynamics 3. (8 points) (a) (4 points) Calculate the standard reaction enthalpy for the formation of C 2 (g) from C (g) and 2 (g): C (g) + ½ 2 (g) C 2 (g), given that: C(graphite) + ½ 2 (g) C (g) C(graphite) + 2 (g) C 2 (g) = kj = kj (b) (4 points) Consider C (g). Given that its f is kj/mol and its G f is kj/mol, (i) predict whether C (g) is stable or unstable with respect to decomposition into its elements under standard conditions. (ii) Briefly explain your answer. SLUBILITY 4. (6 points) Circle the molecule(s) that are likely to be polar and therefore water soluble. Electronegativity values: C 2.55: 2.20; N 3.04; C 2 C C 2 2 C C 3 C 2 2 N N C C N
5 5 of 9 Solubility 5. (8 points) Consider the following enry s constants for gases in water at 20 C Gas k (mol L -1 atm -1 ) xygen 1.3 x 10-3 Nitrogen 7.0 x 10-4 ydrogen 8.5 x 10-4 (a) (8 points) Draw a single plot with Molar Solubility (mol L -1 ) on the y-axis and Partial Pressure in atm on the x-axis for each of the three gases in the table above. (i) label the y-axis with the numbers that correspond to Molar Solubility of each gas at 1.0 atm; and (ii) label the curve for each gas.
6 6 of 9 ACID-BASE EQUILIBRIUM 6. (32 points) ml of a M solution of weak acid C 3 C (aq) is titrated with a M solution of Na (aq) at 25 C. The K a for C 3 C is 1.8 x 10-5 at 25 C. (a) (12 points) Calculate the p of the solution after addition of ml of Na titrant. You can assume that [ 3 + ] at equilibrium satisfies the 5% rule without checking. Show your work. (b) (5 points) Calculate the p at the half-equivalence (half-stoichiometric) point. (c) (3 points) Calculate the volume of Na titrant needed to reach the equivalence (stoichiometric) point.
7 7 of 9 (d) (12 points) Calculate the p to 2 decimal places at the equivalence (stoichiometric) point. Check any assumption. Show your work. 7. (6 points) Circle the structure that best represents the ionization state of the amino acid glutamate at p = 5.0 (the p of the lysosome). pk a = N C C pk a = N C C N C C - 2 N C C - 2 N C C 2 N C C C pk a = 4.1 C C - C - C C - Structure A Structure B Structure C Structure D Structure E Structure F
8 8 of 9 r = Σ B(reactants) - Σ B(products) r = Σ f (products) - Σ f (reactants) S r = ΣS (products) - ΣS (reactants) G r = Σ G f (products) - Σ G f (reactants) ΔG = Δ - TΔS ΔG = - RT ln K ΔG = ΔG + RT ln Q G = RT ln (Q/K) ln (K 2 /K 1 ) = - (Δ /R)(1/T 2 1/T 1 ) PV = nrt R = JK -1 mol -1 R = L atm K -1 mol -1 x = [-b ± (b 2 4ac) 1/2 ] / 2a s = k P = p + p at 25 C p = -log [ - ] p = -log [ + 3 ] K w = K a K b K w = 1.00 x at 25 C pk a = -log [K a ] pk b = -log [K b ] p pk a log([a]/[a - ])
9 MIT pencourseware Principles of Chemical Science Fall 2014 For information about citing these materials or our Terms of Use, visit:
1. (24 points) 5. (8 points) 6d. (13 points) 7. (6 points)
 1 of 9 Third our Exam 5.111 Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. The exam is closed notes and closed book. 1. Read all parts of each
1 of 9 Third our Exam 5.111 Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. The exam is closed notes and closed book. 1. Read all parts of each
Practice Exam 2 Key Fall 2014
 Practice Exam 2 Key Fall 2014 Second our Exam 5.111 Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. The exam is closed notes and closed book. 1.
Practice Exam 2 Key Fall 2014 Second our Exam 5.111 Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. The exam is closed notes and closed book. 1.
Thermodynamics 1a, 1b (18 points) page 2. Thermodynamics and Chemical Equilibrium 1c, 1d, 2a (18 points) page 3
 1 of Practice Third Hour Exam for 5.111 FALL 2014 Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. The exam is closed notes and closed book. 1. Read
1 of Practice Third Hour Exam for 5.111 FALL 2014 Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. The exam is closed notes and closed book. 1. Read
Page 1 of 16. Final Exam 5.111
 Final Exam 5.111 Page 1 of 16 Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. 1) Read each part of a problem thoroughly. Many of a problem s latter
Final Exam 5.111 Page 1 of 16 Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. 1) Read each part of a problem thoroughly. Many of a problem s latter
Third Hour Exam 5.111
 Page 1 of 10 pages Third Hour Exam 5.111 Write your name below. This is a closed book exam. Solve all 6 problems. Read all problems thoroughly and read all parts of a problem. Many of the latter parts
Page 1 of 10 pages Third Hour Exam 5.111 Write your name below. This is a closed book exam. Solve all 6 problems. Read all problems thoroughly and read all parts of a problem. Many of the latter parts
Chem 1A, Fall 2015, Midterm Exam 3. Version A November 17, 2015 (Prof. Head-Gordon) 2. Student ID: TA:
 Chem 1A, Fall 2015, Midterm Exam 3. Version A November 17, 2015 (Prof. Head-Gordon) 2 Name: Student ID: TA: Contents: 6 pages A. Multiple choice (10 points) B. Thermochemistry and Equilibria (12 points)
Chem 1A, Fall 2015, Midterm Exam 3. Version A November 17, 2015 (Prof. Head-Gordon) 2 Name: Student ID: TA: Contents: 6 pages A. Multiple choice (10 points) B. Thermochemistry and Equilibria (12 points)
5.111 Principles of Chemical Science
 MIT OpenCourseWare http://ocw.mit.edu 5.111 Principles of Chemical Science Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. Page 1 of 10 pages
MIT OpenCourseWare http://ocw.mit.edu 5.111 Principles of Chemical Science Fall 2008 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. Page 1 of 10 pages
Practice Exam A for Fall 2014 Exam 1
 Practice Exam A for Fall 2014 Exam 1 Page 1 of 8 pages First Hour Exam 5.111 Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. The exam is closed
Practice Exam A for Fall 2014 Exam 1 Page 1 of 8 pages First Hour Exam 5.111 Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. The exam is closed
Unit 12. Thermochemistry
 Unit 12 Thermochemistry A reaction is spontaneous if it will occur without a continuous input of energy However, it may require an initial input of energy to get it started (activation energy) For Thermochemistry
Unit 12 Thermochemistry A reaction is spontaneous if it will occur without a continuous input of energy However, it may require an initial input of energy to get it started (activation energy) For Thermochemistry
Chemistry 1A, Spring 2009 Midterm 3 April 13, 2009
 Chemistry 1A, Spring 2009 Midterm 3 April 13, 2009 (90 min, closed book) Name: SID: TA Name: There are 20 Multiple choice questions worth 3 points each. There are 3, multi-part short answer questions.
Chemistry 1A, Spring 2009 Midterm 3 April 13, 2009 (90 min, closed book) Name: SID: TA Name: There are 20 Multiple choice questions worth 3 points each. There are 3, multi-part short answer questions.
Practice Exam A Answer Key for Fall 2014
 Practice Exam A Answer Key for Fall 2014 First Hour Exam 5.111. Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. The exam is closed notes and closed
Practice Exam A Answer Key for Fall 2014 First Hour Exam 5.111. Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. The exam is closed notes and closed
Department of Chemistry Memorial University of Newfoundland Chemistry 1050
 Department of Chemistry Memorial University of Newfoundland Chemistry 1050 FINAL EXAMINATION Fall 017 TIME: 3 hours READ TE FOLLOWING CAREFULLY 1. This examination consists of 13 pages including a Data
Department of Chemistry Memorial University of Newfoundland Chemistry 1050 FINAL EXAMINATION Fall 017 TIME: 3 hours READ TE FOLLOWING CAREFULLY 1. This examination consists of 13 pages including a Data
Chemistry 1A, Fall 2003 Midterm 2 Oct 14, 2003 (90 min, closed book)
 Name: SID: TA Name: Chemistry 1A, Fall 2003 Midterm 2 Oct 14, 2003 (90 min, closed book) This exam has 45 multiple choice questions. Fill in the Scantron form AND circle your answer on the exam. Each question
Name: SID: TA Name: Chemistry 1A, Fall 2003 Midterm 2 Oct 14, 2003 (90 min, closed book) This exam has 45 multiple choice questions. Fill in the Scantron form AND circle your answer on the exam. Each question
Chemistry 1A, Spring 2007 Midterm Exam 3 April 9, 2007 (90 min, closed book)
 Chemistry 1A, Spring 2007 Midterm Exam 3 April 9, 2007 (90 min, closed book) Name: KEY SID: TA Name: 1.) Write your name on every page of this exam. 2.) This exam has 34 multiple choice questions. Fill
Chemistry 1A, Spring 2007 Midterm Exam 3 April 9, 2007 (90 min, closed book) Name: KEY SID: TA Name: 1.) Write your name on every page of this exam. 2.) This exam has 34 multiple choice questions. Fill
AP Chemistry. Free-Response Questions
 2018 AP Chemistry Free-Response Questions College Board, Advanced Placement Program, AP, AP Central, and the acorn logo are registered trademarks of the College Board. AP Central is the official online
2018 AP Chemistry Free-Response Questions College Board, Advanced Placement Program, AP, AP Central, and the acorn logo are registered trademarks of the College Board. AP Central is the official online
The Second Law of Thermodynamics (Chapter 4)
 The Second Law of Thermodynamics (Chapter 4) First Law: Energy of universe is constant: ΔE system = - ΔE surroundings Second Law: New variable, S, entropy. Changes in S, ΔS, tell us which processes made
The Second Law of Thermodynamics (Chapter 4) First Law: Energy of universe is constant: ΔE system = - ΔE surroundings Second Law: New variable, S, entropy. Changes in S, ΔS, tell us which processes made
Page 1 of 22 Final Exam December 21, PAGE 2 1. TRANSITION METALS (35 points) PAGE 3 2. TRANSITION METALS (10 points)
 Page 1 of 22 Final Exam 5.111 December 21, 2011 Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. 1) Read each part of a problem thoroughly. Many
Page 1 of 22 Final Exam 5.111 December 21, 2011 Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. 1) Read each part of a problem thoroughly. Many
CHEMISTRY 202 Practice Hour Exam II. Dr. D. DeCoste T.A (60 pts.) 21 (40 pts.) 22 (20 pts.)
 CHEMISTRY 202 Practice Hour Exam II Fall 2016 Dr. D. DeCoste Name Signature T.A. This exam contains 22 questions on 7 numbered pages. Check now to make sure you have a complete exam. You have two hours
CHEMISTRY 202 Practice Hour Exam II Fall 2016 Dr. D. DeCoste Name Signature T.A. This exam contains 22 questions on 7 numbered pages. Check now to make sure you have a complete exam. You have two hours
Chem GENERAL CHEMISTRY II
 GENERAL CHEMISTRY II CHEM 206 /2 51 Final Examination December 19, 2006 1900-2200 Dr. Cerrie ROGERS x x programmable calculators must be reset Chem 206 --- GENERAL CHEMISTRY II LAST NAME: STUDENT NUMBER:
GENERAL CHEMISTRY II CHEM 206 /2 51 Final Examination December 19, 2006 1900-2200 Dr. Cerrie ROGERS x x programmable calculators must be reset Chem 206 --- GENERAL CHEMISTRY II LAST NAME: STUDENT NUMBER:
Chemistry 1A, Spring 2007 Midterm Exam 2 March 5, 2007 (90 min, closed book)
 Chemistry 1A, Spring 2007 Midterm Exam 2 March 5, 2007 (90 min, closed book) Name: KEY SID: TA Name: 1.) Write your name on every page of this exam. 2.) This exam has 40 multiple choice questions. Fill
Chemistry 1A, Spring 2007 Midterm Exam 2 March 5, 2007 (90 min, closed book) Name: KEY SID: TA Name: 1.) Write your name on every page of this exam. 2.) This exam has 40 multiple choice questions. Fill
BCIT Fall Chem Exam #2
 BCIT Fall 2017 Chem 3310 Exam #2 Name: Attempt all questions in this exam. Read each question carefully and give a complete answer in the space provided. Part marks given for wrong answers with partially
BCIT Fall 2017 Chem 3310 Exam #2 Name: Attempt all questions in this exam. Read each question carefully and give a complete answer in the space provided. Part marks given for wrong answers with partially
AP Chemistry Big Idea Review
 Name: AP Chemistry Big Idea Review Background The AP Chemistry curriculum is based on 6 Big Ideas and many Learning Objectives associated with each Big Idea. This review will cover all of the Big Ideas
Name: AP Chemistry Big Idea Review Background The AP Chemistry curriculum is based on 6 Big Ideas and many Learning Objectives associated with each Big Idea. This review will cover all of the Big Ideas
with increased Lecture Summary #33 Wednesday, December 3, 2014
 5. Lecture Summary #33 Wednesday, December 3, 204 Reading for Today: 4.-4.3 in 5 th ed and 3.-3.3 in 4 th ed Reading for Lecture #34: 4.4 & 4.6 in 5 th ed and 3.4 & 3.6 in 4 th ed Topic: Kinetics I. Effect
5. Lecture Summary #33 Wednesday, December 3, 204 Reading for Today: 4.-4.3 in 5 th ed and 3.-3.3 in 4 th ed Reading for Lecture #34: 4.4 & 4.6 in 5 th ed and 3.4 & 3.6 in 4 th ed Topic: Kinetics I. Effect
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Chemistry 102 Spring 2016 Discussion #12, Chapter 17 Student name TA name Section. Things you should know when you leave Discussion today: ( G o f
 Chemistry 10 Spring 016 Discussion #1, Chapter 17 Student name TA name Section Things you should know when you leave Discussion today: 1. ΔS sys = Δ r S = Σ [n i (S )] product - Σ [n j (S )] reactants.
Chemistry 10 Spring 016 Discussion #1, Chapter 17 Student name TA name Section Things you should know when you leave Discussion today: 1. ΔS sys = Δ r S = Σ [n i (S )] product - Σ [n j (S )] reactants.
Chemistry 1A, Spring 2006 Midterm Exam II, Version 1 March 6, 2006 (90 min, closed book)
 Chemistry 1A, Spring 2006 Midterm Exam II, Version 1 March 6, 2006 (90 min, closed book) Name: SID: Identification Sticker TA Name: Write your name on every page of this exam. This exam has 38 multiple
Chemistry 1A, Spring 2006 Midterm Exam II, Version 1 March 6, 2006 (90 min, closed book) Name: SID: Identification Sticker TA Name: Write your name on every page of this exam. This exam has 38 multiple
Suggested time minutes (22 points) minutes (16 points) minutes (38 points) 4. 9 minutes (24 points) Total (100 points) Name
 First Hour Exam 5.111 Write your name below. Do not open the exam until the start of the exam is announced. The exam is closed notes and closed book. 1. Read each part of each problem carefully and thoroughly.
First Hour Exam 5.111 Write your name below. Do not open the exam until the start of the exam is announced. The exam is closed notes and closed book. 1. Read each part of each problem carefully and thoroughly.
Chemistry 1A, Fall 2006 Final Exam, Version B Dec 12, 2006 (180 min, closed book)
 Name: SID: GSI Name: Chemistry 1A, Fall 2006 Final Exam, Version B Dec 12, 2006 (180 min, closed book) There are 60 Multiple choice questions worth 4.34 points each. There are 13 short answer questions.
Name: SID: GSI Name: Chemistry 1A, Fall 2006 Final Exam, Version B Dec 12, 2006 (180 min, closed book) There are 60 Multiple choice questions worth 4.34 points each. There are 13 short answer questions.
5.111 Lecture Summary #18 Wednesday, October 22, 2014
 5.111 Lecture Summary #18 Wednesday, October 22, 2014 Reading for Today: Sections 10.1-10.5, 10.9 (Sections 9.1-9.4 in 4 th ed.) Reading for Lecture #19: Sections 10.9-10.13 (Section 9.4-9.5 in 4 th ed.)
5.111 Lecture Summary #18 Wednesday, October 22, 2014 Reading for Today: Sections 10.1-10.5, 10.9 (Sections 9.1-9.4 in 4 th ed.) Reading for Lecture #19: Sections 10.9-10.13 (Section 9.4-9.5 in 4 th ed.)
Chemistry 1A, Spring 2009 Midterm 2, Version March 9, 2009 (90 min, closed book)
 Name: SID: TA Name: Chemistry 1A, Spring 2009 Midterm 2, Version March 9, 2009 (90 min, closed book) There are 20 Multiple choice questions worth 2.5 points each. There are 3, multi-part short answer questions.
Name: SID: TA Name: Chemistry 1A, Spring 2009 Midterm 2, Version March 9, 2009 (90 min, closed book) There are 20 Multiple choice questions worth 2.5 points each. There are 3, multi-part short answer questions.
Chemistry 123: Physical and Organic Chemistry Topic 2: Thermochemistry
 Recall the equation. w = -PΔV = -(1.20 atm)(1.02 L)( = -1.24 10 2 J -101 J 1 L atm Where did the conversion factor come from? Compare two versions of the gas constant and calculate. 8.3145 J/mol K 0.082057
Recall the equation. w = -PΔV = -(1.20 atm)(1.02 L)( = -1.24 10 2 J -101 J 1 L atm Where did the conversion factor come from? Compare two versions of the gas constant and calculate. 8.3145 J/mol K 0.082057
10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics
 Chapter 10 Thermochemistry 10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics OFB Chap. 10 1 Chapter 10 Thermochemistry Heat
Chapter 10 Thermochemistry 10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics OFB Chap. 10 1 Chapter 10 Thermochemistry Heat
Class XI Chapter 6 Thermodynamics Chemistry
 Class XI Chapter 6 Chemistry Question 6.1: Choose the correct answer. A thermodynamic state function is a quantity (i) used to determine heat changes (ii) whose value is independent of path (iii) used
Class XI Chapter 6 Chemistry Question 6.1: Choose the correct answer. A thermodynamic state function is a quantity (i) used to determine heat changes (ii) whose value is independent of path (iii) used
2 NO, has reached a state of dynamic equilibrium, which statement below is true?
 Chemistry 11-014, Vining Exam #3 NAME: Answer Key Take Home Version 1. When the reversible reaction, N + O NO, has reached a state of dynamic equilibrium, which statement below is true? (a) Both the forward
Chemistry 11-014, Vining Exam #3 NAME: Answer Key Take Home Version 1. When the reversible reaction, N + O NO, has reached a state of dynamic equilibrium, which statement below is true? (a) Both the forward
Chemistry 1A, Spring 2008 Midterm Exam III, Version A April 14, 2008 (90 min, closed book)
 Chemistry 1A, Spring 2008 Midterm Exam III, Version A April 14, 2008 (90 min, closed book) Name: KEY SID: A Name: 1.) Write your name on every page of this exam. 2.) his exam has 15 multiple-choice questions
Chemistry 1A, Spring 2008 Midterm Exam III, Version A April 14, 2008 (90 min, closed book) Name: KEY SID: A Name: 1.) Write your name on every page of this exam. 2.) his exam has 15 multiple-choice questions
Exp.3 Determination of the Thermodynamic functions for the Borax Solution
 Exp.3 Determination of the Thermodynamic functions for the Borax Solution Theory: The relationship between Gibb s energy (ΔG), Enthalpy (ΔH), Entropy (ΔS) and the equilibrium constant (K) for a chemical
Exp.3 Determination of the Thermodynamic functions for the Borax Solution Theory: The relationship between Gibb s energy (ΔG), Enthalpy (ΔH), Entropy (ΔS) and the equilibrium constant (K) for a chemical
Chapter 19 Chemical Thermodynamics Entropy and free energy
 Chapter 19 Chemical Thermodynamics Entropy and free energy Learning goals and key skills: Understand the meaning of spontaneous process, reversible process, irreversible process, and isothermal process.
Chapter 19 Chemical Thermodynamics Entropy and free energy Learning goals and key skills: Understand the meaning of spontaneous process, reversible process, irreversible process, and isothermal process.
Chemistry 1A, Spring 2008 Final Exam, Version A May 17, 2008 (180 min, closed book)
 Chemistry 1A, Spring 2008 Final Exam, Version A May 17, 2008 (180 min, closed book) Name: SID: TA Name: 1) Write your name on every page of this exam. 2) This exam has 25 multiple-choice questions and
Chemistry 1A, Spring 2008 Final Exam, Version A May 17, 2008 (180 min, closed book) Name: SID: TA Name: 1) Write your name on every page of this exam. 2) This exam has 25 multiple-choice questions and
Chemical Thermodynamics
 Page III-16-1 / Chapter Sixteen Lecture Notes Chemical Thermodynamics Thermodynamics and Kinetics Chapter 16 Chemistry 223 Professor Michael Russell How to predict if a reaction can occur, given enough
Page III-16-1 / Chapter Sixteen Lecture Notes Chemical Thermodynamics Thermodynamics and Kinetics Chapter 16 Chemistry 223 Professor Michael Russell How to predict if a reaction can occur, given enough
Thermodynamic Connection
 Slide 1 Thermodynamic Connection Q and G and letters other than K Slide 2 Reaction Quotient What do you call an equilibrium constant when you aren t at equilibrium? A Reaction Quotient! (Q) Slide 3 Consider
Slide 1 Thermodynamic Connection Q and G and letters other than K Slide 2 Reaction Quotient What do you call an equilibrium constant when you aren t at equilibrium? A Reaction Quotient! (Q) Slide 3 Consider
Energy is the capacity to do work
 1 of 10 After completing this chapter, you should, at a minimum, be able to do the following. This information can be found in my lecture notes for this and other chapters and also in your text. Correctly
1 of 10 After completing this chapter, you should, at a minimum, be able to do the following. This information can be found in my lecture notes for this and other chapters and also in your text. Correctly
Chemistry 1A, Spring 2009 KEY Midterm 2, Version March 9, 2009 (90 min, closed book)
 Name: SID: TA Name: Chemistry 1A, Spring 2009 KEY Midterm 2, Version March 9, 2009 (90 min, closed book) There are 20 Multiple choice questions worth 2.5 points each. There are 3, multi-part, short answer
Name: SID: TA Name: Chemistry 1A, Spring 2009 KEY Midterm 2, Version March 9, 2009 (90 min, closed book) There are 20 Multiple choice questions worth 2.5 points each. There are 3, multi-part, short answer
EQUILIBRIUM GENERAL CONCEPTS
 017-11-09 WHEN THE REACTION IS IN EQUILIBRIUM EQUILIBRIUM GENERAL CONCEPTS The concentrations of all species remain constant over time, but both the forward and reverse reaction never cease When a system
017-11-09 WHEN THE REACTION IS IN EQUILIBRIUM EQUILIBRIUM GENERAL CONCEPTS The concentrations of all species remain constant over time, but both the forward and reverse reaction never cease When a system
For problems 1-4, circle the letter of the answer that best satisfies the question.
 CHM 106 Exam II For problems 1-4, circle the letter of the answer that best satisfies the question. 1. Which of the following statements is true? I. A weak base has a strong conjugate acid II. The strength
CHM 106 Exam II For problems 1-4, circle the letter of the answer that best satisfies the question. 1. Which of the following statements is true? I. A weak base has a strong conjugate acid II. The strength
CHEMISTRY 202 Hour Exam II. Dr. D. DeCoste T.A (60 pts.) 31 (20 pts.) 32 (40 pts.)
 CHEMISTRY 202 Hour Exam II October 27, 2015 Dr. D. DeCoste Name Signature T.A. This exam contains 32 questions on 11 numbered pages. Check now to make sure you have a complete exam. You have two hours
CHEMISTRY 202 Hour Exam II October 27, 2015 Dr. D. DeCoste Name Signature T.A. This exam contains 32 questions on 11 numbered pages. Check now to make sure you have a complete exam. You have two hours
OKANAGAN UNIVERSITY COLLEGE FINAL EXAMINATION CHEMISTRY 121
 Name (Print) Surname Given Names Student Number Centre OKANAGAN UNIVERSITY COLLEGE FINAL EXAMINATION CHEMISTRY 2 Professor: Nigel Eggers, Renee Van Poppelen, Stephen McNeil April 5, 2004 Duration: 3 hours
Name (Print) Surname Given Names Student Number Centre OKANAGAN UNIVERSITY COLLEGE FINAL EXAMINATION CHEMISTRY 2 Professor: Nigel Eggers, Renee Van Poppelen, Stephen McNeil April 5, 2004 Duration: 3 hours
Chemistry 1A, Fall 2007 KEY Midterm Exam III November 13, 2007 (90 min, closed book)
 Name: SID: Chemistry 1A, Fall 2007 KEY Midterm Exam III November 13, 2007 (90 min, closed book) TA: Section: Please read this first: Write your name and that of your TA on all 8 pages of the exam Test-taking
Name: SID: Chemistry 1A, Fall 2007 KEY Midterm Exam III November 13, 2007 (90 min, closed book) TA: Section: Please read this first: Write your name and that of your TA on all 8 pages of the exam Test-taking
concentrations (molarity) rate constant, (k), depends on size, speed, kind of molecule, temperature, etc.
 #80 Notes Ch. 12, 13, 16, 17 Rates, Equilibriums, Energies Ch. 12 I. Reaction Rates NO 2(g) + CO (g) NO (g) + CO 2(g) Rate is defined in terms of the rate of disappearance of one of the reactants, but
#80 Notes Ch. 12, 13, 16, 17 Rates, Equilibriums, Energies Ch. 12 I. Reaction Rates NO 2(g) + CO (g) NO (g) + CO 2(g) Rate is defined in terms of the rate of disappearance of one of the reactants, but
10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics
 Chapter 10 Thermochemistry 10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics OFB Chap. 10 1 OFB Chap. 10 2 Thermite Reaction
Chapter 10 Thermochemistry 10-1 Heat 10-2 Calorimetry 10-3 Enthalpy 10-4 Standard-State Enthalpies 10-5 Bond Enthalpies 10-6 The First Law of Thermodynamics OFB Chap. 10 1 OFB Chap. 10 2 Thermite Reaction
Chapter 19 Chemical Thermodynamics Entropy and free energy
 Chapter 19 Chemical Thermodynamics Entropy and free energy Learning goals and key skills: Explain and apply the terms spontaneous process, reversible process, irreversible process, and isothermal process.
Chapter 19 Chemical Thermodynamics Entropy and free energy Learning goals and key skills: Explain and apply the terms spontaneous process, reversible process, irreversible process, and isothermal process.
AP CHEMISTRY SCORING GUIDELINES
 Mean 5.64 out of 9 pts AP CHEMISTRY Question 1 CO(g) + 1 2 O 2 (g) CO 2 (g) 1. The combustion of carbon monoxide is represented by the equation above. (a) Determine the value of the standard enthalpy change,
Mean 5.64 out of 9 pts AP CHEMISTRY Question 1 CO(g) + 1 2 O 2 (g) CO 2 (g) 1. The combustion of carbon monoxide is represented by the equation above. (a) Determine the value of the standard enthalpy change,
Name AP CHEM / / Collected AP Exam Essay Answers for Chapter 16
 Name AP CHEM / / Collected AP Exam Essay Answers for Chapter 16 1980 - #7 (a) State the physical significance of entropy. Entropy (S) is a measure of randomness or disorder in a system. (b) From each of
Name AP CHEM / / Collected AP Exam Essay Answers for Chapter 16 1980 - #7 (a) State the physical significance of entropy. Entropy (S) is a measure of randomness or disorder in a system. (b) From each of
CHEMISTRY. Section II (Total time 95 minutes) Part A Time 55 minutes YOU MAY USE YOUR CALCULATOR FOR PART A.
 CHEMISTRY Section II (Total time 95 minutes) Part A Time 55 minutes YOU MAY USE YOUR CALCULATOR FOR PART A. CLEARLY SHOW THE METHOD USED AND THE STEPS INVOLVED IN ARRIVING AT YOUR ANSWERS. It is to your
CHEMISTRY Section II (Total time 95 minutes) Part A Time 55 minutes YOU MAY USE YOUR CALCULATOR FOR PART A. CLEARLY SHOW THE METHOD USED AND THE STEPS INVOLVED IN ARRIVING AT YOUR ANSWERS. It is to your
SIR MICHELANGELO REFALO CENTRE FOR FURTHER STUDIES VICTORIA GOZO
 SIR MICHELANGELO REFALO CENTRE FOR FURTHER STUDIES VICTORIA GOZO Page 1 of 7 Half Yearly Exam 2013 Subject: Chemistry 1 st Year Level: Advanced Time: 3 hrs Answer SEVEN (7) questions. All questions carry
SIR MICHELANGELO REFALO CENTRE FOR FURTHER STUDIES VICTORIA GOZO Page 1 of 7 Half Yearly Exam 2013 Subject: Chemistry 1 st Year Level: Advanced Time: 3 hrs Answer SEVEN (7) questions. All questions carry
6. For the reaction stated below, what the effect of decreasing the volume would be on the amount of product. 2SO 2 (g) + O 2 (g) 2SO 3 (g)
 Molar Concen 1 Chem 13 Fall 013 Practice Questions for EXAM III CCBC-Catonsville This AS BEEN updated after Monday s lecture JUST studying these questions is not sufficient preparation. There certainly
Molar Concen 1 Chem 13 Fall 013 Practice Questions for EXAM III CCBC-Catonsville This AS BEEN updated after Monday s lecture JUST studying these questions is not sufficient preparation. There certainly
5.111 Lecture Summary #30 Monday, November 24, 2014
 5.111 Lecture Summary #30 Monday, November 24, 2014 Reading for Today: 14.114.5 in 5 th ed and 13.113.5 in 4 th ed. Reading for Lecture #31: 14.6, 17.7 in 5 th ed and 13.6, 17.7 in 4 th ed. Topic: Introduction
5.111 Lecture Summary #30 Monday, November 24, 2014 Reading for Today: 14.114.5 in 5 th ed and 13.113.5 in 4 th ed. Reading for Lecture #31: 14.6, 17.7 in 5 th ed and 13.6, 17.7 in 4 th ed. Topic: Introduction
2. Explain why the ph of unbuffered water is generally around 5.7
 CHEM 108b FINAL EXAM The exam is worth a total of 100 points. Each problem is worth five points. Show all work for partial credit. Don t forget to label units and check whether equations are balanced if
CHEM 108b FINAL EXAM The exam is worth a total of 100 points. Each problem is worth five points. Show all work for partial credit. Don t forget to label units and check whether equations are balanced if
Exam 3 Solutions. ClO g. At 200 K and a total pressure of 1.0 bar, the partial pressure ratio for the chlorine-containing compounds is p ClO2
 Chemistry 360 Dr. Jean M. Standard Fall 2016 Name KEY Exam 3 Solutions 1.) (14 points) Consider the gas phase decomposition of chlorine dioxide, ClO 2, ClO 2 ( g) ClO ( g) + O ( g). At 200 K and a total
Chemistry 360 Dr. Jean M. Standard Fall 2016 Name KEY Exam 3 Solutions 1.) (14 points) Consider the gas phase decomposition of chlorine dioxide, ClO 2, ClO 2 ( g) ClO ( g) + O ( g). At 200 K and a total
15/04/2018 EQUILIBRIUM- GENERAL CONCEPTS
 15/04/018 EQUILIBRIUM- GENERAL CONCEPTS When a system is at equilibrium, the forward and reverse reactions are proceeding at the same rate. The concentrations of all species remain constant over time,
15/04/018 EQUILIBRIUM- GENERAL CONCEPTS When a system is at equilibrium, the forward and reverse reactions are proceeding at the same rate. The concentrations of all species remain constant over time,
MCGILL UNIVERSITY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEM 120 MONDAY MARCH 16, :30PM 8:30PM VERSION NUMBER: 1
 MCGILL UNIVERSITY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEM 120 MONDAY MARCH 16, 2009 6:30PM 8:30PM VERSION NUMBER: 1 Instructions: BEFORE YOU BEGIN: Enter your student number and name on the computer
MCGILL UNIVERSITY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEM 120 MONDAY MARCH 16, 2009 6:30PM 8:30PM VERSION NUMBER: 1 Instructions: BEFORE YOU BEGIN: Enter your student number and name on the computer
TODAY 0. Why H = q (if p ext =p=constant and no useful work) 1. Constant Pressure Heat Capacity (what we usually use)
 361 Lec 7 Fri 9sep15 TODAY 0. Why H = q (if p ext =p=constant and no useful work) 1. Constant Pressure Heat Capacity (what we usually use) 2. Heats of Chemical Reactions: r H (mechanics of obtaining from
361 Lec 7 Fri 9sep15 TODAY 0. Why H = q (if p ext =p=constant and no useful work) 1. Constant Pressure Heat Capacity (what we usually use) 2. Heats of Chemical Reactions: r H (mechanics of obtaining from
Final Exam Review-Honors Name Period
 Final Exam Review-Honors Name Period This is not a fully comprehensive review packet. This packet is especially lacking practice of explanation type questions!!! You should study all previous review sheets
Final Exam Review-Honors Name Period This is not a fully comprehensive review packet. This packet is especially lacking practice of explanation type questions!!! You should study all previous review sheets
Chapter Eighteen. Thermodynamics
 Chapter Eighteen Thermodynamics 1 Thermodynamics Study of energy changes during observed processes Purpose: To predict spontaneity of a process Spontaneity: Will process go without assistance? Depends
Chapter Eighteen Thermodynamics 1 Thermodynamics Study of energy changes during observed processes Purpose: To predict spontaneity of a process Spontaneity: Will process go without assistance? Depends
Bonus Final Exam 3. 1 Calculate the heat of reaction,δh 0 rxn, for the following reaction as written at 298 K: g 2H 2 CH 4. g CF 4.
 Bonus Final Exam 3 1 Calculate the heat of reaction,δh rxn, for the following reaction as written at 298 K: CH 4 2F 2 CF 4 2H 2 substance CH 4 CF 4 ΔH f kj/mol 75 68 (A) ΔH rxn 23 kj (B) ΔH rxn 914 kj
Bonus Final Exam 3 1 Calculate the heat of reaction,δh rxn, for the following reaction as written at 298 K: CH 4 2F 2 CF 4 2H 2 substance CH 4 CF 4 ΔH f kj/mol 75 68 (A) ΔH rxn 23 kj (B) ΔH rxn 914 kj
Thermodynamics: Entropy
 Name: Band: Date: Thermodynamics: Entropy Big Idea: Entropy When we were studying enthalpy, we made a generalization: most spontaneous processes are exothermic. This is a decent assumption to make because
Name: Band: Date: Thermodynamics: Entropy Big Idea: Entropy When we were studying enthalpy, we made a generalization: most spontaneous processes are exothermic. This is a decent assumption to make because
This test is closed note/book. One 8.5 x 11 handwritten crib sheet (one sided) is permitted.
 Exam3 TestFormA Chem1310 Fall2008 11/24/2008 Dr.Williams PrintName Signature This test is closed note/book. One 8.5 x 11 handwritten crib sheet (one sided) is permitted. Use a #2 pencil. Calculators are
Exam3 TestFormA Chem1310 Fall2008 11/24/2008 Dr.Williams PrintName Signature This test is closed note/book. One 8.5 x 11 handwritten crib sheet (one sided) is permitted. Use a #2 pencil. Calculators are
Chemistry 2000 Fall 2017 Test 2 Version A Solutions
 Chemistry 2000 Fall 207 Test 2 Version A Solutions. Start with a balanced reaction: (a) liquid water: C 2 H 4(g) + 3 O 2(g) 2 CO 2(g) + 2 H 2 O r S = 2S (CO 2 ) + 2S (H 2 O, l) [S (C 2 H 4 ) + 3S (O 2
Chemistry 2000 Fall 207 Test 2 Version A Solutions. Start with a balanced reaction: (a) liquid water: C 2 H 4(g) + 3 O 2(g) 2 CO 2(g) + 2 H 2 O r S = 2S (CO 2 ) + 2S (H 2 O, l) [S (C 2 H 4 ) + 3S (O 2
Final Exam. CHEM 181: Introduction to Chemical Principles December 14, 2015 Answer Key N C S C
 Final Exam EM 181: Introduction to hemical Principles December 14, 2015 Answer Key 1. The compound 4 5 NO has the connectivity shown below O N On the next page: Draw all reasonable resonance structures
Final Exam EM 181: Introduction to hemical Principles December 14, 2015 Answer Key 1. The compound 4 5 NO has the connectivity shown below O N On the next page: Draw all reasonable resonance structures
UNIT 9 IB MATERIAL KINETICS & THERMODYNAMICS
 UNIT 9 IB MATERIAL KINETICS & THERMODYNAMICS Name: ESSENTIALS: Know, Understand, and Be Able To State that combustion and neutralization are exothermic processes. Calculate the heat energy change when
UNIT 9 IB MATERIAL KINETICS & THERMODYNAMICS Name: ESSENTIALS: Know, Understand, and Be Able To State that combustion and neutralization are exothermic processes. Calculate the heat energy change when
 Class XI Chapter 6 Thermodynamics Question 6.1: Choose the correct answer. A thermodynamic state function is a quantity (i) used to determine heat changes (ii) whose value is independent of path (iii)
Class XI Chapter 6 Thermodynamics Question 6.1: Choose the correct answer. A thermodynamic state function is a quantity (i) used to determine heat changes (ii) whose value is independent of path (iii)
Big Idea 6 Equilibrium
 Big Idea 6 Equilibrium AP CHEMISTRY Course and Exam Description Effective Fall 2013 The College Board New York, NY AP Chemistry Curriculum Framework The Concept Outline The focus of Chapters 15-17 is on
Big Idea 6 Equilibrium AP CHEMISTRY Course and Exam Description Effective Fall 2013 The College Board New York, NY AP Chemistry Curriculum Framework The Concept Outline The focus of Chapters 15-17 is on
Chemistry 1A, Fall 2006 Final Exam, Version B Dec 12, 2006 (180 min, closed book)
 Name: SID: GSI Name: Chemistry 1A, Fall 2006 Final Exam, Version B Dec 12, 2006 (180 min, closed book) There are 60 Multiple choice questions worth 4.34 points each. There are 13 short answer questions.
Name: SID: GSI Name: Chemistry 1A, Fall 2006 Final Exam, Version B Dec 12, 2006 (180 min, closed book) There are 60 Multiple choice questions worth 4.34 points each. There are 13 short answer questions.
Name: Score: /100. Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each
 Name: Score: /100 Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each 1. Which of the following contains the greatest number of moles of O? A) 2.3 mol H 2 O
Name: Score: /100 Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each 1. Which of the following contains the greatest number of moles of O? A) 2.3 mol H 2 O
Last Name: First Name: High School Name: Individual Exam 3 Solutions: Kinetics, Electrochemistry, and Thermodynamics
 Last Name: First Name: High School Name: Washington University Chemistry Tournament April 2, 2016 Individual Exam 3 Solutions: Kinetics, Electrochemistry, and Thermodynamics Please write your full name
Last Name: First Name: High School Name: Washington University Chemistry Tournament April 2, 2016 Individual Exam 3 Solutions: Kinetics, Electrochemistry, and Thermodynamics Please write your full name
KEY: FREE RESPONSE. Question Ammonium carbamate, NH 4CO 2NH 2, reversibly decomposes into CO 2 and NH 3 as shown by the balanced equation below.
 KEY: FREE RESPONSE Question 1 1. Ammonium carbamate, NH 4CO 2NH 2, reversibly decomposes into CO 2 and NH 3 as shown by the balanced equation below. NH 4CO 2NH 2(s) 2 NH 3(g) + CO 2(g) K p =? A student
KEY: FREE RESPONSE Question 1 1. Ammonium carbamate, NH 4CO 2NH 2, reversibly decomposes into CO 2 and NH 3 as shown by the balanced equation below. NH 4CO 2NH 2(s) 2 NH 3(g) + CO 2(g) K p =? A student
FRONT PAGE FORMULA SHEET - TEAR OFF
 FRONT PAGE FORMULA SHEET - TEAR OFF N A = 6.022 x 10 23 C = ( 5 / 9 ) ( F - 32) F = ( 9 / 5 )( C) + 32 1 amu = 1.661 x 10-27 kg C = K - 273.15 K = C + 273.15 1 atm = 760 torr = 760 mm Hg 1 atm = 1.013
FRONT PAGE FORMULA SHEET - TEAR OFF N A = 6.022 x 10 23 C = ( 5 / 9 ) ( F - 32) F = ( 9 / 5 )( C) + 32 1 amu = 1.661 x 10-27 kg C = K - 273.15 K = C + 273.15 1 atm = 760 torr = 760 mm Hg 1 atm = 1.013
CHEMISTRY 107 Section 501 Final Exam Version A December 12, 2016 Dr. Larry Brown
 NAME: (print) UIN #: CHEMISTRY 107 Section 501 Final Exam Version A December 12, 2016 Dr. Larry Brown This is a 2-hour exam, and contains 11 problems. There should be 14 numbered pages, including this
NAME: (print) UIN #: CHEMISTRY 107 Section 501 Final Exam Version A December 12, 2016 Dr. Larry Brown This is a 2-hour exam, and contains 11 problems. There should be 14 numbered pages, including this
5.111 Lecture Summary #20. CHEMICAL EQUILIBRIUM Chapter 9 sections Topics: External effects on K and sig figs for logs.
 20.1 CHEMICAL EQUILIBRIUM Chapter 9 sections 9.10-9.13 Topics: External effects on K and sig figs for logs. Recall Principle of Le Châtelier: A system in equilibrium that is subjected to stress will react
20.1 CHEMICAL EQUILIBRIUM Chapter 9 sections 9.10-9.13 Topics: External effects on K and sig figs for logs. Recall Principle of Le Châtelier: A system in equilibrium that is subjected to stress will react
1. Entropy questions: PICK TWO (6 each)
 1. Entropy questions: PICK TWO (6 each) 1.00 mole of water freezes at 0.00ºC and 1 atm, releasing 6.01 kj of heat. Calculate the change in entropy and free energy for the process. Calculate the entropy
1. Entropy questions: PICK TWO (6 each) 1.00 mole of water freezes at 0.00ºC and 1 atm, releasing 6.01 kj of heat. Calculate the change in entropy and free energy for the process. Calculate the entropy
2/18/2013. Spontaneity, Entropy & Free Energy Chapter 16. The Dependence of Free Energy on Pressure Sample Exercises
 Spontaneity, Entropy & Free Energy Chapter 16 16.7 The Dependence of Free Energy on Pressure Why is free energy dependent on pressure? Isn t H, enthalpy independent of pressure at constant pressure? No
Spontaneity, Entropy & Free Energy Chapter 16 16.7 The Dependence of Free Energy on Pressure Why is free energy dependent on pressure? Isn t H, enthalpy independent of pressure at constant pressure? No
Free-energy change ( G) and entropy change ( S)
 Free-energy change ( G) and entropy change ( S) A SPONTANEOUS PROCESS (e.g. diffusion) will proceed on its own without any external influence. A problem with H A reaction that is exothermic will result
Free-energy change ( G) and entropy change ( S) A SPONTANEOUS PROCESS (e.g. diffusion) will proceed on its own without any external influence. A problem with H A reaction that is exothermic will result
Midterm Exam IV. CHEM 181: Introduction to Chemical Principles November 29, C(s, coal) + O 2 (g) CO 2 (g)
 Midterm Exam IV CHEM 8: Introduction to Chemical Principles November 29, 202. Use some or all of the following data: C(graphite) 0. C(diamond).9 CO 2 (g) 393.5 H 2 O(g) 24.8 CH 4 (g) 74.6 H f (kj mol )
Midterm Exam IV CHEM 8: Introduction to Chemical Principles November 29, 202. Use some or all of the following data: C(graphite) 0. C(diamond).9 CO 2 (g) 393.5 H 2 O(g) 24.8 CH 4 (g) 74.6 H f (kj mol )
Chem 6 sample exam 1 (100 points total)
 Chem 6 sample exam 1 (100 points total) @ This is a closed book exam to which the Honor Principle applies. @ The last page contains several equations which may be useful; you can detach it for easy reference.
Chem 6 sample exam 1 (100 points total) @ This is a closed book exam to which the Honor Principle applies. @ The last page contains several equations which may be useful; you can detach it for easy reference.
Thermodynamics. For the process to occur under adiabatic conditions, the correct condition is: (iii) q = 0. (iv) = 0
 Thermodynamics Choose the correct answer. A thermodynamic state function is a quantity (i) used to determine heat changes (ii) whose value is independent of path (iii) used to determine pressure volume
Thermodynamics Choose the correct answer. A thermodynamic state function is a quantity (i) used to determine heat changes (ii) whose value is independent of path (iii) used to determine pressure volume
CHEM J-2 June 2006 HCO 2. Calculate the osmotic pressure of a solution of 1.0 g of glucose (C 6 H 12 O 6 ) in 1500 ml of water at 37 C.
 CEM1405 2006-J-2 June 2006 Draw Lewis structures of ozone, 3, and the formate anion, C 2, including resonance hybrids where appropriate. 3 C 2 3 C C Calculate the osmotic pressure of a solution of 1.0
CEM1405 2006-J-2 June 2006 Draw Lewis structures of ozone, 3, and the formate anion, C 2, including resonance hybrids where appropriate. 3 C 2 3 C C Calculate the osmotic pressure of a solution of 1.0
Last Name or Student ID
 10/06/08, Chem433 Exam # 1 Last Name or Student ID 1. (3 pts) 2. (3 pts) 3. (3 pts) 4. (2 pts) 5. (2 pts) 6. (2 pts) 7. (2 pts) 8. (2 pts) 9. (6 pts) 10. (5 pts) 11. (6 pts) 12. (12 pts) 13. (22 pts) 14.
10/06/08, Chem433 Exam # 1 Last Name or Student ID 1. (3 pts) 2. (3 pts) 3. (3 pts) 4. (2 pts) 5. (2 pts) 6. (2 pts) 7. (2 pts) 8. (2 pts) 9. (6 pts) 10. (5 pts) 11. (6 pts) 12. (12 pts) 13. (22 pts) 14.
Chemical Thermodynamics
 Quiz A 42.8 ml solution of ammonia (NH 3 ) is titrated with a solution of 0.9713 M hydrochloric acid. The initial reading on the buret containing the HCl was 47.13 ml and the final reading when the endpoint
Quiz A 42.8 ml solution of ammonia (NH 3 ) is titrated with a solution of 0.9713 M hydrochloric acid. The initial reading on the buret containing the HCl was 47.13 ml and the final reading when the endpoint
CHEMISTRY 102B Hour Exam II. Dr. D. DeCoste T.A.
 CHEMISTRY 10B Hour Exam II March 19, 015 Dr. D. DeCoste Name Signature T.A. This exam contains questions on 9 numbered pages. Check now to make sure you have a complete exam. You have one hour and thirty
CHEMISTRY 10B Hour Exam II March 19, 015 Dr. D. DeCoste Name Signature T.A. This exam contains questions on 9 numbered pages. Check now to make sure you have a complete exam. You have one hour and thirty
Midterm Exam III. CHEM 181: Introduction to Chemical Principles November 25, 2014 Answer Key
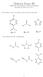 Midterm Exam III EM 181: Introduction to hemical Principles ovember 25, 2014 Answer Key 1. For reference, here are the pk a values for three weak acids: 3 pk a = 7.2 pk a = 4.8 pk a = 15 ow consider this
Midterm Exam III EM 181: Introduction to hemical Principles ovember 25, 2014 Answer Key 1. For reference, here are the pk a values for three weak acids: 3 pk a = 7.2 pk a = 4.8 pk a = 15 ow consider this
Autumn Semester. Basic Chemistry for Engineering/ Fundamental Inorganic Chemistry Final Exam. 18 February Time: 60 minutes
 Basic Chemistry Final Exam 1 Name: Student number: Instructions: Autumn Semester Basic Chemistry for Engineering/ Fundamental Inorganic Chemistry Final Exam 18 February 2013 Time: 60 minutes Answer any
Basic Chemistry Final Exam 1 Name: Student number: Instructions: Autumn Semester Basic Chemistry for Engineering/ Fundamental Inorganic Chemistry Final Exam 18 February 2013 Time: 60 minutes Answer any
Rate Law Summary. Rate Laws vary as a function of time
 Rate Law Summary Measure the instantaneous rate of a reaction: this is a number with units of M/s! Measure the rate of loss of a reactant r... the rate of appearance of a product Repeat the experiment
Rate Law Summary Measure the instantaneous rate of a reaction: this is a number with units of M/s! Measure the rate of loss of a reactant r... the rate of appearance of a product Repeat the experiment
Enthalpy, Entropy, and Free Energy Calculations
 Adapted from PLTL The energies of our system will decay, the glory of the sun will be dimmed, and the earth, tideless and inert, will no longer tolerate the race which has for a moment disturbed its solitude.
Adapted from PLTL The energies of our system will decay, the glory of the sun will be dimmed, and the earth, tideless and inert, will no longer tolerate the race which has for a moment disturbed its solitude.
Energy Ability to produce change or do work. First Law of Thermodynamics. Heat (q) Quantity of thermal energy
 THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
THERMOCHEMISTRY Thermodynamics Study of energy and its interconversions Energy is TRANSFORMED in a chemical reaction (POTENTIAL to KINETIC) HEAT (energy transfer) is also usually produced or absorbed -SYSTEM:
2.1) Name the following 4 compounds: [1 point each, 4 total] 2.2) Draw the following compounds in the box provided [1 point each, 4 total]
![2.1) Name the following 4 compounds: [1 point each, 4 total] 2.2) Draw the following compounds in the box provided [1 point each, 4 total] 2.1) Name the following 4 compounds: [1 point each, 4 total] 2.2) Draw the following compounds in the box provided [1 point each, 4 total]](/thumbs/78/77529351.jpg) The exam will be composed of 3 sections. The first section will contain ~30 multiple choice marked at 1 point each. The second section will consist of ~6 questions of ~4 points each for ~24 points. The
The exam will be composed of 3 sections. The first section will contain ~30 multiple choice marked at 1 point each. The second section will consist of ~6 questions of ~4 points each for ~24 points. The
CHAPTER THERMODYNAMICS
 54 CHAPTER THERMODYNAMICS 1. If ΔH is the change in enthalpy and ΔE the change in internal energy accompanying a gaseous reaction, then ΔHis always greater than ΔE ΔH< ΔE only if the number of moles of
54 CHAPTER THERMODYNAMICS 1. If ΔH is the change in enthalpy and ΔE the change in internal energy accompanying a gaseous reaction, then ΔHis always greater than ΔE ΔH< ΔE only if the number of moles of
CHEMISTRY 202 Practice Hour Exam III. Dr. D. DeCoste T.A (60 pts.) 21 (15 pts.) 22 (20 pts.) 23 (25 pts.) Total (120 pts)
 CHEMISTRY 202 Practice Hour Exam III Fall 2016 Dr. D. DeCoste Name Signature T.A. This exam contains 23 questions on 10 numbered pages. Check now to make sure you have a complete exam. You have two hours
CHEMISTRY 202 Practice Hour Exam III Fall 2016 Dr. D. DeCoste Name Signature T.A. This exam contains 23 questions on 10 numbered pages. Check now to make sure you have a complete exam. You have two hours
Questions 1-2 Consider the atoms of the following elements. Assume that the atoms are in the ground state. a. S b. Ca c. Ga d. Sb e.
 AP Chemistry Fall Semester Practice Exam 5 MULTIPLE CHOICE PORTION: Write the letter for the correct answer to the following questions on the provided answer sheet. Each multiple choice question is worth
AP Chemistry Fall Semester Practice Exam 5 MULTIPLE CHOICE PORTION: Write the letter for the correct answer to the following questions on the provided answer sheet. Each multiple choice question is worth
KEY. Chemistry 1A, Fall2003 Midterm Exam III, Version A November 13, 2003 (90 min, closed book)
 KEY Name: SID: TA Name: hemistry 1A, Fall2003 Midterm Exam III, Version A November 13, 2003 (90 min, closed book) Write your name on every page of this exam. This exam is multiple choice. Fill in the Scantron
KEY Name: SID: TA Name: hemistry 1A, Fall2003 Midterm Exam III, Version A November 13, 2003 (90 min, closed book) Write your name on every page of this exam. This exam is multiple choice. Fill in the Scantron
3/30/2017. Section 17.1 Spontaneous Processes and Entropy Thermodynamics vs. Kinetics. Chapter 17. Spontaneity, Entropy, and Free Energy
 Chapter 17 Spontaneity, Entropy, and Thermodynamics vs. Kinetics Domain of Kinetics Rate of a reaction depends on the pathway from reactants to products. Thermodynamics tells us whether a reaction is spontaneous
Chapter 17 Spontaneity, Entropy, and Thermodynamics vs. Kinetics Domain of Kinetics Rate of a reaction depends on the pathway from reactants to products. Thermodynamics tells us whether a reaction is spontaneous
EXAM III Nov. 1, 2018
 EM 105 Dr. Lammi Name: EXAM III Nov. 1, 2018 You may work until 10:50 to complete this exam. Please show all work in the space provided or on the attached scratch page. Remember to report your final answers
EM 105 Dr. Lammi Name: EXAM III Nov. 1, 2018 You may work until 10:50 to complete this exam. Please show all work in the space provided or on the attached scratch page. Remember to report your final answers
