Final Exam. CHEM 181: Introduction to Chemical Principles Answer Key. 1. The major resonance structure of 1,4-diazepine is shown below:
|
|
|
- Wesley Greene
- 5 years ago
- Views:
Transcription
1 Final Exam EM 181: Introduction to hemical Principles Answer Key 1. The major resonance structure of 1,4-diazepine is shown below: or Show all reasonable resonance structures; label them as major (if equivalent to the one above), minor, or very minor. Label all non-zero formal charges. (If you prefer, you can skip writing the carbons and their attached hydrogens, as shown above on the right.) There are seven possible arrangements that rotate the three double bonds around the ring. There is one minor structure: which places positive and negative formal charges on the two nitrogens. The following five structures are all very minor (positive nitrogen and negative carbon), and roughly though not exactly equivalent: 1
2 2. onsider the following resonance structures of cytosine: There are other (more minor) resonance structures that are not important for this problem. (next page) 2
3 When an acid protonates cytosine, the + is added only at one particular nitrogen. Draw the Lewis structure for protonated cytosine, including resonance structures and formal charges. Explain the reason that this site is protonated while the others are not. A simple approach is to look at the resonance structures for the unprotonated molecule, and note that for both the top and the bottom nitrogens, the lone pair is not present in all resonance structures that is, the lone pair is actually involved in molecular orbitals associated with bonding. This is not true for the center nitrogen. Protonating the center nitrogen leaves the major and minor resonance structures alone: 3
4 Protonating either of the others prevents the formation of either one or two of the minor structures: 4
5 3. itric acid ( ) is a triprotic acid. Abbreviating citrate as it = , the K a values are 3 it(aq) 2 it (aq) + + (aq) 2 it (aq) it 2 (aq) + + (aq) it 2 (aq) it 3 (aq) + + (aq) K a1 = K a2 = K a3 = Given orange juice with a p of 3.5, what are the relative concentrations (expressed as percentages) of 3 it, 2 it, it 2, and it 3? The equilibrium constant expressions are [ + ][ 2 it ] [ 3 it] [ + ][it ] [ 2 it ] [ + ][it 3 ] [it 2 ] = = = and the [ + ] concentration is = Since this is the + concentration at equilibrium, we can plug it in to the above to get [ 2 it ] [it ] = 2.66, [ 3 it] [ 2 it ] = 0.057, and [it3 ] [it 2 ] = If we set [ 3 it] = 1, that gives us [ 2 it ], which gives us [it 2 ], which gives us [it 3 ]: [ 3 it] = 1 [ 2 it ] = 2.66 [it 2 ] = [it 3 ] = Whatever the actual overall concentration, equilibrium concentrations of each species will always have these same proportions. Divide by the sum (3.81) and multiply by 100 to get percentages: 26.2% 3 it, 69.7% 2 it, 4.0% it 2, and 0.05% it 3 5
6 4. Use the following reactions and equilibrium constants: Ag 3 As 4 (s) 3Ag + (aq) + As 3 4 (aq) K sp = AgBr(s) Ag + (aq) + Br (aq) K sp = What are the equilibrium concentrations of Ag + (aq), Br (aq) and As 3 4 (aq) in a solution where there is an excess of both solids? (An approximate answer within 10% of the correct value will receive full credit if you also state whether your approximation is above or below the exact value.) Equilibrium expressions: [Ag + ] 3 [As 3 4 ] = [Ag + ][Br ] = Stoichiometry says that for every three silvers, there must be three Br or one As 4 : [Ag + ] = [Br ] + 3[As 3 4 ] (Arithmetic check: if we just had Ag 3 As 4, the silver concentration would be three times the As 4 concentration.) ow, divide the top equilibrium expression by the cube of the second: [Ag + ] 3 [As 3 4 ] ([Ag + ][Br ]) 3 = ( ) 3 [As 3 4 ] ([Br ]) 3 = [As 3 4 ] = ([Br ]) 3 Plug in to the stoichiometry: [Ag + ] = [Br ] ([Br ]) 3 and plug this into the AgBr equilibrium: [Ag + ][Br ] = ( [Br ] [Br ] 3) [Br ] = [Br ] 4 + [Br ] = 0 6
7 This is a quadratic in [Br ] 2 (or you can solve it with a calculator): Plug and chug [Br ] 2 = and [Br ] = [Br ] = M [Ag + ] = M [As 3 4 ] = M heck the stoichiometry: [Ag + ] = [Br ] + 3[As 3 4 ] = = You can also take an approximate approach. It s important to figure out which reaction to start with first, though; if you try the AgBr reaction, you get [Ag + ][Br ] = [Ag + ] = [Br ] [Ag + ] 2 = [Ag + ] = owever, if you plug this value for [Ag + ] into the other reaction, things do not work out: [Ag + ] 3 [As 3 4 ] = ( ) [As 3 4 ] = [As 3 4 ] = Since there must be at least three silver ions for every As 3 4, this cannot be 7
8 the As 3 4 concentration. Instead, you have to start with the other reaction: [Ag + ] 3 [As 3 4 ] = [Ag + ] = 3[As 3 4 ] ( 3[As 3 4 ] ) 3 [As 3 4 ] = [As 3 4 ] 4 = [As 3 4 ] = [Ag + ] = and then [Br ] = /[Ag + ] = Because this number is less than 10% of the As 3 4 concentration, it s reasonable to say that the amount of Ag + coming from this reaction is (at least) 10 times less than the amount coming from the As 3 4 reaction. By just plugging the Ag + concentration in, as we did above, we re pretending that somehow, producing Br doesn t produce more Ag +. This means that our approximate answer is going to predict less Ag + than there actually will be, and so must also be predicting more Br and As Definitions and constants: (a) The enthalpy of formation for a compound is the heat that goes into the system when pure elements in their most stable forms react to make the compound. The most stable forms for carbon, hydrogen, and oxygen are (s), 2 (g), and 2 (g). (b) The enthalpy of formation of 2 is 116 kj/mol. (c) The enthalpy of sublimation for carbon is the enthalpy change for the reaction: (s) (g) and is equal to 715 kj/mol. (d) The bond dissociation enthalpies for 2 and 2 are the heats that must go into the system to break the or bonds, forming gaseous atomic products. The exact values are bond ( 2 ) = 436 kj/mol and bond ( 2 ) = 498 kj/mol (e) The enthalpy of atomization is the heat that goes into a molecule to break every bond, forming only atomic gas-phase products. 8
9 alculate the enthalpy of atomization of 2. We want the reaction enthalpy for 2 (g) 2(g) + (g) + (g) The reactions we have to work with are 2 (g) + (s) (g) 2 (g) f = 116 kj mol 1 (s) (g) sub = 715 kj mol 1 2 (g) 2(g) bond = 436 kj mol 1 2 (g) 2(g) bond = 498 kj mol 1 What we want instead are 2 (g) 2 (g) + (s) (g) f = 116 kj mol 1 (s) (g) sub = 715 kj mol 1 2 (g) 2(g) bond = 436 kj mol (g) (g) bond = 249 kj mol 1 These add up to Alternately, ( ) kj mol 1 = 1516 kj mol 1 9
10 6. alcium hydroxide, a() 2, is somewhat soluble in water, but dissociates completely when it does dissolve: a() 2 (s) 2 a 2+ (aq) + 2 (aq) A saturated solution (with excess solid) of a() 2 at 25 is measured to have a p of The same solution heated to 50 has a p of What is the enthalpy of solution of a() 2? onvert p values to concentrations: [ ] = = K and [ ] = = K In both cases, [a 2+ ] = [ ]/2 by stoichiometry, giving us K 298 = and K 323 = Plug these values into the van t off equation: ln K 323 K 298 = R ( ) ln = R ( ) R = = R = ( J mol 1 K 1) = J mol 1 = 17.7 kj mol 1 Raising the temperature reduces the concentration, so it must be driving products back to reagents. Le hatelier s principle tells us that for this to be the case, must be negative. 10
11 7. The M diagram for l is formed from the 1s atomic orbital of and the 3s and 3p atomic orbitals of the l, and is shown below (vertical energy scale is not exact): l 4p l 4s l l 3d z 1s y x (or ) l 3p z s l 3s n the same energy scale as the molecular orbitals, draw in (horizontal lines) and label the 3s, 3p, 3d, 4s, and 4p energy levels for atomic chlorine and the 1s energy level for atomic hydrogen. The l 3s and 3p orbitals have to line up with the non-bonding l orbitals (which look exactly like the atomic orbitals). The 1s orbital must lie somewhere between the bonding and antibonding Ms. 11
12 The table below gives the wavelengths for the lowest-energy absorption features of l and l. Because some of the molecular orbitals of l look very much like some of the l atomic orbitals, some of the electronic transitions are very similar between l and l. Each transition corresponds to exciting a single electron from an initial to a final state. Example of how to describe transitions: the 3p 5p transition in chlorine causes an absorption at 105 nm, while the σ z l(5s) transition is at 100 nm. (These values will probably not be used.) λ (nm) l transition λ (nm) l transition 200 π x,y σ z 134 3p 4s 136 π x,y 4s 125 σ z σ z 120 3p 4p 120 π x,y 4p 116 3s 3p Fill in the above blanks with the starting and ending states for each transition. Things to consider when making these assignments: (a) Transitions must start with an orbital with at least one electron, and end in an orbital with at most one. (b) The chlorine 3s and 3p create non-bonding Ms, and for the same reasons so do the chlorine 4s and 4p. These will be at the same or nearly the same energy for l and l, so transitions that are matched in energy for l and l should both start and end from l non-bonding orbitals. (c) Since these are the lowest-energy absorption features, transitions to 4d, 5s, 5p, etc. orbitals should not be considered before transitions to 4s, 4p, etc. (Similarly, transitions from 1s, 2s, and 2p orbitals should not be considered.) 12
13 (d) Transitions that l has but l does not should either start at the σ z state (which is filled) or end at the σ z state (which is empty). (e) Transitions that end at the chlorine 3p orbital are the only ones that won t have a match with l this orbital is not completely filled for l, but the similar π x,y orbital is filled for l. 13
Final Exam. CHEM 181: Introduction to Chemical Principles December 14, 2015 Answer Key N C S C
 Final Exam EM 181: Introduction to hemical Principles December 14, 2015 Answer Key 1. The compound 4 5 NO has the connectivity shown below O N On the next page: Draw all reasonable resonance structures
Final Exam EM 181: Introduction to hemical Principles December 14, 2015 Answer Key 1. The compound 4 5 NO has the connectivity shown below O N On the next page: Draw all reasonable resonance structures
Midterm Exam III. CHEM 181: Introduction to Chemical Principles Solutions C HC N H 3 C. pka = 9.7
 Midterm Exam III EM 181: Introduction to hemical Principles Solutions 1. For reference, here are the pk a values for four weak acids (not all resonance structures shown): N 3 N N 3 3 2 pka = 3.5 pka =
Midterm Exam III EM 181: Introduction to hemical Principles Solutions 1. For reference, here are the pk a values for four weak acids (not all resonance structures shown): N 3 N N 3 3 2 pka = 3.5 pka =
Name: 1: /33 Grade: /100 2: /33 3: /33 +1 free point. Midterm Exam I. CHEM 181: Introduction to Chemical Principles September 20, 2012 Answer Key
 ame: 1: /33 Grade: /100 2: /33 3: /33 +1 free point Directions: Do all three problems. Midterm Exam I EM 181: Introduction to hemical Principles eptember 20, 2012 Answer Key how all of your work neatly
ame: 1: /33 Grade: /100 2: /33 3: /33 +1 free point Directions: Do all three problems. Midterm Exam I EM 181: Introduction to hemical Principles eptember 20, 2012 Answer Key how all of your work neatly
What is the energy of a photon with wavelength 232 nm?
 EMISTRY 110 EXAM 1 February 6, 2012 FRM A 1 ow many single covalent bonds must a sulfur atom form to have a complete octet in its valence shell? A. 3 B. 4. 1 D. 2 E. 0 2. What are the correct numbers of
EMISTRY 110 EXAM 1 February 6, 2012 FRM A 1 ow many single covalent bonds must a sulfur atom form to have a complete octet in its valence shell? A. 3 B. 4. 1 D. 2 E. 0 2. What are the correct numbers of
1. My answers for this Chemistry 102 exam should be graded with the answer sheet associated with: a. Form A b. Form B c. Form C d. Form D e.
 EMISTRY 10 Fall 010 our Exam Page 1 1. My answers for this hemistry 10 exam should be graded with the answer sheet associated with: a. Form A b. Form B c. Form d. Form D e. Form E. Which of the statements
EMISTRY 10 Fall 010 our Exam Page 1 1. My answers for this hemistry 10 exam should be graded with the answer sheet associated with: a. Form A b. Form B c. Form d. Form D e. Form E. Which of the statements
Midterm Exam III. CHEM 181: Introduction to Chemical Principles November 25, 2014 Answer Key
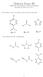 Midterm Exam III EM 181: Introduction to hemical Principles ovember 25, 2014 Answer Key 1. For reference, here are the pk a values for three weak acids: 3 pk a = 7.2 pk a = 4.8 pk a = 15 ow consider this
Midterm Exam III EM 181: Introduction to hemical Principles ovember 25, 2014 Answer Key 1. For reference, here are the pk a values for three weak acids: 3 pk a = 7.2 pk a = 4.8 pk a = 15 ow consider this
Covalent Bonding 10/29/2013
 Bond Energies or Bond Dissociation Energies Tables 8.4 and 8.5 on page 72 gives a list of the energy required to dissociate or break bonds. This value is used to determine whether covalent bonds will form
Bond Energies or Bond Dissociation Energies Tables 8.4 and 8.5 on page 72 gives a list of the energy required to dissociate or break bonds. This value is used to determine whether covalent bonds will form
Chemistry 201 FINAL EXAM Name Illinois Wesleyan University December 13, 2005 Fall 2005
 Chemistry 201 FINAL EXAM Name Illinois Wesleyan University December 13, 2005 Fall 2005 There are 200 points on this exam, and you have exactly two hours to complete it. 1. (12 points) a. Give the name
Chemistry 201 FINAL EXAM Name Illinois Wesleyan University December 13, 2005 Fall 2005 There are 200 points on this exam, and you have exactly two hours to complete it. 1. (12 points) a. Give the name
CH 222 Sample Exam Exam I Name: Lab Section:
 222 Sample Exam Exam I Name: Lab Section: Part I: Multiple hoice Questions (100 Points) Use a scantron sheet for Part I. There is only one best answer for each question. 1. Which of the following statements
222 Sample Exam Exam I Name: Lab Section: Part I: Multiple hoice Questions (100 Points) Use a scantron sheet for Part I. There is only one best answer for each question. 1. Which of the following statements
Fifth Exam CHEM 1A Summer 2017
 ifth Exam EM 1A Summer 2017 Name: Last KEY irst Instructions: Read every problem carefully. Gauge your time. Use the proper number of significant figures on your results. Don t just believe the calculator,
ifth Exam EM 1A Summer 2017 Name: Last KEY irst Instructions: Read every problem carefully. Gauge your time. Use the proper number of significant figures on your results. Don t just believe the calculator,
What we learn from Chap 17
 Acids and Bases hapter 17 What we learn from hap 17 17.2 This chapter concerns the nature and uses of acids and bases. It is the second in our three-chapter h discussion i about equilibrium, and reinforces
Acids and Bases hapter 17 What we learn from hap 17 17.2 This chapter concerns the nature and uses of acids and bases. It is the second in our three-chapter h discussion i about equilibrium, and reinforces
exothermic reaction and that ΔH c will therefore be a negative value. Heat change, q = mcδt q = m(h 2
 Worked solutions hapter 5 Exercises 1 B If the temperature drops, the process must be endothermic. Δ for endothermic reactions is always positive. 2 B All exothermic reactions give out heat. While there
Worked solutions hapter 5 Exercises 1 B If the temperature drops, the process must be endothermic. Δ for endothermic reactions is always positive. 2 B All exothermic reactions give out heat. While there
The reactions we have dealt with so far in chemistry are considered irreversible.
 1. Equilibrium Students: model static and dynamic equilibrium and analyse the differences between open and closed systems investigate the relationship between collision theory and reaction rate in order
1. Equilibrium Students: model static and dynamic equilibrium and analyse the differences between open and closed systems investigate the relationship between collision theory and reaction rate in order
CHM 151 Practice Final Exam
 CM 151 Practice Final Exam 1. ow many significant figures are there in the result of 5.52 divided by 3.745? (a) 1 (b) 2 (c) 3 (d) 4 (e) 5 2. ow many significant figures are there in the answer when 9.021
CM 151 Practice Final Exam 1. ow many significant figures are there in the result of 5.52 divided by 3.745? (a) 1 (b) 2 (c) 3 (d) 4 (e) 5 2. ow many significant figures are there in the answer when 9.021
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Take Home Exam Chem 1A Fall 2008 - Chapters 6 to 9: You may us any resource you wish accept people. On your honor, you may not ask another person for help. Show your work on every answer. Partial credit
Take Home Exam Chem 1A Fall 2008 - Chapters 6 to 9: You may us any resource you wish accept people. On your honor, you may not ask another person for help. Show your work on every answer. Partial credit
Some Basic Concepts of Chemistry
 0 Some Basic Concepts of Chemistry Chapter 0: Some Basic Concept of Chemistry Mass of solute 000. Molarity (M) Molar mass volume(ml).4 000 40 500 0. mol L 3. (A) g atom of nitrogen 8 g (B) 6.03 0 3 atoms
0 Some Basic Concepts of Chemistry Chapter 0: Some Basic Concept of Chemistry Mass of solute 000. Molarity (M) Molar mass volume(ml).4 000 40 500 0. mol L 3. (A) g atom of nitrogen 8 g (B) 6.03 0 3 atoms
4. Which of the following gas molecules will have the highest average velocity at 500K? a. H 2 b. He c. CH 4 d. C 2H 6
 Multiple hoice (3 pts each) 1. In which of the following processes will work be positive? Assume the pressure remains constant for all examples. a. 2(l) 2(g) at constant temperature b. 2 8 18(l) + 25 2(g)
Multiple hoice (3 pts each) 1. In which of the following processes will work be positive? Assume the pressure remains constant for all examples. a. 2(l) 2(g) at constant temperature b. 2 8 18(l) + 25 2(g)
Chemistry 231 Fall 2014 Oregon State University Final Exam December 8, 2014 Drs. Nafshun, Watson, Nyman, Barth, Burand
 Chemistry 231 Fall 2014 Oregon State University Final Exam December 8, 2014 Drs. Nafshun, Watson, Nyman, Barth, Burand Instructions: You should have with you several number two pencils, an eraser, your
Chemistry 231 Fall 2014 Oregon State University Final Exam December 8, 2014 Drs. Nafshun, Watson, Nyman, Barth, Burand Instructions: You should have with you several number two pencils, an eraser, your
Chem 127, Final Exam December 10, 2003
 I. (55 points) This part of the final corresponds to Exam I. It covers the material in Chapters 1, 2 and 3. A. (10 points) Answer the following questions by writing your answers on the blanks provided.
I. (55 points) This part of the final corresponds to Exam I. It covers the material in Chapters 1, 2 and 3. A. (10 points) Answer the following questions by writing your answers on the blanks provided.
Final Exam Review Chem 110 MJ Bojan
 Final Exam Review hem 110 MJ Bojan EM 110 EXAM Final Exam Fri. May 3 10:10am noon Locations are assigned by section and are posted on the Web. (IGNORE e-lion listings) http://courses.chem.psu.edu/chem110spring!
Final Exam Review hem 110 MJ Bojan EM 110 EXAM Final Exam Fri. May 3 10:10am noon Locations are assigned by section and are posted on the Web. (IGNORE e-lion listings) http://courses.chem.psu.edu/chem110spring!
CHAPTER 9 COVALENT BONDING: ORBITALS 323
 APTER 9 OVALET BODIG: ORBITALS 323 2 3 2 2 2 3 3 2 2 3 2 3 O * * 2 o; most of the carbons are not in the same plane since a majority of carbon atoms exhibit a tetrahedral structure (19.5 bond angles).
APTER 9 OVALET BODIG: ORBITALS 323 2 3 2 2 2 3 3 2 2 3 2 3 O * * 2 o; most of the carbons are not in the same plane since a majority of carbon atoms exhibit a tetrahedral structure (19.5 bond angles).
CHEM 341: Organic Chemistry I at North Dakota State University Midterm Exam 01 - Fri, Feb 10, 2012!! Name:!
 CEM 341: rganic Chemistry I at North Dakota State University Midterm Exam 01 - Fri, Feb 10, 2012!! Name:! Please read through each question carefully and answer in the spaces provided. A good strategy
CEM 341: rganic Chemistry I at North Dakota State University Midterm Exam 01 - Fri, Feb 10, 2012!! Name:! Please read through each question carefully and answer in the spaces provided. A good strategy
Chemistry 250A -- Exam #1 Answer Key -- September 23, 2008 (There are 5 pages.)
 ame: 1 hemistry 250 -- Exam #1 nswer Key -- September 23, 2008 (There are 5 pages.) 1. (15 pts) Show the major product(s) from the following acid-base reactions. If there is no reaction then say o Reaction.
ame: 1 hemistry 250 -- Exam #1 nswer Key -- September 23, 2008 (There are 5 pages.) 1. (15 pts) Show the major product(s) from the following acid-base reactions. If there is no reaction then say o Reaction.
Name AP CHEM / / Collected AP Exam Essay Answers for Chapter 16
 Name AP CHEM / / Collected AP Exam Essay Answers for Chapter 16 1980 - #7 (a) State the physical significance of entropy. Entropy (S) is a measure of randomness or disorder in a system. (b) From each of
Name AP CHEM / / Collected AP Exam Essay Answers for Chapter 16 1980 - #7 (a) State the physical significance of entropy. Entropy (S) is a measure of randomness or disorder in a system. (b) From each of
Chem 101 General Chemistry Practice Final Exam
 Name h = 6.626 x 10-34 J s (Planck s Constant) c = 3.00 x 10 8 m/s (speed of light) R H = 1.097 x 10-7 m -1 (Rydberg Constant) Chem 101 General Chemistry Practice Final Exam Multiple Choice (5 points each)
Name h = 6.626 x 10-34 J s (Planck s Constant) c = 3.00 x 10 8 m/s (speed of light) R H = 1.097 x 10-7 m -1 (Rydberg Constant) Chem 101 General Chemistry Practice Final Exam Multiple Choice (5 points each)
Learning Guide for Chapter 1 - Atoms and Molecules
 Learning Guide for hapter 1 - Atoms and Molecules I. Introduction to organic chemistry - p 1 II. Review of atomic structure - p 3 Elementary particles Periodic Table of Elements Electronegativity Atomic
Learning Guide for hapter 1 - Atoms and Molecules I. Introduction to organic chemistry - p 1 II. Review of atomic structure - p 3 Elementary particles Periodic Table of Elements Electronegativity Atomic
CHEMISTRY - TRO 4E CH.9 - CHEMICAL BONDING I: THE LEWIS MODEL
 !! www.clutchprep.com CONCEPT: ATOMIC PROPERTIES AND CHEMICAL BONDS Before we examine the types of chemical bonding, we should ask why atoms bond at all. Generally, the reason is that ionic bonding the
!! www.clutchprep.com CONCEPT: ATOMIC PROPERTIES AND CHEMICAL BONDS Before we examine the types of chemical bonding, we should ask why atoms bond at all. Generally, the reason is that ionic bonding the
Chem. 1A Final Practice Test 1
 Chem. 1A Final Practice Test 1 All work must be shown on the exam for partial credit. Points will be taken off for incorrect or no units. Calculators are allowed. Cell phones may not be used for calculators.
Chem. 1A Final Practice Test 1 All work must be shown on the exam for partial credit. Points will be taken off for incorrect or no units. Calculators are allowed. Cell phones may not be used for calculators.
CHEMISTRY 110 Final EXAM Dec 17, 2012 FORM A
 CEMISTRY 110 Final EXAM Dec 17, 2012 FORM A 1. Given the following reaction which of the following statements is true? Fe(s) + CuCl 2 (aq)! Cu(s) + FeCl 2 (aq) A. Iron is oxidized and copper is reduced.
CEMISTRY 110 Final EXAM Dec 17, 2012 FORM A 1. Given the following reaction which of the following statements is true? Fe(s) + CuCl 2 (aq)! Cu(s) + FeCl 2 (aq) A. Iron is oxidized and copper is reduced.
HourEx4 Practice Chapt 8 & 9 Kotz
 HourEx4 Practice Chapt 8 & 9 Kotz Multiple Choice 5 pts apiece Identify the letter of the choice that best completes the statement or answers the question. PUT YOUR ANSWER ON THE BUBBLE SHEET PROVIDED.You
HourEx4 Practice Chapt 8 & 9 Kotz Multiple Choice 5 pts apiece Identify the letter of the choice that best completes the statement or answers the question. PUT YOUR ANSWER ON THE BUBBLE SHEET PROVIDED.You
CHEMISTRY 15 EXAM IV-Version A (White)
 CHEMISTRY 15 EXAM IV-Version A (White) Dr. M. Richards-Babb June 26, 2001 An optical scoring machine will grade this examination. The machine is not programmed to accept the correct one of two sensed answers
CHEMISTRY 15 EXAM IV-Version A (White) Dr. M. Richards-Babb June 26, 2001 An optical scoring machine will grade this examination. The machine is not programmed to accept the correct one of two sensed answers
CH 222 Chapter Seven Concept Guide
 CH 222 Chapter Seven Concept Guide 1. Lewis Structures Draw the Lewis Dot Structure for cyanide ion, CN -. 1 C at 4 electrons = 4 electrons 1 N at 5 electrons = 5 electrons -1 charge = + 1 electron Total
CH 222 Chapter Seven Concept Guide 1. Lewis Structures Draw the Lewis Dot Structure for cyanide ion, CN -. 1 C at 4 electrons = 4 electrons 1 N at 5 electrons = 5 electrons -1 charge = + 1 electron Total
You might find the following useful. CHEMISTRY 1A Fall 2008 EXAM 3 Key CHAPTERS 7, 8, 9 & part 10
 You might find the following useful. CHEMISTRY 1A Fall 2008 EXAM 3 Key CHAPTERS 7, 8, 9 & part 10 1 For each of the following, write the word, words, or number in each blank that best completes each sentence.
You might find the following useful. CHEMISTRY 1A Fall 2008 EXAM 3 Key CHAPTERS 7, 8, 9 & part 10 1 For each of the following, write the word, words, or number in each blank that best completes each sentence.
CHERRY HILL TUITION AQA CHEMISTRY A2 PAPER Section A. Answer all questions in the spaces provided.
 2 Section A Answer all questions in the spaces provided. 1 This question is about bond dissociation enthalpies and their use in the calculation of enthalpy changes. 1 (a) Define bond dissociation enthalpy
2 Section A Answer all questions in the spaces provided. 1 This question is about bond dissociation enthalpies and their use in the calculation of enthalpy changes. 1 (a) Define bond dissociation enthalpy
Name:. Correct Questions = Wrong Questions =.. Unattempt Questions = Marks =
 Name:. Correct Questions = Wrong Questions =.. Unattempt Questions = Marks = 1. (12%) Compound X contains 2.239% hydrogen, 26.681% carbon and 71.080 % oxygen by mass. The titration of 0.154 g of this compound
Name:. Correct Questions = Wrong Questions =.. Unattempt Questions = Marks = 1. (12%) Compound X contains 2.239% hydrogen, 26.681% carbon and 71.080 % oxygen by mass. The titration of 0.154 g of this compound
Chemistry: The Central Science
 Chemistry: The Central Science Fourteenth Edition Chapter 8 Basic Concepts of Chemical Bonding Chemical Bonds Three basic types of bonds Ionic Electrostatic attraction between ions Covalent Sharing of
Chemistry: The Central Science Fourteenth Edition Chapter 8 Basic Concepts of Chemical Bonding Chemical Bonds Three basic types of bonds Ionic Electrostatic attraction between ions Covalent Sharing of
CHEM J-2 June 2006 HCO 2. Calculate the osmotic pressure of a solution of 1.0 g of glucose (C 6 H 12 O 6 ) in 1500 ml of water at 37 C.
 CEM1405 2006-J-2 June 2006 Draw Lewis structures of ozone, 3, and the formate anion, C 2, including resonance hybrids where appropriate. 3 C 2 3 C C Calculate the osmotic pressure of a solution of 1.0
CEM1405 2006-J-2 June 2006 Draw Lewis structures of ozone, 3, and the formate anion, C 2, including resonance hybrids where appropriate. 3 C 2 3 C C Calculate the osmotic pressure of a solution of 1.0
Chapter The Ionic Bond. Why are ionic compounds stable? Spontaneous Processes. Chemical Bonding I: Basic Concepts.
 Lewis Dot ymbols hapter 9 hemical Bonding I: Basic oncepts Introduced by G.. Lewis Element symbol plus 1 dot for each valence e Elements tend to form octets, noble gas configurations Useful for representative
Lewis Dot ymbols hapter 9 hemical Bonding I: Basic oncepts Introduced by G.. Lewis Element symbol plus 1 dot for each valence e Elements tend to form octets, noble gas configurations Useful for representative
Chemistry *P45044A0128* Pearson Edexcel P45044A
 Write your name here Surname ther names Pearson Edexcel International Advanced Level entre Number andidate Number hemistry Advanced Unit 4: General Principles of hemistry I Rates, Equilibria and Further
Write your name here Surname ther names Pearson Edexcel International Advanced Level entre Number andidate Number hemistry Advanced Unit 4: General Principles of hemistry I Rates, Equilibria and Further
Practice Test Questions 4 Molecular Orbital Theory: Polyatomic Molecules
 Practice Test Questions 4 Molecular rbital Theory: Polyatomic Molecules 1. The images below show the valence molecular orbitals obtained for the carbonate ion via a semiempirical calculation. Both side
Practice Test Questions 4 Molecular rbital Theory: Polyatomic Molecules 1. The images below show the valence molecular orbitals obtained for the carbonate ion via a semiempirical calculation. Both side
Chem. 1B Final Practice First letter of last name
 Chem. 1B Final Practice First letter of last name Name Student Number All work must be shown on the exam for partial credit. Points will be taken off for incorrect or no units. Calculators are allowed.
Chem. 1B Final Practice First letter of last name Name Student Number All work must be shown on the exam for partial credit. Points will be taken off for incorrect or no units. Calculators are allowed.
Chem. 1C Midterm 1 Version B April 26, 2017
 First initial of last name Chem. C Midterm Version B April 6, 07 Name: Print Neatly. You will lose point if I cannot read your name or perm number. Perm Number: All work must be shown on the exam for partial
First initial of last name Chem. C Midterm Version B April 6, 07 Name: Print Neatly. You will lose point if I cannot read your name or perm number. Perm Number: All work must be shown on the exam for partial
Chem. 1C Midterm 1 Version A April 26, 2017
 First initial of last name Chem. C Midterm Version A April 6, 07 Name: Print Neatly. You will lose point if I cannot read your name or perm number. Perm Number: All work must be shown on the exam for partial
First initial of last name Chem. C Midterm Version A April 6, 07 Name: Print Neatly. You will lose point if I cannot read your name or perm number. Perm Number: All work must be shown on the exam for partial
Chemistry 122 (Tyvoll) ANSWERS TO PRACTICE EXAMINATION I Fall 2005
 hemistry 122 (Tyvoll) ANSWERS T PRATIE EXAMINATIN I Fall 2005 1. Which statement is not correct? 1) A volatile liquid has a high boiling point. 2. Which of the following compounds is predicted to have
hemistry 122 (Tyvoll) ANSWERS T PRATIE EXAMINATIN I Fall 2005 1. Which statement is not correct? 1) A volatile liquid has a high boiling point. 2. Which of the following compounds is predicted to have
Chapter 3. Stoichiometry
 1 hapter 3 Stoichiometry 2 Atomic Mass Avogadro s Number and Molar Mass 3 Atomic Mass: Mass of 1 atom in atomic mass units 1 amu = 1/12 of the mass of 1-12 atom = 1.661X10-24 g Naturally occurring carbon
1 hapter 3 Stoichiometry 2 Atomic Mass Avogadro s Number and Molar Mass 3 Atomic Mass: Mass of 1 atom in atomic mass units 1 amu = 1/12 of the mass of 1-12 atom = 1.661X10-24 g Naturally occurring carbon
Bonding and Dynamics. Outline Bonding and Dynamics Water Interactions Self Ionization of Water Homework
 Liquid Water Structure In liquid water, most of the water molecules have the same local environment as in ice but the long range structure of ice disappears due to motion of the molecules. Bonds between
Liquid Water Structure In liquid water, most of the water molecules have the same local environment as in ice but the long range structure of ice disappears due to motion of the molecules. Bonds between
UNIVERSITY OF VICTORIA. CHEMISTRY 101 Mid-Term Test 2, November
 NAME Student No. SECTIN (circle one): A01 (Codding) A02 (Sirk) A03 (Briggs) Version A UNIVERSITY F VICTRIA CEMISTRY 101 Mid-Term Test 2, November 19 2010 Version A This test has two parts and 8 pages,
NAME Student No. SECTIN (circle one): A01 (Codding) A02 (Sirk) A03 (Briggs) Version A UNIVERSITY F VICTRIA CEMISTRY 101 Mid-Term Test 2, November 19 2010 Version A This test has two parts and 8 pages,
Lewis Formulas CHAPTER 7.
 ATER 7. Lewis ormulas 7-1. See the text. Because silicon is below carbon in the periodic table, we can determine the Lewis formula of Si 4 in the same manner as for 4. 7-2. Because phosphorus is the unique
ATER 7. Lewis ormulas 7-1. See the text. Because silicon is below carbon in the periodic table, we can determine the Lewis formula of Si 4 in the same manner as for 4. 7-2. Because phosphorus is the unique
Level 2 Chemistry, 2014
 91164 911640 2SUPERVISOR S Level 2 Chemistry, 2014 91164 Demonstrate understanding of bonding, structure, properties and energy changes 2.00 pm Tuesday 11 November 2014 Credits: Five Achievement Achievement
91164 911640 2SUPERVISOR S Level 2 Chemistry, 2014 91164 Demonstrate understanding of bonding, structure, properties and energy changes 2.00 pm Tuesday 11 November 2014 Credits: Five Achievement Achievement
CHEMISTRY 107 Section 501 Exam #3 Version A November 16, 2016 Dr. Larry Brown
 NAME: (print) UIN #: CHEMISTRY 107 Section 501 Exam #3 Version A November 16, 2016 Dr. Larry Brown This is a 50-minute exam, and contains 7 problems. There should be 10 numbered pages, including this one.
NAME: (print) UIN #: CHEMISTRY 107 Section 501 Exam #3 Version A November 16, 2016 Dr. Larry Brown This is a 50-minute exam, and contains 7 problems. There should be 10 numbered pages, including this one.
Name:. Correct Questions = Wrong Questions =.. Unattempt Questions = Marks =
 Name:. orrect Questions = Wrong Questions =.. Unattempt Questions = Marks = 1. (11%) A(g) + 3B(g) r 2(g) Use the tabulated data to answer the questions about this reaction, which is carried out in a 1.0
Name:. orrect Questions = Wrong Questions =.. Unattempt Questions = Marks = 1. (11%) A(g) + 3B(g) r 2(g) Use the tabulated data to answer the questions about this reaction, which is carried out in a 1.0
CHEMISTRY. Section II (Total time 95 minutes) Part A Time 55 minutes YOU MAY USE YOUR CALCULATOR FOR PART A.
 CHEMISTRY Section II (Total time 95 minutes) Part A Time 55 minutes YOU MAY USE YOUR CALCULATOR FOR PART A. CLEARLY SHOW THE METHOD USED AND THE STEPS INVOLVED IN ARRIVING AT YOUR ANSWERS. It is to your
CHEMISTRY Section II (Total time 95 minutes) Part A Time 55 minutes YOU MAY USE YOUR CALCULATOR FOR PART A. CLEARLY SHOW THE METHOD USED AND THE STEPS INVOLVED IN ARRIVING AT YOUR ANSWERS. It is to your
MITOCW MIT3_091SCF10Final_Exam_A_Prob_10_300k
 MITOCW MIT3_091SCF10Final_Exam_A_Prob_10_300k SPEAKER: The following content is provided under a Creative Commons License. Your support will help MIT OpenCourseWare continue to offer high quality educational
MITOCW MIT3_091SCF10Final_Exam_A_Prob_10_300k SPEAKER: The following content is provided under a Creative Commons License. Your support will help MIT OpenCourseWare continue to offer high quality educational
a. N b. As c. C d. O e. Br f. Be g. S h. Se 3. Which compound in each of the following pairs should require the higher temperature to melt?
 C 222 Practice Problem Set #1 This is a practice problem set and not the actual graded problem set that you will turn in for credit. Answers to each problem can be found at the end of this assignment.
C 222 Practice Problem Set #1 This is a practice problem set and not the actual graded problem set that you will turn in for credit. Answers to each problem can be found at the end of this assignment.
Chapter 6 Molecular Structure
 hapter 6 Molecular Structure 1. Draw the Lewis structure of each of the following ions, showing all nonzero formal charges. Indicate whether each ion is linear or bent. If the ion is bent, what is the
hapter 6 Molecular Structure 1. Draw the Lewis structure of each of the following ions, showing all nonzero formal charges. Indicate whether each ion is linear or bent. If the ion is bent, what is the
Chapter 8 Basic Concepts of Chemical Bonding
 hapter 8 Basic oncepts of hemical Bonding An Important Principle in hemistry The microscopic structure defines the properties of matter at our mesoscopic level. Ex. Graphite and Diamond (both are pure
hapter 8 Basic oncepts of hemical Bonding An Important Principle in hemistry The microscopic structure defines the properties of matter at our mesoscopic level. Ex. Graphite and Diamond (both are pure
Chem. 1A Midterm 1 Version A October 19, 2018
 First initial of last name Chem. 1A Midterm 1 Version A October 19, 2018 Name: Print Neatly. You will lose 1 point if I cannot read your name or perm number. Perm Number: All work must be shown on the
First initial of last name Chem. 1A Midterm 1 Version A October 19, 2018 Name: Print Neatly. You will lose 1 point if I cannot read your name or perm number. Perm Number: All work must be shown on the
17/11/2010. Lewis structures
 Reading assignment: 8.5-8.8 As you read ask yourself: How can I use Lewis structures to account for bonding in covalent molecules? What are the differences between single, double and triple bonds in terms
Reading assignment: 8.5-8.8 As you read ask yourself: How can I use Lewis structures to account for bonding in covalent molecules? What are the differences between single, double and triple bonds in terms
Chapter 13. Conjugated Unsaturated Systems. +,., - Allyl. What is a conjugated system? AllylicChlorination (High Temperature)
 What is a conjugated system? Chapter 13 Conjugated Unsaturated Systems Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital may be empty (a carbocation The
What is a conjugated system? Chapter 13 Conjugated Unsaturated Systems Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital may be empty (a carbocation The
CHEMISTRY. Section II (Total time 95 minutes) Part A Time 55 minutes YOU MAY USE YOUR CALCULATOR FOR PART A.
 CHEMISTRY Section II (Total time 95 minutes) Part A Time 55 minutes YOU MAY USE YOUR CALCULATOR FOR PART A. CLEARLY SHOW THE METHOD USED AND THE STEPS INVOLVED IN ARRIVING AT YOUR ANSWERS. It is to your
CHEMISTRY Section II (Total time 95 minutes) Part A Time 55 minutes YOU MAY USE YOUR CALCULATOR FOR PART A. CLEARLY SHOW THE METHOD USED AND THE STEPS INVOLVED IN ARRIVING AT YOUR ANSWERS. It is to your
Version 001 Practice Final Exam 1
 Version 001 Practice Final Exam 1 This print-out should have 50 questions. Multiple-choice questions may continue on the next column or page find all choices before answering. 001 2.0 points ow many moles
Version 001 Practice Final Exam 1 This print-out should have 50 questions. Multiple-choice questions may continue on the next column or page find all choices before answering. 001 2.0 points ow many moles
Chapter In each case the conjugate base is obtained by removing a proton from the acid: (a) OH (b) I (c)
 Practice Exercises 16.1 Conjugate acid base pairs (a), (c), and (f) (b) The conjugate base of I is I (d) The conjugate base of N 2 is N 2 and the conjugate base of N 4 is N 3 (e) The conjugate acid of
Practice Exercises 16.1 Conjugate acid base pairs (a), (c), and (f) (b) The conjugate base of I is I (d) The conjugate base of N 2 is N 2 and the conjugate base of N 4 is N 3 (e) The conjugate acid of
Ionic Bond TRANSFER of electrons between atoms. Ionic Bonding. Ionic Bonding. Ionic Bonding. Attraction that holds atoms together
 BONDING Chemical Bond Attraction that holds atoms together Types include IONIC, METALLIC, or COVALENT Differences in electronegativity determine the bond type Ionic Bond TRANSFER of electrons between atoms
BONDING Chemical Bond Attraction that holds atoms together Types include IONIC, METALLIC, or COVALENT Differences in electronegativity determine the bond type Ionic Bond TRANSFER of electrons between atoms
Chemical Bonding I: Basic Concepts
 Chapter 8 Chemical Bonding I: Basic Concepts Dr. A. Al-Saadi 1 Chapter 8 Preview Ionic Bonding vs. covalent bonding. Electronegativity and dipole moment. Bond polarity. Lewis structure: ow to draw a Lewis
Chapter 8 Chemical Bonding I: Basic Concepts Dr. A. Al-Saadi 1 Chapter 8 Preview Ionic Bonding vs. covalent bonding. Electronegativity and dipole moment. Bond polarity. Lewis structure: ow to draw a Lewis
2 Examiner SECTION A. Answer all the questions in the spaces provided.
 2 SECTION A Answer all the questions in the spaces provided. 1. An isotope of magnesium, 27 Mg, is used to detect leaks in water pipes. (a) It decays by β emission with a half life of 9 5 minutes. (i)
2 SECTION A Answer all the questions in the spaces provided. 1. An isotope of magnesium, 27 Mg, is used to detect leaks in water pipes. (a) It decays by β emission with a half life of 9 5 minutes. (i)
Chem. 1B Final Practice
 Chem. 1B Final Practice Name Student Number All work must be shown on the exam for partial credit. Points will be taken off for incorrect or no units and for the incorrect number of significant figures.
Chem. 1B Final Practice Name Student Number All work must be shown on the exam for partial credit. Points will be taken off for incorrect or no units and for the incorrect number of significant figures.
1. How many electrons, protons and neutrons does 87 Sr 2+ have?
 ***This is a sample exam is lacking some questions over chapter 12 as this is a new chapter for the general chemistry sequence this semester. For a sampling of some chapter 12 problems, see the additional
***This is a sample exam is lacking some questions over chapter 12 as this is a new chapter for the general chemistry sequence this semester. For a sampling of some chapter 12 problems, see the additional
Practice Hour Examination # 1-1
 CHEM 346 Organic Chemistry I Fall 2013 Practice Hour Examination # 1-1 Solutions Key Page 1 of 12 CHEM 346 Organic Chemistry I (for Majors) Instructor: Paul J. Bracher Practice Hour Examination # 1-1 Monday,
CHEM 346 Organic Chemistry I Fall 2013 Practice Hour Examination # 1-1 Solutions Key Page 1 of 12 CHEM 346 Organic Chemistry I (for Majors) Instructor: Paul J. Bracher Practice Hour Examination # 1-1 Monday,
Chem 105 Final Exam. Here is the summary of the total 225 points plus 10 bonus points. Carefully read the questions. Good luck!
 May 3 rd, 2012 Name: CLID: Score: Chem 105 Final Exam There are 50 multiple choices that are worth 3 points each. There are 4 problems and 1 bonus problem. Try to answer the questions, which you know first,
May 3 rd, 2012 Name: CLID: Score: Chem 105 Final Exam There are 50 multiple choices that are worth 3 points each. There are 4 problems and 1 bonus problem. Try to answer the questions, which you know first,
AP Chemistry Summer Review Assignment
 Name: Period: Chem I Teacher/year: AP Chemistry Summer Review Assignment Due on the FIRST DAY OF SCHOOL! A. Chemical Foundations 1. The beakers shown below have different precisions. a. Label the amount
Name: Period: Chem I Teacher/year: AP Chemistry Summer Review Assignment Due on the FIRST DAY OF SCHOOL! A. Chemical Foundations 1. The beakers shown below have different precisions. a. Label the amount
May 09, Ksp.notebook. Ksp = [Li + ] [F + ] Find the Ksp for the above reaction.
![May 09, Ksp.notebook. Ksp = [Li + ] [F + ] Find the Ksp for the above reaction. May 09, Ksp.notebook. Ksp = [Li + ] [F + ] Find the Ksp for the above reaction.](/thumbs/85/92550924.jpg) example: Constant Product K sp Solubility Product Constant Some compounds dissolve in water Some compounds dissolve better than others The more that a compound can dissolve, the more soluble the compound
example: Constant Product K sp Solubility Product Constant Some compounds dissolve in water Some compounds dissolve better than others The more that a compound can dissolve, the more soluble the compound
CHE1502. Tutorial letter 201/1/2016. General Chemistry 1B. Semester 1. Department of Chemistry CHE1502/201/1/2016
 CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
CE1502/201/1/2016 Tutorial letter 201/1/2016 General Chemistry 1B CE1502 Semester 1 Department of Chemistry This tutorial letter contains the answers to the questions in assignment 1. FIRST SEMESTER: KEY
Learning Guide for Chapter 7 - Organic Reactions I
 Learning Guide for Chapter 7 - rganic Reactions I I. Introduction to Reactions II. Principles of Kinetics III. Principles of Thermodynamics IV. Nucleophiles and Electrophiles V. Acids and Bases What a
Learning Guide for Chapter 7 - rganic Reactions I I. Introduction to Reactions II. Principles of Kinetics III. Principles of Thermodynamics IV. Nucleophiles and Electrophiles V. Acids and Bases What a
Deduce the following information from the structure of estradiol, a phenol contain compound.
 A2 Chemistry: F324 Rings, Polymers and Analysis The Chemistry of Phenol Learning Outcomes: All (E) State the uses of phenols Most (C) Describe the reactions of phenol with aqueous alkalis and with sodium
A2 Chemistry: F324 Rings, Polymers and Analysis The Chemistry of Phenol Learning Outcomes: All (E) State the uses of phenols Most (C) Describe the reactions of phenol with aqueous alkalis and with sodium
Chapter 14 Acids and Bases
 Properties of Acids and Bases Chapter 14 Acids and Bases Svante Arrhenius (1859-1927) First to develop a theory for acids and bases in aqueous solution Arrhenius Acids Compounds which dissolve (dissociate)
Properties of Acids and Bases Chapter 14 Acids and Bases Svante Arrhenius (1859-1927) First to develop a theory for acids and bases in aqueous solution Arrhenius Acids Compounds which dissolve (dissociate)
4 Diatomic molecules
 s manual for Burrows et.al. Chemistry 3 Third edition 4 Diatomic molecules Answers to worked examples WE 4.1 The Lewis model (on p. 174 in Chemistry 3 ) Use the Lewis model to describe the bonding in (a)
s manual for Burrows et.al. Chemistry 3 Third edition 4 Diatomic molecules Answers to worked examples WE 4.1 The Lewis model (on p. 174 in Chemistry 3 ) Use the Lewis model to describe the bonding in (a)
Overview of Photosynthesis
 Overview of Photosynthesis In photosynthesis, green plants absorb energy from the sun and use the energy to drive an endothermic reaction, the reaction between carbon dioxide and water that produces glucose
Overview of Photosynthesis In photosynthesis, green plants absorb energy from the sun and use the energy to drive an endothermic reaction, the reaction between carbon dioxide and water that produces glucose
tert-butyl alcohol n-butyl alcohol methyl propyl ether (c) Explain briefly why n-butyl alcohol has a much higher bp than methyl propyl ether.
 1. (15 points) Three 4 10 isomers are shown below, along with their boiling points. ( 3 ) 3 3 2 2 2 3 2 2 3 tert-butyl alcohol n-butyl alcohol methyl propyl ether bp: 82 118 39 (a) Based on their boiling
1. (15 points) Three 4 10 isomers are shown below, along with their boiling points. ( 3 ) 3 3 2 2 2 3 2 2 3 tert-butyl alcohol n-butyl alcohol methyl propyl ether bp: 82 118 39 (a) Based on their boiling
DO NOT OPEN UNTIL INSTRUCTED TO DO SO. CHEM 110 Dr. McCorkle Exam #5. While you wait, please complete the following information:
 DO NOT OPEN UNTIL INSTRUCTED TO DO SO CHEM 110 Dr. McCorkle Exam #5 While you wait, please complete the following information: Name: Student ID: Turn off cellphones and stow them away. No headphones, mp3
DO NOT OPEN UNTIL INSTRUCTED TO DO SO CHEM 110 Dr. McCorkle Exam #5 While you wait, please complete the following information: Name: Student ID: Turn off cellphones and stow them away. No headphones, mp3
Lecture 21 Cations, Anions and Hydrolysis in Water:
 2P32 Principles of Inorganic Chemistry Dr. M. Pilkington Lecture 21 Cations, Anions and ydrolysis in Water: 1. ydration.energy 2. ydrolysis of metal cations 3. Categories of acidity and observable behavior
2P32 Principles of Inorganic Chemistry Dr. M. Pilkington Lecture 21 Cations, Anions and ydrolysis in Water: 1. ydration.energy 2. ydrolysis of metal cations 3. Categories of acidity and observable behavior
MODEL QUESTION PAPER FOR SUMMATIVE ASSESSMENT CHEMISTRY PART-A
 MODEL QUESTION PAPER FOR SUMMATIVE ASSESSMENT CHEMISTRY Time: 3 Hours Max Marks: 70 INSTRUCTIONS: i) The question paper has five parts A.B.C.D and E. All the parts are compulsory. Write balanced chemical
MODEL QUESTION PAPER FOR SUMMATIVE ASSESSMENT CHEMISTRY Time: 3 Hours Max Marks: 70 INSTRUCTIONS: i) The question paper has five parts A.B.C.D and E. All the parts are compulsory. Write balanced chemical
Chem. 1A Midterm 1 Version A October 14, 2016
 Chem. 1A Midterm 1 Version A October 14, 2016 First initial of last name Name: Print Neatly. You will lose 1 point if I cannot read your name or perm number. Perm Number: All work must be shown on the
Chem. 1A Midterm 1 Version A October 14, 2016 First initial of last name Name: Print Neatly. You will lose 1 point if I cannot read your name or perm number. Perm Number: All work must be shown on the
Chem. 1A Midterm 1 Version B October 14, 2016
 Chem. 1A Midterm 1 Version B October 14, 2016 First initial of last name Name: Print Neatly. You will lose 1 point if I cannot read your name or perm number. Perm Number: All work must be shown on the
Chem. 1A Midterm 1 Version B October 14, 2016 First initial of last name Name: Print Neatly. You will lose 1 point if I cannot read your name or perm number. Perm Number: All work must be shown on the
Name: Class: Date: 3. How many lone pairs of electrons are assigned to the carbon atom in carbon monoxide? a. 0 b. 1 c. 2 d. 3
 Class: Date: Midterm 3, Fall 2009 Record your name on the top of this exam and on the scantron form. Record the test ID letter in the top right box of the scantron form. Record all of your answers on the
Class: Date: Midterm 3, Fall 2009 Record your name on the top of this exam and on the scantron form. Record the test ID letter in the top right box of the scantron form. Record all of your answers on the
CHEMISTRY 107 Section 501 Final Exam Version A December 12, 2016 Dr. Larry Brown
 NAME: (print) UIN #: CHEMISTRY 107 Section 501 Final Exam Version A December 12, 2016 Dr. Larry Brown This is a 2-hour exam, and contains 11 problems. There should be 14 numbered pages, including this
NAME: (print) UIN #: CHEMISTRY 107 Section 501 Final Exam Version A December 12, 2016 Dr. Larry Brown This is a 2-hour exam, and contains 11 problems. There should be 14 numbered pages, including this
CHEM1101 Worksheet 6: Lewis Structures
 CHEM1101 Worksheet 6: Lewis Structures Model 1: Simple Compounds of C, N, O and F The octet rule tells us that C, N, O and F will form covalent bonds so that they are surrounded by eight electrons. For
CHEM1101 Worksheet 6: Lewis Structures Model 1: Simple Compounds of C, N, O and F The octet rule tells us that C, N, O and F will form covalent bonds so that they are surrounded by eight electrons. For
1. How many protons, electrons, and neutrons are in one atom of the following isotopes (6 points)?
 Chemistry 11 Department of Physical Sciences Kingsborough Community College City University of New York NAME Exam 1: Chapters 1-3 50 points 1. How many protons, electrons, and neutrons are in one atom
Chemistry 11 Department of Physical Sciences Kingsborough Community College City University of New York NAME Exam 1: Chapters 1-3 50 points 1. How many protons, electrons, and neutrons are in one atom
Name: Chemistry 151 INSTRUCTIONS: Complete each question and the answers to the questions are on the last part of the exam
 Practice Final Exam Name: Chemistry 151 INSTRUCTIONS: Complete each question and the answers to the questions are on the last part of the exam 1. How many protons, neutrons, and electrons are present in
Practice Final Exam Name: Chemistry 151 INSTRUCTIONS: Complete each question and the answers to the questions are on the last part of the exam 1. How many protons, neutrons, and electrons are present in
Name AP CHEM / / Chapter 8 Outline Bonding: General Concepts
 Name AP CHEM / / Chapter 8 Outline Bonding: General Concepts Types of Chemical Bonds Information about the strength of a bonding interaction is obtained by measuring the bond energy, which is the energy
Name AP CHEM / / Chapter 8 Outline Bonding: General Concepts Types of Chemical Bonds Information about the strength of a bonding interaction is obtained by measuring the bond energy, which is the energy
VOCABULARY Define. 1. chemical bond. 2. covalent bond. 3. ionic bonding. 4. polar-covalent bond
 Name Date lass Modern hemistry APTER 6 OMEWORK 6-1 (pp. 161 163) VOABULARY Define. 1. chemical bond 2. covalent bond 3. ionic bonding 4. polar-covalent bond SKILL BUILDER Use the electronegativity values
Name Date lass Modern hemistry APTER 6 OMEWORK 6-1 (pp. 161 163) VOABULARY Define. 1. chemical bond 2. covalent bond 3. ionic bonding 4. polar-covalent bond SKILL BUILDER Use the electronegativity values
SUPPLEMENTAL HOMEWORK SOLUTIONS WEEK 8
 SUPPLEMETAL MEWRK SLUTIS WEEK 8 Assignment for Tuesday, March 7 th 7.36 a) + + b) + + c) 6 14 4 + 6 14 4 + + d) 6 5 3 7 + 6 5 7 + Be sure to write the correct charges for the products. 7.4 a) l + l + b)
SUPPLEMETAL MEWRK SLUTIS WEEK 8 Assignment for Tuesday, March 7 th 7.36 a) + + b) + + c) 6 14 4 + 6 14 4 + + d) 6 5 3 7 + 6 5 7 + Be sure to write the correct charges for the products. 7.4 a) l + l + b)
Final Exam Review Chem 101
 Final Exam Review Chem 101 1. Know your nomenclature. a) Know how to go from the name to the formula. b) Know how to go from the formula to the name. 1. Ionic compounds (binary and ternary) a. Example:
Final Exam Review Chem 101 1. Know your nomenclature. a) Know how to go from the name to the formula. b) Know how to go from the formula to the name. 1. Ionic compounds (binary and ternary) a. Example:
M09/4/CHEMI/SP2/ENG/TZ1/XX+ CHEMISTRY. Monday 18 May 2009 (afternoon) Candidate session number. 1 hour 15 minutes INSTRUCTIONS TO CANDIDATES
 M09/4/EMI/SP2/ENG/TZ1/XX+ 22096111 EMISTRY standard level Paper 2 Monday 18 May 2009 (afternoon) 1 hour 15 minutes 0 0 andidate session number INSTRUTINS T ANDIDATES Write your session number in the boxes
M09/4/EMI/SP2/ENG/TZ1/XX+ 22096111 EMISTRY standard level Paper 2 Monday 18 May 2009 (afternoon) 1 hour 15 minutes 0 0 andidate session number INSTRUTINS T ANDIDATES Write your session number in the boxes
Unit 1, Lesson 07: Introduction to Covalent Bonding and the Octet Rule
 Unit 1, Lesson 07: Introduction to Covalent Bonding and the Octet Rule non-metals (except Noble gases) have high electronegativity and high ionization energy. They have a strong pull on new electrons if
Unit 1, Lesson 07: Introduction to Covalent Bonding and the Octet Rule non-metals (except Noble gases) have high electronegativity and high ionization energy. They have a strong pull on new electrons if
Ionic and Covalent Bonding
 Chapter 9 Ionic and Covalent Bonding Concept Check 9.1 The following are electron configurations of some ions. Which ones would you expect to see in chemical compounds? State the concept or rule you used
Chapter 9 Ionic and Covalent Bonding Concept Check 9.1 The following are electron configurations of some ions. Which ones would you expect to see in chemical compounds? State the concept or rule you used
Department of Chemistry Memorial University Chemistry 1050
 Department of Chemistry Memorial University Chemistry 1050 Fall 2013 Deferred Examination Time 3 hours NAME: MUN Student Number: Circle your professor s name: Dr. R. Davis Dr. T. Fridgen Dr. C. Kozak Read
Department of Chemistry Memorial University Chemistry 1050 Fall 2013 Deferred Examination Time 3 hours NAME: MUN Student Number: Circle your professor s name: Dr. R. Davis Dr. T. Fridgen Dr. C. Kozak Read
CHEM 1411 SAMPLE FINAL EXAM
 PART I - Multiple Choice (2 points each) CHEM 1411 SAMPLE FINAL EXAM 1. The distance between carbon atoms in ethylene is 134 picometers. Which of the following expresses this distance in meters? A. 1.34
PART I - Multiple Choice (2 points each) CHEM 1411 SAMPLE FINAL EXAM 1. The distance between carbon atoms in ethylene is 134 picometers. Which of the following expresses this distance in meters? A. 1.34
Na Cl Wants to lose ONE electron! Na Cl Ionic Bond TRANSFER of electrons between atoms. Ionic Bonding. Ionic Bonding.
 BONDING Chemical Bond Attraction that holds atoms together Types include IONIC, METALLIC, or COVALENT Differences in electronegativity determine the bond type Ionic Bond TRANSFER of electrons between atoms
BONDING Chemical Bond Attraction that holds atoms together Types include IONIC, METALLIC, or COVALENT Differences in electronegativity determine the bond type Ionic Bond TRANSFER of electrons between atoms
9 STRUCTURE & BONDING
 9 STRUCTURE & BONDING 9.1 REVISION It is assumed that you know the following: the solar system model for the structure of the atom what atoms try to do to become stable how atoms form ionic bonds how atoms
9 STRUCTURE & BONDING 9.1 REVISION It is assumed that you know the following: the solar system model for the structure of the atom what atoms try to do to become stable how atoms form ionic bonds how atoms
