Arsenic(V) incorporation in vivianite during microbial reduction of arsenic(v)-bearing biogenic Fe(III) (oxyhydr)oxides
|
|
|
- Dinah Stewart
- 5 years ago
- Views:
Transcription
1 Arsenic(V) incorporation in vivianite during microbial reduction of arsenic(v)-bearing biogenic Fe(III) (oxyhydr)oxides E. Marie Muehe 1,2*, Guillaume Morin 3, Lukas Scheer 1, Pierre Le Pape 3, Imène Esteve 3, Birgit Daus 4, Andreas Kappler 1 1 Geomicrobiology, Center for Applied Geosciences, University of Tuebingen, Germany 2 Now: Environmental Soil Biogeochemistry, Earth System Science, Stanford University, USA 3 Environmental Mineralogy, Institut de Minéralogie, de Physique des Matériaux et de Cosmochimie (IMPMC), UMR CNRS - UPMC, France 4 UFZ Helmholtz Centre for Environmental Research, Department Analytical Chemistry, Leipzig, Germany SUPPORTING INFORMATION *To whom correspondence should be sent: E. Marie Muehe, Environmental Soil Biogeochemistry, Earth System Science, Stanford University, 473 a Ortega, Stanford, CA 94305, USA Phone: , mmuehe@stanford.edu
2 Content: Number of pages: 13 List of Tables: Table S1: Rietveld refined Unit-cell parameters of vivianite in the biogenic samples compared to the 1 wt% As(V) substituted vivianite model compound, and available crystallographic data for vivianite and symplesite. Page S2 Table S2: Shell-by-shell EXAFS fitting parameters for the 1 wt% As(V)-substituted vivianite model compound EXAFS data, compared to crystallographic data for the refined structure of vivianite by Fejdi et al. (1980) 3. Page S3 Table S3: Solubility product and saturation index calculation Page S4 List of Figures: Figure S1. Synchrotron beam damage after a ~40 min long EXAFS scan at K on the incubated sample with a Fe to As(V) ratio of 50 to 1 (w/w) (left) and on the 1 wt% As(V)- substituted vivianite model compound..page S5 Figure S2: Powder X-ray diffraction patterns of microbially reduced As(V)-bearing biogenic Fe(III) (oxyhydr)oxides..page S6 Figure S3: Rietveld refinement of the Powder X-ray diffraction patterns of microbially reduced As(V)-bearing biogenic Fe(III) (oxyhydr)oxides (with Fe to As ratios of 50 to 1 and 250 to 1, respectively), and of the 1 wt% As-substituted vivianite reference compound. Page S7 Figure S4: Shell-by-shell fitting of the 1 wt% As(V)-substituted vivianite model compound EXAFS data using parameters reported in Table S1. Page S8 Figure S5: Local structure around the PO4 group in the vivianite structure according to Fejdi et al. (1980) 3. Page S9 Figure S6: Alternative Linear Combination Fit solutions for the As K-edge data of As-bearing biogenic Fe(III) (hydroxide) with a Fe to As ratio of 50 to 1 (w/w) after 5 days of reduction. Page S10 Figure S7: Ability of Shewanella oneidensis MR-1 to reduce As(V) when grown on mineral medium..page S11 Figure S8: Reduction of As(V) by AH2QDS..Page S12 References..Page S13 S1
3 Table S1: Rietveld refined Unit-cell parameters of vivianite in the biogenic samples compared to the 1 wt% As(V) substituted vivianite model compound, and available crystallographic data for vivianite and symplesite. Arsenic substitution rate in the biogenic and model compound samples could not be reliably derived from a Vegard law approach because of imprecision in the structural data reported by Mori and Ito, for symplesite, i.e. <As-O> ~1.655 ± 0.047, instead of an expected <As-O> = ± Å (Hawthorne, ). a (Å) b (Å) c (Å) (Å) Sample Fe:As(V) 50:1-5 days 10.13(2) 13.46(2) 4.70(1) 104.6(1) Sample Fe:As(V) 250:1-5 days 10.12(4) 13.46(2) 4.70(1) 104.4(3) Model compound 1 wt% As*(V)-vivianite 10.13(2) 13.48(3) 4.72(1) 104.4(1) vianite (Mori and Ito, ) vianite (Fedji et al., ) Symplesite (Mori and Ito, ) S2
4 Table S2: Shell-by-shell EXAFS fitting parameters for the 1 wt% As(V)-substituted vivianite model compound EXAFS data, compared to crystallographic data for the refined structure of vivianite by Fejdi et al., * fixed parameters. (-) parameter linked to the parameter in the Table S1. R (Å) shell (Å) E 0 (ev) vivianite As(V) 1wt% 1.695(2) 4* As-O 0.060(1) 11.3(8) 3.1* 12* As-O-O 0.05* (5) 3* As-Fe 0.067(3) (5) 2* As-Fe 0.051(3) - vivianite (Fejdi et al., ) 1.54(2) 4 P-O 3.23(1) 3 P-Fe 3.41(1) 2 P-Fe S3
5 Table S3: Solubility products, ion activity products and saturation index calculations for the formation of vivianite-symplesite mixed mineral phases in the experiment. (at 25 C). Reaction: Fe3(PO4)1.75(AsO4)0.25 = 3 Fe 2+ + [1.75 PO AsO4 3- ] Solubility product Ksp = [Fe 2+ ] 3 [PO AsO4 3- ] ( ) [Fe3(PO4)1.75(AsO4)0.25] Ksp (vianite) Ksp (Symplesite) Ksp (vianite-symplesite) ~ mol 2 L -2 (Johnston and Singer, ) mol 2 L -2 (Johnston and Singer, ) mol 2 L -2 (expected from vivianite and symplesite) Fe to As ratio 50:1 [w/w] Fe to As ratio 250:1 [w/w] Ion concentration in the aqueous phase at day 5 Fe mol L mol L -1 As(V)-species 66% of aqueous As is As(V) = mg L -1 = mol L -1 66% of aqueous As is As(V) = mg L -1 = mol L -1 PO4 3- -species ~ mol L -1 ~ mol L -1 Ion activity product IAP = [Fe 2+ ] 3 [PO AsO4 3- ] ( ) Saturation index SI = log(iap/ksp) = mol 2 L -2 = mol 2 L -2 = 17.7 = 18.1 IAP = K sp or SI = 0 solution is saturated IAP < K sp or SI < 0 solution is undersaturated (dissolution of mineral) IAP > K sp or SI > 0 solution is supersaturated (mineral precipitation) S4
6 Figure S1. Synchrotron beam damage observed on the XANES region after a ~40 min long EXAFS scan at K on the incubated sample with a Fe to As(V) ratio of 50 to 1 (w/w) (left) and on the 1 wt% As(V)-substituted vivianite model compound. The blue and red lines display two successive scans on the same spot. The green line display a scan on another spot after having shifted the sample. Since the samples were found to be sensitive to photo-reduction of As(V) to As(III), the beam position was shifted between each EXAFS scan to merge 7 and 3 scans for the biogenic and model compound sample, respectively. S5
7 Gt Gt Counts Gt Gt Sd Gt Gt Gt Gt Gt Gt Gt Fe:As(V) 50:1 5d Fe:As(V) 250:1 5d theta ( ) Figure S2: Powder X-ray diffraction patterns of As(V)-bearing biogenic Fe(III) (oxyhydr)oxides synthesized with a Fe to As ratio of 50 to 1 and 250 to 1 (w/w) after 5 days of reduction by S. oneidensis strain MR-1. The strongest Bragg reflections of vivianite (), goethite (Gt), and siderite (Sd) are depicted in grey. S6
8 Yobs Ycal 0 1 Yobs Ycal 0 1 Yobs Ycal 0 Fe:As 50:1-5 days counts Fe:As 250:1-5 days vivianite:as 1 wt% theta ( ) Figure S3: Rietveld refinement of the Powder X-ray diffraction patterns of microbially reduced As(V)-bearing biogenic Fe(III) (oxyhydr)oxides (with Fe to As ratios of 50 to 1 and 250 to 1, respectively), and of the 1 wt% As-substituted vivianite reference compound. Experimental data are plotted in black line. The calculated patterns (red) include vivianite (green line) and goethite (orange line). Refined unit-cell parameters for vivianite in the samples are reported in Table S1. Rietveld refinement was performed using the XND1.3 code (Berar et al., ). S7
9 unfiltered k 3 c(k) 5 10 k 3 -weighted EXAFS filtered k 3 c(k) Fast Fourier Transform k(å -1 ) R + r (Å) Figure S4: Shell-by-shell fitting of the 1 wt% As(V)-substituted vivianite model compound EXAFS data using parameters reported in Table S1. The fit curve (red) is shown for experimental data (black line), either unfiltered or filtered within the R+ r range. The Fast Fourier Transform of the unfiltered data and of the fit is plotted on the right side of the figure, uncorrected for phase shift. The unfitted long distance peaks in the FT at R+ r ~ 4.5 and 6 Å are assigned to contributions from multiple scattering within the vivianite structure. S8
10 Figure S5: Local structure around the PO4 3- group in the vivianite structure according to Fejdi et al., S9
11 Figure S6: Alternative Linear Combination Fit solutions for the As K-edge data of As-bearing biogenic Fe(III) (hydroxide) with a Fe to As ratio of 50 to 1 (w/w) after 5 days of reduction. (A) Fit without As(V)-substituted vivianite (As(V):vivianite); (B) Fit with As(V)-substituted vivianite retained as the best fit solution; (C) Fit with As(V)-substituted vivianite and with As(III)-sorbed goethite (As(V)/Gt) instead of As(III)-sorbed ferrihydrite (As(III)/Fh). The vertical arrows indicate contributions from multiple scattering within the vivianite structure (see Fig. S3). S10
12 Figure S7: The ability of Shewanella oneidensis MR-1 to reduce As(V) when grown on mineral medium in the presence of 100 µm As(V) as electron acceptor and 20 mm lactate (lac) as electron donor. For some setups, 40 mm fumarate (fum) was amended as an additional electron acceptor. Growth was determined via quantification of the absorption at 660 nm at 0 and 4 days (A); aqueous As(V) (black) and As(III) (white) concentrations after 0 and 4 days are given in µm (B). No reduction of As(V) was observed in any setup, so no white bars are shown in B. n=3. S11
13 Figure S8: Reduction of As(V) by AH2QDS. This control setup was conducted under the same experimental conditions as applied in the main experiment (mineral medium at ph 7, 20 mm lactate, 40 mm fumarate, incubation in the dark at 28 C). Approximately 3 mg L -1 of aqueous As(V) were added, as this was in the range of the aqueous As concentration present at the end of incubation of the main experiment. 100 µm of AH2QDS was used (the AH2QDS was synthesized from AQDS by chemical reduction at ph 7 using H2 and a palladium catalyst). Additional controls were: no AQDS/AH2QDS, 100 µm of AQDS, and 100 µm of AQDS plus Shewanella oneidensis MR-1. Total As was quantified by ICP-MS, the speciation of As was determined with HPLC-ICP-MS. Aqueous As(V) (grey) and As(III) (white) concentrations after 1 and 4 days are given in mg L -1. No reduction of As(V) was observed in any setup, so no white bars are shown. n=3. S12
14 Supporting Information References 1 Mori, H.; Ito, T., The structure of vivianite and symplesite. Acta Crystallogr. 1950, 3, Hawthorne, F. C., Hydrogen positions in scorodite. Acta Crystallogr. 1976, 32, Fejdi, P.; Poullen, J. F.; Gasperin, M., Affinement de la structure de la vivianite Fe3(PO4)2*8H2O. Bull. Mineral. 1980, 103, Johnston, R. B.; Singer, P. C., Solubility of symplesite (ferrous arsenate): Implications for reduced groundwaters and other geochemical environments. Soil Sci. Soc. Am. J. 2007, 71(1), Berar, J. F.; Baldinozzi, G., XND code: From X-ray laboratory data to incommensurately modulated phases. Rietveld modeling of complex materials. Int. Un. Crystallogr., Commis.Powder Diffract. Newsl. 1998, 20, 3 5. S13
Redox transformation of arsenic. by Fe(II)-activated goethite (α-feooh)
 -SUPPORTING INFORMATION- Redox transformation of arsenic by Fe(II)-activated goethite (α-feooh) Katja Amstaetter 1,2, Thomas Borch 3, Philip Larese-Casanova 1, Andreas Kappler 1 * 1 Geomicrobiology, Center
-SUPPORTING INFORMATION- Redox transformation of arsenic by Fe(II)-activated goethite (α-feooh) Katja Amstaetter 1,2, Thomas Borch 3, Philip Larese-Casanova 1, Andreas Kappler 1 * 1 Geomicrobiology, Center
Supporting Information
 Supporting Information Enhancement of Arsenic Adsorption during Mineral Transformation from Siderite to Goethite: Mechanism and Application Huaming Guo 1, 2, *, Yan Ren 2, Qiong Liu 2, Kai Zhao 1, 2, Yuan
Supporting Information Enhancement of Arsenic Adsorption during Mineral Transformation from Siderite to Goethite: Mechanism and Application Huaming Guo 1, 2, *, Yan Ren 2, Qiong Liu 2, Kai Zhao 1, 2, Yuan
Stabilization of Mercury and Methyl Mercury by Biochars in Water/Sediment Microcosms
 Stabilization of Mercury and Methyl Mercury by Biochars in Water/Sediment Microcosms Peng Liu, Carol Ptacek, David Blowes, Krista Paulson, Jing Ma, and Alana Ou Wang Introduction Department of Earth and
Stabilization of Mercury and Methyl Mercury by Biochars in Water/Sediment Microcosms Peng Liu, Carol Ptacek, David Blowes, Krista Paulson, Jing Ma, and Alana Ou Wang Introduction Department of Earth and
EXAFS. Extended X-ray Absorption Fine Structure
 AOFSRR Cheiron School 2010, SPring-8 EXAFS Oct. 14th, 2010 Extended X-ray Absorption Fine Structure Iwao Watanabe Ritsumeikan University EXAFS Theory Quantum Mechanics Models Approximations Experiment
AOFSRR Cheiron School 2010, SPring-8 EXAFS Oct. 14th, 2010 Extended X-ray Absorption Fine Structure Iwao Watanabe Ritsumeikan University EXAFS Theory Quantum Mechanics Models Approximations Experiment
Green rust articles (key and from the consortium marked with *)
 Green rust articles (key and from the consortium marked with *) Stability, structure, formation and transformation Hansen et (1989) Composition, stabilization, and light absorption of Fe(II)Fe(III) hydroxy-carbonate
Green rust articles (key and from the consortium marked with *) Stability, structure, formation and transformation Hansen et (1989) Composition, stabilization, and light absorption of Fe(II)Fe(III) hydroxy-carbonate
Supporting Information
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2018 Supporting Information In situ generated catalyst: Copper (II) oxide and
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2018 Supporting Information In situ generated catalyst: Copper (II) oxide and
Fe II oxidation by molecular O 2 during HCl extraction
 Accessory publication Fe II oxidation by molecular O 2 during HCl extraction Katharina Porsch A,B Andreas Kappler A,C A Geomicrobiology, Center for Applied Geosciences, University of Tuebingen, Sigwartstrasse
Accessory publication Fe II oxidation by molecular O 2 during HCl extraction Katharina Porsch A,B Andreas Kappler A,C A Geomicrobiology, Center for Applied Geosciences, University of Tuebingen, Sigwartstrasse
SUPPLEMENTARY INFORMATION
 DOI: 1.138/NGEO1825 Hydrogen generation from low temperature water-rock reactions L.E. Mayhew* 1, E.T. Ellison 1, T.M. McCollom 2, T.P. Trainor 3, and A.S. Templeton 1 *Corresponding author tel: (33) 492
DOI: 1.138/NGEO1825 Hydrogen generation from low temperature water-rock reactions L.E. Mayhew* 1, E.T. Ellison 1, T.M. McCollom 2, T.P. Trainor 3, and A.S. Templeton 1 *Corresponding author tel: (33) 492
X-ray Absorption Spectroscopy
 X-ray Absorption Spectroscopy Matthew Newville Center for Advanced Radiation Sources University of Chicago 12-Sept-2014 SES VI SES VI 12-Sept-2014 SES VI What Is XAFS? X-ray Absorption Fine-Structure (XAFS)
X-ray Absorption Spectroscopy Matthew Newville Center for Advanced Radiation Sources University of Chicago 12-Sept-2014 SES VI SES VI 12-Sept-2014 SES VI What Is XAFS? X-ray Absorption Fine-Structure (XAFS)
SUPPLEMENTAL MATERIAL
 SUPPLEMENTAL MATERIAL Zerovalent Iron with High Sulfur Content Enhances the Formation of Cadmium Sulfide in Reduced Paddy Soils Yohey Hashimoto 1*, Mitsuhiro Furuya 1, Noriko Yamaguchi 2*, and Tomoyuki
SUPPLEMENTAL MATERIAL Zerovalent Iron with High Sulfur Content Enhances the Formation of Cadmium Sulfide in Reduced Paddy Soils Yohey Hashimoto 1*, Mitsuhiro Furuya 1, Noriko Yamaguchi 2*, and Tomoyuki
ENVIRONMENTAL FACTORS IMPACTING THE FORMATION AND KINETICS OF FE(II) LAYERED HYDROXIDES ON MINERALS AND SOILS. Autumn Nichole Starcher
 ENVIRONMENTAL FACTORS IMPACTING THE FORMATION AND KINETICS OF FE(II) LAYERED HYDROXIDES ON MINERALS AND SOILS by Autumn Nichole Starcher A dissertation submitted to the Faculty of the University of Delaware
ENVIRONMENTAL FACTORS IMPACTING THE FORMATION AND KINETICS OF FE(II) LAYERED HYDROXIDES ON MINERALS AND SOILS by Autumn Nichole Starcher A dissertation submitted to the Faculty of the University of Delaware
Solid State Spectroscopy Problem Set 7
 Solid State Spectroscopy Problem Set 7 Due date: June 29th, 2015 Problem 5.1 EXAFS Study of Mn/Fe substitution in Y(Mn 1-x Fe x ) 2 O 5 From article «EXAFS, XANES, and DFT study of the mixed-valence compound
Solid State Spectroscopy Problem Set 7 Due date: June 29th, 2015 Problem 5.1 EXAFS Study of Mn/Fe substitution in Y(Mn 1-x Fe x ) 2 O 5 From article «EXAFS, XANES, and DFT study of the mixed-valence compound
ADVANCED APPLICATIONS OF SYNCHROTRON RADIATION IN CLAY SCIENCE
 CMS WORKSHOP LECTURES Volume 19 ADVANCED APPLICATIONS OF SYNCHROTRON RADIATION IN CLAY SCIENCE THE CLAY MINERALS SOCIETY Joseph W. Stucki, Series Editor and Editor in Chief University of Illinois Urbana,
CMS WORKSHOP LECTURES Volume 19 ADVANCED APPLICATIONS OF SYNCHROTRON RADIATION IN CLAY SCIENCE THE CLAY MINERALS SOCIETY Joseph W. Stucki, Series Editor and Editor in Chief University of Illinois Urbana,
Simultaneous Microbial Reduction of Iron(III) and Arsenic(V) in Suspensions of Hydrous Ferric Oxide
 5-1 Chapter 5 Simultaneous Microbial Reduction of Iron(III) and Arsenic(V) in Suspensions of Hydrous Ferric Oxide * Adapted from Campbell, et al. 2006. Environmental Science and Technology, vol. 40, no.
5-1 Chapter 5 Simultaneous Microbial Reduction of Iron(III) and Arsenic(V) in Suspensions of Hydrous Ferric Oxide * Adapted from Campbell, et al. 2006. Environmental Science and Technology, vol. 40, no.
Muffin-tin potentials in EXAFS analysis
 J. Synchrotron Rad. (5)., doi:.7/s6577555 Supporting information Volume (5) Supporting information for article: Muffin-tin potentials in EXAFS analysis B. Ravel Supplemental materials: Muffin tin potentials
J. Synchrotron Rad. (5)., doi:.7/s6577555 Supporting information Volume (5) Supporting information for article: Muffin-tin potentials in EXAFS analysis B. Ravel Supplemental materials: Muffin tin potentials
Synchrotron Radiation & Environmental Science
 Scuola SILS, Grado, settembre 2013 Synchrotron Radiation & Environmental Science Pierfranco Lattanzi Università di Cagliari What is environmental science? very broad umbrella covering any phenomenon that
Scuola SILS, Grado, settembre 2013 Synchrotron Radiation & Environmental Science Pierfranco Lattanzi Università di Cagliari What is environmental science? very broad umbrella covering any phenomenon that
A kinetic model for bacterial Fe(III) oxide reduction in batch cultures
 WATER RESOURCES RESEARCH, VOL. 39, NO. 4, 098, doi:0.029/2002wr0032, 2003 A kinetic model for bacterial Fe(III) oxide reduction in batch cultures Eric L. Hacherl and David S. Kosson Department of Civil
WATER RESOURCES RESEARCH, VOL. 39, NO. 4, 098, doi:0.029/2002wr0032, 2003 A kinetic model for bacterial Fe(III) oxide reduction in batch cultures Eric L. Hacherl and David S. Kosson Department of Civil
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry
Electrochemical Water Splitting by Layered and 3D Cross-linked Manganese Oxides: Correlating Structural Motifs and Catalytic Activity
 Electronic Supplementary Information Electrochemical Water Splitting by Layered and 3D Cross-linked Manganese Oxides: Correlating Structural Motifs and Catalytic Activity Arno Bergmann,* a Ivelina Zaharieva,*
Electronic Supplementary Information Electrochemical Water Splitting by Layered and 3D Cross-linked Manganese Oxides: Correlating Structural Motifs and Catalytic Activity Arno Bergmann,* a Ivelina Zaharieva,*
Part II. Fundamentals of X-ray Absorption Fine Structure: data analysis
 Part II Fundamentals of X-ray Absorption Fine Structure: data analysis Sakura Pascarelli European Synchrotron Radiation Facility, Grenoble, France Page 1 S. Pascarelli HERCULES 2016 Data Analysis: EXAFS
Part II Fundamentals of X-ray Absorption Fine Structure: data analysis Sakura Pascarelli European Synchrotron Radiation Facility, Grenoble, France Page 1 S. Pascarelli HERCULES 2016 Data Analysis: EXAFS
Supporting Information to
 Supporting Information to 'Bisulfide reaction with natural organic matter enhances arsenite sorption: Insights from X-ray absorption spectroscopy' Martin Hoffmann, Christian Mikutta* and Ruben Kretzschmar
Supporting Information to 'Bisulfide reaction with natural organic matter enhances arsenite sorption: Insights from X-ray absorption spectroscopy' Martin Hoffmann, Christian Mikutta* and Ruben Kretzschmar
Synthesis of 12 nm iron oxide nanoparticles
 Electronic Supporting Information for Dendronized iron oxide nanoparticles as contrast agent for MRI Brice Basly, a Delphine Felder-Flesch,* a Pascal Perriat, b Claire Billotey, c Jacqueline Taleb, c Geneviève
Electronic Supporting Information for Dendronized iron oxide nanoparticles as contrast agent for MRI Brice Basly, a Delphine Felder-Flesch,* a Pascal Perriat, b Claire Billotey, c Jacqueline Taleb, c Geneviève
REMOVAL OF ARSENIC, CHROMIUM AND LEAD FROM SIMULATED GROUNDWATER WITH REACTIVE NANOSCALE IRON PARTICLES
 REMOVAL OF ARSENIC, CHROMIUM AND LEAD FROM SIMULATED GROUNDWATER WITH REACTIVE NANOSCALE IRON PARTICLES Kenji Okinaka (Kenji_Okinaka@todakogyo.co.jp) (Toda Kogyo Corporation, Yamaguchi, Japan) Andreas
REMOVAL OF ARSENIC, CHROMIUM AND LEAD FROM SIMULATED GROUNDWATER WITH REACTIVE NANOSCALE IRON PARTICLES Kenji Okinaka (Kenji_Okinaka@todakogyo.co.jp) (Toda Kogyo Corporation, Yamaguchi, Japan) Andreas
Electronic Supplementary Information (ESI)
 Electronic Supplementary Information (ESI) S1 Experimental Section: Materials and methods: All commercially available chemicals were used as supplied without further purification. The Q[5] was synthesized
Electronic Supplementary Information (ESI) S1 Experimental Section: Materials and methods: All commercially available chemicals were used as supplied without further purification. The Q[5] was synthesized
Speciation of Actinides Using XAFS
 Speciation of Actinides Using XAFS Part I Tobias Reich Johannes Gutenberg-Universität Mainz Institut für Kernchemie Ringvorlesung des GRK Elementspeziation im SS 2006 Mainz, 4.9.2006 Outline Introduction
Speciation of Actinides Using XAFS Part I Tobias Reich Johannes Gutenberg-Universität Mainz Institut für Kernchemie Ringvorlesung des GRK Elementspeziation im SS 2006 Mainz, 4.9.2006 Outline Introduction
Potential Impacts of Tailings and Tailings Cover. Fertilization on Arsenic Mobility in Surface and. Ground Waters
 Potential Impacts of Tailings and Tailings Cover Fertilization on Arsenic Mobility in Surface and Ground Waters Sierra Rayne * and Kaya Forest Water Treatment Technology Program, Thompson Rivers University,
Potential Impacts of Tailings and Tailings Cover Fertilization on Arsenic Mobility in Surface and Ground Waters Sierra Rayne * and Kaya Forest Water Treatment Technology Program, Thompson Rivers University,
Search and Discovery Article #50999 (2014)** Posted August 18, Abstract
 Oil Degradation in the Gullfaks Field (Norway): How Hydrogeochemical Modeling can Help to Decipher Organic- Inorganic Interactions Controlling CO 2 Fate and Behavior* Wolfgang van Berk 1, Yunjiao Fu 2,
Oil Degradation in the Gullfaks Field (Norway): How Hydrogeochemical Modeling can Help to Decipher Organic- Inorganic Interactions Controlling CO 2 Fate and Behavior* Wolfgang van Berk 1, Yunjiao Fu 2,
Removing arsenic from synthetic groundwater with iron electrocoagulation: An Fe and As K-edge EXAFS study
 Article Subscriber access provided by - UC Berkeley Library Removing arsenic from synthetic groundwater with iron electrocoagulation: An Fe and As K-edge EXAFS study Case Michael van Genuchten, Susan E.
Article Subscriber access provided by - UC Berkeley Library Removing arsenic from synthetic groundwater with iron electrocoagulation: An Fe and As K-edge EXAFS study Case Michael van Genuchten, Susan E.
Supplementary Information For: Cu, Pb, and Zn Sorption to Bacteriogenic Iron Oxyhydr(oxides) Formed in Circumneutral Environments
 Supplementary Information For: Cu, Pb, and Zn Sorption to Bacteriogenic Iron Oxyhydr(oxides) Formed in Circumneutral Environments Andrew H. Whitaker 1 and Owen W. Duckworth 1, * 1 Department of Crop and
Supplementary Information For: Cu, Pb, and Zn Sorption to Bacteriogenic Iron Oxyhydr(oxides) Formed in Circumneutral Environments Andrew H. Whitaker 1 and Owen W. Duckworth 1, * 1 Department of Crop and
Supplementary Figure 1. TEM analysis of Co0.5 showing (a) a SAED pattern, and (b-f) bright-field images of the microstructure. Only two broad rings
 Supplementary Figure 1. TEM analysis of Co0.5 showing (a) a SAED pattern, and (bf) brightfield images of the microstructure. Only two broad rings were observed in the SAED pattern, as expected for amorphous
Supplementary Figure 1. TEM analysis of Co0.5 showing (a) a SAED pattern, and (bf) brightfield images of the microstructure. Only two broad rings were observed in the SAED pattern, as expected for amorphous
Basics of XRD part III
 Basics of XRD part III Dr. Peter G. Weidler Institute of Functional Interfaces IFG 1 10/31/17 KIT The Research University of the Helmholtz Association Name of Institute, Faculty, Department www.kit.edu
Basics of XRD part III Dr. Peter G. Weidler Institute of Functional Interfaces IFG 1 10/31/17 KIT The Research University of the Helmholtz Association Name of Institute, Faculty, Department www.kit.edu
Raffinate Neutralization Experiments at the McClean Lake Mill Removal of Arsenic and Nickel
 Raffinate Neutralization Experiments at the McClean Lake Mill Removal of Arsenic and Nickel John Mahoney 1, Donald Langmuir 2, Maynard Slaughter 3, John Rowson 4 1 Hydrologic Consultants, Inc. Lakewood,
Raffinate Neutralization Experiments at the McClean Lake Mill Removal of Arsenic and Nickel John Mahoney 1, Donald Langmuir 2, Maynard Slaughter 3, John Rowson 4 1 Hydrologic Consultants, Inc. Lakewood,
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and
 This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
XAS studies in Environmental and Materials Sciences
 XAS studies in Environmental and Materials Sciences Giuliana Aquilanti giuliana.aquilanti@elettra.eu November 18, 2014 2 Table of Contents XAS: basic concepts Applications to Environmental sciences Applications
XAS studies in Environmental and Materials Sciences Giuliana Aquilanti giuliana.aquilanti@elettra.eu November 18, 2014 2 Table of Contents XAS: basic concepts Applications to Environmental sciences Applications
K.A. Terzi 1,2, I. Bountas 1,2 C.A. Aggelopoulos 1, C.D. Tsakiroglou 1
 K.A. Terzi 1,2, I. Bountas 1,2 C.A. Aggelopoulos 1, C.D. Tsakiroglou 1 1 Foundation for Research and Technology Hellas Institute of Chemical Engineering Sciences 2 Department of Chemical Engineering, Univ.
K.A. Terzi 1,2, I. Bountas 1,2 C.A. Aggelopoulos 1, C.D. Tsakiroglou 1 1 Foundation for Research and Technology Hellas Institute of Chemical Engineering Sciences 2 Department of Chemical Engineering, Univ.
Metal Release and Speciation Changes during Wet Aging of Coal Fly Ashes
 Supporting Information for: Metal Release and Speciation Changes during Wet Aging of Coal Fly Ashes Jeffrey G. Catalano 1 *, Brittany L. Huhmann 1, Yun Luo 1,2, Elizabeth H. Mitnick 1, Adam Slavney 1,
Supporting Information for: Metal Release and Speciation Changes during Wet Aging of Coal Fly Ashes Jeffrey G. Catalano 1 *, Brittany L. Huhmann 1, Yun Luo 1,2, Elizabeth H. Mitnick 1, Adam Slavney 1,
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL Characterisation of hydrous ferric oxides derived from iron-rich groundwaters and their contribution to the suspended sediment of streams Stijn Baken*, Carin Sjöstedt, Jon Petter
SUPPLEMENTARY MATERIAL Characterisation of hydrous ferric oxides derived from iron-rich groundwaters and their contribution to the suspended sediment of streams Stijn Baken*, Carin Sjöstedt, Jon Petter
J. Am. Chem. Soc., 1998, 120(7), , DOI: /ja972816e
 J. Am. Chem. Soc., 1998, 120(7), 1430-1433, DOI:10.1021/ja972816e Terms & Conditions Electronic Supporting Information files are available without a subscription to ACS Web Editions. The American Chemical
J. Am. Chem. Soc., 1998, 120(7), 1430-1433, DOI:10.1021/ja972816e Terms & Conditions Electronic Supporting Information files are available without a subscription to ACS Web Editions. The American Chemical
Supporting Information
 Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2018 Supporting Information The chemical identity, state and structure of catalytically active
Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2018 Supporting Information The chemical identity, state and structure of catalytically active
Low-activated Li-Ion Mobility and Metal to Semiconductor Transition in CdP Phases
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Low-activated Li-Ion Mobility and Metal to Semiconductor Transition in
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2015 Low-activated Li-Ion Mobility and Metal to Semiconductor Transition in
Effect of Chloride Anions on the Synthesis and. Enhanced Catalytic Activity of Silver Nanocoral
 Supporting Information Effect of Chloride Anions on the Synthesis and Enhanced Catalytic Activity of Silver Nanocoral Electrodes for CO 2 Electroreduction Polyansky* Yu-Chi Hsieh, Sanjaya D. Senanayake,
Supporting Information Effect of Chloride Anions on the Synthesis and Enhanced Catalytic Activity of Silver Nanocoral Electrodes for CO 2 Electroreduction Polyansky* Yu-Chi Hsieh, Sanjaya D. Senanayake,
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Anion-induced Ag I self-assemblies with electron
Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Anion-induced Ag I self-assemblies with electron
XUV 773: X-Ray Fluorescence Analysis of Gemstones
 Fischer Application report vr118 HELM UT FISCHER GMBH + CO. KG Institut für Elektronik und Messtechnik Industriestrasse 21-7169 Sindelfingen, Germany Tel.: (+49) 731 33- - Fax: (+49) 731 33-79 E-Mail:
Fischer Application report vr118 HELM UT FISCHER GMBH + CO. KG Institut für Elektronik und Messtechnik Industriestrasse 21-7169 Sindelfingen, Germany Tel.: (+49) 731 33- - Fax: (+49) 731 33-79 E-Mail:
Introduction to XAFS. Grant Bunker Associate Professor, Physics Illinois Institute of Technology. Revised 4/11/97
 Introduction to XAFS Grant Bunker Associate Professor, Physics Illinois Institute of Technology Revised 4/11/97 2 tutorial.nb Outline Overview of Tutorial 1: Overview of XAFS 2: Basic Experimental design
Introduction to XAFS Grant Bunker Associate Professor, Physics Illinois Institute of Technology Revised 4/11/97 2 tutorial.nb Outline Overview of Tutorial 1: Overview of XAFS 2: Basic Experimental design
Supplementary Information
 Supplementary Information Supplementary Figure 1. Photographs show the titration experiments by dropwise adding ~5 times number of moles of (a) LiOH and LiOH+H 2 O, (b) H 2 O 2 and H 2 O 2 +LiOH, (c) Li
Supplementary Information Supplementary Figure 1. Photographs show the titration experiments by dropwise adding ~5 times number of moles of (a) LiOH and LiOH+H 2 O, (b) H 2 O 2 and H 2 O 2 +LiOH, (c) Li
Supplementary Information. Structure and Electrochemical Performance
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2014 Supplementary Information P-type Na x Ni 0.22 Co 0.11 Mn 0.66 O 2 Materials:
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2014 Supplementary Information P-type Na x Ni 0.22 Co 0.11 Mn 0.66 O 2 Materials:
How Arsenic Chemistry Determines Remediation Efficacy as well as Fate and Transport Russ Gerads Business Development Director
 Northwest Remediation Conference October 5, 2017 How Arsenic Chemistry Determines Remediation Efficacy as well as Fate and Transport Russ Gerads Business Development Director www.brooksapplied.com Arsenic
Northwest Remediation Conference October 5, 2017 How Arsenic Chemistry Determines Remediation Efficacy as well as Fate and Transport Russ Gerads Business Development Director www.brooksapplied.com Arsenic
rinds for 8 Loihi rocks. Sample spectra, fits and model compounds are shown in Figure S5. All
 SUPPLEMENTARY INFORMATION Supplementary Table 1 Sample Location %Mats (M34, M39) 2L-FH (III)- smectite Vivianite Basalt glass red chi 2 J2-369-R2 Marker 17a 30% 21% - 11% 38% 0.06 J2-369-R5 Marker 17a
SUPPLEMENTARY INFORMATION Supplementary Table 1 Sample Location %Mats (M34, M39) 2L-FH (III)- smectite Vivianite Basalt glass red chi 2 J2-369-R2 Marker 17a 30% 21% - 11% 38% 0.06 J2-369-R5 Marker 17a
General theory of diffraction
 General theory of diffraction X-rays scatter off the charge density (r), neutrons scatter off the spin density. Coherent scattering (diffraction) creates the Fourier transform of (r) from real to reciprocal
General theory of diffraction X-rays scatter off the charge density (r), neutrons scatter off the spin density. Coherent scattering (diffraction) creates the Fourier transform of (r) from real to reciprocal
An Introduction to XAFS
 An Introduction to XAFS Matthew Newville Center for Advanced Radiation Sources The University of Chicago 21-July-2018 Slides for this talk: https://tinyurl.com/larch2018 https://millenia.cars.aps.anl.gov/gsecars/data/larch/2018workshop
An Introduction to XAFS Matthew Newville Center for Advanced Radiation Sources The University of Chicago 21-July-2018 Slides for this talk: https://tinyurl.com/larch2018 https://millenia.cars.aps.anl.gov/gsecars/data/larch/2018workshop
Engineering - CLS Day. Thomas Ellis Director of Research Canadian Light Source
 Engineering - CLS Day Thomas Ellis Director of Research Canadian Light Source E-gun & Linear Accelerator 250 MeV Canadian Light Source Transfer Line & Booster Ring Commissioned Storage Ring 2.9 GeV 200
Engineering - CLS Day Thomas Ellis Director of Research Canadian Light Source E-gun & Linear Accelerator 250 MeV Canadian Light Source Transfer Line & Booster Ring Commissioned Storage Ring 2.9 GeV 200
Supplementary Information
 Supplementary Information Supplementary Table 1. Atomic details for the crystal structures of silver closo-boranes. See Table 1 for further details. α Ag 2 B 10 H 10 Wyckoff x y z U / Å 2 Occ. Ag 4d 0.250
Supplementary Information Supplementary Table 1. Atomic details for the crystal structures of silver closo-boranes. See Table 1 for further details. α Ag 2 B 10 H 10 Wyckoff x y z U / Å 2 Occ. Ag 4d 0.250
RESULTS EXPERIMENTAL SECTION. Environmental Science & Technology
 pubs.acs.org/est Differential Pair Distribution Function Study of the Structure of Arsenate Adsorbed on Nanocrystalline γ-alumina Wei Li,*,,^ Richard Harrington,*,, Yuanzhi Tang,*,,z James D. Kubicki,
pubs.acs.org/est Differential Pair Distribution Function Study of the Structure of Arsenate Adsorbed on Nanocrystalline γ-alumina Wei Li,*,,^ Richard Harrington,*,, Yuanzhi Tang,*,,z James D. Kubicki,
Supporting Information
 Supporting Information Hydrogen Storage in the Dehydrated Prussian Blue Analogues M 3 [Co(CN) 6 ] 2 (M = Mn, Fe, Co, Ni, Cu, Zn) Steven S. Kaye and Jeffrey R. Long* Dept. of Chemistry, University of California,
Supporting Information Hydrogen Storage in the Dehydrated Prussian Blue Analogues M 3 [Co(CN) 6 ] 2 (M = Mn, Fe, Co, Ni, Cu, Zn) Steven S. Kaye and Jeffrey R. Long* Dept. of Chemistry, University of California,
Strain, Stress and Cracks Klaus Attenkofer PV Reliability Workshop (Orlando) April 7-8, 2015
 Strain, Stress and Cracks Klaus Attenkofer PV Reliability Workshop (Orlando) April 7-8, 2015 1 BROOKHAVEN SCIENCE ASSOCIATES Overview Material s response to applied forces or what to measure Definitions
Strain, Stress and Cracks Klaus Attenkofer PV Reliability Workshop (Orlando) April 7-8, 2015 1 BROOKHAVEN SCIENCE ASSOCIATES Overview Material s response to applied forces or what to measure Definitions
ARSENIC SPECIATION AND IDENTIFICATION ON ACTIVE IRON ADSORBENT SITES BY XAFS TECHNOLOGY
 ARSENIC SPECIATION AND IDENTIFICATION ON ACTIVE IRON ADSORBENT SITES BY XAFS TECHNOLOGY Dilshad Masih, Ken-ichi Aika and Yasuo Izumi (Tokyo Institute of Technology, Midori-ku, Yokohama 226-8502, Japan)
ARSENIC SPECIATION AND IDENTIFICATION ON ACTIVE IRON ADSORBENT SITES BY XAFS TECHNOLOGY Dilshad Masih, Ken-ichi Aika and Yasuo Izumi (Tokyo Institute of Technology, Midori-ku, Yokohama 226-8502, Japan)
1. Forming a Precipitate 2. Solubility Product Constant (One Source of Ions)
 Chemistry 12 Solubility Equilibrium II Name: Date: Block: 1. Forming a Precipitate 2. Solubility Product Constant (One Source of Ions) Forming a Precipitate Example: A solution may contain the ions Ca
Chemistry 12 Solubility Equilibrium II Name: Date: Block: 1. Forming a Precipitate 2. Solubility Product Constant (One Source of Ions) Forming a Precipitate Example: A solution may contain the ions Ca
In situ speciation studies of copper in the electroplating sludge under an electric field
 Spectrochimica Acta Part A 6 (24) 2387 2391 In situ speciation studies of copper in the electroplating sludge under an electric field S.-H. Liu, H. Paul Wang Department of Environmental Engineering, National
Spectrochimica Acta Part A 6 (24) 2387 2391 In situ speciation studies of copper in the electroplating sludge under an electric field S.-H. Liu, H. Paul Wang Department of Environmental Engineering, National
and biogenic ligands present in solution and spectroscopic evidence of ligand
 Electronic Supplementary Material (ESI) for Metallomics. This journal is The Royal Society of Chemistry 2014 Supporting Information: Hg(II) bacterial biouptake: The role of anthropogenic and biogenic ligands
Electronic Supplementary Material (ESI) for Metallomics. This journal is The Royal Society of Chemistry 2014 Supporting Information: Hg(II) bacterial biouptake: The role of anthropogenic and biogenic ligands
RESULTS AND DISCUSSION Characterization of pure CaO and Zr-TiO 2 /CaO nanocomposite
 RESULTS AND DISCUSSION 4.1. Characterization of pure CaO and Zr-TiO 2 /CaO nanocomposite 4.1.1. Scanning electron microscopy analysis (SEM) SEM images of prepared CaO are shown in Fig. 4.1 (a and b). CaO
RESULTS AND DISCUSSION 4.1. Characterization of pure CaO and Zr-TiO 2 /CaO nanocomposite 4.1.1. Scanning electron microscopy analysis (SEM) SEM images of prepared CaO are shown in Fig. 4.1 (a and b). CaO
NEW CORRECTION PROCEDURE FOR X-RAY SPECTROSCOPIC FLUORESCENCE DATA: SIMULATIONS AND EXPERIMENT
 Copyright JCPDS - International Centre for Diffraction Data 2005, Advances in X-ray Analysis, Volume 48. 266 NEW CORRECTION PROCEDURE FOR X-RAY SPECTROSCOPIC FLUORESCENCE DATA: SIMULATIONS AND EXPERIMENT
Copyright JCPDS - International Centre for Diffraction Data 2005, Advances in X-ray Analysis, Volume 48. 266 NEW CORRECTION PROCEDURE FOR X-RAY SPECTROSCOPIC FLUORESCENCE DATA: SIMULATIONS AND EXPERIMENT
Supplementary Figure S1: Particle size distributions of the Pt ML /Pd 9 Au 1 /C
 a 2 15 before cycle test mean particle size: 3.8 ± 1.2 nm b 2 15 after.6v - 1.V 1k cycle test mean particle size: 4.1 ± 1.5 nm Number 1 total number: 558 Number 1 total number: 554 5 5 1 2 3 4 5 6 7 8
a 2 15 before cycle test mean particle size: 3.8 ± 1.2 nm b 2 15 after.6v - 1.V 1k cycle test mean particle size: 4.1 ± 1.5 nm Number 1 total number: 558 Number 1 total number: 554 5 5 1 2 3 4 5 6 7 8
THE IMPORTANCE OF THE SPECIMEN DISPLACEMENT CORRECTION IN RIETVELD PATTERN FITTING WITH SYMMETRIC REFLECTION-OPTICS DIFFRACTION DATA
 Copyright(c)JCPDS-International Centre for Diffraction Data 2001,Advances in X-ray Analysis,Vol.44 96 THE IMPORTANCE OF THE SPECIMEN DISPLACEMENT CORRECTION IN RIETVELD PATTERN FITTING WITH SYMMETRIC REFLECTION-OPTICS
Copyright(c)JCPDS-International Centre for Diffraction Data 2001,Advances in X-ray Analysis,Vol.44 96 THE IMPORTANCE OF THE SPECIMEN DISPLACEMENT CORRECTION IN RIETVELD PATTERN FITTING WITH SYMMETRIC REFLECTION-OPTICS
Hassan Osseili, Debabrata Mukherjee, Klaus Beckerle, Thomas P. Spaniol, and Jun Okuda*
 Supporting Information Me6TREN-Supported Alkali Metal Hydridotriphenylborates [(L)M][HBPh3] (M = Li, Na, K): Synthesis, Structure, and Reactivity Hassan Osseili, Debabrata Mukherjee, Klaus Beckerle, Thomas
Supporting Information Me6TREN-Supported Alkali Metal Hydridotriphenylborates [(L)M][HBPh3] (M = Li, Na, K): Synthesis, Structure, and Reactivity Hassan Osseili, Debabrata Mukherjee, Klaus Beckerle, Thomas
HOW TO ANALYZE SYNCHROTRON DATA
 HOW TO ANALYZE SYNCHROTRON DATA 1 SYNCHROTRON APPLICATIONS - WHAT Diffraction data are collected on diffractometer lines at the world s synchrotron sources. Most synchrotrons have one or more user facilities
HOW TO ANALYZE SYNCHROTRON DATA 1 SYNCHROTRON APPLICATIONS - WHAT Diffraction data are collected on diffractometer lines at the world s synchrotron sources. Most synchrotrons have one or more user facilities
Adsorption Mechanisms of Cadmium onto Pillared Clays and Chalcogenides
 Journal of Sciences, Islamic Republic of Iran 23(3): 231-236 (2012) University of Tehran, ISSN 1016-1104 http://jsciences.ut.ac.ir Adsorption Mechanisms of Cadmium onto Pillared Clays and Chalcogenides
Journal of Sciences, Islamic Republic of Iran 23(3): 231-236 (2012) University of Tehran, ISSN 1016-1104 http://jsciences.ut.ac.ir Adsorption Mechanisms of Cadmium onto Pillared Clays and Chalcogenides
Coprecipitation of Large Scorodite Particles from Aqueous Fe(II) and As(V) Solution by Oxygen Injection
 Materials Transactions, Vol. 50, No. 5 (2009) pp. 1196 to 1201 #2009 The Mining and Materials Processing Institute of Japan Coprecipitation of Large Scorodite Particles from Aqueous Fe(II) and As(V) Solution
Materials Transactions, Vol. 50, No. 5 (2009) pp. 1196 to 1201 #2009 The Mining and Materials Processing Institute of Japan Coprecipitation of Large Scorodite Particles from Aqueous Fe(II) and As(V) Solution
Supplementary Information. Hydrogen absorption in 1 nm Pd clusters confined in. MIL-101(Cr)
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supplementary Information Hydrogen absorption in 1 nm Pd clusters confined
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supplementary Information Hydrogen absorption in 1 nm Pd clusters confined
INTRODUCTION TO GEOCHEMICAL AND REACTIVE TRANSPORT MODELING. Ondra Sracek
 INTRODUCTION TO GEOCHEMICAL AND REACTIVE TRANSPORT MODELING Ondra Sracek Principal types of geochemical modeling: 1. Speciation (for exemple, for lead); 2. Inverse geochemical modeling; 3. Direct geochemical
INTRODUCTION TO GEOCHEMICAL AND REACTIVE TRANSPORT MODELING Ondra Sracek Principal types of geochemical modeling: 1. Speciation (for exemple, for lead); 2. Inverse geochemical modeling; 3. Direct geochemical
Pamela Reilly and Julia Barringer
 Pamela Reilly and Julia Barringer U.S. Geological Survey New Jersey Water Science Center This information is preliminary and is subject to revision. It is being provided to meet the need for timely best
Pamela Reilly and Julia Barringer U.S. Geological Survey New Jersey Water Science Center This information is preliminary and is subject to revision. It is being provided to meet the need for timely best
X-ray Fluorescence Imaging Following Synchrotron Beam Excitation
 Conference on Applied Digital Imaging Techniques for Understanding the Palimpsest X-ray Fluorescence Imaging Following Synchrotron Beam Excitation Uwe Bergmann Stanford Synchrotron Radiation Laboratory
Conference on Applied Digital Imaging Techniques for Understanding the Palimpsest X-ray Fluorescence Imaging Following Synchrotron Beam Excitation Uwe Bergmann Stanford Synchrotron Radiation Laboratory
Arsenic and Other Trace Elements in Groundwater in the Southern San Joaquin Valley of California
 Arsenic and Other Trace Elements in Groundwater in the Southern San Joaquin Valley of California Dirk Baron Geological Sciences California State University, Bakersfield Trace Element Maximum Contaminant
Arsenic and Other Trace Elements in Groundwater in the Southern San Joaquin Valley of California Dirk Baron Geological Sciences California State University, Bakersfield Trace Element Maximum Contaminant
Supporting Information
 Supporting Information Dynamic Interaction between Methylammonium Lead Iodide and TiO 2 Nanocrystals Leads to Enhanced Photocatalytic H 2 Evolution from HI Splitting Xiaomei Wang,, Hong Wang,, Hefeng Zhang,,
Supporting Information Dynamic Interaction between Methylammonium Lead Iodide and TiO 2 Nanocrystals Leads to Enhanced Photocatalytic H 2 Evolution from HI Splitting Xiaomei Wang,, Hong Wang,, Hefeng Zhang,,
Env/CE 543 Aquatic Chemistry. Sample Midterm Exam
 Env/CE 543 Aquatic Chemistry Sample Midterm Exam Note: The questions on this exam were taken from previous midterm and final exams. This sample exam is about 1.5 to 2.0 times longer than the midterm exam
Env/CE 543 Aquatic Chemistry Sample Midterm Exam Note: The questions on this exam were taken from previous midterm and final exams. This sample exam is about 1.5 to 2.0 times longer than the midterm exam
Lecture 3. Applications of x-ray spectroscopy to inorganic chemistry
 Lecture 3. Applications of x-ray spectroscopy to inorganic chemistry 1. Bioinorganic chemistry/enzymology. Organometallic Chemistry 3. Battery materials MetE (cobalamin independent MetSyn) contains Zn
Lecture 3. Applications of x-ray spectroscopy to inorganic chemistry 1. Bioinorganic chemistry/enzymology. Organometallic Chemistry 3. Battery materials MetE (cobalamin independent MetSyn) contains Zn
Role of Salts in Phase Transformation of Clathrate Hydrates under Brine Environments
 Supporting Information for Role of Salts in Phase Transformation of Clathrate Hydrates under Brine Environments Donghoon Shin, Jong-Won Lee, Yesol Woo, Minjun Cha, Yongjae Lee, Seen Ae Chae, Sun Ha Kim,
Supporting Information for Role of Salts in Phase Transformation of Clathrate Hydrates under Brine Environments Donghoon Shin, Jong-Won Lee, Yesol Woo, Minjun Cha, Yongjae Lee, Seen Ae Chae, Sun Ha Kim,
Supplemental Information (SI): Cobalt-iron (oxy)hydroxide oxygen evolution electrocatalysts: The role of
 Supplemental Information (SI: Cobalt-iron (oxyhydroxide oxygen evolution electrocatalysts: The role of structure and composition on activity, stability, and mechanism Michaela S. Burke, Matthew G. Kast,
Supplemental Information (SI: Cobalt-iron (oxyhydroxide oxygen evolution electrocatalysts: The role of structure and composition on activity, stability, and mechanism Michaela S. Burke, Matthew G. Kast,
Deciphering and predicting spatial and temporal concentrations of arsenic within the Mekong Delta aquifer
 Environ. Chem. 2014, 11, 579-594 CSIRO 2014 Supplementary material Deciphering and predicting spatial and temporal concentrations of arsenic within the Mekong Delta aquifer Benjamin Kocar, A Shawn Benner
Environ. Chem. 2014, 11, 579-594 CSIRO 2014 Supplementary material Deciphering and predicting spatial and temporal concentrations of arsenic within the Mekong Delta aquifer Benjamin Kocar, A Shawn Benner
Part 1: What is XAFS? What does it tell us? The EXAFS equation. Part 2: Basic steps in the analysis Quick overview of typical analysis
 Introduction to XAFS Part 1: What is XAFS? What does it tell us? The EXAFS equation Part 2: Basic steps in the analysis Quick overview of typical analysis Tomorrow Measurement methods and examples The
Introduction to XAFS Part 1: What is XAFS? What does it tell us? The EXAFS equation Part 2: Basic steps in the analysis Quick overview of typical analysis Tomorrow Measurement methods and examples The
Supporting Information. High Selectivity of Supported Ru Catalysts in the Selective. CO Methanation - Water Makes the Difference
 S1 Supporting Information High Selectivity of Supported Ru Catalysts in the Selective CO Methanation - Water Makes the Difference Ali M. Abdel-Mageed,, Stephan Eckle, and R. Ju rgen Behm *, Institute of
S1 Supporting Information High Selectivity of Supported Ru Catalysts in the Selective CO Methanation - Water Makes the Difference Ali M. Abdel-Mageed,, Stephan Eckle, and R. Ju rgen Behm *, Institute of
Crystal structure dependent in vitro antioxidant activity of biocompatible calcium gallate MOFs
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry B. This journal is The Royal Society of Chemistry 2017 Crystal structure dependent in vitro antioxidant activity of biocompatible
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry B. This journal is The Royal Society of Chemistry 2017 Crystal structure dependent in vitro antioxidant activity of biocompatible
Department of Geological Sciences and Geological Engineering, Queen s University, Kingston, ON 2
 SOLID-PHASE SPECIATION AND POST-DEPOSITIONAL MOBILITY OF ARSENIC IN LAKE SEDIMENTS IMPACTED BY ORE ROASTING AT LEGACY GOLD MINES NEAR YELLOWKNIFE, NT, CANADA Christopher E. Schuh 1, Heather E. Jamieson
SOLID-PHASE SPECIATION AND POST-DEPOSITIONAL MOBILITY OF ARSENIC IN LAKE SEDIMENTS IMPACTED BY ORE ROASTING AT LEGACY GOLD MINES NEAR YELLOWKNIFE, NT, CANADA Christopher E. Schuh 1, Heather E. Jamieson
Unusual Molecular Material formed through Irreversible Transformation and Revealed by 4D Electron Microscopy
 26 March 2013 Unusual Molecular Material formed through Irreversible Transformation and Revealed by 4D Electron Microscopy Renske M. van der Veen, Antoine Tissot, Andreas Hauser, Ahmed H. Zewail Physical
26 March 2013 Unusual Molecular Material formed through Irreversible Transformation and Revealed by 4D Electron Microscopy Renske M. van der Veen, Antoine Tissot, Andreas Hauser, Ahmed H. Zewail Physical
sodium ion battery synthesized by a soft chemistry route
 Supporting Information Title: γ-na 0.96 V 2 O 5 : a new competitive cathode material for sodium ion battery synthesized by a soft chemistry route Nicolas Emery 1,, Rita Baddour-Hadjean 1, Dauren Batyrbekuly
Supporting Information Title: γ-na 0.96 V 2 O 5 : a new competitive cathode material for sodium ion battery synthesized by a soft chemistry route Nicolas Emery 1,, Rita Baddour-Hadjean 1, Dauren Batyrbekuly
Supporting Information
 Electronic Supplementary Material (ESI) for CrystEngComm. This journal is The Royal Society of Chemistry 2015 Supporting Information Single-Crystal-to-Single-Crystal Transformation of an Anion Exchangeable
Electronic Supplementary Material (ESI) for CrystEngComm. This journal is The Royal Society of Chemistry 2015 Supporting Information Single-Crystal-to-Single-Crystal Transformation of an Anion Exchangeable
Source: Tokyo Institute of Technology, Center for Public Affairs and Communications
 PRESS RELEASE Source: Tokyo Institute of Technology, Center for Public Affairs and Communications For immediate release: 22 February 2017 Tokyo Institute of Technology research: "Quartz" crystals at the
PRESS RELEASE Source: Tokyo Institute of Technology, Center for Public Affairs and Communications For immediate release: 22 February 2017 Tokyo Institute of Technology research: "Quartz" crystals at the
Geochemical study of arsenic release mechanisms in the Bengal Basin groundwater
 Geochemical study of arsenic release mechanisms in the Bengal Basin groundwater Carolyn B. Dowling, Robert J. Poreda, Asish R. Basu, and Scott L. Peters Sampling Sixty-eight groundwater samples Bangladesh
Geochemical study of arsenic release mechanisms in the Bengal Basin groundwater Carolyn B. Dowling, Robert J. Poreda, Asish R. Basu, and Scott L. Peters Sampling Sixty-eight groundwater samples Bangladesh
Supporting Information
 Supporting Information Synchrotron-Based In Situ Characterization of Carbon-Supported Platinum and Platinum Monolayer Electrocatalysts Kotaro Sasaki 1*, Nebojsa Marinkovic 2, Hugh S. Isaacs 1, Radoslav
Supporting Information Synchrotron-Based In Situ Characterization of Carbon-Supported Platinum and Platinum Monolayer Electrocatalysts Kotaro Sasaki 1*, Nebojsa Marinkovic 2, Hugh S. Isaacs 1, Radoslav
Controllable Growth of Bulk Cubic-Phase CH 3 NH 3 PbI 3 Single Crystal with Exciting Room-Temperature Stability
 Electronic Supplementary Material (ESI) for CrystEngComm. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information Controllable Growth of Bulk Cubic-Phase CH 3 NH 3 PbI
Electronic Supplementary Material (ESI) for CrystEngComm. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information Controllable Growth of Bulk Cubic-Phase CH 3 NH 3 PbI
Supporting Information for. Fast Direct Synthesis and Compaction of Phase Pure. Thermoelectric ZnSb
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry C. This journal is The Royal Society of Chemistry 2015 Supporting Information for Fast Direct Synthesis and Compaction of Phase
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry C. This journal is The Royal Society of Chemistry 2015 Supporting Information for Fast Direct Synthesis and Compaction of Phase
4. Other diffraction techniques
 4. Other diffraction techniques 4.1 Reflection High Energy Electron Diffraction (RHEED) Setup: - Grazing-incidence high energy electron beam (3-5 kev: MEED,
4. Other diffraction techniques 4.1 Reflection High Energy Electron Diffraction (RHEED) Setup: - Grazing-incidence high energy electron beam (3-5 kev: MEED,
Protein crystallography. Garry Taylor
 Protein crystallography Garry Taylor X-ray Crystallography - the Basics Grow crystals Collect X-ray data Determine phases Calculate ρ-map Interpret map Refine coordinates Do the biology. Nitrogen at -180
Protein crystallography Garry Taylor X-ray Crystallography - the Basics Grow crystals Collect X-ray data Determine phases Calculate ρ-map Interpret map Refine coordinates Do the biology. Nitrogen at -180
X-ray Absorption Spectroscopy Eric Peterson 9/2/2010
 X-ray Absorption Spectroscopy Eric Peterson 9/2/2010 Outline Generation/Absorption of X-rays History Synchrotron Light Sources Data reduction/analysis Examples Crystallite size from Coordination Number
X-ray Absorption Spectroscopy Eric Peterson 9/2/2010 Outline Generation/Absorption of X-rays History Synchrotron Light Sources Data reduction/analysis Examples Crystallite size from Coordination Number
The Iron Wheel in Lac Pavin: Interaction with Phosphorus Cycle
 1 2 The Iron Wheel in Lac Pavin: Interaction with Phosphorus Cycle 12 3 4 5 V. Busigny, Didier Jézéquel, J. Cosmidis, E. Viollier, K. Benzerara, N.J. Planavsky, Patrick Albéric, O. Lebeau, G. Sarazin,
1 2 The Iron Wheel in Lac Pavin: Interaction with Phosphorus Cycle 12 3 4 5 V. Busigny, Didier Jézéquel, J. Cosmidis, E. Viollier, K. Benzerara, N.J. Planavsky, Patrick Albéric, O. Lebeau, G. Sarazin,
Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c,
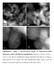 Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c, 2 nm; d, 2 nm. The low magnification images demonstrate
Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c, 2 nm; d, 2 nm. The low magnification images demonstrate
7-4 Systematic Treatment of Equilibrium
 7-4 Systematic Treatment of Equilibrium The equilibrium problem can be solved by working n equations and n unknowns. n equations n-2 : chemical equilibrium conditions 2 : Charge balance Mass balance 1)
7-4 Systematic Treatment of Equilibrium The equilibrium problem can be solved by working n equations and n unknowns. n equations n-2 : chemical equilibrium conditions 2 : Charge balance Mass balance 1)
Supporting Information (SI) for
 Supporting Information (SI) for Cryst. Growth Des. DOI: 10.1021/acs.cgd.7b00892 Modulator effect in UiO-66-NDC (1,4-naphthalenedicarboxilic acid) synthesis and comparison with UiO-67-NDC isoreticular MOFs
Supporting Information (SI) for Cryst. Growth Des. DOI: 10.1021/acs.cgd.7b00892 Modulator effect in UiO-66-NDC (1,4-naphthalenedicarboxilic acid) synthesis and comparison with UiO-67-NDC isoreticular MOFs
Supporting Information. using EXAFS, micro-xrf and micro-xrd
 Supporting Information Lead speciation in house dust from Canadian urban homes using EXAFS, micro-xrf and micro-xrd Lachlan C.W. MacLean 1, Suzanne Beauchemin, and Pat E. Rasmussen 1, * 8 1. Health Canada,
Supporting Information Lead speciation in house dust from Canadian urban homes using EXAFS, micro-xrf and micro-xrd Lachlan C.W. MacLean 1, Suzanne Beauchemin, and Pat E. Rasmussen 1, * 8 1. Health Canada,
Correlating Transport and Structural Properties in Li1+xAlxGe2 x(po4)3 (LAGP) Prepared from Aqueous Solution
 Supporting Information for Correlating Transport and Structural Properties in Li1+xAlxGe2 x(po4)3 (LAGP) Prepared from Aqueous Solution Manuel Weiss a, Dominik A. Weber a, Anatoliy Senyshyn b, Jürgen Janek
Supporting Information for Correlating Transport and Structural Properties in Li1+xAlxGe2 x(po4)3 (LAGP) Prepared from Aqueous Solution Manuel Weiss a, Dominik A. Weber a, Anatoliy Senyshyn b, Jürgen Janek
WM 04 Conference, February 29 March 4, 2004, Tucson AZ ESTIMATING SITE CONCENTRATIONS IN SOILS FOR SURFACE COMPLEXATION MODELING OF SORPTION
 ESTIMATING SITE CONCENTRATIONS IN SOILS FOR SURFACE COMPLEXATION MODELING OF SORPTION M. Ewanic, M. North-Abbott, D. Reichhardt, M. H. Zaluski MSE Technology Applications, Inc. ABSTRACT MSE Technology
ESTIMATING SITE CONCENTRATIONS IN SOILS FOR SURFACE COMPLEXATION MODELING OF SORPTION M. Ewanic, M. North-Abbott, D. Reichhardt, M. H. Zaluski MSE Technology Applications, Inc. ABSTRACT MSE Technology
