Teacher: check the web. He isn t here, so I will have to yell at him.
|
|
|
- Allyson Price
- 5 years ago
- Views:
Transcription
1 September 9, 2003 (Reading from page of announcements) working offline! Remember neo accts are important. (Reading from page of NOTES) I am answering your s; it just may take some time. I have information of how to work with your packet that is easier to work with than the information from your packet. I went to the bookstore; they can order the old editions. I put books on reserve at the Annex. There is a backside to the green sheet, which is organic nomenclature. For the first exam: you must know the Names and formulas, not the structure this time. That is all on the back of that sheet. Review by Silvia, this Thursday from 7-9, for the lab. My review on Sunday was just background for the lab, coming up, which was for the ionic equation. Thursday she will go over that again. These reviews will help you write up the laboratories you have done and prepare for the next one. (Reading from page notes, you can dye your goggles if you go by room 400 on Friday). Female Student: is there an SI-? Teacher: check the web. He isn t here, so I will have to yell at him. Page 1 of 1
2 Male Student: where do you buy goggles? Teacher: well the goggle people will come by and sell them in your labs. If you miss that time, graduate students will sell them between times or I can sell you some. Male Student: does the skill builder require a cd? Teacher: you will have to buy it from the bookstore; you must ask for it as it s under the counter. If you bought it from the web, then you don t need the CD. This will be the last year to be able to buy it on the web. Female Student: what if I bought it from a friend? Teacher: you need to come see me. Female Student: are we doing CPR? Teacher: we are waiting for information on that. I am hoping we will Any questions about the CHEM, skill builder? Then, come and see me and we will work it out We talked about thermodynamics and other things. Lets go through them one by one Page 2 of 2
3 Internal energy, E E cannot be measured directly, but we can measure delta e, or the change. That is final minus initial. The IE, of a system, can be changed in 2 ways. 1. Add heat to the system, with a q, or generic heat, or remove heat from a system. 2. System does work; w, or work is done on a system. Know that q and w are not state functions. Using plus (+) or minus ( ) helps convey information. So let s look at sign conventions for q and w. If, q+, then heat is added to system, but if q-, then heat is removed from a system. Then, w+ is work is done on the system by the surrounding, but if w-, then work is done by the system to the surrounding. So E (i) and add heat to the system and do work on the system then we end up with E (f). There (Example on board.) is the formula. Delta E has a sign convention. E+ energy has been absorbed by the system. E -, energy is released from the system. If 1200 J of heat are removed from the system and system does 800 J of work then: (a) what is delta E for the system? Delta E is q + w, then delta E = -1200J 800J = J (b) for the surrounding delta E = J You can start with problem 72, which deals with E. You don t have to do BOPs in order, but label well. Page 3 of 3
4 Work in chemical reactions is due to changes in volume; we will only be considering gases. For example 1 mole of liquid (H2O) changing to 1 mole of gas (H2O) How much mass is one mol of water? 18g. Water as a gas still has 18g of gas. What is the volume or mils, density, of water do we have? Density of water is 1g/m. 1 mole of water as a gas, at STP, then temperature is 0^C, P is one atmosphere, that means it occupies 22.4L. That number you should memorize. What happens to the volume? The volume has expanded from 18 to 22+; in this situation, the system does work. The system, water, has pushed out against the surroundings. In this case, the sign is (-) negative, in this system. (Example on board.). PV = nrt Work done on gas equals -P (delta) V; calculate the work done on the following system done at 127C and 1 atm, given 4 moles of H2. (Example on board.) Here is the formula. Given 4 moles, here are 2 mol and we made 4 mol. Our reactants, in equation: 3 mol of gas going to 2 mol of gas. Consider STP, volume, etc. 3 mol 2 mol gas, then the surroundings are doing work on the system. The work is (+) positive. Page 4 of 4
5 Larger volume to a smaller volume here V(f) V(i) would seem to be negative, that is why we have the sign convention, because we know the work must be positive. (delta)ngas = ngas product ngas reactants, using our data 4 mol (2 mol and 4 mol) = 4 6; = 2. By the in front of the P, you can see this will be positive. The R, 8.314J/molK; the K that shows me what measurement for my temperature, with no degree symbol. (Example on board.). We know that work is equal to P delta V, then at constant T and P: (Example on board.) In some cases, no work is done. Examples of that would be: 1. Delta ngas = 0. W = -delta ngas RT. W=0 (Example on board.) using Cgraphite, etc. 2. Work depends on change in volume of system. That is w = -P delta V. If delta V=0 no change in volume w=0; if rxn is done in a closed container, constant V, then no work is done. Page 5 of 5
6 How is E measured? Using calorimeters. There are 2 types. 1. Bomb calorimeter rxn occurs at const V, it s a closed system. But pressure can build up. (Example on board.) Del E = q = w, = q + Here is the picture of one. There are ignition wires and we do combustion rxn. Heat is being released into the surrounding which is all this water. In the water there is a thermometer to measure the change in water. Process is for next time 2. Coffee cup calorimeter system can change volume and the rxn occurs at cont pressure. Quiz 3 (Example on board.). Open book, open notes and talk to your neighbor, but write your own work Page 6 of 6
Laws versus Theories
 Announcements Text HW due tomorrow (Friday) Online HW #3 (Type 1) due Monday, September 17 by 7:00 p.m. Online HW #3 (Type 2) due Wednesday, September 19 by 7:00 p.m. Lab write-up for Gases Lab due Wednesday,
Announcements Text HW due tomorrow (Friday) Online HW #3 (Type 1) due Monday, September 17 by 7:00 p.m. Online HW #3 (Type 2) due Wednesday, September 19 by 7:00 p.m. Lab write-up for Gases Lab due Wednesday,
Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat.
 CHEM134- F18 Dr. Al- Qaisi Chapter 06: Thermodynamics Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat. Energy is anything that has the capacity
CHEM134- F18 Dr. Al- Qaisi Chapter 06: Thermodynamics Thermochemistry is the study of the relationships between chemical reactions and energy changes involving heat. Energy is anything that has the capacity
The Nature of Energy. Chapter Six: Kinetic vs. Potential Energy. Energy and Work. Temperature vs. Heat
 The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
Don't forget to. Mastering Chemistry everyday! October 9 th, Next week in Lab: Next week in disc: 10/9/2014.
 Solutions of nitric acid, sodium bicarbonate, silver nitrate and iron (III) acetate are mixed. Write all NIE s. There are 4! H + (aq) + HCO 2 3 (aq) H 2 O(l) + CO 2 () (g) H + (aq) + C 2 H 3 O 2 (aq) HC
Solutions of nitric acid, sodium bicarbonate, silver nitrate and iron (III) acetate are mixed. Write all NIE s. There are 4! H + (aq) + HCO 2 3 (aq) H 2 O(l) + CO 2 () (g) H + (aq) + C 2 H 3 O 2 (aq) HC
Gas Laws and Thermochemistry Review Packet
 Gas Laws and Thermochemistry Review Packet Introduction to Gas Laws Gas Laws Earlier in your science education you learned to describe the gas state as the state of matter with no definite shape, no definite
Gas Laws and Thermochemistry Review Packet Introduction to Gas Laws Gas Laws Earlier in your science education you learned to describe the gas state as the state of matter with no definite shape, no definite
L = 6.02 x mol Determine the number of particles and the amount of substance (in moles)
 1.1 The Mole 1.1.1 - Apply the mole concept to substances A mole is the name given to a certain quantity. It represents 6.02 x 10 23 particles. This number is also known as Avogadro's constant, symbolised
1.1 The Mole 1.1.1 - Apply the mole concept to substances A mole is the name given to a certain quantity. It represents 6.02 x 10 23 particles. This number is also known as Avogadro's constant, symbolised
DETERMINING AND USING H
 DETERMINING AND USING H INTRODUCTION CHANGES IN CHEMISTRY Chemistry is the science that studies matter and the changes it undergoes. Changes are divided into two categories: physical and chemical. During
DETERMINING AND USING H INTRODUCTION CHANGES IN CHEMISTRY Chemistry is the science that studies matter and the changes it undergoes. Changes are divided into two categories: physical and chemical. During
Chapter 10 Study Guide The Mole Section 10 1
 Chapter 10 Study Guide The Mole Section 10 1 Measuring Matter The electron configuration of hydrogen is like that of Group 1 metals. 4. Study Guide - Chapter 10 The Mole Section 10.1 Measuring Matter 1.
Chapter 10 Study Guide The Mole Section 10 1 Measuring Matter The electron configuration of hydrogen is like that of Group 1 metals. 4. Study Guide - Chapter 10 The Mole Section 10.1 Measuring Matter 1.
Chapter 6. Energy Thermodynamics
 Chapter 6 Energy Thermodynamics 1 Energy is... The ability to do work. Conserved. made of heat and work. a state function. independent of the path, or how you get from point A to B. Work is a force acting
Chapter 6 Energy Thermodynamics 1 Energy is... The ability to do work. Conserved. made of heat and work. a state function. independent of the path, or how you get from point A to B. Work is a force acting
Chemical Thermodynamics
 Quiz A 42.8 ml solution of ammonia (NH 3 ) is titrated with a solution of 0.9713 M hydrochloric acid. The initial reading on the buret containing the HCl was 47.13 ml and the final reading when the endpoint
Quiz A 42.8 ml solution of ammonia (NH 3 ) is titrated with a solution of 0.9713 M hydrochloric acid. The initial reading on the buret containing the HCl was 47.13 ml and the final reading when the endpoint
Chapter 8. Thermochemistry
 Chapter 8 Thermochemistry Copyright 2001 by Harcourt, Inc. All rights reserved. Requests for permission to make copies of any part of the work should be mailed to the following address: Permissions Department,
Chapter 8 Thermochemistry Copyright 2001 by Harcourt, Inc. All rights reserved. Requests for permission to make copies of any part of the work should be mailed to the following address: Permissions Department,
Enthalpies of Reaction
 Enthalpies of Reaction Enthalpy is an extensive property Magnitude of H is directly related to the amount of reactant used up in a process. CH 4 (g) + 2O 2 (g) CO 2 (g) + 2H 2 O(l) H = 890 kj 2CH 4 (g)
Enthalpies of Reaction Enthalpy is an extensive property Magnitude of H is directly related to the amount of reactant used up in a process. CH 4 (g) + 2O 2 (g) CO 2 (g) + 2H 2 O(l) H = 890 kj 2CH 4 (g)
Chapter 6: Thermochemistry
 Chapter 6: Thermochemistry 1. Light the Furnace: The Nature of Energy and Its Transformations a. Thermochemistry is the study of the relationships between chemistry and energy i. This means that we will
Chapter 6: Thermochemistry 1. Light the Furnace: The Nature of Energy and Its Transformations a. Thermochemistry is the study of the relationships between chemistry and energy i. This means that we will
Contents and Concepts
 Contents and Concepts 1. First Law of Thermodynamics Spontaneous Processes and Entropy A spontaneous process is one that occurs by itself. As we will see, the entropy of the system increases in a spontaneous
Contents and Concepts 1. First Law of Thermodynamics Spontaneous Processes and Entropy A spontaneous process is one that occurs by itself. As we will see, the entropy of the system increases in a spontaneous
Ideal Gas Law. Deduced from Combination of Gas Relationships: PV = nrt. where R = universal gas constant
 Ideal Gas Law Deduced from Combination of Gas Relationships: V 1/P, Boyle's Law V T, Charles's Law V n, Avogadro's Law Therefore, V nt/p or PV nt PV = nrt where R = universal gas constant The empirical
Ideal Gas Law Deduced from Combination of Gas Relationships: V 1/P, Boyle's Law V T, Charles's Law V n, Avogadro's Law Therefore, V nt/p or PV nt PV = nrt where R = universal gas constant The empirical
Quantities in Chemical Reactions
 Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
General Chemistry I Office: Chem
 General Chemistry I Office: Chem 122 Fall 2009 email: pdoucette@elcamino.edu Office Hours: Tu & Th: 1:00 2:00, W: 12:00 12:30, or by appointment Meeting times and locations: Lectures: T Th 2:00 4:05 Chem
General Chemistry I Office: Chem 122 Fall 2009 email: pdoucette@elcamino.edu Office Hours: Tu & Th: 1:00 2:00, W: 12:00 12:30, or by appointment Meeting times and locations: Lectures: T Th 2:00 4:05 Chem
Name Class Date = + 1 S atom 32.1 amu +
 Molar Mass 10. What is the atomic mass of an element? 11. Circle the letter of the phrase that completes this sentence correctly. The atomic masses of all elements a. are the same. b. are based on the
Molar Mass 10. What is the atomic mass of an element? 11. Circle the letter of the phrase that completes this sentence correctly. The atomic masses of all elements a. are the same. b. are based on the
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Energy -Very much a chemistry topic Every chemical change has an accompanying change of. Combustion of fossil fuels The discharging a battery Metabolism of foods If we are to
Chapter 5 Thermochemistry Energy -Very much a chemistry topic Every chemical change has an accompanying change of. Combustion of fossil fuels The discharging a battery Metabolism of foods If we are to
1. State in your own terms what is the first law of thermodynamics, a closed system, an isolated system, surroundings, heat, work, and energy.
 Worksheet 1 1. State in your own terms what is the first law of thermodynamics, a closed system, an isolated system, surroundings, heat, work, and energy. The first law of thermodynamics is the conservation
Worksheet 1 1. State in your own terms what is the first law of thermodynamics, a closed system, an isolated system, surroundings, heat, work, and energy. The first law of thermodynamics is the conservation
Ch. 7: Thermochemistry
 Thermodynamics and Thermochemistry Thermodynamics concerns itself with energy and its relationship to the large scale bulk properties of a system that are measurable: Volume, Temperature, Pressure, Heat
Thermodynamics and Thermochemistry Thermodynamics concerns itself with energy and its relationship to the large scale bulk properties of a system that are measurable: Volume, Temperature, Pressure, Heat
Contents and Concepts
 Contents and Concepts 1. First Law of Thermodynamics Spontaneous Processes and Entropy A spontaneous process is one that occurs by itself. As we will see, the entropy of the system increases in a spontaneous
Contents and Concepts 1. First Law of Thermodynamics Spontaneous Processes and Entropy A spontaneous process is one that occurs by itself. As we will see, the entropy of the system increases in a spontaneous
Contents and Concepts
 Contents and Concepts 1. First Law of Thermodynamics Spontaneous Processes and Entropy A spontaneous process is one that occurs by itself. As we will see, the entropy of the system increases in a spontaneous
Contents and Concepts 1. First Law of Thermodynamics Spontaneous Processes and Entropy A spontaneous process is one that occurs by itself. As we will see, the entropy of the system increases in a spontaneous
47 people in recitation yesterday. Expect quizzes there and in class.
 Announcements 47 people in recitation yesterday. Expect quizzes there and in class. Chapter 6 Problems: 6.9, 6.11, 6.13(except c), 6.19, 6.23, 6.34, 6.38, 6.42, 6.51, 6.53, 6.54, 6.57, 6.64, 6.66, 6.69,
Announcements 47 people in recitation yesterday. Expect quizzes there and in class. Chapter 6 Problems: 6.9, 6.11, 6.13(except c), 6.19, 6.23, 6.34, 6.38, 6.42, 6.51, 6.53, 6.54, 6.57, 6.64, 6.66, 6.69,
2012 AP CHEMISTRY FREE-RESPONSE QUESTIONS
 01 AP CHEMISTRY FREE-RESPONSE QUESTIONS. A sample of a pure, gaseous hydrocarbon is introduced into a previously evacuated rigid 1.00 L vessel. The pressure of the gas is 0.00 atm at a temperature of 17C.
01 AP CHEMISTRY FREE-RESPONSE QUESTIONS. A sample of a pure, gaseous hydrocarbon is introduced into a previously evacuated rigid 1.00 L vessel. The pressure of the gas is 0.00 atm at a temperature of 17C.
Unit 13 Gas Laws. Gases
 Unit 13 Gas Laws Gases The Gas Laws Kinetic Theory Revisited 1. Particles are far apart and have negligible volume. 2. Move in rapid, random, straight-line motion. 3. Collide elastically. 4. No attractive
Unit 13 Gas Laws Gases The Gas Laws Kinetic Theory Revisited 1. Particles are far apart and have negligible volume. 2. Move in rapid, random, straight-line motion. 3. Collide elastically. 4. No attractive
Thermochemistry Chapter 4
 Thermochemistry Chapter 4 Thermochemistry is the study of energy changes that occur during chemical reactions Focus is on heat and matter transfer between the system and the surroundings Energy The ability
Thermochemistry Chapter 4 Thermochemistry is the study of energy changes that occur during chemical reactions Focus is on heat and matter transfer between the system and the surroundings Energy The ability
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Chemistry 163B. Thermochemistry. Chapter 4 Engel & Reid
 Chemistry 163B Thermochemistry Chapter 4 Engel & Reid 3 heats of reactions (constant volume; bomb calorimeter) ΔU V = q V ΔU= U products -U reactants 4 heats of reactions (constant volume; fig 4.3 E&R)
Chemistry 163B Thermochemistry Chapter 4 Engel & Reid 3 heats of reactions (constant volume; bomb calorimeter) ΔU V = q V ΔU= U products -U reactants 4 heats of reactions (constant volume; fig 4.3 E&R)
Quantities in Chemical Reactions
 Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
THERMOCHEMISTRY & DEFINITIONS
 THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
Enthalpy. Enthalpy. Enthalpy. Enthalpy. E = q + w. Internal Energy at Constant Volume SYSTEM. heat transfer in (endothermic), +q
 heat transfer in (endothermic), +q heat transfer out (exothermic), -q SYSTEM E = q + w w transfer in (+w) w transfer out (-w) Internal Energy at Constant Volume E = KE + PE ΔE = q + w Because most systems,
heat transfer in (endothermic), +q heat transfer out (exothermic), -q SYSTEM E = q + w w transfer in (+w) w transfer out (-w) Internal Energy at Constant Volume E = KE + PE ΔE = q + w Because most systems,
First Law of Thermodynamics: energy cannot be created or destroyed.
 1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
Let a system undergo changes from a state 1 to state 2.
 ME 410 Day 11 Topics First Law of Thermodynamics Applied to Combustion Constant olume Combustion Constant ressure Combustion Enthalpy of Formation Heating alues of Fuels 1. The First Law of Thermodynamics
ME 410 Day 11 Topics First Law of Thermodynamics Applied to Combustion Constant olume Combustion Constant ressure Combustion Enthalpy of Formation Heating alues of Fuels 1. The First Law of Thermodynamics
Lesson Plan Book-stacking Activity
 T o g o d i r e c t l y t o a l e s s o n, c l i c k o n e o f t h e f o l l o w i n g l i n k s : B o o k - s t a c k i n g A c t i v i t y B a l l o o n A c t i v i t y H y d r o g e n G a s L a b F
T o g o d i r e c t l y t o a l e s s o n, c l i c k o n e o f t h e f o l l o w i n g l i n k s : B o o k - s t a c k i n g A c t i v i t y B a l l o o n A c t i v i t y H y d r o g e n G a s L a b F
Chemistry: The Central Science. Chapter 5: Thermochemistry
 Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
Exam 4, Enthalpy and Gases
 CHEM 1100 Dr. Stone November 8, 2017 Name_ G Exam 4, Enthalpy and Gases Equations and constants you may need: ΔE system = q + w PV = nrt R = 0.0821 (L*atm)/(mole*K) w = -PΔV K.E. = 1 2 m *µ 2 rms µ rms=
CHEM 1100 Dr. Stone November 8, 2017 Name_ G Exam 4, Enthalpy and Gases Equations and constants you may need: ΔE system = q + w PV = nrt R = 0.0821 (L*atm)/(mole*K) w = -PΔV K.E. = 1 2 m *µ 2 rms µ rms=
8.6 The Thermodynamic Standard State
 8.6 The Thermodynamic Standard State The value of H reported for a reaction depends on the number of moles of reactants...or how much matter is contained in the system C 3 H 8 (g) + 5O 2 (g) > 3CO 2 (g)
8.6 The Thermodynamic Standard State The value of H reported for a reaction depends on the number of moles of reactants...or how much matter is contained in the system C 3 H 8 (g) + 5O 2 (g) > 3CO 2 (g)
Physics 9 Monday, February 13, 2012
 Physics 9 Monday, February 13, 2012 learningcatalytics.com class session ID: 927092 This week and next week: heat, thermal physics, etc. It s an important topic for you, so we re reading two authors presentations
Physics 9 Monday, February 13, 2012 learningcatalytics.com class session ID: 927092 This week and next week: heat, thermal physics, etc. It s an important topic for you, so we re reading two authors presentations
WARMUP. Draw the Lewis dot diagram and determine the VSEPR shape for the following compounds. This is practice for the quiz. OF2
 WARMUP Draw the Lewis dot diagram and determine the VSEPR shape for the following compounds. This is practice for the quiz. OF2 HBr SiO2 We will take a quiz over VSEPR and assemble a foldable. I will complete
WARMUP Draw the Lewis dot diagram and determine the VSEPR shape for the following compounds. This is practice for the quiz. OF2 HBr SiO2 We will take a quiz over VSEPR and assemble a foldable. I will complete
CHEM 1105 S10 March 11 & 14, 2014
 CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
Physics 121, April 15, Temperature/Heat and the Ideal Gas Law.
 Physics 121, April 15, 2008. Temperature/Heat and the Ideal Gas Law. http://www.brickinfo.org/bia/technotes/t18.htm Physics 121. April 15, 2008. Course information Topics to be discussed today: Temperature
Physics 121, April 15, 2008. Temperature/Heat and the Ideal Gas Law. http://www.brickinfo.org/bia/technotes/t18.htm Physics 121. April 15, 2008. Course information Topics to be discussed today: Temperature
Chapter 5. Chemistry for Changing Times, Chemical Accounting. Lecture Outlines. John Singer, Jackson Community College. Thirteenth Edition
 Chemistry for Changing Times, Thirteenth Edition Lecture Outlines Chemical Accounting John Singer, Jackson Community College Chemical Sentences: Equations Chemical equations represent the sentences in
Chemistry for Changing Times, Thirteenth Edition Lecture Outlines Chemical Accounting John Singer, Jackson Community College Chemical Sentences: Equations Chemical equations represent the sentences in
Study Guide Chapter 5
 Directions: Answer the following 1. When writing a complete ionic equation, a. what types of substances should be shown as dissociated/ionized? soluble ionic compounds, acids, bases b. What types of substances
Directions: Answer the following 1. When writing a complete ionic equation, a. what types of substances should be shown as dissociated/ionized? soluble ionic compounds, acids, bases b. What types of substances
ALE 26. Energy Changes ( E) and Enthalpy Changes ( H) in Chemical Reactions
 Name Chem 161, Section: Group Number: ALE 26. Energy Changes ( E) and Enthalpy Changes ( H) in Chemical Reactions (Reference: Chapter 6 - Silberberg 5 th edition) Important!! For answers that involve a
Name Chem 161, Section: Group Number: ALE 26. Energy Changes ( E) and Enthalpy Changes ( H) in Chemical Reactions (Reference: Chapter 6 - Silberberg 5 th edition) Important!! For answers that involve a
Chapter 5 - Thermochemistry
 Chapter 5 - Thermochemistry Study of energy changes that accompany chemical rx s. I) Nature of Energy Energy / Capacity to do work Mechanical Work w = F x d Heat energy - energy used to cause the temperature
Chapter 5 - Thermochemistry Study of energy changes that accompany chemical rx s. I) Nature of Energy Energy / Capacity to do work Mechanical Work w = F x d Heat energy - energy used to cause the temperature
1.3: Mole Ratio, Limiting & Excess Reactants, Percent Yield. Ms. Kiely Coral Gables Senior High IB Chemistry SL
 1.3: Mole Ratio, Limiting & Excess Reactants, Percent Yield Ms. Kiely Coral Gables Senior High IB Chemistry SL Bell-Ringer #4 Which of the following are empirical formulas? I. C₆H₆ II. C₃H₈ III. N₂O₄ IV.
1.3: Mole Ratio, Limiting & Excess Reactants, Percent Yield Ms. Kiely Coral Gables Senior High IB Chemistry SL Bell-Ringer #4 Which of the following are empirical formulas? I. C₆H₆ II. C₃H₈ III. N₂O₄ IV.
= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)
![= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj) = (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)](/thumbs/89/101004910.jpg) CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
1. (24 points) 5. (8 points) 6d. (13 points) 7. (6 points)
 1 of 9 Third our Exam 5.111 Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. The exam is closed notes and closed book. 1. Read all parts of each
1 of 9 Third our Exam 5.111 Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. The exam is closed notes and closed book. 1. Read all parts of each
1. Making the invisible, visible. 2. Enabling the students to witness experiments that they cannot conduct themselves.
 Lesson Plan Lesson: Gas Calculations Aim: To investigate the molar and reacting volumes of gases. Learning Outcomes : At the end of the lesson, students will be able to : 1. state the molar volumes of
Lesson Plan Lesson: Gas Calculations Aim: To investigate the molar and reacting volumes of gases. Learning Outcomes : At the end of the lesson, students will be able to : 1. state the molar volumes of
Thermodynamics: Entropy
 Name: Band: Date: Thermodynamics: Entropy Big Idea: Entropy When we were studying enthalpy, we made a generalization: most spontaneous processes are exothermic. This is a decent assumption to make because
Name: Band: Date: Thermodynamics: Entropy Big Idea: Entropy When we were studying enthalpy, we made a generalization: most spontaneous processes are exothermic. This is a decent assumption to make because
Friday, November 2, 2018 GLE/Standard: The fact that atoms are conserved, together
 Mrs. Chausse s Physical Science Gifted Lesson Plan: Unit 7 Chemical Reactions November 1 16, 2018 Thursday, November 1, 2018 Objective: SWBAT balance a chemical equation that satisfies the Law of Conservation
Mrs. Chausse s Physical Science Gifted Lesson Plan: Unit 7 Chemical Reactions November 1 16, 2018 Thursday, November 1, 2018 Objective: SWBAT balance a chemical equation that satisfies the Law of Conservation
Kwantlen Polytechnic University Chemistry 1105 S10 Spring Term Test No. 3 Thursday, April 4, 2013
 Kwantlen Polytechnic University Chemistry 1105 S10 Spring Term Test No. 3 Thursday, April 4, 2013 Name: Student Number Instructions: Ensure that this exam contains all eight pages including this page.
Kwantlen Polytechnic University Chemistry 1105 S10 Spring Term Test No. 3 Thursday, April 4, 2013 Name: Student Number Instructions: Ensure that this exam contains all eight pages including this page.
Energy Heat Work Heat Capacity Enthalpy
 Energy Heat Work Heat Capacity Enthalpy 1 Prof. Zvi C. Koren 20.07.2010 Thermodynamics vs. Kinetics Thermodynamics Thermo = Thermo + Dynamics E (Note: Absolute E can never be determined by humans!) Can
Energy Heat Work Heat Capacity Enthalpy 1 Prof. Zvi C. Koren 20.07.2010 Thermodynamics vs. Kinetics Thermodynamics Thermo = Thermo + Dynamics E (Note: Absolute E can never be determined by humans!) Can
Obj: Observe and describe states of matter.
 Do Now Date: March 6, 2017 Obj: Observe and describe states of matter. Copy and Solve: 1. If Dr. B has a 5.0L container at a pressure of 2 atm, how much pressure would it place on the walls of a 2.5L container?
Do Now Date: March 6, 2017 Obj: Observe and describe states of matter. Copy and Solve: 1. If Dr. B has a 5.0L container at a pressure of 2 atm, how much pressure would it place on the walls of a 2.5L container?
ENTHALPY, INTERNAL ENERGY, AND CHEMICAL REACTIONS: AN OUTLINE FOR CHEM 101A
 ENTHALPY, INTERNAL ENERGY, AND CHEMICAL REACTIONS: AN OUTLINE FOR CHEM 101A PART 1: KEY TERMS AND SYMBOLS IN THERMOCHEMISTRY System and surroundings When we talk about any kind of change, such as a chemical
ENTHALPY, INTERNAL ENERGY, AND CHEMICAL REACTIONS: AN OUTLINE FOR CHEM 101A PART 1: KEY TERMS AND SYMBOLS IN THERMOCHEMISTRY System and surroundings When we talk about any kind of change, such as a chemical
Thermodynamics of Salt Dissolution
 1 Thermodynamics of Salt Dissolution ORGANIZATION Mode: Part A groups of 3 or 4; Part B individual work; Part C back to groups Grading: lab notes, lab performance, and post-lab report Safety: goggles,
1 Thermodynamics of Salt Dissolution ORGANIZATION Mode: Part A groups of 3 or 4; Part B individual work; Part C back to groups Grading: lab notes, lab performance, and post-lab report Safety: goggles,
CHEMISTRY 102B Hour Exam II. Dr. D. DeCoste T.A.
 CHEMISTRY 10B Hour Exam II March 19, 015 Dr. D. DeCoste Name Signature T.A. This exam contains questions on 9 numbered pages. Check now to make sure you have a complete exam. You have one hour and thirty
CHEMISTRY 10B Hour Exam II March 19, 015 Dr. D. DeCoste Name Signature T.A. This exam contains questions on 9 numbered pages. Check now to make sure you have a complete exam. You have one hour and thirty
PHY101: Major Concepts in Physics I
 Welcome back to PHY101: Major Concepts in Physics I Photo: J. M. Schwarz Announcements In class today we will finish Chapter 20 (sections 3, 4, and 7). and then move to Chapter 13 (the first six sections).
Welcome back to PHY101: Major Concepts in Physics I Photo: J. M. Schwarz Announcements In class today we will finish Chapter 20 (sections 3, 4, and 7). and then move to Chapter 13 (the first six sections).
Properties of Gases. Properties of Gases. Pressure. Three phases of matter. Definite shape and volume. solid. Definite volume, shape of container
 Properties of Gases Properties of Gases Three phases of matter solid Definite shape and volume liquid Definite volume, shape of container gas Shape and volume of container Properties of Gases A gas is
Properties of Gases Properties of Gases Three phases of matter solid Definite shape and volume liquid Definite volume, shape of container gas Shape and volume of container Properties of Gases A gas is
Enthalpy Chapter 5.3-4,7
 Enthalpy Chapter 5.3-4,7 heat transfer in (endothermic), +q heat transfer out (exothermic), -q SYSTEM E = q + w w transfer in (+w) w transfer out (-w) Internal Energy at Constant Volume E = E K + E P ΔE
Enthalpy Chapter 5.3-4,7 heat transfer in (endothermic), +q heat transfer out (exothermic), -q SYSTEM E = q + w w transfer in (+w) w transfer out (-w) Internal Energy at Constant Volume E = E K + E P ΔE
Chapter 5 Thermochemistry. 許富銀 ( Hsu Fu-Yin)
 Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
Thermochemistry Ch. 8
 Definitions I. Energy (E): capacity to do work. II. Heat (q): transfer of energy from a body at a high temp. to a body at a low temp. III. Reaction perspectives: A. System: the focus. B. Surroundings:
Definitions I. Energy (E): capacity to do work. II. Heat (q): transfer of energy from a body at a high temp. to a body at a low temp. III. Reaction perspectives: A. System: the focus. B. Surroundings:
1. (24 points) 2. (15 points) 4. (6 points) 6a,b,c. (20 points)
 1 of 9 Third our Exam 5.111 Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. The exam is closed notes and closed book. 1. Read all parts of each
1 of 9 Third our Exam 5.111 Write your name and your TA's name below. Do not open the exam until the start of the exam is announced. The exam is closed notes and closed book. 1. Read all parts of each
Edexcel Chemistry A-level
 Edexcel Chemistry A-level Topic 5 - Formulae, Equations and Amounts of Substance Flashcards What is the symbol for amount of substance? What is the symbol for amount of substance? n What is the unit used
Edexcel Chemistry A-level Topic 5 - Formulae, Equations and Amounts of Substance Flashcards What is the symbol for amount of substance? What is the symbol for amount of substance? n What is the unit used
2 Standards of Measurement
 What You ll Learn the SI units and symbols for length, volume, mass, density, time, and temperature how to convert related SI units 2 Standards of Measurement (A), 2(D), 2(C), 2(E) Before You Read If someone
What You ll Learn the SI units and symbols for length, volume, mass, density, time, and temperature how to convert related SI units 2 Standards of Measurement (A), 2(D), 2(C), 2(E) Before You Read If someone
CHEMISTRY Matter and Change. Chapter 13: Gases
 CHEMISTRY Matter and Change Chapter 13: Gases CHAPTER 13 Table Of Contents Section 13.1 Section 13.2 Section 13.3 The Gas Laws The Ideal Gas Law Gas Stoichiometry Click a hyperlink to view the corresponding
CHEMISTRY Matter and Change Chapter 13: Gases CHAPTER 13 Table Of Contents Section 13.1 Section 13.2 Section 13.3 The Gas Laws The Ideal Gas Law Gas Stoichiometry Click a hyperlink to view the corresponding
Gas UNIT - READINESS ASSESSMENT QUIZ (RAQ) THIS QUIZ WILL BE PACED WITH CLICKER QUESTIONS
 Gas UNIT - READINESS ASSESSMENT QUIZ (RAQ) THIS QUIZ WILL BE PACED WITH CLICKER QUESTIONS 1. A 5.0 mol sample of Ne is confined in a 3.14 L vessel at a pressure of 2.5 atm. What is the number density of
Gas UNIT - READINESS ASSESSMENT QUIZ (RAQ) THIS QUIZ WILL BE PACED WITH CLICKER QUESTIONS 1. A 5.0 mol sample of Ne is confined in a 3.14 L vessel at a pressure of 2.5 atm. What is the number density of
Advanced Chemistry: Chemical Reactions
 ILLINOIS MATHEMATICS & SCIENCE ACADEMY Teacher: Dave DeVol Advanced Chemistry: Chemical Reactions January 2014 Unit 1: Equilibrium Theme: Equilibrium is a dynamic process that involves change at the molecular
ILLINOIS MATHEMATICS & SCIENCE ACADEMY Teacher: Dave DeVol Advanced Chemistry: Chemical Reactions January 2014 Unit 1: Equilibrium Theme: Equilibrium is a dynamic process that involves change at the molecular
Unit 7 Thermochemistry Chemistry 020, R. R. Martin
 Unit 7 Thermochemistry Chemistry 020, R. R. Martin 1. Thermochemistry Heat is a form of energy - which may take many forms: - Kinetic energy due to motion, ½ mv 2 - Potential energy due to position - Electrical
Unit 7 Thermochemistry Chemistry 020, R. R. Martin 1. Thermochemistry Heat is a form of energy - which may take many forms: - Kinetic energy due to motion, ½ mv 2 - Potential energy due to position - Electrical
**VII-1 C NC I-3 C NC II-2 C NC *VII-2 C NC I-4 C NC. CHEMISTRY 131 Quiz 5 Fall 2010 Form B
 **VII-1 N I-3 N II-2 N *VII-2 N I-4 N EMISTRY 131 Quiz 5 Fall 2010 Form B NAME: Key hapter 11: States of Matter A. (2 pts) onsider the structures of the compounds shown below, and determine which intermolecular
**VII-1 N I-3 N II-2 N *VII-2 N I-4 N EMISTRY 131 Quiz 5 Fall 2010 Form B NAME: Key hapter 11: States of Matter A. (2 pts) onsider the structures of the compounds shown below, and determine which intermolecular
Thermodynamics - Energy Relationships in Chemical Reactions:
 Thermodynamics - Energy Relationships in Chemical Reactions: energy - The capacity to do work. Types of Energy: radiant-energy from the sun. potential-energy due to an objects position. chemical-energy
Thermodynamics - Energy Relationships in Chemical Reactions: energy - The capacity to do work. Types of Energy: radiant-energy from the sun. potential-energy due to an objects position. chemical-energy
Chemistry : General Chemistry, Fall 2013 Department of Chemistry and Biochemistry California State University East Bay
 Chemistry 1101 01: General Chemistry, Fall 2013 Department of Chemistry and Biochemistry California State University East Bay Lecture instructor: Patrick Huang Lectures: MWF 8:15 9:05 am in VBT124 Email:
Chemistry 1101 01: General Chemistry, Fall 2013 Department of Chemistry and Biochemistry California State University East Bay Lecture instructor: Patrick Huang Lectures: MWF 8:15 9:05 am in VBT124 Email:
SAVE THIS SYLLABUS FOR REFERENCE DURING THE SEMESTER.
 SYLLABUS Course: General Chemistry I: (call #31437) Lecture: 8:30-10:00AM Mon.-Wed.; Room 6006 Recitation: 1 hour per week: Mon.; 12:00-1:00 Room 3066 Laboratory: 3 hours per week: Mon;1:00-4:00 Room 3066
SYLLABUS Course: General Chemistry I: (call #31437) Lecture: 8:30-10:00AM Mon.-Wed.; Room 6006 Recitation: 1 hour per week: Mon.; 12:00-1:00 Room 3066 Laboratory: 3 hours per week: Mon;1:00-4:00 Room 3066
8 Enthalpy of Reaction
 E x p e r i m e n t Enthalpy of Reaction Lecture and Lab Skills Emphasized Calculating the heat and enthalpy of reactions. Writing net ionic equations. Using Hess s law to determine the enthalpy of a reaction.
E x p e r i m e n t Enthalpy of Reaction Lecture and Lab Skills Emphasized Calculating the heat and enthalpy of reactions. Writing net ionic equations. Using Hess s law to determine the enthalpy of a reaction.
SAVE THIS SYLLABUS FOR REFERENCE DURING THE SEMESTER.
 SYLLABUS Course: General Chemistry I: (call #16279) Lecture: 9:00-10:25AM Mon.-Wed.; Room 6068 Recitation: 1 hour per week: Mon.; 12:00-1:00 Room 3066 Laboratory: 3 hours per week: Mon.; 1:00-4:00 Room
SYLLABUS Course: General Chemistry I: (call #16279) Lecture: 9:00-10:25AM Mon.-Wed.; Room 6068 Recitation: 1 hour per week: Mon.; 12:00-1:00 Room 3066 Laboratory: 3 hours per week: Mon.; 1:00-4:00 Room
Objective: SWBAT identify a compound as either ionic, covalent or an acid after reviewing terminology at 85% accuracy.
 Mrs. Chausse s Chemistry Gifted Lesson Plan: Unit 11 Chemical Reactions January 4 18, 2018 Wednesday, January 3, 2018 Naming and writing formulas Objective: SWBAT identify a compound as either ionic, covalent
Mrs. Chausse s Chemistry Gifted Lesson Plan: Unit 11 Chemical Reactions January 4 18, 2018 Wednesday, January 3, 2018 Naming and writing formulas Objective: SWBAT identify a compound as either ionic, covalent
Enthalpy. Slide 1. Slide 2 Reaction Energies. Slide 3 Enthalpy of Reactions. Calorimetry of Chemistry
 Slide 1 Enthalpy Calorimetry of Chemistry Slide 2 Reaction Energies In our earlier discussions of calorimetry, we used physical sources of heat (hot metal slug). It is also possible to use chemical sources
Slide 1 Enthalpy Calorimetry of Chemistry Slide 2 Reaction Energies In our earlier discussions of calorimetry, we used physical sources of heat (hot metal slug). It is also possible to use chemical sources
Calorimetry. Enthalpy of Neutralization
 Calorimetry Enthalpy of Neutralization Introduction A calorimeter is a device that can measure the heat absorbed or released by a reaction (Petrucci, 2011). A calorimeter is thermally insulated from its
Calorimetry Enthalpy of Neutralization Introduction A calorimeter is a device that can measure the heat absorbed or released by a reaction (Petrucci, 2011). A calorimeter is thermally insulated from its
Chemical Thermodynamics. Chemical Thermodynamics. Changes of State. Chemical Thermodynamics. State Functions. State Functions 11/25/13
 Chemical Thermodynamics n Thermodynamics is the study of the energetics and order of a system. n A system is the thing we want to study it can be a chemical reaction, a solution, an automobile, or the
Chemical Thermodynamics n Thermodynamics is the study of the energetics and order of a system. n A system is the thing we want to study it can be a chemical reaction, a solution, an automobile, or the
For the reaction: A B R f = R r. Chemical Equilibrium Chapter The Concept of Equilibrium. The Concept of Equilibrium
 Chemical Equilibrium Chapter 15.1-4 This is the last unit of the year, and it contains quite a lot of material. Do not wait until the end of the unit to begin studying. Use what you have learned about
Chemical Equilibrium Chapter 15.1-4 This is the last unit of the year, and it contains quite a lot of material. Do not wait until the end of the unit to begin studying. Use what you have learned about
Limiting Reactants How do you know if there will be enough of each chemical to make your desired product?
 Limiting Reactants How do you know if there will be enough of each chemical to make your desired product? Why? If a factory runs out of tires while manufacturing a car, all production stops. No more cars
Limiting Reactants How do you know if there will be enough of each chemical to make your desired product? Why? If a factory runs out of tires while manufacturing a car, all production stops. No more cars
Summary of Gas Laws V T. Boyle s Law (T and n constant) Charles Law (p and n constant) Combined Gas Law (n constant) 1 =
 Summary of Gas Laws Boyle s Law (T and n constant) p 1 V 1 = p 2 V 2 Charles Law (p and n constant) V 1 = T 1 V T 2 2 Combined Gas Law (n constant) pv 1 T 1 1 = pv 2 T 2 2 1 Ideal Gas Equation pv = nrt
Summary of Gas Laws Boyle s Law (T and n constant) p 1 V 1 = p 2 V 2 Charles Law (p and n constant) V 1 = T 1 V T 2 2 Combined Gas Law (n constant) pv 1 T 1 1 = pv 2 T 2 2 1 Ideal Gas Equation pv = nrt
MONDAY MORNING. Prior Knowledge and Fundamental Concepts Pre-AP Expectations AP Chemistry in the science sequence
 MONDAY MORNING Introduction to AP Chemistry AP Chemistry Syllabus and Redesign o Why the redesign? o What s new? What s out? Breadth and depth What is assessed. MC and FRQ on the exam. National Scoring
MONDAY MORNING Introduction to AP Chemistry AP Chemistry Syllabus and Redesign o Why the redesign? o What s new? What s out? Breadth and depth What is assessed. MC and FRQ on the exam. National Scoring
Lecture 10. What is energy? Professor Hicks Inorganic Chemistry (CHE151) Ability to do work. Work means moving something against a force
 Lecture 10 Professor Hicks Inorganic Chemistry (CHE151) Ability to do work What is energy? Work means moving something against a force Energy thought of as an imaginary liquid that gets moved from one
Lecture 10 Professor Hicks Inorganic Chemistry (CHE151) Ability to do work What is energy? Work means moving something against a force Energy thought of as an imaginary liquid that gets moved from one
EXAM III Nov. 1, 2018
 EM 105 Dr. Lammi Name: EXAM III Nov. 1, 2018 You may work until 10:50 to complete this exam. Please show all work in the space provided or on the attached scratch page. Remember to report your final answers
EM 105 Dr. Lammi Name: EXAM III Nov. 1, 2018 You may work until 10:50 to complete this exam. Please show all work in the space provided or on the attached scratch page. Remember to report your final answers
Chapter 8 Thermochemistry
 William L Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 8 Thermochemistry Edward J. Neth University of Connecticut Outline 1. Principles of heat flow 2. Measurement
William L Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 8 Thermochemistry Edward J. Neth University of Connecticut Outline 1. Principles of heat flow 2. Measurement
Adiabatic Expansion/Compression
 Adiabatic Expansion/Compression Calculate the cooling in a the reversible adiabatic expansion of an ideal gas. P P 1, 1, T 1 A du q w First Law: Since the process is adiabatic, q = 0. Also w = -p ex d
Adiabatic Expansion/Compression Calculate the cooling in a the reversible adiabatic expansion of an ideal gas. P P 1, 1, T 1 A du q w First Law: Since the process is adiabatic, q = 0. Also w = -p ex d
9/18/2013. Scientists represent atoms by using different colored circles, called a model.
 Pre-Lab Notes Lab Title: Behavior of Gases: Molar Mass of a Vapor Purpose: To determine the molar mass of a gas from a knowledge of its mass, temperature, pressure, and volume. Each element is unique.
Pre-Lab Notes Lab Title: Behavior of Gases: Molar Mass of a Vapor Purpose: To determine the molar mass of a gas from a knowledge of its mass, temperature, pressure, and volume. Each element is unique.
DO NOT OPEN UNTIL INSTRUCTED TO DO SO. CHEM 110 Dr. McCorkle Exam #3. While you wait, please complete the following information:
 DO NOT OPEN UNTIL INSTRUCTED TO DO SO CHEM 110 Dr. McCorkle Exam #3 While you wait, please complete the following information: Name: Student ID: Turn off cellphones and stow them away. No headphones, mp3
DO NOT OPEN UNTIL INSTRUCTED TO DO SO CHEM 110 Dr. McCorkle Exam #3 While you wait, please complete the following information: Name: Student ID: Turn off cellphones and stow them away. No headphones, mp3
This reaction is ENDOTHERMIC. Energy is being transferred from the room/flask/etc. (the SURROUNDINGS) to the reaction itself (the SYSTEM).
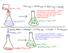 151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
He measures the steady temperature of the water before adding the lithium iodide.
 1 A student uses this apparatus to measure the temperature change when lithium iodide dissolves in water. 100 g of water He measures the steady temperature of the water before adding the lithium iodide.
1 A student uses this apparatus to measure the temperature change when lithium iodide dissolves in water. 100 g of water He measures the steady temperature of the water before adding the lithium iodide.
1. What is the value of the quantity PV for one mole of an ideal gas at 25.0 C and one atm?
 Real Gases Thought Question: How does the volume of one mole of methane gas (CH4) at 300 Torr and 298 K compare to the volume of one mole of an ideal gas at 300 Torr and 298 K? a) the volume of methane
Real Gases Thought Question: How does the volume of one mole of methane gas (CH4) at 300 Torr and 298 K compare to the volume of one mole of an ideal gas at 300 Torr and 298 K? a) the volume of methane
Red Sox - Yankees. Baseball can not get more exciting than these games. Physics 121, April 17, Kinetic theory of gases.
 Red Sox - Yankees. Baseball can not get more exciting than these games. Physics 121, April 17, 2008. Kinetic theory of gases. http://eml.ou.edu/physics/module/thermal/ketcher/idg4.avi Physics 121. April
Red Sox - Yankees. Baseball can not get more exciting than these games. Physics 121, April 17, 2008. Kinetic theory of gases. http://eml.ou.edu/physics/module/thermal/ketcher/idg4.avi Physics 121. April
Energy, Heat and Chemical Change
 Energy, Heat and Chemical Change Chemistry 35 Fall 2000 Thermochemistry A part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? -will a reaction
Energy, Heat and Chemical Change Chemistry 35 Fall 2000 Thermochemistry A part of Thermodynamics dealing with energy changes associated with physical and chemical reactions Why do we care? -will a reaction
Obj: Describe and predict the relationship b/t P, T, and V with the combined gas law.
 Do Now Date: February 29, 2016 Obj: Describe and predict the relationship b/t P, T, and V with the combined gas law. Solve: A gas at STP occupies 28 ml of space. If the pressure changes to 3.8 atm and
Do Now Date: February 29, 2016 Obj: Describe and predict the relationship b/t P, T, and V with the combined gas law. Solve: A gas at STP occupies 28 ml of space. If the pressure changes to 3.8 atm and
Chapter 11. Thermochemistry. 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages
 Chapter 11 Thermochemistry 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages 293-94 The Flow of energy - heat Thermochemistry concerned with the heat changes that occur
Chapter 11 Thermochemistry 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages 293-94 The Flow of energy - heat Thermochemistry concerned with the heat changes that occur
General Chemistry I. Dr. PHAN TẠI HUÂN Faculty of Food Science and Technology Nong Lam University. Module 4: Chemical Thermodynamics
 General Chemistry I Dr. PHAN TẠI HUÂN Faculty of Food Science and Technology Nong Lam University Module 4: Chemical Thermodynamics Zeroth Law of Thermodynamics. First Law of Thermodynamics (state quantities:
General Chemistry I Dr. PHAN TẠI HUÂN Faculty of Food Science and Technology Nong Lam University Module 4: Chemical Thermodynamics Zeroth Law of Thermodynamics. First Law of Thermodynamics (state quantities:
Chapter 17.3 Entropy and Spontaneity Objectives Define entropy and examine its statistical nature Predict the sign of entropy changes for phase
 Chapter 17.3 Entropy and Spontaneity Objectives Define entropy and examine its statistical nature Predict the sign of entropy changes for phase changes Apply the second law of thermodynamics to chemical
Chapter 17.3 Entropy and Spontaneity Objectives Define entropy and examine its statistical nature Predict the sign of entropy changes for phase changes Apply the second law of thermodynamics to chemical
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
