E. Toukoniitty, J. Wärnå, D. Yu. Murzin and T. Salmi. Common in fine chemicals production: Three-phase applications, reactant, solvent (l) -
|
|
|
- Sybil Lane
- 5 years ago
- Views:
Transcription
1 Transient methods in three-phase catalysis E. Toukoniitty, J. Wärnå, D. Yu. Murzin and T. Salmi Three-phase systems Common in fine chemicals production: Three-phase applications, H 2, 2 (g) - reactant, solvent (l) - catalyst (s) Batch- / Semi-batch reactors utilized typically Complex reactions where regio-, diastereo- and enantioselectivity can be involved High selectivity crucial 1
2 Typical reaction scheme H H (I) H (E) H (B) (A) H H H (F) (D) Complex reaction scheme (parallel and consecutive) Pt/Al 2 3 catalyst Toluene solvent 2 C, 1 bar H 2 Main product used for the synthesis of several pharmaceuticals e.g. ephedrine... H H H H H (H) (C) (G) Hydrogenation of 1-phenylpropane-1,2-dione over a Pt/Al 2 3 catalyst Examples of three-phase reactions for fine chemicals production Substrate Catalyst Reference Citral Ni/Si 2 (fibrous) T. Salmi et al. Appl. Catal. A. Gen. 196 (2) 193. Glucose Ru/C (pellets) P. Gallezot et al. J. Catal. 18 (1998) 51 Ethyl pyruvate Pt/Al 2 3 (powder) + CD N. Kunzle et al. J. Catal. 186 (1999) 239 Ketopantolactone 1-Phenyl-1,2- propanedione Pt/ Si 2 (fibrous) E. Toukoniitty et al. 216 (2) 73 2
3 Transient methods Well established tools in mechanistic studies of two-phase reactions (gas-solid) e.g. Temporal Analysis of Products (TAP), Steady State Isotopic Transient Kinetic Analysis (SSITKA) TAP reactor set up (1%D 2 )/Ar (1%H 2 )/Ar Rahkamaa-Tolonen, J. Catal. 21 (22) 17 Transient methods Transient methods are seldom used in three-phase reactions Experimentation and analysis is demanding Transients are introduced into a system by varying e.g. pressure, temperature, concentration etc. Typically step and pulse change experiments ee (%) Time-on-stream (min) 3
4 Setup in transient experiments - batch reactor Required for transient experiments Setup in transient experiments fixed bed reactor 6-way injection valve Pulse or Step change H 2 HPLC pump H 2 d i = 9 mm Catalyst bed H 2,Ar, 2 or liquid MS (gas phase) GC (Liquid phase) H 2, Ar or 2 saturation 4
5 Setup in transient experiments - product analysis n-line methods (MS, IR, UV-VIS, etc.) In the presence of liquid-phase and complex product mixtures containing e.g. mixtures of enantiomers conventional on-line methods cannot be utilized Practical solution Small liquid-phase samples are collected and analyzed off-line with e.g. GC, HPLC, etc. Analysis can be a bottle neck and a rate limiting factor in threephase transient experiments Transient experiments in a batch reactor Step change in modifier concentration/composition during reaction Successfully applied in investigations of nonlinear behavior of binary modifier mixtures Practical limitations due to slow transient responses and short reaction times V L changes, however, often ΔV L is small and has insignificant influence on the results 5
6 Transient experiments in a fixed bed reactor Step change and pulse experiments More versatile compared to batch reactor: Reaction time does not limit the experiments in case of slow responses Change of solvent, reactant, concentration, etc. possible More demanding experimentation compared to batch reactor (kinetic regime, flow conditions) Enantiomers Mirror plane H H (R)-enantiomer H H (S)-enantiomer Non-superimposible mirror image structures e.g. left and right hands 6
7 Enantiomers (R)- and (S)- enantiomers behave differently in the human body (chiral environment) Enantiopure chemicals play a central role in the areas of Pharmaceuticals Agrochemicals Fragrances Living organisms Asymmetric heterogeneous catalysis The demand for enantiopure chemicals is increasing and technically simple and inexpensive production methods are needed. Chirally modified metals (Ni, Pt, Pd ) easy catalyst separation and handling continuous operation inexpensive Research aims to increased mechanistic understanding 7
8 Asymmetric heterogeneous catalysis over cinchona alkaloid modified Pt catalysts Cinchona alkaloid modified Pt catalyst were discovered by rito et al for hydrogenation of α-keto esters Trace amounts of modifier (monolayer) can induce high enantioselectivity (ee=98%) and up to 1-fold rate acceleration Extensively studied in batch reactors N H H N Cinchonidine (CD) Catalyst modifier anchoring part quinuclidine N atom chirality N H H N Cinchonidine (CD) 8
9 Principle of a chirally modified catalyst Unmodified catalyst: 5% (R) and 5% (S) (R)-enantiomer product (S)-enantiomer product + H 2 + H 2 ee = Reactant pro-r Pt particle [ R] [ S] [ R] + [ S] 1 % = % Reactant pro-s Principle of a chirally modified catalyst (R)- enantiomer product Modified catalyst: 9% (R) and 1% (S) 1-to-11 reactant- modifier interaction (S)-enantiomer product Modifier Modifier ee = Reactant [ R] [ S] [ R] + [ S] 1 % = 8% Pt particle Reactant 9
10 Catalysts D = 27% d Pt = 4 nm Structure sensitive reactions Relatively large metal particles are needed for optimum selectivity and reaction rate (~ 4 nm) 1 mm μm particles 4 nm D = 4% d Pt = 2.5 nm SEM image of the 5 wt.% Pt/Si 2 fiber catalyst TEM image of the 5 wt.% Pt/Al 2 3 (Strem Chemicals) catalyst Mechanistic questions Is there any deactivation? Catalyst regeneration and prevention of deactivation? Mechanism of nonlinear behavior of binary modifier mixtures? Mechanism of ligand acceleration? Can one increase selectivity under transient conditions? 1
11 Catalyst deactivation.3.25 M (racemic) 1 Possible catalyst deactivation difficult to distinguish.25 8 Ethyl pyruvate c (mol/l) EP (R )-EL (S )-EL 6 4 ee(%) 2.5 ee Time (min) + H 2 Typical batch reactor kinetics. H Conditions: c (EP).25 mol L -1, c (CD)= mol L -1, 5 mg 5 wt.% Pt/Al 2 catalyst, toluene, 15 o C, 1 bar H 2 (R)-Ethyl lactate Catalyst deactivation 1 ee or conversion (%) 8 Conversion Enantiomeric excess (ee) Time-on-stream (min) Identical conditions as in batch reactor => Relatively rapid catalyst deactivation noticeable Conditions: c (EP).25 mol L -1, c (CD)= mol L -1, 25 mg 5 wt.% Pt/Al 2 3 catalyst, toluene, 15 o C, 1 bar H 2, V L 3. ml min -1, V H ml min -1 11
12 Step change: c(modifier) Appl. Catal. 235 (22) 125 ee CD stop CD on 2.5E-2 2.E-2 1.5E-2 1.E-2 5.E-3 Hydrogen uptake (mol dm -3 ) No rate acceleration Slow decrease of ee Time-on-stream (min).e+ 5%Pt/Si 2 fiber, T=25 o C, Toluene, c DINE =.25M Pulse of 2 Catal. Lett. 93 (24) 171 Increase of ee and rate Pulse of 2 at 4 and 8 min C (Ads) +½ 2 -> C 2 12
13 Change of solvent toluene -> acetic acid acetic acid -> toluene 1 Enantiomeric excess (%) Toluene Acetic acid Change of solvent during reaction Toluene Acetic acid Time-on-stream (min) c(modifier inlet) liquid flow rate Instantaneous drop of ee (R)-1 Increased selectivity under transient operation ee increased under transient operation conversion decreased 13
14 rigin ligand acceleration (LA) 5-5 fold higher TF over modified site due to reactantmodifier interactions => ligand acceleration ver 1 articles state that LA is an intrinsic kinetic feature The LA concept is included in all previous kinetic models rate (mol L -1 min -1 g -1 ) LA c(ep) / mol L ee (%) Batch reactor, 1 bar H 2 15 C Journal of Catalysis 241 (26) 96 rigin of ligand acceleration Increased activity Reduced deactivation LA requires a times higher TF which is induced by reactant-modifier modifier- interactions Modifier prevents deactivation and maintains high initial activity Journal of Catalysis 241 (26) 96 14
15 rigin of ligand acceleration Increased activity Reduced deactivation LA requires a times higher TF which is induced by reactant-modifier modifier- interactions Modifier prevents deactivation and maintains initial activity Journal of Catalysis 241 (26) 96 Transient experiment 1. Conversion of EP M.25 M.5 M Racemic Enantioselective The deactivation rate proportional to reactant inlet concentration Modifier restores initial activity and prevents deactivation Time-on-stream (min) Fixed bed reactor, 1 bar H 2 15 C Steady-state and transient experiments revealed that ligand acceleration originates from reduced catalyst deactivation!!! Journal of Catalysis 241 (26) 96 15
16 Kinetic modeling Journal of Catalysis. 213 (23) 7 Non-steady state and steady state kinetic models were tested Parallel and tilted adsorption modes for modifier Different number of adsorption sites for reactant (two adsorption modes) and modifier (two adsorption modes). θ T Tilted Parallel θ P Mechanism 16
17 Mechanism steps 1 and 2 : adsorption of the reactant (A) A1m and A2n : the adsorption modes of 1- and 2- carbonyls n, m the number of sites required for adsorption of them CH 3 Mechanism steps 3 and 4: adsorption of the modifier Mp : the parallel adsorption mode of the cinchonidine,, interacts with An or Am. Mq : the tilted adsorption mode; a spectator. 17
18 Mechanism Racemic hydrogenation on unmodified sites Enantioselective hydrogenation to (R) Some hydrogenation to (S) may take place on the modified sites Kinetic model Impossible to obtain explicit rate expressions, but fractional coverages are solved numerically by Newton s method c A (mk 1 (1+ k 7 /k 8 )c (m-1) Θ m + nk 2 (1+ k 9 /k 8 )c (n-1) Θ n ) + c M (pk 3 c (p-1) Θ p + qk 4 c (q-1) Θ q ) + (m+p+f)k 1 K 3 K 5 (1+k 11 /k 12 )c A c M c (m+p+f-1) + (n+p+l)k 2 K 3 K 6 (1+ k 13 /k 14 )c A c M c (n+p+l-1) + Θ = 1 18
19 Rates Mass balances Kinetic modelling :slurry mod_1.txt.1 c (mol dm -3 ) A B time Time (min) C D + E Response simulation (Dump file) 19
20 Kinetic modelling: parameters E.Toukoniitty, B. Sevcikova, P. Mäki-Arvela, J. Wärnå, T.Salmi, D.Yu. Murzin, Journal of Catalysis, 23, 213, 7 Reactor modeling dc r Dynamic axial dispersion model for i dt continuous fixed bed reactor Peclet number from impulse experiments with an inert tracer 2 1 d cli = ρ B + ( PeLε Lτ L ) ( ε Lτ L ) 2 dz Li 1 Injection 2 μl (H 2 + NaCl) H 2 dcli dz H 2 response 2
21 Transient modelling :dione kinetic parameters from batch non-steady adsorption (dynamics) C B. B time. C Catalysis Today 79-8 (23) time Problems with by- porducts description More recent modelling x C C c (mol/l) c (mol/l) time (min) 5 x C time (min) 3 C c (mol/l) c (mol/l) time (min) time (min) 21
22 Conclusions Three-phase pulse and step change experiments are technically possible and give reproducible results Continuous operation and transient experiments give valuable mechanistic information about catalyst regeneration and deactivation mechanisms Suitable for investigation of solvent effects Ideal for studying competitive adsorption Transient operation can also increase selectivity Conclusions Transient three-phase experiments give valuable information about reaction mechanisms and catalyst deactivation, which cannot be obtained from steady state experiments 22
Multiscale modelling: from surface chemistry to reactors
 Multiscale modelling: from surface chemistry to reactors T.Salmi, E. Toukoniitty, J. Wärnå, A. Taskinen, D. Yu. Murzin Laboratory of Industrial Chemistry and Reaction Engineering, Johan Gadolin Process
Multiscale modelling: from surface chemistry to reactors T.Salmi, E. Toukoniitty, J. Wärnå, A. Taskinen, D. Yu. Murzin Laboratory of Industrial Chemistry and Reaction Engineering, Johan Gadolin Process
Transient modelling and catalyst deactivation in reaction engineering
 Transient modelling and catalyst deactivation in reaction engineering Tapio Salmi, Dmitry Murzin, Johan Wärnå, Esa Toukoniitty, Fredrik Sandelin Åbo Akademi utline Modelling of transient experiments Why
Transient modelling and catalyst deactivation in reaction engineering Tapio Salmi, Dmitry Murzin, Johan Wärnå, Esa Toukoniitty, Fredrik Sandelin Åbo Akademi utline Modelling of transient experiments Why
Aspects on reaction intensification
 Aspects on reaction intensification Tapio Salmi Åbo AkademiLaboratory of Industrial Chemistry and Reaction Engineering FI-20500 Turku / Åbo Finland Process intensification Raw material Process Products
Aspects on reaction intensification Tapio Salmi Åbo AkademiLaboratory of Industrial Chemistry and Reaction Engineering FI-20500 Turku / Åbo Finland Process intensification Raw material Process Products
A Compact Reactor-Pump-Cell-Injection System for In-Situ / On- Line Spectroscopic Studies
 A Compact Reactor-Pump-Cell-Injection System for In-Situ / On- Line Spectroscopic Studies Feng Gao, Li Chuanzhao and Marc Garland Department of Chemical and Biomolecular Engineering 4 Engineering Drive
A Compact Reactor-Pump-Cell-Injection System for In-Situ / On- Line Spectroscopic Studies Feng Gao, Li Chuanzhao and Marc Garland Department of Chemical and Biomolecular Engineering 4 Engineering Drive
Part A: Operando FT-IR Studies of heterogeneous catalytic reactions: pitfalls and benefits.
 Part A: Operando FT-IR Studies of heterogeneous catalytic reactions: pitfalls and benefits. Fred Meunier fcm@ircelyon.univ-lyon1.fr Institut de Recherche sur la Catalyse et l Environnement de Lyon Villeurbanne,
Part A: Operando FT-IR Studies of heterogeneous catalytic reactions: pitfalls and benefits. Fred Meunier fcm@ircelyon.univ-lyon1.fr Institut de Recherche sur la Catalyse et l Environnement de Lyon Villeurbanne,
Heterogeneous chiral catalysis on surfaces, in nanopores and with emulsions
 Prof. Can Li's Laboratory Heterogeneous chiral catalysis on surfaces, in nanopores and with emulsions Chiral catalysis is of great industrial interest for the production of enantiomerically pure compounds.
Prof. Can Li's Laboratory Heterogeneous chiral catalysis on surfaces, in nanopores and with emulsions Chiral catalysis is of great industrial interest for the production of enantiomerically pure compounds.
Aqueous-phase reforming a pathway to chemicals and fuels
 Alexey Kirilin Aqueous-phase reforming a pathway to chemicals and fuels Laboratory of Industrial Chemistry and Reaction Engineering Process Chemistry Centre Åbo Akademi Agenda 2 Short Introduction, biomass,
Alexey Kirilin Aqueous-phase reforming a pathway to chemicals and fuels Laboratory of Industrial Chemistry and Reaction Engineering Process Chemistry Centre Åbo Akademi Agenda 2 Short Introduction, biomass,
BAE 820 Physical Principles of Environmental Systems
 BAE 820 Physical Principles of Environmental Systems Catalysis of environmental reactions Dr. Zifei Liu Catalysis and catalysts Catalysis is the increase in the rate of a chemical reaction due to the participation
BAE 820 Physical Principles of Environmental Systems Catalysis of environmental reactions Dr. Zifei Liu Catalysis and catalysts Catalysis is the increase in the rate of a chemical reaction due to the participation
Bimetallic ruthenium-copper nanoparticles embedded in. mesoporous carbon as an effective hydrogenation catalyst
 Supporting Information Bimetallic ruthenium-copper nanoparticles embedded in mesoporous carbon as an effective hydrogenation catalyst Jiajia Liu, *a Li Li Zhang, b Jiatao Zhang, a Tao Liu, c and X. S.
Supporting Information Bimetallic ruthenium-copper nanoparticles embedded in mesoporous carbon as an effective hydrogenation catalyst Jiajia Liu, *a Li Li Zhang, b Jiatao Zhang, a Tao Liu, c and X. S.
Ionic liquid-supported Pt nanoparticles as catalysts for enantioselective hydrogenation
 Supporting information Ionic liquid-supported Pt nanoparticles as catalysts for enantioselective hydrogenation Matthias Josef Beier, Jean-Michel Andanson, Tamas Mallat, Frank Krumeich and Alfons Baiker*
Supporting information Ionic liquid-supported Pt nanoparticles as catalysts for enantioselective hydrogenation Matthias Josef Beier, Jean-Michel Andanson, Tamas Mallat, Frank Krumeich and Alfons Baiker*
achiral amine additives Conversion
 Enantioselective hydrogenation of activated ketones in the presence of Pt-Cinchona catalysts. Is the proton transfer concept valid? József L. Margitfalvi, Emília Tálas Catalysis Communications 46 (2014),
Enantioselective hydrogenation of activated ketones in the presence of Pt-Cinchona catalysts. Is the proton transfer concept valid? József L. Margitfalvi, Emília Tálas Catalysis Communications 46 (2014),
A New Model for Asymmetric Amplification in Amino Acid Catalysis - Supporting information
 A New Model for Asymmetric Amplification in Amino Acid Catalysis - Supporting information Martin Klussmann, Hiroshi Iwamura, Suju P. Mathew, David H. Wells, Urvish Pandya, Alan Armstrong and Donna G. Blackmond
A New Model for Asymmetric Amplification in Amino Acid Catalysis - Supporting information Martin Klussmann, Hiroshi Iwamura, Suju P. Mathew, David H. Wells, Urvish Pandya, Alan Armstrong and Donna G. Blackmond
Topical Workshop MOF Catalysis. Microkinetics in Heterogeneous Catalysis. DFG Priority Program 1362
 Topical Workshop MOF Catalysis Microkinetics in Heterogeneous Catalysis DFG Priority Program 1362 Roger Gläser Institut für Technische Chemie Institut für Nichtklassische Chemie e.v. Universität Leipzig
Topical Workshop MOF Catalysis Microkinetics in Heterogeneous Catalysis DFG Priority Program 1362 Roger Gläser Institut für Technische Chemie Institut für Nichtklassische Chemie e.v. Universität Leipzig
Enantioselective Transamination in Continuous Flow Mode with Transaminase Immobilized in a Macrocellular Silica Monolith. Supporting Information
 Enantioselective Transamination in Continuous Flow Mode with Transaminase Immobilized in a Macrocellular Silica Monolith Ludivine van den Biggelaar 1, Patrice Soumillion 2 and Damien P. Debecker 1, * 1
Enantioselective Transamination in Continuous Flow Mode with Transaminase Immobilized in a Macrocellular Silica Monolith Ludivine van den Biggelaar 1, Patrice Soumillion 2 and Damien P. Debecker 1, * 1
Effects of Different Processing Parameters on Divinylbenzene (DVB) Production Rate
 1 Effects of Different Processing Parameters on Divinylbenzene (DVB) Production Rate ME Zeynali Petrochemical Synthesis Group, Petrochemical Faculty, Iran Polymer and Petrochemical Institute (IPPI), P.O.
1 Effects of Different Processing Parameters on Divinylbenzene (DVB) Production Rate ME Zeynali Petrochemical Synthesis Group, Petrochemical Faculty, Iran Polymer and Petrochemical Institute (IPPI), P.O.
Glycerol conversion to 1,2-propanediol in mild conditions
 Glycerol conversion to 1,2-propanediol in mild conditions Els D Hondt CK, KULeuven, Belgium Poitiers, March 13, 2008 Content Introduction Use of 1,2-propanediol Results with better literature catalysts
Glycerol conversion to 1,2-propanediol in mild conditions Els D Hondt CK, KULeuven, Belgium Poitiers, March 13, 2008 Content Introduction Use of 1,2-propanediol Results with better literature catalysts
Wilkinson s other (ruthenium) catalyst
 Wilkinson s other (ruthenium) catalyst Cl 3 ; 2 h 3, reflux 3h h 3 Cl h 3 h Cl 3 Good catalyst especially for 2 1-alkenes 2, base toluene Cl h 3 h 3 h 3 Et 3 Cl h 3 Cl h 3 h 3 R h 3 h 3 Cl h 3 R RC 2 C
Wilkinson s other (ruthenium) catalyst Cl 3 ; 2 h 3, reflux 3h h 3 Cl h 3 h Cl 3 Good catalyst especially for 2 1-alkenes 2, base toluene Cl h 3 h 3 h 3 Et 3 Cl h 3 Cl h 3 h 3 R h 3 h 3 Cl h 3 R RC 2 C
Asymmetric Transfer Hydrogenation: A Suitable Tool for the Synthesis of the Precursors of Pharmaceutical Substances
 Department of Organic Technology Specialized Laboratory for Drug Production programme (N111049) and Organic Technology programme (N111025) Asymmetric Transfer Hydrogenation: A Suitable Tool for the Synthesis
Department of Organic Technology Specialized Laboratory for Drug Production programme (N111049) and Organic Technology programme (N111025) Asymmetric Transfer Hydrogenation: A Suitable Tool for the Synthesis
A Thermoregulated Phase-Separable Chiral Pt Nanocatalyst for Recyclable Asymmetric Hydrogenation of α-ketoesters
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information (ESI) for A Thermoregulated Phase-Separable Chiral Pt Nanocatalyst
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information (ESI) for A Thermoregulated Phase-Separable Chiral Pt Nanocatalyst
University of Szeged Doctoral School of Pharmaceutical Sciences. Pharmaceutical Chemistry and Drug Research
 University of Szeged Doctoral School of Pharmaceutical Sciences Pharmaceutical Chemistry and Drug Research Programme director: Prof. Dr. Ferenc Fülöp Institute of Pharmaceutical Chemistry Lenke Kovács
University of Szeged Doctoral School of Pharmaceutical Sciences Pharmaceutical Chemistry and Drug Research Programme director: Prof. Dr. Ferenc Fülöp Institute of Pharmaceutical Chemistry Lenke Kovács
5. Collection and Analysis of. Rate Data
 5. Collection and nalysis of o Objectives Rate Data - Determine the reaction order and specific reaction rate from experimental data obtained from either batch or flow reactors - Describe how to analyze
5. Collection and nalysis of o Objectives Rate Data - Determine the reaction order and specific reaction rate from experimental data obtained from either batch or flow reactors - Describe how to analyze
CHE 611 Advanced Chemical Reaction Engineering
 CHE 611 Advanced Chemical Reaction Engineering Dr. Muhammad Rashid Usman Institute of Chemical Engineering and Technology University of the Punjab, Lahore 54590 mrusman.icet@pu.edu.pk 1 Course contents
CHE 611 Advanced Chemical Reaction Engineering Dr. Muhammad Rashid Usman Institute of Chemical Engineering and Technology University of the Punjab, Lahore 54590 mrusman.icet@pu.edu.pk 1 Course contents
Direct Synthesis of H 2 O 2 on AgPt Octahedra: The Importance of Ag-Pt Coordination for High H 2 O 2 Selectivity
 Supporting Information Direct Synthesis of H 2 O 2 on AgPt Octahedra: The Importance of Ag-Pt Coordination for High H 2 O 2 Selectivity Neil M. Wilson, 1 Yung-Tin Pan, 1 Yu-Tsun Shao, 2 Jian-Min Zuo, 2
Supporting Information Direct Synthesis of H 2 O 2 on AgPt Octahedra: The Importance of Ag-Pt Coordination for High H 2 O 2 Selectivity Neil M. Wilson, 1 Yung-Tin Pan, 1 Yu-Tsun Shao, 2 Jian-Min Zuo, 2
Shi Asymmetric Epoxidation
 Shi Asymmetric Epoxidation Chiral dioxirane strategy: R 3 + 1 xone, ph 10.5, K 2 C 3, H 2, C R 3 formed in situ catalyst (10-20 mol%) is prepared from D-fructose, and its enantiomer from L-sorbose oxone,
Shi Asymmetric Epoxidation Chiral dioxirane strategy: R 3 + 1 xone, ph 10.5, K 2 C 3, H 2, C R 3 formed in situ catalyst (10-20 mol%) is prepared from D-fructose, and its enantiomer from L-sorbose oxone,
Homogeneous Catalysis - B. List
 omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
Continuous flow nanocatalysis: reaction pathways in the conversion of levulinic acid to valuable chemicals
 Electronic Supplementary Material (ESI) for Green Chemistry ESI Continuous flow nanocatalysis: reaction pathways in the conversion of levulinic acid to valuable chemicals Jose M. Bermudez, J. Angel Menéndez,
Electronic Supplementary Material (ESI) for Green Chemistry ESI Continuous flow nanocatalysis: reaction pathways in the conversion of levulinic acid to valuable chemicals Jose M. Bermudez, J. Angel Menéndez,
Synthesis of jet fuel range cycloalkanes with diacetone alcohol. from lignocellulose
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Supporting Information Synthesis of jet fuel range cycloalkanes with diacetone alcohol from
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Supporting Information Synthesis of jet fuel range cycloalkanes with diacetone alcohol from
1. Introduction. Olatunde Jogunola 1,*, Tapio Salmi 1, Johan Wärnå 1, Sébastien Leveneur 1,2, Jyri-Pekka Mikkola 1,3
 Modelling of Simultaneous Reaction and Diffusion in hemical Reactors with Particle Size Distributions: Application of Ion-exchange Resins in Heterogeneous atalysis Olatunde Jogunola,*, Tapio Salmi, Johan
Modelling of Simultaneous Reaction and Diffusion in hemical Reactors with Particle Size Distributions: Application of Ion-exchange Resins in Heterogeneous atalysis Olatunde Jogunola,*, Tapio Salmi, Johan
Supplementary Figure 1. Schematic layout of set-up for operando NMR studies.
 Supplementary Figure 1. Schematic layout of set-up for operando NMR studies. Supplementary Figure 2. Correlations between different ratios of D2O/H2O and 1 H chemical shifts of HDO. The spectra were acquired
Supplementary Figure 1. Schematic layout of set-up for operando NMR studies. Supplementary Figure 2. Correlations between different ratios of D2O/H2O and 1 H chemical shifts of HDO. The spectra were acquired
Abstract Process Economics Program Report 232 CHIRAL INTERMEDIATES (March 2001)
 Abstract Process Economics Program Report 232 CHIRAL INTERMEDIATES (March 2001) Chiral chemicals are a unique class of compounds that, although chemically identical, exist as mirror images of each other
Abstract Process Economics Program Report 232 CHIRAL INTERMEDIATES (March 2001) Chiral chemicals are a unique class of compounds that, although chemically identical, exist as mirror images of each other
Engineering and. Tapio Salmi Abo Akademi Abo-Turku, Finland. Jyri-Pekka Mikkola. Umea University, Umea, Sweden. Johan Warna.
 Chemical Reaction Engineering and Reactor Technology Tapio Salmi Abo Akademi Abo-Turku, Finland Jyri-Pekka Mikkola Umea University, Umea, Sweden Johan Warna Abo Akademi Abo-Turku, Finland CRC Press is
Chemical Reaction Engineering and Reactor Technology Tapio Salmi Abo Akademi Abo-Turku, Finland Jyri-Pekka Mikkola Umea University, Umea, Sweden Johan Warna Abo Akademi Abo-Turku, Finland CRC Press is
FHI Lecture Series Modern Methods in Heterogeneous Catalysis: Transient Infrared Spectroscopy
 FHI Lecture Series Modern Methods in Heterogeneous Catalysis: Transient Infrared Spectroscopy Prof. Guido Mul University of Twente Thanks to : Dr. Gerben Hamminga (now BASF) Dr. Dirk Renckens (now ASML)
FHI Lecture Series Modern Methods in Heterogeneous Catalysis: Transient Infrared Spectroscopy Prof. Guido Mul University of Twente Thanks to : Dr. Gerben Hamminga (now BASF) Dr. Dirk Renckens (now ASML)
CHAPTER 5 SYNTHESIS OF 7-HYDROXY-4-METHYLCOUMARIN OVER ZAPO-5 AND LEWIS ACID METAL ION-EXCHANGED ZAPO-5 MOLECULAR SIEVES
 79 CHAPTER 5 SYNTHESIS F 7-HYDRXY-4-METHYLCUMARIN VER ZAP-5 AND LEWIS ACID METAL IN-EXCHANGED ZAP-5 MLECULAR SIEVES 5.1 INTRDUCTIN Coumarins are an important group of naturally occurring compounds widely
79 CHAPTER 5 SYNTHESIS F 7-HYDRXY-4-METHYLCUMARIN VER ZAP-5 AND LEWIS ACID METAL IN-EXCHANGED ZAP-5 MLECULAR SIEVES 5.1 INTRDUCTIN Coumarins are an important group of naturally occurring compounds widely
To increase the concentration of product formed in a PFR, what should we do?
 To produce more moles of product per time in a flow reactor system, what can we do? a) Use less catalyst b) Make the reactor bigger c) Make the flow rate through the reactor smaller To increase the concentration
To produce more moles of product per time in a flow reactor system, what can we do? a) Use less catalyst b) Make the reactor bigger c) Make the flow rate through the reactor smaller To increase the concentration
Effect of Ni Loading and Reaction Conditions on Partial Oxidation of Methane to Syngas
 Journal of Natural Gas Chemistry 12(2003)205 209 Effect of Ni Loading and Reaction Conditions on Partial Oxidation of Methane to Syngas Haitao Wang, Zhenhua Li, Shuxun Tian School of Chemical Engineering
Journal of Natural Gas Chemistry 12(2003)205 209 Effect of Ni Loading and Reaction Conditions on Partial Oxidation of Methane to Syngas Haitao Wang, Zhenhua Li, Shuxun Tian School of Chemical Engineering
Chiral Amplification. Literature Talk Fabian Schneider Konstanz, Universität Konstanz
 Chiral Amplification Literature Talk Fabian Schneider Konstanz, 18.10.2017 Overview 1) Motivation 2) The nonlinear Effect in asymmetric catalysis - First encounters - Basic principles - Formalization and
Chiral Amplification Literature Talk Fabian Schneider Konstanz, 18.10.2017 Overview 1) Motivation 2) The nonlinear Effect in asymmetric catalysis - First encounters - Basic principles - Formalization and
FlowCAT a versatile continuous flow reactor system focusing on reactions under elevated pressure and/or involving heterogeneous catalysis.
 1 FlowCAT - a versatile continuous flow reactor system FlowCAT a versatile continuous flow reactor system focusing on reactions under elevated pressure and/or involving heterogeneous catalysis. By Dr Jasbir
1 FlowCAT - a versatile continuous flow reactor system FlowCAT a versatile continuous flow reactor system focusing on reactions under elevated pressure and/or involving heterogeneous catalysis. By Dr Jasbir
Overcoming ammonia synthesis scaling relations with plasma-enabled catalysis
 SUPPLEMENTARY INFORMATION Articles https://doi.org/10.1038/s41929-018-0045-1 In the format provided by the authors and unedited. Overcoming ammonia synthesis scaling relations with plasma-enabled catalysis
SUPPLEMENTARY INFORMATION Articles https://doi.org/10.1038/s41929-018-0045-1 In the format provided by the authors and unedited. Overcoming ammonia synthesis scaling relations with plasma-enabled catalysis
Supporting Information for. Discovery of Multicomponent Heterogeneous Catalysts via Admixture Screening:
 Supporting Information for Discovery of Multicomponent Heterogeneous Catalysts via Admixture Screening: PdBiTe Catalysts for Aerobic xidative Esterification of Primary Alcohols David S. Mannel a, Maaz
Supporting Information for Discovery of Multicomponent Heterogeneous Catalysts via Admixture Screening: PdBiTe Catalysts for Aerobic xidative Esterification of Primary Alcohols David S. Mannel a, Maaz
BAE 820 Physical Principles of Environmental Systems
 BAE 820 Physical Principles of Environmental Systems Acquisition of reaction rate data Dr. Zifei Liu Uncertainties in real world reaction rate data Most interesting reaction systems involves multiple reactions,
BAE 820 Physical Principles of Environmental Systems Acquisition of reaction rate data Dr. Zifei Liu Uncertainties in real world reaction rate data Most interesting reaction systems involves multiple reactions,
Combinatorial Heterogeneous Catalysis
 Combinatorial Heterogeneous Catalysis 650 μm by 650 μm, spaced 100 μm apart Identification of a new blue photoluminescent (PL) composite material, Gd 3 Ga 5 O 12 /SiO 2 Science 13 March 1998: Vol. 279
Combinatorial Heterogeneous Catalysis 650 μm by 650 μm, spaced 100 μm apart Identification of a new blue photoluminescent (PL) composite material, Gd 3 Ga 5 O 12 /SiO 2 Science 13 March 1998: Vol. 279
Steady-State Molecular Diffusion
 Steady-State Molecular Diffusion This part is an application to the general differential equation of mass transfer. The objective is to solve the differential equation of mass transfer under steady state
Steady-State Molecular Diffusion This part is an application to the general differential equation of mass transfer. The objective is to solve the differential equation of mass transfer under steady state
Study on the Selective Hydrogenation of Nitroaromatics to N-aryl hydroxylamines using a Supported Pt nanoparticle Catalyst
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 204 Supporting Information Study on the Selective Hydrogenation of Nitroaromatics
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 204 Supporting Information Study on the Selective Hydrogenation of Nitroaromatics
Supporting Text Synthesis of (2 S ,3 S )-2,3-bis(3-bromophenoxy)butane (3). Synthesis of (2 S ,3 S
 Supporting Text Synthesis of (2S,3S)-2,3-bis(3-bromophenoxy)butane (3). Under N 2 atmosphere and at room temperature, a mixture of 3-bromophenol (0.746 g, 4.3 mmol) and Cs 2 C 3 (2.81 g, 8.6 mmol) in DMS
Supporting Text Synthesis of (2S,3S)-2,3-bis(3-bromophenoxy)butane (3). Under N 2 atmosphere and at room temperature, a mixture of 3-bromophenol (0.746 g, 4.3 mmol) and Cs 2 C 3 (2.81 g, 8.6 mmol) in DMS
ISOC Catalytic Mechanisms and High Pressure, Operando Kinetic Studies of Homogeneous Catalysts
 ISOC 2017 Catalytic Mechanisms and High Pressure, Operando Kinetic Studies of Homogeneous Catalysts Clark Landis Department of Chemistry University of Wisconsin-Madison Clark Landis - Modern Methods...
ISOC 2017 Catalytic Mechanisms and High Pressure, Operando Kinetic Studies of Homogeneous Catalysts Clark Landis Department of Chemistry University of Wisconsin-Madison Clark Landis - Modern Methods...
CHAPTER 4. LIQUID PHASE AEROBIC OXIDATION OF ETHYLBENZENE OVER PrAlPO-5
 106 CHAPTER 4 LIQUID PHASE AEROBIC OXIDATION OF ETHYLBENZENE OVER PrAlPO-5 4.1 INTRODUCTION Selective catalytic oxidation of alkyl aromatics is a viable technology to functionalize saturated and unsaturated
106 CHAPTER 4 LIQUID PHASE AEROBIC OXIDATION OF ETHYLBENZENE OVER PrAlPO-5 4.1 INTRODUCTION Selective catalytic oxidation of alkyl aromatics is a viable technology to functionalize saturated and unsaturated
Chapter 14 Chemical Kinetics
 7/10/003 Chapter 14 Chemical Kinetics 14-1 Rates of Chemical Reactions 14- Reaction Rates and Concentrations 14-3 The Dependence of Concentrations on Time 14-4 Reaction Mechanisms 14-5 Reaction Mechanism
7/10/003 Chapter 14 Chemical Kinetics 14-1 Rates of Chemical Reactions 14- Reaction Rates and Concentrations 14-3 The Dependence of Concentrations on Time 14-4 Reaction Mechanisms 14-5 Reaction Mechanism
Supplementary Figure 2. (a) XRD patterns of the MOF and the simulated Ni-MOF-74
 Supplementary Figure 1. Low-magnification TEM image of Pt-Ni frame @ MOF. The scale bar is 200 nm. Supplementary Figure 2. (a) XRD patterns of the Pt-Ni @ MOF and the simulated Ni-MOF-74 pattern. (b) XRD
Supplementary Figure 1. Low-magnification TEM image of Pt-Ni frame @ MOF. The scale bar is 200 nm. Supplementary Figure 2. (a) XRD patterns of the Pt-Ni @ MOF and the simulated Ni-MOF-74 pattern. (b) XRD
Molybdenum-Catalyzed Asymmetric Allylic Alkylation
 Molybdenum-Catalyzed Asymmetric Allylic Alkylation X MoL n u u * Tommy Bui 9/14/04 Asymmetric Allylic Alkylation from a Synthetic Viewpoint X X M u u * and/or u form a C-C bond with the creation of a new
Molybdenum-Catalyzed Asymmetric Allylic Alkylation X MoL n u u * Tommy Bui 9/14/04 Asymmetric Allylic Alkylation from a Synthetic Viewpoint X X M u u * and/or u form a C-C bond with the creation of a new
Asymmetric Hydrogenation of α, β-unsaturated Carboxylic Acid Over Chiral-Modified Palladium Catalyst
 University of South Carolina Scholar Commons Theses and Dissertations 1-1-2013 Asymmetric Hydrogenation of α, β-unsaturated Carboxylic Acid Over Chiral-Modified Palladium Catalyst Shuai Tan University
University of South Carolina Scholar Commons Theses and Dissertations 1-1-2013 Asymmetric Hydrogenation of α, β-unsaturated Carboxylic Acid Over Chiral-Modified Palladium Catalyst Shuai Tan University
Chapter 14. Principles of Catalysis
 Organometallics Study Meeting 2011/08/28 Kimura Chapter 14. Principles of Catalysis 14. 1. General Principles 14.1.1. Definition of a Catalyst 14.1.2. Energetics of Catalysis 14.1.3. Reaction Coordinate
Organometallics Study Meeting 2011/08/28 Kimura Chapter 14. Principles of Catalysis 14. 1. General Principles 14.1.1. Definition of a Catalyst 14.1.2. Energetics of Catalysis 14.1.3. Reaction Coordinate
Enantioselective Hydrogenation of Ethyl Pyruvate and 1-Phenyl-1,2-propanedione on Catalysts Prepared by Impregnation of Colloidal Platinum on SiO 2
 J. Braz. Chem. Soc., Vol. 21, No. 2, 262-269, 2010. Printed in Brazil - 2010 Sociedade Brasileira de Química 0103-5053 $6.00+0.00 Article Enantioselective Hydrogenation of Ethyl Pyruvate and 1-Phenyl-1,2-propanedione
J. Braz. Chem. Soc., Vol. 21, No. 2, 262-269, 2010. Printed in Brazil - 2010 Sociedade Brasileira de Química 0103-5053 $6.00+0.00 Article Enantioselective Hydrogenation of Ethyl Pyruvate and 1-Phenyl-1,2-propanedione
Chiral Catalysis. Chiral Catalyst. Substrate. Chiral Catalyst
 Chiral Catalysis Chiral (stoichiometric) reagents are a very important class of compound but... eed a stoichiometric quantity of the chiral component Unless it is cheap or recoverable this is not very
Chiral Catalysis Chiral (stoichiometric) reagents are a very important class of compound but... eed a stoichiometric quantity of the chiral component Unless it is cheap or recoverable this is not very
AUTOMOTIVE EXHAUST AFTERTREATMENT
 AUTOMOTIVE EXHAUST AFTERTREATMENT CATALYST FUNDAMENTLS Catalyst in its simplest term is a material that increase the rate (molecules converted by unit time) of a chemical reaction while itself not undergoing
AUTOMOTIVE EXHAUST AFTERTREATMENT CATALYST FUNDAMENTLS Catalyst in its simplest term is a material that increase the rate (molecules converted by unit time) of a chemical reaction while itself not undergoing
SILP (Supported Ionic Liquid Phase) catalysis in Gas-Phase Hydroformylation and Carbonylation
 SIL (Supported Ionic Liquid hase) catalysis in Gas-hase Hydroformylation and Carbonylation Anders Riisager, Rasmus Fehrmann Department of Chemistry, Technical University of Denmark, Denmark Marco Haumann,
SIL (Supported Ionic Liquid hase) catalysis in Gas-hase Hydroformylation and Carbonylation Anders Riisager, Rasmus Fehrmann Department of Chemistry, Technical University of Denmark, Denmark Marco Haumann,
2017 Reaction of cinnamic acid chloride with ammonia to cinnamic acid amide
 217 Reaction of cinnamic acid chloride with ammonia to cinnamic acid amide O O Cl NH 3 NH 2 C 9 H 7 ClO (166.6) (17.) C 9 H 9 NO (147.2) Classification Reaction types and substance classes reaction of
217 Reaction of cinnamic acid chloride with ammonia to cinnamic acid amide O O Cl NH 3 NH 2 C 9 H 7 ClO (166.6) (17.) C 9 H 9 NO (147.2) Classification Reaction types and substance classes reaction of
Catalytic hydrogenation of liquid alkenes with a silica grafted hydride. pincer iridium(iii) complex: Support for a heterogeneous mechanism
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 215 Electronic Supplementary Information for Catalysis Science & Technology Catalytic
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 215 Electronic Supplementary Information for Catalysis Science & Technology Catalytic
Palladium-Catalyzed Asymmetric Benzylic Alkylation Reactions
 Palladium-Catalyzed Asymmetric Benzylic Alkylation Reactions Reporter: Hong-Qiang Shen Checker: Cong Liu Date: 2016/07/12 Masahiro Miura et al. Angew. Chem. Int. Ed. 2016, 55, 6973. Masahiro Miura Osaka
Palladium-Catalyzed Asymmetric Benzylic Alkylation Reactions Reporter: Hong-Qiang Shen Checker: Cong Liu Date: 2016/07/12 Masahiro Miura et al. Angew. Chem. Int. Ed. 2016, 55, 6973. Masahiro Miura Osaka
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) Multi-scale promoting effects
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) Multi-scale promoting effects
Solid-Supported DNA for Asymmetric Synthesis: a Stepping Stone toward Practical Applications
 Solid-Supported DA for Asymmetric Synthesis: a Stepping Stone toward Practical Applications Soyoung Park, * a Keiichi Ikehata, a and iroshi Sugiyama*,a,b,c a Department of Chemistry, Graduate School of
Solid-Supported DA for Asymmetric Synthesis: a Stepping Stone toward Practical Applications Soyoung Park, * a Keiichi Ikehata, a and iroshi Sugiyama*,a,b,c a Department of Chemistry, Graduate School of
APPLICATION OF CHEMICAL KINETICS IN THE HETEROGENEOUS CATALYSIS STUDIES
 ALICATION OF CHEMICAL KINETICS IN THE HETEROGENEOUS CATALYSIS STUDIES L. A. ETROV SABIC Chair in Heterogeneous Catalysis Chemical and Materials Engineering Department College of Engineering, King Abdulaziz
ALICATION OF CHEMICAL KINETICS IN THE HETEROGENEOUS CATALYSIS STUDIES L. A. ETROV SABIC Chair in Heterogeneous Catalysis Chemical and Materials Engineering Department College of Engineering, King Abdulaziz
4023 Synthesis of cyclopentanone-2-carboxylic acid ethyl ester from adipic acid diethyl ester
 NP 4023 Synthesis of cyclopentanone-2-carboxylic acid ethyl ester from adipic acid diethyl ester NaEt C 10 H 18 4 Na C 2 H 6 C 8 H 12 3 (202.2) (23.0) (46.1) (156.2) Classification Reaction types and substance
NP 4023 Synthesis of cyclopentanone-2-carboxylic acid ethyl ester from adipic acid diethyl ester NaEt C 10 H 18 4 Na C 2 H 6 C 8 H 12 3 (202.2) (23.0) (46.1) (156.2) Classification Reaction types and substance
Evaluation of kinetic data. From simple plots to advanced computations
 Evaluation of kinetic data From simple plots to advanced computations Intrinsic kinetics What is intrinsic kinetics? Real kinetics, free from mass and heat transfer effects Kinetics of universal value,
Evaluation of kinetic data From simple plots to advanced computations Intrinsic kinetics What is intrinsic kinetics? Real kinetics, free from mass and heat transfer effects Kinetics of universal value,
Chemical Reactions and Kinetics of the Carbon Monoxide Coupling in the Presence of Hydrogen
 Journal of Natural Gas Chemistry 11(2002)145 150 Chemical Reactions and Kinetics of the Carbon Monoxide Coupling in the Presence of Hydrogen Fandong Meng 1,2, Genhui Xu 1, Zhenhua Li 1, Pa Du 1 1. State
Journal of Natural Gas Chemistry 11(2002)145 150 Chemical Reactions and Kinetics of the Carbon Monoxide Coupling in the Presence of Hydrogen Fandong Meng 1,2, Genhui Xu 1, Zhenhua Li 1, Pa Du 1 1. State
Highly Diastereo- and Enantioselective Organocatalytic Domino Michael/Aldol Reaction of Acyclic 3- Halogeno- 1,2- Diones to α,β- Unsaturated Aldehydes
 Supporting Information for Highly Diastereo- and Enantioselective rganocatalytic Domino Michael/Aldol Reaction of Acyclic 3- Halogeno- 1,2- Diones to α,β- Unsaturated Aldehydes Alice Lefranc 1, Laure Guénée
Supporting Information for Highly Diastereo- and Enantioselective rganocatalytic Domino Michael/Aldol Reaction of Acyclic 3- Halogeno- 1,2- Diones to α,β- Unsaturated Aldehydes Alice Lefranc 1, Laure Guénée
BASIC DESIGN EQUATIONS FOR MULTIPHASE REACTORS
 BASIC DESIGN EQUATIONS FOR MULTIPHASE REACTORS Starting Reference 1. P. A. Ramachandran and R. V. Chaudhari, Three-Phase Catalytic Reactors, Gordon and Breach Publishers, New York, (1983). 2. Nigam, K.D.P.
BASIC DESIGN EQUATIONS FOR MULTIPHASE REACTORS Starting Reference 1. P. A. Ramachandran and R. V. Chaudhari, Three-Phase Catalytic Reactors, Gordon and Breach Publishers, New York, (1983). 2. Nigam, K.D.P.
Supporting information. Enantioselective synthesis of 2-methyl indoline by palladium catalysed asymmetric C(sp 3 )-H activation/cyclisation.
 Supporting information Enantioselective synthesis of 2-methyl indoline by palladium catalysed asymmetric C(sp 3 )-H activation/cyclisation Saithalavi Anas, Alex Cordi and Henri B. Kagan * Institut de Chimie
Supporting information Enantioselective synthesis of 2-methyl indoline by palladium catalysed asymmetric C(sp 3 )-H activation/cyclisation Saithalavi Anas, Alex Cordi and Henri B. Kagan * Institut de Chimie
Lecture # 3, 4 Selecting a Catalyst (Non-Kinetic Parameters), Review of Enzyme Kinetics, Selectivity, ph and Temperature Effects
 1.492 - Integrated Chemical Engineering (ICE Topics: Biocatalysis MIT Chemical Engineering Department Instructor: Professor Kristala Prather Fall 24 Lecture # 3, 4 Selecting a Catalyst (Non-Kinetic Parameters,
1.492 - Integrated Chemical Engineering (ICE Topics: Biocatalysis MIT Chemical Engineering Department Instructor: Professor Kristala Prather Fall 24 Lecture # 3, 4 Selecting a Catalyst (Non-Kinetic Parameters,
"-Amino Acids: Function and Synthesis
 "-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
"-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
Lecture 11 Kjemisk reaksjonsteknikk Chemical Reaction Engineering
 Lecture Kjemisk reaksjonsteknikk Chemical Reaction Engineering Review of previous lectures Kinetic data analysis of heterogeneous reactions. Characterization of t catalysts. Kinetic study, find a kinetic
Lecture Kjemisk reaksjonsteknikk Chemical Reaction Engineering Review of previous lectures Kinetic data analysis of heterogeneous reactions. Characterization of t catalysts. Kinetic study, find a kinetic
ISCHIA ADVANCED SCHOOL OF ORGANIC CHEMISTRY
 ewis acid activation ewis base activation ISCIA ADVACED SC F GAIC CEMISTY Dual in Enantioselective Synthesis of Cyanohydrins Me A A Me UM ewis base catalysis is the process by which an electronpair donor
ewis acid activation ewis base activation ISCIA ADVACED SC F GAIC CEMISTY Dual in Enantioselective Synthesis of Cyanohydrins Me A A Me UM ewis base catalysis is the process by which an electronpair donor
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: favored. disfavored. favored. disfavored
 The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c,
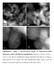 Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c, 2 nm; d, 2 nm. The low magnification images demonstrate
Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c, 2 nm; d, 2 nm. The low magnification images demonstrate
Chapter 14 Chemical Kinetics
 4//004 Chapter 4 Chemical Kinetics 4- Rates of Chemical Reactions 4- Reaction Rates and Concentrations 4-3 The Dependence of Concentrations on Time 4-4 Reaction Mechanisms 4-5 Reaction Mechanism and Rate
4//004 Chapter 4 Chemical Kinetics 4- Rates of Chemical Reactions 4- Reaction Rates and Concentrations 4-3 The Dependence of Concentrations on Time 4-4 Reaction Mechanisms 4-5 Reaction Mechanism and Rate
Racemic catalysis through asymmetric activation*
 Pure Appl. Chem., Vol. 73, No. 2, pp. 255 259, 2001. 2001 IUPAC Racemic catalysis through asymmetric activation* Koichi Mikami, Toshinobu Korenaga, Yousuke Matsumoto, Makoto Ueki, Masahiro Terada, and
Pure Appl. Chem., Vol. 73, No. 2, pp. 255 259, 2001. 2001 IUPAC Racemic catalysis through asymmetric activation* Koichi Mikami, Toshinobu Korenaga, Yousuke Matsumoto, Makoto Ueki, Masahiro Terada, and
Supporting Information
 Supporting Information Wiley-VCH 2008 69451 Weinheim, Germany Supporting Information for Chiral Brönsted Acid Catalyzed Asymmetric Baeyer-Villiger Reaction of 3-Substituted Cyclobutanones Using Aqueous
Supporting Information Wiley-VCH 2008 69451 Weinheim, Germany Supporting Information for Chiral Brönsted Acid Catalyzed Asymmetric Baeyer-Villiger Reaction of 3-Substituted Cyclobutanones Using Aqueous
Magnetic Silica Particles for Catalysis
 4 Magnetic Silica Particles for atalysis Abstract Monodisperse magnetizable colloidal silica particles in a stable dispersion have been functionalized with a homogeneous catalyst: a PP-pincer Pd-complex.
4 Magnetic Silica Particles for atalysis Abstract Monodisperse magnetizable colloidal silica particles in a stable dispersion have been functionalized with a homogeneous catalyst: a PP-pincer Pd-complex.
Name: CHEM 633: Advanced Organic Chemistry: Physical Final Exam. Please answer the following questions clearly and concisely.
 Name: 1 CEM 633: Advanced rganic Chemistry: Physical Final Exam Please answer the following questions clearly and concisely. You may write your answers in the space provided and/or on additional pages.
Name: 1 CEM 633: Advanced rganic Chemistry: Physical Final Exam Please answer the following questions clearly and concisely. You may write your answers in the space provided and/or on additional pages.
Chapter 14 Chemical Kinetics
 How fast do chemical processes occur? There is an enormous range of time scales. Chapter 14 Chemical Kinetics Kinetics also sheds light on the reaction mechanism (exactly how the reaction occurs). Why
How fast do chemical processes occur? There is an enormous range of time scales. Chapter 14 Chemical Kinetics Kinetics also sheds light on the reaction mechanism (exactly how the reaction occurs). Why
dissolved into methanol (20 ml) to form a solution. 2-methylimidazole (263 mg) was dissolved in
 Experimental section Synthesis of small-sized ZIF-8 particles (with average diameter of 50 nm): Zn(NO 3 ) 2 (258 mg) was dissolved into methanol (20 ml) to form a solution. 2-methylimidazole (263 mg) was
Experimental section Synthesis of small-sized ZIF-8 particles (with average diameter of 50 nm): Zn(NO 3 ) 2 (258 mg) was dissolved into methanol (20 ml) to form a solution. 2-methylimidazole (263 mg) was
SCREENING OF N,N-BIDENTATE PYRIDINE-BASED LIGANDS IN COPPER AND PALLADIUM CATALYSED REACTIONS MAURIZIO SOLINAS
 SCREEIG OF,-BIDETATE PYRIDIE-BASED LIGADS I COPPER AD PALLADIUM CATALYSED REACTIOS MAURIZIO SOLIAS ITRO: Green Chemistry Principle #9 Catalytic reagents (as selective as possible) are superior to stoichiometric
SCREEIG OF,-BIDETATE PYRIDIE-BASED LIGADS I COPPER AD PALLADIUM CATALYSED REACTIOS MAURIZIO SOLIAS ITRO: Green Chemistry Principle #9 Catalytic reagents (as selective as possible) are superior to stoichiometric
Enhancing Stability of Platinum on Silica by Surface Modification - Application to CO Oxidation -
 2012 CLEERS Workshop Enhancing Stability of Platinum on Silica by Surface Modification - Application to CO Oxidation - Mi-Young Kim, Jae-Soon Choi, Todd J. Toops Emissions and Catalysis Research Group
2012 CLEERS Workshop Enhancing Stability of Platinum on Silica by Surface Modification - Application to CO Oxidation - Mi-Young Kim, Jae-Soon Choi, Todd J. Toops Emissions and Catalysis Research Group
Principles of Gas- Chromatography (GC)
 Principles of Gas- Chromatography (GC) Mohammed N. Sabir January 2017 10-Jan-17 1 GC is a chromatographic technique utilizes gas as the mobile phase which is usually an inert gas (Hydrogen, Helium, Nitrogen
Principles of Gas- Chromatography (GC) Mohammed N. Sabir January 2017 10-Jan-17 1 GC is a chromatographic technique utilizes gas as the mobile phase which is usually an inert gas (Hydrogen, Helium, Nitrogen
cis,cis-muconic acid: Separation and catalysis to bioadipic acid for nylon-6,6 polymerization
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information for: cis,cis-muconic acid: Separation and catalysis
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information for: cis,cis-muconic acid: Separation and catalysis
Ir-Re Alloy as a highly active catalyst for the hydrogenolysis
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2014 Supporting Information Ir-Re Alloy as a highly active catalyst for the hydrogenolysis
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2014 Supporting Information Ir-Re Alloy as a highly active catalyst for the hydrogenolysis
Supporting Information
 Supporting Information S1 Reversible stereodivergent cycloaddition of racemic helicenes to [60]fullerene: a chiral resolution strategy Rosa M. Girón, Jiangkun Ouyang, Ludovic Favereau, Nicolas Vanthuyne,
Supporting Information S1 Reversible stereodivergent cycloaddition of racemic helicenes to [60]fullerene: a chiral resolution strategy Rosa M. Girón, Jiangkun Ouyang, Ludovic Favereau, Nicolas Vanthuyne,
Supplementary information
 Supplementary information doi: 10.1038/nchem.1025 Platinum nanocrystals selectively shaped using facet-specific peptide sequences Chin-Yi Chiu 1, Yujing Li 1, Lingyan Ruan 1, Xingchen Ye 2, Christopher
Supplementary information doi: 10.1038/nchem.1025 Platinum nanocrystals selectively shaped using facet-specific peptide sequences Chin-Yi Chiu 1, Yujing Li 1, Lingyan Ruan 1, Xingchen Ye 2, Christopher
Measuring enzyme (enantio)selectivity
 Measuring enzyme (enantio)selectivity Types of selectivity - review stereoisomers Stereoselective synthesis (create) vs. resolutions (separate) Enantioselectivity & enantiomeric purity Ways to measure
Measuring enzyme (enantio)selectivity Types of selectivity - review stereoisomers Stereoselective synthesis (create) vs. resolutions (separate) Enantioselectivity & enantiomeric purity Ways to measure
Hydrogen Peroxide Direct Synthesis: from Catalyst Preparation to Continuous Reactors
 Hydrogen Peroxide Direct Synthesis: from Catalyst Preparation to Continuous Reactors Pierdomenico Biasi 1, *, Sergio Zancanella 2, Francesco Pinna 3, Paolo Canu 2 and Tapio O. Salmi 1 1 Process Chemistry
Hydrogen Peroxide Direct Synthesis: from Catalyst Preparation to Continuous Reactors Pierdomenico Biasi 1, *, Sergio Zancanella 2, Francesco Pinna 3, Paolo Canu 2 and Tapio O. Salmi 1 1 Process Chemistry
Electronic Supplementary Information
 Electronic Supplementary Information Synthesis Procedure PVP-Pd colloid 0.034 g PdCl 2 and 0.3011 g KBr were dissolved in 6 ml of H 2 O. 0.1068 g PVP and 0.0619 g ascorbic acid were dissolved in 5 ml H
Electronic Supplementary Information Synthesis Procedure PVP-Pd colloid 0.034 g PdCl 2 and 0.3011 g KBr were dissolved in 6 ml of H 2 O. 0.1068 g PVP and 0.0619 g ascorbic acid were dissolved in 5 ml H
Kinetics of liquid-phase hydrogenation reactions over supported metal catalysts areview
 Applied Catalysis A: General 213 (2001) 1 24 Review Kinetics of liquid-phase hydrogenation reactions over supported metal catalysts areview Utpal K. Singh, M. Albert Vannice Department of Chemical Engineering,
Applied Catalysis A: General 213 (2001) 1 24 Review Kinetics of liquid-phase hydrogenation reactions over supported metal catalysts areview Utpal K. Singh, M. Albert Vannice Department of Chemical Engineering,
Dehydrogenation of propane with selective hydrogen combustion: A mechanistic study by transient analysis of products
 Dehydrogenation of propane with selective hydrogen combustion: A mechanistic study by transient analysis of products Oliver Czuprat a, Jürgen Caro a, V.A. Kondratenko b, E.V. Kondratenko b,* a Institute
Dehydrogenation of propane with selective hydrogen combustion: A mechanistic study by transient analysis of products Oliver Czuprat a, Jürgen Caro a, V.A. Kondratenko b, E.V. Kondratenko b,* a Institute
D. X. Hu Towards Catalytic Enantioselective Halogenation of Alkenes Burns Group
 D. X. Hu Towards Catalytic Enantioselective Halogenation of Alkenes Burns Group Literature Review Organic Synthesis 10, 20, 50 Years from Now? Catalytic Enantioselective Halogenation October 6 th, 2012
D. X. Hu Towards Catalytic Enantioselective Halogenation of Alkenes Burns Group Literature Review Organic Synthesis 10, 20, 50 Years from Now? Catalytic Enantioselective Halogenation October 6 th, 2012
CHAPTER 7 SELECTIVE OXIDATION OF ETHYL BENZENE
 104 CHAPTER 7 SELECTIVE OXIDATION OF ETHYL BENZENE 7.1 INTRODUCTION Aromatic ketones such as acetophenone are important intermediates for the synthesis of drugs and pharmaceuticals (Choudhary et al 2004).
104 CHAPTER 7 SELECTIVE OXIDATION OF ETHYL BENZENE 7.1 INTRODUCTION Aromatic ketones such as acetophenone are important intermediates for the synthesis of drugs and pharmaceuticals (Choudhary et al 2004).
Application Note. Authors. Abstract. Energy & Chemicals, Biofuels & Alternative Energy
 Analytical Quantification of Deoxygenated Compounds in Catalytic Reaction Samples as an Evaluation Technique to Detere Reaction Conversion from a Bioderived Oil in Novel Biofuel Testing Application Note
Analytical Quantification of Deoxygenated Compounds in Catalytic Reaction Samples as an Evaluation Technique to Detere Reaction Conversion from a Bioderived Oil in Novel Biofuel Testing Application Note
Chemical Engineering
 Chemical Engineering Basic Principles: Energy and material balances Transport Processes Momentum Transfer: Fluid Flow Energy Transfer: Heat Mass Transfer: mixing and separation processes Physical and Chemical
Chemical Engineering Basic Principles: Energy and material balances Transport Processes Momentum Transfer: Fluid Flow Energy Transfer: Heat Mass Transfer: mixing and separation processes Physical and Chemical
Examination paper for TKP 4155 / KP 8903 REACTION KINETICS AND CATALYSIS
 Department of Chemical Engineering Examination paper for TKP 4155 / KP 8903 REACTION KINETICS AND CATALYSIS Academic contact during examination: Professor Magnus Rønning Phone: 918 97 585 Examination date:
Department of Chemical Engineering Examination paper for TKP 4155 / KP 8903 REACTION KINETICS AND CATALYSIS Academic contact during examination: Professor Magnus Rønning Phone: 918 97 585 Examination date:
An extraordinarily stable catalyst: Pt NPs supported on two-dimensional Ti 3 C 2 X 2 (X=OH, F) nanosheets for Oxygen Reduction Reaction
 An extraordinarily stable catalyst: Pt NPs supported on two-dimensional Ti 3 X 2 (X=OH, F) nanosheets for Oxygen Reduction Reaction Xiaohong Xie, Siguo Chen*, Wei Ding, Yao Nie, and Zidong Wei* Experimental
An extraordinarily stable catalyst: Pt NPs supported on two-dimensional Ti 3 X 2 (X=OH, F) nanosheets for Oxygen Reduction Reaction Xiaohong Xie, Siguo Chen*, Wei Ding, Yao Nie, and Zidong Wei* Experimental
Engineering. Green Chemical. S. Suresh and S. Sundaramoorthy. and Chemical Processes. An Introduction to Catalysis, Kinetics, CRC Press
 I i Green Chemical Engineering An Introduction to Catalysis, Kinetics, and Chemical Processes S. Suresh and S. Sundaramoorthy CRC Press Taylor & Francis Group Boca Raton London New York CRC Press is an
I i Green Chemical Engineering An Introduction to Catalysis, Kinetics, and Chemical Processes S. Suresh and S. Sundaramoorthy CRC Press Taylor & Francis Group Boca Raton London New York CRC Press is an
Table of Contents 1. General procedure for the chiral phosphoric acid catalyzed asymmetric reductive amination using benzothiazoline
 Enantioselective Organocatalytic Reductive Amination of Aliphatic Ketones by Benzothiazoline as Hydrogen Donor Kodai Saito, Takahiko Akiyama* Department of Chemistry, Faculty of Science, Gakushuin University,
Enantioselective Organocatalytic Reductive Amination of Aliphatic Ketones by Benzothiazoline as Hydrogen Donor Kodai Saito, Takahiko Akiyama* Department of Chemistry, Faculty of Science, Gakushuin University,
