Supporting Information for. Discovery of Multicomponent Heterogeneous Catalysts via Admixture Screening:
|
|
|
- Percival Pope
- 5 years ago
- Views:
Transcription
1 Supporting Information for Discovery of Multicomponent Heterogeneous Catalysts via Admixture Screening: PdBiTe Catalysts for Aerobic xidative Esterification of Primary Alcohols David S. Mannel a, Maaz S. Ahmed b, Thatcher W. Root a, *, and Shannon S. Stahl b, * a Department of Chemical and Biological Engineering b Department of Chemistry University of Wisconsin-Madison, Madison Wisconsin, 5706 * thatcher@engr.wisc.edu, stahl@chem.wisc.edu Table of Contents 1) Response-Surface Methodology Data for PdBiTe Catalyst ptimization ) rigin of Catalyst Poisoning by 1-ctanol in the Flow Reactor ) Reaction Dependence on K 2 C ) Catalyst Stability in Batch and Flow ) SEM-EDX of PBT-1 and PBT-2 Catalysts ) ptimization of oxygen flow-rate and pressure in the continuous flow reactor ) Leaching of Pd, Bi, and Te from 5 day continuous flow run ) xidation of 1-ctanol with Single Promoter Catalysts ) Base Dependence with 1-ctanol in Flow ) Experimental Data for Arrhenius Plot S1
2 1) Response-Surface Methodology Data for PdBiTe Catalyst ptimization. Scheme S1. General reaction conditions for the oxidative methyl esterification of 1-octanol by PdBiTe catalysts. 1 M H 1 mol % Pd (PdBi x Te y /C, 5 wt. % Pd) 25 mol % K 2 C, 60 C 1 bar 2, MeH, 12 h Me In the development of catalysts with defined catalyst compositions, paths of steepest ascent were followed from a starting point of Pd 1 Bi 1 Te 1 /C towards an optimum catalyst. Data from two independent efforts (Paths A and B) are presented below. All experiments were performed under the conditions in Scheme S1. Path A (Figure S1) was carried out by taking constant step sizes of 0.1 unit, and path B (Figure S2) was carried out by taking larger step sizes of approximately half the distance to an axis. Similar paths were taken for both with the path of steepest ascent first decreasing x Bi of approx. 0., then decreasing x Te to approx Both paths ended at a region of high activity (Figure S4) from which PdBi 0.5 Te 0.2 /C (PBT-2) and PdBi 0.47 Te 0.09 /C (PBT-1) were chosen. S2
3 Experimental Yields from Each Step (Path A): 1) 2) ) Calculated Path of Steepest Ascent (Path A) 1) 2) ) Experimental Yields from Each Step (Path A) (cont'd): 4) 5) Calculated Path of Steepest Ascent (Path A) (cont'd) 4) 5) Figure S1. Experimental yields (left) for catalysts along path A and the calculated path of steepest ascent (right). Reaction conditions given in Scheme S1. Color axis is yield. S
4 Experimental Yields from Each Step (Path B): 1) 2) ) Calculated Path of Steepest Ascent (Path B) 1) Experimental Yield and Calculated Path of Steepest Ascent (Path B) (cont'd): 4) Figure S2. Experimental yields (left) for catalysts along path B and the calculated path of steepest ascent (right). Reaction conditions given in Scheme S1. Color axis is yield. Figure S. Paths of steepest ascent followed for Path A and Path B. S4
5 2) rigin of Catalyst Poisoning by 1-ctanol in the Flow Reactor. Catalyst poisoning under flow reaction conditions was manifested as a limit to the maximum attainable conversion of 1-octanol. Recycling of reactor effluent back through the reactor showed no increase in concentration of methyl octanoate (Scheme S2). In addition, spiking the feed solution with methyl octanoate or octanoic acid yielded 80% conversion of 1-octanol to methyl octanoate, showing that neither of these species poisons the catalyst (Scheme S). Scheme S2. Recycling of Reactor Effluent. C 7 H 15 H 0.25M PBT-2 (40 mg) MeH, 60 C, 0.1 ml/min (WHSV = 9.6 h -1 ) 0.56 bar 2 (9 % 2 in N 2 ) 8:1 2 :sub 20 % mesitylene as I.S. 25 mol % K 2 C C 7 H 15 Me 0.2 M + C 7 H MH PBT-2 (40 mg) MeH, 60 C, 0.1 ml/min (WHSV = 9.6 h -1 ) 0.56 bar 2 (9 % 2 in N 2 ) 8:1 2 :sub 20 % mesitylene as I.S. 25 mol % K 2 C C 7 H 15 Me 0.2 M + C 7 H MH Scheme S. Reaction Conversion with Added Methyl ctanoate or ctanoic Acid. C 7 H 15 H M PBT-2 (40 mg) + C 7 H 15 H + C 7 H 15 MeH, 60 C, 0.1 ml/min liquid M M 0.56 bar 2 (9 % 2 in N 2 ) 8:1 2 :sub 20 % mesitylene as I.S., M K 2 C (85 % conv.) C 7 H 15 Me 0.25 M C 7 H 15 H M PBT-2 (40 mg) + C 7 H 15 H + C 7 H 15 H MeH, 60 C, 0.1 ml/min liquid 0.04 M M 0.56 bar 2 (9 % 2 in N 2 ) 8:1 2 :sub 20 % mesitylene as I.S., M K 2 C (8 % conv.) + C 7 H 15 H M C 7 H 15 Me 0.17 M In contrast, use of octyl aldehyde as the starting material afforded only 50% yield of methyl octanoate at 100% conversion of octyl aldehyde. A fresh sample of 1-octanol was added to the reactor effluent and the mixture was re-fed through the reactor under standard reaction conditions. This run afforded only 7.5% conversion of the alcohol, indicating that a catalyst S5
6 poison is generated during the oxidation of octyl aldehyde (Scheme S4). The missing mass from the initial reaction of octyl aldehyde was identified as 2-hexyl-2-decenal and methyl 2-hexyl-2- decenoate by GC-MS, 1 H NMR, and 1 C NMR, reflecting aldol self-condensation of octyl aldehyde. Scheme S4. Catalyst Poisoning After Reaction of ctyl Aldehyde. C 7 H M PBT-2 (40 mg) MeH, 60 C, 0.1 ml/min liquid 0.56 bar 2 (9 % 2 in N 2 ) 8:1 2 :sub 20 % mesitylene as I.S., M K 2 C C 7 H 15 Me 0.12 M (100 % Conv) M 1-octanol PBT-2 (40 mg) MeH, 60 C, 0.1 ml/min liquid 0.56 bar 2 (9 % 2 in N 2 ) 8:1 2 :sub 20 % mesitylene as I.S., M K 2 C C 7 H 15 H 0.2 M (7.5 % conv) + C 7 H 15 Me 0.1 M 2-Hexyl-2-decenal (aldol condensation product) was independently synthesized (Scheme S5) to confirm the identity of the catalyst poison. Inclusion of the aldol condensation species in a batch oxidation reaction of 1-octanol resulted in significant reduction in the yield and conversion of the reaction (Figure S4). Scheme S5. Synthesis of 2-hexyl-decenal and ligomers from ctyl Aldehyde. 200 µl 1 M NaH in H ml 20 mg PBT-2 80 C, 22 hours + oligomers >71 % conv. S6
7 Figure S4. Reaction poisoning by aldol condensation products in the batch reaction aerobic methyl esterification of 1-octanol. 1 M 1-octanol, 1 mol % Pd (PdBi 0.47 Te 0.09 /C, 5 wt. % Pd), 60 C, 1 bar 2, MeH, 1 ml total volume, 20 mol % Mesitylene. 0 or 10 µl aldol condensation products added. ) Reaction Dependence on K 2 C. Batch reactions with different concentrations of K 2 C revealed that the yield maximized at 25 mol % K 2 C (Figure S5). 1 M H 1 mol % PBT-2 25 mol % K 2 C, 60 C 1 bar 2, MeH, 24 h Me Figure S5. Yield of methyl octanoate obtained at various [K 2 C ] showing maximum yield being reached at 0.25 M. S7
8 4) Catalyst Stability in Batch and Flow. To assess the activity and long-term stability of the two PBT catalysts, a continuous flow run was performed at different WHSV s and over an extended reaction time at a constant WHSV (Figure S6A and B). The PBT-2 catalyst has higher activity for the oxidation of benzyl alcohol in the flow reactor with quantitative yields obtained at a WHSV of 500 h -1 (1/WHSV = 7.2 s). The catalyst stability was measured by holding the reactor at a constant elevated WHSV of 700 h -1 (1/WHSV ~ 5 s), and the product yield was monitored over an extended time period. After h and 22,000 turnovers, the yield of methyl benzoate produced from the reaction with PBT-1 decreased from 7 to 50%. In contrast, the reaction with PBT-2 showed no loss in activity after 4 h and 27,000 turnovers. Therefore, PBT-2 was chosen for the demonstration of a 5 day continuous process. 0.5 M H PdBiTe 25 mol % K 2 C, MeH, 60 C 0.54 bar 2 (9% 2 in N 2 ) 8:1 mol 2 :mol substrate Me A. B. Figure S6. Continuous flow oxidation of benzyl alcohol to methyl benzoate by PdBiTe catalysts. (A) WHSV scan of PBT-1 and PBT-2 catalysts showing higher activity of PBT-2. (B) Stability of PBT-1 and PBT-2 catalysts over extended times in the flow reactor measured from yield of methyl benzoate at the highlighted WHSV (1/WHSV = 5 s). S8
9 5) SEM-EDX of PBT-1 and PBT-2 Catalysts. SEM-EDX mapping of fresh and used PdBiTe catalysts showed higher concentrations of Bi and Te on Pd (Figures S7-S9). No strong changes were observed between the fresh and used catalyst suggesting that leaching of Bi and Te was relatively uniform from both the Pd surface and the carbon support. The molar ratios of Bi and Te to Pd are in agreement between the catalyst synthesis, the SEM-EDX integrated signal, and the total metal content analyzed from ICP-AES (Table S1). Both PBT-1 and PBT-2 showed significant losses of both Bi and Te after use in the continuous flow reactor. Figure S7. SEM-EDX mapping of Pd Bi and Te on freshly prepared PBT-1. Figure S8. SEM-EDX mapping of Pd Bi and Te on freshly prepared PBT-2. Figure S9. SEM-EDX mapping of Pd, Bi and Te on PBT-2 after a 5 day continuous run. S9
10 Table S1. Elemental Composition of PBT Catalysts from Different Analysis Techniques. Catalyst Detection Method Bi:Pd Te:Pd PBT-1 (Fresh) From Catalyst Synthesis SEM-EDX PBT-1 (Used) SEM-EDX From Catalyst Synthesis PBT-2 (Fresh) SEM-EDX ICP-AES PBT-2 (Used) SEM-EDX ICP-AES Fresh Catalysts are as-prepared prior to any reactions. PBT-1 (Used) was recovered from flow reactor after undergoing 22,000 turnovers (see Figure S6B). PBT-2 (Used) was recovered from flow reactor after undergoing 58,000 turnovers (see Figure 6). 6) ptimization of oxygen flow-rate and pressure in the continuous flow reactor. With higher pressures obtainable in the flow reactor, additional screening with benzyl alcohol was done to optimize the catalysts performance. Varying the 2 pressure and the 2 to substrate ratio landed on optimum values of 0.5 bar 2 and 8 mol 2 /mol substrate (Table S2). Table S2. ptimization of p 2 and 2 Flow Rate in the xidation of Benzyl Alcohol. 0.5 M H PBT-2 (4.6 mg) WHSV =,500 h -1, MeH, 60 C x bar 2 (9% 2 in N 2 ) y:1 2 :sub 25 mol % K 2 C Me Entry mol 2 :mol substrate p 2 (bar) Yield (%) :1 4:1 4:1 8:1 8:1 8:1 16:1 16:1 16: ) Leaching of Pd, Bi, and Te from 5 day continuous flow run. Effluent concentrations of Pd, Bi, and Te were measured from different fractions of the fiveday continuous flow run (Table S). The recovered mass of Pd, Bi, and Te in combination with the quantities leached from the reactor were within 5% of the original mass of Pd, Bi, and Te. S10
11 Table S. Average effluent concentrations of Pd, Bi, and Te between 0-1, 1-10, and h, and the corresponding mass of Pd, Bi, and Te lost from original catalyst. Element Pd Bi Te Time on Stream (h) Effluent Concentration (ppb) % Lost from Initial Catalyst ) xidation of 1-ctanol with Single Promoter Catalysts xidation of single promoter catalysts (PdBi x and PdTe y ) was done to compare the effect of additional promoter amounts (Table S4). With both Bi and Te containing catalysts increased amounts of Bi or Te did not increase the reaction yield, relative to those observed with the dual promoter PBT-1 and PBT-2 catalysts. Table S4. Yield of methyl octanoate with single promoter catalysts. a Catalyst Yield (%) PdBi PdBi PdBi 5 5 PdTe PdTe PdTe a 1 M 1-octanol, 1 eq. KMe, 1 bar 2, 24 h, 60 C, MeH solvent, 1 mol % Pd. 9) Base Dependence with 1-ctanol in Flow The oxidation of 1-octanol to methyl octanoate in a continuous process shows increased yields with decreasing [K 2 C ] (Figure S10). During the screening of the [K 2 C ] in the S11
12 oxidation of 1-octanol a single packed bed reactor was used showing sustained activity of the PBT-1 catalyst over a 180 h period with varying reaction conditions. This corresponds with the PBT-1 catalyst undergoing nearly 800 turnovers. Figure S10. Aerobic oxidative methyl esterification of 1-octanol with various concentrations of K 2 C. Catalyst activity is maintained over the 180 h run. 10) Experimental Data for Arrhenius Plot The activation energy and pre-exponential factor for the oxidation of benzyl alcohol were calculated from the conversion of the substrate at different temperatures and a constant flow rate (Figure S11). Rate constants were calculated assuming first order kinetics using equation S1 (Figure S12). S12
13 Figure S11. Yield of methyl benzoate at different reaction temperatures. Reaction Conditions: 0.5 M benzyl alcohol, 4.6 mg PBT-2, 25 mol % K 2 C, 0.54 bar 2 (9% 2 in N 2 ), 8:1 mol 2 :mol substrate, WHSV =,500 h -1. k!"" = ln 1 Conversion 1/WHSV (S1) Figure S12. Time course for the aerobic oxidative methyl esterification of benzyl alcohol showing conversion of benzyl alcohol (blue circles) to benzaldehyde intermediate (red diamonds) and subsequent reaction of benzaldehyde to methyl benzoate (green triangles) over PdBiTe catalysts. Conditions: 1 M benzyl alcohol and 0.25 M K 2 C in 1 ml MeH, 0.1 mol % PBT-2, 60 C, 1 bar 2. Fits are from treating both oxidation reactions as pseudo-first order reactions. S1
Nanocatalysis in Continuous Flow: Supported Iron. Oxidation of Benzyl Alcohol
 This journal is The Royal Society of Chemistry 13 1 Supporting Information Nanocatalysis in Continuous Flow: Supported Iron xide Nanoparticles for the Heterogeneous Aerobic xidation of Benzyl Alcohol David
This journal is The Royal Society of Chemistry 13 1 Supporting Information Nanocatalysis in Continuous Flow: Supported Iron xide Nanoparticles for the Heterogeneous Aerobic xidation of Benzyl Alcohol David
Student Manual for Aerobic Alcohol Oxidation Using a Copper(I)/TEMPO Catalyst System
 Student Manual for Aerobic Alcohol Oxidation Using a Copper(I)/TEMPO Catalyst System icholas J. Hill, Jessica M. Hoover and Shannon S. Stahl* Department of Chemistry, University of Wisconsin-Madison, 1101
Student Manual for Aerobic Alcohol Oxidation Using a Copper(I)/TEMPO Catalyst System icholas J. Hill, Jessica M. Hoover and Shannon S. Stahl* Department of Chemistry, University of Wisconsin-Madison, 1101
Development of Safe and Scalable Continuous-Flow Methods for. Palladium-Catalyzed Aerobic Oxidation Reactions
 Supplementary Data for Development of Safe and Scalable Continuous-Flow Methods for Palladium-Catalyzed Aerobic Oxidation Reactions Xuan Ye, a Martin D. Johnson, *b Tianning Diao, a Matthew H. Yates *b
Supplementary Data for Development of Safe and Scalable Continuous-Flow Methods for Palladium-Catalyzed Aerobic Oxidation Reactions Xuan Ye, a Martin D. Johnson, *b Tianning Diao, a Matthew H. Yates *b
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/4/10/eaas9319/dc1 Supplementary Materials for Transformation of alcohols to esters promoted by hydrogen bonds using oxygen as the oxidant under metal-free conditions
advances.sciencemag.org/cgi/content/full/4/10/eaas9319/dc1 Supplementary Materials for Transformation of alcohols to esters promoted by hydrogen bonds using oxygen as the oxidant under metal-free conditions
Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c,
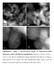 Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c, 2 nm; d, 2 nm. The low magnification images demonstrate
Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c, 2 nm; d, 2 nm. The low magnification images demonstrate
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/3/6/e1603180/dc1 Supplementary Materials for MnTiO3-driven low-temperature oxidative coupling of methane over TiO2-doped Mn2O3-Na2WO4/SiO2 catalyst This PDF file
advances.sciencemag.org/cgi/content/full/3/6/e1603180/dc1 Supplementary Materials for MnTiO3-driven low-temperature oxidative coupling of methane over TiO2-doped Mn2O3-Na2WO4/SiO2 catalyst This PDF file
Pd-P nanoalloys supported on porous carbon frame as efficient catalyst for benzyl alcohol oxidation
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2018 Supporting information Pd-P nanoalloys supported on porous carbon frame as
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2018 Supporting information Pd-P nanoalloys supported on porous carbon frame as
Aerobic Oxidation of 2-Phenoxyethanol Lignin Model. Compounds Using Vanadium and Copper Catalysts
 Electronic Supporting Information for: Aerobic Oxidation of 2-Phenoxyethanol Lignin Model Compounds Using Vanadium and Copper Catalysts Christian Díaz-Urrutia, Baburam Sedai, Kyle C. Leckett, R. Tom Baker,,*
Electronic Supporting Information for: Aerobic Oxidation of 2-Phenoxyethanol Lignin Model Compounds Using Vanadium and Copper Catalysts Christian Díaz-Urrutia, Baburam Sedai, Kyle C. Leckett, R. Tom Baker,,*
ChE 344 Winter 2013 Mid Term Exam I Tuesday, February 26, Closed Book, Web, and Notes. Honor Code
 ChE 344 Winter 2013 Mid Term Exam I Tuesday, February 26, 2013 Closed Book, Web, and Notes Name Honor Code (Sign at the end of exam period) 1) / 5 pts 2) / 5 pts 3) / 5 pts 4) / 5 pts 5) / 5 pts 6) / 5
ChE 344 Winter 2013 Mid Term Exam I Tuesday, February 26, 2013 Closed Book, Web, and Notes Name Honor Code (Sign at the end of exam period) 1) / 5 pts 2) / 5 pts 3) / 5 pts 4) / 5 pts 5) / 5 pts 6) / 5
Electronic Supplementary Information. Alcohols
 Electronic Supplementary Information An Excellent Au/meso-γ-Al 2 O 3 Catalyst for Aerobic Selective Oxidation of Alcohols Guofeng Zhao, Min Ling, Huanyun Hu, Miaomiao Deng, Qingsong Xue, Yong Lu * Shanghai
Electronic Supplementary Information An Excellent Au/meso-γ-Al 2 O 3 Catalyst for Aerobic Selective Oxidation of Alcohols Guofeng Zhao, Min Ling, Huanyun Hu, Miaomiao Deng, Qingsong Xue, Yong Lu * Shanghai
Supporting Information
 Supporting Information Highly Cross-Linked Imidazolium Salts Entrapped Magnetic Particles Preparation and Applications Paola Agrigento, a Matthias Josef Beier, b Jesper T. N. Knijnenburg, c Alfons Baiker
Supporting Information Highly Cross-Linked Imidazolium Salts Entrapped Magnetic Particles Preparation and Applications Paola Agrigento, a Matthias Josef Beier, b Jesper T. N. Knijnenburg, c Alfons Baiker
Supporting Information
 Supporting Information Mechanistic Study of Alcohol Oxidation by the / /DMSO Catalyst System and Implications for the Development of Improved Aerobic Oxidation Catalysts Bradley A. Steinhoff, Shannon R.
Supporting Information Mechanistic Study of Alcohol Oxidation by the / /DMSO Catalyst System and Implications for the Development of Improved Aerobic Oxidation Catalysts Bradley A. Steinhoff, Shannon R.
CHAPTER 7 SELECTIVE OXIDATION OF ETHYL BENZENE
 104 CHAPTER 7 SELECTIVE OXIDATION OF ETHYL BENZENE 7.1 INTRODUCTION Aromatic ketones such as acetophenone are important intermediates for the synthesis of drugs and pharmaceuticals (Choudhary et al 2004).
104 CHAPTER 7 SELECTIVE OXIDATION OF ETHYL BENZENE 7.1 INTRODUCTION Aromatic ketones such as acetophenone are important intermediates for the synthesis of drugs and pharmaceuticals (Choudhary et al 2004).
 MEEBAL Exam 1 October 2012 Show all work in your blue book. Points will be deducted if steps leading to answers are not shown. No work outside blue books (such as writing on the flow sheets) will be considered.
MEEBAL Exam 1 October 2012 Show all work in your blue book. Points will be deducted if steps leading to answers are not shown. No work outside blue books (such as writing on the flow sheets) will be considered.
CSTR 1 CSTR 2 X A =?
 hemical Engineering HE 33 F Applied Reaction Kinetics Fall 014 Problem Set 3 Due at the dropbox located in the hallway outside of WB 5 by Monday, Nov 3 rd, 014 at 5 pm Problem 1. onsider the following
hemical Engineering HE 33 F Applied Reaction Kinetics Fall 014 Problem Set 3 Due at the dropbox located in the hallway outside of WB 5 by Monday, Nov 3 rd, 014 at 5 pm Problem 1. onsider the following
Supporting Information
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2015 Supporting Information Experimental details 1) Preparation of the catalytic materials 1,
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2015 Supporting Information Experimental details 1) Preparation of the catalytic materials 1,
Supplementary Figure 1. Protonation equilibria of the CO 2 in water.
 Supplementary Figure 1. Protonation equilibria of the CO 2 in water. 1 Supplementary Figure 2. Hydrogenation of CO 2 with [RuCl 2 (PTA) 4 ]. 1 H NMR signal of HCOOH as a function of time in the hydrogenation
Supplementary Figure 1. Protonation equilibria of the CO 2 in water. 1 Supplementary Figure 2. Hydrogenation of CO 2 with [RuCl 2 (PTA) 4 ]. 1 H NMR signal of HCOOH as a function of time in the hydrogenation
Continuous flow nanocatalysis: reaction pathways in the conversion of levulinic acid to valuable chemicals
 Electronic Supplementary Material (ESI) for Green Chemistry ESI Continuous flow nanocatalysis: reaction pathways in the conversion of levulinic acid to valuable chemicals Jose M. Bermudez, J. Angel Menéndez,
Electronic Supplementary Material (ESI) for Green Chemistry ESI Continuous flow nanocatalysis: reaction pathways in the conversion of levulinic acid to valuable chemicals Jose M. Bermudez, J. Angel Menéndez,
Supporting Information. Copper(II)-catalyzed Aerobic Oxidation of Primary Alcohols to Aldehydes in Ionic Liquid [bmpy]pf 6
![Supporting Information. Copper(II)-catalyzed Aerobic Oxidation of Primary Alcohols to Aldehydes in Ionic Liquid [bmpy]pf 6 Supporting Information. Copper(II)-catalyzed Aerobic Oxidation of Primary Alcohols to Aldehydes in Ionic Liquid [bmpy]pf 6](/thumbs/89/99017119.jpg) Supporting Information Copper(II)-catalyzed Aerobic Oxidation of Primary Alcohols to Aldehydes in Ionic Liquid [bmpy]pf 6 Nan Jiang and Arthur J. Ragauskas * Department of Chemistry, Georgia Institute
Supporting Information Copper(II)-catalyzed Aerobic Oxidation of Primary Alcohols to Aldehydes in Ionic Liquid [bmpy]pf 6 Nan Jiang and Arthur J. Ragauskas * Department of Chemistry, Georgia Institute
Supporting Information
 Supporting Information MgFeCe ternary layered double hydroxide as highly efficient and recyclable heterogeneous base catalyst for synthesis of dimethyl carbonate by transesterification Nayana T. Nivangune
Supporting Information MgFeCe ternary layered double hydroxide as highly efficient and recyclable heterogeneous base catalyst for synthesis of dimethyl carbonate by transesterification Nayana T. Nivangune
CHAPTER 4. LIQUID PHASE AEROBIC OXIDATION OF ETHYLBENZENE OVER PrAlPO-5
 106 CHAPTER 4 LIQUID PHASE AEROBIC OXIDATION OF ETHYLBENZENE OVER PrAlPO-5 4.1 INTRODUCTION Selective catalytic oxidation of alkyl aromatics is a viable technology to functionalize saturated and unsaturated
106 CHAPTER 4 LIQUID PHASE AEROBIC OXIDATION OF ETHYLBENZENE OVER PrAlPO-5 4.1 INTRODUCTION Selective catalytic oxidation of alkyl aromatics is a viable technology to functionalize saturated and unsaturated
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) Multi-scale promoting effects
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) Multi-scale promoting effects
Green Chemistry and the United States Environmental Protection Agency A Postdoc s Perspective James T. Ciszewski & Michael A.
 Green Chemistry and the United States Environmental Protection Agency A Postdoc s Perspective James T. Ciszewski & Michael A. Gonzalez United States Environmental Protection Agency Office of Research and
Green Chemistry and the United States Environmental Protection Agency A Postdoc s Perspective James T. Ciszewski & Michael A. Gonzalez United States Environmental Protection Agency Office of Research and
Azaphosphatranes as Structurally Tunable Organocatalysts for Carbonate Synthesis from CO 2 and Epoxides
 Azaphosphatranes as Structurally Tunable Organocatalysts for Carbonate Synthesis from CO 2 and Epoxides Bastien Chatelet, Lionel Joucla, Jean-Pierre Dutasta, Alexandre Martinez *, Kai C. Szeto and Véronique
Azaphosphatranes as Structurally Tunable Organocatalysts for Carbonate Synthesis from CO 2 and Epoxides Bastien Chatelet, Lionel Joucla, Jean-Pierre Dutasta, Alexandre Martinez *, Kai C. Szeto and Véronique
Supporting Information
 Supporting Information Wiley-VCH 2007 69451 Weinheim, Germany 1 Dihydrogen Reduction of Carboxylic Esters to Alcohols Catalyzed by Homogeneous Ruthenium Complexes: High Efficiency and Unprecedented Chemoselectivity.
Supporting Information Wiley-VCH 2007 69451 Weinheim, Germany 1 Dihydrogen Reduction of Carboxylic Esters to Alcohols Catalyzed by Homogeneous Ruthenium Complexes: High Efficiency and Unprecedented Chemoselectivity.
Synthesis of mixed alcohols over K-Ni-MoS 2 catalysts
 Synthesis of mixed alcohols over K-Ni-MoS 2 catalysts Rodrigo Suárez París Supervisors: Magali Boutonnet, Sven Järås Division of Chemical Technology, KTH OUTLINE Introduction and objective Experimental
Synthesis of mixed alcohols over K-Ni-MoS 2 catalysts Rodrigo Suárez París Supervisors: Magali Boutonnet, Sven Järås Division of Chemical Technology, KTH OUTLINE Introduction and objective Experimental
Aqueous-phase reforming a pathway to chemicals and fuels
 Alexey Kirilin Aqueous-phase reforming a pathway to chemicals and fuels Laboratory of Industrial Chemistry and Reaction Engineering Process Chemistry Centre Åbo Akademi Agenda 2 Short Introduction, biomass,
Alexey Kirilin Aqueous-phase reforming a pathway to chemicals and fuels Laboratory of Industrial Chemistry and Reaction Engineering Process Chemistry Centre Åbo Akademi Agenda 2 Short Introduction, biomass,
Kinetic investigations on the esterification of phthalic anhydride with n-heptyl, n-nonyl or n-undecyl alcohol over sulfuric acid catalyst
 Reac Kinet Mech at (211) 14:9 15 DI 1.17/s11144-11-337-9 Kinetic investigations on the esterification of phthalic anhydride with n-heptyl, n-nonyl or n-undecyl alcohol over sulfuric acid catalyst Maria
Reac Kinet Mech at (211) 14:9 15 DI 1.17/s11144-11-337-9 Kinetic investigations on the esterification of phthalic anhydride with n-heptyl, n-nonyl or n-undecyl alcohol over sulfuric acid catalyst Maria
Supporting Information
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 ucleophilic addition of amines, alcohols, and thiophenol with epoxide/olefin using highly efficient
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 ucleophilic addition of amines, alcohols, and thiophenol with epoxide/olefin using highly efficient
CHEM N-4 November 2014
 CHEM1002 2014-N-4 November 2014 The reaction order for a chemical reaction is given by the sum of the powers in the rate law. Why is the reaction order usually given by a small positive integer, i.e. 2
CHEM1002 2014-N-4 November 2014 The reaction order for a chemical reaction is given by the sum of the powers in the rate law. Why is the reaction order usually given by a small positive integer, i.e. 2
Process Design Decisions and Project Economics Prof. Dr. V. S. Moholkar Department of Chemical Engineering Indian Institute of Technology, Guwahati
 Process Design Decisions and Project Economics Prof. Dr. V. S. Moholkar Department of Chemical Engineering Indian Institute of Technology, Guwahati Module - 2 Flowsheet Synthesis (Conceptual Design of
Process Design Decisions and Project Economics Prof. Dr. V. S. Moholkar Department of Chemical Engineering Indian Institute of Technology, Guwahati Module - 2 Flowsheet Synthesis (Conceptual Design of
Evidence for an HX-Reductive-Elimination Pathway
 Supporting Information Reaction of Molecular Oxygen with a Pd II Hydride to Produce a Pd II Hydroperoxide: Experimental Evidence for an HX-Reductive-Elimination Pathway Michael M. Konnick and Shannon S.
Supporting Information Reaction of Molecular Oxygen with a Pd II Hydride to Produce a Pd II Hydroperoxide: Experimental Evidence for an HX-Reductive-Elimination Pathway Michael M. Konnick and Shannon S.
CHAPTER 6 SELECTIVE OXIDATION OF DIPHEYLMETHANE TO BENZOPHENONE
 110 CHAPTER 6 SELECTIVE OXIDATION OF DIPHEYLMETHANE TO BENZOPHENONE 6.1 INTRODUCTION Oxidation of diphenylmethane (DPM) to benzophenone is an industrially important reaction as the product benzophenone
110 CHAPTER 6 SELECTIVE OXIDATION OF DIPHEYLMETHANE TO BENZOPHENONE 6.1 INTRODUCTION Oxidation of diphenylmethane (DPM) to benzophenone is an industrially important reaction as the product benzophenone
Supporting Information. Highly Efficient Aerobic Oxidation of Various Amines Using Pd 3 Pb Intermetallic Compound Catalysts
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supporting Information Highly Efficient Aerobic Oxidation of Various Amines Using Pd 3 Pb Intermetallic
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supporting Information Highly Efficient Aerobic Oxidation of Various Amines Using Pd 3 Pb Intermetallic
Supporting Information
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2017 Supporting Information Iodine Catalyzed xidation of Alcohols and Aldehydes to Carboxylic
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2017 Supporting Information Iodine Catalyzed xidation of Alcohols and Aldehydes to Carboxylic
Supplementary Information
 Supplementary Information Supplementary Figure 1. X-ray diffraction patterns of (a) pure LDH, (b) AuCl 4 ion-exchanged LDH and (c) the Au/LDH hybrid catalyst. The refined cell parameters for pure, ion-exchanged,
Supplementary Information Supplementary Figure 1. X-ray diffraction patterns of (a) pure LDH, (b) AuCl 4 ion-exchanged LDH and (c) the Au/LDH hybrid catalyst. The refined cell parameters for pure, ion-exchanged,
Aerial oxidation of substituted aromatic hydrocarbons catalyzed by Co/Mn/Br in water-dioxane medium
 Catalysis Communications 5 (2004) 9 13 www.elsevier.com/locate/catcom Aerial oxidation of substituted aromatic hydrocarbons catalyzed by Co/Mn/Br in water-dioxane medium Kala Nair, Dhanashri P. Sawant,
Catalysis Communications 5 (2004) 9 13 www.elsevier.com/locate/catcom Aerial oxidation of substituted aromatic hydrocarbons catalyzed by Co/Mn/Br in water-dioxane medium Kala Nair, Dhanashri P. Sawant,
cis,cis-muconic acid: Separation and catalysis to bioadipic acid for nylon-6,6 polymerization
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information for: cis,cis-muconic acid: Separation and catalysis
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information for: cis,cis-muconic acid: Separation and catalysis
SELECTIVE OXIDATION OF TOLUENE TO BENZALDEHYDE USING Cu/Sn/Br CATALYST SYSTEM
 Int. J. Chem. Sci.: 9(2), 211, 545-552 ISSN 972-768X www.sadgurupublications.com SELECTIVE OXIDATION OF TOLUENE TO BENZALDEHYDE USING Cu/Sn/Br CATALYST SYSTEM KALPENDRA RAJURKAR *, NILESH KULKARNI, VILAS
Int. J. Chem. Sci.: 9(2), 211, 545-552 ISSN 972-768X www.sadgurupublications.com SELECTIVE OXIDATION OF TOLUENE TO BENZALDEHYDE USING Cu/Sn/Br CATALYST SYSTEM KALPENDRA RAJURKAR *, NILESH KULKARNI, VILAS
Ligand-free coupling of phenols and alcohols with aryl halides by a recyclable heterogeneous copper catalyst
 Supporting Information Ligand-free coupling of phenols and alcohols with aryl halides by a recyclable heterogeneous copper catalyst Man Wang, Bizhen Yuan, Tongmei Ma, Huanfeng Jiang and Yingwei Li* School
Supporting Information Ligand-free coupling of phenols and alcohols with aryl halides by a recyclable heterogeneous copper catalyst Man Wang, Bizhen Yuan, Tongmei Ma, Huanfeng Jiang and Yingwei Li* School
Supporting Information: Synthesis of Boron Doped Carbon Nitride Solids and Their Use as Metal Free Catalyst for Aliphatic C-H Bond Oxidation
 Supporting Information: Synthesis of Boron Doped Carbon itride Solids and Their Use as Metal Free Catalyst for Aliphatic C-H Bond Oxidation Yong Wang, * Haoran Li, Jia Yao, Xinchen Wang, and Markus Antonietti
Supporting Information: Synthesis of Boron Doped Carbon itride Solids and Their Use as Metal Free Catalyst for Aliphatic C-H Bond Oxidation Yong Wang, * Haoran Li, Jia Yao, Xinchen Wang, and Markus Antonietti
E. Toukoniitty, J. Wärnå, D. Yu. Murzin and T. Salmi. Common in fine chemicals production: Three-phase applications, reactant, solvent (l) -
 Transient methods in three-phase catalysis E. Toukoniitty, J. Wärnå, D. Yu. Murzin and T. Salmi Three-phase systems Common in fine chemicals production: Three-phase applications, H 2, 2 (g) - reactant,
Transient methods in three-phase catalysis E. Toukoniitty, J. Wärnå, D. Yu. Murzin and T. Salmi Three-phase systems Common in fine chemicals production: Three-phase applications, H 2, 2 (g) - reactant,
Simulation of Butyl Acetate and Methanol Production by Transesterification Reaction via Conventional Distillation Process
 Simulation of Butyl Acetate and Methanol Production by Transesterification Reaction via Conventional Distillation Process Nikhil V. Sancheti Department of Chemical Engineering L.I.T., Nagpur, Maharashtra,
Simulation of Butyl Acetate and Methanol Production by Transesterification Reaction via Conventional Distillation Process Nikhil V. Sancheti Department of Chemical Engineering L.I.T., Nagpur, Maharashtra,
1 Answer. 2 Answer A B C D
 216 W10-Exam #1 Page 1 of 9. I. (8 points) 1) Given below are infrared (IR) spectra of four compounds. The structures of compounds are given below. Assign each spectrum to its compound by putting the letter
216 W10-Exam #1 Page 1 of 9. I. (8 points) 1) Given below are infrared (IR) spectra of four compounds. The structures of compounds are given below. Assign each spectrum to its compound by putting the letter
Heterogeneously catalyzed selective aerobic oxidative cross-coupling of terminal alkynes and amides with simple copper(ii) hydroxide
 Electronic Supplementary Information (ESI) for Heterogeneously catalyzed selective aerobic oxidative cross-coupling of terminal alkynes and amides with simple copper(ii) hydroxide Xiongjie Jin, Kazuya
Electronic Supplementary Information (ESI) for Heterogeneously catalyzed selective aerobic oxidative cross-coupling of terminal alkynes and amides with simple copper(ii) hydroxide Xiongjie Jin, Kazuya
Study on the Selective Hydrogenation of Nitroaromatics to N-aryl hydroxylamines using a Supported Pt nanoparticle Catalyst
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 204 Supporting Information Study on the Selective Hydrogenation of Nitroaromatics
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 204 Supporting Information Study on the Selective Hydrogenation of Nitroaromatics
SBA-15-functionalized sulfonic acid confined acidic ionic liquid: a powerful and water-tolerant catalyst for solvent-free esterifications
 SBA-15-functionalized sulfonic acid confined acidic ionic liquid: a powerful and water-tolerant catalyst for solvent-free esterifications Babak Karimi* a, Majid Vafaeezadeh a a Department of Chemistry,
SBA-15-functionalized sulfonic acid confined acidic ionic liquid: a powerful and water-tolerant catalyst for solvent-free esterifications Babak Karimi* a, Majid Vafaeezadeh a a Department of Chemistry,
Cannizzaro Reaction. Part I
 annizzaro eaction Part I annizzaro eaction In the presence of concentrated alkali, aldehydes containing no α - hydrogens undergo self oxidation & reduction (edox) reaction to yield a mixture of an alcohol
annizzaro eaction Part I annizzaro eaction In the presence of concentrated alkali, aldehydes containing no α - hydrogens undergo self oxidation & reduction (edox) reaction to yield a mixture of an alcohol
Initial carbon carbon bond formation during synthesis gas conversion to higher alcohols on K Cu Mg 5 CeO x catalysts
 Catalysis Letters 51 (1998) 47 52 47 Initial carbon carbon bond formation during synthesis gas conversion to higher alcohols on K Cu Mg 5 CeO x catalysts Mingting Xu and Enrique Iglesia Department of Chemical
Catalysis Letters 51 (1998) 47 52 47 Initial carbon carbon bond formation during synthesis gas conversion to higher alcohols on K Cu Mg 5 CeO x catalysts Mingting Xu and Enrique Iglesia Department of Chemical
Highly Efficient and Robust Au/MgCuCr 2 O 4 Catalyst for Gas-Phase Oxidation of Ethanol to Acetaldehyde
 Highly Efficient and Robust Au/MgCuCr O 4 Catalyst for Gas-Phase Oxidation of Ethanol to Acetaldehyde Peng Liu,*, and Emiel J. M. Hensen*, Department of Chemical Engineering and Chemistry, Eindhoven University
Highly Efficient and Robust Au/MgCuCr O 4 Catalyst for Gas-Phase Oxidation of Ethanol to Acetaldehyde Peng Liu,*, and Emiel J. M. Hensen*, Department of Chemical Engineering and Chemistry, Eindhoven University
Strategies to Synthesize Supported Bimetallic Catalysts
 Strategies to Synthesize Supported Bimetallic Catalysts M. Sankar Cardiff Catalysis Institute School of Chemistry Cardiff University, Cardiff United Kingdom sankar@cardiff.ac.uk Outline Ø Supported Bimetallic
Strategies to Synthesize Supported Bimetallic Catalysts M. Sankar Cardiff Catalysis Institute School of Chemistry Cardiff University, Cardiff United Kingdom sankar@cardiff.ac.uk Outline Ø Supported Bimetallic
Technical Resource Package 1
 Technical Resource Package 1 Green Chemistry Impacts in Batch Chemical Processing UNIDO IAMC Toolkit Images may not be copied, transmitted or manipulated 1/5 The following list provides an overview of
Technical Resource Package 1 Green Chemistry Impacts in Batch Chemical Processing UNIDO IAMC Toolkit Images may not be copied, transmitted or manipulated 1/5 The following list provides an overview of
FlowCAT a versatile continuous flow reactor system focusing on reactions under elevated pressure and/or involving heterogeneous catalysis.
 1 FlowCAT - a versatile continuous flow reactor system FlowCAT a versatile continuous flow reactor system focusing on reactions under elevated pressure and/or involving heterogeneous catalysis. By Dr Jasbir
1 FlowCAT - a versatile continuous flow reactor system FlowCAT a versatile continuous flow reactor system focusing on reactions under elevated pressure and/or involving heterogeneous catalysis. By Dr Jasbir
Supporting information. for. hydrophobic pockets for acylation reactions in water
 Supporting information for Functionalized organocatalytic nanoreactors: hydrophobic pockets for acylation reactions in water Pepa Cotanda, Annhelen Lu, Joseph P. Patterson, Nikos Petzetakis, Rachel K.
Supporting information for Functionalized organocatalytic nanoreactors: hydrophobic pockets for acylation reactions in water Pepa Cotanda, Annhelen Lu, Joseph P. Patterson, Nikos Petzetakis, Rachel K.
Supporting Information
 Supporting Information Wiley-VCH 25 69451 Weinheim, Germany Direct Asymmetric α-fluorination of Aldehydes [**] Derek D. Steiner, Nobuyuki Mase, Carlos F. Barbas III* [*] Prof. Dr. C. F. Barbas III, Derek
Supporting Information Wiley-VCH 25 69451 Weinheim, Germany Direct Asymmetric α-fluorination of Aldehydes [**] Derek D. Steiner, Nobuyuki Mase, Carlos F. Barbas III* [*] Prof. Dr. C. F. Barbas III, Derek
Oxidative Dehydrogenation of Olefin*
 Surface Heterogenity of Bismuth-Molybdate Catalyst in Oxidative Dehydrogenation of Olefin* by Toru Watanabe** and Etsuro Echigoya** Summary: The oxidative dehydrogenation of C4, C5 olefins over bismuth
Surface Heterogenity of Bismuth-Molybdate Catalyst in Oxidative Dehydrogenation of Olefin* by Toru Watanabe** and Etsuro Echigoya** Summary: The oxidative dehydrogenation of C4, C5 olefins over bismuth
Silica-supported sulfonic acids as recyclable catalyst. for esterification of levulinic acid with stoichiometric
 Supporting information for Silica-supported sulfonic acids as recyclable catalyst for esterification of levulinic acid with stoichiometric amounts of alcohols Raimondo Maggi* 1, N. Raveendran Shiju* 2,
Supporting information for Silica-supported sulfonic acids as recyclable catalyst for esterification of levulinic acid with stoichiometric amounts of alcohols Raimondo Maggi* 1, N. Raveendran Shiju* 2,
Supplementary Figure 1. Schematic layout of set-up for operando NMR studies.
 Supplementary Figure 1. Schematic layout of set-up for operando NMR studies. Supplementary Figure 2. Correlations between different ratios of D2O/H2O and 1 H chemical shifts of HDO. The spectra were acquired
Supplementary Figure 1. Schematic layout of set-up for operando NMR studies. Supplementary Figure 2. Correlations between different ratios of D2O/H2O and 1 H chemical shifts of HDO. The spectra were acquired
The aldol condensation of benzaldehyde and acetone is a classical example of a spontaneous, exothermic reaction. To avoid controlled reagent addition
 The aldol condensation of benzaldehyde and acetone is a classical example of a spontaneous, exothermic reaction. To avoid controlled reagent addition and cooling, the reaction was successfully translated
The aldol condensation of benzaldehyde and acetone is a classical example of a spontaneous, exothermic reaction. To avoid controlled reagent addition and cooling, the reaction was successfully translated
Plasma driven ammonia decomposition on Fe-catalyst: eliminating surface nitrogen poisoning
 Supporting Information for Plasma driven ammonia decomposition on Fe-catalyst: eliminating surface nitrogen poisoning Contents: 1. Scheme of the DBD plasma-driven catalysis reactor, Scheme S1. 2. XRF analysis
Supporting Information for Plasma driven ammonia decomposition on Fe-catalyst: eliminating surface nitrogen poisoning Contents: 1. Scheme of the DBD plasma-driven catalysis reactor, Scheme S1. 2. XRF analysis
Poly(4-vinylimidazolium)s: A Highly Recyclable Organocatalyst Precursor for. Benzoin Condensation Reaction
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 24 Supporting Information Poly(4-vinylimidazolium)s: A Highly Recyclable rganocatalyst Precursor
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 24 Supporting Information Poly(4-vinylimidazolium)s: A Highly Recyclable rganocatalyst Precursor
High Activity Redox Catalysts Synthesised by Chemical Vapour Impregnation
 Supporting Online Information High Activity Redox Catalysts Synthesised by Chemical Vapour Impregnation Michael M. Forde, 1 Lokesh Kesavan, 1 Mohd Izham bin Saiman, 1 Qian He, 2 Nikolaos Dimitratos, 1
Supporting Online Information High Activity Redox Catalysts Synthesised by Chemical Vapour Impregnation Michael M. Forde, 1 Lokesh Kesavan, 1 Mohd Izham bin Saiman, 1 Qian He, 2 Nikolaos Dimitratos, 1
Supplementary Figure 2. (a) XRD patterns of the MOF and the simulated Ni-MOF-74
 Supplementary Figure 1. Low-magnification TEM image of Pt-Ni frame @ MOF. The scale bar is 200 nm. Supplementary Figure 2. (a) XRD patterns of the Pt-Ni @ MOF and the simulated Ni-MOF-74 pattern. (b) XRD
Supplementary Figure 1. Low-magnification TEM image of Pt-Ni frame @ MOF. The scale bar is 200 nm. Supplementary Figure 2. (a) XRD patterns of the Pt-Ni @ MOF and the simulated Ni-MOF-74 pattern. (b) XRD
CHAPTER 4: CATALYTIC PROPERTIES OF ZSM-5 ZEOLITES AND CUBIC MESOPOROUS MATERIALS
 102 CHAPTER 4: CATALYTIC PROPERTIES OF ZSM-5 ZEOLITES AND CUBIC MESOPOROUS MATERIALS Chapter summary The role of heterogeneous catalysts in organic reactions is included in this chapter. Two organic reactions,
102 CHAPTER 4: CATALYTIC PROPERTIES OF ZSM-5 ZEOLITES AND CUBIC MESOPOROUS MATERIALS Chapter summary The role of heterogeneous catalysts in organic reactions is included in this chapter. Two organic reactions,
Titanium Nitride-Nickel Nanocomposite as Heterogeneous Catalyst for the Hydrogenolysis of Aryl Ethers
 Titanium Nitride-Nickel Nanocomposite as Heterogeneous Catalyst for the Hydrogenolysis of Aryl Ethers Valerio Molinari, Cristina Giordano, Markus Antonietti and Davide Esposito* Max-Planck-Institute of
Titanium Nitride-Nickel Nanocomposite as Heterogeneous Catalyst for the Hydrogenolysis of Aryl Ethers Valerio Molinari, Cristina Giordano, Markus Antonietti and Davide Esposito* Max-Planck-Institute of
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/NCHEM.1066 Facile removal of stabiliser-ligands from supported gold nanoparticles Jose A. Lopez-Sanchez 1, Nikolaos Dimitratos 1, Ceri Hammond 1, Gemma L. Brett 1, Lokesh Kesavan 1, Saul White
DOI: 10.1038/NCHEM.1066 Facile removal of stabiliser-ligands from supported gold nanoparticles Jose A. Lopez-Sanchez 1, Nikolaos Dimitratos 1, Ceri Hammond 1, Gemma L. Brett 1, Lokesh Kesavan 1, Saul White
Electronic Supplementary Information (ESI)
 Electronic Supplementary Information (ESI) Cu-SSZ-39, an active and hydrothermally stable catalyst for the selective catalytic reduction of NOx Manuel Moliner, *a Cristina Franch, a Eduardo Palomares,
Electronic Supplementary Information (ESI) Cu-SSZ-39, an active and hydrothermally stable catalyst for the selective catalytic reduction of NOx Manuel Moliner, *a Cristina Franch, a Eduardo Palomares,
Synthesis gas production via the biogas reforming reaction over Ni/MgO-Al 2 O 3 and Ni/CaO-Al 2 O 3 catalysts
 Synthesis gas production via the biogas reforming reaction over Ni/MgO-Al 2 O 3 and Ni/CaO-Al 2 O 3 catalysts N.D. Charisiou 1,2, A. Baklavaridis 1, V.G. Papadakis 2, M.A. Goula 1 1 Department of Environmental
Synthesis gas production via the biogas reforming reaction over Ni/MgO-Al 2 O 3 and Ni/CaO-Al 2 O 3 catalysts N.D. Charisiou 1,2, A. Baklavaridis 1, V.G. Papadakis 2, M.A. Goula 1 1 Department of Environmental
Sub-10-nm Au-Pt-Pd Alloy Trimetallic Nanoparticles with. High Oxidation-Resistant Property as Efficient and Durable
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Sub-10-nm Au-Pt-Pd Alloy Trimetallic Nanoparticles with High
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Sub-10-nm Au-Pt-Pd Alloy Trimetallic Nanoparticles with High
Magnetic Silica Particles for Catalysis
 4 Magnetic Silica Particles for atalysis Abstract Monodisperse magnetizable colloidal silica particles in a stable dispersion have been functionalized with a homogeneous catalyst: a PP-pincer Pd-complex.
4 Magnetic Silica Particles for atalysis Abstract Monodisperse magnetizable colloidal silica particles in a stable dispersion have been functionalized with a homogeneous catalyst: a PP-pincer Pd-complex.
INFLUENCE OF SUPERCRITICAL CO 2 IN THE REACTIVITY OF A REACTIVE DYE WITH A MODEL ALCOHOL IN A TUBULAR REACTOR
 IFLUECE OF SUPERCRITICAL CO 2 I THE REACTIVITY OF A REACTIVE DYE WITH A MODEL ALCOHOL I A TUBULAR REACTOR M.V. Fernandez Cid* a, M. van der Kraan a, W.J.T. Veugelers b, G.F. Woerlee b, G.J. Witkamp a a
IFLUECE OF SUPERCRITICAL CO 2 I THE REACTIVITY OF A REACTIVE DYE WITH A MODEL ALCOHOL I A TUBULAR REACTOR M.V. Fernandez Cid* a, M. van der Kraan a, W.J.T. Veugelers b, G.F. Woerlee b, G.J. Witkamp a a
Effects of Different Processing Parameters on Divinylbenzene (DVB) Production Rate
 1 Effects of Different Processing Parameters on Divinylbenzene (DVB) Production Rate ME Zeynali Petrochemical Synthesis Group, Petrochemical Faculty, Iran Polymer and Petrochemical Institute (IPPI), P.O.
1 Effects of Different Processing Parameters on Divinylbenzene (DVB) Production Rate ME Zeynali Petrochemical Synthesis Group, Petrochemical Faculty, Iran Polymer and Petrochemical Institute (IPPI), P.O.
The GO was synthesized by oxidation of purified natural small graphite and graphite
 Jing-He Yang, a,b Geng Sun, a Yongjun Gao, a Huabo Zhao, a Pei Tang, a Juan Tan, b Lu b and Ding Ma*,a An-Hui a Beijing National Laboratory for Molecular Sciences, College of Chemistry and Molecular Engineering,
Jing-He Yang, a,b Geng Sun, a Yongjun Gao, a Huabo Zhao, a Pei Tang, a Juan Tan, b Lu b and Ding Ma*,a An-Hui a Beijing National Laboratory for Molecular Sciences, College of Chemistry and Molecular Engineering,
Rh 3d. Co 2p. Binding Energy (ev) Binding Energy (ev) (b) (a)
 Co 2p Co(0) 778.3 Rh 3d Rh (0) 307.2 810 800 790 780 770 Binding Energy (ev) (a) 320 315 310 305 Binding Energy (ev) (b) Supplementary Figure 1 Photoemission features of a catalyst precursor which was
Co 2p Co(0) 778.3 Rh 3d Rh (0) 307.2 810 800 790 780 770 Binding Energy (ev) (a) 320 315 310 305 Binding Energy (ev) (b) Supplementary Figure 1 Photoemission features of a catalyst precursor which was
One-pot Solvent-free Synthesis of Sodium Benzoate from the Oxidation of Benzyl Alcohol over Novel Efficient AuAg/TiO 2 Catalysts
 Electronic Supplementary Information One-pot Solvent-free Synthesis of Sodium Benzoate from the Oxidation of Benzyl Alcohol over Novel Efficient AuAg/TiO 2 Catalysts Ying Wang, Jia-Min Zheng, Kangnian
Electronic Supplementary Information One-pot Solvent-free Synthesis of Sodium Benzoate from the Oxidation of Benzyl Alcohol over Novel Efficient AuAg/TiO 2 Catalysts Ying Wang, Jia-Min Zheng, Kangnian
Mechanistic Studies of Wacker-Type Amidocyclization. and Stereochemical Implications of Proton Transfer
 Supporting Information Mechanistic Studies of Wacker-Type Amidocyclization of Alkenes Catalyzed by (IMes)Pd(TFA) 2 (H 2 O): Kinetic and Stereochemical Implications of Proton Transfer Xuan Ye, Paul B. White
Supporting Information Mechanistic Studies of Wacker-Type Amidocyclization of Alkenes Catalyzed by (IMes)Pd(TFA) 2 (H 2 O): Kinetic and Stereochemical Implications of Proton Transfer Xuan Ye, Paul B. White
Supporting Information. for. A Sustainable Protocol for the Spontaneous Synthesis of Zinc-Glutamate. Wet Conditions
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Supporting Information for A Sustainable Protocol for the Spontaneous Synthesis of Zinc-Glutamate
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Supporting Information for A Sustainable Protocol for the Spontaneous Synthesis of Zinc-Glutamate
Chemical Reaction Engineering. Multiple Reactions. Dr.-Eng. Zayed Al-Hamamre
 Chemical Reaction Engineering Multiple Reactions Dr.-Eng. Zayed Al-Hamamre 1 Content Types of Reactions Selectivity Reaction Yield Parallel Reactions Series Reactions Net Rates of Reaction Complex Reactions
Chemical Reaction Engineering Multiple Reactions Dr.-Eng. Zayed Al-Hamamre 1 Content Types of Reactions Selectivity Reaction Yield Parallel Reactions Series Reactions Net Rates of Reaction Complex Reactions
Supporting Information
 Electronic Supplementary Material (ESI) for CrystEngComm. This journal is The Royal Society of Chemistry 2018 Supporting Information 2-Methylimidazole-Assisted Synthesis of Two-Dimensional MOF-5 Catalyst
Electronic Supplementary Material (ESI) for CrystEngComm. This journal is The Royal Society of Chemistry 2018 Supporting Information 2-Methylimidazole-Assisted Synthesis of Two-Dimensional MOF-5 Catalyst
Optimization of Batch Distillation Involving Hydrolysis System
 273 Optimization of Batch Distillation Involving Hydrolysis System Elmahboub A. Edreder 1, Iqbal M. Mujtaba 2, Mansour Emtir 3* 1 Libyan Petroleum Institute, P.O. Box 6431, Tripoli, Libya 2 School of Engineering
273 Optimization of Batch Distillation Involving Hydrolysis System Elmahboub A. Edreder 1, Iqbal M. Mujtaba 2, Mansour Emtir 3* 1 Libyan Petroleum Institute, P.O. Box 6431, Tripoli, Libya 2 School of Engineering
Strategic use of CuAlO 2 as a sustained release catalyst for production of hydrogen from methanol steam reforming
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Strategic use of CuAlO 2 as a sustained release catalyst for
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Strategic use of CuAlO 2 as a sustained release catalyst for
Total: 10 pages, 4 figures and 4 tables.
 Supporting Information Catalysis in flow: Nickel-catalyzed synthesis of primary amines from alcohols and NH 3 Andrew Yuk Keung Leung, Klaus Hellgardt and Mimi (King Kuok) Hii Total: 10 pages, 4 figures
Supporting Information Catalysis in flow: Nickel-catalyzed synthesis of primary amines from alcohols and NH 3 Andrew Yuk Keung Leung, Klaus Hellgardt and Mimi (King Kuok) Hii Total: 10 pages, 4 figures
Supporting Information
 Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2016 Supporting Information Single-atom dispersed Co--C catalyst: structure identification and
Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2016 Supporting Information Single-atom dispersed Co--C catalyst: structure identification and
PRODUCTION OF ETHYL ACETATE USING CATALYTIC REACTION METHOD
 PRODUCTION OF ETHYL ACETATE USING CATALYTIC REACTION METHOD Bamunusingha Arachchige Nadeeka Niroshinie Bamunusingha (108001 T) Thesis submitted in partial fulfillment of the requirements for the degree
PRODUCTION OF ETHYL ACETATE USING CATALYTIC REACTION METHOD Bamunusingha Arachchige Nadeeka Niroshinie Bamunusingha (108001 T) Thesis submitted in partial fulfillment of the requirements for the degree
CHEMICAL and BIOMOLECULAR ENGINEERING 140 Exam 1 Friday, September 28, 2018 Closed Book. Name: Section:
 CHEMICAL and BIOMOLECULAR ENGINEERING 140 Exam 1 Friday, September 28, 2018 Closed Book Name: Section: Total score: /100 Problem 1: /30 Problem 2: /35 Problem 3: /35 1. (30 points) Short answer questions:
CHEMICAL and BIOMOLECULAR ENGINEERING 140 Exam 1 Friday, September 28, 2018 Closed Book Name: Section: Total score: /100 Problem 1: /30 Problem 2: /35 Problem 3: /35 1. (30 points) Short answer questions:
ORGANIC SYNTHESIS: MICROWAVE-ASSISTED FISCHER ESTERIFICATION
 EXPERIMENT 7 ORGANIC SYNTHESIS: MICROWAVE-ASSISTED FISCHER ESTERIFICATION Materials Needed 1.0-2.0 ml of an alcohol to be chosen from the following: 3-methyl 1-butanol (isoamyl alcohol, isopentyl alcohol),
EXPERIMENT 7 ORGANIC SYNTHESIS: MICROWAVE-ASSISTED FISCHER ESTERIFICATION Materials Needed 1.0-2.0 ml of an alcohol to be chosen from the following: 3-methyl 1-butanol (isoamyl alcohol, isopentyl alcohol),
PHASE EQUILIBRIUM ENGINEERING OF SUPERCRITICAL REACTORS
 PHASE EQUILIBRIUM ENGINEERING OF SUPERCRITICAL REACTORS S. Pereda, L. J. Rovetto, S. B. Bottini and E. A. Brignole PLAPIQUI - Universidad Nacional del Sur CONICET Camino La Carrindanga Km 7, 8 Bahía Blanca,
PHASE EQUILIBRIUM ENGINEERING OF SUPERCRITICAL REACTORS S. Pereda, L. J. Rovetto, S. B. Bottini and E. A. Brignole PLAPIQUI - Universidad Nacional del Sur CONICET Camino La Carrindanga Km 7, 8 Bahía Blanca,
Copper-Catalyzed Oxidative Amination of Benzoxazoles via C-H and C-N Bond Activation: A
 Supporting Information for: Copper-Catalyzed xidative Amination of Benzoxazoles via C-H and C- Bond Activation: A ew Strategy for Using Tertiary Amines as itrogen Group Sources Shengmei Guo, Bo Qian, Yinjun
Supporting Information for: Copper-Catalyzed xidative Amination of Benzoxazoles via C-H and C- Bond Activation: A ew Strategy for Using Tertiary Amines as itrogen Group Sources Shengmei Guo, Bo Qian, Yinjun
Supporting Information
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Supporting Information One-of-A-Kind: A Microporous Metal-Organic Framework
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Supporting Information One-of-A-Kind: A Microporous Metal-Organic Framework
Supporting Information for
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2017 Supporting Information for Microwave-enhanced catalytic wet peroxide oxidation of quinoline:
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2017 Supporting Information for Microwave-enhanced catalytic wet peroxide oxidation of quinoline:
Supporting information. Stability Issues in Pd-based Catalysts: The Role of Surface Pt in Improving the Stability
 Supporting information Stability Issues in Pd-based Catalysts: The Role of Surface Pt in Improving the Stability and Oxygen Reduction Reaction (ORR) Activity R. K. Singh, R. Rahul, M. Neergat 1 Department
Supporting information Stability Issues in Pd-based Catalysts: The Role of Surface Pt in Improving the Stability and Oxygen Reduction Reaction (ORR) Activity R. K. Singh, R. Rahul, M. Neergat 1 Department
Catalytic Vapor Phase Hydration Acetylene and Its Derivatives
 Catalytic Vapor Phase Hydration Acetylene and Its Derivatives N.I. Fayzullaev, Samarkand State University. Alisher Navoi Abstract The reactions of the synthesis of acetylene from direct hydration of acetone
Catalytic Vapor Phase Hydration Acetylene and Its Derivatives N.I. Fayzullaev, Samarkand State University. Alisher Navoi Abstract The reactions of the synthesis of acetylene from direct hydration of acetone
Experiment 2 Solvent-free Aldol Condensation between 3,4-dimethoxybenzaldehyde and 1-indanone
 Experiment 2 Solvent-free Aldol Condensation between 3,4-dimethoxybenzaldehyde and 1-indanone Chemical Concepts Carbonyl chemistry, base catalyzed aldol reaction, melting point, recrystallization Green
Experiment 2 Solvent-free Aldol Condensation between 3,4-dimethoxybenzaldehyde and 1-indanone Chemical Concepts Carbonyl chemistry, base catalyzed aldol reaction, melting point, recrystallization Green
Experimental and Simulation Study on the Reactive Distillation Process for the Production of Ethyl Acetate
 Experimental and Simulation Study on the Reactive Distillation Process for the Production of Ethyl Acetate Jongkee Park, Na-Hyun Lee, So-Jin Park, and Jungho Cho, Separation Process Research Center, Korea
Experimental and Simulation Study on the Reactive Distillation Process for the Production of Ethyl Acetate Jongkee Park, Na-Hyun Lee, So-Jin Park, and Jungho Cho, Separation Process Research Center, Korea
Investigation of Petasis and Ugi Reactions in Series in an Automated Microreactor System
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supplementary Information Investigation of Petasis and Ugi Reactions in Series in an Automated
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supplementary Information Investigation of Petasis and Ugi Reactions in Series in an Automated
Chemistry 263 Laboratory Experiment 3: The Cannizzaro Disproportionation of Benzaldehyde to Benzoic Acid and Benzyl Alcohol 1 4/19/18, 4/20/18
 Chemistry 263 Laboratory Experiment 3: The Cannizzaro Disproportionation of Benzaldehyde to Benzoic Acid and Benzyl Alcohol 1 4/19/18, 4/20/18 Introduction If an aldehyde has no -hydrogens that can be
Chemistry 263 Laboratory Experiment 3: The Cannizzaro Disproportionation of Benzaldehyde to Benzoic Acid and Benzyl Alcohol 1 4/19/18, 4/20/18 Introduction If an aldehyde has no -hydrogens that can be
Microwave-Assisted Biginelli Reaction
 Boston University penbu Chemistry http://open.bu.edu rganic Chemistry Laboratory Experiments 2011-07-14 Microwave-Assisted Biginelli Reaction Mulcahy, Seann P. https://hdl.handle.net/2144/1416 Boston University
Boston University penbu Chemistry http://open.bu.edu rganic Chemistry Laboratory Experiments 2011-07-14 Microwave-Assisted Biginelli Reaction Mulcahy, Seann P. https://hdl.handle.net/2144/1416 Boston University
Aspects on reaction intensification
 Aspects on reaction intensification Tapio Salmi Åbo AkademiLaboratory of Industrial Chemistry and Reaction Engineering FI-20500 Turku / Åbo Finland Process intensification Raw material Process Products
Aspects on reaction intensification Tapio Salmi Åbo AkademiLaboratory of Industrial Chemistry and Reaction Engineering FI-20500 Turku / Åbo Finland Process intensification Raw material Process Products
Supporting Information. Photocatalytic C-H activation of Hydrocarbons over 3 N 4
 Supporting Information Photocatalytic C-H activation of Hydrocarbons over VO@g-C 3 N 4 Sanny Verma a, R. B. Nasir Baig a, Mallikarjuna N. Nadagouda b and Rajender S. Varma a* a Sustainable Technology Division,
Supporting Information Photocatalytic C-H activation of Hydrocarbons over VO@g-C 3 N 4 Sanny Verma a, R. B. Nasir Baig a, Mallikarjuna N. Nadagouda b and Rajender S. Varma a* a Sustainable Technology Division,
Supporting Information
 Supporting Information Conversion and Kinetics Study of Fructose-to-5- Hydroxymethylfurfural (HMF) Using Sulfonic and Ionic Liquid Groups Bi-functionalized Mesoporous Silica Nanoparticles as Recyclable
Supporting Information Conversion and Kinetics Study of Fructose-to-5- Hydroxymethylfurfural (HMF) Using Sulfonic and Ionic Liquid Groups Bi-functionalized Mesoporous Silica Nanoparticles as Recyclable
