Supplementary Materials for
|
|
|
- Morgan Jackson
- 5 years ago
- Views:
Transcription
1 advances.sciencemag.org/cgi/content/full/3/6/e /dc1 Supplementary Materials for MnTiO3-driven low-temperature oxidative coupling of methane over TiO2-doped Mn2O3-Na2WO4/SiO2 catalyst This PDF file includes: Pengwei Wang, Guofeng Zhao, Yu Wang, Yong Lu Published 9 June 2017, Sci. Adv. 3, e (2017) DOI: /sciadv Supplementary Text fig. S1. CH4 conversion and C2-C3 selectivity for the pure supports and the supported Mn2O3-Na2WO4 catalysts. fig. S2. SEM and EDX mapping images. fig. S3. XRD patterns and testing results of the 2.7Mn2O3-5.0Na2WO4/Ti-MWW and 2.7Mn2O3-5.0Na2WO4/TS-1 catalysts under different reaction conditions. fig. S4. XRD patterns and testing results for various samples with different Si:Ti molar ratio (or Ti content). fig. S5. XPS spectra of various samples. fig. S6. Raman spectra of various samples. fig. S7. Testing results of the catalysts with different active components. fig. S8. Ea calculations. fig. S9. Testing results of the catalysts with different active components prepared by the grinding method. fig. S10. H2-TPR profiles and XRD patterns. fig. S11. Effects of Mn2O3 plus TiO2, and Na2WO4 loadings on the OCM performance for the Mn2O3-TiO2-Na2WO4/SiO2 catalyst. fig. S12. Stability testing of the 6.2Mn2O3-6.3TiO2-10Na2WO4/SiO2 catalyst. fig. S13. Testing results and XRD patterns for the 6.2Mn2O3-6.3TiO2-10Na2WO4/SiO2 catalyst under different reaction conditions. table S1. Detailed treatment history of some catalysts for XRD and Raman measurements. table S2. Specific surface areas and real contents of Mn, W, Na, and Ti of all used catalysts.
2 table S3. Surface contents of W, Mn, Na, Ti, Si, O, and C measured by XPS for the used catalysts. table S4. CH4 conversion and C2-C3 selectivity over representative catalysts. References (38 49)
3 Supplementary Text Activation energy (Ea) calculations According to the reaction network of Stansch et al. (38), methane is converted in three parallel reactions, the formation of C2-C3 products (C2H6, C2H4, C3H8, and C3H6) by oxidative coupling of methane, the nonselective total oxidation of methane to carbon dioxide, and the partial oxidation of methane to carbon monoxide. Methane has been recognized to be oxidized on the catalyst surface through an adsorption step followed by a single hydrogen abstraction, C-H bond cleavage and methyl (CH3 ) radical coupling to form C2-C3 products in the OCM process. The pathway from CH4 to C2-C3 products (equation 1) was used to calculate the Ea of the OCM process CH4 + O2 C2H6 + C2H4 + C3H8 + C3H6 + H2O (1) The reaction rate of the OCM process on the Mn2O3-Na2WO4/Ti-MWW and Mn2O3- Na2WO4/SiO2 catalysts was determined according to equation 1 in the temperature range of o C, in which kinetics limit the rate (39). The reaction rate is defined as (2) where FCH4,in (mol s -1 ) is the molar flow rate of CH4 at the inlet and W (g) the mass of catalyst filled in the reactor. The kinetic experiments can be divided into two parts to determine the Ea. During the experiments, we kept the total gas flow constant and changed the weight of the catalysts. Fig. S8A shows the variation of the conversion of CH4 to C2-C3 products (i.e., C2-C3 products yield) against W/FCH4,in. The CH4 conversion to C2-C3 products changed linearly with W/FCH4,in and the reaction rate was calculated on the basis of the slope of the straight line at each temperature. Fig. S8B also shows the linear relationship of the Ln function of the reaction rate with the reciprocal of temperature for the Mn2O3-Na2WO4/SiO2 catalyst. According to the Arrhenius equation (Lnk = LnA - Ea/RT), Ea is the slope of the straight line in fig. S8B and was estimated to be kj mol -1, consistent with the reported results for the Mn2O3-Na2WO4/SiO2 catalyst ( 200 kj mol -1 ) (19, 38). For the Mn2O3-Na2WO4/Ti-MWW catalyst, Ea was estimated to be kj mol -1 (fig. S8, C and D).
4 Evolution of Mn species from MnTiO3 (or MnWO4) to Mn2O3 in an O2 stream and Mn2O3 reversed into MnTiO3 (or MnWO4) in a CH4 stream The Mn 2+ in the Mn2O3-Na2WO4/SiO2 catalyst reacted exclusively to MnWO4, while in the Mn2O3-Na2WO4/Ti-MWW catalyst Mn 2+ reacted to MnTiO3 and to a trace of MnWO4 (Fig. 2A). Mn 3+ on the other hand, reacted exclusively to Mn2O3 for both catalysts. Therefore, we chose the change in Mn 3+ amount to show the evolution of Mn species of the two catalysts in the stream of O2 or CH4. The Mn 3+ content was calculated from the height of the Mn2O3 XRD peak at 2θ = 33 o. All Mn species of the Mn2O3-Na2WO4/Ti-MWW catalyst (mainly MnTiO3 and some MnWO4), transformed fully and fast into Mn2O3 in the O2 stream (i.e., in 1 min at 800 o C, 2 min at 760 o C, and 4 min at 720 o C), and the height of the Mn2O3 XRD peak of this sample was taken as reference height (Mn 3+ content of 100%). The Mn 3+ contents of the other samples treated in the O2 stream was calculated by dividing the height of their Mn2O3 XRD peaks by the height of the reference peak. Subsequently, the Mn2O3 was mainly reduced to MnTiO3 as well as to a trace of MnWO4 in the CH4 stream, and the Mn 3+ contents of these samples treated in CH4 stream was calculated in the same way from the heights of the XRD peaks. All MnWO4 species of the Mn2O3-Na2WO4/SiO2 catalyst could be fully transformed into Mn2O3 in the O2 stream, but relatively slowly (i.e., 3 min at 800 o C, 15 min at 760 o C, and 30 min at 720 o C). The height of the Mn2O3 XRD peak of this sample was taken as reference height (Mn 3+ content of 100%). The Mn 3+ contents of the other samples treated in the O2 stream were calculated by dividing the height of their Mn2O3 XRD peaks by the height of the reference peak. Subsequently, the Mn2O3 was reduced to MnWO4 in the CH4 stream, and the Mn 3+ contents of the resulting samples was calculated in the same way from the heights of the XRD peaks. Transition rate of Mn 2+ to Mn 3+ The half-cycle transition rate of Mn 2+ to Mn 3+ (%/min) was defined as the change of Mn 3+ (%) per minute within the linear portion of the curve (Fig. 2, D to F) in the O2 stream. For example, the transition rate of Mn 2+ to Mn 3+ for the Mn2O3-Na2WO4/Ti-MWW catalyst at 760 o C (Fig. 2E) was calculated from the linear portion of this curve (i.e., the
5 time range from 0 to 2 min). With a change of Mn 3+ (%) of 74% in this range, the transition rate was 37%/min (74% divided by 2 min). Similarly, for the Mn2O3- Na2WO4/SiO2 catalyst at 760 o C (Fig. 2E), the linear portion is from 0 to 10 min with a Mn 3+ (%) change of 70%, so that the transition rate is 7%/min (70% divided by 10 min). The transition rates of the Mn 3+ species for both catalysts at 720 and 800 o C were obtained by the same method. Notably, selecting the data within the linear portion is to make the calculation results more accurate. H2-TPR and XRD analysis The Mn2O3-TiO2-Na2WO4/SiO2 catalysts with different Mn2O3 loadings (4.3, 7.1, and 10 wt%) were characterized by H2-TPR (fig. S10A). Two peaks at o C and o C were generated for each Mn2O3-TiO2-Na2WO4/SiO2 catalyst. Na2WO4 contributes to the peak at o C because pure MnWO4 (not shown) and the Na2WO4/SiO2 sample generated only one H2-TPR-peak in this range (purple line in fig. S10A). Moreover, clear MnWO4 diffraction (2θ of 29.9 and 30.3 o ) was shown in the XRD patterns of the three catalysts (fig. S10B). Therefore, the peaks at o C of our catalysts should be generated by both MnWO4 and Na2WO4. The peaks at o C exhibited an increased area with increasing XRD-intensity of Mn2O3 (fig. S10B) and, accordingly, Mn2O3 should contribute mainly to these peaks. In addition, the XRD patterns in fig. S10B showed clear MnTiO3 (2θ of 32.2 and 35 o ) and TiO2 (2θ of 25.3 o ) diffractions. As a reference, the pure sample of MnTiO3 as well as Mn2O3 and TiO2, was also characterized by H2-TPR (fig. S10C). Their reduction temperatures are much lower than that of Na2WO4. Combining the fact that there was only one peak at o C, the Mn2O3 and MnTiO3 as well as TiO2 should be reduced at the same temperature. Therefore, the peaks at o C should be mainly generated by Mn2O3 as well as by MnTiO3 and TiO2. However, the reduction temperature of o C is much higher than those of pure Mn2O3 at 542 o C and MnTiO3 at 473 o C. Such increases in Mn2O3 and MnTiO3 reduction temperatures are likely caused by the Mn2O3- Na2WO4 and MnTiO3-Na2WO4 interactions, which increase their reduction temperatures with the result of improved catalyst selectivity (9).
6 fig. S1. CH4 conversion and C2-C3 selectivity for the pure supports and the supported Mn2O3-Na2WO4 catalysts. The pure supports include Ti-MWW and TS-1 zeolites (Si:Ti molar ratio of 40:1), pure anatase-tio2, Ti2O3, amorphous SiO2-gel, and perovskite CaTiO3. The catalysts were prepared by loading 2.7 wt% Mn2O3 and 5.0 wt% Na2WO4 on these pure supports. Reaction conditions: GHSV of 8000 ml gcat. -1 h -1 of a feed of 50% CH4 in air. Note: The C3 selectivity was 3-5%, 2-3%, and 0-2% for all catalysts at a C2-C3 total selectivity above 60%, between 40 and 60%, and below 40%, respectively.
7 fig. S2. SEM and EDX mapping images. SEM and EDX mapping images of W, Mn, and Na elements of the used Mn2O3-Na2WO4/Ti-MWW (denoted as Cat-Ti-MWW), Mn2O3-Na2WO4/TS- 1 (denoted as Cat-TS-1), and Mn2O3-Na2WO4/SiO2 (denoted as Cat-SiO2) catalysts (see detailed treatment history in table S1). The morphologies of the three catalysts are different, but the EDX mapping images indicate that their dispersions are similar.
8 fig. S3. XRD patterns and testing results of the 2.7Mn2O3-5.0Na2WO4/Ti-MWW and 2.7Mn2O3-5.0Na2WO4/TS-1 catalysts under different reaction conditions. XRD patterns of the 2.7Mn2O3-5.0Na2WO4/Ti-MWW catalyst (red line) and the 2.7Mn2O3-5.0Na2WO4/TS-1 catalyst (light dark line) after directly undergoing OCM reaction at 720 o C, and the 2.7Mn2O3-5.0Na2WO4/TS-1 catalyst after undergoing OCM reaction at 800 o C and subsequently at 720 o C (navy line), as well as CH4 conversion (CCH4) and C2-C3 selectivity (SC2-3) at 720 o C over these catalysts. Reaction conditions: GHSV of 8000 ml gcat. -1 h -1 of a feed of 50% CH4 in air. Note: the C3 selectivity was 4% for the 2.7Mn2O3-5.0Na2WO4/Ti-MWW catalyst when directly tested at 720 o C, 1-2% for the 2.7Mn2O3-5.0Na2WO4/TS-1 catalyst when directly tested at 720 o C, and 3-4% for the 2.7Mn2O3-5.0Na2WO4/TS-1 catalyst after reaction at 800 o C followed by testing at 720 o C.
9 fig. S4. XRD patterns and testing results for various samples with different Si:Ti molar ratio (or Ti content). (A) XRD patterns of the as-prepared Ti-MWW zeolites with Si:Ti molar ratio from 80:1 to 40:1 as well as the full Si zeolite also with MWW structure. (B) XRD patterns of the as-prepared TS-1 zeolites with Si:Ti molar ratio from 80:1 to 40:1 as well as the full Si zeolite also with MFI structure. (C) The Mn2O3-Na2WO4/TS-1 catalysts with different Si:Ti molar ratio in TS-1 zeolites after directly running at 720 o C. (D) The Mn2O3-Na2WO4/TS-1 catalysts with different Si:Ti molar ratio in TS-1 zeolites after running at 800 o C for 2 hours and subsequently at 720 o C for 0.5 hour. (E) CH4 conversion for the two series of catalysts in fig. S4, C and D at 720 o C. Reaction conditions: GHSV of 8000 ml gcat. -1 h -1 of a feed of 50% CH4 in air.
10 fig. S5. XPS spectra of various samples. (A) Mn 2p XPS of pure Mn2O3 and MnTiO3. (B) Mn 2p XPS, (C) W 3d XPS, (D) Na 1s XPS, (E) O 1s XPS, and (F) Si 1s XPS of the Cat-Ti-MWW, Cat-TS-1, and Cat-SiO2 catalysts (see detailed treatment history in table S1). Reaction conditions: GHSV of 8000 ml gcat. -1 h -1 of a feed of 50% CH4 in air.
11 fig. S6. Raman spectra of various samples. Raman spectra of pure MnOx (MnO, Mn3O4, Mn2O3 and MnO2), TiOx (Ti2O3, rutile-tio2 and anatase-tio2), Na2WO4, WO3, MnWO4, and MnTiO3, and of the Mn2O3-Na2WO4/SiO2 (denoted as Cat-SiO2), Mn2O3-Na2WO4/Ti-MWW (denoted as Cat-Ti-MWW), and Mn2O3-Na2WO4/TS-1 catalysts. Note: the Mn2O3-Na2WO4/SiO2 (denoted as Cat-SiO2) and Mn2O3-Na2WO4/Ti-MWW (denoted as Cat-Ti-MWW) catalysts experienced the OCM reaction at 800 o C for 2 hours and subsequently at 760 and 720 o C for 0.5 hour; the Mn2O3- Na2WO4/TS-1 catalyst experienced the OCM reaction directly at 720 o C for 0.5 hour.
12 fig. S7. Testing results of the catalysts with different active components. CH4 conversion (blue bar) and C2-C3 selectivity (gray bar) over the MnTiO3/SiO2, Mn2O3-Na2WO4/SiO2, Mn2O3/SiO2, Na2WO4/SiO2, and Mn2O3-Na2WO4/Ti-MWW catalysts. Fresh catalyst was employed for each testing at the appointed temperature. Reaction conditions: GHSV of 8000 ml gcat. -1 h -1 of a feed of 50% CH4 in air. Note: The C3 selectivity was 3-5%, 2-3%, and 0-2% for all catalysts at a C2-C3 total selectivity above 60%, between 40 and 60%, and below 40%, respectively.
13 fig. S8. Ea calculations. (A) Variation of the fractional conversion of CH4 to C2-C3 products (i.e., C2-C3 yield) against W/FCH4, and (B) Ln(Rate) as a function of 1000/T for the Mn2O3- Na2WO4/SiO2 catalyst. (C) Variation of fractional conversion of CH4 to C2-C3 products (i.e., C2- C3 yield) against W/FCH4, and (D) Ln(Rate) as a function of 1000/T for the Mn2O3-Na2WO4/Ti- MWW catalyst. Reaction conditions: a feed of 50% CH4 in air.
14 fig. S9. Testing results of the catalysts with different active components prepared by the grinding method. CH4 conversion (blue bar) and C2-C3 selectivity (gray bar) over the following catalysts prepared by the grinding method: MnTiO3-Mn2O3-Na2WO4, MnWO4-Mn2O3-Na2WO4, MnTiO3-Mn2O3, and MnWO4-Mn2O3. Reaction conditions: GHSV of 8000 ml gcat. -1 h -1 of a feed of 50% CH4 in air. Note: The C3 selectivity was 3-5%, 2-3%, and 0-2% for all catalysts at a C2-C3 total selectivity above 60%, between 40 and 60%, and below 40%, respectively.
15 fig. S10. H2-TPR profiles and XRD patterns. (A) H2-TPR profiles and (B) XRD patterns for the Mn2O3-TiO2-Na2WO4/SiO2 catalysts prepared by the grinding method (different Mn2O3 loadings of 4.3, 7.1, and 10 wt%). The loading of Na2WO4 was 10 wt%, and amorphous SiO2-gel as well as TiO2 made up the balance (with Si:Ti molar ratio of 20:1). (C) H2-TPR profiles for the pure Mn2O3, MnTiO3, and rutile-tio2.
16 fig. S11. Effects of Mn2O3 plus TiO2, and Na2WO4 loadings on the OCM performance for the Mn2O3-TiO2-Na2WO4/SiO2 catalyst. The catalysts with varied Mn2O3 plus TiO2 loadings (i.e., with stoichiometric ratio of Mn2O3 to TiO2 to be fully transformed into MnTiO3) and Na2WO4 loadings for the Mn2O3-TiO2-Na2WO4/SiO2 catalysts were prepared by grinding method. Reaction conditions: GHSV of 8000 ml gcat. -1 h -1 of a feed of 50% CH4 in air, 720 o C. Note: The C3 selectivity was 3-5%, 2-3%, and 0-2% for all catalysts at a C2-C3 total selectivity above 60%, between 40 and 60%, and below 40%, respectively.
17 fig. S12. Stability testing of the 6.2Mn2O3-6.3TiO2-10Na2WO4/SiO2 catalyst. CH4 conversion and C2-C3 selectivity along with the time on stream at 800 o C. Reaction conditions: GHSV of 8000 ml gcat. -1 h -1 of a feed of 50% CH4 in air. The C3 selectivity was 3-5%.
18
19 fig. S13. Testing results and XRD patterns for the 6.2Mn2O3-6.3TiO2-10Na2WO4/SiO2 catalyst under different reaction conditions. (A) CH4 conversion (blue bar) and C2-C3 selectivity (gray bar) for the 6.2Mn2O3-6.3TiO2-10Na2WO4/SiO2 catalyst directly running at 650, 680, 700, and 720 o C (fresh sample was employed for each testing experiment), and then 650 o C again (i.e., after reaction at 720 o C for 1 h and then at 650 o C for another 1 h). (B) XRD patterns of the 6.2Mn2O3-6.3TiO2-10Na2WO4/SiO2 catalyst after directly running at 650 o C (for 1, 4, 8, and 12 hours) and 720 o C (for 1 and 8 hours). Note: The C3 selectivity was 3-5%, 2-3%, and 0-2% for all catalysts at a C2-C3 total selectivity above 60%, between 40 and 60%, and below 40%, respectively.
20 table S1. Detailed treatment history of some catalysts for XRD and Raman measurements. Catalyst Treatment history The catalysts in the section of In-situ MnTiO3 formation dependent low-temperature activity improvement Mn2O3-Na2WO4/Ti-MWW denoted as Cat-Ti-MWW Mn2O3-Na2WO4/TS-1 denoted as Cat-TS-1 Mn2O3-Na2WO4/SiO2 denoted as Cat-SiO2 These catalysts firstly reacted in the OCM reaction at 800 o C for 2 hours. Then, the catalyst bed temperature was gradually reduced to 720 o C, and these catalysts reacted for 0.5 hour. The catalysts in the section of MnTiO3-triggered Mn 2+ Mn 3+ chemical cycle the nature of low-temperature OCM catalysis Mn2O3-Na2WO4/Ti-MWW denoted as Ti-MWW catalyst in Fig. 2A-C,G-I Mn2O3-Na2WO4/SiO2 denoted as SiO2 catalyst in Fig. 2A-C,G-I The two catalysts (under working state) were reduced at 800 o C for 0.5 hour in CH4 stream. Then, the catalysts were treated respectively at 800, 760, 720 o C for some time in O2 stream. Finally, the catalysts were reduced respectively at 800, 760, 720 o C for some time in CH4 stream. table S2. Specific surface areas and real contents of Mn, W, Na, and Ti of all used catalysts. Catalyst a SSA (m 2 /g) Pore Size (nm) Contents (wt%) b Mn W Na Ti Mn2O3-Na2WO4/Ti-MWW (Cat-Ti-MWW) Mn2O3-Na2WO4/TS-1 (Cat-TS-1) Mn2O3-Na2WO4/SiO2 (Cat-SiO2) a The OCM reaction was run over all catalysts for 2 hours at 800 o C followed by at 720 o C for 0.5 hour using 50 vol% CH 4 in air at 720 o C with a total GHSV of 8000 ml g -1 h -1. b All contents were determined by AES-ICP measurements.
21 table S3. Surface contents of W, Mn, Na, Ti, Si, O, and C measured by XPS for the used catalysts. Catalyst a Elements contents (mol %) Na Mn W Ti Si O C Mn2O3-Na2WO4/Ti-MWW Mn2O3-Na2WO4/TS Mn2O3-Na2WO4/SiO a The OCM reaction was run over all catalysts at 800 o C for 2 hours and subsequently at 720 o C for 0.5 hour using a feed of 50 vol% CH 4 in air with a total GHSV of 8000 ml g -1 h -1.
22 table S4. CH4 conversion and C2-C3 selectivity over representative catalysts. Catalyst Temp. ( o C) v a (ml/(min g)) C:O:X b Conv. (%) Select. (%) C2-3Yield (%) Ref. Li/MgO :8: Ba/MgO :1: NaMnO4/MgO :10: La2O :2: La2O3/MgO :20: SrLa2O :9: LiSrLa2O :15: LiCa2Bi3O4Cl :10: Bi1.5Y0.3Sm0.2O3-x :10: Rb2WO4/SiO : Mn-Na2WO4/SiO :2: Mn-Na2WO4/SiO :1: Mn-Na2WO4/SBA :1: Mn2O3-6.3TiO2-10Na2WO4/SiO :1: c 20.5 p.w. d :1: c 17.5 p.w. d :1: c 13.6 p.w. d a Gas hourly space velocity. b CH 4/O 2/balance (N 2, He, or Ar in the corresponding reference). c Including C 3 selectivity of 3-5%. d Present work.
Plasma driven ammonia decomposition on Fe-catalyst: eliminating surface nitrogen poisoning
 Supporting Information for Plasma driven ammonia decomposition on Fe-catalyst: eliminating surface nitrogen poisoning Contents: 1. Scheme of the DBD plasma-driven catalysis reactor, Scheme S1. 2. XRF analysis
Supporting Information for Plasma driven ammonia decomposition on Fe-catalyst: eliminating surface nitrogen poisoning Contents: 1. Scheme of the DBD plasma-driven catalysis reactor, Scheme S1. 2. XRF analysis
Strategic use of CuAlO 2 as a sustained release catalyst for production of hydrogen from methanol steam reforming
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Strategic use of CuAlO 2 as a sustained release catalyst for
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Strategic use of CuAlO 2 as a sustained release catalyst for
Catalysis Science & Technology
 Catalysis Science & Technology Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted
Catalysis Science & Technology Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted
Rh 3d. Co 2p. Binding Energy (ev) Binding Energy (ev) (b) (a)
 Co 2p Co(0) 778.3 Rh 3d Rh (0) 307.2 810 800 790 780 770 Binding Energy (ev) (a) 320 315 310 305 Binding Energy (ev) (b) Supplementary Figure 1 Photoemission features of a catalyst precursor which was
Co 2p Co(0) 778.3 Rh 3d Rh (0) 307.2 810 800 790 780 770 Binding Energy (ev) (a) 320 315 310 305 Binding Energy (ev) (b) Supplementary Figure 1 Photoemission features of a catalyst precursor which was
Ch 13 Rates of Reaction (Chemical Kinetics)
 Ch 13 Rates of Reaction (Chemical Kinetics) Reaction Rates and Kinetics - The reaction rate is how fast reactants are converted to products. - Chemical kinetics is the study of reaction rates. Kinetics
Ch 13 Rates of Reaction (Chemical Kinetics) Reaction Rates and Kinetics - The reaction rate is how fast reactants are converted to products. - Chemical kinetics is the study of reaction rates. Kinetics
By Rogéria Amaral and Sébastien Thomas
 Kinetics of CO 2 methanation over a Ni/alumina industrial catalyst By Rogéria Amaral and Sébastien Thomas Laboratoire de Matériaux, Surfaces et Procédés pour la Catalyse, Groupe Energie et Carburants pour
Kinetics of CO 2 methanation over a Ni/alumina industrial catalyst By Rogéria Amaral and Sébastien Thomas Laboratoire de Matériaux, Surfaces et Procédés pour la Catalyse, Groupe Energie et Carburants pour
Supporting Information
 Supporting Information Towards rational design of Cu/SSZ-13 selective catalytic reduction catalysts: implications from atomic-level understanding of hydrothermal stability James Song, a,c Yilin Wang, a
Supporting Information Towards rational design of Cu/SSZ-13 selective catalytic reduction catalysts: implications from atomic-level understanding of hydrothermal stability James Song, a,c Yilin Wang, a
Supporting Information
 Supporting Information Insight into the Formation of Co@Co 2 C Catalysts for Direct Synthesis of Higher Alcohols and Olefins from Syngas Ziang Zhao, 1,2, Wei Lu, 1, Ruoou Yang, 2,4 Hejun Zhu, 1,* Wenda
Supporting Information Insight into the Formation of Co@Co 2 C Catalysts for Direct Synthesis of Higher Alcohols and Olefins from Syngas Ziang Zhao, 1,2, Wei Lu, 1, Ruoou Yang, 2,4 Hejun Zhu, 1,* Wenda
Synthesis gas production via the biogas reforming reaction over Ni/MgO-Al 2 O 3 and Ni/CaO-Al 2 O 3 catalysts
 Synthesis gas production via the biogas reforming reaction over Ni/MgO-Al 2 O 3 and Ni/CaO-Al 2 O 3 catalysts N.D. Charisiou 1,2, A. Baklavaridis 1, V.G. Papadakis 2, M.A. Goula 1 1 Department of Environmental
Synthesis gas production via the biogas reforming reaction over Ni/MgO-Al 2 O 3 and Ni/CaO-Al 2 O 3 catalysts N.D. Charisiou 1,2, A. Baklavaridis 1, V.G. Papadakis 2, M.A. Goula 1 1 Department of Environmental
Studies on Mo/HZSM-5 Complex catalyst for Methane Aromatization
 Journal of Natural Gas Chemistry 13(2004)36 40 Studies on Mo/HZSM-5 Complex catalyst for Methane Aromatization Qun Dong 1, Xiaofei Zhao 1, Jian Wang 1, M Ichikawa 2 1. Department of Petrochemical Engineering,
Journal of Natural Gas Chemistry 13(2004)36 40 Studies on Mo/HZSM-5 Complex catalyst for Methane Aromatization Qun Dong 1, Xiaofei Zhao 1, Jian Wang 1, M Ichikawa 2 1. Department of Petrochemical Engineering,
unique electronic structure for efficient hydrogen evolution
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supplementary Information Atom-scale dispersed palladium in conductive
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supplementary Information Atom-scale dispersed palladium in conductive
Supplementary Text and Figures
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Text and Figures NaCl Induced Nickel-Cobalt Inverse Spinel
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2018 Supplementary Text and Figures NaCl Induced Nickel-Cobalt Inverse Spinel
Supplementary Figure S1 Reactor setup Calcined catalyst (0.40 g) and silicon carbide powder (0.4g) were mixed thoroughly and inserted into a 4 mm
 Supplementary Figure S1 Reactor setup Calcined catalyst (.4 g) and silicon carbide powder (.4g) were mixed thoroughly and inserted into a 4 mm diameter silica reactor (G). The powder mixture was sandwiched
Supplementary Figure S1 Reactor setup Calcined catalyst (.4 g) and silicon carbide powder (.4g) were mixed thoroughly and inserted into a 4 mm diameter silica reactor (G). The powder mixture was sandwiched
Supporting Information for. Discovery of Multicomponent Heterogeneous Catalysts via Admixture Screening:
 Supporting Information for Discovery of Multicomponent Heterogeneous Catalysts via Admixture Screening: PdBiTe Catalysts for Aerobic xidative Esterification of Primary Alcohols David S. Mannel a, Maaz
Supporting Information for Discovery of Multicomponent Heterogeneous Catalysts via Admixture Screening: PdBiTe Catalysts for Aerobic xidative Esterification of Primary Alcohols David S. Mannel a, Maaz
Supporting Information
 Supporting Information Remarkable performance of Ir 1 /FeO x single-atom catalyst in water gas shift reaction Jian Lin, Aiqin Wang, Botao Qiao, Xiaoyan Liu, Xiaofeng Yang, Xiaodong Wang, Jinxia Liang,
Supporting Information Remarkable performance of Ir 1 /FeO x single-atom catalyst in water gas shift reaction Jian Lin, Aiqin Wang, Botao Qiao, Xiaoyan Liu, Xiaofeng Yang, Xiaodong Wang, Jinxia Liang,
Sub-10-nm Au-Pt-Pd Alloy Trimetallic Nanoparticles with. High Oxidation-Resistant Property as Efficient and Durable
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Sub-10-nm Au-Pt-Pd Alloy Trimetallic Nanoparticles with High
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Sub-10-nm Au-Pt-Pd Alloy Trimetallic Nanoparticles with High
Visible-light Driven Plasmonic Photocatalyst Helical Chiral TiO 2 Nanofibers
 Visible-light Driven Plasmonic Photocatalyst Ag/AgCl @ Helical Chiral TiO 2 Nanofibers Dawei Wang, Yi Li*, Gianluca Li Puma, Chao Wang, Peifang Wang, Wenlong Zhang, and Qing Wang Fig. S1. The reactor of
Visible-light Driven Plasmonic Photocatalyst Ag/AgCl @ Helical Chiral TiO 2 Nanofibers Dawei Wang, Yi Li*, Gianluca Li Puma, Chao Wang, Peifang Wang, Wenlong Zhang, and Qing Wang Fig. S1. The reactor of
Selective oxidation of methane to carbon monoxide on supported palladium catalyst
 Applied Catalysis A: General, 80 (1992) Ll-L5 Elsevier Science Publishers B.V., Amsterdam Ll APCAT 2187 Selective oxidation of methane to carbon monoxide on supported palladium catalyst A.K. Bhattacharya*,
Applied Catalysis A: General, 80 (1992) Ll-L5 Elsevier Science Publishers B.V., Amsterdam Ll APCAT 2187 Selective oxidation of methane to carbon monoxide on supported palladium catalyst A.K. Bhattacharya*,
Supporting Information for Atomic layer deposited TiO 2 on nitrogen-doped graphene/sulfur electrode for high performance lithiumsulfur
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2016 Supporting Information for Atomic layer deposited TiO 2 on nitrogen-doped
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2016 Supporting Information for Atomic layer deposited TiO 2 on nitrogen-doped
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/2/6/e1501764/dc1 Supplementary Materials for Efficient solar-driven water splitting by nanocone BiVO4-perovskite tandem cells Yongcai Qiu, Wei Liu, Wei Chen, Wei
advances.sciencemag.org/cgi/content/full/2/6/e1501764/dc1 Supplementary Materials for Efficient solar-driven water splitting by nanocone BiVO4-perovskite tandem cells Yongcai Qiu, Wei Liu, Wei Chen, Wei
Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c,
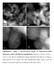 Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c, 2 nm; d, 2 nm. The low magnification images demonstrate
Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c, 2 nm; d, 2 nm. The low magnification images demonstrate
The GO was synthesized by oxidation of purified natural small graphite and graphite
 Jing-He Yang, a,b Geng Sun, a Yongjun Gao, a Huabo Zhao, a Pei Tang, a Juan Tan, b Lu b and Ding Ma*,a An-Hui a Beijing National Laboratory for Molecular Sciences, College of Chemistry and Molecular Engineering,
Jing-He Yang, a,b Geng Sun, a Yongjun Gao, a Huabo Zhao, a Pei Tang, a Juan Tan, b Lu b and Ding Ma*,a An-Hui a Beijing National Laboratory for Molecular Sciences, College of Chemistry and Molecular Engineering,
Supporting information
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2017 Supporting information Cu Supported on Thin Carbon Layer Coated Porous SiO
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2017 Supporting information Cu Supported on Thin Carbon Layer Coated Porous SiO
AP Chemistry - Notes - Chapter 12 - Kinetics Page 1 of 7 Chapter 12 outline : Chemical kinetics
 AP Chemistry - Notes - Chapter 12 - Kinetics Page 1 of 7 Chapter 12 outline : Chemical kinetics A. Chemical Kinetics - chemistry of reaction rates 1. Reaction Rates a. Reaction rate- the change in concentration
AP Chemistry - Notes - Chapter 12 - Kinetics Page 1 of 7 Chapter 12 outline : Chemical kinetics A. Chemical Kinetics - chemistry of reaction rates 1. Reaction Rates a. Reaction rate- the change in concentration
REACTION KINETICS. Catalysts substances that increase the rates of chemical reactions without being used up. e.g. enzymes.
 REACTION KINETICS Study of reaction rates Why? Rates of chemical reactions are primarily controlled by 5 factors: the chemical nature of the reactants 2 the ability of the reactants to come in contact
REACTION KINETICS Study of reaction rates Why? Rates of chemical reactions are primarily controlled by 5 factors: the chemical nature of the reactants 2 the ability of the reactants to come in contact
cis,cis-muconic acid: Separation and catalysis to bioadipic acid for nylon-6,6 polymerization
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information for: cis,cis-muconic acid: Separation and catalysis
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information for: cis,cis-muconic acid: Separation and catalysis
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/4/6/eaap9360/dc1 Supplementary Materials for Oxygen-deficient triple perovskites as highly active and durable bifunctional electrocatalysts for oxygen electrode
advances.sciencemag.org/cgi/content/full/4/6/eaap9360/dc1 Supplementary Materials for Oxygen-deficient triple perovskites as highly active and durable bifunctional electrocatalysts for oxygen electrode
RESULTS AND DISCUSSION Characterization of pure CaO and Zr-TiO 2 /CaO nanocomposite
 RESULTS AND DISCUSSION 4.1. Characterization of pure CaO and Zr-TiO 2 /CaO nanocomposite 4.1.1. Scanning electron microscopy analysis (SEM) SEM images of prepared CaO are shown in Fig. 4.1 (a and b). CaO
RESULTS AND DISCUSSION 4.1. Characterization of pure CaO and Zr-TiO 2 /CaO nanocomposite 4.1.1. Scanning electron microscopy analysis (SEM) SEM images of prepared CaO are shown in Fig. 4.1 (a and b). CaO
Supporting Information. Highly Selective Non-oxidative Coupling of Methane. over Pt-Bi Bimetallic Catalysts
 Supporting Information Highly Selective Non-oxidative Coupling of Methane over Pt-Bi Bimetallic Catalysts Yang Xiao and Arvind Varma Davidson School of Chemical Engineering, Purdue University, West Lafayette,
Supporting Information Highly Selective Non-oxidative Coupling of Methane over Pt-Bi Bimetallic Catalysts Yang Xiao and Arvind Varma Davidson School of Chemical Engineering, Purdue University, West Lafayette,
Reaction Chemistry of W-Mn/SiO 2 Catalyst for the Oxidative Coupling of Methane
 Journal of Natural Gas Chemistry 12(2003)1 9 Reaction Chemistry of W-Mn/SiO 2 Catalyst for the Oxidative Coupling of Methane Shuben Li State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou
Journal of Natural Gas Chemistry 12(2003)1 9 Reaction Chemistry of W-Mn/SiO 2 Catalyst for the Oxidative Coupling of Methane Shuben Li State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou
Characterization of Re-Pd/SiO 2 catalysts for hydrogenation of stearic acid
 Supporting Information: Characterization of Re-Pd/SiO 2 catalysts for hydrogenation of stearic acid Yasuyuki Takeda, [a] Masazumi Tamura, [a] Yoshinao Nakagawa, [a] Kazu Okumura, [b] and Keiichi Tomishige*
Supporting Information: Characterization of Re-Pd/SiO 2 catalysts for hydrogenation of stearic acid Yasuyuki Takeda, [a] Masazumi Tamura, [a] Yoshinao Nakagawa, [a] Kazu Okumura, [b] and Keiichi Tomishige*
Adjustment and Matching of Energy band of TiO 2 -based Photocatalysts by Metal Ions (Pd, Cu, Mn) for Photoreduction of CO 2 into CH 4.
 Supplementary Information Adjustment and Matching of Energy band of -based Photocatalysts by Metal Ions (Pd, Cu, Mn) for Photoreduction of CO 2 into CH 4. Yabin Yan a, Yanlong Yu c, Shaolong Huang d, Yajun
Supplementary Information Adjustment and Matching of Energy band of -based Photocatalysts by Metal Ions (Pd, Cu, Mn) for Photoreduction of CO 2 into CH 4. Yabin Yan a, Yanlong Yu c, Shaolong Huang d, Yajun
Scientific report 2016 January September. Designing composite nanoarchitectures for hydrogen production and environmental depollution
 Scientific report 2016 January September Designing composite nanoarchitectures for hydrogen production and environmental depollution Synthesis of the spherical Pt nanoparticles We have chosen to synthesize
Scientific report 2016 January September Designing composite nanoarchitectures for hydrogen production and environmental depollution Synthesis of the spherical Pt nanoparticles We have chosen to synthesize
Supporting Information High Activity and Selectivity of Ag/SiO 2 Catalyst for Hydrogenation of Dimethyloxalate
 Supporting Information High Activity and Selectivity of Ag/SiO 2 Catalyst for Hydrogenation of Dimethyloxalate An-Yuan Yin, Xiao-Yang Guo, Wei-Lin Dai*, Kang-Nian Fan Shanghai Key Laboratory of Molecular
Supporting Information High Activity and Selectivity of Ag/SiO 2 Catalyst for Hydrogenation of Dimethyloxalate An-Yuan Yin, Xiao-Yang Guo, Wei-Lin Dai*, Kang-Nian Fan Shanghai Key Laboratory of Molecular
Hexagonal Boron Nitride supported mesosio 2 -confined Ni Catalysts. for Dry Reforming of Methane
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information (ESI): Hexagonal Boron Nitride supported mesosio 2 -confined
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information (ESI): Hexagonal Boron Nitride supported mesosio 2 -confined
Electronic Supplementary Information (ESI) Tunable Phase and Visible-Light Photocatalytic Activity
 Electronic Supplementary Information (ESI) Metallic-Zinc Assistant Synthesis of Ti 3+ Self-Doped TiO 2 with Tunable Phase and Visible-Light Photocatalytic Activity Zhaoke Zheng, a Baibiao Huang,* a Xiaodong
Electronic Supplementary Information (ESI) Metallic-Zinc Assistant Synthesis of Ti 3+ Self-Doped TiO 2 with Tunable Phase and Visible-Light Photocatalytic Activity Zhaoke Zheng, a Baibiao Huang,* a Xiaodong
Supplementary Figure 2. (a) XRD patterns of the MOF and the simulated Ni-MOF-74
 Supplementary Figure 1. Low-magnification TEM image of Pt-Ni frame @ MOF. The scale bar is 200 nm. Supplementary Figure 2. (a) XRD patterns of the Pt-Ni @ MOF and the simulated Ni-MOF-74 pattern. (b) XRD
Supplementary Figure 1. Low-magnification TEM image of Pt-Ni frame @ MOF. The scale bar is 200 nm. Supplementary Figure 2. (a) XRD patterns of the Pt-Ni @ MOF and the simulated Ni-MOF-74 pattern. (b) XRD
Chapter 12. Chemical Kinetics
 Chapter 12 Chemical Kinetics Chapter 12 Table of Contents 12.1 Reaction Rates 12.2 Rate Laws: An Introduction 12.3 Determining the Form of the Rate Law 12.4 The Integrated Rate Law 12.5 Reaction Mechanisms
Chapter 12 Chemical Kinetics Chapter 12 Table of Contents 12.1 Reaction Rates 12.2 Rate Laws: An Introduction 12.3 Determining the Form of the Rate Law 12.4 The Integrated Rate Law 12.5 Reaction Mechanisms
KMUTNB Int J Appl Sci Technol, Vol. 9, No. 4, pp , 2016
 KMUTNB Int J Appl Sci Technol, Vol. 9, No. 4, pp. 255 259, 216 Research Article Effect of Strong Metal Support Interactions of Supported Ni and Ni-Co Catalyst on Metal Dispersion and Catalytic Activity
KMUTNB Int J Appl Sci Technol, Vol. 9, No. 4, pp. 255 259, 216 Research Article Effect of Strong Metal Support Interactions of Supported Ni and Ni-Co Catalyst on Metal Dispersion and Catalytic Activity
Reactions in Aqueous Solutions
 Reactions in Aqueous Solutions Chapter 4 Copyright McGraw-Hill Education. All rights reserved. No reproduction or distribution without the prior written consent of McGraw-Hill Education. Titrations In
Reactions in Aqueous Solutions Chapter 4 Copyright McGraw-Hill Education. All rights reserved. No reproduction or distribution without the prior written consent of McGraw-Hill Education. Titrations In
CHEM N-4 November 2014
 CHEM1002 2014-N-4 November 2014 The reaction order for a chemical reaction is given by the sum of the powers in the rate law. Why is the reaction order usually given by a small positive integer, i.e. 2
CHEM1002 2014-N-4 November 2014 The reaction order for a chemical reaction is given by the sum of the powers in the rate law. Why is the reaction order usually given by a small positive integer, i.e. 2
Supporting Information: Synthesis of Boron Doped Carbon Nitride Solids and Their Use as Metal Free Catalyst for Aliphatic C-H Bond Oxidation
 Supporting Information: Synthesis of Boron Doped Carbon itride Solids and Their Use as Metal Free Catalyst for Aliphatic C-H Bond Oxidation Yong Wang, * Haoran Li, Jia Yao, Xinchen Wang, and Markus Antonietti
Supporting Information: Synthesis of Boron Doped Carbon itride Solids and Their Use as Metal Free Catalyst for Aliphatic C-H Bond Oxidation Yong Wang, * Haoran Li, Jia Yao, Xinchen Wang, and Markus Antonietti
Chapter 3. Distinguishing between Reaction Intermediates and. Spectators: A Kinetic Study of Acetone Oxidation Using
 Chapter 3 Distinguishing between Reaction Intermediates and Spectators: A Kinetic Study of Acetone Oxidation Using Ozone on a Silica-Supported Manganese Oxide Catalyst 3.1 Introduction This chapter concentrates
Chapter 3 Distinguishing between Reaction Intermediates and Spectators: A Kinetic Study of Acetone Oxidation Using Ozone on a Silica-Supported Manganese Oxide Catalyst 3.1 Introduction This chapter concentrates
Chemistry 112 Midterm January 30, 2006
 1. (35 points) The reaction of A and B to form products is thought to go according to the following mechanism: A + 2B 2C + D k -1 2C 2C 2C + M k 2 k 3 k 4 G H J + M (a) (5) Identify the products in this
1. (35 points) The reaction of A and B to form products is thought to go according to the following mechanism: A + 2B 2C + D k -1 2C 2C 2C + M k 2 k 3 k 4 G H J + M (a) (5) Identify the products in this
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Experimental section Synthesis of Ni-Co Prussian
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Experimental section Synthesis of Ni-Co Prussian
Chlorohydrination of Allyl Chloride with HCl and H 2 O 2 to Produce. Dichloropropanols Catalyzed by Hollow TS-1 Zeolite
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 216 Chlorohydrination of Allyl Chloride with and 2 O 2 to Produce Dichloropropanols Catalyzed
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 216 Chlorohydrination of Allyl Chloride with and 2 O 2 to Produce Dichloropropanols Catalyzed
Chapter 12. Chemical Kinetics
 Chapter 12 Chemical Kinetics Section 12.1 Reaction Rates Section 12.1 Reaction Rates Section 12.1 Reaction Rates Section 12.1 Reaction Rates Section 12.1 Reaction Rates Section 12.1 Reaction Rates Section
Chapter 12 Chemical Kinetics Section 12.1 Reaction Rates Section 12.1 Reaction Rates Section 12.1 Reaction Rates Section 12.1 Reaction Rates Section 12.1 Reaction Rates Section 12.1 Reaction Rates Section
The role of various iron species in Fe-Beta catalysts with low. iron loadings for NH 3 -SCR
 Electronic Supplementary Material (ESI) for Catalysis Science. This journal is The Royal Society of Chemistry 2014 Supplementary Information: The role of various iron species in Fe-Beta catalysts with
Electronic Supplementary Material (ESI) for Catalysis Science. This journal is The Royal Society of Chemistry 2014 Supplementary Information: The role of various iron species in Fe-Beta catalysts with
Research on Direct Epoxidation of Propylene over Modified Au/Ts-1. Catalysts. Lina Wang1, a
 Advances in Engineering Research (AER), volume 107 2nd International Conference on Materials Engineering and Information Technology Applications (MEITA 2016) Research on Direct Epoxidation of Propylene
Advances in Engineering Research (AER), volume 107 2nd International Conference on Materials Engineering and Information Technology Applications (MEITA 2016) Research on Direct Epoxidation of Propylene
Consequences of Surface Oxophilicity of Ni, Ni-Co, and Co Clusters on Methane. Activation
 Supporting Information for: Consequences of Surface Oxophilicity of Ni, Ni-Co, and Co Clusters on Methane Activation Weifeng Tu, 1 Mireille Ghoussoub, Chandra Veer Singh,,3** and Ya-Huei (Cathy) Chin 1,*
Supporting Information for: Consequences of Surface Oxophilicity of Ni, Ni-Co, and Co Clusters on Methane Activation Weifeng Tu, 1 Mireille Ghoussoub, Chandra Veer Singh,,3** and Ya-Huei (Cathy) Chin 1,*
Reaction Coordinates. Activation Energy. Catalysis
 Today Reaction Coordinates Activation Energy Catalysis We have a balloon with H2 and O2 why is not reacting? 2H2(g) + O2(g) 2H2O(g) We have a balloon with H2 and O2 why is not reacting? 2H2(g) + O2(g)
Today Reaction Coordinates Activation Energy Catalysis We have a balloon with H2 and O2 why is not reacting? 2H2(g) + O2(g) 2H2O(g) We have a balloon with H2 and O2 why is not reacting? 2H2(g) + O2(g)
Supplementary data Methanolysis of Ammonia Borane by Shape-Controlled Mesoporous Copper Nanostructures for Hydrogen Generation
 Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2014 Supplementary data Methanolysis of Ammonia Borane by Shape-Controlled Mesoporous Copper
Electronic Supplementary Material (ESI) for Dalton Transactions. This journal is The Royal Society of Chemistry 2014 Supplementary data Methanolysis of Ammonia Borane by Shape-Controlled Mesoporous Copper
Supplementary Figure 1 Morpholigical properties of TiO 2-x SCs. The statistical particle size distribution (a) of the defective {001}-TiO 2-x SCs and
 Supplementary Figure 1 Morpholigical properties of TiO 2-x s. The statistical particle size distribution (a) of the defective {1}-TiO 2-x s and their typical TEM images (b, c). Quantity Adsorbed (cm 3
Supplementary Figure 1 Morpholigical properties of TiO 2-x s. The statistical particle size distribution (a) of the defective {1}-TiO 2-x s and their typical TEM images (b, c). Quantity Adsorbed (cm 3
Chapter 12. Chemical Kinetics
 Chapter 12 Chemical Kinetics Section 12.1 Reaction Rates Reaction Rate Change in concentration of a reactant or product per unit time. Rate = concentration of A at time t t 2 1 2 1 concentration of A at
Chapter 12 Chemical Kinetics Section 12.1 Reaction Rates Reaction Rate Change in concentration of a reactant or product per unit time. Rate = concentration of A at time t t 2 1 2 1 concentration of A at
Methane production from CO2 over Ni-Hydrotalcite derived catalysts
 Methane production from CO2 over Ni-Hydrotalcite derived catalysts Keerthivarman Veerappanchatram Kaliappan vkkeerthivarman@gmail.com Instituto Superior Tecnico, Universidade de Lisboa, Portugal. October
Methane production from CO2 over Ni-Hydrotalcite derived catalysts Keerthivarman Veerappanchatram Kaliappan vkkeerthivarman@gmail.com Instituto Superior Tecnico, Universidade de Lisboa, Portugal. October
CHEMISTRY - CLUTCH CH.13 - CHEMICAL KINETICS.
 !! www.clutchprep.com CONCEPT: RATES OF CHEMICAL REACTIONS is the study of reaction rates, and tells us the change in concentrations of reactants or products over a period of time. Although a chemical
!! www.clutchprep.com CONCEPT: RATES OF CHEMICAL REACTIONS is the study of reaction rates, and tells us the change in concentrations of reactants or products over a period of time. Although a chemical
Pd-P nanoalloys supported on porous carbon frame as efficient catalyst for benzyl alcohol oxidation
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2018 Supporting information Pd-P nanoalloys supported on porous carbon frame as
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2018 Supporting information Pd-P nanoalloys supported on porous carbon frame as
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/NCHEM.2522 Catalyst design for natural-gas upgrading through oxybromination chemistry Vladimir Paunović, Guido Zichittella, Maximilian Moser, Amol P. Amrute, and Javier Pérez-Ramírez* Further
DOI: 10.1038/NCHEM.2522 Catalyst design for natural-gas upgrading through oxybromination chemistry Vladimir Paunović, Guido Zichittella, Maximilian Moser, Amol P. Amrute, and Javier Pérez-Ramírez* Further
2/23/2018. Familiar Kinetics. ...and the not so familiar. Chemical kinetics is the study of how fast reactions take place.
 CHEMICAL KINETICS & REACTION MECHANISMS Readings, Examples & Problems Petrucci, et al., th ed. Chapter 20 Petrucci, et al., 0 th ed. Chapter 4 Familiar Kinetics...and the not so familiar Reaction Rates
CHEMICAL KINETICS & REACTION MECHANISMS Readings, Examples & Problems Petrucci, et al., th ed. Chapter 20 Petrucci, et al., 0 th ed. Chapter 4 Familiar Kinetics...and the not so familiar Reaction Rates
Chemical Kinetics -- Chapter 14
 Chemical Kinetics -- Chapter 14 1. Factors that Affect Reaction Rate (a) Nature of the reactants: molecular structure, bond polarity, physical state, etc. heterogeneous reaction: homogeneous reaction:
Chemical Kinetics -- Chapter 14 1. Factors that Affect Reaction Rate (a) Nature of the reactants: molecular structure, bond polarity, physical state, etc. heterogeneous reaction: homogeneous reaction:
SUPPORTING INFORMATION. Framework and Extraframework Tin Sites in Zeolite Beta React Glucose Differently
 SUPPORTING INFORMATION Framework and Extraframework Tin Sites in Zeolite Beta React Glucose Differently Ricardo Bermejo-Deval, Rajamani Gounder and Mark E. Davis* Chemical Engineering, California Institute
SUPPORTING INFORMATION Framework and Extraframework Tin Sites in Zeolite Beta React Glucose Differently Ricardo Bermejo-Deval, Rajamani Gounder and Mark E. Davis* Chemical Engineering, California Institute
Insights into Interfacial Synergistic Catalysis over Catalyst toward Water-Gas Shift Reaction
 Supporting Information Insights into Interfacial Synergistic Catalysis over Ni@TiO2-x Catalyst toward Water-Gas Shift Reaction Ming Xu, 1 Siyu Yao, 2 Deming Rao, 1 Yiming Niu, 3 Ning Liu, 1 Mi Peng, 2
Supporting Information Insights into Interfacial Synergistic Catalysis over Ni@TiO2-x Catalyst toward Water-Gas Shift Reaction Ming Xu, 1 Siyu Yao, 2 Deming Rao, 1 Yiming Niu, 3 Ning Liu, 1 Mi Peng, 2
Supplementary Figure S1: Solution NMR spectra for D-glucose with a 4:1 sugar:sb molar ratio in H 2 O. a) 13 C NMR of D-(1-13 C)glucose, b) 13 C NMR
 a c b d Supplementary Figure S1: Solution NMR spectra for D-glucose with a 4:1 sugar:sb molar ratio in H 2 O. a) 13 C NMR of D-(1-13 C)glucose, b) 13 C NMR of D-glucose, c) 11 B NMR, d) 1 H NMR a c b d
a c b d Supplementary Figure S1: Solution NMR spectra for D-glucose with a 4:1 sugar:sb molar ratio in H 2 O. a) 13 C NMR of D-(1-13 C)glucose, b) 13 C NMR of D-glucose, c) 11 B NMR, d) 1 H NMR a c b d
Alkali α-mno 2 /Na x MnO 2 collaboratively catalyzed ammoxidation-pinner tandem reaction of aldehydes
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information for Alkali α-mno 2 /Na x MnO 2 collaboratively
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information for Alkali α-mno 2 /Na x MnO 2 collaboratively
Electronic Supplementary Information. Alcohols
 Electronic Supplementary Information An Excellent Au/meso-γ-Al 2 O 3 Catalyst for Aerobic Selective Oxidation of Alcohols Guofeng Zhao, Min Ling, Huanyun Hu, Miaomiao Deng, Qingsong Xue, Yong Lu * Shanghai
Electronic Supplementary Information An Excellent Au/meso-γ-Al 2 O 3 Catalyst for Aerobic Selective Oxidation of Alcohols Guofeng Zhao, Min Ling, Huanyun Hu, Miaomiao Deng, Qingsong Xue, Yong Lu * Shanghai
Electronic Supplementary Material
 Electronic Supplementary Material Synthesis and characterization of mesoporous Si MCM-41 materials and their application as solid acid catalysts in some esterification reactions by Tarun F Parangi (pp
Electronic Supplementary Material Synthesis and characterization of mesoporous Si MCM-41 materials and their application as solid acid catalysts in some esterification reactions by Tarun F Parangi (pp
Supplementary Figure 1 A schematic representation of the different reaction mechanisms
 Supplementary Figure 1 A schematic representation of the different reaction mechanisms observed in electrode materials for lithium batteries. Black circles: voids in the crystal structure, blue circles:
Supplementary Figure 1 A schematic representation of the different reaction mechanisms observed in electrode materials for lithium batteries. Black circles: voids in the crystal structure, blue circles:
Enhanced formaldehyde selectivity in catalytic methane oxidation by vanadia on Ti-doped SBA-15
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) for the paper entitled: Enhanced formaldehyde selectivity
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) for the paper entitled: Enhanced formaldehyde selectivity
Supporting Information
 Supporting Information Single-atom and Nano-clustered Pt Catalysts for Selective CO 2 Reduction Yuan Wang, a Hamidreza Arandiyan,* a,b Jason Scott,* a Kondo-Francois Aguey-Zinsou, c and Rose Amal* a Miss
Supporting Information Single-atom and Nano-clustered Pt Catalysts for Selective CO 2 Reduction Yuan Wang, a Hamidreza Arandiyan,* a,b Jason Scott,* a Kondo-Francois Aguey-Zinsou, c and Rose Amal* a Miss
Synthesis of highly b-oriented zeolite MFI films by suppressing. twin crystal growth during the secondary growth
 Supplementary Information Synthesis of highly b-oriented zeolite MFI films by suppressing twin crystal growth during the secondary growth Xianming Li, a Yong Peng, a Zhengbao Wang,* a and Yushan Yan a,
Supplementary Information Synthesis of highly b-oriented zeolite MFI films by suppressing twin crystal growth during the secondary growth Xianming Li, a Yong Peng, a Zhengbao Wang,* a and Yushan Yan a,
Effects of sodium on the catalytic performance of CoMn catalysts for Fischer-Tropsch to olefins
 Supporting Information Effects of sodium on the catalytic performance of CoMn catalysts for Fischer-Tropsch to olefins Zhengjia Li a,b, Liangshu Zhong b,*, Fei Yu b,c, Yunlei An b,d, Yuanyuan Dai b,c,
Supporting Information Effects of sodium on the catalytic performance of CoMn catalysts for Fischer-Tropsch to olefins Zhengjia Li a,b, Liangshu Zhong b,*, Fei Yu b,c, Yunlei An b,d, Yuanyuan Dai b,c,
Supporting Information
 Supporting Information Identification of the nearby hydroxyls role in promoting HCHO oxidation over a Pt catalyst Ying Huo #, Xuyu Wang #, Zebao Rui *, Xiaoqing Yang, Hongbing Ji * School of Chemical Engineering
Supporting Information Identification of the nearby hydroxyls role in promoting HCHO oxidation over a Pt catalyst Ying Huo #, Xuyu Wang #, Zebao Rui *, Xiaoqing Yang, Hongbing Ji * School of Chemical Engineering
Hydrogen Titanium Oxide Hydrate: Excellent Performance. on Degradation of Methyl Blue in Aqueous Solutions
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supplementary information Hydrogen Titanium Oxide Hydrate: Excellent Performance on Degradation
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Supplementary information Hydrogen Titanium Oxide Hydrate: Excellent Performance on Degradation
AP CHEMISTRY NOTES 7-1 KINETICS AND RATE LAW AN INTRODUCTION
 AP CHEMISTRY NOTES 7-1 KINETICS AND RATE LAW AN INTRODUCTION CHEMICAL KINETICS the study of rates of chemical reactions and the mechanisms by which they occur FACTORS WHICH AFFECT REACTION RATES 1. Nature
AP CHEMISTRY NOTES 7-1 KINETICS AND RATE LAW AN INTRODUCTION CHEMICAL KINETICS the study of rates of chemical reactions and the mechanisms by which they occur FACTORS WHICH AFFECT REACTION RATES 1. Nature
Synthesis of mixed alcohols over K-Ni-MoS 2 catalysts
 Synthesis of mixed alcohols over K-Ni-MoS 2 catalysts Rodrigo Suárez París Supervisors: Magali Boutonnet, Sven Järås Division of Chemical Technology, KTH OUTLINE Introduction and objective Experimental
Synthesis of mixed alcohols over K-Ni-MoS 2 catalysts Rodrigo Suárez París Supervisors: Magali Boutonnet, Sven Järås Division of Chemical Technology, KTH OUTLINE Introduction and objective Experimental
Efficient Hydrogen Evolution. University of Central Florida, 4000 Central Florida Blvd. Orlando, Florida, 32816,
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2017 MoS 2 /TiO 2 Heterostructures as Nonmetal Plasmonic Photocatalysts for Highly
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2017 MoS 2 /TiO 2 Heterostructures as Nonmetal Plasmonic Photocatalysts for Highly
Temperature dependence of electrocatalytic and. photocatalytic oxygen evolution reaction rates
 Supporting information Temperature dependence of electrocatalytic and photocatalytic oxygen evolution reaction rates using NiFe oxide Ela Nurlaela, Tatsuya Shinagawa, Muhammad Qureshi, Dattatray S. Dhawale,
Supporting information Temperature dependence of electrocatalytic and photocatalytic oxygen evolution reaction rates using NiFe oxide Ela Nurlaela, Tatsuya Shinagawa, Muhammad Qureshi, Dattatray S. Dhawale,
Asian Journal on Energy and Environment
 As. J. Energy Env. 2006, 7(01), 241-245 Asian Journal on Energy and Environment ISSN 1513-4121 Available online at www.asian-energy-journal.info Hydrogen Production from Carbon Dioxide Reforming of Methane
As. J. Energy Env. 2006, 7(01), 241-245 Asian Journal on Energy and Environment ISSN 1513-4121 Available online at www.asian-energy-journal.info Hydrogen Production from Carbon Dioxide Reforming of Methane
Key for Unit Exam: Kinetics
 Key for Unit Exam: Kinetics On the following pages are problems covering material in kinetics. Read each question carefully and consider how you will approach it before you put pen or pencil to paper.
Key for Unit Exam: Kinetics On the following pages are problems covering material in kinetics. Read each question carefully and consider how you will approach it before you put pen or pencil to paper.
Supporting Information for. Size-Dependent Oxidation State and CO Oxidation Activity of Tin
 Supporting Information for Size-Dependent Oxidation State and CO Oxidation Activity of Tin Oxide Clusters Yusuke Inomata, Ken Albrecht,, Kimihisa Yamamoto *,, Laboratory for Chemistry and Life Science,
Supporting Information for Size-Dependent Oxidation State and CO Oxidation Activity of Tin Oxide Clusters Yusuke Inomata, Ken Albrecht,, Kimihisa Yamamoto *,, Laboratory for Chemistry and Life Science,
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information MoS 2 nanosheet/mo 2 C-embedded N-doped
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information MoS 2 nanosheet/mo 2 C-embedded N-doped
Electronic Supplementary Information (ESI)
 Electronic Supplementary Information (ESI) Cu-SSZ-39, an active and hydrothermally stable catalyst for the selective catalytic reduction of NOx Manuel Moliner, *a Cristina Franch, a Eduardo Palomares,
Electronic Supplementary Information (ESI) Cu-SSZ-39, an active and hydrothermally stable catalyst for the selective catalytic reduction of NOx Manuel Moliner, *a Cristina Franch, a Eduardo Palomares,
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information (ESI) LiTi 2 (PO 4 ) 3 /reduced graphene oxide nanocomposite
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information (ESI) LiTi 2 (PO 4 ) 3 /reduced graphene oxide nanocomposite
CHAPTER 4. SYNTHESIS, CHARACTERIZATION OF TiO 2 NANOTUBES AND THEIR APPLICATION IN DYE SENSITIZED SOLAR CELL
 93 CHAPTER 4 SYNTHESIS, CHARACTERIZATION OF TiO 2 NANOTUBES AND THEIR APPLICATION IN DYE SENSITIZED SOLAR CELL 4.1 INTRODUCTION TiO 2 -derived nanotubes are expected to be applicable for several applications,
93 CHAPTER 4 SYNTHESIS, CHARACTERIZATION OF TiO 2 NANOTUBES AND THEIR APPLICATION IN DYE SENSITIZED SOLAR CELL 4.1 INTRODUCTION TiO 2 -derived nanotubes are expected to be applicable for several applications,
Highly doped and exposed Cu(I)-N active sites within graphene towards. efficient oxygen reduction for zinc-air battery
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) for Energy & Environmental Science.
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) for Energy & Environmental Science.
11/2/ and the not so familiar. Chemical kinetics is the study of how fast reactions take place.
 Familiar Kinetics...and the not so familiar Reaction Rates Chemical kinetics is the study of how fast reactions take place. Some happen almost instantaneously, while others can take millions of years.
Familiar Kinetics...and the not so familiar Reaction Rates Chemical kinetics is the study of how fast reactions take place. Some happen almost instantaneously, while others can take millions of years.
PROJECT 20: SUPPORTED METALS NANOPARTICLES AS CATALYST FOR THE PROX REACTION
 PROJECT 20: SUPPORTED METALS NANOPARTICLES AS CATALYST FOR THE PROX REACTION Prof. Elisabete M. Assaf, PhD IQSC - USP Prof. José M. Assaf, PhD; Janaina F. Gomes, PhD; Aline R. L. Miranda, Ms DEQ - UFSCar
PROJECT 20: SUPPORTED METALS NANOPARTICLES AS CATALYST FOR THE PROX REACTION Prof. Elisabete M. Assaf, PhD IQSC - USP Prof. José M. Assaf, PhD; Janaina F. Gomes, PhD; Aline R. L. Miranda, Ms DEQ - UFSCar
METHANOL OXIDATION OVER AU/ γ -AL 2 O 3 CATALYSTS
 Bajopas Volume 2 Number 2 December, 29 Bayero Journal of Pure and Applied Sciences, 2(2): 149-154 Received: May, 29 Accepted: July, 29 METHANOL OXIDATION OVER AU/ γ -AL 2 O 3 CATALYSTS Abdullahi Nuhu Kano
Bajopas Volume 2 Number 2 December, 29 Bayero Journal of Pure and Applied Sciences, 2(2): 149-154 Received: May, 29 Accepted: July, 29 METHANOL OXIDATION OVER AU/ γ -AL 2 O 3 CATALYSTS Abdullahi Nuhu Kano
AP Chem Chapter 14 Study Questions
 Class: Date: AP Chem Chapter 14 Study Questions 1. A burning splint will burn more vigorously in pure oxygen than in air because a. oxygen is a reactant in combustion and concentration of oxygen is higher
Class: Date: AP Chem Chapter 14 Study Questions 1. A burning splint will burn more vigorously in pure oxygen than in air because a. oxygen is a reactant in combustion and concentration of oxygen is higher
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Supporting Information High-Density Platinum Nanoparticle-Decorated Titanium
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Supporting Information High-Density Platinum Nanoparticle-Decorated Titanium
Preparation of TiO2-Bamboo Leaves Ash Composite as Photocatalyst for Dye Photodegradation
 Preparation of TiO2-Bamboo Leaves Ash Composite as Photocatalyst for Dye Photodegradation Atin Prihatiningsih*, LitaDewi Pertiwi, Is Fatimah Chemistry Department, Universitas Islam Indonesia KampusTerpadu
Preparation of TiO2-Bamboo Leaves Ash Composite as Photocatalyst for Dye Photodegradation Atin Prihatiningsih*, LitaDewi Pertiwi, Is Fatimah Chemistry Department, Universitas Islam Indonesia KampusTerpadu
Kinetics - Chapter 14. reactions are reactions that will happen - but we can t tell how fast. - the steps by which a reaction takes place.
 The study of. Kinetics - Chapter 14 reactions are reactions that will happen - but we can t tell how fast. - the steps by which a reaction takes place. Factors that Affect Rx Rates 1. The more readily
The study of. Kinetics - Chapter 14 reactions are reactions that will happen - but we can t tell how fast. - the steps by which a reaction takes place. Factors that Affect Rx Rates 1. The more readily
Depressing the hydrogenation and decomposition. nanoparticles on oxygen functionalized. carbon nanofibers. Supporting Information
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2015 Depressing the hydrogenation and decomposition reaction in H 2 O 2 synthesis
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2015 Depressing the hydrogenation and decomposition reaction in H 2 O 2 synthesis
Supporting Information
 Copyright WILEY-VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2018. Supporting Information for Small, DOI: 10.1002/smll.201801523 Ultrasensitive Surface-Enhanced Raman Spectroscopy Detection Based
Copyright WILEY-VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2018. Supporting Information for Small, DOI: 10.1002/smll.201801523 Ultrasensitive Surface-Enhanced Raman Spectroscopy Detection Based
, but bursts into flames in pure oxygen.
 Chemical Kinetics Chemical kinetics is concerned with the speeds, or rates of chemical reactions Chemical kinetics is a subject of broad importance. How quickly a medicine can work The balance of ozone
Chemical Kinetics Chemical kinetics is concerned with the speeds, or rates of chemical reactions Chemical kinetics is a subject of broad importance. How quickly a medicine can work The balance of ozone
Supporting Information. Size-tunable Ni nanoparticles supported on surface-modified, cage-type mesoporous
 Supporting Information Size-tunable Ni nanoparticles supported on surface-modified, cage-type mesoporous silica as highly active catalysts for CO 2 hydrogenation Ching-Shiun Chen, a,b* Canggih Setya Budi,
Supporting Information Size-tunable Ni nanoparticles supported on surface-modified, cage-type mesoporous silica as highly active catalysts for CO 2 hydrogenation Ching-Shiun Chen, a,b* Canggih Setya Budi,
Highly Efficient and Robust Au/MgCuCr 2 O 4 Catalyst for Gas-Phase Oxidation of Ethanol to Acetaldehyde
 Highly Efficient and Robust Au/MgCuCr O 4 Catalyst for Gas-Phase Oxidation of Ethanol to Acetaldehyde Peng Liu,*, and Emiel J. M. Hensen*, Department of Chemical Engineering and Chemistry, Eindhoven University
Highly Efficient and Robust Au/MgCuCr O 4 Catalyst for Gas-Phase Oxidation of Ethanol to Acetaldehyde Peng Liu,*, and Emiel J. M. Hensen*, Department of Chemical Engineering and Chemistry, Eindhoven University
Chapter: Chemical Kinetics
 Chapter: Chemical Kinetics Rate of Chemical Reaction Question 1 Nitrogen pentaoxide decomposes according to equation: This first order reaction was allowed to proceed at 40 o C and the data below were
Chapter: Chemical Kinetics Rate of Chemical Reaction Question 1 Nitrogen pentaoxide decomposes according to equation: This first order reaction was allowed to proceed at 40 o C and the data below were
Sintering-resistant Ni-based Reforming Catalysts via. the Nanoconfinement Effect
 Supporting Information Sintering-resistant Ni-based Reforming Catalysts via the Nanoconfinement Effect Chengxi Zhang a,b, Wancheng Zhu c, Shuirong Li a,b, Gaowei Wu a,b, Xinbin Ma a,b, Xun Wang c, and
Supporting Information Sintering-resistant Ni-based Reforming Catalysts via the Nanoconfinement Effect Chengxi Zhang a,b, Wancheng Zhu c, Shuirong Li a,b, Gaowei Wu a,b, Xinbin Ma a,b, Xun Wang c, and
Zeolite-supported rhodium sub-nano cluster catalyst for low-temperature
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information Zeolite-supported rhodium sub-nano cluster
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2017 Electronic Supplementary Information Zeolite-supported rhodium sub-nano cluster
