Characterization of Re-Pd/SiO 2 catalysts for hydrogenation of stearic acid
|
|
|
- Cecilia Lang
- 5 years ago
- Views:
Transcription
1 Supporting Information: Characterization of Re-Pd/SiO 2 catalysts for hydrogenation of stearic acid Yasuyuki Takeda, [a] Masazumi Tamura, [a] Yoshinao Nakagawa, [a] Kazu Okumura, [b] and Keiichi Tomishige* [a] [a] Department of Applied Chemistry, School of Engineering, Tohoku University, 6-6-7, Aoba, Aramaki, Aoba-ku, Sendai , Japan [b] Department of Applied Chemistry, Faculty of Engineering, Kogakuin University, Nishi-Shinjuku, Shinjuku-ku, Tokyo , Japan * Corresponding author: Keiichi Tomishige School of Engineering, Tohoku University, 6-6-7, Aoba, Aramaki, Aoba-ku, Sendai, , Japan tomi@erec.che.tohoku.ac.jp
2 Table S1. The normalization data for XRD on the catalysts. Entry Spectra (Figure 1) Catalyst Re/Pd Amount of SiO 2 / wt% SiO 2 area (8 35 ) / 1 4 degree counts raw normalized 1 (a) Pd(L, 413, Reaction) (b) Re-Pd(L, 413, Reaction) (c) Re-Pd(L, 413, Reaction) (d) Re-Pd(L, 413, Reaction) (e) Re-Pd(L, 413, Reaction) (f) Re-Pd(L, 413, Reaction) (g) Re(L, 413, Reaction) (h) Re-Pd(G, 473) (i) Re(G, 473) (j) Re(G, 773)
3 1 (n) White line area / a.u (k) (c) (e) (l) (g) (h) (f) (a) (d) (b) Valence of Re Figure S1. Relation between white line areas of Re L 3 -edge XANES spectra and valence of Re. (a) Re(L, 413, Reaction) (Re = 14 wt%), (b) Re(G, 473) (Re = 14 wt%), (c) Re(G, 773) (Re = 14 wt%), (d) Re(G, 473, Reaction) (Re = 14 wt%), (e) Re(G, 773, Reaction) (Re = 14 wt%), (f) Re-Pd (L, 413, Reaction) (Re/Pd = 8), (g) Re-Pd(G, 473) (Re/Pd = 8), (h) Re-Pd(G, 473, Reaction) (Re/Pd = 8), (i) Re(Calcination), (j) Re-Pd(Calcination), (k) Re powder, (l) ReO 2, (m) ReO 3, (n) Re 2 O 7. (m) (i) (j)
4 Table S2. The parameter of XRD fitting method i A i ratio x, i / Re(HCP) Re(FCC) 1. (39.48).48 (45.92) Pd(FCC) 1. (4.2).47 (46.66)
5 Table S3. Effect of the reduction methods over Re-Pd catalysts reduced in liquid phase at 413 K in the STA hydrogenation. Entry Conversion Selectivity / % Conversion rate TOF / % C 18 H 37 OH C 18 H 38 C 17 H 36 Others / mmol g-cat -1 h -1 / h -1 1 a b Reaction conditions: 5 wt% STA solution 2 g (STA 1 g, 1,4-dioxane 19 g), Re-Pd(L, 413) (Re/Pd = 8) 1 mg, reaction temperature 413 K, H 2 pressure 8. MPa, reaction time 4 h, Others: CO 2, CH 4 and C 2 H 6. a Standard procedure for Re-Pd(L, 413), and the catalyst was reduced during the reaction. b The catalyst was reduced in only the solvent (1,4-dioxane, 19 g) at 413 K. After cooling down to room temperature, the autoclave was opened in N 2 atmosphere and stearic acid (1 g) was added to the autoclave reactor, where all the procedures were carried out without exposure to air.
6 (f) (e) (d) (c) (b) (a) Temperature / K Figure S2. TPR profiles of the calcined Pd, Re and Re-Pd catalysts (a) Pd(Calcination), (b) Re-Pd(Calcination) (Re/Pd = 2). (c) Re-Pd(Calcination) (Re/Pd = 4), (d) Re-Pd(Calcination) (Re/Pd = 8), (e) Re-Pd(Calcination) (Re/Pd = 12), (f) Re(Calcination). Conditions: Catalyst.5 g, 5% H 2 /Ar 3 ml/min, 1 K/min. Experimental method for TPR Temperature-programmed reduction (TPR) was carried out in a fixed-bed reactor equipped with a thermal conductivity detector using 5% H 2 diluted with Ar (3 ml/min). The amount of catalyst was.5 g, and the temperature was increased from room temperature to 1123 K at a heating rate of 1 K/min. Reduction degree of Re on Re-Pd catalyst was determined by using the consumed H 2 amount of Pd/SiO 2 catalyst (entry 1). In general, Pd metal species can be easily reduced at room temperature, and therefore the consumed amount of H 2 is lower than the total Pd amount.
7 Table S4. Results for TPR profiles of the calcined Pd, Re and Re-Pd catalysts. Loading amount H 2 consumption a Reduction degree of Re b Re valence c Entry Catalyst Re/Pd / mmol g-cat -1 / mmol g-cat -1 Pd Re K K K K K K 1 Pd(Calcination) d.4 d Re-Pd(Calcination) Re-Pd(Calcination) Re-Pd(Calcination) Re-Pd(Calcination) Re(Calcination) a H 2 consumption was estimated in the range of or K. b Reduction degree of Re = (H 2 consumption [mol] * 2 H 2 consumption of Pd (entry 1) [mol]) / ((Re amount [mol]) * 7). c Re valence was calculated from H 2 consumption. Initial valence of Re before TPR is +7. Valence of Re after TPR is calculated by 7 (H 2 consumption [mol] * 2) / (Re amount [mol] ). d This amount is lower than the total amount of Pd, which is due to the reduction of Pd species at room temperature.
8 1 Conversion / % filtration Time / h Figure S3. Activity tests of the STA hydrogenation using Re-Pd(L,413) (Re/Pd = 8) catalyst without or with the removal of the catalyst by filtration a. Reaction conditions: 5 wt% STA solution 2 g (STA 1 g, 1,4-dioxane 19 g), catalyst amount 1 mg, reaction temperature 413 K, H 2 pressure 8. MPa. Solid line, : without filtration; broken line, : after filtration. a The catalyst was removed after 2 h reaction time by filtration
9 (a) (b) RMSE = 29 RMSE = θ / degree (c) θ / degree 5 55 (d) RMSE = 25 RMSE = θ / degree (e) θ / degree 5 55 (f) RMSE = 29 RMSE = θ / degree θ / degree 55 (h) (g) RMSE = 57 RMSE = θ / degree θ / degree 5 55
10 (i) RMSE = 53 (j) RMSE = θ / degree θ / degree Figure S4. The fitting results of XRD peaks of Re, Pd and Re-Pd catalysts using Re(HCP), Re(FCC) and Pd metal species. Brown line: raw data (Figure 1), yellow dotted line: background, red line: Re(HCP), blue line: Pd, green line: Re(FCC), black dotted line: the result of curve fitting. Fitting range: 2θ = (a) Re(L, 413, Reaction) (Re = 14 wt%), (b) Pd(L, 413, Reaction) (Pd = 1 wt%), (c) Re-Pd (L, 413, Reaction) (Re/Pd = 2), (d) Re-Pd (L, 413, Reaction) (Re/Pd = 4), (e) Re-Pd (L, 413, Reaction) (Re/Pd = 6), (f) Re-Pd (L, 413, Reaction) (Re/Pd = 8), (g) Re-Pd (L, 413, Reaction) (Re/Pd = 12), (h) Re-Pd(G, 473) (Re/Pd = 8), (i) Re (G, 473) (Re = 14 wt%), (j) Re (G, 773) (Re = 14 wt%).
11 RMSE = θ / degree Figure S5. A fitting result of XRD peaks of Re (L, 413, Reaction) (Re=14 wt%) using Re(HCP) metal species. Brown line: raw data (Figure 1 (g)), yellow dotted line: background, red line: Re(HCP), black dotted line: the result of curve fitting. Fitting range: 2θ =
12 (f) (e) Normalized absorbance (d) (c) (b) (a) E / ev Figure S6. Pd K-edge XANES spectra of Pd and Re-Pd catalysts after the reaction or reduction. (a) Pd foil, (b) Pd(L, 413, Reaction), (c) Re-Pd(L, 413, Reaction) (Re/Pd = 8), (d) Re-Pd(G, 473) (Re/Pd = 8), (e) Re-Pd(G, 473, Reaction) (Re/Pd = 8), (f) PdO.
13 . (I) 3 f e d (II) 2 f e d k 3 χ(k) c b a F(r) c b a k / 1 nm (III) 2 e 8 d 6 k 3 χ(k) 4 c b Distance /.1 nm k / 1 nm -1. Figure S7. Results of Pd K-edge EXAFS analysis of Pd and Re-Pd (Re/Pd = 8) catalysts after reaction and flow reduction. (I) k 3 -Weighted EXAFS oscillations. (II) Fourier transform of k 3 -weighted Pd K-edge EXAFS, FT range: 3 14 nm 1. (III) Fourier filtered EXAFS data (solid line) and calculated data (dotted line), Fourier filtering range: nm. (a) Pd foil, (b) Pd(L, 413, Reaction), (c) Re-Pd(L, 413, Reaction) (Re/Pd = 8), (d) Re-Pd(G, 473) (Re/Pd = 8), (e) Re-Pd(G, 473, Reaction) (Re/Pd = 8), (f) PdO.
14 Table S5 Curve fitting results for the Pd K-edge EXAFS analysis of Pd and Re-Pd (Re/Pd = 8) catalysts after the reaction and H 2 reduction treatment a. Entry Catalyst Re/Pd Shells CN b R / 1-1 nm c σ / 1-1 nm d E / ev e R f / % f PdO Pd-O g Pd(L, 413, Reaction) h Pd-Pd g Re-Pd(L, 413, Reaction) i 8 Pd-Pd j Re-Pd(G, 473) i 8 Pd-Pd g, j Re-Pd(G, 473, Reaction) i 8 Pd-Pd Pd foil Pd-Pd a Fourier filtering range: nm. b Coordination number. c Bond distance. d Debye Waller factor. e Difference in the origin of photoelectron energy between the reference and the sample. f Residual factor. g Reaction conditions: 5 wt% STA solution 2 g (STA 1 g, 1,4-dioxane 19 g), Cat. 2 mg, 413 K, 8 MPa, 2 h. h Pd = 1 wt%. i Re: 14 wt%, Pd = 1 wt%. j Reduction conditions: 1% H 2 3 ml/min, time 1 h.
15 Conversion rate / mmol h -1 g-cat (e) (d) (g) (c) (b) (a) (f) (h) (i) (j) Effective XRD area / 1 4 degree counts Figure S8. Conversion rate of STA hydrogenation as a function of effective XRD area of Pd, Re-Pd and Re catalysts. (a) Pd(L, 413, Reaction), (b) Re-Pd(L, 413, Reaction) (Re/Pd = 2). (c) Re-Pd(L, 413, Reaction) (Re/Pd = 4), (d) Re-Pd(L, 413, Reaction) (Re/Pd = 6), (e) Re-Pd(L, 413, Reaction) (Re/Pd = 8), (f) Re-Pd(L, 413, Reaction) (Re/Pd = 12), (g) Re(L, 413, Reaction), (h) Re-Pd(G, 473) (Re/Pd = 8), (i) Re(G, 473), (j) Re(G, 473). Effective XRD area = XRD area Re dispersion (Figure 1, Table 4). Conversion rate is based on Table 1 and 2.
16 Table S6 Effect of STA concentration in the hydrogenation of STA over Re-Pd(L, 413) (Re/Pd = 8) and Re(L, 413) catalysts. Entry Catalyst Cat. amount STA 1,4-dioxane Initial STA conc. Time Conv. Selectivity / % Conversion rate / g / g / g / wt% / h / % C 18 H 37 OH C 18 H 38 C 17 H 36 Others / mmol g-cat -1 h -1 1 Re-Pd(L, 413) Re/Pd = Re = 14 wt% 3 Pd = 1 wt% Re(L, 413) (Re = 14 wt%) Reaction conditions: 3~15 wt% STA solution, 1,4-dioxane solvent g, STA.6-1. g, catalyst.6-.1 g, reaction temperature 413 K, H 2 pressure 8. MPa, Others: CO 2, CH 4 and C 2 H 6.
17 Table S7 The calculation result for reaction order with respect to initial STA concentration over Re-Pd(L, 413) catalysts. Entry Run time Calculated initial conversion rate / mmol g-cat -1 h -1 Calculated Figure S9 (3 wt%) a (5 wt%) a (7 wt%) a (1 wt%) a (15 wt%) a reaction order 1 1st (a) (zero order assumption) 2 2nd (b) (+.87 order assumption) 3 3rd (c) (+.97 order assumption) Reaction conditions: 3~15 wt% STA solution, 1,4-dioxane solvent g, STA.6-1. g, catalyst.6-.1 g, reaction temperature 413 K, H 2 pressure 8. MPa. a STA concentration Calculation method for reaction order: (1) The reaction order was decided from the slope of double logarithmic plot for conversion rate vs initial STA concentration by using least-square approach, where the conversion rate was calculated assuming the zero reaction order (the slope obtained from the data at the specific reaction time and the origin) (entry 1). (2) The reaction rate constant in this reaction order was evaluated from each data point at the specific reaction time and concentration, and the initial conversion rate was estimated again from the rate constant (entry 2). (3) The calculations of (1) and (2) were repeated until the reaction order converged (entries 2-4).
18 Table S8 The calculation result for reaction order with respect to initial STA concentration over Re(L, 413) catalysts. Entry Run time Calculated initial conversion rate / mmol g-cat -1 h -1 Calculated Figure S1 (3 wt%) a (5 wt%) a (7 wt%) a (1 wt%) a (15 wt%) a reaction order 1 1st (a) (zero order assumption) 2 2nd (b) (+.79 order assumption) 3 3rd (c) (+.82 order assumption) 4 4th (d) (+.83 order assumption) Reaction conditions: 3~15 wt% STA solution, 1,4-dioxane solvent g, STA.6-1. g, catalyst.6-.1 g, reaction temperature 413 K, H 2 pressure 8. MPa. a STA concentration Calculation method for reaction order: (1) The reaction order was decided from the slope of double logarithmic plot for conversion rate vs initial STA concentration by using least-square approach, where the conversion rate was calculated assuming the zero reaction order (the slope obtained from the data at the specific reaction time and the origin) (entry 1). (2) The reaction rate constant in this reaction order was evaluated from each data point at the specific reaction time and concentration, and the initial conversion rate was estimated again from the rate constant (entry 2). (3) The calculations of (1) and (2) were repeated until the reaction order converged (entries 2-4).
19 ln (Reaction rate / mmol g-cat -1 h -1 ) 2 (a) y =.87x ln ( Initial STA concentration / wt%) ln (Reaction rate / mmol g-cat -1 h -1 ) 2 (b) y =.97x ln ( Initial STA concentration / wt%) ln (Reaction rate / mmol g-cat -1 h -1 ) 2 (c) y =.97x ln ( Initial STA concentration / wt%) Figure S9. The comparison of reaction orders with respect to the initial STA concentration over Re-Pd(L, 413) catalysts. Reac tion conditions: 3~15 wt% STA solution, 1,4-dioxane solvent g, STA.6-1. g, catalyst.6-.1 g, reaction temperature 413 K, H 2 pressure 8. MPa. (a) zero order assumption (Table S7, entry 1), (b) +.93 order assumption (Table S7, entry 2), (c) +.94 order assumption (Table S7, entry 3).
20 ln (Reaction rate / mmol g-cat -1 h -1 ) 2 (b) 1.5 y =.82 x ln ( Initial STA concentration / wt%) ln (Reaction rate / mmol g-cat -1 h -1 ) 2 (b) 1.5 y =.82 x ln ( Initial STA concentration / wt%) ln (Reaction rate / mmol g-cat -1 h -1 ) 2 (c) 1.5 y =.83 x ln ( Initial STA concentration / wt%) ln (Reaction rate / mmol g-cat -1 h -1 ) 2 (d) 1.5 y =.83 x ln ( Initial STA concentration / wt%) Figure S1. The comparison of reaction orders with respect to the initial STA concentration over Re(L, 413) catalysts. Reaction conditions: 3~15 wt% STA solution, 1,4-dioxane solvent g, STA.6-1. g, catalyst.6-.1 g, reaction temperature 413 K, H 2 pressure 8. MPa. (a) zero order assumption (Table S8, entry 1), (b) +.74 order assumption (Table S8, entry 2), (c) +.82 order assumption (Table S8, entry 3), (d) +.83 order assumption (Table S8, entry 4).
21 Conversion / % Time / h Figure S1. Time dependence for hydrogenation of STA over Re(L, 413) and the determination of the initial reaction rate. Reaction conditions: 5 wt% STA solution 2 g (STA 1 g, 1,4-dioxane 19 g), catalyst amount.1 g, reaction temperature 413 K, H 2 pressure 8. MPa. : conversion; blue line: zero order line for STA concentration; red line:.74 order line for STA concentration; green line: tangent line for red line at origin; black broken line: the converged order line (.83) for STA concentration.
22 Table S9 Effect of H 2 pressure in the hydrogenation of STA over Re-Pd(L, 413) (Re/Pd = 8) and Re(L, 413) catalysts. Entry Catalyst H 2 pressure Time Conversion Selectivity / % Conversion rate / MPa / h / % C 18 H 37 OH C 18 H 38 C 17 H 36 Others / mmol g-cat -1 h -1 1 Re-Pd(L, 413) Re/Pd = Re = 14 wt% 3 Pd = 1 wt% Re(L, 413) (Re = 14 wt%) Reaction conditions: 5 wt% STA solution 2 g (STA 1 g, 1,4-dioxane 19 g), catalyst amount.1 g, reaction temperature 413 K, Others: CO 2, CH 4 and C 2 H 6.
6-6-07, Aoba, Aramaki, Aoba-ku, Sendai , Japan Nakano-machi, Hachioji, Tokyo , Japan
 Supporting Information: Performance, Structure and Mechanism of Re x Pd/Ce 2 Catalyst for Simultaneous Removal of Vicinal H Groups with H 2 Nobuhiko ta, [a] Masazumi Tamura, [a, c] Yoshinao Nakagawa,*
Supporting Information: Performance, Structure and Mechanism of Re x Pd/Ce 2 Catalyst for Simultaneous Removal of Vicinal H Groups with H 2 Nobuhiko ta, [a] Masazumi Tamura, [a, c] Yoshinao Nakagawa,*
Catalytic Hydrogenation of Amino Acids to Amino Alcohols with Complete Retention of Configuration
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Catalytic Hydrogenation of Amino Acids to Amino Alcohols with
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Catalytic Hydrogenation of Amino Acids to Amino Alcohols with
Supporting information. Formation of A New Strong Basic Nitrogen Anion by Metal Oxide Modification
 Supporting information Formation of A ew Strong Basic itrogen Anion by Metal Oxide Modification Masazumi Tamura,*,, Ryota Kishi, Akira akayama,*,, Yoshinao akagawa, Jun-ya Hasegawa, Keiichi Tomishige*,
Supporting information Formation of A ew Strong Basic itrogen Anion by Metal Oxide Modification Masazumi Tamura,*,, Ryota Kishi, Akira akayama,*,, Yoshinao akagawa, Jun-ya Hasegawa, Keiichi Tomishige*,
Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c,
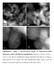 Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c, 2 nm; d, 2 nm. The low magnification images demonstrate
Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c, 2 nm; d, 2 nm. The low magnification images demonstrate
Supporting information
 Supporting information Cu Sub-nanoparticles on Cu/CeO 2 as an Effective Catalyst for Methanol Synthesis from Organic Carbonate by Hydrogenation Masazumi Tamura* Takahisa Kitanaka, Yoshinao Nakagawa and
Supporting information Cu Sub-nanoparticles on Cu/CeO 2 as an Effective Catalyst for Methanol Synthesis from Organic Carbonate by Hydrogenation Masazumi Tamura* Takahisa Kitanaka, Yoshinao Nakagawa and
Table S1. Structural parameters of shell-by-shell fitting of the EXAFS spectrum for reduced and oxidized samples at room temperature (RT)
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting information Table S1. Structural parameters of shell-by-shell
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting information Table S1. Structural parameters of shell-by-shell
Experimental Section
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Supplementary Material (ESI) for Chemical Communications Modification of Ga 2 O 3 by Ag Cr Core
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Supplementary Material (ESI) for Chemical Communications Modification of Ga 2 O 3 by Ag Cr Core
Supporting Information
 Supporting Information Insight into the Formation of Co@Co 2 C Catalysts for Direct Synthesis of Higher Alcohols and Olefins from Syngas Ziang Zhao, 1,2, Wei Lu, 1, Ruoou Yang, 2,4 Hejun Zhu, 1,* Wenda
Supporting Information Insight into the Formation of Co@Co 2 C Catalysts for Direct Synthesis of Higher Alcohols and Olefins from Syngas Ziang Zhao, 1,2, Wei Lu, 1, Ruoou Yang, 2,4 Hejun Zhu, 1,* Wenda
Strategic use of CuAlO 2 as a sustained release catalyst for production of hydrogen from methanol steam reforming
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Strategic use of CuAlO 2 as a sustained release catalyst for
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Strategic use of CuAlO 2 as a sustained release catalyst for
Supporting Information High Activity and Selectivity of Ag/SiO 2 Catalyst for Hydrogenation of Dimethyloxalate
 Supporting Information High Activity and Selectivity of Ag/SiO 2 Catalyst for Hydrogenation of Dimethyloxalate An-Yuan Yin, Xiao-Yang Guo, Wei-Lin Dai*, Kang-Nian Fan Shanghai Key Laboratory of Molecular
Supporting Information High Activity and Selectivity of Ag/SiO 2 Catalyst for Hydrogenation of Dimethyloxalate An-Yuan Yin, Xiao-Yang Guo, Wei-Lin Dai*, Kang-Nian Fan Shanghai Key Laboratory of Molecular
Depressing the hydrogenation and decomposition. nanoparticles on oxygen functionalized. carbon nanofibers. Supporting Information
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2015 Depressing the hydrogenation and decomposition reaction in H 2 O 2 synthesis
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2015 Depressing the hydrogenation and decomposition reaction in H 2 O 2 synthesis
Supplementary Information
 Supplementary Information Supplementary Figure 1. X-ray diffraction patterns of (a) pure LDH, (b) AuCl 4 ion-exchanged LDH and (c) the Au/LDH hybrid catalyst. The refined cell parameters for pure, ion-exchanged,
Supplementary Information Supplementary Figure 1. X-ray diffraction patterns of (a) pure LDH, (b) AuCl 4 ion-exchanged LDH and (c) the Au/LDH hybrid catalyst. The refined cell parameters for pure, ion-exchanged,
doi: /j.cattod
 doi: 10.1016/j.cattod.2007.12.031 Catalytic performance and QXAFS analysis of Ni catalysts modified with Pd for oxidative steam reforming of methane Yuya Mukainakano a, Kaori Yoshida a, Kazu Okumura b,
doi: 10.1016/j.cattod.2007.12.031 Catalytic performance and QXAFS analysis of Ni catalysts modified with Pd for oxidative steam reforming of methane Yuya Mukainakano a, Kaori Yoshida a, Kazu Okumura b,
Supporting Information Fe-containing magnesium aluminate support for stability and carbon control during methane reforming
 Supporting Information Fe-containing magnesium aluminate support for stability and carbon control during methane reforming Stavros Alexandros Theofanidis 1, Vladimir V. Galvita 1*, Hilde Poelman 1, N.V.R.
Supporting Information Fe-containing magnesium aluminate support for stability and carbon control during methane reforming Stavros Alexandros Theofanidis 1, Vladimir V. Galvita 1*, Hilde Poelman 1, N.V.R.
EXAFS. Extended X-ray Absorption Fine Structure
 AOFSRR Cheiron School 2010, SPring-8 EXAFS Oct. 14th, 2010 Extended X-ray Absorption Fine Structure Iwao Watanabe Ritsumeikan University EXAFS Theory Quantum Mechanics Models Approximations Experiment
AOFSRR Cheiron School 2010, SPring-8 EXAFS Oct. 14th, 2010 Extended X-ray Absorption Fine Structure Iwao Watanabe Ritsumeikan University EXAFS Theory Quantum Mechanics Models Approximations Experiment
The active sites of supported silver particle catalysts in formaldehyde. oxidation
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) The active sites of supported silver particle catalysts
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) The active sites of supported silver particle catalysts
Electronic Supplementary Information. Pd(diimine)Cl 2 Embedded Heterometallic Compounds with Porous Structures as Efficient Heterogeneous Catalysts
 Electronic Supplementary Information Pd(diimine)Cl 2 Embedded Heterometallic Compounds with Porous Structures as Efficient Heterogeneous Catalysts Sheng-Li Huang, Ai-Quan Jia and Guo-Xin Jin* Experimental
Electronic Supplementary Information Pd(diimine)Cl 2 Embedded Heterometallic Compounds with Porous Structures as Efficient Heterogeneous Catalysts Sheng-Li Huang, Ai-Quan Jia and Guo-Xin Jin* Experimental
Thermodynamic and Kinetic Investigations for Redox Reactions of Nickel Species Supported on Silica
 Thermodynamic and Kinetic Investigations for Redox Reactions of Nickel Species Supported on Silica Shohei Yamashita, Misaki Katayama, Yasuhiro Inada Graduate School of Life Sciences, Ritsumeikan University,
Thermodynamic and Kinetic Investigations for Redox Reactions of Nickel Species Supported on Silica Shohei Yamashita, Misaki Katayama, Yasuhiro Inada Graduate School of Life Sciences, Ritsumeikan University,
Ir-Re Alloy as a highly active catalyst for the hydrogenolysis
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2014 Supporting Information Ir-Re Alloy as a highly active catalyst for the hydrogenolysis
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2014 Supporting Information Ir-Re Alloy as a highly active catalyst for the hydrogenolysis
Supplementary Figure 1 Result from XRD measurements. Synchrotron radiation XRD patterns of the as-prepared gold-ceria samples.
 Supplementary Figure 1 Result from XRD measurements. Synchrotron radiation XRD patterns of the as-prepared gold-ceria samples. The detailed information on XRD measurement is seen in the Supplementary Methods.
Supplementary Figure 1 Result from XRD measurements. Synchrotron radiation XRD patterns of the as-prepared gold-ceria samples. The detailed information on XRD measurement is seen in the Supplementary Methods.
Supporting Information. Cyanamide Route to Calcium-Manganese Oxide Foams for Water Oxidation
 Supporting Information Cyanamide Route to Calcium-Manganese Oxide Foams for Water Oxidation Elham Baktash a, Ivelina Zaharieva* b, Marc Schröder c, Caren Goebel a, Holger Dau* b and Arne Thomas* a a Technische
Supporting Information Cyanamide Route to Calcium-Manganese Oxide Foams for Water Oxidation Elham Baktash a, Ivelina Zaharieva* b, Marc Schröder c, Caren Goebel a, Holger Dau* b and Arne Thomas* a a Technische
Supporting Information. Highly Efficient Aerobic Oxidation of Various Amines Using Pd 3 Pb Intermetallic Compound Catalysts
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supporting Information Highly Efficient Aerobic Oxidation of Various Amines Using Pd 3 Pb Intermetallic
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Supporting Information Highly Efficient Aerobic Oxidation of Various Amines Using Pd 3 Pb Intermetallic
Supplementary Information. ZIF-8 Immobilized Ni(0) Nanoparticles: Highly Effective Catalysts for Hydrogen Generation from Hydrolysis of Ammonia Borane
 Supplementary Information ZIF-8 Immobilized Ni() Nanoparticles: Highly Effective Catalysts for Hydrogen Generation from Hydrolysis of Ammonia Borane Pei-Zhou Li, a,b Kengo Aranishi, a and Qiang Xu* a,b
Supplementary Information ZIF-8 Immobilized Ni() Nanoparticles: Highly Effective Catalysts for Hydrogen Generation from Hydrolysis of Ammonia Borane Pei-Zhou Li, a,b Kengo Aranishi, a and Qiang Xu* a,b
Highly Efficient and Robust Au/MgCuCr 2 O 4 Catalyst for Gas-Phase Oxidation of Ethanol to Acetaldehyde
 Highly Efficient and Robust Au/MgCuCr O 4 Catalyst for Gas-Phase Oxidation of Ethanol to Acetaldehyde Peng Liu,*, and Emiel J. M. Hensen*, Department of Chemical Engineering and Chemistry, Eindhoven University
Highly Efficient and Robust Au/MgCuCr O 4 Catalyst for Gas-Phase Oxidation of Ethanol to Acetaldehyde Peng Liu,*, and Emiel J. M. Hensen*, Department of Chemical Engineering and Chemistry, Eindhoven University
Study on the Selective Hydrogenation of Nitroaromatics to N-aryl hydroxylamines using a Supported Pt nanoparticle Catalyst
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 204 Supporting Information Study on the Selective Hydrogenation of Nitroaromatics
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 204 Supporting Information Study on the Selective Hydrogenation of Nitroaromatics
cis,cis-muconic acid: Separation and catalysis to bioadipic acid for nylon-6,6 polymerization
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information for: cis,cis-muconic acid: Separation and catalysis
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information for: cis,cis-muconic acid: Separation and catalysis
Genesis of a highly active Cerium Oxide-Supported Gold Catalyst for CO Oxidation
 Supporting information Genesis of a highly active Cerium Oxide-Supported Gold Catalyst for CO Oxidation Veronica Aguilar-Guerrero and Bruce C. Gates* Experimental A. Sample preparation Samples were synthesized
Supporting information Genesis of a highly active Cerium Oxide-Supported Gold Catalyst for CO Oxidation Veronica Aguilar-Guerrero and Bruce C. Gates* Experimental A. Sample preparation Samples were synthesized
One-pass Selective Conversion of Syngas to para-xylene
 Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2017 Supporting Information One-pass Selective Conversion of Syngas to para-xylene Peipei Zhang,
Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2017 Supporting Information One-pass Selective Conversion of Syngas to para-xylene Peipei Zhang,
Supporting Information
 Supporting Information Highly Active and Selective Hydrogenation of CO 2 to Ethanol by Ordered Pd-Cu Nanoparticles Shuxing Bai, Qi Shao, Pengtang Wang, Qiguang Dai, Xingyi Wang, Xiaoqing Huang * College
Supporting Information Highly Active and Selective Hydrogenation of CO 2 to Ethanol by Ordered Pd-Cu Nanoparticles Shuxing Bai, Qi Shao, Pengtang Wang, Qiguang Dai, Xingyi Wang, Xiaoqing Huang * College
Supporting Information for. Size-Dependent Oxidation State and CO Oxidation Activity of Tin
 Supporting Information for Size-Dependent Oxidation State and CO Oxidation Activity of Tin Oxide Clusters Yusuke Inomata, Ken Albrecht,, Kimihisa Yamamoto *,, Laboratory for Chemistry and Life Science,
Supporting Information for Size-Dependent Oxidation State and CO Oxidation Activity of Tin Oxide Clusters Yusuke Inomata, Ken Albrecht,, Kimihisa Yamamoto *,, Laboratory for Chemistry and Life Science,
Insights into Interfacial Synergistic Catalysis over Catalyst toward Water-Gas Shift Reaction
 Supporting Information Insights into Interfacial Synergistic Catalysis over Ni@TiO2-x Catalyst toward Water-Gas Shift Reaction Ming Xu, 1 Siyu Yao, 2 Deming Rao, 1 Yiming Niu, 3 Ning Liu, 1 Mi Peng, 2
Supporting Information Insights into Interfacial Synergistic Catalysis over Ni@TiO2-x Catalyst toward Water-Gas Shift Reaction Ming Xu, 1 Siyu Yao, 2 Deming Rao, 1 Yiming Niu, 3 Ning Liu, 1 Mi Peng, 2
Role of Re and Ru in Re Ru/C Bimetallic Catalysts for the
 Role of Re and Ru in Re Ru/C Bimetallic Catalysts for the Aqueous Hydrogenation of Succinic Acid Xin Di a, Chuang Li a, Bingsen Zhang b, Ji Qi a, Wenzhen Li c, Dangsheng Su b, Changhai Liang a, * a Laboratory
Role of Re and Ru in Re Ru/C Bimetallic Catalysts for the Aqueous Hydrogenation of Succinic Acid Xin Di a, Chuang Li a, Bingsen Zhang b, Ji Qi a, Wenzhen Li c, Dangsheng Su b, Changhai Liang a, * a Laboratory
Surface Oxidation Mechanism of Ni(0) Particle Supported on Silica
 Surface Oxidation Mechanism of Ni(0) Particle Supported on Silica Shohei Yamashita, Yusaku Yamamoto, Misaki Katayama, and Yasuhiro Inada Department of Applied Chemistry, Graduate School of Life Sciences,
Surface Oxidation Mechanism of Ni(0) Particle Supported on Silica Shohei Yamashita, Yusaku Yamamoto, Misaki Katayama, and Yasuhiro Inada Department of Applied Chemistry, Graduate School of Life Sciences,
Supporting Information
 Supporting Information Remarkable performance of Ir 1 /FeO x single-atom catalyst in water gas shift reaction Jian Lin, Aiqin Wang, Botao Qiao, Xiaoyan Liu, Xiaofeng Yang, Xiaodong Wang, Jinxia Liang,
Supporting Information Remarkable performance of Ir 1 /FeO x single-atom catalyst in water gas shift reaction Jian Lin, Aiqin Wang, Botao Qiao, Xiaoyan Liu, Xiaofeng Yang, Xiaodong Wang, Jinxia Liang,
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/3/6/e1603180/dc1 Supplementary Materials for MnTiO3-driven low-temperature oxidative coupling of methane over TiO2-doped Mn2O3-Na2WO4/SiO2 catalyst This PDF file
advances.sciencemag.org/cgi/content/full/3/6/e1603180/dc1 Supplementary Materials for MnTiO3-driven low-temperature oxidative coupling of methane over TiO2-doped Mn2O3-Na2WO4/SiO2 catalyst This PDF file
Sub-10-nm Au-Pt-Pd Alloy Trimetallic Nanoparticles with. High Oxidation-Resistant Property as Efficient and Durable
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Sub-10-nm Au-Pt-Pd Alloy Trimetallic Nanoparticles with High
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Sub-10-nm Au-Pt-Pd Alloy Trimetallic Nanoparticles with High
Determination of Metal Dispersion of Pt/CeO2 Catalyst by CO-pulse Method
 Journal of the Japan Petroleum Institute, 48, (3), 173 177 (2005) 173 [Research Note] Determination of Metal Dispersion of Pt/CeO2 Catalyst by CO-pulse Method Shin ichi KOMAI 1), Yoshiteru YAZAWA 1), Atsushi
Journal of the Japan Petroleum Institute, 48, (3), 173 177 (2005) 173 [Research Note] Determination of Metal Dispersion of Pt/CeO2 Catalyst by CO-pulse Method Shin ichi KOMAI 1), Yoshiteru YAZAWA 1), Atsushi
Sintering-resistant Ni-based Reforming Catalysts via. the Nanoconfinement Effect
 Supporting Information Sintering-resistant Ni-based Reforming Catalysts via the Nanoconfinement Effect Chengxi Zhang a,b, Wancheng Zhu c, Shuirong Li a,b, Gaowei Wu a,b, Xinbin Ma a,b, Xun Wang c, and
Supporting Information Sintering-resistant Ni-based Reforming Catalysts via the Nanoconfinement Effect Chengxi Zhang a,b, Wancheng Zhu c, Shuirong Li a,b, Gaowei Wu a,b, Xinbin Ma a,b, Xun Wang c, and
A Tunable Process: Catalytic Transformation of Renewable Furfural with. Aliphatic Alcohols in the Presence of Molecular Oxygen. Supporting Information
 Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 A Tunable Process: Catalytic Transformation of Renewable Furfural with Aliphatic
Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 A Tunable Process: Catalytic Transformation of Renewable Furfural with Aliphatic
Supporting Information
 Electronic Supplementary Material (ESI) for Materials Chemistry Frontiers. This journal is the Partner Organisations 2018 Supporting Information Single palladium site catalyst as a bridge for converting
Electronic Supplementary Material (ESI) for Materials Chemistry Frontiers. This journal is the Partner Organisations 2018 Supporting Information Single palladium site catalyst as a bridge for converting
X-ray absorption spectroscopy
 X-ray absorption spectroscopy Jagdeep Singh Jeroen A. van Bokhoven Absorption as function of energy of the x-ray Data-analysis Absorption (a.u.) 2.0 Pre-edge subtraction 1.5 1.0 0.5 0.0-0.5 8800 9000 9200
X-ray absorption spectroscopy Jagdeep Singh Jeroen A. van Bokhoven Absorption as function of energy of the x-ray Data-analysis Absorption (a.u.) 2.0 Pre-edge subtraction 1.5 1.0 0.5 0.0-0.5 8800 9000 9200
TEM image of derivative 1 and fluorescence spectra of derivative 1 upon addition of
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Supramolecular ensemble of PBI derivative and Cu 2 O NPs: Potential photo catalysts for
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Supramolecular ensemble of PBI derivative and Cu 2 O NPs: Potential photo catalysts for
A Highly efficient Iron doped BaTiO 3 nanocatalyst for the catalytic reduction of nitrobenzene to azoxybenzene
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 A Highly efficient Iron doped BaTiO 3 nanocatalyst for the catalytic reduction of nitrobenzene
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 A Highly efficient Iron doped BaTiO 3 nanocatalyst for the catalytic reduction of nitrobenzene
Electronic Supplementary Information
 Electronic Supplementary Information Simultaneous Production of p-tolualdehyde and Hydrogen Peroxide in Photocatalytic Oxygenation of p-xylene and Reduction of Oxygen with 9-Mesityl-1-Methylacridinium
Electronic Supplementary Information Simultaneous Production of p-tolualdehyde and Hydrogen Peroxide in Photocatalytic Oxygenation of p-xylene and Reduction of Oxygen with 9-Mesityl-1-Methylacridinium
Supporting Information. CdS/mesoporous ZnS core/shell particles for efficient and stable photocatalytic hydrogen evolution under visible light
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2014 Supporting Information CdS/mesoporous ZnS core/shell particles for efficient
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2014 Supporting Information CdS/mesoporous ZnS core/shell particles for efficient
Heterogeneously catalyzed selective aerobic oxidative cross-coupling of terminal alkynes and amides with simple copper(ii) hydroxide
 Electronic Supplementary Information (ESI) for Heterogeneously catalyzed selective aerobic oxidative cross-coupling of terminal alkynes and amides with simple copper(ii) hydroxide Xiongjie Jin, Kazuya
Electronic Supplementary Information (ESI) for Heterogeneously catalyzed selective aerobic oxidative cross-coupling of terminal alkynes and amides with simple copper(ii) hydroxide Xiongjie Jin, Kazuya
Plasma driven ammonia decomposition on Fe-catalyst: eliminating surface nitrogen poisoning
 Supporting Information for Plasma driven ammonia decomposition on Fe-catalyst: eliminating surface nitrogen poisoning Contents: 1. Scheme of the DBD plasma-driven catalysis reactor, Scheme S1. 2. XRF analysis
Supporting Information for Plasma driven ammonia decomposition on Fe-catalyst: eliminating surface nitrogen poisoning Contents: 1. Scheme of the DBD plasma-driven catalysis reactor, Scheme S1. 2. XRF analysis
Supporting Information. High Selectivity of Supported Ru Catalysts in the Selective. CO Methanation - Water Makes the Difference
 S1 Supporting Information High Selectivity of Supported Ru Catalysts in the Selective CO Methanation - Water Makes the Difference Ali M. Abdel-Mageed,, Stephan Eckle, and R. Ju rgen Behm *, Institute of
S1 Supporting Information High Selectivity of Supported Ru Catalysts in the Selective CO Methanation - Water Makes the Difference Ali M. Abdel-Mageed,, Stephan Eckle, and R. Ju rgen Behm *, Institute of
Photocatalytic degradation of dyes over graphene-gold nanocomposites under visible light irradiation
 Photocatalytic degradation of dyes over graphene-gold nanocomposites under visible light irradiation Zhigang Xiong, Li Li Zhang, Jizhen Ma, X. S. Zhao* Department of Chemical and Biomolecular Engineering,
Photocatalytic degradation of dyes over graphene-gold nanocomposites under visible light irradiation Zhigang Xiong, Li Li Zhang, Jizhen Ma, X. S. Zhao* Department of Chemical and Biomolecular Engineering,
Xiufang Chen, Jinshui Zhang, Xianzhi Fu, Markus Antonietti, and Xinchen Wang*
 -Catalyzed Oxidation of Benzene to Phenol Using Hydrogen Peroxide and Visible Light Xiufang Chen, Jinshui Zhang, Xianzhi Fu, Markus Antonietti, and Xinchen Wang* Supporting Information: Synthesis of :
-Catalyzed Oxidation of Benzene to Phenol Using Hydrogen Peroxide and Visible Light Xiufang Chen, Jinshui Zhang, Xianzhi Fu, Markus Antonietti, and Xinchen Wang* Supporting Information: Synthesis of :
Electronic Supplementary Information. Alcohols
 Electronic Supplementary Information An Excellent Au/meso-γ-Al 2 O 3 Catalyst for Aerobic Selective Oxidation of Alcohols Guofeng Zhao, Min Ling, Huanyun Hu, Miaomiao Deng, Qingsong Xue, Yong Lu * Shanghai
Electronic Supplementary Information An Excellent Au/meso-γ-Al 2 O 3 Catalyst for Aerobic Selective Oxidation of Alcohols Guofeng Zhao, Min Ling, Huanyun Hu, Miaomiao Deng, Qingsong Xue, Yong Lu * Shanghai
Muffin-tin potentials in EXAFS analysis
 J. Synchrotron Rad. (5)., doi:.7/s6577555 Supporting information Volume (5) Supporting information for article: Muffin-tin potentials in EXAFS analysis B. Ravel Supplemental materials: Muffin tin potentials
J. Synchrotron Rad. (5)., doi:.7/s6577555 Supporting information Volume (5) Supporting information for article: Muffin-tin potentials in EXAFS analysis B. Ravel Supplemental materials: Muffin tin potentials
Supplemental Information. In Situ Electrochemical Production. of Ultrathin Nickel Nanosheets. for Hydrogen Evolution Electrocatalysis
 Chem, Volume 3 Supplemental Information In Situ Electrochemical Production of Ultrathin Nickel Nanosheets for Hydrogen Evolution Electrocatalysis Chengyi Hu, Qiuyu Ma, Sung-Fu Hung, Zhe-Ning Chen, Daohui
Chem, Volume 3 Supplemental Information In Situ Electrochemical Production of Ultrathin Nickel Nanosheets for Hydrogen Evolution Electrocatalysis Chengyi Hu, Qiuyu Ma, Sung-Fu Hung, Zhe-Ning Chen, Daohui
Supporting Information. for. Gold Nanoparticles Supported on Alumina as a Catalyst for Surface Plasmon-Enhanced Selective Reductions of Nitrobenzene
 Supporting Information for Gold Nanoparticles Supported on Alumina as a Catalyst for Surface Plasmon-Enhanced Selective Reductions of Nitrobenzene Kittichai Chaiseeda, Shun Nishimura, and Kohki Ebitani
Supporting Information for Gold Nanoparticles Supported on Alumina as a Catalyst for Surface Plasmon-Enhanced Selective Reductions of Nitrobenzene Kittichai Chaiseeda, Shun Nishimura, and Kohki Ebitani
Supplementary Figure S1: Particle size distributions of the Pt ML /Pd 9 Au 1 /C
 a 2 15 before cycle test mean particle size: 3.8 ± 1.2 nm b 2 15 after.6v - 1.V 1k cycle test mean particle size: 4.1 ± 1.5 nm Number 1 total number: 558 Number 1 total number: 554 5 5 1 2 3 4 5 6 7 8
a 2 15 before cycle test mean particle size: 3.8 ± 1.2 nm b 2 15 after.6v - 1.V 1k cycle test mean particle size: 4.1 ± 1.5 nm Number 1 total number: 558 Number 1 total number: 554 5 5 1 2 3 4 5 6 7 8
EXAFS data analysis. Extraction of EXAFS signal
 EXAFS data analysis Extraction of EXAFS signal 1 Experimental EXAFS spectrum Φ x Φ µ (ω) source monochromator sample detectors 1 6 1 5 32 - Ge 1 4 1 3 1 2 1 1 1 1 1 Photon energy hω (kev) 1-1 Ge, 1 K µ
EXAFS data analysis Extraction of EXAFS signal 1 Experimental EXAFS spectrum Φ x Φ µ (ω) source monochromator sample detectors 1 6 1 5 32 - Ge 1 4 1 3 1 2 1 1 1 1 1 Photon energy hω (kev) 1-1 Ge, 1 K µ
Operando Time-resolved XAFS Study for Surface Events on a Pt 3 Co/C. Cathode Catalyst in a PEFC during Voltage-Operating Processes
 Operando Time-resolved XAFS Study for Surface Events on a Pt 3 Co/C Cathode Catalyst in a PEFC during Voltage-Operating Processes Nozomu Ishiguro ǁ, Takahiro Saida ǁ, Tomoya Uruga, Shin-ichi Nagamatsu,
Operando Time-resolved XAFS Study for Surface Events on a Pt 3 Co/C Cathode Catalyst in a PEFC during Voltage-Operating Processes Nozomu Ishiguro ǁ, Takahiro Saida ǁ, Tomoya Uruga, Shin-ichi Nagamatsu,
Supplementary Figure 1. TEM analysis of Co0.5 showing (a) a SAED pattern, and (b-f) bright-field images of the microstructure. Only two broad rings
 Supplementary Figure 1. TEM analysis of Co0.5 showing (a) a SAED pattern, and (bf) brightfield images of the microstructure. Only two broad rings were observed in the SAED pattern, as expected for amorphous
Supplementary Figure 1. TEM analysis of Co0.5 showing (a) a SAED pattern, and (bf) brightfield images of the microstructure. Only two broad rings were observed in the SAED pattern, as expected for amorphous
Electronic Supplementary Information (ESI) Efficient synthesis of the Cu-SSZ-39 catalyst for DeNOx applications
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) Efficient synthesis of the Cu-SSZ-39 catalyst for DeNOx
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) Efficient synthesis of the Cu-SSZ-39 catalyst for DeNOx
Supporting Information
 Supporting Information An Extremely Active and General Catalyst for Suzuki Coupling Reactions of Unreactive Aryl Chlorides Dong-Hwan Lee and Myung-Jong Jin* School of Chemical Science and Engineering,
Supporting Information An Extremely Active and General Catalyst for Suzuki Coupling Reactions of Unreactive Aryl Chlorides Dong-Hwan Lee and Myung-Jong Jin* School of Chemical Science and Engineering,
Supporting information. Fuel Cells Fabricated by A Selective Electrochemical Sn Deposition Method
 Supporting information Surface-regulated Nano-SnO2/Pt3Co/C Cathode Catalysts for Polymer Electrolyte Fuel Cells Fabricated by A Selective Electrochemical Sn Deposition Method Kensaku Nagasawa a, Shinobu
Supporting information Surface-regulated Nano-SnO2/Pt3Co/C Cathode Catalysts for Polymer Electrolyte Fuel Cells Fabricated by A Selective Electrochemical Sn Deposition Method Kensaku Nagasawa a, Shinobu
X-ray Spectroscopy. Interaction of X-rays with matter XANES and EXAFS XANES analysis Pre-edge analysis EXAFS analysis
 X-ray Spectroscopy Interaction of X-rays with matter XANES and EXAFS XANES analysis Pre-edge analysis EXAFS analysis Element specific Sensitive to low concentrations (0.01-0.1 %) Why XAS? Applicable under
X-ray Spectroscopy Interaction of X-rays with matter XANES and EXAFS XANES analysis Pre-edge analysis EXAFS analysis Element specific Sensitive to low concentrations (0.01-0.1 %) Why XAS? Applicable under
Electrochemical Water Splitting by Layered and 3D Cross-linked Manganese Oxides: Correlating Structural Motifs and Catalytic Activity
 Electronic Supplementary Information Electrochemical Water Splitting by Layered and 3D Cross-linked Manganese Oxides: Correlating Structural Motifs and Catalytic Activity Arno Bergmann,* a Ivelina Zaharieva,*
Electronic Supplementary Information Electrochemical Water Splitting by Layered and 3D Cross-linked Manganese Oxides: Correlating Structural Motifs and Catalytic Activity Arno Bergmann,* a Ivelina Zaharieva,*
Method and process for combustion synthesized supported cobalt catalysts for fixed bed Fischer Tropsch reaction
 Method and process for combustion synthesized supported cobalt catalysts for fixed bed Fischer Tropsch reaction Center for Sustainable Technologies Indian Institute of Science Bangalore IDF presentation
Method and process for combustion synthesized supported cobalt catalysts for fixed bed Fischer Tropsch reaction Center for Sustainable Technologies Indian Institute of Science Bangalore IDF presentation
Synthesis of jet fuel range cycloalkanes with diacetone alcohol. from lignocellulose
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Supporting Information Synthesis of jet fuel range cycloalkanes with diacetone alcohol from
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2016 Supporting Information Synthesis of jet fuel range cycloalkanes with diacetone alcohol from
Rh/ZrP 2 O 7 as an Efficient Automotive Catalyst for NO x Reduction under Slightly Lean Conditions
 Supporting Information Rh/ZrP 2 O 7 as an Efficient Automotive Catalyst for NO x Reduction under Slightly Lean Conditions Yuki Nagao, * Yunosuke Nakahara, Takahiro Sato, Hironori Iwakura, Shoya Takeshita,
Supporting Information Rh/ZrP 2 O 7 as an Efficient Automotive Catalyst for NO x Reduction under Slightly Lean Conditions Yuki Nagao, * Yunosuke Nakahara, Takahiro Sato, Hironori Iwakura, Shoya Takeshita,
Metal-free and Solvent-free Oxidative Coupling of Amines to Imines with Mesoporous Carbon from Macrocyclic Compounds
 1 Supporting Information Metal-free and Solvent-free Oxidative Coupling of Amines to Imines with Mesoporous Carbon from Macrocyclic Compounds Bo Chen, 1, Lianyue Wang, 1 Wen Dai, 1 Sensen Shang, 1, Ying
1 Supporting Information Metal-free and Solvent-free Oxidative Coupling of Amines to Imines with Mesoporous Carbon from Macrocyclic Compounds Bo Chen, 1, Lianyue Wang, 1 Wen Dai, 1 Sensen Shang, 1, Ying
Supporting Information. Size-tunable Ni nanoparticles supported on surface-modified, cage-type mesoporous
 Supporting Information Size-tunable Ni nanoparticles supported on surface-modified, cage-type mesoporous silica as highly active catalysts for CO 2 hydrogenation Ching-Shiun Chen, a,b* Canggih Setya Budi,
Supporting Information Size-tunable Ni nanoparticles supported on surface-modified, cage-type mesoporous silica as highly active catalysts for CO 2 hydrogenation Ching-Shiun Chen, a,b* Canggih Setya Budi,
Visible-light Driven Plasmonic Photocatalyst Helical Chiral TiO 2 Nanofibers
 Visible-light Driven Plasmonic Photocatalyst Ag/AgCl @ Helical Chiral TiO 2 Nanofibers Dawei Wang, Yi Li*, Gianluca Li Puma, Chao Wang, Peifang Wang, Wenlong Zhang, and Qing Wang Fig. S1. The reactor of
Visible-light Driven Plasmonic Photocatalyst Ag/AgCl @ Helical Chiral TiO 2 Nanofibers Dawei Wang, Yi Li*, Gianluca Li Puma, Chao Wang, Peifang Wang, Wenlong Zhang, and Qing Wang Fig. S1. The reactor of
photo-mineralization of 2-propanol under visible light irradiation
 Electronic Supplementary Information for WO 3 modified titanate network film: highly efficient photo-mineralization of 2-propanol under visible light irradiation Experimental Preparation of STN, and WO
Electronic Supplementary Information for WO 3 modified titanate network film: highly efficient photo-mineralization of 2-propanol under visible light irradiation Experimental Preparation of STN, and WO
Supplementary Material (ESI) for Chemical Communications This journal is (c) The Royal Society of Chemistry 2008
 Supplementary Information for: Scrambling Reaction between Polymers Prepared by Step-growth and Chain-growth Polymerizations: Macromolecular Cross-metathesis between 1,4-Polybutadiene and Olefin-containing
Supplementary Information for: Scrambling Reaction between Polymers Prepared by Step-growth and Chain-growth Polymerizations: Macromolecular Cross-metathesis between 1,4-Polybutadiene and Olefin-containing
Solid State Spectroscopy Problem Set 7
 Solid State Spectroscopy Problem Set 7 Due date: June 29th, 2015 Problem 5.1 EXAFS Study of Mn/Fe substitution in Y(Mn 1-x Fe x ) 2 O 5 From article «EXAFS, XANES, and DFT study of the mixed-valence compound
Solid State Spectroscopy Problem Set 7 Due date: June 29th, 2015 Problem 5.1 EXAFS Study of Mn/Fe substitution in Y(Mn 1-x Fe x ) 2 O 5 From article «EXAFS, XANES, and DFT study of the mixed-valence compound
Part 1: What is XAFS? What does it tell us? The EXAFS equation. Part 2: Basic steps in the analysis Quick overview of typical analysis
 Introduction to XAFS Part 1: What is XAFS? What does it tell us? The EXAFS equation Part 2: Basic steps in the analysis Quick overview of typical analysis Tomorrow Measurement methods and examples The
Introduction to XAFS Part 1: What is XAFS? What does it tell us? The EXAFS equation Part 2: Basic steps in the analysis Quick overview of typical analysis Tomorrow Measurement methods and examples The
Supporting Information of
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information of Synthesis of Hard Carbons from Argan Shell for
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information of Synthesis of Hard Carbons from Argan Shell for
Structural and Catalytic Investigation of Active-Site Isolation in Pd-Ga Intermetallic Compounds
 Structural and Catalytic Investigation of Active-Site Isolation in Pd-Ga Intermetallic Compounds, Rainer Giedigkeit, Kirill Kovnir, Rolf E. Jentoft, Marc Armbrüster, Yuri Grin, Robert Schlögl, Thorsten
Structural and Catalytic Investigation of Active-Site Isolation in Pd-Ga Intermetallic Compounds, Rainer Giedigkeit, Kirill Kovnir, Rolf E. Jentoft, Marc Armbrüster, Yuri Grin, Robert Schlögl, Thorsten
Supplementary Information. Pd L-series. Supplementary Figure 1. Energy Dispersive Spectroscopy (EDS). The analysis is of the boxed
 Supplementary Figures Supplementary Information 400 C 2hr 5% H 2 /N 2 10 nm Pd Pd L-series La La L-series Supplementary Figure 1. Energy Dispersive Spectroscopy (EDS). The analysis is of the boxed region
Supplementary Figures Supplementary Information 400 C 2hr 5% H 2 /N 2 10 nm Pd Pd L-series La La L-series Supplementary Figure 1. Energy Dispersive Spectroscopy (EDS). The analysis is of the boxed region
Chemical tuning of electrochemical properties of Ptskin surface for highly active oxygen reduction reactions
 Chemical tuning of electrochemical properties of Ptskin surface for highly active oxygen reduction reactions Namgee Jung, a Young-Hoon Chung, b Dong-Young Chung, b Kwang-Hyun Choi, b Hee- Young Park, a
Chemical tuning of electrochemical properties of Ptskin surface for highly active oxygen reduction reactions Namgee Jung, a Young-Hoon Chung, b Dong-Young Chung, b Kwang-Hyun Choi, b Hee- Young Park, a
Rh 3d. Co 2p. Binding Energy (ev) Binding Energy (ev) (b) (a)
 Co 2p Co(0) 778.3 Rh 3d Rh (0) 307.2 810 800 790 780 770 Binding Energy (ev) (a) 320 315 310 305 Binding Energy (ev) (b) Supplementary Figure 1 Photoemission features of a catalyst precursor which was
Co 2p Co(0) 778.3 Rh 3d Rh (0) 307.2 810 800 790 780 770 Binding Energy (ev) (a) 320 315 310 305 Binding Energy (ev) (b) Supplementary Figure 1 Photoemission features of a catalyst precursor which was
Supporting Information
 Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2016 Supporting Information Single-atom dispersed Co--C catalyst: structure identification and
Electronic Supplementary Material (ESI) for Chemical Science. This journal is The Royal Society of Chemistry 2016 Supporting Information Single-atom dispersed Co--C catalyst: structure identification and
Electronic Supplementary Information for. Biomimetic aerobic oxidative hydroxylation of arylboronic acids to phenols catalysed by a flavin derivative
 Electronic Supplementary Material (ESI) for Organic. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information for Biomimetic aerobic oxidative hydroxylation of arylboronic
Electronic Supplementary Material (ESI) for Organic. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information for Biomimetic aerobic oxidative hydroxylation of arylboronic
Supplementary Figure 2. (a) XRD patterns of the MOF and the simulated Ni-MOF-74
 Supplementary Figure 1. Low-magnification TEM image of Pt-Ni frame @ MOF. The scale bar is 200 nm. Supplementary Figure 2. (a) XRD patterns of the Pt-Ni @ MOF and the simulated Ni-MOF-74 pattern. (b) XRD
Supplementary Figure 1. Low-magnification TEM image of Pt-Ni frame @ MOF. The scale bar is 200 nm. Supplementary Figure 2. (a) XRD patterns of the Pt-Ni @ MOF and the simulated Ni-MOF-74 pattern. (b) XRD
Facile Fabrication of Shape-controlled Co x Mn y O β Nanocatalysts for Benzene Oxidation at Low Temperature
 Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2018 Facile Fabrication of Shape-controlled Co x Mn y O β Nanocatalysts for Benzene Oxidation
Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2018 Facile Fabrication of Shape-controlled Co x Mn y O β Nanocatalysts for Benzene Oxidation
Fe 2 O 3 and Co-Co 3 O 4 hydrogenation of nitroarenes under mild conditions
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2016 Supporting information for Fe 2 O 3 /NGr@C- and Co-Co 3 O 4 /NGr@C-catalysed
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2016 Supporting information for Fe 2 O 3 /NGr@C- and Co-Co 3 O 4 /NGr@C-catalysed
Efficient H2 generation from formic acid using azole complexes in water
 Electronic Supplementary Information (ESI) Efficient H2 generation from formic acid using azole complexes in water Yuichi Manaka, a Wan-Hui Wang,* a,b Yuki Suna, a Hide Kambayashi, a James T. Muckerman,
Electronic Supplementary Information (ESI) Efficient H2 generation from formic acid using azole complexes in water Yuichi Manaka, a Wan-Hui Wang,* a,b Yuki Suna, a Hide Kambayashi, a James T. Muckerman,
Electronic Supplementary Information
 Electronic Supplementary Information General and highly active catalyst for mono and double Hiyama coupling reactions of unreactive aryl chlorides in water Dong-Hwan Lee, Ji-Young Jung, and Myung-Jong
Electronic Supplementary Information General and highly active catalyst for mono and double Hiyama coupling reactions of unreactive aryl chlorides in water Dong-Hwan Lee, Ji-Young Jung, and Myung-Jong
Supporting Information
 Supporting Information of highly concentrated cellulose to 1, 2-propanediol and ethylene glycol over high efficient CuCr catalysts Zihui Xiao, Shaohua Jin, Min Pang and Changhai Liang Experimental The
Supporting Information of highly concentrated cellulose to 1, 2-propanediol and ethylene glycol over high efficient CuCr catalysts Zihui Xiao, Shaohua Jin, Min Pang and Changhai Liang Experimental The
Electronic Supplementary Information (ESI)
 Electronic Supplementary Information (ESI) Cu-SSZ-39, an active and hydrothermally stable catalyst for the selective catalytic reduction of NOx Manuel Moliner, *a Cristina Franch, a Eduardo Palomares,
Electronic Supplementary Information (ESI) Cu-SSZ-39, an active and hydrothermally stable catalyst for the selective catalytic reduction of NOx Manuel Moliner, *a Cristina Franch, a Eduardo Palomares,
Supporting Information
 Supporting Information Au 25 -Loaded BaLa 4 Ti 4 O 15 Water-Splitting Photocatalyst with Enhanced Activity and Durability Produced Using New Chromium Oxide Shell Formation Method Wataru Kurashige, 1 Rina
Supporting Information Au 25 -Loaded BaLa 4 Ti 4 O 15 Water-Splitting Photocatalyst with Enhanced Activity and Durability Produced Using New Chromium Oxide Shell Formation Method Wataru Kurashige, 1 Rina
Electron-hopping brings lattice strain and high catalytic activity in low temperature oxidative coupling of methane in an electric field
 Supporting information Electron-hopping brings lattice strain and high catalytic activity in low temperature oxidative coupling of methane in an electric field Shuhei Ogo*, Hideaki Nakatsubo, Kousei Iwasaki,
Supporting information Electron-hopping brings lattice strain and high catalytic activity in low temperature oxidative coupling of methane in an electric field Shuhei Ogo*, Hideaki Nakatsubo, Kousei Iwasaki,
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry
Chemicals All chemicals were of analytical grade and used as purchased without further purification.
 Supplementary Methods Chemicals All chemicals were of analytical grade and used as purchased without further purification. Synthesis of referenced catalysts Synthesis of Au/ZSM-5. As a typical run, 1 g
Supplementary Methods Chemicals All chemicals were of analytical grade and used as purchased without further purification. Synthesis of referenced catalysts Synthesis of Au/ZSM-5. As a typical run, 1 g
Supporting Information
 Supporting Information Highly Cross-Linked Imidazolium Salts Entrapped Magnetic Particles Preparation and Applications Paola Agrigento, a Matthias Josef Beier, b Jesper T. N. Knijnenburg, c Alfons Baiker
Supporting Information Highly Cross-Linked Imidazolium Salts Entrapped Magnetic Particles Preparation and Applications Paola Agrigento, a Matthias Josef Beier, b Jesper T. N. Knijnenburg, c Alfons Baiker
Enhancing Stability of Platinum on Silica by Surface Modification - Application to CO Oxidation -
 2012 CLEERS Workshop Enhancing Stability of Platinum on Silica by Surface Modification - Application to CO Oxidation - Mi-Young Kim, Jae-Soon Choi, Todd J. Toops Emissions and Catalysis Research Group
2012 CLEERS Workshop Enhancing Stability of Platinum on Silica by Surface Modification - Application to CO Oxidation - Mi-Young Kim, Jae-Soon Choi, Todd J. Toops Emissions and Catalysis Research Group
Supporting Information:
 Supporting Information: In Situ Synthesis of Magnetically Recyclable Graphene Supported Pd@Co Core-Shell Nanoparticles as Efficient Catalysts for Hydrolytic Dehydrogenation of Ammonia Borane Jun Wang,
Supporting Information: In Situ Synthesis of Magnetically Recyclable Graphene Supported Pd@Co Core-Shell Nanoparticles as Efficient Catalysts for Hydrolytic Dehydrogenation of Ammonia Borane Jun Wang,
Polymer Semiconductors for Artificial Photosynthesis: Hydrogen Evolution by Mesoporous Graphitic Carbon Nitride with Visible Light
 Polymer Semiconductors for Artificial Photosynthesis: Hydrogen Evolution by Mesoporous Graphitic Carbon Nitride with Visible Light Xinchen Wang*, Kazuhiko Maeda, Xiufang Chen, Kazuhiro Takanabe, Kazunari
Polymer Semiconductors for Artificial Photosynthesis: Hydrogen Evolution by Mesoporous Graphitic Carbon Nitride with Visible Light Xinchen Wang*, Kazuhiko Maeda, Xiufang Chen, Kazuhiro Takanabe, Kazunari
3. EXAFS Data Analysis using Athena 2012 년 2 월 29 일 13:30 14:20
 3. EXAFS Data Analysis using Athena 2012 년 2 월 29 일 13:30 14:20 IFEFFIT package FEFFIT Fit χ(k) data to the theoretical calculations of FEFF, and assess the errors in the fitting parameters. The fitting
3. EXAFS Data Analysis using Athena 2012 년 2 월 29 일 13:30 14:20 IFEFFIT package FEFFIT Fit χ(k) data to the theoretical calculations of FEFF, and assess the errors in the fitting parameters. The fitting
XAFS Analysis for Calcination Process of Supported Mn Catalysts on Silica
 XAFS Analysis for Calcination Process of Supported Mn Catalysts on Silica Kazutaka Furusato, Misaki Katayama, and Yasuhiro Inada Department of Applied Chemistry, Graduate School of Life Sciences, Ritsumeikan
XAFS Analysis for Calcination Process of Supported Mn Catalysts on Silica Kazutaka Furusato, Misaki Katayama, and Yasuhiro Inada Department of Applied Chemistry, Graduate School of Life Sciences, Ritsumeikan
Supporting Information
 Supporting Information Ag.1 Pd.9 /rgo: An Efficient Catalyst for Hydrogen Generation from Formic Acid/Sodium Formate Yun Ping, Jun-Min Yan*, Zhi-Li Wang, Hong-Li Wang, Qing Jiang Key Laboratory of Automobile
Supporting Information Ag.1 Pd.9 /rgo: An Efficient Catalyst for Hydrogen Generation from Formic Acid/Sodium Formate Yun Ping, Jun-Min Yan*, Zhi-Li Wang, Hong-Li Wang, Qing Jiang Key Laboratory of Automobile
INVESTIGATION OF SURFACE CHEMISTRY PROPERTIES OF Ga 2 O 3 /Al 2 O 3 CATALYSTS BY FT-IR SPECTROSCOPY
 INVESTIGATION OF SURFACE CHEMISTRY PROPERTIES OF Ga 2 O 3 /Al 2 O 3 CATALYSTS BY FT-IR SPECTROSCOPY Balázs Szabó 1, Tamás Ollár 1, Ákos Rédey 1 1 Department of Environmental Engineering and Chemical Technology,
INVESTIGATION OF SURFACE CHEMISTRY PROPERTIES OF Ga 2 O 3 /Al 2 O 3 CATALYSTS BY FT-IR SPECTROSCOPY Balázs Szabó 1, Tamás Ollár 1, Ákos Rédey 1 1 Department of Environmental Engineering and Chemical Technology,
A green and efficient oxidation of alcohols by supported gold. conditions
 A green and efficient oxidation of alcohols by supported gold catalysts using aqueous H 2 O 2 under organic solvent-free conditions Ji Ni, Wen-Jian Yu, Lin He, Hao sun, Yong Cao,* He-Yong He, and Kang-Nian
A green and efficient oxidation of alcohols by supported gold catalysts using aqueous H 2 O 2 under organic solvent-free conditions Ji Ni, Wen-Jian Yu, Lin He, Hao sun, Yong Cao,* He-Yong He, and Kang-Nian
