Enhancing Stability of Platinum on Silica by Surface Modification - Application to CO Oxidation -
|
|
|
- Britney Wells
- 5 years ago
- Views:
Transcription
1 2012 CLEERS Workshop Enhancing Stability of Platinum on Silica by Surface Modification - Application to CO Oxidation - Mi-Young Kim, Jae-Soon Choi, Todd J. Toops Emissions and Catalysis Research Group Oak Ridge National Laboratory May 1, 2012
2 Outline 1. The aim of this study Preparation of highly dispersed platinum catalyst with sulfur tolerance and hydrothermal stability 2. Results Characterization & catalytic performance Fresh catalysts Sulfated catalysts Hydrothermal aged catalysts 3. Conclusions Results summary Application possibility for automotive catalysts 2
3 1. The aim of this study Preparation of highly dispersed platinum catalyst with sulfur tolerance and hydrothermal stability 3
4 Designing active & stable Pt catalyst using silica Alumina as a support for diesel oxidation catalyst - Advantage: good interaction with platinum - Disadvantage: the formation of aluminum sulfate (Al 2 (SO 4 ) 3 ) induces severe catalyst deactivation. Silica as a support for platinum metal - Advantage: chemical inertness (no S adsorption) high surface area and thermal stability processibility owing to surface hydroxyl group - Disadvantage: Pt can easily sinter on silica due to weak platinum interaction. To have both good dispersion and sulfur tolerance Two approach methods for the preparation of platinum catalysts - Incorporation of metal oxide layer (Ti, Zr ) on silica - Treatment of impregnated platinum precursor with hydrogen peroxide 4
5 First method: incorporation of metal oxide layer on silica Reducibility introduction on silica - Reducible metal oxide: Ti, Zr, Ce, V 2 O 5 cf. γ-al 2 O 3, Si Acid strength increase Acidity increase Metal oxide: Chemistry and applications, 2006, CRC, 273 p. γ-al 2 O 3 (SL/B), Ti -Si (SB), Zr -Si (SB), > Ti (ML) > Zr (MWL) Si (MWB) S: Strong; M: Medium; W: Weak; B: Brönsted acid; L: Lewis acid Reducibility and acidity of support induce strong platinum-support interaction. Acid strength and electronegativity of binary oxides New Solid Acid and Bases, 1989, Elsevier, 113 p. 5
6 Preparation methods of M -Si (M = Ti or Zr) Type Preparation method Remark Physically mixed Solid state mixing Weak Van der Waals forces Interaction Impregnation Si powder + M(NO 3 ) 4 aqueous solutions (M = Ti or Zr) Chemically bonded Co-precipitation Sol-gel Si precursor + M precursor ph adjustment Si precursor + M alkoxide hydrolysis, condensation Si powder + M alkoxide reaction between alkoxide and OH group of silica Among these methods, the sol-gel method is the most effective method for controlling textural and surface characteristics of mixed oxide. Preparation method in this study 6
7 Second method: hydrogen peroxide treatment Electron property change of support Ti O O O Ti OH H 2 Catal. Lett., 16 (1992) 109, H 2 O O O O H 2 O Ti OO- H 2 O Zr Appl. Catal. A, 369 (2009) 27 H 2 Oxygen linkage generation Pt Ti Oxygen linkage Si The oxo-species generated by hydrogen peroxide treatment change the electronic property of support and make oxygen linkage, result in further improvement of platinum dispersion on silica. 7
8 Our preparation procedure of highly dispersive platinum catalysts Sol-gel process Ti(OR) 4 + Si OH RO O Ti Si OR O Si Incorporation of Ti or Zr Si Si Ti, Zr Pt Oxygen HO OH O O O Ti Ti Ti O O or O O O Si Si Si Impregnation of Pt precursor Treatment of hydrogen peroxide Ti H 2 O O O Si Ti O OH Si O - Si Treatment of H 2 Si Reduction Si 8
9 Catalysts characterized after sulfation and hydrothermal aging Preparation of platinum catalysts (1wt%) - Pt/Ti-Si-H 2, Pt/Zr-Si-H 2, Pt/Si, Pt/Al Catalyst characterization - XRD, TEM, EXAFS, XANES, TPD, TPR Consecutive catalytic performances: CO oxidation - To verify the sulfur tolerance and hydrothermal stability of platinum catalysts Fresh catalysts Sulfated catalysts 400 o C, 50 ppm S, 3h Hydrothermal aged catalysts 800 o C, w/ H2O, 2 h Reaction condition: ml/min (1% CO, 4%, 5% H 2 O, Ar) g catalyst o C, 2 o C/min 9
10 2. Results Characterization & catalytic performance Fresh catalysts Sulfated catalysts Hydrothermal aged catalysts 10
11 Intensity Highly dispersed platinum on Ti -, Zr - incorporated silica XRD results of fresh catalysts 100 cps Fresh catalysts Pt/Al Pt peak on Pt/Si - Aggregation of platinum Pt/Si No Pt peak on Pt/Ti-Si-H 2 and Pt/Zr-Si-H 2 - Improvement of platinum dispersion due to metal oxide incorporation Pt/Ti-Si-H 2 Pt/Zr-Si-H Theta (degree) Pt Zr Ti Al 2 O 3 Si 11
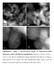
12 Average D p (nm) Dispersion improvement through incorporating Ti and Zr Pt/Al Pt/Si Pt/Ti-Si-H 2 Pt/Zr-Si-H 2 10 nm 10 nm 10 nm 10 nm Aggregated platinum particle Large Pt particles on Pt/Si - Diameter of around 20 nm Medium Pt particles on Pt/Al - Diameter ranged in 5~7 nm 0 Pt/Al Pt/Si Pt/Ti-Si-H 2 Pt/Zr-Si-H 2 Small platinum particles on Pt/Ti-Si-H 2 and Pt/Zr-Si-H 2 - Diameter ranged in 1~4 nm 12
13 Zr-Si-H 2 support enhances adsorption of oxygen on Pt Normalized Absorbance Normalized Absorbance Pt L III -edge XANES spectra from Advanced Photon Source at ANL Pt/Al Pt/Zr-Si-H 2 higher electron deficiency than Pt/Al in oxidative condition Pt/Al_H Energy (ev) Pt/Al_ 0.4 Pt/Zr-Si-H 2 _H 0.2 Pt/Zr-Si-H 2 _ Energy (ev) High whiteline intensity: high electron deficiency High electron deficiency: high oxygen adsorption property and high sulfur tolerance [X-ray Absorption Fine Structure for Catalysts and Surface, World Scientific, 1996; Oxygen Complexes and Oxygen Activation by Transition Metals, Plenum Press, 1988; Catalyst Deactivation, Vol. 6, Elsevier, 1980.] Pt/Al and Pt/Zr-Si-H 2 had same XANES spectra in reductive condition, however Pt/Zr-Si-H 2 had higher whiteline intensity than Pt/Al in oxidative condition. Higher whiteline intensity of Pt/Zr-Si-H 2 means stronger adsorption property of oxygen and higher sulfur tolerance in oxidative condition. 13
14 Pt/Ti-Si-H 2 showed high catalytic activity in fresh catalysts CO conversion (%) 1 st CO oxidation over fresh catalysts Pt/Al Pt/Si Pt/Ti-Si-H 2 Pt/Zr-Si-H 2 Fresh catalysts Catalytic activity in CO oxidation (T 50%,o C) - Pt/Ti-Si-H 2 (148 o C) > Pt/Zr-Si-H 2 (165 o C) > Pt/Al Pt/Si (218 o C) Temperature ( o C) Higher catalytic activity over Pt/Ti-Si-H 2 and Pt/Zr-Si-H 2 - Consistent w/ higher Pt dispersion - Apparently due to enhanced oxygen adsorption on Pt (see XANES result) 14
15 Impact of surface modification on surface acidobasicity Detector signal (a.u.) TPD (Temperature Programmed Desorption) Relative amount = 1 NH 3 TPD_acidity Pt/Al C TPD_basicity Relative amount = 1 Pt/Al 0 Pt/Si 0 Pt/Zr-Si-H Pt/Si Pt/Ti-Si-H 2 0 Pt/Ti-Si-H Pt/Zr-Si-H Pt/Zr-Si-H Pt/Si Temperature ( o C) Temperature ( o C) High acidity: high platinum dispersion, High basicity: high sulfur adsorption - Pt/Al had strong acidic and basic sites, while Pt/Si had neither. - Pt/Zr-Si-H 2 had more acidic and basic sites than Pt/Ti-Si-H 2. - Thus, Pt/Ti-Si-H 2 & Pt/Zr-Si-H 2 show good Pt dispersion and better sulfur tolerance. 15
16 Pt/Ti-Si-H 2 showed high catalytic activity after sulfation CO conversion (%) 2 nd CO oxidation over sulfated catalysts 100 Pt/Al Pt/Si Pt/Ti-Si-H 2 80 Pt/Zr-Si-H Sulfated catalysts Catalytic activity in CO oxidation (T 50%, o C) - Pt/Ti-Si-H 2 (197 o C) > Pt/Zr-Si-H 2 (204 o C) > Pt/Al (236 o C) > Pt/Si (256 o C) Decrease of catalytic activity over all catalysts - T 50% increases ~ 50 o C vs. fresh catalysts Temperature ( o C) Relatively high catalytic activity over Pt/Ti-Si-H 2 and Pt/Zr-Si-H 2 - Consistent w/ sulfur tolerance property due to low basicity and high electron deficiency in oxidative condition 16
17 Ti & Zr-incorporated catalysts exhibit better S tolerance than Pt/Al Amount of desorbed sulfur (μmol/g cat min) Temperature ( o C) TPR (Temperature Programmed Reduction): Desulfation with 1% H Pt/Al Pt/Si Pt/Ti-Si-H Desorption peak area (μmol/g cat min) - Pt/Al (726) > Pt/Zr-Si-H 2 (370) > Pt/Ti-Si-H 2 (171) > Pt/Si (37) Pt/Al had most S species which are more stable Pt/Zr-Si-H Time (min) The relative low amount of sulfur desorbed and low temperature desorption peak on Pt/Ti-Si-H 2 and Pt/Zr-Si-H 2 - Low basicity and high platinum dispersion, resulting in high catalytic activity after sulfation No sulfur desorption peak on Pt/Si - Although no sulfur adsorbed on Pt/Si (no basicity), its low platinum dispersion (no acidity) induces low catalytic activity after sulfation. 17
18 CO conversion (%) Pt/Zr-Si-H 2 showed high catalytic activity after hydrothermal aging 3 rd CO oxidation over hydrothermal aged catalysts Hydrothermal aged catalysts Pt/Al Pt/Si Pt/Ti-Si-H 2 Pt/Zr-Si-H Temperature ( o C) Catalytic activity in CO oxidation (T o 50%, C) - Pt/Zr-Si-H 2 (218 o C) > Pt/Ti-Si-H 2 (237 o C) > Pt/Al (241 o C) > Pt/Si (256 o C) Higher catalytic activity of Pt/Zr-Si-H 2 after hydrothermal aging - Due to its hydrothermal stability (see next page XRD results) 18
19 Intensity Pt/Zr-Si-H 2 maintains its platinum dispersion after hydrothermal aging XRD results of hydrothermal aged catalysts 100 cps Fresh catalysts Pt/Al Pt Zr 200 cps Aged catalysts Pt/Al Ti Pt/Si Al 2 O 3 Si Pt/Si HT aging Pt/Ti-Si-H 2 Pt/Ti-Si-H 2 Pt/Zr-Si-H 2 Pt/Zr-Si-H Theta (degree) Theta (degree) Platinum peak increase of all catalysts means the platinum sintering of hydrothermal aged catalysts - However, Pt/Zr-Si-H 2 had the smallest platinum peak. Relatively high stability of Pt/Zr-Si-H 2 after hydrothermal aging results in the best catalytic activity. 19
20 4. Conclusions Results summary Application possibility for automotive catalysts 20
21 Results summary Various contributing factors to dispersion and S tolerance of Pt catalyst Dispersion (XRD, TEM, EXAFS) Fresh Aged Adsorption amount of sulfur (TPR) Basicity (C TPD) Acidity (NH 3 TPD) Electron deficiency (XANES) Catalytic activity (CO oxidation) Pt/Zr-Si-H 2 high high medium low high high high even after aging Pt/Ti-Si-H 2 high low low none medium medium high after sulfation Pt/Si low very low very low none none very low low Pt/Al medium low high high very high very low medium after aging Correlation Catalytic activity Adsorption of acidic S on basic supports Dispersion, Oxygen Adsorption, S tolerance The highest catalytic activity of Pt/Zr-Si-H 2 Merit Demerit 21
22 Conclusions 1. Platinum catalysts on Ti -, Zr -incorporated silica show higher catalytic activity than Pt/Al and Pt/Si in CO oxidation. 2. Pt/Ti-Si-H 2 has the best sulfur tolerance property and Pt/Zr-Si-H 2 shows the best hydrothermal stability. 3. Pt/Zr-Si-H 2 has thermal stability, electron deficiency, high acidity and low basicity, resulting in high platinum dispersion even after hydrothermal aging and suppression of the adsorption of acidic S. Pt/Zr-Si-H 2 represents its application possibility for the diesel oxidation catalysts 22
23 Acknowledgement Financial supports Research sponsored by DOE, Vehicle Technologies Program - Program Managers: Ken Howden, Gurpreet Singh Korea Research Foundation: Postdoctoral scholarship Oak Ridge National Laboratory: Post-graduate research associates program Research supports Chonnam National University: Gon Seo FEERC: Bill Partridge, William J. Johnson, Josh A. Pihl Chonbuk National University: Sang-Wook Han, Eun-Suk Jung CNMS: Viviane Schwartz, Jihua Chen APS: Trudy Bolin 23
24 Thank you! Mi-Young Kim, Ph.D. Emissions and Catalysis Research Group Fuels, Engines and Emissions Research Center Oak Ridge National Laboratory 2360 Cherahala Blvd. Knoxville, TN Office: (865) Fax: (865)
Correlation between LNT NH 3 and N 2 O selectivities under fast cycling conditions
 Correlation between LNT and N 2 O selectivities under fast cycling conditions Jae-Soon Choi, Josh Pihl, Miyoung Kim, Bill Partridge, Stuart Daw Oak Ridge National Laboratory Petr Kočí Institute of Chemical
Correlation between LNT and N 2 O selectivities under fast cycling conditions Jae-Soon Choi, Josh Pihl, Miyoung Kim, Bill Partridge, Stuart Daw Oak Ridge National Laboratory Petr Kočí Institute of Chemical
A new transient CLEERS SCR protocol
 A new transient CLEERS SCR protocol Josh Pihl, Todd Toops, Stuart Daw Oak Ridge National Laboratory presented to: 2010 DOE Crosscut Workshop on Lean Emissions Reduction Simulation Dearborn, MI April 20,
A new transient CLEERS SCR protocol Josh Pihl, Todd Toops, Stuart Daw Oak Ridge National Laboratory presented to: 2010 DOE Crosscut Workshop on Lean Emissions Reduction Simulation Dearborn, MI April 20,
XAFS Analysis for Calcination Process of Supported Mn Catalysts on Silica
 XAFS Analysis for Calcination Process of Supported Mn Catalysts on Silica Kazutaka Furusato, Misaki Katayama, and Yasuhiro Inada Department of Applied Chemistry, Graduate School of Life Sciences, Ritsumeikan
XAFS Analysis for Calcination Process of Supported Mn Catalysts on Silica Kazutaka Furusato, Misaki Katayama, and Yasuhiro Inada Department of Applied Chemistry, Graduate School of Life Sciences, Ritsumeikan
An in-situ infrared and XAS study on NO adsorption on Pd/zeolite under complex gas feed
 An in-situ infrared and XAS study on NO adsorption on Pd/zeolite under complex gas feed Donna Liu, Loredana Mantorosie, H. Islam, V. Novak, M. Sarwar, H.Y. Chen, J. E. Collier and D. Thompsett Outline
An in-situ infrared and XAS study on NO adsorption on Pd/zeolite under complex gas feed Donna Liu, Loredana Mantorosie, H. Islam, V. Novak, M. Sarwar, H.Y. Chen, J. E. Collier and D. Thompsett Outline
Structural and Electronic properties of platinum nanoparticles studied by diffraction and absorption spectroscopy
 The 4 th SUNBEAM Workshop Structural and Electronic properties of platinum nanoparticles studied by in situ x-ray x diffraction and in situ x-ray x absorption spectroscopy Hideto Imai Fundamental and Environmental
The 4 th SUNBEAM Workshop Structural and Electronic properties of platinum nanoparticles studied by in situ x-ray x diffraction and in situ x-ray x absorption spectroscopy Hideto Imai Fundamental and Environmental
Strategic use of CuAlO 2 as a sustained release catalyst for production of hydrogen from methanol steam reforming
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Strategic use of CuAlO 2 as a sustained release catalyst for
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Strategic use of CuAlO 2 as a sustained release catalyst for
A STUDY ON PRODUCTION OF OXIDANT BY DECOMPOSITION OF H 2
 Jr. of Industrial Pollution Control 34(1)(218) pp 1811-1817 www.icontrolpollution.com Research Article A STUDY ON PRODUCTION OF OXIDANT BY DECOMPOSITION OF ON MN BASED CATALYST AND NO OXIDATION JUNG HEE
Jr. of Industrial Pollution Control 34(1)(218) pp 1811-1817 www.icontrolpollution.com Research Article A STUDY ON PRODUCTION OF OXIDANT BY DECOMPOSITION OF ON MN BASED CATALYST AND NO OXIDATION JUNG HEE
Deposition of Titania Nanoparticles on Spherical Silica
 Journal of Sol-Gel Science and Technology 26, 489 493, 2003 c 2003 Kluwer Academic Publishers. Manufactured in The Netherlands. Deposition of Titania Nanoparticles on Spherical Silica DONG HWAN RYU, SEONG
Journal of Sol-Gel Science and Technology 26, 489 493, 2003 c 2003 Kluwer Academic Publishers. Manufactured in The Netherlands. Deposition of Titania Nanoparticles on Spherical Silica DONG HWAN RYU, SEONG
Surface Oxidation Mechanism of Ni(0) Particle Supported on Silica
 Surface Oxidation Mechanism of Ni(0) Particle Supported on Silica Shohei Yamashita, Yusaku Yamamoto, Misaki Katayama, and Yasuhiro Inada Department of Applied Chemistry, Graduate School of Life Sciences,
Surface Oxidation Mechanism of Ni(0) Particle Supported on Silica Shohei Yamashita, Yusaku Yamamoto, Misaki Katayama, and Yasuhiro Inada Department of Applied Chemistry, Graduate School of Life Sciences,
PRODUCTION HYDROGEN AND NANOCARBON VIA METHANE DECOMPOSITION USING Ni-BASED CATALYSTS. EFFECT OF ACIDITY AND CATALYST DIAMETER
 MAKARA, TEKNOLOGI, VOL. 9, NO. 2, NOPEMBER 25: 48-52 PRODUCTION HYDROGEN AND NANOCARBON VIA METHANE DECOMPOSITION USING BASED CATALYSTS. EFFECT OF ACIDITY AND CATALYST DIAMETER Widodo W. Purwanto, M. Nasikin,
MAKARA, TEKNOLOGI, VOL. 9, NO. 2, NOPEMBER 25: 48-52 PRODUCTION HYDROGEN AND NANOCARBON VIA METHANE DECOMPOSITION USING BASED CATALYSTS. EFFECT OF ACIDITY AND CATALYST DIAMETER Widodo W. Purwanto, M. Nasikin,
X-ray absorption spectroscopy
 X-ray absorption spectroscopy Jagdeep Singh Jeroen A. van Bokhoven Absorption as function of energy of the x-ray Data-analysis Absorption (a.u.) 2.0 Pre-edge subtraction 1.5 1.0 0.5 0.0-0.5 8800 9000 9200
X-ray absorption spectroscopy Jagdeep Singh Jeroen A. van Bokhoven Absorption as function of energy of the x-ray Data-analysis Absorption (a.u.) 2.0 Pre-edge subtraction 1.5 1.0 0.5 0.0-0.5 8800 9000 9200
Deactivation of a Cu- CHA NH 3 -SCR catalyst by SO 2 and SO 3
 Deactivation of a Cu- CHA NH 3 -SCR catalyst by SO 2 and SO 3 217 CLEERS workshop October 217 Peter S. Hammershøi, Yasser Jangjou, William S. Epling, Anker D. Jensen, Ton V.W. Janssens Submitted to Appl.
Deactivation of a Cu- CHA NH 3 -SCR catalyst by SO 2 and SO 3 217 CLEERS workshop October 217 Peter S. Hammershøi, Yasser Jangjou, William S. Epling, Anker D. Jensen, Ton V.W. Janssens Submitted to Appl.
Sintering-resistant Ni-based Reforming Catalysts via. the Nanoconfinement Effect
 Supporting Information Sintering-resistant Ni-based Reforming Catalysts via the Nanoconfinement Effect Chengxi Zhang a,b, Wancheng Zhu c, Shuirong Li a,b, Gaowei Wu a,b, Xinbin Ma a,b, Xun Wang c, and
Supporting Information Sintering-resistant Ni-based Reforming Catalysts via the Nanoconfinement Effect Chengxi Zhang a,b, Wancheng Zhu c, Shuirong Li a,b, Gaowei Wu a,b, Xinbin Ma a,b, Xun Wang c, and
Supporting Information High Activity and Selectivity of Ag/SiO 2 Catalyst for Hydrogenation of Dimethyloxalate
 Supporting Information High Activity and Selectivity of Ag/SiO 2 Catalyst for Hydrogenation of Dimethyloxalate An-Yuan Yin, Xiao-Yang Guo, Wei-Lin Dai*, Kang-Nian Fan Shanghai Key Laboratory of Molecular
Supporting Information High Activity and Selectivity of Ag/SiO 2 Catalyst for Hydrogenation of Dimethyloxalate An-Yuan Yin, Xiao-Yang Guo, Wei-Lin Dai*, Kang-Nian Fan Shanghai Key Laboratory of Molecular
Hydrogen production by DME steam reforming over copper catalysts prepared using the sol-gel method
 Hydrogen production by DME steam reforming over copper catalysts prepared using the sol-gel method Kaoru TAKEISHI (武石 薫) E-mail: tcktake ipc.shizuoka.ac.jp Faculty of Engineering, Shizuoka University (Japan)
Hydrogen production by DME steam reforming over copper catalysts prepared using the sol-gel method Kaoru TAKEISHI (武石 薫) E-mail: tcktake ipc.shizuoka.ac.jp Faculty of Engineering, Shizuoka University (Japan)
Department of Chemical and Petroleum Engineering Sharif University of Technology, Iran
 Tayebeh Hamzehlouyan Department of Chemical and Petroleum Engineering Sharif University of Technology, Iran ETH-Conference on Combustion Generated Nanoparticles June 0, 018 1. Motivation. Experimental
Tayebeh Hamzehlouyan Department of Chemical and Petroleum Engineering Sharif University of Technology, Iran ETH-Conference on Combustion Generated Nanoparticles June 0, 018 1. Motivation. Experimental
Supplementary Figure 1 Morpholigical properties of TiO 2-x SCs. The statistical particle size distribution (a) of the defective {001}-TiO 2-x SCs and
 Supplementary Figure 1 Morpholigical properties of TiO 2-x s. The statistical particle size distribution (a) of the defective {1}-TiO 2-x s and their typical TEM images (b, c). Quantity Adsorbed (cm 3
Supplementary Figure 1 Morpholigical properties of TiO 2-x s. The statistical particle size distribution (a) of the defective {1}-TiO 2-x s and their typical TEM images (b, c). Quantity Adsorbed (cm 3
Nanostructured Ti 0.7 Mo 0.3 O 2 Support Enhances Electron Transfer to Pt : High-Performance Catalyst for Oxygen Reduction Reaction
 Nanostructured Ti 0.7 Mo 0.3 O 2 Support Enhances Electron Transfer to Pt : High-Performance Catalyst for Oxygen Reduction Reaction Seonbaek Ha Professor : Carlo U. Segre 12. 06. 2013 Department of Chemical
Nanostructured Ti 0.7 Mo 0.3 O 2 Support Enhances Electron Transfer to Pt : High-Performance Catalyst for Oxygen Reduction Reaction Seonbaek Ha Professor : Carlo U. Segre 12. 06. 2013 Department of Chemical
KMUTNB Int J Appl Sci Technol, Vol. 9, No. 4, pp , 2016
 KMUTNB Int J Appl Sci Technol, Vol. 9, No. 4, pp. 255 259, 216 Research Article Effect of Strong Metal Support Interactions of Supported Ni and Ni-Co Catalyst on Metal Dispersion and Catalytic Activity
KMUTNB Int J Appl Sci Technol, Vol. 9, No. 4, pp. 255 259, 216 Research Article Effect of Strong Metal Support Interactions of Supported Ni and Ni-Co Catalyst on Metal Dispersion and Catalytic Activity
Effects of Tungsten Promoter on Catalytic Performance of Pt/CeO 2 -ZrO 2 -Al 2 O 3 catalyst for the Diesel Exhaust Purification
 Effects of Tungsten Promoter on Catalytic Performance of Pt/CeO 2 -ZrO 2 -Al 2 O 3 catalyst for the Diesel Exhaust Purification Zhengzheng Yang College of Environmental Science and Technology, China West
Effects of Tungsten Promoter on Catalytic Performance of Pt/CeO 2 -ZrO 2 -Al 2 O 3 catalyst for the Diesel Exhaust Purification Zhengzheng Yang College of Environmental Science and Technology, China West
An extraordinarily stable catalyst: Pt NPs supported on two-dimensional Ti 3 C 2 X 2 (X=OH, F) nanosheets for Oxygen Reduction Reaction
 An extraordinarily stable catalyst: Pt NPs supported on two-dimensional Ti 3 X 2 (X=OH, F) nanosheets for Oxygen Reduction Reaction Xiaohong Xie, Siguo Chen*, Wei Ding, Yao Nie, and Zidong Wei* Experimental
An extraordinarily stable catalyst: Pt NPs supported on two-dimensional Ti 3 X 2 (X=OH, F) nanosheets for Oxygen Reduction Reaction Xiaohong Xie, Siguo Chen*, Wei Ding, Yao Nie, and Zidong Wei* Experimental
The impacts of Pdin BEA zeolite on decreasing cold start HC emission of an E85 vehicle
 CLEERS presentation October, 2017 The impacts of Pdin BEA zeolite on decreasing cold start HC emission of an E85 vehicle Lifeng Xu*, Jason Lupescu, Jeffery Hepburn, Giovanni Cavataio, Kevin Guo, Paul Laing,
CLEERS presentation October, 2017 The impacts of Pdin BEA zeolite on decreasing cold start HC emission of an E85 vehicle Lifeng Xu*, Jason Lupescu, Jeffery Hepburn, Giovanni Cavataio, Kevin Guo, Paul Laing,
Acidic Water Monolayer on Ruthenium(0001)
 Acidic Water Monolayer on Ruthenium(0001) Youngsoon Kim, Eui-seong Moon, Sunghwan Shin, and Heon Kang Department of Chemistry, Seoul National University, 1 Gwanak-ro, Seoul 151-747, Republic of Korea.
Acidic Water Monolayer on Ruthenium(0001) Youngsoon Kim, Eui-seong Moon, Sunghwan Shin, and Heon Kang Department of Chemistry, Seoul National University, 1 Gwanak-ro, Seoul 151-747, Republic of Korea.
CHAPTER 6. SOLVENT-FREE SELECTIVE OXIDATION OF -PINENE OVER Co-SBA-15 CATALYST
 135 CHAPTER 6 SOLVENT-FREE SELECTIVE OXIDATION OF -PINENE OVER Co-SBA-15 CATALYST 6.1 INTRODUCTION -Pinene is a terpenoid family of organic compound which is inexpensive, readily available and renewable
135 CHAPTER 6 SOLVENT-FREE SELECTIVE OXIDATION OF -PINENE OVER Co-SBA-15 CATALYST 6.1 INTRODUCTION -Pinene is a terpenoid family of organic compound which is inexpensive, readily available and renewable
Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c,
 Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c, 2 nm; d, 2 nm. The low magnification images demonstrate
Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c, 2 nm; d, 2 nm. The low magnification images demonstrate
1. Introduction The supported metal catalysts are widely used for applications such as purification of harmful gas generated in industrial plants for
 Characterization of Supported Mn Species During the Preparation Process of Mn/SiO2 Catalyst Shohei Yamashita, Tomohiro Seigai, Yusaku Yamamoto, Naoki Tsutsumi, Misaki Katayama, and Yasuhiro Inada Department
Characterization of Supported Mn Species During the Preparation Process of Mn/SiO2 Catalyst Shohei Yamashita, Tomohiro Seigai, Yusaku Yamamoto, Naoki Tsutsumi, Misaki Katayama, and Yasuhiro Inada Department
TPR, TPO and TPD Examination of Cu 0.15 Ce 0.85 O 2-y Mixed Oxide Catalyst Prepared by Co-precipitation Synthesis
 TPR, TPO and TPD Examination of Cu 0.15 Ce 0.85 O 2-y Mixed Oxide Catalyst Prepared by Co-precipitation Synthesis Albin Pintar *, Jurka Batista, Stanko Hočevar Laboratory for Catalysis and Chemical Reaction
TPR, TPO and TPD Examination of Cu 0.15 Ce 0.85 O 2-y Mixed Oxide Catalyst Prepared by Co-precipitation Synthesis Albin Pintar *, Jurka Batista, Stanko Hočevar Laboratory for Catalysis and Chemical Reaction
Plasma and catalysts. Part-financed by the European Union (European Regional Development Fund
 Plasma and catalysts David Cameron Professor of Material Technology Advanced Surface technology Research Laboratory (ASTRaL) University of Lappeenranta Finland Part-financed by the European Union (European
Plasma and catalysts David Cameron Professor of Material Technology Advanced Surface technology Research Laboratory (ASTRaL) University of Lappeenranta Finland Part-financed by the European Union (European
Thermodynamic and Kinetic Investigations for Redox Reactions of Nickel Species Supported on Silica
 Thermodynamic and Kinetic Investigations for Redox Reactions of Nickel Species Supported on Silica Shohei Yamashita, Misaki Katayama, Yasuhiro Inada Graduate School of Life Sciences, Ritsumeikan University,
Thermodynamic and Kinetic Investigations for Redox Reactions of Nickel Species Supported on Silica Shohei Yamashita, Misaki Katayama, Yasuhiro Inada Graduate School of Life Sciences, Ritsumeikan University,
Catalysis Science & Technology
 Catalysis Science & Technology Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted
Catalysis Science & Technology Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted
Insights into Interfacial Synergistic Catalysis over Catalyst toward Water-Gas Shift Reaction
 Supporting Information Insights into Interfacial Synergistic Catalysis over Ni@TiO2-x Catalyst toward Water-Gas Shift Reaction Ming Xu, 1 Siyu Yao, 2 Deming Rao, 1 Yiming Niu, 3 Ning Liu, 1 Mi Peng, 2
Supporting Information Insights into Interfacial Synergistic Catalysis over Ni@TiO2-x Catalyst toward Water-Gas Shift Reaction Ming Xu, 1 Siyu Yao, 2 Deming Rao, 1 Yiming Niu, 3 Ning Liu, 1 Mi Peng, 2
Chemical tuning of electrochemical properties of Ptskin surface for highly active oxygen reduction reactions
 Chemical tuning of electrochemical properties of Ptskin surface for highly active oxygen reduction reactions Namgee Jung, a Young-Hoon Chung, b Dong-Young Chung, b Kwang-Hyun Choi, b Hee- Young Park, a
Chemical tuning of electrochemical properties of Ptskin surface for highly active oxygen reduction reactions Namgee Jung, a Young-Hoon Chung, b Dong-Young Chung, b Kwang-Hyun Choi, b Hee- Young Park, a
Catalytic Oxidation of Benzene with Ozone Over Nanoporous Mn/MCM-48 Catalyst
 Copyright 12 American Scientific Publishers All rights reserved Printed in the United States of America Journal of Nanoscience and Nanotechnology Vol. 12, 5942 5946, 12 Catalytic Oxidation of Benzene with
Copyright 12 American Scientific Publishers All rights reserved Printed in the United States of America Journal of Nanoscience and Nanotechnology Vol. 12, 5942 5946, 12 Catalytic Oxidation of Benzene with
Simultaneous removal of SO 2 and NO x from flue gas using a CuO/Al 2 O 3 catalyst sorbent I. Deactivation of SCR activity by SO 2 at low temperatures
 Journal of Catalysis 224 (2004) 36 41 www.elsevier.com/locate/jcat Simultaneous removal of SO 2 and NO x from flue gas using a CuO/Al 2 O 3 catalyst sorbent I. Deactivation of SCR activity by SO 2 at low
Journal of Catalysis 224 (2004) 36 41 www.elsevier.com/locate/jcat Simultaneous removal of SO 2 and NO x from flue gas using a CuO/Al 2 O 3 catalyst sorbent I. Deactivation of SCR activity by SO 2 at low
INVESTIGATION OF SURFACE CHEMISTRY PROPERTIES OF Ga 2 O 3 /Al 2 O 3 CATALYSTS BY FT-IR SPECTROSCOPY
 INVESTIGATION OF SURFACE CHEMISTRY PROPERTIES OF Ga 2 O 3 /Al 2 O 3 CATALYSTS BY FT-IR SPECTROSCOPY Balázs Szabó 1, Tamás Ollár 1, Ákos Rédey 1 1 Department of Environmental Engineering and Chemical Technology,
INVESTIGATION OF SURFACE CHEMISTRY PROPERTIES OF Ga 2 O 3 /Al 2 O 3 CATALYSTS BY FT-IR SPECTROSCOPY Balázs Szabó 1, Tamás Ollár 1, Ákos Rédey 1 1 Department of Environmental Engineering and Chemical Technology,
Supporting Information
 Supporting Information Towards rational design of Cu/SSZ-13 selective catalytic reduction catalysts: implications from atomic-level understanding of hydrothermal stability James Song, a,c Yilin Wang, a
Supporting Information Towards rational design of Cu/SSZ-13 selective catalytic reduction catalysts: implications from atomic-level understanding of hydrothermal stability James Song, a,c Yilin Wang, a
Molecular-Level Insight into Selective Catalytic Reduction of NO x with NH 3 to N 2
 Supporting Information Molecular-Level Insight into Selective Catalytic Reduction of NO x with to N 2 over Highly Efficient Bifunctional V a Catalyst at Low Temperature Ying Xin, Hao Li, Nana Zhang, Qian
Supporting Information Molecular-Level Insight into Selective Catalytic Reduction of NO x with to N 2 over Highly Efficient Bifunctional V a Catalyst at Low Temperature Ying Xin, Hao Li, Nana Zhang, Qian
Supplementary information
 Supplementary information Supplementary Figures Supplementary Figure 1. CO 2 light off curve obtained from the 5 wt% Pt/Al 2 O 3 catalyst obtained through heating the catalyst under a 50 ml.min -1 flow
Supplementary information Supplementary Figures Supplementary Figure 1. CO 2 light off curve obtained from the 5 wt% Pt/Al 2 O 3 catalyst obtained through heating the catalyst under a 50 ml.min -1 flow
Synthesis of isoalkanes over core (Fe-Zn-Zr)-shell (zeolite) catalyst
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) Synthesis of isoalkanes over core (Fe-Zn-Zr)-shell (zeolite)
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) Synthesis of isoalkanes over core (Fe-Zn-Zr)-shell (zeolite)
PROJECT 20: SUPPORTED METALS NANOPARTICLES AS CATALYST FOR THE PROX REACTION
 PROJECT 20: SUPPORTED METALS NANOPARTICLES AS CATALYST FOR THE PROX REACTION Prof. Elisabete M. Assaf, PhD IQSC - USP Prof. José M. Assaf, PhD; Janaina F. Gomes, PhD; Aline R. L. Miranda, Ms DEQ - UFSCar
PROJECT 20: SUPPORTED METALS NANOPARTICLES AS CATALYST FOR THE PROX REACTION Prof. Elisabete M. Assaf, PhD IQSC - USP Prof. José M. Assaf, PhD; Janaina F. Gomes, PhD; Aline R. L. Miranda, Ms DEQ - UFSCar
Surface modification of silica particles with organoalkoxysilanes through two-step (acid-base) process in aqueous solution
 Journal of Ceramic Processing Research. Vol. 3, No. 3, pp. 216~221 (2002) J O U R N A L O F Surface modification of silica particles with organoalkoxysilanes through two-step (acid-base) process in aqueous
Journal of Ceramic Processing Research. Vol. 3, No. 3, pp. 216~221 (2002) J O U R N A L O F Surface modification of silica particles with organoalkoxysilanes through two-step (acid-base) process in aqueous
Oxygen Reduction Reaction
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Oxygen Reduction Reaction Oxygen is the most common oxidant for most fuel cell cathodes simply
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 Oxygen Reduction Reaction Oxygen is the most common oxidant for most fuel cell cathodes simply
CHAPTER 4. SYNTHESIS, CHARACTERIZATION OF TiO 2 NANOTUBES AND THEIR APPLICATION IN DYE SENSITIZED SOLAR CELL
 93 CHAPTER 4 SYNTHESIS, CHARACTERIZATION OF TiO 2 NANOTUBES AND THEIR APPLICATION IN DYE SENSITIZED SOLAR CELL 4.1 INTRODUCTION TiO 2 -derived nanotubes are expected to be applicable for several applications,
93 CHAPTER 4 SYNTHESIS, CHARACTERIZATION OF TiO 2 NANOTUBES AND THEIR APPLICATION IN DYE SENSITIZED SOLAR CELL 4.1 INTRODUCTION TiO 2 -derived nanotubes are expected to be applicable for several applications,
Catalytic Effects and Characteristics of Ni-based Catalysts Supported on TiO 2 -SiO 2 Xerogel
 2288 Bull. Korean Chem. Soc. 2007, Vol. 28, No. 12 Jong-Woo Jeong et al. Catalytic Effects and Characteristics of Ni-based Catalysts Supported on TiO 2 -SiO 2 Xerogel Jong-Woo Jeong, a Jong-Hui Park, Sung-Woo
2288 Bull. Korean Chem. Soc. 2007, Vol. 28, No. 12 Jong-Woo Jeong et al. Catalytic Effects and Characteristics of Ni-based Catalysts Supported on TiO 2 -SiO 2 Xerogel Jong-Woo Jeong, a Jong-Hui Park, Sung-Woo
Synthesis of TiO 2 Photocatalyst Nanoparticles by Thermal Plasmas.
 2012 International Conference on Future Environment and Energy IPCBEE vol.28(2012) (2012)IACSIT Press, Singapoore Synthesis of TiO 2 Photocatalyst Nanoparticles by Thermal Plasmas. Nguyen Hoang Hai, Kyo-Seon
2012 International Conference on Future Environment and Energy IPCBEE vol.28(2012) (2012)IACSIT Press, Singapoore Synthesis of TiO 2 Photocatalyst Nanoparticles by Thermal Plasmas. Nguyen Hoang Hai, Kyo-Seon
CHAPTER 7 SELECTIVE OXIDATION OF ETHYL BENZENE
 104 CHAPTER 7 SELECTIVE OXIDATION OF ETHYL BENZENE 7.1 INTRODUCTION Aromatic ketones such as acetophenone are important intermediates for the synthesis of drugs and pharmaceuticals (Choudhary et al 2004).
104 CHAPTER 7 SELECTIVE OXIDATION OF ETHYL BENZENE 7.1 INTRODUCTION Aromatic ketones such as acetophenone are important intermediates for the synthesis of drugs and pharmaceuticals (Choudhary et al 2004).
Deactivation and Regeneration of Titania Catalyst Supported on Glass Fiber in the Photocatalytic Degradation of Toluene
 Korean J. Chem. Eng., 20(1), 58-64 (2003) Deactivation and Regeneration of Titania Catalyst Supported on Glass Fiber in the Photocatalytic Degradation of Toluene Young San You, Kyeong-Hwan Chung, Young
Korean J. Chem. Eng., 20(1), 58-64 (2003) Deactivation and Regeneration of Titania Catalyst Supported on Glass Fiber in the Photocatalytic Degradation of Toluene Young San You, Kyeong-Hwan Chung, Young
5. Surface species and reactions on WO 3 -based powders studied by DRIFTS and TPD
 5. Surface species and reactions on -based powders 5. Surface species and reactions on -based powders studied by DRIFTS and TPD Introduction...148 5.1 DRIFTS studies...149 5.1.0 Experimental procedure...149
5. Surface species and reactions on -based powders 5. Surface species and reactions on -based powders studied by DRIFTS and TPD Introduction...148 5.1 DRIFTS studies...149 5.1.0 Experimental procedure...149
Supporting Information. Size-tunable Ni nanoparticles supported on surface-modified, cage-type mesoporous
 Supporting Information Size-tunable Ni nanoparticles supported on surface-modified, cage-type mesoporous silica as highly active catalysts for CO 2 hydrogenation Ching-Shiun Chen, a,b* Canggih Setya Budi,
Supporting Information Size-tunable Ni nanoparticles supported on surface-modified, cage-type mesoporous silica as highly active catalysts for CO 2 hydrogenation Ching-Shiun Chen, a,b* Canggih Setya Budi,
EFFECTS OF POST-TREATMENT STEAMING ON CATALYTIC PERFORMANCE OF MODIFIED HZSM-5 CATALYSTS FOR THE CONVERSION OF n-pentane TO AROMATICS
 EFFECTS OF POST-TREATMENT STEAMING ON CATALYTIC PERFORMANCE OF MODIFIED HZSM-5 CATALYSTS FOR THE CONVERSION OF n-pentane TO AROMATICS Chaninwut Kalajuck a, Siriporn Jongpatiwut*,a,b, Thirasak Rirksomboon
EFFECTS OF POST-TREATMENT STEAMING ON CATALYTIC PERFORMANCE OF MODIFIED HZSM-5 CATALYSTS FOR THE CONVERSION OF n-pentane TO AROMATICS Chaninwut Kalajuck a, Siriporn Jongpatiwut*,a,b, Thirasak Rirksomboon
Solar desalination coupled with water remediation and molecular hydrogen production: A novel solar water-energy nexus
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 17 Supporting Information Solar desalination coupled with water remediation and
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 17 Supporting Information Solar desalination coupled with water remediation and
Synchrotron radiation and catalysis. A. Martorana - X School on Synchrotron Radiation - Duino, September 16th 2009
 Synchrotron radiation and catalysis A. Martorana - X School on Synchrotron Radiation - Duino, September 16th 2009 1 What is a catalyst Why synchrotron light is suitable in catalysis Some details of instrumentation
Synchrotron radiation and catalysis A. Martorana - X School on Synchrotron Radiation - Duino, September 16th 2009 1 What is a catalyst Why synchrotron light is suitable in catalysis Some details of instrumentation
A test reaction for titanium silicalite catalysts
 A test reaction for titanium silicalite catalysts Kraushaar, B.; van Hooff, J.H.C. Published in: Catalysis Letters DOI: 10.1007/BF00765329 Published: 01/01/1989 Document Version Publisher s PDF, also known
A test reaction for titanium silicalite catalysts Kraushaar, B.; van Hooff, J.H.C. Published in: Catalysis Letters DOI: 10.1007/BF00765329 Published: 01/01/1989 Document Version Publisher s PDF, also known
A Tunable Process: Catalytic Transformation of Renewable Furfural with. Aliphatic Alcohols in the Presence of Molecular Oxygen. Supporting Information
 Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 A Tunable Process: Catalytic Transformation of Renewable Furfural with Aliphatic
Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 A Tunable Process: Catalytic Transformation of Renewable Furfural with Aliphatic
Electronic Supplementary Information (ESI)
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) Multi-scale promoting effects
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) Multi-scale promoting effects
Supplementary Information. ZIF-8 Immobilized Ni(0) Nanoparticles: Highly Effective Catalysts for Hydrogen Generation from Hydrolysis of Ammonia Borane
 Supplementary Information ZIF-8 Immobilized Ni() Nanoparticles: Highly Effective Catalysts for Hydrogen Generation from Hydrolysis of Ammonia Borane Pei-Zhou Li, a,b Kengo Aranishi, a and Qiang Xu* a,b
Supplementary Information ZIF-8 Immobilized Ni() Nanoparticles: Highly Effective Catalysts for Hydrogen Generation from Hydrolysis of Ammonia Borane Pei-Zhou Li, a,b Kengo Aranishi, a and Qiang Xu* a,b
Supporting Information. CdS/mesoporous ZnS core/shell particles for efficient and stable photocatalytic hydrogen evolution under visible light
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2014 Supporting Information CdS/mesoporous ZnS core/shell particles for efficient
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2014 Supporting Information CdS/mesoporous ZnS core/shell particles for efficient
Supporting Information
 Supporting Information Single-atom and Nano-clustered Pt Catalysts for Selective CO 2 Reduction Yuan Wang, a Hamidreza Arandiyan,* a,b Jason Scott,* a Kondo-Francois Aguey-Zinsou, c and Rose Amal* a Miss
Supporting Information Single-atom and Nano-clustered Pt Catalysts for Selective CO 2 Reduction Yuan Wang, a Hamidreza Arandiyan,* a,b Jason Scott,* a Kondo-Francois Aguey-Zinsou, c and Rose Amal* a Miss
Supporting Information
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2017 Supporting formation Selective Hydrogenation of Unsaturated Carbonyls by
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2017 Supporting formation Selective Hydrogenation of Unsaturated Carbonyls by
Supporting Information. Highly Selective Non-oxidative Coupling of Methane. over Pt-Bi Bimetallic Catalysts
 Supporting Information Highly Selective Non-oxidative Coupling of Methane over Pt-Bi Bimetallic Catalysts Yang Xiao and Arvind Varma Davidson School of Chemical Engineering, Purdue University, West Lafayette,
Supporting Information Highly Selective Non-oxidative Coupling of Methane over Pt-Bi Bimetallic Catalysts Yang Xiao and Arvind Varma Davidson School of Chemical Engineering, Purdue University, West Lafayette,
Sub-10-nm Au-Pt-Pd Alloy Trimetallic Nanoparticles with. High Oxidation-Resistant Property as Efficient and Durable
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Sub-10-nm Au-Pt-Pd Alloy Trimetallic Nanoparticles with High
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Sub-10-nm Au-Pt-Pd Alloy Trimetallic Nanoparticles with High
Preparation of Colloidal Sols and Gels
 Preparation of Colloidal Sols and Gels Objective This laboratory examines the preparation of silica suspensions and gels by the sol-gel processing of silicate solution under hydrolytic conditions using
Preparation of Colloidal Sols and Gels Objective This laboratory examines the preparation of silica suspensions and gels by the sol-gel processing of silicate solution under hydrolytic conditions using
Supporting Information
 Supporting Information Wiley-VCH 2007 69451 Weinheim, Germany Efficient Bifunctional Nanocatalysts by Simple Postgrafting of Spatially-Isolated Catalytic Groups on Mesoporous Materials By Krishna K. Sharma
Supporting Information Wiley-VCH 2007 69451 Weinheim, Germany Efficient Bifunctional Nanocatalysts by Simple Postgrafting of Spatially-Isolated Catalytic Groups on Mesoporous Materials By Krishna K. Sharma
Adsorption and conversion of various hydrocarbons on monolithic hydrocarbon adsorber
 Journal of Colloid and Interface Science 274 (2004) 538 542 www.elsevier.com/locate/jcis Adsorption and conversion of various hydrocarbons on monolithic hydrocarbon adsorber Dae Jung Kim, a Ji Man Kim,
Journal of Colloid and Interface Science 274 (2004) 538 542 www.elsevier.com/locate/jcis Adsorption and conversion of various hydrocarbons on monolithic hydrocarbon adsorber Dae Jung Kim, a Ji Man Kim,
METHANOL OXIDATION OVER AU/ γ -AL 2 O 3 CATALYSTS
 Bajopas Volume 2 Number 2 December, 29 Bayero Journal of Pure and Applied Sciences, 2(2): 149-154 Received: May, 29 Accepted: July, 29 METHANOL OXIDATION OVER AU/ γ -AL 2 O 3 CATALYSTS Abdullahi Nuhu Kano
Bajopas Volume 2 Number 2 December, 29 Bayero Journal of Pure and Applied Sciences, 2(2): 149-154 Received: May, 29 Accepted: July, 29 METHANOL OXIDATION OVER AU/ γ -AL 2 O 3 CATALYSTS Abdullahi Nuhu Kano
Deactivation of V 2 O 5 /Sulfated TiO 2 Catalyst Used in Diesel Engine for NO X Reduction with Urea
 Deactivation of V 2 O 5 /Sulfated TiO 2 Catalyst Used in Diesel Engine for NO X Reduction with Urea In-Young Lee a*, Jung-Bin Lee a, Kwang-Kyu Park a, Jeong-Hee Hong b a Korea Electric Power Corporation,
Deactivation of V 2 O 5 /Sulfated TiO 2 Catalyst Used in Diesel Engine for NO X Reduction with Urea In-Young Lee a*, Jung-Bin Lee a, Kwang-Kyu Park a, Jeong-Hee Hong b a Korea Electric Power Corporation,
Supplementary Figure S1 Reactor setup Calcined catalyst (0.40 g) and silicon carbide powder (0.4g) were mixed thoroughly and inserted into a 4 mm
 Supplementary Figure S1 Reactor setup Calcined catalyst (.4 g) and silicon carbide powder (.4g) were mixed thoroughly and inserted into a 4 mm diameter silica reactor (G). The powder mixture was sandwiched
Supplementary Figure S1 Reactor setup Calcined catalyst (.4 g) and silicon carbide powder (.4g) were mixed thoroughly and inserted into a 4 mm diameter silica reactor (G). The powder mixture was sandwiched
The GO was synthesized by oxidation of purified natural small graphite and graphite
 Jing-He Yang, a,b Geng Sun, a Yongjun Gao, a Huabo Zhao, a Pei Tang, a Juan Tan, b Lu b and Ding Ma*,a An-Hui a Beijing National Laboratory for Molecular Sciences, College of Chemistry and Molecular Engineering,
Jing-He Yang, a,b Geng Sun, a Yongjun Gao, a Huabo Zhao, a Pei Tang, a Juan Tan, b Lu b and Ding Ma*,a An-Hui a Beijing National Laboratory for Molecular Sciences, College of Chemistry and Molecular Engineering,
-:Vijay Singh(09CEB023)
 Heterogeneous Semiconductor Photocatalyst -:Vijay Singh(09CEB023) Guided by Azrina Abd Aziz Under Dr. Saravanan Pichiah Preparation of TiO 2 Nanoparticle TiO 2 was prepared by hydrolysis and poly-condensation
Heterogeneous Semiconductor Photocatalyst -:Vijay Singh(09CEB023) Guided by Azrina Abd Aziz Under Dr. Saravanan Pichiah Preparation of TiO 2 Nanoparticle TiO 2 was prepared by hydrolysis and poly-condensation
Supporting Information
 Supporting Information A Simple Descriptor to Rapidly Screen CO Oxidation Activity on Rare- Earth Metal Doped CeO 2 : from Experiment to First-Principles Kyeounghak Kim a,, Jeong Do Yoo b,, Siwon Lee b,
Supporting Information A Simple Descriptor to Rapidly Screen CO Oxidation Activity on Rare- Earth Metal Doped CeO 2 : from Experiment to First-Principles Kyeounghak Kim a,, Jeong Do Yoo b,, Siwon Lee b,
Rh 3d. Co 2p. Binding Energy (ev) Binding Energy (ev) (b) (a)
 Co 2p Co(0) 778.3 Rh 3d Rh (0) 307.2 810 800 790 780 770 Binding Energy (ev) (a) 320 315 310 305 Binding Energy (ev) (b) Supplementary Figure 1 Photoemission features of a catalyst precursor which was
Co 2p Co(0) 778.3 Rh 3d Rh (0) 307.2 810 800 790 780 770 Binding Energy (ev) (a) 320 315 310 305 Binding Energy (ev) (b) Supplementary Figure 1 Photoemission features of a catalyst precursor which was
The Curious Case of Au Nanoparticles
 The Curious Case of Au Nanoparticles Industrial reactions performed by metals 1 Low Au reactivity Predictions are typically based on d-band model Hold well for polycrystalline materials Coinage metals
The Curious Case of Au Nanoparticles Industrial reactions performed by metals 1 Low Au reactivity Predictions are typically based on d-band model Hold well for polycrystalline materials Coinage metals
Catalytic activity of liquid phase prepared Cu-Zn-Al catalyst for CO hydrogenation in a fixed bed reactor
 Indian Journal of Chemistry Vol. 51A, December 2012, pp. 1663-1668 Catalytic activity of liquid phase prepared Cu-Zn-Al catalyst for CO hydrogenation in a fixed bed reactor Chunhui Luan a, b, Anrong Zhang
Indian Journal of Chemistry Vol. 51A, December 2012, pp. 1663-1668 Catalytic activity of liquid phase prepared Cu-Zn-Al catalyst for CO hydrogenation in a fixed bed reactor Chunhui Luan a, b, Anrong Zhang
Selective Activation of Methane on Single-Atom Catalyst of Rhodium Dispersed on Zirconia for Direct Conversion
 Supporting Information Selective Activation of Methane on Single-Atom Catalyst of Rhodium Dispersed on Zirconia for Direct Conversion Yongwoo Kwon a, Tae Yong Kim b, Gihun Kwon a, Jongheop Yi b *, and
Supporting Information Selective Activation of Methane on Single-Atom Catalyst of Rhodium Dispersed on Zirconia for Direct Conversion Yongwoo Kwon a, Tae Yong Kim b, Gihun Kwon a, Jongheop Yi b *, and
Block Copolymer Based Hybrid Nanostructured Materials As Key Elements In Green Nanotechnology
 The 7 th Korea-U.S. Nano Forum Block Copolymer Based Hybrid Nanostructured Materials As Key Elements In Green Nanotechnology Dong Ha Kim Department of Chemistry and Nano Science, Ewha Womans University
The 7 th Korea-U.S. Nano Forum Block Copolymer Based Hybrid Nanostructured Materials As Key Elements In Green Nanotechnology Dong Ha Kim Department of Chemistry and Nano Science, Ewha Womans University
Supporting Information
 Supporting Information Robust Co-Catalytic Performance of Nanodiamonds Loaded on WO 3 for the Decomposition of Volatile Organic Compounds under Visible Light Hyoung il Kim, a Hee-na Kim, a Seunghyun Weon,
Supporting Information Robust Co-Catalytic Performance of Nanodiamonds Loaded on WO 3 for the Decomposition of Volatile Organic Compounds under Visible Light Hyoung il Kim, a Hee-na Kim, a Seunghyun Weon,
Electronic Supplementary Information. Direct synthesis of H 2 O 2 catalyzed by Pd nanoparticles encapsulated in multi-layered
 Electronic Supplementary Information Direct synthesis of H 2 O 2 catalyzed by Pd nanoparticles encapsulated in multi-layered polyelectrolyte nanoreactors on a charged sphere Young-Min Chung,* a Yong-Tak
Electronic Supplementary Information Direct synthesis of H 2 O 2 catalyzed by Pd nanoparticles encapsulated in multi-layered polyelectrolyte nanoreactors on a charged sphere Young-Min Chung,* a Yong-Tak
Emissions Catalyst Design using GT-SUITE. Dr. Chaitanya Sampara Mr. Dominik Artukovic
 Emissions Catalyst Design using GT-SUITE Dr. Chaitanya Sampara Mr. Dominik Artukovic Viridis Chemicals Background Specialize in developing catalyst specific reaction kinetics Chemistries Gasoline TWC Diesel
Emissions Catalyst Design using GT-SUITE Dr. Chaitanya Sampara Mr. Dominik Artukovic Viridis Chemicals Background Specialize in developing catalyst specific reaction kinetics Chemistries Gasoline TWC Diesel
EFFECTS OF ADDITIONAL GASES ON THE CATALYTIC DECOMPOSITION OF N20 OVER Cu-ZSM-5
 Jointly published by Elsevier Science B.V., Amsterdam and Akad~miai Kiad6, Budapest RKCL3296 Reaet.Kinet. Catal.Lett. Vol. 64, No. 2, 215-220 (1998) EFFECTS OF ADDITIONAL GASES ON THE CATALYTIC DECOMPOSITION
Jointly published by Elsevier Science B.V., Amsterdam and Akad~miai Kiad6, Budapest RKCL3296 Reaet.Kinet. Catal.Lett. Vol. 64, No. 2, 215-220 (1998) EFFECTS OF ADDITIONAL GASES ON THE CATALYTIC DECOMPOSITION
Supplementary Information for
 Supplementary Information for Facile transformation of low cost thiourea into nitrogen-rich graphitic carbon nitride nanocatalyst with high visible light photocatalytic performance Fan Dong *a, Yanjuan
Supplementary Information for Facile transformation of low cost thiourea into nitrogen-rich graphitic carbon nitride nanocatalyst with high visible light photocatalytic performance Fan Dong *a, Yanjuan
Supports, Zeolites, Mesoporous Materials - Chapter 9
 Supports, Zeolites, Mesoporous Materials - Chapter 9 Krijn P. de Jong Inorganic Chemistry and Catalysis Utrecht University NIOK CAIA Course, Schiermonnikoog, December 4 th, 2009 1 Overview of lecture Introduction
Supports, Zeolites, Mesoporous Materials - Chapter 9 Krijn P. de Jong Inorganic Chemistry and Catalysis Utrecht University NIOK CAIA Course, Schiermonnikoog, December 4 th, 2009 1 Overview of lecture Introduction
Characterization of Re-Pd/SiO 2 catalysts for hydrogenation of stearic acid
 Supporting Information: Characterization of Re-Pd/SiO 2 catalysts for hydrogenation of stearic acid Yasuyuki Takeda, [a] Masazumi Tamura, [a] Yoshinao Nakagawa, [a] Kazu Okumura, [b] and Keiichi Tomishige*
Supporting Information: Characterization of Re-Pd/SiO 2 catalysts for hydrogenation of stearic acid Yasuyuki Takeda, [a] Masazumi Tamura, [a] Yoshinao Nakagawa, [a] Kazu Okumura, [b] and Keiichi Tomishige*
Manganese promotion in cobalt-based Fischer-Tropsch catalysis
 Manganese promotion in cobalt-based Fischer-Tropsch catalysis F. Morales Cano, O.L.J. Gijzeman, F.M.F. de Groot and B.M. Weckhuysen Department of Inorganic Chemistry and Catalysis, Debye Institute, Utrecht
Manganese promotion in cobalt-based Fischer-Tropsch catalysis F. Morales Cano, O.L.J. Gijzeman, F.M.F. de Groot and B.M. Weckhuysen Department of Inorganic Chemistry and Catalysis, Debye Institute, Utrecht
Determination of Metal Dispersion of Pt/CeO2 Catalyst by CO-pulse Method
 Journal of the Japan Petroleum Institute, 48, (3), 173 177 (2005) 173 [Research Note] Determination of Metal Dispersion of Pt/CeO2 Catalyst by CO-pulse Method Shin ichi KOMAI 1), Yoshiteru YAZAWA 1), Atsushi
Journal of the Japan Petroleum Institute, 48, (3), 173 177 (2005) 173 [Research Note] Determination of Metal Dispersion of Pt/CeO2 Catalyst by CO-pulse Method Shin ichi KOMAI 1), Yoshiteru YAZAWA 1), Atsushi
Oxydation sélective des composés organiques par H 2 O 2 catalysée par xerogels mésoporeux SiO 2 -TiO 2
 UMR 53 - Institut de Chimie Moléculaire et des Matériaux de Montpellier xydation sélective des composés organiques par H 2 2 catalysée par xerogels mésoporeux i 2 -Ti 2 Dr. Ana Mihaela Cojocariu P.H. Mutin,
UMR 53 - Institut de Chimie Moléculaire et des Matériaux de Montpellier xydation sélective des composés organiques par H 2 2 catalysée par xerogels mésoporeux i 2 -Ti 2 Dr. Ana Mihaela Cojocariu P.H. Mutin,
Highly doped and exposed Cu(I)-N active sites within graphene towards. efficient oxygen reduction for zinc-air battery
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) for Energy & Environmental Science.
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2016 Electronic Supplementary Information (ESI) for Energy & Environmental Science.
McCord CH301 Exam 2 Oct 10, 2017
 452 version last name first name signature McCord CH301 Exam 2 Oct 10, 2017 50070 Tuesday Remember to refer to the Periodic Table handout that is separate from this exam copy. There are many conversion
452 version last name first name signature McCord CH301 Exam 2 Oct 10, 2017 50070 Tuesday Remember to refer to the Periodic Table handout that is separate from this exam copy. There are many conversion
Supporting Information
 Supporting Information of highly concentrated cellulose to 1, 2-propanediol and ethylene glycol over high efficient CuCr catalysts Zihui Xiao, Shaohua Jin, Min Pang and Changhai Liang Experimental The
Supporting Information of highly concentrated cellulose to 1, 2-propanediol and ethylene glycol over high efficient CuCr catalysts Zihui Xiao, Shaohua Jin, Min Pang and Changhai Liang Experimental The
International Journal of Scientific Research and Innovative Technology ISSN: Vol. 4 No. 6; June 2017
 Effect of Sulfur Poisoning on the Catalytic Performance of Pt/Al 2 O 3 Diesel Oxidation Catalyst: Study on Sulfur Dioxide Adsorption and Sulfates Accumulation Zhengzheng Yang* College of Environmental
Effect of Sulfur Poisoning on the Catalytic Performance of Pt/Al 2 O 3 Diesel Oxidation Catalyst: Study on Sulfur Dioxide Adsorption and Sulfates Accumulation Zhengzheng Yang* College of Environmental
RKCL5155 PREPARATION AND EVALUATION OF AMMONIA DECOMPOSITION CATALYSTS BY HIGH-THROUGHPUT TECHNIQUE
 Jointly published by React.Kinet.Catal.Lett. Akadémiai Kiadó, Budapest Vol. 93, No. 1, 11 17 (2008) and Springer, Dordrecht 10.1007/s11144-008-5155-3 RKCL5155 PREPARATION AND EVALUATION OF AMMONIA DECOMPOSITION
Jointly published by React.Kinet.Catal.Lett. Akadémiai Kiadó, Budapest Vol. 93, No. 1, 11 17 (2008) and Springer, Dordrecht 10.1007/s11144-008-5155-3 RKCL5155 PREPARATION AND EVALUATION OF AMMONIA DECOMPOSITION
Methane production from CO2 over Ni-Hydrotalcite derived catalysts
 Methane production from CO2 over Ni-Hydrotalcite derived catalysts Keerthivarman Veerappanchatram Kaliappan vkkeerthivarman@gmail.com Instituto Superior Tecnico, Universidade de Lisboa, Portugal. October
Methane production from CO2 over Ni-Hydrotalcite derived catalysts Keerthivarman Veerappanchatram Kaliappan vkkeerthivarman@gmail.com Instituto Superior Tecnico, Universidade de Lisboa, Portugal. October
Applied Catalysis B: Environmental
 Applied Catalysis B: Environmental 106 (2011) 529 539 Contents lists available at ScienceDirect Applied Catalysis B: Environmental jo ur n al homepage: www.elsevier.com/locate/apcatb Effect of Ti(IV) loading
Applied Catalysis B: Environmental 106 (2011) 529 539 Contents lists available at ScienceDirect Applied Catalysis B: Environmental jo ur n al homepage: www.elsevier.com/locate/apcatb Effect of Ti(IV) loading
Surface Chemistry & States of Matter
 Surface Chemistry & States of Matter S. Sunil Kumar Lecturer in Chemistry 1. Adsorption is a. Colligative property b. Oxidation process c. Reduction process d. Surface phenomenon Ans. d 2. When adsorption
Surface Chemistry & States of Matter S. Sunil Kumar Lecturer in Chemistry 1. Adsorption is a. Colligative property b. Oxidation process c. Reduction process d. Surface phenomenon Ans. d 2. When adsorption
Supplementary Figure 1 Result from XRD measurements. Synchrotron radiation XRD patterns of the as-prepared gold-ceria samples.
 Supplementary Figure 1 Result from XRD measurements. Synchrotron radiation XRD patterns of the as-prepared gold-ceria samples. The detailed information on XRD measurement is seen in the Supplementary Methods.
Supplementary Figure 1 Result from XRD measurements. Synchrotron radiation XRD patterns of the as-prepared gold-ceria samples. The detailed information on XRD measurement is seen in the Supplementary Methods.
Synthesis, Characterization and Catalytic Activity of MCM-41 Catalyst for Nitration of Phenol
 http://www.e-journals.in Chemical Science Transactions DOI:10.7598/cst2015.952 2015, 4(2), 438-442 RESEARCH ARTICLE Synthesis, Characterization and Catalytic Activity of MCM-41 Catalyst for Nitration of
http://www.e-journals.in Chemical Science Transactions DOI:10.7598/cst2015.952 2015, 4(2), 438-442 RESEARCH ARTICLE Synthesis, Characterization and Catalytic Activity of MCM-41 Catalyst for Nitration of
Catalysis Letters, 79 (1-4): Kluwer Academic Publishers-Plenum Publishers.
 Provided by the author(s) and University College Dublin Library in accordance with publisher policies. Please cite the published version when available. Title The effect of support and copper precursor
Provided by the author(s) and University College Dublin Library in accordance with publisher policies. Please cite the published version when available. Title The effect of support and copper precursor
Special Properties of Au Nanoparticles
 Special Properties of Au Nanoparticles Maryam Ebrahimi Chem 7500/750 March 28 th, 2007 1 Outline Introduction The importance of unexpected electronic, geometric, and chemical properties of nanoparticles
Special Properties of Au Nanoparticles Maryam Ebrahimi Chem 7500/750 March 28 th, 2007 1 Outline Introduction The importance of unexpected electronic, geometric, and chemical properties of nanoparticles
Synthesis gas production via the biogas reforming reaction over Ni/MgO-Al 2 O 3 and Ni/CaO-Al 2 O 3 catalysts
 Synthesis gas production via the biogas reforming reaction over Ni/MgO-Al 2 O 3 and Ni/CaO-Al 2 O 3 catalysts N.D. Charisiou 1,2, A. Baklavaridis 1, V.G. Papadakis 2, M.A. Goula 1 1 Department of Environmental
Synthesis gas production via the biogas reforming reaction over Ni/MgO-Al 2 O 3 and Ni/CaO-Al 2 O 3 catalysts N.D. Charisiou 1,2, A. Baklavaridis 1, V.G. Papadakis 2, M.A. Goula 1 1 Department of Environmental
Topic 12 Transition Metals Revision Notes
 Topic 12 Transition Metals Revision Notes 1) Introduction Transition metals have 4 characteristic properties: they form complexes, they form coloured compounds, they have more than one oxidation state
Topic 12 Transition Metals Revision Notes 1) Introduction Transition metals have 4 characteristic properties: they form complexes, they form coloured compounds, they have more than one oxidation state
Characterization of Pt-impregnated MCM-41 and MCM-48 and their catalytic performances in selective catalytic reduction for NO x
 Characterization of Pt-impregnated MCM-41 and MCM-48 and their catalytic performances in selective catalytic reduction for NO x D-J Kim, J-K Cho, J-H Jang, S-C Lee, M Kang 1, and S-J Choung * Department
Characterization of Pt-impregnated MCM-41 and MCM-48 and their catalytic performances in selective catalytic reduction for NO x D-J Kim, J-K Cho, J-H Jang, S-C Lee, M Kang 1, and S-J Choung * Department
