6-6-07, Aoba, Aramaki, Aoba-ku, Sendai , Japan Nakano-machi, Hachioji, Tokyo , Japan
|
|
|
- Ralf Leonard
- 5 years ago
- Views:
Transcription
1 Supporting Information: Performance, Structure and Mechanism of Re x Pd/Ce 2 Catalyst for Simultaneous Removal of Vicinal H Groups with H 2 Nobuhiko ta, [a] Masazumi Tamura, [a, c] Yoshinao Nakagawa,* [a, c] Kazu kumura, [b] [a, c] Keiichi Tomishige* [a] Department of Applied Chemistry, School of Engineering, Tohoku University, , Aoba, Aramaki, Aoba-ku, Sendai , Japan [b] Department of Applied Chemistry, Faculty of Engineering, Kogakuin University, Nakano-machi, Hachioji, Tokyo , Japan [c] Research Center for Rare Metal and Green Innovation, Tohoku University, 468-1, Aoba, Aramaki, Aoba-ku, Sendai , Japan *Corresponding author: Keiichi Tomishige School of Engineering, Tohoku University, , Aoba, Aramaki, Aoba-ku, Sendai, , Japan tomi@erec.che.tohoku.ac.jp *Corresponding author: Yoshinao Nakagawa School of Engineering, Tohoku University, , Aoba, Aramaki, Aoba-ku, Sendai, , Japan yoshinao@erec.che.tohoku.ac.jp
2 Table of Contents i. Figure S1, Screening of active metals (M 1 in M 1 x Pd/Ce 2 ) for simultaneous removal of H groups of 1,4-AHERY... 1 ii. Figure S2, Screening of additives (M 2 in Re x M 2 /Ce 2 ) for simultaneous removal of H groups of 1,4-AHERY... 2 iii. Figure S3, Detail of Figure 4-upper (effect of hydrogen pressure)... 3 iv. Figure S4, Detail of Figure 4-lower (effect of substrate concentration)... 4 v. Table S1, Comparison of hydrogen and 3-pentanol as a reductant in the reaction of 1,4-AHERY over Re x Pd/Ce 2 catalyst... 5 vi. Table S2, Effect of reaction temperature on simultaneous removal of H groups of 1,4-AHERY over Re x Pd/Ce 2 catalyst... 6 vii. Figure S5, X-ray diffraction patterns of Re x Pd/Ce viii. Figure S6, TEM images of calcined Re x Pd/Ce 2 (Re = 2 wt%, Pd/Re = 0.25)... 8 ix. Figure S7, XPS of Re x Pd/Ce 2 in Pd 3d region... 9 x. Figure S8, XPS of reacted catalysts in Re 4f region xi. Figure S9, Relation between white line area and valence of Re xii. Figure S10, Pd K-edge EXAFS spectra xiii. Table S3 and Table S3, Curve fitting results of Pd K-edge EXAFS of Re x Pd/Ce xiv. Figure S11, Ce cluster model for DFT calculation xv. xvi. Figure S12, Calculated energy diagram of DDH of 1,4-AHERY over monomeric Re species on Ce model cluster Figure S13, Raman spectra of Re x Pd/Ce 2, Re x /Ce 2, Pd/Ce 2 after calcination at 773 K for 3 h xvii. Table S4, Suppliers of the reagents xviii. Table S5, Comparison of calculation levels between this and literature studies for methyltrioxorhenium-catalyzed DDH of ethylene glycol... 18
3 Conv. or Sel. / % thers THF Conv. Re W Mo Cr Nb Mn V Active matal (M 1 in M 1 x Pd/Ce 2 ) Figure S1 Screening of active metals (M 1 in M 1 x Pd/Ce 2 ) for simultaneous removal of H groups of 1,4-AHERY. Conditions: 1,4-AHERY 1 g, 1,4-dioxane 4 g, W cat = 150 mg (2 wt% M 1, Pd/M 1 = 0.25), PH 2 = 8 MPa, T = 413 K, t = 4 h. AHERY = anhydroerythritol, THF = tetrahydrofuran. 1
4 Conv. or Sel. / % H H 100 1,4-AHTHR H 1-BuH thers DHF None Co Ni Cu Ru Rh Pd Ir Pt Additives (M 2 in Re x M 2 /Ce 2 ) THF Figure S2 Screening of additives (M 2 in Re x M 2 /Ce 2 ) for simultaneous removal of H groups of 1,4-AHERY. Conditions: 1,4-AHERY 1 g, 1,4-dioxane 4 g, W cat = 150 mg (2 wt% Re, M 2 /Re = 0.25), PH 2 = 8 MPa, T = 413 K, t = 4 h. AHERY = anhydroerythritol, THF = tetrahydrofuran, BuH = butanol, AHTHR = anhydrothreitol, DHF = dihydrofuran. 2
5 Amount of THF / mmol Amount of THF / mmol Amount of THF / mmol Amount of THF / mmol 2.0 (a) 2.0 (b) y = x R² = Time / h (c) y = x R² = Time / h (d) y = x R² = y = x R² = Time / h Time / h Figure S3 Detail of Figure 4-upper (effect of hydrogen pressure). Conditions: 1,4-AHERY 0.5 g, 1,4-dioxane 2 g, W cat = 50 mg (2 wt% Re, 0.3 wt% Pd), PH 2 = MPa, T = 413 K. (a) PH 2 = 0.5 MPa, (b) PH 2 = 1 MPa, (c) PH 2 = 4 MPa, (d) PH 2 = 8 MPa. 3
6 Amount of THF / mmol Amount of THF / mmol Amount of THF / mmol Amount of THF / mmol 2.0 (a) 2.0 (b) y = x R² = y = x R² = Time / h (c) Time / h 2.0 (d) y = x R² = y = x R² = Time / h Time / h Figure S4 Detail of Figure 4-lower (effect of substrate concentration). Conditions: 1,4-AHERY 0.5 g, 1,4-dioxane g, W cat = 50 mg (2 wt% Re, 0.3 wt% Pd), PH 2 = 8 MPa, T = 413 K. (a) C = 5 wt%, (b) C = 10 wt%, (c) C = 15 wt%, (d) C = 20 wt%. 4
7 Table S1: Comparison of hydrogen and 3-pentanol as a reductant in the reaction of 1,4-AHERY over Re x Pd/Ce 2 (2 wt% Re, 0.3 wt% Pd) catalyst. Entry Reductant Conv. / % Selectivity / % Tetrahydrofuran Dihydrofurans thers 1 hydrogen 38 >99 <1 <1 2 a 3-pentanol 2 <1 27 b 73 Conditions: 1,4-AHERY 1 g, 1,4-dioxane 4 g, W cat = 150 mg (2 wt% Re, 0.3 wt% Pd), PH 2 = 8 MPa, T = 413 K, t = 4 h. a 3-pentanol 4 g (as a solvent and reductant), P Ar = 4 MPa (at room temperature). b Ratio of 2,3-dihydrofuran/2,5-dihydrofuran=1/3. 5
8 Table S2: Effect of reaction temperature on simultaneous removal of H groups of 1,4-AHERY over Re x Pd/Ce 2 (2 wt% Re, 0.3 wt% Pd) catalyst. Entry Reaction temp. /K Conv. / % Selectivity / % 3-Hydroxy- Tetrahydrofuran BuDs BuHs Tetrahydrofuran 1,4-Anhydrothreitol >99 <1 <1 <1 <1 < >99 <1 <1 <1 <1 < <1 >99 <1 <1 <1 <1 <1 thers Conditions: 1,4-AHERY 0.5 g, 1,4-dioxane 2 g, W cat = 50 mg, PH 2 = 8 MPa, t = 2 h. BuD = bunanediol, BuH = butanol. 6
9 Intensity / counts Ce 2 (e) (d) (c) (b) (a) degree / 2q Figure S5 X-ray diffraction patterns of Re x Pd/Ce 2. (a) Re x Pd/Ce 2 (2 wt% Re, 0.3 wt% Pd) after calcination, (b) Re x Pd/Ce 2 (10 wt% Re, 1.5 wt% Pd) after calcination, (c) Re x Pd/Ce 2 (2 wt% Re, 0.3 wt% Pd) after reduction in H 2 flow (30 cc/min) at 423 K for 1 h (at a heating rate 10 K/min), (d) Re x Pd/Ce 2 (2 wt% Re, 0.3 wt% Pd) after reaction, (e) Re x Pd/Ce 2 (10 wt% Re, 1.5 wt% Pd) after reduction in H 2 flow (30 cc/min) at 423 K for 1 h (at a heating rate 10 K/min) 7
10 Figure S6 TEM images of calcined Re x Pd/Ce 2 (2 wt% Re, 0.3 wt% Pd). 8
11 Intensity / a.u. Pd II 3d 5/2 Pd 0 3d 5/2 a b c Binding Energy / ev Figure S7 XPS of Re x Pd/Ce 2 (2 wt% Re, 0.3 wt% Pd) in Pd 3d region. (a) Re x Pd/Ce 2 before stoichiometric reaction (Reduction temp. = 423 K), (b) Re x Pd/Ce 2 after stoichiometric reaction (Reaction temp. = 353 K, t = 4 h), (c) Re x Pd/Ce 2 after reaction (Reaction temp. = 413 K, t = 4 h). Raw XPS data (black solid line), calculated data (black dotted line). Reference: C 1s = ev. Normalized by area of Ce 3d XPS. 9
12 Intensity / a.u. Re VII Re VI 4f 7/2 4f 7/2 Re IV 4f 7/2 Re 0 4f 7/2 a Valence of Re from XPS 5.0 b Binding Energy / ev 38 Figure S8 XPS of reacted catalysts in Re 4f region. (a) Re x Pd/Ce 2 after reaction (2 wt% Re, 0.3 wt% Pd, Reaction temp. = 413 K, t = 4 h), (b) Re x Pd/Si 2 after reaction (2 wt% Re, 0.3 wt% Pd, Reaction temp. = 413 K, t = 4 h). Raw XPS data (black solid line), calculated data (black dotted line). Reference: C 1s = ev. 10
13 White line area / a.u (e) (f) (c) (d) (b) (a) Valence of Re Figure S9 Relation between white line area and valence of Re. (a) Re powder, (b) Re 2, (c) Re 3, (d) Re x Pd/Ce 2 (2 wt% Re, 0.3 wt% Pd) after reduction in H 2 flow (30 cc/min) at 423 K for 1 h, (e) Re x Pd/Ce 2 (2 wt% Re, 0.3 wt% Pd) after stoichiometric reaction for 4 h, (f) Re x Pd/Ce 2 (2 wt% Re, 0.3 wt% Pd) after catalytic use under the standard conditions. 11
14 k 3 χ(k) k 3 χ(k) F(r). (I) 20. (II) 20 c 5 c b b a a k / 10 nm -1.. (III) Distance / 0.1 nm. c k / 10 nm -1. Figure S10 Pd K-edge EXAFS spectra. (I) k 3 -Weighted EXAFS oscillations. (II) Fourier transform of k 3 -weighted Pd K-edge EXAFS, FT range: nm 1. (III) Fourier filtered EXAFS data (solid line) and calculated data (dotted line), Fourier filtering range: nm. (a)pd, (b) Pd foil, (c) Re x Pd/Ce 2 (2 wt% Re, 0.3 wt% Pd) after catalytic use under standard conditions. 12
15 Table S3: Curve fitting results of Pd K-edge EXAFS of Re x Pd/Ce 2 (2 wt% Re, 0.3 wt% Pd). Sample Shells CN a R / 10-1 nm b / 10-1 nm c E 0 / ev d R f / % e Re x Pd/Ce 2 after catalytic use under the standard conditions Pd Pd 3.1± ± ± ± Pd Re 1.6± ± ± ±0.9 Pd foil Pd Pd a CN = Coordination number, b R = Bond distance, c = Debye-Waller factor, d E 0 = Difference in origin of photoelectron energy between the reference and the sample, e R f = Residual factor. Table S3 : Curve fitting results of Pd K-edge EXAFS of Rex Pd/Ce2 (2 wt% Re, 0.3 wt% Pd) after catalytic use under the standard conditions. Model Shells CN a R / 10-1 nm b / 10-1 nm c E 0 / ev d R f / % e Two shells model Pd Pd Pd Re ne shell model Pd Pd a CN = Coordination number, b R = Bond distance, c = Debye-Waller factor, d E 0 = Difference in origin of photoelectron energy between the reference and the sample, e R f = Residual factor. The value of Residual factor on one shell model is unacceptably high. Therefore, the curve fitting analysis of Pd K-edge EXAFS is conducted using two shells model. 13
16 (a) Top view (b) Side view (c) Front view (d) Model construction scheme (2.5,2.5,5) (2.5,5,2.5) (2.5,5,2.5) (2.5,3.75,3.75) (3.75, ) (1.25,3.75,2.5) (2.5,1.25,1.25) (5,2.5,2.5) (0,2.5,2.5) (5,2.5,2.5) (1.25,2.5,1.25) (3.75,1.25,3.75) (2.5,0,2.5) (2.5,2.5,0) (2.5,2.5,0) Figure S11 Ce cluster model for DFT calculation. The cluster is sculpted from the 5x5x5 supercell of Ce 2 crystal with fluorite structure. First, an octahedron composed of (111) surface is sculpted from the supercell by cutting down the vertexes of the supercell. The atoms on the boundary are included except the 6 cerium atoms at the vertexes of the octahedron, because these cerium atoms have a too low (4) coordination number. Next, the octahedron is cut in half. The cross section becomes a large (111) surface which is used as the model surface. 14
17 Re IV Re VI Re IV Re IV Re Re IV Figure S12 Calculated energy diagram of DDH of 1,4-anhydroerythritol over monomeric Re species on Ce model cluster. Energies are shown in kj/mol. sub = Doubly deprotonated 1,4-anhydroerythritol. Calculation level: PW91/DND-3.5+DSPP, gas phase. Note 1: Although the four states shown in the right side are in high energy level, the Re species of these states are coordinated with only one oxygen atom on the Ce 2 surface. These states can be somewhat stabilized by adsorption of another alcoholic molecule (substrate) on the unoccupied oxygen site which was occupied in Re 2 /Ce 2. Adsorption of methanol on the Ce model cluster is calculated to be exothermic by 56 kj/mol. Note 2: Because a pure GGA-DFT (PW91) was used, the calculated activation barriers will be lower by ~30% than those calculated with more common B3LYP functional. See Table S5 for the comparison using model system with methyltrioxorhenium catalyst. Note 3: Re 2 /Ce 2 with only one Re bond was calculated to be in much higher energy level (+186 kj/mol) than the standard structure with two Re bonds as shown above. Structure of Re 3 /Ce 2 with two Re bonds was not found; geometry optimization leads to the structure with one Re bond as shown above. 15
18 Intensity / a.u. Intensity / a.u. a b c d e f g / Raman Shift / cm -1 Raman Shift / cm -1 Figure S13 Raman spectra of Re x Pd/Ce 2, Re x /Ce 2, Pd/Ce 2 after calcination at 773 K for 3 h. The intensity was normalized by that of Ce 2 -derived 464 cm -1 peak. (a) Ce 2 (after calcination at 873 K for 3 h), (b) Re x Pd/Ce 2 (10 wt% Re, 1.5 wt% Pd), (c) Re x Pd/Ce 2 (4 wt% Re, 0.6 wt% Pd), (d) Re x Pd/Ce 2 (2 wt% Re, 0.3 wt% Pd), (e) Re x Pd/Ce 2 (0.5 wt% Re, 0.07 wt% Pd), (f) Re x /Ce 2 (2 wt% Re), (g) Pd/Ce 2 (0.3 wt% Pd) 16
19 Table S4: Suppliers of the reagents. Reagent Supplier NH 4 V 3 Cr(N 3 ) 3 9H 2 Mn(N 3 ) 2 6H 2 (NH 4 ) 6 Mo H 2 (NH 4 )Nb(C 2 4 ) 2 xh 2 Aldrich Co., Ltd. (NH 4 ) 6 H 2 W xh 2 Strem Chemicals Inc. NH 4 Re 4 Soekawa Chemicals Co., Ltd. Pd(N 3 ) 2 N.E. Chemcat Corp. Co(N 3 ) 2 6H 2 Ni(N 3 ) 2 6H 2 Cu(N 3 ) 2 3H 2 Ru(N)(N 3 ) 3-x (H) x Aldrich Co., Ltd. RhCl 3 3H 2 H 2 IrCl 6 Furuya Metals Co., Ltd. H 2 PtCl 6 6H 2 Soekawa Chemicals Co., Ltd. Ce 2 Daiichi Kigenso Co., Ltd. Si 2 Fuji Silysia Chemical Ltd. 1,4-anhydroerythritol Aldrich Co., Ltd. 1,4-dioxane hydrogen Showa Denko K.K. 1,4-anhydrothreitol Astatech, Inc. cis-1,2-cyclopentanediol Frinton Laboratories, Inc. trans-1,2-cyclopentanediol Aldrich Co., Ltd. cis-1,2-cyclohexanediol Sigma Aldrich, Co. LLC. trans-1,2-cyclohexanediol Tokyo Chemical Industry Co., Ltd. 2,3-butanediol Tokyo Chemical Industry Co., Ltd. 3-hydroxytetrahydrofuran Tokyo Chemical Industry Co., Ltd. tetrahydrofuran 1,2-butanediol 1,4-butanediol 1,3-butanediol 1,2-hexanediol erythritol 1,2-dimethoxyethane dodecane 1-pentanol 3-pentanol 17
20 Table S5: Comparison of calculation levels between this and literature [S1] studies for methyltrioxorhenium(mt)-catalyzed DDH of ethylene glycol (EG) Parameters (lengths in Å; energies in kj/mol) This study Program: DMol 3 Functional: PW91 Basis set & ECP: DND3.5, DSPP Solvent: none Program: DMol 3 Functional: B3LYP Basis set & ECP: DND3.5, DSPP Solvent: none Ref. S1 Program: Gaussian09 Functional: B3LYP Basis set & ECP: 6-31G(d), LanL2DZ Solvent: C 6 H 6 (CPCM) Structure of EG diolate of reduced MT Re- 1 : 1.93, Re- 2 : C: 1.46, 2-2 C: 1.46 C- 2 C: 1.52 Re- 1 : 1.93, Re- 2 : C: 1.43, 2-2 C: 1.43 C- 2 C: 1.51 Re- 1 : 1.92, Re- 2 : C: 1.44, 2-2 C: 1.44 C- 2 C: 1.53 Re- 3 : 1.72 Re- 3 : 1.71 Re- 3 : 1.70 Structure of TS of Re- 1 : 1.81, Re- 2 : 1.80 Re- 1 : 1.79, Re- 2 : 1.79 Re- 1 : 1.78, Re- 2 : 1.79 DDH (MT catalyst) 1-1 C: 1.81, 2-2 C: C: 1.86, 2-2 C: C: 1.83, 2-2 C: C- 2 C: C- 2 C: C- 2 C: 1.42 Re- 3 : 1.73 Re- 3 : 1.73 Re- 3 : 1.72 Activation barrier (MT catalyst) Structure of EG diolate of reduced MT + H 2 Ea: 49 Ea: 91 Ea: 74 Ga: 65 (298 K, 1 atm) Re- 1 : 1.95, Re- 2 : 1.94 Re- 1 : 1.97, Re- 2 : 1.94 Re- 1 : 1.94, Re- 2 : C: 1.44, 2-2 C: C: 1.42, 2-2 C: C: 1.43, 2-2 C: C- 2 C: C- 2 C: C- 2 C: 1.53 Re- 3 : 1.72 Re- 3 : 1.70 Re- 3 : 1.70 Re- 4 : 2.27 Re- 4 : 2.21 Re- 4 : 2.16 Structure of TS of Re- 1 : 1.81, Re- 2 : 1.82 Re- 1 : 1.79, Re- 2 : 1.82 Re- 1 : 1.78, Re- 2 : 1.78 DDH (MT + H C: 1.83, 2-2 C: C: 1.92, 2-2 C: C: 1.90, 2-2 C: 1.89 catalyst) 1 C- 2 C: C- 2 C: C- 2 C: 1.41 Re- 3 : 1.74 Re- 3 : 1.73 Re- 3 : 1.72 Re- 4 : 2.42 Re- 4 : 2.33 Re- 4 : 2.33 Activation barrier (MT + H 2 catalyst) Ea: 86 Ea: 141 Ea: 129 Ga: 113 (298 K, 1 atm) Continues to next page [S1] Liu, P.; Nicholas, K. M. rganometallics 2013, 32,
21 Table S5 (continued) Parameters This study (lengths in Å; energies Program: DMol 3 in kj/mol) Functional: PW91 Basis set & ECP: DND3.5, DSPP Solvent: none Structure of MT Re- 1 : 1.73, Re- 2 : 1.73 Re- 3 : 1.73 Program: DMol 3 Functional: B3LYP Basis set & ECP: DND3.5, DSPP Solvent: none Re- 1 : 1.73, Re- 2 : 1.73 Re- 3 : 1.72 Ref. S1 Program: Gaussian09 Functional: B3LYP Basis set & ECP: 6-31G(d), LanL2DZ Solvent: C 6 H 6 (CPCM) Re- 1 : 1.71, Re- 2 : 1.71 Re- 3 : 1.71 Structure of MT + Re- 1 : 1.74, Re- 2 : 1.74 Re- 1 : 1.74, Re- 2 : 1.74 Re- 1 : 1.72, Re- 2 : 1.72 H 2 Re- 3 : 1.73 Re- 3 : 1.73 Re- 3 : 1.71 Re- 4 : 2.64 Re- 4 : 2.58 Re- 4 : 2.64 E of coordination of MT with H 2 Structure of EG C- 2 C: C- 2 C: C- 2 C: C- 1 : C- 1 : C- 1 : C- 2 : C- 2 : C- 2 : 1.43 Structure of EG diolate Re- 1 : 1.99, Re- 2 : 1.93 Re- 1 : 1.98, Re- 2 : 1.92 Re- 1 : 1.97, Re- 2 : 1.91 of MT 1-1 C: 1.43, 2-2 C: C: 1.47, 2-2 C: C: 1.42, 2-2 C: C- 2 C: C- 2 C: C- 2 C: 1.52 Re- 3 : 1.73, Re- 4 : 1.73 Re- 3 : 1.72, Re- 4 : 1.72 Re- 3 : 1.71, Re- 4 : 1.70 E of diolate formation from MT and EG Structure of EG diolate of MT + H Re- 1 : 2.02, Re- 2 : 1.93 Re- 1 : 2.01, Re- 2 : 1.91 Re- 1 : 2.01, Re- 2 : C: 1.43, 2-2 C: C: 1.42, 2-2 C: C: 1.42, 2-2 C: C- 2 C: C- 2 C: C- 2 C: 1.53 Re- 3 : 1.72, Re- 4 : 1.74 Re- 5 : 2.69 Re- 3 : 1.71, Re- 4 : 1.73 Re- 5 : 2.69 Re- 3 : 1.72, Re- 4 : 1.70 Re- 5 : 2.59 E of diolate formation from MT + H 2 and EG [S1] Liu, P.; Nicholas, K. M. rganometallics 2013, 32,
Characterization of Re-Pd/SiO 2 catalysts for hydrogenation of stearic acid
 Supporting Information: Characterization of Re-Pd/SiO 2 catalysts for hydrogenation of stearic acid Yasuyuki Takeda, [a] Masazumi Tamura, [a] Yoshinao Nakagawa, [a] Kazu Okumura, [b] and Keiichi Tomishige*
Supporting Information: Characterization of Re-Pd/SiO 2 catalysts for hydrogenation of stearic acid Yasuyuki Takeda, [a] Masazumi Tamura, [a] Yoshinao Nakagawa, [a] Kazu Okumura, [b] and Keiichi Tomishige*
Catalytic Hydrogenation of Amino Acids to Amino Alcohols with Complete Retention of Configuration
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Catalytic Hydrogenation of Amino Acids to Amino Alcohols with
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Catalytic Hydrogenation of Amino Acids to Amino Alcohols with
Supplementary Information
 Supplementary Information Supplementary Figure 1. X-ray diffraction patterns of (a) pure LDH, (b) AuCl 4 ion-exchanged LDH and (c) the Au/LDH hybrid catalyst. The refined cell parameters for pure, ion-exchanged,
Supplementary Information Supplementary Figure 1. X-ray diffraction patterns of (a) pure LDH, (b) AuCl 4 ion-exchanged LDH and (c) the Au/LDH hybrid catalyst. The refined cell parameters for pure, ion-exchanged,
Supporting information
 Supporting information Cu Sub-nanoparticles on Cu/CeO 2 as an Effective Catalyst for Methanol Synthesis from Organic Carbonate by Hydrogenation Masazumi Tamura* Takahisa Kitanaka, Yoshinao Nakagawa and
Supporting information Cu Sub-nanoparticles on Cu/CeO 2 as an Effective Catalyst for Methanol Synthesis from Organic Carbonate by Hydrogenation Masazumi Tamura* Takahisa Kitanaka, Yoshinao Nakagawa and
Yali Liu, Pengfei Zhang, Junmin Liu, Tao Wang, Qisheng Huo, Li Yang, Lei. Sun,*, Zhen-An Qiao,*, and Sheng Dai *, ASSOCIATED CONTENT
 ASSOCIATED CONTENT Supporting Information Gold Cluster-CeO 2 Nanostructured Hybrid Architectures as Catalysts for Selective Oxidation of Inert Hydrocarbons Yali Liu, Pengfei Zhang, Junmin Liu, Tao Wang,
ASSOCIATED CONTENT Supporting Information Gold Cluster-CeO 2 Nanostructured Hybrid Architectures as Catalysts for Selective Oxidation of Inert Hydrocarbons Yali Liu, Pengfei Zhang, Junmin Liu, Tao Wang,
Experimental Section
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Supplementary Material (ESI) for Chemical Communications Modification of Ga 2 O 3 by Ag Cr Core
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2017 Supplementary Material (ESI) for Chemical Communications Modification of Ga 2 O 3 by Ag Cr Core
Chapter 3: Stoichiometry
 Chapter 3: Stoichiometry Chem 6A Michael J. Sailor, UC San Diego 1 Announcements: Thursday (Sep 29) quiz: Bring student ID or we cannot accept your quiz! No notes, no calculators Covers chapters 1 and
Chapter 3: Stoichiometry Chem 6A Michael J. Sailor, UC San Diego 1 Announcements: Thursday (Sep 29) quiz: Bring student ID or we cannot accept your quiz! No notes, no calculators Covers chapters 1 and
Supporting information. Formation of A New Strong Basic Nitrogen Anion by Metal Oxide Modification
 Supporting information Formation of A ew Strong Basic itrogen Anion by Metal Oxide Modification Masazumi Tamura,*,, Ryota Kishi, Akira akayama,*,, Yoshinao akagawa, Jun-ya Hasegawa, Keiichi Tomishige*,
Supporting information Formation of A ew Strong Basic itrogen Anion by Metal Oxide Modification Masazumi Tamura,*,, Ryota Kishi, Akira akayama,*,, Yoshinao akagawa, Jun-ya Hasegawa, Keiichi Tomishige*,
CHEM 172 EXAMINATION 1. January 15, 2009
 CHEM 17 EXAMINATION 1 January 15, 009 Dr. Kimberly M. Broekemeier NAME: Circle lecture time: 9:00 11:00 Constants: c = 3.00 X 10 8 m/s h = 6.63 X 10-34 J x s J = kg x m /s Rydberg Constant = 1.096776 x
CHEM 17 EXAMINATION 1 January 15, 009 Dr. Kimberly M. Broekemeier NAME: Circle lecture time: 9:00 11:00 Constants: c = 3.00 X 10 8 m/s h = 6.63 X 10-34 J x s J = kg x m /s Rydberg Constant = 1.096776 x
Supporting Information for. Size-Dependent Oxidation State and CO Oxidation Activity of Tin
 Supporting Information for Size-Dependent Oxidation State and CO Oxidation Activity of Tin Oxide Clusters Yusuke Inomata, Ken Albrecht,, Kimihisa Yamamoto *,, Laboratory for Chemistry and Life Science,
Supporting Information for Size-Dependent Oxidation State and CO Oxidation Activity of Tin Oxide Clusters Yusuke Inomata, Ken Albrecht,, Kimihisa Yamamoto *,, Laboratory for Chemistry and Life Science,
Ch. 9 NOTES ~ Chemical Bonding NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics.
 Ch. 9 NOTES ~ Chemical Bonding NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Review: Comparison of ionic and molecular compounds Molecular compounds Ionic
Ch. 9 NOTES ~ Chemical Bonding NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Review: Comparison of ionic and molecular compounds Molecular compounds Ionic
All chemical bonding is based on the following relationships of electrostatics: 2. Each period on the periodic table
 UNIT VIII ATOMS AND THE PERIODIC TABLE 25 E. Chemical Bonding 1. An ELECTROSTATIC FORCE is All chemical bonding is based on the following relationships of electrostatics: The greater the distance between
UNIT VIII ATOMS AND THE PERIODIC TABLE 25 E. Chemical Bonding 1. An ELECTROSTATIC FORCE is All chemical bonding is based on the following relationships of electrostatics: The greater the distance between
Supporting Information
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2019 Supporting Information Atomically dispersed Ni as the active site towards selective hydrogenation
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2019 Supporting Information Atomically dispersed Ni as the active site towards selective hydrogenation
Last 4 Digits of USC ID:
 Chemistry 05 B Practice Exam Dr. Jessica Parr First Letter of last Name PLEASE PRINT YOUR NAME IN BLOCK LETTERS Name: Last 4 Digits of USC ID: Lab TA s Name: Question Points Score Grader 8 2 4 3 9 4 0
Chemistry 05 B Practice Exam Dr. Jessica Parr First Letter of last Name PLEASE PRINT YOUR NAME IN BLOCK LETTERS Name: Last 4 Digits of USC ID: Lab TA s Name: Question Points Score Grader 8 2 4 3 9 4 0
Supplementary Figure S1 Stable structures of I, I', II, and II' optimized at the
 H C Si 2.492 (2.348) 1.867 (2.005) Fe O 2.106 (2.077) 2.360 (2.497) N 1.126 (1.115) 2.105 (2.175) I quintet (triplet) 0.0 (+4.1) kcal/mol II triplet (quintet) 0.0 (+4.3) kcal/mol 1.857 (2.076) 2.536 (2.351)
H C Si 2.492 (2.348) 1.867 (2.005) Fe O 2.106 (2.077) 2.360 (2.497) N 1.126 (1.115) 2.105 (2.175) I quintet (triplet) 0.0 (+4.1) kcal/mol II triplet (quintet) 0.0 (+4.3) kcal/mol 1.857 (2.076) 2.536 (2.351)
The Periodic Table of Elements
 The Periodic Table of Elements 8 Uuo Uus Uuh (9) Uup (88) Uuq (89) Uut (8) Uub (8) Rg () 0 Ds (9) 09 Mt (8) 08 Hs (9) 0 h () 0 Sg () 0 Db () 0 Rf () 0 Lr () 88 Ra () 8 Fr () 8 Rn () 8 At (0) 8 Po (09)
The Periodic Table of Elements 8 Uuo Uus Uuh (9) Uup (88) Uuq (89) Uut (8) Uub (8) Rg () 0 Ds (9) 09 Mt (8) 08 Hs (9) 0 h () 0 Sg () 0 Db () 0 Rf () 0 Lr () 88 Ra () 8 Fr () 8 Rn () 8 At (0) 8 Po (09)
Lewis dot structures for molecules
 1 Lewis dot structures for molecules In the dot structure of a molecule, - SHARED valence electrons are shown with dashes - one per pair. - UNSHARED valence electrons ("lone pairs") are represented by
1 Lewis dot structures for molecules In the dot structure of a molecule, - SHARED valence electrons are shown with dashes - one per pair. - UNSHARED valence electrons ("lone pairs") are represented by
Supporting Information
 Supporting Information MgFeCe ternary layered double hydroxide as highly efficient and recyclable heterogeneous base catalyst for synthesis of dimethyl carbonate by transesterification Nayana T. Nivangune
Supporting Information MgFeCe ternary layered double hydroxide as highly efficient and recyclable heterogeneous base catalyst for synthesis of dimethyl carbonate by transesterification Nayana T. Nivangune
Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Speed of light c = m/s. x n e a x d x = 1. 2 n+1 a n π a. He Li Ne Na Ar K Ni 58.
 Physical Chemistry II Test Name: KEY CHEM 464 Spring 18 Chapters 7-11 Average = 1. / 16 6 questions worth a total of 16 points Planck's constant h = 6.63 1-34 J s Speed of light c = 3. 1 8 m/s ħ = h π
Physical Chemistry II Test Name: KEY CHEM 464 Spring 18 Chapters 7-11 Average = 1. / 16 6 questions worth a total of 16 points Planck's constant h = 6.63 1-34 J s Speed of light c = 3. 1 8 m/s ħ = h π
Supplementary Information. ZIF-8 Immobilized Ni(0) Nanoparticles: Highly Effective Catalysts for Hydrogen Generation from Hydrolysis of Ammonia Borane
 Supplementary Information ZIF-8 Immobilized Ni() Nanoparticles: Highly Effective Catalysts for Hydrogen Generation from Hydrolysis of Ammonia Borane Pei-Zhou Li, a,b Kengo Aranishi, a and Qiang Xu* a,b
Supplementary Information ZIF-8 Immobilized Ni() Nanoparticles: Highly Effective Catalysts for Hydrogen Generation from Hydrolysis of Ammonia Borane Pei-Zhou Li, a,b Kengo Aranishi, a and Qiang Xu* a,b
Supporting Information For Pt Monolayer on Porous Pd-Cu Alloys as Oxygen Reduction Electrocatalysts
 Supporting Information For Pt Monolayer on Porous Pd-Cu Alloys as Oxygen Reduction Electrocatalysts Minhua Shao, *, Krista Shoemaker, Amra Peles, Keiichi Kaneko #, Lesia Protsailo UTC Power, South Windsor,
Supporting Information For Pt Monolayer on Porous Pd-Cu Alloys as Oxygen Reduction Electrocatalysts Minhua Shao, *, Krista Shoemaker, Amra Peles, Keiichi Kaneko #, Lesia Protsailo UTC Power, South Windsor,
4.1 Atomic structure and the periodic table. GCSE Chemistry
 4.1 Atomic structure and the periodic table GCSE Chemistry All substances are made of atoms this is cannot be chemically broken down it is the smallest part of an element. Elements are made of only one
4.1 Atomic structure and the periodic table GCSE Chemistry All substances are made of atoms this is cannot be chemically broken down it is the smallest part of an element. Elements are made of only one
CHEM 130 Exp. 8: Molecular Models
 CHEM 130 Exp. 8: Molecular Models In this lab, we will learn and practice predicting molecular structures from molecular formulas. The Periodic Table of the Elements IA 1 H IIA IIIA IVA VA VIA VIIA 3 5
CHEM 130 Exp. 8: Molecular Models In this lab, we will learn and practice predicting molecular structures from molecular formulas. The Periodic Table of the Elements IA 1 H IIA IIIA IVA VA VIA VIIA 3 5
8. Relax and do well.
 CHEM 1314 3;30 pm Theory Exam III John III. Gelder November 13, 2002 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last page include a periodic
CHEM 1314 3;30 pm Theory Exam III John III. Gelder November 13, 2002 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last page include a periodic
Local Electronic Structures and Chemical Bonds in Zr-Based Metallic Glasses
 Materials Transactions, Vol. 45, No. 4 (2004) pp. 1172 to 1176 Special Issue on Bulk Amorphous, Nano-Crystalline and Nano-Quasicrystalline Alloys-V #2004 The Japan Institute of Metals Local Electronic
Materials Transactions, Vol. 45, No. 4 (2004) pp. 1172 to 1176 Special Issue on Bulk Amorphous, Nano-Crystalline and Nano-Quasicrystalline Alloys-V #2004 The Japan Institute of Metals Local Electronic
 lectures accompanying the book: Solid State Physics: An Introduction, by Philip ofmann (2nd edition 2015, ISBN-10: 3527412824, ISBN-13: 978-3527412822, Wiley-VC Berlin. www.philiphofmann.net 1 Bonds between
lectures accompanying the book: Solid State Physics: An Introduction, by Philip ofmann (2nd edition 2015, ISBN-10: 3527412824, ISBN-13: 978-3527412822, Wiley-VC Berlin. www.philiphofmann.net 1 Bonds between
ORBITAL DIAGRAM - A graphical representation of the quantum number "map" of electrons around an atom.
 178 (MAGNETIC) SPIN QUANTUM NUMBER: "spin down" or "spin up" - An ORBITAL (region with fixed "n", "l" and "ml" values) can hold TWO electrons. ORBITAL DIAGRAM - A graphical representation of the quantum
178 (MAGNETIC) SPIN QUANTUM NUMBER: "spin down" or "spin up" - An ORBITAL (region with fixed "n", "l" and "ml" values) can hold TWO electrons. ORBITAL DIAGRAM - A graphical representation of the quantum
Atomic Structure & Interatomic Bonding
 Atomic Structure & Interatomic Bonding Chapter Outline Review of Atomic Structure Atomic Bonding Atomic Structure Atoms are the smallest structural units of all solids, liquids & gases. Atom: The smallest
Atomic Structure & Interatomic Bonding Chapter Outline Review of Atomic Structure Atomic Bonding Atomic Structure Atoms are the smallest structural units of all solids, liquids & gases. Atom: The smallest
... but using electron configurations to describe how aluminum bromide forms is a bit cumbersome! Can we simplify the picture a bit?
 193... but using electron configurations to describe how aluminum bromide forms is a bit cumbersome! Can we simplify the picture a bit? LEWIS NOTATION / ELECTRON-DOT NOTATION - Lewis notation represents
193... but using electron configurations to describe how aluminum bromide forms is a bit cumbersome! Can we simplify the picture a bit? LEWIS NOTATION / ELECTRON-DOT NOTATION - Lewis notation represents
Modified from: Larry Scheffler Lincoln High School IB Chemistry 1-2.1
 Modified from: Larry Scheffler Lincoln High School IB Chemistry 1-2.1 The development of the periodic table brought a system of order to what was otherwise an collection of thousands of pieces of information.
Modified from: Larry Scheffler Lincoln High School IB Chemistry 1-2.1 The development of the periodic table brought a system of order to what was otherwise an collection of thousands of pieces of information.
CHEM 172 EXAMINATION 2. February 12, Dr. Kimberly M. Broekemeier NAME: l = 2r l = 8 1/2 r l = (4/3 1/2 )r. h = 6.
 EM 17 EXAMINATION February 1, 009 Dr. Kimberly M. Broekemeier NAME: P 1 1 P1 R T T1 ln = - ( - ) l = r l = 8 1/ r l = (4/3 1/ )r onstants: c = 3.00 X 10 8 m/s h = 6.63 X 10-34 J x s R = 0.0806 L x atm/mol
EM 17 EXAMINATION February 1, 009 Dr. Kimberly M. Broekemeier NAME: P 1 1 P1 R T T1 ln = - ( - ) l = r l = 8 1/ r l = (4/3 1/ )r onstants: c = 3.00 X 10 8 m/s h = 6.63 X 10-34 J x s R = 0.0806 L x atm/mol
Bonding/Lewis Dots Lecture Page 1 of 12 Date. Bonding. What is Coulomb's Law? Energy Profile: Covalent Bonds. Electronegativity and Linus Pauling
 Bonding/Lewis Dots Lecture Page 1 of 12 Date Bonding What is Coulomb's Law? Energy Profile: Covalent Bonds Electronegativity and Linus Pauling 2.1 H 1.0 Li 0.9 Na 0.8 K 0.8 Rb 0.7 Cs 0.7 Fr 1.5 Be 1.2
Bonding/Lewis Dots Lecture Page 1 of 12 Date Bonding What is Coulomb's Law? Energy Profile: Covalent Bonds Electronegativity and Linus Pauling 2.1 H 1.0 Li 0.9 Na 0.8 K 0.8 Rb 0.7 Cs 0.7 Fr 1.5 Be 1.2
PART CHAPTER2. Atomic Bonding
 PART O N E APTER2 Atomic Bonding The scanning tunneling microscope (Section 4.7) allows the imaging of individual atoms bonded to a material surface. In this case, the microscope was also used to manipulate
PART O N E APTER2 Atomic Bonding The scanning tunneling microscope (Section 4.7) allows the imaging of individual atoms bonded to a material surface. In this case, the microscope was also used to manipulate
Solutions and Ions. Pure Substances
 Class #4 Solutions and Ions CHEM 107 L.S. Brown Texas A&M University Pure Substances Pure substance: described completely by a single chemical formula Fixed composition 1 Mixtures Combination of 2 or more
Class #4 Solutions and Ions CHEM 107 L.S. Brown Texas A&M University Pure Substances Pure substance: described completely by a single chemical formula Fixed composition 1 Mixtures Combination of 2 or more
Supporting Information
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2014 Supporting Information Acceptorless dehydrogenative coupling of primary alcohols
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2014 Supporting Information Acceptorless dehydrogenative coupling of primary alcohols
INSTRUCTIONS: Exam III. November 10, 1999 Lab Section
 CHEM 1215 Exam III John III. Gelder November 10, 1999 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and
CHEM 1215 Exam III John III. Gelder November 10, 1999 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and
Earth Materials I Crystal Structures
 Earth Materials I Crystal Structures Isotopes same atomic number, different numbers of neutrons, different atomic mass. Ta ble 1-1. Su mmar y of quantu m num bers Name Symbol Values Principal n 1, 2,
Earth Materials I Crystal Structures Isotopes same atomic number, different numbers of neutrons, different atomic mass. Ta ble 1-1. Su mmar y of quantu m num bers Name Symbol Values Principal n 1, 2,
2 (27) 3 (26) 4 (21) 5 (18) 6 (8) Total (200) Periodic Table
 Chem 3311 Sammakia Fall 2009 Midterm 1 Student ID page points: 2 (27) 3 (26) 4 (21) 5 (18) 6 (8) Total (200) Periodic Table e Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn
Chem 3311 Sammakia Fall 2009 Midterm 1 Student ID page points: 2 (27) 3 (26) 4 (21) 5 (18) 6 (8) Total (200) Periodic Table e Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn
Xiufang Chen, Jinshui Zhang, Xianzhi Fu, Markus Antonietti, and Xinchen Wang*
 -Catalyzed Oxidation of Benzene to Phenol Using Hydrogen Peroxide and Visible Light Xiufang Chen, Jinshui Zhang, Xianzhi Fu, Markus Antonietti, and Xinchen Wang* Supporting Information: Synthesis of :
-Catalyzed Oxidation of Benzene to Phenol Using Hydrogen Peroxide and Visible Light Xiufang Chen, Jinshui Zhang, Xianzhi Fu, Markus Antonietti, and Xinchen Wang* Supporting Information: Synthesis of :
Recent applications of nanostructured materials - from solar cells and batteries to biological markers
 Recent applications of nanostructured materials - from solar cells and batteries to biological markers Prof. Jan Augustyński o Origins of nanotechnology - limits of miniaturization. o Nanoparticle size
Recent applications of nanostructured materials - from solar cells and batteries to biological markers Prof. Jan Augustyński o Origins of nanotechnology - limits of miniaturization. o Nanoparticle size
Room Temperature Hydrogen Generation from Hydrous Hydrazine for Chemical Hydrogen Storage
 (Supporting Information) Room Temperature Hydrogen Generation from Hydrous Hydrazine for Chemical Hydrogen Storage Sanjay Kumar Singh, Xin-Bo Zhang, and Qiang Xu* National Institute of Advanced Industrial
(Supporting Information) Room Temperature Hydrogen Generation from Hydrous Hydrazine for Chemical Hydrogen Storage Sanjay Kumar Singh, Xin-Bo Zhang, and Qiang Xu* National Institute of Advanced Industrial
Supporting information for Mesoporous Nitrogen-Doped Carbons with High Nitrogen Content and
 Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2015 Supporting information for Mesoporous Nitrogen-Doped Carbons with High Nitrogen Content
Electronic Supplementary Material (ESI) for Green Chemistry. This journal is The Royal Society of Chemistry 2015 Supporting information for Mesoporous Nitrogen-Doped Carbons with High Nitrogen Content
Catalytic Activity of IrO 2 (110) Surface: A DFT study
 Catalytic Activity of IrO 2 (110) Surface: A DFT study Jyh-Chiang Jiang Department of Chemical Engineering, National Taiwan University of Science and Technology (NTUST) NCTS-NCKU 9/7, 2010 Computational
Catalytic Activity of IrO 2 (110) Surface: A DFT study Jyh-Chiang Jiang Department of Chemical Engineering, National Taiwan University of Science and Technology (NTUST) NCTS-NCKU 9/7, 2010 Computational
Large-Scale Synthesis of Transition-metal Doped TiO 2 Nanowires. with Controllable Overpotential
 Large-Scale Synthesis of Transition-metal Doped TiO 2 Nanowires with Controllable Overpotential Bin Liu 1, Hao Ming Chen, 1 Chong Liu 1,3, Sean C. Andrews 1,3, Chris Hahn 1, Peidong Yang 1,2,3,* 1 Department
Large-Scale Synthesis of Transition-metal Doped TiO 2 Nanowires with Controllable Overpotential Bin Liu 1, Hao Ming Chen, 1 Chong Liu 1,3, Sean C. Andrews 1,3, Chris Hahn 1, Peidong Yang 1,2,3,* 1 Department
02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr
 Chemistry 05 B First Letter of PLEASE PRINT YOUR NAME IN BLOCK LETTERS Exam last Name Name: 02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr Lab TA s Name: Question Points Score Grader 2 2 9 3 9 4 2
Chemistry 05 B First Letter of PLEASE PRINT YOUR NAME IN BLOCK LETTERS Exam last Name Name: 02/05/09 Last 4 Digits of USC ID: Dr. Jessica Parr Lab TA s Name: Question Points Score Grader 2 2 9 3 9 4 2
1 Electrons and Chemical Bonding
 CHAPTER 13 1 Electrons and Chemical Bonding SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is chemical bonding? What are valence
CHAPTER 13 1 Electrons and Chemical Bonding SECTION Chemical Bonding BEFORE YOU READ After you read this section, you should be able to answer these questions: What is chemical bonding? What are valence
Korrelationsfunktionen in Flüssigkeiten oder Gasen
 Korrelationsfunktionen in Flüssigkeiten oder Gasen mittlere Dichte Relaxationszeit T 0 L. Van Hove, Phys. Rev. 95, 249 (1954) Inelastische und quasielastische Streuung M. Bée, Chem. Phys. 292, 121 (2003)
Korrelationsfunktionen in Flüssigkeiten oder Gasen mittlere Dichte Relaxationszeit T 0 L. Van Hove, Phys. Rev. 95, 249 (1954) Inelastische und quasielastische Streuung M. Bée, Chem. Phys. 292, 121 (2003)
Depressing the hydrogenation and decomposition. nanoparticles on oxygen functionalized. carbon nanofibers. Supporting Information
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2015 Depressing the hydrogenation and decomposition reaction in H 2 O 2 synthesis
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2015 Depressing the hydrogenation and decomposition reaction in H 2 O 2 synthesis
If anything confuses you or is not clear, raise your hand and ask!
 CHM 1045 Dr. Light s Section December 10, 2002 FINAL EXAM Name (please print) Recitation Section Meeting Time This exam consists of six pages. Make sure you have one of each. Print your name at the top
CHM 1045 Dr. Light s Section December 10, 2002 FINAL EXAM Name (please print) Recitation Section Meeting Time This exam consists of six pages. Make sure you have one of each. Print your name at the top
K. 27 Co. 28 Ni. 29 Cu Rb. 46 Pd. 45 Rh. 47 Ag Cs Ir. 78 Pt.
 1 IA 1 ydrogen 1.01 Atomic number Element symbol Element name Atomic mass VIIIA 1 1.01 IIA IIIA IVA VA VIA VIIA 2 e 4.00 Metalloids 3 Li 6.94 4 Be 9.01 5 B 10.81 6 C 12.01 7 N 14.01 8 O 16.00 9 F 19.00
1 IA 1 ydrogen 1.01 Atomic number Element symbol Element name Atomic mass VIIIA 1 1.01 IIA IIIA IVA VA VIA VIIA 2 e 4.00 Metalloids 3 Li 6.94 4 Be 9.01 5 B 10.81 6 C 12.01 7 N 14.01 8 O 16.00 9 F 19.00
Chapter 3: Elements and Compounds. 3.1 Elements
 Chapter 3: Elements and Compounds 3.1 Elements An element is a fundamental substance that cannot be broken down by chemical or physical methods to simpler substances. The 118 known elements are nature
Chapter 3: Elements and Compounds 3.1 Elements An element is a fundamental substance that cannot be broken down by chemical or physical methods to simpler substances. The 118 known elements are nature
Molybdenum compound MoP as an efficient. electrocatalyst for hydrogen evolution reaction
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2014 Molybdenum compound MoP as an efficient electrocatalyst for hydrogen evolution
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2014 Molybdenum compound MoP as an efficient electrocatalyst for hydrogen evolution
Supporting Information
 Supporting Information Selective synthesis of organogold magic clusters Au54(C CPh)26 Prasenjit Maity, a Tomonari Wakabayashi, b Nobuyuki Ichikuni, c Hironori Tsunoyama, a Songhai Xie, a Miho Yamauchi,
Supporting Information Selective synthesis of organogold magic clusters Au54(C CPh)26 Prasenjit Maity, a Tomonari Wakabayashi, b Nobuyuki Ichikuni, c Hironori Tsunoyama, a Songhai Xie, a Miho Yamauchi,
8. Relax and do well.
 CHEM 1225 Exam I John I. Gelder February 4, 1999 Name KEY TA's Name Lab Section Please sign your name below to give permission to post your course scores on homework, laboratories and exams. If you do
CHEM 1225 Exam I John I. Gelder February 4, 1999 Name KEY TA's Name Lab Section Please sign your name below to give permission to post your course scores on homework, laboratories and exams. If you do
Guide to the Extended Step-Pyramid Periodic Table
 Guide to the Extended Step-Pyramid Periodic Table William B. Jensen Department of Chemistry University of Cincinnati Cincinnati, OH 452201-0172 The extended step-pyramid table recognizes that elements
Guide to the Extended Step-Pyramid Periodic Table William B. Jensen Department of Chemistry University of Cincinnati Cincinnati, OH 452201-0172 The extended step-pyramid table recognizes that elements
Lecture 6 - Bonding in Crystals
 Lecture 6 onding in Crystals inding in Crystals (Kittel Ch. 3) inding of atoms to form crystals A crystal is a repeated array of atoms Why do they form? What are characteristic bonding mechanisms? How
Lecture 6 onding in Crystals inding in Crystals (Kittel Ch. 3) inding of atoms to form crystals A crystal is a repeated array of atoms Why do they form? What are characteristic bonding mechanisms? How
Stoichiometry. Mole Concept. Balancing Chemical Equations
 Stoichiometry The story so far The structure of an atom protons, neutrons & electrons Electron structure & the Periodic Table Shapes of electron orbitals (Quantum Numbers) Essential and toxic elements
Stoichiometry The story so far The structure of an atom protons, neutrons & electrons Electron structure & the Periodic Table Shapes of electron orbitals (Quantum Numbers) Essential and toxic elements
Pt-Ni alloyed nanocrystals with controlled archtectures for enhanced. methanol oxidation
 Supplementary Information Pt-Ni alloyed nanocrystals with controlled archtectures for enhanced methanol oxidation Xiao-Jing Liu, Chun-Hua Cui, Ming Gong, Hui-Hui Li, Yun Xue, Feng-Jia Fan and Shu-Hong
Supplementary Information Pt-Ni alloyed nanocrystals with controlled archtectures for enhanced methanol oxidation Xiao-Jing Liu, Chun-Hua Cui, Ming Gong, Hui-Hui Li, Yun Xue, Feng-Jia Fan and Shu-Hong
Chemistry 185 Exam #2 - A November 5, Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Table S1. Structural parameters of shell-by-shell fitting of the EXAFS spectrum for reduced and oxidized samples at room temperature (RT)
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting information Table S1. Structural parameters of shell-by-shell
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting information Table S1. Structural parameters of shell-by-shell
- A CHEMICAL BOND is a strong attractive force between the atoms in a compound. attractive forces between oppositely charged ions
 191 CHEMICAL BONDS - A CHEMICAL BOND is a strong attractive force between the atoms in a compound. 3 TYPES OF CHEMICAL BOND Ionic bonds attractive forces between oppositely charged ions sodium chloride
191 CHEMICAL BONDS - A CHEMICAL BOND is a strong attractive force between the atoms in a compound. 3 TYPES OF CHEMICAL BOND Ionic bonds attractive forces between oppositely charged ions sodium chloride
Molecular-Level Insight into Selective Catalytic Reduction of NO x with NH 3 to N 2
 Supporting Information Molecular-Level Insight into Selective Catalytic Reduction of NO x with to N 2 over Highly Efficient Bifunctional V a Catalyst at Low Temperature Ying Xin, Hao Li, Nana Zhang, Qian
Supporting Information Molecular-Level Insight into Selective Catalytic Reduction of NO x with to N 2 over Highly Efficient Bifunctional V a Catalyst at Low Temperature Ying Xin, Hao Li, Nana Zhang, Qian
EXAFS. Extended X-ray Absorption Fine Structure
 AOFSRR Cheiron School 2010, SPring-8 EXAFS Oct. 14th, 2010 Extended X-ray Absorption Fine Structure Iwao Watanabe Ritsumeikan University EXAFS Theory Quantum Mechanics Models Approximations Experiment
AOFSRR Cheiron School 2010, SPring-8 EXAFS Oct. 14th, 2010 Extended X-ray Absorption Fine Structure Iwao Watanabe Ritsumeikan University EXAFS Theory Quantum Mechanics Models Approximations Experiment
Supporting Information for
 Supporting Information for Flowerlike Mesoporous Silica: A Bifunctionalized Catalyst for Rhodium-Catalyzed Asymmetric Transfer Hydrogenation of Aromatic Ketones in Aqueous Medium Fei Gao, Ronghua Jin,
Supporting Information for Flowerlike Mesoporous Silica: A Bifunctionalized Catalyst for Rhodium-Catalyzed Asymmetric Transfer Hydrogenation of Aromatic Ketones in Aqueous Medium Fei Gao, Ronghua Jin,
5 questions, 3 points each, 15 points total possible. 26 Fe Cu Ni Co Pd Ag Ru 101.
 Physical Chemistry II Lab CHEM 4644 spring 2017 final exam KEY 5 questions, 3 points each, 15 points total possible h = 6.626 10-34 J s c = 3.00 10 8 m/s 1 GHz = 10 9 s -1. B= h 8π 2 I ν= 1 2 π k μ 6 P
Physical Chemistry II Lab CHEM 4644 spring 2017 final exam KEY 5 questions, 3 points each, 15 points total possible h = 6.626 10-34 J s c = 3.00 10 8 m/s 1 GHz = 10 9 s -1. B= h 8π 2 I ν= 1 2 π k μ 6 P
1. Following Dalton s Atomic Theory, 2. In 1869 Russian chemist published a method. of organizing the elements. Mendeleev showed that
 20 CHEMISTRY 11 D. Organizing the Elements The Periodic Table 1. Following Dalton s Atomic Theory, By 1817, chemists had discovered 52 elements and by 1863 that number had risen to 62. 2. In 1869 Russian
20 CHEMISTRY 11 D. Organizing the Elements The Periodic Table 1. Following Dalton s Atomic Theory, By 1817, chemists had discovered 52 elements and by 1863 that number had risen to 62. 2. In 1869 Russian
Solid State Spectroscopy Problem Set 7
 Solid State Spectroscopy Problem Set 7 Due date: June 29th, 2015 Problem 5.1 EXAFS Study of Mn/Fe substitution in Y(Mn 1-x Fe x ) 2 O 5 From article «EXAFS, XANES, and DFT study of the mixed-valence compound
Solid State Spectroscopy Problem Set 7 Due date: June 29th, 2015 Problem 5.1 EXAFS Study of Mn/Fe substitution in Y(Mn 1-x Fe x ) 2 O 5 From article «EXAFS, XANES, and DFT study of the mixed-valence compound
Supporting Information High Activity and Selectivity of Ag/SiO 2 Catalyst for Hydrogenation of Dimethyloxalate
 Supporting Information High Activity and Selectivity of Ag/SiO 2 Catalyst for Hydrogenation of Dimethyloxalate An-Yuan Yin, Xiao-Yang Guo, Wei-Lin Dai*, Kang-Nian Fan Shanghai Key Laboratory of Molecular
Supporting Information High Activity and Selectivity of Ag/SiO 2 Catalyst for Hydrogenation of Dimethyloxalate An-Yuan Yin, Xiao-Yang Guo, Wei-Lin Dai*, Kang-Nian Fan Shanghai Key Laboratory of Molecular
8. Relax and do well.
 CHEM 1215 Exam III John III. Gelder November 11, 1998 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and
CHEM 1215 Exam III John III. Gelder November 11, 1998 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 7 different pages. The last page includes a periodic table and
Supporting Information. for. Gold Nanoparticles Supported on Alumina as a Catalyst for Surface Plasmon-Enhanced Selective Reductions of Nitrobenzene
 Supporting Information for Gold Nanoparticles Supported on Alumina as a Catalyst for Surface Plasmon-Enhanced Selective Reductions of Nitrobenzene Kittichai Chaiseeda, Shun Nishimura, and Kohki Ebitani
Supporting Information for Gold Nanoparticles Supported on Alumina as a Catalyst for Surface Plasmon-Enhanced Selective Reductions of Nitrobenzene Kittichai Chaiseeda, Shun Nishimura, and Kohki Ebitani
Supporting Information
 Supporting Information Insight into the Formation of Co@Co 2 C Catalysts for Direct Synthesis of Higher Alcohols and Olefins from Syngas Ziang Zhao, 1,2, Wei Lu, 1, Ruoou Yang, 2,4 Hejun Zhu, 1,* Wenda
Supporting Information Insight into the Formation of Co@Co 2 C Catalysts for Direct Synthesis of Higher Alcohols and Olefins from Syngas Ziang Zhao, 1,2, Wei Lu, 1, Ruoou Yang, 2,4 Hejun Zhu, 1,* Wenda
Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c,
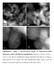 Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c, 2 nm; d, 2 nm. The low magnification images demonstrate
Supplementary Figure 1 HAADF-STEM images of 0.08%Pt/FeO x -R200 single-atom catalyst with different magnifications. Scale bar: a, 20 nm; b, 10 nm; c, 2 nm; d, 2 nm. The low magnification images demonstrate
Heterogeneous catalysis: the fundamentals Kinetics
 www.catalysiscourse.com Heterogeneous catalysis: the fundamentals Kinetics Prof dr J W (Hans) Niemantsverdriet Schuit Institute of Catalysis Catalysis is a cycle A B separation P catalyst P bonding catalyst
www.catalysiscourse.com Heterogeneous catalysis: the fundamentals Kinetics Prof dr J W (Hans) Niemantsverdriet Schuit Institute of Catalysis Catalysis is a cycle A B separation P catalyst P bonding catalyst
Ir-Re Alloy as a highly active catalyst for the hydrogenolysis
 Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2014 Supporting Information Ir-Re Alloy as a highly active catalyst for the hydrogenolysis
Electronic Supplementary Material (ESI) for Catalysis Science & Technology. This journal is The Royal Society of Chemistry 2014 Supporting Information Ir-Re Alloy as a highly active catalyst for the hydrogenolysis
Chemistry 2 Exam Roane State Academic Festival. Name (print neatly) School
 Name (print neatly) School There are fifteen question on this exam. Each question is weighted equally. n the answer sheet, write your name in the space provided and your answers in the blanks provided.
Name (print neatly) School There are fifteen question on this exam. Each question is weighted equally. n the answer sheet, write your name in the space provided and your answers in the blanks provided.
M09/4/CHEMI/SPM/ENG/TZ1/XX+ CHEMISTRY. Monday 18 May 2009 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES
 M09/4/CHEMI/SPM/ENG/TZ1/XX+ 22096110 CHEMISTRY standard level Paper 1 Monday 18 May 2009 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so.
M09/4/CHEMI/SPM/ENG/TZ1/XX+ 22096110 CHEMISTRY standard level Paper 1 Monday 18 May 2009 (afternoon) 45 minutes INSTRUCTIONS TO CANDIDATES Do not open this examination paper until instructed to do so.
How many grams of sodium metal is required to completely react with 2545 grams of chlorine gas?
 146 EXAMPLE PROBLEM: How many grams of sodium metal is required to completely react with 2545 grams of chlorine gas? 1 - Convert 2545 grams of chlorine gas to moles. Use formula weight. 2 - Convert moles
146 EXAMPLE PROBLEM: How many grams of sodium metal is required to completely react with 2545 grams of chlorine gas? 1 - Convert 2545 grams of chlorine gas to moles. Use formula weight. 2 - Convert moles
- A CHEMICAL BOND is a strong attractive force between the atoms in a compound. attractive forces between oppositely charged ions
 CHEMICAL BONDS - A CHEMICAL BOND is a strong attractive force between the atoms in a compound. 3 TYPES OF CHEMICAL BOND Ionic bonds attractive forces between oppositely charged ions sodium chloride Covalent
CHEMICAL BONDS - A CHEMICAL BOND is a strong attractive force between the atoms in a compound. 3 TYPES OF CHEMICAL BOND Ionic bonds attractive forces between oppositely charged ions sodium chloride Covalent
Unit 1 Part 2 Atomic Structure and The Periodic Table Introduction to the Periodic Table UNIT 1 ATOMIC STRUCTURE AND THE PERIODIC TABLE
 UNIT 1 ATOMIC STRUCTURE AND THE PERIODIC TABLE PART 2 INTRODUCTION TO THE PERIODIC TABLE Contents 1. The Structure of the Periodic Table 2. Trends in the Periodic Table Key words: group, period, block,
UNIT 1 ATOMIC STRUCTURE AND THE PERIODIC TABLE PART 2 INTRODUCTION TO THE PERIODIC TABLE Contents 1. The Structure of the Periodic Table 2. Trends in the Periodic Table Key words: group, period, block,
CHEM 171 EXAMINATION 1. October 9, Dr. Kimberly M. Broekemeier. NAME: Key
 CHEM 171 EXAMINATION 1 October 9, 008 Dr. Kimberly M. Broekemeier NAME: Key I A II A III B IV B V B VI B VII B VIII I B II B III A IV A V A VI A VII A inert gase s 1 H 1.008 Li.941 11 Na.98 19 K 9.10 7
CHEM 171 EXAMINATION 1 October 9, 008 Dr. Kimberly M. Broekemeier NAME: Key I A II A III B IV B V B VI B VII B VIII I B II B III A IV A V A VI A VII A inert gase s 1 H 1.008 Li.941 11 Na.98 19 K 9.10 7
Lab Day and Time: Instructions. 1. Do not open the exam until you are told to start.
 Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Name: Lab Day and Time: Instructions 1. Do not open the exam until you are told to start. 2. This exam is closed note and closed book. You are not allowed to use any outside material while taking this
Reversible dioxygen binding on asymmetric dinuclear rhodium centres
 Electronic Supporting Information for Reversible dioxygen binding on asymmetric dinuclear rhodium centres Takayuki Nakajima,* Miyuki Sakamoto, Sachi Kurai, Bunsho Kure, Tomoaki Tanase* Department of Chemistry,
Electronic Supporting Information for Reversible dioxygen binding on asymmetric dinuclear rhodium centres Takayuki Nakajima,* Miyuki Sakamoto, Sachi Kurai, Bunsho Kure, Tomoaki Tanase* Department of Chemistry,
Reporting Category 1: Matter and Energy
 Name: Science Teacher: Reporting Category 1: Matter and Energy Atoms 8.5A Fill in the missing information to summarize what you know about atomic structure. Name of Subatomic Particle Location within the
Name: Science Teacher: Reporting Category 1: Matter and Energy Atoms 8.5A Fill in the missing information to summarize what you know about atomic structure. Name of Subatomic Particle Location within the
Supporting Information
 Supporting Information Dynamic Interaction between Methylammonium Lead Iodide and TiO 2 Nanocrystals Leads to Enhanced Photocatalytic H 2 Evolution from HI Splitting Xiaomei Wang,, Hong Wang,, Hefeng Zhang,,
Supporting Information Dynamic Interaction between Methylammonium Lead Iodide and TiO 2 Nanocrystals Leads to Enhanced Photocatalytic H 2 Evolution from HI Splitting Xiaomei Wang,, Hong Wang,, Hefeng Zhang,,
Insights into Interfacial Synergistic Catalysis over Catalyst toward Water-Gas Shift Reaction
 Supporting Information Insights into Interfacial Synergistic Catalysis over Ni@TiO2-x Catalyst toward Water-Gas Shift Reaction Ming Xu, 1 Siyu Yao, 2 Deming Rao, 1 Yiming Niu, 3 Ning Liu, 1 Mi Peng, 2
Supporting Information Insights into Interfacial Synergistic Catalysis over Ni@TiO2-x Catalyst toward Water-Gas Shift Reaction Ming Xu, 1 Siyu Yao, 2 Deming Rao, 1 Yiming Niu, 3 Ning Liu, 1 Mi Peng, 2
Name Date Class STUDY GUIDE FOR CONTENT MASTERY. covalent bond molecule sigma bond exothermic pi bond
 Covalent Bonding Section 9.1 The Covalent Bond In your textbook, read about the nature of covalent bonds. Use each of the terms below just once to complete the passage. covalent bond molecule sigma bond
Covalent Bonding Section 9.1 The Covalent Bond In your textbook, read about the nature of covalent bonds. Use each of the terms below just once to complete the passage. covalent bond molecule sigma bond
Element Cube Project (x2)
 Element Cube Project (x2) Background: As a class, we will construct a three dimensional periodic table by each student selecting two elements in which you will need to create an element cube. Helpful Links
Element Cube Project (x2) Background: As a class, we will construct a three dimensional periodic table by each student selecting two elements in which you will need to create an element cube. Helpful Links
ORBITAL DIAGRAM - A graphical representation of the quantum number "map" of electrons around an atom.
 160 ORBITAL DIAGRAM - A graphical representation of the quantum number "map" of electrons around an atom. 4p 3d 4s 3p 3s 2p 2s 1s Each blank represents an ORBITAL, and can hold two electrons. The 4s subshell
160 ORBITAL DIAGRAM - A graphical representation of the quantum number "map" of electrons around an atom. 4p 3d 4s 3p 3s 2p 2s 1s Each blank represents an ORBITAL, and can hold two electrons. The 4s subshell
Chem 112, Fall 05 Exam 2a
 Name: YOU MUST: Put your name and student ID on the bubble sheet correctly. Put all your answers on the bubble sheet. Please sign the statement on the last page of the exam. Please make sure your exam
Name: YOU MUST: Put your name and student ID on the bubble sheet correctly. Put all your answers on the bubble sheet. Please sign the statement on the last page of the exam. Please make sure your exam
Covalent Bonding & Molecular Structure
 ovalent Bonding & Molecular Structure I. Electronic onfiguration and e! sharing. A. The Periodic Table s shape helps you understand outer- (and inner-) shell e! configuration. Which e! were of greatest
ovalent Bonding & Molecular Structure I. Electronic onfiguration and e! sharing. A. The Periodic Table s shape helps you understand outer- (and inner-) shell e! configuration. Which e! were of greatest
CLASS TEST GRADE 11. PHYSICAL SCIENCES: CHEMISTRY Test 4: Matter and materials 1
 CLASS TEST GRADE PHYSICAL SCIENCES: CHEMISTRY Test 4: Matter and materials MARKS: 45 TIME: hour INSTRUCTIONS AND INFORMATION. Answer ALL the questions. 2. You may use non-programmable calculators. 3. You
CLASS TEST GRADE PHYSICAL SCIENCES: CHEMISTRY Test 4: Matter and materials MARKS: 45 TIME: hour INSTRUCTIONS AND INFORMATION. Answer ALL the questions. 2. You may use non-programmable calculators. 3. You
100% ionic compounds do not exist but predominantly ionic compounds are formed when metals combine with non-metals.
 2.21 Ionic Bonding 100% ionic compounds do not exist but predominantly ionic compounds are formed when metals combine with non-metals. Forming ions Metal atoms lose electrons to form +ve ions. Non-metal
2.21 Ionic Bonding 100% ionic compounds do not exist but predominantly ionic compounds are formed when metals combine with non-metals. Forming ions Metal atoms lose electrons to form +ve ions. Non-metal
-"l" also contributes ENERGY. Higher values for "l" mean the electron has higher energy.
 175 - Giving the four parameters will uniquely identify an electron around an atom. No two electrons in the same atom can share all four. These parameters are called QUANTUM NUMBERS. PRINCIPAL QUANTUM
175 - Giving the four parameters will uniquely identify an electron around an atom. No two electrons in the same atom can share all four. These parameters are called QUANTUM NUMBERS. PRINCIPAL QUANTUM
- A polar molecule has an uneven distribution of electron density, making it have ends (poles) that are slightly charged.
 14 POLARITY and shape: - A polar molecule has an uneven distribution of electron density, making it have ends (poles) that are slightly charged. POLARITY influences several easily observable properties.
14 POLARITY and shape: - A polar molecule has an uneven distribution of electron density, making it have ends (poles) that are slightly charged. POLARITY influences several easily observable properties.
Why all the repeating Why all the repeating Why all the repeating Why all the repeating
 Why all the repeating Why all the repeating Why all the repeating Why all the repeating Patterns What Patterns have you observed in your life? Where to Get Help If you don t understand concepts in chapter
Why all the repeating Why all the repeating Why all the repeating Why all the repeating Patterns What Patterns have you observed in your life? Where to Get Help If you don t understand concepts in chapter
8. Relax and do well.
 CHEM 1515 Exam II John II. Gelder October 14, 1993 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last two pages include a periodic table, a
CHEM 1515 Exam II John II. Gelder October 14, 1993 Name TA's Name Lab Section INSTRUCTIONS: 1. This examination consists of a total of 8 different pages. The last two pages include a periodic table, a
CHEM Come to the PASS workshop with your mock exam complete. During the workshop you can work with other students to review your work.
 It is most beneficial to you to write this mock midterm UNDER EXAM CONDITIONS. This means: Complete the midterm in 1.5 hours. Work on your own. Keep your notes and textbook closed. Attempt every question.
It is most beneficial to you to write this mock midterm UNDER EXAM CONDITIONS. This means: Complete the midterm in 1.5 hours. Work on your own. Keep your notes and textbook closed. Attempt every question.
The Periodic Table of the Elements
 The Periodic Table of the Elements All matter is composed of elements. All of the elements are composed of atoms. An atom is the smallest part of an element which still retains the properties of that element.
The Periodic Table of the Elements All matter is composed of elements. All of the elements are composed of atoms. An atom is the smallest part of an element which still retains the properties of that element.
The Periodic Table. Periodic Properties. Can you explain this graph? Valence Electrons. Valence Electrons. Paramagnetism
 Periodic Properties Atomic & Ionic Radius Energy Electron Affinity We want to understand the variations in these properties in terms of electron configurations. The Periodic Table Elements in a column
Periodic Properties Atomic & Ionic Radius Energy Electron Affinity We want to understand the variations in these properties in terms of electron configurations. The Periodic Table Elements in a column
