Chemistry of Ring-Retaining Products I. Barnes Bergische University Wuppertal
|
|
|
- Egbert Hodges
- 5 years ago
- Views:
Transcription
1 Mechanism of the Photooxidation of Aromatic Hydrocarbons: Chemistry of Ring-Retaining Products I. Barnes Bergische University Wuppertal
2 Photoreactor in Wuppertal Germany 6 Meter or 1080 l reactor (~ 1990) In situ FITR, GC and DMA Base optical path 6 meter, total optical path 484,7 m
3 utdoor Photoreactor, Valencia, Spain Mixing Fan Actinometers for J ( 1 D) and J (N 2) Clean Air Inlet Ports for Reactant Inlet and Sampling LIF Mounting Position Mixing Fan White System for DAS White System for FT-IR EUPHRE chamber at CEAM, Valencia, Spain
4 Primary oxidation steps CH 2 CH 2 CH CH 3 2 / N 2 abstraction + H CH 3 M H H various mechanisms to form ring-retaining and ring-cleavage products addition (+ other isomers) Recent review of the kinetics of aromatic-h-adducts with 2 and N 2 in: R. Koch, R. Knispel, M. Elend, M. Siese und C. Zetzsch, Atmos. Chem. Phys. Discuss. 2006, 6, 7623
5 Gas phase H radical photooxidation of aromatic hydrocarbons + H + 2 H. H ADDITIN N (i) + 2 (ii) (iii) (vi) 2 N 2 H 2 H-ABSTRACTIN H 2 XEPIN H N 2 N 2 H 2 H 2 H PHENLIC H (iv) N 2 N H. (v) H 2 H N 2 H N 2 H 2 β-hydrxy PERXY H H 2 EPXY-XY 1,4- ADDITIN PERXIDE BICYCLIC Simplified photooxidation mechanism from the EXACT final report Recent quantification of dienedials by IFG and EUPHRE (Berndt and Böge, PCCP 2006, 8, )
6 H oxidation of benzene / toluene Ring-retaining products H H 50-60%* *(Berndt and Böge, Phys. Chem. Chem. Phys., 2006, 8, ) CH 3 CH 3 H H + isomers 16-18%
7 H oxidation products of phenol and cresols Reactant Product Yield (% molar) Yield (lit) Major products are phenol o-cresol 1,2-dihyroxybenzene 1,4-benzoquinone 2-nitrophenol 1,2-dihyroxy-3-methylbenzene methyl-1,4-benzoquinone 6-methyl-2-nitrophenol 78.1 ± ± ± ± ± ± ± ± 1.5 1,2-dihydroxybenzenes H H and m-cresol 1,2-dihyroxy-3-methylbenzene methyl-1,4-benzoquinone 3-methyl-2-nitrophenol 5-methyl-2-nitrophenol 70.0 ± ± ± ± ± 0.1 p-cresol 1,2-dihydroxy-4-methylbenzene 4-metyl-2-nitrophenol 64.1 ± ± ± 4 benzoquinones lariu et al. Atmos. Environ., 2002, 36, Product study H + phenol: Berndt and Böge, Phys. Chem. Chem. Phys., 2003, 5,
8 H + 2 H Simplified mechanism for the reaction of phenol/cresols with H -H 2 R + N 2 R H + 2 H H R + H H + N 2 R N 2 R nitrophenols benzoquinones + 2 M R H H 2 R 1,2-dihydroxybenzenes ring opening-products
9 H oxidation of benzene / toluene Ring-retaining products H H H H H 50-60%* up to 80% CH 3 CH 3 CH 3 H H H H 16-18% + isomers up to 80% H *(Berndt and Böge, Phys. Chem. Chem. Phys., 2006, 8, )
10 Rate coefficients for the reactions of H radicals with dihydroxybenzenes and benzoquinone (Relative kinetic technique at 298 K) Compound k (10-11 cm 3 s -1 ) τ i = 1/k[H] H H H H CH 3 1,2-dihydroxybenzene 10.4 ± min 1,2-dihydroxy- 3-methylbenzene 20.5 ± min 1,4-benzoquinone (k = (0.46 ± 0.09) x ) (37 h) CH 3 H CH 3 H 1,2-dihydroxy- 4-methylbenzene 15.6 ± min (lariu et al. Int. J. Chem. Kinet., 2000, 32, ) methyl-1,4-benzoquinone (k = (2.35 ± 0.47) x ) (7.4 h)
11 Rate coefficients at 298 K for the reactions of 3 with Dihydroxybenzenes (relative kinetic technique at 298 K) Compound k (10-17 cm 3 s -1 ) H H 1,2- dihydroxybenzene 0.96 ± 0.0 H H CH 3 H CH 3 H 1,2-dihydroxy- 3-methylbenzene 2.80 ± ,2-dihydroxy- 4-methylbenzene 2.63 ± 0.34 (Tomas et al, Int. J. Chem. Kinet., 2003, 35, )
12 Rate coefficients at 298 K for the reactions of N 3 radicals with dihydroxybenzenes Compound 1080 l reactor k (10-11 cm 3 s -1 ) EUPHRE k (10-11 cm 3 s -1 ) k(average) (10-11 cm 3 s -1 ) H H 1,2- dihydroxybenzene 9.03 ± ± ± 5.0 H H CH 3 H CH 3 H 1,2-dihydroxy- 3-methylbenzene 17.3 ± ± ± 5.6 1,2-dihydroxy- 4-methylbenzene 16.0 ± ± ± 6.5 (lariu et al. Int. J. Chem. Kinetics, 2004,36, )
13 Lifetimes of aromatic compounds H H H τ(h) 9.5 d 5.1 h 1.3 h τ( 3 ) >4.5 yr d τ(n 3 ) >4 yr 8.8 min 20.4 s Atmospheric lifetimes calculated using: [H] a 12-h daytime average of 2 x 10 6 molecule cm -3 [ 3 ] a 24-h average of 7 x molecule cm -3 [N 3 ] a 12-h nightime average of 5 x 10 8 molecule cm -3
14 Trend of rate coefficients for the reaction of methylated hydroxyaromatic compounds with N 3 radicals. 1.8e e e e-11 K N3 (cm 3 s -1 ) 6.0e e e e e ls methylcatechols catechol dimethylphenols cresols phenol trimethylbenzenes xylenes toluene benzene For the isomers an average of the rate coefficients has been used. For benzene an upper limit of k N3 = cm 3 s 1 has been used.
15 Rate coefficients for the reaction of Cl with catechols (relative kinetic technique at 298 K) compound reference k 1 /k 2 k 1 (10-10 cm 3 s -1 ) k 1 (average) (10-10 cm 3 s -1 ) H H ethene 3.8± ±0.1 isoprene 1.8± ±0.1 (6.6 ± 0.1) 1,2-dihydroxybenezene H H ethene 3.7± ±0.3 3-methyl-1,2- dihydroxybenzene H H CH 3 CH 3 4-methyl-1,2- dihydroxybenzene isoprene 2.1± ±0.7 ethene 3.1± ±0.8 (6.7 ± 0.8) (6.2 ± 0.8)
16 Dihydroxybenzene (catechol) H oxidation products (analysis with long path FTIR) H 2 / -H 2 o-benzoquinone abstraction + H 2 evidence for formation* H H H H ring-opening / other products C maleic anhydride addition H H H H observed H H 2 / -H 2 ther Products? -H / -H 2 H H H H H not formed Ipso H addition? An intermediate ketene absorption is observed (*4-methyl-ortho-benzoquinone identified in H + 4-methyl-1,2-dihydroxybenzene)
17 Dihydroxybenzene (catechol) H oxidation products Main observations: 1,2,3-trihydroxybenzene and 1,2,4-trihydroxybenzene not formed An intermediate infrared absorption due to a ketene group (-C=C=) is observed Infrared evidence for the formation of o-benzoquinone (and methylated derivatives) Positive identification of maleic anhydride (4-7%) (probable precursor butenedial) maleic anhydride Formation of C observed, yield increases with time, Detection of C shows ring opening is occurring maleic anyhdride may also indicate ring-opening Identification of 4-nitrocatechol
18 Dihydroxybenzene (catechol) H oxidation products (C and maleic anhydride) 0.25 maleic acid anhydride C maleic acid anhydride and C yield catechol
19 Photolysis of butenedials CH 3 ~20% C R 9-10% 0-18% (R=CH 3 ) 18-20% 0-10% (R=H) ~80% R R C H C From: Thüner, Rea and Wenger 4th EUPHRE Report 2001 R? Z-4-oxo-pent-2-enal system (R= CH 3 ) E-butendial system (R = H ) α-angelicalactone 31-35% (3H)-Furan-2-one 45-55% Maleic Anhydride 4-5% Maleic Anhydride 20-21% Unknown C= 20% Unknown C= 6-14%
20 Ketene and benzoquinone formation work on Cl + catechols has shown H-atom abstraction from an H group Is a major channels to ketene and benzoquinone formation H H H H H + Cl - HCl H 2 H 2 2 N N 2 2 H 2 2 N N 2 2 H 2 H H KETENE formation
21 Generation of phenoxy radicals H H H H CH + Cl H H N 2 N 2 + Cl main product
22 bservation of intermediate ketene absorptions Phenoxy radicals generated from benzaldehyde, phenol and catechol by reaction with Cl atoms a - Benzaldehyde reaction with Cl b - Catechol reaction with Cl c - Phenol reaction with Cl Initial dark Visible lamps Absorbance ketene (c) Wavelenght (cm -1 ) Absorbance ketene (b) Absorbance ketene (a) Phenol (ppm), Ketene (factor) Phenol (ppm) 0.2 Ketene (factor) HCl (ppm) C (ppm) Final dark Time (s) T 1/2 =90s HCl, C (ppm)
23 Ketene identity? H H H H Cl H H HCl H ring opening 2 + H 2 2-butene-1,4-diketene H C ring size reduction + H 1,4-cyclopentadienyl-5-ketene
24 Dihydroxybenzene (catechol) - N 3 reaction products (work 2006) H H H N 3 H + HN 3 N 2 nly observe 4-nitro-1,2-dihydroxybenzene and nitric acid as gas phase products No indication of other products in the residual infrared spectrum Aerosol is formed still to be quantified
25 Dihydroxybenzene (catechol) - N 3 reaction Mechanism? (work 2006) H H N 3 H 2N H -HN 3 H H H N 2 H H H N 2 2 N H
26 xidation of aromatics to nitrophenols CH 3 H/Nx H N 2 H H 3 C N 2 H CH 3 H CH 3 H H N 3 H CH 3 H H H H/Nx N 2 N 2 H Nitrophenol formation
27 Gas phas chemistry of nitrophenols Status up to 2005: nly a rate coefficient available for the reaction of H with 2-nitrophenol obtained by Zetzsch (1985 Report) using FP-RF Estimated rate coefficients for H with 3-, 4- and 5-methyl-2-nitrophenol Photolysis in solution was reported to be very slow
28 Plot of the kinetic data for the reaction of 3-methyl-2-nitrophenols (3M2N) with H radicals 0.3 H N 2 Ln([3M2N] t0 /[3M2N] t )-k (wall, hν) *t CH Ln([reference] t0 /[reference] t )/k H (reference)(10 12 cm -3 s) Data obtained at 1000 mbar total pressure of synthetic air by 298 K using with the relative kinetic technique and ( ) ethene as reference hydrocarbon
29 Rate coefficients for the reactions of H with 2-nitrophenols (NP) at 298 K obtained with the relative kinetic technique Compound k H (10-12 cm 3 s -1 ) k (literature) (10-12 cm 3 s -1 ) H N 2 NP ~ a H N 2 3M2NP (3.69±0.16) 11.2 b CH 3 H N 2 4M2NP (3.46±0.18) 5.38 b H CH 3 N 2 5M2NP (7.34±0.52) 11.2 b H 3 C H 3 C H N 2 6M2NP (2.70±0.17) - a) Zetzsch and coworkers using FP-RF; b) computer estimate by Meylan and Howard, Chemosphere, 1993, 26(12)
30 UV spectra of 2-nitrophenols
31 UV spectra of 3-methyl-2-nitrocresol 2.0E log10 (σ, cm 2 /molec -1 )( ) 1.6E E E E Absorbance ( ) 0.0E Wavelength (nm)
32 N 2 + hν Proposed nitrophenol atmospheric photolysis pathways H N + ( 3 P) (minimum energy 389 KJ mol-1 = threshold of 307 nm H H + N 2 (minimum energy 297 kj mol-1 = 403 nm) N 2 + H (minimum energy 362 kj mol-1 = 330 nm)
33 H-bonding in 2-nitrophenols H H N 2 + hν N + HN photolytic assisted H-atom transfer radical product + nitrous acid H N H N H N H N I I II III
34 Flow system used for study of nitrophenol photolysis alternative to FTIR LPAP (HN) Nx - monitor (N and N 2 ) optical cell FTIR (nitrocresols) excess nitrocresol source fan flowcontr. lamps lamps photoreactor heating system with temperature controlled syn. air, N 2, 2 Ar He
35 For Harvey (and others) LPAP (HN) instrument costs around EUR. More information can be found at:
36 Experiments on the photolysis of 2-nitrophenols (Bejan, et al., J. Phys. Chem. Chem. Phys., , ) HN [ppbv] HN 3M2N blank: lamps on lamps off nitrophenol source connected lamps on lamps off M2N [ppmv] :00 15:00 18:00 time [hh:mm] 0
37 Photolysis of 2-nitrophenols Extensive tests performed to test mechanism of HN formation Tested effects of: Number of photolysis lamps HN(large photoreactor) [ppbv] large photoreactor small photoreactor [HN] (large) = x [3M2N] [HN] (small) = x [3M2N] M2N [ppmv] HN(small photoreactor) [ppbv] Concentration of the nitrophenols 8 7 Change in surface to volume ratio Bath gas (Ar, N 2, 2, syn air) HN [ppbv] No. of lamps
38 Photolysis of 2-nitrophenols H N 2 CH 3 7 H HN [ppbv] H 3 C H N 2 H N 2 N ortho-nitrohydroxylated aromatic compounds [ppmv] CH 3 All observations are in line with a purely photolytic production of HN No formation of N 2 observed 2 is not only a quench gas but appears to be active in the mechanism
39 Photolysis of 2-nitrophenols 80 CH 3 nitrotoluene N 2 HN-formation [ppt ppm-1 s-1] H N 2 CH 3 3-Methyl-2- nitrophenol H N 2 2-Nitrophenol H 3 C H 5-Methyl-2- nitrophenol N 2 H CH 3 N 2 4-Methyl-2- nitrophenol HN formation rate (ppt ppm -1 s -1 ) from the different ortho-nitrohydroxylated monoaromatic compounds observed in the flow system.
40 Photolysis of 2-nitrophenols products + HN d)uni-molecular decomposition H N a) + hν b) + M 2 > N 2 > Ar > He H N * * H * c) energy transfer N II III III * e) + 2 Mechanism proposed to explain HN formation + HN (Bejan, et al., J. Phys. Chem. Chem. Phys., , ) products
41 Atmospheric lifetimes of 2-nitrophenols Approximate atmospheric lifetimes of the 2-nitrocresols with respect to photolysis and reaction with H radicals. compound 2-nitrophenol 3-methyl-2- nitrophenol 4-methyl-2- nitrophenol 5-methyl-2- nitrophenol τ hν (a) (min) ~ 25 min ~ 23 min ~ 44 min ~ 37 min τ H (b) 7.2 d ~ 47 h ~ 50 h ~ 24 h (a) Calculated for atmospheric conditions against J(N 2 ) (b) Daily average H = 1.6 x 10 6 cm-3 (Prinn et al., 1995).
42 Assuming 1 ppb total 2-nitrophenols in the atmosphere and a J(N 2 ) of 10-2 s -1 would support a gas phase HN product rate of 100 pptv/h
43 Time dependence of the aerosol size distribution measured during a typical photolysis experiment on 2-nitrophenol Background 5.0e+8 1.0e+9 1.5e+9 2.0e+9 2.5e+9 Lamps FF Dp, nm 150 Lamps N :48:00 20:08:00 20:28:00 20:48:00 21:08:00 Time Conversion of ver low concentrations (1 ppb) results in aerosol formation
44 Rate coefficients for the reactions of Cl atoms with 2-nitrophenol, methyl-2-nitrophenols and 2-chlorophenol at 298 K Compound k (cm 3 molecule -1 s -1 ) 2-nitrophenol (0.69 ± 0.02) 3-methyl-2-nitrophenol (14.97 ± 0.38) 4-methyl-2-nitrophenol (4.92 ± 0.03) 5-methyl-2-nitrophenol (2.13 ± 0.03) 6-methyl-2-nitrophenol (3.04 ± 0.07) 2-chlorophenol (0.63 ± 0.05)
45 THANKS FR LISTENING
46 Ketene identity? Absorbance (Base 10) Ketene formation from: 1. E,E-2-Methyl-2.4-hexadiendial UV-photolysis 2. E,E-2.4-hexadiendial UV-photolysis 3. E.Z-2,4-hexadiendial UV-photolysis 4. Benzene oxid-oxepin VIS-photolysis Z-oxopentenal UV-photolysis 6. Benzoloxid UV-photolysis 7. Catechol H/Nx system Wavenumber (cm -1 ) 4 7 6
47 Laboratory photolysis rates and steady-state H radical concentrations observed for 2-nitrophenols in a photoreactor. Compound 2-nitrophenol 3-methyl-2- nitrophenol 4-methyl-2- nitrophenol 5-methyl-2- nitrophenol Photolysis (a) (s -1 ) (1.5 ± 0.5) 10-4 (1.67 ± 0.11) 10-4 (8.86 ± 1.07) 10-5 (1.07 ± 0.14) 10-4 H concentration (cm -3 ) ~ ~ ~ ~ (a) Values for the photolysis rate obtained in the 1080 L quartz glass reactor. Lamps: Philips TL05 40W superactinic lamps, emit in the range nm and have a maximum intensity at 360 nm. Value for 2-nitrotoulene s -1 )
48 N H N H N N N N H H H H CC TC CT TT Matrix-isolation infrared spectroscopy in combination with density Function (DFT) calculations Identification of the aci-nitro form of 2-nitrophenol (Nagaya, Kudoh and Nakata, Chem. Phys. Letter, 2006, 427, 67-71) hν
49 H H H k(h) cm 3 molecule -1 s -1 k( 3 ) < not known cm 3 molecule -1 s -1 k(n 3 ) < cm 3 molecule -1 s -1 CH 3 CH 3 CH 3 H H k(h) cm 3 molecule -1 s -1 k( 3 ) < cm 3 molecule -1 s -1 k(n 3 ) cm 3 molecule -1 s -1 H
50 Primary oxidation products 1 H H 4 H H 2 hν H 13 2 H 14 bzw. 15 H H 2 2,N H N 2 H N N 2 H 2 H 2 H, 2 2 H 2 N N 2 2 H H 2 H 2 Recent quantification of dienedials by IFG and EUPHRE (Berndt and Böge, PCCP 2006, 8, )
51 Comparison of estimated atmospheric J-values for 2-nitrocresols with those measured by Bardini (2006) in the EUPHRE chamber, Valencia, Spain. 3.0E-04 Photolysis frequency (s -1 ) 2.0E E E+00 Bardini, M2N this study 4M2N 5M2N 6M2N
52 Dark, with chlorine VIS lamps ( nm ) Dark Nitrophenol, 2Cl-phenol, HCl(ppm Dark initial, without chlorine 2NitroPhenol 2ClPhenol HCl C ClN2 N ClN 2, C, N 2 (ppm) Time (s)
53 Comparison between the product spectrum obtained from the reaction of catechol with chlorine atoms with that from the reaction with H radicals. The lower trace is a spectrum of 4-nitrocatechol identified as product.
54 * C N 2 N 2 Formation of cyclopentadiene ketene from the phototransformation of 2-nitrophenol in aqueous solution (Alif et al., 1991)
55 Aerosol yields from the ozonolysis of dihydroxybenzenes (DHB) Reaction Mass catechol reacted (mg m3) Mass aerosol (mg m3) Aerosol yield (%) 1,2-DHB (cyclohexane) ,2-DHB (cyclohexane) ,2-DHB ,2-DHB ,2-DHB M-1,2-DHB (cyclohexane) M-1,2-DHB M-1,2-DHB (cyclohexane) M-1,2-DHB
56 (Benzene) Toluene + H Adduct (+ 2 ) (+ 2 ) (Phenol) Cresols Adducts- 2 (+ H) (+ N 3 ) N N 2 Catechol /Methylcatechols (+ H)? Nitrophenols (+ H) Ring Fragmentation Semi Volatile Products? Non Volatile Products Semi Volatile Products ther Products
Photolysis of Butenedial and 4-Oxo-2-pentenal Under Atmospheric Conditions
 Photolysis of Butenedial and 4-xo-2-pentenal Under Atmospheric onditions Gerard Rea, Lars Thüner and John Wenger entre for Research into Atmospheric hemistry (RA) Department of hemistry University ollege
Photolysis of Butenedial and 4-xo-2-pentenal Under Atmospheric onditions Gerard Rea, Lars Thüner and John Wenger entre for Research into Atmospheric hemistry (RA) Department of hemistry University ollege
Development of a detailed chemical mechanism (MCMv3.1) for the atmospheric oxidation of aromatic hydrocarbons
 Atmos. Chem. Phys., 5, 641 664, 25 SRef-ID: 168-7324/acp/25-5-641 European Geosciences Union Atmospheric Chemistry and Physics Development of a detailed chemical mechanism (MCMv3.1) for the atmospheric
Atmos. Chem. Phys., 5, 641 664, 25 SRef-ID: 168-7324/acp/25-5-641 European Geosciences Union Atmospheric Chemistry and Physics Development of a detailed chemical mechanism (MCMv3.1) for the atmospheric
Studies on Oxygenated Fuel Additives: Ethers and Acetals
 Studies on xygenated Fuel Additives: Ethers and Acetals I. Barnes, K.. Becker, L. Thüner and T. Maurer Bergische Universität Gesamthochschule Wuppertal, Physikalische hemie / Fachbereich 9, Gaußstraße
Studies on xygenated Fuel Additives: Ethers and Acetals I. Barnes, K.. Becker, L. Thüner and T. Maurer Bergische Universität Gesamthochschule Wuppertal, Physikalische hemie / Fachbereich 9, Gaußstraße
Aromatic Hydrocarbon Research in the European Photoreactor (EUPHORE)
 Aromatic Hydrocarbon Research in the European Photoreactor (EUPHORE) Björn Klotz Bergische Universität, Physikalische Chemie, Gaußstraße 20, D-42097 Wuppertal, Germany Present address: The National Institute
Aromatic Hydrocarbon Research in the European Photoreactor (EUPHORE) Björn Klotz Bergische Universität, Physikalische Chemie, Gaußstraße 20, D-42097 Wuppertal, Germany Present address: The National Institute
Atmospheric Oxidation of Selected Aromatic Hydrocarbons
 Atmospheric xidation of Selected Aromatic Hydrocarbons Thesis submitted to the Faculty of Chemistry Bergische Universität Gesamthochschule Wuppertal for the Degree of Doctor of Science (Dr. rerum naturalium)
Atmospheric xidation of Selected Aromatic Hydrocarbons Thesis submitted to the Faculty of Chemistry Bergische Universität Gesamthochschule Wuppertal for the Degree of Doctor of Science (Dr. rerum naturalium)
New Product and Aerosol Studies On The Photooxidation Of Dimethylsulfide
 New Product and Aerosol Studies On The Photooxidation Of Dimethylsulfide C. Arsene, I. Barnes and K.H. Becker Physikalische Chemie / Fachbereich 9, Bergische Universität-GH Wuppertal Gaußstraße, 97 Wuppertal,
New Product and Aerosol Studies On The Photooxidation Of Dimethylsulfide C. Arsene, I. Barnes and K.H. Becker Physikalische Chemie / Fachbereich 9, Bergische Universität-GH Wuppertal Gaußstraße, 97 Wuppertal,
Lecture 13: Gas Phase Organic- NO x + UV Reactions II
 Lecture 13: Gas Phase rganic- N x + UV Reactions II Required Reading: FP&P Chapter 6 (except as noted next) Additional Reading: S&P Chapter 5 Catching-Up Reading: Jacob Chapters 11 & 12 (free online) Atmospheric
Lecture 13: Gas Phase rganic- N x + UV Reactions II Required Reading: FP&P Chapter 6 (except as noted next) Additional Reading: S&P Chapter 5 Catching-Up Reading: Jacob Chapters 11 & 12 (free online) Atmospheric
Investigations on the Gas Phase Atmospheric Chemistry of Nitrophenols and Catechols
 Investigations on the Gas Phase Atmospheric Chemistry of Nitrophenols and Catechols Thesis submitted to the Faculty of Mathematics and Natural Sciences Bergische Universität Wuppertal for the Degree of
Investigations on the Gas Phase Atmospheric Chemistry of Nitrophenols and Catechols Thesis submitted to the Faculty of Mathematics and Natural Sciences Bergische Universität Wuppertal for the Degree of
The SAPRC Chemical Mechanisms
 The SAPR hemical Mechanisms utline William P. L. arter E-ERT, University of alifornia, Riverside December 6, 2006 Major components of the SAPR mechanisms Mechanism generation system Status of SAPR mechanism
The SAPR hemical Mechanisms utline William P. L. arter E-ERT, University of alifornia, Riverside December 6, 2006 Major components of the SAPR mechanisms Mechanism generation system Status of SAPR mechanism
Supplement of Formation of highly oxygenated low-volatility products from cresol oxidation
 Supplement of Atmos. Chem. Phys., 17, 3453 3474, 2017 http://www.atmos-chem-phys.net/17/3453/2017/ doi:10.5194/acp-17-3453-2017-supplement Author(s) 2017. CC Attribution 3.0 License. Supplement of Formation
Supplement of Atmos. Chem. Phys., 17, 3453 3474, 2017 http://www.atmos-chem-phys.net/17/3453/2017/ doi:10.5194/acp-17-3453-2017-supplement Author(s) 2017. CC Attribution 3.0 License. Supplement of Formation
Paul Ziemann Department of Chemistry & CIRES University of Colorado at Boulder
 Gas- and Particle-Phase Products and their Mechanisms of Formation from the Reactions of Monoterpenes with N 3 Radicals: Comprehensive Measurements and Modeling Paul Ziemann Department of Chemistry & CIRES
Gas- and Particle-Phase Products and their Mechanisms of Formation from the Reactions of Monoterpenes with N 3 Radicals: Comprehensive Measurements and Modeling Paul Ziemann Department of Chemistry & CIRES
Atmospheric Oxidation Mechanisms of Unsaturated Oxygenated VOCs
 Atmospheric Oxidation Mechanisms of Unsaturated Oxygenated VOCs R. Thévenet, G. Thiault, E. Vésine, G. Laverdet, A. Mellouki, G. Le Bras LCSR-CNRS-1C, Avenue de la recherche scientifique 4571, Orléans,
Atmospheric Oxidation Mechanisms of Unsaturated Oxygenated VOCs R. Thévenet, G. Thiault, E. Vésine, G. Laverdet, A. Mellouki, G. Le Bras LCSR-CNRS-1C, Avenue de la recherche scientifique 4571, Orléans,
( -pinene) products. Rate coefficient data. Comments
 IUPAC Task Group on Atmospheric Chemical Kinetic Data Evaluation Data Sheet Hx_VC9 Datasheets can be downloaded for personal use only and must not be retransmitted or disseminated either electronically
IUPAC Task Group on Atmospheric Chemical Kinetic Data Evaluation Data Sheet Hx_VC9 Datasheets can be downloaded for personal use only and must not be retransmitted or disseminated either electronically
ATMOSPHERIC CHEMISTRY OF SELECTED HYDROXYCARBONYLS. Sara M. Aschmann, Janet Arey and Roger Atkinson
 ATMOSPHERIC CHEMISTRY OF SELECTED HYDROXYCARBONYLS Sara M. Aschmann, Janet Arey and Roger Atkinson Air Pollution Research Center University of California Riverside, CA 92521, U.S.A. Introduction Volatile
ATMOSPHERIC CHEMISTRY OF SELECTED HYDROXYCARBONYLS Sara M. Aschmann, Janet Arey and Roger Atkinson Air Pollution Research Center University of California Riverside, CA 92521, U.S.A. Introduction Volatile
2B Technologies, Inc. An InDevR Company
 2B Technologies, Inc. An InDevR Company Technical Note No. 40 UV-Absorbing Interferences in Ozone Monitors Date: 22 April 2015 Author: John Birks Background Ozone measurements by absorbance of the 253.7-nm
2B Technologies, Inc. An InDevR Company Technical Note No. 40 UV-Absorbing Interferences in Ozone Monitors Date: 22 April 2015 Author: John Birks Background Ozone measurements by absorbance of the 253.7-nm
Influence of Biogenic VOCs on Photooxidant Formation: Simulation Experiments in EUPHORE and Comparison with Model Calculations
 Introduction Influence of Biogenic VOCs on Photooxidant Formation: Simulation Experiments in EUPHORE and Comparison with Model Calculations Fraunhofer Institut Atmosphärische Umweltforschung, IFU Kreuzeckbahnstr.
Introduction Influence of Biogenic VOCs on Photooxidant Formation: Simulation Experiments in EUPHORE and Comparison with Model Calculations Fraunhofer Institut Atmosphärische Umweltforschung, IFU Kreuzeckbahnstr.
Chemical Mechanisms for Representation of Aromatic Hydrocarbons in Airshed Models: Effects of Structure on Ozone Reactivity
 Chemical Mechanisms for Representation of Aromatic Hydrocarbons in Airshed Models: Effects of Structure on Ozone Reactivity William P. L. Carter College of Engineering Center for Environmental Research
Chemical Mechanisms for Representation of Aromatic Hydrocarbons in Airshed Models: Effects of Structure on Ozone Reactivity William P. L. Carter College of Engineering Center for Environmental Research
Tropospheric OH chemistry
 Tropospheric OH chemistry CO Oxidation mechanism: CO + OH CO 2 + H, H + O 2 + M HO 2 + M, HO 2 + NO OH + NO 2 NO 2 + hν (+O 2 ) NO + O 3 Initiation step Propagation Net: CO + 2 O 2 CO 2 + O 3 HO 2 + HO
Tropospheric OH chemistry CO Oxidation mechanism: CO + OH CO 2 + H, H + O 2 + M HO 2 + M, HO 2 + NO OH + NO 2 NO 2 + hν (+O 2 ) NO + O 3 Initiation step Propagation Net: CO + 2 O 2 CO 2 + O 3 HO 2 + HO
Supplement of Secondary formation of nitrated phenols: insights from observations during the Uintah Basin Winter Ozone Study (UBWOS) 2014
 Supplement of Atmos. Chem. Phys., 16, 2139 2153, 2016 http://www.atmos-chem-phys.net/16/2139/2016/ doi:10.5194/acp-16-2139-2016-supplement Author(s) 2016. CC Attribution 3.0 License. Supplement of Secondary
Supplement of Atmos. Chem. Phys., 16, 2139 2153, 2016 http://www.atmos-chem-phys.net/16/2139/2016/ doi:10.5194/acp-16-2139-2016-supplement Author(s) 2016. CC Attribution 3.0 License. Supplement of Secondary
Protocol for the development of the Master Chemical Mechanism, MCM v3 (Part B): tropospheric degradation of aromatic volatile organic compounds
 Atmos. Chem. Phys., 3, 181 193, 2003 Atmospheric Chemistry and Physics Protocol for the development of the Master Chemical Mechanism, MCM v3 (Part B): tropospheric degradation of aromatic volatile organic
Atmos. Chem. Phys., 3, 181 193, 2003 Atmospheric Chemistry and Physics Protocol for the development of the Master Chemical Mechanism, MCM v3 (Part B): tropospheric degradation of aromatic volatile organic
1.1 Is the following molecule aromatic or not aromatic? Give reasons for your answer.
 Page 1 QUESTION ONE 1.1 Is the following molecule aromatic or not aromatic? Give reasons for your answer. 1.2 List four criteria which compounds must meet in order to be considered aromatic. Page 2 QUESTION
Page 1 QUESTION ONE 1.1 Is the following molecule aromatic or not aromatic? Give reasons for your answer. 1.2 List four criteria which compounds must meet in order to be considered aromatic. Page 2 QUESTION
Heterogeneous Process Studies on the Formation of Secondary Aerosol
 2016 BC workshop Heterogeneous Process Studies on the Formation of Secondary Aerosol Maofa Ge Institute of Chemistry Chinese Academy of Sciences June-27-2016 Aerosol Heterogeneous Chemistry Atmospheric
2016 BC workshop Heterogeneous Process Studies on the Formation of Secondary Aerosol Maofa Ge Institute of Chemistry Chinese Academy of Sciences June-27-2016 Aerosol Heterogeneous Chemistry Atmospheric
12. Structure Determination: Mass Spectrometry and Infrared Spectroscopy
 12. Structure Determination: Mass Spectrometry and Infrared Spectroscopy Determining the Structure of an Organic Compound The analysis of the outcome of a reaction requires that we know the full structure
12. Structure Determination: Mass Spectrometry and Infrared Spectroscopy Determining the Structure of an Organic Compound The analysis of the outcome of a reaction requires that we know the full structure
PCCP. OH-initiated oxidation of benzene. Part II.y Influence of elevated NO x concentrations. 1. Introduction
 PCCP OH-initiated oxidation of benzene Part II.y Influence of elevated NO x concentrations Björn Klotz,*z a Rainer Volkamer, b Michael D. Hurley, c Mads P. Sulbaek Andersen, d Ole John Nielsen, e Ian Barnes,
PCCP OH-initiated oxidation of benzene Part II.y Influence of elevated NO x concentrations Björn Klotz,*z a Rainer Volkamer, b Michael D. Hurley, c Mads P. Sulbaek Andersen, d Ole John Nielsen, e Ian Barnes,
Chapter 21. Phenols and Aryl Halides. Nucleophilic Aromatic Substitution. Ch. 21-1
 Chapter 21 Phenols and Aryl alides Nucleophilic Aromatic Substitution Ch. 21-1 1. Structure and Nomenclature of Phenols Phenol 1-Naphthol (α-naphthol) 9-Phenanthrol Ch. 21-2 1A. Nomenclature of Phenols
Chapter 21 Phenols and Aryl alides Nucleophilic Aromatic Substitution Ch. 21-1 1. Structure and Nomenclature of Phenols Phenol 1-Naphthol (α-naphthol) 9-Phenanthrol Ch. 21-2 1A. Nomenclature of Phenols
UV3000. Accurate, precise, and portable ambient gas point analyzer
 UV3000 Accurate, precise, and portable ambient gas point analyzer The Cerex UV3000 is a multifunction analyzer designed to detect part per billion (ppb) to percent level concentrations of multiple gases
UV3000 Accurate, precise, and portable ambient gas point analyzer The Cerex UV3000 is a multifunction analyzer designed to detect part per billion (ppb) to percent level concentrations of multiple gases
EVALUATION OF ATMOSPHERIC PROCESSES FOR OZONE FORMATION FROM VEHICLE EMISSIONS
 EVALUATION OF ATMOSPHERIC PROCESSES FOR OZONE FORMATION FROM VEHICLE EMISSIONS by WILLIAM P. L. CARTER STATEWIDE AIR POLLUTION RESEARCH CENTER, and COLLEGE OF ENGINEERING CENTER FOR ENVIRONMENTAL RESEARCH
EVALUATION OF ATMOSPHERIC PROCESSES FOR OZONE FORMATION FROM VEHICLE EMISSIONS by WILLIAM P. L. CARTER STATEWIDE AIR POLLUTION RESEARCH CENTER, and COLLEGE OF ENGINEERING CENTER FOR ENVIRONMENTAL RESEARCH
Supporting Information
 Supporting Information Han et al. 10.1073/pnas.1212690110 SI Materials and Methods Aging of Soot by O 3. Soot particles were deposited on the ZnSe crystal. The sample in the in situ reactor was purged
Supporting Information Han et al. 10.1073/pnas.1212690110 SI Materials and Methods Aging of Soot by O 3. Soot particles were deposited on the ZnSe crystal. The sample in the in situ reactor was purged
Oxidation of Phenolic Wastewater by Fenton's Reagent
 Iraqi Journal of Chemical and Petroleum Engineering Iraqi Journal of Chemical and Petroleum Engineering Vol.0 No. ( June 009) 35-4 ISSN: 997-4884 University of Baghdad College of Engineering xidation of
Iraqi Journal of Chemical and Petroleum Engineering Iraqi Journal of Chemical and Petroleum Engineering Vol.0 No. ( June 009) 35-4 ISSN: 997-4884 University of Baghdad College of Engineering xidation of
CURRENT STATUS OF THE DEVELOPMENT AND EVALUATION OF AN UPDATED DETAILED MECHANISM FOR VOC OXIDATION
 CURRENT STATUS OF THE DEVELOPMENT AND EVALUATION OF AN UPDATED DETAILED MECHANISM FOR VOC OXIDATION William P. L. Carter Statewide Air Pollution Research Center and College of Engineering, Center for Environmental
CURRENT STATUS OF THE DEVELOPMENT AND EVALUATION OF AN UPDATED DETAILED MECHANISM FOR VOC OXIDATION William P. L. Carter Statewide Air Pollution Research Center and College of Engineering, Center for Environmental
Electronic supplementary information to Heterogeneous OH oxidation. of biomass burning organic aerosol surrogate compounds: Assessment
 Electronic supplementary information to Heterogeneous OH oxidation of biomass burning organic aerosol surrogate compounds: Assessment of volatilisation products and the role of OH concentration on the
Electronic supplementary information to Heterogeneous OH oxidation of biomass burning organic aerosol surrogate compounds: Assessment of volatilisation products and the role of OH concentration on the
UNIVERSITY OF CALGARY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEMISTRY 353 READ ALL THE INSTRUCTIONS CAREFULLY
 WEDNESDAY MARCH 9th, 2016 UNIVERSITY OF CALGARY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEMISTRY 353 Version 1 Time: 2 Hours READ ALL THE INSTRUCTIONS CAREFULLY PLEASE WRITE YOUR NAME, STUDENT I.D. NUMBER
WEDNESDAY MARCH 9th, 2016 UNIVERSITY OF CALGARY FACULTY OF SCIENCE MIDTERM EXAMINATION CHEMISTRY 353 Version 1 Time: 2 Hours READ ALL THE INSTRUCTIONS CAREFULLY PLEASE WRITE YOUR NAME, STUDENT I.D. NUMBER
Atmospheric Fate of Unsubstituted Alkoxy and Carbonyl Radicals
 Atmospheric Fate of Unsubstituted Alkoxy and Carbonyl Radicals O. Shestakov, S. Jagiella, J. Theloke, H. G. Libuda, and F. Zabel Institut für Physikalische Chemie, Universität Stuttgart, Pfaffenwaldring
Atmospheric Fate of Unsubstituted Alkoxy and Carbonyl Radicals O. Shestakov, S. Jagiella, J. Theloke, H. G. Libuda, and F. Zabel Institut für Physikalische Chemie, Universität Stuttgart, Pfaffenwaldring
CHEMISTRY Topic #3: Using Spectroscopy to Identify Molecules: Radicals and Mass Spectrometry (MS) Spring 2018 Dr.
 CHEMISTRY 2600 Topic #3: Using Spectroscopy to Identify Molecules: Radicals and Mass Spectrometry (MS) Spring 2018 Dr. Susan Findlay Mass Spectrometry: How Does It Work? In CHEM 1000, you saw that mass
CHEMISTRY 2600 Topic #3: Using Spectroscopy to Identify Molecules: Radicals and Mass Spectrometry (MS) Spring 2018 Dr. Susan Findlay Mass Spectrometry: How Does It Work? In CHEM 1000, you saw that mass
Unique nature of Earth s atmosphere: O 2 present photosynthesis
 Atmospheric composition Major components N 2 78% O 2 21% Ar ~1% Medium components CO 2 370 ppmv (rising about 1.5 ppmv/year) CH 4 1700 ppbv H 2 O variable Trace components H 2 600 ppbv N 2 O 310 ppbv CO
Atmospheric composition Major components N 2 78% O 2 21% Ar ~1% Medium components CO 2 370 ppmv (rising about 1.5 ppmv/year) CH 4 1700 ppbv H 2 O variable Trace components H 2 600 ppbv N 2 O 310 ppbv CO
WP3: In-situ chemical, physical and optical properties of aerosols. WP4: Trace gases networking: Volatile organic carbon and nitrogen oxides
 WP3: In-situ chemical, physical and optical properties of aerosols WP4: Trace gases networking: Volatile organic carbon and nitrogen oxides WP3: In-situ chemical, physical and optical properties of aerosols
WP3: In-situ chemical, physical and optical properties of aerosols WP4: Trace gases networking: Volatile organic carbon and nitrogen oxides WP3: In-situ chemical, physical and optical properties of aerosols
Chemistry Standard level Paper 1
 M15/4/CHEMI/SPM/ENG/TZ1/XX Chemistry Standard level Paper 1 Thursday 14 May 2015 (afternoon) 45 minutes Instructions to candidates Do not open this examination paper until instructed to do so. Answer all
M15/4/CHEMI/SPM/ENG/TZ1/XX Chemistry Standard level Paper 1 Thursday 14 May 2015 (afternoon) 45 minutes Instructions to candidates Do not open this examination paper until instructed to do so. Answer all
CHEM4. (JAN12CHEM401) WMP/Jan12/CHEM4. General Certificate of Education Advanced Level Examination January 2012
 Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials General Certificate of Education Advanced Level Examination January 2012 Question 1 2 Mark
Centre Number Surname Candidate Number For Examiner s Use Other Names Candidate Signature Examiner s Initials General Certificate of Education Advanced Level Examination January 2012 Question 1 2 Mark
Advanced oxidation of organic pollutants in air non-thermal plasmas
 UNIVERSITÀ DEGLI STUDI DI PADVA Department of Chemical Sciences Advanced oxidation of organic pollutants in air non-thermal plasmas Ester Marotta and Cristina Paradisi PlasTEP, Berlin, December 5-6, 212
UNIVERSITÀ DEGLI STUDI DI PADVA Department of Chemical Sciences Advanced oxidation of organic pollutants in air non-thermal plasmas Ester Marotta and Cristina Paradisi PlasTEP, Berlin, December 5-6, 212
Chapter 13 Conjugated Unsaturated Systems
 Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Stable carbon isotope ratios of ambient secondary organic aerosols in Toronto
 Atmos. Chem. Phys., 15, 1825 1838, 215 www.atmos-chem-phys.net/15/1825/215/ doi:1.5194/acp-15-1825-215 Author(s) 215. CC Attribution 3. License. Stable carbon isotope ratios of ambient secondary organic
Atmos. Chem. Phys., 15, 1825 1838, 215 www.atmos-chem-phys.net/15/1825/215/ doi:1.5194/acp-15-1825-215 Author(s) 215. CC Attribution 3. License. Stable carbon isotope ratios of ambient secondary organic
ALE 2. Rate Laws: Expressing and Quantifying the Rate of Reaction
 Name Chem 163 Section: Team Number: ALE 2. Rate Laws: Expressing and Quantifying the Rate of Reaction (Reference: 16.3 Silberberg 5 th edition) How are concentrations of reactants related to the rate of
Name Chem 163 Section: Team Number: ALE 2. Rate Laws: Expressing and Quantifying the Rate of Reaction (Reference: 16.3 Silberberg 5 th edition) How are concentrations of reactants related to the rate of
New particle formation during α- and β-pinene oxidation by O 3, OH and NO 3, and the influence of water vapour: particle size distribution studies
 Atmos. Chem. Phys., 2, 183 196, 2002 Atmospheric Chemistry and Physics New particle formation during α- and β-pinene oxidation by O 3, OH and NO 3, and the influence of water vapour: particle size distribution
Atmos. Chem. Phys., 2, 183 196, 2002 Atmospheric Chemistry and Physics New particle formation during α- and β-pinene oxidation by O 3, OH and NO 3, and the influence of water vapour: particle size distribution
Topic 10 Organic Chemistry. Ms. Kiely IB Chemistry (SL) Coral Gables Senior High School
 Topic 10 Organic Chemistry Ms. Kiely IB Chemistry (SL) Coral Gables Senior High School -Alkanes: have low reactivity and undergo free radical substitution. -Alkenes: are more reactive than alkanes, since
Topic 10 Organic Chemistry Ms. Kiely IB Chemistry (SL) Coral Gables Senior High School -Alkanes: have low reactivity and undergo free radical substitution. -Alkenes: are more reactive than alkanes, since
Supporting Information for. Suppression of OH Generation from the Photo-Fenton Reaction in the Presence of α-pinene Secondary Organic Aerosol Material
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Supporting Information for Suppression of OH Generation from the Photo-Fenton Reaction in the Presence of α-pinene Secondary Organic Aerosol Material Rachel F. Hems *,
1 2 3 4 5 6 7 8 9 10 11 12 13 14 Supporting Information for Suppression of OH Generation from the Photo-Fenton Reaction in the Presence of α-pinene Secondary Organic Aerosol Material Rachel F. Hems *,
C h a p t e r F o u r t e e n: Structure Determination: Mass Spectrometry and Infrared Spectroscopy
 C h a p t e r F o u r t e e n: Structure Determination: Mass Spectrometry and Infrared Spectroscopy Cl OH Cl An electron ionization mass spectrum of 2,5-dichlorophenol CHM 323: Summary of Important Concepts
C h a p t e r F o u r t e e n: Structure Determination: Mass Spectrometry and Infrared Spectroscopy Cl OH Cl An electron ionization mass spectrum of 2,5-dichlorophenol CHM 323: Summary of Important Concepts
Introduction. The analysis of the outcome of a reaction requires that we know the full structure of the products as well as the reactants
 Introduction The analysis of the outcome of a reaction requires that we know the full structure of the products as well as the reactants Spectroscopy and the Electromagnetic Spectrum Unlike mass spectrometry,
Introduction The analysis of the outcome of a reaction requires that we know the full structure of the products as well as the reactants Spectroscopy and the Electromagnetic Spectrum Unlike mass spectrometry,
Chem 263 Oct. 6, Single bonds, σ. e - donating Activate Activate ortho and para directing ortho and para directing
 Chem 263 ct. 6, 2009 lectrophilic Substitution of Substituted Benzenes Resonance ffect Inductive ffect C=C, π system Single bonds, σ Strong Weak e - donating Activate Activate ortho and para directing
Chem 263 ct. 6, 2009 lectrophilic Substitution of Substituted Benzenes Resonance ffect Inductive ffect C=C, π system Single bonds, σ Strong Weak e - donating Activate Activate ortho and para directing
Chapter 17 Reactions of Aromatic Compounds. Electrophilic Aromatic Substitution
 Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Electrophile substitutes for a hydrogen on the benzene ring. Chapter 17: Aromatics 2-Reactions Slide 17-2 1 Mechanism Step
Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Electrophile substitutes for a hydrogen on the benzene ring. Chapter 17: Aromatics 2-Reactions Slide 17-2 1 Mechanism Step
2. Sketch a plot of R vs. z. Comment on the shape. Explain physically why R(z) has a maximum in the atmospheric column.
 190 PROBLEMS 10. 1 Shape of the ozone layer Consider a beam of solar radiation of wavelength λ propagating downward in the vertical direction with an actinic flux I at the top of the atmosphere. Assume
190 PROBLEMS 10. 1 Shape of the ozone layer Consider a beam of solar radiation of wavelength λ propagating downward in the vertical direction with an actinic flux I at the top of the atmosphere. Assume
A Theoretical Study of Oxidation of Phenoxy and Benzyl Radicals by HO 2
 A Theoretical Study of xidation of Phenoxy and Benzyl adicals by 2 S. Skokov, A. Kazakov, and F. L. Dryer Department of Mechanical and Aerospace Engineering Princeton University, Princeton, NJ 08544 We
A Theoretical Study of xidation of Phenoxy and Benzyl adicals by 2 S. Skokov, A. Kazakov, and F. L. Dryer Department of Mechanical and Aerospace Engineering Princeton University, Princeton, NJ 08544 We
Class XI Chapter 13 Hydrocarbons Chemistry
 Question 13.1: How do you account for the formation of ethane during chlorination of methane? Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the
Question 13.1: How do you account for the formation of ethane during chlorination of methane? Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the
Real-time ppb CO 2 Impurity Detection by an Advanced FTIR- UVF System
 Real-time ppb CO 2 Impurity Detection by an Advanced FTIR- UVF System Presented at the BevTech Conference, Albuquerque, NM 2018 by Charles M. Phillips Ph.D., Max Analytical Technologies Mark Taylor, Vice
Real-time ppb CO 2 Impurity Detection by an Advanced FTIR- UVF System Presented at the BevTech Conference, Albuquerque, NM 2018 by Charles M. Phillips Ph.D., Max Analytical Technologies Mark Taylor, Vice
Aromatic Compounds and Amines
 Aromatic Compounds and Amines 22 8 Consider compound P shown below that is formed by the reaction of benzene with an electrophile. O C CH 2 CH 3 P 8 (a) Give the two substances that react together to form
Aromatic Compounds and Amines 22 8 Consider compound P shown below that is formed by the reaction of benzene with an electrophile. O C CH 2 CH 3 P 8 (a) Give the two substances that react together to form
Supplemental Material for Elemental Composition and Oxidation of Chamber Organic Aerosol
 Supplemental Material for Elemental Composition and Oxidation of Chamber Organic Aerosol P. S. Chhabra 1,N.L.Ng 2, M. R. Canagaratna 2, A. L. Corrigan 3, L. M. Russell 3, D. R. Worsnop 2, R. C. Flagan
Supplemental Material for Elemental Composition and Oxidation of Chamber Organic Aerosol P. S. Chhabra 1,N.L.Ng 2, M. R. Canagaratna 2, A. L. Corrigan 3, L. M. Russell 3, D. R. Worsnop 2, R. C. Flagan
UNIVERSITY OF CAMBRIDGE INTERNATIONAL EXAMINATIONS General Certifi cate of Education Advanced Level
 *3126331034* UNIVERSITY F CAMBRIDGE INTERNATINAL EXAMINATINS General Certifi cate of Education Advanced Level CHEMISTRY 9701/42 Paper 4 Structured Questions May/June 2013 2 hours Candidates answer on the
*3126331034* UNIVERSITY F CAMBRIDGE INTERNATINAL EXAMINATINS General Certifi cate of Education Advanced Level CHEMISTRY 9701/42 Paper 4 Structured Questions May/June 2013 2 hours Candidates answer on the
SPECTROSCOPY MEASURES THE INTERACTION BETWEEN LIGHT AND MATTER
 SPECTROSCOPY MEASURES THE INTERACTION BETWEEN LIGHT AND MATTER c = c: speed of light 3.00 x 10 8 m/s (lamda): wavelength (m) (nu): frequency (Hz) Increasing E (J) Increasing (Hz) E = h h - Planck s constant
SPECTROSCOPY MEASURES THE INTERACTION BETWEEN LIGHT AND MATTER c = c: speed of light 3.00 x 10 8 m/s (lamda): wavelength (m) (nu): frequency (Hz) Increasing E (J) Increasing (Hz) E = h h - Planck s constant
RS DYNAMICS ECOPROBE 5. Portable IR/PID Gas Analyzer PID. PID and IR Analyzers
 RS DYNAMICS ECOPROBE 5 Portable IR/PID Gas Analyzer PID + IR PID and IR Analyzers General ECOPROBE 5 has two autonomous analyzers in one case. The combination of analyzers provides a set of data designed
RS DYNAMICS ECOPROBE 5 Portable IR/PID Gas Analyzer PID + IR PID and IR Analyzers General ECOPROBE 5 has two autonomous analyzers in one case. The combination of analyzers provides a set of data designed
BENZENE & AROMATIC COMPOUNDS
 BENZENE & AROMATIC COMPOUNDS Dr. Zainab M Almarhoon 2 Learning Objectives By the end of chapter four the students will: Understand the resonance description of structure of benzene Understand the hybridization
BENZENE & AROMATIC COMPOUNDS Dr. Zainab M Almarhoon 2 Learning Objectives By the end of chapter four the students will: Understand the resonance description of structure of benzene Understand the hybridization
Welcome to Organic Chemistry II
 Welcome to Organic Chemistry II Erika Bryant, Ph.D. erika.bryant@hccs.edu Class Syllabus 3 CHAPTER 12: STRUCTURE DETERMINATION 4 What is this solution Soda Tea Coffee??? 5 What is this solution Soda Tea
Welcome to Organic Chemistry II Erika Bryant, Ph.D. erika.bryant@hccs.edu Class Syllabus 3 CHAPTER 12: STRUCTURE DETERMINATION 4 What is this solution Soda Tea Coffee??? 5 What is this solution Soda Tea
Simulating chemistry on interstellar dust grains in the laboratory
 From Stars to Life, April 3-6 2013, Gainesville FL Simulating chemistry on interstellar dust grains in the laboratory Nicolas Polfer University of Florida Department of Chemistry http://www.cosmotography.com/images/cosmic_nurseries.html
From Stars to Life, April 3-6 2013, Gainesville FL Simulating chemistry on interstellar dust grains in the laboratory Nicolas Polfer University of Florida Department of Chemistry http://www.cosmotography.com/images/cosmic_nurseries.html
18.1 Arenes benzene compounds Answers to Exam practice questions
 Pages 230 232 1 a) Benzene has a planar molecule ; with six carbon atoms in a regular hexagon. Each carbon atom forms a normal covalent ( ) bond with its two adjacent carbons atoms and a hydrogen atom.
Pages 230 232 1 a) Benzene has a planar molecule ; with six carbon atoms in a regular hexagon. Each carbon atom forms a normal covalent ( ) bond with its two adjacent carbons atoms and a hydrogen atom.
Radiant energy is proportional to its frequency (cycles/s = Hz) as a wave (Amplitude is its height) Different types are classified by frequency or
 CHEM 241 UNIT 5: PART B INFRA-RED RED SPECTROSCOPY 1 Spectroscopy of the Electromagnetic Spectrum Radiant energy is proportional to its frequency (cycles/s = Hz) as a wave (Amplitude is its height) Different
CHEM 241 UNIT 5: PART B INFRA-RED RED SPECTROSCOPY 1 Spectroscopy of the Electromagnetic Spectrum Radiant energy is proportional to its frequency (cycles/s = Hz) as a wave (Amplitude is its height) Different
Chapter 12 Structure Determination: Mass Spectrometry and Infrared Spectroscopy
 Chapter 12 Structure Determination: Mass Spectrometry and Infrared Spectroscopy Figure 12.1 - The electron-ionization, magneticsector mass spectrometer Representing the Mass Spectrum Base Peak Parent
Chapter 12 Structure Determination: Mass Spectrometry and Infrared Spectroscopy Figure 12.1 - The electron-ionization, magneticsector mass spectrometer Representing the Mass Spectrum Base Peak Parent
CHEM Chapter 12 Infrared and Mass Spec (homework). Stafford. S18
 Exhibit 12-4 The following question(s) refer to the mass spectrum shown below. 1. Refer to Exhibit 12-4. This compound contains C, H, and one other atom. Identify the other atom from the mass spectrum
Exhibit 12-4 The following question(s) refer to the mass spectrum shown below. 1. Refer to Exhibit 12-4. This compound contains C, H, and one other atom. Identify the other atom from the mass spectrum
Supplement of A new source of methylglyoxal in the aqueous phase
 Supplement of Atmos. Chem. Phys., 16, 2689 2702, 2016 http://www.atmos-chem-phys.net/16/2689/2016/ doi:10.5194/acp-16-2689-2016-supplement Author(s) 2016. CC Attribution 3.0 License. Supplement of A new
Supplement of Atmos. Chem. Phys., 16, 2689 2702, 2016 http://www.atmos-chem-phys.net/16/2689/2016/ doi:10.5194/acp-16-2689-2016-supplement Author(s) 2016. CC Attribution 3.0 License. Supplement of A new
235 Organic II. Final Exam Review REACTIONS OF CONJUGATED DIENES 1,2 VS 1,4 ADDITION REACTIONS OF CONJUGATED DIENES
 b. the ompound 7 i 1 Spectral Data: singlet, 196.5 ppm singlet, 14.1 ppm singlet, 14.4 ppm doublet, 19.1 ppm doublet, 18.5 ppm 1 MR Mass Spectrum Absorbance Intensity Infrared Spectrum 65 91 9. Structure:
b. the ompound 7 i 1 Spectral Data: singlet, 196.5 ppm singlet, 14.1 ppm singlet, 14.4 ppm doublet, 19.1 ppm doublet, 18.5 ppm 1 MR Mass Spectrum Absorbance Intensity Infrared Spectrum 65 91 9. Structure:
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS
 AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
Formation of Peroxy Radicals from OH-Toluene Adducts and O 2
 6092 J. Phys. Chem. A 2001, 105, 6092-6101 Formation of Peroxy Radicals from OH-Toluene Adducts and O 2 Birger Bohn Institut für Chemie und Dynamik der Geosphäre, Institut II: Troposphäre, Forschungszentrum
6092 J. Phys. Chem. A 2001, 105, 6092-6101 Formation of Peroxy Radicals from OH-Toluene Adducts and O 2 Birger Bohn Institut für Chemie und Dynamik der Geosphäre, Institut II: Troposphäre, Forschungszentrum
GCE A level 1094/01 CHEMISTRY CH4
 Surname ther Names Centre 2 Candidate GCE A level 1094/01 CHEMISTRY CH4 P.M. MNDAY, 14 January 2013 1¾ hours ADDITINAL MATERIALS In addition to this examination paper, you will need: Data Sheet Periodic
Surname ther Names Centre 2 Candidate GCE A level 1094/01 CHEMISTRY CH4 P.M. MNDAY, 14 January 2013 1¾ hours ADDITINAL MATERIALS In addition to this examination paper, you will need: Data Sheet Periodic
UV-Vis Spectroscopy. Chem 744 Spring Gregory R. Cook, NDSU Thursday, February 14, 13
 UV-Vis Spectroscopy Chem 744 Spring 2013 UV-Vis Spectroscopy Every organic molecule absorbs UV-visible light Energy of electronic transitions saturated functionality not in region that is easily accessible
UV-Vis Spectroscopy Chem 744 Spring 2013 UV-Vis Spectroscopy Every organic molecule absorbs UV-visible light Energy of electronic transitions saturated functionality not in region that is easily accessible
Measurement of VOC Reactivities Using a Photochemical Flow Reactor
 Environ. Sci. Technol. 1998, 32, 1913-1919 Measurement of VOC Reactivities Using a Photochemical Flow Reactor MICHAEL D. HURLEY,* TAI Y. CHANG, STEVEN M. JAPAR, AND TIMOTHY J. WALLINGTON Ford Research
Environ. Sci. Technol. 1998, 32, 1913-1919 Measurement of VOC Reactivities Using a Photochemical Flow Reactor MICHAEL D. HURLEY,* TAI Y. CHANG, STEVEN M. JAPAR, AND TIMOTHY J. WALLINGTON Ford Research
Visible and IR Absorption Spectroscopy. Andrew Rouff and Kyle Chau
 Visible and IR Absorption Spectroscopy Andrew Rouff and Kyle Chau The Basics wavelength= (λ) original intensity= Ι o sample slab thickness= dl Final intensity= I f ε = molar extinction coefficient -di=
Visible and IR Absorption Spectroscopy Andrew Rouff and Kyle Chau The Basics wavelength= (λ) original intensity= Ι o sample slab thickness= dl Final intensity= I f ε = molar extinction coefficient -di=
CHEM 241 UNIT 5: PART A DETERMINATION OF ORGANIC STRUCTURES BY SPECTROSCOPIC METHODS [MASS SPECTROMETRY]
![CHEM 241 UNIT 5: PART A DETERMINATION OF ORGANIC STRUCTURES BY SPECTROSCOPIC METHODS [MASS SPECTROMETRY] CHEM 241 UNIT 5: PART A DETERMINATION OF ORGANIC STRUCTURES BY SPECTROSCOPIC METHODS [MASS SPECTROMETRY]](/thumbs/83/88348834.jpg) CHEM 241 UNIT 5: PART A DETERMINATION OF ORGANIC STRUCTURES BY SPECTROSCOPIC METHODS [MASS SPECTROMETRY] 1 Introduction Outline Mass spectrometry (MS) 2 INTRODUCTION The analysis of the outcome of a reaction
CHEM 241 UNIT 5: PART A DETERMINATION OF ORGANIC STRUCTURES BY SPECTROSCOPIC METHODS [MASS SPECTROMETRY] 1 Introduction Outline Mass spectrometry (MS) 2 INTRODUCTION The analysis of the outcome of a reaction
Chapter 12 Mass Spectrometry and Infrared Spectroscopy
 Organic Chemistry, 6 th Edition L. G. Wade, Jr. Chapter 12 Mass Spectrometry and Infrared Spectroscopy Jo Blackburn Richland College, Dallas, TX Dallas County Community College District 2006, Prentice
Organic Chemistry, 6 th Edition L. G. Wade, Jr. Chapter 12 Mass Spectrometry and Infrared Spectroscopy Jo Blackburn Richland College, Dallas, TX Dallas County Community College District 2006, Prentice
* * Cambridge International Examinations Cambridge International Advanced Level
 *3272601861* Cambridge International Examinations Cambridge International Advanced Level CHEMISTRY 9701/42 Paper 4 Structured Questions ctober/november 2014 2 hours Candidates answer on the Question Paper.
*3272601861* Cambridge International Examinations Cambridge International Advanced Level CHEMISTRY 9701/42 Paper 4 Structured Questions ctober/november 2014 2 hours Candidates answer on the Question Paper.
Increasing energy. ( 10 4 cm -1 ) ( 10 2 cm -1 )
 The branch of science which deals with the interaction of electromagnetic radiation with matter is called spectroscopy The energy absorbed or emitted in each transition corresponds to a definite frequency
The branch of science which deals with the interaction of electromagnetic radiation with matter is called spectroscopy The energy absorbed or emitted in each transition corresponds to a definite frequency
Spectroscopy. Page 1 of 8 L.Pillay (2012)
 Spectroscopy Electromagnetic radiation is widely used in analytical chemistry. The identification and quantification of samples using electromagnetic radiation (light) is called spectroscopy. Light has
Spectroscopy Electromagnetic radiation is widely used in analytical chemistry. The identification and quantification of samples using electromagnetic radiation (light) is called spectroscopy. Light has
CH-442. Photochemistry I. Prof. Jacques-E. Moser.
 CH-442 Photochemistry I Prof. Jacques-E. Moser http://photochemistry.epfl.ch/pc.html Content PHOTOCHEMISTRY I 1. Basic principles 1.1 Introduction 1.2 Laws of light absorption 1.3 Radiation and molecular
CH-442 Photochemistry I Prof. Jacques-E. Moser http://photochemistry.epfl.ch/pc.html Content PHOTOCHEMISTRY I 1. Basic principles 1.1 Introduction 1.2 Laws of light absorption 1.3 Radiation and molecular
Hydrogen Abstraction/Acetylene Addition Revealed
 Hydrogen Abstraction/Acetylene Addition Revealed Dorian S. N. Parker, Ralf I. Kaiser,* Tyler P. Troy, and Musahid Ahmed* University of Hawaii at Manoa, USA Lawrence Berkeley National Laboratory, USA Angew.
Hydrogen Abstraction/Acetylene Addition Revealed Dorian S. N. Parker, Ralf I. Kaiser,* Tyler P. Troy, and Musahid Ahmed* University of Hawaii at Manoa, USA Lawrence Berkeley National Laboratory, USA Angew.
Terms used in UV / Visible Spectroscopy
 Terms used in UV / Visible Spectroscopy Chromophore The part of a molecule responsible for imparting color, are called as chromospheres. OR The functional groups containing multiple bonds capable of absorbing
Terms used in UV / Visible Spectroscopy Chromophore The part of a molecule responsible for imparting color, are called as chromospheres. OR The functional groups containing multiple bonds capable of absorbing
Infrared Spectroscopy
 Infrared Spectroscopy IR Spectroscopy Used to identify organic compounds IR spectroscopy provides a 100% identification if the spectrum is matched. If not, IR at least provides information about the types
Infrared Spectroscopy IR Spectroscopy Used to identify organic compounds IR spectroscopy provides a 100% identification if the spectrum is matched. If not, IR at least provides information about the types
Atomic Absorption Spectroscopy
 CH 2252 Instrumental Methods of Analysis Unit IV Atomic Absorption Spectroscopy Dr. M. Subramanian Associate Professor Department of Chemical Engineering Sri Sivasubramaniya Nadar College of Engineering
CH 2252 Instrumental Methods of Analysis Unit IV Atomic Absorption Spectroscopy Dr. M. Subramanian Associate Professor Department of Chemical Engineering Sri Sivasubramaniya Nadar College of Engineering
Mass Spectrometry Instrumentation
 Mass Spectrometry Instrumentation A mass spectrometer is composed of an inlet system (which introduces the sample to the instrument and vaporizes the sample) A molecular leak (which produces a steady stream
Mass Spectrometry Instrumentation A mass spectrometer is composed of an inlet system (which introduces the sample to the instrument and vaporizes the sample) A molecular leak (which produces a steady stream
Training Coarse ECOPROBE 5 CHEMISTRY RS DYNAMICS
 Training Coarse ECOPROBE 5 CHEMISTRY Contents Ecoprobe analytical principles Photoionization Infrared spectroscopy Oxygen measurement Pollutants Types and responses Head space New application of Ecoprobe
Training Coarse ECOPROBE 5 CHEMISTRY Contents Ecoprobe analytical principles Photoionization Infrared spectroscopy Oxygen measurement Pollutants Types and responses Head space New application of Ecoprobe
Jee Advance 2014 PART II - CHEMISTRY
 R Prerna Tower, Road No - 2, Contractors Area, Bistupur, Jamshedpur - 831001, Tel - (0657)2221892, www.prernaclasses.com Jee Advance 2014 Paper- II PART II - CHEMISTRY 24. For the identification of -napthol
R Prerna Tower, Road No - 2, Contractors Area, Bistupur, Jamshedpur - 831001, Tel - (0657)2221892, www.prernaclasses.com Jee Advance 2014 Paper- II PART II - CHEMISTRY 24. For the identification of -napthol
AP Chem Chapter 14 Study Questions
 Class: Date: AP Chem Chapter 14 Study Questions 1. A burning splint will burn more vigorously in pure oxygen than in air because a. oxygen is a reactant in combustion and concentration of oxygen is higher
Class: Date: AP Chem Chapter 14 Study Questions 1. A burning splint will burn more vigorously in pure oxygen than in air because a. oxygen is a reactant in combustion and concentration of oxygen is higher
Nitration of (Trifluoromethyl( Trifluoromethyl)benzene CF 3 HNO 3 + +
 Effect on Rate Rate and Regioselectivity in Electrophilic Aromatic Substitution A substituent already present on the ring affects both the rate and regioselectivity of electrophilic aromatic substitution.
Effect on Rate Rate and Regioselectivity in Electrophilic Aromatic Substitution A substituent already present on the ring affects both the rate and regioselectivity of electrophilic aromatic substitution.
Chapter 17 Reactions of Aromatic Compounds
 rganic Chemistry, 6 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds Jo Blackburn Richland College, Dallas, TX Dallas County Community College District 2006, Prentice all Electrophilic
rganic Chemistry, 6 th Edition L. G. Wade, Jr. Chapter 17 Reactions of Aromatic Compounds Jo Blackburn Richland College, Dallas, TX Dallas County Community College District 2006, Prentice all Electrophilic
Chemical kinetics in the gas phase
 Chemical kinetics in the gas phase Chemical kinetics is the study of the rates of transformation of chemical compounds from reactant species into products. The rate of a reaction is defined to be the rate
Chemical kinetics in the gas phase Chemical kinetics is the study of the rates of transformation of chemical compounds from reactant species into products. The rate of a reaction is defined to be the rate
Infrared Spectroscopy
 Infrared Spectroscopy Introduction Spectroscopy is an analytical technique which helps determine structure. It destroys little or no sample. The amount of light absorbed by the sample is measured as wavelength
Infrared Spectroscopy Introduction Spectroscopy is an analytical technique which helps determine structure. It destroys little or no sample. The amount of light absorbed by the sample is measured as wavelength
1. How do you account for the formation of ethane during chlorination of methane?
 1. How do you account for the formation of ethane during chlorination of methane? The formation of ethane is due to the side reaction in termination step by the combination of two CH 3 free radicals. 2.
1. How do you account for the formation of ethane during chlorination of methane? The formation of ethane is due to the side reaction in termination step by the combination of two CH 3 free radicals. 2.
More information can be found in Chapter 12 in your textbook for CHEM 3750/ 3770 and on pages in your laboratory manual.
 CHEM 3780 rganic Chemistry II Infrared Spectroscopy and Mass Spectrometry Review More information can be found in Chapter 12 in your textbook for CHEM 3750/ 3770 and on pages 13-28 in your laboratory manual.
CHEM 3780 rganic Chemistry II Infrared Spectroscopy and Mass Spectrometry Review More information can be found in Chapter 12 in your textbook for CHEM 3750/ 3770 and on pages 13-28 in your laboratory manual.
Ozone Formation in Coastal Urban Atmospheres: The Role of Anthropogenic Sources of Chlorine
 Ozone Formation in Coastal Urban Atmospheres: The Role of Anthropogenic Sources of Chlorine, Sarah Oldfield, Charles B. Mullins, David T. Allen In this communication, we present experimental results from
Ozone Formation in Coastal Urban Atmospheres: The Role of Anthropogenic Sources of Chlorine, Sarah Oldfield, Charles B. Mullins, David T. Allen In this communication, we present experimental results from
IR, MS, UV, NMR SPECTROSCOPY
 CHEMISTRY 318 IR, MS, UV, NMR SPECTROSCOPY PROBLEM SET All Sections CHEMISTRY 318 IR, MS, UV, NMR SPECTROSCOPY PROBLEM SET General Instructions for the 318 Spectroscopy Problem Set Consult the Lab Manual,
CHEMISTRY 318 IR, MS, UV, NMR SPECTROSCOPY PROBLEM SET All Sections CHEMISTRY 318 IR, MS, UV, NMR SPECTROSCOPY PROBLEM SET General Instructions for the 318 Spectroscopy Problem Set Consult the Lab Manual,
Current Research in European Environmental Chambers: Measurements and Models
 Current Research in European Environmental Chambers: Measurements and Models Theo Brauers ICG-II (Tropospäre) Forschungszentrum Jülich, Germany contribution to International Conference Atmospheric Chemistry
Current Research in European Environmental Chambers: Measurements and Models Theo Brauers ICG-II (Tropospäre) Forschungszentrum Jülich, Germany contribution to International Conference Atmospheric Chemistry
Infrared Spectroscopy: Identification of Unknown Substances
 Infrared Spectroscopy: Identification of Unknown Substances Suppose a white powder is one of the four following molecules. How can they be differentiated? H N N H H H H Na H H H H H A technique that is
Infrared Spectroscopy: Identification of Unknown Substances Suppose a white powder is one of the four following molecules. How can they be differentiated? H N N H H H H Na H H H H H A technique that is
Ch.16 Chemistry of Benzene: Electrophilic Aromatic Substitution
 Ch.16 Chemistry of Benzene: Electrophilic Aromatic Substitution Electrophilic aromatic substitution: E + E + + Some electrophilic aromatic substitution: X N 2 S 3 R C R alogenation Nitration Sulfonation
Ch.16 Chemistry of Benzene: Electrophilic Aromatic Substitution Electrophilic aromatic substitution: E + E + + Some electrophilic aromatic substitution: X N 2 S 3 R C R alogenation Nitration Sulfonation
Time Allowed: 60 minutes MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 INTRODUCTION TO ORGANIC AND BIOCHEMISTRY QUIZ 5 Time Allowed: 60 minutes MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What is the IUPAC name
INTRODUCTION TO ORGANIC AND BIOCHEMISTRY QUIZ 5 Time Allowed: 60 minutes MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) What is the IUPAC name
Molecular Spectroscopy. H 2 O e -
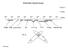 Molecular Spectroscopy ν (cm -1 ) λ (cm) 10 6 10 8 10 10 10 12 10 14 10 16 10 18 10 20 10 22 ν (Hz) NMR ESR microwave IR UV/Vis VUV X-Ray Gamma Ray H 2 e - UV/Vis Spectroscopy absorption technique X hν
Molecular Spectroscopy ν (cm -1 ) λ (cm) 10 6 10 8 10 10 10 12 10 14 10 16 10 18 10 20 10 22 ν (Hz) NMR ESR microwave IR UV/Vis VUV X-Ray Gamma Ray H 2 e - UV/Vis Spectroscopy absorption technique X hν
