Determination of Calories in Food Via Adiabatic Bomb Calorimeter
|
|
|
- Millicent Daniel
- 6 years ago
- Views:
Transcription
1 The Corinthian Volume 6 Article Determination of Calories in Food Via Adiabatic Bomb Calorimeter Kenneth C. McGill Georgia College Michelle L. Yasechko Georgia College Wendy K. Nkari Georgia College Follow this and additional works at: Part of the Chemistry Commons Recommended Citation McGill, Kenneth C.; Yasechko, Michelle L.; and Nkari, Wendy K. (2004) "Determination of Calories in Food Via Adiabatic Bomb Calorimeter," The Corinthian: Vol. 6, Article 9. Available at: This Article is brought to you for free and open access by Knowledge Box. It has been accepted for inclusion in The Corinthian by an authorized editor of Knowledge Box.
2 The Corinthian: The Journal of Student Research at GC&SU Determination of Calories in Food Via Adiabatic Bomb Calorimeter Dr. Kenneth C. McGill Michelle L. Yasechko Chemistry Major Wendy K. Nkari Chemistry Major Dr. Kenneth C. McGill Faculty Sponsor The adiabatic bomb calorimeter has been an effective tool in facili tating heat transfer between molecules via combustion reaction.1 Heat is released from the substance combusted and transferred into another, usually water. The heat transferred is measured and the enthalpy of combustion determined for the combusted material. One such classic experiment is the determination of combustion enthalpy of sucrose, obtaining the calorimeter constant with benzoic acid. The results of an adiabatic combustion experiment can be taken one step further to determine the combustion enthalpy in kcal/g by a simple conversion calculation. Since food nutrition labels report calories per grams of serving (note: 1 food calorie" 1 kcal), food can be combusted in an adiabatic bomb calorimeter and the results compared to its corresponding nutrition label. The food chosen for this experiment consisted of marshmallows and cheddar cheese. Introduction In 1881, a scientist named Berthelot invented a closed vessel in which a controlled combustion could take place, which he referred to as a bomb. He knew that many substances, particularly hydrocarbons, wi ll com bust in the presence of oxygen to form water and carbon dioxide. A general hydrocarbon combustion reaction can be written as follows: The balanced version of this reaction would be: Commonly, fuels such as gasolines usually come to mind when mentioning hydrocarbons. However, any organic compound can be referred to as fuel because any organic compound, whether gasoline, carbohydrates, sugars, or proteins, can be combusted in an oxygen rich environment, releasing energy as heat to its surroundings. Just as gasoline provides the energy to propel vehicles, food provides the energy to drive muscular functions. The common reaction that occurs in all fuel combustions follows: 92
3 Determination of Calories in Food Via Adiabatic Bomb Calorimeter organic compound + oxygen _ carbon dioxide + water + energy And for proteins: protein + oxygen _ carbon dioxide + water + ammonia + energy The energy resulting from combustion reactions can be measured in an adiabatic bomb calorimeter. In an adiabatic bomb calorimeter the bomb, which is the vessel containing the sample and where the combustion takes place, is pressurized with 02 to ensure complete combustion, and then submersed in a known quantity of water. Both the bomb and the water are brought to temperature equilibrium before initiating combustion. To achieve sufficient heat for the combustion, the sample is in contact with a fuse wire, which ignites the sample when current is passed through the wire. The heat resulting from the combustion is transferred to the water surrounding the bomb and is measured with a thermometer or resistive temperature device, RTD. Operation of the actiabatic bomb calorimeter is based on the First Law of Thermodynamics: heat lost (by the sample) = heat gained (by the water). 3 The heat gained by the water from the complete combustion of the sample is proportional to the temperature change of the water. Therefore, accurately recorcting the temperature of the water before and after the combustion is the key to success of this experiment. Since the temperature change,.1t, will be determined, along with the calorimeter constant, C, the heat given off by the sample in the combustion, Q, can be calculated from the relation: Q = C.1T ( equation 1) In order to compare the heat of combustion of food to its corresponding nutrition label value, its enthalpy of combustion must first be calculated. A.n equation for calculating the heat of combustion of food can be derived ft-0111 equation 1. Since the combustion occurs at constant volume, equation 1 can be written as: ( equation 2) And, since at constant volume.1u = Cvl, equation 2 can be written as: U = Cv.1T ( equation 3) Also, enthalpy and internal energy are related in the expression: H =.1U +.1(pV) (equation 4 ) 93
4 The Corinthian: The Journal of Student Research at GC&SU Since the contribution to Li(pV) from the net change in pv of solids going from reactants to products is generally negligible, equation 4 can be written as: H = LiU ( equation 5) Now, substituting LiH in for LiU in equation 3 gives: H = CLiT ( equation 6) For enhanced accuracy of data, an RTD was used instead of a thermometer. An RTD outputs data in resistance ( as the temperature increases, its resistance increases). It follows that equation 6 becomes: H= C_R ( equation 7) where LiR is the change in resistance proportional to change in temperature. Benzoic Acid Combustion As mentioned previously, a sample with a known heat of combustion, benzoic acid, was combusted in order to obtain the calorimeter constant, C, in equations 1-7. Therefore, equation 7 is rearranged: C = LiH/LiU ( equation 8) Since the sample must be in contact with a fuse wire for the combustion to take place, the fuse wire is combusted with the sample. The fuse wire therefore must be accounted for in all calculations. To incorporate the fuse wire into each combustion, equation 8 can be expanded: C =.(mbw- m~)*lihb...±jm.~ - mwa)*lih~ Rf- Rj (equation 9) Where mbw is the mass of benzoic acid + wire, mw is the mass of the wire, _Hb is the enthalpy of combustion of benzoic acid, mwa is the mass of the wire after the combustion, _Hw is the enthalpy of combustion of the wire and is also a known value, Rj is the initial resistance, and Rf is the final resistance. 94
5 Determination of Calories in Food Via Adiabatic Bomb Calorimeter Food Combustion In this experiment marshmallows and cheese were combusted. To calculate each combustion, 11Hmm and _Heh takes the place of 11Hh respectively, and mmmw and mchw takes the place of mbw respectively in eq uation 9. Then, equation 9 is rearranged to give and, Hmm= -(C*Rf- C*~ - 11Hwmw + 11H~ *mwa) (equation IO ) (-mmmw + mw) Heh= -(C*Rf- C*~ - 11Hwmw + 11Hw*mwa) ( -mchw + mw) respectively. Finally, the resulting enthalpy of combustion, calculated in units of kilojoules/gram is converted to kilocalories/gram. Experimental Benzoic acid was employed to obtain the heat capacity of the calorimeter. First, approximately 1.00 g of benzoic acid was weighed, pressed into a pellet, and then re -weighed. A 10 cm piece of ignition wire was cut, cleaned with acetone, and also weighed. The fuse wire was carefully fused into the center of the benzoic acid pellet using a 9-volt battery. N.ext, the sample holder, previously cleaned with soap and water and then with acetone, was removed from the likewise cleaned bomb vessel. The benzoic acid pellet was then connected to the two terminals of the sample holder by carefully threading each end of the fuse wire through the terminals and then wrapping the excess fuse wire around the terminals on the sample holder. Caution was taken so that the ends of the fuse wire were turned inward and not touching the sides of bomb vessel in order to prevent shorting against the bomb vessel. The sample holder was gently lowered into the vessel and the bomb lid was properly assembled onto the bomb vessel, ensuring a good seal. The hose from an oxygen tank was irmly connected to the inlet valve located on top of the bomb. The bomb as slowly pressurized to 30 ± latm. By carefully opening the release alve, the bomb was slowly vented to O ±1 atm. The bomb was then reressurized and re-vented two more times. Finally, the bomb was pressurzed again to 30 ± 1 atm to ensure that as much pure oxygen as possible as contained in the bomb. Then the shutoff valve on the oxygen bottle as turned off, the oxygen line was vented, and removed from tl1e bomb. 95
6 The Corinthian: The Journal of Student Research at GC&SU Using a digital multimeter, the continuity of the two terminals located on top of the bomb was read. The reading obtained should be 1 ohm or less. Next, the water bucket is filled with approximately 2000 ±1 ml of tap water, obtained by using a 1 L volumetric flask. To ensure the exact mass of water was for every bomb ignition, the water bucket was placed on a balance and balanced with weights. The weights needed to balance the water were recorded and the bucket removed. The bucket of water was then placed inside of the calorimeter. Bomb tongs were attached to the bomb vessel, and the bomb was lowered into the water. Electrodes of the apparatus were then plugged into their appropriate positions on top of the bomb. The bomb tongs were then carefully removed. (Note: ensure that no bubbling appears from the bomb, which could signal a leak.) The adiabatic jacket cover was secured and its attached RTD was lowered into position. The electric stirrer was then lowered and the system was allowed to reach temperature equilibrium (approximately 10 minutes). Resistance readings were monitored via applicable software. The charging switch of the calorimeter was turned on. Then the ignition button was depressed until illumination of the ignition indicating light was observed. The button was then released (Note: the ignition button should not be depressed for more than 4 seconds otherwise damage may occur). The resistance was then observed for an increase, indicating successful combustion. Resistance readings were allowed to stabilize for approximately 10 ±1 minutes. The system was then turned off and the bomb was removed from the apparatus and vented. Using Microsoft Excel, the data collected were saved. The fuse wire remaining was then weighed. The bomb was cleaned and the procedure was repeated two more times employing benzoic acid, three times with marshmallows, and three times with cheddar cheese cubes (Note: the weight of marshmallows was approximately 2 grams while that of cheese cubes was approximately 1 gram). Employing the saved Microsoft Excel file, the output of the RTD was formatted into a graph. From the minimum and maximum resistance values on the graph, the initial and final resistance values were determined, along with standard deviations. In the absence of a minimum on any of the graphs, a linear regression was performed. Such a graph is displayed in Figure 1. 96
7 Determination of Calories in Food Via Adiabatic Bomb Calorimeter Table 1: "Marshmallow Combustion Graph" Marshmallow Combustion, Run 1 U) f t l g t J.l9 ; t GI ~ Time (sec) Results and Discussion T he calorimeter constant calculated from equation 9 was found to be (± 0.986) kj/q. The data employed for this calculation was recorded from the Benzoic Acid combustions and is shown in Table 1. Table 1: Benzoic Acid Combustion Data!Run 1:!Run 2:!Run~: Mass of Benzoic Acid + wire mb (±0.001 ) (±0.001 ) (±0.001) ' w.(grams): h. Mass of wire m ' w (grams): (± ) (±0.0001) (±0.0009) h.. Mass of wire after wa (grams): ' (±0.0007) (±0.0006) (±0.0007) h.. I.. nit,al resistance, R; (±0.0024) (±0.0018) (±0.0017) (ohms ):?-- 1 nal resistance, 1~ (± ) (±0.0016) l (±0.0021) (ohms): ~ 97
8 The Corinthian: The Journal of Student Research at GC&SU The enthalpy of combustion from the label attached to the nichrome fuse wire and the literature value for enthalpy of combustion of Benzoic Acidl were recorded as follows and incorporated into equation 9 as well : Hw: (±1 ) caljg Hb: (±1 ) kj/ mol Marshmallow Combustion The results of the marshmallow combustion experiment successfully elucidated the approximate kilocalories/ gram of marshmallows. The values from the following table, Table 2, were recorded and incorporated into equation 10: Table 2: Marshmallow Combustion Data Run 1 Run 2 Run3 J Mass of wire before, (± ) (±0.0004) (± ) mw (grams): Mass of wire after, mwa (grams) : (±0.0006) (±0.0008) (±0.0009) Mass of marshmalow + wire mmmw (±0.0007) (±0.0007) (0.0003) (grams): Initial resistance, Rj (ohms): (±0.0023) (±0.0024) (±0.0030) Final resistance, Rf (ohms): (±0.0030) (±0.0018) (±0.0020) I Table 3 shows the results calculated from equation 10. Table 3: Marshmallow Combustion Enthalpy KJ/g: Kcal/g: Runl : 20.1(±1.7 ) 4.8(±1.7) Run 2: 17. 5(±1.7) 4.2(±1.7) - Run 3: 23.1 (±2.1) 5.5(±2.1) 98
9 Determination of Calories in Food Via Adiabatic Bomb Calorimeter An average enthalpy of combustion for marshmallows was calculated to be 3.23(±0.99) kcal/ g. Prior to comparing this result to the nutrition label, the theoretical value was obtained by dividing the calories per serving by the grams per serving found on the nutrition label. The calories per serving were 100, and the grams per serving were 28, giving a theoretical value of3.6(±1.4) kcal/g and a percent difference of9.5 percent. Cheese Combustion The result obtained for the cheese combustion proved to be less successful than the marshmallow experiment. A larger percent difference was calculated from the results. Contributing to the decreased success of this combustion experiment was the observation of a residue in the bomb after the cheese combustion consisting of hard, tiny, white beads, presumed to be calcium. Further analysis is required to properly account for the non-combustible material found in food, such as minerals. Continuation of the cheese experiment would consist of analyzing the water formed in the combustion as well as analysis of the residue. The analysis of water would be best accomplished using Gas Chromatography Mass Spectrometry methods to identif)r the other compounds present in the water formed from the combustion. Analysis of the residue can be done by doing various tests depending on the food label, for example in this case, a protein test and a lipid test can be done4.. The values from the following table, Table 4, were recorded and lllcorporated into equation 10: Table 4: Cheese Combustion Data Run 1 Rw12 RW13 Mass of wire before m ' w (± ) (±0.0008) (±0.0008) (grams): Mass of wire after, mwa (grams ): (±0.0002) (± ) (±0.0008) Mass of cheese + Mrire, mchw (±0.0009) (± ) (±0.0014) (grams ): Initial resistance 2\(ohms): ' (±0.0014) (±0.0013) (±0.0016) Final resistance Rt (ohms) : ' (±0.0018) (±0.0020) (±0.0020) - 99
10 The Corinthian: The Journal of Student Research at GC&SU Table 5 shows the results calculated from equation 10. Table 5: Cheese Combustion Enthalpy KJ/g: Kcal/g: Run 1: (±0.99) 3.47(±0.99) Run 2: (±0.99) 3.37(±0.99 ) Run 3: 11.89(±0.99 ) 2.84(±0.99 ) An average enthalpy of combustion for cheese was calculated to be 4.8(±1.8 ) kcal/g. Prior to comparing this result to the nutrition label, the theoretical value was obtained by dividing the calories per serving by the grams per serving found on the nutrition label. The calories per serving were llo, and the grams per serving were 28, giving a theoretical value of 3.9(±1.4) kcal/g and a percent difference of22.9 percent. The results of both marshmallow and cheese combustion experiments are shown in Table 6: Table 6: Combustion Enthalpy Comparison Experimental Nutrition Label Combustion: Kcal/g: Kcal/g Marshmallow 3.2(±1.7) 3.6 (±1.4) Cheese 4.8(±3.3) 3.9(±1.4) NB: The error analysis is an absolute value Conclusion The adiabatic bomb calorimeter provides a safe, simple, and effective means of measuring energies of combustion or many types of fuels, including food. Determination of calories in food, especially sugars and carbohydrates, is successfully accomplished via adiabatic calorimetry. LOO
11 Determination of Calories in Food Via Adiabatic Bomb Calorimeter Works Cited Atkins, P., et al. Physical Chemistry, 7th Ed., W.H. Freeman and Company, New York, 2002; pp 42-43, Campbell, N.A.; Reece, J.B. Biology, 6th Edition. Benjamin Cummings: San Francisco, 2002; pp , 172. Horm, Detton T., Basic Electronics Theory, 4th Edition, lab books, McGraw Hill, Inc., 1994, pp 430. Mader, S. S.; Biology Laboratory Manual, 7th Ed., Mc Graw Hill, New York, 2001; pp 28, 37. Oxtoby, D.W.; Gillis, H.P.; Nachtrieb, N.H. Principles of Modern Chemistry, 4th Edition. Saunders College Publishing, 1999; pp
Heat of Combustion. Parr oxygen bomb calorimeter (or equivalent), pellet press, thermometer (0.01 C), fuse wire.
 Heat of Combustion PURPOSE The purposes of this experiment are to determine the heats of combustion of several related substances using a bomb calorimeter and relate differences in heats of combustion
Heat of Combustion PURPOSE The purposes of this experiment are to determine the heats of combustion of several related substances using a bomb calorimeter and relate differences in heats of combustion
Bomb Calorimetry. Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise.
 Experiment 12 Bomb Calorimetry Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. Purpose A bomb calorimeter will
Experiment 12 Bomb Calorimetry Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. Purpose A bomb calorimeter will
Physical Chemistry Laboratory [CHEM 335] BOMB CALORIMETRY Adriana Biney and Silmilly Toribio
![Physical Chemistry Laboratory [CHEM 335] BOMB CALORIMETRY Adriana Biney and Silmilly Toribio Physical Chemistry Laboratory [CHEM 335] BOMB CALORIMETRY Adriana Biney and Silmilly Toribio](/thumbs/87/96015679.jpg) Physical Chemistry Laboratory [CHEM 335] BOMB CALORIMETRY Adriana Biney and Silmilly Toribio ABSTRACT In this experiment a Parr 1341 Plain Jacket Oxygen Bomb Calorimeter was employed to determine the enthalpy
Physical Chemistry Laboratory [CHEM 335] BOMB CALORIMETRY Adriana Biney and Silmilly Toribio ABSTRACT In this experiment a Parr 1341 Plain Jacket Oxygen Bomb Calorimeter was employed to determine the enthalpy
Experiment 1: Adiabatic Bomb Calorimeter (Dated: August 25, 2009)
 Experiment 1: Adiabatic Bomb Calorimeter (Dated: August 25, 2009) I. INTRODUCTION Heat released in a chemical reaction can be determined experimentally by using an adiabatic calorimeter. The reaction must
Experiment 1: Adiabatic Bomb Calorimeter (Dated: August 25, 2009) I. INTRODUCTION Heat released in a chemical reaction can be determined experimentally by using an adiabatic calorimeter. The reaction must
The Enthalpy of Formation of Camphor by Bomb Calorimetry 1
 The Enthalpy of Formation of Camphor by Bomb Calorimetry 1 Purpose: The enthalpy of combustion of camphor will be determined in a bomb calorimeter. The enthalpy of formation of camphor will be calculated
The Enthalpy of Formation of Camphor by Bomb Calorimetry 1 Purpose: The enthalpy of combustion of camphor will be determined in a bomb calorimeter. The enthalpy of formation of camphor will be calculated
Determination of Resonance Stabilization Energy of Benzene Jonathan Smith (With Small Adaptations by Amanda Nienow)
 Determination of Resonance Stabilization Energy of Benzene Jonathan Smith (With Small Adaptations by Amanda Nienow) Abstract In this investigation we will measure the heat evolved during the combustion
Determination of Resonance Stabilization Energy of Benzene Jonathan Smith (With Small Adaptations by Amanda Nienow) Abstract In this investigation we will measure the heat evolved during the combustion
Lab 2. Molar enthalpy of combustion
 Lab 2. Molar enthalpy of combustion Introduction Chemical reactions like burning an organic compound typically produce heat. In this experiment you will be measuring the amount of heat produced by burning
Lab 2. Molar enthalpy of combustion Introduction Chemical reactions like burning an organic compound typically produce heat. In this experiment you will be measuring the amount of heat produced by burning
PROPULSION LAB MANUAL
 PROPULSION LAB MANUAL Measurement of Calorific Value of a Solid Fuel Sample using a Bomb Calorimeter DEPARTMENT OF AEROSPACE ENGINEERING Indian Institute of Technology Kharagpur CONTENTS 1. Introduction
PROPULSION LAB MANUAL Measurement of Calorific Value of a Solid Fuel Sample using a Bomb Calorimeter DEPARTMENT OF AEROSPACE ENGINEERING Indian Institute of Technology Kharagpur CONTENTS 1. Introduction
Chemistry: The Central Science. Chapter 5: Thermochemistry
 Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
This reaction is ENDOTHERMIC. Energy is being transferred from the room/flask/etc. (the SURROUNDINGS) to the reaction itself (the SYSTEM).
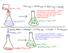 151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
- Joule (J): SI unit for energy. It's defined based on the equation for kinetic energy. from. mass. velocity
 153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
MEEN 3242 MEE LAB II
 MEEN 3242 MEE LAB II Experiment Report Lab # 2 Bomb Calorimeter Group Members: Hugo Diaz Reed Greenwood Juan Orona Alan Sanoja Micheal Shackelford Date: September 27, 2013 DEPARTMENT OF MECHANICAL AND
MEEN 3242 MEE LAB II Experiment Report Lab # 2 Bomb Calorimeter Group Members: Hugo Diaz Reed Greenwood Juan Orona Alan Sanoja Micheal Shackelford Date: September 27, 2013 DEPARTMENT OF MECHANICAL AND
ALE 26. Energy Changes ( E) and Enthalpy Changes ( H) in Chemical Reactions
 Name Chem 161, Section: Group Number: ALE 26. Energy Changes ( E) and Enthalpy Changes ( H) in Chemical Reactions (Reference: Chapter 6 - Silberberg 5 th edition) Important!! For answers that involve a
Name Chem 161, Section: Group Number: ALE 26. Energy Changes ( E) and Enthalpy Changes ( H) in Chemical Reactions (Reference: Chapter 6 - Silberberg 5 th edition) Important!! For answers that involve a
- Kinetic energy: energy of matter in motion. gravity
 148 2500 L of chlorine gas at 25.0 C and 1.00 atm are used to make hydrochloric acid. How many grams of hydrochloric acid could be produced if all the chlorine reacts? 1 - Convert 2500 L chlorine gas to
148 2500 L of chlorine gas at 25.0 C and 1.00 atm are used to make hydrochloric acid. How many grams of hydrochloric acid could be produced if all the chlorine reacts? 1 - Convert 2500 L chlorine gas to
Bomb Calorimetry: Heat of Combustion of Naphthalene
 Bomb Calorimetry: Heat of Combustion of Naphthalene Most tabulated H values of highly exothermic reactions come from bomb calorimeter experiments. Heats of combustion are most common, in which the combustible
Bomb Calorimetry: Heat of Combustion of Naphthalene Most tabulated H values of highly exothermic reactions come from bomb calorimeter experiments. Heats of combustion are most common, in which the combustible
Thermochemistry/Calorimetry. Determination of the enthalpy of combustion with a calorimetric bomb LEC 02. What you need:
 LEC 02 Thermochemistry/Calorimetry with a calorimetric bomb What you can learn about 1st law of thermodynamics Hess law Enthalpy of combustion Enthalpy of formation Heat capacity Principle and tasks The
LEC 02 Thermochemistry/Calorimetry with a calorimetric bomb What you can learn about 1st law of thermodynamics Hess law Enthalpy of combustion Enthalpy of formation Heat capacity Principle and tasks The
How many ml of 0.250M potassium permangenate are needed to react with 3.36 g of iron(ii) sulfate?
 115 How many ml of 0.250M potassium permangenate are needed to react with 3.36 g of iron(ii) sulfate? 1) Convert 3.36 grams iron(ii) sulfate to moles. Use FORMULA WEIGHT. 2) Convert moles iron(ii) sulfate
115 How many ml of 0.250M potassium permangenate are needed to react with 3.36 g of iron(ii) sulfate? 1) Convert 3.36 grams iron(ii) sulfate to moles. Use FORMULA WEIGHT. 2) Convert moles iron(ii) sulfate
Calculate the mass of L of oxygen gas at 25.0 C and 1.18 atm pressure.
 148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
AC : PC-BASED MEASUREMENT OF THE HEAT OF COMBUSTION OF A SOLID FUEL USING OXYGEN BOMB CALORIMETER
 AC 2007-2839: PC-BASED MEASUREMENT OF THE HEAT OF COMBUSTION OF A SOLID FUEL USING OXYGEN BOMB CALORIMETER Ramesh Prasad, University of New Brunswick-St. John Ramesh C. Prasad, Professor of Mechanical
AC 2007-2839: PC-BASED MEASUREMENT OF THE HEAT OF COMBUSTION OF A SOLID FUEL USING OXYGEN BOMB CALORIMETER Ramesh Prasad, University of New Brunswick-St. John Ramesh C. Prasad, Professor of Mechanical
4 BOMB CALORIMETRY. Chapter 4. Bomb Calorimetry 24 I. PREPARATION
 Chapter 4. Bomb Calorimetry 24 4 BOMB CALORIMETRY I. PREPARATION Read "Principles of Calorimetry" in Ch. VI of Shoemaker, Garland, and Nibler (SGN, pp. 145-151), as well as Experiment 6 (pp. 152-158).
Chapter 4. Bomb Calorimetry 24 4 BOMB CALORIMETRY I. PREPARATION Read "Principles of Calorimetry" in Ch. VI of Shoemaker, Garland, and Nibler (SGN, pp. 145-151), as well as Experiment 6 (pp. 152-158).
How bad is that snack anyway?
 Physical Sciences 11 Experiment 1 How bad is that snack anyway? Monday, 2/10 Wednesday, 2/12 Science Center Room 117 Please read this entire document and complete the attached prelab before your lab. This
Physical Sciences 11 Experiment 1 How bad is that snack anyway? Monday, 2/10 Wednesday, 2/12 Science Center Room 117 Please read this entire document and complete the attached prelab before your lab. This
Calorimetry and Hess s Law Prelab
 Calorimetry and Hess s Law Prelab Name Total /10 1. What is the purpose of this experiment? 2. Make a graph (using some kind of graphing computer software) of temperature vs. time for the following data:
Calorimetry and Hess s Law Prelab Name Total /10 1. What is the purpose of this experiment? 2. Make a graph (using some kind of graphing computer software) of temperature vs. time for the following data:
Thermochemistry. Using Heats of Reaction - Hess s Law - Standard Enthalpies of Formation - Fuels Foods, Commercial Fuels, and Rocket Fuels
 Thermochemistry Understanding Heats of Reaction - Energy and Its Units - Heat of Reaction - Enthalpy and Enthalpy Change - Thermochemical Equations - Applying Stoichiometry to Heats of Reaction - Measuring
Thermochemistry Understanding Heats of Reaction - Energy and Its Units - Heat of Reaction - Enthalpy and Enthalpy Change - Thermochemical Equations - Applying Stoichiometry to Heats of Reaction - Measuring
- The empirical gas laws (including the ideal gas equation) do not always apply.
 145 At 300 C, ammonium nitrate violently decomposes to produce nitrogen gas, oxygen gas, and water vapor. What is the total volume of gas that would be produced at 1.00 atm by the decomposition of 15.0
145 At 300 C, ammonium nitrate violently decomposes to produce nitrogen gas, oxygen gas, and water vapor. What is the total volume of gas that would be produced at 1.00 atm by the decomposition of 15.0
Experiment 2: THE DENSITY OF A SOLID UNKNOWN AND CALIBRATION WITH DATASTUDIO SOFTWARE
 Experiment 2: THE DENSITY OF A SOLID UNKNOWN AND CALIBRATION WITH DATASTUDIO SOFTWARE Concepts: Density Equipment Calibration Approximate time required: 90 minutes for density 90 minutes for two thermometers
Experiment 2: THE DENSITY OF A SOLID UNKNOWN AND CALIBRATION WITH DATASTUDIO SOFTWARE Concepts: Density Equipment Calibration Approximate time required: 90 minutes for density 90 minutes for two thermometers
EXPERIMENT 5. THE ENTHALPY OF FORMATION OF CAMPHOR BY BOMB CALORIMETRY 1
 EXPERIMENT 5. THE ENTHALPY OF FORMATION OF CAMPHOR BY BOMB CALORIMETRY 1 The enthalpies of combustion and formation for camphor will be determined using a bomb calorimeter and tabulated values for the
EXPERIMENT 5. THE ENTHALPY OF FORMATION OF CAMPHOR BY BOMB CALORIMETRY 1 The enthalpies of combustion and formation for camphor will be determined using a bomb calorimeter and tabulated values for the
First Law of Thermodynamics: energy cannot be created or destroyed.
 1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
Stoichiometry Rockets
 Stoichiometry Rockets The objective of this lab is to to: calculate the needed volume of fuel to react with a given volume of gas and result in a productive explosion determine the heat of the reaction
Stoichiometry Rockets The objective of this lab is to to: calculate the needed volume of fuel to react with a given volume of gas and result in a productive explosion determine the heat of the reaction
First Law of Thermodynamics
 Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Thermochemistry AP Chemistry Lecture Outline
 Thermochemistry AP Chemistry Lecture Outline Name: thermodynamics: the study of energy and its transformations -- thermochemistry: the subdiscipline involving chemical reactions and energy changes Energy
Thermochemistry AP Chemistry Lecture Outline Name: thermodynamics: the study of energy and its transformations -- thermochemistry: the subdiscipline involving chemical reactions and energy changes Energy
Thermochemistry Chapter 8
 Thermochemistry Chapter 8 Thermochemistry First law of thermochemistry: Internal energy of an isolated system is constant; energy cannot be created or destroyed; however, energy can be converted to different
Thermochemistry Chapter 8 Thermochemistry First law of thermochemistry: Internal energy of an isolated system is constant; energy cannot be created or destroyed; however, energy can be converted to different
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
Chapter 5 Thermochemistry Learning Outcomes: Interconvert energy units Distinguish between the system and the surroundings in thermodynamics Calculate internal energy from heat and work and state sign
PHYS320 ilab (O) Experiment 2 Instructions Conservation of Energy: The Electrical Equivalent of Heat
 PHYS320 ilab (O) Experiment 2 Instructions Conservation of Energy: The Electrical Equivalent of Heat Objective: The purpose of this activity is to determine whether the energy dissipated by a heating resistor
PHYS320 ilab (O) Experiment 2 Instructions Conservation of Energy: The Electrical Equivalent of Heat Objective: The purpose of this activity is to determine whether the energy dissipated by a heating resistor
COPYRIGHT FOUNTAINHEAD PRESS
 Calorimetry: Heats of Solution Objective: Use calorimetric measurements to determine heats of solution of two ionic compounds. Materials: Solid ammonium nitrate (NH 4 NO 3 ) and anhydrous calcium chloride
Calorimetry: Heats of Solution Objective: Use calorimetric measurements to determine heats of solution of two ionic compounds. Materials: Solid ammonium nitrate (NH 4 NO 3 ) and anhydrous calcium chloride
Gilbert Kirss Foster. Chapter 9. Thermochemistry. Energy Changes in Chemical Reactions
 Gilbert Kirss Foster Chapter 9 Thermochemistry Energy Changes in Chemical Reactions Chapter Outline 9.1 Energy as a Reactant or Product 9.2 Transferring Heat and Doing Work 9.3 Enthalpy and Enthalpy Changes
Gilbert Kirss Foster Chapter 9 Thermochemistry Energy Changes in Chemical Reactions Chapter Outline 9.1 Energy as a Reactant or Product 9.2 Transferring Heat and Doing Work 9.3 Enthalpy and Enthalpy Changes
Laboratory 12: Three Thermodynamics Experiments
 Laboratory 12: Three Thermodynamics Experiments Experiment 1: Coefficient of Linear Expansion of Metals The fact that most objects expand when heated is common knowledge. The change in the linear dimensions
Laboratory 12: Three Thermodynamics Experiments Experiment 1: Coefficient of Linear Expansion of Metals The fact that most objects expand when heated is common knowledge. The change in the linear dimensions
Calorimetry. Summary. Contents
 Summary Calorimetry Calorimetry can be used to determine the heat flow in chemical reactions, to measure heats of solvation, characterize phase transitions and ligand binding to proteins. Theoretical predictions
Summary Calorimetry Calorimetry can be used to determine the heat flow in chemical reactions, to measure heats of solvation, characterize phase transitions and ligand binding to proteins. Theoretical predictions
CHEMISTRY. Chapter 5 Thermochemistry
 CHEMISTRY The Central Science 8 th Edition Chapter 5 Thermochemistry Dr. Kozet YAPSAKLI The Nature of Energy Kinetic and Potential Energy Potential energy can be converted into kinetic energy. E p = mgh
CHEMISTRY The Central Science 8 th Edition Chapter 5 Thermochemistry Dr. Kozet YAPSAKLI The Nature of Energy Kinetic and Potential Energy Potential energy can be converted into kinetic energy. E p = mgh
Chapter 6 Energy and Chemical Change. Brady and Senese 5th Edition
 Chapter 6 Energy and Chemical Change Brady and Senese 5th Edition Index 6.1 An object has energy if it is capable of doing work 6.2 Internal energy is the total energy of an object s molecules 6.3 Heat
Chapter 6 Energy and Chemical Change Brady and Senese 5th Edition Index 6.1 An object has energy if it is capable of doing work 6.2 Internal energy is the total energy of an object s molecules 6.3 Heat
11B, 11E Temperature and heat are related but not identical.
 Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Quantities in Chemical Reactions
 Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Selected Questions on Chapter 5 Thermochemistry
 Selected Questions on Chapter 5 Thermochemistry Circle the correct answer: 1) At what velocity (m/s) must a 20.0 g object be moving in order to possess a kinetic energy of 1.00 J? A) 1.00 B) 100 10 2 C)
Selected Questions on Chapter 5 Thermochemistry Circle the correct answer: 1) At what velocity (m/s) must a 20.0 g object be moving in order to possess a kinetic energy of 1.00 J? A) 1.00 B) 100 10 2 C)
Chapter 5. Thermochemistry
 Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 5 John D. Bookstaver St. Charles Community College Cottleville, MO 2009, Prentice-Hall,
Chemistry, The Central Science, 11th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 5 John D. Bookstaver St. Charles Community College Cottleville, MO 2009, Prentice-Hall,
Ch 6. Energy and Chemical Change. Brady & Senese, 5th Ed.
 Ch 6. Energy and Chemical Change Brady & Senese, 5th Ed. Energy Is The Ability To Do Work Energy is the ability to do work (move mass over a distance) or transfer heat Types: kinetic and potential kinetic:
Ch 6. Energy and Chemical Change Brady & Senese, 5th Ed. Energy Is The Ability To Do Work Energy is the ability to do work (move mass over a distance) or transfer heat Types: kinetic and potential kinetic:
Matter & Energy: Temperature & Heat in Physical Processes
 Matter & Energy: Temperature & Heat in Physical Processes Objectives: 1) To observe changes in temperature and heat energy which occur during physical processes such as dissolving. 2) To become familiar
Matter & Energy: Temperature & Heat in Physical Processes Objectives: 1) To observe changes in temperature and heat energy which occur during physical processes such as dissolving. 2) To become familiar
Thermochemistry/Calorimetry. Determination of the enthalpy of vaporization of liquids LEC 02. What you need: What you can learn about
 LEC 02 Thermochemistry/Calorimetry Determination of the enthalpy of vaporization of liquids What you can learn about Enthalpy of vaporisation Entropy of vaporisation Trouton s rule Calorimetry Heat capacity
LEC 02 Thermochemistry/Calorimetry Determination of the enthalpy of vaporization of liquids What you can learn about Enthalpy of vaporisation Entropy of vaporisation Trouton s rule Calorimetry Heat capacity
Gravity is a force which keeps us stuck to the earth. The Electrostatic force attracts electrons to protons in an atom.
 Energy Relations in Chemistry: Thermochemistry The Nature of Energy Sugar you eat is "combusted" by your body to produce CO 2 and H 2 O. During this process energy is also released. This energy is used
Energy Relations in Chemistry: Thermochemistry The Nature of Energy Sugar you eat is "combusted" by your body to produce CO 2 and H 2 O. During this process energy is also released. This energy is used
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy of motion:
Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy of motion:
1. State in your own terms what is the first law of thermodynamics, a closed system, an isolated system, surroundings, heat, work, and energy.
 Worksheet 1 1. State in your own terms what is the first law of thermodynamics, a closed system, an isolated system, surroundings, heat, work, and energy. The first law of thermodynamics is the conservation
Worksheet 1 1. State in your own terms what is the first law of thermodynamics, a closed system, an isolated system, surroundings, heat, work, and energy. The first law of thermodynamics is the conservation
Quantities in Chemical Reactions
 Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
The following gas laws describes an ideal gas, where
 Alief ISD Chemistry STAAR Review Reporting Category 4: Gases and Thermochemistry C.9.A Describe and calculate the relations between volume, pressure, number of moles, and temperature for an ideal gas as
Alief ISD Chemistry STAAR Review Reporting Category 4: Gases and Thermochemistry C.9.A Describe and calculate the relations between volume, pressure, number of moles, and temperature for an ideal gas as
COMBUSTION OF FUEL 12:57:42
 COMBUSTION OF FUEL The burning of fuel in presence of air is known as combustion. It is a chemical reaction taking place between fuel and oxygen at temperature above ignition temperature. Heat is released
COMBUSTION OF FUEL The burning of fuel in presence of air is known as combustion. It is a chemical reaction taking place between fuel and oxygen at temperature above ignition temperature. Heat is released
Calorimetry Measurements of Fusion, Hydration and Neutralization - Hess Law
 Calorimetry Measurements of Fusion, Hydration and Neutralization - Hess Law EXPERIMENT 9 Prepared by Edward L. Brown, Lee University and Verrill M. Norwood, Cleveland State Community College To become
Calorimetry Measurements of Fusion, Hydration and Neutralization - Hess Law EXPERIMENT 9 Prepared by Edward L. Brown, Lee University and Verrill M. Norwood, Cleveland State Community College To become
Heat of Solution for Aqueous Potassium Nitrate
 CHEM 331L Physical Chemistry Laboratory Revision 2.2 Heat of Solution for Aqueous Potassium Nitrate In this laboratory exercise we will measure the Integral of Solution for the solvation of Potassium Nitrate
CHEM 331L Physical Chemistry Laboratory Revision 2.2 Heat of Solution for Aqueous Potassium Nitrate In this laboratory exercise we will measure the Integral of Solution for the solvation of Potassium Nitrate
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Energy -Very much a chemistry topic Every chemical change has an accompanying change of. Combustion of fossil fuels The discharging a battery Metabolism of foods If we are to
Chapter 5 Thermochemistry Energy -Very much a chemistry topic Every chemical change has an accompanying change of. Combustion of fossil fuels The discharging a battery Metabolism of foods If we are to
DETERMINING AND USING H
 DETERMINING AND USING H INTRODUCTION CHANGES IN CHEMISTRY Chemistry is the science that studies matter and the changes it undergoes. Changes are divided into two categories: physical and chemical. During
DETERMINING AND USING H INTRODUCTION CHANGES IN CHEMISTRY Chemistry is the science that studies matter and the changes it undergoes. Changes are divided into two categories: physical and chemical. During
Chemistry Slide 1 of 33
 Chemistry 17.2 1 of 33 17.2 Measuring and Expressing Enthalpy Changes A burning match releases heat to its surroundings in all directions. How much heat does this exothermic reaction release? You will
Chemistry 17.2 1 of 33 17.2 Measuring and Expressing Enthalpy Changes A burning match releases heat to its surroundings in all directions. How much heat does this exothermic reaction release? You will
AP* Chemistry THERMOCHEMISTRY
 AP* Chemistry THERMOCHEMISTRY Terms for you to learn that will make this unit understandable: Energy (E) the ability to do work or produce heat ; the sum of all potential and kinetic energy in a system
AP* Chemistry THERMOCHEMISTRY Terms for you to learn that will make this unit understandable: Energy (E) the ability to do work or produce heat ; the sum of all potential and kinetic energy in a system
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
Enthalpy of Formation of Ammonium Chloride Version 6.2.5
 Enthalpy of Formation of Ammonium Chloride Version 6.2.5 Michael J. Vitarelli Jr. Department of Chemistry and Chemical Biology Rutgers University, 60 Taylor Road, Piscataway, NJ 08854 I. INTRODUCTION Enthalpy
Enthalpy of Formation of Ammonium Chloride Version 6.2.5 Michael J. Vitarelli Jr. Department of Chemistry and Chemical Biology Rutgers University, 60 Taylor Road, Piscataway, NJ 08854 I. INTRODUCTION Enthalpy
1.4 Enthalpy. What is chemical energy?
 1.4 Enthalpy What is chemical energy? Chemical energy is a form of potential energy which is stored in chemical bonds. Chemical bonds are the attractive forces that bind atoms together. As a reaction takes
1.4 Enthalpy What is chemical energy? Chemical energy is a form of potential energy which is stored in chemical bonds. Chemical bonds are the attractive forces that bind atoms together. As a reaction takes
AP CHEMISTRY SCORING GUIDELINES
 Mean 5.64 out of 9 pts AP CHEMISTRY Question 1 CO(g) + 1 2 O 2 (g) CO 2 (g) 1. The combustion of carbon monoxide is represented by the equation above. (a) Determine the value of the standard enthalpy change,
Mean 5.64 out of 9 pts AP CHEMISTRY Question 1 CO(g) + 1 2 O 2 (g) CO 2 (g) 1. The combustion of carbon monoxide is represented by the equation above. (a) Determine the value of the standard enthalpy change,
Slide 2 / 118. Thermochemistry
 Slide 1 / 118 Slide 2 / 118 Thermochemistry Slide 3 / 118 Table of Contents The Nature of Energy State Functions** Click on the topic to go to that section Enthalpy Measuring Enthalpy Changes: Calorimetry
Slide 1 / 118 Slide 2 / 118 Thermochemistry Slide 3 / 118 Table of Contents The Nature of Energy State Functions** Click on the topic to go to that section Enthalpy Measuring Enthalpy Changes: Calorimetry
Lecture Presentation. Chapter 5. Thermochemistry Pearson Education, Inc.
 Lecture Presentation Chapter 5 Energy Energy is the ability to do work or transfer heat. Energy used to cause an object that has mass to move is called work. Energy used to cause the temperature of an
Lecture Presentation Chapter 5 Energy Energy is the ability to do work or transfer heat. Energy used to cause an object that has mass to move is called work. Energy used to cause the temperature of an
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Chapter 8 Thermochemistry
 William L Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 8 Thermochemistry Edward J. Neth University of Connecticut Outline 1. Principles of heat flow 2. Measurement
William L Masterton Cecile N. Hurley http://academic.cengage.com/chemistry/masterton Chapter 8 Thermochemistry Edward J. Neth University of Connecticut Outline 1. Principles of heat flow 2. Measurement
Exercise 2-4. Titration of a Buffer Solution EXERCISE OBJECTIVES
 Exercise 2-4 Titration of a Buffer Solution EXERCISE OBJECTIVES To define the terms buffer solution and buffer capacity; To titrate a buffer solution with a weak acid solution; To plot a graph using the
Exercise 2-4 Titration of a Buffer Solution EXERCISE OBJECTIVES To define the terms buffer solution and buffer capacity; To titrate a buffer solution with a weak acid solution; To plot a graph using the
Chemistry 123: Physical and Organic Chemistry Topic 2: Thermochemistry
 Topic 2: Introduction, Topic 2: Thermochemistry Text: Chapter 7 and 19 (~ 3 weeks) 2.0 Introduction, terminology and scope 2.1 Enthalapy and Energy Change in a chemical process; 1st law of Thermodynamics
Topic 2: Introduction, Topic 2: Thermochemistry Text: Chapter 7 and 19 (~ 3 weeks) 2.0 Introduction, terminology and scope 2.1 Enthalapy and Energy Change in a chemical process; 1st law of Thermodynamics
Thermochemistry. Chapter 6. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Thermochemistry Chapter 6 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Energy is the capacity to do work. Radiant energy comes from the sun and is earth s
Thermochemistry Chapter 6 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Energy is the capacity to do work. Radiant energy comes from the sun and is earth s
s Traditionally, we use the calorie as a unit of energy. The nutritional Calorie, Cal = 1000 cal. Kinetic Energy and Potential Energy
 AP Chemistry: Thermochemistry Lecture Outline 5.1 The Nature of Energy Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationships between chemical
AP Chemistry: Thermochemistry Lecture Outline 5.1 The Nature of Energy Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationships between chemical
Measuring and Expressing Enthalpy Changes. Copyright Pearson Prentice Hall. Measuring and Expressing Enthalpy Changes. Calorimetry
 Measuring and Expressing Enthalpy Changes A burning match releases heat to its surroundings in all directions. How much heat does this exothermic reaction release? You will learn to measure heat flow in
Measuring and Expressing Enthalpy Changes A burning match releases heat to its surroundings in all directions. How much heat does this exothermic reaction release? You will learn to measure heat flow in
8.6 The Thermodynamic Standard State
 8.6 The Thermodynamic Standard State The value of H reported for a reaction depends on the number of moles of reactants...or how much matter is contained in the system C 3 H 8 (g) + 5O 2 (g) > 3CO 2 (g)
8.6 The Thermodynamic Standard State The value of H reported for a reaction depends on the number of moles of reactants...or how much matter is contained in the system C 3 H 8 (g) + 5O 2 (g) > 3CO 2 (g)
Experiment 3. Electrical Energy. Calculate the electrical power dissipated in a resistor.
 Experiment 3 Electrical Energy 3.1 Objectives Calculate the electrical power dissipated in a resistor. Determine the heat added to the water by an immersed heater. Determine if the energy dissipated by
Experiment 3 Electrical Energy 3.1 Objectives Calculate the electrical power dissipated in a resistor. Determine the heat added to the water by an immersed heater. Determine if the energy dissipated by
Chapter 5 - Thermochemistry
 Chapter 5 - Thermochemistry Study of energy changes that accompany chemical rx s. I) Nature of Energy Energy / Capacity to do work Mechanical Work w = F x d Heat energy - energy used to cause the temperature
Chapter 5 - Thermochemistry Study of energy changes that accompany chemical rx s. I) Nature of Energy Energy / Capacity to do work Mechanical Work w = F x d Heat energy - energy used to cause the temperature
Energy and Energy Conversion Minneapolis Community and Tech. College Principles of Chemistry 1 v q water = m water C water T water (Equation 1)
 Energy and Energy Conversion Minneapolis Community and Tech. College Principles of Chemistry 1 v.6.13 Energy Energy is defined by most textbooks as the capacity to do work. However, the true usefulness
Energy and Energy Conversion Minneapolis Community and Tech. College Principles of Chemistry 1 v.6.13 Energy Energy is defined by most textbooks as the capacity to do work. However, the true usefulness
Chapter 5 Practice Multiple Choice & Free
 Name Response 1. A system has an increase in internal energy, E, of 40 kj. If 20 kj of work, w, is done on the system, what is the heat change, q? a) +60 kj d) -20 kj b) +40 kj e) -60 kj c) +20 kj 2. Which
Name Response 1. A system has an increase in internal energy, E, of 40 kj. If 20 kj of work, w, is done on the system, what is the heat change, q? a) +60 kj d) -20 kj b) +40 kj e) -60 kj c) +20 kj 2. Which
EXPERIMENT. Stoichiometry of a Precipitation Reaction
 EXPERIMENT Stoichiometry of a Precipitation Reaction Hands-On Labs, Inc. Version 42-0201-00-02 Review the safety materials and wear goggles when working with chemicals. Read the entire exercise before
EXPERIMENT Stoichiometry of a Precipitation Reaction Hands-On Labs, Inc. Version 42-0201-00-02 Review the safety materials and wear goggles when working with chemicals. Read the entire exercise before
CHEM 103 CHEMISTRY I
 CHEM 103 CHEMISTRY I CHAPTER 5 THERMOCHEMISTRY Inst. Dr. Dilek IŞIK TAŞGIN Inter-Curricular Courses Department Çankaya University, Inc. Energy Energy is the ability to do work or transfer heat. Energy
CHEM 103 CHEMISTRY I CHAPTER 5 THERMOCHEMISTRY Inst. Dr. Dilek IŞIK TAŞGIN Inter-Curricular Courses Department Çankaya University, Inc. Energy Energy is the ability to do work or transfer heat. Energy
Chapter 5. Thermochemistry
 Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 5 John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice Hall,
Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 5 John D. Bookstaver St. Charles Community College St. Peters, MO 2006, Prentice Hall,
Chapter 6 Thermochemistry
 Chapter 6 Thermochemistry Contents and Concepts Understanding Heats of Reaction The first part of the chapter lays the groundwork for understanding what we mean by heats of reaction. 1. Energy and Its
Chapter 6 Thermochemistry Contents and Concepts Understanding Heats of Reaction The first part of the chapter lays the groundwork for understanding what we mean by heats of reaction. 1. Energy and Its
Lab #9- Calorimetry/Thermochemistry to the Rescue
 Chesapeake Campus Chemistry 111 Laboratory Lab #9- Calorimetry/Thermochemistry to the Rescue Objectives Determine whether a reaction is endothermic or exothermic. Determine the best ionic compound of to
Chesapeake Campus Chemistry 111 Laboratory Lab #9- Calorimetry/Thermochemistry to the Rescue Objectives Determine whether a reaction is endothermic or exothermic. Determine the best ionic compound of to
AP Chemistry. Free-Response Questions
 2018 AP Chemistry Free-Response Questions College Board, Advanced Placement Program, AP, AP Central, and the acorn logo are registered trademarks of the College Board. AP Central is the official online
2018 AP Chemistry Free-Response Questions College Board, Advanced Placement Program, AP, AP Central, and the acorn logo are registered trademarks of the College Board. AP Central is the official online
Chapter 6. Energy Thermodynamics
 Chapter 6 Energy Thermodynamics 1 Energy is... The ability to do work. Conserved. made of heat and work. a state function. independent of the path, or how you get from point A to B. Work is a force acting
Chapter 6 Energy Thermodynamics 1 Energy is... The ability to do work. Conserved. made of heat and work. a state function. independent of the path, or how you get from point A to B. Work is a force acting
The Nature of Energy. Chapter Six: Kinetic vs. Potential Energy. Energy and Work. Temperature vs. Heat
 The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
SAMPLE CHEMISTRY QUESTIONS MIXTURE OF UNIT 3 & 4 MATERIALS
 SAMPLE CHEMISTRY QUESTIONS MIXTURE OF UNIT 3 & 4 MATERIALS QUESTION 1 The equation describing the production of butyl ethanoate is given below. Catalyst C4H 9OH CH 3COOH CH 3COOC 4H 9 H 2O( l ) 0.0500
SAMPLE CHEMISTRY QUESTIONS MIXTURE OF UNIT 3 & 4 MATERIALS QUESTION 1 The equation describing the production of butyl ethanoate is given below. Catalyst C4H 9OH CH 3COOH CH 3COOC 4H 9 H 2O( l ) 0.0500
If neither volume nor pressure of the system changes, w = 0 and ΔE = q = ΔH. The change in internal energy is equal to the change in enthalpy.
 5 Thermochemistry Visualizing Concepts 5.1 The book s potential energy is due to the opposition of gravity by an object of mass m at a distance d above the surface of the earth. Kinetic energy is due to
5 Thermochemistry Visualizing Concepts 5.1 The book s potential energy is due to the opposition of gravity by an object of mass m at a distance d above the surface of the earth. Kinetic energy is due to
In this experiment, the concept of electric field will be developed by
 Physics Equipotential Lines and Electric Fields Plotting the Electric Field PURPOSE MATERIALS 5 alligator clip leads 2 batteries, 9 V 2 binder clips, large computer In this experiment, the concept of electric
Physics Equipotential Lines and Electric Fields Plotting the Electric Field PURPOSE MATERIALS 5 alligator clip leads 2 batteries, 9 V 2 binder clips, large computer In this experiment, the concept of electric
Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions
 Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions Jeffrey Mack California State University, Sacramento Energy & Chemistry Questions that need to be addressed: How do we measure
Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions Jeffrey Mack California State University, Sacramento Energy & Chemistry Questions that need to be addressed: How do we measure
Name Class Date. As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings.
 Name Class Date Thermochemistry 17.1 The Flow of Energy As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings. Process Cause Effect endothermic
Name Class Date Thermochemistry 17.1 The Flow of Energy As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings. Process Cause Effect endothermic
Don't forget to. Mastering Chemistry everyday! October 9 th, Next week in Lab: Next week in disc: 10/9/2014.
 Solutions of nitric acid, sodium bicarbonate, silver nitrate and iron (III) acetate are mixed. Write all NIE s. There are 4! H + (aq) + HCO 2 3 (aq) H 2 O(l) + CO 2 () (g) H + (aq) + C 2 H 3 O 2 (aq) HC
Solutions of nitric acid, sodium bicarbonate, silver nitrate and iron (III) acetate are mixed. Write all NIE s. There are 4! H + (aq) + HCO 2 3 (aq) H 2 O(l) + CO 2 () (g) H + (aq) + C 2 H 3 O 2 (aq) HC
Thermochemistry. Energy (and Thermochemistry) World of Chemistry Chapter 10. Energy. Energy
 Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
c. K 2 CO 3 d. (NH 4 ) 2 SO 4 Answer c
 Chem 130 Name Exam 2, Ch 4-6 July 7, 2016 100 Points Please follow the instructions for each section of the exam. Show your work on all mathematical problems. Provide answers with the correct units and
Chem 130 Name Exam 2, Ch 4-6 July 7, 2016 100 Points Please follow the instructions for each section of the exam. Show your work on all mathematical problems. Provide answers with the correct units and
Chapter 5 Thermochemistry. 許富銀 ( Hsu Fu-Yin)
 Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
Chapter 5 Thermochemistry 許富銀 ( Hsu Fu-Yin) 1 Thermodynamics The study of energy and its transformations is known as thermodynamics The relationships between chemical reactions and energy changes that
CHAPTER 16: REACTION ENERGY AND CHAPTER 17: REACTION KINETICS. Honors Chemistry Ms. Agostine
 CHAPTER 16: REACTION ENERGY AND CHAPTER 17: REACTION KINETICS Honors Chemistry Ms. Agostine 16.1 Thermochemistry Definition: study of the transfers of energy as heat that accompany chemical reactions and
CHAPTER 16: REACTION ENERGY AND CHAPTER 17: REACTION KINETICS Honors Chemistry Ms. Agostine 16.1 Thermochemistry Definition: study of the transfers of energy as heat that accompany chemical reactions and
Chemistry Stoichiometry and Heat Exam (ver.1) Mr. Thaler. Please do not write on this exam. Mark your answers on the scantron only.
 1. Identify from the unbalanced equations below the one that does not represent a redox reaction. a. H 2O 2(aq) + MnO 4 - (aq) O 2(g) + Mn 2+ (aq) b. H 2(g) + N 2(g) NH 3(g) c. NaCl (aq) + AgNO 3(aq) NaNO
1. Identify from the unbalanced equations below the one that does not represent a redox reaction. a. H 2O 2(aq) + MnO 4 - (aq) O 2(g) + Mn 2+ (aq) b. H 2(g) + N 2(g) NH 3(g) c. NaCl (aq) + AgNO 3(aq) NaNO
EXPERIMENT. Titration for Acetic Acid in Vinegar
 EXPERIMENT Titration for Acetic Acid in Vinegar Hands-On Labs, Inc. Version 42-0208-00-02 Review the safety materials and wear goggles when working with chemicals. Read the entire exercise before you begin.
EXPERIMENT Titration for Acetic Acid in Vinegar Hands-On Labs, Inc. Version 42-0208-00-02 Review the safety materials and wear goggles when working with chemicals. Read the entire exercise before you begin.
Chapter 15 Energy and Chemical Change
 Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Chapter 15 Energy and Chemical Change Chemical reactions usually absorb or release energy. Section 1: Energy Section 2: Heat Section 3: Thermochemical Equations Section 4: Calculating Enthalpy Change Section
Name: Section: Score: /10 PRE LABORATORY ASSIGNMENT EXPERIMENT 7
 Name: Section: Score: /10 PRE LABORATORY ASSIGNMENT EXPERIMENT 7 1. Is the sign of Δ r H for an exothermic reaction positive or negative? Why? 2. When 4.21 grams of potassium hydroxide are added to 250.
Name: Section: Score: /10 PRE LABORATORY ASSIGNMENT EXPERIMENT 7 1. Is the sign of Δ r H for an exothermic reaction positive or negative? Why? 2. When 4.21 grams of potassium hydroxide are added to 250.
Chapter 5 THERMO. THERMO chemistry. 5.4 Enthalpy of Reactions 5.5 Calorimetry 5.6 Hess s Law 5.7 Enthalpies of Formation
 Chapter 5 THERMO THERMO chemistry 5.4 Enthalpy of Reactions 5.5 Calorimetry 5.6 Hess s Law 5.7 Enthalpies of Formation Chemical Equations 1 st WRITE the Chemical Equation 2 nd BALANCE the Chemical Equation
Chapter 5 THERMO THERMO chemistry 5.4 Enthalpy of Reactions 5.5 Calorimetry 5.6 Hess s Law 5.7 Enthalpies of Formation Chemical Equations 1 st WRITE the Chemical Equation 2 nd BALANCE the Chemical Equation
Thermodynamic Processes and Thermochemistry
 General Chemistry Thermodynamic Processes and Thermochemistry 박준원교수 ( 포항공과대학교화학과 ) 이번시간에는! Systems, states, and processes The first law of thermodynamics: internal energy, work, and heat Heat capacity,
General Chemistry Thermodynamic Processes and Thermochemistry 박준원교수 ( 포항공과대학교화학과 ) 이번시간에는! Systems, states, and processes The first law of thermodynamics: internal energy, work, and heat Heat capacity,
