Bomb Calorimetry. Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise.
|
|
|
- Augustine Bryan
- 5 years ago
- Views:
Transcription
1 Experiment 12 Bomb Calorimetry Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. Purpose A bomb calorimeter will be calibrated using a sample of known benzoic acid and then used to determine the calorie content of an unknown corn chip. This value will be compared to the tabulated nutritional facts. Background Combustion is one of the most important types of reaction studied by means of calorimetry. We can measure the amount of heat produced from a combustion reaction by measuring the temperature change effected on the water-filled container surrounding it. A bomb calorimeter is a device specifically designed to contain combustion reactions and the heat they produce. It is an insulated container of water in which a metal bomb (reaction chamber) is placed with a stirrer to circulate the water and a thermometer to measure the temperature (Fig. 1). The bomb is a strong metal container that can withstand the high pressures associated with the rapid combustion of a material and transmit the heat to its surroundings very quickly. Inside the bomb is a sample holder, an electrical ignition system to start the reaction, and enough oxygen gas to achieve complete and rapid combustion (Fig. 2). Figure 2: Bomb Set-Up Figure 1: Bomb Calorimeter Set-Up The sample is placed in the sample holder with sufficient oxygen pumped into the bomb to allow complete combustion. The reaction chamber is connected to an electrical circuit to allow external ignition and then immersed in the water and the initial temperature measured over a length of time to ensure that it is stable. Ignition sends a spark to begin combustion. The heat produced is trapped by the water, 12-1
2 causing an increase in temperature, and the final temperature reach is recorded over a length of time. This increase in temperature corresponds to the amount of heat generated (evolved) by the combustion reaction. To calculate the heat of combustion for an unknown, the relationship between the temperature change of the water inside the calorimeter and the heat produced from a known reaction must be measured first in a process called calibration. The relationship is called the heat capacity of the calorimeter (C cal), which includes the bomb, water, stirrer, thermometer, and entire container. The heat absorbed by the calorimeter is dependent on this constant and the change in temperature, as seen in Eqn. 1. q cal = C cal x DT cal Eqn. 1 Inside the calorimeter, a small piece of string is used to hold the sample in place. This string also fully combusts, adding heat, with a known heat of combustion of 3.59 cal./cm. Thus the heat the calorimeter absorbs due to both the reaction and the string releasing heat during combustion is shown in Eqn. 2. q cal = -(q rxn + q string) Eqn. 2 Calibration is performed by using a sample of a substance with a known heat of combustion, such as benzoic acid, and observing the resulting temperature change of the calorimeter when a known amount of the substance is combusted. As each calorimeter is different, calibration must be performed for each instrument individually. The initial and final temperatures will be determined graphically. The temperature inside the calorimeter will be recorded over time and linear trendlines will be used to find an accurate average for both. 12-2
3 Example Problem: Calibration of CCal The combustion of g of benzoic acid (C7H6O2) will produce kj of heat. A g sample of benzoic acid and 10.0 cm of string with a heat of combustion of cal/cm was combusted in a bomb calorimeter. The trendlines for before ignition was y = x and after ignition was y = x with an intercept of x = 5.2 min., calculate the heat capacity of the calorimeter (Ccal) in kj/ o C. Step 1: Solving for CCal from Eqn. 1 Since qcal = CCal DT, rearranging for Ccal gives: C cal = q cal T Step 2: Find the heat released by the reaction, qrxn g benzoic acid kj g benzoic acid 1000 J 1 kj Step 3: Find the heat released by the string, qstring 1 cal J = cal. evolved 10.0 cm cal 1 cm = cal. evolved Step 4: Find the heat absorbed by the calorimeter, qcal qcal = - (qrxn + qstring) = -( cal cal.) = cal. Step 5: Find the change in temperature, DT Before ignition: y = (5.2) = C After ignition: y = (0.0132)(5.2) = C DT = (Tfinal Tinitial) = C 20.1 C = C Step 5: Find Ccal C cal = cal. #.833 = 3269 cal./o C This relationship means that for the apparatus in the example, a 1 C temperature increase corresponds to the reaction and string producing 1260 cal. of heat. A 2 C increase would indicate that the reaction produced 2520 cal. of heat (2 C x 1260 cal./ C), etc. In the first half of this experiment, you will calibrate your bomb calorimeter with a known quantity of benzoic acid to find C cal. In the second half of this experiment, you will use your calculated C cal to find the heat of combustion (calorie content) for a food item provided by your instructor and compare it to the tabulated values on the nutrition label. The example shown is of a corn chip. 12-3
4 Example Problem: Finding the Caloric Content for a Food Item Using the same calorimeter as in the previous example, a g corn chip and 10.0 cm of string are combusted. The trendlines before ignition was y = x and after ignition was y = x with an intercept of x = 5.3 min. Calculate the caloric content for the chip in Cal./serving. Step 1: Find the change in temperature, ΔT Before ignition: y = (5.3) = C After ignition: y = (5.3) = C ΔT = C C = C Step 2: Find the heat absorbed by the calorimeter, qcal qcal = (3269 cal./ C)(7.801 C) = cal. Step 3: Find the heat released by the string, qstring 10.0 cm cal. 1 cm = cal Step 4: Find the heat released by the reaction, qrxn qcal = -(qstring + qrxn) so qrxn = -qcal - qstring = cal. (-35.9 cal.) = cal. Step 5: Converting qrxn cal. 1 Calorie 1000 calories = Calories Step 4: Find heat of combustion. ΔHrxn Calories g = Cal./gram Step 5: Converting heat of combustion Cal. 1 gram 28 grams = -250 Cal./serving or 250 Cal./serving evolved 1 serving 12-4
5 Procedure Part I: Calibration of the Calorimeter with Benzoic Acid 1. Fill the calorimeter water bucket with 2.00 L of distilled water using a volumetric flask. Place the water bucket inside the calorimeter. 2. Obtain one pellet of benzoic acid. Weigh it accurately and record its weight on your data sheet. 3. Cut a 10.0 cm length of string. Tie one end of the string around the benzoic acid tablet and the other to the ignition wire between the electrodes in the bomb. The tablet may either rest in the sample holder or hang in the air above it. The string must not come untied during any part of the experiment. A loose string will not carry the ignition spark and no combustion will occur. 4. Close the bomb by carefully lowering the lid with sample vertically into the bottom portion. Place the large O-ring cover over the top and hand-tighten it fully. 5. Ensure that the pressure valve is closed by screwing it down by hand. Pressurize the bomb to 30 atm of oxygen from the provided tank (Caution! Do not exceed 35 atm!). 6. Using the provided clamps, carefully place the bomb into the water bucked into the calorimeter, ensuring no water is spilled or removed. Connect the electrodes to the wire connectors in the bomb s lid (it does not matter which). If any continuous gas bubbles are observed, then the bomb is leaking and will need to be removed and reassembled before continuing. 7. Place the calorimeter s lid over the top, being careful to insert the stirrer in the water. Connect the two wheels by pulley and start the motor to begin stirring. Connect the outer wires to the ignition trigger around the ground and 10 cm knobs. Lower the steel thermometer into its slot on the calorimeter s lid. 8. Turn on the temperature reader and begin recording the temperature every 30 seconds in the data table. The reader is both a timer and a thermometer. 9. At four minutes, ignite the bomb by pressing the ignition button. The indicator light should be on while the ignition trigger is pressed. Hold the trigger for exactly three seconds and no longer, then release. 10. Continue monitoring the temperature. By the next reading at 4.5 minutes, the temperature should have increased by a degree Celsius or more. 11. Continue to record the temperature every 30 seconds until 12 minutes total. 12. Stop the stirrer. Carefully remove the pulley, the thermometer, and then the calorimeter s lid. Remove the bomb and the water. 13. Release the pressure in the bomb by opening the pressure valve. Open the bomb and clean any residue that may remain. If any sample remains behind, weigh it. Dry the bomb thoroughly inside and out. 14. Pour out the water and dry the bucket thoroughly inside and out. 12-5
6 Part II: Finding the Heat of Combustion for a Food Item 1. Repeat Steps 1-14 in Part I. For Step 2, use the food item assigned by your instructor instead of the benzoic acid pellet. Part III: Finding DT for Both Trials 1. Prepare two graphs of time (min.) versus temperature ( C), one for Part I and one for Part II. 2. For each graph, find the change in temperature by adding two trendlines: before ignition and after ignition. On Excel: a. Right click on any data point on the graph. From the pop-up menu, click Select Data. b. Under Legend Entries (Series), click Add. c. In Series name, type Before Ignition. d. In Series X values, click the button at the right end of the box and then drag and select all x-values that correspond to the times before ignition. Click the button on the right of the box to go back. e. In Series Y values, delete what is typed there, click the button on the right end of the box, and then drag and select all y- values that correspond to the temperatures before ignition. These should be ordered pairs with what you selected in Step D. Click the button on the right of the box to go back. Click OK. f. Under Legend Entries (Series), click Add. g. In Series name, type After Ignition. h. Repeat Steps D-E to select the times and temperatures that correspond to your data after ignition. Note: not ALL data on your graph should be included in these trendlines. i. Click OK. j. On the graph, add a trendline to the data highlighted as Before Ignition and the data After Ignition. Be sure to show equations for both. 3. Print out each graph. 12-6
7 4. Using a ruler and a pencil, extrapolate both trendlines to the edges of the graph. Then turn the ruler vertically and draw a line that intersects both extrapolated trendlines in such a way that the area of the upper and lower triangles created by the line, the trendline, and the curve itself have approximately the same area (Fig. 4). Continue this line down to the x-axis and estimate the value. 5. Plug in your x-value from Step 4 to both trendline equations to solve for y. These y-values correspond to your initial and final temperatures for each trial. Figure 4: Finding initial and final temperature graphically Part IV: Finding Heat of Combustion for a Food Item 1. Using your initial and final temperatures from your calibration with benzoic acid graph, find C cal in cal./ C. Note: remember that the string burned inside the bomb with the sample. You will need to subtract the heat evolved from the burning string (the heat of combustion for the string is cal./cm). 2. Using your C cal calculated in Step 1 and your initial and final temperatures from your food item graph, find the heat of combustion for the food item in cal./gram, then convert to Calories/gram and Calories/serving. 3. Compare your calculated value in Step 2 to the tabulated value listed on the nutrition label. 12-7
8 Experiment 12 Data Sheet Name: Calorimeter: Partners: Part I: Calibration of the Calorimeter with Benzoic Acid 1. Mass of benzoic acid (g) 2. Heat released by the reaction (q rxn, cal) 3. Initial length of string (cm) 4. Final length of string (cm) 5. Length of string burned (cm) 6. Heat released by string burning (q string, cal) 7. Heat absorbed by the calorimeter (q cal, cal) 8. Initial temperature of the calorimeter (T initial, C) show calculation (use graph equation): 9. Final temperature of the calorimeter (T final, C) show calculation (use graph equation): 12-8
9 10. Change in Temperature (ΔT, C) 11. Heat Capacity of the calorimeter (C cal, cal/ C) Part II: Finding the Heat of Combustion for a Food Item 1. Mass of food item (g) 2. Initial length of string (cm) 3. Final length of string (cm) 4. Length of string burned (cm) 5. Initial temperature of the calorimeter (T initial, C) show calculation (use graph equation): 6. Final temperature of the calorimeter (T final, C) show calculation (use graph equation): 7. Change in Temperature (ΔT, C) 8. Heat absorbed by the calorimeter (q cal, cal) 12-9
10 9. Heat released by string burning (q string, cal) 10. Heat released by the reaction (q rxn, cal) 11. Heat of Combustion of food item (DH rxn, Cal/g) 12. Serving Size (from label) 13. Heat of Combustion per serving (DH rxn, Cal/serving) 13. Percent Error of Heat of Combustion (%) 12-10
11 Temperature Readings for Combustion of Benzoic Acid Time (min) Temperature ( C) Time (min) Temperature ( C) Ignition
12 Temperature Readings for Combustion of Corn Chip Time (min) Temperature ( C) Time (min) Temperature ( C) Ignition
13 Experiment 12 Post-Lab Assignment 1. Rank by increasing size, starting with the smallest unit: Joule, kilojoule, calorie, Calorie (kcal). 2. Using your data from Part II, calculate the heat of combustion of the food item in Cal./serving while ignoring the heat produced by the combustion of the string (q string). Find the percent error. 3. From the food item s packaging, determine the percentage of Calories/serving that come from carbohydrates, fats, and proteins (hint: you will need to look up the values for the Calories per gram of each produced in the human body)
14 4. Bomb calorimeters are also common calibrated by combusting naphthalene instead of benzoic acid. If a g sample of naphthalene (heat of combustion: kj/g) causes the temperature of a bomb calorimeter to rise by 7.81 C, calculate the C cal of the calorimeter in cal./ C. You may ignore the heat from the combustion of the string (q string). 5. Was your percent error for the heat of combustion of the food item high or low? Does this indicate accurate or precise results? Give three experimental reasons to account for the difference between your value and the label s
15 Experiment 12 Pre-Lab Assignment Name: For all calculations, show all work and draw a box around the final answers. 1. A g sample of benzoic acid and 10.0 cm of string are combusted in a bomb calorimeter and the temperature of the system increases from C to C. What is the heat capacity of the bomb calorimeter in (a) kj/ C and (b) cal./ C? 2. Calculate the moles of O 2(g) needed to completely burn 1.00 grams of sucrose (C 12H 22O 22). (hint: you will need the balanced combustion equation for sucrose). 3. Given that a bomb calorimeter has a volume of 1.5 L and an initial temperature of 25.0 C, what pressure of O 2(g) in atm is required to completely combust 1.00 g sucrose? 12-15
16 4. Based on your answer in (3), why is the bomb pressure set to 30 atm in this experiment? 5. A 1.00 g sample of sucrose is combusted in the same calorimeter described in (1) and its temperature increased from C to C. Calculate the heat of combustion for sucrose in (a) kj/mol and (b) Cal./mol 12-16
Heat of Combustion. Parr oxygen bomb calorimeter (or equivalent), pellet press, thermometer (0.01 C), fuse wire.
 Heat of Combustion PURPOSE The purposes of this experiment are to determine the heats of combustion of several related substances using a bomb calorimeter and relate differences in heats of combustion
Heat of Combustion PURPOSE The purposes of this experiment are to determine the heats of combustion of several related substances using a bomb calorimeter and relate differences in heats of combustion
Bomb Calorimetry: Heat of Combustion of Naphthalene
 Bomb Calorimetry: Heat of Combustion of Naphthalene Most tabulated H values of highly exothermic reactions come from bomb calorimeter experiments. Heats of combustion are most common, in which the combustible
Bomb Calorimetry: Heat of Combustion of Naphthalene Most tabulated H values of highly exothermic reactions come from bomb calorimeter experiments. Heats of combustion are most common, in which the combustible
The Enthalpy of Formation of Camphor by Bomb Calorimetry 1
 The Enthalpy of Formation of Camphor by Bomb Calorimetry 1 Purpose: The enthalpy of combustion of camphor will be determined in a bomb calorimeter. The enthalpy of formation of camphor will be calculated
The Enthalpy of Formation of Camphor by Bomb Calorimetry 1 Purpose: The enthalpy of combustion of camphor will be determined in a bomb calorimeter. The enthalpy of formation of camphor will be calculated
Experiment 6: Using Calorimetry to Determine the Enthalpy of Formation of Magnesium Oxide
 Experiment 6: Using Calorimetry to Determine the Enthalpy of Formation of Magnesium Oxide Reading: Chapter sections 5.4 5.7 of your textbook and this lab handout. Ongoing Learning Goals: To use a scientific
Experiment 6: Using Calorimetry to Determine the Enthalpy of Formation of Magnesium Oxide Reading: Chapter sections 5.4 5.7 of your textbook and this lab handout. Ongoing Learning Goals: To use a scientific
Determination of Resonance Stabilization Energy of Benzene Jonathan Smith (With Small Adaptations by Amanda Nienow)
 Determination of Resonance Stabilization Energy of Benzene Jonathan Smith (With Small Adaptations by Amanda Nienow) Abstract In this investigation we will measure the heat evolved during the combustion
Determination of Resonance Stabilization Energy of Benzene Jonathan Smith (With Small Adaptations by Amanda Nienow) Abstract In this investigation we will measure the heat evolved during the combustion
Experiment 1: Adiabatic Bomb Calorimeter (Dated: August 25, 2009)
 Experiment 1: Adiabatic Bomb Calorimeter (Dated: August 25, 2009) I. INTRODUCTION Heat released in a chemical reaction can be determined experimentally by using an adiabatic calorimeter. The reaction must
Experiment 1: Adiabatic Bomb Calorimeter (Dated: August 25, 2009) I. INTRODUCTION Heat released in a chemical reaction can be determined experimentally by using an adiabatic calorimeter. The reaction must
c H2 O = J (g H 2 O)( C change)
 Calorimetry 1 CHM120 Introduction: Have you ever noticed the nutrition label located on the packaging of the food you buy? One of the first things listed on the label are the calories per serving. How
Calorimetry 1 CHM120 Introduction: Have you ever noticed the nutrition label located on the packaging of the food you buy? One of the first things listed on the label are the calories per serving. How
PROPULSION LAB MANUAL
 PROPULSION LAB MANUAL Measurement of Calorific Value of a Solid Fuel Sample using a Bomb Calorimeter DEPARTMENT OF AEROSPACE ENGINEERING Indian Institute of Technology Kharagpur CONTENTS 1. Introduction
PROPULSION LAB MANUAL Measurement of Calorific Value of a Solid Fuel Sample using a Bomb Calorimeter DEPARTMENT OF AEROSPACE ENGINEERING Indian Institute of Technology Kharagpur CONTENTS 1. Introduction
Specific Heat of a Metal
 Specific Heat of a Metal Purpose The objective of this experiment is to determine the specific heat of zinc sample using coffeecup calorimeter. Theory In a chemical reaction, the quantity of heat that
Specific Heat of a Metal Purpose The objective of this experiment is to determine the specific heat of zinc sample using coffeecup calorimeter. Theory In a chemical reaction, the quantity of heat that
Physical Chemistry Laboratory [CHEM 335] BOMB CALORIMETRY Adriana Biney and Silmilly Toribio
![Physical Chemistry Laboratory [CHEM 335] BOMB CALORIMETRY Adriana Biney and Silmilly Toribio Physical Chemistry Laboratory [CHEM 335] BOMB CALORIMETRY Adriana Biney and Silmilly Toribio](/thumbs/87/96015679.jpg) Physical Chemistry Laboratory [CHEM 335] BOMB CALORIMETRY Adriana Biney and Silmilly Toribio ABSTRACT In this experiment a Parr 1341 Plain Jacket Oxygen Bomb Calorimeter was employed to determine the enthalpy
Physical Chemistry Laboratory [CHEM 335] BOMB CALORIMETRY Adriana Biney and Silmilly Toribio ABSTRACT In this experiment a Parr 1341 Plain Jacket Oxygen Bomb Calorimeter was employed to determine the enthalpy
CHM201 General Chemistry and Laboratory I Laboratory 7 Thermochemistry and Hess s Law May 2, 2018
 Purpose: CHM201 General Chemistry and Laboratory I Laboratory 7 Thermochemistry and Hess s Law May 2, 2018 In this laboratory, you will measure heat changes arising from chemical reactions. You will use
Purpose: CHM201 General Chemistry and Laboratory I Laboratory 7 Thermochemistry and Hess s Law May 2, 2018 In this laboratory, you will measure heat changes arising from chemical reactions. You will use
EXPERIMENT 14 SPECIFIC HEAT OF WATER. q = m s T
 EXPERIMENT 14 SPECIFIC HEAT OF WATER INTRODUCTION: Heat is a form of energy which can pass from an object of relatively high temperature to an object of relatively low temperature. One physical property
EXPERIMENT 14 SPECIFIC HEAT OF WATER INTRODUCTION: Heat is a form of energy which can pass from an object of relatively high temperature to an object of relatively low temperature. One physical property
Thermochemistry: Calorimetry and Hess s Law
 Thermochemistry: Calorimetry and Hess s Law Some chemical reactions are endothermic and proceed with absorption of heat while others are exothermic and proceed with an evolution of heat. The magnitude
Thermochemistry: Calorimetry and Hess s Law Some chemical reactions are endothermic and proceed with absorption of heat while others are exothermic and proceed with an evolution of heat. The magnitude
Name: Section: Score: /10 PRE LABORATORY ASSIGNMENT EXPERIMENT 7
 Name: Section: Score: /10 PRE LABORATORY ASSIGNMENT EXPERIMENT 7 1. Is the sign of Δ r H for an exothermic reaction positive or negative? Why? 2. When 4.21 grams of potassium hydroxide are added to 250.
Name: Section: Score: /10 PRE LABORATORY ASSIGNMENT EXPERIMENT 7 1. Is the sign of Δ r H for an exothermic reaction positive or negative? Why? 2. When 4.21 grams of potassium hydroxide are added to 250.
MEEN 3242 MEE LAB II
 MEEN 3242 MEE LAB II Experiment Report Lab # 2 Bomb Calorimeter Group Members: Hugo Diaz Reed Greenwood Juan Orona Alan Sanoja Micheal Shackelford Date: September 27, 2013 DEPARTMENT OF MECHANICAL AND
MEEN 3242 MEE LAB II Experiment Report Lab # 2 Bomb Calorimeter Group Members: Hugo Diaz Reed Greenwood Juan Orona Alan Sanoja Micheal Shackelford Date: September 27, 2013 DEPARTMENT OF MECHANICAL AND
Experiment 2: THE DENSITY OF A SOLID UNKNOWN AND CALIBRATION WITH DATASTUDIO SOFTWARE
 Experiment 2: THE DENSITY OF A SOLID UNKNOWN AND CALIBRATION WITH DATASTUDIO SOFTWARE Concepts: Density Equipment Calibration Approximate time required: 90 minutes for density 90 minutes for two thermometers
Experiment 2: THE DENSITY OF A SOLID UNKNOWN AND CALIBRATION WITH DATASTUDIO SOFTWARE Concepts: Density Equipment Calibration Approximate time required: 90 minutes for density 90 minutes for two thermometers
ALE 26. Energy Changes ( E) and Enthalpy Changes ( H) in Chemical Reactions
 Name Chem 161, Section: Group Number: ALE 26. Energy Changes ( E) and Enthalpy Changes ( H) in Chemical Reactions (Reference: Chapter 6 - Silberberg 5 th edition) Important!! For answers that involve a
Name Chem 161, Section: Group Number: ALE 26. Energy Changes ( E) and Enthalpy Changes ( H) in Chemical Reactions (Reference: Chapter 6 - Silberberg 5 th edition) Important!! For answers that involve a
Name Partner. Thermal Physics. Part I: Heat of Vaporization of Nitrogen. Introduction:
 Name Partner Thermal Physics Part I: Heat of Vaporization of Nitrogen Introduction: The heat of vaporization of a liquid, L v, is the energy required to vaporize (boil) a unit mass of substance. Thus if
Name Partner Thermal Physics Part I: Heat of Vaporization of Nitrogen Introduction: The heat of vaporization of a liquid, L v, is the energy required to vaporize (boil) a unit mass of substance. Thus if
COPYRIGHT FOUNTAINHEAD PRESS
 Calorimetry: Heats of Solution Objective: Use calorimetric measurements to determine heats of solution of two ionic compounds. Materials: Solid ammonium nitrate (NH 4 NO 3 ) and anhydrous calcium chloride
Calorimetry: Heats of Solution Objective: Use calorimetric measurements to determine heats of solution of two ionic compounds. Materials: Solid ammonium nitrate (NH 4 NO 3 ) and anhydrous calcium chloride
College Chem I 2045C Specific Heat of a Metal-SL. Objective: In this lab, you will use calorimetry to determine the specific heat of a metal.
 Student Name Partner s Name Date College Chem I 2045C Specific Heat of a Metal-SL Objective: In this lab, you will use calorimetry to determine the specific heat of a metal. Materials: Metal Sample Bunsen
Student Name Partner s Name Date College Chem I 2045C Specific Heat of a Metal-SL Objective: In this lab, you will use calorimetry to determine the specific heat of a metal. Materials: Metal Sample Bunsen
Lab 2. Molar enthalpy of combustion
 Lab 2. Molar enthalpy of combustion Introduction Chemical reactions like burning an organic compound typically produce heat. In this experiment you will be measuring the amount of heat produced by burning
Lab 2. Molar enthalpy of combustion Introduction Chemical reactions like burning an organic compound typically produce heat. In this experiment you will be measuring the amount of heat produced by burning
EXPERIMENT 5. THE ENTHALPY OF FORMATION OF CAMPHOR BY BOMB CALORIMETRY 1
 EXPERIMENT 5. THE ENTHALPY OF FORMATION OF CAMPHOR BY BOMB CALORIMETRY 1 The enthalpies of combustion and formation for camphor will be determined using a bomb calorimeter and tabulated values for the
EXPERIMENT 5. THE ENTHALPY OF FORMATION OF CAMPHOR BY BOMB CALORIMETRY 1 The enthalpies of combustion and formation for camphor will be determined using a bomb calorimeter and tabulated values for the
4 BOMB CALORIMETRY. Chapter 4. Bomb Calorimetry 24 I. PREPARATION
 Chapter 4. Bomb Calorimetry 24 4 BOMB CALORIMETRY I. PREPARATION Read "Principles of Calorimetry" in Ch. VI of Shoemaker, Garland, and Nibler (SGN, pp. 145-151), as well as Experiment 6 (pp. 152-158).
Chapter 4. Bomb Calorimetry 24 4 BOMB CALORIMETRY I. PREPARATION Read "Principles of Calorimetry" in Ch. VI of Shoemaker, Garland, and Nibler (SGN, pp. 145-151), as well as Experiment 6 (pp. 152-158).
Experiment 12 Determination of an Enthalpy of Reaction, Using Hess s Law
 Experiment 12 Determination of an Enthalpy of Reaction, Using Hess s Law Object: To measure the standard heat of formation, f, of MgO (s), and to become familiar with calorimetry as a toll for measuring
Experiment 12 Determination of an Enthalpy of Reaction, Using Hess s Law Object: To measure the standard heat of formation, f, of MgO (s), and to become familiar with calorimetry as a toll for measuring
Quantities in Chemical Reactions
 Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
EXPERIMENT A8: CALORIMETRY. Learning Outcomes. Introduction. Upon completion of this lab, the student will be able to:
 1 EXPERIMENT A8: CALORIMETRY Learning Outcomes Upon completion of this lab, the student will be able to: 1) Measure the heat of a reaction under constant pressure conditions. 2) Calculate the enthalpy
1 EXPERIMENT A8: CALORIMETRY Learning Outcomes Upon completion of this lab, the student will be able to: 1) Measure the heat of a reaction under constant pressure conditions. 2) Calculate the enthalpy
Chemistry 212 THE ENTHALPY OF FORMATION OF MAGNESIUM OXIDE LEARNING OBJECTIVES
 Chemistry 212 THE ENTHALPY OF FORMATION OF MAGNESIUM OXIDE The learning objectives of this experiment are LEARNING OBJECTIVES A simple coffee cup calorimeter will be used to determine the enthalpy of formation
Chemistry 212 THE ENTHALPY OF FORMATION OF MAGNESIUM OXIDE The learning objectives of this experiment are LEARNING OBJECTIVES A simple coffee cup calorimeter will be used to determine the enthalpy of formation
Calculate the mass of L of oxygen gas at 25.0 C and 1.18 atm pressure.
 148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
PHYS320 ilab (O) Experiment 2 Instructions Conservation of Energy: The Electrical Equivalent of Heat
 PHYS320 ilab (O) Experiment 2 Instructions Conservation of Energy: The Electrical Equivalent of Heat Objective: The purpose of this activity is to determine whether the energy dissipated by a heating resistor
PHYS320 ilab (O) Experiment 2 Instructions Conservation of Energy: The Electrical Equivalent of Heat Objective: The purpose of this activity is to determine whether the energy dissipated by a heating resistor
Chemistry: The Central Science. Chapter 5: Thermochemistry
 Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
This reaction is ENDOTHERMIC. Energy is being transferred from the room/flask/etc. (the SURROUNDINGS) to the reaction itself (the SYSTEM).
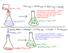 151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
Quantities in Chemical Reactions
 Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Quantities in Chemical Reactions 6-1 6.1 The Meaning of a Balanced Equation C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O (g) The balanced equation tells us: 1 molecule of propane reacts with 5 molecules of
Name: Chemistry 103 Laboratory University of Massachusetts Boston HEATS OF REACTION PRELAB ASSIGNMENT
 Name: Chemistry 103 Laboratory University of Massachusetts Boston HEATS OF REACTION PRELAB ASSIGNMENT Chemical and physical changes usually involve the absorption or liberation of heat, given the symbol
Name: Chemistry 103 Laboratory University of Massachusetts Boston HEATS OF REACTION PRELAB ASSIGNMENT Chemical and physical changes usually involve the absorption or liberation of heat, given the symbol
Energy and Energy Conversion Minneapolis Community and Tech. College Principles of Chemistry 1 v q water = m water C water T water (Equation 1)
 Energy and Energy Conversion Minneapolis Community and Tech. College Principles of Chemistry 1 v.6.13 Energy Energy is defined by most textbooks as the capacity to do work. However, the true usefulness
Energy and Energy Conversion Minneapolis Community and Tech. College Principles of Chemistry 1 v.6.13 Energy Energy is defined by most textbooks as the capacity to do work. However, the true usefulness
Thermodynamics. Equations to use for the calculations:
 Thermodynamics Introduction: Gibbs Free Energy, G, can be used to determine if a reaction is spontaneous or not. A negative value of G indicates that a given reaction is spontaneous at the measured conditions
Thermodynamics Introduction: Gibbs Free Energy, G, can be used to determine if a reaction is spontaneous or not. A negative value of G indicates that a given reaction is spontaneous at the measured conditions
- Joule (J): SI unit for energy. It's defined based on the equation for kinetic energy. from. mass. velocity
 153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
Experiment #7 CALORIMETRY AND THE SPECIFIC HEAT OF METALS
 PURPOSE: Experiment #7 CALORIMETRY AND THE SPECIFIC HEAT OF METALS To experimentally determine the specific heat of a metal. INTRODUCTION When a substance is heated, the motion of its individual particles
PURPOSE: Experiment #7 CALORIMETRY AND THE SPECIFIC HEAT OF METALS To experimentally determine the specific heat of a metal. INTRODUCTION When a substance is heated, the motion of its individual particles
Chemistry 123: Physical and Organic Chemistry Topic 2: Thermochemistry
 Topic 2: Introduction, Topic 2: Thermochemistry Text: Chapter 7 and 19 (~ 3 weeks) 2.0 Introduction, terminology and scope 2.1 Enthalapy and Energy Change in a chemical process; 1st law of Thermodynamics
Topic 2: Introduction, Topic 2: Thermochemistry Text: Chapter 7 and 19 (~ 3 weeks) 2.0 Introduction, terminology and scope 2.1 Enthalapy and Energy Change in a chemical process; 1st law of Thermodynamics
Thermodynamics. Equations to use for the calculations:
 Thermodynamics Introduction: Gibbs Free Energy, G, can be used to determine if a reaction is spontaneous or not. A negative value of G indicates that a given reaction is spontaneous at the measured conditions
Thermodynamics Introduction: Gibbs Free Energy, G, can be used to determine if a reaction is spontaneous or not. A negative value of G indicates that a given reaction is spontaneous at the measured conditions
EXPERIMENT 9 ENTHALPY OF REACTION HESS S LAW
 EXPERIMENT 9 ENTHALPY OF REACTION HESS S LAW INTRODUCTION Chemical changes are generally accompanied by energy changes; energy is absorbed or evolved, usually as heat. Breaking chemical bonds in reactants
EXPERIMENT 9 ENTHALPY OF REACTION HESS S LAW INTRODUCTION Chemical changes are generally accompanied by energy changes; energy is absorbed or evolved, usually as heat. Breaking chemical bonds in reactants
Matter & Energy: Temperature & Heat in Physical Processes
 Matter & Energy: Temperature & Heat in Physical Processes Objectives: 1) To observe changes in temperature and heat energy which occur during physical processes such as dissolving. 2) To become familiar
Matter & Energy: Temperature & Heat in Physical Processes Objectives: 1) To observe changes in temperature and heat energy which occur during physical processes such as dissolving. 2) To become familiar
Finding the Constant K c 4/21/15 Maya Parks Partners: Ben Seufert, Caleb Shumpert. Abstract:
 Finding the Constant K c 4/21/15 Maya Parks Partners: Ben Seufert, Caleb Shumpert Abstract: This lab was performed to find the chemical equilibrium constant K c for the reaction Fe 3+ + SCN FeSCN 2+ using
Finding the Constant K c 4/21/15 Maya Parks Partners: Ben Seufert, Caleb Shumpert Abstract: This lab was performed to find the chemical equilibrium constant K c for the reaction Fe 3+ + SCN FeSCN 2+ using
How bad is that snack anyway?
 Physical Sciences 11 Experiment 1 How bad is that snack anyway? Monday, 2/10 Wednesday, 2/12 Science Center Room 117 Please read this entire document and complete the attached prelab before your lab. This
Physical Sciences 11 Experiment 1 How bad is that snack anyway? Monday, 2/10 Wednesday, 2/12 Science Center Room 117 Please read this entire document and complete the attached prelab before your lab. This
Chapter 5 Thermochemistry
 244 Chapter 5 Thermochemistry Figure 5.10 (a) The Ivanpah solar thermal plant uses 170,000 mirrors to concentrate sunlight on water-filled towers. (b) It covers 4000 acres of public land near the Mojave
244 Chapter 5 Thermochemistry Figure 5.10 (a) The Ivanpah solar thermal plant uses 170,000 mirrors to concentrate sunlight on water-filled towers. (b) It covers 4000 acres of public land near the Mojave
5-Sep-15 PHYS101-2 GRAPHING
 GRAPHING Objectives 1- To plot and analyze a graph manually and using Microsoft Excel. 2- To find constants from a nonlinear relation. Exercise 1 - Using Excel to plot a graph Suppose you have measured
GRAPHING Objectives 1- To plot and analyze a graph manually and using Microsoft Excel. 2- To find constants from a nonlinear relation. Exercise 1 - Using Excel to plot a graph Suppose you have measured
Measuring Energy Changes. Introducing Heat Capacity and Specific Heat
 Measuring Energy Changes Introducing Heat Capacity and Specific Heat Before Today s Discussion Begins Remember the differences between temperature, thermal energy, and heat Temperature is the average kinetic
Measuring Energy Changes Introducing Heat Capacity and Specific Heat Before Today s Discussion Begins Remember the differences between temperature, thermal energy, and heat Temperature is the average kinetic
Lab #9- Calorimetry/Thermochemistry to the Rescue
 Chesapeake Campus Chemistry 111 Laboratory Lab #9- Calorimetry/Thermochemistry to the Rescue Objectives Determine whether a reaction is endothermic or exothermic. Determine the best ionic compound of to
Chesapeake Campus Chemistry 111 Laboratory Lab #9- Calorimetry/Thermochemistry to the Rescue Objectives Determine whether a reaction is endothermic or exothermic. Determine the best ionic compound of to
Expt 10, Thermochemistry. Name: Lab Partner: Date: Introduction. Part A. Determination of Heat Capacity of the Calorimeter
 Expt 10, Thermochemistry Name: Lab Partner: Date: Introduction Commented [1]: Use a nice title page better Commented [2]: What are you going to do and why are you doing it. Add a couple of sentences outlining
Expt 10, Thermochemistry Name: Lab Partner: Date: Introduction Commented [1]: Use a nice title page better Commented [2]: What are you going to do and why are you doing it. Add a couple of sentences outlining
Chemical Equilibrium: Finding a Constant, Kc
 Chemical Equilibrium: Finding a Constant, Kc Experiment 20 The purpose of this lab is to experimentally determine the equilibrium constant, K c, for the following chemical reaction: Fe 3+ (aq) + SCN (aq)
Chemical Equilibrium: Finding a Constant, Kc Experiment 20 The purpose of this lab is to experimentally determine the equilibrium constant, K c, for the following chemical reaction: Fe 3+ (aq) + SCN (aq)
Hess' Law: Calorimetry
 Exercise 9 Page 1 Illinois Central College CHEMISTRY 130 Name: Hess' Law: Calorimetry Objectives The objectives of this experiment are to... - measure the heats of reaction for two chemical reactions.
Exercise 9 Page 1 Illinois Central College CHEMISTRY 130 Name: Hess' Law: Calorimetry Objectives The objectives of this experiment are to... - measure the heats of reaction for two chemical reactions.
Density of an Unknown
 Experiment 3 Density of an Unknown Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. Purpose The density of an
Experiment 3 Density of an Unknown Pre-Lab Assignment Before coming to lab: Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. Purpose The density of an
11B, 11E Temperature and heat are related but not identical.
 Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
CHEM 1105 S10 March 11 & 14, 2014
 CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
Laboratory 12: Three Thermodynamics Experiments
 Laboratory 12: Three Thermodynamics Experiments Experiment 1: Coefficient of Linear Expansion of Metals The fact that most objects expand when heated is common knowledge. The change in the linear dimensions
Laboratory 12: Three Thermodynamics Experiments Experiment 1: Coefficient of Linear Expansion of Metals The fact that most objects expand when heated is common knowledge. The change in the linear dimensions
C q T q C T. Heat is absorbed by the system H > 0 endothermic Heat is released by the system H < 0 exothermic
 PLEASE REORD ALL DATA DIRETLY INTO YOUR LAB NOTEBOOKS Introduction Heating a substance is one of the simplest processes carried out in the chemical laboratory, and is usually accompanied by a rise in the
PLEASE REORD ALL DATA DIRETLY INTO YOUR LAB NOTEBOOKS Introduction Heating a substance is one of the simplest processes carried out in the chemical laboratory, and is usually accompanied by a rise in the
Determination of Calories in Food Via Adiabatic Bomb Calorimeter
 The Corinthian Volume 6 Article 9 2004 Determination of Calories in Food Via Adiabatic Bomb Calorimeter Kenneth C. McGill Georgia College Michelle L. Yasechko Georgia College Wendy K. Nkari Georgia College
The Corinthian Volume 6 Article 9 2004 Determination of Calories in Food Via Adiabatic Bomb Calorimeter Kenneth C. McGill Georgia College Michelle L. Yasechko Georgia College Wendy K. Nkari Georgia College
AREN 2110: Thermodynamics
 AREN 2110: Thermodynamics ITLL LAB #1: MEASUREMENT OF SPECIFIC HEAT IN A CALORIMETER Calorimetry is a basic technique for measuring thermodynamic quantities, especially those involving heat transfer: specific
AREN 2110: Thermodynamics ITLL LAB #1: MEASUREMENT OF SPECIFIC HEAT IN A CALORIMETER Calorimetry is a basic technique for measuring thermodynamic quantities, especially those involving heat transfer: specific
CHEMISTRY 130 General Chemistry I. Thermochemistry
 CHEMISTRY 130 General Chemistry I Thermochemistry The burning of a match, shown above [1], is a chemical reaction between oxygen and sulfur. [2] Intuitively, we know that this reaction releases heat enough
CHEMISTRY 130 General Chemistry I Thermochemistry The burning of a match, shown above [1], is a chemical reaction between oxygen and sulfur. [2] Intuitively, we know that this reaction releases heat enough
Name Group # Date Partners. Specific Heat and Calorimetry
 Specific Heat and Calorimetry Experimental Objective The objective of this experiment is to determine the specific heat of two metals using a calorimetric procedure called the method of mixtures. Theory
Specific Heat and Calorimetry Experimental Objective The objective of this experiment is to determine the specific heat of two metals using a calorimetric procedure called the method of mixtures. Theory
Calorimetry and Hess s Law Prelab
 Calorimetry and Hess s Law Prelab Name Total /10 1. What is the purpose of this experiment? 2. Make a graph (using some kind of graphing computer software) of temperature vs. time for the following data:
Calorimetry and Hess s Law Prelab Name Total /10 1. What is the purpose of this experiment? 2. Make a graph (using some kind of graphing computer software) of temperature vs. time for the following data:
CALORIMETRY: Heat of Fusion of Ice
 Pre-Lab Discussion CALORIMETRY: Heat of Fusion of Ice When a chemical or physical change takes place, heat is either given off or absorbed That is, the change is either exothermic or endothermic It is
Pre-Lab Discussion CALORIMETRY: Heat of Fusion of Ice When a chemical or physical change takes place, heat is either given off or absorbed That is, the change is either exothermic or endothermic It is
Calorimetry Measurements of Fusion, Hydration and Neutralization - Hess Law
 Calorimetry Measurements of Fusion, Hydration and Neutralization - Hess Law EXPERIMENT 9 Prepared by Edward L. Brown, Lee University and Verrill M. Norwood, Cleveland State Community College To become
Calorimetry Measurements of Fusion, Hydration and Neutralization - Hess Law EXPERIMENT 9 Prepared by Edward L. Brown, Lee University and Verrill M. Norwood, Cleveland State Community College To become
Rate law Determination of the Crystal Violet Reaction Using the Isolation Method
 Rate law Determination of the Crystal Violet Reaction Using the Isolation Method Introduction A common challenge in chemical kinetics is to determine the rate law for a reaction with multiple reactants.
Rate law Determination of the Crystal Violet Reaction Using the Isolation Method Introduction A common challenge in chemical kinetics is to determine the rate law for a reaction with multiple reactants.
THER Mo CHEMISTRY: HEAT OF Ne UTRALIZATION
 Experiment 11 Name: 42 THER Mo CHEMISTRY: HEAT OF Ne UTRALIZATION In this experiment, you will use calorimetry to experimentally determine the heat of neutralization of NaOH-HCl, or the enthalpy of the
Experiment 11 Name: 42 THER Mo CHEMISTRY: HEAT OF Ne UTRALIZATION In this experiment, you will use calorimetry to experimentally determine the heat of neutralization of NaOH-HCl, or the enthalpy of the
Ch. 14 Notes ENERGY AND CHEMICAL CHANGE NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics.
 Ch. 14 Notes ENERGY AND CHEMICAL CHANGE NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Energy the capacity to do work or produce heat A. two basic types of
Ch. 14 Notes ENERGY AND CHEMICAL CHANGE NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Energy the capacity to do work or produce heat A. two basic types of
Chesapeake Campus Chemistry 111 Laboratory
 Chesapeake Campus Chemistry 111 Laboratory Objectives Calculate the density of a sugar solution. Evaluate lab sources of error and their effect on an experiment. Introduction The density of an object is
Chesapeake Campus Chemistry 111 Laboratory Objectives Calculate the density of a sugar solution. Evaluate lab sources of error and their effect on an experiment. Introduction The density of an object is
Chemical Equilibrium: Finding a Constant, Kc
 Chemical Equilibrium: Finding a Constant, Kc Experiment 20 The purpose of this lab is to experimentally determine the equilibrium constant, K c, for the following chemical reaction: Fe 3+ (aq) + SCN -
Chemical Equilibrium: Finding a Constant, Kc Experiment 20 The purpose of this lab is to experimentally determine the equilibrium constant, K c, for the following chemical reaction: Fe 3+ (aq) + SCN -
In fact, we are going to be sneaky and use Hess s Law to determine the heat of magnesium combustion indirectly. Go to the website:
 Chemistry 161 Please prepare your notebook though the data tables before class on Wednesday, October 27. Write an abstract and place it at the front of your individual report. The abstract and the report
Chemistry 161 Please prepare your notebook though the data tables before class on Wednesday, October 27. Write an abstract and place it at the front of your individual report. The abstract and the report
Part 1: Equivalence of Energy: Heat, Mechanical Part 2: Equivalence of Energy: Heat, Electrical, Light
 Name: Laboratory Section: Laboratory Section Date: Partners Names: Grade: Last Revised on November 15, 2017 EXPERIMENT 5 - New Part 1: Equivalence of Energy: Heat, Mechanical Part 2: Equivalence of Energy:
Name: Laboratory Section: Laboratory Section Date: Partners Names: Grade: Last Revised on November 15, 2017 EXPERIMENT 5 - New Part 1: Equivalence of Energy: Heat, Mechanical Part 2: Equivalence of Energy:
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy of motion:
Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy of motion:
- Kinetic energy: energy of matter in motion. gravity
 148 2500 L of chlorine gas at 25.0 C and 1.00 atm are used to make hydrochloric acid. How many grams of hydrochloric acid could be produced if all the chlorine reacts? 1 - Convert 2500 L chlorine gas to
148 2500 L of chlorine gas at 25.0 C and 1.00 atm are used to make hydrochloric acid. How many grams of hydrochloric acid could be produced if all the chlorine reacts? 1 - Convert 2500 L chlorine gas to
MEASUREMENT: PART II
 1 MEASUREMENT: PART II Copyright: Department of Chemistry, University of Idaho, Moscow, ID 83844-2343, 2013. INTRODUCTION Read and/or review Section 1.7 and Figure 7.5 in your textbook. The first part
1 MEASUREMENT: PART II Copyright: Department of Chemistry, University of Idaho, Moscow, ID 83844-2343, 2013. INTRODUCTION Read and/or review Section 1.7 and Figure 7.5 in your textbook. The first part
Thermodynamics Enthalpy of Reaction and Hess s Law
 P.O. Box 219 Batavia, Illinois 60510 1-800-452-1261 flinn@flinnsci.com Visit our website at: www.flinnsci.com 2003 Flinn Scientific, Inc. All Rights Reserved. Your Safer Source for Science Supplies Thermodynamics
P.O. Box 219 Batavia, Illinois 60510 1-800-452-1261 flinn@flinnsci.com Visit our website at: www.flinnsci.com 2003 Flinn Scientific, Inc. All Rights Reserved. Your Safer Source for Science Supplies Thermodynamics
Exp. B Bomb Calorimetry
 Exp. B Bomb Calorimetry PRELAB: The prelab. must completed prior to the beginning of the lab period and handed in before any lab work may be started. Make sure you read the safety instructions. See the
Exp. B Bomb Calorimetry PRELAB: The prelab. must completed prior to the beginning of the lab period and handed in before any lab work may be started. Make sure you read the safety instructions. See the
Modification of Procedure for Experiments 17 and 18. everything with distilled water and dry thoroughly. (Note: Do not use acetone to rinse cups.
 Modification of Procedure for Experiments 17 and 18 I. Calorimeter Constant Obtain two polystyrene cups, a lid for one of the cups and a magnetic stirrer. Rinse everything with distilled water and dry
Modification of Procedure for Experiments 17 and 18 I. Calorimeter Constant Obtain two polystyrene cups, a lid for one of the cups and a magnetic stirrer. Rinse everything with distilled water and dry
Thermochemistry/Calorimetry. Determination of the enthalpy of combustion with a calorimetric bomb LEC 02. What you need:
 LEC 02 Thermochemistry/Calorimetry with a calorimetric bomb What you can learn about 1st law of thermodynamics Hess law Enthalpy of combustion Enthalpy of formation Heat capacity Principle and tasks The
LEC 02 Thermochemistry/Calorimetry with a calorimetric bomb What you can learn about 1st law of thermodynamics Hess law Enthalpy of combustion Enthalpy of formation Heat capacity Principle and tasks The
Chemical Equilibrium: Finding a Constant, Kc
 Lab12 Chemical Equilibrium: Finding a Constant, Kc The purpose of this lab is to experimentally determine the equilibrium constant, K c, for the following chemical reaction: Fe 3+ (aq) + SCN (aq) FeSCN
Lab12 Chemical Equilibrium: Finding a Constant, Kc The purpose of this lab is to experimentally determine the equilibrium constant, K c, for the following chemical reaction: Fe 3+ (aq) + SCN (aq) FeSCN
8 Enthalpy of Reaction
 E x p e r i m e n t Enthalpy of Reaction Lecture and Lab Skills Emphasized Calculating the heat and enthalpy of reactions. Writing net ionic equations. Using Hess s law to determine the enthalpy of a reaction.
E x p e r i m e n t Enthalpy of Reaction Lecture and Lab Skills Emphasized Calculating the heat and enthalpy of reactions. Writing net ionic equations. Using Hess s law to determine the enthalpy of a reaction.
The Determination of an Equilibrium Constant
 The Determination of an Equilibrium Constant Chemistry 102 10 Chemical reactions occur to reach a state of equilibrium. The equilibrium state can be characterized by quantitatively defining its equilibrium
The Determination of an Equilibrium Constant Chemistry 102 10 Chemical reactions occur to reach a state of equilibrium. The equilibrium state can be characterized by quantitatively defining its equilibrium
DETERMINING AND USING H
 DETERMINING AND USING H INTRODUCTION CHANGES IN CHEMISTRY Chemistry is the science that studies matter and the changes it undergoes. Changes are divided into two categories: physical and chemical. During
DETERMINING AND USING H INTRODUCTION CHANGES IN CHEMISTRY Chemistry is the science that studies matter and the changes it undergoes. Changes are divided into two categories: physical and chemical. During
Introduction to Spectroscopy: Analysis of Copper Ore
 Introduction to Spectroscopy: Analysis of Copper Ore Using a Buret and Volumetric Flask: 2.06 ml of solution 2.47 ml of solution 50.00 ml delivered delivered Volumetric Flask Reading a buret: Burets are
Introduction to Spectroscopy: Analysis of Copper Ore Using a Buret and Volumetric Flask: 2.06 ml of solution 2.47 ml of solution 50.00 ml delivered delivered Volumetric Flask Reading a buret: Burets are
Experiment 3. Electrical Energy. Calculate the electrical power dissipated in a resistor.
 Experiment 3 Electrical Energy 3.1 Objectives Calculate the electrical power dissipated in a resistor. Determine the heat added to the water by an immersed heater. Determine if the energy dissipated by
Experiment 3 Electrical Energy 3.1 Objectives Calculate the electrical power dissipated in a resistor. Determine the heat added to the water by an immersed heater. Determine if the energy dissipated by
Just a reminder that everything you do related to lab should be entered directly into your lab notebook. Calorimetry
 Just a reminder that everything you do related to lab should be entered directly into your lab notebook. Objectives: Calorimetry After completing this lab, you should be able to: - Assemble items of common
Just a reminder that everything you do related to lab should be entered directly into your lab notebook. Objectives: Calorimetry After completing this lab, you should be able to: - Assemble items of common
UNIT 3: CHEMICAL EQUILIBRIUM (TEXT: Chap 14-pg 627 & Chap 18 pg )
 UNIT 3: CHEMICAL EQUILIBRIUM (TEXT: Chap 14-pg 627 & Chap 18 pg 818-829) *Remedial questions on Concentration of Solutions (3.10 pg 130-135) 3:1. ATTEMPT QUESTIONS a) 3.109 b) 3.113 c) 3.115 d) 3.118 on
UNIT 3: CHEMICAL EQUILIBRIUM (TEXT: Chap 14-pg 627 & Chap 18 pg 818-829) *Remedial questions on Concentration of Solutions (3.10 pg 130-135) 3:1. ATTEMPT QUESTIONS a) 3.109 b) 3.113 c) 3.115 d) 3.118 on
Introduction to Spectroscopy: Analysis of Copper Ore
 Introduction to Spectroscopy: Analysis of Copper Ore Using a Buret and Volumetric Flask: 2.06 ml of solution delivered 2.47 ml of solution delivered 50.00 ml Volumetric Flask Reading a buret: Burets are
Introduction to Spectroscopy: Analysis of Copper Ore Using a Buret and Volumetric Flask: 2.06 ml of solution delivered 2.47 ml of solution delivered 50.00 ml Volumetric Flask Reading a buret: Burets are
Energy and Chemical Change
 Energy and Chemical Change Section 15.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Energy and Chemical Change Section 15.1 Energy In your textbook, read about the nature of energy. In the space at the left, write true if the statement is true; if the statement is false, change the italicized
Experiment #13. Enthalpy of Hydration of Sodium Acetate.
 Experiment #13 Enthalpy of Hydration of Sodium Acetate Goal To determine the enthalpy (ΔH) for the following process: NaC 2 H 3 O 2 (s) + 3 H 2 O(l) à NaC 2 H 3 O 2 3H 2 O(s) Introduction Most chemical
Experiment #13 Enthalpy of Hydration of Sodium Acetate Goal To determine the enthalpy (ΔH) for the following process: NaC 2 H 3 O 2 (s) + 3 H 2 O(l) à NaC 2 H 3 O 2 3H 2 O(s) Introduction Most chemical
Thermochemistry. Questions to ponder. Because 4/20/14. an ice-cube? an ice-cube? Part 2: Calorimetry. But I KNOW. Q=mc T, but T=0
 Thermochemistry Part 2: Calorimetry p p If you leave your keys and your chemistry book sitting in the sun on a hot summer day, which one is hotter? Why is there a difference in temperature between the
Thermochemistry Part 2: Calorimetry p p If you leave your keys and your chemistry book sitting in the sun on a hot summer day, which one is hotter? Why is there a difference in temperature between the
Calorimetery and Hess s Law
 Calorimetery and Hess s Law Overview: Calorimetry is the technique used to measure the heat required or evolved during a chemical reaction. Heat has units of joules, so one might expect to be using a joule
Calorimetery and Hess s Law Overview: Calorimetry is the technique used to measure the heat required or evolved during a chemical reaction. Heat has units of joules, so one might expect to be using a joule
To use calorimetry results to calculate the specific heat of an unknown metal. To determine heat of reaction ( H) from calorimetry measurements.
 Calorimetry PURPOSE To determine if a Styrofoam cup calorimeter provides adequate insulation for heat transfer measurements, to identify an unknown metal by means of its heat capacity and to determine
Calorimetry PURPOSE To determine if a Styrofoam cup calorimeter provides adequate insulation for heat transfer measurements, to identify an unknown metal by means of its heat capacity and to determine
M61 1 M61.1 PC COMPUTER ASSISTED DETERMINATION OF ANGULAR ACCELERATION USING TORQUE AND MOMENT OF INERTIA
 M61 1 M61.1 PC COMPUTER ASSISTED DETERMINATION OF ANGULAR ACCELERATION USING TORQUE AND MOMENT OF INERTIA PRELAB: Before coming to the lab, you must write the Object and Theory sections of your lab report
M61 1 M61.1 PC COMPUTER ASSISTED DETERMINATION OF ANGULAR ACCELERATION USING TORQUE AND MOMENT OF INERTIA PRELAB: Before coming to the lab, you must write the Object and Theory sections of your lab report
Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise.
 Experiment 10 Stoichiometry- Gravimetric Analysis Pre-lab Assignment Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. Purpose The purpose this experiment
Experiment 10 Stoichiometry- Gravimetric Analysis Pre-lab Assignment Read the lab thoroughly. Answer the pre-lab questions that appear at the end of this lab exercise. Purpose The purpose this experiment
SPECIFIC HEAT OF WATER LAB 11-2
 CONCEPT Heat of Fusion Changes of state (phase changes) involve the conversion or transition of matter from one of the common states (solid, liquid or gas) to another. Examples include fusion or melting
CONCEPT Heat of Fusion Changes of state (phase changes) involve the conversion or transition of matter from one of the common states (solid, liquid or gas) to another. Examples include fusion or melting
Chemical Equilibrium: Finding a Constant, Kc
 Chemical Equilibrium: Finding a Constant, Kc Computer 20 The purpose of this lab is to experimentally determine the equilibrium constant, K c, for the following chemical reaction: Fe 3+ (aq) + SCN (aq)
Chemical Equilibrium: Finding a Constant, Kc Computer 20 The purpose of this lab is to experimentally determine the equilibrium constant, K c, for the following chemical reaction: Fe 3+ (aq) + SCN (aq)
AP Chemistry Lab #10- Hand Warmer Design Challenge (Big Idea 5) Figure 1
 www.pedersenscience.com AP Chemistry Lab #10- Hand Warmer Design Challenge (Big Idea 5) 5.A.2: The process of kinetic energy transfer at the particulate scale is referred to in this course as heat transfer,
www.pedersenscience.com AP Chemistry Lab #10- Hand Warmer Design Challenge (Big Idea 5) 5.A.2: The process of kinetic energy transfer at the particulate scale is referred to in this course as heat transfer,
I. The Nature of Energy A. Energy
 I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
Additivity of Heats of Reaction: Hess s Law
 Additivity of Heats of Reaction: Hess s Law Experiment 21 In this experiment, you will use a Styrofoam-cup calorimeter to measure the heat released by three reactions. One of the reactions is the same
Additivity of Heats of Reaction: Hess s Law Experiment 21 In this experiment, you will use a Styrofoam-cup calorimeter to measure the heat released by three reactions. One of the reactions is the same
Learning Objectives: Use a calorimeter to test the specific heat of an unknown metal and to identify it
 And there shall be a tabernacle for a shade in the daytime from the heat, and for a security and covert from the whirlwind, and from rain. Is 4:6 Thermal physics defines heat as the transfer of energy
And there shall be a tabernacle for a shade in the daytime from the heat, and for a security and covert from the whirlwind, and from rain. Is 4:6 Thermal physics defines heat as the transfer of energy
Experiment 2 Heat of Combustion: Magnesium
 Experiment 2 Heat of Combustion: Magnesium Purpose Hess s Law states that when are going from a particular set of reactants to a particular set of products, the heat of reaction is the same whether the
Experiment 2 Heat of Combustion: Magnesium Purpose Hess s Law states that when are going from a particular set of reactants to a particular set of products, the heat of reaction is the same whether the
Thermochemistry: The Heat of Neutralization
 Thermochemistry: The Heat of Neutralization Safety Solid NaOH is a severe contact hazard. Avoid touching it! HCl and NaOH solutions are both contact hazards. Wear goggles at all times since NaOH is a severe
Thermochemistry: The Heat of Neutralization Safety Solid NaOH is a severe contact hazard. Avoid touching it! HCl and NaOH solutions are both contact hazards. Wear goggles at all times since NaOH is a severe
