The following gas laws describes an ideal gas, where
|
|
|
- Easter West
- 5 years ago
- Views:
Transcription
1 Alief ISD Chemistry STAAR Review Reporting Category 4: Gases and Thermochemistry C.9.A Describe and calculate the relations between volume, pressure, number of moles, and temperature for an ideal gas as described by Boyles s law, Charles law, Avogadro s law, Dalton s law of partial pressure, and the ideal gas law. Gas Laws The following gas laws describes an ideal gas, where P = pressure of gas (atm), V = volume of gas (L), n = moles of gas, T = absolute temperature of gas (K), and R = the gas constant, L atm/mol K. The gas law that contains all four variables----p, V, T, and n is called the ideal gas law. Gas Law Equation Description Dalton s Law of Partial P T = P 1 + P 2 + P 3 + Pressures The total pressure of a mixture of gases equals the sum of the individual gas pressures Boyle s Law P 1 V 1 = P 2 V 2 P is inversely proportional to V at constant T (and at constant n) Charles Law V 1 = V 2 T 1 T 2 Avogadro s Law V 1 = V 2 n 1 n 2 V is directly proportional to T at constant P (and at constant n) V is directly proportional to n at constant T and P Ideal Gas Law PV = nrt P V is directly proportional to n T Standard Temperature and Pressure, STP: conditions of 0 o C (273 K) and 1 atm or kpa (760 mm Hg); at STP, 1 mole of an ideal gas has a volume of 22.4L To solve problems using gas laws, convert each unit to those in R L atm mol K Sample Problem 1: The pressure on a balloon with a volume of 300 ml increases from 1.10 to 2.00 atmospheres (atm). Use Boyle s law to calculate the new volume of the balloon. 1. First, identify the variables. P 1 = 1.10 atm V 1 = 300 ml P 2 = 2.00 atm V 2 =? 2. Now, rearrange the equation algebraically to isolate the unknown. V 2 = P 1 x V 1 P 2
2 3. Insert the known values and solve. V 2 = 1.10 atm x 300mL 2.00 atm V 2 = 165 ml Sample Problem 2: The air in a balloon with a volume of 25.0 liters (L) is heated from 20 o C to 60 o C. If the pressure stays the same, what will be the new volume of the balloon? 1. First, identify the variables. V 1 = 25.0 L T 1 = 20 o C (convert o C to K by adding 273) = 293K T 2 = 60 o C = 333K V 2 =? 2. Rearrange the equation algebraically to isolate the unknown. V 2 = V 1 x T 2 T 1 3. Insert the known values and solve. V 2 = 25.0 L x 333 K 293 K V 2 = 28.4 L Sample Problem 3: A sample of gas in a 1.00 L flask at 1.50 atm contains 75.0 percent CO 2 and 25.0 percent H 2 O gas. Calculate the partial pressures of each gas. P CO2 = (0.750 x 1.50 atm) = atm P H2O = (0.250 x 1.50 atm) = atm Sample Problem 4: Calculate the temperature of 5.85 mol N 2 gas in a 12.0 L steel bottle under 10.0 atm of pressure. 1. First, identify the variables. P = 10.0 atm V = 12.0 L n = 5.85 mol R = atm/mol K T =? 2. Rearrange the equation algebraically to isolate the unknown. T = P x V n x R 3. Insert known values and solve. T = 10.0 atm x 12.0 L 5.85 mol x L atm/mol K T = 250 K = o C
3 C.9.B Perform stoichiometric calculations, including determination of mass and volume relationships between reactants and products for reactions involving gases. Gas Stoichiometry Reactant and product relationships, including determination of mass and volume relationships, can be found for reactions involving gases. For example, suppose you want to determine the mass relationships between reactants and products for a reaction involving gases. You first convert the given mass to moles. Then you use the mole ratio from the balanced equation to calculate the number of moles of the wanted substance. Finally, you convert the moles of the wanted substance to mass. Sample Problem 1: Mass relationship between reactants and products for reactions involving gases What is the mass of oxygen gas produced when 29.2 g of water is decomposed by electrolysis according to this balanced equation? 2H 2 0 2H 2 + O 2 In order to solve this equation, you have to perform the following calculations: g H 2 O mole H 2 O mol O 2 g O 2 You need to find the mole ratio of H 2 O to O 2 using the balanced equation: 2H 2 0 2H 2 + O 2 Mole Ratio : 2 mol H 2 0 to 1 mol O 2 Can be written as: 2 mol H 2 0 OR 1 mol O 2 depending on problem 1 mol O 2 2 mol H First, start with the given quantity and convert from mass to moles g H 2 0 x 1 mol H g H Then use your mole ratio from the balanced equation to convert moles of reactant to moles of product 29.2 g H 2 O x 1 mol H 2 0 x 1 mol O g H mol H Finish by converting from moles of the product to mass of the product g H 2 O x 1 mol H 2 0 x 1 mol O 2 x 32.0 g O 2 = 26.0 g O g H mol H mol O 2
4 Sample Problem 2: Volume relationships between reactants and products for reactions involving gases Nitrogen monoxide and oxygen gas combine to form the brown gas nitrogen dioxide, which contributes to photochemical smog. What volume (in L) of nitrogen dioxide gas is produced when 34 L of oxygen gas react with an excess of nitrogen monoxide? Assume conditions are at STP. 2NO + O 2 2NO 2 Recall that the following calculations need to be performed: L O 2 mol O 2 mol NO 2 L NO 2 1. First, start with the given quantity and convert from volume to moles by using the mole-volume ratio. 34 L O 2 x 1 mol O L O 2 2. Then, convert from moles of reactant to moles of product by using the correct mole ratio from balance equation. 34 L O 2 x 1 mol O 2 x 2 mol NO L O 2 1 mol O 2 3. Finish by converting from moles of the product to liters of the product. 34 L O 2 x 1 mol O 2 x 2 mol NO 2 x 22.4 L NO 2 = 68 L NO L O 2 1 mol O 2 1 mol NO 2 C.9.C Describe the postulates of kinetic molecular theory Kinetic Molecular Theory Postulates of kinetic molecular theory perfectly describe an ideal gas: 1. Gases are made of molecules or atoms in constant, random motion 2. The volume of these molecules or atoms is extremely small. 3. Collisions among these molecules, atoms, and the container walls are elastic (no net energy loss or gain) 4. Attractive or repulsive forces among these molecules or atoms are extremely small 5. The average kinetic energy of these molecules or atoms is proportional to the absolute temperature of the gas in Kelvin (K).
5 C.11.A Understand energy and its forms, including kinetic, potential, chemical, and thermal energies Energy and Its Forms What is energy? Energy is the capacity to do work or produce heat. Energy does not have mass or volume. Units called joules (J) are used to measure energy. A common non-si unit of energy is the calorie. One calorie (cal) is the quantity of heat that raises the temperature of 1 g of water by 1 degree Celsius. The conversion relationships for joules and calories are: 1 J = cal 1 cal = J Energy exists in different forms: Form Description Example Potential Stored energy Rock resting on a hill Kinetic Energy of motion Rock rolling down a hill Chemical Potential energy stored in Gasoline in car Thermal chemical bonds Kinetic energy of atoms or molecules motion A toaster transforms electrical energy to thermal energy Other forms of energy: light, heat, sound, mechanical motion, nuclear energy, gravitational potential energy, mechanical potential energy. Energy is always being transformed from one form to another. Some energy changes involve single transformations, while others involve many transformations. A toaster performs a single transformation as it transforms electrical energy to thermal energy. In a car engine, a series of transformations are required to make the car move. The chemical energy in the gasoline is ignited, producing light, heat, and sound in the engine. The ignition of the gasoline also pushes a piston, which makes the car move, resulting in mechanical motion. C.11.B Understand the law of conservation of energy and the processes of heat transfer Conservation of Energy and Heat Transfer Law of Conservation of Energy: Energy cannot be created or destroyed in an ordinary chemical reaction, but the form of energy can change. Example: A campfire transforms energy stored in the wood s chemical bonds (Potential energy) into thermal energy (kinetic energy), causing molecules in the air near the fire to move faster (temperature increases). Heat can be transferred 3 ways: 1. Conduction: objects in contact, like a warm hand melting ice 2. Convection: fluid motion or currents, like warm air rising 3. Radiation: electromagnetic waves, like those moving through empty space from the sun or like those made in a kitchen microwave.
6 C.11.C Use thermochemical equations to calculate energy changes that occur in chemical reactions and classify reactions as exothermic or endothermic. Energy Changes in Chemical Reactions Standard state: reference state for a substance (typically 1 atm, 25 o C or 298 K, and 1 mole); defining a property at standard state can allow it to be intensive (independent of size); indicated by a superscript of 0. Standard molar enthalpy of formation, H o f : difference in energy that occurs when one mole of a substance is formed from its pure elements (all at standard state conditions); H o f for any element = 0. means change or difference Enthalpy of reaction, H: energy released or absorbed during a chemical reaction. At standard state, H = H o f (products) H o f (reactants), where means sum of all (multiply each H o f by its coefficient. Reaction Enthalpy Change Description Exothermic H < 0 (negative) Heat is released Endothermic H > 0 (positive) Heat is absorbed Sample Problem 1: Calculate the change in energy for the following reaction at standard conditions. Is this reaction endothermic or exothermic? CH 4 (g) + 2O 2 (g) CO 2 (g) + 2H 2 O (g) Substance H o f (kj/mol) CH O 2 0 CO H 2 O Use the following formula to calculate the change in energy: H = H o f (products) H o f (reactants) H o f (products) = (1 mol)( kj/mol) + (2 mol)( kj/mol) = kj H o f (reactants) = (1 mol)( kj/mol) + (2 mol)(0 kj/mol) = kj H = kj ( kj) = kj (EXOTHERMIC, because H < 0)
7 Sample Problem 2: Calculate the change in energy for the following reaction at standard conditions. Is this reaction endothermic or exothermic? CaCO 3 (s) CaO (s) + CO 2 (g) Substance H o f (kj/mol) CaCO CaO CO Use the following formula to calculate the change in energy: H = H o f (products) H o f (reactants) H o f (products) = (1 mol)( kj/mol) + (1 mol)( kj/mol) = kj H o f (reactants) =(1 mol)( kj/mol) = kj H = kj (-1207 kj) = kj (ENDOTHERMIC, because H > 0) C.11.D Perform calculations involving heat, mass, temperature change, and specific heat. Heat, Mass, Temperature Change, and Specific Heat Specific Heat: Specific heat is an intensive property. It does not vary with the amount of the substance. The specific heat of a substance is the amount of heat needed to raise the temperature of one gram of the substance by one degree Celsius. The unit of specific heat is joules per gram-degree Celsius (J/g o C). The equation for specific heat is: Q = mc p T, Q represents the heat input, m is for mass, c p is for specific heat, and T is for the change in temperature. Sample Problem 1: Given that c p of copper = J/ g o C, calculate the heat absorbed by a kg piece of copper metal that is heated from 25 o C to 125 o C. Note: Convert to common units so units will cancel (change kg to g). So, kg = 20 g Q = mc p T = (20 g)( J/ g o C)( 125 o C - 25 o C) = 770 J
8 Sample Problem 2: The specific heat of water is J/ g o C. A 1,200 g water sample at 19 o C loses 10kJ of heat. What is the final temperature? Note: Convert to common units so units will cancel (change kj to J). So, 10kJ = 10,000 J T = Q = -10,000 J = -2 o C mc p (1,200 g)( J/ g o C) (Q is negative since energy flows out of system) T = T final - T initial, so T final = T + T initial = -2 o C + 19 o C = 17 o C C.11.E Use calorimetry to calculate the heat of a chemical process Calorimetry The Science of Measuring Heat Flow Calorimeter: tool used to measure heat of a chemical process ( H); heat flows into a substance with known c p (specific heat) and the temperature change is recorded: H = -Q ; where Q = mc p T A calorimeter usually contains a carefully measured mass of a substance of known specific heat, such as water. As the reaction absorbs or releases energy, the temperature of the water will change. From that temperature change, the energy that the water absorbed or released can be calculated. Energy released by the reaction = Energy absorbed by the solution Sample Problem 1: A student is using a calorimeter to measure the molar heat of salvation of calcium chloride (CaCl 2 ). The calorimeter contains g of water at 25.1 o C. After 10.0 g of CaCl 2 is fully dissolved, the temperature of water rises to 42.7 o C. (Hint: Remember that the specific heat of water is 4.18 J/g o C. 1. First, write the process as a chemical equation: CaCl 2 (s) Ca 2+ (aq) + 2Cl - (aq) H = H solution 2. Then calculate the temperature change of the water. T = 42.7 o C o C = 17.6 o C 3. Next, calculate the energy absorbed by the water using the following equation. Q water = mass water x c pwater x T Q water = (100.0 g) (4.18 J/ g o C) (17.6 o C) Q water = 7360 J Q water is positive, indicating that the water absorbs energy from the reaction. In a perfect calorimeter, Q water equals the amount of energy released by the dissolving of calcium chloride
9 However, Q water is the energy released when 10.0 g CaCl 2 dissolves, not 1 mol CaCl 2. To calculate the heat per mole, first divide 10.0 g CaCl 2 by the molar mass of CaCl 2, which is g/mol g CaCl 2 x 1 mol CaCl 2 = mol CaCl g CaCl 2 Then divide the heat change of the water by mol CaCl 2. Notice that because the heat change of the water is positive, the heat change of forming the solution is negative. H solution = -Q water / number of mol CaCl 2 H solution = J/ mol CaCl 2 H solution = J/mol CaCl 2 H solution = kj/mol CaCl 2
2. If the volume of a container holding a gas is reduced, what will happen to the presure within the container?
 1. Which gas law states that the volume of a fixed mass of a gas is directly proportional to its Kelvin temperature if the pressure is kept constant? A. Boyle s law B. Charles law C. Dalton s law D. Gay-Lussac
1. Which gas law states that the volume of a fixed mass of a gas is directly proportional to its Kelvin temperature if the pressure is kept constant? A. Boyle s law B. Charles law C. Dalton s law D. Gay-Lussac
Thermochemistry. Energy. 1st Law of Thermodynamics. Enthalpy / Calorimetry. Enthalpy of Formation
 THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
THERMOCHEMISTRY Thermochemistry Energy 1st Law of Thermodynamics Enthalpy / Calorimetry Hess' Law Enthalpy of Formation The Nature of Energy Kinetic Energy and Potential Energy Kinetic energy is the energy
Gas Laws. Gas Properties. Gas Properties. Gas Properties Gases and the Kinetic Molecular Theory Pressure Gas Laws
 Gas Laws Gas Properties Gases and the Kinetic Molecular Theory Pressure Gas Laws Gas Properties 1) Gases have mass - the density of the gas is very low in comparison to solids and liquids, which make it
Gas Laws Gas Properties Gases and the Kinetic Molecular Theory Pressure Gas Laws Gas Properties 1) Gases have mass - the density of the gas is very low in comparison to solids and liquids, which make it
THERMOCHEMISTRY & DEFINITIONS
 THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
THERMOCHEMISTRY & DEFINITIONS Thermochemistry is the study of the study of relationships between chemistry and energy. All chemical changes and many physical changes involve exchange of energy with the
The Nature of Energy. Chapter Six: Kinetic vs. Potential Energy. Energy and Work. Temperature vs. Heat
 The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
The Nature of Energy Chapter Six: THERMOCHEMISTRY Thermodynamics is the study of energy and its transformations. Thermochemistry is the study of the relationship between chemical reactions and energy changes
17.2 Thermochemical Equations
 17.2. Thermochemical Equations www.ck12.org 17.2 Thermochemical Equations Lesson Objectives Define enthalpy, and know the conditions under which the enthalpy change in a reaction is equal to the heat absorbed
17.2. Thermochemical Equations www.ck12.org 17.2 Thermochemical Equations Lesson Objectives Define enthalpy, and know the conditions under which the enthalpy change in a reaction is equal to the heat absorbed
Thermochemistry (chapter 5)
 Thermochemistry (chapter 5) Basic Definitions: Thermochemistry = the study of the energy changes that accompany physical and chemical changes of matter. Energy is defined as the ability to do work or the
Thermochemistry (chapter 5) Basic Definitions: Thermochemistry = the study of the energy changes that accompany physical and chemical changes of matter. Energy is defined as the ability to do work or the
First Law of Thermodynamics
 Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Thermochemistry-Part 1
 Brad Collins Thermochemistry-Part 1 Chapter 7 Thermochemistry Thermodynamics: The study of energy Thermochemistry: The study of energy in chemical reactions Energy: The capacity to do work Work = force
Brad Collins Thermochemistry-Part 1 Chapter 7 Thermochemistry Thermodynamics: The study of energy Thermochemistry: The study of energy in chemical reactions Energy: The capacity to do work Work = force
Thermochemistry. Energy (and Thermochemistry) World of Chemistry Chapter 10. Energy. Energy
 Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Name Date Class THE FLOW OF ENERGY HEAT AND WORK
 17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
17.1 THE FLOW OF ENERGY HEAT AND WORK Section Review Objectives Explain the relationship between energy, heat, and work Distinguish between exothermic and endothermic processes Distinguish between heat
Apparatus for Studying the Relationship Between Pressure and Volume of a Gas
 The Gas Laws Apparatus for Studying the Relationship Between Pressure and Volume of a Gas As P (h) increases V decreases Boyle s Law P x V = constant P 1 x V 1 = P 2 x V 2 Constant temperature Constant
The Gas Laws Apparatus for Studying the Relationship Between Pressure and Volume of a Gas As P (h) increases V decreases Boyle s Law P x V = constant P 1 x V 1 = P 2 x V 2 Constant temperature Constant
Name Class Date. As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings.
 Name Class Date Thermochemistry 17.1 The Flow of Energy As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings. Process Cause Effect endothermic
Name Class Date Thermochemistry 17.1 The Flow of Energy As you read Lesson 17.1, use the cause and effect chart below. Complete the chart with the terms system and surroundings. Process Cause Effect endothermic
Accelerated Chemistry Study Guide Chapter 12, sections 1 and 2: Heat in Chemical Reactions
 Accelerated Chemistry Study Guide Chapter 12, sections 1 and 2: Heat in Chemical Reactions Terms, definitions, topics Joule, calorie (Re-read p 57-58) Thermochemistry Exothermic reaction Endothermic reaction
Accelerated Chemistry Study Guide Chapter 12, sections 1 and 2: Heat in Chemical Reactions Terms, definitions, topics Joule, calorie (Re-read p 57-58) Thermochemistry Exothermic reaction Endothermic reaction
Energy Transformations
 Thermochemistry Energy Transformations Thermochemistry - concerned with heat changes that occur during chemical reactions Energy - capacity for doing work or supplying heat weightless, odorless, tasteless
Thermochemistry Energy Transformations Thermochemistry - concerned with heat changes that occur during chemical reactions Energy - capacity for doing work or supplying heat weightless, odorless, tasteless
Name: General Chemistry Chapter 11 Thermochemistry- Heat and Chemical Change
 Name: General Chemistry Chapter 11 Thermochemistry- Heat and Chemical Change Notepack 1 Section 11.1: The Flow of Energy Heat (Pages 293 299) 1. Define the following terms: a. Thermochemistry b. Energy
Name: General Chemistry Chapter 11 Thermochemistry- Heat and Chemical Change Notepack 1 Section 11.1: The Flow of Energy Heat (Pages 293 299) 1. Define the following terms: a. Thermochemistry b. Energy
11B, 11E Temperature and heat are related but not identical.
 Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Thermochemistry Key Terms thermochemistry heat thermochemical equation calorimeter specific heat molar enthalpy of formation temperature enthalpy change enthalpy of combustion joule enthalpy of reaction
Gases. Measuring Temperature Fahrenheit ( o F): Exceptions to the Ideal Gas Law. Kinetic Molecular Theory
 Ideal gas: a gas in which all collisions between atoms or molecules are perfectly elastic (no energy lost) there are no intermolecular attractive forces Think of an ideal gas as a collection of perfectly
Ideal gas: a gas in which all collisions between atoms or molecules are perfectly elastic (no energy lost) there are no intermolecular attractive forces Think of an ideal gas as a collection of perfectly
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
Chapter 5 Thermochemistry Dr. A. Al-Saadi 1 Preview Introduction to thermochemistry: Potential energy and kinetic energy. Chemical energy. Internal energy, work and heat. Exothermic vs. endothermic reactions.
Chapter 6. Thermochemistry
 Chapter 6. Thermochemistry 1 1. Terms to Know: thermodynamics thermochemistry energy kinetic energy potential energy heat heat vs. temperature work work of expanding gases work of expanding gases under
Chapter 6. Thermochemistry 1 1. Terms to Know: thermodynamics thermochemistry energy kinetic energy potential energy heat heat vs. temperature work work of expanding gases work of expanding gases under
Topic 05 Energetics : Heat Change. IB Chemistry T05D01
 Topic 05 Energetics 5.1-5.2: Heat Change IB Chemistry T05D01 5.1 Exothermic and endothermic reactions - 1 hour 5.1.1 Define the terms exothermic reaction, endothermic reaction and standard enthalpy change
Topic 05 Energetics 5.1-5.2: Heat Change IB Chemistry T05D01 5.1 Exothermic and endothermic reactions - 1 hour 5.1.1 Define the terms exothermic reaction, endothermic reaction and standard enthalpy change
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Energy -Very much a chemistry topic Every chemical change has an accompanying change of. Combustion of fossil fuels The discharging a battery Metabolism of foods If we are to
Chapter 5 Thermochemistry Energy -Very much a chemistry topic Every chemical change has an accompanying change of. Combustion of fossil fuels The discharging a battery Metabolism of foods If we are to
CHEM 1105 S10 March 11 & 14, 2014
 CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
CHEM 1105 S10 March 11 & 14, 2014 Today s topics: Thermochemistry (Chapter 6) Basic definitions Calorimetry Enthalpy Thermochemical equations Calculating heats of reaction Hess s Law Energy and Heat Some
Name Date Class SECTION 16.1 PROPERTIES OF SOLUTIONS
 SOLUTIONS Practice Problems In your notebook, solve the following problems. SECTION 16.1 PROPERTIES OF SOLUTIONS 1. The solubility of CO 2 in water at 1.22 atm is 0.54 g/l. What is the solubility of carbon
SOLUTIONS Practice Problems In your notebook, solve the following problems. SECTION 16.1 PROPERTIES OF SOLUTIONS 1. The solubility of CO 2 in water at 1.22 atm is 0.54 g/l. What is the solubility of carbon
AP CHEMISTRY NOTES 4-1 THERMOCHEMISTRY: ENTHALPY AND ENTROPY
 AP CHEMISTRY NOTES 4-1 THERMOCHEMISTRY: ENTHALPY AND ENTROPY Reaction Rate how fast a chemical reaction occurs Collision Theory In order for a chemical reaction to occur, the following conditions must
AP CHEMISTRY NOTES 4-1 THERMOCHEMISTRY: ENTHALPY AND ENTROPY Reaction Rate how fast a chemical reaction occurs Collision Theory In order for a chemical reaction to occur, the following conditions must
IB Chemistry Solutions Gasses and Energy
 Solutions A solution is a homogeneous mixture it looks like one substance. An aqueous solution will be a clear mixture with only one visible phase. Be careful with the definitions of clear and colourless.
Solutions A solution is a homogeneous mixture it looks like one substance. An aqueous solution will be a clear mixture with only one visible phase. Be careful with the definitions of clear and colourless.
Name: Score: /100. Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each
 Name: Score: /100 Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each 1. Which of the following contains the greatest number of moles of O? A) 2.3 mol H 2 O
Name: Score: /100 Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each 1. Which of the following contains the greatest number of moles of O? A) 2.3 mol H 2 O
Ch 6. Energy and Chemical Change. Brady & Senese, 5th Ed.
 Ch 6. Energy and Chemical Change Brady & Senese, 5th Ed. Energy Is The Ability To Do Work Energy is the ability to do work (move mass over a distance) or transfer heat Types: kinetic and potential kinetic:
Ch 6. Energy and Chemical Change Brady & Senese, 5th Ed. Energy Is The Ability To Do Work Energy is the ability to do work (move mass over a distance) or transfer heat Types: kinetic and potential kinetic:
Thermochemistry (chapter 5)
 Thermochemistry (chapter 5) Is the study of the energy changes that accompany physical and chemical changes. Energy is defined as the ability to do work or the capacity to produce change. The forms of
Thermochemistry (chapter 5) Is the study of the energy changes that accompany physical and chemical changes. Energy is defined as the ability to do work or the capacity to produce change. The forms of
Section Using Gas Laws to Solve Problems
 Gases and Gas Laws Section 13.2 Using Gas Laws to Solve Problems Kinetic Molecular Theory Particles of matter are ALWAYS in motion Volume of individual particles is zero. Consists of large number of particles
Gases and Gas Laws Section 13.2 Using Gas Laws to Solve Problems Kinetic Molecular Theory Particles of matter are ALWAYS in motion Volume of individual particles is zero. Consists of large number of particles
Chemistry Chapter 16. Reaction Energy
 Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Chemistry Reaction Energy Section 16.1.I Thermochemistry Objectives Define temperature and state the units in which it is measured. Define heat and state its units. Perform specific-heat calculations.
Thermochemistry. Mr.V
 Thermochemistry Mr.V Introduction to Energy changes System Surroundings Exothermic Endothermic Internal energy Enthalpy Definitions System A specified part of the universe which is under investigation
Thermochemistry Mr.V Introduction to Energy changes System Surroundings Exothermic Endothermic Internal energy Enthalpy Definitions System A specified part of the universe which is under investigation
Thermochemistry: Heat and Chemical Change
 Thermochemistry: Heat and Chemical Change 1 Heat or Thermal Energy (q) Heat is a form of energy Is heat the same as temperature? Heat flows between two objects at different temperatures. Hot Cold 2 Chemical
Thermochemistry: Heat and Chemical Change 1 Heat or Thermal Energy (q) Heat is a form of energy Is heat the same as temperature? Heat flows between two objects at different temperatures. Hot Cold 2 Chemical
Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93
 Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93 Chapter 6 Thermochemistry The study of chemical reactions and the energy changes
Chapter 6 Problems: 9, 19, 24, 25, 26, 27, 31-33, 37, 39, 43, 45, 47, 48, 53, 55, 57, 59, 65, 67, 73, 78-82, 85, 89, 93 Chapter 6 Thermochemistry The study of chemical reactions and the energy changes
Introduction to Thermochemistry. Thermochemistry Unit. Definition. Terminology. Terminology. Terminology 07/04/2016. Chemistry 30
 Thermochemistry Unit Introduction to Thermochemistry Chemistry 30 Definition Thermochemistry is the branch of chemistry concerned with the heat produced and used in chemical reactions. Most of thermochemistry
Thermochemistry Unit Introduction to Thermochemistry Chemistry 30 Definition Thermochemistry is the branch of chemistry concerned with the heat produced and used in chemical reactions. Most of thermochemistry
- The empirical gas laws (including the ideal gas equation) do not always apply.
 145 At 300 C, ammonium nitrate violently decomposes to produce nitrogen gas, oxygen gas, and water vapor. What is the total volume of gas that would be produced at 1.00 atm by the decomposition of 15.0
145 At 300 C, ammonium nitrate violently decomposes to produce nitrogen gas, oxygen gas, and water vapor. What is the total volume of gas that would be produced at 1.00 atm by the decomposition of 15.0
Gases. What are the four variables needed to describe a gas?
 Gases What are the four variables needed to describe a gas? 1 Gases The simplest state of matter K.E. >> intermolecular forces Random motion Predictable behavior 2 Gases at STP Few Elements: H 2 N 2 O
Gases What are the four variables needed to describe a gas? 1 Gases The simplest state of matter K.E. >> intermolecular forces Random motion Predictable behavior 2 Gases at STP Few Elements: H 2 N 2 O
Thermochemistry: Energy Flow and Chemical Reactions
 Thermochemistry: Energy Flow and Chemical Reactions Outline thermodynamics internal energy definition, first law enthalpy definition, energy diagrams, calorimetry, theoretical calculation (heats of formation
Thermochemistry: Energy Flow and Chemical Reactions Outline thermodynamics internal energy definition, first law enthalpy definition, energy diagrams, calorimetry, theoretical calculation (heats of formation
CHAPTER 17 Thermochemistry
 CHAPTER 17 Thermochemistry Thermochemistry The study of the heat changes that occur during chemical reactions and physical changes of state. Chemical Change: new substances created during chemical reaction
CHAPTER 17 Thermochemistry Thermochemistry The study of the heat changes that occur during chemical reactions and physical changes of state. Chemical Change: new substances created during chemical reaction
Chapter 5. Thermochemistry
 Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Chapter 5 Thermochemistry Energy Thermodynamics Study of the relationship between heat, work, and other forms of energy Thermochemistry A branch of thermodynamics Focuses on the study of heat given off
Chapter 6 Energy and Chemical Change. Brady and Senese 5th Edition
 Chapter 6 Energy and Chemical Change Brady and Senese 5th Edition Index 6.1 An object has energy if it is capable of doing work 6.2 Internal energy is the total energy of an object s molecules 6.3 Heat
Chapter 6 Energy and Chemical Change Brady and Senese 5th Edition Index 6.1 An object has energy if it is capable of doing work 6.2 Internal energy is the total energy of an object s molecules 6.3 Heat
CHAPTER 16: REACTION ENERGY AND CHAPTER 17: REACTION KINETICS. Honors Chemistry Ms. Agostine
 CHAPTER 16: REACTION ENERGY AND CHAPTER 17: REACTION KINETICS Honors Chemistry Ms. Agostine 16.1 Thermochemistry Definition: study of the transfers of energy as heat that accompany chemical reactions and
CHAPTER 16: REACTION ENERGY AND CHAPTER 17: REACTION KINETICS Honors Chemistry Ms. Agostine 16.1 Thermochemistry Definition: study of the transfers of energy as heat that accompany chemical reactions and
Exam 4, Enthalpy and Gases
 CHEM 1100 Dr. Stone November 8, 2017 Name_ G Exam 4, Enthalpy and Gases Equations and constants you may need: ΔE system = q + w PV = nrt R = 0.0821 (L*atm)/(mole*K) w = -PΔV K.E. = 1 2 m *µ 2 rms µ rms=
CHEM 1100 Dr. Stone November 8, 2017 Name_ G Exam 4, Enthalpy and Gases Equations and constants you may need: ΔE system = q + w PV = nrt R = 0.0821 (L*atm)/(mole*K) w = -PΔV K.E. = 1 2 m *µ 2 rms µ rms=
Thermochemistry Chapter 4
 Thermochemistry Chapter 4 Thermochemistry is the study of energy changes that occur during chemical reactions Focus is on heat and matter transfer between the system and the surroundings Energy The ability
Thermochemistry Chapter 4 Thermochemistry is the study of energy changes that occur during chemical reactions Focus is on heat and matter transfer between the system and the surroundings Energy The ability
Gases. Properties of Gases Kinetic Molecular Theory of Gases Pressure Boyle s and Charles Law The Ideal Gas Law Gas reactions Partial pressures.
 Gases Properties of Gases Kinetic Molecular Theory of Gases Pressure Boyle s and Charles Law The Ideal Gas Law Gas reactions Partial pressures Gases Properties of Gases All elements will form a gas at
Gases Properties of Gases Kinetic Molecular Theory of Gases Pressure Boyle s and Charles Law The Ideal Gas Law Gas reactions Partial pressures Gases Properties of Gases All elements will form a gas at
Calculate the mass of L of oxygen gas at 25.0 C and 1.18 atm pressure.
 148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
I. The Nature of Energy A. Energy
 I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
I. The Nature of Energy A. Energy is the ability to do work or produce heat. It exists in 2 forms: 1. Potential energy is energy due to the composition or position of an object. 2. Kinetic energy is energy
Chapter 3. Thermochemistry: Energy Flow and Chemical Change. 5.1 Forms of Energy and Their Interconversion
 Chapter 3 Thermochemistry: Energy Flow and Chemical Change 5.1 Forms of Energy and Their Interconversion 5.2 Enthalpy: Chemical Change at Constant Pressure 5.3 Calorimetry: Measuring the Heat of a Chemical
Chapter 3 Thermochemistry: Energy Flow and Chemical Change 5.1 Forms of Energy and Their Interconversion 5.2 Enthalpy: Chemical Change at Constant Pressure 5.3 Calorimetry: Measuring the Heat of a Chemical
First Law of Thermodynamics: energy cannot be created or destroyed.
 1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
Chapter 5 Thermochemistry
 Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 5 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Unit 6. Unit Vocabulary: Distinguish between the three phases of matter by identifying their different
 *STUDENT* Unit Objectives: Absolute Zero Avogadro s Law Normal Boiling Point Compound Cooling Curve Deposition Energy Element Evaporation Heat Heat of Fusion Heat of Vaporization Unit 6 Unit Vocabulary:
*STUDENT* Unit Objectives: Absolute Zero Avogadro s Law Normal Boiling Point Compound Cooling Curve Deposition Energy Element Evaporation Heat Heat of Fusion Heat of Vaporization Unit 6 Unit Vocabulary:
Chapter 17 Thermochemistry
 Chapter 17 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
Chapter 17 Thermochemistry Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Explain how energy, heat, and work are related. 2 Section 17.1 The Flow of Energy Heat and Work OBJECTIVES: Classify
This reaction is ENDOTHERMIC. Energy is being transferred from the room/flask/etc. (the SURROUNDINGS) to the reaction itself (the SYSTEM).
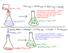 151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
This should serve a s a study guide as you go on to do the problems in Sapling and take the quizzes and exams.
 CHM 111 Chapter 9 Worksheet and Study Guide Purpose: This is a guide for your as you work through the chapter. The major topics are provided so that you can write notes on each topic and work the corresponding
CHM 111 Chapter 9 Worksheet and Study Guide Purpose: This is a guide for your as you work through the chapter. The major topics are provided so that you can write notes on each topic and work the corresponding
Chapter 5 The Gaseous State
 Chapter 5 The Gaseous State Contents and Concepts Gas Laws We will investigate the quantitative relationships that describe the behavior of gases. 1. Gas Pressure and Its Measurement 2. Empirical Gas Laws
Chapter 5 The Gaseous State Contents and Concepts Gas Laws We will investigate the quantitative relationships that describe the behavior of gases. 1. Gas Pressure and Its Measurement 2. Empirical Gas Laws
Name: Thermochemistry. Practice Test C. General Chemistry Honors Chemistry
 Name: Thermochemistry C Practice Test C General Chemistry Honors Chemistry 1 Objective 1: Use the relationship between mass, specific heat, and temperature change to calculate the heat flow during a chemical
Name: Thermochemistry C Practice Test C General Chemistry Honors Chemistry 1 Objective 1: Use the relationship between mass, specific heat, and temperature change to calculate the heat flow during a chemical
Chapter 11. Thermochemistry. 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages
 Chapter 11 Thermochemistry 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages 293-94 The Flow of energy - heat Thermochemistry concerned with the heat changes that occur
Chapter 11 Thermochemistry 1. Let s begin by previewing the chapter (Page 292). 2. We will partner read Pages 293-94 The Flow of energy - heat Thermochemistry concerned with the heat changes that occur
Apply the concept of percent yield to stoichiometric problems. Methanol can be produced through the reaction of CO and H 2 in the presence of a
 Apply the concept of percent yield to stoichiometric problems. Methanol can be produced through the reaction of CO and H 2 in the presence of a catalyst. CO (g) + H 2 (g) CH 3 OH (l) If 75.0 g of CO reacts
Apply the concept of percent yield to stoichiometric problems. Methanol can be produced through the reaction of CO and H 2 in the presence of a catalyst. CO (g) + H 2 (g) CH 3 OH (l) If 75.0 g of CO reacts
12.2. The Ideal Gas Law. Density and Molar Mass of Gases SECTION. Key Terms
 SECTION 12.2 The Ideal Gas Law You have related the combined gas law to Avogadro s volume-mole gas relationship using two sets of conditions. This enabled you to make calculations of pressure, temperature,
SECTION 12.2 The Ideal Gas Law You have related the combined gas law to Avogadro s volume-mole gas relationship using two sets of conditions. This enabled you to make calculations of pressure, temperature,
CHEMISTRY Matter and Change. Chapter 13: Gases
 CHEMISTRY Matter and Change Chapter 13: Gases CHAPTER 13 Table Of Contents Section 13.1 Section 13.2 Section 13.3 The Gas Laws The Ideal Gas Law Gas Stoichiometry Click a hyperlink to view the corresponding
CHEMISTRY Matter and Change Chapter 13: Gases CHAPTER 13 Table Of Contents Section 13.1 Section 13.2 Section 13.3 The Gas Laws The Ideal Gas Law Gas Stoichiometry Click a hyperlink to view the corresponding
CHAPTER 13 Gases The Gas Laws
 CHAPTER 13 Gases 13.1 The Gas Laws The gas laws apply to ideal gases, which are described by the kinetic theory in the following five statements. Gas particles do not attract or repel each other. Gas particles
CHAPTER 13 Gases 13.1 The Gas Laws The gas laws apply to ideal gases, which are described by the kinetic theory in the following five statements. Gas particles do not attract or repel each other. Gas particles
Gases! n Properties! n Kinetic Molecular Theory! n Variables! n The Atmosphere! n Gas Laws!
 Gases n Properties n Kinetic Molecular Theory n Variables n The Atmosphere n Gas Laws Properties of a Gas n No definite shape or volume n Gases expand to fill any container n Thus they take the shape of
Gases n Properties n Kinetic Molecular Theory n Variables n The Atmosphere n Gas Laws Properties of a Gas n No definite shape or volume n Gases expand to fill any container n Thus they take the shape of
CHEMISTRY. Chapter 5 Thermochemistry
 CHEMISTRY The Central Science 8 th Edition Chapter 5 Thermochemistry Dr. Kozet YAPSAKLI The Nature of Energy Kinetic and Potential Energy Potential energy can be converted into kinetic energy. E p = mgh
CHEMISTRY The Central Science 8 th Edition Chapter 5 Thermochemistry Dr. Kozet YAPSAKLI The Nature of Energy Kinetic and Potential Energy Potential energy can be converted into kinetic energy. E p = mgh
Chemistry I Practice Exam
 Chemistry I Practice Exam Name Multiple Choice: Choose the best answer that completes each statement. Put all answers on your answer sheet. 1. The mass of one mole of NaCl is a..53 g b. 22.99 g c. 5.44
Chemistry I Practice Exam Name Multiple Choice: Choose the best answer that completes each statement. Put all answers on your answer sheet. 1. The mass of one mole of NaCl is a..53 g b. 22.99 g c. 5.44
Gas Volumes and the Ideal Gas Law
 SECTION 11.3 Gas Volumes and the Ideal Gas Law Section 2 presented laws that describe the relationship between the pressure, temperature, and volume of a gas. The volume of a gas is also related to the
SECTION 11.3 Gas Volumes and the Ideal Gas Law Section 2 presented laws that describe the relationship between the pressure, temperature, and volume of a gas. The volume of a gas is also related to the
CST Review Part 2. Liquid. Gas. 2. How many protons and electrons do the following atoms have?
 CST Review Part 2 1. In the phase diagram, correctly label the x-axis and the triple point write the names of all six phases transitions in the arrows provided. Liquid Pressure (ATM) Solid Gas 2. How many
CST Review Part 2 1. In the phase diagram, correctly label the x-axis and the triple point write the names of all six phases transitions in the arrows provided. Liquid Pressure (ATM) Solid Gas 2. How many
1. Determine the mass of water that can be produced when 10.0g of hydrogen is combined with excess oxygen. 2 H 2 + O 2 2 H 2 O
 Pre-AP Chemistry Spring 2016 Final Review Objective 6.1: Students will recognize indicators of chemical change write balanced chemical equations to describe them based on common reactivity patterns. [S.12.C.1,
Pre-AP Chemistry Spring 2016 Final Review Objective 6.1: Students will recognize indicators of chemical change write balanced chemical equations to describe them based on common reactivity patterns. [S.12.C.1,
Name Date Class THERMOCHEMISTRY
 Name Date Class 17 THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY HEAT AND WORK (pages 505 510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
Name Date Class 17 THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY HEAT AND WORK (pages 505 510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
Gas Volumes and the Ideal Gas Law
 Section 3, 9B s Gases react in whole-number ratios. Equal volumes of gases under the same conditions contain equal numbers of molecules. All gases have a volume of 22.4 L under standard conditions. In
Section 3, 9B s Gases react in whole-number ratios. Equal volumes of gases under the same conditions contain equal numbers of molecules. All gases have a volume of 22.4 L under standard conditions. In
Thermochemistry. Chapter 6. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Thermochemistry Chapter 6 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Energy is the capacity to do work. Radiant energy comes from the sun and is earth s
Thermochemistry Chapter 6 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Energy is the capacity to do work. Radiant energy comes from the sun and is earth s
A Gas Uniformly fills any container. Easily compressed. Mixes completely with any other gas. Exerts pressure on its surroundings.
 Chapter 5 Gases Chapter 5 A Gas Uniformly fills any container. Easily compressed. Mixes completely with any other gas. Exerts pressure on its surroundings. Copyright Cengage Learning. All rights reserved
Chapter 5 Gases Chapter 5 A Gas Uniformly fills any container. Easily compressed. Mixes completely with any other gas. Exerts pressure on its surroundings. Copyright Cengage Learning. All rights reserved
Chapter 8 Thermochemistry: Chemical Energy. Chemical Thermodynamics
 Chapter 8 Thermochemistry: Chemical Energy Chapter 8 1 Chemical Thermodynamics Chemical Thermodynamics is the study of the energetics of a chemical reaction. Thermodynamics deals with the absorption or
Chapter 8 Thermochemistry: Chemical Energy Chapter 8 1 Chemical Thermodynamics Chemical Thermodynamics is the study of the energetics of a chemical reaction. Thermodynamics deals with the absorption or
Chapter 5 - Thermochemistry
 Chapter 5 - Thermochemistry Study of energy changes that accompany chemical rx s. I) Nature of Energy Energy / Capacity to do work Mechanical Work w = F x d Heat energy - energy used to cause the temperature
Chapter 5 - Thermochemistry Study of energy changes that accompany chemical rx s. I) Nature of Energy Energy / Capacity to do work Mechanical Work w = F x d Heat energy - energy used to cause the temperature
Although different gasses may differ widely in their chemical properties, they share many physical properties
 IV. Gases (text Chapter 9) A. Overview of Chapter 9 B. Properties of gases 1. Ideal gas law 2. Dalton s law of partial pressures, etc. C. Kinetic Theory 1. Particulate model of gases. 2. Temperature and
IV. Gases (text Chapter 9) A. Overview of Chapter 9 B. Properties of gases 1. Ideal gas law 2. Dalton s law of partial pressures, etc. C. Kinetic Theory 1. Particulate model of gases. 2. Temperature and
Chapter Objectives. Chapter 9 Energy and Chemistry. Chapter Objectives. Energy Use and the World Economy. Energy Use and the World Economy
 Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
Chapter Objectives Larry Brown Tom Holme www.cengage.com/chemistry/brown Chapter 9 Energy and Chemistry Explain the economic importance of conversions between different forms of energy and the inevitability
Unit 15 Energy and Thermochemistry Notes
 Name KEY Period CRHS Academic Chemistry Unit 15 Energy and Thermochemistry Notes Quiz Date Exam Date Lab Dates Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic Chemistry Website: https://cincochem.pbworks.com
Name KEY Period CRHS Academic Chemistry Unit 15 Energy and Thermochemistry Notes Quiz Date Exam Date Lab Dates Notes, Homework, Exam Reviews and Their KEYS located on CRHS Academic Chemistry Website: https://cincochem.pbworks.com
Ch. 17 Thermochemistry
 Ch. 17 Thermochemistry 17.1 The Flow of Energy Energy Transformations Thermochemistry: study of energy changes in chemical reactions and changes in state Chemical potential energy: energy stored in bonds
Ch. 17 Thermochemistry 17.1 The Flow of Energy Energy Transformations Thermochemistry: study of energy changes in chemical reactions and changes in state Chemical potential energy: energy stored in bonds
Energy Changes in Reactions p
 Energy Changes in Reactions p.126 210 Heat vs. temperature: Heat is a form of energy, it is transferred from one system to another Temperature is an indication of the intensity of heat, it measures the
Energy Changes in Reactions p.126 210 Heat vs. temperature: Heat is a form of energy, it is transferred from one system to another Temperature is an indication of the intensity of heat, it measures the
Gases: Their Properties & Behavior. Chapter 09 Slide 1
 9 Gases: Their Properties & Behavior Chapter 09 Slide 1 Gas Pressure 01 Chapter 09 Slide 2 Gas Pressure 02 Units of pressure: atmosphere (atm) Pa (N/m 2, 101,325 Pa = 1 atm) Torr (760 Torr = 1 atm) bar
9 Gases: Their Properties & Behavior Chapter 09 Slide 1 Gas Pressure 01 Chapter 09 Slide 2 Gas Pressure 02 Units of pressure: atmosphere (atm) Pa (N/m 2, 101,325 Pa = 1 atm) Torr (760 Torr = 1 atm) bar
Unit 7 Kinetics and Thermodynamics
 17.1 The Flow of Energy Heat and Work Unit 7 Kinetics and Thermodynamics I. Energy Transformations A. Temperature 1. A measure of the average kinetic energy of the particles in a sample of matter B. Heat
17.1 The Flow of Energy Heat and Work Unit 7 Kinetics and Thermodynamics I. Energy Transformations A. Temperature 1. A measure of the average kinetic energy of the particles in a sample of matter B. Heat
THERMOCHEMISTRY. This section explains the relationship between energy and heat, and distinguishes between heat capacity and specific heat.
 I Name _ Date _ Class _ THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY-HEAT AND WORK (pages 505-510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
I Name _ Date _ Class _ THERMOCHEMISTRY SECTION 17.1 THE FLOW OF ENERGY-HEAT AND WORK (pages 505-510) This section explains the relationship between energy and heat, and distinguishes between heat capacity
Name: Score: /100. Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each
 Name: Score: /100 Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each 1. Which of the following contains the greatest number of moles of O? A) 2.3 mol H 2 O
Name: Score: /100 Part I. Multiple choice. Write the letter of the correct answer for each problem. 3 points each 1. Which of the following contains the greatest number of moles of O? A) 2.3 mol H 2 O
CHEMISTRY CP Name: Period:
 CHEMISTRY CP Name: Period: CHEMISTRY SPRING FINAL REVIEW SHEET NOTE: Below are concepts that we have covered in class throughout the second semester. Questions are organized by chapter/concept to help
CHEMISTRY CP Name: Period: CHEMISTRY SPRING FINAL REVIEW SHEET NOTE: Below are concepts that we have covered in class throughout the second semester. Questions are organized by chapter/concept to help
c. K 2 CO 3 d. (NH 4 ) 2 SO 4 Answer c
 Chem 130 Name Exam 2, Ch 4-6 July 7, 2016 100 Points Please follow the instructions for each section of the exam. Show your work on all mathematical problems. Provide answers with the correct units and
Chem 130 Name Exam 2, Ch 4-6 July 7, 2016 100 Points Please follow the instructions for each section of the exam. Show your work on all mathematical problems. Provide answers with the correct units and
Heat. Heat Terminology 04/12/2017. System Definitions. System Definitions
 System Definitions Heat Physical Science 20 Ms. Hayduk Heat Terminology System: the part of the universe being studied (big Earth, or small one atom) Surroundings: the part of the universe outside the
System Definitions Heat Physical Science 20 Ms. Hayduk Heat Terminology System: the part of the universe being studied (big Earth, or small one atom) Surroundings: the part of the universe outside the
Energetics. Topic
 Energetics Topic 5.1 5.2 Topic 5.1 Exothermic and Endothermic Reactions?? total energy of the universe is a constant if a system loses energy, it must be gained by the surroundings, and vice versa Enthalpy
Energetics Topic 5.1 5.2 Topic 5.1 Exothermic and Endothermic Reactions?? total energy of the universe is a constant if a system loses energy, it must be gained by the surroundings, and vice versa Enthalpy
Chapter 6: Thermochemistry
 Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
Chapter 6: Thermochemistry Section 6.1: Introduction to Thermochemistry Thermochemistry refers to the study of heat flow or heat energy in a chemical reaction. In a study of Thermochemistry the chemical
Chapter 6 Thermochemistry 許富銀
 Chapter 6 Thermochemistry 許富銀 6.1 Chemical Hand Warmers Thermochemistry: the study of the relationships between chemistry and energy Hand warmers use the oxidation of iron as the exothermic reaction: Nature
Chapter 6 Thermochemistry 許富銀 6.1 Chemical Hand Warmers Thermochemistry: the study of the relationships between chemistry and energy Hand warmers use the oxidation of iron as the exothermic reaction: Nature
Chapter 5: Thermochemistry. Molecular Kinetic Energy -Translational energy E k, translational = 1/2mv 2 -Rotational energy 5.
 Chapter 5: Thermochemistry 1. Thermodynamics 2. Energy 3. Specific Heat 4. Enthalpy 5. Enthalpies of Reactions 6. Hess s Law 7. State Functions 8. Standard Enthalpies of Formation 9. Determining Enthalpies
Chapter 5: Thermochemistry 1. Thermodynamics 2. Energy 3. Specific Heat 4. Enthalpy 5. Enthalpies of Reactions 6. Hess s Law 7. State Functions 8. Standard Enthalpies of Formation 9. Determining Enthalpies
Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions
 Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions Jeffrey Mack California State University, Sacramento Energy & Chemistry Questions that need to be addressed: How do we measure
Chapter 5 Principles of Chemical Reactivity: Energy and Chemical Reactions Jeffrey Mack California State University, Sacramento Energy & Chemistry Questions that need to be addressed: How do we measure
CP Chapter 17 Thermochemistry
 CP Chapter 17 Thermochemistry Thermochemistry Thermochemistry is the study of energy that occur during chemical reactions and phase changes (changes of state) The Nature of Energy Energy is the ability
CP Chapter 17 Thermochemistry Thermochemistry Thermochemistry is the study of energy that occur during chemical reactions and phase changes (changes of state) The Nature of Energy Energy is the ability
= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)
![= (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj) = (25.0 g)(0.137 J/g C)[61.2 C - (-31.4 C)] = 317 J (= kj)](/thumbs/89/101004910.jpg) CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
CHEM 101A ARMSTRONG SOLUTIONS TO TOPIC D PROBLEMS 1) For all problems involving energy, you may give your answer in either joules or kilojoules, unless the problem specifies a unit. (In general, though,
Chapter 8 Thermochemistry: Chemical Energy
 Chapter 8 Thermochemistry: Chemical Energy 國防醫學院生化學科王明芳老師 2011-11-8 & 2011-11-15 Chapter 8/1 Energy and Its Conservation Conservation of Energy Law: Energy cannot be created or destroyed; it can only be
Chapter 8 Thermochemistry: Chemical Energy 國防醫學院生化學科王明芳老師 2011-11-8 & 2011-11-15 Chapter 8/1 Energy and Its Conservation Conservation of Energy Law: Energy cannot be created or destroyed; it can only be
Thermochemistry. Section The flow of energy
 Thermochemistry Section 17.1 - The flow of energy What is Energy? Energy is the capacity for doing work or supplying heat Energy does not have mass or volume, and it can only be detected because of its
Thermochemistry Section 17.1 - The flow of energy What is Energy? Energy is the capacity for doing work or supplying heat Energy does not have mass or volume, and it can only be detected because of its
Ch10.4 Attractive Forces
 Ch10.4 Attractive Forces Intermolecular Forces are the forces holding molecules to each other. Solids have strong forces Gases (vapor) have weak forces Intermolecular forces determine the phase of matter.
Ch10.4 Attractive Forces Intermolecular Forces are the forces holding molecules to each other. Solids have strong forces Gases (vapor) have weak forces Intermolecular forces determine the phase of matter.
CHAPTER 14: The Behavior of Gases
 Name: CHAPTER 14: The Behavior of Gases Period: RELATIONSHIPS BETWEEN PRESSURE, VOLUME & TEMPERATURE OF A GAS Boyle s Law-Pressure and Volume Volume (ml) Pressure ( ) 60 50 40 30 20 10 Practice problem:
Name: CHAPTER 14: The Behavior of Gases Period: RELATIONSHIPS BETWEEN PRESSURE, VOLUME & TEMPERATURE OF A GAS Boyle s Law-Pressure and Volume Volume (ml) Pressure ( ) 60 50 40 30 20 10 Practice problem:
Thermochemistry. Energy and Chemical Change
 Thermochemistry Energy and Chemical Change Energy Energy can change for and flow, but it is always conserved. The Nature of Energy Energy the ability to do work or produce heat Potential energy Kinetic
Thermochemistry Energy and Chemical Change Energy Energy can change for and flow, but it is always conserved. The Nature of Energy Energy the ability to do work or produce heat Potential energy Kinetic
AP CHEMISTRY. Unit 5 Thermochemistry. Jeff Venables Northwestern High School
 AP CHEMISTRY Unit 5 Thermochemistry Jeff Venables Northwestern High School Kinetic Energy and Potential Energy Kinetic energy - the energy of motion: Ek = 1 mv 2 Potential energy - the energy an object
AP CHEMISTRY Unit 5 Thermochemistry Jeff Venables Northwestern High School Kinetic Energy and Potential Energy Kinetic energy - the energy of motion: Ek = 1 mv 2 Potential energy - the energy an object
Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy
 Unit 7: Energy Outline Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy Energy Energy is the ability to do work or produce heat. The energy
Unit 7: Energy Outline Types of Energy Calorimetry q = mc T Thermochemical Equations Hess s Law Spontaneity, Entropy, Gibb s Free energy Energy Energy is the ability to do work or produce heat. The energy
Slide 2 / 118. Thermochemistry
 Slide 1 / 118 Slide 2 / 118 Thermochemistry Slide 3 / 118 Table of Contents The Nature of Energy State Functions** Click on the topic to go to that section Enthalpy Measuring Enthalpy Changes: Calorimetry
Slide 1 / 118 Slide 2 / 118 Thermochemistry Slide 3 / 118 Table of Contents The Nature of Energy State Functions** Click on the topic to go to that section Enthalpy Measuring Enthalpy Changes: Calorimetry
