Chem 400. Inorganic Chemistry. Exam 1. Σ of of of of of of of of 10. Name (please print)
|
|
|
- Marian Mason
- 6 years ago
- Views:
Transcription
1 Chem 400 Inorganic Chemistr Eam 1 1 of 10 2 of 10 3 of 30 4 of 10 5 of 10 6 of 20 7 of 10 Σ of 100 % Name (please print)
2 1. Sketch out the molecular orbital energ level diagram for B 3 appling the LGO approach. Define the spatial orientation of B 3 using,,z directions and identif all atomic and ligand group orbitals involved in bonding. (10 points) Planar B 3 ψ 7 node z 2p z 2p 2p ψ 5 ψ 4 ψ 6 LGO(3) LGO(2) LGO(1) LGO(2) LGO(3) Energ ψ 2 ψ 3 LGO(1) 2s B ψ 1 B B 3 All atoms of B 3 lie in the plane, coincides with principal rotation ais. LGO s of 3 fragment are derived b taking linear combinations of the three 1 s orbitals. 1
3 2. Draw schematic representations for all of the bonding and antibonding molecular orbitals (MO s) of B 3 (use the information derived in Problem 1). (10 points) ψ 1 ψ 2 ψ 3 ψ 4 p z ψ 7 ψ 5 ψ 6 2
4 3. Draw Lewis structures for each of the following molecules. (2 points each) Based on our Lewis structure, develop threedimensional structures for each compound using VSEPR theor. (2 points each) inall, determine the appropriate point group for each of our three dimensional drawings. (2 points each) a. Br 4 Br Br D 4h b. I 7 I I D 5h c. SiClBrI Cl Cl Si I Br Si I Br C 1 d. S 5 S S C 4v 3
5 e. 2 O 2 O O O O C 2h 4. ow man normal modes of vibration does each of the following molecules possess, and how man of those are IR active for (2 points each): a. CCl 4 CCl 4 is tetrahedral and nonpolar; T d smmetr. our modes of vibrational freedom are IR active and give rise to two bands in the IR. b. BCl 3 BCl 3 has a trigonal planar structure and is nonpolar. Therefore, the smmetric stretch is IR inactive. ive modes of vibrational freedom give rise to three bands in the IR spectrum. c. CS 2 CS 2 is linear and nonpolar. Thus the asmteric stretch and the doubl degenerate deformation are IR active, giving rise to two bands in the IR spectrum. d. 2 S 2 S is polar and possesses a bent molecular shape. All three modes of vibration are IR active. e. N 3 N 3 has trigonal pramidal structure and is polar. There are si modes of vibrational freedom giving rise to four bands in the IR spectrum. 4
6 6 5. Give the epected possible covalent MX n compounds ( where X = Cl or Br ) of Sn, At, and Ra. Assume onl σbonding, and predict the geometr of each molecule. int: To predict possible covalent states, start with the ground state electron configurations of the atom and obtain the simplest possible covalent state b utilizing onl the initiall unpaired electrons in covalent bond formation. The partial table below indicates would tpe of answer I epect. (10 points) Valence State Configuration bridization ormula Geometr Sn sp 2 SnX 2 sp 3 SnX 4 bent tetrahedral At sp 3 AtX linear sp 3 d sp 3 d 2 AtX 3 AtX 5 Tshaped square pramidal sp 3 d 3 AtX 5 pentagonal bipramidal Ra sd RaX 2 bent
7 6.a Draw an MO energlevel diagram for the MOs that would arise in a diatomic molecule from the combination of unhbridized d orbitals. Label the AOs being combined and the resulting MOs. Let the z ais lie along the σbond. (10 points) σ* π 1 * π 2 * Energ δ 1 * δ 2 * d 2 2 d 2 2 d d d d z z d z d z d d z 2 z 2 Note: dorbitals are degenerate, drawn at different energies to simplif figure. δ 1 δ 2 π 1 π 2 σ AO M MO MM AO M b. Sketch the shape of the resulting MO s. (10 points) σ π 1 z z π 2 δ 1 d z 2 d z 2 d z d z d z d z d 2 2 d 2 2 δ 2 π 1 * z π 2 * z δ 1 * d d d z d z d z d z d 2 2 d 2 2 δ 2 * σ* d d d z 2 d z 2 6
8 8 7. Assign (1) formal charges, (2) oidation numbers, and (3) evaluate the relative importance of the three Lewis structures of OCN, the canate ion. Compare these (i.e., do the same) to the Lewis structures of the fulminate ion, CNO. (10 points) or canate ion: a) O C N b) O C N c) O C N ormal charges Oidation numbers II IV III II IV III II IV III Best charge distribution is in b). Structure b) should be the most important, with structure a) net. Structure c) should be unimportant because of poor charge distribution. or fulminate ion: a) O N C b) O N C c) O N C ormal charges Oidation numbers II IV 0 II I II II III I The best structure is b), the second best a). Structure c) should be unimportant because of poor charge distribution. Etra Credit: Eplain the observation that the canate ion is stable, the fulminate ion is eplosive, and CON does not eist. (2 points) The poor charge distribution in the fulminate ion is the source of its eplosiveness.
Chem 400. Inorganic Chemistry. Practice Exam 1. 1 of 5. 2 of 5. 3 of of of of of of of of 10.
 Chem 400 Inorganic Chemistr Practice Eam 1 1 of 5 2 of 5 3 of 10 4 of 10 5 of 10 6 of 10 7 of 10 8 of 10 9 of 10 10 of 10 11 of 10 Σ of 100 Name KEY (please print) 1. a. Predict the structure of 4 using
Chem 400 Inorganic Chemistr Practice Eam 1 1 of 5 2 of 5 3 of 10 4 of 10 5 of 10 6 of 10 7 of 10 8 of 10 9 of 10 10 of 10 11 of 10 Σ of 100 Name KEY (please print) 1. a. Predict the structure of 4 using
Figure 1 Correlation diagram for BeH 2
 Self-Stud Problems / Eam Preparation revise our computational chemistr workshop from last ear: http://www.huntresearchgroup.org.uk/teaching/ear1_lab_start.html o make sure ou have checked that the molecule
Self-Stud Problems / Eam Preparation revise our computational chemistr workshop from last ear: http://www.huntresearchgroup.org.uk/teaching/ear1_lab_start.html o make sure ou have checked that the molecule
CHEMISTRY - MCMURRY 7E CH.7 - COVALENT BONDING AND ELECTRON DOT STRUCTURES
 !! www.clutchprep.com CONCEPT: ELECTRONIC GEOMETRY When drawing a compound you have to take into account two different systems of geometrical shape. The simpler system known as electronic geometry or shape
!! www.clutchprep.com CONCEPT: ELECTRONIC GEOMETRY When drawing a compound you have to take into account two different systems of geometrical shape. The simpler system known as electronic geometry or shape
CHEMISTRY - ZUMDAHL 2E CH.4 - MOLECULAR STRUCTURE AND ORBITALS.
 !! www.clutchprep.com CONCEPT: ELECTRONIC GEOMETRY When drawing a compound you have to take into account two different systems of geometrical shape. The simpler system known as electronic geometry or shape
!! www.clutchprep.com CONCEPT: ELECTRONIC GEOMETRY When drawing a compound you have to take into account two different systems of geometrical shape. The simpler system known as electronic geometry or shape
Molecular Geometry and Bonding Theories. Chapter 9
 Molecular Geometry and Bonding Theories Chapter 9 Molecular Shapes CCl 4 Lewis structures give atomic connectivity; The shape of a molecule is determined by its bond angles VSEPR Model Valence Shell Electron
Molecular Geometry and Bonding Theories Chapter 9 Molecular Shapes CCl 4 Lewis structures give atomic connectivity; The shape of a molecule is determined by its bond angles VSEPR Model Valence Shell Electron
CHM2045 F13--Exam # MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 CHM2045 F13--Exam #2 2013.10.18 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) A valid Lewis structure of cannot be drawn without violating the
CHM2045 F13--Exam #2 2013.10.18 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) A valid Lewis structure of cannot be drawn without violating the
Fragment Orbitals for Transition Metal Complexes
 Fragment Orbitals for Transition Metal Complees Introduction so far we have concentrated on the MO diagrams for main group elements, and the first 4 lectures will link into our Main Group chemistr course
Fragment Orbitals for Transition Metal Complees Introduction so far we have concentrated on the MO diagrams for main group elements, and the first 4 lectures will link into our Main Group chemistr course
Chapter 9. Covalent Bonding: Orbitals
 Chapter 9. Covalent onding: Orbitals Models to explain the structures and/or energies of the covalent molecules Localized Electron (LE) onding Model Lewis Structure Valence Shell Electron Pair Repulsion
Chapter 9. Covalent onding: Orbitals Models to explain the structures and/or energies of the covalent molecules Localized Electron (LE) onding Model Lewis Structure Valence Shell Electron Pair Repulsion
Chapter 10 Chemical Bonding II
 Chapter 10 Chemical Bonding II Valence Bond Theory Valence Bond Theory: A quantum mechanical model which shows how electron pairs are shared in a covalent bond. Bond forms between two atoms when the following
Chapter 10 Chemical Bonding II Valence Bond Theory Valence Bond Theory: A quantum mechanical model which shows how electron pairs are shared in a covalent bond. Bond forms between two atoms when the following
Molecular Orbitals in Inorganic Chemistry
 Outline Molecular Orbitals in Inorganic hemistr Dr. P. unt p.hunt@imperial.ac.uk Rm 167 (hemistr) http://www.ch.ic.ac.uk/hunt/ choosing fragments orbital smmetr (again!) bonding and antibonding character
Outline Molecular Orbitals in Inorganic hemistr Dr. P. unt p.hunt@imperial.ac.uk Rm 167 (hemistr) http://www.ch.ic.ac.uk/hunt/ choosing fragments orbital smmetr (again!) bonding and antibonding character
Polarity in Molecules!
 Chapter 9 part 2: Polarity in Molecules, Valence Bond Theory! Read:!!BLB 9.3 5! W:!!BLB 9.33, 35, 38!!!Packet 9:8-11! Know:! bond angles and geometry! polarity of molecules! Polarity in Molecules! " Just
Chapter 9 part 2: Polarity in Molecules, Valence Bond Theory! Read:!!BLB 9.3 5! W:!!BLB 9.33, 35, 38!!!Packet 9:8-11! Know:! bond angles and geometry! polarity of molecules! Polarity in Molecules! " Just
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9. Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 9. Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
CHAPTER TEN MOLECULAR GEOMETRY MOLECULAR GEOMETRY V S E P R CHEMICAL BONDING II: MOLECULAR GEOMETRY AND HYBRIDIZATION OF ATOMIC ORBITALS
 CHAPTER TEN CHEMICAL BONDING II: AND HYBRIDIZATION O ATOMIC ORBITALS V S E P R VSEPR Theory In VSEPR theory, multiple bonds behave like a single electron pair Valence shell electron pair repulsion (VSEPR)
CHAPTER TEN CHEMICAL BONDING II: AND HYBRIDIZATION O ATOMIC ORBITALS V S E P R VSEPR Theory In VSEPR theory, multiple bonds behave like a single electron pair Valence shell electron pair repulsion (VSEPR)
Hybridization and Molecular Orbital (MO) Theory
 ybridization and Molecular Orbital (MO) Theory Chapter 10 istorical Models G.N.Lewis and I. Langmuir (~1920) laid out foundations Ionic species were formed by electron transfer Covalent molecules arise
ybridization and Molecular Orbital (MO) Theory Chapter 10 istorical Models G.N.Lewis and I. Langmuir (~1920) laid out foundations Ionic species were formed by electron transfer Covalent molecules arise
Chemistry: The Central Science. Chapter 9: Molecular Geometry and Bonding Theory
 Chemistry: The Central Science Chapter 9: Molecular Geometry and Bonding Theory The shape and size of a molecule of a particular substance, together with the strength and polarity of its bonds, largely
Chemistry: The Central Science Chapter 9: Molecular Geometry and Bonding Theory The shape and size of a molecule of a particular substance, together with the strength and polarity of its bonds, largely
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals
 Chemical Bonding II: and ybridization of Atomic rbitals Chapter 10 Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the electrostatic repulsions between the
Chemical Bonding II: and ybridization of Atomic rbitals Chapter 10 Valence shell electron pair repulsion (VSEPR) model: Predict the geometry of the molecule from the electrostatic repulsions between the
Molecular Geometry. Dr. Williamson s Molecular Geometry Notes. VSEPR: Definition of Terms. Dr. V.M. Williamson Texas A & M University Student Version
 Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Geometry. Dr. Williamson s Molecular Geometry Notes. VSEPR: Definition of Terms. VSEPR: Electronic Geometries VSEPR
 Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Geometry Dr. V.M. Williamson Texas A & M University Student Version Valence Shell Electron Pair Repulsion- VSEPR 1. Valence e- to some extent 2. Electron pairs move as far away as possible to
Molecular Orbitals in Inorganic Chemistry. Dr. P. Hunt Rm 167 (Chemistry)
 Molecular Orbitals in Inorganic Chemistr Dr. P. unt p.hunt@imperial.ac.uk Rm 167 (Chemistr) http://www.ch.ic.ac.uk/hunt/ Outline choosing fragments orbital smmetr (again!) bonding and antibonding character
Molecular Orbitals in Inorganic Chemistr Dr. P. unt p.hunt@imperial.ac.uk Rm 167 (Chemistr) http://www.ch.ic.ac.uk/hunt/ Outline choosing fragments orbital smmetr (again!) bonding and antibonding character
Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model.
 Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model. Determine whether a molecule is polar or nonpolar based
Chapter 9: Molecular Geometries and Bonding Theories Learning Outcomes: Predict the three-dimensional shapes of molecules using the VSEPR model. Determine whether a molecule is polar or nonpolar based
Chapter 9: Molecular Geometry and Bonding Theories
 Chapter 9: Molecular Geometry and Bonding Theories 9.1 Molecular Geometries -Bond angles: angles made by the lines joining the nuclei of the atoms in a molecule -Bond angles determine overall shape of
Chapter 9: Molecular Geometry and Bonding Theories 9.1 Molecular Geometries -Bond angles: angles made by the lines joining the nuclei of the atoms in a molecule -Bond angles determine overall shape of
Form J. Test #4 Last Name First Name Zumdahl, Chapters 8 and 9 November 23, 2004
 Form J Chemistry 1441-023 Name (please print) Test #4 Last Name First Name Zumdahl, Chapters 8 and 9 November 23, 2004 Instructions: 1. This exam consists of 27 questions. 2. No scratch paper is allowed.
Form J Chemistry 1441-023 Name (please print) Test #4 Last Name First Name Zumdahl, Chapters 8 and 9 November 23, 2004 Instructions: 1. This exam consists of 27 questions. 2. No scratch paper is allowed.
Topic 2. Structure and Bonding Models of Covalent Compounds of p-block Elements
 Topic 2 2-1 Structure and Bonding Models of Covalent Compounds of p-block Elements Bonding 2-2 Many different approaches to describe bonding: Ionic Bonding: Elements with large electronegativity differences;
Topic 2 2-1 Structure and Bonding Models of Covalent Compounds of p-block Elements Bonding 2-2 Many different approaches to describe bonding: Ionic Bonding: Elements with large electronegativity differences;
Adapted from CHM 130 Maricopa County, AZ Molecular Geometry and Lewis Dot Formulas Introduction
 Adapted from CHM 130 Maricopa County, AZ Molecular Geometry and Lewis Dot Formulas Introduction A chemical bond is an intramolecular (within the molecule) force holding two or more atoms together. Covalent
Adapted from CHM 130 Maricopa County, AZ Molecular Geometry and Lewis Dot Formulas Introduction A chemical bond is an intramolecular (within the molecule) force holding two or more atoms together. Covalent
x find all of the symmetry operations/elements: o character table headings: E, 2C φ σ v, i, S φ C 2 φ
 Construct and annotate a valence molecular orbital diagram for the linear molecule Mg 2. Assume the 3pAOs lie deeper in energ than the Mg 3sAO and that the Mg and orbitals interact onl slightl. Mg is a
Construct and annotate a valence molecular orbital diagram for the linear molecule Mg 2. Assume the 3pAOs lie deeper in energ than the Mg 3sAO and that the Mg and orbitals interact onl slightl. Mg is a
Bonding/Lewis Dots Lecture Page 1 of 12 Date. Bonding. What is Coulomb's Law? Energy Profile: Covalent Bonds. Electronegativity and Linus Pauling
 Bonding/Lewis Dots Lecture Page 1 of 12 Date Bonding What is Coulomb's Law? Energy Profile: Covalent Bonds Electronegativity and Linus Pauling 2.1 H 1.0 Li 0.9 Na 0.8 K 0.8 Rb 0.7 Cs 0.7 Fr 1.5 Be 1.2
Bonding/Lewis Dots Lecture Page 1 of 12 Date Bonding What is Coulomb's Law? Energy Profile: Covalent Bonds Electronegativity and Linus Pauling 2.1 H 1.0 Li 0.9 Na 0.8 K 0.8 Rb 0.7 Cs 0.7 Fr 1.5 Be 1.2
Organic Chemistry. Review Information for Unit 1. VSEPR Hybrid Orbitals Polar Molecules
 rganic hemistry Review Information for Unit 1 VSEPR ybrid rbitals Polar Molecules VSEPR The valence shell electron pair repulsion model (VSEPR) can be used to predict the geometry around a particular atom
rganic hemistry Review Information for Unit 1 VSEPR ybrid rbitals Polar Molecules VSEPR The valence shell electron pair repulsion model (VSEPR) can be used to predict the geometry around a particular atom
Chapter One MULTIPLE CHOICE QUESTIONS. Topic: General Section: 1.1 Difficulty Level: Easy
 Chapter ne MULTIPLE CICE QUESTIS Topic: General Section: 1.1 1. Credit for the first synthesis of an organic compound from an inorganic precursor is usually given to: A) Berzelius B) Arrhenius C) Kekule
Chapter ne MULTIPLE CICE QUESTIS Topic: General Section: 1.1 1. Credit for the first synthesis of an organic compound from an inorganic precursor is usually given to: A) Berzelius B) Arrhenius C) Kekule
CHEMICAL BONDING. Chemical Bonds. Ionic Bonding. Lewis Symbols
 CHEMICAL BONDING Chemical Bonds Lewis Symbols Octet Rule whenever possible, valence electrons in covalent compounds distribute so that each main-group element is surrounded by 8 electrons (except hydrogen
CHEMICAL BONDING Chemical Bonds Lewis Symbols Octet Rule whenever possible, valence electrons in covalent compounds distribute so that each main-group element is surrounded by 8 electrons (except hydrogen
Lewis Structure. Lewis Structures & VSEPR. Octet & Duet Rules. Steps for drawing Lewis Structures
 Lewis Structure Lewis Structures & VSEPR Lewis Structures shows how the are arranged among the atoms of a molecule There are rules for Lewis Structures that are based on the formation of a Atoms want to
Lewis Structure Lewis Structures & VSEPR Lewis Structures shows how the are arranged among the atoms of a molecule There are rules for Lewis Structures that are based on the formation of a Atoms want to
Molecular shape is determined by the number of bonds that form around individual atoms.
 Chapter 9 CH 180 Major Concepts: Molecular shape is determined by the number of bonds that form around individual atoms. Sublevels (s, p, d, & f) of separate atoms may overlap and result in hybrid orbitals
Chapter 9 CH 180 Major Concepts: Molecular shape is determined by the number of bonds that form around individual atoms. Sublevels (s, p, d, & f) of separate atoms may overlap and result in hybrid orbitals
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Take Home Exam Chem 1A Fall 2008 - Chapters 6 to 9: You may us any resource you wish accept people. On your honor, you may not ask another person for help. Show your work on every answer. Partial credit
Take Home Exam Chem 1A Fall 2008 - Chapters 6 to 9: You may us any resource you wish accept people. On your honor, you may not ask another person for help. Show your work on every answer. Partial credit
Chapter 6 PRETEST: Chemical Bonding
 Chapter 6 PRETEST: Chemical In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1.The charge on an ion is a. always positive.
Chapter 6 PRETEST: Chemical In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1.The charge on an ion is a. always positive.
Chapter 13: Phenomena
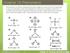 Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Chapter 13: Phenomena Phenomena: Scientists measured the bond angles of some common molecules. In the pictures below each line represents a bond that contains 2 electrons. If multiple lines are drawn together
Chapter 10: Chemical Bonding II: Molecular Shapes; VSEPR, Valence Bond and Molecular Orbital Theories
 C h e m i s t r y 1 A : C h a p t e r 1 0 P a g e 1 Chapter 10: Chemical Bonding II: Molecular Shapes; VSEPR, Valence Bond and Molecular Orbital Theories Homework: Read Chapter 10: Work out sample/practice
C h e m i s t r y 1 A : C h a p t e r 1 0 P a g e 1 Chapter 10: Chemical Bonding II: Molecular Shapes; VSEPR, Valence Bond and Molecular Orbital Theories Homework: Read Chapter 10: Work out sample/practice
Experiment 15. The Valence Shell Electron Pair Repulsion (VSEPR) Theory of Directed Valency: An exercise
 Experiment 15 The Valence Shell Electron Pair Repulsion (VSEPR) Theory of Directed Valency: An exercise Attempts to understand and predict the shapes of molecules using either the valencebond theory or
Experiment 15 The Valence Shell Electron Pair Repulsion (VSEPR) Theory of Directed Valency: An exercise Attempts to understand and predict the shapes of molecules using either the valencebond theory or
Chapter 8. Molecular Shapes. Valence Shell Electron Pair Repulsion Theory (VSEPR) What Determines the Shape of a Molecule?
 PowerPoint to accompany Molecular Shapes Chapter 8 Molecular Geometry and Bonding Theories Figure 8.2 The shape of a molecule plays an important role in its reactivity. By noting the number of bonding
PowerPoint to accompany Molecular Shapes Chapter 8 Molecular Geometry and Bonding Theories Figure 8.2 The shape of a molecule plays an important role in its reactivity. By noting the number of bonding
Chemical Bonding Chapter 8
 Chemical Bonding Chapter 8 Get your Clicker, 2 magnets, goggles and your handouts Nov 15 6:15 PM Recall that: Ionic-Involves the transfer of electrons - forms between a metal and a nonmetal Covalent-Involves
Chemical Bonding Chapter 8 Get your Clicker, 2 magnets, goggles and your handouts Nov 15 6:15 PM Recall that: Ionic-Involves the transfer of electrons - forms between a metal and a nonmetal Covalent-Involves
Lecture 17 - Covalent Bonding. Lecture 17 - VSEPR and Molecular Shape. Lecture 17 - Introduction. Lecture 17 - VSEPR and Molecular Shape
 Chem 103, Section F0F Unit VI - Compounds Part II: Covalent Compounds Lecture 17 Using the Valence-Shell Electron-Pair Repulsion (VSEPR) Theory to predict molecular shapes Molecular shape and polarity
Chem 103, Section F0F Unit VI - Compounds Part II: Covalent Compounds Lecture 17 Using the Valence-Shell Electron-Pair Repulsion (VSEPR) Theory to predict molecular shapes Molecular shape and polarity
Molecular Geometry and Chemical Bonding Theory
 Molecular Geometry and Chemical Bonding Theory The Valence -Shell Electron -Pair Repulsion (VSEPR) Model predicts the shapes of the molecules and ions by assuming that the valence shell electron pairs
Molecular Geometry and Chemical Bonding Theory The Valence -Shell Electron -Pair Repulsion (VSEPR) Model predicts the shapes of the molecules and ions by assuming that the valence shell electron pairs
Molecular shape is only discussed when there are three or more atoms connected (diatomic shape is obvious).
 Chapter 10 Molecular Geometry (Ch9 Jespersen, Ch10 Chang) The arrangement of the atoms of a molecule in space is the molecular geometry. This is what gives the molecules their shape. Molecular shape is
Chapter 10 Molecular Geometry (Ch9 Jespersen, Ch10 Chang) The arrangement of the atoms of a molecule in space is the molecular geometry. This is what gives the molecules their shape. Molecular shape is
Molecular Structure Chapter 26: Molecular Structure Problems
 Molecular Structure Chapter 26: Molecular Structure Problems 1 1. Draw the Lewis dot resonance structures for the carbonate ion, CO3 2-. Are the electrons delocalized? Give the average bond order for the
Molecular Structure Chapter 26: Molecular Structure Problems 1 1. Draw the Lewis dot resonance structures for the carbonate ion, CO3 2-. Are the electrons delocalized? Give the average bond order for the
Inorganic Chemistry A. Cl and 37 Cl are and
 S e l f - s t u d y e x e r c i s e s 1 Inorganic Chemistry A Self-study exercises Chapters 1 and 2 1. Calculate the value of A r for naturally occurring chlorine if the distribution of isotopes is 75.77%
S e l f - s t u d y e x e r c i s e s 1 Inorganic Chemistry A Self-study exercises Chapters 1 and 2 1. Calculate the value of A r for naturally occurring chlorine if the distribution of isotopes is 75.77%
Chapter 9 Molecular Geometry and Bonding Theories
 Lecture Presentation Chapter 9 Geometry James F. Kirby Quinnipiac University Hamden, CT Shapes Lewis Structures show bonding and lone pairs, but do not denote shape. However, we use Lewis Structures to
Lecture Presentation Chapter 9 Geometry James F. Kirby Quinnipiac University Hamden, CT Shapes Lewis Structures show bonding and lone pairs, but do not denote shape. However, we use Lewis Structures to
Subtopic 4.2 MOLECULAR SHAPE AND POLARITY
 Subtopic 4.2 MOLECULAR SHAPE AND POLARITY 1 LEARNING OUTCOMES (covalent bonding) 1. Draw the Lewis structure of covalent molecules (octet rule such as NH 3, CCl 4, H 2 O, CO 2, N 2 O 4, and exception to
Subtopic 4.2 MOLECULAR SHAPE AND POLARITY 1 LEARNING OUTCOMES (covalent bonding) 1. Draw the Lewis structure of covalent molecules (octet rule such as NH 3, CCl 4, H 2 O, CO 2, N 2 O 4, and exception to
Valence Bond Theory - Description
 Bonding and Molecular Structure - PART 2 - Valence Bond Theory and Hybridization 1. Understand and be able to describe the Valence Bond Theory description of covalent bond formation. 2. Understand and
Bonding and Molecular Structure - PART 2 - Valence Bond Theory and Hybridization 1. Understand and be able to describe the Valence Bond Theory description of covalent bond formation. 2. Understand and
Exam 2 October 29, time finished. Notes on exam format:
 Chemistry 417-01 Inorganic Chemistry Exam 2 October 29, 2001 GROUND RULES: a. Closed book; closed notes; no consulting b. allowed: calculator; model set; point group flowchart; ruler c. 180 minute (3 h)
Chemistry 417-01 Inorganic Chemistry Exam 2 October 29, 2001 GROUND RULES: a. Closed book; closed notes; no consulting b. allowed: calculator; model set; point group flowchart; ruler c. 180 minute (3 h)
EXPERIMENT 12: MOLECULAR ARCHITECTURE
 Name Section EXPERIMENT 12: MLECULAR ARCITECTURE PRE-LABRATRY QUESTINS The following preparatory questions should be answered before coming to lab. They are intended to introduce you to several ideas important
Name Section EXPERIMENT 12: MLECULAR ARCITECTURE PRE-LABRATRY QUESTINS The following preparatory questions should be answered before coming to lab. They are intended to introduce you to several ideas important
A Simple Model for Chemical Bonds
 A Simple Model for hemical Bonds Multiple hoice 1. Modern organic chemistry a. is the study of carbon-containing compounds. b. is the study of compounds from living organisms. c. deals exclusively with
A Simple Model for hemical Bonds Multiple hoice 1. Modern organic chemistry a. is the study of carbon-containing compounds. b. is the study of compounds from living organisms. c. deals exclusively with
Ch. 9- Molecular Geometry and Bonding Theories
 Ch. 9- Molecular Geometry and Bonding Theories 9.0 Introduction A. Lewis structures do not show one of the most important aspects of molecules- their overall shapes B. The shape and size of molecules-
Ch. 9- Molecular Geometry and Bonding Theories 9.0 Introduction A. Lewis structures do not show one of the most important aspects of molecules- their overall shapes B. The shape and size of molecules-
SUPeR Chemistry CH 222 Practice Exam
 SUPeR Chemistry CH 222 Practice Exam This exam has been designed to help you practice working multiple choice problems over the material that will be covered on the first CH 222 midterm. The actual exams
SUPeR Chemistry CH 222 Practice Exam This exam has been designed to help you practice working multiple choice problems over the material that will be covered on the first CH 222 midterm. The actual exams
Chapter 9. Molecular Geometry and Bonding Theories
 Chapter 9. Molecular Geometry and Bonding Theories PART I Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Chapter 9. Molecular Geometry and Bonding Theories PART I Molecular Shapes Lewis structures give atomic connectivity: they tell us which atoms are physically connected to which atoms. The shape of a molecule
Lecture Presentation. Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory
 Lecture Presentation Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory Predicting Molecular Geometry 1. Draw the Lewis structure. 2. Determine the number
Lecture Presentation Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory Predicting Molecular Geometry 1. Draw the Lewis structure. 2. Determine the number
CHM 151LL: Geometry of Covalent Compounds
 CM 151LL: Geometry of Covalent Compounds Introduction Octet Rule A Lewis structure (or electrondot formula) is a twodimensional structural formula showing the arrangement of electrons around atoms in covalently
CM 151LL: Geometry of Covalent Compounds Introduction Octet Rule A Lewis structure (or electrondot formula) is a twodimensional structural formula showing the arrangement of electrons around atoms in covalently
Name: Period: Date: What Is VSEPR? Now explore the Compare Two Structures link. Try changing the display to explore different combinations.
 Name: Period: Date: What Is VSEPR? Exploring The Valence Shell Electron Pair Repulsion (VSEPR) model. Go to the Purdue University website to explore VSEPR theory. http://www.chem.purdue.edu/gchelp/vsepr/structur2.html
Name: Period: Date: What Is VSEPR? Exploring The Valence Shell Electron Pair Repulsion (VSEPR) model. Go to the Purdue University website to explore VSEPR theory. http://www.chem.purdue.edu/gchelp/vsepr/structur2.html
Carbon Compounds. Chemical Bonding Part 1b
 Carbon Compounds Chemical Bonding Part 1b Board Notes Introduction to VSEPR Organic Formulas Various Representations " dimethyl ether C 2 H 6 O " propyl alcohol C 3 H 8 O 3D representations " Wedges and
Carbon Compounds Chemical Bonding Part 1b Board Notes Introduction to VSEPR Organic Formulas Various Representations " dimethyl ether C 2 H 6 O " propyl alcohol C 3 H 8 O 3D representations " Wedges and
structure prediction, chemical bonding
 1 structure prediction, chemical bonding 2 Lewis structures Atoms listed in order of increasing EN, no connectivity implied CSPF (PNF 2 ) 4 3 after the Lewis Structure determine the steric number number
1 structure prediction, chemical bonding 2 Lewis structures Atoms listed in order of increasing EN, no connectivity implied CSPF (PNF 2 ) 4 3 after the Lewis Structure determine the steric number number
SKILL-BUILDING EXERCISE
 4. O and H Solution 1. Carbon has an electronegativity of 2.5, while the value for hydrogen is 2.1. The difference is 0.3, which is rather small. The C H bond is therefore considered nonpolar. 2. Both
4. O and H Solution 1. Carbon has an electronegativity of 2.5, while the value for hydrogen is 2.1. The difference is 0.3, which is rather small. The C H bond is therefore considered nonpolar. 2. Both
Activity Formal Charge and VSEPR Theory for Expanded Octets
 Activity 201 7 Formal Charge and VSEPR Theory for Expanded Octets Directions: This Guided Learning Activity (GLA) goes over formal charge and the structures of molecules with expanded octets. Part A introduces
Activity 201 7 Formal Charge and VSEPR Theory for Expanded Octets Directions: This Guided Learning Activity (GLA) goes over formal charge and the structures of molecules with expanded octets. Part A introduces
Group 1 Group 2 Group 3 Group 4 Group 5 Group 6 Group 7 Group 8. Na Mg Al Si P S Cl Ar
 CHM 111 Chapters 7 and 8 Worksheet and Study Guide Purpose: This is a guide for your as you work through the chapter. The major topics are provided so that you can write notes on each topic and work the
CHM 111 Chapters 7 and 8 Worksheet and Study Guide Purpose: This is a guide for your as you work through the chapter. The major topics are provided so that you can write notes on each topic and work the
Chem 111 Exam #2 November 8, J h = c E = h E. ΔH = energies of bonds broken - energies of bonds formed SHOW ALL WORK
 General Chemistry I NAME: Answer Key Chem 111 Exam #2 November 8, 2013 Some Equations and Constants for your use: -18-2.18 10 J h = c E = h E n = = 2 n mv o ΔH = energies of bonds broken - energies of
General Chemistry I NAME: Answer Key Chem 111 Exam #2 November 8, 2013 Some Equations and Constants for your use: -18-2.18 10 J h = c E = h E n = = 2 n mv o ΔH = energies of bonds broken - energies of
2. (10%) Correct answers are given below. Every correct answer gives a student 1⅔=1.666 point. There are no negative points for incorrect answers
 1. (9%) There are 9 correct answers: (0,0), (1,-1), (1,0), (1,1), (2,-2), (2,-1), (2,0), (2,1), and (2,2). Each correct answer gives a student +1 point. All other answers are incorrect. Every incorrect
1. (9%) There are 9 correct answers: (0,0), (1,-1), (1,0), (1,1), (2,-2), (2,-1), (2,0), (2,1), and (2,2). Each correct answer gives a student +1 point. All other answers are incorrect. Every incorrect
Copyrighted by Gabriel Tang B.Ed., B.Sc.
 Unit 2: Chemical Bonding and rganic Chemistr Chemistr AP Chapter 9: Covalent Bonding: rbitals 9.1: bridiation and the Localied lectron Model bridiation: - the combining of orbitals of different atomic
Unit 2: Chemical Bonding and rganic Chemistr Chemistr AP Chapter 9: Covalent Bonding: rbitals 9.1: bridiation and the Localied lectron Model bridiation: - the combining of orbitals of different atomic
Chapter 10 Chemical Bonding II: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory
 10.1 Artificial Sweeteners: Fooled by Molecular Shape 425 10.2 VSEPR Theory: The Five Basic Shapes 426 10.3 VSEPR Theory: The Effect of Lone Pairs 430 10.4 VSEPR Theory: Predicting Molecular Geometries
10.1 Artificial Sweeteners: Fooled by Molecular Shape 425 10.2 VSEPR Theory: The Five Basic Shapes 426 10.3 VSEPR Theory: The Effect of Lone Pairs 430 10.4 VSEPR Theory: Predicting Molecular Geometries
Bonding Test pg 1 of 4 Name: Pd. Date:
 Bonding Test pg 1 of 4 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) How many electrons are shared in a single covalent bond? 1. A) 2 B) 3 C)
Bonding Test pg 1 of 4 MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) How many electrons are shared in a single covalent bond? 1. A) 2 B) 3 C)
A REVIEW OF GENERAL CHEMISTRY: ELECTRONS, BONDS AND MOLECULAR PROPERTIES
 A REVIEW OF GENERAL CEMISTRY: ELECTRONS, BONDS AND MOLECULAR PROPERTIES A STUDENT SOULD BE ABLE TO: 1. Draw Lewis (electron dot and line) structural formulas for simple compounds and ions from molecular
A REVIEW OF GENERAL CEMISTRY: ELECTRONS, BONDS AND MOLECULAR PROPERTIES A STUDENT SOULD BE ABLE TO: 1. Draw Lewis (electron dot and line) structural formulas for simple compounds and ions from molecular
Lewis Theory of Shapes and Polarities of Molecules
 Lewis Theory of Shapes and Polarities of Molecules Sulfanilamide Lewis Structures and the Real 3D-Shape of Molecules Molecular Shape or Geometry The way in which atoms of a molecule are arranged in space
Lewis Theory of Shapes and Polarities of Molecules Sulfanilamide Lewis Structures and the Real 3D-Shape of Molecules Molecular Shape or Geometry The way in which atoms of a molecule are arranged in space
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals 1 Chemical Bonding II Molecular Geometry (10.1) Dipole Moments (10.2) Valence Bond Theory (10.3) Hybridization of Atomic Orbitals
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals 1 Chemical Bonding II Molecular Geometry (10.1) Dipole Moments (10.2) Valence Bond Theory (10.3) Hybridization of Atomic Orbitals
Bonding in Molecules Covalent Bonding
 Bonding in Molecules Covalent Bonding The term covalent implies sharing of electrons between atoms. Valence electrons and valence shell orbitals - nly valence electrons are used for bonding: ns, np, nd
Bonding in Molecules Covalent Bonding The term covalent implies sharing of electrons between atoms. Valence electrons and valence shell orbitals - nly valence electrons are used for bonding: ns, np, nd
4/25/2017. VSEPR Theory. Two Electron Groups. Shapes of Molecules. Two Electron Groups with Double Bonds. Three Electron Groups.
 Chapter 10 Lecture Chapter 10 Bonding and Properties of Solids and Liquids 10.3 Shapes of Molecules and Ions (VSEPR Theory) Learning Goal Predict the three-dimensional structure of a molecule or a polyatomic
Chapter 10 Lecture Chapter 10 Bonding and Properties of Solids and Liquids 10.3 Shapes of Molecules and Ions (VSEPR Theory) Learning Goal Predict the three-dimensional structure of a molecule or a polyatomic
Carbon-based molecules are held together by covalent bonds between atoms
 hapter 1: hemical bonding and structure in organic compounds arbon-based molecules are held together by covalent bonds between atoms omposition: Mainly nonmetals; especially,, O, N, S, P and the halogens
hapter 1: hemical bonding and structure in organic compounds arbon-based molecules are held together by covalent bonds between atoms omposition: Mainly nonmetals; especially,, O, N, S, P and the halogens
Chapter 10. Structure Determines Properties! Molecular Geometry. Chemical Bonding II
 Chapter 10 Chemical Bonding II Structure Determines Properties! Properties of molecular substances depend on the structure of the molecule The structure includes many factors, including: the skeletal arrangement
Chapter 10 Chemical Bonding II Structure Determines Properties! Properties of molecular substances depend on the structure of the molecule The structure includes many factors, including: the skeletal arrangement
CHAPTER 5: Bonding Theories - Explaining Molecular Geometry. Chapter Outline
 CHAPTER 5: Bonding Theories - Explaining Molecular Geometry Chapter Outline 5.1 Molecular Shape 5.2 Valence-Shell Electron-Pair Repulsion Theory (VSEPR) 5.3 Polar Bonds and Polar Molecules» What Makes
CHAPTER 5: Bonding Theories - Explaining Molecular Geometry Chapter Outline 5.1 Molecular Shape 5.2 Valence-Shell Electron-Pair Repulsion Theory (VSEPR) 5.3 Polar Bonds and Polar Molecules» What Makes
ADVANCED INORGANIC CHEMISTRY February 17, 2009 INSTRUCTIONS: PRINT YOUR NAME > NAME.
 ADVANCED INORGANIC CHEMISTRY QUIZ I February 17, 2009 INSTRUCTIONS: PRINT YOUR NAME > NAME. WORK 4 of the 5 problems SHOW YOUR WORK FOR PARTIAL CREDIT THE LAST PAGE IS A Periodic Table R = 0.08206 lit-atm/mol-k
ADVANCED INORGANIC CHEMISTRY QUIZ I February 17, 2009 INSTRUCTIONS: PRINT YOUR NAME > NAME. WORK 4 of the 5 problems SHOW YOUR WORK FOR PARTIAL CREDIT THE LAST PAGE IS A Periodic Table R = 0.08206 lit-atm/mol-k
Please pass in only this completed answer sheet on the day of the test. LATE SUBMISSIONS WILL NOT BE ACCEPTED
 CHM-201 General Chemistry and Laboratory I Unit #4 Take Home Test Due December 13, 2018 Please pass in only this completed answer sheet on the day of the test. LATE SUBMISSIONS WILL NOT BE ACCEPTED CHM-201
CHM-201 General Chemistry and Laboratory I Unit #4 Take Home Test Due December 13, 2018 Please pass in only this completed answer sheet on the day of the test. LATE SUBMISSIONS WILL NOT BE ACCEPTED CHM-201
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Valence shell electron
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 1 Valence shell electron
CHEMISTRY 110 EXAM 2 Feb 25, 2013 FORM A
 EMISTRY 110 EXAM 2 Feb 25, 2013 FORM A 1. ow many valence electrons and lone pairs are in the structure of the ammonium ion? # valence electrons # lone pairs A. 8 0 B. 10 1. 8 1 D. 10 2 E. 12 3 2. Which
EMISTRY 110 EXAM 2 Feb 25, 2013 FORM A 1. ow many valence electrons and lone pairs are in the structure of the ammonium ion? # valence electrons # lone pairs A. 8 0 B. 10 1. 8 1 D. 10 2 E. 12 3 2. Which
General Chemistry. Contents. Chapter 12: Chemical Bonding II: Additional Aspects What a Bonding Theory Should Do. Potential Energy Diagram
 General Chemistry Principles and Modern Applications Petrucci Harwood Herring 8 th Edition Chapter 12: Chemical Bonding II: Additional Aspects Philip Dutton University of Windsor, Canada N9B 3P4 Contents
General Chemistry Principles and Modern Applications Petrucci Harwood Herring 8 th Edition Chapter 12: Chemical Bonding II: Additional Aspects Philip Dutton University of Windsor, Canada N9B 3P4 Contents
Test bank for Chemistry The Central Science 10th Edition by Brown, LeMay, Bursten
 Test bank for Chemistry The Central Science 10th Edition by Brown, LeMay, Bursten Chapter 9, Molecular Geometry and Bonding Theories Multiple-Choice and Bimodal 1) For a molecule with the formula A) linear
Test bank for Chemistry The Central Science 10th Edition by Brown, LeMay, Bursten Chapter 9, Molecular Geometry and Bonding Theories Multiple-Choice and Bimodal 1) For a molecule with the formula A) linear
2. Write the electron configuration notation and the electron dot notation for each: (a) Ni atom (b) Ni 2+ ion (c) Ni 3+ ion
 EXTRA HOMEWORK 2A 1. Predict whether each of the following types of matter will be bonded with ionic, covalent, or metallic bonds, and identify whether each will be composed of atoms, ions, or molcules
EXTRA HOMEWORK 2A 1. Predict whether each of the following types of matter will be bonded with ionic, covalent, or metallic bonds, and identify whether each will be composed of atoms, ions, or molcules
UNIVERSITY OF VICTORIA. CHEMISTRY 101 Mid-Term Test 2, November
 NAME Student No. SECTIN (circle one): A01 (Codding) A02 (Sirk) A03 (Briggs) Version A UNIVERSITY F VICTRIA CEMISTRY 101 Mid-Term Test 2, November 19 2010 Version A This test has two parts and 8 pages,
NAME Student No. SECTIN (circle one): A01 (Codding) A02 (Sirk) A03 (Briggs) Version A UNIVERSITY F VICTRIA CEMISTRY 101 Mid-Term Test 2, November 19 2010 Version A This test has two parts and 8 pages,
Chapter 10: Chemical Bonding II. Bonding Theories
 Chapter 10: Chemical Bonding II Dr. Chris Kozak Memorial University of Newfoundland, Canada Bonding Theories Previously, we saw how the shapes of molecules can be predicted from the orientation of electron
Chapter 10: Chemical Bonding II Dr. Chris Kozak Memorial University of Newfoundland, Canada Bonding Theories Previously, we saw how the shapes of molecules can be predicted from the orientation of electron
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Linear Trigonal 180 o planar 120 o Tetrahedral 109.5 o Trigonal Bipyramidal 120 and 90 o Octahedral 90 o linear Linear
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 10 Linear Trigonal 180 o planar 120 o Tetrahedral 109.5 o Trigonal Bipyramidal 120 and 90 o Octahedral 90 o linear Linear
5.111 Lecture Summary #13 Monday, October 6, 2014
 5.111 Lecture Summary #13 Monday, October 6, 2014 Readings for today: Section 3.8 3.11 Molecular Orbital Theory (Same in 5 th and 4 th ed.) Read for Lecture #14: Sections 3.4, 3.5, 3.6 and 3.7 Valence
5.111 Lecture Summary #13 Monday, October 6, 2014 Readings for today: Section 3.8 3.11 Molecular Orbital Theory (Same in 5 th and 4 th ed.) Read for Lecture #14: Sections 3.4, 3.5, 3.6 and 3.7 Valence
Constitutional isomers. Two bracelets with the same structural parts assembled differently
 Constitutional isomers 1 6 6 Two bracelets with the same structural parts assembled differently Constitutional isomers C C N C C N C 2 7 N Two molecules with the same constituent atoms assembled differently
Constitutional isomers 1 6 6 Two bracelets with the same structural parts assembled differently Constitutional isomers C C N C C N C 2 7 N Two molecules with the same constituent atoms assembled differently
Chapter 9. Molecular Geometries and Bonding Theories. Lecture Presentation. John D. Bookstaver St. Charles Community College Cottleville, MO
 Lecture Presentation Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The shape of a molecule plays an important role in its reactivity. By noting the number of
Lecture Presentation Chapter 9 Theories John D. Bookstaver St. Charles Community College Cottleville, MO Shapes The shape of a molecule plays an important role in its reactivity. By noting the number of
Instant download Test bank for Chemistry The Central Science 10th Edition by Brown, LeMay, Bursten CLICK HERE
 Chemistry, 10e (Brown) Chapter 9, Molecular Geometry and Bonding Theories Instant download Test bank for Chemistry The Central Science 10th Edition by Brown, LeMay, Bursten CLICK HERE http://testbankair.com/download/test-bank-for-chemistry-the-central-science-10th-edition-by-brown-lemay-bursten/
Chemistry, 10e (Brown) Chapter 9, Molecular Geometry and Bonding Theories Instant download Test bank for Chemistry The Central Science 10th Edition by Brown, LeMay, Bursten CLICK HERE http://testbankair.com/download/test-bank-for-chemistry-the-central-science-10th-edition-by-brown-lemay-bursten/
Chapter 9 Molecular Geometry and Bonding Theories
 Chapter 9 Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity (which atoms are physically connected). By noting the number of bonding and nonbonding electron
Chapter 9 Molecular Geometry and Bonding Theories 9.1 Molecular Shapes Lewis structures give atomic connectivity (which atoms are physically connected). By noting the number of bonding and nonbonding electron
AP Chemistry - Problem Drill 15: Lewis Structures and VSEPR Theory
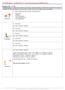 AP Chemistry - Problem Drill 15: Lewis Structures and VSEPR Theory No. 1 of 10 1. Which shape would have sp 3 hybridization? (A) Linear (B) Bent (C) Tetrahedron (D) Trigonal planar (E) Octahedron C. Correct.
AP Chemistry - Problem Drill 15: Lewis Structures and VSEPR Theory No. 1 of 10 1. Which shape would have sp 3 hybridization? (A) Linear (B) Bent (C) Tetrahedron (D) Trigonal planar (E) Octahedron C. Correct.
1. There are paired and unpaired electrons in the Lewis symbol for a phosphorus atom. a. 4, 2 b. 2, 4 c. 2, 3 d. 4, 3 e. 0, 3
 Name: Score: 0 / 42 points (0%) [2 open ended questions not graded] C8&9Practice Multiple Choice Identify the choice that best completes the statement or answers the question. 1. There are paired and unpaired
Name: Score: 0 / 42 points (0%) [2 open ended questions not graded] C8&9Practice Multiple Choice Identify the choice that best completes the statement or answers the question. 1. There are paired and unpaired
Name (printed): Signature:
 CHEM Lab Section Number: Name (printed): Signature: This exam consists of 36 questions all of equal value for a total of 225 points. Make sure that your test has all of the pages. Please read each problem
CHEM Lab Section Number: Name (printed): Signature: This exam consists of 36 questions all of equal value for a total of 225 points. Make sure that your test has all of the pages. Please read each problem
SMK SULTAN ISMAIL JB, NUR FATHIN SUHANA BT AYOB
 SMK SULTAN ISMAIL JB, NUR FATHIN SUHANA BT AYOB POLAR AND NON POLAR BONDS BOND POLARITY 1. Atoms with different electronegative from polar bonds (difference in EN) 2. Depicted as polar arrow : 3. Example
SMK SULTAN ISMAIL JB, NUR FATHIN SUHANA BT AYOB POLAR AND NON POLAR BONDS BOND POLARITY 1. Atoms with different electronegative from polar bonds (difference in EN) 2. Depicted as polar arrow : 3. Example
Name: Class: Date: 3. How many lone pairs of electrons are assigned to the carbon atom in carbon monoxide? a. 0 b. 1 c. 2 d. 3
 Class: Date: Midterm 3, Fall 2009 Record your name on the top of this exam and on the scantron form. Record the test ID letter in the top right box of the scantron form. Record all of your answers on the
Class: Date: Midterm 3, Fall 2009 Record your name on the top of this exam and on the scantron form. Record the test ID letter in the top right box of the scantron form. Record all of your answers on the
Figure 1 Setting the charge in HF 2 -.
 Self-Stud Problems / Eam Preparation compute the orbitals for (linear) -, draw and annotate a MO diagram consistent with the MOs ou observe o if ou have problems setting up the calculation, select Be and
Self-Stud Problems / Eam Preparation compute the orbitals for (linear) -, draw and annotate a MO diagram consistent with the MOs ou observe o if ou have problems setting up the calculation, select Be and
Chapter 9 Molecular Geometries. and Bonding Theories
 Chapter 9 Molecular Geometries and Bonding Theories Coverage of Chapter 9 9.1 All 9.2 All 9.3 All 9.4 All 9.5 Omit Hybridization Involving d Orbitals 9.6 All 9.7 and 9.8 Omit ALL MOLECULAR SHAPES The shape
Chapter 9 Molecular Geometries and Bonding Theories Coverage of Chapter 9 9.1 All 9.2 All 9.3 All 9.4 All 9.5 Omit Hybridization Involving d Orbitals 9.6 All 9.7 and 9.8 Omit ALL MOLECULAR SHAPES The shape
Chapter 9. Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory
 Chapter 9 Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory Problems with Lewis Theory Lewis theory generally predicts trends in properties, but does not give good numerical predictions.
Chapter 9 Lewis Theory-VSEPR Valence Bond Theory Molecular Orbital Theory Problems with Lewis Theory Lewis theory generally predicts trends in properties, but does not give good numerical predictions.
1. Sodium nitrite is an ionic compound containing a polyatomic ion. Answer the following questions relative to nitrite.
 Ch 10-11 Practice Problems - KEY The following problems are intended to provide you with additional practice in preparing for the exam. Questions come from the textbook, previous quizzes, previous exams,
Ch 10-11 Practice Problems - KEY The following problems are intended to provide you with additional practice in preparing for the exam. Questions come from the textbook, previous quizzes, previous exams,
GHW#3 Louisiana Tech University, Chemistry 281. POGIL exercise on Chapter 2. Covalent Bonding: VSEPR, VB and MO Theories. How and Why?
 GHW#3 Louisiana Tech University, Chemistry 281. POGIL exercise on Chapter 2. Covalent Bonding: VSEPR, VB and MO Theories. How and Why? How is Valence Shell Electron Pair Repulsion Theory developed from
GHW#3 Louisiana Tech University, Chemistry 281. POGIL exercise on Chapter 2. Covalent Bonding: VSEPR, VB and MO Theories. How and Why? How is Valence Shell Electron Pair Repulsion Theory developed from
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 1
 Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. How to get the book of
Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals Chapter 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. How to get the book of
At the end of this lesson, students should be able to :
 At the end of this lesson, students should be able to : (a) Explain Valence Shell Electron Pair Repulsion theory (VSEPR) (b) Draw the basic molecular shapes: linear, planar, tetrahedral, and octahedral.
At the end of this lesson, students should be able to : (a) Explain Valence Shell Electron Pair Repulsion theory (VSEPR) (b) Draw the basic molecular shapes: linear, planar, tetrahedral, and octahedral.
