X SCIENCE (THEORY) BLUE PRINT II. Form of Questions VSA SA - I SA - II LA Total Unit (1 Mark) (2 Marks) (3 Marks) (5 Marks)
|
|
|
- Stanley Banks
- 6 years ago
- Views:
Transcription
1 X SCIENCE (THEORY) BLUE PRINT II Form of Questions VSA SA - I SA - II LA Total Unit (1 Mark) (2 Marks) (3 Marks) (5 Marks) Chemical Substances 3(3) 4(2) 6(2) 5(1) 18(8) World of Living 2(2) 6(3) 3(1) 5(1) 16(7) Effects of current 1(1) 4(2) - 5(1) 10(4) Light 2(2) - 6(2) - 8(4) Natural Resources 1(1) 4(2) 3(1) - 8(4) Total 9(9) 18(9) 18(6) 15(3) 60(27) 316
2 SAMPLE QUESTION PAPER II X - SCIENCE (Theory) Time:2 Hours Max. Marks: 60 General Instructions 1. The question paper comprises of two sections A and B. You have to attempt both the sections. 2. All questions are compulsory. 3. There is no overall choice. However, internal choice has been provided in all the three questions of five marks category. Only one option in such questions is to be attempted. 4. All questions of section A and all questions of section B to be attempted separately. 5. Questions 1 to 6 in section A and 19 to 21 in section B are very short answer questions. These carry one mark each. 6. Questions 7 to 12 in section A and 22 to 24 in section B are short answer type questions and carry two marks each. 7. Questions 13 to 16 in section A and 25 to 26 in section B are also short answer type questions and carry three marks each. 8. Questions 17 and 18 in section A and question 27 in section B are long answer type questions and carry five marks each. Section A 1. A ray of light AM is incident on a spherical mirror as shown in the diagram. Redraw the diagram on the answer sheet and show the path of reflected ray. 1. A metal M belongs to 13 th group in the modern periodic table. Write the valency of the metal. 2. The ciliary muscles of a normal eye are in their (i) most relaxed (ii) most contracted state. In which of the two cases is the focal length of the eye-lens more? 3. Tap water conducts electricity whereas distilled water does not. Why? 4. An electric geyser has the ratings 2000W, 220V marked on it. What should be the minimum rating, in whole number of a fuse wire, that may be required for safe use with this geyser? 5. Kerosene burns with a sooty flame. Is it a saturated or an un saturated compound? 6. Two metallic wires A and B of the same material are connected in parallel. Wire A has length l and radius r, wire B has a length 2l and radius 2r. Calculate the ratio of the equivalent resistance in parallel combination and the resistance of wire A. 7. A student performs an experiment to study the magnetic effect of current around a current 317
3 carrying straight conductor. He reports that (i) for a given battery, the degree of deflection of a N pole decreases when the compass is kept at a point farther away from the conductor. (ii) the direction of deflection of the north pole of a compass needle kept at a given point near the conductor remains unaffected even when the terminals of the same battery sending current in the wire are inter changed. Which of the above observations of the student appears to be wrong and why? 8. A housewife wanted her house to be whitewashed. She bought 10kg of quick lime from the market and added it to 30 litres of water. On adding lime to water she noticed that the water appeared to be boiling even when it was not being heated. Give reason for her observation. Write the corresponding chemical equation and name the product formed. 9. Write the electron- dot structure for sodium and chlorine atoms. How do these form a chemical bond? Name the type of bond so formed. Why does a compound so formed have high melting point? 10. Why are many thermal power plants set up near coal or oil fields? 11. How is charcoal obtained from wood? Write two advantages of using charcoal as a fuel over wood? 12. Two carbon compounds A and B have the molecular formula C 3 H 8 and C 3 H 6 respectively. Which one of the two is most likely to show addition reaction? Justify your answer. Explain with the help of a chemical equation, how an addition reaction is useful in vegetable ghee industry. 13. A beam of white light falling on a glass prism gets split up into seven colours marked 1 to 7 as shown in the diagram. A student makes the following statements about the spectrum observed on the screen. 14. A beam of white light falling on a glass prism gets split up into seven colours marked 1 to 7 as shown in the diagram. A student makes the following statements about the spectrum observed on the screen. (a) The colours at positions marked 3 and 5 are similar to the colour of the sky and the colour of gold metal respectively. Is the above statement made by the student correct or incorrect? Justify. (b) Which two positions correspond closely to the colour of (i) a brinjal (ii) danger or stop signal lights? 15. Baking soda is used in small amount in making bread and cake. It helps to make these soft and spongy. An aqueous solution of baking soda turns red litmus blue. 318
4 It is also used in soda acid fire extinguisher. Use this information to answer the following questions:- (i) How does Baking Soda help to make cakes and bread soft and spongy? (ii) How does it help in extinguishing fire? (iii) Is the ph value of baking soda solution lower than or higher than 7? 16. (i) A concave mirror produces three times enlarged image of an object placed at 10 cm in front of it. Calculate the focal length of the mirror. (ii) Show the formation of the image with the help of a ray diagram when the object is placed 6 cm away from the pole of the mirror. 17. (a) How does the atomic radius change as you go (i) from left to right in a period? (ii) down a group in the periodic table (b) Two elements X and Y have atomic numbers 12 and 16 respectively. Write the electronic configuration for these elements. To which period of the modern periodic table do these two elements belong? What type of bond will be formed between them and why? (a) How would the tendency to lose electrons change as you go (i) from left to right across a period (ii) down a group Or (b) An element X (2, 8,2) combines separately with (NO 3 ) 1-, (SO 4 ) 2-, and (PO 4 ) 3- radicals. Write the formulae of the three compounds so formed. To which group of the periodic table does the element X belong? Will it form covalent or ionic compound? Why? 18. (a) The electric power consumed by a device may be calculated by using either of the two expressions P = 1 2 R or P =. The first expression indicates that it is directly proportional to R whereas the second expression indicates inverse proportionality. How can the seemingly different dependence of P on R in these expressions be explained? (b) (i) A 100 W electric bulb is connected to 220 V mains power supply. Calculate the strength of the electric current passing through the bulb. (ii) If the same bulb is taken to U.S.A where the main power supply is 110 V, how much electric current will pass through the bulb when connected to mains? OR Explain the meaning of the word electromagnetic and induction in the termelectromagnetic induction. On what factors does the value of induced current produced in a circuit depend? Name and state the rule used for determination of direction of induced current. State one practical application of this phenomenon in every day life. 319
5 SECTION B Q.19 What will be the impact on ecosystems if Bacteria, fungi/microorganism are remove from the environment? 1 Q.20 Why is sexual reproduction considered to be superior to asexual reproduction is terms of evolution? 1 Q.21 Name one organ where growth hormone is synthesized in case of plants and man. 1 Q.22 The given experimental set up establishes the response of different plant parts towards gravity. a) Give the scientific term used for such response/movement. 1 b) How is shoot response different from root response/movement. 1 Q.23 Name the parts of brain which control following activities. Blood Pressure Riding Bicycle Hearing Centre associated with hunger x 4 = 2 Q.24 Given below is the experimental set up to establish that one of the atmospheric gases is essential for photosynthesis is plants. a. Name the atmospheric gas which is essential for photosynthesis. 2 b. What is kept is watch glass in figure a and why? Q.25 Mrs. Joshi is a house wife and wants to contribute for conservation of natural resources. List any six activities that she can do on her own
6 Q.26 (i) Label any 4 parts in the above diagram. (ii) What are the two functions represented in this diagram? 3 Q.27 Plants absorb water from the soil. How does this water reach the tree tops? Explain in detail. OR Complete the glucose breakdown pathway in case of aerobic respiration by filling the blanks. 6 =3 a) Name the molecule in the cell which stores the energy produced at the end of the path way. b) Why do we get cramps during sudden muscular activity? + 321
7 MARKING SCHEME SAMPLE QUESTION PAPER II X SCIENCE (THEORY) Q.No. Value Point Marks The valency of the metal is In the first case Tap water contains dissolved salts and minerals which ionise in water and hence conducts electricity. Distilled water is a covalent compound so it does not conduct electricity A 1 6. Unsaturated Observation (ii) is wrong. 1 When the direction of flow of current is changed, the direction of the magnetic field and hence the direction of force also changes The reaction of lime with water is an exothermic reaction in which lot of heat is evolved
8 CaO +H 2 O Ca (OH) Na Ca(OH) 2 is called slaked lime or calcium hydroxide 1 Sodium atom loses one electron to attain octet configuration and forms sodium ion. Chlorine atom has 7 electrons in the outermost shell, so it gains the electron lost by the sodium atom to form the chloride ion. The two oppositely charged ions are held together by strong electrostatic force of attraction to form the ionic bond. 1 Ionic compounds have high melting points because a considerable amount of energy is required to break the strong inter ionic attractions. 11. Coal or Petroleum is required to heat water to produce steam for running the turbines in thermal power plants. Cost of transportation is reduced if the thermal power plants are located near coal or oil fields Charcoal is obtained by burning wood in a limited supply of oxygen 1 (i) combus.tion of charcoal is comparatively smokeless (ii) It has a higher heat generation efficiency 2 x = C 3 H 6 will show addition reaction. C 3 H 6 is an unsaturated compound with a double bond. Vegetable oils have long unsaturated carbon chains which on addition of hydrogen in the presence of catalyst Nickel, change in to saturated carbon chains. This is called hydrogenation of oils (a) Incorrect. The student is stating the nature of colours in reverse order. 1 (b) (i) Colour marked 7 1 (ii) Colour marked 1 323
9 15. (i) On heating, baking soda gives out CO 2 which makes cakes and bread rise up, so they become soft and spongy. 1 (ii) CO 2 being heavier than air settles down and forms a layer between the burning substance and the air, thus cutting off the supply of oxygen. 1 (iii) Its ph value is greater than (i) (ii) 17. (a) (i) Atomic radius decreases 1 (ii) Atomic radius increases 1 (b) Atomic number X, Z= 12 2, 8, 2 Y, Z = 16 2, 8, 6 1 They belong to the third period. They will form ionic bond because X is a metal and Y is a non metal. X loses 2 electrons which will be gained by Y. 2 OR (a) (i) Tendency to lose electrons decreases 1 (ii) increases. 324
10 (b) X 3 (PO 4 ) 2 X (NO 3 ) 2 X SO 4 X belongs to Group II in the periodic table. It will form ionic compounds because it will readily lose 2 electrons (a) Both the expressions are correct. In the first case, I remains constant whereas the second expression is true when V remains constant. 2 (b) 1 1 OR (i) Meaning of the terms 1 (ii) Rate of change of magnetic flux 1 (iii) Fleming s Right Hand Rule 1 (iv) Statement of the rule 1 (v) Electric Generator 1 SECTION -B 19. Complex organic molecules will not breakdown in to simple inorganic substances, Preventing replenishment of soil Sexual mode of reproduction is a source of variation (in a population of organisms) which ensures survival of the species Shoot tip in plants (or any other appropriate example), pituitary gland (anterior) in man a) Geotropism 1 b) Root shows positive while shoot shows negative geotropic movement a) Hind brain b) Hind brain (cerebellum) c) Fore brain d) Fore brain 4=
11 24. a) CO 2 Carbon dioxide. b) KOH it absorbs CO 2 to create an atmosphere which is devoid of CO 2. ( + ) 25. 1) Using solar cooker 2) Collecting waste separately 3) Using public transport 4) Using CFL 5) Avoid using lift or AC 6) Getting leaking taps repaired immediately 7) Use of pressure cooker 8) Switching off engine at traffic light (any other suitable examples). 6=3 26. (i) a) Pulmonary artery to lungs b) Lung capillaries c) Pulmonary vein from lungs d) Aorta to body e) Capillaries in body organs f ) Venacava from body. (ii) Transport of oxygen and carbon dioxide Exchange of oxygen and carbon dioxide 27. Xylem (vessels) of roots, stems and leaves are interconnected to form a continuous column. Roots also take up mineral salts actively, water moves in as a result creating pressure - the root pressure that pushes the water up. Stomatal transpiration creates suction force, pulling up the water from root xylem / transpiration pull. 1x5 = ) Pyruvate (3 carbon molecules) 2) Energy 3) Presence of oxygen 4) In Mitochondria 5) Carbon dioxide OR 6) Water 6 = 3 a) ATP 1 b) Lactic acid accumulation, in the absence of oxygen(anaerobic respiration) + 326
KENDRIYA VIDYALAYA GACHIBOWLI, GPRA CAMPUS, HYD 32
 KENDRIYA VIDYALAYA GACHIBOWLI, GPRA CAMPUS, HYD 32 SAMPLE PAPER 03 (2018-19) SUBJECT: SCIENCE (086) BLUE PRINT : CLASS X UNIT Chemical Substances - Nature and Behaviour World of Living Natural Phenomen
KENDRIYA VIDYALAYA GACHIBOWLI, GPRA CAMPUS, HYD 32 SAMPLE PAPER 03 (2018-19) SUBJECT: SCIENCE (086) BLUE PRINT : CLASS X UNIT Chemical Substances - Nature and Behaviour World of Living Natural Phenomen
KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION
 KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION SAMPLE PAPER 03 (2017-18) SUBJECT: SCIENCE (086) BLUE PRINT : CLASS X UNIT Chemical Substances - Nature and Behaviour World of Living Natural Phenomen a Effects
KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION SAMPLE PAPER 03 (2017-18) SUBJECT: SCIENCE (086) BLUE PRINT : CLASS X UNIT Chemical Substances - Nature and Behaviour World of Living Natural Phenomen a Effects
KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION
 KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION SAMPLE PAPER 05 (2017-18) SUBJECT: SCIENCE (086) BLUE PRINT : CLASS X UNIT Chemical Substances - Nature and Behaviour World of Living Natural Phenomen a Effects
KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION SAMPLE PAPER 05 (2017-18) SUBJECT: SCIENCE (086) BLUE PRINT : CLASS X UNIT Chemical Substances - Nature and Behaviour World of Living Natural Phenomen a Effects
KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION
 KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION SAMPLE PAPER 09 (2017-18) SUBJECT: SCIENCE (086) BLUE PRINT : CLASS X UNIT Chemical Substances - Nature and Behaviour World of Living Natural Phenomen a Effects
KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION SAMPLE PAPER 09 (2017-18) SUBJECT: SCIENCE (086) BLUE PRINT : CLASS X UNIT Chemical Substances - Nature and Behaviour World of Living Natural Phenomen a Effects
KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION
 KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION SAMPLE PAPER 05 FOR SA I (2016-17) SUBJECT: SCIENCE BLUE PRINT : SA-I CLASS X Unit/Topic Chemical Reactions and Equations Acids, Bases and Salts Metals and
KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION SAMPLE PAPER 05 FOR SA I (2016-17) SUBJECT: SCIENCE BLUE PRINT : SA-I CLASS X Unit/Topic Chemical Reactions and Equations Acids, Bases and Salts Metals and
KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION
 KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION SAMPLE PAPER 01 FOR PERIODIC TEST II EXAM (2017-18) SUBJECT: SCIENCE (086) BLUE PRINT FOR HALF YEARLY EXAM: CLASS X Chapter Chemical Reactions and Equations
KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION SAMPLE PAPER 01 FOR PERIODIC TEST II EXAM (2017-18) SUBJECT: SCIENCE (086) BLUE PRINT FOR HALF YEARLY EXAM: CLASS X Chapter Chemical Reactions and Equations
KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION
 KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION SAMPLE PAPER 08 (2017-18) SUBJECT: SCIENCE (086) BLUE PRINT : CLASS X UNIT Chemical Substances - Nature and Behaviour World of Living Natural Phenomen a Effects
KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION SAMPLE PAPER 08 (2017-18) SUBJECT: SCIENCE (086) BLUE PRINT : CLASS X UNIT Chemical Substances - Nature and Behaviour World of Living Natural Phenomen a Effects
KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION
 KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION SAMPLE PAPER 10 (2017-18) SUBJECT: SCIENCE (086) BLUE PRINT : CLASS X UNIT Chemical Substances - Nature and Behaviour World of Living Natural Phenomen a Effects
KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION SAMPLE PAPER 10 (2017-18) SUBJECT: SCIENCE (086) BLUE PRINT : CLASS X UNIT Chemical Substances - Nature and Behaviour World of Living Natural Phenomen a Effects
6 (i)name the hormones that are released in human males and females when they reach puberty.
 PRACTICE PAPER FOR SUMMATIVE ASSESSMENT I, 0 Class X SCIENCE Time Allowed : hours Maximum Marks : 90 General Instructions :. The question paper comprises of two Sections, A and B. You are to attempt both
PRACTICE PAPER FOR SUMMATIVE ASSESSMENT I, 0 Class X SCIENCE Time Allowed : hours Maximum Marks : 90 General Instructions :. The question paper comprises of two Sections, A and B. You are to attempt both
CBSE X Science
 Time allowed: 3 hours; Maximum Marks: 90 General Instructions: 1. The question paper comprises of two Sections, A and B. You are to attempt both the sections. 2. All questions are compulsory. 3. There
Time allowed: 3 hours; Maximum Marks: 90 General Instructions: 1. The question paper comprises of two Sections, A and B. You are to attempt both the sections. 2. All questions are compulsory. 3. There
Sub- Science (Physics and Chemistry ) Important questions for board purpose
 Class -10 Sub- Science (Physics and Chemistry ) Important questions for board purpose Note: Students are requested to solve these questions in separate notebook/a-4 sheets. 1. Burning of fossil fuels result
Class -10 Sub- Science (Physics and Chemistry ) Important questions for board purpose Note: Students are requested to solve these questions in separate notebook/a-4 sheets. 1. Burning of fossil fuels result
Roll No. SCIENCE (Theory) Time allowed: General Instructions:
 Series LRH/2 Roll No Code no. Candidates must write the code on the title page of the answer book. Please check that this question paper contains 12 printed pages. Code number given on the right hand side
Series LRH/2 Roll No Code no. Candidates must write the code on the title page of the answer book. Please check that this question paper contains 12 printed pages. Code number given on the right hand side
CBSE Class X Science Sample Paper - 1 Time: 3 hrs Total Marks: 80. Section A
 CBSE Class X Science Sample Paper - 1 Time: 3 hrs Total Marks: 80 General Instructions: 1. The question paper comprises of two Sections, A and B. You are to attempt both the sections. 2. All questions
CBSE Class X Science Sample Paper - 1 Time: 3 hrs Total Marks: 80 General Instructions: 1. The question paper comprises of two Sections, A and B. You are to attempt both the sections. 2. All questions
KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION
 KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION SAMPLE PAPER 06 (2017-18) SUBJECT: SCIENCE (086) BLUE PRINT : CLASS X UNIT Chemical Substances - Nature and Behaviour World of Living Natural Phenomen a Effects
KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION SAMPLE PAPER 06 (2017-18) SUBJECT: SCIENCE (086) BLUE PRINT : CLASS X UNIT Chemical Substances - Nature and Behaviour World of Living Natural Phenomen a Effects
Unit 3 Lesson 4 Ionic, Covalent, and Metallic Bonding. Copyright Houghton Mifflin Harcourt Publishing Company
 Opposites Attract What is an ion? An atom has a neutral charge because it has an equal number of electrons and protons. An ion is a particle with a positive or negative charge. An ion forms when an atom
Opposites Attract What is an ion? An atom has a neutral charge because it has an equal number of electrons and protons. An ion is a particle with a positive or negative charge. An ion forms when an atom
A. MOLECULE: B. CHEMICAL BOND:
 What is a molecule? A. MOLECULE: a group of atoms bonded together 1. Molecules can be made of one kind of atom or many different kinds of atoms. Oxygen we breathe is an example of one kind of atom in a
What is a molecule? A. MOLECULE: a group of atoms bonded together 1. Molecules can be made of one kind of atom or many different kinds of atoms. Oxygen we breathe is an example of one kind of atom in a
Ashwani Gupta Mb:
 Class X Question bank Semester 1 1. Write the reaction that takes place when limestone is heated. 2. Give the characteristics of chemical changes 3. Why is a magnesium ribbon cleaned with sandpaper before
Class X Question bank Semester 1 1. Write the reaction that takes place when limestone is heated. 2. Give the characteristics of chemical changes 3. Why is a magnesium ribbon cleaned with sandpaper before
2. Name the part of the brain which controls posture and balance of the body?
 . Give two characteristics of magnetic field lines. SECTION-A 2. Name the part of the brain which controls posture and balance of the body?. Mention two different ways of harnessing energy from ocean.
. Give two characteristics of magnetic field lines. SECTION-A 2. Name the part of the brain which controls posture and balance of the body?. Mention two different ways of harnessing energy from ocean.
CLASS X GUESS PAPER SCIENCE
 CLASS X GUESS PAPER SCIENCE TIME ALLOWED: 3 HOURS MAXIMUM MARKS: 60 GENERAL INSTRUCTIONS: 1. All questions are compulsory. 2. This question paper consist of two sections A and B. Questions 1-6 in Section
CLASS X GUESS PAPER SCIENCE TIME ALLOWED: 3 HOURS MAXIMUM MARKS: 60 GENERAL INSTRUCTIONS: 1. All questions are compulsory. 2. This question paper consist of two sections A and B. Questions 1-6 in Section
Science and Technology Material World Periodic Table and Solutions
 Science and Technology Material World Periodic Table and Solutions Peridoic table is grouped by broad categories of elements, groups and periods. Broad categories: metals, non-metals and metalloids o Metals
Science and Technology Material World Periodic Table and Solutions Peridoic table is grouped by broad categories of elements, groups and periods. Broad categories: metals, non-metals and metalloids o Metals
Important Instructions for the School Principal. (Not to be printed with the question paper)
 Important Instructions for the School Principal (Not to be printed with the question paper) ) This question paper is strictly meant for use in school based SA-I, September-202 only. This question paper
Important Instructions for the School Principal (Not to be printed with the question paper) ) This question paper is strictly meant for use in school based SA-I, September-202 only. This question paper
Channa Asela
 Reproducing the following contents by amending or deleting the author s name and contact numbers is prohibited. You may email or print without any amendment. Underline the most suitable answer 1995-I-2
Reproducing the following contents by amending or deleting the author s name and contact numbers is prohibited. You may email or print without any amendment. Underline the most suitable answer 1995-I-2
CBSE Sample Papers for Class 10 Science Solved 2016 (Set 1)
 CBSE Sample Papers for Class 10 Science Solved 2016 (Set 1) Time Duartions:3Hrs Maximum Marks:90 General Instructions: 1. The question paper comprises of two Sections, A and B. You are to attempt both
CBSE Sample Papers for Class 10 Science Solved 2016 (Set 1) Time Duartions:3Hrs Maximum Marks:90 General Instructions: 1. The question paper comprises of two Sections, A and B. You are to attempt both
Atoms, Elements, Atoms, Elements, Compounds and Mixtures. Compounds and Mixtures. Atoms and the Periodic Table. Atoms and the.
 Atoms, Elements, Compounds and Mixtures Explain how fractional distillation can be used to separate a mixture. 1 Atoms, Elements, Compounds and Mixtures Fractional distillation is used to separate components
Atoms, Elements, Compounds and Mixtures Explain how fractional distillation can be used to separate a mixture. 1 Atoms, Elements, Compounds and Mixtures Fractional distillation is used to separate components
Chapter: Cell Processes
 Table of Contents Chapter: Cell Processes Section 1: Chemistry of Life Section 2: Moving Cellular Materials Section 3: Energy for Life 1 Chemistry of Life The Nature of Matter Matter is anything that has
Table of Contents Chapter: Cell Processes Section 1: Chemistry of Life Section 2: Moving Cellular Materials Section 3: Energy for Life 1 Chemistry of Life The Nature of Matter Matter is anything that has
Page 1 of 14. Website: Mobile:
 Question 1: You have been provided with three test tubes. One of them contains distilled water and the other two contain an acidic solution and a basic solution, respectively. If you are given only red
Question 1: You have been provided with three test tubes. One of them contains distilled water and the other two contain an acidic solution and a basic solution, respectively. If you are given only red
6. Differentiate: a) Nuclear fission & nuclear fusion. b) Geothermal Energy & Ocean thermal Energy. c) Biogas & Biomass. (3)
 Std. 10 SCIENCE Time : 3 hrs. M. Marks : 90 General Instructions : 1. The question paper comprises of four sections A, B, C and D. You are to attempt all the four sections. 2. All questions are compulsory.
Std. 10 SCIENCE Time : 3 hrs. M. Marks : 90 General Instructions : 1. The question paper comprises of four sections A, B, C and D. You are to attempt all the four sections. 2. All questions are compulsory.
KS3 Science PERSONAL LEARNING CHECKLIST. YEAR 88 CONTENT Use this document to monitor your revision and target specific areas.
 KS3 Science PERSONAL LEARNING CHECKLIST YEAR 88 CONTENT Use this document to monitor your revision and target specific areas. Topic Name BIOLOGY the human skeleton Analysing the skeleton the role of skeletal
KS3 Science PERSONAL LEARNING CHECKLIST YEAR 88 CONTENT Use this document to monitor your revision and target specific areas. Topic Name BIOLOGY the human skeleton Analysing the skeleton the role of skeletal
Visit For All NCERT Solutions, CSBE Sample papers, Question, papers, Notes For Class 6 to 12 SUMMATIVE ASSESSMENT-II [2013] SCIENCE
![Visit For All NCERT Solutions, CSBE Sample papers, Question, papers, Notes For Class 6 to 12 SUMMATIVE ASSESSMENT-II [2013] SCIENCE Visit For All NCERT Solutions, CSBE Sample papers, Question, papers, Notes For Class 6 to 12 SUMMATIVE ASSESSMENT-II [2013] SCIENCE](/thumbs/83/87854604.jpg) SUMMATIVE ASSESSMENT-II [2013] SCIENCE Time allowed: 3 hours] [Maximum marks :90 General Instructions: (i) The question paper comprises of two sections, A and B. You are to attempt both the sections. (ii)
SUMMATIVE ASSESSMENT-II [2013] SCIENCE Time allowed: 3 hours] [Maximum marks :90 General Instructions: (i) The question paper comprises of two sections, A and B. You are to attempt both the sections. (ii)
0654 CO-ORDINATED SCIENCES
 CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2013 series 0654 CO-ORDINATED SCIENCES 0654/22 Paper 2 (Core Theory), maximum
CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2013 series 0654 CO-ORDINATED SCIENCES 0654/22 Paper 2 (Core Theory), maximum
Coimisiún na Scrúduithe Stáit State Examinations Commission
 2009. M 37 Coimisiún na Scrúduithe Stáit State Examinations Commission LEAVING CERTIFICATE EXAMINATION 2009 PHYSICS AND CHEMISTRY ORDINARY LEVEL MONDAY, 15 JUNE MORNING 9:30 TO 12:30 Six questions to be
2009. M 37 Coimisiún na Scrúduithe Stáit State Examinations Commission LEAVING CERTIFICATE EXAMINATION 2009 PHYSICS AND CHEMISTRY ORDINARY LEVEL MONDAY, 15 JUNE MORNING 9:30 TO 12:30 Six questions to be
CBSE Class X Science Board Paper 2018 (Set 2)
 CBSE Class X Science Board Paper 2018 (Set 2) Time allowed: 3 hours Maximum marks: 80 General Instructions: 1. The question paper comprises of two Sections, A and B. You are to attempt both the sections.
CBSE Class X Science Board Paper 2018 (Set 2) Time allowed: 3 hours Maximum marks: 80 General Instructions: 1. The question paper comprises of two Sections, A and B. You are to attempt both the sections.
Chemical Reactions. Unit 4
 Chemical Reactions Unit 4 Lesson 1: Chemical Bonds Unit 4: Reactions Compounds Most substances around you are NOT elements. There are around 100 elements, but millions of different substances. Most substances
Chemical Reactions Unit 4 Lesson 1: Chemical Bonds Unit 4: Reactions Compounds Most substances around you are NOT elements. There are around 100 elements, but millions of different substances. Most substances
 Question 1: What would be the electron dot structure of carbon dioxide which has the formula CO 2? Electron dot structure of CO 2 is What would be the electron dot structure of a molecule of sulphur which
Question 1: What would be the electron dot structure of carbon dioxide which has the formula CO 2? Electron dot structure of CO 2 is What would be the electron dot structure of a molecule of sulphur which
4 Energy and Rates of Chemical Reactions
 CHAPTER 14 4 and Rates of Chemical Reactions SECTION Chemical Reactions BEFORE YOU READ After you read this section, you should be able to answer these questions: How is energy involved in a chemical reaction?
CHAPTER 14 4 and Rates of Chemical Reactions SECTION Chemical Reactions BEFORE YOU READ After you read this section, you should be able to answer these questions: How is energy involved in a chemical reaction?
SUMMATIVE ASSESSMENT-II, SCIENCE Time : 3½ hrs. Class X M.M. : 90
 SUMMATIVE ASSESSMENT-II, 2014 SCIENCE Time : 3½ hrs. Class X M.M. : 90 Date 08.2.2014 General Instructions : The question paper comprises of two Sections, A and B. You are to attempt both the sections.
SUMMATIVE ASSESSMENT-II, 2014 SCIENCE Time : 3½ hrs. Class X M.M. : 90 Date 08.2.2014 General Instructions : The question paper comprises of two Sections, A and B. You are to attempt both the sections.
Downloaded from
 Subject: Chemistry Class: XI Chapter: The s-block Elements Top concepts. The s-block elements of the periodic table are those in which the last electron enters the outermost s-orbital. Elements of group
Subject: Chemistry Class: XI Chapter: The s-block Elements Top concepts. The s-block elements of the periodic table are those in which the last electron enters the outermost s-orbital. Elements of group
Subject: Science-83E
 KARNATAKA SECONDARY EDUCATION EXAMINATION BOARD MODEL QUESTION PAPER 2018-19 Subject: Science-83E Time: 3 hours Marks: 80 I. Four alternatives are provided for each question. Choose the most appropriate
KARNATAKA SECONDARY EDUCATION EXAMINATION BOARD MODEL QUESTION PAPER 2018-19 Subject: Science-83E Time: 3 hours Marks: 80 I. Four alternatives are provided for each question. Choose the most appropriate
PAF Chapter Comprehensive Worksheet May 2016 Science Class 7 (Answering Key)
 The City School PAF Chapter Comprehensive Worksheet May 2016 Science Class 7 (Answering Key) The City School/ PAF Chapter/ Comprehensive Worksheet/ May 2016/ Science/ Class 7 /Ans Key Page 1 of 7 OBJECTIVE
The City School PAF Chapter Comprehensive Worksheet May 2016 Science Class 7 (Answering Key) The City School/ PAF Chapter/ Comprehensive Worksheet/ May 2016/ Science/ Class 7 /Ans Key Page 1 of 7 OBJECTIVE
2-2 Properties of Water. Copyright Pearson Prentice Hall
 2-2 Properties of Water Water Water is the most important molecule on earth. Because of its unique shape and chemical behavior it easily bonds with other molecules, and itself. Water: Covalent Bond Water
2-2 Properties of Water Water Water is the most important molecule on earth. Because of its unique shape and chemical behavior it easily bonds with other molecules, and itself. Water: Covalent Bond Water
8 th Grade Science. Directed Reading Packet. Chemistry. Name: Teacher: Period:
 8 th Grade Science Directed Reading Packet Chemistry Name: Teacher: Period: Chapter 1, Section 1: Inside the Atom Introduction 1. Atoms are the particles of an element that still have the element s. 2.
8 th Grade Science Directed Reading Packet Chemistry Name: Teacher: Period: Chapter 1, Section 1: Inside the Atom Introduction 1. Atoms are the particles of an element that still have the element s. 2.
2018 Version. Photosynthesis Junior Science
 2018 Version Photosynthesis Junior Science 1 Plants fill the role of Producers in a community Plants are special because they have leaves and are able to produce their own food by the process of photosynthesis
2018 Version Photosynthesis Junior Science 1 Plants fill the role of Producers in a community Plants are special because they have leaves and are able to produce their own food by the process of photosynthesis
SCIENCE HIGHER LEVEL
 J.37 PRE-JUNIOR CERTIFICATE EXAMINATION, 2014 SCIENCE HIGHER LEVEL TIME: 2 HOURS INSTRUCTIONS 1. Write your name, school s name and teacher s name in the boxes provided on this page. 2. Answer all questions.
J.37 PRE-JUNIOR CERTIFICATE EXAMINATION, 2014 SCIENCE HIGHER LEVEL TIME: 2 HOURS INSTRUCTIONS 1. Write your name, school s name and teacher s name in the boxes provided on this page. 2. Answer all questions.
Elements and Their Oxides
 Elements and Their Oxides An oxide is a. Oxides can form when an element reacts with oxygen, often in air. This reaction can be rapid with the release of a great deal of energy, as in the combustion of
Elements and Their Oxides An oxide is a. Oxides can form when an element reacts with oxygen, often in air. This reaction can be rapid with the release of a great deal of energy, as in the combustion of
ICSE Board. Class X Chemistry. Board Paper Time: 1½ hrs Total Marks: 80
 ICSE Board Class X Chemistry Board Paper 2013 Time: 1½ hrs Total Marks: 80 General Instructions: 1. Answers to this paper must be written on the paper provided separately. 2. You will NOT be allowed to
ICSE Board Class X Chemistry Board Paper 2013 Time: 1½ hrs Total Marks: 80 General Instructions: 1. Answers to this paper must be written on the paper provided separately. 2. You will NOT be allowed to
video 6.1 types of bonds
 video 6.1 types of bonds what is a bond? Intramolecular force that holds one to another in a compound The energy stored in a bond is energy 1 why do atoms bond? Atoms bond together to get 8 valence electrons
video 6.1 types of bonds what is a bond? Intramolecular force that holds one to another in a compound The energy stored in a bond is energy 1 why do atoms bond? Atoms bond together to get 8 valence electrons
2.1-2 Chemistry and Water
 Prepared by Kim Foglia. Adapted and modified by Nhan Pham. 2.1-2 Chemistry and Water Objectives Discuss why we study chemistry in biology Review structure of an atom Explain the role of valence electrons
Prepared by Kim Foglia. Adapted and modified by Nhan Pham. 2.1-2 Chemistry and Water Objectives Discuss why we study chemistry in biology Review structure of an atom Explain the role of valence electrons
The City School PAF Chapter
 The City School PAF Chapter Comprehensive Worksheet May 2016 Science Class 7 Candidate Name: Index Number: Section: Branch/Campus: Date: INSTRUCTIONS: Write your name, index number, section, branch/campus
The City School PAF Chapter Comprehensive Worksheet May 2016 Science Class 7 Candidate Name: Index Number: Section: Branch/Campus: Date: INSTRUCTIONS: Write your name, index number, section, branch/campus
II, SUMMATIVE ASSESSMENT II, / SCIENCE. X / Class X. Time Allowed : 3 hours Maximum Marks : 90
 ... II, 06-7 SUMMATIVE ASSESSMENT II, 06-7 / SCIENCE X / Class X 90 Time Allowed : hours Maximum Marks : 90 4.. 4 6 0-0 6. 7 8 0-0 7. 9 4 70-70 8. 9. 4 6 K8CBTT General Instructions :. The question paper
... II, 06-7 SUMMATIVE ASSESSMENT II, 06-7 / SCIENCE X / Class X 90 Time Allowed : hours Maximum Marks : 90 4.. 4 6 0-0 6. 7 8 0-0 7. 9 4 70-70 8. 9. 4 6 K8CBTT General Instructions :. The question paper
SET 2 Series : HRK/C H$moS> Z.
 SET 2 Series : HRK/C H$moS> Z. 31/2. Roll No. - 12 Code No. - - Candidates must write the Code on the title page of the answer-book. - - - - 36, - 15-10.15 10.15 10.30 - - Please check that this question
SET 2 Series : HRK/C H$moS> Z. 31/2. Roll No. - 12 Code No. - - Candidates must write the Code on the title page of the answer-book. - - - - 36, - 15-10.15 10.15 10.30 - - Please check that this question
Y8 Science Controlled Assessment Topics & Keywords
 Y8 Science Controlled Assessment Topics & Biology Respiration. Know that respiration in living organisms can be aerobic or anaerobic The word equation for aerobic respiration The process of anaerobic respiration
Y8 Science Controlled Assessment Topics & Biology Respiration. Know that respiration in living organisms can be aerobic or anaerobic The word equation for aerobic respiration The process of anaerobic respiration
KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION
 KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION SAMPLE PAPER 01 F PERIODIC TEST III EXAM (2017-18) SUBJECT: SCIENCE (086) BLUE PRINT : CLASS X UNIT Chapter VSA (1 mark) SA I (2 marks) SA II (3 marks) LA
KENDRIYA VIDYALAYA SANGATHAN, HYDERABAD REGION SAMPLE PAPER 01 F PERIODIC TEST III EXAM (2017-18) SUBJECT: SCIENCE (086) BLUE PRINT : CLASS X UNIT Chapter VSA (1 mark) SA I (2 marks) SA II (3 marks) LA
SCIENCE AND TECHNOLOGY
 Total No. of Printed Pages 11 X/13/S & T 2 0 1 3 SCIENCE AND TECHNOLOGY ( CANDIDATES WITH PRACTICAL/INTERNAL ASSESSMENT ) Full Marks : 80 Pass Marks : 24 ( CANDIDATES WITHOUT PRACTICAL/INTERNAL ASSESSMENT
Total No. of Printed Pages 11 X/13/S & T 2 0 1 3 SCIENCE AND TECHNOLOGY ( CANDIDATES WITH PRACTICAL/INTERNAL ASSESSMENT ) Full Marks : 80 Pass Marks : 24 ( CANDIDATES WITHOUT PRACTICAL/INTERNAL ASSESSMENT
Chemical Bonds & Reactions
 Chemical Bonds & Reactions Chemical Bonding Do you understand how it works? What do you think when I pull out a bag of candy? I want that candy cause I don t have any! Does everyone think the same thing?
Chemical Bonds & Reactions Chemical Bonding Do you understand how it works? What do you think when I pull out a bag of candy? I want that candy cause I don t have any! Does everyone think the same thing?
Science Year 10 Unit 1 Biology
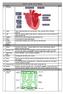 Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
Week 1: 1. The Heart Science Year 10 Unit 1 Biology RAG 2. Artery Takes oxygenated blood away from the heart. Thick, muscular walls to withstand pressure. 3. Vein Takes deoxygenated blood towards the heart.
ExamLearn.ie. Chemical Bonding
 ExamLearn.ie Chemical Bonding Chemical Bonding A molecule is a group of atoms joined together. It is the smallest particle of an element or compound that can exist independently. Eg: Molecule of water
ExamLearn.ie Chemical Bonding Chemical Bonding A molecule is a group of atoms joined together. It is the smallest particle of an element or compound that can exist independently. Eg: Molecule of water
NORTH CENTRAL HIGH SCHOOL NOTE & STUDY GUIDE. Honors Biology I
 NOTE/STUDY GUIDE: Unit 1-2, Biochemistry Honors Biology I, Mr. Doc Miller, M.Ed. North Central High School Name: Period: Seat #: Date: NORTH CENTRAL HIGH SCHOOL NOTE & STUDY GUIDE Honors Biology I Unit
NOTE/STUDY GUIDE: Unit 1-2, Biochemistry Honors Biology I, Mr. Doc Miller, M.Ed. North Central High School Name: Period: Seat #: Date: NORTH CENTRAL HIGH SCHOOL NOTE & STUDY GUIDE Honors Biology I Unit
Chemical bonding & structure
 Chemical bonding & structure Ionic bonding and structure Covalent bonding Covalent structures Intermolecular forces Metallic bonding Ms. Thompson - SL Chemistry Wooster High School Topic 4.1 Ionic bonding
Chemical bonding & structure Ionic bonding and structure Covalent bonding Covalent structures Intermolecular forces Metallic bonding Ms. Thompson - SL Chemistry Wooster High School Topic 4.1 Ionic bonding
Please check that this question paper contains 12 printed pages. Code number given on the right hand side of the question paper should be written
 SET-3 Series TYM Code No. 31/3 Roll No. Candidates must write the Code on the title page of the answer-book Please check that this question paper contains 12 printed pages. Code number given on the right
SET-3 Series TYM Code No. 31/3 Roll No. Candidates must write the Code on the title page of the answer-book Please check that this question paper contains 12 printed pages. Code number given on the right
Chapter 15 Study Questions
 Chapter 15 Study Questions Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Which of the following describes lipids? a. used to store energy
Chapter 15 Study Questions Multiple Choice Identify the letter of the choice that best completes the statement or answers the question. 1. Which of the following describes lipids? a. used to store energy
Indicate the answer choice that best completes the statement or answers the question.
 Indicate the answer choice that best completes the statement or answers the question. 1. Which of the following bonds is polar? a. F F b. O H c. O O d. H H 2. In the compound, H 2 O, the electrons in the
Indicate the answer choice that best completes the statement or answers the question. 1. Which of the following bonds is polar? a. F F b. O H c. O O d. H H 2. In the compound, H 2 O, the electrons in the
Chapter 6: Chemical Bonding
 Chapter 6: Chemical Bonding Learning Objectives Describe the formation of ions by electron loss/gain to obtain the electronic configuration of a noble gas. Describe the formation of ionic bonds between
Chapter 6: Chemical Bonding Learning Objectives Describe the formation of ions by electron loss/gain to obtain the electronic configuration of a noble gas. Describe the formation of ionic bonds between
5.1 How Atoms Form Compounds. compound chemical formula molecule chemical bond ionic bond valence covalent bond
 5.1 How Atoms Form Compounds compound chemical formula molecule chemical bond ionic bond valence covalent bond What is a compound? 5.1 How Atoms Form Compounds A compound is a pure substance that contains
5.1 How Atoms Form Compounds compound chemical formula molecule chemical bond ionic bond valence covalent bond What is a compound? 5.1 How Atoms Form Compounds A compound is a pure substance that contains
Life is a chemical process
 CHEMISTRY FOR LIFE Life is a chemical process Relies on and is subject to chemistry Must obey the laws of physics Biologists study Chemistry because all living things are made of matter. Matter undergoes
CHEMISTRY FOR LIFE Life is a chemical process Relies on and is subject to chemistry Must obey the laws of physics Biologists study Chemistry because all living things are made of matter. Matter undergoes
0620 CHEMISTRY. Mark schemes should be read in conjunction with the question paper and the Principal Examiner Report for Teachers.
 CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2014 series 0620 CHEMISTRY 0620/32 Paper 3 (Extended Theory), maximum raw mark
CAMBRIDGE INTERNATIONAL EXAMINATIONS International General Certificate of Secondary Education MARK SCHEME for the May/June 2014 series 0620 CHEMISTRY 0620/32 Paper 3 (Extended Theory), maximum raw mark
Science Mock Test Paper CBSE Class 10
 Science Mock Test Paper CBSE Class 10 Time: 3 hrs Code - 180101 Max. Marks: 90 General Instructions 1. The question paper comprises of two sections, A and B. You are to attempt both the sections. 2. All
Science Mock Test Paper CBSE Class 10 Time: 3 hrs Code - 180101 Max. Marks: 90 General Instructions 1. The question paper comprises of two sections, A and B. You are to attempt both the sections. 2. All
Chapter 2. The Chemistry of Life
 Chapter 2 The Chemistry of Life Introduction Cells, tissues and organs composed of chemicals Chemical reactions important for function Chemistry is the study of elements, compounds, chemical reactions,
Chapter 2 The Chemistry of Life Introduction Cells, tissues and organs composed of chemicals Chemical reactions important for function Chemistry is the study of elements, compounds, chemical reactions,
ANDHRA PRADESH SSC EXAMINATIONS - MARCH 2017 IGNITE PHYSICS TARGET MODEL PAPER - 02 GENERAL SCIENCE, Paper I
 ANDHRA PRADESH SSC EXAMINATIONS - MARCH 2017 IGNITE PHYSICS TARGET MODEL PAPER - 02 GENERAL SCIENCE, Paper I (Physical Sciences) (English Version) Time: 2 Hours 45 Min. Parts A and B Maximum Marks : 40
ANDHRA PRADESH SSC EXAMINATIONS - MARCH 2017 IGNITE PHYSICS TARGET MODEL PAPER - 02 GENERAL SCIENCE, Paper I (Physical Sciences) (English Version) Time: 2 Hours 45 Min. Parts A and B Maximum Marks : 40
GCSE OCR Revision Chemistry. GCSE OCR Revision Chemistry. GCSE OCR Revision Chemistry. Bonding. GCSE OCR Revision Chemistry
 Particle Model and Atomic Structure The following symbols describe two different substances. Deduce all the information you can from these symbols. 13 C 12 6 6 C 1 Particle Model and Atomic Structure The
Particle Model and Atomic Structure The following symbols describe two different substances. Deduce all the information you can from these symbols. 13 C 12 6 6 C 1 Particle Model and Atomic Structure The
Paper Reference. Sample Assessment Material Time: 2 hours
 Centre No. Candidate No. Paper Reference(s) 4CH0/1C Edexcel IGCSE Chemistry Chemistry Paper 1 Sample Assessment Material Time: 2 hours Materials required for examination Nil Items included with question
Centre No. Candidate No. Paper Reference(s) 4CH0/1C Edexcel IGCSE Chemistry Chemistry Paper 1 Sample Assessment Material Time: 2 hours Materials required for examination Nil Items included with question
CBSE 10th Science 2014 Unsolved Paper All India
 Perfect solution to all problems Tips, Tricks, General Knowledge, Current Affairs, Latest Sample, Previous Year, Practice Papers with solutions. CBSE 10th Science 2014 Unsolved Paper All India Buy solution:
Perfect solution to all problems Tips, Tricks, General Knowledge, Current Affairs, Latest Sample, Previous Year, Practice Papers with solutions. CBSE 10th Science 2014 Unsolved Paper All India Buy solution:
Additional Science Chemistry
 Additional Science Chemistry C2 Core Questions and Keywords and Definitions Question How did Mendeleev arrange the elements known at the time into a periodic table? How did Mendeleev use his table? Where
Additional Science Chemistry C2 Core Questions and Keywords and Definitions Question How did Mendeleev arrange the elements known at the time into a periodic table? How did Mendeleev use his table? Where
Describe how the inter-conversion of solids, liquids and gases are achieved and recall names used for these inter-conversions
 Understand the arrangements, movements and energy of the particle in each of the 3 states of matter : solid, liquid and gas Describe how the inter-conversion of solids, liquids and gases are achieved and
Understand the arrangements, movements and energy of the particle in each of the 3 states of matter : solid, liquid and gas Describe how the inter-conversion of solids, liquids and gases are achieved and
Class-X Science-086 SAMPLE QUESTION PAPER-19
 Class-X Science-086 SAMPLE QUESTION PAPER-19 TIME: 3 Hrs. M.M.:80 General Instructions: (i) The question paper comprises of five sections A, B, C, D and E. You are to attempt all the sections. (ii) All
Class-X Science-086 SAMPLE QUESTION PAPER-19 TIME: 3 Hrs. M.M.:80 General Instructions: (i) The question paper comprises of five sections A, B, C, D and E. You are to attempt all the sections. (ii) All
SECTION - I (Marks : 15)
 OCTOBER 2014 SCIENCE Time Allowed: 2 1/2 Hours Maximum Marks : 75 Note: This question paper contains three Sections. SECTION - I (Marks : 15) Note: (i) Answer all the 15 questions. (ii) Choose the correct
OCTOBER 2014 SCIENCE Time Allowed: 2 1/2 Hours Maximum Marks : 75 Note: This question paper contains three Sections. SECTION - I (Marks : 15) Note: (i) Answer all the 15 questions. (ii) Choose the correct
BUT FIRST LET S REVIEW IONS AND BONDING. What is the Lewis dot diagram for Magnesium? 2+ 2-
 ELECTROLYTES BUT FIRST LET S REVIEW IONS AND BONDING What is the Lewis dot diagram for Magnesium? Mg 2 2- S What is the Lewis dot diagram for Sulfur? How would these 2 elements complete the octet rule?
ELECTROLYTES BUT FIRST LET S REVIEW IONS AND BONDING What is the Lewis dot diagram for Magnesium? Mg 2 2- S What is the Lewis dot diagram for Sulfur? How would these 2 elements complete the octet rule?
2 Ionic and Covalent Bonding
 CHAPTER 6 2 Ionic and Covalent Bonding SECTION The Structure of Matter KEY IDEAS As you read this section, keep these questions in mind: Why do atoms form bonds? How do ionic bonds and covalent bonds differ?
CHAPTER 6 2 Ionic and Covalent Bonding SECTION The Structure of Matter KEY IDEAS As you read this section, keep these questions in mind: Why do atoms form bonds? How do ionic bonds and covalent bonds differ?
Chemical Bonding: Chemical Formulas OL
 Name: Chemical Bonding 5. Chemical Bonding: Chemical Formulas Ionic Bonding Covalent Bonding Electronegativity Shapes of Molecules and Intermolecular Forces Objectives -understand that compounds can be
Name: Chemical Bonding 5. Chemical Bonding: Chemical Formulas Ionic Bonding Covalent Bonding Electronegativity Shapes of Molecules and Intermolecular Forces Objectives -understand that compounds can be
2. What property of cooperating valence electrons is mostly responsible for creating domains in special elements? CHOOSE ONE ANSWER ONLY
 T/TE Pretest 3.2 v 2015 (note that even though nitrogen cycle is officially part of T, it involves material closely related to TE, so it will count for TE on the test. For magnetism, TE includes all material
T/TE Pretest 3.2 v 2015 (note that even though nitrogen cycle is officially part of T, it involves material closely related to TE, so it will count for TE on the test. For magnetism, TE includes all material
Chapter 1 Section 1- Pages 4-7: Electrons and Chemical Bonding COMBINING ATOMS THROUGH CHEMICAL BONDING
 Study Guide Chapter 1 and 2 Interactions of Matter Chapter 1 Section 1- Pages 4-7: Electrons and Chemical Bonding COMBINING ATOMS THROUGH CHEMICAL BONDING 1. Which of these substances is a combination
Study Guide Chapter 1 and 2 Interactions of Matter Chapter 1 Section 1- Pages 4-7: Electrons and Chemical Bonding COMBINING ATOMS THROUGH CHEMICAL BONDING 1. Which of these substances is a combination
Elements and Chemical Bonds. Chapter 11
 Elements and Chemical Bonds Chapter 11 Essential Question How does understanding periodic trends allow us to predict properties of different elements? Vocabulary Ionic bond Covalent bond Compounds, Chemical
Elements and Chemical Bonds Chapter 11 Essential Question How does understanding periodic trends allow us to predict properties of different elements? Vocabulary Ionic bond Covalent bond Compounds, Chemical
5 Energy from chemicals
 5 Energy from chemicals Content 5.1 Enthalpy 5.2 Hydrogen fuel cell Learning Outcomes Candidates should be able to: (a) (b) (c) (d) (e) describe the meaning of enthalpy change in terms of exothermic (H
5 Energy from chemicals Content 5.1 Enthalpy 5.2 Hydrogen fuel cell Learning Outcomes Candidates should be able to: (a) (b) (c) (d) (e) describe the meaning of enthalpy change in terms of exothermic (H
Cell Structure. Cell Structure. Investigating Cells. Investigating Cells. Cell Division. Cell Division. Transport In and Out of Cells
 Cell Structure What are the two main types of cell? Cell Structure The two main types of cell are prokaryotic and eukaryotic. 1 1 Investigating Cells How is magnification calculated? 2 Investigating Cells
Cell Structure What are the two main types of cell? Cell Structure The two main types of cell are prokaryotic and eukaryotic. 1 1 Investigating Cells How is magnification calculated? 2 Investigating Cells
MARKING SCHEME CLASS X DELHI. Q2. Each piece regenerates into a new Planaria. 1 1
 MARKING SCHEME CLASS X DELHI Code No. // Expected Answer/ Value point Marks Total SECTION A Q. C 4 H 0 ; C 6 H 4, Q. Each piece regenerates into a new Planaria. Q. Because the green plants prepare food
MARKING SCHEME CLASS X DELHI Code No. // Expected Answer/ Value point Marks Total SECTION A Q. C 4 H 0 ; C 6 H 4, Q. Each piece regenerates into a new Planaria. Q. Because the green plants prepare food
Atoms, molecules, bonding, periodic table
 Atoms, molecules, bonding, periodic table Atoms Modern Atom Model Nucleus-Protons and Neutrons Electrons around nucleus, never know the true location Protons Positively charged In nucleus Neutrons Neutral
Atoms, molecules, bonding, periodic table Atoms Modern Atom Model Nucleus-Protons and Neutrons Electrons around nucleus, never know the true location Protons Positively charged In nucleus Neutrons Neutral
INDIAN SCHOOL MUSCAT MIDDLE SECTION FINAL EXAMINATION SCIENCE
 Name Roll Number CLASS: VIII INDIAN SCHOOL MUSCAT MIDDLE SECTION FINAL EXAMINATION 07-8 SCIENCE Time Allotted: ½ Hrs..0.08 Max.Marks: 80 General Instructions:. The question paper comprises of two Sections,
Name Roll Number CLASS: VIII INDIAN SCHOOL MUSCAT MIDDLE SECTION FINAL EXAMINATION 07-8 SCIENCE Time Allotted: ½ Hrs..0.08 Max.Marks: 80 General Instructions:. The question paper comprises of two Sections,
MONICA COACHING CENTRE An Institute For Science Classes JALANDHAR, PUNJAB CARBON AND COMPOUNDS
 CARBON AND COMPOUNDS The atmosphere has only 0.03% of carbon dioxide. The number of electrons lost or gained by an atom to complete its octet (or duplet) is called its valency. It depends on the number
CARBON AND COMPOUNDS The atmosphere has only 0.03% of carbon dioxide. The number of electrons lost or gained by an atom to complete its octet (or duplet) is called its valency. It depends on the number
CBSE Class X Science Board Paper 2011 (Set 1) Term II
 CBSE Class X Science Board Paper 2011 (Set 1) Term II Total time: 3 hrs Total marks: 80 General instructions: 1. The question paper comprises of two Sections, A and B. You are to attempt both the sections.
CBSE Class X Science Board Paper 2011 (Set 1) Term II Total time: 3 hrs Total marks: 80 General instructions: 1. The question paper comprises of two Sections, A and B. You are to attempt both the sections.
4. Which of the following compounds is polar? A. CCl 4 B. BF 3 C. H 2 CCH 2 D. CO 2 E. NH 3 *
 Exam2 Name Part A Follow the directions and select the BEST answer for each section. Mark your answers on the scantron answer sheet carefully. Make sure your scantron answer sheet is filled out properly-
Exam2 Name Part A Follow the directions and select the BEST answer for each section. Mark your answers on the scantron answer sheet carefully. Make sure your scantron answer sheet is filled out properly-
Edexcel Chemistry Checklist
 Topic 1. Key concepts in chemistry Video: Developing the atomic model Describe how and why the atomic model has changed over time. Describe the difference between the plum-pudding model of the atom and
Topic 1. Key concepts in chemistry Video: Developing the atomic model Describe how and why the atomic model has changed over time. Describe the difference between the plum-pudding model of the atom and
There are two main requirements for atoms to form a covalent bond and make a molecule:
 HOW ATOMS BOND TO EACH OTHER Covalent bonding Remember that a hydrogen atom has 1 proton and 1 electron and that the electron and the proton are attracted to each other. But if the atoms get close enough
HOW ATOMS BOND TO EACH OTHER Covalent bonding Remember that a hydrogen atom has 1 proton and 1 electron and that the electron and the proton are attracted to each other. But if the atoms get close enough
Important Instructions for the School Principal. (Not to be printed with the question paper)
 Important Instructions for the School Principal (Not to be printed with the question paper) ) This question paper is strictly meant for use in school based SA-I, September-202 only. This question paper
Important Instructions for the School Principal (Not to be printed with the question paper) ) This question paper is strictly meant for use in school based SA-I, September-202 only. This question paper
Physical Science 1 Chapter 12 THE MODERN ATOM
 THE MODERN ATOM The modern model of the atom describes the electron cloud consisting of separate energy levels, each containing a fixed number of electrons. The energy levels increase in energy based on
THE MODERN ATOM The modern model of the atom describes the electron cloud consisting of separate energy levels, each containing a fixed number of electrons. The energy levels increase in energy based on
Biology. Chapter 2 Notes
 Biology Chapter 2 Notes Section 1: Nature of Matter Objectives: 1) Differentiate between atoms and elements 2) Analyze how compounds are formed 3) Distinguish between covalent bonds, hydrogen bonds and
Biology Chapter 2 Notes Section 1: Nature of Matter Objectives: 1) Differentiate between atoms and elements 2) Analyze how compounds are formed 3) Distinguish between covalent bonds, hydrogen bonds and
How do Elements Combine to Form Compounds?
 How do Elements Combine to Form Compounds? ACTIVITY What is it made of? Think about the calcium atom vs the calcium ion Compounds account for the huge variety of matter on Earth All the compounds that
How do Elements Combine to Form Compounds? ACTIVITY What is it made of? Think about the calcium atom vs the calcium ion Compounds account for the huge variety of matter on Earth All the compounds that
Lab Activity:To show that light is necessary for photosynthesis. Lab Activity:To prepare temporary mount of stomata.
 The Orchid School Baner Syllabus Overview 2015-2016 Std X Subject : Science Month Lesson / Content / Name of the Book Expected Learning Objective Activities/FAs Planned Chemical Reactions and Equations:
The Orchid School Baner Syllabus Overview 2015-2016 Std X Subject : Science Month Lesson / Content / Name of the Book Expected Learning Objective Activities/FAs Planned Chemical Reactions and Equations:
CAREER POINT. PRE FOUNDATON DIVISION FACULTY SELECTION TEST CHEMISTRY [Time : 2 Hr.] [Max. Marks : 60]
![CAREER POINT. PRE FOUNDATON DIVISION FACULTY SELECTION TEST CHEMISTRY [Time : 2 Hr.] [Max. Marks : 60] CAREER POINT. PRE FOUNDATON DIVISION FACULTY SELECTION TEST CHEMISTRY [Time : 2 Hr.] [Max. Marks : 60]](/thumbs/88/117231520.jpg) CAREER POINT PRE FOUNDATON DIVISION FACULTY SELECTION TEST CHEMISTRY [Time : 2 Hr.] [Max. Marks : 60] INSTRUCTIONS : 1. Attempt all questions. 2. Indicate your answer on the question paper itself. 3. Each
CAREER POINT PRE FOUNDATON DIVISION FACULTY SELECTION TEST CHEMISTRY [Time : 2 Hr.] [Max. Marks : 60] INSTRUCTIONS : 1. Attempt all questions. 2. Indicate your answer on the question paper itself. 3. Each
4.1.1 A simple model of the atom, symbols, relative atomic mass, electronic charge and isotopes. Unit 1 Unit 2 Unit 3. C2.1.1a Structure and bonding
 Summary of changes This resource outlines the main changes that have been made to the assessment and subject content from our previous GCSE Chemistry (4402) to the new specification (8462). Our new specifications
Summary of changes This resource outlines the main changes that have been made to the assessment and subject content from our previous GCSE Chemistry (4402) to the new specification (8462). Our new specifications
Matter mass space atoms solid, a liquid, a gas, or plasm elements compounds mixtures atoms Compounds chemically combined Mixtures not chemically
 SOL PS.2 THE NATURE OF MATTER Matter is anything that has mass and occupies space. All matter is made up of small particles called atoms. Matter can exist as a solid, a liquid, a gas, or plasma. Matter
SOL PS.2 THE NATURE OF MATTER Matter is anything that has mass and occupies space. All matter is made up of small particles called atoms. Matter can exist as a solid, a liquid, a gas, or plasma. Matter
How do Elements Combine to Form Compounds?
 How do Elements Combine to Form Compounds? ACTIVITY What is it made of? Compounds account for the huge variety of matter on Earth All the compounds that exist on Earth are built from elements 118 elements
How do Elements Combine to Form Compounds? ACTIVITY What is it made of? Compounds account for the huge variety of matter on Earth All the compounds that exist on Earth are built from elements 118 elements
