What s Your Reaction? Types of Reactions
|
|
|
- Edgar Bryan
- 6 years ago
- Views:
Transcription
1 LESSON 6 What s Your Reaction? Types of Reactions Name Date Period ACTIVITY Purpose To find patterns in the types of chemical equations and to classify a reaction by type. Materials Toxic Reactions cards Part 1: Sorting Chemical Equations Use the Toxic Reactions cards from Lesson 1: Toxic Reactions. Sort the cards according to the directions for Questions 1 through 4, then answer the questions. 1. Find all the cards that have only one product formed in the reaction. List the letters on the cards. a. Describe how the atoms rearrange in these reactions. b. Why do you think these reactions are called combination reactions? 2. Find all the cards that have a reactant that is an elemental metal. List the letters on the cards. a. Describe how the atoms rearrange in these reactions. b. Why do you think these reactions are called single exchange reactions? 3. Consider all the cards that have a reactant that is an ionic compound. List the letters on the cards. a. Describe how the atoms rearrange in these reactions. b. Why do you think these reactions are called double exchange reactions? 20 Unit 4 Toxins Living By Chemistry Teaching and Classroom Masters: Units 4 6 Lesson 6 Worksheet 2010 Key Curriculum Press
2 4. Examine all the remaining cards. a. Verify that these reactions involve reactants that are molecules. List the letters on the cards. b. Describe how the atoms rearrange in these reactions. c. Classify these reactions to the best of your ability. 5. 2H 2 O 2 (l) 2H 2 O(l) O 2 (g) Why do you think this reaction is called a decomposition reaction? Part 2: Classifying Reactions 1. Classify each reaction as combination, decomposition, single exchange, or double exchange. a. Fill in any missing reactants or products. b. Balance the equation if necessary. Reaction Type N 2 (g) H 2 (g) 2NH 3 (g) C 2 H 4 (g) H 2 (g) CaCO 3 (s) CaO(s) CO 2 (g) Cl 2 (g) CaI 2 (s) I 2 (s) NaOH(aq) HCl(aq) H 2 O(l) 2KClO 3 (s) Mg(s) 2HCl(aq) 2KCl(s) 3O 2 (g) H 2 (g) AgNO 3 (aq) KCl(aq) AgCl(s) 2. List six molecules in the reactions in the table. 3. List six ionic compounds in the reactions in the table. 4. Making Sense You can remove the toxin carbon monoxide, CO(g), from the air through a reaction with oxygen, O 2, to produce carbon dioxide, CO 2 (g). Write a balanced chemical equation for this combination reaction. Living By Chemistry Teaching and Classroom Masters: Units 4 6 Unit 4 Toxins Key Curriculum Press Lesson 6 Worksheet
3 TOXIC REACTIONS CARDS A Phosgene, COCl 2 B Formaldehyde, CH 2 O Biological weapon in World War I In the production of plywood and carpeting Damages eyes, nose, throat, and lungs COCl 2 (g) H 2 O(l) 2HCl(aq) CO 2 (g) Phosgene gas reacts with water from tears, saliva, or mucus to produce aqueous hydrochloric acid and carbon dioxide gas. 2CH 2 O(aq) O 2 (g) 2CH 2 O 2 (aq) Aqueous formaldehyde reacts with oxygen gas to produce aqueous formic acid in the blood. C Thallium oxide, Tl 2 O D Ammonia, NH 3 In the creation of clay pottery and ceramics Often found in household cleaning supplies Damages eyes, nose, throat, lungs Tl 2 O(s) 2HCl(aq) 2TlCl(aq) H 2 O(l) Solid thallium (I) oxide reacts with aqueous hydrochloric acid (stomach acid) to form aqueous thallium (I) chloride and water. NH 3 (g) H 2 O(l) NH 4 OH(aq) Ammonia gas reacts with water (tears, saliva, mucus) to produce aqueous ammonium hydroxide. 4 Unit 4 Toxins Living By Chemistry Teaching and Classroom Masters: Units 4 6 Lesson 1 Card Masters 2010 Key Curriculum Press
4 E Nitric oxide, NO F Ethanol, C 2 H 6 O Produced by automobile engines and lightning As automobile fuel; found in alcoholic beverages Damages eyes, nose, throat, lungs 4NO(g) O 2 (g) 2H 2 O(l) 4HNO 2 (aq) C 2 H 6 O(aq) O 2 (g) C 2 H 4 O 2 (aq) H 2 O(l) Nitric oxide gas reacts with water (tears, saliva, mucus) and oxygen gas to produce aqueous nitrous acid. Aqueous ethanol reacts with oxygen gas to produce aqueous acetic acid and water in the blood. G Chlorine, Cl 2 H Mercury sulfide, HgS In water purification, disinfectants, and bleach Damages eyes, nose, throat, and lungs Cl 2 (g) H 2 O(l) HOCl(aq) HCl(aq) As a red paint pigment HgS(s) 2HCl(aq) HgCl 2 (s) H 2 S(aq) Chlorine gas reacts with water (tears, saliva, mucus) to produce aqueous hypochlorous acid and aqueous hydrochloric acid. Solid mercury (II) sulfide reacts with aqueous hydrochloric acid (stomach acid) to produce solid mercury (II) chloride and aqueous hydrogen sulfide. Living By Chemistry Teaching and Classroom Masters: Units 4 6 Unit 4 Toxins Key Curriculum Press Lesson 1 Card Masters
5 I Ethylene glycol, C 2 H 6 O 2 J Lead carbonate, PbCO 3 As antifreeze in automobiles In house paint until 1978 C 2 H 6 O 2 (aq) O 2 (g) C 2 H 4 O 3 (aq) H 2 O(l) PbCO 3 (s) 2HCl(aq) PbCl 2 (aq) H 2 CO 3 (aq) Aqueous ethylene glycol reacts with oxygen gas to produce aqueous glycolic acid and water in the blood. Solid lead (II) carbonate reacts with aqueous hydrochloric acid (stomach acid) to produce aqueous lead (II) chloride and carbonic acid. K Sodium oxalate, Na 2 C 2 O 4 L Lead, Pb In certain foods: chocolate, peanuts, spinach, beets, rhubarb, berries Formerly in household paint, toys, plumbing, and car bodies Kidney stones Na 2 C 2 O 4 (aq) CaCl 2 (aq) CaC 2 O 4 (s) 2NaCl(aq) Aqueous sodium oxalate reacts with aqueous calcium chloride to produce solid calcium oxalate and aqueous sodium chloride. Pb(s) 2HCl(aq) PbCl 2 (aq) H 2 (g) Solid lead reacts with aqueous hydrochloric acid (stomach acid) to produce aqueous lead (II) chloride and hydrogen gas. 6 Unit 4 Toxins Living By Chemistry Teaching and Classroom Masters: Units 4 6 Lesson 1 Card Masters 2010 Key Curriculum Press
6 M Arsenic, As N Oxalic acid, C 2 H 2 O 4 In agricultural insecticides; found in contaminated groundwater Natural ingredient of many plants and foods, including black pepper, parsley, and rhubarb Kidney stones 2As(s) 6HCl(aq) 2AsCl 3 (aq) 3H 2 (g) C 2 H 2 O 4 (aq) CaCl 2 (aq) CaC 2 O 4 (s) 2HCl(aq) Solid arsenic reacts with aqueous hydrochloric acid (stomach acid) to produce aqueous arsenic trichloride and hydrogen gas. Aqueous oxalic acid reacts with aqueous calcium chloride to produce solid calcium oxalate and aqueous hydrochloric acid. O Methanol, CH 4 O P Sodium phosphate, Na 3 PO 4 As a fuel in dragsters, sprint cars, and model airplanes CH 4 O(aq) O 2 (g) CH 2 O 2 (aq) H 2 O(l) Aqueous methanol reacts with oxygen to produce aqueous formic acid and water in the blood. As a cleaning agent, degreaser, and laxative Kidney stones 2Na 3 PO 4 (aq) 3CaCl 2 (aq) Ca 3 PO 4 2 (s) 6NaCl(aq) Aqueous sodium phosphate reacts with aqueous calcium chloride to produce solid calcium phosphate and aqueous sodium chloride. Living By Chemistry Teaching and Classroom Masters: Units 4 6 Unit 4 Toxins Key Curriculum Press Lesson 1 Card Masters
Toxic Reactions Chemical Equations
 LESSON 1 Toxic Reactions Chemical Equations Name Date Period Activity Purpose To interpret chemical equations involving toxins. Materials J Toxic Reactions cards Part 1: Interpreting Chemical Equations
LESSON 1 Toxic Reactions Chemical Equations Name Date Period Activity Purpose To interpret chemical equations involving toxins. Materials J Toxic Reactions cards Part 1: Interpreting Chemical Equations
2/24/2010. Mr. Puccetti Spring What toxins have you encountered in your life? 2. How can toxins enter the body? 3. How can toxins harm you?
 Mr. Puccetti Spring 2010 how toxins are defined how chemists determine toxicity the mechanisms by which toxic substances act in our bodies and what this has to do with chemical reactions 1. What toxins
Mr. Puccetti Spring 2010 how toxins are defined how chemists determine toxicity the mechanisms by which toxic substances act in our bodies and what this has to do with chemical reactions 1. What toxins
Chapter 8 Chemical Reactions
 Chemistry/ PEP Name: Date: Chapter 8 Chemical Reactions Chapter 8: 1 7, 9 18, 20, 21, 24 26, 29 31, 46, 55, 69 Practice Problems 1. Write a skeleton equation for each chemical reaction. Include the appropriate
Chemistry/ PEP Name: Date: Chapter 8 Chemical Reactions Chapter 8: 1 7, 9 18, 20, 21, 24 26, 29 31, 46, 55, 69 Practice Problems 1. Write a skeleton equation for each chemical reaction. Include the appropriate
Chapter 6. Chemical Reactions. Sodium reacts violently with bromine to form sodium bromide.
 Chapter 6 Chemical Reactions Sodium reacts violently with bromine to form sodium bromide. Evidence of Chemical Reactions Chemical Equations Reactants Products Reactant(s): Substance(s) present before the
Chapter 6 Chemical Reactions Sodium reacts violently with bromine to form sodium bromide. Evidence of Chemical Reactions Chemical Equations Reactants Products Reactant(s): Substance(s) present before the
Which of the following answers is correct and has the correct number of significant figures?
 Avogadro s Number, N A = 6.022 10 23 1. [7 points] Carry out the following mathematical operation: 6.06 10 3 + 1.1 10 2 Which of the following answers is correct and has the correct number of significant
Avogadro s Number, N A = 6.022 10 23 1. [7 points] Carry out the following mathematical operation: 6.06 10 3 + 1.1 10 2 Which of the following answers is correct and has the correct number of significant
Chemical reactions describe processes involving chemical change
 1.1 Chemical Reactions 1.2 Chemical Equations Chemical reactions describe processes involving chemical change The chemical change involves rearranging matter Converting one or more pure substances into
1.1 Chemical Reactions 1.2 Chemical Equations Chemical reactions describe processes involving chemical change The chemical change involves rearranging matter Converting one or more pure substances into
NET IONIC REACTIONS in AQUEOUS SOLUTIONS AB + CD AD + CB
 NET IONIC REACTIONS in AQUEOUS SOLUTIONS Double replacements are among the most common of the simple chemical reactions. Consider the hypothetical reaction: AB + CD AD + CB where AB exists as A + and B
NET IONIC REACTIONS in AQUEOUS SOLUTIONS Double replacements are among the most common of the simple chemical reactions. Consider the hypothetical reaction: AB + CD AD + CB where AB exists as A + and B
Chapter 3 & 4: Reactions Part 1
 Chapter 3 & 4: Reactions Part 1 Read: BLB 3.1 3.2; 4.2 4.4 HW: BLB 3:1, 11a, b, e, 13 4:19, 24, 39, 49a, c, e, f, 51b, d Supplemental: Rxns:1, 2, 6 11 Know: Chapter 3 Reactions Combustion Decomposition
Chapter 3 & 4: Reactions Part 1 Read: BLB 3.1 3.2; 4.2 4.4 HW: BLB 3:1, 11a, b, e, 13 4:19, 24, 39, 49a, c, e, f, 51b, d Supplemental: Rxns:1, 2, 6 11 Know: Chapter 3 Reactions Combustion Decomposition
Chem 110 Acids, Bases, ph, and Redox
 Chem 110 Acids, Bases, ph, and Redox 1. If 10.0 ml of 0.100 M HCl is titrated with 0.200 M NaOH, what volume of sodium hydroxide solution is required to neutralize the acid? HCl(aq) + NaOH(aq) NaCl(aq)
Chem 110 Acids, Bases, ph, and Redox 1. If 10.0 ml of 0.100 M HCl is titrated with 0.200 M NaOH, what volume of sodium hydroxide solution is required to neutralize the acid? HCl(aq) + NaOH(aq) NaCl(aq)
Chemical Reactions CHAPTER Reactions and Equations
 CHAPTER 9 Chemical Reactions 9.1 Reactions and Equations The process by which atoms of one or more substances are rearranged to form different substances is called a chemical reaction. There are a number
CHAPTER 9 Chemical Reactions 9.1 Reactions and Equations The process by which atoms of one or more substances are rearranged to form different substances is called a chemical reaction. There are a number
Basic Principles of Chemistry Lecture Notes #11 Types of Chemical Reactions
 Basic Principles of Chemistry Lecture Notes #11 Types of Chemical Reactions Types of Chemical Reactions Part 1 Precipitation Reactions In a precipitation reaction, a solid substance is formed in a solution
Basic Principles of Chemistry Lecture Notes #11 Types of Chemical Reactions Types of Chemical Reactions Part 1 Precipitation Reactions In a precipitation reaction, a solid substance is formed in a solution
Chapter 7 Chemical Reactions
 Chapter 7 Chemical Reactions Evidence of Chemical Change Release or Absorption of Heat Color Change Emission of Light Formation of a Gas Formation of Solid Precipitate Tro's "Introductory 2 How Do We Represent
Chapter 7 Chemical Reactions Evidence of Chemical Change Release or Absorption of Heat Color Change Emission of Light Formation of a Gas Formation of Solid Precipitate Tro's "Introductory 2 How Do We Represent
Page 1. Exam 2 Review Summer A 2002 MULTIPLE CHOICE. 1. Consider the following reaction: CaCO (s) + HCl(aq) CaCl (aq) + CO (g) + H O(l)
 Page 1 MULTIPLE CHOICE 1. Consider the following reaction: CaCO (s) + HCl(aq) CaCl (aq) + CO (g) + H O(l) The coefficient of HCl(aq) in the balanced reaction is. a) 1 b) 2 c) 3 d) 4 e) 0 2. Given the information
Page 1 MULTIPLE CHOICE 1. Consider the following reaction: CaCO (s) + HCl(aq) CaCl (aq) + CO (g) + H O(l) The coefficient of HCl(aq) in the balanced reaction is. a) 1 b) 2 c) 3 d) 4 e) 0 2. Given the information
Unit 7: Stoichiometry Homework Packet (85 points)
 Name: Period: By the end of the Unit 7, you should be able to: Chapter 12 1. Use stoichiometry to determine the amount of substance in a reaction 2. Determine the limiting reactant of a reaction 3. Determine
Name: Period: By the end of the Unit 7, you should be able to: Chapter 12 1. Use stoichiometry to determine the amount of substance in a reaction 2. Determine the limiting reactant of a reaction 3. Determine
Chem 110 General Principles of Chemistry
 Chem 110 General Principles of Chemistry Chapter 3 (Page 88) Aqueous Reactions and Solution Stoichiometry In this chapter you will study chemical reactions that take place between substances that are dissolved
Chem 110 General Principles of Chemistry Chapter 3 (Page 88) Aqueous Reactions and Solution Stoichiometry In this chapter you will study chemical reactions that take place between substances that are dissolved
CHE 105 FA17 Exam 2. How many moles of beryllium are in 15.0 grams of Be?
 CHE 105 FA17 Exam 2 Your Name: Your ID: Question #: 1 How many moles of beryllium are in 150 grams of Be? A 66 B 13515 C 901 D 0601 Question #: 2 Vanillin, C8H8O3, is the molecule responsible for the vanilla
CHE 105 FA17 Exam 2 Your Name: Your ID: Question #: 1 How many moles of beryllium are in 150 grams of Be? A 66 B 13515 C 901 D 0601 Question #: 2 Vanillin, C8H8O3, is the molecule responsible for the vanilla
Slide 1 / 90. Stoichiometry HW. Grade:«grade» Subject: Date:«date»
 Slide 1 / 90 Stoichiometry HW Grade:«grade» Subject: Date:«date» Slide 2 / 90 1 The calculation of quantities in chemical equations is called. A B C D E accuracy and precision dimensional analysis percent
Slide 1 / 90 Stoichiometry HW Grade:«grade» Subject: Date:«date» Slide 2 / 90 1 The calculation of quantities in chemical equations is called. A B C D E accuracy and precision dimensional analysis percent
A reaction in which a solid forms is called a precipitation reaction. Solid = precipitate
 Chapter 7 Reactions in Aqueous Solutions 1 Section 7.1 Predicting Whether a Reaction Will Occur Four Driving Forces Favor Chemical Change 1. Formation of a solid 2. Formation of water 3. Transfer of electrons
Chapter 7 Reactions in Aqueous Solutions 1 Section 7.1 Predicting Whether a Reaction Will Occur Four Driving Forces Favor Chemical Change 1. Formation of a solid 2. Formation of water 3. Transfer of electrons
The photograph in the textbook provides evidence that an exothermic chemical reaction is occurring.
 Preview Lesson Starter Objectives Indications of a Chemical Reaction Characteristics of Chemical Equations Significance of a Chemical Equation Balancing Chemical Equations Section 1 Describing Chemical
Preview Lesson Starter Objectives Indications of a Chemical Reaction Characteristics of Chemical Equations Significance of a Chemical Equation Balancing Chemical Equations Section 1 Describing Chemical
Indicators of chemical reactions
 Indicators of chemical reactions Emission of light or heat Formation of a gas Formation of a precipitate Color change Emission of odor All chemical reactions: have two parts Reactants - the substances
Indicators of chemical reactions Emission of light or heat Formation of a gas Formation of a precipitate Color change Emission of odor All chemical reactions: have two parts Reactants - the substances
Chapter 7: Stoichiometry in Chemical Reactions
 Chapter 7: Stoichiometry in Chemical Reactions Mini Investigation: Precipitating Ratios, page 315 A. ZnCl 2 (aq) + Na 2 CO 3 (aq) ZnCO 3 (s) + 2 NaCl(aq) 3 AgNO 3 (aq) + Na 3 PO 4 (aq) Ag 3 PO 4 (s) +
Chapter 7: Stoichiometry in Chemical Reactions Mini Investigation: Precipitating Ratios, page 315 A. ZnCl 2 (aq) + Na 2 CO 3 (aq) ZnCO 3 (s) + 2 NaCl(aq) 3 AgNO 3 (aq) + Na 3 PO 4 (aq) Ag 3 PO 4 (s) +
Electrolytes do conduct electricity, in proportion to the concentrations of their ions in solution.
 Chapter 4 (Hill/Petrucci/McCreary/Perry Chemical Reactions in Aqueous Solutions This chapter deals with reactions that occur in aqueous solution these solutions all use water as the solvent. We will look
Chapter 4 (Hill/Petrucci/McCreary/Perry Chemical Reactions in Aqueous Solutions This chapter deals with reactions that occur in aqueous solution these solutions all use water as the solvent. We will look
Types of Chemical Reactions
 Types of Chemical Reactions 1) Combination (Synthesis) Reaction 2) Decomposition 3) Single Replacement 4) Double Replacement 5) Combustion 6) Oxidation-Reduction (Redox) Combination (Synthesis) Reactions
Types of Chemical Reactions 1) Combination (Synthesis) Reaction 2) Decomposition 3) Single Replacement 4) Double Replacement 5) Combustion 6) Oxidation-Reduction (Redox) Combination (Synthesis) Reactions
Practice Problems: Set #3-Solutions
 Practice Problems: Set #3-Solutions IIa) Balance the following equations:(10) 1) Zn (s) + H 3 PO 4 (aq) Zn 3 (PO 4 ) 2 (s) + H 2 (g) 3Zn (s) + 2H 3 PO 4 (aq) Zn 3 (PO 4 ) 2 (s) + 3H 2 (g) 2. Mg 3 N 2 (s)
Practice Problems: Set #3-Solutions IIa) Balance the following equations:(10) 1) Zn (s) + H 3 PO 4 (aq) Zn 3 (PO 4 ) 2 (s) + H 2 (g) 3Zn (s) + 2H 3 PO 4 (aq) Zn 3 (PO 4 ) 2 (s) + 3H 2 (g) 2. Mg 3 N 2 (s)
Chapter 4 Electrolytes and Aqueous Reactions. Dr. Sapna Gupta
 Chapter 4 Electrolytes and Aqueous Reactions Dr. Sapna Gupta Aqueous Solutions Solution - a homogeneous mixture of solute + solvent Solute: the component that is dissolved Solvent: the component that does
Chapter 4 Electrolytes and Aqueous Reactions Dr. Sapna Gupta Aqueous Solutions Solution - a homogeneous mixture of solute + solvent Solute: the component that is dissolved Solvent: the component that does
Ch 9 Stoichiometry Practice Test
 Ch 9 Stoichiometry Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A balanced chemical equation allows one to determine the a. mole ratio
Ch 9 Stoichiometry Practice Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. A balanced chemical equation allows one to determine the a. mole ratio
AIIMS,CBSE,AIPMT, & PMT,
 1 Which of the following solutions has the highest boiling point? (a) 5.85% solution of NaCl (b) 18.0% solution of glucose (c) 6.0% solution of urea (d) all have same boiling point 2 Two solutions of NaCl
1 Which of the following solutions has the highest boiling point? (a) 5.85% solution of NaCl (b) 18.0% solution of glucose (c) 6.0% solution of urea (d) all have same boiling point 2 Two solutions of NaCl
Chemical Reactions and Equations
 Chemical Reactions and Equations 5-1 5.1 What is a Chemical Reaction? A chemical reaction is a chemical change. A chemical reaction occurs when one or more substances is converted into one or more new
Chemical Reactions and Equations 5-1 5.1 What is a Chemical Reaction? A chemical reaction is a chemical change. A chemical reaction occurs when one or more substances is converted into one or more new
CHEMICAL REACTIONS. There are three ways we write chemical equations. 1. Molecular Equations 2. Full Ionic Equations 3. Net Ionic Equations
 CHEMICAL REACTIONS Reactants: Zn + I 2 Product: Zn I 2 Unit 2 Chemical Reactions The unit 2 exam will cover material from multiple chapters. You are responsible for the following from your text on exam
CHEMICAL REACTIONS Reactants: Zn + I 2 Product: Zn I 2 Unit 2 Chemical Reactions The unit 2 exam will cover material from multiple chapters. You are responsible for the following from your text on exam
Chapter 9. Vocabulary Ch Kick Off Activity. Objectives. Interpreting Formulas. Interpreting Formulas
 Chapter 9 Chemical Vocabulary Ch. 9.1 Chemical reaction Reactant Product Word Equation Skeleton Equation Chemical equation Coefficient 1 2 Objectives Write chemical equations to describe chemical reactions
Chapter 9 Chemical Vocabulary Ch. 9.1 Chemical reaction Reactant Product Word Equation Skeleton Equation Chemical equation Coefficient 1 2 Objectives Write chemical equations to describe chemical reactions
Ch 7 Chemical Reactions Study Guide Accelerated Chemistry SCANTRON
 Ch 7 Chemical Reactions Study Guide Accelerated Chemistry SCANTRON Name /80 TRUE/FALSE. Write 'T' if the statement is true and 'F' if the statement is false. Correct the False statments by changing the
Ch 7 Chemical Reactions Study Guide Accelerated Chemistry SCANTRON Name /80 TRUE/FALSE. Write 'T' if the statement is true and 'F' if the statement is false. Correct the False statments by changing the
1. Hydrochloric acid is mixed with aqueous sodium bicarbonate Molecular Equation
 NAME Hr Chapter 4 Aqueous Reactions and Solution Chemistry Practice A (Part 1 = Obj. 1-3) (Part 2 = Obj. 4-6) Objective 1: Electrolytes, Acids, and Bases a. Indicate whether each of the following is strong,
NAME Hr Chapter 4 Aqueous Reactions and Solution Chemistry Practice A (Part 1 = Obj. 1-3) (Part 2 = Obj. 4-6) Objective 1: Electrolytes, Acids, and Bases a. Indicate whether each of the following is strong,
CHAPTER 11 Stoichiometry Defining Stoichiometry
 CHAPTER 11 Stoichiometry 11.1 Defining Stoichiometry Stoichiometry is the study of quantitative relationships between amounts of reactants used and products formed by a chemical reaction. Stoichiometry
CHAPTER 11 Stoichiometry 11.1 Defining Stoichiometry Stoichiometry is the study of quantitative relationships between amounts of reactants used and products formed by a chemical reaction. Stoichiometry
Types of Reactions. There are five types of chemical reactions we observed in the lab:
 Chemical Reactions Acids and Bases Acids: Form hydrogen ions (H + ) when dissolved in water. HCl (aq) H + (aq) + Cl - (aq) Examples: HCl (hydrochloric acid), HNO 3 (nitric acid), H 2 SO 4 (sulfuric acid),
Chemical Reactions Acids and Bases Acids: Form hydrogen ions (H + ) when dissolved in water. HCl (aq) H + (aq) + Cl - (aq) Examples: HCl (hydrochloric acid), HNO 3 (nitric acid), H 2 SO 4 (sulfuric acid),
Chemical Reactions Unit
 Name: Hour: Teacher: ROZEMA / Chemistry Chemical Reactions Unit 1 P a g e 2 P a g e 3 P a g e 4 P a g e 5 P a g e 6 P a g e Chemistry Balancing Equations Balance the following equations by inserting the
Name: Hour: Teacher: ROZEMA / Chemistry Chemical Reactions Unit 1 P a g e 2 P a g e 3 P a g e 4 P a g e 5 P a g e 6 P a g e Chemistry Balancing Equations Balance the following equations by inserting the
General Chemistry. Contents. Chapter 5: Introduction to Reactions in Aqueous Solutions. Electrolytes. 5.1 The Nature of Aqueous Solutions
 General Chemistry Principles and Modern Applications Petrucci Harwood Herring 8 th Edition Chapter 5: Introduction to Reactions in Aqueous Solutions Philip Dutton University of Windsor, Canada N9B 3P4
General Chemistry Principles and Modern Applications Petrucci Harwood Herring 8 th Edition Chapter 5: Introduction to Reactions in Aqueous Solutions Philip Dutton University of Windsor, Canada N9B 3P4
2. Relative molecular mass, M r - The relative molecular mass of a molecule is the average mass of the one molecule when compared with
 Chapter 3: Chemical Formulae and Equations 1. Relative atomic mass, A r - The relative atomic mass of an element is the average mass of one atom of an element when compared with mass of an atom of carbon-12
Chapter 3: Chemical Formulae and Equations 1. Relative atomic mass, A r - The relative atomic mass of an element is the average mass of one atom of an element when compared with mass of an atom of carbon-12
General Chemistry. Chapter 5: Introduction to Reactions in Aqueous Solutions. Principles and Modern Applications Petrucci Harwood Herring 8 th Edition
 General Chemistry Principles and Modern Applications Petrucci Harwood Herring 8 th Edition Chapter 5: Introduction to Reactions in Aqueous Solutions Philip Dutton University of Windsor, Canada N9B 3P4
General Chemistry Principles and Modern Applications Petrucci Harwood Herring 8 th Edition Chapter 5: Introduction to Reactions in Aqueous Solutions Philip Dutton University of Windsor, Canada N9B 3P4
4.2. Double Displacement Reactions. Characteristics of Double Displacement Reactions SECTION. Key Terms
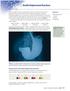 SECTION 4.2 Double Displacement Reactions X rays are highly useful for creating images of bones in the human body, but they generally do not show soft tissues clearly. To help doctors diagnose conditions
SECTION 4.2 Double Displacement Reactions X rays are highly useful for creating images of bones in the human body, but they generally do not show soft tissues clearly. To help doctors diagnose conditions
Reaction Classes. Precipitation Reactions
 Reaction Classes Precipitation: synthesis of an ionic solid a solid precipitate forms when aqueous solutions of certain ions are mixed AcidBase: proton transfer reactions acid donates a proton to a base,
Reaction Classes Precipitation: synthesis of an ionic solid a solid precipitate forms when aqueous solutions of certain ions are mixed AcidBase: proton transfer reactions acid donates a proton to a base,
The names of the reactants are: The names of the products are: The name of the product is:
 CLASSIFICATION OF EQUATIONS WORKSHEET Chemical reactions produce new substances with new properties. The starting materials in a chemical reaction are called reactants while the materials that are formed
CLASSIFICATION OF EQUATIONS WORKSHEET Chemical reactions produce new substances with new properties. The starting materials in a chemical reaction are called reactants while the materials that are formed
CH 221 Chapter Four Part II Concept Guide
 CH 221 Chapter Four Part II Concept Guide 1. Solubility Why are some compounds soluble and others insoluble? In solid potassium permanganate, KMnO 4, the potassium ions, which have a charge of +1, are
CH 221 Chapter Four Part II Concept Guide 1. Solubility Why are some compounds soluble and others insoluble? In solid potassium permanganate, KMnO 4, the potassium ions, which have a charge of +1, are
EXAM 3 CHEM 1310 WS09 Key Version #2
 EXAM 3 CHEM 1310 WS09 Key Version #2 1. (p. 116) Select the correct name and chemical formula for the precipitate that forms when the following reactants are mixed. CuCl 2 (aq) + Na 2 CO 3 (aq) A. copper(ii)
EXAM 3 CHEM 1310 WS09 Key Version #2 1. (p. 116) Select the correct name and chemical formula for the precipitate that forms when the following reactants are mixed. CuCl 2 (aq) + Na 2 CO 3 (aq) A. copper(ii)
2) Solve for protons neutrons and electrons for the bromide ION.
 1) Write the formulas for the following a) Calcium nitride c)lithium hydroxide b) Iron (III) sulfide d) sulfuric acid 2) Solve for protons neutrons and electrons for the bromide ION. 3) Write the electron
1) Write the formulas for the following a) Calcium nitride c)lithium hydroxide b) Iron (III) sulfide d) sulfuric acid 2) Solve for protons neutrons and electrons for the bromide ION. 3) Write the electron
Chapter 3 Stoichiometry: Calculations with Chemical Formulas and Equations
 Chapter 3 Stoichiometry: Calculations with Chemical Formulas and Equations 3.1 Chemical Reactions What happens during a chemical reaction? What distinguishes a chemical change from changes in physical
Chapter 3 Stoichiometry: Calculations with Chemical Formulas and Equations 3.1 Chemical Reactions What happens during a chemical reaction? What distinguishes a chemical change from changes in physical
11/3/09. Aqueous Solubility of Compounds. Aqueous Solubility of Ionic Compounds. Aqueous Solubility of Ionic Compounds
 Aqueous Solubility of Compounds Not all compounds dissolve in water. Solubility varies from compound to compound. Chapter 5: Chemical Reactions Soluble ionic compounds dissociate. Ions are solvated Most
Aqueous Solubility of Compounds Not all compounds dissolve in water. Solubility varies from compound to compound. Chapter 5: Chemical Reactions Soluble ionic compounds dissociate. Ions are solvated Most
Which other compounds react with acids to produce salts? Acids can also react with metals and carbonates to produce salts.
 Salts Textbook pages 234 243 Section 5.2 Summary Before You Read How many different uses for salts can you name? Write your answers on the lines below. What are salts? In chemistry, salts are a class of
Salts Textbook pages 234 243 Section 5.2 Summary Before You Read How many different uses for salts can you name? Write your answers on the lines below. What are salts? In chemistry, salts are a class of
CHEMICAL REACTIONS. Types of Reactions. Steps to Writing Reactions
 Types of Reactions CHEMICAL REACTIONS There are five types of chemical reactions we will talk about: 1. Synthesis reactions 2. reactions 3. Single displacement reactions 4. reactions 5. Combustion reactions
Types of Reactions CHEMICAL REACTIONS There are five types of chemical reactions we will talk about: 1. Synthesis reactions 2. reactions 3. Single displacement reactions 4. reactions 5. Combustion reactions
Lesson 13: Ionic Equations & Intro to the Mole with Conversions
 NOTES Name: Date: Class: Lesson 13: Ionic Equations & Intro to the Mole with Conversions Box 1: Balance: 1. Mg + O2 MgO 2. KClO3 KCl + O2 3. C2H6 + O2 CO2 + H2O Write and balance the equation that represents
NOTES Name: Date: Class: Lesson 13: Ionic Equations & Intro to the Mole with Conversions Box 1: Balance: 1. Mg + O2 MgO 2. KClO3 KCl + O2 3. C2H6 + O2 CO2 + H2O Write and balance the equation that represents
Chapter 5 Chemical Reactions
 Chapter 5 Chemical Reactions 5.1 Chemical Equations A chemical equation shows the chemical change taking place. The state of each substance is written in parentheses after the formula: s for solids, l
Chapter 5 Chemical Reactions 5.1 Chemical Equations A chemical equation shows the chemical change taking place. The state of each substance is written in parentheses after the formula: s for solids, l
Topic 8: Chemical Reactions Chemical Equations & Reactions
 Topic 8: Chemical Reactions Chemical Equations & Reactions (Chapter 8 in Modern Chemistry) Describing Chemical Reactions A chemical reaction is the process by which one or more substances are changed into
Topic 8: Chemical Reactions Chemical Equations & Reactions (Chapter 8 in Modern Chemistry) Describing Chemical Reactions A chemical reaction is the process by which one or more substances are changed into
7/16/2012. Chapter Four: Like Dissolve Like. The Water Molecule. Ionic Compounds in Water. General Properties of Aqueous Solutions
 General Properties of Aqueous Solutions Chapter Four: TYPES OF CHEMICAL REACTIONS AND SOLUTION STOICHIOMETRY A solution is a homogeneous mixture of two or more substances. A solution is made when one substance
General Properties of Aqueous Solutions Chapter Four: TYPES OF CHEMICAL REACTIONS AND SOLUTION STOICHIOMETRY A solution is a homogeneous mixture of two or more substances. A solution is made when one substance
Types of Chemical Reactions. Synthesis, Combustion, Decomposition and Replacement
 Types of Chemical Reactions Synthesis, Combustion, Decomposition and Replacement You can think of atoms as people getting together as couples... Analogy One person A couple Switching partners Chemical
Types of Chemical Reactions Synthesis, Combustion, Decomposition and Replacement You can think of atoms as people getting together as couples... Analogy One person A couple Switching partners Chemical
AP Chapter 3 Study Questions
 Class: Date: AP Chapter 3 Study Questions True/False Indicate whether the statement is true or false. 1. The mass of a single atom of an element (in amu) is numerically EQUAL to the mass in grams of 1
Class: Date: AP Chapter 3 Study Questions True/False Indicate whether the statement is true or false. 1. The mass of a single atom of an element (in amu) is numerically EQUAL to the mass in grams of 1
2 Amount of substance Exam-style questions. AQA Chemistry
 1 From AQA Chemistry Unit 1 Foundation Chemistry CHEM1 January 2009 (Question 3) Titanium(IV) oxide (TiO 2, M r = 79.9) is used as a white pigment in some paints. The pigment can be made as shown in the
1 From AQA Chemistry Unit 1 Foundation Chemistry CHEM1 January 2009 (Question 3) Titanium(IV) oxide (TiO 2, M r = 79.9) is used as a white pigment in some paints. The pigment can be made as shown in the
Chapter 4 Reactions in Aqueous Solution
 Chapter 4 Reactions in Aqueous Solution Homework Chapter 4 11, 15, 21, 23, 27, 29, 35, 41, 45, 47, 51, 55, 57, 61, 63, 73, 75, 81, 85 1 2 Chapter Objectives Solution To understand the nature of ionic substances
Chapter 4 Reactions in Aqueous Solution Homework Chapter 4 11, 15, 21, 23, 27, 29, 35, 41, 45, 47, 51, 55, 57, 61, 63, 73, 75, 81, 85 1 2 Chapter Objectives Solution To understand the nature of ionic substances
Unit 4: Reactions and Stoichiometry
 Unit 4: Reactions and Stoichiometry Reactions Chemical equation Expression representing a chemical reaction Formulas of reactants on the left side Formulas of products on the right side Arrow(s) connect(s)
Unit 4: Reactions and Stoichiometry Reactions Chemical equation Expression representing a chemical reaction Formulas of reactants on the left side Formulas of products on the right side Arrow(s) connect(s)
Study Guide: Stoichiometry
 Name: Study Guide: Stoichiometry Period: **YOUR ANSWERS MUST INCLUDE THE PROPER NUMBER OF SIG FIGS AND COMPLETE UNITS IN ORDER TO RECEIVE CREDIT FOR THE PROBLEM.** BALANCE THE FOLLOWING EQUATIONS TO USE
Name: Study Guide: Stoichiometry Period: **YOUR ANSWERS MUST INCLUDE THE PROPER NUMBER OF SIG FIGS AND COMPLETE UNITS IN ORDER TO RECEIVE CREDIT FOR THE PROBLEM.** BALANCE THE FOLLOWING EQUATIONS TO USE
Precipitation Reactions
 Precipitation Reactions Precipitation reactions are reactions in which a solid forms when we mix two solutions reactions between aqueous solutions of ionic compounds produce an ionic compound that is insoluble
Precipitation Reactions Precipitation reactions are reactions in which a solid forms when we mix two solutions reactions between aqueous solutions of ionic compounds produce an ionic compound that is insoluble
Fe(s) + O2(g) Chapter 11 Chemical Reactions. Chemical Equations. Fe + O2. January 26, What is a chemical reaction?
 Chapter 11 Chemical Reactions What is a chemical reaction? Chemical Reaction: process by which one or more substances are changed into one or more different substances. Indications of a chemical reaction
Chapter 11 Chemical Reactions What is a chemical reaction? Chemical Reaction: process by which one or more substances are changed into one or more different substances. Indications of a chemical reaction
Classifying Chemical Reactions
 1 Classifying Chemical Reactions Analyzing and Predicting Products Introduction The power of chemical reactions to transform our lives is visible all around us-in our cars, even in our bodies. Chemists
1 Classifying Chemical Reactions Analyzing and Predicting Products Introduction The power of chemical reactions to transform our lives is visible all around us-in our cars, even in our bodies. Chemists
During photosynthesis, plants convert carbon dioxide and water into glucose (C 6 H 12 O 6 ) according to the reaction:
 Example 4.1 Stoichiometry During photosynthesis, plants convert carbon dioxide and water into glucose (C 6 H 12 O 6 ) according to the reaction: Suppose that a particular plant consumes 37.8 g of CO 2
Example 4.1 Stoichiometry During photosynthesis, plants convert carbon dioxide and water into glucose (C 6 H 12 O 6 ) according to the reaction: Suppose that a particular plant consumes 37.8 g of CO 2
CHE 105 Spring 2018 Exam 2
 CHE 105 Spring 2018 Exam 2 Your Name: Your ID: Question #: 1 A penny contains 373 x 10 2 moles of zinc How many atoms of zinc are in a penny? A 225 x 10 22 atoms B 221 x 10 23 atoms C 375 x 10 22 atoms
CHE 105 Spring 2018 Exam 2 Your Name: Your ID: Question #: 1 A penny contains 373 x 10 2 moles of zinc How many atoms of zinc are in a penny? A 225 x 10 22 atoms B 221 x 10 23 atoms C 375 x 10 22 atoms
Chapter 4 Electrolytes Acid-Base (Neutralization) Oxidation-Reduction (Redox) Reactions. Dr. Sapna Gupta
 Chapter 4 Electrolytes Acid-Base (Neutralization) Oxidation-Reduction (Redox) Reactions Dr. Sapna Gupta Types of Reactions Two classifications: one how atoms are rearrangement and the other is chemical
Chapter 4 Electrolytes Acid-Base (Neutralization) Oxidation-Reduction (Redox) Reactions Dr. Sapna Gupta Types of Reactions Two classifications: one how atoms are rearrangement and the other is chemical
Chapter 4 Notes Types of Chemical Reactions and Solutions Stoichiometry A Summary
 Chapter 4 Notes Types of Chemical Reactions and Solutions Stoichiometry A Summary 4.1 Water, the Common Solvent A. Structure of water 1. Oxygen s electronegativity is high (3.5) and hydrogen s is low (2.1)
Chapter 4 Notes Types of Chemical Reactions and Solutions Stoichiometry A Summary 4.1 Water, the Common Solvent A. Structure of water 1. Oxygen s electronegativity is high (3.5) and hydrogen s is low (2.1)
Chapter 3 Chemical Reactions
 Chapter 3 Chemical Reactions Jeffrey Mack California State University, Sacramento Chemical Reactions Reactants: Zn + I 2 Product: ZnI 2 Chemical Reactions Evidence of a chemical reaction: Gas Evolution
Chapter 3 Chemical Reactions Jeffrey Mack California State University, Sacramento Chemical Reactions Reactants: Zn + I 2 Product: ZnI 2 Chemical Reactions Evidence of a chemical reaction: Gas Evolution
Chapter 4: Stoichiometry of Chemical Reactions. 4.1 Writing and Balancing Chemical Equations
 Chapter 4: Stoichiometry of Chemical Reactions 4.1 Writing and Balancing Chemical Equations A chemical equation represents or symbolizes a chemical reaction. o Substances are represents by their chemical
Chapter 4: Stoichiometry of Chemical Reactions 4.1 Writing and Balancing Chemical Equations A chemical equation represents or symbolizes a chemical reaction. o Substances are represents by their chemical
Chemical Equations and Chemical Reactions
 Chemical Equations Chemical Equations and Chemical Reactions Chemical equations are concise representations of chemical reactions. Chemical Equations Symbols Used in Chemical Equations The formulas of
Chemical Equations Chemical Equations and Chemical Reactions Chemical equations are concise representations of chemical reactions. Chemical Equations Symbols Used in Chemical Equations The formulas of
10.3 Types of Chemical Reactions
 10.3 Types of Chemical Reactions Chemical equations follow certain patterns Combination (synthesis ) The number of possible chemical is very large. However, there are a limited number of structural patterns
10.3 Types of Chemical Reactions Chemical equations follow certain patterns Combination (synthesis ) The number of possible chemical is very large. However, there are a limited number of structural patterns
Review Material for Exam #2
 Review Material for Exam #2 1. a. Calculate the molarity of a solution made with 184.6 mg sample of potassium dichromate dissolved in enough water to give 500.0 ml of solution. b. What is the molarity
Review Material for Exam #2 1. a. Calculate the molarity of a solution made with 184.6 mg sample of potassium dichromate dissolved in enough water to give 500.0 ml of solution. b. What is the molarity
UNIT (4) CALCULATIONS AND CHEMICAL REACTIONS
 UNIT (4) CALCULATIONS AND CHEMICAL REACTIONS 4.1 Formula Masses Recall that the decimal number written under the symbol of the element in the periodic table is the atomic mass of the element. Atomic mass
UNIT (4) CALCULATIONS AND CHEMICAL REACTIONS 4.1 Formula Masses Recall that the decimal number written under the symbol of the element in the periodic table is the atomic mass of the element. Atomic mass
Reaction Writing Sheet #1 Key
 Reaction Writing Sheet #1 Key Write and balance each of the following reactions and indicate the reaction type(s) present: 1. zinc + sulfur zinc sulfide 8 Zn (s) + S 8 (s) 8 ZnS (s) synthesis 2. potassium
Reaction Writing Sheet #1 Key Write and balance each of the following reactions and indicate the reaction type(s) present: 1. zinc + sulfur zinc sulfide 8 Zn (s) + S 8 (s) 8 ZnS (s) synthesis 2. potassium
Chapter 4. The Major Classes of Chemical Reactions 4-1
 Chapter 4 The Major Classes of Chemical Reactions 4-1 The Major Classes of Chemical Reactions 4.1 The Role of Water as a Solvent 4.2 Writing Equations for Aqueous Ionic Reactions 4.3 Precipitation Reactions
Chapter 4 The Major Classes of Chemical Reactions 4-1 The Major Classes of Chemical Reactions 4.1 The Role of Water as a Solvent 4.2 Writing Equations for Aqueous Ionic Reactions 4.3 Precipitation Reactions
Dr. Rick Fletcher. Clearly print your name in the name section of the Scantron answer sheet.
 Dr. Rick Fletcher Exam 2 Chemistry 101 March 7, 2011 Clearly print your name in the name section of the Scantron answer sheet. Clearly place your student ID number in the SUBJECT section of the Scantron
Dr. Rick Fletcher Exam 2 Chemistry 101 March 7, 2011 Clearly print your name in the name section of the Scantron answer sheet. Clearly place your student ID number in the SUBJECT section of the Scantron
Chapter 7 - Chemical Reactions
 Chapter 7 - Chemical Reactions Evidence of a Chemical Reaction If we could see the atoms and molecules that compose matter, we could easily identify a chemical reaction: Atoms combine with other atoms
Chapter 7 - Chemical Reactions Evidence of a Chemical Reaction If we could see the atoms and molecules that compose matter, we could easily identify a chemical reaction: Atoms combine with other atoms
TYPES OF CHEMICAL REACTIONS SYNTHESIS (COMPOSITION), DECOMPOSITION AND REPLACEMENT (SINGLE AND DOUBLE), AND COMBUSTION
 TYPES OF CHEMICAL REACTIONS SYNTHESIS (COMPOSITION), DECOMPOSITION AND REPLACEMENT (SINGLE AND DOUBLE), AND COMBUSTION YOU CAN THINK OF ATOMS AS PEOPLE GETTING TOGETHER AS COUPLES... Analogy One person
TYPES OF CHEMICAL REACTIONS SYNTHESIS (COMPOSITION), DECOMPOSITION AND REPLACEMENT (SINGLE AND DOUBLE), AND COMBUSTION YOU CAN THINK OF ATOMS AS PEOPLE GETTING TOGETHER AS COUPLES... Analogy One person
Salts Soluble Insoluble Nitrate salts - All nitrate salts - Carbonate salts - Potassium carbonate, K 2 CO. Except
 Chapter 8: Salts 1. Salts - A salt is an ionic compound. - The anion part comes from the acid while the cation part comes from a base. - Example: KCl, KOH(aq) + HCl(aq) KCl(aq) + H 2 O(l) - A salt is a
Chapter 8: Salts 1. Salts - A salt is an ionic compound. - The anion part comes from the acid while the cation part comes from a base. - Example: KCl, KOH(aq) + HCl(aq) KCl(aq) + H 2 O(l) - A salt is a
Reactions. Chapter 3 Combustion Decomposition Combination. Chapter 4 Reactions. Exchange reactions (Metathesis) Formation of a precipitate
 Reactions Chapter 3 Combustion Decomposition Combination Chapter 4 Reactions Exchange reactions (Metathesis) Formation of a precipitate Formation of a gas Formation of a week or nonelectrolyte Single Displacement
Reactions Chapter 3 Combustion Decomposition Combination Chapter 4 Reactions Exchange reactions (Metathesis) Formation of a precipitate Formation of a gas Formation of a week or nonelectrolyte Single Displacement
Name: Class: Date: SHORT ANSWER Answer the following questions in the space provided.
 CHAPTER 14 REVIEW Acids and Bases SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Name the following compounds as acids: a. H 2 SO 4 b. H 2 SO 3 c. H 2 S d. HClO 4 e. hydrogen
CHAPTER 14 REVIEW Acids and Bases SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Name the following compounds as acids: a. H 2 SO 4 b. H 2 SO 3 c. H 2 S d. HClO 4 e. hydrogen
Science 1206 Unit 3: Chemical Reactions Page 1 of 15
 Science 1206 Unit 3: Chemical Reactions Page 1 of 15 Introduction to Chemical Reactions Notes Part II TEXT p. 218-219 (word equations) There are many chemical reactions too many to count in fact! Like
Science 1206 Unit 3: Chemical Reactions Page 1 of 15 Introduction to Chemical Reactions Notes Part II TEXT p. 218-219 (word equations) There are many chemical reactions too many to count in fact! Like
Chapter 5 Classification and Balancing of Chemical Reactions
 Chapter 5 Classification and Balancing of Chemical Reactions 5.1 Chemical Equations Chemical equations describe chemical reactions. - As words: hydrogen plus oxygen combine to form water - As a chemical
Chapter 5 Classification and Balancing of Chemical Reactions 5.1 Chemical Equations Chemical equations describe chemical reactions. - As words: hydrogen plus oxygen combine to form water - As a chemical
CHAPTER 8 SALTS. NaCl. A salt is an ionic substance produced when the hydrogen ion of the acid is replaced by metal ion or an ammonium ion.
 CHAPTER 8 SALTS A salt is an ionic substance produced when the hydrogen ion of the acid is replaced by metal ion or an ammonium ion. The salt consists of two parts, cation from base and anion from acid.
CHAPTER 8 SALTS A salt is an ionic substance produced when the hydrogen ion of the acid is replaced by metal ion or an ammonium ion. The salt consists of two parts, cation from base and anion from acid.
UNIT 3 IB MATERIAL BONDING, MOLES & STOICHIOMETRY
 UNIT 3 IB MATERIAL Name: BONDING, MOLES & STOICHIOMETRY ESSENTIALS: Know, Understand, and Be Able To Apply the mole concept to substances. Determine the number of particles and the amount of substance
UNIT 3 IB MATERIAL Name: BONDING, MOLES & STOICHIOMETRY ESSENTIALS: Know, Understand, and Be Able To Apply the mole concept to substances. Determine the number of particles and the amount of substance
Funsheet 3.0 [WRITING & BALANCING EQUATIONS] Gu/R. 2017
![Funsheet 3.0 [WRITING & BALANCING EQUATIONS] Gu/R. 2017 Funsheet 3.0 [WRITING & BALANCING EQUATIONS] Gu/R. 2017](/thumbs/80/82478584.jpg) Funsheet 3.0 [WRITING & BALANCING EQUATIONS] Gu/R. 2017 Balance the following chemical equations. Remember, it is not necessary to write "1" if the coefficient is one. 1. N 2 + H 2 NH 3 2. KClO 3 KCl +
Funsheet 3.0 [WRITING & BALANCING EQUATIONS] Gu/R. 2017 Balance the following chemical equations. Remember, it is not necessary to write "1" if the coefficient is one. 1. N 2 + H 2 NH 3 2. KClO 3 KCl +
Chem!stry. Assignment on Acids, Bases and Salts #
 Chem!stry Name: ( ) Class: Date: / / Assignment on Acids, Bases and Salts #5 Write your answers in the spaces below: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 1. Which of the
Chem!stry Name: ( ) Class: Date: / / Assignment on Acids, Bases and Salts #5 Write your answers in the spaces below: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 1. Which of the
Name Period CH 180 Practice Test: Chapters 3 and 4
 Name Period CH 180 Practice Test: Chapters 3 and 4 Multiple Choice: 1. 11. 2. 12. 3. 13. 4. 14. 5. 15. 6. 16. 7. 17. 8. 18. 9. 19. 10. 20. 21. 22. 23. 24. 25. 26. 27. 1 Directions: Answer all questions.
Name Period CH 180 Practice Test: Chapters 3 and 4 Multiple Choice: 1. 11. 2. 12. 3. 13. 4. 14. 5. 15. 6. 16. 7. 17. 8. 18. 9. 19. 10. 20. 21. 22. 23. 24. 25. 26. 27. 1 Directions: Answer all questions.
4. Magnesium has three natural isotopes with the following masses and natural abundances:
 Exercise #1. Determination of Weighted Average Mass 1. The average mass of pennies minted after 1982 is 2.50 g and the average mass of pennies minted before 1982 is 3.00 g. Suppose that a bag of pennies
Exercise #1. Determination of Weighted Average Mass 1. The average mass of pennies minted after 1982 is 2.50 g and the average mass of pennies minted before 1982 is 3.00 g. Suppose that a bag of pennies
Last Lecture. K 2 SO 4 (aq) + Ba(NO 3 ) 2 (aq) AgNO 3 (aq) + KCl(aq) NaNO 3 (aq) + KCl(aq) What will happen when these are mixed together?
 Announcements Precipitation lab write-up due tomorrow at the start of discussion Text HW due tomorrow in discussion Lon-capa HW #4 Type 1 due Monday, Oct 15 th at 7:00pm Lon-capa HW #4 Type 2 due Wednesday,
Announcements Precipitation lab write-up due tomorrow at the start of discussion Text HW due tomorrow in discussion Lon-capa HW #4 Type 1 due Monday, Oct 15 th at 7:00pm Lon-capa HW #4 Type 2 due Wednesday,
26. N 2 + H 2 NH N 2 + O 2 N 2 O 28. CO 2 + H 2 O C 6 H 12 O 6 + O SiCl 4 + H 2 O H 4 SiO 4 + HCl 30. H 3 PO 4 H 4 P 2 O 7 + H 2 O
 Balance the following chemical equations: (Some may already be balanced.) 1. H 2 + O 2 H 2 O 2. S 8 + O 2 SO 3 3. HgO Hg + O 2 4. Zn + HCl ZnCl 2 + H 2 5. Na + H 2 O NaOH + H 2 6. C 10 H 16 + Cl 2 C +
Balance the following chemical equations: (Some may already be balanced.) 1. H 2 + O 2 H 2 O 2. S 8 + O 2 SO 3 3. HgO Hg + O 2 4. Zn + HCl ZnCl 2 + H 2 5. Na + H 2 O NaOH + H 2 6. C 10 H 16 + Cl 2 C +
11.3 Reactions in Aqueous Essential Understanding Reactions that occur in aqueous solutions are double-replacement
 13. Is the following sentence true or false? Hydrocarbons, compounds of hydrogen and carbon, are often the reactants in combustion reactions. 14. Circle the letter of each compound that can be produced
13. Is the following sentence true or false? Hydrocarbons, compounds of hydrogen and carbon, are often the reactants in combustion reactions. 14. Circle the letter of each compound that can be produced
LISTA DE EXERCÍCIOS AULA 06/10/2016
 LISTA DE EXERCÍCIOS AULA 06/10/2016 1- Sodium hypochlorite, the active ingredient in Clorox, can be made by the following reaction: 2 NaOH(aq) + Cl 2 (g) NaCl(aq) + NaClO(aq) + H 2 O(l) If chlorine gas
LISTA DE EXERCÍCIOS AULA 06/10/2016 1- Sodium hypochlorite, the active ingredient in Clorox, can be made by the following reaction: 2 NaOH(aq) + Cl 2 (g) NaCl(aq) + NaClO(aq) + H 2 O(l) If chlorine gas
Activity Predicting Products of Double Displacement Reactions
 KEY Activity 151-6 Predicting Products of Double Displacement Reactions Directions: This GLA worksheet is focused on predicting products and writing balanced equations for double displacement reactions.
KEY Activity 151-6 Predicting Products of Double Displacement Reactions Directions: This GLA worksheet is focused on predicting products and writing balanced equations for double displacement reactions.
October 19, 1999 Page 1. Chapter 4 Practice Worksheet Dr. Palmer Graves, Instructor MULTIPLE CHOICE
 October 19, 1999 Page 1 MULTIPLE CHOICE Section 4.1 Some Ways that Chemical Reactions Occur 1. The reaction of HNO (aq) + KOH(aq) KNO (aq) + H O(l) is best classified as a(n) a) acid-base neutralization
October 19, 1999 Page 1 MULTIPLE CHOICE Section 4.1 Some Ways that Chemical Reactions Occur 1. The reaction of HNO (aq) + KOH(aq) KNO (aq) + H O(l) is best classified as a(n) a) acid-base neutralization
CHEMICAL EQUATIONS WHAT BALANCING AN EQUATION MEANS
 17 CHEMICAL EQUATIONS WHAT BALANCING AN EQUATION MEANS WHAT IS A CHEMICAL EQUATION? A chemical equation is a way of representing a chemical reaction in symbolic form. For example, when hydrochloric acid
17 CHEMICAL EQUATIONS WHAT BALANCING AN EQUATION MEANS WHAT IS A CHEMICAL EQUATION? A chemical equation is a way of representing a chemical reaction in symbolic form. For example, when hydrochloric acid
UNIT 1 Chemical Reactions Part II Workbook. Name:
 UNIT 1 Chemical Reactions Part II Workbook Name: 1 Molar Volume 1. How many moles of a gas will occupy 2.50 L at STP? 2. Calculate the volume that 0.881 mol of gas at STP will occupy. 3. Determine the
UNIT 1 Chemical Reactions Part II Workbook Name: 1 Molar Volume 1. How many moles of a gas will occupy 2.50 L at STP? 2. Calculate the volume that 0.881 mol of gas at STP will occupy. 3. Determine the
Name Date Class STUDY GUIDE FOR CONTENT MASTERY
 Chemical Reactions Section 10.1 Reactions and Equations In your textbook, read about evidence of chemical reactions. For each statement, write yes if evidence of a chemical reaction is present. Write no
Chemical Reactions Section 10.1 Reactions and Equations In your textbook, read about evidence of chemical reactions. For each statement, write yes if evidence of a chemical reaction is present. Write no
BALANCING EQUATIONS NOTES
 BALANCING EQUATIONS NOTES WHY DO WE NEED TO BALANCE CHEMICAL EQUATIONS? The LAW OF CONSERVATION OF MASS says that matter cannot be created or destroyed. In other words, you cannot end up with any more
BALANCING EQUATIONS NOTES WHY DO WE NEED TO BALANCE CHEMICAL EQUATIONS? The LAW OF CONSERVATION OF MASS says that matter cannot be created or destroyed. In other words, you cannot end up with any more
Gizmo: Balancing Chemical Equations
 Name: Date: Gizmo: Balancing Chemical Equations Part A: Prior Knowledge Questions (Do these BEFORE using the Gizmo.) The scouts are making s mores out of toasted marshmallows, chocolate, and graham crackers.
Name: Date: Gizmo: Balancing Chemical Equations Part A: Prior Knowledge Questions (Do these BEFORE using the Gizmo.) The scouts are making s mores out of toasted marshmallows, chocolate, and graham crackers.
Chemical Reaction Types
 Chemical Reactions Chemical Reaction Types There are 5 types of chemical reactions that you need to know Combination reaction Decomposition reaction Combustion reaction Single replacement (redox) reaction
Chemical Reactions Chemical Reaction Types There are 5 types of chemical reactions that you need to know Combination reaction Decomposition reaction Combustion reaction Single replacement (redox) reaction
Chapter 8 Chemical Equations and Reactions
 Chapter 8 Chemical Equations and Reactions 8-1 Describing Chemical Reactions Chemical reactions Matter undergoes 2 types of changes: 1.Physical changes no new substance produced 2.Chemical changes new/different
Chapter 8 Chemical Equations and Reactions 8-1 Describing Chemical Reactions Chemical reactions Matter undergoes 2 types of changes: 1.Physical changes no new substance produced 2.Chemical changes new/different
