Interfaces Between Gas Phase Mechanisms and Aqueous Chemistry
|
|
|
- Leo Rogers
- 6 years ago
- Views:
Transcription
1 Interfaces Between Gas Phase Mechanisms and Aqueous Chemistry Hartmut Herrmann with Andreas Tilgner, Paolo Barzaghi, Dirk Hoffmann, Yoshi Iinuma and laf Böge Leibniz-Institut für Troposphärenforschung (IfT), Leipzig UC Davis,
2 (A) Motivation (B) The IfT Chemistry Dept. Aqueous Phase Kinetics Laboratory Recent results on the reactivity of atmospheric aqueous phase radicals (e.g. H, N 3 ) (C) The CAPRAM mechanism line Recent developments: rganic chemistry extension, halogen module (D) The IfT LEAK Aerosol chamber experiments Recent results from chamber experiments on the formation of macromolecular compounds utline
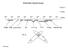
3 The Atmospheric multiphase system (Jaeschke, 1986)
4 Why Radicals? Even though the concentrations of radicals in clouds and deliquescent aerosols are thought to be small they are able to convert both inorganic and especially organic particle constituents into chemically different reaction products. Tropospheric aqueous phase radical chemistry
5 Lab studies of H aqueous phase reactions
6 Facilities at the aqueous phase process laboratory of the IfT
7 Blue laser 473nm mirror Ecximer Laser KrF λ = 248 nm Trigger unit mirror Photodiode Computer Thermostated Reaction Cell lens Solution inlet Digital oscilloscope To pump Excimer mirror Laser photolysis / long path laser absorption (LP-LPLA) set-up
8 H hν (248 nm) 2 H H + SCN - SCNH - SCNH - SCN +H - SCN + SCN - (SCN) - 2 (R-a) (R-b) (R-c) (R-d) H + reactant product (R-e) k b/c (T) = exp (-15.8 kj mol -1 /RT) M -1 s -1 (Chin and Wine, 1992) k b/c (T) = exp (-11.1 kj mol -1 /RT) M -1 s -1 (Elliot and Simsons, 1984) k b/c (T) = exp (-13.8 kj mol -1 /RT) M -1 s -1 (Chin and Wine, 1994) Details: Ervens, Gligorovski and Herrmann, Phys. Chem. Chem. Phys. 2003, 5, H Competition kinetics: Experimental method
9 Compound Formule k 298K ( M -1 s -1 ) Acetone CH 3 CCH 3 (2.1 ± 0.6) 10 8 (this study) (1.7 ± 0.5) 10 8 (Monod et al., 2002) 2-Butanone CH 3 CCH 2 CH 3 (1.5 ± 0.7 ) 10 9 (this study) (George et al., 2002) Acetonylacetone CH 3 CCH 2 CH 2 CCH 3 (7.6 ± 1.1 ) 10 8 (this study) Isobutyraldehyde (CH 3 ) 2 CHCH (2.9 ± 1.0 ) 10 9 (this study) Ethyl Formate HCC 2 H 5 (3.2 ± 0.8 ) 10 8 (this study) (Adams et al., 1965) bserved rate constants for reactions of H with organic compounds in aqueous solution at 298 K
10 T-Dependency studies of H reactions with diols in aqueous solution
11 Compound A l/mol s E A kj/mol G kj/mol H kj/mol S J/K mol Carbonyl Compounds Acetone (2.8 ± 0.5) (18 ± 11) (26 ± 20) (16 ± 10) -(34 ± 6) 2-Butanone (1.2 ± 0.2) (23 ± 10) (21 ± 12) (20 ± 9) -(3 ± 0.4) Acetonylacetone (5.1 ± 0.1) (16 ± 8) (22 ± 13) (14 ± 7) -(29 ± 3) Isobutyraldehyde (3.0 ± 0.1) (6 ± 3) (19 ± 10) (3 ± 2) -(53 ± 3) Diacetyl (8.7 ± 1.2) (20 ± 10) (24 ± 11) (7 ± 3) -(57 ± 4) Diols 1,2-Ethanediol (9.1 ± 0.2) (10 ± 2) (20 ± 4) 7 ± 1 -(43 ± 1) 1,2-Propanediol (2.0 ± 0.3) (12 ± 8) (20 ± 17) 9 ± 7 -(37 ± 5) 1,3-Propanediol (2.7 ± 0.3) (12 ± 6) (19 ± 12) 9 ± 5 -(34 ± 3) 1,2-Butanediol (5.2 ± 0.5) (13 ± 7) (19 ± 12) 11 ± 6 -(29 ± 3) 1,4-Butanediol (1.9 ± 0.1) (10 ± 2) (19 ± 4) 7 ± 1 -(37 ± 1) Activation parameters for the reactions of H with organic compounds
12 E A = -(46 ± 32) + (0.15 ± 0.08) BDE (C-H) (n = 20; r = 0.63) : Gligorovski and Herrmann, 2004 : Weigert et al. 2006, in preparation Evans-Polanyi plot for H-atom abstraction reactions of H in aqueous solution
13 12,0 N o 1 Reactant acetone 11,5 2 tert-butanol log k H / l mol -1 s -1 11,0 10,5 10,0 9,5 9,0 8,5 8,0 7, * * * lg (k H /M -1 s -1 ) = (33 ± 3) - (0.06 ± 0.01) BDE /kj mol -1 n = 10; r = 0.99; 381 < BDE < 411 aqueous phase lg (k H /M -1 s -1 ) = (32 ± 4) - (0.06 ± 0.01) BDE /kj mol -1 n = 11; r = 0.98; 373 < BDE < 411 gas phase * 7, BDE / kj mol acetonylacetone ethyl formate 2-butanone ethanol 1-propanol 1-butanol 2-propanol 2-butanol acetaldehyde propionaldehyde butyraldehyde isobutyraldehyde Comparison of Evans-Polanyi plot for H-atom abstraction reactions of H in aqueous solution and in gas phase (Gligorovski and Herrmann, 2004)
14 Controller ST133 Spektrograph + ICCD-Camera L Moveable mirror Deuterium -Lamp Photodiode Moveable mirror Irisblende Argon-Ion-Laser (λ = 244 nm) WS 1 PC Cell V=28ml WS 2 L S Excimer Laser Pulse-Delay/ Trigger scilloscope λ = 193 nm λ = 248 nm Set-up for H/R 2 kinetic and spectroscopic studies in aqueous solution
15 Modelled aqueous phase concentrations of the H radical for the urban and remote scenario
16 Spectra Nr. Parent Compound ph λ max (nm) ε 10 (l mol -1 cm -1 ) 1 a 2-Propanol b 1-Butanol c 2-Butanol d Propionaldehyde e Butyraldehyde f Acetone g 2-Hydroxy-3-butanone ε 10 [l mol -1 cm -1 ] a b c ε 10 [l mol -1 cm -1 ] d e f g Wavelength [nm] Wavelength [nm] Spectroscopic investigations: Alcohols and ketones
17 Spectra Nr. Parent Compound ph λ max (nm) ε 10 (l mol -1 cm -1 ) A a Glutaric acid b Adipic acid c Pimelic acid d Suberic acid e Glutarate (dianion) f Adipate (dianion) g Pimelate (dianion) h Suberate (dianion) B i Tartronic acid j D,L-Malic acid k Tartronate (dianion) l Malate (dianion) Spectroscopic investigations: Carboxylic acids and their anions forms
18 Electron Transfer (ETR): ED + X > ED + + X - e.g.: Mono- and Diacids: R-C()- - + X > R-C()- + X- Then decarboxylation might occur leading to C-Chain shortening: R-C()- > R + C 2 Note: ETR involves also much transition metal chemistry Solution phase non-radical reactions, e.g. ozonolysis rganic chemistry non-radical reactions. e.g. acidcatalysed condensations ther Special Aqueous Phase Reactions Note: The decarboxylation rate constants are often grossly overstimated!!
19 The CAPRAM mechanism line: Recent developments
20 CAPRAM 3.0i verview Box model results The air parcel model SPACCIM verview Simulation results Study case Summary utline part II
21 Gas phase mechanism consists of an updated and revised RACM mechanism, lumped mechanism Uptake processes of soluble species following the approach by Schwartz (1986) Multiphase mechanism incorporates the chemical aqueous phase radical mechanism CAPRAM 3.0 (Herrmann et al., 2005) Multiphase chemistry mechanism RACM-MIM2ext/CAPRAM3.0i
22 1. Initiation reaction RH + X R + HX ( X = H, N ) 3 2. Peroxyl radical formation 2 R R 3a. Peroxyl radical unimolecular decomposition R R 2 R H C C + H (R- = alkyl, H, H) R 3b. Peroxyl radical bimolecular decomposition (after von Sonntag and Schuchmann, 1997) R R R Basic organic chemistry in CAPRAM R C + R CHH HC CH 2 R (R- = alkyl, H, H) 4 2C + H 2 2 R R 2 R 2CH + 2
23 CH 3 CC H H H 2 Hydroxyacetone H 2 CH 3 CCH. H CH 3 CCH H H CH 3 CCH H.. H H 2 CH 3 CCH. Pyruvic Acid Gas phase input H 2 2 H CH 3 CCH 2 H CH 3 CC H H 2 H 2 H CH 3 CCH H H H CH 3 CCH. 2 H 2 2 H CH 3 CCH 3 H CH 3 CCH 2. CH 3 CCH 2 CH 3 CCH 2. Methylgloxal H. H 2 H. CH 3 CCH 3 2-Propanol 2 CH 3 CCH. 2 2 H 2 H CH 3 CCH 3. H 2 H CH3CCH3 Acetone In the remote scenario, the aqueous phase oxidation of acetone can contribute up to 40% to the production fluxes of Methyl Glyoxal (MGly) in the aqueous phase. Aqueous phase oxidation of acetone in CAPRAM 3.0
24 2.5e e-13 conc. [moles / l] 1.5e e e H CAPRAM 2.4 H CAPRAM 3.0 Aqueous phase concentrations of H obtained with CAPRAM 2.4 and 3.0 for the remote scenario
25 2.5e e-14 conc. [moles / l] 1.5e e e N3 CAPRAM 2.4 N3 CAPRAM 3.0 Aqueous phase concentrations of N 3 obtained with CAPRAM 2.4 and 3.0 for the remote scenario
26 Comparison of sinks and sources for H (aq) (remote scenario, noon 2.day)
27 meteorological scenario based on the calculations of Pruppacher and Jaenicke (1995) 8 cloud passages of about 2 hours within 4.5 days ( 4 day- and 4 nighttime clouds) intermediate aerosol state at 90 % relative humidity assumption: well diluted particles simulations for three atmospheric scenarios:[start: 0:00 19 th June, 45 N] urban, remote and marine with and without aqueous phase chemistry interactions (used acronym: wocloud) Scheme of the meteorological scenario (air parcel model trajectory)
28 Modelled gas phase concentrations of the H radical for the urban and remote scenario
29 H [aq] sinks and source fluxes (urban scenario)
30 A Case Study
31 Important multiphase aqueous phase organic oxidations in CAPRAM
32 Modelled aqueous phase sinks and sources of oxalic acid (remote case)
33 +130 ng m ng m -3 Modelled aqueous phase C 3 chemistry productions
34 Modelled aqueous phase C 3 chemistry productions
35 Significant effects on the tropospheric oxidation budget Influenced oxidation of VC s due to the changed oxidation budget Significant differences in the aqueous phase chemistry of cloud droplets and wet particles The potential importance of the deliquescent particles as a reactive chemical medium due to the in-situ production of radical oxidants and no-radical oxidants in the aqueous phase The importance of the aqueous phase for the formation substituted mono- and diacids Size-resolved organic mass productions and spectral processing of the particle distributions The importance of the consideration of aqueous phase processes in future higher scale models Summary: CAPRAM 3.0i + SPACCIM
36 Goto : More about CAPRAM
37 Recent results from laboratory experiments on SA formation following ozonolysis of terpenes
38 Volume: 17.7 m 3 S/V ratio: 2.1 m -1 Temperature range: K Material: FEP-Teflon Block Diagram of the new IfT Aerosol Chamber LEAK (Leipziger Aerosol Kammer)
39 The LEAK Building
40 Material: Volume: S/V Ratio: Temperature Range: Humidity Range: UV Lamps: Gas Measurements: Particle Measurements: FEP-Teflon 17.7 m m K (16-35 C) 10-85% sram Eversun 5.6kW total 3 monitor, nline GC/FID, PTR/MS Denuder-Filter (CE/MS, HPLC/MS, GC/MS), TF-AMS, DMPS The new IfT Aerosol Chamber LEAK (Leipziger Aerosol Kammer)
41 β-pinene Limonene Initial HC Conc. [ppbv] [ppbv] RH% Temp [ºC] Reaction Time [h] Sampling Time [h] No. of Exp No. of Samples (for each condition) (2 m 3 ) 5 x Neutral 5 x Acidic 5 x Strongly Acidic 3 x QF ( TC) 2 x PTFE (Speciation) (2 m 3 ) 5 x Neutral 5 x Acidic 5 x Strongly Acidic 3 x QF ( TC) 2 x PTFE (Speciation) Experimental Condition
42 Thermography Quantification of TC on seed particle CE/ESI-ITMS Quantification of monomeric compounds 1D or 2D HPLC/ESI-TFMS&/ESI-ITMS Characterisation of dimers/oligomers Analytical Methods
43 Formation of Dimers and ligomers
44 TC [µgc m -3 ] Neutral 650&850 C Acidic 650&850 C Strongly Acidic 650 C Strongly Acidic 850 C 20 0 Methylencyclohexane β-pinene Limonene No significant difference found between neutral and acidic seed particles. Much higher TC was observed for methylenecyclohexane and β-pinene in presence of strongly acidic particles whereas limonene did not exhibit this behaviour. The effect possibly hidden by extremely high SA yield of limonene Not all organics evaporated at 650 C for strongly acidic cases. 850 C was required. Indirect evidence of influence of strong acidity on particle phase products? TC
45 Limonene
46 Total Ion Chromatogram No significant difference except lower intensities for the strongly acidic case (confirms the CE/ESI-ITMS result) Mass Spectra min m/z 281 under acidic conditions min m/z under acidic conditions min m/z 465, under acidic conditions Limonene Dimer/ligomer Analysis
47 201 Base Peak Chromatogram (m/z ) m/z 281 detected only under acidic conditions , Base Peak Chromatogram (m/z ) Clear influence of acidity Larger peaks under acidic conditions Although TIC was smaller for the strongly acidic case, no such trend was observed for BPC Limonene Dimer/ligomer Analysis
48 m/z TF Suggested Elemental Composition 281 C 10 H 17 5 S- 453 C 18 H S- 465 C 20 H S- 481 C 20 H S- 483 C 23 H 31 9 S- Bond Neutral Acidic R S H R S R R S R R S R R S R Strongly Acidic x x x x x m/z TF Suggested Elemental Composition 369 C 19 H C 18 H C 19 H Bond Neutral Acidic R R/ H R C CH R R R/ H R C CH R R R/ H R C CH R Strongly Acidic 387 C 22 H R R 401 C 19 H C 20 H R R/ H R C CH R R R/ H R C CH R 355 C 18 H R R/ H R C CH R >500???? Influence of seed particle acidity on m/z 281 and compounds > m/z 400 TFMS suggested elemental compositions and MS/MS fragments suggests sulphate ester structures for these compounds Most dimers between m/z are detected regardless of seed particle acidities (i.e. no influence of acidity possibly peroxycarboxylic acid dimers) Summary of Limonene Dimer/ligomer Products
49 Influence of Acidity on Compounds > m/z 500
50 3mer 3mer 4mer 4mer 3mer 3mer 4mer 4mer 5mer 3mer 3mer 4mer 4mer 5mer Much higher amounts of compounds over m/z 500 are found under acidic conditions Acidity does influence the Mw of oligomeric compounds acid catalysed reactions still important Much larger molecules were observed in endo-exo-cyclic limonene than β-pinene or α-pinene (previous work) It seems more complex the structure is (i.e. reaction mechanisms), higher the molecular weights of early generation oligomers The distribution of compounds > m/z 500 can be explained by the combination of Cn, Cn-1, Cn-2, Cn-3 etc. oxidation products (hence 14 or 16 differences) and a number of sulphate ester units or peroxyacid units. There is not a DINSAUR. Compounds beyond m/z 500
51 A possible formation pathway for limonene sulphate ester m/z 281 S 2 H 2 HS 3 - S 3 2- xidation (l) CH (g) CH Limononaldehyde 3 H 2 S 4 HS 4 - S HS 4 CH S H H H + hydroxylimononaldehyde sulphate ester H 2 2 or RC()H (l) CH (g) CH Epoxylimononaldehyde or 3-(2-methyloxiran-2-yl)-6-oxoheptanal Potential Atmospheric Relevance? Bridging Inorganic and rganic Chemistry
52 Cn-1 compounds accounts 60-70wt% of the total detected monomers for β-pinene and a model compound (methylenecyclohexane) whereas Cn and Cn-1 monomers were found in similar amounts in limonene SA Dimers found regardless of seed particle acidities appear to be peroxycarboxylic acid dimers Sulphate esters (inc. m/z < 300) were detected only from terpenes (or a model compound) with an exocyclic double bond under our experimental conditions (the location of double bond important) The exact formation mechanisms of sulphate esters are not still clear though it seems to involve acid catalysed nucleophilic addition of sulphate Sulphate esters from terpene oxidation are not only chamber compounds. There is evidence of sulphate esters in atmosphere. Summary oligomer studies
53 The troposphere is a multiphase system. Complex chemistry occurs in aqueous particles. Radical chemistry is studied with emphasis on organics oxidation further to pure gas phase reactions Lab results are being used to further develop CAPRAM (and other schemes). Emphasis is on organics, currently up to C 4 Further development of schemes such as CAPRAM might be performed applying reactivity correlations for the estimation of rate constants rather than applying directly measured data only rganic reactions in the tropospheric particle phase are able to produce high-molecular weight products including organic sulphate esters verall Summary
54 Particle phase chemistry should be better described There are coupling to important issues in atmospheric chemistry: Acid production Change of atmospheric oxidation capacity Production of particle mass and, especially, organic mass Particle radiative properties Particle hygroscopicity and CCN efficiency, ACI In both cloud drops as well as in deliquescent particles aqueous phase reactions occur VC chemistry is linked to particle chemistry both for AVC and BVC Wider Issues and utlook
55 H H + + H 2 H+ H + H H H H Aerosol Chamber: Yoshi Iinuma, laf Böge, Yunkun Miao, Conny Müller Aqueous Phase Kinetics: Paolo Barzaghi, Dirk Hoffmann, Kshama Parajuli, Saso Gligorovski (now Marseille) CAPRAM: Andreas Tilgner Funding: EC (MST (EVK2-CT ) BMBF (BEWA, MDMEP, FEBUK) DFG (HE-3086/4-1) Acknowledgements
CAPRAM MODELLING OF THE PHYSICO-CHEMICAL CLOUD PROCESSING OF TROPOSPHERIC AEROSOLS
 CAPRAM MODELLING OF THE PHYSICO-CHEMICAL CLOUD PROCESSING OF TROPOSPHERIC AEROSOLS A. Tilgner 1 *, R. Wolke 1 and H. Herrmann 1 1 Leibniz-Institut für Troposphärenforschung, Permoserstr. 15, D-04318 Leipzig,
CAPRAM MODELLING OF THE PHYSICO-CHEMICAL CLOUD PROCESSING OF TROPOSPHERIC AEROSOLS A. Tilgner 1 *, R. Wolke 1 and H. Herrmann 1 1 Leibniz-Institut für Troposphärenforschung, Permoserstr. 15, D-04318 Leipzig,
Paul Ziemann Department of Chemistry & CIRES University of Colorado at Boulder
 Gas- and Particle-Phase Products and their Mechanisms of Formation from the Reactions of Monoterpenes with N 3 Radicals: Comprehensive Measurements and Modeling Paul Ziemann Department of Chemistry & CIRES
Gas- and Particle-Phase Products and their Mechanisms of Formation from the Reactions of Monoterpenes with N 3 Radicals: Comprehensive Measurements and Modeling Paul Ziemann Department of Chemistry & CIRES
An alcohol is a compound obtained by substituting a hydoxyl group ( OH) for an H atom on a carbon atom of a hydrocarbon group.
 Derivatives of Hydrocarbons A functional group is a reactive portion of a molecule that undergoes predictable reactions. All other organic compounds can be considered as derivatives of hydrocarbons (i.e.,
Derivatives of Hydrocarbons A functional group is a reactive portion of a molecule that undergoes predictable reactions. All other organic compounds can be considered as derivatives of hydrocarbons (i.e.,
Laboratory simulation for the aqueous OH-oxidation of methyl vinyl ketone and methacrolein: Significance to the in-cloud SOA production
 Laboratory simulation for the aqueous OHoxidation of methyl vinyl ketone and methacrolein: Significance to the incloud SOA production X. Zhang, Z. M. Chen, and Y. Zhao State Key Laboratory of Environmental
Laboratory simulation for the aqueous OHoxidation of methyl vinyl ketone and methacrolein: Significance to the incloud SOA production X. Zhang, Z. M. Chen, and Y. Zhao State Key Laboratory of Environmental
Tropospheric Aqueous Phase Chemistry Laboratory and Modelling Studies
 Tropospheric Aqueous Phase Chemistry Laboratory and Modelling Studies Hartmut Herrmann, B. Ervens, J. Hesper and F. Wicktor Institut für Troposphärenforschung Permoserstr.15, 04318 Leipzig Overview Results
Tropospheric Aqueous Phase Chemistry Laboratory and Modelling Studies Hartmut Herrmann, B. Ervens, J. Hesper and F. Wicktor Institut für Troposphärenforschung Permoserstr.15, 04318 Leipzig Overview Results
Structure-activity relationships for the development of MCM/GECKOA mechanisms
 Atmospheric Chemical Mechanisms, Davis, December 2018 Structure-activity relationships for the development of MCM/GECKOA mechanisms Acknowledgement: Bernard Aumont Richard Valorso, Marie Camredon LISA
Atmospheric Chemical Mechanisms, Davis, December 2018 Structure-activity relationships for the development of MCM/GECKOA mechanisms Acknowledgement: Bernard Aumont Richard Valorso, Marie Camredon LISA
Lecture 13: Gas Phase Organic- NO x + UV Reactions II
 Lecture 13: Gas Phase rganic- N x + UV Reactions II Required Reading: FP&P Chapter 6 (except as noted next) Additional Reading: S&P Chapter 5 Catching-Up Reading: Jacob Chapters 11 & 12 (free online) Atmospheric
Lecture 13: Gas Phase rganic- N x + UV Reactions II Required Reading: FP&P Chapter 6 (except as noted next) Additional Reading: S&P Chapter 5 Catching-Up Reading: Jacob Chapters 11 & 12 (free online) Atmospheric
Aromatic Hydrocarbons
 Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
CHAPTER 24 Organic Chemistry
 CHAPTER 24 rganic Chemistry 1. The general formula for alkenes is A. C n H 2n+2 B. C 2n H 2n C. C n H n+2 D. C n H 2n E. C n H 2n 2 2. The general formula of an alkane is A. C n H 2n B. C n H 2n+2 C. C
CHAPTER 24 rganic Chemistry 1. The general formula for alkenes is A. C n H 2n+2 B. C 2n H 2n C. C n H n+2 D. C n H 2n E. C n H 2n 2 2. The general formula of an alkane is A. C n H 2n B. C n H 2n+2 C. C
Chapter Eight: Conclusions and Future Work
 2004 PhD Thesis 202 Chapter Eight: Conclusions and Future Work 8.1 Conclusions The Aerodyne aerosol mass spectrometer is capable of providing quantitative information on the chemical composition of the
2004 PhD Thesis 202 Chapter Eight: Conclusions and Future Work 8.1 Conclusions The Aerodyne aerosol mass spectrometer is capable of providing quantitative information on the chemical composition of the
Appendix G: Low-Molecular-Weight and Oligomeric Components in Secondary Organic Aerosol from the Ozonolysis of Cycloalkenes and Alpha-pinene *
 273 Appendix G: Low-Molecular-Weight and Oligomeric Components in Secondary Organic Aerosol from the Ozonolysis of Cycloalkenes and Alpha-pinene * * This chapter is reproduced by permission from Low-Molecular-Weight
273 Appendix G: Low-Molecular-Weight and Oligomeric Components in Secondary Organic Aerosol from the Ozonolysis of Cycloalkenes and Alpha-pinene * * This chapter is reproduced by permission from Low-Molecular-Weight
More information can be found in Chapter 12 in your textbook for CHEM 3750/ 3770 and on pages in your laboratory manual.
 CHEM 3780 rganic Chemistry II Infrared Spectroscopy and Mass Spectrometry Review More information can be found in Chapter 12 in your textbook for CHEM 3750/ 3770 and on pages 13-28 in your laboratory manual.
CHEM 3780 rganic Chemistry II Infrared Spectroscopy and Mass Spectrometry Review More information can be found in Chapter 12 in your textbook for CHEM 3750/ 3770 and on pages 13-28 in your laboratory manual.
Review of the SAPRC-16 Chemical Mechanism and Comparison with the Regional Atmospheric Chemistry Mechanism, Version-2
 VOC O 3 NO NO NO 2 O 3 NO 2 Review of the SAPRC-16 Chemical Mechanism and Comparison with the Regional Atmospheric Chemistry Mechanism, Version-2 NO 2 William R. Stockwell a, Emily Saunders b, Rosa Fitzgerald
VOC O 3 NO NO NO 2 O 3 NO 2 Review of the SAPRC-16 Chemical Mechanism and Comparison with the Regional Atmospheric Chemistry Mechanism, Version-2 NO 2 William R. Stockwell a, Emily Saunders b, Rosa Fitzgerald
Nomenclature of Aldehydes and Ketones
 Aldehydes and Ketones Nomenclature of Aldehydes and Ketones Common aldehydes H H Methanl (formaldehyde) H3C H CH3CH2 ethanl (acetaldehyde) H propanal (propionaldehyde) CH3CH2CH2 butanal (n-butyraldehyde)
Aldehydes and Ketones Nomenclature of Aldehydes and Ketones Common aldehydes H H Methanl (formaldehyde) H3C H CH3CH2 ethanl (acetaldehyde) H propanal (propionaldehyde) CH3CH2CH2 butanal (n-butyraldehyde)
Chapter 23 Aldehydes and Ketones
 Chapter 23 Aldehydes and Ketones Ketones are common solvents for quickdrying paints. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris Hein, Scott Pattison, and Susan
Chapter 23 Aldehydes and Ketones Ketones are common solvents for quickdrying paints. Introduction to General, Organic, and Biochemistry, 10e John Wiley & Sons, Inc Morris Hein, Scott Pattison, and Susan
Role of Quantum Chemistry in Atmospheric Chemical Mechanism Development
 Role of Quantum Chemistry in Atmospheric Chemical Mechanism Development Renyi Zhang and Jun Zhao Department of Atmospheric Sciences Texas A&M University College Station, TX 77843 Presented at the international
Role of Quantum Chemistry in Atmospheric Chemical Mechanism Development Renyi Zhang and Jun Zhao Department of Atmospheric Sciences Texas A&M University College Station, TX 77843 Presented at the international
Heterogeneous Process Studies on the Formation of Secondary Aerosol
 2016 BC workshop Heterogeneous Process Studies on the Formation of Secondary Aerosol Maofa Ge Institute of Chemistry Chinese Academy of Sciences June-27-2016 Aerosol Heterogeneous Chemistry Atmospheric
2016 BC workshop Heterogeneous Process Studies on the Formation of Secondary Aerosol Maofa Ge Institute of Chemistry Chinese Academy of Sciences June-27-2016 Aerosol Heterogeneous Chemistry Atmospheric
DEVELOPMENT, EXTENSION AND VALIDATION OF (THEORY-BASED) SARS DECEMBER 2018 I LUC VEREECKEN
 DEVELOPMENT, EXTENSION AND VALIDATION OF (THEORY-BASED) SARS DECEMBER 2018 I LUC VEREECKEN THE NEED FOR DATABASES AND SARS Models for atmospheric chemistry are ever more complex - Air quality : more, and
DEVELOPMENT, EXTENSION AND VALIDATION OF (THEORY-BASED) SARS DECEMBER 2018 I LUC VEREECKEN THE NEED FOR DATABASES AND SARS Models for atmospheric chemistry are ever more complex - Air quality : more, and
pinene (at 2 and 50 Torr) and β-pinene (at 200 Torr) with OH have been determined in varied conditions.
 ABSTRACT The composition of the troposphere is strongly affected by biogenic and anthropogenic emissions of chemical compounds, including hydrocarbons, carbon monoxide, and the nitrogen oxides. The emissions
ABSTRACT The composition of the troposphere is strongly affected by biogenic and anthropogenic emissions of chemical compounds, including hydrocarbons, carbon monoxide, and the nitrogen oxides. The emissions
Tropospheric OH chemistry
 Tropospheric OH chemistry CO Oxidation mechanism: CO + OH CO 2 + H, H + O 2 + M HO 2 + M, HO 2 + NO OH + NO 2 NO 2 + hν (+O 2 ) NO + O 3 Initiation step Propagation Net: CO + 2 O 2 CO 2 + O 3 HO 2 + HO
Tropospheric OH chemistry CO Oxidation mechanism: CO + OH CO 2 + H, H + O 2 + M HO 2 + M, HO 2 + NO OH + NO 2 NO 2 + hν (+O 2 ) NO + O 3 Initiation step Propagation Net: CO + 2 O 2 CO 2 + O 3 HO 2 + HO
Supplementary Material for: A Comparison of the chemical sinks of atmospheric organics in the gas and aqueous phase
 Supplementary Material for: A Comparison of the chemical sinks of atmospheric organics in the gas and aqueous phase S. A. Epstein and S. A. Nizkorodov, * Department of Chemistry, University of California,
Supplementary Material for: A Comparison of the chemical sinks of atmospheric organics in the gas and aqueous phase S. A. Epstein and S. A. Nizkorodov, * Department of Chemistry, University of California,
Interactive comment on Reactive uptake of ammonia to secondary organic aerosols: kinetics of organonitrogen formation by Y. Liu et al.
 Atmos. Chem. Phys. Discuss., 15, C7412 C7424, 215 www.atmos-chem-phys-discuss.net/15/c7412/215/ Author(s) 215. This work is distributed under the Creative Commons Attribute 3. License. Atmospheric Chemistry
Atmos. Chem. Phys. Discuss., 15, C7412 C7424, 215 www.atmos-chem-phys-discuss.net/15/c7412/215/ Author(s) 215. This work is distributed under the Creative Commons Attribute 3. License. Atmospheric Chemistry
Reactions of Chapter 10 Worksheet and Key
 1) Alcohol Fermentation Reactions of Chapter 10 Worksheet and Key Alcohol fermentation is a series of chemical reaction that convert sugar molecules, such a glucose, into ethanol and C 2. The overall reaction
1) Alcohol Fermentation Reactions of Chapter 10 Worksheet and Key Alcohol fermentation is a series of chemical reaction that convert sugar molecules, such a glucose, into ethanol and C 2. The overall reaction
CHAPTER OUTLINE. I. Elemental Carbon II. Crude Oil : the Basic Resource III. Hydrocarbons IV. Separating Hydrocarbons by Fractional Distillation
 Carbon Chapter 12 CHAPTER UTLINE 9.2 I. Elemental Carbon II. Crude il : the Basic Resource III. Hydrocarbons IV. Separating Hydrocarbons by Fractional Distillation V. Processing Hydrocarbons VI. Typical
Carbon Chapter 12 CHAPTER UTLINE 9.2 I. Elemental Carbon II. Crude il : the Basic Resource III. Hydrocarbons IV. Separating Hydrocarbons by Fractional Distillation V. Processing Hydrocarbons VI. Typical
Atmospheric Oxidation Mechanisms of Unsaturated Oxygenated VOCs
 Atmospheric Oxidation Mechanisms of Unsaturated Oxygenated VOCs R. Thévenet, G. Thiault, E. Vésine, G. Laverdet, A. Mellouki, G. Le Bras LCSR-CNRS-1C, Avenue de la recherche scientifique 4571, Orléans,
Atmospheric Oxidation Mechanisms of Unsaturated Oxygenated VOCs R. Thévenet, G. Thiault, E. Vésine, G. Laverdet, A. Mellouki, G. Le Bras LCSR-CNRS-1C, Avenue de la recherche scientifique 4571, Orléans,
14.1 Aldehydes and Ketones Copyright 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings
 Chapter 14 Aldehydes, Ketones, and Chiral Molecules 14.1 Aldehydes and Ketones Copyright 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings Carbonyl Group in Aldehydes A carbonyl group and
Chapter 14 Aldehydes, Ketones, and Chiral Molecules 14.1 Aldehydes and Ketones Copyright 2007 by Pearson Education, Inc. Publishing as Benjamin Cummings Carbonyl Group in Aldehydes A carbonyl group and
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 6 Dr Ali El-Agamey 1 Oxidation States Easy for inorganic salts: CrO 4 2- reduced to Cr 2 O 3. KMnO 4 reduced to MnO 2. Oxidation: Gain of O,
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 6 Dr Ali El-Agamey 1 Oxidation States Easy for inorganic salts: CrO 4 2- reduced to Cr 2 O 3. KMnO 4 reduced to MnO 2. Oxidation: Gain of O,
Química Orgânica I. Ciências Farmacêuticas Bioquímica Química AFB QO I 2007/08 1
 Química rgânica I Ciências Farmacêuticas Bioquímica Química AFB Q I 2007/08 1 alcohols Adaptado de rganic Chemistry, 6th Edition; Wade rganic Chemistry, 6 th Edition; McMurry AFB Q I 2007/08 2 Typical
Química rgânica I Ciências Farmacêuticas Bioquímica Química AFB Q I 2007/08 1 alcohols Adaptado de rganic Chemistry, 6th Edition; Wade rganic Chemistry, 6 th Edition; McMurry AFB Q I 2007/08 2 Typical
On the Photolysis of simple Anions and neutral Molecules as Sources of O-/ OH, SOx- and Cl in Aqueous Solution
 1 Electronic Supplementary Material (ESM) to On the Photolysis of simple Anions and neutral Molecules as Sources of O/ OH, SOx and Cl in Aqueous Solution Hartmut Herrmann LeibnizInstitut für Troposphärenforschung
1 Electronic Supplementary Material (ESM) to On the Photolysis of simple Anions and neutral Molecules as Sources of O/ OH, SOx and Cl in Aqueous Solution Hartmut Herrmann LeibnizInstitut für Troposphärenforschung
Name/CG: 2012 Term 2 Organic Chemistry Revision (Session II) Deductive Question
 Name/G: 2012 Term 2 rganic hemistry Revision (Session II) Deductive Question 1(a) A yellow liquid A, 7 7 N 2, reacts with alkaline potassium manganate (VII) and on acidification gives a yellow solid B,
Name/G: 2012 Term 2 rganic hemistry Revision (Session II) Deductive Question 1(a) A yellow liquid A, 7 7 N 2, reacts with alkaline potassium manganate (VII) and on acidification gives a yellow solid B,
Objective 14. Develop synthesis strategies for organic synthesis.
 Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Role of Water and Phase in the Heterogeneous Oxidation of Solid and Aqueous Succinic Acid Aerosol by Hydroxyl Radicals
 pubs.acs.org/jpcc Role of Water and Phase in the Heterogeneous Oxidation of Solid and Aqueous Succinic Acid Aerosol by Hydroxyl Radicals Man Nin Chan, Haofei Zhang,, Allen H. Goldstein,,, and Kevin R.
pubs.acs.org/jpcc Role of Water and Phase in the Heterogeneous Oxidation of Solid and Aqueous Succinic Acid Aerosol by Hydroxyl Radicals Man Nin Chan, Haofei Zhang,, Allen H. Goldstein,,, and Kevin R.
A coupled field and modelling study on aerosol - cloud interaction
 A coupled field and modelling study on aerosol - cloud interaction (AFO 2000 projects FEBUKO (FKZ 07ATF01) and MODMEP (FKZ 07ATF40)) Hartmut Herrmann*, Ralf Wolke* and the FEBUKO/MODMEP-team** *: Leibniz-Institut
A coupled field and modelling study on aerosol - cloud interaction (AFO 2000 projects FEBUKO (FKZ 07ATF01) and MODMEP (FKZ 07ATF40)) Hartmut Herrmann*, Ralf Wolke* and the FEBUKO/MODMEP-team** *: Leibniz-Institut
Essential Organic Chemistry. Chapter 9
 Essential Organic Chemistry Paula Yurkanis Bruice Chapter 9 Substitution and Elimination Reactions of Alkyl Halides 9.1 How Alkyl Halides React Substitution Reactions One group takes the place of another.
Essential Organic Chemistry Paula Yurkanis Bruice Chapter 9 Substitution and Elimination Reactions of Alkyl Halides 9.1 How Alkyl Halides React Substitution Reactions One group takes the place of another.
Advanced oxidation of organic pollutants in air non-thermal plasmas
 UNIVERSITÀ DEGLI STUDI DI PADVA Department of Chemical Sciences Advanced oxidation of organic pollutants in air non-thermal plasmas Ester Marotta and Cristina Paradisi PlasTEP, Berlin, December 5-6, 212
UNIVERSITÀ DEGLI STUDI DI PADVA Department of Chemical Sciences Advanced oxidation of organic pollutants in air non-thermal plasmas Ester Marotta and Cristina Paradisi PlasTEP, Berlin, December 5-6, 212
Formation, Speciation, and Chemical Processing of Atmospheric Particulate Matter Kara E. Huff Hartz
 Formation, Speciation, and Chemical Processing of Atmospheric Particulate Matter Kara E. Huff Hartz Department of Chemistry and Biochemistry Southern Illinois University Carbondale Carbondale, IL USA khuffhartz@chem.siu.edu
Formation, Speciation, and Chemical Processing of Atmospheric Particulate Matter Kara E. Huff Hartz Department of Chemistry and Biochemistry Southern Illinois University Carbondale Carbondale, IL USA khuffhartz@chem.siu.edu
CONTENTS 1 MEASURES OF ATMOSPHERIC COMPOSITION
 i CONTENTS 1 MEASURES OF ATMOSPHERIC COMPOSITION 1 1.1 MIXING RATIO 1 1.2 NUMBER DENSITY 2 1.3 PARTIAL PRESSURE 6 PROBLEMS 10 1.1 Fog formation 10 1.2 Phase partitioning of water in cloud 10 1.3 The ozone
i CONTENTS 1 MEASURES OF ATMOSPHERIC COMPOSITION 1 1.1 MIXING RATIO 1 1.2 NUMBER DENSITY 2 1.3 PARTIAL PRESSURE 6 PROBLEMS 10 1.1 Fog formation 10 1.2 Phase partitioning of water in cloud 10 1.3 The ozone
15.1: Hydrocarbon Reactions
 15.1: Hydrocarbon Reactions Halogenation An alkane will react with a halogen to produce a halalkane and the corresponding hydrogen halide. The catalyst is ultraviolet radiation. Reaction 1 methane chlorine
15.1: Hydrocarbon Reactions Halogenation An alkane will react with a halogen to produce a halalkane and the corresponding hydrogen halide. The catalyst is ultraviolet radiation. Reaction 1 methane chlorine
TOWARDS A MORE DETAILED DESCRIPTION OF TROPOSPHERIC AQUEOUS PHASE ORGANIC CHEMISTRY: CAPRAM 3.0 ELECTRONIC SUPPLEMENTAL MATERIAL (ESM)
 TWARDS A MRE DETAILED DESRIPTIN F TRPSPERI AQUEUS PASE RGANI EMISTRY: APRAM 30 errmann 1 *, A Tilgner 1, P Barzaghi 1, Z Majdik 1, S Gligorovski 1, L Poulain 2 and A Monod 2 1 Leibniz Institut für Troposphärenforschung,
TWARDS A MRE DETAILED DESRIPTIN F TRPSPERI AQUEUS PASE RGANI EMISTRY: APRAM 30 errmann 1 *, A Tilgner 1, P Barzaghi 1, Z Majdik 1, S Gligorovski 1, L Poulain 2 and A Monod 2 1 Leibniz Institut für Troposphärenforschung,
CHEM 263 Oct 11, Lecture Outline 3: Alcohols, Ethers, Stereochemistry, Ketones, and Aldehydes. Ethanol
 CEM 263 ct 11, 2016 Lecture utline 3: Alcohols, Ethers, Stereochemistry, Ketones, and Aldehydes Nomenclature of Alcohols Alcohols are compounds that have a hydroxyl group (-) bonded to a carbon atom (but
CEM 263 ct 11, 2016 Lecture utline 3: Alcohols, Ethers, Stereochemistry, Ketones, and Aldehydes Nomenclature of Alcohols Alcohols are compounds that have a hydroxyl group (-) bonded to a carbon atom (but
MASS SPECTROMETRY: BASIC EXPERIMENT
 http://science.widener.edu/svb/massspec/ei.html relative abundance Pavia 8.1-8.5 MASS SPECTROMETRY: BASIC EXPERIMENT scienceaid.co.uk -e Molecule Molecule +. + 2e base peak [Fragments] +. fragment peaks
http://science.widener.edu/svb/massspec/ei.html relative abundance Pavia 8.1-8.5 MASS SPECTROMETRY: BASIC EXPERIMENT scienceaid.co.uk -e Molecule Molecule +. + 2e base peak [Fragments] +. fragment peaks
Theophylline (TH), the structure of which is presented below, is a bronchial-dilator used for the treatment of asthma.
 1 EXAM SCIETIIC CULTURE CEMISTRY PRBLEM 1: Theophylline (T), the structure of which is presented below, is a bronchial-dilator used for the treatment of asthma. 3 C 7 1 3 9 1.1 Structural study and acid-base
1 EXAM SCIETIIC CULTURE CEMISTRY PRBLEM 1: Theophylline (T), the structure of which is presented below, is a bronchial-dilator used for the treatment of asthma. 3 C 7 1 3 9 1.1 Structural study and acid-base
DEVELOPMENT AND EVALUATION OF THE SAPRC-99 CHEMICAL MECHANISM
 DEVELOPMENT AND EVALUATION OF THE SAPRC-99 CHEMICAL MECHANISM W. P. L. Carter Air Pollution Research Center University of California, Riverside, CA 92521 ALL MECHANISMS DESIGN OBJECTIVES CAN BE USED IN
DEVELOPMENT AND EVALUATION OF THE SAPRC-99 CHEMICAL MECHANISM W. P. L. Carter Air Pollution Research Center University of California, Riverside, CA 92521 ALL MECHANISMS DESIGN OBJECTIVES CAN BE USED IN
Monoterpene and Sesquiterpene Emissions from Ponderosa Pine: Implications for Secondary Organic Aerosol Formation
 Monoterpene and Sesquiterpene Emissions from Ponderosa Pine: Implications for Secondary Organic Aerosol Formation Anita Lee, Gunnar Schade, Allen Goldstein UC Berkeley GCEP Workshop: August 19, 2002 What
Monoterpene and Sesquiterpene Emissions from Ponderosa Pine: Implications for Secondary Organic Aerosol Formation Anita Lee, Gunnar Schade, Allen Goldstein UC Berkeley GCEP Workshop: August 19, 2002 What
Atmospheric Chemistry Aqueous Phase Chemistry
 Atmospheric Chemistry Aqueous Phase Chemistry Final report Tel-Tek report no. 2211030-AQ05 v2 Hartmut Herrmann and Christian Weller 23.11.2011 Tel-Tek Kjølnes ring 30 NO-3918 Porsgrunn NORWAY About the
Atmospheric Chemistry Aqueous Phase Chemistry Final report Tel-Tek report no. 2211030-AQ05 v2 Hartmut Herrmann and Christian Weller 23.11.2011 Tel-Tek Kjølnes ring 30 NO-3918 Porsgrunn NORWAY About the
Carbonyl Group in Aldehydes and Ketones
 Lecture 4: Aldehydes, Ketones, and Chiral Molecules 14.1 Aldehydes and Ketones Carbonyl Group in Aldehydes and Ketones A carbonyl group (C=) In an aldehyde is attached to at least one atom. In a ketone
Lecture 4: Aldehydes, Ketones, and Chiral Molecules 14.1 Aldehydes and Ketones Carbonyl Group in Aldehydes and Ketones A carbonyl group (C=) In an aldehyde is attached to at least one atom. In a ketone
Subject Overview Curriculum pathway
 Subject Overview Curriculum pathway Course Summary Course: A Level Chemistry Overall Summary Unit / Module Exam / Controlled % of course UMS allocation Marks available UMS / RAW mark grade boundaries from
Subject Overview Curriculum pathway Course Summary Course: A Level Chemistry Overall Summary Unit / Module Exam / Controlled % of course UMS allocation Marks available UMS / RAW mark grade boundaries from
Physical Chemistry Chemical Physics
 Physical Chemistry Chemical Physics www.rsc.org/pccp Volume 13 Number 26 14 July 2011 Pages 12101 12336 ISSN 1463-9076 COVER ARTICLE Nizkorodov et al. Photolytic processing of secondary organic aerosols
Physical Chemistry Chemical Physics www.rsc.org/pccp Volume 13 Number 26 14 July 2011 Pages 12101 12336 ISSN 1463-9076 COVER ARTICLE Nizkorodov et al. Photolytic processing of secondary organic aerosols
CURRENT STATUS OF THE DEVELOPMENT AND EVALUATION OF AN UPDATED DETAILED MECHANISM FOR VOC OXIDATION
 CURRENT STATUS OF THE DEVELOPMENT AND EVALUATION OF AN UPDATED DETAILED MECHANISM FOR VOC OXIDATION William P. L. Carter Statewide Air Pollution Research Center and College of Engineering, Center for Environmental
CURRENT STATUS OF THE DEVELOPMENT AND EVALUATION OF AN UPDATED DETAILED MECHANISM FOR VOC OXIDATION William P. L. Carter Statewide Air Pollution Research Center and College of Engineering, Center for Environmental
A New Mechanism for Regional Atmospheric Chemistry Modelling. A contribution to subproject CMD. W.R. Stockwell', F Kirchner^ M. Kuhn' and S.
 A New Mechanism for Regional Atmospheric Chemistry Modelling A contribution to subproject CMD W.R. Stockwell', F Kirchner^ M. Kuhn' and S. Seefeld* *Fraunhofer Institute for Atmospheric Environmental Research
A New Mechanism for Regional Atmospheric Chemistry Modelling A contribution to subproject CMD W.R. Stockwell', F Kirchner^ M. Kuhn' and S. Seefeld* *Fraunhofer Institute for Atmospheric Environmental Research
CHEM 1412 SAMPLE FINAL EXAM
 CHEM 1412 SAMPLE FINAL EXAM PART I - Multiple Choice (2 points each) 1. In which colligative property(ies) does the value decrease as more solute is added? A. boiling point B. freezing point and osmotic
CHEM 1412 SAMPLE FINAL EXAM PART I - Multiple Choice (2 points each) 1. In which colligative property(ies) does the value decrease as more solute is added? A. boiling point B. freezing point and osmotic
Chapter 11. Introduction to Organic Chemistry
 hapter 11 Introduction to rganic hemistry Properties of arbon and its compounds 2 Properties of arbon and its compounds 3 Properties of arbon and its compounds 4 Properties of arbon and its compounds 5
hapter 11 Introduction to rganic hemistry Properties of arbon and its compounds 2 Properties of arbon and its compounds 3 Properties of arbon and its compounds 4 Properties of arbon and its compounds 5
1. What is the major organic product obtained from the following sequence of reactions?
 CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
Naming Organic Halides. Properties of Organic Halides
 Organic Compounds Organic Halides A hydrocarbon in which one or more hydrogen atoms have been replaced by halogen atoms Freons (chlorofluorocarbons) in refrigeration and air conditioning Teflon (polytetrafluoroethane)
Organic Compounds Organic Halides A hydrocarbon in which one or more hydrogen atoms have been replaced by halogen atoms Freons (chlorofluorocarbons) in refrigeration and air conditioning Teflon (polytetrafluoroethane)
2005 UPDATES TO THE CARBON BOND MECHANISM: CB05
 2005 UPDATES TO THE CARBON BOND MECHANISM: CB05 Gary Z. Whitten, Smog Reyes Greg Yarwood, ENVIRON smogreyes@yahoo.com International Conference on Chemical Mechanisms December 6, 2006 Ackowledgements EPA:
2005 UPDATES TO THE CARBON BOND MECHANISM: CB05 Gary Z. Whitten, Smog Reyes Greg Yarwood, ENVIRON smogreyes@yahoo.com International Conference on Chemical Mechanisms December 6, 2006 Ackowledgements EPA:
( -pinene) products. Rate coefficient data. Comments
 IUPAC Task Group on Atmospheric Chemical Kinetic Data Evaluation Data Sheet Hx_VC9 Datasheets can be downloaded for personal use only and must not be retransmitted or disseminated either electronically
IUPAC Task Group on Atmospheric Chemical Kinetic Data Evaluation Data Sheet Hx_VC9 Datasheets can be downloaded for personal use only and must not be retransmitted or disseminated either electronically
ORGANIC - BRUICE 8E CH MASS SPECT AND INFRARED SPECTROSCOPY
 !! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
!! www.clutchprep.com CONCEPT: PURPOSE OF ANALYTICAL TECHNIQUES Classical Methods (Wet Chemistry): Chemists needed to run dozens of chemical reactions to determine the type of molecules in a compound.
L.7. Mass Spectrum Interpretation
 L.7. Mass Spectrum Interpretation Fragmentation reactions Spectrum interpretation Confirmation of ion structural assignment Biomolecule dissociation Fragmentation reactions 1. Fragmentation reactions of
L.7. Mass Spectrum Interpretation Fragmentation reactions Spectrum interpretation Confirmation of ion structural assignment Biomolecule dissociation Fragmentation reactions 1. Fragmentation reactions of
(A) 10 (B) 10 (C) 140 (D) 140 (E) 70 7.Calculate the ionization energy, in kj/mol, of a mole of hydrogen atoms in the ground state.
 80 1.The term that describes the following series Ne, F -, 2-, and N 3- is (A) Isotopic (C) isobar anionic 2.Which oxide of chlorine is unstable? (B) isoelectronic allotropic (A) Cl 2 (B) Cl 2 3 (C) Cl
80 1.The term that describes the following series Ne, F -, 2-, and N 3- is (A) Isotopic (C) isobar anionic 2.Which oxide of chlorine is unstable? (B) isoelectronic allotropic (A) Cl 2 (B) Cl 2 3 (C) Cl
Abstract. 1 Introduction
 The influence of cloud chemistry on the budget of photo-oxidants G. Mauersberger Brandenberg Technical University Cottbus, Rudower Chausse 5, D-12489 Berlin, Germany Abstract The RADM II gas-phase system
The influence of cloud chemistry on the budget of photo-oxidants G. Mauersberger Brandenberg Technical University Cottbus, Rudower Chausse 5, D-12489 Berlin, Germany Abstract The RADM II gas-phase system
Nucleophiles: nucleus liking species. Nucleophilic substitution
 1 Nucleophiles: nucleus liking species Electron rich Possesses negative charge or non bonded valence electrons Large abundance of nucleophiles in the environment (water itself is a nucleophile) Nucleophilic
1 Nucleophiles: nucleus liking species Electron rich Possesses negative charge or non bonded valence electrons Large abundance of nucleophiles in the environment (water itself is a nucleophile) Nucleophilic
PAPER No.12 :Organic Spectroscopy MODULE No.30: Combined problem on UV, IR, 1 H NMR, 13 C NMR and Mass - Part II
 Subject Chemistry Paper No and Title Module No and Title Module Tag 12 : rganic Spectroscopy 30: Combined problem on UV, IR, 1 H NMR, 13 C NMR and Mass Part-II CHE_P12_M30 TABLE F CNTENTS 1. Learning utcomes
Subject Chemistry Paper No and Title Module No and Title Module Tag 12 : rganic Spectroscopy 30: Combined problem on UV, IR, 1 H NMR, 13 C NMR and Mass Part-II CHE_P12_M30 TABLE F CNTENTS 1. Learning utcomes
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
CHE 325 SPECTROSCOPY (A) CHAP 13A ASSIGN CH 2 CH CH 2 CH CHCH 3
 CE 325 SPECTRSCPY (A) CAP 13A ASSIGN 1. Which compound would have a UV absorption band at longest wavelength? A. I B. II C. III D. IV E. V C CC 3 CC C 2 C CC 3 I II III C 2 C C 2 C CC 3 IV V 2. Select
CE 325 SPECTRSCPY (A) CAP 13A ASSIGN 1. Which compound would have a UV absorption band at longest wavelength? A. I B. II C. III D. IV E. V C CC 3 CC C 2 C CC 3 I II III C 2 C C 2 C CC 3 IV V 2. Select
High-resolution mass spectrometric analysis of secondary organic aerosol produced by ozonation of limonenew
 PAPER www.rsc.org/pccp Physical Chemistry Chemical Physics High-resolution mass spectrometric analysis of secondary organic aerosol produced by ozonation of limonenew Maggie L. Walser, a Yury Desyaterik,
PAPER www.rsc.org/pccp Physical Chemistry Chemical Physics High-resolution mass spectrometric analysis of secondary organic aerosol produced by ozonation of limonenew Maggie L. Walser, a Yury Desyaterik,
Photolysis of Butenedial and 4-Oxo-2-pentenal Under Atmospheric Conditions
 Photolysis of Butenedial and 4-xo-2-pentenal Under Atmospheric onditions Gerard Rea, Lars Thüner and John Wenger entre for Research into Atmospheric hemistry (RA) Department of hemistry University ollege
Photolysis of Butenedial and 4-xo-2-pentenal Under Atmospheric onditions Gerard Rea, Lars Thüner and John Wenger entre for Research into Atmospheric hemistry (RA) Department of hemistry University ollege
Gas Particle Partitioning to OA
 Gas Particle Partitioning to OA Advanced Atmospheric chemistry CHEM 5152 Prof. J.L. Jimenez Last updated: Spring 2017 1 Gas Phase vs Aerosol Compounds For monofunctional compounds, alkanes beyond C 20
Gas Particle Partitioning to OA Advanced Atmospheric chemistry CHEM 5152 Prof. J.L. Jimenez Last updated: Spring 2017 1 Gas Phase vs Aerosol Compounds For monofunctional compounds, alkanes beyond C 20
Chapter 19: Alkenes and Alkynes
 Chapter 19: Alkenes and Alkynes The vast majority of chemical compounds that we know anything about and that we synthesize in the lab or the industrial plant are organic compounds. The simplest organic
Chapter 19: Alkenes and Alkynes The vast majority of chemical compounds that we know anything about and that we synthesize in the lab or the industrial plant are organic compounds. The simplest organic
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
PROBLEMS Sources of CO Sources of tropospheric ozone
 220 PROBLEMS 11. 1 Sources of CO The two principal sources of CO to the atmosphere are oxidation of CH 4 and combustion. Mean rate constants for oxidation of CH 4 and CO by OH in the troposphere are k
220 PROBLEMS 11. 1 Sources of CO The two principal sources of CO to the atmosphere are oxidation of CH 4 and combustion. Mean rate constants for oxidation of CH 4 and CO by OH in the troposphere are k
3) Oxidation of tertiary alcohol yields A) Aldehyde B) No reaction C) Ketone D) Carboxylic acid
 ALKYL HALIDES 18- The reaction of Propyl bromide with Na is A) Nucleophilic addition. B) Nucleophilic substitution. C) Electrophilic substitution. D) Electrophilic addition. 25) Which of the following
ALKYL HALIDES 18- The reaction of Propyl bromide with Na is A) Nucleophilic addition. B) Nucleophilic substitution. C) Electrophilic substitution. D) Electrophilic addition. 25) Which of the following
(Neither an oxidation or reduction: Addition or loss of H +, H 2 O, HX).
 eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
Structural Determination Of Compounds
 EXPERIMENT 10 Mass Spectroscopy Structural Determination Of Compounds. Introduction - In mass spectrometry, a substance is bombarded with an electron beam having sufficient energy to fragment the molecule.
EXPERIMENT 10 Mass Spectroscopy Structural Determination Of Compounds. Introduction - In mass spectrometry, a substance is bombarded with an electron beam having sufficient energy to fragment the molecule.
Chemistry 110. Bettelheim, Brown, Campbell & Farrell. Ninth Edition
 Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, rganic and Biochemistry Chapter 14 Alcohols, Ethers and Thiols Alcohols have a ydroxyl Group, -, bonded to tetrahedral
Chemistry 110 Bettelheim, Brown, Campbell & Farrell Ninth Edition Introduction to General, rganic and Biochemistry Chapter 14 Alcohols, Ethers and Thiols Alcohols have a ydroxyl Group, -, bonded to tetrahedral
1. Following are structural formulas for two steroid hormones.
 CHEM 122: Introduction to rganic Chemistry Chapter 9: Aldehydes and Ketones. 1. Following are structural formulas for two steroid hormones. Cortisone Aldosterone Name the functional groups in each. b)
CHEM 122: Introduction to rganic Chemistry Chapter 9: Aldehydes and Ketones. 1. Following are structural formulas for two steroid hormones. Cortisone Aldosterone Name the functional groups in each. b)
ATMOSPHERIC CHEMISTRY OF SELECTED HYDROXYCARBONYLS. Sara M. Aschmann, Janet Arey and Roger Atkinson
 ATMOSPHERIC CHEMISTRY OF SELECTED HYDROXYCARBONYLS Sara M. Aschmann, Janet Arey and Roger Atkinson Air Pollution Research Center University of California Riverside, CA 92521, U.S.A. Introduction Volatile
ATMOSPHERIC CHEMISTRY OF SELECTED HYDROXYCARBONYLS Sara M. Aschmann, Janet Arey and Roger Atkinson Air Pollution Research Center University of California Riverside, CA 92521, U.S.A. Introduction Volatile
Multistep Synthesis of 5-isopropyl-1,3-cyclohexanedione
 Multistep Synthesis of 5-isopropyl-1,3-cyclohexanedione The purpose of this experiment was to synthesize 5-isopropyl-1,3-cyclohexanedione from commercially available compounds. To do this, acetone and
Multistep Synthesis of 5-isopropyl-1,3-cyclohexanedione The purpose of this experiment was to synthesize 5-isopropyl-1,3-cyclohexanedione from commercially available compounds. To do this, acetone and
GCE A level 1094/01 CHEMISTRY CH4
 Surname ther Names Centre 2 Candidate GCE A level 1094/01 CHEMISTRY CH4 P.M. MNDAY, 14 January 2013 1¾ hours ADDITINAL MATERIALS In addition to this examination paper, you will need: Data Sheet Periodic
Surname ther Names Centre 2 Candidate GCE A level 1094/01 CHEMISTRY CH4 P.M. MNDAY, 14 January 2013 1¾ hours ADDITINAL MATERIALS In addition to this examination paper, you will need: Data Sheet Periodic
Aldehydes and Ketones Reactions. Dr. Sapna Gupta
 Aldehydes and Ketones Reactions Dr. Sapna Gupta Reactions of Aldehydes and Ketones Nucleophilic Addition A strong nucleophile attacks the carbonyl carbon, forming an alkoxide ion that is then protonated.
Aldehydes and Ketones Reactions Dr. Sapna Gupta Reactions of Aldehydes and Ketones Nucleophilic Addition A strong nucleophile attacks the carbonyl carbon, forming an alkoxide ion that is then protonated.
Ch 22 Carbonyl Alpha ( ) Substitution
 Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Write your name and date on the cover page Do not open exam until instructed to do so
 Write your name and date on the cover page Do not open exam until instructed to do so Name: Date: Exam III hem. 210 Do not open exam until told to do so. Get out your pencil, eraser, and scientific nongraphing
Write your name and date on the cover page Do not open exam until instructed to do so Name: Date: Exam III hem. 210 Do not open exam until told to do so. Get out your pencil, eraser, and scientific nongraphing
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 Ch16_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which type of compound does not contain a carbonyl group? ketone B) aldehyde C) amine D)
Ch16_PT MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which type of compound does not contain a carbonyl group? ketone B) aldehyde C) amine D)
Gas, Cloudwater, and Rain Hydrogen Peroxide and Methylhydroperoxide Measurements in RICO
 Gas, Cloudwater, and Rain Hydrogen Peroxide and Methylhydroperoxide Measurements in RICO Brian G. Heikes, Center for Atmospheric Chemical Studies, Graduate School of Oceanography, University of Rhode Island
Gas, Cloudwater, and Rain Hydrogen Peroxide and Methylhydroperoxide Measurements in RICO Brian G. Heikes, Center for Atmospheric Chemical Studies, Graduate School of Oceanography, University of Rhode Island
Chapter 8: Alkene Structure and Preparation via Elimination Reactions
 1. Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C bond length bond strength 3 C C 3 3 C C 3 3 C C 3 3 C 2 C C 2 3 C a C=C double bond
1. Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C bond length bond strength 3 C C 3 3 C C 3 3 C C 3 3 C 2 C C 2 3 C a C=C double bond
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
Chapter 16. Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group. Physical Properties of Aldehydes and Ketones. Synthesis of Aldehydes
 Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS
 AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
Molecular Spectroscopy. H 2 O e -
 Molecular Spectroscopy ν (cm -1 ) λ (cm) 10 6 10 8 10 10 10 12 10 14 10 16 10 18 10 20 10 22 ν (Hz) NMR ESR microwave IR UV/Vis VUV X-Ray Gamma Ray H 2 e - UV/Vis Spectroscopy absorption technique X hν
Molecular Spectroscopy ν (cm -1 ) λ (cm) 10 6 10 8 10 10 10 12 10 14 10 16 10 18 10 20 10 22 ν (Hz) NMR ESR microwave IR UV/Vis VUV X-Ray Gamma Ray H 2 e - UV/Vis Spectroscopy absorption technique X hν
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Carbonyl Group. Chemistry 121(01) Winter Chapter 15: Aldehyde and Ketones. Chapter 15: Aldehyde and Ketones
 Chemistry 121(01) Winter 2012 Introduction to rganic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. hio State) E-mail: upali@chem.latech.edu ffice: 311 Carson Taylor Hall ; Phone: 318-257-4941;
Chemistry 121(01) Winter 2012 Introduction to rganic Chemistry and Biochemistry Instructor Dr. Upali Siriwardane (Ph.D. hio State) E-mail: upali@chem.latech.edu ffice: 311 Carson Taylor Hall ; Phone: 318-257-4941;
CAPRAM 2.4 (MODAC mechanism): An extended and condensed tropospheric aqueous phase mechanism and its application
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D14, 4426, doi:10.1029/2002jd002202, 2003 CAPRAM 2.4 (MODAC mechanism): An extended and condensed tropospheric aqueous phase mechanism and its application
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 108, NO. D14, 4426, doi:10.1029/2002jd002202, 2003 CAPRAM 2.4 (MODAC mechanism): An extended and condensed tropospheric aqueous phase mechanism and its application
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2013 Dr. Rainer Glaser
 Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2013 Dr. Rainer Glaser Examination #4 Enols and Aldol Reaction, Carboxylic Acids and Carboxylic Acid Derivatives. Thursday, November 14, 2013,
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2013 Dr. Rainer Glaser Examination #4 Enols and Aldol Reaction, Carboxylic Acids and Carboxylic Acid Derivatives. Thursday, November 14, 2013,
Reaction mechanism 1/17/19. HCl. HCl. 3 radical can form here. Br 2. hint!
 If the reagents or reaction conditions favor radicals, then it is likely to be a radical reaction. Look for hints like peroxide, AIBN, NBS, hn (UV light). 3 radical can form here Reaction mechanism How
If the reagents or reaction conditions favor radicals, then it is likely to be a radical reaction. Look for hints like peroxide, AIBN, NBS, hn (UV light). 3 radical can form here Reaction mechanism How
Lecture notes in EI-Mass spectrometry. By Torben Lund
 1 Lecture notes in EI-Mass spectrometry By Torben Lund RUC 2015 1. Basic advise: 1) Identify the mole peak M +. 2) Nitogen rule: Determine number of N 3) A+2 elements: Cl, Br, S, Si 4) N C = 100 [M+1]/([M]
1 Lecture notes in EI-Mass spectrometry By Torben Lund RUC 2015 1. Basic advise: 1) Identify the mole peak M +. 2) Nitogen rule: Determine number of N 3) A+2 elements: Cl, Br, S, Si 4) N C = 100 [M+1]/([M]
Worksheet Chapter 10: Organic chemistry glossary
 Worksheet 10.1 Chapter 10: Organic chemistry glossary Addition elimination reaction A reaction in which two molecules combine with the release of a small molecule, often water. This type of reaction is
Worksheet 10.1 Chapter 10: Organic chemistry glossary Addition elimination reaction A reaction in which two molecules combine with the release of a small molecule, often water. This type of reaction is
Peter J. Gallimore et al. Correspondence to: Markus Kalberer
 Supplement of Atmos. Chem. Phys., 17, 983 9868, 2017 https://doi.org/.194/acp-17-983-2017-supplement Author(s) 2017. This work is distributed under the Creative Commons Attribution 3.0 License. Supplement
Supplement of Atmos. Chem. Phys., 17, 983 9868, 2017 https://doi.org/.194/acp-17-983-2017-supplement Author(s) 2017. This work is distributed under the Creative Commons Attribution 3.0 License. Supplement
Chapter 8: Alkene Structure and Preparation via Elimination Reactions
 Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C 3 C C 3 bond length bond strength 2 C C 2 a C=C double bond is stronger than a C C single
Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C 3 C C 3 bond length bond strength 2 C C 2 a C=C double bond is stronger than a C C single
Supporting Information
 Supporting Information Reactive processing of formaldehyde and acetaldehyde in aqueous aerosol mimics: Surface tension depression and secondary organic products Zhi Li, Allison N. Schwier, Neha Sareen,
Supporting Information Reactive processing of formaldehyde and acetaldehyde in aqueous aerosol mimics: Surface tension depression and secondary organic products Zhi Li, Allison N. Schwier, Neha Sareen,
Supplemental material
 Supplemental material Supplemental discussion of internal and its possible removal by C 3 F 6 addition chem represents the real and wave-chem represents an instrument interference only if no internal is
Supplemental material Supplemental discussion of internal and its possible removal by C 3 F 6 addition chem represents the real and wave-chem represents an instrument interference only if no internal is
Chapter Four: Laboratory Characterisation of the Aerodyne Aerosol Mass Spectrometer
 24 PhD Thesis 79 Chapter Four: Laboratory Characterisation of the Aerodyne Aerosol Mass Spectrometer Laboratory characterisation of the Aerodyne aerosol mass spectrometer has been an important part of
24 PhD Thesis 79 Chapter Four: Laboratory Characterisation of the Aerodyne Aerosol Mass Spectrometer Laboratory characterisation of the Aerodyne aerosol mass spectrometer has been an important part of
