Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2013 Dr. Rainer Glaser
|
|
|
- Brice Chapman
- 5 years ago
- Views:
Transcription
1 Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2013 Dr. Rainer Glaser Examination #4 Enols and Aldol Reaction, Carboxylic Acids and Carboxylic Acid Derivatives. Thursday, November 14, 2013, 8:25-9:15 am Name: Question 1. Enols and Aldol Reactions 20 Question 2. Carboxylic Acids 20 Question 3. Carboxylic Acid Esters 20 Question 4. Carboxylic Acid Halides and Anhydrides 20 Question 5. Carboxylic Acid Amides 20 Total 100 ALLOWED: Periodic System of the Elements (printed, w/o handwriting on it). Molecular models (you can bring pre-made models). Simple, non-programmable calculator (not really needed). NOT ALLOWED: Books. Notes. Electronic devices of any kind (other than a simple calculator). 1
2 Question 1. Enols and Aldol Reactions. (20 points) (a) Butanal (H 3 C CH 2 CH 2 CHO) is an enolizable aldehyde. Draw the two important resonance forms of the anion formed by deprotonation of butanal. Show all lone pairs and formal charges; use the correct resonance arrow. Use curved arrows to show the electron flow that converts one resonance forms into the other. (6 points) (b) Draw the structure of the mosquito repellent 2-ethylhexane-1,3-diol (a.k.a ). This pesticide can be prepared in two steps beginning with butanal. The first reaction is a base-catalyzed aldol reaction of butanal. Provide structural formula of butanal aldol. The second reaction is a and requires a catalyst such as. (6 points) 6-12, 2-ethylhexane-1,3-diol Butanal Aldol (c) Cinnamaldehyde (H 5 C 6 CH=CH CHO) and dibenzalacetone (H 5 C 6 CH=CH CO CH=CH C 6 H 5 ) can be prepared in two-step sequences consisting of an aldol reaction and thermal condensation. These syntheses involve a aldol reaction between the non-enolizable aldehyde with either the enolizable aldehyde or the enolizable ketone. (4 points) (d) Draw the structure of the product of the aldol condensation reaction of cinnamaldehyde with acetone in the presence of NaOH. (4 points) Expected Product 2
3 Question 2. Carboxylic Acids. (20 points) (a) Carboxylic acids form strongly bonded dimeric aggregates (homodimers). The electrostatic potential surface plot is shown of the homodimer of formic acid (HCOOH)2. In very dilute solution, formic acid will be monomeric and form hydrogen bonds with water molecules. One structure (HCOOH)(H2O) is particularly interesting because the water molecule acts both as HB-donor and HB acceptor to the same carboxylic acid and the electrostatic potential surface plot of this aggregate also is shown. Draw the complete structural formula of both aggregates in the space above the plot. Your structure drawing should be aligned as much as possible with the orientation of the molecules in the ESP plots. Indicate H-bonds as dashed lines. (8 points) (b) Syntheses of carboxylic acids. Provide substrate, reagent or product as requested. (12 points) Product formed by oxidation of Product formed by oxidation of o-xylene, with hot KMnO4: propylbenzene, C6H5 C3H7 with hot KMnO4: Reagent used for the oxidation of glyoxal, OHC CHO to oxalic acid: Reagent that reacts with the Grignard reagent formed from tert-butylbromide (H3C)3CBr to form (H3C)3C COOH after workup with dilute acid: 3
4 Question 3. Carboxylic Acid Esters. (20 points) (a) Ethyl acetate H 3 C CO O CH 2 CH 3 can be made by Fischer esterification of the carboxylic acid (formic acid, acetic acid, benzoic acid) and the alcohol (methanol, ethanol, phenol). Consider the mechanism of the formation of ethyl acetate by Fischer esterification, that is, the ester formation catalyzed by a strong mineral acid. Draw the structural formulas of the species indicated (show all lone pairs and formal charges). You may abbreviate the methyl group as H 3 C or as Me and the ethyl group as H 5 C 2 or as Et. (14 points) 1: Protonated carboxylic acid, i.e., the actual substrate for alcohol addition 2: Product formed by addition of alcohol to the protonated carboxylic acid 3: Neutral gem tri-functional intermediate 4: Protonated gem tri-functional intermediate that will lead to water elimination (b) γ-hydroxybutyric acid (GHB), a.k.a. Lollipops, Liquid Ecstasy, Liquid X, Liquid G, and Fantasy, is one of the three most common date rape drugs. GHB is easily synthesized by reaction of γ-butyrolactone (GBL) with NaOH. GBL itself is a prodrug because it is converted to GHB in vivo. Draw the structural formulas of the species indicate (show lone pairs, formal charges). (6 points) Structure of GHB Geminal tri-functional intermediate in the ester hydrolysis of GBL with NaOH Structure of GBL 4
5 Question 4. Carboxylic Acid Halides and Anhydrides. (20 points) (a) Consider the preparation of acetyl chloride from acetic acid using (PCl 3, PCl 4, PCl 5, (HO) 3 PCl 2, (HO) 2 PCl 3, OPCl 3 ). Draw structural formulas of the substrate, of the reagent, of the organic product (the acetyl chloride), and also of the inorganic products. (8 points) (b) Succinic acid is the trivial name of butanedioic acid (note butane ). Succinic anhydride is the intramolecular anhydride of succinic acid. Draw the structure of succinic anhydride in the box to the right. (2 points) (c) Malic acid is the trivial name of Z-butenedioic acid (note butene ). Draw the structure of malic anhydride in the box to the right. Maleic anhydride (is, is not) an intramolecular anhydride. (2 points) (d) Fumaric acid is the trivial name of E-butenedioic acid (note butene ). Draw the structure of fumaric anhydride in the box to the right. Fumaric anhydride (is, is not) an intramolecular anhydride. (2 points) (e) Suggest a synthesis of benzoic acetic anhydride. Your synthesis should result only in the mixed anhydride without formations of acetic anhydride or benzoic anhydride. Draw structural formulas of the substrates and of the organic product (the mixed anhydride). (6 points) 5
6 Question 5. Carboxylic Acid Amides. (20 points) (a) Consider the electronic structure of N-methylacetamide, H 3 C CO NH CH 3. This amide is a very simple model of a peptide bond. Provide complete Lewis-Kekule structures of the major resonance forms of the molecule and indicate their relative contributions (i.e., minor or major ). Specify the preferred N-hybridization for each of the resonance forms. Discuss whether the amino group is pyramidal or whether it is planar. (8 points) (b) Draw the structure of the product of hydrolysis of alanine dipeptide, HOOC CH(CH 3 ) NH CO CH(CH 3 ) NH 2 in the presence of NaOH. [Hint: Account for salt formation.] (4 points) (c) Consider the formation of N,N-dimethylacetamide, H 3 C CO N(CH 3 ) 2 by reaction of acetic anhydride with dimethylamine. Two molecules of the amine are required for every molecule of acetic anhydride used. Show reaction equations for the reaction A of the first amine with the anhydride and for the reaction B of the second amine with the formed in the preceding reaction. (8 points) 6
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser
 Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Thursday, November 15, 2012, 8:25 9:15 am Name: Question 1. Aldehydes
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Thursday, November 15, 2012, 8:25 9:15 am Name: Question 1. Aldehydes
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser
 Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Wednesday, November 16, 2011, 10 10:50 am Name: Answer Key Question 1.
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Wednesday, November 16, 2011, 10 10:50 am Name: Answer Key Question 1.
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser
 Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Make-Up Carbonyl Compounds and Amines. Wednesday, November 30, 2011, 10 10:50 am Name: Answer Key Question
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Make-Up Carbonyl Compounds and Amines. Wednesday, November 30, 2011, 10 10:50 am Name: Answer Key Question
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser
 Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Practice Edition Carbonyl Compounds and Amines. Wednesday, November 16, 2011, 10 10:50 am Name: Question
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Practice Edition Carbonyl Compounds and Amines. Wednesday, November 16, 2011, 10 10:50 am Name: Question
Chemistry 2030, FS17, Dr. Rainer Glaser Introduction to Organic Chemistry
 Chemistry 2030, FS17, Dr. Rainer Glaser Introduction to Organic Chemistry Examination #4 Aldehydes & Ketones, Carboxylic Acids & Carboxylic Acid Derivatives, Lipids & Detergents, and Amines. Handout: Tuesday,
Chemistry 2030, FS17, Dr. Rainer Glaser Introduction to Organic Chemistry Examination #4 Aldehydes & Ketones, Carboxylic Acids & Carboxylic Acid Derivatives, Lipids & Detergents, and Amines. Handout: Tuesday,
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2017 Dr. Rainer Glaser
 Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2017 Dr. Rainer Glaser Examination #3 Nucleophilic Substitutions & Eliminations, Alcohols, and Ethers Thursday, November 2, 2017, 8:25-9:15
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2017 Dr. Rainer Glaser Examination #3 Nucleophilic Substitutions & Eliminations, Alcohols, and Ethers Thursday, November 2, 2017, 8:25-9:15
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser
 Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser Examination #3 Nucleophilic Substitutions & Eliminations, Alcohols, and Ethers Thursday, November 5, 2015, 8:25-9:15
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser Examination #3 Nucleophilic Substitutions & Eliminations, Alcohols, and Ethers Thursday, November 5, 2015, 8:25-9:15
Ch 20 Carboxylic Acids and Nitriles
 Ch 20 Carboxylic Acids and Nitriles Carboxylic Acids (RCO 2 H) are compounds with an OH attached to a carbonyl. Nitriles (RC N) are compounds a carbon-nitrogen triple bond. Naming Carboxylic Acids 1. Replace
Ch 20 Carboxylic Acids and Nitriles Carboxylic Acids (RCO 2 H) are compounds with an OH attached to a carbonyl. Nitriles (RC N) are compounds a carbon-nitrogen triple bond. Naming Carboxylic Acids 1. Replace
NH 2 O O O O OCH (6 pts) Provide an acceptable name for each of the following compounds: NH 2 COOH HOOC COOH. piperidine
 CEMISTRY 314-01 MIDTERM # 4 April 1, 2003 Statistics: Average: 87 pts (72%); ighest: 116 pts (97%); Lowest: 49 pts (41%) umber of students performing at or above average: 13 (62%) 1. (9 pts) Mark as true
CEMISTRY 314-01 MIDTERM # 4 April 1, 2003 Statistics: Average: 87 pts (72%); ighest: 116 pts (97%); Lowest: 49 pts (41%) umber of students performing at or above average: 13 (62%) 1. (9 pts) Mark as true
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser
 Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #3 Alcohols, Ethers, Aldehydes & Ketones. Wednesday, October 26, 2011, 10 10:50 am Name: Question 1. Names,
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #3 Alcohols, Ethers, Aldehydes & Ketones. Wednesday, October 26, 2011, 10 10:50 am Name: Question 1. Names,
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser
 Chemistry 2030 Survey of Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser Examination #2 Reactions of Alkenes & Alkynes, Chemistry of Aromatic Compounds, and Stereochemistry Thursday, October 8,
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser Examination #2 Reactions of Alkenes & Alkynes, Chemistry of Aromatic Compounds, and Stereochemistry Thursday, October 8,
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser
 Chemistry 2030 Survey of Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser Examination #2 Reactions of Alkenes & Alkynes, Chemistry of Aromatic Compounds, and Stereochemistry Thursday, October 8,
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser Examination #2 Reactions of Alkenes & Alkynes, Chemistry of Aromatic Compounds, and Stereochemistry Thursday, October 8,
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2013 Dr. Rainer Glaser
 Chemistry 2030 Survey of Organic Chemistry Fall Semester 2013 Dr. Rainer Glaser Examination #1 Bonding, Alkanes, Alkenes & Alkynes Thursday, September 12, 2013, 8:00 8:50 am Question 1. Atomic Structure
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2013 Dr. Rainer Glaser Examination #1 Bonding, Alkanes, Alkenes & Alkynes Thursday, September 12, 2013, 8:00 8:50 am Question 1. Atomic Structure
CHM 292 Final Exam Answer Key
 CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
CHM 292 Final Exam Answer Key 1. Predict the product(s) of the following reactions (5 points each; 35 points total). May 7, 2013 Acid catalyzed elimination to form the most highly substituted alkene possible
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2014 Dr. Rainer Glaser
 Chemistry 2030 Survey of Organic Chemistry Fall Semester 2014 Dr. Rainer Glaser Examination #1 Bonding, Alkanes, Alkenes & Alkynes Thursday, September 18, 2014, 8:00 8:50 am Question 1. Atomic Structure
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2014 Dr. Rainer Glaser Examination #1 Bonding, Alkanes, Alkenes & Alkynes Thursday, September 18, 2014, 8:00 8:50 am Question 1. Atomic Structure
CHEM Chapter 21. Carboxylic Acid Derivatives_Nucleophilic Acyl Substitution Reactions (homework) W
 Short Answer IUPAC Naming Instructions: Provide proper IUPAC names. 1. Name: 2. Name: 3. Name: Drawing Instructions: Draw structures corresponding to each of the given names. 4. Draw: acetic formic anhydride
Short Answer IUPAC Naming Instructions: Provide proper IUPAC names. 1. Name: 2. Name: 3. Name: Drawing Instructions: Draw structures corresponding to each of the given names. 4. Draw: acetic formic anhydride
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 20: Carboxylic Acids
 1 Chapter 20: Carboxylic Acids I. Introduction: Carboxylic acid structure: Classification of carboxylic acids: A carboxylic acid donates protons by the heterocyclic cleavage of the O-H bond, generating
1 Chapter 20: Carboxylic Acids I. Introduction: Carboxylic acid structure: Classification of carboxylic acids: A carboxylic acid donates protons by the heterocyclic cleavage of the O-H bond, generating
Chem 263 March 28, 2006
 Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
ALCOHOLS AND PHENOLS
 ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
ALCOHOLS AND PHENOLS ALCOHOLS AND PHENOLS Alcohols contain an OH group connected to a a saturated C (sp3) They are important solvents and synthesis intermediates Phenols contain an OH group connected to
CHEM 343 Principles of Organic Chemistry II Summer Instructor: Paul J. Bracher. Quiz # 3. Monday, July 21 st, :30 a.m.
 CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2017 Dr. Rainer Glaser
 Chemistry 2030 Survey of Organic Chemistry Fall Semester 2017 Dr. Rainer Glaser Examination #2 Reactions of Alkenes & Alkynes, Chemistry of Aromatic Compounds, and Stereochemistry Thursday, October 5,
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2017 Dr. Rainer Glaser Examination #2 Reactions of Alkenes & Alkynes, Chemistry of Aromatic Compounds, and Stereochemistry Thursday, October 5,
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
ORGANIC CHEMISTRY II
 ORGANIC EMISTRY II. CARBOXYLIC ACIDS Saturated mono carboxylic s are called fatty s. group is COOH which is made up of carbonyl and hydroxy groups. General molecular formula is CnH n O or C n H n+1 COOH.
ORGANIC EMISTRY II. CARBOXYLIC ACIDS Saturated mono carboxylic s are called fatty s. group is COOH which is made up of carbonyl and hydroxy groups. General molecular formula is CnH n O or C n H n+1 COOH.
Chemistry 210 Organic Chemistry I Fall Semester 2000 Dr. Rainer Glaser
 Chemistry 210 Organic Chemistry I Fall Semester 2000 Dr. Rainer Glaser Examination #3 Alkenes and Alkynes. Structure, Synthesis and Reactions. Friday, November 17, 2000, 9:00-9:50 Name: Question 1. Alkenes
Chemistry 210 Organic Chemistry I Fall Semester 2000 Dr. Rainer Glaser Examination #3 Alkenes and Alkynes. Structure, Synthesis and Reactions. Friday, November 17, 2000, 9:00-9:50 Name: Question 1. Alkenes
Alkyl phenyl ketones are usually named by adding the acyl group as prefix to phenone.
 Aldehydes, Ketones and Carboxylic Acids Nomenclature of aldehydes and ketones Aldehydes: Often called by their common names instead of IUPAC names. Ketones: Derived by naming two alkyl or aryl groups bonded
Aldehydes, Ketones and Carboxylic Acids Nomenclature of aldehydes and ketones Aldehydes: Often called by their common names instead of IUPAC names. Ketones: Derived by naming two alkyl or aryl groups bonded
CH 320/328 N Summer II 2018
 CH 320/328 N Summer II 2018 HW 1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. (5 pts each) 1. Which
CH 320/328 N Summer II 2018 HW 1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. (5 pts each) 1. Which
CHEM 343 Principles of Organic Chemistry II Summer Instructor: Paul J. Bracher. Quiz # 3. Monday, July 21 st, :30 a.m.
 CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Problem Booklet Page 1 of 7 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21
CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Problem Booklet Page 1 of 7 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21
1. What is the major organic product obtained from the following sequence of reactions?
 CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
Lecture 3: Aldehydes and ketones
 Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Carboxylic Acids and Nitriles
 Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Carboxylic Acids and Nitriles Why this Chapter? Carboxylic acids present in many industrial processes and most biological processes They are the starting materials from which other acyl derivatives are
Chapter 1 Reactions of Organic Compounds. Reactions Involving Hydrocarbons
 Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chem 263 Nov 24, Properties of Carboxylic Acids
 Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
COURSE UNIT DESCRIPTION. Dept. Organic Chemistry, Vilnius University. Type of the course unit
 Course unit title Organic Chemistry II Lecturer(s) Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery Period
Course unit title Organic Chemistry II Lecturer(s) Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery Period
Assessment Schedule 2016 Chemistry: Demonstrate understanding of the properties of organic compounds (91391)
 NCEA Level 3 Chemistry (91391) 2016 page 1 of 6 Assessment Schedule 2016 Chemistry: Demonstrate understanding of the properties of organic compounds (91391) Evidence Statement Q Evidence Achievement Achievement
NCEA Level 3 Chemistry (91391) 2016 page 1 of 6 Assessment Schedule 2016 Chemistry: Demonstrate understanding of the properties of organic compounds (91391) Evidence Statement Q Evidence Achievement Achievement
N_HW1 N_HW1. 1. What is the purpose of the H 2 O in this sequence?
 N_HW1 N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. 1. What is the purpose of the H 2 O in this
N_HW1 N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. 1. What is the purpose of the H 2 O in this
CATEDRA DE BIOCHIMIE ȘI BIOCHIMIE CLINICA INDICAȚIE METODICA FACULTATEA SĂNĂTATE PUBLICĂ, ANUL I Pag. 1 / 6
 FACULTATEA SĂNĂTATE PUBLICĂ, ANUL I Pag. 1 / 6 Analizată și aprobată la ședința catedrei din, proces verbal nr șeful catedrei de Biochimie și Biochimie Clinică, conferențiar universitar, doctor habilitat
FACULTATEA SĂNĂTATE PUBLICĂ, ANUL I Pag. 1 / 6 Analizată și aprobată la ședința catedrei din, proces verbal nr șeful catedrei de Biochimie și Biochimie Clinică, conferențiar universitar, doctor habilitat
The carbonyl C = O H C = O C = O O R C - O. acetone. aldehyde. ketone H + Carboxylic acids. d - d + Phosgene! COCl 2 Chemical weapon
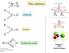 d + d - C = O The carbonyl acetone H C = O aldehyde R R C = O ketone R O R C - O Paolo Sarti 2011 Department of Biochemistry Sapienza H + Carboxylic acids Phosgene! COCl 2 Chemical weapon Paolo Sarti 2011
d + d - C = O The carbonyl acetone H C = O aldehyde R R C = O ketone R O R C - O Paolo Sarti 2011 Department of Biochemistry Sapienza H + Carboxylic acids Phosgene! COCl 2 Chemical weapon Paolo Sarti 2011
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
For more info visit Aldehyde and Ketones. Preparation of Aldehydes. a. Oxidation of primary alcohols
 Aldehyde and Ketones Preparation of Aldehydes a. Oxidation of primary alcohols Preparation of Ketones: a) Oxidation of Secondary alcohols: d) With Organometallics Reactions of Aldehydes and Ketones: a)
Aldehyde and Ketones Preparation of Aldehydes a. Oxidation of primary alcohols Preparation of Ketones: a) Oxidation of Secondary alcohols: d) With Organometallics Reactions of Aldehydes and Ketones: a)
C h a p t e r T w e n t y : Carboxylic Acids & Their Derivatives
 C h a p t e r T w e n t y : Carboxylic Acids & Their Derivatives N C3 N Lysergic acid, the depressant used to make LSD, the famous acid of the 1960s LSD and the Search for God, a San Francisco-based band
C h a p t e r T w e n t y : Carboxylic Acids & Their Derivatives N C3 N Lysergic acid, the depressant used to make LSD, the famous acid of the 1960s LSD and the Search for God, a San Francisco-based band
Spring Term 2012 Dr. Williams (309 Zurn, ex 2386)
 Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
Chemistry 242 Organic Chemistry II Spring Term 2012 Dr. Williams (309 Zurn, ex 2386) Web Page: http://math.mercyhurst.edu/~jwilliams/ jwilliams@mercyhurst.edu (or just visit Department web site and look
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2013 Dr. Rainer Glaser
 Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2013 Dr. Rainer Glaser Examination #5: The Final Amines, Lipids, Carbohydrates, and Nucleobases Wednesday, December 11, 2013, 7:30 9:30 am.
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2013 Dr. Rainer Glaser Examination #5: The Final Amines, Lipids, Carbohydrates, and Nucleobases Wednesday, December 11, 2013, 7:30 9:30 am.
Chem 263 Nov 14, e.g.: Fill the reagents to finish the reactions (only inorganic reagents)
 hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Chapter 19. Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions. ß-dicarbonyl compounds. Why are ß-dicarbonyls useful?
 Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
2. An aldehyde can be obtained by the dehydrogenation of an alcohol. The catalyst used in the reaction is
 Class: 12 Subject: Chemistry Topic: Organic Chemistry of O compounds No. of Questions: 20 Duration: 60 Min Maximum Marks: 60 1. Rectified spirit is converted to absolute alcohol taking advantage of the
Class: 12 Subject: Chemistry Topic: Organic Chemistry of O compounds No. of Questions: 20 Duration: 60 Min Maximum Marks: 60 1. Rectified spirit is converted to absolute alcohol taking advantage of the
But in organic terms: Oxidation: loss of H 2 ; addition of O or O 2 ; addition of X 2 (halogens).
 Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
Reactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. Normally: Oxidation
Class: 12 Subject: chemistry Topic: Organic Chemistry of O compounds No. of Questions: 20 Duration: 60 Min Maximum Marks: 60
 Class: 12 Subject: chemistry Topic: Organic Chemistry of O compounds No. of Questions: 20 Duration: 60 Min Maximum Marks: 60 1. Ethylene is passed through conc. H 2 SO 4 and the product obtained is diluted
Class: 12 Subject: chemistry Topic: Organic Chemistry of O compounds No. of Questions: 20 Duration: 60 Min Maximum Marks: 60 1. Ethylene is passed through conc. H 2 SO 4 and the product obtained is diluted
Carboxylic Acids O R C + H + O - Chemistry 618B
 arboxylic Acids R H R + H + - R - Nomenclature - IUPA IUPA names: drop the -e from the parent alkane and add the suffix -oic acid If the compound contains a carbon-carbon double bond, change the infix
arboxylic Acids R H R + H + - R - Nomenclature - IUPA IUPA names: drop the -e from the parent alkane and add the suffix -oic acid If the compound contains a carbon-carbon double bond, change the infix
Chemistry 212 and 300 Organic Chemistry II Winter Semester 2000 Dr. Rainer Glaser
 Chemistry 212 and 300 rganic Chemistry II Winter Semester 2000 Dr. Rainer Glaser Examination #2 Amines, Carboxylic Acids & Carboxylic Acid Derivatives Thursday, March 9, 2000, 9:00-9:50 Your Name: Answer
Chemistry 212 and 300 rganic Chemistry II Winter Semester 2000 Dr. Rainer Glaser Examination #2 Amines, Carboxylic Acids & Carboxylic Acid Derivatives Thursday, March 9, 2000, 9:00-9:50 Your Name: Answer
CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher. Quiz # 4. Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m.
 CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser
 Chemistry 2030 Survey of Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser Examination #1 Bonding, Alkanes, Alkenes & Alkynes Thursday, September 17, 2015, 8:25 9:15 am Question 1. Atomic Structure,
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2015 Dr. Rainer Glaser Examination #1 Bonding, Alkanes, Alkenes & Alkynes Thursday, September 17, 2015, 8:25 9:15 am Question 1. Atomic Structure,
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2
 Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Chapter 20: Carboxylic Acids and Nitriles شیمی آلی 2 Dr M. Mehrdad University of Guilan, Department of Chemistry, Rasht, Iran m-mehrdad@guilan.ac.ir Based on McMurry s Organic Chemistry, 7 th edition The
Reactions at α-position
 Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Dr. Mohamed El-Newehy
 By Dr. Mohamed El-Newehy Chemistry Department, College of Science, King Saud University http://fac.ksu.edu.sa/melnewehy Carboxylic acids and Their Derivatives 1 Structure of Carboxylic Acids -The functional
By Dr. Mohamed El-Newehy Chemistry Department, College of Science, King Saud University http://fac.ksu.edu.sa/melnewehy Carboxylic acids and Their Derivatives 1 Structure of Carboxylic Acids -The functional
Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution Reactions. McMurray Text Chapter 21
 Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution eactions McMurray Text Chapter 21 Carboxylic Acid Derivatives X Acid alide Ester ' Acid Anhydride ' N 2 Amide (1 ) Nomenclature Acid alides
Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution eactions McMurray Text Chapter 21 Carboxylic Acid Derivatives X Acid alide Ester ' Acid Anhydride ' N 2 Amide (1 ) Nomenclature Acid alides
The Claisen Condensation
 Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
MULTIPLE CHOICE 2 points each
 Name: Date: Score: / 110 Chapter 1/ TEST 1 OPEN BOOK KEY Organic Chemistry MULTIPLE CHOICE 2 points each 1. An atom of which element would have an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 1? a.
Name: Date: Score: / 110 Chapter 1/ TEST 1 OPEN BOOK KEY Organic Chemistry MULTIPLE CHOICE 2 points each 1. An atom of which element would have an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 1? a.
Chem 22 Final Exam Practice
 Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
21.1 Introduction Carboxylic Acids Nomenclature of Carboxylic Acids. Acids Structure and Properties of Carboxylic Acids.
 21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
Chapter 17. Carbonyl Compounds I Nucleophilic Acyl Substitution
 Chapter 17. Carbonyl Compounds I Nucleophilic Acyl Substitution Carbonyl compounds: compounds containing C=O group. 1. Class I carbonyl compounds: Carboxylic acids and derivatives containing a group (
Chapter 17. Carbonyl Compounds I Nucleophilic Acyl Substitution Carbonyl compounds: compounds containing C=O group. 1. Class I carbonyl compounds: Carboxylic acids and derivatives containing a group (
video 14.4 isomers isomers Isomers have the molecular formula but are rearranged in a structure with different properties. Example: Both C 4 H 10
 video 14.4 isomers isomers Isomers have the molecular formula but are rearranged in a structure with different properties. Example: Both C 4 H 10 Butane Methylpropane 1 match the isomers drawing an isomer
video 14.4 isomers isomers Isomers have the molecular formula but are rearranged in a structure with different properties. Example: Both C 4 H 10 Butane Methylpropane 1 match the isomers drawing an isomer
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding.
 Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
Chapter 19 Substitutions at the Carbonyl Group
 Chapter 19 Substitutions at the Carbonyl Group In Chapter 18 Additions to the Carbonyl Groups In Chapter 19 Substitutions at the Carbonyl Group O O - - O - O R Y R C+ Y R Y Nu -Ȳ R N u + Y=goodleavinggroup
Chapter 19 Substitutions at the Carbonyl Group In Chapter 18 Additions to the Carbonyl Groups In Chapter 19 Substitutions at the Carbonyl Group O O - - O - O R Y R C+ Y R Y Nu -Ȳ R N u + Y=goodleavinggroup
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
When we deprotonate we generate enolates or enols. Mechanism for deprotonation: Resonance form of the anion:
 Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
Chapter 19. Organic Chemistry. Carbonyl Compounds III. Reactions at the a-carbon. 4 th Edition Paula Yurkanis Bruice
 Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
State University of New York at Stony Brook Department of Chemistry. CHE 322, Organic Chemistry II Exam II March 17, 2004 Form 1
 State University of New York at Stony Brook Department of Chemistry CHE 22, rganic Chemistry II Exam II March 17, 2004 Form 1 Please answer all questions specifically, concisely, and readably in the spaces
State University of New York at Stony Brook Department of Chemistry CHE 22, rganic Chemistry II Exam II March 17, 2004 Form 1 Please answer all questions specifically, concisely, and readably in the spaces
Ch 19 Aldehydes and Ketones
 Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
Acyl-Transfer Reactions
 hem 215-216 W13 otes Dr. Masato Koreeda - Page 1 of 17. Date: March 18, 2013 hapter 15: arboxylic Acids and Their Derivatives and 21.3, /21.5 A Acyl-Transfer eactions I. Introduction Examples: note: could
hem 215-216 W13 otes Dr. Masato Koreeda - Page 1 of 17. Date: March 18, 2013 hapter 15: arboxylic Acids and Their Derivatives and 21.3, /21.5 A Acyl-Transfer eactions I. Introduction Examples: note: could
Organic Compounds Containing C, H and O
 Organic Compounds Containing C, H and O Long Answer Questions: **1. i. Explain the acidic nature of phenol and Compare with that of alcohols. ii. Describe the following a. Cannizaro reaction b. Decarboxylation.
Organic Compounds Containing C, H and O Long Answer Questions: **1. i. Explain the acidic nature of phenol and Compare with that of alcohols. ii. Describe the following a. Cannizaro reaction b. Decarboxylation.
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Class XII - Chemistry Aldehydes, Ketones and Carboxylic Acid Chapter-wise Problems
 Class XII - Chemistry Aldehydes, Ketones and Carboxylic Acid Chapter-wise Problems I. Multiple Choice Questions (Type-I) 1. Addition of water to alkynes occurs in acidic medium and in the presence of Hg
Class XII - Chemistry Aldehydes, Ketones and Carboxylic Acid Chapter-wise Problems I. Multiple Choice Questions (Type-I) 1. Addition of water to alkynes occurs in acidic medium and in the presence of Hg
Chemistry 210 Organic Chemistry I Fall Semester 2000 Dr. Rainer Glaser
 hemistry 210 rganic hemistry I all Semester 2000 Dr. Rainer Glaser xamination #3 Alkenes and Alkynes. Structure, Synthesis and Reactions. riday, November 17, 2000, 9:00-9:50 Name: Answer Key Question 1.
hemistry 210 rganic hemistry I all Semester 2000 Dr. Rainer Glaser xamination #3 Alkenes and Alkynes. Structure, Synthesis and Reactions. riday, November 17, 2000, 9:00-9:50 Name: Answer Key Question 1.
(1) Recall the different types of intermolecular interactions. (2) Look at the structure and determine the correct answer.
 MCAT rganic Chemistry - Problem Drill 11: Carboxylic Acids Question No. 1 of 10 Question 1. What kinds of interactions are holding together the carboxylic acid dimer shown? Question #01 3 C C 3 (A) Van
MCAT rganic Chemistry - Problem Drill 11: Carboxylic Acids Question No. 1 of 10 Question 1. What kinds of interactions are holding together the carboxylic acid dimer shown? Question #01 3 C C 3 (A) Van
Instructions: Cut individually and fold/glue. Can be either laminated or folded around cardboard.
 NCEA Chemistry 3.5 Flash Cards Instructions: Cut individually and fold/glue. Can be either laminated or folded around cardboard. Ideas for Use: 1. Group reactants (or products) into functional groups 2.
NCEA Chemistry 3.5 Flash Cards Instructions: Cut individually and fold/glue. Can be either laminated or folded around cardboard. Ideas for Use: 1. Group reactants (or products) into functional groups 2.
Chapter 12: Carbonyl Compounds II
 Chapter 12: Carbonyl Compounds II Learning bjectives: 1. Recognize and assign names to aldehydes and ketones. 2. Write the mechanism for nucleophilic addition and nucleophilic addition-elimination reactions
Chapter 12: Carbonyl Compounds II Learning bjectives: 1. Recognize and assign names to aldehydes and ketones. 2. Write the mechanism for nucleophilic addition and nucleophilic addition-elimination reactions
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Nucleophilic Addition Reactions of Carboxylic Acid Derivatives
 Lecture 5: bjectives: Nucleophilic Addition eactions of Carboxylic Acid Derivatives By the end of this lecture you will be able to: draw the mechanism of a nucleophilic addition-elimination reaction with
Lecture 5: bjectives: Nucleophilic Addition eactions of Carboxylic Acid Derivatives By the end of this lecture you will be able to: draw the mechanism of a nucleophilic addition-elimination reaction with
CHAPTER 22 HW: CO 2 H DERIVATIVES
 CHAPTER 22 HW: C 2 H DERIVATIVES MECLATURE 1. Give the name for each compound (IUPAC or common name). Use R/S naming where needed. Structure Br ame Structure ame 2. Give the name for each ester. Structure
CHAPTER 22 HW: C 2 H DERIVATIVES MECLATURE 1. Give the name for each compound (IUPAC or common name). Use R/S naming where needed. Structure Br ame Structure ame 2. Give the name for each ester. Structure
Chem 3719 Klein Chapter Practice Problems
 Chem 379 Klein Chapter Practice Problems Dr. Peter Norris, 208 Klein Chapter Problems : Review of General Chemistry. Draw viable structures for molecules with the following molecular formulae. Remember
Chem 379 Klein Chapter Practice Problems Dr. Peter Norris, 208 Klein Chapter Problems : Review of General Chemistry. Draw viable structures for molecules with the following molecular formulae. Remember
Chapter 1: Atomic and Molecular Structure
 Chapter 1: Atomic and Molecular Structure LEARNING OBJECTIVES Determine the number of valence and/or core electrons for an atom or ion. Multiple Choice: 1, 6, 11 Interpret the electron configuration and
Chapter 1: Atomic and Molecular Structure LEARNING OBJECTIVES Determine the number of valence and/or core electrons for an atom or ion. Multiple Choice: 1, 6, 11 Interpret the electron configuration and
Chapter 17: Alcohols and Phenols. Based on McMurry s Organic Chemistry, 7 th edition
 Chapter 17: Alcohols and Phenols Based on McMurry s Organic Chemistry, 7 th edition Alcohols and Phenols Alcohols contain an OH group connected to a a saturated C (sp 3 ) They are important solvents and
Chapter 17: Alcohols and Phenols Based on McMurry s Organic Chemistry, 7 th edition Alcohols and Phenols Alcohols contain an OH group connected to a a saturated C (sp 3 ) They are important solvents and
Ch 22 Carbonyl Alpha ( ) Substitution
 Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Chemistry 210 Organic Chemistry I Winter Semester 2002 Dr. Rainer Glaser
 hemistry 210 Organic hemistry I Winter Semester 2002 Dr. Rainer Glaser Examination #6 Reactions of Alkenes & Alkyne hemistry. Friday, March 26, 2002, 9 9:50 am Name: Question 1. alogenation Reactions.
hemistry 210 Organic hemistry I Winter Semester 2002 Dr. Rainer Glaser Examination #6 Reactions of Alkenes & Alkyne hemistry. Friday, March 26, 2002, 9 9:50 am Name: Question 1. alogenation Reactions.
CARBOXYLIC ACIDS and their Derivatives Nucleophilic Acyl substitution - Review the nomenclature for these compounds in your textbook
 CARBXYLIC ACIDS and their Derivatives Nucleophilic Acyl substitution - Review the nomenclature for these compounds in your textbook R Z R Z R Z - the basicity of Z determines the relative stability of
CARBXYLIC ACIDS and their Derivatives Nucleophilic Acyl substitution - Review the nomenclature for these compounds in your textbook R Z R Z R Z - the basicity of Z determines the relative stability of
Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry
 Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry 30 Questions (5 pages); Time limit = 45 minutes Use of books or notes is not permitted. 1. When analyzed with a polarimeter, which of the
Hyperlearning MCAT Instructor Qualifying Exam Organic Chemistry 30 Questions (5 pages); Time limit = 45 minutes Use of books or notes is not permitted. 1. When analyzed with a polarimeter, which of the
ORGANIC - BROWN 8E CH CARBOXYLIC ACIDS.
 RGANIC - BRWN 8E CH. 17 - CARBXYLIC ACIDS!! www.clutchprep.com RGANIC - BRWN 8E CH. 17 - CARBXYLIC ACIDS CNCEPT: CARBXYLIC ACID NMENCLATURE IUPAC: Replace alkane -e with Substituents are located using
RGANIC - BRWN 8E CH. 17 - CARBXYLIC ACIDS!! www.clutchprep.com RGANIC - BRWN 8E CH. 17 - CARBXYLIC ACIDS CNCEPT: CARBXYLIC ACID NMENCLATURE IUPAC: Replace alkane -e with Substituents are located using
Naming Organic Halides. Properties of Organic Halides
 Organic Compounds Organic Halides A hydrocarbon in which one or more hydrogen atoms have been replaced by halogen atoms Freons (chlorofluorocarbons) in refrigeration and air conditioning Teflon (polytetrafluoroethane)
Organic Compounds Organic Halides A hydrocarbon in which one or more hydrogen atoms have been replaced by halogen atoms Freons (chlorofluorocarbons) in refrigeration and air conditioning Teflon (polytetrafluoroethane)
Loudon Chapter 20 & 21 Review: Carboxylic Acids & Derivatives CHEM 3331, Jacquie Richardson, Fall Page 1
 Loudon Chapter 20 & 21 eview: Carboxylic Acids & Derivatives CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 These two chapters cover compounds which are all at the three bonds to more electronegative
Loudon Chapter 20 & 21 eview: Carboxylic Acids & Derivatives CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 These two chapters cover compounds which are all at the three bonds to more electronegative
Chemistry 210 Organic Chemistry I Winter Semester 2001 Dr. Rainer Glaser
 hemistry 210 rganic hemistry I Winter Semester 2001 Dr. Rainer Glaser Examination #3 Alkenes and Alkynes. Structure, Synthesis and Reactions. Friday, April 20, 2001, 9:00-9:50 Name: Answer Key Question
hemistry 210 rganic hemistry I Winter Semester 2001 Dr. Rainer Glaser Examination #3 Alkenes and Alkynes. Structure, Synthesis and Reactions. Friday, April 20, 2001, 9:00-9:50 Name: Answer Key Question
OCR (A) Chemistry A-level. Module 6: Organic Chemistry and Analysis
 OCR (A) Chemistry A-level Module 6: Organic Chemistry and Analysis Organic Synthesis Notes by Adam Robertson DEFINITIONS Heterolytic fission: The breaking of a covalent bond when one of the bonded atoms
OCR (A) Chemistry A-level Module 6: Organic Chemistry and Analysis Organic Synthesis Notes by Adam Robertson DEFINITIONS Heterolytic fission: The breaking of a covalent bond when one of the bonded atoms
Name: Date: I. Multiple Choice (Circle the letter for the best answer)
 Organic Chemistry II Sample Exam 3 You should also be able to name compounds, draw structures from names, and complete reactions given the reactants and conditions of the reaction. Name: Date: I. Multiple
Organic Chemistry II Sample Exam 3 You should also be able to name compounds, draw structures from names, and complete reactions given the reactants and conditions of the reaction. Name: Date: I. Multiple
ORGANIC - CLUTCH CH ALCOHOLS AND CARBONYL COMPOUNDS.
 !! www.clutchprep.com CONCEPT: INTRO TO REDOX Oxidation reactions involve an increase in the content of a molecule Reduction reactions involve an increase in the content of a molecule EXAMPLE: Label the
!! www.clutchprep.com CONCEPT: INTRO TO REDOX Oxidation reactions involve an increase in the content of a molecule Reduction reactions involve an increase in the content of a molecule EXAMPLE: Label the
Name. Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry II Examination #3 - March 31, 2003
 INSTRUTINS Name Department of hemistry SUNY/neonta hem 322 - rganic hemistry II Examination #3 - March 31, 2003 This examination has two parts. Part I is in multiple choice format and the answers should
INSTRUTINS Name Department of hemistry SUNY/neonta hem 322 - rganic hemistry II Examination #3 - March 31, 2003 This examination has two parts. Part I is in multiple choice format and the answers should
Definition: A carboxylic acid derivative undergoes hydrolysis (bond breaking with water) to form a carboxylic acid R C N + H 2 O + H O R
 arboxylic Acid Derivatives Addition/Elimination Definition: A carboxylic acid derivative undergoes hydrolysis (bond breaking with water) to form a carboxylic acid X = l, Br addition/elimination X acid
arboxylic Acid Derivatives Addition/Elimination Definition: A carboxylic acid derivative undergoes hydrolysis (bond breaking with water) to form a carboxylic acid X = l, Br addition/elimination X acid
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2017 Dr. Rainer Glaser
 Chemistry 2030 Survey of Organic Chemistry Fall Semester 2017 Dr. Rainer Glaser Examination #1 Bonding, Alkanes, Alkenes & Alkynes Thursday, September 14, 2017, 8:25 9:15 am Name: Answer Key Question 1.
Chemistry 2030 Survey of Organic Chemistry Fall Semester 2017 Dr. Rainer Glaser Examination #1 Bonding, Alkanes, Alkenes & Alkynes Thursday, September 14, 2017, 8:25 9:15 am Name: Answer Key Question 1.
