NH 2 O O O O OCH (6 pts) Provide an acceptable name for each of the following compounds: NH 2 COOH HOOC COOH. piperidine
|
|
|
- Jocelyn Watson
- 5 years ago
- Views:
Transcription
1 CEMISTRY MIDTERM # 4 April 1, 2003 Statistics: Average: 87 pts (72%); ighest: 116 pts (97%); Lowest: 49 pts (41%) umber of students performing at or above average: 13 (62%) 1. (9 pts) Mark as true (T) or false (F) the following statements. Do not explain! (F) Fischer esterification occurs only in strongly basic conditions; (F) Amides are less reactive than acid chlorides but more reactive than esters; (T) Saponification is the process of base-catalyzed hydrolysis of esters; (F) Lactones are intramolecular amides; (F) Primary amines are molecules in which the amino group is bound to a primary carbon; (T) The value of pk b decreases with an increasing strength of the base; (T) Primary amines can serve as hydrogen bond donors and hydrogen bond acceptors; (T) Secondary amines are stronger bases than primary amines; (F) Michael addition reactions give 1,2-addition products; 2. Circle all that apply: A. (3 pts) The following reactions CAT be used to generate esters: a. Base-catalyzed reaction of carboxylic acid and alcohol; b. Reaction of acid chloride and alcohol; c. Reaction of amide and alcohol; d. Baeyer-Villiger oxidation of ketones; B. (3 pts) The following reactions D T occur with an intermediate formation of enolates: a. Fischer esterification; b. Claisen condensation; c. Dieckmann cyclization; d. Michael addition; C. (3 pts) The following compounds D T decarboxylate upon ing: a. β-ketoacids; b. γ-dicarboxylic acids; c. β-dicarboxylic acids; d. γ -hydroxyacids; 3. (6 pts) Provide an acceptable name for each of the following compounds: 2 C 3 cis-3-aminocyclopentanol phthalic anhydride 6-hexanolide methyl acetoacetate -ethyl-methylcyclohexylamine butylmethylmalonic acid 4. ( pts) Provide the structure of each of the following compounds: C C 2 C C C (E)-2,3-dimethylbutenedioic acid 2,2-dibromocyclopropanamine piperidine 4-methyl-2,6-pyridinedicarboxylic acid -(dimethylamino)-3-hexynoic acid
2 . ( pts) Rank the following compounds in order of increasing basicity. Do not explain! A. Cyclopentylamine; B. -methylcyclopentylamine; C. Aniline; D. 4-nitroaniline; E. Sodium amide; D < C < A < B < E 6. ( pts) Rank the following compounds in order of increasing acidity. Do not explain! A. Trichloroacetic acid; B. Trifluoroacetic acid; C. Propanoic acid; D. Isopropanol; E. Isopropylamine; D < C < A < B < E 7. Write a complete equation for each of the processes indicated below (TE: Do not write mechanisms!!). A. (4 pts) Reaction of maleic anhydride (cis-butenedioic acid anhydride) with ethanol. + C 2 C 2 B. (4 pts) Saponification of γ-butyrolactone. K 2 K C. (4 pts) Reaction of n-butyl bromide with sodium cyanide, followed by acid hydrolysis; 1. ac C , D. (4 pts) Reaction of ethyl acetate with ethyl formate in the presence of ac 2 /C 2 ; ac 2 + C 2 C 2 C 2 C 2 8. (18 pts) Predict the principal organic product in each of the following reactions: 1. Mg/ether 2. C C C 1. LiAl C 2 C C C 1. ac 2 /C 2 2. C 1. 2 /P C Cl C 3 2 pyridine C 3 C C 3 C 3 2 S 4 CC 3 C 3 1. C 3 Mg
3 CC 2 CC 2 1. ac 2 / C 2 2. C 2 C CC 2 1. LDA, 3 C C 3 CC 3 9. (16 pts) Fill in the blanks in the following scheme: 1. ac/dms , C 3 2 S 4 I C CC 3 1. ac 3 / C C 3 SCl 2 CCl pyr 1. a , 1. ac 3 / C 3 2. C 3 I C (6 pts) Propose a mechanism for the following reaction: C 2 C 3 + C C 2 C 3 Cl C C 3 + C C 3 C C 3 C 2 C C C 2 C C C 2 C C 3 - C Suggest a plausible synthetic sequence for each of the following transformations. Use any necessary organic or inorganic reagents. A. ( pts)?? C 3
4 1. 2 ac/dmf , 3. 2 C 3, 2 S 4 3 CC?? C 2 C 2 CC 3 ac 3 C 3 C 3 B. ( pts) 1. ac/dmf , 3. C 2, 2 S 4 CC2 ac 2 C 2 C 2 C 2 C Use the acetoacetic or malonic ester synthesis to prepare each of the following compounds. Employ any other necessary organic or inorganic reagents. A. ( pts) 3-ethyl--hexen-2-one; C 2 1. ac 2 /C 2 2. C 2 3. ac 2 /C 2 4. B. (4 pts) Cyclopentanecarboxylic acid; C 2 1. K , - C 2 C 2 C 2 1. ac 2 /C 2 2. C 2 C CC 2 1. K , - C 2 C 13. (6 pts) The 1 MR spectra of two isomeric compounds, A and B, with molecular formula C are shown below. Assign the correct structure for each isomer. Compound A C
5 Compound B C (4 pts) BUS PRBLEM (In order to receive credit for this problem, it has to be solved entirely!!). In the Stobbe condensation an ester of succinic acid (such as diethyl succinate below) is subjected to base deprotonation and then reacted with a nonenolizable aldehyde or ketone to give a product with a lactone ring. Propose a plausible, detailed mechanism for the Stobbe condensation below. + CC 2 CC 2 ac 2 C 2 CC 2 CC 2 CC 2 ac 2 C 2 CC 2 CC 2 C 2 CC 2 CC 2 CC 2 C 2
CHEMISTRY MIDTERM # 4 ANSWER KEY April 16, 2002
 CEMISTY 314-01 MIDTEM # 4 ASWE KEY April 16, 00 1. (6 pts) Mark as true (T) or false (F) the following statements. Do not explain! (F) Amides are less reactive than acid chlorides but more reactive than
CEMISTY 314-01 MIDTEM # 4 ASWE KEY April 16, 00 1. (6 pts) Mark as true (T) or false (F) the following statements. Do not explain! (F) Amides are less reactive than acid chlorides but more reactive than
Score: Homework Problem Set 9 Iverson CH320N Due Monday, April 17. NAME (Print): Chemistry 320N Dr. Brent Iverson 9th Homework April 10, 2017
 omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
CHEMISTRY MIDTERM # 2 November 02, The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck!
 CEMISTRY 314-01 MIDTERM # 2 November 02, 2009 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (8 pts) Mark as true (T) or false (F) the following
CEMISTRY 314-01 MIDTERM # 2 November 02, 2009 Name... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (8 pts) Mark as true (T) or false (F) the following
Chapter 19. Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions. ß-dicarbonyl compounds. Why are ß-dicarbonyls useful?
 Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser
 Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Thursday, November 15, 2012, 8:25 9:15 am Name: Question 1. Aldehydes
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2012 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Thursday, November 15, 2012, 8:25 9:15 am Name: Question 1. Aldehydes
Practice Synthetic Problems: CHEM 235 Page 2
 Practice Synthetic Problems: CM 235 Page 2 Syntheses based on diethyl malonate, ethyl acetoacetate, etc. Using diethyl malonate and any other necessary organic reagents, show a synthesis of: a) 2,2-dimethyl-1,3-propanediamine
Practice Synthetic Problems: CM 235 Page 2 Syntheses based on diethyl malonate, ethyl acetoacetate, etc. Using diethyl malonate and any other necessary organic reagents, show a synthesis of: a) 2,2-dimethyl-1,3-propanediamine
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2013 Dr. Rainer Glaser
 Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2013 Dr. Rainer Glaser Examination #4 Enols and Aldol Reaction, Carboxylic Acids and Carboxylic Acid Derivatives. Thursday, November 14, 2013,
Chemistry 2030 Introduction to Organic Chemistry Fall Semester 2013 Dr. Rainer Glaser Examination #4 Enols and Aldol Reaction, Carboxylic Acids and Carboxylic Acid Derivatives. Thursday, November 14, 2013,
Chemistry 234 Practice Exam 3. The Periodic Table
 ame: Last First MI Chemistry 234 Practice Exam 3 Instructions: The first 15 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron sheet.
ame: Last First MI Chemistry 234 Practice Exam 3 Instructions: The first 15 questions of this exam should be answered on the provided Scantron. You must use a pencil for filling in the Scantron sheet.
CHEMISTRY MIDTERM # 1 answer key February 12, 2009
 CEMSTRY 313-01 MDTERM # 1 answer key February 12, 2009 Statistics: Average: 78 pts (78%); ighest: 97 pts (97%); Lowest: 43 pts (43%) umber of students performing at or above average: 28 (62%) umber of
CEMSTRY 313-01 MDTERM # 1 answer key February 12, 2009 Statistics: Average: 78 pts (78%); ighest: 97 pts (97%); Lowest: 43 pts (43%) umber of students performing at or above average: 28 (62%) umber of
CHEMISTRY MIDTERM # 1 answer key September 29, 2005
 CEMISTRY 313-01 MIDTERM # 1 answer key September 29, 2005 Statistics: Average: 75 pts (75%); ighest: 99 pts (99%); Lowest: 31 pts (31%) Number of students performing at or above average: 28 (57%) Number
CEMISTRY 313-01 MIDTERM # 1 answer key September 29, 2005 Statistics: Average: 75 pts (75%); ighest: 99 pts (99%); Lowest: 31 pts (31%) Number of students performing at or above average: 28 (57%) Number
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
20.3 Alkylation of Enolate Anions
 864 APTER 20 ELATE AD TER ARB ULEPILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone The reaction can also be used in synthesis to convert a methyl
864 APTER 20 ELATE AD TER ARB ULEPILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone The reaction can also be used in synthesis to convert a methyl
CHEM 234: Organic Chemistry II Reaction Sheets
 EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
CHAPTER 19: CARBONYL COMPOUNDS III
 CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
CHEMISTRY MIDTERM # 1 answer key October 05, 2010
 CEMISTRY 313-03 MIDTERM # 1 answer key ctober 05, 2010 Statistics: Average: 73 pts (73%); ighest: 99 pts (99%); Lowest: 31 pts (31%) Number of students performing at or above average: 61 (52%) Number of
CEMISTRY 313-03 MIDTERM # 1 answer key ctober 05, 2010 Statistics: Average: 73 pts (73%); ighest: 99 pts (99%); Lowest: 31 pts (31%) Number of students performing at or above average: 61 (52%) Number of
C h a p t e r T w e n t y : Carboxylic Acids & Their Derivatives
 C h a p t e r T w e n t y : Carboxylic Acids & Their Derivatives N C3 N Lysergic acid, the depressant used to make LSD, the famous acid of the 1960s LSD and the Search for God, a San Francisco-based band
C h a p t e r T w e n t y : Carboxylic Acids & Their Derivatives N C3 N Lysergic acid, the depressant used to make LSD, the famous acid of the 1960s LSD and the Search for God, a San Francisco-based band
Ch 22 Carbonyl Alpha ( ) Substitution
 Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution Reactions. McMurray Text Chapter 21
 Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution eactions McMurray Text Chapter 21 Carboxylic Acid Derivatives X Acid alide Ester ' Acid Anhydride ' N 2 Amide (1 ) Nomenclature Acid alides
Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution eactions McMurray Text Chapter 21 Carboxylic Acid Derivatives X Acid alide Ester ' Acid Anhydride ' N 2 Amide (1 ) Nomenclature Acid alides
Summary of π Bond Chemistry
 Chapter 10 Summary of π Bond Chemistry to π C=C 13.01 Chapter 11 at π C=C to π C=C Chapter 1 LG at π C=C Chapter 13 at α position C= activates position ow does C= activation α position? Via or. E base
Chapter 10 Summary of π Bond Chemistry to π C=C 13.01 Chapter 11 at π C=C to π C=C Chapter 1 LG at π C=C Chapter 13 at α position C= activates position ow does C= activation α position? Via or. E base
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser
 Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Make-Up Carbonyl Compounds and Amines. Wednesday, November 30, 2011, 10 10:50 am Name: Answer Key Question
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Make-Up Carbonyl Compounds and Amines. Wednesday, November 30, 2011, 10 10:50 am Name: Answer Key Question
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions
 Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Chemistry Organic Chemistry II: Reactivity and Synthesis ANSWERS FOR FINAL EXAM Winter 2004
 ASWER KEY Page 1 of 18 Chemistry 2.222 rganic Chemistry II: Reactivity and Synthesis ASWERS FR FIAL EXAM Winter 2004 Paper umber 631 Thursday April 22, 2004 1:30 4:30 pm University Centre Rm 210-224 Students
ASWER KEY Page 1 of 18 Chemistry 2.222 rganic Chemistry II: Reactivity and Synthesis ASWERS FR FIAL EXAM Winter 2004 Paper umber 631 Thursday April 22, 2004 1:30 4:30 pm University Centre Rm 210-224 Students
Carbonyl Chemistry. aldehydes ketones. carboxylic acid and derivatives. Wednesday, April 29, 2009
 Carbonyl Chemistry X aldehydes ketones carboxylic acid and derivatives Electrophiles (eg. + ) Nucleophiles (eg. C 3 MgBr) an enolate Base β β β α α α 1 2 3 4 Nuc- 1 2 Nuc 3 4 1,2-addition 1 2 3 4 Nuc-
Carbonyl Chemistry X aldehydes ketones carboxylic acid and derivatives Electrophiles (eg. + ) Nucleophiles (eg. C 3 MgBr) an enolate Base β β β α α α 1 2 3 4 Nuc- 1 2 Nuc 3 4 1,2-addition 1 2 3 4 Nuc-
I pledge on my honor that I have neither given nor received unauthorized aid on this examination
 Chemistry 220b, Section 1 Exam 2 (100 pts) Thursday, ebruary 26, 2015 Chapters 13, 15-19 ame Write and sign the VU onor Pledge: I pledge on my honor that I have neither given nor received unauthorized
Chemistry 220b, Section 1 Exam 2 (100 pts) Thursday, ebruary 26, 2015 Chapters 13, 15-19 ame Write and sign the VU onor Pledge: I pledge on my honor that I have neither given nor received unauthorized
CHEM 343 Principles of Organic Chemistry II Summer Instructor: Paul J. Bracher. Quiz # 3. Monday, July 21 st, :30 a.m.
 CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
B. (3 pts) The following values are independent of the operating frequency of the NMR: a. Coupling constant; c. Chemical shift; b. Gyromagnetic ratio;
 CEMISTRY 314-01 MIDTERM # 1 answer key September 29, 2009 Statistics: Average: 70 pts (70%); ighest: 96 pts (96%); Lowest: 37 pts (37%) Number of students performing at or above average: 16 (57%) Number
CEMISTRY 314-01 MIDTERM # 1 answer key September 29, 2009 Statistics: Average: 70 pts (70%); ighest: 96 pts (96%); Lowest: 37 pts (37%) Number of students performing at or above average: 16 (57%) Number
Loudon Chapter 20 & 21 Review: Carboxylic Acids & Derivatives CHEM 3331, Jacquie Richardson, Fall Page 1
 Loudon Chapter 20 & 21 eview: Carboxylic Acids & Derivatives CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 These two chapters cover compounds which are all at the three bonds to more electronegative
Loudon Chapter 20 & 21 eview: Carboxylic Acids & Derivatives CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 These two chapters cover compounds which are all at the three bonds to more electronegative
OCH 2 CH 3. A) 2-chlorohexyl ethanoate C) ethyl 2-chlorohexanoate B) 1-chlorohexyl ethanoate D) ethyl 1-chlorohexanoate
 (2 points each) Write your answer in the box to the right of the question. 1. A mixture of 1-hexanol and hexanoic acid in diethyl ether is shaken with an aqueous sodium bicarbonate solution. Which line
(2 points each) Write your answer in the box to the right of the question. 1. A mixture of 1-hexanol and hexanoic acid in diethyl ether is shaken with an aqueous sodium bicarbonate solution. Which line
A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation:
 1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
Chemistry 2030, FS17, Dr. Rainer Glaser Introduction to Organic Chemistry
 Chemistry 2030, FS17, Dr. Rainer Glaser Introduction to Organic Chemistry Examination #4 Aldehydes & Ketones, Carboxylic Acids & Carboxylic Acid Derivatives, Lipids & Detergents, and Amines. Handout: Tuesday,
Chemistry 2030, FS17, Dr. Rainer Glaser Introduction to Organic Chemistry Examination #4 Aldehydes & Ketones, Carboxylic Acids & Carboxylic Acid Derivatives, Lipids & Detergents, and Amines. Handout: Tuesday,
CHAPTER 22 HW: CO 2 H DERIVATIVES
 APTER 22 W: 2 DERIVATIVES MELATURE 1. Give the name for each compound (IUPA or common name). Use R/S naming where needed. Structure 2 3 1 3 ame 2,2-dimethylpropanoyl chloride (R)-3-methylpentanoyl bromide
APTER 22 W: 2 DERIVATIVES MELATURE 1. Give the name for each compound (IUPA or common name). Use R/S naming where needed. Structure 2 3 1 3 ame 2,2-dimethylpropanoyl chloride (R)-3-methylpentanoyl bromide
CHEM 343 Principles of Organic Chemistry II Summer Instructor: Paul J. Bracher. Quiz # 3. Monday, July 21 st, :30 a.m.
 CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Problem Booklet Page 1 of 7 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21
CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Problem Booklet Page 1 of 7 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21
COURSE UNIT DESCRIPTION. Dept. Organic Chemistry, Vilnius University. Type of the course unit
 Course unit title Organic Chemistry II Lecturer(s) Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery Period
Course unit title Organic Chemistry II Lecturer(s) Rimantas Vaitkus COURSE UNIT DESCRIPTION Department Dept. Organic Chemistry, Vilnius University Cycle First Type of the course unit Mode of delivery Period
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser
 Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Wednesday, November 16, 2011, 10 10:50 am Name: Answer Key Question 1.
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Carbonyl Compounds and Amines. Wednesday, November 16, 2011, 10 10:50 am Name: Answer Key Question 1.
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
Chem 342 Organic Chemistry II Final Exam 13 May 2009
 hem 342 rganic hemistry II Final Exam 13 May 2009 KEY Please read through each question carefully and answer in the spaces provided. A good strategy is to go through the test and answer all the questions
hem 342 rganic hemistry II Final Exam 13 May 2009 KEY Please read through each question carefully and answer in the spaces provided. A good strategy is to go through the test and answer all the questions
THE UNIVERSITY OF CALGARY FACULTY OF SCIENCE FINAL EXAMINATION CHEMISTRY 350
 Page 1 of 16 TE UNIVERSITY F CALGARY FACULTY F SCIENCE FINAL EXAMINATIN CEMISTRY 350 April, 1997 Time: 3 ours PLEASE WRITE YUR NAME, STUDENT I.D. NUMBER AND SECTIN NUMBER (01 for MWF lectures and 02 for
Page 1 of 16 TE UNIVERSITY F CALGARY FACULTY F SCIENCE FINAL EXAMINATIN CEMISTRY 350 April, 1997 Time: 3 ours PLEASE WRITE YUR NAME, STUDENT I.D. NUMBER AND SECTIN NUMBER (01 for MWF lectures and 02 for
CHAPTER 23 HW: ENOLS + ENOLATES
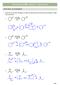 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
Chapter 19. Organic Chemistry. Carbonyl Compounds III. Reactions at the a-carbon. 4 th Edition Paula Yurkanis Bruice
 Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Michael and Aldol CH391 December 4, 2002
 Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser
 Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Practice Edition Carbonyl Compounds and Amines. Wednesday, November 16, 2011, 10 10:50 am Name: Question
Chemistry 2050 Introduction to Organic Chemistry Fall Semester 2011 Dr. Rainer Glaser Examination #4 Practice Edition Carbonyl Compounds and Amines. Wednesday, November 16, 2011, 10 10:50 am Name: Question
Carboxylic Acids O R C + H + O - Chemistry 618B
 arboxylic Acids R H R + H + - R - Nomenclature - IUPA IUPA names: drop the -e from the parent alkane and add the suffix -oic acid If the compound contains a carbon-carbon double bond, change the infix
arboxylic Acids R H R + H + - R - Nomenclature - IUPA IUPA names: drop the -e from the parent alkane and add the suffix -oic acid If the compound contains a carbon-carbon double bond, change the infix
B. (2 pts) The regiochemistry of alcohol dehydration is determined by the: a. Zaitsev rule; b. Hammond postulate;
 CEMISTRY 313-01 MIDTERM # answer key ctober 1, 008 Statistics: Average: 73 pts (73%); ighest: 98 pts (98%); Lowest: 3 pts (3%) Number of students performing at or above average: 36 (58%) Number of students
CEMISTRY 313-01 MIDTERM # answer key ctober 1, 008 Statistics: Average: 73 pts (73%); ighest: 98 pts (98%); Lowest: 3 pts (3%) Number of students performing at or above average: 36 (58%) Number of students
Lecture 23. The Aldol Condensation. an Aldol! April 12, 2018 O H O H - Chemistry 328N
 Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
Final Exam Version 1. Chemistry 140C. Fall Good Luck! Dec 5, :30 am 2:30 pm This exam accounts for 50% of the final grade.
 Chemistry 140C Final Exam Version 1 Fall 2006 Dec 5, 2006 11:30 am 2:30 pm This exam accounts for 50% of the final grade. Mark your final answer clearly. Completely erase irrelevant information! Exams
Chemistry 140C Final Exam Version 1 Fall 2006 Dec 5, 2006 11:30 am 2:30 pm This exam accounts for 50% of the final grade. Mark your final answer clearly. Completely erase irrelevant information! Exams
ORGANIC - BRUICE 8E CH CARBONYL COMPOUNDS III: REACTIONS AT THE ALPHA-CARBON
 !! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
!! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
CARBOXYLIC ACIDS and their Derivatives Nucleophilic Acyl substitution - Review the nomenclature for these compounds in your textbook
 CARBXYLIC ACIDS and their Derivatives Nucleophilic Acyl substitution - Review the nomenclature for these compounds in your textbook R Z R Z R Z - the basicity of Z determines the relative stability of
CARBXYLIC ACIDS and their Derivatives Nucleophilic Acyl substitution - Review the nomenclature for these compounds in your textbook R Z R Z R Z - the basicity of Z determines the relative stability of
Suggested solutions for Chapter 28
 s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
20.10 Conjugate Additions
 894 APTER 20 ELATE AD THER CARB UCLEPHILES 2010 Conjugate Additions The conjugate addition of nucleophiles to,-unsaturated carbonyl compounds at the -position was described in Section 1810 Enolate and
894 APTER 20 ELATE AD THER CARB UCLEPHILES 2010 Conjugate Additions The conjugate addition of nucleophiles to,-unsaturated carbonyl compounds at the -position was described in Section 1810 Enolate and
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Lecture 24 Two Germans and an Englishman
 Lecture 24 Two Germans and an Englishman Robert Robinson 1886-1975 Nobel Laureate 1947 April 17, 2018 tto Paul Hermann Diels 1876-1954 Nobel Laureates 1950 Kurt Alder 1902-1958 Exam III Tomorrow Wed April
Lecture 24 Two Germans and an Englishman Robert Robinson 1886-1975 Nobel Laureate 1947 April 17, 2018 tto Paul Hermann Diels 1876-1954 Nobel Laureates 1950 Kurt Alder 1902-1958 Exam III Tomorrow Wed April
The Claisen Condensation
 Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
CARBOXYLIC ACIDS AND THEIR DERIVATIVES. 3. Predict the relative acidity and basicity of compounds and ions. Important criteria include:
 CARBXYLIC ACIDS AND TEIR DERIVATIVES A STUDENT SULD BE ABLE T: 1. Give the IUPAC name given the structure, and draw the structure given the name, of carboxylic acids and their metal salts, acyl chlorides,
CARBXYLIC ACIDS AND TEIR DERIVATIVES A STUDENT SULD BE ABLE T: 1. Give the IUPAC name given the structure, and draw the structure given the name, of carboxylic acids and their metal salts, acyl chlorides,
Chem 2320 Final 210 points Dr. Luther Giddings
 Chem 2320 Final 210 points Dr. Luther Giddings Name Phone or E-Mail Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam,
Chem 2320 Final 210 points Dr. Luther Giddings Name Phone or E-Mail Instructions: This is a closed book, closed notebook test. You may not discuss this exam with anyone, either during or after the exam,
Chapter 21 Ester Enolates
 hapter 21 Ester Enolates Introduction R R' H H The preparation and reactions of β-dicarbonyl compounds, especially β-keto esters, is the main focus of this chapter. A proton on the carbon flanked by the
hapter 21 Ester Enolates Introduction R R' H H The preparation and reactions of β-dicarbonyl compounds, especially β-keto esters, is the main focus of this chapter. A proton on the carbon flanked by the
Objective 14. Develop synthesis strategies for organic synthesis.
 Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Objective 14. Develop synthesis strategies for organic synthesis. Skills: Draw structure ID structural features and reactive sites (alpha C, beta C, LG, etc.) ID Nu - and E + use curved arrows to show
Lecture 23. Amines. Chemistry 328N. April 12, 2016
 Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chapter 12: Carbonyl Compounds II
 Chapter 12: Carbonyl Compounds II Learning bjectives: 1. Recognize and assign names to aldehydes and ketones. 2. Write the mechanism for nucleophilic addition and nucleophilic addition-elimination reactions
Chapter 12: Carbonyl Compounds II Learning bjectives: 1. Recognize and assign names to aldehydes and ketones. 2. Write the mechanism for nucleophilic addition and nucleophilic addition-elimination reactions
Keynotes in Organic Chemistry
 Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
Keynotes in Organic Chemistry Second Edition ANDREW F. PARSONS Department of Chemistry, University of York, UK Wiley Contents Preface xi 1 Structure and bonding 1 1.1 Ionic versus covalent bonds 1 1.2
State University of New York at Stony Brook Department of Chemistry. CHE 322, Organic Chemistry II Exam II March 17, 2004 Form 1
 State University of New York at Stony Brook Department of Chemistry CHE 22, rganic Chemistry II Exam II March 17, 2004 Form 1 Please answer all questions specifically, concisely, and readably in the spaces
State University of New York at Stony Brook Department of Chemistry CHE 22, rganic Chemistry II Exam II March 17, 2004 Form 1 Please answer all questions specifically, concisely, and readably in the spaces
Note: You must have your answers written in pen if you want a regrade!!!!
 AME (Print): SIGATURE: hemistry 310 Dr. Brent Iverson 2nd Midterm March 29, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
AME (Print): SIGATURE: hemistry 310 Dr. Brent Iverson 2nd Midterm March 29, 2007 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there
21.1 Introduction Carboxylic Acids Nomenclature of Carboxylic Acids. Acids Structure and Properties of Carboxylic Acids.
 21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
CH310N Spring Anslyn. March 23, Exam 2
 C310N Spring 2010 Anslyn March 23, 2010 Exam 2 Please PRINT the first three letters of your last name in the boxes below. PRINT Full Name UT-EID 1) ( 8 pts ) 2) ( 5 pts ) 3) ( 5 pts ) 4) ( 22 pts ) 5)
C310N Spring 2010 Anslyn March 23, 2010 Exam 2 Please PRINT the first three letters of your last name in the boxes below. PRINT Full Name UT-EID 1) ( 8 pts ) 2) ( 5 pts ) 3) ( 5 pts ) 4) ( 22 pts ) 5)
CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher. Quiz # 4. Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m.
 CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
CHEM 347 Quiz # 4 Spring 2014 Page 1 of 9 CHEM 347 Organic Chemistry II (for Majors) Instructor: Paul J. Bracher Quiz # 4 Due in Monsanto Hall 103 by: Friday, April 4 th, 2014, 7:00 p.m. Student Name (Printed)
trans-cyclooctene 4-fluoro-1-butanol 2-cyclohexenol 4. (5 pts) Provide structural formula for each of the following molecules: Br OH Cl OH
 CEMISTRY 313-01 MIDTERM # 2 answer key March 12, 2009 Statistics: Average: 72 pts (72%); ighest: 98 pts (98%); Lowest: 21 pts (21%) umber of students performing at or above average: 22 (50%) umber of students
CEMISTRY 313-01 MIDTERM # 2 answer key March 12, 2009 Statistics: Average: 72 pts (72%); ighest: 98 pts (98%); Lowest: 21 pts (21%) umber of students performing at or above average: 22 (50%) umber of students
OChem2 Course Pack. Practice Problems by Chapter. Practice Exams
 Chem2 Course Pack Practice Problems by Chapter Practice Exams Chemistry 3720 Problem Sets Chemistry 3720 Ch. 13-19 Synthesis Problems These problems are typical of those that will be on the upcoming exams
Chem2 Course Pack Practice Problems by Chapter Practice Exams Chemistry 3720 Problem Sets Chemistry 3720 Ch. 13-19 Synthesis Problems These problems are typical of those that will be on the upcoming exams
Final Exam ASU ID or Posting ID 8 /18... Extra Credit /5
 EM 234, Spring 2008 PRITED FIRST AME PRITED LAST AME Final Exam ASU ID or Posting ID Ian R. Gould Person on your LEFT (or Aisle)!PRIT YUR AME EA PAGE! READ TE DIRETIS AREFULLY! USE BLAK PAGES AS SRAT PAPER
EM 234, Spring 2008 PRITED FIRST AME PRITED LAST AME Final Exam ASU ID or Posting ID Ian R. Gould Person on your LEFT (or Aisle)!PRIT YUR AME EA PAGE! READ TE DIRETIS AREFULLY! USE BLAK PAGES AS SRAT PAPER
1. What is the major organic product obtained from the following sequence of reactions?
 CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
CH320 N N_HW1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. Carefully record your answers on the Scantron
CHEM Chapter 21. Carboxylic Acid Derivatives_Nucleophilic Acyl Substitution Reactions (homework) W
 Short Answer IUPAC Naming Instructions: Provide proper IUPAC names. 1. Name: 2. Name: 3. Name: Drawing Instructions: Draw structures corresponding to each of the given names. 4. Draw: acetic formic anhydride
Short Answer IUPAC Naming Instructions: Provide proper IUPAC names. 1. Name: 2. Name: 3. Name: Drawing Instructions: Draw structures corresponding to each of the given names. 4. Draw: acetic formic anhydride
Chapter 1 Reactions of Organic Compounds. Reactions Involving Hydrocarbons
 Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chapter 1 Reactions of Organic Compounds Reactions Involving Hydrocarbons Reactions of Alkanes Single bonds (C-C) are strong and very hard to break, therefore these compounds are relatively unreactive
Chem 22 Final Exam Practice
 Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
Chemistry 52 Exam #2. Name: 5 February This exam has six (6) questions, two cover pages, six exam pages, and three scratch pages.
 Chemistry 52 Exam #2 Name: 5 February 2003 This exam has six (6) questions, two cover pages, six exam pages, and three scratch pages. Please check before beginning to make sure no questions are missing.
Chemistry 52 Exam #2 Name: 5 February 2003 This exam has six (6) questions, two cover pages, six exam pages, and three scratch pages. Please check before beginning to make sure no questions are missing.
Name Date Class. aryl halides substitution reaction
 23.1 INTRODUCTION TO FUNCTIONAL GROUPS Section Review Objectives Explain how organic compounds are classified Identify the IUPAC rules for naming halocarbons Describe how halocarbons can be prepared Vocabulary
23.1 INTRODUCTION TO FUNCTIONAL GROUPS Section Review Objectives Explain how organic compounds are classified Identify the IUPAC rules for naming halocarbons Describe how halocarbons can be prepared Vocabulary
TOK: The relationship between a reaction mechanism and the experimental evidence to support it could be discussed. See
 Option G: Further organic chemistry (15/22 hours) SL students study the core of these options and HL students study the whole option (the core and the extension material). TOK: The relationship between
Option G: Further organic chemistry (15/22 hours) SL students study the core of these options and HL students study the whole option (the core and the extension material). TOK: The relationship between
video 14.4 isomers isomers Isomers have the molecular formula but are rearranged in a structure with different properties. Example: Both C 4 H 10
 video 14.4 isomers isomers Isomers have the molecular formula but are rearranged in a structure with different properties. Example: Both C 4 H 10 Butane Methylpropane 1 match the isomers drawing an isomer
video 14.4 isomers isomers Isomers have the molecular formula but are rearranged in a structure with different properties. Example: Both C 4 H 10 Butane Methylpropane 1 match the isomers drawing an isomer
3. (6 pts) Provide an acceptable name for each of the following molecules: OH
 CEMISTRY 313-61 MIDTERM # 2 answer key June 07, 2005 Statistics: Average: 71 pts (71%); ighest: 99 pts (99%); Lowest: 45 pts (45%) Number of students performing at or above average: 15 (48%) 1. (11 pts)
CEMISTRY 313-61 MIDTERM # 2 answer key June 07, 2005 Statistics: Average: 71 pts (71%); ighest: 99 pts (99%); Lowest: 45 pts (45%) Number of students performing at or above average: 15 (48%) 1. (11 pts)
CHEM J-8 June Complete the following table. Make sure you give the name of the starting material where indicated. REAGENTS/ CONDITIONS
 CEM1102 2014-J-8 June 2014 Complete the following table. Make sure you give the name of the starting material where indicated. STARTIG MATERIAL REAGETS/ CDITIS STRUCTURAL FRMULA(S) F MAJR RGAIC PRDUCT(S)
CEM1102 2014-J-8 June 2014 Complete the following table. Make sure you give the name of the starting material where indicated. STARTIG MATERIAL REAGETS/ CDITIS STRUCTURAL FRMULA(S) F MAJR RGAIC PRDUCT(S)
Lecture 3: Aldehydes and ketones
 Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
Lecture 3: Aldehydes and ketones I want to start by talking about the mechanism of hydroboration/ oxidation, which is a way to get alcohols from alkenes. This gives the anti-markovnikov product, primarily
ORGANIC - BROWN 8E CH CARBOXYLIC ACIDS.
 RGANIC - BRWN 8E CH. 17 - CARBXYLIC ACIDS!! www.clutchprep.com RGANIC - BRWN 8E CH. 17 - CARBXYLIC ACIDS CNCEPT: CARBXYLIC ACID NMENCLATURE IUPAC: Replace alkane -e with Substituents are located using
RGANIC - BRWN 8E CH. 17 - CARBXYLIC ACIDS!! www.clutchprep.com RGANIC - BRWN 8E CH. 17 - CARBXYLIC ACIDS CNCEPT: CARBXYLIC ACID NMENCLATURE IUPAC: Replace alkane -e with Substituents are located using
Answers to Hour Examination #3, Chemistry 302X, 2006
 Answers to our Examination #, Chemistry 02X, 2006 1. (a). That SN2 shown just can t happen with the required inversion. The back of that methyl group is not reachable by the putative nucleophile, the pi
Answers to our Examination #, Chemistry 02X, 2006 1. (a). That SN2 shown just can t happen with the required inversion. The back of that methyl group is not reachable by the putative nucleophile, the pi
Theophylline (TH), the structure of which is presented below, is a bronchial-dilator used for the treatment of asthma.
 1 EXAM SCIETIIC CULTURE CEMISTRY PRBLEM 1: Theophylline (T), the structure of which is presented below, is a bronchial-dilator used for the treatment of asthma. 3 C 7 1 3 9 1.1 Structural study and acid-base
1 EXAM SCIETIIC CULTURE CEMISTRY PRBLEM 1: Theophylline (T), the structure of which is presented below, is a bronchial-dilator used for the treatment of asthma. 3 C 7 1 3 9 1.1 Structural study and acid-base
Lecture 15. More Carbonyl Chemistry. Alcohols React with Aldehydes and Ketones in two steps first O R'OH, H + OR" 2R"OH R + H 2 O OR" 3/8/16
 Lecture 15 More Carbonyl Chemistry R" R C + R' 2R" R C R" R' + 2 March 8, 2016 Alcohols React with Aldehydes and Ketones in two steps first R', + R R 1 emiacetal reacts further in acid to yield an acetal
Lecture 15 More Carbonyl Chemistry R" R C + R' 2R" R C R" R' + 2 March 8, 2016 Alcohols React with Aldehydes and Ketones in two steps first R', + R R 1 emiacetal reacts further in acid to yield an acetal
CHEMISTRY MIDTERM # 1 October 06, The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck!
 CEMISTRY 313-02 MIDTERM # 1 ctober 06, 2009 ame... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (12 pts) Mark as true (T) or false (F) the following
CEMISTRY 313-02 MIDTERM # 1 ctober 06, 2009 ame... The total number of points in this midterm is 100. The total exam time is 120 min (2 h). Good luck! 1. (12 pts) Mark as true (T) or false (F) the following
CHEMISTRY 3371 (ORG CHEM FOR MAJORS)
 1 Student Name (first, last): Student Number: CHEMISTRY 3371 (ORG CHEM FOR MAJORS) Josef Michl FINAL EXAMINATION May 8, 2007 1. (40 points) Check the correct statements only (make no other marks): ( )
1 Student Name (first, last): Student Number: CHEMISTRY 3371 (ORG CHEM FOR MAJORS) Josef Michl FINAL EXAMINATION May 8, 2007 1. (40 points) Check the correct statements only (make no other marks): ( )
Reactions at α-position
 Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
CHAPTER 22 HW: CO 2 H DERIVATIVES
 CHAPTER 22 HW: C 2 H DERIVATIVES MECLATURE 1. Give the name for each compound (IUPAC or common name). Use R/S naming where needed. Structure Br ame Structure ame 2. Give the name for each ester. Structure
CHAPTER 22 HW: C 2 H DERIVATIVES MECLATURE 1. Give the name for each compound (IUPAC or common name). Use R/S naming where needed. Structure Br ame Structure ame 2. Give the name for each ester. Structure
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS
 AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
AQA A2 CHEMISTRY TOPIC 4.10 ORGANIC SYNTHESIS AND ANALYSIS TOPIC 4.11 STRUCTURE DETERMINATION BOOKLET OF PAST EXAMINATION QUESTIONS 1 1. Consider the following reaction sequence. CH 3 CH 3 CH 3 Step 1
A. B. C. D. 2. Which compound does not give a stable Grignard reagent when reacting with Mg metal?
 Practice Test, Chemistry 2220 Final Exam 1. Which structure has the MST signals for its proton NMR? 2. Which compound does not give a stable Grignard reagent when reacting with Mg metal? 3. Which of these
Practice Test, Chemistry 2220 Final Exam 1. Which structure has the MST signals for its proton NMR? 2. Which compound does not give a stable Grignard reagent when reacting with Mg metal? 3. Which of these
CH 320/328 N Summer II 2018
 CH 320/328 N Summer II 2018 HW 1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. (5 pts each) 1. Which
CH 320/328 N Summer II 2018 HW 1 Multiple Choice Identify the choice that best completes the statement or answers the question. There is only one correct response for each question. (5 pts each) 1. Which
What is in Common for the Following Reactions, and How Do They Work?
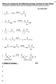 What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
Note: You must have your answers written in pen if you want a regrade!!!!
 AME (Print): SIGATURE: Chemistry 310 Dr. Brent Iverson Final May 11, 2006 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there is
AME (Print): SIGATURE: Chemistry 310 Dr. Brent Iverson Final May 11, 2006 Please print the first three letters of your last name in the three boxes Please ote: This test may be a bit long, but there is
18: Reactions of Enolate Ions and Enols
 18: Reactions of Enolate Ions and Enols 18.1 Enolate Ions and Enols 18-3 Halogenation, Alkylation, and Condensation Reactions (18.1A) 18-3 Acidity of α-c-h's (18.1B) 18-4 Resonance Stabilization Enol Form
18: Reactions of Enolate Ions and Enols 18.1 Enolate Ions and Enols 18-3 Halogenation, Alkylation, and Condensation Reactions (18.1A) 18-3 Acidity of α-c-h's (18.1B) 18-4 Resonance Stabilization Enol Form
Section Practice Exam II Solutions
 Paul Bracher Chem 30 Section 7 Section Practice Exam II s Whether problems old r problems new, You d better practice, r you ll fail exam II. -- Anonymous TF Problem 1 a) Rank the following series of electrophiles
Paul Bracher Chem 30 Section 7 Section Practice Exam II s Whether problems old r problems new, You d better practice, r you ll fail exam II. -- Anonymous TF Problem 1 a) Rank the following series of electrophiles
Aromatic Hydrocarbons
 Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
Aromatic Hydrocarbons Aromatic hydrocarbons contain six-membered rings of carbon atoms with alternating single and double carbon-carbon bonds. The ring is sometimes shown with a circle in the center instead
Aldol Reactions pka of a-h ~ 20
 Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
