GAS PHASE AND SURFACE MODELING OF CHEMICAL VAPOR DEPOSITION OF PYROLYTIC CARBON ON SILICON CARBIDE FIBERS USING A PURE METHANE PRECURSOR.
|
|
|
- Ethan Dorsey
- 6 years ago
- Views:
Transcription
1 GAS PHASE AND SURFACE MODELING OF CHEMICAL VAPOR DEPOSITION OF PYROLYTIC CARBON ON SILICON CARBIDE FIBERS USING A PURE METHANE PRECURSOR A Thesis Presented to The Graduate Faculty of The University of Akron In Partial Fulfillment of the Requirements for the Degree Master of Science Rajesh Balachandran May, 2011
2 GAS PHASE AND SURFACE MODELING OF CHEMICAL VAPOR DEPOSITION OF PYROLYTIC CARBON ON SILICON CARBIDE FIBERS USING A PURE METHANE PRECURSOR Rajesh Balachandran Thesis Approved: Accepted: Advisor Dr. Edward Evans Committee Member Dr. Bi-min Zhang Newby Committee Member Dr. Gang Cheng Dean of the College Dr. George K Haritos Dean of the Graduate School Dr. George R. Newkome Date Department Chair Dr. Lu-Kwang Ju ii
3 ABSTRACT Ceramic matrix composites (CMC) constitute a category of composite materials widely used in the aerospace industry as they satisfy the thermal, chemical and mechanical requirements of a good composite material with the drawback being in the high processing costs. Chemical vapor deposition is a process long known for its importance in aerospace and structural applications. Chemical vapor deposition can be used to develop thin interfacial coatings on fibers, which are reinforced in to a matrix according to the application. The interfacial layers are useful for preventing reactions between the fibers and the matrix material at high temperatures. Interfacial coatings can also deflect crack propagation once the composite is put into service. This work focuses on developing a suitable gas phase and surface kinetics model for obtaining the deposition profiles in the chemical vapor deposition of pyrocarbon on silicon carbide fibers at the given conditions of temperature, pressure and feed rate. The model uses an existing gas phase mechanism (National Institute for Standards and Technology and Gas Research Institute). There are combinations of species in the gas phase, but all of them do not lead to the deposition of pyrocarbon. This raises a question as to which of them would affect the rate of deposition and why. For the given conditions of temperature, pressure and flow rate and from the information available in different literature sources acetylene is assumed to be the major depositing specie of pyrocarbon. iii
4 Also, the gas phase mechanism was modified to simplify and suit the needs of the given conditions. The importance behind modeling the surface kinetics is that it could help understand the actual process in the formation of pyrolytic carbon. The mechanism of the pyrocarbon deposition could be important in knowing the type of carbon being deposited, which is of utmost importance in its applications. The modeling data is validated by means of using data obtained from experiments. iv
5 ACKNOWLEDGMENTS I would like to profoundly thank Dr. Edward A. Evans for his support and valuable suggestions throughout my tenure here, particularly, to his patience and critique of this research work. I would also like to thank Dr. Bi-min Zhang Newby and Dr. Gang Cheng for serving on my committee and giving valuable inputs. The support of Dr. Mark P. Dyko and Mr. Bryan Howdyshell from Meggitt PLC is highly appreciated. I express my sincere gratitude to the Department of Chemical and Biomolecular Engineering at The University of Akron for their support. Finally, I would like to thank my family and friends for their love, moral support and encouragement throughout my stay here. v
6 TABLE OF CONTENTS Page LIST OF TABLES..ix LIST OF FIGURES...xi CHAPTER I. INTRODUCTION Composites Fibers Fiber-Reinforced Composites Chemical Vapor Deposition/Chemical Vapor Infiltration Importance of Interface Coatings Types of Pyrolytic Carbon and their Importance Objective of this Research...11 II. LITERATURE REVIEW Development of Pyrolytic Carbon Development of Pyrolytic Carbon on Fiber Surfaces Development of Pyrolytic Carbon on Silicon Carbide Fibers Chemical Vapor Deposition Techniques Characterization of Flow in LPCVD reactors 19 vi
7 2.5.1 Significance of Reynolds Number Boundary Layer Thickness Estimation Estimation of Diffusivity Determination of Surface Reaction Rate Modeling Background Gas Phase Kinetics Model Surface Kinetics Model..23 III. EXPERIMENTAL SECTION Experimental Setup Reactor Setup Sample Preparation Weight Measurement (CAHN Microbalance) Microbalance Calibration Reactor Pump Down Reactor Start-Up Flow Control and Display Pressure Measurement, Display and Control Temperature Measurement and Display Mass Spectrometer.37 IV. EXPERIMENTAL RESULTS AND DISCUSSION Experimental Results Effect of Flow Rate Statistical Significance of the Deposition Results.42 vii
8 4.1.3 Effect of Residence Time Effect of Temperature Activation Energy Determination Reactor Model for CHEMKIN Modeling Results Gas Phase Modeling Reduced Reaction Mechanism Rate Analysis Sensitivity Analysis...69 V. DEVELOPMENT OF SURFACE KINETICS Surface Kinetics Modeling Development of Simple Surface Model-CHEMKIN Kinetic Explanation of the CHEMKIN Model Effect of Reactor Pressure Surface Kinetics with Hydrogen Adsorption and Reaction 5.2 Comprehensive Surface Mechanism..98 VI. CONCLUSION..99 VII REFERENCES 101 VIII APPENDICES.106 viii
9 LIST OF TABLES Table Page 1.1 Comparison of Metals, Monolithic Ceramic and Fiber-Reinforced Composites Properties of Carbon and Silicon Carbide Fibers Properties of Monofilament and Multifilament Silicon Carbide Fibers Deposition Data with Standard Deviation Statistical Analysis of Deposition Rates Activation Energies Equilibrium Concentrations of various species at T=1348K and P=12Torr Rate Analysis for selecting dominant reactions for CH 3 species Modified Rate Analysis for selecting dominant reactions for CH 3 species Rate Analysis for selecting dominant reactions for C 2 H 3 species Modified Rate Analysis for selecting dominant reactions for C 2 H 3 species Reduced Gas Phase Reactions Set from Birakayala et al Percentage Deviation from Original Gas Phase Reaction Mechanism Sensitivity Analysis for Birakayala Reduced Reaction Mechanism..71 ix
10 4.12 Significant Gas Phase Reactions from Sensitivity Analysis Corrected Modeling Deposition Rates (mg/hr) for a pure methane (99.97%) feed at different flow rates using a sticking coefficient of 5*10-5 for acetylene adsorption Rate of formation of Pyrocarbon as a function of Methyl and Acetylene Concentrations at a temperature and pressure of 1075 C and 12 Torr...84 x
11 LIST OF FIGURES Figure Page 1.1 Fiber Reinforced Composites (a) continuous and aligned fibers, (b) discontinuous and aligned fibers and (c) discontinuous and randomly oriented fibers Modes of Crack Propagation subject to tensile loading (a) normal to fibers (b) crack deflection along the fibers or splitting Homogeneous and Heterogeneous reactions indicating the deposition of various types of pyrocarbons Experimental Setup for pyrolytic carbon deposition Sequence of events during Chemical Vapor Deposition Inter dependence of process parameters, coating properties and reaction chemistry in chemical vapor deposition process Pyrocarbon Deposition Rate (cm/sec) profiles obtained from Bammidipati Surface Reaction Model 3.1 Schematic of the Experimental Setup Sample preparation (a) sample in the form of eight, (b) extreme ends tied xi
12 Together Overall Deposition Curve Smoothed weight (dw/dt) as a function of time Schematic of the Calibration Process Temperature Profile along the length of the reactor Schematic of Mass Spectrometer Deposition Rate profiles at different temperatures Deposition Rate Profiles at different residence times Deposition Rate with varying residence times with linear fit Deposition Rate Profiles at different flow rates Plot of deposition rate versus the inverse of temperature Relationship between software and user input files in CHEMKIN code Schematic of reactor in CHEMKIN Kinetic and Equilibrium Concentrations of hydrogen and methane at T=1348K, P=12Torr and CH 4 =100% Kinetic and Equilibrium Concentrations of acetylene and benzene at T=1348K, P=12Torr and CH 4 =100%...56 xii
13 4.10 Rate of Production of CH 3 species Total rate of production of CH 3 species Rate of production of C 2 H 3 species Rate of production of C 2 H 5 species Concentration profiles of (a) methane, (b) acetylene and (c) ethylene for different gas phase reaction schemes Gas concentrations of various species at reactor temperature of 1075 C and methane inlet flow rate of 50 SCCM Development of Correction factor Corrected Modeling and Experimental Deposition Rates (mg/hr) as a function of inlet methane flow rates using a sticking coefficient of 5*10-5 for acetylene adsorption Comparison of Deposition rates obtained from CHEMKIN and Eley Rideal kinetics at a reactor temperature and pressure of 1075 C and 12 Torr Comparison of Concentrations of Methyl and Acetylene with deposition rate trending at reactor temperature and pressure of 1075 C and 12 Torr (a) Modeling deposition rate trending as a function of reactor pressure using a xiii
14 sticking coefficient of 5*10-5 for acetylene adsorption (b) experimental deposition rate as a function of methane inlet flow rate at different reactor pressures at operating temperature of 1075 C Surface site concentration of adsorbed acetylene as a function of different reactor pressures at an operating temperature and flow rate of 1075 C and 50 SCCM Ratio of acetylene to hydrogen as a function of methane inlet flow rate at an Operating temperature of 1075 C (a) Deposition rate trending of pyrocarbon with the inclusion of molecular adsorption of hydrogen at 1075 C, (b) comparison of experimental and modeling rates at 1075 C and 12 Torr (a) Deposition rate trending of pyrocarbon with the inclusion of dissociative adsorption of hydrogen at 1075 C, (b) comparison of experimental and modeling rates at 1075 C and 12 Torr Modeling deposition rate trending with dissociative adsorption of hydrogen and its surface reaction at different reactor pressures at 1075 C...97 xiv
15 CHAPTER I INTRODUCTION The goal of this research is to develop a suitable (1) gas phase and (2) surface kinetic model to predict the chemical vapor deposition of pyrocarbon interface coating on silicon carbide fibers at different reactor conditions. Silicon carbide fibers are used as reinforcements in composites in the aerospace industry, refractory industry, and nuclear fusion reactor lining to name a few (20-22). The importance of interface coatings in fiberreinforced composites is given in the sections to come. 1.1 Composites This section describes the importance and applications of composites in various industries. Composites in aerospace applications require good mechanical properties like high strength to weight ratio, shear strength and tensile strength. These requirements led to the production of metal matrix composites as they have superior mechanical properties in comparison to other substances. Metals possess high strength, but with the limitation of lower strength to weight ratio resulting from their high densities. The other drawback about metals is that they undergo failure at high temperatures (14). These limitations of metal matrix composites led to the development of ceramics and ceramic matrix composites which fulfill the requirements of an ideal aerospace material. Monolithic 1
16 ceramics contributed to the category of non-metallic materials used in energy applications, with limitations in its use in the aerospace industry. The drawback of monolithic ceramics in the aerospace industry was realized in the form of anisotropy in the load bearing capacity. The limitations mentioned above in the traditional materials encouraged the development of fiber reinforced composites. The fiber reinforced composites have good strength to weight ratio, low thermal expansion coefficient and uniform load bearing capacity. The differences in properties of the various materials are tabulated in Table 1.1 which clearly shows the advantages of using fiber reinforcement over other materials. Table 1.1 Comparison of metals, monolithic ceramic and fiber-reinforced composites (14) Type of composite Metal Monolithic ceramic Fiber reinforced Material Steel Al 2 O 3 C-C Density (g/cc) Thermal Conductivity (W/m-K) Thermal Expansion (K -1 x 10-6 ) Tensile Strength (GPa) Young s Modulus (GPa)
17 1.2 Fibers Fibers are used as reinforcements owing mainly to their strength. The strength of fibrous materials is superior in comparison to the same material in its bulk form. This higher strength of fibrous materials is a result of the fact that the grain and other microstructural units are bigger in comparison to the fiber diameter; thereby allowing a higher fraction of theoretical strength to be achieved than that possible with the bulk form of the same material (32). Therefore, it could be deduced that the probability of finding an imperfection of a critical size leading to the material s failure becomes lower with decreasing size. This effect is known as the size effect (32). The other advantage behind using fibers as reinforcing materials is their flexibility. Although materials like silicon carbide (32) are brittle in the bulk form, they become quite flexible in their fibrous form. The flexibility is of importance in developing fiber preforms for CVI. Also fibers have a high aspect ratio. Aspect ratio could be defined as the ratio of the length to diameter of the fiber (32). This high aspect ratio accounts for the load transfer in fiber-reinforced composites. These unique properties give rise to the use of fibers as a reinforcing agent in the composites. Both oxide and non-oxide fibers could be used as reinforcements (15). The first such fiber-reinforced composite was made of glass fibers. Carbon and silicon carbide fibers are widely used in the field of ceramic matrix composites. The uniqueness of silicon carbide fibers in comparison to other fibers is that they have a greater resistance to oxidation at higher temperatures, higher compressive strengths and higher electrical resistance (1, 20-22). 3
18 1.3 Fiber-Reinforced Composites The concept behind using reinforcements was developed because of the load sharing capability of reinforcements (Figure 1.1). The reinforcement of cement using iron bars serves as a good example in understanding the significance of the reinforcing agent. A fiber reinforced composite essentially consists of three components namely: matrix, reinforcement and interfacial coating (32). The fiber serves as a reinforcing material. An interfacial coating is deposited on the fiber to provide a chemical barrier between the fiber and the matrix. It also enhances mechanical strength of the resulting composite. The matrix is infiltrated in to the fiber preform which eventually gives rise to the three dimensional structure of the composite. Figure 1.1 Fiber Reinforced Composites (a) continuous and aligned fibers, (b) discontinuous and aligned fibers and (c) discontinuous and randomly oriented fibers The latest developments have led to the use of fibers (carbon, silicon carbide) as the reinforcement materials and substances like pyrolytic carbon, silicon carbide as the 4
19 matrix material. Reinforcements are mainly made in order to strengthen the resulting composite. For instance, a silicon carbide fiber- silicon carbide matrix composite was developed with a pyrolytic carbon interface as a candidate for the first wall, divertor and blanket material in fusion reactors (15). As will be explained in the next sections, pyrocarbon interface (15) has a layered structure which is of primary importance in debonding of interfaces. Also, pyrocarbon coatings firmly bond to the surface of fiber thereby becoming the primary barrier to crack propagation. 1.4 Chemical Vapor Deposition/Chemical Vapor Infiltration Fiber-reinforced composites have been produced mainly by chemical vapor deposition/ chemical vapor infiltration processes. Chemical vapor deposition is a process for depositing films on to surfaces from a gas phase precursor. The selection of the precursor depends on the application for which the material is to be used. For instance, a pyrocarbon coating on carbon fibers provides for de-bonding in carbon fiber reinforced carbon composites (38). The importance of de-bonding and its effect on toughness of composite is explained in the further sections. Chemical vapor infiltration is the process of infiltrating a pre-formed structure with the required matrix material. The major difference between the above two processes is that the former is a surface phenomenon whereas the latter is a bulk phenomenon. Chemical vapor deposition finds its uses in various fields such as interfacial coatings in aerospace materials, refractory materials, nuclear fusion reactor linings and thin films in semiconductors. To the contrary chemical vapor infiltration is useful in the manufacture of lightweight aerospace composites(8). 5
20 Silicon carbide fibers were available in two types, namely, monofilament and multifilament. A comparison of the properties of carbon and silicon carbide fibers is given in Table 1.2. The multifilament silicon carbide fibers are about twice the diameter of that of the carbon fibers. Apart from the good intrinsic properties like high strength, stiffness and high temperature stability, silicon carbide fibers possess high oxidation resistance (28) which encourages its use in composites for aerospace applications. Silicon carbide fibers also have high thermal conductivities (0.25 W/cm C) (28) and low coefficient of thermal expansion, thereby giving it a good thermal shock resistance. Table 1.2 Properties of Carbon and Silicon Carbide Fibers (2) Properties Carbon fiber (high strength) Silicon carbide (multifilament) Fiber diameter (µm) 8 15 Specific gravity Stiffness (GPa) Specific stiffness Ultimate strain (%) Strength (GPa) Specific strength Coefficient of thermal expansion ( *10-6 m/m/ C) Maximum use temperature ( C) 2000 and greater
21 Table 1.3 Properties of Monofilament and Multifilament Silicon Carbide Fibers (2) Properties Silicon carbide (monofilament) Silicon carbide (multifilament) Fiber diameter (µm) Specific gravity Stiffness (GPa) Specific stiffness Ultimate strain (%) Strength (GPa) Specific strength Coefficient of thermal expansion ( *10-6 m/m/ C) Maximum use temperature ( C) Importance of Interface Coatings The importance of coating the fiber surface with an interfacial layer is that it enhances the strength of the resulting composite when the fibers are reinforced into the matrix. The interfacial layers are formed by chemical vapor deposition. The requirements of a good interfacial coating lies in its virtue of loosely bonding with the fiber surface, in other words it should be able to de-bond when a crack approaches the fiber and deflect the crack (5, 15). The usual crack propagation mechanism in the fiber/matrix interface is that the crack propagates through the matrix and approaches the interfacial coating. Once it reaches the coating the crack is deflected by it and becomes parallel to the fiber surface. 7
22 In brittle solids failure occurs by the crack propagation through the matrix of the material whereas in the case cited above (fiber/matrix composites) the failure is as shown in the Fig 1.2. The crack propagation is terminated by the fiber-pull out mechanisms in composites (2). Figure 1.2 Modes of crack propagation subject to tensile loading (a) normal to fibers, (b) crack deflection along the fibers or splitting (2) The strengthening process is achieved by means of distributing the applied load on the fiber and matrix (7). Toughening refers to the energy dissipation in the matrix due to the presence of fiber/matrix interfaces. Toughening is enhanced when the fiber/matrix inter layers easily de-bond i.e. the inter layer needs to be weakly bound so that the crack is deflected thereby increasing the amount of energy dissipated per unit area. In contrast to this, high strength of the composite is achieved by the strong bonding of the interface to the fiber as well as the matrix so that the load transfer in between them is efficient. 1.6 Types of Pyrolytic Carbon and their importance 8
23 The importance of interface coatings has been explained in the previous section. This research work aims at developing pyrolytic carbon interfaces on silicon carbide fiber surfaces; therefore the types of pyrolytic carbon and their importance need to be known to have a good insight. Initial developments in using pyrocarbon as a space material were done by studying the structure and properties of pyrocarbon with respect to their deposition conditions (like temperature, pressure, concentrations, residence times and reactor geometry). In contrast to the graphitic lattice, pyrocarbon exhibits a turbostratic structure. The turbostratic structure is produced as a result of incomplete dehydrogenation or presence of branching in the pyrocarbon layers. The turbostratic structure bears a lot of defects resulting from the conditions of pyrocarbon deposition. The defects have been classified into three distinguishable categories namely rough laminar (RL), smooth laminar (SL) and isotropic (ISO) from their respective optical characterizations. The fluidized bed reactor studies by Bokros and tubular flow studies by Pierson and Liebermann were initially used to justify the structures of pyrocarbon (8). They correlated the formed microstructures of pyrocarbon with the reaction gas phase chemistry. The types of pyrocarbons vary in the fact that they have different angles of extinction, interlayer spacing, crystalline size and eventually densities. In contrast to the isotropic carbon, the rough laminar carbon has the closest interlayer spacing and largest crystalline size indicating the presence of minimal defects, thereby making it the desirable form of pyrocarbon. 9
24 Tesner (9) proposed that the deposition of carbon could occur in two ways namely, (1) by homogeneous gas phase reactions which cause nucleation of different hydrocarbons thereby producing soot, or (2) by the diffusion of the hydrocarbon species on to the substrate surface and its decomposition into carbon. The residence time of the hydrocarbon precursor in the reaction chamber is of importance. The longer the precursor stays in the chamber, the greater is the probability for the formation of soot or other poly aromatic products. Dimitrijevic and Marinkovic (8) observed that the deposition of soot was a major product for residence times of seconds for a propylene precursor. Soot is formed as a result of the inelastic collisions of large hydrocarbon particles favored by conditions of high initial concentrations of propylene, low temperatures and longer residence times. As illustrated by Marinkovic and Dimitrijevic (10) soot is formed at high residence times. Soot is an isotropic form of deposited pyrocarbon and also one of the unwanted forms of carbon for interfacial coatings. Figure 1.3 clearly depicts the types of pyrocarbons formed from different kinds of hydrocarbons, which are a result of the operating conditions. The aim of this research work is to model the gas phase and surface reactions which eventually lead to the formation of the various textures of pyrocarbon. 10
25 Figure 1.3 Homogeneous and heterogeneous reactions indicating the deposition of various types of pyrocarbons (13). 1.7 Objective of this Research As discussed in the previous sections, the pyrocarbon deposition and type of pyrocarbon being formed is dependent on the kinetics (both homogeneous and heterogeneous) of incoming precursor gas. The motivation behind this research is also based on studying the kinetics for specific operating conditions. A brief outline of this research work is given as follows, The determination of deposition rates of carbon from pure methane precursor using silicon carbide fibers as the substrate. The determination of activation energy (E a ) of methane by using different flow rates of the precursor gas. Analysis of the effluent gases using the mass spectrometer. 11
26 Developing a reduced gas phase mechanism for the conditions of temperature and pressure. Developing a surface reaction model and its validation. Comparison of the results from the Model and from Experiments. 12
27 CHAPTER II LITERATURE REVIEW This chapter compiles the information available in literature about the deposition of pyrocarbon on silicon carbide fibers and the gas phase and surface kinetics involved in the deposition of pyrocarbon from various precursors so that a comprehensive model could be derived for the requirements of this research work. Chemical vapor deposition of pyrocarbon on silicon carbide fibers finds its importance in aerospace and nuclear research (16, 20, 21). Pyrocarbon coatings on silicon carbide fibers find their uses in both metal matrix (21) and ceramic matrix composites (20, 37). The pyrocarbon coating on silicon carbide fibers has many advantages such as increase in tensile strength up to two times, preventive coating for interaction in metal matrix composites and weak interface to provide de-bonding thereby reducing the chances of catastrophic failure of composite (21). As already explained in the previous chapter, the type of pyrocarbon (8-10, 13) being deposited depends on the reaction pathways of homogenous and heterogeneous reactions occurring during the chemical vapor deposition process. 13
28 2.1 Development of Pyrolytic Carbon Although pyrocarbon was known since the beginning of the 20 th century, its technical importance was realized in the 1960 s (23). In the 1960 s pyrocarbon was used in space vehicles and fabrication of nuclear fuel particles (23). Pyrolytic carbon as already discussed in the previous chapter essentially exists in three different microstructures (8) namely rough laminar, smooth laminar and isotropic. As the name says, the isotropic microstructure exhibits isotropy (properties are same in all directions), while the first two exhibit anisotropy. Each microstructure has its own properties based on which it could be used in different applications. Rough laminar pyrocarbon has been widely used as interface coatings and matrix material in aerospace applications (rocket nozzle cones, engines, sheaths, brakes etc). Isotropic pyrocarbon finds its uses in the biomedical industry (implants, heart valves etc). 2.2 Development of Pyrolytic Carbon films on fiber surfaces The idea of depositing pyrolytic carbon on surfaces of fibers and also its further infiltration into the pores of fibers, thereby developing a three dimensional composite was founded in the late 1960 s due to the large availability of carbon fibers. The endurance of carbon fiber reinforced composites still ensures them to be among the high grade materials to be used in aerospace materials (23). 14
29 2.3 Development of Pyrolytic Carbon films on Silicon Carbide Fibers Silenko and co-researchers have used a continuous CVD setup in their experiments as shown in Figure 2.1. The temperatures used here are in between K and the precursor gases include toluene, n-heptane, acetone, carbon tetrachloride and methane with argon as a carrier gas. The silicon carbide fiber is continuously dispensed from a spool and collected by means of a take up spool. The fibers are heated by means of mercury contacts at both ends of the reactor through which the required current is passed Figure 2.1 Experimental setup (17) for pyrolytic carbon deposition (1) SiC fiber feed spool, (2) mercury contacts, (3) inlet tube, (4) deposition chamber, (5) outlet tube, (6) C/SiC fiber take up spool, (7) power supply The emphasis of their (17) work was on evaluating the apparent activation energies of the precursor gases used in the deposition of pyrocarbon on silicon carbide fibers. The given range of the operating conditions for the above research work is narrow. The time taken to conduct experiments at wide ranges of temperatures, pressures, flow rates and surface/volume ratios is cumbersome and costly. In order to determine the pyrocarbon deposition at other operating conditions a suitable and comprehensive kinetic model of the resultant reactions needs to be developed. The kinetic model for chemical 15
30 vapor deposition essentially consists of homogeneous and heterogeneous reactions for a particular precursor. A lot of literature could be obtained for the pyrolysis of methane (NIST and GRI) and the required set of homogeneous reactions could be shortlisted for the required operating conditions. The scarcity in the available literature for modeling the surface reactions is one of the main motivations behind this work. To better understand the concept behind developing a fitting model, a thorough knowledge of chemical vapor deposition and the factors affecting this process should be discussed. The following section describes in detail the types of chemical vapor deposition and how each one is different from the other. 2.4 Chemical Vapor Deposition Techniques As already explained in the last chapter, chemical vapor deposition is a process of depositing films on surfaces by flowing precursor gas/gases over it. Conventional chemical vapor deposition processes were carried out in either hot-wall or cold-wall reactors and over a sub-atmospheric to atmospheric pressure range (14). A variety of application specific chemical vapor deposition processes have been developed. The different types of CVD could be listed as, Atmospheric Pressure CVD Low Pressure CVD Plasma Enhanced CVD Laser CVD The main differences between the different types of CVD are the operating conditions which govern the transport and reactions. A low pressure chemical vapor deposition 16
31 (LPCVD) reactor was used for the purpose of this thesis (Figure 3.1). The importance behind using LPCVD reactors is explained in the following sections. A good knowledge of the different types of transport and reactions occurring in a CVD reactor furthers the understanding of the process. The following steps (Figure 2.2) basically describe the overall phenomenon of chemical vapor deposition of carbon on to the surface of substrate (36), Precursor pyrolysis at high temperatures Transport (diffusion) of the resulting gas from bulk phase to the surface of substrate Adsorption of gas molecules on to free surface sites on the substrate surface Reaction of adsorbed molecules with the gas phase molecules to deposit carbon Desorption of the by-products of the surface reaction Transport of by-products into the bulk Figure 2.2 Sequence of events during Chemical Vapor Deposition (36) 17
32 Figure 2.3 Inter-dependence of process parameters, coating properties and reaction chemistry in chemical vapor deposition process (24) There is a significance of using a particular temperature, pressure and flow in reactors. Figure 2.3 explains the inter-dependence of the various process parameters, reaction chemistry and thereby the coating properties. Temperature and pressure influence the rate of diffusion (36) which in turn influences the reaction rate. Flow, on the other hand defines the effective residence time of the species, which affects the deposition rate. The detailed effect of flow with respect to various parameters is discussed in the next section. 18
33 2.5 Characterization of Flow in LPCVD reactors The following section describes the importance of temperature, pressure and flow and how each of them contributes to the deposition rate of required species on to the substrate Significance of Reynolds Number Chemical vapor deposition reactors are usually operated in flow regimes where the Reynolds number of the flow is below Reynolds number for a pipe or tube flow is given by the expression, Re = ρvd/µ Where, ρ = density of the fluid, v = velocity of the fluid, d = diameter of the pipe, µ = viscosity of the fluid The density, viscosity and velocity are dependent on pressure and temperature of the process. 19
34 2.5.2 Boundary Layer Thickness Estimation The conditions used in LPCVD reactors enable the surface reactions to be rate limiting, thereby reducing the effects of boundary layer. The boundary layer thickness could be calculated from the expression (36), Δ = Where, Δ = boundary layer thickness, x = distance from inlet, Re = Reynolds number The above expression clearly suggests that the boundary layer thickness is inversely proportional to the Reynolds number. The lower, the boundary layer thickness, the lower is the effect of diffusion. For the case of a plug flow reactor, the boundary layer thickness is considered to be very small (almost zero) Estimation of Diffusivity Pressure is the driving force for diffusivity of a gas. The Chapman-Enskog equation indicates that diffusivity is inversely proportional to the pressure. For a decrease in pressure from 760 torr to 1 torr, the diffusivity of reactants and products of the surface reaction increases by more than 100 times (36). The diffusivities of the dominant species could be estimated by, 20
35 D = (1.86*10-3 ) (T 3/2 ) [ (1/M 1 +1/M 2 )] / Pσ 12 2 Ω Where, D = diffusivity, cm 2 /sec T = temperature, K M 1, M 2 = molar masses of the molecules, a.m.u P = pressure, atm σ 12 = average collision diameter of the molecules, A Ω = temperature dependent collision integral Low pressures prevent the nucleation of species formed in the gas phase, thereby reducing the chances of formation and condensation of higher aromatic species, which in turn affect the type of pyrocarbon formed Determination of Surface Reaction Rate Low pressures insure that the chemical vapor deposition is surface limiting with respect to diffusion. Sticking coefficients of the various depositing species determines the rate of surface reaction. Sticking coefficient is the ratio of number of molecules that stick or react with the substrate surface to the number of molecules that impinge on the surface of the substrate. Sticking coefficient (γ) = Sticking coefficient is related to the surface reaction rate constant by the expression (19), 21
36 K s = Where, K s = surface reaction rate constant, (cm 3, mol, s) τ = number of active sites, mol/cm 2, m = sum of all stoichiometric coefficients of the surface species R = universal gas constant, gm-cm 2 /mol-k-s 2 T = temperature of the reactor, K W = weight of the reacting gas phase species, gm/mol The surface reaction rate could be calculated by having knowledge of surface reaction rate constant, concentration of the depositing species and the surface area of the substrate. The surface reaction rate could be represented by the following expression, R s = K s * C d * A s Where, R s = surface reaction rate, C d = concentration of depositing species, mol/cm 3, A s = surface area of the substrate 2.6 Modeling Background In order to compare the operating conditions (temperature, pressure and flow rate) in the reactor a suitable gas phase and surface kinetic model needs to be established. 22
37 2.6.1 Gas Phase Kinetics Model This section describes about how suitable gas phase models were developed in the past by other researchers. A comprehensive gas phase kinetic model was determined by Birakayala (14) for the conditions of T=1373K and P=40 Torr. The reduced gas phase model (14) was obtained by thorough analysis of the existing original gas phase mechanism developed from the NIST and GRI database. The analysis was done in three steps (1) sensitivity analysis, (2) rate analysis and (3) Damkohler analysis. The resulting reduced gas phase mechanism was used in conjunction with a surface reaction scheme to predict the deposition rates in the CVD/CVI of pyrocarbon using methane (with and without dilution) as the precursor. After developing a good gas phase reaction mechanism, the need arises for the establishment of a good surface kinetic reaction set. The following section describes the development of one such comprehensive surface kinetic model Surface Kinetic Model One of the comprehensive developments in the modeling of diamond (and also graphite) deposition was done by Dandy and Coltrin (19) as they took into consideration the various conditions used by other authors. They studied the deposition of diamond in subatmospheric dc plasma-gun reactors using methane as feed gas. The methane feed is diluted so as to contain less than 1% methane. The reactor was maintained at a pressure of 30 Torr, while two different inlet temperatures (2500 and 3300K) and a constant substrate temperature (1200K) were used. These conditions favor the deposition of 23
38 diamond as the major product. The reason behind the high deposition rate of diamond could be attributed to the abundant formation of CH 3 radicals (for high inlet model fractions of H) and their subsequent abstraction reactions to form CH 2, CH and C. the depositing species for diamond are C, CH 3 and C 2 H 2, but under the given conditions only C and CH 3 largely contribute to the diamond deposition. Here, graphite is formed as a byproduct. The deposition of graphite increases with the increase in mole fraction of inlet methane. This result indicates that for the scope of this research work (pure methane feed, 1075 C and 12 Torr), acetylene could be cited as the major deposition species and the surface reactions used for the deposition of graphite (19) could be adopted for modeling. Bammidipati and co-researchers (18) established sticking coefficients for the deposition of pyrolytic carbon on graphite rods by using pure methane as precursor for a substrate temperature and reactor pressure range of C and Torr. They indicate the use of different sticking at different pressures and temperatures as given by the following surface reaction rate constant expression, K s = γ Where, K s is the surface reaction rate constant, γ is the sticking coefficient and M is the molecular weight of the pyrocarbon depositing species. For a particular temperature, the sticking coefficient decreases with increase in pressure (18). They observed that the deposition rate trending did not significantly vary by varying the sticking coefficient for a particular temperature and pressure. 24
39 Once the surface kinetic model has been developed, it needs to be compared with the available literature and experimental data to check for consistency. The chapters to come have described in detail about the required experimental setup, comprehensive gas phase and surface kinetic model and its validity. Figure 2.4 Pyrocarbon Deposition Rate (cm/sec) profiles obtained from Bammidipati Surface Reaction Model The surface model developed by Bammidipati (18) was initially used with the respective sticking coefficients at different reactor pressures and the results were compared to the experimental results. The trends observed by using the Bammidipati surface model from CHEMKIN are compared to the experimental deposition rate trending as shown in the Figure 2.4. The peak (18) in the pyrocarbon deposition rate is observed at low inlet flow rates of methane (<10 SCCM) while the peaks observed from 25
40 experiments were observed at higher flow rates (>25 SCCM). This shift in deposition could be accounted for by the inclusion of a hydrogen inhibition mechanism which is developed and tested in the Chapter V. 26
41 CHAPTER III EXPERIMENTAL SECTION 3.1 Experimental Setup This chapter describes in detail the experimental setup used to coat pyrolytic carbon on to silicon carbide fibers Reactor Setup The experimental setup consists of a low pressure chemical vapor deposition (LPCVD) reactor with an alumina tube. The alumina tube is 2 inches in diameter and 47 cm in length (Figure 3.1). The inlet and outlet lines of the reactor are one inch in diameter. The reactor tube is surrounded by a cooling jacket through which water is flowed. Water flow through the jacket is not in direct contact with the reactor tube, but flowed through copper tubing. The alumina tube and jacket are independently connected to two vacuum pumps (Edwards). The reactor is isolated from the atmosphere by means of rubber O-rings (Viton) and pumped down to vacuum. As shown in the experimental setup (Fig 3.1), the pure methane gas (99.97% pure, Grade 3.7, Advanced Gas Technologies), is metered into the reactor by means of flow controller (MKS Type 247B 4 channel readout) at various temperatures ( C) 27
42 and flow rates ( SCCM) at constant reactor pressure of 12 Torr. The methane gas enters the reactor through the left end of a four way connection, while the pressure in the chamber is measured by means of a baratron gauge (MKS Type 222BA) and thermocouple gauge (DST-531) connected to the other end. The baratron and thermocouple gauges are in turn connected to a pressure display (PDR-C-2) and multimeter respectively for indicating the pressures. The pressure display indicates the pressure of the reactor and can read as low as 10-2 Torr. The upper end of the vertical part of the four way connection is connected to the microbalance while the lower end is connected to the reactor. The silicon carbide fiber sample is suspended from the microbalance by means of a nichrome wire. The microbalance (CAHN-2000) is used to measure the change in weight of the sample as deposition occurs. As the name says, microbalance is used to quantify weight in micrograms (µg). The microbalance is connected to a digital weight recorder by means of an electrical tether. This digital recorder is in turn connected to a personal computer which displays the change in weight with respect to time. The details of the microbalance calibration and sample preparation are given in the following sections. The incoming hydrocarbon gas (methane) flows into the reactor and undergoes homogeneous and heterogeneous reactions thereby depositing pyrocarbon on the silicon carbide fiber and then exits the reactor at the bottom by means of a vacuum pump. The effluent then passes through a filter through a valve into a vacuum pump. The valve is placed above the pump in order to regulate the pressure in the reactor. A secondary line is provided to analyze the concentrations of effluent by means of mass spectrometer. 28
43 Fig 3.1 Schematic of the Experimental Setup Sample preparation The polycrystalline silicon carbide fiber sample is about 70 inches in length and 10 µm in diameter. Since it is longer than the reaction chamber it needs to be bound in such a way that it is essentially within the radial and longitudinal limits of the reactor. The configuration used in the setup was as shown in Figure 3.2. The fiber sample is folded four times and tied at the center as shown. The sample is in the form of an eight. The extreme ends are tied together and suspended from the microbalance. The advantage 29
44 of this configuration is that it effectively lies in the pathway of the precursor gas and there lies a good probability for the deposition reaction. Figure 3.2 Sample Preparation (a) sample in the form of eight, (b) extreme ends tied together The fiber sample is suspended from the microbalance by means of a nichrome wire. The length of the nichrome wire needs to be such that the sample is placed exactly in the center of the reaction zone. The reaction zone essentially is that portion of the reactor where the homogeneous reactions take place. The sample essentially needs to be in the center of the reaction zone. Before the sample is loaded, the microbalance needs to be calibrated so that it is measuring accurately in the desired weight range. The details about the weight measurement and microbalance calibration are given in the following sections. 30
45 3.1.3 Weight Measurement (CAHN microbalance) The sample is suspended in the center of the reactor by means of a nichrome wire. The other end of the nichrome wire is hooked on to the CAHN microbalance (Figure 3.1). The microbalance has an accuracy of up to 0.1 µg. The maximum weight of the sample is about 1.5 gm to get the best accuracy. Smoothing of the deposition (weight) values is done using the CAHN software (35). Caution must be taken when the smoothing region is taken into consideration. The smoothing region (Figure 3.4) which has less chatter is considered and the average value of the deposition rate is obtained. For consistency of the measurements, the average value of deposition for a time period of 10 minutes is considered. The standard deviation should be within a range of ±10% of the average deposition rate for the chosen region. Deposition Cool down Heat up Figure 3.3 Overall deposition curve 31
46 As shown in Figure 3.3, the overall deposition curve consists of three regions namely heat-up, deposition and cool down. The heat-up region is the period in which the reactor is heated to the required temperature. Heat-up of the reactor usually takes between four to five hours depending on the temperature to be achieved. Once the required temperature is reached, the reactor is allowed to stabilize and then the gases are introduced in the chamber. Further details and precautions about the heat-up process are given in section The initial jump in weight between the heat up and deposition phases is due to the force exerted by the sudden flow of gases into reactor. The weight of the sample further increases linearly indicative of a deposition. The microbalance records the weight of the sample in regular intervals of 10 seconds. The average deposition rate is found by averaging the smoothed deposition data. Figure 3.4 indicates that the smoothed weight is constant for the time period of collection with the exception of a few peaks occurring due to the presence of noise from external sources. Once the deposition is complete, the gas flow is turned off and the valve to the vacuum pump is opened up completely. The reactor is now under cool down and maintains a constant sample weight throughout the process (Figure 3.3). 32
47 Figure 3.4 Smoothed weight (dw/dt) as a function of time Microbalance Calibration The mass of the sample needs to be within the weight limits (±1.5gm) of the microbalance in order to collect and record the deposition data. To ensure this, the microbalance needs to be calibrated to the weight of sample. First, the weight of the fiber sample was weighed on a simple weight balance. Then an equivalent of fiber sample weight is taken in the form of fractional weights and is used to calibrate the microbalance. The fractional weights were placed on either arms (sample and tare pans) of the microbalance (Figure 3.5). The resultant weight would be 33
48 approximately (highly sensitive to vibrations of the pump) zero milligrams indicating equal weights on both sides. Figure 3.5 Schematic of the calibration process Now, a 100 mg weight is placed on the sample pan (Figure 3.5), thereby showing a weight reading of +100 mg on the display. Then the microbalance is calibrated for the weight of 100 mg. The 100 mg weight is then removed and the sample is loaded on to the sample pan of the microbalance. A typical sample weight of 320 mg was used. Once the sample is loaded, the lid of the microbalance is placed on top of the apparatus and sealed by means of tightening screws. The reactor is then sealed and the pump down is started. 34
49 3.1.5 Reactor Pump Down The reactor is pumped down once the sample is loaded in the reactor. The sample loading and reactor pump down are usually started the day before actually starting the reactor run (heat-up). The pump down ensures that the reactor is under good vacuum thereby reducing the content of air and moisture, which are not desirable for the concerned conditions. After pumping down the reactor for about eight hours, the heat-up is started Reactor Start-Up The reactor is heated to the required temperature by means of heating coils connected to a high voltage transformer. The controls to the transformer are setup in the control panel shown alongside the reactor in Figure 3.1. The reactor is heated in steps of 0.5 V after an initial soak at 5 V for a time period of one hour. The heating rate is about 200 C/hour. This gradual heating of the reactor prevents any damage resulting from thermal shocks. After reaching the required temperature the reactor is allowed to equilibrate at that condition for about 20 min and then the flow of precursor gas is started. There is an initial jump in temperature that could result from the various homogeneous reactions taking place as the gas enters the reaction chamber. After some time the temperature stabilizes and comes back to its original set value. 35
50 3.1.7 Flow Control and Display The reactor is fed with methane (99.97% pure, Grade-3.7, Advanced Gas Technologies) as the precursor gas at the required temperature ( K) and pressure (12 Torr). The flow rate of methane is set using an MKS mass flow controller (Figure 3.1) connected to mass flow meter/readout (MKS Type 247B 4 channel readout). The set point for the flow rate must be given to the mass flow meter before the gas is introduced into the reactor. Once the gas is introduced, the pressure of the reactor is regulated by means of a valve. The position of the valve is varied with respect to the flow rates to maintain a constant pressure of 12 Torr. The valve is turned inwards when the flow rates are stepped down (decreased). The gases are flowed in the descending order of flow rates i.e. 200, 100, 75, 50 SCCM. This sequence was maintained for all the experimental runs. The significance behind maintaining the same sequence is that the deposition rate calculations become independent on the sequence of flow rates Pressure Measurement, Display and Control The pressure in the reactor is measured using a Baratron gauge (MKS Type 222BA) and a Thermocouple gauge (DST-531). The output of the Baratron and Thermocouple gauges are read by means of MKS PDR-C-2 and multimeter display respectively (Figure 3.1). After the reactor attains the reaction temperature, the reactor pressure needs to be maintained at about 12 Torr and this is achieved by using a vacuum regulating valve in tandem with the Baratron gauge. 36
51 The jacket pressure is also measured using a Thermocouple gauge (DST-531). Water is continuously flowed through the jacket of the reactor for constant cooling of the outer containment of the reactor. This continuous flow of water is required for the uniform heating and also to prevent damage to the reactor Temperature Measurement and Display The temperature is measured by means of an N type thermocouple. The thermocouple is placed about 2cm below the sample. The temperature varies as a function of the length of the reactor. The observed temperature profile as shown in Figure 3.6 is a parabola with the average high temperature being at the center of the reactor. The advantage of using a hot-wall reactor with a temperature gradient is that there is a gradual variation in temperature because of which the turbulent forces decrease (42). Fig 3.6 Temperature profile along the length of the reactor 37
52 3.2 Mass spectrometer The outlet composition here is measured in-situ by the mass spectrometer which is connected to the outlet stream of the reactor upstream of the pressure control valve (Figure 3.7). The startup of the mass spectrometer is a very methodical process. The valves on either side of the system are closed initially and the system is pumped down. The system is pumped down to a very low pressure using a roughing pump (10-3 Torr) and turbo pump (10-8 Torr). Then the gate valve or the roughing valve to the mass spectrometer is opened slowly. The pressure is allowed to stabilize (10-8 Torr) and then the valves to the reactor are opened one by one. Care must be taken in not allowing the vacuum to increase greater than 10-5 Torr; else it causes the mass spectrometer filament to shut down just as a precaution. The mass spectrometer is used in the multiplier mode. The current is input in steps of 0.25 ma by monitoring the pressure. A peak current of 2.1 ma is supplied to the filament. The scanning range is set to an m/z ratio of 34 with a scan center at 17. The frequency of the scans could be adjusted by using a variable scan setting. The output peaks could be recorded in the range of 10-5 to amps. The output of the mass spectrometer is collected using a data acquisition device (DI-158U) and software (WINDAQ). The collected data is in the form of peaks. Therefore, for a given m/z ratio the peaks would be repeated according to the scan frequency. The higher the frequency, the higher is the number of peaks for a given 38
53 amount of time and for a given m/z ratio. The output file from WINDAQ is processed in MATLAB and the average values for the intensities of peaks are calculated. Figure 3.7 Schematic of Mass Spectrometer Having explained the experimental setup in detail, the obtained experimental results from the LPCVD reactor need to be discussed. The following chapter explains the experimental results and their statistical significance. Also, the establishment of a suitable gas phase and surface kinetic model is described. 39
54 CHAPTER IV EXPERIMENTAL RESULTS AND DISCUSSION 4.1 Experimental Results Results were obtained by performing experiments at a particular temperature for a given run, by changing the flow rate every 40 minutes. The flow rates were stepped down from 200 to 50 SCCM and this sequence was maintained for every experiment. As described in the previous chapter the deposition rate is measured with respect to the weight deposited per unit time. The resulting deposition rate data is smoothed by the CAHN software and the deposition rate for a given flow rate of methane is obtained. The deposition data was obtained from three sets of experiments for reproducibility. Care is taken to ensure that the sample size and other operating conditions are the same in all experiments. The standard deviation in between the sets of data is plotted on the deposition profile to indicate the accuracy of results. Table 4.1 shows the obtained results for deposition data. An important point to be noted here is that the deposition data for the low temperature (980 C) produced a large measurement deviation in all three experiments. The deposition rates at 980 C were obtained by both smoothing as well as R 2 fitting of the deposition data. The smoothed deposition data of the 980 C produced large standard 40
55 deviations, so the deposition data is recorded as the slope of the linear trendline to weight deposited versus time. The large standard deviation in the low temperature deposition data could be attributed to the operating conditions of the reactor. At temperatures lower than that of pyrolysis temperatures of methane, the probability of deposition is less and the data collected could be that of the weight of deposited carbon and also the weight exerted by the flowing gas (dominant). Table 4.1 Deposition data with Standard Deviation Flow rates of pure methane, SCCM Temperature, C ± ± ± ± ± ± ± ± ± ± ± ± Effect of Flow Rate The temperature profile as shown in the Fig 3.6 is parabolic along the length of the reactor. The highest temperature is however in the center of the reactor. Fig 4.1 shows the deposition rate profiles of carbon using a pure methane (99.97%) precursor at four different temperatures. Murphy suggested that carbon film deposition from methane is initiated at temperatures of about 1000 C. The deposition curves at 980 C and 1030 C are a clear indication that methane pyrolysis or carbon film deposition from pure methane takes place in between these temperatures as suggested by Murphy (1). 41
56 Fig 4.1 Deposition Rate profiles at Different Temperatures By using pure methane as feed, the probability of the reaction of any methane molecule becomes the same. The methane closest to the wall or the methane molecule at a higher temperature tends to react and form products. The low residence times used in this research work indicate that the methane molecules have lower probability to stay close to the wall, thereby leading to a very minute conversion in methane. As the flow rate changes the molar rate of the reacting species also changes as per the given equation. F ai = µ i * C ai The observed trending in the deposition rates of pyrocarbon on silicon carbide fibers with respect to different flow rates (50, 75,100, 200 SCCM) is also illustrated in the Figure 4.1. The trending suggests that the deposition rate of pyrocarbon decreases 42
57 with increasing flow rate. The statistical significance of the obtained deposition results is discussed in the next section Statistical Significance of the Deposition Results The obtained deposition data needs to be statistically analyzed to check the significance of the operating conditions (temperature, pressure, temperature and pressure) on the deposition rate values. By observing the deposition data (equal rows and columns); Latin squares (34) could be cited as the most suitable statistical analysis method. The sum of squares (SS) of the treatments, temperature and flow is calculated to evaluate the sum of squares of error (34). Then the mean of squares (MS), F o and p values are calculated using their respective formulas (Appendix B). Table 4.2 Statistical Analysis of Deposition Rates Source SS df MS F o p Treatments Temperature Flow Error Total It could be clearly seen from Table 4.2 that the p-value for the treatment is , which is greater than 0.05, thus making the effect of treatments insignificant. The treatments represent the deposition rate obtained at a given value of flow rate and temperature. The independent effects of temperature and flow rates are significant (0.008 and ). 43
58 4.1.3 Effect of Residence Time The deposition data plotted for the different flow rates could be otherwise represented in the form of residence times (Fig 4.2) corresponding to their respective flow rates. It is clear from the standard deviation data that the error bars corresponding to the flow rates (residence times) do not overlap on one another indicating that the deposition rates are significantly different from each other. Figure 4.2 Deposition Rate profiles at different Residence Times Fig 4.2 shows that the slope for the deposition rate curve at 1030 C and 1050 C have a small slope up to the residence time of 3 seconds beyond which the slope rapidly increases. The interesting point to be noted here is that the slope of the deposition rate curve at 1075 C does not change as rapidly as that at 1030 and 1050 C. Another possible way to analyze the data given in Fig 4.2 is by linearly trend fitting the curves. Fig 4.3 shows the data of the linearly fit deposition data with their respective error bars. 44
59 Fig 4.3 Deposition rate with varying Residence times with linear fit The linearity in the fit increases as the temperature is increased from 1030 C. It is important to note that the coordinates representing the residence times of 1 and 2 seconds line on their respective trend lines, while the other two coordinates fall off. This deviation in the fit is indicative of a change in the amount of available deposition species. At higher temperatures, the amount of carbon growth species is high enough that the deposition rate changes constantly with the change in temperature, whereas at temperatures lower than 1075 C, the amount of species available for surface reactions may be the limiting factor Effect of Temperature Figure 4.4 illustrates that as the temperature increases, the deposition rate increases as expected. The trending observed at a constant flow rate is almost linear. It is evident from the graph that the deposition rate at 980 C is almost negligible as observed 45
60 from Table 4.1. The other important observation from the data is that as the temperature is increased beyond 1030 C, the deposition rate doubles with every step. Figure 4.4 Deposition rate profiles at different flow rates Activation Energy Determination This section deals with the arrival at the activation energy for the experimental deposition of pyrocarbon from pure methane. The activation energy for the overall reaction could be determined from the obtained deposition data. The rate of carbon deposition could be written as R = dc p /dt = kc p n A s (2.1) Where, R is the rate of carbon deposition, k is the reaction rate constant, C p is the concentration of the precursor, n is the reaction order and A s is the surface area of the substrate. For a given weight of substrate and flow rate of precursor gas, the reaction rate 46
61 predominantly depends on the reaction temperature [2]. The expression for rate constant is given by Arrhenius equation, k = A exp(-e/rt) (2.2) Where, A is the pre-exponential factor and E is the activation energy. Now substituting equation 2.2 into equation 2.1 gives the rate expression in terms of Arrhenius constants. The equation can be rearranged and written as R = A exp (-E/RT) C p n A s First order reaction was assumed and a plot of ln(r) versus 1/T was plotted, the slope of which gives the activation energy (E) as shown in Figure 4.5. Fig 4.5 Plot of deposition rate versus the inverse of temperature 47
62 Table 4.3 Activation Energies Flow rate (SCCM) Activation Energy (Kcal/mol) The average value of activation energy is about 115 ± 5.9 Kcal/mol which is close to the minimum energy required to break a C-H bond in a methane molecule (104.3 Kcal/mol). Also, Silenko and co-workers have mentioned activation energy of about 95 Kcal/mol for methane pyrolysis at temperatures of K (3). Having analyzed the obtained experimental deposition rate data, the focus needs to shift towards the establishment of a suitable gas phase and surface model for the above operating conditions. 4.2 Reactor Model for CHEMKIN The concentrations of various gas phase species for the actual reactor specifications need to be calculated so as to compare the model to the real case scenario. The geometry and operating conditions of the reactor have already been explained in the previous sections. The CHEMKIN package is used to model the gas phase and surface kinetics. CHEMKIN has the advantage of being able to input the gas phase and surface reaction chemistry files in the same software package which is not the case in 48
63 the older versions of CHEMKIN. The general working of a CHEMKIN software package is elucidated in the Figure 4.6. The newer version of CHEMKIN (4.1.1) has the CHEMKIN, SURFACE CHEMKIN and TRANSPORT packages merged into a single interface. Figure 4.6 Relationship between software (boxes) and user input files (ovals) in CHEMKIN code (30) A similar model (Fig 4.7) needs to be developed in CHEMKIN so as to get considerably good comparison. Gas phase and surface reactions are written according to the syntax given in CHEMKIN manual (29) and their respective input (text) files are created. The thermodynamic (therm.dat) and transport properties (tran.dat) are adopted 49
64 from the CHEMKIN library (29). A brief explanation as to how the software calculates the reaction parameters needs to be given to understand its functioning. For a thermal system with reversible reactions (29), the forward reaction rate constant is calculated by means of, K fi = A i T β exp (-E i /R c T) Where A i is the pre-exponential factor, T is the gas temperature, β is the temperature exponent and E i is the activation energy for the reaction. The reverse reaction rate constant is related to the forward rate constant by the following expression, K ri = K fi /K ci Where, K ci is the equilibrium constant. From thermodynamics it is well known that, the equilibrium constant is related Gibbs free energy by, ΔG i = -RT ln (K ci ) ΔG i is the change in Gibbs free energy and this value is calculated from the change in enthalpy (ΔH) and entropy (ΔS) values. ΔG i = ΔH i - TΔS i The change in enthalpy and entropy values for given reaction (i) could be arrived at by using the thermodynamic properties (seven coefficients a 1 -a 7 ) (29) defined in the gas phase input file. The simplest consideration of the experimental reactor would be that of a plug flow reactor due to its simplicity. Therefore, the PLUG flow package of CHEMKIN is 50
65 used to determine the concentrations of the species. The PLUG flow package assumes that the thickness of boundary layer is constant at 0.01 cm throughout the length of the reactor. The assumption of plug flow is reasonable for the operating conditions of this research work, as the Peclet number is very small (~0.6). A variable boundary layer thickness could be given from the user interface. The user defined inputs include the gas phase and surface reaction chemistry, physical dimensions (diameter, length), temperature of the surface and gas phase (temperature profile if any), inlet flow rate, inlet concentrations and initial guess for the surface site fractions. To start with, a very simple geometry of the reactor (Figure 4.7) is considered. The reactor is considered to be a cluster which includes all the various flow and reaction variables. The reactor physical properties, species-specific properties and bulk-phase properties were defined in the cluster properties. A reactor length of 18 inches and a diameter of 2 inches were used to specify the reactor physical dimensions. The pressure in the reactor was taken as 12 Torr. The species-specific properties tab was used to define the surface fraction, bulk activity and surface site density for surface species. Figure 4.7 Schematic of the reactor in CHEMKIN 51
66 A temperature profile (Fig 3.6) was employed along the length of the reactor. The center of the reactor is maintained at the target temperature i.e. the average substrate temperature. The average substrate temperature is varied between C. A fixed gas temperature problem type is considered for a given profile. The temperature profile is defined for the gas and in the real case also the temperature of the gas is measured. The surface temperature is considered to be the same as the gas temperature. The inlet specifications of the precursor gas could be defined in the inlet panel. The flow rate was varied in the range of SCCM with no diluent. The inlet mole fractions of methane, ethane and propane were taken as , and respectively. An absolute and relative tolerance of 1*10-6 was considered for the solver properties. They define the convergence criteria for the calculations of the solver. Lower the convergence criteria, higher is the error in the output of the model. The modified Arrhenius expression is used to indicate the temperature dependence for various reactions. For the calculations involving pressure dependent rate constants the TROE expressions were employed. All the required Arrhenius parameters have been obtained from the NIST and GRI database. The required thermodynamic properties were taken from CHEMKIN and JANAF databases. Assumptions in the Model the model. The following assumptions were taken into consideration to effectively simplify 52
67 Plug flow Cylindrical flow channel Activation energy of surface reactions are zero (refer to chapter V) Active sites already exist C(R,G) = 1 The next section describes the development of a suitable gas phase model for the above given reactor setup. As the maximum possible conversion or in other words pyrocarbon deposition could be observed in a plug flow this assumption defines the maximum limit of the deposition values and therefore could be used as a starting point. 4.3 Modeling Results The different techniques used to develop a simplified reaction mechanism are described in the sections to come Gas phase Modeling This section deals with the various gas phase models developed previously and optimizing their use in the development of a suitable mechanism for the needs of this thesis. A detailed knowledge of the homogeneous or volumetric reaction pathways is required to model the deposition of pyrocarbon. Natural gas is used as a common precursor in the carbon CVD/CVI processes (14). Methane is the major constituent of natural gas and could be conveniently used as a precursor for carbon CVD/CVI. The main emphasis behind modeling the gas phase is dependent on modeling the pyrolysis of methane at the given conditions of temperature, 53
68 pressure and flow rate. One of the important developments in modeling the gas phase chemistry of methane was based on Gas Research Institute s (GRI) mechanism for combustion (GRI-Mech 2.11). The GRI mechanism essentially consists of 49 species and their resulting 277 elementary reactions. The mechanism gives a detailed description about the rate expressions used to model the species mainly consisting of Carbon, Hydrogen, Nitrogen and Oxygen. The gas phase mechanism required for the purpose of this research work limits the use of only the reactions containing the species with carbon and hydrogen and the rest could be neglected. The GRI mechanism used here is therefore condensed from the original form and consists of 13 species participating in 31 reversible reactions. This mechanism illustrates reactions with one or two carbon atoms, whereas the possibility of the presence of higher carbon containing species could not be neglected (Murphy et al, 1996). This necessitated the addition of reactions with C1-C6 species to make the gas phase model comparable to the real case scenario. The final mechanism included 19 species with 47 reversible reactions. Birakayala showed that the number of reactions in the mechanism could be further reduced by carrying out a sensitivity analysis. The condensed set of gas phase reactions consisted of 28 reactions and was adopted for modeling the gas phase for the purpose of this thesis. It was necessary to have an idea of the equilibrium concentrations (31) of various gas phase species and decide the dominant species for deposition of carbon. An equilibrium calculation was done with the help of EQUIL functionality of CHEMKIN for the given precursor and the resulting equilibrium concentrations are tabulated in Table 54
69 4.4. The table clearly indicates that acetylene, ethylene and benzene are the major components of pyrolysis at equilibrium conditions or at very high residence times. Table 4.4 Equilibrium concentrations of various species at T = 1348K and P = 12 Torr Species Initial Equilibrium H 0.00E E-05 H E E-01 CH E E-06 CH E E-02 C 2 H E E-01 C 2 H E E-07 C 2 H E E-03 C 2 H E E-09 C 2 H E E-07 C 3 H E E-10 C 4 H E E-03 C 4 H E E-04 C 4 H E E-08 C 4 H E E-08 C 6 H E E-02 The above obtained equilibrium concentrations are in agreement with those calculated by Birakayala (14). The gas concentration profiles along the length for an 55
70 isothermal plug flow reactor at 1348K, 12 Torr and residence times of 400 seconds have been plotted in Figure 4.8 and Figure 4.9. Residence time of 400 seconds was chosen in order to provide enough time to the gas phase to achieve equilibrium, but it could be observed that the species approach equilibrium and do not attain equilibrium except for the C 2 H 2 species. Fig 4.8 Kinetic and Equilibrium Concentrations of hydrogen and methane at T=1348K, P=12 Torr, CH 4 = 100% 56
71 Fig 4.9 Kinetic and Equilibrium Concentrations of acetylene and benzene at T=1348K, P=12 Torr, CH 4 = 100% Reduced Reaction Mechanism 57
72 The complete reaction mechanism developed by Birakayala (14) from the Gas Research Institute and NIST database consisted of 47 reactions with 19 species. The drawback of using this reaction set is that it consists of many species with unknown concentrations which when combined with the transport equations increase the difficulties in convergence. In order to avoid this difficulty in convergence, the reaction set needs to be condensed. The reduced set of reactions should represent the complete reaction set within an acceptable deviation limit of 10%. The sensitivity analysis technique is used in order to reduce the reaction set. The following section describes the techniques used to reduce the original reaction set. The complete reaction set was condensed by using different reduction techniques. The species which really do not affect the further rate of production of the deposition species need to be eliminated. The reduction techniques used here are sensitivity analysis and rate analysis. Eventually the results of these reduction techniques are compared for consistency Rate Analysis Birakayala (14) developed the reduced reaction set for the temperature and pressure of 1373K and 40 Torr. As already explained, the higher the pressure, the higher is the chance for the formation of the higher aromatic species. A higher pressure therefore hints at the inclusion of aromatic species in gas phase reaction mechanism. The operating conditions used for the purpose of this thesis were T=1348K and P=12Torr. A suitable gas phase reaction needs to be developed for this purpose. 58
73 A reduced gas phase reaction was developed for the conditions of T=1348K, P=12 Torr and Q=50sccm, since the maximum deposition occurs (14) at these conditions. A reaction feed with 100% methane is considered as the inlet. A gas phase mechanism satisfying the above operating conditions would be good for other temperatures and flow rates. As a starting point the model is run for the complete reaction mechanism consisting of 47 reactions and 19 species (17). The rate of production of dominant species from the complete mechanism is compared to that with the condensed reaction mechanism consisting of 28 reactions with 15 species. The sensitivities and rates are identical, thereby indicating the use of the condensed reaction mechanism as a representative of the complete mechanism. The reduced gas phase reaction set developed by Birakayala (17) consists of 28 reactions with 15 species. The rate analysis is based on the rate of production of a specific species from different reactions to its total rate of production. A reaction is considered superfluous if the rate of production of the particular species is significantly small i.e. lower than 5% of the total rate of production. The rates of production of all the species having high concentrations are measured (14). Thereby a reduced reaction set could be derived by using this approach. The species that is absent in the reduced mechanism is considered superfluous and is eliminated. If r ik could be defined as the rate of production/dissociation of species k from the reaction i, then the total production/dissociation rate of the species k could be given by R k, therefore α ik could be written as, 59
74 α ik = r ik /R k A reaction could be considered insignificant if the α ik is lower than This means that reactions contributing 5% or more end up representing the complete mechanism. From the model the dominant gas species are identified as H 2, CH 3, CH 4, C 2 H 2, C 2 H 4, and C 2 H 6. The rate of production of radical species like C 3 H 3, C 3 H 4, C 4 H 3 and C 4 H 4 are very small in comparison to other radical and dominant species, thereby these species could be eliminated and also the reactions in which they appear. Elimination of the above radical species reduces the number of reactions to 17 from the actual set of 28. Although, C 2 H participates in reactions that produce C 2 H 2, it could be neglected on the basis of rate analysis i.e. the rate of production/dissociation of C 2 H is very small for the given operating conditions. The reactions consisting of C 2 H are eliminated from the reaction mechanism, thereby yielding a mechanism with 15 reactions and 9 species. The total rate of production/dissociation of radical species CH 3, C 2 H 3 and C 2 H 5 are very small in comparison to individual reaction rates contributing to their production/dissociation. Although the above radical species have a low rate of production, their respective reactions they cannot be eliminated from the reaction set since the individual reactions are very sensitive. Figure 4.10 shows the rates of production of CH 3 species for different reactions. The total rate of production of CH 3 is higher than the rate of production of CH 3 from individual reactions. It is interesting to know that the CH 3 species exhibits a maximum total rate of production as well as dissociation (Figure 4.11), implying that the rates of 60
75 production and dissociation are highly competitive. The actual α i values for CH 3 from the modeling data is shown in Table 4.5. The α i values thus obtained for the individual reactions contributing to the production/dissociation of CH 3 are higher than 1, thereby indicating the requirement of normalization with respect to the highest rate of production/dissociation. The modified rate analysis (14) could be used to troubleshoot the issue of α i being greater than one. Modified rate analysis considers the maximum rates of production/dissociation of a particular species and the modified α i values are calculated keeping this as a basis. H+CH 4 = CH 3 +H 2 2CH 3 = C 2 H 5 +H From Figure 4.10 it could clearly be seen that the first reaction (H+CH 4 = CH 3 +H 2 ) has the highest rate of production of the CH 3 species, while the second reaction (2CH 3 = C 2 H 5 +H) has the highest rate of dissociation. Therefore, the modified α i values have been calculated with respect to the above reactions and displayed in Table 4.6. The reactions in bold indicate the significant or sensitive reactions for the CH 3 species. From Table 4.6 it is clear that all the reactions contributing to the production/dissociation of CH 3 are important and cannot be eliminated. Similar rate analysis could be done for the C 2 species (C 2 H 3, C 2 H 5 ). 61
76 Figure 4.10 Rate of Production of CH 3 species Further rate analysis of the rate of production/dissociation of the C 2 H 3 and C 2 H 5 radical reactions in the condensed reaction mechanism yields, H + C 2 H 2 (+M) = C 2 H 3 (+M) CH 3 + C 2 H 4 = C 2 H 3 + CH 4 The first reaction represents the maximum dissociation rate of C 2 H 3 among all the reactions, while the second represents the maximum rate of production of C 2 H 3. The maximum rate of production/dissociation of C 2 H 3 is higher than that of the total production rate. This overall rate implies that although the C 2 H 3 radical is not dominant specie, it furthers the formation of dominant species (C 2 H 2, CH 4 ). Similar explanation could be applied for the C 2 H 5 radicals. 62
77 The rate analysis was applied to the other reactions consisting C 2 H 3 radicals and the actual and modified α i values were established (Tables 4.7 and 4.8). The rate analysis predicts that reactions other than the above cited have an α ik value lower than 0.05 thereby eliminating them. A similar approach could be applied to reactions consisting C 2 H 5 species and a further reduced reaction set could be achieved. The reactions in bold indicate the sensitive reactions for the C 2 H 3 species. Table 4.5 Rate Analysis for selecting dominant reactions for CH 3 species Rate of Production (50sccm) Rate (mole/cm3-sec) α ik = r ik /R k Total Rate of Production 8E-11 - Total Rate of Dissociation -6E-11 - H+CH 3 (+M) = CH 4 (+M) 1.5E H+CH 4 = CH 3 +H 2 1.4E CH 3 (+M) = C 2 H 6 (+M) -3E CH 3 = C2H 5 +H -1E CH 3 +C 2 H 4 = C 2 H 3 +CH 4-1.5E CH 3 +C 2 H 6 = C 2 H 5 +CH 4-1E
78 Table 4.6 Modified Rate Analysis for selecting dominant reactions for CH 3 species Rate of Production (50sccm) Rate (mole/cm3-sec) α i = r i /R Total Rate of Production 8E-11 - Total Rate of Dissociation -6E-11 - H+CH 3 (+M) = CH 4 (+M) 1.5E H+CH 4 = CH 3 +H 2 1.4E CH 3 (+M) = C 2 H 6 (+M) -3E CH 3 = C2H 5 +H -1E-09 1 CH 3 +C 2 H 4 = C 2 H 3 +CH 4-1.5E CH 3 +C 2 H 6 = C 2 H 5 +CH 4-1E Table 4.7 Rate Analysis for selecting dominant reactions for C 2 H 3 species Rate of Production (50sccm) Rate (mole/cm 3 -sec) α i = r i /R Total Rate 6E-14 - H+C 2 H 2 (+M) = C 2 H 3 (+M) -1.5E H+C 2 H 3 (+M) = C 2 H 4 (+M) 1.4E H+C 2 H 3 = H 2 +C 2 H 2-1E H+C 2 H 4 = C 2 H 3 +H 2 2.5E CH 3 +C 2 H 4 = C 2 H 3 +CH 4 1.5E
79 Table 4.8 Modified Rate Analysis for selecting dominant reactions for C 2 H 3 species Rate of Production (50sccm) Rate (mole/cm 3 -sec) α i = r i /R Total Rate 6E-14 - H+C 2 H 2 (+M) = C 2 H 3 (+M) -1.5E-10 1 H+C 2 H 3 (+M) = C 2 H 4 (+M) 1.4E E-05 H+C 2 H 3 = H 2 +C 2 H 2-1E E-05 H+C 2 H 4 = C 2 H 3 +H 2 2.5E CH 3 +C 2 H 4 = C 2 H 3 +CH 4 1.5E-10 1 Figure 4.11 Total Rate of Production of CH 3 species 65
80 Figure 4.12 Rate of Production of C 2 H 3 species Figure 4.13 Rate of Production of C 2 H 5 species 66
81 Table 4.9 Reduced Gas Phase Reaction Set from Birakayala (17) Reaction Number Reaction Reactions to Predict C1-C2 Arrhenius Expression (mol, cm, s) A*T β *exp(-e a /RT) 1 H+CH 3 (+M) = CH 4 (+M) 1.27E16*T -0.6 *exp(192.7/t) 2 H+CH 4 = CH 3 +H 2 6.6E8*T 1.6 *exp(5454.9/t) 3 H+C 2 H 2 (+M) = C 2 H 3 (+M) 5.6E12*exp(1207.7/T) 4 H+C 2 H 3 = H 2 +C 2 H E13 5 H+C 2 H 4 (+M) = C 2 H 5 (+M) 1.08E12*T -0.5 *exp(915.9/t) 6 H+C 2 H 4 = C 2 H 3 +H E6*T 2.5 *exp(6159.4/t) 7 H+C 2 H 6 = C 2 H 5 +H E8*T 1.9 *exp(3789.3/t) 8 2CH 3 (+M) = C 2 H 6 (+M) 2.12E16*T 1.0 *exp(312/t) 9 2CH 3 = H+C 2 H E12*T -0.1 *exp(5334.1/t) 10 CH 3 +C 2 H 4 = C 2 H 3 +CH E5*T 2.0 *exp(4629.6/t) Reactions to Predict C2-C6 11 C 2 H+H 2 = H+C 2 H E5*T 2.4 *exp(100.6/t) 12 H+C 2 H (+M) = C 2 H 2 (+M) 1.00E17*T C 4 H 3 +C 2 H 3 = C 6 H E14*exp(412.6/T) 14 C 3 H 3 +C 3 H 3 = C 6 H 6 3.0E11 15 C 3 H 4 = C 3 H 3 +H 1.00E17*exp( /T) 16 CH 3 +C 2 H 2 = C 3 H 4 +H 6.74E19*T -2.1 *exp( /t) 17 C 2 H+C 2 H 2 = C 4 H E13 67
82 18 C 2 H 2 +C 2 H 2 = C 4 H E14*exp( /T) 19 C 2 H 3 +C 2 H 2 = C 4 H 4 +H 2.0E12*exp(2516.1/T) 20 C 2 H+C 2 H 4 = C 4 H 4 +H 1.21E13 21 CH 3 +C 2 H 6 = C 2 H 5 +CH E6*T 1.7 *exp(5258.7/t) 22 C 3 H 4 +C 3 H 3 = C 6 H 6 +H 2.2E11*exp(1006.4/T) 23 C 2 H 4 (+M) = H 2 +C 2 H 2 (+M) 8.0E12*T 0.4 *exp( /t) 24 C 4 H 4 +C 2 H 2 = C 6 H E11*exp( /T) 25 C 3 H 4 +C 2 H = C 3 H 3 +C 2 H E13 26 H+C 2 H 5 (+M) = C 2 H 6 (+M) 5.21E17*T -1.0 *exp(795.1/t) 27 H+C 2 H 3 (+M) = C 2 H 4 (+M) 6.08E12*T -0.3 *exp(140.9/t) 28 H+C 2 H 5 = H 2 +C 2 H 4 2.0E12 For the present operating conditions T= K, P=12 Torr and Q= sccm, the concentration of benzene is very low in comparison to acetylene (Figure 4.9). This less concentration of benzene could be attributed to the low residence time of the reacting gases in the reactor. Therefore the reactions contributing to the production/dissociation of benzene could be eliminated. For a 99.97% pure methane feed the following reaction was added to the gas phase reaction set as its rate was greater than 5% of the total rate value. CH 3 + C 2 H 5 = C 3 H 8 The amount of error or the deviation in the concentration of species data is illustrated in Figs (a), (b), (c). The plots show the concentration profiles of the 68
83 dominating species present during the chemical vapor deposition of carbon from methane for the given operating conditions (17-18). The percentage deviation in the concentration data for different gas phase reaction schemes is also tabulated in Table It could be clearly seen that the deviation in concentration of various species is lower than 1%, when the gas phase mechanism is reduced from 110 to 10 reactions. 69
84 Figure 4.14 Concentration Profiles of (a) methane, (b) acetylene and (c) ethylene for different gas phase reaction schemes Table 4.10 Percentage Deviation from the Original Gas Phase Reaction Mechanism number of reactions species H CH C 2 H C 2 H C 2 H C 3 H Sensitivity Analysis This section describes deriving a reduced gas phase reaction set using sensitivity analysis. Sensitivity analysis as the name says measures the sensitivity of kinetics of the species concentration to the changes in the reaction kinetics. The expression for a first order sensitivity coefficient matrix is given by, 70
85 Z ki = δy k / δa i Where, Z ki is the sensitivity coefficient, Y k is the mole fraction of species k and A i is the Arrhenius parameter of the i th reaction. The units of sensitivity coefficient depend on the order of the reaction. The sensitivity coefficients need to be normalized for each reaction to maintain dimensional consistency in units. The normalized sensitivity coefficient could be expressed as Z ki,n, Z ki,n = (A i /Y k ) (δy k / δa i ) The concept behind the evaluation of sensitivity coefficients is that the CHEMKIN package changes the pre-exponential factor (independent variable) A i by a small value and calculates the change in concentration (mole fraction) Y k of a particular species (k) for a particular reaction (i). The sensitivity values calculated by the CHEMKIN software are displayed in Table From Table 4.11 it could be observed that for the given operating conditions (T=1348K, P=12 Torr, Q= 50sccm), the reactions contributing to the production/dissociation of CH 4 species are tabulated in decreasing order of sensitivities with respect to species of homogeneous reactions in chemical vapor deposition of pyrocarbon. Reactions with sensitivity coefficients less than ±0.5 are considered to be less significant. The above gas phase mechanism could be reduced based on number of times a particular reaction occurs in the sensitivity analysis data (Table 4.11) corresponding to each species. The significant reactions thereby deduced are listed in Table
86 Table 4.11 Sensitivity analysis for Birakayala Reduced Reaction Mechanism Species H H CH CH C 2 H C 2 H C 2 H C 2 H C 2 H C 2 H C 3 H C 3 H C 3 H C 4 H C 4 H C 6 H The numbers in the first row indicate the sensitivities in descending order with respect to various species of the gas phase reaction. The numbers in the boxes denote the serial number of the reactions in the reduced gas phase mechanism obtained by Birakayala (17). 72
87 Table 4.12 Significant Gas Phase Reactions from Sensitivity Analysis H + CH 3 (+M) = CH 4 (+M) CH 3 + C 2 H 5 = C 3 H 8 2CH 3 (+M) = C 2 H 6 (+M) CH 3 + C 2 H 6 = C 2 H 5 + CH 4 2CH 3 = H + C 2 H 5 H + C 2 H 4 (+M) = C 2 H 5 (+M) H + C 2 H 2 (+M) = C 2 H 3 (+M) H + CH 4 = CH 3 + H 2 CH 3 + C 2 H 4 = C 2 H 3 + CH 4 C 2 H 4 (+M) = H 2 + C 2 H 2 (+M) Now that a suitable gas phase mechanism has been developed it could be used in CHEMKIN to calculate the concentrations of various gas phase species. The obtained model is used to predict the concentration of gas phase species which therein contribute to the deposition of pyrocarbon. Figure 4.15 shows the trend in the concentrations of acetylene, ethylene and ethane with respect to the reactor length. Figure 4.15 Gas Concentrations of Various Species from the CHEMKIN gas phase model at a reactor temperature of 1075 C and methane inlet flow rate of 50 SCCM 73
88 This chapter concludes with having obtained a suitable reduced gas phase reaction set using two different techniques (rate and sensitivity analysis) and thereby prediction of gas phase concentrations of various species. 74
89 CHAPTER V DEVELOPMENT OF SURFACE KINETICS After having obtained a suitable comprehensive homogeneous reaction mechanism, the need for developing a corresponding heterogeneous model arises. This chapter focuses on the steps involved in developing a good surface kinetic model. The information available in literature is combined with the experimental results obtained in the previous chapter to develop the heterogeneous model. The following section explains the development of a suitable surface kinetic model starting with a simple reaction mechanism. 5.1 Surface Kinetics Modeling The development of surface reaction mechanism and therefore the results obtained are discussed in this section Development of Simple Surface Model - CHEMKIN This section aims at the development of a simple surface kinetic model which could predict the observed experimental deposition rate with the help of information available from literature. From literature (17-19) it is known that for the given operating 75
90 conditions (T= C, P=12 Torr, Q= SCCM) that acetylene and ethylene are the dominant species for the deposition of pyrocarbon from a methane precursor. The gas phase modeling also supports this fact by indicating a high production of acetylene, ethylene and ethane in comparison to the other species (Figure 4.15). Initially, a simple two step surface reaction model as given below (19) is developed using acetylene as the dominant pyrocarbon growth species. To start with, the simple two step surface reaction mechanism was suggested so as to decrease the difficulties involved with the tediousness in calculations and therefore the convergence of the model. C 2 H 2 + C(R, G) => C (G) + C 2 H 2 (S, R) C 2 H 2 (S, R) + CH 3 => C(R, G) + C 2 H 5 The first reaction describes the adsorption of acetylene on to a carbon active site (19). The nomenclature of the species is important in understanding the concept behind the reaction mechanism. C 2 H 2 (S, R) denotes a radical species with one dangling bond and the rest normal bonds (19). C (G) and C(R, G) denote deposited pyrolytic carbon and carbon active site, respectively. The second reaction shows the regeneration of active free sites from the acetylene adsorbed sites. The actual feed used in our experiments consists of 99.97% pure methane. Therefore the model should be designed to be used with a methane feed with a purity of 99.97%. The remainder 0.03% is assumed to be ethane and propane in equal amounts. The rate analysis is performed again to determine the sensitive homogeneous reactions. As already explained in the previous chapter, the gas phase reaction chemistry set is the 76
91 same as that used for the 100% pure methane feed with the addition of the following reaction which accounts for production/dissociation of propane. CH 3 + C 2 H 5 = C 3 H 8 The kinetic parameters (pre-exponential factor, temperature coefficient and activation energy) for the surface reactions were adopted from literature (17-19). The preexponential factor was input in terms of sticking coefficient. Bammidipati (18) used a sticking coefficient of (0.7-50)*10-6 for the carbon deposition from methane pyrolysis for temperatures ranging from C and pressures of 10 and 40 Torr. Dandy and Coltrin (19) have used a value of 3.3*10-2 for the acetylene adsorption reaction and thereby the graphite deposition occurring during diamond growth in subatmospheric dc plasma-gun reactors at substrate temperature of 1200K, inlet temperatures of 2500 and 3300K and reactor pressure of 30 Torr. To start with, the value of 5*10-5 was chosen as the sticking coefficient for the acetylene adsorption reaction because the pyrocarbon deposition rate trending observed was similar to that observed from literature (18). The value of K s for the second surface reaction is assumed to be the same as the first reaction thereby enabling the calculation of sticking coefficient as 4*10-5 by goal seeking. Goal seeking was done in Microsoft Excel so as to equalize the reaction rate constants for the given two surface reactions. The rate constants are assumed to be equal since it becomes easier while deriving the rate expression for the surface model (Section 5.1.2). As already explained in chapter II, the value of sticking coefficient (19) for the second reaction was obtained by using the rate constant equation. 77
92 K s = Where, K s = surface reaction rate constant, (cm 3, mol, s) τ = number of active sites, mol/cm 2, m = sum of all stoichiometric coefficients of the surface species R = universal gas constant, gm-cm 2 /mol-k-s 2 T = temperature of the reactor, K W = weight of the reacting gas phase species, gm/mol The activation energies for the surface reactions are taken as zero. The importance of activation energy needs to be explained here. Activation energy is the minimum energy required for the reactants to undergo reaction and yield products. The higher the activation energy, the lower is the probability of a reaction to occur. The assumption of zero activation energy is plausible because it states that there is no threshold energy for the reaction to occur (19). The above surface reactions along with the gas phase mechanism are used in CHEMKIN to determine the deposition rate profiles of pyrocarbon at different temperatures and flow rates. The model yields deposition rate results with units of cm/sec. A detailed calculation of the conversion of deposition rate from cm/sec to mg/hr so as to compare with the experimental deposition values is given in the Appendix C. The issue that arises from the calculation of pyrocarbon deposition rate from the CHEMKIN model is that it measures the deposition rate at the surface of the inner wall of the reactor 78
93 and not on the surface of the silicon carbide fiber, whose area is significantly different from that at the wall surface of the reactor. This difference in the deposition rate could be accounted for by developing a correction factor that relates the surface area of the silicon carbide fiber to that of the reactor wall. This section describes how to relate the magnitudes of the rate calculated by the model with the experimental rate. As shown in the Figure 5.1, the molar flux (mol/cm 2 - sec) entering along the radial section of a plug flow reactor remains constant or in other words the flux at the wall of the reactor (J Aw ) is equal to the flux at the surface of the fiber (J Af ). Therefore the experimental and modeling pyrocarbon deposition rate could be related to the molar flux, with surface area of the substrate being the variable. The significance of using a fiber substrate is that it has a large specific surface area. One important assumption is that the amount of pyrocarbon collected on the surface of the fiber is considered to be uniform. The correction factor (η) essentially is the ratio of surface area of fiber to that of the inner wall of the reactor. Figure 5.1 Development of Correction Factor 79
Transient modeling of chemical vapor infiltration of methane using multi-step reaction and deposition models
 Chemical Engineering Science 62 (2007) 4976 4982 www.elsevier.com/locate/ces Transient modeling of chemical vapor infiltration of methane using multi-step reaction and deposition models Aijun Li, Olaf
Chemical Engineering Science 62 (2007) 4976 4982 www.elsevier.com/locate/ces Transient modeling of chemical vapor infiltration of methane using multi-step reaction and deposition models Aijun Li, Olaf
Vacuum Pumps. Two general classes exist: Gas transfer physical removal of matter. Mechanical, diffusion, turbomolecular
 Vacuum Technology Vacuum Pumps Two general classes exist: Gas transfer physical removal of matter Mechanical, diffusion, turbomolecular Adsorption entrapment of matter Cryo, sublimation, ion Mechanical
Vacuum Technology Vacuum Pumps Two general classes exist: Gas transfer physical removal of matter Mechanical, diffusion, turbomolecular Adsorption entrapment of matter Cryo, sublimation, ion Mechanical
Lecture 3 Vacuum Science and Technology
 Lecture 3 Vacuum Science and Technology Chapter 3 - Wolf and Tauber 1/56 Announcements Homework will be online from noon today. This is homework 1 of 4. 25 available marks (distributed as shown). This
Lecture 3 Vacuum Science and Technology Chapter 3 - Wolf and Tauber 1/56 Announcements Homework will be online from noon today. This is homework 1 of 4. 25 available marks (distributed as shown). This
Lecture 1: Vapour Growth Techniques
 PH3EC2 Vapour Growth and Epitaxial Growth Lecturer: Dr. Shinoj V K Lecture 1: Vapour Growth Techniques 1.1 Vapour growth The growth of single crystal materials from the vapour phase. Deposition from the
PH3EC2 Vapour Growth and Epitaxial Growth Lecturer: Dr. Shinoj V K Lecture 1: Vapour Growth Techniques 1.1 Vapour growth The growth of single crystal materials from the vapour phase. Deposition from the
PHYSICAL VAPOR DEPOSITION OF THIN FILMS
 PHYSICAL VAPOR DEPOSITION OF THIN FILMS JOHN E. MAHAN Colorado State University A Wiley-Interscience Publication JOHN WILEY & SONS, INC. New York Chichester Weinheim Brisbane Singapore Toronto CONTENTS
PHYSICAL VAPOR DEPOSITION OF THIN FILMS JOHN E. MAHAN Colorado State University A Wiley-Interscience Publication JOHN WILEY & SONS, INC. New York Chichester Weinheim Brisbane Singapore Toronto CONTENTS
Plasma Deposition (Overview) Lecture 1
 Plasma Deposition (Overview) Lecture 1 Material Processes Plasma Processing Plasma-assisted Deposition Implantation Surface Modification Development of Plasma-based processing Microelectronics needs (fabrication
Plasma Deposition (Overview) Lecture 1 Material Processes Plasma Processing Plasma-assisted Deposition Implantation Surface Modification Development of Plasma-based processing Microelectronics needs (fabrication
TMT4320 Nanomaterials November 10 th, Thin films by physical/chemical methods (From chapter 24 and 25)
 1 TMT4320 Nanomaterials November 10 th, 2015 Thin films by physical/chemical methods (From chapter 24 and 25) 2 Thin films by physical/chemical methods Vapor-phase growth (compared to liquid-phase growth)
1 TMT4320 Nanomaterials November 10 th, 2015 Thin films by physical/chemical methods (From chapter 24 and 25) 2 Thin films by physical/chemical methods Vapor-phase growth (compared to liquid-phase growth)
An Introduction to Chemical Kinetics
 An Introduction to Chemical Kinetics Michel Soustelle WWILEY Table of Contents Preface xvii PART 1. BASIC CONCEPTS OF CHEMICAL KINETICS 1 Chapter 1. Chemical Reaction and Kinetic Quantities 3 1.1. The
An Introduction to Chemical Kinetics Michel Soustelle WWILEY Table of Contents Preface xvii PART 1. BASIC CONCEPTS OF CHEMICAL KINETICS 1 Chapter 1. Chemical Reaction and Kinetic Quantities 3 1.1. The
Fabrication Technology, Part I
 EEL5225: Principles of MEMS Transducers (Fall 2004) Fabrication Technology, Part I Agenda: Microfabrication Overview Basic semiconductor devices Materials Key processes Oxidation Thin-film Deposition Reading:
EEL5225: Principles of MEMS Transducers (Fall 2004) Fabrication Technology, Part I Agenda: Microfabrication Overview Basic semiconductor devices Materials Key processes Oxidation Thin-film Deposition Reading:
COMBUSTION OF FUEL 12:57:42
 COMBUSTION OF FUEL The burning of fuel in presence of air is known as combustion. It is a chemical reaction taking place between fuel and oxygen at temperature above ignition temperature. Heat is released
COMBUSTION OF FUEL The burning of fuel in presence of air is known as combustion. It is a chemical reaction taking place between fuel and oxygen at temperature above ignition temperature. Heat is released
The Vacuum Sorption Solution
 The Total Sorption Solution The Vacuum Sorption Solution The Vacuum Sorption Solution www.thesorptionsolution.com About the Technique DVS Vacuum - the only gravimetric system that supports static and dynamic
The Total Sorption Solution The Vacuum Sorption Solution The Vacuum Sorption Solution www.thesorptionsolution.com About the Technique DVS Vacuum - the only gravimetric system that supports static and dynamic
Vacuum Technology and film growth. Diffusion Resistor
 Vacuum Technology and film growth Poly Gate pmos Polycrystaline Silicon Source Gate p-channel Metal-Oxide-Semiconductor (MOSFET) Drain polysilicon n-si ion-implanted Diffusion Resistor Poly Si Resistor
Vacuum Technology and film growth Poly Gate pmos Polycrystaline Silicon Source Gate p-channel Metal-Oxide-Semiconductor (MOSFET) Drain polysilicon n-si ion-implanted Diffusion Resistor Poly Si Resistor
Vacuum Gas Carburizing with Acetylene - Gas Phase Modeling of a Bench Scale Reactor
 25 Vacuum Gas Carburizing with Acetylene - Gas Phase Modeling of a Bench Scale Reactor R.U. Khan, M. Saleem and A. Shafeeq Abstrac Vacuum gas carburizing is an important industrial process used for hardening
25 Vacuum Gas Carburizing with Acetylene - Gas Phase Modeling of a Bench Scale Reactor R.U. Khan, M. Saleem and A. Shafeeq Abstrac Vacuum gas carburizing is an important industrial process used for hardening
Diamond deposition by atmospheric pressure induction plasma: effects of impinging jet fluid mechanics on film formation
 19 Diamond and Related Materials, 2 (1993) 19-195 Diamond deposition by atmospheric pressure induction plasma: effects of impinging jet fluid mechanics on film formation S. L. Girshick, B. W. Yu, C. Li
19 Diamond and Related Materials, 2 (1993) 19-195 Diamond deposition by atmospheric pressure induction plasma: effects of impinging jet fluid mechanics on film formation S. L. Girshick, B. W. Yu, C. Li
Adsorption Processes. Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad
 Adsorption Processes Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad Contents Introduction Principles of adsorption Types of adsorption Definitions Brief history Adsorption isotherms Mechanism
Adsorption Processes Ali Ahmadpour Chemical Eng. Dept. Ferdowsi University of Mashhad Contents Introduction Principles of adsorption Types of adsorption Definitions Brief history Adsorption isotherms Mechanism
Complex Compounds Background of Complex Compound Technology
 Complex Compounds For more than 20 years, Rocky Research has been a pioneer in the field of sorption refrigeration utilizing complex compounds. Our technology earned special recognition from NASA in 1999.
Complex Compounds For more than 20 years, Rocky Research has been a pioneer in the field of sorption refrigeration utilizing complex compounds. Our technology earned special recognition from NASA in 1999.
K n. III. Gas flow. 1. The nature of the gas : Knudsen s number. 2. Relative flow : Reynold s number R = ( dimensionless )
 III. Gas flow. The nature of the gas : Knudsen s number K n λ d 2. Relative flow : U ρ d η U : stream velocity ρ : mass density Reynold s number R ( dimensionless ) 3. Flow regions - turbulent : R > 2200
III. Gas flow. The nature of the gas : Knudsen s number K n λ d 2. Relative flow : U ρ d η U : stream velocity ρ : mass density Reynold s number R ( dimensionless ) 3. Flow regions - turbulent : R > 2200
Modern Methods in Heterogeneous Catalysis Research: Preparation of Model Systems by Physical Methods
 Modern Methods in Heterogeneous Catalysis Research: Preparation of Model Systems by Physical Methods Methods for catalyst preparation Methods discussed in this lecture Physical vapour deposition - PLD
Modern Methods in Heterogeneous Catalysis Research: Preparation of Model Systems by Physical Methods Methods for catalyst preparation Methods discussed in this lecture Physical vapour deposition - PLD
DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD
 Chapter 4 DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD 4.1 INTRODUCTION Sputter deposition process is another old technique being used in modern semiconductor industries. Sputtering
Chapter 4 DEPOSITION OF THIN TiO 2 FILMS BY DC MAGNETRON SPUTTERING METHOD 4.1 INTRODUCTION Sputter deposition process is another old technique being used in modern semiconductor industries. Sputtering
CHEMISTRY Matter and Change. Chapter 12: States of Matter
 CHEMISTRY Matter and Change Chapter 12: States of Matter CHAPTER 12 States of Matter Section 12.1 Section 12.2 Section 12.3 Section 12.4 Gases Forces of Attraction Liquids and Solids Phase Changes Click
CHEMISTRY Matter and Change Chapter 12: States of Matter CHAPTER 12 States of Matter Section 12.1 Section 12.2 Section 12.3 Section 12.4 Gases Forces of Attraction Liquids and Solids Phase Changes Click
Chemical Vapor Deposition *
 OpenStax-CNX module: m25495 1 Chemical Vapor Deposition * Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 note: This module was developed
OpenStax-CNX module: m25495 1 Chemical Vapor Deposition * Andrew R. Barron This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 note: This module was developed
DARS Digital Analysis of Reactive Systems
 DARS Digital Analysis of Reactive Systems Introduction DARS is a complex chemical reaction analysis system, developed by DigAnaRS. Our latest version, DARS V2.0, was released in September 2008 and new
DARS Digital Analysis of Reactive Systems Introduction DARS is a complex chemical reaction analysis system, developed by DigAnaRS. Our latest version, DARS V2.0, was released in September 2008 and new
VACUUM PUMPING METHODS
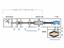 VACUUM PUMPING METHODS VACUUM PUMPS (METHODS) Positive Displacement Vacuum Gas Transfer Vacuum Kinetic Vacuum Entrapment Vacuum Adsorption Reciprocating Displacement Rotary Drag Fluid Entrainment Ion Transfer
VACUUM PUMPING METHODS VACUUM PUMPS (METHODS) Positive Displacement Vacuum Gas Transfer Vacuum Kinetic Vacuum Entrapment Vacuum Adsorption Reciprocating Displacement Rotary Drag Fluid Entrainment Ion Transfer
Micro Chemical Vapor Deposition System: Design and Verification
 Micro Chemical Vapor Deposition System: Design and Verification Q. Zhou and L. Lin Berkeley Sensor and Actuator Center, Department of Mechanical Engineering, University of California, Berkeley 2009 IEEE
Micro Chemical Vapor Deposition System: Design and Verification Q. Zhou and L. Lin Berkeley Sensor and Actuator Center, Department of Mechanical Engineering, University of California, Berkeley 2009 IEEE
Numerical simulation of Vibrationally Active Ar-H2 Microwave Plasma
 Numerical simulation of Vibrationally Active Ar-H2 Microwave Plasma F. Bosi 1, M. Magarotto 2, P. de Carlo 2, M. Manente 2, F. Trezzolani 2, D. Pavarin 2, D. Melazzi 2, P. Alotto 1, R. Bertani 1 1 Department
Numerical simulation of Vibrationally Active Ar-H2 Microwave Plasma F. Bosi 1, M. Magarotto 2, P. de Carlo 2, M. Manente 2, F. Trezzolani 2, D. Pavarin 2, D. Melazzi 2, P. Alotto 1, R. Bertani 1 1 Department
9 MECHANICAL PROPERTIES OF SOLIDS
 9 MECHANICAL PROPERTIES OF SOLIDS Deforming force Deforming force is the force which changes the shape or size of a body. Restoring force Restoring force is the internal force developed inside the body
9 MECHANICAL PROPERTIES OF SOLIDS Deforming force Deforming force is the force which changes the shape or size of a body. Restoring force Restoring force is the internal force developed inside the body
High-Pressure Volumetric Analyzer
 High-Pressure Volumetric Analyzer High-Pressure Volumetric Analysis HPVA II Benefits Dual free-space measurement for accurate isotherm data Free space can be measured or entered Correction for non-ideality
High-Pressure Volumetric Analyzer High-Pressure Volumetric Analysis HPVA II Benefits Dual free-space measurement for accurate isotherm data Free space can be measured or entered Correction for non-ideality
Chapter 6. Summary and Conclusions
 Chapter 6 Summary and Conclusions Plasma deposited amorphous hydrogenated carbon films (a-c:h) still attract a lot of interest due to their extraordinary properties. Depending on the deposition conditions
Chapter 6 Summary and Conclusions Plasma deposited amorphous hydrogenated carbon films (a-c:h) still attract a lot of interest due to their extraordinary properties. Depending on the deposition conditions
Thermodynamics. Atoms are in constant motion, which increases with temperature.
 Thermodynamics SOME DEFINITIONS: THERMO related to heat DYNAMICS the study of motion SYSTEM an object or set of objects ENVIRONMENT the rest of the universe MICROSCOPIC at an atomic or molecular level
Thermodynamics SOME DEFINITIONS: THERMO related to heat DYNAMICS the study of motion SYSTEM an object or set of objects ENVIRONMENT the rest of the universe MICROSCOPIC at an atomic or molecular level
Lecture (9) Reactor Sizing. Figure (1). Information needed to predict what a reactor can do.
 Lecture (9) Reactor Sizing 1.Introduction Chemical kinetics is the study of chemical reaction rates and reaction mechanisms. The study of chemical reaction engineering (CRE) combines the study of chemical
Lecture (9) Reactor Sizing 1.Introduction Chemical kinetics is the study of chemical reaction rates and reaction mechanisms. The study of chemical reaction engineering (CRE) combines the study of chemical
LECTURE 5 SUMMARY OF KEY IDEAS
 LECTURE 5 SUMMARY OF KEY IDEAS Etching is a processing step following lithography: it transfers a circuit image from the photoresist to materials form which devices are made or to hard masking or sacrificial
LECTURE 5 SUMMARY OF KEY IDEAS Etching is a processing step following lithography: it transfers a circuit image from the photoresist to materials form which devices are made or to hard masking or sacrificial
EE C247B / ME C218 INTRODUCTION TO MEMS DESIGN SPRING 2014 C. Nguyen PROBLEM SET #4
 Issued: Wednesday, Mar. 5, 2014 PROBLEM SET #4 Due (at 9 a.m.): Tuesday Mar. 18, 2014, in the EE C247B HW box near 125 Cory. 1. Suppose you would like to fabricate the suspended cross beam structure below
Issued: Wednesday, Mar. 5, 2014 PROBLEM SET #4 Due (at 9 a.m.): Tuesday Mar. 18, 2014, in the EE C247B HW box near 125 Cory. 1. Suppose you would like to fabricate the suspended cross beam structure below
Applied Fluid Mechanics
 Applied Fluid Mechanics 1. The Nature of Fluid and the Study of Fluid Mechanics 2. Viscosity of Fluid 3. Pressure Measurement 4. Forces Due to Static Fluid 5. Buoyancy and Stability 6. Flow of Fluid and
Applied Fluid Mechanics 1. The Nature of Fluid and the Study of Fluid Mechanics 2. Viscosity of Fluid 3. Pressure Measurement 4. Forces Due to Static Fluid 5. Buoyancy and Stability 6. Flow of Fluid and
Well Stirred Reactor Stabilization of flames
 Well Stirred Reactor Stabilization of flames Well Stirred Reactor (see books on Combustion ) Stabilization of flames in high speed flows (see books on Combustion ) Stabilization of flames Although the
Well Stirred Reactor Stabilization of flames Well Stirred Reactor (see books on Combustion ) Stabilization of flames in high speed flows (see books on Combustion ) Stabilization of flames Although the
INTERMEDIATE PHASES OF PYROLYTIC CARBON OBSERVED BY ATOMIC FORCE MICROSCOPY
 Carbon 24, Rhode Island, USA INTERMEDIATE PHASES OF PYROLYTIC CARBON OBSERVED BY ATOMIC FORCE MICROSCOPY Andreas Pfrang 1, Thomas Schimmel 1,2 1 Institute of Applied Physics, University of Karlsruhe, D-76128
Carbon 24, Rhode Island, USA INTERMEDIATE PHASES OF PYROLYTIC CARBON OBSERVED BY ATOMIC FORCE MICROSCOPY Andreas Pfrang 1, Thomas Schimmel 1,2 1 Institute of Applied Physics, University of Karlsruhe, D-76128
Introduction to Thin Film Processing
 Introduction to Thin Film Processing Deposition Methods Many diverse techniques available Typically based on three different methods for providing a flux of atomic or molecular material Evaporation Sputtering
Introduction to Thin Film Processing Deposition Methods Many diverse techniques available Typically based on three different methods for providing a flux of atomic or molecular material Evaporation Sputtering
Properties of Gases. The perfect gas. States of gases Gas laws Kinetic model of gases (Ch th ed, th ed.) Real gases
 Properties of Gases Chapter 1 of Physical Chemistry - 6th Edition P.W. Atkins. Chapter 1 and a little bit of Chapter 24 of 7th Edition. Chapter 1 and a little bit of Chapter 21 of 8th edition. The perfect
Properties of Gases Chapter 1 of Physical Chemistry - 6th Edition P.W. Atkins. Chapter 1 and a little bit of Chapter 24 of 7th Edition. Chapter 1 and a little bit of Chapter 21 of 8th edition. The perfect
Lecture 4. Ultrahigh Vacuum Science and Technology
 Lecture 4 Ultrahigh Vacuum Science and Technology Why do we need UHV? 1 Atmosphere = 760 torr; 1 torr = 133 Pa; N ~ 2.5 10 19 molecules/cm 3 Hertz-Knudsen equation p ZW 1/ 2 ( 2mk T) At p = 10-6 Torr it
Lecture 4 Ultrahigh Vacuum Science and Technology Why do we need UHV? 1 Atmosphere = 760 torr; 1 torr = 133 Pa; N ~ 2.5 10 19 molecules/cm 3 Hertz-Knudsen equation p ZW 1/ 2 ( 2mk T) At p = 10-6 Torr it
Distinguish quantitatively between the adsorption isotherms of Gibbs, Freundlich and Langmuir.
 Module 8 : Surface Chemistry Lecture 36 : Adsorption Objectives After studying this lecture, you will be able to Distinguish between physisorption and chemisorption. Distinguish between monolayer adsorption
Module 8 : Surface Chemistry Lecture 36 : Adsorption Objectives After studying this lecture, you will be able to Distinguish between physisorption and chemisorption. Distinguish between monolayer adsorption
Chapter 3 Engineering Science for Microsystems Design and Fabrication
 Lectures on MEMS and MICROSYSTEMS DESIGN and MANUFACTURE Chapter 3 Engineering Science for Microsystems Design and Fabrication In this Chapter, we will present overviews of the principles of physical and
Lectures on MEMS and MICROSYSTEMS DESIGN and MANUFACTURE Chapter 3 Engineering Science for Microsystems Design and Fabrication In this Chapter, we will present overviews of the principles of physical and
Computational Analysis for Composites
 Computational Analysis for Composites Professor Johann Sienz and Dr. Tony Murmu Swansea University July, 011 The topics covered include: OUTLINE Overview of composites and their applications Micromechanics
Computational Analysis for Composites Professor Johann Sienz and Dr. Tony Murmu Swansea University July, 011 The topics covered include: OUTLINE Overview of composites and their applications Micromechanics
A Multistep Surface Mechanism for Ethane Oxidative Dehydrogenation on Pt- and Pt/Sn-Coated Monoliths
 Ind. Eng. Chem. Res. 2005, 44, 3453-3470 3453 A Multistep Surface Mechanism for Ethane Oxidative Dehydrogenation on Pt- and Pt/Sn-Coated Monoliths Francesco Donsì, Kenneth A. Williams, and Lanny D. Schmidt*
Ind. Eng. Chem. Res. 2005, 44, 3453-3470 3453 A Multistep Surface Mechanism for Ethane Oxidative Dehydrogenation on Pt- and Pt/Sn-Coated Monoliths Francesco Donsì, Kenneth A. Williams, and Lanny D. Schmidt*
Applied Fluid Mechanics
 Applied Fluid Mechanics 1. The Nature of Fluid and the Study of Fluid Mechanics 2. Viscosity of Fluid 3. Pressure Measurement 4. Forces Due to Static Fluid 5. Buoyancy and Stability 6. Flow of Fluid and
Applied Fluid Mechanics 1. The Nature of Fluid and the Study of Fluid Mechanics 2. Viscosity of Fluid 3. Pressure Measurement 4. Forces Due to Static Fluid 5. Buoyancy and Stability 6. Flow of Fluid and
Chapter 5: Nanoparticle Production from Cathode Sputtering. in High-Pressure Microhollow Cathode and Arc Discharges
 96 Chapter 5: Nanoparticle Production from Cathode Sputtering in High-Pressure Microhollow Cathode and Arc Discharges 5.1. Introduction Sputtering is a fundamental aspect of plasma operation and has been
96 Chapter 5: Nanoparticle Production from Cathode Sputtering in High-Pressure Microhollow Cathode and Arc Discharges 5.1. Introduction Sputtering is a fundamental aspect of plasma operation and has been
CHAPTER 13. States of Matter. Kinetic = motion. Polar vs. Nonpolar. Gases. Hon Chem 13.notebook
 CHAPTER 13 States of Matter States that the tiny particles in all forms of matter are in constant motion. Kinetic = motion A gas is composed of particles, usually molecules or atoms, with negligible volume
CHAPTER 13 States of Matter States that the tiny particles in all forms of matter are in constant motion. Kinetic = motion A gas is composed of particles, usually molecules or atoms, with negligible volume
Chapter 10: States of Matter. Concept Base: Chapter 1: Properties of Matter Chapter 2: Density Chapter 6: Covalent and Ionic Bonding
 Chapter 10: States of Matter Concept Base: Chapter 1: Properties of Matter Chapter 2: Density Chapter 6: Covalent and Ionic Bonding Pressure standard pressure the pressure exerted at sea level in dry air
Chapter 10: States of Matter Concept Base: Chapter 1: Properties of Matter Chapter 2: Density Chapter 6: Covalent and Ionic Bonding Pressure standard pressure the pressure exerted at sea level in dry air
Investigation of CNT Growth Regimes in a Tubular CVD Reactor Considering Growth Temperature
 ICHMT2014-XXXX Investigation of CNT Growth Regimes in a Tubular CVD Reactor Considering Growth Temperature B. Zahed 1, T. Fanaei Sheikholeslami 2,*, A. Behzadmehr 3, H. Atashi 4 1 PhD Student, Mechanical
ICHMT2014-XXXX Investigation of CNT Growth Regimes in a Tubular CVD Reactor Considering Growth Temperature B. Zahed 1, T. Fanaei Sheikholeslami 2,*, A. Behzadmehr 3, H. Atashi 4 1 PhD Student, Mechanical
k T m 8 B P m k T M T
 I. INTRODUCTION AND OBJECTIVE OF THE EXPERIENT The techniques for evaporation of chemicals in a vacuum are widely used for thin film deposition on rigid substrates, leading to multiple applications: production
I. INTRODUCTION AND OBJECTIVE OF THE EXPERIENT The techniques for evaporation of chemicals in a vacuum are widely used for thin film deposition on rigid substrates, leading to multiple applications: production
AE 3051, Lab #16. Investigation of the Ideal Gas State Equation. By: George P. Burdell. Group E3
 AE 3051, Lab #16 Investigation of the Ideal Gas State Equation By: George P. Burdell Group E3 Summer Semester 000 Abstract The validity of the ideal gas equation of state was experimentally tested for
AE 3051, Lab #16 Investigation of the Ideal Gas State Equation By: George P. Burdell Group E3 Summer Semester 000 Abstract The validity of the ideal gas equation of state was experimentally tested for
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January ISSN
 International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-214 29 An Experimental Analysis of Stress Relaxation in Nonwoven Fabrics Sajid Ahmed Qureshi ABSTRACT - The current
International Journal of Scientific & Engineering Research, Volume 5, Issue 1, January-214 29 An Experimental Analysis of Stress Relaxation in Nonwoven Fabrics Sajid Ahmed Qureshi ABSTRACT - The current
ANSWERS 391. Chapter 9
 ANSWERS 391 ANSWERS Chapter 9 9.1 1.8 9. (a) From the given graph for a stress of 150 10 6 N m - the strain is 0.00 Approximate yield strength of the material is 3 10 8 N m - 9.3 (a) Material A Strength
ANSWERS 391 ANSWERS Chapter 9 9.1 1.8 9. (a) From the given graph for a stress of 150 10 6 N m - the strain is 0.00 Approximate yield strength of the material is 3 10 8 N m - 9.3 (a) Material A Strength
Thermal Methods of Analysis Theory, General Techniques and Applications. Prof. Tarek A. Fayed
 Thermal Methods of Analysis Theory, General Techniques and Applications Prof. Tarek A. Fayed 1- General introduction and theory: Thermal analysis (TA) is a group of physical techniques in which the chemical
Thermal Methods of Analysis Theory, General Techniques and Applications Prof. Tarek A. Fayed 1- General introduction and theory: Thermal analysis (TA) is a group of physical techniques in which the chemical
Earlier Lecture. In the earlier lecture, we have seen non metallic sensors like Silicon diode, Cernox and Ruthenium Oxide.
 41 1 Earlier Lecture In the earlier lecture, we have seen non metallic sensors like Silicon diode, Cernox and Ruthenium Oxide. Silicon diodes have negligible i 2 R losses. Cernox RTDs offer high response
41 1 Earlier Lecture In the earlier lecture, we have seen non metallic sensors like Silicon diode, Cernox and Ruthenium Oxide. Silicon diodes have negligible i 2 R losses. Cernox RTDs offer high response
Differential Mobility Particle Sizer (Aerosol measurements)
 Institute for Atmospheric and Climate Science - IACETH Atmospheric Physics Lab Work Differential Mobility Particle Sizer (Aerosol measurements) Abstract A differential mobility particle sizer (DMPS) is
Institute for Atmospheric and Climate Science - IACETH Atmospheric Physics Lab Work Differential Mobility Particle Sizer (Aerosol measurements) Abstract A differential mobility particle sizer (DMPS) is
DETAILED MODELLING OF SHORT-CONTACT-TIME REACTORS
 DETAILED MODELLING OF SHORT-CONTACT-TIME REACTORS Olaf Deutschmann 1, Lanny D. Schmidt 2, Jürgen Warnatz 1 1 Interdiziplinäres Zentrum für Wissenschaftliches Rechnen, Universität Heidelberg Im Neuenheimer
DETAILED MODELLING OF SHORT-CONTACT-TIME REACTORS Olaf Deutschmann 1, Lanny D. Schmidt 2, Jürgen Warnatz 1 1 Interdiziplinäres Zentrum für Wissenschaftliches Rechnen, Universität Heidelberg Im Neuenheimer
12/8/2009. Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka
 Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Introduction and classes of properties Case studies showing selection of the right material for the job Deformation of material under the action of a
Prof. A.K.M.B. Rashid Department of MME BUET, Dhaka Introduction and classes of properties Case studies showing selection of the right material for the job Deformation of material under the action of a
Investigation of the fluidized bed-chemical vapor deposition (FB- CVD) process using CFD-DEM method
 Investigation of the fluidized bed-chemical vapor deposition (FB- CVD) process using CFD-DEM method Malin Liu 1, Rongzheng Liu, Yuanyun Wen, Bing Liu, Youlin Shao (Institute of Nuclear and New Energy Technology,
Investigation of the fluidized bed-chemical vapor deposition (FB- CVD) process using CFD-DEM method Malin Liu 1, Rongzheng Liu, Yuanyun Wen, Bing Liu, Youlin Shao (Institute of Nuclear and New Energy Technology,
Influence of residual stresses in the structural behavior of. tubular columns and arches. Nuno Rocha Cima Gomes
 October 2014 Influence of residual stresses in the structural behavior of Abstract tubular columns and arches Nuno Rocha Cima Gomes Instituto Superior Técnico, Universidade de Lisboa, Portugal Contact:
October 2014 Influence of residual stresses in the structural behavior of Abstract tubular columns and arches Nuno Rocha Cima Gomes Instituto Superior Técnico, Universidade de Lisboa, Portugal Contact:
Chemical Kinetics of Ethane Oxidation and Methane Oxidation with Platinum
 Abstract Chemical Kinetics of Ethane Oxidation and Methane Oxidation with Platinum Jerry J. Zhang University of Southern California Professor Kenneth Brezinsky University of Illinois at Chicago Aleksandr
Abstract Chemical Kinetics of Ethane Oxidation and Methane Oxidation with Platinum Jerry J. Zhang University of Southern California Professor Kenneth Brezinsky University of Illinois at Chicago Aleksandr
Name: Class: Date: SHORT ANSWER Answer the following questions in the space provided.
 CHAPTER 10 REVIEW States of Matter SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Identify whether the descriptions below describe an ideal gas or a real gas. a. The gas
CHAPTER 10 REVIEW States of Matter SECTION 1 SHORT ANSWER Answer the following questions in the space provided. 1. Identify whether the descriptions below describe an ideal gas or a real gas. a. The gas
Research and Development of Parylene Thin-Film Deposition and Application for Water-Proofing
 Advanced Materials Research Online: 2012-06-14 ISSN: 1662-8985, Vols. 538-541, pp 23-28 doi:10.4028/www.scientific.net/amr.538-541.23 2012 Trans Tech Publications, Switzerland Research and Development
Advanced Materials Research Online: 2012-06-14 ISSN: 1662-8985, Vols. 538-541, pp 23-28 doi:10.4028/www.scientific.net/amr.538-541.23 2012 Trans Tech Publications, Switzerland Research and Development
CHAPTER 6: Etching. Chapter 6 1
 Chapter 6 1 CHAPTER 6: Etching Different etching processes are selected depending upon the particular material to be removed. As shown in Figure 6.1, wet chemical processes result in isotropic etching
Chapter 6 1 CHAPTER 6: Etching Different etching processes are selected depending upon the particular material to be removed. As shown in Figure 6.1, wet chemical processes result in isotropic etching
Chapter 10. Lesson Starter. Why did you not smell the odor of the vapor immediately? Explain this event in terms of the motion of molecules.
 Preview Lesson Starter Objectives The Kinetic-Molecular Theory of Gases The Kinetic-Molecular Theory and the Nature of Gases Deviations of Real Gases from Ideal Behavior Section 1 The Kinetic-Molecular
Preview Lesson Starter Objectives The Kinetic-Molecular Theory of Gases The Kinetic-Molecular Theory and the Nature of Gases Deviations of Real Gases from Ideal Behavior Section 1 The Kinetic-Molecular
CHEM. Ch. 12 Notes ~ STATES OF MATTER
 CHEM. Ch. 12 Notes ~ STATES OF MATTER NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. 12.1 topics States of Matter: SOLID, LIQUID, GAS, PLASMA I. Kinetic Theory
CHEM. Ch. 12 Notes ~ STATES OF MATTER NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. 12.1 topics States of Matter: SOLID, LIQUID, GAS, PLASMA I. Kinetic Theory
ANALYSIS OF TRANSIENT HEAT CONDUCTION IN DIFFERENT GEOMETRIES BY POLYNOMIAL APPROXIMATION METHOD
 Int. J. Mech. Eng. & Rob. Res. Devanshu Prasad, Research Paper ISSN 78 9 www.ijmerr.com Vol., No., April IJMERR. All Rights Reserved ANALYSIS OF TRANSIENT HEAT CONDUCTION IN DIFFERENT GEOMETRIES Y POLYNOMIAL
Int. J. Mech. Eng. & Rob. Res. Devanshu Prasad, Research Paper ISSN 78 9 www.ijmerr.com Vol., No., April IJMERR. All Rights Reserved ANALYSIS OF TRANSIENT HEAT CONDUCTION IN DIFFERENT GEOMETRIES Y POLYNOMIAL
CHE-201. I n t r o d u c t i o n t o Chemical E n g i n e e r i n g. I N S T R U CTOR: D r. N a b e e l S a l i m A b o - Ghander.
 I n t r o d u c t i o n t o Chemical E n g i n e e r i n g CHE-201 I N S T R U CTOR: D r. N a b e e l S a l i m A b o - Ghander C h a p t e r 3 Processes and Process Variables Introduction What is a process?
I n t r o d u c t i o n t o Chemical E n g i n e e r i n g CHE-201 I N S T R U CTOR: D r. N a b e e l S a l i m A b o - Ghander C h a p t e r 3 Processes and Process Variables Introduction What is a process?
Chapter 11. Freedom of Motion. Comparisons of the States of Matter. Liquids, Solids, and Intermolecular Forces
 Liquids, Solids, and Intermolecular Forces Chapter 11 Comparisons of the States of Matter The solid and liquid states have a much higher density than the gas state The solid and liquid states have similar
Liquids, Solids, and Intermolecular Forces Chapter 11 Comparisons of the States of Matter The solid and liquid states have a much higher density than the gas state The solid and liquid states have similar
TEST METHOD FOR DETERMINING BREAKAWAY FORCE OF A MAGNET
 STANDARD MDFA 101 95 TEST METHOD FOR DETERMINING BREAKAWAY FORCE OF A MAGNET DISCLAIMER: This test method was developed by representative members of the Magnet Distributor and Fabricators Association (MDFA)
STANDARD MDFA 101 95 TEST METHOD FOR DETERMINING BREAKAWAY FORCE OF A MAGNET DISCLAIMER: This test method was developed by representative members of the Magnet Distributor and Fabricators Association (MDFA)
LBS 172 Exam 1 Review
 Chapter 12- Gases LBS 172 Exam 1 Review I. What is a gas? a. Properties i. Non-definite volume, fills container, can flow, spread out, can be compressed b. Air is a gas composed of many gases i. Relatively
Chapter 12- Gases LBS 172 Exam 1 Review I. What is a gas? a. Properties i. Non-definite volume, fills container, can flow, spread out, can be compressed b. Air is a gas composed of many gases i. Relatively
Introduction to Engineering Materials ENGR2000. Dr. Coates
 Introduction to Engineering Materials ENGR2 Chapter 6: Mechanical Properties of Metals Dr. Coates 6.2 Concepts of Stress and Strain tension compression shear torsion Tension Tests The specimen is deformed
Introduction to Engineering Materials ENGR2 Chapter 6: Mechanical Properties of Metals Dr. Coates 6.2 Concepts of Stress and Strain tension compression shear torsion Tension Tests The specimen is deformed
Mass Transfer Operations
 College of Engineering Tutorial # 1 Chemical Engineering Dept. 14/9/1428 1. Methane and helium gas mixture is contained in a tube at 101.32 k Pa pressure and 298 K. At one point the partial pressure methane
College of Engineering Tutorial # 1 Chemical Engineering Dept. 14/9/1428 1. Methane and helium gas mixture is contained in a tube at 101.32 k Pa pressure and 298 K. At one point the partial pressure methane
Micromechanical analysis of FRP hybrid composite lamina for in-plane transverse loading
 Indian Journal of Engineering & Materials Sciences Vol. 15, October 2008, pp. 382-390 Micromechanical analysis of FRP hybrid composite lamina for in-plane transverse loading K Sivaji Babu a *, K Mohana
Indian Journal of Engineering & Materials Sciences Vol. 15, October 2008, pp. 382-390 Micromechanical analysis of FRP hybrid composite lamina for in-plane transverse loading K Sivaji Babu a *, K Mohana
Chemical Vapor Deposition (CVD)
 Chemical Vapor Deposition (CVD) source chemical reaction film substrate More conformal deposition vs. PVD t Shown here is 100% conformal deposition ( higher temp has higher surface diffusion) t step 1
Chemical Vapor Deposition (CVD) source chemical reaction film substrate More conformal deposition vs. PVD t Shown here is 100% conformal deposition ( higher temp has higher surface diffusion) t step 1
( KS A ) (1) , vapour, vapor (USA) , saturation vapour pressure. , standard reference conditions for gases. , degree of saturation
 ( KS A 3014-91 ) (1), standard reference conditions for gases 0, 101325 Pa (1 =760mmHg ), vacuum, low ( rough ) vacuum 100Pa, medium vacuum 100 01 Pa, high vacuum 01 10 5 Pa, ultra high vacuum ( UHV )
( KS A 3014-91 ) (1), standard reference conditions for gases 0, 101325 Pa (1 =760mmHg ), vacuum, low ( rough ) vacuum 100Pa, medium vacuum 100 01 Pa, high vacuum 01 10 5 Pa, ultra high vacuum ( UHV )
SUPPORTING INFORMATION. Si wire growth. Si wires were grown from Si(111) substrate that had a low miscut angle
 SUPPORTING INFORMATION The general fabrication process is illustrated in Figure 1. Si wire growth. Si wires were grown from Si(111) substrate that had a low miscut angle of 0.1. The Si was covered with
SUPPORTING INFORMATION The general fabrication process is illustrated in Figure 1. Si wire growth. Si wires were grown from Si(111) substrate that had a low miscut angle of 0.1. The Si was covered with
Composite Materials. Fibre-Matrix Interfaces. There is nothing there really except the two of you (or the fiber and matrix).
 Composite Materials Fibre-Matrix Interfaces There is nothing there really except the two of you (or the fiber and matrix). Composite Parameters Fibre properties Composite Interfaces Matrix properties Fibre
Composite Materials Fibre-Matrix Interfaces There is nothing there really except the two of you (or the fiber and matrix). Composite Parameters Fibre properties Composite Interfaces Matrix properties Fibre
A Novel Approach to the Layer Number-Controlled and Grain Size- Controlled Growth of High Quality Graphene for Nanoelectronics
 Supporting Information A Novel Approach to the Layer Number-Controlled and Grain Size- Controlled Growth of High Quality Graphene for Nanoelectronics Tej B. Limbu 1,2, Jean C. Hernández 3, Frank Mendoza
Supporting Information A Novel Approach to the Layer Number-Controlled and Grain Size- Controlled Growth of High Quality Graphene for Nanoelectronics Tej B. Limbu 1,2, Jean C. Hernández 3, Frank Mendoza
Study of thermal and adsorption characteristics of composite ammonia adsorber with expanded graphite and using nano fluids.
 International Engineering Research Journal Special Edition PGCON-MECH-2017 Study of thermal and adsorption characteristics of composite ammonia adsorber with expanded graphite and using nano fluids. Swapnil
International Engineering Research Journal Special Edition PGCON-MECH-2017 Study of thermal and adsorption characteristics of composite ammonia adsorber with expanded graphite and using nano fluids. Swapnil
Study of DC Cylindrical Magnetron by Langmuir Probe
 WDS'2 Proceedings of Contributed Papers, Part II, 76 8, 22. ISBN 978-737825 MATFYZPRESS Study of DC Cylindrical Magnetron by Langmuir Probe A. Kolpaková, P. Kudrna, and M. Tichý Charles University Prague,
WDS'2 Proceedings of Contributed Papers, Part II, 76 8, 22. ISBN 978-737825 MATFYZPRESS Study of DC Cylindrical Magnetron by Langmuir Probe A. Kolpaková, P. Kudrna, and M. Tichý Charles University Prague,
The fundamental difference between. particles.
 Gases, Liquids and Solids David A. Katz Department of Chemistry Pima Community College States of Matter The fundamental difference between states t of matter is the distance between particles. States of
Gases, Liquids and Solids David A. Katz Department of Chemistry Pima Community College States of Matter The fundamental difference between states t of matter is the distance between particles. States of
Hydrothermal Stability Analysis of Carbonised Template Molecular Sieve Silica Membranes
 Refereed Proceedings Separations Technology VI: New Perspectives on Very Large-Scale Operations Engineering Conferences International Year 2004 Hydrothermal Stability Analysis of Carbonised Template Molecular
Refereed Proceedings Separations Technology VI: New Perspectives on Very Large-Scale Operations Engineering Conferences International Year 2004 Hydrothermal Stability Analysis of Carbonised Template Molecular
High Tech High Top Hat Technicians. An Introduction to Solid Mechanics. Is that supposed to bend there?
 High Tech High Top Hat Technicians An Introduction to Solid Mechanics Or Is that supposed to bend there? Why don't we fall through the floor? The power of any Spring is in the same proportion with the
High Tech High Top Hat Technicians An Introduction to Solid Mechanics Or Is that supposed to bend there? Why don't we fall through the floor? The power of any Spring is in the same proportion with the
UNIT I SIMPLE STRESSES AND STRAINS
 Subject with Code : SM-1(15A01303) Year & Sem: II-B.Tech & I-Sem SIDDHARTH GROUP OF INSTITUTIONS :: PUTTUR Siddharth Nagar, Narayanavanam Road 517583 QUESTION BANK (DESCRIPTIVE) UNIT I SIMPLE STRESSES
Subject with Code : SM-1(15A01303) Year & Sem: II-B.Tech & I-Sem SIDDHARTH GROUP OF INSTITUTIONS :: PUTTUR Siddharth Nagar, Narayanavanam Road 517583 QUESTION BANK (DESCRIPTIVE) UNIT I SIMPLE STRESSES
Experiences in an Undergraduate Laboratory Using Uncertainty Analysis to Validate Engineering Models with Experimental Data
 Experiences in an Undergraduate Laboratory Using Analysis to Validate Engineering Models with Experimental Data W. G. Steele 1 and J. A. Schneider Abstract Traditionally, the goals of engineering laboratory
Experiences in an Undergraduate Laboratory Using Analysis to Validate Engineering Models with Experimental Data W. G. Steele 1 and J. A. Schneider Abstract Traditionally, the goals of engineering laboratory
Class XI Physics Syllabus One Paper Three Hours Max Marks: 70
 Class XI Physics Syllabus 2013 One Paper Three Hours Max Marks: 70 Class XI Weightage Unit I Physical World & Measurement 03 Unit II Kinematics 10 Unit III Laws of Motion 10 Unit IV Work, Energy & Power
Class XI Physics Syllabus 2013 One Paper Three Hours Max Marks: 70 Class XI Weightage Unit I Physical World & Measurement 03 Unit II Kinematics 10 Unit III Laws of Motion 10 Unit IV Work, Energy & Power
Micro- and Nano-structure Characterization of Isotropic Pyrocarbon Obtained via Chemical Vapor Deposition in Hot Wall Reactor
 J. Mater. Sci. Technol., 2010, 26(12), 1133-1138. Micro- and Nano-structure Characterization of Isotropic Pyrocarbon Obtained via Chemical Vapor Deposition in Hot Wall Reactor Kezhi Li, Dongsheng Zhang,
J. Mater. Sci. Technol., 2010, 26(12), 1133-1138. Micro- and Nano-structure Characterization of Isotropic Pyrocarbon Obtained via Chemical Vapor Deposition in Hot Wall Reactor Kezhi Li, Dongsheng Zhang,
Thermal properties of Engineering Materials
 Thermal properties of Engineering Materials Engineering materials are important in everyday life because of their versatile structural properties. Other than these properties, they do play an important
Thermal properties of Engineering Materials Engineering materials are important in everyday life because of their versatile structural properties. Other than these properties, they do play an important
CVD: General considerations.
 CVD: General considerations. PVD: Move material from bulk to thin film form. Limited primarily to metals or simple materials. Limited by thermal stability/vapor pressure considerations. Typically requires
CVD: General considerations. PVD: Move material from bulk to thin film form. Limited primarily to metals or simple materials. Limited by thermal stability/vapor pressure considerations. Typically requires
Lecture Outline. 5.1 The Nature of Energy. Kinetic Energy and Potential Energy. 1 mv
 Chapter 5. Thermochemistry Common Student Misconceptions Students confuse power and energy. Students confuse heat with temperature. Students fail to note that the first law of thermodynamics is the law
Chapter 5. Thermochemistry Common Student Misconceptions Students confuse power and energy. Students confuse heat with temperature. Students fail to note that the first law of thermodynamics is the law
Hydrogen addition to the Andrussow process for HCN synthesis
 Applied Catalysis A: General 201 (2000) 13 22 Hydrogen addition to the Andrussow process for HCN synthesis A.S. Bodke, D.A. Olschki, L.D. Schmidt Department of Chemical Engineering and Materials Science,
Applied Catalysis A: General 201 (2000) 13 22 Hydrogen addition to the Andrussow process for HCN synthesis A.S. Bodke, D.A. Olschki, L.D. Schmidt Department of Chemical Engineering and Materials Science,
Liquids and Solids. H fus (Heat of fusion) H vap (Heat of vaporization) H sub (Heat of sublimation)
 Liquids and Solids Phase Transitions All elements and compounds undergo some sort of phase transition as their temperature is increase from 0 K. The points at which these phase transitions occur depend
Liquids and Solids Phase Transitions All elements and compounds undergo some sort of phase transition as their temperature is increase from 0 K. The points at which these phase transitions occur depend
Solutions for Assignment-6
 Solutions for Assignment-6 Q1. What is the aim of thin film deposition? [1] (a) To maintain surface uniformity (b) To reduce the amount (or mass) of light absorbing materials (c) To decrease the weight
Solutions for Assignment-6 Q1. What is the aim of thin film deposition? [1] (a) To maintain surface uniformity (b) To reduce the amount (or mass) of light absorbing materials (c) To decrease the weight
AE 205 Materials and Energy Balances Asst. Prof. Dr. Tippabust Eksangsri. Chapter 4 Stoichiometry and MB with Reactions
 AE 205 Materials and Energy Balances Asst. Prof. Dr. Tippabust Eksangsri Chapter 4 Stoichiometry and MB with Reactions Stoichiometry Stoichiometry provides a quantitative means of relating the amount of
AE 205 Materials and Energy Balances Asst. Prof. Dr. Tippabust Eksangsri Chapter 4 Stoichiometry and MB with Reactions Stoichiometry Stoichiometry provides a quantitative means of relating the amount of
Interactions between oxygen permeation and homogeneous-phase fuel conversion on the sweep side of an ion transport membrane
 Interactions between oxygen permeation and homogeneous-phase fuel conversion on the sweep side of an ion transport membrane The MIT Faculty has made this article openly available. Please share how this
Interactions between oxygen permeation and homogeneous-phase fuel conversion on the sweep side of an ion transport membrane The MIT Faculty has made this article openly available. Please share how this
Chemical Reaction Engineering - Part 16 - more reactors Richard K. Herz,
 Chemical Reaction Engineering - Part 16 - more reactors Richard K. Herz, rherz@ucsd.edu, www.reactorlab.net More reactors So far we have learned about the three basic types of reactors: Batch, PFR, CSTR.
Chemical Reaction Engineering - Part 16 - more reactors Richard K. Herz, rherz@ucsd.edu, www.reactorlab.net More reactors So far we have learned about the three basic types of reactors: Batch, PFR, CSTR.
Gravity is a force which keeps us stuck to the earth. The Electrostatic force attracts electrons to protons in an atom.
 Energy Relations in Chemistry: Thermochemistry The Nature of Energy Sugar you eat is "combusted" by your body to produce CO 2 and H 2 O. During this process energy is also released. This energy is used
Energy Relations in Chemistry: Thermochemistry The Nature of Energy Sugar you eat is "combusted" by your body to produce CO 2 and H 2 O. During this process energy is also released. This energy is used
Effect of Spiral Microwave Antenna Configuration on the Production of Nano-crystalline Film by Chemical Sputtering in ECR Plasma
 THE HARRIS SCIENCE REVIEW OF DOSHISHA UNIVERSITY, VOL. 56, No. 1 April 2015 Effect of Spiral Microwave Antenna Configuration on the Production of Nano-crystalline Film by Chemical Sputtering in ECR Plasma
THE HARRIS SCIENCE REVIEW OF DOSHISHA UNIVERSITY, VOL. 56, No. 1 April 2015 Effect of Spiral Microwave Antenna Configuration on the Production of Nano-crystalline Film by Chemical Sputtering in ECR Plasma
Compressible Gas Flow
 Compressible Gas Flow by Elizabeth Adolph Submitted to Dr. C. Grant Willson CHE53M Department of Chemical Engineering The University of Texas at Austin Fall 008 Compressible Gas Flow Abstract In this lab,
Compressible Gas Flow by Elizabeth Adolph Submitted to Dr. C. Grant Willson CHE53M Department of Chemical Engineering The University of Texas at Austin Fall 008 Compressible Gas Flow Abstract In this lab,
Enhanced Boiling Heat Transfer by using micropin-finned surfaces for Electronic Cooling
 Enhanced Boiling Heat Transfer by using micropin-finned surfaces for Electronic Cooling JinJia Wei State Key Laboratory of Multiphase Flow in Power Engineering Xi an Jiaotong University Contents 1. Background
Enhanced Boiling Heat Transfer by using micropin-finned surfaces for Electronic Cooling JinJia Wei State Key Laboratory of Multiphase Flow in Power Engineering Xi an Jiaotong University Contents 1. Background
Gases. Properties of Gases Kinetic Molecular Theory of Gases Pressure Boyle s and Charles Law The Ideal Gas Law Gas reactions Partial pressures.
 Gases Properties of Gases Kinetic Molecular Theory of Gases Pressure Boyle s and Charles Law The Ideal Gas Law Gas reactions Partial pressures Gases Properties of Gases All elements will form a gas at
Gases Properties of Gases Kinetic Molecular Theory of Gases Pressure Boyle s and Charles Law The Ideal Gas Law Gas reactions Partial pressures Gases Properties of Gases All elements will form a gas at
