AP Chemistry 1: Summer 2015 Assignment
|
|
|
- Edwin Malone
- 6 years ago
- Views:
Transcription
1 AP Chemistry 1: Summer 2015 Assignment TEXTBOOK: Chemistry, 2 nd Edition CK-12 Foundation (Berwick & Parsons); customized as AP Chemistry 1 Summer Reading Assignment The textbook & content outline can be accessed through: **If you cannot access the textbook, please contact Dr. Best at bests@tesd.net** This is the textbook for the summer assignment. You will be given an additional hardcopy textbook on the first day of school that we will use for the duration of the AP Chemistry 1 course. The textbook seems long at first glance (98 pages), but it is a relatively easy read. Each page in the Chemistry e-textbook are shorter than most pages in a traditional textbook and cover less material (~2 pages in the e-book to 1 page in a normal Chemistry text). There are also lots of YouTube videos that you can access as well as practice problems included for each of the sections to enhance your understanding of the material. CONTENT OUTLINE: Read the textbook and fill out the notes outline by HAND as you go along. NO typewritten notes outlines will be accepted! Sometimes you will just need to fill in a blank, while other times you may need to glean the most important concepts from the textbook. In general, the notes outline follows the textbook with a few noted exceptions. If you cannot find something, feel free to access the additional resources noted in the textbook and/or the internet. SUMMER ASSIGNMENT QUIZ: There will be a quiz on the material covered in the summer assignment that will occur on the Friday before Labor Day, September 4 th, You may use your packet to take this quiz. In addition, you will also turn in your packet at the end of the quiz. These packets will be graded for completeness, not accuracy, and will count as a homework grade. NO typewritten notes outlines may be used for the quiz or turned in for homework points! REVIEW PROBLEMS: There are review problems at the end of each section in the textbook. Please complete these problems in your notes packet. Remember that you will be able to use your packet for the quiz, so if you think it would be helpful to write out the questions/statements, you may do so. Also feel free to include additional handwritten pages if you need additional space to complete the problems. REMINDER: **Packet Due Date & Quiz: Friday, September 4, 2015** Pg.1
2 CHAPTER 1: INTRODUCTION TO CHEMISTRY 1.1 What is Chemistry? A. Chemistry is. B. The man who would greatly advance the development of modern chemistry was. Considered the father of modern chemistry, (seen in Figure 1.2) discovered that although matter may change its shape or form, its always remains the. As a result, he would state the first version of the. C. Chemists apply information about and the to improve our lives in many different ways. D. Using complete sentences, answer the review questions on the bottom of p. 4 in the space below: The Process of Science A. Describe why Aristotle s methods were unsuitable for describing the nature of the physical world. B. The is a method of investigation involving experimentation and observation to. Scientists frequently list the as a. Some scientists oppose this listing of because not all occur in every case, nor do they always. The is listed here as a series of, but you should remember that you are not required to rigidly follow this list. Instead, the is a valuable tool that provides a basic and adaptable. C. List the steps in the scientific method: Pg.2
3 D. Define the following: 1. Hypothesis 2. Theory 3. Scientific Law 4. Experiment 5. Controlled experiment 6. Control sample 7. Model E. List two examples of scientific laws as described in the reading: F. From your previous science courses or general knowledge, list one example of a scientific theory: Pg.3
4 D. Answer the review questions on p. 12 to p. 16. Place the answer to each multiple choice question in the numbered space below: CHAPTER 2: Measurement in Chemistry 2.1 Making Observations A. Define the following: 1. Observation 2. Quantitative observations 3. Qualitative observations D. Answer the review questions on p. 20 to 21. Label each of the following observations as qualitative or quantitative. Label each observation as qualitative or quantitative. 1. The temperature of this room is 25_C. 2. It is comfortably warm in this room. 3. Most people have removed their coats. 4. The building is 25 stories high. 5. It is a very tall building. 6. The building is taller than any nearby trees. 7. The bottle is green. 8. The bottle contains 250 milliliters of liquid. 9. Robert bought his son a small car. 10. The car is smaller than his hand. 11. The car is about three inches long. 12. The race is about 27 miles long. Pg.4
5 2.2 Measurement Systems A. The is an international -based system of measurement. Because the metric system is a system, making conversions between different units of the metric system are always done with. B. Explain why the metric system is more useful and easier to manipulate than the US customary system: C. Fill in the missing blanks in Table 2.4 below: Prefix Meaning Symbol piconanomicromillicentidecikilo- D. Fill in the missing blanks below: Length: millimeters = 1 meter centimeters = 1 meter millimeters = 1 centimeter Mass: milligrams = 1 gram grams = 1 kilogram Volume: 1 liter = milliliters 2.3 The SI System of Measurement A. The International System of Units, abbreviated from the French Le Système International d Unites, is the main system of measurement units used in science. Since the 1960s, the International System of Units has been agreed upon internationally as the. The SI are based on standards. The definitions of the SI base units have been and continue to be modified, and new base units are added as advancements in science are made. Each SI, except the, is described by stable properties of the universe. B. Mass Pg.5
6 Define Mass: Define weight: Explain how mass and weight differ: The basic unit of mass in the SI system is the. C. Length Define Length: The basic unit of length in the SI system is the. D. Volume Define Volume: Volume is a, meaning that. The is the SI unit of length and is based on the. More common units of volume used in chemistry are the: Liter; 1 liter = meter = quart Milliliter; 1 milliliter = liter = cm 3 E. Temperature Define Temperature: Define heat: Explain how temperature and heat differ: F. Temperature Scales Pg.6
7 1. Comparison of Fahrenheit, Celsius, and Kelvin scales 2. Describe what the Fahrenheit temperature scale is based on: 3. Describe what the Celsius temperature scale is based on: 4. Describe what the Kelvin temperature scale is based on: 5. List the formula used to convert between degrees C and Kelvin: 6. Define absolute zero: 7. It should be noted that many mathematical calculations in chemistry involve the between two temperatures, symbolized by (pronounced delta T). Since the is the same in and in, the will be the for either scale. For example, 20 o C = K and 50 o C = K; the difference between the Celsius temperatures is, and the difference between the Kelvin temperatures is. When the calculations involve, it is not necessary to convert Celsius to Kelvin, but when the in an equation, it is necessary to convert Celsius to Kelvin. G. Time The SI unit for time is the. Pg.7
8 H. Using complete sentences, answer the review questions on p. 30 in the space below: Significant Figures A. Measurements do not produce numbers; the only numbers in science are such as. Since measurements are fundamental to science, science does not. It is very important to recognize and report the of a measurement along with the and of the measurement.. B. Significant figures, also known as, are C. In the first ruler illustration shown on p. 31, the measurement is (estimated to the place). D. In the second ruler illustration shown on the top of p. 32, the measurement is (estimated to the place). E. In the third ruler illustration shown on p. 32, the measurement is (estimated to the place). Pg.8
9 F. In the fourth ruler illustration shown on p. 32, the measurement is (estimated to the place). G. List the rules for determining the Number of Significant Figures: H. Equipment Determines Significant Figures: 1. Examine the two graduated cylinders found on the bottom of p. 33. The one on the left can be estimated to place, while the one on the right can be estimated to place. 2. Examine the two balances found on p. 34. The one on the left can measure to, while the one on the right can measure to. I. Significant Figures in Calculations 1. List the rule for determining the number of significant figures in an addition or subtraction problem: 2. List the rule for determining the number of significant figures in a multiplication or division problem: J. Using the significant figures rules, answer the review questions on pp in the space below: 1. How many significant figures are in the following numbers? (a) (b) (c) (d) Pg.9
10 (e) (f) (g) 2. Perform the following calculations and give your answer with the correct number of significant figures: (a) (b) (c) 3. Perform the following calculations and give your answer with the correct number of significant figures: (a) (b) (c) (d) 2.5 Using Algebra in Chemistry A. The formula for density is D (density) = B. Examine the examples given on the bottom of p. 37 and the top of p. 38. Solve the following problems: Problem #1 What is the density of Hg (mercury) if g occupy a volume of 12.1cm 3? Problem #2 Using the density you solved for in Problem #1, what is the mass of 2.15 cm 3 of Hg? Problem #3 Using the density you solved for in Problem #3, what is the volume of 94.2 g of Hg? Pg.10
11 C. A conversion factor is. D. Dimensional analysis is. E. Review the dimensional analysis problems shown on p. 39, and answer the following review questions from p. 40. Show all of your work, and use significant figures in your final answers. 1. For the equation PV = nrt, re-write it so that it is in the form of T =. 2. The equation for density is D = mv. If D is 12:8 g/cm3 and m is 46:1 g, solve for V, keeping significant figures in mind. 3. The equation P 1 xv 1 = P 2 x V 2, known as Boyle s law, shows that gas pressure is inversely proportional to its volume. Re-write Boyle s law so it is in the form of V 1 =. 4. The density of a certain solid is measured and found to be 12:68 g/ml. Convert this measurement into kg/l. 5. In a nuclear chemistry experiment, an alpha particle is found to have a velocity of 14, 285 m/s. Convert this measurement into miles/hour (mi/h). 2.6 Scientific Notation A. In, very large and very small numbers are expressed as the of a number between multiplied by some power of. Pg.11
12 B. As you can see from the examples in Table 2.7, to convert a number from decimal form into scientific notation, you needed to move the decimal, and that number becomes the. If you are moving the decimal to the, the exponent is, and if you are moving the decimal to the, the exponent is. You should note that are maintained in scientific notation. C. Scientific Notation in Calculations 1. Addition and Subtraction: When numbers in exponential form are added or subtracted, the exponents must be the. If the exponents are the, the coefficients are and the exponent remains the. 2. Multiplication and Division: When multiplying or dividing numbers in exponential form, the numbers. To exponential numbers, the coefficients and the exponents. To exponential numbers, the coefficients and the exponents. D. Review the scientific notation examples shown on pp , and answer the following review questions from p a) b) c) d) Evaluating Measurements A. Precision vs. Accuracy Pg.12
13 Definitions: Accuracy how close measurement is to. Precision how close values in a set of measurements are to. B. Percent Error: Note that % error is always positive, so take the absolute value of any answer you obtain! Equation: C. Answer the review questions on pp in the space below: Graphing A. Define the following: Pg.13
14 1. Graph 2. Interpolation 3. Extrapolation 4. Slope D. Review the data tables and graphs shown on pp , and answer the following review questions from p. 54.For questions #2 and #3, either use the graph paper grids provided, or graph the data using Excel and paste copies of the graph onto the page a) b) c) Pg.14
15 3. Relationship: Slope: Meaning of slope: CHAPTER 3: Matter and Energy 3.1 What is Matter? A. Define the following: 1. matter Pg.15
16 2. atom 3. pure substance 4. mixture 5. homogeneous mixture 6. heterogeneous mixture 7. element 8. compound 9. molecule 10. periodic table 11. chemical symbol 12. chemical formula B. Answer the review questions on p. 61 to 62. Place the answer to each multiple choice question in the numbered space below: Pg.16
17 3.2 Properties and Changes of Matter A. Define the following: 1. physical properties 2. chemical properties 3. physical changes 4. chemical changes B. Answer the review questions on p. 65 to 66. Place the answer to each multiple choice question in the numbered space below: Energy A. Define the following: 1. kinetic energy 2. potential energy 3. chemical potential energy 4. Law of Conservation of Energy Pg.17
18 B. Answer the review questions on p. 69. Place the answer to each multiple choice question in the numbered space below: CHAPTER 4: ATOMIC THEORY 4.1 THE ATOMIC THEORY 1. ~ BC, Democritus atomos 2. Middle Ages, Alchemists (see Chapter 1.1) John Dalton 4. Law of Definite Proportions (define and give an example) 5. Law of Multiple Proportions (define and give an example) 6. Dalton s Atomic Theory & Model of the Atom Pg.18
19 Pg.19
20 Chapter 4.1 Review Questions on Pages : Pg.20
21 4.2 FURTHER UNDERSTANDING OF THE ATOM JJ Thomson a. Cathode ray tubes (Crooke s tubes) b. mass to charge ratio (how did mass & charge relate to Thompson s study) c. Plum Pudding Model of the Atom Robert Millikan Oil Drop Experiment Ernest Rutherford a. Gold Foil Experiment: what it was and how it was done b. Main Conclusions of Gold Foil Experiment c. Planetary Model of the Atom (Solar System Model of the Atom) Pg.21
22 Chapter 4.2 Review Questions on Pages : Pg.22
23 4.3 ATOMIC STRUCTURES Antoine Lavoisier -Chemist, Biologist, Economist, & Royal Tax Collector -Made quantitative observations (to.0001 g) -Articulated the Law of Conservation of Matter a. Law of Conservation of Matter (Chapter 3.3) b. Law of Constant Composition (Law of Definite Composition) - All samples of a compound have the same composition and have the same proportions by mass of the elements present Ex. All H2O molecules are exactly the same! Henri Becquerel, and 1898 Marie Curie Radioactivity -Observed elements that could darken photographic plates even though they were covered = Radioactivity! -3 Types: Alpha ( ), Beta ( ), Gamma ( ) -Contradicted Dalton s idea that atoms are indivisible since they determined that there must be particles smaller than atoms (sub-atomic) 3. Composition of the Nucleus a. Electrons (e - ) b. Protons (p + ) c. Neutrons (n o ): 1932, Chadwick Pg.23
24 d. Properties of subatomic particles: symbol, mass, charge, location Particle Symbol Mass Charge Location Protons Neutrons Electrons 4. Atomic Number (Z) 5. Mass Number (A) 6. Atomic Symbol -Unique combination of one, two, or three letters that represents each element *Capital letter is always written first -Names usually indicate properties or discoverers Ex. Fe = Iron (Ferrous) 7. Isotopes -Ex. Hydrogen Protium Deuterium Tritium 1p + 1p + and 1n o 1p + and 2n o 8. Mass Spectrometer and Percent Abundance -Uses mass-charge ratio to break apart and separate fragments of compounds -Shows isotopic abundance for elements % Abundance = (# atoms-isotope)/(total # atoms-all isotopes)*100 Chapter 4.3 Review Questions on Pages : 1. Pg.24
25 Pg.25
26 Pg.26
Glencoe: Chapter 4. The Structure of the Atom
 Glencoe: Chapter 4 The Structure of the Atom Section One: Early Ideas about Matter Atomists and Democritus : 400 B.C. From Thrace in Greece. Atoms- Uncut-Table Indivisible parts which cannot be broken
Glencoe: Chapter 4 The Structure of the Atom Section One: Early Ideas about Matter Atomists and Democritus : 400 B.C. From Thrace in Greece. Atoms- Uncut-Table Indivisible parts which cannot be broken
Chapter 4 Atomic Structure. Chemistry- Lookabaugh Moore High School
 Chapter 4 Atomic Structure Chemistry- Lookabaugh Moore High School Section 4.1 Defining the Atom Democritus (460 B.C 370 B.C.) first used the term atomon to describe the smallest particle of matter possible.
Chapter 4 Atomic Structure Chemistry- Lookabaugh Moore High School Section 4.1 Defining the Atom Democritus (460 B.C 370 B.C.) first used the term atomon to describe the smallest particle of matter possible.
History of Atomic Theory
 Unit 2 The Atom History of Atomic Theory A. Democritus and Aristotle Democritus named the "atom" - means indivisible Dalton (with work of Lavoisier, Proust, and Gay-Lussac) 1. atomic theory - first based
Unit 2 The Atom History of Atomic Theory A. Democritus and Aristotle Democritus named the "atom" - means indivisible Dalton (with work of Lavoisier, Proust, and Gay-Lussac) 1. atomic theory - first based
Early Atomic Theory. Alchemy. The atom
 Early Atomic Theory Chapter 3 Democritus 460 BC- ~ 370 BC Nothing exists except atoms and empty space; everything else is opinion. Matter is composed of small indivisible particles, atomos meaning Indivisible
Early Atomic Theory Chapter 3 Democritus 460 BC- ~ 370 BC Nothing exists except atoms and empty space; everything else is opinion. Matter is composed of small indivisible particles, atomos meaning Indivisible
Chapter 4. The structure of the atom. AL-COS Objectives 1, 2,3,4,7, 10, 15, 20, 21, 22, 27and 28
 Chapter 4 The structure of the atom AL-COS Objectives 1, 2,3,4,7, 10, 15, 20, 21, 22, 27and 28 You ll learn to Identify the experiments that led to the development of the nuclear model of atomic structure
Chapter 4 The structure of the atom AL-COS Objectives 1, 2,3,4,7, 10, 15, 20, 21, 22, 27and 28 You ll learn to Identify the experiments that led to the development of the nuclear model of atomic structure
3. SI Base Units used in Chemistry: Quantity Unit Name Abbreviation Tool to Measure Length Mass Time Temperature Amount of Substance
 Honors Chemistry Unit 1 Intro and Atomic Theory Notes Intro Scientific Measurements: 1. Chemistry is the study of. 2. Why do scientists worldwide use the SI system of measurement? 3. SI Base Units used
Honors Chemistry Unit 1 Intro and Atomic Theory Notes Intro Scientific Measurements: 1. Chemistry is the study of. 2. Why do scientists worldwide use the SI system of measurement? 3. SI Base Units used
Atomic Structure. How do you discover and study something you can t see?
 Atomic Structure How do you discover and study something you can t see? WHAT IS A THEORY? A hypothesis is a proposed explanation made as a starting point for further investigation (It s bright outside
Atomic Structure How do you discover and study something you can t see? WHAT IS A THEORY? A hypothesis is a proposed explanation made as a starting point for further investigation (It s bright outside
CHEMISTRY. CHM201 Class #2 CHEMISTRY. Chapter 1 Continued. Chapter 1 Outline for Class #2. Particles of Matter: Measurements and the Tools of Science
 CHEMISTRY Fifth Edition Gilbert Kirss Foster Bretz Davies CHM201 Class #2 Chemistry, 5 th Edition Copyright 2017, W. W. Norton & Company CHEMISTRY Fifth Edition Gilbert Kirss Foster Bretz Davies Chapter
CHEMISTRY Fifth Edition Gilbert Kirss Foster Bretz Davies CHM201 Class #2 Chemistry, 5 th Edition Copyright 2017, W. W. Norton & Company CHEMISTRY Fifth Edition Gilbert Kirss Foster Bretz Davies Chapter
Accelerated Chemistry Study Guide Atomic Structure, Chapter 3
 Accelerated Chemistry Study Guide Atomic Structure, Chapter 3 Terms and definitions atom ion law of constant composition isotope atomic theory of matter mass number cathode ray tube atomic mass electron
Accelerated Chemistry Study Guide Atomic Structure, Chapter 3 Terms and definitions atom ion law of constant composition isotope atomic theory of matter mass number cathode ray tube atomic mass electron
CHEMISTRY 11 UNIT REVIEW: ATOMIC THEORY & PERIODIC TRENDS
 CHEMISTRY 11 UNIT REVIEW: ATOMIC THEORY & PERIODIC TRENDS Atoms Atoms have protons and neutrons located in the nucleus of the atom. Electrons orbit around the nucleus in well-defined paths. Protons have
CHEMISTRY 11 UNIT REVIEW: ATOMIC THEORY & PERIODIC TRENDS Atoms Atoms have protons and neutrons located in the nucleus of the atom. Electrons orbit around the nucleus in well-defined paths. Protons have
THE BIG IDEA: ELECTRONS AND THE STRUCTURE OF ATOMS
 HONORS CHEMISTRY - CHAPTER 4 ATOMIC STRUCTURE OBJECTIVES AND NOTES - V10 NAME: DATE: PAGE: THE BIG IDEA: ELECTRONS AND THE STRUCTURE OF ATOMS Essential Questions 1. What components make up an atom? 2.
HONORS CHEMISTRY - CHAPTER 4 ATOMIC STRUCTURE OBJECTIVES AND NOTES - V10 NAME: DATE: PAGE: THE BIG IDEA: ELECTRONS AND THE STRUCTURE OF ATOMS Essential Questions 1. What components make up an atom? 2.
Democritus 460 BC 370 BC. First scholar to suggest that atoms existed. Believed that atoms were indivisible and indestructible.
 Democritus 460 BC 370 BC First scholar to suggest that atoms existed. Believed that atoms were indivisible and indestructible. Democritus 460 BC 370 BC Problems with theory: 1. Did not explain chemical
Democritus 460 BC 370 BC First scholar to suggest that atoms existed. Believed that atoms were indivisible and indestructible. Democritus 460 BC 370 BC Problems with theory: 1. Did not explain chemical
CHAPTER 3. Atoms: The Building Blocks of Matter
 CHAPTER 3 Atoms: The Building Blocks of Matter Origins of the Atom Democritus: Greek philosopher (460 BC - 370 BC) Coined the term atom from the Greek word atomos Democritus believes that atoms were indivisible
CHAPTER 3 Atoms: The Building Blocks of Matter Origins of the Atom Democritus: Greek philosopher (460 BC - 370 BC) Coined the term atom from the Greek word atomos Democritus believes that atoms were indivisible
Early Atomic Theories and the Origins of Quantum Theory. Chapter 3.1
 Early Atomic Theories and the Origins of Quantum Theory Chapter 3.1 What is Matter Made of? People have wondered about the answer to this question for thousands of years Philosophers Matter is composed
Early Atomic Theories and the Origins of Quantum Theory Chapter 3.1 What is Matter Made of? People have wondered about the answer to this question for thousands of years Philosophers Matter is composed
Atomic Theory: Early Models of the Atom:
 Atomic Theory: Our next job in Chemistry 11 is to learn about what matter is made of. After we have done this, we can start to understand why matter behaves the way it does. Everything that has volume
Atomic Theory: Our next job in Chemistry 11 is to learn about what matter is made of. After we have done this, we can start to understand why matter behaves the way it does. Everything that has volume
Name: Block Unit 3- The Atom
 Name: Block Unit 3- The Atom DEMOCRITUS 1. Was Democritus a scientist? Notes 2. In what time of history did he live? 3. Describe Democritus thoughts about gold. 4. What was Democritus word for something
Name: Block Unit 3- The Atom DEMOCRITUS 1. Was Democritus a scientist? Notes 2. In what time of history did he live? 3. Describe Democritus thoughts about gold. 4. What was Democritus word for something
Chapter 3 Atoms: The Building Blocks of Matter. Honors Chemistry 412
 Chapter 3 Atoms: The Building Blocks of Matter Honors Chemistry 412 Foundations of Atomic Theory Democritus Greek Philosopher 460-370 B.C. Stated Matter could be divided into smaller & smaller particles
Chapter 3 Atoms: The Building Blocks of Matter Honors Chemistry 412 Foundations of Atomic Theory Democritus Greek Philosopher 460-370 B.C. Stated Matter could be divided into smaller & smaller particles
Nuclear Chemistry. Atomic Structure Notes Start on Slide 20 from the second class lecture
 Nuclear Chemistry Atomic Structure Notes Start on Slide 20 from the second class lecture The Birth of an Idea Democritus, 400 B.C. coined the term atom If you divide matter into smaller and smaller pieces,
Nuclear Chemistry Atomic Structure Notes Start on Slide 20 from the second class lecture The Birth of an Idea Democritus, 400 B.C. coined the term atom If you divide matter into smaller and smaller pieces,
Chapter 4: Atomic Structure Section 4.1 Defining the Atom
 Chapter 4: Atomic Structure Section 4.1 Defining the Atom Early Models of the Atom atom the smallest particle of an element that retains its identity in a chemical reaction Democritus s Atomic Philosophy
Chapter 4: Atomic Structure Section 4.1 Defining the Atom Early Models of the Atom atom the smallest particle of an element that retains its identity in a chemical reaction Democritus s Atomic Philosophy
What is a theory? An organized system of accepted knowledge that applies in a variety of circumstances to explain a specific set of phenomena
 Atomic Structure What is a theory? An organized system of accepted knowledge that applies in a variety of circumstances to explain a specific set of phenomena Early Theories Democritus: 4 B.C.: atom He
Atomic Structure What is a theory? An organized system of accepted knowledge that applies in a variety of circumstances to explain a specific set of phenomena Early Theories Democritus: 4 B.C.: atom He
Chapter Two: Early History of Chemistry. Three Important Laws. Dalton s Atomic Theory (1808) Three Important Laws (continued) Greek Explanation
 Greek Explanation Chapter Two: ATOMS, MOLECULES, AND IONS Notes 2.1 In the Greek model they theorized there were four elements earth, water, air, and fire. These elements were characterized by the following
Greek Explanation Chapter Two: ATOMS, MOLECULES, AND IONS Notes 2.1 In the Greek model they theorized there were four elements earth, water, air, and fire. These elements were characterized by the following
The Atom. protons, neutrons, and electrons oh my!
 The Atom protons, neutrons, and electrons oh my! What s an Atom? An atom is the smallest physical particle of an element that still retains the properties of that element. How Big is an Atom? At sea level,
The Atom protons, neutrons, and electrons oh my! What s an Atom? An atom is the smallest physical particle of an element that still retains the properties of that element. How Big is an Atom? At sea level,
AP Atomic Structure Models
 AP Atomic Structure Models What is a Model? On a scrap piece of paper, write down your definition of a model with at least two examples. A model is a representation of an object, idea, action, or concept.
AP Atomic Structure Models What is a Model? On a scrap piece of paper, write down your definition of a model with at least two examples. A model is a representation of an object, idea, action, or concept.
DescribeDemocritus s Democritus s ideas
 Atomic Structure Section 4.1 Defining the Atom DescribeDemocritus s Democritus s ideas about atoms. Section 4.1 Defining the Atom Explain Dalton s atomic theory. Section 4.1 Defining the Atom Identifywhat
Atomic Structure Section 4.1 Defining the Atom DescribeDemocritus s Democritus s ideas about atoms. Section 4.1 Defining the Atom Explain Dalton s atomic theory. Section 4.1 Defining the Atom Identifywhat
4-1 Notes. Defining the Atom
 4-1 Notes Defining the Atom Early Models of the Atom All matter is composed of atoms Atoms are the smallest particles of an element that retains their identity in a chemical reaction Greek philosopher
4-1 Notes Defining the Atom Early Models of the Atom All matter is composed of atoms Atoms are the smallest particles of an element that retains their identity in a chemical reaction Greek philosopher
Democritus of Abdera. John Dalton. Dalton s Atom. Dalton s Atomic Theory Ancient Greece - 4th century BC. Eaglesfield, England
 Democritus of Abdera Ancient Greece - 4th century BC first suggested the existence of tiny fundamental particles that make up matter. atoms = indestructible did not agree with the current sci theory -
Democritus of Abdera Ancient Greece - 4th century BC first suggested the existence of tiny fundamental particles that make up matter. atoms = indestructible did not agree with the current sci theory -
Atoms and their structure
 Atoms and their structure History of atomic theory Not the history of atom, but the idea of the atom Original idea Ancient Greece (400 B.C..) Democritus and Leucippus Greek philosophers Another Greek Aristotle
Atoms and their structure History of atomic theory Not the history of atom, but the idea of the atom Original idea Ancient Greece (400 B.C..) Democritus and Leucippus Greek philosophers Another Greek Aristotle
Atomic Structure. ppst.com
 Atomic Structure ppst.com Defining the Atom The Greek philosopher (460 B.C. 370 B.C.) was among the first to suggest the existence of atoms (from the Greek word ) He believed that atoms were and His ideas
Atomic Structure ppst.com Defining the Atom The Greek philosopher (460 B.C. 370 B.C.) was among the first to suggest the existence of atoms (from the Greek word ) He believed that atoms were and His ideas
Chapter 4. Atomic Structure
 Chapter 4 Atomic Structure Warm Up We have not yet discussed this material, but what do you know already?? What is an atom? What are electron, neutrons, and protons? Draw a picture of an atom from what
Chapter 4 Atomic Structure Warm Up We have not yet discussed this material, but what do you know already?? What is an atom? What are electron, neutrons, and protons? Draw a picture of an atom from what
Scientist wanted to understand how the atom looked. It was known that matter was neutral. It was known that matter had mass
 Atom Models Scientist wanted to understand how the atom looked It was known that matter was neutral It was known that matter had mass They used these ideas to come up with their models, however science
Atom Models Scientist wanted to understand how the atom looked It was known that matter was neutral It was known that matter had mass They used these ideas to come up with their models, however science
Atomic Structure. For thousands of years, people had many ideas about matter Ancient Greeks believed that everything was made up of the four elements
 An atom is the smallest particle of an element that retains its identity in a chemical reaction. Although early philosophers and scientists could not observe individual atoms, they were still able to propose
An atom is the smallest particle of an element that retains its identity in a chemical reaction. Although early philosophers and scientists could not observe individual atoms, they were still able to propose
Introduction and Fundamental Concepts
 Introduction and Fundamental Concepts Chemistry 35 Fall 2000 Introductory Stuff Chemistry is an experimental science How is science done? - Observation - Hypothesis - EXPERIMENT - Observation - Revise
Introduction and Fundamental Concepts Chemistry 35 Fall 2000 Introductory Stuff Chemistry is an experimental science How is science done? - Observation - Hypothesis - EXPERIMENT - Observation - Revise
Honors Chemistry Unit 2: The Atom & Its Nucleus
 Honors Chemistry Unit 2: The Atom & Its Nucleus (2017-2018) Bunsen, I must tell you how excellent your study of chemical spectroscopy is, as is your pioneer work in photochemistry but what really impresses
Honors Chemistry Unit 2: The Atom & Its Nucleus (2017-2018) Bunsen, I must tell you how excellent your study of chemical spectroscopy is, as is your pioneer work in photochemistry but what really impresses
Chapter 3 https://youtu.be/thndxfdkzzs?list=pl8dpuualjx tphzzyuwy6fyeax9mqq8ogr
 Chapter 3 https://youtu.be/thndxfdkzzs?list=pl8dpuualjx tphzzyuwy6fyeax9mqq8ogr The smallest particle of an element that retains the chemical properties of that element. Regions: Nucleus: very small region
Chapter 3 https://youtu.be/thndxfdkzzs?list=pl8dpuualjx tphzzyuwy6fyeax9mqq8ogr The smallest particle of an element that retains the chemical properties of that element. Regions: Nucleus: very small region
CHAPTER 4 Atomic Structure
 CHAPTER 4 Atomic Structure 4.1 Early Theories of Matter Earth, Water, Air, Fire Matter was thought to be infinitely divisible No method was available to test theories Democritus (460 B.C. 370 B.C.) First
CHAPTER 4 Atomic Structure 4.1 Early Theories of Matter Earth, Water, Air, Fire Matter was thought to be infinitely divisible No method was available to test theories Democritus (460 B.C. 370 B.C.) First
A = number of protons + number of neutrons Z = number of protons
 Worksheet 3 Fundamentals Objectives To understand and be able to apply the fundamental laws. The Law of Conservation of Mass Mass can be neither created nor destroyed in a chemical reaction. The Law of
Worksheet 3 Fundamentals Objectives To understand and be able to apply the fundamental laws. The Law of Conservation of Mass Mass can be neither created nor destroyed in a chemical reaction. The Law of
tomic tructure Chapter 3
 tomic tructure Chapter 3 Early Theories of Matter 460 BC Democritus Proposed the matter was not infinitely divisible. Believed matter composed of particles called atoms. Early Theories of Matter Aristotle
tomic tructure Chapter 3 Early Theories of Matter 460 BC Democritus Proposed the matter was not infinitely divisible. Believed matter composed of particles called atoms. Early Theories of Matter Aristotle
Chemistry Chapter 3. Atoms: The Building Blocks of Matter
 Chemistry Chapter 3 Atoms: The Building Blocks of Matter I. From Philosophical Idea to Scientific Theory History of the Atom The Ancient Greeks were the first to come up with the idea of the atom. Democritus
Chemistry Chapter 3 Atoms: The Building Blocks of Matter I. From Philosophical Idea to Scientific Theory History of the Atom The Ancient Greeks were the first to come up with the idea of the atom. Democritus
Honor Chemistry: Quarter 1 Interim Exam Review Sheet
 Teacher: Dr. P. Powell 2014-2015 Honor Chemistry: Quarter 1 Interim Exam Review Sheet The interim exam counts as 10% of your quarter grade and will consist of problems and multiple-choice questions. You
Teacher: Dr. P. Powell 2014-2015 Honor Chemistry: Quarter 1 Interim Exam Review Sheet The interim exam counts as 10% of your quarter grade and will consist of problems and multiple-choice questions. You
Name Chemistry-PAP Per. Notes: Atomic Structure
 Name Chemistry-PAP Per. I. Historical Development of the Atomic Model Ancient Greek Model Notes: Atomic Structure Democritus (460-370 BC) was an ancient Greek philosopher credited with the first particle
Name Chemistry-PAP Per. I. Historical Development of the Atomic Model Ancient Greek Model Notes: Atomic Structure Democritus (460-370 BC) was an ancient Greek philosopher credited with the first particle
Smoking at an early age may make it more difficult to quit smoking later. Which of the above statements is an opinion and which is a theory?
 Preview Lesson Starter Objectives Foundations of Atomic Theory Law of Conservation of Mass Law of Multiple Proportions Dalton s Atomic Theory Modern Atomic Theory Section 1 The Atom: From Philosophical
Preview Lesson Starter Objectives Foundations of Atomic Theory Law of Conservation of Mass Law of Multiple Proportions Dalton s Atomic Theory Modern Atomic Theory Section 1 The Atom: From Philosophical
Unit 1 review. Chapter 1, chapter , 2.4
 Unit 1 review Chapter 1, chapter 2.1-2.2, 2.4 The Organization of Matter Matter Mixtures: a) Homogeneous (Solutions) b) Heterogeneous Pure Substances Elements Compounds Atoms Nucleus Protons Quarks Electrons
Unit 1 review Chapter 1, chapter 2.1-2.2, 2.4 The Organization of Matter Matter Mixtures: a) Homogeneous (Solutions) b) Heterogeneous Pure Substances Elements Compounds Atoms Nucleus Protons Quarks Electrons
Unit 2 Atomic Structure and Nuclear Chemistry
 Chemistry 1 West Linn High School Unit 2 Packet and Goals Name: Period: Unit 2 Atomic Structure and Nuclear Chemistry Unit Goals: As you work through this unit, you should be able to: 1. describe Dalton
Chemistry 1 West Linn High School Unit 2 Packet and Goals Name: Period: Unit 2 Atomic Structure and Nuclear Chemistry Unit Goals: As you work through this unit, you should be able to: 1. describe Dalton
CHEM 101 Mock Exam 1 Spring For the following questions, please select the most correct answer.
 For the following questions, please select the most correct answer. 1.) Matter is a. Something important. b. Something that has mass and occupies space. c. Something that has weight and occupies space.
For the following questions, please select the most correct answer. 1.) Matter is a. Something important. b. Something that has mass and occupies space. c. Something that has weight and occupies space.
CHEMISTRY 11 UNIT REVIEW: ATOMIC THEORY & PERIODIC TRENDS
 CHEMISTRY 11 UNIT REVIEW: ATOMIC THEORY & PERIODIC TRENDS Atoms Atoms have protons and neutrons located in the nucleus of the atom. Electrons orbit around the nucleus in well-defined paths. Protons have
CHEMISTRY 11 UNIT REVIEW: ATOMIC THEORY & PERIODIC TRENDS Atoms Atoms have protons and neutrons located in the nucleus of the atom. Electrons orbit around the nucleus in well-defined paths. Protons have
An atom is the smallest physical particle of an element that still retains the properties of that element.
 Unit 3.1 An atom is the smallest physical particle of an element that still retains the properties of that element. At sea level, one cubic centimeter of air (size of a sugar cube, or marble) will have
Unit 3.1 An atom is the smallest physical particle of an element that still retains the properties of that element. At sea level, one cubic centimeter of air (size of a sugar cube, or marble) will have
Name Period Date Engage-Atoms 1. What does Bill cut in half?
 Name Period Date Engage-Atoms https://www.youtube.com/watch?v=fmuskig2exi 1. What does Bill cut in half? 2. By cutting this item in half he tries to prove that there are pieces that are uncut- table called
Name Period Date Engage-Atoms https://www.youtube.com/watch?v=fmuskig2exi 1. What does Bill cut in half? 2. By cutting this item in half he tries to prove that there are pieces that are uncut- table called
1. What is the difference between a qualitative and quantitative observation? Give at least one example of each.
 1 st 9wks Exam Review Name Per. Chemistry 1H Scientific Skills Unit - Lab Safety & Equipment: KEY EQUIPMENT TO KNOW: +beaker +Erlenmeyer flask +beaker tongs +balance +graduated cylinder +test tube +test
1 st 9wks Exam Review Name Per. Chemistry 1H Scientific Skills Unit - Lab Safety & Equipment: KEY EQUIPMENT TO KNOW: +beaker +Erlenmeyer flask +beaker tongs +balance +graduated cylinder +test tube +test
Chapter 4. History of the atom. History of Atom Smallest possible piece? Atomos - not to be cut. Atoms and their structure
 Chapter 4 Atoms and their structure History of the atom Not the history of atom, but the idea of the atom. Original idea Ancient Greece (400 B.C.) Democritus and Leucippus Greek philosophers. Looked at
Chapter 4 Atoms and their structure History of the atom Not the history of atom, but the idea of the atom. Original idea Ancient Greece (400 B.C.) Democritus and Leucippus Greek philosophers. Looked at
Get out your diagram from your research paper. Get out a sheet of paper to take some notes on.
 Bellwork: Get out your diagram from your research paper. Get out a sheet of paper to take some notes on. Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons
Bellwork: Get out your diagram from your research paper. Get out a sheet of paper to take some notes on. Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons
Name Date Class ATOMIC STRUCTURE
 4 ATOMIC STRUCTURE SECTION 4.1 DEFINING THE ATOM (pages 101 103) This section describes early atomic theories of matter and provides ways to understand the tiny size of individual atoms. Early Models of
4 ATOMIC STRUCTURE SECTION 4.1 DEFINING THE ATOM (pages 101 103) This section describes early atomic theories of matter and provides ways to understand the tiny size of individual atoms. Early Models of
Chemistry of Our Environment Notes. Chapter 1
 Chemistry of Our Environment Notes Introduction: Purpose of Course: 1. Meet Lib Ed Requirement 2. Fire Science & Dental Hygiene How we will meet both needs. Exams & Grading Homework Assignments Chapter
Chemistry of Our Environment Notes Introduction: Purpose of Course: 1. Meet Lib Ed Requirement 2. Fire Science & Dental Hygiene How we will meet both needs. Exams & Grading Homework Assignments Chapter
The History of the Atom. How did we learn about the atom?
 The History of the Atom How did we learn about the atom? The Atomic Theory of Matter All matter is made up of fundamental particles. What does fundamental mean? The Greek Philosophers, 400 B.C. Democritus
The History of the Atom How did we learn about the atom? The Atomic Theory of Matter All matter is made up of fundamental particles. What does fundamental mean? The Greek Philosophers, 400 B.C. Democritus
CHEMISTRY. Matter and Change. Table Of Contents. Section 4.1 Early Ideas About Matter. Unstable Nuclei and Radioactive Decay
 CHEMISTRY 4 Table Of Contents Matter and Change Section 4.1 Early Ideas About Matter Chapter 4: The Structure of the Atom Section 4.2 Section 4.3 Section 4.4 Defining the Atom How Atoms Differ Unstable
CHEMISTRY 4 Table Of Contents Matter and Change Section 4.1 Early Ideas About Matter Chapter 4: The Structure of the Atom Section 4.2 Section 4.3 Section 4.4 Defining the Atom How Atoms Differ Unstable
Chapter 4 The Atom. Philosophers and scientists have proposed many ideas on the structure of atoms.
 Chapter4 TheAtom 4.1 Early Models of the Atom An atom is the smallest particle of an element that retains its identity in a chemical reaction. Philosophers and scientists have proposed many ideas on the
Chapter4 TheAtom 4.1 Early Models of the Atom An atom is the smallest particle of an element that retains its identity in a chemical reaction. Philosophers and scientists have proposed many ideas on the
Early Atomic Models. Atoms: the smallest particle of an element that retains the properties of that element.
 Chapter 5 Early Atomic Models Atoms: the smallest particle of an element that retains the properties of that element. (Greek: atomos = indivisible) Democritus (Greek teacher in the 4 th century BC) First
Chapter 5 Early Atomic Models Atoms: the smallest particle of an element that retains the properties of that element. (Greek: atomos = indivisible) Democritus (Greek teacher in the 4 th century BC) First
1.5 Measurement Uncertainty, Accuracy, and Precision 1.6 Mathematical Treatment of Measurement Results
 1.5 Measurement Uncertainty, Accuracy, and Precision 1.6 Mathematical Treatment of Measurement Results Let s recap what we saw in lab: o A measurement is a quantitative observation; a measurement is never
1.5 Measurement Uncertainty, Accuracy, and Precision 1.6 Mathematical Treatment of Measurement Results Let s recap what we saw in lab: o A measurement is a quantitative observation; a measurement is never
Chemistry Basic Science Concepts. Observations: are recorded using the senses. Examples: the paper is white; the air is cold; the drink is sweet.
 Note Packet # 1 1 Chemistry: the study of matter. Chemistry Basic Science Concepts Matter: anything that has mass and occupies space. Observations: are recorded using the senses. Examples: the paper is
Note Packet # 1 1 Chemistry: the study of matter. Chemistry Basic Science Concepts Matter: anything that has mass and occupies space. Observations: are recorded using the senses. Examples: the paper is
THE ATOM Pearson Education, Inc.
 THE ATOM Title and Highlight Right Side NOTES ONLY TN Ch 4.1-4.2 Topic: EQ: Date Reflect Question: Reflect on the material by asking a question (its not suppose to be answered from notes) NOTES: Write
THE ATOM Title and Highlight Right Side NOTES ONLY TN Ch 4.1-4.2 Topic: EQ: Date Reflect Question: Reflect on the material by asking a question (its not suppose to be answered from notes) NOTES: Write
CH4 HOMEWORK : ATOMIC STRUCTURE
 Name Date Class 4 CH4 HOMEWORK : ATOMIC STRUCTURE SECTION 4.1 DEFINING THE ATOM (pages 101 103) This section describes early atomic theories of matter and provides ways to understand the tiny size of individual
Name Date Class 4 CH4 HOMEWORK : ATOMIC STRUCTURE SECTION 4.1 DEFINING THE ATOM (pages 101 103) This section describes early atomic theories of matter and provides ways to understand the tiny size of individual
CHEM 1211K Test I. 2) Which one of the following is a pure substance? A) salt water B) concrete C) milk D) wood E) elemental copper
 CHEM 1211K Test I MULTIPLE CHOICE. (3 points each) 1) Consider a mixture consisting of sand in salt water. This mixture could be separated into its three components (sand, salt, and water) by first the
CHEM 1211K Test I MULTIPLE CHOICE. (3 points each) 1) Consider a mixture consisting of sand in salt water. This mixture could be separated into its three components (sand, salt, and water) by first the
Unit 3 Atomic Structure Chapter 3 of your book.
 Unit 3 Atomic Structure Chapter 3 of your book. Early Booklet E.C.: / 2 Unit 3 Hwk. Pts: / 24 Unit 3 Lab Pts: / 16 Late, Incomplete, No Work, No Units Fees? Y / N Learning Targets for Unit 3 1.1 I can
Unit 3 Atomic Structure Chapter 3 of your book. Early Booklet E.C.: / 2 Unit 3 Hwk. Pts: / 24 Unit 3 Lab Pts: / 16 Late, Incomplete, No Work, No Units Fees? Y / N Learning Targets for Unit 3 1.1 I can
Vocabulary QUIZ: 1. The total number of particles in the nucleus 2. 1 / 12
 Sep 29 11:29 AM Vocabulary QUIZ: 1. The total number of particles in the nucleus 2. 1 / 12 th of the mass of a carbon atom 3. The weighted average mass of all the isotopes of a particular element 4. A
Sep 29 11:29 AM Vocabulary QUIZ: 1. The total number of particles in the nucleus 2. 1 / 12 th of the mass of a carbon atom 3. The weighted average mass of all the isotopes of a particular element 4. A
ATOMS AND ELEMENTS. Democritus 400 B.C. Atomic Theory of Matter. Dalton s Postulates (1803) Page 1
 ATOMS AND ELEMENTS Democritus 400 BC Believed that matter was composed of invisible particles of matter he called atoms According to Democritus, atoms could not be broken into smaller particles Atomic
ATOMS AND ELEMENTS Democritus 400 BC Believed that matter was composed of invisible particles of matter he called atoms According to Democritus, atoms could not be broken into smaller particles Atomic
Unit 1 Introduction to Chemistry
 Chemistry 1 West Linn High School Unit 1 Packet and Goals Name: Period: Unit 1 Introduction to Chemistry Unit Goals: As you work through this unit, you should be able to: 1. Identify characteristics of
Chemistry 1 West Linn High School Unit 1 Packet and Goals Name: Period: Unit 1 Introduction to Chemistry Unit Goals: As you work through this unit, you should be able to: 1. Identify characteristics of
Origins of the Atom. Atoms: The Building Blocks of Matter. Let s Get Ready to Rumble. Aristotle s Theory of the Atom CHAPTER 3
 Origins of the Atom CHAPTER 3 Atoms: The Building Blocks of Matter Let s Get Ready to Rumble The idea of the atom was met with great skepticism, especially among great thinkers. The most vocal critic of
Origins of the Atom CHAPTER 3 Atoms: The Building Blocks of Matter Let s Get Ready to Rumble The idea of the atom was met with great skepticism, especially among great thinkers. The most vocal critic of
Chapter 3. Table of Contents. Section 1 The Atom: From Philosophical Idea to Scientific Theory. Section 2 The Structure of the Atom
 Atoms: The Building Blocks of Matter Table of Contents Section 1 The Atom: From Philosophical Idea to Scientific Theory Section 2 The Structure of the Atom Section 1 The Atom: From Philosophical Idea to
Atoms: The Building Blocks of Matter Table of Contents Section 1 The Atom: From Philosophical Idea to Scientific Theory Section 2 The Structure of the Atom Section 1 The Atom: From Philosophical Idea to
Ch. 4 Notes THE STRUCTURE OF THE ATOM NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics.
 Ch. 4 Notes THE STRUCTURE OF THE ATOM NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Early Ideas About Matter A. atom the smallest particle of an element retaining
Ch. 4 Notes THE STRUCTURE OF THE ATOM NOTE: Vocabulary terms are in boldface and underlined. Supporting details are in italics. I. Early Ideas About Matter A. atom the smallest particle of an element retaining
Atomic Theory. Past and Present: pieces of a puzzle
 Atomic Theory Past and Present: pieces of a puzzle The First Atomic Hypothesis Democritus (460 370 BC): Greek philosopher Speculated that matter is composed of atoms which move through empty space Atoms
Atomic Theory Past and Present: pieces of a puzzle The First Atomic Hypothesis Democritus (460 370 BC): Greek philosopher Speculated that matter is composed of atoms which move through empty space Atoms
Notes:&&Unit&4:&Atomics& & & & & & & & & & & & & & & & &
 Name: RegentsChemistry:Mr.Palermo Notes:Unit4:Atomics! www.mrpalermo.com Name: $ Key$Ideas$ Themodernmodeloftheatomhasevolvedoveralongperiodoftimethroughtheworkofmany scientists.(3.1a) Eachatomhasanucleus,withanoverallpositivecharge,surroundedbyoneormorenegatively
Name: RegentsChemistry:Mr.Palermo Notes:Unit4:Atomics! www.mrpalermo.com Name: $ Key$Ideas$ Themodernmodeloftheatomhasevolvedoveralongperiodoftimethroughtheworkofmany scientists.(3.1a) Eachatomhasanucleus,withanoverallpositivecharge,surroundedbyoneormorenegatively
Chap 4 Bell -Ringers
 Chap 4 Bell -Ringers The Structure of the Atom The Atom has a Structure What we ve seen so far Chapter 1 The Science of Chemistry - Chemistry is about discovering and understanding natural laws using the
Chap 4 Bell -Ringers The Structure of the Atom The Atom has a Structure What we ve seen so far Chapter 1 The Science of Chemistry - Chemistry is about discovering and understanding natural laws using the
Ms. Agostine s College Prep Chemistry Midterm Exam Review Packet for
 Ms. Agostine s College Prep Chemistry Midterm Exam Review Packet for 2014-2015 MIDTERM EXAM SCHEDULE for 2014-2015: Review Day: FRIDAY, JANUARY 16, 2015 Tuesday, January 20, 2015: Midterm Exams Wednesday,
Ms. Agostine s College Prep Chemistry Midterm Exam Review Packet for 2014-2015 MIDTERM EXAM SCHEDULE for 2014-2015: Review Day: FRIDAY, JANUARY 16, 2015 Tuesday, January 20, 2015: Midterm Exams Wednesday,
3.01 Understanding Atoms
 3.01 Understanding Atoms The Events Leading to the Discovery of the Building Block of Matter Dr. Fred Omega Garces Chemistry 111 Miramar College 1 3.02 Atomic Evolution Environmental Problems in our Lifetime
3.01 Understanding Atoms The Events Leading to the Discovery of the Building Block of Matter Dr. Fred Omega Garces Chemistry 111 Miramar College 1 3.02 Atomic Evolution Environmental Problems in our Lifetime
Chapter 3. Chapter 3. Objectives. Table of Contents. Chapter 3. Chapter 3. Foundations of Atomic Theory, continued. Foundations of Atomic Theory
 Atoms: The Building Blocks of Matter Table of Contents Philosophical Idea to Scientific Theory Objectives Explain the law of conservation of mass, the law of definite proportions, and the law of multiple
Atoms: The Building Blocks of Matter Table of Contents Philosophical Idea to Scientific Theory Objectives Explain the law of conservation of mass, the law of definite proportions, and the law of multiple
Democritus & Leucippus (~400 BC) Greek philosophers: first to propose that matter is made up of particles called atomos, the Greek word for atoms
 AP Chemistry Ms. Ye Name Date Block The Evolution of the Atomic Model Since atoms are too small to see even with a very powerful microscope, scientists rely upon indirect evidence and models to help them
AP Chemistry Ms. Ye Name Date Block The Evolution of the Atomic Model Since atoms are too small to see even with a very powerful microscope, scientists rely upon indirect evidence and models to help them
Atomic Structure. History of Atomic Theory
 Atomic Structure History of Atomic Theory Democritus (460-370 BC) Was the to come up with the idea of atom Believed that all matter was composed of Which is derived from the Greek word Atomos meaning He
Atomic Structure History of Atomic Theory Democritus (460-370 BC) Was the to come up with the idea of atom Believed that all matter was composed of Which is derived from the Greek word Atomos meaning He
Chapter 3. Atoms: Building Blocks of Matter
 Chapter 3 Atoms: Building Blocks of Matter Atom: means, from Democritus (Greek, 400BC) Atom: smallest particle of an element that retains the of that element Chemical Reaction: transformation of substances
Chapter 3 Atoms: Building Blocks of Matter Atom: means, from Democritus (Greek, 400BC) Atom: smallest particle of an element that retains the of that element Chemical Reaction: transformation of substances
Matter and Energy. Chapter 3
 Matter and Energy Chapter 3 Matter Anything that has mass and takes up space Two categories Pure substances Mixtures Pure Substances Matter with a fixed composition Either an element or compound Element
Matter and Energy Chapter 3 Matter Anything that has mass and takes up space Two categories Pure substances Mixtures Pure Substances Matter with a fixed composition Either an element or compound Element
Honors Chemistry Chapter 2 Problem Handout Solve the following on separate sheets of paper. Where appropriate, show all work. 1. Convert each of the
 Honors Chemistry Chapter 2 Problem Handout Solve the following on separate sheets of paper. Where appropriate, show all work. 1. Convert each of the following quantities to the required unit. a. 12.75
Honors Chemistry Chapter 2 Problem Handout Solve the following on separate sheets of paper. Where appropriate, show all work. 1. Convert each of the following quantities to the required unit. a. 12.75
) The nucleus of an atom, when compared to the entire atom, is (Circle two).
 Unit 3: The Atom Review Packet Directions: Answer the following questions WITHOUT using your notes first. This will be a great way to study for your test. Then, get out your notes and go back and fill
Unit 3: The Atom Review Packet Directions: Answer the following questions WITHOUT using your notes first. This will be a great way to study for your test. Then, get out your notes and go back and fill
AP Chemistry Summer Homework 2018
 AP Chemistry Summer Homework 2018 Welcome to AP Chem!!!! In early May next year, you will sit for the AP exam in chemistry. This exam covers the content of an introductory college-level chemistry course.
AP Chemistry Summer Homework 2018 Welcome to AP Chem!!!! In early May next year, you will sit for the AP exam in chemistry. This exam covers the content of an introductory college-level chemistry course.
Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons Electrons Neutrons Atomic # Mass # 24 Na
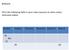 Bellwork: Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons Electrons Neutrons Atomic # Mass # 24 Na + 11 29 63 Bellwork: Fill in the Following Table in
Bellwork: Fill in the Following Table in your notes (assume an atom unless otherwise stated: Symbol Protons Electrons Neutrons Atomic # Mass # 24 Na + 11 29 63 Bellwork: Fill in the Following Table in
Dalton Thompson Rutherford Bohr Modern Model ("Wave. Models of the Atom
 Dalton Thompson Rutherford Bohr Modern Model ("Wave Models of the Atom Mechanical" Model) Aim: To discuss the scientists and their contributions to the current atomic model. Focus: Rutherford's Gold Foil
Dalton Thompson Rutherford Bohr Modern Model ("Wave Models of the Atom Mechanical" Model) Aim: To discuss the scientists and their contributions to the current atomic model. Focus: Rutherford's Gold Foil
How to Use This Presentation
 How to Use This Presentation To View the presentation as a slideshow with effects select View on the menu bar and click on Slide Show. To advance through the presentation, click the right-arrow key or
How to Use This Presentation To View the presentation as a slideshow with effects select View on the menu bar and click on Slide Show. To advance through the presentation, click the right-arrow key or
SNC1D1 History of the Atom
 SNC1D1 History of the Atom What is the atom? Atoms are the building block for all matter: Atoms make up elements! Elements combine to make compounds!2 ATOMIC MODEL TIMELINE 400 B.C PRESENT DAY ATOMIC MODEL
SNC1D1 History of the Atom What is the atom? Atoms are the building block for all matter: Atoms make up elements! Elements combine to make compounds!2 ATOMIC MODEL TIMELINE 400 B.C PRESENT DAY ATOMIC MODEL
DESCRIBING MATTER. Matter is anything that has mass and volume
 DESCRIBING MATTER Matter is anything that has mass and volume Mass the amount of matter in an object measured with a balance Units are grams, kilograms (SI), centigrams Weight the measurement of gravitational
DESCRIBING MATTER Matter is anything that has mass and volume Mass the amount of matter in an object measured with a balance Units are grams, kilograms (SI), centigrams Weight the measurement of gravitational
Understanding the Atom
 Name Date Period 3.1 Discovering Parts of an Atom Directions: On the line before each statement, write correct if the statement is correct or not correct if the statement is not correct. If the statement
Name Date Period 3.1 Discovering Parts of an Atom Directions: On the line before each statement, write correct if the statement is correct or not correct if the statement is not correct. If the statement
7.1 Development of a Modern Atomic Theory
 7.1 Development of a Modern Atomic Theory Development of the Atomic Theory Many scientists in different countries have contributed to the understanding of matter - atoms John Dalton Credited with developing
7.1 Development of a Modern Atomic Theory Development of the Atomic Theory Many scientists in different countries have contributed to the understanding of matter - atoms John Dalton Credited with developing
Mass number i. Example U (uranium 235) and U (uranium 238) atomic number e. Average atomic mass weighted of the isotopes of that element i.
 CP NT Ch. 4&25 I. Atomic Theory and Structure of the Atom a. Democritus all matter consists of very small, indivisible particles, which he named i. Atom smallest particle of an element that retains all
CP NT Ch. 4&25 I. Atomic Theory and Structure of the Atom a. Democritus all matter consists of very small, indivisible particles, which he named i. Atom smallest particle of an element that retains all
Atomic Structure. Chapters 4, 8, Bravo 15,000 kilotons
 Atomic Structure Chapters 4, 8, 18.1-18.3 Bravo 15,000 kilotons What is an atom? Smallest unit of an element that retains all the properties of the element Can combine with other atoms to form compound
Atomic Structure Chapters 4, 8, 18.1-18.3 Bravo 15,000 kilotons What is an atom? Smallest unit of an element that retains all the properties of the element Can combine with other atoms to form compound
Chapter 2 Atoms, Ions, and the Periodic Table. Law of Conservation of Mass. Law of Conservation of Mass
 Chapter 2 Atoms, Ions, and the Periodic Table Dalton s Atomic Theory Structure of the Atom Ions Atomic Mass The Periodic Table Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
Chapter 2 Atoms, Ions, and the Periodic Table Dalton s Atomic Theory Structure of the Atom Ions Atomic Mass The Periodic Table Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction
Notes: Unit 1: Math and Measurement
 Name: Regents Chemistry: Notes: Unit 1: Math and Measurement www.chempride.weebly.com Key Ideas Major Understandings: o Chemistry is the study of matter: Matter takes up space and has mass. (K- 4, 3.1a)
Name: Regents Chemistry: Notes: Unit 1: Math and Measurement www.chempride.weebly.com Key Ideas Major Understandings: o Chemistry is the study of matter: Matter takes up space and has mass. (K- 4, 3.1a)
Notes: Unit 1: Math and Measurement
 Name: Regents Chemistry: Notes: Unit 1: Math and Measurement www.chempride.weebly.com Key Ideas Major Understandings: o Chemistry is the study of matter: Matter takes up space and has mass. (K- 4, 3.1a)
Name: Regents Chemistry: Notes: Unit 1: Math and Measurement www.chempride.weebly.com Key Ideas Major Understandings: o Chemistry is the study of matter: Matter takes up space and has mass. (K- 4, 3.1a)
CHEM134, Fall 2018 Dr. Al-Qaisi Chapter 1 review
 Upon completion of this chapter, you should be able to: Ø Know the Scientific approach to knowledge Ø Define Mater, atom and molecule ü Explain and give examples of the following: element, mixture, mixture
Upon completion of this chapter, you should be able to: Ø Know the Scientific approach to knowledge Ø Define Mater, atom and molecule ü Explain and give examples of the following: element, mixture, mixture
Chemistry. Robert Taggart
 Chemistry Robert Taggart Table of Contents To the Student..................................................v Unit 1: Matter and Measurement Lesson 1: Chemistry and the Scientific Method...................3
Chemistry Robert Taggart Table of Contents To the Student..................................................v Unit 1: Matter and Measurement Lesson 1: Chemistry and the Scientific Method...................3
The Story of the Atom. A history of atomic theory over many years
 The Story of the Atom A history of atomic theory over many years Democritus Many years ago, between 460BC and 370BC the Greek philosophers wondered what we were made of. Leucippus and Democritus came up
The Story of the Atom A history of atomic theory over many years Democritus Many years ago, between 460BC and 370BC the Greek philosophers wondered what we were made of. Leucippus and Democritus came up
5 Early Atomic Theory and Structure
 5 Early Atomic Theory and Structure Chapter Outline 5.1 5.2 Electric Charge A. Discovery of Ions 5.3 Subatomic Parts of the Atom Lightning occurs when electrons move to neutralize charge difference between
5 Early Atomic Theory and Structure Chapter Outline 5.1 5.2 Electric Charge A. Discovery of Ions 5.3 Subatomic Parts of the Atom Lightning occurs when electrons move to neutralize charge difference between
BEGINNING OF ATOMIC THEORY
 Skills Worksheet Directed Reading B Section: Development of the Atomic Theory THE BEGINNING OF ATOMIC THEORY 1. What does the word atom mean? a. dividable b. invisible c. hard particles d. not able to
Skills Worksheet Directed Reading B Section: Development of the Atomic Theory THE BEGINNING OF ATOMIC THEORY 1. What does the word atom mean? a. dividable b. invisible c. hard particles d. not able to
Elements and Compounds. Composition of Matter
 Elements and Compounds Composition of Matter Elements Element Identification Source of Light Elements Solar Spectra Detecting Elements Molecular Spectrum of CO Chemical and Physical Properties Elements
Elements and Compounds Composition of Matter Elements Element Identification Source of Light Elements Solar Spectra Detecting Elements Molecular Spectrum of CO Chemical and Physical Properties Elements
Pre-Lab 0.2 Reading: Measurement
 Name Block Pre-Lab 0.2 Reading: Measurement section 1 Description and Measurement Before You Read Weight, height, and length are common measurements. List at least five things you can measure. What You
Name Block Pre-Lab 0.2 Reading: Measurement section 1 Description and Measurement Before You Read Weight, height, and length are common measurements. List at least five things you can measure. What You
