The linear, nonlinear optical properties and quantum chemical parameters of some sudan dyes
|
|
|
- Clinton Bates
- 5 years ago
- Views:
Transcription
1 The linear, nonlinear optical properties and quantum chemical parameters of some sudan dyes Aslı EŞME 1,*, Seda GÜNEŞDOĞDU SAĞDINÇ 2 1 Department of Elementary Science Education, Kocaeli University, Umuttepe campus Kocaeli, Turkey. 2 Department of Physics, Science and Art Faculty, Kocaeli University, Umuttepe campus Kocaeli, Turkey. Abstract In this study, the polarizability (<α>), the anisotropy of the polarizability (<Δα>), groundstate dipole moment (µ) and the first-order hyperpolarizability (β) of the Sudan III (SIII) [1-({4-[(phenyl)diazenyl] phenyl}diazenyl) naphthalen-2-ol], Sudan Red G (SRG) [1-(2- Methoxyphenylazo)-2-naphthol] and Sudan Orange G (SOG) [4-(Phenylazo)resorcinol] are studied at the Hartree-Fock (HF) and Density Functional theory (DFT/) levels of the theory with 3-21G, 6-31G, 6-31G(d), 6-31G(d,p), 6-31G+(d,p), 6-31G++(d,p), 6-311G, 6-311G(d), 6-311G(d,p), 6-311G++(d,p) basis sets. Also, E HOMO (the highest occupied molecular orbital energy), E LUMO (the lowest unoccupied molecular orbital energy), HOMO-LUMO energy gap (ΔE), electron affinity (A), ionization potential (I), global hardness (η), softness (σ), electronegativity (χ), chemical potential (Pi), global electrophilicity index (ω) are investigated. All quantum chemical parameters, in general, are dependent on the choice of the basis sets, and are clearly influenced after the addition of polarization and diffusion functions. Keywords : Sudan dyes, nonlinear optics, hyperpolarizability, polarizability, DFT, HF. Bazı sudan boyalarının lineer, lineer olmayan optik özellikleri ve kuantum kimyasal parametreleri Özet Bu çalışmada, Sudan III (SIII) [1-({4-[(fenil)diazenil] fenil}diazenil) naftalin-2-ol], Sudan Kırmızı G (SKG) [1-(2-Metoksifenilazo)-2-naftol] ve Sudan Turuncu G (STG) [4- (fenilazo)rezorsinol] moleküllerinin polarizabilite (<α>), anizotropi polarizabilite (<Δα>), * Aslı EŞME, asliesme@gmail.com, Tel: (262)
2 EŞME A., SAĞDINÇ S. G. taban-durum dipol moment (µ) ve birinci-derece hiperpolarizabilite (β) değerleri Hartree- Fock (HF) metodu ve Yoğunluk Fonksiyonel Teorisi (DFT/) metodu ile 3-21G, 6-31G, 6-31G(d), 6-31G(d,p), 6-31G+(d,p), 6-31G++(d,p), 6-311G, 6-311G(d), 6-311G(d,p), 6-311G++(d,p) temel setleri kullanılarak incelendi. Ayrıca, E HOMO (en yüksek dolu moleküler orbital enerji), E LUMO (en düşük boş moleküler orbital enerji), HOMO- LUMO enerji farkı (ΔE), elektron ilgisi (A), iyonizasyon potansiyeli (I), global sertlik (η), yumuşaklık (σ), elektronegatiflik (χ), kimyasal potansiyel (Pi), global elektrofilik indis (ω) değerleri araştırıldı. Tüm kuantum kimyasal parametreler, genelde, temel setlerin seçiminden bağımsızdır, ve açıkça polarizasyon ve difüz fonksiyonlarının eklenmesinden sonra etkilenmektedir. Anahtar kelimeler: Sudan boyaları, lineer olmayan optik, hiperpolarizabilite, polarizabilite, DFT, HF. 1. Introduction Colorants are generally added into food to enhance its visual aesthetics, and to promote sales [1]. Although the allowable amount of synthetic colorants is reduced for consumer health reasons in recent years, many kinds of synthetic food dyes are still widely used all over the world due to their low price, high effectiveness, and excellent stability [2]. Azocompounds are widely used as synthetic organic colorants. Generally, synthetic colorants can be classified as water-soluble or fat-soluble colorants based on their solubility. Most fat-soluble synthetic colorants present in the market are azo compounds, such as Sudan III (SIII) [3]. Belonging to the azo-dye class, sudan dyes are non-ionic fat-soluble dyes used in the gasoline, diesel, lubricating grease and polymer dye production, and as dye for food (chilli) and tattoos. SIII [1-({4-[(phenyl)diazenyl] phenyl}diazenyl) naphthalen-2-ol] is fatsoluble dye predominantly used for demonstrating the presence of triglycerides in frozen sections. In addition, SIII is commonly used for coloring waxes, oils and spirit varnishes [4]. Sudan Red G (SRG) [1-(2-Methoxyphenylazo)-2-naphthol] is a yellowish red lysochrome azo dye. It has the appearance of an odorless reddish-orange powder with melting point 225 C. It is soluble in fats and used for coloring of fats, oils, and waxes, including the waxes used in turpentine-based polishes [5]. Sudan Orange G (SOG) [4- (Phenylazo)resorcinol] is useful for staining triglycerides in animal tissues (frozen sections) [6]. About 50% of the total world colorant production belongs to the so-called azo dyes compounds [7]. The main feature of this dye family is the presence of the azo group (- N=N-) which gives the possibility of providing a more extended electronic conjugation of π electrons, and consequently allowing for a strong light absorption in the visible region of the electromagnetic spectrum. It is known that organic molecules formed by a donor acceptor pair connected to a π- delocalized framework present attractive non-linear optics (NLO) characteristics, which can be estimated from their hyperpolarizabilities [8-11]. Recently, there has been a growing interest in the nonlinear optical (NLO) properties of azo materials with donor-acceptor groups for their large nonlinear refraction [12], which are interesting for the application in 48
3 optical storage, optical-limiting and optical switching application [13, 14]. Their nonlinear optical response may result from electronic and/or nonelectronic process. Electronic nonlinearities occur as the result of the nonlinear response of bound electrons on an applied optical field. Furthermore, the usually good planarity of the azo bridge plays important role to larger π electron transmission effects [15-18]. The first hyperpolarizability, (β), gives information about the material capability to generate second order non-linear effects [11]. Also, the experimental and theoretical studies have been expanded to understand many aspects of molecular hyperpolarizabilities [15]. The use of quantum chemical methods as Hartree-Fock (HF) and density functional theory (DFT) for molecular hyperpolarizabilities is expected to supply a guidance and accelarate subsequent experimental studies [19,20]. Thus, in the present work, the molecular structures, E HOMO (the highest occupied molecular orbital energy), E LUMO (the lowest unoccupied molecular orbital energy), HOMO-LUMO energy gap (ΔE), dipole moments (μ), polarizabilities (<α>), the anisotropy of the polarizabilities (<Δα>) and first-order hyperpolarizabilities (β) are investigated using HF and methods with different basis sets on some sudan azo dyes, such as SIII, SOG and SRG (Fig. 1). Dyes Chemical structures Name of dyes SIII [1-({4-[(phenyl)diazenyl] phenyl}diazenyl) naphthalen-2-ol] SOG [4-(Phenylazo)resorcinol] SRG [1-(2-Methoxyphenylazo)- 2-naphthol] Fig 1. Chemical structures of azo-dyes investigated. Although the X-ray studies of SIII and SOG has not been reported till now, SIII has been calculated structural parameters by HF and DFT methods for investigation of the tautomerism in it [21]. Also, the nonlinear optical (NLO) parameters such as the polarizability (<α>), the anisotropy of the polarizability (<Δα>), ground-state dipole moment (µ) and the first-order hyperpolarizability (β) of SIII have been extensively studied 49
4 EŞME A., SAĞDINÇ S. G. density functional theory (DFT) calculation with 6-311G(d,p) basis set [22]. The experimental structure of SRG has been reported in the literature [23] and the molecular structural values of this molecule have been repoted in Cambridge Crystallographic Database (CSD code: JATJIX). In that study, SRG has analysed of the structure obtained for azo-form [23]. The other objective of this paper is to find effective quantum chemical methods (HF and DFT methods) that would offer a certainty of finding molecular parameters. The HF and DFT studies were initiated with the minimal basis set 3-21G and moved to higher basis sets for checking both (d), (p), (d,p) polarization function and +, ++ diffuse function effects. The basis sets incorporated in this study include 3-21G, 6-31G, 6-311G, 6-31G(d), 6-311G(d), 6-31G(d,p), 6-311G(d,p), 6-31G+(d,p), 6-31G++(d,p), 6-311G++(d,p) [24-30]. Also, the molecular hardness (η), global softness (σ) electronegativity (χ), chemical potential (Pi) and global electrophilicty index (ω) have been obtained from molecular frontier orbital energies using ab initio methods at different basis sets. 2. Computational Details All calculations were performed using the GAUSSIAN-09W [31] software package and GaussView, Rev [32] molecular visualization programs. The molecular geometries of SIII, SOG and SRG are restricted. The DFT calculations were performed using Becke s three-parameter hybrid functional [33] with the Lee-Yang-Parr correlation functional [34], a combination that gives rise to the well-known method. In addition, the HF method was also used to obtain the NLO properties and energies (E HOMO, E LUMO, E= E LUMO -E HOMO ) of SIII, SOG and SRG for comparison with results. The effects of basis sets on calculations are studied at HF and levels with 3-21G, 6-31G, 6-311G, 6-31G(d), 6-311G(d), 6-31G(d,p), 6-311G(d,p), 6-31G+(d,p), 6-31G++(d,p), 6-311G++(d,p) basis sets. In the context of the HF theorem, the E HOMO and E LUMO is used to approximate the ionization potential (I) and electron affinity (A) given by Koopmans theorem [35], respectively. Although no formal proof of this theorem exists within DFT, its validity is generally accepted. I and A are related to I EHOMO A E LUMO. (1) If we assume that these relations are valid within the DFT frame, the chemical potential (Pi) known as the negative of electronegativity (χ), and hardness (η) can be estimated with I A ; 2 I A Pi ; 2 I A. (2) 2 Recently, several researches [36-39] have introduced an global electrophilicity index (ω) defined as 2 ( Pi) / 2. (3) This was proposed as a measure of the electrophilic power of a molecule and global softness (σ) is given by [40] 1/ (4) 50
5 Polarizabilities were calculated at the same level of theory using the standard GAUSSIAN- 09W keyword Polar [41]. This keyword means that the polarizabilities were obtained analytically rather than by numerical differentiation. The energy of an uncharged molecule under a weak, general electric field can be expressed by Buckingham type expansion [42-44] E E0 i Fi (1/ 2) ij Fi F j (1/ 6) ijk Fi F j Fk... (5) where E is the energy of a molecule under the electric field F, E 0 is the unperturbed energy of a free molecule, F i is the vector component of the electric field in the i direction, and i, ij, ijk are the dipole moment, linear polarizability and first-order hyperpolarizability, respectively. Here, each subscript of i, j and k denotes the indices of the Cartesian axes x, y, z, and a repeated subscript means a summation over the Cartesian indices x, y, z. The ground state dipole moment μ, the polarizability <α>, the anisotropy of the polarizability <Δα> and the first-order hyperpolarizability β, using the x, y, z components they are defined as [45, 46] / 2 (6) x y z xx yy zz (7) xx yy yy zz zz xx xy xz yz (8) xxx xyy 2 xzz yyy yzz yxx zzz zxx zyy 1/ 2. (9) Since the values of the polarizabilities (α) and first-order hyperpolarizability (β) of GAUSSIAN-09W output [30] are reported in atomic units (a.u.), the calculated values have been converted into electrostatic units (esu) (α: 1 a.u. = x10-24 esu; β: 1 a.u.= x10-33 esu) [47]. 3. Results and Discussion In general, sudan dyes display two possible tautomeric forms, the azo (O-H) and hydrazo (N-H) forms, as shown in Fig. 2 for SRG [22, 23]. Depending on the tautomers, two types of intermolecular hydrogen bonds are observed in sudan dyes: O-H N in azo and N-H O in hydrazo tautomer. Several researchers have studied the azo/hydrazo form of sudan dyes [21, 23, 48]. The position and nature of the equilibrium depends on the solvent utilized. In this study, we have not used any solvents to determine the molecular parameters of sudan dyes. Therefore, we decided to study only the azo (OH) form of the sudan dyes because of their molecular parameters and non-linear optical properties. The molecular structures of SIII, SOG and SRG have been completely optimized at the HF and levels with 3-21G, 6-31G, 6-31G(d), 6-31G(d,p), 6-31G+(d,p), 6-31G++(d,p), 6-311G, 6-311G(d), 6-311G(d,p), 6-311G++(d,p) basis sets. The optimized structures of the studied molecules at the DFT/ level using the 6-31G(d,p) basis set are shown in Fig
6 EŞME A., SAĞDINÇ S. G. CH 3 O N H N O CH 3 O N H N O Fig 2. Tautomeric equilibrium for the SRG. Fig. 3. The optimized structures obtained using /6-31G(d,p) level of (a) SIII, (b) SOG and (c) SRG. The calculated N-N and C-N lengths of the three compounds are presented in Table 1, and are compared with the experimental values of SRG values from X-ray diffraction data [23]. Also, the experimental structure of SIII has not been reported until now, therefore we have compared the calculated bond lengths of SIII with its experimentally available parent compound trans-azobenzene from the X-ray study [49]. From the crystalline structure described for trans-azobenzene, the N 1 -N 2 bond length was Å. In the present study, the calculated N-N bond distances for SIII was found to be between and Å by HF and between and Å by level for N 1 -N 2 and 6-31G basis set for OH isomer structure of SIII. In that study, the N 1 -N 2 and N 3 -N 4 bonds were found to be 0.02 Å shorter than our calculated values with same basis set. Due to the lack of experimental data in SOG, we compared the experimental N-N bond length of SRG with the theoretical value obtained with several basis sets of SOG. From the theoretical values, it can be stated that N-N bond lengths of SRG and SOG are lower than the experimental value of SRG [23]. The discrepancy between the experimental and calculated bond lengths of SRG might result from the different forms of SRG (experimentally in the hydrazo form and theoretically in the azo form). Going from 3-21G to 6-31G decreases the N-N bond lengths of the studied molecules, and using the 6-311G basis set shows little change in this bond length. This suggests that increasing the size of the orbitals does not improve the description of this bond length. The same conclusion can be arrived at with respect to the addition of polarization (d, (d,p)) and diffuse (+, ++) functions. The calculated C-N bond lengths for sudan dyes compare with those corresponding to data reported in the literature [23, 49]. As shown in Table 1, the calculated values correspond well to those within the literature [21] (1.404 Å for HF/6-31G and Å for /6-31G) and for our study (between and Å for HF and between and Å 52
7 for ) values of C 2 -N 1 bonds for SIII, but there was a discrepancy in the experimental results regarding the structure of trans-azobenzene (1.428 Å) [49]. The deviations in the C 2 -N 1 and N 2 -C 11 bond lengths for SIII are less than and Å for HF/6-31G and and Å for /6-31G when compared to the trans-azobenzene, respectively. The differences of bond lengths between the experimental [23] and the calculated values for SRG are found in the C 2 -N 1 and N 2 -C 11 bonds, with the different values being and Å for HF/6-31G and and Å for /6-31G, respectively. For the optimized SOG structure, the difference of C 2 -N 1 and N 2 -C 11 bond lengths are found to be and Å for HF/6-31G and and Å for /6-31G, respectively, as compared to the observed value of and Å in the X-Ray data for SRG [23]. The use of several basis sets for sudan dyes has no effect on the value of these bond lengths. Analyses of the conformation of individual rings for azo dyes have been important [50], thus the dihedral angles around N-N moiety of studied molecules have been investigated. The values of N 1 -N 2 -C 11 -C 12 and N 2 -N 1 -C 2 -C 1 dihedral angles are given in Table 2. It can be seen that all the rings for sudan dyes were found planar (~180 ). Also, the experimental values of N 1 -N 2 -C 11 -C 12 and N 2 -N 1 -C 2 -C 1 for SRG [22] are 0.8º and 178º (X-ray) which are closer to the dihedral angles calculated using the HF and levels. The O-H N distances were 1.62, 2.07 and 1.61 Å (with /6-31G(d,p)) for SIII, SOG and SRG, respectively. These distances are significantly smaller than the summation of the Van der Waals radii (~2.6 Å), by just confirming the presence of a very strong hydrogen interaction in these compounds [48,50]. The highest occupied molecular orbital (HOMO), and the lowest unoccupied molecular orbital (LUMO) orbitals are called frontier molecular orbitals as they lie at the outermost boundaries of the electrons of the molecules. The HOMO and LUMO are the main orbitals responsible for chemical stability. The HOMO-LUMO orbital pictures of SIII, SOG and SRG molecules are given in Fig. 4. The HOMO of SIII and SOG molecules are delocalized over the N-N bond and the HOMO of SRG is delocalized over the C-C and N-N bonds. In contrast, the LUMO of SIII, SOG and SRG molecules are located in all of the molecules. 53
8 EŞME A., SAĞDINÇ S. G. Table 1. Selected bond lengths (in Å) calculated for SIII, SOG and SRG at HF and levels and literature values for comparison. SIII SOG SRG Basis Sets C 2 -N 1 N 1 -N 2 N 2 -C 11 C 14 -N 3 N 3 -N 4 N 4 -C 17 C 2 -N 1 N 1 -N 2 N 2 -C 7 C 2 -N 1 N 1 -N 2 N 2 -C G HF G HF G(d) HF G(d,p) HF G(d,p) HF G(d,p) HF G HF G(d) HF G(d,p) HF G(d,p) HF G a HF X-ray b X-ray c a Taken from Ref [23] b Taken from Ref [51] Taken from Ref [24, 25] 54
9 Table 2. Selected calculated and experimental dihedral angles ( o ) of SIII, SOG and SRG. Basis Sets SIII SOG SRG N 1 -N 2 -C 11 -C 12 N 2 -N 1 -C 2 -C 1 N 3 -N 4 -C 17 -C 18 N 4 -N 3 -C 14 -C 13 N 1 =N 2 =C 7 =C 8 N 2 -N 1 -C 2 -C 1 N 1 =N 2 =C 11 =C 12 N 2 =N 1 =C 2 =C G HF G HF G(d) HF G(d,p) HF G(d,p) HF G(d,p) HF G HF G(d) HF G(d,p) HF G(d,p) HF X-Ray (a) (a) Taken from Ref [23] 55
10 EŞME A., SAĞDINÇ S. G. The calculated values for the E HOMO and E LUMO and the frontier molecular orbital energy gap ( E) with several basis sets are given in Table 3. E HOMO is often associated with the electron-donating ability of a molecule, whereas E LUMO indicates its ability to accept electrons. The frontier orbital gap helps characterize the chemical reactivity and kinetic stability of the molecule. A molecule with a small frontier orbital gap is generally associated with a high chemical reactivity, low kinetic stability, and is also defined as a LUMO energy gaps with /6-31G for SIII, SOG and SRG decrease in the order soft molecule [40]. The E HOMO and E LUMO energies for SIII were calculated using the DFT calculation with the functional and the 6-31G basis set by Silva et al. [51]. The E HOMO and E LUMO energies for the SIII molecule obtained using /6-31G level were found to be and ev, respectively [52]. In this study, these energies for SIII, SOG and SRG have been calculated to be and ev for SIII, and ev for SOG, and ev for SRG (E HOMO ) and and ev for SIII, and ev for SOG, and ev for SRG (E LUMO ) by HF/6-31-G and /6-31G levels. According to these results obtained from the HF and DFT methods, the values of E HOMO and E LUMO show the decreasing trend of the properties: SOG < S3 < SRG and S3 < SRG < SOG, respectively. As can be seen from Table 3, the E HOMO and E LUMO values with 6-31G at HF and levels follow the same trend as other theoretical basis sets. SOG has more E LUMO than SRG for two basis sets results [6-31++G(d,p) and 6-311G]. Silva et al. [51] were calculated at the Fermi level (approximately ev) located at the center of the E HOMO and E LUMO levels of the SIII, so the ΔE value from this energy was found to be 2.97 ev. In this study, the HOMO (SOG) > 3.05 (SRG) > 2.74 (SIII) ev, which are consistent with the ability of the electrondonating of the group N 2 C 6 H 10 > CH 3 O > H. Concerning the value of the energy of the gap ΔE, larger values of the energy difference will also provide low reactivity to chemical types. As seen in Table 3, the addition of diffuse and polarization functions, and the calculated values of E were found to be almost the same. Fig. 5(a)-(c) show the variation of the calculated energy levels of E HOMO, E LUMO and E values at HF and methods using different basis sets. It can be seen in Fig. 5(a)-(c) that there is an overlap between the different methods and the basis sets of E HOMO, E LUMO and E values. In the most common case, ionization potential (I) and electron affinity (A) are related to E HOMO and E LUMO respectively. The low I creates a better electron donor, and the large A makes a better electron acceptor. Using all the methods, the I of SRG is the lowest [7.332 ev (HF/6-31G(d,p)) and ev (/3-21 G)]. The A is found to be the highest for SIII with ev (HF/6-311G) and for SOG with ev (/6-311G). The obtained values of I and A (Table 4) were considered for the calculation of global hardness (η), softness (σ), electronegativity (χ), chemical potential (Pi), and electrophilicity index (ω). These quantum chemical parameters were evaluated using Eqs. (2-4) and were listed as these values calculated with several basis sets in Table 5. 56
11 Fig. 4. 3D plots of HOMO and LUMO of studied molecules by /6-31G(d,p) with energies. 57
12 EŞME A., SAĞDINÇ S. G. Table 3. Calculated energy levels (in ev) of the HOMO, LUMO and ΔE. SIII SOG SRG Basis Sets E HOMO (ev) E LUMO (ev) ΔE (ev) E HOMO (ev) E LUMO (ev) ΔE (ev) E HOMO (ev) E LUMO (ev) ΔE (ev) 3-21G HF G HF G(d) HF G(d,p) HF G(d,p) HF G(d,p) HF G HF G(d) HF G(d,p) HF G(d,p) HF
13 Fig. 5. Variation of (a) E HOMO (ev) (b) E LUMO (ev) (c) HOMO-LUMO energy gap (ΔE, in ev ) (d) hardness (η, in ev) (e) softness (σ, in 1/eV) (f) global electrophilicity index (ω, in ev) of sudan dyes with HF and methods and different basis sets. 59
14 EŞME A., SAĞDINÇ S. G. Table 4: Electron affinities (A) and ionization potentials (I) values of SIII, SOG and SRG. SIII SOG SRG Basis Sets I=-E HOMO A=-E LUMO A=-E LUMO I=-E HOMO A=-E LUMO I=-E HOMO 3-21G HF G HF G(d) HF G(d,p) HF G(d,p) HF G(d,p) HF G HF G(d) HF G(d,p) HF G(d,p) HF
15 Table 5. Global hardness (η), softness (σ), electronegativity (χ), chemical potential (Pi) and electrophilicity index (ω) of SIII, SOG and SRG. SIII SOG SRG Basis Sets η (ev) σ (1/eV) χ (ev) Pi (ev) ω (ev) η (ev) σ (1/eV) χ (ev) Pi (ev) ω (ev) η (ev) σ (1/eV) χ (ev) Pi (ev) ω (ev) 3-21G HF G HF G(d) HF G(d,p) HF G(d,p) HF G(d,p) HF G HF G(d) HF G(d,p) HF G(d,p) HF
16 EŞME A., SAĞDINÇ S. G. The global hardness (η), softness (σ), electronegativity (χ), chemical potential (Pi) and electrophilicity index (ω) have been used by a number of workers [37, 53, 54] to assess a priori of the reactivity of chemical properties from their intrinsic electrical properties. Global hardness and softness are important properties to measure the molecular stability and reactivity. A hard molecule has a large energy gap, and a soft molecule has a small one. Soft molecules are more reactive than hard ones because they could easily offer electrons to an acceptor. For the simplest transfer of electrons, absoption could occur at the part of the molecule where softness, which is a localised, has the highest value. Evaluating the values of the hardness in Table 5 shows that SOG has the greatest. This means that SOG has the largest potential chemical resistance to change the number of electrons among the other molecules. It can be noted that the hardness of the molecules follows the order SOG > SRG > SIII using all the methods. Also, it can be seen in Table 5 that SIII is the compound that displays the greater reactivity in relationship to the others as a result of the high value of global softness. The softness of the molecules follows the order SIII > SRG > SOG using all the methods. The electrophilicity index, ω, encompasses both; the propensity of the electrophile to acquire an additional electronic charge driven by Pi 2 (the square of chemical potential) and the resistance of the system to exchange an electronic charge with the environment described by η simultaneously. Therefore, a good electrophile is characterized by a high value of Pi and a low value of η. We have computed the electrophilicity indexes and the corresponding values are shown in Table 5. SIII indicates the highest value of electrophilicity, which confirms its high capacity to accept electrons. For the other two molecules, the differences in the electrophilicity index remain relatively constant, with only minor variations. From Table 5, the variations of the ground state electrophilicity indexes of the studied compounds are similar to the different basis sets and methods. Global hardness, softness and electrophilicity index have been found to remain unchanged at the different basis sets (see Fig. 5(d)-(f)). Another important molecular feature of its electronic properties is its polarizability. The π electrons of unsubstitued aromatic molecules do not contribute to the polarizability in a direction perpendicular to the plane; however, in the case of sudan dyes the π electrons may contribute to the polarizability via OH group [54]. It has been documented that the hydroxy group is an electron donor via a π-bond and an electron acceptor via σ-bond. However, the polarizability of molecules in the perpendicular direction is mainly due to the polarizability of the σ-bonds. This would suggest that certain orientations of dipoles can have a disadvantageous effect on the order parameter. On the other hand, the dipole moment of the C-OH bond seems to be significant because the C-OH dipole may lead to both attraction and repulsion, and the net effect may be very small [54]. Ghanadzadeh et al. [54] investigated the experimental parameters (e.g. dichroic ratio R and order parameter) of five sudan dye solutions including SIII by measuring the intensity of the absorption bands in the visible region of parallel aligned samples. In that study, the polarized absorption of the Sudan dyes were measured, but no data on the polarizability of those dyes was provided [54]. Therefore, we have not compared this experimental data with our theoretical values for the polarizabilities of sudan dyes. The calculated components of the polarizability tensor α, the polarizability <α> and the anisotropy of the polarizability <Δα> for studying the molecules are listed in Table 6. The 62
17 variation of <α>, <Δα> in the atomic units and <α> in esu (x10-24 ) for studying the molecules are in the same order as SOG < SRG < SIII with HF and levels. The concepts of hardness and softness of atoms and molecules are however, intimately linked with their polarizabilities and also the sizes. Softness and polarizability are assumed to be related: a soft species is also more polarizable. Thus, a hard (soft) species is known to correspond to a low (high) value of the polarizability as well as a small (large) size [54]. Indeed, one expects the SIII, the most polarizable member of the family, to be the softest. In contrast, the SOG, the less polarizable molecule of the family, is expected to be the hardest. As seen in Fig. 6(a)-(b), the variation of <α> and <Δα> values for SIII, SOG, and SRG, and that it decreases from the largest molecular structure (SIII) to the smallest molecular structure (SOG) is obvious. The dipole moment in a molecule is an important property, which is mainly used to study the intermolecular interactions involving the nonbonded type dipole dipole interactions, because the higher the dipole moment, the stronger the intermolecular interactions will be. The dipole moments of the studied dyes obtained using HF and DFT calculations are summarized in Table 7. The higher values of dipole moments in the cases of SIII (1.72 D), SOG (3.59 D) and SRG (2.84 D) using /3-21G, HF/6-31G and HF/6-31 basis sets, respectively, is mainly attributed to an overall imbalance in the charge from one side of a molecule to the other. The variations of the ground state dipole moments of studied sudan dyes are shown in Fig. 6(c). As can be seen Fig. 6(c), results show that there is approximately increase in μ when the calculation is done at 6-31G and 6-311G basis sets when compared to other basis sets. In general, the stronger the donor, the smaller the energy difference between ground and excited states, and the longer the wavelength of UV-visible absorption. This red shift suggests an increase of molecular hyperpolarizability, according to theoretical and experimental NLO studies [55]. In previous studies, the UV-visible absorption spectra of azo dyes in different solvents were investigated by several authors [12, 21, 54]. Sudan dyes contain intramolecular charge-transfer chromophores which have large and stable NLO responses. The absorption spectrum of Sudan I was recorded by the UV-VIS-NIR spectrometer [12]. The absorption bands of Sudan I in solution is strong at 488 and 532 nm in the visible region. He and Wang [12] have investigated the nonlinear optical property of Sudan I under pulse 532 nm. They found the second hyperpolarizability to be 1.83x10-30 esu [12]. Santos et al. [21] have reported the experimental UV-visible spectrum of SIII, but they have given any information regarding the solution for the recorded spectrum. Two absorption bands of SIII were observed at 351 and 513 nm in the experimental spectrum. The band close to 350 nm was not affected by the tautomeric equilibrium [52]. The lowest energy transition, responsible for the absorption band at 484 nm obtained with, involves the highest occupied MO (HOMO) and the lowest unoccupied MO (LUMO) with the Configuration interaction (CI) contribution equal to 86% (HOMO LUMO). Also, Ghanadzadeh et al. [54] investigated the maximum absorption wavelengths of SIII in isotropic and anisotropic solvents. They found these wavelengths to be between 495 and 520 nm. The absorption maxima of SOG were observed at 254 and 382 nm in all solvents. 63
18 EŞME A., SAĞDINÇ S. G. For DHAB, the absorption maxima at 256 and 382 nm in a non-polar solvent suggests the presence of an intramolecular hydrogen bonding interaction (IHB) between OH N=N groups [57]. The investigation of the first static hyperpolarizability is explained by the calculation of the frontier molecular orbital energies, which helps to use intramolecular charge transfer to explain the hyperpolarizability. Therefore previous and present calculations show the inverse relationship between the polarizability and HOMO-LUMO energy gaps [58]. As the experimental values for the first hyperpolarizability of sudan dyes in the literature are not reported, it is difficult to conclude which basis set computes reliable values of β. The 6-31G basis set has been a common strategy for study in many previous theoretical investigations of the NLO properties of organic molecules [59, 60]. Though it is well established that diffuse and polarization functions are required for a quantitative description of both the electronic and NR (hyper) polarizabilities of medium size organic molecules [61], it has previously been noted that the 6-31G basis is adequate in obtaining semiquantitative results [62-64]. In this study, the values of the first hyperpolarizability obtained using the above Eq.9 have been calculated using HF and methods with different basis sets and are given in Table 8. From Table 6-8, it was suggested that these compounds are polar having non-zero dipole moment, hyperpolarizabilities, and hence have positive microscopic NLO behavior [65, 66]. On the other hand, the first polarizability values obtained using HF/6-31G level for SIII and SOG are generally lower than the other basis sets. In this sense, due to the deficiency of the electron correlation, we expect that the results obtained from level are larger than ab initio HF level calculations using different basis sets. This study reveals that the SIII and SOG have large first static hyperpolarizability, and have the potential applications for the development of NLO devices. Urea is one of the prototypical molecules used in this study for the NLO properties of the molecular systems for comparative purposes. It can be seen from Table 8, the calculated β values of SIII, SOG and SRG using HF/6-31G(d,p) and /6-31G(d,p) levels (the β of urea is x10-30 esu) were found nearly 42, 46 and 14 (with HF ) and 225, 87 and 12 (with ) times more than that of urea, respectively. Fig. 6(d) shows that the variation of the first hyperpolarizability for studying Sudan dyes. It can be seen from Fig. 6(d) that the relative changes from one basis set to another are nearly the same for studying Sudan dyes. The variation of β for SIII, SOG and SRG are the order of SRG < SIII < SOG with HF levels, and SRG < SOG < SIII with levels although the results of obtained with level are bigger than ab initio HF level calculations using different basis sets. However, as seen in Figure 6(d), when diffuse functions are added to these basis sets on heavy atoms and hydrogen atoms, the magnitude of β increases significantly. The β values obtained using the DFT method with different exchange and correlation functionals are higher than those from the HF methods. Figure 6(d) shows that the value of β obtained using /3-21G for SRG is slightly different from those of other methods. 64
19 Table 6. Calculated components of the polarizability tensor α, the polarizability <α> and the anisotropy of the polarizability <Δα> of studied molecules using different basis sets from HF and DFT calculations. Basis Sets α xx α xy α yy α yz α zz α xz <α> (a.u.) <Δα> (a.u.) < α>x10-24 (esu) <α>x10-24 (esu) SIII HF 3-21G HF 6-31G HF 6-31G(d) HF 6-31G(d.p) HF 6-31G+(d,p) HF G(d,p) HF 6-311G HF 6-311G(d) HF 6-311G(d.p) HF 6-311G++(d.p) SOG HF 3-21G HF 6-31G HF 6-31G(d) HF 6-31G(d.p) HF 6-31G+(d.p) HF 6-31G++(d,p) HF 6-311G
20 EŞME A., SAĞDINÇ S. G. Table 6 Continued. Calculated components of the polarizability tensor α, the polarizability <α> and the anisotropy of the polarizability <Δα> of studied molecules using different basis sets from HF and DFT calculations. Basis Sets α xx α xy α yy α yz α zz α xz <α> (a.u.) <Δα> (a.u.) < α>x10-24 (esu) <α>x10-24 (esu) HF 6-311G(d) HF 6-311G(d.p) HF 6-311G++(d.p) SRG HF 3-21G HF 6-31G HF 6-31G(d) HF 6-31G(d.p) HF 6-31G+(d.p) HF 6-31G++(d.p) HF 6-311G HF 6-311G(d) HF 6-311G(d.p) HF 6-311G++(d.p)
21 Fig. 6. Variation of (a) polarizability (<α>, in esu) (b) the anisotropy of the polarizability (<Δα>, in esu) (c) ground-state dipole moment (µ, in D) and (d) the first-order hyperpolarizability (β, in esu) of sudan dyes with HF and methods and different basis sets. 67
22 EŞME A., SAĞDINÇ S. G. Table 7. The electric dipole moments, µ(d) of studied molecules derived from HF and DFT calculations. SIII SOG SRG Basis Sets µ x µ y µ z µ(d) µ x µ y µ z µ(d) µ x µ y µ z µ(d) 3-21G HF G HF G(d) HF G(d.p) HF G(d.p) HF G(d.p) HF G HF G(d) HF G(d.p) HF G(d.p) HF
23 Table 8. All β (a.u.) components and β (esu) values calculated using HF and DFT levels of theory for all compounds. Basis Sets β xxx β xxy β xyy β yyy β xxz β xyz β yyz β xzz β yzz β zzz βx (esu) SIII HF 3-21G HF 6-31G HF 6-31G(d) HF 6-31G(d.p) HF 6-31G+(d,p) HF G(d,p) HF 6-311G HF 6-311G(d) HF 6-311G(d.p) HF 6-311G++(d.p) SOG HF 3-21G HF 6-31G HF 6-31G(d) HF 6-31G(d.p) HF 6-31G+(d.p) HF 6-31G++(d,p) HF 6-311G HF 6-311G(d)
CHAPTER 8 REPORT ON HIGHER SHG EFFICIENCY IN BIS (CINNAMIC ACID): HEXAMINE COCRYSTAL
 CHAPTER 8 REPORT ON HIGHER SHG EFFICIENCY IN BIS (CINNAMIC ACID): HEXAMINE COCRYSTAL 8.1. Introduction In recent times higher Second Harmonic Generation (SHG) efficiency organic materials receive great
CHAPTER 8 REPORT ON HIGHER SHG EFFICIENCY IN BIS (CINNAMIC ACID): HEXAMINE COCRYSTAL 8.1. Introduction In recent times higher Second Harmonic Generation (SHG) efficiency organic materials receive great
First Order Hyperpolarizability and Homo-Lumo Analysis of L-Arginine Maleate (LArM) by Density Functional Theory Methods
 51 Sciencia Acta Xaveriana An International Science Journal ISSN. 0976-1152 Volume 4 No. 2 pp. 51-58 September 2013 First Order Hyperpolarizability and Homo-Lumo Analysis of L-Arginine Maleate (LArM) by
51 Sciencia Acta Xaveriana An International Science Journal ISSN. 0976-1152 Volume 4 No. 2 pp. 51-58 September 2013 First Order Hyperpolarizability and Homo-Lumo Analysis of L-Arginine Maleate (LArM) by
Quantum chemical studies on the structures of some heterocyclic azo disperse dyes
 Quantum chemical studies on the structures of some heterocyclic azo disperse dyes Nesrin Tokay, a* Zeynel Seferoğlu, b Cemil Öğretir, c and Nermin Ertan b a Hacettepe University, Faculty of Science, Chemistry
Quantum chemical studies on the structures of some heterocyclic azo disperse dyes Nesrin Tokay, a* Zeynel Seferoğlu, b Cemil Öğretir, c and Nermin Ertan b a Hacettepe University, Faculty of Science, Chemistry
Excited States Calculations for Protonated PAHs
 52 Chapter 3 Excited States Calculations for Protonated PAHs 3.1 Introduction Protonated PAHs are closed shell ions. Their electronic structure should therefore be similar to that of neutral PAHs, but
52 Chapter 3 Excited States Calculations for Protonated PAHs 3.1 Introduction Protonated PAHs are closed shell ions. Their electronic structure should therefore be similar to that of neutral PAHs, but
Structure, Electronic and Nonlinear Optical Properties of Furyloxazoles and Thienyloxazoles
 Journal of Physics: Conference Series PAPER OPEN ACCESS Structure, Electronic and Nonlinear Optical Properties of Furyls and Thienyls To cite this article: Ozlem Dagli et al 6 J. Phys.: Conf. Ser. 77 View
Journal of Physics: Conference Series PAPER OPEN ACCESS Structure, Electronic and Nonlinear Optical Properties of Furyls and Thienyls To cite this article: Ozlem Dagli et al 6 J. Phys.: Conf. Ser. 77 View
The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then
 1 The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then filled with the available electrons according to
1 The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then filled with the available electrons according to
Chapter 13. Conjugated Unsaturated Systems. +,., - Allyl. What is a conjugated system? AllylicChlorination (High Temperature)
 What is a conjugated system? Chapter 13 Conjugated Unsaturated Systems Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital may be empty (a carbocation The
What is a conjugated system? Chapter 13 Conjugated Unsaturated Systems Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital may be empty (a carbocation The
( ) R kj. = y k y j. y A ( ) z A. y a. z a. Derivatives of the second order electrostatic tensor with respect to the translation of ( ) δ yβ.
 Supporting information Derivatives of R with respect to the translation of fragment along the y and z axis: y = y k y j (S1) z ( = z z k j) (S2) Derivatives of S with respect to the translation of fragment
Supporting information Derivatives of R with respect to the translation of fragment along the y and z axis: y = y k y j (S1) z ( = z z k j) (S2) Derivatives of S with respect to the translation of fragment
Australian Journal of Basic and Applied Sciences
 AENSI Journals Australian Journal of Basic and Applied Sciences ISSN:1991-8178 Journal home page: www.ajbasweb.com Theoretical Study for the Effect of Hydroxyl Radical on the Electronic Properties of Cyclobutadiene
AENSI Journals Australian Journal of Basic and Applied Sciences ISSN:1991-8178 Journal home page: www.ajbasweb.com Theoretical Study for the Effect of Hydroxyl Radical on the Electronic Properties of Cyclobutadiene
Vibrational spectra, nonlinear optical properties, NBO analysis and thermodynamic properties of N-phenylethanolamine by density functional method
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 016, 8(6):184-05 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Vibrational spectra, nonlinear optical properties,
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 016, 8(6):184-05 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Vibrational spectra, nonlinear optical properties,
AB INITIO MODELING OF THE STRUCTURAL DEFECTS IN AMIDES
 Int. J. Chem. Sci.: 9(4), 2011, 1564-1568 ISSN 0972-768X www.sadgurupublications.com AB INITIO MODELING OF THE STRUCTURAL DEFECTS IN AMIDES M. FATHIMA BEGUM, HEMA TRESA VARGHESE a, Y. SHEENA MARY a, C.
Int. J. Chem. Sci.: 9(4), 2011, 1564-1568 ISSN 0972-768X www.sadgurupublications.com AB INITIO MODELING OF THE STRUCTURAL DEFECTS IN AMIDES M. FATHIMA BEGUM, HEMA TRESA VARGHESE a, Y. SHEENA MARY a, C.
Electronegativity is a very useful concept for the explanation or understanding of chemical reactivity throughout the periodic table.
 1.6. Review of Electronegativity (χ) CONCEPT: Electronegativity is a very useful concept for the explanation or understanding of chemical reactivity throughout the periodic table. There are many definitions
1.6. Review of Electronegativity (χ) CONCEPT: Electronegativity is a very useful concept for the explanation or understanding of chemical reactivity throughout the periodic table. There are many definitions
CHAPTER-IV. FT-IR and FT-Raman investigation on m-xylol using ab-initio HF and DFT calculations
 4.1. Introduction CHAPTER-IV FT-IR and FT-Raman investigation on m-xylol using ab-initio HF and DFT calculations m-xylol is a material for thermally stable aramid fibers or alkyd resins [1]. In recent
4.1. Introduction CHAPTER-IV FT-IR and FT-Raman investigation on m-xylol using ab-initio HF and DFT calculations m-xylol is a material for thermally stable aramid fibers or alkyd resins [1]. In recent
Chapter 13 Conjugated Unsaturated Systems
 Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Chapter 13 Conjugated Unsaturated Systems Introduction Conjugated unsaturated systems have a p orbital on a carbon adjacent to a double bond The p orbital can come from another double or triple bond The
Journal of Chemical and Pharmaceutical Research, 2015, 7(2): Research Article
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(2):641-645 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Synthesis, characterization and theoretical study
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(2):641-645 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Synthesis, characterization and theoretical study
Tuning Color Through Substitution
 1 Tuning Color Through Substitution Introduction In this experiment, the effect of substituents on the absorbance spectra of molecules will be related to the structure of the molecular orbitals involved
1 Tuning Color Through Substitution Introduction In this experiment, the effect of substituents on the absorbance spectra of molecules will be related to the structure of the molecular orbitals involved
Terms used in UV / Visible Spectroscopy
 Terms used in UV / Visible Spectroscopy Chromophore The part of a molecule responsible for imparting color, are called as chromospheres. OR The functional groups containing multiple bonds capable of absorbing
Terms used in UV / Visible Spectroscopy Chromophore The part of a molecule responsible for imparting color, are called as chromospheres. OR The functional groups containing multiple bonds capable of absorbing
Andrew Rosen *Note: If you can rotate a molecule to have one isomer equal to another, they are both the same
 *Note: If you can rotate a molecule to have one isomer equal to another, they are both the same *Note: For hybridization, if an SP 2 is made, there is one unhybridized p orbital (because p usually has
*Note: If you can rotate a molecule to have one isomer equal to another, they are both the same *Note: For hybridization, if an SP 2 is made, there is one unhybridized p orbital (because p usually has
Competition between Alkalide Characteristics and Nonlinear Optical Properties in OLi 3 M Li 3 O (M = Li, Na, and K) Complexes
 Competition between Alkalide Characteristics and Nonlinear Optical Properties in OLi 3 M Li 3 O (M = Li, Na, and K) Complexes Ambrish Kumar Srivastava and Neeraj Misra * Department of Physics, University
Competition between Alkalide Characteristics and Nonlinear Optical Properties in OLi 3 M Li 3 O (M = Li, Na, and K) Complexes Ambrish Kumar Srivastava and Neeraj Misra * Department of Physics, University
Advanced Analytical Chemistry
 84.514 Advanced Analytical Chemistry Part III Molecular Spectroscopy (continued) Website http://faculty.uml.edu/david_ryan/84.514 http://www.cem.msu.edu/~reusch/virtualtext/ Spectrpy/UV-Vis/spectrum.htm
84.514 Advanced Analytical Chemistry Part III Molecular Spectroscopy (continued) Website http://faculty.uml.edu/david_ryan/84.514 http://www.cem.msu.edu/~reusch/virtualtext/ Spectrpy/UV-Vis/spectrum.htm
with the larger dimerization energy also exhibits the larger structural changes.
 A7. Looking at the image and table provided below, it is apparent that the monomer and dimer are structurally almost identical. Although angular and dihedral data were not included, these data are also
A7. Looking at the image and table provided below, it is apparent that the monomer and dimer are structurally almost identical. Although angular and dihedral data were not included, these data are also
A very brief history of the study of light
 1. Sir Isaac Newton 1672: A very brief history of the study of light Showed that the component colors of the visible portion of white light can be separated through a prism, which acts to bend the light
1. Sir Isaac Newton 1672: A very brief history of the study of light Showed that the component colors of the visible portion of white light can be separated through a prism, which acts to bend the light
Lesmahagow High School CfE Advanced Higher Chemistry. Unit 2 Organic Chemistry and Instrumental Analysis. Molecular Orbitals and Structure
 Lesmahagow High School CfE Advanced Higher Chemistry Unit 2 Organic Chemistry and Instrumental Analysis Molecular Orbitals and Structure 1 Molecular Orbitals Orbitals can be used to explain the bonding
Lesmahagow High School CfE Advanced Higher Chemistry Unit 2 Organic Chemistry and Instrumental Analysis Molecular Orbitals and Structure 1 Molecular Orbitals Orbitals can be used to explain the bonding
General Physical Chemistry II
 General Physical Chemistry II Lecture 13 Aleksey Kocherzhenko October 16, 2014" Last time " The Hückel method" Ø Used to study π systems of conjugated molecules" Ø π orbitals are treated separately from
General Physical Chemistry II Lecture 13 Aleksey Kocherzhenko October 16, 2014" Last time " The Hückel method" Ø Used to study π systems of conjugated molecules" Ø π orbitals are treated separately from
Valence bond theory accounts, at least qualitatively, for the stability of the covalent bond in terms of overlapping atomic orbitals.
 Molecular Orbital Theory Valence bond theory accounts, at least qualitatively, for the stability of the covalent bond in terms of overlapping atomic orbitals. Using the concept of hybridization, valence
Molecular Orbital Theory Valence bond theory accounts, at least qualitatively, for the stability of the covalent bond in terms of overlapping atomic orbitals. Using the concept of hybridization, valence
Chapter 5. Molecular Orbitals
 Chapter 5. Molecular Orbitals MO from s, p, d, orbitals: - Fig.5.1, 5.2, 5.3 Homonuclear diatomic molecules: - Fig. 5.7 - Para- vs. Diamagnetic Heteronuclear diatomic molecules: - Fig. 5.14 - ex. CO Hybrid
Chapter 5. Molecular Orbitals MO from s, p, d, orbitals: - Fig.5.1, 5.2, 5.3 Homonuclear diatomic molecules: - Fig. 5.7 - Para- vs. Diamagnetic Heteronuclear diatomic molecules: - Fig. 5.14 - ex. CO Hybrid
Conjugated Systems, Orbital Symmetry and UV Spectroscopy
 Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Conjugated Systems, Orbital Symmetry and UV Spectroscopy Introduction There are several possible arrangements for a molecule which contains two double bonds (diene): Isolated: (two or more single bonds
Growth and Characterization of an Organic Crystal and DFT Studies of 2-amino 5-methyl Pyridinium Salicylate
 ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2016, Vol. 32, No. (4): Pg. 1937-1945 Growth and Characterization
ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2016, Vol. 32, No. (4): Pg. 1937-1945 Growth and Characterization
Coordination Chemistry: Bonding Theories. Molecular Orbital Theory. Chapter 20
 Coordination Chemistry: Bonding Theories Molecular Orbital Theory Chapter 20 Review of the Previous Lecture 1. Discussed magnetism in coordination chemistry and the different classification of compounds
Coordination Chemistry: Bonding Theories Molecular Orbital Theory Chapter 20 Review of the Previous Lecture 1. Discussed magnetism in coordination chemistry and the different classification of compounds
Downloaded from
 I.I.T.Foundation - XI Chemistry MCQ #4 Time: 45 min Student's Name: Roll No.: Full Marks: 90 Chemical Bonding I. MCQ - Choose Appropriate Alternative 1. The energy required to break a chemical bond to
I.I.T.Foundation - XI Chemistry MCQ #4 Time: 45 min Student's Name: Roll No.: Full Marks: 90 Chemical Bonding I. MCQ - Choose Appropriate Alternative 1. The energy required to break a chemical bond to
2x (x 2 + y 2 + 1) 2 2y. (x 2 + y 2 + 1) 4. 4xy. (1, 1)(x 1) + (1, 1)(y + 1) (1, 1)(x 1)(y + 1) 81 x y y + 7.
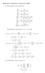 Homework 8 Solutions, November 007. (1 We calculate some derivatives: f x = f y = x (x + y + 1 y (x + y + 1 x = (x + y + 1 4x (x + y + 1 4 y = (x + y + 1 4y (x + y + 1 4 x y = 4xy (x + y + 1 4 Substituting
Homework 8 Solutions, November 007. (1 We calculate some derivatives: f x = f y = x (x + y + 1 y (x + y + 1 x = (x + y + 1 4x (x + y + 1 4 y = (x + y + 1 4y (x + y + 1 4 x y = 4xy (x + y + 1 4 Substituting
Ethene. Introduction. The ethene molecule is planar (i.e. all the six atoms lie in the same plane) and has a high degree of symmetry:
 FY1006 Innføring i kvantefysikk og TFY4215 Kjemisk fysikk og kvantemekanikk Spring 2012 Chemical Physics Exercise 1 To be delivered by Friday 27.04.12 Introduction Ethene. Ethylene, C 2 H 4, or ethene,
FY1006 Innføring i kvantefysikk og TFY4215 Kjemisk fysikk og kvantemekanikk Spring 2012 Chemical Physics Exercise 1 To be delivered by Friday 27.04.12 Introduction Ethene. Ethylene, C 2 H 4, or ethene,
Periodic Trends in Properties of Homonuclear
 Chapter 8 Periodic Trends in Properties of Homonuclear Diatomic Molecules Up to now, we have discussed various physical properties of nanostructures, namely, two-dimensional - graphene-like structures:
Chapter 8 Periodic Trends in Properties of Homonuclear Diatomic Molecules Up to now, we have discussed various physical properties of nanostructures, namely, two-dimensional - graphene-like structures:
17.1 Classes of Dienes
 17.1 Classes of Dienes There are three categories for dienes: Cumulated: pi bonds are adjacent. Conjugated: pi bonds are separated by exactly ONE single bond. Isolated: pi bonds are separated by any distance
17.1 Classes of Dienes There are three categories for dienes: Cumulated: pi bonds are adjacent. Conjugated: pi bonds are separated by exactly ONE single bond. Isolated: pi bonds are separated by any distance
Problem Set 2 Due Thursday, October 1, & & & & # % (b) Construct a representation using five d orbitals that sit on the origin as a basis:
 Problem Set 2 Due Thursday, October 1, 29 Problems from Cotton: Chapter 4: 4.6, 4.7; Chapter 6: 6.2, 6.4, 6.5 Additional problems: (1) Consider the D 3h point group and use a coordinate system wherein
Problem Set 2 Due Thursday, October 1, 29 Problems from Cotton: Chapter 4: 4.6, 4.7; Chapter 6: 6.2, 6.4, 6.5 Additional problems: (1) Consider the D 3h point group and use a coordinate system wherein
Computer Laboratory DFT and Frequencies
 Computer Laboratory DFT and Frequencies 1. DFT KEYWORDS FOR DFT METHODS Names for the various pure DFT models are given by combining the names for the exchange and correlation functionals. In some cases,
Computer Laboratory DFT and Frequencies 1. DFT KEYWORDS FOR DFT METHODS Names for the various pure DFT models are given by combining the names for the exchange and correlation functionals. In some cases,
Chapter 6. Electronic spectra and HOMO-LUMO studies on Nickel, copper substituted Phthalocyanine for solar cell applications
 Chapter 6 Electronic spectra and HOMO-LUMO studies on Nickel, copper substituted Phthalocyanine for solar cell applications 6.1 Structures of Ni, Cu substituted Phthalocyanine Almost all of the metals
Chapter 6 Electronic spectra and HOMO-LUMO studies on Nickel, copper substituted Phthalocyanine for solar cell applications 6.1 Structures of Ni, Cu substituted Phthalocyanine Almost all of the metals
Conjugated Systems. With conjugated double bonds resonance structures can be drawn
 Conjugated Systems Double bonds in conjugation behave differently than isolated double bonds With conjugated double bonds resonance structures can be drawn With isolated double bonds cannot draw resonance
Conjugated Systems Double bonds in conjugation behave differently than isolated double bonds With conjugated double bonds resonance structures can be drawn With isolated double bonds cannot draw resonance
Lecture Presentation. Chapter 11. Liquids and Intermolecular Forces. John D. Bookstaver St. Charles Community College Cottleville, MO
 Lecture Presentation Chapter 11 Liquids and Intermolecular Forces John D. Bookstaver St. Charles Community College Cottleville, MO Properties of Gases, Liquids, and Solids State Volume Shape of State Density
Lecture Presentation Chapter 11 Liquids and Intermolecular Forces John D. Bookstaver St. Charles Community College Cottleville, MO Properties of Gases, Liquids, and Solids State Volume Shape of State Density
General Chemistry I (2012) Lecture by B. H. Hong
 3.8 The Limitations of Lewis's Theory 3.9 Molecular Orbitals The valence-bond (VB) and molecular orbital (MO) theories are both procedures for constructing approximate wavefunctions of electrons. The MO
3.8 The Limitations of Lewis's Theory 3.9 Molecular Orbitals The valence-bond (VB) and molecular orbital (MO) theories are both procedures for constructing approximate wavefunctions of electrons. The MO
ELEMENTARY BAND THEORY
 ELEMENTARY BAND THEORY PHYSICIST Solid state band Valence band, VB Conduction band, CB Fermi energy, E F Bloch orbital, delocalized n-doping p-doping Band gap, E g Direct band gap Indirect band gap Phonon
ELEMENTARY BAND THEORY PHYSICIST Solid state band Valence band, VB Conduction band, CB Fermi energy, E F Bloch orbital, delocalized n-doping p-doping Band gap, E g Direct band gap Indirect band gap Phonon
EJL R Sβ. sum. General objective: Reduce the complexity of the analysis by exploiting symmetry. Garth J. Simpson
 e sum = EJL R Sβ General objective: Reduce the complexity of the analysis by exploiting symmetry. Specific Objectives: 1. The molecular symmetry matrix S. How to populate it.. Relationships between the
e sum = EJL R Sβ General objective: Reduce the complexity of the analysis by exploiting symmetry. Specific Objectives: 1. The molecular symmetry matrix S. How to populate it.. Relationships between the
Chapter 14: Conjugated Dienes
 Chapter 14: Conjugated Dienes Coverage: 1. Conjugated vs Nonconjugated dienes and Stability 2. MO picture of 1,3-butadiene 3. Electrophilic addition to Dienes 4. Kinetic vs Thermodynamic Control 5. Diels-Alder
Chapter 14: Conjugated Dienes Coverage: 1. Conjugated vs Nonconjugated dienes and Stability 2. MO picture of 1,3-butadiene 3. Electrophilic addition to Dienes 4. Kinetic vs Thermodynamic Control 5. Diels-Alder
2. Polar Covalent Bonds: Acids and Bases
 2. Polar Covalent Bonds: Acids and Bases Based on McMurry s Organic Chemistry, 6 th edition, Chapter 2 2003 Ronald Kluger Department of Chemistry University of Toronto 2.1 Polar Covalent Bonds: Electronegativity!
2. Polar Covalent Bonds: Acids and Bases Based on McMurry s Organic Chemistry, 6 th edition, Chapter 2 2003 Ronald Kluger Department of Chemistry University of Toronto 2.1 Polar Covalent Bonds: Electronegativity!
The broad topic of physical metallurgy provides a basis that links the structure of materials with their properties, focusing primarily on metals.
 Physical Metallurgy The broad topic of physical metallurgy provides a basis that links the structure of materials with their properties, focusing primarily on metals. Crystal Binding In our discussions
Physical Metallurgy The broad topic of physical metallurgy provides a basis that links the structure of materials with their properties, focusing primarily on metals. Crystal Binding In our discussions
Carbon Compounds. Electronegativity. Chemical Bonding Part 1c. Bond Polarity. Bond Polarity
 Electronegativity Carbon Compounds Electronegativity is a relative measure on the pull of electrons by an atom in a bond. Most bonds fall somewhere in between and these bonds are considered polar. Chemical
Electronegativity Carbon Compounds Electronegativity is a relative measure on the pull of electrons by an atom in a bond. Most bonds fall somewhere in between and these bonds are considered polar. Chemical
Session 1. Introduction to Computational Chemistry. Computational (chemistry education) and/or (Computational chemistry) education
 Session 1 Introduction to Computational Chemistry 1 Introduction to Computational Chemistry Computational (chemistry education) and/or (Computational chemistry) education First one: Use computational tools
Session 1 Introduction to Computational Chemistry 1 Introduction to Computational Chemistry Computational (chemistry education) and/or (Computational chemistry) education First one: Use computational tools
UNIT TWO BOOKLET 1. Molecular Orbitals and Hybridisation
 DUNCANRIG SECONDARY ADVANCED HIGHER CHEMISTRY UNIT TWO BOOKLET 1 Molecular Orbitals and Hybridisation In the inorganic unit we learned about atomic orbitals and how they could be used to write the electron
DUNCANRIG SECONDARY ADVANCED HIGHER CHEMISTRY UNIT TWO BOOKLET 1 Molecular Orbitals and Hybridisation In the inorganic unit we learned about atomic orbitals and how they could be used to write the electron
Molecular Geometry and intermolecular forces. Unit 4 Chapter 9 and 11.2
 1 Molecular Geometry and intermolecular forces Unit 4 Chapter 9 and 11.2 2 Unit 4.1 Chapter 9.1-9.3 3 Review of bonding Ionic compound (metal/nonmetal) creates a lattice Formula doesn t tell the exact
1 Molecular Geometry and intermolecular forces Unit 4 Chapter 9 and 11.2 2 Unit 4.1 Chapter 9.1-9.3 3 Review of bonding Ionic compound (metal/nonmetal) creates a lattice Formula doesn t tell the exact
Jack Smith. Center for Environmental, Geotechnical and Applied Science. Marshall University
 Jack Smith Center for Environmental, Geotechnical and Applied Science Marshall University -- Division of Science and Research WV Higher Education Policy Commission WVU HPC Summer Institute June 20, 2014
Jack Smith Center for Environmental, Geotechnical and Applied Science Marshall University -- Division of Science and Research WV Higher Education Policy Commission WVU HPC Summer Institute June 20, 2014
Computational Chemistry Workshops West Ridge Research Building-UAF Campus 9:00am-4:00pm, Room 009
 Computational Chemistry Workshops West Ridge Research Building-UAF Campus 9:00am-4:00pm, Room 009 Electronic Structure - July 19-21, 2016 Molecular Dynamics - July 26-28, 2016 Why are the Azulene and Naphthalene
Computational Chemistry Workshops West Ridge Research Building-UAF Campus 9:00am-4:00pm, Room 009 Electronic Structure - July 19-21, 2016 Molecular Dynamics - July 26-28, 2016 Why are the Azulene and Naphthalene
Conjugated Dienes and Ultraviolet Spectroscopy
 Conjugated Dienes and Ultraviolet Spectroscopy Key Words Conjugated Diene Resonance Structures Dienophiles Concerted Reaction Pericyclic Reaction Cycloaddition Reaction Bridged Bicyclic Compound Cyclic
Conjugated Dienes and Ultraviolet Spectroscopy Key Words Conjugated Diene Resonance Structures Dienophiles Concerted Reaction Pericyclic Reaction Cycloaddition Reaction Bridged Bicyclic Compound Cyclic
Available online at WSN 61(2) (2017) EISSN
 Available online at www.worldscientificnews.com WSN 61(2) (2017) 150-185 EISSN 2392-2192 FT-IR, FT-Raman, NMR and U-V Spectral investigation: Computation of vibrational frequency, chemical shifts and electronic
Available online at www.worldscientificnews.com WSN 61(2) (2017) 150-185 EISSN 2392-2192 FT-IR, FT-Raman, NMR and U-V Spectral investigation: Computation of vibrational frequency, chemical shifts and electronic
Homework 08 - Bonding Theories & IMF
 HW08 - Bonding Theories & IMF This is a preview of the published version of the quiz Started: Jun 4 at 11:4am Quiz Instructions Homework 08 - Bonding Theories & IMF Question 1 A sigma bond... stems from
HW08 - Bonding Theories & IMF This is a preview of the published version of the quiz Started: Jun 4 at 11:4am Quiz Instructions Homework 08 - Bonding Theories & IMF Question 1 A sigma bond... stems from
CHEM 344 Molecular Modeling
 CHEM 344 Molecular Modeling The Use of Computational Chemistry to Support Experimental Organic Chemistry Part 1: Molecular Orbital Theory, Hybridization, & Formal Charge * all calculation data obtained
CHEM 344 Molecular Modeling The Use of Computational Chemistry to Support Experimental Organic Chemistry Part 1: Molecular Orbital Theory, Hybridization, & Formal Charge * all calculation data obtained
A Combined Optical and EPR Spectroscopy Study: Azobenzene-Based Biradicals as Reversible Molecular Photoswitches
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2017 A Combined Optical and EPR Spectroscopy Study: Azobenzene-Based Biradicals as Reversible
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2017 A Combined Optical and EPR Spectroscopy Study: Azobenzene-Based Biradicals as Reversible
Electronic structure Crystal-field theory Ligand-field theory. Electronic-spectra electronic spectra of atoms
 Chapter 19 d-metal complexes: electronic structure and spectra Electronic structure 19.1 Crystal-field theory 19.2 Ligand-field theory Electronic-spectra 19.3 electronic spectra of atoms 19.4 electronic
Chapter 19 d-metal complexes: electronic structure and spectra Electronic structure 19.1 Crystal-field theory 19.2 Ligand-field theory Electronic-spectra 19.3 electronic spectra of atoms 19.4 electronic
Same idea for polyatomics, keep track of identical atom e.g. NH 3 consider only valence electrons F(2s,2p) H(1s)
 XIII 63 Polyatomic bonding -09 -mod, Notes (13) Engel 16-17 Balance: nuclear repulsion, positive e-n attraction, neg. united atom AO ε i applies to all bonding, just more nuclei repulsion biggest at low
XIII 63 Polyatomic bonding -09 -mod, Notes (13) Engel 16-17 Balance: nuclear repulsion, positive e-n attraction, neg. united atom AO ε i applies to all bonding, just more nuclei repulsion biggest at low
Why Is Molecular Interaction Important in Our Life
 Why Is Molecular Interaction Important in ur Life QuLiS and Graduate School of Science iroshima University http://www.nabit.hiroshima-u.ac.jp/iwatasue/indexe.htm Suehiro Iwata Sept. 29, 2007 Department
Why Is Molecular Interaction Important in ur Life QuLiS and Graduate School of Science iroshima University http://www.nabit.hiroshima-u.ac.jp/iwatasue/indexe.htm Suehiro Iwata Sept. 29, 2007 Department
Computational Methods. Chem 561
 Computational Methods Chem 561 Lecture Outline 1. Ab initio methods a) HF SCF b) Post-HF methods 2. Density Functional Theory 3. Semiempirical methods 4. Molecular Mechanics Computational Chemistry " Computational
Computational Methods Chem 561 Lecture Outline 1. Ab initio methods a) HF SCF b) Post-HF methods 2. Density Functional Theory 3. Semiempirical methods 4. Molecular Mechanics Computational Chemistry " Computational
Chem 263 Notes Sept. 26, 2013
 Chem 263 Notes Sept. 26, 2013 Example of Predicting Stereochemistry The following example uses 2Z, 4E-hexadiene (diene) and cyclopentene (dienophile) to produce an endo product. As shown before with the
Chem 263 Notes Sept. 26, 2013 Example of Predicting Stereochemistry The following example uses 2Z, 4E-hexadiene (diene) and cyclopentene (dienophile) to produce an endo product. As shown before with the
RDCH 702 Lecture 4: Orbitals and energetics
 RDCH 702 Lecture 4: Orbitals and energetics Molecular symmetry Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating
RDCH 702 Lecture 4: Orbitals and energetics Molecular symmetry Bonding and structure Molecular orbital theory Crystal field theory Ligand field theory Provide fundamental understanding of chemistry dictating
Problem Set 2 Due Tuesday, September 27, ; p : 0. (b) Construct a representation using five d orbitals that sit on the origin as a basis: 1
 Problem Set 2 Due Tuesday, September 27, 211 Problems from Carter: Chapter 2: 2a-d,g,h,j 2.6, 2.9; Chapter 3: 1a-d,f,g 3.3, 3.6, 3.7 Additional problems: (1) Consider the D 4 point group and use a coordinate
Problem Set 2 Due Tuesday, September 27, 211 Problems from Carter: Chapter 2: 2a-d,g,h,j 2.6, 2.9; Chapter 3: 1a-d,f,g 3.3, 3.6, 3.7 Additional problems: (1) Consider the D 4 point group and use a coordinate
2.1 Experimental and theoretical studies
 Chapter 2 NiO As stated before, the first-row transition-metal oxides are among the most interesting series of materials, exhibiting wide variations in physical properties related to electronic structure.
Chapter 2 NiO As stated before, the first-row transition-metal oxides are among the most interesting series of materials, exhibiting wide variations in physical properties related to electronic structure.
Practical 1: Structure and electronic properties of organic molecules. B/ Structure, electronic and vibrational properties of the water molecule
 D1CH9116 - MMCO Molecular Modelling applied to organic chemistry Practical 1: tructure and electronic properties of organic molecules B/ tructure, electronic and vibrational properties of the water molecule
D1CH9116 - MMCO Molecular Modelling applied to organic chemistry Practical 1: tructure and electronic properties of organic molecules B/ tructure, electronic and vibrational properties of the water molecule
Structural Properties, Natural Bond Orbital, Theory Functional Calculations (DFT), and Energies for the α Halorganic Compounds
 Current World Environment Vol. 7(2), 221-226 (2012) Structural Properties, Natural Bond Orbital, Theory Functional Calculations (DFT), and Energies for the α Halorganic Compounds NAJLA SEIDY and SHAHRIAR
Current World Environment Vol. 7(2), 221-226 (2012) Structural Properties, Natural Bond Orbital, Theory Functional Calculations (DFT), and Energies for the α Halorganic Compounds NAJLA SEIDY and SHAHRIAR
17.1 Classes of Dienes
 W 2/1 Due: HW14, spec03 Due: n/a M 2/6 Lecture HW14 grading Lect17a Conjugated π systems Lecture quiz Lect17b Lab Lab02 Qual Analysis II (cont) 7-1 17.1 Classes of Dienes There are three categories for
W 2/1 Due: HW14, spec03 Due: n/a M 2/6 Lecture HW14 grading Lect17a Conjugated π systems Lecture quiz Lect17b Lab Lab02 Qual Analysis II (cont) 7-1 17.1 Classes of Dienes There are three categories for
Chapter 2 Structure and Properties of Organic Molecules. Advanced Bonding: Review
 hapter 2 Structure and Properties of Organic Molecules hemistry 231 Organic hemistry I Fall 2007 Advanced Bonding: Review Atomic Quantum Mechanics cannot explain how molecules like 4 form: Valence Bond
hapter 2 Structure and Properties of Organic Molecules hemistry 231 Organic hemistry I Fall 2007 Advanced Bonding: Review Atomic Quantum Mechanics cannot explain how molecules like 4 form: Valence Bond
Theoretical Chemistry - Level II - Practical Class Molecular Orbitals in Diatomics
 Theoretical Chemistry - Level II - Practical Class Molecular Orbitals in Diatomics Problem 1 Draw molecular orbital diagrams for O 2 and O 2 +. E / ev dioxygen molecule, O 2 dioxygenyl cation, O 2 + 25
Theoretical Chemistry - Level II - Practical Class Molecular Orbitals in Diatomics Problem 1 Draw molecular orbital diagrams for O 2 and O 2 +. E / ev dioxygen molecule, O 2 dioxygenyl cation, O 2 + 25
A Eşme* Department of Elementary Science Education, Kocaeli University, Kocaeli 41380, Turkey
 Indian Journal of Pure & Applied Physics Vol. 55, July 2017, pp. 478-489 Quantum chemical calculations on the geometrical, conformational, spectroscopic (FTIR, FT-Raman) analysis and NLO activity of milrinone
Indian Journal of Pure & Applied Physics Vol. 55, July 2017, pp. 478-489 Quantum chemical calculations on the geometrical, conformational, spectroscopic (FTIR, FT-Raman) analysis and NLO activity of milrinone
Chapter 2 Polar Covalent Bonds; Acids and Bases SAMPLE. Chapter Outline
 Chapter 2 Polar Covalent Bonds; Acids and Bases Chapter utline I. Polar covalent bonds (Sections 2.1 2.3). A. Electronegativity (Section 2.1). 1. Although some bonds are totally ionic and some are totally
Chapter 2 Polar Covalent Bonds; Acids and Bases Chapter utline I. Polar covalent bonds (Sections 2.1 2.3). A. Electronegativity (Section 2.1). 1. Although some bonds are totally ionic and some are totally
Chapter 3. Orbitals and Bonding
 Chapter 3. Orbitals and Bonding What to master Assigning Electrons to Atomic Orbitals Constructing Bonding and Antibonding Molecular Orbitals with Simple MO Theory Understanding Sigma and Pi Bonds Identifying
Chapter 3. Orbitals and Bonding What to master Assigning Electrons to Atomic Orbitals Constructing Bonding and Antibonding Molecular Orbitals with Simple MO Theory Understanding Sigma and Pi Bonds Identifying
Be H. Delocalized Bonding. Localized Bonding. σ 2. σ 1. Two (sp-1s) Be-H σ bonds. The two σ bonding MO s in BeH 2. MO diagram for BeH 2
 The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
The Delocalized Approach to Bonding: The localized models for bonding we have examined (Lewis and VBT) assume that all electrons are restricted to specific bonds between atoms or in lone pairs. In contrast,
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding.
 Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
Molecular Geometry: VSEPR model stand for valence-shell electron-pair repulsion and predicts the 3D shape of molecules that are formed in bonding. Sigma and Pi Bonds: All single bonds are sigma(σ), that
Spectroscopic properties of dipicolinic acid and its dianion
 Chemical Physics 322 (2006) 254 268 www.elsevier.com/locate/chemphys Spectroscopic properties of dipicolinic acid and its dianion John Rui-Hua Xie a, Vedene H. Smith Jr. b, Roland E. Allen a, * a Department
Chemical Physics 322 (2006) 254 268 www.elsevier.com/locate/chemphys Spectroscopic properties of dipicolinic acid and its dianion John Rui-Hua Xie a, Vedene H. Smith Jr. b, Roland E. Allen a, * a Department
THEORETICAL INVESTIGATION OF ELECTROSTATIC POTENTIAL AND NON LINEAR OPTICAL PROPERTIES OF M NITROACETANILIDE
 ISSN: 0974-1496 e-issn: 0976-0083 CODEN: RJCABP http://www.rasayanjournal.com http://www.rasayanjournal.co.in THEORETICAL INVESTIGATION OF ELECTROSTATIC POTENTIAL AND NON LINEAR OPTICAL PROPERTIES OF N.
ISSN: 0974-1496 e-issn: 0976-0083 CODEN: RJCABP http://www.rasayanjournal.com http://www.rasayanjournal.co.in THEORETICAL INVESTIGATION OF ELECTROSTATIC POTENTIAL AND NON LINEAR OPTICAL PROPERTIES OF N.
Electronic structures of one-dimension carbon nano wires and rings
 IOP Publishing Journal of Physics: Conference Series 61 (2007) 252 256 doi:10.1088/1742-6596/61/1/051 International Conference on Nanoscience and Technology (ICN&T 2006) Electronic structures of one-dimension
IOP Publishing Journal of Physics: Conference Series 61 (2007) 252 256 doi:10.1088/1742-6596/61/1/051 International Conference on Nanoscience and Technology (ICN&T 2006) Electronic structures of one-dimension
Hints on Using the Orca Program
 Computational Chemistry Workshops West Ridge Research Building-UAF Campus 9:00am-4:00pm, Room 009 Electronic Structure - July 19-21, 2016 Molecular Dynamics - July 26-28, 2016 Hints on Using the Orca Program
Computational Chemistry Workshops West Ridge Research Building-UAF Campus 9:00am-4:00pm, Room 009 Electronic Structure - July 19-21, 2016 Molecular Dynamics - July 26-28, 2016 Hints on Using the Orca Program
Helical Structure and Circular Dichroism Spectra of DNA: A Theoretical Study
 pubs.acs.org/jpca Helical Structure and Circular Dichroism Spectra of DNA: A Theoretical Study Tomoo Miyahara, Hiroshi Nakatsuji,*, and Hiroshi Sugiyama Quantum Chemistry Research Institute, JST, CREST,
pubs.acs.org/jpca Helical Structure and Circular Dichroism Spectra of DNA: A Theoretical Study Tomoo Miyahara, Hiroshi Nakatsuji,*, and Hiroshi Sugiyama Quantum Chemistry Research Institute, JST, CREST,
Covalent bonds can have ionic character These are polar covalent bonds
 Polar Covalent Bonds: Electronegativity Covalent bonds can have ionic character These are polar covalent bonds Bonding electrons attracted more strongly by one atom than by the other Electron distribution
Polar Covalent Bonds: Electronegativity Covalent bonds can have ionic character These are polar covalent bonds Bonding electrons attracted more strongly by one atom than by the other Electron distribution
Organic Chemistry: CHEM2322
 Conjugated Systems Organic Chemistry: We met in Chem 2321 unsaturated bonds as either a C=C bond or C C bond. If these unsaturated bonds are well separated then they react independently however if there
Conjugated Systems Organic Chemistry: We met in Chem 2321 unsaturated bonds as either a C=C bond or C C bond. If these unsaturated bonds are well separated then they react independently however if there
Department of Physics, A.V.C. College, Mayiladuthurai, Tamilnadu, India.
 IOSR Journal of Applied Physics (IOSR-JAP) e-issn: 2278-4861.Volume 8, Issue 3 Ver. II (May. - Jun. 2016), PP 01-14 www.iosrjournals.org The Spectroscopic (FT-IR, FT-Raman, NMR & UV-Vis) and first order
IOSR Journal of Applied Physics (IOSR-JAP) e-issn: 2278-4861.Volume 8, Issue 3 Ver. II (May. - Jun. 2016), PP 01-14 www.iosrjournals.org The Spectroscopic (FT-IR, FT-Raman, NMR & UV-Vis) and first order
MOLECULAR STRUCTURE. Molecular Structure - B. Molecular Structure - B. Molecular Structure - B. Molecular Structure - B. Molecular Structure - B
 MOLECULAR STRUCTURE Molecular Orbital all orbitals of the appropriate symmetry contribute to a molecular orbital. Bundet Boekfa Chem Div, Faculty Lib Arts & Sci Kasetsart University Kamphaeng Saen Campus
MOLECULAR STRUCTURE Molecular Orbital all orbitals of the appropriate symmetry contribute to a molecular orbital. Bundet Boekfa Chem Div, Faculty Lib Arts & Sci Kasetsart University Kamphaeng Saen Campus
INTERMOLECULAR FORCES: Polarity of Molecules. Seventh Course (General Chemistry) by Dr. Istadi
 INTERMOLECULAR FORCES: Polarity of Molecules Seventh Course (General Chemistry) by Dr. Istadi 1 Types of Intermolecular Forces The nature of the phases and their changes are due primarily to forces among
INTERMOLECULAR FORCES: Polarity of Molecules Seventh Course (General Chemistry) by Dr. Istadi 1 Types of Intermolecular Forces The nature of the phases and their changes are due primarily to forces among
Terms used in UV / Visible Spectroscopy
 Terms used in UV / Visible Spectroscopy Chromophore The part of a molecule responsible for imparting color, are called as chromospheres. OR The functional groups containing multiple bonds capable of absorbing
Terms used in UV / Visible Spectroscopy Chromophore The part of a molecule responsible for imparting color, are called as chromospheres. OR The functional groups containing multiple bonds capable of absorbing
Review Outline Chemistry 1B, Fall 2012
 Review Outline Chemistry 1B, Fall 2012 -------------------------------------- Chapter 12 -------------------------------------- I. Experiments and findings related to origin of quantum mechanics A. Planck:
Review Outline Chemistry 1B, Fall 2012 -------------------------------------- Chapter 12 -------------------------------------- I. Experiments and findings related to origin of quantum mechanics A. Planck:
Journal of Advanced Scientific Research DFT APPROACH ON CORROSION INHIBITION PERFORMANCE OF THIOSEMICARBAZONE DERIVATIVES ON METALLIC IRON
 Rajendran et al., J Adv Sci Res, 2016, 7(1): 32-37 32 Journal of Advanced Scientific Research Available online through http://www.sciensage.info/jasr ISSN 0976-9595 Research Article DFT APPROACH ON CORROSION
Rajendran et al., J Adv Sci Res, 2016, 7(1): 32-37 32 Journal of Advanced Scientific Research Available online through http://www.sciensage.info/jasr ISSN 0976-9595 Research Article DFT APPROACH ON CORROSION
Atomic Structure. Atomic Structure. Atomic Structure. Atomic Structure. Electron Configuration. Electron Configuration
 Atomic Electron onfiguration Atomic Electron onfiguration z z z E 3rd SELL 3d 3p 3s y 2p x x y 2p y x y 2p z x 2nd SELL t SELL 2p x y z 2nd SELL 2p x y z y z x Atomic Ground State onfiguration Lowest energy
Atomic Electron onfiguration Atomic Electron onfiguration z z z E 3rd SELL 3d 3p 3s y 2p x x y 2p y x y 2p z x 2nd SELL t SELL 2p x y z 2nd SELL 2p x y z y z x Atomic Ground State onfiguration Lowest energy
Density Functional Theory Study of Exohedral Carbon Atoms Effect on Electrophilicity of Nicotine: Comparative Analysis
 Computational Chemistry, 2016, 4, 17-31 Published Online January 2016 in SciRes. http://www.scirp.org/journal/cc http://dx.doi.org/10.4236/cc.2016.41003 Density Functional Theory Study of Exohedral Carbon
Computational Chemistry, 2016, 4, 17-31 Published Online January 2016 in SciRes. http://www.scirp.org/journal/cc http://dx.doi.org/10.4236/cc.2016.41003 Density Functional Theory Study of Exohedral Carbon
Inorganic Pharmaceutical Chemistry
 Inorganic Pharmaceutical Chemistry Lecture No. 4 Date :25/10 /2012 Dr. Mohammed Hamed --------------------------------------------------------------------------------------------------------------------------------------
Inorganic Pharmaceutical Chemistry Lecture No. 4 Date :25/10 /2012 Dr. Mohammed Hamed --------------------------------------------------------------------------------------------------------------------------------------
Chapter 2 Polar Covalent Bonds; Acids and Bases. Chapter Outline
 rganic Chemistry 9th Edition McMurry SLUTINS MANUAL Full clear download at: https://testbankreal.com/download/organic-chemistry-9th-edition-mcmurrysolutions-manual/ rganic Chemistry 9th Edition McMurry
rganic Chemistry 9th Edition McMurry SLUTINS MANUAL Full clear download at: https://testbankreal.com/download/organic-chemistry-9th-edition-mcmurrysolutions-manual/ rganic Chemistry 9th Edition McMurry
Chapter 2 Alkanes and Cycloalkanes: Introduction to Hydrocarbons
 Chapter 2 Alkanes and Cycloalkanes: Introduction to Hydrocarbons 2.1 Classes of Hydrocarbons Classes of Hydrocarbons Hydrocarbons only contain carbon and hydrogen atoms. Hydrocarbons are either classed
Chapter 2 Alkanes and Cycloalkanes: Introduction to Hydrocarbons 2.1 Classes of Hydrocarbons Classes of Hydrocarbons Hydrocarbons only contain carbon and hydrogen atoms. Hydrocarbons are either classed
Paper: 12, Organic Spectroscopy Module: 5, Applications of UV spectroscopy
 Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy Applications of UV-visible Spectroscopy CHE_P12_M5 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction
Subject Chemistry Paper No and Title Module No and Title Module Tag Paper 12: Organic Spectroscopy Applications of UV-visible Spectroscopy CHE_P12_M5 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction
FT-IR, FT-Raman And UV Spectra and Ab-Initio HF and DFT Vibrational Study of 1-Propyl 4-Piperidone
 FT-IR, FT-Raman And UV Spectra and Ab-Initio HF and DFT Vibrational Study of 1-Propyl 4-Piperidone K. Rajalakshmi 1 and E.Elumalai 2 1 Department of Physics, Sri Chandrasekharendra Saraswathi Viswa Mahavidyalaya,
FT-IR, FT-Raman And UV Spectra and Ab-Initio HF and DFT Vibrational Study of 1-Propyl 4-Piperidone K. Rajalakshmi 1 and E.Elumalai 2 1 Department of Physics, Sri Chandrasekharendra Saraswathi Viswa Mahavidyalaya,
Quantum Chemistry. NC State University. Lecture 5. The electronic structure of molecules Absorption spectroscopy Fluorescence spectroscopy
 Quantum Chemistry Lecture 5 The electronic structure of molecules Absorption spectroscopy Fluorescence spectroscopy NC State University 3.5 Selective absorption and emission by atmospheric gases (source:
Quantum Chemistry Lecture 5 The electronic structure of molecules Absorption spectroscopy Fluorescence spectroscopy NC State University 3.5 Selective absorption and emission by atmospheric gases (source:
Quantum Chemical Simulations and Descriptors. Dr. Antonio Chana, Dr. Mosè Casalegno
 Quantum Chemical Simulations and Descriptors Dr. Antonio Chana, Dr. Mosè Casalegno Classical Mechanics: basics It models real-world objects as point particles, objects with negligible size. The motion
Quantum Chemical Simulations and Descriptors Dr. Antonio Chana, Dr. Mosè Casalegno Classical Mechanics: basics It models real-world objects as point particles, objects with negligible size. The motion
Structure of Coordination Compounds
 Chapter 22 COORDINATION CHEMISTRY (Part II) Dr. Al Saadi 1 Structure of Coordination Compounds The geometry of coordination compounds plays a significant role in determining their properties. The structure
Chapter 22 COORDINATION CHEMISTRY (Part II) Dr. Al Saadi 1 Structure of Coordination Compounds The geometry of coordination compounds plays a significant role in determining their properties. The structure
Chapter 10: Chemical Bonding II: Molecular Shapes; VSEPR, Valence Bond and Molecular Orbital Theories
 C h e m i s t r y 1 A : C h a p t e r 1 0 P a g e 1 Chapter 10: Chemical Bonding II: Molecular Shapes; VSEPR, Valence Bond and Molecular Orbital Theories Homework: Read Chapter 10: Work out sample/practice
C h e m i s t r y 1 A : C h a p t e r 1 0 P a g e 1 Chapter 10: Chemical Bonding II: Molecular Shapes; VSEPR, Valence Bond and Molecular Orbital Theories Homework: Read Chapter 10: Work out sample/practice
CHEM 2010 Symmetry, Electronic Structure and Bonding Winter Numbering of Chapters and Assigned Problems
 CHEM 2010 Symmetry, Electronic Structure and Bonding Winter 2011 Numbering of Chapters and Assigned Problems The following table shows the correspondence between the chapter numbers in the full book (Physical
CHEM 2010 Symmetry, Electronic Structure and Bonding Winter 2011 Numbering of Chapters and Assigned Problems The following table shows the correspondence between the chapter numbers in the full book (Physical
The wavefunction that describes a bonding pair of electrons:
 4.2. Molecular Properties from VB Theory a) Bonding and Bond distances The wavefunction that describes a bonding pair of electrons: Ψ b = a(h 1 ) + b(h 2 ) where h 1 and h 2 are HAOs on adjacent atoms
4.2. Molecular Properties from VB Theory a) Bonding and Bond distances The wavefunction that describes a bonding pair of electrons: Ψ b = a(h 1 ) + b(h 2 ) where h 1 and h 2 are HAOs on adjacent atoms
