Computer Laboratory DFT and Frequencies
|
|
|
- Junior Stevens
- 6 years ago
- Views:
Transcription
1 Computer Laboratory DFT and Frequencies 1. DFT KEYWORDS FOR DFT METHODS Names for the various pure DFT models are given by combining the names for the exchange and correlation functionals. In some cases, standard synonyms used in the field are also available as keywords. Exchange Functionals. The following exchange functionals are available in Gaussian 03: Slater: ρ 4/3 with theoretical coefficient of 2/3, also referred to as Local Spin Density exchange [75,76,77]. Keyword: Used Alone: HFS, Comb. Form: S Xαρ 4/3 with the empirical coefficient of 0.7, usually used when this exchange functional is used without a correlation functional [75,76,77]. Keyword: Used Alone: XAlpha, Comb. Form: XA. Becke 88: Becke's 1988 functional, which includes the Slater exchange along with corrections involving the gradient of the density [462]. Keyword: Used Alone: HFB, Comb.Form: B. Perdew-Wang 91: The exchange component of Perdew and Wang's 1991 functional [463,464,465,466,467]. Keyword: Used Alone: N/A, Comb. Form: PW91. Barone's Modified PW91: The Perdew-Wang 1991 exchange functional as modified by Adamo and Barone [468]. Keyword: Used Alone: N/A, Comb. Form: MPW. Gill 96: The 1996 exchange functional of Gill [469,470]. Keyword: Used Alone: N/A, Comb. Form: G96. PBE: The 1996 functional of Perdew, Burke and Ernzerhof [471,472]. Keyword: Used Alone: N/A, Comb. Form: PBE. MPBE: Adamo and Barone's modification of PBE [473]. Alone: N/A, Comb. Form: MPBE. OPTX: Handy's OPTX modification of Becke's exchange functional [474]. Keyword: Comb. Form: O. The combination forms are used when one of these exchange functionals is used in combination with a correlation functional (see below).
2 Correlation Functionals. The following correlation functionals are available, listed by their corresponding keyword component: VWN: Vosko, Wilk, and Nusair 1980 correlation functional(iii) fitting the RPA solution to the uniform electron gas, often referred to as Local Spin Density (LSD) correlation [475] (functional III in the paper). VWN V(VWN5): Functional V from the 1980 paper which fits the Ceperly-Alder solution to the uniform electron gas (this is the functional recommended in the paper) [475]. LYP: The correlation functional of Lee, Yang, and Parr which includes both local and non-local terms [476,477]. PL (Perdew Local): The local (non-gradient corrected) functional of Perdew (1981) [478]. P86 (Perdew 86): The gradient corrections of Perdew, along with his 1981 local correlation functional [479]. PW91 (Perdew/Wang 91): Perdew and Wang's 1991 gradient-corrected correlation functional [463,464,465,466,467]. B95 (Becke 95): Becke's τ-dependent gradient-corrected correlation functional (defined as part of his one parameter hybrid functional [480]. PBE: The 1996 gradient-corrected correlation functional of Perdew, Burke and Ernzerhof [471,472]. MPBE: Adamo and Barone's modification of PBE [473]. All of the keywords for these correlation functionals must be combined with the keyword for the desired exchange functional. For example, BLYP requests the Becke exchange functional and the LYP correlation functional. SVWN requests the Slater exchange and the VWN correlation functional, and is known in the literature by its synonym LSDA (Local Spin Density Approximation). LSDA is a synonym for SVWN. Some other software packages with DFT facilities use the equivalent of SVWN5 when "LSDA" is requested. Check the documentation carefully for all packages when making comparisons. Correlation Functional Variations. The following correlation functionals combine local and non-local terms from different correlation functionals: VP86: VWN5 local and P86 non-local correlation functional. V5LYP: VWN5 local and LYP non-local correlation functional.
3 Standalone Functionals. The following functionals are self-contained and are not combined with any other functional keyword components: VSXC: van Voorhis and Scuseria's τ-dependant gradient-corrected correlation functional [481]. HCTH/*: Handy's family functional including gradient-corrected correlation [482,483,484]. HCTH refers to HCTH/407, HCTH93 to HCTH/93, HCTH147 to HCTH/147, and HCTH407 to HCTH/407. Note that the related HCTH/120 functional is not implemented. Hybrid Functionals. Three hybrid functionals, which include a mixture of Hartree-Fock exchange with DFT exchange-correlation, are available via keywords: Becke Three Parameter Hybrid Functionals. These functionals have the form devised by Becke in 1993 [79]: A*E Slater X +(1-A)*E HF X +B*ΔE Becke X +E VWN non-local C +C*ΔE C where A, B, and C are the constants determined by Becke via fitting to the G1 molecule set. There are several variations of this hybrid functional. B3LYP uses the non-local correlation provided by the LYP expression, and VWN functional III for local correlation (not functional V). Note that since LYP includes both local and nonlocal terms, the correlation functional used is actually: C*E LYP VWN C +(1-C)*E C In other words, VWN is used to provide the excess local correlation required, since LYP contains a local term essentially equivalent to VWN. B3P86 specifies the same functional with the non-local correlation provided by Perdew 86, and B3PW91 specifies this functional with the non-local correlation provided by Perdew/Wang 91. Becke One Parameter Hybrid Functionals. The B1B95 keyword is used to specify Becke's one-parameter hybrid functional as defined in the original paper [480]. The program also provides other, similar one parameter hybrid functionals, as implemented by Adamo and Barone [480,485]. In one variation, B1LYP, the LYP correlation functional is used (as described for B3LYP above). Another version, MPW1PW91, uses modified Perdew-Wang exchange and Perdew-Wang 91 correlation [468 ]. Becke's 1998 revisions to B97 [486,487]. The keyword is B98, and it implements equation 2c in reference [487]. Handy, Tozer and coworkers modification to B97: B971 [482]. Wilson, Bradley and Tozer's modification to B97: B972 [488]. The 1997 hybrid functional of Perdew, Burke and Ernzerhof [472]. The keyword is PBE1PBE. This functional uses 25% exchange and 75% correlation weighting. Half-and-half Functionals, which implement the following functionals: BHandH: 0.5*E HF X + 0.5*E LSDA LYP X + E C BHandHLYP: 0.5*E HF X + 0.5*E LSDA X + 0.5*ΔE Becke88 LYP X + E C
4 ACCURACY CONSIDERATIONS A DFT calculation adds an additional step to each major phase of a Hartree-Fock calculation. This step is a numerical integration of the functional (or various derivatives of the functional). Thus in addition to the sources of numerical error in Hartree-Fock calculations (integral accuracy, SCF convergence, CPHF convergence), the accuracy of DFT calculations also depends on number of points used in the numerical integration. The "fine" integration grid (corresponding to Integral=FineGrid) is the default in Gaussian 03. This grid greatly enhances calculation accuracy at minimal additional cost. We do not recommend using any smaller grid in production DFT calculations. Note also that it is important to use the same grid for all calculations where you intend to compare energies (e.g., computing energy differences, heats of formation, and so on). Larger grids are available when needed (e.g. tight optimization of certain kinds of systems). An alternate grid may be selected by including Integral=(Grid=N) in the route section (see the discussion of the Integral keyword for details). 2. Frequencies This calculation type keyword computes force constants and the resulting vibrational frequencies. Intensities are also computed. By default, the force constants are determined analytically if possible (for RHF, UHF, MP2, CIS, all DFT methods, and CASSCF), by single numerical differentiation for methods for which only first derivatives are available (MP3, MP4(SDQ), CID, CISD, CCD, QCISD, and all semi-empirical methods), and by double numerical differentiation for those methods for which only energies are available. Vibrational frequencies are computed by determining the second derivatives of the energy with respect to the Cartesian nuclear coordinates and then transforming to massweighted coordinates. This transformation is only valid at a stationary point! Thus, it is meaningless to compute frequencies at any geometry other than a stationary point for the method used for frequency determination. The recommended practice is to compute frequencies following a previous geometry optimization using the same method. This may be accomplished automatically by specifying both Opt and Freq within the route section for a job.
5 Specifying #P in the route section produces some additional output for frequency calculations. Of most importance are the polarizability and hyperpolarizability tensors (they still may be found in the archive entry in normal print-level jobs). They are presented in lower triangular and lower tetrahedral order, respectively (i.e., α XX,α XY, α YY, α XZ, α YZ,α ZZ and β XXX, β XXY, β XYY, β YYY, β XXZ, β XYZ, β YYZ, β XZZ, β YZZ, β ZZZ ), in the standard orientation: Dipole = D D D-01 Polarizability= D D D D D D+00 HyperPolar = D D D D D D D D D D+01 #P also produces a bar-graph of the simulated spectra for small cases. 3. Vibrations in GaussView Displaying Vibrational Modes and Spectra The Results=>Vibrations menu item is used to access various spectra results (except NMR). It allows calculated vibrational data to be displayed as dynamic screen motions, based on information from a frequency calculation. This menu item displays the Display Vibrations window, as illustrated in Figure 64. The Display Vibration dialog box shows which vibration is currently selected for dynamic display. You can start this display by selecting the Start button, and halt it by clicking on the Stop button. The molecule will begin a cyclical displacement showing the motions corresponding to the vibration selected. You can select other modes by selecting the desired mode on the scrolling list in the Display Vibrations dialog. You can select a new mode without halting the previous one. You can also rotate and move the vibrating molecule, using the normal mouse buttons. Structure and frequencies of F 3 - Method R Sym.stretch Bend Asymm.stretch LSDA MP B3LYP Exp 440 +/ / /-20
6 MP2: %chk=c:\documents and Settings\dmitry\My Documents\f3mp2.chk %mem=6mw %nproc=1 # opt freq rmp2/lanl2dz geom=connectivity F3- Mp2-1 1 F F 1 B1 F 1 B2 2 A1 B B A B3LYP: %chk=c:\documents and Settings\dmitry\My Documents\f3b3lyp.chk %mem=6mw %nproc=1 # opt freq rb3lyp geom=connectivity d95v+(d) F3- B3LYP -1 1 F F 1 B1 F 1 B2 2 A1 B B A
7 Method Scale Frequenc Factor ZPE/Therm HF/3-21G l HF/6-31G(d) MP2(Full)/ MP2(FC)/ SVWN/6-31G(d) BLYP/6-31G(d) B3LYP/6-31G(d) Computed values of the intensities should not be taken too literally. However, tit j relative values of the intensities for each frequency may be reliably compared. Scaling Frequencies and Zero-Point Energies Frequencies computed with methods other than Hartree-Fock are also scaled to similarly eliminate known systematic errors in calculated frequencies. The followng table lists the recommended scale factors for frequencies and for zero-point energies and for use in computing thermal energy corrections (the latter two items are discussed later in this chapter), for several important calculation types. As the table indicates, the optimal scaling factors for the frequencies themselves and for the zero-point energies and for use in computing thermal energy corrections are slightly different. However, it is also common practice to use the same factor for both of them ( in the case of Hartree-Fock). For example, the G2 high accuracy energy method scales computed HF/6-31G(d) zero-point energy corrections by (see Chapter 7). You should be aware that the optimal scaling factors vary by basis set. For example, Bauschlicher and Partridge computed the B3LYP/6-311+G(3df,2p) ZPE/thermal energy correction scaling factor to be * Additional scaling factors have also been computed by Wong and by Scott and Radom. Consult the references for detailed discussions of these issues. Most of the scale factors in this table are from the recent paper of Wong. The HF/6-31G(d) and MP2(Full) scale factors are the traditional ones computed by Pople and coworkers and cited by Wong. Note that the MP2 scale factor used in this book is the one for MP2(Full) even though our jobs are run using the (default) frozen core approximation. Scott and Radom computed the MP2(FC) and HF/3-21G entries in the table, but this work came to our attention only just as this book was going to press. Their value is for the 6-31G(d) basis set. Note that published scale factors often vary slightly from one another due primarily to differences in the molecule sets used to compute them.
8
Advanced Quantum Chemistry III: Part 3. Haruyuki Nakano. Kyushu University
 Advanced Quantum Chemistry III: Part 3 Haruyuki Nakano Kyushu University 2013 Winter Term 1. Hartree-Fock theory Density Functional Theory 2. Hohenberg-Kohn theorem 3. Kohn-Sham method 4. Exchange-correlation
Advanced Quantum Chemistry III: Part 3 Haruyuki Nakano Kyushu University 2013 Winter Term 1. Hartree-Fock theory Density Functional Theory 2. Hohenberg-Kohn theorem 3. Kohn-Sham method 4. Exchange-correlation
Density Func,onal Theory (Chapter 6, Jensen)
 Chem 580: DFT Density Func,onal Theory (Chapter 6, Jensen) Hohenberg- Kohn Theorem (Phys. Rev., 136,B864 (1964)): For molecules with a non degenerate ground state, the ground state molecular energy and
Chem 580: DFT Density Func,onal Theory (Chapter 6, Jensen) Hohenberg- Kohn Theorem (Phys. Rev., 136,B864 (1964)): For molecules with a non degenerate ground state, the ground state molecular energy and
NWChem: Hartree-Fock, Density Functional Theory, Time-Dependent Density Functional Theory
 NWChem: Hartree-Fock, Density Functional Theory, Time-Depent Density Functional Theory Hartree-Fock! Functionality! Input! Wavefunctions! Initial MO vectors! Direct and semidirect algorithms! Convergence,
NWChem: Hartree-Fock, Density Functional Theory, Time-Depent Density Functional Theory Hartree-Fock! Functionality! Input! Wavefunctions! Initial MO vectors! Direct and semidirect algorithms! Convergence,
Appendix D Simulating Spectroscopic Bands Using Gaussian and PGopher
 429 Appendix D Simulating Spectroscopic Bands Using Gaussian and PGopher This appendix contains methods for using Gaussian 09 121 and PGopher 120 to simulate vibrational and electronic bands of molecules.
429 Appendix D Simulating Spectroscopic Bands Using Gaussian and PGopher This appendix contains methods for using Gaussian 09 121 and PGopher 120 to simulate vibrational and electronic bands of molecules.
DFT calculations of NMR indirect spin spin coupling constants
 DFT calculations of NMR indirect spin spin coupling constants Dalton program system Program capabilities Density functional theory Kohn Sham theory LDA, GGA and hybrid theories Indirect NMR spin spin coupling
DFT calculations of NMR indirect spin spin coupling constants Dalton program system Program capabilities Density functional theory Kohn Sham theory LDA, GGA and hybrid theories Indirect NMR spin spin coupling
NMR and IR spectra & vibrational analysis
 Lab 5: NMR and IR spectra & vibrational analysis A brief theoretical background 1 Some of the available chemical quantum methods for calculating NMR chemical shifts are based on the Hartree-Fock self-consistent
Lab 5: NMR and IR spectra & vibrational analysis A brief theoretical background 1 Some of the available chemical quantum methods for calculating NMR chemical shifts are based on the Hartree-Fock self-consistent
Computational Methods. Chem 561
 Computational Methods Chem 561 Lecture Outline 1. Ab initio methods a) HF SCF b) Post-HF methods 2. Density Functional Theory 3. Semiempirical methods 4. Molecular Mechanics Computational Chemistry " Computational
Computational Methods Chem 561 Lecture Outline 1. Ab initio methods a) HF SCF b) Post-HF methods 2. Density Functional Theory 3. Semiempirical methods 4. Molecular Mechanics Computational Chemistry " Computational
Note that if DFTTYP=NONE, an ab initio calculation will be performed, rather than density functional theory.
 Input Description $DFT 2-52 ========================================================== $DFT group (relevant if DFTTYP is chosen) (relevant if SCFTYP=RHF,UHF,ROHF) Note that if DFTTYP=NONE, an ab initio
Input Description $DFT 2-52 ========================================================== $DFT group (relevant if DFTTYP is chosen) (relevant if SCFTYP=RHF,UHF,ROHF) Note that if DFTTYP=NONE, an ab initio
Hong Zhou, Guang-Xiang Liu,* Xiao-Feng Wang and Yan Wang * Supporting Information
 Three cobalt(ii) coordination polymers based on V-shaped aromatic polycarboxylates and rigid bis(imidazole) ligand: Syntheses, crystal structures, physical properties and theoretical studies Hong Zhou,
Three cobalt(ii) coordination polymers based on V-shaped aromatic polycarboxylates and rigid bis(imidazole) ligand: Syntheses, crystal structures, physical properties and theoretical studies Hong Zhou,
Molecular Vibrations C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology
 Molecular Vibrations C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology Why Estimate Molecular Vibrations? Simulation of vibrational spectrum (identification of molecules)
Molecular Vibrations C. David Sherrill School of Chemistry and Biochemistry Georgia Institute of Technology Why Estimate Molecular Vibrations? Simulation of vibrational spectrum (identification of molecules)
Density functional studies of molecular polarizabilities. Part 3; ethene, buta-1,3-diene and hexa-1,3,5-triene
 ELECTRONIC JOURNAL OF THEORETICAL CHEMISTRY, VOL. 2, 315 324 (1997) Density functional studies of molecular polarizabilities. Part 3; ethene, buta-1,3-diene and hexa-1,3,5-triene ALAN HINCHLIFFE 1 AND
ELECTRONIC JOURNAL OF THEORETICAL CHEMISTRY, VOL. 2, 315 324 (1997) Density functional studies of molecular polarizabilities. Part 3; ethene, buta-1,3-diene and hexa-1,3,5-triene ALAN HINCHLIFFE 1 AND
Transition states and reaction paths
 Transition states and reaction paths Lab 4 Theoretical background Transition state A transition structure is the molecular configuration that separates reactants and products. In a system with a single
Transition states and reaction paths Lab 4 Theoretical background Transition state A transition structure is the molecular configuration that separates reactants and products. In a system with a single
Introduction to the OCTOPUS code
 Introduction to the OCTOPUS code Esa Räsänen Nanoscience Center, University of Jyväskylä, Finland www.tddft.org Brief history Origins in the fixed nucleus code of Bertsch and Yabana [1] and in the real
Introduction to the OCTOPUS code Esa Räsänen Nanoscience Center, University of Jyväskylä, Finland www.tddft.org Brief history Origins in the fixed nucleus code of Bertsch and Yabana [1] and in the real
MO Calculation for a Diatomic Molecule. /4 0 ) i=1 j>i (1/r ij )
 MO Calculation for a Diatomic Molecule Introduction The properties of any molecular system can in principle be found by looking at the solutions to the corresponding time independent Schrodinger equation
MO Calculation for a Diatomic Molecule Introduction The properties of any molecular system can in principle be found by looking at the solutions to the corresponding time independent Schrodinger equation
Short Course on Density Functional Theory and Applications III. Implementations
 Short Course on Density Functional Theory and Applications III. Implementations Samuel B. Trickey Sept. 2008 Quantum Theory Project Dept. of Physics and Dept. of Chemistry trickey@qtp.ufl.edu KS E xc and
Short Course on Density Functional Theory and Applications III. Implementations Samuel B. Trickey Sept. 2008 Quantum Theory Project Dept. of Physics and Dept. of Chemistry trickey@qtp.ufl.edu KS E xc and
What correlation eœects are covered by density functional theory?
 MOLECULAR PHYSICS, 2000, VOL. 98, NO. 20, 1639 ± 1658 What correlation eœects are covered by density functional theory? YUAN HE, JU È RGEN GRA È FENSTEIN, ELFI KRAKA and DIETER CREMER* Department of Theoretical
MOLECULAR PHYSICS, 2000, VOL. 98, NO. 20, 1639 ± 1658 What correlation eœects are covered by density functional theory? YUAN HE, JU È RGEN GRA È FENSTEIN, ELFI KRAKA and DIETER CREMER* Department of Theoretical
IFM Chemistry Computational Chemistry 2010, 7.5 hp LAB2. Computer laboratory exercise 1 (LAB2): Quantum chemical calculations
 Computer laboratory exercise 1 (LAB2): Quantum chemical calculations Introduction: The objective of the second computer laboratory exercise is to get acquainted with a program for performing quantum chemical
Computer laboratory exercise 1 (LAB2): Quantum chemical calculations Introduction: The objective of the second computer laboratory exercise is to get acquainted with a program for performing quantum chemical
THE PERFORMANCE OF DENSITY FUNCTIONALS WITH RESPECT TO THE CORRELATION CONSISTENT BASIS SETS. Xuelin Wang, B.S., M.S
 THE PERFORMANCE OF DENSITY FUNCTIONALS WITH RESPECT TO THE CORRELATION CONSISTENT BASIS SETS Xuelin Wang, B.S., M.S Dissertation Prepared for the Degree of DOCTOR OF PHILOSOPHY UNIVERSITY OF NORTH TEXAS
THE PERFORMANCE OF DENSITY FUNCTIONALS WITH RESPECT TO THE CORRELATION CONSISTENT BASIS SETS Xuelin Wang, B.S., M.S Dissertation Prepared for the Degree of DOCTOR OF PHILOSOPHY UNIVERSITY OF NORTH TEXAS
Electron Correlation
 Electron Correlation Levels of QM Theory HΨ=EΨ Born-Oppenheimer approximation Nuclear equation: H n Ψ n =E n Ψ n Electronic equation: H e Ψ e =E e Ψ e Single determinant SCF Semi-empirical methods Correlation
Electron Correlation Levels of QM Theory HΨ=EΨ Born-Oppenheimer approximation Nuclear equation: H n Ψ n =E n Ψ n Electronic equation: H e Ψ e =E e Ψ e Single determinant SCF Semi-empirical methods Correlation
The role of the basis set: Assessing density functional theory
 JOURNAL OF CHEMICAL PHYSICS VOLUME 119, NUMBER 6 8 AUGUST 2003 The role of the basis set: Assessing density functional theory A. Daniel Boese and Jan M. L. Martin Department of Organic Chemistry, Weizmann
JOURNAL OF CHEMICAL PHYSICS VOLUME 119, NUMBER 6 8 AUGUST 2003 The role of the basis set: Assessing density functional theory A. Daniel Boese and Jan M. L. Martin Department of Organic Chemistry, Weizmann
Introduction to Computational Chemistry Computational (chemistry education) and/or. (Computational chemistry) education
 Introduction to Computational Chemistry Computational (chemistry education) and/or (Computational chemistry) education First one: Use computational tools to help increase student understanding of material
Introduction to Computational Chemistry Computational (chemistry education) and/or (Computational chemistry) education First one: Use computational tools to help increase student understanding of material
Oslo node. Highly accurate calculations benchmarking and extrapolations
 Oslo node Highly accurate calculations benchmarking and extrapolations Torgeir Ruden, with A. Halkier, P. Jørgensen, J. Olsen, W. Klopper, J. Gauss, P. Taylor Explicitly correlated methods Pål Dahle, collaboration
Oslo node Highly accurate calculations benchmarking and extrapolations Torgeir Ruden, with A. Halkier, P. Jørgensen, J. Olsen, W. Klopper, J. Gauss, P. Taylor Explicitly correlated methods Pål Dahle, collaboration
Advanced Electronic Structure Theory Density functional theory. Dr Fred Manby
 Advanced Electronic Structure Theory Density functional theory Dr Fred Manby fred.manby@bris.ac.uk http://www.chm.bris.ac.uk/pt/manby/ 6 Strengths of DFT DFT is one of many theories used by (computational)
Advanced Electronic Structure Theory Density functional theory Dr Fred Manby fred.manby@bris.ac.uk http://www.chm.bris.ac.uk/pt/manby/ 6 Strengths of DFT DFT is one of many theories used by (computational)
计算物理作业二. Excercise 1: Illustration of the convergence of the dissociation energy for H 2 toward HF limit.
 计算物理作业二 Excercise 1: Illustration of the convergence of the dissociation energy for H 2 toward HF limit. In this exercise, basis indicates one of the following basis sets: STO-3G, cc-pvdz, cc-pvtz, cc-pvqz
计算物理作业二 Excercise 1: Illustration of the convergence of the dissociation energy for H 2 toward HF limit. In this exercise, basis indicates one of the following basis sets: STO-3G, cc-pvdz, cc-pvtz, cc-pvqz
ASSESSMENT OF DFT METHODS FOR SOLIDS
 MSSC2009 - Ab Initio Modeling in Solid State Chemistry ASSESSMENT OF DFT METHODS FOR SOLIDS Raffaella Demichelis Università di Torino Dipartimento di Chimica IFM 1 MSSC2009 - September, 10 th 2009 Table
MSSC2009 - Ab Initio Modeling in Solid State Chemistry ASSESSMENT OF DFT METHODS FOR SOLIDS Raffaella Demichelis Università di Torino Dipartimento di Chimica IFM 1 MSSC2009 - September, 10 th 2009 Table
Using Web-Based Computations in Organic Chemistry
 10/30/2017 1 Using Web-Based Computations in Organic Chemistry John Keller UAF Department of Chemistry & Biochemistry The UAF WebMO site Practical aspects of computational chemistry theory and nomenclature
10/30/2017 1 Using Web-Based Computations in Organic Chemistry John Keller UAF Department of Chemistry & Biochemistry The UAF WebMO site Practical aspects of computational chemistry theory and nomenclature
2x (x 2 + y 2 + 1) 2 2y. (x 2 + y 2 + 1) 4. 4xy. (1, 1)(x 1) + (1, 1)(y + 1) (1, 1)(x 1)(y + 1) 81 x y y + 7.
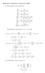 Homework 8 Solutions, November 007. (1 We calculate some derivatives: f x = f y = x (x + y + 1 y (x + y + 1 x = (x + y + 1 4x (x + y + 1 4 y = (x + y + 1 4y (x + y + 1 4 x y = 4xy (x + y + 1 4 Substituting
Homework 8 Solutions, November 007. (1 We calculate some derivatives: f x = f y = x (x + y + 1 y (x + y + 1 x = (x + y + 1 4x (x + y + 1 4 y = (x + y + 1 4y (x + y + 1 4 x y = 4xy (x + y + 1 4 Substituting
Supporting information for: First hyperpolarizability of collagen using the. point dipole approximation
 Supporting information for: First hyperpolarizability of collagen using the point dipole approximation Ignat Harczuk, Olav Vahtras, and Hans Ågren KTH Royal Institute of Technology, School of Biotechnology,
Supporting information for: First hyperpolarizability of collagen using the point dipole approximation Ignat Harczuk, Olav Vahtras, and Hans Ågren KTH Royal Institute of Technology, School of Biotechnology,
CLIMBING THE LADDER OF DENSITY FUNCTIONAL APPROXIMATIONS JOHN P. PERDEW DEPARTMENT OF PHYSICS TEMPLE UNIVERSITY PHILADELPHIA, PA 19122
 CLIMBING THE LADDER OF DENSITY FUNCTIONAL APPROXIMATIONS JOHN P. PERDEW DEPARTMENT OF PHYSICS TEMPLE UNIVERSITY PHILADELPHIA, PA 191 THANKS TO MANY COLLABORATORS, INCLUDING SY VOSKO DAVID LANGRETH ALEX
CLIMBING THE LADDER OF DENSITY FUNCTIONAL APPROXIMATIONS JOHN P. PERDEW DEPARTMENT OF PHYSICS TEMPLE UNIVERSITY PHILADELPHIA, PA 191 THANKS TO MANY COLLABORATORS, INCLUDING SY VOSKO DAVID LANGRETH ALEX
The Role of the Basis Set: Assessing Density Functional Theory. Abstract
 The Role of the Basis Set: Assessing Density Functional Theory A. Daniel Boese and Jan M. L. Martin Department of Organic Chemistry, Weizmann Institute of Science, IL-76100 Reḥovot, Israel Nicholas C.
The Role of the Basis Set: Assessing Density Functional Theory A. Daniel Boese and Jan M. L. Martin Department of Organic Chemistry, Weizmann Institute of Science, IL-76100 Reḥovot, Israel Nicholas C.
Institut Néel Institut Laue Langevin. Introduction to electronic structure calculations
 Institut Néel Institut Laue Langevin Introduction to electronic structure calculations 1 Institut Néel - 25 rue des Martyrs - Grenoble - France 2 Institut Laue Langevin - 71 avenue des Martyrs - Grenoble
Institut Néel Institut Laue Langevin Introduction to electronic structure calculations 1 Institut Néel - 25 rue des Martyrs - Grenoble - France 2 Institut Laue Langevin - 71 avenue des Martyrs - Grenoble
Electronic structure theory: Fundamentals to frontiers. 2. Density functional theory
 Electronic structure theory: Fundamentals to frontiers. 2. Density functional theory MARTIN HEAD-GORDON, Department of Chemistry, University of California, and Chemical Sciences Division, Lawrence Berkeley
Electronic structure theory: Fundamentals to frontiers. 2. Density functional theory MARTIN HEAD-GORDON, Department of Chemistry, University of California, and Chemical Sciences Division, Lawrence Berkeley
Publicat ion I. Publication I
 Publicat ion I I Publication I K. J. Jalkanen, V. W. Jurgensen and I. M. Degtyarenko, Linear response properties required to simulate vibrational spectra of biomolecules in various media: (R)-Phenyloxirane
Publicat ion I I Publication I K. J. Jalkanen, V. W. Jurgensen and I. M. Degtyarenko, Linear response properties required to simulate vibrational spectra of biomolecules in various media: (R)-Phenyloxirane
Comparison of DFT Methods for Molecular Orbital Eigenvalue Calculations
 1554 J. Phys. Chem. A 2007, 111, 1554-1561 Comparison of DFT Methods for Molecular Orbital Eigenvalue Calculations Gang Zhang and Charles B. Musgrave* Department of Chemical Engineering, Stanford UniVersity,
1554 J. Phys. Chem. A 2007, 111, 1554-1561 Comparison of DFT Methods for Molecular Orbital Eigenvalue Calculations Gang Zhang and Charles B. Musgrave* Department of Chemical Engineering, Stanford UniVersity,
Supporting Information for. Predicting the Stability of Fullerene Allotropes Throughout the Periodic Table MA 02139
 Supporting Information for Predicting the Stability of Fullerene Allotropes Throughout the Periodic Table Qing Zhao 1, 2, Stanley S. H. Ng 1, and Heather J. Kulik 1, * 1 Department of Chemical Engineering,
Supporting Information for Predicting the Stability of Fullerene Allotropes Throughout the Periodic Table Qing Zhao 1, 2, Stanley S. H. Ng 1, and Heather J. Kulik 1, * 1 Department of Chemical Engineering,
9.6 Electronic Excitation Energies and the Singlet/Triplet Splitting in Carbenes
 9 Relative Energies and Thermochemistry Table 9-13. Compilation of mean absolute deviations (maximum deviation in parentheses) for electron affinities [ev] of small main group molecules from different
9 Relative Energies and Thermochemistry Table 9-13. Compilation of mean absolute deviations (maximum deviation in parentheses) for electron affinities [ev] of small main group molecules from different
5 Density Functional Theory
 Computational Chemistry: A Practical Guide for Applying Techniques to Real-World Problems. David C. Young Copyright ( 2001 John Wiley & Sons, Inc. ISBNs: 0-471-33368-9 (Hardback); 0-471-22065-5 (Electronic)
Computational Chemistry: A Practical Guide for Applying Techniques to Real-World Problems. David C. Young Copyright ( 2001 John Wiley & Sons, Inc. ISBNs: 0-471-33368-9 (Hardback); 0-471-22065-5 (Electronic)
Rapid and precise thermochemical calculations by quantum chemical methods
 Rapid and precise thermochemical calculations by quantum chemical methods Ph.D. thesis By: Adrienn Ruzsinszky Supervisor: Dr. Gábor Csonka Budapest University of Technology and Economics Department of
Rapid and precise thermochemical calculations by quantum chemical methods Ph.D. thesis By: Adrienn Ruzsinszky Supervisor: Dr. Gábor Csonka Budapest University of Technology and Economics Department of
Orbital dependent correlation potentials in ab initio density functional theory
 Orbital dependent correlation potentials in ab initio density functional theory noniterative - one step - calculations Ireneusz Grabowski Institute of Physics Nicolaus Copernicus University Toruń, Poland
Orbital dependent correlation potentials in ab initio density functional theory noniterative - one step - calculations Ireneusz Grabowski Institute of Physics Nicolaus Copernicus University Toruń, Poland
CHAPTER-IV. FT-IR and FT-Raman investigation on m-xylol using ab-initio HF and DFT calculations
 4.1. Introduction CHAPTER-IV FT-IR and FT-Raman investigation on m-xylol using ab-initio HF and DFT calculations m-xylol is a material for thermally stable aramid fibers or alkyd resins [1]. In recent
4.1. Introduction CHAPTER-IV FT-IR and FT-Raman investigation on m-xylol using ab-initio HF and DFT calculations m-xylol is a material for thermally stable aramid fibers or alkyd resins [1]. In recent
MU, Chemistry Computational Chemistry Spring Semester 2009 Dr. Rainer Glaser
 Name: MU, Chemistry8 330 ComputationalChemistry SpringSemester2009 Dr.RainerGlaser Third1 HourExamination Friday,April24,2009,11:00 11:50am ANSWERKEY Question1.ElectronCorrelation 20 Question2.PerturbationTheory
Name: MU, Chemistry8 330 ComputationalChemistry SpringSemester2009 Dr.RainerGlaser Third1 HourExamination Friday,April24,2009,11:00 11:50am ANSWERKEY Question1.ElectronCorrelation 20 Question2.PerturbationTheory
One-Electron Hamiltonians
 One-Electron Hamiltonians Hartree-Fock and Density Func7onal Theory Christopher J. Cramer @ChemProfCramer 2017 MSSC, July 10, 2017 REVIEW A One-Slide Summary of Quantum Mechanics Fundamental Postulate:
One-Electron Hamiltonians Hartree-Fock and Density Func7onal Theory Christopher J. Cramer @ChemProfCramer 2017 MSSC, July 10, 2017 REVIEW A One-Slide Summary of Quantum Mechanics Fundamental Postulate:
QUANTUM CHEMISTRY PROJECT 3: PARTS B AND C
 Chemistry 460 Fall 2017 Dr. Jean M. Standard November 6, 2017 QUANTUM CHEMISTRY PROJECT 3: PARTS B AND C PART B: POTENTIAL CURVE, SPECTROSCOPIC CONSTANTS, AND DISSOCIATION ENERGY OF DIATOMIC HYDROGEN (20
Chemistry 460 Fall 2017 Dr. Jean M. Standard November 6, 2017 QUANTUM CHEMISTRY PROJECT 3: PARTS B AND C PART B: POTENTIAL CURVE, SPECTROSCOPIC CONSTANTS, AND DISSOCIATION ENERGY OF DIATOMIC HYDROGEN (20
Introduction to Computational Quantum Chemistry: Theory
 Introduction to Computational Quantum Chemistry: Theory Dr Andrew Gilbert Rm 118, Craig Building, RSC 3108 Course Lectures 2007 Introduction Hartree Fock Theory Configuration Interaction Lectures 1 Introduction
Introduction to Computational Quantum Chemistry: Theory Dr Andrew Gilbert Rm 118, Craig Building, RSC 3108 Course Lectures 2007 Introduction Hartree Fock Theory Configuration Interaction Lectures 1 Introduction
Density Functional Theory
 Chemistry 380.37 Fall 2015 Dr. Jean M. Standard October 28, 2015 Density Functional Theory What is a Functional? A functional is a general mathematical quantity that represents a rule to convert a function
Chemistry 380.37 Fall 2015 Dr. Jean M. Standard October 28, 2015 Density Functional Theory What is a Functional? A functional is a general mathematical quantity that represents a rule to convert a function
Calculating Bond Enthalpies of the Hydrides
 Proposed Exercise for the General Chemistry Section of the Teaching with Cache Workbook: Calculating Bond Enthalpies of the Hydrides Contributed by James Foresman, Rachel Fogle, and Jeremy Beck, York College
Proposed Exercise for the General Chemistry Section of the Teaching with Cache Workbook: Calculating Bond Enthalpies of the Hydrides Contributed by James Foresman, Rachel Fogle, and Jeremy Beck, York College
Density Functional Theory - II part
 Density Functional Theory - II part antonino.polimeno@unipd.it Overview From theory to practice Implementation Functionals Local functionals Gradient Others From theory to practice From now on, if not
Density Functional Theory - II part antonino.polimeno@unipd.it Overview From theory to practice Implementation Functionals Local functionals Gradient Others From theory to practice From now on, if not
Calculating NMR Chemical Shifts for beta-ionone O
 Calculating NMR Chemical Shifts for beta-ionone O Molecular orbital calculations can be used to get good estimates for chemical shifts. In this exercise we will calculate the chemical shifts for beta-ionone.
Calculating NMR Chemical Shifts for beta-ionone O Molecular orbital calculations can be used to get good estimates for chemical shifts. In this exercise we will calculate the chemical shifts for beta-ionone.
INVITED PAPER Density functional theory: coverage of dynamic and non-dynamic electron correlation e ects
 MOLECULAR PHYSICS, 2001, VOL. 99, NO. 23, 1899± 1940 INVITED PAPER Density functional theory: coverage of dynamic and non-dynamic electron correlation e ects DIETER CREMER* Department of Theoretical Chemistry,
MOLECULAR PHYSICS, 2001, VOL. 99, NO. 23, 1899± 1940 INVITED PAPER Density functional theory: coverage of dynamic and non-dynamic electron correlation e ects DIETER CREMER* Department of Theoretical Chemistry,
( ) R kj. = y k y j. y A ( ) z A. y a. z a. Derivatives of the second order electrostatic tensor with respect to the translation of ( ) δ yβ.
 Supporting information Derivatives of R with respect to the translation of fragment along the y and z axis: y = y k y j (S1) z ( = z z k j) (S2) Derivatives of S with respect to the translation of fragment
Supporting information Derivatives of R with respect to the translation of fragment along the y and z axis: y = y k y j (S1) z ( = z z k j) (S2) Derivatives of S with respect to the translation of fragment
Walter Kohn was awarded with the Nobel Prize in Chemistry in 1998 for his development of the density functional theory.
 Walter Kohn was awarded with the Nobel Prize in Chemistry in 1998 for his development of the density functional theory. Walter Kohn receiving his Nobel Prize from His Majesty the King at the Stockholm
Walter Kohn was awarded with the Nobel Prize in Chemistry in 1998 for his development of the density functional theory. Walter Kohn receiving his Nobel Prize from His Majesty the King at the Stockholm
Introduction to Hartree-Fock calculations in Spartan
 EE5 in 2008 Hannes Jónsson Introduction to Hartree-Fock calculations in Spartan In this exercise, you will get to use state of the art software for carrying out calculations of wavefunctions for molecues,
EE5 in 2008 Hannes Jónsson Introduction to Hartree-Fock calculations in Spartan In this exercise, you will get to use state of the art software for carrying out calculations of wavefunctions for molecues,
Scaled Density Functional Theory Correlation Functionals
 J. Phys. Chem. A: Robert E. Wyatt Festschrift jp0728353 Submitted April 11, 2007; Revised May 21, 2007 Scaled Density Functional Theory Correlation Functionals Mohammed M. Ghouri, a Saurabh Singh, a and
J. Phys. Chem. A: Robert E. Wyatt Festschrift jp0728353 Submitted April 11, 2007; Revised May 21, 2007 Scaled Density Functional Theory Correlation Functionals Mohammed M. Ghouri, a Saurabh Singh, a and
Teoría del Funcional de la Densidad (Density Functional Theory)
 Teoría del Funcional de la Densidad (Density Functional Theory) Motivation: limitations of the standard approach based on the wave function. The electronic density n(r) as the key variable: Functionals
Teoría del Funcional de la Densidad (Density Functional Theory) Motivation: limitations of the standard approach based on the wave function. The electronic density n(r) as the key variable: Functionals
QUANTUM CHEMISTRY PROJECT 3: ATOMIC AND MOLECULAR STRUCTURE
 Chemistry 460 Fall 2017 Dr. Jean M. Standard November 1, 2017 QUANTUM CHEMISTRY PROJECT 3: ATOMIC AND MOLECULAR STRUCTURE OUTLINE In this project, you will carry out quantum mechanical calculations of
Chemistry 460 Fall 2017 Dr. Jean M. Standard November 1, 2017 QUANTUM CHEMISTRY PROJECT 3: ATOMIC AND MOLECULAR STRUCTURE OUTLINE In this project, you will carry out quantum mechanical calculations of
Project 2. Chemistry of Transient Species in Planetary Atmospheres: Exploring the Potential Energy Surfaces of CH 2 S
 Chemistry 362 Spring 2018 Dr. Jean M. Standard March 21, 2018 Project 2. Chemistry of Transient Species in Planetary Atmospheres: Exploring the Potential Energy Surfaces of CH 2 S In this project, you
Chemistry 362 Spring 2018 Dr. Jean M. Standard March 21, 2018 Project 2. Chemistry of Transient Species in Planetary Atmospheres: Exploring the Potential Energy Surfaces of CH 2 S In this project, you
Chemistry 4560/5560 Molecular Modeling Fall 2014
 Final Exam Name:. User s guide: 1. Read questions carefully and make sure you understand them before answering (if not, ask). 2. Answer only the question that is asked, not a different question. 3. Unless
Final Exam Name:. User s guide: 1. Read questions carefully and make sure you understand them before answering (if not, ask). 2. Answer only the question that is asked, not a different question. 3. Unless
QUANTUM CHEMISTRY WITH GAUSSIAN : A VERY BRIEF INTRODUCTION (PART 2)
 QUANTUM CHEMISTRY WITH GAUSSIAN : A VERY BRIEF INTRODUCTION (PART 2) TARAS V. POGORELOV AND MIKE HALLOCK SCHOOL OF CHEMICAL SCIENCES, UIUC This tutorial continues introduction to Gaussian [2]. Here we
QUANTUM CHEMISTRY WITH GAUSSIAN : A VERY BRIEF INTRODUCTION (PART 2) TARAS V. POGORELOV AND MIKE HALLOCK SCHOOL OF CHEMICAL SCIENCES, UIUC This tutorial continues introduction to Gaussian [2]. Here we
Lec20 Fri 3mar17
 564-17 Lec20 Fri 3mar17 [PDF]GAUSSIAN 09W TUTORIAL www.molcalx.com.cn/wp-content/uploads/2015/01/gaussian09w_tutorial.pdf by A Tomberg - Cited by 8 - Related articles GAUSSIAN 09W TUTORIAL. AN INTRODUCTION
564-17 Lec20 Fri 3mar17 [PDF]GAUSSIAN 09W TUTORIAL www.molcalx.com.cn/wp-content/uploads/2015/01/gaussian09w_tutorial.pdf by A Tomberg - Cited by 8 - Related articles GAUSSIAN 09W TUTORIAL. AN INTRODUCTION
Basics of DFT. Kieron Burke and Lucas Wagner. Departments of Physics and of Chemistry, University of California, Irvine, CA 92697, USA
 Basics of DFT Kieron Burke and Lucas Wagner Departments of Physics and of Chemistry, University of California, Irvine, CA 92697, USA October 10-19th, 2012 Kieron (UC Irvine) Basics of DFT Lausanne12 1
Basics of DFT Kieron Burke and Lucas Wagner Departments of Physics and of Chemistry, University of California, Irvine, CA 92697, USA October 10-19th, 2012 Kieron (UC Irvine) Basics of DFT Lausanne12 1
1 Density functional theory (DFT)
 1 Density functional theory (DFT) 1.1 Introduction Density functional theory is an alternative to ab initio methods for solving the nonrelativistic, time-independent Schrödinger equation H Φ = E Φ. The
1 Density functional theory (DFT) 1.1 Introduction Density functional theory is an alternative to ab initio methods for solving the nonrelativistic, time-independent Schrödinger equation H Φ = E Φ. The
Conformational energy analysis
 Lab 3 Conformational energy analysis Objective This computational project deals with molecular conformations the spatial arrangement of atoms of molecules. Conformations are determined by energy, so the
Lab 3 Conformational energy analysis Objective This computational project deals with molecular conformations the spatial arrangement of atoms of molecules. Conformations are determined by energy, so the
AN INTRODUCTION TO QUANTUM CHEMISTRY. Mark S. Gordon Iowa State University
 AN INTRODUCTION TO QUANTUM CHEMISTRY Mark S. Gordon Iowa State University 1 OUTLINE Theoretical Background in Quantum Chemistry Overview of GAMESS Program Applications 2 QUANTUM CHEMISTRY In principle,
AN INTRODUCTION TO QUANTUM CHEMISTRY Mark S. Gordon Iowa State University 1 OUTLINE Theoretical Background in Quantum Chemistry Overview of GAMESS Program Applications 2 QUANTUM CHEMISTRY In principle,
ABC of ground-state DFT
 ABC of ground-state DFT Kieron Burke and Lucas Wagner Departments of Physics and of Chemistry, University of California, Irvine, CA 92697, USA January 5-9th, 2014 Kieron (UC Irvine) ABC of ground-state
ABC of ground-state DFT Kieron Burke and Lucas Wagner Departments of Physics and of Chemistry, University of California, Irvine, CA 92697, USA January 5-9th, 2014 Kieron (UC Irvine) ABC of ground-state
Vibrational Analysis in Gaussian
 Vibrational Analysis in Gaussian Joseph W. Ochterski, Ph.D. help@gaussian.com October 9, 1999 Minor corrections 14 June 018 Abstract One of the most commonly asked questions about Gaussian is What is the
Vibrational Analysis in Gaussian Joseph W. Ochterski, Ph.D. help@gaussian.com October 9, 1999 Minor corrections 14 June 018 Abstract One of the most commonly asked questions about Gaussian is What is the
Ab-initio Electronic Structure Calculations β and γ KNO 3 Energetic Materials
 ISSN 0974-9373 Vol. 15 No.3 (2011) Journal of International Academy of Physical Sciences pp. 337-344 Ab-initio Electronic Structure Calculations of α, β and γ KNO 3 Energetic Materials Pradeep Jain and
ISSN 0974-9373 Vol. 15 No.3 (2011) Journal of International Academy of Physical Sciences pp. 337-344 Ab-initio Electronic Structure Calculations of α, β and γ KNO 3 Energetic Materials Pradeep Jain and
Module 6 1. Density functional theory
 Module 6 1. Density functional theory Updated May 12, 2016 B A DDFT C K A bird s-eye view of density-functional theory Authors: Klaus Capelle G http://arxiv.org/abs/cond-mat/0211443 R https://trac.cc.jyu.fi/projects/toolbox/wiki/dft
Module 6 1. Density functional theory Updated May 12, 2016 B A DDFT C K A bird s-eye view of density-functional theory Authors: Klaus Capelle G http://arxiv.org/abs/cond-mat/0211443 R https://trac.cc.jyu.fi/projects/toolbox/wiki/dft
Gaussian: Basic Tutorial
 Input file: # hf sto-g pop=full Water - Single Point Energy 0 H.0 H.0 H 04.5 Route Section Start with # Contains the keywords Gaussian: Basic Tutorial Route Section Title Section Charge-Multiplicity Molecule
Input file: # hf sto-g pop=full Water - Single Point Energy 0 H.0 H.0 H 04.5 Route Section Start with # Contains the keywords Gaussian: Basic Tutorial Route Section Title Section Charge-Multiplicity Molecule
Density functional theory in the solid state
 Density functional theory in the solid state Ari P Seitsonen IMPMC, CNRS & Universités 6 et 7 Paris, IPGP Department of Applied Physics, Helsinki University of Technology Physikalisch-Chemisches Institut
Density functional theory in the solid state Ari P Seitsonen IMPMC, CNRS & Universités 6 et 7 Paris, IPGP Department of Applied Physics, Helsinki University of Technology Physikalisch-Chemisches Institut
Session 1. Introduction to Computational Chemistry. Computational (chemistry education) and/or (Computational chemistry) education
 Session 1 Introduction to Computational Chemistry 1 Introduction to Computational Chemistry Computational (chemistry education) and/or (Computational chemistry) education First one: Use computational tools
Session 1 Introduction to Computational Chemistry 1 Introduction to Computational Chemistry Computational (chemistry education) and/or (Computational chemistry) education First one: Use computational tools
Example: H 2 O (the car file)
 Example: H 2 O (the car file) As a practical example of DFT methods we calculate the energy and electronic properties of the water molecule. In order to carry out the DFT calculation you will need a set
Example: H 2 O (the car file) As a practical example of DFT methods we calculate the energy and electronic properties of the water molecule. In order to carry out the DFT calculation you will need a set
Theoretical study of electronic and atomic structures of (MnO)n
 Theoretical study of electronic and atomic structures of (MnO)n Hiori Kino, a Lucas K. Wagner b and Lubos Mitas c a National Institute for Materials Science, 1-2-1 Sengen, Tsukuba, Ibaraki 305-0047, Japan.
Theoretical study of electronic and atomic structures of (MnO)n Hiori Kino, a Lucas K. Wagner b and Lubos Mitas c a National Institute for Materials Science, 1-2-1 Sengen, Tsukuba, Ibaraki 305-0047, Japan.
Density-functional theory in quantum chemistry. Trygve Helgaker. From Quarks to the Nuclear Many-Body Problem
 1 Density-functional theory in quantum chemistry Trygve Helgaker Centre for Theoretical and Computational Chemistry, University of Oslo, Norway From Quarks to the Nuclear Many-Body Problem A conference
1 Density-functional theory in quantum chemistry Trygve Helgaker Centre for Theoretical and Computational Chemistry, University of Oslo, Norway From Quarks to the Nuclear Many-Body Problem A conference
Supporting Information
 Supporting Information Roles of Zeolite Confinement and Cu O Cu Angle on the Direct Conversion of Methane to Methanol by [Cu 2 (µ-o)] 2+ -exchanged AEI, CHA, AFX, and MFI Zeolites M. Haris Mahyuddin,,
Supporting Information Roles of Zeolite Confinement and Cu O Cu Angle on the Direct Conversion of Methane to Methanol by [Cu 2 (µ-o)] 2+ -exchanged AEI, CHA, AFX, and MFI Zeolites M. Haris Mahyuddin,,
Introduction to Computational Chemistry: Theory
 Introduction to Computational Chemistry: Theory Dr Andrew Gilbert Rm 118, Craig Building, RSC andrew.gilbert@anu.edu.au 3023 Course Lectures Introduction Hartree Fock Theory Basis Sets Lecture 1 1 Introduction
Introduction to Computational Chemistry: Theory Dr Andrew Gilbert Rm 118, Craig Building, RSC andrew.gilbert@anu.edu.au 3023 Course Lectures Introduction Hartree Fock Theory Basis Sets Lecture 1 1 Introduction
CHAPTER 8 REPORT ON HIGHER SHG EFFICIENCY IN BIS (CINNAMIC ACID): HEXAMINE COCRYSTAL
 CHAPTER 8 REPORT ON HIGHER SHG EFFICIENCY IN BIS (CINNAMIC ACID): HEXAMINE COCRYSTAL 8.1. Introduction In recent times higher Second Harmonic Generation (SHG) efficiency organic materials receive great
CHAPTER 8 REPORT ON HIGHER SHG EFFICIENCY IN BIS (CINNAMIC ACID): HEXAMINE COCRYSTAL 8.1. Introduction In recent times higher Second Harmonic Generation (SHG) efficiency organic materials receive great
Performance of B3PW91, PBE1PBE and OPBE Functionals in Comparison to B3LYP for 13C NMR Chemical Shift Calculations
 Journal of Science and Technology Ubon Ratchathani University : Special Issue November 17 Performance of B3PW91, PBE1PBE and OPBE Functionals in Comparison to B3LYP for 13C NMR Chemical Shift Calculations
Journal of Science and Technology Ubon Ratchathani University : Special Issue November 17 Performance of B3PW91, PBE1PBE and OPBE Functionals in Comparison to B3LYP for 13C NMR Chemical Shift Calculations
This is a very succinct primer intended as supplementary material for an undergraduate course in physical chemistry.
 1 Computational Chemistry (Quantum Chemistry) Primer This is a very succinct primer intended as supplementary material for an undergraduate course in physical chemistry. TABLE OF CONTENTS Methods...1 Basis
1 Computational Chemistry (Quantum Chemistry) Primer This is a very succinct primer intended as supplementary material for an undergraduate course in physical chemistry. TABLE OF CONTENTS Methods...1 Basis
June 12, 2004 Prepared for J. Phys. Chem. A
 June 12, 2004 Prepared for J. Phys. Chem. A Hybrid Meta Density Functional Theory Methods for Thermochemistry, Thermochemical Kinetics, and Noncovalent Interactions: The MPW1B95 and MPWB1K Models and Comparative
June 12, 2004 Prepared for J. Phys. Chem. A Hybrid Meta Density Functional Theory Methods for Thermochemistry, Thermochemical Kinetics, and Noncovalent Interactions: The MPW1B95 and MPWB1K Models and Comparative
HECToR CSE technical meeting, Oxford Parallel Algorithms for the Materials Modelling code CRYSTAL
 HECToR CSE technical meeting, Oxford 2009 Parallel Algorithms for the Materials Modelling code CRYSTAL Dr Stanko Tomi Computational Science & Engineering Department, STFC Daresbury Laboratory, UK Acknowledgements
HECToR CSE technical meeting, Oxford 2009 Parallel Algorithms for the Materials Modelling code CRYSTAL Dr Stanko Tomi Computational Science & Engineering Department, STFC Daresbury Laboratory, UK Acknowledgements
Finite-Temperature Hartree-Fock Exchange and Exchange- Correlation Free Energy Functionals. Travis Sjostrom. IPAM 2012 Workshop IV
 1 of 45 Finite-Temperature Hartree-Fock Exchange and Exchange- Correlation Free Energy Functionals Travis Sjostrom Quantum Theory Project Depts. of Physics and Chemistry IPAM 2012 Workshop IV 2012 2 of
1 of 45 Finite-Temperature Hartree-Fock Exchange and Exchange- Correlation Free Energy Functionals Travis Sjostrom Quantum Theory Project Depts. of Physics and Chemistry IPAM 2012 Workshop IV 2012 2 of
CHAPTER 5 FT INFRARED, FT RAMAN, VIBRATIONAL ASSIGNMENTS AND QUANTUM CHEMICAL STUDIES OF NICOTINIC ACID (NIASIN)
 CHAPTER 5 FT INFRARED, FT RAMAN, VIBRATIONAL ASSIGNMENTS AND QUANTUM CHEMICAL STUDIES OF NICOTINIC ACID (NIASIN) 5.1. INTRODUCTION Nicotinic acid widely known as Niacin are also known as vitamin B 3 and
CHAPTER 5 FT INFRARED, FT RAMAN, VIBRATIONAL ASSIGNMENTS AND QUANTUM CHEMICAL STUDIES OF NICOTINIC ACID (NIASIN) 5.1. INTRODUCTION Nicotinic acid widely known as Niacin are also known as vitamin B 3 and
Vibrations of Carbon Dioxide and Carbon Disulfide
 Vibrations of Carbon Dioxide and Carbon Disulfide Purpose Vibration frequencies of CO 2 and CS 2 will be measured by Raman and Infrared spectroscopy. The spectra show effects of normal mode symmetries
Vibrations of Carbon Dioxide and Carbon Disulfide Purpose Vibration frequencies of CO 2 and CS 2 will be measured by Raman and Infrared spectroscopy. The spectra show effects of normal mode symmetries
Quantum Chemical and Dynamical Tools for Solving Photochemical Problems
 2.165430 3.413060 3.889592 9 H 3.413060 2.165430 1.099610 2.165430 3.413060 10 H 3.889592 3.413060 2.165430 1.099610 2.165430 11 H 3.413060 3.889592 3.413060 2.165430 1.099610 12 H 2.165430 3.413060 3.889592
2.165430 3.413060 3.889592 9 H 3.413060 2.165430 1.099610 2.165430 3.413060 10 H 3.889592 3.413060 2.165430 1.099610 2.165430 11 H 3.413060 3.889592 3.413060 2.165430 1.099610 12 H 2.165430 3.413060 3.889592
Ab initio methods: The e-e cusp and DFT
 Ab initio methods: The e-e cusp and DFT Alston J. Misquitta Centre for Condensed Matter and Materials Physics Queen Mary, University of London March 20, 2012 Density-Functional Theory I Hohenberg & Kohn
Ab initio methods: The e-e cusp and DFT Alston J. Misquitta Centre for Condensed Matter and Materials Physics Queen Mary, University of London March 20, 2012 Density-Functional Theory I Hohenberg & Kohn
Selective formation of a zwitterion adduct and. N-benzyl cyclic guanidine under dry and wet
 Supporting Information for Selective formation of a zwitterion adduct and bicarbonate salt in the efficient CO 2 fixation by N-benzyl cyclic guanidine under dry and wet conditions Yoshiaki Yoshida, Naoto
Supporting Information for Selective formation of a zwitterion adduct and bicarbonate salt in the efficient CO 2 fixation by N-benzyl cyclic guanidine under dry and wet conditions Yoshiaki Yoshida, Naoto
Electron Affinities of Selected Hydrogenated Silicon Clusters (Si x H y, x ) 1-7, y ) 0-15) from Density Functional Theory Calculations
 J. Phys. Chem. A 2000, 104, 6083-6087 6083 Electron Affinities of Selected Hydrogenated Silicon Clusters (Si x H y, x ) 1-7, y ) 0-15) from Density Functional Theory Calculations Mark T. Swihart Department
J. Phys. Chem. A 2000, 104, 6083-6087 6083 Electron Affinities of Selected Hydrogenated Silicon Clusters (Si x H y, x ) 1-7, y ) 0-15) from Density Functional Theory Calculations Mark T. Swihart Department
An Approximate DFT Method: The Density-Functional Tight-Binding (DFTB) Method
 Fakultät für Mathematik und Naturwissenschaften - Lehrstuhl für Physikalische Chemie I / Theoretische Chemie An Approximate DFT Method: The Density-Functional Tight-Binding (DFTB) Method Jan-Ole Joswig
Fakultät für Mathematik und Naturwissenschaften - Lehrstuhl für Physikalische Chemie I / Theoretische Chemie An Approximate DFT Method: The Density-Functional Tight-Binding (DFTB) Method Jan-Ole Joswig
Study of Ozone in Tribhuvan University, Kathmandu, Nepal. Prof. S. Gurung Central Department of Physics, Tribhuvan University, Kathmandu, Nepal
 Study of Ozone in Tribhuvan University, Kathmandu, Nepal Prof. S. Gurung Central Department of Physics, Tribhuvan University, Kathmandu, Nepal 1 Country of the Mt Everest 2 View of the Mt Everest 3 4 5
Study of Ozone in Tribhuvan University, Kathmandu, Nepal Prof. S. Gurung Central Department of Physics, Tribhuvan University, Kathmandu, Nepal 1 Country of the Mt Everest 2 View of the Mt Everest 3 4 5
Minnesota Functional Module Version 1.8
 1 Minnesota Functional Module Version 1.8 Subroutines for evaluating the M05, M05-2X, M06-L, M06-HF, M06, M06-2X, M08-HX, M08-SO, M11, M11-L, MN12-L, SOGGA, SOGGA11, SOGGA11-X, N12, N12-SX Functionals
1 Minnesota Functional Module Version 1.8 Subroutines for evaluating the M05, M05-2X, M06-L, M06-HF, M06, M06-2X, M08-HX, M08-SO, M11, M11-L, MN12-L, SOGGA, SOGGA11, SOGGA11-X, N12, N12-SX Functionals
Session 7 Overview: Part A I. Prediction of Vibrational Frequencies (IR) Part B III. Prediction of Electronic Transitions (UV-Vis) IV.
 Session 7 Overview: Part A I. Prediction of Vibrational Frequencies (IR) II. Thermochemistry Part B III. Prediction of Electronic Transitions (UV-Vis) IV. NMR Predictions 1 I. Prediction of Vibrational
Session 7 Overview: Part A I. Prediction of Vibrational Frequencies (IR) II. Thermochemistry Part B III. Prediction of Electronic Transitions (UV-Vis) IV. NMR Predictions 1 I. Prediction of Vibrational
Analysis of Permanent Electric Dipole Moments of Aliphatic Amines.
 Analysis of Permanent Electric Dipole Moments of Aliphatic Amines. Boris Lakard* LPUB, UMR CNRS 5027, University of Bourgogne, F-21078, Dijon, France Internet Electronic Conference of Molecular Design
Analysis of Permanent Electric Dipole Moments of Aliphatic Amines. Boris Lakard* LPUB, UMR CNRS 5027, University of Bourgogne, F-21078, Dijon, France Internet Electronic Conference of Molecular Design
Exchange Correlation Functional Investigation of RT-TDDFT on a Sodium Chloride. Dimer. Philip Straughn
 Exchange Correlation Functional Investigation of RT-TDDFT on a Sodium Chloride Dimer Philip Straughn Abstract Charge transfer between Na and Cl ions is an important problem in physical chemistry. However,
Exchange Correlation Functional Investigation of RT-TDDFT on a Sodium Chloride Dimer Philip Straughn Abstract Charge transfer between Na and Cl ions is an important problem in physical chemistry. However,
QMC dissociation energy of the water dimer: Time step errors and backflow calculations
 QMC dissociation energy of the water dimer: Time step errors and backflow calculations Idoia G. de Gurtubay and Richard J. Needs TCM group. Cavendish Laboratory University of Cambridge Idoia G. de Gurtubay.
QMC dissociation energy of the water dimer: Time step errors and backflow calculations Idoia G. de Gurtubay and Richard J. Needs TCM group. Cavendish Laboratory University of Cambridge Idoia G. de Gurtubay.
Theoretical and experimental analysis on vibrational spectra of formate species adsorbed on Cu Al 2 O 3 catalyst
 Journal of Molecular Structure: THEOCHEM 857 (2008) 38 43 www.elsevier.com/locate/theochem Theoretical and experimental analysis on vibrational spectra of formate species adsorbed on Cu Al 2 O 3 catalyst
Journal of Molecular Structure: THEOCHEM 857 (2008) 38 43 www.elsevier.com/locate/theochem Theoretical and experimental analysis on vibrational spectra of formate species adsorbed on Cu Al 2 O 3 catalyst
Appendix C Calculating Excited States using Gaussian
 Appendix C Calculating Excited States using Gaussian 403 This appendix contains methods for using Gaussian 03 78 and Gaussian 09 121 to calculate excited states of molecules. Such methods are useful for
Appendix C Calculating Excited States using Gaussian 403 This appendix contains methods for using Gaussian 03 78 and Gaussian 09 121 to calculate excited states of molecules. Such methods are useful for
High-power Broadband Organic THz Generator
 Supplementary Information High-power Broadband Organic THz Generator Jae-Hyeok Jeong,1, Bong-Joo Kang,2, Ji-Soo Kim 1, Mojca Jazbinsek 3, Seung-Heon Lee 1, Seung-Chul Lee 1, In-Hyung Baek 2, Hoseop Yun
Supplementary Information High-power Broadband Organic THz Generator Jae-Hyeok Jeong,1, Bong-Joo Kang,2, Ji-Soo Kim 1, Mojca Jazbinsek 3, Seung-Heon Lee 1, Seung-Chul Lee 1, In-Hyung Baek 2, Hoseop Yun
Computational Chemistry I
 Computational Chemistry I Text book Cramer: Essentials of Quantum Chemistry, Wiley (2 ed.) Chapter 3. Post Hartree-Fock methods (Cramer: chapter 7) There are many ways to improve the HF method. Most of
Computational Chemistry I Text book Cramer: Essentials of Quantum Chemistry, Wiley (2 ed.) Chapter 3. Post Hartree-Fock methods (Cramer: chapter 7) There are many ways to improve the HF method. Most of
Introduction to Ab Initio Quantum Chemical Computation
 c:\374-17\computation\computation17.doc for 9mar17 Prof. Patrik Callis 8mar17 Introduction to Ab Initio Quantum Chemical Computation Purpose: 1. To become acquainted with basic concepts of ab initio quantum
c:\374-17\computation\computation17.doc for 9mar17 Prof. Patrik Callis 8mar17 Introduction to Ab Initio Quantum Chemical Computation Purpose: 1. To become acquainted with basic concepts of ab initio quantum
Dalton Quantum Chemistry Program
 1 Quotation from home page: Dalton Quantum Chemistry Program Dalton QCP represents a powerful quantum chemistry program for the calculation of molecular properties with SCF, MP2, MCSCF or CC wave functions.
1 Quotation from home page: Dalton Quantum Chemistry Program Dalton QCP represents a powerful quantum chemistry program for the calculation of molecular properties with SCF, MP2, MCSCF or CC wave functions.
