2 - ALDEHYDES, KETONES AND DERIVATIVES
|
|
|
- Judith Warren
- 5 years ago
- Views:
Transcription
1 2 - ALDEYDES, KETNES AND DEIVATIVES Carbonyl chemistry is one of the most important areas of organic chemistry. You have been introduced to the chemistry of carboxylic acids in CEM*2700, and in this course we will focus on aldehydes and ketones. ence the compounds that we will study in this chapter will possess a carbonyl group which has either hydrogens or carbons attached to it. C carbonyl group C ketone ( ) C aldehyde The reactivity of aldehydes and ketones is based on two important reactions of them. These species are prone to nucleophilic attack at the carbonyl carbon and one can make a nucleophile on a carbon to the carbonyl group. C Nu- Nu C - C 2 C base - C C This chapter will first demonstrate some synthetic routes to aldehydes and ketones, and then will describe some of their vast chemistry based on the two principal modes of reaction shown above. 1. Synthetic outes to Aldehydes and Ketones (SF/SFS 16.4, 16.5) ne of the main methods for the preparation of aldehydes and ketones is oxidation of an alcohol as shown in a general sense below. C 2 [] C ' [] ' The following are some popular routes to aldehydes and ketones and of course include oxidation of an alcohol. Most should be familiar to you. CEM*3750 CUSE NTES 1 of 41
2 a) Aldehydes Me Me CC 2 C 2 C 2 PCC Me CC C 2 Cl 2 C 2 C 2 Me primary alcohol aldehyde, 75% PCC = N - ClCr 3 pyridinium chlorochromate b) Aldehydes and Ketones 3, C 2 Cl 2-78 C Zn Ac c) Ketones C 2 62% Na 2 Cr 2 7 2, 2 S 4 menthol menthone, 84% Ph 1. PhMgBr [] 2. Ph 3 Ph Ph Ph Grignard chemistry followed by oxidation CEM*3750 CUSE NTES 2 of 41
3 Me 1. Me 2. Cl, 2, AlCl 3 93% Cl Et 2 CuLi -78 C ether 2. Acidity and Enolization of Aldehydes and Ketones (SF/SFS and SF ; SFS ) As stated, one of the origins of the reactivity of aldehydes and ketones is their inherent acidity, which is based on the electronic properties of the carbonyl group. The carbonyl group is an electron-withdrawing group by both induction and resonance. So, hydrogens to the group possess increased acidity simply due to their proximity and the incipient anion can be stabilized through -resonance with the carbonyl group. base - - acidic hydrogen, pk a = ca resonance structures There is a geometric requirement for facile proton removal in this chemistry. The C- bond that holds the removable hydrogen must be aligned with C the -orbitals of the C= bond. The required conformation is trivial to achieve in a freely rotating system, but may not be accessible in cyclic or all aligned, so is constrained molecules. Without the proper orbital ideal for removal overlap, then the -resonance stabilization of the anion is not possible. The result is that the acidity of the hydrogen is greatly reduced. CEM*3750 CUSE NTES 3 of 41
4 Bases suitable for deprotonation of an aldehyde or ketone must meet two principal requirements: a) The base must be strong enough to remove the hydrogen. In this regard, any base whose conjugate acid has a pk a greater than 20 will be suitable. Sometimes a weaker base will work, but the reaction must proceed under equilibrium deprotonation conditions. b) The base has to perform selective attack at hydrogen and cannot have properties that will promote nucleophilic attack at the carbonyl carbon. A sterically hindered base usually provides the desired chemoselectivity. LDA, lithium diisopropylamide ([Me 2 C] 2 N - Li ) is often the base of choice. The resonance stabilized anion that results from these deprotonation reactions is called an enolate, which means that it is the anion of an acid called an enol. - enolate enol These species are of course interconvertible through the transfer of one proton. As we will see shortly, the enolate and the enol can be functionalized at the -carbon. ence one can use an enol or an enolate for derivatizing the compound next to its carbonyl group. Enols can be generated through a reversible acid catalyzed reaction. 3 base (= 2 ) is much more acidic since the protonated carbonyl is a much better electron sink The methods of forming enols and enolates are fully reversible. So the carbonyl compound can be regenerated from an enol by simply adding acid and isolating the substrate (except in specialized instances). Attempted isolation of the enol from acid solution will provide the aldehyde or ketone. CEM*3750 CUSE NTES 4 of 41
5 Mechanistically, regeneration of the carbonyl compound can be the reverse of enolate formation, or may involve the enol. - Under these conditions, the keto form of the compound is regenerated upon isolation. The equilibrium between enol and keto shown above is called tautomerism, since the compounds are tautomers of one another. The carbonyl compound is usually the thermodynamically favoured form, based on the carbonyl bond strength, but there are exceptions to this rule. A number of equilibrium constants have been determined for keto-enol tautomerism. They are calculated as shown, and are of course dependent on the medium in which the measurement is made. If the substrate is neat, the tautomer ratio depends [enol] on the origin of the material. Keq = [keto] The K eq (keto-enol) for acetone in water is 1.5 X It should be noted that despite the fact that the ratio can significantly favor the keto form, reactions selective for the enol form can still take place, as we shall see. The relative acidity of the hydrogens next to the carbonyl groups of aldehyde or ketones has both advantages and disadvantages. For one advantage, the acidity allows for deuterium incorporation into the molecule. The protocol involves simple addition of the carbonyl compound to D 2 containing a trace amount of acid or base. Any hydrogens in the -position that can adopt the required conformation for enol or enolate formation will become fully deuterated, given sufficient time. C 3 C 3 D 2, - D C 3 CD 3 D D (Me) 2 C D 2, D 3 (Me) 2 CD Another consequence of enol formation, having negative and positive implications, is the ease with which chiral -carbons can invert their stereochemistry. ence if you have taken the time to make an important, optically pure product possessing stereochemistry at the -carbon, exposure to acid or bases may racemize the material (SF/SFS 18.3A). For example, optically active 3-phenyl-2-butanone in basic ethanol (r.t.) racemizes within minutes. CEM*3750 CUSE NTES 5 of 41
6 Ph C 3 C 3 Et - /Et Ph 3 C - C 3 Et - /Et Ph 3 C C 3 (S)-3-Phenyl- 2-butanone Achiral, planar isolation ()-3-Phenyl- 2-butanone racemic ketone form. There are instances of advantageous isomerism of -carbon, through their planar C 2 C=C 2 10% K Et - K C 2 C=C 2 C 2 C=C 2 C 3 C 3 C 3 In the anionic form, the -carbon is planar and in theory can be protonated from either face. owever, the configuration of the -carbon which bears the methyl group is a constant and that substituent creates a bias in the reprotonation reaction. The allyl and methyl substituents prefer to be trans to one another for steric reasons and trans isomer is produced in >95% yield under these thermodynamic conditions. CEM*3750 CUSE NTES 6 of 41
7 3. alogenation of Ketones and Aldehydes (SF/SFS 18.3B-18.3D) The following general reaction is the topic of this section. X 2 acid or base X As noted, halogenation of these carbonyl compounds may proceed under acidic or basic conditions. Under either of these conditions the mechanism for generation of the enol or enolate is the same as previous. The reactive compound then attacks the halogen rather than any other electrophile such as a proton. via enolate - X X X - via enol X X X ate studies have shown that initial rates of the halogenation are independent of halogen concentration. This is interpreted to mean that introduction of halogen into the substrate is not rate determining. Furthermore, experiments have shown that the rate is dependent on concentration of carbonyl compound and acid (or base). From this evidence, it is generally believed that enol or enolate formation is rate determining. Cl 2, 70 C Cl 85% CEM*3750 CUSE NTES 7 of 41
8 Sometimes as shown below, the reaction is autocatalytic. That is, it is very slow until some halogenation occurs which produces acid as a byproduct. The rate of halogenation then accelerates due to the presence of acid. ence the term autocatalytic, since the reaction provides the means to promote itself. Br 2 Me Br Br 70% Under basic conditions, multiple halogenation is often a problem and therefore basic conditions are not recommended if one is striving for only the monohalogenated product. Note that this reactive character can be used to one s advantage, as shown below. For the introduction of only one halogen, the reaction is usually performed under acidic conditions since introduction of one halogen into the molecule slows further reaction. For synthetic efficiency, monohalogenation reaction must be performed under acidic conditions. X In the halogenated form, the electron withdrawing nature of the halogen retards the initial step of enol formation and hence slows the introduction of more halogens. halogen pulls electron density away from the carbonyl group Multiple halogenation of methyl ketones provides chemistry that has been used as a functional group identification technique, although spectroscopic methods are now more popular. Multiple halogenation of methyl ketones leads to the haloform reaction and iodine is the more common halogen employed (iodoform reaction). The mechanism involves triple iodination of the methyl group of the ketone under basic conditions. base I 2 I base I 2 (2X) I I I Then, hydroxide attacks at the carbonyl group to make a tetrahedral intermediate which expels - CI 3. CEM*3750 CUSE NTES 8 of 41
9 Complete mechanism: Interaction with the solvent provides the products: iodoform and carboxylate. - CI 3 is a good leaving group in this case because the three electronegative halogens help to stabilize the negative charge on the carbon atom. The electronegativity also accounts for the multiple halogenation at a single site. nce one halogen is introduced, the remaining hydrogens on that same carbon atom have enhanced acidity. Acidic workup affords the carboxylic acid if desired as opposed to the carboxylate. The iodoform is a bright yellow precipitate that serves as a useful indicator. If a compound is suspected of being a methyl ketone, simply add excess hydroxide and I 2 and look for a yellow precipitate for confirmation of the suspected structure. The reaction can also be synthetically useful in that it can be used to achieve the overall conversion of methyl ketone to carboxylic acid. CEM*3750 CUSE NTES 9 of 41
10 1. Cl 2 /Na 2. Cl/ 2 CCl 3 5-methyl-3-hexen-2-one 5-methyl-2-pentenoic acid chloroform 4. Alkylation eactions and Enamines (SF 16.8C, 18.4A-B, 18.9; SFS 16.8D, 18.4A- B, 18.9) Generating an enolate from a ketone or aldehyde and quenching it with an alkylating agent affords a method for the synthesis of compounds bearing an alkyl substituent next to the carbonyl group. Simple reactions can occur in some cases. 1. Na, C Me 2 C=CC 2 Br C 2 C=CMe 2 88% via - Br (SF/SFS 18.4A-B) Et LDA cold Et - Li MeI warm to r.t. Et Me N(iPr) 2 93% CEM*3750 CUSE NTES 10 of 41
11 There can be problems associated with this simple approach. ne is that there are two sites for deprotonation when the compound bears -hydrogens on either side of the carbonyl group. There can also be a problem with multiple alkylations, depending on the substrate and reaction conditions. For this reason, other methods to achieve simple efficient alkylation have been developed. ne useful method for overcoming some of the difficulties involves the chemistry of enamines. As their name indicates enamines contain a double bond (ene) and an amine and the name is applied to systems where the two are in conjugation. N - N Gilbert Stork of Columbia University developed the chemistry shown here and it still bears his name. Enamines are prepared by condensing a secondary amine with a ketone or an aldehyde. If the amine is a primary amine the result is an imine, which is actually the more thermodynamically stable form of an imine/enamine tautomeric equilibrium (SF 16.8C; SFS 16.8D). N ' (' = ) N - 2 N ' enamine imine The following mechanism accounts for the enamine formation. CEM*3750 CUSE NTES 11 of 41
12 /toluene N N N N 2 N N N N N iminium ion note with 1re amines EtN 2-2 N N enamine via deprotonation of the N at the iminium ion stage Water must be driven from the reaction vessel in order to force the equilibrium reaction to completion. This is usually achieved through the use of a Dean Stark apparatus: evaporated compounds condense, fall into graduated tube and separate. Water stays on lower layer and never return to the flask while the benzene (or toluene) rises to the level where it runs back into the reaction vessel. reaction mixture of benzene or toluene and maybe water, which also is created by the reaction CEM*3750 CUSE NTES 12 of 41
13 As shown above the enamine has nucleophilicity at carbon and it is this atom that is preferentially functionalized in a simple S N 2 reaction. X N S N 2 X N I - C 3 C 3 I usually X = -C 2 C 2 - (pyrrolidine) -C 2 C 2 C 2 - (piperidine) -C 2 C 2 - (morpholine) 3 C 3 In this reaction the -carbon of the enamine ( -carbon of the ketone) bears the new substituent. To remove the nitrogen auxiliary and recover the new ketone, the iminium salt is hydrolyzed under aqueous acid conditions. The amine is lost in the acidic medium and can be recycled if desired. This method is preferred over simple deprotonation and alkylation since it minimizes double or multiple alkylation. The reaction occurs best with reactive halo compounds such as methyl iodide, benzyl halides, allyl halides, -halocarbonyl compounds and acyl halides. Below is an example of the overall reaction sequence. 1. pyrrolidine, 2. BrC 2 C 3 3., 2 C 2 C 3 67% CEM*3750 CUSE NTES 13 of 41
14 ther examples: N 1. Br 2. 3 N 1. Cl The Aldol Condensation (SF/SFS ) To this point we have examined the reactions of enols and enolates with a number of reactive electrophiles. ne can also react them with the very substrates that provide enols and enolates: CABNYL CMPUNDS. Under the proper conditions the carbonyl group is sufficiently reactive to accept electron density from an enol or enolate. The archetypal example in many texts is the base catalyzed reaction of two molecules of acetaldehyde. The name aldol originates from the presence of an aldehyde and an alcohol in the product molecule. This name is used even if ketones are involved in the chemistry. 2 C 3 10% Na, 2 5 C C 3 C 2 portion that acted as an electrophile portion that was enolate The mechanism for the reaction is straightforward and is the basis of many other reactions yet to be introduced in this chapter. It begins with the reversible generation of CEM*3750 CUSE NTES 14 of 41
15 an enolate. The enolate then reacts as indicated. Note that all the steps are reversible and sometimes aldol condensations are difficult to complete because of a propensity to revert to starting materials. Sometimes the aldol product will lose water spontaneously to afford a double bond. The conjugation of the double bond with the carbonyl group is the driving force in the dehydration. This can often be achieved simply by heating the reaction mixture during or after the bond forming process. Sometimes making the solution acidic will achieve the same purpose. The term condensation arises from the loss-of-water step. In synthetic chemistry, condensation means: the loss of water or its equivalent. Sometimes when the equilibrium constant for aldol formation is small, the reaction can still be driven by pushing the product through to the dehydrated product, the formation of which proceeds efficiently and is less reversible. Basic conditions: C 3 C C 3 C ften, if the elimination of water does not proceed efficiently under basic condition, one converts the mixture to an acidic one. Under acidic conditions, loss of water is an easier process. CEM*3750 CUSE NTES 15 of 41
16 Acidic conditions: 2 C 3 C 2 C 3 2 C C 3 C 2 2 The whole aldol condensation can also be performed under acidic conditions, where an enol rather than an enolate is the nucleophile. The acid initially induces enol formation by the means shown earlier in this chapter. This enol is a weak nucleophile and will only undergo aldol chemistry when the electron accepting carbonyl group has been protonated. Mechanism for acidic aldol reaction with loss of water: CEM*3750 CUSE NTES 16 of 41
17 It is very difficult to isolate the true aldol product under acidic conditions. The reactions usually carries through to the unsaturated material. Both ketones and aldehydes can undergo the aldol condensation. Aldehydes are more reactive since nucleophilic attack at the ketone carbonyl is more sterically hindered. Also, the extra electron donating substituent makes the ketone less electrophilic. Aldol condensations as shown above with two molecules of acetaldehyde are the simplest possible examples. Complications quickly arise when two different aldehydes are used, or when ketones with two sets of -hydrogens are employed. For instance with two different aldehydes, four products are available. The list includes two compounds from self condensation and two different products from a crossed aldol reaction. ' ' ' ' ' Selectivity can be achieved by choosing one reactant that does not have - hydrogens. C Na, 2 25 C, 4 hr. 100 % C C 3 C 2 C Na, 2 C C C C 3 72% CEM*3750 CUSE NTES 17 of 41
18 C 3 (C 3 ) 3 C C Na, 2 CC(C 3 ) 3 C 81% Sometimes, when there is a large number of -hydrogens in the systems, it may be possible to selectively generate a particular enolate and then quench that enolate with another carbonyl compound. Such a protocol requires a method for preparation of specific enolates. The use of LDA is recommended in these circumstances. ecall that treatment of an aldehyde or ketone with LDA is a reliable method for the complete and irreversible formation of a lithium enolate. With asymmetric ketones, LDA will remove a proton from the least sterically hindered position, to afford the kinetic enolate. 1 This is generally believed to be the best method for carrying out crossed aldol condensations. LDA, TF - Li C 3 C 2 C 3-78 C C 2 C 2 C 3 C 3 C 2 - Li C 3 C 2 C 2 C 3 C 3 C 2 C 2 C 3 If a given substrate possesses two carbonyl groups, it is possible for the compound to undergo an intramolecular aldol condensation. This form of the reaction is only suitable for the synthesis of 5, 6, and 7 membered rings. There are some rules that can be applied at this stage. ne is a reminder that aldehydes are more electrophilic than ketones. The other is that 5 membered rings will form more readily that 7- membered rings while 6-membered rings are the best. ecall that aldol condensations are reversible and there is opportunity to form the most thermodynamically stable product. Intramolecular aldol condensations will virtually always proceed through with loss of water to form the a,b-unsaturated ketone or aldehyde. When deciding the product of this cyclization, an important step in the analysis is determining when water can be readily lost from the aldol. If not, aldol formation often reverses itself. 1 f the two possible enolates formed from a ketone such as methyl ethyl ketone, the one with the least substituents on the double bond of the enolate is termed the kinetic enolate. The kinetic enolate is formed by deprotonation of the least hindered site. Deprotonation from the more hindered site (which can be achieved under different conditions) gives the thermodynamic enolate, which is more stable because it has more substituents around the double bond form of the enolate. See SF/SFS CEM*3750 CUSE NTES 18 of 41
19 Na, C not Na, C Before we leave the aldol condensation, the role of the carbonyl group in this chemistry should be emphasized. The polarity and resonance ability of the carbonyl group enhances the acidity of the hydrogens to it, allowing for the formation of enolates. That creates the nucleophilic component of the two reactants. For the electrophilic component the carbonyl group is polarized such that the carbon can accept attack by a nucleophile and the oxygen can hold the negative charge. 6. ther elated Condensation eactions Whereas the Aldol condensation involves the reaction of an enolate of an aldehyde or ketone with another aldehyde or ketone, the Claisen Condensation (SF/SFS 19.2) involves the reaction of an enolate of an ester and a carboxylic acid derivative, usually another ester. ne can view the initial steps of the Claisen condensation as analogous to ketone chemistry. Enolates of esters are less acidic than enolates of ketones or aldehydes and can therefore undergo chemistry with less reactive compounds. The archetypal example in this condensation involves the reaction of two molecules of ethyl acetate induced by ethoxide ion. The mechanism of the reaction is shown below. CEM*3750 CUSE NTES 19 of 41
20 Although the scheme above shows the -ketoester as the product, it should be realized that under the reaction conditions, the material actually rests as the deprotonated form until acted upon by the addition of acid which returns the hydrogen. That is, the Claisen reaction mixture must be quenched with acid before isolation of the product. Acetic acid or aq. ammonium chloride are often employed. Note that anions derived from the -ketoester have two carbonyl groups available for conjugation. ence the pk a of the -ketoester is much lower than that of a simple ketone or ester, since anion stabilization is offer by both of these carbonyl groups in a single molecule. ence, the -ketoester is readily deprotonated by ethoxide. CEM*3750 CUSE NTES 20 of 41
21 - C 3 C 2 C C 3 C 3 C 2 C 2 C 3 Et - Na C 3 C 2 C C 3 - C 3 C 2 C C 3 The deprotonation step as shown above is key to the completion of the reaction. The -ketoester anion is essentially inert and furthermore, in anionic form, the species is captured and frozen and cannot succumb to the reversibility of the reaction. The overall reaction only works well when there are 2 or 3 hydrogens on the starting ester. The - ketoester drawn above is known by the common name of ethyl acetoacetate and hence the self-condensation of esters is sometimes known as the acetoacetate ester condensation. Example: 2 Me 1. Me - Na 2. Me Crossed Claisen condensations are possible when one of the esters does not possess -hydrogens, much like the ideal situation for crossed aldol reactions. CEM*3750 CUSE NTES 21 of 41
22 diethyl succinate diethyl oxalate C 3 C 3 - Na toluene 90% 1. C 3 C 3 - Na ethanol 2. ethyl benzoate 71% ethyl formate C 3 1. C 3 C 3 - Na ethanol 2. 80% Note that in each of the three examples above, one of the reacting esters does not contain -hydrogens. Carbonate esters which also lack -hydrogens will successfully partake in crossed Claisen reactions. Another reaction that simplifies potentially complicated reactions is the eformatsky reaction. It begins with an -halo ester * and uses metallic zinc to establish an ester enolate. In this reaction, the halo ester and zinc are mixed together to create a solution containing a zinc enolate, to which the ketone or aldehyde is added. As with aldol condensations, the product of eformatsky reactions can be readily converted to the unsaturated material by treatment with acid. This achieves the dehydration process. * -alo esters can be prepared by the ell-volhard-zellinsky reaction whereby a carboxylic acid is treated with molecular halogen and elemental phosphorus and then water. See SF/SFS 18.3D for details of this preparation. The acid can then simply be esterified. CEM*3750 CUSE NTES 22 of 41
23 Br Zn - (ZnBr) - (ZnBr) C 60% Br 1. Zn, toluene % If two ester groups are in the same molecule and are separated by 4 or 5 carbon atoms, then one can achieve an intramolecular Claisen condensation. This reaction is called the Dieckmann (SF/SFS 19.2A) condensation. Again there is an archetypal system that exemplifies the reaction. CEM*3750 CUSE NTES 23 of 41
24 As with the Claisen condensation, the equilibrium is frozen at the desired product by deprotonation of the acidic hydrogen between the carbonyl groups. The Dieckmann condensation is only useful for 5 and 6 membered rings. C 2 Et C2Et C 2 Et Et - A form of the Claisen Condensation is very prevalent in biological systems: it is vital to the construction of many naturally occurring and biologically important chemicals. In this case the carboxylic acid being attacked is a thiolester (textbooks will tell you thioester), and the sulfur and the remainder of coenzyme A is lost in a potentially reversible step. The nucleophile is not exactly the enolate of an ester, but one that bears an extra -C 2 - unit. The release of C 2, concurrent with nucleophilic attack, assists the Claisen Condensation over its kinetic barrier. - C 2 SCoA 3 C SCoA -C 2 - C 3 CoAS C 2 SCoA - - SCoA 3 C C 2 SCoA reduce, dehydrate, reduce C 3 C 2 C 2 SCoA more repetitions C 3 (C 2 C 2 )n SCoA steroidal hormones bile acids terpenes CEM*3750 CUSE NTES 24 of 41
25 7. Synthetic Applications of Condensation eactions a) Acetoacetic Ester Synthesis (SF/SFS 18.6) This synthesis, named after the starting material employed is a valuable means of preparing substituted acetones (methyl ketones). The approach utilizes two key qualities of the starting material, MeC()C 2 C 2 Et. ne is that the compound possess a C 2 group with low acidity that can be readily functionalized. The other is that the ester can be converted to an acid which is then completely removed. The general equation for the overall transformation is as follows, where = a non-hydrogen group and ' may be hydrogen or another group. ' ' substituted acetone In detail, the first reaction is similar to those you have seen before. ne of the acidic hydrogens of the dicarbonyl compound is easily deprotonated by an alkoxide and the anion is captured at carbon by addition of an electrophile. Such chemistry will lead to a mono-substituted acetone. ne can repeat the chemistry and introduce a second electrophile and the result would eventually be a di-substituted acetone. pk a = M - M - M X ' - M CEM*3750 CUSE NTES 25 of 41
26 The next step is alkaline hydrolysis of the ethyl ester, a process called saponification. The solution is quenched with acid to allow for the isolation of the -keto acid. ' 1. Na, 2 2. Cl, 2 ' The last step in the overall transformation has its origin in the structure of the - keto acid. -Carbonyl carboxylic acids often adopt a conformation where the acid hydrogen can hydrogen bond in an intramolecular sense with the other carbonyl group. The hydrogen bonding interaction is idealized by the fact that the system forms a six membered ring. hydrogen bond ' ' The hydrogen bonded arrangement allows the -carbonyl carboxylic acids to undergo a thermally induced decarboxylation reaction. The arrows in the diagram below help to assign the re-positioning of the electrons. nly when the system form a 6- membered cyclic form is the decarboxylation facilitated, as indicated by the arrows. ' The decarboxylation is effected by heating the substrate at 100 C. The immediate product of the carboxylation is actually an enol but upon isolation, the material tautomerizes to the ketone. ' heat -C 2 ' ' CEM*3750 CUSE NTES 26 of 41
27 The same chemistry takes place if the target molecule is a di-substituted acetone. The only difference is that after the introduction of the first alkyl group and before the saponification, the substrate is deprotonated again and quenched again with another electrophile. nce the introduction of two groups between the carbonyl is complete then the conversion to acid and subsequent decarboxylation can proceed. Some examples: Et 1. NaEt, Et 1. Na, 2 2. (Me) 2 CC 2 Br Et heat, -C 2 1. NaEt, Et 2. Cl Ph Ph 1. Na, 2, then 2. heat, -C 2 Ph CEM*3750 CUSE NTES 27 of 41
28 Sometimes the acid and thermolysis treatments can be addressed simultaneously: 1. NaEt, Et 2. C 3 C 2 C 2 C 2 Br 1. Na, , 2 S 4, heat 1. NaEt Et 2. MeI 1. NaEt Et 2. Br 1. Na, 2, then 2. heat, -C 2 CEM*3750 CUSE NTES 28 of 41
29 The same chemistry can be performed on a wide range of -keto esters. C 2 Et 1. NaEt, Et C2 C 2 C 2 Br C 2 Et 2. excess Br(C 2 ) 3 Br 1. saponification heat C 2 C 2 C 2 Br 60% b) Malonic Ester Synthesis (SF/SFS 18.7) The steps required in the malonic ester synthesis are analogous to those of the acetoacetic ester synthesis. ne difference is the starting material, changing the methyl ketone to an ethyl ester creates a difference in the product: a substituted acetic acid derivative is obtained rather than a methyl ketone. Starting material: vs Final product: vs CEM*3750 CUSE NTES 29 of 41
30 verall reaction of malonate synthesis Et Et Et ' Et ' substituted acetic acid Diethyl malonate * is the usual starting material. The anion of it is quenched with an electrophile. The product, also a malonate, is hydrolyzed to a diacid, which is then thermally decarboxylated. The result is the monosubstituted acetic acid. Disubstituted acetic acids are also available and can be obtained by following the same protocol as for disubstituted acetones. Diethyl malonate is slightly less acidic than acetyl acetone, with a pk a of 13.3, but the same chemistry can take place anyway. C 2 (C 2 Et) 2 1. NaEt, Et 2. allyl bromide C 2 Et C C 2 Et 1. Na, heat C 2 C 2 (C 2 Et) 2 2 eq. NaEt Et Br(C 2 ) 3 Cl C(C 2 Et) 2 1. K, , heat C 2 cyclobutanecarboxylic acid, 43% * 1,3-Propanedioic acid is commonly known as malonic acid and so diesters are known as malonates. CEM*3750 CUSE NTES 30 of 41
31 C 2 (C 2 Et) 2 1. NaEt, Et 2. Br C 2 Et C C 2 Et 1. NaEt, Et 2. EtBr C C 2 1. K, , heat C C 2 Et C 2 Et Clearly, the prominent structural feature in the acetoacetic esters and the malonates is the methylene group surrounded by two electron stabilizing functionalities. Although there is a detailed presentation here of the chemistry of the systems with two esters and with a ketone and an ester, other functional groups may also be present. Indeed, a number of groups can serve the role of electron withdrawing groups in the general structure Z-C 2 -Z' (SF/SFS 18.8). Typical ones include of course esters and ketones, but one may also encounter other carbonyl containing compounds as well as nitriles, nitro compounds and sulfur or phosphorus containing groups. The final step of thermal decarboxylation is not always possible. Indeed, sometimes one wishes to maintain both of the groups in the molecule. Furthermore, there may be other methods for removing the particular functional groups once they have served their role. Me 1 eq. Na MeI, 2 70% Me Me 2 eq. NaMe, MeI, Me 80% CEM*3750 CUSE NTES 31 of 41
32 c) obinson Annulation (SF/SFS 19.7B) Before introducing the chemistry and overall value associated with the obinson Annulation, it is important to introduce a very important reaction known as the Michael Addition (SF/SF 19.7A). The Michael addition was originally defined as the addition of a malonate to the position of an, -unsaturated ketone, ester or nitrile. This strict definition has subsided and a Michael addition is now defined as the addition of any nucleophile to the position of an alkene bearing one or more electron withdrawing groups. Below are some examples using nucleophiles that have recently been introduced to you. ther carbon nucleophiles can be used in this chemistry as can amines, alcohols and sulfur nucleophiles. Examples pka C 2 (C 2 Et) ca. 9 catalytic - in C(C 2 Et) 2 ca. 11 CN and others Note that the product of these reaction possesses two strong electron withdrawing groups and may well contain one or two carbonyl groups. The obinson annulation begins with a Michael addition and is completed by an intramolecular aldol condensation. In general, there are other materials that can participate in Michael additions (but not necessarily the full obinson annulation). NUCLEPILES: -diketones, -keto esters, dialkyl cuprates, enamines, -keto nitriles, -nitro ketones amines, alcohols, thiols MICAEL ACCEPTS: conjugated aldehydes, conjugated ketones, conjugated esters, conjugated amides, conjugated nitriles, conjugated nitro compounds CEM*3750 CUSE NTES 32 of 41
33 Mechanism of Michael addition: _ - Et - Et Et acidify Et Using the example in the mechanism above, where = C 3, exposure of that material to base (usually from the original Michael addition reaction mixture), provides a means for the intramolecular aldol reaction. In this particular case, the anion of the methyl group may attack one of two equivalent ketone groups. In many cases there is a lone ketone and the reaction works perfectly well. From this point the regular aldol and its dehydration occur. CEM*3750 CUSE NTES 33 of 41
34 base _ C 3 C 3 C 2 _ protonation & dehydration - The thermodynamics of the reaction make the formation of 6 membered ring most favorable. ence the obinson annulation is ideal for the formation of cyclohexenones including those fused to other rings. verall, as stated, the obinson annelation involves, first a Michael addition and then an aldol condensation. Michael addition C 2 aldol condensation cyclohexenone Some examples: C 2 Et methyl vinyl ketone (MVK) Na Et C 2 Et CEM*3750 CUSE NTES 34 of 41
35 Me ethyl vinyl ketone NaMe Me Me Michael addition product Me obinson annulation product.. More, general examples of the annulation. Et Michael Et Aldol C()Et CEM*3750 CUSE NTES 35 of 41
36 Michael Aldol d) ther Conjugate Additions (SF/SFS 19.7) As you know, a number of nucleophiles add to the carbonyl carbon of aldehydes and ketones. The presence of a conjugated double bond creates an additional electrophilic site, and above it was shown that stabilized carbon anions will perform Michael additions on these substrates. Simple carbon nucleophiles such as Grignards will still attack at the C= group. rganolithium reagents can attack at either electrophilic site and are not synthetically useful for this chemistry. rganocuprate reagents attack in a conjugate fashion and are easily generated using Grignard reagents in the presence of CuI. Et 1. EtMgBr 1. EtMgBr Et 2 2. / 2 CuI, Et 2 2. / 2 Et A number of other nucleophiles are also useful in conjugate addition reactions. The list includes amines (N 2, N), alcohols (), thiols (S) and CN (prepared via KCN/ ). The addition of a nitrile is particularly synthetically useful since it can be converted to a carboxylic acid by hydrolysis. NMe MeN 2 Ph KCN/ NC Ph acid or base hydrolysis Ph CEM*3750 CUSE NTES 36 of 41
37 A significant example of a Michael addition has recently been found in nature. The Michael reaction is critical to the operation of a rather promising anticancer drug, calicheamicin. In the first step in its operation the trisulfide bond is broken (1). The nucleophilic sulfide then adds in Michael fashion to give enolate 2. This addition changes the shape of the molecule, bringing the ends of the two acetylenes closer together. A cyclization occurs to give a di-radical (3), and this diradical abstracts hydrogen from the cancer cell's DNA, killing it. Calicheamicin depends for its action on the change of shape. Before the Michael reaction, the ends of the two acetylenes are too far apart to cyclize. They are freed to do so when the sulfur adds to the a,b unsaturated carbonyl. NCMe SSSMe NCMe S - - NCMe S 1 2 dead cancer cells - NCMe S DNA - NCMe S 3 = series of sugar moieties CEM*3750 CUSE NTES 37 of 41
38 8. The Wittig lefination of Aldehydes and Ketones (SF/SFS 16.10) The most popular method for the conversion of aldehydes and ketone to alkenes is the Wittig eaction. Generally speaking it involves the reaction of a carbonyl compound with a phosphorus ylide. * The result is an alkene and a phosphine oxide. _ " 3 P C " 3 P - C ' ' ylide alkene " 3 P A specific procedure is usually required to prepare the Wittig reagent. First one treats the halogen in an alkyl halide with a phosphine, usually triphenylphosphine. The result is an alkyltriphenylphosphonium salt. The salt is then treated with a strong base, usually butyllithium in order to generate the ylide. Ph 3 P C 2 X C 6 6 Ph 3 P C 2 X - Ph 3 P C 2 C - Li (n-buli) _ Ph 3 P C butane LiX phosphonium ylide Deprotonation of the phosphonium salt may also be achieved using alkoxides or Na. Some ylides are stable. Most often the ylide is prepared in the flask and then is brought together with the carbonyl compound. Under these circumstances, the carbon anion portion of the ylide attacks the carbonyl group. The result is a phosphonium betaine, which readily closes to a cyclic isomer, an oxaphosphetane. To conclude the synthesis, the oxaphosphetane fragments to make the alkene and triphenylphosphine oxide. ne of the driving forces of the reaction is the formation of the strong P= bond. * An ylide was originally defined as a compound that possesses a carbon anion directly beside a heteroatomic cation, where the formation of a multiple bond is usually not possible. The definition has eased in recent years and now encompasses any compound bearing adjacent positive and negative charges in situations where a formal multiple bond may or may not be possible. ther popular methods for olefination may involve silicon or sulfur atoms rather than a phosphorus atom. CEM*3750 CUSE NTES 38 of 41
39 The Wittig reaction can be carried out in the presence of ether, ester, halogen and other multiple bond functionalities. It often affords a mixture of geometric isomers about the double bond, although sometimes the reaction is selective. Some examples: _ Ph C(C2 ) 3 C 3 P C(C 2 ) 3 C 3 3 Ph 3 P= PhC 2 _ Ph 3 P CC 3 CC 3 Ph3 P= PhC 2 87:13 = Z:E PhC 2 _ Ph3 P C PhC 2 C C Ph 3 P= E isomer only CEM*3750 CUSE NTES 39 of 41
40 PPh 3 X - NaMe Me PPh 3 C()Me C()Me -, 2 (saponification) Vitamin A 1 PPh 3 Bu - Li PPh3 - C Ph 3 P C C CEM*3750 CUSE NTES 40 of 41
41 9. eductive Conversion of C= to C 2 A final important transformation of aldehydes and ketones is their conversion to methylene groups. The following two reagent systems are observed routinely with ketones and occasionally aldehydes. The Wolff-Kishner approach (SFS 15.9, 16.8C) utilizes 2 NN 2 /high boiling solvent/na/heat while the Clemmensen reduction (SF/SFS 15.9) involves refluxing the ketone in hydrochloric acid with a zinc amalgam [Zn(g)]. Clearly the Wolff-Kishner protocol is best for substrates that are not base sensitive while the Clemmensen procedure is preferred when there are no acid sensitive functionalities in the ketone. Each of these procedures are part of the family of reductions known as deoxygenations. 2 NN 2, Na (C 2 C 2 ) 2 heat 47% Me 2 NN 2, Na Me (C 2 C 2 ) 2 2, heat 69% C C 3 Cl, Zn(g) C 3 heat C 3 Both methods are particularly effective in tandem with electrophilic aromatic acylation reactions. CEM*3750 CUSE NTES 41 of 41
Reactions at α-position
 Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Reactions at α-position In preceding chapters on carbonyl chemistry, a common reaction mechanism observed was a nucleophile reacting at the electrophilic carbonyl carbon site NUC NUC Another reaction that
Alpha Substitution and Condensations of Enols and Enolate Ions. Alpha Substitution
 Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
Alpha Substitution and ondensations of Enols and Enolate Ions hap 23 W: 27, 28, 30, 31, 37, 39, 42-44, 47, 51, 54-56 Alpha Substitution Replacement of a hydrogen on the carbon adjacent to the carbonyl,
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
CHAPTER 19: CARBONYL COMPOUNDS III
 CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
CHAPTER 19: CARBONYL COMPOUNDS III A hydrogen bonded to a carbon adjacent to a carbonyl carbon is sufficiently acidic to be removed by a strong base. The carbon adjacent to a carbonyl carbon is called
Enols and Enolates. A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl)
 Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Enols and Enolates A type of reaction with carbonyl compounds is an α-substitution (an electrophile adds to the α carbon of a carbonyl) E+ E In the preceding chapters, we primarily studied nucleophiles
Aldehydes and Ketones : Aldol Reactions
 Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Aldehydes and Ketones : Aldol Reactions The Acidity of the a Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons a to carbonyls are unusually acidic The resulting anion is stabilized by
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon
 Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Chapter 19. Carbonyl Compounds III Reaction at the α-carbon There is a basic hydrogen (α hydrogen) on α carbon, which can be removed by a strong base. 19.1 The Acidity of α-hydrogens A hydrogen bonded
Ch 22 Carbonyl Alpha ( ) Substitution
 Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
Ch 22 Carbonyl Alpha () Substitution The overall reaction replaces an H with an E + The acid-catalyzed reaction has an enol intermediate The base-catalyzed reaction has an enolate intermediate Keto-Enol
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions
 Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Lecture Notes Chem 51C S. King Chapter 24 Carbonyl Condensation Reactions I. Reaction of Enols & Enolates with ther Carbonyls Enols and enolates are electron rich nucleophiles that react with a number
Aldol Reactions pka of a-h ~ 20
 Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Enolate Anions Chapter 17 Hydrogen on a carbons a to a carbonyl is unusually acidic The resulting anion is stabilized by resonance to the carbonyl Aldehydes and Ketones II Aldol Reactions pka of a-h ~
Chapter 19. Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions. ß-dicarbonyl compounds. Why are ß-dicarbonyls useful?
 Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
Chapter 19 Synthesis and Reactions of b-dicarbonyl Compounds: More Chemistry of Enolate Anions ß-dicarbonyl compounds Two carbonyl groups separated by a carbon Three common types ß-diketone ß-ketoester
A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation:
 1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
1 Chapter 22: Reactions of Enols and Enolates I. Alpha Substitution verview: A. Review of Acidity and pk a Common way to examine acidity is to use the Bronsted-Lowry acid-base equation: Recall that the
Suggested solutions for Chapter 28
 s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
s for Chapter 28 28 PBLEM 1 ow would you make these four compounds? Give your disconnections, explain why you chose them and then give reagents for the. 2 2 Me S Exercises in basic one- group C X disconnections.
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chem 263 Nov 14, e.g.: Fill the reagents to finish the reactions (only inorganic reagents)
 hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
ORGANIC - BRUICE 8E CH CARBONYL COMPOUNDS III: REACTIONS AT THE ALPHA-CARBON
 !! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
!! www.clutchprep.com CNCEPT: ALPHA CARBNS AND TAUTMERIZATIN We have discussed the high reactivity of the carbonyl carbon. However, carbonyls contain another highly reactive component. What is the acidity
20.3 Alkylation of Enolate Anions
 864 APTER 20 ELATE AD TER ARB ULEPILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone The reaction can also be used in synthesis to convert a methyl
864 APTER 20 ELATE AD TER ARB ULEPILES which precipitates as a yellow solid, provides a positive test for the presence of a methyl ketone The reaction can also be used in synthesis to convert a methyl
Organic Chemistry, Third Edition. Chapter 24 Carbonyl condensations
 rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
rganic Chemistry, Third Edition Chapter 24 Carbonyl condensations 1 Review: enolates LDA, -78 C TF kinetic RX R = Me, 1 alkyl R Na TF, RT RX R = Me, 1 alkyl thermodynamic R enolates = nucleophiles React
Summary of π Bond Chemistry
 Chapter 10 Summary of π Bond Chemistry to π C=C 13.01 Chapter 11 at π C=C to π C=C Chapter 1 LG at π C=C Chapter 13 at α position C= activates position ow does C= activation α position? Via or. E base
Chapter 10 Summary of π Bond Chemistry to π C=C 13.01 Chapter 11 at π C=C to π C=C Chapter 1 LG at π C=C Chapter 13 at α position C= activates position ow does C= activation α position? Via or. E base
Additions to the Carbonyl Groups
 Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
Chapter 18 Additions to the Carbonyl Groups Nucleophilic substitution (S N 2andS N 1) reaction occurs at sp3 hybridized carbons with electronegative leaving groups Why? The carbon is electrophilic! Addition
What is in Common for the Following Reactions, and How Do They Work?
 What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
What is in Common for the Following Reactions, and ow Do They Work? You should eventually be able to draw the mechanism for these (and other) reactions 13 Key Intermediate 1 Br-Br Na Br 2 C 3 -I Me NaMe
Chapter 19. Organic Chemistry. Carbonyl Compounds III. Reactions at the a-carbon. 4 th Edition Paula Yurkanis Bruice
 Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
Organic Chemistry 4 th Edition Paula Yurkanis Bruice Chapter 19 Carbonyl Compounds III Reactions at the a-carbon Disampaikan oleh: Dr. Sri Handayani 2013 Irene Lee Case Western Reserve University Cleveland,
2.222 Practice Problems 2003
 2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
2.222 Practice Problems 2003 Set #1 1. Provide the missing starting compound(s), reagent/solvent, or product to correctly complete each of the following. Most people in the class have not done this type
New bond. ph 4.0. Fischer esterification. New bond 2 O * New bond. New bond H 2N. New C-C bond. New C-C bond. New C-C bond. O Cl.
 Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Iverson C 0N KRE Table: For use in synthesis problems, count carbons in products and starting materials then identify location(s) of new s, especially C-C or C=C s. With that information, use the following
Chem 263 Nov 19, Cl 2
 Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
Chem 263 Nov 19, 2013 eactions of Enolates: X X alogenation X C 2 Alkylation C Aldol eaction X C Acylation Example: halogenation LDA 2 Chloroacetone is used in tear gas. chloroacetone In this reaction,
MCAT Organic Chemistry Problem Drill 10: Aldehydes and Ketones
 MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
MCAT rganic Chemistry Problem Drill 10: Aldehydes and Ketones Question No. 1 of 10 Question 1. Which of the following is not a physical property of aldehydes and ketones? Question #01 (A) Hydrogen bonding
Aldehydes and Ketones Reactions. Dr. Sapna Gupta
 Aldehydes and Ketones Reactions Dr. Sapna Gupta Reactions of Aldehydes and Ketones Nucleophilic Addition A strong nucleophile attacks the carbonyl carbon, forming an alkoxide ion that is then protonated.
Aldehydes and Ketones Reactions Dr. Sapna Gupta Reactions of Aldehydes and Ketones Nucleophilic Addition A strong nucleophile attacks the carbonyl carbon, forming an alkoxide ion that is then protonated.
Chapter 22 Enols and Enolates
 Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
Chapter Enols and Enolates Acidity of the α hydrogen o The position next door to a carbonyl is called the α position o When an α proton is abstracted, the resulting carbanion is resonancestabilized. This
Chapter 23. Alpha Substitution of Carbonyl Compounds.
 1 Chapter 23. Alpha Substitution of Carbonyl Compounds. 1. Enolates. a) Carbonyl compounds are acidic at the α C, can be deprotonated by bases to give enolates. i) Ketones and aldehydes have pk a = 18
1 Chapter 23. Alpha Substitution of Carbonyl Compounds. 1. Enolates. a) Carbonyl compounds are acidic at the α C, can be deprotonated by bases to give enolates. i) Ketones and aldehydes have pk a = 18
Module No and Title. PAPER No: 5 ; TITLE : Organic Chemistry-II MODULE No: 25 ; TITLE: S E 1 reactions
 Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 25; S E 1 reactions CHE_P5_M25 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. S E 1 reactions 3.1
Subject Chemistry Paper No and Title Module No and Title Module Tag 5; Organic Chemistry-II 25; S E 1 reactions CHE_P5_M25 TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. S E 1 reactions 3.1
Loudon Chapter 19 Review: Aldehydes and Ketones CHEM 3331, Jacquie Richardson, Fall Page 1
 Loudon Chapter 19 eview: Aldehydes and Ketones CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 Beginning with this chapter, we re looking at a very important functional group: the carbonyl. We ve seen
Loudon Chapter 19 eview: Aldehydes and Ketones CEM 3331, Jacquie ichardson, Fall 2010 - Page 1 Beginning with this chapter, we re looking at a very important functional group: the carbonyl. We ve seen
Background Information
 ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
ackground nformation ntroduction to Condensation eactions Condensation reactions occur between the α-carbon of one carbonyl-containing functional group and the carbonyl carbon of a second carbonyl-containing
Practice Synthetic Problems: CHEM 235 Page 2
 Practice Synthetic Problems: CM 235 Page 2 Syntheses based on diethyl malonate, ethyl acetoacetate, etc. Using diethyl malonate and any other necessary organic reagents, show a synthesis of: a) 2,2-dimethyl-1,3-propanediamine
Practice Synthetic Problems: CM 235 Page 2 Syntheses based on diethyl malonate, ethyl acetoacetate, etc. Using diethyl malonate and any other necessary organic reagents, show a synthesis of: a) 2,2-dimethyl-1,3-propanediamine
CHAPTER 23 HW: ENOLS + ENOLATES
 CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
CAPTER 23 W: ENLS + ENLATES KET-ENL TAUTMERSM 1. Draw the curved arrow mechanism to show the interconversion of the keto and enol form in either trace acid or base. trace - 2 trace 3 + 2 + E1 2 c. trace
Synthesis of Nitriles a. dehydration of 1 amides using POCl 3 : b. SN2 reaction of cyanide ion on halides:
 I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
I. Nitriles Nitriles consist of the CN functional group, and are linear with sp hybridization on C and N. Nitriles are non-basic at nitrogen, since the lone pair exists in an sp orbital (50% s character
CHEM 234: Organic Chemistry II Reaction Sheets
 EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
ORGANIC - BROWN 8E CH ALDEHYDES AND KETONES.
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chem 263 March 28, 2006
 Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 March 28, 2006 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Nucleophilic Addition Reactions of Carboxylic Acid Derivatives
 Lecture 5: bjectives: Nucleophilic Addition eactions of Carboxylic Acid Derivatives By the end of this lecture you will be able to: draw the mechanism of a nucleophilic addition-elimination reaction with
Lecture 5: bjectives: Nucleophilic Addition eactions of Carboxylic Acid Derivatives By the end of this lecture you will be able to: draw the mechanism of a nucleophilic addition-elimination reaction with
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution
 Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Chapter 20 Carboxylic Acid Derivatives Nucleophilic Acyl Substitution Nomenclature: In carboxylic acid chlorides, anhydrides, esters and amides, the parent is the carboxylic acid. In each case be sure
Topic 9. Aldehydes & Ketones
 Chemistry 2213a Fall 2012 Western University Topic 9. Aldehydes & Ketones A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is the most important group in bio-organic
Chemistry 2213a Fall 2012 Western University Topic 9. Aldehydes & Ketones A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is the most important group in bio-organic
18: Reactions of Enolate Ions and Enols
 18: Reactions of Enolate Ions and Enols 18.1 Enolate Ions and Enols 18-3 Halogenation, Alkylation, and Condensation Reactions (18.1A) 18-3 Acidity of α-c-h's (18.1B) 18-4 Resonance Stabilization Enol Form
18: Reactions of Enolate Ions and Enols 18.1 Enolate Ions and Enols 18-3 Halogenation, Alkylation, and Condensation Reactions (18.1A) 18-3 Acidity of α-c-h's (18.1B) 18-4 Resonance Stabilization Enol Form
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group
 Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Chapter 16 Aldehydes and Ketones I Nucleophilic Addition to the Carbonyl Group Nomenclature of Aldehydes and Ketones Aldehydes are named by replacing the -e of the corresponding parent alkane with -al
Lecture Notes Chem 51C S. King. Chapter 20 Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation & Reduction
 Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
21.1 Introduction Carboxylic Acids Nomenclature of Carboxylic Acids. Acids Structure and Properties of Carboxylic Acids.
 21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
21.1 Introduction Carboxylic Acids Carboxylic acids are abundant in nature and in pharmaceuticals. 21.1 Introduction Carboxylic Acids The US produces over 2.5 million tons of acetic acid per year, which
Lecture 23. Amines. Chemistry 328N. April 12, 2016
 Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
Lecture 23 Amines April 12, 2016 Michael Reaction Michael reaction: conjugate addition of an enolate Arthur Michael anion to an, -unsaturated carbonyl compound!! Following are two examples in the first,
Michael and Aldol CH391 December 4, 2002
 Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Michael and Aldol H391 December 4, 2002 RH 2 H pk a = 16-20 + H RHH + Enolate anions... So.a basic solution contains comparable amounts of the aldehyde and its enolate. Which gives rise to..the Aldol ondensation
Chem 263 Nov 24, Properties of Carboxylic Acids
 Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
Chem 263 ov 24, 2009 Properties of Carboxylic Acids Since carboxylic acids are structurally related to both ketones and aldehydes, we would expect to see some similar structural properties. The carbonyl
CHEM 330. Topics Discussed on Oct 5. Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on Oct 2
 CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
CEM 330 Topics Discussed on ct 5 Irreversible nature of the reaction of carbonyl enolates with the electrophiles discussed on ct 2 Kinetic control in an irreversible reaction: the product that is obtained
Answers to Hour Examination #3, Chemistry 302X, 2006
 Answers to our Examination #, Chemistry 02X, 2006 1. (a). That SN2 shown just can t happen with the required inversion. The back of that methyl group is not reachable by the putative nucleophile, the pi
Answers to our Examination #, Chemistry 02X, 2006 1. (a). That SN2 shown just can t happen with the required inversion. The back of that methyl group is not reachable by the putative nucleophile, the pi
Chapter 16. Aldehydes and Ketones I. Nucleophilic Addition to the Carbonyl Group. Physical Properties of Aldehydes and Ketones. Synthesis of Aldehydes
 Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
Nomenclature of Aldehydes and Ketones Chapter 16 Aldehydes and Ketones I. Aldehydes replace the -e of the parent alkane with -al The functional group needs no number Nucleophilic Addition to the Carbonyl
When we deprotonate we generate enolates or enols. Mechanism for deprotonation: Resonance form of the anion:
 Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
Lecture 5 Carbonyl Chemistry III September 26, 2013 Ketone substrates form tertiary alcohol products, and aldehyde substrates form secondary alcohol products. The second step (treatment with aqueous acid)
Name. Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry II Examination #3 - March 31, 2003
 INSTRUTINS Name Department of hemistry SUNY/neonta hem 322 - rganic hemistry II Examination #3 - March 31, 2003 This examination has two parts. Part I is in multiple choice format and the answers should
INSTRUTINS Name Department of hemistry SUNY/neonta hem 322 - rganic hemistry II Examination #3 - March 31, 2003 This examination has two parts. Part I is in multiple choice format and the answers should
Chem 22 Final Exam Practice
 Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
Chem 22 Final Exam Practice Questions taken from regular tests given during the previous semesters. Only one answer is correct unless the question says otherwise. The questions are somewhat scrambled with
Carbonyl Chemistry. aldehydes ketones. carboxylic acid and derivatives. Wednesday, April 29, 2009
 Carbonyl Chemistry X aldehydes ketones carboxylic acid and derivatives Electrophiles (eg. + ) Nucleophiles (eg. C 3 MgBr) an enolate Base β β β α α α 1 2 3 4 Nuc- 1 2 Nuc 3 4 1,2-addition 1 2 3 4 Nuc-
Carbonyl Chemistry X aldehydes ketones carboxylic acid and derivatives Electrophiles (eg. + ) Nucleophiles (eg. C 3 MgBr) an enolate Base β β β α α α 1 2 3 4 Nuc- 1 2 Nuc 3 4 1,2-addition 1 2 3 4 Nuc-
CHEM Chapter 23. Carbonyl Condensation Reactions (quiz) W25
 CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
CHEM 2425. Chapter 23. Carbonyl Condensation Reactions (quiz) W25 Student: 1. Which of the following statements about Aldol reactions with either aldehydes or ketones is true? Equilibrium favors the starting
Carbonyl Chemistry IV + C O C. Lecture 10. Chemistry /30/02
 arbonyl hemistry IV Ō - + Lecture 10 Addition of Nitrogen Nucleophiles Primary Amines RN 2 Imines Secondary Amines R 2 N Enamines ydrazine derivatives RNN 2 ydrazones ydroxyl Amine N 2 ximes Imine Formation
arbonyl hemistry IV Ō - + Lecture 10 Addition of Nitrogen Nucleophiles Primary Amines RN 2 Imines Secondary Amines R 2 N Enamines ydrazine derivatives RNN 2 ydrazones ydroxyl Amine N 2 ximes Imine Formation
Tips for taking exams in 852
 Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
ORGANIC - CLUTCH CH ALDEHYDES AND KETONES: NUCLEOPHILIC ADDITION
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
MITOCW watch?v=gboyppj9ok4
 MITOCW watch?v=gboyppj9ok4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
MITOCW watch?v=gboyppj9ok4 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To
(Neither an oxidation or reduction: Addition or loss of H +, H 2 O, HX).
 eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
eactions of Alcohols Alcohols are versatile organic compounds since they undergo a wide variety of transformations the majority of which are either oxidation or reduction type reactions. xidation is a
Lecture 23. The Aldol Condensation. an Aldol! April 12, 2018 O H O H - Chemistry 328N
 Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
Lecture 23 The Aldol Condensation - b a an Aldol! April 12, 2018 Exam III - Wed April 18 WEL 2.122 7-9 PM Covers thru today omework ydrolysis Reactions Synthesis Get an A!!! Lithium diisopropylamide (LDA)
First Year Organic Chemistry THE CHEMISTRY OF THE CARBONYL GROUP: CORE CARBONYL CHEMISTRY
 First Year rganic Chemistry TE CEMISTY F TE CABNYL GUP: CE CABNYL CEMISTY Professor Tim Donohoe 8 lectures, T, weeks 1-4, 2015 Wednesdays at 9am; Fridays at 10am (Dyson Perrins) andout A You will be able
First Year rganic Chemistry TE CEMISTY F TE CABNYL GUP: CE CABNYL CEMISTY Professor Tim Donohoe 8 lectures, T, weeks 1-4, 2015 Wednesdays at 9am; Fridays at 10am (Dyson Perrins) andout A You will be able
Ch 19 Aldehydes and Ketones
 Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
Ch 19 Aldehydes and Ketones Aldehydes (RCHO), with the exception of formaldehyde (H 2 CO), are compounds with both an H and an organic group attached to a carbonyl. Ketones (R 2 CO) are compounds with
Lecture 24 Two Germans and an Englishman
 Lecture 24 Two Germans and an Englishman Robert Robinson 1886-1975 Nobel Laureate 1947 April 17, 2018 tto Paul Hermann Diels 1876-1954 Nobel Laureates 1950 Kurt Alder 1902-1958 Exam III Tomorrow Wed April
Lecture 24 Two Germans and an Englishman Robert Robinson 1886-1975 Nobel Laureate 1947 April 17, 2018 tto Paul Hermann Diels 1876-1954 Nobel Laureates 1950 Kurt Alder 1902-1958 Exam III Tomorrow Wed April
ENOLATES IN ORGANIC SYNTHESIS
 ENLATES IN RGANIC SYNTHESIS 1 ENLATES IN RGANIC SYNTHESIS Recall Enolate alkylation, Aldol addition and condensation can provide access to a wide variety of multi-functional compounds, which can lend themselves
ENLATES IN RGANIC SYNTHESIS 1 ENLATES IN RGANIC SYNTHESIS Recall Enolate alkylation, Aldol addition and condensation can provide access to a wide variety of multi-functional compounds, which can lend themselves
Chapter 25: The Chemistry of Life: Organic and Biological Chemistry
 Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Chemistry: The Central Science Chapter 25: The Chemistry of Life: Organic and Biological Chemistry The study of carbon compounds constitutes a separate branch of chemistry known as organic chemistry The
Exam 1 (Monday, July 6, 2015)
 Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
Chem 231 Summer 2015 Assigned Homework Problems Last updated: Friday, July 24, 2015 Problems Assigned from Essential Organic Chemistry, 2 nd Edition, Paula Yurkanis Bruice, Prentice Hall, New York, NY,
12. Aldehydes & Ketones (text )
 2009, Department of Chemistry, The University of Western ntario 12.1 12. Aldehydes & Ketones (text 13.1 13.11) A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is
2009, Department of Chemistry, The University of Western ntario 12.1 12. Aldehydes & Ketones (text 13.1 13.11) A. Structure and Nomenclature The carbonyl group is present in aldehydes and ketones and is
Chem 263 Notes March 2, 2006
 Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Lecture 19. Carboxylic Acids O C OH O R C O - + H + O - Chemistry 328N
 Lecture 19 arboxylic Acids R R - + + R - March 29, 2018 Review: Selectivity in Reduction LiAl 4 reduces any and all carbonyl compounds to the corresponding alcohols NaB 4 only reduces aldehydes and ketone
Lecture 19 arboxylic Acids R R - + + R - March 29, 2018 Review: Selectivity in Reduction LiAl 4 reduces any and all carbonyl compounds to the corresponding alcohols NaB 4 only reduces aldehydes and ketone
Chapter 10: Carboxylic Acids and Their Derivatives
 Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 10: Carboxylic Acids and Their Derivatives The back of the white willow tree (Salix alba) is a source of salicylic acid which is used to make aspirin (acetylsalicylic acid) The functional group
Chapter 20 Carboxylic Acid Derivatives. Nucleophilic Acyl Substitution
 ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
75. A This is a Markovnikov addition reaction. In these reactions, the pielectrons in the alkene act as a nucleophile. The strongest electrophile will
 71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
71. B SN2 stands for substitution nucleophilic bimolecular. This means that there is a bimolecular rate-determining step. Therefore, the reaction will follow second-order kinetics based on the collision
3 - CONJUGATION. More than one double bond can be in a given compound: n=0
 3 - NJUGATIN 1. Terminology and Nomenclature (SF 13.1 13.6; SFS 13.1 13.6) A compound containing a double bond is called an alkene, olefin or maybe simply "ene". There are often other names associated
3 - NJUGATIN 1. Terminology and Nomenclature (SF 13.1 13.6; SFS 13.1 13.6) A compound containing a double bond is called an alkene, olefin or maybe simply "ene". There are often other names associated
Answers To Chapter 7 Problems.
 Answers To Chapter Problems.. Most of the Chapter problems appear as end-of-chapter problems in later chapters.. The first reaction is an ene reaction. When light shines on in the presence of light and
Answers To Chapter Problems.. Most of the Chapter problems appear as end-of-chapter problems in later chapters.. The first reaction is an ene reaction. When light shines on in the presence of light and
I5 ELECTROPHILIC SUBSTITUTIONS OF
 Section I Aromatic chemistry I5 ELECTPILIC SUBSTITUTINS F MN-SUBSTITUTED AMATIC INGS Key Notes ortho, meta and para substitution Substituent effect eaction profile Activating groups inductive o/p Deactivating
Section I Aromatic chemistry I5 ELECTPILIC SUBSTITUTINS F MN-SUBSTITUTED AMATIC INGS Key Notes ortho, meta and para substitution Substituent effect eaction profile Activating groups inductive o/p Deactivating
Important Concepts. Problems. Chapter Problems. Cuprate additions followed by enolate alkylations. 15. Michael Addition (Section 18-11)
 Problems hapter 18 861 uprate additions followed by enolate alkylations P 1. R 2uLi, TF 2. RX A A R R N 15. Michael Addition (Section 18-11) D P D R P R A RR 16. Robinson Annulation (Section 18-11) Important
Problems hapter 18 861 uprate additions followed by enolate alkylations P 1. R 2uLi, TF 2. RX A A R R N 15. Michael Addition (Section 18-11) D P D R P R A RR 16. Robinson Annulation (Section 18-11) Important
CH 3 CHCH 3 CH 3 CHCH 3 Isopropyl cation. Oxomium ion intermediate. intermediate (an electrophile)
 Understanding (as opposed to memorizing) mechanisms is critical to mastering organic chemistry. Although the mechanisms you encounter throughout the course may seem entirely different, they are actually
Understanding (as opposed to memorizing) mechanisms is critical to mastering organic chemistry. Although the mechanisms you encounter throughout the course may seem entirely different, they are actually
A. Loupy, B.Tchoubar. Salt Effects in Organic and Organometallic Chemistry
 A. Loupy, B.Tchoubar Salt Effects in Organic and Organometallic Chemistry 1 Introduction - Classification of Specific Salt Effects 1 1.1 Specific Salt Effects Involving the Salt's Lewis Acid or Base Character
A. Loupy, B.Tchoubar Salt Effects in Organic and Organometallic Chemistry 1 Introduction - Classification of Specific Salt Effects 1 1.1 Specific Salt Effects Involving the Salt's Lewis Acid or Base Character
b.p.=100 C b.p.=65 C b.p.=-25 C µ=1.69 D µ=2.0 D µ=1.3 D
 Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 6 Dr Ali El-Agamey 1 Oxidation States Easy for inorganic salts: CrO 4 2- reduced to Cr 2 O 3. KMnO 4 reduced to MnO 2. Oxidation: Gain of O,
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 6 Dr Ali El-Agamey 1 Oxidation States Easy for inorganic salts: CrO 4 2- reduced to Cr 2 O 3. KMnO 4 reduced to MnO 2. Oxidation: Gain of O,
Carboxylic Acids O R C + H + O - Chemistry 618B
 arboxylic Acids R H R + H + - R - Nomenclature - IUPA IUPA names: drop the -e from the parent alkane and add the suffix -oic acid If the compound contains a carbon-carbon double bond, change the infix
arboxylic Acids R H R + H + - R - Nomenclature - IUPA IUPA names: drop the -e from the parent alkane and add the suffix -oic acid If the compound contains a carbon-carbon double bond, change the infix
How to make pyridines: the Hantzsch pyridine synthesis
 ow to make pyridines: the antzsch pyridine synthesis 1191 Zinc in acetic acid (Chapter 24) reduces the oxime to the amine and we can start the synthesis by doing the conjugate addition and then reducing
ow to make pyridines: the antzsch pyridine synthesis 1191 Zinc in acetic acid (Chapter 24) reduces the oxime to the amine and we can start the synthesis by doing the conjugate addition and then reducing
THE CHEMISTRY OF THE CARBONYL GROUP
 TE CEMISTY F TE CABYL GUP Professor Tim Donohoe 8 lectures, T, weeks 1-4, 2007 andout A C C You will be able to download copies of the handouts from this course at http://users.ox.ac.uk/~magd1571/teaching/teaching.htm
TE CEMISTY F TE CABYL GUP Professor Tim Donohoe 8 lectures, T, weeks 1-4, 2007 andout A C C You will be able to download copies of the handouts from this course at http://users.ox.ac.uk/~magd1571/teaching/teaching.htm
Lecture 13A 05/11/12. Amines. [Sn2; Hofmann elimination; reduction of alkyl azides, amides, nitriles, imines; reductive amination; Gabriel synthesis]
![Lecture 13A 05/11/12. Amines. [Sn2; Hofmann elimination; reduction of alkyl azides, amides, nitriles, imines; reductive amination; Gabriel synthesis] Lecture 13A 05/11/12. Amines. [Sn2; Hofmann elimination; reduction of alkyl azides, amides, nitriles, imines; reductive amination; Gabriel synthesis]](/thumbs/86/93838793.jpg) Lecture 13A 05/11/12 Amines [Sn2; ofmann elimination; reduction of alkyl azides, amides, nitriles, imines; reductive amination; Gabriel synthesis] Curtius and ofmann rearrangements Both of these, in principle,
Lecture 13A 05/11/12 Amines [Sn2; ofmann elimination; reduction of alkyl azides, amides, nitriles, imines; reductive amination; Gabriel synthesis] Curtius and ofmann rearrangements Both of these, in principle,
Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013
 Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013 Lecture 9 Biochemical Transformations I. Carbon-carbon bond forming and cleaving reactions in Biology (see the Lexicon). Enzymes catalyze a limited
Chemistry 5.07SC Biological Chemistry I Fall Semester, 2013 Lecture 9 Biochemical Transformations I. Carbon-carbon bond forming and cleaving reactions in Biology (see the Lexicon). Enzymes catalyze a limited
Chapter 15. Reactions of Aromatic Compounds. Electrophilic Aromatic Substitution on Arenes. The first step is the slow, rate-determining step
 Electrophilic Aromatic Substitution on Arenes Chapter 15 Reactions of Aromatic Compounds The characteristic reaction of aromatic rings is substitution initiated by an electrophile halogenation nitration
Electrophilic Aromatic Substitution on Arenes Chapter 15 Reactions of Aromatic Compounds The characteristic reaction of aromatic rings is substitution initiated by an electrophile halogenation nitration
Carbon-heteroatom single bonds basic C N C X. X= F, Cl, Br, I Alkyl Halide C O. epoxide Chapter 14 H. alcohols acidic H C S C. thiols.
 hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
hapter 13: Alcohols and Phenols 13.1 Structure and Properties of Alcohols Alkanes arbon - arbon Multiple Bonds arbon-heteroatom single bonds basic Alkenes X X= F, l,, I Alkyl alide amines hapter 23 nitro
18.8 Oxidation. Oxidation by silver ion requires an alkaline medium
 18.8 Oxidation Oxidation by silver ion requires an alkaline medium Test for detecting aldehydes Tollens reagent to prevent precipitation of the insoluble silver oxide, a complexing agent is added: ammonia
18.8 Oxidation Oxidation by silver ion requires an alkaline medium Test for detecting aldehydes Tollens reagent to prevent precipitation of the insoluble silver oxide, a complexing agent is added: ammonia
CHAPTER 24 HW: CARBONYL CONDENSATIONS
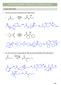 CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
CAPTER 24 W: CARBNYL CNDENSATINS ALDL REACTIN 1. Draw the curved arrow mechanism for each aldol reaction. Na 2 product Na Et prod. 2. Give the curved arrow mechanism for this aldol reaction (and dehydration
Chapter 11 Reaction of Alcohols
 Chapter 11 eaction of Alcohols xidation of alcohols Alcohols are at the same oxidation level as alkenes Therefore alkenes can be converted to alcohols with acidic water PDC or PCC 2 C C 2 3 + X 3 C 3 C
Chapter 11 eaction of Alcohols xidation of alcohols Alcohols are at the same oxidation level as alkenes Therefore alkenes can be converted to alcohols with acidic water PDC or PCC 2 C C 2 3 + X 3 C 3 C
The Claisen Condensation
 Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
Lecture 22 The Claisen Condensation CH 3 CCH 2 CH 3 CH 2 CCH 2 CH 3 April 10, 2018 Hydrolysis of Amides Hydrolysis of amides is irreversible. In acid solution the amine product is protonated to give an
Ketones and Aldehydes Reading Study Problems Key Concepts and Skills Lecture Topics: Structure of Ketones and Aldehydes Structure:
 Ketones and Aldehydes Reading: Wade chapter 18, sections 18-1- 18-21 Study Problems: 18-43, 18-44,18-50, 18-51, 18-52, 18-59, 18-60, 18-62, 18-64, 18-72. Key Concepts and Skills: Interpret the IR, NMR,
Ketones and Aldehydes Reading: Wade chapter 18, sections 18-1- 18-21 Study Problems: 18-43, 18-44,18-50, 18-51, 18-52, 18-59, 18-60, 18-62, 18-64, 18-72. Key Concepts and Skills: Interpret the IR, NMR,
Chapter 17 Reactions of Aromatic Compounds. Electrophilic Aromatic Substitution
 Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Electrophile substitutes for a hydrogen on the benzene ring. Chapter 17: Aromatics 2-Reactions Slide 17-2 1 Mechanism Step
Chapter 17 Reactions of Aromatic Compounds Electrophilic Aromatic Substitution Electrophile substitutes for a hydrogen on the benzene ring. Chapter 17: Aromatics 2-Reactions Slide 17-2 1 Mechanism Step
Score: Homework Problem Set 9 Iverson CH320N Due Monday, April 17. NAME (Print): Chemistry 320N Dr. Brent Iverson 9th Homework April 10, 2017
 omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
omework Problem Set 9 Iverson C0N Due Monday, April 7 NAME (Print): SIGNATURE: Chemistry 0N Dr. ent Iverson 9th omework April 0, 07 Please print the first three letters of your last name in the three boxes
Chem 263 March 7, 2006
 Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
Chem 263 March 7, 2006 Aldehydes and Ketones Aldehydes and ketones contain a carbonyl group, in which the carbon atom is doubly bonded to an oxygen atom. The carbonyl group is highly polarized, with a
CHEM 343 Principles of Organic Chemistry II Summer Instructor: Paul J. Bracher. Quiz # 3. Monday, July 21 st, :30 a.m.
 CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
CHEM 343 Principles of Organic Chemistry II Summer 2014 Quiz # 3 Solutions Key Page 1 of 9 CHEM 343 Principles of Organic Chemistry II Summer 2014 Instructor: Paul J. Bracher Quiz # 3 Monday, July 21 st,
