Prediction of Vapour Pressures of Dioxin Congeners. R & D Center, Baoshan Iron & Steel Co., Ltd., Fujin Rd., Baoshan District, Shanghai , China
|
|
|
- Albert Watkins
- 5 years ago
- Views:
Transcription
1 Prediction of Vaour Pressures of Dioxin Congeners Xian-Wei Li, 1 Etsuro Shibata, 2 Eiki Kasai, 2 Takashi Nakamura 2 1 R & D Center, Baoshan Iron & Steel Co., Ltd., Fujin Rd., Baoshan District, Shanghai , China 2 Institute of Multidiscilinary Research for Advanced Material, Tohoku University, 1,1 Katahira, 2-Chome, Aobaku, Sendai , Jaan Abstract: The vaour ressures and thermal roerties of non-measured olychlorinated dibenzo--dioxins and furans (PCDDs/PCDFs) are redicted by using a correlation method based on the exerimental vaor ressures obtained by the Knudsen effusion method. The vaor ressures of all the 59 PCDDs and 131 PCDFs redicted here are more or less higher than the data redicted by Rordorf, although the calculation method is the same. For the most toxic 2,3,7,8-TeCDD, the vaour ressure at 298 K redicted in this study is Pa, 31 times higher than the value rovided by Rordorf. Keywords: PCDDs/PCDFs, Dioxins, Vaour ressure 1 Introduction Vaour ressures are fundamental roerties of ersistent organic ollutants and are imortant in determining their distributions and results in the environment. Due to large number of dioxin congeners and of other POPs, exerimental measurements can only cover a minor fraction of them, so that the rediction of vaour ressures of dioxin congeners by semi emirical correlations is useful and necessary. Prediction methods thus lay an imortant role in environmental research, roviding data otherwise inaccessible, and those more doubtful in testing. Numerous equations and correlations for estimating vaour ressure are resented in literature. Most methods are emirical and the derived results are very rough. [1-4] Rordorf [5-7] suggested a good correlation method to redict vaour ressure, which derives from thermodynamic theory. Based on exerimental vaour 1.
2 ressure data, this method gives not only the enthaly and entroy of sublimation but also the enthaly of melting and boiling oint. Based on our exerimental data reorted in the recently aer [8], this research emloys the correlation method to redict the vaour ressure of all PCDDs/PCDFs. Correlation methods are of articular imortance when dealing with dioxin congeners since there are thousands of ossible homologues and isomers. Correlation methods also allow testing of exerimental data set for self-consistency. Calculation of related substance roerties (i.e. boiling oints and enthalies of fusion) is ossible starting from vaour ressure data. 2 Prediction methods Equations that relate vaour ressure to temerature are commonly derived by integration of the Clausius-Claeyron equation: [1] d ln /dt= H v /( Z R T 2 ) (I) where is the vaour ressure, H v is the heat of vaorization, R is the gas constant, T is the temerature, and Z is a comressibility factor, given by Z = V/(R T) (II) where V is the volume difference between vaour and liquid. The correlation method uses the liquid hase as a reference state, as has been the case for most redictive vaour ressure methods. Suersaturated liquid hases are usually defined at the temerature of interest. A full use is made of the measured temerature deendence of the vaour ressure of the solid. The data are extraolated to the melting oint and the liquid hase is brought in at this oint. Enthalies ( H m ) and entroies of fusion ( S m ) are defined for the melting temerature (T m ) and unknown temerature deendencies of these functions are not involved. The two arameters are determined for the different comounds and are found to vary significantly between these comounds. The redicted method is not limited by assumtion of either temerature or substance indeendence of the entroies of fusion. Entroies of fusion are affected by symmetry relations between molecule and crystal lattice. Figure 1 illustrates the calculation rocedure of vaour ressure correlation method suggested by Rordorf. 2.
3 These correlations ermit the estimation of boiling oints and enthalies of evaoration for dioxins and the solid-state vaour ressures can be redicted by the equations of Fig 1 for dioxin congeners of known melting oints. ln (/Pa) T b (T b )=101,325 Pa Liquid T b = boiling oint G = molar Gibbs energy T m = melting oint T m liqud = solid H = molar enthaly S = molar entroy Subcooled liquid C = molar heat caacity R = gaas constant Solid (T) = vaour ressure at T Subscrit s : sublimation Subscrit v : vaorization K/T Subscrit m : fusion At the boiling oint: H v (T b ) = T b K F (36.61+R ln T b ) (1) where K F =1.01 S v (T b ) = H v (T b )/T b + R ln (2) where =101,325 Pa In the liquid hase: H ( T v 2 ) = H v ( T ) + C 1 T2 T1 gas liq. dt gives after integration for T 1 =T b and T 2 =T: H v (T) = H v (T b ) [1 + K ( 1 T/T b )] (4) with K = -T b C gas-liq. / H v (T b ) (5) Setting formula (4) into the Clausius-Claeyron equation, d ln ( T ) = T b T H v ( T b )[1 + K(1 T / T b (3) 2 )]/ RT dt gives after integration from the melting to boiling oint: ln (T m ) = ln - ( H v (T b )/R T b )[(1+K)(T b /T m -1)-K ln(t b /T m ) (7) At the melting oint: From G s = G m + G v (8) H m = H s - H v (9) and G = H - T S (10) follows S m = S s - S v (11) For T=T m : G m (T m )= 0 (12) and S m (T m ) = H m (T m )/ T m (13) From G v = - RT ln liq. = H v - T S v (14) follows for T = T m : S v (T m ) = R ln liq (T m ). + H v (T m )/T m (15) From equation (4), for T=T m : H v (T m ) = H v (T b ) [1 + K ( 1 T m /T b )] (4) In the solid hase: G s (T) = -RT ln solid (T) = H s (T) T S s (T) (16) R ln solid ( T ) = [ S s ( T m ) + T Tm C g s / TdT ] [ H R ln solid (T) = S s (T m ) - H s (T m )/T (18) s ( T m ) / T + Fig 1 The calculation rocedure of correlation method suggested by Rordorf [5]. (Relationshi between the vaour ressures of the solid and the liquid hase) (6) T Tm C g s dt ] (17) 3.
4 3 Predicted vaour ressure results for PCDDs/PCDFs Enthalies and entroies of sublimation of the 22 PCDDs/PCDFs were obtained by linear regression of the exerimental vaour ressures over the indicated temerature intervals in the aer ublished recently [8]. They hold for the mid temerature, T mid =2(T max T min )/(T max + T min ), of the investigated temerature ranges, and are recalculated for the melting oints and room temerature by means of bracket exressions of equation (17). The melting oint (T m ) data come from literature [9]. The boiling oint (T b ) aears in several exressions and is determined in an iterative rocedure. EXCEL is used for the solution of the equations shown in Fig 1 by an iterative rocedure. Figures 2 and 3 show that the enthaly of sublimation and derived boiling oint of PCDDs and PCDFs correlate well with the substitution number of chlorine, while the melting oint and entroy of sublimation show a great deal of scatter PCDDs 350 PCDDs H sub(298k), kj/mol S sub, J/mol/K y = x R 2 = y = x R 2 = (a) (b) PCDFs PCDFs H sub(298k), kj/mol S sub(298k), J/mol/K y = x R 2 = y = x R 2 =
5 (c) (d) Fig 2 Chlorine correlations of thermal roerties, (a) enthaly of sublimation of PCDDs, (b) entroy of sublimation of PCDDs, (c) enthaly of sublimation of PCDFs, (d) entroy of sublimation of PCDFs PCDDs 800 PCDFs Tb (K) Tb (K) y = x R 2 = y = x R 2 = (a) 500 Cl substituion number (b) Fig 3 Chlorine correlations of boiling oint of PCDDs and PCDFs The determined boiling oints are then set for correct rediction of the vaour ressures ( liquid (T m )= solid (T m )) at the melting oints. The determined boiling oints and enthalies of fusion of the dioxin congeners are also correlated with the degree of chlorine substitution. Figures 3 and 4 show the correlations of the redicted boiling oints and enthalies of fusion with the degree of chlorination (x). The relation is as the following. For PCDDs: T b = x ( K) H m (T m ) = 3.808x ( kj mol -1 ) For PCDFs: T b = x (K) H m (T m ) = 3.553x (kj mol -1 ) Using these formulas, boiling oints and enthalies of fusion are redicted for unknown dioxin congeners, and then the solid-state vaour ressures are calculated by the equations of Fig 1. 5.
6 The thermal roerties and vaour ressures for 59 PCDDs and 131 PCDFs are redicted. 100 PCDDs 100 PCDFs H (T m), kj/mol H (T m), kj/mol Hv(Tm) Hm(Tm) 0 20 Hv(Tm) Hm(Tm) 0 (a) (b) Fig 4 Chlorine correlations of enthalies of evaoration and fusion of PCDDs and PCDFs at melting oint All the vaour ressures of PCDDs/PCDFs redicted here are more or less higher than the data redicted by Rordorf [7] for the same comounds. Figure 5 shows the vaour ressure differences between Rordorf s study and this study for the toxic congeners, which are all substituted at the ositions 2, 3, 7, and 8. For the most toxic 2,3,7,8-TeCDD, the redicted vaour ressure at 25 by this study is Pa, which is 31 times higher than the value given by Rordorf. Therefore, the concentration of air saturated with 2,3,7,8-TeCDD at 25 can arrive at 805 ng/m 3. 6.
7 ln ( /Pa) -10 ln ( /Pa) ,3,7,8-TeCDD,This study 2,3,7,8-TeCDD, Rordorf 1,2,3,7,8-PeCDD,This study 1,2,3,7,8-PeCDD, Rordorf 1,2,3,4,7,8-HxCDD,This study 1,2,3,4,7,8-HxCDD, Rordorf (K/T ) 1,2,3,7,8,9-HxCDD,This study -17 1,2,3,7,8,9-HxCDD, Rordorf 1,2,3,6,7,8-HxCDD,This study 1,2,3,6,7,8-HxCDD, Rordorf 1,2,3,4,6,7,8-HCDD,This study 1,2,3,4,6,7,8-HCDD, Rordorf (K/T ) (a) (b) ln ( /Pa) -10 ln ( /Pa) ,3,7,8-TeCDF, This study 2,3,7,8-TeCDF, Rordorf 1,2,3,7,8-PeCDF, This study 1,2,3,7,8-PeCDF, Rordorf 1,2,3,4,7,8-HxCDF, This study 1,2,3,4,7,8-HxCDF, Rordorf ,3,4,7,8-PeCDF, This study 2,3,4,7,8-PeCDF, Rordorf 2,3,4,6,7,8-HxCDF, This study 2,3,4,6,7,8-HxCDF, Rordorf 1,2,3,4,7,8,9-HCDF, This study 1,2,3,4,7,8,9-HCDF, Rordorf (K/T ) 1000 (K/T ) (c) (d) Fig 5 Comarison of vaour ressure of high chlorinated PCDDs/PCDFs redicted by Rordorf s study and this study 4 Conclusions Based on our measured vaour ressure data set of a number of dioxin congeners, the vaour ressure and thermal roerties of non-measured PCDDs/PCDFs are redicted by using a correlation method. The enthaly of 7.
8 sublimation and calculated boiling oint correlate well with the degree of chlorination, while the melting oint and entroy of sublimation show a great deal of scatter. All vaour ressures of the 59 PCDDs and 131 PCDFs redicted here are more or less higher than the data redicted by Rordorf, although the calculated method is the same. For the most toxic 2,3,7,8-TeCDD, the vaour ressure at 25 redicted by this study is Pa, which is 31 times higher than the value given by Rordorf. The concentration of air saturated with 2,3,7,8-TeCDD at 25 can hence attain 805 ng/m 3. References 1 Lyman WJ, Reehl WF, Rosenblatt DH. Handbook of Chemical Proerty Estimation Methods, American Chemical Society, Washington, DC, Miller DG. Estimating vaor ressures a comarison of equations. Ind. Eng. Chem., 56 (1964), Miller DG. Derivation of two equations for the estimation of vaor ressures, J. Phys. Chem., 68 (1964), Partington JR. An Advanced Treatise on Physical Chemistry( Vol. 2). Longmans, Green and Co., London, Rordorf BF. Thermal roerties of dioxins, furans and related comounds. Chemoshere, 15 (1986), Rordorf BF. Prediction of vaor ressures, boiling oints and enthalies of fusion for twenty-nine halogenated dibenzo--dioxines. Thermochim. Acta, 112 (1987), Rordorf BF. Prediction of vaour ressures, boiling oints and enthalies of fusion for twenty-nine halogenated dibenzo--dioxins and fifty-five dibenzofurans by a vaor ressure correlation method. Chemoshere, 18 8.
9 (1989), Li X-W, Shibata E, Kasai E, and Nakamura T.. Vaor ressures and enthalies of sublimation of 17 olychlorinated dibenzo--dioxins and 5 olychlorinated dibenzofurans. Environmental Toxicology and Chemistry,23 (2004), Shiu W-Y, Ma K-C. Temerature deendence of hysical-chemical roerties of selected chemicals of environmental interest. II. Chlorobenzenes, olychlorinated bihenyls, olychlorinated dibenzo--dioxins, and dibenzofurans. J. Phys. Chem. Ref. Data,29 (2000)
dn i where we have used the Gibbs equation for the Gibbs energy and the definition of chemical potential
 Chem 467 Sulement to Lectures 33 Phase Equilibrium Chemical Potential Revisited We introduced the chemical otential as the conjugate variable to amount. Briefly reviewing, the total Gibbs energy of a system
Chem 467 Sulement to Lectures 33 Phase Equilibrium Chemical Potential Revisited We introduced the chemical otential as the conjugate variable to amount. Briefly reviewing, the total Gibbs energy of a system
δq T = nr ln(v B/V A )
 hysical Chemistry 007 Homework assignment, solutions roblem 1: An ideal gas undergoes the following reversible, cyclic rocess It first exands isothermally from state A to state B It is then comressed adiabatically
hysical Chemistry 007 Homework assignment, solutions roblem 1: An ideal gas undergoes the following reversible, cyclic rocess It first exands isothermally from state A to state B It is then comressed adiabatically
Phase transition. Asaf Pe er Background
 Phase transition Asaf Pe er 1 November 18, 2013 1. Background A hase is a region of sace, throughout which all hysical roerties (density, magnetization, etc.) of a material (or thermodynamic system) are
Phase transition Asaf Pe er 1 November 18, 2013 1. Background A hase is a region of sace, throughout which all hysical roerties (density, magnetization, etc.) of a material (or thermodynamic system) are
The Second Law: The Machinery
 The Second Law: The Machinery Chater 5 of Atkins: The Second Law: The Concets Sections 3.7-3.9 8th Ed, 3.3 9th Ed; 3.4 10 Ed.; 3E 11th Ed. Combining First and Second Laws Proerties of the Internal Energy
The Second Law: The Machinery Chater 5 of Atkins: The Second Law: The Concets Sections 3.7-3.9 8th Ed, 3.3 9th Ed; 3.4 10 Ed.; 3E 11th Ed. Combining First and Second Laws Proerties of the Internal Energy
FUGACITY. It is simply a measure of molar Gibbs energy of a real gas.
 FUGACITY It is simly a measure of molar Gibbs energy of a real gas. Modifying the simle equation for the chemical otential of an ideal gas by introducing the concet of a fugacity (f). The fugacity is an
FUGACITY It is simly a measure of molar Gibbs energy of a real gas. Modifying the simle equation for the chemical otential of an ideal gas by introducing the concet of a fugacity (f). The fugacity is an
Institute of Multidisciplinary Research for Advanced Materials (IMRAM), Tohoku University, Sendai , Japan
 Materials Transactions, Vol. 43, No. 11 (2002) pp. 2903 to 2907 c 2002 The Japan Institute of Metals EXPRESS REGULAR ARTICLE Vapour Pressure Determination for Dibenzo- p-dioxin, Dibenzofuran, Octachlorodibenzo-
Materials Transactions, Vol. 43, No. 11 (2002) pp. 2903 to 2907 c 2002 The Japan Institute of Metals EXPRESS REGULAR ARTICLE Vapour Pressure Determination for Dibenzo- p-dioxin, Dibenzofuran, Octachlorodibenzo-
3. High Temperature Gases FPK1 2009/MZ 1/23
 3. High Temerature Gases FP 9/MZ /3 Terms and Concets Dissociation Diatomic element gases Bond energy, dissociation energy (enthali) Flood s dissociation diagram Vaorization, eaoration, boiling, sublimation
3. High Temerature Gases FP 9/MZ /3 Terms and Concets Dissociation Diatomic element gases Bond energy, dissociation energy (enthali) Flood s dissociation diagram Vaorization, eaoration, boiling, sublimation
/ p) TA,. Returning to the
 Toic2610 Proerties; Equilibrium and Frozen A given closed system having Gibbs energy G at temerature T, ressure, molecular comosition (organisation ) and affinity for sontaneous change A is described by
Toic2610 Proerties; Equilibrium and Frozen A given closed system having Gibbs energy G at temerature T, ressure, molecular comosition (organisation ) and affinity for sontaneous change A is described by
Chemistry 531 Spring 2009 Problem Set 6 Solutions
 Chemistry 531 Sring 2009 Problem Set 6 Solutions 1. In a thermochemical study of N 2, the following heat caacity data were found: t 0 C,m d 27.2Jmol 1 K 1 f t b f C,m d 23.4Jmol 1 K 1 C,m d 11.4Jmol 1
Chemistry 531 Sring 2009 Problem Set 6 Solutions 1. In a thermochemical study of N 2, the following heat caacity data were found: t 0 C,m d 27.2Jmol 1 K 1 f t b f C,m d 23.4Jmol 1 K 1 C,m d 11.4Jmol 1
Phase Equilibrium Calculations by Equation of State v2
 Bulletin of Research Center for Comuting and Multimedia Studies, Hosei University, 7 (3) Published online (htt://hdl.handle.net/4/89) Phase Equilibrium Calculations by Equation of State v Yosuke KATAOKA
Bulletin of Research Center for Comuting and Multimedia Studies, Hosei University, 7 (3) Published online (htt://hdl.handle.net/4/89) Phase Equilibrium Calculations by Equation of State v Yosuke KATAOKA
Liquid water static energy page 1/8
 Liquid water static energy age 1/8 1) Thermodynamics It s a good idea to work with thermodynamic variables that are conserved under a known set of conditions, since they can act as assive tracers and rovide
Liquid water static energy age 1/8 1) Thermodynamics It s a good idea to work with thermodynamic variables that are conserved under a known set of conditions, since they can act as assive tracers and rovide
4. A Brief Review of Thermodynamics, Part 2
 ATMOSPHERE OCEAN INTERACTIONS :: LECTURE NOTES 4. A Brief Review of Thermodynamics, Part 2 J. S. Wright jswright@tsinghua.edu.cn 4.1 OVERVIEW This chater continues our review of the key thermodynamics
ATMOSPHERE OCEAN INTERACTIONS :: LECTURE NOTES 4. A Brief Review of Thermodynamics, Part 2 J. S. Wright jswright@tsinghua.edu.cn 4.1 OVERVIEW This chater continues our review of the key thermodynamics
I affirm that I have never given nor received aid on this examination. I understand that cheating in the exam will result in a grade F for the class.
 Chem340 Physical Chemistry for Biochemists Exam Mar 16, 011 Your Name _ I affirm that I have never given nor received aid on this examination. I understand that cheating in the exam will result in a grade
Chem340 Physical Chemistry for Biochemists Exam Mar 16, 011 Your Name _ I affirm that I have never given nor received aid on this examination. I understand that cheating in the exam will result in a grade
602 ZHANG Zhi and CHEN Li-Rong Vol Gibbs Free Energy From the thermodynamics, the Gibbs free energy of the system can be written as G = U ; TS +
 Commun. Theor. Phys. (Beijing, China) 37 (2002) 601{606 c International Academic Publishers Vol. 37, No. 5, May 15, 2002 The 2D Alternative Binary L J System: Solid-Liquid Phase Diagram ZHANG Zhi and CHEN
Commun. Theor. Phys. (Beijing, China) 37 (2002) 601{606 c International Academic Publishers Vol. 37, No. 5, May 15, 2002 The 2D Alternative Binary L J System: Solid-Liquid Phase Diagram ZHANG Zhi and CHEN
General Physical Chemistry I
 General Physical Cheistry I Lecture 12 Aleksey Kocherzhenko Aril 2, 2015" Last tie " Gibbs free energy" In order to analyze the sontaneity of cheical reactions, we need to calculate the entroy changes
General Physical Cheistry I Lecture 12 Aleksey Kocherzhenko Aril 2, 2015" Last tie " Gibbs free energy" In order to analyze the sontaneity of cheical reactions, we need to calculate the entroy changes
Phase transitions. Lectures in Physical Chemistry 4. Tamás Turányi Institute of Chemistry, ELTE. Phases
 Phase transitions Lectures in Physical Cheistry 4 Taás Turányi Institute of Cheistry, ELTE Phases DEF a syste is hoogeneous, if () it does not contain arts searated by acroscoic surfaces, and () all intensive
Phase transitions Lectures in Physical Cheistry 4 Taás Turányi Institute of Cheistry, ELTE Phases DEF a syste is hoogeneous, if () it does not contain arts searated by acroscoic surfaces, and () all intensive
AT 25 C! CH10090 Thermodynamics (part 2) Enthalpy changes during reactions. Let s remember what we did in CH10089
 CH10090 hermodynamics (art ) Let s remember what we did in CH10089 Enthaly changes during reactions o o o H98 ( reaction) = νi Hf, 98( roducts) νi Hf, 98( reactants) ν i reresents the stoichiometric coefficient.
CH10090 hermodynamics (art ) Let s remember what we did in CH10089 Enthaly changes during reactions o o o H98 ( reaction) = νi Hf, 98( roducts) νi Hf, 98( reactants) ν i reresents the stoichiometric coefficient.
F = U TS G = H TS. Availability, Irreversibility S K Mondal s Chapter 5. max. actual. univ. Ns =
 Availability, Irreversibility S K Mondal s Chater 5 I = W W 0 max ( S) = Δ univ actual 7. Irreversibility rate = I = rate of energy degradation = rate of energy loss (Wlost) = 0 S gen for all rocesses
Availability, Irreversibility S K Mondal s Chater 5 I = W W 0 max ( S) = Δ univ actual 7. Irreversibility rate = I = rate of energy degradation = rate of energy loss (Wlost) = 0 S gen for all rocesses
Speed of sound measurements in liquid Methane at cryogenic temperature and for pressure up to 10 MPa
 LNGII - raining Day Delft, August 07 Seed of sound measurements in liquid Methane at cryogenic temerature and for ressure u to 0 MPa Simona Lago*, P. Alberto Giuliano Albo INRiM Istituto Nazionale di Ricerca
LNGII - raining Day Delft, August 07 Seed of sound measurements in liquid Methane at cryogenic temerature and for ressure u to 0 MPa Simona Lago*, P. Alberto Giuliano Albo INRiM Istituto Nazionale di Ricerca
The Role of Water Vapor. atmosphere (we will ignore the solid phase here) Refer to the phase diagram in the web notes.
 The Role of Water Vaor Water can exist as either a vaor or liquid in the atmoshere (we will ignore the solid hase here) under a variety of Temerature and ressure conditions. Refer to the hase diagram in
The Role of Water Vaor Water can exist as either a vaor or liquid in the atmoshere (we will ignore the solid hase here) under a variety of Temerature and ressure conditions. Refer to the hase diagram in
arxiv:cond-mat/ v2 25 Sep 2002
 Energy fluctuations at the multicritical oint in two-dimensional sin glasses arxiv:cond-mat/0207694 v2 25 Se 2002 1. Introduction Hidetoshi Nishimori, Cyril Falvo and Yukiyasu Ozeki Deartment of Physics,
Energy fluctuations at the multicritical oint in two-dimensional sin glasses arxiv:cond-mat/0207694 v2 25 Se 2002 1. Introduction Hidetoshi Nishimori, Cyril Falvo and Yukiyasu Ozeki Deartment of Physics,
Chapter 2 Solutions. 2.1 (D) Pressure and temperature are dependent during phase change and independent when in a single phase.
 Chater Solutions.1 (D) Pressure and temerature are deendent during hase change and indeendent when in a single hase.. (B) Sublimation is the direct conversion of a solid to a gas. To observe this rocess,
Chater Solutions.1 (D) Pressure and temerature are deendent during hase change and indeendent when in a single hase.. (B) Sublimation is the direct conversion of a solid to a gas. To observe this rocess,
Carrying out thermodynamic calculations and definition of the main reactions of decomposition of vapours of ethyl alcohol
 Carrying out thermodynamic calculations and definition of the main reactions of decomosition of vaours of ethyl alcohol A I Sechin, O S Kyrmakova, T A Ivanova National Research Tomsk olytechnic university,
Carrying out thermodynamic calculations and definition of the main reactions of decomosition of vaours of ethyl alcohol A I Sechin, O S Kyrmakova, T A Ivanova National Research Tomsk olytechnic university,
The extreme case of the anisothermal calorimeter when there is no heat exchange is the adiabatic calorimeter.
 .4. Determination of the enthaly of solution of anhydrous and hydrous sodium acetate by anisothermal calorimeter, and the enthaly of melting of ice by isothermal heat flow calorimeter Theoretical background
.4. Determination of the enthaly of solution of anhydrous and hydrous sodium acetate by anisothermal calorimeter, and the enthaly of melting of ice by isothermal heat flow calorimeter Theoretical background
Homework #11. (Due December 5 at the beginning of the class.) Numerical Method Series #6: Engineering applications of Newton-Raphson Method to
 Homework #11 (Due December 5 at the beginning of the class.) Numerical Method Series #6: Engineering alications of Newton-Rahson Method to solving systems of nonlinear equations Goals: 1. Understanding
Homework #11 (Due December 5 at the beginning of the class.) Numerical Method Series #6: Engineering alications of Newton-Rahson Method to solving systems of nonlinear equations Goals: 1. Understanding
THERMODYNAMICS. Contents
 THERMODYAMICS EREST YEUG ERESTYALUMI@GMAIL.COM Contents Part 1. otes and Solutions for Thermal Physics by Ralh Baierlein 1 1. Background 1 1.1. Heating and Temerature 1 1.2. Some dilute gas relationshis
THERMODYAMICS EREST YEUG ERESTYALUMI@GMAIL.COM Contents Part 1. otes and Solutions for Thermal Physics by Ralh Baierlein 1 1. Background 1 1.1. Heating and Temerature 1 1.2. Some dilute gas relationshis
Comparison of R744 and R134a heat transfer coefficients during flow boiling in a horizontal circular smooth tube
 Euroean Association for the Develoment of Renewable Energies, Environment and Power Quality International Conference on Renewable Energies and Power Quality (ICREPQ 9) Valencia (Sain), 15th to 17th Aril,
Euroean Association for the Develoment of Renewable Energies, Environment and Power Quality International Conference on Renewable Energies and Power Quality (ICREPQ 9) Valencia (Sain), 15th to 17th Aril,
Mathematics as the Language of Physics.
 Mathematics as the Language of Physics. J. Dunning-Davies Deartment of Physics University of Hull Hull HU6 7RX England. Email: j.dunning-davies@hull.ac.uk Abstract. Courses in mathematical methods for
Mathematics as the Language of Physics. J. Dunning-Davies Deartment of Physics University of Hull Hull HU6 7RX England. Email: j.dunning-davies@hull.ac.uk Abstract. Courses in mathematical methods for
Thermodynamics in combustion
 Thermodynamics in combustion 2nd ste in toolbox Thermodynamics deals with a equilibrium state and how chemical comosition can be calculated for a system with known atomic or molecular comosition if 2 indeendent
Thermodynamics in combustion 2nd ste in toolbox Thermodynamics deals with a equilibrium state and how chemical comosition can be calculated for a system with known atomic or molecular comosition if 2 indeendent
1 Entropy 1. 3 Extensivity 4. 5 Convexity 5
 Contents CONEX FUNCIONS AND HERMODYNAMIC POENIALS 1 Entroy 1 2 Energy Reresentation 2 3 Etensivity 4 4 Fundamental Equations 4 5 Conveity 5 6 Legendre transforms 6 7 Reservoirs and Legendre transforms
Contents CONEX FUNCIONS AND HERMODYNAMIC POENIALS 1 Entroy 1 2 Energy Reresentation 2 3 Etensivity 4 4 Fundamental Equations 4 5 Conveity 5 6 Legendre transforms 6 7 Reservoirs and Legendre transforms
ANALYTICAL MODEL FOR THE BYPASS VALVE IN A LOOP HEAT PIPE
 ANALYTICAL MODEL FOR THE BYPASS ALE IN A LOOP HEAT PIPE Michel Seetjens & Camilo Rindt Laboratory for Energy Technology Mechanical Engineering Deartment Eindhoven University of Technology The Netherlands
ANALYTICAL MODEL FOR THE BYPASS ALE IN A LOOP HEAT PIPE Michel Seetjens & Camilo Rindt Laboratory for Energy Technology Mechanical Engineering Deartment Eindhoven University of Technology The Netherlands
whether a process will be spontaneous, it is necessary to know the entropy change in both the
 93 Lecture 16 he entroy is a lovely function because it is all we need to know in order to redict whether a rocess will be sontaneous. However, it is often inconvenient to use, because to redict whether
93 Lecture 16 he entroy is a lovely function because it is all we need to know in order to redict whether a rocess will be sontaneous. However, it is often inconvenient to use, because to redict whether
not to be republished NCERT STATES OF MATTER UNIT 5 INTRODUCTION
 32 CHEMISRY UNI 5 SAES OF MAER After studying this unit you will be able to exlain the existence of different states of matter in terms of balance between intermolecular forces and thermal energy of articles;
32 CHEMISRY UNI 5 SAES OF MAER After studying this unit you will be able to exlain the existence of different states of matter in terms of balance between intermolecular forces and thermal energy of articles;
Internal Energy in terms of Properties
 Lecture #3 Internal Energy in terms of roerties Internal energy is a state function. A change in the state of the system causes a change in its roerties. So, we exress the change in internal energy in
Lecture #3 Internal Energy in terms of roerties Internal energy is a state function. A change in the state of the system causes a change in its roerties. So, we exress the change in internal energy in
Chapter III: Statistical mechanics
 Chater III: Statistical mechanics Section 3: Temerature and heat caacity... Schottky model... Relation between heat and entroy...5 From fundamentals functions to exerimental measurable...6 What drive heat
Chater III: Statistical mechanics Section 3: Temerature and heat caacity... Schottky model... Relation between heat and entroy...5 From fundamentals functions to exerimental measurable...6 What drive heat
Chemistry 420/523 Chemical Thermodynamics (Spring ) Examination 1
 Chemistry 420/523 Chemical hermodynamics (Sring 2001-02) Examination 1 1 Boyle temerature is defined as the temerature at which the comression factor Z m /(R ) of a gas is exactly equal to 1 For a gas
Chemistry 420/523 Chemical hermodynamics (Sring 2001-02) Examination 1 1 Boyle temerature is defined as the temerature at which the comression factor Z m /(R ) of a gas is exactly equal to 1 For a gas
Standard partial molal properties of aqueous alkylphenols and alkylanilines over a wide range of temperatures and pressures
 Geochimica et Cosmochimica Acta 71 (2007) 580 603 www.elsevier.com/locate/gca Standard artial molal roerties of aqueous alkylhenols and alkylanilines over a wide range of temeratures and ressures Miroslav
Geochimica et Cosmochimica Acta 71 (2007) 580 603 www.elsevier.com/locate/gca Standard artial molal roerties of aqueous alkylhenols and alkylanilines over a wide range of temeratures and ressures Miroslav
I have not proofread these notes; so please watch out for typos, anything misleading or just plain wrong.
 hermodynamics I have not roofread these notes; so lease watch out for tyos, anything misleading or just lain wrong. Please read ages 227 246 in Chater 8 of Kittel and Kroemer and ay attention to the first
hermodynamics I have not roofread these notes; so lease watch out for tyos, anything misleading or just lain wrong. Please read ages 227 246 in Chater 8 of Kittel and Kroemer and ay attention to the first
Formation and transformation of volatile nanoparticles from a diesel engine during exhaust dilution
 Article Mechanical Engineering March 2012 Vol.57 No.8: 948954 doi: 10.1007/s11434-011-4927-8 Formation and transformation of volatile nanoarticles from a diesel engine during exhaust dilution LI XinLing
Article Mechanical Engineering March 2012 Vol.57 No.8: 948954 doi: 10.1007/s11434-011-4927-8 Formation and transformation of volatile nanoarticles from a diesel engine during exhaust dilution LI XinLing
STATES OF MATTER UNIT 5
 32 32 CHEMISTRY UNIT 5 After studying this unit you will be able to exlain the existence of different states of matter in terms of balance between intermolecular forces and thermal energy of articles;
32 32 CHEMISTRY UNIT 5 After studying this unit you will be able to exlain the existence of different states of matter in terms of balance between intermolecular forces and thermal energy of articles;
Chapter 9 Practical cycles
 Prof.. undararajan Chater 9 Practical cycles 9. Introduction In Chaters 7 and 8, it was shown that a reversible engine based on the Carnot cycle (two reversible isothermal heat transfers and two reversible
Prof.. undararajan Chater 9 Practical cycles 9. Introduction In Chaters 7 and 8, it was shown that a reversible engine based on the Carnot cycle (two reversible isothermal heat transfers and two reversible
The temperature dependence of the isothermal bulk modulus at 1 bar pressure
 he temerature deendence of the isothermal bulk modulus at bar ressure J. Garai a) Det. of Earth Sciences, Florida International University, University Park, PC 344, Miami, FL 3399, USA A. Laugier IdPCES
he temerature deendence of the isothermal bulk modulus at bar ressure J. Garai a) Det. of Earth Sciences, Florida International University, University Park, PC 344, Miami, FL 3399, USA A. Laugier IdPCES
Mixture Homogeneous Mixtures (air, sea water ) same composition, no chemical bond components are NOT distinguishable
 BASIC CONCEPTAND DEFINITIONS1 2 THERMODYNAMICS CHAPTER 2 Thermodynamic Concets Lecturer Axel GRONIEWSKY, PhD 11 th of February2019 Thermodynamics study of energy and its transformation describes macroscoic
BASIC CONCEPTAND DEFINITIONS1 2 THERMODYNAMICS CHAPTER 2 Thermodynamic Concets Lecturer Axel GRONIEWSKY, PhD 11 th of February2019 Thermodynamics study of energy and its transformation describes macroscoic
Eric M. Yezdimer, and Robert H. Wood
 J. Chem. hermodynamics 1997, 29, 517 531 Aarent molar heat caacities of aqueous solutions of roylamine, 1,4-butanediamine, 1,6-hexanediamine, roylamine hydrochloride, roionamide, yridine, and sodium benzenesulfonate
J. Chem. hermodynamics 1997, 29, 517 531 Aarent molar heat caacities of aqueous solutions of roylamine, 1,4-butanediamine, 1,6-hexanediamine, roylamine hydrochloride, roionamide, yridine, and sodium benzenesulfonate
Principles of Food and Bioprocess Engineering (FS 231) Solutions to Example Problems on Evaporation
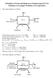 Princiles of Food and Biorocess Engineering (FS 231) Solutions to Examle Problems on Eaoration 1. The system diagram is as follows: Solids balance equation: 0.1 (m f) = 0.5 (1,000) Thus, m f = 5,000 kg/hr
Princiles of Food and Biorocess Engineering (FS 231) Solutions to Examle Problems on Eaoration 1. The system diagram is as follows: Solids balance equation: 0.1 (m f) = 0.5 (1,000) Thus, m f = 5,000 kg/hr
Casimir Force Between the Two Moving Conductive Plates.
 Casimir Force Between the Two Moving Conductive Plates. Jaroslav Hynecek 1 Isetex, Inc., 95 Pama Drive, Allen, TX 751 ABSTRACT This article resents the derivation of the Casimir force for the two moving
Casimir Force Between the Two Moving Conductive Plates. Jaroslav Hynecek 1 Isetex, Inc., 95 Pama Drive, Allen, TX 751 ABSTRACT This article resents the derivation of the Casimir force for the two moving
Chapter 20: Exercises: 3, 7, 11, 22, 28, 34 EOC: 40, 43, 46, 58
 Chater 0: Exercises:, 7,,, 8, 4 EOC: 40, 4, 46, 8 E: A gasoline engine takes in.80 0 4 and delivers 800 of work er cycle. The heat is obtained by burning gasoline with a heat of combustion of 4.60 0 4.
Chater 0: Exercises:, 7,,, 8, 4 EOC: 40, 4, 46, 8 E: A gasoline engine takes in.80 0 4 and delivers 800 of work er cycle. The heat is obtained by burning gasoline with a heat of combustion of 4.60 0 4.
Compressible Flow Introduction. Afshin J. Ghajar
 36 Comressible Flow Afshin J. Ghajar Oklahoma State University 36. Introduction...36-36. he Mach Number and Flow Regimes...36-36.3 Ideal Gas Relations...36-36.4 Isentroic Flow Relations...36-4 36.5 Stagnation
36 Comressible Flow Afshin J. Ghajar Oklahoma State University 36. Introduction...36-36. he Mach Number and Flow Regimes...36-36.3 Ideal Gas Relations...36-36.4 Isentroic Flow Relations...36-4 36.5 Stagnation
The International Association for the Properties of Water and Steam
 IAPWS SR5-05(2016) he International Association for the Proerties of Water and Steam Moscow, Russia June 2014 Revised Sulementary Release on Backward Equations for Secific Volume as a Function of Pressure
IAPWS SR5-05(2016) he International Association for the Proerties of Water and Steam Moscow, Russia June 2014 Revised Sulementary Release on Backward Equations for Secific Volume as a Function of Pressure
Calculators are permitted. Computers, PDAs, and other electronic devices with a keyboard are not. Cell phones may not be used as calculators.
 Chem. 30 Sring 008 Exam 3 VERSION A Professors Williams/Whetten his test is closed note/book One 8.5 x handwritten crib sheet (one sided) is ermitted. Please turn off your cell hone. Use a # encil. Calculators
Chem. 30 Sring 008 Exam 3 VERSION A Professors Williams/Whetten his test is closed note/book One 8.5 x handwritten crib sheet (one sided) is ermitted. Please turn off your cell hone. Use a # encil. Calculators
Effect of hole-trapping on mass/charge transport properties in acceptor-doped BaTiO 3
 RESEARCH PAPER Effect of hole-traing on mass/charge transort roerties in accetor-doed BaTiO 3 H.-I. Yoo* a and K. D. Becker b PCCP www.rsc.org/cc a School of Materials Science and Engineering, Seoul National
RESEARCH PAPER Effect of hole-traing on mass/charge transort roerties in accetor-doed BaTiO 3 H.-I. Yoo* a and K. D. Becker b PCCP www.rsc.org/cc a School of Materials Science and Engineering, Seoul National
PHYS1001 PHYSICS 1 REGULAR Module 2 Thermal Physics Chapter 17 First Law of Thermodynamics
 PHYS1001 PHYSICS 1 REGULAR Module Thermal Physics Chater 17 First Law of Thermodynamics References: 17.1 to 17.9 Examles: 17.1 to 17.7 Checklist Thermodynamic system collection of objects and fields. If
PHYS1001 PHYSICS 1 REGULAR Module Thermal Physics Chater 17 First Law of Thermodynamics References: 17.1 to 17.9 Examles: 17.1 to 17.7 Checklist Thermodynamic system collection of objects and fields. If
ONE. The Earth-atmosphere system CHAPTER
 CHAPTER ONE The Earth-atmoshere system 1.1 INTRODUCTION The Earth s atmoshere is the gaseous enveloe surrounding the lanet. Like other lanetary atmosheres, it figures centrally in transfers of energy between
CHAPTER ONE The Earth-atmoshere system 1.1 INTRODUCTION The Earth s atmoshere is the gaseous enveloe surrounding the lanet. Like other lanetary atmosheres, it figures centrally in transfers of energy between
First law of thermodynamics (Jan 12, 2016) page 1/7. Here are some comments on the material in Thompkins Chapter 1
 First law of thermodynamics (Jan 12, 2016) age 1/7 Here are some comments on the material in Thomkins Chater 1 1) Conservation of energy Adrian Thomkins (eq. 1.9) writes the first law as: du = d q d w
First law of thermodynamics (Jan 12, 2016) age 1/7 Here are some comments on the material in Thomkins Chater 1 1) Conservation of energy Adrian Thomkins (eq. 1.9) writes the first law as: du = d q d w
Quasi-particle Contribution in Thermal Expansion and Thermal Conductivity in Metals
 e-issn:-459 -ISSN:47-6 Quasi-article Contribution in Thermal Exansion and Thermal Conductivity in Metals Edema OG * and Osiele OM ederal Polytechnic, Auchi, Nigeria Delta State University, Abraka, Nigeria
e-issn:-459 -ISSN:47-6 Quasi-article Contribution in Thermal Exansion and Thermal Conductivity in Metals Edema OG * and Osiele OM ederal Polytechnic, Auchi, Nigeria Delta State University, Abraka, Nigeria
16. CHARACTERISTICS OF SHOCK-WAVE UNDER LORENTZ FORCE AND ENERGY EXCHANGE
 16. CHARACTERISTICS OF SHOCK-WAVE UNDER LORENTZ FORCE AND ENERGY EXCHANGE H. Yamasaki, M. Abe and Y. Okuno Graduate School at Nagatsuta, Tokyo Institute of Technology 459, Nagatsuta, Midori-ku, Yokohama,
16. CHARACTERISTICS OF SHOCK-WAVE UNDER LORENTZ FORCE AND ENERGY EXCHANGE H. Yamasaki, M. Abe and Y. Okuno Graduate School at Nagatsuta, Tokyo Institute of Technology 459, Nagatsuta, Midori-ku, Yokohama,
First Name Last Name CIRCLE YOUR LECTURE BELOW: Div. 1 10:30 am Div. 2 2:30 pm Div. 3 4:30 pm Prof. Gore Prof. Udupa Prof. Chen
 CIRCLE YOUR LECURE BELOW: Div. 1 10:30 am Div. :30 m Div. 3 4:30 m Prof. Gore Prof. Udua Prof. Chen EXAM # 3 INSRUCIONS 1. his is a closed book examination. All needed roerty tables are rovided.. Do not
CIRCLE YOUR LECURE BELOW: Div. 1 10:30 am Div. :30 m Div. 3 4:30 m Prof. Gore Prof. Udua Prof. Chen EXAM # 3 INSRUCIONS 1. his is a closed book examination. All needed roerty tables are rovided.. Do not
Modelling heat transfer and fluid flow inside a pressure cooker
 17 th Euroean Symosium on Comuter Aided Process Engineering ESCAPE17 V. Plesu and P.S. Agachi (Editors) 2007 Elsevier B.V. All rights reserved. 1 Modelling heat transfer and fluid flow inside a ressure
17 th Euroean Symosium on Comuter Aided Process Engineering ESCAPE17 V. Plesu and P.S. Agachi (Editors) 2007 Elsevier B.V. All rights reserved. 1 Modelling heat transfer and fluid flow inside a ressure
ENTROPY GENERATION AND THERMAL PERFORMANCE OF A PULSATING HEAT PIPE. A Thesis. presented to. the Faculty of the Graduate School
 ENTROPY GENERATION AND THERMAL PERFORMANCE OF A PULSATING HEAT PIPE A Thesis resented to the Faculty of the Graduate School at the University of Missouri-Columbia In Partial Fulfillment of the Requirements
ENTROPY GENERATION AND THERMAL PERFORMANCE OF A PULSATING HEAT PIPE A Thesis resented to the Faculty of the Graduate School at the University of Missouri-Columbia In Partial Fulfillment of the Requirements
Chemical Kinetics and Equilibrium - An Overview - Key
 Chemical Kinetics and Equilibrium - An Overview - Key The following questions are designed to give you an overview of the toics of chemical kinetics and chemical equilibrium. Although not comrehensive,
Chemical Kinetics and Equilibrium - An Overview - Key The following questions are designed to give you an overview of the toics of chemical kinetics and chemical equilibrium. Although not comrehensive,
MODULE 2: DIFFUSION LECTURE NO DIFFUSION COEFFICIENT: MEASUREMENT AND PREDICTION
 NPTEL Chemical ass Transfer Oeration OULE : IFFUSION LECTURE NO. 4.4 IFFUSION COEFFICIENT: ESUREENT N PREICTION The roortionality factor of Fick s law is called diffusivity or diffusion coefficient which
NPTEL Chemical ass Transfer Oeration OULE : IFFUSION LECTURE NO. 4.4 IFFUSION COEFFICIENT: ESUREENT N PREICTION The roortionality factor of Fick s law is called diffusivity or diffusion coefficient which
Chapter 1 Fundamentals
 Chater Fundamentals. Overview of Thermodynamics Industrial Revolution brought in large scale automation of many tedious tasks which were earlier being erformed through manual or animal labour. Inventors
Chater Fundamentals. Overview of Thermodynamics Industrial Revolution brought in large scale automation of many tedious tasks which were earlier being erformed through manual or animal labour. Inventors
Thermodynamic Properties are Measurements p,t,v, u,h,s - measure directly -measure by change
 Thermodynamic Proerties are Measurements,T,, u,h,s - measure directly -measure by change s Tables T T Proerty Data Cure its Tables Correlation's, Boyles Law Tables c@tc limited hand calculations Equations
Thermodynamic Proerties are Measurements,T,, u,h,s - measure directly -measure by change s Tables T T Proerty Data Cure its Tables Correlation's, Boyles Law Tables c@tc limited hand calculations Equations
Study of Current Decay Time during Disruption in JT-60U Tokamak
 1 EX/7-Rc Study of Current Decay Time during Disrution in JT-60U Tokamak M.Okamoto 1), K.Y.Watanabe ), Y.Shibata 3), N.Ohno 3), T.Nakano 4), Y.Kawano 4), A.Isayama 4), N.Oyama 4), G.Matsunaga 4), K.Kurihara
1 EX/7-Rc Study of Current Decay Time during Disrution in JT-60U Tokamak M.Okamoto 1), K.Y.Watanabe ), Y.Shibata 3), N.Ohno 3), T.Nakano 4), Y.Kawano 4), A.Isayama 4), N.Oyama 4), G.Matsunaga 4), K.Kurihara
Entransy analysis of open thermodynamic systems
 Article Engineering hermohysics August 0 Vol.57 No.: 934940 doi: 0.007/s434-0-54-x Entransy analysis of oen thermodynamic systems CHENG Xueao, WANG WenHua & LIANG XinGang * Key Laboratory for hermal Science
Article Engineering hermohysics August 0 Vol.57 No.: 934940 doi: 0.007/s434-0-54-x Entransy analysis of oen thermodynamic systems CHENG Xueao, WANG WenHua & LIANG XinGang * Key Laboratory for hermal Science
Equilibrium Thermodynamics
 Part I Equilibrium hermodynamics 1 Molecular hermodynamics Perhas the most basic equation in atmosheric thermodynamics is the ideal gas law = rr where is ressure, r is the air density, is temerature, and
Part I Equilibrium hermodynamics 1 Molecular hermodynamics Perhas the most basic equation in atmosheric thermodynamics is the ideal gas law = rr where is ressure, r is the air density, is temerature, and
Lab 4 Module α 2. Miscibility Gaps
 3.014 Materials Laboratory Dec. 9 th Dec. 14 st, 2005 Lab 4 Module α 2 Miscibility Gas OBJECTIVES Review miscibility gas in binary systems Introduce statistical thermodynamics of olymer solutions Learn
3.014 Materials Laboratory Dec. 9 th Dec. 14 st, 2005 Lab 4 Module α 2 Miscibility Gas OBJECTIVES Review miscibility gas in binary systems Introduce statistical thermodynamics of olymer solutions Learn
HEAT, WORK, AND THE FIRST LAW OF THERMODYNAMICS
 HET, ORK, ND THE FIRST L OF THERMODYNMIS 8 EXERISES Section 8. The First Law of Thermodynamics 5. INTERPRET e identify the system as the water in the insulated container. The roblem involves calculating
HET, ORK, ND THE FIRST L OF THERMODYNMIS 8 EXERISES Section 8. The First Law of Thermodynamics 5. INTERPRET e identify the system as the water in the insulated container. The roblem involves calculating
Optical self-energy of superconducting Pb in the terahertz region
 Otical self-energy of suerconducting Pb in the terahertz region T. Mori, 1 E. J. Nicol, 2, * S. Shiizuka, 1 K. Kuniyasu, 3 T. Nojima, 3 N. Toyota, 1 and J. P. Carbotte 4 1 Physics Deartment, Graduate School
Otical self-energy of suerconducting Pb in the terahertz region T. Mori, 1 E. J. Nicol, 2, * S. Shiizuka, 1 K. Kuniyasu, 3 T. Nojima, 3 N. Toyota, 1 and J. P. Carbotte 4 1 Physics Deartment, Graduate School
df da df = force on one side of da due to pressure
 I. Review of Fundamental Fluid Mechanics and Thermodynamics 1. 1 Some fundamental aerodynamic variables htt://en.wikiedia.org/wiki/hurricane_ivan_(2004) 1) Pressure: the normal force er unit area exerted
I. Review of Fundamental Fluid Mechanics and Thermodynamics 1. 1 Some fundamental aerodynamic variables htt://en.wikiedia.org/wiki/hurricane_ivan_(2004) 1) Pressure: the normal force er unit area exerted
THERMODYNAMICS. Prepared by Sibaprasad Maity Asst. Prof. in Chemistry For any queries contact at
 HERMODYNAMIS reared by Sibarasad Maity Asst. rof. in hemistry For any queries contact at 943445393 he word thermo-dynamic, used first by illiam homson (later Lord Kelvin), has Greek origin, and is translated
HERMODYNAMIS reared by Sibarasad Maity Asst. rof. in hemistry For any queries contact at 943445393 he word thermo-dynamic, used first by illiam homson (later Lord Kelvin), has Greek origin, and is translated
Minimum Fluidization Velocities of Binary- Solid Mixtures: Model Comparison
 Vol:4, No:3, 00 Minimum Fluidization Velocities of Binary- Solid Mixtures: Model Comarison Mohammad Asif International Science Index, Chemical and Molecular Engineering Vol:4, No:3, 00 waset.org/publication/5950
Vol:4, No:3, 00 Minimum Fluidization Velocities of Binary- Solid Mixtures: Model Comarison Mohammad Asif International Science Index, Chemical and Molecular Engineering Vol:4, No:3, 00 waset.org/publication/5950
Pressure-sensitivity Effects on Toughness Measurements of Compact Tension Specimens for Strain-hardening Solids
 American Journal of Alied Sciences (9): 19-195, 5 ISSN 1546-939 5 Science Publications Pressure-sensitivity Effects on Toughness Measurements of Comact Tension Secimens for Strain-hardening Solids Abdulhamid
American Journal of Alied Sciences (9): 19-195, 5 ISSN 1546-939 5 Science Publications Pressure-sensitivity Effects on Toughness Measurements of Comact Tension Secimens for Strain-hardening Solids Abdulhamid
What is Physical Chemistry?
 Chemistry 313 Dr. Caleb Arrington 10:30 am - 11:20 am M,W,&F Lab: Wednesday 2:00-5:00 Office RMSC 306-A What do we do the first day of every class? syllabus What is Physical Chemistry? Mathematically redictive
Chemistry 313 Dr. Caleb Arrington 10:30 am - 11:20 am M,W,&F Lab: Wednesday 2:00-5:00 Office RMSC 306-A What do we do the first day of every class? syllabus What is Physical Chemistry? Mathematically redictive
Dioxins & PCBs concerns
 Dioxins & PCBs concerns Properties, Sources and Formation RIGHT S O L U T I O N S RIGHT PARTNER Program Introduction to dioxins and PCBs Sources and formation Fate in environmental media Exposure pathways
Dioxins & PCBs concerns Properties, Sources and Formation RIGHT S O L U T I O N S RIGHT PARTNER Program Introduction to dioxins and PCBs Sources and formation Fate in environmental media Exposure pathways
Phase Diagrams. NC State University
 Chemistry 433 Lecture 18 Phase Diagrams NC State University Definition of a phase diagram A phase diagram is a representation of the states of matter, solid, liquid, or gas as a function of temperature
Chemistry 433 Lecture 18 Phase Diagrams NC State University Definition of a phase diagram A phase diagram is a representation of the states of matter, solid, liquid, or gas as a function of temperature
Thermodynamic equations of state of polymers and conversion processing
 Polimery, No.,,. 66 hermodynamic equations of state of olymers and conversion rocessing B. Kowalska Selected from International Polymer Science and echnology, 9, No.,, reference P //66; transl. serial
Polimery, No.,,. 66 hermodynamic equations of state of olymers and conversion rocessing B. Kowalska Selected from International Polymer Science and echnology, 9, No.,, reference P //66; transl. serial
Foundations of thermodynamics Fundamental thermodynamical concepts
 Foundations of thermodynamics Fundamental thermodynamical concets System is the macrohysical entity under consideration Surrounding is the wld outside of the system Oen system can exchange matter heat
Foundations of thermodynamics Fundamental thermodynamical concets System is the macrohysical entity under consideration Surrounding is the wld outside of the system Oen system can exchange matter heat
The International Association for the Properties of Water and Steam
 IAP AN5-13(2016) he International Association for the Proerties of ater and team London, United Kindom etember 2013 Advisory Note No. 5: Industrial Calculation of the hermodynamic Proerties of eawater
IAP AN5-13(2016) he International Association for the Proerties of ater and team London, United Kindom etember 2013 Advisory Note No. 5: Industrial Calculation of the hermodynamic Proerties of eawater
The Second Law of Thermodynamics (Chapter 4)
 The Second Law of Thermodynamics (Chapter 4) First Law: Energy of universe is constant: ΔE system = - ΔE surroundings Second Law: New variable, S, entropy. Changes in S, ΔS, tell us which processes made
The Second Law of Thermodynamics (Chapter 4) First Law: Energy of universe is constant: ΔE system = - ΔE surroundings Second Law: New variable, S, entropy. Changes in S, ΔS, tell us which processes made
CHAPTER 2 THERMODYNAMICS
 CHAPER 2 HERMODYNAMICS 2.1 INRODUCION herodynaics is the study of the behavior of systes of atter under the action of external fields such as teerature and ressure. It is used in articular to describe
CHAPER 2 HERMODYNAMICS 2.1 INRODUCION herodynaics is the study of the behavior of systes of atter under the action of external fields such as teerature and ressure. It is used in articular to describe
Thickness and refractive index measurements using multiple beam interference fringes (FECO)
 Journal of Colloid and Interface Science 264 2003 548 553 Note www.elsevier.com/locate/jcis Thickness and refractive index measurements using multile beam interference fringes FECO Rafael Tadmor, 1 Nianhuan
Journal of Colloid and Interface Science 264 2003 548 553 Note www.elsevier.com/locate/jcis Thickness and refractive index measurements using multile beam interference fringes FECO Rafael Tadmor, 1 Nianhuan
Actual exergy intake to perform the same task
 CHAPER : PRINCIPLES OF ENERGY CONSERVAION INRODUCION Energy conservation rinciles are based on thermodynamics If we look into the simle and most direct statement of the first law of thermodynamics, we
CHAPER : PRINCIPLES OF ENERGY CONSERVAION INRODUCION Energy conservation rinciles are based on thermodynamics If we look into the simle and most direct statement of the first law of thermodynamics, we
We now turn to considerations of mixtures. To keep our discussion reasonably simple,
 143 Lecture 23 We now turn to considerations of mixtures. To kee our discussion reasonably simle, we will limit our discussion to non-reacting systems, and to non-ionic systems. In other words, we will
143 Lecture 23 We now turn to considerations of mixtures. To kee our discussion reasonably simle, we will limit our discussion to non-reacting systems, and to non-ionic systems. In other words, we will
Distribution of populations in excited states of electrodeless discharge lamp of Rb atoms
 Article Atomic & Molecular Physics June 2013 Vol.58 No.16: 18761881 doi: 10.1007/s11434-013-5789-z Distribution of oulations in excited states of electrodeless discharge lam of Rb atoms TAO ZhiMing 1,2,
Article Atomic & Molecular Physics June 2013 Vol.58 No.16: 18761881 doi: 10.1007/s11434-013-5789-z Distribution of oulations in excited states of electrodeless discharge lam of Rb atoms TAO ZhiMing 1,2,
Evaluation of New Dual-Layer Carbon Reversible Column in Analysis of PCDDs/PCDFs and Co-Planar PCBs
 Evaluation of New Dual-Layer Carbon Reversible Column in Analysis of PCDDs/PCDFs and Co-Planar PCBs Yukihiro Nishimura, Masaji Yoshida, Mikihiro Kawabata, Atsushi Maemura, Koji Funakoshi, Katsuya Higuchi,
Evaluation of New Dual-Layer Carbon Reversible Column in Analysis of PCDDs/PCDFs and Co-Planar PCBs Yukihiro Nishimura, Masaji Yoshida, Mikihiro Kawabata, Atsushi Maemura, Koji Funakoshi, Katsuya Higuchi,
On the q-deformed Thermodynamics and q-deformed Fermi Level in Intrinsic Semiconductor
 Advanced Studies in Theoretical Physics Vol. 11, 2017, no. 5, 213-223 HIKARI Ltd, www.m-hikari.com htts://doi.org/10.12988/ast.2017.61138 On the q-deformed Thermodynamics and q-deformed Fermi Level in
Advanced Studies in Theoretical Physics Vol. 11, 2017, no. 5, 213-223 HIKARI Ltd, www.m-hikari.com htts://doi.org/10.12988/ast.2017.61138 On the q-deformed Thermodynamics and q-deformed Fermi Level in
Thermodynamic Modeling and Analysis of an Optical Electric-Field Sensor
 Sensors 15, 15, 715-715; doi:1.9/s154715 Article OPEN ACCESS sensors ISSN 144-8 www.mdi.com/journal/sensors Thermodynamic Modeling and Analysis of an Otical Electric-Field Sensor Xia Xiao *, Yan Xu and
Sensors 15, 15, 715-715; doi:1.9/s154715 Article OPEN ACCESS sensors ISSN 144-8 www.mdi.com/journal/sensors Thermodynamic Modeling and Analysis of an Otical Electric-Field Sensor Xia Xiao *, Yan Xu and
A Simulation Model for the Application of Nanofluids as Condenser Coolants in Vapor Compression Heat Pumps
 Purdue University Purdue e-pubs International Refrigeration and Air Conditioning Conference School of Mechanical Engineering 2012 A Simulation Model for the Alication of Nanofluids as Condenser Coolants
Purdue University Purdue e-pubs International Refrigeration and Air Conditioning Conference School of Mechanical Engineering 2012 A Simulation Model for the Alication of Nanofluids as Condenser Coolants
Chapter-6: Entropy. 1 Clausius Inequality. 2 Entropy - A Property
 hater-6: Entroy When the first law of thermodynamics was stated, the existence of roerty, the internal energy, was found. imilarly, econd law also leads to definition of another roerty, known as entroy.
hater-6: Entroy When the first law of thermodynamics was stated, the existence of roerty, the internal energy, was found. imilarly, econd law also leads to definition of another roerty, known as entroy.
Determination of Pressure Losses in Hydraulic Pipeline Systems by Considering Temperature and Pressure
 Paer received: 7.10.008 UDC 61.64 Paer acceted: 0.04.009 Determination of Pressure Losses in Hydraulic Pieline Systems by Considering Temerature and Pressure Vladimir Savi 1,* - Darko Kneževi - Darko Lovrec
Paer received: 7.10.008 UDC 61.64 Paer acceted: 0.04.009 Determination of Pressure Losses in Hydraulic Pieline Systems by Considering Temerature and Pressure Vladimir Savi 1,* - Darko Kneževi - Darko Lovrec
A numerical tool for plasma spraying. Part II: Model of statistic distribution of alumina multi particle powder.
 A numerical tool for lasma sraying. Part II: Model of statistic distribution of alumina multi article owder. G. Delluc, L. Perrin, H. Ageorges, P. Fauchais, B. Pateyron Science des Procédés Céramiques
A numerical tool for lasma sraying. Part II: Model of statistic distribution of alumina multi article owder. G. Delluc, L. Perrin, H. Ageorges, P. Fauchais, B. Pateyron Science des Procédés Céramiques
Modeling Volume Changes in Porous Electrodes
 Journal of The Electrochemical Society, 53 A79-A86 2006 003-465/2005/53/A79/8/$20.00 The Electrochemical Society, Inc. Modeling olume Changes in Porous Electrodes Parthasarathy M. Gomadam*,a,z John W.
Journal of The Electrochemical Society, 53 A79-A86 2006 003-465/2005/53/A79/8/$20.00 The Electrochemical Society, Inc. Modeling olume Changes in Porous Electrodes Parthasarathy M. Gomadam*,a,z John W.
NOTICE: This is the author's version of a work that was accepted for publication in Chemical Engineering Research and Design. Changes resulting from
 NOTICE: This is the author's version of a work that was acceted for ublication in Chemical Engineering Research and Design. Changes resulting from the ublishing rocess, such as eer review, editing, corrections,
NOTICE: This is the author's version of a work that was acceted for ublication in Chemical Engineering Research and Design. Changes resulting from the ublishing rocess, such as eer review, editing, corrections,
The characteristic curves of water
 he characteristic curves of water Arnold Neumaier 1 Deartment of Mathematics, University of ienna, Oskar-Morgenstern-Platz 1, A-1090 Wien, Austria Ulrich K. Deiters Institute of Physical Chemistry, University
he characteristic curves of water Arnold Neumaier 1 Deartment of Mathematics, University of ienna, Oskar-Morgenstern-Platz 1, A-1090 Wien, Austria Ulrich K. Deiters Institute of Physical Chemistry, University
Journal of Thermal Science and Technology
 Science and Technology Design Considerations of Porous Gas Enthaly Radiation Converters for Exhaust-Heat Recovery Systems Preecha KHANTIKOMOL and Kouichi KAMIUTO High-Temerature Heat Transfer Laboratory,
Science and Technology Design Considerations of Porous Gas Enthaly Radiation Converters for Exhaust-Heat Recovery Systems Preecha KHANTIKOMOL and Kouichi KAMIUTO High-Temerature Heat Transfer Laboratory,
12.808: Some Physical Properties of Sea Water or, More than you ever wanted to know about the basic state variables of the ocean
 12.88: Some Physical Proerties of Sea Water or, More than you ever wanted to know about the basic state variables of the ocean Salinity Various salt constituents in 1 m 3 of seawater having (t, S) = (2,
12.88: Some Physical Proerties of Sea Water or, More than you ever wanted to know about the basic state variables of the ocean Salinity Various salt constituents in 1 m 3 of seawater having (t, S) = (2,
Paper C Exact Volume Balance Versus Exact Mass Balance in Compositional Reservoir Simulation
 Paer C Exact Volume Balance Versus Exact Mass Balance in Comositional Reservoir Simulation Submitted to Comutational Geosciences, December 2005. Exact Volume Balance Versus Exact Mass Balance in Comositional
Paer C Exact Volume Balance Versus Exact Mass Balance in Comositional Reservoir Simulation Submitted to Comutational Geosciences, December 2005. Exact Volume Balance Versus Exact Mass Balance in Comositional
