Thermodynamic Properties are Measurements p,t,v, u,h,s - measure directly -measure by change
|
|
|
- Winfred Carpenter
- 6 years ago
- Views:
Transcription
1 Thermodynamic Proerties are Measurements,T,, u,h,s - measure directly -measure by change s Tables T T Proerty Data Cure its Tables Correlation's, Boyles Law Tables c@tc limited hand calculations Equations o State, RT Tables Calculation Modules NIST, EES, HYSYM interactie, callable
2 P atm aor liquid Q
3
4 Gien T and P Suer Heat Region i, < sat Comressed Liquid Region i, T sat sat
5
6 STEAM PRESUE AND TEMPERATURE TABLES
7 T Saturated Liquid Line Saturated Vaor Line h u s h u s g g g g
8 Three Tables Temerature Table at saced T s Pressure Table at saced P s Suerheat Table at saced T and P 6 Proerties Temerature Pressure Volume Internal Energy Enthaly Entroy
9 Solid-Liquid-Gas Phase Diagram
10 Saturation liquid internal energy at 0 C 0. Table Base Saturated liquid enthaly at 5 C kj/kg Saturated aor entroy at 5 C kj/kg K Enthaly at 0 C, 00 kpa assume saturated liquid enthaly at 0 C 8.96 kj/kg Temerature o saturated aor with an internal energy o 96. kj/kg 5 C Enthaly o aorization at 0 C kj/kg
11 Table A-7 Metric T, Table A-7E English T, Table A-4 Metric, T Table A-5 Metric, Table A-4E English, T Table A-5E English, TEMPERATURE TABLE PRESSURE TABLE Table A-6, Metric, T, Table A-6E, English, T, SUPERHEAT TABLE
12 water T saturation saturation at 400ka and 00 ka 00C kPa R 4a at 500 kpa and 6 C Tsaturation@ 500 ka 6 C 6.ka saturation T 00C kPa ka C constant T suerheated 5.7C 500 kpa 6.ka 6 C subcooled constant
13 Two Phase Real Gas Proerties ( ) g g g g g g g x x x x Quality m m x m m m V V V g g g g x u x u u h x h h + + +
14 Quality, x g x.64 (64%) u u + x (u u ) g u u + x u g u u BTU/lb h h + x h g h h 75.4 BTU/lb T 90 o Find the roerties o F Temerature Table u58.07 h58.07 ( ) s.65 water at 90 ( g ) o u h s F, 00 t g g g /lb. u 040. h 07.7 s.008 s s + x s s s g o.54 BTU/lb R.06t / lb 00. t /lb g 467.7t / lb
15 Engineering Equation Soler - EES Fluid Proerty Inormation - 69 luids aailable Thermohysical Functions - 5 roerties calculated Equations Window henthaly(steam, T00.,P00) suerheated aor henthaly(steam,t00.,x) saturated aor uintenergy(steam,t00.,x0.) saturated liquid ressure(steam,t00.,x0.) saturation ressure Thermohysical Functions entroy intenergy ressure quality density enthaly isidealgas temerature olume Function Arguments H seciic enthaly P ressure S seciic entroy T temerature U seciic internal energy V seciic olume X quality
16 EES FLUIDS FUNCTIONS
17 Table EES Program Saturation internal energy at 4 kpa u l u Pressure Table g.45kj/kg 45.kJ/kg u u u u l l g g intenergy(steam, P 4.,X 0).kJ/kg intenergy(steam, P , X.) Enthaly and olume o water at 50 kpa and 0 C Internal energy u o water at 0 MPa u and 00 C l l Suerheat Table h h saturated 0 h l l l l 5.75 kj/kg saturated liquid@ m /kg Temerature Table u saturated 00.0 kj/kg o o o C C C u u l l h h l l l l enthaly(steam,t 5.67kJ/kg olume(steam,t.00004m /kg intenergy(steam,t.446 kj/kg 0., 0., 00., 500.) 500.) 0000.)
18 Sole Windows Equations
19 Windows Arrays
20 Windows Plot Window
21
22 0 MPa saturated steam has an enthaly o 00 kj/kg. What is its internal energy? h h + x h g 00 kj/kg x 7. x.4574 u u + x u g u u kj/kg EES Program x quality(steam,p 0000.,h 00) x.4576 u intenergy(steam,p 0000.,h 00) u kj/kg
23 Steam at 0 C has an enthaly o 800 kj/kg. What is the internal energy? h h + x h 800 kj/kg x 454. x.7 u u + x u g g u u kj/kg
24 STEAM SUPERHEAT TABLE
25 SUPERHEAT TABLE Enthaly at 700 C and.0 Ma 98. kj/kg Temerature at entroy o and.05 Ma 400 C Enthaly at.05 MPa and entroy o kj/kg C kj/kg
26 Steam initially at a temerature o 00 C and a ressure o.0 MPa undergoes a rocess during which its entroy remains constant to a ressure o.0 MPa. What is the enthaly and temerature o the steam at the end o the rocess? T Entroy at 00 C,. MPa 0.659kJ/kg K Enthaly at.0 MPa, entroy kj/kg Temerature at.0 MPa, entroy C.0 MPa s
27 Linear Interolation with Variables 450 C, 7 MPa) Steam Suerheat Table Table Values 5 mpa 7 MPa 0 MPa T 450 h h h For the ressure table entry, The desired ressure, 7 kpa, is 40 % o the dierence between table alues. All the other roerties at 7 kpa must be at the same dierence. EES h enthaly(steam,t 450., 7000) h 90.7 kj/kg.% dierence, table and interolation
28 SPREAD SHEET WORLD EXCELL ADD-IN 40 luids 4 sets o units
29 SPREADSHEET WORLD THERMAL FLUIDS PROPERTIES CALL A B C D E F G H I J T 00 P 5 TTPros("AIR","EE_F", "P", $C$,"T", $C$) P T u s h X STATE ERROR RETURN erheated a 0 sia t^/lbm deg F BTU/lbm BTU/lbm-R BTU/lbm nd
30 A saturated mixture o kg water and kg aor in contained in a iston cylinder deice at 00 ka. Heat is added and the iston, initially resting on stos, begins to moe at a ressure o 00 ka. Heating is stoed when the total olume in increased by 0%. Find: a) the initial and inal temeratures. b) the mass o liquid water when the ressure reaches 00 kpa and the iston starts to moe. c) the work done by the exansion. at 00kPa, T 99.6 x kg aor.6 5kg total u u ( ).068m /kg u + x + x u g g kJ/kg Q 00 ka kg kg.88
31 Point V Interolation romtablea 6 u u h h V h h g W 5 kg m u@ h@. V at. MPa, (.06,P. MPa) 6.kJ/kg (.068,P. MPa) 86.47kJ/kg.8857 suerheated Point at. MPa (.9,P. MPa) kj/kg dv W kpa m 5 kg.9 dv 0 + ) ( V V ) ( m 5.08m ) 0.kJ constant,- Q E+ W Q E+ V Q H Q m h Q W - - ( h ) alteratiely, Q - Q 5 ( ) kj m ( u u ) constant,- Q E + W W 0 Q E U Q m u ( u ) Q 5 ( ) Q kj.88
32 Interolation or h Suerheat TableA -6 (. MPa,.9) T h ratio o h kj/kg.7
33 Sread Sheet World module solution water aor Proerties at gien and x x ,000 P T u s h X STATE Mixed regio Pascals m^/kg deg C kj/kg kj/kg-k kj/kg nd Proerties at gien and 00,000 P T u s h X STATE erheated a Pascals m^/kg deg C kj/kg kj/kg-k kj/kg nd Vm x V. * V V/m.08 00,000 Proerties at gien and P T u s h X STATE erheated a Pascals m^/kg deg C kj/kg kj/kg-k kj/kg nd Q -m*(h-h) W - Q --m*(u-u) 0.6 Q -m*(u-u)
34 EES Solution Q 00 ka kg kg
35 kg aor and kg liquid R-4a is contained in a rigid tank at 0 C. What is the olume o the tank? I the tank is heated until the ressure? reaches.6 MPa? What is the quality, and enthaly o the mixture o liquid and aor? V V V V Ater heating, V constant, m constant constant x h h V m kg kg m V m + 0 C + m.08 m 4 MPa + x h + x h g g.07 m /kg.787(78.7%) kj/kg g 0 C age 84, TableA MPa age 84, Table T 0 C A - Q.6 MPa.576 MPa
36 kg o aor and kg o liquid R-4a is contained in a iston cylinder deice. The olume o the aor is.074 cubic meters. What is the temerature and ressure? I the cylinder and its contents are heated until olume is.5 cubic meters what is the quality? x g g at V V m g g.058 m 0 C,.5m.0 m /kg g.074 m kg, m /kg.058 m /kg.576 MPa During the heating rocess the ressure V 0 C + x 0 C is constant (8.4%) age 84, TableA 0 C T x roerty (roerty) (roerty) g roerty (roerty) + x (roerty) g Q
37 Thermodynamic Problem Soling Technique. Problem Statement Carbon dioxide is contained in a cylinder with a iston. The carbon dioxide is comressed with heat remoal rom T, to T,. The gas is then heated rom T, to T, at constant olume and then exanded without heat transer to the original state oint.. Schematic. Select Thermodynamic System oen - closed - control olume a closed thermodynamic system comosed to the mass o carbon dioxide in the cylinder CO 4. Proerty Diagram state oints - rocesses - cycle Q T, T, W Q W T, 5. Proerty Determination T, T u h s 6. Laws o Thermodynamics Q? W? E? material lows?
38 AVOGADRO' S LAW Ideal Gas Law IDEAL (PERFECT) GAS LAW One() moleo any gas.4 liters o STP (atm and 0 C) BOLYES LAW molecules/moleo gas at CHARLES LAW T T RT V mrt - absolute ressure, sia,kpa o o T - absolute temerature, R, K * R R molecular weight * R o t lb R lbmole kj kmole K * R 8.4 or o kpa m o kmole K T T mass moles Molecular Weight m n Molecular Weight V * R T * nr T
39 water Ideal Gas RT constant seciic heat
40 o What is the mass o. m o oxygen at 4 C and a gage ressure o 500 kpa. Atmosheric ressure is 97 kpa kpa + 97 kpa 597 kpa gage atmoshere o o 8.4 kj/kmole K or kpa m /kmole K R O.598 kpa m /kg alsotablea V 597 kpa. m m 9.8 kg o o RT.598 kpa m /kg 4 C K ( ) o What is the olume mass o. lbm o air at 4 F and a gage ressure o 500 sia. Atmosheric ressure is 4. 7 sia sia sia 54 sia air gage atmoshere t lb / lbmole R t lb R 5.6 also Table A E in BTU and si units m R T V V.5047t o 8.97 lbm R ( 9R o ) o o. lbm 5.6 t lb/ lbm R 4 F sia 44 t /in
41 IDEAL GAS EQUATION FORMS - For Air P m R T kpa m O kpa m kmole 8.4 K O kmole K kpa m O kpa m kpa m kg.87 K R 8.4 / 8.96 O o kg K kmole K lb t lb O t lbmole R O t lbmole R lb t lb O t lb t lbm 5.5 R R /8.96 O O t lbm R lbmole R si lb O t lb si t lbm.704 R O R /44/8.96 O lbm R lbmole R lb BTU O t lb t lbm R R /8.96/ O 778 O t lbm R lbmole R
42 The seciic olume o R.476 t The seciic olume o kpa m R 8.4 kg mole air / t lb/lbm R RT RT /lb.847 m /kg O air at t lb/lbm R 4.7 lb/in 44 in air at 4 / 8.96 K.87 kpa m /kg 0.5 kpa o F and4.7 sia o o ( R + 75 F) o /t C and0.5 kpa o o ( 7.5 K + 4 F)
43 Air initially at a olume o m and a ressure o 5 kpa exands at a constant temerature to a olume o m. What is the inal ressure? mass constant, RT m V R T m V R T V m 5 kpa m 7.9 kpa R T V R T V ( T ) T constant.86 4 kpa m V V m mconst Tconst m
44 SPECIFIC HEATS FOR GASSES
45 h SPECIFIC HEAT C T const T h c ( T) dt u c ( T) h c dh c dt dt u c subsistuting or dh and du c with same units and k FOR IDEAL GAS ONLY du c dt c dt dt c c c c c const For an ideal gas seciic heats are assumed constant By deinition h u + substituting RT h u + RT dierentiating dh du + RdT C u dt + R c dt + RdT R k R c
46 IDEAL GAS IMPROVEMENTS Enthaly, h, internal energy, u, and entroy, s. are not absolute but Dierences rom a base. h u c T c c Table Base State T c Table Base State ( T) ( T) dt dt ( T),c c ( T) Tables A -7, A -7 to A -, A - E
47 IDEAL GAS WITH VARIBLE SPECIFIC HEAT
48 b) a) Table A - a, h + O h e)ees K to000 seciic heat at the aerage temerature Table A - b, d) ( ) c T dt, c a + bt + ct + dt 8.9( ) +.5(.00057)( ) ( )( ) ( )( ) b)aerage seciic heat oer the temerature range, c)room temerature seciic heat, d) TableA 8E h h@000 Determine the enthaly change, h, o nitrogen in kj/kg as it is heated rom O K (76 C,40 Table A 8E, O O K h@600 O O F) using : e) 4 c a) h c O emirical seciic heat equation Table A c, h 544 T.kJ/kg K K kj/kg h kj/kg K. kj/kgk ( ) ( ) 448.5kJ/kg 45.6 kj/kg K 0,9 kj/kgmole 756 kj/kgmole 566 kj/kgmole h 566kJ/kgmole/8.0kg/kgmole kj/kg h enthaly(nitrogen, T 000., 0.5) enthaly(nitrogen, T 600., 0.5) c c) O seciic heat at room temerature 544 kj/kmole kj/kg h kJ/kg
49 PRINCIPAL OF CORRERSPONDING STATES COMPRESSIBILITY FACTOR Z Z is about the same or all gasses at the same reduced temerature and the same reduced ressure where: P Z Z RT V mrt P T R R T critical T critical ( 0)
50 VAN DER WAALS EQUATION OF STATE - 87 a + ( b) RT ( - ) a intermolecular orces b olume o gas molecules d a RT critical T critical critical 7 R T 64 b 0 critical critical a critical d b T critical R T 8 critical critical 0 T 0 0 critical oint T
51 THERMODYNAMIC PROPERTY MEASURMENT Thermodynamic roerties are indeendent o ath or rocess and are exact dierentials. Heat and Work are not exact dierentials but are deendent on rocess or ath. gas real alue or a gas,a 0 or an ideal T Joule Tomson Coeicient T c T u c T h 60 equations time, at a 6 thermodynamic roerites d u ds s u du ) (s, u h h s + T T s used with the First Law
52 T JT Coeicient h hconstant
53 Course Proerty Sources ) Ideal Gas Law with constant seciic heats ) Tables Steam Rerigerant Air ) EES CD NIST or home work conenience
54 EQUATION OF STATE ERRORS nitrogen
55 NIST Webbook Proerties tt://webbook.nist/go/chemistry/luid Temerature Table or Water in. degree increments rom 40 to 40 degrees. Select Units Select Table Tye
56 Select luid
57 Set low and high temerature and temerature increment.
58
59
Chapter 2 Solutions. 2.1 (D) Pressure and temperature are dependent during phase change and independent when in a single phase.
 Chater Solutions.1 (D) Pressure and temerature are deendent during hase change and indeendent when in a single hase.. (B) Sublimation is the direct conversion of a solid to a gas. To observe this rocess,
Chater Solutions.1 (D) Pressure and temerature are deendent during hase change and indeendent when in a single hase.. (B) Sublimation is the direct conversion of a solid to a gas. To observe this rocess,
P=1 atm. vapor. liquid
 P1 atm ar liqid Q Gien and P Ser Heat Regin i, P < P sat > sat Cmressed Liqid Regin i, P > P sat < sat SEAM PRESUE AND EMPERAURE ABLES Satrated Liqid Line Satrated Var Line s s g g g g ree ables emeratre
P1 atm ar liqid Q Gien and P Ser Heat Regin i, P < P sat > sat Cmressed Liqid Regin i, P > P sat < sat SEAM PRESUE AND EMPERAURE ABLES Satrated Liqid Line Satrated Var Line s s g g g g ree ables emeratre
Outline. Example. Solution. Property evaluation examples Specific heat Internal energy, enthalpy, and specific heats of solids and liquids Examples
 Outline Property ealuation examples Specific heat Internal energy, enthalpy, and specific heats of solids and liquids s A piston-cylinder deice initially contains 0.5m of saturated water apor at 00kPa.
Outline Property ealuation examples Specific heat Internal energy, enthalpy, and specific heats of solids and liquids s A piston-cylinder deice initially contains 0.5m of saturated water apor at 00kPa.
Chapter 4 Practice Problems
 Chater 4 ractice roblems (4.) (a) the number of microstates is N (b) 3 articles total 3! {, } 3 number microstates of secific arrangement (macrostate)!*! robability = (# microstates of secific arrangement)/(total
Chater 4 ractice roblems (4.) (a) the number of microstates is N (b) 3 articles total 3! {, } 3 number microstates of secific arrangement (macrostate)!*! robability = (# microstates of secific arrangement)/(total
ENERGY ANALYSIS: CLOSED SYSTEM
 ENERGY ANALYSIS: CLOSED SYSTEM A closed system can exchange energy with its surroundings through heat and work transer. In other words, work and heat are the orms that energy can be transerred across the
ENERGY ANALYSIS: CLOSED SYSTEM A closed system can exchange energy with its surroundings through heat and work transer. In other words, work and heat are the orms that energy can be transerred across the
Chapter 4: Properties of Pure Substances. Pure Substance. Phases of a Pure Substance. Phase-Change Processes of Pure Substances
 Chapter 4: roperties o ure Substances ure Substance A substance that has a ixed chemical composition throughout is called a pure substance such as water, air, and nitrogen A pure substance does not hae
Chapter 4: roperties o ure Substances ure Substance A substance that has a ixed chemical composition throughout is called a pure substance such as water, air, and nitrogen A pure substance does not hae
Thermodynamics I Chapter 2 Properties of Pure Substances
 Thermodynamics I Chapter 2 Properties of Pure Substances Mohsin Mohd Sies Fakulti Kejuruteraan Mekanikal, Universiti Teknologi Malaysia Properties of Pure Substances (Motivation) To quantify the changes
Thermodynamics I Chapter 2 Properties of Pure Substances Mohsin Mohd Sies Fakulti Kejuruteraan Mekanikal, Universiti Teknologi Malaysia Properties of Pure Substances (Motivation) To quantify the changes
ME 201 Thermodynamics
 8 ME 0 Thermodynamics Practice Problems or Property Evaluation For each process described below provide the temperature, pressure, and speciic volume at each state and the change in enthalpy, internal
8 ME 0 Thermodynamics Practice Problems or Property Evaluation For each process described below provide the temperature, pressure, and speciic volume at each state and the change in enthalpy, internal
Thermodynamics I Spring 1432/1433H (2011/2012H) Saturday, Wednesday 8:00am - 10:00am & Monday 8:00am - 9:00am MEP 261 Class ZA
 Thermodynamics I Spring 1432/1433H (2011/2012H) Saturday, Wednesday 8:00am - 10:00am & Monday 8:00am - 9:00am MEP 261 Class ZA Dr. Walid A. Aissa Associate Professor, Mech. Engg. Dept. Faculty of Engineering
Thermodynamics I Spring 1432/1433H (2011/2012H) Saturday, Wednesday 8:00am - 10:00am & Monday 8:00am - 9:00am MEP 261 Class ZA Dr. Walid A. Aissa Associate Professor, Mech. Engg. Dept. Faculty of Engineering
The Second Law: The Machinery
 The Second Law: The Machinery Chater 5 of Atkins: The Second Law: The Concets Sections 3.7-3.9 8th Ed, 3.3 9th Ed; 3.4 10 Ed.; 3E 11th Ed. Combining First and Second Laws Proerties of the Internal Energy
The Second Law: The Machinery Chater 5 of Atkins: The Second Law: The Concets Sections 3.7-3.9 8th Ed, 3.3 9th Ed; 3.4 10 Ed.; 3E 11th Ed. Combining First and Second Laws Proerties of the Internal Energy
Chapter 1 Fundamentals
 Chater Fundamentals. Overview of Thermodynamics Industrial Revolution brought in large scale automation of many tedious tasks which were earlier being erformed through manual or animal labour. Inventors
Chater Fundamentals. Overview of Thermodynamics Industrial Revolution brought in large scale automation of many tedious tasks which were earlier being erformed through manual or animal labour. Inventors
300 kpa 77 C. (d) If we neglect kinetic energy in the calculation of energy transport by mass
 6-6- Air flows steadily a ie at a secified state. The diameter of the ie, the rate of flow energy, and the rate of energy transort by mass are to be determed. Also, the error oled the determation of energy
6-6- Air flows steadily a ie at a secified state. The diameter of the ie, the rate of flow energy, and the rate of energy transort by mass are to be determed. Also, the error oled the determation of energy
AE301 Aerodynamics I UNIT A: Fundamental Concepts
 AE301 Aerodynamics I UNIT A: Fundamental Concets ROAD MAP... A-1: Engineering Fundamentals Reiew A-: Standard Atmoshere A-3: Goerning Equations of Aerodynamics A-4: Airseed Measurements A-5: Aerodynamic
AE301 Aerodynamics I UNIT A: Fundamental Concets ROAD MAP... A-1: Engineering Fundamentals Reiew A-: Standard Atmoshere A-3: Goerning Equations of Aerodynamics A-4: Airseed Measurements A-5: Aerodynamic
KNOWN: Data are provided for a closed system undergoing a process involving work, heat transfer, change in elevation, and change in velocity.
 Problem 44 A closed system of mass of 10 kg undergoes a process during which there is energy transfer by work from the system of 0147 kj per kg, an elevation decrease of 50 m, and an increase in velocity
Problem 44 A closed system of mass of 10 kg undergoes a process during which there is energy transfer by work from the system of 0147 kj per kg, an elevation decrease of 50 m, and an increase in velocity
C H A P T E R ,1752'8&7,21
 CHAPTER The first law of thermodynamics is a secial case of the fundamental and general law of conseration of energy, which is alied to thermal henomena in thermodynamic system. The basic law of energy
CHAPTER The first law of thermodynamics is a secial case of the fundamental and general law of conseration of energy, which is alied to thermal henomena in thermodynamic system. The basic law of energy
ME 201 Thermodynamics
 Spring 01 ME 01 Thermodynamics Property Evaluation Practice Problems II Solutions 1. Air at 100 K and 1 MPa goes to MPa isenthapically. Determine the entropy change. Substance Type: Ideal Gas (air) Process:
Spring 01 ME 01 Thermodynamics Property Evaluation Practice Problems II Solutions 1. Air at 100 K and 1 MPa goes to MPa isenthapically. Determine the entropy change. Substance Type: Ideal Gas (air) Process:
GAS. Outline. Experiments. Device for in-class thought experiments to prove 1 st law. First law of thermodynamics Closed systems (no mass flow)
 Outline First law of thermodynamics Closed systems (no mass flow) Device for in-class thought experiments to prove 1 st law Rubber stops GAS Features: Quasi-equlibrium expansion/compression Constant volume
Outline First law of thermodynamics Closed systems (no mass flow) Device for in-class thought experiments to prove 1 st law Rubber stops GAS Features: Quasi-equlibrium expansion/compression Constant volume
Example problems. Chapter 3: The Kinetic Theory of Gases. Homework: 13, 18, 20, 23, 25, 27 (p )
 Examle roblems Chater : he Kinetic heory o Gases Homework:, 8,,, 5, 7 (. 5-5) 9. An automobile tire has a volume o.64 x m and contains air at a gauge ressure (above atmosheric ressure) o 65 kpa when the
Examle roblems Chater : he Kinetic heory o Gases Homework:, 8,,, 5, 7 (. 5-5) 9. An automobile tire has a volume o.64 x m and contains air at a gauge ressure (above atmosheric ressure) o 65 kpa when the
PHYS1001 PHYSICS 1 REGULAR Module 2 Thermal Physics Chapter 17 First Law of Thermodynamics
 PHYS1001 PHYSICS 1 REGULAR Module Thermal Physics Chater 17 First Law of Thermodynamics References: 17.1 to 17.9 Examles: 17.1 to 17.7 Checklist Thermodynamic system collection of objects and fields. If
PHYS1001 PHYSICS 1 REGULAR Module Thermal Physics Chater 17 First Law of Thermodynamics References: 17.1 to 17.9 Examles: 17.1 to 17.7 Checklist Thermodynamic system collection of objects and fields. If
TDEC202 - Energy II (Recitation #3)
 TDEC202 - Energy II (Recitation #3) Summer Term, 2007 M. Carchidi Solution to Problem 3-029 We are given that the substance is R-134a and metric units so that Tables A-11, A-12 and A-13 in Appendix 1 will
TDEC202 - Energy II (Recitation #3) Summer Term, 2007 M. Carchidi Solution to Problem 3-029 We are given that the substance is R-134a and metric units so that Tables A-11, A-12 and A-13 in Appendix 1 will
To receive full credit all work must be clearly provided. Please use units in all answers.
 Exam is Open Textbook, Open Class Notes, Computers can be used (Computer limited to class notes, lectures, homework, book material, calculator, conversion utilities, etc. No searching for similar problems
Exam is Open Textbook, Open Class Notes, Computers can be used (Computer limited to class notes, lectures, homework, book material, calculator, conversion utilities, etc. No searching for similar problems
Earlier Lecture. Basics of Refrigeration/Liquefaction, coefficient of performance and importance of Carnot COP.
 9 1 Earlier Lecture Basics o Rerigeration/Liqueaction, coeicient o perormance and importance o Carnot COP. Throttling, heat exchanger, compression/expansion systems. Deinition o a rerigerator, liqueier
9 1 Earlier Lecture Basics o Rerigeration/Liqueaction, coeicient o perormance and importance o Carnot COP. Throttling, heat exchanger, compression/expansion systems. Deinition o a rerigerator, liqueier
δq T = nr ln(v B/V A )
 hysical Chemistry 007 Homework assignment, solutions roblem 1: An ideal gas undergoes the following reversible, cyclic rocess It first exands isothermally from state A to state B It is then comressed adiabatically
hysical Chemistry 007 Homework assignment, solutions roblem 1: An ideal gas undergoes the following reversible, cyclic rocess It first exands isothermally from state A to state B It is then comressed adiabatically
Chemistry 531 Spring 2009 Problem Set 6 Solutions
 Chemistry 531 Sring 2009 Problem Set 6 Solutions 1. In a thermochemical study of N 2, the following heat caacity data were found: t 0 C,m d 27.2Jmol 1 K 1 f t b f C,m d 23.4Jmol 1 K 1 C,m d 11.4Jmol 1
Chemistry 531 Sring 2009 Problem Set 6 Solutions 1. In a thermochemical study of N 2, the following heat caacity data were found: t 0 C,m d 27.2Jmol 1 K 1 f t b f C,m d 23.4Jmol 1 K 1 C,m d 11.4Jmol 1
Thermodynamics in combustion
 Thermodynamics in combustion 2nd ste in toolbox Thermodynamics deals with a equilibrium state and how chemical comosition can be calculated for a system with known atomic or molecular comosition if 2 indeendent
Thermodynamics in combustion 2nd ste in toolbox Thermodynamics deals with a equilibrium state and how chemical comosition can be calculated for a system with known atomic or molecular comosition if 2 indeendent
Phase transition. Asaf Pe er Background
 Phase transition Asaf Pe er 1 November 18, 2013 1. Background A hase is a region of sace, throughout which all hysical roerties (density, magnetization, etc.) of a material (or thermodynamic system) are
Phase transition Asaf Pe er 1 November 18, 2013 1. Background A hase is a region of sace, throughout which all hysical roerties (density, magnetization, etc.) of a material (or thermodynamic system) are
Chapter 3 PROPERTIES OF PURE SUBSTANCES
 Thermodynamics: An Engineering Approach Seventh Edition Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2011 Chapter 3 PROPERTIES OF PURE SUBSTANCES Copyright The McGraw-Hill Companies, Inc. Permission
Thermodynamics: An Engineering Approach Seventh Edition Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2011 Chapter 3 PROPERTIES OF PURE SUBSTANCES Copyright The McGraw-Hill Companies, Inc. Permission
Chapter 3 First Law of Thermodynamics and Energy Equation
 Fundamentals of Thermodynamics Chapter 3 First Law of Thermodynamics and Energy Equation Prof. Siyoung Jeong Thermodynamics I MEE0-0 Spring 04 Thermal Engineering Lab. 3. The energy equation Thermal Engineering
Fundamentals of Thermodynamics Chapter 3 First Law of Thermodynamics and Energy Equation Prof. Siyoung Jeong Thermodynamics I MEE0-0 Spring 04 Thermal Engineering Lab. 3. The energy equation Thermal Engineering
I affirm that I have never given nor received aid on this examination. I understand that cheating in the exam will result in a grade F for the class.
 Chem340 Physical Chemistry for Biochemists Exam Mar 16, 011 Your Name _ I affirm that I have never given nor received aid on this examination. I understand that cheating in the exam will result in a grade
Chem340 Physical Chemistry for Biochemists Exam Mar 16, 011 Your Name _ I affirm that I have never given nor received aid on this examination. I understand that cheating in the exam will result in a grade
ME 201 Thermodynamics
 Fall 204 ME 20 Thermodynamics Homework #8 Solutions. A rigid wall, insulated container is divided into two regions by a removable wall. One region contains 0. lb m of CO at 00 F, while the other region
Fall 204 ME 20 Thermodynamics Homework #8 Solutions. A rigid wall, insulated container is divided into two regions by a removable wall. One region contains 0. lb m of CO at 00 F, while the other region
Chapter 4. Energy Analysis of Closed Systems
 Chapter 4 Energy Analysis of Closed Systems The first law of thermodynamics is an expression of the conservation of energy principle. Energy can cross the boundaries of a closed system in the form of heat
Chapter 4 Energy Analysis of Closed Systems The first law of thermodynamics is an expression of the conservation of energy principle. Energy can cross the boundaries of a closed system in the form of heat
Chemistry 420/523 Chemical Thermodynamics (Spring ) Examination 1
 Chemistry 420/523 Chemical hermodynamics (Sring 2001-02) Examination 1 1 Boyle temerature is defined as the temerature at which the comression factor Z m /(R ) of a gas is exactly equal to 1 For a gas
Chemistry 420/523 Chemical hermodynamics (Sring 2001-02) Examination 1 1 Boyle temerature is defined as the temerature at which the comression factor Z m /(R ) of a gas is exactly equal to 1 For a gas
GEF2200 vår 2017 Løsningsforslag sett 1
 GEF2200 vår 2017 Løsningsforslag sett 1 A.1.T R is the universal gas constant, with value 8.3143JK 1 mol 1. R is the gas constant for a secic gas, given by R R M (1) where M is the molecular weight of
GEF2200 vår 2017 Løsningsforslag sett 1 A.1.T R is the universal gas constant, with value 8.3143JK 1 mol 1. R is the gas constant for a secic gas, given by R R M (1) where M is the molecular weight of
COMPENDIUM OF EQUATIONS Unified Engineering Thermodynamics
 COMPENDIUM OF EQUAIONS Unified Engineering hermodynamics Note: It is with some reseration that I suly this comendium of equations. One of the common itfalls for engineering students is that they sole roblems
COMPENDIUM OF EQUAIONS Unified Engineering hermodynamics Note: It is with some reseration that I suly this comendium of equations. One of the common itfalls for engineering students is that they sole roblems
(b) The heat transfer can be determined from an energy balance on the system
 8-5 Heat is transferred to a iston-cylinder device wit a set of stos. e work done, te eat transfer, te exergy destroyed, and te second-law efficiency are to be deterined. Assutions e device is stationary
8-5 Heat is transferred to a iston-cylinder device wit a set of stos. e work done, te eat transfer, te exergy destroyed, and te second-law efficiency are to be deterined. Assutions e device is stationary
CHAPTER. Properties of Pure Substances
 CHAPTER 2 Properties of Pure Substances A Pure Substance Is a substance that is chemically homogenous and fixed in chemical composition.(e.g. water, nitrogen, air & etc.) mixture of oil and water is not
CHAPTER 2 Properties of Pure Substances A Pure Substance Is a substance that is chemically homogenous and fixed in chemical composition.(e.g. water, nitrogen, air & etc.) mixture of oil and water is not
Chapter 5. The Properties of Gases. Gases and Their Properties. Why Study Gases? Gas Pressure. some very common elements exist in a gaseous state
 Chapter 5 Gases and Their Properties Why Study Gases? some very common elements exist in a gaseous state our gaseous atmosphere provides one means of transferring energy and material throughout the globe
Chapter 5 Gases and Their Properties Why Study Gases? some very common elements exist in a gaseous state our gaseous atmosphere provides one means of transferring energy and material throughout the globe
Thermodynamics I. Properties of Pure Substances
 Thermodynamics I Properties of Pure Substances Dr.-Eng. Zayed Al-Hamamre 1 Content Pure substance Phases of a pure substance Phase-change processes of pure substances o Compressed liquid, Saturated liquid,
Thermodynamics I Properties of Pure Substances Dr.-Eng. Zayed Al-Hamamre 1 Content Pure substance Phases of a pure substance Phase-change processes of pure substances o Compressed liquid, Saturated liquid,
Chapter 1 Solutions Engineering and Chemical Thermodynamics 2e Wyatt Tenhaeff Milo Koretsky
 Chapter 1 Solutions Engineering and Chemical Thermodynamics 2e Wyatt Tenhaeff Milo Koretsky School of Chemical, Biological, and Enironmental Engineering Oregon State Uniersity 1.1 (b) The olume of water
Chapter 1 Solutions Engineering and Chemical Thermodynamics 2e Wyatt Tenhaeff Milo Koretsky School of Chemical, Biological, and Enironmental Engineering Oregon State Uniersity 1.1 (b) The olume of water
d dt T R m n p 1. (A) 4. (4) Carnot engine T Refrigerating effect W COPref. = 1 4 kw 5. (A)
 . (A). (C) 5. (C) 7. ( to 5) 9. (C) 6. (C). (C). (D) 6. (A) 8. (0.6 to 0.66) 50. (D) 6. (C). (A) 5. (C) 7. (A) 9. (C) 5. (D) 6. (C). () 6. (C) 8. (600) 0. (D) 5. (B) 6. (D) 5. (A) 7. (A) 9. (D). (C) 5.
. (A). (C) 5. (C) 7. ( to 5) 9. (C) 6. (C). (C). (D) 6. (A) 8. (0.6 to 0.66) 50. (D) 6. (C). (A) 5. (C) 7. (A) 9. (C) 5. (D) 6. (C). () 6. (C) 8. (600) 0. (D) 5. (B) 6. (D) 5. (A) 7. (A) 9. (D). (C) 5.
CHAPTER 2 ENERGY INTERACTION (HEAT AND WORK)
 CHATER ENERGY INTERACTION (HEAT AND WORK) Energy can cross the boundary of a closed system in two ways: Heat and Work. WORK The work is done by a force as it acts upon a body moving in direction of force.
CHATER ENERGY INTERACTION (HEAT AND WORK) Energy can cross the boundary of a closed system in two ways: Heat and Work. WORK The work is done by a force as it acts upon a body moving in direction of force.
Unit code: H/ QCF level: 5 Credit value: 15 OUTCOME 1 - THERMODYNAMIC SYSTEMS TUTORIAL 2
 Unit 43: Plant and Process Princiles Unit code: H/60 44 QCF level: 5 Credit value: 5 OUCOME - HERMODYNAMIC SYSEMS UORIAL Understand thermodynamic systems as alied to lant engineering rocesses hermodynamic
Unit 43: Plant and Process Princiles Unit code: H/60 44 QCF level: 5 Credit value: 5 OUCOME - HERMODYNAMIC SYSEMS UORIAL Understand thermodynamic systems as alied to lant engineering rocesses hermodynamic
Chapter 3 PROPERTIES OF PURE SUBSTANCES. Thermodynamics: An Engineering Approach, 6 th Edition Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2008
 Chapter 3 PROPERTIES OF PURE SUBSTANCES Thermodynamics: An Engineering Approach, 6 th Edition Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2008 Objectives Introduce the concept of a pure substance. Discuss
Chapter 3 PROPERTIES OF PURE SUBSTANCES Thermodynamics: An Engineering Approach, 6 th Edition Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2008 Objectives Introduce the concept of a pure substance. Discuss
SOLUTION MANUAL ENGLISH UNIT PROBLEMS CHAPTER 5 SONNTAG BORGNAKKE VAN WYLEN. FUNDAMENTALS of. Thermodynamics. Sixth Edition
 SOLUTION MANUAL ENGLISH UNIT ROBLEMS CHATER 5 SONNTAG BORGNAKKE VAN WYLEN FUNDAMENTALS of Thermodynamics Sixth Edition CHATER 5 SUBSECTION ROB NO. Concept-Study Guide roblems 39-44 Kinetic and otential
SOLUTION MANUAL ENGLISH UNIT ROBLEMS CHATER 5 SONNTAG BORGNAKKE VAN WYLEN FUNDAMENTALS of Thermodynamics Sixth Edition CHATER 5 SUBSECTION ROB NO. Concept-Study Guide roblems 39-44 Kinetic and otential
Pure Substance. Properties of Pure Substances & Equations of State. Vapour-Liquid-Solid Phase Equilibrium
 Pure Substance Properties of Pure Substances & Equations of State Dr. d. Zahurul Haq Professor Department of echanical Engineering Bangladesh University of Engineering & Technology (BUET) Dhaka-1000, Bangladesh
Pure Substance Properties of Pure Substances & Equations of State Dr. d. Zahurul Haq Professor Department of echanical Engineering Bangladesh University of Engineering & Technology (BUET) Dhaka-1000, Bangladesh
Pure Substance. Properties of Pure Substances & Equations of State. Vapour-Liquid-Solid Phase Equilibrium
 Pure Substance Properties of Pure Substances & Equations of State Dr. d. Zahurul Haq Professor Department of echanical Engineering Bangladesh University of Engineering & Technology (BUET) Dhaka-1000, Bangladesh
Pure Substance Properties of Pure Substances & Equations of State Dr. d. Zahurul Haq Professor Department of echanical Engineering Bangladesh University of Engineering & Technology (BUET) Dhaka-1000, Bangladesh
Internal Energy in terms of Properties
 Lecture #3 Internal Energy in terms of roerties Internal energy is a state function. A change in the state of the system causes a change in its roerties. So, we exress the change in internal energy in
Lecture #3 Internal Energy in terms of roerties Internal energy is a state function. A change in the state of the system causes a change in its roerties. So, we exress the change in internal energy in
Basic Thermodynamics Module 1
 Basic Thermodynamics Module 1 Lecture 9: Thermodynamic Properties of Fluids Thermodynamic Properties of fluids Most useful properties: Properties like pressure, volume and temperature which can be measured
Basic Thermodynamics Module 1 Lecture 9: Thermodynamic Properties of Fluids Thermodynamic Properties of fluids Most useful properties: Properties like pressure, volume and temperature which can be measured
3. High Temperature Gases FPK1 2009/MZ 1/23
 3. High Temerature Gases FP 9/MZ /3 Terms and Concets Dissociation Diatomic element gases Bond energy, dissociation energy (enthali) Flood s dissociation diagram Vaorization, eaoration, boiling, sublimation
3. High Temerature Gases FP 9/MZ /3 Terms and Concets Dissociation Diatomic element gases Bond energy, dissociation energy (enthali) Flood s dissociation diagram Vaorization, eaoration, boiling, sublimation
Theory of turbomachinery. Chapter 1
 Theory of turbomachinery Chater Introduction: Basic Princiles Take your choice of those that can best aid your action. (Shakeseare, Coriolanus) Introduction Definition Turbomachinery describes machines
Theory of turbomachinery Chater Introduction: Basic Princiles Take your choice of those that can best aid your action. (Shakeseare, Coriolanus) Introduction Definition Turbomachinery describes machines
Introduction to Thermodynamic Cycles Part 1 1 st Law of Thermodynamics and Gas Power Cycles
 Introduction to Thermodynamic Cycles Part 1 1 st Law of Thermodynamics and Gas Power Cycles by James Doane, PhD, PE Contents 1.0 Course Oeriew... 4.0 Basic Concepts of Thermodynamics... 4.1 Temperature
Introduction to Thermodynamic Cycles Part 1 1 st Law of Thermodynamics and Gas Power Cycles by James Doane, PhD, PE Contents 1.0 Course Oeriew... 4.0 Basic Concepts of Thermodynamics... 4.1 Temperature
Efficiencies. Damian Vogt Course MJ2429. Nomenclature. Symbol Denotation Unit c Flow speed m/s c p. pressure c v. Specific heat at constant J/kgK
 Turbomachinery Lecture Notes 1 7-9-1 Efficiencies Damian Vogt Course MJ49 Nomenclature Subscrits Symbol Denotation Unit c Flow seed m/s c Secific heat at constant J/kgK ressure c v Secific heat at constant
Turbomachinery Lecture Notes 1 7-9-1 Efficiencies Damian Vogt Course MJ49 Nomenclature Subscrits Symbol Denotation Unit c Flow seed m/s c Secific heat at constant J/kgK ressure c v Secific heat at constant
B 2, C 2, N 2. O 2, F 2, Ne 2. Energy order of the p 2p and s 2p orbitals changes across the period.
 Chapter 11 Gases Energy order of the p p and s p orbitals changes across the period. Due to lower nuclear charge of B, C & N there is no s-p orbitals interaction Due to high nuclear charge of O, F& Ne
Chapter 11 Gases Energy order of the p p and s p orbitals changes across the period. Due to lower nuclear charge of B, C & N there is no s-p orbitals interaction Due to high nuclear charge of O, F& Ne
ME 2322 Thermodynamics I PRE-LECTURE Lesson 10 Complete the items below Name:
 Lesson 10 1. (5 pt) If P > P sat (T), the phase is a subcooled liquid. 2. (5 pt) if P < P sat (T), the phase is superheated vapor. 3. (5 pt) if T > T sat (P), the phase is superheated vapor. 4. (5 pt)
Lesson 10 1. (5 pt) If P > P sat (T), the phase is a subcooled liquid. 2. (5 pt) if P < P sat (T), the phase is superheated vapor. 3. (5 pt) if T > T sat (P), the phase is superheated vapor. 4. (5 pt)
Chapter 11 Gases 1 Copyright McGraw-Hill 2009
 Chapter 11 Gases Copyright McGraw-Hill 2009 1 11.1 Properties of Gases The properties of a gas are almost independent of its identity. (Gas molecules behave as if no other molecules are present.) Compressible
Chapter 11 Gases Copyright McGraw-Hill 2009 1 11.1 Properties of Gases The properties of a gas are almost independent of its identity. (Gas molecules behave as if no other molecules are present.) Compressible
SOLUTION MANUAL ENGLISH UNIT PROBLEMS CHAPTER 3 SONNTAG BORGNAKKE VAN WYLEN. FUNDAMENTALS of. Thermodynamics. Sixth Edition
 SOLUTION MANUAL ENGLISH UNIT PROBLEMS CHAPTER 3 SONNTAG BORGNAKKE VAN WYLEN FUNDAMENTALS of Thermodynamics Sixth Edition CHAPTER 3 SUBSECTION PROB NO. Concept-Study Guide Problems 128-132 Phase diagrams
SOLUTION MANUAL ENGLISH UNIT PROBLEMS CHAPTER 3 SONNTAG BORGNAKKE VAN WYLEN FUNDAMENTALS of Thermodynamics Sixth Edition CHAPTER 3 SUBSECTION PROB NO. Concept-Study Guide Problems 128-132 Phase diagrams
MAE 110A. Homework 3: Solutions 10/20/2017
 MAE 110A Homework 3: Solutions 10/20/2017 3.10: For H 2O, determine the specified property at the indicated state. Locate the state on a sketch of the T-v diagram. Given a) T 140 C, v 0.5 m 3 kg b) p 30MPa,
MAE 110A Homework 3: Solutions 10/20/2017 3.10: For H 2O, determine the specified property at the indicated state. Locate the state on a sketch of the T-v diagram. Given a) T 140 C, v 0.5 m 3 kg b) p 30MPa,
df da df = force on one side of da due to pressure
 I. Review of Fundamental Fluid Mechanics and Thermodynamics 1. 1 Some fundamental aerodynamic variables htt://en.wikiedia.org/wiki/hurricane_ivan_(2004) 1) Pressure: the normal force er unit area exerted
I. Review of Fundamental Fluid Mechanics and Thermodynamics 1. 1 Some fundamental aerodynamic variables htt://en.wikiedia.org/wiki/hurricane_ivan_(2004) 1) Pressure: the normal force er unit area exerted
ME 201 Thermodynamics
 ME 0 Thermodynamics Solutions First Law Practice Problems. Consider a balloon that has been blown up inside a building and has been allowed to come to equilibrium with the inside temperature of 5 C and
ME 0 Thermodynamics Solutions First Law Practice Problems. Consider a balloon that has been blown up inside a building and has been allowed to come to equilibrium with the inside temperature of 5 C and
Week 5. Energy Analysis of Closed Systems. GENESYS Laboratory
 Week 5. Energy Analysis of Closed Systems Objectives 1. Examine the moving boundary work or PdV work commonly encountered in reciprocating devices such as automotive engines and compressors 2. Identify
Week 5. Energy Analysis of Closed Systems Objectives 1. Examine the moving boundary work or PdV work commonly encountered in reciprocating devices such as automotive engines and compressors 2. Identify
SPC 407 Sheet 6 - Solution Compressible Flow Fanno Flow
 SPC 407 Sheet 6 - Solution Comressible Flow Fanno Flow 1. What is the effect of friction on flow velocity in subsonic and suersonic Fanno flow? Friction increases the flow velocity in subsonic Fanno flow,
SPC 407 Sheet 6 - Solution Comressible Flow Fanno Flow 1. What is the effect of friction on flow velocity in subsonic and suersonic Fanno flow? Friction increases the flow velocity in subsonic Fanno flow,
Fundamentals of Thermodynamics SI Version
 Solution Manual Chapter 2 Fundamentals of hermodynamics SI Version Borgnakke Sonntag 8e Updated July 2013 2 Borgnakke and Sonntag CONEN CHAPER 2 SUBSECION PROB NO. Concept problems 1-15 Phase diagrams,
Solution Manual Chapter 2 Fundamentals of hermodynamics SI Version Borgnakke Sonntag 8e Updated July 2013 2 Borgnakke and Sonntag CONEN CHAPER 2 SUBSECION PROB NO. Concept problems 1-15 Phase diagrams,
ME Thermodynamics I. Lecture Notes and Example Problems
 ME 227.3 Thermodynamics I Lecture Notes and Example Problems James D. Bugg September 2018 Department of Mechanical Engineering Introduction Part I: Lecture Notes This part contains handout versions of
ME 227.3 Thermodynamics I Lecture Notes and Example Problems James D. Bugg September 2018 Department of Mechanical Engineering Introduction Part I: Lecture Notes This part contains handout versions of
Dr Ali Jawarneh. Hashemite University
 Dr Ali Jawarneh Department of Mechanical Engineering Hashemite University Examine the moving boundary work or P d work commonly encountered in reciprocating devices such as automotive engines and compressors.
Dr Ali Jawarneh Department of Mechanical Engineering Hashemite University Examine the moving boundary work or P d work commonly encountered in reciprocating devices such as automotive engines and compressors.
Gases. Characteristics of Gases. Unlike liquids and solids, gases
 Gases Characteristics of Gases Unlike liquids and solids, gases expand to fill their containers; are highly compressible; have extremely low densities. 1 Pressure Pressure is the amount of force applied
Gases Characteristics of Gases Unlike liquids and solids, gases expand to fill their containers; are highly compressible; have extremely low densities. 1 Pressure Pressure is the amount of force applied
CHAPTER 7 ENTROPY. Copyright Hany A. Al-Ansary and S. I. Abdel-Khalik (2014) 1
 CHAPTER 7 ENTROPY S. I. Abdel-Khalik (2014) 1 ENTROPY The Clausius Inequality The Clausius inequality states that for for all cycles, reversible or irreversible, engines or refrigerators: For internally-reversible
CHAPTER 7 ENTROPY S. I. Abdel-Khalik (2014) 1 ENTROPY The Clausius Inequality The Clausius inequality states that for for all cycles, reversible or irreversible, engines or refrigerators: For internally-reversible
Chapter 3 PROPERTIES OF PURE SUBSTANCES
 Thermodynamics: An Engineering Approach Seventh Edition in SI Units Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2011 Chapter 3 PROPERTIES OF PURE SUBSTANCES Copyright The McGraw-Hill Companies, Inc.
Thermodynamics: An Engineering Approach Seventh Edition in SI Units Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2011 Chapter 3 PROPERTIES OF PURE SUBSTANCES Copyright The McGraw-Hill Companies, Inc.
Chapter 7. Entropy. by Asst.Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn
 Chapter 7 Entropy by Asst.Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn Reference: Cengel, Yunus A. and Michael A. Boles, Thermodynamics: An Engineering Approach, 5th ed.,
Chapter 7 Entropy by Asst.Prof. Dr.Woranee Paengjuntuek and Asst. Prof. Dr.Worarattana Pattaraprakorn Reference: Cengel, Yunus A. and Michael A. Boles, Thermodynamics: An Engineering Approach, 5th ed.,
HEAT, WORK, AND THE FIRST LAW OF THERMODYNAMICS
 HET, ORK, ND THE FIRST L OF THERMODYNMIS 8 EXERISES Section 8. The First Law of Thermodynamics 5. INTERPRET e identify the system as the water in the insulated container. The roblem involves calculating
HET, ORK, ND THE FIRST L OF THERMODYNMIS 8 EXERISES Section 8. The First Law of Thermodynamics 5. INTERPRET e identify the system as the water in the insulated container. The roblem involves calculating
THERMODYNAMICS. Contents
 THERMODYAMICS EREST YEUG ERESTYALUMI@GMAIL.COM Contents Part 1. otes and Solutions for Thermal Physics by Ralh Baierlein 1 1. Background 1 1.1. Heating and Temerature 1 1.2. Some dilute gas relationshis
THERMODYAMICS EREST YEUG ERESTYALUMI@GMAIL.COM Contents Part 1. otes and Solutions for Thermal Physics by Ralh Baierlein 1 1. Background 1 1.1. Heating and Temerature 1 1.2. Some dilute gas relationshis
Common Terms, Definitions and Conversion Factors
 1 Common Terms, Definitions and Conversion Factors 1. Force: A force is a push or pull upon an object resulting from the object s interaction with another object. It is defined as Where F = m a F = Force
1 Common Terms, Definitions and Conversion Factors 1. Force: A force is a push or pull upon an object resulting from the object s interaction with another object. It is defined as Where F = m a F = Force
Comparison of Solids, Liquids, and Gases
 CHAPTER 8 GASES Comparison of Solids, Liquids, and Gases The density of gases is much less than that of solids or liquids. Densities (g/ml) Solid Liquid Gas H O 0.97 0.998 0.000588 CCl 4.70.59 0.00503
CHAPTER 8 GASES Comparison of Solids, Liquids, and Gases The density of gases is much less than that of solids or liquids. Densities (g/ml) Solid Liquid Gas H O 0.97 0.998 0.000588 CCl 4.70.59 0.00503
Some Fundamental Definitions:
 Lecture 2. The GAS LAWS Some Fundamental Definitions: SYSTEM: the part of the universe being the subject of study 1 Some Fundamental Definitions: State of the System: condition of a system at any given
Lecture 2. The GAS LAWS Some Fundamental Definitions: SYSTEM: the part of the universe being the subject of study 1 Some Fundamental Definitions: State of the System: condition of a system at any given
First Law of Thermodynamics
 First Law of Thermodynamics During an interaction between a system and its surroundings, the amount of energy gained by the system must be exactly equal to the amount of energy lost by the surroundings.
First Law of Thermodynamics During an interaction between a system and its surroundings, the amount of energy gained by the system must be exactly equal to the amount of energy lost by the surroundings.
Problem 1.6 Make a guess at the order of magnitude of the mass (e.g., 0.01, 0.1, 1.0, 10, 100, or 1000 lbm or kg) of standard air that is in a room 10
 Problem 1.6 Make a guess at the order of magnitude of the mass (e.g., 0.01, 0.1, 1.0, 10, 100, or 1000 lbm or kg) of standard air that is in a room 10 ft by 10 ft by 8 ft, and then compute this mass in
Problem 1.6 Make a guess at the order of magnitude of the mass (e.g., 0.01, 0.1, 1.0, 10, 100, or 1000 lbm or kg) of standard air that is in a room 10 ft by 10 ft by 8 ft, and then compute this mass in
Lecture 2 PROPERTIES OF GASES
 Lecture 2 PROPERTIES OF GASES Reference: Principles of General Chemistry, Silberberg Chapter 6 SOME FUNDAMENTAL DEFINITIONS: SYSTEM: the part of the universe being the subject of study 1 SOME FUNDAMENTAL
Lecture 2 PROPERTIES OF GASES Reference: Principles of General Chemistry, Silberberg Chapter 6 SOME FUNDAMENTAL DEFINITIONS: SYSTEM: the part of the universe being the subject of study 1 SOME FUNDAMENTAL
CHAPTER 12 GASES AND KINETIC-MOLECULAR THEORY
 . Pressure CHAPER GASES AND KINEIC-MOLECULAR HEORY. Boyle s Law: he -P Relationship 3. Charles Law: he - Relationship 4. Standard &P 5. he Combined Gas Law Equation 6. Avogadro s Law and the Standard Molar
. Pressure CHAPER GASES AND KINEIC-MOLECULAR HEORY. Boyle s Law: he -P Relationship 3. Charles Law: he - Relationship 4. Standard &P 5. he Combined Gas Law Equation 6. Avogadro s Law and the Standard Molar
ATMOS Lecture 7. The First Law and Its Consequences Pressure-Volume Work Internal Energy Heat Capacity Special Cases of the First Law
 TMOS 5130 Lecture 7 The First Law and Its Consequences Pressure-Volume Work Internal Energy Heat Caacity Secial Cases of the First Law Pressure-Volume Work Exanding Volume Pressure δw = f & dx δw = F ds
TMOS 5130 Lecture 7 The First Law and Its Consequences Pressure-Volume Work Internal Energy Heat Caacity Secial Cases of the First Law Pressure-Volume Work Exanding Volume Pressure δw = f & dx δw = F ds
Properties of Gases. Gases have four main characteristics compared with solids and liquids:
 1 Properties of Gases Gases have four main characteristics compared with solids and liquids: Gases take the volume and shape of their containers. Mix completely (homogeneously) with any other gas. Compressible:
1 Properties of Gases Gases have four main characteristics compared with solids and liquids: Gases take the volume and shape of their containers. Mix completely (homogeneously) with any other gas. Compressible:
School of Chemical & Biological Engineering, Konkuk University
 School of Chemical & Biological Engineering, Konkuk University Chemistry is the science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical
School of Chemical & Biological Engineering, Konkuk University Chemistry is the science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical
Thermodynamics Introduction and Basic Concepts
 Thermodynamics Introduction and Basic Concepts by Asst. Prof. Channarong Asavatesanupap Mechanical Engineering Department Faculty of Engineering Thammasat University 2 What is Thermodynamics? Thermodynamics
Thermodynamics Introduction and Basic Concepts by Asst. Prof. Channarong Asavatesanupap Mechanical Engineering Department Faculty of Engineering Thammasat University 2 What is Thermodynamics? Thermodynamics
Chapter 19 Thermal Properties of Matter. A PowerPoint Presentation by Paul E. Tippens, Professor of Physics Southern Polytechnic State University
 Chapter 19 Thermal Properties of Matter A PowerPoint Presentation by Paul E. Tippens, Professor of Physics Southern Polytechnic State University 2007 Objectives: After finishing this unit, you should be
Chapter 19 Thermal Properties of Matter A PowerPoint Presentation by Paul E. Tippens, Professor of Physics Southern Polytechnic State University 2007 Objectives: After finishing this unit, you should be
FUGACITY. It is simply a measure of molar Gibbs energy of a real gas.
 FUGACITY It is simly a measure of molar Gibbs energy of a real gas. Modifying the simle equation for the chemical otential of an ideal gas by introducing the concet of a fugacity (f). The fugacity is an
FUGACITY It is simly a measure of molar Gibbs energy of a real gas. Modifying the simle equation for the chemical otential of an ideal gas by introducing the concet of a fugacity (f). The fugacity is an
Engineering Thermodynamics
 David Ng Summer 2017 Contents 1 July 5, 2017 3 1.1 Thermodynamics................................ 3 2 July 7, 2017 3 2.1 Properties.................................... 3 3 July 10, 2017 4 3.1 Systems.....................................
David Ng Summer 2017 Contents 1 July 5, 2017 3 1.1 Thermodynamics................................ 3 2 July 7, 2017 3 2.1 Properties.................................... 3 3 July 10, 2017 4 3.1 Systems.....................................
Chapter 10 Gases. Measurement of pressure: Barometer Manometer Units. Relationship of pressure and volume (Boyle s Law)
 Chapter 10 Gases Conditions of ideal gases: Ideal gases have no attractive forces between the molecules. the atoms volume taken into account when looking at the volume a gas occupies. Low pressure and
Chapter 10 Gases Conditions of ideal gases: Ideal gases have no attractive forces between the molecules. the atoms volume taken into account when looking at the volume a gas occupies. Low pressure and
Pure Substances Phase Change, Property Tables and Diagrams
 Pure Substances Phase Change, Property Tables and Diagrams In this chapter we consider the property values and relationships of a pure substance (such as water) which can exist in three phases - solid,
Pure Substances Phase Change, Property Tables and Diagrams In this chapter we consider the property values and relationships of a pure substance (such as water) which can exist in three phases - solid,
Chapter 3 PROPERTIES OF PURE SUBSTANCES SUMMARY
 Chapter 3 PROPERTIES OF PURE SUBSTANCES SUMMARY PURE SUBSTANCE Pure substance: A substance that has a fixed chemical composition throughout. Compressed liquid (sub-cooled liquid): A substance that it is
Chapter 3 PROPERTIES OF PURE SUBSTANCES SUMMARY PURE SUBSTANCE Pure substance: A substance that has a fixed chemical composition throughout. Compressed liquid (sub-cooled liquid): A substance that it is
Chapter 7. Entropy: A Measure of Disorder
 Chapter 7 Entropy: A Measure of Disorder Entropy and the Clausius Inequality The second law of thermodynamics leads to the definition of a new property called entropy, a quantitative measure of microscopic
Chapter 7 Entropy: A Measure of Disorder Entropy and the Clausius Inequality The second law of thermodynamics leads to the definition of a new property called entropy, a quantitative measure of microscopic
Readings for this homework assignment and upcoming lectures
 Homework #3 (group) Tuesday, February 13 by 4:00 pm 5290 exercises (individual) Thursday, February 15 by 4:00 pm extra credit (individual) Thursday, February 15 by 4:00 pm Readings for this homework assignment
Homework #3 (group) Tuesday, February 13 by 4:00 pm 5290 exercises (individual) Thursday, February 15 by 4:00 pm extra credit (individual) Thursday, February 15 by 4:00 pm Readings for this homework assignment
Pure Substance Properties and Equation of State
 Pure Substance Properties and Equation of State Pure Substance Content Pure Substance A substance that has a fixed chemical composition throughout is called a pure substance. Water, nitrogen, helium, and
Pure Substance Properties and Equation of State Pure Substance Content Pure Substance A substance that has a fixed chemical composition throughout is called a pure substance. Water, nitrogen, helium, and
Ideal Gas Law. September 2, 2014
 Ideal Gas Law Setember 2, 2014 Thermodynamics deals with internal transformations of the energy of a system and exchanges of energy between that system and its environment. A thermodynamic system refers
Ideal Gas Law Setember 2, 2014 Thermodynamics deals with internal transformations of the energy of a system and exchanges of energy between that system and its environment. A thermodynamic system refers
First Name Last Name CIRCLE YOUR LECTURE BELOW: Div. 1 10:30 am Div. 2 2:30 pm Div. 3 4:30 pm Prof. Gore Prof. Udupa Prof. Chen
 CIRCLE YOUR LECURE BELOW: Div. 1 10:30 am Div. :30 m Div. 3 4:30 m Prof. Gore Prof. Udua Prof. Chen EXAM # 3 INSRUCIONS 1. his is a closed book examination. All needed roerty tables are rovided.. Do not
CIRCLE YOUR LECURE BELOW: Div. 1 10:30 am Div. :30 m Div. 3 4:30 m Prof. Gore Prof. Udua Prof. Chen EXAM # 3 INSRUCIONS 1. his is a closed book examination. All needed roerty tables are rovided.. Do not
PROPERTIES OF PURE SUBSTANCES. Chapter 3. Mehmet Kanoglu. Thermodynamics: An Engineering Approach, 6 th Edition. Yunus A. Cengel, Michael A.
 Thermodynamics: An Engineering Approach, 6 th Edition Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2008 Chapter 3 PROPERTIES OF PURE SUBSTANCES Mehmet Kanoglu Copyright The McGraw-Hill Companies, Inc.
Thermodynamics: An Engineering Approach, 6 th Edition Yunus A. Cengel, Michael A. Boles McGraw-Hill, 2008 Chapter 3 PROPERTIES OF PURE SUBSTANCES Mehmet Kanoglu Copyright The McGraw-Hill Companies, Inc.
Principles of Food and Bioprocess Engineering (FS 231) Solutions to Example Problems on Evaporation
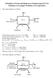 Princiles of Food and Biorocess Engineering (FS 231) Solutions to Examle Problems on Eaoration 1. The system diagram is as follows: Solids balance equation: 0.1 (m f) = 0.5 (1,000) Thus, m f = 5,000 kg/hr
Princiles of Food and Biorocess Engineering (FS 231) Solutions to Examle Problems on Eaoration 1. The system diagram is as follows: Solids balance equation: 0.1 (m f) = 0.5 (1,000) Thus, m f = 5,000 kg/hr
Chapter 5: The First Law of Thermodynamics: Closed Systems
 Chapter 5: The First Law of Thermodynamics: Closed Systems The first law of thermodynamics can be simply stated as follows: during an interaction between a system and its surroundings, the amount of energy
Chapter 5: The First Law of Thermodynamics: Closed Systems The first law of thermodynamics can be simply stated as follows: during an interaction between a system and its surroundings, the amount of energy
QUIZZES RIEPJCPIγPJEJJJY
 Che 3021 Thermodynamics I QUIZZES RIEPJCPIγPJEJJJY QUIZ 1. Find Molecular Weights: 1 1 CO 2 2 NaCl 3 Aspirin C 9 H 8 O 4 CO2 = NaCl = C9H8O4 = PIgPJC Quiz 1. Temperature conversion 1 Convert 94 o F, to
Che 3021 Thermodynamics I QUIZZES RIEPJCPIγPJEJJJY QUIZ 1. Find Molecular Weights: 1 1 CO 2 2 NaCl 3 Aspirin C 9 H 8 O 4 CO2 = NaCl = C9H8O4 = PIgPJC Quiz 1. Temperature conversion 1 Convert 94 o F, to
Chapter 11. Molecular Composition of Gases
 Chapter 11 Molecular Composition of Gases PART 1 Volume-Mass Relationships of Gases Avogadro s Law Equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. Recall
Chapter 11 Molecular Composition of Gases PART 1 Volume-Mass Relationships of Gases Avogadro s Law Equal volumes of gases at the same temperature and pressure contain equal numbers of molecules. Recall
Chapter 5 MASS AND ENERGY ANALYSIS OF CONTROL VOLUMES
 5- Chapter 5 MASS AND ENERGY ANALYSIS OF CONTROL OLUMES Conseration of Mass 5-C Mass, energy, momentum, and electric charge are consered, and olume and entropy are not consered durg a process. 5-C Mass
5- Chapter 5 MASS AND ENERGY ANALYSIS OF CONTROL OLUMES Conseration of Mass 5-C Mass, energy, momentum, and electric charge are consered, and olume and entropy are not consered durg a process. 5-C Mass
Part One: The Gas Laws. gases (low density, easy to compress)
 CHAPTER FIVE: THE GASEOUS STATE Part One: The Gas Laws A. Introduction. 1. Comparison of three states of matter: fluids (flow freely) solids condensed states liquids (high density, hard to compress) gases
CHAPTER FIVE: THE GASEOUS STATE Part One: The Gas Laws A. Introduction. 1. Comparison of three states of matter: fluids (flow freely) solids condensed states liquids (high density, hard to compress) gases
+ m B1 = 1. u A1. u B1. - m B1 = V A. /v A = , u B1 + V B. = 5.5 kg => = V tot. Table B.1.
 5.6 A rigid tank is divided into two rooms by a membrane, both containing water, shown in Fig. P5.6. Room A is at 200 kpa, v = 0.5 m3/kg, VA = m3, and room B contains 3.5 kg at 0.5 MPa, 400 C. The membrane
5.6 A rigid tank is divided into two rooms by a membrane, both containing water, shown in Fig. P5.6. Room A is at 200 kpa, v = 0.5 m3/kg, VA = m3, and room B contains 3.5 kg at 0.5 MPa, 400 C. The membrane
