Asymmetric Deprotonation
|
|
|
- Antony Paul Hall
- 5 years ago
- Views:
Transcription
1 ( )-sparteine i-pr, t 2, -98 C 84% ee Asymmetric Deprotonation gands, Bases, and Applications CF 3 TMS t-bu TMSCl, MPA TF, -100 C t-bu 93% ee Fe (i-pr) 2 1. n-bu ( )-sparteine t 2, -78 C 2. 2 PCl P 2 Fe (i-pr) 2 90% ee David bner Stoltz Group terature Presentation March 28, 2005
2 nantioselective Deprotonation eaction Pathways retention b - a M b electrophile ' ' inversion b a b base ' possible anion inversion retention a ' - b a M electrophile ' ' inversion a '
3 nantioselective Deprotonation eaction Pathways retention b - a M b electrophile ' ' inversion b a b base ' possible anion inversion retention a ' - b a M electrophile ' ' inversion a '
4 nantioselective Deprotonation What Will T Be Covered retention b - a M b electrophile ' ' inversion b a b base ' possible anion inversion retention a ' - b a M electrophile ' ' inversion a ' Great Chemistry ot Covered Today: - Chiral Auxiliary-Directed Deprotonation - nantioselective Additions of Achiral Anions to Chiral or omochiral lectrophiles Aldols Carbolithiations Achiral Alkyllithium Additions to lectrophiles - nantioselective eactions of acemic or quilibrating Anions Deprotonation Adjacent to S, P, Se, (usually) Wittig earrangements
5 nantioselective Deprotonation I. meso-ketone Deprotonations II. meso-poxide Deprotonations a. -Deprotonation b. -Deprotonation III. Deprotonation Adjacent to eteroatoms a. : Alkyl and Allylic Systems b. : Alkyl, Benzylic, and Allylic Systems IV. ther nantioselective Deprotonations a. limination of X b. Aromatic thiation utline and Key Players K. Koga Tokyo Ketones D. odgson xford poxides. Simpkins ottingham Ketones and Aromatics D. oppe Muenster eteroatoms: M. Majewski Saskatchewan Ketones P. Beak Illinois, Urbana-Champaign eteroatoms: General eviews: 1. Majewski, "nantioselective Deprotonation of Cyclic Ketones," in Advances in Asymmetric Synthesis, Vol. 3, pp 39-76, JAI Press, London: Waldmann, "nantioselective Deprotonation and Protonation," in rganic Synthesis ighlights II,. Waldmann, ed. pp 19-28, VC, Weinheim: rganolithiums in nantioselective Synthesis, D. odgson, ed. Springer, ew York: (Whole book is good, especially pp ) 4. 'Brien, "ecent Advances in Asymmetric Synthesis Using Chiral thium Amide Bases," J. Chem. Soc., Perkin Trans. 1, 1998, oppe and ense, "nantioselective Synthesis with thium/( )-Sparteine Carbanion Pairs," Angew. Chem. Int. d. ngl. 1997, 36, odgson, Gibbs, and Lee, "nantioselective Desymmetrisation of Achiral poxides," Tetrahedron, 1996, 52 (46), Cox and Simpkins, "Asymmetric Synthesis Using omochiral thium Amide Bases," Tetrahedron: Asymmetry, 1991, 2 (1), ames, "ecent Developments in nantioselective Deprotonation Mediated by Sub-Stoichiometric Quantities of Chiral Bases," ur. J. rg. Chem., 2002, Beak, Basu, Gallagher, Park and Thayumanavan, "egioselective, Diastereoselective, and nantioselective thiation-substitution Sequences: eaction Pathways and Synthetic Applicaitons," Acc. Chem. es. 1996, 29,
6 Asymmetric Deprotonation of meso Cyclic Ketones General Considerations -amide Base (Additives) Protons Differentiated by Chiral Base L n '' ' L n ' '' + -amide Base (Additives)?
7 Chiral thium Amides Used in Ketone Deprotonations nly a Few of the Many! CF 3 Me Me CF 3 t-bu
8 Prochiral 4-Substituted Cyclohexanones nantioselective Deprotonation of Simple Substrates -amide TMSCl TMS -amide TMSCl TMS MPA TF, -100 C t-bu TF t-bu CF 3 % Yield % ee Temp ( C) Quench Cl (equiv) % ee Me Internal 0 69 i-pr Internal xternal 0 23 t-bu xternal Koga, et. al. Tetrahedron 1997, 53, Simpkins, et. al. J. Chem. Soc., Perkin Trans , 3113
9 Tropinone Derivatives Different Conditions and lectrophiles Lead to a Variety of Products Cl, TF, -78 C t-bu C Me 2 CC 95% ee Me 2 C ClC 2 Bn Bn 2 C Majewski, et. al. Tetrahedron Lett. 1994, 35, 3653 Tetrahedron Lett. 1995, 36, 5465
10 ther Prochiral Cyclic Substrates Similar gands Yield Good ee's with a Variety of lectrophiles TS TMS C 2 Me 67% 92% ee 84% ee TMS 72% 91% ee 97% ee 81% 71% ee TMS 60% ee t-bu Bn 96% 84% ee TMS t-bu TMS n n = 3: >99% ee n = 5: 95% ee 62% 85% ee TMS TBS 90% 90% ee TMS 95% 83% ee TMS
11 Catalytic Asymmetric Ketone Deprotonations arly fforts Lead to Moderate Selectivity 1. Base, TF, -78 C MPA (2.4 equiv) 2. TMSCl TMS CF equiv CF equiv 2.4 equiv 1.5 equiv DABC Stoichiometric, 1 h % Yield % ee Catalytic, 1.5 h % Yield % ee Me i-pr t-bu Koga, et. al. Tetrahedron 1997, 53, 1698
12 Chiral Magnesium Amides in Ketone Deprotonation Less eactive Alternative to thium Amides 2 Mg TMS i-pr i-pr Mg 2 i-pr TMS i-pr MPA (0.5 equiv), TMSCl TF, -78 C, 6 h MPA (0.5 equiv) TMSCl, TF % Conv. % ee Temp ( C) Time (h) % Conv. % ee Me >99 n-pr i-pr rt t-bu i-pr (±)- i-pr 2 Mg MPA (0.5 equiv), TMSCl TF, -40 C, 67 h TMS i-pr i-pr i-pr i-pr + 66%, 62% ee 34%, 88% ee Kerr, enderson, et. al. Tetrahedron, 2002, 58, 4573
13 Deprotonation of poxides arly Surprising esults t 2 t 2, -10 C Proposed Mechanism: :B 95% Cope and Tiffany, J. Am. Chem. Soc. 1951, 73, 4158 t 2 t 2, C % 55-60% 10-15% :B :B Cope, et. al. J. Am. Chem. Soc. 1958, 80, 2849
14 Deprotonation of poxides arly Surprising esults t 2 t 2, -10 C Proposed Mechanism: :B 95% Cope and Tiffany, J. Am. Chem. Soc. 1951, 73, 4158 t 2 t 2, C % 55-60% 10-15% :B :B Cope, et. al. J. Am. Chem. Soc. 1958, 80, 2849
15 Deprotonation of poxides arly Surprising esults t 2 t 2, -10 C Proposed Mechanism: :B 95% Cope and Tiffany, J. Am. Chem. Soc. 1951, 73, 4158 t 2 t 2, C % 55-60% 10-15% :B :B Cope, et. al. J. Am. Chem. Soc. 1958, 80, 2849
16 Deprotonation of poxides arly Surprising esults t 2 t 2, -10 C Proposed Mechanism: :B 95% Cope and Tiffany, J. Am. Chem. Soc. 1951, 73, 4158 t 2 t 2, C % 55-60% 10-15% :B :B Cope, et. al. J. Am. Chem. Soc. 1958, 80, 2849
17 Deprotonation of poxides arly Surprising esults t 2 t 2, -10 C Proposed Mechanism: :B 95% Cope and Tiffany, J. Am. Chem. Soc. 1951, 73, 4158 t 2 t 2, C % 55-60% 10-15% :B :B Cope, et. al. J. Am. Chem. Soc. 1958, 80, 2849
18 Asymmetric Deprotonation of poxides Can eactivity Be Controlled? Base Addition lectrophile Trapping lectrocyclic -ing pening 1,2-ydride Shift C- Insertion 2 C- or C=C Insertion eductive Alkylation odgson and Gras, Synthesis, 2002, 12, 1625
19 Asymmetric ß-Deprotonation of poxides Allylic Alcohols Using thium Aminoamide Bases TF, 0 C 77% 79% ee Proposed Selectivity Model: amide TBS TBS, 4 C 92% 90% ee Favored Disfavored C TBS amide C 3, 0 C C TBS 75% 89% ee Asami, et. al. Tetrahedron, 1995, 51, TBS TBS, 0 C 30 h 66% 97% ee Singh, et. al. J. rg. Chem, 1996, 61, 6108
20 Asymmetric ß-Deprotonation of poxides Use of Commercially-Available phedrine-based Aminoalcohols = Bn 91%, 86% ee = C 2 57%, 95% ee Murphy, et. al. J. Chem. Soc., Chem. Commun., 1993, 884 = Me 63%, 99% ee = Bu 67%, 96% ee = BnC 2 76%, 89% ee odgson, et. al. Tetrahedron: Asymmetry, 1996, 7, 407
21 Catalytic Asymmetric xamples ecent Studies Allow Similar Levels of Selectivity amide (20 mol %) LDA, TF, rt + 1 equiv LDA + 6 equiv DBU 12 h, 71%, 75% ee equiv LDA 6 h, 95%, 88% ee Asami, et. al. Tetrahedron: Asymmetry 1994, 5, 793 Tetrahedron Lett. 1997, 38, 6425 n catalyst (5 mol%) LDA (2 equiv) TF/DBU (95:5) 0 C, 24 h n n = 1 91%, 96% ee n = 2 89%, 96% ee n = 3 81%, 78% ee catalyst (20 mol%) LDA (1.25 equiv) TF, 0 C, 15 h 77% 95% ee Andersson, et. al. J. Am. Chem. Soc., 1998, 120, Malhotra, Tetrahedron: Asymmetry, 2003, 14, 645
22 nantioselective -Deprotonation of poxides Transannular C- Insertion With Alkyllithium Diamine Complexes 86% 84% ee ( )-sparteine (2.5 equiv) i-pr (2.4 equiv) t 2, -98 C to rt, 20 h 77% 83% ee 97% 77% ee odgson and Lee, Chem. Commun. 1996, 1015 ( )- -isosparteine (24 mol %) i-pr (2.4 equiv) t 2, -98 C, 40 h t-bu 54%, 89% ee odgson, et. al. J. Chem. Soc., Perkin Trans 1, 2001, 2161
23 More Transannular poxide earrangements eactivity Differences in Bicyclic Systems chiral base ydride Shift amide t 2 0 C to rt ( )-sparteine s-bu pentane -78 C to rt 73%, 49% ee 73%, 52% ee a + b 40%, 32% ee 20%, 38% ee a Shift b Shift b a odgson and Marriott,Tetrahedron: Asymmetry, 1997, 8, 519
24 Trapping of thiated poxides nantioselective poxide Substitution with Various lectrophiles TMS 72%, 74% ee 80%, 76% ee 68%, 77% ee TMSCl C CMe 2 Conditions Sn 3 = Bu; 72%, 74% ee = Me; 65%, 78% ee 3 SnCl tc t 74%, 76% ee s-bu (1.25 equiv) ( )-sparteine (1.3 equiv) t 2, -90 C, 3 h then electrophile (1.5 equiv) -90 C to rt over 5 h tccl tcme 2 t 2 C t t t t 58%, 81% ee 75%, 77% ee 67%, 78% ee odgson, et. al. rg. Biomol. Chem. 2003, 1, 4293
25 Deprotonation Adjacent to eteroatoms A eturn to eaction Pathways retention b - a M b electrophile X X inversion b a b base X = (usually) X = S, P, Se, etc. retention a X - b a M electrophile X X inversion a X Asymmetric Deprotonation Adjacent to eteroatoms X =, = Alkyl X = C=C''', = Aryl, Alkyl X =, = Alkyl, Aryl, Allyl
26 Deprotonation Adjacent to xygen Alkyl Carbamates S Cby 1. s-bu ( )-sparteine 2. lectrophile Cby Cby = Me Cby Cby Bn 2 Cby Me % Yield % ee % Yield % ee % Yield % ee D 86 > 96 3 C(C 2 ) C 2 Me 56 > 95 PbMe C 2 C=C Bu 3 Sn 70 > 95 Me 3 Si 70 > 95 Cby 85 > 95 Me 72 > 95 Fe Cby TBS Cby Cby Cby P 2 SnBu 3 C 2 Me 80% > 95% ee 85% > 95% ee 80% > 95% ee oppe, et. al. Angew. Chem., Int. d. ngl. 1990, 29, 1422 Angew. Chem., Int. d. ngl. 1992, 31, 1505 Synthesis 1999, 1573 rg. Lett. 2002, 4, 2189
27 Asymmetric Deprotonation of Alkyl Carbamates (i-pr) 2 Intramolecular eactions s-bu ( )-sparteine t 2-78 C to rt (i-pr) 2 46% 96% ee -78 C to rt (i-pr) 2 (i-pr) 2 Tomooka, Shimizu, Inoue, Shibata,l akai, Chem. Lett. 1999, 759 Cby Cby s-bu ( )-sparteine t 2, -78 C Cby Cby (sparteine) Cby = TMSCl or ZnCl 2 TBSTf or BF 3 t 2 Cby Cby > 95% ee 74% ee oppe, et. al. Synlett 1994, 1034
28 Silyl Alkenyl Carbamates Configurationally Stable Allyllithiums TMS Cbx n-bu ( )-sparteine C 3, -78 C (i-pr) 2 TMS electrophile TMS Cbx Cbx = (i-pr) 2 = TMS Cbx ' ' ' ' TMS Cbx TMS TMS TMS Cbx Cbx Cbx % Yield % ee Me 3 Si Bu 3 Sn Sn MeC() t-buc() % Yield % ee Me 3 Si Bu 3 Sn Sn % Yield % ee MeC() t-buc() Me 2 C cc oppe, et. al. rg. Lett. 2004, 6, 783
29 nantioselective omoaldols Metal xchange to Ti Leads to a ighly Diastereoselective Process TMS Cbx n-bu ( )-sparteine C 3, -78 C (i-pr) 2 TMS TiCl(t 2 ) 3 TMS Ti(t 2 ) 3 Cbx Cbx = (i-pr) 2 'C ' TMS Cbx ' TMS ' TMS Me Cbx Cbx ' % Yield % ee ' % Yield % ee C 3 (C 2 ) C 3 (C 2 ) (C 3 ) 2 C (C 3 ) 2 C (C 3 ) 3 C (C 3 ) 3 C cc cc C BrC oppe, et. al. rg. Lett. 2004, 6, 783
30 Deprotonation Adjacent to itrogen Alkyl Carbamates evisited S s-bu ( )-sparteine electrophile S t 2, -78 C t-bu % Yield % ee Me % 0-88% ee Coldham, et. al. rg. Lett. 2001, 3, 3799 Me 3 Si Bu 3 Sn C Beak, et. al. J. Am. Chem. Soc. 1994, 116, % 94-98% ee Beak, et. al. J. rg. Chem. 1997, 62, CuC Cl 2. alkenyl iodide % 86-90% ee Dieter, et. al. J. Am. Chem. Soc. 2001, 123, 5132
31 Benzylic Carbamates Configurationally Stable Benzyllithiums Possible Most Benzylic Deprotonations: Configurationally Unstable Anions X chiral base X M* enantioselective electrophile substitution X X = Ar: nantiomeric Anions Do ot Interconvert at Low Temp! Ar chiral base enantioselective deprotonation Ar M* electrophile substitution Ar Me 1. n-bu ( )-sparteine 2. Tf 3. CA % Yield % ee Me t Allyl Bn Beak, et. al. J. Am. Chem. Soc. 1996, 118, 3757
32 More Benzylic Carbamates Applications and lectrophiles Ar 1. n-bu ( )-sparteine 2. Michael Acceptor Ar Ar = p-mec 6 4 Ar = : 72%, 94% ee = Me: 63%, 94% ee Ar n n = 1: 86%, >99:1 dr, 92% ee n = 2: 82%, >99:1 dr, 92% ee C C Ar 91%, 92:8 dr, 90% ee Beak, et. al. J. Am. Chem. Soc. 1997, 119, J. rg. Chem. 1999, 64, 2996 Cl Ar s-bu ( )-sparteine C 3, -78 C Ar Ar % Yield % ee aphthyl thienyl furyl Beak, et. al. J. Am. Chem. Soc. 1996, 118, 715
33 thium Amides in Benzylic Deprotonation Cyclization of Arylamides Leads to Isoindolones -78 to 0 C Me Me Me 1. 4 Cl, 2 2. Cl, 2 88%, 81% ee C 2 C 2 ( )-kainic acid 2 C C 2 C 2 ( )-isodomoic acid C Clayden, et. al. Tetrahedron 2002, 58, 4727 J. Am. Chem. Soc. 2005, 127, 2412
34 nantioselective -Allylcarbamate Deprotonation Ar n-bu ( )-sparteine Ar electrophile Ar Ar = p-mec 6 4 % Yield % ee Me Bn Allyl i-bu 73%, >94% ee 98:2 dr Ar t 2 C t 2 C 86%, 96% ee 94:6 dr Ar C C Cy 80%, 88% ee 63:44 dr Ar Ar 1. n-bu ( )-sparteine 2. Carbonyl or Michael Acceptor ' Ar '' ' '' Ar 77%, 98% ee Ar Ar 80%, 86% ee 90:10 dr Me Ar 62%, 96% ee 80:20 dr 80%, 92% ee 89:11 dr Ar Beak, et. al. J. Am. Chem. Soc. 1996, 118, J. Am. Chem. Soc. 2001, 123, 1004
35 nantioselective Synthesis by Loss of X Chiral Amides Lead to nantioenriched Alkenes X Tf t 2, -70 C X X = 44% ee X = (C 2 ) 2 99% ee Sakai, et. al. Tetrahedron Lett. 1987, 28, 6489 = Me 80% ee Cl 2 C TF, -78 C 2 C = t-bu 82% ee Me Me 75% ee Cl C 2 TF, -78 C C 2 Duhamel, et. al. Tetrahedron: Asymmetry, 1990, 1, 347
36 More nantioselective Loss of X Chiral Alkoxides Allow Catalytic eactions Br Br Me 2 (10 mol %) Me (4 mol %) K, TF -80 C, 72 h Br t-bu % Yield % ee n-pr i-pr p-mec >98 78 >98 p- 2 C 6 4 K + - p-c Br Br Me 2 Me K 10 mol % 4 mol % 2.5 equiv Br MeK 2 Me 2 Duhamel, et. al. J. rg. Chem., 1998, 63, 5541
37 nantioselective thiation of Aromatics Generation of Planar Chirality with thium Amide Bases Fe P 2 TMSCl, TF -78 C Fe P 2 TMS 95% 54% ee Simpkins and Price, Tetrahedron Lett., 1995, 36, 6135 Me Me Me Cl, TF -78 C, 15 min C 67% 65% ee Cr(C) 3 Cr(C) 3 Cr(C) 3 Simpkins, et. al. J. Chem. Soc., Perkin Trans 1, 1997, 401
38 nantioselective thiation of Aromatics Generation of Planar Chirality with Alkyllithium / Sparteine % Yield % ee Fe (i-pr) 2 1. n-bu ( )-sparteine t 2, -78 C 2. electrophile Fe (i-pr) 2 TMS Me C() I P B() Snieckus, et. al. J. Am. Chem. Soc., 1996, 118, 685 Me 2 Me 2 t-bu C 3, -78 C 2 PCl 85% 75% ee P 2 Cr(C) 3 Cr(C) 3 Cr(C) 3 Uemura, et. al. Tetrahedron: Asymmetry, 1994, 5, 1427
Highlights of Schmidt Reaction in the Last Ten Years
 ighlights of Schmidt eaction in the Last Ten Years Dendrobates histrionicus Jack Liu ov. 18, 2003 Introduction Classical Schmidt reaction of aldehydes and carboxylic acids Classical Schmidt reaction of
ighlights of Schmidt eaction in the Last Ten Years Dendrobates histrionicus Jack Liu ov. 18, 2003 Introduction Classical Schmidt reaction of aldehydes and carboxylic acids Classical Schmidt reaction of
Lecture 6: Transition-Metal Catalysed C-C Bond Formation
 Lecture 6: Transition-Metal Catalysed C-C Bond Formation (a) Asymmetric allylic substitution 1 u - d u (b) Asymmetric eck reaction 2 3 Ar- d (0) Ar 2 3 (c) Asymmetric olefin metathesis alladium π-allyl
Lecture 6: Transition-Metal Catalysed C-C Bond Formation (a) Asymmetric allylic substitution 1 u - d u (b) Asymmetric eck reaction 2 3 Ar- d (0) Ar 2 3 (c) Asymmetric olefin metathesis alladium π-allyl
Electrophilic Carbenes
 Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Chiral Brønsted Acid Catalysis
 Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
"-Amino Acids: Function and Synthesis
 "-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
"-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
Asymmetric Nucleophilic Catalysis
 Asymmetric ucleophilic Catalysis Chiral catalyst X 2 Chiral catalyst X = alkyl, X 1 2 1 Vedejs, E.; Daugulis,. J. Am. Chem. Soc. 2003, 125, 4166-4173 Shaw, S. A.; Aleman,.; Vedejs, E. J. Am. Chem. Soc.
Asymmetric ucleophilic Catalysis Chiral catalyst X 2 Chiral catalyst X = alkyl, X 1 2 1 Vedejs, E.; Daugulis,. J. Am. Chem. Soc. 2003, 125, 4166-4173 Shaw, S. A.; Aleman,.; Vedejs, E. J. Am. Chem. Soc.
Asymmetric Catalysis by Lewis Acids and Amines
 Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
Asymmetric Catalysis by Lewis Acids and Amines Asymmetric Lewis acid catalysis - Chiral (bisooxazoline) copper (II) complexes - Monodentate Lewis acids: the formyl -bond Amine catalysed reactions Asymmetric
Chiral Proton Catalysis in Organic Synthesis. Samantha M. Frawley Organic Seminar September 14 th, 2005
 Chiral Proton Catalysis in rganic Synthesis Samantha M. Frawley rganic Seminar September 14 th, 2005 Seminar utline Introduction Lewis Acid-assisted Chiral Brønsted Acids Enantioselective protonation for
Chiral Proton Catalysis in rganic Synthesis Samantha M. Frawley rganic Seminar September 14 th, 2005 Seminar utline Introduction Lewis Acid-assisted Chiral Brønsted Acids Enantioselective protonation for
A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols
 A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols B() 2 H H B() 2 H H Hu, X.-D.; Fan, C.-A.; Zhang, F.-M.; Tu, Y.
A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols B() 2 H H B() 2 H H Hu, X.-D.; Fan, C.-A.; Zhang, F.-M.; Tu, Y.
Total synthesis of Spongistatin
 Literature Semminar 1. Introduction: Total synthesis of Spongistatin Chen Zhihua (M2) Isolation: Pettit et al. J. rg. Chem. 1993, 58, 1302. Kitagawa et al. Tetrahedron Lett. 1993, 34, 1993. Fusetani et
Literature Semminar 1. Introduction: Total synthesis of Spongistatin Chen Zhihua (M2) Isolation: Pettit et al. J. rg. Chem. 1993, 58, 1302. Kitagawa et al. Tetrahedron Lett. 1993, 34, 1993. Fusetani et
Synthetic Methodology. Using Tertiary Phosphines. as Nucleophilic Catalysts
 Synthetic Methodology Using Tertiary osphines as Nucleophilic Catalysts 1 3 2 u 2 (P 3 ) 3 4 1 2 D. Ma, X. Lu 1988 1 2 Pd 2 (dba) 3.CCl 3 /P 3 /Ac or Pd(Ac) 2 /P 3 1 2 B. M. Trost 1988 1 3 2 u 2 (P 3 )
Synthetic Methodology Using Tertiary osphines as Nucleophilic Catalysts 1 3 2 u 2 (P 3 ) 3 4 1 2 D. Ma, X. Lu 1988 1 2 Pd 2 (dba) 3.CCl 3 /P 3 /Ac or Pd(Ac) 2 /P 3 1 2 B. M. Trost 1988 1 3 2 u 2 (P 3 )
Rhodium Catalyzed Alkyl C-H Insertion Reactions
 Rhodium Catalyzed Alkyl C-H Insertion Reactions Rh Rh Jeff Kallemeyn 5/17/05 1. Cyclopropanation The Versatile and Reactive Rhodium Carbene R + Et Rh 2 (Ac) 4 R C 2 Et N 2 2. [2,3] sigmatropic rearrangement
Rhodium Catalyzed Alkyl C-H Insertion Reactions Rh Rh Jeff Kallemeyn 5/17/05 1. Cyclopropanation The Versatile and Reactive Rhodium Carbene R + Et Rh 2 (Ac) 4 R C 2 Et N 2 2. [2,3] sigmatropic rearrangement
Use of Cp 2 TiCl in Synthesis
 Use of 2 TiCl in Synthesis eagent Control of adical eactions Jeff Kallemeyn May 21, 2002 eactions of 2 TiCl 1. Pinacol Coupling H H H 2. Epoxide pening H H E H Chemoselectivity Activated aldehydes (aromatic,
Use of 2 TiCl in Synthesis eagent Control of adical eactions Jeff Kallemeyn May 21, 2002 eactions of 2 TiCl 1. Pinacol Coupling H H H 2. Epoxide pening H H E H Chemoselectivity Activated aldehydes (aromatic,
R 2 R 4 Ln catalyst. This manuscript describes the methods for the synthesis and application of group 4 metallocene bis(trimethylsilyl)acetylene
 VII Abstracts 2011 p1 2.12.15 rganometallic Complexes of Scandium, Yttrium, and the Lanthanides P. Dissanayake, D. J. Averill, and M. J. Allen This manuscript is an update to the existing Science of Synthesis
VII Abstracts 2011 p1 2.12.15 rganometallic Complexes of Scandium, Yttrium, and the Lanthanides P. Dissanayake, D. J. Averill, and M. J. Allen This manuscript is an update to the existing Science of Synthesis
Ynolate Chemistry. Jeff Kallemeyn October 22, 2002
 Ynolate Chemistry While enolates have numbered among the most important reagents of organic chemistry for more than a century, ynolates have hitherto remained unknown although their chemistry should be
Ynolate Chemistry While enolates have numbered among the most important reagents of organic chemistry for more than a century, ynolates have hitherto remained unknown although their chemistry should be
Mechanism Problem. 1. NaH allyl bromide, THF N H
 Mechanism Problem 1. a allyl bromide, TF 2. 9-BB (1.2 equiv), TF, rt; ame (1.2 equiv); t-buli (2.4 equiv), TMEDA (2.4 equiv) 30 to rt; allyl bromide; 30% 2 2, aq. a, 0 C (58% yield) Mechanism Problem 9-BB
Mechanism Problem 1. a allyl bromide, TF 2. 9-BB (1.2 equiv), TF, rt; ame (1.2 equiv); t-buli (2.4 equiv), TMEDA (2.4 equiv) 30 to rt; allyl bromide; 30% 2 2, aq. a, 0 C (58% yield) Mechanism Problem 9-BB
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: favored. disfavored. favored. disfavored
 The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of H X 2. Addition of H OH and addition of Y X 3. Addition to allene and
 Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of X 2. Addition of and addition of Y X 3. Addition to allene and alkyne 4. Substitution at α-carbon 5. eactions via organoborane
Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of X 2. Addition of and addition of Y X 3. Addition to allene and alkyne 4. Substitution at α-carbon 5. eactions via organoborane
Keisuke Suzuki. Baran lab Group Meeting 6/11/16. Shigenobu Umemiya. Akira Suzuki. Takanori Suzuki (Hokkaido University)
 197.D., Teruaki Mukaiyama, University of Tokyo 193 Assistant Professor, Keio University 197 Lecturer, Keio University 199 Assocate Professor, Keio University 1990 Visiting Professor, ET 1994 ull Professor,
197.D., Teruaki Mukaiyama, University of Tokyo 193 Assistant Professor, Keio University 197 Lecturer, Keio University 199 Assocate Professor, Keio University 1990 Visiting Professor, ET 1994 ull Professor,
Strategies for Catalytic Asymmetric Electrophilic a Halogenation of Carbonyl Compounds
 Strategies for Catalytic Asymmetric Electrophilic a alogenation of Carbonyl Compounds 1 2 Y Catalyst [X + ] 1 X! 2 Y intermann, L. ; Togni, A. Angew. Chem. Int. Ed. 2000, 39, 4359 4362 amashima, Y.; Sodeoka,
Strategies for Catalytic Asymmetric Electrophilic a alogenation of Carbonyl Compounds 1 2 Y Catalyst [X + ] 1 X! 2 Y intermann, L. ; Togni, A. Angew. Chem. Int. Ed. 2000, 39, 4359 4362 amashima, Y.; Sodeoka,
Chiral Catalyst II. Palladium Catalysed Allylic Displacement ( -allyl complexes) 1. L n Pd(0) 2. Nuc
 Chiral Catalyst II ast lecture we looked at asymmetric catalysis for oxidation and reduction Many other organic transformations, this has led to much investigation Today we will look at some others...
Chiral Catalyst II ast lecture we looked at asymmetric catalysis for oxidation and reduction Many other organic transformations, this has led to much investigation Today we will look at some others...
transmetallate displace ox. add. M + (insert) (β-elim.)
 Chapter IV. Transition Metal σ-alkyl Complexes I. General For much of the rest of this course it will be necessary to understand how σ-alkyl metal complexes are formed and how they react. This is summarized
Chapter IV. Transition Metal σ-alkyl Complexes I. General For much of the rest of this course it will be necessary to understand how σ-alkyl metal complexes are formed and how they react. This is summarized
CHEM 330. Final Exam December 5, 2014 ANSWERS. This a closed-notes, closed-book exam. The use of molecular models is allowed
 CEM 330 Final Exam December 5, 2014 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam consists of 12 pages Time: 2h 30 min 1. / 30 2. / 30 3. / 30
CEM 330 Final Exam December 5, 2014 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam consists of 12 pages Time: 2h 30 min 1. / 30 2. / 30 3. / 30
Carbonyl Ylide Cycloadditions
 Carbonyl Ylide Cycloadditions cond. icholas Anderson Denmark Group eting 07/13/10 Carbonyl Ylides Uncharged 1,3-Dipole Conjugated π-system ighly reactive on-isolable Generate in-situ Carbonyl Ylide Stability
Carbonyl Ylide Cycloadditions cond. icholas Anderson Denmark Group eting 07/13/10 Carbonyl Ylides Uncharged 1,3-Dipole Conjugated π-system ighly reactive on-isolable Generate in-situ Carbonyl Ylide Stability
[3,3]-sigmatropic Processes. [2,3]-sigmatropic Processes. Ene Reactions. Generalized Sigmatropic Processes X,Y=C, N, O, S X,Y=C, N, O, S
![[3,3]-sigmatropic Processes. [2,3]-sigmatropic Processes. Ene Reactions. Generalized Sigmatropic Processes X,Y=C, N, O, S X,Y=C, N, O, S [3,3]-sigmatropic Processes. [2,3]-sigmatropic Processes. Ene Reactions. Generalized Sigmatropic Processes X,Y=C, N, O, S X,Y=C, N, O, S](/thumbs/94/119475672.jpg) Generalized igmatropic Processes [3,3]-sigmatropic Processes 1 3,=C,,, 1 3 3,=C,,, 3 [2,3]-sigmatropic Processes 1 3,=C,,, 1 3 Ene eactions 1 3 1 3 Cope earrangement [3,3]- igmatropic earrangements Transition
Generalized igmatropic Processes [3,3]-sigmatropic Processes 1 3,=C,,, 1 3 3,=C,,, 3 [2,3]-sigmatropic Processes 1 3,=C,,, 1 3 Ene eactions 1 3 1 3 Cope earrangement [3,3]- igmatropic earrangements Transition
Denmark s Base Catalyzed Aldol/Allylation
 Denmark s Base Catalyzed Aldol/Allylation Evans Group Seminar ovember 1th, 003 Jimmy Wu Lead eferences: Denmark, S. E. Acc. Chem. es., 000, 33, 43 Denmark, S. E. Chem. Comm. 003, 167 Denmark, S. E. Chem.
Denmark s Base Catalyzed Aldol/Allylation Evans Group Seminar ovember 1th, 003 Jimmy Wu Lead eferences: Denmark, S. E. Acc. Chem. es., 000, 33, 43 Denmark, S. E. Chem. Comm. 003, 167 Denmark, S. E. Chem.
[3,3]-Sigmatropic rearrangements
![[3,3]-Sigmatropic rearrangements [3,3]-Sigmatropic rearrangements](/thumbs/75/71504906.jpg) 1 [3,3]-Sigmatropic rearrangements heat R 1 R 3 R 1 R 3 R 1 R 3 A class of pericyclic reactions whose stereochemical outcome is governed by the geometric requirements of the cyclic transition state Reactions
1 [3,3]-Sigmatropic rearrangements heat R 1 R 3 R 1 R 3 R 1 R 3 A class of pericyclic reactions whose stereochemical outcome is governed by the geometric requirements of the cyclic transition state Reactions
Organocopper Reagents
 rganocopper eagents General Information!!! why organocopper reagents? - Efficient method of C-C bond formation - Cu less electropositive than Li or Mg, so -Cu bond less polarized - consequences: 1. how
rganocopper eagents General Information!!! why organocopper reagents? - Efficient method of C-C bond formation - Cu less electropositive than Li or Mg, so -Cu bond less polarized - consequences: 1. how
Stereoselective Organic Synthesis
 Stereoselective rganic Synthesis Prabhat Arya Professor and Leader, Chemical Biology Program Dean, Academic Affairs, Institute of Life Sciences (An Associate Institute of University of yderabad Supported
Stereoselective rganic Synthesis Prabhat Arya Professor and Leader, Chemical Biology Program Dean, Academic Affairs, Institute of Life Sciences (An Associate Institute of University of yderabad Supported
Additions to Metal-Alkene and -Alkyne Complexes
 Additions to tal-alkene and -Alkyne Complexes ecal that alkenes, alkynes and other π-systems can be excellent ligands for transition metals. As a consequence of this binding, the nature of the π-system
Additions to tal-alkene and -Alkyne Complexes ecal that alkenes, alkynes and other π-systems can be excellent ligands for transition metals. As a consequence of this binding, the nature of the π-system
Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02
![Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02](/thumbs/93/114018431.jpg) Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Xiao, W.-J. et al. J. Am. Chem. Soc. 2016, 138, 8360.
Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Xiao, W.-J. et al. J. Am. Chem. Soc. 2016, 138, 8360.
Nickel-Catalyzed Multicomponent Coupling of Alkynes. -Recent development in methodologies and applications. Zhenjie Lu. Department of Chemistry, MSU
 28 58.69 i ickel ickel-catalyzed Multicomponent Coupling of Alkynes -ecent development in methodologies and applications Zhenjie u Department of Chemistry, MSU January 28, 2004 Background Introduction
28 58.69 i ickel ickel-catalyzed Multicomponent Coupling of Alkynes -ecent development in methodologies and applications Zhenjie u Department of Chemistry, MSU January 28, 2004 Background Introduction
VI. Metal alkyls from oxidative addition / insertion
 V. Metal alkyls from oxidative addition / insertion A. Carbonylation - C insertion very facile, metal acyls easily cleaved, all substrates which undergo oxidative addition can in principle be carbonylated.
V. Metal alkyls from oxidative addition / insertion A. Carbonylation - C insertion very facile, metal acyls easily cleaved, all substrates which undergo oxidative addition can in principle be carbonylated.
Corey-Bakshi. Bakshi-Shibata Reduction. Name Reaction Nilanjana Majumdar
 Corey-Bakshi Bakshi-Shibata Reduction Name Reaction Nilanjana Majumdar 02.27.09 utline Introduction Background CBS Reaction Application to Synthesis Introduction Born: 12 th July, 1928 in Methuen, Massachusetts,
Corey-Bakshi Bakshi-Shibata Reduction Name Reaction Nilanjana Majumdar 02.27.09 utline Introduction Background CBS Reaction Application to Synthesis Introduction Born: 12 th July, 1928 in Methuen, Massachusetts,
Short Literature Presentation 10/4/2010 Erika A. Crane
 Copper-Catalyzed Enantioselective Synthesis of trans-1- Alkyl-2-substituted Cyclopropanes via Tandem Conjugate Additions-Intramolecular Enolate Trapping artog, T. D.; Rudolph, A.; Macia B.; Minnaard, A.
Copper-Catalyzed Enantioselective Synthesis of trans-1- Alkyl-2-substituted Cyclopropanes via Tandem Conjugate Additions-Intramolecular Enolate Trapping artog, T. D.; Rudolph, A.; Macia B.; Minnaard, A.
Chiral Diol Promoted Boronates Addi3on Reac3ons. Lu Yan Morken Group Boston College
 Chiral Diol Promoted Boronates Addi3on Reac3ons Lu Yan Morken Group Boston College Main Idea R R B or R R B Ar * exchange B * * or B Ar R 1 R 1 R 2 R 1 R 2 Products not nucleophilic enough nucleophilic
Chiral Diol Promoted Boronates Addi3on Reac3ons Lu Yan Morken Group Boston College Main Idea R R B or R R B Ar * exchange B * * or B Ar R 1 R 1 R 2 R 1 R 2 Products not nucleophilic enough nucleophilic
Organic Electron Donors
 rganic Electron onors Yang Li Zakarian esearch Group epartment of Chemistry and Biochemistry University of California, anta Barbara 11/15/2018 2 2 2 2 2 2 TAF1 TAE TAF2 TTF BPL utlines rganic Electron
rganic Electron onors Yang Li Zakarian esearch Group epartment of Chemistry and Biochemistry University of California, anta Barbara 11/15/2018 2 2 2 2 2 2 TAF1 TAE TAF2 TTF BPL utlines rganic Electron
Pericyclic Reactions - Continued
 Dr. P. Wipf Page 1 of 7 10/21/2009 Pericyclic eactions - Continued Sigmatropic earrangements Definition: A sigmatropic rearrangement is defined as an intramolecular rearrangement of a σ bond, adjacent
Dr. P. Wipf Page 1 of 7 10/21/2009 Pericyclic eactions - Continued Sigmatropic earrangements Definition: A sigmatropic rearrangement is defined as an intramolecular rearrangement of a σ bond, adjacent
eatles Oasis - 199
 eatles - 1964 asis - 199 Biography 2001-present: University of 1997-2000: Professor, Shef 1988-1997: Various Reader 1986-1988: Post-doc with G 1983-1986: D at Universi 1983: Undergrad at Cambrid Enantioselective
eatles - 1964 asis - 199 Biography 2001-present: University of 1997-2000: Professor, Shef 1988-1997: Various Reader 1986-1988: Post-doc with G 1983-1986: D at Universi 1983: Undergrad at Cambrid Enantioselective
Metal Catalyzed Outer Sphere Alkylations of Unactivated Olefins and Alkynes
 Metal Catalyzed uter Sphere Alkylations of Unactivated lefins and Alkynes Stephen Goble rganic Super-Group Meeting Literature Presentation ctober 6, 2004 1 utline I. Background Introduction to Carbometallation
Metal Catalyzed uter Sphere Alkylations of Unactivated lefins and Alkynes Stephen Goble rganic Super-Group Meeting Literature Presentation ctober 6, 2004 1 utline I. Background Introduction to Carbometallation
Memory of Chirality: A Strategy for Asymmetric Synthesis
 Memory of Chirality: A trategy for Asymmetric ynthesis David J. Richard eptember 14, 2005 Two Forms of Chirality Absolute (tatic) Chirality 2 - Absolute chirality - orientation of functional groups at
Memory of Chirality: A trategy for Asymmetric ynthesis David J. Richard eptember 14, 2005 Two Forms of Chirality Absolute (tatic) Chirality 2 - Absolute chirality - orientation of functional groups at
Asymmetric Lewis Base Strategies for Heterocycle Synthesis
 Asymmetric Lewis Base trategies for eterocycle ynthesis Dr Andrew mith EatCEM, chool of Chemistry, University of t Andrews 1st cottish-japanese ymposium of rganic Chemistry, University of Glasgow Friday
Asymmetric Lewis Base trategies for eterocycle ynthesis Dr Andrew mith EatCEM, chool of Chemistry, University of t Andrews 1st cottish-japanese ymposium of rganic Chemistry, University of Glasgow Friday
Lewis Base Catalysis: the Aldol Reaction (Scott Denmark) Tom Blaisdell Friday, January 17 th 2014 Topic Talk
 Lewis Base Catalysis: the Aldol eaction (Scott Denmark) Tom Blaisdell Friday, January 17 th 2014 Topic Talk Scott E. Denmark 1975 - S.B. in Chemistry MIT (ichard. olm and Daniel S. Kemp) 1980 - D.Sc in
Lewis Base Catalysis: the Aldol eaction (Scott Denmark) Tom Blaisdell Friday, January 17 th 2014 Topic Talk Scott E. Denmark 1975 - S.B. in Chemistry MIT (ichard. olm and Daniel S. Kemp) 1980 - D.Sc in
Stereoselective reactions of enolates
 1 Stereoselective reactions of enolates Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones These are
1 Stereoselective reactions of enolates Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones These are
Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group.
 Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group. akatani, Y.; Koizumi, Y.; Yamasaki, R.; Saito, S. rg. Lett. 2008, 10, 2067-2070. An Annulation Reaction for the Synthesis
Copper-Catalyzed Synthesis of Esters from Ketones. Alkyl Group as a Leaving Group. akatani, Y.; Koizumi, Y.; Yamasaki, R.; Saito, S. rg. Lett. 2008, 10, 2067-2070. An Annulation Reaction for the Synthesis
Spiro Monophosphite and Monophosphoramidite Ligand Kit
 Spiro Monophosphite and Monophosphoramidite Ligand Kit metals inorganics organometallics catalysts ligands custom synthesis cgm facilities nanomaterials 15-5162 15-5150 15-5156 15-5163 15-5151 15-5157
Spiro Monophosphite and Monophosphoramidite Ligand Kit metals inorganics organometallics catalysts ligands custom synthesis cgm facilities nanomaterials 15-5162 15-5150 15-5156 15-5163 15-5151 15-5157
Tips for taking exams in 852
 Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
o-palladated cat. [Chem. Comm (1999)] [Org. Lett. 2, 1826 (2000)] [Org. Lett. 2, 2881 (2000)] [JACS 41, 9550 (1999)]
![o-palladated cat. [Chem. Comm (1999)] [Org. Lett. 2, 1826 (2000)] [Org. Lett. 2, 2881 (2000)] [JACS 41, 9550 (1999)] o-palladated cat. [Chem. Comm (1999)] [Org. Lett. 2, 1826 (2000)] [Org. Lett. 2, 2881 (2000)] [JACS 41, 9550 (1999)]](/thumbs/85/92754396.jpg) 3. Boron -- eview [Suzuki Chem. ev. 95, 2457 (1995)] U77b ydroboration also attractive but B Pd transmetallation difficult - must produce stable B product - solved (by Suzuki) by adding base to make Borates
3. Boron -- eview [Suzuki Chem. ev. 95, 2457 (1995)] U77b ydroboration also attractive but B Pd transmetallation difficult - must produce stable B product - solved (by Suzuki) by adding base to make Borates
Organocopper Chemistry
 rganocopper Chemistry ave a great historical importance, but still remain highly useful reactions. If not the first organometallic reactions developed they are among the first. Most often used in conjugate
rganocopper Chemistry ave a great historical importance, but still remain highly useful reactions. If not the first organometallic reactions developed they are among the first. Most often used in conjugate
Stereoselective reactions of the carbonyl group
 1 Stereoselective reactions of the carbonyl group We have seen many examples of substrate control in nucleophilic addition to the carbonyl group (Felkin-Ahn & chelation control) If molecule does not contain
1 Stereoselective reactions of the carbonyl group We have seen many examples of substrate control in nucleophilic addition to the carbonyl group (Felkin-Ahn & chelation control) If molecule does not contain
A 1,3 Strain and the Anomeric Effect. Michael Shaghafi Chem. Topics Feb. 6, 2012
 A 1,3 Strain and the Anomeric Effect Michael Shaghafi Chem. Topics Feb. 6, 2012 Introduction: Definition of A 1,3 Strain m L L m m 3 L 3 1 1 otation about σ-bond between α-stereocenter and olefin is associated
A 1,3 Strain and the Anomeric Effect Michael Shaghafi Chem. Topics Feb. 6, 2012 Introduction: Definition of A 1,3 Strain m L L m m 3 L 3 1 1 otation about σ-bond between α-stereocenter and olefin is associated
Catalytic Asymmetric Acyl Halide-Aldehyde Cyclocondensation Reactions of Substituted Ketenes
 Catalytic Asymmetric Acyl Halide-Aldehyde Cyclocondensation eactions of Substituted Ketenes Scott G. elson, Cheng Zhu, and Xiaoqiang Shen J. Am. Chem Soc. 2004, 126, 14-15. Michael C. Myers, Literature
Catalytic Asymmetric Acyl Halide-Aldehyde Cyclocondensation eactions of Substituted Ketenes Scott G. elson, Cheng Zhu, and Xiaoqiang Shen J. Am. Chem Soc. 2004, 126, 14-15. Michael C. Myers, Literature
A New Strategy for Efficient Synthesis of Medium and Large Ring Lactones without High Dilution or Slow Addition
 A ew Strategy for Efficient Synthesis of Medium and Large ing Lactones without High Dilution or Slow Addition BF 3 Et 2 TIPS 2,4,6-collidine n + n+2 ' ' Zhao, W.; Li, Z; Sun, J. J. Am. Chem. Soc. 2013
A ew Strategy for Efficient Synthesis of Medium and Large ing Lactones without High Dilution or Slow Addition BF 3 Et 2 TIPS 2,4,6-collidine n + n+2 ' ' Zhao, W.; Li, Z; Sun, J. J. Am. Chem. Soc. 2013
ANSWER GUIDE APRIL/MAY 2006 EXAMINATIONS CHEMISTRY 249H
 AWER GUIDE APRIL/MAY 2006 EXAMIATI CEMITRY 249 1. (a) PDC / C 2 2 (b) t-bume 2 i (1 equiv) / imidazole (1 equiv) i TBDM protection of the less sterically hindered alcohol (c) BuLi (1 equiv) then (d) 2
AWER GUIDE APRIL/MAY 2006 EXAMIATI CEMITRY 249 1. (a) PDC / C 2 2 (b) t-bume 2 i (1 equiv) / imidazole (1 equiv) i TBDM protection of the less sterically hindered alcohol (c) BuLi (1 equiv) then (d) 2
O or R E + R. - Keto-Enol Tautomerization (enol form usually very minor for simple ketones)
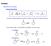 General eactivity base or acid or E + E - Keto-Enol Tautomerization (enol form usually very minor for simple ketones) - Can enhance rate / concentration by addition of acid or base + catalyzed + + + base
General eactivity base or acid or E + E - Keto-Enol Tautomerization (enol form usually very minor for simple ketones) - Can enhance rate / concentration by addition of acid or base + catalyzed + + + base
PhD research with Prof. Lutz H. Gade at the Univ. of Strasbourg. Postdoc in 2003 with Andreas Pfaltz (Basel Switzerland)
 idium-catalyzed Asymmetric Isomerization of rimary Allylic Alcohols Mantilli, L., Gerard, D., Torche, S., Besnard, C., Mazet * C. Angew. Chem. Int. Ed., 2009, 48, 1-6 1,n-Glycols as Dialdehyde Equivalents
idium-catalyzed Asymmetric Isomerization of rimary Allylic Alcohols Mantilli, L., Gerard, D., Torche, S., Besnard, C., Mazet * C. Angew. Chem. Int. Ed., 2009, 48, 1-6 1,n-Glycols as Dialdehyde Equivalents
Synthesis and Utility of α Silylamines: A Brief Overview. Literature Presentation 4/6/2K4 Dave Ballweg
 Synthesis and Utility of α Silylamines: A Brief verview Literature Presentation 4/6/2K4 Dave Ballweg Utility of Amines 1. Amines are ubiquitous in organic/medicinal chemistry 2. Important precursers to
Synthesis and Utility of α Silylamines: A Brief verview Literature Presentation 4/6/2K4 Dave Ballweg Utility of Amines 1. Amines are ubiquitous in organic/medicinal chemistry 2. Important precursers to
Direct, Catalytic Hydroaminoalkylation of Unactivated Olefins with N-Alkyl Arylamines
 Current Literature - May 12, 2007 Direct, Catalytic ydroaminoalkylation of Unactivated lefins with -Alkyl ylamines ' '' Ta[ 2 ] 5 (4-8 mol%), 160-165 o C 24-67h 66-95% ' '' S. B. erzon and J. F. artwig,
Current Literature - May 12, 2007 Direct, Catalytic ydroaminoalkylation of Unactivated lefins with -Alkyl ylamines ' '' Ta[ 2 ] 5 (4-8 mol%), 160-165 o C 24-67h 66-95% ' '' S. B. erzon and J. F. artwig,
Three Type Of Carbene Complexes
 Three Type f arbene omplexes arbene complexes have formal metal-to-carbon double bonds. Several types are known. The reactivity of the carbene and how it contributes to the overall electron counting is
Three Type f arbene omplexes arbene complexes have formal metal-to-carbon double bonds. Several types are known. The reactivity of the carbene and how it contributes to the overall electron counting is
Stereoselective reactions of enolates: auxiliaries
 1 Stereoselective reactions of enolates: auxiliaries Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones
1 Stereoselective reactions of enolates: auxiliaries Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones
Approaches to the Synthesis. of Tetrahydropyrans. (and closely related heterocycles)
 Approaches to the Synthesis of Tetrahydropyrans (and closely related heterocycles) William Morris Literature Presentation July 6, 2004 I. Intro A Nomenclature B Prevalence in Nature C Biosynthetic Considerations
Approaches to the Synthesis of Tetrahydropyrans (and closely related heterocycles) William Morris Literature Presentation July 6, 2004 I. Intro A Nomenclature B Prevalence in Nature C Biosynthetic Considerations
CuI CuI eage lic R tal ome rgan gbr ommon
 Common rganometallic eagents Li Et 2 Li Mg Et 2 Li alkyllithium rignard Mg Mg Li Zn TF ZnCl 2 TF dialkylzinc Zn 2 2 Zn Li CuI TF ganocuprate CuI 2 2 CuI common electrophile pairings ' Cl ' '' ' ' ' ' '
Common rganometallic eagents Li Et 2 Li Mg Et 2 Li alkyllithium rignard Mg Mg Li Zn TF ZnCl 2 TF dialkylzinc Zn 2 2 Zn Li CuI TF ganocuprate CuI 2 2 CuI common electrophile pairings ' Cl ' '' ' ' ' ' '
Enantioselective Protonations
 Enantioselective Protonations Marc Timo Gieseler 25.02.2013 15.03.2013 Group Seminar AK Kalesse 1 verview Introduction Enantioselective Protonation of Cyclic Substrates Enantioselective Protonation of
Enantioselective Protonations Marc Timo Gieseler 25.02.2013 15.03.2013 Group Seminar AK Kalesse 1 verview Introduction Enantioselective Protonation of Cyclic Substrates Enantioselective Protonation of
Total Synthesis of Oxazolomycin A
 Total Synthesis of xazolomycin A Me xazolomycin A Me Eto, K.; Yoshino, M.; Takahashi K.; Ishihara, J.; atakeyama S. rg. Lett. 2011, 13, 5398 Dimas Paz Wipf group- Current Literature ctober 8, 2011 Dimas
Total Synthesis of xazolomycin A Me xazolomycin A Me Eto, K.; Yoshino, M.; Takahashi K.; Ishihara, J.; atakeyama S. rg. Lett. 2011, 13, 5398 Dimas Paz Wipf group- Current Literature ctober 8, 2011 Dimas
s-buli (1.2 eq.), ( )-sparteine (1.2 eq.) Et 2 O, 78 ºC ; 1-2 (1.2 eq.), 78 ºC ; solvent switch to CHCl 3 *, reflux
 Problem Session (4) ) Please provide the reaction mechanisms. ) Please fill in the blank -6. - TDPS - TI - (. eq.), TF, 78 ºC to rt ; - (. eq.), 78 ºC to rt ; a 4 ( eq.) solvent switch to CD * 65 ºC 8%,
Problem Session (4) ) Please provide the reaction mechanisms. ) Please fill in the blank -6. - TDPS - TI - (. eq.), TF, 78 ºC to rt ; - (. eq.), 78 ºC to rt ; a 4 ( eq.) solvent switch to CD * 65 ºC 8%,
Mild Cobalt-Catalyzed Hydrocyanation of Olefins with Tosyl Cyanide
 Mild Cobalt-Catalyzed ydrocyanation of lefins with Tosyl Cyanide 1 3 2 + Ts Co cat., Si 3 Et, 1-3 h, T 1 2 3 Gaspar, B.; Carreira, E. M. Angew. Chem. Int. Ed. ASAP Current Literature Kalyani Patil 12 May
Mild Cobalt-Catalyzed ydrocyanation of lefins with Tosyl Cyanide 1 3 2 + Ts Co cat., Si 3 Et, 1-3 h, T 1 2 3 Gaspar, B.; Carreira, E. M. Angew. Chem. Int. Ed. ASAP Current Literature Kalyani Patil 12 May
Asymmetric Radical Reactions. Zhen Liu 08/30/2018
 Asymmetric adical eactions Zhen Liu 08/30/2018 Contents Introduction eactions Using Chiral Auxiliary Chiral Lewis Acid-diated eactions Transition tal-catalyzed eactions eactions Using Chiral rganocatalysts
Asymmetric adical eactions Zhen Liu 08/30/2018 Contents Introduction eactions Using Chiral Auxiliary Chiral Lewis Acid-diated eactions Transition tal-catalyzed eactions eactions Using Chiral rganocatalysts
a-aminoallylation of Aldehydes with Ammonia: Stereoselective Synthesis of Homoallylic Primary Amines
 a-aminoallylation of Aldehydes with Ammonia: Stereoselective Synthesis of omoallylic Primary Amines 1 3 2 3 ML n 1 2 2 3 Masaharu Sugiura, Keiichi irano and Shu Kobayashi JACS ASAP ryan Wakefield @ Wipf
a-aminoallylation of Aldehydes with Ammonia: Stereoselective Synthesis of omoallylic Primary Amines 1 3 2 3 ML n 1 2 2 3 Masaharu Sugiura, Keiichi irano and Shu Kobayashi JACS ASAP ryan Wakefield @ Wipf
A Modular Approach to Polyketide Building Blocks: Cycloadditions of Nitrile Oxides and Homoallylic Alcohols
 A Modular Approach to Polyketide Building Blocks: Cycloadditions of itrile xides and Homoallylic Alcohols rganic Letters, 2005, ASAP ina Lohse-Fraefel and Erick M. Carreira * H H H + ' 1. t-bucl, -78 C
A Modular Approach to Polyketide Building Blocks: Cycloadditions of itrile xides and Homoallylic Alcohols rganic Letters, 2005, ASAP ina Lohse-Fraefel and Erick M. Carreira * H H H + ' 1. t-bucl, -78 C
Molybdenum-Catalyzed Asymmetric Allylic Alkylation
 Molybdenum-Catalyzed Asymmetric Allylic Alkylation X MoL n u u * Tommy Bui 9/14/04 Asymmetric Allylic Alkylation from a Synthetic Viewpoint X X M u u * and/or u form a C-C bond with the creation of a new
Molybdenum-Catalyzed Asymmetric Allylic Alkylation X MoL n u u * Tommy Bui 9/14/04 Asymmetric Allylic Alkylation from a Synthetic Viewpoint X X M u u * and/or u form a C-C bond with the creation of a new
ISCHIA ADVANCED SCHOOL OF ORGANIC CHEMISTRY
 ewis acid activation ewis base activation ISCIA ADVACED SC F GAIC CEMISTY Dual in Enantioselective Synthesis of Cyanohydrins Me A A Me UM ewis base catalysis is the process by which an electronpair donor
ewis acid activation ewis base activation ISCIA ADVACED SC F GAIC CEMISTY Dual in Enantioselective Synthesis of Cyanohydrins Me A A Me UM ewis base catalysis is the process by which an electronpair donor
Stereoselective Synthesis of Configurationally Stable Functionalized Ethano-Bridged Tröger Bases
 Stereoselective Synthesis of Configurationally Stable Functionalized Ethano-Bridged Tröger Bases Michon, C.; Sharma, A.; Bernardinelli, G.; Francotte, E.; Lacour, J. Chem. Commun., 2010, 46, 2206-2208
Stereoselective Synthesis of Configurationally Stable Functionalized Ethano-Bridged Tröger Bases Michon, C.; Sharma, A.; Bernardinelli, G.; Francotte, E.; Lacour, J. Chem. Commun., 2010, 46, 2206-2208
When something goes wrong. Goya: Mother showing her derformed child to two women Louvre, Paris
 1 ew Catalytic Asymmestric eactions Karl Anker Jørgensen Danish ational eserach Foundation: Center for Catalysis Department of Chemistry, Aarhus University Denmark kaj@chem.au.dk When something goes wrong
1 ew Catalytic Asymmestric eactions Karl Anker Jørgensen Danish ational eserach Foundation: Center for Catalysis Department of Chemistry, Aarhus University Denmark kaj@chem.au.dk When something goes wrong
Radical Reactions. Radical Stability!!! bond dissociation energies X Y X + Y. bond BDE (kcal/mol) bond BDE (kcal/mol) CH 3 CH 3 CH 2 95 O H R 2 C H
 adical eactions adical Stability!!! bond dissociation energies X Y X Y bond BDE (kcal/mol) bond BDE (kcal/mol) C 3 104 108 C 3 C 2 98 110 95 2 C 102 (-) 93 (C-) 92 C 3 C 3 36 89 85 C 3 C 3 80 adical eactions
adical eactions adical Stability!!! bond dissociation energies X Y X Y bond BDE (kcal/mol) bond BDE (kcal/mol) C 3 104 108 C 3 C 2 98 110 95 2 C 102 (-) 93 (C-) 92 C 3 C 3 36 89 85 C 3 C 3 80 adical eactions
Organocatalysis Enabled by N-Heterocyclic Carbenes
 rganocatalysis Enabled by -eterocyclic Carbenes Acyl Anions X Y omoenolates Acylazolium Azolium enolate Base Catalysis Jiaming Li 2018/04/27 tability of -heterocyclic Carbenes 2p x,y,z p π 2p x,y,z p π
rganocatalysis Enabled by -eterocyclic Carbenes Acyl Anions X Y omoenolates Acylazolium Azolium enolate Base Catalysis Jiaming Li 2018/04/27 tability of -heterocyclic Carbenes 2p x,y,z p π 2p x,y,z p π
Suggested solutions for Chapter 41
 s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
Asymmetric Alklylation of Enolates
 Asymmetric Alklylation of Enolates M with material from A G Meyers http://faculty.chemistry.harvard.edu/myers/pages/chem-215-handouts 745 rganic Synthesis Spring 2015 Asymmetric Alkylation - eed to control
Asymmetric Alklylation of Enolates M with material from A G Meyers http://faculty.chemistry.harvard.edu/myers/pages/chem-215-handouts 745 rganic Synthesis Spring 2015 Asymmetric Alkylation - eed to control
Chap 11. Carbonyl Alpha-Substitution Reactions and Condensation Reactions
 Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Chap 11. Carbonyl Alpha-Substitution eactions and Condensation eactions Four fundamental reactions of carbonyl compounds 1) Nucleophilic addition (aldehydes and ketones) ) Nucleophilic acyl substitution
Total Synthesis of ( )-Virginiamycin M2
 Total Synthesis of ( )-Virginiamycin M2 Jie Wu and James S. Panek, Angewandte Chemie International Edition, 2010, 49, 6165-6168 btained from the CDC Public ealth Image Library. Image credit: CDC/Dr. David
Total Synthesis of ( )-Virginiamycin M2 Jie Wu and James S. Panek, Angewandte Chemie International Edition, 2010, 49, 6165-6168 btained from the CDC Public ealth Image Library. Image credit: CDC/Dr. David
Suggested solutions for Chapter 40
 s for Chapter 40 40 PBLEM 1 Suggest mechanisms for these reactions, explaining the role of palladium in the first step. Ac Et Et BS () 4 2 1. 2. K 2 C 3 evision of enol ethers and bromination, the Wittig
s for Chapter 40 40 PBLEM 1 Suggest mechanisms for these reactions, explaining the role of palladium in the first step. Ac Et Et BS () 4 2 1. 2. K 2 C 3 evision of enol ethers and bromination, the Wittig
Denmark Group Meeting. & Electrophilic rearrangement of amides
 Denmark Group Meeting Palladium catalyzed Dearomatizationeaction & Electrophilic rearrangement of amides 11 th Bo Peng th Feb. 2014 1 https://maps.google.com 2 Palladium catalyzed Dearomatization eaction
Denmark Group Meeting Palladium catalyzed Dearomatizationeaction & Electrophilic rearrangement of amides 11 th Bo Peng th Feb. 2014 1 https://maps.google.com 2 Palladium catalyzed Dearomatization eaction
Homogeneous Catalysis - B. List
 omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
omogeneous Catalysis - B. List 2.2.2 Research Area "rganocatalytic Asymmetric α-alkylation of Aldehydes" (B. List) Involved:. Vignola, A. Majeed Seayad bjective: α-alkylations of carbonyl compounds are
Domino Reactions in Total Synthesis! Reporter: Tianhe Yang! Supervisors: Prof. Yang! Prof. Chen! Prof. Tang!
 1! Domino Reactions in Total Synthesis! Reporter: Tianhe Yang! Supervisors: Prof. Yang! Prof. Chen! Prof. Tang! 2! utline! 1. Brief Introduction! 2. ucleophilic Dominoes! 3. Electrophilc Dominoes! 4. Radical
1! Domino Reactions in Total Synthesis! Reporter: Tianhe Yang! Supervisors: Prof. Yang! Prof. Chen! Prof. Tang! 2! utline! 1. Brief Introduction! 2. ucleophilic Dominoes! 3. Electrophilc Dominoes! 4. Radical
Direct Organocatalytic Enantioselective Mannich Reactions of Ketimines: An Approach to Optically Active Quaternary α-amino Acid Derivatives
 Direct rganocatalytic Enantioselective Mannich eactions of Ketimines: An Approach to ptically Active Quaternary α-amino Acid Derivatives Wei Zhang, Steen Saaby, and Karl Anker Jorgensen The Danish ational
Direct rganocatalytic Enantioselective Mannich eactions of Ketimines: An Approach to ptically Active Quaternary α-amino Acid Derivatives Wei Zhang, Steen Saaby, and Karl Anker Jorgensen The Danish ational
Functionalization of C(sp 3 ) H Bonds Using a Transient Directing Group
 Literature eport Functionalization of C(sp 3 ) Bonds Using a Transient Directing Group eporter: Mu-Wang Chen Checker: Yue Ji Date: 2016-04-05 Yu, J.-Q. et al. Science 2016, 351, 252-256. Scripps esearch
Literature eport Functionalization of C(sp 3 ) Bonds Using a Transient Directing Group eporter: Mu-Wang Chen Checker: Yue Ji Date: 2016-04-05 Yu, J.-Q. et al. Science 2016, 351, 252-256. Scripps esearch
Recent Development in. Tandem Radical Reactions (TRR)
 ecent Development in Tandem adical eactions (T) Feng u Jan. 13, 2006 Contents Brief Introduction of the istory of T Definition of T Intramolecular T Intermolecular T T as Key Steps in Total Synthesis of
ecent Development in Tandem adical eactions (T) Feng u Jan. 13, 2006 Contents Brief Introduction of the istory of T Definition of T Intramolecular T Intermolecular T T as Key Steps in Total Synthesis of
Facile preparation of α-amino ketones from oxidative ring-opening of aziridines by pyridine N-oxide
 Facile preparation of α-amino ketones from oxidative ring-opening of aziridines by pyridine -oxide rg. Biomol. Chem., 2007, 5, 3428 Luo, Z.-B.; Wu, J.-Y.; ou, X.-L.; Dai, L.-X. Ts toluene Ts 80 o C John
Facile preparation of α-amino ketones from oxidative ring-opening of aziridines by pyridine -oxide rg. Biomol. Chem., 2007, 5, 3428 Luo, Z.-B.; Wu, J.-Y.; ou, X.-L.; Dai, L.-X. Ts toluene Ts 80 o C John
Catalytic Asymmetric Intramolecular. Reactions
 Catalytic Asymmetric Intramolecular Pauson-Khand and Pauson-Khand-Type eactions Steven Ballmer CEM 535 Seminar ctober 9, 2008 University of Illinois at Urbana-Champaign pyright 2008 by Steven Ballmer Synthetic
Catalytic Asymmetric Intramolecular Pauson-Khand and Pauson-Khand-Type eactions Steven Ballmer CEM 535 Seminar ctober 9, 2008 University of Illinois at Urbana-Champaign pyright 2008 by Steven Ballmer Synthetic
Chapter 5 Three and Four-Membered Ring Systems
 Chapter 5 Three and Four-mbered ing ystems 5.1 Aziridines Aziridines are good alkylating agents because of their tendency to undergo ring-opening reaction with nucleophiles 例 mitomycin C antibiotic and
Chapter 5 Three and Four-mbered ing ystems 5.1 Aziridines Aziridines are good alkylating agents because of their tendency to undergo ring-opening reaction with nucleophiles 例 mitomycin C antibiotic and
Chiral Bronsted Acids as Catalysts
 Chiral Bronsted Acids as Catalysts Short Literature Seminar 6/3/08 Dustin aup BIL Derived osphoric Acids - First reported in 1992 as a ligand by irrung and coworkers. 4 h 2 irrung Tet. Lett. 1992, 33,
Chiral Bronsted Acids as Catalysts Short Literature Seminar 6/3/08 Dustin aup BIL Derived osphoric Acids - First reported in 1992 as a ligand by irrung and coworkers. 4 h 2 irrung Tet. Lett. 1992, 33,
!"#$%&&'!&(!)*+,-./!01"2.3$*4!"!#$!%$!%&'(') *+,!-$!%&'(').!'/ *&%&*,$.&-!"!3$!4$!5)01+!.*!06'2
 !"#$%&&'!&(!)*+,-./!01"2.3$*4!"!#$!%$!%&'(') *+,!-$!%&'(').!'/012 546*&%&*,$.&-!"!3$!4$!5)01+!.*!06'2 C-C Bond Formation: Cross-coupling Reaction of rganometal Compounds with rganic alids M C-m + X-C C-C
!"#$%&&'!&(!)*+,-./!01"2.3$*4!"!#$!%$!%&'(') *+,!-$!%&'(').!'/012 546*&%&*,$.&-!"!3$!4$!5)01+!.*!06'2 C-C Bond Formation: Cross-coupling Reaction of rganometal Compounds with rganic alids M C-m + X-C C-C
Topic 18: Nucleophilic Sigma Bonds
 Professor David L. Van Vranken Chemistry 201: rganic eaction Mechanisms I Topic 18: ucleophilic Sigma Bonds E E C E eferences: terature cited ecall the Six Types of Canonical Frontier rbitals We ve already
Professor David L. Van Vranken Chemistry 201: rganic eaction Mechanisms I Topic 18: ucleophilic Sigma Bonds E E C E eferences: terature cited ecall the Six Types of Canonical Frontier rbitals We ve already
Strained Molecules in Organic Synthesis
 Strained Molecules in rganic Synthesis 0. Introduction ~ featuring on three-membered rings ~ Tatsuya itabaru (M) Lit. Seminar 08068 for cyclobutadienes : see Mr. Yamatsugu's Lit. Sem. 069 eat of Formation
Strained Molecules in rganic Synthesis 0. Introduction ~ featuring on three-membered rings ~ Tatsuya itabaru (M) Lit. Seminar 08068 for cyclobutadienes : see Mr. Yamatsugu's Lit. Sem. 069 eat of Formation
Synthesis of 1,3-Diols via Controlled, Radical-Mediated C-H Functionalization
 Synthesis of 1,3-Diols via Controlled, Radical-Mediated C- Functionalization Chen, K.; Richter, J. M.; Baran, P. S. J. Amer. Chem. Soc. 2008, 130, 7247-7249. Literature Group Presentation Wynter Gilson
Synthesis of 1,3-Diols via Controlled, Radical-Mediated C- Functionalization Chen, K.; Richter, J. M.; Baran, P. S. J. Amer. Chem. Soc. 2008, 130, 7247-7249. Literature Group Presentation Wynter Gilson
Journal Club Presentation by Remond Moningka 04/17/2006
 β-alkyl-α-allylation of Michael Acceptors through the Palladium-Catalyzed Three-Component Coupling between Allylic Substrate, Trialkylboranes, and Activated lefins Yoshinori Yamamoto, et al. J. rg. Chem.
β-alkyl-α-allylation of Michael Acceptors through the Palladium-Catalyzed Three-Component Coupling between Allylic Substrate, Trialkylboranes, and Activated lefins Yoshinori Yamamoto, et al. J. rg. Chem.
Stable gold(iii) catalysts by oxidative addition of a carboncarbon
 Stable gold(iii) catalysts by oxidative addition of a carboncarbon bond Chung-Yeh Wu, Takahiro oribe, Christian Borch Jacobsen & F. Dean Toste ature, 517, 449-454 (2015) presented by Ian Crouch Literature
Stable gold(iii) catalysts by oxidative addition of a carboncarbon bond Chung-Yeh Wu, Takahiro oribe, Christian Borch Jacobsen & F. Dean Toste ature, 517, 449-454 (2015) presented by Ian Crouch Literature
Organocatalytic Umpolung via N- Heterocyclic Carbenes. Qinghe Liu Hu Group Meeting August 20 th 2015
 rganocatalytic Umpolung via N- Heterocyclic Carbenes Qinghe Liu Hu Group Meeting August 20 th 2015 Contents Part 1: Introduction Part 2: N-Heterocyclic carbene-catalyzed umpolung: classical umpolung, conjugated
rganocatalytic Umpolung via N- Heterocyclic Carbenes Qinghe Liu Hu Group Meeting August 20 th 2015 Contents Part 1: Introduction Part 2: N-Heterocyclic carbene-catalyzed umpolung: classical umpolung, conjugated
Total Synthesis of (+)-Suaveolindole
 1 Total Synthesis of (+)-Suaveolindole 15 2 C 16 11 Emile J. Velthuisen and Samuel J. Danishefsky J. Am. Chem. Soc. 2007, 9, 10640-10641 Julia Vargas September 15, 2007 2 utline Isolation and Elucidation
1 Total Synthesis of (+)-Suaveolindole 15 2 C 16 11 Emile J. Velthuisen and Samuel J. Danishefsky J. Am. Chem. Soc. 2007, 9, 10640-10641 Julia Vargas September 15, 2007 2 utline Isolation and Elucidation
CHO. OMe. endo. xylene, 140 o C, 2 h 70% 1. CH 2 (OMe) 2, MeOH TsOH, rt 2. Bu 2 O, 1,2-dichloroethane 140 o C, 2 h 3. 6 M HCl, THF, rt 44%
 VII Abstracts 2010 p1 2.4.12 Arene rganometallic Complexes of Chromium, Molybdenum, and Tungsten M. Uemura This review is an update to Section 2.4 and covers the literature from 1999 to 2010. (h 6 -Arene)chromium
VII Abstracts 2010 p1 2.4.12 Arene rganometallic Complexes of Chromium, Molybdenum, and Tungsten M. Uemura This review is an update to Section 2.4 and covers the literature from 1999 to 2010. (h 6 -Arene)chromium
Roxanne Atienza Short Literature Presentation January 24, 2011
 Aza-xy-Carbanion elay via on-brook earrangement: Efficient ynthesis of Furo[3,2-c] pyridinones Liang, F.; Lin,.; Wei, Y. J. Am. Chem. oc. 2011, AAP. Double Isocyanide Cyclization: A ynthetic trategy for
Aza-xy-Carbanion elay via on-brook earrangement: Efficient ynthesis of Furo[3,2-c] pyridinones Liang, F.; Lin,.; Wei, Y. J. Am. Chem. oc. 2011, AAP. Double Isocyanide Cyclization: A ynthetic trategy for
