Synthesis and Utility of α Silylamines: A Brief Overview. Literature Presentation 4/6/2K4 Dave Ballweg
|
|
|
- Jasmine Harvey
- 6 years ago
- Views:
Transcription
1 Synthesis and Utility of α Silylamines: A Brief verview Literature Presentation 4/6/2K4 Dave Ballweg
2 Utility of Amines 1. Amines are ubiquitous in organic/medicinal chemistry 2. Important precursers to imines, amides, sulfonamides 3. Main component in amino acid synthesis 4. Useful in library construction 5. itrogen containing ligands in organometallic chemistry 6. itrogen bases provide important pka range (32-37) 7. Additive for breaking lithium aggregates (TMEDA)
3 verview 1) General synthesis 2) adical pathways 3) Amide precursors A) Brook rearrangement B) Peterson olefination 4) Iminium precursors 5) eterocycle synthesis 6) Carbocycle synthesis A) Sommelet auser rearrangement B) Stevens rearrangement 7) Disilylation 8) Biological use MA inhibitor 9) Useful deprotonations
4 General Synthesis First synthesis of an α silylamine Cl Si 3 Li 1 3 (l) 3 C Si 3 can be alkyl, alkoxyl, oxy, and/or aryl 1 can be, or alkyl Later expanded to include disubstituted amine igh heat can become nessecary for addition oll, J. E.; Speier, J. L.; Daubert, B. F. J. Am. Chem. Soc. 1951, C MgBr Cl 3 C C Cl Cl 3 C C 3 C 80 o 2 C C C Si 2 C 3 3 C 3 C 3 C Chan, T..; orvath,. F. J. rg. Chem. 1989, 54, S
5 adical Pathways 3 C a, TMSCl Toluene 3 C SiMe 3 69% a eaction mechanism not clearly understood, but first step is presumed to be a radical anion +e (3 F/mol), TMSCl LiCl 4 / TF Mg electrode SiMe 3 64% aving alkyl substituents instead of aryl groups yields a different product n -C 3 7 +e (3 F/mol), TMSCl LiCl 4 / TF Mg electrode 3 C n -C 3 7 n -C 3 7 TMS 70% Kashimura, S.; Ishifune, M.; Murai, Y.; Murase,.; Shimomura, M.; Shono, T. Tetrahedron Lett. 1998, 39,
6 adical Pathways Electrophile + e TMSCl (3 eq.) E Electrophiles: aliphatic aldehydes, aryl aldehydes, anhydrides, amides, esters If reaction done without electrophiles, then the below mixture of products are obtained: TMS 30% 21% 31% Shono, T.; Kise,.; Kunimi,.; omura,. Chem. Lett. 1991,
7 Amide Precurser 3 C Me 2 Me 2 SiLi (1.2 equiv.) TF, 78 o C 3 C Me 2 SiMe2 1) Me 2 SiLi (1.2 eqiuv.) TF, 20 o C 2) ac 3 / 2 3 C Me 2 SiMe 2 96% Brook 2 Me 2 SiMe 2 SiMe 2 3 C 3 C SiMe 2 Me 2 3 C Me 2 igh yielding eeds phenyl groups either on amide, or on silyl anion to promote Brook rearrangement Product is dialkyl amine, limits further functionalization on nitrogen Fleming, I.; Mack, S..; Clark, B.P. Chem. Commun. 1998, 713.
8 Amide Precurser Me 2 SiMe2 Me 2 SiMe2 E D D 2 MeI or AllylBr E = Me (54%) E = Allyl (63%) Me 2 Me 2 SiLi (2.4 eq.) TF, 78 o C Me 2 SiMe 2 Li 3 C Me 2 SiMe 2 3 C Peterson Me 2 Cl, 2 3 C 3 C 73% Fleming, I.; Mack, S..; Clark, B.P. Chem. Commun. 1998, 713.
9 Amide eduction Me 2 SiCl 3 (2.4 eq.) n Pr 3 (1.2 eq.) 64% Me 2 SiCl3 K Et eat 90% Me 2 SiCl 3 MgI Et 2 SiCl 3 Me 2 SiCl 3 Cl Cl Si SiCl 3 Me 2 2 Me 2 SiMe3 Mozdzen, E. C.; Li, G. S.; Benkeser,. A. J. rganomet. Chem. 1979, 178, 21-28
10 Iminium Precursors Me 3 Si' 2 LiCl 4 /ether rt ' ' 1) Me 2 SiLi 2) Me 2 SiLi 3) 3 SiLi rt ' 2 Si" 3 Dialkyl substituted amine severly hinders further functionalization eaction doesn't proceed with trimethylsilyl lithium species Yields vary from 70-95% with aromatic aldehydes, 50% with isobutyraldehyde Convenent one pot synthesis Dangers involved with using lithium perchlorate aimi-jamal, M..; Mojtahedi, M. M.; Ipaktschi, J.; Saidi, M.. J. Chem. Soc., Perkin Trans. 1, 1999,
11 Iminium Precursors ' Cl ' 1 2 MeSiLi 2 1 Si Me 2 1 Si Me n-buli 2 1 Si Li SS 2 1 Si S 2 1 Si S n-buli 2 1 Si Li S Strohmann, C.; Abele, B. C. in rganosilicon Chemistry III, Eds.. Auner and J. Weis, VC, Weinheim, 1997,
12 Pyrrole/Aziridine Synthesis TMS eat TMS 1) DEAD 2) 2 Et 2 C C 2 Et TMS Et 2 C C 2 Et hshiro, Y.; Tsuno, S.; hno, M.; Komatsu, M. Chem. Lett. 1990,
13 Pyrrole Synthesis TMS eat " TMS " ' ' ovel generation of azomethine ylide TMS ' " Me 2 C C 2 Me Toluene eflux, 5h ' " Me 2 C C 2 Me Proposed intermediate ' " TMS Me 2 C C 2 Me eactions also run with dimethyl maleate (84% of one isomer-trans) and dimethyl fumarate (47% trans, 46% cis) hshiro, Y.; Miyata,.; Komatsu, M.; hno, M. Tetrahedron Lett. 1991, 32,
14 ucleophilic Attack by itrogen 1) ClC 2 Et, K 2 C 3, TF, 99% IC 2 TMS, MeC 2 2) LiAl 4, TF, reflux, 99% C3 reflux, 88% SiMe3 Cl 4, Me 2 a/ 3, Et TF, 78 o C 94% SiMe3 SiMe3 reflux, 95% SiMe3 Mariano, P. S.; Zhang, x.; Xu, W. J. Am. Chem. Soc. 1991, 113,
15 Carbocycle Formation hν, Me A C2 SiMe 3 37h 91% of a 40:51 Η A = α : β 1% C2 SiMe 3 air-purged Me 14.5h 9,10-dicyanoanthracene in C 2 Cl 2 15% A = α 31% A = β 34% exclusively Solvent C2 SiMe 3 hν, MeC 25h SiMe3 C2 50%, 3:3:2 isomers minor Mariano, P. S.; Zhang, x.; Xu, W. J. Am. Chem. Soc. 1991, 113,
16 earrangements Me 2 CsF Me 2 C 2 SiMe 3 Me 2 DBU hν Me Me 2 Me 2 Sommelet auser rearrangement product Stevens rearrangement products = = Alkyl [2,3] sigmatropic rearrangement [1,2] shift of ylide through radical intermediate Sato, Y.; Maeda, Y. J. Chem. Soc., Perkin Trans. 1, 1997, Sato, Y.; Shirai,.; Sugimori, J. Tanaka, T. J. rg. Chem. 1992, 57,
17 ing Enlargement eactions SiMe 3 Ar Li SiMe 3 LiAl 4 SiMe 3 MeI Me 1) Me 3 SiC 2 Tf Me SiMe 3 2) KI Me Me Me MPA CeF Different ratios of cis-trans were studied, and yielded different product ratios Sato, Y.; Kurono, Y.; atano, K.; Shirai,.; Sakuragi, A. J. rg. Chem. 1994, 59,
18 Disilylation Alkyl FMe 2Si Alkyl FSiMe 2 SiMe 2 F Pd(P 3 ) 4 (2%) 60 or 120 o C SiMe 2 F igh temperatures in sealed tubes Substituents on nitrogen affect product, Me and n-bu yield disilylation while benzyl yields unidentified products The palladium catalyst choosen affects disilylation vs. ortho-disilylation The combination f disilane and catalyst is important for yields Quantitative yields Tanaka, M.; Uchimaru, Y.; Williams,. A. J. Chem. Soc., Dalton Trans., 2003,
19 Monoamine xidase Inactivation 3 C 3 C Cl 4 Et SiMe 3 2 MeC 3 C 3 C Et SiMe 3 3-methyl-5-ethyllumiflavinium perchlorate MA Flavin u 3 SiC C MA u 3 Si C 2 -Acid MA Flavin 2 u Si 3 Mariano, P. S.; oegy, S. E.; Kim, J. J. Am. Chem. Soc. 1995, 117,
20 Deprotonation Between Silicon and itrogen 'Li Li SiMe 3 X Boc 1 SiMe 3 Boc SiMe 3 SiMe 3 Li Boc SiMe 3 X isolated yield 1 : 2 D 97% 0.9 : 1 2 Br 59% 0.7 : 1 Et Cl 76% 1 : 1 Me 3 Si Cl 50% 1 : 0 'are,. K.; Somers, J. J.; Sieburth, S. M. Tetrahedron, 1996, 52,
21 Enantioenriched α Silylamines Li Si 3 Si 3 s BuLi sparteine Si 3 Si 3 = TMS, TIPS Yields ranging from 51% to 67% and ee's from 35% to 70% Voyer,.; Barberis, C. Tetrahedron Lett. 1998, 39, Me s BuLi sparteine Li Me TMSCl ( ) 3 Si Me 48% yield and 68% ee Voyer,.; oby, J. Tetrahedron Lett. 1995, 37,
22 Enantioenriched α Silylamines Si 2 1) Boc 2 (100%) 3 2) 3 SiTf (80%) Boc s BuLi sparteine 82% Boc Si 3 >90% ee Boc Si 3 s BuLi sparteine Boc Si 3 88%, 90-95% ee Boc Si 3 Boc Si 3 s BuLi sparteine 38%, 98% ee Boc SiMe 2 p-clc Boc SiMe 2 2:1 mix of diastereomers C 6 4 Cl Boc Si Grubbs Boc 82% Si Sieburth, S. M.; 'are,. K.; Xu, J.; Chen, Y.; Liu, G. rg. Lett. 2003, 5,
23 Conclusions α Silylamines are easily synthesized from commercailly available starting materials Wide variety of silicon substituents Synthesis of many different amines, with and without protecting groups Methods availibe for synthesizing heterocycles and carbocycle Potential biological applications Use of sparteine to obtain chiral molecules Coming soon, Addition of Silyl anions to imines
"-Amino Acids: Function and Synthesis
 "-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
"-Amino Acids: Function and Synthesis # Conformations of "-Peptides # Biological Significance # Asymmetric Synthesis Sean Brown MacMillan Group eting ovember 14, 2001 Lead eferences: Cheng,. P.; Gellman,
Highlights of Schmidt Reaction in the Last Ten Years
 ighlights of Schmidt eaction in the Last Ten Years Dendrobates histrionicus Jack Liu ov. 18, 2003 Introduction Classical Schmidt reaction of aldehydes and carboxylic acids Classical Schmidt reaction of
ighlights of Schmidt eaction in the Last Ten Years Dendrobates histrionicus Jack Liu ov. 18, 2003 Introduction Classical Schmidt reaction of aldehydes and carboxylic acids Classical Schmidt reaction of
Chiral Brønsted Acid Catalysis
 Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
Chiral Brønsted Acid Catalysis Aryl Aryl Aryl Aryl S CF 3 2 P Fe CF 3 CF 3 2 Jack Liu ov. 16, 2004 CF 3 Introduction Chiral Brønsted acid catalysis in nature: enzymes and peptides Chiral Brønsted acid
Electrophilic Carbenes
 Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Electrophilic Carbenes The reaction of so-called stabilized diazo compounds with late transition metals produces a metal carbene intermediate that is electrophilic. The most common catalysts are Cu(I)
Chapter 5 Three and Four-Membered Ring Systems
 Chapter 5 Three and Four-mbered ing ystems 5.1 Aziridines Aziridines are good alkylating agents because of their tendency to undergo ring-opening reaction with nucleophiles 例 mitomycin C antibiotic and
Chapter 5 Three and Four-mbered ing ystems 5.1 Aziridines Aziridines are good alkylating agents because of their tendency to undergo ring-opening reaction with nucleophiles 例 mitomycin C antibiotic and
Chapter 22: Amines. Organic derivatives of ammonia, NH 3. Nitrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic
 hapter 22: Amines. rganic derivatives of ammonia, 3. itrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic 22.1: Amines omenclature. (please read) sp 3 Amines are classified
hapter 22: Amines. rganic derivatives of ammonia, 3. itrogen atom have a lone pair of electrons, making the amine both basic and nucleophilic 22.1: Amines omenclature. (please read) sp 3 Amines are classified
Direct, Catalytic Hydroaminoalkylation of Unactivated Olefins with N-Alkyl Arylamines
 Current Literature - May 12, 2007 Direct, Catalytic ydroaminoalkylation of Unactivated lefins with -Alkyl ylamines ' '' Ta[ 2 ] 5 (4-8 mol%), 160-165 o C 24-67h 66-95% ' '' S. B. erzon and J. F. artwig,
Current Literature - May 12, 2007 Direct, Catalytic ydroaminoalkylation of Unactivated lefins with -Alkyl ylamines ' '' Ta[ 2 ] 5 (4-8 mol%), 160-165 o C 24-67h 66-95% ' '' S. B. erzon and J. F. artwig,
Catalytic Reactions in Organic Synthesis
 17 April, 2008 Catalytic eactions in rganic Synthesis hodium Complexes and edox Catalysts Koichi AASAKA, Motoki YAMAE, Shunsuke CIBA Division of Chemistry and Biological Chemistry, School of ysical and
17 April, 2008 Catalytic eactions in rganic Synthesis hodium Complexes and edox Catalysts Koichi AASAKA, Motoki YAMAE, Shunsuke CIBA Division of Chemistry and Biological Chemistry, School of ysical and
a-aminoallylation of Aldehydes with Ammonia: Stereoselective Synthesis of Homoallylic Primary Amines
 a-aminoallylation of Aldehydes with Ammonia: Stereoselective Synthesis of omoallylic Primary Amines 1 3 2 3 ML n 1 2 2 3 Masaharu Sugiura, Keiichi irano and Shu Kobayashi JACS ASAP ryan Wakefield @ Wipf
a-aminoallylation of Aldehydes with Ammonia: Stereoselective Synthesis of omoallylic Primary Amines 1 3 2 3 ML n 1 2 2 3 Masaharu Sugiura, Keiichi irano and Shu Kobayashi JACS ASAP ryan Wakefield @ Wipf
Strained Molecules in Organic Synthesis
 Strained Molecules in rganic Synthesis 0. Introduction ~ featuring on three-membered rings ~ Tatsuya itabaru (M) Lit. Seminar 08068 for cyclobutadienes : see Mr. Yamatsugu's Lit. Sem. 069 eat of Formation
Strained Molecules in rganic Synthesis 0. Introduction ~ featuring on three-membered rings ~ Tatsuya itabaru (M) Lit. Seminar 08068 for cyclobutadienes : see Mr. Yamatsugu's Lit. Sem. 069 eat of Formation
R 4. metathesis. reductive elimination
 VII Abstracts 2011 p1 2.10.18 rganometallic Complexes of Titanium T. Takeda and A. Tsubouchi This manuscript is an update to the earlier Science of Synthesis contribution describing methods for the preparation
VII Abstracts 2011 p1 2.10.18 rganometallic Complexes of Titanium T. Takeda and A. Tsubouchi This manuscript is an update to the earlier Science of Synthesis contribution describing methods for the preparation
CHEM 330. Final Exam December 5, 2014 ANSWERS. This a closed-notes, closed-book exam. The use of molecular models is allowed
 CEM 330 Final Exam December 5, 2014 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam consists of 12 pages Time: 2h 30 min 1. / 30 2. / 30 3. / 30
CEM 330 Final Exam December 5, 2014 Your name: ASWERS This a closed-notes, closed-book exam The use of molecular models is allowed This exam consists of 12 pages Time: 2h 30 min 1. / 30 2. / 30 3. / 30
Suggested solutions for Chapter 40
 s for Chapter 40 40 PBLEM 1 Suggest mechanisms for these reactions, explaining the role of palladium in the first step. Ac Et Et BS () 4 2 1. 2. K 2 C 3 evision of enol ethers and bromination, the Wittig
s for Chapter 40 40 PBLEM 1 Suggest mechanisms for these reactions, explaining the role of palladium in the first step. Ac Et Et BS () 4 2 1. 2. K 2 C 3 evision of enol ethers and bromination, the Wittig
Chapter 20 Carboxylic Acid Derivatives. Nucleophilic Acyl Substitution
 ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
ucleophilic Acyl Substitution hapter 20 arboxylic Acid Derivatives ucleophilic Acyl Substitution Y (1) need to have Y as a u Y u u + Y (2) could not happen with aldehydes or ketones as : and : are poor
Spiro Monophosphite and Monophosphoramidite Ligand Kit
 Spiro Monophosphite and Monophosphoramidite Ligand Kit metals inorganics organometallics catalysts ligands custom synthesis cgm facilities nanomaterials 15-5162 15-5150 15-5156 15-5163 15-5151 15-5157
Spiro Monophosphite and Monophosphoramidite Ligand Kit metals inorganics organometallics catalysts ligands custom synthesis cgm facilities nanomaterials 15-5162 15-5150 15-5156 15-5163 15-5151 15-5157
Ynolate Chemistry. Jeff Kallemeyn October 22, 2002
 Ynolate Chemistry While enolates have numbered among the most important reagents of organic chemistry for more than a century, ynolates have hitherto remained unknown although their chemistry should be
Ynolate Chemistry While enolates have numbered among the most important reagents of organic chemistry for more than a century, ynolates have hitherto remained unknown although their chemistry should be
A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols
 A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols B() 2 H H B() 2 H H Hu, X.-D.; Fan, C.-A.; Zhang, F.-M.; Tu, Y.
A Tandem Semipinacol Rearrangement/Alkylation of a-epoxy Alcohols: An Efficient and Stereoselective Approach to Multifunctional 1,3-Diols B() 2 H H B() 2 H H Hu, X.-D.; Fan, C.-A.; Zhang, F.-M.; Tu, Y.
Chem 316/422 Beauchamp 1 Match the step number in the synthesis with the letter of the reagents listed just below.
 hem 316/422 Beauchamp 1 Match the step number in the synthesis with the letter of the reagents listed just below. TP l Et step 8 4 5 eagents used in synthesis A B D E F a DMF (solvent) 1. (i-pr) 2 Li (LDA)/TF
hem 316/422 Beauchamp 1 Match the step number in the synthesis with the letter of the reagents listed just below. TP l Et step 8 4 5 eagents used in synthesis A B D E F a DMF (solvent) 1. (i-pr) 2 Li (LDA)/TF
Roxanne Atienza Short Literature Presentation January 24, 2011
 Aza-xy-Carbanion elay via on-brook earrangement: Efficient ynthesis of Furo[3,2-c] pyridinones Liang, F.; Lin,.; Wei, Y. J. Am. Chem. oc. 2011, AAP. Double Isocyanide Cyclization: A ynthetic trategy for
Aza-xy-Carbanion elay via on-brook earrangement: Efficient ynthesis of Furo[3,2-c] pyridinones Liang, F.; Lin,.; Wei, Y. J. Am. Chem. oc. 2011, AAP. Double Isocyanide Cyclization: A ynthetic trategy for
Asymmetric Deprotonation
 ( )-sparteine i-pr, t 2, -98 C 84% ee Asymmetric Deprotonation gands, Bases, and Applications CF 3 TMS t-bu TMSCl, MPA TF, -100 C t-bu 93% ee Fe (i-pr) 2 1. n-bu ( )-sparteine t 2, -78 C 2. 2 PCl P 2 Fe
( )-sparteine i-pr, t 2, -98 C 84% ee Asymmetric Deprotonation gands, Bases, and Applications CF 3 TMS t-bu TMSCl, MPA TF, -100 C t-bu 93% ee Fe (i-pr) 2 1. n-bu ( )-sparteine t 2, -78 C 2. 2 PCl P 2 Fe
Denmark Group Meeting. & Electrophilic rearrangement of amides
 Denmark Group Meeting Palladium catalyzed Dearomatizationeaction & Electrophilic rearrangement of amides 11 th Bo Peng th Feb. 2014 1 https://maps.google.com 2 Palladium catalyzed Dearomatization eaction
Denmark Group Meeting Palladium catalyzed Dearomatizationeaction & Electrophilic rearrangement of amides 11 th Bo Peng th Feb. 2014 1 https://maps.google.com 2 Palladium catalyzed Dearomatization eaction
Direct Organocatalytic Enantioselective Mannich Reactions of Ketimines: An Approach to Optically Active Quaternary α-amino Acid Derivatives
 Direct rganocatalytic Enantioselective Mannich eactions of Ketimines: An Approach to ptically Active Quaternary α-amino Acid Derivatives Wei Zhang, Steen Saaby, and Karl Anker Jorgensen The Danish ational
Direct rganocatalytic Enantioselective Mannich eactions of Ketimines: An Approach to ptically Active Quaternary α-amino Acid Derivatives Wei Zhang, Steen Saaby, and Karl Anker Jorgensen The Danish ational
Stereoselective reactions of the carbonyl group
 1 Stereoselective reactions of the carbonyl group We have seen many examples of substrate control in nucleophilic addition to the carbonyl group (Felkin-Ahn & chelation control) If molecule does not contain
1 Stereoselective reactions of the carbonyl group We have seen many examples of substrate control in nucleophilic addition to the carbonyl group (Felkin-Ahn & chelation control) If molecule does not contain
Asymmetric Nucleophilic Catalysis
 Asymmetric ucleophilic Catalysis Chiral catalyst X 2 Chiral catalyst X = alkyl, X 1 2 1 Vedejs, E.; Daugulis,. J. Am. Chem. Soc. 2003, 125, 4166-4173 Shaw, S. A.; Aleman,.; Vedejs, E. J. Am. Chem. Soc.
Asymmetric ucleophilic Catalysis Chiral catalyst X 2 Chiral catalyst X = alkyl, X 1 2 1 Vedejs, E.; Daugulis,. J. Am. Chem. Soc. 2003, 125, 4166-4173 Shaw, S. A.; Aleman,.; Vedejs, E. J. Am. Chem. Soc.
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: favored. disfavored. favored. disfavored
 The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
The aldol reaction with metal enolates proceeds by a chair-like, pericyclic process: Z-enolates: M 2 M 2 syn 2 C 2 favored 2 M 2 anti disfavored E-enolates: M 2 2 C 3 C 3 C 2 favored 2 M M disfavored In
CHEM 234: Organic Chemistry II Reaction Sheets
 EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
EM234:rganichemistry eactionsheets ucleophilic addition at carbonyl groups: Grignards and reducing agents u: u u u: u u = or = or l u u u ucleophilic addition at carbonyl groups: oxygen and nitrogen nucleophiles:
Total Synthesis of (+/-)-Goniomitine via a Formal Nitrile/Donor-Acceptor Cyclopropane [3 + 2] Cyclization
![Total Synthesis of (+/-)-Goniomitine via a Formal Nitrile/Donor-Acceptor Cyclopropane [3 + 2] Cyclization Total Synthesis of (+/-)-Goniomitine via a Formal Nitrile/Donor-Acceptor Cyclopropane [3 + 2] Cyclization](/thumbs/82/85235286.jpg) Total Synthesis of (+/-)-Goniomitine via a Formal itrile/donor-acceptor Cyclopropane [3 + 2] Cyclization (-)-Goniomitine Christian L. Morales and Brian Pagenkopf* rganic Letters, ASAP Current Literature
Total Synthesis of (+/-)-Goniomitine via a Formal itrile/donor-acceptor Cyclopropane [3 + 2] Cyclization (-)-Goniomitine Christian L. Morales and Brian Pagenkopf* rganic Letters, ASAP Current Literature
Additions to Metal-Alkene and -Alkyne Complexes
 Additions to tal-alkene and -Alkyne Complexes ecal that alkenes, alkynes and other π-systems can be excellent ligands for transition metals. As a consequence of this binding, the nature of the π-system
Additions to tal-alkene and -Alkyne Complexes ecal that alkenes, alkynes and other π-systems can be excellent ligands for transition metals. As a consequence of this binding, the nature of the π-system
CHEM 203. Final Exam December 15, 2010 ANSWERS. This a closed-notes, closed-book exam. You may use your set of molecular models
 CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
CEM 203 Final Exam December 15, 2010 Your name: ANSWERS This a closed-notes, closed-book exam You may use your set of molecular models This test contains 15 pages Time: 2h 30 min 1. / 16 2. / 15 3. / 24
Three Type Of Carbene Complexes
 Three Type f arbene omplexes arbene complexes have formal metal-to-carbon double bonds. Several types are known. The reactivity of the carbene and how it contributes to the overall electron counting is
Three Type f arbene omplexes arbene complexes have formal metal-to-carbon double bonds. Several types are known. The reactivity of the carbene and how it contributes to the overall electron counting is
Suggested solutions for Chapter 41
 s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
s for Chapter 41 41 PBLEM 1 Explain how this synthesis of amino acids, starting with natural proline, works. Explain the stereoselectivity of each step after the first. C 2 C 2 3 CF 3 C 2 2 Pd 2 C 2 +
Shi Asymmetric Epoxidation
 Shi Asymmetric Epoxidation Chiral dioxirane strategy: R 3 + 1 xone, ph 10.5, K 2 C 3, H 2, C R 3 formed in situ catalyst (10-20 mol%) is prepared from D-fructose, and its enantiomer from L-sorbose oxone,
Shi Asymmetric Epoxidation Chiral dioxirane strategy: R 3 + 1 xone, ph 10.5, K 2 C 3, H 2, C R 3 formed in situ catalyst (10-20 mol%) is prepared from D-fructose, and its enantiomer from L-sorbose oxone,
Molybdenum-Catalyzed Asymmetric Allylic Alkylation
 Molybdenum-Catalyzed Asymmetric Allylic Alkylation X MoL n u u * Tommy Bui 9/14/04 Asymmetric Allylic Alkylation from a Synthetic Viewpoint X X M u u * and/or u form a C-C bond with the creation of a new
Molybdenum-Catalyzed Asymmetric Allylic Alkylation X MoL n u u * Tommy Bui 9/14/04 Asymmetric Allylic Alkylation from a Synthetic Viewpoint X X M u u * and/or u form a C-C bond with the creation of a new
Reduction. Boron based reagents. NaBH 4 / NiCl 2. Uses: Zn(BH 4 ) 2. Preparation: Good for base sensitive groups Chelation control model.
 Uses: Ar N 2 Ar N 2 Ar N Ar N 2 eduction Boron based reagents NaB 4 / NiCl 2 2 Ar C N Ar C N 2 Preparation: Zn(B 4 ) 2 ZnCl 2 (Ether) NaB 4 Zn(B 4 ) 2 Good for base sensitive groups Chelation control model
Uses: Ar N 2 Ar N 2 Ar N Ar N 2 eduction Boron based reagents NaB 4 / NiCl 2 2 Ar C N Ar C N 2 Preparation: Zn(B 4 ) 2 ZnCl 2 (Ether) NaB 4 Zn(B 4 ) 2 Good for base sensitive groups Chelation control model
Lecture 6: Transition-Metal Catalysed C-C Bond Formation
 Lecture 6: Transition-Metal Catalysed C-C Bond Formation (a) Asymmetric allylic substitution 1 u - d u (b) Asymmetric eck reaction 2 3 Ar- d (0) Ar 2 3 (c) Asymmetric olefin metathesis alladium π-allyl
Lecture 6: Transition-Metal Catalysed C-C Bond Formation (a) Asymmetric allylic substitution 1 u - d u (b) Asymmetric eck reaction 2 3 Ar- d (0) Ar 2 3 (c) Asymmetric olefin metathesis alladium π-allyl
Organocopper Chemistry
 rganocopper Chemistry ave a great historical importance, but still remain highly useful reactions. If not the first organometallic reactions developed they are among the first. Most often used in conjugate
rganocopper Chemistry ave a great historical importance, but still remain highly useful reactions. If not the first organometallic reactions developed they are among the first. Most often used in conjugate
CHAPTER 22: Amines R 2 H N N N R 1. cadeverine N CH 3. nicotine
 Based on notes by JZ updated 4/2018 Amines are Biologically Important CAPTE 22: Amines Amino acids are the basis of all peptides and proteins. These are the tissue building blocks and nature's catalysts
Based on notes by JZ updated 4/2018 Amines are Biologically Important CAPTE 22: Amines Amino acids are the basis of all peptides and proteins. These are the tissue building blocks and nature's catalysts
Suggested solutions for Chapter 29
 s for Chapter 29 29 PRBLEM 1 or each of the following reactions (a) state what kind of substitution is suggested and (b) suggest what product might be formed if monosubstitution occured. Br 2 3 2 S 4 S
s for Chapter 29 29 PRBLEM 1 or each of the following reactions (a) state what kind of substitution is suggested and (b) suggest what product might be formed if monosubstitution occured. Br 2 3 2 S 4 S
Chiral Proton Catalysis in Organic Synthesis. Samantha M. Frawley Organic Seminar September 14 th, 2005
 Chiral Proton Catalysis in rganic Synthesis Samantha M. Frawley rganic Seminar September 14 th, 2005 Seminar utline Introduction Lewis Acid-assisted Chiral Brønsted Acids Enantioselective protonation for
Chiral Proton Catalysis in rganic Synthesis Samantha M. Frawley rganic Seminar September 14 th, 2005 Seminar utline Introduction Lewis Acid-assisted Chiral Brønsted Acids Enantioselective protonation for
Sonogashira: in situ, metal assisted deprotonation
 M.C. White, Chem 253 Cross-Coupling -120- Week of ctober 11, 2004 Sonogashira: in situ, metal assisted deprotonation catalytic cycle: ' (h 3 ) n d II The first report: h Sonogashira T 1975 (50) 4467. h
M.C. White, Chem 253 Cross-Coupling -120- Week of ctober 11, 2004 Sonogashira: in situ, metal assisted deprotonation catalytic cycle: ' (h 3 ) n d II The first report: h Sonogashira T 1975 (50) 4467. h
Functionalization of C O Bonds. Stefan McCarver. MacMillan Lab Group Meeting
 Functionalization of C Bonds Stefan McCarver MacMillan Lab Group eting November 23 rd, 2016 Functionalization of C Bonds "X" X homolytic cleavage catalyst M oxidative addition Why is C Bond Manipulation
Functionalization of C Bonds Stefan McCarver MacMillan Lab Group eting November 23 rd, 2016 Functionalization of C Bonds "X" X homolytic cleavage catalyst M oxidative addition Why is C Bond Manipulation
Catalytic Asymmetric Pauson-Khand Reaction. Won-jin Chung 02/25/2003
 Catalytic Asymmetric Pauson-Khand eaction U. Khand; G.. Knox; P. L. Pauson; W. E. Watts J. Chem. Soc. Chem. Commun. 1971, 36 Won-jin Chung 02/25/2003 The General Pattern of the Pauson-Khand eaction Co
Catalytic Asymmetric Pauson-Khand eaction U. Khand; G.. Knox; P. L. Pauson; W. E. Watts J. Chem. Soc. Chem. Commun. 1971, 36 Won-jin Chung 02/25/2003 The General Pattern of the Pauson-Khand eaction Co
ANSWER GUIDE APRIL/MAY 2006 EXAMINATIONS CHEMISTRY 249H
 AWER GUIDE APRIL/MAY 2006 EXAMIATI CEMITRY 249 1. (a) PDC / C 2 2 (b) t-bume 2 i (1 equiv) / imidazole (1 equiv) i TBDM protection of the less sterically hindered alcohol (c) BuLi (1 equiv) then (d) 2
AWER GUIDE APRIL/MAY 2006 EXAMIATI CEMITRY 249 1. (a) PDC / C 2 2 (b) t-bume 2 i (1 equiv) / imidazole (1 equiv) i TBDM protection of the less sterically hindered alcohol (c) BuLi (1 equiv) then (d) 2
Discussion Addendum for: Trifluoromethylation at the -Position of, -Unsaturated Ketones: 4-Phenyl-3-Trifluoromethyl-2-Butanone
 DI:10.15227/orgsyn.089.0374 Discussion Addendum for: Trifluoromethylation at the -Position of, -Unsaturated Ketones: 4-Phenyl-3-Trifluoromethyl-2-Butanone C 3 Ph hcl(pph 3 ) 3 C 3 I C 3 Ph T C 3 Prepared
DI:10.15227/orgsyn.089.0374 Discussion Addendum for: Trifluoromethylation at the -Position of, -Unsaturated Ketones: 4-Phenyl-3-Trifluoromethyl-2-Butanone C 3 Ph hcl(pph 3 ) 3 C 3 I C 3 Ph T C 3 Prepared
Total Synthesis of (+)-Sieboldine A J. Am. Chem. Soc. 2010, 132,
 Total Synthesis of (+)-Sieboldine A J. Am. Chem. Soc. 2010, 132, 7876 7877. Current Literature Presentation 10JUL2010 Michael Yang Mike Yang @ Wipf Group Page 1 of 15 7/10/2010 Sieboldine A Background
Total Synthesis of (+)-Sieboldine A J. Am. Chem. Soc. 2010, 132, 7876 7877. Current Literature Presentation 10JUL2010 Michael Yang Mike Yang @ Wipf Group Page 1 of 15 7/10/2010 Sieboldine A Background
Stereoselective reactions of enolates: auxiliaries
 1 Stereoselective reactions of enolates: auxiliaries Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones
1 Stereoselective reactions of enolates: auxiliaries Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones
Tips for taking exams in 852
 Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Comprehensive Tactical Methods in rganic Synthesis W. D. Wulff 1) Know the relative reactivity of carbonyl compounds Tips for taking exams in 852 Cl > > ' > > ' N2 eg: 'Mg Et ' 1equiv. 1equiv. ' ' Et 50%
Stereoselective reactions of enolates
 1 Stereoselective reactions of enolates Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones These are
1 Stereoselective reactions of enolates Chiral auxiliaries are frequently used to allow diastereoselective enolate reactions Possibly the most extensively studied are the Evan s oxazolidinones These are
1/4/2011. Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds
 Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Chapter 18 Aldehydes and Ketones Reaction at the -carbon of carbonyl compounds The Acidity of the Hydrogens of Carbonyl Compounds: Enolate Anions Hydrogens on carbons to carbonyls are unusually acidic
Total Syntheses of Minfiensine
 Total Syntheses of Minfiensine Douany, A. B.; umphreys, P. G.; verman, L. E.*; Wrobelski, A. D., J. Am. Chem. Soc. 2008, ASAP. D: 10.1021/ja800163v Shen, L.; Zhang, M.; Wu, Y.; Qin, Y.*, Angew. Chem. nt.
Total Syntheses of Minfiensine Douany, A. B.; umphreys, P. G.; verman, L. E.*; Wrobelski, A. D., J. Am. Chem. Soc. 2008, ASAP. D: 10.1021/ja800163v Shen, L.; Zhang, M.; Wu, Y.; Qin, Y.*, Angew. Chem. nt.
TMSCl imidazole DMF. Ph Ph OTMS. Michael reaction. Michael reaction Ph R 3. epoxidation O R
 eaction using diarylprolinol silyl ether derivatives as catalyst 1) C Et K C 3, ) MgBr, TF TMS hexane, 0 o C TBS p- C 6 4, T C Et 85%, 99% ee Angew. Chem., nt. Ed., 44, 41 (005). rg. Synth., 017, 94, 5.
eaction using diarylprolinol silyl ether derivatives as catalyst 1) C Et K C 3, ) MgBr, TF TMS hexane, 0 o C TBS p- C 6 4, T C Et 85%, 99% ee Angew. Chem., nt. Ed., 44, 41 (005). rg. Synth., 017, 94, 5.
[3,3]-Sigmatropic rearrangements
![[3,3]-Sigmatropic rearrangements [3,3]-Sigmatropic rearrangements](/thumbs/75/71504906.jpg) 1 [3,3]-Sigmatropic rearrangements heat R 1 R 3 R 1 R 3 R 1 R 3 A class of pericyclic reactions whose stereochemical outcome is governed by the geometric requirements of the cyclic transition state Reactions
1 [3,3]-Sigmatropic rearrangements heat R 1 R 3 R 1 R 3 R 1 R 3 A class of pericyclic reactions whose stereochemical outcome is governed by the geometric requirements of the cyclic transition state Reactions
Use of Cp 2 TiCl in Synthesis
 Use of 2 TiCl in Synthesis eagent Control of adical eactions Jeff Kallemeyn May 21, 2002 eactions of 2 TiCl 1. Pinacol Coupling H H H 2. Epoxide pening H H E H Chemoselectivity Activated aldehydes (aromatic,
Use of 2 TiCl in Synthesis eagent Control of adical eactions Jeff Kallemeyn May 21, 2002 eactions of 2 TiCl 1. Pinacol Coupling H H H 2. Epoxide pening H H E H Chemoselectivity Activated aldehydes (aromatic,
Intramolecular Ene Reactions Utilizing Oxazolones and Enol Ethers Fisk, J.S. and Tepe, J..J J. Am. Chem. Soc., 2007, 129,
 Intramolecular Ene Reactions Utilizing xazolones and Enol Ethers Fisk, J.S. and Tepe, J..J J. Am. Chem. Soc., 2007, 129, 3058-3059 - versus -Arylation of Aminoalcohols: rthogonal Selectivity in Copper-Based
Intramolecular Ene Reactions Utilizing xazolones and Enol Ethers Fisk, J.S. and Tepe, J..J J. Am. Chem. Soc., 2007, 129, 3058-3059 - versus -Arylation of Aminoalcohols: rthogonal Selectivity in Copper-Based
Total Synthesis of Oxazolomycin A
 Total Synthesis of xazolomycin A Me xazolomycin A Me Eto, K.; Yoshino, M.; Takahashi K.; Ishihara, J.; atakeyama S. rg. Lett. 2011, 13, 5398 Dimas Paz Wipf group- Current Literature ctober 8, 2011 Dimas
Total Synthesis of xazolomycin A Me xazolomycin A Me Eto, K.; Yoshino, M.; Takahashi K.; Ishihara, J.; atakeyama S. rg. Lett. 2011, 13, 5398 Dimas Paz Wipf group- Current Literature ctober 8, 2011 Dimas
Synthetic Methodology. Using Tertiary Phosphines. as Nucleophilic Catalysts
 Synthetic Methodology Using Tertiary osphines as Nucleophilic Catalysts 1 3 2 u 2 (P 3 ) 3 4 1 2 D. Ma, X. Lu 1988 1 2 Pd 2 (dba) 3.CCl 3 /P 3 /Ac or Pd(Ac) 2 /P 3 1 2 B. M. Trost 1988 1 3 2 u 2 (P 3 )
Synthetic Methodology Using Tertiary osphines as Nucleophilic Catalysts 1 3 2 u 2 (P 3 ) 3 4 1 2 D. Ma, X. Lu 1988 1 2 Pd 2 (dba) 3.CCl 3 /P 3 /Ac or Pd(Ac) 2 /P 3 1 2 B. M. Trost 1988 1 3 2 u 2 (P 3 )
Copper-Catalyzed Reaction of Alkyl Halides with Cyclopentadienylmagnesium Reagent
 Copper-Catalyzed eaction of Alkyl Halides with Cyclopentadienylmagnesium eagent Mg 1) cat. Cu(Tf) 2 i Pr 2, 25 o C, 3 h 2) H 2, Pt 2 Masahiro Sai, Hidenori Someya, Hideki Yorimitsu, and Koichiro shima
Copper-Catalyzed eaction of Alkyl Halides with Cyclopentadienylmagnesium eagent Mg 1) cat. Cu(Tf) 2 i Pr 2, 25 o C, 3 h 2) H 2, Pt 2 Masahiro Sai, Hidenori Someya, Hideki Yorimitsu, and Koichiro shima
Requirements for an Effective Chiral Auxiliary Enolate Alkylation
 Requirements for an Effective Chiral Auxiliary Enolate Alkylation 1. Xc must be low cost, and available in both enentiomeric forms 2. The cleavage of Xc from the substrate must occur under mild enough
Requirements for an Effective Chiral Auxiliary Enolate Alkylation 1. Xc must be low cost, and available in both enentiomeric forms 2. The cleavage of Xc from the substrate must occur under mild enough
Direct Catalytic Cross-Coupling of Organolithium
 Literature report Direct Catalytic Cross-Coupling of Organolithium Compounds Reporter: Zhang-Pei Chen Checker: Mu-Wang Chen Date: 02/07/2013 Feringa, B.L.et al. Feringa, B. L. et al. Nature Chem. 2013,
Literature report Direct Catalytic Cross-Coupling of Organolithium Compounds Reporter: Zhang-Pei Chen Checker: Mu-Wang Chen Date: 02/07/2013 Feringa, B.L.et al. Feringa, B. L. et al. Nature Chem. 2013,
O or R E + R. - Keto-Enol Tautomerization (enol form usually very minor for simple ketones)
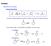 General eactivity base or acid or E + E - Keto-Enol Tautomerization (enol form usually very minor for simple ketones) - Can enhance rate / concentration by addition of acid or base + catalyzed + + + base
General eactivity base or acid or E + E - Keto-Enol Tautomerization (enol form usually very minor for simple ketones) - Can enhance rate / concentration by addition of acid or base + catalyzed + + + base
eatles Oasis - 199
 eatles - 1964 asis - 199 Biography 2001-present: University of 1997-2000: Professor, Shef 1988-1997: Various Reader 1986-1988: Post-doc with G 1983-1986: D at Universi 1983: Undergrad at Cambrid Enantioselective
eatles - 1964 asis - 199 Biography 2001-present: University of 1997-2000: Professor, Shef 1988-1997: Various Reader 1986-1988: Post-doc with G 1983-1986: D at Universi 1983: Undergrad at Cambrid Enantioselective
Lecture Notes Chem 51C S. King. Chapter 20 Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation & Reduction
 Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Lecture Notes Chem 51C S. King Chapter 20 Introduction to Carbonyl Chemistry; rganometallic Reagents; xidation & Reduction I. The Reactivity of Carbonyl Compounds The carbonyl group is an extremely important
Chromium Arene Complexes
 Go through Reviews Chem. Reviews Chem. Soc. Reviews Book by Prof. A. J. Elias Chromium Arene Complexes Complexation of Cr(CO) 3 with ARENES Chromium arene complexes Metal complexation is appealing in organic
Go through Reviews Chem. Reviews Chem. Soc. Reviews Book by Prof. A. J. Elias Chromium Arene Complexes Complexation of Cr(CO) 3 with ARENES Chromium arene complexes Metal complexation is appealing in organic
CH 3 TMG, DMF N H 3 CO 2 S. (PPh 3 ) 2 Pd 0
 1. (a) rovide a reasonable mechanism for the following transformation. I S 2 C 3 C 3 ( 3 ) 2 2, CuI C 3 TMG, DMF 3 C 2 S TMG = Me 2 Me 2 ICu ( 3 ) 2 0 I S 2 C 3 S 2 C 3 Cu I 3 3 3 C 2 S I 3 3 3 C 2 S 3
1. (a) rovide a reasonable mechanism for the following transformation. I S 2 C 3 C 3 ( 3 ) 2 2, CuI C 3 TMG, DMF 3 C 2 S TMG = Me 2 Me 2 ICu ( 3 ) 2 0 I S 2 C 3 S 2 C 3 Cu I 3 3 3 C 2 S I 3 3 3 C 2 S 3
Topic 18: Nucleophilic Sigma Bonds
 Professor David L. Van Vranken Chemistry 201: rganic eaction Mechanisms I Topic 18: ucleophilic Sigma Bonds E E C E eferences: terature cited ecall the Six Types of Canonical Frontier rbitals We ve already
Professor David L. Van Vranken Chemistry 201: rganic eaction Mechanisms I Topic 18: ucleophilic Sigma Bonds E E C E eferences: terature cited ecall the Six Types of Canonical Frontier rbitals We ve already
CHO. OMe. endo. xylene, 140 o C, 2 h 70% 1. CH 2 (OMe) 2, MeOH TsOH, rt 2. Bu 2 O, 1,2-dichloroethane 140 o C, 2 h 3. 6 M HCl, THF, rt 44%
 VII Abstracts 2010 p1 2.4.12 Arene rganometallic Complexes of Chromium, Molybdenum, and Tungsten M. Uemura This review is an update to Section 2.4 and covers the literature from 1999 to 2010. (h 6 -Arene)chromium
VII Abstracts 2010 p1 2.4.12 Arene rganometallic Complexes of Chromium, Molybdenum, and Tungsten M. Uemura This review is an update to Section 2.4 and covers the literature from 1999 to 2010. (h 6 -Arene)chromium
Keisuke Suzuki. Baran lab Group Meeting 6/11/16. Shigenobu Umemiya. Akira Suzuki. Takanori Suzuki (Hokkaido University)
 197.D., Teruaki Mukaiyama, University of Tokyo 193 Assistant Professor, Keio University 197 Lecturer, Keio University 199 Assocate Professor, Keio University 1990 Visiting Professor, ET 1994 ull Professor,
197.D., Teruaki Mukaiyama, University of Tokyo 193 Assistant Professor, Keio University 197 Lecturer, Keio University 199 Assocate Professor, Keio University 1990 Visiting Professor, ET 1994 ull Professor,
Pyrrole reaction. Assis.Prof.Dr.Mohammed Hassan Lecture 4
 Pyrrole reaction Assis.Prof.Dr.Mohammed assan Lecture 4 Acidic properties of pyrrole Due to participation of lone pair in aromaticity), pyrrole has exceptionally strong acidic properties It can react with
Pyrrole reaction Assis.Prof.Dr.Mohammed assan Lecture 4 Acidic properties of pyrrole Due to participation of lone pair in aromaticity), pyrrole has exceptionally strong acidic properties It can react with
Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02
![Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates. Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02](/thumbs/93/114018431.jpg) Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Xiao, W.-J. et al. J. Am. Chem. Soc. 2016, 138, 8360.
Catalytic Asymmetric [4+1] Annulation of Sulfur Ylides with Copper Allenylidene Intermediates Reporter: Jie Wang Checker: Shubo Hu Date: 2016/08/02 Xiao, W.-J. et al. J. Am. Chem. Soc. 2016, 138, 8360.
Molecular Rearrangements
 Ø Migration of one the molecule group from one atom to another within Ø Generally the migrating group never leaves the molecule Ø There are five types of skeletal rearrangements- 1. Electron deficient
Ø Migration of one the molecule group from one atom to another within Ø Generally the migrating group never leaves the molecule Ø There are five types of skeletal rearrangements- 1. Electron deficient
Pericyclic Reactions 6 Lectures Year 3 Handout 2 Michaelmas 2017
 Pericyclic eactions 6 Lectures Year 3 andout 2 Michaelmas 27 π6 a σ2 s Prof Martin Smith CL 3.87 martin.smith@chem.ox.ac.uk http://msmith.chem.ox.ac.uk/ Cycloadditions: oxyallyl cation P46 ω s σ2 s odd
Pericyclic eactions 6 Lectures Year 3 andout 2 Michaelmas 27 π6 a σ2 s Prof Martin Smith CL 3.87 martin.smith@chem.ox.ac.uk http://msmith.chem.ox.ac.uk/ Cycloadditions: oxyallyl cation P46 ω s σ2 s odd
Mechanistic Studies of Allylsilane Rearrangement
 chanistic Studies of Allylsilane Rearrangement Thesis: The goal of our project is to determine the operating mechanism in the transformation of α-siloxy allylsilanes to vinyl silanes. Elucidating the mechanism
chanistic Studies of Allylsilane Rearrangement Thesis: The goal of our project is to determine the operating mechanism in the transformation of α-siloxy allylsilanes to vinyl silanes. Elucidating the mechanism
Recent Development in. Tandem Radical Reactions (TRR)
 ecent Development in Tandem adical eactions (T) Feng u Jan. 13, 2006 Contents Brief Introduction of the istory of T Definition of T Intramolecular T Intermolecular T T as Key Steps in Total Synthesis of
ecent Development in Tandem adical eactions (T) Feng u Jan. 13, 2006 Contents Brief Introduction of the istory of T Definition of T Intramolecular T Intermolecular T T as Key Steps in Total Synthesis of
Total Synthesis of (+)-Suaveolindole
 1 Total Synthesis of (+)-Suaveolindole 15 2 C 16 11 Emile J. Velthuisen and Samuel J. Danishefsky J. Am. Chem. Soc. 2007, 9, 10640-10641 Julia Vargas September 15, 2007 2 utline Isolation and Elucidation
1 Total Synthesis of (+)-Suaveolindole 15 2 C 16 11 Emile J. Velthuisen and Samuel J. Danishefsky J. Am. Chem. Soc. 2007, 9, 10640-10641 Julia Vargas September 15, 2007 2 utline Isolation and Elucidation
Problem session (3) Daiki Kuwana. Please fill in the blank and explain reaction mechanisms and stereoselectivities.
 Problem session (3) Daiki Kuwana Please fill in the blank and explain reaction mechanisms and stereoselectivities. 1. 1-1 1. (Ac) 2 (10 mol%), DPEphos (20 mol%) Et 3, toluene, 90 C 2. s 4 (14 mol%), M;
Problem session (3) Daiki Kuwana Please fill in the blank and explain reaction mechanisms and stereoselectivities. 1. 1-1 1. (Ac) 2 (10 mol%), DPEphos (20 mol%) Et 3, toluene, 90 C 2. s 4 (14 mol%), M;
Studies on Heck and Suzuki Reactions Catalyzed by Palladium(0) and Wacker- Type Oxidative Cyclization Catalyzed by Palladium(II)
 Studies on eck and Suzuki Reactions Catalyzed by Palladium(0) and Wacker- Type xidative Cyclization Catalyzed by Palladium() Zuhui Zhang enmark Group Meeting 10/21/2008 1 Part ne: Palladium(0)-Catalyzed
Studies on eck and Suzuki Reactions Catalyzed by Palladium(0) and Wacker- Type xidative Cyclization Catalyzed by Palladium() Zuhui Zhang enmark Group Meeting 10/21/2008 1 Part ne: Palladium(0)-Catalyzed
Chem 263 Nov 14, e.g.: Fill the reagents to finish the reactions (only inorganic reagents)
 hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
hem 263 ov 14, 2013 More examples: e.g.: Fill the reagents to finish the reactions (only inorganic reagents) Br 2 hv Br a 2 r 4 S 2 or swern oxidation Mg Li 0 0 MgBr Li e.g. : Fill the reagents (any reagents
Mechanism Problem. 1. NaH allyl bromide, THF N H
 Mechanism Problem 1. a allyl bromide, TF 2. 9-BB (1.2 equiv), TF, rt; ame (1.2 equiv); t-buli (2.4 equiv), TMEDA (2.4 equiv) 30 to rt; allyl bromide; 30% 2 2, aq. a, 0 C (58% yield) Mechanism Problem 9-BB
Mechanism Problem 1. a allyl bromide, TF 2. 9-BB (1.2 equiv), TF, rt; ame (1.2 equiv); t-buli (2.4 equiv), TMEDA (2.4 equiv) 30 to rt; allyl bromide; 30% 2 2, aq. a, 0 C (58% yield) Mechanism Problem 9-BB
Rhenium-Catalyzed Synthesis of Multisubstituted Aromatic Compounds via C-C Single-Bond Cleavage
 henium-catalyzed Synthesis of Multisubstituted Aromatic Compounds via C-C Single-Bond Cleavage Kuninobu, Y.; Takata,.; Kawata, A.; Takai, K. rg. Lett. ASAP Et 5 6 cat. [ebr(c) 3 (thf)] 2 5 6 Current Literature
henium-catalyzed Synthesis of Multisubstituted Aromatic Compounds via C-C Single-Bond Cleavage Kuninobu, Y.; Takata,.; Kawata, A.; Takai, K. rg. Lett. ASAP Et 5 6 cat. [ebr(c) 3 (thf)] 2 5 6 Current Literature
Chem 263 Notes March 2, 2006
 Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
Chem 263 Notes March 2, 2006 Average for the midterm is 102.5 / 150 (approx. 68%). Preparation of Aldehydes and Ketones There are several methods to prepare aldehydes and ketones. We will only deal with
CHEMISTRY 850 Exam I Saturday, October 20, 2012 NAME (Print)
 CEMISTRY 850 Exam I Saturday, ctober 20, 2012 AME (Print) Question Max Points Score 1 12 2 8 3 12 4 10 5 14 6 10 7 12 8 24 9 16 10 8 11 8 12 14 13 16 14 36 BUS 8 Exam Total 1 1) Draw the detailed arrow
CEMISTRY 850 Exam I Saturday, ctober 20, 2012 AME (Print) Question Max Points Score 1 12 2 8 3 12 4 10 5 14 6 10 7 12 8 24 9 16 10 8 11 8 12 14 13 16 14 36 BUS 8 Exam Total 1 1) Draw the detailed arrow
Catalytic Asymmetric Acyl Halide-Aldehyde Cyclocondensation Reactions of Substituted Ketenes
 Catalytic Asymmetric Acyl Halide-Aldehyde Cyclocondensation eactions of Substituted Ketenes Scott G. elson, Cheng Zhu, and Xiaoqiang Shen J. Am. Chem Soc. 2004, 126, 14-15. Michael C. Myers, Literature
Catalytic Asymmetric Acyl Halide-Aldehyde Cyclocondensation eactions of Substituted Ketenes Scott G. elson, Cheng Zhu, and Xiaoqiang Shen J. Am. Chem Soc. 2004, 126, 14-15. Michael C. Myers, Literature
New Catalysts for Reductive Amination
 ew Catalysts for eductive Amination - Ver.4 - From a carbonyl compound, -PA4 -PA2 a primary amine, a secondary amine, a tertiary amine are easily and conveniently synthesized in a one-pot synthesis ew
ew Catalysts for eductive Amination - Ver.4 - From a carbonyl compound, -PA4 -PA2 a primary amine, a secondary amine, a tertiary amine are easily and conveniently synthesized in a one-pot synthesis ew
Graphical Abstract. Tandem Epoxysilane Rearrangement/Wittig-Type Reactions Using γ- Phosphinoyl- and γ-phosphonio-α β-epoxysilane
 Graphical Abstract Tandem Epoxysilane earrangement/wittig-type eactions Using γ- Phosphinoyl- and γ-phosphonio-α β-epoxysilane Michiko Sasaki, Mai orai, Kei Takeda * Tf t BuMe Si PPh. n-buli. C SiMe Bu
Graphical Abstract Tandem Epoxysilane earrangement/wittig-type eactions Using γ- Phosphinoyl- and γ-phosphonio-α β-epoxysilane Michiko Sasaki, Mai orai, Kei Takeda * Tf t BuMe Si PPh. n-buli. C SiMe Bu
Memory of Chirality: A Strategy for Asymmetric Synthesis
 Memory of Chirality: A trategy for Asymmetric ynthesis David J. Richard eptember 14, 2005 Two Forms of Chirality Absolute (tatic) Chirality 2 - Absolute chirality - orientation of functional groups at
Memory of Chirality: A trategy for Asymmetric ynthesis David J. Richard eptember 14, 2005 Two Forms of Chirality Absolute (tatic) Chirality 2 - Absolute chirality - orientation of functional groups at
Organic Electron Donors
 rganic Electron onors Yang Li Zakarian esearch Group epartment of Chemistry and Biochemistry University of California, anta Barbara 11/15/2018 2 2 2 2 2 2 TAF1 TAE TAF2 TTF BPL utlines rganic Electron
rganic Electron onors Yang Li Zakarian esearch Group epartment of Chemistry and Biochemistry University of California, anta Barbara 11/15/2018 2 2 2 2 2 2 TAF1 TAE TAF2 TTF BPL utlines rganic Electron
Application of Two Direct C(sp 3 )-H Functionalizations for the Total Synthesis of (+)-Lactacystin
 Application of Two Direct C(sp 3 )- Functionalizations for the Total Synthesis of (+)-Lactacystin two stereoselective C(sp 3 )- functionalisations 2 C S Ac (S)-pyroglutaminol (+)-lactacystin S. Yoshioka,
Application of Two Direct C(sp 3 )- Functionalizations for the Total Synthesis of (+)-Lactacystin two stereoselective C(sp 3 )- functionalisations 2 C S Ac (S)-pyroglutaminol (+)-lactacystin S. Yoshioka,
b.p.=100 C b.p.=65 C b.p.=-25 C µ=1.69 D µ=2.0 D µ=1.3 D
 Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Alcohols I eading: Wade chapter 10, sections 10-1- 10-12 Study Problems: 10-35, 10-37, 10-38, 10-39, 10-40, 10-42, 10-43 Key Concepts and Skills: Show how to convert alkenes, alkyl halides, and and carbonyl
Chem 345 Reaction List: Chem 343 Reactions: Page 1 (You do not need to know the mechanism for the reactions in the boxes).
 Chem 345 eaction List: Chem 343 eactions: Page 1 (You do not need to know the mechanism for the reactions in the boxes). uc - optically active LG 2 optically active uc Alpha carbon (carbon attached to
Chem 345 eaction List: Chem 343 eactions: Page 1 (You do not need to know the mechanism for the reactions in the boxes). uc - optically active LG 2 optically active uc Alpha carbon (carbon attached to
Indoles. A. Fischer Indole Synthesis
 Indoles Indoles are very commonly encountered in nature and many, many individual examples which have biological implcations. Below is a very small selection of examples. C 2 2 2 Me Ac Me C 2 tryptophan
Indoles Indoles are very commonly encountered in nature and many, many individual examples which have biological implcations. Below is a very small selection of examples. C 2 2 2 Me Ac Me C 2 tryptophan
Chem 253 Problem Set 7 Due: Friday, December 3, 2004
 Chem 253 roblem Set 7 ue: Friday, ecember 3, 2004 Name TF. Starting with the provided starting material, provide a concise synthesis of. You may use any other reagents for your synthesis. It can be assumed
Chem 253 roblem Set 7 ue: Friday, ecember 3, 2004 Name TF. Starting with the provided starting material, provide a concise synthesis of. You may use any other reagents for your synthesis. It can be assumed
Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of H X 2. Addition of H OH and addition of Y X 3. Addition to allene and
 Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of X 2. Addition of and addition of Y X 3. Addition to allene and alkyne 4. Substitution at α-carbon 5. eactions via organoborane
Chapter 4 Electrophilic Addition to Carbon Carbon Multiple Bonds 1. Addition of X 2. Addition of and addition of Y X 3. Addition to allene and alkyne 4. Substitution at α-carbon 5. eactions via organoborane
ζ ε δ γ β α α β γ δ ε ζ
 hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
hem 263 Nov 17, 2016 eactions at the α-arbon The alpha carbon is the carbon adjacent to the carbonyl carbon. Beta is the next one, followed by gamma, delta, epsilon, and so on. 2 ε 2 δ 2 γ 2 2 β α The
Ch.17 Alcohols and Phenols
 Ch.17 Alcohols and Phenols C An alcohol A phenol An enol - Me: wood alcohol: made from wood - industrial preparation of methanol: C + 2 2 400 o C Zinc oxide/chromia C 3 - Ethanol: fermentation of grains
Ch.17 Alcohols and Phenols C An alcohol A phenol An enol - Me: wood alcohol: made from wood - industrial preparation of methanol: C + 2 2 400 o C Zinc oxide/chromia C 3 - Ethanol: fermentation of grains
R N R N R N. primary secondary tertiary
 Chapter 19 Amines omenclature o assification of amines Amines are classified as 1, 2, or 3 based on how many R groups are attached to the nitrogen R R R R R R primary secondary tertiary When there are
Chapter 19 Amines omenclature o assification of amines Amines are classified as 1, 2, or 3 based on how many R groups are attached to the nitrogen R R R R R R primary secondary tertiary When there are
Suggested solutions for Chapter 30
 s for Chapter 30 30 PRBLEM 1 uggest a mechanism for this synthesis of a tricyclic aromatic heterocycle. 2 Cl base A simple exercise in the synthesis of a pyridine fused to a pyrrole (or an indole with
s for Chapter 30 30 PRBLEM 1 uggest a mechanism for this synthesis of a tricyclic aromatic heterocycle. 2 Cl base A simple exercise in the synthesis of a pyridine fused to a pyrrole (or an indole with
Chapter 17: Alcohols and Phenols
 hapter 17: Alcohols and Phenols sp 3 alcohol phenol (aromatic alcohol) pka~ 16-18 pka~ 10 Alcohols contain an group connected to a saturated carbon (sp 3 ) Phenols contain an group connected to a carbon
hapter 17: Alcohols and Phenols sp 3 alcohol phenol (aromatic alcohol) pka~ 16-18 pka~ 10 Alcohols contain an group connected to a saturated carbon (sp 3 ) Phenols contain an group connected to a carbon
ORGANIC - CLUTCH CH ALDEHYDES AND KETONES: NUCLEOPHILIC ADDITION
 !! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
!! www.clutchprep.com CONCEPT: ALDEHYDE NOMENCLATURE Replace the suffix of the alkane -e with the suffix On the parent chain, the carbonyl is always terminal, and receive a location As substituents, they
Facile preparation of α-amino ketones from oxidative ring-opening of aziridines by pyridine N-oxide
 Facile preparation of α-amino ketones from oxidative ring-opening of aziridines by pyridine -oxide rg. Biomol. Chem., 2007, 5, 3428 Luo, Z.-B.; Wu, J.-Y.; ou, X.-L.; Dai, L.-X. Ts toluene Ts 80 o C John
Facile preparation of α-amino ketones from oxidative ring-opening of aziridines by pyridine -oxide rg. Biomol. Chem., 2007, 5, 3428 Luo, Z.-B.; Wu, J.-Y.; ou, X.-L.; Dai, L.-X. Ts toluene Ts 80 o C John
Lecture Notes Chemistry Mukund P. Sibi Lecture 36 Synthesis of Amines
 Lecture otes hemistry 42-2008 Mukund P. Sibi Synthesis of Amines Amines can be prepared from a variety of starting materials. All of these methods involve functional group transformations. The main methods
Lecture otes hemistry 42-2008 Mukund P. Sibi Synthesis of Amines Amines can be prepared from a variety of starting materials. All of these methods involve functional group transformations. The main methods
