A critical investigation of the Tanford-Kirkwood scheme by means of Monte Carlo simulations
|
|
|
- Amice Mason
- 5 years ago
- Views:
Transcription
1 A critical investigation of the Tanford-Kirkwood scheme by means of Monte Carlo simulations FERNANDO LUÍS B. DA SILVA, 1,3 BO JÖNSSON, 1 AND ROBERT PENFOLD 2 1 Theoretical Chemistry, Chemical Centre, Lund University, S Lund, Sweden 2 Institute of Food Research, Norwich Research Park, Colney, Norwich NR4 7UA, United Kindgom 3 Group of Biomolecular Physics, Department of Physics, Faculty of Science, UNESP/Bauru, Bauru, São Paulo, Brazil (RECEIVED OCTOBER 10, 2000; FINAL REVISION MARCH 15, 2001; ACCEPTED APRIL 23, 2001) Abstract Monte Carlo simulations are used to assess the adequacy of the Tanford-Kirkwood prescription for electrostatic interactions in macromolecules. Within a continuum dielectric framework, the approach accurately describes salt screening of electrostatic interactions for moderately charged systems consistent with common proteins at physiological conditions. The limitations of the Debye-Hückel theory, which forms the statistical mechanical basis for the Tanford-Kirkwood result, become apparent for highly charged systems. It is shown, both by an analysis of the Debye-Hückel theory and by numerical simulations, that the difference in dielectric permittivity between macromolecule and surrounding solvent does not play a significant role for salt effects if the macromolecule is highly charged. By comparison to experimental data, the continuum dielectric model (combined with either an approximate effective Hamiltonian as in the Tanford-Kirkwood treatment or with exact Monte Carlo simulations) satisfactorily predicts the effects of charge mutation on metal ion binding constants, but only if the macromolecule and solvent are assigned the same or similar permittivities. Keywords: Electrostatic interactions; Debye-Hückel; low dielectric cavity; computer simulations; continuum model; proteins model Reprint requests to: Fernando Luís B. da Silva, Theoretical Chemistry, Chemical Centre, Lund University, POB 124, S Lund, Sweden; fernando@signe.teokem.lu.se; fax: Article and publication are at /ps It seems reasonable to believe that the binding of a charged ligand to a protein or some other macromolecule will depend, at least in part, on purely electrostatic interactions. All chemically interesting interactions for molecular systems ultimately stem from an electrostatic origin, of course, as only Coulombic potential terms enter the Schrödinger equation. Very often, however, to render a many-body problem tractable, an effective Hamiltonian is introduced where large classes of microscopic interactions are replaced by a suitable average and not accounted for explicitly. Partitioning an effective interaction into an electrostatic (ionic) term, which dominates at long range, and other terms of covalent character with much shorter range is, then, a useful heuristic and computational device. Consider, for example, the titration of a residue side chain in a protein. The free energy change for proton transfer will be broadly similar to that of the corresponding process in the isolated amino acid, but further augmented by an electrostatic term that depends on the overall charge and charge distribution of the protein. In such cases, the binding constant shift arising solely from electrostatic interactions can amount to several pk units. The role of electrostatic interactions in protein structure and function has long attracted interest. In one of the first theoretical attempts to deal with the problem, Tanford and Kirkwood assumed that the long-range part could be described within a dielectric continuum model (Tanford and Kirkwood 1957). This effective Hamiltonian model has been invoked extensively to study the interaction between charged ligands and proteins, membranes, and other macromolecules (Hill 1955, 1956; Warwicker and Watson 1982; Warshel et al. 1984; Harvey 1986; Bashford et al. 1988) (the reference list given is by no means exhaustive). The original Tanford-Kirkwood (TK) scheme considers the macromolecule as a sphere of low dielectric permittivity Protein Science (2001), 10: Published by Cold Spring Harbor Laboratory Press. Copyright 2001 The Protein Society 1415
2 da Silva et al. different from the surrounding (aqueous) solution of high permittivity. With respect to the rigid macromolecular reference frame supporting fixed charged groups, the salt ions and other charged ligands are supposed to be in relative motion throughout the solvent medium. Tanford and Kirkwood avoided the formidable statistical thermodynamics problem this motion implies by again appealing to the effective interaction construct, eliminating explicit reference to the mobile particles and introducing the Debye-Hückel potential. Despite the evident success of the TK result, it suffers from two significant limitations: (1) closed form analytical solutions are only available in simple geometric configurations (usually spherical); and (2) nonlinear effects and explicit ion ion correlations are ignored. At the cost of computational simplicity, the first constraint has been removed in a number of calculations by several groups (Warwicker and Watson 1982; Bashford et al. 1988; Bashford and Karplus 1990; Beroza et al. 1991; Juffer et al. 1999). The second limitation has only been partially tested by replacing the DH equation with the nonlinear Poisson-Boltzmann equation (Fushiki et al. 1991), though the description remains at the mean-field level ignoring ion ion correlations. Monte Carlo (MC) simulations have addressed the full statistical mechanical problem but then only for a model with a uniform dielectric permittivity (Fushiki et al. 1991; Kesvatera et al. 1994; Penfold et al. 1998). An additional simplification of the TK prescription, which may prove unrealistic at low ionic strength, lies in the assumption of infinitesimally small macromolecule concentration (Linse et al. 1995). In NMR studies of proteins the typical protein concentration is around 1 mm, whereas the concentration of accompanying counterions can be an order of magnitude larger or more. The original application of the TK scheme dealt with titrating groups in proteins and how the pk a for an ionizable amino acid is affected by the rest of the protein. The quantity calculated is the shift in pk a between the free amino acid and the same residue in the protein. It became clear at an early stage that the scheme invoking a low dielectric interior for the protein did not properly reflect the difference in solvation energy between the free amino acid and when it is part of a protein (Tanford and Roxby 1972; Shire et al. 1974; Warshel et al. 1984; Papazyan and Warshel 1997). It seems, however, as there is no consensus of how to best tackle the problem (King et al. 1991; Warshel and Åqvist 1991; Antonsiewicz et al. 1994a,b; Simonson and Perahia 1995; Simonson and Brooks 1996; Löffler et al. 1997; Sham et al. 1997; Baptista and Soares, unpubl.) without invoking additional empirical parameters describing solvation and water penetration (Dwyer et al. 2000). A simple alternative is to assume a uniform dielectric response throughout the solution, including the macromolecule, equal to that of pure water. For titrating groups at or close to the protein surface this latter ansatz seems to give better agreement with experiment (Spassov and Bashford 1998; Kesvatera et al. 1999, 2001). Another application of the TK scheme is to the binding of a charged ligand to a protein at different conditions, that is, mutation of charged amino acids and/or addition of inert salt. The pk for such a process does not invoke the problematic solvation term mentioned above, and we will use the binding of divalent cations to a negatively charged protein as a model throughout this work. By using MC simulation to resolve the statistical mechanical problem, we will present a critical investigation of the dielectric continuum model originally studied by Tanford and Kirkwood. The simulations will be performed for a model including the dielectric discontinuity and the mobile species in the solution will comprise of salt particles as well as additional counterions in order to maintain electroneutrality. Model systems We investigate a model system mimicking a globular protein with a net charge, which is supposed to bind divalent ions. It could, for example, be a caricature of calbindin D 9k (Linse et al. 1988), and consists of a rigid sphere with radius R p and a centrally located charge of valency Z. A surrounding electrolyte solution, including neutralizing counterions, is described by the so-called restricted primitive model (Levesque et al. 1986). Each uniformly charged mobile ion k with valency z k is treated as a hard sphere of radius R k ; the solvent degrees of freedom are characterized only by an average dielectric permittivity s that is also assigned to the ionic interior. Internal structural details of the biopolymer are neglected so that the protein interior is represented by a concentric spherical cavity of radius R d (referred to as the dielectric radius) with a continuum dielectric permittivity p < s ). Typically, R d <R p though this is not a constraint of the model. In any case, max{r d,r p } defines an exclusion boundary impenetrable by mobile salt particles and counterions. The entire system is placed in a spherical cell of radius R c determined by the protein concentration (see Fig. 1). Although the approach is quite general, the model parameters are chosen here to roughly suggest the calcium binding protein calbindin. Calbindin D 9k is a small globular protein with 722 atoms (46 charged for the wild-type apo form giving a net charge of 8) that binds two calcium ions Ca +2 with high affinity (Linse et al. 1988) at sites located about 10.34Å and 11.08Å from the protein center of mass with cartesian coordinates (7.340, 7.114, 1.607) and (10.503, 3.509, 0.364), respectively, in Å units. The binding properties of calbindin have been studied in detail both experimentally and theoretically (Linse et al. 1988, 1991; Svensson et al. 1991). Effects arising from mutations of charged residues as well as varying salt and protein concentrations have been investigated (Linse et al. 1995). The agreement 1416 Protein Science, vol. 10
3 Critical investigation of Tanford-Kirkwood scheme u el r ij = z iz j e p 1 r ij 1 R d n=0 u el r ij = z iz j e s 1 r ij n max s p P n cos ij s + p nn + 1 r ir j R d n max R d s p P n cos ij n=1 s 1 + 1n + p R d 2 r i r j n+1, n, r i R d r j R d, (2) r i R d r j R d, (3) Fig. 1. Schematic representation of the model systems (see also Table 1). A spherical protein in a spherical cell with radii R p and R C, respectively. The protein interior with a low dielectric permittivity is shown as a shaded region of radius R d < R p. A central charge of valency Z and two binding sites marked as black dots. between experiments and MC simulations for this protein is excellent provided that the protein is treated as a medium of high dielectric permittivity (Svensson et al. 1990, 1991). Poisson-Boltzmann and DH calculations using a low dielectric response for the protein predict binding constant shifts that are qualitatively different from those observed by experiment (Penfold et al. 1998; Spassov and Bashford 1998; Kesvatera et al. 2001). Classical techniques suggest a straightforward development of the electrostatic potential at any point (because of a fixed charge distribution and a dielectric discontinuity) in an orthogonal polynomial basis (Böttcher 1973), that is, the multipole expansion. This power series is not ideally suited for computer simulations, though, as the number of terms required for satisfactory convergence increases sharply when sources lie very close to the dielectric interface. Nevertheless, if the charges are more than 0.5Å from this boundary, it is feasible to achieve a precision of about 10 10, with an acceptable number of terms, that is, <1000. Given the elementary charge e and the vacuum permittivity 0, two ionised sites of valency z i,z j (either fixed or mobile) located at radial positions r i,r j and subtending an angle ij, contribute an electrostatic potential energy given by, u el r ij = z i z j e 2 1 r ij n max s p P n cos ij 2 0 s + p 1 r i n=0 2 s n p n r j r i n, r i R d r j R d, (1) where r 2 ij r i 2 + r j 2 2r i r j cos ij if the spatial separation, P n is the Legendre polynomial of order n, and n max is the maximum number of terms included in the sum. The first term in Equations 1 3 is the direct Coulomb interaction, whereas the second describes the reaction field accounting for induced polarization charge at the dielectric discontinuity. In the absence of a dielectric inhomogeneity ( p s ), it is easy to verify that Equations 1 3 reduce to the ordinary Coulomb potential, u el r ij = z iz j e s r ij, (4) with the characteristic divergence at small separation (r ij 0). On the other hand, for a nonuniform dielectric response, the reaction field term converges in this limit to yield a nonvanishing contribution, that is, the so-called self-image energy. Together with electrostatic interactions, the simulation Hamiltonian also includes a short-ranged hard-core overlap restriction among the mobile ions (thereby preventing Coulomb collapse in the configurational Markov chain), u hs r ij =, r ij R i + R j 0, otherwise, (5) as well as a one-body external field that accounts for the protein excluded volume and imposes the cell boundary constraint acting as a hard wall, v ex r i = 0, R i + maxr p, R d r i R c, otherwise. (6)
4 da Silva et al. The full configurational energy of the system then becomes, Ur k = i=1 N c +N s v ex r i + 1 N 2 i=1 N u el r ij + u hs r ij, (7) j=1 where N N c + N s + N p is the total number of charges comprising N c mobile counterions, N s mobile added salt ions, and N p fixed protein charges. The calbindin-like model, introduced above, with a central net charge and two binding sites located at the usual positions, will be denoted SC8 (Spherical model with a Central charge of 8). Three other variations of this model, listed in Table 1, have been considered. A mutation that neutralizes a charged amino acid of the wild type will, in this model, simply lead to a change of the protein net charge, that is, we will refer to this mutant as SC7. Slightly more realistic is the Spherical model with a central charge of 7 and a Peripheral charge of 1 close to the protein surface, referred to as SP8 (see Table 1 for further details). One purpose of this work is to investigate the limitations of the DH approximation in a biophysical context. We have, therefore, also included a model protein that is otherwise identical to SC8 but with a variable central charge (ranging from 4 to 24). The most highly charged model system with Z 24, SC24, has been studied in greater detail. Results and Discussion Gauging the validity range of the linear DH approximation is the primary objective of this work. In addition, the extent to which the ion binding characteristics of a protein is affected by the presence of a low dielectric interior region is also of interest. Not only does this notion underlie the original TK description, but it has also become a staple modeling supposition widely implemented in a number of generalized program packages used to study biomolecular phenomena (Warwicker and Watson 1982; Bashford and Gerwert 1992; Honig and Nicholls 1995). Table 1. Summary of the main model systems Model Z 1 position Z 2 position N c SC8 8 (0,0,0) 8 SC24 24 (0,0,0) 24 SC7 7 (0,0,0) 7 SP8 7 (0,0,0) 1 (13.5,0,0) 8 The coordinates (in Å units) of the fixed charges Z k are listed along with the number N c of positive counterions added to the system to maintain global electroneutrality. All four models have two divalent cation binding sites located at coordinates (7.340, 7.114, 1.607) and (10.503, 3.509, 0.364), respectively. Fig. 2. The reduced excess chemical potential for a free divalent cation in a20m protein solution is indicated as a function of monovalent salt concentration. The (superimposed) solid lines refer to the SC8 model and the broken lines to the SC24 model. Lines marked by solid circles are calculated with a dielectric boundary radius R d 14Å; lines without symbols refer to R d 0Å. The thin broken line shows the excess chemical potential of a divalent ion in a bulk salt solution. The binding constant shift pk is related to the free energy cost for the protein to incorporate a mobile ion from the solution, see Equations 9 and 10. To calculate pk, therefore, estimates of ex for both free and bound ions are required. The free ion contribution F ex is usually minor, but can become significant in some circumstances. Figure 2 illustrates the dependence of F ex on salt concentration c s for a protein solution at experimental conditions (c p 0.02 mm) typically encountered. A moderately charged protein (model SC8) has only a small effect on the free ion chemical potential at low c s, whereas it is efficiently screened at higher salt concentrations where F ex closely follows the ionic excess chemical potential of a bulk electrolyte solution. Moreover, without a very substantial net protein charge, F ex is entirely insensitive to the dielectric properties of the protein interior, as Figure 2 demonstrates, where any shifts on exchanging a solvent filled cavity for a vacuum are below the statistical noise level, regardless of salt content. On the other hand, a highly charged protein (model SC24) dramatically lowers F ex by several kt in dilute electrolyte. Added salt will screen the strong electrostatic field from the protein, however, effecting an increase in the excess chemical potential with increasing c s, contrary to the behaviour in a bulk electrolyte solution at least up to physiological concentrations. A dielectric discontinuity now contributes a small but noticeable effect, amounting to a few tenths of kt, as displayed in Figure 2. To complement the picture, Figure 3 demonstrates how F ex varies as a function of protein concentration c p at a constant level of salt. At low salt content, a non-negligible effect of increasing c p is observed for model SC8, but it is entirely screened out at c s 100 mm. This effect is, of 1418 Protein Science, vol. 10
5 Critical investigation of Tanford-Kirkwood scheme Fig. 3. The reduced excess chemical potential for a divalent cation as a function of protein concentration with a homogeneous dielectric response (dielectric radius R d 0). The solid lines refer to the SC8 model and the broken lines to the SC24 model. Lines marked by circles denote a monovalent simple electrolyte concentration of c s 100 mm; lines without symbols are for c s 1 mm. The arrows indicate the value of F ex in the corresponding bulk salt solutions. The upper arrow indicates the system at c s 1mM( F ex 0.156). course, more pronounced for a highly charged protein, where the curve for c s 1 mm even exhibits non-monotonic behaviour. For a protein/salt solution, Figures 2 and 3 clearly demonstrate that all charged species present will contribute to the Coulomb shielding. Should the protein be only weakly charged, then the screening effects can be described, at least qualitatively, by the inverse length within the DH approximation. A highly charged protein, however, requires a more sophisticated theoretical treatment that can account for both nonlinear and ion ion correlation terms. On the other hand, the chemical potential of a bound ion is only marginally affected by changes in protein concentration. To mimic the calcium binding property of calbindin D 9k, the protein model SC8 binds two divalent ions. The stoichiometric ion binding constant varies with the amount of salt present and provides a suitable test of theoretical predictions for the shift pk with respect to c s. Figure 4a shows a very significant shift of 3 4 pk units on increasing c s from 1 mm (reference state) to 500 mm. The simple TK calculation performs very satisfactorily in predicting pk, disagreeing with MC simulation results by less than a few tenths of a pk unit. More significantly, it appears that the presence of a low dielectric protein interior has practically no influence on pk. With a dielectric radius R d 12Å, pk is 3.5, whereas a shift of 3.8 is obtained within a uniform dielectric model (see Fig. 4b). Increasing R d further to 18Å reduces the shift down to 3.2 but because the protein radius is 14Å this corresponds to suggesting that the contact water layer, about 4Å thick, is both impenetrable to salt ions and unpolarizable with a relative dielectric permittivity of unity. Experimental studies have measured pk 4.6 for aqueous solutions of calbindin D 9k on raising the salt concentration from 1 mm to 500 mm (Kesvatera et al. 1994). Although model SC8, with the net charge contracted to a single point and no higher electric moments, is surely too crude a description for any real protein, the salt dependence of cation binding constant shifts for a model with eight randomly placed negative charges is almost indistinguishable from the results obtained with model SC8, as illustrated in Figure 4c. For the highly charged model SC24, the corresponding results are shown in Figure 5a. In this case, the TK scheme breaks down regardless of the dielectric permittivity assigned to the protein interior. The discrepancy between calculated and simulated shifts can reach 5 pk units, implying an error five orders of magnitude in the binding constant! Obviously, the linear approximation is not capable of sensibly accounting for strong coupling and ion ion correlations. In defence of the TK analysis, however, a protein as highly charged as SC24 is rare in biological systems. Because the binding constant shift depends on the difference between B ex and F ex, it is possible that the failure of the TK approximation is related to only one of these terms. Both are incorrectly predicted by the linear theory, it turns out, as demonstrated in Figure 5b and might have been guessed on the basis of Figure 2. The error arises from the neglect of both nonlinear effects and ion ion correlations. The effect of different protein charges is explored in more detail in Figure 6. For moderately charged proteins (Z < 12), the analytical theory correctly predicts the salt pk shifts. On further increasing the protein charge, however, nonlinear effects and ion ion correlations progressively appear that result in a gradual breakdown of the TK scheme. This occurs at approximately the same protein charge irrespective of whether a uniform or inhomogeneous dielectric continuum model is used. Thus, Figure 6 serves a practical purpose by indicating the validity range of the TK prescription. In case the mean field is not small everywhere compared with the average thermal energy, the nonlinear part of the error can be recovered by using the Poisson-Boltzmann equation instead of the DH equation. At equilibrium, the motion of mobile salt and counterion charges is such that the average distribution of particles will follow the exponential Boltzmann law with the potential of mean force (pmf). Ordinarily, with a uniform dielectric response, say, where the protein surface charge density is small and salt concentrations/valencies are low, the pmf is well approximated by the mean electro- static potential. On the other hand, with a dielectric discontinuity the mobile ions will see strong correlations, at least with their own images, just as the fixed charges do. This suggests that the pmf could be very different from the electrostatic potential, even if all the mean field conditions appropriate to a primitive dielectric
6 da Silva et al. Fig. 4. (a) Total shift in ion binding constant pk, owing to solution ionic strength, is plotted as a function of salt concentration c s for the SC8 model. A reference state at c s 1 mm is chosen and the protein is allowed to bind two divalent cations. Results from MC simulations and TK calculations are compared as solid and broken lines, respectively. Lines marked by solid circles correspond to a dielectric boundary radius R d 18Å and those marked by open squares denote R d 12Å; unmarked lines refer to a homogeneous dielectric response (R d = 0Å). (b) The shift of pk arising from the presence of an interior vacuum pk pk (R d 0Å) pk (R d 14Å) is demonstrated as a function of salt concentration c s. In the solvent region, the relative dielectric permittivity is s Solid line shows MC data and broken line indicates TK results. (c) Total shift in ion binding constant pk, owing to solution ionic strength, is plotted as a function of salt concentration c s for a spherical protein model with eight randomly placed negative charges. Results from MC simulations and TK calculations are compared as solid line and solid circles, respectively. The protein dielectric boundary radius was R d 12Å in all calculations. model are satisfied. That is, when setting up the equations describing an inhomogeneous dielectric model, it is not generally sufficient to write the mobile ion distributions in terms of the electrostatic potentials, even though these do contain effects from the surface polarization induced by the fixed charges. There is the possibility of a sizeable correlation term (i.e. the self image correlation) that should be included, or its neglect needs to be justified. The effect of this correlation, a desolvation penalty, would be to remove mobile ions from the neighborhood of the dielectric boundary. Turning now to the consequences of a dielectric boundary, even for weakly charged proteins, enlarging the interior region of low permittivity has only a marginal effect on the salt shift of the binding constant as depicted in Figure 7 for the SC8 model. Although both binding sites remain in the highly polarizable region, a small reduction in pk is observed on expanding the dielectric radius R d from zero to about 10Å. Further increasing R d to incorporate the binding sites within the dielectric boundary has no effect at all on the calculated shift. When R d extends beyond the protein radius (R p 14Å) a decrease of pk is again seen. The latter is a trivial effect caused primarily by salt exclusion, as mobile ions are very strongly repelled from the low dielectric region. For the highly charged model SC24, see Figure 8, MC data is almost constant in this scale and TK com Protein Science, vol. 10
7 Critical investigation of Tanford-Kirkwood scheme Fig. 6. Total shift in ion binding constant pk, owing to solution ionic strength, is plotted as a function of the protein net charge (Z). A reference state at salt concentration c s 1 mm is chosen and the final salt concentration is 500 mm. Results from MC simulations and TK calculations are compared as solid and broken lines, respectively. Lines marked by solid circles correspond to a dielectric boundary radius R d 12Å; unmarked lines refer to a homogeneous dielectric response (R d 0Å). Fig. 5. (a) Total shift in ion binding constant pk, owing to solution ionic strength, is plotted as a function of salt concentration c s for the SC24 model. Other details are as for Fig. 4A. (b) The difference of excess chemical potentials B ex B ex (c s ) B ex (c s 1 mm) for a divalent cation in a bulk monovalent electrolyte solution is plotted as a function of salt concentration c s. pletely overpredicts the shifts, although it behaves in a qualitatively similar manner to TK results for model SC8. In view of these results, it can be concluded that (1) the shifts in binding equilibrium attributable to changes in aqueous salt content are well described by DH theory, provided the protein is not too highly charged; and (2) the existence of a weakly polarizable region within the protein has no significant effect on the salt concentration dependence of ion binding, even if the binding sites are located within the low dielectric boundary. To assess the impact on ligand binding equilibria of perturbations in protein charge and charge distribution (realized experimentally by site directed mutagenesis of ionizable residues), a closely related model is also considered. By suggesting the replacement of, say, a carboxylic acid group by the corresponding amine, model SC7 represents a mutant protein obtained in either of two ways. First, the SC7 variant of model SC8 is formed by simply increasing the valency of the central charge from 8 to 7. Second, the peripheral charge of model SP8 is neutralized again decreasing the overall charge to 7 units. Figure 9 shows the change in pk of divalent cation binding attributable to both these mutations as a function of dielectric radius. In both cases, the shift pk is very nearly independent of R d as long as the binding sites are in the high dielectric region. Moreover, the calculated value of pk is also in reasonable agreement with experimental observations (Kesvatera et al. 1994) of calbindin, where a single charge mutation typically alters the calcium binding constant with pk units at Fig. 7. Total shift in ion binding constant pk, owing to solution ionic strength, is plotted as a function of the dielectric radius R d for the SC8 model. A reference state at salt concentration c s 1 mm is chosen and the protein (of radius R p 14Å) is allowed to bind two divalent cations. Results from MC simulations and TK calculations are compared as solid and broken lines, respectively
8 da Silva et al. entropy lost on binding, but the ligand is exposed to the bare protein charge without dielectric shielding and therefore incurs a significant electrostatic energy. Although this desolvation cost also appears in the pk shift because of variation in salt content, the energy term exactly cancels when computing the excess chemical potential difference, compare with Equation 9, as there is no change in overall protein charge. Fig 8. Total shift in ion binding constant pk, owing to solution ionic strength, is plotted as a function of the dielectric radius R d for the SC24 model. A reference state at salt concentration c s 1 mm is chosen and the protein (of radius Rp 14Å) is allowed to bind two divalent cations. The final salt concentration is 500 mm. Results from MC simulations and TK calculations are compared as open and solid symbols, respectively, joined by straight line segments to guide the eye. low salt and protein concentration (Svensson et al. 1990). Once the low dielectric cavity incorporates the binding sites and the point of mutation, however, pk climbs steeply and more so for the SP8 variant. When R d matches the protein radius (R d 14Å), clearly unphysical binding constant shift around 25 pk units is apparent. Because the simulation results are faithfully tracked by the TK predictions over the full range of R d, this failure must rest with inadequacies of the model rather than theoretical approximations. The large pk increase arises from the loss of solvation free energy as the charged ligand passes from a polarizable medium to vacuum. Not only is configurational Fig. 9. Total shift in ion binding constant pk, owing to a single charge mutation, is plotted as a function of the dielectric radius R d for the SC8 and SP8 models. The salt concentration is c s 1 mm and the protein concentration is c p 0.02 mm. MC results are shown with solid (SC8) and broken (SP8) lines; TK data are denoted by solid circles. Conclusion The TK prescription based on the DH theory is an excellent approximation for studies of the binding of charged ligands to macromolecules. For sufficiently charged systems the linearization in the DH equation is unjustified and one must invoke a more accurate theory. Whether or not the nonlinear Poisson-Boltzmann theory is sufficient will depend on the system. For example, the description of highly charged aggregates, such as DNA in the presence of multivalent ions, where ion ion correlations become important requires a correlated theory or numerical simulations (Guldbrand et al. 1986; Mel nikov et al. 1999). The TK theory also becomes less reliable at high protein and low salt concentration, mainly because it approximates the excess chemical potential of the ligand with its value in the corresponding bulk salt solution. Typically this will happen when N c c p c s. The relative permittivity of the macromolecule is usually assumed to be much smaller than the surrounding aqueous solvent. For a highly charged macromolecule this is immaterial, when studying effects caused by salt concentration, as the leading term in the free energy difference is proportional to the net charge and the dielectric effect only enters in higher order terms. This can be demonstrated from the TK theory but is also found in MC simulations. In a more general sense, any type of protein/solvent model that proposes an effective interaction potential by partitioning space into a finite number of dielectric continuna with different permittivities can be traced back to the intuitive design of Tanford and Kirkwood. Of course, the original TK analysis also simplifies the geometry of the ensuing electrostatic problem, but the essential idea of dielectrically distinct background regions remains common to more sophisticated models. The major weakness of applying such a scheme is the lack of an a priori prescription for locating the intervening dielectric boundaries and a method for estimating the dielectric response of the macromolecule. Charged ligand binding equilibria are highly sensitive to this aspect of electrostatic interactions, hence it may not always be possible to generate biologically relevant predictions without an independent method to sensibly determine these parameters. This is most clearly seen when studying the effect of charge mutations on the ligand binding, where models invoking a low dielectric response for the protein in many cases fail completely Protein Science, vol. 10
9 Critical investigation of Tanford-Kirkwood scheme Materials and methods Numerical simulations Although the protein remains fixed at the center of the cell, the surrounding counterions and monovalent salt ions sample equilibrium configurations from the canonical (NVT) ensemble generated by the standard Metropolis MC algorithm (Metropolis et al. 1953). At high bulk protein concentrations, the cell radius is determined straightforwardly to obtain the appropriate volume per macromolecule, whereas for low protein and high salt concentrations the number of mobile particles required in the simulation rapidly becomes prohibitive. Tests have indicated, though, that the configuration-dependent part of the ligand chemical potential at the binding sites is rather insensitive to the cell size and that salt pairs are typically sufficient to ensure convergence on the sizeconsistent limit. The excess chemical potential of the free calcium ion F ex is, however, greatly influenced by the macromolecular electrostatic field so that a naive reduction of the cell radius R c would yield an erroneous result. Nevertheless, in low concentration, the protein will have only a marginal influence on F ex, particularly at high salt concentration where the electrostatic interactions are efficiently screened. Under these conditions it becomes more appropriate to calculate F ex from a bulk salt solution at the corresponding concentration. The number of counterions, N c, was set to comply with overall electroneutrality, the temperature was fixed at T K and the solvent dielectric constant was taken to be s The protein radius was chosen to be equal to R p 14Å whereas each mobile ion was assigned a common radius of R k R s 2Å for all k. The low dielectric response of the protein was explored in the extreme limit by introducing a spherical (radius R d ) vacuum cavity ( p 1) centered on the cell origin. Commonly, the dielectric permittivity of a protein is taken to be comparable with that of a pure hydrocarbon phase, though there is no clear consensus on this issue (Warshel and Aqvist 1991; Antonsiewicz et al. 1994b; Simonson and Perahia 1995; Simonoson and Brooks 1996; Löffler et al. 1997; Papazyan and Washel 1997; Warshel and Papazyan 1998; Sham et al. 1998; Warwicker 1999; Baptista and Soares, unpubl.). To focus on qualitative physical mechanisms rather than quantitative prediction, we adopt the vacuum value here to exaggerate any effects arising from the dielectric discontinuity, although the consequences of high protein permittivity are examined in the following section. Note that the penetration of a real ion into a region of weak dielectric response is associated with a high energy, thereby implying a vanishingly low probability for the process. This is accounted for in the simulations by treating the dielectric discontinuity as an impenetrable boundary to the mobile ions. Normally we will set R p > R d so that the protein excluded volume overrides the dielectric depletion mechanism, though we admit the general condition R s + max{r p,r d } defining the mobile ion exclusion sphere, see Equation 5. Values of R d in the range of 0 18Å were studied, with the salt concentration c s between 1 mm and 500 mm. For both equilibration and production runs, 10 5 trial configurations per particle were generated. Direct evaluation of the explicit TK equations (Tanford and Kirkwood 1957) was also carried out for the same model systems, with the limitation that all the protein charges and binding sites must lie inside the dielectric cavity. Moreover, these calculations were confirmed by numerical finite differece solutions obtained using the MEADpackage (Bashford and Gerwert 1992). Free energy calculations When an ion binds to a protein, the changes in the free energy can be separated into two parts, G = G el + G rest, (8) where the first term represents the electrostatic interactions and the second accounts for all the remaining contributions (structural changes, etc.). If G rest is assumed to be independent of the salt concentration or charge mutations, then the logarithmic change in the stoichiometric binding constant is given by pk = G el G el ref ln10, (9) where, for all the cases and models examined here, G el ref identifies a reference electrostatic free energy for the system at c s 1 mm with respect to a standard state and 1/kT (k is the Boltzmann constant). In terms of the excess chemical potentials of protein (P), protein + ligand (P + L) and free ions (F), the electrostatic free energy can be expressed as G el ex = P+L ex P ex F = ex ex B F. (10) where µ B ex is the excess chemical potential of the bound ion(ligand). If several ligands are involved in the binding process, than µ F ex should, besides the excess chemical potential of each bound ligand, also include the interaction between the ligands. Similarly, F ex should contain the excess chemical potential of the free ligands. Widom s (1963) test particle insertion method is an effective way to evaluate chemical potentials in continuum simulations. When applying the Widom technique to Coulomb fluids, where the explicit particle density is not too high, it should be realized that insertion of a single ion means that the total system becomes nonelectroneutral, possibly introducing spurious systematic errors. Most of this error can be corrected for by rescaling some or all of the charges in the system in such a way that the total system including the ghost particle becomes electroneutral. Such a scheme, referred to as the modified Widom technique, has been implemented and described in detail elsewhere (Svensson and Woodward 1988). The chemical potential of a free calcium ion is obtained by random particle insertions over the entire system, whereas B ex is calculated from an insertion directly into the binding site. This is done at every fifth particle move throughout the simulation to obtain a typical statistical error on the excess chemical potential around kt. TK analysis Instead of directly computing the excess chemical potential at a binding site as an ensemble average, it is conveniently expressed in terms of the total electrostatic free energy change on detaching a ligand from the protein, B ex = G el Protein + Ligand G el Protein. (11) Consistent with the linear response analysis of Tanford and Kirkwood, the chemical potential for a free charged hard sphere ion with valency z and radius R s is approximated using the DH theory of strong electrolytes,
10 da Silva et al. z 2 e 2 ex F = 8 0 s kt1 + 2R s, (12) where is the inverse DH screening length, which is proportional to the square root of the salt concentration. Following Tanford and Kirkwood, the electrostatic free energy of the neutral macromolecule is set to zero, and G el for a protein containing N p fixed charges becomes with, G el = e2 Np Np 8 0 i=1 z i z j A ij B ij C ij, (13) j=1 A ij = 1 p r ij, (14) B ij = 1 p R d n=0 s p s + p n n + 1 r n ir j R d 2 P n cos ij, s 1 x C ij = R p + R s 1 + x + 2n + 1 x2 n=1 2n 1 s r n + 1 s + n p 2 i r n j P n cos ij K n+1x R d R p + R s R p + R s 2 (15) K n 1 x + n s p n + 1 s + n p x2 2n+1. (16) 4n 2 1 The solution ionic strength enters only through the variable x (R p +R s ) and the auxiliary functions, n K n x = p=0 n p 2n p 2xp p!, (17) described in an early paper by Kirkwood (1934). Notwithstanding the thorough discussion of results in the original work by Tanford and Kirkwood (1957), it is instructive here to consider the three terms of Equation 13 in some detail. The direct Coulombic interaction between the charges of the protein is accounted for by the A ij s and is independent of salt concentration, whereas the B ij s are just the reaction field components (see Equation 2) contributing the effects from induced polarization charge at the dielectric boundary between protein and solvent. In the case of uniform dielectric permittivity, that is, s p, all the B ij s will vanish. The C ij terms describe the effect of mobile counterions and added salt. Now suppose a ligand of valency z l binds to the protein at radial position r l subtending angles kl with all other charges k, and consider the shift in its excess chemical potential on altering the salt concentration from x 1 to x 2, so that, B ex = G el x 2 G el x 1. (18) It is easy to see that only the C ij terms contribute to ex B. Writing the net charge of the apo protein Z= Np i=1 z i and retaining only the leading terms of the multipole expansion (16) yield, x G el x = + z 8 0 s R p + R s Z l 2 Z x N z l r p l + R p + R s 2z l r l + 2 i=1 z i r i cos ilgx. e 2 (19) With the additional geometric simplification R p + R s =R d, then g(x) takes the form, 2 K 2x gx = 3x 2 s 2 s + p + s p K 0 x 2 s + p 3 x2. (20) Further, noting that K 0 1 and K 2 (x) 1+x + x 2 /3, the limiting cases of interest are obtained, gx = gx = 3x x + x 2 2 for s p, (21) x x + x 2 3 for s = p, (22) so that the excess chemical potential of the bound ion ex B finally becomes, e 2 ex B = 8 0 s R p + R s z l + 2Z x 1 x x x 2 + m l m l + 2M l gx 1 gx 2. (23) For a weakly charged protein and a simple ligand (both Z and z l small), the monopole term may well be dominated by dipolar contributions describing the first moment of the charge distribution with m i z i r i /(R p + R s ) and M l Np i1 m i cos il. The ionic strength dependent factor of the dipole term is also sensitive to the location of the dielectric boundary as well as the ratio of permittivities and will be approximately doubled on changing from a high to a low dielectric interior. Acknowledgments This work has been supported by the Conselho Nacional de Desenvolvimento Científico e Technológico (CNPq/Brazil and FAPESP/Brazil), whom F.L.B.D.S. wishes to thank. We also thank Don Bashford for the access to MEAD. It is also a pleasure to acknowledge fruitful discussions with Henry S. Ashbaugh and Herman J.C. Berendsen. The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 USC section 1734 solely to indicate this fact. References Antonsiewicz, J., McCammon, J.A., and Gilson, M.K. 1994a. The determinants of pk a s in proteins. Biochemistry 35: Protein Science, vol. 10
11 Critical investigation of Tanford-Kirkwood scheme. 1994b. Prediction of ph-dependence properties of proteins. J. Mol. Biol. 238: Bashford, D. and Gerwert, K Electrostatic calculations of the pk a values of ionizable groups in bacteriorhodopsin. J. Mol. Biol. 224: Bashford, D. and Karplus, M pk a s of ionizable groups in proteins: Atomic detail from a continuum electrostatic model. Biochemistry 29: Bashford, D., Karplus, M., and Canters, G.W Electrostatic effects of charge pertubations introduced by metal oxidation in proteins a theoretical analysis. J. Mol. Biol. 203: Beroza, P., Fredkin, D.R., Okamura, M.Y., and Feher, G Protonation of interacting residues in a protein by a Monte Carlo method: Application to lysozyme and the photosynthetic reaction center of rhodobacter sphaeroides. Proc. Natl. Acad. Sci. 88: Böttcher, C.J.F Theory of Electric Polarization. Elsevier, Amsterdam, The Netherlands. Fushiki, M., Svensson, B., Jönsson, B., and Woodward, C.E Electrostatic interactions in protein solution A comparison between Poisson-Boltzmann and Monte Carlo calculations. Biopolymers 31: Guldbrand, L., Nilsson, L., and Nordenskiöld, L A Monte Carlo simulation study of electrostatic forces between hexagonally packed DNA double helices. J. Chem. Phys. 85: Harvey, S.C Treatment of electrostatic effects in marcomolecular modeling. Proteins: Struct., Func. Genet. 5: Hill, T.L Approximate calculations of the electrostatic free energy of nucleic acids and other cylindrical macromolecules. Arch. Biochem. Biophys. 57: Influence of electrolyte on effective dielectric constants in enzymes, proteins and other molecules. J. Chem. Phys. 60: Honig, B. and Nicholls, A Classical electrostatics in biology and chemistry. Science 268: Kesvatera, T., Jönsson, B., Thulin, E., and Linse, S Binding of Ca 2 to Calbindin D 9K : Structural stability and function at high salt concentration. Biochemistry 33: Ionization behaviour of acidic residues in calbindin D 9k. Proteins: Struc., Func. Genet. 37: Focusing the electrostatic potential at EF-hands of Calbindin D 9K. Titration of acidic residues. King, G., Lee, F.S., and Warshel, A Microscopic simulations of macroscopic dielectric constants of solvated proteins. J. Chem. Phys. 95: Kirkwood, J.G Theory of solutions of molecules containing widely separated charges with special applications to zwitterions. J. Chem. Phys. 2: Levesque, D., Weis, J.J., and Hansen, J.P Simulation of classical fluids. Monte Carlo Methods Stat. Phys. 5: Linse, S., Brodin, P., Johansson, C., Thulin, E., Grundström, T., and Forsen, S The role of protein surface changes in ion binding. Nature 335: Linse, S., Johansson, C., Brodin, P., Grundström, T., Drakenberg, T., and Forsen, S Electrostatic contribution to the binding of calcium in calbindin D 9K. Biochemistry 30: Linse, S., Jönsson, B., and Chazin, W.J The effect of protein concentration on ion binding. Proc. Natl. Acad. Sci. 92: Löffler, G., Screiber, H., and Steinhauser, O Calculation of the dielectric properties of a protein and its solvent: Theory and a case study. J. Mol. Biol. 270: Mel nikov, S., Lindman, B., Khan, M.O., and Jönsson, B Phase behaviour of a single DNA in mixed solvents. J. Am. Chem. Soc. 121: Metropolis, N.A., Rosenbluth, A.W., Rosenbluth, M.N., Teller, A., and Teller, E Equation of state calculations by fast computing machines. J. Chem. Phys. 21: Papazyan, A. and Warshel, A Continuum and dipole-lattice models of solvation. J. Phys. Chem. B 101: Penfold, R., Warwicker, J., and Jönsson, B Electrostatic models for calcium binding proteins. J. Phys. Chem. B 108: Sham, Y.Y., Chu, Z.T., and Warshel, A Consistent calculations of pk a s of ionizable residues in proteins: Semi-microscopic and microscopic approaches. J. Phys. Chem. B 101: Sham, Y.Y., Muegge, I., and Warshel, A The effect of protein relaxation on charge-charge interactions and dielectric constants of proteins. Biophys. J. 74: Shire, S.J., Hanania, G.I.H., and Gurd, F.R.N Electrostatic effects in myoglobin. Hydrogen ion equilibria in sperm whale Ferrimyoglobin. Biochemistry 13: Simonson, T. and Brooks III, C.L Charge screening and the dielectric constant of proteins: Insights from molecular dynamics. J. Am. Chem. Soc. 118: Simonson, T. and Perahia, D Microscopic dielectric properties of cytochrome c from molecular dynamics simulations in aqueous solution. J. Am. Chem. Soc. 117: Spassov, V. and Bashford, D Electrostatic coupling to ph-titrating sites as a source of cooperativity in protein-ligand binding. Prot. Sci. 7: Svensson, B.R. and Woodward, C.E Widom s method for uniform and non-uniform electrolyte solutions. Mol. Phys. 64: Svensson, B., Jönsson, B., and Woodward, C.E Electrostatic contributions of the binding of Ca 2+ in calbindin mutants. A Monte Carlo study. Biophys. Chem. 38: Svensson, B., Jönsson, B., Woodward, C.E., and Linse, S Ion binding properties of calbindin D 9k A Monte Carlo simulation study. Biochemistry 30: Tanford, C. and Kirkwood, J.G Theory of protein titration curves. I. General equations for impenetrable spheres. J. Am. Chem. Soc. 79: Tanford, C. and Roxby, R Interpretation of protein titration curves. Application to lysozyme. Biochemistry 11: Warshel, A. and Aqvist, J Electrostatic energy and macromolecular function. Annu. Rev. Biophys. Biophys. Chem. 20: Warshel, A. and Papazyan, A Electrostatic effects in macromolecules: Fundamental concepts and practical modeling. Curr. Opin. Struct. Biol. 8: Warshel, A., Russel, S.T., and Churg, A.K Macroscopic models for studies of electrostatic interactions in proteins: Limitations and applicability. Proc. Natl. Acad. Sci. 81: Warwicker, J Simplified methods for pk a and acid ph-dependent stability estimation in proteins: Removing dielectric and counterion boundaries. Prot. Sci. 8: Warwicker, J. and Watson, H.C Calculation of the electric potential in the active site cleft due to -helix dipoles. J. Mol. Biol. 157: Widom, B Some topics in the theory of fluids. J. Chem. Phys. 39:
Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015,
 Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015, Course,Informa5on, BIOC%530% GraduateAlevel,discussion,of,the,structure,,func5on,,and,chemistry,of,proteins,and, nucleic,acids,,control,of,enzyma5c,reac5ons.,please,see,the,course,syllabus,and,
Biochemistry,530:,, Introduc5on,to,Structural,Biology, Autumn,Quarter,2015, Course,Informa5on, BIOC%530% GraduateAlevel,discussion,of,the,structure,,func5on,,and,chemistry,of,proteins,and, nucleic,acids,,control,of,enzyma5c,reac5ons.,please,see,the,course,syllabus,and,
Electrostatics of membrane adhesion
 Electrostatics of membrane adhesion S. Marcelja Department of Applied Mathematics, Research School of Physical Sciences and Engineering, The Australian National University, Canberra ACT 6, Australia ABSTRACT
Electrostatics of membrane adhesion S. Marcelja Department of Applied Mathematics, Research School of Physical Sciences and Engineering, The Australian National University, Canberra ACT 6, Australia ABSTRACT
Free Energy of Solvation, Interaction, and Binding of Arbitrary Charge Distributions Imbedded in a Dielectric Continuum
 J. Phys. Chem. 1994, 98, 5113-5111 5773 Free Energy of Solvation, Interaction, and Binding of Arbitrary Charge Distributions Imbedded in a Dielectric Continuum B. Jayaram Department of Chemistry, Indian
J. Phys. Chem. 1994, 98, 5113-5111 5773 Free Energy of Solvation, Interaction, and Binding of Arbitrary Charge Distributions Imbedded in a Dielectric Continuum B. Jayaram Department of Chemistry, Indian
Supporting Text Z = 2Γ 2+ + Γ + Γ [1]
![Supporting Text Z = 2Γ 2+ + Γ + Γ [1] Supporting Text Z = 2Γ 2+ + Γ + Γ [1]](/thumbs/91/105230015.jpg) Supporting Text RNA folding experiments are typically carried out in a solution containing a mixture of monovalent and divalent ions, usually MgCl 2 and NaCl or KCl. All three species of ions, Mg, M +
Supporting Text RNA folding experiments are typically carried out in a solution containing a mixture of monovalent and divalent ions, usually MgCl 2 and NaCl or KCl. All three species of ions, Mg, M +
A Mesoscopic Model for Protein-Protein Interactions in Solution
 A Mesoscopic Model for Protein-Protein Interactions in Solution Lund, Mikael; Jönsson, Bo Published in: Biophysical Journal 2003 Link to publication Citation for published version (APA): Lund, M., & Jönsson,
A Mesoscopic Model for Protein-Protein Interactions in Solution Lund, Mikael; Jönsson, Bo Published in: Biophysical Journal 2003 Link to publication Citation for published version (APA): Lund, M., & Jönsson,
Peptide folding in non-aqueous environments investigated with molecular dynamics simulations Soto Becerra, Patricia
 University of Groningen Peptide folding in non-aqueous environments investigated with molecular dynamics simulations Soto Becerra, Patricia IMPORTANT NOTE: You are advised to consult the publisher's version
University of Groningen Peptide folding in non-aqueous environments investigated with molecular dynamics simulations Soto Becerra, Patricia IMPORTANT NOTE: You are advised to consult the publisher's version
2 Structure. 2.1 Coulomb interactions
 2 Structure 2.1 Coulomb interactions While the information needed for reproduction of living systems is chiefly maintained in the sequence of macromolecules, any practical use of this information must
2 Structure 2.1 Coulomb interactions While the information needed for reproduction of living systems is chiefly maintained in the sequence of macromolecules, any practical use of this information must
 Title Super- and subcritical hydration of Thermodynamics of hydration Author(s) Matubayasi, N; Nakahara, M Citation JOURNAL OF CHEMICAL PHYSICS (2000), 8109 Issue Date 2000-05-08 URL http://hdl.handle.net/2433/50350
Title Super- and subcritical hydration of Thermodynamics of hydration Author(s) Matubayasi, N; Nakahara, M Citation JOURNAL OF CHEMICAL PHYSICS (2000), 8109 Issue Date 2000-05-08 URL http://hdl.handle.net/2433/50350
Phase Equilibria and Molecular Solutions Jan G. Korvink and Evgenii Rudnyi IMTEK Albert Ludwig University Freiburg, Germany
 Phase Equilibria and Molecular Solutions Jan G. Korvink and Evgenii Rudnyi IMTEK Albert Ludwig University Freiburg, Germany Preliminaries Learning Goals Phase Equilibria Phase diagrams and classical thermodynamics
Phase Equilibria and Molecular Solutions Jan G. Korvink and Evgenii Rudnyi IMTEK Albert Ludwig University Freiburg, Germany Preliminaries Learning Goals Phase Equilibria Phase diagrams and classical thermodynamics
THE TANGO ALGORITHM: SECONDARY STRUCTURE PROPENSITIES, STATISTICAL MECHANICS APPROXIMATION
 THE TANGO ALGORITHM: SECONDARY STRUCTURE PROPENSITIES, STATISTICAL MECHANICS APPROXIMATION AND CALIBRATION Calculation of turn and beta intrinsic propensities. A statistical analysis of a protein structure
THE TANGO ALGORITHM: SECONDARY STRUCTURE PROPENSITIES, STATISTICAL MECHANICS APPROXIMATION AND CALIBRATION Calculation of turn and beta intrinsic propensities. A statistical analysis of a protein structure
Density Functional Theory for the Nonspecific Binding of Salt to Polyelectrolytes: Thermodynamic Properties
 Biophysical Journal Volume 78 February 2000 699 706 699 Density Functional Theory for the Nonspecific Binding of Salt to Polyelectrolytes: Thermodynamic Properties Chandra N. Patra and Arun Yethiraj Department
Biophysical Journal Volume 78 February 2000 699 706 699 Density Functional Theory for the Nonspecific Binding of Salt to Polyelectrolytes: Thermodynamic Properties Chandra N. Patra and Arun Yethiraj Department
A new algorithm for Reverse Monte Carlo simulations
 A new algorithm for Reverse Monte Carlo simulations Fernando Lus B. da Silva, Bo Svensson, Torbjörn Åkesson, and Bo Jönsson Citation: The Journal of Chemical Physics 109, 2624 (1998); doi: 10.1063/1.476861
A new algorithm for Reverse Monte Carlo simulations Fernando Lus B. da Silva, Bo Svensson, Torbjörn Åkesson, and Bo Jönsson Citation: The Journal of Chemical Physics 109, 2624 (1998); doi: 10.1063/1.476861
Chapter 2. Dielectric Theories
 Chapter Dielectric Theories . Dielectric Theories 1.1. Introduction Measurements of dielectric properties of materials is very important because it provide vital information regarding the material characteristics,
Chapter Dielectric Theories . Dielectric Theories 1.1. Introduction Measurements of dielectric properties of materials is very important because it provide vital information regarding the material characteristics,
we1 = j+dq + = &/ET, (2)
 EUROPHYSICS LETTERS Europhys. Lett., 24 (S), pp. 693-698 (1993) 10 December 1993 On Debye-Huckel s Theory. I. M. MLADENOV Central Laboratory of Biophysics, Bulgarian Academy of Sciences Acad. G. Bonchev
EUROPHYSICS LETTERS Europhys. Lett., 24 (S), pp. 693-698 (1993) 10 December 1993 On Debye-Huckel s Theory. I. M. MLADENOV Central Laboratory of Biophysics, Bulgarian Academy of Sciences Acad. G. Bonchev
Supporting Information. The hinge region strengthens the nonspecific interaction. between lac-repressor and DNA: a computer simulation.
 Supporting Information The hinge region strengthens the nonspecific interaction between lac-repressor and DNA: a computer simulation study Lili Sun 1, Marcin Tabaka 1, Sen Hou 1, Lin Li 2, Krzysztof Burdzy
Supporting Information The hinge region strengthens the nonspecific interaction between lac-repressor and DNA: a computer simulation study Lili Sun 1, Marcin Tabaka 1, Sen Hou 1, Lin Li 2, Krzysztof Burdzy
Research Statement. Shenggao Zhou. November 3, 2014
 Shenggao Zhou November 3, My research focuses on: () Scientific computing and numerical analysis (numerical PDEs, numerical optimization, computational fluid dynamics, and level-set method for interface
Shenggao Zhou November 3, My research focuses on: () Scientific computing and numerical analysis (numerical PDEs, numerical optimization, computational fluid dynamics, and level-set method for interface
Aqueous solutions. Solubility of different compounds in water
 Aqueous solutions Solubility of different compounds in water The dissolution of molecules into water (in any solvent actually) causes a volume change of the solution; the size of this volume change is
Aqueous solutions Solubility of different compounds in water The dissolution of molecules into water (in any solvent actually) causes a volume change of the solution; the size of this volume change is
Modeling Viscosity of Multicomponent Electrolyte Solutions 1
 International Journal of Thermophysics, Vol. 19, No. 2, 1998 Modeling Viscosity of Multicomponent Electrolyte Solutions 1 M. M. Lencka, 2 A. Anderko, 2,3 S. J. Sanders, 2 and R. D. Young 2 A comprehensive
International Journal of Thermophysics, Vol. 19, No. 2, 1998 Modeling Viscosity of Multicomponent Electrolyte Solutions 1 M. M. Lencka, 2 A. Anderko, 2,3 S. J. Sanders, 2 and R. D. Young 2 A comprehensive
Electrolytes. Chapter Basics = = 131 2[ ]. (c) From both of the above = = 120 8[
![Electrolytes. Chapter Basics = = 131 2[ ]. (c) From both of the above = = 120 8[ Electrolytes. Chapter Basics = = 131 2[ ]. (c) From both of the above = = 120 8[](/thumbs/83/88791608.jpg) Chapter 1 Electrolytes 1.1 Basics Here we consider species that dissociate into positively and negatively charged species in solution. 1. Consider: 1 H (g) + 1 Cl (g) + ()+ () = { } = (+ )+ ( ) = 167[
Chapter 1 Electrolytes 1.1 Basics Here we consider species that dissociate into positively and negatively charged species in solution. 1. Consider: 1 H (g) + 1 Cl (g) + ()+ () = { } = (+ )+ ( ) = 167[
 Title Theory of solutions in the energy r of the molecular flexibility Author(s) Matubayasi, N; Nakahara, M Citation JOURNAL OF CHEMICAL PHYSICS (2003), 9702 Issue Date 2003-11-08 URL http://hdl.handle.net/2433/50354
Title Theory of solutions in the energy r of the molecular flexibility Author(s) Matubayasi, N; Nakahara, M Citation JOURNAL OF CHEMICAL PHYSICS (2003), 9702 Issue Date 2003-11-08 URL http://hdl.handle.net/2433/50354
The change in free energy on transferring an ion from a medium of low dielectric constantε1 to one of high dielectric constant ε2:
 The Born Energy of an Ion The free energy density of an electric field E arising from a charge is ½(ε 0 ε E 2 ) per unit volume Integrating the energy density of an ion over all of space = Born energy:
The Born Energy of an Ion The free energy density of an electric field E arising from a charge is ½(ε 0 ε E 2 ) per unit volume Integrating the energy density of an ion over all of space = Born energy:
V. Electrostatics Lecture 24: Diffuse Charge in Electrolytes
 V. Electrostatics Lecture 24: Diffuse Charge in Electrolytes MIT Student 1. Poisson-Nernst-Planck Equations The Nernst-Planck Equation is a conservation of mass equation that describes the influence of
V. Electrostatics Lecture 24: Diffuse Charge in Electrolytes MIT Student 1. Poisson-Nernst-Planck Equations The Nernst-Planck Equation is a conservation of mass equation that describes the influence of
1. Poisson-Boltzmann 1.1. Poisson equation. We consider the Laplacian. which is given in spherical coordinates by (2)
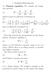 1. Poisson-Boltzmann 1.1. Poisson equation. We consider the Laplacian operator (1) 2 = 2 x + 2 2 y + 2 2 z 2 which is given in spherical coordinates by (2) 2 = 1 ( r 2 ) + 1 r 2 r r r 2 sin θ θ and in
1. Poisson-Boltzmann 1.1. Poisson equation. We consider the Laplacian operator (1) 2 = 2 x + 2 2 y + 2 2 z 2 which is given in spherical coordinates by (2) 2 = 1 ( r 2 ) + 1 r 2 r r r 2 sin θ θ and in
Lecture 2 and 3: Review of forces (ctd.) and elementary statistical mechanics. Contributions to protein stability
 Lecture 2 and 3: Review of forces (ctd.) and elementary statistical mechanics. Contributions to protein stability Part I. Review of forces Covalent bonds Non-covalent Interactions: Van der Waals Interactions
Lecture 2 and 3: Review of forces (ctd.) and elementary statistical mechanics. Contributions to protein stability Part I. Review of forces Covalent bonds Non-covalent Interactions: Van der Waals Interactions
Lecture 2-3: Review of forces (ctd.) and elementary statistical mechanics. Contributions to protein stability
 Lecture 2-3: Review of forces (ctd.) and elementary statistical mechanics. Contributions to protein stability Part I. Review of forces Covalent bonds Non-covalent Interactions Van der Waals Interactions
Lecture 2-3: Review of forces (ctd.) and elementary statistical mechanics. Contributions to protein stability Part I. Review of forces Covalent bonds Non-covalent Interactions Van der Waals Interactions
8.592J HST.452J: Statistical Physics in Biology
 Assignment # 4 8.592J HST.452J: Statistical Physics in Biology Coulomb Interactions 1. Flory Theory: The Coulomb energy of a ball of charge Q and dimension R in d spacial dimensions scales as Q 2 E c.
Assignment # 4 8.592J HST.452J: Statistical Physics in Biology Coulomb Interactions 1. Flory Theory: The Coulomb energy of a ball of charge Q and dimension R in d spacial dimensions scales as Q 2 E c.
T H E J O U R N A L O F G E N E R A L P H Y S I O L O G Y. jgp
 S u p p l e m e n ta l m at e r i a l jgp Lee et al., http://www.jgp.org/cgi/content/full/jgp.201411219/dc1 T H E J O U R N A L O F G E N E R A L P H Y S I O L O G Y S u p p l e m e n ta l D I S C U S
S u p p l e m e n ta l m at e r i a l jgp Lee et al., http://www.jgp.org/cgi/content/full/jgp.201411219/dc1 T H E J O U R N A L O F G E N E R A L P H Y S I O L O G Y S u p p l e m e n ta l D I S C U S
On the local and nonlocal components of solvation thermodynamics and their relation to solvation shell models
 JOURNAL OF CHEMICAL PHYSICS VOLUME 109, NUMBER 12 22 SEPTEMBER 1998 On the local and nonlocal components of solvation thermodynamics and their relation to solvation shell models Nobuyuki Matubayasi Institute
JOURNAL OF CHEMICAL PHYSICS VOLUME 109, NUMBER 12 22 SEPTEMBER 1998 On the local and nonlocal components of solvation thermodynamics and their relation to solvation shell models Nobuyuki Matubayasi Institute
Biophysical Journal, Volume 99. Supporting Material. Chromatin ionic atmosphere analyzed by a mesoscale electrostatic approach
 Biophysical Journal, Volume 99 Supporting Material Chromatin ionic atmosphere analyzed by a mesoscale electrostatic approach Hin Hark Gan and Tamar Schlick Supporting Material Chromatin ionic atmosphere
Biophysical Journal, Volume 99 Supporting Material Chromatin ionic atmosphere analyzed by a mesoscale electrostatic approach Hin Hark Gan and Tamar Schlick Supporting Material Chromatin ionic atmosphere
lim = F F = F x x + F y y + F z
 Physics 361 Summary of Results from Lecture Physics 361 Derivatives of Scalar and Vector Fields The gradient of a scalar field f( r) is given by g = f. coordinates f g = ê x x + ê f y y + ê f z z Expressed
Physics 361 Summary of Results from Lecture Physics 361 Derivatives of Scalar and Vector Fields The gradient of a scalar field f( r) is given by g = f. coordinates f g = ê x x + ê f y y + ê f z z Expressed
with a proper choice of the potential U(r). Clearly, we should include the potential of the ions, U ion (r):.
 The Hartree Equations So far we have ignored the effects of electron-electron (e-e) interactions by working in the independent electron approximation. In this lecture, we shall discuss how this effect
The Hartree Equations So far we have ignored the effects of electron-electron (e-e) interactions by working in the independent electron approximation. In this lecture, we shall discuss how this effect
The effect of surface dipoles and of the field generated by a polarization gradient on the repulsive force
 Journal of Colloid and Interface Science 263 (2003) 156 161 www.elsevier.com/locate/jcis The effect of surface dipoles and of the field generated by a polarization gradient on the repulsive force Haohao
Journal of Colloid and Interface Science 263 (2003) 156 161 www.elsevier.com/locate/jcis The effect of surface dipoles and of the field generated by a polarization gradient on the repulsive force Haohao
Bchem 675 Lecture 9 Electrostatics-Lecture 2 Debye-Hückel: Continued Counter ion condensation
 Bchem 675 Lecture 9 Electrostatics-Lecture 2 Debye-Hückel: Continued Counter ion condensation Ion:ion interactions What is the free energy of ion:ion interactions ΔG i-i? Consider an ion in a solution
Bchem 675 Lecture 9 Electrostatics-Lecture 2 Debye-Hückel: Continued Counter ion condensation Ion:ion interactions What is the free energy of ion:ion interactions ΔG i-i? Consider an ion in a solution
Electrostatic correlations and fluctuations for ion binding to a finite length polyelectrolyte
 THE JOURNAL OF CHEMICAL PHYSICS 122, 044903 2005 Electrostatic correlations and fluctuations for ion binding to a finite length polyelectrolyte Zhi-Jie Tan and Shi-Jie Chen a) Department of Physics and
THE JOURNAL OF CHEMICAL PHYSICS 122, 044903 2005 Electrostatic correlations and fluctuations for ion binding to a finite length polyelectrolyte Zhi-Jie Tan and Shi-Jie Chen a) Department of Physics and
Dielectrics. Lecture 20: Electromagnetic Theory. Professor D. K. Ghosh, Physics Department, I.I.T., Bombay
 What are dielectrics? Dielectrics Lecture 20: Electromagnetic Theory Professor D. K. Ghosh, Physics Department, I.I.T., Bombay So far we have been discussing electrostatics in either vacuum or in a conductor.
What are dielectrics? Dielectrics Lecture 20: Electromagnetic Theory Professor D. K. Ghosh, Physics Department, I.I.T., Bombay So far we have been discussing electrostatics in either vacuum or in a conductor.
CE 530 Molecular Simulation
 1 CE 530 Molecular Simulation Lecture 1 David A. Kofke Department of Chemical Engineering SUNY Buffalo kofke@eng.buffalo.edu 2 Time/s Multi-Scale Modeling Based on SDSC Blue Horizon (SP3) 1.728 Tflops
1 CE 530 Molecular Simulation Lecture 1 David A. Kofke Department of Chemical Engineering SUNY Buffalo kofke@eng.buffalo.edu 2 Time/s Multi-Scale Modeling Based on SDSC Blue Horizon (SP3) 1.728 Tflops
Density-functional theory for the structures and thermodynamic properties of highly asymmetric electrolyte and neutral component mixtures
 PHYSICAL REVIEW E 70, 031109 (2004) Density-functional theory for the structures and thermodynamic properties of highly asymmetric electrolyte and neutral component mixtures Zhidong Li and Jianzhong Wu*
PHYSICAL REVIEW E 70, 031109 (2004) Density-functional theory for the structures and thermodynamic properties of highly asymmetric electrolyte and neutral component mixtures Zhidong Li and Jianzhong Wu*
INTERMOLECULAR AND SURFACE FORCES
 INTERMOLECULAR AND SURFACE FORCES SECOND EDITION JACOB N. ISRAELACHVILI Department of Chemical & Nuclear Engineering and Materials Department University of California, Santa Barbara California, USA ACADEMIC
INTERMOLECULAR AND SURFACE FORCES SECOND EDITION JACOB N. ISRAELACHVILI Department of Chemical & Nuclear Engineering and Materials Department University of California, Santa Barbara California, USA ACADEMIC
arxiv:cond-mat/ v1 2 Feb 94
 cond-mat/9402010 Properties and Origins of Protein Secondary Structure Nicholas D. Socci (1), William S. Bialek (2), and José Nelson Onuchic (1) (1) Department of Physics, University of California at San
cond-mat/9402010 Properties and Origins of Protein Secondary Structure Nicholas D. Socci (1), William S. Bialek (2), and José Nelson Onuchic (1) (1) Department of Physics, University of California at San
Phase transitions of quadrupolar fluids
 Phase transitions of quadrupolar fluids Seamus F. O Shea Department of Chemistry, University of Lethbridge, Lethbridge, Alberta, Canada, T1K 3M4 Girija S. Dubey Brookhaven National Laboratory, Upton, New
Phase transitions of quadrupolar fluids Seamus F. O Shea Department of Chemistry, University of Lethbridge, Lethbridge, Alberta, Canada, T1K 3M4 Girija S. Dubey Brookhaven National Laboratory, Upton, New
Solvation and Macromolecular Structure. The structure and dynamics of biological macromolecules are strongly influenced by water:
 Overview Solvation and Macromolecular Structure The structure and dynamics of biological macromolecules are strongly influenced by water: Electrostatic effects: charges are screened by water molecules
Overview Solvation and Macromolecular Structure The structure and dynamics of biological macromolecules are strongly influenced by water: Electrostatic effects: charges are screened by water molecules
16 years ago TODAY (9/11) at 8:46, the first tower was hit at 9:03, the second tower was hit. Lecture 2 (9/11/17)
 16 years ago TODAY (9/11) at 8:46, the first tower was hit at 9:03, the second tower was hit By Anthony Quintano - https://www.flickr.com/photos/quintanomedia/15071865580, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=38538291
16 years ago TODAY (9/11) at 8:46, the first tower was hit at 9:03, the second tower was hit By Anthony Quintano - https://www.flickr.com/photos/quintanomedia/15071865580, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=38538291
Gas-liquid phase separation in oppositely charged colloids: stability and interfacial tension
 7 Gas-liquid phase separation in oppositely charged colloids: stability and interfacial tension We study the phase behaviour and the interfacial tension of the screened Coulomb (Yukawa) restricted primitive
7 Gas-liquid phase separation in oppositely charged colloids: stability and interfacial tension We study the phase behaviour and the interfacial tension of the screened Coulomb (Yukawa) restricted primitive
Many proteins spontaneously refold into native form in vitro with high fidelity and high speed.
 Macromolecular Processes 20. Protein Folding Composed of 50 500 amino acids linked in 1D sequence by the polypeptide backbone The amino acid physical and chemical properties of the 20 amino acids dictate
Macromolecular Processes 20. Protein Folding Composed of 50 500 amino acids linked in 1D sequence by the polypeptide backbone The amino acid physical and chemical properties of the 20 amino acids dictate
510 Subject Index. Hamiltonian 33, 86, 88, 89 Hamilton operator 34, 164, 166
 Subject Index Ab-initio calculation 24, 122, 161. 165 Acentric factor 279, 338 Activity absolute 258, 295 coefficient 7 definition 7 Atom 23 Atomic units 93 Avogadro number 5, 92 Axilrod-Teller-forces
Subject Index Ab-initio calculation 24, 122, 161. 165 Acentric factor 279, 338 Activity absolute 258, 295 coefficient 7 definition 7 Atom 23 Atomic units 93 Avogadro number 5, 92 Axilrod-Teller-forces
Biophysics II. Hydrophobic Bio-molecules. Key points to be covered. Molecular Interactions in Bio-molecular Structures - van der Waals Interaction
 Biophysics II Key points to be covered By A/Prof. Xiang Yang Liu Biophysics & Micro/nanostructures Lab Department of Physics, NUS 1. van der Waals Interaction 2. Hydrogen bond 3. Hydrophilic vs hydrophobic
Biophysics II Key points to be covered By A/Prof. Xiang Yang Liu Biophysics & Micro/nanostructures Lab Department of Physics, NUS 1. van der Waals Interaction 2. Hydrogen bond 3. Hydrophilic vs hydrophobic
Distance Constraint Model; Donald J. Jacobs, University of North Carolina at Charlotte Page 1 of 11
 Distance Constraint Model; Donald J. Jacobs, University of North Carolina at Charlotte Page 1 of 11 Taking the advice of Lord Kelvin, the Father of Thermodynamics, I describe the protein molecule and other
Distance Constraint Model; Donald J. Jacobs, University of North Carolina at Charlotte Page 1 of 11 Taking the advice of Lord Kelvin, the Father of Thermodynamics, I describe the protein molecule and other
arxiv: v1 [q-bio.bm] 6 Apr 2016
![arxiv: v1 [q-bio.bm] 6 Apr 2016 arxiv: v1 [q-bio.bm] 6 Apr 2016](/thumbs/88/115645706.jpg) Multi-shell model of ion-induced nucleic acid condensation Igor S. Tolokh Department of Computer Science, Virginia Tech, Blacksburg, VA 24061, USA Aleksander Drozdetski Department of Physics, Virginia
Multi-shell model of ion-induced nucleic acid condensation Igor S. Tolokh Department of Computer Science, Virginia Tech, Blacksburg, VA 24061, USA Aleksander Drozdetski Department of Physics, Virginia
 CHAPTER 6 CHEMICAL BONDING SHORT QUESTION WITH ANSWERS Q.1 Dipole moments of chlorobenzene is 1.70 D and of chlorobenzene is 2.5 D while that of paradichlorbenzene is zero; why? Benzene has zero dipole
CHAPTER 6 CHEMICAL BONDING SHORT QUESTION WITH ANSWERS Q.1 Dipole moments of chlorobenzene is 1.70 D and of chlorobenzene is 2.5 D while that of paradichlorbenzene is zero; why? Benzene has zero dipole
Generalizations for the Potential of Mean Force between Two Isolated Colloidal Particles from Monte Carlo Simulations
 Journal of Colloid and Interface Science 252, 326 330 (2002) doi:10.1006/jcis.2002.8497 Generalizations for the Potential of Mean Force between Two Isolated Colloidal Particles from Monte Carlo Simulations
Journal of Colloid and Interface Science 252, 326 330 (2002) doi:10.1006/jcis.2002.8497 Generalizations for the Potential of Mean Force between Two Isolated Colloidal Particles from Monte Carlo Simulations
Molecular Interactions F14NMI. Lecture 4: worked answers to practice questions
 Molecular Interactions F14NMI Lecture 4: worked answers to practice questions http://comp.chem.nottingham.ac.uk/teaching/f14nmi jonathan.hirst@nottingham.ac.uk (1) (a) Describe the Monte Carlo algorithm
Molecular Interactions F14NMI Lecture 4: worked answers to practice questions http://comp.chem.nottingham.ac.uk/teaching/f14nmi jonathan.hirst@nottingham.ac.uk (1) (a) Describe the Monte Carlo algorithm
AP PHYSICS 2 FRAMEWORKS
 1 AP PHYSICS 2 FRAMEWORKS Big Ideas Essential Knowledge Science Practices Enduring Knowledge Learning Objectives ELECTRIC FORCE, FIELD AND POTENTIAL Static Electricity; Electric Charge and its Conservation
1 AP PHYSICS 2 FRAMEWORKS Big Ideas Essential Knowledge Science Practices Enduring Knowledge Learning Objectives ELECTRIC FORCE, FIELD AND POTENTIAL Static Electricity; Electric Charge and its Conservation
Chapter 4. Electric Fields in Matter
 Chapter 4. Electric Fields in Matter 4.1.2 Induced Dipoles What happens to a neutral atom when it is placed in an electric field E? The atom now has a tiny dipole moment p, in the same direction as E.
Chapter 4. Electric Fields in Matter 4.1.2 Induced Dipoles What happens to a neutral atom when it is placed in an electric field E? The atom now has a tiny dipole moment p, in the same direction as E.
Consider a point P on the line joining the two charges, as shown in the given figure.
 Question 2.1: Two charges 5 10 8 C and 3 10 8 C are located 16 cm apart. At what point(s) on the line joining the two charges is the electric potential zero? Take the potential at infinity to be zero.
Question 2.1: Two charges 5 10 8 C and 3 10 8 C are located 16 cm apart. At what point(s) on the line joining the two charges is the electric potential zero? Take the potential at infinity to be zero.
Supporting Information for Lysozyme Adsorption in ph-responsive Hydrogel Thin-Films: The non-trivial Role of Acid-Base Equilibrium
 Electronic Supplementary Material (ESI) for Soft Matter. This journal is The Royal Society of Chemistry 215 Supporting Information for Lysozyme Adsorption in ph-responsive Hydrogel Thin-Films: The non-trivial
Electronic Supplementary Material (ESI) for Soft Matter. This journal is The Royal Society of Chemistry 215 Supporting Information for Lysozyme Adsorption in ph-responsive Hydrogel Thin-Films: The non-trivial
Electrolyte Solutions
 Chapter 8 Electrolyte Solutions In the last few chapters of this book, we will deal with several specific types of chemical systems. The first one is solutions and equilibria involving electrolytes, which
Chapter 8 Electrolyte Solutions In the last few chapters of this book, we will deal with several specific types of chemical systems. The first one is solutions and equilibria involving electrolytes, which
Finite-Difference Solution of the Poisson-Boltzmann Equation:
 Finite-Difference Solution of the Poisson-Boltzmann Equation: C om p 1 e t e E 1 im in a t i on of S e 1 f = Ene r gy ZHONGXIANG ZHOU," PHILIP PAYNE, and MAX VASQUEZ Protein Design Labs, lnc., 2375 Garcia
Finite-Difference Solution of the Poisson-Boltzmann Equation: C om p 1 e t e E 1 im in a t i on of S e 1 f = Ene r gy ZHONGXIANG ZHOU," PHILIP PAYNE, and MAX VASQUEZ Protein Design Labs, lnc., 2375 Garcia
Electrostatic Models for Calcium Binding Proteins
 J. Phys. Chem. B 1998, 102, 8599-8610 8599 Electrostatic Models for Calcium Binding Proteins Robert Penfold*, and James Warwicker Institute of Food Research, Reading Laboratory, Earley Gate, Whiteknights
J. Phys. Chem. B 1998, 102, 8599-8610 8599 Electrostatic Models for Calcium Binding Proteins Robert Penfold*, and James Warwicker Institute of Food Research, Reading Laboratory, Earley Gate, Whiteknights
Molecular Dynamics Simulation Study of the Ionic Mobility of OH Using the OSS2 Model
 1154 Bull. Korean Chem. Soc. 2006, Vol. 27, No. 8 Song Hi Lee Molecular Dynamics Simulation Study of the Ionic Mobility of OH Using the OSS2 Model Song Hi Lee Department of Chemistry, Kyungsung University,
1154 Bull. Korean Chem. Soc. 2006, Vol. 27, No. 8 Song Hi Lee Molecular Dynamics Simulation Study of the Ionic Mobility of OH Using the OSS2 Model Song Hi Lee Department of Chemistry, Kyungsung University,
Initial position, x p (0)/L
 .4 ) xp().2 ) ( 2L 2 xp Dc ( Displacement, /L.2.4.5.5 Initial position, x p ()/L Supplementary Figure Computed displacements of (red) positively- and (blue) negatively-charged particles at several CO 2
.4 ) xp().2 ) ( 2L 2 xp Dc ( Displacement, /L.2.4.5.5 Initial position, x p ()/L Supplementary Figure Computed displacements of (red) positively- and (blue) negatively-charged particles at several CO 2
CE 530 Molecular Simulation
 1 CE 530 Molecular Simulation Lecture 14 Molecular Models David A. Kofke Department of Chemical Engineering SUNY Buffalo kofke@eng.buffalo.edu 2 Review Monte Carlo ensemble averaging, no dynamics easy
1 CE 530 Molecular Simulation Lecture 14 Molecular Models David A. Kofke Department of Chemical Engineering SUNY Buffalo kofke@eng.buffalo.edu 2 Review Monte Carlo ensemble averaging, no dynamics easy
Convergence of replica exchange molecular dynamics
 THE JOURNAL OF CHEMICAL PHYSICS 123, 154105 2005 Convergence of replica exchange molecular dynamics Wei Zhang and Chun Wu Department of Chemistry and Biochemistry, University of Delaware, Newark, Delaware
THE JOURNAL OF CHEMICAL PHYSICS 123, 154105 2005 Convergence of replica exchange molecular dynamics Wei Zhang and Chun Wu Department of Chemistry and Biochemistry, University of Delaware, Newark, Delaware
Center for Theoretical Physics, Department of Applied Physics, Twente University, P.O. Box 217, 7500 AE Enschede, The Netherlands
 Physica A 193 (1993) 413-420 North-Holland Distribution of ions around a charged sphere P. Strating and F.W. Wiegel Center for Theoretical Physics, Department of Applied Physics, Twente University, P.O.
Physica A 193 (1993) 413-420 North-Holland Distribution of ions around a charged sphere P. Strating and F.W. Wiegel Center for Theoretical Physics, Department of Applied Physics, Twente University, P.O.
Statistical Theory and Learning from Molecular Simulations
 Statistical Theory and Learning from Molecular Simulations Lawrence R. Pratt 1 and Susan B. Rempe 2 1 Department of Chemical & Biomolecular Engineering 2 Center for Biological and Material Sciences, Sandia
Statistical Theory and Learning from Molecular Simulations Lawrence R. Pratt 1 and Susan B. Rempe 2 1 Department of Chemical & Biomolecular Engineering 2 Center for Biological and Material Sciences, Sandia
Monte Carlo Simulation Study of a System with a Dielectric Boundary: Application to Calcium Channel Selectivity
 Molecular Simulation, Vol. 30 (2 3), 15 February 15 March 2004, pp. 89 96 Monte Carlo Simulation Study of a System with a Dielectric Boundary: Application to Calcium Channel Selectivity DEZSŐ BODA a, *,
Molecular Simulation, Vol. 30 (2 3), 15 February 15 March 2004, pp. 89 96 Monte Carlo Simulation Study of a System with a Dielectric Boundary: Application to Calcium Channel Selectivity DEZSŐ BODA a, *,
Some properties of water
 Some properties of water Hydrogen bond network Solvation under the microscope 1 Water solutions Oil and water does not mix at equilibrium essentially due to entropy Substances that does not mix with water
Some properties of water Hydrogen bond network Solvation under the microscope 1 Water solutions Oil and water does not mix at equilibrium essentially due to entropy Substances that does not mix with water
Entropy enthalpy compensation: Fact or artifact?
 FOR THE RECORD Entropy enthalpy compensation: Fact or artifact? KIM SHARP Johnson Research Foundation, Department of Biochemistry and Biophysics, University of Pennsylvania, Philadelphia, Pennsylvania
FOR THE RECORD Entropy enthalpy compensation: Fact or artifact? KIM SHARP Johnson Research Foundation, Department of Biochemistry and Biophysics, University of Pennsylvania, Philadelphia, Pennsylvania
Molecular Origin of Hydration Heat Capacity Changes of Hydrophobic Solutes: Perturbation of Water Structure around Alkanes
 J. Phys. Chem. B 1997, 101, 11237-11242 11237 Molecular Origin of Hydration Heat Capacity Changes of Hydrophobic Solutes: Perturbation of Water Structure around Alkanes Bhupinder Madan and Kim Sharp* The
J. Phys. Chem. B 1997, 101, 11237-11242 11237 Molecular Origin of Hydration Heat Capacity Changes of Hydrophobic Solutes: Perturbation of Water Structure around Alkanes Bhupinder Madan and Kim Sharp* The
Solvation and reorganization energies in polarizable molecular and continuum solvents
 Solvation and reorganization energies in polarizable molecular and continuum solvents Joel S. Bader CuraGen Corporation, 322 East Main Street, Branford, Connecticut 06405 Christian M. Cortis Department
Solvation and reorganization energies in polarizable molecular and continuum solvents Joel S. Bader CuraGen Corporation, 322 East Main Street, Branford, Connecticut 06405 Christian M. Cortis Department
Lecture 5: Electrostatic Interactions & Screening
 Lecture 5: Electrostatic Interactions & Screening Lecturer: Prof. Brigita Urbanc (brigita@drexel.edu) PHYS 461 & 561, Fall 2009-2010 1 A charged particle (q=+1) in water, at the interface between water
Lecture 5: Electrostatic Interactions & Screening Lecturer: Prof. Brigita Urbanc (brigita@drexel.edu) PHYS 461 & 561, Fall 2009-2010 1 A charged particle (q=+1) in water, at the interface between water
3. Solutions W = N!/(N A!N B!) (3.1) Using Stirling s approximation ln(n!) = NlnN N: ΔS mix = k (N A lnn + N B lnn N A lnn A N B lnn B ) (3.
 3. Solutions Many biological processes occur between molecules in aqueous solution. In addition, many protein and nucleic acid molecules adopt three-dimensional structure ( fold ) in aqueous solution.
3. Solutions Many biological processes occur between molecules in aqueous solution. In addition, many protein and nucleic acid molecules adopt three-dimensional structure ( fold ) in aqueous solution.
V = 2ze 2 n. . a. i=1
 IITS: Statistical Physics in Biology Assignment # 3 KU Leuven 5/29/2013 Coulomb Interactions & Polymers 1. Flory Theory: The Coulomb energy of a ball of charge Q and dimension R in d spacial dimensions
IITS: Statistical Physics in Biology Assignment # 3 KU Leuven 5/29/2013 Coulomb Interactions & Polymers 1. Flory Theory: The Coulomb energy of a ball of charge Q and dimension R in d spacial dimensions
Critical parameters of unrestricted primitive model electrolytes with charge asymmetries up to 10:1
 JOURNAL OF CHEMICAL PHYSICS VOLUME 119, NUMBER 16 22 OCTOBER 2003 Critical parameters of unrestricted primitive model electrolytes with charge asymmetries up to 10:1 Daniel W. Cheong and Athanassios Z.
JOURNAL OF CHEMICAL PHYSICS VOLUME 119, NUMBER 16 22 OCTOBER 2003 Critical parameters of unrestricted primitive model electrolytes with charge asymmetries up to 10:1 Daniel W. Cheong and Athanassios Z.
Physics (
 Question 2.12: A charge of 8 mc is located at the origin. Calculate the work done in taking a small charge of 2 10 9 C from a point P (0, 0, 3 cm) to a point Q (0, 4 cm, 0), via a point R (0, 6 cm, 9 cm).
Question 2.12: A charge of 8 mc is located at the origin. Calculate the work done in taking a small charge of 2 10 9 C from a point P (0, 0, 3 cm) to a point Q (0, 4 cm, 0), via a point R (0, 6 cm, 9 cm).
Space Charges in Insulators
 1 Space Charges in Insulators Summary. The space charges in insulators directly determine the built-in field and electron energy distribution, as long as carrier transport can be neglected. In this chapter
1 Space Charges in Insulators Summary. The space charges in insulators directly determine the built-in field and electron energy distribution, as long as carrier transport can be neglected. In this chapter
Protein Folding Prof. Eugene Shakhnovich
 Protein Folding Eugene Shakhnovich Department of Chemistry and Chemical Biology Harvard University 1 Proteins are folded on various scales As of now we know hundreds of thousands of sequences (Swissprot)
Protein Folding Eugene Shakhnovich Department of Chemistry and Chemical Biology Harvard University 1 Proteins are folded on various scales As of now we know hundreds of thousands of sequences (Swissprot)
UB association bias algorithm applied to the simulation of hydrogen fluoride
 Fluid Phase Equilibria 194 197 (2002) 249 256 UB association bias algorithm applied to the simulation of hydrogen fluoride Scott Wierzchowski, David A. Kofke Department of Chemical Engineering, University
Fluid Phase Equilibria 194 197 (2002) 249 256 UB association bias algorithm applied to the simulation of hydrogen fluoride Scott Wierzchowski, David A. Kofke Department of Chemical Engineering, University
Multimedia : Boundary Lubrication Podcast, Briscoe, et al. Nature , ( )
 3.05 Nanomechanics of Materials and Biomaterials Thursday 04/05/07 Prof. C. Ortiz, MITDMSE I LECTURE 14: TE ELECTRICAL DOUBLE LAYER (EDL) Outline : REVIEW LECTURE #11 : INTRODUCTION TO TE ELECTRICAL DOUBLE
3.05 Nanomechanics of Materials and Biomaterials Thursday 04/05/07 Prof. C. Ortiz, MITDMSE I LECTURE 14: TE ELECTRICAL DOUBLE LAYER (EDL) Outline : REVIEW LECTURE #11 : INTRODUCTION TO TE ELECTRICAL DOUBLE
Proteins polymer molecules, folded in complex structures. Konstantin Popov Department of Biochemistry and Biophysics
 Proteins polymer molecules, folded in complex structures Konstantin Popov Department of Biochemistry and Biophysics Outline General aspects of polymer theory Size and persistent length of ideal linear
Proteins polymer molecules, folded in complex structures Konstantin Popov Department of Biochemistry and Biophysics Outline General aspects of polymer theory Size and persistent length of ideal linear
Introduction to molecular dynamics
 1 Introduction to molecular dynamics Yves Lansac Université François Rabelais, Tours, France Visiting MSE, GIST for the summer Molecular Simulation 2 Molecular simulation is a computational experiment.
1 Introduction to molecular dynamics Yves Lansac Université François Rabelais, Tours, France Visiting MSE, GIST for the summer Molecular Simulation 2 Molecular simulation is a computational experiment.
Interference effects between ion induced solvent polarisations in Liquid water
 Interference effects between ion induced solvent polarisations in Liquid water Puja Banerjee and Biman Bagchi* Solid State and Structural Chemistry Unit, Indian Institute of Science, Bangalore, Karnataka-
Interference effects between ion induced solvent polarisations in Liquid water Puja Banerjee and Biman Bagchi* Solid State and Structural Chemistry Unit, Indian Institute of Science, Bangalore, Karnataka-
Inorganic Pharmaceutical Chemistry
 Inorganic Pharmaceutical Chemistry Lecture No. 4 Date :25/10 /2012 Dr. Mohammed Hamed --------------------------------------------------------------------------------------------------------------------------------------
Inorganic Pharmaceutical Chemistry Lecture No. 4 Date :25/10 /2012 Dr. Mohammed Hamed --------------------------------------------------------------------------------------------------------------------------------------
Molecular dynamics simulation of Aquaporin-1. 4 nm
 Molecular dynamics simulation of Aquaporin-1 4 nm Molecular Dynamics Simulations Schrödinger equation i~@ t (r, R) =H (r, R) Born-Oppenheimer approximation H e e(r; R) =E e (R) e(r; R) Nucleic motion described
Molecular dynamics simulation of Aquaporin-1 4 nm Molecular Dynamics Simulations Schrödinger equation i~@ t (r, R) =H (r, R) Born-Oppenheimer approximation H e e(r; R) =E e (R) e(r; R) Nucleic motion described
Using Fundamental Measure Theory to Treat the Correlation Function of the Inhomogeneous Hard-Sphere Fluid
 Using Fundamental Measure Theory to Treat the Correlation Function of the Inhomogeneous Hard-Sphere Fluid Jeff B. Schulte, Patrick A. Kreitzberg, Chris V. Haglund, and David Roundy Department of Physics,
Using Fundamental Measure Theory to Treat the Correlation Function of the Inhomogeneous Hard-Sphere Fluid Jeff B. Schulte, Patrick A. Kreitzberg, Chris V. Haglund, and David Roundy Department of Physics,
A simple technique to estimate partition functions and equilibrium constants from Monte Carlo simulations
 A simple technique to estimate partition functions and equilibrium constants from Monte Carlo simulations Michal Vieth Department of Chemistry, The Scripps Research Institute, 10666 N. Torrey Pines Road,
A simple technique to estimate partition functions and equilibrium constants from Monte Carlo simulations Michal Vieth Department of Chemistry, The Scripps Research Institute, 10666 N. Torrey Pines Road,
Theoretische Physik 2: Elektrodynamik (Prof. A-S. Smith) Home assignment 11
 WiSe 22..23 Prof. Dr. A-S. Smith Dipl.-Phys. Matthias Saba am Lehrstuhl für Theoretische Physik I Department für Physik Friedrich-Alexander-Universität Erlangen-Nürnberg Problem. Theoretische Physik 2:
WiSe 22..23 Prof. Dr. A-S. Smith Dipl.-Phys. Matthias Saba am Lehrstuhl für Theoretische Physik I Department für Physik Friedrich-Alexander-Universität Erlangen-Nürnberg Problem. Theoretische Physik 2:
Solutions and Non-Covalent Binding Forces
 Chapter 3 Solutions and Non-Covalent Binding Forces 3.1 Solvent and solution properties Molecules stick together using the following forces: dipole-dipole, dipole-induced dipole, hydrogen bond, van der
Chapter 3 Solutions and Non-Covalent Binding Forces 3.1 Solvent and solution properties Molecules stick together using the following forces: dipole-dipole, dipole-induced dipole, hydrogen bond, van der
INTRODUCTION TO FINITE ELEMENT METHODS ON ELLIPTIC EQUATIONS LONG CHEN
 INTROUCTION TO FINITE ELEMENT METHOS ON ELLIPTIC EQUATIONS LONG CHEN CONTENTS 1. Poisson Equation 1 2. Outline of Topics 3 2.1. Finite ifference Method 3 2.2. Finite Element Method 3 2.3. Finite Volume
INTROUCTION TO FINITE ELEMENT METHOS ON ELLIPTIC EQUATIONS LONG CHEN CONTENTS 1. Poisson Equation 1 2. Outline of Topics 3 2.1. Finite ifference Method 3 2.2. Finite Element Method 3 2.3. Finite Volume
Statistical Geometry and Lattices
 Journal of Statistical Physics, Vol. 96, Nos. 56, 1999 Statistical Geometry and Lattices Gerardo Soto-Campos, 1 Richard Bowles, 1 Andrey Itkin, 1 and Howard Reiss 1 Received January 11, 1999; final May
Journal of Statistical Physics, Vol. 96, Nos. 56, 1999 Statistical Geometry and Lattices Gerardo Soto-Campos, 1 Richard Bowles, 1 Andrey Itkin, 1 and Howard Reiss 1 Received January 11, 1999; final May
Atomic and molecular interaction forces in biology
 Atomic and molecular interaction forces in biology 1 Outline Types of interactions relevant to biology Van der Waals interactions H-bond interactions Some properties of water Hydrophobic effect 2 Types
Atomic and molecular interaction forces in biology 1 Outline Types of interactions relevant to biology Van der Waals interactions H-bond interactions Some properties of water Hydrophobic effect 2 Types
On the Chemical Free Energy of the Electrical Double Layer
 1114 Langmuir 23, 19, 1114-112 On the Chemical Free Energy of the Electrical Double Layer Marian Manciu and Eli Ruckenstein* Department of Chemical Engineering, State University of New York at Buffalo,
1114 Langmuir 23, 19, 1114-112 On the Chemical Free Energy of the Electrical Double Layer Marian Manciu and Eli Ruckenstein* Department of Chemical Engineering, State University of New York at Buffalo,
q 2 Da This looks very similar to Coulomb doesn t it? The only difference to Coulomb is the factor of 1/2.
 Born Lets now think about a somewhat different, but related problem that is associated with the name Born. Using a name (usually the name of the guy who first discovered or solved the problem) to describe
Born Lets now think about a somewhat different, but related problem that is associated with the name Born. Using a name (usually the name of the guy who first discovered or solved the problem) to describe
Big Idea #5: The laws of thermodynamics describe the essential role of energy and explain and predict the direction of changes in matter.
 KUDs for Unit 6: Chemical Bonding Textbook Reading: Chapters 8 & 9 Big Idea #2: Chemical and physical properties of materials can be explained by the structure and the arrangement of atoms, ion, or molecules
KUDs for Unit 6: Chemical Bonding Textbook Reading: Chapters 8 & 9 Big Idea #2: Chemical and physical properties of materials can be explained by the structure and the arrangement of atoms, ion, or molecules
Molecular dynamics simulations of anti-aggregation effect of ibuprofen. Wenling E. Chang, Takako Takeda, E. Prabhu Raman, and Dmitri Klimov
 Biophysical Journal, Volume 98 Supporting Material Molecular dynamics simulations of anti-aggregation effect of ibuprofen Wenling E. Chang, Takako Takeda, E. Prabhu Raman, and Dmitri Klimov Supplemental
Biophysical Journal, Volume 98 Supporting Material Molecular dynamics simulations of anti-aggregation effect of ibuprofen Wenling E. Chang, Takako Takeda, E. Prabhu Raman, and Dmitri Klimov Supplemental
Density of states for electrons and holes. Distribution function. Conduction and valence bands
 Intrinsic Semiconductors In the field of semiconductors electrons and holes are usually referred to as free carriers, or simply carriers, because it is these particles which are responsible for carrying
Intrinsic Semiconductors In the field of semiconductors electrons and holes are usually referred to as free carriers, or simply carriers, because it is these particles which are responsible for carrying
Where are the protons? Measuring and modelling proton equilibria in complex macromolecular systems.
 Frans Mulder PhD course Jyväskylä 2017 Where are the protons? Measuring and modelling proton equilibria in complex macromolecular systems. Frans Mulder Lecture 3 Application of NMR spectroscopy to study
Frans Mulder PhD course Jyväskylä 2017 Where are the protons? Measuring and modelling proton equilibria in complex macromolecular systems. Frans Mulder Lecture 3 Application of NMR spectroscopy to study
MMGBSA: Thermodynamics of Biomolecular Systems
 MMGBSA: Thermodynamics of Biomolecular Systems The MMGBSA approach employs molecular mechanics, the generalized Born model and solvent accessibility method to elicit free energies from structural information
MMGBSA: Thermodynamics of Biomolecular Systems The MMGBSA approach employs molecular mechanics, the generalized Born model and solvent accessibility method to elicit free energies from structural information
Introduction The gramicidin A (ga) channel forms by head-to-head association of two monomers at their amino termini, one from each bilayer leaflet. Th
 Abstract When conductive, gramicidin monomers are linked by six hydrogen bonds. To understand the details of dissociation and how the channel transits from a state with 6H bonds to ones with 4H bonds or
Abstract When conductive, gramicidin monomers are linked by six hydrogen bonds. To understand the details of dissociation and how the channel transits from a state with 6H bonds to ones with 4H bonds or
Chapter 4. Electrostatic Fields in Matter
 Chapter 4. Electrostatic Fields in Matter 4.1. Polarization 4.2. The Field of a Polarized Object 4.3. The Electric Displacement 4.4. Linear Dielectrics 4.5. Energy in dielectric systems 4.6. Forces on
Chapter 4. Electrostatic Fields in Matter 4.1. Polarization 4.2. The Field of a Polarized Object 4.3. The Electric Displacement 4.4. Linear Dielectrics 4.5. Energy in dielectric systems 4.6. Forces on
8.333: Statistical Mechanics I Problem Set # 5 Due: 11/22/13 Interacting particles & Quantum ensembles
 8.333: Statistical Mechanics I Problem Set # 5 Due: 11/22/13 Interacting particles & Quantum ensembles 1. Surfactant condensation: N surfactant molecules are added to the surface of water over an area
8.333: Statistical Mechanics I Problem Set # 5 Due: 11/22/13 Interacting particles & Quantum ensembles 1. Surfactant condensation: N surfactant molecules are added to the surface of water over an area
