Photosensitization of TiO 2 Inverse Opal Adsorbed with. CdSe Quantum Dots and Its Application to Solar Cell LINA JAYA DIGUNA
|
|
|
- Barnard Blake
- 5 years ago
- Views:
Transcription
1 Photosensitization of TiO 2 Inverse Opal Adsorbed with CdSe Quantum Dots and Its Application to Solar Cell LINA JAYA DIGUNA THE UNIVERSITY OF ELECTRO-COMMUNICATIONS MARCH 2009
2 Photosensitization of TiO 2 Inverse Opal Adsorbed with CdSe Quantum Dots and Its Application to Solar Cell LINA JAYA DIGUNA DEPARTMENT OF APPLIED PHYSICS AND CHEMISTRY THE UNIVERSITY OF ELECTRO-COMMUNICATIONS MARCH 2009
3 Photosensitization of TiO 2 Inverse Opal Adsorbed with CdSe Quantum Dots and Its Application to Solar Cell APPROVED BY THE SUPERVISORY COMMITTEE: CHAIRPERSON: PROF. Taro Toyoda MEMBER: PROF. Shigeo Hayashi MEMBER: PROF. Naoki Kobayashi MEMBER: PROF. Kohji Abe MEMBER: PROF. Tsuyoshi Okuno
4 Copyright By LINA JAYA DIGUNA 2009
5 CdSe 量子ドットによる TiO 2 逆オパール電極の光増感と 太陽電池への応用 近年 深刻化する環境問題やエネルギー資源問題の解決策の一つとして 太陽電池の開発が活発に行われている 現在の太陽電池の主流はシリコン (Si) 系太陽電池であるが 原料供給や製造工程の複雑性 高コストなどが 依然問題となっている そのなかで 製造プロセスが簡便で 安価に作製可能な色素増感太陽電池が注目されている 現在 色素増感太陽電池で用いられている増感剤は主に有機色素系が適用され 数多くの研究が進行しているが 新たな増感剤の候補として半導体量子ドットが大きな注目を集めている 半導体量子ドットの特徴として 1 光吸収係数が大きい 2 双極子モーメントが大きく電荷分離が急速に進行する 3 粒径を制御することで光吸収領域を任意に設定することが可能 4 多重励起子生成による量子効率の増大が期待される といった有機色素系には無い特徴を持っている また TiO 2 基板電極においては 従来の不規則性ナノ粒子系の代わりに 規則性ナノ構造を持つ逆オパール構造のTiO 2 電極を適用し それに伴う光電流の向上が期待できる 本研究ではこれらの特徴をふまえ 逆オパール構造 TiO 2 光電極を作製し 増感剤としてCdSe 量子ドットを適用した系を対象とし 光吸収や分光増感特性および光励起キャリアの過渡応答特性 さらに光電変換特性の評価を行い 高効率太陽電池への応用について検討した 本論文ではまず逆オパール構造 TiO 2 光電極の作製とその基本構造と各特性 ( 光吸収 光透過 光反射と光電流量子変換効率 (IPCE) スペクトル および過渡光電流応答特性 ) について論じた ポリスチレン (PS) ラテックス粒子の自己組織化によるオパール状のテンプレートを作製し TiCl 4 を充填前駆体として適用し TiO 2 逆オパールナノ構造電極を形成した X 線粉末回折測定から作製されたTiO 2 逆オパールナノ構造はanatase 構造を持っていることが分かった 透過スペクトルと拡散反射スペクトルから フォトニック結晶 ( すなわち 逆オパール構造 ) に特有の光子の存在することができない領域であるフォトニックバンドギャップ (PBG) の存在が確認された 光音響 (PA) 分光法によって測定した光吸収スペクトルから TiO 2 逆オパールのバンドギャップが 3.2eVであることが i
6 確認された 過渡光電流応答から得られたTiO 2 逆オパールの電子拡散係数は従来の不規則性ナノ粒子 TiO 2 電極のより大きいことが判明した 次に CdSe 量子ドットのTiO 2 逆オパール電極への吸着 さらにCdSe 量子ドットの構造とCdSe 量子ドット吸着したTiO 2 逆オパール電極の各特性 ( 光吸収とIPCEスペクトル ) について調べた CdSe 量子ドットの吸着には CdSO 4 溶液 N(CH 2 COONa) 3 溶液 Na 2 SeSO 3 溶液を混合した液にTiO 2 電極を浸し 一定温度で浸漬時間をパラメータとして CdSe 量子ドット吸着を行った 光吸収スペクトルから CdSe 量子ドットが吸着時間の増加と共に成長することが確認できた PAスペクトルの肩の位置をCdSe 量子ドットの第一励起エネルギーと仮定し 有効質量近似法からCdSe 量子ドットの平均粒径を見積ったところ 吸着時間の増加と共にCdSe 量子ドットのサイズが 4nmからおよそ 7 nmまで成長したことが分かった IPCEスペクトルから CdSe 量子ドットによるTiO 2 逆オパール電極の分光増感が確認できた さらに 光励起キャリアのダイナミクスについて調べた 光励起キャリアのダイナミクスの評価には過渡回折格子 (TG) 法を適用した TG 応答特性から CdSe 量子ドットにおける光励起電子とホールのダイナミクスを同時に測定することに成功した その結果より CdSe 量子ドットにおける光励起ホールが 2ps 以内に緩和し 光励起電子が数 10psから数 100psまで緩和することが判明した さらに ガラス基板に吸着したCdSe 量子ドットとTiO 2 逆オパールに吸着したCdSe 量子ドットでの光励起電子の緩和時間の比較から CdSe 量子ドットからTiO 2 への電子移動速度を測定できた 最後に CdSeを吸着したTiO 2 逆オパール電極の光電変換特性について調べた 半導体量子ドット表面への適切な修飾 (ZnSとFの吸着) により 光電変換特性 ( 短絡電流 開放電圧 fill factor 光電変換効率) が向上したことを見出した 過渡電圧応答測定より 表面修飾による表面欠陥の減少が示唆された さらに 適切な対極の適用やCdSの吸着により 最高の光電変換効率が 3.7% になった この結果は 半導体量子ドット増感太陽電池では比較的に高い値である ii
7 Photosensitization of TiO 2 Inverse Opal Adsorbed with CdSe Quantum Dots and Its Application to Solar Cell ABSTRACT Recently, dye-sensitized solar cells (DSSCs) has been attracted much interest in replacing silicon solar cells due to its low cost and simple fabrication method. The improvement of the performance of dye-sensitized solar cell (DSSC) must be achieved by considering the morphology of TiO 2 film and the choice of sensitizers. TiO 2 inverse opal could offer a promising solution in search for enhancing the light harvesting efficiency of dye-sensitized solar cell (DSSC) due to its large interconnected pores for better penetration of dye and photon localization in the red edge of photonic band gap for significant enhancement of dye absorption. In view of sensitizers, semiconductor quantum dots (QDs) have attracted much attention as dye substitute due to its tunable optical properties to maximize the solar absorption and capability of carrier multiplication through impact ionization. In this study, TiO 2 inverse opal with its interconnected marcroporous structure and CdSe quantum dots (QDs) having tunable optical properties were investigated to be potential as novel light harvesting system. Inverse opals TiO 2 electrodes could be replicated from colloidal latex crystals through the simple and relative fast bottom up method with only using one precursor solution, i.e. TiCl 4 in methanol solution. CdSe QDs were then chemically adsorbed on inverse opal TiO 2 electrodes through chemical bath deposition method. The photosensitization of TiO 2 inverse opal with CdSe QDs adsorption was mainly studied by characterizing its optical absorption, photocurrent and photoexcited carrier dynamics. Moreover, its application to solar cell was introduced resulting efficient QD-sensitized solar cell. Grown CdSe QDs in situ on TiO 2 inverse opal cause the red-shift in optical absorption and photocurrent properties. The higher energy of first excitation energy for each adsorption times relative to the band gap of bulk CdSe shows the occurrence of quantum confinement effect. The optimum photocurrent for certain adsorption times describes the appropriate condition for sufficient carrier generation, electron injection at CdSe/TiO 2 iii
8 interface and hole injection at CdSe/electrolyte interface (affected by the porous size in TiO 2 inverse opal to allow the penetration of the electrolyte across the matrix). As the initial mechanism in photosensitization providing the vital comprehension of charge separation, ultrafast carrier dynamics in CdSe QD-adsorbed TiO 2 inverse opal was characterized by transient grating technique. By comparing the measurement in air and electrolyte, the simultaneous detection of photoexcited hole and electron dynamics in CdSe QDs was successfully detected. The hole decay was found to be faster than the electron decay, that is in opposite to the mechanism of usual dye-sensitized solar cells. By changing the substrate, from TiO 2 to glass, the hole decay time in the faster process is almost constant and the slower decay time with respect to electron changed greatly due to the electron injection from CdSe to TiO 2 which does not occur in case of glass substrate. Thus the electron injection from CdSe QD to the TiO 2 electrode could be measured. Furthermore, for same CdSe QDs and larger quantity of adsorbed CdSe QDs (forming larger cluster of CdSe QDs), different decay times were observed indicating the dominance of the role of CdSe-CdSe interfaces in carrier dynamics. These CdSe-CdSe interfaces provide the carrier transport along the clusters of CdSe QDs and this phenomena should be accounted in the ultrafast carrier dynamics overall. In sensitized solar cell application, TiO 2 inverse opal structure matched very well with in situ growth of CdSe QD allowing the sufficient quantity of QD to absorb solar light on thinner electrode towards high efficiency. Relative to the CdSe QD-sensitized TiO 2 nanocrystalline solar cells, CdSe QD-sensitized TiO 2 inverse opal solar cells typically have higher open circuit voltage and fill factor. The former indicates the larger fraction of electron injection at TiO 2 inverse opal surfaces rather than the common TiO 2 nanocrystalline surfaces thus highly increasing the quasi Fermi level in TiO 2 inverse opal conduction band. The latter implies the better penetration of electrolyte through the entire TiO 2 electrode due to its macroporous structure resulting in the efficient hole transfer to the electrolyte. Moreover, the photovoltaic performances enhanced significantly by considering the surface modification on QD-sensitized TiO 2 inverse opal electrode. By means of surface modification, the reduction of surface state was achieved in which confirmed by transient photovoltage. Further improvement could be achieved by using the proper porous counter electrode with large surface area against the used polysulfide electrolyte. Finally a power conversion efficiency of about 3.7% has been attained, under solar illumination of 100mW/cm 2. This value is relatively high for QD-sensitized solar cells. iv
9 Contents Japanese abstract i Abstract iii Contents v List of figures viii List of tables xii 1 Introduction Background Purposes Brief outline of the thesis 5 2 Theoretical backgrounds Dye-sensitized solar cells (DSSCs) Inverse opal structured electrode Semiconductor quantum dot sensitizer Large extinction coefficient and dipole moment Quantum confinement effect Hot carrier and multiple exciton generation Material properties Titanium Dioxide (TiO 2 ) Cadnium Selenide (CdSe) Photonic crystal 19 3 Experimental procedures Sample preparation TiO 2 inverse opal Adsorption of CdSe QDs on TiO 2 inverse opal Characterization techniques Scanning electron microscopy X-ray diffraction spectrometry Reflectance spectroscopy Photo acoustic spectroscopy Incident photon to current efficiency (IPCE) spectroscopy 31 v
10 3.2.6 Solar simulators Transient photocurrent or photovoltage spectroscopy Heterodyne detection transient grating spectroscopy 35 4 TiO 2 inverse opal electrode Introduction Experimental Results and discussion Crystal structure and morphology Transmission and reflection spectra Optical absorption and photocurrent properties Electron diffusivity property Thermal diffusivity property Conclusions 48 5 Photosensitization of TiO 2 inverse opal with CdSe quantum dots Introduction Experimental Results and discussion Crystal structure and morphology Reflectance spectra Optical absorption Photocurrent properties Photovoltaic properties Conclusions 61 6 Ultrafast carrier dynamics of TiO 2 inverse opal with CdSe quantum dots Introduction Experimental Results and discussion Hole dynamics of TiO 2 inverse opal Simultaneous electron and hole detection in CdSe QD-adsorbed TiO 2 inverse opal Role of the interfaces in ultrafast carrier dynamics Electron transfer at TiO 2 /CdSe QD interface Conclusions 76 7 Efficient CdSe QD-sensitized TiO 2 inverse opal solar cell 78 vi
11 7.1 Introduction Experimental Results and discussion Typical characteristics of QD-sensitized TiO 2 inverse opal solar cell Surface modification with ZnS and Fluoride ions Counter electrode improvement Further photoanode electrode with Lithium ions and CdS Conclusions 93 8 Summary 96 Publications and conference visits 98 Acknowledgements 101 Biography 103 vii
12 List of figures Figure 1.1 Basic operation of solar cells. 2 Figure 1.2 Classification of solar cells. 3 Figure 2.1 Schematic representation of dye-sensitized solar cell. 8 Figure 2.2 Requirements for TiO 2 sensitizers. 10 Figure 2.3 Quantum confinement effect on electron energy levels. 12 Figure 2.4 Representation of multiple exciton generation (E 1 relating to first excitation energy in quantum dot, likewise E g relating to energy band gap of bulk semiconductor). 14 Figure 2.5 Schematic diagram of photocatalytic reaction by TiO Figure 2.6 Figure 2.7 Figure 2.8 Figure 2.9 Figure 3.1 Band edges position of several semiconductors in contact with aqueous electrolyte at ph 1. Solid state structure of CdSe. (a) Wurtzite structure (b) hexagonal structure. Real space representation of a photonic crystal and reciprocal space regions for which propagation is shielded. Energy dispersion relations for free electron and electron in a (1D) solid and for a free photon and a photon in a photonic crystal. Schematic procedure of the fabrication TiO 2 inverse opal. (a) Template preparation, (b) filling the template with TiO 2 (c) calcination of template and annealing of TiO Figure 3.2 Flow chart of chemical solution preparation for CdSe adsorption. 26 Figure 3.3 CdSe quantum dots adsorption. 26 Figure 3.4 Schematic diagram of reflectance spectroscopy. 28 Figure 3.5 Cross-sectional view of a photoacoustic cell. 29 Figure 3.6 Schematic diagram of photoacoustic spectroscopy. 30 viii
13 Figure 3.7 Typical PA spectrum of carbon black. 31 Figure 3.8 Schematic diagram of IPCE spectroscopy. 32 Figure 3.9 Typical J-V characteristics of a solar cell. 32 Figure 3.10 Air mass or optical path length of sunlight through Earth s atmosphere. 33 Figure 3.11 Schematic diagram of transient photocurrent/photovoltage spectroscopy. 34 Figure 3.12 Figure 4.1 Figure 4.2 Figure 4.3 Figure 4.4 Principle of the lens-free heterodyne transient grating (LF-HD- TG) technique (improved TG). X-ray diffraction pattern for the TiO 2 photonic crystal for different filling process (TiCl 4 drops). SEM images of a latex template (diameter of 309 nm) (a) surface, (b) cross section, together with the replicated inverse opal TiO 2 (c) surface, (d) cross section. Transmission spectra of nanocrystalline TiO 2 with diameter 15 nm (a) and those of TiO 2 inverse opal for 3 (b) times in filling process (TiCl 4 drops). Reflection spectra of TiO 2 inverse opal made from latex spheres with diameters of (a) 309 nm and (b) 394 nm Figure 4.5 Typical PA spectrum of TiO 2 inverse opal. 44 Figure 4.6 IPCE spectrum of TiO 2 inverse opal electrode. 45 Figure 4.7 Photocurrent transients of (a) TiO 2 inverse opal (made from 309 nm sized latex) and (b) 40 nm sized nanocrystalline TiO 2 with similar thickness of about 3 µm. 46 Figure 4.8 Figure 5.1 Figure 5.2 Transient grating responses of TiO 2 inverse opal (a) at various pump intensities (b) and nanocrystalline in diameter of 15 nm at 2.67 mj/pulse pump intensity. X-ray diffraction patterns for TiO 2 inverse opal and that of with 24h CdSe QDs adsorption. SEM images of TiO 2 inverse opal electrodes with various CdSe adsorption times ix
14 Figure 5.3 Figure 5.4 Figure 5.5 Fig. 5.6 Fig. 5.7 Figure 5.8 SEM cross section images of TiO 2 inverse opal electrodes with 8h CdSe adsorption time. Reflection spectra of TiO 2 inverse opal with various CdSe adsorption times. Normalized PA spectra of TiO 2 inverse opal with various CdSe QDs adsorption times at 10 O C. Dependence of CdSe QDs diameter on adsorption times at various temperature. IPCE spectra of TiO 2 inverse opal with CdSe QDs for different deposition times at 10 O C. J-V characteristic of TiO 2 inverse opal made from latex diameter of 309 nm with various CdSe adsorption times at 10 O C Figure 6.1 LF-HD-TG response of TiO 2 inverse opal. 66 Figure 6.2 Transient grating responses of 8h CdSe adsorption onto TiO 2 inverse opal in air and electrolyte (S 2- ) measurements. 68 Figure 6.3 Figure 6.4 Figure 6.5 Figure 6.6 Figure 6.7 Figure 6.8 Figure 6.9 Schematic illustrations of photoexcited electron and hole dynamics in CdSe QD-adsorbed onto TiO 2 inverse opal in air and electrolyte (S 2- ) measurements. Experimental and theoretical fitting results of the LF-HD-TG responses of TiO 2 inverse opal with various CdSe adsorption times (4, 8 and 24 h). Dependences of the time constants of fast (τ 1 ) and slow (τ 2 ) decay processes on the CdSe adsorption time. Normalized PA spectra of TiO 2 inverse opal and nanocrystalline TiO 2 with 4h CdSe adsorption time. (The vertical dotted line is shown to ensure definitely the E 1 determination). Absorbance of 4h CdSe QDs adsorbed TiO 2 inverse opal and nanocrystallinetio 2 after being dissolved in HNO 3 solution. Experimental and theoretical fitting results of the LF-HD-TG responses of the TiO 2 inverse opal and nanocrystalline TiO 2 with CdSe adsorption time of 4 h. TG responses of 5.5 nm sized CdSe QDs on TiO 2 inverse opal and glass x
15 Figure 7.1 Figure 7.2 Figure 7.3 Figure 7.4 Figure 7.5 Figure 7.6 Figure 7.7 Figure 7.8 J-V characteristic of typical CdSe QD-sensitized TiO 2 inverse opal and nanocrystalline TiO 2 solar cells. J-V characteristic of four different TiO 2 inverse opal electrodes made from a latex template with a diameter of 309 nm (TiO 2 /CdSe, TiO 2 /CdSe/ZnS, TiO 2 /F/CdSe/F and TiO 2 /F/CdSe/F/ZnS). IPCE spectra of two different TiO 2 inverse opal electrodes made from a latex template with a diameter of 204 nm (TiO 2 /F/CdSe/F/ZnS and TiO 2 /CdSe). Photo-voltage transient responses in (a) linear scale and (b) logarithmic scale of two different TiO 2 inverse opal electrodes made from a latex template with a diameter of 204 nm (TiO 2 /F/CdSe/F/ZnS and TiO 2 /CdSe). The inset (in (a)) shows the typical experimental and theoretical fitting results of the photovoltage transient responses of the TiO 2 /CdSe where the units of the inset are same as the main figure. J-V characteristic of two different TiO 2 inverse opal electrodes made from latex template with diameter of 309 and 394 nm (TiO 2 /CdSe and TiO 2 /F/CdSe/F/ZnS). Anodic and cathodic polarization curves of Pt-coated FTO and Cu 2 S electrodes in polysulfide electrolyte (1M S 2-, 1M S). J-V characteristic of different TiO 2 inverse opal electrodes (TiO 2 /CdSe, TiO 2 /F/CdSe/F/ZnS and TiO 2 /F/CdSe/F/ZnS/Li) with different used counter electrodes (Pt and Cu 2 S). J-V characteristic of different TiO 2 inverse opal electrodes with Cu 2 S counter electrode xi
16 List of tables Table 2.1 List of some properties of rutile and anatase structures. 15 Table 2.2 List of some properties of CdSe (Wurtzite structure) Table 4.1 Table 5:1 Table 7.1 Table 7.2 Table 7.3 Time constants of fast (τ 1 ) and slow (τ 2 ) transient grating decay processes on thetio 2 inverse opal at various pump intensities. Photovoltaic properties of TiO 2 inverse opal electrodes made from latex diameter of 309 nm with various CdSe adsorption times at 10 o C. Photovoltaic properties of different TiO 2 inverse opal electrodes made from latex templates of 309 and 394 nm in diameter. Photovoltaic properties of different TiO 2 inverse opal electrodes used with different counter electrodes (Pt, Cu 2 S). Photovoltaic properties of improved TiO 2 inverse opal electrodes used with Cu 2 S counter electrode xii
17 INTRODUCTION 1 Introduction 1.1 Background At the present time, most of the world s consumption of energy is based on coal, oil and natural gas. However, the use of this fossil fuel could result in pollution. Burning coal produces sulphur dioxide, an acidic gas that contributes to the formation of acid rain and burning any fossil fuel produces carbon dioxide, which leds to the "greenhouse effect", warming the Earth. There has been an enormous increase in the demand for energy since the middle of the last century as a result of industrial development and population growth. In the 1970s, Middeast oil crisis panicked world due to petroleum shortage and high gasoline prices 1. The supply shortage and environmental problems have attracted our interest to find another energy source beyond fossil fuel. Solar energy is an alternative renewable energy without any environmental damage. The following is several advantages of solar energy, Inexhaustible fuel source No pollution (enviromentally friendly) Readily availability (in a sunny enough climate) Often an excellent supplement to other renewable sources Versatile, is used for powering items as diverse as solar cars and satellites A Solar cell is a semiconductor device that converts photons (solar light) into electricity. Photovoltaic effect Photovoltaic effect is the basic process through which a solar cell converts solar radiation into electricity, which was first observed by Henri Becquerel 2 in He generated electricity between two electrodes attached to a liquid system upon irradiating light onto 1
18 INTRODUCTION this system. The next significant photovoltaic development arose from the first demonstration of photovoltaic effects in selenium (solid-state system) by Adam and Day 3 in They observed the photovoltaic effect by illuminating a junction between the platinum and selenium. The next significant step forward came with the work of Fritts 4 in He was able to prepare a selenium solar cell, the first "thin-film" photovoltaic devices, consisted of thin selenium films which adhered to one metal plate (e.g brass) and gold leaf. Fundamentally, there are three basic requirements for the photovoltaic effect (shown in Fig. 1.1). First, upon irradiation, light or photon should be absorbed creating electron-hole pairs. Then these electron-hole pairs should be separated so that their recombination is inhibited. In the end, these electrons and holes should be collected separately by each of collecting electrodes. Anode is the electrode collecting electrons and cathode is electrode collecting holes. So that current can be induced to flow in a external circuit. Cathode Anode - + Light absorbing material Figure 1.1: Basic operation of solar cells. Classification of solar cells Generally, solar cell technologies are classified into three generation (shown in Fig. 1.2) according to Martin Green from University of New South Wales (UNSW). The first generation solar cells is aimed to obtain high efficiency and mainly based on crystalline silicon, which is currently used worldwide with the best laboratory energy conversion efficiency achieved 25%. The theoretical limit of the solar cell using single junction is calculated by Shockley and Queisser 5 to be about 31%, limited to the thermalization loss of hot carriers (heat loss of the excess kinetic energy of hot photogenerated carriers 2
19 INTRODUCTION created by the absorption of photon with high energy) and no absorption of photon with energy less than band gap. In fact to synthesis such high purity silicon, it requires high cost, energy-intensive high-temperature (more than 1000 O C) and high-vacuum processes. The cost of silicon solar cell may be reduced by thin film technology (know as second generation solar cells), in which thin film silicon solar cells are mainly deposited by chemical vapour deposition (CVD) from silane gas (SiH 4 ) and hydrogen in 200 O C. This process produces amorphous silicon (no crystalline orientation) with lower conversion efficiency. The best laboratory efficiencies could be achieved 20% for polycrystalline silicon and 15% for amorphous silicon. The other second generation solar cells include the chalcogenide material, such as cadmium telluride (CdTe), copper indium sulfide (CIS) and copper indium gallium selenide (CIGS). These materials are applied in a thin film to a supporting substrate such as glass or ceramics reducing material mass and therefore costs. These technologies, particularly CIGS-CIS, DSC and CdTe offer significantly cheaper production costs. Dye-sensitized solar cells (DSSCs) and organic solar cells are the advanced thin film photovoltaic with low cost and simple production based on photoelectrochemical method. So far, the energy conversion efficiency of DSSCs could exceed 11%. 6 Third generation solar cells aim to enhance the performance of second generation (thin-film technologies) while maintaining very low production costs. Current research purposes to obtain the energy conversion efficiencies of 30-60% while retaining low cost materials and manufacturing techniques. 7 This may be achieved by exploiting the hot photogenerated carriers in quantum dots (QD) to produce higher photovoltages or Generations of Solar 1 st high η Crystalline Silicon Silicon (amorphous, Polycrystalline) 2 nd Low-cost thin Chalcogenide (CdTe, CIS, CIGS) Dye-sensitized Organic Figure 1.2: Classification of solar cells. 3 rd low-cost, Unique properties Quantum dot 3
20 INTRODUCTION higher photocurrents. 8 The former is based on miniband transport and collection of hot carriers in QD array photoelectrodes before their relaxation to the band edges through photon emission and heat loss consequently. The latter is based on generation and collection additional electron-hole pairs by utilizing hot carriers in QD solar cells through enhanced impact ionization processes. Further, the scope in this study concentrates on the DSSCs and the application of semiconductor QD (i.e. CdSe QD) as photosensitizer replacing dye. Dye-sensitized solar cells (DSSCs) Dye Sensitized TiO 2 Nanocrystalline solar cells (DSSCs) has attracted much attention after the pioneering work of dye-sensitized nanocrystalline TiO 2 by Grätzel and coworkers. 9 In this cell, the use of dye molecules as photosensitizers, nanostructured TiO 2 as the electron transport layer and I /I 3 redox couple as hole transport layer dramatically improve light harvesting efficiency. In order to achieve efficient solar energy conversion as well as long-term photostability, some researchers have applied quantum dot semiconductors as dye substitute for sensitizers. 10, 11 Due to the quantum confinement effect, 12, 13 the bandgap of semiconductor nanoparticles and, hence its optical absorption, can be modulated over a wide spectral range by controlling their size to match the distribution of solar light. Moreover, semiconductor nanocrystals have robust inorganic nature so these particles are more stable against photodegradation than the usual organic dyes. 14 The relatively low efficiency obtained in DSSC is assigned to the poor penetration of material into the thick TiO 2 film, and the detachment of hole transport layer from TiO 2 electrode. 15 In order to address the penetration of both sensitizers and redox couples, a novel approach has been proposed using mesoporous inverse opal titania starting from self-organizing systems, such as opal of polystyrene latex, as template. 16 This inverse opal (volumetric inverse of opal) titania has large interconnected pores lead to better infiltration. In addition, it also exhibits photonic band gap (frequency range that will not allow the propagation of particular wavelengths because of multiple Bragg reflection), which depends on the filling fraction of TiO 2 in the inverse opal structure. On the red-edge of the photonic band gap, the photon will be localized in high refractive index layer of sensitized TiO 2 inverse opal thus could significantly enhance the sensitizer absorption, especially in the edge of optical absorption where the sensitizer absorbs weakly solar light. 17 4
21 INTRODUCTION 1.2 Purposes Based on the background mentioned above, this study mainly focuses on the study and application of inverse opal structured electrode and CdSe QD replacing nanocrystalline electrode and dye sensitizer, respectively. CdSe is selected among the other semiconductors due to the possible electron injection from CdSe to TiO 2 and its wellknown properties. The purposes of this study are as follows: 1. Synthesis three dimensional TiO 2 inverse opal and characterize its physical properties. 2. Adsorb CdSe quantum dots on TiO 2 inverse opal and characterize the photosensitization of TiO 2 inverse opal with CdSe QDs 3. Study the ultrafast carrier dynamics of CdSe QD-adsorbed TiO 2 inverse opal. 4. Investigate the photovoltaic properties of CdSe QD-adsorbed TiO 2 inverse opal and introduce suggestions for better solar cells. 1.3 Brief outline of the thesis This thesis consists of a total of eight chapters. The first chapter shows the introduction to this field, purpose and outline of this thesis. Chapter two is aimed to provide a general overview of some of the concepts that are needed for reading this thesis. It is by no means a complete overview, thus many references are necessary to provide the comprehensive background to this field. Chapter three explains the experimental procedures, i.e. sample preparations and used characterization techniques. The former includes the TiO 2 inverse opal preparation and the method to adsorb the CdSe QD in situ on TiO 2 inverse opal. The latter includes the used technique to characterize its structure, morphology, reflection, optical absorption, photocurrent, photovoltaic properties. Characterization of carrier dynamics in both CdSe and TiO 2 inverse opal is performed by using femto laser and nanosecond laser. Chapter four describes the structure, morphology and optical absorption of TiO 2 inverse opal. Its unique properties due to the Bragg reflection are characterized using reflection and transmittance spectroscopy. The studied photocurrent and morphology dependent thermal conductivity of TiO 2 later open its wide applications. Chapter five shows the adsorption of CdSe in situ on TiO 2 inverse opal and its effect on the morphology and reflection properties. Photosensitization of TiO 2 inverse opal with 5
22 INTRODUCTION CdSe QDs is studied by characterizing its optical absorption, photocurrent as well as photovoltaic performances as a function of adsorption times. Chapter six discusses the ultrafast carrier dynamics of CdSe QD-adsorbed TiO 2 inverse opal. Effects of the presence of electrolyte, CdSe adsorption times and morphology of TiO 2 on the ultrafast carrier dynamics are included. Chapter seven covers characterization of the photovoltaic properties of CdSe QDsensitized TiO 2 inverse opal in solar cell application. Its typical photovoltaic properties will be compared with those of CdSe QD-sensitized nanocrystalline TiO 2 solar cells. To increase solar cell performance, the surface modification with ZnS and F ions on CdSe QD-sensitized inverse opal electrode is introduced including the determination of this effect by using transient photovoltage. Metal sulfide electrode is then used as active counter electrode against the polysulfide electrolyte, usually used as redox couple in QDsensitized solar cells, in replacing platinum counter electrode. Further improvements are suggested resulting in efficient QD-sensitized solar cells. Chapter eight, the final chapter in this thesis, summarizes the whole results in this study. The chapter ends with general feedback for further investigation in order to realize its wide ranged and useful applications. References A. E. Becquerel, Comt Rend. Academie d. Sciences 9 (1839) W. G. Adams and R. E. Day, Proc. Roy. Soc. London Ser. A 25 (1876) C. E. Fritts, Amer. J. Sci. 26 (1883) W. Shockley and H. J. Queisser, J. Appl. Phys. 32 (1961) A. Islam, Y. Chiba, Y. Watanabe, R. Komiya, N. Koide and L. Han, Jpn. J. Appl. Phys., 45 (2006) L M. A. Green, Physica E 14 (2002) A. J. Nozik, Physica E 14 (2002) B. O Regan and M. Grätzel, Nature 353 (1991) R. Vogel, P. Hoyer and H. Weller, J. Phys. Chem. 98 (1994) L. M. Peter, D. J. Riley, E. J. Tull and K. G. V. Wijayanta, Chem. Commun. 10 (2002) L. E. Brus, J. Chem. Phys. 79 (1983)
23 INTRODUCTION 13 L. E. Brus, J. Chem. Phys. 80 (1984) D. F. Underwood, T. Kippeny and S. J. Rosenthal: Eur. Phys. J. D 16 (2001) S. Tanaka, Jpn. J. Appl. Phys. 40 (2001) J. Wijnhoven, W. Vos, Science 281 (1998) S. Nishimura, N. Abrams, B. A. Lewis, L. I. Halaoui, T. E. Mallouk, K. D. Benkstein, J. van de Lagemaat and A. J. Frank, J. Am. Chem. Soc. 125 (2003)
24 THEORETICAL BACKGROUNDS 2 Theoretical backgrounds 2.1 Dye-sensitized solar cells (DSSCs) Dye-sensitized solar cells 1 (shown in Fig. 2.1) are based on the mechanism of regenerative cell, which converts light to electric power leaving no net chemical change behind. The main difference of this type of solar cell compared with conventional cells is that the functional element responsible for light absorption (the dye) is separated from the charge carrier transport itself. In the case of the n-type semiconductor TiO 2, this results in working cycle starting with the dye excitation by an absorbed photon and an electron injection into the conduction band of TiO 2. The injected electrons may migrate to the back contact and can be extracted as an external current. The dye is subsequently reduced by a redox electrolyte, based on an organic solvent and the redox couple. The redox electrolyte e - Conduction band e - Light Back contact Fermi level TiO 2 Dye Reduction ΔV - I 3 I - Oxidation e - Pt Valence band Electrolyte Figure 2.1: Schematic representation of dye-sensitized solar cell. 8
25 THEORETICAL BACKGROUNDS also accomplishes the charge transport between the counter electrode and the dye molecule. For a low-resistant electron transfer, the counter electrode is covered with some Pt, which acts as a catalyst for the redox reaction. 2 On DSSC, only dye molecules directly attached to the semiconductor surface are able to efficiently inject charge carriers into the semiconductor with a quantum yield of more than 90%. As the overall light absorption of a dye monolayer is only small, this limits the photocurrent efficiency with respect to the incident light to a value well below 1%. This mechanism could be evaded by the preparation of titanium dioxide electrodes with large surface area. The pioneering example of the dye-sensitized nanocrystalline TiO 2 solar cell by Grätzel and co-workers has attracted great interest in nanostructures photoelectrodes as an alternative to conventional single-crystal solar cells. 2 In this cell, a nanocrystalline titanium dioxide layer dramatically improves light harvesting, relative to a nonporous electrode, because its surface area is approximately a thousand times higher. Ru-based dyes are used for light absorption and electron injection in the conduction band of the nanocrystalline TiO 2 whereas a triiodide/iodide redox couple regenerates the dyes. 2.2 Inverse opal structured electrode To enhance the red response of DSSCs, an approach on confining photons to the high refractive index dye-sensitized TiO 2 has been proposed instead of altering the dye absorption. 3 It could be realized by utilizing the localization of light in the high index portion of the photonic crystal by means of TiO 2 photonic crystal. TiO 2 inverse opal (or face centered cubic crystals of air spheres in TiO 2 ) is one of the photonic crystals. The photonic crystals also have photonic band gap forbidding the propagation of certain frequency range of light through the structure due to the occurrence of Bragg reflection in its periodic dielectric structure, which is not mainly aimed in solar cell application. In fact, the nonlinear dispersion of light in a periodic dielectric of TiO 2 inverse opal may be used to increase the light harvesting efficiency of dye-sensitized TiO 2 inverse opal. Near the wavelength of photonic band gap, the group velocity of the light becomes anomalously small and the light can be described increasingly as a standing wave. On the blue edge of photonic band gap, the peaks of this wave are primarily localized in the low dielectric part of the photonic crystal, and on the red edge, they are localized in the high dielectric part. This implies that an absorber in the high dielectric medium should interact more strongly with light at wavelength to the red edge of photonic band gap, and less strongly to the blue. 9
26 THEORETICAL BACKGROUNDS Moreover, TiO 2 inverse opal electrode has been proposed to substitute nanocrystalline TiO 2. 4 However, the reported photovoltaic conversion efficiency of dye-sensitized TiO 2 inverse opal is only 0.6%, which is much lower than found with common DSSCs, possibly because of the difference in film thickness. In the case of solid state DSSCs, higher efficiency could be obtained by using TiO 2 inverse opal rather than nanocrystalline TiO 2 due to the fact that its large interconnected pores lead to a better penetration of both sensitizers and hole transporting material, throughout the whole depth of TiO 2 film. 5 In solid state DSSCs, the used of electrolyte or redox couple is not in the liquid system but in solid or quasi solid system and it is preferable to its practical use. 2.3 Semiconductor quantum dot sensitizer Instead of using organic dye, we use inorganic semiconductor nanoparticles, such as quantum dots (QDs), as a sensitizer of TiO 2, where these semiconductors QDs have to fulfill the following requirement (as shown in Fig. 2.2), 1. Broad absorption in visible and near-ir region. 2. Higher energetic LUMO of semiconductor QDs than the edge of TiO 2 conduction band. 3. High stability in the oxidized state against oxidation agent (TiO 2 ). e - Energy level of electron small Figure 2.2: Requirements for TiO 2 sensitizers. Semiconductors QDs have other potentialities that promise to exceed the capability of DSSCs. Its large extinction coefficient and dipole moment relative to the dye could enhance the absorption strength and the photogenerated carrier lifetime. Due to the quantum confinement effect, the band gap of a semiconductor nanoparticle can be 10
27 THEORETICAL BACKGROUNDS modulated over a wide spectral range and, hence, its optical absorption can be tuned to match the spectral distribution of sunlight. Moreover, the possibility also exists for efficient hot-carrier injection due to slow cooling of hot electrons in quantum dots and carrier multiplication by impact ionization Large extinction coefficient and dipole moment Semiconductor QDs, such as CdTe, CdSe and CdS QDs have the extinction coefficient per mole of quantum dots at the first excitonic absorption in the order of 10 5 cm -1 M -1 and it strongly depend on the size of the quantum dots. 6 This is ten times higher than the extinction coefficient per mole of Ru-based dye molecules at the metal to ligand charge transfer transition (MLCT) band in the order of 10 4 cm -1 M CdSe QD exhibit large dipole moments ( Debye for the diameters of nm) originated by the highly polar character of Cd-Se ionic bond, polar bonding geometry, surface strain and number of surface states (surface localized charges). 8 This leads to rapid charge separation inside CdSe QD upon photoexcitation. On the other hand, Ru-complex dyes (usually used as photosensitizer) have lower dipole moments than that of QD varying from 10 to 30 Debye depending on its conjugated ligands and directions Quantum confinement effect The band gap (E g ) of a semiconductor is defined as the energy difference between the highest energy valence band states and the lowest energy conduction band states. The excitation of an electron from the valence band to the conduction band leaves a hole in the valance band. The electron and hole can form a bound state through Coulombic interactions. This bound electron-hole pair is called an exciton 10 (in this case a Wannier exciton). The bound state has energy slightly less than the energy of the band gap. When the radius of the nanoparticle approaches the size of the exciton Bohr radius, the motion of the electrons and holes become confined in the nanoparticle. The Bohr radius (a B ) of the exciton is given by a B = 4πε ε h m 1 m * * 0e e mh (2.1) where ε is the high frequency relative dielectric constant of the medium m e is the effective mass of the electron (in m 0 units) 11
28 THEORETICAL BACKGROUNDS m h is the effective mass of the hole(in m 0 units) m 0 is the mass of the electron at rest. Energy Level HOMO LUMO E g Conduction band Valence band Atom Diatomic molecule Cluster Quantum Dots Bulk Particle size (nm) Figure 2.3: Quantum confinement effect on electron energy levels. This resulting Bohr radius for excitons in semiconductors is much larger than that of hydrogen atom. A created electron-hole pair can only fit into a nanoparticle when the charge carriers are in a state of higher energy. As a consequence of this, the band gap increases with decreasing particle size. In this regime of spatial confinement, the kinetic energy becomes quantized and the energy bands split into discrete levels shown in Fig Regarding this, both the absorption and emission spectra of the material shift to higher energies with decreasing particle size. One possible way to explain the quantum confinement effect is through the use of the 11, 12 effective mass approximation approach (EMA). Here, the size dependency of the band gap of the nanoparticle can be derived as: E E g = π 2m 2 h 2 o R 2 1 m * e 1 + m * h 2 1.8e 4πε oε R (2.2) 12
29 THEORETICAL BACKGROUNDS where R is the radius of the semiconductor particle and E g is the band gap of the bulk semiconductor. The first term in the equation above represents the particle in box quantum localization energy and has a 1/R 2 dependence. The second term represents the Coulomb energy having a 1/R dependence. For large R values, E approaches E g Hot carrier and multiple exciton generation Photoexcitation of a semiconductor with photons having energies above the band gap of semiconductor creates electron and holes with a total excess kinetic energy equal to the difference between the photon energy and band gap. This excess kinetic energy causes the effective temperature of carriers much higher than the lattice temperature, called as hot electrons and hot holes. To enhance the energy conversion efficiency, two fundamental ways to utilize hot carriers has been proposed by Nozik, 13 i.e. enhanced photovoltage and enhanced photocurrent. By utilizing hot photogenerated carriers to produce higher photovoltages and photocurrents, the maximum attainable thermodynamic conversion efficiency of solar photon could increase up to about 66 % from 31% for Schokley- Queissar limit 14 of single band gap cells. The former requires the extraction of the hot carriers before they cool through their respective carrier-carrier collisions (called carrier thermalization) and carrier-phonon interactions (phonon emission occurs as the result of the cooling of the carriers and heating of the lattice until carrier and lattice temperatures become equal; the phonons involved in the process are the longitudinal optical phonons). In order to achieve this, the rates of the photogenerated carrier separation, transport and interfacial transfer across the semiconductor interface must all be fast compared with the rate of carrier cooling. The latter requires the energetic hot carriers to produce a second or more electron-hole pairs (multiple exciton generation, MEG) through impact ionization due to strong carrier-carrier interactions, as shown in Fig This is the inverse of an Auger process whereby two electron-hole pairs recombine to produce a single highly energetic electron-hole pair. Thus for enhanced photocurrent, the rate of impact ionization (i.e. inverse Auger effect) should be greater than the rate of Auger process or carrier cooling. In bulk semiconductor, this MEG is inefficient because of the relatively weak Coulomb interactions, the restrictions imposed by energy and translational momentum conservation, as well as fast energy loss due to phonon emission. Strict selection rules and competing processes in the bulk permit MEG at energies of n x E g where E g is the band gap of bulk 13
30 THEORETICAL BACKGROUNDS semiconductorand n > 3; however, efficient MEG is observed only for n > 5 as a matter of fact. 15, 16 On the other hand, MEG become efficient in zero-dimensional quantum dots with the lower values of n (typically 2-3) 17 with respect to E 1 (first excitation energy and not Eg any longer in quantum confinement regime) because of wide separation between discrete electronic states inhibiting phonon emission due to phonon bottleneck, stronger Coulomb interactions and relaxation in translational momentum conservation. In phonon bottleneck, 18, 19 a large population of hot carriers produces a no equilibrium distribution of phonon (in particular, LO phonon that are the type involved in the electron-phonon interactions at high carrier energies) because the LO phonons cannot equilibrate fast enough with crystal bath; these hot LO phonons can be reabsorbed by the electron plasma to keep it hot. Besides Auger mechanism; electron-hole scattering, deep-level trapping and acoustical-optical photon interactions are other possible mechanisms for breaking the phonon bottleneck. hυ ne 1 E Figure 2.4: Representation of multiple exciton generation (E 1 relating to first excitation energy in quantum dot, likewise Eg relating to energy band gap of bulk semiconductor). The first experimental evidence for high efficiency MEG in quantum dot was detected in PbSe QDs. 20 Later, MEG was also observed for QDs of other semiconductor, such as PbTe, 21 CdSe, 22 InAs 23 including an important photovoltaic material Si. 24 Moreover, MEG in photocurrent was indicated in PbSe QD device structure. 25 However, more recent studies have questioned the efficiency of MEG particularly for CdSe 26 and InAs 27 QDs. Synthesis differences between samples may left some with the QD surfaces that affect the efficiency of MEG. So engineering QDs is the key factor to optimize the potential MEG in solar cell applications. 14
31 THEORETICAL BACKGROUNDS 2.4 Material properties Titanium Dioxide (TiO 2 ) Generally, titanium dioxide (TiO 2 ) is a fine white powder. Pure titanium dioxide does not occur in nature but is derived from ilmenite (FeTiO 3 ) or leuxocene ores. It is also readily mined in one of the purest forms, rutile beach sand. TiO 2 exists in a number of crystalline forms, anatase, rutile and brookite, but the most important are anatase and rutile. At higher temperatures, about 915 O C, anatase will automatically revert to the rutile structure, while about 750 O C is for brookite. Table 2.1 shows some properties of rutile and anatase structures. Table 2.1: List of some properties of rutile and anatase structures. Crystal structure Rutile Anatase Unit cell (Å) A C Refractive index Dielectric constant Density Melting point( ) 1858 Change to rutile on high temperature Band gap(ev) Arrangement of atoms 28 15
32 THEORETICAL BACKGROUNDS Titanium Dioxide (TiO 2 ) Photocatalyst Photocatalysis effect 29 was first investigated by A. Fujishima and K. Honda in 1972 where ultraviolet light-induced water has been achieved by using titanium dioxide (TiO 2 ) photoanode in combination with a platinum counter electrode soaked in electrolyte aqueous solution. TiO 2 is close to being an ideal photocatalyst because it is relatively inexpensive, highly stable chemically, the photogenerated holes are highly oxidizing and photogenerated electrons are reducing enough to produce superoxide from dioxygen. Since the bandgap of TiO 2 is 3 ev, upon UV irradiation, holes can react with water and produce hydroxyl radical ( OH). Although some electrons can become trapped and lose some of their reducing power, a significant number are still able to reduce dioxygen to superoxide O - 2. Depending upon the exact conditions, the holes, OH radicals, O - 2, H 2 O 2 and O 2 itself can play important roles in the photocatalytic reaction mechanisms. 30 As a photocatalyst, TiO 2 has been used in diverse fields, such as: 1. fog proof, and self cleaning glass 2. anti-bacterial, anti-viral, fungicidal 3. anti-soiling, self cleaning 4. deodorizing, air purification (removing nitrogen oxide from air) 5. water treatment, water purification Energy of Electron Conduction Band UV light Valence Band e - O 2 <Reduction> O - 2 (superoxide anion) OH(hydroxide radical) h + <Oxidation> H 2 O Figure 2.5: Schematic diagram of photocatalytic reaction by TiO 2. The photocatalysis invention has opened up the possibility of photochemical solar energy conversion by semiconductor or sensitizers as a new energy source to overcome the crisis energy and global warming. Its photoelectrochemical process has been aimed as alternative approach to that of the solid state junction photovoltaic cell. 16
33 THEORETICAL BACKGROUNDS Cadmium Selenide (CdSe) Cadmium selenide or cadmium (II) selenide, sometimes written as the chemical formula of CdSe, belongs to the class of II-IV semiconductors. CdSe is selected as the photosensitizer of TiO 2 due to its smaller band gap of CdSe relative to TiO 2 extending the optical absorption to visible region as well as its well-known properties (shown in Table 2.2). Moreover, the more negative energetic bottom level in conduction band of CdSe than that of TiO 2 and the more positive energetic top level in valence band of CdSe than that of TiO 2 (shown in Fig ) indicate the possible injection of photoexcited electrons from CdSe to TiO 2 conduction band and no injection of photoexcited holes from CdSe to TiO 2 valence band making the charge separation upon photoexcitation in CdSe feasible. Figure 2.6: Band edges position of several semiconductors in contact with aqueous electrolyte at ph 1. CdSe is generally yellow to red crystalline solid with melting point at 1268 C. This material can be crystallized in either the wurtzite or hexagonal structure, as shown in Fig The formula weight is gram/mol. It is an intrinsic semiconductor with a band gap of 1.70 ev at 300 K. Some characteristics of CdSe in wurtzite structure are presented in Table
34 THEORETICAL BACKGROUNDS Table 2.2: List of some properties of CdSe (Wurtzite structure). 33 Density: 5.81 g/cm 3 Lattice parameter a = Å c = Å Dielectric constant 10.2 Young s Modulus dyne/cm 2 Electron mobility (300 K) < 650 cm 2 V -1 s -1 Coefficient of thermal expansion (500 K) Specific Heat Thermal conductivity (at 25 o C) α 1 = /K α 3 = /K 0.49 J/gK 0.04 W/cmK Max. Transmittance (λ = μm) 71 % Absorption coefficient (λ =10.6 μm) cm -1 (including 2 surfaces) Refractive index (λ =10.6 μm) 2.4 Solubility in water Insoluble In nano size, CdSe exhibits the quantum confinement effect where CdSe nanocrystals of different sizes exhibit different colors. With decreasing crystal size the band gap of the crystal increases and the dot emits more energetic or bluer photons. CdSe QDs which is grown by using method of colloidal chemistry, (core-shell) CdSe/ZnS with TOPO as surface stabilizing molecule has high luminescence efficiency (65 %) at room temperature. (a) (b) Cd Se Figure 2.7: Solid state structure of CdSe. (a) Wurtzite structure (b) hexagonal structure. 18
35 THEORETICAL BACKGROUNDS Recently this nanocrystal has found important applications in Biology. QDs are coupled to biological molecules for use in ultrasensitive biological detection at the single-dot level. Quantum dots are used there as fluorescent tags capable of tracing specific proteins within cells and in the future it is hoped to develop lighting up DNA or viruses by QDs Photonic crystal Figure 2.8: Real space representation of a photonic crystal and reciprocal space regions for which propagation is shielded. Photonic crystals (PC) are periodically dielectric structure along one, two or three directions of space that enables to control the flow of photons by means of photonic band gap (PBG), ranges of frequency in which light cannot propagate through the structure. The idea of a three-dimensional photonic band gap crystal was proposed by Yablonovich in In a composite formed by two dielectrics, light will propagate more slowly in the scattering centers, i.e, that of the higher dielectric constant, ε. If the scattering centers are regularly arranged in a medium, light is coherently scattered. In this case, interference will eventually cause that some frequencies will not be allowed to propagate, giving rise to forbidden and allowed bands. Under certain conditions, regions of frequency may appear that are forbidden regardless of the propagation direction in the PC, shown in Fig In such case, this PC is said to present a full PBG. On the red edge of the PBG, lights are primarily localized in the high dielectric part of the photonic crystal, and on the blue edge, they are localized in the low dielectric part. 19
36 THEORETICAL BACKGROUNDS Figure 2.9: Energy dispersion relations for free electron and electron in a (1D) solid and for a free photon and a photon in a photonic crystal. The term of photonic band gaps is in analogy with the electronic band gap. The energy dispersion relation (i.e., the frequency-wavefactor relation) for an electron and photon in both vacuum and periodic dielectric is shown in Fig The energy dispersion for electron in vacuum is parabolic with no gaps. When a periodic potential is present gaps open and electrons with energies therein have localized (non-propagating) wavefunctions as opposed to those of electrons in allowed bands which have extended (propagating) wavefunctions. In a similar way, a periodic dielectric medium will present frequency regions where propagating photons are not allowed and will find it impossible to travel the crystal. PGB is appeared as a result of Bragg reflection at the Brillouin zone boundary, preventing the propagation of electromagnetic waves (photon). Considering photon in medium with energy λ/n (λ=the wavelength in vacuum, n=index refractive of material) and lattice distance, a, the Bragg reflection can be given by: mλ 2 a = (2.3) n 20
37 THEORETICAL BACKGROUNDS Where m, the order of the reflection is an integer 1, 2, 3... For a material which is periodically structured in air, the average refractive index will be given by: n a 2 2 = n f + n (1 f ) (2.4) air where f is a filling fraction, which is the percentage of total crystal volume occupied by any one of the materials. The optical features of a PC will depend on parameters indicated below: 1. The type of symmetry of the structure. 2. Dielectric constant contrast (e 1 / e 2 ). 3. Filling factor, that is, the ratio between the volumes occupied by each dielectric with respect to the total volume of the composite. 4. The topology, which can be either cermet (discontinuous topology of separated clusters in a matrix) where scattering centers are isolated from each other; or network where scattering centers are connected between them. 5. The shape of the scattering centers. All these factors determine the photonic band structure of the PC and, therefore, its optical properties. References 1 B. O Regan and M. Grätzel, Nature 353 (1991) A. Goetzberger and V. U. Hoffman, Photovoltaic Solar Energy Generation, Springer, Germany, (2005) p S. Nishimura, N. Abrams, B. A. Lewis, L. I. Halaoui, T. E. Mallouk, K. D. Benkstein, J. van de Lagemaat and A. J. Frank, J. Am. Chem. Soc. 125 (2003) C. L. Huisman, J. Schoonman and A. Goossens, Sol. Energy Mater. Sol. Cells 85 (2005) P. R. Somani, C. Dionigi, M. Murgia, D. Palles, P. Nozar and G. Ruani, Sol. Energy Mater. Sol. Cells 87 (2005) W. W. Yu, L. Qu, W. Guo and X. Peng, Chem. Mater. 15 (2003) M. K. Nazeeruddin, T. Bessho, L. Ceveya, S. Ito, C. Klein, F. De Angelis, S. Fantacci, P. Comte, P. Liska, H. Imai and M. Graetzel, J. Photochem. Photobiol. A : Chem. 185 (2007) M. Shim and P. Guyot-Sionnest, J. Chem. Phys. 111 (1999)
38 THEORETICAL BACKGROUNDS 9 F. de Angelis, S. Fantacci, A. Selloni, M. Graetzel and M. K. Nazeeruddin, Nanoletters 7 (2007) C. Kittel, Introduction to Solid State Physics (6 th edition), John Wiley and Sons, New York, 1986) p L. E. Brus, J. Chem. Phys. 79 (1983) L. E. Brus, J. Chem. Phys. 80 (1984) A. J. nozik, Annu. Rev. Phys. Chem. 52 (2001) W. Shockley, H. J. Queisser, J. Appl. Phys. 32 (1961) O. Christensen, J. Appl. Phys. 47 (1976) S. Kolodinski, J. H. Werner, T. Wittchen and H. Queisser, Appl. Phys. Lett. 63 (1993) A. J. Nozik, Phyisica E 14 (2002) P. Lugli and S. M. Goodnick, Phys. Rev. Lett. 59 (1987) R. P. Joshi and D. K. Ferry, Phys. Rev. B 39 (1989) R. D. Schaller and V. I. Klimov, Phys. Rev. Lett. 92 (2004) J. E. Murphy, M. C. Beard, A. G. Norman, S. P. Ahrenkiel, J. C. Johnson, P. Yu, O. I. Micic, R. J. Ellingson and A. J. Nozik, J. Am. Chem. Soc. 128 (2006) R. D. Schaller, M. A. Petruska and V. I. Klimov, Appl. Phys. Lett. 87 (2005) R. D. Schaller, J. M. Pietryga and V. I. Klimov, Nano Lett. 7 (2007) M. C. Beard, K. P. Knutsen, P. Yu, J. M. Luther, Q. Song, W. K. Metzger, R. J. Ellingon and A. J. Nozik, Nano Lett. 7 (2007) S. J. Kim, W. J. Kim, Y. Sahoo, A. N. Cartwright and P. N. Prasad, Appl. Lett. 92 (2008) G. Nair and M. G. Bawendi, Phys. Rev. B. 76 (2007) M. Ben-Lulu, D. Mocatta, M. Bonn, U. Banin and S. Ruhman, Nano Lett. 8 (2008) A. Fujishima and K. Honda, Nature 238 (1972) M. Kaneko and I. Okura, Photocatalysis Science and Technology, Springer, Japan, (2002) p A. Hagfeldt and M. Graetzel, Chem. Rev. 95 (1995)
39 THEORETICAL BACKGROUNDS 35 Yablonovich, E. Phys. Rev. Lett., 58 (1987)
40 EXPERIMENTAL PROCEDURES 3 Experimental procedures 3.1 Sample preparation TiO 2 inverse opal The TiO 2 inverse opal was prepared by filling the void in an artificial template and subsequently removing the template. Generally speaking, it can be grouped into three steps as follows, as briefly described in Fig. 3.1: (1) Template preparation 1 1. Conductive fluorine-doped tin oxide (FTO) coated glass of 4.5 cm length and 2.3 cm width was cleaned ultrasonically with soap, concentrated KOH, distilled water and methanol, respectively wt. % of monodisperse hydrophobic polystyrene latex from Seradyn Co. was diluted with distillated water to a concentration of 0.1 wt. %. The suspension was then dispersed ultrasonically for 30 min. 3. The FTO glass was immersed vertically in 30 ml beaker containing 15 ml latex suspension. 4. The beaker was then kept in an oven at 40 O C for 1 to 2 days until the suspension was fully evaporated. (2) Filling the void in template with TiO % TiCl 4 (from WAKO, 99 %) in methanol (from WAKO, 99.8 %) as TiO 2 source was prepared by mixing 2 ml TiCl 4 and 98 ml methanol % TiCl 4 in methanol was vertically dropped on to a 1.3 x 2.3 cm template by using a 10μl sized micropipette. 3. The template was then kept in a desiccators (humidity %, temperature 24-25%) for 30 min. 4. The template was heated in an oven at 80 O C for 10 min. 5. The 2-4 processes were repeated for few times. 24
41 EXPERIMENTAL PROCEDURES In this step, heat treatment time at 80 O C is necessary to make a compact structure before the further addition of TiCl 4. (3) Calcinations of the template and annealing of TiO 2 Calcinations of the template and annealing of TiO 2 were conducted together in which the sample was heated at 450 O C with 0.5 O C/min heating rate. After 1 hour heating at 450 O C, the temperature was cooled down to room temperature within 3 hours. FTO Slow Evaporated at 40 o C Polystyrene latex colloidal (wt 0.1%) (a) Self-assembling latex template Dropping 2% TiCl 4 in CH 3 OH few times Hydrolysis 30min Heating at 450 o C Self-assembling template (b) Heat treatment at 80 o C (10 min) (c) Inverse Opal TiO 2 Figure 3.1: Schematic procedure of the fabrication TiO 2 inverse opal. (a) Template preparation, (b) filling the template with TiO 2 (c) calcination of template and annealing of TiO Adsorption of CdSe QDs on TiO 2 inverse opal All chemicals used in this deposition process are 3CdSO 4 H 2 O 99.0 %, N(CH 2 COONa) 3 H 2 O 97 %, NaSO 3 97 %, Se 99.0 % obtained from WAKO. For this study, CdSe QDs adsorption on TiO 2 inverse opal was conducted by chemical deposition method similar to what G. Hodes, et. al. 3 has done. The adsorption procedure is as follows: mm CdSO 4 was made by dissolving gr CdSO 4 in 25 ml disttiled water and 0.72 M Sodium nitrilotriacetate {N(CH 2 COONa) 3 } was made by mixing gr N(CH 2 COONa) 3 in 25 ml distilled water. 25
42 EXPERIMENTAL PROCEDURES 0.48 mm CdSO 4 solution(25 ml) 0.72 mm N(CH 2 COONa) 3 solution(25 ml) 2 mm NaSO 3 (100ml) mol Se (0.95gr) heated at 70 O C 0.12 mm Na 2 SeSO 3 solution(100ml) Chemical solution (80 mm CdSO 4, 120 mm N(CH 2 COONa) 3 and 80 mm Na 2 SeSO 3 ) Figure 3.2: Flow chart of chemical solution preparation for CdSe adsorption M sodium selenosulfate (Na 2 SeSO 3 ) in excess Na 2 SO 3 was made by stirring gr Na 2 SO 3 in 100 ml distilled water, resulting 2 M Na 2 SO 3, and subsequently adding mol Se (0.95 gr) and then mixing it at 70 O C for several hours). 3. The deposition solution was prepared by adding N(CH 2 COONa) 3 solution to CdSO 4 solution and finally Na 2 SeSO 3 solution was added. 4. TiO 2 inverse opal was then immersed for some times in the final chemical solution where placed in the water bath to keep the temperature constantly. After deposition, the sample was rinsed with distilled water and dried in nitrogen gas flow. CdSe QDs were adsorbed on TiO 2 inverse opal in various times in order to determine the best adsorption time. TiO 2 TiO 2 +CdSe For some times Chemical solution (before) Chemical solution (after) Figure 3.3: CdSe quantum dots adsorption. 26
43 EXPERIMENTAL PROCEDURES 3.2 Characterization techniques Scanning electron microscopy 4 Scanning electron microscopy (SEM) is the best known and most widely-used of the surface analytical techniques. The first Scanning Electron Microscope (SEM) debuted in 1942 with the first commercial instruments around SEM is considered a relatively rapid, inexpensive, and basically non-destructive approach to surface analysis. High resolution images of surface topography, with excellent depth of field are produced using a highly-focused, scanning (primary) electron beam. The primary electrons enter a surface with an energy of kev, and generate many low energy secondary electrons. The intensity of these secondary electrons is largely governed by the surface topography of the sample. An image of the sample surface can thus be constructed by measuring secondary electron intensity as a function of the position of the scanning primary electron beam. High spatial resolution is possible because the primary electron beam can be focused to a very small spot (< 10 nm). High sensitivity to topographic features on the outermost surface (< 5 nm) is achieved when using a primary electron beam with an energy of < 1 kev. In addition to low energy secondary electrons, backscattered electrons and X-rays are also generated by primary electron bombardment. The intensity of backscattered electrons can be correlated to the atomic number of the element within the sampling volume. Hence, some qualitative elemental information can be obtained. The analysis of characteristic X- rays emitted from the sample gives more quantitative elemental information. Such X-ray analysis can be confined to analytical volumes as small as 1 cubic micron X-Ray diffraction spectrometry In 1895, the German physicist Roentgen discovered X-rays. Even before this method was understood, people have already begun to use it. Its earliest use was for radiographic method. Later, it was put to use by engineers wanting to study the internal structure of opaque objects. It wasn t until 1912 that the phenomenon of X-rays diffraction by crystal was discovered, and this simultaneously proved the wave nature of X-rays and provided a new method for investigating the fine structure of matter. X-ray diffraction (XRD) is the coherent scattering of X-rays by a crystalline material. This technique can be used to obtain a wide range of structural information. The X-ray diffraction peaks from a polycrystalline solid can be used to determine the crystalline 27
44 EXPERIMENTAL PROCEDURES phase of the material, average grain size, residual stress, and preferred crystalline orientation (texture). High-resolution x-ray diffraction can be used to obtain the orientation and quality of single crystals and the composition and relaxation of epitaxial layers. XRD is a non-destructive technique and requires no special sample preparation. When the size of the individual crystals is less than 0.1μm (1000 Å), the term particle size is used 5. Crystals that have small sizes causes a broadening in the Debye rings. The extent of this broadening is given by the following equation (called the Debye-Scherrer formula): 0.9λ B = (3.1) t cosθ where B is the broadening of the diffraction line, measured at half its maximum intensity (radians) t is the diameter of the crystal particle λ is the X-ray wavelength θ is the angle of incidence of the X-ray beam at the maximum of the diffraction beam. When the particle size exceeds 1000 Å, B is essentially zero Reflectance spectroscopy Halogen haloge lamp n Monochromator White light Reflected light Computer DATA A Sample White light Reflected light Figure 3.4: Schematic diagram of reflectance spectroscopy. Reflection spectra were measured with fiber optic spectrometer equipped with reflection probe providing the illumination and detection from the same direction, as shown in Fig A 20 W tungsten halogen lamp was used as source light. The spectra were taken at room temperature in the wavelength range of 400 to 850 nm. 28
45 EXPERIMENTAL PROCEDURES Photoacoustic spectroscopy One of the most effective means for studying the properties of matter nondestructively is to observe how photons interact with the material. This is known as optical spectroscopy. This can be done by absorption and reflection spectroscopy. Another usable technique involves the measurement of photoacoustic signals. The term of photoacoustic usually refers to the generation of acoustic waves by modulated optical radiation 6. In a broader sense, photoacoustic means the generation of acoustic waves or other thermoelastic effects by any type of energetic radiation, including electromagnetic radiation from radio frequency to x ray, electrons, protons, ions and other particles. In 1976, Rosencwaig and Gersho derived the analysis of the photoacoustic process in solid. They found that the primary source of the acoustic signal in the photoacoustic cell arises from the periodic heat flow from the solid to the surrounding gas when the solid is cyclically heated by chopped light. Only a relatively thin layer of air adjacent to the surface of the solid responds thermally to that periodic heat flow. This boundary layer of air can be regarded as a vibratory piston, creating the acoustic signal detected in the cell. Since the magnitude of the periodic pressure fluctuations in the cell is proportional to the amount of heating coming from the solid absorber, there is a close correspondence between the strength of the acoustic signal and the amount of light absorbed by the solid. Backing material Sample Boundary layer of gas Gas (air) Quartz window D Incident Light L -(l+l b ) -l 0 2π/a g l g Figure 3.5: Cross-sectional view of a photoacoustic cell. x Consider a one-dimensional model of heat flow in the cell resulting from the absorbed light energy as shown in Fig The cell has a diameter D and length L where L is small 29
46 EXPERIMENTAL PROCEDURES compared to the wavelength of the acoustic signal. The sample has a diameter D and thickness l and the back surface has a thickness l b. The photoacoustic signal is ultimately governed by the magnitude of the optical absorption and thermal diffusion length of the solid. Since the thermal diffusion length can be changed by altering the chopping frequency, it is possible to obtain optical absorption spectra on any, but the most highly opaque, solids. If μ s <l then sample is thermally thick and the photoacoustic signal determined by its thermal properties, and if μ s >l then the sample is thermally thin and the photo acoustic signal becomes dependent on the thermal properties of the backing. For the optically transparent (optical absorption length, μ β >l) and thermally thin (μ s >l) solid, the photoacoustic signal (Q) is proportional to the optical absorption coefficient (β) as shown by the following equation, ( 1 j) βl μb Q ( ) Y (3.2) 2a k where a g is thermal diffusivity of enclosed gas. k b is thermal conductivity of backing material μ b is thermal diffusion length of backing material Y is constant. g b Monochromator Optical Lens PA Cell Xe-Lamp Sample Microphone Chopper Data Pre-Amplifier Computer Lock-in Amplifier Figure 3.6: Schematic diagram of photoacoustic spectroscopy. Figure 3.6 shows a system for solid state photoacoustic spectroscopy. A high-powered arc discharge lamp (i.e. Xe lamp) is used as the source of illumination and a scanning grating monochromator is used to achieve the spectral desired. A mechanical chopper 30
47 EXPERIMENTAL PROCEDURES modulates the monochromated light. Optical lenses are used for focusing. The PA cell hand-crafted from aluminum is a gas tight cell equipped with a microphone for signal detection. Quartz glass was used for the windows (since it is optically transparent in the low wavelength region). The sample is placed in the airtight cavity. In the presence of modulated light, the gas inside the cavity (air) is periodically heated. This causes slight changes in pressure inside the cell that is detected as an audible signal. The microphone detects this signal and it is amplified and stored in the computer. The reference sample used for normalization is a black absorber (carbon black sheet) to eliminate the spectral variations of the illumination source. Shown in Fig. 3.7 is a typical spectrum of a carbon black sheet PA intensity (a. u.) Photon energy (ev) Figure 3.7: Typical PA spectrum of carbon black Incident photon to current efficiency (IPCE) spectroscopy IPCE is the ratio of injected electron numbers to incident photon numbers which indicate the quantum yield for electron injection from the exited sensitizer (in this study: CdSe quantum dots) to the conduction band of the semiconductor (TiO 2 ) and the collection efficiency of the electron to the back-contact (FTO). IPCE or incident photon to current efficiency which corresponds to the quantum efficiency is calculated from Eq Injected electron numbers 1240 x I sc IPCE(%) = = x100 (3.3) Incident photon numbers λ x P I sc is the measured short current (ma), P is the incident light power (mw) and λ is wavelength of incident light (nm). 31
48 EXPERIMENTAL PROCEDURES Zero-Shuntmeter Data Xe-Lamp Monochromator Computer Electrolyte Sample Pt Optical Lens Quartz Cell FTO Figure 3.8: Schematic diagram of IPCE spectroscopy. Figure 3.8 shows the schematic diagram of IPCE (Incident Photon to Conversion Efficiency) spectrometer. In IPCE spectroscopy the incident light is directly focused on the sample, without modulation through chopper. The intensity of incident light is measured using power meter and the distribution of incident light is obtained by using photoacoustic spectrum of carbon Solar simulators Under illumination J sc Ideal Power Current density Dark Max Voltage V oc Figure 3.9: Typical J-V characteristics of a solar cell. 32
49 EXPERIMENTAL PROCEDURES The photovoltaic performances or I-V characteristics of solar cells are characterized using solar simulator. It yields important operational parameters that are the short-circuit current density (J sc ), the open-circuit voltage (V oc ), the current (I max ) and voltage (V max ) at the maximum power point (P max ). The fill factor combines these terms and is defined as FF P V J oc sc V V oc I J max max max = = (3.4) The fill factor does not depend on the area of the cell and is essentially a measure of quality of the solar cell. FF can also be interpreted graphically as the ratio of the rectangular areas depicted in Fig The energy conversion efficiency is the ratio of the electrical power output P out, compared to the solar power input, P in, into the PV cell. FF V in sc oc sc η = (3.5) P J AM 0 Atmosphere AM 1 AM = 1.5 sinθ θ = 41.8 O Figure 3.10: Air mass or optical path length of sunlight through Earth s atmosphere. The most frequently used experimental conditions are the irradiance of 100 mw/cm 2, which is defined as the standard 1 sun value with spectrum consistent to an air mass (AM) global value of 1.5 at ambient temperature 25 0 C. AM 1.5 is a standard reference solar spectral irradiance after the solar radiation has traveled through the atmosphere with the Sun at an altitude angle of 41.8º, which simulates a longer optical path through the Earth s atmosphere relative to the Sun at zenith above the Earth s atmosphere (AM 1), as shown in Fig The use of this standard irradiance value is almost universally used to characterize terrestrial solar panels and particularly convenient because the cell efficiency in percent is then numerically equal to the power output from the cell in mw/cm 2. 33
50 EXPERIMENTAL PROCEDURES Transient photocurrent or photovoltage spectroscopy Time-resolved measurements of the potential and current are methods to characterize the electron dynamics in photoanode. Photocurrent transient is used to determine the diffusion coefficient. 7 Photovoltage transient method measures the decay of free electrons in the conduction band of photoanode including not only the loss of electrons in the back reaction but also the changes in electron trap occupancy. 8 The schematic diagram of transient photocurrent/photovoltage spectroscopy is shown in Fig The irradiating illumination was obtained by the second harmonic generation of a Nd:YAG laser (GAIA, Rayture Systems) with a wavelength of 532 nm, repetition rate of 1 Hz and pulse width of 4 ns. Laser beam illuminates the sample generating carriers on the sample. The short circuit current density transients or open circuit voltage transients were monitored by potensiostat or galvanostat and transferred to a computer for data treatment. Part of the laser beam irradiates the photodiode and is used as trigger signal. Computer DATA Osilloscope Potensiostat/Galvanostat Potenstat Photodiode Electrolyte Nd:YAG Laser Nd: YAP Laser Splitter Optical Lens PEC Cell Sample Pt Figure 3.11: Schematic diagram of transient photocurrent/photovoltage spectroscopy. Transient photocurrent Transient photocurrent is mainly used to investigate the electron transport in nanostructured TiO 2 in contact with electrolyte. Diffusion current is derived by solving the time-dependent diffusion equation (Fick s second law) 7 for electrons defined in Eq n( x, t) t = D 2 n( x, t) 2 x (3.6) 34
51 EXPERIMENTAL PROCEDURES D is the electron diffusion coefficient, x is distance from the back contact, t is time and n is electron recombination. At t = 0 with the initial distribution at electrode surface ΔN, the solution with thickness of electrode W is n( x, t) = 2 ΔN πdt e ( x W ) 2 / 4 Dt (3.7) The current is the electron concentration gradient times the diffusion coefficient and the elementary charge q. By deriving the expression for current, the diffusion current I diff at the back contact x = 0 is I diff = 2 qwδn πdt 3 2 e W 2 / 4 Dt (3.8) At di diff /dt = 0, the current is maximum and the time for current maxima (t peak ) appears when 2 t peak = W / 6D (3.9) Thus there is linear dependence between the square of the film thickness and t peak. By measuring the TiO 2 film thickness and the t peak, the diffusion coefficient could be estimates. Transient photovoltage Transient photovoltage is used to investigate the relaxation time constant for electrons in the TiO 2 conduction band, referred as electron lifetime. It is determined not only by the rate of loss of electrons in the back-reaction (electron injection to electrolyte) but also by changes in electron trap occupancy. For quasi static condition in which trapping and detrapping govern the time response, the electron relaxation constant is defined as 9 = (3.10) τ L n τ n0 n nc where nl / n c is the kinetic factors for trapping and detrapping, τ n0 is the rate constant for charge transfer, for any kind or concentration of the acceptor species Heterodyne detection transient grating spectroscopy Transient grating method 10 is a powerful tool to detect various kinds of dynamics, such as molecular and lattice vibrations, bulk and surface acoustic waves, diffusion of molecules or heat, and relaxation of carriers or excited species. This method concerns the decay of refractive-index grating produced by interfering two optical beams and the related time constants depending on the physical effects involved ranging from 35
52 EXPERIMENTAL PROCEDURES millisecond down to femtoseconds. Recently, heterodyne detection 11 has been developed in transient grating technique, where pump and probe beams are coaxially incident on a special transmission grating called a phase mask and each divided into two beams. The two divided pump beams are focused on the sample and used for the two interfering pump beams. One of the two divided probe beams is used as probe beam and the other is used for a reference beam for the heterodyne detection. Heterodyne transient grating has several advantages, like simple and compact configuration, high sensitivity, easy finding of a signal and selective measurement of the real and imaginary parts of the dielectric function change. Excitation of sample Sample Detection of signal Pump Probe Signal Heterodyne Detection Transmission Reference Figure 3.12: Principle of the lens-free heterodyne transient grating (LF-HD-TG) technique (improved TG). The enhanced heterodyne transient grating, i.e. lens-free heterodyne transient grating (LF-HD-TG) technique, 12 is used in this study, where the interference pattern induced by two interfering pump beams is replaced by the transmission grating. Its principle is shown in Fig Before being incident on the transmission grating and sample, pump and probe beams are set coaxially by dichroic mirror. The pump beam propagates through the transmission grating and is diffracted by transmission grating resulting in the spatial intensity profile having an interference pattern in the vicinity of the other side of transmission grating surface. This interference pattern having same grating spacing as that of transmission grating in principle excites the sample and produces a transient grating in sample. The delayed probe beam also propagates into the transmission grating and diffracted by the transmission grating and also diffracted by the transiently excited grating in sample. These two diffractions are principally progress in the same direction, and these two mixed diffraction signals are then detected by detector set at the point of the visible +1 diffraction by the transmission grating. It automatically 36
53 EXPERIMENTAL PROCEDURES satisfies the condition for heterodyne detections and thus the changes of the refractive index versus time can be monitored by the diffraction signal. This improved technique features 1. simple and compact optical equipment and easy optical alignment as no lens is used for focusing beams on sample, 2. easy control of the phase difference between the probe and reference beams, and 3. high stability of phase due to the short optical path length of the probe and reference beams. The detected signal intensity (I s ) is expressed as 13 I I ~ 2 = + Δε I I + 2( Δε cos Δφ + Δε sin Δφ) I I (3.11) s R ex p Where I R, I ex and I p are the intensities of reference, pump and probe beams, respectively, Δ ~ ε = Δε + i Δε is the dielectric function change of the sample, and φ is the phase 1 2 difference between the probe and reference beams. The second and third terms in Eq are the homodyne and heterodyne components. I R is removed by lock-in amplifier in the measurement. Detection of real ( Δ ε1 ) and imaginary ( Δ ε ex p ) in the dielectric function change, or refractive index as consequence, can be selectively detected simply by changing the sample grating distance. In the experiments, the difference in the optical path length between the probe and pump beams satisfies the following condition, D = nλ / 4 (3.12) where λ is the probe wavelength and n is a positive integer including zero. Δ ε1 is selectively detected when n is an even number. Depending on the pulse width, e.g. femto or nano second lasers, different physical phenomena could be detected with time constants ranging from milliseconds to femtoseconds. References 1 青井芳史他, 日本材料科会読, 53 (2004) R.Vogel, K.Pohl and H. Weller, Chem. Phys. Lett. 174 (1990) S.Gorer and G.Hodes, J. Phys. Chem. 98 (1994) B.D. Cullity, Elements of X-Ray Diffraction 2 nd ed., Addison-Wesley Publishing Co. Inc., (1978) p
54 EXPERIMENTAL PROCEDURES 6 A. C. Tam, Rev. Mod. Phys. 58 (1996) A. Solbrand, H. Lindström, H. Rensmo, A. Hagfeldt and S. E. Lindquist, J. Phys. Chem. B 101 (1997) M. Bailes, P. J. Cameron, K. Lobato and L. M. Peter, J. Phys. Chem. B 109 (2005) J. Bisquert and V. S. Vikhrenko, J. Phys. Chem. B 108 (2004) H. J. Eichler, P. Gunter and D. W. Pohl, Laser-Induced Dynamic Gratings (Springer, Berlin, 1986). 11 A. A. Maznev, K. A. Nelson and J. A. Rogers, Opt. Lett. 23 (2002) K. Katayama, M. Yamaguchi and T. Sawada, Appl. Phys. Lett. 82 (2003) M. Terazima, J. Phys. Chem. A 103 (1999)
55 TiO 2 INVERSE OPAL ELECTRODE 4 TiO 2 inverse opal electrode 4.1 Introduction Photonic crystals, spatially periodic dielectric composites with lattice spacing of the order of wavelength of light, have attracted much interest due to the photonic band gaps, or the frequency ranges where no propagation of light exits. 1 The photonic band gap plays important role in low threshold lasers, 2 waveguide structures that cause light to curve at acute angles, 3 and perfect dielectric mirrors. At the edges of photonic band gap, photon dispersion curve strongly deviates from linear behavior due to the slow group velocity. This unusual property could lead to an enhancement of optical gain 4 and so-called superprism phenomenon. 5 Recently, the slowing and localization of photon or light in the high refractive index of photonic crystal have been proposed to enhance the red response of dye adsorption in dye-sensitized solar cells by changing the nanocrystalline TiO 2 structure to TiO 2 photonic crystal with inverse opal structure. 6 Although the enhancement of dye absorption at wavelength matching the red edge of the photonic band gap in TiO 2 photonic crystal has been observed, its direct relation on the photocurrent generation was not clearly investigated. Complete photonic band gap in the visible region could be achieved when the dielectric contrast between two materials must be 2.8 or more. 7 Having high refractive index of 2.5 in anatase phase and 2.9 in rutile, TiO 2 inverse opal is desired to lead to a more complete band gap. TiO 2 inverse opal is a three-dimensional (3D) photonic crystals, fabricated by the replication of a colloidal crystal (or synthetic opal) template. This template can be easily be fabricated by the crystallization of latex spheres into face-centered cubic (fcc) structures. After filling the template with TiO 2, inverse opal structure could be obtained by removing the template. However, filling the voids in the template with TiO 2 by the reported liquid phase deposition method 8 involves the use of several precursor solutions, so it is not practice. 39
56 TiO 2 INVERSE OPAL ELECTRODE In this chapter, a simple preparation method of TiO 2 inverse opal electrode is introduced with only using one precursor solution, i.e. TiCl 4 in methanol solution, in the replication step of opal template. The crystal structure of TiO 2 and morphology of inverse opal are studied by investigating its X-ray diffraction and SEM. The behavior of photonic band gap in TiO 2 inverse opal is confirmed from its reflection and transmission. The optical absorption, photocurrent and thermal conductivity properties are also characterized using photoacoustic, IPCE, transient current and transient grating spectroscopies. 4.2 Experimental Inverse opal TiO 2 films were prepared on fluorine-doped tin oxide (FTO)-coated glass by the replication of a self-organizing material used as a template. 9 Substrates were cleaned ultrasonically with soap, KOH, water, and methanol. Monodisperse polystyrene latex (309 nm in diameter) suspensions were sonicated for 30 min to break down the aggregared particles. The synthetic opal templates were assembled by immersing the FTO substrate vertically in 0.1 wt % monodisperse polystyrene (309 nm in diameter) suspension and evaporating the solvent in an oven at 40 O C for 1 to 2 days until the solvent completely disappeared, leaving behind a colloidal crystal film on the substrate. Then TiO 2 was brought into the void of the template by the following method. 10 μl drop of 2% TiCl 4 in methanol was added via a micropipette onto the 1.3 x 2.3 cm 2 colloidal crystal surface. After hydrolysis for 30 min, the sample was subsequently heated to 80 O C in air. This process was repeated three times to ensure the filling of all the voids. To investigate the effect of heat treatment in this step, two heat treatment times (10 and 30 min) were applied. Finally, the sample was subsequently heated up to 450 O C for 1 h in air with a heating rate of 0.5 O C /min to calcinate the template and anneal the TiO 2. The transmission spectra were taken by using photoacoustic technique where the sample was stuck on the outside of PA cell window (quartz glass) with grease while the carbon black was put inside the PA cell. In this case, the incident light will pass through the sample and then be absorbed by sample. The unabsorbed light is transmitted through the sample and subsequently absorbed by carbon black. The light absorbed by the carbon black is proportional to the incident light transmitted by sample. Similar to the PA spectra, the transmittance spectra of the sample is also normalized by PA spectrum of carbon black to eliminate the spectral variation of illumination source. 40
57 TiO 2 INVERSE OPAL ELECTRODE 4.3 Results and discussion Three dimensional TiO 2 inverse opals were simply fabricated by replication of selforganizing polystyrene latex as template wherein the voids in artificial opal latex are filled with nanosized TiO 2 particles through adding TiCl 4 drop into the latex matrix, hydrolyzing and heating Crystal structure and morphology The resulted inverse opal structure was determined by using XRD. The XRD patterns for TiO 2 inverse opal with 1, 2, 3, 5 TiCl 4 drops were seen in Fig The XRD pattern for the only FTO was also shown together for comparison. In XRD patterns of inverse opal TiO 2, a peak appeared at 26 degree indicating (101) crystal plane of anatase structure. The second peak for (200) crystal plane of anatase structure was observed for 3 and 5 TiCl 4 drops sample. The peak intensity increases with TiCl 4 addition. From this data, all the resulted inverse opal TiO 2 is definitely crystalline. As the calcinations temperature is 450 O C, the TiO 2 is expected to be annealed, resulting in anatase crystal structure. So at 450 O C, the calcinations of the template and annealing of TiO 2 were conducted together. TiO 2 (101) TiO 2 (200) Intensity (a. u.) FTO TiO 2 Filling process (TiCl 4 drops) θ (Degrees) Figure 4.1: X-ray diffraction pattern for the TiO 2 photonic crystal for different filling process (TiCl 4 drops). 41
58 TiO 2 INVERSE OPAL ELECTRODE Typical scanning electron microscopy (SEM) images of the latex template, with a diameter of 309 nm, and the obtained TiO 2 inverse opal are shown in Fig The latex particles were arranged in the fcc lattice (Fig. 4.2 (a)) with the thickness (Fig. 4.2 (b)), depending on the concentration of original colloidal latex suspension, before the vertical immersion of FTO substrate and evaporation of the aqueous solvent. After the calcination process, the replication of this template with the titanium oxide resulted in a honeycomb structure with an ordered hexagonal pattern of spherical pores that connected each sphere to its nearest neighbors, as shown in Fig. 4.2 (c). The diameter or center to center distance of the pores, referred to as the periodic lattice constant of inverse opal TiO 2, was determined to be ~270 nm (Fig. 4.2 (c)) which shows the shrinkage of the polystyrene latex particles with a diameter of 309 nm. This structure also consists of several layers, connected to each other, as shown in Fig. 4.2 (d) with a possible maximum thickness similar to the initial latex film. The thickness of obtained TiO 2 inverse opal film was measured to be the same as those of the latex film, while the thinner inverse opal films could be made from a smaller amount of TiCl 4. (a) (b) (c) (d) Figure 4.2: SEM images of a latex template (diameter of 309 nm) (a) surface, (b) cross section, together with the replicated inverse opal TiO 2 (c) surface, (d) cross section. 42
59 TiO 2 INVERSE OPAL ELECTRODE Transmission and reflection spectra Fig. 4.3 shows the typical transmission spectra for the nanocrystalline TiO 2 (anatase, 15 nm) and TiO 2 inverse opal with different addition of TiCl 4 drops. In Fig. 4.3 (a), the transmission spectrum for nanocrystalline TiO 2 shows low transmittance percentage below 360 nm indicating the absorption of TiO 2 since 360 nm light has higher energy than the energy gap of anatase typed TiO 2, 3.2 ev, enabling electron excitation from valence band to conduction band upon light absorption. Beyond 360 nm, the transmittance percentage will increase exponentially. The typical transmission spectrum for TiO 2 inverse opal (Fig. 4.3 (b)) shows a drop at ~ 425 nm. This drop in which where the light can not propagate through the structure resulting in lower transmittance percentage. 1.0 Photon energy (ev) Photon energy (ev) Transmittance (%) (a) Transmittance (%) (b) Wavelength (nm) Wavelength (nm) Figure 4.3: Transmission spectra of nanocrystalline TiO 2 with diameter 15 nm (a) and those of TiO 2 inverse opal for 3 (b) times in filling process (TiCl 4 drops). The reflection spectra of TiO 2 inverse opal made from different sizes of latex templates are shown in Fig For TiO 2 inverse opal made from a latex template with a diameter of 309 nm, the reflection spectrum (a) reveals a peak at 425 nm. This reflection peak that is in the same position of the observed drop in transmittance spectrum corresponds to the Bragg reflection peak of the hexagonal air-sphere layers [the (111) reflection peak of the fcc air crystals] 9. Due to this Bragg reflection, the photon with certain frequency range will not propagate through the inverse opal structure creating photonic band gaps. The photon frequency of Bragg reflection is inversely proportional to the lattice spacing. So with the larger lattice spacing, this photon frequency will become smaller. By using larger latex spheres, for example those with a diameter of 394 nm, the larger center-center 43
60 TiO 2 INVERSE OPAL ELECTRODE distance of the pores (lattice spacing) in TiO 2 inverse opal will be produced and the position of the reflection peak was tuned to lower photon energy at 620 nm as result. 500 Photon energy (ev) Reflection intensity (a. u.) a b Wavelength (nm) Figure 4.4: Reflection spectra of TiO 2 inverse opal made from latex spheres with diameters of (a) 309 nm and (b) 394 nm Optical absorption and photocurrent properties 1.2 Wavelength (nm) PA intensity (a. u.) Photon energy (ev) Figure 4.5: Typical PA spectrum of TiO 2 inverse opal. The optical absorption of TiO 2 inverse opal was characterized using photoacoustic technique. A typical PA spectrum for the resulting TiO 2 inverse opal is shown in Fig The band gap deduced from PA intensity is 3.2 ev, which corresponds to the energy gap of anatase-type TiO 2. There is no observed change on the optical absorption by changing 44
61 TiO 2 INVERSE OPAL ELECTRODE the latex spheres from 308 to 398 nm since the energy gap of anatase-typed TiO 2 inverse opal is not changed by varying its porous size. 15 Wavelength (nm) IPCE (%) Photon energy (ev) Figure 4.6: IPCE spectrum of TiO 2 inverse opal electrode. Incident-photon to current conversion efficiency (IPCE) spectrum of inverse opal TiO 2 is shown in Fig As one can notice, the IPCE spectrum starts from about 3.2 ev, similar to the optical absorption, and increases for higher photon energy and eventually forms a peak at about 3.6 ev. The decrease of IPCE value for photon energy higher than about 3.6 ev may be caused by the absorption of FTO at this photon energy, resulting in the decrease of TiO 2 absorption. This occurred since the TiO 2 inverse opal electrode was illuminated by monochromatic light from the back wall (through an FTO/TiO 2 interface), in the opposite direction with PA measurement where light illumination was performed directly on TiO 2 inverse opal. Moreover, IPCE value could be increased by more addition of TiCl 4 since the quantity of absorbed light was increased by larger amount of TiO 2. The highest IPCE value is obtained for 3 drops TiCl Electron diffusivity property The electron diffusion in TiO 2 inverse opal was characterized in comparison to nanocrystalline TiO 2 by using nanosecond laser. Figure 4.7 shows the photocurrent decays of TiO 2 inverse opal made from latex diameter of 309 nm and nanocrystalline TiO 2 in diameter of 40 nm with the same thickness of about 3 µm. The laser intensity was controlled to be the same in order to obtain the comparable electron density for both electrodes. By assuming that the electrons are diffused in a one-dimensional space, the electron diffusion coefficients (denoted as D) 10 could be calculated from 45
62 TiO 2 INVERSE OPAL ELECTRODE D 2 W = (4.1) 6τ peak where W is the film thickness and τ peak is the time peak of the photocurrent transient. The τ peak for TiO 2 inverse opal and nanocryatalline TiO 2 were obtained to be about 0.09 and 20.4 ms, so by using Eq. 4.1 the electron diffusion coefficients were calculated to be 1.67 x 10-4 and 7.35 x 10-7 cm 2 /s, respectively. The higher electron diffusion coefficient for TiO 2 inverse opal relative to nanocrystalline TiO 2 suggested that electron in TiO 2 inverse opal spend much smaller fraction of their transit time in trap sites located at TiO 2 surface, compared to those in nanocrsytalline TiO 2. However taking into account the macroporous in TiO 2 inverse opal structure, the optical absorption length of TiO 2 inverse opal may be much larger than nanocrystalline TiO 2. In consequence, the excited electron distribution will be broad along the TiO 2 inverse opal electrode and narrow in the outermost layer of nanocrystalline TiO 2 electrode. If this case happens, the Eq. 4.1 could not be used. Thus further investigations are required to determine the other electron diffusion equation, especially for TiO 2 inverse opal. Norm. TC signal (a. u.) Inverse opal TiO 2 originated from 309 nm sized latex (a) Norm. TC signal (a. u.) Nanocrystalline TiO 2 in diameter of 40 nm (b) Decay time (ms) Decay time (ms) Figure 4.7: Photocurrent transients of (a) TiO 2 inverse opal (made from 309 nm sized latex) and (b) 40 nm sized nanocrystalline TiO 2 with similar thickness of about 3 µ m Thermal diffusivity property The thermal properties of TiO 2 inverse opal was characterized by transient grating technique using nanosecond pulse laser. The transient grating responses for TiO 2 inverse opal made from latex diameter of 308 nm with different pulse intensities are shown in Fig. 4.8 (a). For measurement at 0.40 and 1.53 mj/pulse, the decays were fitted to be 46
63 TiO 2 INVERSE OPAL ELECTRODE Norm. TG signal (a. u.) mj/pulse 1.53 mj/pulse 2.67 mj/pulse 4.77 mj/pulse Norm. TG signal (a. u.) inverse opal nanocrystalline Delay time (ms) Delay time (ms) Figure 4.8: Transient grating responses of TiO 2 inverse opal (a) at various pump intensities (b) and nanocrystalline in diameter of 15 nm at 2.67 mj/pulse pump intensity. single exponential decay, while the decays measured at 2.67 and 4.77 mj/pulse were fitted to be double exponential decay. y t / τ1 t / τ 2 = A e + A e (4.2) 1 where y is the LF-HD-TG signal, A1 and A2 are constants. τ 1 and τ 2 are time constants of the two decay processes, respectively. The details of time constants at various pump intensities are shown in Table 4.1. The time constant of 22.0 ms at pump intensity of 0.4 mj/pulse corresponds to the thermal conductivity process. Then as increasing the pump intensity to 1.53 mj/pulse causing the longer time constant to be 23.6 ms. At pump intensity of 2.67 mj/pulse, the fast decay appears with the time constant of 3.4 ms and the slow decay with time constant of 26.7 (longer than the time constant of 23.6 measured at pump intensity of 1.53 mj/pulse). Further increasing the pump intensity of 4.77 mj/pulse, these fast and slow time constants become 6.4 and 51.8, respectively. It was found that increasing pump intensity causes the longer decay process and at certain pump intensity, the faster decay process appears. This may be caused by the larger concentration of excited carriers with stronger pump intensity and larger heat generation in consequence, and then resulting in the non linear thermal properties, e.g. bottleneck phenomena. Further investigations are necessary to know the exact physical phenomena. 2 Table 4.1: Time constants of fast (τ 1 ) and slow (τ 2 ) transient grating decay processes on thetio 2 inverse opal at various pump intensities. Pump intensities τ 1 τ 2 47
64 TiO 2 INVERSE OPAL ELECTRODE In order to determine the effect of TiO 2 morphology on this thermal property, the transient grating responses of TiO 2 inverse opal were compared to those of nanocrystalline TiO 2 at pump intensity of 2.67 mj/pulse as shown in Fig. 4.8 (b). The decays then were fitted to be double exponential decays (Eg. 4.2). For nanocrystalline TiO 2, these time constants (τ 1 and τ 1 ) seem to be faster than those of TiO 2 inverse opal (as shown in Table 4.1), i.e. 2.7 and 19.5 ms. This indicates that thermal diffusivity of TiO 2 inverse opal is worse than nanocrystalline TiO 2 due to the macroporous in inverse opal structure thus the generated heat could not be freely transferred to the surrounding easily. This result may be applied to the electronic circuit which uses the thermal properties of the different TiO 2 morphology. 4.4 Conclusions TiO 2 inverse opal has been successfully synthesized by a simple method. The anatase structured TiO 2 inverse opal with ordered macroporous structure was confirmed by X-ray diffraction pattern and SEM images. The transmittance and reflection spectra indicate the position of photonic band gap due to the Bragg reflection. Changing with the larger latex size shifted the position of the photonic band gap to the lower energy region due to the larger center-center distance of the porous or the lattice spacing in TiO 2 inverse opal. Approaching from lower energy region, the optical absorption with band gap of 3.2 ev and the photocurrent properties started at 3.2 ev were shown by the PA and IPCE methods. The photocurrent transient of TiO 2 inverse opal was found to be much faster than nanocrystalline TiO 2 for the same electrode thickness and pulse intensity. Using transient grating technique with nanosecond laser as pump, the thermal diffusivity of TiO 2 inverse opal was found to be lower than nanocrystalline TiO 2, indicating morphologies dependent thermal properties. 48
65 TiO 2 INVERSE OPAL ELECTRODE References 1 E. Yablonovitch, J. Opt. Soc. Am. 10 (1993) Y. Yamamoto and R. E. Slusher, Phys. Today 46 (1993) A. Mekis, J. C. Chen, I. Kurland, S. Fan, P. R. Villeneuve and J. D. Joannopoulos, Phys. Rev. Lett. 77 (1996) J. P. Dowling, M. Scalora, M. J. Bloemer and C. W. Bowden, J. Appl. Phys. 75 (1994) H. Kosaka, Phys. Rev. B 58 (1998) R S. Nishimura, N. Abrams, B. A. Lewis, L. I. Halaoui, T. E. Mallouk, K. D. Benkstein, J. van de Lagemaat and A. J. Frank, J. Am. Chem. Soc 125 (2003) K. Busch and S. John, Phys. Rev. E 58 (1998) S. Nishimura, A. Shishido, N. Abrams and T. E. Mallouk, Appl. Phys. Lett. 81 (2002) E. G. Judith, J. Wijnhoven and W. L. Vos., Science 281 (1998) A. Solbrand, H. Lindström, H. Rensmo, A. Hagfeldt, S. E. Lindquist and S. Södergren, J. Phys. Chem. B 101 (1997)
66 PHOTOSENSITIZATION OF TiO 2 INVERSE OPAL WITH CdSe QUANTUM DOTS 5 Photosensitization of TiO 2 inverse opal with CdSe Quantum Dots 5.1 Introduction Dye-sensitized solar cells (DSSCs) have been attracting much attention as an alternative to conventional silicon solar cells since the pioneering work on dye-sensitized nanocrystalline TiO 2 by O Regan and Grätzel. 1 In these cells, the use of dye molecules as photosensitizers, nanostructured TiO 2 as the electron transport layer, and, the I /I 3 redox couple as a hole transport layer dramatically improve the light-harvesting efficiency. Rubased dyes attached to mesoporous TiO 2 with large surface areas absorb solar energy efficiently. The electrons injected by the optically excited dye into the TiO 2 conduction band diffuse across the semiconductor film layer and reach the back contact. Redox couples diffusing in solution, which are in turn reduced at the counter electrode, regenerate the oxidized dye. To achieve efficient solar energy conversion as well as long-term photostability, some researchers have applied quantum-dot semiconductors as dye substitutes for sensitizers. 2, 3 These semiconductor nanocrystals have a robust inorganic nature, so these particles are more stable against photodegradation than the usual organic dyes. 4 Moreover, owing to the quantum confinement effect, the band gap of semiconductor quantum dots and, hence, their optical absorption, can be modulated over a wide spectral range by controlling their size to match the distribution of solar light. The relatively low efficiency compared to the theoretical value obtained in DSSCs is ascribed to the poor penetration of materials into thick TiO 2 films, and the detachment of hole transport layers from TiO 2 electrodes. 5 To address the penetration of both sensitizers and redox couples, a novel approach has been proposed using mesoporous inverse opal titania originating from self-organizing systems, such as polystyrene, as templates. 6 This inverse opal titania has large interconnected pores that lead to a better infiltration. In addition, it also exhibits a photonic band gap, which depends on the filling fraction of 50
67 PHOTOSENSITIZATION OF TiO 2 INVERSE OPAL WITH CdSe QUANTUM DOTS TiO 2 in the inverse opal structure. At the wavelength near the photonic band gap, the group velocity of photons becomes anomalously small. This phenomenon can be understood by considering the bending of the photon dispersion curve (E vs k) in a periodic dielectric. 7 At wavelengths approaching the photonic band gap, light can be described as a standing wave. 8 The peaks of this standing wave are localized in the high dielectric part of the photonic crystal on the red edge of the photonic band gap, whereas they are localized in the low dielectric part of the photonic crystal on the blue edge. Taking note of the sensitizer in the high dielectric part of photonic crystal, it may be possible to significantly enhance solar energy absorption by adjusting the red edge of the photonic band gap to fit with the absorption of the sensitizers used, particularly for quantum dots that originally have large extinction coefficients. In this chapter, CdSe QDs are adsorbed in situ on TiO 2 inverse opal and its effect on the morphology and reflection properties are characterized. Photosensitization of TiO 2 inverse opal with CdSe QDs is studied by characterizing its optical absorption, photocurrent as well as photovoltaic performances as a function of adsorption times. 5.2 Experimental TiO 2 inverse opal films were prepared on fluorine-doped tin oxide (FTO)-coated glass by the replication of a self-organizing material used as a template. 9 Substrates were cleaned ultrasonically with soap, KOH, water, and methanol. Monodisperse polystyrene latex (309 nm in diameter) suspensions were sonicated for 30 min to break down the aggregared particles. The synthetic opal templates were assembled by immersing the FTO substrate vertically in 0.1 wt % monodisperse polystyrene (309 nm in diameter) suspension and evaporating the solvent in an oven at 40 O C for 1 to 2 days until the solvent completely disappeared, leaving behind a colloidal crystal film on the substrate. Then TiO 2 was brought into the void of the template by the following method. 10 μl drop of 2% TiCl 4 in methanol was added via a micropipette onto the 1.3 x 2.3 cm 2 colloidal crystal surface. After hydrolysis for 30 min, the sample was subsequently heated to 80 O C in air for 10 min in order to make a compact structure before further addition of TiCl 4. This process was repeated three times to ensure the filling of all the voids. Finally, the samples were subsequently heated at 450 O C for 1 h in air with a heating rate of 0.5 O C /min to calcinate the template and anneal the TiO 2. 51
68 PHOTOSENSITIZATION OF TiO 2 INVERSE OPAL WITH CdSe QUANTUM DOTS CdSe quantum dots were deposited on inverse opal TiO 2 by chemical deposition. 9 The deposition solution was prepared by adding 0.7 M sodium nitrilotriacetate [N(CH 2 COONa) 3 or NTA] to 0.5 M CdSO 4. Then 0.2 M sodium selenosulfate (Na 2 SeSO 3 ) in excess Na 2 SO 3, prepared by stirring 0.2 M Se with 0.5 M Na 2 SO 3 at 70 O C, was added, resulting in a final composition of 80 mm CdSO 4, 80 mm Na 2 SeSO 3 (which includes 0.12 M free Na 2 SO 3 ), and 120 mm NTA. During the deposition, the inverse opal TiO 2 was placed in the solution, which was put in a thermostat chamber to control temperature at 10 O C and kept in the dark. Afterwards the samples were rinsed with water and dried in a N 2 flow. 5.3 Results and discussion Crystal structure and morphology TiO 2 /FTO CdSe/TiO 2 /FTO Intensity (a. u.) CdSe (111) FTO TiO 2 FTO (101) FTO CdSe (220) θ (Degrees) Figure 5.1: X-ray diffraction patterns for TiO 2 inverse opal and that of with 24h CdSe QDs adsorption. X-ray diffraction was taken in order to confirm the formation of CdSe QDs on the TiO 2 electrodes, which could be clearly seen for long CdSe adsorption time, i.e. 24h. The XRD pattern for CdSe-adsorbed TiO 2 inverse opal is shown in Fig The XRD pattern of only TiO 2 inverse opal (without CdSe deposition) is also presented together for 52
69 PHOTOSENSITIZATION OF TiO 2 INVERSE OPAL WITH CdSe QUANTUM DOTS comparison. As can be seen, the peak at 2θ = 25.5 O is substantially broadened at the base and a new diffraction peak appeared at 2θ = 42.2 O. These two peaks correspond to the (111) and (220) lattice planes reflection of cubic CdSe crystal. The broadening of the peaks implies that the size of CdSe particles is very small. The average size of CdSe crystals was estimated to be approximately 6 nm from the full-width at half maximum of diffraction peaks, using the Scherrer equation. For initial deposition, these peaks can not be seen clearly. It is because just small amount of CdSe has been deposited on TiO 2 inverse opal. However, these peaks should appear after some times while the intensity of these peaks will gradually increase by increasing deposition time. X-ray diffraction (XRD) pattern show that crystal structure of TiO 2 inverse opal is anatase structure and cubic structure of CdSe QDs. 0 h 4 h 200 nm 200 nm 12 h 24 h 200 nm 200 nm Figure 5.2: SEM images of TiO 2 inverse opal electrodes with various CdSe adsorption times. Figure 5.2 shows SEM images of TiO 2 inverse opal electrodes originated from latex diameter of 308 nm, with and without CdSe QD adsorption. For 4h adsorption time, CdSe QDs formation has been initiated on the TiO 2 surface. During the adsorption, CdSe QDs grow onto TiO 2 inverse opal surface increasingly, indicated by the thicker wall in the inverse opal structure. The thickness of adsorbed CdSe surrounding TiO 2 inverse opal wall could reach higher than the thickness of TiO 2 inverse opal wall itself. Furthermore, increasing CdSe adsorption time results in smaller porous size consequently, especially for 53
70 PHOTOSENSITIZATION OF TiO 2 INVERSE OPAL WITH CdSe QUANTUM DOTS 24 h adsorption. From the cross section (shown in Fig. 5.3), CdSe adsorption was confirmed not only on the portion near to the surface of inverse opal structure but also in the entirely inverse opal structure. 0 h 8 h 2 µm 1.5 µm Figure 5.3: SEM cross section images of TiO 2 inverse opal electrodes with 8h CdSe adsorption time Reflectance spectra Figure 5.4 shows the reflection spectra of TiO 2 inverse opal (originated from latex diameter of 308 nm) with CdSe adsorption. A typical reflection spectrum of inverse opal structure reveals a peak that is a Bragg reflection peak of the hexagonal air-sphere layers [the (111) reflection peak of the fcc air crystals]. 10 Due to this Bragg reflection, the photon with certain frequency range will not propagate through the inverse opal structure creating photonic band gaps. In strongly photonic crystal, the lattice spacing and the average refractive index (n a ) of the crystal are inversely proportional to the photon frequency of Bragg reflection, c d = m n a υ 2, (5.1) where d is lattice spacing, m is the integer (1,2,3 ), c is the speed of light, n a is the average refractive index of photonic crystal and υ is the photon frequency of Bragg reflection. This average refractive index is given by n a 2 2 = n f + n (1 f ), (5.2) air where n is refractive index of certain material (in this case TiO 2 ), n air is the air refractive index, f is the filling fraction (which is the percentage of total crystal volume occupied by TiO 2 ). As shown in Fig. 5.4, the red shift of the reflection peak to the longer wavelength could be observed clearly as increasing adsorption time. Since the lattice spacing is found to be unchanged during CdSe adsorption shown in the SEM images, the red shift in the 54
C-H Activation in Total Synthesis Masayuki Tashiro (M1)
 1 C-H Activation in Total Synthesis Masayuki Tashiro (M1) 21 st Jun. 2014 Does a late-stage activation of unactivated C-H bond shorten a total synthesis?? 2 3 Contents 1. Comparison of Two Classical Strategies
1 C-H Activation in Total Synthesis Masayuki Tashiro (M1) 21 st Jun. 2014 Does a late-stage activation of unactivated C-H bond shorten a total synthesis?? 2 3 Contents 1. Comparison of Two Classical Strategies
車載用高効率燃焼圧センサー基板に最適なランガサイト型結晶の開発 結晶材料化学研究部門 シチズンホールディングス ( 株 )* 宇田聡 八百川律子 * Zhao Hengyu 前田健作 野澤純 藤原航三
 車載用高効率燃焼圧センサー基板に最適なランガサイト型結晶の開発 結晶材料化学研究部門 シチズンホールディングス ( 株 )* 宇田聡 八百川律子 * Zhao Hengyu 前田健作 野澤純 藤原航三 概要車載用燃焼圧センサー用として高抵抗を示すランガサイト型圧電結晶をデザインした すなわち4 元系のランガサイト型結晶 CNGS の適正組成をイオン半径 結晶構成要素の結晶サイト存在に関する自由度 および
車載用高効率燃焼圧センサー基板に最適なランガサイト型結晶の開発 結晶材料化学研究部門 シチズンホールディングス ( 株 )* 宇田聡 八百川律子 * Zhao Hengyu 前田健作 野澤純 藤原航三 概要車載用燃焼圧センサー用として高抵抗を示すランガサイト型圧電結晶をデザインした すなわち4 元系のランガサイト型結晶 CNGS の適正組成をイオン半径 結晶構成要素の結晶サイト存在に関する自由度 および
Reactive Fluid Dynamics 1 G-COE 科目 複雑システムのデザイン体系 第 1 回 植田利久 慶應義塾大学大学院理工学研究科開放環境科学専攻 2009 年 4 月 14 日. Keio University
 Reactive Fluid Dynamics 1 G-COE 科目 複雑システムのデザイン体系 第 1 回 植田利久 慶應義塾大学大学院理工学研究科開放環境科学専攻 2009 年 4 月 14 日 Reactive Fluid Dynamics 2 1 目的 本 G-COE で対象とする大規模複雑力学系システムを取り扱うにあたって, 非線形力学の基本的な知識と応用展開力は不可欠である. そこで,
Reactive Fluid Dynamics 1 G-COE 科目 複雑システムのデザイン体系 第 1 回 植田利久 慶應義塾大学大学院理工学研究科開放環境科学専攻 2009 年 4 月 14 日 Reactive Fluid Dynamics 2 1 目的 本 G-COE で対象とする大規模複雑力学系システムを取り扱うにあたって, 非線形力学の基本的な知識と応用展開力は不可欠である. そこで,
CMB の温度 偏光揺らぎにおける弱い重力レンズ効果 並河俊弥 ( 東京大学 )
 CMB の温度 偏光揺らぎにおける弱い重力レンズ効果 再構築法の開発 並河俊弥 ( 東京大学 ) 201 1 第 41 回天文 天体物理若手夏の学校 2011/08/01-04 CMB lensing の宇宙論への応用 暗黒エネルギー ニュートリノ質量など 比較的高赤方偏移の揺らぎに感度をもつ 光源の性質がよく分かっている 他の観測と相補的 原始重力波 TN+ 10 重力レンズ由来の B-mode
CMB の温度 偏光揺らぎにおける弱い重力レンズ効果 再構築法の開発 並河俊弥 ( 東京大学 ) 201 1 第 41 回天文 天体物理若手夏の学校 2011/08/01-04 CMB lensing の宇宙論への応用 暗黒エネルギー ニュートリノ質量など 比較的高赤方偏移の揺らぎに感度をもつ 光源の性質がよく分かっている 他の観測と相補的 原始重力波 TN+ 10 重力レンズ由来の B-mode
Q. Shen 1,2) and T. Toyoda 1,2)
 Photosensitization of nanostructured TiO 2 electrodes with CdSe quntum dots: effects of microstructure in substrates Q. Shen 1,2) and T. Toyoda 1,2) Department of Applied Physics and Chemistry 1), and
Photosensitization of nanostructured TiO 2 electrodes with CdSe quntum dots: effects of microstructure in substrates Q. Shen 1,2) and T. Toyoda 1,2) Department of Applied Physics and Chemistry 1), and
Agilent 4263B LCR Meter Operation Manual. Manual Change. Change 1 Add TAR in Test Signal Frequency Accuracy Test (Page 9-38) as follows.
 Agilent 4263B LCR Meter Operation Manual Manual Change Agilent Part No. N/A Jun 2009 Change 1 Add TAR in Test Signal Frequency Accuracy Test (Page 9-38) as follows. Test Signal Frequency Accuracy Frequency
Agilent 4263B LCR Meter Operation Manual Manual Change Agilent Part No. N/A Jun 2009 Change 1 Add TAR in Test Signal Frequency Accuracy Test (Page 9-38) as follows. Test Signal Frequency Accuracy Frequency
The unification of gravity and electromagnetism.
 The unification of gravity and electromagnetism. (The physics of dark energy.) Terubumi Honjou The 9 chapter. The unification of gravity and electromagnetism. [1]... to the goal of modern physics and the
The unification of gravity and electromagnetism. (The physics of dark energy.) Terubumi Honjou The 9 chapter. The unification of gravity and electromagnetism. [1]... to the goal of modern physics and the
むらの定量化について IEC-TC110 HHG2 への提案をベースに ソニー株式会社冨岡聡 フラットパネルディスプレイの人間工学シンポジウム
 むらの定量化について IEC-TC110 HHG2 への提案をベースに ソニー株式会社冨岡聡 むら を 定量化する とは? どちらも輝度分布の最大最小の差は 22% 画面の 4 隅に向かって暗くなる状態は 気にならない 気になるもの むら むら とは人の主観である 気になる要素 を見出し 計測可能にする SEMU: 既存の定量評価方法 SEMI: 半導体 FPD ナノテクノロジー MEMS 太陽光発電
むらの定量化について IEC-TC110 HHG2 への提案をベースに ソニー株式会社冨岡聡 むら を 定量化する とは? どちらも輝度分布の最大最小の差は 22% 画面の 4 隅に向かって暗くなる状態は 気にならない 気になるもの むら むら とは人の主観である 気になる要素 を見出し 計測可能にする SEMU: 既存の定量評価方法 SEMI: 半導体 FPD ナノテクノロジー MEMS 太陽光発電
Day 5. A Gem of Combinatorics 組合わせ論の宝石. Proof of Dilworth s theorem Some Young diagram combinatorics ヤング図形の組合せ論
 Day 5 A Gem of Combinatorics 組合わせ論の宝石 Proof of Dilworth s theorem Some Young diagram combinatorics ヤング図形の組合せ論 Recall the last lecture and try the homework solution 復習と宿題確認 Partially Ordered Set (poset)
Day 5 A Gem of Combinatorics 組合わせ論の宝石 Proof of Dilworth s theorem Some Young diagram combinatorics ヤング図形の組合せ論 Recall the last lecture and try the homework solution 復習と宿題確認 Partially Ordered Set (poset)
京都 ATLAS meeting 田代. Friday, June 28, 13
 京都 ATLAS meeting 0.06.8 田代 Exotic search GUT heavy gauge boson (W, Z ) Extra dimensions KK particles Black hole Black hole search (A 模型による )Extra dimension が存在する場合 parton 衝突で black hole が生成される Hawking
京都 ATLAS meeting 0.06.8 田代 Exotic search GUT heavy gauge boson (W, Z ) Extra dimensions KK particles Black hole Black hole search (A 模型による )Extra dimension が存在する場合 parton 衝突で black hole が生成される Hawking
シリコンベース新材料を用いた薄膜結晶太陽電池を目指して
 シリコンベース新材料を用いた薄膜結晶太陽電池を目指して 筑波大数理物質科学研究科電子 物理工学専攻 JST-PRESTO 末益 崇 BaSi 2 c=1.158nm b=0.680nm a=0.892nm Jan. 25, 2010 日本板硝子工学助成会 1 太陽電池の分類 単結晶シリコン 材料による分類 シリコン系 結晶シリコン アモルファス 多結晶シリコン 微結晶シリコン 太陽電池 化合物系 III-V
シリコンベース新材料を用いた薄膜結晶太陽電池を目指して 筑波大数理物質科学研究科電子 物理工学専攻 JST-PRESTO 末益 崇 BaSi 2 c=1.158nm b=0.680nm a=0.892nm Jan. 25, 2010 日本板硝子工学助成会 1 太陽電池の分類 単結晶シリコン 材料による分類 シリコン系 結晶シリコン アモルファス 多結晶シリコン 微結晶シリコン 太陽電池 化合物系 III-V
Interface Modification and Interfacial Charge Dynamics in Quantum Dot Solar Cells and Perovskite Solar Cells
 Interface Modification and Interfacial Charge Dynamics in Quantum Dot Solar Cells and Perovskite Solar Cells YAOHONG ZHANG THE UNIVERSITY OF ELECTRO-COMMUNICATIONS SEPTEMBER 2017 Interface Modification
Interface Modification and Interfacial Charge Dynamics in Quantum Dot Solar Cells and Perovskite Solar Cells YAOHONG ZHANG THE UNIVERSITY OF ELECTRO-COMMUNICATIONS SEPTEMBER 2017 Interface Modification
非弾性散乱を利用した不安定核 核構造研究 佐藤義輝東京工業大学
 非弾性散乱を利用した不安定核 核構造研究 佐藤義輝東京工業大学 Unbound excited states in,17 C Ground state deformation property of carbon isotopes Y.Satou et al., Phys. Lett. B 660, 320(2008). Be BC Li H He Ne O F N N=8 C 17 C Neutron
非弾性散乱を利用した不安定核 核構造研究 佐藤義輝東京工業大学 Unbound excited states in,17 C Ground state deformation property of carbon isotopes Y.Satou et al., Phys. Lett. B 660, 320(2008). Be BC Li H He Ne O F N N=8 C 17 C Neutron
英語問題 (60 分 ) 受験についての注意 3. 時計に組み込まれたアラーム機能 計算機能 辞書機能などを使用してはならない 4. 試験開始前に 監督から指示があったら 解答用紙の受験番号欄の番号が自身の受験番号かどうかを確認し 氏名を記入すること
 (2018 年度一般入試 A) 英語問題 (60 分 ) ( この問題冊子は 8 ページである ) 受験についての注意 1. 監督の指示があるまで 問題を開いてはならない 2. 携帯電話 PHS の電源は切ること 3. 時計に組み込まれたアラーム機能 計算機能 辞書機能などを使用してはならない 4. 試験開始前に 監督から指示があったら 解答用紙の受験番号欄の番号が自身の受験番号かどうかを確認し 氏名を記入すること
(2018 年度一般入試 A) 英語問題 (60 分 ) ( この問題冊子は 8 ページである ) 受験についての注意 1. 監督の指示があるまで 問題を開いてはならない 2. 携帯電話 PHS の電源は切ること 3. 時計に組み込まれたアラーム機能 計算機能 辞書機能などを使用してはならない 4. 試験開始前に 監督から指示があったら 解答用紙の受験番号欄の番号が自身の受験番号かどうかを確認し 氏名を記入すること
重力波天体の多様な観測による 宇宙物理学の新展開 勉強会 熱海 銀河における元素量の観測. 青木和光 Wako Aoki. 国立天文台 National Astronomical Observatory of Japan
 重力波天体の多様な観測による 宇宙物理学の新展開 勉強会 2013.1.8. 熱海 銀河における元素量の観測 青木和光 Wako Aoki 国立天文台 National Astronomical Observatory of Japan 銀河における元素量の観測 中性子星合体によってどのような元素合成が起こ るか とくにr-processのサイトか 化学進化への影響は 化学組成と金属量 化学組成 元素量
重力波天体の多様な観測による 宇宙物理学の新展開 勉強会 2013.1.8. 熱海 銀河における元素量の観測 青木和光 Wako Aoki 国立天文台 National Astronomical Observatory of Japan 銀河における元素量の観測 中性子星合体によってどのような元素合成が起こ るか とくにr-processのサイトか 化学進化への影響は 化学組成と金属量 化学組成 元素量
超新星残骸からの陽子起源ガンマ線 放射スペクトルの変調機構
 超新星残骸からの陽子起源ガンマ線 放射スペクトルの変調機構 名古屋大学理学研究科 井上剛志 今日の話とは別の成果 : Inoue, Hennebelle, Fukui, Matsumoto, Iwasaki, Inutsuka 2018, PASJ Special issue, arxiv: 1707.02035 IntroducGon to RX J1713.7-3946 p SNR: RX J1713.7-3946:
超新星残骸からの陽子起源ガンマ線 放射スペクトルの変調機構 名古屋大学理学研究科 井上剛志 今日の話とは別の成果 : Inoue, Hennebelle, Fukui, Matsumoto, Iwasaki, Inutsuka 2018, PASJ Special issue, arxiv: 1707.02035 IntroducGon to RX J1713.7-3946 p SNR: RX J1713.7-3946:
WHO 飲料水水質ガイドライン第 4 版 ( 一部暫定仮訳 ) 第 9 章放射線学的観点 9.4 飲料水中で一般的に検出される放射性核種のガイダンスレベル 過去の原子力緊急事態に起因する長期被ばく状況に関連する可能性のある人工の放射性核種のみならず 飲料水供給で最も一般的に検出される自然由来及び人工
 WHO 飲料水水質ガイドライン第 4 版 ( 一部暫定仮訳 ) 第 9 章放射線学的観点 9.4 飲料水中で一般的に検出される放射性核種のガイダンスレベル 過去の原子力緊急事態に起因する長期被ばく状況に関連する可能性のある人工の放射性核種のみならず 飲料水供給で最も一般的に検出される自然由来及び人工の放射性核種について定められたガイダンスレベルを表 9.2 に示す それぞれの成人の線量換算係数もまた示されている
WHO 飲料水水質ガイドライン第 4 版 ( 一部暫定仮訳 ) 第 9 章放射線学的観点 9.4 飲料水中で一般的に検出される放射性核種のガイダンスレベル 過去の原子力緊急事態に起因する長期被ばく状況に関連する可能性のある人工の放射性核種のみならず 飲料水供給で最も一般的に検出される自然由来及び人工の放射性核種について定められたガイダンスレベルを表 9.2 に示す それぞれの成人の線量換算係数もまた示されている
May 7, /05/07 Advanced Course in Environmental Catalytic Chemistry I 1
 May 7, 2015 2015/05/07 Advanced Course in Environmental Catalytic Chemistry I 1 Advanced Course in Environmental Catalytic Chemistry I understanding chemistry by understanding photocatalysis understanding
May 7, 2015 2015/05/07 Advanced Course in Environmental Catalytic Chemistry I 1 Advanced Course in Environmental Catalytic Chemistry I understanding chemistry by understanding photocatalysis understanding
シミュレーション物理 6 運動方程式の方法 : 惑星の軌道 出席のメール ( 件名に学生番号と氏名 ) に, 中点法をサブルーチンを使って書いたプログラムを添付
 シミュレーション物理 6 運動方程式の方法 : 惑星の軌道 出席のメール ( 件名に学生番号と氏名 ) に, 中点法をサブルーチンを使って書いたプログラムを添付 今回の授業の目的 4 次のRunge-Kutta 法を用いて, 惑星の軌道のシミュレーションを行う 2 d r GMm m = r 2 3 dt r 2 d r GM = 2 (using angular momentum cons.) 2
シミュレーション物理 6 運動方程式の方法 : 惑星の軌道 出席のメール ( 件名に学生番号と氏名 ) に, 中点法をサブルーチンを使って書いたプログラムを添付 今回の授業の目的 4 次のRunge-Kutta 法を用いて, 惑星の軌道のシミュレーションを行う 2 d r GMm m = r 2 3 dt r 2 d r GM = 2 (using angular momentum cons.) 2
2011/12/25
 Outline [] Control Technologies for Ocean Engineering Control technologies as the second axis Kyushu University Prof. H.Kajiwara (.8,at PNU) LQI Control Linear-Quadratic- Integral Design of Linear-Time-
Outline [] Control Technologies for Ocean Engineering Control technologies as the second axis Kyushu University Prof. H.Kajiwara (.8,at PNU) LQI Control Linear-Quadratic- Integral Design of Linear-Time-
Photoacclimation Strategy in Photosystem II of Prymnesiophyceae Isochrysis galbana
 Photoacclimation Strategy in Photosystem II of Prymnesiophyceae Isochrysis galbana プリムネシウム藻綱 Isochrysis galbana の光化学系 II における光適応戦略 6D551 小幡光子 指導教員山本修一 SYNOPSIS 海洋に生息する藻類は 海水の鉛直混合や昼夜により 弱光から強光までの様々な光強度
Photoacclimation Strategy in Photosystem II of Prymnesiophyceae Isochrysis galbana プリムネシウム藻綱 Isochrysis galbana の光化学系 II における光適応戦略 6D551 小幡光子 指導教員山本修一 SYNOPSIS 海洋に生息する藻類は 海水の鉛直混合や昼夜により 弱光から強光までの様々な光強度
May 18, /05/18 Advanced Course in Environmental Catalytic Chemistry I 1
 May 18, 2017 2017/05/18 Advanced Course in Environmental Catalytic Chemistry I 1 Advanced Course in Environmental Catalytic Chemistry I understanding chemistry by understanding photocatalysis understanding
May 18, 2017 2017/05/18 Advanced Course in Environmental Catalytic Chemistry I 1 Advanced Course in Environmental Catalytic Chemistry I understanding chemistry by understanding photocatalysis understanding
高分解能原子核乾板を用いた暗黒物質探索 中竜大 名古屋大学基本粒子研究室 (F 研 ) ICEPP 白馬
 高分解能原子核乾板を用いた暗黒物質探索 中竜大 名古屋大学基本粒子研究室 (F 研 ) ICEPP シンポジウム @ 白馬 2010.2.14-17 χ Direct Detection χ q q Indirect Detection χ γ, ν, lepton Accelerator Detection q χ χ γ, ν, lepton q χ Target : Xe, Ge, Si, NaI,
高分解能原子核乾板を用いた暗黒物質探索 中竜大 名古屋大学基本粒子研究室 (F 研 ) ICEPP シンポジウム @ 白馬 2010.2.14-17 χ Direct Detection χ q q Indirect Detection χ γ, ν, lepton Accelerator Detection q χ χ γ, ν, lepton q χ Target : Xe, Ge, Si, NaI,
Development of a High-Resolution Climate Model for Model-Observation Integrating Studies from the Earth s Surface to the Lower Thermosphere
 Chapter 1 Earth Science Development of a High-Resolution Climate Model for Model-Observation Integrating Studies from the Earth s Surface to the Lower Thermosphere Project Representative Shingo Watanabe
Chapter 1 Earth Science Development of a High-Resolution Climate Model for Model-Observation Integrating Studies from the Earth s Surface to the Lower Thermosphere Project Representative Shingo Watanabe
11/13 Diagonalization. Theorem: The set R of all real numbers is not enumerable. 12/13. 0.a k1 a k2 a k3... where a ij {0, 1,...
 対角線論法 可算無限集合 : 自然数全体の集合との間に 対 対応がある集合のこと. 可算集合 : 有限または可算無限である集合のこと. つまり, つずつ要素を取り出してきて, もれなく書き並べられるもの 例. 正の偶数全体の集合 E は可算無限である. 自然数全体の集合 N の要素 と,E の要素 2 を対とする 対 対応がある. 例 2. 整数全体の集合 Z は可算無限である. 対 対応がある.
対角線論法 可算無限集合 : 自然数全体の集合との間に 対 対応がある集合のこと. 可算集合 : 有限または可算無限である集合のこと. つまり, つずつ要素を取り出してきて, もれなく書き並べられるもの 例. 正の偶数全体の集合 E は可算無限である. 自然数全体の集合 N の要素 と,E の要素 2 を対とする 対 対応がある. 例 2. 整数全体の集合 Z は可算無限である. 対 対応がある.
一体型地上気象観測機器 ( ) の風計測性能評価 EVALUATION OF WIND MEASUREMENT PERFORMANCE OF COMPACT WEATHER SENSORS
 第 23 回風工学シンポジウム (2014) 一体型地上気象観測機器 ( ) の風計測性能評価 EVALUATION OF WIND MEASUREMENT PERFORMANCE OF COMPACT WEATHER SENSORS 1) 2) 3) 4) 5) 吉田大紀 林 泰一 伊藤芳樹 林 夕路 小松亮介 1) 4) 4) 1) 1) 寺地雄輔 太田行俊 田村直美 橋波伸治 渡邉好弘 Daiki
第 23 回風工学シンポジウム (2014) 一体型地上気象観測機器 ( ) の風計測性能評価 EVALUATION OF WIND MEASUREMENT PERFORMANCE OF COMPACT WEATHER SENSORS 1) 2) 3) 4) 5) 吉田大紀 林 泰一 伊藤芳樹 林 夕路 小松亮介 1) 4) 4) 1) 1) 寺地雄輔 太田行俊 田村直美 橋波伸治 渡邉好弘 Daiki
Fast response silicon pixel detector using SOI. 2016/08/10 Manabu Togawa
 Fast response silicon pixel detector using SOI 2016/08/10 Manabu Togawa 1 100 ps High intensity fixed target NA62200 ps J-PARC MEG TOF-PET TOF-PET 400 ps α K + -> π + νν : NA62 expriment At CERN SPS Goal
Fast response silicon pixel detector using SOI 2016/08/10 Manabu Togawa 1 100 ps High intensity fixed target NA62200 ps J-PARC MEG TOF-PET TOF-PET 400 ps α K + -> π + νν : NA62 expriment At CERN SPS Goal
69 地盤の水分変化モニタリング技術 比抵抗モニタリングシステムの概要 * 小林剛 Monitoring Technology for a Moisture Change of Subsurface Outline of the Resistivity Monitoring System Tsuyo
 69 比抵抗モニタリングシステムの概要 * 小林剛 Monitoring Technology for a Moisture Change of Subsurface Outline of the Resistivity Monitoring System Tsuyoshi KOBAYASHI * Abstract Resistivity monitoring system is a visualization
69 比抵抗モニタリングシステムの概要 * 小林剛 Monitoring Technology for a Moisture Change of Subsurface Outline of the Resistivity Monitoring System Tsuyoshi KOBAYASHI * Abstract Resistivity monitoring system is a visualization
近距離重力実験実験室における逆二乗則の法則の検証. Jiro Murata
 近距離重力実験実験室における逆二乗則の法則の検証? Jiro Murata Rikkyo University / TRIUMF 第 29 回理論懇シンポジウム 重力が織りなす宇宙の諸階層 2016/12/20-22 東北大学天文学教室 1. 重力実験概観 Newton 実験の紹介 2. 加速器実験の解釈 比較 1 ATLAS よくある質問 Q 重力実験はどのくらいの距離までいってるんですか? A
近距離重力実験実験室における逆二乗則の法則の検証? Jiro Murata Rikkyo University / TRIUMF 第 29 回理論懇シンポジウム 重力が織りなす宇宙の諸階層 2016/12/20-22 東北大学天文学教室 1. 重力実験概観 Newton 実験の紹介 2. 加速器実験の解釈 比較 1 ATLAS よくある質問 Q 重力実験はどのくらいの距離までいってるんですか? A
結合および相互作用エネルギーの定量的 評価法の開発と新規典型元素化合物の構築
 結合および相互作用エネルギーの定量的 評価法の開発と新規典型元素化合物の構築 ( 課題番号 :17350021) 平成 17 年度 ~ 平成 18 年度科学研究費補助金 ( 基盤研究 (B) ) 研究成果報告書 平成 19 年 5 月 研究代表者山本陽介 広島大学大学院理学研究科教授 目次 1. 課題番号 2. 研究課題 3. 研究組織 4. 研究経費 5. 研究発表 原著論文 総説類 国内学会における特別講演
結合および相互作用エネルギーの定量的 評価法の開発と新規典型元素化合物の構築 ( 課題番号 :17350021) 平成 17 年度 ~ 平成 18 年度科学研究費補助金 ( 基盤研究 (B) ) 研究成果報告書 平成 19 年 5 月 研究代表者山本陽介 広島大学大学院理学研究科教授 目次 1. 課題番号 2. 研究課題 3. 研究組織 4. 研究経費 5. 研究発表 原著論文 総説類 国内学会における特別講演
谷本俊郎博士の研究業績概要 谷本俊郎博士は これまで地球内部の大規模なマントルの対流運動を解明するための研究 および 大気 - 海洋 - 固体地球の相互作用に関する研究を様々な角度から進めてきた これらのうち主要な研究成果は 以下の様にまとめることができる
 谷本俊郎博士の研究業績概要 谷本俊郎博士は これまで地球内部の大規模なマントルの対流運動を解明するための研究 および 大気 - 海洋 - 固体地球の相互作用に関する研究を様々な角度から進めてきた これらのうち主要な研究成果は 以下の様にまとめることができる 1. 地球内部の全球的 3 次元構造とマントル対流プレートテクトニクスによる地球表面の運動が GPS 等の測地観測によってリアルタイムでわかるようになってきた現在
谷本俊郎博士の研究業績概要 谷本俊郎博士は これまで地球内部の大規模なマントルの対流運動を解明するための研究 および 大気 - 海洋 - 固体地球の相互作用に関する研究を様々な角度から進めてきた これらのうち主要な研究成果は 以下の様にまとめることができる 1. 地球内部の全球的 3 次元構造とマントル対流プレートテクトニクスによる地球表面の運動が GPS 等の測地観測によってリアルタイムでわかるようになってきた現在
On Attitude Control of Microsatellite Using Shape Variable Elements 形状可変機能を用いた超小型衛星の姿勢制御について
 The 4th Workshop on JAXA: Astrodynamics and Flight Mechanics, Sagamihara, July 015. On Attitude Control of Microsatellite Using Shape Variable Elements By Kyosuke Tawara 1) and Saburo Matunaga ) 1) Department
The 4th Workshop on JAXA: Astrodynamics and Flight Mechanics, Sagamihara, July 015. On Attitude Control of Microsatellite Using Shape Variable Elements By Kyosuke Tawara 1) and Saburo Matunaga ) 1) Department
Mathematics 数理科学専修. welcome to 統合数理科学を 目 指 す 基礎理工学専攻
 数理科学専修 統合数理科学を 目 指 す welcome to Mathematics 数理科学 とは 数学および数学と諸科学との関係領域に構築 された学問分野の総称であり 数学理論 いわゆる純粋数学 の探求ともに 現実現象の記述 抽象化 定式化 モデル化 の開発にも重点を置くものです 1981年 慶應義塾は他大学にさきがけてを設置し ましたが 数理科学はあらゆる科学技術を語る共通の言葉とし て 理工学はもちろん
数理科学専修 統合数理科学を 目 指 す welcome to Mathematics 数理科学 とは 数学および数学と諸科学との関係領域に構築 された学問分野の総称であり 数学理論 いわゆる純粋数学 の探求ともに 現実現象の記述 抽象化 定式化 モデル化 の開発にも重点を置くものです 1981年 慶應義塾は他大学にさきがけてを設置し ましたが 数理科学はあらゆる科学技術を語る共通の言葉とし て 理工学はもちろん
Yutaka Shikano. Visualizing a Quantum State
 Yutaka Shikano Visualizing a Quantum State Self Introduction http://qm.ims.ac.jp 東京工業大学 ( 大岡山キャンパス ) 学部 4 年間 修士課程 博士課程と通ったところです 宇宙物理学理論研究室というところにいました マサチューセッツ工科大学 博士課程の際に 機械工学科に留学していました (1 年半 ) チャップマン大学
Yutaka Shikano Visualizing a Quantum State Self Introduction http://qm.ims.ac.jp 東京工業大学 ( 大岡山キャンパス ) 学部 4 年間 修士課程 博士課程と通ったところです 宇宙物理学理論研究室というところにいました マサチューセッツ工科大学 博士課程の際に 機械工学科に留学していました (1 年半 ) チャップマン大学
Report on the experiment of vibration measurement of Wire Brushes. mounted on hand held power tools ワイヤ ブラシ取付け時の手持動力工具振動測定調査の実施について
 Report on the experiment of vibration measurement of Wire Brushes mounted on hand held power tools ワイヤ ブラシ取付け時の手持動力工具振動測定調査の実施について In this report, the purpose of the experiment is to clarify the vibration
Report on the experiment of vibration measurement of Wire Brushes mounted on hand held power tools ワイヤ ブラシ取付け時の手持動力工具振動測定調査の実施について In this report, the purpose of the experiment is to clarify the vibration
Illustrating SUSY breaking effects on various inflation models
 7/29 基研研究会素粒子物理学の進展 2014 Illustrating SUSY breaking effects on various inflation models 山田悠介 ( 早稲田大 ) 共同研究者安倍博之 青木俊太朗 長谷川史憲 ( 早稲田大 ) H. Abe, S. Aoki, F. Hasegawa and Y. Y, arxiv:1408.xxxx 7/29 基研研究会素粒子物理学の進展
7/29 基研研究会素粒子物理学の進展 2014 Illustrating SUSY breaking effects on various inflation models 山田悠介 ( 早稲田大 ) 共同研究者安倍博之 青木俊太朗 長谷川史憲 ( 早稲田大 ) H. Abe, S. Aoki, F. Hasegawa and Y. Y, arxiv:1408.xxxx 7/29 基研研究会素粒子物理学の進展
ロタキサンの超高速初期過程の研究 : シクロデキストリンに包摂されたアゾベンゼン誘導体の光異性化ダイナミクス
 1P031 ロタキサンの超高速初期過程の研究 : シクロデキストリンに包摂されたアゾベンゼン誘導体の光異性化ダイナミクス ( 理研 田原分子分光 1 理研 光量子工学 2 ) Matthew Sartin 1 大澤正久 1 竹内佐年 1,2 田原太平 1,2 Ultrafast initial motions of a rotaxane consisting of cyclodextrin threaded
1P031 ロタキサンの超高速初期過程の研究 : シクロデキストリンに包摂されたアゾベンゼン誘導体の光異性化ダイナミクス ( 理研 田原分子分光 1 理研 光量子工学 2 ) Matthew Sartin 1 大澤正久 1 竹内佐年 1,2 田原太平 1,2 Ultrafast initial motions of a rotaxane consisting of cyclodextrin threaded
Analysis of shale gas production performance by SGPE
 447 77 6 24 11 447 453 Journal of the Japanese Association for Petroleum Technology Vol. 77, No. 6 Nov., 2012 pp. 447 453 Lecture Analysis of shale gas production performance by SGPE Kanji Kato **, Mahmood
447 77 6 24 11 447 453 Journal of the Japanese Association for Petroleum Technology Vol. 77, No. 6 Nov., 2012 pp. 447 453 Lecture Analysis of shale gas production performance by SGPE Kanji Kato **, Mahmood
2015 年度研究活動報告理工学術院 先進理工 応用物理学科小澤徹 Department of Applied Physics, Waseda University
 2015 年度研究活動報告理工学術院 先進理工 応用物理学科小澤徹 Tohru OZAWA Department of Applied Physics, Waseda University 出版された論文 R. Carles, T. Ozawa Finite time extinction for nonlinear Schrödinger equation in 1D and 2D, Commun.
2015 年度研究活動報告理工学術院 先進理工 応用物理学科小澤徹 Tohru OZAWA Department of Applied Physics, Waseda University 出版された論文 R. Carles, T. Ozawa Finite time extinction for nonlinear Schrödinger equation in 1D and 2D, Commun.
PRESENTED BY: PROF. S. Y. MENSAH F.A.A.S; F.G.A.A.S UNIVERSITY OF CAPE COAST, GHANA.
 SOLAR CELL AND ITS APPLICATION PRESENTED BY: PROF. S. Y. MENSAH F.A.A.S; F.G.A.A.S UNIVERSITY OF CAPE COAST, GHANA. OUTLINE OF THE PRESENTATION Objective of the work. A brief introduction to Solar Cell
SOLAR CELL AND ITS APPLICATION PRESENTED BY: PROF. S. Y. MENSAH F.A.A.S; F.G.A.A.S UNIVERSITY OF CAPE COAST, GHANA. OUTLINE OF THE PRESENTATION Objective of the work. A brief introduction to Solar Cell
Advance Publication by J-STAGE. 日本機械学会論文集 Transactions of the JSME (in Japanese)
 Advance Publication by J-STAGE 日本機械学会論文集 Transactions of the JSME (in Japanese) DOI:10.1299/transjsme.16-00058 Received date : 24 February, 2016 Accepted date : 6 September, 2016 J-STAGE Advance Publication
Advance Publication by J-STAGE 日本機械学会論文集 Transactions of the JSME (in Japanese) DOI:10.1299/transjsme.16-00058 Received date : 24 February, 2016 Accepted date : 6 September, 2016 J-STAGE Advance Publication
28 th Conference on Severe Local Storms 11 Nov Eigo Tochimoto and Hiroshi Niino (AORI, The Univ. of Tokyo)
 L 28 th Conference on Severe Local Storms 11 Nov. 2016 Eigo Tochimoto and Hiroshi Niino (AORI, The Univ. of Tokyo) Introduction In the warm sector of extratropical cyclones (ECs), there are strong upper-level
L 28 th Conference on Severe Local Storms 11 Nov. 2016 Eigo Tochimoto and Hiroshi Niino (AORI, The Univ. of Tokyo) Introduction In the warm sector of extratropical cyclones (ECs), there are strong upper-level
電離によるエネルギー損失. β δ. Mean ioniza9on energy. 物質のZ/Aに比例 Z/A~1/2, β~1 1.5MeV/(g cm 2 ) 入射粒子の速度 (β) に依存粒子識別が可能低速では1/β 2. 高速ではβ 2 /(1- β 2 ) で上昇 1.
 放射線検出器の基礎 - 2 飯嶋徹 電離によるエネルギー損失 2 2 de 2 Z 1 2mc 2 2 ln e β δ = Kz β (1 2 dx A β I β ) 2 K = 4π N r m c = 0.3071 MeV cm / gr 2 2 2 0 0 e I Mean ioniza9on energy I 7 = 12 + ev Z Z Z < 13 I 1.19 = 9.76 +
放射線検出器の基礎 - 2 飯嶋徹 電離によるエネルギー損失 2 2 de 2 Z 1 2mc 2 2 ln e β δ = Kz β (1 2 dx A β I β ) 2 K = 4π N r m c = 0.3071 MeV cm / gr 2 2 2 0 0 e I Mean ioniza9on energy I 7 = 12 + ev Z Z Z < 13 I 1.19 = 9.76 +
熊本大学学術リポジトリ. Kumamoto University Repositor
 熊本大学学術リポジトリ Kumamoto University Repositor Title 新興薬剤耐性菌が産生するメタロ -β- ラクタマーゼの加 水分解機構の解明と非可逆的阻害剤の開発 Author(s) 山口, 佳宏 Citation Issue date 2005-10-06 Type URL Thesis or Dissertation http://hdl.handle.net/2298/12170
熊本大学学術リポジトリ Kumamoto University Repositor Title 新興薬剤耐性菌が産生するメタロ -β- ラクタマーゼの加 水分解機構の解明と非可逆的阻害剤の開発 Author(s) 山口, 佳宏 Citation Issue date 2005-10-06 Type URL Thesis or Dissertation http://hdl.handle.net/2298/12170
SOLID STATE PHYSICAL CHEMISTRY
 SOLID STATE PHYSICAL CHEMISTRY Annual Research Highlights (1) ε-al x Fe 2 x O 3 : an advanced nanomagnets exhibiting millimeter-wave absorption In this work, aluminum-substituted epsilon-phase iron oxides,
SOLID STATE PHYSICAL CHEMISTRY Annual Research Highlights (1) ε-al x Fe 2 x O 3 : an advanced nanomagnets exhibiting millimeter-wave absorption In this work, aluminum-substituted epsilon-phase iron oxides,
October 7, Shinichiro Mori, Associate Professor
 The areas of landslides and affected buildings and houses within those areas, in and around Palu City during Sulawesi Earthquake on September 28, 2018 specified by an analysis based on satellite imagery
The areas of landslides and affected buildings and houses within those areas, in and around Palu City during Sulawesi Earthquake on September 28, 2018 specified by an analysis based on satellite imagery
Thermal Safety Software (TSS) series
 Ready-solutions Thermal Safety Software (TSS) series 各種の反応危険性評価ソフト The subset for adiabatic calorimetry ( 断熱測定データ解析ソリューション ) 断熱熱量計 (ARC VSP DEWAR) 等は 化学反応の熱的危険性評価に有効であることが知られています このソリューションは 断熱下で測定した熱量データを多目的に解析するために
Ready-solutions Thermal Safety Software (TSS) series 各種の反応危険性評価ソフト The subset for adiabatic calorimetry ( 断熱測定データ解析ソリューション ) 断熱熱量計 (ARC VSP DEWAR) 等は 化学反応の熱的危険性評価に有効であることが知られています このソリューションは 断熱下で測定した熱量データを多目的に解析するために
Spontaneous magnetization of quark matter in the inhomogeneous chiral phase
 Spontaneous agnetization of uar atter in the inhoogeneous chiral phase arxiv:7. [hep-ph] Ryo Yoshiie (Kyoto University) collaborator:kazuya Nishiyaa oshitaa atsui XQCD 9/@CCNU QCD phase diagra and chiral
Spontaneous agnetization of uar atter in the inhoogeneous chiral phase arxiv:7. [hep-ph] Ryo Yoshiie (Kyoto University) collaborator:kazuya Nishiyaa oshitaa atsui XQCD 9/@CCNU QCD phase diagra and chiral
Mesoporous titanium dioxide electrolyte bulk heterojunction
 Mesoporous titanium dioxide electrolyte bulk heterojunction The term "bulk heterojunction" is used to describe a heterojunction composed of two different materials acting as electron- and a hole- transporters,
Mesoporous titanium dioxide electrolyte bulk heterojunction The term "bulk heterojunction" is used to describe a heterojunction composed of two different materials acting as electron- and a hole- transporters,
統合シミュレーションコードによる高速点火実験解析大阪大学レーザーエネルギー学研究センター中村龍史
 統合シミュレーションコードによる高速点火実験解析大阪大学レーザーエネルギー学研究センター中村龍史 概要 高速点火核融合ではターゲット爆縮から追加熱用レーザーによる高速電子発生まで時間 空間スケールにおいて非常に広範な領域の現象を理解する必要がある そこで本プロジェクトでは輻射流体コード 粒子コード Fokker-Planck コード の開発が進められており これらのコードをネットワークを介して結合することで統合コードの構築を目指している
統合シミュレーションコードによる高速点火実験解析大阪大学レーザーエネルギー学研究センター中村龍史 概要 高速点火核融合ではターゲット爆縮から追加熱用レーザーによる高速電子発生まで時間 空間スケールにおいて非常に広範な領域の現象を理解する必要がある そこで本プロジェクトでは輻射流体コード 粒子コード Fokker-Planck コード の開発が進められており これらのコードをネットワークを介して結合することで統合コードの構築を目指している
高エネルギーニュートリノ : 理論的な理解 の現状
 第 24 回 宇宙ニュートリノ 研究会 高エネルギーニュートリノ : 理論的な理解 の現状 京都大学基礎物理学研究所 長滝重博 Kaji Yasuyuki 9th March 2011, ICRR, Tokyo Overview History of the Universe UHECRs? VHE-ν? When? Who? How? CMB 3000k -> 2.75k Mysteries of
第 24 回 宇宙ニュートリノ 研究会 高エネルギーニュートリノ : 理論的な理解 の現状 京都大学基礎物理学研究所 長滝重博 Kaji Yasuyuki 9th March 2011, ICRR, Tokyo Overview History of the Universe UHECRs? VHE-ν? When? Who? How? CMB 3000k -> 2.75k Mysteries of
Network of Evolutionary Trends and Maturity assessment through contradiction analysis 進化トレンドのネットワークと矛盾解析による成熟度評価
 Knowledge Aided Engineering Manufacturing and Related Technologies 和訳 : 井上淳 ( 東芝 ) 中川徹 ( 大阪学院大 ) Network of Evolutionary Trends and Maturity assessment through contradiction analysis 進化トレンドのネットワークと矛盾解析による成熟度評価
Knowledge Aided Engineering Manufacturing and Related Technologies 和訳 : 井上淳 ( 東芝 ) 中川徹 ( 大阪学院大 ) Network of Evolutionary Trends and Maturity assessment through contradiction analysis 進化トレンドのネットワークと矛盾解析による成熟度評価
ATLAS 実験における荷電ヒッグス粒子の探索
 1 ATLAS 実験における荷電ヒッグス粒子の探索 筑波大学大学院数理物質科学研究科物理学専攻博士後期課程 3 年永田和樹 2016 年 1 月 19 日 CiRfSE workshop 重い荷電ヒッグス粒子探索 2 もし 荷電ヒッグス粒子の発見できたら 標準理論を超える物理の存在の証明 例 :Minimal Supersymmetric Standard Model (MSSM) 重い荷電ヒッグス粒子探索
1 ATLAS 実験における荷電ヒッグス粒子の探索 筑波大学大学院数理物質科学研究科物理学専攻博士後期課程 3 年永田和樹 2016 年 1 月 19 日 CiRfSE workshop 重い荷電ヒッグス粒子探索 2 もし 荷電ヒッグス粒子の発見できたら 標準理論を超える物理の存在の証明 例 :Minimal Supersymmetric Standard Model (MSSM) 重い荷電ヒッグス粒子探索
2018 年 ( 平成 30 年 ) 7 月 13 日 ( 金曜日 ) Fri July 13, 2018
 2018 年 ( 平成 30 年 ) 13 日 ( 金曜日 ) Fri July 13, 2018 発行所 (Name) : 株式会社東京証券取引所 所在地 (Address) : 103-8220 東京都中央日本橋兜町 2-1 ホームヘ ーシ (URL) : https://www.jpx.co.jp/ 電話 (Phone) : 03-3666-0141 2-1 Nihombashi Kabutocho,
2018 年 ( 平成 30 年 ) 13 日 ( 金曜日 ) Fri July 13, 2018 発行所 (Name) : 株式会社東京証券取引所 所在地 (Address) : 103-8220 東京都中央日本橋兜町 2-1 ホームヘ ーシ (URL) : https://www.jpx.co.jp/ 電話 (Phone) : 03-3666-0141 2-1 Nihombashi Kabutocho,
Photovoltaic Energy Conversion. Frank Zimmermann
 Photovoltaic Energy Conversion Frank Zimmermann Solar Electricity Generation Consumes no fuel No pollution No greenhouse gases No moving parts, little or no maintenance Sunlight is plentiful & inexhaustible
Photovoltaic Energy Conversion Frank Zimmermann Solar Electricity Generation Consumes no fuel No pollution No greenhouse gases No moving parts, little or no maintenance Sunlight is plentiful & inexhaustible
Numerical Simulation of Seismic Wave Propagation and Strong Motions in 3D Heterogeneous Structure
 Numerical Simulation of Seismic Wave Propagation and Strong Motions in 3D Heterogeneous Structure Project Representative Takashi Furumura Author Takashi Furumura Center for Integrated Disaster Information
Numerical Simulation of Seismic Wave Propagation and Strong Motions in 3D Heterogeneous Structure Project Representative Takashi Furumura Author Takashi Furumura Center for Integrated Disaster Information
一般化川渡り問題について. 伊藤大雄 ( 京都大学 ) Joint work with Stefan Langerman (Univ. Libre de Bruxelles) 吉田悠一 ( 京都大学 ) 組合せゲーム パズルミニ研究集会
 一般化川渡り問題について 伊藤大雄 ( 京都大学 ) Joint work with Stefan Langerman (Univ. Libre de Bruxelles) 吉田悠一 ( 京都大学 ) 12.3.8 組合せゲーム パズルミニ研究集会 1 3 人の嫉妬深い男とその妹の問題 [Alcuin of York, 青年達を鍛えるための諸問題 より ] 3 人の男がそれぞれ一人ずつ未婚の妹を連れて川にさしかかった
一般化川渡り問題について 伊藤大雄 ( 京都大学 ) Joint work with Stefan Langerman (Univ. Libre de Bruxelles) 吉田悠一 ( 京都大学 ) 12.3.8 組合せゲーム パズルミニ研究集会 1 3 人の嫉妬深い男とその妹の問題 [Alcuin of York, 青年達を鍛えるための諸問題 より ] 3 人の男がそれぞれ一人ずつ未婚の妹を連れて川にさしかかった
質量起源 暗黒物質 暗黒エネルギー 宇宙線 陽子崩壊 ニュートリノ質量 米国 P5 ニュートリノ CPV 宇宙背景ニュートリノクォーク レプトンマヨラナ粒子 ニュートリノ測定器 陽子崩壊探索. Diagram courtesy of P5. Origin of Mass.
 Diagram courtesy of P5 The Energy Frontier Origin of Mass Matter/Antimatter Asymmetry Dark Matter Origin of Universe Unification of Forces New Physics Beyond the Standard Model ニュートリノ質量 質量起源 The Intensity
Diagram courtesy of P5 The Energy Frontier Origin of Mass Matter/Antimatter Asymmetry Dark Matter Origin of Universe Unification of Forces New Physics Beyond the Standard Model ニュートリノ質量 質量起源 The Intensity
Effects of pairing correlation on the low-lying quasiparticle resonance in neutron drip-line nuclei
 2016/09/13 INPC2016 @ Adelaide 1 Effects of pairing correlation on the low-lying quasiparticle resonance in neutron drip-line nuclei Back ground Prog. Theor. Exp. Phys. 2016, 013D01 Yoshihiko Kobayashi
2016/09/13 INPC2016 @ Adelaide 1 Effects of pairing correlation on the low-lying quasiparticle resonance in neutron drip-line nuclei Back ground Prog. Theor. Exp. Phys. 2016, 013D01 Yoshihiko Kobayashi
XENON SHORT ARC LAMPS キセノンショートアークランプ
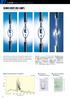 光源事業 / キセノンショートアークランプ XENON SHORT ARC LAMPS キセノンショートアークランプ 当社のキセノンショートアークランプは 自然太陽光に近似し た光波長 分光分布を有する特性を生かし 太陽電池の性能評 価 基礎研究のための弊社製ソーラシミュレータ用標準光源と して採用しているだけでなく 高輝度 点光源 高演色という 特性を生かし 分光器用光源 映写機用光源 プラネタリウム
光源事業 / キセノンショートアークランプ XENON SHORT ARC LAMPS キセノンショートアークランプ 当社のキセノンショートアークランプは 自然太陽光に近似し た光波長 分光分布を有する特性を生かし 太陽電池の性能評 価 基礎研究のための弊社製ソーラシミュレータ用標準光源と して採用しているだけでなく 高輝度 点光源 高演色という 特性を生かし 分光器用光源 映写機用光源 プラネタリウム
第 6 回 スペースデブリワークショップ 講演資料集 291 E3 デオービット用膜面展開機構の開発 Development of Membran Deployment mechanism for Deorbiting 高井元 ( 宇宙航空研究開発機構 ), 古谷寛, 坂本啓 ( 東京工業大学 ),
 第 6 回 スペースデブリワークショップ 講演資料集 291 E3 デオービット用膜面展開機構の開発 Development of Membran Deployment mechanism for Deorbiting 高井元 ( 宇宙航空研究開発機構 ), 古谷寛, 坂本啓 ( 東京工業大学 ), 奥泉信克, 宮崎英治, 井上浩一 ( 宇宙航空研究開発機構 ) Moto Takai(JAXA),
第 6 回 スペースデブリワークショップ 講演資料集 291 E3 デオービット用膜面展開機構の開発 Development of Membran Deployment mechanism for Deorbiting 高井元 ( 宇宙航空研究開発機構 ), 古谷寛, 坂本啓 ( 東京工業大学 ), 奥泉信克, 宮崎英治, 井上浩一 ( 宇宙航空研究開発機構 ) Moto Takai(JAXA),
BCR30AM-12LB. RJJ03G Rev I T(RMS) 30 A V DRM 600 V I FGT I, I RGT I, I RGT III 50 ma : PRSS0004ZE-A ( : TO-3P) 4 2, 4
 BCRAM-1LB ( 1 C ) RJJG- Rev...11. I T(RMS) A V DRM 6 V I FGT I, I RGT I, I RGT III ma : PRSSZE-A ( : TO-P), 1 1. T 1. T.. T 1 1.. 1 C 1 C 1 * 1 V DRM 6 V * 1 V DSM V I T(RMS) A Tc = C I TSM A 6Hz 1 I t
BCRAM-1LB ( 1 C ) RJJG- Rev...11. I T(RMS) A V DRM 6 V I FGT I, I RGT I, I RGT III ma : PRSSZE-A ( : TO-P), 1 1. T 1. T.. T 1 1.. 1 C 1 C 1 * 1 V DRM 6 V * 1 V DSM V I T(RMS) A Tc = C I TSM A 6Hz 1 I t
Safer Building and Urban Development ( 安全な建物づくり まちづくりづ ) Contents ( 内容 ) 1)Lessons from building damage by earthquake motions and/or tsunami ( 振動被害または
 Safer Building and Urban Development ( 安全な建物づくり まちづくりづ ) Contents ( 内容 ) 1)Lessons from building damage by earthquake motions and/or tsunami ( 振動被害または津波被害からの教訓 ) 2)Way of thinking for reconstruction (
Safer Building and Urban Development ( 安全な建物づくり まちづくりづ ) Contents ( 内容 ) 1)Lessons from building damage by earthquake motions and/or tsunami ( 振動被害または津波被害からの教訓 ) 2)Way of thinking for reconstruction (
Multiple Exciton Generation in Quantum Dots. James Rogers Materials 265 Professor Ram Seshadri
 Multiple Exciton Generation in Quantum Dots James Rogers Materials 265 Professor Ram Seshadri Exciton Generation Single Exciton Generation in Bulk Semiconductors Multiple Exciton Generation in Bulk Semiconductors
Multiple Exciton Generation in Quantum Dots James Rogers Materials 265 Professor Ram Seshadri Exciton Generation Single Exciton Generation in Bulk Semiconductors Multiple Exciton Generation in Bulk Semiconductors
Youhei Uchida 1, Kasumi Yasukawa 1, Norio Tenma 1, Yusaku Taguchi 1, Jittrakorn Suwanlert 2 and Somkid Buapeng 2
 Bulletin of the Geological Subsurface Survey of Japan, Thermal vol.60 Regime (9/10), in the p.469-489, Chao-Phraya 2009 Plain, Thailand (Uchida et al.) Subsurface Thermal Regime in the Chao-Phraya Plain,
Bulletin of the Geological Subsurface Survey of Japan, Thermal vol.60 Regime (9/10), in the p.469-489, Chao-Phraya 2009 Plain, Thailand (Uchida et al.) Subsurface Thermal Regime in the Chao-Phraya Plain,
Product Specification
 This information may be changed without a previous notice. Specification No. JECXDE-9004E Product Specification Issued Date: 17 / Dec. / 2014 Part Description: Supercapacitor (EDLC) Customer Part No.:
This information may be changed without a previous notice. Specification No. JECXDE-9004E Product Specification Issued Date: 17 / Dec. / 2014 Part Description: Supercapacitor (EDLC) Customer Part No.:
Neutron-Insensitive Gamma-Ray Detector with Aerogel for Rare Neutral-Kaon Decay Experiment
 Neutron-Insensitive Gamma-Ray Detector with Aerogel for Rare Neutral-Kaon Decay Experiment Kyoto University E-mail: maeda_y@scphys.kyoto-u.ac.jp A novel γ-ray detector insensitive to neutrons is developed
Neutron-Insensitive Gamma-Ray Detector with Aerogel for Rare Neutral-Kaon Decay Experiment Kyoto University E-mail: maeda_y@scphys.kyoto-u.ac.jp A novel γ-ray detector insensitive to neutrons is developed
Development of Advanced Simulation Methods for Solid Earth Simulations
 Chapter 1 Earth Science Development of Advanced Simulation Methods for Solid Earth Simulations Project Representative Mikito Furuichi Department of Mathematical Science and Advanced Technology, Japan Agency
Chapter 1 Earth Science Development of Advanced Simulation Methods for Solid Earth Simulations Project Representative Mikito Furuichi Department of Mathematical Science and Advanced Technology, Japan Agency
東ソー株式会社 - 明日のしあわせを化学する - TOSOH CORPORATION The Chemistry of Innovation. TOSOH CORPORATION The Chemistry of Innovation
 東ソー株式会社 - 明日のしあわせを化学する - TOSOH CORPORATION The Chemistry of Innovation TOSOH CORPORATION The Chemistry of Innovation 目次 - Contents 1) 東ソー株式会社の概要 Outline of Tosoh Corporation. 2) Tosoh Advanced Materials
東ソー株式会社 - 明日のしあわせを化学する - TOSOH CORPORATION The Chemistry of Innovation TOSOH CORPORATION The Chemistry of Innovation 目次 - Contents 1) 東ソー株式会社の概要 Outline of Tosoh Corporation. 2) Tosoh Advanced Materials
2A01 Fourier-transform spectroscopy of D + 2 using intense near-infrared few-cycle laser pulses [Abstract] [Introduction] [Methods]
![2A01 Fourier-transform spectroscopy of D + 2 using intense near-infrared few-cycle laser pulses [Abstract] [Introduction] [Methods] 2A01 Fourier-transform spectroscopy of D + 2 using intense near-infrared few-cycle laser pulses [Abstract] [Introduction] [Methods]](/thumbs/95/124160086.jpg) 2A01 Fourier-transform spectroscopy of D2 + using intense near-infrared few-cycle laser pulses Toshiaki Ando, Atsushi Iwasaki, Kaoru Yamanouchi Department of Chemistry, School of Science, The University
2A01 Fourier-transform spectroscopy of D2 + using intense near-infrared few-cycle laser pulses Toshiaki Ando, Atsushi Iwasaki, Kaoru Yamanouchi Department of Chemistry, School of Science, The University
( 主査 ) 教授髙橋秀幸教授山口信次郎准教授佐藤修正
 氏名 ( 本籍地 ) 学 位 の 種 類 学 位 記 番 号 学位授与年月日 学位授与の要件 研究科, 専攻 論 文 題 目 ぱんれい 庞磊博士 ( 生命科学 ) 生博第 337 号平成 29 年 3 月 24 日学位規則第 4 条第 1 項該当東北大学大学院生命科学研究科 ( 博士課程 ) 生態システム生命科学専攻 Analyses of Functional Tissues and Hormonal
氏名 ( 本籍地 ) 学 位 の 種 類 学 位 記 番 号 学位授与年月日 学位授与の要件 研究科, 専攻 論 文 題 目 ぱんれい 庞磊博士 ( 生命科学 ) 生博第 337 号平成 29 年 3 月 24 日学位規則第 4 条第 1 項該当東北大学大学院生命科学研究科 ( 博士課程 ) 生態システム生命科学専攻 Analyses of Functional Tissues and Hormonal
CH676 Physical Chemistry: Principles and Applications. CH676 Physical Chemistry: Principles and Applications
 CH676 Physical Chemistry: Principles and Applications Crystal Structure and Chemistry Synthesis of Tetrahexahedral Platinum Nanocrystals with High-Index Facets and High Electro-Oxidation Activity Na Tian
CH676 Physical Chemistry: Principles and Applications Crystal Structure and Chemistry Synthesis of Tetrahexahedral Platinum Nanocrystals with High-Index Facets and High Electro-Oxidation Activity Na Tian
D j a n g o と P H P の仲間たち ( 改変済 ) サイボウズ ラボ株式会社 TSURUOKA Naoya
 D j a n g o と P H P の仲間たち ( 改変済 ) サイボウズ ラボ株式会社 TSURUOKA Naoya D j a n g o と P H P の仲間たち What's Django? W h a t ' s D j a n g o Pythonのフレームワーク インストール簡単 コマンドを実行するだけで自動生成しまくり
D j a n g o と P H P の仲間たち ( 改変済 ) サイボウズ ラボ株式会社 TSURUOKA Naoya D j a n g o と P H P の仲間たち What's Django? W h a t ' s D j a n g o Pythonのフレームワーク インストール簡単 コマンドを実行するだけで自動生成しまくり
Y. Okayasu for the Jlab E collaboration Department of Physics. Tohoku University
 Analysis status of the Λ hyernuclear sectroscoic exeriment @ Jlab Hall C (E01-011) Contents 1: Characteristics of the (e,e K ) reaction 2: Physics goals of Jlab E01-011 3: Thomas Jefferson National Accelerator
Analysis status of the Λ hyernuclear sectroscoic exeriment @ Jlab Hall C (E01-011) Contents 1: Characteristics of the (e,e K ) reaction 2: Physics goals of Jlab E01-011 3: Thomas Jefferson National Accelerator
井出哲博士の研究業績概要 井出哲博士はこれまで データ解析や数値シミュレーションの手法を用いることによって 地震の震源で起きている現象を様々な角度から研究してきた その主な研究成果は 以下の 3 つに大別される
 井出哲博士の研究業績概要 井出哲博士はこれまで データ解析や数値シミュレーションの手法を用いることによって 地震の震源で起きている現象を様々な角度から研究してきた その主な研究成果は 以下の 3 つに大別される 1. 地震の動的破壊プロセスの解明地下の岩盤の破壊を伴う摩擦すべりである地震を理解するには 地下で何が起きたかを正確に知る必要がある 井出博士は そのための手法である 断層すべりインバージョン法
井出哲博士の研究業績概要 井出哲博士はこれまで データ解析や数値シミュレーションの手法を用いることによって 地震の震源で起きている現象を様々な角度から研究してきた その主な研究成果は 以下の 3 つに大別される 1. 地震の動的破壊プロセスの解明地下の岩盤の破壊を伴う摩擦すべりである地震を理解するには 地下で何が起きたかを正確に知る必要がある 井出博士は そのための手法である 断層すべりインバージョン法
Oxford, Vol. 9, pp (2004).. (3) 微小空間を活用する多相系有機合成反応 小林重太 森雄一朗 小林修 化学と工業 59, pp (2006).
 森雄一朗博士の研究業績概要 現代科学において環境は最も重要なキーワードの一つであり 化学分野では環境にやさしい化学 すなわちグリーン サステイナブルケミストリー (GSC) が世界的に重要な研究領域として位置づけられている GSCという言葉が生み出されて約 10 年であるが 化学と環境の共存という大きな目標に向けて今後益々の発展が必要な分野である 森雄一朗博士はこれまでGSC 分野を中心に基礎から応用まで幅広く研究活動を行い成果を挙げている
森雄一朗博士の研究業績概要 現代科学において環境は最も重要なキーワードの一つであり 化学分野では環境にやさしい化学 すなわちグリーン サステイナブルケミストリー (GSC) が世界的に重要な研究領域として位置づけられている GSCという言葉が生み出されて約 10 年であるが 化学と環境の共存という大きな目標に向けて今後益々の発展が必要な分野である 森雄一朗博士はこれまでGSC 分野を中心に基礎から応用まで幅広く研究活動を行い成果を挙げている
XAFS Spectroscopy X-ray absorption fine structure
 17/7/9 XAFS Spectroscopy X-ray absorpton fne structure K.Asakura, ICAT Hokuda 1 17/7/9 X 線とは X 線とは波長の短い電磁波 ( 横波 ) である. 4 17/7/9 X 線と物質との相互作用 3 図 1.3 X 線の吸収によって引き起こされる様々な現象. 図 1.4 1 個の原子内での X 線吸収現象. 3 17/7/9
17/7/9 XAFS Spectroscopy X-ray absorpton fne structure K.Asakura, ICAT Hokuda 1 17/7/9 X 線とは X 線とは波長の短い電磁波 ( 横波 ) である. 4 17/7/9 X 線と物質との相互作用 3 図 1.3 X 線の吸収によって引き起こされる様々な現象. 図 1.4 1 個の原子内での X 線吸収現象. 3 17/7/9
SML-811x/812x/813x Series
 SML-811x/812x/813x Series Data Sheet 特 背面実装可能タイプ 外観図 サイズ情報 3412 (15) 3.4 1.25mm (t=1.1mm) Color Type V U D W M B WB 外形寸法図 3.4 はんだ付け推奨パターン 1.2 R 1.25 1.25 φ2.4 4-R.3 レジスト +.5 1.1 -.1 Hole + 1.25.28 カソードインデックス
SML-811x/812x/813x Series Data Sheet 特 背面実装可能タイプ 外観図 サイズ情報 3412 (15) 3.4 1.25mm (t=1.1mm) Color Type V U D W M B WB 外形寸法図 3.4 はんだ付け推奨パターン 1.2 R 1.25 1.25 φ2.4 4-R.3 レジスト +.5 1.1 -.1 Hole + 1.25.28 カソードインデックス
Influence of MJO on Asian Climate and its Performance of JMA Monthly Forecast Model
 Influence of MJO on Asian Climate and its Performance of JMA Monthly Forecast Model Satoko Matsueda, Yuhei Takaya and Kengo Miyaoka Climate Prediction Division, Japan Meteorological Agency Madden-Julian
Influence of MJO on Asian Climate and its Performance of JMA Monthly Forecast Model Satoko Matsueda, Yuhei Takaya and Kengo Miyaoka Climate Prediction Division, Japan Meteorological Agency Madden-Julian
More Efficient Solar Cells via Multi Exciton Generation
 More Efficient Solar Cells via Multi Exciton Generation By: MIT Student Instructor: Gang Chen May 14, 2010 1 Introduction Sunlight is the most abundant source of energy available on Earth and if properly
More Efficient Solar Cells via Multi Exciton Generation By: MIT Student Instructor: Gang Chen May 14, 2010 1 Introduction Sunlight is the most abundant source of energy available on Earth and if properly
化学気相成長 (CVD) 法による カーボン薄膜の成長制御
 化学気相成長 (CVD) 法による カーボン薄膜の成長制御 吉村雅満豊田工業大学表面科学研究室 yoshi@toyota-ti.ac.jp R. Tiwari, 鈴木誠也, 松岡佑樹, D. Nguyen 1. カーボンナノチューブーグラフェン複合体 2. グラフェン Background & MoJvaJon Discovery of Nano Carbons 1985 1991 1993 2004
化学気相成長 (CVD) 法による カーボン薄膜の成長制御 吉村雅満豊田工業大学表面科学研究室 yoshi@toyota-ti.ac.jp R. Tiwari, 鈴木誠也, 松岡佑樹, D. Nguyen 1. カーボンナノチューブーグラフェン複合体 2. グラフェン Background & MoJvaJon Discovery of Nano Carbons 1985 1991 1993 2004
日本政府 ( 文部科学省 ) 奨学金留学生申請書
 A PP L IC AT IO N J AP A N ESE G O VE R NMENT ( M ONB UK AG AK US HO :M EXT)SC H OL A RS H IP F O R 2 0 1 6 日本政府 ( 文部科学省 ) 奨学金留学生申請書 Undergraduate Students ( 学部留学生 ) INSTRUCTIONS( 記入上の注意 ) 1.Type application,
A PP L IC AT IO N J AP A N ESE G O VE R NMENT ( M ONB UK AG AK US HO :M EXT)SC H OL A RS H IP F O R 2 0 1 6 日本政府 ( 文部科学省 ) 奨学金留学生申請書 Undergraduate Students ( 学部留学生 ) INSTRUCTIONS( 記入上の注意 ) 1.Type application,
マテリアルズインフォマティクスの最前線 吉田 亮. サイエンティフィック システム研究会 科学技術計算分科会 2017年度会合
 サイエンティフィック システム研究会 科学技術計算分科会 2017年度会合 マテリアルズインフォマティクスの最前線 吉田 亮 1,2,3,4 yoshidar@ism.ac.jp 情報 システム研究機構 統計数理研究所 モデリング研究系 准教授 情報 システム研究機構 統計数理研究所 ものづくりデータ科学研究センター センター長 3 総合研究大学院大学 複合科学研究科 統計科学専攻 准教授 4 物質
サイエンティフィック システム研究会 科学技術計算分科会 2017年度会合 マテリアルズインフォマティクスの最前線 吉田 亮 1,2,3,4 yoshidar@ism.ac.jp 情報 システム研究機構 統計数理研究所 モデリング研究系 准教授 情報 システム研究機構 統計数理研究所 ものづくりデータ科学研究センター センター長 3 総合研究大学院大学 複合科学研究科 統計科学専攻 准教授 4 物質
din Linguistics 一 一 St1'uctur~s a proposals representagrammati squite incomplete. Although generate al
 G D M G Nw B C 英語教授法通信 第 号 ι 年 月 日勺 WHA RANSFORM AONALYNAX 一一 A R! L 一 一 ず 併 j ~ ;! ~も Wm Y m m m x N~w B~ mx j (; ; qw m M w SC mj m S~ Cm mx mm C J q m A H CmS S m m m mm m Cm R P S ; x mm w A と w R
G D M G Nw B C 英語教授法通信 第 号 ι 年 月 日勺 WHA RANSFORM AONALYNAX 一一 A R! L 一 一 ず 併 j ~ ;! ~も Wm Y m m m x N~w B~ mx j (; ; qw m M w SC mj m S~ Cm mx mm C J q m A H CmS S m m m mm m Cm R P S ; x mm w A と w R
Crustal Deformation Associated with the 2005 West Off Fukuoka Prefecture Earthquake Derived from ENVISAT/InSAR and Fault- slip Modeling
 Crustal Deformation Associated with the 2005 West Off Fukuoka Prefecture Earthquake Derived from ENVISAT/InSAR and Fault- slip Modeling Taku OZAWA, Sou NISHIMURA, and Hiroshi OHKURA Volcano Research Department,
Crustal Deformation Associated with the 2005 West Off Fukuoka Prefecture Earthquake Derived from ENVISAT/InSAR and Fault- slip Modeling Taku OZAWA, Sou NISHIMURA, and Hiroshi OHKURA Volcano Research Department,
Effects of Different Solvents on Hydrogenation of Acetic Acid over Pt/TiO 2 for Bioethanol Production
 162 J. Jpn. Inst. Energy, Vol. Journal 95, No. of 2, the 2016 Japan Institute of Energy, 95, 162-166(2016) Effects of Different Solvents on Hydrogenation of Acetic Acid over Pt/TiO 2 for Bioethanol Production
162 J. Jpn. Inst. Energy, Vol. Journal 95, No. of 2, the 2016 Japan Institute of Energy, 95, 162-166(2016) Effects of Different Solvents on Hydrogenation of Acetic Acid over Pt/TiO 2 for Bioethanol Production
Introduction to Multi-hazard Risk-based Early Warning System in Japan
 Introduction to Multi-hazard Risk-based Early Warning System in Japan Yasuo SEKITA (Mr) Director-General, Forecast Department Japan Meteorological Agency (JMA) Natural Disasters in Asia Source: Disasters
Introduction to Multi-hazard Risk-based Early Warning System in Japan Yasuo SEKITA (Mr) Director-General, Forecast Department Japan Meteorological Agency (JMA) Natural Disasters in Asia Source: Disasters
Estimation of Gravel Size Distribution using Contact Time
 ISSN 386-1678 Report of the Research Institute of Industrial Technology, Nihon University Number 97, 212 Estimation of Gravel Size Distribution using Contact Time Akira ODA*, Yuya HIRANO**, Masanori WATANABE***
ISSN 386-1678 Report of the Research Institute of Industrial Technology, Nihon University Number 97, 212 Estimation of Gravel Size Distribution using Contact Time Akira ODA*, Yuya HIRANO**, Masanori WATANABE***
Fusion neutron production with deuterium neutral beam injection and enhancement of energetic-particle physics study in the Large Helical Device
 15 th IAEA Technical Meeting on Energetic Particles in Magnetic Confinement Systems, 5-8 September, PPPL Session 8 : Diagnostic Development : O-14 Fusion neutron production with deuterium neutral beam
15 th IAEA Technical Meeting on Energetic Particles in Magnetic Confinement Systems, 5-8 September, PPPL Session 8 : Diagnostic Development : O-14 Fusion neutron production with deuterium neutral beam
Photocatalysis: semiconductor physics
 Photocatalysis: semiconductor physics Carlos J. Tavares Center of Physics, University of Minho, Portugal ctavares@fisica.uminho.pt www.fisica.uminho.pt 1 Guimarães Where do I come from? 3 Guimarães 4 Introduction>>
Photocatalysis: semiconductor physics Carlos J. Tavares Center of Physics, University of Minho, Portugal ctavares@fisica.uminho.pt www.fisica.uminho.pt 1 Guimarães Where do I come from? 3 Guimarães 4 Introduction>>
(n, γ) 法による 99 Mo/ 99m Tc 製造用照射ターゲットの製造技術開発と特性評価
 JAEA-Technology 2014-034 (n, γ) 法による 99 Mo/ 99m Tc 製造用照射ターゲットの製造技術開発と特性評価 Fabrication Technology Development and Characterization of Irradiation Targets for 99 Mo/ 99m Tc Production by (n, g) Method 西方香緒里木村明博石田卓也椎名孝行
JAEA-Technology 2014-034 (n, γ) 法による 99 Mo/ 99m Tc 製造用照射ターゲットの製造技術開発と特性評価 Fabrication Technology Development and Characterization of Irradiation Targets for 99 Mo/ 99m Tc Production by (n, g) Method 西方香緒里木村明博石田卓也椎名孝行
21 点 15 点 3 解答用紙に氏名と受検番号を記入し, 受検番号と一致したマーク部分を塗りつぶすこと 受検番号が 0( ゼロ ) から始まる場合は,0( ゼロ ) を塗りつぶすこと
 平成 29 年度入学者選抜学力検査問題 英 語 ( 配点 ) 1 2 3 4 5 6 10 点 15 点 24 点 15 点 15 点 21 点 ( 注意事項 ) 1 問題冊子は指示があるまで開かないこと 2 問題冊子は 1 ページから 8 ページまでです 検査開始の合図のあとで確かめること 3 解答用紙に氏名と受検番号を記入し, 受検番号と一致したマーク部分を塗りつぶすこと 受検番号が 0( ゼロ
平成 29 年度入学者選抜学力検査問題 英 語 ( 配点 ) 1 2 3 4 5 6 10 点 15 点 24 点 15 点 15 点 21 点 ( 注意事項 ) 1 問題冊子は指示があるまで開かないこと 2 問題冊子は 1 ページから 8 ページまでです 検査開始の合図のあとで確かめること 3 解答用紙に氏名と受検番号を記入し, 受検番号と一致したマーク部分を塗りつぶすこと 受検番号が 0( ゼロ
Chapter 13 Properties of Solutions
 Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 13 Properties of Solutions Adapted by K. Kasatani from: John D. Bookstaver St. Charles
Chemistry, The Central Science, 10th edition Theodore L. Brown; H. Eugene LeMay, Jr.; and Bruce E. Bursten Chapter 13 Properties of Solutions Adapted by K. Kasatani from: John D. Bookstaver St. Charles
Evaluation of IGS Reprocessed Precise Ephemeris Applying the Analysis of the Japanese Domestic GPS Network Data
 Evaluation of IGS Reprocessed Precise Ephemeris Applying the Analysis of the Japanese Domestic GPS Network Data Seiichi SHIMADA * Abstract The IGS reprocessed GPS precise ephemeris (repro1) is evaluated
Evaluation of IGS Reprocessed Precise Ephemeris Applying the Analysis of the Japanese Domestic GPS Network Data Seiichi SHIMADA * Abstract The IGS reprocessed GPS precise ephemeris (repro1) is evaluated
757G-V2 Series. For General Lighting Application. NF2x757G-F1, NFSx757G COB Series. Ra 90. Ra 80 Cove Light. Surface Mount Type
 DETAIL DETAIL For General Lighting High color rendering Ra 95 option High color rendering option Ra 95 is now available, even in higher color temperatures. Illuminated objects can appear more natural than
DETAIL DETAIL For General Lighting High color rendering Ra 95 option High color rendering option Ra 95 is now available, even in higher color temperatures. Illuminated objects can appear more natural than
Kyoko Kagohara 1, Tomio Inazaki 2, Atsushi Urabe 3 and Yoshinori Miyachi 4 楮原京子
 地質調査総合センター速報 No.54, 平成 21 年度沿岸域の地質 活断層調査研究報告,p.61-67,2010 長岡平野西縁断層帯における浅層反射法地震探査 - 新潟市松野尾地区の地下構造 Subsurface structure of the Western boundary fault zone of Nagaoka Plain, based on high-resolution seismic
地質調査総合センター速報 No.54, 平成 21 年度沿岸域の地質 活断層調査研究報告,p.61-67,2010 長岡平野西縁断層帯における浅層反射法地震探査 - 新潟市松野尾地区の地下構造 Subsurface structure of the Western boundary fault zone of Nagaoka Plain, based on high-resolution seismic
ADEOS-II ミッションと海洋学 海洋気象学 (ADEOS-II Mission and Oceanography/Marine Meteorology)
 ADEOS-II ミッションと海洋学 海洋気象学 (ADEOS-II Mission and Oceanography/Marine Meteorology) 川村宏 ( 東北大学大気海洋変動観測研究センター教授 ) Hiroshi Kawamura (Tohoku University, Center for Atmospheric and Oceanic Studies, Professor)
ADEOS-II ミッションと海洋学 海洋気象学 (ADEOS-II Mission and Oceanography/Marine Meteorology) 川村宏 ( 東北大学大気海洋変動観測研究センター教授 ) Hiroshi Kawamura (Tohoku University, Center for Atmospheric and Oceanic Studies, Professor)
新技術説明会 ラマン分光 必見! AFM- ラマンによるナノイメージの世界 株式会社堀場製作所
 新技術説明会 ラマン分光 必見! AFM- ラマンによるナノイメージの世界 株式会社堀場製作所 2013 HORIBA Scientific. All rights reserved. Ultra fast simultaneous AFM and hyper-spectral imaging: chemical and physical investigation of nano-materials
新技術説明会 ラマン分光 必見! AFM- ラマンによるナノイメージの世界 株式会社堀場製作所 2013 HORIBA Scientific. All rights reserved. Ultra fast simultaneous AFM and hyper-spectral imaging: chemical and physical investigation of nano-materials
Hetty Triastuty, Masato IGUCHI, Takeshi TAMEGURI, Tomoya Yamazaki. Sakurajima Volcano Research Center, DPRI, Kyoto University
 Hypocenters, Spectral Analysis and Source Mechanism of Volcanic Earthquakes at Kuchinoerabujima: High-frequency, Low-frequency and Monochromatic Events Hetty Triastuty, Masato IGUCHI, Takeshi TAMEGURI,
Hypocenters, Spectral Analysis and Source Mechanism of Volcanic Earthquakes at Kuchinoerabujima: High-frequency, Low-frequency and Monochromatic Events Hetty Triastuty, Masato IGUCHI, Takeshi TAMEGURI,
