CHM 233 : Fall 2018 Quiz #12 - Answer Key Question 1 MCalkenesIn Which are the best reagents/conditions for the following reaction?
|
|
|
- Brandon James
- 5 years ago
- Views:
Transcription
1 M 233 : Fall 2018 Quiz #12 - nswer Key Question 1 MalkenesIn Which are the best reagents/conditions for the following reaction? 2 / 2 S 4 (cat.) conc. 2 S 4 /heat 1. 3.TF 2. Na/ g(c) 2 / 2 2. Na 4 2 / 2 S 4 (cat.) Markovnikov-like addition of 2 across the = bond WIT rearrangement, requires a carbocation intermediate and this a RNSTE acid catalyst
2 Question 2 M26n Which is MST LIKELY to be the major product of the following reaction???? 3 Me Me Me Me L/ /L = Me Me L/ /L Me L Me L
3 Question 3 MalkenesIh Which is the expected major organic product of the following reaction 2??? Et (solvent) Et 2 is a Lewis acid that is strong enough to react with a = bond, Et is not, thus: the Et MUST add from the Et "top" Et Et Et assume the 2 adds to the "bottom of the = bond (if it added to the top a stereoisomer would result that is not important in the present context) Et adds to the bromonium and not because there are many more Et molecules than
4 Question 4 MalkenesIp Which are the best reagents/conditions to perform the following reaction (±) 2 / 2 S 4 (cat.) conc. 2 S 4 /heat 1. 3.TF 2. Na/ g(c) 2 / 2 2. Na TF 2. Na/ 2 2 (±) NTI-Markovnikov addition of 2 across the = bond WITUT rearrangement Requires hydroboration
5 Question 5 M26s Which are the reagents and conditions that are most likely to give the products of the following reactions? 1) 1. g(c) 2 / 2 2. Na 4 2) 3) 2 2 S 4 (cat.)/heat TF / Reaction 1) 2 / 2 S 4 (cat.)/heat Reaction 2) 1. g(c) 2 / 2, 2. Na 4 Reaction 3) TF, / Reaction 1) TF, / Reaction 2) 1. g(c) 2 / 2, 2. Na 4 Reaction 3) 2 / 2 S 4 (cat.)/heat Reaction 1) 1. g(c) 2 / 2, 2. Na 4 Reaction 2) 2 / 2 S 4 (cat.)/heat Reaction 3) TF, / Reaction 1) TF, / Reaction 2) 2 / 2 S 4 (cat.)/heat Reaction 3) 1. g(c) 2 / 2, 2. Na 4 2 / 2 S 4 (cat.)/heat results in Markovnikov-type addition of 2, but involves a carbocation intermediate that will rearrange if possible, as in these reactions 1. g(c) 2 / 2, 2. Na 4 results in Markovnikov addition of 2 across the = bond, but avoids a carbocation intermediate, rearrangements do not occur, this is the preferred method for Markovnikov water addition, do NT use aqueous acid in a synthesis problem TF, / results in NTI-Markovnikov addition of 2 across the = bond, avoids a carbocation intermediate, rearrangements do not occur
6 Question 6 MalkenesIu Give the best reagents/conditions to perform the following reaction??? 2 / 2 S 4 (cat.) conc. 2 S 4 /heat 1. 3.TF 2. Na/ g(c) 2 / 2 2. Na 4 1. g(c) 2 / 2 2. Na 4 Markovnikov addition WITUT RERRNGEMENT must be oxymercuration
7 Question 7 M20q Using the homolytic bond dissociation energies provided, which statement best describes the following reaction? + +..E. (kcal/mol) The reaction is exothermic by 41 kcal/mol The reaction is exothermic by 5 kcal/mol The reaction is endothermic by 5 kcal/mol The reaction is endothermic by 41 kcal/mol Even though the reaction involves heterolytic bond cleavage in Lewis acid/base reactions, we are only comparing the reactants and products The bond dissocaition energies measure the energies of the electrons in bonds, and reactions involve breaking bonds and making new ones ll we need to do is to compare the energy cost of breaking the bonds that are broken to the energy gain of the bonds that are made Energy "cost" due to breaking bonds = 86 (-) + 88 (-) Total = 174 kcal/mol Energy "gain" due to making bonds = 68 (-) (-) Total = 179 kcal/mol Electron energy in products lower than in reactants (the bonds are stronger in the products than in the reactants, thus the reaction is EXTERMI by = 5 kcal/mol
8 Question 8 MalkenesIs nly one of the following reactions actually gives the provide product, the other reactions actually give different products. Identify the correct reaction. (ignore stereochemistry in this problem) 4-ethyl-3-heptene 1. 3.TF 2. Na/ ethyl-2-heptene 1. 2 /g(c) 2 2. Na 4 3-ethyl-3-heptene 1. 3.TF 2. Na/ ethyl-3-heptene 1. 2 /g(c) 2 2. Na 4 4-ethyl-3-heptene 1. 3.TF 2. Na/ ethyl-2-heptene 1. 2 /g(c) 2 2. Na 4 3-ethyl-3-heptene 1. 2 /g(c) 2 2. Na 4
CHM 233 : Fall 2017 Quiz #11 - Answer Key Question 1
 CM 233 : Fall 2017 Quiz #11 - Answer Key Question 1 MC26k Which best describes the stereochemistry of the C=C double bonds 1 and 2 in the following structure? 1 2 Br A) 1 = E, 2 = E B) 1 = E, 2 = Z Br
CM 233 : Fall 2017 Quiz #11 - Answer Key Question 1 MC26k Which best describes the stereochemistry of the C=C double bonds 1 and 2 in the following structure? 1 2 Br A) 1 = E, 2 = E B) 1 = E, 2 = Z Br
C C. sp 2. π M.O. atomic. orbitals. carbon 1. σ M.O. molecular. orbitals. H C C rotate D. D H zero overlap of p orbitals: π bond broken!
 Alkenes Electrophilic Addition 1 Alkene Structures chemistry of double bond σ BDE ~ 80 kcal/mol π = BDE ~ 65 kcal/mol The p-bond is weaker than the sigma-bond The, electrons in the p-bond are higher in
Alkenes Electrophilic Addition 1 Alkene Structures chemistry of double bond σ BDE ~ 80 kcal/mol π = BDE ~ 65 kcal/mol The p-bond is weaker than the sigma-bond The, electrons in the p-bond are higher in
ORGANIC - CLUTCH CH ADDITION REACTIONS.
 !! www.clutchprep.com CONCEPT: GENERAL MECHANISM Addition reactions are ones in which 1 bond is broken and 2 new bonds are formed. They are the inverse of reactions EXAMPLE: Provide the mechanism for the
!! www.clutchprep.com CONCEPT: GENERAL MECHANISM Addition reactions are ones in which 1 bond is broken and 2 new bonds are formed. They are the inverse of reactions EXAMPLE: Provide the mechanism for the
Chemistry 231 Organic I Exam 2 Dr. Gallo (Brown & Foote) October 29, 2004
 hemistry 231 rganic I Exam 2 Dr. Gallo (Brown & Foote) ctober 29, 2004 1 Name: I(24). ircle the correct answer for each of the following multiple choice questions. 1.) Which alkene would yield 3-methylpentane
hemistry 231 rganic I Exam 2 Dr. Gallo (Brown & Foote) ctober 29, 2004 1 Name: I(24). ircle the correct answer for each of the following multiple choice questions. 1.) Which alkene would yield 3-methylpentane
LECTURE #14 Thurs., Oct.20, Midterm exam: Tues.Oct.25 during class Ch.1, , 7.10, 2, Sections
 CHEM 221 section 01 LECTURE #14 Thurs., Oct.20, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSIGNED READINGS: TODAY S CLASS: NEXT LECTURE: Sections 4.7-4.10 finish Ch.4,
CHEM 221 section 01 LECTURE #14 Thurs., Oct.20, 2005 Midterm exam: Tues.Oct.25 during class Ch.1, 7.2-7.5, 7.10, 2, 3.1-3.5 ASSIGNED READINGS: TODAY S CLASS: NEXT LECTURE: Sections 4.7-4.10 finish Ch.4,
C C. sp 2. π M.O. atomic. orbitals. carbon 1. σ M.O. molecular. orbitals. H C C rotate D. D H zero overlap of p orbitals: π bond broken!
 Alkenes Electrophilic Addition 1 Alkene Structures chemistry of double bond σ BE ~ 80 kcal/mol π = BE ~ 65 kcal/mol The p-bond is weaker than the sigma-bond The, electrons in the p-bond are higher in energy
Alkenes Electrophilic Addition 1 Alkene Structures chemistry of double bond σ BE ~ 80 kcal/mol π = BE ~ 65 kcal/mol The p-bond is weaker than the sigma-bond The, electrons in the p-bond are higher in energy
ORGANIC - BROWN 8E CH ALKENES AND REACTIONS OF ALKENES
 !! www.clutchprep.com CONCEPT: ALKENES and ALKYNES Alkenes/Alkynes are named by adding the suffix modifier (- /- ) to the end of the root. Alkenes/alkynes receive in numbering alkanes Location is assigned
!! www.clutchprep.com CONCEPT: ALKENES and ALKYNES Alkenes/Alkynes are named by adding the suffix modifier (- /- ) to the end of the root. Alkenes/alkynes receive in numbering alkanes Location is assigned
REACTION AND SYNTHESIS REVIEW
 REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
REACTION AND SYNTHESIS REVIEW A STUDENT SHOULD BE ABLE TO PREDICT PRODUCTS, IDENTIFY REACTANTS, GIVE REACTION CONDITIONS, PROPOSE SYNTHESES, AND PROPOSE MECHANISMS (AS LISTED BELOW). REVIEW THE MECHANISM
**YOU ARE NOT ALLOWED TO TAKE SPARE COPIES OF THIS EXAM FROM THE TESTING ROOM**
 EM 233, Fall 2017 Midterm #3 Ian R. Gould MPLETE TIS SETIN : Up to TW PINTS will be removed for incorrect/missing information! PRINTED FIRST NME answer key PRINTED LST NME Person on your LEFT (or Empty
EM 233, Fall 2017 Midterm #3 Ian R. Gould MPLETE TIS SETIN : Up to TW PINTS will be removed for incorrect/missing information! PRINTED FIRST NME answer key PRINTED LST NME Person on your LEFT (or Empty
1. Addition of HBr to alkenes
 eactions of Alkenes I eading: Wade chapter 8, sections 8-1- 8-8 tudy Problems: 8-47, 8-48, 8-55, 8-66, 8-67, 8-70 Key Concepts and kills: Predict the products of additions to alkenes, including regiochemistry
eactions of Alkenes I eading: Wade chapter 8, sections 8-1- 8-8 tudy Problems: 8-47, 8-48, 8-55, 8-66, 8-67, 8-70 Key Concepts and kills: Predict the products of additions to alkenes, including regiochemistry
Preparation of alkenes
 Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
Lecture 11 אלקנים הכנה ותגובות של אלקנים: הידרוגנציה, סיפוח הידרוהלוגנים )כלל מארקובניקוב(, סיפוח הלוגנים והסטראוכימיה של תוצרי הסיפוח, הידרובורציה, אפוקסידציה, אוזונוליזה. 1 Preparation of alkenes 1.
2. Hydrohalogenation: Propylene reacts with HBr to form 2-bromopropane.
 Objective 12. Apply reactivity principles to Electrophilic Addition reactions 1: alkenes identify structural features (pi bond) and electrophiles, use curved arrows to predict product. Structural features:
Objective 12. Apply reactivity principles to Electrophilic Addition reactions 1: alkenes identify structural features (pi bond) and electrophiles, use curved arrows to predict product. Structural features:
Chemistry 210 Organic Chemistry I Winter Semester 2001 Dr. Rainer Glaser
 hemistry 210 rganic hemistry I Winter Semester 2001 Dr. Rainer Glaser Examination #3 Alkenes and Alkynes. Structure, Synthesis and Reactions. Friday, April 20, 2001, 9:00-9:50 Name: Answer Key Question
hemistry 210 rganic hemistry I Winter Semester 2001 Dr. Rainer Glaser Examination #3 Alkenes and Alkynes. Structure, Synthesis and Reactions. Friday, April 20, 2001, 9:00-9:50 Name: Answer Key Question
1. Use appropriate curved arrows to indicate the complete mechanism of each of these reactions. KH (1 equiv.) + KCl THF. + HBr.
 1. Use appropriate curved arrows to indicate the complete mechanism of each of these reactions. K (1 equiv.) TF K 3 2 2 3 enantiomer While writing the mechanism, justify both the regiochemistry the relative
1. Use appropriate curved arrows to indicate the complete mechanism of each of these reactions. K (1 equiv.) TF K 3 2 2 3 enantiomer While writing the mechanism, justify both the regiochemistry the relative
An Overview of Organic Reactions. Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants:
 An Overview of Organic Reactions Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants: 1. Addition (forward) Gain of atoms across a bond Example:
An Overview of Organic Reactions Reaction types: Classification by outcome Most reactions produce changes in the functional group of the reactants: 1. Addition (forward) Gain of atoms across a bond Example:
Chapter 6 Chemical Reactivity and Mechanisms
 Chapter 6 Chemical Reactivity and Mechanisms 6.1 Enthalpy Enthalpy (ΔH or q) is the heat energy exchange between the reaction and its surroundings at constant pressure Breaking a bond requires the system
Chapter 6 Chemical Reactivity and Mechanisms 6.1 Enthalpy Enthalpy (ΔH or q) is the heat energy exchange between the reaction and its surroundings at constant pressure Breaking a bond requires the system
Homework Problem Set 8 Iverson CH320M/328M Due Friday, Nov. 9
 omework Problem Set 8 Iverson 320M/328M Due Friday, Nov. 9 NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson 8th omework November 2, 2018 Please print the first three letters of your last name
omework Problem Set 8 Iverson 320M/328M Due Friday, Nov. 9 NAME (Print): SIGNATURE: hemistry 320M/328M Dr. ent Iverson 8th omework November 2, 2018 Please print the first three letters of your last name
Learning Guide for Chapter 10 - Alkyl Halides II
 Learning Guide for Chapter 10 - Alkyl Halides II I. Elimination Reactions of Alkyl Halides Introduction Mechanisms Beta hydrogens, constitutional isomers, and stereoisomers E2 vs E1 Strong and Weak Bases
Learning Guide for Chapter 10 - Alkyl Halides II I. Elimination Reactions of Alkyl Halides Introduction Mechanisms Beta hydrogens, constitutional isomers, and stereoisomers E2 vs E1 Strong and Weak Bases
The carbon-carbon double bond is the distinguishing feature of alkenes.
 Alkenes: Structure & Properties Alkane (acyclic): n 2n+2 > saturated. Alkene (acyclic): n 2n > unsaturated. eg ethylene (IUPA: ethene), 2 4 : 2 = 2 The carbon-carbon double bond is the distinguishing feature
Alkenes: Structure & Properties Alkane (acyclic): n 2n+2 > saturated. Alkene (acyclic): n 2n > unsaturated. eg ethylene (IUPA: ethene), 2 4 : 2 = 2 The carbon-carbon double bond is the distinguishing feature
p Bonds as Nucleophiles
 Chapter 8 p Bonds as Nucleophiles REACTIONS OF ALKENES, ALKYNES, DIENES, AND ENOLS Copyright 2018 by Nelson Education Limited 1 8.2.1 Orbital structure of alkenes Geometry: Electrostatic potential: Electron-rich
Chapter 8 p Bonds as Nucleophiles REACTIONS OF ALKENES, ALKYNES, DIENES, AND ENOLS Copyright 2018 by Nelson Education Limited 1 8.2.1 Orbital structure of alkenes Geometry: Electrostatic potential: Electron-rich
CONCERTED sp 2 H. HO Et
 Alkyl alides Substitution and Elimination 1 Substitutions (Quick Review) 1.1 SN2 Reactions LB nucleophile "backside attack!" NERTED reaction This is fundamentally just a Lewis acid/base reaction, the Lewis
Alkyl alides Substitution and Elimination 1 Substitutions (Quick Review) 1.1 SN2 Reactions LB nucleophile "backside attack!" NERTED reaction This is fundamentally just a Lewis acid/base reaction, the Lewis
Organic Chemistry I Lesson Objectives, Lesson Problems, Course Outline Spring 2008
 Organic Chemistry I Lesson Objectives, Lesson Problems, Course Outline Spring 2008 Lesson Date Assignment Lesson Objective Description Lesson Problems 4 14-Jan Chapter 1 Quiz Describe how bond polarity
Organic Chemistry I Lesson Objectives, Lesson Problems, Course Outline Spring 2008 Lesson Date Assignment Lesson Objective Description Lesson Problems 4 14-Jan Chapter 1 Quiz Describe how bond polarity
Detailed Course Content
 Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Detailed Course Content Chapter 1: Carbon Compounds and Chemical Bonds The Structural Theory of Organic Chemistry 4 Chemical Bonds: The Octet Rule 6 Lewis Structures 8 Formal Charge 11 Resonance 14 Quantum
Chapter 8 Alkenes and Alkynes II: Addition Reactions "
 Chapter 8 Alkenes and Alkynes II: Addition Reactions Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The π
Chapter 8 Alkenes and Alkynes II: Addition Reactions Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The π
6.10 Acid-Catalyzed Hydration of Alkenes. Acid-Catalyzed Hydration of Alkenes
 Acid-atalyzed ydration of Alkenes 6.10 Acid-atalyzed ydration of Alkenes O O reaction is acid catalyzed; typical hydration medium is 50% 2 SO 4-50% 2 O Follows Markovnikov's Rule Follows Markovnikov's
Acid-atalyzed ydration of Alkenes 6.10 Acid-atalyzed ydration of Alkenes O O reaction is acid catalyzed; typical hydration medium is 50% 2 SO 4-50% 2 O Follows Markovnikov's Rule Follows Markovnikov's
Chapter 8 Alkenes and Alkynes II: Addition Reactions. Alkenes are electron rich. Additions to Alkenes
 Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
Additions to Alkenes Chapter 8 Alkenes and Alkynes II: Addition Reactions Generally the reaction is exothermic because one p and one s bond are converted to two s bonds Alkenes are electron rich The carbocation
4.15 Halogenation of Alkanes RH + X 2 RX + HX
 4.15 alogenation of Alkanes R + X 2 RX + X Energetics R + X 2 RX + X explosive for F 2 exothermic for Cl 2 and Br 2 endothermic for I 2 4.16 Chlorination of Methane Chlorination of Methane carried out
4.15 alogenation of Alkanes R + X 2 RX + X Energetics R + X 2 RX + X explosive for F 2 exothermic for Cl 2 and Br 2 endothermic for I 2 4.16 Chlorination of Methane Chlorination of Methane carried out
3. (6 pts) Provide an acceptable name for each of the following molecules: OH
 CEMISTRY 313-61 MIDTERM # 2 answer key June 07, 2005 Statistics: Average: 71 pts (71%); ighest: 99 pts (99%); Lowest: 45 pts (45%) Number of students performing at or above average: 15 (48%) 1. (11 pts)
CEMISTRY 313-61 MIDTERM # 2 answer key June 07, 2005 Statistics: Average: 71 pts (71%); ighest: 99 pts (99%); Lowest: 45 pts (45%) Number of students performing at or above average: 15 (48%) 1. (11 pts)
Name: Chapter 3: The Nature Of Organic Reactions: Alkenes
 Name: Chapter 3: The Nature Of Organic Reactions: Alkenes 1 Vocabulary cis-trans isomerism: E,Z designation: Addition: Elimination: Substitution: Rearrangement: Homolytic: Heterolytic Homogenic: Heterogenic:
Name: Chapter 3: The Nature Of Organic Reactions: Alkenes 1 Vocabulary cis-trans isomerism: E,Z designation: Addition: Elimination: Substitution: Rearrangement: Homolytic: Heterolytic Homogenic: Heterogenic:
CHEMISTRY Topic #4: Electrophilic Addition Reactions of Alkenes and Alkynes Fall 2018 Dr. Susan Findlay
 EMISTRY 2600 Topic #4: Electrophilic Addition Reactions of Alkenes and Alkynes Fall 2018 Dr. Susan Findlay rganic Reactions (EM 2500 Review) Most reactions in organic chemistry fall into one (or more)
EMISTRY 2600 Topic #4: Electrophilic Addition Reactions of Alkenes and Alkynes Fall 2018 Dr. Susan Findlay rganic Reactions (EM 2500 Review) Most reactions in organic chemistry fall into one (or more)
CHEM 263 Oct 25, stronger base stronger acid weaker acid weaker base
 CEM 263 ct 25, 2016 Reactions and Synthesis (Preparation) of R- Breaking the - Bond of R- with Metals R + Li 0 or Na 0 or K 0 metal R Li + 1/2 2 alkoxide Breaking the - Bond of R- by Acid-Base Reaction
CEM 263 ct 25, 2016 Reactions and Synthesis (Preparation) of R- Breaking the - Bond of R- with Metals R + Li 0 or Na 0 or K 0 metal R Li + 1/2 2 alkoxide Breaking the - Bond of R- by Acid-Base Reaction
ORGANIC - BROWN 8E CH.8 - HALOALKANES, HALOGENATION AND RADICALS
 !! www.clutchprep.com CONCEPT: ALKYL HALIDES Alkyl halides are named by naming them as a substituent before the root chain and indicating their location. Prefixes: -F, -Cl -Br -I Alkyl halides have NO
!! www.clutchprep.com CONCEPT: ALKYL HALIDES Alkyl halides are named by naming them as a substituent before the root chain and indicating their location. Prefixes: -F, -Cl -Br -I Alkyl halides have NO
HONORS ORGANIC CHEM. HAHS MRS. RICHARDS
 NRS RGANIC CEM. AS MRS. RICARDS RGANIC CEMISTRY: FINAL EXAM REVIEW List of Topics: While the exam will specifically focus on material from Quarter 2, an understanding of several important concepts from
NRS RGANIC CEM. AS MRS. RICARDS RGANIC CEMISTRY: FINAL EXAM REVIEW List of Topics: While the exam will specifically focus on material from Quarter 2, an understanding of several important concepts from
Chapter 8 Alkenes and Alkynes II: Addition Reactions. Alkenes are electron rich. Additions to Alkenes
 Additions to Alkenes hapter 8 Alkenes and Alkynes II: Addition eactions Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The carbocation
Additions to Alkenes hapter 8 Alkenes and Alkynes II: Addition eactions Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds Alkenes are electron rich The carbocation
QUESTION 1 MC29f Give the product of the following reaction sequence with the starting material shown
 M 234, Spring 2018 QUIZ #5 NSWER KEY (hit the RETURN utton to return to the Main Quiz Page) QUESTIN 1 M29f Give the product of the following reaction sequence with the starting material shown 3 3 1. Excess
M 234, Spring 2018 QUIZ #5 NSWER KEY (hit the RETURN utton to return to the Main Quiz Page) QUESTIN 1 M29f Give the product of the following reaction sequence with the starting material shown 3 3 1. Excess
What is the major product of the following reaction?
 What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
What is the major product of the following reaction? Predict the major product of the following reaction: 2-methylbutane + Br 2 /light energy? A) 1-bromo-2-methylbutane B) 2-bromo-2-methylbutane C) 2-bromo-3-methylbutane
Classification of Reactions by:
 lassification of Reactions by: 1) Functional group 2) Kind a) Addition: A + B > b) Elimination: A > B + c) Substitution: A-B + -D > A- + B-D d) Rearrangement: A > B, where B is a constitutional isomer
lassification of Reactions by: 1) Functional group 2) Kind a) Addition: A + B > b) Elimination: A > B + c) Substitution: A-B + -D > A- + B-D d) Rearrangement: A > B, where B is a constitutional isomer
Chapter 8 - Alkenes and Alkynes II Addition Reactions of Alkenes - Electrons in the π bond of alkenes react with electrophiles
 Andrew Rosen Chapter 8 - Alkenes and Alkynes II 8.1 - Addition Reactions of Alkenes - Electrons in the π bond of alkenes react with electrophiles 8.2 - Electrophilic Addition of Hydrogen Halides to Alkenes:
Andrew Rosen Chapter 8 - Alkenes and Alkynes II 8.1 - Addition Reactions of Alkenes - Electrons in the π bond of alkenes react with electrophiles 8.2 - Electrophilic Addition of Hydrogen Halides to Alkenes:
Chapter 8 Alkenes and Alkynes II: Addition Reactions
 Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
Chapter 8 Alkenes and Alkynes II: Addition Reactions Introduction: Additions to Alkenes Generally the reaction is exothermic because one π and one σ bond are converted to two σ bonds The π electrons of
UCF - ORGANIC CHEMISTRY 1 - PROF. DAOUDI UCF PROF. DAOUDI EXAM 3 REVIEW.
 UCF PROF. DAOUDI EXAM 3 REVIEW www.clutchprep.com 1 PRACTICE: Identify the most stable and the least stable alkene PRACTICE: Create the full arrow pushing mechanism which shows all intermediates and all
UCF PROF. DAOUDI EXAM 3 REVIEW www.clutchprep.com 1 PRACTICE: Identify the most stable and the least stable alkene PRACTICE: Create the full arrow pushing mechanism which shows all intermediates and all
use iso-, tert-, sec- prefixes in your substituent names, where necessary
 Chapter 07.2: 1 1) Name. Include E/Z stereochemistry in name, if necessary. a) 3 C b) c) d) e) f) use iso-, tert-, sec- prefixes in your substituent names, where necessary g) Do NT use iso-, tert-, sec-
Chapter 07.2: 1 1) Name. Include E/Z stereochemistry in name, if necessary. a) 3 C b) c) d) e) f) use iso-, tert-, sec- prefixes in your substituent names, where necessary g) Do NT use iso-, tert-, sec-
Chapter 7: Alkenes: Reactions and Synthesis
 hapter 7: Alkenes: Reactions and Synthesis alcohol alkane halohydrin 1,2-diol 1,2-dihalide carbonyl halide halide Addition Y Y Elimination Electrophilic Addition Dehydrohalogenation: loss of from an alkyl
hapter 7: Alkenes: Reactions and Synthesis alcohol alkane halohydrin 1,2-diol 1,2-dihalide carbonyl halide halide Addition Y Y Elimination Electrophilic Addition Dehydrohalogenation: loss of from an alkyl
Chapter 7: Alkene reactions conversion to new functional groups
 hapter 7: Alkene reactions conversion to new functional groups Preparation of alkenes: two common elimination reactions 1. Dehydration of alcohols Dehydration is a common biochemical reaction in carbohydrate
hapter 7: Alkene reactions conversion to new functional groups Preparation of alkenes: two common elimination reactions 1. Dehydration of alcohols Dehydration is a common biochemical reaction in carbohydrate
A) I B) II C) III D) IV E) V
 Exam Name SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. 1) Complete the following reaction and provide a detailed, step-by-step mechanism for the process.
Exam Name SHORT ANSWER. Write the word or phrase that best completes each statement or answers the question. 1) Complete the following reaction and provide a detailed, step-by-step mechanism for the process.
Chapter 19: Alkenes and Alkynes
 Chapter 19: Alkenes and Alkynes The vast majority of chemical compounds that we know anything about and that we synthesize in the lab or the industrial plant are organic compounds. The simplest organic
Chapter 19: Alkenes and Alkynes The vast majority of chemical compounds that we know anything about and that we synthesize in the lab or the industrial plant are organic compounds. The simplest organic
Chapter 8. Alkenes and Alkynes II: Addition Reactions. Ch. 8-1
 hapter 8 Alkenes and Alkynes II: Addition Reactions h. 8-1 1. Addition Reactions of Alkenes E + E Nu Nu h. 8-2 1A. ow To Understand Additions to Alkenes This is an addition reaction: E Nu added across
hapter 8 Alkenes and Alkynes II: Addition Reactions h. 8-1 1. Addition Reactions of Alkenes E + E Nu Nu h. 8-2 1A. ow To Understand Additions to Alkenes This is an addition reaction: E Nu added across
JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I. 5 Credit Hours. Prepared by: Richard A. Pierce
 JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I 5 Credit Hours Prepared by: Richard A. Pierce Revised Date: January 2008 by Ryan H. Groeneman Arts & Science Education Dr. Mindy Selsor, Dean
JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I 5 Credit Hours Prepared by: Richard A. Pierce Revised Date: January 2008 by Ryan H. Groeneman Arts & Science Education Dr. Mindy Selsor, Dean
Alkenes (Olefins) Chapters 7 & 8 Organic Chemistry, 8 th Edition John McMurry
 Alkenes (Olefins) Chapters 7 & 8 Organic Chemistry, 8 th Edition John McMurry 1 Structure and Bonding 2 Structure and Bonding Rotation around the C=C bond is restricted 90 rotation The p orbitals are orthogonal
Alkenes (Olefins) Chapters 7 & 8 Organic Chemistry, 8 th Edition John McMurry 1 Structure and Bonding 2 Structure and Bonding Rotation around the C=C bond is restricted 90 rotation The p orbitals are orthogonal
CHEM 2311 HW1. COMPLETELY FILL THE BOXES IN DARK PENCIL, i.e.:, NOT or or STRUCTURE
 EM 2311 W1 Provide answers to the following multiple choice questions on the Scantron form provided in lecture. Make sure that you put your name and bubble in your GTID # on the answer form. MPLETELY FILL
EM 2311 W1 Provide answers to the following multiple choice questions on the Scantron form provided in lecture. Make sure that you put your name and bubble in your GTID # on the answer form. MPLETELY FILL
CHAPTER 9. ALKYNES: AN INTRODUCTION TO ORGANIC SYNTHESIS
 CAPTER 9. ALKYNES: AN INTRDUCTIN T RGANIC SYNTESIS Alkyne Nomenclature. Like alkenes, number so the alkyne gets the lowest number. Name the following two molecules: 3 C C CC 2 C 2 C(C 3 ) 2 Cl If, however,
CAPTER 9. ALKYNES: AN INTRDUCTIN T RGANIC SYNTESIS Alkyne Nomenclature. Like alkenes, number so the alkyne gets the lowest number. Name the following two molecules: 3 C C CC 2 C 2 C(C 3 ) 2 Cl If, however,
Organic Chemistry, Second Edition. Janice Gorzynski Smith University of Hawai i. Chapter 10 Alkenes
 Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Alkenes Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
Organic Chemistry, Second Edition Janice Gorzynski Smith University of Hawai i Chapter 10 Alkenes Prepared by Rabi Ann Musah State University of New York at Albany Copyright The McGraw-Hill Companies,
CH 3 Cl + Cl 2 CH 2 Cl 2 + HCl
 Energetics 414 alogenation of Alkanes X 2 X X X 2 X X explosive for F 2 exothermic for l 2 and Br 2 endothermic for I 2 hlorination of Methane carried out at high temperature (400 ) 415 hlorination of
Energetics 414 alogenation of Alkanes X 2 X X X 2 X X explosive for F 2 exothermic for l 2 and Br 2 endothermic for I 2 hlorination of Methane carried out at high temperature (400 ) 415 hlorination of
More Tutorial at
 1. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question (50 pts). 1) Which of the following statements about propene, CH3CH CH2, is 1) correct? A) There
1. MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question (50 pts). 1) Which of the following statements about propene, CH3CH CH2, is 1) correct? A) There
Alkenes are prepared by the reverse of the electrophilic reactions in the last chapter. That is, they are prepared by elimination reactions.
 Ch 8 Alkene Reactions and Syntheses Alkenes are prepared by the reverse of the electrophilic reactions in the last chapter. That is, they are prepared by elimination reactions. Dehydrohalogenation - Mechanism
Ch 8 Alkene Reactions and Syntheses Alkenes are prepared by the reverse of the electrophilic reactions in the last chapter. That is, they are prepared by elimination reactions. Dehydrohalogenation - Mechanism
Chapter 8 Alkenes: Reactions
 Chamras Glendale Community College Organic Chemistry 105 Chapter 8 Alkenes: Reactions Name Type 1. alo-hydrogenation (Ionic & Radical) Electrophilic Addition 2. ydration (Acid-Catalyzed) Electrophilic
Chamras Glendale Community College Organic Chemistry 105 Chapter 8 Alkenes: Reactions Name Type 1. alo-hydrogenation (Ionic & Radical) Electrophilic Addition 2. ydration (Acid-Catalyzed) Electrophilic
+ HBr!H = OH CH CH 2. HgOAc
 radical substitution Cl hv + Cl 2 + Cl! = Energy radical addition RR + electrophilic addition + C3 C3! = Energy Energy + 2 acetone water + 2 2 +! =! = Energy Energy acid catalyzed hydration + 2 +! = Energy
radical substitution Cl hv + Cl 2 + Cl! = Energy radical addition RR + electrophilic addition + C3 C3! = Energy Energy + 2 acetone water + 2 2 +! =! = Energy Energy acid catalyzed hydration + 2 +! = Energy
Homework - Review of Chem 2310
 omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
omework - Review of Chem 2310 Chapter 1 - Atoms and Molecules Name 1. What is organic chemistry? 2. Why is there an entire one year course devoted to the study of organic compounds? 3. Give 4 examples
Practice Multiple-Choice Questions for Ending Chem 21 and/or Starting Chem 22
 Practice Multiple-Choice Questions for Ending Chem 21 and/or Starting Chem 22 Includes information from chapters 5-10 of the third edition of Klein s Organic Chemistry text. 1. Which of the following compounds
Practice Multiple-Choice Questions for Ending Chem 21 and/or Starting Chem 22 Includes information from chapters 5-10 of the third edition of Klein s Organic Chemistry text. 1. Which of the following compounds
UNIT #10: Reaction Rates Heat/Energy in Chemical Reactions Le Chatlier s Principle Potential Energy Diagrams
 UNIT #10: Reaction Rates Heat/Energy in Chemical Reactions Le Chatlier s Principle Potential Energy Diagrams NAME: 1. REACTION RATES a) The speed of a chemical reaction determined by the change in concentration
UNIT #10: Reaction Rates Heat/Energy in Chemical Reactions Le Chatlier s Principle Potential Energy Diagrams NAME: 1. REACTION RATES a) The speed of a chemical reaction determined by the change in concentration
ORGANIC CHEMISTRY- 1
 ORGANIC CEMISTRY- 1 ALKENES Alkenes are also called Olefins (C n 2n ) unsaturated hydrocarbons. Alkenes occur abundantly in nature. Ethylene ( 2 C=C 2 ) is a plant hormone that induces ripening in fruit.
ORGANIC CEMISTRY- 1 ALKENES Alkenes are also called Olefins (C n 2n ) unsaturated hydrocarbons. Alkenes occur abundantly in nature. Ethylene ( 2 C=C 2 ) is a plant hormone that induces ripening in fruit.
Learning Guide for Chapter 7 - Organic Reactions I
 Learning Guide for Chapter 7 - rganic Reactions I I. Introduction to Reactions II. Principles of Kinetics III. Principles of Thermodynamics IV. Nucleophiles and Electrophiles V. Acids and Bases What a
Learning Guide for Chapter 7 - rganic Reactions I I. Introduction to Reactions II. Principles of Kinetics III. Principles of Thermodynamics IV. Nucleophiles and Electrophiles V. Acids and Bases What a
Synthesis and Retrosynthesis
 Synthesis and Retrosynthesis opyright, Arizona State University Putting Reactions Together A large part of organic chemistry involves building more complex molecules from smaller ones using a designed
Synthesis and Retrosynthesis opyright, Arizona State University Putting Reactions Together A large part of organic chemistry involves building more complex molecules from smaller ones using a designed
Chapter 7 An Introduction to Chemical Reactions. An Introduction to Chemistry by Mark Bishop
 Chapter 7 An Introduction to Chemical Reactions An Introduction to Chemistry by Mark Bishop Chapter Map Chemical Reaction A chemical change or chemical reaction is a process in which one or more pure substances
Chapter 7 An Introduction to Chemical Reactions An Introduction to Chemistry by Mark Bishop Chapter Map Chemical Reaction A chemical change or chemical reaction is a process in which one or more pure substances
Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I. Examination #2 - October 18, 2004 ANSWERS
 Department of hemistry SUNY/Oneonta hem 221 - Organic hemistry I Examination #2 - October 18 2004 ANSWERS INSTRUTIONS This examination is in multiple choice format; the questions are in this Exam Booklet
Department of hemistry SUNY/Oneonta hem 221 - Organic hemistry I Examination #2 - October 18 2004 ANSWERS INSTRUTIONS This examination is in multiple choice format; the questions are in this Exam Booklet
10/26/2010. An Example of a Polar Reaction: Addition of H 2 O to Ethylene. to Ethylene
 6.5 An Example of a Polar Reaction: Addition of H 2 O to Ethylene Addition of water to ethylene Typical polar process Acid catalyzed addition reaction (Electophilic addition reaction) Polar Reaction All
6.5 An Example of a Polar Reaction: Addition of H 2 O to Ethylene Addition of water to ethylene Typical polar process Acid catalyzed addition reaction (Electophilic addition reaction) Polar Reaction All
Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I. Examination #2 - October 14, 2002
 Department of hemistry SUNY/Oneonta hem 221 - Organic hemistry Examination #2 - October 14, 2002 ANSWERS NSTRUTONS This examination is in multiple choice format; the questions are in this Exam Booklet
Department of hemistry SUNY/Oneonta hem 221 - Organic hemistry Examination #2 - October 14, 2002 ANSWERS NSTRUTONS This examination is in multiple choice format; the questions are in this Exam Booklet
DAMIETTA UNIVERSITY. Energy Diagram of One-Step Exothermic Reaction
 DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
DAMIETTA UNIVERSITY CHEM-103: BASIC ORGANIC CHEMISTRY LECTURE 5 Dr Ali El-Agamey 1 Energy Diagram of One-Step Exothermic Reaction The vertical axis in this graph represents the potential energy. The transition
Chapter 7. Alkenes: Reactions and Synthesis
 Chapter 7. Alkenes: Reactions and Synthesis 1 Synthesis of Alkenes: Elimination Reactions 1. Dehydrohalogenation of alkyl halides. loss of requires CH 2 CH 2 Cl Zaitsev s Rule: CH 2 C 2. Dehydration of
Chapter 7. Alkenes: Reactions and Synthesis 1 Synthesis of Alkenes: Elimination Reactions 1. Dehydrohalogenation of alkyl halides. loss of requires CH 2 CH 2 Cl Zaitsev s Rule: CH 2 C 2. Dehydration of
O Enolate. = Electrophile/ Lewis acid. = Electrophile/ Lewis acid O B. Enolate. = Electrophile/ Lewis acid. Enolate. O O Enolate
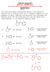 CM 234, Spring 2017 QUIZ #12 ANSWER KEY (hit the RETURN Button to return to the Main Quiz Page) QUESTIN 1 MC34r The following Aldol condensation product was formed by reaction of an enolate anion and a
CM 234, Spring 2017 QUIZ #12 ANSWER KEY (hit the RETURN Button to return to the Main Quiz Page) QUESTIN 1 MC34r The following Aldol condensation product was formed by reaction of an enolate anion and a
Chapter 4. Reactions of alkenes. Addition reactions Carbocations Selectivity of reactions
 Chapter 4 Reactions of alkenes Addition reactions Carbocations Selectivity of reactions Prob 47 p192. Give the reagents that would be required (including catalyst). Ch 4 #2 Electrophilic addition Ch 4
Chapter 4 Reactions of alkenes Addition reactions Carbocations Selectivity of reactions Prob 47 p192. Give the reagents that would be required (including catalyst). Ch 4 #2 Electrophilic addition Ch 4
Only five of the molecules below may be prepared as the sole product of allylic halogenation of the respective alkene. Circle those five.
 Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 6 Answers Question 1. nly five of the molecules below may be prepared as the sole product of allylic halogenation of the respective alkene. Circle those
Chem 232 D. J. Wardrop wardropd@uic.edu Problem Set 6 Answers Question 1. nly five of the molecules below may be prepared as the sole product of allylic halogenation of the respective alkene. Circle those
2. Which of the following is an intermediate in the Markovnikov addition of HBr to 2- methylcyclohexene?
 Quiz 7.1 Alkene Additions and Hydrohalogenations 1. Which is the most stable carbocation? 2. Which of the following is an intermediate in the Markovnikov addition of HBr to 2- methylcyclohexene? 3. What
Quiz 7.1 Alkene Additions and Hydrohalogenations 1. Which is the most stable carbocation? 2. Which of the following is an intermediate in the Markovnikov addition of HBr to 2- methylcyclohexene? 3. What
Chemistry 6A F2007. Dr. J.A. Mack 11/19/07. Chemical Kinetics measure the rate of appearance of products or the rate of disappearance of reactants.
 Chemistry 6A F2007 Dr. J.A. Mack Chemical Kinetics measure the rate of appearance of products or the rate of disappearance of reactants. Reactants Products Reactants go away with time. Products appear
Chemistry 6A F2007 Dr. J.A. Mack Chemical Kinetics measure the rate of appearance of products or the rate of disappearance of reactants. Reactants Products Reactants go away with time. Products appear
B. (2 pts) The regiochemistry of alcohol dehydration is determined by the: a. Zaitsev rule; b. Hammond postulate;
 CEMISTRY 313-01 MIDTERM # answer key ctober 1, 008 Statistics: Average: 73 pts (73%); ighest: 98 pts (98%); Lowest: 3 pts (3%) Number of students performing at or above average: 36 (58%) Number of students
CEMISTRY 313-01 MIDTERM # answer key ctober 1, 008 Statistics: Average: 73 pts (73%); ighest: 98 pts (98%); Lowest: 3 pts (3%) Number of students performing at or above average: 36 (58%) Number of students
CH2710-Exam #5 (Katz)
 C2710-Exam #5 (Katz) Name: Score: Section - Multiple Choice-Choose the BEST answer from the choices given and write the letter for that answer in the space provided. (50 pts) Consider the reaction below
C2710-Exam #5 (Katz) Name: Score: Section - Multiple Choice-Choose the BEST answer from the choices given and write the letter for that answer in the space provided. (50 pts) Consider the reaction below
Chapter 10 Radical Reactions
 Chapter 10 Radical Reactions Introduction Homolytic bond cleavage leads to the formation of radicals (also called free radicals) Radicals are highly reactive, short-lived species Single-barbed arrows are
Chapter 10 Radical Reactions Introduction Homolytic bond cleavage leads to the formation of radicals (also called free radicals) Radicals are highly reactive, short-lived species Single-barbed arrows are
Reactivity of C=C. Chapter 8 Reactions of Alkenes. Types of Additions. Electrophilic Addition. Addition of HX (1) Addition of HX (2)
 rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 8 Reactions of Alkenes Jo Blackburn Richland ollege, allas, TX allas ounty ommunity ollege istrict 2003, Prentice all Reactivity of = Electrons in pi
rganic hemistry, 5 th Edition L. G. Wade, Jr. hapter 8 Reactions of Alkenes Jo Blackburn Richland ollege, allas, TX allas ounty ommunity ollege istrict 2003, Prentice all Reactivity of = Electrons in pi
Chapter 8. Substitution reactions of Alkyl Halides
 Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
Chapter 8. Substitution reactions of Alkyl Halides There are two types of possible reaction in organic compounds in which sp 3 carbon is bonded to an electronegative atom or group (ex, halides) 1. Substitution
What are radicals? H. Cl. Chapter 10 Radical Reactions. Production of radicals. Reactions of radicals. Electronic structure of methyl radical
 What are radicals? Radicals are intermediates with an unpaired electron Chapter 10 Radical Reactions H. Cl. Hydrogen radical Chlorine radical Methyl radical Often called free radicals Formed by homolytic
What are radicals? Radicals are intermediates with an unpaired electron Chapter 10 Radical Reactions H. Cl. Hydrogen radical Chlorine radical Methyl radical Often called free radicals Formed by homolytic
Chapter 8: Alkene Structure and Preparation via Elimination Reactions
 1. Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C bond length bond strength 3 C C 3 3 C C 3 3 C C 3 3 C 2 C C 2 3 C a C=C double bond
1. Nature of the pi bond Chapter 8: Alkene Structure and Preparation via Elimination eactions [Sections: 8.1-8.13] C C bond length bond strength 3 C C 3 3 C C 3 3 C C 3 3 C 2 C C 2 3 C a C=C double bond
JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I. 5 Credit Hours. Prepared by: Richard A. Pierce. Revised by: Sean Birke October, 2013
 JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I 5 Credit Hours Prepared by: Richard A. Pierce Revised by: Sean Birke October, 2013 Ms. Linda Abernathy, Math, Science & Business Division Chair
JEFFERSON COLLEGE COURSE SYLLABUS CHM200 ORGANIC CHEMISTRY I 5 Credit Hours Prepared by: Richard A. Pierce Revised by: Sean Birke October, 2013 Ms. Linda Abernathy, Math, Science & Business Division Chair
California State Polytechnic University, Pomona Nomenclature (one structure) 25
 alifornia State Polytechnic University, Pomona 1 hem 314 Final Exam Spring, 2005 Beauchamp ame Problem Points redit 1. omenclature (one structure) 25 2. 2D Lewis structure (large structure with 20 possible
alifornia State Polytechnic University, Pomona 1 hem 314 Final Exam Spring, 2005 Beauchamp ame Problem Points redit 1. omenclature (one structure) 25 2. 2D Lewis structure (large structure with 20 possible
Chapter 3. Alkenes And Alkynes
 Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
Chapter 3 Alkenes And Alkynes Alkenes ydrocarbons containing double bonds C C double bond the functional group center of reactivity Molecular Formula of Alkene Acyclic alkene: C n 2n Cyclic alkene: C n
Chapter 10 Radical Reactions"
 Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
Chapter 10 Radical Reactions Radicals are intermediates with an unpaired electron H. Cl. Hydrogen radical t Often called free radicals What are radicals? Chlorine radical t Formed by homolytic bond cleavage
Synthesis and Retrosynthesis
 Synthesis and Retrosynthesis Putting Reactions Together A large part of organic chemistry involves building more complex molecules from smaller ones using a designed sequence of reactions, i.e. chemical
Synthesis and Retrosynthesis Putting Reactions Together A large part of organic chemistry involves building more complex molecules from smaller ones using a designed sequence of reactions, i.e. chemical
Solutions. Department of Chemistry SUNY/Oneonta. Chem Organic Chemistry I. Examination #2 - October 23, 2000
 Solutions Department of hemistry SUNY/Oneonta hem 221 - Organic hemistry I Examination #2 - October 23, 2000 INSTRUTIONS --- This examination has two parts. The first part is in multiple choice format;
Solutions Department of hemistry SUNY/Oneonta hem 221 - Organic hemistry I Examination #2 - October 23, 2000 INSTRUTIONS --- This examination has two parts. The first part is in multiple choice format;
Section 8.1 The Covalent Bond
 Section 8.1 The Covalent Bond Apply the octet rule to atoms that form covalent bonds. Describe the formation of single, double, and triple covalent bonds. Contrast sigma and pi bonds. Relate the strength
Section 8.1 The Covalent Bond Apply the octet rule to atoms that form covalent bonds. Describe the formation of single, double, and triple covalent bonds. Contrast sigma and pi bonds. Relate the strength
Midterm #3. Ian R. Gould PRINTED FIRST NAME. Extra Credit /5
 EM 233, Fall 2014 Midterm #3 Ian R. Gould PRITED FIRST ME SWER PRITED LST ME KEY SU ID or Posting ID Person on your LEFT (or isle) PRIT YUR ME E PGE! RED TE DIRETIS REFULLY! USE LK PGES S SRT PPER work
EM 233, Fall 2014 Midterm #3 Ian R. Gould PRITED FIRST ME SWER PRITED LST ME KEY SU ID or Posting ID Person on your LEFT (or isle) PRIT YUR ME E PGE! RED TE DIRETIS REFULLY! USE LK PGES S SRT PPER work
CH Organic Chemistry I (Katz) Practice Exam #3- Fall 2013
 C2710 - rganic Chemistry I (Katz) Practice Exam #3- all 2013 Name: Score: Part I - Choose the best answer and write the letter of your choice in the space provided. (32 pts) 1. f the following, which reaction
C2710 - rganic Chemistry I (Katz) Practice Exam #3- all 2013 Name: Score: Part I - Choose the best answer and write the letter of your choice in the space provided. (32 pts) 1. f the following, which reaction
Química Orgânica I. Organic Reactions
 Química Orgânica I 2008/09 w3.ualg.pt\~abrigas QOI 0809 A6 1 Organic Reactions Addition two molecules combine Elimination one molecule splits Substitution parts from two molecules exchange Rearrangement
Química Orgânica I 2008/09 w3.ualg.pt\~abrigas QOI 0809 A6 1 Organic Reactions Addition two molecules combine Elimination one molecule splits Substitution parts from two molecules exchange Rearrangement
/16 /12 /12 /16 /20 /18 /6 /100
 Chem 2300 Exam 2 ctober 19, 2001 Name: (first) (last) Page Score 2 3 4 5 6 7 8 Total score /16 /12 /12 /16 /20 /18 /6 /100 1 . Multiple choice questions (4 points each) Base on the potential energy diagram
Chem 2300 Exam 2 ctober 19, 2001 Name: (first) (last) Page Score 2 3 4 5 6 7 8 Total score /16 /12 /12 /16 /20 /18 /6 /100 1 . Multiple choice questions (4 points each) Base on the potential energy diagram
Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections
 Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections Fischer Projections are 2-dimensional representations of 3-dimensional
Once familiar with chiral centers, models, drawings and mental images NOW: Final representation of chiral centers: Fischer Projections Fischer Projections are 2-dimensional representations of 3-dimensional
Elimination Reactions. Chapter 6 1
 Elimination Reactions Chapter 6 1 E1 Mechanism Step 1: halide ion leaves, forming a carbocation. Step 2: Base abstracts H + from adjacent carbon forming the double bond. Chapter 6 2 E1 Energy Diagram E1:
Elimination Reactions Chapter 6 1 E1 Mechanism Step 1: halide ion leaves, forming a carbocation. Step 2: Base abstracts H + from adjacent carbon forming the double bond. Chapter 6 2 E1 Energy Diagram E1:
Physical Properties: Structure:
 Nomenclature: Functional group suffix = -ol Functional group prefix = hydroxy- Primary, secondary or tertiary? Alcohols are described as primary (1 o ), secondary (2 o ) or tertiary (3 o ) depending on
Nomenclature: Functional group suffix = -ol Functional group prefix = hydroxy- Primary, secondary or tertiary? Alcohols are described as primary (1 o ), secondary (2 o ) or tertiary (3 o ) depending on
3. A forward reaction has an activation energy of 50 kj and a H of 100 kj. The PE. diagram, which describes this reaction, is
 Kinetics Quiz 4 Potential Energy Diagrams 1. A catalyst increases the rate of a reaction by A. Increasing the concentration of the reactant(s) B. Decreasing the concentration of the reactant(s) C. Increasing
Kinetics Quiz 4 Potential Energy Diagrams 1. A catalyst increases the rate of a reaction by A. Increasing the concentration of the reactant(s) B. Decreasing the concentration of the reactant(s) C. Increasing
Chem Lecture 23 Page- 1 -
 Chem 230-001 Lecture 23 Page- 1 - ydroboration/ oxidation - acemic trans-2-methylcyclohexanol may be neatly synthesized from 1-methyl- 1-cyclohexene by using a reagent called borane, 3. 0.33 equivs. 3,
Chem 230-001 Lecture 23 Page- 1 - ydroboration/ oxidation - acemic trans-2-methylcyclohexanol may be neatly synthesized from 1-methyl- 1-cyclohexene by using a reagent called borane, 3. 0.33 equivs. 3,
C h a p t e r N i n e: Addition Reactions of Alkenes
 C h a p t e r N i n e: Addition Reactions of Alkenes. H C 2 H Biosynthesis of a prostaglandin from arachidonic acid: intermediate intramolecular radical addition CHM 321: Summary of Important Concepts
C h a p t e r N i n e: Addition Reactions of Alkenes. H C 2 H Biosynthesis of a prostaglandin from arachidonic acid: intermediate intramolecular radical addition CHM 321: Summary of Important Concepts
Chapter 8: Chemistry of Alkynes (C n H 2n-2 )
 hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
hapter 8: hemistry of Alkynes ( n 2n-2 ) Bonding & hybridization Both are sp-hybridized Bond angles = 180 o 1 σ + 2 π bonds Linear around lassification R R R' σ bond energy: 88 kcal/mol π bond energy:
exothermic reaction and that ΔH c will therefore be a negative value. Heat change, q = mcδt q = m(h 2
 Worked solutions hapter 5 Exercises 1 B If the temperature drops, the process must be endothermic. Δ for endothermic reactions is always positive. 2 B All exothermic reactions give out heat. While there
Worked solutions hapter 5 Exercises 1 B If the temperature drops, the process must be endothermic. Δ for endothermic reactions is always positive. 2 B All exothermic reactions give out heat. While there
Study of Chemical Reactions
 Study of Chemical Reactions Introduction to Mechanisms There are four different types of organic reactions: Additions Eliminations Substitutions Rearrangements 149 Addition Reactions Occur when 2 reactants
Study of Chemical Reactions Introduction to Mechanisms There are four different types of organic reactions: Additions Eliminations Substitutions Rearrangements 149 Addition Reactions Occur when 2 reactants
