Phase change and the regulation of trichome distribution in Arabidopsis
|
|
|
- Clifford Lambert
- 5 years ago
- Views:
Transcription
1 Development 124, (1997) Printed in Great Britain The Company of Biologists Limited 1997 DEV Phase change and the regulation of trichome distribution in Arabidopsis thaliana Abby Telfer, Krista M. Bollman and R. Scott Poethig Plant Science Institute, Department of Biology, University of Pennsylvania, Philadelphia, PA , USA SUMMARY Higher plants pass through several phases of shoot growth during which they may produce morphologically distinct vegetative structures. In Arabidopsis thaliana this phenomenon is apparent in the distribution of trichomes on the leaf surface. Leaves produced early in rosette development lack trichomes on their abaxial (lower) surface, leaves produced later have trichomes on both surfaces, and leaves in the inflorescence (bracts) may have few or no trichomes on their adaxial (upper) surface. Here we describe some of the factors that regulate this distribution pattern. We found that the timing of abaxial trichome production and the extent to which bracts lack adaxial trichomes varies in different ecotypes. The production of abaxial trichomes appears to be regulated by the age, rather than the size of the plant. This conclusion is based on the observation that mutations that affect either the rate (altered meristem programming1) or onset (paused) of leaf initiation respectively increase or decrease the number of leaves that lack abaxial trichomes, but have only a minor effect on the time at which the first leaf with abaxial trichomes is produced. The production of abaxial trichomes is coordinated with the reproductive development of the shoot as this trait is delayed by photoperiodic conditions and some mutations that delay flowering. The loss of adaxial trichomes is likely to be a consequence of floral induction, and is accelerated by terminal flower1-10, a mutation that accelerates inflorescence development. We demonstrate that gibberellins promote trichome production in Arabidopsis and present evidence indicating that abaxial trichome production is regulated by both the level of a trichome inducer and the competence of the abaxial epidermis to respond to this inducer. Key words: phase change, trichomes, paused, Arabidopsis, shoot development INTRODUCTION All higher plants undergo a process of shoot maturation during which they develop reproductive competence (see reviews by Hackett, 1985; Zimmerman et al., 1985; Poethig, 1990; Hackett and Murray, 1993; Greenwood, 1995; Lawson and Poethig, 1995). In many plants, the change in reproductive competence is preceded by changes in the anatomy, morphology and physiology of vegetative parts of the shoot, with the result that vegetative structures produced during the juvenile and adult phases may be strikingly different. Little is known about how these changes in the vegetative character of the shoot (known as vegetative phase change) are regulated, nor has the nature of the relationship between vegetative phase change and reproductive aspects of shoot maturation been defined (Longman, 1976; Schwabe, 1976; Zimmerman et al., 1985; Hackett, 1985). The present study represents the first step in our genetic and physiological analysis of vegetative phase change in Arabidopsis thaliana. In order to conduct a thorough analysis of phase change it is necessary to have unambiguous markers for the different phases in shoot development. In many plants, the most obvious marker in this respect is leaf shape (Allsopp, 1967). Arabidopsis leaves do vary in shape during shoot growth (Röbbelen, 1957; Medford et al., 1992; Martínez- Zapater et al., 1995), but the variation is gradual and it is difficult to identify factors that affect vegetative phase change simply by their effects on this trait. Another trait that distinguishes juvenile and adult leaves in some species is the density and/or distribution of trichomes (Schaffalitzky de Muckadell, 1954; Wareing and Frydman, 1976; Brand and Lineberger, 1992; Lawson and Poethig, 1995). This trait is obvious and easily quantifiable. In maize, for example, trichomes are confined to the margin of juvenile leaves, but are present on both the leaf margin and on the adaxial (upper) surface of adult leaves (Freeling and Lane, 1994; Lawson and Poethig, 1995). Trichome production in maize is a useful marker of phase change because it is correlated with the expression of many other phase-specific traits, both in wild-type plants and in mutants in which phase change is accelerated or delayed. In Arabidopsis, the total number of trichomes on the adaxial leaf surface changes with leaf position (Martínez-Zapater et al., 1995), although like leaf shape, the variation in this trait is gradual, limiting its utility as a phase marker. A better marker may be found in the changing spatial distribution of trichomes on the abaxial and adaxial leaf surfaces (Telfer and Poethig, 1994; Chien and Sussex, 1996). Here we describe variation in the expression of this trait in different ecotypes, and investigate how it is affected by factors such as gibberellins and flowering time mutations that are known to affect vegetative phase change and/or reproductive development in other species. We demonstrate that abaxial trichome development is
2 646 A. Telfer, K. M. Bollman and R. S. Poethig independent of the growth rate of the shoot. We also describe the failure of bracts to develop adaxial trichomes and discuss the relationship between this trait and inflorescence development. MATERIALS AND METHODS Genetic stocks and growth conditions Mutant seed stocks were generously provided by the following individuals: amp1-1, A. Chaudhury; amp1(cop2), A. Lehman and J. Ecker; tfl1-10, R. Meeks-Wagner; spy-4, S. Jacobsen. spy-3, ga1-3, ga4, ga5, gai, and the late flowering mutant strains were obtained from the Arabidopsis Biological Resource Center at Ohio State University (Columbus, Ohio). The paused(psd) mutation was isolated by K. Barton in our laboratory in a screen of EMS-mutagenized Landsberg erecta plants. Except where noted otherwise, seeds were sown on Metromix 200 (Scotts) in 4 inch pots (5-10 seeds per pot) and placed at 4 C for 2 days before transfer to growth chambers (Conviron) maintained at 22 C. High humidity was maintained during germination and early growth by placing pots in covered flats. The lids were removed after about 2 weeks. In each experiment, pots containing the strains to be compared were interspersed in the same flats to compensate for microclimate differences that could affect growth rate or trichome development. Light-dark regimens and the number of plants examined are noted for each experiment. Short day conditions represent cycles of 8 hours of light and 16 hours of darkness. The late flowering mutants and their Landsberg erecta control population were grown as above but without cold treatment. The requirement of ga1-3 seeds for exogenous GA for germination was circumvented by dissecting the seeds to remove the seed coats. Dry ga1-3 and control seeds were imbibed overnight on water-soaked filter paper at 4 C in the dark. The seed coats were removed by scraping the testa with dissecting needles and applying gentle pressure to pop out the embryos. Dissected embryos were transferred to soil and grown as above, but without further cold treatment. GA3 treatments Wild-type Columbia plants grown in soil under 16 hour days at 22 C were divided into developmentally staged sub-populations and treated with 10 5 mm GA 3 (Sigma) dissolved in 0.05% ethanol. Seedlings treated beginning at germination had partially emerged radicles. Plants treated beginning later in development were staged according to the number of leaves and leaf primordia visible under a dissecting microscope. 100 µl of GA 3 solution was applied to the soil at the base of the hypocotyl every 2 or 3 days. The amount of GA 3 solution applied was increased to 300 µl when six leaves and leaf primordia were visible. Control plants were treated with 0.05% ethanol from the time of germination. To measure the response of ga1-3 mutants to different levels of GA 3, seeds were imbibed in water, embryos were dissected as described above, and transferred to Petri dishes containing filter paper moistened with GA 3 solution or water. The Petri dishes were sealed with parafilm and placed in a growth chamber on 16 hour days, 22 C. After 2 days undamaged seedlings with green cotyledons were transferred to soil and grown under constant illumination at 22 C without further GA treatment. Growth rate analysis The rate of leaf initiation was determined by counting leaves and leaf primordia visible under a dissecting microscope at various times after planting (i.e. when cold-treated seed was transferred to the growth chamber). Therefore, these rates do not actually reflect when leaves are produced by the shoot meristem, but rather when they become visible in the apex. Populations to be compared consisted of plants grown from seed that had been sown, cold-treated, and transferred to the growth chamber at the same time and had germinated on the same day. The exception to this was the comparison of cop2, amp1 and their Columbia control population. These mutants uniformly germinated a day earlier than wild-type sown on the same day. amp1-1 and amp1(cop2) mutant plants often produce abnormal rosettes in which multiple leaves appear to be initiated simultaneously (Chaudhury et al., 1993). Because the aim of this analysis was to estimate when a particular leaf was initiated, we only examined individuals that produced leaves individually and formed normal rosettes. In the case of psd, leaf initiation rates were determined using plants that had at least one primordium visible by 4 days after germination and which went on to produce additional leaves individually, in a rosette (64% of the population). Histological analysis Seedlings were fixed overnight in 3% glutaraldehyde in 0.05 M phosphate buffer (ph 6.8), and postfixed for 2 hours in 2% OsO 4. They were then dehydrated in ethanol, transferred to acetone and embedded in Spurr s resin. 1-2 µm sections were stained with 0.5% methylene blue in 0.5% Na 2B 4O 7 and mounted in immersion oil. Sections were photographed with Kodak technical pan film RESULTS The distribution of trichomes on the adaxial and abaxial leaf surfaces defines two phases of vegetative development Arabidopsis thaliana produces leaves in a compact rosette during the vegetative phase of shoot development. These leaves exhibit differences in the distribution of trichomes on their abaxial leaf surfaces (Fig. 1). The first few leaves in the rosette possess trichomes on their adaxial surface, but do not develop abaxial trichomes. Leaves produced later in rosette development have both adaxial and abaxial trichomes. Abaxial trichomes usually appear first on the midrib or at the base of the leaf, and are initially few in number. The distribution of abaxial trichomes gradually becomes more extensive on successive leaves, until the entire abaxial surface is uniformly covered with trichomes. Based on this variation in trichome production, we have subdivided vegetative development into two phases: a juvenile phase marked by the production of leaves without abaxial trichomes, and an adult phase defined by the production of leaves that have trichomes on both upper and lower surfaces. Abaxial trichome production varies in different ecotypes The Columbia (Col), Landsberg erecta (Ler) and Wassilewskja (Ws) ecotypes produce abaxial trichomes at different leaf positions. When plants were grown in constant light (CL), the first abaxial trichomes were produced on leaf three in Ws, on leaf three or four in most Ler plants, and on leaf five or six in most Col plants (Fig. 2A, Table 1 section a). Thus, Ws had the fewest number of leaves without abaxial trichomes (two), and Col has the most (four or five). The first leaf with abaxial trichomes varies somewhat in different experiments (see Table 1), presumably due to slight differences in growth conditions. However, the relative numbers of leaves that lack abaxial trichomes in these three ecotypes does not change in different growth conditions, nor have trichomes been observed under the first or second leaf of these ecotypes.
3 Phase change in Arabidopsis 647 Fig. 1. Trichome distribution on developing rosette leaves. (A) Landsberg erecta photographed from above to show adaxial trichomes. Numbers refer to the positions of leaves as determined by their order of appearance. (B) Abaxial view of the same plant. Trichomes first appear on the midrib of leaf 3 and 4 (arrow), but are not produced in large numbers until leaf 7. Variation in the number of leaves lacking abaxial trichomes could reflect differences in the temporal duration of the juvenile phase in different ecotypes. It could also arise if the ecotypes have juvenile phases of the same duration but initiate leaves at different rates. To distinguish between these possibilities, the timecourse of leaf initiation in each ecotype was determined (Fig. 2B). All three ecotypes were found to initiate leaves at approximately the same rate under CL. Thus, the difference in abaxial trichome production in these ecotypes is likely to reflect differences in the temporal duration of the juvenile phase. The initiation of the first leaf with abaxial trichomes was estimated to occur 6-7 days after planting in Ws, 7-8 days after planting in Ler, and 9-10 days after planting in Col. The development of abaxial trichomes may be temporally regulated In many plants, developmental changes in morphology are thought to be associated with changes in the size of the shoot (Allsopp, 1967; Hackett, 1985). To determine if shoot size affects trichome distribution in Arabidopsis, we examined the phenotype of mutations that affect the onset or rate of leaf initiation. Plants homozygous for a new recessive mutation paused (psd) germinate normally, but initiation of the first leaves is delayed relative to wild type. Whereas enlarged leaf primordia are readily seen in longitudinal sections of the shoot meristems of germinating Ler seedlings made 3 days after planting, psd seedlings have no detectable primordia at this stage, presumably as a result of the death of cells in the central region of the shoot apical meristem (Fig. 3A,B). Most psd plants recover after several days and begin to initiate leaves. The remainder of the plants, which were not analyzed further, produce no leaves initially, or produce only a single leaf or leaflike structure, but then initiate multiple leaves simultaneously after a delay of 1-2 weeks. Longitudinal sections of the shoot meristem of 8-day old wild-type and recovered psd seedlings reveal that the layered structure of the psd meristem is relatively disorganized (Fig. 3C,D), but it appears to be otherwise normal. Despite their delayed initiation, the first leaf primoridia develop in the proper location in psd plants. The first two leaves of recovered psd plants differ from the first leaves of wild-type plants in that they have an oval shape more typical of leaves produced during the adult phase of development and may not be initiated simultaneously (Fig. 3E). Furthermore, over 70% of these plants produced abaxial trichomes on leaf one or two, whereas wild-type Ler plants produce at least two leaves without abaxial trichomes (Table 1, section b). Thus, psd plants make few, if any, leaves lacking abaxial trichomes. We determined the timecourse of leaf production in psd and wild-type plants and estimated the time of appearance of the first leaf with abaxial trichomes (Fig. 4). The two populations produced leaves with abaxial trichomes at approximately the same time, about 7 days after planting. In contrast to psd mutants, plants homozygous for altered meristem programming1 (amp1) mutant alleles initiate leaves at an accelerated rate (Chaudhury et al., 1993). Both amp1-1 and cop2 (Hou et al., 1993) which we have shown to be allelic (data not shown) cause plants to develop more than twice as many leaves without abaxial trichomes as wild-type plants Table 1. The position of the first leaf with abaxial trichomes in different ecotypes and mutants of Arabidopsis thaliana Table First leaf with section Strain Day length abaxial trichomes* n a Ws CL 3.0± Ler CL 3.7± Col CL 5.4± b Wild type (Ler) CL 3.2± psd CL 1.9± c Wild type (Col) CL 5.3± amp1(cop2) CL 12.5± amp1-1 CL 12.7± d Ws SD 5.2± Ler SD 6.0± Col SD 7.1± e Wild type (Col) CL 6.4± tfl1-10 CL 6.5± f Wild type (Ws) CL 3.0± spy-4 CL 3.0± g Wild type (Ler) CL 3.9± ga4 CL 5.7± ga5 CL 4.4± gai CL 6.1±0.5 8 h Wild type (Ler) CL 4.3± ga1-3 CL none observed 13 *Each value represents the average ± 2 s.e.m. Unless otherwise indicated, plants within each group are significantly different from each other (sections a and e) or from the wild-type control (P<0.05, Student s t-test). Not significantly different from wild type (P>0.05, Student s t-test).
4 648 A. Telfer, K. M. Bollman and R. S. Poethig A 100 Percent of plants Col Ws Ler First leaf with abaxial trichomes B Number of leaves visible Col Ler Ws Ler Ws Col Days after planting Fig. 2. Comparison of the rate of leaf initiation, and the leaf position and time at which abaxial trichomes are first produced in the Col, Ler and Ws ecotypes. (A) Frequency distributions for the position of the first leaf with abaxial trichomes. The percentage of each population (n=20-30) that first developed abaxial trichomes at each leaf position is indicated in the graph. Plants were grown in CL. (B) The rate of leaf initiation of the plants in A. The arrows indicate the average position of the first leaf with abaxial trichomes in each ecotype (see Table 1). From this point, the average time at which abaxial trichome development was initiated was estimated (as indicated by the vertical lines). Error bars indicate 2 s.e.m. (Fig. 5, Table 1, section c). However, as in the case of psd, these mutations have very little effect on the timing of abaxial trichome production. amp1-1 and amp1(cop2) plants produced leaves with abaxial trichomes approximately 10 days after planting, 1 day later than the Col controls. Thus, psd, amp1-1 and amp1(cop2) plants produce leaves Fig. 3. Comparison of psd and wild-type Ler plants. (A,B) Longitudinal section through the shoot apical meristem of Ler (A) and psd (B) seedlings 3 days after planting. The Ler seedling has 2 leaf primordia, whereas no leaf primoridia are obvious in the psd seedling. psd seedlings typically have a group of dead cells (arrow) at the center of the meristem. (C,D) Longitudinal section through the shoot apical meristem of Ler (C) and psd (D) seedlings 8 days after planting. The Ler seedling has initiated 5-6 leaves, whereas the psd seedling has initiated 3-4 leaves. Leaves one and two are indicated by *. (E) Ler (left) and psd (right) seedlings 15 days after planting. Note that the first two leaves of the psd seedling are more elongated than the first two leaves of the Ler seedling. scale bar, 50 µm. with abaxial trichomes at the correct time in shoot development, despite the fact that psd plants make few or no leaves lacking abaxial trichomes and amp1-1 and amp1(cop2) plants make a large number of such leaves. This observation is inconsistent with the hypothesis that abaxial trichome production is regulated by the size of the shoot. Rather, it supports the hypothesis that this trait is regulated either by a temporal change in the character of the shoot meristem, or by factors that change independently of the growth of the shoot. Abaxial trichome production is sensitive to photoperiod and some flowering time mutations Vegetative phase change has been correlated with the attainment of reproductive competence in other species (Hackett, 1985; Zimmerman et al., 1985). If this is also true in Arabidopsis, and if abaxial trichome development is a marker for the vegetative phase of the shoot, then this trait is likely to be influenced by factors that regulate flowering. We found that abaxial trichome production is indeed affected by environmental conditions and some mutations that affect flowering time. Arabidopsis plants grown in short days (SD) flower later, with more rosette leaves, than those grown in long days or CL (Martínez-Zapater et al., 1994). Ws, Ler and Col plants grown
5 Phase change in Arabidopsis 649 Number of leaves visible Ler psd Number of leaves visible Col amp1-1 amp1(cop2) Days after planting Fig. 4. Comparison of the rate of leaf initiation and the time at which abaxial trichomes are first produced in psd and wild-type Ler plants. Leaf initiation was measured in 45 Ler and 41 psd plants. The arrows indicate the average position of the first leaf with abaxial trichomes in each genotype (see Table 1). From this point, the average time at which abaxial trichome development was initiated was estimated (as indicated by the vertical lines). Error bars indicate 2 s.e.m. in SD developed abaxial trichomes at significantly later leaf positions than plants grown in CL (Table 1, section d). Furthermore, six of seven late flowering mutations we examined delayed abaxial trichome production. Data from two separate experiments are shown (Table 2). co-2, fd-1, gi-3, fca-1, fve-1 and fpa-1 produced significantly more juvenile leaves than wild-type plants in both experiments, while ft-1 did not. The additional leaves must reflect a true temporal delay in abaxial trichome production because none of these mutations was found to affect the rate of leaf initiation (data not shown). The extent of the delay varied considerably. fd-1 delayed abaxial trichome production by one leaf, while fpa-1 plants doubled the number of leaves without abaxial trichomes. Further differences between the mutations become apparent when their relative effects on trichome production and flowering time are compared. fpa-1, for example, had a smaller delay in flowering (as indicated by total rosette leaf number), than gi-3, fca-1 and fve-1 although it produced more leaves without abaxial trichomes than any of these mutants. The observation that abaxial trichome development is delayed in late flowering mutants supports the use of abaxial trichome production as a marker for vegetative phase change, and indicates that vegetative phase change and reproductive development have some regulatory factors in common. However, the lack of a general correlation between the delay in abaxial trichome production in different mutants and the extent to which flowering is delayed indicates that there are additional components unique to the regulation of each of these processes. The conclusion that abaxial trichome production and flower induction are separable processes is supported by the phenotype of recessive mutations in the TERMINAL FLOWER1 (TFL1) gene. tfl1 mutations accelerate the transition within the inflorescence from the production of nodes Days after planting Fig. 5. Comparison of leaf initiation and the time at which abaxial trichomes are first produced in amp1-1, amp1(cop2), and wild-type Col plants. Leaf initiation was measured in 11 Col, 23 amp1-1, and 36 amp1(cop2) plants. The arrows indicate the average position of the first leaf with abaxial trichomes in each genotype (see Table 1). From this point, the average time at which abaxial trichome development was initiated was estimated (as indicated by the vertical lines). Error bars indicate 2 s.e.m. with bracts and inflorescence branches to the production of nodes with single flowers, and also accelerate flowering (Shannon and Meeks-Wagner, 1991, 1993; Schultz and Haughn, 1993). To determine if TFL1 controls the timing of vegetative phase change, we examined the effect of the tfl1-10 mutation on abaxial trichome production. No significant difference was observed in the number of leaves without abaxial trichomes in tfl1-10 and wild-type (Col) plants (Table 1, section e), or in their rate of leaf initiation (data not shown). A related result was reported by Weigel and Nilsson (1995), who assessed abaxial trichome development in transgenic plants overexpressing LEAFY, a gene that is negatively regulated by TFL1 (Weigel et al., 1992). They found that these plants, which resemble tfl1 mutants in many respects, produced abaxial trichomes at the same time as untransformed controls. Table 2. The effect of late flowering mutations on rosette development Experiment 1 Experiment 2 Juvenile Total Juvenile Total Genotype leaves* rosette leaves* leaves* rosette leaves* Ler 2.7± ± ± ±0.2 ft-1 3.0± ± ± ±0.7 co-2 3.4± ± ± ±0.5 fd-1 4.1± ± ± ±0.7 gi-3 4.5± ± ± ±2.5 fca-1 5.0± ± ± ±1.5 fve-1 5.1± ± ± ±2.0 fpa-1 7.3± ± ± ±0.4 *Each value is the average ± 2 s.e.m. All values are significantly different from the control (P<0.05, Student s t-test) unless otherwise indicated. Not significantly different from Ler (P>0.05, Student s t-test).
6 650 A. Telfer, K. M. Bollman and R. S. Poethig The regulation of trichome production by gibberellins Gibberellins (GA) have been found to regulate phase change in several species (Hackett, 1985; Zimmermann et al., 1985; Evans and Poethig, 1995). To examine the role of GA in abaxial trichome production, groups of Col plants were given regular applications of GA 3 beginning at different times in their development (Fig. 6). All plants responded by initiating abaxial trichomes precociously, with those receiving the earliest initial treatments producing the earliest trichomes. There was, however, a limit to this response in that plants never made abaxial trichomes earlier than leaf three, even when treated with GA 3 from the time of germination. This result indicates that GA promotes abaxial trichome production but suggests that leaves 1 and 2, unlike later rosette leaves, are incompetent to respond to this signal. This hypothesis is supported by the phenotype of spindly mutants, which undergo a constitutive GA response (Jacobsen and Olszewski, 1993). The spy-3 allele caused abaxial trichomes to occur significantly earlier than wild type (Col) controls, but never earlier than leaf 3 (data not shown). In light of this effect, the phenotype of spy-4 was striking. This mutation was isolated in Ws, an ecotype that normally makes abaxial trichomes under leaf 3. spy-4 enhanced abaxial trichome production in that significantly more abaxial trichomes developed on leaf three in the mutants than in wild type (28.7±9.5 vs. 4.9±2.3 (P<0.001), but the mutation did not accelerate abaxial trichome production; all spy-4 and Ws control plants formed abaxial trichomes on leaf three (Table 1, section f). Thus, both exogenously supplied GA 3 and the activated GA response conferred by spy mutations enhance abaxial trichome production on leaf 3 and later leaves, but neither has an effect on leaves 1 and 2. Consistent with the stimulatory effect of GA on abaxial trichome production, we found that mutations that block different steps in the GA biosynthetic pathway (ga1-3, ga4-1 and ga5-1) (Koornneef and van der Veen, 1980) and a mutation that confers insensitivity to GA (gai) (Koornneef et al., 1985) significantly delay abaxial trichome production (Table 1, sections g and h). In ga1-3 mutants, which have the most extreme GA-deficient phenotype, abaxial trichomes did not develop on any of the rosette leaves or on bracts. We also found that the ga1-3 mutation repressed adaxial trichome production (Fig. 7A). No adaxial trichomes formed on the first few rosette leaves of most mutant plants and although later rosette leaves developed adaxial trichomes in increasing numbers, the trichomes were restricted to the apical regions of the leaf blade. The normal distribution of both abaxial and adaxial trichomes was restored in ga1-3 mutants by exogenous applications of GA 3, although a higher dose of this hormone was required to Position of first leaf with abaxial trichomes Untreated Control Germinating 1-2 leaves induce trichomes on the abaxial surface than on the adaxial surface. In plants treated with 10 7 M or higher concentrations of GA 3, the number of adaxial trichomes and the portion of the adaxial surface covered with trichomes increased at all positions (Fig. 7B). By contrast, abaxial trichomes only formed on ga1-3 plants treated with at least 10 5 M GA 3. These results suggest that GA promotes trichome production on both surfaces of the leaf, but that the abaxial surface is less sensitive to this hormone than is the adaxial surface. Adaxial trichome loss In addition to the changes in trichome distribution that occur during vegetative development, characteristic changes also occur 3-4 leaves Average for group Earliest plant in group Latest plant in group 5-6 leaves 7-8 leaves Developmental stage at time of first GA treatment 3 Fig. 6. Effects of GA 3 on abaxial trichome development in wild-type plants. The position of the first leaf with abaxial trichomes was assayed in groups of plants treated with 10 5 M GA 3 beginning at different stages of development. The circles and diamonds indicate the range of this leaf position in each group. Error bars on the average values are 2 s.e.m leaves Fig. 7. Comparison of trichome development on the abaxial and adaxial surfaces of leaf 5 in wild-type Ler and ga1-3 mutant plants and the response of ga1-3 plants to GA 3 treatment. (A) Untreated plants. (B) Plants treated with GA 3. Each diagram shows the average number of trichomes observed on each surface (n = 5-10). The portion of each surface covered in trichomes is representative of genotype and treatment group. Note that average leaf size was not the same for each group and thus differences in trichome densities should not be inferred. The apical end of each leaf is to the right in this diagram. Genotype Adaxial Surface Abaxial Surface GA Treatment A WT (Ler) No GA 3 ga1-3 B ga1-3 No GA M GA M GA 3
7 Phase change in Arabidopsis 651 Trichome Distribution on Adaxial Surface: 100% 50% 25% 5% 0 A B Fig. 8. Schematic diagram comparing adaxial trichome distributions on the bracts of wild-type Col, Ler and Ws plants and tfl1-10 mutants. (A) Col, (B) Ler, (C) Ws, (D) tfl1-10 (Col background). Vertical lines represent the primary inflorescence stems and diagonal lines represent coflorescence stems. Inflorescences and coflorescences from approximately ten plants of each strain were examined. The trichome distribution shown for each bract is representative of the bracts found at that position in each group of plants. C D during the reproductive phase of shoot development. In Arabidopsis, modified leaves (bracts) are formed at the basal nodes of the primary inflorescence (primary bracts) and at the basal nodes of each inflorescence branch, or coflorescence (coflorescence bracts). The structure of a typical coflorescence is shown in Fig. 8A. All bracts produce abaxial trichomes, but bracts located at apical nodes of the inflorescence usually lack trichomes on all or part of their adaxial surface. Adaxial trichomes disappear first from the basal and medial region of the bract, and this region expands towards the tip of the bract on bracts located at successively higher nodes. This pattern of trichome loss also occurs within each coflorescence. However, coflorescences differ from each other in that the bracts on basal coflorescences have more adaxial trichomes than bracts at identical positions on apical coflorescences. Thus, the character of coflorescence bracts is correlated with the position of the coflorescence within the inflorescence. The Ws, Col and Ler ecotypes differ in the rate at which adaxial trichomes are lost from successive bracts (Fig. 8A-C), with Ws losing adaxial trichomes more rapidly than Col and Ler. The loss of adaxial trichomes appears to be tied to the process of inflorescence development. We have never observed this phenomenon on the rosette leaves of wild-type plants under any growth conditions. Because other morphological changes that occur during inflorescence development are accelerated by mutations in TFL1, we examined tfl1-10 mutants to determine if this mutation also accelerates adaxial trichome loss. While the first bract of wild-type Col plants was completely covered with adaxial trichomes, the first bract in about 70% of tfl1-10 plants was at least partially devoid of adaxial trichomes (Fig. 8D). None of the second bracts in mutant plants had adaxial trichomes, compared with the 50% coverage normally seen on the second bract of control plants. The observation that tfl1-10 accelerates both flower production and loss of adaxial trichomes from bracts supports the hypothesis that this latter event is triggered by floral induction. DISCUSSION The regulation of trichome differentiation in Arabidopsis thaliana has been the subject of intensive genetic analysis (Marks et al., 1981; Hülskamp et al., 1994; Larkin et al., 1996). Less is known about the basis for developmental variation in leaf trichome production (Telfer and Poethig, 1994; Chien and Sussex, 1996). Leaves produced early in rosette development do not have trichomes on their abaxial surface, whereas leaves produced later possess trichomes on both their adaxial and abaxial surfaces. Leaves that develop after the plant has been induced to flower (bracts) possess abaxial trichomes, but frequently lack trichomes on all or part of their adaxial surface. We believe that abaxial trichome production reflects global changes in the character of the shoot related to shoot maturation, and can thus serve as a marker of the adult phase in studies of the regulation of vegetative phase change in Arabidopsis. This conclusion is based on the following observations. In many plants, the types and the distribution of epidermal hairs vary according to the developmental phase of the shoot and are correlated with the appearance of many other less obvious phase-specific traits (Schaffalitzky de Muckadell, 1954; Wareing and Frydman, 1976; Brand and Lineberger, 1992; Lawson and Poethig, 1995). Furthermore, as we have shown here, abaxial trichome production in Arabidopsis is influenced by factors known to affect phase change in other species. These include genes involved in floral initiation, and GA. We suggest that adaxial trichome loss from bracts occurs as a consequence of floral induction and reflects the suppression of vegetative development in the inflorescence. Changes in trichome distribution may be temporally regulated Developmental variation in vegetative traits have been proposed to occur in response to a variety of developmental cues. These include the attainment of a critical overall size, the production of a set number of leaves, and the age of the shoot. The morphology of vegetative structures might also be determined by positional information within the meristem, as has been proposed for the determination of floral organ identity. By analogy to the positional information model of flower morphogenesis (Coen and Meyerowitz, 1991; Weigel and Meyerowitz, 1994), the vegetative shoot meristem could be divided into discrete domains by the overlapping expression of regulatory genes, with the character of a leaf being determined by the domain in which it arose.
8 652 A. Telfer, K. M. Bollman and R. S. Poethig To investigate these possibilities, we asked if mutations that affect the rate of shoot growth also affect the number of leaves that lack abaxial trichomes. We found that psd, a mutation that delays leaf production, results in the production of fewer leaves lacking abaxial trichomes than wild type. Conversely, mutations of amp1, which accelerate leaf production, cause an increase in the number of leaves without abaxial trichomes. In spite of their abnormal rate of leaf initiation, both psd and amp1 mutants begin producing leaves with abaxial trichomes at approximately the same time as wild-type plants. If these mutations can be assumed to have no direct effect on trichome production, this result suggests that the production of abaxial trichomes does not depend on the prior production of a specific number of leaves or the attainment of a specific overall shoot size. It is also inconsistent with models in which the character of a leaf is influenced by its distance from the root since the production of abaxial trichomes occurs at a lower node in the case of psd, and at a higher node in the case of amp1. The phenotype of psd also fails to support the predictions of the positional information model. If the trichome pattern on a leaf were specified by where it arose within the shoot apical meristem, psd would not be expected to affect trichome production on the first leaves because they appear to arise at normal positions on the basal perimeter of the meristem. The phenotypes of psd and amp1 are consistent with the temporal regulation of abaxial trichome production driven, perhaps, by temporal changes in factors produced by the shoot apical meristem or a specific organ (e.g. cotyledons or the root system). A model for the regulation of trichome development One way to account for the distribution of trichomes on rosette leaves is to postulate that there is a global inductive signal for trichome production, the level of which increases during the development of the rosette, and that the abaxial epidermis is less sensitive to this signal than the adaxial epidermis. Early in shoot development the level of this factor is sufficient to induce trichome production on the adaxial surface of the leaf, but not on the abaxial surface. Production of abaxial trichomes begins in developing leaves when the level of the inducer increases above the threshold sensitivity of the abaxial epidermis. This model implies that all rosette leaf primordia have the same sensitivity to the inductive signal but differ in their expression of abaxial trichomes according to the level of the inductive signal in the plant. An alternative is that leaves initiated at different times in development have different capacities to respond to the signal. These two models are not mutually exclusive, and other explanations are possible. Fig. 9 illustrates a case in which the pattern of abaxial trichome development in the rosette depends on both the changing level of an inductive signal and on differences in the competence of the first two and later rosette leaves to respond to that signal. Our results, and those of Chien and Sussex (1996) suggest that the trichome inducer may be GA and that the abaxial and abaxial surfaces do indeed differ in their sensitivity to this hormone. ga1-3 mutants, which contain a null mutation in the enzyme that controls the first committed step in GA-biosynthesis (Sun et al., 1992; Zeevaart and Talón, 1992), produce no abaxial trichomes, and have few or no adaxial trichomes on rosette leaves. This phenotype is corrected by exogenous applications of GA 3, although a higher concentration of this hormone is required to restore trichomes to the abaxial surface of a given leaf than to the adaxial surface. A Competence of abaxial surface to respond to inducer B Adaxial trichomes Abaxial trichomes Inducer level INCOMPETENT Developmental time Abaxial threshold Adaxial threshold COMPETENT Fig. 9. A model of phase change regulation in Arabidopsis. (A) The level of a trichome inducer increases in the plant with time, and must exceed certain threshold levels before the abaxial and adaxial surfaces of developing leaves can respond and produce trichomes. The competence of the abaxial surfaces of leaves to respond to this inducer also changes. The first two leaves are not competent to respond to inducer levels above the threshold, but later leaves are. (B) The pattern of trichome development on rosette leaves predicted by the parameters shown in A. Our observation that leaves one and two do not produce abaxial trichomes in response to exogenously applied GA 3 or to mutations in SPINDLY suggests that abaxial trichome production on these leaves is regulated in a qualitatively different way than it is on later leaves. This could arise if changes in the developmental state of the shoot apex cause the first two leaf primordia to be inherently different from later leaf primordia in their developmental potential (i.e. have different identities). Cells in the abaxial epidermis of the first two leaf primordia might, for example, lack a critical component of the GA signal transduction pathway. It is also possible that all leaf primordia have the same developmental potential, but that the global levels of other developmentally regulated stimuli or inhibitors within the plant influence their sensitivity to GA. The effects of GA on trichome production in Arabidopsis are in agreement with the effect of GA on phase-specific traits in other herbaceous species. In maize, for example, GAdeficient mutations delay the appearance of adult vegetative traits, including epidermal hairs on the leaf blade, and prolong the expression of many juvenile traits; exogenous applications of GA 3 have the opposite effect on these traits (Evans and Poethig, 1995). GA is also regarded as an important regulator of phase change in woody species (Zimmerman et al., 1985; Pharis, 1990). Thus, while the model described here is couched in terms of trichome development, it may be applicable to other phase-specific traits as well. The relationship between trichome production and reproductive development Short day conditions delay flowering in Arabidopsis and also delay the production of leaves with abaxial trichomes. This
9 Phase change in Arabidopsis 653 result is in agreement with that of Chien and Sussex (1996), and is consistent with previous observation that other developmental changes in leaf morphology are delayed in SD (Martínez-Zapater et al., 1995). Most of the late flowering mutations that we examined also delay the production of abaxial trichomes. These results imply that vegetative phase change and reproductive development are coordinated in Arabidopsis, as they appear to be in many other plants (Zimmerman et al., 1985; Hackett, 1985). Based on physiological experiments and double mutant analyses, the late flowering genes FCA, FVE and FPA have been proposed to promote flowering by increasing the level of, or response to, GA (reviewed in Martínez-Zapater et al., 1994). Given that GA promotes abaxial trichome production, it is not surprising that mutations in these genes delay abaxial trichome production as well as flowering. The lack of correlation in these mutants between the extent of delays in flowering and abaxial trichome production could reflect the involvement of different GA species in these two processes. The effects of SD conditions on abaxial trichome production may also be mediated by GA, as photoperiodic conditions have been shown to affect GA biosynthesis and/or the sensitivity to this hormone in a number of species (Zeevaart, 1983; Gilmour et al., 1986; Pharis et al., 1987). The other late flowering mutations that we examined (ft-1, co-2, fd-1, and gi-3) are in genes proposed to function independently of GA (reviewed by Martínez-Zapater et al., 1994). The observation that three of these mutations delay abaxial trichome production is significant because it implies that GA is not the only factor common to the regulation of vegetative phase change and flowering. The mechanism by which flowering and abaxial trichome production are linked remains unclear. Coordination of flowering and abaxial trichome production would occur if (a) the two processes were independent but were regulated by common factors, (b) the factors responsible for abaxial trichome production either induced reproductive maturation or were a prerequisite for it, or (c) floral induction or the development of reproductive competence induced abaxial trichome production. The identification of mutants that affect both of these processes provides an entrée into the genetic analysis of this problem. We anticipate that the analysis of these and other flowering time mutants, in combination with mutants isolated on the basis of their effect on trichome distribution, will allow the nature of the coordination to be determined. Floral initiation affects trichome development on bracts Bracts can be distinguished from rosette leaves not only by their position on the inflorescence stem, but also by their relatively small size, lack of petiole and by the reduced cover of trichomes on their adaxial surface. The phenotypes of wild type and tfl1-10 mutants indicate that the disappearance of adaxial trichomes in the inflorescence is likely to occur as a result of floral induction. Indeed, adaxial trichome loss might serve as a useful marker of floral induction under conditions in which flowers do not develop, or when the inflorescence develops abnormally. Hempel and Feldman (1994) showed that primary bracts develop from leaf primordia present on the meristem at the time of floral induction and proposed that variation in the character of bracts within the inflorescence could reflect the differences in the age of these primoridia at the time of floral induction. The pattern of adaxial trichome loss from bracts is consistent with this model. Leaf tissues mature in a apicalbasal gradient (reviewed by Telfer and Poethig, 1994), with differentiated structures such as trichomes appearing first in apical regions of developing leaf primordia. Thus, differences in the maturation state of tissues within a leaf could lead to the production of bracts in which adaxial trichomes are suppressed to varying extents. The region devoid of trichomes would be expected to be more extensive on apical bracts than on basal bracts because the primordia of apical bracts would have been younger, and therefore less determined, at the time of floral induction. The observation that coflorescence bracts can have more adaxial trichomes than the most apical bract on the main stem is unexpected because coflorescence bracts are initiated later than the primary bracts (Hempel and Feldman, 1994). If axillary buds become florally induced at the same time and to the same extent as the primary meristem, then coflorescence bracts should always have fewer adaxial trichomes than primary bracts. The observation that this is not the case supports the argument that axillary meristems present at the shoot apex at the time of floral induction become induced after the primary meristem, and perhaps independently of this event (Hempel and Feldman, 1994). Prospects for the genetic analysis of phase change using trichome distribution as a phase marker Our studies have shown that trichome distribution is regulated at a number of different levels. Because of this complexity, the simple division of rosette development into juvenile and adult phases based on abaxial trichome production may not accurately reflect the full range of developmental programs expressed during vegetative development. By this single criterion, wild-type Col plants grown in CL, for example, produce 4 or 5 juvenile leaves. However, we have shown that the first two leaves can be distinguished from other juvenile leaves in that they cannot be induced to produce abaxial trichomes by any of the experimental or environmental treatments described in this paper. This distinction can be interpreted to mean that the juvenile phase is composed of two subphases: an early juvenile phase during which abaxial trichomes cannot be induced, and a late juvenile phase during which leaves are competent to produce abaxial trichomes if exposed to the correct stimuli. This is consistent with the observations that in maize the first juvenile leaves are morphologically and anatomically different from later juvenile leaves (Bongard- Pierce et al., 1996) and that gl15, a maize mutation that accelerates the appearance of adult vegetative traits, does not affect these first juvenile leaves (Evans et al., 1994; Moose and Sisco, 1994). It remains to be determined if variation in the production of abaxial trichomes on competent Arabidopsis leaves (leaves 3 and above) is dictated solely by the level of an inducer such as GA, or if other, possibly inherent, differences distinguish late juvenile leaves from adult leaves. We recognize that mutations affecting trichome development can alter trichome distribution in ways that are not phasespecific, complicating the use of trichome distribution as a phase marker in mutant genetic screens. However, we have been able to identify genes that affect other key phase-specific traits (e.g. leaf shape, floral initiation) in screens for mutations
10 654 A. Telfer, K. M. Bollman and R. S. Poethig that accelerate or delay abaxial trichome production (A. Telfer, K. M. Bollman and R. S. Poethig, unpublished). Genes that influence the timing of adaxial trichome loss have also been identified. The information presented in this paper provides a framework for interpreting the role of these and other genes in vegetative phase change and inflorescence development. We gratefully acknowledge support from an American Cancer Society Postdoctoral Fellowship (A. T.), NIH Predoctoral Training Grant GM07229 (K. B.), and NIH Grant R01-GM51893 (R. S. P.). We thank Laura Conway, who initially observed that early rosette leaves do not produce abaxial trichomes, Kathy Barton, who isolated the paused mutation, and Samara Reck-Peterson, who made additional observations of late flowering mutants. We are grateful to current and former members of the laboratory for their discussions and insights, and thank Emily Lawson, Nelleke Kreike and Allan Jones for their critical comments on the manuscript. REFERENCES Allsopp, A. (1967). Heteroblastic development in vascular plants. Adv. Morphol. 6, Bongard-Pierce, D. K., Evans, M. M. S. and Poethig, R. S. (1996). Heteroblastic features of leaf anatomy in maize and their genetic regulation. Int. J. Plant Sci. 157, Brand, M. H. and Lineberger, R. D. (1992). In vitro rejuvenation of Betulaceae: morphological evaluation. Am. J. Bot. 79, Chaudhury, A. M., Letham, S., Craig, S. and Dennis, E. S. (1993). amp1-a mutant with high cytokinin levels and altered embryonic pattern, faster vegetative growth, constitutive photomorphogenesis and precocious flowering. Plant J. 4, Chien, J. C. and Sussex, I. M. (1996). Differential regulation of trichome formation on the adaxial and abaxial leaf surfaces by gibberellins and photoperiod in Arabidopsis thaliana (L.) Heynh. Plant Physiol. 111, Coen, E. S. and Meyerowitz, E. M. (1991). The war of the whorls: Genetic interactions controlling flower development. Nature 353, Evans, M. M. S., Passas, H. and Poethig, R. S. (1994). Heterochronic effects of glossy15 mutations on epidermal cell identity in maize. Development 120, Evans, M. M. S. and Poethig, R. S. (1995). Gibberellins promote vegetative phase change and reproductive maturity in maize. Plant Physiol. 108, Freeling, M. and Lane, B. (1994). The maize leaf. In The Maize Handbook (eds. M. Freeling and V. Walbot), pp Springer-Verlag, New York. Gilmour, S. J., Zeevaart, J. A. D., Schwenen, L. and Graebe, J. E. (1986). Gibberellin metabolism in cell-free extracts from spinach leaves in relation to photoperiod. Plant Physiol. 82, Greenwood, M. S. (1995). Juvenility and maturation in conifers: current concepts. Tree Physiol. 15, Hackett, W. P. (1985). Juvenility, maturation and rejuvenation in woody plants. Hort. Rev. 7, Hackett, W. P. and Murray, J. R. (1993). Maturation and rejuvenation in woody species. In Micropropagation of Woody Plants (ed. M. R. Ahuja), pp Kluwer, Netherlands. Hempel, F. D. and Feldman, L. J. (1994). Bi-directional inflorescence development in Arabidopsis thaliana: Acropetal initiation of flowers and basipetal initiation of paraclades. Planta 192, Hou, Y., von Arnim, A. G. and Deng, X. W. (1993). A new class of Arabidopsis constitutive photomorphogenic genes involved in regulating cotyledon development. Plant Cell 5, Hülskamp, M., Miséra, S. and Jürgens, G. (1994). Genetic dissection of trichome cell development in Arabidopsis. Cell 76, Jacobsen, S. E. and Olszewski, N. E. (1993). Mutations in the SPINDLY locus of Arabidopsis alter gibberellin signal transduction. Plant Cell. 5, Koornneef, M. and van der Veen, J. H. (1980). Induction and analysis of gibberellin sensitive mutants in Arabidopsis thaliana (L.) Heynh. Theor. Appl. Genet. 58, Koornneef, M., Elgersma, A., Hanhart, C. J., Van Loenen-Martinet, E. P. Van Rijn, L. and Zeevaart, J. A. D. (1985). A gibberellin insensitive mutant of Arabidopsis thaliana. Physiol. Plant.. 65, Larkin, J. C., Young, N., Prigge, M. and Marks, M. D. (1996). The control of trichome spacing and number in Arabidopsis. Development 122, Lawson, E. and Poethig, R. S. (1995). Shoot development in plants: time for a change. Trends Genet. 11, Longman, K. A. (1976). Some experimental approaches to the problem of phase change in forest trees. Acta Hort. 56, Marks, M. D., Esch, J., Herman, P., Sivakumaran, S. and Oppenheimer, D. (1991). A model for cell-type determination in differentiation in plants. In Molecular Biology of Plant Development (eds. G. Jenkins and W. Schuch), pp SEB Symposia Vol. XLV. Martínez-Zapater, J. M., Coupland, G., Dean, C. and Koornneef, M. (1994). The transition to flowering in Arabidopsis. In Arabidopsis (eds. E. M. Meyerowitz and C. R. Somerville), pp Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. Martínez-Zapater, J. M., Jarillo, J. A., Cruz-Alvarez, M., Roldán, M. and Salinas, J. (1995). Arabidopsis late-flowering fve mutants are affected in both vegetative and reproductive development. Plant J. 7, Medford, J. I., Behringer, F. J., Callos, J. D. and Feldmann, K. A. (1992). Normal and abnormal development in the Arabidopsis vegetative shoot apex. Plant Cell 4, Moose, S. P. and Sisco, P. H. (1994). Glossy15 controls the epidermal juvenileto-adult transition in maize. Plant Cell 6, Pharis, R. P. (1990). Physiology of gibberellins in relation to floral initiation and early floral differentiation. In Gibberellins (eds. N. Takahashi, B. O. Phinney and J. MacMillan), pp Springer-Verlag, Heidelberg. Pharis, R. P., Evans, L. T., King, R. W. and Mander, L. N. (1987). Gibberellins, endogenous and applied, in relation to flower induction in the long-day plant Lolium temulentum. Plant Physiol. 84, Poethig, R. S. (1990). Phase change and the regulation of shoot morphogenesis in plants. Science 250, Röbbelen, G. (1957). Über Heterophyllie bei Arabidopsis thaliana (L.) Heynh. Ber. Dtsch. Bot. Ges. 70, Schaffalitzky de Muckadell, M. (1954). Juvenile stages in woody plants. Physiol. Plant. 7, Schultz, E. A. and Haughn, G. W. (1993). Genetic analysis of the floral initiation process (FLIP) in Arabidopsis. Development 119, Schwabe, W. W. (1976). Applied aspects of juvenility and some theoretical considerations. Acta Hort. 56, Shannon, S. and Meeks-Wagner, D. R. (1991). A mutation in the Arabidopsis TFL1 gene affects inflorescence meristem development. Plant Cell 3, Shannon, S. and Meeks-Wagner, D. R. (1993). Genetic interactions that regulate inflorescence development in Arabidopsis. Plant Cell 5, Sun, T., Goodman, H. M. and Ausubel, F. M. (1992). Cloning the Arabidopsis GA1 locus by genomic subtraction. Plant Cell 4, Telfer, A. and Poethig, R. S. (1994). Leaf development in Arabidopsis. In Arabidopsis (eds. E. M. Meyerowitz and C. R. Somerville), pp Cold Spring Harbor Press, Cold Spring Harbor, NY. Wareing, P. F. and Frydman, V. M. (1976). General aspects of phase change, with special reference to Hedera helix L. Acta Hort. 56, Weigel, D., Alvarez, J., Smyth, D. R. and Yanofsky, M. F. (1992). LEAFY controls floral meristem identity in Arabidopsis. Cell 69, Weigel, D. and Meyerowitz, E. M. (1994). The ABCs of floral homeotic genes. Cell 78, Weigel, D. and Nilsson, O. (1995). A developmental switch sufficient for flower initiation in diverse plants. Nature 377, Zeevaart, J. A. D. (1983). Gibberellins and flowering. In The Biochemistry and Physiology of Gibberellin. Vol. 2 (ed. A. Crozier), pp Praeger, New York. Zeevaart, J. A. D. and Talón, M. (1992). Gibberellin mutants in Arabidopsis thaliana. In Progress in Plant Growth Regulation (eds. C. M. Kaarsen, L. C. Van Loon and D. Vreugdenhil), pp Kluwer Academic Pub., Amsterdam, Netherlands. Zimmerman, R. H., Hackett, W. P. and Pharis, R. P. (1985). Hormonal aspects of phase change and precocious flowering. Encycl. Plant Physiol. 11, (Accepted 5 November 1996)
The early-flowering mutant efs is involved in the autonomous promotion pathway of Arabidopsis thaliana
 Development 126, 4763-477 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV248 4763 The early-flowering mutant efs is involved in the autonomous promotion pathway of Arabidopsis
Development 126, 4763-477 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV248 4763 The early-flowering mutant efs is involved in the autonomous promotion pathway of Arabidopsis
Genetic interactions of the Arabidopsis flowering time gene FCA, with genes regulating floral initiation
 The Plant Journal (1999) 17(3), 231 239 Genetic interactions of the Arabidopsis flowering time gene FCA, with genes regulating floral initiation Tania Page 1,, Richard Macknight 1,, Chang-Hsien Yang 2
The Plant Journal (1999) 17(3), 231 239 Genetic interactions of the Arabidopsis flowering time gene FCA, with genes regulating floral initiation Tania Page 1,, Richard Macknight 1,, Chang-Hsien Yang 2
Useful Propagation Terms. Propagation The application of specific biological principles and concepts in the multiplication of plants.
 Useful Propagation Terms Propagation The application of specific biological principles and concepts in the multiplication of plants. Adventitious Typically describes new organs such as roots that develop
Useful Propagation Terms Propagation The application of specific biological principles and concepts in the multiplication of plants. Adventitious Typically describes new organs such as roots that develop
Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated
 Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated form by recombinant UGT74E2. The naturally occurring auxin
Figure 1. Identification of UGT74E2 as an IBA glycosyltransferase. (A) Relative conversion rates of different plant hormones to their glucosylated form by recombinant UGT74E2. The naturally occurring auxin
Outline. Leaf Development. Leaf Structure - Morphology. Leaf Structure - Morphology
 Outline 1. Leaf Structure: Morphology & Anatomy 2. Leaf Development A. Anatomy B. Sector analysis C. Leaf Development Leaf Structure - Morphology Leaf Structure - Morphology 1 Leaf Structure - Morphology
Outline 1. Leaf Structure: Morphology & Anatomy 2. Leaf Development A. Anatomy B. Sector analysis C. Leaf Development Leaf Structure - Morphology Leaf Structure - Morphology 1 Leaf Structure - Morphology
early in short days 4, a mutation in Arabidopsis that causes early flowering
 Development 129, 5349-5361 2002 The Company of Biologists Ltd doi:10.1242/dev.00113 5349 early in short days 4, a mutation in Arabidopsis that causes early flowering and reduces the mrna abundance of the
Development 129, 5349-5361 2002 The Company of Biologists Ltd doi:10.1242/dev.00113 5349 early in short days 4, a mutation in Arabidopsis that causes early flowering and reduces the mrna abundance of the
Temperature-sensitive mutations that arrest Arabidopsis shoot development
 Development 122, 3799-3807 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV0078 3799 Temperature-sensitive mutations that arrest Arabidopsis shoot development F. Bryan Pickett,
Development 122, 3799-3807 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV0078 3799 Temperature-sensitive mutations that arrest Arabidopsis shoot development F. Bryan Pickett,
The mode of development in animals and plants is different
 The mode of development in animals and plants is different Outcome of animal embryogenesis is a mini edition of the adult Outcome of plant embryogenesis is a simple structure with -root apical meristem
The mode of development in animals and plants is different Outcome of animal embryogenesis is a mini edition of the adult Outcome of plant embryogenesis is a simple structure with -root apical meristem
Primary Plant Body: Embryogenesis and the Seedling
 BIOL 221 Concepts of Botany Primary Plant Body: Embryogenesis and the Seedling (Photo Atlas: Figures 1.29, 9.147, 9.148, 9.149, 9.150, 9.1, 9.2) A. Introduction Plants are composed of fewer cell types,
BIOL 221 Concepts of Botany Primary Plant Body: Embryogenesis and the Seedling (Photo Atlas: Figures 1.29, 9.147, 9.148, 9.149, 9.150, 9.1, 9.2) A. Introduction Plants are composed of fewer cell types,
Supplementary Figure 1. Phenotype of the HI strain.
 Supplementary Figure 1. Phenotype of the HI strain. (A) Phenotype of the HI and wild type plant after flowering (~1month). Wild type plant is tall with well elongated inflorescence. All four HI plants
Supplementary Figure 1. Phenotype of the HI strain. (A) Phenotype of the HI and wild type plant after flowering (~1month). Wild type plant is tall with well elongated inflorescence. All four HI plants
Plant Structure, Growth, and Development
 Plant Structure, Growth, and Development Plant hierarchy: Cells Tissue: group of similar cells with similar function: Dermal, Ground, Vascular Organs: multiple kinds of tissue, very diverse function Organ
Plant Structure, Growth, and Development Plant hierarchy: Cells Tissue: group of similar cells with similar function: Dermal, Ground, Vascular Organs: multiple kinds of tissue, very diverse function Organ
Molecular Genetics of. Plant Development STEPHEN H. HOWELL CAMBRIDGE UNIVERSITY PRESS
 Molecular Genetics of Plant Development STEPHEN H. HOWELL CAMBRIDGE UNIVERSITY PRESS Contents Preface A Word on Genetic Nomenclature page xiii xvii 1 Approaches to the Study of Plant Development 1 Pattern
Molecular Genetics of Plant Development STEPHEN H. HOWELL CAMBRIDGE UNIVERSITY PRESS Contents Preface A Word on Genetic Nomenclature page xiii xvii 1 Approaches to the Study of Plant Development 1 Pattern
CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E
 CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E The development of a plant the series of progressive changes that take place throughout its life is regulated in complex ways. Factors take part
CONTROL OF PLANT GROWTH AND DEVELOPMENT BI-2232 RIZKITA R E The development of a plant the series of progressive changes that take place throughout its life is regulated in complex ways. Factors take part
EMF Genes Regulate Arabidopsis lnflorescence Development
 The Plant Cell, Vol. 9, 201 1-2024, November 1997 O 1997 American Society of Plant Physiologists EMF Genes Regulate Arabidopsis lnflorescence Development Lingjing Chen, Jin-Chen Cheng, Linda Castle, and
The Plant Cell, Vol. 9, 201 1-2024, November 1997 O 1997 American Society of Plant Physiologists EMF Genes Regulate Arabidopsis lnflorescence Development Lingjing Chen, Jin-Chen Cheng, Linda Castle, and
The effect of the floral repressor FLC on the timing and progression of vegetative phase change in Arabidopsis
 RESEARCH ARTICLE 677 Development 138, 677-685 (2011) doi:10.1242/dev.057448 2011. Published by The Company of Biologists Ltd The effect of the floral repressor FLC on the timing and progression of vegetative
RESEARCH ARTICLE 677 Development 138, 677-685 (2011) doi:10.1242/dev.057448 2011. Published by The Company of Biologists Ltd The effect of the floral repressor FLC on the timing and progression of vegetative
Supplemental Data. Wang et al. (2014). Plant Cell /tpc
 Supplemental Figure1: Mock and NPA-treated tomato plants. (A) NPA treated tomato (cv. Moneymaker) developed a pin-like inflorescence (arrowhead). (B) Comparison of first and second leaves from mock and
Supplemental Figure1: Mock and NPA-treated tomato plants. (A) NPA treated tomato (cv. Moneymaker) developed a pin-like inflorescence (arrowhead). (B) Comparison of first and second leaves from mock and
GENETIC CONTROL OF FLOWERING TIME IN ARABIDOPSIS
 Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998. 49:345 70 Copyright c 1998 by Annual Reviews. All rights reserved GENETIC CONTROL OF FLOWERING TIME IN ARABIDOPSIS Maarten Koornneef, Carlos Alonso-Blanco,
Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998. 49:345 70 Copyright c 1998 by Annual Reviews. All rights reserved GENETIC CONTROL OF FLOWERING TIME IN ARABIDOPSIS Maarten Koornneef, Carlos Alonso-Blanco,
Pathways for inflorescence and floral induction in Antirrhinum
 Development 122, 1535-1544 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV3397 1535 Pathways for inflorescence and floral induction in Antirrhinum Desmond Bradley, Coral Vincent,
Development 122, 1535-1544 (1996) Printed in Great Britain The Company of Biologists Limited 1996 DEV3397 1535 Pathways for inflorescence and floral induction in Antirrhinum Desmond Bradley, Coral Vincent,
GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL
 GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL Kelsey Hoth 1 Dr. Maria Ivanchenko 2 Bioresourse Research 1, Department of Botany and Plant Physiology 2, Oregon State University, Corvallis,
GENETIC ANALYSES OF ROOT SYSTEM DEVELOPMENT IN THE TOMATO CROP MODEL Kelsey Hoth 1 Dr. Maria Ivanchenko 2 Bioresourse Research 1, Department of Botany and Plant Physiology 2, Oregon State University, Corvallis,
Epigenetics and Flowering Any potentially stable and heritable change in gene expression that occurs without a change in DNA sequence
 Epigenetics and Flowering Any potentially stable and heritable change in gene expression that occurs without a change in DNA sequence www.plantcell.org/cgi/doi/10.1105/tpc.110.tt0110 Epigenetics Usually
Epigenetics and Flowering Any potentially stable and heritable change in gene expression that occurs without a change in DNA sequence www.plantcell.org/cgi/doi/10.1105/tpc.110.tt0110 Epigenetics Usually
16. TRANSMISSION OF STIMULUS - THEORIES OF FLOWERING.
 16. TRANSMISSION OF STIMULUS - THEORIES OF FLOWERING. Photoperiodic Induction The influence of the length of day and night on the initiation of flowering is called photoperiodic induction or photo induction.
16. TRANSMISSION OF STIMULUS - THEORIES OF FLOWERING. Photoperiodic Induction The influence of the length of day and night on the initiation of flowering is called photoperiodic induction or photo induction.
Plant Structure and Organization - 1
 Plant Structure and Organization - 1 In our first unit of Biology 203 we will focus on the structure and function of the higher plants, in particular the angiosperms, or flowering plants. We will look
Plant Structure and Organization - 1 In our first unit of Biology 203 we will focus on the structure and function of the higher plants, in particular the angiosperms, or flowering plants. We will look
23-. Shoot and root development depend on ratio of IAA/CK
 Balance of Hormones regulate growth and development Environmental factors regulate hormone levels light- e.g. phototropism gravity- e.g. gravitropism temperature Mode of action of each hormone 1. Signal
Balance of Hormones regulate growth and development Environmental factors regulate hormone levels light- e.g. phototropism gravity- e.g. gravitropism temperature Mode of action of each hormone 1. Signal
FURCA loci. Genetic control of trichome branch number in Arabidopsis: the roles of the. Danlin Luo and David G. Oppenheimer* SUMMARY
 Development 126, 5547-5557 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV0265 5547 Genetic control of trichome branch number in Arabidopsis: the roles of the FURCA loci Danlin
Development 126, 5547-5557 (1999) Printed in Great Britain The Company of Biologists Limited 1999 DEV0265 5547 Genetic control of trichome branch number in Arabidopsis: the roles of the FURCA loci Danlin
Plant Growth and Development
 Plant Growth and Development Concept 26.1 Plants Develop in Response to the Environment Factors involved in regulating plant growth and development: 1. Environmental cues (e.g., day length) 2. Receptors
Plant Growth and Development Concept 26.1 Plants Develop in Response to the Environment Factors involved in regulating plant growth and development: 1. Environmental cues (e.g., day length) 2. Receptors
Plant Growth and Development Part I. Levels of Organization
 Plant Growth and Development Part I Levels of Organization Whole Plant Organs Tissues Cells Organelles Macromolecules Levels of Organization Whole Plant Organs Tissues Cells Organelles Macromolecules 1
Plant Growth and Development Part I Levels of Organization Whole Plant Organs Tissues Cells Organelles Macromolecules Levels of Organization Whole Plant Organs Tissues Cells Organelles Macromolecules 1
ROLES OF THE AF AND TL GENES IN PEA LEAF
 American Journal of Botany 84(10): 1323 1336. 1997. ROLES OF THE AF AND TL GENES IN PEA LEAF MORPHOGENESIS: CHARACTERIZATION OF THE DOUBLE MUTANT (AFAFTLTL) 1 PHILIP J. VILLANI 2 AND DARLEEN A. DEMASON
American Journal of Botany 84(10): 1323 1336. 1997. ROLES OF THE AF AND TL GENES IN PEA LEAF MORPHOGENESIS: CHARACTERIZATION OF THE DOUBLE MUTANT (AFAFTLTL) 1 PHILIP J. VILLANI 2 AND DARLEEN A. DEMASON
Plant Structure. Lab Exercise 24. Objectives. Introduction
 Lab Exercise Plant Structure Objectives - Be able to identify plant organs and give their functions. - Learn distinguishing characteristics between monocot and dicot plants. - Understand the anatomy of
Lab Exercise Plant Structure Objectives - Be able to identify plant organs and give their functions. - Learn distinguishing characteristics between monocot and dicot plants. - Understand the anatomy of
CBSE Quick Revision Notes (Class-11 Biology) CHAPTER-15 PLANT GROWTH AND DEVELOPMENT
 CBSE Quick Revision Notes (Class-11 Biology) CHAPTER-15 PLANT GROWTH AND DEVELOPMENT Root, stem leaves, flower, fruits and seeds arise in orderly manner in plants. The sequence of growth is as follows-
CBSE Quick Revision Notes (Class-11 Biology) CHAPTER-15 PLANT GROWTH AND DEVELOPMENT Root, stem leaves, flower, fruits and seeds arise in orderly manner in plants. The sequence of growth is as follows-
CONTROL SYSTEMS IN PLANTS
 AP BIOLOGY PLANTS FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROL SYSTEMS IN PLANTS HORMONES MECHANISM FOR HORMONE ACTION Plant Form and Function Activity #5 page 1 CONTROL OF CELL ELONGATION Plant
AP BIOLOGY PLANTS FORM & FUNCTION ACTIVITY #5 NAME DATE HOUR CONTROL SYSTEMS IN PLANTS HORMONES MECHANISM FOR HORMONE ACTION Plant Form and Function Activity #5 page 1 CONTROL OF CELL ELONGATION Plant
Other funding Sources Agency Name: MSU Agricultural Experiment Station /Project GREEEN Amount requested or awarded: 30,000
 FINAL PROJECT REPORT Project Title: Functional genomics of flowering in apple PI: Herb Aldwinckle Co-PI(2): Steve VanNocker Organization: Cornell University Organization: Michigan State University Telephone/email:
FINAL PROJECT REPORT Project Title: Functional genomics of flowering in apple PI: Herb Aldwinckle Co-PI(2): Steve VanNocker Organization: Cornell University Organization: Michigan State University Telephone/email:
Nature Genetics: doi: /ng Supplementary Figure 1. The phenotypes of PI , BR121, and Harosoy under short-day conditions.
 Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
Supplementary Figure 1 The phenotypes of PI 159925, BR121, and Harosoy under short-day conditions. (a) Plant height. (b) Number of branches. (c) Average internode length. (d) Number of nodes. (e) Pods
DEVELOPMENTAL GENETICS OF ARABIDOPSIS THALIANA
 DEVELOPMENTAL GENETICS OF ARABIDOPSIS THALIANA CHASE BALLARD LINDA EAN HECTOR LOPEZ DR. JOANNA WERNER-FRACZEK IN COLLABORATION WITH DR. PATRICIA SPRINGER S LAB AT UCR AND ROBERT KOBLE PURPOSE OF RESEARCH
DEVELOPMENTAL GENETICS OF ARABIDOPSIS THALIANA CHASE BALLARD LINDA EAN HECTOR LOPEZ DR. JOANNA WERNER-FRACZEK IN COLLABORATION WITH DR. PATRICIA SPRINGER S LAB AT UCR AND ROBERT KOBLE PURPOSE OF RESEARCH
Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family
 Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family GENES & DEVELOPMENT (2000) 14: 108 117 INTRODUCTION Flower Diagram INTRODUCTION Abscission In plant, the process by which a plant
Leucine-rich repeat receptor-like kinases (LRR-RLKs), HAESA, ERECTA-family GENES & DEVELOPMENT (2000) 14: 108 117 INTRODUCTION Flower Diagram INTRODUCTION Abscission In plant, the process by which a plant
Levels of Organization
 Plant Growth and Development Part I Levels of Organization Whole Plant Organs Tissues Cells Organelles Macromolecules Levels of Organization Whole Plant Organs Tissues Cells Organelles Macromolecules Plant
Plant Growth and Development Part I Levels of Organization Whole Plant Organs Tissues Cells Organelles Macromolecules Levels of Organization Whole Plant Organs Tissues Cells Organelles Macromolecules Plant
Leaf and Internode. Introduction. Parts of the Monocot and Dicot Leaf. Introductory article
 Andrew Hudson, University of Edinburgh, Edinburgh, UK Christopher Jeffree, University of Edinburgh, Edinburgh, UK Leaves of different species show wide variation in morphology and anatomy, usually associated
Andrew Hudson, University of Edinburgh, Edinburgh, UK Christopher Jeffree, University of Edinburgh, Edinburgh, UK Leaves of different species show wide variation in morphology and anatomy, usually associated
Apical dominance models can generate basipetal patterns of bud activation
 Apical dominance models can generate basipetal patterns of bud activation Przemyslaw Prusinkiewicz 1, Richard S. Smith 1 and Ottoline Leyser 2 1 Department of Computer Science, University of Calgary 2
Apical dominance models can generate basipetal patterns of bud activation Przemyslaw Prusinkiewicz 1, Richard S. Smith 1 and Ottoline Leyser 2 1 Department of Computer Science, University of Calgary 2
Growth Regulator Effects on Flowering in Maize
 Growth Regulator Effects on Flowering in Maize Eric Bumann July 14, 2008 My Background Research Associate at Pioneer Hi-Bred in Johnston, IA Production research 5 years in greenhouse research B.S. in Horticulture
Growth Regulator Effects on Flowering in Maize Eric Bumann July 14, 2008 My Background Research Associate at Pioneer Hi-Bred in Johnston, IA Production research 5 years in greenhouse research B.S. in Horticulture
Life Science Journal 2014;11(9) Cryptochrome 2 negatively regulates ABA-dependent seed germination in Arabidopsis
 Cryptochrome 2 negatively regulates ABA-dependent seed germination in Arabidopsis Sung-Il Kim 1, Sang Ik Song 3, Hak Soo Seo 1, 2, 4 * 1 Department of Plant Science and Research Institute of Agriculture
Cryptochrome 2 negatively regulates ABA-dependent seed germination in Arabidopsis Sung-Il Kim 1, Sang Ik Song 3, Hak Soo Seo 1, 2, 4 * 1 Department of Plant Science and Research Institute of Agriculture
Shoot Apex Development at Various Stages of Flowering in Sugarcane (Saccharum spp. hybrid)
 2008 The Japan Mendel Society Cytologia 73(2): 173 177, 2008 Shoot Apex Development at Various Stages of Flowering in Sugarcane (Saccharum spp. hybrid) M. Swapna* and Praveen Kumer Singh Division of Crop
2008 The Japan Mendel Society Cytologia 73(2): 173 177, 2008 Shoot Apex Development at Various Stages of Flowering in Sugarcane (Saccharum spp. hybrid) M. Swapna* and Praveen Kumer Singh Division of Crop
Supplementary Figure S1. Amino acid alignment of selected monocot FT-like and TFL-like sequences. Sequences were aligned using ClustalW and analyzed
 Supplementary Figure S1. Amino acid alignment of selected monocot FT-like and TFL-like sequences. Sequences were aligned using ClustalW and analyzed using the Geneious software. Accession numbers of the
Supplementary Figure S1. Amino acid alignment of selected monocot FT-like and TFL-like sequences. Sequences were aligned using ClustalW and analyzed using the Geneious software. Accession numbers of the
DIFFERENTIATION OF AVOCADO BLOSSOM BUDS IN FLORIDA
 Reprinted for private circulation from the Botanical Gazette, Vol. 104, No. 2, December, 1942. DIFFERENTIATION OF AVOCADO BLOSSOM BUDS IN FLORIDA PHILIP C. REECE 1 (WITH THIRTEEN FIGURES) Subtropical Fruit
Reprinted for private circulation from the Botanical Gazette, Vol. 104, No. 2, December, 1942. DIFFERENTIATION OF AVOCADO BLOSSOM BUDS IN FLORIDA PHILIP C. REECE 1 (WITH THIRTEEN FIGURES) Subtropical Fruit
Interactions between jointless and Wild-Type Tomato Tissues during Development of the Pedicel Abscission Zone and the Inflorescence Meristem
 The Plant Cell, Vol. 11, 159 175, February 1999, www.plantcell.org 1999 American Society of Plant Physiologists Interactions between jointless and Wild-Type Tomato Tissues during Development of the Pedicel
The Plant Cell, Vol. 11, 159 175, February 1999, www.plantcell.org 1999 American Society of Plant Physiologists Interactions between jointless and Wild-Type Tomato Tissues during Development of the Pedicel
GLABRA1 and TRIPTYCHON in Arabidopsis
 Development 125, 2283-2289 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV0160 2283 Tissue layer and organ specificity of trichome formation are regulated by GLABRA1 and TRIPTYCHON
Development 125, 2283-2289 (1998) Printed in Great Britain The Company of Biologists Limited 1998 DEV0160 2283 Tissue layer and organ specificity of trichome formation are regulated by GLABRA1 and TRIPTYCHON
Photo and Hormonal Control of Meristem ldentity in the Arabidopsis Flower Mutants apetala2 and apetala 1
 The Plant Cell, Vol. 9, 37-47, Januaty 1997 O 1997 American Society of Plant Physiologists Photo and Hormonal Control of Meristem ldentity in the Arabidopsis Flower Mutants apetala2 and apetala 1 Jack
The Plant Cell, Vol. 9, 37-47, Januaty 1997 O 1997 American Society of Plant Physiologists Photo and Hormonal Control of Meristem ldentity in the Arabidopsis Flower Mutants apetala2 and apetala 1 Jack
Plant Stimuli pp Topic 3: Plant Behaviour Ch. 39. Plant Behavioural Responses. Plant Hormones. Plant Hormones pp
 Topic 3: Plant Behaviour Ch. 39 Plants exist in environments that are constantly changing. Like animals, plants must be able to detect and react to stimuli in the environment. Unlike animals, plants can
Topic 3: Plant Behaviour Ch. 39 Plants exist in environments that are constantly changing. Like animals, plants must be able to detect and react to stimuli in the environment. Unlike animals, plants can
Electromagenetic spectrum
 Light Controls of Plant Development 1 Electromagenetic spectrum 2 Light It is vital for photosynthesis and is also necessary to direct plant growth and development. It acts as a signal to initiate and
Light Controls of Plant Development 1 Electromagenetic spectrum 2 Light It is vital for photosynthesis and is also necessary to direct plant growth and development. It acts as a signal to initiate and
Plant Development. Chapter 31 Part 1
 Plant Development Chapter 31 Part 1 Impacts, Issues Foolish Seedlings, Gorgeous Grapes Gibberellin and other plant hormones control the growth and development of plants environmental cues influence hormone
Plant Development Chapter 31 Part 1 Impacts, Issues Foolish Seedlings, Gorgeous Grapes Gibberellin and other plant hormones control the growth and development of plants environmental cues influence hormone
INFLUENCE OF PHOTOPERIOD ON IMPROVED 'WHITE SIM' CARNATION (DIANTHUS C A R Y O P H Y L L U S L.) BRANCHING AND FLOWERING
 INFLUENCE OF PHOTOPERIOD ON IMPROVED 'WHITE SIM' CARNATION (DIANTHUS C A R Y O P H Y L L U S L.) BRANCHING AND FLOWERING R. D. Heins and H. F. Wilkins Department of Horticultural Science University of
INFLUENCE OF PHOTOPERIOD ON IMPROVED 'WHITE SIM' CARNATION (DIANTHUS C A R Y O P H Y L L U S L.) BRANCHING AND FLOWERING R. D. Heins and H. F. Wilkins Department of Horticultural Science University of
Genetic and Molecular Regulation by DELLA Proteins of Trichome Development in Arabidopsis 1[W][OA]
![Genetic and Molecular Regulation by DELLA Proteins of Trichome Development in Arabidopsis 1[W][OA] Genetic and Molecular Regulation by DELLA Proteins of Trichome Development in Arabidopsis 1[W][OA]](/thumbs/94/120779971.jpg) Genetic and Molecular Regulation by DELLA Proteins of Trichome Development in Arabidopsis 1[W][OA] Yinbo Gan, Hao Yu, Jinrong Peng, and Pierre Broun* Centre for Novel Agricultural Products, Department
Genetic and Molecular Regulation by DELLA Proteins of Trichome Development in Arabidopsis 1[W][OA] Yinbo Gan, Hao Yu, Jinrong Peng, and Pierre Broun* Centre for Novel Agricultural Products, Department
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
SUPPLEMENTARY INFORMATION doi:10.1038/nature12791 Supplementary Figure 1 (1/3) WWW.NATURE.COM/NATURE 1 RESEARCH SUPPLEMENTARY INFORMATION Supplementary Figure 1 (2/3) 2 WWW.NATURE.COM/NATURE SUPPLEMENTARY
Actions of auxin. Hormones: communicating with chemicals History: Discovery of a growth substance (hormone- auxin)
 Hormones: communicating with chemicals History- discovery of plant hormone. Auxin Concepts of hormones Auxin levels are regulated by synthesis/degradation, transport, compartmentation, conjugation. Polar
Hormones: communicating with chemicals History- discovery of plant hormone. Auxin Concepts of hormones Auxin levels are regulated by synthesis/degradation, transport, compartmentation, conjugation. Polar
Topic 15. The Shoot System
 Topic 15. The Shoot System Introduction. This is the second of two lab topics that focus on the three plant organs (root, stem, leaf). In these labs we want you to recognize how tissues are organized in
Topic 15. The Shoot System Introduction. This is the second of two lab topics that focus on the three plant organs (root, stem, leaf). In these labs we want you to recognize how tissues are organized in
Germinating sunflowers, turgor and nutation. From:
 Germinating sunflowers, turgor and nutation From: http://sunflower.bio.indiana.edu/~rhangart/plantmotion Nutation is Sunflower due to unequal Germination rates of growth in that continuous is dependent
Germinating sunflowers, turgor and nutation From: http://sunflower.bio.indiana.edu/~rhangart/plantmotion Nutation is Sunflower due to unequal Germination rates of growth in that continuous is dependent
Different Roles of Flowering-Time Genes in the Activation of Floral lnitiation Genes in Arabidopsis
 The Plant Cell, Vol. 9, 1921-1934, November 1997 O 1997 American Society of Plant Physiologists RESEARCH ARTICLE Different Roles of Flowering-Time Genes in the Activation of Floral lnitiation Genes in
The Plant Cell, Vol. 9, 1921-1934, November 1997 O 1997 American Society of Plant Physiologists RESEARCH ARTICLE Different Roles of Flowering-Time Genes in the Activation of Floral lnitiation Genes in
Reproduction, Seeds and Propagation
 Reproduction, Seeds and Propagation Diploid (2n) somatic cell Two diploid (2n) somatic cells Telophase Anaphase Metaphase Prophase I One pair of homologous chromosomes (homologues) II Homologues condense
Reproduction, Seeds and Propagation Diploid (2n) somatic cell Two diploid (2n) somatic cells Telophase Anaphase Metaphase Prophase I One pair of homologous chromosomes (homologues) II Homologues condense
Identification of multiple stages in the conversion of maize meristems from vegetative to floral development
 Development 112, 891-898 (1991) Printed in Great Britain The Company of Biologists Limited 1991 891 Identification of multiple stages in the conversion of maize meristems from vegetative to floral development
Development 112, 891-898 (1991) Printed in Great Britain The Company of Biologists Limited 1991 891 Identification of multiple stages in the conversion of maize meristems from vegetative to floral development
Lab Exercise 4: Primary Growth and Tissues in Stems
 Lab Exercise 4: Primary Growth and Tissues in Stems Tissues of the plant body can be classified in a variety of ways: functionally (based on the tissue function, e.g. vascular tissue ), morphologically
Lab Exercise 4: Primary Growth and Tissues in Stems Tissues of the plant body can be classified in a variety of ways: functionally (based on the tissue function, e.g. vascular tissue ), morphologically
Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering
 Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering by Valverde et. Al Published in Science 2004 Presented by Boyana Grigorova CBMG 688R Feb. 12, 2007 Circadian Rhythms: The Clock Within
Photoreceptor Regulation of Constans Protein in Photoperiodic Flowering by Valverde et. Al Published in Science 2004 Presented by Boyana Grigorova CBMG 688R Feb. 12, 2007 Circadian Rhythms: The Clock Within
Pea Compound Leaf Architecture Is Regulated by Interactions among the Genes UNIFOLIATA, COCHLEATA, AFILA, and TENDRIL-LESS
 The Plant Cell, Vol. 12, 1279 1294, August 2000, www.plantcell.org 2000 American Society of Plant Physiologists Pea Compound Leaf Architecture Is Regulated by Interactions among the Genes UNIFOLIATA, COCHLEATA,
The Plant Cell, Vol. 12, 1279 1294, August 2000, www.plantcell.org 2000 American Society of Plant Physiologists Pea Compound Leaf Architecture Is Regulated by Interactions among the Genes UNIFOLIATA, COCHLEATA,
Crop Development and Components of Seed Yield. Thomas G Chastain CSS 460/560 Seed Production
 Crop Development and Components of Seed Yield Thomas G Chastain CSS 460/560 Seed Production White clover seed field Seed Yield Seed yield results from the interaction of the following factors: 1. Genetic
Crop Development and Components of Seed Yield Thomas G Chastain CSS 460/560 Seed Production White clover seed field Seed Yield Seed yield results from the interaction of the following factors: 1. Genetic
Plant Anatomy and Tissue Structures
 Plant Anatomy and Tissue Structures The Two Major Plant Systems Reproductive shoot (flower) Terminal bud Node Internode Angiosperm plants have threse major organs: Roots Stems Leaves & Flowers Terminal
Plant Anatomy and Tissue Structures The Two Major Plant Systems Reproductive shoot (flower) Terminal bud Node Internode Angiosperm plants have threse major organs: Roots Stems Leaves & Flowers Terminal
Plant Juvenility Text Pages: 15 18,
 45 Plant Juvenility Text Pages: 15 18, 613 619. Objectives: 1. Be able to describe and explain terms related to plant aging. 2. Be able to explain how a woody plant contains tissue of different ontogenetic
45 Plant Juvenility Text Pages: 15 18, 613 619. Objectives: 1. Be able to describe and explain terms related to plant aging. 2. Be able to explain how a woody plant contains tissue of different ontogenetic
Plant Propagation PLS 3221/5222
 Plant Propagation PLS 3221/5222 Dr. Sandra Wilson Dr. Mack Thetford Chapter 2 Introduction to the Biology of Plant Propagation -A review- 1 5. Plant Hormones and Plant development Phytohormones Nt Naturally
Plant Propagation PLS 3221/5222 Dr. Sandra Wilson Dr. Mack Thetford Chapter 2 Introduction to the Biology of Plant Propagation -A review- 1 5. Plant Hormones and Plant development Phytohormones Nt Naturally
Studies on the Light Controlling Flower Initiation of Pharbitis Nil. VI. Effect of Natural Twilight. by Atsushi TAKIMOTO* and Katsuhiko IKEVA*
 Studies on the Light Controlling Flower Initiation of Pharbitis Nil. Received September 9, 1959 VI. Effect of Natural Twilight by Atsushi TAKIMOTO* and Katsuhiko IKEVA* Many investigators consider that
Studies on the Light Controlling Flower Initiation of Pharbitis Nil. Received September 9, 1959 VI. Effect of Natural Twilight by Atsushi TAKIMOTO* and Katsuhiko IKEVA* Many investigators consider that
Plants. Tissues, Organs, and Systems
 Plants Tissues, Organs, and Systems Meristematic cells Specialized cells that are responsible for producing specialized cells, they produce three types of tissue in the body of a plant. Meristematic Cells
Plants Tissues, Organs, and Systems Meristematic cells Specialized cells that are responsible for producing specialized cells, they produce three types of tissue in the body of a plant. Meristematic Cells
Life Science Chapter 11 SEED PLANTS PART 2
 Life Science Chapter 11 SEED PLANTS PART 2 Advanced Seed Producing Advanced Seed Producing Vascular Plants Class: Gymnospermae Class: Angiospermae» Subclass: Monocotyledoneae» Subclass: Dicotyledoneae
Life Science Chapter 11 SEED PLANTS PART 2 Advanced Seed Producing Advanced Seed Producing Vascular Plants Class: Gymnospermae Class: Angiospermae» Subclass: Monocotyledoneae» Subclass: Dicotyledoneae
Topic 14. The Root System. II. Anatomy of an Actively Growing Root Tip
 Topic 14. The Root System Introduction. This is the first of two lab topics that focus on the three plant organs (root, stem, leaf). In these labs we want you to recognize how tissues are organized in
Topic 14. The Root System Introduction. This is the first of two lab topics that focus on the three plant organs (root, stem, leaf). In these labs we want you to recognize how tissues are organized in
Supplemental Data. Perrella et al. (2013). Plant Cell /tpc
 Intensity Intensity Intensity Intensity Intensity Intensity 150 50 150 0 10 20 50 C 150 0 10 20 50 D 0 10 20 Distance (μm) 50 20 40 E 50 F 0 10 20 50 0 15 30 Distance (μm) Supplemental Figure 1: Co-localization
Intensity Intensity Intensity Intensity Intensity Intensity 150 50 150 0 10 20 50 C 150 0 10 20 50 D 0 10 20 Distance (μm) 50 20 40 E 50 F 0 10 20 50 0 15 30 Distance (μm) Supplemental Figure 1: Co-localization
THE ROLE OF THE PHYTOCHROME B PHOTORECEPTOR IN THE REGULATION OF PHOTOPERIODIC FLOWERING. AnitaHajdu. Thesis of the Ph.D.
 THE ROLE OF THE PHYTOCHROME B PHOTORECEPTOR IN THE REGULATION OF PHOTOPERIODIC FLOWERING AnitaHajdu Thesis of the Ph.D. dissertation Supervisor: Dr. LászlóKozma-Bognár - senior research associate Doctoral
THE ROLE OF THE PHYTOCHROME B PHOTORECEPTOR IN THE REGULATION OF PHOTOPERIODIC FLOWERING AnitaHajdu Thesis of the Ph.D. dissertation Supervisor: Dr. LászlóKozma-Bognár - senior research associate Doctoral
A. Stimulus Response:
 Plant Hormones A. Stimulus Response: A house plant on a windowsill grows light. If you rotate the plant, it reorients its growth until its leaves face the window again. The growth of a shoot towards light
Plant Hormones A. Stimulus Response: A house plant on a windowsill grows light. If you rotate the plant, it reorients its growth until its leaves face the window again. The growth of a shoot towards light
Integrative Biology 200A "PRINCIPLES OF PHYLOGENETICS" Spring 2012 University of California, Berkeley
 Integrative Biology 200A "PRINCIPLES OF PHYLOGENETICS" Spring 2012 University of California, Berkeley B.D. Mishler Feb. 7, 2012. Morphological data IV -- ontogeny & structure of plants The last frontier
Integrative Biology 200A "PRINCIPLES OF PHYLOGENETICS" Spring 2012 University of California, Berkeley B.D. Mishler Feb. 7, 2012. Morphological data IV -- ontogeny & structure of plants The last frontier
* School of Biological Sciences, Carslaw Building, University of Sydney, Sydney, N.S.W By VERONICA H. K. Low*
 Aust. J. biol. Sci., 1971, 24, 187-95 * School of Biological Sciences, Carslaw Building, University of Sydney, Sydney, N.S.W. 2006.. NTRODUCTON A detailed survey of the morphological and anatomical effects
Aust. J. biol. Sci., 1971, 24, 187-95 * School of Biological Sciences, Carslaw Building, University of Sydney, Sydney, N.S.W. 2006.. NTRODUCTON A detailed survey of the morphological and anatomical effects
Ulrike Folkers 1, Jürgen Berger 2 and Martin Hülskamp 1, * SUMMARY
 Development 124, 3779-3786 (1997) Printed in Great Britain The Company of Biologists Limited 1997 DEV0088 3779 Cell morphogenesis of trichomes in Arabidopsis: differential control of primary and secondary
Development 124, 3779-3786 (1997) Printed in Great Britain The Company of Biologists Limited 1997 DEV0088 3779 Cell morphogenesis of trichomes in Arabidopsis: differential control of primary and secondary
Interaction between GA and Ethrel in Inducing Female Flowers in Jatropha Curcas
 International Journal of Biotechnology and Bioengineering Research. ISSN 2231-1238, Volume 4, Number 5 (2013), pp. 465-472 Research India Publications http://www.ripublication.com/ ijbbr.htm Interaction
International Journal of Biotechnology and Bioengineering Research. ISSN 2231-1238, Volume 4, Number 5 (2013), pp. 465-472 Research India Publications http://www.ripublication.com/ ijbbr.htm Interaction
SCANNING ELECTRON MICROSCOPY OF FLORAL INITIATION AND DEVELOPMENTAL STAGES IN SWEET CHERRY (PRUNUS AVIUM) UNDER WATER DEFICITS HAKAN ENGIN
 Bangladesh J. Bot. 37(1): 15-19, 2008 (June) SCANNING ELECTRON MICROSCOPY OF FLORAL INITIATION AND DEVELOPMENTAL STAGES IN SWEET CHERRY (PRUNUS AVIUM) UNDER WATER DEFICITS HAKAN ENGIN Department of Horticulture,
Bangladesh J. Bot. 37(1): 15-19, 2008 (June) SCANNING ELECTRON MICROSCOPY OF FLORAL INITIATION AND DEVELOPMENTAL STAGES IN SWEET CHERRY (PRUNUS AVIUM) UNDER WATER DEFICITS HAKAN ENGIN Department of Horticulture,
The Coch gene controls the subsequent differentiation of pea axial meristems into lateral structures
 The Coch gene controls the subsequent differentiation of pea axial meristems into lateral structures Rozov, S.M. 1, Institute of Cytology and Genetics SD RAS, Novosibirsk, Russia Voroshilova, V.A. 2, 2
The Coch gene controls the subsequent differentiation of pea axial meristems into lateral structures Rozov, S.M. 1, Institute of Cytology and Genetics SD RAS, Novosibirsk, Russia Voroshilova, V.A. 2, 2
Plant hormones: a. produced in many parts of the plant b. have many functions
 Plant hormones: a. produced in many parts of the plant b. have many functions Illustrated with 4 plant hormones: Gibberellins Auxin Cytokinins Ethylene Gibberellins Gibberellins illustrate how plant hormones
Plant hormones: a. produced in many parts of the plant b. have many functions Illustrated with 4 plant hormones: Gibberellins Auxin Cytokinins Ethylene Gibberellins Gibberellins illustrate how plant hormones
APICAL DOMINANCE IN TUBERS OF POTATO (SOLANUM TUBEROSUM L. )
 MAURI ORA, 1976, 4: 53-59 53 APICAL DOMINANCE IN TUBERS OF POTATO (SOLANUM TUBEROSUM L. ) N. LALLU and J.A. McWHA Department of Botany, University of Canterbury, Christchurch, New Zealand. ABSTRACT Apical
MAURI ORA, 1976, 4: 53-59 53 APICAL DOMINANCE IN TUBERS OF POTATO (SOLANUM TUBEROSUM L. ) N. LALLU and J.A. McWHA Department of Botany, University of Canterbury, Christchurch, New Zealand. ABSTRACT Apical
Biological Roles of Cytokinins
 Direct Control of Shoot Meristem Activity by a Cytokinin-Activating Enzyme By Kurakawa et. Al. Published in Nature Presented by Boyana Grigorova Biological Roles of Cytokinins Cytokinins are positive regulators
Direct Control of Shoot Meristem Activity by a Cytokinin-Activating Enzyme By Kurakawa et. Al. Published in Nature Presented by Boyana Grigorova Biological Roles of Cytokinins Cytokinins are positive regulators
Reflexions, le site de vulgarisation de l'université de Liège
 When tomatoes flower 3/13/12 Understanding the mechanisms responsible for tomato plant flowering will enable new selection procedures to be developed in order to obtain even more productive varieties.
When tomatoes flower 3/13/12 Understanding the mechanisms responsible for tomato plant flowering will enable new selection procedures to be developed in order to obtain even more productive varieties.
The REVOLUTA gene is necessary for apical meristem development and for limiting cell divisions in the leaves and stems of Arabidopsis thaliana
 Development 121, 2723-2735 (1995) Printed in Great Britain The Company of Biologists Limited 1995 2723 The REVOLUTA gene is necessary for apical meristem development and for limiting cell divisions in
Development 121, 2723-2735 (1995) Printed in Great Britain The Company of Biologists Limited 1995 2723 The REVOLUTA gene is necessary for apical meristem development and for limiting cell divisions in
Introduction. Populus trichocarpa TORR. and GRAY. By M. G. R. CANNELL and S. C. WILLETT
 Shoot Growth Phenology, Dry Matter Distribution and Root: Shoot Ratios of Provenances of Populus trichocarpa, Picea sitchensis and Pinus contorta growing in Scotland By M. G. R. CANNELL and S. C. WILLETT
Shoot Growth Phenology, Dry Matter Distribution and Root: Shoot Ratios of Provenances of Populus trichocarpa, Picea sitchensis and Pinus contorta growing in Scotland By M. G. R. CANNELL and S. C. WILLETT
Plant. Responses and Adaptations. Plant Hormones. Plant Hormones. Auxins. Auxins. Hormones tell plants:
 Plant Responses and Adaptations Plant Hormones Hormone - a substance that is produced in 1 part of an organism & affects another part of the same individual (a chemical messenger) Plant hormones are chemical
Plant Responses and Adaptations Plant Hormones Hormone - a substance that is produced in 1 part of an organism & affects another part of the same individual (a chemical messenger) Plant hormones are chemical
Hairy s Inheritance: Investigating Variation, Selection, and Evolution with Wisconsin Fast Plants
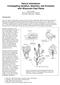 Introduction Hairy s Inheritance: Investigating Variation, Selection, and Evolution with Wisconsin Fast Plants Daniel Lauffer Wisconsin Fast Plants Program University of Wisconsin - Madison Since the dawn
Introduction Hairy s Inheritance: Investigating Variation, Selection, and Evolution with Wisconsin Fast Plants Daniel Lauffer Wisconsin Fast Plants Program University of Wisconsin - Madison Since the dawn
TOPIC 9.3 GROWTH IN PLANTS
 TOPIC 9.3 GROWTH IN PLANTS 9.3 A Growth INTRO http://cdn2.hubspot.net/hubfs/18130/social-suggested-images/plant_growing.jpeg IB BIO 9.3 3 In general, plants are able to grow indeterminately. This means
TOPIC 9.3 GROWTH IN PLANTS 9.3 A Growth INTRO http://cdn2.hubspot.net/hubfs/18130/social-suggested-images/plant_growing.jpeg IB BIO 9.3 3 In general, plants are able to grow indeterminately. This means
The significance of bolting and floral transitions as indicators of reproductive phase change in Arabidopsis
 Journal of Experimental Botany, Vol. 60, No. 12, pp. 3367 3377, 2009 doi:10.1093/jxb/erp173 Advance Access publication 5 June, 2009 RESEARCH PAPER The significance of bolting and floral transitions as
Journal of Experimental Botany, Vol. 60, No. 12, pp. 3367 3377, 2009 doi:10.1093/jxb/erp173 Advance Access publication 5 June, 2009 RESEARCH PAPER The significance of bolting and floral transitions as
Class XI Chapter 15 Plant Growth and Development Biology
 Question 1: Define growth, differentiation, development, dedifferentiation, redifferentiation, determinate growth, meristem and growth rate. (a) Growth It is an irreversible and permanent process, accomplished
Question 1: Define growth, differentiation, development, dedifferentiation, redifferentiation, determinate growth, meristem and growth rate. (a) Growth It is an irreversible and permanent process, accomplished
Horticulture 201H Spring, 2002 Exam 2 Name:
 Horticulture 201H Spring, 2002 Exam 2 Name: Section 1. In the space to the left of the statements below, write the word(s) that best fit the definition or description. (20 pts) Vegetative reproduction
Horticulture 201H Spring, 2002 Exam 2 Name: Section 1. In the space to the left of the statements below, write the word(s) that best fit the definition or description. (20 pts) Vegetative reproduction
Class XI Chapter 15 Plant Growth and Development Biology
 Question 1: Define growth, differentiation, development, dedifferentiation, redifferentiation, determinate growth, meristem and growth rate. (a) Growth It is an irreversible and permanent process, accomplished
Question 1: Define growth, differentiation, development, dedifferentiation, redifferentiation, determinate growth, meristem and growth rate. (a) Growth It is an irreversible and permanent process, accomplished
PLANTS FORM AND FUNCTION PLANT MORPHOLOGY PART I: BASIC MORPHOLOGY. Plant Form & Function Activity #1 page 1
 AP BIOLOGY PLANTS FORM AND FUNCTION ACTIVITY #1 NAME DATE HOUR PLANT MORPHOLOGY PART I: BASIC MORPHOLOGY Plant Form & Function Activity #1 page 1 PART II: ROOTS 1. Examine the examples of the two root
AP BIOLOGY PLANTS FORM AND FUNCTION ACTIVITY #1 NAME DATE HOUR PLANT MORPHOLOGY PART I: BASIC MORPHOLOGY Plant Form & Function Activity #1 page 1 PART II: ROOTS 1. Examine the examples of the two root
Plant Growth and Development
 1. Define plasticity. Give an example? A: Plant Growth and Development The ability of the plants to follow different pathways in response to the environment or phases of life to form different kinds of
1. Define plasticity. Give an example? A: Plant Growth and Development The ability of the plants to follow different pathways in response to the environment or phases of life to form different kinds of
AP Biology Summer 2017
 Directions: Questions 1 and 2 are long free response questions that require about 22 minutes to answer and are worth 10 points each. Questions 3-6 are short free- response questions that require about
Directions: Questions 1 and 2 are long free response questions that require about 22 minutes to answer and are worth 10 points each. Questions 3-6 are short free- response questions that require about
EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA
 EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA Item Type text; Electronic Thesis Authors Bergstrand, Lauren Janel Publisher The University of Arizona. Rights Copyright is held by the author. Digital
EXPRESSION OF THE FIS2 PROMOTER IN ARABIDOPSIS THALIANA Item Type text; Electronic Thesis Authors Bergstrand, Lauren Janel Publisher The University of Arizona. Rights Copyright is held by the author. Digital
NATURAL VARIATION IN THE CYTOKININ METABOLIC NETWORK IN ARABIDOPSIS THALIANA
 NATURAL VARIATION IN THE CYTOKININ METABOLIC NETWORK IN ARABIDOPSIS THALIANA PŘÍRODNÍ VARIACE METABOLISMU CYTOKININŮ U ARABIDOPSIS THALIANA Samsonová Z. 1, 2, 3, Kuklová A. 1, 2, Mazura P. 1, 2, Rotková
NATURAL VARIATION IN THE CYTOKININ METABOLIC NETWORK IN ARABIDOPSIS THALIANA PŘÍRODNÍ VARIACE METABOLISMU CYTOKININŮ U ARABIDOPSIS THALIANA Samsonová Z. 1, 2, 3, Kuklová A. 1, 2, Mazura P. 1, 2, Rotková
Seed Development and Yield Components. Thomas G Chastain CROP 460/560 Seed Production
 Seed Development and Yield Components Thomas G Chastain CROP 460/560 Seed Production The Seed The zygote develops into the embryo which contains a shoot (covered by the coleoptile) and a root (radicle).
Seed Development and Yield Components Thomas G Chastain CROP 460/560 Seed Production The Seed The zygote develops into the embryo which contains a shoot (covered by the coleoptile) and a root (radicle).
Plants are sessile. 10d-17/giraffe-grazing.jpg
 Plants are sessile www.mccullagh.org/db9/ 10d-17/giraffe-grazing.jpg Plants have distinct requirements because of their sessile nature Organism-level requirements Must adjust to environment at given location
Plants are sessile www.mccullagh.org/db9/ 10d-17/giraffe-grazing.jpg Plants have distinct requirements because of their sessile nature Organism-level requirements Must adjust to environment at given location
can affect division, elongation, & differentiation of cells to another region of plant where they have an effect
 Note that the following is a rudimentary outline of the class lecture; it does not contain everything discussed in class. Plant Hormones Plant Hormones compounds regulators growth or can affect division,
Note that the following is a rudimentary outline of the class lecture; it does not contain everything discussed in class. Plant Hormones Plant Hormones compounds regulators growth or can affect division,
Chapter 35: Plant Structure, Growth and Development - No two Plants Are Alike Plant structure
 Chapter 35: Plant Structure, Growth and Development - No two Plants Are Alike Plant structure Systems Root and Shoot system Organs Roots, Stems, Leaves Tissues Dermal, Vascular, Ground Cells parencyma,
Chapter 35: Plant Structure, Growth and Development - No two Plants Are Alike Plant structure Systems Root and Shoot system Organs Roots, Stems, Leaves Tissues Dermal, Vascular, Ground Cells parencyma,
A MicroRNA as a Translational Repressor of APETALA2 in Arabidopsis Flower Development
 A MicroRNA as a Translational Repressor of APETALA2 in Arabidopsis Flower Development Xuemei Chen Waksman Institute, Rutgers University, Piscataway, NJ 08854, USA. E-mail: xuemei@waksman.rutgers.edu Plant
A MicroRNA as a Translational Repressor of APETALA2 in Arabidopsis Flower Development Xuemei Chen Waksman Institute, Rutgers University, Piscataway, NJ 08854, USA. E-mail: xuemei@waksman.rutgers.edu Plant
