Odor Perception on the Two Sides of the Brain: Consistency Despite Randomness
|
|
|
- Egbert Roberts
- 5 years ago
- Views:
Transcription
1 Report Odor Perception on the Two Sides of the Brain: Consistency Despite Randomness Highlights d A random model predicts observed preservation of correlations in piriform responses d d The model supports consistent agreement about odor quality among individuals Consistent generalization may require the full complement of piriform neurons Authors Evan S. Schaffer, Dan D. Stettler, Daniel Kato, Gloria B. Choi, Richard Axel, L.F. Abbott Correspondence ess229@columbia.edu In Brief Random connectivity from the olfactory bulb to piriform implies that the representation of odors will differ in different cortices. Schaffer et al. demonstrate in a model that consistent agreement about odor quality occurs but requires the full complement of piriform neurons. Schaffer et al., 28, Neuron 98, May 6, 28 ª 28 Elsevier Inc.
2 Neuron Report Odor Perception on the Two Sides of the Brain: Consistency Despite Randomness Evan S. Schaffer,,7, * Dan D. Stettler,,5 Daniel Kato, Gloria B. Choi,,6 Richard Axel,,2,3 and L.F. Abbott,4 Mortimer B. Zuckerman Mind Brain Behavior Institute, Department of Neuroscience, Columbia University, New York, NY 27, USA 2 Department of Biochemistry and Molecular Biophysics, Columbia University, New York, NY 32, USA 3 Howard Hughes Medical Institute, Columbia University, New York, NY 32, USA 4 Department of Physiology and Cellular Biophysics, Columbia University, New York, NY 32, USA 5 Present address: Kallyope, Inc., 43 East 29 th Street, th floor, New York, NY 6, USA 6 Present address: McGovern Institute for Brain Research, Department of Brain and Cognitive Sciences, Massachusetts Institute of Technology, Cambridge, MA 239, USA 7 Lead Contact *Correspondence: ess229@columbia.edu SUMMARY Neurons in piriform cortex receive input from a random collection of glomeruli, resulting in odor representations that lack the stereotypic organization of the olfactory bulb. We have performed in vivo optical imaging and mathematical modeling to demonstrate that correlations are retained in the transformation from bulb to piriform cortex, a feature essential for generalization across odors. Random connectivity also implies that the piriform representation of a given odor will differ among different individuals and across brain hemispheres in a single individual. We show that these different representations can nevertheless support consistent agreement about odor quality across a range of odors. Our model also demonstrates that, whereas odor discrimination and categorization require far fewer neurons than reside in piriform cortex, consistent generalization may require the full complement of piriform neurons. INTRODUCTION Olfactory perception involves both odor discrimination and generalization. Discrimination relies on the detection of differences between odors, whereas generalization requires the identification of similarities and dissimilarities across odors. The ability to generalize is vital to performance in any olfactory task because natural variability implies that the same stimulus is never experienced twice. Perceptual consistency across odors requires that generalization be similar among individuals. We thus explore whether the unstructured representation of odors in piriform cortex can support consistent generalization. Individual olfactory sensory neurons in the mouse express one of,5 receptor genes (Buck and Axel, 99; Godfrey et al., 24; Zhang and Firestein, 22). Neurons expressing a given receptor project with precision to two spatially invariant glomeruli within the olfactory bulb (Mombaerts et al., 996; Ressler et al., 993, 994; Vassar et al., 993, 994). Odors elicit distributed neural activity in the sensory epithelium that is transformed in the olfactory bulb, where each odor evokes a distinct spatial pattern of glomerular activity (Davison and Katz, 27; Igarashi and Mori, 25; Ma et al., 22; Meister and Bonhoeffer, 2; Soucy et al., 29; Takahashi et al., 24). A second transformation occurs in the piriform cortex, where individual odors activate unique ensembles of neurons that lack discernable spatial patterning (Illig and Haberly, 23; Iurilli and Datta, 27; Poo and Isaacson, 29; Rennaker et al., 27; Stettler and Axel, 29; Sugai et al., 25; Zhan and Luo, 2). Representations of individual odors are distributed across the entire piriform with no apparent large-scale (Stettler and Axel, 29) or local spatial structure (Figure S). One model consistent with both the anatomy and the physiology assumes that each piriform neuron samples a random combination of glomerular inputs (Choi et al., 2; Davison and Ehlers, 2; Ghosh et al., 2; Miyamichi et al., 2; Sosulski et al., 2). Random connectivity implies that the piriform representation of a given odor will differ among different individuals and across brain hemispheres in a single individual. Does random connectivity from the olfactory bulb to piriform cortex support generalization and allow shared experiences to align the perception of odor quality in different individuals across a wide range of odors? We have combined mathematical modeling with analysis of in vivo optical imaging of odor responses in piriform cortex and find that many properties of the piriform odor representation are in good accord with predictions of a model with random input. Consistent with previous models (Babadi and Sompolinsky, 24; Barak et al., 23; Cho and Saul, 29; Litwin-Kumar et al., 27), we observe that piriform representations simulated with random input are less correlated than the bulb representations but maintain sufficient correlation between odors to support generalization. We consider how individuals with different piriform odor representations can agree consistently about odor quality across a range of odors and how conflicts are avoided between the two sides of a single individual s brain. Our model demonstrates that whereas odor discrimination and categorization require far fewer neurons than reside in piriform cortex (Babadi and Sompolinsky, 24), consistent generalization requires the full complement of piriform neurons. 736 Neuron 98, , May 6, 28 ª 28 Elsevier Inc.
3 A 3% RESULTS B 7% Piriform Neuron Piriform Correlation Glomerulus nonclass Nonclass 3% overlap 7% overlap.5 Odor.5 C D Model.2. Data.3 Observed Frequency Observed Frequency Chance Frequency..2.3 Chance Frequency F Fraction of Odors.8 Prob. Density E Prob. Density Glomerular Correlation Fraction of Odors Figure. The Transformation from Model Bulb to Model Piriform Partially Preserves Stimulus Similarity (A) The representation of a model odor in the bulb is transformed through random connections to a representation in the piriform. Example model odors in the bulb (top) and piriform (bottom), with three different values of the excess overlap parameter f: nonclass odors (f =, black), slightly overlapping odors (f = :3, blue), and very overlapping odors (f = :7, green). For visual clarity, just 5 glomeruli and 5 piriform neurons are shown. Color scale is normalized; white, max response; red, weak response; black, no response. (B) Correlation in piriform representation versus correlation in glomerulus representation. Each dot corresponds to the correlation coefficient between two odors with the same level of overlap (f = :3 or f = :7), color-coded according to the conventions in (A). For reference, the full spectrum of values of f ranging from to is shown in gray. Distributions of correlation in the glomeruli and in piriform are shown along their respective axes. (C) Observed probability of a piriform cell responding to two odors in the model versus the expected probability if odor representations were statistically independent (f = :, f = :3, and f = :7 shown in black, blue, and green, respectively). In order to recapitulate the variability in representation sparseness observed in vivo, model odors were generated with a range of sparseness values from Sx = :5 to Sx = :2. (D) Same as (C), but for in vivo odor responses (average ± SEM). (E) Class selectivity of a neuron is quantified as the difference in that neuron s mean response to class odors versus nonclass odors. Shown is the probability distribution over the fraction of class (green) and nonclass (black) odors eliciting a response for model neurons in the 4th percentile for class selectivity. (F) Same as (E), but for a class defined by an arbitrary collection of odors not necessarily activating similar glomeruli. Selected class members in gray and nonclass members in black. Piriform Responses to Odor Pairs Exhibit Correlations Predicted by a Randomly Connected Model Similar odors evoke correlated patterns of activity in the olfactory bulb (Davison and Katz, 27; Igarashi and Mori, 25; Ma et al., 22; Meister and Bonhoeffer, 2; Soucy et al., 29; Takahashi et al., 24). Random wiring from the bulb to piriform might suggest that these correlations would be absent in cortex. However, a number of theoretical studies have shown that correlations in a set of inputs are reduced, but not eliminated, when activity is transmitted through random synaptic connections (Babadi and Sompolinsky, 24; Barak et al., 23; Cho and Saul, 29). Indeed, piriform responses to pairs of odors activating similar glomeruli can show considerable overlap (Figure D; octanal and hexanal, 24% overlap in piriform [Stettler and Axel, 29]; 6% 7% overlap in bulb [Meister and Bonhoeffer, 2; Igarashi and Mori 25]; see also 26% in bulb [Ma et al., 22]). To explore the implications of random connectivity, we constructed a model in which the connectivity between the olfactory bulb and piriform cortex is random (STAR Methods). The model has an input layer of Nx = ; glomeruli and a cortical layer with Ny neurons. Model odors activate a sparse ensemble of Sx Nx glomeruli, with sparseness Sx = :, unless otherwise noted. Bulb activity both excites and inhibits the cortical layer in a balanced manner, so that the average bulb-derived input across the cortex is zero. Connectivity from bulb to piriform is sparse ðsc = :2Þ, so each piriform neuron receives input from Sc Nx = 2 random glomeruli, and approximately 2 of these are activated by a given odor. Model piriform neuron responses are a threshold-linear function of the sum of their inputs with the threshold chosen so that, on average, Sy Ny cortical neurons respond to an odor with Sy = :6; matching the sparseness of responses observed in vivo. Firing rates of the active glomeruli are chosen from a lognormal distribution. We defined classes of model odors with a mean shared fraction f of active glomeruli (STAR Methods), and we focus our analysis on three sets of odors (Figure A), defined by f = (nonclass), f = :3 (weak class), and f = :7 (strong class). Correlations between simulated piriform activities for odor pairs are related to but smaller than correlations of glomerular activities (Figure B), in agreement with previous results (Babadi and Sompolinsky, 24; Cho and Saul, 29). The shape of this curve follows from the observation that sparse activity patterns can more faithfully preserve positive correlations than negative correlations. Whereas input correlations close to tend to be largely preserved, negative input correlations are transformed into piriform correlations near zero because uncorrelated sparse representations have very little overlap, leaving minimal dynamic range for further anticorrelation. Consequently, the curve relating input correlation to output correlation has a shallow slope for low input correlation. Comparison of model results with data is facilitated by considering the fraction of neurons responding to pairs of odors, rather than the correlation. The fraction of neurons responding to pairs of simulated odors, drawn from all three classes, exceeds the value expected from an uncorrelated odor representation Neuron 98, , May 6,
4 expected for independent representations respond to odor pairs that do not share active glomeruli (Figure C). This correlation arises from common glomerular inputs that occur by chance despite random connectivity (see also Litwin-Kumar et al., 27). The model results make two predictions about correlations in the piriform representations of odor pairs. First, the average correlation between molecularly unrelated odor pairs should be positive, not zero. Second, odors that activate similar sets of glomeruli should evoke correlated representations in piriform, but these correlations should be smaller than in the bulb. Twophoton calcium imaging of layer 2 of piriform cortex (STAR Methods) reveals that the fractions of neurons responding to odor pairs (Figure D) are consistent with the model (Figure C). Most pairs produce larger fractional responses than would be expected for statistically independent activity (p <.2, Wilcoxon signed-rank test). In our model, correlations in the bulb are the major source of correlations in piriform. Adequate data from imaging of the response to the same odor pairs in both bulb and piriform are unavailable. Therefore, we cannot at present experimentally compare correlations across these two olfactory areas. Figure 2. Agreement between Readout Units Requires a Large Piriform (A) Response of a readout unit versus the response of a second readout connected to an independent set of piriform neurons, either before training (top) or after training to a single odor (bottom), for a panel of odors with 7%, with 3%, or at chance levels of overlap with the trained odor, color coded as in Figure. Gray boxes denote the regions in which the readouts do not agree for q = :5 and f = :7. (B) Scaling with N y of readout agreement (with f = ) versus other measures of readout performance: readout agreement with a threshold of q = :5 (brown) or q = :9 (orange), readout correlation (black), SNR (gray), and accuracy (magenta). All quantities except SNR are defined from to (Max[SNR] = 3.4). We normalize SNR such that the minimum value is and the maximum value is to enable comparison to other quantities. (C) Comparison with experimental data from the Drosophila mushroom body. The correlation between two model MBONs as a function of the number of inputs they receive (black). Published data (Hige et al., 25b) showing the correlation across odors in three conjugate pairs of MBONs versus the number of KCs that innervate each MBON (gray; mean ± SEM). (D) Readout agreement is smaller than readout correlation (top), while readout accuracy is greater than normalized readout SNR (bottom). (E) Intuition for why readout agreement is smaller than readout correlation. As the correlation between two readouts grows, their joint probability distribution becomes elongated (gray), but even when this distribution is very elongated, an appreciable portion remains in the off-diagonal quadrants (orange). (Figure C). For independent representations, the fraction of cells responding to both odor A and odor B would be p A p B, where p A and p B are the probabilities for responses to A or B alone. For simulated odors that activate shared sets of glomeruli (Figure C), this is a direct result of input-derived correlations maintained despite random connectivity (Babadi and Sompolinsky, 24; Cho and Saul, 29). In addition, more neurons than Random Connectivity Can Produce Odor-Class Selective Piriform Neurons We next asked whether piriform neurons that respond selectively to a set of similar odors can arise from random input wiring. We define class selectivity as the difference between the mean responses of a neuron to class and nonclass odors and then ranked the neurons in our model according to this selectivity. We illustrate the results by showing the probability distribution over the fraction of odors eliciting a response for model neurons in the 4 th percentile for class selectivity (Figures E and F). On average, these neurons respond near chance levels ðs y = 6%Þ to nonclass odors, but they respond to 4% of class odors (Figure E). We show that this selectivity reflects overlap in the bulb by constructing a nonsensical class defined as a random collection of, odors with f =. Neurons in the 4 th percentile of selectivity to this artificial class respond near chance levels to both class and nonclass odors (Figure F). Thus, even a randomly connected piriform will have some cells with selectivity to a class of similar odors. Learning Single-Odor Associations Can Align Population Readouts from Different Piriform Representations Given the randomness of the input to piriform, the same odor will be represented differently in different brains. We now consider how these individuals can nevertheless align the qualities they assign across a wide range of odors. A similar problem arises in considering the alignment of odor qualities inferred from the two sides of a single individual s brain. To define an odor quality in our model, we introduce a linear readout that characterizes the output of an entire population of piriform neurons. The readout is a weighted sum of piriform activities z = P i w iy i, where y i is the activity of piriform neuron i and w i is its weight. Initially, the readout weights are chosen randomly, and we examine the resulting readout responses from two different randomly wired model piriform cortices (Figure 2A, top). Readout responses obtained from two different model piriform cortices using the same 738 Neuron 98, , May 6, 28
5 weights are uncorrelated (correlation coefficient =.3,.3, and.5, for odors with f = :7,.3, and, respectively) because the two piriform representations are completely different. We next asked whether shared training to a single odor in two different individuals results in readouts that agree across a wide range of odors. In this simulation, we set the weights for each readout equal to the activity produced by the odor being learned in the corresponding piriform (W i = yi, where the piriform response to the trained odor is yi ; STAR Methods). This implements a form of Hebbian learning (Oja, 982). This single shared experience, corresponding to associative learning with a common odor, does an excellent job of aligning the two readouts in response to other odors (Figure 2A, bottom). Simulated odors sharing either 3% or 7% of activated glomeruli with the trained odor evoke significantly larger responses in the readouts than all nonclass odors. Two readouts not only distinguish class from nonclass, but can also rank the similarity of a panel of odors extremely well, even if the odors are not similar to one another or to the trained odor (the f = case). Moreover, the responses of the two readouts across all simulated odors are well correlated (correlation coefficient =.98,.97, and.86, for f = :7,.3, and, respectively). It is worth emphasizing that the observation that this works for all odors implies that the identity of the training odor is inconsequential. Thus, after training with a single exemplar odor, two readouts can generalize in a similar way across other odors. The degree of correlation between two readouts due to singleodor learning depends on the number of piriform neurons. This is of interest because of the observation that the number of piriform neurons exceeds the number required for efficient categorization (Babadi and Sompolinsky, 24). The readout correlation increases and saturates as a function of the number of piriform neurons (Figures 2B and S2). The correlation coefficient between readouts modified by a shared training experience provides a measure of consistency. The ability of a single readout to categorize odors, on the other hand, is characterized by its signal-tonoise ratio (SNR; Babadi and Sompolinsky, 24; Litwin-Kumar et al., 27). As a function of the number of piriform neurons, the SNR saturates (Babadi and Sompolinsky, 24; Litwin-Kumar et al., 27), and in our model, this saturation occurs when roughly, piriform neurons provide input to the readout. It has been noted that this is an order of magnitude less than the actual number of piriform neurons (Babadi and Sompolinsky, 24). The curve showing the correlation coefficient for two readouts (black curve in Figure 2B) overlaps with the curve for the SNR scaled so that its maximum value is (gray curve in Figure 2B). We discuss the reasons for this overlap in the STAR Methods. To provide a test of our calculation of readout alignment, we exploit experimental data from the Drosophila mushroom body. The fly affords an opportunity to examine genetically defined neurons that readout from the two different mushroom bodies in the two hemispheres of the fly brain. In Drosophila, projection neurons connect glomeruli in the antennal lobe (the insect analog of the olfactory bulb) to Kenyon cells (KCs) that form the mushroom body (the piriform analog) (Marin et al., 22; Wong et al., 22). As in the piriform, glomerular inputs to the KCs show no apparent structure (Caron et al., 23; Gruntman and Turner, 23; Murthy et al., 28). Further support for the random nature of these connections is provided by electron microscopy (EM) data from a Drosophila larva (Eichler et al., 27), indicating that input connections to the KCs are not only unstructured, but are also completely different on the two sides of the brain. KCs synapse onto mushroom body output neurons (MBONs) that are analogous to the model readout we have introduced. Learned associations are established by modifying the strength of the KC to MBON synapses (Aso and Rubin, 26; Hige et al., 25a; Séjourné et al., 2). Thus, homologous MBONs on the two sides of the brain of a single fly should exhibit the response alignment that we have discussed. Correlations were determined for three MBON types that receive different numbers of KC inputs (Hige et al., 25b). This allows us to test not only the values of the correlations we computed, but also their dependence on the number of neurons driving the readout. We constructed a fly analog of our piriform model, adjusting the numbers of neurons and synapses appropriately (STAR Methods). The correlation coefficient between two model MBONs as a function of the number of inputs (Figure 2C) resembles the piriform correlation curve in Figure 2B. The fly model correlation saturates at a value of roughly 2, KC inputs, the actual number of KCs in the mushroom body. Hige et al. (25b) have analyzed the responses of left-right pairs of MBONs that receive different numbers of KC inputs to a panel of odorants. The correlation coefficient between MBON pairs determined experimentally shows a striking match with the predictions of the model (Figure 2C). Readout of a Sufficiently Large Piriform Population Supports Consistent Choice Odor-guided behavior typically involves making a binary choice; for example, to act or not to act. We have described a readout that provides a continuous measure of the piriform response. We now model choices by comparing this readout value to a threshold. Readout values greater than the threshold are interpreted as a choice to act; those below threshold as a choice not to act. This allows us to compute the accuracy with which a trained readout can guide choice (Figure 2B; accuracy is defined as fraction correct). Accuracy is an increasing function of the SNR that saturates before the SNR reaches its maximum (Figure 2D, top). As a result, accuracy saturates at a lower number of piriform neurons than does the SNR (Figure 2B), further emphasizing the disparity between the actual number of piriform neurons and the number needed to support categorization and choice. The threshold we have introduced allows us to investigate the consistency of choice across individuals or brain hemispheres. We trained two readouts, each connected to an independent model piriform cortex ( 6 neurons), on a single odor. We then compared the readouts to a threshold and examined the choice agreement across a large number of nonclass odors. Good agreement between readouts is substantially more difficult to obtain than is accuracy in a typical categorization task such as distinguishing class from nonclass odors. We characterize the value of the threshold with a parameter q that is equal to the fraction of odors that produce a subthreshold readout response. At a Neuron 98, , May 6,
6 threshold for which odors are equally divided between supraand subthreshold (q = :5), 95% of the choices are the same for two trained readouts, but agreement is at chance levels (5%) for two untrained readouts (Figure 2A). Note that this consistency is observed for odors that are both similar to and different from the trained odor. Thus, readouts receiving input from different piriform representations can support consistent choices after a single shared training experience. We next asked how the consistency of choices depends on the number of neurons in piriform. For this purpose, we introduce a quantity A q called the agreement. In defining the agreement, we correct for the bias introduced by thresholds different from.5 by subtracting the fraction of choices due to chance from the fraction of choices that agree. Normalizing the resulting quantity to a maximum value of yields our measure of agreement A q (STAR Methods). Whereas accuracy is determined by SNR, agreement is a function of the readout correlation coefficient and is always the smaller quantity (Figure 2D, bottom). A q saturates at higher numbers of piriform neurons than the accuracy, SNR, or readout correlation coefficient (Figures 2B). This is illustrated graphically in Figure 2E. As the number of piriform neurons grows, the values of the two readouts become more correlated and fall in an increasingly elongated elliptical distribution (Figure 2E, gray). For q = :5, readout agreement corresponds to the fraction of the distribution not in the off-diagonal quadrants (Figure 2E, orange). Even when the distribution is very elongated (high correlation), an appreciable portion remains in these quadrants. The slow increase of A q as a function of the number of piriform neurons is a general property that is relatively insensitive to the threshold used because A q has been corrected for bias and normalized. Our conclusions concerning the agreement between two readouts hold when we extend our analysis to multiple readouts. We introduce an additional parameter to specify the fraction of similarly responding readouts required to say that the ensemble agrees (STAR Methods). For small values of this parameter, the many readout case looks very similar to the two-readout case; for large values, maximal agreement requires at least an order of magnitude more inputs from piriform (Figure S2). Furthermore, the number of piriform inputs required for maximal readout agreement reduces only slightly for odors that more densely activate the olfactory bulb (Figure S2). Thus, a single piriform cortex can support accurate categorization and choice with fewer than 5 neurons, an order of magnitude less than the actual number. However, 6 piriforms are required for choice agreement between different individuals or across the two sides of the brain. This may provide a rationale for the large number of piriform neurons. DISCUSSION Neurons in piriform cortex receive input from a random collection of glomeruli, resulting in an odor representation that lacks the stereotypy of the olfactory bulb. Random connectivity implies that each piriform cortex is unique, posing the problem as to how different individuals generalize consistently across stimuli. Generalization implies that different individuals, after learning a conditioned response to odor A, will generate the learned behavior in response to odors with glomerular representations similar to A, but not to odors that activate different glomeruli. Consistent generalization is also necessary across the two differently wired piriform cortices of a single individual if inter-hemispheric conflicts are to be avoided. Our model demonstrates that readouts from two different randomly wired cortices can be highly correlated after a single shared experience. In this manner, perceptual consistency will not require individuals to have lived identical lives. The degree of correlation between model readouts depends on the number of randomly wired neurons, generating predictions that are in excellent agreement with data from the mushroom body output neurons of the fly. Moreover, the ability of the model piriform to support consistent choices across individuals requires that the piriform cortex contain a large number of neurons, a number in accord with that observed in the mouse. Modeling studies using more traditional performance measures such as dimensionality and readout SNR have suggested that piriform has an excess of neurons (Babadi and Sompolinsky, 24). We find that consistent decision-making may require the full complement of piriform neurons. The model we have considered is based on an assumption of random wiring. We investigated this assumption further by examining in vivo data for evidence of structure. We could detect no local spatial structure, no periodic structure, and no clustering across the piriform surface. We did find positive correlations in the responses of individual neurons to odor pairs, but these are consistent with expectations given random wiring from the bulb to piriform. The fact that piriform odor representations generated by random connectivity can inherit correlations from bulb representations is essential for generalization across odors. A number of studies have examined the impact of random connectivity on the ability of a sensory representation to support categorization (Babadi and Sompolinsky, 24; Barak et al., 23; Cortes and Vapnik, 995; Litwin-Kumar et al., 27; Marr, 969; Rigotti et al., 23). In these studies, categorization consists of dividing stimuli (odors) into two classes based on arbitrarily assigned valences. This type of categorization task emphasizes the importance of decorrelation because correlations in piriform responses have a negative impact. In our model, associations are learned for a small subset of odors and behavioral decisions are made on the basis of generalization. In this task, correlations between piriform responses to odors are essential for assessing odor similarity, and similarity provides the basis for generalization. Correlations are also essential for the consistency of generalization across individuals and between brain hemispheres despite the presence of different representations in different cortices. Lastly, in tasks in which structure in the stimulus space can be exploited, plasticity on the random layer itself can further aid the ability to categorize (Babadi and Sompolinsky, 24; Litwin-Kumar et al., 27; Pehlevan and Chklovskii, 24). Correlations between odor representations in the bulb reflect correlations between the binding of odors to receptors in the nose. These correlations are retained in the piriform despite the random connectivity from the bulb. Since individuals in a species express similar repertoires of receptors, correlations will be preserved across neuronal populations in different 74 Neuron 98, , May 6, 28
7 piriform cortices, but the neurons responsible for these correlations will be different. In our model, individuals with different piriform cortices can nevertheless consistently assess the similarity of odors. In this manner, a rose by any other name would smell as sweet (Shakespeare, 25). STAR+METHODS Detailed methods are provided in the online version of this paper and include the following: d KEY RESOURCES TABLE d CONTACT FOR REAGENT AND RESOURCE SHARING d METHOD DETAILS B Piriform Model B Fly Model B Similar Scaling of the SNR and Two-readout Correlation Coefficient as a Function of the Number of Piriform Neurons B Agreement for Two Readouts B Agreement for a Population of Many Readouts B Piriform Data Analysis d QUANTIFICATION AND STATISTICAL ANALYSIS d DATA AND SOFTWARE AVAILABILITY SUPPLEMENTAL INFORMATION Supplemental Information includes two figures and can be found with this article online at ACKNOWLEDGMENTS We thank Mike Shadlen and Torsten Wiesel for constructive skepticism that motivated this work, Toshi Hige and Glenn Turner for generously sharing data, Ashok Litwin-Kumar for helpful comments and suggestions, Phyllis Kisloff and Clayton Eccard for assistance in preparation of the manuscript, and Miriam Gutierrez and Adriana Nemes for general laboratory support. This work was supported by the Howard Hughes Medical Institute (453), the Gatsby Charitable Foundation, NSF NeuroNex Award DBI-77398, and the Simons Collaboration on the Global Brain (SCGB) Award E.S.S. was supported by SCGB postdoctoral fellowship AUTHOR CONTRIBUTIONS Conceptualization, E.S.S., D.D.S., R.A., and L.F.A.; Software, E.S.S. and D.D.S.; Methodology, E.S.S., D.D.S., and G.B.C.; Formal Analysis, E.S.S., D.D.S., D.K., and L.F.A.; Investigation, E.S.S. and D.D.S.; Data Curation, D.D.S. and D.K.; Writing Original Draft, E.S.S., D.D.S., R.A., and L.F.A.; Writing Review & Editing, E.S.S., D.D.S., R.A., and L.F.A. DECLARATION OF INTERESTS The authors declare no competing interests. Received: October 6, 27 Revised: February 3, 28 Accepted: April 3, 28 Published: April 26, 28 REFERENCES Aso, Y., and Rubin, G.M. (26). Dopaminergic neurons write and update memories with cell-type-specific rules. elife 5, e635. Babadi, B., and Sompolinsky, H. (24). Sparseness and expansion in sensory representations. Neuron 83, Barak, O., Rigotti, M., and Fusi, S. (23). The sparseness of mixed selectivity neurons controls the generalization-discrimination trade-off. J. Neurosci. 33, Buck, L., and Axel, R. (99). A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 65, Caron, S.J.C., Ruta, V., Abbott, L.F., and Axel, R. (23). Random convergence of olfactory inputs in the Drosophila mushroom body. Nature 497, 3 7. Cho, Y., and Saul, L.K. (29). Kernel methods for deep learning. NIPS 9, Choi, G.B., Stettler, D.D., Kallman, B.R., Bhaskar, S.T., Fleischmann, A., and Axel, R. (2). Driving opposing behaviors with ensembles of piriform neurons. Cell 46, 4 5. Cortes, C., and Vapnik, V. (995). Support-vector networks. Mach. Learn. 2, Davison, I.G., and Ehlers, M.D. (2). Neural circuit mechanisms for pattern detection and feature combination in olfactory cortex. Neuron 7, Davison, I.G., and Katz, L.C. (27). Sparse and selective odor coding by mitral/tufted neurons in the main olfactory bulb. J. Neurosci. 27, Eichler, K., Li, F., Litwin-Kumar, A., Park, Y., Andrade, I., Schneider-Mizell, C.M., Saumweber, T., Huser, A., Eschbach, C., Gerber, B., et al. (27). The complete connectome of a learning and memory centre in an insect brain. Nature 548, Ghosh, S., Larson, S.D., Hefzi, H., Marnoy, Z., Cutforth, T., Dokka, K., and Baldwin, K.K. (2). Sensory maps in the olfactory cortex defined by longrange viral tracing of single neurons. Nature 472, Godfrey, P.A., Malnic, B., and Buck, L.B. (24). The mouse olfactory receptor gene family. Proc. Natl. Acad. Sci. USA, Gruntman, E., and Turner, G.C. (23). Integration of the olfactory code across dendritic claws of single mushroom body neurons. Nat. Neurosci. 6, Hige, T., Aso, Y., Modi, M.N., Rubin, G.M., and Turner, G.C. (25a). Heterosynaptic plasticity underlies aversive olfactory learning in Drosophila. Neuron 88, Hige, T., Aso, Y., Rubin, G.M., and Turner, G.C. (25b). Plasticity-driven individualization of olfactory coding in mushroom body output neurons. Nature 526, Igarashi, K.M., and Mori, K. (25). Spatial representation of hydrocarbon odorants in the ventrolateral zones of the rat olfactory bulb. J. Neurophysiol. 93, 7 9. Illig, K.R., and Haberly, L.B. (23). Odor-evoked activity is spatially distributed in piriform cortex. J. Comp. Neurol. 457, Iurilli, G., and Datta, S.R. (27). Population coding in an innately relevant olfactory area. Neuron 93, 8 97.e7. Litwin-Kumar, A., Harris, K.D., Axel, R., Sompolinsky, H., and Abbott, L.F. (27). Optimal degrees of synaptic connectivity. Neuron 93, e7. Ma, L., Qiu, Q., Gradwohl, S., Scott, A., Yu, E.Q., Alexander, R., Wiegraebe, W., and Yu, C.R. (22). Distributed representation of chemical features and tunotopic organization of glomeruli in the mouse olfactory bulb. Proc. Natl. Acad. Sci. USA 9, Marin, E.C., Jefferis, G.S.X.E., Komiyama, T., Zhu, H., and Luo, L. (22). Representation of the glomerular olfactory map in the Drosophila brain. Cell 9, Marr, D. (969). A theory of cerebellar cortex. J. Physiol. 22, Meister, M., and Bonhoeffer, T. (2). Tuning and topography in an odor map on the rat olfactory bulb. J. Neurosci. 2, Miyamichi, K., Amat, F., Moussavi, F., Wang, C., Wickersham, I., Wall, N.R., Taniguchi, H., Tasic, B., Huang, Z.J., He, Z., et al. (2). Cortical representations of olfactory input by trans-synaptic tracing. Nature 472, Neuron 98, , May 6, 28 74
8 Mombaerts, P., Wang, F., Dulac, C., Chao, S.K., Nemes, A., Mendelsohn, M., Edmondson, J., and Axel, R. (996). Visualizing an olfactory sensory map. Cell 87, Murthy, M., Fiete, I., and Laurent, G. (28). Testing odor response stereotypy in the Drosophila mushroom body. Neuron 59, Oja, E. (982). A simplified neuron model as a principal component analyzer. J. Math. Biol. 5, Papadopoulou, M., Cassenaer, S., Nowotny, T., and Laurent, G. (2). Normalization for sparse encoding of odors by a wide-field interneuron. Science 332, Pehlevan, C., and Chklovskii, D.B. (24). A Hebbian/anti-Hebbian network derived from online non-negative matrix factorization can cluster and discover sparse features. 48th Asilomar Conference on Signals, Systems and Computers, IEEE, Poo, C., and Isaacson, J.S. (29). Odor representations in olfactory cortex: sparse coding, global inhibition, and oscillations. Neuron 62, Rennaker, R.L., Chen, C.-F.F., Ruyle, A.M., Sloan, A.M., and Wilson, D.A. (27). Spatial and temporal distribution of odorant-evoked activity in the piriform cortex. J. Neurosci. 27, Ressler, K.J., Sullivan, S.L., and Buck, L.B. (993). A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell 73, Ressler, K.J., Sullivan, S.L., and Buck, L.B. (994). Information coding in the olfactory system: evidence for a stereotyped and highly organized epitope map in the olfactory bulb. Cell 79, Rigotti, M., Barak, O., Warden, M.R., Wang, X.-J., Daw, N.D., Miller, E.K., and Fusi, S. (23). The importance of mixed selectivity in complex cognitive tasks. Nature 497, Séjourné, J., Plaçais, P.-Y., Aso, Y., Siwanowicz, I., Trannoy, S., Thoma, V., Tedjakumala, S.R., Rubin, G.M., Tchénio, P., Ito, K., et al. (2). Mushroom body efferent neurons responsible for aversive olfactory memory retrieval in Drosophila. Nat. Neurosci. 4, Shakespeare, W. (25). Romeo and Juliet. In The Norton Shakespeare, S. Greenblatt, ed. (W.W. Norton & Co.). Sosulski, D.L., Bloom, M.L., Cutforth, T., Axel, R., and Datta, S.R. (2). Distinct representations of olfactory information in different cortical centres. Nature 472, Soucy, E.R., Albeanu, D.F., Fantana, A.L., Murthy, V.N., and Meister, M. (29). Precision and diversity in an odor map on the olfactory bulb. Nat. Neurosci. 2, Stettler, D.D., and Axel, R. (29). Representations of odor in the piriform cortex. Neuron 63, Sugai, T., Miyazawa, T., Fukuda, M., Yoshimura, H., and Onoda, N. (25). Odor-concentration coding in the guinea-pig piriform cortex. Neuroscience 3, Takahashi, Y.K., Kurosaki, M., Hirono, S., and Mori, K. (24). Topographic representation of odorant molecular features in the rat olfactory bulb. J. Neurophysiol. 92, Vassar, R., Ngai, J., and Axel, R. (993). Spatial segregation of odorant receptor expression in the mammalian olfactory epithelium. Cell 74, Vassar, R., Chao, S.K., Sitcheran, R., Nuñez, J.M., Vosshall, L.B., and Axel, R. (994). Topographic organization of sensory projections to the olfactory bulb. Cell 79, Wang, J.W., Wong, A.M., Flores, J., Vosshall, L.B., and Axel, R. (23). Twophoton calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell 2, Wong, A.M., Wang, J.W., and Axel, R. (22). Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell 9, Zhan, C., and Luo, M. (2). Diverse patterns of odor representation by neurons in the anterior piriform cortex of awake mice. J. Neurosci. 3, Zhang, X., and Firestein, S. (22). The olfactory receptor gene superfamily of the mouse. Nat. Neurosci. 5, Neuron 98, , May 6, 28
9 STAR+METHODS KEY RESOURCES TABLE REAGENT or RESOURCE SOURCE IDENTIFIER Deposited Data Calcium imaging data of odor responses in mouse Stettler and Axel, 29 N/A piriform cortex Calcium imaging and electrophysiological data of odor responses in Drosophila mushroom body output neurons Hige et al., 25b N/A Software and Algorithms Algorithms for calculation of readout correlation and agreement This paper coherentgeneralization CONTACT FOR REAGENT AND RESOURCE SHARING Further requests for resources should be directed to and will be fulfilled by the Lead Contact, Evan S. Schaffer columbia.edu). METHOD DETAILS Piriform Model Our network model has feedforward connectivity and consists of three layers that we refer to as the glomerular layer, cortical layer, and readout layer, respectively, as shown in Figure. A fraction S x of the N x neurons in the glomerular layer are activated by a given odor. Glomerular representations of class odors with excess overlap f are chosen such that all such odors activate a common set of fn x S x glomeruli and an additional randomly selected ð fþn x S x glomeruli. The case with no glomeruli designated as shared ðf = Þ corresponds to independent or nonclass odors. Response magnitudes of glomeruli whose responses are nonzero are chosen from an N o -dimensional multivariate lognormal distribution, expðn ðm g ;S g ÞÞ, where the diagonal elements of the covariance matrix S g all equal s 2 g, and off-diagonal elements equal ln½fðexp½s2 g Š Þ + Š, and N o is the number of class odors. This yields response magnitudes whose average cross-odor correlation is f. Each of N y cortical neurons receives S ce N x excitatory and S ci N x inhibitory synaptic inputs from a random selection of glomeruli with weights, represented by matrix elements J ij, set to and S ce =S ci, respectively. The response of each cortical neuron is a thresholdlinear function of the sum of its inputs, y i = Q½ P j J ijx j qš, where QðxÞ = x if x>, and QðxÞ = otherwise. The threshold q is chosen such that an average of 6.2% of piriform neurons are activated by each odor, matching our imaging data. For the parameters chosen, this results in an excess of 9.2 excitatory inputs being required, on average, for a piriform neuron to be activated, i.e., qz9:2 exp½m g + ðs 2 g =2ÞŠ. Finally, the readout unit receives a weighted input from every piriform neuron, z = P i W iy i, where W i = y i after training, where the piriform response to the trained odor is y i. Before training, W i is chosen randomly with the same statistics as y. For all simulation results shown, N x =, m g = :, s g = :5, S x = :, S ce = :2, S ci = :4, q = :9. In all quantities computed from the performance of one or multiple trained readout units, a single odor is trained, and the readout is tested with a panel of unrelated odors. Thus, readout accuracy is calculated as the fraction of novel odors correctly rejected as different from the trained odor. A more symmetric task in which the stimulus test set also includes noisy presentations of the trained odor, to which the readout should respond with the same output as the trained odor, gives qualitatively similar results. Fly Model Our fly model differs from our piriform model in the following parameters: N x = 5, S x = :2 (see Wang et al., 23), S ce = 7=5 (see Caron et al., 23), S ci = (see Papadopoulou et al., 2). The only other difference between these models is that in our fly model, the readout plasticity is anti-hebbian (rather than Hebbian), so that synapses from KCs responsive to the trained odor are set to zero, and synapses from KCs not responsive to the trained odor remain strong. This feature is not essential for our results but is motivated by the experimental observation that KC-MBON synapses depress upon pairing of an odor with an unconditioned stimulus (Aso and Rubin, 26; Hige et al., 25a; Séjourné et al., 2). Similar Scaling of the SNR and Two-readout Correlation Coefficient as a Function of the Number of Piriform Neurons As seen in Figure 2B, the signal-to-noise ratio (SNR) of a single readout and the correlation coefficient (CC) between two readouts, all trained by a Hebbian rule, have virtually identical scaling as a function of the number of piriform neurons supporting the readout. We Neuron 98, e e3, May 6, 28 e
10 denote the response of the single readout to odor a by z a and the valence of this odor by v a. Similarly, the responses of the two readouts are denoted by z ðþ a and z ð2þ a. The signal-to-noise ratio for the single readout is given by SNR = hv az a i 2 a Varðz a Þ ; where the average in the numerator is over odors and the denominator is the variance across odors. The correlation coefficient between two readouts is CC = Var D z ðþ a z ðþ a E z ð2þ a a The numerators of these two expressions depend only weakly (as OðÞ + OðNy =2 Þ) on the number of piriform neurons when this number is significantly greater than. The dependences of the denominators on this number thus determine the scaling we are discussing. Because the single readout z a and the pair of readouts z ðþ a and z ð2þ a all have the same statistics, Varðz ðþ a ÞVarðz ð2þ a Þ = Varðz a Þ 2 and thus the denominators of SNR and CC are identical. These two facts explain their similar scaling. Agreement for Two Readouts Agreement A q is calculated by subtracting the chance probability of agreement b from the raw fraction of similarly-responding readouts, a, and then normalizing to one, so that A q = ða bþ=ð bþ, where b = q 2 + ð qþ 2. The relationship between A q and q can be seen more easily in the Gaussian approximation of A q, which makes explicit the dependence on the correlation s, ZZ A Gauss q = p p ffiffiffiffiffiffiffiffiffiffiffiffiffi s 2 Var dx dy exp x>q; y<q z ð2þ a : 2 x 2 2sxy + y 2 : 2s 2 2 Agreement for a Population of Many Readouts Although the case of two readouts is of particular interest because of the application to modeling opposing hemispheres of the same brain, the agreement between more than two readouts is also of interest, with applications to readouts in multiple brain areas or in f; Nz multiple individuals. We define the agreement of a population of N z readouts, Aq, with a threshold criterion f on the fraction of readouts giving the same response (either or ); in other words, a yes choice results when the fraction of readouts with suprathreshold values is greater than a number f. Our original definition of A q in the two-readout case can be seen as population agreement with f =. As in the two-readout case, we subtract the probability of agreement occurring by chance and normalize to a maximum of. More precisely, we subtract the chance probability of agreement b from the raw fraction of similarly-responding readouts, a, and then normalizing to one, so that A f;nz q = ða bþ=ð bþ, where b is the sum of binomial cumulative density functions, b = Nz q i ð qþ Nz i + P N z Nz i = N zf q i ð qþ Nz i. For N z = 2 and f =, this reduces to b = q 2 + ð qþ 2. P Nzð fþ i = i i To properly compare the required piriform resources for readout populations of varying size, we examine population agreement as a function of the total number of piriform neurons the number of piriform inputs per readout, multiplied by the number of readout units. This is an assumption of nonoverlapping inputs to the readouts. Population agreement, A f;nz q, for a range of f values with q = :5 and a population of readouts is qualitatively similar to the original A q curves (Figure S2A, left); independent of the value of the choice threshold f, A f; :5 requires in excess of million piriform inputs for performance to saturate. With a larger readout population ðn z = 5Þ, a dependence on the value of the choice threshold f emerges, such that A f;5 :5 looks very similar to A q when f = :55 but requires several orders of magnitude more piriform neurons when f = :95 (Figure S2A, right). Intuitively, the appearance of an effect of choice threshold when the readout population is large is a consequence of the ability of a large readout population to detect smaller deviations from chance: low values of the choice threshold f make the population agree more often and therefore require less piriform inputs, but it also makes the chance probability of agreement higher. Only a sufficiently large readout population can distinguish between real and chance agreement and take advantage of this lower threshold. Most importantly, even in this case, performance does not saturate until the number of piriform inputs reaches million, suggesting that the importance of a large piriform cortex is general. Piriform Data Analysis Our analyses are based aggregated data from calcium imaging of layer 2 of piriform cortex expressing GCaMP3, GCaMP5, and Oregon Green 488 BAPTA- AM (Stettler and Axel, 29). The responses of thousands of neurons to odor delivery in freely-breathing anesthetized animals were compared to assess response selectivity. Approximately 6% of piriform neurons respond selectively to odors at concentrations of - ppm. A small fraction of the neurons (less than %) respond to most or all presented odors. These cells may represent a separate functional class and have been excluded from further analyses. Cells responsive to a given odor are found across the piriform with no spatial preference, and the representations of different odors exhibit considerable spatial overlap, in e2 Neuron 98, e e3, May 6, 28
11 agreement with prior work (Illig and Haberly, 23; Iurilli and Datta, 27; Stettler and Axel, 29). Cells responsive to the ethologically relevant odors TMT and mouse urine were also found to be distributed without spatial preference (Illig and Haberly, 23; Stettler and Axel, 29). Cells and odor responses were identified and quantified using custom software written in MATLAB as previously described (Stettler and Axel, 29). Nearest-neighbor statistics were calculated using the distance from each cell responsive to a specific odor within a field to the nearest other cell responsive to that odor. We then compared the distribution of these values with the distribution derived from Monte Carlo simulations in which responsive cells were drawn randomly from all the cells in the field, and nearest-neighbor statistics for each cell were then computed in the same manner. In Figure SF, 5 th and 95 th percentiles of the Monte Carlo-derived distribution were computed separately for each distance bin. If responsive cells were clustered, they would tend to have smaller nearest-neighbor separations than randomly distributed cells. At a representative imaging site, the in vivo nearest neighbor distances closely match randomly generated distances (Figure SF). The site-averaged nearest neighbor distances in observed and randomly shuffled data are closely matched across all imaging sites and odors (Wilcoxon signed-rank test, p >.3; Figure SG), providing no evidence for clustering. We also inspected in vivo piriform odor representations for local patterning by analyzing their spatial wavelengths. The spatial power spectra of in vivo response patterns were compared to spectra from simulations for the same sites. No significant discrepancies between the in vivo and randomly generated spectra are apparent for any spatial period across the sites (Figure SH). QUANTIFICATION AND STATISTICAL ANALYSIS Results for readout accuracy, SNR, correlation, and agreement are averaged over input patterns and over instantiations of the network architecture. DATA AND SOFTWARE AVAILABILITY Software was written in MATLAB ( Code used to compute quantities presented in this study is available at: Neuron 98, e e3, May 6, 28 e3
12 Neuron, Volume 98 Supplemental Information Odor Perception on the Two Sides of the Brain: Consistency Despite Randomness Evan S. Schaffer, Dan D. Stettler, Daniel Kato, Gloria B. Choi, Richard Axel, and L.F. Abbott
13 Supplemental Figures A B octanal (5.4%) E acetophenone (7.6%) both (.9%) weakly selective (.8%) F Probability.2. Observed Random G Observed N.N. Dist. (μm) G-CaMP3 C μm octanal D 3% F/F 3% H 3 6 Nearest Neighbor Dist. (µm) Power.5 Observed Random Random Shuffled N.N. Dist. (μm) acetophenone 3% 3% octanal or acetophenone Normalized Period (µm) Figure S, related to Figure. Piriform odor representations exhibit no consistent global or local patterning. A. Cells in layer 2 expressing GCaMP3. B. F/F response to octanal. C. F/F response to acetophenone. D. Cells responsive to octanal (red), acetophenone (green), or both (yellow). E. Montage of several imaging sites produced according to the methods described in Stettler and Axel (29). Cells responsive to >5% of tested odors (blue) are distributed across the piriform in the same unclustered manner as cells responsive to individual odors. F. Nearest neighbor distances for cells responsive to octanal in one animal, aggregated from 7 sites. In vivo values are purple and randomly generated mean values black, with 5 th and 95 th percentiles of randomly shuffled data indicated by the gray region. G. Observed nearest neighbor distances across imaging sites versus the same quantity from randomly shuffled data. Each point corresponds to the mean across all active cells for one odor and one imaging site (data from 4 odors, obtained from 27 sites in 6 animals, Wilcoxon signed-rank test, p>.3). H. Power spectra of observed octanal responses versus the spectra of Monte Carlo patterns generated for the same imaging sites (random means are blue and observed mean SEM are purple). Data were obtained from Stettler & Axel (29) and additional optical recordings with GCaMP3 and GCaMP5 using the same imaging methods.
Sensory Encoding of Smell in the Olfactory System of Drosophila
 Sensory Encoding of Smell in the Olfactory System of Drosophila (reviewing Olfactory Information Processing in Drosophila by Masse et al, 2009) Ben Cipollini COGS 160 May 11, 2010 Smell Drives Our Behavior...
Sensory Encoding of Smell in the Olfactory System of Drosophila (reviewing Olfactory Information Processing in Drosophila by Masse et al, 2009) Ben Cipollini COGS 160 May 11, 2010 Smell Drives Our Behavior...
arxiv:physics/ v1 [physics.bio-ph] 19 Feb 1999
![arxiv:physics/ v1 [physics.bio-ph] 19 Feb 1999 arxiv:physics/ v1 [physics.bio-ph] 19 Feb 1999](/thumbs/83/88796750.jpg) Odor recognition and segmentation by coupled olfactory bulb and cortical networks arxiv:physics/9902052v1 [physics.bioph] 19 Feb 1999 Abstract Zhaoping Li a,1 John Hertz b a CBCL, MIT, Cambridge MA 02139
Odor recognition and segmentation by coupled olfactory bulb and cortical networks arxiv:physics/9902052v1 [physics.bioph] 19 Feb 1999 Abstract Zhaoping Li a,1 John Hertz b a CBCL, MIT, Cambridge MA 02139
How to read a burst duration code
 Neurocomputing 58 60 (2004) 1 6 www.elsevier.com/locate/neucom How to read a burst duration code Adam Kepecs a;, John Lisman b a Cold Spring Harbor Laboratory, Marks Building, 1 Bungtown Road, Cold Spring
Neurocomputing 58 60 (2004) 1 6 www.elsevier.com/locate/neucom How to read a burst duration code Adam Kepecs a;, John Lisman b a Cold Spring Harbor Laboratory, Marks Building, 1 Bungtown Road, Cold Spring
Identification of Odors by the Spatiotemporal Dynamics of the Olfactory Bulb. Outline
 Identification of Odors by the Spatiotemporal Dynamics of the Olfactory Bulb Henry Greenside Department of Physics Duke University Outline Why think about olfaction? Crash course on neurobiology. Some
Identification of Odors by the Spatiotemporal Dynamics of the Olfactory Bulb Henry Greenside Department of Physics Duke University Outline Why think about olfaction? Crash course on neurobiology. Some
A quantitative description of the mouse piriform cortex Shyam Srinivasan 1,2,*, Charles F Stevens 1,2,*
 A quantitative description of the mouse piriform cortex Shyam Srinivasan 1,2,*, Charles F Stevens 1,2,* 1 Kavli Institute for Brain and Mind, University of California, San Diego, CA 92093, USA 2 Salk Institute
A quantitative description of the mouse piriform cortex Shyam Srinivasan 1,2,*, Charles F Stevens 1,2,* 1 Kavli Institute for Brain and Mind, University of California, San Diego, CA 92093, USA 2 Salk Institute
High-dimensional geometry of cortical population activity. Marius Pachitariu University College London
 High-dimensional geometry of cortical population activity Marius Pachitariu University College London Part I: introduction to the brave new world of large-scale neuroscience Part II: large-scale data preprocessing
High-dimensional geometry of cortical population activity Marius Pachitariu University College London Part I: introduction to the brave new world of large-scale neuroscience Part II: large-scale data preprocessing
Sensory/ Motor Systems March 10, 2008
 Sensory/ Motor Systems March 10, 2008 Greg Suh greg.suh@med.nyu.edu Title: Chemical Senses- periphery Richard Axel and Linda Buck Win Nobel Prize for Olfactory System Research 2 3 After Leslie Vosshal
Sensory/ Motor Systems March 10, 2008 Greg Suh greg.suh@med.nyu.edu Title: Chemical Senses- periphery Richard Axel and Linda Buck Win Nobel Prize for Olfactory System Research 2 3 After Leslie Vosshal
Neuro-based Olfactory Model for Artificial Organoleptic Tests
 Neuro-based Olfactory odel for Artificial Organoleptic Tests Zu Soh,ToshioTsuji, Noboru Takiguchi,andHisaoOhtake Graduate School of Engineering, Hiroshima Univ., Hiroshima 739-8527, Japan Graduate School
Neuro-based Olfactory odel for Artificial Organoleptic Tests Zu Soh,ToshioTsuji, Noboru Takiguchi,andHisaoOhtake Graduate School of Engineering, Hiroshima Univ., Hiroshima 739-8527, Japan Graduate School
Lecture 9 Olfaction (Chemical senses 2)
 Lecture 9 Olfaction (Chemical senses 2) All lecture material from the following links unless otherwise mentioned: 1. http://wws.weizmann.ac.il/neurobiology/labs/ulanovsky/sites/neurobiology.labs.ulanovsky/files/uploads/kandel_ch32_smell_taste.pd
Lecture 9 Olfaction (Chemical senses 2) All lecture material from the following links unless otherwise mentioned: 1. http://wws.weizmann.ac.il/neurobiology/labs/ulanovsky/sites/neurobiology.labs.ulanovsky/files/uploads/kandel_ch32_smell_taste.pd
Representations of Odor in the Piriform Cortex
 Article Dan D. Stettler 1 and Richard Axel 1, * 1 Department of Neuroscience and Howard Hughes Medical Institute, College of Physicians and Surgeons, Columbia University, New York, NY 10032, USA *Correspondence:
Article Dan D. Stettler 1 and Richard Axel 1, * 1 Department of Neuroscience and Howard Hughes Medical Institute, College of Physicians and Surgeons, Columbia University, New York, NY 10032, USA *Correspondence:
Analysis of non-olfactory sensory pathways that are necessary for the olfactory associative learning of the Drosophila mushroom body
 15 12601 B 2009 2011 21300117 Analysis of non-olfactory sensory pathways that are necessary for the olfactory associative learning of the Drosophila mushroom body ITO, Kei 00311192 The mushroom body is
15 12601 B 2009 2011 21300117 Analysis of non-olfactory sensory pathways that are necessary for the olfactory associative learning of the Drosophila mushroom body ITO, Kei 00311192 The mushroom body is
NEURAL RELATIVITY PRINCIPLE
 NEURAL RELATIVITY PRINCIPLE Alex Koulakov, Cold Spring Harbor Laboratory 1 3 Odorants are represented by patterns of glomerular activation butyraldehyde hexanone Odorant-Evoked SpH Signals in the Mouse
NEURAL RELATIVITY PRINCIPLE Alex Koulakov, Cold Spring Harbor Laboratory 1 3 Odorants are represented by patterns of glomerular activation butyraldehyde hexanone Odorant-Evoked SpH Signals in the Mouse
Tuning tuning curves. So far: Receptive fields Representation of stimuli Population vectors. Today: Contrast enhancment, cortical processing
 Tuning tuning curves So far: Receptive fields Representation of stimuli Population vectors Today: Contrast enhancment, cortical processing Firing frequency N 3 s max (N 1 ) = 40 o N4 N 1 N N 5 2 s max
Tuning tuning curves So far: Receptive fields Representation of stimuli Population vectors Today: Contrast enhancment, cortical processing Firing frequency N 3 s max (N 1 ) = 40 o N4 N 1 N N 5 2 s max
"That which we call a rose by any other name would smell as sweet": How the Nose knows!
 "That which we call a rose by any other name would smell as sweet": How the Nose knows! Nobel Prize in Physiology or Medicine 2004 Sayanti Saha, Parvathy Ramakrishnan and Sandhya S Vis'Wes'Wariah Richard
"That which we call a rose by any other name would smell as sweet": How the Nose knows! Nobel Prize in Physiology or Medicine 2004 Sayanti Saha, Parvathy Ramakrishnan and Sandhya S Vis'Wes'Wariah Richard
Marr's Theory of the Hippocampus: Part I
 Marr's Theory of the Hippocampus: Part I Computational Models of Neural Systems Lecture 3.3 David S. Touretzky October, 2015 David Marr: 1945-1980 10/05/15 Computational Models of Neural Systems 2 Marr
Marr's Theory of the Hippocampus: Part I Computational Models of Neural Systems Lecture 3.3 David S. Touretzky October, 2015 David Marr: 1945-1980 10/05/15 Computational Models of Neural Systems 2 Marr
An Introductory Course in Computational Neuroscience
 An Introductory Course in Computational Neuroscience Contents Series Foreword Acknowledgments Preface 1 Preliminary Material 1.1. Introduction 1.1.1 The Cell, the Circuit, and the Brain 1.1.2 Physics of
An Introductory Course in Computational Neuroscience Contents Series Foreword Acknowledgments Preface 1 Preliminary Material 1.1. Introduction 1.1.1 The Cell, the Circuit, and the Brain 1.1.2 Physics of
Announcements: Test4: Wednesday on: week4 material CH5 CH6 & NIA CAPE Evaluations please do them for me!! ask questions...discuss listen learn.
 Announcements: Test4: Wednesday on: week4 material CH5 CH6 & NIA CAPE Evaluations please do them for me!! ask questions...discuss listen learn. The Chemical Senses: Olfaction Mary ET Boyle, Ph.D. Department
Announcements: Test4: Wednesday on: week4 material CH5 CH6 & NIA CAPE Evaluations please do them for me!! ask questions...discuss listen learn. The Chemical Senses: Olfaction Mary ET Boyle, Ph.D. Department
Synchronized Oscillatory Discharges of Mitral/Tufted Cells With Different Molecular Receptive Ranges in the Rabbit Olfactory Bulb
 Synchronized Oscillatory Discharges of Mitral/Tufted Cells With Different Molecular Receptive Ranges in the Rabbit Olfactory Bulb HIDEKI KASHIWADANI, 1,2 YASNORY F. SASAKI, 1 NAOSHIGE UCHIDA, 1 AND KENSAKU
Synchronized Oscillatory Discharges of Mitral/Tufted Cells With Different Molecular Receptive Ranges in the Rabbit Olfactory Bulb HIDEKI KASHIWADANI, 1,2 YASNORY F. SASAKI, 1 NAOSHIGE UCHIDA, 1 AND KENSAKU
!) + log(t) # n i. The last two terms on the right hand side (RHS) are clearly independent of θ and can be
 Supplementary Materials General case: computing log likelihood We first describe the general case of computing the log likelihood of a sensory parameter θ that is encoded by the activity of neurons. Each
Supplementary Materials General case: computing log likelihood We first describe the general case of computing the log likelihood of a sensory parameter θ that is encoded by the activity of neurons. Each
Optimal Adaptation Principles In Neural Systems
 University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 2017 Optimal Adaptation Principles In Neural Systems Kamesh Krishnamurthy University of Pennsylvania, kameshkk@gmail.com
University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 2017 Optimal Adaptation Principles In Neural Systems Kamesh Krishnamurthy University of Pennsylvania, kameshkk@gmail.com
The sense of smell Outline Main Olfactory System Odor Detection Odor Coding Accessory Olfactory System Pheromone Detection Pheromone Coding
 The sense of smell Outline Main Olfactory System Odor Detection Odor Coding Accessory Olfactory System Pheromone Detection Pheromone Coding 1 Human experiment: How well do we taste without smell? 2 Brief
The sense of smell Outline Main Olfactory System Odor Detection Odor Coding Accessory Olfactory System Pheromone Detection Pheromone Coding 1 Human experiment: How well do we taste without smell? 2 Brief
References. Adler, E., Hoon, M. A., Mueller, K. L., Chandrashekar, J., Ryba, N. J., & Zuker, C. S. (2000). A
 References Adler, E., Hoon, M. A., Mueller, K. L., Chandrashekar, J., Ryba, N. J., & Zuker, C. S. (2000). A novel family of mammalian taste receptors. Cell, 100(6), 693 702. Bakalyar, H. A., & Reed, R.
References Adler, E., Hoon, M. A., Mueller, K. L., Chandrashekar, J., Ryba, N. J., & Zuker, C. S. (2000). A novel family of mammalian taste receptors. Cell, 100(6), 693 702. Bakalyar, H. A., & Reed, R.
Consider the following spike trains from two different neurons N1 and N2:
 About synchrony and oscillations So far, our discussions have assumed that we are either observing a single neuron at a, or that neurons fire independent of each other. This assumption may be correct in
About synchrony and oscillations So far, our discussions have assumed that we are either observing a single neuron at a, or that neurons fire independent of each other. This assumption may be correct in
arxiv: v4 [q-bio.nc] 22 Jan 2019
![arxiv: v4 [q-bio.nc] 22 Jan 2019 arxiv: v4 [q-bio.nc] 22 Jan 2019](/thumbs/95/124112575.jpg) Adaptation of olfactory receptor abundances for efficient coding Tiberiu Teşileanu 1,2,3, Simona Cocco 4, Rémi Monasson 5, and Vijay Balasubramanian 2,3 arxiv:181.93v4 [q-bio.nc] 22 Jan 219 1 Center for
Adaptation of olfactory receptor abundances for efficient coding Tiberiu Teşileanu 1,2,3, Simona Cocco 4, Rémi Monasson 5, and Vijay Balasubramanian 2,3 arxiv:181.93v4 [q-bio.nc] 22 Jan 219 1 Center for
Linking connectivity, dynamics and computations in low-rank recurrent neural networks
 Linking connectivity, dynamics and computations in low-rank recurrent neural networks Francesca Mastrogiuseppe 1,2, Srdjan Ostojic 1 * 1 Laboratoire de Neurosciences Cognitives, INSERM U960 and 2 Laboratoire
Linking connectivity, dynamics and computations in low-rank recurrent neural networks Francesca Mastrogiuseppe 1,2, Srdjan Ostojic 1 * 1 Laboratoire de Neurosciences Cognitives, INSERM U960 and 2 Laboratoire
Discriminative Direction for Kernel Classifiers
 Discriminative Direction for Kernel Classifiers Polina Golland Artificial Intelligence Lab Massachusetts Institute of Technology Cambridge, MA 02139 polina@ai.mit.edu Abstract In many scientific and engineering
Discriminative Direction for Kernel Classifiers Polina Golland Artificial Intelligence Lab Massachusetts Institute of Technology Cambridge, MA 02139 polina@ai.mit.edu Abstract In many scientific and engineering
The Bayesian Brain. Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester. May 11, 2017
 The Bayesian Brain Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester May 11, 2017 Bayesian Brain How do neurons represent the states of the world? How do neurons represent
The Bayesian Brain Robert Jacobs Department of Brain & Cognitive Sciences University of Rochester May 11, 2017 Bayesian Brain How do neurons represent the states of the world? How do neurons represent
Neuro-based Olfactory Model for Estimation of Sensory Characteristic of Mice
 Proceedings of the 2008 IEEE International Conference on Robotics and Biomimetics Bangkok, Thailand, February 21-26, 2009 Neuro-based Olfactory Model for Estimation of Sensory Characteristic of Mice Zu
Proceedings of the 2008 IEEE International Conference on Robotics and Biomimetics Bangkok, Thailand, February 21-26, 2009 Neuro-based Olfactory Model for Estimation of Sensory Characteristic of Mice Zu
SUPPLEMENTARY INFORMATION
 Supplementary discussion 1: Most excitatory and suppressive stimuli for model neurons The model allows us to determine, for each model neuron, the set of most excitatory and suppresive features. First,
Supplementary discussion 1: Most excitatory and suppressive stimuli for model neurons The model allows us to determine, for each model neuron, the set of most excitatory and suppresive features. First,
Olfactory Information Processing in Drosophila
 Current Biology 19, R7 R713, August 25, 29 ª29 Elsevier Ltd All rights reserved DOI 1.116/j.cub.29.6.26 Olfactory Information Processing in Drosophila Review Nicolas Y. Masse 1, Glenn C. Turner 2, and
Current Biology 19, R7 R713, August 25, 29 ª29 Elsevier Ltd All rights reserved DOI 1.116/j.cub.29.6.26 Olfactory Information Processing in Drosophila Review Nicolas Y. Masse 1, Glenn C. Turner 2, and
Comparing integrate-and-fire models estimated using intracellular and extracellular data 1
 Comparing integrate-and-fire models estimated using intracellular and extracellular data 1 Liam Paninski a,b,2 Jonathan Pillow b Eero Simoncelli b a Gatsby Computational Neuroscience Unit, University College
Comparing integrate-and-fire models estimated using intracellular and extracellular data 1 Liam Paninski a,b,2 Jonathan Pillow b Eero Simoncelli b a Gatsby Computational Neuroscience Unit, University College
7 Rate-Based Recurrent Networks of Threshold Neurons: Basis for Associative Memory
 Physics 178/278 - David Kleinfeld - Fall 2005; Revised for Winter 2017 7 Rate-Based Recurrent etworks of Threshold eurons: Basis for Associative Memory 7.1 A recurrent network with threshold elements The
Physics 178/278 - David Kleinfeld - Fall 2005; Revised for Winter 2017 7 Rate-Based Recurrent etworks of Threshold eurons: Basis for Associative Memory 7.1 A recurrent network with threshold elements The
Sparse Coding as a Generative Model
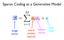 Sparse Coding as a Generative Model image vector neural activity (sparse) feature vector other stuff Find activations by descending E Coefficients via gradient descent Driving input (excitation) Lateral
Sparse Coding as a Generative Model image vector neural activity (sparse) feature vector other stuff Find activations by descending E Coefficients via gradient descent Driving input (excitation) Lateral
Processing of Time Series by Neural Circuits with Biologically Realistic Synaptic Dynamics
 Processing of Time Series by Neural Circuits with iologically Realistic Synaptic Dynamics Thomas Natschläger & Wolfgang Maass Institute for Theoretical Computer Science Technische Universität Graz, ustria
Processing of Time Series by Neural Circuits with iologically Realistic Synaptic Dynamics Thomas Natschläger & Wolfgang Maass Institute for Theoretical Computer Science Technische Universität Graz, ustria
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/319/5869/1543/dc1 Supporting Online Material for Synaptic Theory of Working Memory Gianluigi Mongillo, Omri Barak, Misha Tsodyks* *To whom correspondence should be addressed.
www.sciencemag.org/cgi/content/full/319/5869/1543/dc1 Supporting Online Material for Synaptic Theory of Working Memory Gianluigi Mongillo, Omri Barak, Misha Tsodyks* *To whom correspondence should be addressed.
1
 http://photos1.blogger.com/img/13/2627/640/screenhunter_047.jpg 1 The Nose Knows http://graphics.jsonline.com/graphics/owlive/img/mar05/sideways.one0308_big.jpg 2 http://www.stlmoviereviewweekly.com/sitebuilder/images/sideways-253x364.jpg
http://photos1.blogger.com/img/13/2627/640/screenhunter_047.jpg 1 The Nose Knows http://graphics.jsonline.com/graphics/owlive/img/mar05/sideways.one0308_big.jpg 2 http://www.stlmoviereviewweekly.com/sitebuilder/images/sideways-253x364.jpg
Transformation of stimulus correlations by the retina
 Transformation of stimulus correlations by the retina Kristina Simmons (University of Pennsylvania) and Jason Prentice, (now Princeton University) with Gasper Tkacik (IST Austria) Jan Homann (now Princeton
Transformation of stimulus correlations by the retina Kristina Simmons (University of Pennsylvania) and Jason Prentice, (now Princeton University) with Gasper Tkacik (IST Austria) Jan Homann (now Princeton
Exercises. Chapter 1. of τ approx that produces the most accurate estimate for this firing pattern.
 1 Exercises Chapter 1 1. Generate spike sequences with a constant firing rate r 0 using a Poisson spike generator. Then, add a refractory period to the model by allowing the firing rate r(t) to depend
1 Exercises Chapter 1 1. Generate spike sequences with a constant firing rate r 0 using a Poisson spike generator. Then, add a refractory period to the model by allowing the firing rate r(t) to depend
Olfactory coding from the periphery to higher brain centers in the Drosophila brain
 Seki et al. BMC Biology (2017) 15:56 DOI 10.1186/s12915-017-0389-z RESEARCH ARTICLE Open Access Olfactory coding from the periphery to higher brain centers in the Drosophila brain Yoichi Seki 1,3*, Hany
Seki et al. BMC Biology (2017) 15:56 DOI 10.1186/s12915-017-0389-z RESEARCH ARTICLE Open Access Olfactory coding from the periphery to higher brain centers in the Drosophila brain Yoichi Seki 1,3*, Hany
Synaptic Plasticity. Introduction. Biophysics of Synaptic Plasticity. Functional Modes of Synaptic Plasticity. Activity-dependent synaptic plasticity:
 Synaptic Plasticity Introduction Dayan and Abbott (2001) Chapter 8 Instructor: Yoonsuck Choe; CPSC 644 Cortical Networks Activity-dependent synaptic plasticity: underlies learning and memory, and plays
Synaptic Plasticity Introduction Dayan and Abbott (2001) Chapter 8 Instructor: Yoonsuck Choe; CPSC 644 Cortical Networks Activity-dependent synaptic plasticity: underlies learning and memory, and plays
7 Recurrent Networks of Threshold (Binary) Neurons: Basis for Associative Memory
 Physics 178/278 - David Kleinfeld - Winter 2019 7 Recurrent etworks of Threshold (Binary) eurons: Basis for Associative Memory 7.1 The network The basic challenge in associative networks, also referred
Physics 178/278 - David Kleinfeld - Winter 2019 7 Recurrent etworks of Threshold (Binary) eurons: Basis for Associative Memory 7.1 The network The basic challenge in associative networks, also referred
A MEAN FIELD THEORY OF LAYER IV OF VISUAL CORTEX AND ITS APPLICATION TO ARTIFICIAL NEURAL NETWORKS*
 683 A MEAN FIELD THEORY OF LAYER IV OF VISUAL CORTEX AND ITS APPLICATION TO ARTIFICIAL NEURAL NETWORKS* Christopher L. Scofield Center for Neural Science and Physics Department Brown University Providence,
683 A MEAN FIELD THEORY OF LAYER IV OF VISUAL CORTEX AND ITS APPLICATION TO ARTIFICIAL NEURAL NETWORKS* Christopher L. Scofield Center for Neural Science and Physics Department Brown University Providence,
Global Layout Optimization of Olfactory Cortex and of Amygdala of Rat
 UMIACS-TR-2007-14 CS-TR-4861 Global Layout Optimization of Olfactory Cortex and of Amygdala of Rat Raul Rodriguez-Esteban and Christopher Cherniak June 2005 Committee for Philosophy and the Sciences, Department
UMIACS-TR-2007-14 CS-TR-4861 Global Layout Optimization of Olfactory Cortex and of Amygdala of Rat Raul Rodriguez-Esteban and Christopher Cherniak June 2005 Committee for Philosophy and the Sciences, Department
Smell is perhaps our most evocative
 The Molecular Logic of Smell Mammals can recognize thousands of odors, some of which prompt powerful responses. Recent experiments illuminate how the nose and brain may perceive scents by Richard Axel
The Molecular Logic of Smell Mammals can recognize thousands of odors, some of which prompt powerful responses. Recent experiments illuminate how the nose and brain may perceive scents by Richard Axel
Neurology, neurophysiology and neuropsychology: olfactory clues to brain development and disorder
 Section I Neurology, neurophysiology and neuropsychology: olfactory clues to brain development and disorder 1 Structure and function of the olfactory system Alan Mackay-Sim and Jean-Pierre Royet Introduction
Section I Neurology, neurophysiology and neuropsychology: olfactory clues to brain development and disorder 1 Structure and function of the olfactory system Alan Mackay-Sim and Jean-Pierre Royet Introduction
Nature Neuroscience: doi: /nn Supplementary Figure 1. Expression of GCaMP6s in LGN and their axons in V1.
 Supplementary Figure 1 Expression of GCaMP6s in LGN and their axons in V1. (a, b) Coronal section of LGN and V1 expressing GCaMP6s. a Coronal slice (bregma -2.3 mm) including thalamus confirmed that GCaMP6s
Supplementary Figure 1 Expression of GCaMP6s in LGN and their axons in V1. (a, b) Coronal section of LGN and V1 expressing GCaMP6s. a Coronal slice (bregma -2.3 mm) including thalamus confirmed that GCaMP6s
Unsupervised Learning with Permuted Data
 Unsupervised Learning with Permuted Data Sergey Kirshner skirshne@ics.uci.edu Sridevi Parise sparise@ics.uci.edu Padhraic Smyth smyth@ics.uci.edu School of Information and Computer Science, University
Unsupervised Learning with Permuted Data Sergey Kirshner skirshne@ics.uci.edu Sridevi Parise sparise@ics.uci.edu Padhraic Smyth smyth@ics.uci.edu School of Information and Computer Science, University
The Spike Response Model: A Framework to Predict Neuronal Spike Trains
 The Spike Response Model: A Framework to Predict Neuronal Spike Trains Renaud Jolivet, Timothy J. Lewis 2, and Wulfram Gerstner Laboratory of Computational Neuroscience, Swiss Federal Institute of Technology
The Spike Response Model: A Framework to Predict Neuronal Spike Trains Renaud Jolivet, Timothy J. Lewis 2, and Wulfram Gerstner Laboratory of Computational Neuroscience, Swiss Federal Institute of Technology
A Multivariate Time-Frequency Based Phase Synchrony Measure for Quantifying Functional Connectivity in the Brain
 A Multivariate Time-Frequency Based Phase Synchrony Measure for Quantifying Functional Connectivity in the Brain Dr. Ali Yener Mutlu Department of Electrical and Electronics Engineering, Izmir Katip Celebi
A Multivariate Time-Frequency Based Phase Synchrony Measure for Quantifying Functional Connectivity in the Brain Dr. Ali Yener Mutlu Department of Electrical and Electronics Engineering, Izmir Katip Celebi
3.3 Population Decoding
 3.3 Population Decoding 97 We have thus far considered discriminating between two quite distinct stimulus values, plus and minus. Often we are interested in discriminating between two stimulus values s
3.3 Population Decoding 97 We have thus far considered discriminating between two quite distinct stimulus values, plus and minus. Often we are interested in discriminating between two stimulus values s
Neural Networks 1 Synchronization in Spiking Neural Networks
 CS 790R Seminar Modeling & Simulation Neural Networks 1 Synchronization in Spiking Neural Networks René Doursat Department of Computer Science & Engineering University of Nevada, Reno Spring 2006 Synchronization
CS 790R Seminar Modeling & Simulation Neural Networks 1 Synchronization in Spiking Neural Networks René Doursat Department of Computer Science & Engineering University of Nevada, Reno Spring 2006 Synchronization
Neuronal Tuning: To Sharpen or Broaden?
 NOTE Communicated by Laurence Abbott Neuronal Tuning: To Sharpen or Broaden? Kechen Zhang Howard Hughes Medical Institute, Computational Neurobiology Laboratory, Salk Institute for Biological Studies,
NOTE Communicated by Laurence Abbott Neuronal Tuning: To Sharpen or Broaden? Kechen Zhang Howard Hughes Medical Institute, Computational Neurobiology Laboratory, Salk Institute for Biological Studies,
Comparison of receptive fields to polar and Cartesian stimuli computed with two kinds of models
 Supplemental Material Comparison of receptive fields to polar and Cartesian stimuli computed with two kinds of models Motivation The purpose of this analysis is to verify that context dependent changes
Supplemental Material Comparison of receptive fields to polar and Cartesian stimuli computed with two kinds of models Motivation The purpose of this analysis is to verify that context dependent changes
Methods for Estimating the Computational Power and Generalization Capability of Neural Microcircuits
 Methods for Estimating the Computational Power and Generalization Capability of Neural Microcircuits Wolfgang Maass, Robert Legenstein, Nils Bertschinger Institute for Theoretical Computer Science Technische
Methods for Estimating the Computational Power and Generalization Capability of Neural Microcircuits Wolfgang Maass, Robert Legenstein, Nils Bertschinger Institute for Theoretical Computer Science Technische
How Complex Cells Are Made in a Simple Cell Network
 How Complex Cells Are Made in a Simple Cell Network Louis Tao Courant Institute, New York University Collaborators: Michael Shelley (Courant, NYU) Robert Shapley (CNS, NYU) David McLaughlin (Courant, NYU)
How Complex Cells Are Made in a Simple Cell Network Louis Tao Courant Institute, New York University Collaborators: Michael Shelley (Courant, NYU) Robert Shapley (CNS, NYU) David McLaughlin (Courant, NYU)
Synaptic Integration of Olfactory Information in Mouse Anterior Olfactory Nucleus
 The Journal of Neuroscience, November 15, 2006 26(46):12023 12032 12023 Behavioral/Systems/Cognitive Synaptic Integration of Olfactory Information in Mouse Anterior Olfactory Nucleus Huimeng Lei, 1,2 Richard
The Journal of Neuroscience, November 15, 2006 26(46):12023 12032 12023 Behavioral/Systems/Cognitive Synaptic Integration of Olfactory Information in Mouse Anterior Olfactory Nucleus Huimeng Lei, 1,2 Richard
Divisive Inhibition in Recurrent Networks
 Divisive Inhibition in Recurrent Networks Frances S. Chance and L. F. Abbott Volen Center for Complex Systems and Department of Biology Brandeis University Waltham MA 2454-911 Abstract Models of visual
Divisive Inhibition in Recurrent Networks Frances S. Chance and L. F. Abbott Volen Center for Complex Systems and Department of Biology Brandeis University Waltham MA 2454-911 Abstract Models of visual
Lecture 4: Feed Forward Neural Networks
 Lecture 4: Feed Forward Neural Networks Dr. Roman V Belavkin Middlesex University BIS4435 Biological neurons and the brain A Model of A Single Neuron Neurons as data-driven models Neural Networks Training
Lecture 4: Feed Forward Neural Networks Dr. Roman V Belavkin Middlesex University BIS4435 Biological neurons and the brain A Model of A Single Neuron Neurons as data-driven models Neural Networks Training
Synaptic plasticity in neuromorphic hardware. Stefano Fusi Columbia University
 Synaptic plasticity in neuromorphic hardware Stefano Fusi Columbia University The memory problem Several efficient memory models assume that the synaptic dynamic variables are unbounded, or can be modified
Synaptic plasticity in neuromorphic hardware Stefano Fusi Columbia University The memory problem Several efficient memory models assume that the synaptic dynamic variables are unbounded, or can be modified
Subthreshold cross-correlations between cortical neurons: Areference model with static synapses
 Neurocomputing 65 66 (25) 685 69 www.elsevier.com/locate/neucom Subthreshold cross-correlations between cortical neurons: Areference model with static synapses Ofer Melamed a,b, Gilad Silberberg b, Henry
Neurocomputing 65 66 (25) 685 69 www.elsevier.com/locate/neucom Subthreshold cross-correlations between cortical neurons: Areference model with static synapses Ofer Melamed a,b, Gilad Silberberg b, Henry
The Molecular Logic of Olfaction in Drosophila
 Chem. Senses 26: 207 213, 2001 The Molecular Logic of Olfaction in Drosophila Leslie B. Vosshall Center for Neurobiology and Behavior, Columbia University, 701 West 168th Street, New York, NY 10032, USA
Chem. Senses 26: 207 213, 2001 The Molecular Logic of Olfaction in Drosophila Leslie B. Vosshall Center for Neurobiology and Behavior, Columbia University, 701 West 168th Street, New York, NY 10032, USA
Hopfield Neural Network and Associative Memory. Typical Myelinated Vertebrate Motoneuron (Wikipedia) Topic 3 Polymers and Neurons Lecture 5
 Hopfield Neural Network and Associative Memory Typical Myelinated Vertebrate Motoneuron (Wikipedia) PHY 411-506 Computational Physics 2 1 Wednesday, March 5 1906 Nobel Prize in Physiology or Medicine.
Hopfield Neural Network and Associative Memory Typical Myelinated Vertebrate Motoneuron (Wikipedia) PHY 411-506 Computational Physics 2 1 Wednesday, March 5 1906 Nobel Prize in Physiology or Medicine.
Similarity alignment a new theory of neural computation. Dmitri Mitya Chklovskii
 Similarity alignment a new theory of neural computation Dmitri Mitya Chklovskii Flatiron Institute Neuroscience Institute Simons Foundation NYU Medical Center Imaging neural population activity 50μm Raw
Similarity alignment a new theory of neural computation Dmitri Mitya Chklovskii Flatiron Institute Neuroscience Institute Simons Foundation NYU Medical Center Imaging neural population activity 50μm Raw
Fast neural network simulations with population density methods
 Fast neural network simulations with population density methods Duane Q. Nykamp a,1 Daniel Tranchina b,a,c,2 a Courant Institute of Mathematical Science b Department of Biology c Center for Neural Science
Fast neural network simulations with population density methods Duane Q. Nykamp a,1 Daniel Tranchina b,a,c,2 a Courant Institute of Mathematical Science b Department of Biology c Center for Neural Science
A Dual Algorithm for Olfactory Computation in the Locust Brain
 A Dual Algorithm for Olfactory Computation in the Locust Brain Sina Tootoonian st582@eng.cam.ac.uk Máté Lengyel m.lengyel@eng.cam.ac.uk Computational & Biological Learning Laboratory Department of Engineering,
A Dual Algorithm for Olfactory Computation in the Locust Brain Sina Tootoonian st582@eng.cam.ac.uk Máté Lengyel m.lengyel@eng.cam.ac.uk Computational & Biological Learning Laboratory Department of Engineering,
CSE/NB 528 Final Lecture: All Good Things Must. CSE/NB 528: Final Lecture
 CSE/NB 528 Final Lecture: All Good Things Must 1 Course Summary Where have we been? Course Highlights Where do we go from here? Challenges and Open Problems Further Reading 2 What is the neural code? What
CSE/NB 528 Final Lecture: All Good Things Must 1 Course Summary Where have we been? Course Highlights Where do we go from here? Challenges and Open Problems Further Reading 2 What is the neural code? What
Lecture 2: Connectionist approach Elements and feedforward networks
 Short Course: Computation of Olfaction Lecture 2 Lecture 2: Connectionist approach Elements and feedforward networks Dr. Thomas Nowotny University of Sussex Connectionist approach The connectionist approach
Short Course: Computation of Olfaction Lecture 2 Lecture 2: Connectionist approach Elements and feedforward networks Dr. Thomas Nowotny University of Sussex Connectionist approach The connectionist approach
Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests
 Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests J. Benda, M. Bethge, M. Hennig, K. Pawelzik & A.V.M. Herz February, 7 Abstract Spike-frequency adaptation is a common feature of
Spike-Frequency Adaptation: Phenomenological Model and Experimental Tests J. Benda, M. Bethge, M. Hennig, K. Pawelzik & A.V.M. Herz February, 7 Abstract Spike-frequency adaptation is a common feature of
Rate- and Phase-coded Autoassociative Memory
 Rate- and Phase-coded Autoassociative Memory Máté Lengyel Peter Dayan Gatsby Computational Neuroscience Unit, University College London 7 Queen Square, London WCN 3AR, United Kingdom {lmate,dayan}@gatsby.ucl.ac.uk
Rate- and Phase-coded Autoassociative Memory Máté Lengyel Peter Dayan Gatsby Computational Neuroscience Unit, University College London 7 Queen Square, London WCN 3AR, United Kingdom {lmate,dayan}@gatsby.ucl.ac.uk
Linearization of F-I Curves by Adaptation
 LETTER Communicated by Laurence Abbott Linearization of F-I Curves by Adaptation Bard Ermentrout Department of Mathematics, University of Pittsburgh, Pittsburgh, PA 15260, U.S.A. We show that negative
LETTER Communicated by Laurence Abbott Linearization of F-I Curves by Adaptation Bard Ermentrout Department of Mathematics, University of Pittsburgh, Pittsburgh, PA 15260, U.S.A. We show that negative
Decoding olfaction in Drosophila Andreas Keller and Leslie B Vosshall y
 103 Decoding olfaction in Drosophila Andreas Keller and Leslie B Vosshall y Recent experiments in Drosophila demonstrate striking stereotypy in the neural architecture of the olfactory system. Functional
103 Decoding olfaction in Drosophila Andreas Keller and Leslie B Vosshall y Recent experiments in Drosophila demonstrate striking stereotypy in the neural architecture of the olfactory system. Functional
Neuroscience Introduction
 Neuroscience Introduction The brain As humans, we can identify galaxies light years away, we can study particles smaller than an atom. But we still haven t unlocked the mystery of the three pounds of matter
Neuroscience Introduction The brain As humans, we can identify galaxies light years away, we can study particles smaller than an atom. But we still haven t unlocked the mystery of the three pounds of matter
Modelling stochastic neural learning
 Modelling stochastic neural learning Computational Neuroscience András Telcs telcs.andras@wigner.mta.hu www.cs.bme.hu/~telcs http://pattern.wigner.mta.hu/participants/andras-telcs Compiled from lectures
Modelling stochastic neural learning Computational Neuroscience András Telcs telcs.andras@wigner.mta.hu www.cs.bme.hu/~telcs http://pattern.wigner.mta.hu/participants/andras-telcs Compiled from lectures
THE LOCUST OLFACTORY SYSTEM AS A CASE STUDY FOR MODELING DYNAMICS OF NEUROBIOLOGICAL NETWORKS: FROM DISCRETE TIME NEURONS TO CONTINUOUS TIME NEURONS
 1 THE LOCUST OLFACTORY SYSTEM AS A CASE STUDY FOR MODELING DYNAMICS OF NEUROBIOLOGICAL NETWORKS: FROM DISCRETE TIME NEURONS TO CONTINUOUS TIME NEURONS B. QUENET 1 AND G. HORCHOLLE-BOSSAVIT 2 1 Equipe de
1 THE LOCUST OLFACTORY SYSTEM AS A CASE STUDY FOR MODELING DYNAMICS OF NEUROBIOLOGICAL NETWORKS: FROM DISCRETE TIME NEURONS TO CONTINUOUS TIME NEURONS B. QUENET 1 AND G. HORCHOLLE-BOSSAVIT 2 1 Equipe de
Les sens chimiques Prof. Alan Carleton Département de Neurosciences Fondamentales
 Les sens chimiques Prof. Alan Carleton Département de Neurosciences Fondamentales Les sens chimiques Le système olfactif principal Organe voméronasal et détection des phéromones La perception des goûts
Les sens chimiques Prof. Alan Carleton Département de Neurosciences Fondamentales Les sens chimiques Le système olfactif principal Organe voméronasal et détection des phéromones La perception des goûts
Lateral organization & computation
 Lateral organization & computation review Population encoding & decoding lateral organization Efficient representations that reduce or exploit redundancy Fixation task 1rst order Retinotopic maps Log-polar
Lateral organization & computation review Population encoding & decoding lateral organization Efficient representations that reduce or exploit redundancy Fixation task 1rst order Retinotopic maps Log-polar
Left over from units..
 Left over from units.. Hodgkin-Huxleymodel Inet = g Na m 3 h(vm E Na )+g k n 4 (Vm E k )+(Vm E l ) m, h, n: voltage gating variables with their own dynamics that determine when channels open and close
Left over from units.. Hodgkin-Huxleymodel Inet = g Na m 3 h(vm E Na )+g k n 4 (Vm E k )+(Vm E l ) m, h, n: voltage gating variables with their own dynamics that determine when channels open and close
3 Neural Decoding. 3.1 Encoding and Decoding. (r 1, r 2,..., r N ) for N neurons is a list of spike-count firing rates, although,
 3 Neural Decoding 3.1 Encoding and Decoding In chapters 1 and 2, we considered the problem of predicting neural responses to known stimuli. The nervous system faces the reverse problem, determining what
3 Neural Decoding 3.1 Encoding and Decoding In chapters 1 and 2, we considered the problem of predicting neural responses to known stimuli. The nervous system faces the reverse problem, determining what
Excitatory Interactions between Olfactory Processing Channels in the Drosophila Antennal Lobe
 Article Excitatory Interactions between Olfactory Processing Channels in the Drosophila Antennal Lobe Shawn R. Olsen, 1,2 Vikas Bhandawat, 1,2 and Rachel I. Wilson 1, * 1 Department of Neurobiology, Harvard
Article Excitatory Interactions between Olfactory Processing Channels in the Drosophila Antennal Lobe Shawn R. Olsen, 1,2 Vikas Bhandawat, 1,2 and Rachel I. Wilson 1, * 1 Department of Neurobiology, Harvard
Probabilistic Models in Theoretical Neuroscience
 Probabilistic Models in Theoretical Neuroscience visible unit Boltzmann machine semi-restricted Boltzmann machine restricted Boltzmann machine hidden unit Neural models of probabilistic sampling: introduction
Probabilistic Models in Theoretical Neuroscience visible unit Boltzmann machine semi-restricted Boltzmann machine restricted Boltzmann machine hidden unit Neural models of probabilistic sampling: introduction
Hierarchy. Will Penny. 24th March Hierarchy. Will Penny. Linear Models. Convergence. Nonlinear Models. References
 24th March 2011 Update Hierarchical Model Rao and Ballard (1999) presented a hierarchical model of visual cortex to show how classical and extra-classical Receptive Field (RF) effects could be explained
24th March 2011 Update Hierarchical Model Rao and Ballard (1999) presented a hierarchical model of visual cortex to show how classical and extra-classical Receptive Field (RF) effects could be explained
1/12/2017. Computational neuroscience. Neurotechnology.
 Computational neuroscience Neurotechnology https://devblogs.nvidia.com/parallelforall/deep-learning-nutshell-core-concepts/ 1 Neurotechnology http://www.lce.hut.fi/research/cogntech/neurophysiology Recording
Computational neuroscience Neurotechnology https://devblogs.nvidia.com/parallelforall/deep-learning-nutshell-core-concepts/ 1 Neurotechnology http://www.lce.hut.fi/research/cogntech/neurophysiology Recording
Statistical model of evolution of brain parcellation. Daniel D. Ferrante, Yi Wei, and Alexei A. Koulakov
 1 Statistical model of evolution of brain parcellation Daniel D. Ferrante, Yi Wei, and Alexei A. Koulakov Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, 11724. USA. We study the distribution of
1 Statistical model of evolution of brain parcellation Daniel D. Ferrante, Yi Wei, and Alexei A. Koulakov Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, 11724. USA. We study the distribution of
1 Balanced networks: Trading speed for noise
 Physics 178/278 - David leinfeld - Winter 2017 (Corrected yet incomplete notes) 1 Balanced networks: Trading speed for noise 1.1 Scaling of neuronal inputs An interesting observation is that the subthresold
Physics 178/278 - David leinfeld - Winter 2017 (Corrected yet incomplete notes) 1 Balanced networks: Trading speed for noise 1.1 Scaling of neuronal inputs An interesting observation is that the subthresold
Effects of Interactive Function Forms in a Self-Organized Critical Model Based on Neural Networks
 Commun. Theor. Phys. (Beijing, China) 40 (2003) pp. 607 613 c International Academic Publishers Vol. 40, No. 5, November 15, 2003 Effects of Interactive Function Forms in a Self-Organized Critical Model
Commun. Theor. Phys. (Beijing, China) 40 (2003) pp. 607 613 c International Academic Publishers Vol. 40, No. 5, November 15, 2003 Effects of Interactive Function Forms in a Self-Organized Critical Model
Bayesian Inference. Will Penny. 24th February Bayesian Inference. Will Penny. Bayesian Inference. References
 24th February 2011 Given probabilities p(a), p(b), and the joint probability p(a, B), we can write the conditional probabilities p(b A) = p(a B) = p(a, B) p(a) p(a, B) p(b) Eliminating p(a, B) gives p(b
24th February 2011 Given probabilities p(a), p(b), and the joint probability p(a, B), we can write the conditional probabilities p(b A) = p(a B) = p(a, B) p(a) p(a, B) p(b) Eliminating p(a, B) gives p(b
High-conductance states in a mean-eld cortical network model
 Neurocomputing 58 60 (2004) 935 940 www.elsevier.com/locate/neucom High-conductance states in a mean-eld cortical network model Alexander Lerchner a;, Mandana Ahmadi b, John Hertz b a Oersted-DTU, Technical
Neurocomputing 58 60 (2004) 935 940 www.elsevier.com/locate/neucom High-conductance states in a mean-eld cortical network model Alexander Lerchner a;, Mandana Ahmadi b, John Hertz b a Oersted-DTU, Technical
Plasticity and Learning
 Chapter 8 Plasticity and Learning 8.1 Introduction Activity-dependent synaptic plasticity is widely believed to be the basic phenomenon underlying learning and memory, and it is also thought to play a
Chapter 8 Plasticity and Learning 8.1 Introduction Activity-dependent synaptic plasticity is widely believed to be the basic phenomenon underlying learning and memory, and it is also thought to play a
Synchrony in Neural Systems: a very brief, biased, basic view
 Synchrony in Neural Systems: a very brief, biased, basic view Tim Lewis UC Davis NIMBIOS Workshop on Synchrony April 11, 2011 components of neuronal networks neurons synapses connectivity cell type - intrinsic
Synchrony in Neural Systems: a very brief, biased, basic view Tim Lewis UC Davis NIMBIOS Workshop on Synchrony April 11, 2011 components of neuronal networks neurons synapses connectivity cell type - intrinsic
Binary synapses: better than expected
 October 13, 2008. version 8. 1 Binary synapses: better than expected 1 Introduction Memory is an absolutely critical component in any biological system. Without it, learning would be impossible, and behavior
October 13, 2008. version 8. 1 Binary synapses: better than expected 1 Introduction Memory is an absolutely critical component in any biological system. Without it, learning would be impossible, and behavior
Chemosensory System. Spring 2013 Royce Mohan, PhD Reading: Chapter 15, Neuroscience by Purves et al; FiGh edihon (Sinauer Publishers)
 Chemosensory System Spring 2013 Royce Mohan, PhD mohan@uchc.edu Reading: Chapter 15, Neuroscience by Purves et al; FiGh edihon (Sinauer Publishers) Learning ObjecHves Anatomical and funchonal organizahon
Chemosensory System Spring 2013 Royce Mohan, PhD mohan@uchc.edu Reading: Chapter 15, Neuroscience by Purves et al; FiGh edihon (Sinauer Publishers) Learning ObjecHves Anatomical and funchonal organizahon
Several ways to solve the MSO problem
 Several ways to solve the MSO problem J. J. Steil - Bielefeld University - Neuroinformatics Group P.O.-Box 0 0 3, D-3350 Bielefeld - Germany Abstract. The so called MSO-problem, a simple superposition
Several ways to solve the MSO problem J. J. Steil - Bielefeld University - Neuroinformatics Group P.O.-Box 0 0 3, D-3350 Bielefeld - Germany Abstract. The so called MSO-problem, a simple superposition
A Model for Real-Time Computation in Generic Neural Microcircuits
 A Model for Real-Time Computation in Generic Neural Microcircuits Wolfgang Maass, Thomas Natschläger Institute for Theoretical Computer Science Technische Universitaet Graz A-81 Graz, Austria maass, tnatschl
A Model for Real-Time Computation in Generic Neural Microcircuits Wolfgang Maass, Thomas Natschläger Institute for Theoretical Computer Science Technische Universitaet Graz A-81 Graz, Austria maass, tnatschl
OLFACTORY NETWORK DYNAMICS AND THE CODING OF MULTIDIMENSIONAL SIGNALS
 OLFACTORY NETWORK DYNAMICS AND THE CODING OF MULTIDIMENSIONAL SIGNALS Gilles Laurent The brain faces many complex problems when dealing with odorant signals. Odours are multidimensional objects, which
OLFACTORY NETWORK DYNAMICS AND THE CODING OF MULTIDIMENSIONAL SIGNALS Gilles Laurent The brain faces many complex problems when dealing with odorant signals. Odours are multidimensional objects, which
ECE521 week 3: 23/26 January 2017
 ECE521 week 3: 23/26 January 2017 Outline Probabilistic interpretation of linear regression - Maximum likelihood estimation (MLE) - Maximum a posteriori (MAP) estimation Bias-variance trade-off Linear
ECE521 week 3: 23/26 January 2017 Outline Probabilistic interpretation of linear regression - Maximum likelihood estimation (MLE) - Maximum a posteriori (MAP) estimation Bias-variance trade-off Linear
Recipes for the Linear Analysis of EEG and applications
 Recipes for the Linear Analysis of EEG and applications Paul Sajda Department of Biomedical Engineering Columbia University Can we read the brain non-invasively and in real-time? decoder 1001110 if YES
Recipes for the Linear Analysis of EEG and applications Paul Sajda Department of Biomedical Engineering Columbia University Can we read the brain non-invasively and in real-time? decoder 1001110 if YES
Linear and Non-Linear Responses to Dynamic Broad-Band Spectra in Primary Auditory Cortex
 Linear and Non-Linear Responses to Dynamic Broad-Band Spectra in Primary Auditory Cortex D. J. Klein S. A. Shamma J. Z. Simon D. A. Depireux,2,2 2 Department of Electrical Engineering Supported in part
Linear and Non-Linear Responses to Dynamic Broad-Band Spectra in Primary Auditory Cortex D. J. Klein S. A. Shamma J. Z. Simon D. A. Depireux,2,2 2 Department of Electrical Engineering Supported in part
Iterative face image feature extraction with Generalized Hebbian Algorithm and a Sanger-like BCM rule
 Iterative face image feature extraction with Generalized Hebbian Algorithm and a Sanger-like BCM rule Clayton Aldern (Clayton_Aldern@brown.edu) Tyler Benster (Tyler_Benster@brown.edu) Carl Olsson (Carl_Olsson@brown.edu)
Iterative face image feature extraction with Generalized Hebbian Algorithm and a Sanger-like BCM rule Clayton Aldern (Clayton_Aldern@brown.edu) Tyler Benster (Tyler_Benster@brown.edu) Carl Olsson (Carl_Olsson@brown.edu)
Learning features by contrasting natural images with noise
 Learning features by contrasting natural images with noise Michael Gutmann 1 and Aapo Hyvärinen 12 1 Dept. of Computer Science and HIIT, University of Helsinki, P.O. Box 68, FIN-00014 University of Helsinki,
Learning features by contrasting natural images with noise Michael Gutmann 1 and Aapo Hyvärinen 12 1 Dept. of Computer Science and HIIT, University of Helsinki, P.O. Box 68, FIN-00014 University of Helsinki,
Modeling retinal high and low contrast sensitivity lters. T. Lourens. Abstract
 Modeling retinal high and low contrast sensitivity lters T. Lourens Department of Computer Science University of Groningen P.O. Box 800, 9700 AV Groningen, The Netherlands E-mail: tino@cs.rug.nl Abstract
Modeling retinal high and low contrast sensitivity lters T. Lourens Department of Computer Science University of Groningen P.O. Box 800, 9700 AV Groningen, The Netherlands E-mail: tino@cs.rug.nl Abstract
