Denser Sensor 4 th Grade Kelly Krupa
|
|
|
- Dorothy Lee
- 6 years ago
- Views:
Transcription
1 Denser Sensor 4 th Grade Kelly Krupa Benchmark: (4 th ) SLC 3: Students will use metric measurements given for two and three-dimensional objects to determine a size relationship between those objects. (4 th ) SLC 8: A.) Students will evaluate and provide written observations of experiments (including measurements, attributes, etc.) from a variety of sources (i.e., other students, books, media, etc.) and determine if results are derived from direct observations or inferences. B.) Students will use observations and data when explaining answers and formulating conclusions. Objectives: Students will be able to calculate density, compare densities of solids and liquids, measure liquids, weigh using a balance, and discuss the importance of density. Materials: Clear containers (with a flat bottom that will hold at least 200ml of a liquid) Molasses Corks Water Erasers Oil Triple Beam Balance Marbles Beaker that measures 50 ml Procedure: For a one-day procedure, give the students the raw numbers in the chart below and let them figure out the rest. Pour measured liquids into a container and record observations, then continue with Day 2. I. Mass of Empty Test Tube in grams II. Mass of Container with Liquid (grams) III. Mass of Liquid (II-I) in grams IV. Volume of Liquid (ml) V. Density Mass Volume (III IV) g/ml Liquid s Name A Molasses B Water C Oil Day 1: Give each table 3 containers. Have them label the containers A, B, and C. Have the students measure 50ml of water to pour into each container. Have them mark this height with a marker or tape (if you want this to go faster, pre-mark each container with a 50ml line). The students must empty and dry each container before weighing them. Weigh each container, recording results in chart. Add 50ml of molasses to A, 50ml of water to B, and 50ml of oil to C. Reweigh each container separately and record results. Have students do the math to figure out the mass of the liquid, then the density. Discuss which liquid is the densest, which is the least dense. Have students leave the molasses in the container and add the second densest liquid, water. Then have them add the oil on top of that. Record observations on sheet. Day 2: Review what density means in simple words (thickness, how crowded together things are) and the order of the liquids densities. Give each table 3 solids to identify (marble, cork, and eraser). One at a time, carefully drop into container. Let the solids come to rest and compare their densities by filling in #10 on
2 their sheet. Have them draw and label their containers in #11. This can be done first to help with #10 if they need. Answer the next questions and have a discussion of their answers. Discussion/Summary: After discussing that density is mass/volume or how thick something is (#12), review the order of densities found out (#13). Students should be able to tell you that oil floats on water because it is less dense (#14). The additional questions can be held as a discussion or written out. These help to tie in why we care to know about density. A real world tie from what seems like math is always best to make them see a connection. Target Observations: The molasses is the densest liquid, then water, then oil The order of densities is: marble, molasses, eraser, water, oil, cork Target Model: -Density is the thickness of something. For example if there are lots of people in a room, we could say the crowd is dense. -Objects that are less dense float or sit on top of objects that are more dense.
3 Name: Date: Denser Sensor! 1. Circle the densest liquid: water oil molasses 2. Name an object that will float on water: 3. Label the 3 containers A, B, and C. Weigh each one empty. 4. Pour 50 ml of liquid into each. 5. Weigh the full containers, subtract, and compute the density of each liquid. I. Mass of Empty Test Tube in grams II. Mass of Container with Liquid (grams) III. Mass of Liquid (II-I) in grams IV. Volume of Liquid (ml) V. Density Mass Volume (III IV) g/ml Liquid s Name A B C 6. Keep the densest liquid in its container. Pour the next densest on top of it. Pour the least dense liquid on top of that. Record your observations.
4 7. Identify the solids: #1: #2: #3: 8. Drop the solids (one at a time) into the container with the liquids. 9. Allow them to come to rest. 10. Compare the solids to the liquids by completing the following statements. The density of solid #1 is greater than and less than. The density of solid #2 is greater than and less than. The density of solid #3 is greater than and less than.
5 11. Draw and label the solids and liquids after you poured and dropped them into your container. Solids Liquids 12. What is the density of an object? 13. Sort the 6 objects/liquids in order of density. Most dense Least dense 14. Why does oil float on water?
6 Additional questions: 15. Under #13, write down your calculated densities for the 3 liquids. Estimate what the densities might be for the 3 solids based on where they fell and what the densities are around them. 16. How do weather and density work together? In cold fronts? In warm fronts? When a large ship spilled lots of oil on the ocean near Alaska, what happened to the oil? What are some things we could do to clean up the spilled oil?
Date: / Page #: 4. The diagram below show an enlarged view of the beams of a triple-beam balance.
 Name: Review Packet - Unit 2 1. Two objects A and B were placed in two vials with different liquids C and D in them. This diagram shows what happened to each object when placed in the vial. Date: / Page
Name: Review Packet - Unit 2 1. Two objects A and B were placed in two vials with different liquids C and D in them. This diagram shows what happened to each object when placed in the vial. Date: / Page
Why does a hot air balloon rise up in the air? Record your ideas on the lines below. Why are gases less dense than liquids?
 Fluids and Density Before You Read Why does a hot air balloon rise up in the air? Record your ideas on the lines below. What are fluids? A fluid is any form of matter that can flow. Liquids and gases are
Fluids and Density Before You Read Why does a hot air balloon rise up in the air? Record your ideas on the lines below. What are fluids? A fluid is any form of matter that can flow. Liquids and gases are
KEY 1 = PAN 2 = RIDERS 3 = BEAMS 4 = POINTER ~ Metric Measurement Scientist
 Metric Measurement Scientist Mass Lab Class Date 2015 Objective: To use a triple-beam balance to these 3 together = 1 pt KEY a) to measure mass directly usually a SOLID b) to find mass by difference usually
Metric Measurement Scientist Mass Lab Class Date 2015 Objective: To use a triple-beam balance to these 3 together = 1 pt KEY a) to measure mass directly usually a SOLID b) to find mass by difference usually
1. measure of how a measurement comes to the or true value of whatever is Example: 2. measure of how close a of measurements are to Example:
 Chemistry Chapter 3: Metric, Measuring, Scientific Notation & Significant Figures. Name: 3-1 Measurements A. Scientific Notation: A number written as the product of two numbers: a and raised to a power.
Chemistry Chapter 3: Metric, Measuring, Scientific Notation & Significant Figures. Name: 3-1 Measurements A. Scientific Notation: A number written as the product of two numbers: a and raised to a power.
Chesapeake Campus Chemistry 111 Laboratory
 Chesapeake Campus Chemistry 111 Laboratory Objectives Calculate the density of a sugar solution. Evaluate lab sources of error and their effect on an experiment. Introduction The density of an object is
Chesapeake Campus Chemistry 111 Laboratory Objectives Calculate the density of a sugar solution. Evaluate lab sources of error and their effect on an experiment. Introduction The density of an object is
Part I: How Dense Is It? Fundamental Question: What is matter, and how do we identify it?
 Part I: How Dense Is It? Fundamental Question: What is matter, and how do we identify it? Everything on Earth is made of matter. Matter is as simple as a single element or as complex as the entire planet.
Part I: How Dense Is It? Fundamental Question: What is matter, and how do we identify it? Everything on Earth is made of matter. Matter is as simple as a single element or as complex as the entire planet.
Mass, Volume, & Density
 Mass, Volume, & Density Short Informational Videos Mass Volume & Density Buoyancy Mass Measurement of the amount of matter (or stuff) in an object Measured in grams (g) There are 3 states of matter: Solid
Mass, Volume, & Density Short Informational Videos Mass Volume & Density Buoyancy Mass Measurement of the amount of matter (or stuff) in an object Measured in grams (g) There are 3 states of matter: Solid
Physics. Practical 5: Density. Practical Objective. Content Objective. Apparatus. Your teacher may watch to see if you can:
 The density of a substance is the mass of a unit volume of that substance. Almost all substances are most dense when they are solids and least dense when they are gases. The arrangement of particles can
The density of a substance is the mass of a unit volume of that substance. Almost all substances are most dense when they are solids and least dense when they are gases. The arrangement of particles can
Measurements in the Laboratory
 Measurements in the Laboratory Objectives The objectives of this laboratory are: a) Use standard laboratory measurement devices to measure length, volume and mass amounts. b) Use these measurements to
Measurements in the Laboratory Objectives The objectives of this laboratory are: a) Use standard laboratory measurement devices to measure length, volume and mass amounts. b) Use these measurements to
Lab 1: Precision and accuracy in glassware, and the determination of density
 Chemistry 140 Please have the following pages ready before class on Monday, October 2. Note that the different parts will be standard divisions in all lab writeups. For this particular writeup, please
Chemistry 140 Please have the following pages ready before class on Monday, October 2. Note that the different parts will be standard divisions in all lab writeups. For this particular writeup, please
Activity Sheet Chapter 3, Lesson 3 Density of water
 Activity Sheet Chapter 3, Lesson 3 Density of water Name Date DEMONSTRATION 1. One of your classmates lifted different amounts of water. The largest amount of water also had the most mass. You know how
Activity Sheet Chapter 3, Lesson 3 Density of water Name Date DEMONSTRATION 1. One of your classmates lifted different amounts of water. The largest amount of water also had the most mass. You know how
Limiting Reactants Lab
 Name: Teacher s Name: Class: Block: Date: Partners: Limiting Reactants Lab Purpose: Through experimentation, determine the limiting reactant and the percent yield in a chemical reaction that generates
Name: Teacher s Name: Class: Block: Date: Partners: Limiting Reactants Lab Purpose: Through experimentation, determine the limiting reactant and the percent yield in a chemical reaction that generates
Cut here
 LAB SAFETY MINI BOOK RUBRIC Self and Guardian Evaluation Sheet Directions: Students will make a mini book that tells a creative story which incorporates 7 important lab safety rules. Student must NOT simply
LAB SAFETY MINI BOOK RUBRIC Self and Guardian Evaluation Sheet Directions: Students will make a mini book that tells a creative story which incorporates 7 important lab safety rules. Student must NOT simply
DENSITY CALCULATIONS
 PRACTICE Name: Date: Class: DENSITY CALCULATIONS Density is a measure of how much matter exists within a given volume. The mass of an object tells you how many atoms exist within a certain substance (amount
PRACTICE Name: Date: Class: DENSITY CALCULATIONS Density is a measure of how much matter exists within a given volume. The mass of an object tells you how many atoms exist within a certain substance (amount
Grade 3. Everyday Math: Unit. Fractions. Study Guide. EDM Version 4
 Grade Everyday Math: Unit Fractions Study Guide EDM Version Thank you! Catherine Wiist @ Abc2isme http://www.teacherspayteachers.com/store/abc2isme (All new products are discounted for the first 8 hours!
Grade Everyday Math: Unit Fractions Study Guide EDM Version Thank you! Catherine Wiist @ Abc2isme http://www.teacherspayteachers.com/store/abc2isme (All new products are discounted for the first 8 hours!
Weather Tanks. NC Standards 5.E.1, 5.P.2.1 Page 3. Grade 5 Earth Science, Physical Science. Activity Description & Estimated Class Time.
 Weather Tanks NC Standards 5.E.1, 5.P.2.1 Page 3 Grade 5 Earth Science, Physical Science Throughout the guide, teaching tips are in red. Activity Description & Estimated Class Time Objectives This activity
Weather Tanks NC Standards 5.E.1, 5.P.2.1 Page 3 Grade 5 Earth Science, Physical Science Throughout the guide, teaching tips are in red. Activity Description & Estimated Class Time Objectives This activity
The Molecular Weight of Carbon Dioxide
 The Molecular Weight of Carbon Dioxide Objectives The objectives of this laboratory are as follows: To generate and collect a sample of carbon dioxide gas, then measure its pressure, volume, temperature
The Molecular Weight of Carbon Dioxide Objectives The objectives of this laboratory are as follows: To generate and collect a sample of carbon dioxide gas, then measure its pressure, volume, temperature
Geology Rocks Minerals Earthquakes Natural Resources. Meteorology. Oceanography. Astronomy. Weather Storms Warm fronts Cold fronts
 Geology Rocks Minerals Earthquakes Natural Resources Meteorology Weather Storms Warm fronts Cold fronts Oceanography Mid ocean ridges Tsunamis Astronomy Space Stars Planets Moon Prologue 1 Prologue I.
Geology Rocks Minerals Earthquakes Natural Resources Meteorology Weather Storms Warm fronts Cold fronts Oceanography Mid ocean ridges Tsunamis Astronomy Space Stars Planets Moon Prologue 1 Prologue I.
PREPARE FOR THE ACTIVITY. Activity Sheet Chapter 6, Lesson 8 ph and Color Change
 Activity Sheet Chapter 6, Lesson 8 ph and Color Change Name Date DEMONSTRATION 1. Your teacher poured green universal indicator into each of two cups. What does the change in color of the indicator solution
Activity Sheet Chapter 6, Lesson 8 ph and Color Change Name Date DEMONSTRATION 1. Your teacher poured green universal indicator into each of two cups. What does the change in color of the indicator solution
Test Review: Scientific Method and Measurement ANSWER KEY
 Test Review: Scientific Method and Measurement ANSWER KEY Remember that in order to be eligible for a retake you must complete this review sheet before the test. Scientific Method: Problem: Must be a question,
Test Review: Scientific Method and Measurement ANSWER KEY Remember that in order to be eligible for a retake you must complete this review sheet before the test. Scientific Method: Problem: Must be a question,
Name: Period: Air Masses Notes 7 Minutes Page 2 Watch the air masses video. Make sure you follow along.
 Air Masses and Fronts Activity Guide Component 8.3.3, 8.3.4, & 8.3.5 Guiding Questions (be able to answer these questions at the end of packet) 1. How are air masses formed? 2. What are the differences
Air Masses and Fronts Activity Guide Component 8.3.3, 8.3.4, & 8.3.5 Guiding Questions (be able to answer these questions at the end of packet) 1. How are air masses formed? 2. What are the differences
LESSON 4: Buoyant Butter ESTIMATED TIME Setup: 5 minutes Procedure: 5 10 minutes
 LESSON 4: Buoyant Butter ESTIMATED TIME Setup: 5 minutes Procedure: 5 10 minutes DESCRIPTION Calculate the density of a stick of butter to determine if it will sink or float in water. OBJECTIVE This lesson
LESSON 4: Buoyant Butter ESTIMATED TIME Setup: 5 minutes Procedure: 5 10 minutes DESCRIPTION Calculate the density of a stick of butter to determine if it will sink or float in water. OBJECTIVE This lesson
Be a Scientist Notebook. Student Journal. Grade 5
 Be a Scientist Notebook Student Journal Grade 5 MODULE OPENER Name Date Structure and Properties of Matter Science in Our World Barges can be used to transport large quantities of goods from one place
Be a Scientist Notebook Student Journal Grade 5 MODULE OPENER Name Date Structure and Properties of Matter Science in Our World Barges can be used to transport large quantities of goods from one place
Density and Differentiation. Science Starter and Vocabulary
 Density and Differentiation Science Starter and Vocabulary Science Starter Answer the following and turn in. You have 5 minutes to complete. Use complete sentences to answer the following question. Be
Density and Differentiation Science Starter and Vocabulary Science Starter Answer the following and turn in. You have 5 minutes to complete. Use complete sentences to answer the following question. Be
Measuring Matter - Study Guide
 Name Hour Measuring Matter - Study Guide Goal 1: 1. Measure the lines to the nearest millimeter and to the nearest tenth of a centimeter (2.3 cm). 71 mm 7.1_cm Within 1mm is acceptable answer 136 mm _13.6_cm
Name Hour Measuring Matter - Study Guide Goal 1: 1. Measure the lines to the nearest millimeter and to the nearest tenth of a centimeter (2.3 cm). 71 mm 7.1_cm Within 1mm is acceptable answer 136 mm _13.6_cm
Topic 1: Scientific Method Observation vs. Inference 1. Write 2 observations about this cartoon.
 Name: Section: Earth Science Review Topic 1: Scientific Method Observation vs. Inference 1. Write 2 observations about this cartoon. Observation 1: Observation 2: 2. Write 2 inferences about this cartoon.
Name: Section: Earth Science Review Topic 1: Scientific Method Observation vs. Inference 1. Write 2 observations about this cartoon. Observation 1: Observation 2: 2. Write 2 inferences about this cartoon.
MASS, VOLUME, AND DENSITY HOW TO MAKE LIQUIDS LAYERED!
 MASS, VOLUME, AND DENSITY HOW TO MAKE LIQUIDS LAYERED! MASS A measurement of the amount of matter in an object Can be measured with a triple beam balance or electronic balance It is measured in grams!
MASS, VOLUME, AND DENSITY HOW TO MAKE LIQUIDS LAYERED! MASS A measurement of the amount of matter in an object Can be measured with a triple beam balance or electronic balance It is measured in grams!
Name Class Date. How do mixtures differ from elements and compounds? How can mixtures be separated? What are solutions?
 CHAPTER 3 3 Mixtures SECTION Elements, Compounds, and Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: How do mixtures differ from elements and compounds?
CHAPTER 3 3 Mixtures SECTION Elements, Compounds, and Mixtures BEFORE YOU READ After you read this section, you should be able to answer these questions: How do mixtures differ from elements and compounds?
After you read this section, you should be able to answer these questions:
 CHAPTER 3 12 SECTION Properties of Matter Physical Properties California Science Standards 8.7.c, 8.8.a, 8.8.b, 8.8.d BEFORE YOU READ After you read this section, you should be able to answer these questions:
CHAPTER 3 12 SECTION Properties of Matter Physical Properties California Science Standards 8.7.c, 8.8.a, 8.8.b, 8.8.d BEFORE YOU READ After you read this section, you should be able to answer these questions:
Measuring SKILLS INTRODUCTION
 SKILLS INTRODUCTION Measuring If you enjoy sports, you know how exciting it is when an athlete swims faster, runs longer, or hits a ball farther than other competitors. You also know that people aren t
SKILLS INTRODUCTION Measuring If you enjoy sports, you know how exciting it is when an athlete swims faster, runs longer, or hits a ball farther than other competitors. You also know that people aren t
MEASUREMENT IN THE LABORATORY
 1 MEASUREMENT IN THE LABORATORY INTRODUCTION Today's experiment will introduce you to some simple but important types of measurements commonly used by the chemist. You will measure lengths of objects,
1 MEASUREMENT IN THE LABORATORY INTRODUCTION Today's experiment will introduce you to some simple but important types of measurements commonly used by the chemist. You will measure lengths of objects,
2. Can you describe how temperature and dissolved solids changes the density of water?
 Unit 4: Oceanography LT 4.1 Density: I can explain the role density plays to help form some currents. #1 Yes I can: 1. Can you explain what density is and how you calculate it? 2. Can you describe how
Unit 4: Oceanography LT 4.1 Density: I can explain the role density plays to help form some currents. #1 Yes I can: 1. Can you explain what density is and how you calculate it? 2. Can you describe how
Chapter 3, Lesson 1: What is Density?
 Chapter 3, Lesson 1: What is Density? Key Concepts Density is a characteristic property of a substance. The density of a substance is the relationship between the mass of the substance and how much space
Chapter 3, Lesson 1: What is Density? Key Concepts Density is a characteristic property of a substance. The density of a substance is the relationship between the mass of the substance and how much space
MS20 Laboratory Seawater Salinity and Density
 MS20 Laboratory Seawater Salinity and Density Introduction As you perform these experiments, pay particular attention to the results different methods produce different levels of precision and accuracy.
MS20 Laboratory Seawater Salinity and Density Introduction As you perform these experiments, pay particular attention to the results different methods produce different levels of precision and accuracy.
AT SEA: INVESTIGATING SOME OCEAN DYNAMICS
 NAME DATE PARTNER(S) AT SEA: INVESTIGATING SOME OCEAN DYNAMICS About three-quarters of the Earth s surface are covered by water in the form of oceans. These giant bodies of water have fascinated and intimidated
NAME DATE PARTNER(S) AT SEA: INVESTIGATING SOME OCEAN DYNAMICS About three-quarters of the Earth s surface are covered by water in the form of oceans. These giant bodies of water have fascinated and intimidated
Station 1: measuring & graphing short lengths
 Station 1: measuring & graphing short lengths 1. Read the task carefully. 2. Make a TABLE to collect your data. 3. Collect your data. 4. Graph the data using a bar graph. 5. Clean up the station & put
Station 1: measuring & graphing short lengths 1. Read the task carefully. 2. Make a TABLE to collect your data. 3. Collect your data. 4. Graph the data using a bar graph. 5. Clean up the station & put
Simulation: Density FOR THE TEACHER
 Simulation: Density FOR THE TEACHER Summary In this simulation, students will investigate the effect of changing variables on both the volume and the density of a solid, a liquid and a gas sample. Students
Simulation: Density FOR THE TEACHER Summary In this simulation, students will investigate the effect of changing variables on both the volume and the density of a solid, a liquid and a gas sample. Students
EQ: How do we use the metric system in science?
 #2 EQ: How do we use the metric system in science? Introduction to the Metric System In science class, we will be using the International System (SI) for measurements. (SI is French for Systeme Internationale)
#2 EQ: How do we use the metric system in science? Introduction to the Metric System In science class, we will be using the International System (SI) for measurements. (SI is French for Systeme Internationale)
Section 3. What Drives the Plates? What Do You See? Think About It. Investigate. Learning Outcomes
 Section 3 What Drives the Plates? What Do You See? Learning Outcomes In this section, you will Calculate the density of liquids and compare their densities with their position in a column of liquid. Observe
Section 3 What Drives the Plates? What Do You See? Learning Outcomes In this section, you will Calculate the density of liquids and compare their densities with their position in a column of liquid. Observe
Science Skills. Graduated Cylinder. Triple Beam Balance Hershey s Chocolate Candy Bar Hershey s Chocolate Candy Bar with Almonds
 Science Skills Never taste anything or put anything in your mouth without permission! Background Information: All matter takes up space (we call this volume, the amount of space something takes up) and
Science Skills Never taste anything or put anything in your mouth without permission! Background Information: All matter takes up space (we call this volume, the amount of space something takes up) and
CHM 130LL: The Metric System
 CHM 130LL: The Metric System In this experiment you will: Determine the volume of a drop of water using a graduated cylinder Determine the volume of an object by measuring its dimensions Determine the
CHM 130LL: The Metric System In this experiment you will: Determine the volume of a drop of water using a graduated cylinder Determine the volume of an object by measuring its dimensions Determine the
Test Review: Scientific Method and Measurement
 Test Review: Scientific Method and Measurement Remember that in order to be eligible for a retake you must complete this review sheet before the test. Scientific Method: Problem: Must be a question, must
Test Review: Scientific Method and Measurement Remember that in order to be eligible for a retake you must complete this review sheet before the test. Scientific Method: Problem: Must be a question, must
NAME: Log onto YouTube and search for jocrisci channel. All video listed with numbers below and sorted into playlists for easy access.
 NAME: Log onto YouTube and search for jocrisci channel. All video listed with numbers below and sorted into playlists for easy access. GRAPHING (Video 1.2) 1. Look at a data table: a. Determine which column
NAME: Log onto YouTube and search for jocrisci channel. All video listed with numbers below and sorted into playlists for easy access. GRAPHING (Video 1.2) 1. Look at a data table: a. Determine which column
Name: Period: Date: CHEMISTRY LAB #4 THE ILLUSION OF BLING: Using Density to Identify an Unknown Metal 90 MINUTES
 Name: Period: Date: KIPP NYC College Prep General Chemistry CHEMISTRY LAB #4 THE ILLUSION OF BLING: Using Density to Identify an Unknown Metal 90 MINUTES Do Now Pre- Lab Information: Lab Equipment and
Name: Period: Date: KIPP NYC College Prep General Chemistry CHEMISTRY LAB #4 THE ILLUSION OF BLING: Using Density to Identify an Unknown Metal 90 MINUTES Do Now Pre- Lab Information: Lab Equipment and
Process Skills Review
 Process Skills Review Warm-Up Define function Match the following C. 1. Puts out fire 2. Curved line of liquid in a graduated cylinder 3. Used to observe insects A. B. Which of the following describes
Process Skills Review Warm-Up Define function Match the following C. 1. Puts out fire 2. Curved line of liquid in a graduated cylinder 3. Used to observe insects A. B. Which of the following describes
Read & Learn. Read the provided article. Use the information in the reading to answer the questions on the task cards on your answer sheet.
 Read & Learn Read the provided article. Use the information in the reading to answer the questions on the task cards on your answer sheet. Make sure your answers in the correct spot on the answer sheet.
Read & Learn Read the provided article. Use the information in the reading to answer the questions on the task cards on your answer sheet. Make sure your answers in the correct spot on the answer sheet.
Floaters and Sinkers. Synopsis. Objectives. Materials
 Floaters and Sinkers Synopsis Students will gain an intuitive understanding of density by comparing objects of equal volumes but which have different masses. They will then use two different methods to
Floaters and Sinkers Synopsis Students will gain an intuitive understanding of density by comparing objects of equal volumes but which have different masses. They will then use two different methods to
Density Destiny Data Analysis Worksheet
 Density Destiny Data Analysis Worksheet Name: Teacher: Date: Background Review 1. Density means: 2. To find the density of an object, you need to calculate the object s mass(g) and divide it by the object
Density Destiny Data Analysis Worksheet Name: Teacher: Date: Background Review 1. Density means: 2. To find the density of an object, you need to calculate the object s mass(g) and divide it by the object
ELEMENTARY SCIENCE PROGRAM MATH, SCIENCE & TECHNOLOGY EDUCATION. A Collection of Learning Experiences Density Revised July 2006
 ELEMENTARY SCIENCE PROGRAM MATH, SCIENCE & TECHNOLOGY EDUCATION A Collection of Learning Experiences Density Revised July 2006 CATTARAUGUS-ALLEGANY BOCES GRADE 4 TABLE OF CONTENTS Unit Overview...2 Format
ELEMENTARY SCIENCE PROGRAM MATH, SCIENCE & TECHNOLOGY EDUCATION A Collection of Learning Experiences Density Revised July 2006 CATTARAUGUS-ALLEGANY BOCES GRADE 4 TABLE OF CONTENTS Unit Overview...2 Format
FIGURE 1: INTERCONVERSION MAP
 The Mole For he shall be great before the Lord.And he shall convert many of the children of Israel to the Lord their God.. Luke 1:15-16 Introduction In the lab Counting by Measuring Mass, we explored the
The Mole For he shall be great before the Lord.And he shall convert many of the children of Israel to the Lord their God.. Luke 1:15-16 Introduction In the lab Counting by Measuring Mass, we explored the
Metric System. An Overview of the Concepts of Mass, Volume, Length, Temperature, and Density
 Metric System An Overview of the Concepts of Mass, Volume, Length, Temperature, and Density Length Definition The distance between two points along a straight line Meters (m) base unit Measuring track
Metric System An Overview of the Concepts of Mass, Volume, Length, Temperature, and Density Length Definition The distance between two points along a straight line Meters (m) base unit Measuring track
Measurement and Density
 Measurement and Density Goals q q q Learn to record accurate measurements from a variety of devices. Measure the density of solids and solutions. Use the property of density and measurement to calculate
Measurement and Density Goals q q q Learn to record accurate measurements from a variety of devices. Measure the density of solids and solutions. Use the property of density and measurement to calculate
Significant Figures a.k.a. Significant DIGITS
 1. Two students measure the liquid at right. Zoe says 20.3 ml, Brian says 21.50 ml. Describe why EACH student is incorrect. 2. Why do objects float? (tell me what you think) 3. Predict: If I put two cans
1. Two students measure the liquid at right. Zoe says 20.3 ml, Brian says 21.50 ml. Describe why EACH student is incorrect. 2. Why do objects float? (tell me what you think) 3. Predict: If I put two cans
Experiment Two Laboratory Balance: Mass Calculations
 Name: Lab Section: Experiment Two Laboratory Balance: Mass Calculations Objective The balance is used almost every day in the chemistry lab, understanding the proper use and care for the balance is essential
Name: Lab Section: Experiment Two Laboratory Balance: Mass Calculations Objective The balance is used almost every day in the chemistry lab, understanding the proper use and care for the balance is essential
Mahopac Central School District Curriculum Introduction to Science 8
 Introduction to Science 8 A. The goal of science is to understand the natural world 1. As you make new observations and test new explanations your view of the natural world may change again and again 2.
Introduction to Science 8 A. The goal of science is to understand the natural world 1. As you make new observations and test new explanations your view of the natural world may change again and again 2.
2 The Way Science Works
 CHAPTER 1 Introduction to Science 2 The Way Science Works SECTION KEY IDEAS As you read this section, keep these questions in mind: How can you use critical thinking to solve problems? What are scientific
CHAPTER 1 Introduction to Science 2 The Way Science Works SECTION KEY IDEAS As you read this section, keep these questions in mind: How can you use critical thinking to solve problems? What are scientific
BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN. SC.F The student knows that living things are different but share similar structures.
 activities 38&39 Stomata and Transpiration (Sessions I and II) BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN Grade 5 Quarter 4 Activities 38 & 39 SC.F.1.2.3 The student knows that living things are
activities 38&39 Stomata and Transpiration (Sessions I and II) BROWARD COUNTY ELEMENTARY SCIENCE BENCHMARK PLAN Grade 5 Quarter 4 Activities 38 & 39 SC.F.1.2.3 The student knows that living things are
grams, kilograms, milliliters and liters By L. Langton The Applicious Teacher
 : grams, kilograms, milliliters and liters By L. Langton The Applicious Teacher Thank you for purchasing this mini unit! These pages are designed to be quick prints to supplement your capacity and mass
: grams, kilograms, milliliters and liters By L. Langton The Applicious Teacher Thank you for purchasing this mini unit! These pages are designed to be quick prints to supplement your capacity and mass
ASSESSMENT CHART FOR INVESTIGATIONS 1 AND 2 STUDENT NAME
 ASSESSMENT CHART FOR INVESTIGATIONS 1 AND 2 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. STUDENT NAME Weather Measurements (TO)
ASSESSMENT CHART FOR INVESTIGATIONS 1 AND 2 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. STUDENT NAME Weather Measurements (TO)
Unit 2. Phases of Matter and Density
 Name Pd Unit 2 Phases of Matter and Density Name Pd Name Pd Homework for Unit 2 1. Vocab for Unit 2; due: 2. Pg 17 (1-5), pg 19 (1-5), pg21 (1-5) complete sentences; due: 3. Pg 23 (1-6), pg 27 (1-6) complete
Name Pd Unit 2 Phases of Matter and Density Name Pd Name Pd Homework for Unit 2 1. Vocab for Unit 2; due: 2. Pg 17 (1-5), pg 19 (1-5), pg21 (1-5) complete sentences; due: 3. Pg 23 (1-6), pg 27 (1-6) complete
Density. Go through the powerpoint and take notes on the back of your Density Webquest.
 Density Go through the powerpoint and take notes on the back of your Density Webquest. Which do you think would have the greater volume and mass? Why? 1 kg of feathers 1 kg of rock What the heck is density?
Density Go through the powerpoint and take notes on the back of your Density Webquest. Which do you think would have the greater volume and mass? Why? 1 kg of feathers 1 kg of rock What the heck is density?
Lab: Respiration and Photosynthesis in Plants
 Bio 101 Name: Lab: Respiration and Photosynthesis in Plants OBJECTIVES In this laboratory exploration, you will Use a ph probe to measure the ph of water. Use ph measurements to make inferences on the
Bio 101 Name: Lab: Respiration and Photosynthesis in Plants OBJECTIVES In this laboratory exploration, you will Use a ph probe to measure the ph of water. Use ph measurements to make inferences on the
Moles Lab Activity 1: PCU (Popcorn Counting Units)
 Moles Lab Activity 1: PCU (Popcorn Counting Units) Materials: A container of each of the following: Popcorn kernels Another type of beans A large unopened bag of popcorn Kernels Balance Safety goggles
Moles Lab Activity 1: PCU (Popcorn Counting Units) Materials: A container of each of the following: Popcorn kernels Another type of beans A large unopened bag of popcorn Kernels Balance Safety goggles
7 th Grade S2O1&2 Earth s Layers and Density concepts
 7 th Grade S2O1&2 Earth s Layers and Density concepts Whether something floats in a liquid or not depends on the density of both! If the object placed into the liquid has a higher or larger density than
7 th Grade S2O1&2 Earth s Layers and Density concepts Whether something floats in a liquid or not depends on the density of both! If the object placed into the liquid has a higher or larger density than
3. How many millimeters are in a centimeter? 10. The prefix milli- means a thousand. How many millimeters are in a meter? 1000.
 Name: Answer Key Period: Date: Measuring in Metric Purpose: The purpose of this activity is to practice using the metric system. To conduct a scientific investigation, a researcher must be able to make
Name: Answer Key Period: Date: Measuring in Metric Purpose: The purpose of this activity is to practice using the metric system. To conduct a scientific investigation, a researcher must be able to make
DO NOW LABEL LEFT AND RIGHT PAGES PROPERTIES OF MATTER: DENSITY
 DO NOW LABEL LEFT AND RIGHT PAGES PROPERTIES OF MATTER: DENSITY LAB DEBRIEF What was the independent (test) variable? What was the dependent (outcome) variable? Which trial was solid, liquid, gas? Explain.
DO NOW LABEL LEFT AND RIGHT PAGES PROPERTIES OF MATTER: DENSITY LAB DEBRIEF What was the independent (test) variable? What was the dependent (outcome) variable? Which trial was solid, liquid, gas? Explain.
Density Bundle Contents
 Density Bundle Contents Click the items in the list below to jump to that part of the PDF. Unit Bundle Directions... 2 Printing Orientation... 6 Foldable: Density... 7 Foldable: Density Observed in Objects...11
Density Bundle Contents Click the items in the list below to jump to that part of the PDF. Unit Bundle Directions... 2 Printing Orientation... 6 Foldable: Density... 7 Foldable: Density Observed in Objects...11
TORNADO IN A BOTTLE (1 Hour)
 (1 Hour) Addresses NGSS Level of Difficulty: 3 Grade Range: K-2 OVERVIEW In this activity, students will learn about tornadoes and simulate one inside a small bottle. Topic: Severe Weather Real-World Science
(1 Hour) Addresses NGSS Level of Difficulty: 3 Grade Range: K-2 OVERVIEW In this activity, students will learn about tornadoes and simulate one inside a small bottle. Topic: Severe Weather Real-World Science
1 What Is Matter? Math Focus
 CHAPTER 1 1 What Is Matter? SECTION The Properties of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is matter? What is volume and mass? What are
CHAPTER 1 1 What Is Matter? SECTION The Properties of Matter BEFORE YOU READ After you read this section, you should be able to answer these questions: What is matter? What is volume and mass? What are
Name: Period: V = lwh
 Density Unit Packet Name: Period: To begin we are going to start with volume. Volume is the amount of space something takes up. It is measured in units like cubic centimeters or milliliters. Those units
Density Unit Packet Name: Period: To begin we are going to start with volume. Volume is the amount of space something takes up. It is measured in units like cubic centimeters or milliliters. Those units
Lesson 1 Matter and Its Properties
 Lesson 1 Student Labs and Activities Page Launch Lab 8 Content Vocabulary 9 Lesson Outline 10 MiniLab 12 Content Practice A 13 Content Practice B 14 Math Skills 15 School to Home 16 Key Concept Builders
Lesson 1 Student Labs and Activities Page Launch Lab 8 Content Vocabulary 9 Lesson Outline 10 MiniLab 12 Content Practice A 13 Content Practice B 14 Math Skills 15 School to Home 16 Key Concept Builders
Station 1 Water is a polar molecule and has a very unique structure
 Station 1 Water is a polar molecule and has a very unique structure A water molecule, because of its shape, is a polar molecule. That is, it has one side that is positively charged and one side that is
Station 1 Water is a polar molecule and has a very unique structure A water molecule, because of its shape, is a polar molecule. That is, it has one side that is positively charged and one side that is
Density of Brass: Accuracy and Precision
 Density of Brass: Accuracy and Precision Introduction Density is a measure of a substance s mass-to-volume ratio. For liquids and solids, density is usually expressed in units of g/ml or g/cm 3 ; these
Density of Brass: Accuracy and Precision Introduction Density is a measure of a substance s mass-to-volume ratio. For liquids and solids, density is usually expressed in units of g/ml or g/cm 3 ; these
Matter and Its Properties. Unit 2
 Matter and Its Properties Unit 2 Lesson 1: Physical & Chemical Properties & Changes Unit 2: Matter and Its Properties Section 1: Physical Properties & Change Lesson 1: Physical & Chemical Properties &
Matter and Its Properties Unit 2 Lesson 1: Physical & Chemical Properties & Changes Unit 2: Matter and Its Properties Section 1: Physical Properties & Change Lesson 1: Physical & Chemical Properties &
5.1/4.1 Scientific Investigation, Reasoning, and Logic Question/Answer Packet #1
 5.1/4.1 Scientific Investigation, Reasoning, and Logic Question/Answer Packet #1 The student will demonstrate an understanding of scientific reasoning, logic, and the nature of science by planning and
5.1/4.1 Scientific Investigation, Reasoning, and Logic Question/Answer Packet #1 The student will demonstrate an understanding of scientific reasoning, logic, and the nature of science by planning and
Unit Wun. Version A. 10. The sphere was dropped into water in a graduated cylinder as shown below.
 Unit Wun 1. In order to make observations, an observer must always use (1) proportions (2) the senses (3) mathematical calculations (4) experiments 2. Using a ruler to measure the length of a stick is
Unit Wun 1. In order to make observations, an observer must always use (1) proportions (2) the senses (3) mathematical calculations (4) experiments 2. Using a ruler to measure the length of a stick is
Lab: Density of Substances
 Name: Date: Unit 1: Measuring the Earth - 2 Lab Hours Period: Lab: Density of Substances Introduction: You often hear statements like lead is heavier than water, or gold is the heaviest material on earth.
Name: Date: Unit 1: Measuring the Earth - 2 Lab Hours Period: Lab: Density of Substances Introduction: You often hear statements like lead is heavier than water, or gold is the heaviest material on earth.
Chromatography Lab # 4
 Chromatography Lab # 4 Chromatography is a method for separating mixtures based on differences in the speed at which they migrate over or through a stationary phase which means that a complex mixture will
Chromatography Lab # 4 Chromatography is a method for separating mixtures based on differences in the speed at which they migrate over or through a stationary phase which means that a complex mixture will
1. Mass and volume: a. What is the volume of the liquid in the graduated cylinder pictured to the right? (1 point)
 Potter Name: Date: Hour: Score: /7 Learning Check 4.1 LT 4.1 Density: I can explain the role density plays to help form some currents. 1. Mass and volume: a. What is the volume of the liquid in the graduated
Potter Name: Date: Hour: Score: /7 Learning Check 4.1 LT 4.1 Density: I can explain the role density plays to help form some currents. 1. Mass and volume: a. What is the volume of the liquid in the graduated
Chapter 5, Lesson 1: Water is a Polar Molecule
 Chapter 5, Lesson 1: Water is a Polar Molecule Key Concepts The water molecule, as a whole, has 10 protons and 10 electrons, so it is neutral. In a water molecule, the oxygen atom and hydrogen atoms share
Chapter 5, Lesson 1: Water is a Polar Molecule Key Concepts The water molecule, as a whole, has 10 protons and 10 electrons, so it is neutral. In a water molecule, the oxygen atom and hydrogen atoms share
Working with Solutions. (and why that s not always ideal)
 Page 1 of 13 Working with Solutions (and why that s not always ideal) Learning Objectives: Solutions are prepared by dissolving a solute into a solvent A solute is typically a solid, but may also be a
Page 1 of 13 Working with Solutions (and why that s not always ideal) Learning Objectives: Solutions are prepared by dissolving a solute into a solvent A solute is typically a solid, but may also be a
Density. weight: a measure of the pull of gravity on an object
 Imagine that it is a very hot day. You decide to cool a glass of water by placing several ice cubes in the drink. What happens when you drop the ice into the water? Likely, when you place the first ice
Imagine that it is a very hot day. You decide to cool a glass of water by placing several ice cubes in the drink. What happens when you drop the ice into the water? Likely, when you place the first ice
d. a possible answer to a scientific question.
 A hypothesis is a. a way to share ideas with other scientists. b. a summary of what was learned. c. the observations made during an experiment. d. a possible answer to a scientific question. A hypothesis
A hypothesis is a. a way to share ideas with other scientists. b. a summary of what was learned. c. the observations made during an experiment. d. a possible answer to a scientific question. A hypothesis
THIRD GRADE OCEANS 1 WEEK LESSON PLANS AND ACTIVITIES
 THIRD GRADE OCEANS 1 WEEK LESSON PLANS AND ACTIVITIES WATER CYCLE OVERVIEW OF THIRD GRADE WATER WEEK 1. PRE: Comparing the different components of the water cycle. LAB: Contrasting water with hydrogen
THIRD GRADE OCEANS 1 WEEK LESSON PLANS AND ACTIVITIES WATER CYCLE OVERVIEW OF THIRD GRADE WATER WEEK 1. PRE: Comparing the different components of the water cycle. LAB: Contrasting water with hydrogen
Bay Area Scientists in Schools Presentation Plan
 Bay Area Scientists in Schools Presentation Plan Lesson Name: We Love Gravity! Presenter(s) Virginia Lehr, Laura Hidrobo Grade Level 5 Standards Connection(s) Solar System and Gravity Teaser: Gravity is
Bay Area Scientists in Schools Presentation Plan Lesson Name: We Love Gravity! Presenter(s) Virginia Lehr, Laura Hidrobo Grade Level 5 Standards Connection(s) Solar System and Gravity Teaser: Gravity is
Movement of Molecules Biology Concepts of Biology 3.1
 Movement of Molecules Biology 100 - Concepts of Biology 3.1 Name Instructor Lab Section Objectives: To gain an understanding of: The basic principles of osmosis and diffusion Brownian motion The effects
Movement of Molecules Biology 100 - Concepts of Biology 3.1 Name Instructor Lab Section Objectives: To gain an understanding of: The basic principles of osmosis and diffusion Brownian motion The effects
Semester One Test Review
 Semester One Test Review Net Forces a. 5N 2N b. 5N 3N c. 3N 3N d. 2N 4N 2N 1. Which forces diagrams above show a Net Force of zero? a. All of them b. None of them c. c only d. c and d 2. Which force diagrams
Semester One Test Review Net Forces a. 5N 2N b. 5N 3N c. 3N 3N d. 2N 4N 2N 1. Which forces diagrams above show a Net Force of zero? a. All of them b. None of them c. c only d. c and d 2. Which force diagrams
Fig. 8.1 illustrates the three measurements. air medium A. ray 1. air medium A. ray 2. air medium A. ray 3. Fig For Examiner s Use
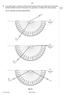 9 9 9 14 8 In an optics lesson, a Physics student traces the paths of three s of light near the boundary between medium A and. The student uses a protractor to measure the various angles. Fig. 8.1 illustrates
9 9 9 14 8 In an optics lesson, a Physics student traces the paths of three s of light near the boundary between medium A and. The student uses a protractor to measure the various angles. Fig. 8.1 illustrates
WONDERING ABOUT WEATHER
 NAME DATE PARTNERS WONDERING ABOUT WEATHER You are lying in the grass enjoying a few relaxing hours in the summer sun. You have your favorite cold drink and magazine close at hand. You close your eyes
NAME DATE PARTNERS WONDERING ABOUT WEATHER You are lying in the grass enjoying a few relaxing hours in the summer sun. You have your favorite cold drink and magazine close at hand. You close your eyes
Physics 2020 Laboratory Manual
 Physics 00 Laboratory Manual Department of Physics University of Colorado at Boulder Spring, 000 This manual is available for FREE online at: http://www.colorado.edu/physics/phys00/ This manual supercedes
Physics 00 Laboratory Manual Department of Physics University of Colorado at Boulder Spring, 000 This manual is available for FREE online at: http://www.colorado.edu/physics/phys00/ This manual supercedes
Ch100: Fundamentals for Chemistry 1 Instructor: Tony Zable. Experiment: Density
 Ch100: Fundamentals for Chemistry 1 Objectives: Experiment: Density To determine the density of a known liquid To identify an unknown liquid by determining its density To determine the density of a regular
Ch100: Fundamentals for Chemistry 1 Objectives: Experiment: Density To determine the density of a known liquid To identify an unknown liquid by determining its density To determine the density of a regular
Talk Science Professional Development
 Talk Science Professional Development Transcript for Grade 4 Scientist Case: The Heavy for Size Investigations 1. The Heavy for Size Investigations, Through the Eyes of a Scientist We met Associate Professor
Talk Science Professional Development Transcript for Grade 4 Scientist Case: The Heavy for Size Investigations 1. The Heavy for Size Investigations, Through the Eyes of a Scientist We met Associate Professor
Unit 1: Introduction to Chemistry
 Unit 1: Introduction to Chemistry I. Observations vs. Inferences Observation: information you gather using your five senses ***You will NEVER use taste in class! o Describes facts Examples You see the
Unit 1: Introduction to Chemistry I. Observations vs. Inferences Observation: information you gather using your five senses ***You will NEVER use taste in class! o Describes facts Examples You see the
Chemistry #3 Notebook States of Matter
 Name Hour Test Date Group # Chemistry #3 Notebook States of Matter LEARNING TARGETS I CAN model the motion and arrangement of particles in typical solids, liquids and gasses. I CAN describe how the motion
Name Hour Test Date Group # Chemistry #3 Notebook States of Matter LEARNING TARGETS I CAN model the motion and arrangement of particles in typical solids, liquids and gasses. I CAN describe how the motion
6.2: Mycorrhizal Fungi Worksheet
 Name Teacher Date 6.2: Mycorrhizal Fungi Worksheet Draw and label arrows that represent the molecules that carbon atoms are in as they move into, through and out of the mycorrhizal fungus as it grows.
Name Teacher Date 6.2: Mycorrhizal Fungi Worksheet Draw and label arrows that represent the molecules that carbon atoms are in as they move into, through and out of the mycorrhizal fungus as it grows.
Front page = 1 point, Questions on back = 1 point each, Mastery Question = 1 point DENSITY OF SOLIDS Sink or Float?
 Last name First name Date Period Front page = 1 point, Questions on back = 1 point each, Mastery Question = 1 point DENSITY OF SOLIDS Sink or? Question to investigate Why does a heavier candle float and
Last name First name Date Period Front page = 1 point, Questions on back = 1 point each, Mastery Question = 1 point DENSITY OF SOLIDS Sink or? Question to investigate Why does a heavier candle float and
Density of Aqueous Sodium Chloride Solutions
 Experiment 3 Density of Aqueous Sodium Chloride Solutions Prepared by Ross S. Nord and Stephen E. Schullery, Eastern Michigan University PURPOSE Determine the concentration of an unknown sodium chloride
Experiment 3 Density of Aqueous Sodium Chloride Solutions Prepared by Ross S. Nord and Stephen E. Schullery, Eastern Michigan University PURPOSE Determine the concentration of an unknown sodium chloride
Matter Practice. 1 What happens if new evidence is discovered about atom that current theories about atoms do not explain?
 Matter Practice 1 What happens if new evidence is discovered about atom that current theories about atoms do not explain? Nothing. urrent theories about atoms are hypotheses and don't need to be supported
Matter Practice 1 What happens if new evidence is discovered about atom that current theories about atoms do not explain? Nothing. urrent theories about atoms are hypotheses and don't need to be supported
Accuracy and Precision of Laboratory Glassware: Determining the Density of Water
 Accuracy and Precision of Laboratory Glassware: Determining the Density of Water During the semester in the general chemistry lab, you will come into contact with various pieces of laboratory glassware.
Accuracy and Precision of Laboratory Glassware: Determining the Density of Water During the semester in the general chemistry lab, you will come into contact with various pieces of laboratory glassware.
Using Scientific Measurements
 Section 3 Main Ideas Accuracy is different from precision. Significant figures are those measured precisely, plus one estimated digit. Scientific notation is used to express very large or very small numbers.
Section 3 Main Ideas Accuracy is different from precision. Significant figures are those measured precisely, plus one estimated digit. Scientific notation is used to express very large or very small numbers.
