Formulas & Constants
|
|
|
- Cassandra Woods
- 5 years ago
- Views:
Transcription
1 Formulas & Constants Net Force F = Fnet The sum of all forces acting on an object is called the net force on the object. The direction of each force must be taken into consideration when summing forces (e.g. forces acting in the same direction add, forces acting in opposite directions subtract). Units: The SI (metric) unit of force, the newton N, is named in honor of Issac Newton. Equilibrium Rule F = 0 When the sum of all forces acting on an object is zero (the net force on the object = 0), the object is in equilibrium. An object in equilibrium is stationary (not moving) or is moving at a constant speed in an unchanging direction. The Equilibrim Rule is basically a restatement of Newton s First Law of Motion. Speed and Velocity speed = distance covered time interval velocity is speed and direction. Units: The SI (metric) unit for speed (and velocity) is a combination of the standard units of distance and time, meters per second, m/s. 1
2 Acceleration acceleration = a = v t = v final v initial t change of velocity time interval Acceleration is a change in velocity during a time interval. Remember that velocity is speed and direction. This means that an object undergoes acceleration anytime its speed or direction changes. Units: The SI (metric) unit for acceleration is a combination of the units of velocity and time, meters per second squared, m/s 2 Newton s 2 nd Law N et F orce = (mass)(acceleration) F net = m a If the net force on an object is not zero, the object will accelerate. This means that the object s speed, direction, or both, will change. How much and object will accelerate depends on its mass - the greater the mass the smaller the acceleration. Units: the SI (metric) unit of mass is the kilogram, kg. Note that 1 kg = 1000 g. Weight and Force F weight = m g where g = 9.8 m s 2 Weight is the attractive force due to gravity on a mass. Your weight is a measure of the force between you and the Earth. The acceleration due to gravity near the Earth s surface is g. Units: Weight is a force and therefore weight is measured in newtons, N.
3 Momentum F t = P = P f P i = m v f m v i where F = force, t = contact time, P = change in momentum, P f = final momentum, P i = initial momentum, v f = final velocity, and v i = initial velocity. This formula is often called the impluse formula. Note that this relationship is useful if you know any three of the following four: force, time, initial momentum, or final momentum. Note that it is important to use the correct SI (metric) units for force, mass, velocity, and time. P f = P i Conservation of Momentum where P f = final momentum of the system, and P i = initial momentum of the system. This relationship is useful when you can determine either the initial or final momentum of a system of objects. Note that it is important to use the correct SI (metric) units for mass and velocity. Energy KE = 1 2 m v2 Kinetic Energy where KE = kinetic energy, m = mass, and v = velocity. Kinetic energy is the energy of motiom. Anything moving has kinetic energy. Units: The SI (metric) unit for energy is the joule, J. Note that it is important to use the correct SI (metric) units for mass and velocity. P E = m g h Potential Energy where P E = potential energy due to Earth s gravity, m = mass, g = acceleration due to gravity, and h = height above the ground. Potential energy is the energy an object has due to its position. Gravitational potential energy is the energy a massive object has due to its position relative to a location on Earth. Units: The SI (metric) unit for energy is the joule, J. Note that it is important to use the correct SI (metric) units for mass, acceleration, and distance.
4 Energy continued... E = KE + P E Total Energy where E = total energy, KE = kinetic energy, and P E = potential energy. E f = E i Conservation of Energy where E f = final energy of the system, and E i = initial energy of the system. This relationship is useful when you can determine either the initial or final energy of a object (or a system of objects). Note that it is important to use the correct SI (metric) units for mass, velocity, acceleration, distance, and energy. Work W = F d where W = work, F = force, and d = distance. Units: The unit of work is the same as the unit of energy, the joule, J. This formula is useful if you know two of the following three quanitities: force, distance, and work. Note that it is important to use the correct SI (metric) units for work, force, and distance. W = KE = (KE f KE i ) where W = work, KE = change in kinetic energy, KE f = final kinetic energy, and KE i = initial kinetic energy. This formula is useful if you know two of the following: work, inital, or final, kinetic energy. Note that it is important to use the correct SI (metric) units for energy, mass, and velocity. Power P = W t Power is work per unit time. Units: Typically, power is measured in watt. One watt is one joule of work performed in one second.
5 Heat Q T = c m T = c m (T f T i ) where Q T = heat transfer needed to change temperature, c = specific heat capacity, m = mass, T = change in temperature, T f = final temperature, and T i = initial temperature. Units: Heat is energy. This fact was not known when heat was first studied, and so now we are stuck with two units in common usage: joules and calories. One calorie is equilivant to Joules. Note that there is another bit of confusing notation: 1 Calorie = 1000 calories = 1 kilocalorie = 1 kcal. Additionally, the unit for specific heat may be specified in joules or calories, and mass is typically specified in grams (or kg). Q v = m L v where Q v = heat transfer needed to change phase between a liquid and a gas, m = mass, and L v = heat of vaporization. To convert a liquid into a gas an amount of heat Q v must be added to the liquid. To convert a gas into a liquid an amount of heat Q v must be removed from the gas. Note: the unit for heat of vaporization may be specified in joules or calories. Q f = m L f where Q f = heat transfer needed to change phase between a liquid and a solid, m = mass, and L f = heat of fusion. To convert a solid into a liquid an amount of heat Q f must be added to the solid. To convert a liquid into a solid an amount of heat Q f must be removed from the liquid. Note: the unit for heat of fusion may be specified in joules or calories. Heat Properties of Water specific heat c = cal/ o C/g = J/ o C/g heat of fusion L f = 334 J/g heat of vaporization L v = 2256 J/g
6 Electric Force F = k Q 1 Q 2 d 2 where k = 9 x 10 9 N m 2 /C 2. Units: Force is in newtons, N, charge is in coulombs, C, and distance in meters, m. This formula allows you to calculate the force between two electric charges. Note that opposite sign charges attract, and same-sign charges repel. Electric Work and Energy voltage = Ohm s Law V = I R electric energy or work amount of charge where V = voltage, I = electric current, and R = resistance. Units: voltage is in volt, V, electric current in ampere, A, and resistance in ohm, Ω Electric Power P = I V where P = power, V = voltage, and I = electric current. Units: Power is in watt. Transformers V 1 = V 2 N 1 N 2 V 1 I 1 = V 2 I 2 V 1 = voltage in primary V 2 = voltage in secondary N 1 = turns in primary N 2 = turns in secondary
7 I 1 = current in primary I 2 = current in secondary Note: be careful to use the correct units for voltage and current. Waves f = 1 T where f = frequency, and T = period. v = λ f where v = wave speed, λ = wavelength, and f = frequency. distance = (speed)(time) speed of sound = 343 m/s speed of light = 300,000 km/s speed of light = 300,000,000 m/s Atomic Mass atomic mass = M isotope ( percent of isotope 100 Percentage of total amount ) % of total amount = 100 (partial amount) total amount Half Life Half life is the amount of time for 1 2 of a substance to undergo nuclear decay. Balancing Chemical Equations The number of atoms of each type must be the same on both sides of a chemical equation.
8 Acids and Bases [H 3 O + ][OH ] = 1.0 x ph = log[h 3 O + ] where [H 3 O + ] = hydronium ion concentration and [OH ] = hydroxide ion concentration. Moles 1 mole of substance = Atomic Mass in grams of substance Examples: 1 mole of C = grams of C 1 mole of H 2 O = grams of H 2 O convert grams to moles: #ofmoles = # of grams # of grams in 1 mole convert moles to grams: # of grams = (# of moles) (# of grams in 1 mole) Mixtures & Solutions concentration in grams/liter concentration in g/l = grams of substance Liters of solution
9 concentration in moles/liter concentration in moles/l = moles of substance Liters of solution
wave speed (metre/second, m/s) = frequency (hertz, Hz) x wavelength (metre, m)
 Physics formulae: Unit P1 (GCSE Science ( Core Science )): The relationship between wave speed, frequency and wavelength: wave speed (metre/second, m/s) = frequency (hertz, Hz) x wavelength (metre, m)
Physics formulae: Unit P1 (GCSE Science ( Core Science )): The relationship between wave speed, frequency and wavelength: wave speed (metre/second, m/s) = frequency (hertz, Hz) x wavelength (metre, m)
100 Physics Facts. 1. The standard international unit (SI unit) for mass (m) is. kg (kilograms) s (seconds)
 100 Physics Facts 1. The standard international unit (SI unit) for mass (m) is. kg (kilograms) 2. The standard international unit (SI unit) for time (t) is. s (seconds) 3. The standard international unit
100 Physics Facts 1. The standard international unit (SI unit) for mass (m) is. kg (kilograms) 2. The standard international unit (SI unit) for time (t) is. s (seconds) 3. The standard international unit
GLOSSARY OF PHYSICS TERMS. v-u t. a =
 GLOSSARY OF PHYSICS TERMS Scalar: A quantity that has magnitude only. Vector: A quantity that has magnitude and direction. Speed is the distance travelled per unit time. OR the rate of change of distance.
GLOSSARY OF PHYSICS TERMS Scalar: A quantity that has magnitude only. Vector: A quantity that has magnitude and direction. Speed is the distance travelled per unit time. OR the rate of change of distance.
This equation is only used when the velocity is constant, or for an average velocity.
 1 2 The speed distance - time equation. d = distance travelled, in metres (m) v = average velocity, in metres per second (m/s) t = time taken for trip, in seconds (s) This equation is only used when the
1 2 The speed distance - time equation. d = distance travelled, in metres (m) v = average velocity, in metres per second (m/s) t = time taken for trip, in seconds (s) This equation is only used when the
- Joule (J): SI unit for energy. It's defined based on the equation for kinetic energy. from. mass. velocity
 153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
Chapter 33 - Electric Fields and Potential. Chapter 34 - Electric Current
 Chapter 33 - Electric Fields and Potential Chapter 34 - Electric Current Electric Force acts through a field An electric field surrounds every electric charge. It exerts a force that causes electric charges
Chapter 33 - Electric Fields and Potential Chapter 34 - Electric Current Electric Force acts through a field An electric field surrounds every electric charge. It exerts a force that causes electric charges
Chemistry Terms. atomic number The atomic number of an element is the number of protons in the nucleus of each atom.
 Chemistry Terms atomic number The atomic number of an element is the number of protons in the nucleus of each atom. chemical reaction A process in which atoms and molecules interact, resulting in the alteration
Chemistry Terms atomic number The atomic number of an element is the number of protons in the nucleus of each atom. chemical reaction A process in which atoms and molecules interact, resulting in the alteration
Equation Sheet For Quizzes and Tests
 hapter : Aluminum.70 g/cm 3 opper 8.96 g/cm 3 Iron 7.87 g/cm 3 Equation Sheet For Quizzes and Tests You must have memorized the unit prefix values and the formulas related to circles, spheres, and rectangles.
hapter : Aluminum.70 g/cm 3 opper 8.96 g/cm 3 Iron 7.87 g/cm 3 Equation Sheet For Quizzes and Tests You must have memorized the unit prefix values and the formulas related to circles, spheres, and rectangles.
Chemistry 104 Chapter Two PowerPoint Notes
 Measurements in Chemistry Chapter 2 Physical Quantities Measurable physical properties such as height, volume, and temperature are called Physical quantity. A number and a unit of defined size is required
Measurements in Chemistry Chapter 2 Physical Quantities Measurable physical properties such as height, volume, and temperature are called Physical quantity. A number and a unit of defined size is required
HONOR S PHYSICS REVIEW
 HONOR S PHYSICS REVIEW 4-28-16 Online Resources (NOTE: Use Link 1 to prepare for exam) Aplus Physics (http://www.aplusphysics.com/courses/honors/honors_physics.html ) Khan Academy (https://www.khanacademy.org/science/physics
HONOR S PHYSICS REVIEW 4-28-16 Online Resources (NOTE: Use Link 1 to prepare for exam) Aplus Physics (http://www.aplusphysics.com/courses/honors/honors_physics.html ) Khan Academy (https://www.khanacademy.org/science/physics
UNITS AND DEFINITIONS RELATED TO BIOMECHANICAL AND ELECTROMYOGRAPHICAL MEASUREMENTS
 APPENDIX B UNITS AND DEFINITIONS RELATED TO BIOMECHANICAL AND ELECTROMYOGRAPHICAL MEASUREMENTS All units used are SI (Système International d Unités). The system is based on seven well-defined base units
APPENDIX B UNITS AND DEFINITIONS RELATED TO BIOMECHANICAL AND ELECTROMYOGRAPHICAL MEASUREMENTS All units used are SI (Système International d Unités). The system is based on seven well-defined base units
This reaction is ENDOTHERMIC. Energy is being transferred from the room/flask/etc. (the SURROUNDINGS) to the reaction itself (the SYSTEM).
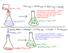 151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
BRCC CHM 101 Class Notes Chapter 1 Page 1 of 7
 BRCC CHM 101 Class Notes Chapter 1 Page 1 of 7 Chemistry - the study of matter, its behavior and interactions. matter - anything that takes up space and has mass mass - the substance which makes up the
BRCC CHM 101 Class Notes Chapter 1 Page 1 of 7 Chemistry - the study of matter, its behavior and interactions. matter - anything that takes up space and has mass mass - the substance which makes up the
Physics Important Terms and their Definitions
 Physics Important Terms and their S.No Word Meaning 1 Acceleration The rate of change of velocity of an object with respect to time 2 Angular Momentum A measure of the momentum of a body in rotational
Physics Important Terms and their S.No Word Meaning 1 Acceleration The rate of change of velocity of an object with respect to time 2 Angular Momentum A measure of the momentum of a body in rotational
- The empirical gas laws (including the ideal gas equation) do not always apply.
 145 At 300 C, ammonium nitrate violently decomposes to produce nitrogen gas, oxygen gas, and water vapor. What is the total volume of gas that would be produced at 1.00 atm by the decomposition of 15.0
145 At 300 C, ammonium nitrate violently decomposes to produce nitrogen gas, oxygen gas, and water vapor. What is the total volume of gas that would be produced at 1.00 atm by the decomposition of 15.0
Energy Conversions. Energy. the ability to do work or produce heat. energy energy due to composition or position of an object
 Energy Energy the ability to do work or produce heat energy energy due to composition or position of an object energy the energy of motion Energy - SI unit for energy 1 J = 1 Kgm 2 / s 2 Energy Conversions
Energy Energy the ability to do work or produce heat energy energy due to composition or position of an object energy the energy of motion Energy - SI unit for energy 1 J = 1 Kgm 2 / s 2 Energy Conversions
Scientific Measurement
 Scientific Measurement A quantity is anything having a measurable size or amount For Example: 5 But 5 what? A unit assigns value to a measured quantity For Example: 5 ft, 5 gal, 5 sec, 5 m, 5 g. Base Units
Scientific Measurement A quantity is anything having a measurable size or amount For Example: 5 But 5 what? A unit assigns value to a measured quantity For Example: 5 ft, 5 gal, 5 sec, 5 m, 5 g. Base Units
Energy, Heat and Temperature. Introduction
 Energy, Heat and Temperature Introduction 3 basic types of energy: Potential (possibility of doing work because of composition or position) Kinetic (moving objects doing work) Radiant (energy transferred
Energy, Heat and Temperature Introduction 3 basic types of energy: Potential (possibility of doing work because of composition or position) Kinetic (moving objects doing work) Radiant (energy transferred
Physical Science Study Guide
 Name: Class: Date: Physical Science Study Guide Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The electrons in a water molecule are gathered nearest
Name: Class: Date: Physical Science Study Guide Multiple Choice Identify the choice that best completes the statement or answers the question. 1. The electrons in a water molecule are gathered nearest
Chapter 2 Heat, Temperature and the First Law of Thermodynamics
 Chapter 2 Heat, Temperature and the First Law of Thermodynamics 2.1. Temperature and the Zeroth Law of Thermodynamics 2.2. Thermal Expansion 2.3. Heat and the Absorption of Heat by Solids and Liquids 2.4.
Chapter 2 Heat, Temperature and the First Law of Thermodynamics 2.1. Temperature and the Zeroth Law of Thermodynamics 2.2. Thermal Expansion 2.3. Heat and the Absorption of Heat by Solids and Liquids 2.4.
N5 H AH Physical Quantity Symbol Unit Unit Abbrev. 5 absorbed dose D gray Gy
 5 absorbed dose D gray Gy 5 absorbed dose rate D gray per second gray per hour gray per year Gys -1 Gyh -1 Gyy -1 5 6 7 acceleration a metre per second per second m s -2 5 6 7 acceleration due to gravity
5 absorbed dose D gray Gy 5 absorbed dose rate D gray per second gray per hour gray per year Gys -1 Gyh -1 Gyy -1 5 6 7 acceleration a metre per second per second m s -2 5 6 7 acceleration due to gravity
DEFINITIONS. Linear Motion. Conservation of Momentum. Vectors and Scalars. Circular Motion. Newton s Laws of Motion
 DEFINITIONS Linear Motion Mass: The mass of a body is the amount of matter in it. Displacement: The displacement of a body from a point is its distance from a point in a given direction. Velocity: The
DEFINITIONS Linear Motion Mass: The mass of a body is the amount of matter in it. Displacement: The displacement of a body from a point is its distance from a point in a given direction. Velocity: The
Electromagnetism. Electricity Electromagnetism Magnetism Optics. In this course we are going to discuss the fundamental concepts of electromagnetism:
 Electromagnetism Electromagnetism is one of the fundamental forces in nature, and the the dominant force in a vast range of natural and technological phenomena The electromagnetic force is solely responsible
Electromagnetism Electromagnetism is one of the fundamental forces in nature, and the the dominant force in a vast range of natural and technological phenomena The electromagnetic force is solely responsible
The number of stars in a galaxy is an example of an estimate that should be expressed in scientific notation.
 3.1 Using and Expressing Measurements A measurement is a quantity that has both a number and a unit. Using and Expressing Measurements In scientific notation, a given number is written as the product of
3.1 Using and Expressing Measurements A measurement is a quantity that has both a number and a unit. Using and Expressing Measurements In scientific notation, a given number is written as the product of
Materials and Energy Balance in Metallurgical Processes. Prof. S. C. Koria. Department of Materials Science and Engineering
 Materials and Energy Balance in Metallurgical Processes Prof. S. C. Koria Department of Materials Science and Engineering Indian Institute of Technology, Kanpur Module No. # 01 Lecture No. # 02 Measurement
Materials and Energy Balance in Metallurgical Processes Prof. S. C. Koria Department of Materials Science and Engineering Indian Institute of Technology, Kanpur Module No. # 01 Lecture No. # 02 Measurement
Test Review Electricity
 Name: Date: 1. An operating television set draws 0.71 ampere of current when connected to a 120-volt outlet. Calculate the time it takes the television to consume 3.0 10 5 joules of electric energy. [Show
Name: Date: 1. An operating television set draws 0.71 ampere of current when connected to a 120-volt outlet. Calculate the time it takes the television to consume 3.0 10 5 joules of electric energy. [Show
Optics Definitions. The apparent movement of one object relative to another due to the motion of the observer is called parallax.
 Optics Definitions Reflection is the bouncing of light off an object Laws of Reflection of Light: 1. The incident ray, the normal at the point of incidence and the reflected ray all lie in the same plane.
Optics Definitions Reflection is the bouncing of light off an object Laws of Reflection of Light: 1. The incident ray, the normal at the point of incidence and the reflected ray all lie in the same plane.
CH. I ME2560 STATICS General Principles GENERAL PRINCIPLES. Rigid body mechanics. Fluid mechanics
 1. MECHANICS GENERAL PRINCIPLES Mechanics is the branch of physics (classic) that studies the state of rest or motion of bodies subjected to the action of forces. Rigid body mechanics Mechanics Deformable
1. MECHANICS GENERAL PRINCIPLES Mechanics is the branch of physics (classic) that studies the state of rest or motion of bodies subjected to the action of forces. Rigid body mechanics Mechanics Deformable
First Law of Thermodynamics: energy cannot be created or destroyed.
 1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
1 CHEMICAL THERMODYNAMICS ANSWERS energy = anything that has the capacity to do work work = force acting over a distance Energy (E) = Work = Force x Distance First Law of Thermodynamics: energy cannot
The SI unit for Energy is the joule, usually abbreviated J. One joule is equal to one kilogram meter squared per second squared:
 Chapter 2 Energy Energy is an extremely loaded term. It is used in everyday parlance to mean a number of different things, many of which bear at most a passing resemblance to the term as used in physical
Chapter 2 Energy Energy is an extremely loaded term. It is used in everyday parlance to mean a number of different things, many of which bear at most a passing resemblance to the term as used in physical
Note: Question/answers in italics are Daily Doubles. They are on the same topic as the category they are found in.
 AP Physics Jeopardy Q&A By Seth Baum August 4, 2007 Note: Question/answers in italics are Daily Doubles. They are on the same topic as the category they are found in. Game 1 Newtonian Mechanics 1) The
AP Physics Jeopardy Q&A By Seth Baum August 4, 2007 Note: Question/answers in italics are Daily Doubles. They are on the same topic as the category they are found in. Game 1 Newtonian Mechanics 1) The
ENERGY UNITS. 1 cal J 1 ev J 1 btu 1055 J 1 kw h J. Your body gets 8,000 J (1900cal=1.9kcal) of energy from eating a
 ENERGY 1 ENERGY UNITS Energy: The ability to do work (make something happen) Joule (J) Calorie (cal) The calories on food packages are really kcal Electron Volt (ev) British Thermal Unit (btu) Kilo watt
ENERGY 1 ENERGY UNITS Energy: The ability to do work (make something happen) Joule (J) Calorie (cal) The calories on food packages are really kcal Electron Volt (ev) British Thermal Unit (btu) Kilo watt
Unit #5- Chapter #6. Types of chemical reactions. Energy: its forms 10/15/2013. Thermodynamics
 Unit #5- Chapter #6 Thermodynamics Types of chemical reactions PRODUCT-FAVORED: when the reaction converts reactants to products completely-it may take a small amount of activation energy but releases
Unit #5- Chapter #6 Thermodynamics Types of chemical reactions PRODUCT-FAVORED: when the reaction converts reactants to products completely-it may take a small amount of activation energy but releases
Grade 7 Science. Enduring Understanding and Essential Questions Competencies Concepts Key Vocabulary
 Unit: The Scientific Method Length: 3 WEEKS Grade 7 Science Enduring Understanding and Essential Questions Competencies Concepts Key Vocabulary The scientific method is problem solving guide based on identification
Unit: The Scientific Method Length: 3 WEEKS Grade 7 Science Enduring Understanding and Essential Questions Competencies Concepts Key Vocabulary The scientific method is problem solving guide based on identification
Beauchamp College Year 11/12 - A- Level Transition Work. Physics.
 Beauchamp College Year 11/1 - A- Level Transition Work Physics Gareth.butcher@beauchamp.org.uk Using S.I. units Specification references.1. a) b) c) d) M0.1 Recognise and make use of appropriate units
Beauchamp College Year 11/1 - A- Level Transition Work Physics Gareth.butcher@beauchamp.org.uk Using S.I. units Specification references.1. a) b) c) d) M0.1 Recognise and make use of appropriate units
8/17/2016. Summary. Summary. Summary. Chapter 1 Quantities and Units. Passive Components. SI Fundamental Units. Some Important Electrical Units
 Passive Components Chapter 1 Quantities and Units Welcome to the Principles of Electric Circuits. You will study important ideas that are used in electronics. You may already be familiar with a few of
Passive Components Chapter 1 Quantities and Units Welcome to the Principles of Electric Circuits. You will study important ideas that are used in electronics. You may already be familiar with a few of
Chapter 07: Kinetic Energy and Work
 Chapter 07: Kinetic Energy and Work Conservation of Energy is one of Nature s fundamental laws that is not violated. Energy can take on different forms in a given system. This chapter we will discuss work
Chapter 07: Kinetic Energy and Work Conservation of Energy is one of Nature s fundamental laws that is not violated. Energy can take on different forms in a given system. This chapter we will discuss work
Define a problem based on a specific body of knowledge. For example: biology, chemistry, physics, and earth/space science.
 Course Name: Physical Science / Physical Science Honors Course Number: 2 0 0 3 3 1 0 / 2 0 0 3 3 2 0 Test : 50 SC.912.L.18.12 Discuss the special properties of water that contribute to Earth's suitability
Course Name: Physical Science / Physical Science Honors Course Number: 2 0 0 3 3 1 0 / 2 0 0 3 3 2 0 Test : 50 SC.912.L.18.12 Discuss the special properties of water that contribute to Earth's suitability
Objects usually are charged up through the transfer of electrons from one object to the other.
 1 Part 1: Electric Force Review of Vectors Review your vectors! You should know how to convert from polar form to component form and vice versa add and subtract vectors multiply vectors by scalars Find
1 Part 1: Electric Force Review of Vectors Review your vectors! You should know how to convert from polar form to component form and vice versa add and subtract vectors multiply vectors by scalars Find
CHAPTER ONE. The Foundations of Chemistry
 CHAPTER ONE The Foundations of Chemistry Why is Chemistry Important? Materials for our homes Components for computers and other electronic devices Cooking Fuel Body functions 2 Some definitions / Vocabulary
CHAPTER ONE The Foundations of Chemistry Why is Chemistry Important? Materials for our homes Components for computers and other electronic devices Cooking Fuel Body functions 2 Some definitions / Vocabulary
b) What is its position when its velocity (magnitude) is largest? When it is at x=0 all the energy is kinetic.
 Question 1. The electrostatic force between two charges, Q 1 and F 1 /4 Q 2 a separated by a distance D, is F 1. What is the force between them after they are moved to a distance 2D apart? (Give in terms
Question 1. The electrostatic force between two charges, Q 1 and F 1 /4 Q 2 a separated by a distance D, is F 1. What is the force between them after they are moved to a distance 2D apart? (Give in terms
ELECTRICITY. Electric Circuit. What do you already know about it? Do Smarty Demo 5/30/2010. Electric Current. Voltage? Resistance? Current?
 ELECTRICITY What do you already know about it? Voltage? Resistance? Current? Do Smarty Demo 1 Electric Circuit A path over which electrons travel, out through the negative terminal, through the conductor,
ELECTRICITY What do you already know about it? Voltage? Resistance? Current? Do Smarty Demo 1 Electric Circuit A path over which electrons travel, out through the negative terminal, through the conductor,
Top 40 Missed Regents Physics Questions Review
 Top 40 Missed Regents Physics Questions - 2015 Review 1. Earth s mass is approximately 81 times the mass of the Moon. If Earth exerts a gravitational force of magnitude F on the Moon, the magnitude of
Top 40 Missed Regents Physics Questions - 2015 Review 1. Earth s mass is approximately 81 times the mass of the Moon. If Earth exerts a gravitational force of magnitude F on the Moon, the magnitude of
Although different gasses may differ widely in their chemical properties, they share many physical properties
 IV. Gases (text Chapter 9) A. Overview of Chapter 9 B. Properties of gases 1. Ideal gas law 2. Dalton s law of partial pressures, etc. C. Kinetic Theory 1. Particulate model of gases. 2. Temperature and
IV. Gases (text Chapter 9) A. Overview of Chapter 9 B. Properties of gases 1. Ideal gas law 2. Dalton s law of partial pressures, etc. C. Kinetic Theory 1. Particulate model of gases. 2. Temperature and
Chapter 1 The Electric Force
 Chapter 1 The Electric Force 1. Properties of the Electric Charges 1- There are two kinds of the electric charges in the nature, which are positive and negative charges. - The charges of opposite sign
Chapter 1 The Electric Force 1. Properties of the Electric Charges 1- There are two kinds of the electric charges in the nature, which are positive and negative charges. - The charges of opposite sign
AP Physics 2 Summer Assignment (2014)
 Name: Date: AP Physics 2 Summer Assignment (2014) Instructions: 1. Read and study Chapter 16 Electric Charge and Electric Field. 2. Answer the questions below. Some questions may require you to use your
Name: Date: AP Physics 2 Summer Assignment (2014) Instructions: 1. Read and study Chapter 16 Electric Charge and Electric Field. 2. Answer the questions below. Some questions may require you to use your
Energy. E d. Energy Power = time. E t P = E t = P
 Energy Forms of energy Energy can never be created or destroyed. It can only be transformed from one type to another (or other types). here are many different forms of energy: Kinetic (movement) Energy
Energy Forms of energy Energy can never be created or destroyed. It can only be transformed from one type to another (or other types). here are many different forms of energy: Kinetic (movement) Energy
First Law of Thermodynamics
 Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
Energy Energy: ability to do work or produce heat. Types of energy 1) Potential energy - energy possessed by objects due to position or arrangement of particles. Forms of potential energy - electrical,
CHEM Thermodynamics. Heat calculations
 Thermodynamics Heat calculations l Internal Energy, E The internal energy of other systems that are more complex than the ideal gas cannot be measured. But the internal energy of the system is still proportional
Thermodynamics Heat calculations l Internal Energy, E The internal energy of other systems that are more complex than the ideal gas cannot be measured. But the internal energy of the system is still proportional
W = Fd cos θ. W = (75.0 N)(25.0 m) cos (35.0º) = 1536 J = J. W 2400 kcal =
 8 CHAPTER 7 WORK, ENERGY, AND ENERGY RESOURCES generator does negative work on the briefcase, thus removing energy from it. The drawing shows the latter, with the force from the generator upward on the
8 CHAPTER 7 WORK, ENERGY, AND ENERGY RESOURCES generator does negative work on the briefcase, thus removing energy from it. The drawing shows the latter, with the force from the generator upward on the
International System of Units 3.2. Slide 1of 33
 International System 3.2 1of 33 3.2 The International System In the signs shown here, the distances are listed as numbers with no units attached. Without the units, it is impossible to communicate the
International System 3.2 1of 33 3.2 The International System In the signs shown here, the distances are listed as numbers with no units attached. Without the units, it is impossible to communicate the
What are the states of Matter?
 What are the states of Matter? Solid Lowest energy/heat Molecules barely moving Definite, uniform shape Example: ice States of Matter Liquid Medium energy/heat Molecules slowly moving Shape of container
What are the states of Matter? Solid Lowest energy/heat Molecules barely moving Definite, uniform shape Example: ice States of Matter Liquid Medium energy/heat Molecules slowly moving Shape of container
Academic Challenge District Physics Exam 1996
 Academic Challenge District Physics Exam 1996 1. 1.73 seconds after being dropped from rest, a freely-falling object near the Earth's surface will have a speed closest to: (a. ) 17.0 meters per second.
Academic Challenge District Physics Exam 1996 1. 1.73 seconds after being dropped from rest, a freely-falling object near the Earth's surface will have a speed closest to: (a. ) 17.0 meters per second.
AP Physics Study Guide Chapter 17 Electric Potential and Energy Name. Circle the vector quantities below and underline the scalar quantities below
 AP Physics Study Guide Chapter 17 Electric Potential and Energy Name Circle the vector quantities below and underline the scalar quantities below electric potential electric field electric potential energy
AP Physics Study Guide Chapter 17 Electric Potential and Energy Name Circle the vector quantities below and underline the scalar quantities below electric potential electric field electric potential energy
In the following information, you will study these three physical quantities as they relate to simple electrical circuits.
 Module 7 Ohm's Law INTRODUCTION In this experiment, you will study Ohm's Law, the most fundamental relation used in the analysis of electrical circuits. Ohm's Law relates the quantities of voltage, electric
Module 7 Ohm's Law INTRODUCTION In this experiment, you will study Ohm's Law, the most fundamental relation used in the analysis of electrical circuits. Ohm's Law relates the quantities of voltage, electric
Maths Skills. Science GCSE (9-1)
 Maths Skills Science GCSE (9-1) Students need to be able to recall. Yes all 24 of them!! distance travelled = average speed time d = s x t acceleration = change in velocity time taken a = (v u) t force
Maths Skills Science GCSE (9-1) Students need to be able to recall. Yes all 24 of them!! distance travelled = average speed time d = s x t acceleration = change in velocity time taken a = (v u) t force
Topic 5: Energetics. Heat & Calorimetry. Thursday, March 22, 2012
 Topic 5: Energetics Heat & Calorimetry 1 Heat is energy that is transferred from one object to another due to a difference in temperature Temperature is a measure of the average kinetic energy of a body
Topic 5: Energetics Heat & Calorimetry 1 Heat is energy that is transferred from one object to another due to a difference in temperature Temperature is a measure of the average kinetic energy of a body
Chapter 5 Energy and States of Matter. Changes of State. Melting and Freezing. Calculations Using Heat of Fusion
 Chapter 5 Energy and States of Matter Changes of State 5.6 Melting and Freezing 5.7 Boiling and Condensation 1 2 Melting and Freezing A substance is melting while it changes from a solid to a liquid. A
Chapter 5 Energy and States of Matter Changes of State 5.6 Melting and Freezing 5.7 Boiling and Condensation 1 2 Melting and Freezing A substance is melting while it changes from a solid to a liquid. A
Which of these particles has an electrical charge?
 Which of these particles has an electrical charge? A. Proton. B. Electron. C. Ion. D. All of the above. Which is the predominant carrier of charge in copper wire? A. Proton. B. Electron. C. Ion. D. All
Which of these particles has an electrical charge? A. Proton. B. Electron. C. Ion. D. All of the above. Which is the predominant carrier of charge in copper wire? A. Proton. B. Electron. C. Ion. D. All
Calculate the mass of L of oxygen gas at 25.0 C and 1.18 atm pressure.
 148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
148 Calculate the mass of 22650 L of oxygen gas at 25.0 C and 1.18 atm pressure. 1 - Convert the volume of oxygen gas to moles using IDEAL GAS EQUATION 2 - Convert moles oxygen gas to mass using formula
structure, properties changes energy ELEMENTS COMPOUNDS PHYSICAL CHEMICAL change MATTER: ATOMS WEIGHT: versus MASS: ELEMENT COMPOUND force amount
 Unit 1a Matter and Energy Chemistry is 1. The study of matter (structure, properties) 2. The changes that matter undergoes and 3. The energy involved in those changes. 1. Classify substances as either
Unit 1a Matter and Energy Chemistry is 1. The study of matter (structure, properties) 2. The changes that matter undergoes and 3. The energy involved in those changes. 1. Classify substances as either
- Kinetic energy: energy of matter in motion. gravity
 148 2500 L of chlorine gas at 25.0 C and 1.00 atm are used to make hydrochloric acid. How many grams of hydrochloric acid could be produced if all the chlorine reacts? 1 - Convert 2500 L chlorine gas to
148 2500 L of chlorine gas at 25.0 C and 1.00 atm are used to make hydrochloric acid. How many grams of hydrochloric acid could be produced if all the chlorine reacts? 1 - Convert 2500 L chlorine gas to
Glossary of Key Symbols and
 Glossary of Key s and Notation Bởi: OpenStaxCollege In this glossary, key symbols and notation are briefly defined. any symbol C Celsius degree F Fahrenheit degree // parallel average (indicated by a bar
Glossary of Key s and Notation Bởi: OpenStaxCollege In this glossary, key symbols and notation are briefly defined. any symbol C Celsius degree F Fahrenheit degree // parallel average (indicated by a bar
DESCRIBING MATTER. Matter is anything that has mass and volume
 DESCRIBING MATTER Matter is anything that has mass and volume Mass the amount of matter in an object measured with a balance Units are grams, kilograms (SI), centigrams Weight the measurement of gravitational
DESCRIBING MATTER Matter is anything that has mass and volume Mass the amount of matter in an object measured with a balance Units are grams, kilograms (SI), centigrams Weight the measurement of gravitational
Physics Curriculum Map - Norwell High School SUBJECT: Physics Grade Level: 11 or 12. Month or Unit: September
 SUBJECT: Physics Grade Level: 11 or 12 Month or Unit: September Scientific Inquiry Skills Scientific literacy can be achieved as students inquire about chemical phenomena. The curriculum should include
SUBJECT: Physics Grade Level: 11 or 12 Month or Unit: September Scientific Inquiry Skills Scientific literacy can be achieved as students inquire about chemical phenomena. The curriculum should include
Energy and Energy Calculations Test Provide the correct answer as a word, phrase or sentence. (3 points each) 1) Define Matter.
 Provide the correct answer as a word, phrase or sentence. (3 points each) 1) Define Matter. 2) What is ENERGY? 3) Give an example of an endothermic process. 4) Give an example of an exothermic process.
Provide the correct answer as a word, phrase or sentence. (3 points each) 1) Define Matter. 2) What is ENERGY? 3) Give an example of an endothermic process. 4) Give an example of an exothermic process.
WORK, POWER & ENERGY
 WORK, POWER & ENERGY Work An applied force acting over a displacement. The force being applied must be parallel to the displacement for work to be occurring. Work Force displacement Units: Newton meter
WORK, POWER & ENERGY Work An applied force acting over a displacement. The force being applied must be parallel to the displacement for work to be occurring. Work Force displacement Units: Newton meter
Study Guide for Physics 1100 Final Exam
 Study Guide for Physics 1100 Final Exam Dr. Fazzini s Physics 1100 Final Exam will take place on Wednesday, May 16 th, 2018 from 9:00AM-10:50AM in Room BIC-3535. Click on the Detailed Class Information
Study Guide for Physics 1100 Final Exam Dr. Fazzini s Physics 1100 Final Exam will take place on Wednesday, May 16 th, 2018 from 9:00AM-10:50AM in Room BIC-3535. Click on the Detailed Class Information
Chemistry: The Central Science. Chapter 5: Thermochemistry
 Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
Chemistry: The Central Science Chapter 5: Thermochemistry Study of energy and its transformations is called thermodynamics Portion of thermodynamics that involves the relationships between chemical and
TEACHER BACKGROUND INFORMATION FORCE
 TEACHER BACKGROUND INFORMATION FORCE WHAT IS FORCE? Force is anything that can change the state of motion of a body. In simpler terms, force is a push or a pull. For example, wind pushing on a flag is
TEACHER BACKGROUND INFORMATION FORCE WHAT IS FORCE? Force is anything that can change the state of motion of a body. In simpler terms, force is a push or a pull. For example, wind pushing on a flag is
PHYSICS FORM 5 ELECTRICAL QUANTITES
 QUANTITY SYMBOL UNIT SYMBOL Current I Amperes A Voltage (P.D.) V Volts V Resistance R Ohm Ω Charge (electric) Q Coulomb C Power P Watt W Energy E Joule J Time T seconds s Quantity of a Charge, Q Q = It
QUANTITY SYMBOL UNIT SYMBOL Current I Amperes A Voltage (P.D.) V Volts V Resistance R Ohm Ω Charge (electric) Q Coulomb C Power P Watt W Energy E Joule J Time T seconds s Quantity of a Charge, Q Q = It
F=ma. Exam 1. Today. Announcements: The average on the first exam was 31/40 Exam extra credit is due by 8:00 am Friday February 20th.
 Today Exam 1 Announcements: The average on the first exam was 31/40 Exam extra credit is due by 8:00 am Friday February 0th. F=ma Electric Force Work, Energy and Power Number 60 50 40 30 0 10 0 17 18 0
Today Exam 1 Announcements: The average on the first exam was 31/40 Exam extra credit is due by 8:00 am Friday February 0th. F=ma Electric Force Work, Energy and Power Number 60 50 40 30 0 10 0 17 18 0
Chapter 1. Chapter 1
 Chapter 1 Scientific and Engineering Notation Very large and very small numbers are represented with scientific and engineering notation. 47,000,000 = 4.7 x 10 7 (Scientific Notation) = 47 x 10 6 (Engineering
Chapter 1 Scientific and Engineering Notation Very large and very small numbers are represented with scientific and engineering notation. 47,000,000 = 4.7 x 10 7 (Scientific Notation) = 47 x 10 6 (Engineering
CHEM What is Energy? Terminology: E = KE + PE. Thermodynamics. Thermodynamics
 Thermodynamics 2 Thermodynamics The study of energy changes accompanying physical and chemical processes. From the laws of thermodynamics, one can: 1. Predict the results of chemical reactions 2. Ascertain
Thermodynamics 2 Thermodynamics The study of energy changes accompanying physical and chemical processes. From the laws of thermodynamics, one can: 1. Predict the results of chemical reactions 2. Ascertain
Unit 2 Electrical Quantities and Ohm s Law
 Electrical Quantities and Ohm s Law Objectives: Define a coulomb. Define an ampere. Define a volt. Define an ohm. Define a watt. Objectives: Compute electrical values using Ohm s law. Discuss basic types
Electrical Quantities and Ohm s Law Objectives: Define a coulomb. Define an ampere. Define a volt. Define an ohm. Define a watt. Objectives: Compute electrical values using Ohm s law. Discuss basic types
welcome to physics! 1.1 Mathematics and Physics
 welcome to physics! 1.1 Mathematics and Physics What is Physics? - study of energy, matter and how they are related - motion, energy of sound waves, electric circuits, etc Mathematics in Physics - use
welcome to physics! 1.1 Mathematics and Physics What is Physics? - study of energy, matter and how they are related - motion, energy of sound waves, electric circuits, etc Mathematics in Physics - use
Electricity Courseware Instructions
 Physics Electricity Courseware Instructions This courseware acts as a supplement to the classroom instruction. The five sections on the following slide link to the topic areas. Following the topic area
Physics Electricity Courseware Instructions This courseware acts as a supplement to the classroom instruction. The five sections on the following slide link to the topic areas. Following the topic area
Physics Spring Final Review C O N C E P T U A L P H Y S I C S : F I R S T & S E C O N D S E M E S T E R
 Name Date Period Physics Spring Final Review C O N C E P T U A L P H Y S I C S : F I R S T & S E C O N D S E M E S T E R Directions: Answer the following questions based on in-class notes, worksheets,
Name Date Period Physics Spring Final Review C O N C E P T U A L P H Y S I C S : F I R S T & S E C O N D S E M E S T E R Directions: Answer the following questions based on in-class notes, worksheets,
High School. Prentice Hall. Conceptual Physics (Hewitt) Correlation to the Mississippi Curriculum Frameworks - Physics (High School)
 Prentice Hall High School C O R R E L A T E D T O Correlation to the Mississippi Curriculum Frameworks - Physics (High School) CONTENT STRANDS: Inquiry Physical Science 1. INQUIRY - Apply inquiry-based
Prentice Hall High School C O R R E L A T E D T O Correlation to the Mississippi Curriculum Frameworks - Physics (High School) CONTENT STRANDS: Inquiry Physical Science 1. INQUIRY - Apply inquiry-based
Thermochemistry-Part 1
 Brad Collins Thermochemistry-Part 1 Chapter 7 Thermochemistry Thermodynamics: The study of energy Thermochemistry: The study of energy in chemical reactions Energy: The capacity to do work Work = force
Brad Collins Thermochemistry-Part 1 Chapter 7 Thermochemistry Thermodynamics: The study of energy Thermochemistry: The study of energy in chemical reactions Energy: The capacity to do work Work = force
Chemistry Section Review 2.2
 Chemistry Section Review 2.2 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Standards of measurement are chosen because they a. can be related to everyday
Chemistry Section Review 2.2 Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Standards of measurement are chosen because they a. can be related to everyday
Introductory College Chemistry
 Introductory College Chemistry This course covers the topics shown below. Students navigate learning paths based on their level of readiness. Institutional users may customize the scope and sequence to
Introductory College Chemistry This course covers the topics shown below. Students navigate learning paths based on their level of readiness. Institutional users may customize the scope and sequence to
Chapter 3: Force, Work and Energy
 Chapter 3: Force and Force Equilibrium Chapter 3: Force, Work and Energy Chapter 3: Force, Work and Energy 3.1 Mass and Weight 3.2 Newton's Law of Gravitation 3.3 Force and Newton's 3 Laws of Motion 3.4
Chapter 3: Force and Force Equilibrium Chapter 3: Force, Work and Energy Chapter 3: Force, Work and Energy 3.1 Mass and Weight 3.2 Newton's Law of Gravitation 3.3 Force and Newton's 3 Laws of Motion 3.4
Page 1. Name:
 Name: 3834-1 - Page 1 1) If a woman runs 100 meters north and then 70 meters south, her total displacement is A) 170 m south B) 170 m north C) 30 m south D) 30 m north 2) The graph below represents the
Name: 3834-1 - Page 1 1) If a woman runs 100 meters north and then 70 meters south, her total displacement is A) 170 m south B) 170 m north C) 30 m south D) 30 m north 2) The graph below represents the
LECTURE 4 - Units Used in Measurements
 LECTURE 4 - Units Used in Measurements Note: Slide numbers refer to the PowerPoint presentation which accompanies the lecture. Units, slide 1 here Introduction Geochemical measurements may be expressed
LECTURE 4 - Units Used in Measurements Note: Slide numbers refer to the PowerPoint presentation which accompanies the lecture. Units, slide 1 here Introduction Geochemical measurements may be expressed
MR. HOLL S PHYSICS FACTS MECHANICS. 1) Velocity is a vector quantity that has both magnitude and direction.
 MR. HOLL S PHYSICS FACTS MECHANICS 1) Velocity is a vector quantity that has both magnitude and direction. 2) Speed is a scalar quantity that has ONLY magnitude. 3) Distance is a scalar and represents
MR. HOLL S PHYSICS FACTS MECHANICS 1) Velocity is a vector quantity that has both magnitude and direction. 2) Speed is a scalar quantity that has ONLY magnitude. 3) Distance is a scalar and represents
Figure 1. In the following information, you will study these three physical quantities as they relate to simple electrical circuits.
 Module 7 Ohm s Law INTRODUCTION In this experiment, you will study Ohm s Law, the most fundamental relation used in the analysis of electrical circuits. Ohm s Law relates the quantities of voltage, electric
Module 7 Ohm s Law INTRODUCTION In this experiment, you will study Ohm s Law, the most fundamental relation used in the analysis of electrical circuits. Ohm s Law relates the quantities of voltage, electric
PREFIXES AND SYMBOLS SI Prefixes you need to know by heart
 PREFIXES AND SYMBOLS SI Prefixes you need to know by heart Prefix Symbol In 10 n in Decimal Forms Giga G 10 9 1,000,000,000 Mega M 10 6 1,000,000 kilo k 10 3 1,000 deci d 10 1 0.1 centi c 10 2 0.01 milli
PREFIXES AND SYMBOLS SI Prefixes you need to know by heart Prefix Symbol In 10 n in Decimal Forms Giga G 10 9 1,000,000,000 Mega M 10 6 1,000,000 kilo k 10 3 1,000 deci d 10 1 0.1 centi c 10 2 0.01 milli
MEASUREMENTS. Dimensions of physical quantities
 The document contains MCQs on Units & Measurements and is aimed at giving the students an idea of how the problems in the unit can be solved speedily MEASUREMENTS Dimensions of physical quantities IKGOGIA
The document contains MCQs on Units & Measurements and is aimed at giving the students an idea of how the problems in the unit can be solved speedily MEASUREMENTS Dimensions of physical quantities IKGOGIA
qq k d Chapter 16 Electric and Magnetic Forces Electric charge Electric charges Negative (electron) Positive (proton)
 Chapter 16 Electric and Magnetic Forces Electric charge Electric charges Negative (electron) Positive (proton) Electrons and protons in atoms/molecules Ions: atoms/molecules with excess of charge Ions
Chapter 16 Electric and Magnetic Forces Electric charge Electric charges Negative (electron) Positive (proton) Electrons and protons in atoms/molecules Ions: atoms/molecules with excess of charge Ions
PHY 101. Work and Kinetic Energy 7.1 Work Done by a Constant Force
 PHY 101 DR M. A. ELERUJA KINETIC ENERGY AND WORK POTENTIAL ENERGY AND CONSERVATION OF ENERGY CENTRE OF MASS AND LINEAR MOMENTUM Work is done by a force acting on an object when the point of application
PHY 101 DR M. A. ELERUJA KINETIC ENERGY AND WORK POTENTIAL ENERGY AND CONSERVATION OF ENERGY CENTRE OF MASS AND LINEAR MOMENTUM Work is done by a force acting on an object when the point of application
Unit 1. ET Unit 1. Quantities, Units, and Electrical Safety. Electronics Fundamentals Circuits, Devices and Applications - Floyd
 ET 115 - Unit 1 Quantities, Units, and Electrical Safety Scientific and Engineering Notation Very large and very small numbers are represented with scientific and engineering notation. 47,000,000 = 4.7
ET 115 - Unit 1 Quantities, Units, and Electrical Safety Scientific and Engineering Notation Very large and very small numbers are represented with scientific and engineering notation. 47,000,000 = 4.7
Prep for AP Chemistry
 Prep for AP Chemistry This course covers the topics shown below. Students navigate learning paths based on their level of readiness. Institutional users may customize the scope and sequence to meet curricular
Prep for AP Chemistry This course covers the topics shown below. Students navigate learning paths based on their level of readiness. Institutional users may customize the scope and sequence to meet curricular
Work- Work done W is defined as the dot product of force F and displacement s.
 Work- Work done W is defined as the dot product of force F and displacement s. Here θ is the angle between and. Work done by the force is positive if the angle between force and displacement is acute (0
Work- Work done W is defined as the dot product of force F and displacement s. Here θ is the angle between and. Work done by the force is positive if the angle between force and displacement is acute (0
UNIT & DIMENSIONS AND MEASUREMENT STRAIGHT LINES
 UNIT & DIMENSIONS AND MEASUREMENT STRAIGHT LINES PHYSICAL QUANTITIES The quantities which can be measured by an instrument and by means of which we can describe the laws of physics are called physical
UNIT & DIMENSIONS AND MEASUREMENT STRAIGHT LINES PHYSICAL QUANTITIES The quantities which can be measured by an instrument and by means of which we can describe the laws of physics are called physical
Basic Math for Relay Technicians. Hands On Relay School 2015 Presented by Charlene Reyes
 Basic Math for Relay Technicians Hands On Relay School 2015 Presented by Charlene Reyes Overview Order of Operations and Order of Magnitude Unit Analysis and Conversions Trigonometry Rectangular and Polar
Basic Math for Relay Technicians Hands On Relay School 2015 Presented by Charlene Reyes Overview Order of Operations and Order of Magnitude Unit Analysis and Conversions Trigonometry Rectangular and Polar
PHYSICS 149: Lecture 26
 PHYSICS 149: Lecture 26 Chapter 14: Heat 14.1 Internal Energy 14.2 Heat 14.3 Heat Capacity and Specific Heat 14.5 Phase Transitions 14.6 Thermal Conduction 14.7 Thermal Convection 14.8 Thermal Radiation
PHYSICS 149: Lecture 26 Chapter 14: Heat 14.1 Internal Energy 14.2 Heat 14.3 Heat Capacity and Specific Heat 14.5 Phase Transitions 14.6 Thermal Conduction 14.7 Thermal Convection 14.8 Thermal Radiation
Electromagnetism Checklist
 Electromagnetism Checklist Elementary Charge and Conservation of Charge 4.1.1A Convert from elementary charge to charge in coulombs What is the charge in coulombs on an object with an elementary charge
Electromagnetism Checklist Elementary Charge and Conservation of Charge 4.1.1A Convert from elementary charge to charge in coulombs What is the charge in coulombs on an object with an elementary charge
Scaler Quantity (definition and examples) Average speed. (definition and examples)
 Newton s First Law Newton s Second Law Newton s Third Law Vector Quantity Scaler Quantity (definition and examples) Average speed (definition and examples) Instantaneous speed Acceleration An object at
Newton s First Law Newton s Second Law Newton s Third Law Vector Quantity Scaler Quantity (definition and examples) Average speed (definition and examples) Instantaneous speed Acceleration An object at
Agenda. Chapter 10, Problem 26. All matter is made of atoms. Atomic Structure 4/8/14. What is the structure of matter? Atomic Terminology
 Agenda Today: HW Quiz, Thermal physics (i.e., heat) Thursday: Finish thermal physics, atomic structure (lots of review from chemistry!) Chapter 10, Problem 26 A boy reaches out of a window and tosses a
Agenda Today: HW Quiz, Thermal physics (i.e., heat) Thursday: Finish thermal physics, atomic structure (lots of review from chemistry!) Chapter 10, Problem 26 A boy reaches out of a window and tosses a
