.edu /~metin Page 1. utdallas. Science of Energy. Traditional Forms of Energy Potential Energy Kinetic Energy Thermal Energy
|
|
|
- Reynard Miller
- 6 years ago
- Views:
Transcription
1 Science of Energy Page 1 Traditional Forms of Energy Potential Energy Kinetic Energy Thermal Energy
2 Forms of Energy Page Fundamental law of Science: Energy and mass are conserved. You cannot waste energy even if you want. Wasting energy is actually transforming energy to an undesirable form Forms of Energy: Traditional Energy: Potential, Kinetic and Thermal Fuel-Based Energy: Chemical and Nuclear Electromagnetic Energy: Electric and Magnetic Energy generation is actually energy transformation from one form to another Dropping a pen: Potential Kinetic Rubbing hands: Kinetic Thermal Boiling water rises: Thermal Potential
3 Potential Energy Page 3 Gravitational force = Weight Weight = Mass m x Gravitational acceleration constant g Mass in kilogram (kg); Acceleration constant in metres per square second (m/s ) Then gravitational force in Newton = kg x m/s Ex: What is the weight (gravitational force applying to) of a person with 80 kilogram mass?» The weight is 784=80(9.8) kilogram meters per square second or 784 newtons. Potential Energy Potential Energy = Gravitational force mg x Height h Gravitational force in Newton & Height in meters Potential energy in Joule = Newton x metre Ex: What is the potential energy gained by a person with 80 kg mass walking stairs up for 10 metres? Would your answer change if the person takes an elevator?» It is 80(9.8)10 kg square meters per square second or simply 7,840 joule. Comparison between metric and imperial units Mass Gravitational Acceleration g Force Metric Kilogram kg 9.81 Metre per (second * second) m/s Newton N Imperial Slug s 1.00 Feet per (second * second) ft/s Pound force lbf Imperial Pound mass lbm 3.0 Feet per (second * second) ft/s Pound force lbf 1 kg = s =.0 lbm kg = 1 s = 3. lbm kg= slug = 1 lbm. Roughly Slug:Kilogram:Pound force ratios are 3 : : 1. If you never hear of slug & wonder where it is used, check crossword puzzles.
4 Kinetic Energy Page 4 Drop an object of mass m from a height of h, which takes time T Initial speed 0 and final speed at the bottom gt, average speed gt/ We must have h=distance=average speed * Time=(gT/)T Furthermore, potential energy becomes kinetic energy at the bottom Potential Energy = mmmmm = mmmm gggg TT = 1 mm gggg = Kinetic energy Kinetic energy is proportional to the mass and square of the speed from above. In general, Kinetic energy= 1 mmvv, where vv is the speed Ex: An accounting book weighs 1 kg and is dropped from 1 meter, what is its kinetic energy at the bottom of the drop? By using the conservation of energy, we can say the kinetic energy gained is equal to the potential energy lost, which is mgh=1(9.8)1=9.8 joule. Ex: Consider a horizontal cylinder which is subject to air flow from left to right. There is a propeller at the right-hand side of the cylinder. The air flow passes 75% of its kinetic energy to the propeller to rotate it. Assuming that the energy conversion is perfect (no energy is lost when air flow's kinetic energy is passed to the propeller), how much does the air flow slows down after passing through the propeller? The kinetic energy of each incoming air particle is mmvv ii at the entry and mmvv ii mmvv ii = 1 3 = 1 mm vv ii mmvv 4 oo at the exit for vv oo = vv ii. Hence, air particles lose half of their speed after transferring their energy to the propeller.
5 Thermal Energy Page 5 Thermal Energy Thermal Energy = Mass m x Heat capacity constant C x Temperature K Mass is in kilogram Heat capacity constant is in joule per (kilogram x celsius) or joule per (kilogram x kelvin) Sometimes temperature is measured in kelvin; 0 kelvin is 73 celsius Heat capacity constant is the ability of a substance to absorb thermal energy For water 400 joule per (kilogram x celsius) > For sand 835 joule per (kilogram x celsius) Ex: To increase the temperature of 0.33 kilogram (330 millilitre, can soda size) of water by 50 celsius, how much thermal energy is needed? The energy needed for water is 0.33(400)50=69,300 joules. For storage and transportation of energy, the industry needs substances with high heat capacity such as water and molten salts. 1 calorie = 4. joule and increases the temperature of 1 gram water by 1 Celsius Ex: A cafeteria sells 1000 calorie salads. How many meters a person with 80 kg mass can climb with this amount of energy? 1000 calorie = 400 joule, which is the potential energy of 80 kg person at 5.35 meters = 4,00/(80 * 9.8). To burn a salad, is it sufficient to climb two floors of a building? No! Dieticians use calorie to mean kilocalories. The salad actually has 1,000,000 calories which can be burnt by climbing a mountain of 5350 meters!
6 Summary Page 6 Potential Energy Kinetic Energy Thermal Energy
7 Which Empire had Imperial Units? A lot of them! British Empire British used pound as the unit of mass. Not only one, but many pounds in use:» London Pound» Merchant Pound» Tower Pound» Troy Pound» Avoir-du-pois (goods-of-weight in French) Pound But these are all shortened as lb, why? French Empire» Livre esterin» Livre de Paris» Livre metrique Pfund in Prussian Empire Pond in the Netherlands Funt in Russian Empire (Skal)Pund in Swedish Empire Litra in Byzantine Empire Litre for volume now Libra Pondo (weights of weighing) in Roman Empire. Libra is shortened as lb. Ponderis: To weigh in Latin. Linguistic Insight: Words Pound & Ponder are relatives. Handle with hook for suspension Cup A chain with head of youthful Mercury Bronze Balance, Naples, Museo Archeologico Nazionale. Libra lb Litra Litre Livre Pondo Pund Funt Pond Pfund Pound Page 7
Energy and the Environment
 Energy and the Environment Energy physics definition the capacity to do work and conjunction used to connect grammatically coordinate words, phrases, or clauses the Environment the aggregate of surrounding
Energy and the Environment Energy physics definition the capacity to do work and conjunction used to connect grammatically coordinate words, phrases, or clauses the Environment the aggregate of surrounding
ENERGY. Unit 12: IPC
 ENERGY Unit 12: IPC WHAT IS ENERGY? Energy- is the ability to do work. Energy is the ability to cause a change. Energy can change an object s: motion shape temperature color THERMAL internal motion of
ENERGY Unit 12: IPC WHAT IS ENERGY? Energy- is the ability to do work. Energy is the ability to cause a change. Energy can change an object s: motion shape temperature color THERMAL internal motion of
Unit Two Chapter Three Forces
 Unit Two Chapter Three Forces Key Concepts After completing this chapter you will be able to distinguish between different types of forces and describe how they affect the velocity and acceleration of
Unit Two Chapter Three Forces Key Concepts After completing this chapter you will be able to distinguish between different types of forces and describe how they affect the velocity and acceleration of
Gravitational. potential energy. Objectives. Assessment. Assessment. Equations. Physics terms 6/3/14
 Gravitational potential energy Objectives Investigate examples of gravitational potential energy. Calculate the potential energy, mass, or height of an object using the gravitational potential energy equation.
Gravitational potential energy Objectives Investigate examples of gravitational potential energy. Calculate the potential energy, mass, or height of an object using the gravitational potential energy equation.
APPLICATIONS OF INTEGRATION
 6 APPLICATIONS OF INTEGRATION APPLICATIONS OF INTEGRATION 6.4 Work In this section, we will learn about: Applying integration to calculate the amount of work done in performing a certain physical task.
6 APPLICATIONS OF INTEGRATION APPLICATIONS OF INTEGRATION 6.4 Work In this section, we will learn about: Applying integration to calculate the amount of work done in performing a certain physical task.
Chemistry 104 Chapter Two PowerPoint Notes
 Measurements in Chemistry Chapter 2 Physical Quantities Measurable physical properties such as height, volume, and temperature are called Physical quantity. A number and a unit of defined size is required
Measurements in Chemistry Chapter 2 Physical Quantities Measurable physical properties such as height, volume, and temperature are called Physical quantity. A number and a unit of defined size is required
UNIT 1 UNITS AND DIMENSIONS
 UNIT 1 UNITS AND DIMENSIONS Unit is any measure or amount used as a standard for measurement. It is a means of the measurable extent of a physical quantity. The derived unit is a combination of primary
UNIT 1 UNITS AND DIMENSIONS Unit is any measure or amount used as a standard for measurement. It is a means of the measurable extent of a physical quantity. The derived unit is a combination of primary
WEP-Work and Power. What is the amount of work done against gravity as an identical mass is moved from A to C? J J J 4.
 1. The work done in accelerating an object along a frictionless horizontal surface is equal to the change in the object s 1. momentum 2. velocity 3. potential energy 4. kinetic energy 2. The graph below
1. The work done in accelerating an object along a frictionless horizontal surface is equal to the change in the object s 1. momentum 2. velocity 3. potential energy 4. kinetic energy 2. The graph below
6.5 Work and Fluid Forces
 6.5 Work and Fluid Forces Work Work=Force Distance Work Work=Force Distance Units Force Distance Work Newton meter Joule (J) pound foot foot-pound (ft lb) Work Work=Force Distance Units Force Distance
6.5 Work and Fluid Forces Work Work=Force Distance Work Work=Force Distance Units Force Distance Work Newton meter Joule (J) pound foot foot-pound (ft lb) Work Work=Force Distance Units Force Distance
Pre Comp Review Questions 7 th Grade
 Pre Comp Review Questions 7 th Grade Section 1 Units 1. Fill in the missing SI and English Units Measurement SI Unit SI Symbol English Unit English Symbol Time second s second s. Temperature Kelvin K Fahrenheit
Pre Comp Review Questions 7 th Grade Section 1 Units 1. Fill in the missing SI and English Units Measurement SI Unit SI Symbol English Unit English Symbol Time second s second s. Temperature Kelvin K Fahrenheit
What is Energy? Energy is the capacity to do work
 What is Energy? Energy is the capacity to do work Work the product of force exerted on an object and the distance the object moves in the direction of the force. W=Fd W = work (Joules, J) F = force (N)
What is Energy? Energy is the capacity to do work Work the product of force exerted on an object and the distance the object moves in the direction of the force. W=Fd W = work (Joules, J) F = force (N)
= W Q H. ɛ = T H T C T H = = 0.20 = T C = T H (1 0.20) = = 320 K = 47 C
 1. Four identical 0.18 kg masses are placed at the corners of a 4.0 x 3.0 m rectangle, and are held there by very light connecting rods which form the sides of the rectangle. What is the moment of inertia
1. Four identical 0.18 kg masses are placed at the corners of a 4.0 x 3.0 m rectangle, and are held there by very light connecting rods which form the sides of the rectangle. What is the moment of inertia
Page 1 SPH3U. Heat. What is Heat? Thermal Physics. Waterloo Collegiate Institute. Some Definitions. Still More Heat
 SPH3U Thermal Physics electrons and holes in semiconductors An Introductory ourse in Thermodynamics converting energy into work magnetism thin films and surface chemistry thermal radiation (global warming)
SPH3U Thermal Physics electrons and holes in semiconductors An Introductory ourse in Thermodynamics converting energy into work magnetism thin films and surface chemistry thermal radiation (global warming)
- Joule (J): SI unit for energy. It's defined based on the equation for kinetic energy. from. mass. velocity
 153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
153 ENERGY UNITS - calorie (cal): the amount of energy required to change the temperature of one gram of water by one degree Celsius (or Kelvin) 1g 1g add one calorie of energy - Calories in food? The
NATIONAL 5 PHYSICS THERMODYNAMICS
 NATIONAL 5 PHYSICS THERMODYNAMICS HEAT AND TEMPERATURE Heat and temperature are not the same thing! Heat Heat is a type of energy. Like all types of energy it is measured in joules (J). The heat energy
NATIONAL 5 PHYSICS THERMODYNAMICS HEAT AND TEMPERATURE Heat and temperature are not the same thing! Heat Heat is a type of energy. Like all types of energy it is measured in joules (J). The heat energy
Temp vs. Heat. Absolute Temperature Scales. Common Temperature Scales. Thermal Energy. Heat and Temperature are not the same!!
 Thermal Energy Heat and Temperature are not the same!! Cold is the absence of heat, not an energy Same concept as light/dark Cold can t come in, heat flows out Heat flows from High Temp Low Temp Temp vs.
Thermal Energy Heat and Temperature are not the same!! Cold is the absence of heat, not an energy Same concept as light/dark Cold can t come in, heat flows out Heat flows from High Temp Low Temp Temp vs.
Work Done by a Constant Force
 Work and Energy Work Done by a Constant Force In physics, work is described by what is accomplished when a force acts on an object, and the object moves through a distance. The work done by a constant
Work and Energy Work Done by a Constant Force In physics, work is described by what is accomplished when a force acts on an object, and the object moves through a distance. The work done by a constant
Chapter 4. Energy. Work Power Kinetic Energy Potential Energy Conservation of Energy. W = Fs Work = (force)(distance)
 Chapter 4 Energy In This Chapter: Work Kinetic Energy Potential Energy Conservation of Energy Work Work is a measure of the amount of change (in a general sense) that a force produces when it acts on a
Chapter 4 Energy In This Chapter: Work Kinetic Energy Potential Energy Conservation of Energy Work Work is a measure of the amount of change (in a general sense) that a force produces when it acts on a
Ch 100: Fundamentals for Chemistry
 Ch 100: Fundamentals for Chemistry Chapter 4: Properties of Matter Lecture Notes Physical & Chemical Properties Physical Properties are the characteristics of matter that can be changed without changing
Ch 100: Fundamentals for Chemistry Chapter 4: Properties of Matter Lecture Notes Physical & Chemical Properties Physical Properties are the characteristics of matter that can be changed without changing
BRCC CHM 101 Class Notes Chapter 1 Page 1 of 7
 BRCC CHM 101 Class Notes Chapter 1 Page 1 of 7 Chemistry - the study of matter, its behavior and interactions. matter - anything that takes up space and has mass mass - the substance which makes up the
BRCC CHM 101 Class Notes Chapter 1 Page 1 of 7 Chemistry - the study of matter, its behavior and interactions. matter - anything that takes up space and has mass mass - the substance which makes up the
Thermodynamics B/C. Rank: Points: Science Olympiad North Regional Tournament at the University of Florida. Name(s): Team Name: School Name:
 Thermodynamics B/C Science Olympiad North Regional Tournament at the University of Florida Rank: Points: Name(s): Team Name: School Name: Team Number: 1. True/False: Boyle s Law relates the volume to the
Thermodynamics B/C Science Olympiad North Regional Tournament at the University of Florida Rank: Points: Name(s): Team Name: School Name: Team Number: 1. True/False: Boyle s Law relates the volume to the
Lecture 1 INTRODUCTION AND BASIC CONCEPTS
 Lecture 1 INTRODUCTION AND BASIC CONCEPTS Objectives Identify the unique vocabulary associated with thermodynamics through the precise definition of basic concepts to form a sound foundation for the development
Lecture 1 INTRODUCTION AND BASIC CONCEPTS Objectives Identify the unique vocabulary associated with thermodynamics through the precise definition of basic concepts to form a sound foundation for the development
INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin
 Lecture INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin The Metric System by Christopher G. Hamaker Illinois State University Basic Units and Symbols The English
Lecture INTRODUCTORY CHEMISTRY Concepts and Critical Thinking Seventh Edition by Charles H. Corwin The Metric System by Christopher G. Hamaker Illinois State University Basic Units and Symbols The English
100 Physics Facts. 1. The standard international unit (SI unit) for mass (m) is. kg (kilograms) s (seconds)
 100 Physics Facts 1. The standard international unit (SI unit) for mass (m) is. kg (kilograms) 2. The standard international unit (SI unit) for time (t) is. s (seconds) 3. The standard international unit
100 Physics Facts 1. The standard international unit (SI unit) for mass (m) is. kg (kilograms) 2. The standard international unit (SI unit) for time (t) is. s (seconds) 3. The standard international unit
Physics 130: Questions to study for midterm #1 from Chapter 7
 Physics 130: Questions to study for midterm #1 from Chapter 7 1. Kinetic energy is defined to be one-half the a. mass times the speed. b. mass times the speed squared. c. mass times the acceleration. d.
Physics 130: Questions to study for midterm #1 from Chapter 7 1. Kinetic energy is defined to be one-half the a. mass times the speed. b. mass times the speed squared. c. mass times the acceleration. d.
To determine the work and power required to walk and then run through one floor stairs. To determine the energy burned during that exercise
 Essentials of Physics: WORK AND POWER Purpose To determine the work and power required to walk and then run through one floor stairs. To determine the energy burned during that exercise Theory In this
Essentials of Physics: WORK AND POWER Purpose To determine the work and power required to walk and then run through one floor stairs. To determine the energy burned during that exercise Theory In this
Chapter 4 Forces Newton s Laws of Motion
 Chapter 4 Forces Newton s Laws of Motion Forces Force A vector quantity that changes the velocity vector of an object. When you hit a baseball, the velocity of the ball changes. Can be a push or a pull
Chapter 4 Forces Newton s Laws of Motion Forces Force A vector quantity that changes the velocity vector of an object. When you hit a baseball, the velocity of the ball changes. Can be a push or a pull
Practice Packet: Energy. Regents Chemistry: Dr. Shanzer. Practice Packet. Chapter 4: Energy.
 Regents Chemistry: Dr. Shanzer Practice Packet Chapter 4: Energy http:/drshanzerchemistry.weebly.com Energy Objectives Define energy. Demonstrate the difference between endothermic and exothermic reactions
Regents Chemistry: Dr. Shanzer Practice Packet Chapter 4: Energy http:/drshanzerchemistry.weebly.com Energy Objectives Define energy. Demonstrate the difference between endothermic and exothermic reactions
CHEM Thermodynamics. Heat calculations
 Thermodynamics Heat calculations l Internal Energy, E The internal energy of other systems that are more complex than the ideal gas cannot be measured. But the internal energy of the system is still proportional
Thermodynamics Heat calculations l Internal Energy, E The internal energy of other systems that are more complex than the ideal gas cannot be measured. But the internal energy of the system is still proportional
International System of Units 3.2. Slide 1of 33
 International System 3.2 1of 33 3.2 The International System In the signs shown here, the distances are listed as numbers with no units attached. Without the units, it is impossible to communicate the
International System 3.2 1of 33 3.2 The International System In the signs shown here, the distances are listed as numbers with no units attached. Without the units, it is impossible to communicate the
KINETIC AND POTENTIAL ENERGY. Chapter 6 (cont.)
 KINETIC AND POTENTIAL ENERGY Chapter 6 (cont.) The Two Types of Mechanical Energy Energy- the ability to do work- measured in joules Potential Energy- energy that arises because of an object s position
KINETIC AND POTENTIAL ENERGY Chapter 6 (cont.) The Two Types of Mechanical Energy Energy- the ability to do work- measured in joules Potential Energy- energy that arises because of an object s position
Topic 5: Energetics. Heat & Calorimetry. Thursday, March 22, 2012
 Topic 5: Energetics Heat & Calorimetry 1 Heat is energy that is transferred from one object to another due to a difference in temperature Temperature is a measure of the average kinetic energy of a body
Topic 5: Energetics Heat & Calorimetry 1 Heat is energy that is transferred from one object to another due to a difference in temperature Temperature is a measure of the average kinetic energy of a body
Properties of Matter. Heat. Summary
 Properties of Matter Heat Summary Heat is a form of energy that is measured in joules (J). The temperature of an object is a measure of the average kinetic energy of the particles in the object and is
Properties of Matter Heat Summary Heat is a form of energy that is measured in joules (J). The temperature of an object is a measure of the average kinetic energy of the particles in the object and is
PE = mgh. Potential energy. What is g here? Let s pick up where we left off last time..the topic was gravitational potential energy
 Let s pick up where we left off last time..the topic was gravitational potential energy Now, let s talk about a second form of energy Potential energy Imagine you are standing on top of half dome in Yosemite
Let s pick up where we left off last time..the topic was gravitational potential energy Now, let s talk about a second form of energy Potential energy Imagine you are standing on top of half dome in Yosemite
Unit 3 Energy and Society Work and Energy
 Unit 3 Energy and Society Work and Energy Today's goal: I can identify energy issues that exist and can articulate the connection between issues and work and energy. Energy Issues: Definitions: Energy
Unit 3 Energy and Society Work and Energy Today's goal: I can identify energy issues that exist and can articulate the connection between issues and work and energy. Energy Issues: Definitions: Energy
SCIENCE STUDENT BOOK. 12th Grade Unit 3
 SCIENCE STUDENT BOOK 12th Grade Unit 3 Unit 3 WORK AND ENERGY SCIENCE 1203 WORK AND ENERGY INTRODUCTION 3 1. TYPE AND SOURCES OF ENERGY 5 MECHANICAL ENERGY 6 FORMS OF ENERGY 9 SELF TEST 1 12 2. CONSERVATION
SCIENCE STUDENT BOOK 12th Grade Unit 3 Unit 3 WORK AND ENERGY SCIENCE 1203 WORK AND ENERGY INTRODUCTION 3 1. TYPE AND SOURCES OF ENERGY 5 MECHANICAL ENERGY 6 FORMS OF ENERGY 9 SELF TEST 1 12 2. CONSERVATION
Chapter 2: Approaches to Problem Solving Lecture notes Math 1030 Section B
 Section B.1: Standardized Unit Systems: U.S. and Metric Different system of standardized units There are different systems of standardized units: the international metric system, called SI (from the French
Section B.1: Standardized Unit Systems: U.S. and Metric Different system of standardized units There are different systems of standardized units: the international metric system, called SI (from the French
Objectives. Power in Translational Systems 298 CHAPTER 6 POWER
 Objectives Explain the relationship between power and work. Explain the relationship between power, force, and speed for an object in translational motion. Calculate a device s efficiency in terms of the
Objectives Explain the relationship between power and work. Explain the relationship between power, force, and speed for an object in translational motion. Calculate a device s efficiency in terms of the
Materials and Energy Balance in Metallurgical Processes. Prof. S. C. Koria. Department of Materials Science and Engineering
 Materials and Energy Balance in Metallurgical Processes Prof. S. C. Koria Department of Materials Science and Engineering Indian Institute of Technology, Kanpur Module No. # 01 Lecture No. # 02 Measurement
Materials and Energy Balance in Metallurgical Processes Prof. S. C. Koria Department of Materials Science and Engineering Indian Institute of Technology, Kanpur Module No. # 01 Lecture No. # 02 Measurement
Chapter 5 Assessment. 164 Chapter 5 Measurements and Calculations. 8. Write each of the following numbers in standard scientific notation. a.
 Chapter 5 Assessment All exercises with blue numbers have answers in the back of this book. 5.1 Scientific Notation and Units A. Scientific Notation 1. When the number 98,145 is written in standard scientific
Chapter 5 Assessment All exercises with blue numbers have answers in the back of this book. 5.1 Scientific Notation and Units A. Scientific Notation 1. When the number 98,145 is written in standard scientific
Physics 2414 Group Exercise 8. Conservation of Energy
 Physics 244 Group Exercise 8 Name : OUID : Name 2: OUID 2: Name 3: OUID 3: Name 4: OUID 4: Section Number: Solutions Solutions Conservation of Energy A mass m moves from point i to point f under the action
Physics 244 Group Exercise 8 Name : OUID : Name 2: OUID 2: Name 3: OUID 3: Name 4: OUID 4: Section Number: Solutions Solutions Conservation of Energy A mass m moves from point i to point f under the action
Unit A-1: List of Subjects
 ES312 Energy Transfer Fundamentals Unit A: Fundamental Concepts ROAD MAP... A-1: Introduction to Thermodynamics A-2: Engineering Properties Unit A-1: List of Subjects What is Thermodynamics? First and
ES312 Energy Transfer Fundamentals Unit A: Fundamental Concepts ROAD MAP... A-1: Introduction to Thermodynamics A-2: Engineering Properties Unit A-1: List of Subjects What is Thermodynamics? First and
MOTOR WIRING DATA From National Electrical Code 3 PHASE SQUIRREL CAGE INDUCTION MOTORS 230 Volt 460 Volt Min. # Max. Rating
 MOTOR WIRING DATA From National Electrical Code PHASE SQUIRREL CAGE INDUCTION MOTORS 20 Volt 0 Volt Min. # Max. Rating Min. Size Size of Full Size Wire Conduit Branch Circuit Load Wire AWG (inches) Fuses
MOTOR WIRING DATA From National Electrical Code PHASE SQUIRREL CAGE INDUCTION MOTORS 20 Volt 0 Volt Min. # Max. Rating Min. Size Size of Full Size Wire Conduit Branch Circuit Load Wire AWG (inches) Fuses
Chapter 3 Metric Units and Conversions
 Chapter 3 Metric Units and Conversions 3.1 The Metric System and Prefixes Metric system: a simple decimal system of measurement that uses the following basic units: Quantity Basic Unit Symbol length meter
Chapter 3 Metric Units and Conversions 3.1 The Metric System and Prefixes Metric system: a simple decimal system of measurement that uses the following basic units: Quantity Basic Unit Symbol length meter
Scientific Measurement
 Scientific Measurement A quantity is anything having a measurable size or amount For Example: 5 But 5 what? A unit assigns value to a measured quantity For Example: 5 ft, 5 gal, 5 sec, 5 m, 5 g. Base Units
Scientific Measurement A quantity is anything having a measurable size or amount For Example: 5 But 5 what? A unit assigns value to a measured quantity For Example: 5 ft, 5 gal, 5 sec, 5 m, 5 g. Base Units
U.S. pound (lb) foot (ft) foot-pounds (ft-lb) pound (lb) inch (in) inch-pounds (in-lb) tons foot (ft) foot-tons (ft-ton)
 Math 1206 Calculus Sec. 6.4: Work I. Work Done by a Constant Force A. Def n : If an object is moved a distance D in the direction of an applied constant force F, then the work W done by the force is defined
Math 1206 Calculus Sec. 6.4: Work I. Work Done by a Constant Force A. Def n : If an object is moved a distance D in the direction of an applied constant force F, then the work W done by the force is defined
Chemistry - the science that describes matter properties physical and chemical changes associated energy changes
 Chemistry - the science that describes matter properties physical and chemical changes associated energy changes Matter - occupies space and has mass. Ex. Textbook Energy is the capacity to do work or
Chemistry - the science that describes matter properties physical and chemical changes associated energy changes Matter - occupies space and has mass. Ex. Textbook Energy is the capacity to do work or
Physics of Energy. Premise of this course in order to come up with such a solution, we need to understand how energy works?
 Physics of Energy As we discussed. Our society needs to find a sustainable energy solution that Fulfills global energy needs in the long term. Doesn t degrade the environment. Premise of this course in
Physics of Energy As we discussed. Our society needs to find a sustainable energy solution that Fulfills global energy needs in the long term. Doesn t degrade the environment. Premise of this course in
Honors Chemistry Energy and Specific Heat Lab
 Honors Chemistry Energy and Lab Name Date Objectives: Calculate the amount of heat absorbed or released by a substance as its temperature changes. Describe how a calorimeter is used to measure energy that
Honors Chemistry Energy and Lab Name Date Objectives: Calculate the amount of heat absorbed or released by a substance as its temperature changes. Describe how a calorimeter is used to measure energy that
is both a Thing and a Process
 ENERGY = Matter + ENERGY is both a Thing and a Process Matter HAS Energy. Energy is usually observable only during transfer. Allows WORK to be done. Matter is bottled-up energy. Energy is the capacity
ENERGY = Matter + ENERGY is both a Thing and a Process Matter HAS Energy. Energy is usually observable only during transfer. Allows WORK to be done. Matter is bottled-up energy. Energy is the capacity
Scaler Quantity (definition and examples) Average speed. (definition and examples)
 Newton s First Law Newton s Second Law Newton s Third Law Vector Quantity Scaler Quantity (definition and examples) Average speed (definition and examples) Instantaneous speed Acceleration An object at
Newton s First Law Newton s Second Law Newton s Third Law Vector Quantity Scaler Quantity (definition and examples) Average speed (definition and examples) Instantaneous speed Acceleration An object at
Lecture 5. > Specific Heat. > Calorimetry. (Source: Cutnell, Giancoli, Tippens) Villacorta--DLSUM-SCIENVP-L Term01
 Lecture 5 > Specific Heat > Calorimetry (Source: Cutnell, Giancoli, Tippens) 1 Heat > An increase in a body's temperature is related to the increase in the kinetic energy of the molecules composing the
Lecture 5 > Specific Heat > Calorimetry (Source: Cutnell, Giancoli, Tippens) 1 Heat > An increase in a body's temperature is related to the increase in the kinetic energy of the molecules composing the
Thermodynamics Test Clio Invitational January 26, 2013
 Thermodynamics Test Clio Invitational January 26, 2013 School Name: Team Number: Variables specified: s = specific heat C = heat capacity H f = heat of fusion H v = heat of vaporization Given information:
Thermodynamics Test Clio Invitational January 26, 2013 School Name: Team Number: Variables specified: s = specific heat C = heat capacity H f = heat of fusion H v = heat of vaporization Given information:
WORK, POWER & ENERGY
 WORK, POWER & ENERGY Work An applied force acting over a displacement. The force being applied must be parallel to the displacement for work to be occurring. Work Force displacement Units: Newton meter
WORK, POWER & ENERGY Work An applied force acting over a displacement. The force being applied must be parallel to the displacement for work to be occurring. Work Force displacement Units: Newton meter
Ch06. Energy. Thermochemistry, understanding energy, heat & work. version 1.5
 Ch06 Energy Thermochemistry, understanding energy, heat & work. version 1.5 Nick DeMello, PhD. 2007-2016 Ch06 Accounting for Energy Energy Definitions Classifications Units Kinetic, Potential, Thermal
Ch06 Energy Thermochemistry, understanding energy, heat & work. version 1.5 Nick DeMello, PhD. 2007-2016 Ch06 Accounting for Energy Energy Definitions Classifications Units Kinetic, Potential, Thermal
CHAPTER 2 Data Analysis
 CHAPTER 2 Data Analysis 2.1 Units of Measurement The standard of measurement used in science are those of the metric system. All the units are based on 10 or multiples of 10. SI Units: The International
CHAPTER 2 Data Analysis 2.1 Units of Measurement The standard of measurement used in science are those of the metric system. All the units are based on 10 or multiples of 10. SI Units: The International
Chapter 1 Introduction
 Fundamentals of Thermodynamics Chapter 1 Introduction Prof. Siyoung Jeong Thermodynamics I MEE2022-01 Thermodynamics : Science of energy and entropy - Science of heat and work and properties related to
Fundamentals of Thermodynamics Chapter 1 Introduction Prof. Siyoung Jeong Thermodynamics I MEE2022-01 Thermodynamics : Science of energy and entropy - Science of heat and work and properties related to
This reaction is ENDOTHERMIC. Energy is being transferred from the room/flask/etc. (the SURROUNDINGS) to the reaction itself (the SYSTEM).
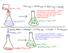 151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
151 This reaction is EXOTHERMIC. Energy is transferred from the reactants and products (the SYSTEM) to the water in the flask, the flask, etc. (the SURROUNDINGS) This reaction is ENDOTHERMIC. Energy is
9.2 Work & Energy Homework - KINETIC, GRAVITATIONAL & SPRING ENERGY
 9. Work & Energy Homework - KINETIC, GRAVITATIONAL & SPRING ENERGY KINETIC ENERGY QUESTIONS 9.H Energy.doc 1. A 500 kilogram car is driving at 15 meters/second. Calculate its kinetic energy? How much does
9. Work & Energy Homework - KINETIC, GRAVITATIONAL & SPRING ENERGY KINETIC ENERGY QUESTIONS 9.H Energy.doc 1. A 500 kilogram car is driving at 15 meters/second. Calculate its kinetic energy? How much does
1 Work, Power, and Machines
 CHAPTER 13 1 Work, Power, and Machines SECTION Work and Energy KEY IDEAS As you read this section, keep these questions in mind: What is work, and how is it measured? How are work and power related? How
CHAPTER 13 1 Work, Power, and Machines SECTION Work and Energy KEY IDEAS As you read this section, keep these questions in mind: What is work, and how is it measured? How are work and power related? How
Chapter 6 Work and Kinetic Energy
 Chapter 6 Work and Kinetic Energy Up until now, we have assumed that the force is constant and thus, the acceleration is constant. Is there a simple technique for dealing with non-constant forces? Fortunately,
Chapter 6 Work and Kinetic Energy Up until now, we have assumed that the force is constant and thus, the acceleration is constant. Is there a simple technique for dealing with non-constant forces? Fortunately,
Unit 5: Energy (Part 2)
 SUPERCHARGED SCIENCE Unit 5: Energy (Part 2) www.sciencelearningspace.com Appropriate for Grades: Lesson 1 (K-12), Lesson 2 (K-12) Duration: 6-15 hours, depending on how many activities you do! We covered
SUPERCHARGED SCIENCE Unit 5: Energy (Part 2) www.sciencelearningspace.com Appropriate for Grades: Lesson 1 (K-12), Lesson 2 (K-12) Duration: 6-15 hours, depending on how many activities you do! We covered
Momentum, Impulse, Work, Energy, Power, and Conservation Laws
 Momentum, Impulse, Work, Energy, Power, and Conservation Laws 1. Cart A has a mass of 2 kilograms and a speed of 3 meters per second. Cart B has a mass of 3 kilograms and a speed of 2 meters per second.
Momentum, Impulse, Work, Energy, Power, and Conservation Laws 1. Cart A has a mass of 2 kilograms and a speed of 3 meters per second. Cart B has a mass of 3 kilograms and a speed of 2 meters per second.
Module VII: Work. Background/Support Information
 Background/Support Information NAME: DATE: Module VII: Work OBJECTIVES/PURPOSE Students will: define the concept of work as force times distance distinguish the relation of work to energy apply the concept
Background/Support Information NAME: DATE: Module VII: Work OBJECTIVES/PURPOSE Students will: define the concept of work as force times distance distinguish the relation of work to energy apply the concept
Honors Chemistry Chapter 2 Problem Handout Solve the following on separate sheets of paper. Where appropriate, show all work. 1. Convert each of the
 Honors Chemistry Chapter 2 Problem Handout Solve the following on separate sheets of paper. Where appropriate, show all work. 1. Convert each of the following quantities to the required unit. a. 12.75
Honors Chemistry Chapter 2 Problem Handout Solve the following on separate sheets of paper. Where appropriate, show all work. 1. Convert each of the following quantities to the required unit. a. 12.75
WORK, POWER, & ENERGY
 WORK, POWER, & ENERGY In physics, work is done when a force acting on an object causes it to move a distance. There are several good examples of work which can be observed everyday - a person pushing a
WORK, POWER, & ENERGY In physics, work is done when a force acting on an object causes it to move a distance. There are several good examples of work which can be observed everyday - a person pushing a
E-BOOK / NEWTON METERS TO FOOT POUNDS EBOOK
 13 March, 2018 E-BOOK / 10000 NEWTON METERS TO FOOT POUNDS EBOOK Document Filetype: PDF 166.9 KB 0 E-BOOK / 10000 NEWTON METERS TO FOOT POUNDS EBOOK Sand, Fine density is equal to 1999 kg/m or 124.793
13 March, 2018 E-BOOK / 10000 NEWTON METERS TO FOOT POUNDS EBOOK Document Filetype: PDF 166.9 KB 0 E-BOOK / 10000 NEWTON METERS TO FOOT POUNDS EBOOK Sand, Fine density is equal to 1999 kg/m or 124.793
Measurement Chapter 1.6-7
 Unit 1 Essential Skills Measurement Chapter 1.6-7 The Unit 1 Test will cover material from the following Chapters and Sections: 1.all 2.5-8 3.all 2 Two types of Data: When we make observations of matter,
Unit 1 Essential Skills Measurement Chapter 1.6-7 The Unit 1 Test will cover material from the following Chapters and Sections: 1.all 2.5-8 3.all 2 Two types of Data: When we make observations of matter,
2 possibilities. 2.) Work is done and... 1.) Work is done and... *** The function of work is to change energy ***
 Work-Energy Theorem and Energy Conservation *** The function of work is to change energy *** 2 possibilities 1.) Work is done and... or 2.) Work is done and... 1 EX: A 100 N box is 10 m above the ground
Work-Energy Theorem and Energy Conservation *** The function of work is to change energy *** 2 possibilities 1.) Work is done and... or 2.) Work is done and... 1 EX: A 100 N box is 10 m above the ground
Motion, Forces, and Energy
 Motion, Forces, and Energy What is motion? Motion - when an object changes position Types of Motion There are 2 ways of describing motion: Distance Displacement Distance Distance is the total path traveled.
Motion, Forces, and Energy What is motion? Motion - when an object changes position Types of Motion There are 2 ways of describing motion: Distance Displacement Distance Distance is the total path traveled.
CHAPTER ONE. The Foundations of Chemistry
 CHAPTER ONE The Foundations of Chemistry Why is Chemistry Important? Materials for our homes Components for computers and other electronic devices Cooking Fuel Body functions 2 Some definitions / Vocabulary
CHAPTER ONE The Foundations of Chemistry Why is Chemistry Important? Materials for our homes Components for computers and other electronic devices Cooking Fuel Body functions 2 Some definitions / Vocabulary
Length is the distance from one point to another. Length has standard units of measurement such as inches or centimeters.
 Page 1 Measurements are a standard set by different cultures to address their own needs. In the United States, we use the U. S. Customary system of units. However, the metric system is used worldwide.
Page 1 Measurements are a standard set by different cultures to address their own needs. In the United States, we use the U. S. Customary system of units. However, the metric system is used worldwide.
P.O.T. GUIDESHEET UNIT 2. - WORK SUBUNIT - WORK IN MECHANICAL SYSTEMS ACTIVITY LESSON DESCRIPTION SCORE/POINTS
 NAME PERIOD P.O.T. GUIDESHEET UNIT 2. - WORK SUBUNIT - WORK IN MECHANICAL SYSTEMS ACTIVITY LESSON DESCRIPTION SCORE/POINTS 1. NT NOTES & STUDY QUESTIONS /20 2. WS PREVIOUS UNITS REVIEW /28 3. TX PP 84-89
NAME PERIOD P.O.T. GUIDESHEET UNIT 2. - WORK SUBUNIT - WORK IN MECHANICAL SYSTEMS ACTIVITY LESSON DESCRIPTION SCORE/POINTS 1. NT NOTES & STUDY QUESTIONS /20 2. WS PREVIOUS UNITS REVIEW /28 3. TX PP 84-89
Pre Comp Review Questions 8 th Grade Answers
 Pre Comp Review Questions 8 th Grade Answers Section 1 Units 1. Fill in the missing SI and English Units Measurement SI Unit SI Symbol English Unit English Symbol Time second s second s. Temperature Kelvin
Pre Comp Review Questions 8 th Grade Answers Section 1 Units 1. Fill in the missing SI and English Units Measurement SI Unit SI Symbol English Unit English Symbol Time second s second s. Temperature Kelvin
Energy Conversions. Energy. the ability to do work or produce heat. energy energy due to composition or position of an object
 Energy Energy the ability to do work or produce heat energy energy due to composition or position of an object energy the energy of motion Energy - SI unit for energy 1 J = 1 Kgm 2 / s 2 Energy Conversions
Energy Energy the ability to do work or produce heat energy energy due to composition or position of an object energy the energy of motion Energy - SI unit for energy 1 J = 1 Kgm 2 / s 2 Energy Conversions
Thermochemistry. Energy (and Thermochemistry) World of Chemistry Chapter 10. Energy. Energy
 Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Thermochemistry Thermodynamics is the science of the relationship between heat and other forms of energy. (and Thermochemistry) World of Chemistry Chapter 10 is defined as the ability to do work or produce
Energy Background Energy Forms and Transformations Integrated Science 4 Honors Name: Per:
 Energy Background Energy Forms and Transformations Integrated Science 4 Honors Name: Per: Humans use energy for a variety of purposes, some that are necessary and some that are not. To address the questions
Energy Background Energy Forms and Transformations Integrated Science 4 Honors Name: Per: Humans use energy for a variety of purposes, some that are necessary and some that are not. To address the questions
WORK, POWER, & ENERGY
 WORK, POWER, & ENERGY In physics, work is done when a force acting on an object causes it to move a distance. There are several good examples of work which can be observed everyday - a person pushing a
WORK, POWER, & ENERGY In physics, work is done when a force acting on an object causes it to move a distance. There are several good examples of work which can be observed everyday - a person pushing a
1 centimeter (cm) 5 10 millimeters (mm) 1 meter (m) centimeters. 1 kilometer (km) 5 1,000 meters. Set up equivalent ratios and cross multiply.
 Domain 2 Lesson 16 Convert Measurements Common Core State Standard: 6.RP.3.d Getting the Idea The tables below show some conversions for units of length in both the customary system and the metric system.
Domain 2 Lesson 16 Convert Measurements Common Core State Standard: 6.RP.3.d Getting the Idea The tables below show some conversions for units of length in both the customary system and the metric system.
Momentum, Impulse, Work, Energy, Power, and Conservation Laws
 Momentum, Impulse, Work, Energy, Power, and Conservation Laws 1. Cart A has a mass of 2 kilograms and a speed of 3 meters per second. Cart B has a mass of 3 kilograms and a speed of 2 meters per second.
Momentum, Impulse, Work, Energy, Power, and Conservation Laws 1. Cart A has a mass of 2 kilograms and a speed of 3 meters per second. Cart B has a mass of 3 kilograms and a speed of 2 meters per second.
Applications of Integration to Physics and Engineering
 Applications of Integration to Physics and Engineering MATH 211, Calculus II J Robert Buchanan Department of Mathematics Spring 2018 Mass and Weight mass: quantity of matter (units: kg or g (metric) or
Applications of Integration to Physics and Engineering MATH 211, Calculus II J Robert Buchanan Department of Mathematics Spring 2018 Mass and Weight mass: quantity of matter (units: kg or g (metric) or
4.1 Energy Energy changes in a system, and the ways energy is stored before and after such changes Energy stores and systems.
 4.1 Energy The concept of energy emerged in the 19th century. The idea was used to explain the work output of steam engines and then generalised to understand other heat engines. It also became a key tool
4.1 Energy The concept of energy emerged in the 19th century. The idea was used to explain the work output of steam engines and then generalised to understand other heat engines. It also became a key tool
Chapter 6: Applications of Integration
 Chapter 6: Applications of Integration Section 6.4 Work Definition of Work Situation There is an object whose motion is restricted to a straight line (1-dimensional motion) There is a force applied to
Chapter 6: Applications of Integration Section 6.4 Work Definition of Work Situation There is an object whose motion is restricted to a straight line (1-dimensional motion) There is a force applied to
CHEM What is Energy? Terminology: E = KE + PE. Thermodynamics. Thermodynamics
 Thermodynamics 2 Thermodynamics The study of energy changes accompanying physical and chemical processes. From the laws of thermodynamics, one can: 1. Predict the results of chemical reactions 2. Ascertain
Thermodynamics 2 Thermodynamics The study of energy changes accompanying physical and chemical processes. From the laws of thermodynamics, one can: 1. Predict the results of chemical reactions 2. Ascertain
WORK, POWER, & ENERGY
 WORK, POWER, & ENERGY In physics, work is done when a force acting on an object causes it to move a distance. There are several good examples of work which can be observed everyday - a person pushing a
WORK, POWER, & ENERGY In physics, work is done when a force acting on an object causes it to move a distance. There are several good examples of work which can be observed everyday - a person pushing a
Applied Fluid Mechanics
 Applied Fluid Mechanics 1. The Nature of Fluid and the Study of Fluid Mechanics 2. Viscosity of Fluid 3. Pressure Measurement 4. Forces Due to Static Fluid 5. Buoyancy and Stability 6. Flow of Fluid and
Applied Fluid Mechanics 1. The Nature of Fluid and the Study of Fluid Mechanics 2. Viscosity of Fluid 3. Pressure Measurement 4. Forces Due to Static Fluid 5. Buoyancy and Stability 6. Flow of Fluid and
F=ma. Exam 1. Today. Announcements: The average on the first exam was 31/40 Exam extra credit is due by 8:00 am Friday February 20th.
 Today Exam 1 Announcements: The average on the first exam was 31/40 Exam extra credit is due by 8:00 am Friday February 0th. F=ma Electric Force Work, Energy and Power Number 60 50 40 30 0 10 0 17 18 0
Today Exam 1 Announcements: The average on the first exam was 31/40 Exam extra credit is due by 8:00 am Friday February 0th. F=ma Electric Force Work, Energy and Power Number 60 50 40 30 0 10 0 17 18 0
the energy of motion!
 What are the molecules of matter doing all the time?! Heat and Temperature! Notes! All matter is composed of continually jiggling atoms or molecules! The jiggling is! If something is vibrating, what kind
What are the molecules of matter doing all the time?! Heat and Temperature! Notes! All matter is composed of continually jiggling atoms or molecules! The jiggling is! If something is vibrating, what kind
Unit 2: Energy THERMAL ENERGY HEAT TRANSFER POTENTIAL VS. KINETIC ENERGY WORK POWER SIMPLE MACHINES
 Unit 2: Energy THERMAL ENERGY HEAT TRANSFER POTENTIAL VS. KINETIC ENERGY WORK POWER SIMPLE MACHINES Bellringer Day 01 1. What is energy? 2. There are different forms of energy. Name two. What is Energy?
Unit 2: Energy THERMAL ENERGY HEAT TRANSFER POTENTIAL VS. KINETIC ENERGY WORK POWER SIMPLE MACHINES Bellringer Day 01 1. What is energy? 2. There are different forms of energy. Name two. What is Energy?
The number of stars in a galaxy is an example of an estimate that should be expressed in scientific notation.
 3.1 Using and Expressing Measurements A measurement is a quantity that has both a number and a unit. Using and Expressing Measurements In scientific notation, a given number is written as the product of
3.1 Using and Expressing Measurements A measurement is a quantity that has both a number and a unit. Using and Expressing Measurements In scientific notation, a given number is written as the product of
Chemistry in Our Lives. Chemistry and Chemicals
 Chemistry in Our Lives Chemistry and Chemicals What is chemistry? Chemistry is the study of substances in terms of Composition Structure Properties Reactions What a material it made of How the elementary
Chemistry in Our Lives Chemistry and Chemicals What is chemistry? Chemistry is the study of substances in terms of Composition Structure Properties Reactions What a material it made of How the elementary
LINEAR KINETICS (PART 2): WORK, ENERGY, AND POWER Readings: McGinnis Chapter 4
 LINEAR KINETICS (PART 2): WORK, ENERGY, AND POWER Readings: McGinnis Chapter 4 1 WORK: Another way of expressing the effect of a force. Mechanically, work is done on an object when a force causes a change
LINEAR KINETICS (PART 2): WORK, ENERGY, AND POWER Readings: McGinnis Chapter 4 1 WORK: Another way of expressing the effect of a force. Mechanically, work is done on an object when a force causes a change
WELCOME TO 1103 PERIOD 6
 WELCOE TO 1103 PERIOD 6 Homework Exercise #5 is due today. Please watch video 2, America Revealed: Electric Nation, for class discussion one week from today. PHYSICS 1103 PERIOD 6 Where is the center of
WELCOE TO 1103 PERIOD 6 Homework Exercise #5 is due today. Please watch video 2, America Revealed: Electric Nation, for class discussion one week from today. PHYSICS 1103 PERIOD 6 Where is the center of
GPE = m g h. GPE = w h. k = f d. PE elastic = ½ k d 2. Work = Force x distance. KE = ½ m v 2
 1 NAME PERIOD PHYSICS GUIDESHEET ENERGY CONVERSIONS POTENTIAL AND KINETIC ENERGY ACTIVITY LESSON DESCRIPTION SCORE/POINTS 1. NT CLASS OVERHEAD NOTES (5 pts/page) (Plus 5 pts/page for sample questions)
1 NAME PERIOD PHYSICS GUIDESHEET ENERGY CONVERSIONS POTENTIAL AND KINETIC ENERGY ACTIVITY LESSON DESCRIPTION SCORE/POINTS 1. NT CLASS OVERHEAD NOTES (5 pts/page) (Plus 5 pts/page for sample questions)
Thermochemistry. The study of energy changes that occur during chemical reactions and changes in state.
 Energy Thermochemistry The study of energy changes that occur during chemical reactions and changes in state. The Nature of Energy Energy - the ability to do work or produce heat Energy is stored in the
Energy Thermochemistry The study of energy changes that occur during chemical reactions and changes in state. The Nature of Energy Energy - the ability to do work or produce heat Energy is stored in the
The Kinetic Theory of Matter. Temperature. Temperature. Temperature. Temperature. Chapter 6 HEAT
 The Kinetic Theory of Matter Hewitt/Lyons/Suchocki/Yeh Conceptual Integrated Science Chapter 6 HEAT Kinetic Theory of Matter: Matter is made up of tiny particles (atoms or molecules) that are always in
The Kinetic Theory of Matter Hewitt/Lyons/Suchocki/Yeh Conceptual Integrated Science Chapter 6 HEAT Kinetic Theory of Matter: Matter is made up of tiny particles (atoms or molecules) that are always in
Tech Tip. Consistent Engineering Units In Finite Element Analysis
 Tech Tip Consistent Engineering Units In Finite Element Analysis Consistent Engineering Units In Finite Element Analysis Depending on the modelling software you use for your Finite Element Analysis (FEA),
Tech Tip Consistent Engineering Units In Finite Element Analysis Consistent Engineering Units In Finite Element Analysis Depending on the modelling software you use for your Finite Element Analysis (FEA),
- The empirical gas laws (including the ideal gas equation) do not always apply.
 145 At 300 C, ammonium nitrate violently decomposes to produce nitrogen gas, oxygen gas, and water vapor. What is the total volume of gas that would be produced at 1.00 atm by the decomposition of 15.0
145 At 300 C, ammonium nitrate violently decomposes to produce nitrogen gas, oxygen gas, and water vapor. What is the total volume of gas that would be produced at 1.00 atm by the decomposition of 15.0
Introduction to Mechanical Engineering Measurements Two Main Purposes of Measurements Engineering experimentation Operational systems
 Introduction, Page 1 Introduction to Mechanical Engineering Measurements Author: John M. Cimbala, Penn State University Latest revision, 19 August 011 Two Main Purposes of Measurements Engineering experimentation
Introduction, Page 1 Introduction to Mechanical Engineering Measurements Author: John M. Cimbala, Penn State University Latest revision, 19 August 011 Two Main Purposes of Measurements Engineering experimentation
Text book. Tenth edition. Walker, Halliday and Resnick. Principles of physics.
 Text book Principles of physics. Tenth edition Walker, Halliday and Resnick Chapter 1 Measurement In this chapter we will explore the following concepts: 1. Measurement of a physical parameter 2. Units,
Text book Principles of physics. Tenth edition Walker, Halliday and Resnick Chapter 1 Measurement In this chapter we will explore the following concepts: 1. Measurement of a physical parameter 2. Units,
