HEAT- I Part - A C D A B. Te m p. Heat input
|
|
|
- Cori Goodman
- 5 years ago
- Views:
Transcription
1 e m p HE- I Part -. solid material is supplied with heat at a constant rate. he temperature of the material is changing with heat input as shown in the graph. Study the graph carefully and answer the following questions ; (a) What do the horizontal regions and represent? (b) if =, what do you infer? (c) What does the slope E represent? (d) he slope of O > slope of. What does this indicate? O E Heat input. steel scale is to be prepared such that the millimeter intervals are to be accurate within mm at a certain temperature. etermine the maximum permissible variation during the ruling of the millimeter marks α = / 0 3. vaccum pump is connected to an insulating flask, containing 50 gm of water at 0 o. s the water vapour is being pumped out, some water evaporates and some water solidifies. If the latent of water of vapourisation at 0 o is. 0 6 J/kg and the latent heat of solidification of water is J/kg.calculate the maximum mass of ice that can be formed by this process. 4. he capacity of air conditioners is sometimes expressed in Watts or tons, the latter being the number of tons of ice that can be frozen from water at 0 o in 4 hrs by the unit. Express the capacity of oneton air conditioner in Watts. 5. lock supports an iron pendulum and another clock supports a brass pendulum. oth the clocks are accurate at 0 o. What will be difference in their readings after one day if the temperature is 40 o? α (steel ) =. 0 5 / 0 and α (brass) = 0 5 / 0 6. wo walls I and II of the same thickness are made of heterogeneous metals, as shown in fig. In what case will the coefficient of thermal conducitivity will be greater? 7. small blackened solid copper sphere of radius m is placed in an evacuated enclosure whose walls are kept at t what rate must energy be supplied to be sphere to keep its temperature constant at 7 0? σ = in S.I. units. nswers : Part -. (a) - melting, - vaporisation (b) L vap. = L fusion (c) slope α specific heat (d) hermal capacity in liquid state > hermal capacity in solid state o /7 gm 4. 35/9 KW sec W 8
2 Part -. open vessel contains 500 gm of ice at - 0 o. he mass of the container can be neglected. Heat is supplied to the vessel at the constant rate of 00 cal/ min for 00 minutes. Plot a curve showing the elapsed time as abscissa and the temperature as ordinate.. copper calorimeter of mass 00 gm contains 50 gm of water and 8 gm of ice in thermal equilibrium at atmospheric pressure. 00 gm of lead at a temperature of 00 o are dropped in the calorimeter. Find the final temperature if no heat is lost to the surrounding. c lead = 0.3 J/gm/ o, c u = 0.39 J/gm/ o. 3. t a temperature of 0 o, the volume of a certain glass flask, upto a reference mark on the stem of the flask, is exactly 00 cm 3. he flask is filled to this point with a liquid whose coefficient of volume expansion is. 0 3 / o, with both flask and liquid at 0 o. he cross-section of the stem is mm and can be considered constant. How far will the liquid rise or fall in the stem if the temperature is raised to 40 o? he coefficient of linear expansion of glass is / o. 4. (a) n aluminum vessel of volume 300 cc is completely filled with glycerine at 0 o. Find the mass of glycerine that will overflow when both are heated to 00 o, ρ (glycerine) at 0 o =.69 g/cc, γ (glycerine) = / o and α (l) = / o (b) glass vessel is partially filled with mercury and when both are heated together, the volume of the unfilled part of the vessel remains constant at all temperatures. Find the initial volume of mercury if the volume of glass vessel is 50 cm 3. α (glass) is / o, α (Hg) = / o. 5. glass flask contains 330 gm of mercury at 0 o, and 35 gm at 00 o. Find the coefficient of linear expansion for flask if the coefficient of cubic expansion of mercury is / o. 6. hree rods of material X and three rods of material Y are connected as shown in fig. ll the rods are of identical length and cross sectional area. If the end is maintained at 60 o, and the junction E at 0 o, calculate the temperatures of the junctions,,. he thermal conductivity of the rod X is 0.9 cal/ o /s/cm and that of Y is 0.46 cal/ o /s/ cm. 7. rod of length l with thermally insulated lateral surface consists of material whose heat conductivity coefficient varies with temperature as K = α/, where α is a constant. he ends of the rod are kept at temperatures and. Find the function (x), where x is the distance from the end whose temperature is, and the heat flow density. 8. wo chunks of metal with heat capacities and are interconneted by a rod of length l and crosssectional area S and fairly low heat conductivity k. he whole system is thermally insulated from the environment. t a moment t = 0, the temperature difference between the two chunks of metal equals ( ) 0. ssuming the heat capacity of the rod to be negligible, find the temperature difference between the chunks as a function of time. 9. constant electric current flows along a uniform wire with cross-sectional radius R and heat conductivity coefficient k. unit volume of the wire generates a thermal power ω. Find the temperature distribution across the wire provided the steady-state temperature at the wire surface is equal to 0. 8
3 0. n isosceles triangle is formed with a thin rod of length l and coefficient of linear expansion α as the base and two thin rods each of length l and coefficient of linear expansion α as the two sides. If the distance between the apex and the mid-point of the base remain unchanged as the temperature is varied show that l l = α α. n aluminum plate is fixed in a horizontal position has a hole of diameter d a = cm. steel sphere of diameter d s =.005 cm rests on this hole. ll the lengths refer to temperature = 0 o. he temperature of the entire system is slowly increased. t what temperature the steel plate cover the hole? α a = / o and α s = 0 6 / o. Petroleum is stored in a cylindrical tank of height h = 0 m. t a temperature t 0 = 0 o, the separation between the level of petroleum and the top of the tank is h = 50 cm. t what temperature t does petroleum overflows from the tank. he coefficient of cubic expansion of petroleum is γ = /K and the diameter of the steel tank is d = 6 m and its coefficient of linear expansion is α s =. 0 5 / o. 3. s a result of a temperature rise of 3 o, a bar with a crack at its centre buckles upward (fig). It the fixed distance L 0 = 3.77 m and the coefficient of linear expansion is / o, find x, the distance to which the centre rises. 4. rod is rotating about an axis passing through its one end and perpendicular to its length with a constant angular velocity ω. alculate the percentage change in the kinetic energy of the rod if the temperature of the rod is changed from 0 o to 50 o. α iron =. 0 5 / o. 5. he length of a copper rod is 00 cm when measured by a steel scale, both being at 0 o. What will be the reading of the scale when both are heated to 50 0? ssume that the scale is accurate at 0 o. α (steel ) =. 0 5 / o α (u) = / o 6. cylindrical metal can 0 cm high and 5 cm in diameter contains liquid helium at 4 K, at which temperature its heat of vepourisation is 0 J/gm. ompletely surrounding the helium can are walls maintained at a temperature of liquid nitrogen, 80 K, the intervening space being evacuated. How much helium is lost per hour? ssuming the radiant emittance of the helium can to be 0. that of a black body at 4 K. ssume only curved surface area of the cylinder. nswers : Part -. 0 o with 0. g ice 3. Raise.38 m g,.4cc / o o, 0 o, 0 o 7. x / l ( x) =, a l q= ln 8. (?) 0 e at ;a = 9. = o + (R -r ) ω/4k. = 8 o. t = 44. o cm + Sk l % cm 6..3 gm/hr 83
4 HE- II Part -. n ideal gas ( γ =.5) has a volume of 0 lt at a pressure of atm and temperature 300 K. It is adiabatically expanded to twice its original volume. alculate (a) no. of moles of the gas (b) the final temperature (c)the work done by the gas.. cylinder contains mole of oxygen at a temperature of 7 o. he cylinder is provided with a frictionless piston which maintains a constant pressure of atm on the gas. he gas is heated until its temperature rises to 7 o. (a) raw a diagram representing the process in the PV plane. (b) How much work is done by the gas? (c) On what is this work done? (d) How much heat was supplied to the gas? (e) What is the change in internal energy of the gas? (f) How much work should have been done if the pressure had been 0.5 atm? 3..0 moles of helium are initially at a temperature of 7 o and occupy a volume of 0 lt. he helium is first expanded at constant pressure until the volume is doubled, and then adiabatically until the temperature returns to its initial value. (a) raw a PV diagram (b) ompute the total heat supplied in the process (c) alculate the change in internal energy of the system. (d) What is the total work done by the gas? (e) What is the final volume? 4. n ideal gas having initial pressure P, volume V and temperature is allowed to expand adiabatically until its volume becomes 5.66 V while its temperature falls to /. (a) How many degrees of freedom do the gas molecules have? (b) Obtain the work done by the gas during theexpansion as a function ofthe initial pressure P and volume V? 5. wo perfect gases at absolute temperatures and are mixed. here is no loss of energy. Find the temperature of the mixture if masses of molecules are m and m and the number of molecules in the gases are n and n respectively. 6. What amount of heat is to be transferred to nitrogen in the isobaric heating process for that gas to perform the work.0 J? 7. wo moles of helium undergo a cyclic process as shown in figure. ssuming the gas to be ideal, calculate the following quantities in this process. (a) he net change in the heat energy. (b) he net work done. (c)he net change in the internal energy. (d) raw the P-V diagram. P atm atm 300 K 400 K 84
5 8. 3 moles of an ideal gas ( p = 7/ R) at pressure P and temperature is isothermally expanded to twice its initial volume. It is then compressed at constant pressure to its original volume. Finally gas is compressed at constant volume to its original pressure P. (a) Sketch the P-V, P - and V - diagrams for the complete process. (b) alculate the net work done by the gas, and net heat supplied to the gas during the complete process. Part -. One mole of a monoatomic ideal gas is taken through the cycle shown : P : adiabatic expansion : cooling at constant volume : adiabatic compression : heating at constant volume he pressure and temperature at, etc. are denoted by P,, P, V etc. respectively. Given that = 000 K, P = (/3) P and P c = (/3) P. alculate the following quantities. (a) he work done by the gas in the process (b) he heat lost by the gas in the process (c) he temperature. (Given : (/3) /5 = 0.85).. n ideal gas is taken through a cyclic thermodynamic process through four steps. he amount of heat involved in these steps are Q = 5960 J. Q = 5585 J, Q 3 = 980 J, Q 4 = 3645 J, W = 00 J, W = 85 J, W 3 = 00 J, and W 4 respectively. (a)find the value of W 4. (b) What is the efficiency of the cycle? 3. horizontal insulated cylinder is provided with frictionless, non-conducting piston. On each side the piston is 50 l t of air at a pressure of atm and 73 K. Heat is slowly suppied to the air at the left hand side, until the piston has compressed the air on the right hand side of.5 atm. Find (a) the final temperature of the air on the right hand side ; (b) work done by the airon the right hand side ; (c) the final temperature of air ; (d) heat added to the air on the left hand side. 4. smooth vertical tube having two different sections is open from both ends and equipped with two pistons of different areas. Each piston slides within a respective tube section. mole of ideal gas is inclosed between the pistons tied with a nonstretchable thread. he cross-sectional area of the upper piston is S = 0 cm greater than that of the lower one. he combined mass of the two pistons is equal to m = 5.0 Kg.he outside air pressure is p 0 =.0 atm. y how many Kelvins must the gas between the pistons be heated so as to move the pistons through = 5.0 cm? P 0 P 0 5. Find the molar heat capacity of an ideal gas in a polytropic process Pv n = const. he adiabatic exponent of the gas is equal to γ. t what values of the polytropic constant n will the heat capacity of the gas be negative? 6. One mole of a certain ideal gas is contained under a weightless piston of a vertical cylinder at a temperature. he space over the piston opens into the atmosphere. What work has to be performed in order to increase isothermally the gas volume under the piston n times by slowly raising the piston? he friction of the piston against the cylinder walls is negligibly small. 85
6 7. he volume of one mole of an ideal gas with adiabatic exponent is varied according to the relation V = a/, where a is a contant. Find the amount of heat obtained by the gas in this process if the gas temperature is increased by. 8. gaseous mixture enclosed in a vessel of volume V. consists of one mole of a gas with γ = 5/3 and another gas with γ= 7/5 at a certain temperature. he relative molar masses of the gases and are 4 and 3 respectively. he gases and do not react with each other and are assumed to be ideal. he gaseous mixture follows the equation : PV 9/3 = constant, in adiabatic processes. (a)find the number of moles of the gas in the gaseous mixture. (b)ompute the speed of sound in the gaseous mixture at = 300 K (c) If is raised by K from 300 K. find the percentage change in the speed of sound in the gaseous mixture. (d) he mixture is compressed adiabatically to /5 of its initial volume V. Find the change in its adiabatic compressibility in terms of the given quantitities. 9. sample of kg of monoatomic Helium (assumed ideal) is taken through the process and another sample of kg of the same gas is taken through the process (see Figure). Given relative molecular mass of Helium = 4. (i) What is the temp.of Helium in each of the stages,, and? (ii) Is there any way of telling afterwards which sample of Helium went 0 4 N/m 0 through the process and which went through the process. Write Yes or No. (iii) How much is the heat involved in each of the processes and? V m 3 0. One mole of a diatomic ideal gas ( γ =.4) is taken through a cyclic process starting from point. he process is an adiabatic compression, is isobaric expansion. is an adiabatic expansion, and is isochoric. he volume ratios are V / V = 6 and V / V = and the temperature at is = 300 K. alculate the temperature of the gas at the points and and find the efficiency of the cycle. nswers Part -. (a) 0.4 (b). K (c) 583 J. (b) 83 J (c) Piston (d) 909 J (e) J (f) Same 3. (b).47 KJ (c) Zero (d).47 KJ (e) 3.4 lt 7. (a) 50.8 J (b) 50.8 J (c) zero 4. (a) 5 (b) PV w = γ ( ( 5.66) ) γ 5. n n + n + n 6. 7 J 8. R( log 8 -.5) Part J J., 500 K J, 0.08 J K, J., 00 K J; γ = K. 5. [R(n - )]/[(n - )(r - )]. Negative for < n < r. 6. R(n - - ln n) 7. R ( -γ )/(γ -) 8. (a) n = mol,(b) 40 ms -. (c) 0.67 (d) ( Pa - )(Km -3.)(V/) 9. (i) 0.8 K, K, 48. K, K, (ii) No. (iii) J, J K, 79.4 K.,
PHYS102 Previous Exam Problems. Temperature, Heat & The First Law of Thermodynamics
 PHYS102 Previous Exam Problems CHAPTER 18 Temperature, Heat & The First Law of Thermodynamics Equilibrium & temperature scales Thermal expansion Exchange of heat First law of thermodynamics Heat conduction
PHYS102 Previous Exam Problems CHAPTER 18 Temperature, Heat & The First Law of Thermodynamics Equilibrium & temperature scales Thermal expansion Exchange of heat First law of thermodynamics Heat conduction
S6. (a) State what is meant by an ideal gas...
 IB PHYSICS Name: DEVIL PHYSICS Period: Date: BADDEST CLASS ON CAMPUS TSOKOS CHAPTER 3 TEST REVIEW S1. Thermal energy is transferred through the glass windows of a house mainly by A. conduction. B. radiation.
IB PHYSICS Name: DEVIL PHYSICS Period: Date: BADDEST CLASS ON CAMPUS TSOKOS CHAPTER 3 TEST REVIEW S1. Thermal energy is transferred through the glass windows of a house mainly by A. conduction. B. radiation.
TEST-11(Solution) TOPIC : HEAT COMPLETE 2400 = m 80 [ Q = ml]
![TEST-11(Solution) TOPIC : HEAT COMPLETE 2400 = m 80 [ Q = ml] TEST-11(Solution) TOPIC : HEAT COMPLETE 2400 = m 80 [ Q = ml]](/thumbs/95/124340082.jpg) entigrade emperature (º) emperature () ES-(Solution) OI : HE OMLEE 400 = m 80 [Q = ml] Q. If specific heat of a substance is infinite, it means- () Heat is given out () Heat is taken in () No change in
entigrade emperature (º) emperature () ES-(Solution) OI : HE OMLEE 400 = m 80 [Q = ml] Q. If specific heat of a substance is infinite, it means- () Heat is given out () Heat is taken in () No change in
Process Nature of Process
 AP Physics Free Response Practice Thermodynamics 1983B. The pv-diagram above represents the states of an ideal gas during one cycle of operation of a reversible heat engine. The cycle consists of the following
AP Physics Free Response Practice Thermodynamics 1983B. The pv-diagram above represents the states of an ideal gas during one cycle of operation of a reversible heat engine. The cycle consists of the following
A thermodynamic system is taken from an initial state X along the path XYZX as shown in the PV-diagram.
 AP Physics Multiple Choice Practice Thermodynamics 1. The maximum efficiency of a heat engine that operates between temperatures of 1500 K in the firing chamber and 600 K in the exhaust chamber is most
AP Physics Multiple Choice Practice Thermodynamics 1. The maximum efficiency of a heat engine that operates between temperatures of 1500 K in the firing chamber and 600 K in the exhaust chamber is most
KINETIC THEORY. was the original mean square velocity of the gas. (d) will be different on the top wall and bottom wall of the vessel.
 Chapter Thirteen KINETIC THEORY MCQ I 13.1 A cubic vessel (with faces horizontal + vertical) contains an ideal gas at NTP. The vessel is being carried by a rocket which is moving at a speed of 500m s 1
Chapter Thirteen KINETIC THEORY MCQ I 13.1 A cubic vessel (with faces horizontal + vertical) contains an ideal gas at NTP. The vessel is being carried by a rocket which is moving at a speed of 500m s 1
Physics 5D PRACTICE FINAL EXAM Fall 2013
 Print your name: Physics 5D PRACTICE FINAL EXAM Fall 2013 Real Exam is Wednesday December 11 Thimann Lecture 3 4:00-7:00 pm Closed book exam two 8.5x11 sheets of notes ok Note: Avogadro s number N A =
Print your name: Physics 5D PRACTICE FINAL EXAM Fall 2013 Real Exam is Wednesday December 11 Thimann Lecture 3 4:00-7:00 pm Closed book exam two 8.5x11 sheets of notes ok Note: Avogadro s number N A =
Chapter 10, Thermal Physics
 CHAPTER 10 1. If it is given that 546 K equals 273 C, then it follows that 400 K equals: a. 127 C b. 150 C c. 473 C d. 1 200 C 2. A steel wire, 150 m long at 10 C, has a coefficient of linear expansion
CHAPTER 10 1. If it is given that 546 K equals 273 C, then it follows that 400 K equals: a. 127 C b. 150 C c. 473 C d. 1 200 C 2. A steel wire, 150 m long at 10 C, has a coefficient of linear expansion
UNIVERSITY COLLEGE LONDON. University of London EXAMINATION FOR INTERNAL STUDENTS. For The Following Qualifications:-
 UNIVERSITY COLLEGE LONDON University of London EXAMINATION FOR INTERNAL STUDENTS For The Following Qualifications:- B.Sc. M.Sci. Physics 1B28: Thermal Physics COURSE CODE : PHYSIB28 UNIT VALUE : 0.50 DATE
UNIVERSITY COLLEGE LONDON University of London EXAMINATION FOR INTERNAL STUDENTS For The Following Qualifications:- B.Sc. M.Sci. Physics 1B28: Thermal Physics COURSE CODE : PHYSIB28 UNIT VALUE : 0.50 DATE
Phase Changes and Latent Heat
 Review Questions Why can a person remove a piece of dry aluminum foil from a hot oven with bare fingers without getting burned, yet will be burned doing so if the foil is wet. Equal quantities of alcohol
Review Questions Why can a person remove a piece of dry aluminum foil from a hot oven with bare fingers without getting burned, yet will be burned doing so if the foil is wet. Equal quantities of alcohol
Module - 1: Thermodynamics
 Thermodynamics: Module - : Thermodynamics Thermodynamics (Greek: thermos = heat and dynamic = change) is the study of the conversion of energy between heat and other forms, mechanical in particular. All
Thermodynamics: Module - : Thermodynamics Thermodynamics (Greek: thermos = heat and dynamic = change) is the study of the conversion of energy between heat and other forms, mechanical in particular. All
The Kinetic Theory of Gases
 PHYS102 Previous Exam Problems CHAPTER 19 The Kinetic Theory of Gases Ideal gas RMS speed Internal energy Isothermal process Isobaric process Isochoric process Adiabatic process General process 1. Figure
PHYS102 Previous Exam Problems CHAPTER 19 The Kinetic Theory of Gases Ideal gas RMS speed Internal energy Isothermal process Isobaric process Isochoric process Adiabatic process General process 1. Figure
Downloaded from
 Chapter 13 (Kinetic Theory) Q1. A cubic vessel (with face horizontal + vertical) contains an ideal gas at NTP. The vessel is being carried by a rocket which is moving at a speed of500 ms in vertical direction.
Chapter 13 (Kinetic Theory) Q1. A cubic vessel (with face horizontal + vertical) contains an ideal gas at NTP. The vessel is being carried by a rocket which is moving at a speed of500 ms in vertical direction.
AP PHYSICS 2 WHS-CH-15 Thermodynamics Show all your work, equations used, and box in your answers!
 AP PHYSICS 2 WHS-CH-15 Thermodynamics Show all your work, equations used, and box in your answers! Nicolas Léonard Sadi Carnot (1796-1832) Sadi Carnot was a French military engineer and physicist, often
AP PHYSICS 2 WHS-CH-15 Thermodynamics Show all your work, equations used, and box in your answers! Nicolas Léonard Sadi Carnot (1796-1832) Sadi Carnot was a French military engineer and physicist, often
Questions Chapter 18 Temperature, Heat, and the First Law of Thermodynamics
 Questions Chapter 18 Temperature, Heat, and the First Law of Thermodynamics 18-1 What is Physics? 18-2 Temperature 18-3 The Zeroth Law of Thermodynamics 18-4 Measuring Temperature 18-5 The Celsius and
Questions Chapter 18 Temperature, Heat, and the First Law of Thermodynamics 18-1 What is Physics? 18-2 Temperature 18-3 The Zeroth Law of Thermodynamics 18-4 Measuring Temperature 18-5 The Celsius and
Entropy & the Second Law of Thermodynamics
 PHYS102 Previous Exam Problems CHAPTER 20 Entropy & the Second Law of Thermodynamics Entropy gases Entropy solids & liquids Heat engines Refrigerators Second law of thermodynamics 1. The efficiency of
PHYS102 Previous Exam Problems CHAPTER 20 Entropy & the Second Law of Thermodynamics Entropy gases Entropy solids & liquids Heat engines Refrigerators Second law of thermodynamics 1. The efficiency of
CHAPTER-11 NCERT SOLUTIONS
 CHAPTER-11 NCERT SOLUTIONS Question 11.1: The triple points of neon and carbon dioxide are 24.57 K and 216.55 K respectively. Express these temperatures on the Celsius and Fahrenheit scales. Kelvin and
CHAPTER-11 NCERT SOLUTIONS Question 11.1: The triple points of neon and carbon dioxide are 24.57 K and 216.55 K respectively. Express these temperatures on the Celsius and Fahrenheit scales. Kelvin and
1985B4. A kilogram sample of a material is initially a solid at a temperature of 20 C. Heat is added to the sample at a constant rate of 100
 1985B4. A 0.020-kilogram sample of a material is initially a solid at a temperature of 20 C. Heat is added to the sample at a constant rate of 100 joules per second until the temperature increases to 60
1985B4. A 0.020-kilogram sample of a material is initially a solid at a temperature of 20 C. Heat is added to the sample at a constant rate of 100 joules per second until the temperature increases to 60
HEAT AND THERMODYNAMICS
 PHT HEAT AND THERMODYNAMICS C C Zeroth law of thermodynamics This law defines the concept of temperature and thermal equilibrium. When two bodies are in thermal equilibrium, their temperature are equal
PHT HEAT AND THERMODYNAMICS C C Zeroth law of thermodynamics This law defines the concept of temperature and thermal equilibrium. When two bodies are in thermal equilibrium, their temperature are equal
Kinetic Theory continued
 Chapter 12 Kinetic Theory continued 12.4 Kinetic Theory of Gases The particles are in constant, random motion, colliding with each other and with the walls of the container. Each collision changes the
Chapter 12 Kinetic Theory continued 12.4 Kinetic Theory of Gases The particles are in constant, random motion, colliding with each other and with the walls of the container. Each collision changes the
Topic 3 &10 Review Thermodynamics
 Name: Date: Topic 3 &10 Review Thermodynamics 1. The kelvin temperature of an object is a measure of A. the total energy of the molecules of the object. B. the total kinetic energy of the molecules of
Name: Date: Topic 3 &10 Review Thermodynamics 1. The kelvin temperature of an object is a measure of A. the total energy of the molecules of the object. B. the total kinetic energy of the molecules of
Answer: The relation between kelvin scale and Celsius scale is TK =TC => TC=TK
 Question The triple points of neon and carbon dioxide are 24.57 K and 216.55 K respectively. Express these temperatures on the Celsius and Fahrenheit scales. Answer: The relation between kelvin scale and
Question The triple points of neon and carbon dioxide are 24.57 K and 216.55 K respectively. Express these temperatures on the Celsius and Fahrenheit scales. Answer: The relation between kelvin scale and
Kinetic Theory continued
 Chapter 12 Kinetic Theory continued 12.4 Kinetic Theory of Gases The particles are in constant, random motion, colliding with each other and with the walls of the container. Each collision changes the
Chapter 12 Kinetic Theory continued 12.4 Kinetic Theory of Gases The particles are in constant, random motion, colliding with each other and with the walls of the container. Each collision changes the
UNIVESITY OF SWAZILAND FACl.JLTY OF SCIENCE AND ENGINEERING DEPARTMENT OF PHYSICS
 UNIVESITY OF SWAZILAND FACl.LTY OF SCIENCE AND ENGINEERING DEPARTMENT OF PHYSICS Main Examination 2016/2017. COURSE NAME: Thermodynamics/Thermofluids COURSE CODE: PHY242/EEE202 TIME ALLOWED: 3 hours ANSWER
UNIVESITY OF SWAZILAND FACl.LTY OF SCIENCE AND ENGINEERING DEPARTMENT OF PHYSICS Main Examination 2016/2017. COURSE NAME: Thermodynamics/Thermofluids COURSE CODE: PHY242/EEE202 TIME ALLOWED: 3 hours ANSWER
 Question 11.1: The triple points of neon and carbon dioxide are 24.57 K and 216.55 K respectively. Express these temperatures on the Celsius and Fahrenheit scales. Kelvin and Celsius scales are related
Question 11.1: The triple points of neon and carbon dioxide are 24.57 K and 216.55 K respectively. Express these temperatures on the Celsius and Fahrenheit scales. Kelvin and Celsius scales are related
MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question.
 CH. 19 PRACTICE Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) When a fixed amount of ideal gas goes through an isobaric expansion, A) its
CH. 19 PRACTICE Name MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) When a fixed amount of ideal gas goes through an isobaric expansion, A) its
Dual Program Level 1 Physics Course
 Dual Program Level 1 Physics Course Assignment 15 Due: 11/Feb/2012 14:00 Assume that water has a constant specific heat capacity of 4190 J/kg K at all temperatures between its melting point and boiling
Dual Program Level 1 Physics Course Assignment 15 Due: 11/Feb/2012 14:00 Assume that water has a constant specific heat capacity of 4190 J/kg K at all temperatures between its melting point and boiling
(2) The volume of molecules is negligible in comparison to the volume of gas. (3) Molecules of a gas moves randomly in all direction.
 9.1 Kinetic Theory of Gases : Assumption (1) The molecules of a gas are identical, spherical and perfectly elastic point masses. (2) The volume of molecules is negligible in comparison to the volume of
9.1 Kinetic Theory of Gases : Assumption (1) The molecules of a gas are identical, spherical and perfectly elastic point masses. (2) The volume of molecules is negligible in comparison to the volume of
CHAPTER 3 TEST REVIEW
 IB PHYSICS Name: Period: Date: # Marks: 52 Raw Score: IB Curve: DEVIL PHYSICS BADDEST CLASS ON CAMPUS CHAPTER 3 TEST REVIEW 1. Water at a temperature of 0 C is kept in a thermally insulated container.
IB PHYSICS Name: Period: Date: # Marks: 52 Raw Score: IB Curve: DEVIL PHYSICS BADDEST CLASS ON CAMPUS CHAPTER 3 TEST REVIEW 1. Water at a temperature of 0 C is kept in a thermally insulated container.
Estimate, for this water, the specific heat capacity, specific heat capacity =... J kg 1 K 1. the specific latent heat of vaporisation.
 1 A kettle is rated as 2.3 kw. A mass of 750 g of water at 20 C is poured into the kettle. When the kettle is switched on, it takes 2.0 minutes for the water to start boiling. In a further 7.0 minutes,
1 A kettle is rated as 2.3 kw. A mass of 750 g of water at 20 C is poured into the kettle. When the kettle is switched on, it takes 2.0 minutes for the water to start boiling. In a further 7.0 minutes,
Chapter 17 Temperature and heat
 Chapter 17 Temperature and heat 1 Temperature and Thermal Equilibrium When we speak of objects being hot and cold, we need to quantify this by some scientific method that is quantifiable and reproducible.
Chapter 17 Temperature and heat 1 Temperature and Thermal Equilibrium When we speak of objects being hot and cold, we need to quantify this by some scientific method that is quantifiable and reproducible.
Speed Distribution at CONSTANT Temperature is given by the Maxwell Boltzmann Speed Distribution
 Temperature ~ Average KE of each particle Particles have different speeds Gas Particles are in constant RANDOM motion Average KE of each particle is: 3/2 kt Pressure is due to momentum transfer Speed Distribution
Temperature ~ Average KE of each particle Particles have different speeds Gas Particles are in constant RANDOM motion Average KE of each particle is: 3/2 kt Pressure is due to momentum transfer Speed Distribution
A) 120 degrees B) 90 degrees C) 60 degrees D) 45 degrees E) 30 degrees
 Phys10 - First Major 071 Zero Version Q1. Two identical sinusoidal traveling waves are sent along the same string in the same direction. What should be the phase difference between the two waves so that
Phys10 - First Major 071 Zero Version Q1. Two identical sinusoidal traveling waves are sent along the same string in the same direction. What should be the phase difference between the two waves so that
CHAPTER - 12 THERMODYNAMICS
 CHAPER - HERMODYNAMICS ONE MARK QUESIONS. What is hermodynamics?. Mention the Macroscopic variables to specify the thermodynamics. 3. How does thermodynamics differ from Mechanics? 4. What is thermodynamic
CHAPER - HERMODYNAMICS ONE MARK QUESIONS. What is hermodynamics?. Mention the Macroscopic variables to specify the thermodynamics. 3. How does thermodynamics differ from Mechanics? 4. What is thermodynamic
First Law of Thermodynamics Second Law of Thermodynamics Mechanical Equivalent of Heat Zeroth Law of Thermodynamics Thermal Expansion of Solids
 Slide 1 / 66 1 What is the name of the following statement: "When two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium with each other"? A B C D E First Law
Slide 1 / 66 1 What is the name of the following statement: "When two systems are in thermal equilibrium with a third system, then they are in thermal equilibrium with each other"? A B C D E First Law
The triple points of neon and carbon dioxide are K and K respectively. Express these temperatures on the Celsius and Fahrenheit scales.
 Question 11.1: The triple points of neon and carbon dioxide are 24.57 K and 216.55 K respectively. Express these temperatures on the Celsius and Fahrenheit scales. Kelvin and Celsius scales are related
Question 11.1: The triple points of neon and carbon dioxide are 24.57 K and 216.55 K respectively. Express these temperatures on the Celsius and Fahrenheit scales. Kelvin and Celsius scales are related
CHEM Thermodynamics. Work. There are two ways to change the internal energy of a system:
 There are two ways to change the internal energy of a system: Thermodynamics Work 1. By flow of heat, q Heat is the transfer of thermal energy between and the surroundings 2. By doing work, w Work can
There are two ways to change the internal energy of a system: Thermodynamics Work 1. By flow of heat, q Heat is the transfer of thermal energy between and the surroundings 2. By doing work, w Work can
Downloaded from
 Chapter 12 (Thermodynamics) Multiple Choice Questions Single Correct Answer Type Q1. An ideal gas undergoes four different processes from the same initial state (figure). Four processes are adiabatic,
Chapter 12 (Thermodynamics) Multiple Choice Questions Single Correct Answer Type Q1. An ideal gas undergoes four different processes from the same initial state (figure). Four processes are adiabatic,
Temperature Thermal Expansion Ideal Gas Law Kinetic Theory Heat Heat Transfer Phase Changes Specific Heat Calorimetry Heat Engines
 Temperature Thermal Expansion Ideal Gas Law Kinetic Theory Heat Heat Transfer Phase Changes Specific Heat Calorimetry Heat Engines Zeroeth Law Two systems individually in thermal equilibrium with a third
Temperature Thermal Expansion Ideal Gas Law Kinetic Theory Heat Heat Transfer Phase Changes Specific Heat Calorimetry Heat Engines Zeroeth Law Two systems individually in thermal equilibrium with a third
Compiled and rearranged by Sajit Chandra Shakya
 1 (a) (i) The kinetic theory of gases leads to the equation m = kt. (b) Explain the significance of the quantity m... the equation to suggest what is meant by the absolute zero of temperature...
1 (a) (i) The kinetic theory of gases leads to the equation m = kt. (b) Explain the significance of the quantity m... the equation to suggest what is meant by the absolute zero of temperature...
TB [103 marks] The damping of the system is now increased. Which describes the change in ƒ 0 and the change in A 0?
![TB [103 marks] The damping of the system is now increased. Which describes the change in ƒ 0 and the change in A 0? TB [103 marks] The damping of the system is now increased. Which describes the change in ƒ 0 and the change in A 0?](/thumbs/87/96952878.jpg) TB [103 marks] 1. A periodic driving force of frequency ƒ acts on a system which undergoes forced oscillations of amplitude A. The graph below shows the variation with ƒ of A. The maximum amplitude A 0
TB [103 marks] 1. A periodic driving force of frequency ƒ acts on a system which undergoes forced oscillations of amplitude A. The graph below shows the variation with ƒ of A. The maximum amplitude A 0
Thermal Conductivity, k
 Homework # 85 Specific Heats at 20 C and 1 atm (Constant Pressure) Substance Specific Heat, c Substance Specific Heat, c kcal/kg C J/kg C kcal/kg C J/kg C Solids Aluminum 0.22 900 Brass 0.090 377 Copper
Homework # 85 Specific Heats at 20 C and 1 atm (Constant Pressure) Substance Specific Heat, c Substance Specific Heat, c kcal/kg C J/kg C kcal/kg C J/kg C Solids Aluminum 0.22 900 Brass 0.090 377 Copper
THERMODYNAMICS. Chapter Twelve MCQ I
 Chapter welve HERMODYNAMICS MCQ I. An ideal gas undergoes four different processes from the same initial state (Fig..). Four processes are adiabatic, isothermal, isobaric and isochoric. Out of,, and 4
Chapter welve HERMODYNAMICS MCQ I. An ideal gas undergoes four different processes from the same initial state (Fig..). Four processes are adiabatic, isothermal, isobaric and isochoric. Out of,, and 4
Version 001 HW 15 Thermodynamics C&J sizemore (21301jtsizemore) 1
 Version 001 HW 15 Thermodynamics C&J sizemore 21301jtsizemore 1 This print-out should have 38 questions. Multiple-choice questions may continue on the next column or page find all choices before answering.
Version 001 HW 15 Thermodynamics C&J sizemore 21301jtsizemore 1 This print-out should have 38 questions. Multiple-choice questions may continue on the next column or page find all choices before answering.
S15--AP Phys Q4--Heat-Thermo Ch13_14_15 PRACTICE
 Name: Class: Date: S5--AP Phys Q4--Heat-Thermo Ch3_4_5 PRACTICE Multiple Choice Identify the choice that best completes the statement or answers the question.. Which of the following is a thermodynamic
Name: Class: Date: S5--AP Phys Q4--Heat-Thermo Ch3_4_5 PRACTICE Multiple Choice Identify the choice that best completes the statement or answers the question.. Which of the following is a thermodynamic
KINETIC THEORY OF GASES
 KINETIC THEORY OF GASES VERY SHORT ANSWER TYPE QUESTIONS ( MARK). Write two condition when real gases obey the ideal gas equation ( nrt). n number of mole.. If the number of molecule in a container is
KINETIC THEORY OF GASES VERY SHORT ANSWER TYPE QUESTIONS ( MARK). Write two condition when real gases obey the ideal gas equation ( nrt). n number of mole.. If the number of molecule in a container is
Speed Distribution at CONSTANT Temperature is given by the Maxwell Boltzmann Speed Distribution
 Temperature ~ Average KE of each particle Particles have different speeds Gas Particles are in constant RANDOM motion Average KE of each particle is: 3/2 kt Pressure is due to momentum transfer Speed Distribution
Temperature ~ Average KE of each particle Particles have different speeds Gas Particles are in constant RANDOM motion Average KE of each particle is: 3/2 kt Pressure is due to momentum transfer Speed Distribution
(Heat capacity c is also called specific heat) this means that the heat capacity number c for water is 1 calorie/gram-k.
 Lecture 23: Ideal Gas Law and The First Law of Thermodynamics 1 (REVIEW) Chapter 17: Heat Transfer Origin of the calorie unit A few hundred years ago when people were investigating heat and temperature
Lecture 23: Ideal Gas Law and The First Law of Thermodynamics 1 (REVIEW) Chapter 17: Heat Transfer Origin of the calorie unit A few hundred years ago when people were investigating heat and temperature
Temperature, Thermal Expansion, and Ideal Gas Law
 Temperature, Thermal Expansion, and Ideal Gas Law The Density of copper is 8.9 E 3 kg/m^3 and each copper atom has a mass of 63 u, where 1u= 1.66 E -27 kg. Estimate the average distance between neighboring
Temperature, Thermal Expansion, and Ideal Gas Law The Density of copper is 8.9 E 3 kg/m^3 and each copper atom has a mass of 63 u, where 1u= 1.66 E -27 kg. Estimate the average distance between neighboring
SHRI RAMSWAROOP MEMORIAL COLLEGE OF ENGG. & MANAGEMENT
 B.Tech. [SEM III (ME-31, 32, 33,34,35 & 36)] QUIZ TEST-1 Time: 1 Hour THERMODYNAMICS Max. Marks: 30 (EME-303) Note: Attempt All Questions. Q1) 2 kg of an ideal gas is compressed adiabatically from pressure
B.Tech. [SEM III (ME-31, 32, 33,34,35 & 36)] QUIZ TEST-1 Time: 1 Hour THERMODYNAMICS Max. Marks: 30 (EME-303) Note: Attempt All Questions. Q1) 2 kg of an ideal gas is compressed adiabatically from pressure
Classification following properties of the system in Intensive and Extensive
 Unit I Classification following properties of the system in Intensive and Extensive Extensive : mass, weight, volume, potential energy, Kinetic energy, Internal energy, entropy, exergy, energy, magnetization
Unit I Classification following properties of the system in Intensive and Extensive Extensive : mass, weight, volume, potential energy, Kinetic energy, Internal energy, entropy, exergy, energy, magnetization
Chapter 10. Thermal Physics. Thermodynamic Quantities: Volume V and Mass Density ρ Pressure P Temperature T: Zeroth Law of Thermodynamics
 Chapter 10 Thermal Physics Thermodynamic Quantities: Volume V and Mass Density ρ Pressure P Temperature T: Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion of Solids and Liquids Ideal
Chapter 10 Thermal Physics Thermodynamic Quantities: Volume V and Mass Density ρ Pressure P Temperature T: Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion of Solids and Liquids Ideal
A) 2.0 atm B) 2.2 atm C) 2.4 atm D) 2.9 atm E) 3.3 atm
 Name: Date: 1. On a cold day ( 3 C), the gauge pressure on a tire reads 2.0 atm. If the tire is heated to 27 C, what will be the absolute pressure of the air inside the tire? A) 2.0 atm B) 2.2 atm C) 2.4
Name: Date: 1. On a cold day ( 3 C), the gauge pressure on a tire reads 2.0 atm. If the tire is heated to 27 C, what will be the absolute pressure of the air inside the tire? A) 2.0 atm B) 2.2 atm C) 2.4
HEAT & THERMODYNAMICS
 Einstein lasses, Unit No. 0, 03, ardhman Ring Road laza, ikas uri Extn., Outer Ring Road New Delhi 0 08, h. : 9369035, 857 HT HEAT & THERMODYNAMIS Syllabus : HEAT Heat, temperature, thermal expansion;
Einstein lasses, Unit No. 0, 03, ardhman Ring Road laza, ikas uri Extn., Outer Ring Road New Delhi 0 08, h. : 9369035, 857 HT HEAT & THERMODYNAMIS Syllabus : HEAT Heat, temperature, thermal expansion;
CHAPTER 17 WORK, HEAT, & FIRST LAW OF THERMODYNAMICS
 CHAPTER 17 WORK, HEAT, and the FIRST LAW OF THERMODYNAMICS In this chapter, we will examine various thermal properties of matter, as well as several mechanisms by which energy can be transferred to and
CHAPTER 17 WORK, HEAT, and the FIRST LAW OF THERMODYNAMICS In this chapter, we will examine various thermal properties of matter, as well as several mechanisms by which energy can be transferred to and
Chapter 19 The First Law of Thermodynamics
 Chapter 19 The First Law of Thermodynamics The first law of thermodynamics is an extension of the principle of conservation of energy. It includes the transfer of both mechanical and thermal energy. First
Chapter 19 The First Law of Thermodynamics The first law of thermodynamics is an extension of the principle of conservation of energy. It includes the transfer of both mechanical and thermal energy. First
Chapter 12. Temperature and Heat. continued
 Chapter 12 Temperature and Heat continued 12.3 The Ideal Gas Law THE IDEAL GAS LAW The absolute pressure of an ideal gas is directly proportional to the Kelvin temperature and the number of moles (n) of
Chapter 12 Temperature and Heat continued 12.3 The Ideal Gas Law THE IDEAL GAS LAW The absolute pressure of an ideal gas is directly proportional to the Kelvin temperature and the number of moles (n) of
CALORIEMETRY. Similar to the other forms of the energy, The S.I unit of heat is joule. joule is represented as J.
 CALORIEMETRY CALORIMETRY Heat is the kinetic energy due to random motion of the molecules of a substance is called heat energy. Heat is a an invisible energy, that causes in us the sensation of hotness
CALORIEMETRY CALORIMETRY Heat is the kinetic energy due to random motion of the molecules of a substance is called heat energy. Heat is a an invisible energy, that causes in us the sensation of hotness
Chapter 18 Heat and the First Law of Thermodynamics
 Chapter 18 Heat and the First Law of Thermodynamics Heat is the transfer of energy due to the difference in temperature. The internal energy is the total energy of the object in its centerofmass reference
Chapter 18 Heat and the First Law of Thermodynamics Heat is the transfer of energy due to the difference in temperature. The internal energy is the total energy of the object in its centerofmass reference
PHYSICS 221, FALL 2010 FINAL EXAM MONDAY, DECEMBER 13, 2010
 PHYSICS 221, FALL 2010 FINAL EXAM MONDAY, DECEMBER 13, 2010 Name (printed): Nine-digit ID Number: Section Number: Recitation Instructor: INSTRUCTIONS: i. Put away all materials except for pens, pencils,
PHYSICS 221, FALL 2010 FINAL EXAM MONDAY, DECEMBER 13, 2010 Name (printed): Nine-digit ID Number: Section Number: Recitation Instructor: INSTRUCTIONS: i. Put away all materials except for pens, pencils,
PURE PHYSICS THERMAL PHYSICS (PART I)
 PURE PHYSICS THERMAL PHYSICS (PART I) 1 The kinetic theory of matter states that all matters are made up of or, which are in and motion. forces hold the atoms or molecules together. The nature of these
PURE PHYSICS THERMAL PHYSICS (PART I) 1 The kinetic theory of matter states that all matters are made up of or, which are in and motion. forces hold the atoms or molecules together. The nature of these
CH 15. Zeroth and First Law of Thermodynamics
 CH 15 Zeroth and First Law of Thermodynamics THERMODYNAMICS Thermodynamics Branch of Physics that is built upon the fundamental laws that heat and work obey. Central Heating Objectives: After finishing
CH 15 Zeroth and First Law of Thermodynamics THERMODYNAMICS Thermodynamics Branch of Physics that is built upon the fundamental laws that heat and work obey. Central Heating Objectives: After finishing
UNIT I Basic concepts and Work & Heat Transfer
 SIDDHARTH GROUP OF INSTITUTIONS :: PUTTUR Siddharth Nagar, Narayanavanam Road 517583 QUESTION BANK (DESCRIPTIVE) Subject with Code: Engineering Thermodynamics (16ME307) Year & Sem: II-B. Tech & II-Sem
SIDDHARTH GROUP OF INSTITUTIONS :: PUTTUR Siddharth Nagar, Narayanavanam Road 517583 QUESTION BANK (DESCRIPTIVE) Subject with Code: Engineering Thermodynamics (16ME307) Year & Sem: II-B. Tech & II-Sem
Atomic Mass and Atomic Mass Number. Moles and Molar Mass. Moles and Molar Mass
 Atomic Mass and Atomic Mass Number The mass of an atom is determined primarily by its most massive constituents: protons and neutrons in its nucleus. The sum of the number of protons and neutrons is called
Atomic Mass and Atomic Mass Number The mass of an atom is determined primarily by its most massive constituents: protons and neutrons in its nucleus. The sum of the number of protons and neutrons is called
Thermodynamic Processes and Thermochemistry
 General Chemistry Thermodynamic Processes and Thermochemistry 박준원교수 ( 포항공과대학교화학과 ) 이번시간에는! Systems, states, and processes The first law of thermodynamics: internal energy, work, and heat Heat capacity,
General Chemistry Thermodynamic Processes and Thermochemistry 박준원교수 ( 포항공과대학교화학과 ) 이번시간에는! Systems, states, and processes The first law of thermodynamics: internal energy, work, and heat Heat capacity,
Topic 5: Energetics. Heat & Calorimetry. Thursday, March 22, 2012
 Topic 5: Energetics Heat & Calorimetry 1 Heat is energy that is transferred from one object to another due to a difference in temperature Temperature is a measure of the average kinetic energy of a body
Topic 5: Energetics Heat & Calorimetry 1 Heat is energy that is transferred from one object to another due to a difference in temperature Temperature is a measure of the average kinetic energy of a body
Cyclic Processes. water
 Name Cyclic Processes Cyclic Processes A fixed quantity of ideal gas is contained within a metal cylinder that is sealed with a movable, frictionless, insulating piston. (The piston can move up or down
Name Cyclic Processes Cyclic Processes A fixed quantity of ideal gas is contained within a metal cylinder that is sealed with a movable, frictionless, insulating piston. (The piston can move up or down
UNIVERSITY OF SOUTHAMPTON
 UNIVERSITY OF SOUTHAMPTON PHYS1013W1 SEMESTER 2 EXAMINATION 2014-2015 ENERGY AND MATTER Duration: 120 MINS (2 hours) This paper contains 8 questions. Answers to Section A and Section B must be in separate
UNIVERSITY OF SOUTHAMPTON PHYS1013W1 SEMESTER 2 EXAMINATION 2014-2015 ENERGY AND MATTER Duration: 120 MINS (2 hours) This paper contains 8 questions. Answers to Section A and Section B must be in separate
Rao IIT Academy/Rao Intelligence Search Examination/2017/Sample Paper / Std. XI. Physics. Single Correct Questions +3 1
 /Rao Intelligence Search Examination/2017/Sample Paper / Std. XI Physics Single Correct Questions +3 1 1. Three conducting rods of same material and cross section are shown in figure. Temperature of A,
/Rao Intelligence Search Examination/2017/Sample Paper / Std. XI Physics Single Correct Questions +3 1 1. Three conducting rods of same material and cross section are shown in figure. Temperature of A,
Thermal Physics. Topics to be covered. Slide 2 / 105. Slide 1 / 105. Slide 3 / 105. Slide 4 / 105. Slide 5 / 105. Slide 6 / 105.
 Slide 1 / 105 Slide 2 / 105 Topics to be covered Thermal Physics Temperature and Thermal quilibrium Gas Laws Internal nergy Heat Work Laws of Thermodynamics Heat ngines Slide 3 / 105 Thermodynamics System
Slide 1 / 105 Slide 2 / 105 Topics to be covered Thermal Physics Temperature and Thermal quilibrium Gas Laws Internal nergy Heat Work Laws of Thermodynamics Heat ngines Slide 3 / 105 Thermodynamics System
Simpo PDF Merge and Split Unregistered Version -
 74. The rate of heat flow by conduction through a slab does NOT depend upon the: A. temperature difference between opposite faces of the slab B. thermal conductivity of the slab C. slab thickness D. cross-sectional
74. The rate of heat flow by conduction through a slab does NOT depend upon the: A. temperature difference between opposite faces of the slab B. thermal conductivity of the slab C. slab thickness D. cross-sectional
Each of the following questions (1-15) is worth 6 points
 Name: ----------------------------------------------- S. I. D.: ------------------------------------ Physics 0 Final Exam (Version A) Summer 06 HIS EXAM CONAINS 36 QUESIONS. ANSWERS ARE ROUNDED. PICK HE
Name: ----------------------------------------------- S. I. D.: ------------------------------------ Physics 0 Final Exam (Version A) Summer 06 HIS EXAM CONAINS 36 QUESIONS. ANSWERS ARE ROUNDED. PICK HE
Homework: 10, 11, 15, 19, 21 (pages ) 25, 29, 30, 32 (page 501)
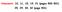 Homework: 1, 11, 15, 19, 1 (pages 5-51) 5, 9, 3, 3 (page 51) 1. An aluminum flagpole is 33m high. By how much does its length increase as the temperature increases by 15 C? For a linear expansion: L LαT
Homework: 1, 11, 15, 19, 1 (pages 5-51) 5, 9, 3, 3 (page 51) 1. An aluminum flagpole is 33m high. By how much does its length increase as the temperature increases by 15 C? For a linear expansion: L LαT
A) 3.1 m/s B) 9.9 m/s C) 14 m/s D) 17 m/s E) 31 m/s
 1. A large tank, open at the top, is filled with water to a depth of 15 m. A spout located 10.0 m above the bottom of the tank is then opened as shown in the drawing. With what speed will water emerge
1. A large tank, open at the top, is filled with water to a depth of 15 m. A spout located 10.0 m above the bottom of the tank is then opened as shown in the drawing. With what speed will water emerge
Lecture 2 - Thursday, May 11 th, 3pm-6pm
 PHYSICS 8A Final Exam Spring 2017 - C. Bordel Lecture 2 - Thursday, May 11 th, 3pm-6pm Student name: Student ID #: Discussion section #: Name of your GSI: Day/time of your DS: Physics Instructions In the
PHYSICS 8A Final Exam Spring 2017 - C. Bordel Lecture 2 - Thursday, May 11 th, 3pm-6pm Student name: Student ID #: Discussion section #: Name of your GSI: Day/time of your DS: Physics Instructions In the
Renewable Energy. Theory: The Ideal Gas Law The equation of state for an ideal gas is written: PV = nrt
 Lab 3 Gas Laws and Heat Engines Fall 2010 Introduction/Purpose: In this exercise you will test some of the aspects of the ideal gas law under conditions of constant pressure, constant temperature, and
Lab 3 Gas Laws and Heat Engines Fall 2010 Introduction/Purpose: In this exercise you will test some of the aspects of the ideal gas law under conditions of constant pressure, constant temperature, and
Name : Applied Physics II Exam One Winter Multiple Choice ( 7 Points ):
 Name : e-mail: Applied Physics II Exam One Winter 2006-2007 Multiple Choice ( 7 Points ): 1. Pure nitrogen gas is contained in a sealed tank containing a movable piston. The initial volume, pressure and
Name : e-mail: Applied Physics II Exam One Winter 2006-2007 Multiple Choice ( 7 Points ): 1. Pure nitrogen gas is contained in a sealed tank containing a movable piston. The initial volume, pressure and
First Law of Thermodynamics Basic Concepts
 236 7 PHYSICAL CHEMISTRY 7 CHAPTER First Law of Thermodynamics Basic Concepts CONTENTS THERMODYNAMIC TERMS SYSTEM, BOUNDARY, SURROUNDINGS HOMOGENEOUS AND HETEROGENEOUS SYSTEMS TYPES OF THERMODYNAMIC SYSTEMS
236 7 PHYSICAL CHEMISTRY 7 CHAPTER First Law of Thermodynamics Basic Concepts CONTENTS THERMODYNAMIC TERMS SYSTEM, BOUNDARY, SURROUNDINGS HOMOGENEOUS AND HETEROGENEOUS SYSTEMS TYPES OF THERMODYNAMIC SYSTEMS
Chapters 17 &19 Temperature, Thermal Expansion and The Ideal Gas Law
 Chapters 17 &19 Temperature, Thermal Expansion and The Ideal Gas Law Units of Chapter 17 & 19 Temperature and the Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion Heat and Mechanical Work
Chapters 17 &19 Temperature, Thermal Expansion and The Ideal Gas Law Units of Chapter 17 & 19 Temperature and the Zeroth Law of Thermodynamics Temperature Scales Thermal Expansion Heat and Mechanical Work
Hence. The second law describes the direction of energy transfer in spontaneous processes
 * Heat and Work The first law of thermodynamics states that: Although energy has many forms, the total quantity of energy is constant. When energy disappears in one form, it appears simultaneously in other
* Heat and Work The first law of thermodynamics states that: Although energy has many forms, the total quantity of energy is constant. When energy disappears in one form, it appears simultaneously in other
Chapter 19: The Kinetic Theory of Gases Questions and Example Problems
 Chapter 9: The Kinetic Theory of Gases Questions and Example Problems N M V f N M Vo sam n pv nrt Nk T W nrt ln B A molar nmv RT k T rms B p v K k T λ rms avg B V M m πd N/V Q nc T Q nc T C C + R E nc
Chapter 9: The Kinetic Theory of Gases Questions and Example Problems N M V f N M Vo sam n pv nrt Nk T W nrt ln B A molar nmv RT k T rms B p v K k T λ rms avg B V M m πd N/V Q nc T Q nc T C C + R E nc
Thermal Physics. Temperature (Definition #1): a measure of the average random kinetic energy of all the particles of a system Units: o C, K
 Thermal Physics Internal Energy: total potential energy and random kinetic energy of the molecules of a substance Symbol: U Units: J Internal Kinetic Energy: arises from random translational, vibrational,
Thermal Physics Internal Energy: total potential energy and random kinetic energy of the molecules of a substance Symbol: U Units: J Internal Kinetic Energy: arises from random translational, vibrational,
Lecture 5. PHYC 161 Fall 2016
 Lecture 5 PHYC 161 Fall 2016 Ch. 19 First Law of Thermodynamics In a thermodynamic process, changes occur in the state of the system. Careful of signs! Q is positive when heat flows into a system. W is
Lecture 5 PHYC 161 Fall 2016 Ch. 19 First Law of Thermodynamics In a thermodynamic process, changes occur in the state of the system. Careful of signs! Q is positive when heat flows into a system. W is
THERMODINAMICS. Tóth Mónika
 THERMODINAMICS Tóth Mónika 2014 monika.a.toth@aok.pte.hu Temperature Temperature: is related to the average energy of the motion of the particles of an object or system. Different temperature scales. Thermometer
THERMODINAMICS Tóth Mónika 2014 monika.a.toth@aok.pte.hu Temperature Temperature: is related to the average energy of the motion of the particles of an object or system. Different temperature scales. Thermometer
R13 SET - 1 '' ''' '' ' '''' Code No RT21033
 SET - 1 II B. Tech I Semester Supplementary Examinations, June - 2015 THERMODYNAMICS (Com. to ME, AE, AME) Time: 3 hours Max. Marks: 70 Note: 1. Question Paper consists of two parts (Part-A and Part-B)
SET - 1 II B. Tech I Semester Supplementary Examinations, June - 2015 THERMODYNAMICS (Com. to ME, AE, AME) Time: 3 hours Max. Marks: 70 Note: 1. Question Paper consists of two parts (Part-A and Part-B)
Classes at: - Topic: Thermodynamics. = E v. = G f T 1
 PHYSICAL CHEMISTRY by: SHAILENDRA KR. Classes at: - SCIENCE TUTORIALS; Opp. Khuda Baksh Library, Ashok Rajpath, Patna PIN POINT STUDY CIRCLE; House No. 5A/65, Opp. Mahual Kothi, Alpana Market, Patna Topic:
PHYSICAL CHEMISTRY by: SHAILENDRA KR. Classes at: - SCIENCE TUTORIALS; Opp. Khuda Baksh Library, Ashok Rajpath, Patna PIN POINT STUDY CIRCLE; House No. 5A/65, Opp. Mahual Kothi, Alpana Market, Patna Topic:
Thermal Physics. Slide 1 / 163. Slide 2 / 163. Slide 3 / 163. Thermal Physics.
 Slide 1 / 163 Slide 2 / 163 Thermal Physics www.njctl.org Thermal Physics Temperature, Thermal Equilibrium and Thermometers Thermal Expansion Heat and Temperature Change Thermal Equilibrium : Heat Calculations
Slide 1 / 163 Slide 2 / 163 Thermal Physics www.njctl.org Thermal Physics Temperature, Thermal Equilibrium and Thermometers Thermal Expansion Heat and Temperature Change Thermal Equilibrium : Heat Calculations
CHAPTER 19: Heat and the First Law of Thermodynamics
 CHAPTER 9: Heat and the First Law of Thermodynamics Responses to Questions. (a) No. Because the ernal energies of solids and liquids are complicated and include potential energies associated with the bonds
CHAPTER 9: Heat and the First Law of Thermodynamics Responses to Questions. (a) No. Because the ernal energies of solids and liquids are complicated and include potential energies associated with the bonds
12. Heat of melting and evaporation of water
 VS 12. Heat of melting and evaporation of water 12.1 Introduction The change of the physical state of a substance in general requires the absorption or release of heat. In this case, one speaks of a first
VS 12. Heat of melting and evaporation of water 12.1 Introduction The change of the physical state of a substance in general requires the absorption or release of heat. In this case, one speaks of a first
Chapter 10: Thermal Physics
 Chapter 10: hermal Physics hermal physics is the study of emperature, Heat, and how these affect matter. hermal equilibrium eists when two objects in thermal contact with each other cease to echange energy.
Chapter 10: hermal Physics hermal physics is the study of emperature, Heat, and how these affect matter. hermal equilibrium eists when two objects in thermal contact with each other cease to echange energy.
Chapter One Reviews of Thermodynamics Update on 2013/9/13
 Chapter One Reviews of Thermodynamics Update on 2013/9/13 (1.1). Thermodynamic system An isolated system is a system that exchanges neither mass nor energy with its environment. An insulated rigid tank
Chapter One Reviews of Thermodynamics Update on 2013/9/13 (1.1). Thermodynamic system An isolated system is a system that exchanges neither mass nor energy with its environment. An insulated rigid tank
Lecture 7, 8 and 9 : Thermodynamic process by: Asst. lect. Karrar Al-Mansoori CONTENTS. 7) Thermodynamic process, path and cycle 2
 CONTENTS Topics pages 7) Thermodynamic process, path and cycle 8) Reversibility and irreversibility 4 9) Thermodynamic processes and calculation of work 5 9.: Constant pressure process or isobaric process
CONTENTS Topics pages 7) Thermodynamic process, path and cycle 8) Reversibility and irreversibility 4 9) Thermodynamic processes and calculation of work 5 9.: Constant pressure process or isobaric process
Physics 1501 Lecture 35
 Physics 1501: Lecture 35 Todays Agenda Announcements Homework #11 (Dec. 2) and #12 (Dec. 9): 2 lowest dropped Honors students: see me after the class! Todays topics Chap.16: Temperature and Heat» Latent
Physics 1501: Lecture 35 Todays Agenda Announcements Homework #11 (Dec. 2) and #12 (Dec. 9): 2 lowest dropped Honors students: see me after the class! Todays topics Chap.16: Temperature and Heat» Latent
LESSON No. 9 WORK TRANSFER: In thermodynamics the work can be defined as follows:
 LESSON No. 9 WORK TRANSFER: In thermodynamics the work can be defined as follows: Work shall be done by the system if the total effect outside the system is equivalent to the raising of weight and this
LESSON No. 9 WORK TRANSFER: In thermodynamics the work can be defined as follows: Work shall be done by the system if the total effect outside the system is equivalent to the raising of weight and this
2. If the volume of a container holding a gas is reduced, what will happen to the presure within the container?
 1. Which gas law states that the volume of a fixed mass of a gas is directly proportional to its Kelvin temperature if the pressure is kept constant? A. Boyle s law B. Charles law C. Dalton s law D. Gay-Lussac
1. Which gas law states that the volume of a fixed mass of a gas is directly proportional to its Kelvin temperature if the pressure is kept constant? A. Boyle s law B. Charles law C. Dalton s law D. Gay-Lussac
Chapter 12. The Laws of Thermodynamics
 Chapter 12 The Laws of Thermodynamics First Law of Thermodynamics The First Law of Thermodynamics tells us that the internal energy of a system can be increased by Adding energy to the system Doing work
Chapter 12 The Laws of Thermodynamics First Law of Thermodynamics The First Law of Thermodynamics tells us that the internal energy of a system can be increased by Adding energy to the system Doing work
Zeroth Law of Thermodynamics
 Thermal Equilibrium When you two systems are placed in contact with each other there is no net energy transfer between them. Consequently, these two systems would be at the same temperature. Zeroth Law
Thermal Equilibrium When you two systems are placed in contact with each other there is no net energy transfer between them. Consequently, these two systems would be at the same temperature. Zeroth Law
Thermal Equilibrium. Zeroth Law of Thermodynamics 2/4/2019. Temperature
 Thermal Equilibrium When you two systems are placed in contact with each other there is no net energy transfer between them. Consequently, these two systems would be at the same temperature. Zeroth Law
Thermal Equilibrium When you two systems are placed in contact with each other there is no net energy transfer between them. Consequently, these two systems would be at the same temperature. Zeroth Law
If the dividing wall were allowed to move, which of the following statements would not be true about its equilibrium position?
 PHYS 213 Exams Database Midterm (A) A block slides across a rough surface, eventually coming to a stop. 1) What happens to the block's internal thermal energy and entropy? a. and both stay the same b.
PHYS 213 Exams Database Midterm (A) A block slides across a rough surface, eventually coming to a stop. 1) What happens to the block's internal thermal energy and entropy? a. and both stay the same b.
Physics 231. Topic 14: Laws of Thermodynamics. Alex Brown Dec MSU Physics 231 Fall
 Physics 231 Topic 14: Laws of Thermodynamics Alex Brown Dec 7-11 2015 MSU Physics 231 Fall 2015 1 8 th 10 pm correction for 3 rd exam 9 th 10 pm attitude survey (1% for participation) 10 th 10 pm concept
Physics 231 Topic 14: Laws of Thermodynamics Alex Brown Dec 7-11 2015 MSU Physics 231 Fall 2015 1 8 th 10 pm correction for 3 rd exam 9 th 10 pm attitude survey (1% for participation) 10 th 10 pm concept
