THEORETICAL INVESTIGATION OF ELECTROSTATIC POTENTIAL AND NON LINEAR OPTICAL PROPERTIES OF M NITROACETANILIDE
|
|
|
- Lionel Gregory
- 5 years ago
- Views:
Transcription
1 ISSN: e-issn: CODEN: RJCABP THEORETICAL INVESTIGATION OF ELECTROSTATIC POTENTIAL AND NON LINEAR OPTICAL PROPERTIES OF N. Boukabcha, N. Benhalima, R. Rahmani, A. Chouaih * and F. Hamzaoui Laboratory of Technology and Solid s Properties, University of Mostaganem , Mostaganem, Algeria. * achouaih@gmail.com ABSTRACT In this paper, we report the electrostatic potential and nonlinear optical properties of meta-nitroacetanilide. Molecular properties were calculated by Hatree Fock (HF) and density functional theory (DFT) methods with the 6-31+G (d) basis set. The molecular electrostatic potential map indicates that the negative potential sites are on oxygen atoms as well as the positive potential sites are around the hydrogen atoms. The second-order nonlinear optical properties based on the first static hyperpolarizability (β) have been investigated. The calculated results show that the title molecule might have nonlinear optical behavior. The thermodynamic functions (entropy, heat capacity and enthalpy) were obtained from spectroscopic data by statistical methods for the range of temperature K. Keywords: Organic compound, dipole-moment, first hyperpolarizability, HOMO-LUMO, Nonlinear optical materials RASĀYAN. All rights reserved INTRODUCTION Nitroacetanilide, commonly known as NAa, an organic nonlinear optical (NLO) compound with formula C 8 H 8 N 2 O 3. Nitroacetanilide is an organic molecule containing extended π-conjugated electrons and characterized by large values of molecular first hyperpolarizabilities showing enhanced NLO properties. As known, the origin of non-linearity in organic molecules is significantly related to the presence of delocalized π-electron system linking donor and acceptor groups, which amplify the obligatory asymmetric polarizability. 1 The NLO properties magnitude of molecules is dependent on the first-order hyperpolarizability. The NLO property of molecules and their hyperpolarizabilities have become an important field of extensive research. 2-6 Several authors already reported synthesis, molecular structure and vibrational spectroscopic investigations of nitroacetanilide. 7-9 More recently, the molecular structure; vibrational spectroscopic and Natural Bond Orbital (NBO) studies of meta-naa have been investigated by Li Xiao-Hong et al. 10 In the present work, we are interested to the Meta form of nitroacetanilide molecule (m-naa). We report here for the first time in literature the electrostatic potential and the nonlinear optical properties of m- NAa. To our knowledge, the NLO properties have not been reported except in our work. The HOMO and LUMO analyses have been used to elucidate information regarding charge transfer within the molecule. Finally, the thermodynamic properties of the optimized structure were obtained theoretically from the harmonic vibrations taken from. 10 EXPERIMENTAL The first task for the computational work was to determine the optimized geometry of the molecule. The molecular structure of m-naa is optimized by Hartree Fock (HF) level and density functional theory (DFT) using 6-31+G(d) basis set, by the Berny method In the DFT method, The effects of electron correlation on the geometry optimization are taken into account by Becke s three parameter hybrid exchange functions and Lee-Young Parr correlation functional (B3LYP) level. 14,15 The optimized structure was then used to calculate the electrostatic potential and nonlinear optical properties of m-naa
2 molecule. The 6-31+G (d) basis set for both HF and DFT levels has been found to be more adequate for obtaining reliable trends in hyperpolarizability (β) values. 16,17 All theoretical calculations were performed using Gaussian 09 program and Gauss-View molecular visualization program package on the personal computer. 18,19 The optimized geometry of m nitroacetanilide which was performed by HF and B3LYP methods is shown in Fig.-1. The obtained structure was used to calculate the different properties of the title molecule. Fig.-1: Optimized geometry of M Nitroacetanilide. RESULTS AND DISCUSSION Molecular electrostatic potential Molecular Electrostatic Potential (MEP) generally presents in the space around the molecule by the charge distribution. The MEP is related to the electronic density and is a very useful descriptor for determining sites for electrophilic attack and nucleophilic reactions as well as hydrogen-bonding interactions The electrostatic potential V(r) are also well suited for analyzing processes based on the recognition of one molecule by another, as in drug receptor, and enzyme substrate interactions, because it is through their potentials that the two species first see each other Being a real physical property V(r) can be determined experimentally by X-ray diffraction or by computational methods. 27 The electrostatic potential V(r), at a given point r(x, y, z) in the space around a molecule (in atomic units) is defined in terms of the interaction energy between the electrical charge generated from the molecule electrons and nuclei and positive test charge (a proton) located at r and can be expressed as: Where Z A is the charge on nucleus A, located at R A and ρ(r) is the electronic density function for the molecule. The first and second terms represent the contributions to the potential due to nuclei and electrons, respectively. V(r) is the resultant electrical potential at each point r, which is the net electrostatic effect produced at the point r by both the electrons and nuclei of the molecule. The molecular electrostatic potential (MEP) serves as a useful quantity to explain hydrogen bonding, reactivity and structure activity relationship of the molecules. 28 In order to predict the molecular reactive sites, the MEP for the title molecule was calculated using 6-31+G (d) basis set as shown in Fig.-2. The different values of the electrostatic potential at the surface are represented by different colors, where blue indicates the highest electrostatic potential energy and red indicates the lowest electrostatic potential energy. As it can be seen from the MEP map of m-naa molecule, the region around oxygen atoms linked with carbon through double bond represents the most negative potential region (red). The most negative V(r) value is associated also with NO 2 group of m- NAa. The hydrogen atoms attached to nitrogen atoms posses the maximum positive charge. The MEP surface provides necessary details about the reactive sites. 510
3 Nonlinear optical properties Theoretical investigations have been done in order to understand the microscopic origin of nonlinear behavior of the studied molecule. The study involves the calculation of dipole moment (µ), polarizability (α) and first hyperpolarizability (β) tensor for the title molecule. First hyperpolarizability is a third rank tensor which can be described by a matrix. Due to the Kleinman symmetry, the 27 components of the 3-D matrix can be reduced to 10 components. 29 The Gaussian 09 output provides 10 components of this matrix as β xxx, β xxy, β xyy, β yyy, β xxz, β xyz, β yyz, β xzz, β yzz, β zzz respectively. Many types of hyperpolarizabilities have been reported in the literature denoted as β vec (β vector), β (β parallel), β tot (β total). 30 β vec is the component along the dipole moment direction. But the theoretical chemists are concerned with β which is the component parallel to the ground state charge transfer direction and the other is the total hyperpolarizability β tot. Using the x, y and z components, and the magnitude of the total static dipole moment (µ) and isotropic polarizability (α), first-order hyperpolarizability β tot tensor can be calculated by the following equations: The complete equation for calculating the magnitude of the first hyeprpolarizability (β) from Gaussian 09 output is given below: To calculate the hyperpolarizability, the origin of the Cartesian coordinate system was chosen as the centre of mass of the molecule. 31 Since the values of the first hyperpolarizability tensors are reported in atomic units (a.u.), the calculated values were converted into electrostatic units. (a) (b) Fig.-2: Molecular electrostatic potential maps of m-nitroacetanilide, (a) 3D plot, (b) contour map. The calculated dipole moment (µ), mean polarizability (α) and first hyperpolarizability (β) of the title molecule have been computed at HF/6-31+G(d) and DFT-B3LYP/6-31+G(d) levels of theory to provide its nonlinear properties and are listed in Table-1. The dipole moment is a basic property of a molecule, used to investigate intermolecular interactions concerning the non-bonded type dipole-dipole interaction. Higher the dipole moment, stronger is the inter-molecular interaction. The small frontier orbital energy gap (between and ev by both methods) and high dipole moment (between and Debye) of m-naa may contribute to multiply its NLO activity. Based on predicted dipole moment values in Table 1, it is found that, in going from the gas phase ( D and D obtained using HF 511
4 and DFT methods, respectively) to the solvent phase ( D and D in methanol obtained using HF and DFT levels of theory, respectively) the dipole moment value increases. The highest value of dipole moment is observed for component µy. In this direction, this value is equal to and Debye by HF level of theory in gas phase and solvent, respectively, and and Debye by DFT level of theory in gas phase and solvent. These values indicate the eventual charge transfer direction within the molecule. Table-1: Calculated dipole moment (µ), polarizability (α) and hyperpolarizability (β total ) in gas and solution of m nitroacetanilide. Parameters Gas Methanol HF/6 31+G(d) DFT/6 31+G(d) HF/6 31+G(d) DFT/6 31+G(d) µ x µ y µ z µ (D) α xx α xy α yy α xz α yz α zz α (a.u) α [10 23 esu] β xxx β xxy β xyy β yyy β xxz β xyz β yyz β xzz β yzz β zzz β (a.u) β [10 30 esu] Polarizability and total hyperpolarizability of the title molecule are calculated to be (between and ) esu and ( and ) esu, respectively (see Table 1). For polarizability, DFT method gives higher values ( and esu) compared to those calculated using HF method ( and esu). Accordingly to the results, polarizability values obtained in solvent phase ( and esu) are most important compared to those obtained in gaseous phase ( and esu). So, from the gas phase to the solvent phase, polarizability values increase. The magnitude of the molecular hyperpolarizability β, is one of key factors in NLO system. The calculated first static hyperpolarisability β value is equal to and and calculated at HF and DFT levels of theory in gas phase, respectively. In solvent phase, these values are and and calculated at HF and DFT levels of theory, respectively. Accordingly to the results, DFT method gives interesting values of β. The calculated first order hyperpolarizability of p-nitroacetanilide (PNA) is esu as reported by T. Gnanasambandan et al. 9 This value is two times greater than the mean β value ( esu) 512
5 of the title molecule. Urea is the reference molecule which is used in the study of the NLO properties of molecular systems. Therefore, it is used commonly as a threshold value for comparative purpose. Calculated β total value for the title molecule ( esu) is found to be 29 times greater than the β total value of urea ( esu), predicting that m-naa is an efficacious candidate for NLO material. HOMO and LUMO analysis Molecular orbitals and their properties such as energy are very useful for scientists and are very important parameters for quantum chemistry. The most important orbitals in a molecule which determine how the molecule interacts with other species are the frontier molecular orbitals, called highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO). They are the key parameters in determining molecular properties and molecular electrical transport properties. 32,33 Moreover, the Eigen values of HOMO (π donor) and LUMO (π acceptor) and their energy gap reflect the chemical activity of the molecules. We have done optimization in order to investigate the energetic behavior of m-naa molecule in gas and solution. The total energy and frontier molecular orbital energies have been calculated with HF/6-31+G (d) and B3LYP/6 31+G (d) levels. Results obtained from solvent (methanol) and gas phase are listed in Table-2. Table-2: Calculated Energies values in gas and solution of m-nitroacetanilide using 6 31+G (d) basis set. Parameters Gas Methanol HF DFT HF DFT Energy E Homo E Lumo E Homo Lumo gap (ev) E Homo E Lumo E Homo1 Lumo+1 gap (ev) The energy gap values are , , and ev in gas and solution for m- nitroacetanilide molecule, respectively. The optical band gap calculated using B3LYP/6 31+G (d) was found to be 4.10 ev which is in accordance with the gap of other nonlinear optical materials. 34,35 The molecular orbital (MO) calculation indicates that m-nitroacetanilide has 47 occupied MOs. We examine the four important molecular orbitals (MOs): the second highest and highest occupied MOs and the lowest and the second lowest unoccupied MOs which we denote HOMO 1, HOMO, LUMO and LUMO+1, respectively. These MOs for gas phase are represented in Fig.-3. The energy gap between HOMO and LUMO indicates molecular chemical stability. In addition, a lower HOMO-LUMO energy gap ( ev) was obtained with DFT/6-31+G (d) using methanol solvent. This value explains the fact that eventual charge transfer interaction is taking place within the molecule. However, HOMO-LUMO energies are influenced not only by the basis set employed in HF and DFT calculations but also by the solvent in which the molecule is contained. From the above results, it can be inferred that the DFT method estimated better the value of the band gap. The distributions of the HOMO 1, HOMO, LUMO and LUMO+1 orbitals computed at the DFT/6-31+G(d) method for the title molecule are shown in Fig.-3. As can be seen from Fig.-3, the HOMO and LUMO+1 are delocalized over the entire molecule. Whereas, the HOMO 1 is delocalized over the NHCOR-CH 3 moiety which acts as electro donor group, while the LUMO is delocalized over the nitrobenzene moiety. The nitro group acts as electro donor. Thermodynamic properties On the basis of vibrational analysis at B3LYP/6 31+G(d,p) level as reported by Li Xiao-Hong et al., the standard statistical thermodynamic functions: entropy, heat capacity and enthalpy changes of m-naa 513
6 molecule were obtained from the theoretical harmonic frequencies and are listed in Table-3. Thermodynamic parameters were obtained using both HF and DFT methods with 6-31+G (d) basis set. From Table-3, we can observe that these thermodynamic functions are increasing with temperature ranging from 100 to 1000 K due to the fact that the molecular vibrational intensities increase with temperature. 36 T(K) Fig.-3: Plots of the frontier orbitals of m nitroacetanilide by B3LYP/6 31+G (d) Table-3: Thermodynamic properties for meta-nitroacetanilide HF/6 31+G (d) DFT/6 31+G (d) S(J/mol.K) Cp (J/mol.K) ddh(kj/mol) S(J/mol.K) Cp(J/mol.K) ddh(kj/mol) The variation of the thermodynamic functions such as entropy, heat capacity and enthalpy with temperature are graphically represented in Fig.-4. As illustrated in Fig.-4, we can see the increase of calculated thermodynamic functions of m-naa molecule with temperature. These results compared to those obtained on the para-nitroacetanilide (PNA) molecule are in good agreement. 9 For example, thermodynamic functions obtained at T = K on m-naa molecule using DFT/6-31+G(d) are J/mol.K, J/mol.K and kj/mol for S, Cp and ddh, respectively. The corresponding 514
7 functions obtained at the same temperature ( K) on PNA molecule using DFT/ G (d, p) are J/mol.K, J/mol.K and kj/mol for S, Cp and ddh, respectively. These slight differences may be are due to the position of NO2 moiety in the molecule. Fig.-4: Correlation graph of thermodynamic parameters of m-nitroacetanilide CONCLUSIONS In this present investigation, we have performed theoretical calculations of molecular electrostatic potential, nonlinear optical properties, HOMO LUMO and thermodynamic properties for the organic molecule meta-nitroacetanilide (m-naa). Molecular properties have been calculated by using ab initio HF and DFT (B3LYP) methods with 6-31+G (d) basis set. The molecular electrostatic potential map shows that the negative potential sites are on the electronegative atoms as well as the positive potential sites are around the hydrogen atoms. These sites give information about the possible regions for inter- and intramolecular hydrogen bonding. The calculated dipole moment mean value of m-naa is 4.65 D which is in agreement with the value (4.9 D) found in litterature. 37 The mean polarizability and total first static hyperpolarizability (βtotal) of the molecule is found to be 17, esu and 5, esu, respectively. The βtotal value of m-naa is nearly 29 times more than βtotal of urea, inferring that m-naa molecule to be a potential candidate for nonlinear optical applications. HOMO-LUMO energy gap explains the eventual charge transfer interactions taking place within the molecule. Furthermore, the firstorder hyperpolarizability and total dipole moment property of the molecule show that the title molecule is an attractive target for future studies of nonlinear optical properties. REFERENCES 1. D.S. Chemala and J. Zyss, Nonlinear Optical Properties of Organic Molecules and Crystals, Academic Press, New York, (1987). 2. L. Xiao-Hong, C. Hong-Ling, Z. Rui-Zhou and Z. Xian-Zhou, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 137, 321(2015). 3. S. Guidara, A. Ben Ahmed, Y. Abid and H. Feki, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 127, 275(2014). 4. B. Koşar, Ç. Albayrak, C. Cüneyt Ersanlı, M. Odabaşoğlu and O. Büyükgüngör, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 93, 1(2012). 515
8 5. J. Chandrasekaran, P. Ilayabarathi and P. Maadeswaran, Rasayan J. Chem., 4, 425(2011). 6. B. Ravi, A. Jegathesan, B. Neelakanda Prasad, C. Sadeshkumar and G. Rajarajan, Rasayan J.Chem., 7, 3(2014). 7. R. Rajendran, T. Harris Freeda, U. Lakshmi Kalasekar and R. Narayana Peruma, Advances in Materials Physics and Chemistry, 1, 39(2011). 8. M. Lenin and P. Ramasamy, Journal of Crystal Growth, 310, 4451(2008). 9. T. Gnanasambandan, S. Gunasekaran and S. Seshadri, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 117, 557(2014). 10. L. Xiao-Hong, L. Tong-Wei, J. Wei-Wei, Y. Yong-Liang and Z. Xian-Zhou, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 118, 503(2014). 11. H.D. Cohen and C.C.J. Roothaan, Journal of Chemical Physics, 43, S34(1965). 12. R. Fletcher and M.J.D. Powell, Comput. J., 6, 163(1963). 13. R.F. Bader, Atoms in Molecules: A Quantum Theory, Clarendon Press, Oxford, (1990). 14. A.D. Becke, J Chem. Phys., 98, 5648(1993). 15. C. Lee, W. Yang and R.G. Parr, J Phys. Chem., 99, 3093(1995). 16. C. Dehu, F. Meyers, E. Hendrickx, K. Clays, A. Parsoons, S.R. Marder and J.L. Bredas, J Am. Chem. Soc., 117, 10127(1995). 17. K.S. Thanthiriwatte and K.M.N. De Silva, J Mol Struct (Theochem), 617, 169(2002). 18. Gaussian 09, Revision B.01, Gaussian Inc., Wallingford CT, (2010). 19. A. Frisch, A.B. Nielsen and A.J. Holder, Gaussview Users Manual, Gaussian Inc., Pittsburgh, (2007). 20. Y. Shyma Mary, P.J. Jojo, C. Yohannan Panicker, Van Alsenoy Christian, Ataei Sanaz and Yildiz Ilkay, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 122, 499(2014). 21. M. Karnan, V. Balachandran and M. Murugan, J Mol Struct, 1039, 197(2013). 22. E. Scrocco and J. Tomasi, Adv Quantum Chem, 11, 115(1978). 23. A. Pullman and H. Berthod, Theoret Chim Acta, 48, 269(1978). 24. S.R. Gadre, C. Kölmel and I.H. Shrivastava, Inorg Chem, 31, 2279(1992). 25. P. Politzer, P.R. Laurence, K. Jayasuriya and J. McKinney, Environ Health Perspect, 61, 191(1985). 26. E. Scrocco and J. Tomasi, Topics in Current Chemistry, Springer, Berlin, (1973). 27. P. Politzer and D.G. Truhlar, Chemical Applications of Atomic and Molecular Electrostatic Potentials, Plenum, New York, (1981). 28. S. Chidangil, M.K. Shukla and P.C. Mishra, J. Mol. Model., 4, 250(1998). 29. D.A. Kleinman, Phys. Rev., 126, 1977(1962). 30. D.R. Kanis, M.A. Ratner and T.J. Marks, Chem. Rev., 94, 195(1994). 31. M. Adant, M. Dupuis and J.L. Bredas, Int. J. Quantum Chem., 56, 497(1995). 32. M. Amalanathan, V.K. Rastogi, I.H. Joe, M.A. Palafox and R. Tomar, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 78, 1437(2011). 33. K. Fukui, Science, 218, 747(1982). 34. A. Jegatheesan, B. Ravi, B. Neelakanda Prasad and G. Rajarajan, Rasayan J. Chem., 7, 353(2014). 35. B. Ravi, A. Jegathesan, B. Neelakanda prasad, C. Sadeshkumar and G. Rajarajan, Rasayan J.Chem., 6, 4(2013). 36. J. Bevan Ott and J. Boerio-Goates, Calculations from Statistical Thermodynamics, Academic Press, New York, (2000). 37. H.X. Cang, W.D. Huang and Y.H. Zhou, Journal of Crystal Growth, 192, 236(1998). [RJC-1358/2015] 516
First Order Hyperpolarizability and Homo-Lumo Analysis of L-Arginine Maleate (LArM) by Density Functional Theory Methods
 51 Sciencia Acta Xaveriana An International Science Journal ISSN. 0976-1152 Volume 4 No. 2 pp. 51-58 September 2013 First Order Hyperpolarizability and Homo-Lumo Analysis of L-Arginine Maleate (LArM) by
51 Sciencia Acta Xaveriana An International Science Journal ISSN. 0976-1152 Volume 4 No. 2 pp. 51-58 September 2013 First Order Hyperpolarizability and Homo-Lumo Analysis of L-Arginine Maleate (LArM) by
FT-IR, FT-Raman And UV Spectra and Ab-Initio HF and DFT Vibrational Study of 1-Propyl 4-Piperidone
 FT-IR, FT-Raman And UV Spectra and Ab-Initio HF and DFT Vibrational Study of 1-Propyl 4-Piperidone K. Rajalakshmi 1 and E.Elumalai 2 1 Department of Physics, Sri Chandrasekharendra Saraswathi Viswa Mahavidyalaya,
FT-IR, FT-Raman And UV Spectra and Ab-Initio HF and DFT Vibrational Study of 1-Propyl 4-Piperidone K. Rajalakshmi 1 and E.Elumalai 2 1 Department of Physics, Sri Chandrasekharendra Saraswathi Viswa Mahavidyalaya,
AB INITIO MODELING OF THE STRUCTURAL DEFECTS IN AMIDES
 Int. J. Chem. Sci.: 9(4), 2011, 1564-1568 ISSN 0972-768X www.sadgurupublications.com AB INITIO MODELING OF THE STRUCTURAL DEFECTS IN AMIDES M. FATHIMA BEGUM, HEMA TRESA VARGHESE a, Y. SHEENA MARY a, C.
Int. J. Chem. Sci.: 9(4), 2011, 1564-1568 ISSN 0972-768X www.sadgurupublications.com AB INITIO MODELING OF THE STRUCTURAL DEFECTS IN AMIDES M. FATHIMA BEGUM, HEMA TRESA VARGHESE a, Y. SHEENA MARY a, C.
THE ELECTROSTATIC PROPERTIES OF 1,2-DIMETHYL-3- NITROBENZENE COMPOUND: ab initio CALCULATION AND X-RAY CHARGE DENSITY ANALYSIS
 ISSN: 0974-1496 e-issn: 0976-0083 CODEN: RJCABP http://www.rasayanjournal.com http://www.rasayanjournal.co.in THE ELECTROSTATIC PROPERTIES OF 1,2-DIMETHYL-3- NITROBENZENE COMPOUND: ab initio CALCULATION
ISSN: 0974-1496 e-issn: 0976-0083 CODEN: RJCABP http://www.rasayanjournal.com http://www.rasayanjournal.co.in THE ELECTROSTATIC PROPERTIES OF 1,2-DIMETHYL-3- NITROBENZENE COMPOUND: ab initio CALCULATION
CHAPTER 8 REPORT ON HIGHER SHG EFFICIENCY IN BIS (CINNAMIC ACID): HEXAMINE COCRYSTAL
 CHAPTER 8 REPORT ON HIGHER SHG EFFICIENCY IN BIS (CINNAMIC ACID): HEXAMINE COCRYSTAL 8.1. Introduction In recent times higher Second Harmonic Generation (SHG) efficiency organic materials receive great
CHAPTER 8 REPORT ON HIGHER SHG EFFICIENCY IN BIS (CINNAMIC ACID): HEXAMINE COCRYSTAL 8.1. Introduction In recent times higher Second Harmonic Generation (SHG) efficiency organic materials receive great
International Journal of Materials Science ISSN Volume 12, Number 2 (2017) Research India Publications
 HF, DFT Computations and Spectroscopic study of Vibrational frequency, HOMO-LUMO Analysis and Thermodynamic Properties of Alpha Bromo Gamma Butyrolactone K. Rajalakshmi 1 and A.Susila 2 1 Department of
HF, DFT Computations and Spectroscopic study of Vibrational frequency, HOMO-LUMO Analysis and Thermodynamic Properties of Alpha Bromo Gamma Butyrolactone K. Rajalakshmi 1 and A.Susila 2 1 Department of
STRUCTURAL DEFECTS IN IMIDATES : AN AB INITIO STUDY
 Int. J. Chem. Sci.: 9(4), 2011, 1763-1767 ISSN 0972-768X www.sadgurupublications.com STRUCTURAL DEFECTS IN IMIDATES : AN AB INITIO STUDY M. FATHIMA BEGUM, HEMA TRESA VARGHESE a, Y. SHEENA MARY a, C. YOHANNAN
Int. J. Chem. Sci.: 9(4), 2011, 1763-1767 ISSN 0972-768X www.sadgurupublications.com STRUCTURAL DEFECTS IN IMIDATES : AN AB INITIO STUDY M. FATHIMA BEGUM, HEMA TRESA VARGHESE a, Y. SHEENA MARY a, C. YOHANNAN
Quantum chemical studies on the structures of some heterocyclic azo disperse dyes
 Quantum chemical studies on the structures of some heterocyclic azo disperse dyes Nesrin Tokay, a* Zeynel Seferoğlu, b Cemil Öğretir, c and Nermin Ertan b a Hacettepe University, Faculty of Science, Chemistry
Quantum chemical studies on the structures of some heterocyclic azo disperse dyes Nesrin Tokay, a* Zeynel Seferoğlu, b Cemil Öğretir, c and Nermin Ertan b a Hacettepe University, Faculty of Science, Chemistry
1 Introduction. ISSN , England, UK World Journal of Modelling and Simulation Vol. 14 (2018) No. 1, pp. 3-11
 ISSN 1 746-7233, England, UK World Journal of Modelling and Simulation Vol. 14 (2018) No. 1, pp. 3-11 Molecular Structure, Mulliken charges, HOMO-LUMO, Electrostatic Potential and Nonlinear Optical Properties
ISSN 1 746-7233, England, UK World Journal of Modelling and Simulation Vol. 14 (2018) No. 1, pp. 3-11 Molecular Structure, Mulliken charges, HOMO-LUMO, Electrostatic Potential and Nonlinear Optical Properties
Theoretical and Experimental Electrostatic Potential around the m-nitrophenol Molecule
 Molecules 2015, 20, 4042-4054; doi:10.3390/molecules20034042 Article OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Theoretical and Experimental Electrostatic Potential around the
Molecules 2015, 20, 4042-4054; doi:10.3390/molecules20034042 Article OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Theoretical and Experimental Electrostatic Potential around the
Competition between Alkalide Characteristics and Nonlinear Optical Properties in OLi 3 M Li 3 O (M = Li, Na, and K) Complexes
 Competition between Alkalide Characteristics and Nonlinear Optical Properties in OLi 3 M Li 3 O (M = Li, Na, and K) Complexes Ambrish Kumar Srivastava and Neeraj Misra * Department of Physics, University
Competition between Alkalide Characteristics and Nonlinear Optical Properties in OLi 3 M Li 3 O (M = Li, Na, and K) Complexes Ambrish Kumar Srivastava and Neeraj Misra * Department of Physics, University
Vibrational spectra, nonlinear optical properties, NBO analysis and thermodynamic properties of N-phenylethanolamine by density functional method
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 016, 8(6):184-05 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Vibrational spectra, nonlinear optical properties,
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 016, 8(6):184-05 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Vibrational spectra, nonlinear optical properties,
CHAPTER-IV. FT-IR and FT-Raman investigation on m-xylol using ab-initio HF and DFT calculations
 4.1. Introduction CHAPTER-IV FT-IR and FT-Raman investigation on m-xylol using ab-initio HF and DFT calculations m-xylol is a material for thermally stable aramid fibers or alkyd resins [1]. In recent
4.1. Introduction CHAPTER-IV FT-IR and FT-Raman investigation on m-xylol using ab-initio HF and DFT calculations m-xylol is a material for thermally stable aramid fibers or alkyd resins [1]. In recent
Structure, Electronic and Nonlinear Optical Properties of Furyloxazoles and Thienyloxazoles
 Journal of Physics: Conference Series PAPER OPEN ACCESS Structure, Electronic and Nonlinear Optical Properties of Furyls and Thienyls To cite this article: Ozlem Dagli et al 6 J. Phys.: Conf. Ser. 77 View
Journal of Physics: Conference Series PAPER OPEN ACCESS Structure, Electronic and Nonlinear Optical Properties of Furyls and Thienyls To cite this article: Ozlem Dagli et al 6 J. Phys.: Conf. Ser. 77 View
Density Functional Theory Study of Exohedral Carbon Atoms Effect on Electrophilicity of Nicotine: Comparative Analysis
 Computational Chemistry, 2016, 4, 17-31 Published Online January 2016 in SciRes. http://www.scirp.org/journal/cc http://dx.doi.org/10.4236/cc.2016.41003 Density Functional Theory Study of Exohedral Carbon
Computational Chemistry, 2016, 4, 17-31 Published Online January 2016 in SciRes. http://www.scirp.org/journal/cc http://dx.doi.org/10.4236/cc.2016.41003 Density Functional Theory Study of Exohedral Carbon
Growth and Characterization of an Organic Crystal and DFT Studies of 2-amino 5-methyl Pyridinium Salicylate
 ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2016, Vol. 32, No. (4): Pg. 1937-1945 Growth and Characterization
ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2016, Vol. 32, No. (4): Pg. 1937-1945 Growth and Characterization
CHEM 344 Molecular Modeling
 CHEM 344 Molecular Modeling The Use of Computational Chemistry to Support Experimental Organic Chemistry Part 1: Molecular Orbital Theory, Hybridization, & Formal Charge * all calculation data obtained
CHEM 344 Molecular Modeling The Use of Computational Chemistry to Support Experimental Organic Chemistry Part 1: Molecular Orbital Theory, Hybridization, & Formal Charge * all calculation data obtained
Department of Physics, A.V.C. College, Mayiladuthurai, Tamilnadu, India.
 IOSR Journal of Applied Physics (IOSR-JAP) e-issn: 2278-4861.Volume 8, Issue 3 Ver. II (May. - Jun. 2016), PP 01-14 www.iosrjournals.org The Spectroscopic (FT-IR, FT-Raman, NMR & UV-Vis) and first order
IOSR Journal of Applied Physics (IOSR-JAP) e-issn: 2278-4861.Volume 8, Issue 3 Ver. II (May. - Jun. 2016), PP 01-14 www.iosrjournals.org The Spectroscopic (FT-IR, FT-Raman, NMR & UV-Vis) and first order
Quantum Chemical Designing of Novel Organic Non-Linear Optical Compounds
 Quantum Chemical Designing of Novel NLO Compounds Bull. Korean Chem. Soc. 2014, Vol. 35, No. 5 1391 http://dx.doi.org/10.5012/bkcs.2014.35.5.1391 Quantum Chemical Designing of Novel Organic Non-Linear
Quantum Chemical Designing of Novel NLO Compounds Bull. Korean Chem. Soc. 2014, Vol. 35, No. 5 1391 http://dx.doi.org/10.5012/bkcs.2014.35.5.1391 Quantum Chemical Designing of Novel Organic Non-Linear
IR, Raman, First Hyperpolarizability and Computational Study of 1-chloroethyl Benzene
 Material Science Research India Vol. 9(1), 117-121 (2012) IR, Raman, First Hyperpolarizability and Computational Study of 1-chloroethyl Benzene HEMA TRESA VARGHESE¹, C.YOHANNAN PANICKER²* and SHEENA MARY
Material Science Research India Vol. 9(1), 117-121 (2012) IR, Raman, First Hyperpolarizability and Computational Study of 1-chloroethyl Benzene HEMA TRESA VARGHESE¹, C.YOHANNAN PANICKER²* and SHEENA MARY
The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then
 1 The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then filled with the available electrons according to
1 The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then filled with the available electrons according to
Visualization in Chemistry. Giovanni Morelli
 Visualization in Chemistry Giovanni Morelli What is Chemistry? If we provide a good answer to this question maybe we can understand what graphics can do for chemistry and chemists What is Chemistry? A
Visualization in Chemistry Giovanni Morelli What is Chemistry? If we provide a good answer to this question maybe we can understand what graphics can do for chemistry and chemists What is Chemistry? A
Hong Zhou, Guang-Xiang Liu,* Xiao-Feng Wang and Yan Wang * Supporting Information
 Three cobalt(ii) coordination polymers based on V-shaped aromatic polycarboxylates and rigid bis(imidazole) ligand: Syntheses, crystal structures, physical properties and theoretical studies Hong Zhou,
Three cobalt(ii) coordination polymers based on V-shaped aromatic polycarboxylates and rigid bis(imidazole) ligand: Syntheses, crystal structures, physical properties and theoretical studies Hong Zhou,
Available online at WSN 61(2) (2017) EISSN
 Available online at www.worldscientificnews.com WSN 61(2) (2017) 150-185 EISSN 2392-2192 FT-IR, FT-Raman, NMR and U-V Spectral investigation: Computation of vibrational frequency, chemical shifts and electronic
Available online at www.worldscientificnews.com WSN 61(2) (2017) 150-185 EISSN 2392-2192 FT-IR, FT-Raman, NMR and U-V Spectral investigation: Computation of vibrational frequency, chemical shifts and electronic
Quantum Chemical Simulations and Descriptors. Dr. Antonio Chana, Dr. Mosè Casalegno
 Quantum Chemical Simulations and Descriptors Dr. Antonio Chana, Dr. Mosè Casalegno Classical Mechanics: basics It models real-world objects as point particles, objects with negligible size. The motion
Quantum Chemical Simulations and Descriptors Dr. Antonio Chana, Dr. Mosè Casalegno Classical Mechanics: basics It models real-world objects as point particles, objects with negligible size. The motion
Quantum Mechanical Study on the Adsorption of Drug Gentamicin onto γ-fe 2
 ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-00 X CODEN: OJCHEG 015, Vol. 31, No. (3): Pg. 1509-1513 Quantum Mechanical
ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-00 X CODEN: OJCHEG 015, Vol. 31, No. (3): Pg. 1509-1513 Quantum Mechanical
Research & Development Centre, Bharathiar University, Coimbatore , India. 2
 EXPERIMENTAL FT-IR, FT-RAMAN AND THEORETICAL QUANTUM CHEMICAL COMPUTATIONS ON THE MOLECULAR STRUCTURE P-BROMOBENZOTRIFLUORIDE V. Anbu 1, K.A. Vijayalakshmi *, T. Karthick 3, Poonam Tandon 4 1 Research
EXPERIMENTAL FT-IR, FT-RAMAN AND THEORETICAL QUANTUM CHEMICAL COMPUTATIONS ON THE MOLECULAR STRUCTURE P-BROMOBENZOTRIFLUORIDE V. Anbu 1, K.A. Vijayalakshmi *, T. Karthick 3, Poonam Tandon 4 1 Research
Quantum chemical origin of high ionization potential and low electron affinity of Tungsten Hexafluoride
 Journal of Computational Methods in Molecular Design, 2015, 5 (4):142-146 Scholars Research Library (http://scholarsresearchlibrary.com/archive.html) ISSN : 2231-3176 CODEN (USA): JCMMDA Quantum chemical
Journal of Computational Methods in Molecular Design, 2015, 5 (4):142-146 Scholars Research Library (http://scholarsresearchlibrary.com/archive.html) ISSN : 2231-3176 CODEN (USA): JCMMDA Quantum chemical
The linear, nonlinear optical properties and quantum chemical parameters of some sudan dyes
 The linear, nonlinear optical properties and quantum chemical parameters of some sudan dyes Aslı EŞME 1,*, Seda GÜNEŞDOĞDU SAĞDINÇ 2 1 Department of Elementary Science Education, Kocaeli University, Umuttepe
The linear, nonlinear optical properties and quantum chemical parameters of some sudan dyes Aslı EŞME 1,*, Seda GÜNEŞDOĞDU SAĞDINÇ 2 1 Department of Elementary Science Education, Kocaeli University, Umuttepe
NITRATE. HOWRAH (West Bengal) INDIA e Thushara, Neethinagar-64, Pattathanam, KOLLAM (Kerala) INDIA ABSTRACT
 Int. J. Chem. Sci.: 8(1), 2010, 176-182 SPECTROSCOIC INVESTIGATIONS OF ANILINIUM NITRATE C. YOHANNAN PANICKER *, HEMA TRESA VARGHESE a, P. E. EAPEN b, K. RAJU c, SUBARNA GANGULI d, FATHIMA BEEGUM and Y.
Int. J. Chem. Sci.: 8(1), 2010, 176-182 SPECTROSCOIC INVESTIGATIONS OF ANILINIUM NITRATE C. YOHANNAN PANICKER *, HEMA TRESA VARGHESE a, P. E. EAPEN b, K. RAJU c, SUBARNA GANGULI d, FATHIMA BEEGUM and Y.
Supporting information for: First hyperpolarizability of collagen using the. point dipole approximation
 Supporting information for: First hyperpolarizability of collagen using the point dipole approximation Ignat Harczuk, Olav Vahtras, and Hans Ågren KTH Royal Institute of Technology, School of Biotechnology,
Supporting information for: First hyperpolarizability of collagen using the point dipole approximation Ignat Harczuk, Olav Vahtras, and Hans Ågren KTH Royal Institute of Technology, School of Biotechnology,
Computer Laboratory DFT and Frequencies
 Computer Laboratory DFT and Frequencies 1. DFT KEYWORDS FOR DFT METHODS Names for the various pure DFT models are given by combining the names for the exchange and correlation functionals. In some cases,
Computer Laboratory DFT and Frequencies 1. DFT KEYWORDS FOR DFT METHODS Names for the various pure DFT models are given by combining the names for the exchange and correlation functionals. In some cases,
MOLECULAR STRUCTURE, NBO AND HOMO-LUMO ANALYSIS OF QUERCETIN ON SINGLE LAYER GRAPHENE BY DENSITY FUNCTIONAL THEORY
 Digest Journal of Nanomaterials and Biostructures Vol. 13, No. 1, January - March 2018, p. 97-105 MOLECULAR STRUCTURE, NBO AND HOMO-LUMO ANALYSIS OF QUERCETIN ON SINGLE LAYER GRAPHENE BY DENSITY FUNCTIONAL
Digest Journal of Nanomaterials and Biostructures Vol. 13, No. 1, January - March 2018, p. 97-105 MOLECULAR STRUCTURE, NBO AND HOMO-LUMO ANALYSIS OF QUERCETIN ON SINGLE LAYER GRAPHENE BY DENSITY FUNCTIONAL
ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal.
 ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2014, Vol. 30, No. (1): Pg. 345-350 DFT Study on 4(5)-Imidazole-carbaldehyde-N(5)-
ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2014, Vol. 30, No. (1): Pg. 345-350 DFT Study on 4(5)-Imidazole-carbaldehyde-N(5)-
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer. Lecture 31, April 12, 2006
 Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 31, April 12, 2006 (Some material in this lecture has been adapted from Cramer, C.
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 31, April 12, 2006 (Some material in this lecture has been adapted from Cramer, C.
Structural and Theoretical Studies of 2-amino-3- nitropyridine
 ISSN: 0973-4945; CODEN ECJHAO E-Journal of Chemistry http://www.ejchem.net 2012, 9(4), 2191-2204 Structural and Theoretical Studies of 2-amino-3- nitropyridine N. S. AL-HOKBANY a, A. A. DAHY b, I. KH.WARAD
ISSN: 0973-4945; CODEN ECJHAO E-Journal of Chemistry http://www.ejchem.net 2012, 9(4), 2191-2204 Structural and Theoretical Studies of 2-amino-3- nitropyridine N. S. AL-HOKBANY a, A. A. DAHY b, I. KH.WARAD
CHAPTER INTRODUCTION
 CHAPTER 3 A SCALED QUANTUM MECHANICAL APPROACH OF VIBRATIONAL ANALYSIS OF O-TOLUNITRILE BASED ON FTIR AND FT RAMAN SPECTRA, AB INITIO, HARTREE FOCK AND DFT METHODS 3.1. INTRODUCTION o-tolunitrile or ortho
CHAPTER 3 A SCALED QUANTUM MECHANICAL APPROACH OF VIBRATIONAL ANALYSIS OF O-TOLUNITRILE BASED ON FTIR AND FT RAMAN SPECTRA, AB INITIO, HARTREE FOCK AND DFT METHODS 3.1. INTRODUCTION o-tolunitrile or ortho
Theoretical Approach on structural aspects of antiepileptic agent indoline-2,3- dione-3-oxime by arguslab 4 software
 Available online at www.scientiaresearchlibrary.com Scientia Research Library ISSN 2348-0408 USA CODEN: JACOGN Journal of Applied Chemistry, 2014, 2 (1):92-101 (http://www.scientiaresearchlibrary.com/arhcive.php)
Available online at www.scientiaresearchlibrary.com Scientia Research Library ISSN 2348-0408 USA CODEN: JACOGN Journal of Applied Chemistry, 2014, 2 (1):92-101 (http://www.scientiaresearchlibrary.com/arhcive.php)
Australian Journal of Basic and Applied Sciences
 AENSI Journals Australian Journal of Basic and Applied Sciences ISSN:1991-8178 Journal home page: www.ajbasweb.com Theoretical Study for the Effect of Hydroxyl Radical on the Electronic Properties of Cyclobutadiene
AENSI Journals Australian Journal of Basic and Applied Sciences ISSN:1991-8178 Journal home page: www.ajbasweb.com Theoretical Study for the Effect of Hydroxyl Radical on the Electronic Properties of Cyclobutadiene
Journal of Computational Methods in Molecular Design, 2013, 3 (1):1-8. Scholars Research Library (
 Journal of Computational Methods in Molecular Design, 2013, 3 (1):1-8 Scholars Research Library (http://scholarsresearchlibrary.com/archive.html) ISSN : 2231-3176 CODEN (USA): JCMMDA Theoretical study
Journal of Computational Methods in Molecular Design, 2013, 3 (1):1-8 Scholars Research Library (http://scholarsresearchlibrary.com/archive.html) ISSN : 2231-3176 CODEN (USA): JCMMDA Theoretical study
Structural Properties, Natural Bond Orbital, Theory Functional Calculations (DFT), and Energies for the α Halorganic Compounds
 Current World Environment Vol. 7(2), 221-226 (2012) Structural Properties, Natural Bond Orbital, Theory Functional Calculations (DFT), and Energies for the α Halorganic Compounds NAJLA SEIDY and SHAHRIAR
Current World Environment Vol. 7(2), 221-226 (2012) Structural Properties, Natural Bond Orbital, Theory Functional Calculations (DFT), and Energies for the α Halorganic Compounds NAJLA SEIDY and SHAHRIAR
Journal of Advanced Scientific Research DFT APPROACH ON CORROSION INHIBITION PERFORMANCE OF THIOSEMICARBAZONE DERIVATIVES ON METALLIC IRON
 Rajendran et al., J Adv Sci Res, 2016, 7(1): 32-37 32 Journal of Advanced Scientific Research Available online through http://www.sciensage.info/jasr ISSN 0976-9595 Research Article DFT APPROACH ON CORROSION
Rajendran et al., J Adv Sci Res, 2016, 7(1): 32-37 32 Journal of Advanced Scientific Research Available online through http://www.sciensage.info/jasr ISSN 0976-9595 Research Article DFT APPROACH ON CORROSION
Molecular Orbital Theory. Molecular Orbital Theory: Electrons are located in the molecule, not held in discrete regions between two bonded atoms
 Molecular Orbital Theory Valence Bond Theory: Electrons are located in discrete pairs between specific atoms Molecular Orbital Theory: Electrons are located in the molecule, not held in discrete regions
Molecular Orbital Theory Valence Bond Theory: Electrons are located in discrete pairs between specific atoms Molecular Orbital Theory: Electrons are located in the molecule, not held in discrete regions
Density Functional Theory Investigation of The Physical Properties of Dicyano Pyridazine Molecules
 Density Functional Theory Investigation of The Physical Properties of Dicyano Pyridazine Molecules Fouad N. Ajeel 1, Alaa M. Khudhair 2, Anees A. Mohammed 3 1,2,3 Department of Physics, College of Sciences,
Density Functional Theory Investigation of The Physical Properties of Dicyano Pyridazine Molecules Fouad N. Ajeel 1, Alaa M. Khudhair 2, Anees A. Mohammed 3 1,2,3 Department of Physics, College of Sciences,
FT-IR, FT-Raman and Computational Study of p-acetylbenzonitrile
 Est. 1984 ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2013, Vol. 29, No. (1): Pg. 291-296 FT-IR, FT-Raman
Est. 1984 ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2013, Vol. 29, No. (1): Pg. 291-296 FT-IR, FT-Raman
Chapter 6. Electronic spectra and HOMO-LUMO studies on Nickel, copper substituted Phthalocyanine for solar cell applications
 Chapter 6 Electronic spectra and HOMO-LUMO studies on Nickel, copper substituted Phthalocyanine for solar cell applications 6.1 Structures of Ni, Cu substituted Phthalocyanine Almost all of the metals
Chapter 6 Electronic spectra and HOMO-LUMO studies on Nickel, copper substituted Phthalocyanine for solar cell applications 6.1 Structures of Ni, Cu substituted Phthalocyanine Almost all of the metals
Chapter 2. Methodology
 Chapter 2 Methodology Chapter 2 Methodology NORMA AUREA RANGEL-VÁZQUEZ 1 FRANCISCO RODRÍGUEZ FÉLIX 2 1 División de Estudios de Posgrado e Investigación del Instituto Tecnológico de Aguascalientes, Ave.
Chapter 2 Methodology Chapter 2 Methodology NORMA AUREA RANGEL-VÁZQUEZ 1 FRANCISCO RODRÍGUEZ FÉLIX 2 1 División de Estudios de Posgrado e Investigación del Instituto Tecnológico de Aguascalientes, Ave.
CHEM 344 Molecular Modeling
 CHEM 344 Molecular Modeling The Use of Computational Chemistry to Support Experimental Organic Chemistry Day 1 all calculation data obtained from Gaussian09 using B3LYP/6-31G(d) unless otherwise noted.
CHEM 344 Molecular Modeling The Use of Computational Chemistry to Support Experimental Organic Chemistry Day 1 all calculation data obtained from Gaussian09 using B3LYP/6-31G(d) unless otherwise noted.
Supporting Information
 Supporting Information Remote Stereoinductive Intramolecular Nitrile Oxide Cycloaddition: Asymmetric Total Synthesis and Structure Revision of ( )-11 -Hydroxycurvularin Hyeonjeong Choe, Thuy Trang Pham,
Supporting Information Remote Stereoinductive Intramolecular Nitrile Oxide Cycloaddition: Asymmetric Total Synthesis and Structure Revision of ( )-11 -Hydroxycurvularin Hyeonjeong Choe, Thuy Trang Pham,
with the larger dimerization energy also exhibits the larger structural changes.
 A7. Looking at the image and table provided below, it is apparent that the monomer and dimer are structurally almost identical. Although angular and dihedral data were not included, these data are also
A7. Looking at the image and table provided below, it is apparent that the monomer and dimer are structurally almost identical. Although angular and dihedral data were not included, these data are also
IFM Chemistry Computational Chemistry 2010, 7.5 hp LAB2. Computer laboratory exercise 1 (LAB2): Quantum chemical calculations
 Computer laboratory exercise 1 (LAB2): Quantum chemical calculations Introduction: The objective of the second computer laboratory exercise is to get acquainted with a program for performing quantum chemical
Computer laboratory exercise 1 (LAB2): Quantum chemical calculations Introduction: The objective of the second computer laboratory exercise is to get acquainted with a program for performing quantum chemical
Electronic structures of one-dimension carbon nano wires and rings
 IOP Publishing Journal of Physics: Conference Series 61 (2007) 252 256 doi:10.1088/1742-6596/61/1/051 International Conference on Nanoscience and Technology (ICN&T 2006) Electronic structures of one-dimension
IOP Publishing Journal of Physics: Conference Series 61 (2007) 252 256 doi:10.1088/1742-6596/61/1/051 International Conference on Nanoscience and Technology (ICN&T 2006) Electronic structures of one-dimension
Molecular Aggregation
 Molecular Aggregation Structure Analysis and Molecular Simulation of Crystals and Liquids ANGELO GAVEZZOTTI University of Milano OXFORD UNIVERSITY PRESS Contents PART I FUNDAMENTALS 1 The molecule: structure,
Molecular Aggregation Structure Analysis and Molecular Simulation of Crystals and Liquids ANGELO GAVEZZOTTI University of Milano OXFORD UNIVERSITY PRESS Contents PART I FUNDAMENTALS 1 The molecule: structure,
2x (x 2 + y 2 + 1) 2 2y. (x 2 + y 2 + 1) 4. 4xy. (1, 1)(x 1) + (1, 1)(y + 1) (1, 1)(x 1)(y + 1) 81 x y y + 7.
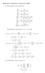 Homework 8 Solutions, November 007. (1 We calculate some derivatives: f x = f y = x (x + y + 1 y (x + y + 1 x = (x + y + 1 4x (x + y + 1 4 y = (x + y + 1 4y (x + y + 1 4 x y = 4xy (x + y + 1 4 Substituting
Homework 8 Solutions, November 007. (1 We calculate some derivatives: f x = f y = x (x + y + 1 y (x + y + 1 x = (x + y + 1 4x (x + y + 1 4 y = (x + y + 1 4y (x + y + 1 4 x y = 4xy (x + y + 1 4 Substituting
Supporting Information
 Supporting Information Tuning of Second-Order Nonlinear Optical Response Properties of Aryl-Substituted Boron-Dipyrromethene Dyes: Unidirectional Charge Transfer Coupled with Structural Tailoring Ramprasad
Supporting Information Tuning of Second-Order Nonlinear Optical Response Properties of Aryl-Substituted Boron-Dipyrromethene Dyes: Unidirectional Charge Transfer Coupled with Structural Tailoring Ramprasad
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for CrystEngComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Configurational and energetical study of the (100) and
Electronic Supplementary Material (ESI) for CrystEngComm. This journal is The Royal Society of Chemistry 2014 Electronic Supplementary Information Configurational and energetical study of the (100) and
Valence bond theory accounts, at least qualitatively, for the stability of the covalent bond in terms of overlapping atomic orbitals.
 Molecular Orbital Theory Valence bond theory accounts, at least qualitatively, for the stability of the covalent bond in terms of overlapping atomic orbitals. Using the concept of hybridization, valence
Molecular Orbital Theory Valence bond theory accounts, at least qualitatively, for the stability of the covalent bond in terms of overlapping atomic orbitals. Using the concept of hybridization, valence
Notes. Rza Abbasoglu*, Abdurrahman Atalay & Ahmet Şenocak. Indian Journal of Chemistry Vol. 53A, March, 2014, pp
 Indian Journal of Chemistry Vol. 53A, March, 2014, pp. 288-293 Notes DFT investigations of the Diels-Alder reaction of fulvene with tetracyclo[6.2.2.1 3,6.0 2,7 ]trideca-4,9,11-triene- 9,10-dicarboxylic
Indian Journal of Chemistry Vol. 53A, March, 2014, pp. 288-293 Notes DFT investigations of the Diels-Alder reaction of fulvene with tetracyclo[6.2.2.1 3,6.0 2,7 ]trideca-4,9,11-triene- 9,10-dicarboxylic
A Computational Investigation of a Series Ofdimeric and Trimetric Tetrathiafulvalenes Using DFT Method
 IOSR Journal of Applied Chemistry (IOSR-JAC) e-issn: 2278-5736.Volume 11, Issue 4 Ver. I (April. 2018), PP 55-64 www.iosrjournals.org A Computational Investigation of a Series Ofdimeric and Trimetric Tetrathiafulvalenes
IOSR Journal of Applied Chemistry (IOSR-JAC) e-issn: 2278-5736.Volume 11, Issue 4 Ver. I (April. 2018), PP 55-64 www.iosrjournals.org A Computational Investigation of a Series Ofdimeric and Trimetric Tetrathiafulvalenes
Journal of Chemical and Pharmaceutical Research
 Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 0975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 2011, 3(4): 589-595 Altering the electronic properties of adamantane
Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 0975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 2011, 3(4): 589-595 Altering the electronic properties of adamantane
High-power Broadband Organic THz Generator
 Supplementary Information High-power Broadband Organic THz Generator Jae-Hyeok Jeong,1, Bong-Joo Kang,2, Ji-Soo Kim 1, Mojca Jazbinsek 3, Seung-Heon Lee 1, Seung-Chul Lee 1, In-Hyung Baek 2, Hoseop Yun
Supplementary Information High-power Broadband Organic THz Generator Jae-Hyeok Jeong,1, Bong-Joo Kang,2, Ji-Soo Kim 1, Mojca Jazbinsek 3, Seung-Heon Lee 1, Seung-Chul Lee 1, In-Hyung Baek 2, Hoseop Yun
CHEM 2010 Symmetry, Electronic Structure and Bonding Winter Numbering of Chapters and Assigned Problems
 CHEM 2010 Symmetry, Electronic Structure and Bonding Winter 2011 Numbering of Chapters and Assigned Problems The following table shows the correspondence between the chapter numbers in the full book (Physical
CHEM 2010 Symmetry, Electronic Structure and Bonding Winter 2011 Numbering of Chapters and Assigned Problems The following table shows the correspondence between the chapter numbers in the full book (Physical
Chapter 5. Molecular Orbitals
 Chapter 5. Molecular Orbitals MO from s, p, d, orbitals: - Fig.5.1, 5.2, 5.3 Homonuclear diatomic molecules: - Fig. 5.7 - Para- vs. Diamagnetic Heteronuclear diatomic molecules: - Fig. 5.14 - ex. CO Hybrid
Chapter 5. Molecular Orbitals MO from s, p, d, orbitals: - Fig.5.1, 5.2, 5.3 Homonuclear diatomic molecules: - Fig. 5.7 - Para- vs. Diamagnetic Heteronuclear diatomic molecules: - Fig. 5.14 - ex. CO Hybrid
Computational quantum chemistry study of a self-assembled Zn(II) porphyrin box
 Internet Electronic Conference of Molecular Design 2003, ovember 23 December 6 Computational quantum chemistry study of a self-assembled (II) porphyrin box Fabio Pichierri 1, * 1 COE Laboratory, Tohoku
Internet Electronic Conference of Molecular Design 2003, ovember 23 December 6 Computational quantum chemistry study of a self-assembled (II) porphyrin box Fabio Pichierri 1, * 1 COE Laboratory, Tohoku
Reaction Mechanism of Polar Diels-Alder Reactions between 3-Nitrofuran and different Dienes. A Theoretical Study.
 Reaction Mechanism of Polar Diels-Alder Reactions between 3-Nitrofuran and different Dienes. A Theoretical Study. MAUR CAINELLI, CARLA RMACHEA, PEDR MANCINI, MARÍA KNEETEMAN* Área Química rgánica Departamento
Reaction Mechanism of Polar Diels-Alder Reactions between 3-Nitrofuran and different Dienes. A Theoretical Study. MAUR CAINELLI, CARLA RMACHEA, PEDR MANCINI, MARÍA KNEETEMAN* Área Química rgánica Departamento
Density Functional Theory Study on Mechanism of Forming Spiro-Geheterocyclic Ring Compound from Me 2 Ge=Ge: and Acetaldehyde
 CHINESE JURNAL F CHEMICAL PHYSICS VLUME 26, NUMBER 1 FEBRUARY 27, 2013 ARTICLE Density Functional Theory Study on Mechanism of Forming Spiro-Geheterocyclic Ring Compound from Me 2 Ge=Ge: and Acetaldehyde
CHINESE JURNAL F CHEMICAL PHYSICS VLUME 26, NUMBER 1 FEBRUARY 27, 2013 ARTICLE Density Functional Theory Study on Mechanism of Forming Spiro-Geheterocyclic Ring Compound from Me 2 Ge=Ge: and Acetaldehyde
NMR and IR spectra & vibrational analysis
 Lab 5: NMR and IR spectra & vibrational analysis A brief theoretical background 1 Some of the available chemical quantum methods for calculating NMR chemical shifts are based on the Hartree-Fock self-consistent
Lab 5: NMR and IR spectra & vibrational analysis A brief theoretical background 1 Some of the available chemical quantum methods for calculating NMR chemical shifts are based on the Hartree-Fock self-consistent
DFT STUDY OF Co 2+ AND Fe 2+ - URACIL COMPLEXES IN THE GAS PHASE
 INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article DFT STUDY OF Co 2+ AND Fe 2+ - URACIL COMPLEXES IN THE GAS PHASE Raghab Parajuli* Department
INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article DFT STUDY OF Co 2+ AND Fe 2+ - URACIL COMPLEXES IN THE GAS PHASE Raghab Parajuli* Department
Electronic structure theory: Fundamentals to frontiers. VI. Analysis and more.
 Electronic structure theory: Fundamentals to frontiers. VI. Analysis and more. MARTIN HEAD-GORDON Department of Chemistry, University of California, Berkeley, and, Chemical Sciences Division, Lawrence
Electronic structure theory: Fundamentals to frontiers. VI. Analysis and more. MARTIN HEAD-GORDON Department of Chemistry, University of California, Berkeley, and, Chemical Sciences Division, Lawrence
Atomic and molecular interaction forces in biology
 Atomic and molecular interaction forces in biology 1 Outline Types of interactions relevant to biology Van der Waals interactions H-bond interactions Some properties of water Hydrophobic effect 2 Types
Atomic and molecular interaction forces in biology 1 Outline Types of interactions relevant to biology Van der Waals interactions H-bond interactions Some properties of water Hydrophobic effect 2 Types
Journal of Chemical and Pharmaceutical Research
 Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 0975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 2011, 3(1):572-583 Ground state properties: Gross orbital charges,
Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 0975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 2011, 3(1):572-583 Ground state properties: Gross orbital charges,
Journal of Chemical and Pharmaceutical Research, 2012, 4(6): Research Article
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2012, 4(6): 3287-3296 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Vibrational, Structural and Electronic properties
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2012, 4(6): 3287-3296 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Vibrational, Structural and Electronic properties
Lecture 4: Band theory
 Lecture 4: Band theory Very short introduction to modern computational solid state chemistry Band theory of solids Molecules vs. solids Band structures Analysis of chemical bonding in Reciprocal space
Lecture 4: Band theory Very short introduction to modern computational solid state chemistry Band theory of solids Molecules vs. solids Band structures Analysis of chemical bonding in Reciprocal space
Journal of Chemical and Pharmaceutical Research, 2015, 7(2): Research Article
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(2):641-645 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Synthesis, characterization and theoretical study
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(2):641-645 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Synthesis, characterization and theoretical study
Solutions and Intermolecular Forces
 Solutions and Intermolecular Forces REVIEW Chemical Bonds Three basic types of bonds: Ionic Electrostatic attraction between ions Covalent Sharing of electrons Metallic Metal atoms bonded to several other
Solutions and Intermolecular Forces REVIEW Chemical Bonds Three basic types of bonds: Ionic Electrostatic attraction between ions Covalent Sharing of electrons Metallic Metal atoms bonded to several other
Structural and Electronic Properties of Folic Acid Adsorption on the Carbon Nanotubes: A Density Functional Theory Study
 ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2015, Vol. 31, No. (1): Pg. 345-351 Structural and Electronic
ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2015, Vol. 31, No. (1): Pg. 345-351 Structural and Electronic
Ab Initio Study of the Dimers of Nodifloridin B
 Ab Initio Study of the Dimers of Nodifloridin B Liliana Mammino Abstract Nodifloridin B is an acylated phloroglucinol derivative in which both the acyl chain and a substituent in meta to the acyl chain
Ab Initio Study of the Dimers of Nodifloridin B Liliana Mammino Abstract Nodifloridin B is an acylated phloroglucinol derivative in which both the acyl chain and a substituent in meta to the acyl chain
Chemistry General Chemistry II Spring 2006 Test #1
 Name: KEY Chemistry 122-04 -- General Chemistry II Spring 2006 Test #1 Organic molecules, molecular structure and bonding theory, solubility, (breathe now) phase transitions, spectroscopy, and kinetics
Name: KEY Chemistry 122-04 -- General Chemistry II Spring 2006 Test #1 Organic molecules, molecular structure and bonding theory, solubility, (breathe now) phase transitions, spectroscopy, and kinetics
NH 3 H 2 O N 2. Why do they make chemical bonds? Molecular Orbitals
 N 2 NH 3 H 2 O Why do they make chemical bonds? 5 Molecular Orbitals Why do they make chemical bonds? Stabilization Bond energy Types of Chemical Bonds Metallic Bond Ionic Bond Covalent Bond Covalent Bond
N 2 NH 3 H 2 O Why do they make chemical bonds? 5 Molecular Orbitals Why do they make chemical bonds? Stabilization Bond energy Types of Chemical Bonds Metallic Bond Ionic Bond Covalent Bond Covalent Bond
Supporting Information
 Supporting Information Three Polymorphic Forms of Ciprofloxacin Maleate: Formation Pathways, Crystal Structures, Calculations and Thermodynamic Stability Aspects Artem O. Surov a, Andrei V. Churakov b,
Supporting Information Three Polymorphic Forms of Ciprofloxacin Maleate: Formation Pathways, Crystal Structures, Calculations and Thermodynamic Stability Aspects Artem O. Surov a, Andrei V. Churakov b,
Why Is Molecular Interaction Important in Our Life
 Why Is Molecular Interaction Important in ur Life QuLiS and Graduate School of Science iroshima University http://www.nabit.hiroshima-u.ac.jp/iwatasue/indexe.htm Suehiro Iwata Sept. 29, 2007 Department
Why Is Molecular Interaction Important in ur Life QuLiS and Graduate School of Science iroshima University http://www.nabit.hiroshima-u.ac.jp/iwatasue/indexe.htm Suehiro Iwata Sept. 29, 2007 Department
First order molecular hyperpolarizabilities and intramolecular charge transfer from vibrational spectra of NLO material: 2,6-dichloro-4-nitroaniline
 Indian Journal of Pure & Applied Physics Vol. 51, September 2013, pp. 601-614 First order molecular hyperpolarizabilities and intramolecular charge transfer from vibrational spectra of NLO material: 2,6-dichloro-4-nitroaniline
Indian Journal of Pure & Applied Physics Vol. 51, September 2013, pp. 601-614 First order molecular hyperpolarizabilities and intramolecular charge transfer from vibrational spectra of NLO material: 2,6-dichloro-4-nitroaniline
Exchange Correlation Functional Investigation of RT-TDDFT on a Sodium Chloride. Dimer. Philip Straughn
 Exchange Correlation Functional Investigation of RT-TDDFT on a Sodium Chloride Dimer Philip Straughn Abstract Charge transfer between Na and Cl ions is an important problem in physical chemistry. However,
Exchange Correlation Functional Investigation of RT-TDDFT on a Sodium Chloride Dimer Philip Straughn Abstract Charge transfer between Na and Cl ions is an important problem in physical chemistry. However,
COMPUTATIONAL STUDY OF H-A-X (A = GROUP TWO ATOMS, X = F, Cl, Br) MOLECULES
 Int. J. Chem. Sci.: 8(2), 2010, 914-922 COMPUTATIONAL STUDY OF H-A-X (A = GROUP TWO ATOMS, X = F, Cl, Br) MOLECULES C. YOHANNAN PANICKER *, S. DEEPTHI, HEMA TRESA VARGHESE a and Y. SHEENA MARY a Department
Int. J. Chem. Sci.: 8(2), 2010, 914-922 COMPUTATIONAL STUDY OF H-A-X (A = GROUP TWO ATOMS, X = F, Cl, Br) MOLECULES C. YOHANNAN PANICKER *, S. DEEPTHI, HEMA TRESA VARGHESE a and Y. SHEENA MARY a Department
Ab initio and DFT study of the thermodynamic properties and nuclear magnetic resonance of (4, 4) armchair AlN nanotubes
 Ab initio and DFT study of the thermodynamic properties and nuclear magnetic resonance of (4, 4) armchair AlN nanotubes Elham Pournamdari * Department of science, Islamshahr Branch, Islamic Azad University,
Ab initio and DFT study of the thermodynamic properties and nuclear magnetic resonance of (4, 4) armchair AlN nanotubes Elham Pournamdari * Department of science, Islamshahr Branch, Islamic Azad University,
MO Calculation for a Diatomic Molecule. /4 0 ) i=1 j>i (1/r ij )
 MO Calculation for a Diatomic Molecule Introduction The properties of any molecular system can in principle be found by looking at the solutions to the corresponding time independent Schrodinger equation
MO Calculation for a Diatomic Molecule Introduction The properties of any molecular system can in principle be found by looking at the solutions to the corresponding time independent Schrodinger equation
Spectroscopic investigations, DFT computations and other molecular properties of 2,4-dimethylbenzoic acid
 Indian Journal of Pure & Applied Physics Vol. 55, August 2017, pp. 541-550 Spectroscopic investigations, DFT computations and other molecular properties of 2,4-dimethylbenzoic acid E Gobinath a *, S Jeyavijayan
Indian Journal of Pure & Applied Physics Vol. 55, August 2017, pp. 541-550 Spectroscopic investigations, DFT computations and other molecular properties of 2,4-dimethylbenzoic acid E Gobinath a *, S Jeyavijayan
Hyperconjugative Effect on the Electronic Wavefunctions of Ethanol. University of Science and Technology of China, Hefei, , China
 Hyperconjugative Effect on the Electronic Wavefunctions of Ethanol Xiangjun Chen, 1,2,a) Fang Wu, 1,2 Mi Yan, 1,2 Hai-Bei Li, 1,3 Shan Xi Tian, 1,3,a) Xu Shan, 1,2 Kedong Wang, 1,2 Zhongjun Li, 1,2 and
Hyperconjugative Effect on the Electronic Wavefunctions of Ethanol Xiangjun Chen, 1,2,a) Fang Wu, 1,2 Mi Yan, 1,2 Hai-Bei Li, 1,3 Shan Xi Tian, 1,3,a) Xu Shan, 1,2 Kedong Wang, 1,2 Zhongjun Li, 1,2 and
2. Polar Covalent Bonds: Acids and Bases
 2. Polar Covalent Bonds: Acids and Bases Based on McMurry s Organic Chemistry, 6 th edition, Chapter 2 2003 Ronald Kluger Department of Chemistry University of Toronto 2.1 Polar Covalent Bonds: Electronegativity!
2. Polar Covalent Bonds: Acids and Bases Based on McMurry s Organic Chemistry, 6 th edition, Chapter 2 2003 Ronald Kluger Department of Chemistry University of Toronto 2.1 Polar Covalent Bonds: Electronegativity!
NWChem: Hartree-Fock, Density Functional Theory, Time-Dependent Density Functional Theory
 NWChem: Hartree-Fock, Density Functional Theory, Time-Depent Density Functional Theory Hartree-Fock! Functionality! Input! Wavefunctions! Initial MO vectors! Direct and semidirect algorithms! Convergence,
NWChem: Hartree-Fock, Density Functional Theory, Time-Depent Density Functional Theory Hartree-Fock! Functionality! Input! Wavefunctions! Initial MO vectors! Direct and semidirect algorithms! Convergence,
Organic Chemistry 6 th Edition Paula Yurkanis Bruice. Chapter 1. Electronic Structure and Bonding. Acids and Bases Pearson Education, Inc.
 Organic Chemistry 6 th Edition Paula Yurkanis Bruice Chapter 1 Electronic Structure and Bonding Acids and Bases 2011 Pearson Education, Inc. 1 Organic Chemistry Carbon-containing compounds were once considered
Organic Chemistry 6 th Edition Paula Yurkanis Bruice Chapter 1 Electronic Structure and Bonding Acids and Bases 2011 Pearson Education, Inc. 1 Organic Chemistry Carbon-containing compounds were once considered
The Boron Buckyball has an Unexpected T h Symmetry
 The Boron Buckyball has an Unexpected T h Symmetry G. Gopakumar, Minh Tho Nguyen, and Arnout Ceulemans* Department of Chemistry and Institute for Nanoscale Physics and Chemistry, University of Leuven,
The Boron Buckyball has an Unexpected T h Symmetry G. Gopakumar, Minh Tho Nguyen, and Arnout Ceulemans* Department of Chemistry and Institute for Nanoscale Physics and Chemistry, University of Leuven,
NATURAL BOND ORBITAL (NBO) POPULATION ANALYSIS OF AN ENERGETIC MOLECULE 1-PHENYL-2- NITROGUANIDINE
 Int. J. Chem. Sci.: 14(4), 2016, 2029-2050 ISSN 0972-768X www.sadgurupublications.com NATURAL BOND ORBITAL (NBO) POPULATION ANALYSIS OF AN ENERGETIC MOLECULE 1-PHENYL-2- NITROGUANIDINE CHINNIAGOUNDER THEIVARASU
Int. J. Chem. Sci.: 14(4), 2016, 2029-2050 ISSN 0972-768X www.sadgurupublications.com NATURAL BOND ORBITAL (NBO) POPULATION ANALYSIS OF AN ENERGETIC MOLECULE 1-PHENYL-2- NITROGUANIDINE CHINNIAGOUNDER THEIVARASU
Spectroscopic properties of dipicolinic acid and its dianion
 Chemical Physics 322 (2006) 254 268 www.elsevier.com/locate/chemphys Spectroscopic properties of dipicolinic acid and its dianion John Rui-Hua Xie a, Vedene H. Smith Jr. b, Roland E. Allen a, * a Department
Chemical Physics 322 (2006) 254 268 www.elsevier.com/locate/chemphys Spectroscopic properties of dipicolinic acid and its dianion John Rui-Hua Xie a, Vedene H. Smith Jr. b, Roland E. Allen a, * a Department
CHAPTER 5 FT INFRARED, FT RAMAN, VIBRATIONAL ASSIGNMENTS AND QUANTUM CHEMICAL STUDIES OF NICOTINIC ACID (NIASIN)
 CHAPTER 5 FT INFRARED, FT RAMAN, VIBRATIONAL ASSIGNMENTS AND QUANTUM CHEMICAL STUDIES OF NICOTINIC ACID (NIASIN) 5.1. INTRODUCTION Nicotinic acid widely known as Niacin are also known as vitamin B 3 and
CHAPTER 5 FT INFRARED, FT RAMAN, VIBRATIONAL ASSIGNMENTS AND QUANTUM CHEMICAL STUDIES OF NICOTINIC ACID (NIASIN) 5.1. INTRODUCTION Nicotinic acid widely known as Niacin are also known as vitamin B 3 and
Chapter 2: Acids and Bases
 1. Which of the following statements is a correct definition for a Brønsted-Lowry acid? A) Proton acceptor C) Electron pair acceptor B) Electron pair donor D) Proton donor 2. Which of the following statements
1. Which of the following statements is a correct definition for a Brønsted-Lowry acid? A) Proton acceptor C) Electron pair acceptor B) Electron pair donor D) Proton donor 2. Which of the following statements
EXAM INFORMATION. Radial Distribution Function: B is the normalization constant. d dx. p 2 Operator: Heisenberg Uncertainty Principle:
 EXAM INFORMATION Radial Distribution Function: P() r RDF() r Br R() r B is the normalization constant., p Operator: p ^ d dx Heisenberg Uncertainty Principle: n ax n! Integrals: xe dx n1 a x p Particle
EXAM INFORMATION Radial Distribution Function: P() r RDF() r Br R() r B is the normalization constant., p Operator: p ^ d dx Heisenberg Uncertainty Principle: n ax n! Integrals: xe dx n1 a x p Particle
Trace Solvent as a Predominant Factor to Tune Dipeptide. Self-Assembly
 Trace Solvent as a Predominant Factor to Tune Dipeptide Self-Assembly Juan Wang,, Kai Liu,,, Linyin Yan,, Anhe Wang, Shuo Bai, and Xuehai Yan *,, National Key Laboratory of Biochemical Engineering, Institute
Trace Solvent as a Predominant Factor to Tune Dipeptide Self-Assembly Juan Wang,, Kai Liu,,, Linyin Yan,, Anhe Wang, Shuo Bai, and Xuehai Yan *,, National Key Laboratory of Biochemical Engineering, Institute
Are the Bader Laplacian and the Bohm Quantum Potential Equivalent?
 Are the Bader Laplacian and the Bohm Quantum Potential Equivalent? Creon Levit & Jack Sarfatti NASA Ames Research Center, creon@nas.nasa.gov Internet Science Eductaion Project, sarfatti@well.com ABSTRACT
Are the Bader Laplacian and the Bohm Quantum Potential Equivalent? Creon Levit & Jack Sarfatti NASA Ames Research Center, creon@nas.nasa.gov Internet Science Eductaion Project, sarfatti@well.com ABSTRACT
Reinvestigation of Hydrogen Bond Effects on the Polarizability and Hyperpolarizability of Urea Molecular Clusters
 8954 J. Phys. Chem. B 2002, 106, 8954-8958 Reinvestigation of Hydrogen Bond Effects on the Polarizability and Hyperpolarizability of Urea Molecular Clusters Kechen Wu* Key State Laboratory of Structural
8954 J. Phys. Chem. B 2002, 106, 8954-8958 Reinvestigation of Hydrogen Bond Effects on the Polarizability and Hyperpolarizability of Urea Molecular Clusters Kechen Wu* Key State Laboratory of Structural
