A Computational Investigation of a Series Ofdimeric and Trimetric Tetrathiafulvalenes Using DFT Method
|
|
|
- Emory Rodgers
- 5 years ago
- Views:
Transcription
1 IOSR Journal of Applied Chemistry (IOSR-JAC) e-issn: Volume 11, Issue 4 Ver. I (April. 2018), PP A Computational Investigation of a Series Ofdimeric and Trimetric Tetrathiafulvalenes Using DFT Method Tahar Abbaz 1*, Amel Bendjeddou 1 and Didier Villemin 2 1 Laboratory of Aquatic and Terrestrial Ecosystems, Org. and Bioorg. Chem. Group, University of Mohamed- Cherif Messaadia, Souk Ahras, 41000, Algeria 2 Laboratory of Molecular and Thio-Organic Chemistry, UMR CNRS 6507, INC3M, FR 3038, Labex EMC3, ensicaen & University of Caen, Caen 14050, France Corresponding author: Tahar Abbaz Abstract: In this perspective article, density functional theory (DFT) calculations are used to investigate themolecular stability and bond strengths of dimeric and trimeric tetrathiafulvalenes 1-4 by applying the natural bond orbital analysis. The indication about the size, shape, charge density distribution and site of chemical reactivity of the molecules has been obtained by mapping electron density with molecular electrostatic potential (MEP).The HOMO and LUMO energies determined showed that the serious charge transfer occurs in the title molecules studied. NLO properties such as dipole moment, polarizability, first static hyperpolarizability were also calculated to predict their Non-linear optical behavior. Keywords: tetrathiafulvalenes; density functional theory; computational chemistry; electronic structure; quantum chemical calculations Date of Subissions: Date of acceptante: I. Introduction Tetrathiafulvalene (TTF) is a fascinating planar π-electron- donor molecule with a broad range of potential applications [1]. Tetrathiafulvalene (TTF) derivatives [2] are very versatile electro-active molecules widely used as building blocks to prepare organic metals, for supramolecular functions [3] and in molecular electronics [4].Recently, donor acceptor type dyads using a tetrathiafulvalene (TTF) framework have received considerable interest as materials for fluorescence switches, chemical sensors, molecular rectification, and photovoltaic and NLO applications [5-10]. Quantum chemical calculation is one of the recent emerging tools in unraveling physical and chemical properties of molecules. Recently, density functional theory (DFT) has been emerged as a powerful tool for study of electronic properties of polyatomic molecules. The application of DFT to chemical systems has received much attention because of faster convergence in time than traditional quantum mechanical correlation methods [11]. In order to understand the electronic properties ofdimeric and trimeric tetrathiafulvalenes 1-4illustrate in literature [12], the theoretical calculations have been computed by DFT/B3LYP method with 6-31G (d,p) basis. This study investigated the characterization of the molecular structures, molecular electrostatic potential (MEP), frontier molecular orbital analysis properties, quantum chemical descriptors,the Fukui functions, second order perturbation theory analysis (NBO) and first order hyperpolarizability (β) using the calculation method quoted above. II. Materials and Methods All calculations have been performed by using Gaussian 09W [13] program package at the DFT level of theory by using the hybrid functional B3LYP [14] with 6-31G (d,p) basis set. The calculated results have been visualized by using Gauss View 5 [15] software. III. Results and Discussion 3.1 Molecular Geometry: The atomic numbering scheme the theoretical geometric structures of dimeric and trimeric tetrathiafulvalenes 1-4 are shown in Figure 1. The optimized parameters (bond lengths, bond angles and dihedral angles) of the title compounds were obtained using DFT and the B3LYP/6-31G (d,p) method. The results are given in Tables 1-4. Figure 1.Optimized molecular structure of dimeric and trimeric tetrathiafulvalenes 1-4 Table 1.Optimized geometric parameters of compound 1 DOI: / Page
2 Bond Length(Å) Bond Angles ( ) Dihedral Angles ( ) R(1,4) A(4,1,17) D(17,1,4,19) R(1,17) A(3,2,24) D(18,1,4,20) R(2,17) A(17,2,24) D(4,1,17,2) R(3,18) A(2,3,23) D(17,2,24,32) R(4,20) A(18,3,23) D(23,3,18,1) R(5,6) A(1,4,20) D(18,3,23,45) R(5,53) A(6,5,53) D(1,4,19,6) R(6,52) A(5,6,52) D(53,5,6,19) R(7,13) A(19,6,52) D(53,5,20,4) R(8,9) A(10,7,13) D(20,5,53,63) R(8,13) A(10,7,14) D(14,7,10,15) R(8,50) A(13,7,14) D(10,7,13,8) R(9,51) A(9,8,13) D(7,10,16,12) R(10,16) A(13,8,50) D(11,22,39,40) R(11,12) A(8,9,14) D(2,24,32,35) Table 2.Optimized geometric parameters of compound 2 Bond Length(Å) Bond Angles ( ) Dihedral Angles ( ) R(1,4) A(4,1,14) D(4,1,13,2) R(1,13) A(13,1,14) D(13,2,3,41) R(2,3) A(3,2,13) D(41,3,14,1) R(2,13) A(3,2,42) D(14,3,41,43) R(3,14) A(13,2,42) D(1,4,16,6) R(4,15) A(2,3,41) D(24,5,6,16) R(5,6) A(14,3,41) D(24,5,15,4) R(5,15) A(1,4,15) D(6,5,24,26) R(6,16) A(1,4,16) D(19,7,10,18) R(7,10) A(6,5,15) D(39,9,20,7) R(8,9) A(15,5,24) D(20,9,39,49) R(8,40) A(5,6,16) D(7,10,18,12) R(9,20) A(16,6,23) D(22,11,17,10) R(9,39) A(10,7,19) D(12,11,22,32) R(10,17) A(19,7,20) D(11,22,32,34) Table 3.Optimized geometric parameters of compound 3 Bond Length(Å) Bond Angles ( ) Dihedral Angles ( ) R(1,18) A(4,1,18) D(18,1,4,20) R(2,3) A(17,1,18) D(4,1,17,2) R(2,17) A(3,2,17) D(17,2,24,32) R(3,18) A(17,2,24) D(23,3,18,1) R(4,19) A(2,3,23) D(1,4,19,6) R(5,6) A(18,3,23) D(51,5,6,19) R(5,20) A(1,4,19) D(51,5,20,4) R(6,50) A(1,4,20) D(20,5,51,61) R(7,13) A(6,5,51) D(10,7,13,8) DOI: / Page
3 R(7,14) A(20,5,51) D(49,9,14,7) R(8,13) A(5,6,50) D(14,9,49,52) R(9,14) A(19,6,50) D(7,10,16,12) R(10,16) A(10,7,13) D(15,11,12,21) R(11,12) A(10,7,14) D(22,11,15,10) R(11,15) A(13,7,14) D(11,22,38,39) Table 4.Optimized geometric parameters of compound 4 Bond Length(Å) Bond Angles ( ) Dihedral Angles ( ) R(1,18) A(4,1,17) D(17,1,4,19) R(2,3) A(17,1,18) D(18,1,4,20) R(2,17) A(3,2,24) D(4,1,17,2) R(3,23) A(17,2,24) D(17,2,24,28) R(4,19) A(2,3,18) D(23,3,18,1) R(5,6) A(18,3,23) D(18,3,23,40) R(5,20) A(1,4,19) D(1,4,19,6) R(5,46) A(19,4,20) D(46,5,6,19) R(6,45) A(6,5,46) D(46,5,20,4) R(7,10) A(20,5,46) D(20,5,46,56) R(8,9) A(5,6,45) D(14,7,10,15) R(8,43) A(19,6,45) D(13,8,9,44) R(9,14) A(10,7,13) D(44,9,14,7) R(9,44) A(13,7,14) D(7,10,16,12) R(10,15) A(9,8,43) D(15,11,12,21) Molecular Electrostatic Potential (MEP): The molecular electrostatic potential (MEP) has been used as a useful method in research of molecular structure with its physicochemical property relationship [16]. The molecular electrostatic potential, V(r), at a given point r (x,y,z) in the vicinity of a molecule, is defined in terms of the interaction energy between the electrical charge generated from the molecule electrons and nuclei and a positive test charge (a proton) located at r. It is defined by: ' ρr ' r r Z ' Vr A dr R A A r In which Z A is the charge of nucleus A, located at R A and ρ(r') is the electronic density function for the molecule and r' is the dummy integration variable [17,18]. The MEP has been used for predicting sites and relative reactivity toward electrophilic attack and in the studies of biological recognition and hydrogen bonding interactions [19].Potential increases in the order red < orange < yellow < green < blue. To predict reactivesites for electrophilic and nucleophilic attack for the title molecules,mep was calculated at the B3LYP/6-31G(d,p) optimized geometries.the negative (red) regions of MEP were related to electrophilicreactivity and the positive (bleu) regions to nucleophilic reactivity illustrated in Figure 2. Figure 2. Molecular electrostatic potential surface of dimeric and trimeric tetrathiafulvalenes 1-4 DOI: / Page
4 As seen from the figure above that, in all molecules, the regions exhibiting the negative electrostatic potential are localized near the TTF core and the nitrile function in compound 1 while the regions presenting the positive potential are localized vicinity of the hydrogen atoms of alkyl and cycled groups. 3.3 Frontier Molecular Orbitals (FMOs): The highest occupied molecular orbitals (HOMOs) and the lowest-lying unoccupied molecular orbitals (LUMOs) are named as Frontier Molecular Orbitals (FMOs). The FMOs play an important role in the optical and electric properties, as well as in quantum chemistry. HOMO, LUMO energy characterizes the ability of electron accepting. The eigenvalues of HOMO and LUMO and their energy gap reflect the biological activity of the molecule. These orbitals are a pair of orbitals in the compound, which allows them to interact more strongly. The frontier orbital gap helps characterize the chemical reactivity and the kinetic stability of the molecule. A molecule having a small frontier orbitals gap is more polarizable and is generally associated with a high chemical reactivity and low kinetic stability [20 22]. The HOMO and LUMO orbitals of compound 3 with a small energy gap were computed by the DFT/B3LYP method with 6-31G (d,p) basis set and visualized in Figure 3. Figure 3. HOMO-LUMO Structure with the energy level diagram of compound Global Reactivity Descriptors: Density functional theory method concept the chemical reactivity and site selectivity of the molecular systems. The energies of frontier molecular orbitals (E HOMO, E LUMO ), energy band gap which explains the eventual charge transfer interaction within the molecule, electronegativity (χ), chemical potential (µ), global hardness (η), global softness (S) and global electrophilicity index (ω) [23,24] of dimeric and trimeric tetrathiafulvalenes 1-4 are computed by the DFT/B3LYP method with 6-31G (d,p) basis set and listed in Table 5. χ 1 2E LUMO E HOMO μ χ 1 2E LUMO E HOMO η 1 2E LUMO E HOMO S 1 2η ω μ 2 2η The large HOMO-LUMO gap means a hard molecule and small HOMO-LUMO gap means a soft molecule. One can also relate the stability of the molecule to hardness, which means that the molecule with least HOMO-LUMO gap means it is more reactive. Table 5.Quantum chemical descriptors of dimeric and trimeric tetrathiafulvalenes 1-4 Parameters Compound 1 Compound 2 Compound 3 Compound 4 E HOMO (ev) E LUMO (ev) ΔE gap (ev) IE (ev) A (ev) µ (ev) DOI: / Page
5 χ (ev) ƞ (ev) S (ev) ω (ev) As presented in table 5, the compound which have the lowest energetic gap is the compound 3 ( E gap = ev). This lower gap allows it to be the softest molecule. The compound that have the highest energy gap is the compound 2 ( E gap = ev).the compound that has the highest HOMO energy is the compound 3 (E HOMO = ev). This higher energy allows it to be the best electron donor. The compound that has the lowest LUMO energy is the compound 3 (E LUMO = ev) which signifies that it can be the best electron acceptor. The two properties like I (potential ionization) and A (affinity) are so important, the determination of these two properties allow us to calculate the absolute electronegativity (χ) and the absolute hardness (η). These two parameters are related to the one-electron orbital energies of the HOMO and LUMO respectively. Compound 3 has lowest value of the potential ionization (I = ev), so that will be the better electron donor. Compound 3 has the largest value of the affinity (A = ev), so it is the better electron acceptor. The chemical reactivity varies with the structural of molecules. Chemical hardness (softness) value of compound 3 (η = ev, S = ev) is lesser (greater) among all the molecules. Thus, compound 3 is found to be more reactive than all the compounds. Compound 1 possesses higher electronegativity value (χ = ev) than all compounds so; it is the best electron acceptor. The value of ω for compound 3 (ω = ev) indicates that it is the stronger electrophiles than all compounds. Compound 3 has the smaller frontier orbital gap so, it is more polarizable and is associated with a high chemical reactivity, low kinetic stability and is also termed as soft molecule. 3.5 Local Reactivity Descriptors: The Fukui function is a local reactivity descriptor that gives the preferred regions where a chemical species will change its density when the number of electrons is changed and it shows the propensity of the electronic density to deform at a given position upon accepting or donating electrons [25-27]. Also, the condensed or atomic Fukui functions on the jth atom site is given as, f q N q N 1 j j fj qj j N 1 q N 0 1 fj qj 2 j N 1 q N 1 j For an electrophilic f - j (r), nucleophilic or free radical attack f + j (r), on the reference molecule, respectively. In these equations, q j is the atomic charge at the jth atomic site is the neutral (N), anionic (N+1) or cationic (N-1) chemical species. Chattarajet al. [28] proposed the concept of generalized philicity and it contains almost all information about the known different global and local reactivity and selectivity descriptor, in addition to the information regarding electrophilic/nucleophilic power of a given atomic site in a molecule. Morell et al. [29] proposed a dual descriptor (Δf(r)), which is defined as the difference between the nucleophilic and electrophilic Fukuifunction and is given by, fr f r f r Δf (r) > 0, then the site is favored for a nucleophilic attack, whereas ifδf(r)< 0, then the site may be favored for an electrophilic attack. Under this situation, dual descriptors Δf(r) gives a clear difference between nucleophilic and electrophilic attack at a particular region with their sign and it provides positive value for site prone for nucleophilic attack and a negative value prone for electrophilic attack. Fukui functions for selected atomic sites dimeric and trimeric tetrathiafulvalenes 1-4 are calculated by the DFT/B3LYP method with 6-31G (d,p) basis set and shown in Tables 6-7. Table 6.Order of the reactive sites on compounds 1 and 2 Compound 1 Compound 2 Atom 10 C 1 C 8 C 5 C Atom 10 C 4 C 26 C 9 C f f Atom 23 S 24 S 52 S 53 S Atom 23 S 24 S 42 S 41 S f f Atom 10 C 1 C 9 C 11 C Atom 10 C 24 S 4 C 8 C f f Table 7.Order of the reactive sites on compounds 3 and 4 Compound 3 Compound 4 Atom 92 C 66 C 14 S 13 S Atom 10 C 1 C 65 C 67 C f f Atom 64 S 81 C 82 C 2 C Atom 25 C 12 C 9C 61 C DOI: / Page
6 f f Atom 66 C 23 S 22 S 32 C Atom 10 C 65 C 1 C 3 C f f From the tables 6-7, the parameters of local reactivity descriptors show that 10C is the more reactive site in compounds 1, 2 and 4 and 92C in compound 3 respectively for nucleophilic attacks. The more reactive sites in radical attacks are 10C for compounds 1, 2 and 4 and 66C for compounds 3 respectively.the more reactive sites for electrophilic attacks are 23S for compounds 1, 2 and 64S, 25C for compounds 3 and 4respectively. 3.6 Natural Bond Orbital Analysis (NBO): NBO analysis helps in identifying individual bonds and the energies associated with lone pair electrons that play a vital role in the chemical processes [30 32]. It helps in studying hybridization, hydrogen bonding as well as hyper-conjugative interactions between occupied Lewis type (bonding or lone pair) and unoccupied (anti-bonding or Rydberg) orbitals which further help in studying inter and intramolecular interactions among various bonds. Stabilization energy (E (2) ) associated as a result of electron delocalization between donor NBO (i) and acceptor NBO (j), is estimated by equation [33,34] Fij2 2 E qi Ej Ei whereq i is the donor orbital occupancy; E(i) and E(j) are orbital energies of donor and acceptor NBO orbitals and F ij is the off-diagonal Fock matrix. Extent of hyper-conjugation depends upon the donating ability of Lewis type orbitals and accepting ability of non Lewis type orbitals and this is measured in terms of the interaction energy E (2).The second-order perturbation theory analysis of Fock matrix in NBO basis of dimeric and trimeric tetrathiafulvalenes 1-4 is given in Tables Table 8.Second order perturbation theory analysis of Fock matrix on NBO of compound 1 Donor(i) ED/e Acceptor(j) ED/e E(2) E(j)-E(i) F(i.j) Kcal/mol a.u a.u LP (2) S π*(c2-c3) LP (2) S π*(c11-c12) LP (2) S π*(c 2-C3) LP (2) S π*(c11-c12) LP (2) S π*(c5-c6) LP (2) S π*(c8-c9) LP (2) S π*(c8-c9) LP (2) S π*(c5-c6) LP (2) S π*(c8-c9) LP (2) S π*(c5-c6) LP (2) S π*(c7-c10) LP (2) S π*(c7-c10) LP (2) S π*(c1-c4) LP (2) S π*(c1-c4) LP (1) N π*(c28-c31) LP (1) N π*(c35-c38) LP (2) S π*(c8-c9) LP (2) S π*(c5-c6) LP (2) S π*(c1-c4) LP (2) S π*(c1-c4) Table 9.Second order perturbation theory analysis of Fock matrix on NBO of compound 2 Donor(i) ED/e Acceptor(j) ED/e E(2) E(j)-E(i) F(i.j) Kcal/mol a.u a.u LP (2) S π*(c8-c9) LP (2) S π*(c2-c3) LP (2) S π*(c11-c12) LP (2) S π*(c2-c3) LP (2) S π*(c8-c9) LP (2) S π*(c11-c12) LP (2) S π*(c5-c6) LP (2) S π*(c5-c6) LP (2) S π*(c2-c3) LP (2) S π*(c8-c9) LP (2) S π*(c7-c10) LP (2) S π*(c7-c10) LP (2) S π*(c1-c4) LP (2) S π*(c1-c4) LP (2) S π*(c1-c4) LP (2) S π*(c1-c4) DOI: / Page
7 LP (2) S π*(c7-c10) LP (2) S π*(c7-c10) LP (2) S π*(c2-c3) LP (2) S π*(c8-c9) Table 10.Second order perturbation theory analysis of Fock matrix on NBO of compound 3 Donor(i) ED/e Acceptor(j) ED/e E(2) E(j)-E(i) F(i.j) Kcal/mol a.u a.u LP (2) S π*(c65-c66) LP (2) S π*(c65-c66) LP (2) S π*(c2-c3) LP (2) S π*(c2-c3) LP (2) S π*(c11-c12) LP (2) S π*(c11-c12) LP (2) S π*(c81-c82) LP (2) S π*(c5-c6) LP (2) S π*(c8-c9) LP (2) S π*(c5-c6) LP (2) S π*(c8-c9) LP (2) S π*(c81-c82) LP (2) S π*(c81-c82) LP (2) S π*(c5-c6) LP (2) S π*(c8-c9) LP (2) S π*(c7-c10) LP (2) S π*(c7-c10) LP (2) S π*(c1-c4) LP (2) S π*(c1-c4) LP (2) S π*(c70-c72) Table 11.Second order perturbation theory analysis of Fock matrix on NBO of compound 4 Donor(i) ED/e Acceptor(j) ED/e E(2) Kcal/mol E(j)-E(i) a.u DOI: / Page F(i.j) a.u LP (2) S π*(c2-c3) LP (2) S π*(c2-c3) LP (2) S π*(c60-c61) LP (2) S π*(c60-c61) LP (2) S π*(c11-c12) LP (2) S π*(c11-c12) LP (2) S π*(c5-c6) LP (2) S π*(c8-c9) LP (2) S π*(c75-c76) LP (2) S π*(c5-c6) LP (2) S π*(c8-c9) LP (2) S π*(c75-c76) LP (2) S π*(c8-c9) LP (2) S π*(c75-c76) LP (2) S π*(c5-c6) LP (2) S π*(c65-c67) LP (2) S π*(c65-c67) LP (2) S π*(c1-c4) LP (2) S π*(c1-c4) LP (2) S π*(c7-c10) The intra molecular interaction for the title compounds is formed by the orbital overlap between: LP(2) S18 orbital to the π*(c2-c3) for compound 1, LP(2) S19 orbital to π*(c8-c9) for compound 2, LP(2) S67 orbital to π*(c65-c66) for compound 3,LP(2) S18 orbital to π*(c2-c3) for compound 4 respectively, show the stabilization energy of 18.51, 16.44, and kj mol -1 respectively. 3.7 Nonlinear Optical Properties (NLO): The NLO activity provide the key functions for frequency shifting, optical modulation, optical switching and optical logic for the developing technologies in areas such as communication, signal processing and optical interconnections [35,36].In the presence of an applied electric field, the energy of a system is a function of the electric field and the first hyperpolarizability is a third rank tensor that can be described by a matrix. The 27 components of the 3D matrix can be reduced to 10 components because of the Kleinman symmetry [37]. The matrix can be given in the lower tetrahedral format. It is obvious that the lower part of the matrices is a tetrahedral. The components of β are defined as the coefficients in the Taylor series expansion of the energy in the external electric field. When the external electric field is weak and homogeneous, this expansion is given below:
8 0 E E μαfα 1 2ααβFα Fβ 1 6βαβγFα Fβ Fγ... wheree 0 is the energy of the unperturbed molecules, F α is the field at the origin, µ α, α αβ and β αβγ are the components of dipole moment, polarizability and first hyperpolarizability, respectively. The total static dipole moment µ, the mean polarizability α 0, the anisotropy of the polarizability Δα and the mean first hyperpolarizability β 0, using the x, y and z components are defined as: Dipole moment is μ μ 1 2 x μy μz Static polarizability is α α α α 3 0 xx yy zz 2 2 6α 1 2 xx yy yy zz zz xx Total polarizability is 1 2 α α α 2 α α 2 α α 2 xz First order hyperpolarizability is β β β β 1 2 Where βx βy βz 0 x y z β xxx βxyy βxzz β yyy βyzz βyxx β zzz βzxx βzyy β β β 2 β β β 2 β β β β0 xxx xyy xzz yyy yzz yxx zzz zxx zyy The first static hyperpolarizability (β 0 ) and its related properties (β, α 0 and Δα)of dimeric and trimeric tetrathiafulvalenes1-4 have been calculated using B3LYP/6-31G (d,p) level based on finite field approach and given in Table 12. Table 12.Thedipole moments µ (D), polarizability α 0 (esu), the anisotropy of the polarizability Δα (esu) and the first hyperpolarizability β 0 (esu) of dimeric and trimeric tetrathiafulvalenes 1-4. Parameters Compound 1 Compound 2 Compound 3 Compound 4 β xxx β yyy β zzz β xyy β xxy β xxz β xzz β yzz β yyz β xyz β 0 (esu)x µ x µ y µ z µ(d) α xx α yy α zz α xy α xz α yz α 0(esu)x α(esu)x Since the values of the polarizabilities ( α) and the hyperpolarizabilities (β tot ) of the GAUSSIAN 09 output are obtained in atomic units (a.u.), the calculated values have been converted into electrostatic units (e.s.u.) (for α; 1 a.u = x e.s.u., for β; 1 a.u = x e.s.u.). The calculated values of dipole moment (µ) for the title compounds were found to be , , and D respectively, which are approximately four times than to the value for urea (µ = D). Urea is one of the prototypical molecules used in the study of the NLO properties of molecular systems. Therefore, it has been used frequently as a threshold value for comparative purposes. The calculated values of polarizability are x 10-24, x 10-24, x and x esu respectively; the values of anisotropy of the polarizability are , , and esu, respectively. The magnitude of the molecular hyperpolarizability (β 0 ) is one of important key factors in a NLO system. The DFT/6-31G(d,p) calculated first hyperpolarizability value (β 0 ) of dimeric and trimeric tetrathiafulvalenes molecules are equal to x 10-33, x 10-33, x and x esu. The first hyperpolarizability of title molecules is approximately 2.16, 1.30, 4.19 and 1.49 times than those of urea (β of urea is x10-33 esu obtained by B3LYP/6-311G (d,p) method). This result indicates that dimeric and trimeric tetrathiafulvalenes 1-4 are nonlinear. DOI: / Page
9 IV. Conclusion In the present study, molecular structure, geometrical parameters, Molecular electrostatic potential map of dimeric and trimeric tetrathiafulvalenes 1-4 has been studied using DFT/B3LYP method with 6-31G (d,p) basis set. The frontier molecular orbital analysis provides information regarding ionization potential, chemical potential and other chemical descriptors.fukui function also computed to predict reactive sites for electrophilic and nucleophilic attacks in the studied molecules. The NBO analysis obviously illustrates the stability of the molecular structure that arises from conjugative interactions, charge delocalization and E(2) energies confirm the occurrence of intra-molecular charge transfer. The calculated dipole moment and first order hyperpolarizability results indicate that the title compounds is a good candidate of NLO material. Acknowledgments This work was generously supported by the (General Directorate for Scientific Research and Technological Development, DGRS-DT) and Algerian Ministry of Scientific Research. References [1] T.Ishiguro, K.Yamaji, G.Saito."Proceedings of the International Conference on Science and Technology of Synthetic Metals, ICSM'98," Synth. Met(1999) [2] M.A. Herranz, L.Sanchez, N.Martin.Tetrathiafulvalene: A Paradigmatic Electron Donor Molecule.Phosphorus, Sulfur Silicon Relat. Elem. 180(2005) DOI: /chin [3] T.Uemura, K.Naka, Y.Chujo. Functional Macromolecules with Electron-Donating Dithiafulvene Unit. Adv.Polym. Sci. 167(2004), DOI: /b [4] G.Maruccio, R.Cingolani, R.Rinaldi.Projecting the Nanoworld: Concepts, Results and Perspectives of Molecular Electronics.J. Mater. Chem. 14(2004) DOI: /B311929G. [5] J.Yamada, T. Sugimoto.TTF Chemistry: Fundamentals and Applications of Tetrathiafulvalene, Eds.; Kodansha-Springer: Tokyo (2004). [6] N.Martín, L.Sánchez, B. Illescas, I.Pérez. C 60-Based Electroactive Organofullerenes. Chem. Rev. 98(1998) DOI: /cr [7] R. M. Metzger.Electrical Rectification by a Molecule: The Advent of Unimolecular Electronic Devices. Acc. Chem. Res. 32(1999) DOI: /ar [8] M.Bendikov, F.Wudl, D.F. Perepichka. Tetrathiafulvalenes, Oligoacenenes, and Their Buckminsterfullerene Derivatives: The Brick and Mortar of Organic Electronics. Chem. Rev. 104(2004) DOI: /cr030666m. [9] A.Gorgues, P.Hudhomme, M.Sallé.Highly Functionalized Tetrathiafulvalenes: Riding along the Synthetic Trail from Electrophilic Alkynes.Chem. Rev. 104 (2004) DOI: /cr [10] J. L.Segura, N.Martín. New Concepts in Tetrathiafulvalene Chemistry.Angew. Chem., Int Ed. 40(2001) DOI: / ( )40:8<1372::AID-ANIE1372>3.0.CO;2-I. [11] C. Ravikumar, I. Hubert Joe, D. Sajan.Vibrational Contributions to the Second-Order Nonlinear Optical Properties of π-conjugated Structure Acetoacetanilide. J.Chem. Phys. 369 (2010) 1-7. DOI: /j.chemphys [12] A. Abd El-Wareth, O. Sarhan. Synthesis and Applications of Tetrathiafulvalenes and Ferrocene-Tetrathiafulvalenes and Related Compounds. Tetrahedron 61 (2005) [13] M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Petersson and al.gaussian 09, Revision C.01; Gaussian Inc.: Wallingford, CT, USA (2010). [14] A.D. Becke. Density Functional Thermochemistry. III. The Role of Exact Exchange.J.Chem. Phys. 98 (1993) DOI: / [15] R. Dennington, T. Keith and J. Millam,GaussView, Version 5, Semichem Inc., Shawnee Mission, KS (2009). [16] S. Muthu, M. Prasath, E. IsacPaulraj, R.A. Balaji.FT-IR, FT-Raman Spectra and ab initio HF and DFT Calculations of 7-chloro-5- (2-chlorophenyl)-3-hydroxy-2,3-dihydro-1H-1,4-benzodiazepin-2-one. Spectrochim. Acta A 120(2014) DOI: /j.saa [17] P. Politzer, D.G. Truhlar (Eds.), Chemical Application of Atomic and Molecular Electrostatic Potentials, Plenum, New York, (1981). [18] P. Politzer, P. Lane. A Computational Study of Some Nitrofluoromethanes. Struct. Chem. 1(1990) [19] N. Gunay, H. Pir, D. Avci, Y. Atalay.NLO and NBO Analysis of Sarcosine-Maleic Acid by Using HF and B3LYP Calculations. J. Chem. (2013) 16. DOI: /2013/ [20] N. Sinha, O. Prasad, V. Narayan, S.R. Shukla. Raman, FT-IR Spectroscopic Analysis and First-order Hyperpolarisability of 3- benzoyl-5-chlorouracil by First Principles.J. Mol. Simul. 37 (2011) DOI: / [21] D.F.V. Lewis, C. Loannides, D.V. Parke. Interaction of aseries of Nitriles with the Alcohol-Inducible Isoform of P450: Computer Analysis of Structure Activity Relationships.Xenobiotica 24 (1994) DOI: / [22] B. Kosar, C. Albayrak.Spectroscopic Investigations and Quantum Chemical Computational Study of (E)-4-methoxy-2-[(ptolylimino)methyl]phenol. Spectrochim. Acta A 78 (2011) DOI : /j.saa [23] R.G. Pearson. Absolute Electronegativity and Hardness: Applications to Organic Chemistry.J. Org. Chem. 54 (1989) DOI: /jo00267a034. [24] J. Padmanabhan, R. Parthasarathi, V. Subramanian, P. Chattaraj.Electrophilicity-Based Charge Transfer Descriptor.J. Phys. Chem. A 111 (2007) DOI: /jp [25] R.G. Parr, W. Yang, Functional Theory of Atoms and Molecules, Oxford University Press, New York, (1989). [26] P.W. Ayers, R.G. Parr. Variational Principles for Describing Chemical Reactions: The Fukui Function and Chemical Hardness Revisited.J. Am. Chem. Soc. 122 (2000) DOI: /ja [27] R.G. Parr, W.J. Yang. Density Functional Approach to the Frontier-Electron Theory of Chemical Reactivity.J.Am. Chem. Soc. 106 (1984) DOI: /ja00326a036. [28] P.K. Chattaraj, B. Maiti, U. Sarkar. Philicity: A Unified Treatment of Chemical Reactivity and Selectivity. J. Phys. Chem. A 107 (2003) DOI: /jp034707u. DOI: / Page
10 [29] C. Morell, A. Grand, A. Toro-Labbe. New Dual Descriptor For Chemical Reactivity.J. Phys. Chem. A 109 (2005) DOI: /jp046577a. [30] V. Pophristic, L. Goodman, N. Guchhait.Role of Lone-Pairs in Internal Rotation Barriers. J. Phy. Chem. A 101 (1997) DOI: /jp971020z. [31] D. Guo, L. Goodman. Nature of Barrier Forces in Acetaldehyde. J. Phys. Chem. 100 (1996) DOI: /jp960182c. [32] F. Weinhold. Chemistry: A New Twist on Molecular Shape.Nature 411 (2001) DOI: / [33] A.E. Reed, L.A. Curtiss, F. Weinhold. Intermolecular Interactions FromaNatural Bond Orbital, Donor-Acceptor Viewpoint.J.Chem. Rev. 88 (1988) DOI: /cr00088a005. [34] C.G. Liu, Z.M. Su, X.H. Guan, S. Muhammad. Redox and Photoisomerization Switching the Second-Order Nonlinear Optical Properties of a Tetrathiafulvalene Derivative Across Six States: A DFT Study.J. Phys. Chem. C 115 (2011) DOI: /jp [35] C. Andraud, T. Brotin, C. Garcia, F. Pelle, P. Goldner, B. Bigot, A. Collet.Theoretical and Experimental Investigations of the Nonlinear Optical Properties of Vanillin, Polyenovanillin, and Bis Vanillin Derivatives. J. Am.Chem. Soc. 116 (1994) DOI: /ja00084a055. [36] V.M. Geskin, C. Lambert, J.L. Bredas. Origin of High Second- and Third-Order Nonlinear Optical Response in Ammonio/BoratoDiphenylpolyene Zwitterions: the Remarkable Role of Polarized Aromatic Groups.J. Am. Chem. Soc. 125 (2003) DOI: /ja035862p. [37] D.A. Kleinman. Nonlinear Dielectric Polarization in Optical Media.Phys. Rev. 126 (1962)1977.DOI: /PhysRev IOSR Journal of Applied Chemistry (IOSR-JAC) is UGC approved Journal with Sl. No. 4031, Journal no Tahar Abbaz "A Computational Investigation of a Series Ofdimeric and Trimetric Tetrathiafulvalenes Using DFT Method." IOSR Journal of Applied Chemistry (IOSR-JAC) 11.4 (2018): DOI: / Page
First Order Hyperpolarizability and Homo-Lumo Analysis of L-Arginine Maleate (LArM) by Density Functional Theory Methods
 51 Sciencia Acta Xaveriana An International Science Journal ISSN. 0976-1152 Volume 4 No. 2 pp. 51-58 September 2013 First Order Hyperpolarizability and Homo-Lumo Analysis of L-Arginine Maleate (LArM) by
51 Sciencia Acta Xaveriana An International Science Journal ISSN. 0976-1152 Volume 4 No. 2 pp. 51-58 September 2013 First Order Hyperpolarizability and Homo-Lumo Analysis of L-Arginine Maleate (LArM) by
Density Functional Theory Study of Exohedral Carbon Atoms Effect on Electrophilicity of Nicotine: Comparative Analysis
 Computational Chemistry, 2016, 4, 17-31 Published Online January 2016 in SciRes. http://www.scirp.org/journal/cc http://dx.doi.org/10.4236/cc.2016.41003 Density Functional Theory Study of Exohedral Carbon
Computational Chemistry, 2016, 4, 17-31 Published Online January 2016 in SciRes. http://www.scirp.org/journal/cc http://dx.doi.org/10.4236/cc.2016.41003 Density Functional Theory Study of Exohedral Carbon
Vibrational spectra, nonlinear optical properties, NBO analysis and thermodynamic properties of N-phenylethanolamine by density functional method
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 016, 8(6):184-05 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Vibrational spectra, nonlinear optical properties,
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 016, 8(6):184-05 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Vibrational spectra, nonlinear optical properties,
CHAPTER 8 REPORT ON HIGHER SHG EFFICIENCY IN BIS (CINNAMIC ACID): HEXAMINE COCRYSTAL
 CHAPTER 8 REPORT ON HIGHER SHG EFFICIENCY IN BIS (CINNAMIC ACID): HEXAMINE COCRYSTAL 8.1. Introduction In recent times higher Second Harmonic Generation (SHG) efficiency organic materials receive great
CHAPTER 8 REPORT ON HIGHER SHG EFFICIENCY IN BIS (CINNAMIC ACID): HEXAMINE COCRYSTAL 8.1. Introduction In recent times higher Second Harmonic Generation (SHG) efficiency organic materials receive great
QUANTUM CHEMICAL DESCRIPTORS OF SOME P- AMINOPHENYL TETRATHIAFULVALENES THROUGH DENSITY FUNCTIONAL THEORY (DFT)
 IN: 0974-1496 e-in: 0976-0083 CODEN: RJCABP http://www.rasayanjournal.com http://www.rasayanjournal.co.in QUANTUM CHEMICAL DECRIPTOR OF OME P- AMINOPHENYL TETRATHIAFULVALENE THROUGH DENITY FUNCTIONAL THEORY
IN: 0974-1496 e-in: 0976-0083 CODEN: RJCABP http://www.rasayanjournal.com http://www.rasayanjournal.co.in QUANTUM CHEMICAL DECRIPTOR OF OME P- AMINOPHENYL TETRATHIAFULVALENE THROUGH DENITY FUNCTIONAL THEORY
Structure, Electronic and Nonlinear Optical Properties of Furyloxazoles and Thienyloxazoles
 Journal of Physics: Conference Series PAPER OPEN ACCESS Structure, Electronic and Nonlinear Optical Properties of Furyls and Thienyls To cite this article: Ozlem Dagli et al 6 J. Phys.: Conf. Ser. 77 View
Journal of Physics: Conference Series PAPER OPEN ACCESS Structure, Electronic and Nonlinear Optical Properties of Furyls and Thienyls To cite this article: Ozlem Dagli et al 6 J. Phys.: Conf. Ser. 77 View
THEORETICAL INVESTIGATION OF ELECTROSTATIC POTENTIAL AND NON LINEAR OPTICAL PROPERTIES OF M NITROACETANILIDE
 ISSN: 0974-1496 e-issn: 0976-0083 CODEN: RJCABP http://www.rasayanjournal.com http://www.rasayanjournal.co.in THEORETICAL INVESTIGATION OF ELECTROSTATIC POTENTIAL AND NON LINEAR OPTICAL PROPERTIES OF N.
ISSN: 0974-1496 e-issn: 0976-0083 CODEN: RJCABP http://www.rasayanjournal.com http://www.rasayanjournal.co.in THEORETICAL INVESTIGATION OF ELECTROSTATIC POTENTIAL AND NON LINEAR OPTICAL PROPERTIES OF N.
Competition between Alkalide Characteristics and Nonlinear Optical Properties in OLi 3 M Li 3 O (M = Li, Na, and K) Complexes
 Competition between Alkalide Characteristics and Nonlinear Optical Properties in OLi 3 M Li 3 O (M = Li, Na, and K) Complexes Ambrish Kumar Srivastava and Neeraj Misra * Department of Physics, University
Competition between Alkalide Characteristics and Nonlinear Optical Properties in OLi 3 M Li 3 O (M = Li, Na, and K) Complexes Ambrish Kumar Srivastava and Neeraj Misra * Department of Physics, University
Quantum chemical origin of high ionization potential and low electron affinity of Tungsten Hexafluoride
 Journal of Computational Methods in Molecular Design, 2015, 5 (4):142-146 Scholars Research Library (http://scholarsresearchlibrary.com/archive.html) ISSN : 2231-3176 CODEN (USA): JCMMDA Quantum chemical
Journal of Computational Methods in Molecular Design, 2015, 5 (4):142-146 Scholars Research Library (http://scholarsresearchlibrary.com/archive.html) ISSN : 2231-3176 CODEN (USA): JCMMDA Quantum chemical
Research & Development Centre, Bharathiar University, Coimbatore , India. 2
 EXPERIMENTAL FT-IR, FT-RAMAN AND THEORETICAL QUANTUM CHEMICAL COMPUTATIONS ON THE MOLECULAR STRUCTURE P-BROMOBENZOTRIFLUORIDE V. Anbu 1, K.A. Vijayalakshmi *, T. Karthick 3, Poonam Tandon 4 1 Research
EXPERIMENTAL FT-IR, FT-RAMAN AND THEORETICAL QUANTUM CHEMICAL COMPUTATIONS ON THE MOLECULAR STRUCTURE P-BROMOBENZOTRIFLUORIDE V. Anbu 1, K.A. Vijayalakshmi *, T. Karthick 3, Poonam Tandon 4 1 Research
AB INITIO MODELING OF THE STRUCTURAL DEFECTS IN AMIDES
 Int. J. Chem. Sci.: 9(4), 2011, 1564-1568 ISSN 0972-768X www.sadgurupublications.com AB INITIO MODELING OF THE STRUCTURAL DEFECTS IN AMIDES M. FATHIMA BEGUM, HEMA TRESA VARGHESE a, Y. SHEENA MARY a, C.
Int. J. Chem. Sci.: 9(4), 2011, 1564-1568 ISSN 0972-768X www.sadgurupublications.com AB INITIO MODELING OF THE STRUCTURAL DEFECTS IN AMIDES M. FATHIMA BEGUM, HEMA TRESA VARGHESE a, Y. SHEENA MARY a, C.
Department of Physics, A.V.C. College, Mayiladuthurai, Tamilnadu, India.
 IOSR Journal of Applied Physics (IOSR-JAP) e-issn: 2278-4861.Volume 8, Issue 3 Ver. II (May. - Jun. 2016), PP 01-14 www.iosrjournals.org The Spectroscopic (FT-IR, FT-Raman, NMR & UV-Vis) and first order
IOSR Journal of Applied Physics (IOSR-JAP) e-issn: 2278-4861.Volume 8, Issue 3 Ver. II (May. - Jun. 2016), PP 01-14 www.iosrjournals.org The Spectroscopic (FT-IR, FT-Raman, NMR & UV-Vis) and first order
Structural Properties, Natural Bond Orbital, Theory Functional Calculations (DFT), and Energies for the α Halorganic Compounds
 Current World Environment Vol. 7(2), 221-226 (2012) Structural Properties, Natural Bond Orbital, Theory Functional Calculations (DFT), and Energies for the α Halorganic Compounds NAJLA SEIDY and SHAHRIAR
Current World Environment Vol. 7(2), 221-226 (2012) Structural Properties, Natural Bond Orbital, Theory Functional Calculations (DFT), and Energies for the α Halorganic Compounds NAJLA SEIDY and SHAHRIAR
Journal of Advanced Scientific Research DFT APPROACH ON CORROSION INHIBITION PERFORMANCE OF THIOSEMICARBAZONE DERIVATIVES ON METALLIC IRON
 Rajendran et al., J Adv Sci Res, 2016, 7(1): 32-37 32 Journal of Advanced Scientific Research Available online through http://www.sciensage.info/jasr ISSN 0976-9595 Research Article DFT APPROACH ON CORROSION
Rajendran et al., J Adv Sci Res, 2016, 7(1): 32-37 32 Journal of Advanced Scientific Research Available online through http://www.sciensage.info/jasr ISSN 0976-9595 Research Article DFT APPROACH ON CORROSION
Global and Reactivity Descriptors Studies of Cyanuric Acid Tautomers in Different Solvents by using of Density Functional Theory (DFT)
 Global and Reactivity Descriptors Studies of Cyanuric Acid Tautomers in Different Solvents by using of Density Functional Theory (DFT) Numbury Surendra Babu 1, Didugu Jayaprakash 1 Department of Chemistry,
Global and Reactivity Descriptors Studies of Cyanuric Acid Tautomers in Different Solvents by using of Density Functional Theory (DFT) Numbury Surendra Babu 1, Didugu Jayaprakash 1 Department of Chemistry,
Growth and Characterization of an Organic Crystal and DFT Studies of 2-amino 5-methyl Pyridinium Salicylate
 ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2016, Vol. 32, No. (4): Pg. 1937-1945 Growth and Characterization
ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2016, Vol. 32, No. (4): Pg. 1937-1945 Growth and Characterization
The linear, nonlinear optical properties and quantum chemical parameters of some sudan dyes
 The linear, nonlinear optical properties and quantum chemical parameters of some sudan dyes Aslı EŞME 1,*, Seda GÜNEŞDOĞDU SAĞDINÇ 2 1 Department of Elementary Science Education, Kocaeli University, Umuttepe
The linear, nonlinear optical properties and quantum chemical parameters of some sudan dyes Aslı EŞME 1,*, Seda GÜNEŞDOĞDU SAĞDINÇ 2 1 Department of Elementary Science Education, Kocaeli University, Umuttepe
CHEM 344 Molecular Modeling
 CHEM 344 Molecular Modeling The Use of Computational Chemistry to Support Experimental Organic Chemistry Part 1: Molecular Orbital Theory, Hybridization, & Formal Charge * all calculation data obtained
CHEM 344 Molecular Modeling The Use of Computational Chemistry to Support Experimental Organic Chemistry Part 1: Molecular Orbital Theory, Hybridization, & Formal Charge * all calculation data obtained
Australian Journal of Basic and Applied Sciences
 AENSI Journals Australian Journal of Basic and Applied Sciences ISSN:1991-8178 Journal home page: www.ajbasweb.com Theoretical Study for the Effect of Hydroxyl Radical on the Electronic Properties of Cyclobutadiene
AENSI Journals Australian Journal of Basic and Applied Sciences ISSN:1991-8178 Journal home page: www.ajbasweb.com Theoretical Study for the Effect of Hydroxyl Radical on the Electronic Properties of Cyclobutadiene
Available online at WSN 61(2) (2017) EISSN
 Available online at www.worldscientificnews.com WSN 61(2) (2017) 150-185 EISSN 2392-2192 FT-IR, FT-Raman, NMR and U-V Spectral investigation: Computation of vibrational frequency, chemical shifts and electronic
Available online at www.worldscientificnews.com WSN 61(2) (2017) 150-185 EISSN 2392-2192 FT-IR, FT-Raman, NMR and U-V Spectral investigation: Computation of vibrational frequency, chemical shifts and electronic
Effects of Solvent Polarity on Solvation Free Energy, Dipole Moment, Polarizability, Hyperpolarizability and Molecular Properties of Metronidazole
 Bangladesh Pharmaceutical Journal 19(1): 9-14, 2016 Effects of Solvent Polarity on Solvation Free Energy, Dipole Moment, Polarizability, Hyperpolarizability and Molecular Properties of Metronidazole Mohammad
Bangladesh Pharmaceutical Journal 19(1): 9-14, 2016 Effects of Solvent Polarity on Solvation Free Energy, Dipole Moment, Polarizability, Hyperpolarizability and Molecular Properties of Metronidazole Mohammad
CHEM 344 Molecular Modeling
 CHEM 344 Molecular Modeling The Use of Computational Chemistry to Support Experimental Organic Chemistry Day 1 all calculation data obtained from Gaussian09 using B3LYP/6-31G(d) unless otherwise noted.
CHEM 344 Molecular Modeling The Use of Computational Chemistry to Support Experimental Organic Chemistry Day 1 all calculation data obtained from Gaussian09 using B3LYP/6-31G(d) unless otherwise noted.
How Partial Atomic Charges and Bonding. Orbitals Affect the Reactivity of Aluminum
 Supporting Information for: How Partial Atomic Charges and Bonding Orbitals Affect the Reactivity of Aluminum Clusters with Water? Anthony M.S Pembere ξ, Xianhu Liu ξ, Weihua Ding, Zhixun Luo * State Key
Supporting Information for: How Partial Atomic Charges and Bonding Orbitals Affect the Reactivity of Aluminum Clusters with Water? Anthony M.S Pembere ξ, Xianhu Liu ξ, Weihua Ding, Zhixun Luo * State Key
1 Introduction. ISSN , England, UK World Journal of Modelling and Simulation Vol. 14 (2018) No. 1, pp. 3-11
 ISSN 1 746-7233, England, UK World Journal of Modelling and Simulation Vol. 14 (2018) No. 1, pp. 3-11 Molecular Structure, Mulliken charges, HOMO-LUMO, Electrostatic Potential and Nonlinear Optical Properties
ISSN 1 746-7233, England, UK World Journal of Modelling and Simulation Vol. 14 (2018) No. 1, pp. 3-11 Molecular Structure, Mulliken charges, HOMO-LUMO, Electrostatic Potential and Nonlinear Optical Properties
FT-IR, FT-Raman And UV Spectra and Ab-Initio HF and DFT Vibrational Study of 1-Propyl 4-Piperidone
 FT-IR, FT-Raman And UV Spectra and Ab-Initio HF and DFT Vibrational Study of 1-Propyl 4-Piperidone K. Rajalakshmi 1 and E.Elumalai 2 1 Department of Physics, Sri Chandrasekharendra Saraswathi Viswa Mahavidyalaya,
FT-IR, FT-Raman And UV Spectra and Ab-Initio HF and DFT Vibrational Study of 1-Propyl 4-Piperidone K. Rajalakshmi 1 and E.Elumalai 2 1 Department of Physics, Sri Chandrasekharendra Saraswathi Viswa Mahavidyalaya,
DFT calculations of effective reactive sites of inositol
 Indian Journal of Chemistry Vol. 56A, July 2017, pp. 786-790 DFT calculations of effective reactive sites of inositol D Jeevitha a, K Sadasivam a, * & R Praveena b a Department of Physics, b Department
Indian Journal of Chemistry Vol. 56A, July 2017, pp. 786-790 DFT calculations of effective reactive sites of inositol D Jeevitha a, K Sadasivam a, * & R Praveena b a Department of Physics, b Department
A Eşme* Department of Elementary Science Education, Kocaeli University, Kocaeli 41380, Turkey
 Indian Journal of Pure & Applied Physics Vol. 55, July 2017, pp. 478-489 Quantum chemical calculations on the geometrical, conformational, spectroscopic (FTIR, FT-Raman) analysis and NLO activity of milrinone
Indian Journal of Pure & Applied Physics Vol. 55, July 2017, pp. 478-489 Quantum chemical calculations on the geometrical, conformational, spectroscopic (FTIR, FT-Raman) analysis and NLO activity of milrinone
with the larger dimerization energy also exhibits the larger structural changes.
 A7. Looking at the image and table provided below, it is apparent that the monomer and dimer are structurally almost identical. Although angular and dihedral data were not included, these data are also
A7. Looking at the image and table provided below, it is apparent that the monomer and dimer are structurally almost identical. Although angular and dihedral data were not included, these data are also
Electronegativity is a very useful concept for the explanation or understanding of chemical reactivity throughout the periodic table.
 1.6. Review of Electronegativity (χ) CONCEPT: Electronegativity is a very useful concept for the explanation or understanding of chemical reactivity throughout the periodic table. There are many definitions
1.6. Review of Electronegativity (χ) CONCEPT: Electronegativity is a very useful concept for the explanation or understanding of chemical reactivity throughout the periodic table. There are many definitions
Quantum chemical studies on the structures of some heterocyclic azo disperse dyes
 Quantum chemical studies on the structures of some heterocyclic azo disperse dyes Nesrin Tokay, a* Zeynel Seferoğlu, b Cemil Öğretir, c and Nermin Ertan b a Hacettepe University, Faculty of Science, Chemistry
Quantum chemical studies on the structures of some heterocyclic azo disperse dyes Nesrin Tokay, a* Zeynel Seferoğlu, b Cemil Öğretir, c and Nermin Ertan b a Hacettepe University, Faculty of Science, Chemistry
Trace Solvent as a Predominant Factor to Tune Dipeptide. Self-Assembly
 Trace Solvent as a Predominant Factor to Tune Dipeptide Self-Assembly Juan Wang,, Kai Liu,,, Linyin Yan,, Anhe Wang, Shuo Bai, and Xuehai Yan *,, National Key Laboratory of Biochemical Engineering, Institute
Trace Solvent as a Predominant Factor to Tune Dipeptide Self-Assembly Juan Wang,, Kai Liu,,, Linyin Yan,, Anhe Wang, Shuo Bai, and Xuehai Yan *,, National Key Laboratory of Biochemical Engineering, Institute
Density Functional Theory Investigation of The Physical Properties of Dicyano Pyridazine Molecules
 Density Functional Theory Investigation of The Physical Properties of Dicyano Pyridazine Molecules Fouad N. Ajeel 1, Alaa M. Khudhair 2, Anees A. Mohammed 3 1,2,3 Department of Physics, College of Sciences,
Density Functional Theory Investigation of The Physical Properties of Dicyano Pyridazine Molecules Fouad N. Ajeel 1, Alaa M. Khudhair 2, Anees A. Mohammed 3 1,2,3 Department of Physics, College of Sciences,
Density functional theory study of the Si 2 H 6 x F x series of molecules q
 Chemical Physics Letters 372 (2003) 815 824 www.elsevier.com/locate/cplett Density functional theory study of the Si 2 H 6 x F x series of molecules q Felipe Valencia a, Aldo H. Romero a, Miguel Kiwi b,
Chemical Physics Letters 372 (2003) 815 824 www.elsevier.com/locate/cplett Density functional theory study of the Si 2 H 6 x F x series of molecules q Felipe Valencia a, Aldo H. Romero a, Miguel Kiwi b,
STRUCTURAL DEFECTS IN IMIDATES : AN AB INITIO STUDY
 Int. J. Chem. Sci.: 9(4), 2011, 1763-1767 ISSN 0972-768X www.sadgurupublications.com STRUCTURAL DEFECTS IN IMIDATES : AN AB INITIO STUDY M. FATHIMA BEGUM, HEMA TRESA VARGHESE a, Y. SHEENA MARY a, C. YOHANNAN
Int. J. Chem. Sci.: 9(4), 2011, 1763-1767 ISSN 0972-768X www.sadgurupublications.com STRUCTURAL DEFECTS IN IMIDATES : AN AB INITIO STUDY M. FATHIMA BEGUM, HEMA TRESA VARGHESE a, Y. SHEENA MARY a, C. YOHANNAN
NATURAL BOND ORBITAL (NBO) POPULATION ANALYSIS OF AN ENERGETIC MOLECULE 1-PHENYL-2- NITROGUANIDINE
 Int. J. Chem. Sci.: 14(4), 2016, 2029-2050 ISSN 0972-768X www.sadgurupublications.com NATURAL BOND ORBITAL (NBO) POPULATION ANALYSIS OF AN ENERGETIC MOLECULE 1-PHENYL-2- NITROGUANIDINE CHINNIAGOUNDER THEIVARASU
Int. J. Chem. Sci.: 14(4), 2016, 2029-2050 ISSN 0972-768X www.sadgurupublications.com NATURAL BOND ORBITAL (NBO) POPULATION ANALYSIS OF AN ENERGETIC MOLECULE 1-PHENYL-2- NITROGUANIDINE CHINNIAGOUNDER THEIVARASU
Andrew Zahrt Denmark Group Meeting
 Andrew Zahrt Denmark Group Meeting 4.28.15 Introduction Early Publications/Hypotheses: Thermodynamic Product Stability Ground State Destabilization Transition State Stabilization Solvent Effects Gas Phase
Andrew Zahrt Denmark Group Meeting 4.28.15 Introduction Early Publications/Hypotheses: Thermodynamic Product Stability Ground State Destabilization Transition State Stabilization Solvent Effects Gas Phase
International Journal of Materials Science ISSN Volume 12, Number 2 (2017) Research India Publications
 HF, DFT Computations and Spectroscopic study of Vibrational frequency, HOMO-LUMO Analysis and Thermodynamic Properties of Alpha Bromo Gamma Butyrolactone K. Rajalakshmi 1 and A.Susila 2 1 Department of
HF, DFT Computations and Spectroscopic study of Vibrational frequency, HOMO-LUMO Analysis and Thermodynamic Properties of Alpha Bromo Gamma Butyrolactone K. Rajalakshmi 1 and A.Susila 2 1 Department of
( ) R kj. = y k y j. y A ( ) z A. y a. z a. Derivatives of the second order electrostatic tensor with respect to the translation of ( ) δ yβ.
 Supporting information Derivatives of R with respect to the translation of fragment along the y and z axis: y = y k y j (S1) z ( = z z k j) (S2) Derivatives of S with respect to the translation of fragment
Supporting information Derivatives of R with respect to the translation of fragment along the y and z axis: y = y k y j (S1) z ( = z z k j) (S2) Derivatives of S with respect to the translation of fragment
Journal of Chemical and Pharmaceutical Research, 2015, 7(2): Research Article
 Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(2):641-645 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Synthesis, characterization and theoretical study
Available online www.jocpr.com Journal of Chemical and Pharmaceutical Research, 2015, 7(2):641-645 Research Article ISSN : 0975-7384 CODEN(USA) : JCPRC5 Synthesis, characterization and theoretical study
Journal of Physical and Theoretical Chemistry
 Journal of Physical and Theoretical Chemistry of Islamic Azad University of Iran, 11 (1) 1-13: Spring 214 (J. Phys. Theor. Chem. IAU Iran) ISSN 1735-2126 Investigation of relationship with electron configuration
Journal of Physical and Theoretical Chemistry of Islamic Azad University of Iran, 11 (1) 1-13: Spring 214 (J. Phys. Theor. Chem. IAU Iran) ISSN 1735-2126 Investigation of relationship with electron configuration
Electronic structure and reactivity analysis of some TTF-donor substituted molecules
 Available online at www.scholarsresearchlibrary.com cholars esearch Library Der Pharmacia Lettre, 2016, 8 (4):151-160 (http://scholarsresearchlibrary.com/archive.html) IN 0975-5071 UA CODEN: DPLEB4 Electronic
Available online at www.scholarsresearchlibrary.com cholars esearch Library Der Pharmacia Lettre, 2016, 8 (4):151-160 (http://scholarsresearchlibrary.com/archive.html) IN 0975-5071 UA CODEN: DPLEB4 Electronic
arxiv:cond-mat/ v1 [cond-mat.mtrl-sci] 23 Jul 2002
![arxiv:cond-mat/ v1 [cond-mat.mtrl-sci] 23 Jul 2002 arxiv:cond-mat/ v1 [cond-mat.mtrl-sci] 23 Jul 2002](/thumbs/94/118745427.jpg) arxiv:cond-mat/0207554v1 [cond-mat.mtrl-sci] 23 Jul 2002 Internal Rotation of Disilane and Related Molecules: a Density Functional Study 1 Abstract Felipe Valencia a Aldo H. Romero a Miguel Kiwi a Ricardo
arxiv:cond-mat/0207554v1 [cond-mat.mtrl-sci] 23 Jul 2002 Internal Rotation of Disilane and Related Molecules: a Density Functional Study 1 Abstract Felipe Valencia a Aldo H. Romero a Miguel Kiwi a Ricardo
NON-LINEAR OPTICAL PROPERTIES STUDY OF TWO HETEROCYCLIC COMPOUNDS ABSTRACT
 Original Research Article Chemistry ISSN 2250-0480 NON-LINEAR OPTICAL PROPERTIES STUDY OF TWO HETEROCYCLIC COMPOUNDS V.T. MUTTANNAVAR a, RAVEENDRA. MELAVANKI b*, P. BHAVYA c, RAVIRAJ KUSANUR d, N. R. PATIL
Original Research Article Chemistry ISSN 2250-0480 NON-LINEAR OPTICAL PROPERTIES STUDY OF TWO HETEROCYCLIC COMPOUNDS V.T. MUTTANNAVAR a, RAVEENDRA. MELAVANKI b*, P. BHAVYA c, RAVIRAJ KUSANUR d, N. R. PATIL
Why Is Molecular Interaction Important in Our Life
 Why Is Molecular Interaction Important in ur Life QuLiS and Graduate School of Science iroshima University http://www.nabit.hiroshima-u.ac.jp/iwatasue/indexe.htm Suehiro Iwata Sept. 29, 2007 Department
Why Is Molecular Interaction Important in ur Life QuLiS and Graduate School of Science iroshima University http://www.nabit.hiroshima-u.ac.jp/iwatasue/indexe.htm Suehiro Iwata Sept. 29, 2007 Department
Atomic and molecular interaction forces in biology
 Atomic and molecular interaction forces in biology 1 Outline Types of interactions relevant to biology Van der Waals interactions H-bond interactions Some properties of water Hydrophobic effect 2 Types
Atomic and molecular interaction forces in biology 1 Outline Types of interactions relevant to biology Van der Waals interactions H-bond interactions Some properties of water Hydrophobic effect 2 Types
FT-IR, FT-Raman, NMR and FMO Analysis of 3-(2- Furyl) Propionic Acid By Quantum Chemical Calculations
 FT-IR, FT-Raman, NMR and FMO Analysis of 3-(2- Furyl) Propionic Acid By Quantum Chemical Calculations G. Velraj, S. Ramachandran, and T. Kavitha* Department of Physics, Periyar University Salem-636 011,
FT-IR, FT-Raman, NMR and FMO Analysis of 3-(2- Furyl) Propionic Acid By Quantum Chemical Calculations G. Velraj, S. Ramachandran, and T. Kavitha* Department of Physics, Periyar University Salem-636 011,
Modes of Neighbouring Group Participation by the Methyl Selenyl Substituent in β-methylselenylmethyl-substituted 1-Phenylethyl Carbenium Ions
 Molecules 2013, 18, 11705-11711; doi:10.3390/molecules181011705 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Article Modes of Neighbouring Group Participation by the Methyl Selenyl
Molecules 2013, 18, 11705-11711; doi:10.3390/molecules181011705 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Article Modes of Neighbouring Group Participation by the Methyl Selenyl
QUANTITATIVE STRUCTURE ACTIVITY RELATIONSHIPSOFSOME ATENOLOL AND PROPRANOLOLDERIVATIVES
 Vol. 9 No. 2 189 202 April June 2016 ISSN: 09741496 eissn: 09760083 CODEN: RJCABP http://www.rasayanjournal.com http://www.rasayanjournal.co.in QUANTITATIVE STRUCTURE ACTIVITY RELATIONSHIPSOFSOME ATENOLOL
Vol. 9 No. 2 189 202 April June 2016 ISSN: 09741496 eissn: 09760083 CODEN: RJCABP http://www.rasayanjournal.com http://www.rasayanjournal.co.in QUANTITATIVE STRUCTURE ACTIVITY RELATIONSHIPSOFSOME ATENOLOL
Journal of Computational Methods in Molecular Design, 2013, 3 (1):1-8. Scholars Research Library (
 Journal of Computational Methods in Molecular Design, 2013, 3 (1):1-8 Scholars Research Library (http://scholarsresearchlibrary.com/archive.html) ISSN : 2231-3176 CODEN (USA): JCMMDA Theoretical study
Journal of Computational Methods in Molecular Design, 2013, 3 (1):1-8 Scholars Research Library (http://scholarsresearchlibrary.com/archive.html) ISSN : 2231-3176 CODEN (USA): JCMMDA Theoretical study
IR, Raman, First Hyperpolarizability and Computational Study of 1-chloroethyl Benzene
 Material Science Research India Vol. 9(1), 117-121 (2012) IR, Raman, First Hyperpolarizability and Computational Study of 1-chloroethyl Benzene HEMA TRESA VARGHESE¹, C.YOHANNAN PANICKER²* and SHEENA MARY
Material Science Research India Vol. 9(1), 117-121 (2012) IR, Raman, First Hyperpolarizability and Computational Study of 1-chloroethyl Benzene HEMA TRESA VARGHESE¹, C.YOHANNAN PANICKER²* and SHEENA MARY
DFT STUDY OF Co 2+ AND Fe 2+ - URACIL COMPLEXES IN THE GAS PHASE
 INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article DFT STUDY OF Co 2+ AND Fe 2+ - URACIL COMPLEXES IN THE GAS PHASE Raghab Parajuli* Department
INTERNATIONAL JOURNAL OF RESEARCH IN PHARMACY AND CHEMISTRY Available online at www.ijrpc.com Research Article DFT STUDY OF Co 2+ AND Fe 2+ - URACIL COMPLEXES IN THE GAS PHASE Raghab Parajuli* Department
Molecular Orbital Theory. Molecular Orbital Theory: Electrons are located in the molecule, not held in discrete regions between two bonded atoms
 Molecular Orbital Theory Valence Bond Theory: Electrons are located in discrete pairs between specific atoms Molecular Orbital Theory: Electrons are located in the molecule, not held in discrete regions
Molecular Orbital Theory Valence Bond Theory: Electrons are located in discrete pairs between specific atoms Molecular Orbital Theory: Electrons are located in the molecule, not held in discrete regions
Quantum chemical calculations of Cr 2 O 3 /SnO 2 using density functional theory method
 Pramana J. Phys. (2018) 90:38 https://doi.org/10.1007/s12043-018-1526-0 Indian Academy of Sciences Quantum chemical calculations of Cr 2 O 3 /SnO 2 using density functional theory method K RACKESH JAWAHER
Pramana J. Phys. (2018) 90:38 https://doi.org/10.1007/s12043-018-1526-0 Indian Academy of Sciences Quantum chemical calculations of Cr 2 O 3 /SnO 2 using density functional theory method K RACKESH JAWAHER
ASIAN JOURNAL OF CHEMISTRY
 Asian Journal of Chemistry; Vol. 3, No. 1 (218), 2255-2263 ASIAN JOURNAL OF CHEMISTRY https://doi.org/1.14233/ajchem.218.21419 Simulation of Structure, Molecular Orbitals, Electron Density and Reactivity
Asian Journal of Chemistry; Vol. 3, No. 1 (218), 2255-2263 ASIAN JOURNAL OF CHEMISTRY https://doi.org/1.14233/ajchem.218.21419 Simulation of Structure, Molecular Orbitals, Electron Density and Reactivity
Excited States Calculations for Protonated PAHs
 52 Chapter 3 Excited States Calculations for Protonated PAHs 3.1 Introduction Protonated PAHs are closed shell ions. Their electronic structure should therefore be similar to that of neutral PAHs, but
52 Chapter 3 Excited States Calculations for Protonated PAHs 3.1 Introduction Protonated PAHs are closed shell ions. Their electronic structure should therefore be similar to that of neutral PAHs, but
Quantum Chemical Designing of Novel Organic Non-Linear Optical Compounds
 Quantum Chemical Designing of Novel NLO Compounds Bull. Korean Chem. Soc. 2014, Vol. 35, No. 5 1391 http://dx.doi.org/10.5012/bkcs.2014.35.5.1391 Quantum Chemical Designing of Novel Organic Non-Linear
Quantum Chemical Designing of Novel NLO Compounds Bull. Korean Chem. Soc. 2014, Vol. 35, No. 5 1391 http://dx.doi.org/10.5012/bkcs.2014.35.5.1391 Quantum Chemical Designing of Novel Organic Non-Linear
EJL R Sβ. sum. General objective: Reduce the complexity of the analysis by exploiting symmetry. Garth J. Simpson
 e sum = EJL R Sβ General objective: Reduce the complexity of the analysis by exploiting symmetry. Specific Objectives: 1. The molecular symmetry matrix S. How to populate it.. Relationships between the
e sum = EJL R Sβ General objective: Reduce the complexity of the analysis by exploiting symmetry. Specific Objectives: 1. The molecular symmetry matrix S. How to populate it.. Relationships between the
Truong Ba Tai, Long Van Duong, Hung Tan Pham, Dang Thi Tuyet Mai and Minh Tho Nguyen*
 Supplementary Information: A Disk-Aromatic Bowl Cluster B 30 : Towards Formation of Boron Buckyballs Truong Ba Tai, Long Van Duong, Hung Tan Pham, Dang Thi Tuyet Mai and Minh Tho Nguyen* The file contains
Supplementary Information: A Disk-Aromatic Bowl Cluster B 30 : Towards Formation of Boron Buckyballs Truong Ba Tai, Long Van Duong, Hung Tan Pham, Dang Thi Tuyet Mai and Minh Tho Nguyen* The file contains
Are the Bader Laplacian and the Bohm Quantum Potential Equivalent?
 Are the Bader Laplacian and the Bohm Quantum Potential Equivalent? Creon Levit & Jack Sarfatti NASA Ames Research Center, creon@nas.nasa.gov Internet Science Eductaion Project, sarfatti@well.com ABSTRACT
Are the Bader Laplacian and the Bohm Quantum Potential Equivalent? Creon Levit & Jack Sarfatti NASA Ames Research Center, creon@nas.nasa.gov Internet Science Eductaion Project, sarfatti@well.com ABSTRACT
Spectroscopic properties of dipicolinic acid and its dianion
 Chemical Physics 322 (2006) 254 268 www.elsevier.com/locate/chemphys Spectroscopic properties of dipicolinic acid and its dianion John Rui-Hua Xie a, Vedene H. Smith Jr. b, Roland E. Allen a, * a Department
Chemical Physics 322 (2006) 254 268 www.elsevier.com/locate/chemphys Spectroscopic properties of dipicolinic acid and its dianion John Rui-Hua Xie a, Vedene H. Smith Jr. b, Roland E. Allen a, * a Department
Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory
 Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory Electronic Structure of Six and Four-Coordinate Complexes Using Crystal Field Theory, we can generate energy level
Chemistry 3211 Coordination Chemistry Part 3 Ligand Field and Molecular Orbital Theory Electronic Structure of Six and Four-Coordinate Complexes Using Crystal Field Theory, we can generate energy level
Electronic structures of one-dimension carbon nano wires and rings
 IOP Publishing Journal of Physics: Conference Series 61 (2007) 252 256 doi:10.1088/1742-6596/61/1/051 International Conference on Nanoscience and Technology (ICN&T 2006) Electronic structures of one-dimension
IOP Publishing Journal of Physics: Conference Series 61 (2007) 252 256 doi:10.1088/1742-6596/61/1/051 International Conference on Nanoscience and Technology (ICN&T 2006) Electronic structures of one-dimension
2x (x 2 + y 2 + 1) 2 2y. (x 2 + y 2 + 1) 4. 4xy. (1, 1)(x 1) + (1, 1)(y + 1) (1, 1)(x 1)(y + 1) 81 x y y + 7.
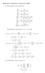 Homework 8 Solutions, November 007. (1 We calculate some derivatives: f x = f y = x (x + y + 1 y (x + y + 1 x = (x + y + 1 4x (x + y + 1 4 y = (x + y + 1 4y (x + y + 1 4 x y = 4xy (x + y + 1 4 Substituting
Homework 8 Solutions, November 007. (1 We calculate some derivatives: f x = f y = x (x + y + 1 y (x + y + 1 x = (x + y + 1 4x (x + y + 1 4 y = (x + y + 1 4y (x + y + 1 4 x y = 4xy (x + y + 1 4 Substituting
XRD, FT-IR, Electronic and Fluorescence Spectroscopic Studies of Benzothiophenesulfone-2-methanol
 XRD, FT-IR, Electronic and Fluorescence Spectroscopic Studies of Benzothiophenesulfone-2-methanol 1 Katta Eswar Srikanth, 2 K. Anand Solomon 1 A. Veeraiah 1 Molecular Spectroscopy Laboratory, Department
XRD, FT-IR, Electronic and Fluorescence Spectroscopic Studies of Benzothiophenesulfone-2-methanol 1 Katta Eswar Srikanth, 2 K. Anand Solomon 1 A. Veeraiah 1 Molecular Spectroscopy Laboratory, Department
Bottlenecks in the prediction of regioselectivity of [4 + 2] cycloaddition reactions: An assessment of reactivity descriptors
![Bottlenecks in the prediction of regioselectivity of [4 + 2] cycloaddition reactions: An assessment of reactivity descriptors Bottlenecks in the prediction of regioselectivity of [4 + 2] cycloaddition reactions: An assessment of reactivity descriptors](/thumbs/95/124122018.jpg) J. Chem. Sci., Vol. 117, No. 5, September 2005, pp. 573 582. Indian Academy of Sciences. Bottlenecks in the prediction of regioselectivity of [4 + 2] cycloaddition reactions: An assessment of reactivity
J. Chem. Sci., Vol. 117, No. 5, September 2005, pp. 573 582. Indian Academy of Sciences. Bottlenecks in the prediction of regioselectivity of [4 + 2] cycloaddition reactions: An assessment of reactivity
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer. Lecture 31, April 12, 2006
 Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 31, April 12, 2006 (Some material in this lecture has been adapted from Cramer, C.
Chem 3502/4502 Physical Chemistry II (Quantum Mechanics) 3 Credits Spring Semester 2006 Christopher J. Cramer Lecture 31, April 12, 2006 (Some material in this lecture has been adapted from Cramer, C.
Dispersion Interactions from the Exchange-Hole Dipole Moment
 Dispersion Interactions from the Exchange-Hole Dipole Moment Erin R. Johnson and Alberto Otero-de-la-Roza Chemistry and Chemical Biology, University of California, Merced E. R. Johnson (UC Merced) Dispersion
Dispersion Interactions from the Exchange-Hole Dipole Moment Erin R. Johnson and Alberto Otero-de-la-Roza Chemistry and Chemical Biology, University of California, Merced E. R. Johnson (UC Merced) Dispersion
Organic Chemistry 6 th Edition Paula Yurkanis Bruice. Chapter 1. Electronic Structure and Bonding. Acids and Bases Pearson Education, Inc.
 Organic Chemistry 6 th Edition Paula Yurkanis Bruice Chapter 1 Electronic Structure and Bonding Acids and Bases 2011 Pearson Education, Inc. 1 Organic Chemistry Carbon-containing compounds were once considered
Organic Chemistry 6 th Edition Paula Yurkanis Bruice Chapter 1 Electronic Structure and Bonding Acids and Bases 2011 Pearson Education, Inc. 1 Organic Chemistry Carbon-containing compounds were once considered
Electronic structure theory: Fundamentals to frontiers. VI. Analysis and more.
 Electronic structure theory: Fundamentals to frontiers. VI. Analysis and more. MARTIN HEAD-GORDON Department of Chemistry, University of California, Berkeley, and, Chemical Sciences Division, Lawrence
Electronic structure theory: Fundamentals to frontiers. VI. Analysis and more. MARTIN HEAD-GORDON Department of Chemistry, University of California, Berkeley, and, Chemical Sciences Division, Lawrence
CHAPTER INTRODUCTION
 CHAPTER 3 A SCALED QUANTUM MECHANICAL APPROACH OF VIBRATIONAL ANALYSIS OF O-TOLUNITRILE BASED ON FTIR AND FT RAMAN SPECTRA, AB INITIO, HARTREE FOCK AND DFT METHODS 3.1. INTRODUCTION o-tolunitrile or ortho
CHAPTER 3 A SCALED QUANTUM MECHANICAL APPROACH OF VIBRATIONAL ANALYSIS OF O-TOLUNITRILE BASED ON FTIR AND FT RAMAN SPECTRA, AB INITIO, HARTREE FOCK AND DFT METHODS 3.1. INTRODUCTION o-tolunitrile or ortho
Hyperconjugative Effect on the Electronic Wavefunctions of Ethanol. University of Science and Technology of China, Hefei, , China
 Hyperconjugative Effect on the Electronic Wavefunctions of Ethanol Xiangjun Chen, 1,2,a) Fang Wu, 1,2 Mi Yan, 1,2 Hai-Bei Li, 1,3 Shan Xi Tian, 1,3,a) Xu Shan, 1,2 Kedong Wang, 1,2 Zhongjun Li, 1,2 and
Hyperconjugative Effect on the Electronic Wavefunctions of Ethanol Xiangjun Chen, 1,2,a) Fang Wu, 1,2 Mi Yan, 1,2 Hai-Bei Li, 1,3 Shan Xi Tian, 1,3,a) Xu Shan, 1,2 Kedong Wang, 1,2 Zhongjun Li, 1,2 and
Quantum Mechanical Study on the Adsorption of Drug Gentamicin onto γ-fe 2
 ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-00 X CODEN: OJCHEG 015, Vol. 31, No. (3): Pg. 1509-1513 Quantum Mechanical
ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-00 X CODEN: OJCHEG 015, Vol. 31, No. (3): Pg. 1509-1513 Quantum Mechanical
Supporting information for: First hyperpolarizability of collagen using the. point dipole approximation
 Supporting information for: First hyperpolarizability of collagen using the point dipole approximation Ignat Harczuk, Olav Vahtras, and Hans Ågren KTH Royal Institute of Technology, School of Biotechnology,
Supporting information for: First hyperpolarizability of collagen using the point dipole approximation Ignat Harczuk, Olav Vahtras, and Hans Ågren KTH Royal Institute of Technology, School of Biotechnology,
Supporting Information
 Supporting Information Ionic Liquid Designed for PEDOT:PSS Conductivity Enhancement Ambroise de Izarra, a,b,1 Seongjin Park, a,1 Jinhee Lee, a Yves Lansac, b,c, * Yun Hee Jang a, * a Department of Energy
Supporting Information Ionic Liquid Designed for PEDOT:PSS Conductivity Enhancement Ambroise de Izarra, a,b,1 Seongjin Park, a,1 Jinhee Lee, a Yves Lansac, b,c, * Yun Hee Jang a, * a Department of Energy
CHAPTER-IV. FT-IR and FT-Raman investigation on m-xylol using ab-initio HF and DFT calculations
 4.1. Introduction CHAPTER-IV FT-IR and FT-Raman investigation on m-xylol using ab-initio HF and DFT calculations m-xylol is a material for thermally stable aramid fibers or alkyd resins [1]. In recent
4.1. Introduction CHAPTER-IV FT-IR and FT-Raman investigation on m-xylol using ab-initio HF and DFT calculations m-xylol is a material for thermally stable aramid fibers or alkyd resins [1]. In recent
Supplementary Information
 Supplementary Information S1. Energy Minimization and ECD Calculations. Figure S1. HRESIMS spectrum of compound 1. Figure S2. IR spectrum of compound 1. Figure S3. 1 H NMR spectrum of compound 1 in CDCl
Supplementary Information S1. Energy Minimization and ECD Calculations. Figure S1. HRESIMS spectrum of compound 1. Figure S2. IR spectrum of compound 1. Figure S3. 1 H NMR spectrum of compound 1 in CDCl
Theoretical and Experimental Electrostatic Potential around the m-nitrophenol Molecule
 Molecules 2015, 20, 4042-4054; doi:10.3390/molecules20034042 Article OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Theoretical and Experimental Electrostatic Potential around the
Molecules 2015, 20, 4042-4054; doi:10.3390/molecules20034042 Article OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Theoretical and Experimental Electrostatic Potential around the
Comparison of molecular structure of alkali metal ortho substituted benzoates
 Spectroscopy 24 (2010) 439 443 439 DOI 10.3233/SPE-2010-0444 IOS Press Comparison of molecular structure of alkali metal ortho substituted benzoates R. Świsłocka Department of Chemistry, Biatystok Technical
Spectroscopy 24 (2010) 439 443 439 DOI 10.3233/SPE-2010-0444 IOS Press Comparison of molecular structure of alkali metal ortho substituted benzoates R. Świsłocka Department of Chemistry, Biatystok Technical
New Bisphosphomide Ligands, 1,3-Phenylenebis((diphenylphosphino)methanone) [1,3-
 New Bisphosphomide Ligands, 1,3-Phenylenebis((diphenylphosphino)methanone) [1,3- Ph 2 PC(O)} 2 C 6 H 4 ] and (2-Bromo-1,3-phenylene)bis((diphenylphosphino)methanone) [1,3- Ph 2 PC(O)} 2 C 6 H 4 Br]: Synthesis,
New Bisphosphomide Ligands, 1,3-Phenylenebis((diphenylphosphino)methanone) [1,3- Ph 2 PC(O)} 2 C 6 H 4 ] and (2-Bromo-1,3-phenylene)bis((diphenylphosphino)methanone) [1,3- Ph 2 PC(O)} 2 C 6 H 4 Br]: Synthesis,
The wavefunction that describes a bonding pair of electrons:
 4.2. Molecular Properties from VB Theory a) Bonding and Bond distances The wavefunction that describes a bonding pair of electrons: Ψ b = a(h 1 ) + b(h 2 ) where h 1 and h 2 are HAOs on adjacent atoms
4.2. Molecular Properties from VB Theory a) Bonding and Bond distances The wavefunction that describes a bonding pair of electrons: Ψ b = a(h 1 ) + b(h 2 ) where h 1 and h 2 are HAOs on adjacent atoms
ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal.
 ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2014, Vol. 30, No. (1): Pg. 345-350 DFT Study on 4(5)-Imidazole-carbaldehyde-N(5)-
ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2014, Vol. 30, No. (1): Pg. 345-350 DFT Study on 4(5)-Imidazole-carbaldehyde-N(5)-
Natural Bond Orbital Analysis of the Bonding in Complexes of Li with Ammonia
 Research Journal of Chemical Sciences ISSN 2231-606X Natural Bond Orbital Analysis of the Bonding in Complexes of Li with Ammonia Françoise Diendere *, Issaka Guiguemde and Abdouraman Bary Laboratoire
Research Journal of Chemical Sciences ISSN 2231-606X Natural Bond Orbital Analysis of the Bonding in Complexes of Li with Ammonia Françoise Diendere *, Issaka Guiguemde and Abdouraman Bary Laboratoire
A theoretical investigation by DFT method monomer that is a plastic polymer commonly used in the manufacture of eyeglass lenses
 American Journal of Optics and Photonics 014; (1): 7-11 Published online March 0, 014 (http://www.sciencepublishinggroup.com/j/ajop) doi: 10.11648/j.ajop.014001.1 A theoretical investigation by DFT method
American Journal of Optics and Photonics 014; (1): 7-11 Published online March 0, 014 (http://www.sciencepublishinggroup.com/j/ajop) doi: 10.11648/j.ajop.014001.1 A theoretical investigation by DFT method
Natural orbital Fukui function and application in understanding cycloaddition reaction mechanisms
 Electronic Supplementary Material (ESI) or Physical Chemistry Chemical Physics atural orbital Fukui unction and application in understanding cycloaddition reaction mechanisms Panpan Zhou, a Paul W. Ayers,
Electronic Supplementary Material (ESI) or Physical Chemistry Chemical Physics atural orbital Fukui unction and application in understanding cycloaddition reaction mechanisms Panpan Zhou, a Paul W. Ayers,
Visualization in Chemistry. Giovanni Morelli
 Visualization in Chemistry Giovanni Morelli What is Chemistry? If we provide a good answer to this question maybe we can understand what graphics can do for chemistry and chemists What is Chemistry? A
Visualization in Chemistry Giovanni Morelli What is Chemistry? If we provide a good answer to this question maybe we can understand what graphics can do for chemistry and chemists What is Chemistry? A
Chapter 5. Molecular Orbitals
 Chapter 5. Molecular Orbitals MO from s, p, d, orbitals: - Fig.5.1, 5.2, 5.3 Homonuclear diatomic molecules: - Fig. 5.7 - Para- vs. Diamagnetic Heteronuclear diatomic molecules: - Fig. 5.14 - ex. CO Hybrid
Chapter 5. Molecular Orbitals MO from s, p, d, orbitals: - Fig.5.1, 5.2, 5.3 Homonuclear diatomic molecules: - Fig. 5.7 - Para- vs. Diamagnetic Heteronuclear diatomic molecules: - Fig. 5.14 - ex. CO Hybrid
DFT study on structure, electronic properties, and reactivity of cis-isomers of [(NC 5 H 4 -S) 2 Fe(CO) 2 ]
![DFT study on structure, electronic properties, and reactivity of cis-isomers of [(NC 5 H 4 -S) 2 Fe(CO) 2 ] DFT study on structure, electronic properties, and reactivity of cis-isomers of [(NC 5 H 4 -S) 2 Fe(CO) 2 ]](/thumbs/92/109989430.jpg) J. Chem. Sci. Vol. 123, No. 5, September 2011, pp. 727 731. c Indian Academy of Sciences. DFT study on structure, electronic properties, and reactivity of cis-isomers of [(NC 5 H 4 -S) 2 Fe(CO) 2 ] CHARLES
J. Chem. Sci. Vol. 123, No. 5, September 2011, pp. 727 731. c Indian Academy of Sciences. DFT study on structure, electronic properties, and reactivity of cis-isomers of [(NC 5 H 4 -S) 2 Fe(CO) 2 ] CHARLES
Computer Laboratory DFT and Frequencies
 Computer Laboratory DFT and Frequencies 1. DFT KEYWORDS FOR DFT METHODS Names for the various pure DFT models are given by combining the names for the exchange and correlation functionals. In some cases,
Computer Laboratory DFT and Frequencies 1. DFT KEYWORDS FOR DFT METHODS Names for the various pure DFT models are given by combining the names for the exchange and correlation functionals. In some cases,
Supporting Information for. Predicting the Stability of Fullerene Allotropes Throughout the Periodic Table MA 02139
 Supporting Information for Predicting the Stability of Fullerene Allotropes Throughout the Periodic Table Qing Zhao 1, 2, Stanley S. H. Ng 1, and Heather J. Kulik 1, * 1 Department of Chemical Engineering,
Supporting Information for Predicting the Stability of Fullerene Allotropes Throughout the Periodic Table Qing Zhao 1, 2, Stanley S. H. Ng 1, and Heather J. Kulik 1, * 1 Department of Chemical Engineering,
Internal rotation of disilane and related molecules: a density functional study
 Chemical Physics Letters 371 (2003) 267 275 www.elsevier.com/locate/cplett Internal rotation of disilane and related molecules: a density functional study Felipe Valencia a,b, Aldo H. Romero a,b, Miguel
Chemical Physics Letters 371 (2003) 267 275 www.elsevier.com/locate/cplett Internal rotation of disilane and related molecules: a density functional study Felipe Valencia a,b, Aldo H. Romero a,b, Miguel
Notes. Rza Abbasoglu*, Abdurrahman Atalay & Ahmet Şenocak. Indian Journal of Chemistry Vol. 53A, March, 2014, pp
 Indian Journal of Chemistry Vol. 53A, March, 2014, pp. 288-293 Notes DFT investigations of the Diels-Alder reaction of fulvene with tetracyclo[6.2.2.1 3,6.0 2,7 ]trideca-4,9,11-triene- 9,10-dicarboxylic
Indian Journal of Chemistry Vol. 53A, March, 2014, pp. 288-293 Notes DFT investigations of the Diels-Alder reaction of fulvene with tetracyclo[6.2.2.1 3,6.0 2,7 ]trideca-4,9,11-triene- 9,10-dicarboxylic
ELECTRON FLOW IN ORGANIC CHEMISTR Y. Paul H. Scudder
 ELECTRON FLOW IN ORGANIC CHEMISTR Y Paul H. Scudder 1 BONDING AND ELECTRON DISTRIBUTION 1 2 THE PROCESS OF BOND FORMATION 3 4 3 STABILITY AND REACTIVITY OF INTERMEDIATES 5 6 4 CLASSIFICATION OF ELECTRON
ELECTRON FLOW IN ORGANIC CHEMISTR Y Paul H. Scudder 1 BONDING AND ELECTRON DISTRIBUTION 1 2 THE PROCESS OF BOND FORMATION 3 4 3 STABILITY AND REACTIVITY OF INTERMEDIATES 5 6 4 CLASSIFICATION OF ELECTRON
FT-IR, FT-Raman and Computational Study of p-acetylbenzonitrile
 Est. 1984 ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2013, Vol. 29, No. (1): Pg. 291-296 FT-IR, FT-Raman
Est. 1984 ORIENTAL JOURNAL OF CHEMISTRY An International Open Free Access, Peer Reviewed Research Journal www.orientjchem.org ISSN: 0970-020 X CODEN: OJCHEG 2013, Vol. 29, No. (1): Pg. 291-296 FT-IR, FT-Raman
A density functional study on equilibrium geometries, stabilities and electronic properties of Au 5 Li binary clusters
 Appl Nanosci (2012) 2:359 364 DOI 10.1007/s13204-012-0092-x ORIGINAL ARTICLE A density functional study on equilibrium geometries, stabilities and electronic properties of Au 5 Li binary clusters Ajanta
Appl Nanosci (2012) 2:359 364 DOI 10.1007/s13204-012-0092-x ORIGINAL ARTICLE A density functional study on equilibrium geometries, stabilities and electronic properties of Au 5 Li binary clusters Ajanta
Analysis of Permanent Electric Dipole Moments of Aliphatic Amines.
 Analysis of Permanent Electric Dipole Moments of Aliphatic Amines. Boris Lakard* LPUB, UMR CNRS 5027, University of Bourgogne, F-21078, Dijon, France Internet Electronic Conference of Molecular Design
Analysis of Permanent Electric Dipole Moments of Aliphatic Amines. Boris Lakard* LPUB, UMR CNRS 5027, University of Bourgogne, F-21078, Dijon, France Internet Electronic Conference of Molecular Design
The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then
 1 The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then filled with the available electrons according to
1 The symmetry properties & relative energies of atomic orbitals determine how they react to form molecular orbitals. These molecular orbitals are then filled with the available electrons according to
Macrocyclic Oligofurans: A Computational Study
 Supporting Information Macrocyclic Oligofurans: A Computational Study Or Dishi and Ori Gidron* Institute of Chemistry, The Hebrew University of Jerusalem, Edmond J. Safra Campus, Givat Ram, Jerusalem,
Supporting Information Macrocyclic Oligofurans: A Computational Study Or Dishi and Ori Gidron* Institute of Chemistry, The Hebrew University of Jerusalem, Edmond J. Safra Campus, Givat Ram, Jerusalem,
Quantum Chemical Simulations and Descriptors. Dr. Antonio Chana, Dr. Mosè Casalegno
 Quantum Chemical Simulations and Descriptors Dr. Antonio Chana, Dr. Mosè Casalegno Classical Mechanics: basics It models real-world objects as point particles, objects with negligible size. The motion
Quantum Chemical Simulations and Descriptors Dr. Antonio Chana, Dr. Mosè Casalegno Classical Mechanics: basics It models real-world objects as point particles, objects with negligible size. The motion
Chemistry III (Organic): An Introduction to Reaction Stereoelectronics. LECTURE 1 Recap of Key Stereoelectronic Principles
 1 Chemistry III (Organic): An Introduction to Reaction Stereoelectronics LCTUR 1 Recap of Key Stereoelectronic Principles Alan C. Spivey a.c.spivey@imperial.ac.uk Nov 2015 2 Format & scope of lecture 1
1 Chemistry III (Organic): An Introduction to Reaction Stereoelectronics LCTUR 1 Recap of Key Stereoelectronic Principles Alan C. Spivey a.c.spivey@imperial.ac.uk Nov 2015 2 Format & scope of lecture 1
Journal of Chemical and Pharmaceutical Research
 Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 0975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 2011, 3(4): 589-595 Altering the electronic properties of adamantane
Available on line www.jocpr.com Journal of Chemical and Pharmaceutical Research ISSN No: 0975-7384 CODEN(USA): JCPRC5 J. Chem. Pharm. Res., 2011, 3(4): 589-595 Altering the electronic properties of adamantane
